Note: Descriptions are shown in the official language in which they were submitted.
CA 02535638 1999-10-18
APPARATUS AND PROCESS FOR MAKING
A CORRUGATION-FREE FOAM
This application is a divisional application of Canadian Patent Application
2,286,450 filed on October 18, 1999.
Field of the Invention
The present invention is directed to a process and an apparatus for producing
a
corrugation-free foam. Specifically, the foaming process includes the
injection and
mixing of a predominantly carbon dioxide physical blowing agent into a
foamable
extrudate and extruding the mixture through an annular die and contacting a
restrictive
surface within a critical time after the foam exits.
Background of the Invention
Low density polymeric foam, such as polystyrene foam, is conventionally made
by combining a physical blowing agent with a molten polymeric mixture under
pressure
and, after thorough mixing, extruding the combination through an appropriate
die into a
lower pressure atmosphere. This type of foam is commonly used to manufacture
plates,
bowls, cups and like items.
From about the 1950's to the present, physical blowing agents of choice have
included halocarbons, hydrocarbons, specific atmospheric gases, or
combinations thereof.
Examples of the halocarbons include commercially available halocarbon
compositions
such as dichlorodifluoromethane (CFC-12), trichlorofluoromethane (CFC-I 1 )
and
mixtures thereof. Examples of the hydrocarbon blowing agents are the C2-C6
alkanes
such as ethane, propane, butane, isobutane, pentane, isopentane, and hexane.
Examples
of the specific atmospheric gases are carbon dioxide and argon.
During the 1980's, the worldwide scientific community presented evidence
linking
halocarbons containing halogens other than fluorine, such as
chlorofluorocarbons (CFCs)
and hydrofluorocarbons (HCFCs) with atmospheric ozone depletion. Consequently,
governments sought to regulate CFCs and HCFCs. As a result of such
regulations,
manufacturers of extruded polymeric foam products were forced to switch to
alternatives
which have had adverse effects that resulted in increased processing costs,
reduced
product quality, and increased safety issues, or combinations thereof.
CA 02535638 1999-10-18
-2-
For example, hydrocarbon blowing agents, particularly the short-chained
alkanes
produce foam with satisfactory physical properties. However, depending upon
the
location of the factory and the amount of the blowing agent used, a
manufacturer may be
required to capture and destroy emissions of the hydrocarbon blowing agents
through a
processing step like incineration. Atmospheric emission of short-chained
hydrocarbons,
which are classified as photoreactive volatile organic compounds (VOCs), when
combined with certain other gases and subjected to sunlight, may result in
"smog".
Moreover, the flammability of the hydrocarbons requires elaborate control
systems and
costly ventilation systems to prevent the exposure of highly flammable blowing
agent-
and-air mixtures to ignition sources. Similar to hydrocarbon blowing agents,
certain
hydrofluorocarbon blowing agents, SuEh as 1,1-difluoroethane (HFC-152a),
produce foam
with satisfactory physical properties, but have the adverse effect of
flammability. In
addition, the nearly ten-fold higher unit pricing of these hydrofluorocarbon
blowing
agents in relation to most of the hydrocarbon blowing agents adversely
increases foam
product costs.
The disadvantages of the prior blowing agents have led to the use of carbon
dioxide as a blowing agent. Carbon dioxide does not have the adverse
environmental and
flam3nability characteristics associated with CFCs and HCFCs. Carbon dioxide
has a
molecular weight that is lower than most of the commercially used hydrocarbons
and the
hydrofluorocarbons and thereby requires lower usage rates. Carbon dioxide also
has
lower unit pricing than the commercially used hydrocarbons and
hydrofluorocarbons.
However, the foams made with higher levels of carbon dioxide have not been
comparable
to foams made with hydrocarbon or with hydrofluorocarbon blowing agents. The
foams
made with blowing agents that are primarily carbon dioxide have generally had
both
increased product cost and decreased product quality. The increased cost is
attributable to
a combination of reduced extrusion rates and limited post-expansion in
secondary
operations, which results in increased product weight. The reduced product
quality is
attributable to both diminished aesthetics and increased variability in the
mechanical
properties.
The diminished aesthetics of foam produced with carbon dioxide is generally
related to larger cell size, often greater than 0.4 mm, which give such foams
a poor,
grainy texture, and to the presence of multiple visible parallel regions of
light and dark in
the foam substrate. These adjacent parallel regions are not only deleterious
to the visible
CA 02535638 1999-10-18
-3-
aesthetics of the foams, but also create significant localized differences to
mechanical
properties.
The physical property that both diminishes aesthetics and increases the
variability
of the mechanical properties of foams made with carbon dioxide is related to
the low
solubility of the carbon dioxide gas in the polymer at ambient atmospheric
conditions.
The low solubility results in a very high volumetric expansion rate of the
foamable
composition at the die. As a consequence of this high volumetric expansion
rate of the
foam at the die, the use of a physical blowing agent comprised primarily of
carbon
dioxide in the production of fine-celled foams having a foam density below
about
100 kg/m3 or a cell size below about 0.40 mm Causes corrugation. The severity
of the
corrugations tends to increase as eithex-the density or the cell size is
decreased. The
corrugations are manifest as periodic bands which are oriented in the machine
direction
within the extruded foam sheet and which differ in cell size, cell shape, and
often foam
thickness from the majority of the foam. The corrugations not only detract
from the
aesthetics but also reduce the overall mechanical properties of parts made
from the foam.
In most food service and beverage applications, it is preferred that the
average cell
size be about 0.20-0.30 mm, which provides the foam with an aesthetically
pleasing,
relatively smooth surface texture while maintaining the requisite mechanical
properties
strength. Smaller cell sizes tend to undesirably sacrifice: a smoother finish
for strength.
Larger cell sizes tend to have an undesirable appearance.
When used as the sole blowing agent, carbon dioxide's very high volumetric
expansion rate typically produces unacceptable levels of corrugation.
Therefore, previous
attempts to use carbon dioxide as a blowing agent to produce a commercially
acceptable
foam product focused on blending the carbon dioxide with another blowing
agent. The
blended blowing agents typically included carbon dioxide as a minor
constituent and
either a hydrocarbon or hydrofluorocarbon blowing agent as the predominant
constituent.
A common blended blowing agent would include carbon dioxide in combination
with
pentane. Typically, the carbon dioxide in the blended blowing agent was
limited to 30
mole percent of the blended blowing agent, which reduced, but did not
eliminate, the use
of a hydrocarbon or hydroflurocarbon-blowing agent. Thus, the blended blowing
agent
still has the disadvantages of the hydrocarbon and hydroflurocarbon blowing
agents.
CA 02535638 1999-10-18
-4-
Attempts were also made to produce a commercially suitable polystyrene foam
with substantially 100 percent carbon dioxide as the blowing agent. Examples
of such
processes and foams are disclosed in U.S. Patents 5,266,605, 5,340,844, and
5,250,577.
Most of these foams had an average cell size of 0.36 mm and still contained
visible
corrugation. Although these foams would be suitable for some applications,
they did not
produce corrugation-free foams with cell sizes in the preferred range.
Referring to FIGS. 1 and 2, Applicants previously produced a corrugation-free
polystyrene foam with 100 percent carbon dioxide as the blowing agent from a
tandem
extruder, which is commonly known in the industry, in combination with a choke
ring
I 0 annularly positioned around an annular die extrusion opening.
The two-stage extruder apparatus comprises a hopper A feeding material into a
first extruder B where polystyrene resin material is heated and melted in
heating zone C,
mixed with a blowing agent delivered by an injector D, further mixed by a
mixing zone E,
and cooled in a second stage extruder in a cooling zone F before delivery to a
die G. The
choke ring H contacts the exiting extrudate before a sizing mandrel I sizes
the sheet.
The previously-used choke ring 10 and die 12 are shown in more detail in FIG.
2.
The choke ring 10 has a smooth temperature-regulated inner surface 11 that is
positioned
to be concentric with the die 12 so that extrudate 13 contacts inner surface
11 before
reaching the sizing mandrel I5.
The previously-used die 12 comprises a first generally converging portion 16
that
terminates at an annular die opening 18. A second converging portion 20
extends from
the annular die opening 18 and terminates at a cylindrical portion 22.
The choke ring surface 11 and the annular die opening 18 are located a radius
of r~
and rd, respectively, from the longitudinal axis 24. The choke ring gap is the
difference
between the radii (r~-rd).
Two choke rings having different diameters were tried. The first choke ring
had a
diameter such that the gap was 0.2375 inches. The second choke ring had a gap
of 0.18
inches, resulting in a contact time of approximately 0.37 ms for the given
operational
parameters. Although both of these choke rings produced corrugation-free foam,
the cell
size remained above 0.40 mm. Therefore, there is still a need for a
polystyrene foam and
method of making a polystyrene foam that is corrugation-free with a cell size
in the
preferred range and using a carbon dioxide blowing agent.
CA 02535638 1999-10-18
-5-
SUMMARY OF THE INVENTION
The invention relates to a corrugation-free foam and an apparatus and method
for
making the corrugation-free foam. The foam is preferably made in an extrusion
apparatus
S that extrudes a polymeric foam from an extrudate comprising a polymeric
resin and a
blowing agent. The blowing agent provides the extrudate with a cellular
structure upon
extrusion. The apparatus can comprise an extruder having an inlet that is
adapted to
receive the extrudate. An extrusion die is provided on the extruder and forms
an outlet
for the extruder. The extrusion die defines a longitudinal axis and has an
annular die
opening that is concentrically oriented relative to the longitudinal axis and
positioned a
first radial distance from the axis. The apparatus further comprises a choke
ring having
an opening defined by an annular choke ring surface. The choke ring is
positioned
relative to the extruder such that at least a portion of the die is received
within the choke
ring opening. The choke ring opening is preferably positioned concentrically
about the
longitudinal axis and is positioned a second radial distance therefrom. The
difference
between the second radial distance and the first radial distance defines the
gap between
the choke ring and the die. The contact time is a function of the line speed
and can be
characterized by the ratio of the gap size in millimeters (mm) relative to the
line speed in
millimeters per second of the extrudate as it leaves the die opening with the
ratio being
between 0.001 and 0.020. The choke ring gap is less than 5.6 mm and is
preferably less
than or equal to 0.8 mm.
The die can be of any suitable shape; however, it is preferred that the die is
symmetrical relative to the longitudinal-axis. It is also preferred that the
die has a
generally cylindrical body with an annular slot defining the die opening. The
generally
cylindrical body can include an annular ridge extending from the cylindrical
body and
terminating in a peak in which the annular slot is located.
In the method according to the invention, a polymeric foam is extruded from an
extrudate that comprises a polymeric resin and a blowing agent, which provides
the
polymeric resin with a cellular structure upon exiting from the extruder. The
extruder has
an annular die with an annular die opening and a choke ring having an internal
opening
formed by an annular choke ring surface. The internal opening is sized to
receive at least
a portion of the annular die. The annular die defines a longitudinal axis from
which the
annular die opening is spaced a first radial distance and the annular choke
ring surface is
spaced a second radial distance, with the difference between the second and
first radial
CA 02535638 1999-10-18
-6-
distances defining a choke ring gap. The method includes forming the extrudate
by
mixing a polymeric resin and a blowing agent and then extruding the extrudate
through
the annular die opening at a rate so that the extrudate leaving the annular
die opening
contacts the annular choke ring surface within a contact time of 1.0-20.0
milliseconds
(ms).
The extrudate is preferably kept constrained against the choke ring surface
for a
constrainment time of 5-7 ms and preferably between 8-50 ms. The blowing agent
can be
a blend but is preferably 100% carbon dioxide. The rate at which the extrudate
is pulled
through the annular choke ring opening can range from 50 mm/second to 250
mm/second. It is preferred that the contact time be less than 8 ms and more
preferably be
5 ms or less.
In an alternate form of the method, ahe polymeric foam is formed by mixing a
polymeric resin and a blowing agent consisting substantially of 100% carbon
dioxide,
extruding the extrudate from the annular die opening and through the choke
ring gap, and
controlling the extruded foam so it has an average cell size of 0.20 mm to
0.25 mm.
In another alternate form, the method relates to forming the extrudate by
mixing a
polymeric resin and a blowing agent comprising a blowing agent blend and
extruding the
extrudate through the choke ring gap.
The invention also relates to a foam, preferably made in accordance with the
above methods, having an average cell size of 0.20 mm-0.35 mm. The average
cell size
is preferably less than 0.30 mm. Such a foam will have no visible corrugation.
Preferably, the foam will be corrugation-free. The foam can be made in a foam
sheet,
which has a thickness preferably of 1-4 mm.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a block diagram illustrating the major functional components of a
prior
art foam extrusion system, including a choke ring surrounding an extrusion
die;
FIG. 2 is an enlarged sectional view of the prior art choke ring and die of
FIG. 1,
illustrating the relationship between the choke ring, die, and sizing mandrel
of the
apparatus and the corresponding interaction of the foam;
FIG. 3 is a view similar to FIG. 2 and illustrating a choke ring and die
according
to the invention; and
FIG. 4 is an enlarged sectional view showing the relationship between the
choke
ring and die according to the invention.
CA 02535638 1999-10-18
_7_
DETAILED DESCRIPTION OF THE INVENTION
The invention is both a corrugation-free foam and an apparatus for making the
corrugation-free foam. The corrugation-free foam is preferably made using the
prior art
tandem extruder with an improved choke ring alone or in combination with an
improved
S die. The improvement in the choke ring permits the production of a
polystyrene foam
with 100% carbon dioxide blowing agent having a fine cell structure with the
average cell
size being between 0.20 mm and 0.35 mm. It heretofore was thought impossible
to
produce such a corrugation-free foam with such a fine cell size by using
carbon dioxide
as a blowing agent.
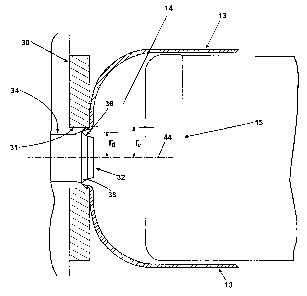
FIGS. 3 and 4 illustrate the improved choke ring 30 along with the new annular
die 32. The choke ring 30 and annular die 32 are disclosed in the context of
the prior art
tandem extruder configuration of FIG: 1. Therefore, similar parts in the
figures will be
identified by the same numerals.
The choke ring 30 has an annular inner surface 31 which is concentrically
oriented
with respect to a longitudinal axis 44 so that the inner surface 31 is located
a radial
distance r~ from the longitudinal axis 44. Similarly, the annular die 32 is
concentrically
oriented with respect to longitudinal axis 44 and the die opening 38 is
positioned a radial
distance rd from the longitudinal axis 44.
Unlike the prior art dies, the die 32 according to the invention does not
taper from
the base to the tip of the die. Instead, the die 32 begins at its base with a
generally
constant cross section portion 34, which transitions into an outwardly
directed radially
converging portion or collar 36, in whose apex the annular die opening 38 is
formed. The
die then ends with a slightly tapered cross section portion 40. The advantage
of the die 32
over the prior art die 12 is that the die outlet opening forms the largest
outer diameter of
the die 32; no other portion of the die 32 can interfere with the insertion of
the die into the
choke ring opening defined by the choke ring inner surface 31, especially for
the very
small gap sizes required by the invention.
The choke ring 30 preferably comprises a smooth, temperature-regulated surface
concentric to the annular foam die positioned in such a manner as to direct
but not reduce
the flow of the foamable extrudate as it leaves the die. Preferably an
adjustment
mechanism of the choke ring apparatus allows the smooth, temperature-regulated
surface
31 to be reproducibly positioned along the extrusion direction axis and be
held concentric
with the exit of die 32. An example of a suitable adjustment mechanism is one
that can
adjust the up/down, side-to-side, and upstream/downstream position of the
choke ring
CA 02535638 1999-10-18
_8_
relative to the annular die 32. Worm gears or similar mechanism can be used to
make the
adjustments.
It is also contemplated that the inner surface of the choke ring of the
present
invention can be constructed from any material that is solid at the
temperature of the
foamable extrudate. It is further contemplated that the inner surface of the
choke ring can
be constructed of any sintered material. The preferred materials of
construction for the
choke ring are thermoplastic polymeric materials with a glass transition
temperature or
melting temperature above about 160 C, thermoset polymeric materials, and
metallic
materials. Examples of suitable thermoplastic materials include
polytetrafluoroethylene,
polyacetal, polyamides, polyesters, and polyoxymethylene, and crosslinked
polyolefins.
Examples of thermoset materials include phenolics and epoxy resins. Examples
of
metallic materials include aluminum, carbon steel, and stainless steel. It is
contemplated
that materials of higher thermal conductivity are more effective as materials
of
construction of the inner surface of the choke ring. The most preferred
material of
construction for the inner surface of the choke ring is aluminum.
It is contemplated that the inner surface of the choke ring of the present
invention
can be configured with any suitably-shaped curve that will allow the expanding
foamable
composition to remain continuously constrained by the temperature-regulated
surface for
a time period of from about 5 to about 75 ms. The choke ring inner surface can
preferably
be configured to have diametrically opposing boundaries of the inner surface
of the choke
ring which are parallel lines, non-parallel lines, convex curves or concave
curves. For
examples in which the diametrically opposing sides of the choke ring are
parallel lines,
the boundaries of the inner surface describe a cylinder. For examples in which
the
diametrically opposing sides of the choke ring are non-parallel, the
boundaries of the
inner surface describe a frustoconical section of a cone.
The apex of the cone formed by a projection of the inner surface boundaries
may
be upstream or downstream of the 'die face. The term "converging angle" is
used herein to
describe the angle formed if the apex of the cone formed by the projection of
the inner
surface boundaries is downstream of the extruder. The term "diverging angle"
is used
herein to describe the angle formed if the apex of the cone formed by the
projection of the
inner surface boundaries is downstream of the extruder. In one example in
which the
diametrically opposing sides of the choke ring form a convex curve, the
boundaries of the
inner surface describe a section of a paraboloid. The vertex of said
paraboloid formed by
projection of the choke ring inner surface boundaries may be upstream or
downstream of
CA 02535638 1999-10-18
-9-
the die face. In one example in which the diametrically opposing sides of the
choke ring
form a concave curve, the boundaries of the inner surface describe an inner
section of a
torus.
Preferred configurations for the inner surface 31 of the choke ring are
frustoconical sections with a diverging angle, cylinders, and frustoconical
sections with a
converging angle. The converging angle or diverging angle is measured using
the
extrusion direction axis as the base. The most preferred configurations for
the choke ring
inner surface are a frustoconical section of a cone having a converging angle
less than
about 20°, a cylinder, and a frustoconical section of a cone having a
diverging angle less
than about 30°. The most highly preferred configurations for the choke
ring surface are a
frustoconical section of a cone having a converging angle less than
10°, a cylinder, and a
frustoconical section of a cone having a diverging angle less than about
10°.
It is contemplated that a downstream end 46 of the inner surface of the choke
ring
can be any configuration that will allow the foam to begin radial expansion
without
tearing of the foam surface. Preferred configurations for the downstream end
of the inner
surface of the choke ring are a radius in the range of from 0.4 mm to about
7.0 mm. The
most preferred configuration for the downstream end 46 of the inner surface of
the choke
ring is a radius in the range of from about 1.5 mm to about 3.5 mm.
It is further contemplated that the convective fluid used for temperature
regulation
of the choke ring can be any fluid that is conventionally used for cooling or
heating
applications as long as said fluid is not subject to thermal decomposition at
the extrudate
temperature and said fluid will not react with the material of construction of
the choke
ring. Examples of convective fluids include water, ethylene glycol-water
mixture in any
proportions, and commercial low viscosity thermal oils commonly used for heat
transfer
application. The preferred convective fluid for the temperature regulation of
the choke
ring is water.
The support for the choke ring inner surface can be any support that will
enable
positioning of the inner surface to be concentric with the annular die. The
support can be
attached to the extruder frame, the die body or the floor. The support is
preferably
attached to the body of the die.
Broadly, the process of the present invention combines an alkenyl aromatic
polymer, a nucleating agent, a physical blowing agent consisting of at least
15 mole
percent carbon dioxide and optionally one or more auxiliary physical blowing
agents, and
CA 02535638 1999-10-18
-10-
optional colorants and additives in the extruder B to form a foamable alkenyl
aromatic
polymer composition or extrudate. The foamable extrudate is pressurized above
a
particular threshold pressure specific to the composition and released to an
area of lower
pressure through an annular die G. The temperature-regulated annular choke
ring H is
positioned so that, within a contact time period of about 20 ms or less after
the foamable
extrudate 13 exits the die G, the outer surface of the extruded material 13 is
forced into
contact with the smooth inner surface 31 of the choke ring 30 in a manner that
deflects
the outer surface of extrudate 13 bur does not restrict the flow through the
die 32 or
damage the surface of the foam. The contact with the choke ring surface 31 is
maintained
for a constrainment time period in the range of from about 5 to about 75 ms.
The foam
extrudate 13 is then allowed to expand freely in the radial direction and is
drawn at
constant line speed over a mandrel 1_5 'which has a radius of about 1.5 to
about 6.0 times
the radius of the annular choke ring 10 to form a substantially corrugation-
free alkenyl
aromatic polymer foam.
The corrugations sought to be eliminated or reduced by the invention are well
known in the art and comprise multiple parallel regions formed on the extruded
sheet that
are oriented in the extrusion direction and which are apparent to the unaided
eye of an
observer through either visible light reflection or visible light
transmission. The least
severe corrugations are those in which the multiple parallel regions are
visible only by
transmitted light. Moderate corrugations are those which are visible by
reflected light and
may have slight localized thickness variations that are perceptible to human
touch. Severe
corrugations are those which also result in significant thickness difference
between
widths that are less than about 4 percent of the overall sheet width.
Extremely severe
corrugation describes the condition when adjacent parallel segments actually
join together
across the width direction to produce an overlap or even "S" folded cross-
section in the
thickness direction.
Mechanical properties of solid materials, such as flexural stiffness and
tensile
strength, are directly relatable to the mass distribution of the substrate.
Thus, mechanical
properties of foam are likewise directly dependent on the, amount of solid
mass or the
localized density of the foam. Corrugated foam generally has localized
variations in
density. Consequently, since lower density means lower strength, corrugations
are thus
deleterious to the overall mechanical properties of the foam.
Looking at the process in more detail and according to one embodiment of the
present invention, the process for producing substantially corrugation-free
alkenyl
CA 02535638 1999-10-18
-11-
aromatic polymer foam begins by feeding pellets of an alkenyl aromatic polymer
into the
extruder hopper A. The polymer along with 0.02 to about 2.0 weight percent of
pelletized
talc nucleating agent and 0 to about 2 weight percent of optional additives,
colorants, and
fire retardants are fed by gravity into the hopper A. (All weight percentages
relate to the
weight of the extrudate, unless otherwise noted.) The polymeric-talc-additives
mixture is
conveyed through the hopper A into the first extruder B and heated at the
heating zone C
to a temperature sufficient to form a polymeric-talc-additives blend.
A physical blowing agent consisting preferably solely of carbon dioxide and
optionally one or more members selected from the group consisting of fully
hydrogenated
hydrocarbon blowing agents, partially fluorinated blowing agents, and
combinations
thereof, is added at the injector D of the extruder in an appropriate ratio to
the target
density. Preferably, the carbon dioxide comprises 1 to 3 weight percent and is
injected in
a liquid state. The polymeric-talc-additives blend and physical blowing agent
are
thoroughly mixed in the mixing zone E, transferred to the second extruder, and
subsequently cooled in a cooling zone F to a temperature sufficient to form
foam. The
cooled foamable extrudate consisting of the polymeric-talc-additives-physical
blowing
agent is extruded through an annular die into a lower pressure region on the
downstream
side of the die G.
It is worth noting that carbon dioxide as used herein refers to commercially
available carbon dioxide. Commercially available carbon dioxide is not pure
and
contains some contaminants. It is preferable to use 100% carbon dioxide.
As shown in FIGS. 3 and 4, the expansion of the extruded foamable composition
is restricted by the choke ring 30, while~making contact with the smooth
temperature-regulated inner surface 31, which is concentric with the annular
die 32. The
inner surface 31 is sized so that the foamable composition contacts the inner
surface of
the choke ring within a contact time period of between about 1 and 20 ms, The
foam is
drawn over the inner surface 31 of the choke ring 30 and held there against it
for a
constrainment time period of between about 5 and 75 ms. The resulting foam 13
is then
allowed to expand freely in a radial direction in the form.of an air bubble 14
of regulated
pressure, drawn over a cylindrical sizing mandrel 1 S that has a diameter that
is 1.5 to 6.0
times that of the annular die, and collected on any conventional sheet
collection device.
The resulting alkenyl aromatic polymer foam of the present invention is free
of visible
corrugations. The foam preferably has no corrugations visible to the eye by
light
transmission.
CA 02535638 1999-10-18
-12-
The polymeric foams produced by the present invention are generally of a
density
of from about 30 kg/m3 to 120 kg/m3. The polymeric foams produced by the
present
invention generally have an average cell size from about 0.20 mm to about 0.35
mm. The
polymeric foams are produced with uniform and consistent physical properties.
The
polymeric foams are substantially free of even the least severe corrugations.
The
polymeric foams are light in weight and can be converted into plates, cups,
and bowls.
Other contemplated applications for the polymeric foams produced by the
present
invention include uses in insulation, toys, and low impact protective
packaging
applications.
The polymeric foam produced by the present invention preferably has a thin
cross
section in the thickness direction of the foamed structure that is less than
about 5 mm.
The preferred dimension in the thickness direction of the foamed product is
from about
1.0 to 4.0 mm.
The "average cell size" as used herein is defined as the mean of the cross
direction
cell size and the extrusion direction cell size. The extrusion direction cell
size and cross
direction cell size are measured in conformance to ASTM Method D3676. The
average
cell size is in the range of about 0.15 to about 0.60 mm. The most preferred
average cell
size is in the range of about 0.20 to about 0.35 mm in order to obtain the
most
commercially desirable combination of finish, strength, and density.
The contact time is a characteristic time period required by an element of the
foamable composition on the outer surface of the expanding foamed structure to
travel the
distance from the annular die outlet opening to the inner surface 11 of the
choke ring 10.
The contact time is calculated by dividing the distance between the choke ring
inner
surface and the die outlet (in units of length such as mm) by the line speed
(in units of
length/time such as mm/sec) used in drawing the foam over the mandrel. That
is:
>~o= (rte ra)/L
where too is the contact time (in sec),
r~ is the mean radius of the choke ring inner surface (mm),
rd is the radius of the annular.die at the die gap (mm), and
L is the mean line speed of downstream equipment (mm/sec)
The preferred contact time is from about 1.0 to about 20 ms. The most
preferred
contact time is from about 1.0 to 8 ms. The most highly preferred contact time
is from
1.0 to 5.0 ms.
CA 02535638 1999-10-18
-13-
The constrainment time is preferably a characteristic time period that an
element
of the foamable composition on the outer surface of the foamed structure
actually travels
along the inner surface of the choke ring. The constrainment time is
calculated by
dividing the length of the choke ring inner surface that is downstream of the
annular die
exit (in units of length such as mm) by the line speed (in units of
length/time such as
mm/sec) used in drawing the foam over the mandrel. That is:
t~~=1~/L
where t~~ is the constrainment time (in sec),
1~ is the contact length of the choke ring inner surface (mm),
L is the mean line speed of downstream equipment (mm/sec)
The preferred constrainment time is from about 5 ms to about 75 ms. The most
preferred constrainment time is from about 8 to about 50 ms.
The alkenyl aromatic polymer preferably includes polymers of aromatic
hydrocarbon molecules that contain an aryl group joined to an olefinic group
with only
double bonds in the linear structure, such as styrene, a-methylstyrene, o-
methylstyrene,
m-methylstyrene, p-methylstyrene, a-ethylstyrene, a-vinylxylene, a-
chlorostyrene,
a-bromostyrene, vinyl toluene and the like. Alkenyl aromatics polymers include
homopolymers of styrene (commonly referred to as polystyrene) and copolymers
of
styrene and butadiene (commonly referred to as impact polystyrene).
The contact time, constrainment time, the choke ring gap size, and the line
speed
are interdependent or, in other words, functions of each other. Although the
contact time
and constrainment time are useful for quantifying operational range of the
process for
obtaining a corrugation-free foam according to.the invention, testing has also
shown that
the currently preferred choke ring gap of 0.03 inches produces corrugation-
free foam
with a 0.20-0.35 mm average cell size for the current range of line speeds.
The polystyrene resin or polystyrenic material preferably includes
homopolymers
of styrene, and styrene copolymers comprised of at least 50 mole percent of a
styrene unit
(preferably at least about 70 mole percent) and a minor (i.e. less than 50
mole percent)
proportion of a monomer copolymerizable with styrene. The term "polystyrene
resin" or
"polystyrenic material" as used herein also includes blends of at least 50
percent by
weight of the styrene homopolymer (preferably at least about 60 weight
percent) with
another predominantly styrenic copolymer. The physical blends are combined in
a dry
form after the blends have been polymerized.
CA 02535638 1999-10-18
-14-
The polystyrene resin that can be used in the polymeric mixture can be any of
those homopolymers obtained by polymerizing styrene to a weight average
molecular
weight (M«) of from about 100,000 to about 450,000 (commonly referred to as
crystal
polystyrene), can be any of those copolymers obtained by polymerizing styrene
and from
about 3 to 20 mole percent butadiene to a weight average molecular weight (M«)
of from
about 100,000 to about 350,000, or can be any of those graft copolymers
obtained by
polymerizing a blend of polymerized styrene upon a nucleus of styrene-
butadiene rubber
(SBR) to a weight average molecular weight of from about 100,000 to about
350,000
(commonly referred to as impact polystyrene).
The preferred crystal polystyrenes are uncrosslinked homopolymers of styrene
and have a melt flow index of from about 0.5 to about 15.0 dg/min. as measured
by
ASTM D1238 (nominal flow rate at2:00 C.and 689.5 kPa). The most preferred
crystal
polystyrene is uncrosslinked polystyrene having a melt flow index from about
1.0 to
3.0 dg/min.
Impact polystyrenes are generally classified as medium impact polystyrene
(MIPS), high impact polystyrene (HIPS) or super high impact polystyrene (S-
HIPS). The
butadiene level of the impact polymer is preferably in the range of from about
3 to about
10 weight percent of the copolymer (polybutadiene and polystyrene). The most
preferred
butadiene level is in the range of from about 5 to 8 weight percent of the
copolymer. The
impact polystyrene generally has a melt flow index of less than about 25
dg/min., and
preferably less than about 8 dg/min. The most preferred impact polystyrenes
are
uncrosslinked HIPSs having a melt flow index of. from about 2.2 to 3.2 dg/min.
as
measured by ASTM D1238 (nominal flew rate at 200 C and 689.5 kPa), and a
Notched
Izod Impact of from about 9 to about 13 kg-cm/cm as measured by ASTM D256. The
Notched Izod Impact is the energy required to break notched specimens under
standard
conditions and is work per unit of notch. Therefore, a higher Notched Izod
Impact
indicates a tougher material.
The alkenyl aromatic polymer of the present invention can be obtained by
blending two or more alkenyl aromatic polymers. For example, blends of crystal
polystyrene and impact polystyrenes such as crystal polystyrene and HIPS, may
be
blended to comprise the alkenyl aromatic polymer of the present invention.
The nucleating agent preferably includes any conventional or useful nucleating
agents) used to adjust the size of the cells in the foamed structure to the
target size
desired. The term "cell size control agent" has also been used interchangeably
in the art.
CA 02535638 1999-10-18
-15-
The amount of nucleating agent to be added depends upon the desired cell size,
the
selected blowing agent, and the density of the alkenyl aromatic polymer foam
composition. The nucleating agent is generally added in amounts of from about
0.02 to
2.0 weight percent of the polymeric composition. Nucleating agents may be
inorganic or
organic compounds and are generally available ~in a small particulate form.
Examples of inorganic nucleating agents include clay, talc, silica, and
diatomaceous earth. The preferred organic nucleating agents include those
compounds
which decompose or react at the heating temperature within the extruder to
evolve gas.
Examples of these preferred organic nucleating agents include polycarboxylic
acids and
alkali metal salts of a polycarboxylic acid in combination with a carbonate or
bicarbonate.
Some specific examples of an alkali metal salt include, but are not limited,
to the
monosodium salt of 2,3-dihydroxy-butanedioic acid (commonly referred to as
sodium
hydrogen tartrate), the monopotassium salt of butanedioic acid (commonly
referred to as
potassium hydrogen succinate), the trisodium and tripotassium salts of 2-
hydroxy-1,2,3-
propanetricarboxylic acid (commonly referred to as sodium and potassium
citrate
respectively), and the disodium salt of ethanedioic acid (commonly referred to
as sodium
oxalate). An example of a polycarboxylic acid is 2-hydroxy-1,2,3-
propanetricarboxylic
acid (commonly referred to as citric acid). Some examples of a carbonate or a
bicarbonate
include, but are not limited to, sodium carbonate, sodium bicarbonate,
potassium
carbonate, and calcium carbonate.
It is contemplated that mixtures of inorganic and organic nucleating agents
can
also be used in the present invention. The most preferred nucleating agent is
talc. Talc is
preferably added in a powder form, but may also be added in a carrier. If
added in a
carrier, the talc concentration is preferably between 20 to 60 weight percent
in an alkenyl
aromatic polymer which is preferably a styrene homopolymer.
The physical blowing agent for this invention includes at least 15 mole
percent,
preferably at least 50 mole percent, carbon dioxide and optionally one or more
auxiliary
physical blowing agents. The most preferred amount is 100 percent carbon
dioxide to
provide the greatest positive environmental and safety characteristics. The
carbon
dioxide blowing agent can be used at a rate of about 0.1 to 4.0 weight
percent, but
preferably about 1.0 to about 3.0 weight percent, of the total extruder feed
rate.
The auxiliary physical blowing agent comprises at least 1 mole percent, and
preferably at least 5 mole percent, but less than 85 mole percent of the total
blowing
agent. More than one auxiliary physical blowing agents may also be included.
Examples
CA 02535638 1999-10-18
-16-
of auxiliary physical blowing agents include but are not limited to organic
physical
blowing agents.
The organic auxiliary physical blowing agents preferably includes organic
chemical compounds that have boiling points less than about 37 C. These
organic
compounds include, but are not limited to, fully hydrogenated hydrocarbons and
partially
fluorinated hydrocarbons which may be considered to be flammable. Flammable as
defined herein generally includes those materials having flashpoints less than
about 37.8
C.
Examples of fully hydrogenated hydrocarbon blowing agents include the initial
members of the alkane series of hydrocarbons that contain up to six carbon
atoms.
Preferably, the hydrogenated hydrocarbon blowing agents are not regulated by
governmental agencies as being specif cally toxic to human or plant life under
normal
exposure. The preferred Ci-Cbalkane compounds include methane, ethane,
propane, n-
butane, isobutane, n-pentane, isopentane, n-hexane, and blends thereof. The
most
preferred fully hydrogenated hydrocarbon auxiliary physical blowing agents are
the C4.C5
and blends thereof.
The preferred partially fluorinated hydrocarbons auxiliary physical blowing
agents
are hydrofluorocarbon gases that have molecules which contain up to three
carbon atoms
without any other halogen atoms other than fluorine. These partially
fluorinated auxiliary
physical blowing agents may be flammable. The most preferred partially
fluorinated
hydrocarbon auxiliary physical blowing agents are 1,1-difluorethane (HFC-152a)
and
1,1,1-trifluoroethane (HFC-143a). It is also contemplated that 1,1-
chloroethane (HFC-
142b) and 1-1-dichloro-2-fluoroethane (HFC-141b) may be added as auxiliary
blowing
agents for non-regulated insulation applications.
The optional additives used in the process preferably provide specific, non-
mechanical physical properties to the foamed product and do not interfere with
or
influence the extrusion of the foamed product. Additives may constitute from
about 0.05
to about 5 weight percent of the total polymeric foam rate. Examples of
additives include
but are not limited to antistats, fire retardants, perfumes, ultra-violet (UV)
light absorber,
UV stabilizers, and infra-red light tracers.
Similarly, colorants used in the process preferably include specific additives
included solely for the purpose of providing a desired color to the foamed
product that is
different from the natural color provided by the alkenyl aromatic polymer when
foamed.
Examples of colorants include various pigments and color concentrates as known
in the
CA 02535638 1999-10-18
-17-
art, such as carbon black and titanium dioxide white. The preferred form of
colorant for
addition to the extruder is in a pellet consisting of about 1 to about 40
weight percent of
the color material compounded,in an alkenyl aromatic polymer such as
polystyrene that
may be different from the alkenyl aromatic polymer used for the foam. The most
preferred form of colorant for addition to the extruder is in a pellet
consisting of about 5
to about 20 weight percent of the color material compounded in the same
alkenyl
aromatic polymer as used for the foam. The most preferred concentration of
additive is
from 0.2 to 2.0 weight percent of the total extruder flow rate.
EXAMPLES
Inventive Example 1
Pellets of a previously extruded mixture of Dart Polymers, Inc. PS 1 O 1 high
heat
crystal polystyrene (specific gravity of about 1.05 g/cm3 and a melt index
(MI) of about
1.8 dg/min.) and an undetermined level, between 1.0 and S.0 weight percent, of
Phillips
K-Resin (styrene-butadiene copolymer) was mixed with 0.50 weight percent of
Huntsman
27678 1 talc concentrate pellets. The pellet mixture was heated to form a
blend in a 32:1
L:D Battenfeld Gloucester Engineering Co., Inc. 2.5-inch (35.3 cm) single-
screw extruder
operating at a screw speed of about 95 rpm. Pressurized commercial-grade
carbon
dioxide (31.0 MPa) was injected at a rate of 2.31 kg/hr. The polymer melt and
carbon
dioxide were mixed and further heated to a melt temperature of about 209 C and
pressurized to 26.9 MPa at the extruder discharge.
The heated mixture was then transferred through a heated pipe to a second,
larger
3.5 inch (89 mm) single-screw cooling extruder operating at 25 rpm.
Subsequently, the
extrudate was cooled to a melt temperature of about 149 C and pressurized to
about 21.4
MPa for delivery at about 75 kg/hr into a 5.40-cm diameter annular die.
The extrudate is pulled from the die by downstream equipment which is
operating
at about 244 mm/sec and is drawn into contact with a choke ring having an
inner surface
diameter of 5.55 cm and a choke ring gap size of 0.762 mm, for a contact time
of 3.13 ms.
The choke ring temperature was regulated by the flow of. cooling water which
was
maintained at 27 C. The foam remained in contact with the inner surface of the
choke ring
for a distance of about 6.4 mm, resulting in a constrainment time of about 26
ms. The
foam was then allowed to expand freely and was subsequently drawn over a
mandrel to
form a foam having a density of 59.0 kg/m3, an average thickness of 2.22 mm,
and an
CA 02535638 1999-10-18
-18-
average cell size of 0.25 mm. The foam is free of corrugations visible to the
unaided eye
by light transmission.
Inventive Example 2
This example is similar to Inventive Example 1 with reduction of the talc
concentrate level to 0.25 weight percent and an increase of the cooling water
temperature
on the choke ring to 63 C and a reduction of the line speed to 199 mm/sec.
The contact time was 3.83 ms. The constrainment time was about 32 ms. The
foam was then allowed to expand freely and was subsequently drawn over a
mandrel to
form a foam having a density of 60.0 kg/m3, an average thickness of 2.57 mm,
and an
average cell size of 0.35 mm. The foam is free of corrugations visible to the
unaided eye
by light transmission. y
Inventive Example 3
This example is similar to Inventive Example 2 with a change to a blowing
agent
blend comprising 80 mole percent carbon dioxide and 20 mole percent Phillips
Chemical
Company commercial grade isopentane at a total injection rate of 2.54 kg/hr.
Other
adjustments were a cooling extruder screw speed of 26 rpm, choke ring cooling
water
temperature to 32 C and line speed to 203 mm/sec.
The contact time was 3.75 ms. The constrainment time was about 31 ms. The
foam was then allowed to expand freely and was subsequently drawn over a
mandrel to
form a foam having a density of 60.6 kg/m3, an average thickness of 2.56 mm,
and an
average cell size of 0.31 mm. The foam is free of corrugations visible to the
unaided eye
by light transmission.
Inventive Example 4
This example is similar to Inventive Example 3 with a change to a blowing
agent
blend comprising 62 mole percent carbon dioxide and 38 mole percent Phillips
Chemical
Company commercial grade isopentane at a total physical blowing agent
injection rate of
2.27 kg/hr. The line speed was changed to 221 mm/sec.
The contact time was 3.45 ms. The constrainment time was about 29 ms. The
foam was then allowed to expand freely and was subsequently drawn over a
mandrel to
form a foam having a density of 82.2 kg/m3, an average thickness of 1.78 mm,
and an
CA 02535638 1999-10-18
-19-
average cell size of 0.31 mm. The foam is free of corrugations visible to the
unaided eye
by light transmission.
Inventive Example 5
This example is similar to Inventive Example 1 with reduction of the carbon
dioxide rate to 1.9S kg/hr, an increase of the choke ring cooling water
temperature to 32 C
and a reduction of the line speed to 156 mm/sec.
The contact time was 4.89 ms. The constrainment time was about 41 ms. The
foam was then allowed to expand freely and was subsequently drawn over a
mandrel to
form a foam having a density of 70.1 kg/m3, an average thickness of 2.87 mm,
and an
average cell size of 0.30 mm. The foam is free of corrugations visible to the
unaided eye
by light transmission. _-
Comparative Example 6
This example is similar to Inventive Example 2 with the elimination of the
choke
ring and change of the nucleating agent from a talc concentrate to a powdered
talc. The
extrusion rate was 80.7 kg/hr.
The foam was allowed to expand from the die freely and was subsequently drawn
over a mandrel to form a foam having a density of 59.9 kg/m3, an average
thickness of
3.25 mm, and an average cell size of 0.59 mm. The foam has large cells and
moderate
corrugations that have visible thickness variations on the surface of the
foam.
Comparative Example 7
This example is similar to Comparative Example 6 with a change of the
nucleating agent to Boehringer Ingelheim Hydrocerol Compound (a mixture of
citric acid
and sodium bicarbonate in a proprietary carrier).
The foam was allowed to expand from the die freely and was subsequently drawn
over a mandrel to form a foam having a density of 85.7 kg/m3, an average
thickness of
1.49 mm, and an average cell size of 0.34 mm. The foam has large cells and
severe
corrugations that have visible thickness variations on the surface of the
foam.
Comparative Example 8
This example is similar to Inventive Example 3 with the elimination of the
choke
ring. The foam was allowed to expand from the die freely and was subsequently
drawn
CA 02535638 1999-10-18
-20-
over a mandrel to form a foam having a density of 74.7 kg/m3, an average
thickness of
1.84 mm, and an average cell size of 0.35 mm. The foam has large cells and
moderate
corrugations that have visible thickness variations on the surface of the
foam.
Comparative Example 9
This example is also similar to Comparative Example 8 with a change of carbon
dioxide/isopentane mole fraction ratio from 80/20 to 70/30. The foam was
allowed to
expand from the die freely and was.subsequently drawn over a mandrel to form a
foam
having a density of 79.8 kg/m3, an average thickness of 1.63 mm, and an
average cell size
of 0.29 mm. The foam has large cells and moderate corrugations that have
visible
thickness variations on the surface of the foam.
Comparative Example 10
This example is similar to Inventive Example 1 except that the choke ring had
an
inner surface diameter of 66.04 mm, resulting in a gap of 6 mm and a contact
time of 38
ms. The resultant foam was corrugation-free and had a thickness of 2.91 mm, a
density of
66.4 kg/m2, and an average cell size of 0.42 mm.
Comparative Example 11
This example is similar to Inventive Example 1 except that a choke ring having
an
inner surface diameter of 63.12 mm, resulting in a gap of 4.6 mm and a contact
time of 20
ms, was used. The resultant foam has severe corrugation and had a thickness of
2.46 mm,
a density of 54.1 kg/m3, and an average cell size of 0.26 mm.
Comparative Example 12
Comparative Example 12 is similar to Comparative Example 11 except that the
contact time was 30 ms, which resulted in a foam having severe corrugation
with a
thickness of 3.08 mm, a density of 65.8 kg/m2, and an average cell size of
0.27 mm.
CA 02535638 1999-10-18
-21-
The key results of the examples are summarized in Table 1.
Table 1.
Physical
Blowing
Agent
ExampleCOZ IsopentaneExtrudedAverage
umber Mole Mole DensityCell ThicknessCorrugation
FractionFraction (kg/m3)Size (mm)
(mm)
INVENTIVE
FOAMS
1 100% 0% 59.0 0.25 2.22 None
2 100% 0% 60.0 0.35 2.57 None
3 80% 20% 60.6 0.31 2.56 None
4 62% 3 8% 82.2 0.27 1.78 None
100% 0% 70.1 0.30 2.87 None
COMPARATIVE
FOAMS
-
6 100% 0% 59.9 0.59 3.25 Moderate
7 100% 0% 85.7 0.34 1.49 Severe
8 80% 20% 74.7 0.35 1.84 Moderate
9 70% 30% 79.8 0.29 1.63 Moderate
100% 0% 66.4 0.42 2.91 None
11 100% 0% 54.1 0.26 2.46 Severe
12 100% 0% 65.8 0.27 3.08 Severe
5
As can be seen, the invention with its choke ring and reduced contact times
result
in a highly desirable commercial foam having a cell size within the preferred
range of
0.20 to 0.35 without any corrugation, regardless of the percentage of carbon
dioxide. The
invention is further advantageous in that the blowing agent can comprise 100
percent COZ
10 which has superior environmental and safety characteristics as compared to
the
hydrocarbon and hydroflurocarbon blowing agents. The prior art foams made with
a
choke ring having contact times greater than 20 ms did not produce a suitable
foam
because either the corrugation or cell size was too great. The prior art foams
made
without a choke ring were not suitable because both the corrugation and the
cell size were
too great.
While the present invention has been described with reference to one or more
particular embodiments, those skilled in the art will recognize that many
changes may be
made thereto without departing from the scope and intentions of the present
invention.
Those variations thereof are contemplated to fall within the scope and
intention of the
described invention.