Note: Descriptions are shown in the official language in which they were submitted.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-1-
METHOD AND APPARATUS FOR CARDIAC RESUSCITATION
The present invention relates generally to the field of cardiac resuscitation
and, in
particular, to a method for restoring cardiac activity after a prolonged
period of asystole or
near-asystole by delivering a combination of circulatory assistance and a
series of
depolarizing cardiac stimulation pulses.
Sudden cardiac death (SCD) takes the lives of an estimated 400,000 to 450,000
people in the United States each year, and the incidence of SCD among young
adults is
increasing. SCD is typically caused by an arrhythmia or coronary artery
disease. Because
of the unexpected nature of SCD, it is difficult to prevent and remains the
attributed cause
of a high proportion of cardiac-related deaths.
Ventricular tachycardia (VT) or ventricular fibrillation (VF) often precede
SCD.
Defibrillation shocks delivered within the first minute of fibrillation onset
can be highly
effective in preventing death and restoring normal heart rhythm. Patients
diagnosed with a
propensity for arrhythmias can benefit from implantable cardioverter
defibrillators, which
provide life-saving electrical stimulation therapies quickly after the onset
of an
arrhythmia.
However, a patient's risk for SCD is often unknown prior to the first
arrhytlnnia
episode, which, unless treated quickly, is often fatal. The chance of
successful
resuscitation from cardiac arrest using cardiopulmonary resuscitation (CPR)
alone is very
low. As few as 1-2% of patients treated with CPR in a hospital are discharged.
CPR
alone will generally not convert VF to sinus rhythm, but even if external
defibrillation
shocks are delivered to terminate VF, the result is often asystole or
pulseless electrical
activity (PEA), also referred to as electro-mechanical dissociation (EMD). The
success
rate of delivering defibrillation shocks decreases dramatically over the first
two to three
minutes following the onset of VF. See August 2003 Annals of Emergency
Medicine
Volume 42 at pages 242-250 entitled, "Optimal Defibrillation Response
Intervals for
Maximum Out-of hospital Cardiac Arrest Survival Rates," by V. DeMaio et al.
The
likelihood of an emergency responder arnving on the scene within four minutes
is small
and so the probability of a successful resuscitation using existing techniques
is low. The
probability of emergency responders arnving on scene within fifteen minutes is
good.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-2-
Thus, new cardiac resuscitation techniques that allow successful reversion to
sinus rhythm
after prolonged VF, fine VF, or asystole are needed.
A consequence of VF that limits the rate of successful resuscitation using
current
methods is the hypoxia that results from impaired perfusion during VF. During
normal
cardiac function, contraction of myocardial fibers occurs when calcium enters
the cell via
calcium channels (L-type channels). Calcium enters the cell when the cell
membrane is
depolarized by a passing action potential to cause an increase in permeability
to calcium
via the L-type channels. Calcium entering the cell causes additional release
of calcium
from the sarcoplasrnic reticulum (SR) through ryanodine channels into the
sarcoplasm.
This "calcium-induced calcium release" increases the sarcoplasma calcium
concentration,
allowing the calcium to interact with the myofilaments to cause mechanical
cycling of the
myofilaments and sarcomere shortening.
Calcium is sequestered back into the SR via intracellular calcium pumps, which
requires the cellular fuel adenosine triphosphate (ATP) to operate and are
lcnown as
"sarcoplasmic or endoplasmic reticulum calcium ATPases" (or "SERCA")..
Myofilament
cycling, in particular during the relaxation phase, also requires ATP. Thus
both of these
operations are affected by hypoxia because oxygen is required for the
production of ATP.
During fibrillation, poor perfusion of the heart leads to hypoxia and a lack
of ATP
available for cellular functions. When a VF, asystolic, or near-asystolic
episode persists
for several minutes, the hypoxic state becomes severe and is a limiting factor
in preventing
external defibrillation attempts from being successful.
However, other mechanisms may play an important role. The ryanodine calcium
channels, which normally release calcium from the SR during calcium-induced
calcium
release, are "leaky," i.e., some calcium is released from the SR in the
absence of the
calcium-induced calcium release mechanism. Normally, SR calcium stores are
replenished during each cardiac cycle via calcium entering the cell through
the L-type
channels and by the SR reuptake of calcium from the sarcoplasma via SERCA. W
the
absence of a regular sequence of action potentials, the SR calcium stores may
be reduced
as calcium is leaked out of the SR and is removed from the cell by sodium-
calcium ion
channels which maintain the cell's normal resting potential. As a result, this
"leaked"
calcium is not available to contribute to sarcomere shortening upon the next
action
potential.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-3-
In the past, this SR calcium leak was considered trivial, however, recent work
suggests that this calcium leak can be substantial. If so, one can theorize
that during
fibrillation, cardiac function becomes increasingly compromised by at least
two
mechanisms. First, hypoxia due to insufficient myocardial perfusion from the
effective
loss of circulation reduces the ATP available for myofilament cycling and SR
calcium
uptake via SERCA. Second, SR calcium depletion due to calcium leaking becomes
significant due to a lack of sufficient cellular depolarization and SERCA
activity to
replenish SR calcium stores thereby reducing calcium available for myofilament
cycling.
Thus, cumulative ATP loss and SR calcium depletion may both contribute to an
exacerbation of loss of function during the first three to five minutes
following cardiac
arrest, precluding successful resuscitation using currently known techniques.
Defibrillation alone may restore a heart beat when the SR calcium stores have
not
been substantially depleted, which theoretically corresponds to the maximum
three to five
minute time course in which defibrillation can be effective. A single high-
voltage
defibrillation shock may act to depolarize a large myocardial mass to allow an
influx in
calcium, which in turn creates a cycle of calcium handling which is able to
regenerate the
normal cardiac cycling process if ATP reserves are not yet depleted. If,
however, SR
calcium stores are substantially depleted, defibrillation alone may not be
sufficient to
restore SR calcium losses without the contribution of extra-cellular calcium
influx through
L-type channels that occurs only with repeated cellular depolarization. If
hypoxia has set
in, ATP is unavailable for myofilament cycling and SERCA. A number of recent
publications have emphasized the importance of CPR administered before shock
delivery.
It has been reported that one minute of CPR delivered before shock delivery
when
fibrillation has been present for longer than four minutes resulted in better
survival than
immediate defibrillation.
Even when CPR has been administered, however, individual defibrillation shocks
typically do not restore sinus rhythm after a prolonged fibrillation or
asystolic episode. A
single depolarizing shock may not be adequate in restoring the cellular
cycling of calcium
handling because the calcium influx occurring during a single depolarization
may be
insufficient to restore normal SR calcium concentration. Early works in the
development
of transcutaneous defibrillation therapies reported that a series of shocks
was more
effective than a single, more energetic, shock. In one study, a series of
shocks were
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-4-
uniformly successful if the initial shock failed to defibrillate completely. A
series of
depolarizations may be necessary to restore normal SR calcium concentration
through the
additive effect of a sequence of depolarizations making intracellular calcium
available for
SR sequestration.
Despite the early observations in defibrillation studies, later work largely
focused
on single shock defibrillation as currently implemented in implantable
defibrillation
devices. Single shoclc defibrillation requires less energy than a series of
shocks and is thus
more feasible to implement in implantable devices. Ultimately, single shock
defibrillation
has proven effective in the scenario of fibrillation detection within seconds
of onset by
arrhythmia detection algorithms available in implantable'devices.
The use of multiple pulses during treatments of cardiac arrhythmias has been
proposed in several patents. A method of reducing the likelihood of onset of
pulseless
electrical activity (PEA) after defibrillation in a subject afflicted with a
fibrillating heart
including a first treatment waveform insufficient to defibrillate the heart
and a second
treatment waveform that defibrillates the heart is generally disclosed in U.S.
Pat. Appl.
Publication No. 200210161407 to Walcott et al. A system and method for
delivering
multiple closely spaced defibrillation pulses to a heart is generally
disclosed in U.S. Pat.
No. 5,620,464, issued to Kroll, et al., which reduces the overall size of the
main energy
delivery capacitor for pulse delivery. A process to apply an electrical
pretreatment to a
fibrillating heart that begins the process of organizing the action of the
chaotically
contracting myocardial cells, so that the defibrillating waveform applied
after the
pretreatment can accomplish its task with less energy than would otherwise be
required is
generally disclosed in U.S. Pat. No. 5,314,448 issued to Kroll et al. In U.S.
Pat. No.
6,314,319 issued to Kroll, et al., an electrical method of stimulating cardiac
cells causing
contraction to force hemodynamic output during fibrillation, hemodynamically
compromising tachycardia, or asystole is generally disclosed in a method
referred to as
"Electrical Cardiac Output Forcing."
A method for treating the heart to restore blood flow where electromechanical
dissociation occurs after termination of a ventricular tachyarrhythmia of
ventricular
fibrillation including identifying electromechanical disassociation after
termination of a
ventricular tachyarrhythmia or a fibrillation and providing electrical
therapy, the therapy
comprising a series of packets of electrical pulses is generally disclosed in
PCT
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-5-
Publication No. WO 00/66222, issued to Rosborough and Deno. The series of
pulse
packets is delivered after ventricular tachyarrhythmia or fibrillation is
terminated and
electromechanical dissociation is detected.
There remains a need, however, to address the problem of cardiac resuscitation
after a prolonged episode of ventricular fibrillation or asystole, after which
the severity of
hypoxia plays an important role in limiting the success of electrical
stimulation based
methods for defibrillating the heart. In the scenario of prolonged
fibrillation or asystole,
e.g., greater than one or two minutes, the inventors of the present invention
propose that
both hypoxia and progressive SR calcium loss contribute to the lower success
rate of
single-shock defibrillation. In order to improve the success of cardiac
resuscitation,
resuscitative methods must therefore address hypoxia and intracellular calcium
loss.
Resuscitative methods are needed, therefore, that include mechanisms for
alleviating
hypoxia for making ATP available for ATP-dependent calcium pumps to allow
normalization of SR calcium storesbecause the repetitive delivery of
electrical stimulation
are believed to make more calcium available to the pumps.
The present invention is directed toward providing a system and method for
performing cardiac resuscitation, in particular after a prolonged episode of
VF, fine VF or
asystole. The present invention is achieved in a system and method that
include the
provision of circulatory assistance, which may be in the form of manual or
automatically
delivered CPR or other perfusion or hemodynamic assistance, and inducing a
series of
cardiac depolarizations after a period of circulatory assistance. In one
embodiment, the
series of cardiac depolarizations are induced by electrical stimulation pulses
delivered at a
pulse energy that depolarizes a mass of myocardial cells. The pulse series may
therefore
contain pulses of relatively low energy, such as the energy normally
associated with
cardiac pacing pulses, referred to herein as "pacing-class pulses," and/or
pulses of
relatively high energy normally associated with defibrillation shocks,
referred to herein as
"defibrillation-class pulses." The series of cardiac stimulation pulses may be
delivered at
a regular or varying rate and regular or varying amplitude for an interval of
time ox
number of pulses. In one embodiment, the series of stimulation pulses is
concluded with a
high-voltage defibrillation shoclc at a specified interval following the last
stimulation pulse
of the series.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-6-
Apparatus for delivering the electrical stimulation portion of the
resuscitation therapy may be embodied as an external stimulation device and
associated
set of electrodes for delivering cardiac stimulation pulses transcutaneously,
percutaneously, or esophogeally. Alternatively, apparatus for delivering
cardiac
stimulation pulses may be embodied as an implantable cardiac stimulation
device and
electrode system capable of delivering cardiac stimulation pulses via
intracardiac
electrodes, epicardial electrodes, or subcutaneously or sub-muscularly placed
electrodes.
In a programmable, implantable device the resuscitation method provided by the
present
invention may be included in a selectable menu of arrhythmia therapies.
In other embodiments, the cardiac stimulation device further includes
cardiac stimulation therapies provided for improving cardiac output following
cardiac
resuscitation. In one embodiment, the cardiac stimulation device includes the
provision of
extra systolic stimulation pulses delivered to achieve the mechanical benefits
of post-extra
systolic potentiation.
In yet other embodiments, a physiological sensor, capable of generating a
signal
related to the delivery of circulatory support, is included in the system. The
sensor may be
provided as a sensor of blood oxygen saturation (Sa02) or a surrogate
therefore such as
lactate or hydrogen peroxide, pH or other metabolic parameter that directly or
indirectly
indicates the degree of hypoxia. In alternative embodiments, the sensor may be
provided
as a mechanical sensor that generates a signal related to the presence of
circulatory support
such as a blood pressure sensor, accelerometer or a mechanical sensor
sensitive to chest
compressions delivered during CPR. The sensor signal may be used by an
implantable
stimulation device for automatically detecting the presence and duration of
circulatory
assistance for use in determining the appropriate time for initiating the
electrical
stimulation portion of the resuscitation therapy.
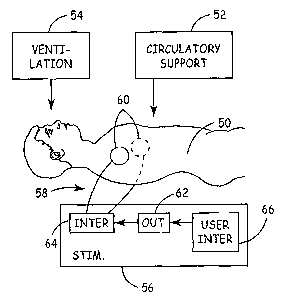
Figure 1 is a schematic diagram of a method for delivering a cardiac
resuscitation
therapy after a prolonged episode of VF, near asystole or asystole.
Figure 2 is a functional block diagram of one embodiment of an external
stimulation device, which may be used in delivering the cardiac resuscitation
therapy.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
Figure 3 is an illustration of an implantable cardiac stimulation device and
associated cardiac lead deployed in a patient's heart, which may be used in
delivering the
cardiac resuscitation therapy.
Figure 4 is a functional block diagram of the implantable cardiac stimulation
device of Figure 3.
Figure 5 is a functional block diagram of an implantable cardiac stimulation
device
that includes a sensor used for determining when the electrical stimulation
portion of the
resuscitation therapy should be initiated.
Figure 6 is a timing diagram of one method for delivering cardiac
resuscitation
according to the methods of the present invention.
Figure 7 is a timing diagram depicting the events occurring during an
alternative
method for delivering cardiac resuscitation according to the present
invention.
Figure 8 is a timing diagram of a method for performing cardiac resuscitation
according to the present invention that includes the delivery of extra
systolic stimulation
for improving hemodynamic function after successfully resuscitating the heart.
Figure 9 is a timing diagram illustrating a method for delivering cardiac
resuscitation that includes delivering both pacing class and defibrillation
class pulses.
Figure 10 is a timing diagram illustrating an alternative method for
performing
cardiac resuscitation according to the present invention.
Figure 11 is a graph ~of experimental results obtained from an isolated
myocyte
preparation.
. Figure 1 is a schematic diagram of a method for delivering a cardiac
resuscitation
therapy after a prolonged episode of VF, fine VF, or asystole. As used herein,
VF refers to
coarse VF which appears on an ECG as relatively high amplitude fibrillation
waves which
are typically readily observable on ECG monitoring equipment; "fine VF" refers
to the
presence of relatively low amplitude fibrillation waves which may not be
observable on
some ECG monitoring equipment. The term "asystole" as used herein refers to
the
complete absence of electrical activity and activity that is sometimes
referred to as
"bradycardia asystole" wherein electrical activity may be present but at a
very low rate of
about 10 depolarizations per minute or less.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
_g-
The method includes delivering circulatory assistance 52, which may be in the
form of external chest compressions as used in CPR. Preferably, the patient 50
is provided
with circulatory assistance 52 to prevent or alleviate hypoxia during a
prolonged episode
of VF, fine VF or asystole. By preventing or alleviating hypoxia, it is
believed that ATP
will be made available for SERCA as well as other cellular functions. In
addition,
maintaining perfusion of the brain is of particular importance during the
prolonged VF,
fme VF, or asystole in order to avoid irreversible cerebral damage upon
successful cardiac
resuscitation.
Chest compressions may be delivered manually by an emergency responder or
automatically using automated resuscitative equipment. If the patient 50 is
not breathing
spontaneously, ventilatory support may also be required. Ventilation 54 may be
delivered
manually, according to known CPR techniques, or with the use of a ventilator.
Depending
on the location of the patient, equipment available at the site, and the skill
of the
emergency responders, the type of ventilation 54 and circulatory assistance 52
applied
may vary. For example, in a hospital or emergency room setting the patient may
be placed
on a ventilator and receive manual or automated chest compressions. In a
surgical setting,
direct heart massage may be provided for circulatory assist or another type of
circulatory
assist mechanism may be in place such as an infra-aortic balloon pump or extra-
corporeal
membrane oxygenation (ECMO). In an out-of hospital setting, manual CPR may be
the
only circulatory assist available.
The resuscitation method further includes the delivery of cardiac stimulation
pulses
using a stimulation device 56, which, in the embodiment shown in Figure 1 is
an external
electrical stimulation device. Device 56 delivers cardiac electrical
stimulation pulses via
an associated set of leads 58 and electrodes 60. Electrodes 60 may be provided
as
cutaneous electrodes, typically placed on the torso and generally in the
thoracic area, for
transcutaneous cardiac stimulation. The depicted positions of electrodes 60
are merely
exemplary and alternate locations of electrodes 60 may be used for delivering
cardiac
stimulating pulses. Electrodes 60 may alternatively be adapted for
percutaneous or
esophogeal placement for stimulating the heart. For infra-operative cardiac
resuscitation
applications, electrodes 60 may take the form of epicardial electrodes that
may be placed
directly on the surface of the heart. Other types of electrodes lcnown for
invasively or non-
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-9-
invasively applying cardiac electrical stimulation may be utilized for
delivering cardiac
stimulation pulses in practicing the present invention.
Device 56 includes an interface 64 for coupling leads 58 to pulse generating
output
circuitry 62. Output circuitry 62 may include high-voltage output circuitry
for delivering
high-voltage, defibrillation-class, shocking pulses and/or low-voltage output
circuitry for
delivering low-voltage, pacing-class pulses. High-energy output circuitry for
use in an
external defibrillator is generally disclosed in U.S. Pat. No. 5,824,017
issued to Sullivan et
al., incorporated herein by reference in its entirety. Device 56 may be a
battery powered
device and may alternatively or additionally include DG input with appropriate
electrical
shielding to allow connection to a wall socket.
In a basic embodiment, the rate, pulse energy, pulse shape and other features
of the
electrical stimulation pulses delivered by device 56 are fixed and delivered
by output
circuitry 62 upon enabling or empowering device 56 via the user interface 66.
In
alternative embodiments, parameters controlling the delivery of a cardiac
electrical
stimulation pulse series may be set by an emergency responder via a user
interface 66
coupled to output circuitry 62. Various output parameters including, but not
limited to,
pulse energy, pulse amplitude, pulse width, pulse rate, andlor duration of the
pulse series
may be set by an emergency responder using the user interface 66.
Figure 2 is a functional block diagram of one embodiment of the cardiac
stimulation device of Figure 1. In this embodiment, the stimulation device 56
is an
external stimulation device that includes sensing circuitry 70 for monitoring
the patient's
ECG. Sensing circuitry 70 is coupled to lead interface 64 for receiving ECG
signals from
electrodes 60. The ECG may be visually displayed on display 68 for viewing by
the
emergency responder and/or used by external stimulation device 56 for
detecting the
presence of cardiac activity and classifying the heart rhythm. Such
information may be
used by device 56 for selecting stimulation pulse parameters and controlling
the time that
a depolarizing stimulation pulse series is initiated. Device 56 may be
provided with
asystole detection capabilities as generally disclosed in U.S. Pat. No.
6,304,773 issued to
Taylor et al., incorporated herein by reference in its entirety. Other rhythm
detection and
classification algorithms known for use in cardiac stimulation or monitoring
devices may
be implemented for detecting and classifying the heart activity and in
recommending
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-10-
andlor automatically selecting a resuscitation therapy based on the detection
of prolonged
VF, fine VF, or asystole.
Display 68 may include visual or audio signals correlating to electrical
activity of
the heart. During or after the delivery of a series of depolarizing stimuli,
the
depolarizations, intrinsic or evolved, are expected to be initially
accompanied by
mechanically weak contractions, which will grow in strength with subsequent
depolarizations. Hemodynamic benefit may be provided by synchronizing chest or
heart
compressions with the weak heartbeats. As such, a depolarization sensed by
sense circuit
70 may cause display 68, under the control of microprocessor 72, to generate a
signal
perceivable by an emergency responder, which may be an acoustic, tactile
and/or visual
signal, indicating the occurrence of a cardiac depolarization or other
triggering event for
the delivery of manual CPR. An emergency responder may then deliver chest or
heart
compressions, or another form of generally pulsatile circulatory assistance,
synchronized
to the depolarizations so as to enhance the cardiac output of the weak
mechanical
contraction.
External stimulation device 56 is shown in Figure 2 as a microprocessor-
controlled
device wherein cardiac stimulation functions may be controlled by
microprocessor 72.
However, it is recognized that device 56 may be provided as other types of
pulse
generating devices that are not microprocessor-based, for example devices that
utilize a
platform of dedicated digital or analog circuitry. User interface 66 may allow
entry of
patient-related data such as time of VF/asystole episode onset and/or duration
of CPR or
other circulatory assistance. Such data may be used by device 56, in
conjunction with the
currently sensed cardiac activity, in automatically selecting or recommending
when and
what type of electrical pulse series should be delivered.
In alternative embodiments, a cardiac stimulation device used in delivering
the
electrical stimulation portion of cardiac resuscitation according to the
present invention
may be provided as an implantable electrical stimulation device. Figure 3 is
an illustration
of an implantable cardiac stimulation device 210 and associated cardiac lead
216 deployed
in a patient's heart 208. Stimulation device 210 includes a connector bloclc
212 for
receiving the proximal end of one or more cardiac leads deployed in operative
relation to a
patient's heart 208. In Figure 3, a right ventricular lead 216 is used for
positioning
electrodes for sensing cardiac activity and delivering cardiac stimulation
pulses which may
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-11-
include relatively low-energy, pacing-class pulses andlor high-energy
cardioversion/defibrillation-class shock pulses. For these purposes, right
ventricular (RV)
lead 216 is equipped with a ring electrode 224, a tip electrode 226,
optionally mounted
retractably within an electrode head 228, an RV coil electrode 220, and a
superior vena
cava (SVC) coil electrode 230, each of which are connected to an insulated
conductor
contained within the body of lead 216. The proximal end of the insulated
conductors are
coupled to corresponding connector terminals carned by lead connector 217 at
the
proximal end of lead 216 for providing electrical connection to the device
210.
The electrodes 224 and 226 may be used as a bipolar pair for sensing cardiac
activity or delivering low-energy stimulation pulses, commonly referred to as
a "tip-to-
ring" configuration, or individually in a unipolar configuration with the
device housing
211 serving as the indifferent electrode, commonly referred to as the "can" or
"case"
electrode. The device housing 211 may also serve as a subcutaneous electrode
in
combination with one or both of the coil electrodes 220 or 230 for delivering
high-energy
stimulation pulses to the atria or ventricles.
The depicted positions of the RV lead 216 and electrodes 224, 226, 220 and 240
shown in Figure 3 in or about the right heart chambers are approximate and
merely
exemplary. Furthermore, it is recognized that alternative leads having other
combinations
of tip, ring, canister-based, and/or coil electrodes provided for stimulating
or sensing at
particular sites in one or more heart chambers may be used in conjunction with
the present
invention. While a particular implantable cardiac stimulation device and lead
system is
illustrated in Figure 3, methodologies included in the present invention may
be applied in
single chamber, dual chamber, or mufti-chamber systems which include unipolar,
bipolar
or multipolar leads positioned endocardially, epicardially or within the
coronary sinus.
The present invention may alternatively be implemented in a system that does
not employ
leads for deploying electrodes within or on the heart. For example, a device
implanted
subcutaneously or sub-muscularly in an operative location relative to the
heart, such as in
the left or right pectoral regions, could use non-intracardiac lead based
methods for
electrical sensing to detect cardiac activity and for delivering electrical
stimulation. Such
systems may employ subcutaneous or submuscular electrodes incorporated in or
on the
device housing.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-12-
Figure 4 is a functional block diagram of the implantable cardiac stimulation
device of Figure 3. Device 210 includes a microprocessor 250, pacing output
circuitry
252, cardioversion/defibrillation output circuitry 254, and stimulation timing
and control
circuitry 256 which are linked by control/data bus 258. In accordance with the
present
invention; device 210 delivers the depolarizing stimulation portion of the
cardiac
resuscitation therapy. A series of depolarizing electrical stimulation pulses
may be
delivered by pacing output circuitry 252 and/or cardioversion/defibrillation
output
circuitry 254 under the control of stimulation timing and control circuitry
256. Device 210
is equipped with terminals 260, 262, 264 and 266 for electrical connection to
electrodes
placed in operative relation to the heart. Terminals 260 and 262 may be
coupled to low-
voltage pacing/sensing electrode for delivering relatively low-voltage, pacing-
class pulses
within the pulse series. Terminals 264 and 266 may be coupled to high-voltage
electrodes
for delivering relatively high-voltage, defibrillation-class pulses within the
pulse series.
Parameters controlling the delivery of the pulse series may be programmed or
stored in
memory associated with microprocessor 250 and communicated to stimulation
timing and
control 256 via data bus 258.
Device 210 may additionally be capable of sensing cardiac activity and
delivering
pacing, cardioversion, defibrillation and/or other cardiac stimulation
therapies according to
methods known in the art. General sensing, pacing and defibrillation function
may be
provided according to the description provided in U.S. Pat. No. 5,117,824
issued to
Keimel, incorporated herein by reference in its entirety.
Typically, electrical signals from terminals 260 and 262 are provided to
sensing
circuitry 280 on input lines 270 and 272. Terminals 260 and 262 provide
electrical
connection to a sensing electrode pair, e.g. a bipolar tip-to-ring pair, which
may be the
same electrode pair used for delivering pacing-class stimulation pulses, and
therefore
terminals 260 and 262 may be additionally coupled to pacing output circuitry
252, as
shown in Figure 4. In response to the detection of a cardiac signal, e.g. a P-
wave or an R-
wave, the sensing circuitry 280 provides a logic signal on output line 274 to
stimulation
timing and control circuitry 256, which serves to reset an escape interval
used to control
the timing of stimulation pulse delivery. Intervals between sensed events may
be used for
detection and classifying the heart rhythm.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-13-
According to general pacing operations, if timing and control circuitry 256
does
not receive a signal on output line 352 for a predetermined period of time
corresponding to
the escape interval set for controlling the timing of cardiac stimulation
pulses, the timing
and control circuitry 256 will trigger the generation of a pacing pulse by
pacing output
circuit 252. A disable signal on line 276 prevents sensing of the pacing pulse
by default
sensing circuitry 280. The gain of sensing circuitry 280 is also controlled by
timing and
control 256 on signal line 278.
Terminals 264 and 266 provide electrical connection to a high-energy
stimulation
electrode configuration, which will generally includes at least one coil
electrode paired
with another coil electrode and/or the device housing. Terminals 264 and 266
are coupled
to cardioversion/defibrillation output circuitry 254 and used for delivering
high-energy,
cardioversion/defibrillation-class pulses.
In response to the detection tachycardia, an anti-tachycardia pacing therapy
may be
delivered if desired by loading a regimen from microprocessor 250 into timing
and control
circuitry 256 according to the type of tachycardia detected. In the event that
higher
voltage cardioversion or defibrillation shock pulses are required,
microprocessor 250
activates the cardioversion and defibrillation output circuitry 254. Timing of
the delivery
of the defibrillation or cardioversion pulse is controlled by timing and
control circuitry
256.
Any ventricular cardioversion or defibrillation pulse control circuitry known
for
use in implantable cardioverter/defibrillators may be usable in conjunction
with the
present invention. In the illustrated device, delivery of cardioversion or
defibrillation
pulses is accomplished by cardioversion/defibrillation output circuit 254,
under control of
timing/control circuitry 256 via control bus 258. Output circuit 254
determines the shock
pulse waveform, e.g. whether a monophasic, biphasic or multiphasic pulse is
delivered,
which electrodes are involved in delivery of the pulse, the pulse shape and
tilt, pulse
energy, etc.
In modern implantable cardioverter defibrillators (ICDs), the particular
therapies
are programmed into the device ahead of time by the physician, and a menu of
therapies is
typically provided. For example, on initial detection of tachycardia, an anti-
tachycardia
pacing therapy may be selected. On redetection of tachycardia, a more
aggressive anti-
tachycardia pacing therapy may be scheduled. If repeated attempts at anti-
tachycardia
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-14-
pacing therapies fail, a higher-level cardioversion pulse therapy may be
selected thereafter.
As in the case of currently available ICDs, the amplitude of the
defibrillation shock may
be incremented in response to failure of an initial shock or shocks to
terminate fibrillation.
Sustained VF, fme VF or asystole may persist or develop when defibrillation
shock
therapies fail to convert VF to sinus rhythm using the single-shock
defibrillation approach
within the first one to two minutes after VF detection. Cardiac resuscitation
therapies
according to the present invention may be programmed to be initiated after an
interval of
unsuccessfully treated VF or upon detection of fme VF or asystole following
attempted
defibrillation therapies. The cardiac resuscitation therapy provided by the
present
invention may therefore be included in a programmable menu of arrhythmia
therapies.
The resuscitative therapy includes the delivery of a series of stimulation
pulses
delivered either before hypoxia has become severe or after circulatory
assistance has been
provided to reverse hypoxia. The stimulation pulse series, which may include
relatively
low energy, pacing-class pulses and/or high-energy defibrillation-class
pulses, is delivered
by the pacing output circuitry 252 and/or the cardioversion/defibrillation
output circuitry
254 under the control of timing and control circuit 256. Parameters
controlling the pulse
series may be programmable and may include, but are not limited to, the type
of pulses
included in the series (pacing class or defibrillation class), pulse
amplitude, pulse width,
pulse shape, pulse rate, and time duration of the pulse series or the total
number of pulses.
These parameters are applied by timing and control circuitry 256 according to
data
received from microprocessor 250 on data bus 258.
Device 210 may further include a cardiac event indicator 284, which includes
circuitry for generating a signal, such as a visual, tactile, and/or audible
signal (such as a
beep or a tone), to indicate to an emergency responder the occurrence of a
heart beat. As
noted previously, during or after the delivery of a series of depolarizing
stimuli, the
depolarizations, intrinsic or evoked, are expected to be accompanied initially
by
mechanically weak contractions, which will grow in strength with subsequent
depolarizations. Hemodynamic benefit may be provided by synchronizing chest or
heart
compressions with the wealc heartbeats. As such, a depolarization sensed by
sense circuit
280 or a depolarizing pulse delivered by pace output circuitry 252 or
cardioversion/defibrillation output 254 may cause event indicator 284, under
the control of
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-15-
microprocessor 72, to generate a signal perceivable by an emergency responder,
indicating
the occurrence of a cardiac event.
Generation of such signals may be automatically or manually enabled after the
stimulating pulse series is initiated such that an emergency responder may
deliver chest or
heart compressions, or another form of pulsatile circulatory assistance,
synchronized to the
cardiac event so as to enhance the cardiac output produced by the weak
mechanical
contraction during and/or after the series of pulses. The generation of
cardiac event
indicator signals may be disabled automatically after a predetermined interval
of time
during which normal cardiac function is expected to be restored and/or
manually disabled
at any time by the emergency responder using an external programming device.
Alleviating hypoxia via circulatory assistance prior to delivering the series
of
depolarizing stimuli is important in preparing the myocytes to benefit from
the stimulation
portion of the resuscitation therapy. As such, a sensor capable of generating
a signal
indicative of the delivery of circulatory assistance or for detecting blood
oxygen levels
may be included in an implantable device provided for practicing the present
invention.
By detecting the delivery and duration of circulatory assistance and/or the
level of or
change in blood oxygen saturation, the irnplantable device can determine when
initiation
of the stimulation portion of the resuscitation therapy is appropriate.
Figure 5 is a functional block diagram of an implantable cardiac electrical
stimulation device that includes a sensor used for determining when the
stimulation
portion of the resuscitation therapy should be initiated. Sensor 290 may be
included
within and/or on the housing of the implantable device 210 or may be located
external to
device 210 but implanted within the body of the patient. Sensor 290 is
connected to
sensor processing circuitry 292 for receiving and processing signals generated
by sensor
290.
Sensor 290 may be embodied as an oxygen sensor used for detecting blood oxygen
saturation levels to indicate the relative level of hypoxia. In this
embodiment, sensor 290
may be positioned on a lead and deployed in an infra-cardiac or infra-arterial
location.
Such a lead may further include cardiac stimulation or sensing electrodes and
be coupled
to device 210 via the connector block.
A.signal that is correlated to blood oxygen saturation levels may be
conditioned
and processed by sensor processing circuitry 292. The resulting oxygen
saturation data is
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-16-
provide to microprocessor 250 for use in determining if the measured oxygen
saturation
level indicates a reversal of hypoxia during a prolonged episode of VF, fine
VF or asystole
as is expected after CPR or other circulatory assistance has been delivered
for a period of
time, e.g, about one minute or more. Upon detection an oxygen saturation level
that is
increased by a specified amount over a previous, hypoxic oxygen saturation
level or upon
detection of an oxygen saturation greater than a specified minimum level, the
stimulation
portion of the resuscitation therapy may be initiated by device 210.
In alternative embodiments, sensor 290 may be provided as a mechanical sensor
capable of generating a signal indicating the presence of circulatory
assistance. In one
embodiment, sensor 290 may be embodied as a pressure sensor capable of
detecting the
increase in blood pressure created during CPR or other circulatory assistance
delivery. In
I another embodiment, sensor 290 may be embodied as an accelerometer or
piezoelectric
sensor capable of generating a signal corresponding to the application of
chest
compressions delivered during manual or automated CPR. The output of a
mechanical
sensor is provided to sensor processing circuitry 292 for signal conditioning
and
processing such that data relating to the mechanical effects of circulatory
assistance can be
provided to microprocessor 250. Microprocessor 250 may initiate the
stimulation portion
of the resuscitation therapy after detecting circulatory assistance for a
sustained interval of
time, e.g., after about one minute.
Figure 6 is a timing diagram of one method for delivering cardiac
resuscitation
according to the methods of the present invention. An ECG signal initially
shows
essentially no cardiac activity during a prolonged episode of asystole 100.
The
resuscitation methods may be administered following a prolonged episode of VF,
fine VF,
or asystole. An emergency responder begins to deliver CPR or another form of
circulatory
support at 102 to alleviate hypoxia. If medical-grade oxygen is available,
ventilation of
medical-grade oxygen will more quickly reverse hypoxic conditions. CPR or
other
circulatory assistance is delivered for a period of time 104 prior to
initiating the
stimulation portion of the resuscitation therapy. Current cardiac
resuscitation techniques
generally emphasize administration of defibrillation shocks as quickly as
possible
following cardiac arrest. However, in order to achieve a successful response
to the
stimulation portion of the resuscitation method, it is expected that ATP must
be available
for powering calcium-handling functions such as SERCA. Therefore, CPR or
another
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-17-
form of circulatory support is provided for an interval of time to alleviate
myocyte
hypoxia and make ATP available to the myocytes for calcium handling functions.
An
appropriate interval of time for delivering circulatory support may be on the
order of one
minute, but may be longer or shorter depending on the severity of the hypoxia
and the
duration of the VF, fine VF or asystolic episode. The duration of circulatory
assistance
may be a nominal interval of time, such as one minute, or be based on sensing
blood
oxygen levels or another physiological indicator of hypoxia such as pH.
After an interval of circulatory assistance 104, the stimulation portion of
the
resuscitation therapy is administered by delivering a series of stimulation
pulses 106. The
stimulation pulses may be delivered by an external device or an implantable
device, such
as the devices described in conjunction with Figures 1 through 5 above. The
stimulation
pulses may be relatively low-voltage, electrical pacing-class pulses that are
of high enough
energy to cause depolarization of a mass of myocardial cells. The pulses may
alternatively
be high-energy electrical shocking pulses. The pulses are delivered at a
predetermined
pulse rate, for example a rate of on the order of 1 Hz. The pulses are
delivered for an
interval of time 108, for example on the order of a minute or longer. Without
intending to
limit the present invention to any particular theory, it is presently believed
that a series of
depolarizing pulses is needed to restore normal SR calcium concentrations by
the influx of
calcium during successive cellular depolarizations and sequestration of that
calcium by the
aerobic function of the calcium pumps.
After the pulse series 106 is delivered, the cardiac activity is monitored to
verify
successful restoration of normal sinus rhythm 112. In some cases, a prolonged
condition
of asystole or fine VF may be reverted to VF rather than sinus rhytlun. Figure
7 is a
timing diagram depicting the events occurring during an alternative method for
delivering
cardiac resuscitation according to the present invention. In this example, a
series of low-
voltage pulses 106 delivered after a period of circulatory support 102 is
terminated by a
defibrillation pulse 120, which may follow the last of pulses 106 by a
predetermined time
interval 118. The defibrillation pulse 120 is provided in order to convert VF
116 to sinus
rhythm 122 when the prolonged asystole or fine VF 100 is converted to VF 116
by the
pulse series 106.
It is further recognized that an implantable or external stimulation device
may
deliver bradycardia pacing to maintain a desired heart rate once normal
electromechanical
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-I8-
association is regained after a successful resuscitation therapy but the
intrinsic rate
remains bradycardic.
While the interval of circulatory assistance 102 is shown to end prior to the
onset
of the pulse series 106 in Figures 6 and 7, it is contemplated that
circulatory assistance
may be delivered continuously during the depolarizing stimulation delivery if
the
circulatory assistance does not interfere with stimulation delivery and the
pulse series is
not composed of high-voltage electrical stimulation pulses that would impose
risk to an
emergency-responder delivering CPR or other circulatory assistance. It is
further
contemplated that intervals of circulatory assistance may be interspersed with
intervals of
stimulation pulses.
A form of circulatory assistance may additionally be continued or restarted
after
the series of stimulation pulses is completed to support post-resuscitation
hemodynamic
recovery. In some embodiments, cardiac stimulation therapies aimed at
improving caxdiac
hemodynamic performance may be delivered by the stimulation device after the
pulse
series in order to increase cardiac output. Such stimulation therapies may
include, but are
not limited to, cardiac resynchronization therapy and/or extra systolic
stimulation. Figure
8 is a timing diagram of a method for performing cardiac resuscitation
according to the
present invention that includes the delivery of extra systolic stimulation for
improving
hemodynamic function after successfully xesuscitating the heart.
A prolonged VF or asystolic episode 100 is first treated With CPR 102 or other
circulatory assistance for alleviating hypoxia. The circulatory assistance is
followed by a
series of depolarizing stimulation pulses 106, presently believed to alleviate
SR calcium
loss. After completing the series of pulses 106 and verifying that sinus
rhytlnn 112 is
restored, or electro-mechanical association is restored and the heart rate is
maintained by
bradycardia pacing, extra-systolic stimulation 140 is delivered to enhance
cardiac pumping
function by achieving a post-extra systolic potentiation effect. Aspects and
benefits of
extra-systolic stimulation for achieving the mechanical benefits of post-extra
systolic
potentiation are described in PCT Patent Publication WO 03/020364 to Deno, et
al.,
incorporated herein by reference in its entirety.
Extra systolic stimulation 140 may be applied by delivering an extra systolic
stimulation pulse 136 following ventricular events (VE) 134, which may be
sensed R-
waves or ventricular pacing pulses. Extra systolic stimulation pulses 140 may
be
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-19-
delivered with every paced or intrinsic cardiac cycle or less frequently,
e.g., at some ratio
of the intrinsic or paced heart rate. In the example of Figure 8, an extra
systolic (ES)
stimulation pulse 136 is delivered after every other R-wave 130 sensed as a
ventricular
event (VE) 134 in order to induce an extra systolic depolarization 132.
Additional details
regarding the control of extra systolic stimulation pulse delivery are
provided in non-
provisional U.S. patent aapplication no 10/ ~~XX,XXX (Atty Dkt No. P-11214.00)
to
Burnes et al., and non-provisional U.S. patent aapplication no 10/ XX~~,XX~~
(Atty Dkt
No. P-11252.00) to Burnes, et al., both of which are incorporated herein by
reference in
their entirety.
The pulse series 106 shown in Figures 6, 7 and 8 is shown to be consisting of
pulses of fixed pulse amplitude delivered at a constant rate. It is recognized
that a pulse
series may consist of pulses of different or varying pulse energies or
amplitudes and may
be delivered at different or varying rates within a pulse series. A series of
pulses delivered
with the intention of restoring normal SR calcium levels and myocyte calcium
handling
may be tailored in order to provide the most effective restoration of normal
cardiac
activity, which may depend in part on the initial cardiac activity present
when the
resuscitation methods are begun and/or the cardiac activity present during or
after the
interval of circulatory support and an initial pulse series.
In one embodiment, the cardiac stimulation device monitors for a return of VF
during resuscitation procedures and alters the stimulation portion of the
resuscitation
therapy if VF is detected. Figure 9 is a timing diagram illustrating a method
for delivering
cardiac resuscitation that includes delivering both pacing-class and
defibrillation-class
pulses. After the pulse series 106 is initiated, the asystolic episode 100 is
converted to VF
i
at 142. Upon detecting VF, a high-energy shocking pulse is delivered in place
of a low-
energy pulse in the pulse series. A number of high-energy shock pulses 146 may
replace
the low-energy pulses in the pulse series, or all remaining pulses in the
pulse series may be
delivered as high-energy shocking pulses to convert and prevent VF.
Alternatively, or
additionally, a pulse series may include a sequence of varying rate pulses
148. A sequence
of varying rate pulses 148 may be delivered at a high rate that is gradually
reduced to a
slower rate in an attempt to convert or prevent VF from resuming during or
after the pulse
series.
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-20-
In the example of Figure 9, circulatory assistance 102 is provided for an
interval of
time 104 prior to the onset of the stimulation pulse series 106 and continues
during
administration of the pulse series until the high-voltage defibrillation-class
pulses 146 are
delivered in response to VF 142 detection. Upon terminating VF, low-energy
pulses 148
are delivered, and circulatory assistance is resumed at 103.
Figure 10 is a timing diagram illustrating an alternative method for
performing
cardiac resuscitation according to the present invention. The fibrillation
waves of a
sustained VF or fme VF episode may contribute to the depletion of
intracellular calcium
since these waves, though mechanically ineffective, still require energy. The
outcome of a
defibrillation shock delivered late after VF or fine VF onset may be asystole.
However,
this conversion to asystole may be advantageous in reducing ATP losses due to
the
generation of fibrillation waves. Therefore, in Figure 10, a prolonged episode
of VF 152,
which may be coarse or fine VF, may first be treated with a defibrillation
shock 154 to
convert the VF to asystole 156. Upon inducing asystole 156, an interval of
circulatory
assistance 102 is delivered followed by a series of depolarizing pulses 106 as
described
previously in conjunction with Figure 6.
Figure 11 is a graph of experimental results obtained from an isolated myocyte
preparation. A continuously-perfused, isolated guinea pig myocyte was
stimulated using
1Hz supra-threshold pulses until reaching a steady-state mechanical response.
Stimulation
was discontinued for intervals of 1, 2.5, 5, 10, 15 and 20 minutes after which
1 Hz
stimulation was resumed. The results of the 20-minute quiescent period
experiment are
shown in the graph of Figure 1. Sarcomere length is plotted over time.
Baseline steady-
state shortening 10 was established during 1Hz stimulation followed by a 20-
minute
quiescent period at 12. Upon re-initiating 1 Hz stimulation at 14, sarcomere
shortening
was initially impaired but recovered to the baseline steady-state response at
16 over the
course of approximately one minute of sustained 1 Hz stimulation. Thus, in the
presence
of adequate oxygenation, recovery of normal myocyte shortening is attainable
even after
20 minutes of no activity.
Of note, is that significant mechanical impairment is present after 20 minutes
of no
depolarizations despite adequate oxygenation. Thus, hypoxia may not be the
only cause of
electromechanical dissociation (EMD) that occurs after sustained fibrillation
or asystole.
Results for shorter quiescent periods of 5 minutes or more were similar to the
results
CA 02536194 2006-02-17
WO 2005/021089 PCT/US2004/026946
-21 -
shown in Figure 1 in that myocyte shortening was reduced to approximately 10
percent of
baseline shortening after the quiescent period, and full recovery of baseline
shortening was
achieved after approximately one minute of sustained 1 Hz stimulation.
Mechanical
impairment was lesser and recovery toward baseline shortening occurred snore
quickly
following quiescent times of less than five minutes. These results support the
theory that
SR calcium losses increase with an increased period of inactivity due to
calcium leaking
and that a sustained series of depolarizations is required in order to
replenish calcium
stores. These results further support the need for resuscitative methods that
reverse both
hypoxia and SR calcium loss.
Of course, the present invention may be readily implemented as instructions
stored
on a computer readable medium and execute under computer control in an
implantable or
external medical device. The computer readable medium includes magnetic,
optical and
other storage medium now known or later developed in all forms such as random-
access,
read-only, serial-access and dynamic and erasable versions thereof (e.g., RAM,
ROM,
SAM, DRAM, EPROM, EEPROM and the like).
Thus, a cardiac resuscitation method has been described that addresses the
need for
resuscitating the heart after a prolonged episode of VF, fme VF, or asystole.
The methods
and apparatus described herein for practicing the invention have been
described according
to specific embodiments. These embodiments are intended to be exemplary, not
limiting,
with regard to the following claims.