Note: Descriptions are shown in the official language in which they were submitted.
CA 02535728 2006-06-21
PREPARATION OF. RISPERIDON~
This application is a divisional of Canadian patent application Serial No.
2,419,314 filed internationally on August 14, 2001 and entered nationally on
February 12, 2003.
FIELD OF THE INVENTION
The present invention relates to novel polymorphic forms of risperidone. The
present invention also relates to methods of making polymorphic forma of
risperidane.
BACKGROUND OF THE INVENTION
RISPE1ZDAL~ (risperidone) is an antipsychotic agent t~elonging to a new
chemical
class, the benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-
fluoro-1,2-
benzisoxazol-3-yl~l piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-iriethyl-4 H
pyrido[1,2-
a]PY~~-.4-one. .
U.S. Patent No. 4,804,663, describes a synthesis of risperidone. Risperidone
may be
prepared by condensation of the following two intermediates, 6-fluoro-3-(4-
piperidinyl)-1,2-
benzisoxazole (Compound I) and 3-(2-cholorethyl)-6,7,8,9-tetrahydro-2-methyl-
4H-pyrido[1,2-
a~pyrimidin-4-one (Compound II) in dimethylformamide (DMF) in basic conditions
(NazC03 or
KZC03) with catalytic amount of potassium iodide (KI). The crude risperidone
product (III) is
crystallized from a mixture of DMF and isopropanol with an overall yield of
46%.
\ . _ W C~I3 .. .
~g Kz~~~~
at 85:901;
2s ~ ~ .~
a:$~~- 3-c~n~,~a-~~-
i,2~~isoxazole. ma~yl-4Ii-pyddoll,2-a~cinstdin-4~on° ,
1
CA 02535728 2001-08-14
Polymorphism is the occurrence of different crystalline forms of a single
compound and it is a properly of some compounds and complexes. Thus,
polymorphs are
distinct solids sharing the same molecular formula, yet each polymorph may
have distinct
physical properties. Therefore, a single compound may give rise to a variety
of
polymorphic forms where each form has different and distinct physical
properties, such as
different solubility profiles, different melting point temperatures and/or
different x-ray
diffraction peaks. Since the solubility of each polymorph may vary,
identifying the
existence of pharmaceutical polymorphs is essential for providing
pharmaceuticals with
predicable solubility profiles. It is desirable to investigate all solid state
forms of a drug,
including all polymorphic forms, and to determine the stability, dissolution
and flow
properties of each polymorphic form. Polymoiphic forms of a compound can be
distinguished in a laboratory byX-ray diffraction spectroscopy and by other
methods such
as, infrared spectrometry. For a general review of polymorphs and the
pharmaceutical
1 S applications of polyrnorphs see G.M. WaII, Pharm Manuf. 3, 33 {I986); J.K.
Haleblian
and W. MeCrone, J. Pharm. Sci., S8, 911 (1969);.and~J.K. Haleblian, J_ Pharm.
Sci., 64,
1269 (1975),
SUIVI11ZARY OF THE INVENTION
An object of the processes of the present invention is to provide more
efllcient and
quicker methods for making pure risperidone. We have now found that the
synthesis of
risperidone from compounds I and II can done in acetonitrile and isopropanol,
without
using DMF, to give an improved and higher yield of about 75%.
The present invention provides a process for the preparation of risperidone
from
fine following two intermediates, 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole
(Compound
I) and 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2~a]pyrimidin-
4-one
(Compound II) in acetonitrile.
it has also been found that the crude risperidone can be e~ciently
crystallized in
high yield from an alcohol, for example, isopropanol, butanol, ethanol, or
methanol; or
from a ketone, for example, acetone or ethyl methyl ketone, without the need
of using
DMF, which is harmful to humans and is a very difficult solvent to remove.
2
CA 02535728 2001-08-14
Polymotphs of risperidone are mentioned in the Summary Basis of Approval
(SBA) of New Drug Application 20-272 and 20-588, however the SBA does not
identify
them by recognized methods of crystal structure identification such as x-ray
diffraction.
The present invention also provides forms of risperidone designated
risperidone
Form A, Form B and Form E.
The present invention further provides a process for making risperidone
comprising reacting Compound I with Compound II to form crude risperidone
(ffl) in a
solvent selected from the group consisting of acetonitrile, isopropanol,
methyl ethyl
ketone and iso-butanol.
In another embodiment, the crude risperidone is recrystallized from an
alcohol; a
mixture of alcohols; a mixture of water and alcohol; or from a ketone, e.g.;
acetone. In
another embodiment, the alcohol is selected from the group consisting of
methanol,
ethanol, isopropanol, propanol, butanol, sec butanol, iso butanol and t
butanol. In another
embodiment, the alcohol is isopropanol. In another embodiment, the alcohol is
acetonitrile. In another embodiment, the alcohol is isopropanol. Tn another
embodiment,
the alcohol is iso-butanol. In another embodiment, the ketone is acetone. In
another
embodiment, the acetone is methyl ethyl ketone.
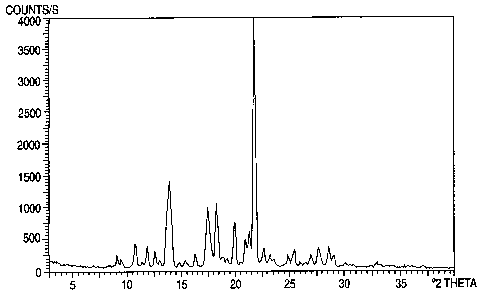
The present invention also provides risperidone Form A which.is characterized
by
x-ray powder diffraction peaks at 14.210.2, 21.3f0.2 degrees two-theta. The
present
invention also provides risperidone Form A of further characterized by x-ray
powder
diffraction peaks at 10.6~0.2,11.410.2,16.4t0.2,18.9~0.2,19.9~0.2, 22.50.2,
23.30.2,
25.40.2, 27.60.2, 29.00.2 degrees two-theta.
The present invention also provides a risperidone polymorph that is
characterized
by a powder x-ray di~-action pattern substantially as depicted in Figure 1.
The present invention also provides risperidone Form B which is characterized
by
x-ray powder diffraction peaks at 14.00.2 and 21.70.2 degrees two-theta.
The present invention also provides a risperidone polymorph that is
characterized
by a powder x-ray diffraction pattern substantially as depicted in Figure 2.
The present invention also provides risperidone Form B which is further
characterized by x-ray powder diffzaction peaks at 10.810.2,11.910.2,12.6f0.2,
3
CA 02535728 2001-08-14
14.0f0.2, l7.St0.2,18.3t0.2,19.9~0.2, 21.0f0.2, 21.70.2 degrees two-theta.
The present invention also provides risperidone Form E which is characterized
by
x-ray powder diffraction peaks at 16.50.2, 2I.7~0.2degrees two-theta.
The present invention also provides risperidone Form E which is further
characterized by x-ray powder diffraction peaks at 16.50.2, 12.60.2, 21.70.2 ,
15.6~0.2,17.0~0.2,18.4~0.2,19.1~0.2, 21.30.2, 24.00.2, 24.910.2, 27.00.2
degrees
two-theta.
The present invention also provides a risperidone polymorph that is
characterized
by a powder x-ray diffraction pattern substantially as depicted in Figure 3.
The present invention also provides a process for preparing risperidone Form B
comprising the steps. of dissolving risperidone in a substantially water
soluble alcohol
having 1 to 4 carbon atoms where the ratio of risperidone to alcohol is about
1:7.5 to
about 1:9; adding water to facilitate precipitation; and isolating risperidone
Form B.
The present invention also provides a process for preparing risperidone Form B
comprising the steps of dissolving risperidone in chloroform; adding
cyclohexane or
hexane to facilitate precipitation; and isolating risperidone Form B.
The present invention also provides a process for preparing risperidone Form B
comprisingthe steps of dissolving risperidone in an aqueous solution of HCI;
adding an
aqueous solution of NaZC03; and isolating risperidone Form B.
The present invention also provides a process for preparing risperidone Form A
comprising the steps of dissolving risperidone in an organic solvent selected
from the
group consisting of dimethylformamide, tetrahydrofuran, acetone, benzene,
ethyl methyl
ketone, n butanol, methanol, isopropanol, absolute ethanol, acetonitrile,
toluene, dimethyl
sulfoxide, iso-butanol, and ethyl acetate or mixtures thereof; heating the
solvent to reflux;.
cooling the solvent to facilitate precipitation; and isolating risperidone
Form A.
The present invention also provides a process for preparing risperidone Form A
comprising the steps of dissolving risperidone in dichloromethane; adding
cyclohexane
or hexane to facilitate precipitation; and isolating risperidone Form A.
The present invention also provides a method for preparing risperidone Form A
comprising the step of heating risperidone Form B at a temperature of about
25°C to
4
CA 02535728 2001-08-14
about 80°C for a time suflxcient to induce to formation of risperidone
Form A; and
isolating risperidone Form A. In another embodiment, the heating takes place
under
reduced pressure or at atmospheric pressure. In another embodiment, the
temperature is
about 80°C. In another embodiment, the time for heating is about 16 to
about 20 hours.
The present invention also provides a process for preparing risperidone Form E
comprising the steps of dissolving risperidone in isopropanol where the ratio
of
risperidone to isopropanol is about 1:12; adding water to facilitate
precipitation; and
isolating risperidone Form E.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a characteristic x-ray powder diffraction spectrum of xisperidone
Form A.
Figure 2 is a characteristic x-ray powder diffraction spectrum of risperidone
Form B.
Figure 3 is a characteristic x-ray powder diffraction spectrum of risperidone
Form E.
DETAILED DESCRIPTION OF THE INVENTION
Synthesis of Risperidone
The present invention provides new processes for preparing risperidone from
the
following two intermediates,'6-fluoro-3-(4-piperidinyl)-1,2 benzisoxazole (n
and 3-(2-
chloroethyl~-6,?,8,9-tetrahydro-2 methyl-4H-pyrido[1,2-a]pyrimidin-4.-one (II)
using
acetonuitrile, isopropanol, iso-butanol, or methyl ethyl ketone as the
solvent, which
eliminates the need to use DMF as a solvent. By the methods of the present
invention,
risperidone is prepared by adding , 3-(2-chloroethyl)-6,7,8,9 tetrahydro-2-
methyl-4H-
pyrido[1,2-a]pyrimidin-4-one (Compound B or "the chlorine derivative"); 6-
fluoro-3-(4-
piperidinyl)-1,2-benzisoxazole (Compound I or "the piperidine derivative");
sodium
carbonate; and potassium iodide (66 mg) into a flask containing the solvent
isopropanol,
acetonitrile, methyl ethyl ketone or iso-butanol. Preferably, the Compound I
and
Compound II are present in a ratio of about 1:1. The reaction mixture is then
heated by
methods known in the art, such as, by placing the flask in an oil bath which
is heated from
about 60°C to about 85°C, and the reaction is allowed to reflux
for a time sufficient to
~0 complete the formation of risperidone, about 9 hours to overnight.
Preferably, the
5
CA 02535728 2001-08-14
reaction mixture is heated to about 60°C to about 67°C.
Preferably the reaction is heated
for about 9 hours when the solvent is isopropanol. Preferably the reaction
mixture is
heated overnight when the solvent is methyl ethyl ketone or iso-butanol.
Preferably the
reaction is heated for about 17 hours when the solvent is acetonitrile. Upon
completion of
the reaction, the mixture is cooled by methods known in the art to induce the
precipitation
of risperidone.
The resulting precipitated risperidone is filtered and the filter cake is
washed in the
filter with a small amount of isopropanol, acetone or a mixture of acetone and
water. The
filter cake is then slurried; filtered and easily dried by conventional
methods to give crude
risperidone in a yield of about 63 to 74 % yield. The present method
eliminates the
difficult step of removing DMF from the crude risperidone.
The present invention also relates to new processes for recrystallizing crude
risperidone firm; an alcohol, such as, methanol, ethanol, isopropanol,
propanol, butanol,
seo-butanol and t-butanol; a mixture of alcohols containing any combination
of, methanol,
ethanol, isopropanol, propanol, butanol, sec-butanol and t-butanol; or a
mixture of water
and alcohol where the alcohol is one or more of the following alcohols,
methanol, etl,~anol;
isopropanol, propanol, butanol, sec-butanol and t-butanol. The present
recrystallization
eliminates the use of the difficult to remove and potentially harmful solvent
DMF.
Preferably, the solvent is isopropanol. By the methods of the present
invention, crude
risperidone is recrystallized by dissolving the crude risperidone in a solvent
which is hot.
Preferably, the olvent is heated to reflux. Preferably the crude risperidone
and solvent
are present in a ratio of about 10 to about 15, more preferably the ratio is
about 11 to 13,
most preferably the ratio is about 11.5 to about 12.5. Preferably the solvent
is
isopropanol. The hot mixture is then filtered hot and allowed to cool where
upon purified
risperidone precipitates. The mixture is filtered by conventional methods to
give high
purity risperidone with a purity of about 99.7 to about 99.8 %. The overall
yield of the
present method of synthesis and recrystallization of risperidone is about 60
to about 63 %.
The present invention also provides new processes for recrystallizing crude
risperidone from a solvent that is a lcetone, such as, acetone. The present
recrystallization
eliminates the use of the difficult to remove and potentially harmful solvent
DMF.
6
CA 02535728 2001-08-14
Preferably, the solvent is acetone. By the methods of the present invention,
crude
risperidone is recrystallized by dissolving the crude risperidone in a ketone,
which is hot.
Preferably, the ketone is heated to reflux. Preferably the crude risperidone
and solvent are
present in a ratio of about 25 to about 40, more preferably the ratio is about
28 to about
5. 32. Preferably the solvent is acetone. The hot mixture is then filtered hot
and allowed to
cool where upon purified risperidone precipitates. The mixture is filtered by
conventional
methods to give high purity risperidone with a purity of about 99.7 to about
99.8 %. The
overall yield of the present method of synthesis and recrystallization of
risperidone is
about 60 to about 63 %.
Risperldone Form A
The present invention also relates to a novel risperidone crystalline form
designated Form A and processes for making risperidone Form A. Risperidone
Form A is
characterized by unique strong powder x-ray diffraction peaks at 14.20.2; and
21.30.2
degrees two-theta and medium intensity peaks at
10.6~0.2,11.4~0.2,16.4~0.2,18.9~0.2,
19.90.2, 22.5t0.2, 23.30.2, 27:60.2, 25.40.2, and 29.0f0.2 degrees two-theta.
Another aspect of this invention is a method of preparing risperidone Form A.
In
the method of preparing risperidone Form A, risperidone Form A is crystallized
from
risperidone at the reffux temperature of an organic solvent, such as, DMF,
tetrahydrofuran
(TIC'), acetone, benzene, ethyl methyl ketone, n-butanol, methanol,
isopropanol, absolute
ethanol, acetonitrile, toluene, dimethyl sulfoxide (DMSO), iso-butanol or
ethyl acetate.
By the methods of the present invention, risperidone is added to in a minimum
amount of
organic solvent by heating the mixture to facilitate dissolution of the
risperidone. Upon
complete dissolution of the risperidone, the solution is left to cool to room
temperature to
induce the precipitation of risperidone Form A. After the solution has reached
room
temperature, it is further cooled in an ice bath and then filtered to isolate
risperidone Form
A. Suitable volumes of solvent required for the present methods are listed
below in
Example 11 and in Table 1.
Another aspect of this invention is a method of preparing risperidone Form A;
or a
mixture of risperidone Form A and other forms of risperidone, including
risperidone Form
B; by dissolving risperidone in dichloromethane and adding cyclohexane or
hexane to
7
CA 02535728 2001-08-14
induce precipitation. By the methods of the present invention, risperidone is
dissolved in
dichloromethane in a ratio of about 1 to about 9. Hexane or cyclohexane is
then added
until a cloudy dispersion is formed. The risperidone Form A is then isolated
by filtration.
Another aspect of this invention is a method of preparing risperidone Form A
by
heating risperidone Form B. By the methods of the present invention,
risperidone Form A
is prepared by heating risperidone Form B, or a mixture of risperidone Form A.
and B at
temperatures above room temperature, preferably at about 80°C, under
either reduced
pressure or at atmospheric pressure, for a period of several minutes to
several hours,
preferably 16-20 hours. One embodiment of the present method for preparing
risperidone
Form A is heating risperidone Form B, or a mixture of risperidone Form B and
risperidone Form A, at 80°C overnight, under reduced pressure or at
ahnospheric
pressure, and isolating the resulting crystals of risperidone Form A. An
alternative
method of preparing risperidone Form A by heating risperidone Form B includes,
heating
risperidone Form B in a differential scanning calorimeter, at the rate of S to
20 degrees
1 S per minute, to yield risperidone Form A_
Risperidone Form B
The present invention also relates to a novel crystalline form of risperidone,
denominated risperidone Form B. Risperidone Form B is characterized by unique
strong
powder x-ray diffiaction peaks at 14.0f02 and 21.70.2 degrees two-theta, and
medium
peaks at 10.8~0.2,11.9~0.2,12.6f0.2,17.5~0.2,~ 18.3~0.2,19.9~0.2, 21.0f0.2,
21.30.2
degrees two-theta, and is well distinguished from risperidone Fonn A. The
presence of
risperidone Form B in a mixture with risperidone Form A is detected by the
appearance
mainly of the strongest peaks at 21.710.2, 17.50.2, 18.40.2, and also by the
other peaks
which appear at 11.90.2, 12.60.2 degrees two theta.
2S The DSC thermogram ofrisperidone Form B is characterized by a solid-solid
transition to risperidone Form A detected in a small endotherm at 164°C
followed by a
small exotherm and a melting endothenri of risperidone Form A at 171
°C.
Another aspect of this invention is a method of preparing risperidone Form B
by
dissolving risperidone in an alcohol having 1 to 4 carbon atoms, followed by
the addition
of water to facilitate the precipitation of risperidone Form B. Preferably the
ratio of
8
CA 02535728 2001-08-14
risperidone to alcohol is about 1:7.5 to about 1:9. Preferably the alcohol is
ethanol or
methanol.
Another aspect of this invention is a method of preparing risperidone Form B
pure
or in a mixture with another form of risperidone, such as, risperidone Form A,
which
includes dissolving risperidone in a hot solution of aqueous HCl followed by
the addition
of aqueous Na2C03 to induce precipitation of risperidone Form B. By the
methods of the
present invention, risperidone is added to 0.5 N HCl in a ratio of about 1:6.
Water is
added in an amount equal to about two thirds the volume of HCl used. The
solution is
heated to induce dissolution of the risperidone. Sodium carbonate is then
added until a pH
of about 8 is reached, to facilitate precipitation. The solution is cooled and
risperidone '
Form B is isolated by filtration.
Another aspect of this invention is a method of preparing risperidone Form B
pure
or in a mixture with another form of risperidone such as risperidone Form A,
wherein
xisperidone is dissolved in chloroform followed by the addition of cyclohexane
or hexane
to facilitate precipitation. By the methods of the present invention,
risperidone is
dissolved in chloroform in a ratio of about 1:6 followed by the addition of
hexane of
cyclohexane in an amount sufficient to produce a cloudy dispersion. The
risperidone
Form B is then isolated upon filtration.
Ris~e~idone Forth E
The present invention also relates to a novel crystalline form of risperidone,
denominated risperidone Form E. Risperidone Form E is characterized by typical
strong
x-ray peaks at 16.50.2, 21.710.2 degrees two-theta, and medium x-ray peaks at
12.60.2,
15.610.2, 17.0f0.2, 18.40.2, 19.10.2, 21.30.2, 24.00.2, 24.90.2, 27.00.2
degrees
two-theta
Another aspect of this invention is a method of preparing risperidone Form E.
By
the methods of the present invention, risperidone is dissolved in isopropanol
in a ratio of
about I to 12. Water is then added until a cloudy dispexsion is formed thereby
facilitating
the precipitation of risperidone Form E. itisperidone Form E is isolated upon
filtration of
the dispersion.
In accordance with the present invention, these new forms of risperidone may
be
9
CA 02535728 2006-06-21
pt~ap~ared as pharimaceutical compositions that are particularly useful for
the manageatent
of the manifestations of psychotic disorders. Such compositions comprise one
of the new.
forms of risparidone with pharmaceutically acceptable carriers and/or
excipients known to
one of skill in the art.
Preferably, these compositions are prepared as mod'tcam~ts to be administered
orally, or intravenously. Suitable forms for off al administration include
tablets,
compressed or coated pills, drageea, sachets, hard or gelatin capsules,
sublingual tablets,
syrups and suspensions. While orie of ordinary skill in the art will
understand~that
dosages will vary according to the indication; age of the patient, etc.,
generally
polymorphic forms of rispcridone of the present invention will be administered
at a daily
dosage of about 4 to about 16 mg per day, and preferably about 4 to shoal 8 mg
per day.
:EXAMPLES
Tlie pre$ent invention wili now be further explained in the following
examples. .
. ' However, the present invention.should not be construed as limited thereby.
Methods
Conditions for obtaining Powder X ray Diffraction (PXRD) patterns: ~ The
powder
' TM
X ray diffraction patterns were obtained by methods known in the art using a
Ph~ips X-
ray powder diffractometer, Phillips Generator TW18~U; Goniometer PW3020;MMPD
. . Control PW3710MX-Ray tube with Cu target anode; Monochromatar proportional
. counter; Divergence slits 1 °, Receiving slit 0.2 mm, Scatter slit
l°; 40KV, 30mA.; and
Scanning speed step 0.05 degrees to 2 degreas/min.
The differential scanning calorimeter thermograms were obtained by me~ods
known in the art using a DSC Mettler 821 Stab M The weight of the samples was
about 3-5
em . The 'erature a of scans was .30°C 250°C at a rate of
10°Grain. S les
g . ~8
were. purged with nitrogen gas at a flaw rate of 40~mI:Jtnin. Standard 40 ~1
aluminum
cruc~'b~es weae used having lids with three small holes.
Synthesis of ltisperidone
Isopropanol (20 mL), 3-{2-chloro~:hyl~6,7,8,9-tE~ahyd~c~O-2 methyl-4~I-
~ pyrido[l,2-a]pyrimidin-~4~one (Compound IIX"the chlorine derivative")(2.63
g,10
CA 02535728 2001-08-14
mmoles, l eq.), 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole (Compound I)("the
piperidine derivative") (2.I7 g, IO mmoles, I eq.), sodium carbonate (3.18 g,
30 mmoles,
3 eq.), and potassium iodide (66 mg) were added to a 100 mL round bottom flask
and
stirred with a magnetic stir bar. The flask was placed in an oil bath at
80°C and allowed
S to reflex for 9 hours. The flask was then cooled in an ice bath and the
contents was
filtered. The filter cake was washed in the filter with a small amount of
isopropanol. The
$lter cake was then slurned 3 times in 20 mL of water and filtered. The
resulting slurry
was dried to give 3 g of material in ?3 % yield. The slurry was recrystallized
by
dissolving in 37 mL of boiling isopropanol, filtered hot and allowed to cool
and filtered to
give material which had a purity of 99.7 % and an overall yield of 60
°/.
Example 2
Synthesis of R3speridone
The same materials and method as in Example 1 with the exception being that
methyl ethyl ketone (MEK) (15 mL) was used instead of 20 mL of isopropanol.
The flask
was put in an oil bath at 79-83 °C overnight, cooled, filtered and
washed with acetone and
water to give 2.19 g, 53 % yield.
Example 3
Synthesis of Risperidone
The same materials and method as in Example 1 with the exception being that 20
mL of acetonitrile was used instead of 20 mL of isopropanol. The flask was put
in an oiI
bath for 17 hours at 79-83°C, then put in the freezer for 2 Sours,
filtered, and the filter
cake washed with acetone until the filtrate had no color. The filter cake was
then slunried
in 25 naL water 3 times and filtered and dried to give 3.03 g, 74 % yield, of
crude
risperidone. The crude risperidone was recrystallized from 35 mL of
isopropanol, filtered
hot, cooled, filtered and dried to give 2.47g of risperidone, 60 % overall
yield, 99.8
pure by HPLC.
Example 4
Synthesis of Risperidone
The same materials and method as in Example 1 with the exception being that 20
mL of acetonitrile was used instead of 20 mL of isopropanol. The flask was put
in an oil
11
CA 02535728 2001-08-14
bath for 17 hours at 79-83°C, then put in the freezer fox 2 hours,
filtered, and the filter
cake washed with acetone until the filtrate had no color. The filter cake was
then sluzxied
in 25 mL water 3 times and filtered and dried to give 3.03 g, 74 % yield, of
crude
risperidone. The crude risperidone was recrystallized from 75 mL of acetone,
filtered hot,
cooled, filtered and dried to give 2.25 g of risperidone; 60 % overall yield,
99.9 % pure by
HPLC.
Example 5
Synthesis of Risperidone
The same materials and method as in Example 1 with the exception being that 20
mL of iso-butanol was used instead of 20 mL of isopropanol followed by
stinting in an oil
bath at 78 °C over night. Risperidone was isolated in 63 % yield.
Exam 1e 6
Preparation of Risperidone Form B
Risperidone (5.3 g) was dissolved in chloroform (30 mL). Cyclohexane (280
mL)was slowly added to the solution until a cloudy dispersion was formed. The
suspension was filtered. The filtrate, analyzed by PXRD, contained risperidone
Form B.
Further heating overnight at 80°C under reduced pressure produced
risperidone Form A,
which was confirmed by PXRD analysis.
Example 7
Preparation of Risperidone Form B
Risperidone (S.0 g) was dissolved in 30 mL chloroform. Hexane (250 mL) was
added to the solution until a cloudy dispersion was formed The suspension was
filtered.
The isolated filtrate, analyzed by PXRD, contained risperidone Form B. Further
heating
of the filtrate overnight at 80°C under reduced pressure produced
risperidone Form A,
2S ' which was confirmed by PXRD analysis.
Example 8
Preparation of Risperidone Form B
R.isperidone (5.3 g) was dissolved in 40 ml ethanol. Water (100 mL) was added
to
the solution until a cloudy dispersion was formed. The resulting suspension
was filtered.
The isolated filtrate, analyzed by PXRD, contained risperidone Form B. Further
heating
12
CA 02535728 2001-08-14
of the filtrate overnight at 80°C, under reduced pressure, produced
risperidone Form A,
which was confirmed by PXRD analysis.
Example 9
Preparation of Risperidone Form B
Risperidone (S.0 g) was dissolved in methanol (45 mL). Water (70 ml) was added
to the solution until a cloudy dispersion was formed. The suspension was
filtered. The
isolated filtrate, analyzed by PXRD, contained risperidone Form B. Further
heating of the
filtrate overnight at 80°C, under reduced pressure, produced
risperidone Form A, which
was confirmed by PXRD analysis.
Example 10
Preparation of Risperidone Form B in Water
Risperidone (6 g) was dissolved at room temperature in 60 mL of 0.5 N HCl and
water (40 mL) was added. The solution was heated in a boiling water bath and
stirred
with a magnetic stir bar. Concentrated aqueous sodium carbonate was added
portion-wise
to the solution to facilitate precipitation until a pH of approximately 8 was
attained. A
precipitate was formed. After cooling to room temperature, the mixture was
cooled in an
ice bath and filtered to give a mixture of risperidone Form A and risperidone
Foam B in
an 82 % yield.
Example 11
Preparation of Risperidone Form A by Crystallization in Organic Solvents
ltisperidone (6 g) was added portion-wise and dissolved in a minimum amount of
solvent by heating in a boiling water bath (about 95°C). Suitable
solvents and the
corresponding suitable volumes are listed below in Table 1. Solvents having a
boiling
point lower than 95°C were heated to their boiling point. The solutions
were left to cool
to room temperature to facilitate precipitation of risperidone Form A. The
mixture was
then further cooled in an ice bath and then filtered. The precipitate was
analyzed by
PXRD and found to be risperidone Form A.
13
CA 02535728 2001-08-14
Table 1. Preparationsperidone Form A
of lti ed per 6 grams of
The volumes of Risperidone
solvents us
DMF: 40 ml
iso-butanol: 35 ml
THF: 40 ml
Acetone: 200 ml
Benzene: 26 ml
methyl ethyl ketone:70 ml
absolute ethanol:35 ml
n-butanol: 45 ml
Methanol: 40 ml
Toluene: 45 ml
Acetonitrile: 100 ml
DMSO: 100 ml
ethyl acetate: ~ 150 ml
Isopropanol: 100 ml
Example 12
Preparation of risperidone Form A
Risperidone (5.6 g) was dissolved in 50 mL dichloromethane. Cyclohexane (170
mL) was added to the solution until a cloudy dispersion was formed. The
resulting
suspension was filtered. The isolated filtrate, analyzed by PXRD, contained
risperidone
Form A and a minor quantity of risperidone Form B.
Example 13
Preparation of Risperidone Form A
Risperidone (5.1 g) was dissolved in 30 mL dichloromethane. n-Hexane (150 mL)
was added to the solution to facilitate precipitation until a cloudy
dispersion Was formed.
The resulting suspension was filtered. The filtrate, analyzed by PXItD,
contained
risperidone Form A and a minor quantity of risperidone Form B.
Example 14
Preparation of Risperidone Form E
lRisperidone (5 g ) was dissolved in 60 mL isopropanol. Water (950 mL) was
added to the solution to facilitate precipitation until a cloudy dispersion
was formed. The
suspension. was filtered. The filtrate, analyzed by PXRD, contained
risperidone Form E.
14
CA 02535728 2001-08-14
Although certain presently prefen~i embodiments of the invention have been
described herein, it will be apparent to those skilled in the art to which the
invention
pertains that variations and modifications of the described embodiment may be
made
without departing from the spirit and scope of the invention. Accordingly, it
is intended
that the invention be limited only to the extent required by the appended
claims and the
applicable rules of law.