Note: Descriptions are shown in the official language in which they were submitted.
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
SYSTEM FOR INLINE PACKAGING AND IRRADIATION OF ARTICLES
Cross-Reference to Related Applications
This application claims priority to United States Provisional Patent
Application No.
60/496,445 filed on August 20, 2003.
Statement Regarding Federally Sponsored Research or Development.
Not Applicable.
Baclcgzouzzd of the Izzvezztion
1. Field of the Izzvezztiozz
This invention relates generally to the process of packaging products in a
conveyor
system and, more particularly, to a process for simultaneously sterilizing a
discrete amount of
the product being packaged.
2. Related AYt
Irradiation processing of materials for the purpose of material modification,
reducing
bioburden and product sterilization has been practiced for many years. This
process involves
passing the material or products through a stream or curtain of electrons or
photons provided by
an electron beam or X-ray accelerator. The delivered ionizing irradiation
energy can, for
example, then effectively cause free radical crosslinking as is done in
polymeric materials,
killing of organic pathogens as is done to reduce e-coli and other food borne
pathogens or the
elimination of harmful bacteria and microorganisms that contaminate medical
devices and cause
illness and infection in patients. This electron or X-ray energy penetrates
deeply and sufficiently
through the material being processed to effectively treat the entire volume of
the product
including the packaging material. Variations in energy of the electron beam
and power of the
accelerator system dictate depth of penetration and processing throughput for
various products
F:\ST LOUIS\DONAHUED\APP\18591?9.01
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
Irradiation processing practices to date have been effectively implemented by
treating
finished goods after they leave the manufacturing/assembly process line. This
process typically
occurs at the end of the process where individual products have been collected
and packed into
the shipping and distribution cartons containing one or in most cases many
individually
packaged C0171pO11eI1tS. These materials are then transferred to a conveyor
system which in turn
t1-ansports the materials to be presented to the irradiator and subsequently
processed. One
embodiment of this process is described in US Patent No. 5,396,074 (Peck et
al.) which consists
of irradiation system and a mufti-stage power and free overhead conveyor
system used to
transport carriers on which the product boxes or materials are loaded and
subsequently
processed. These products which are typically packaged in shipping cartons
would require
electron beam systems of higher energy to sufficiently penetrate the carton
and multiple
products in the carton to deliver the proper sterilization dose. The full
system including the
accelerator, radiation shielding ad material handling conveyor system can be
rather large,
occupying typically thousands of square feet of floor space, and is not
conducive to being placed
into an inline manufacturing layout. This type of system is designed to
irradiation processes for
entire manufacturing plant outputs rather than individual or a few individual
product
manufacturing lines.
Variations in this design concept have been implemented which are physically
smaller
and sized to treat smaller volumes and fit better into a process line but
these are still designed to
treat boxes of products rather than individual products. Examples ,of these
designs can be
reviewed in systems supplied around the globe by Ion Beam Applications (IBA)
of Louvain-la-
Neuve Belgium, Radiation Dynamics hlc. (RDI) of Edgewood, New Yorlc,
Scanditronics (SCX)
of Uppsala, Sweden and Titan Scan Technologies of San Diego, California )
The sterilization processing of materials requires very strict control,
product tracking
-2-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
and many levels of quality assurance to properly qualify the end product
sterility or properties.
An example of the current industry practice implemented can be seen in
irradiation processing
done today in gamma, x-ray and electron beam sites where a typical product
processed are
individual boxes containing about one gross of medical syringes in each box.
This packing box
is typically labeled with a unique identifier such as a bar code label which
would allow
traceability to when the products inside were manufactured, processed etc.
Once this box has
been opened and the syringes are removed this "traceability" is lost as the
individual syringes do
not carry these identifiers. Process qualification will verify that a
particular box was processed
but not the individual contents. An example of a packaged radiation-sterilized
medical device is
shown in US Patent No. 4813,210 (Masuda et al.).
To date there have been a number of different approaches to address the
sterilization/bio-burden reduction in packaged goods. These range fi~om a post
process
sterilization as described above to various means for treating the packaging
materials and
products. An example of prior art described in US Patent No. 4,223,512
(Buchner) uses an
alternating high-frequency electromagnetic field (RF or radio frequency waves)
to sterilize food
products in a form-fill and seal packaging line. The drawback of this method
of treatment is the
packaged goods are heated by the RF and subsequently partially "cooked" in
this process.
Another system is described in US Patent No. 4,983,411 (Tanaka et al.) which
uses ultraviolet
(UV) light as an irradiation means along with heat to sterilize vacuum packed
raw meat. 'hhis
means of irradiation treatment can be used to effectively control surface
pathogens that can be
exposed to the UV but fails to penetrate deeply into and through the material.
Thus pathogens
may still be present in the processed product which then requires that heat is
employed along
with the UV energy to properly treat the product. Other embodiments such as
seen in US Patent
Nos. 4,566,251 (Spisalc et al.), 4,652,763 (Nablo), 4,994,132 (Carlsson et
al.), 5,368,828
-3-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
(Carlson), and S,S49,868 (Carlson II) describe septic sterilization system in
which the packaging
material is sterilized in-line, such as prior to filling the package with
material or goods. Also,
these prior art systems do not fully combine the efficiencies of in-line form-
fill-seal packaging
with the benefits of in-line sterilization of the product within the packaging
before leaving the
form-fill-seal packaging system. To efficiently combine in-line form-fill-seal
packaging with
in-line sterilization, the differences between to types of conveyors must be
overcome. In prior
systems, it is lalown to use an index feed system for the packaging system and
to continuously
supply product to an intermittently operating packaging machine, such as
respectively described
in US Patent Nos. 5,477,660 (Smith) and 5,685,130 (Horsman), which are
incorporated herein
by reference. However, these systems do not perform any treatment proces using
a continuous
feed.
Sufnnzay of the Invention
It is in view of the above problems that the present invention was developed.
The
invention is a form-f 11-seal packaging system with an in-line biological
treatment device. As
described in detail below and recited in the claims, the biological treatment
device uses
irradiation to reduce biological contaminants or sterilize the packaged
products. Preferably. a
low to medium power, mediuln to high energy electron beam or x-ray system is
integrated with
the packaging system for the product packaging and biological treatment
thereof In one
embodiment, a medium to high power, medium to high energy electron beam or X-
ray system is
integrated with an inline form-fill and seal packaging system to provide an
integrated processing
solution and means of packaging and irradiation sterilization or b10-bLlrden
1'edLICt1011 of VarlOUS
materials. Embodiments are shown which have a different types of buffer
systems, including
those in which allow the irradiation device is used before the packages are
cut from their web,
-4-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
such as with a roller accumulator or a slackening trough, as well as those in
which the
irradiation device is placed after the pacleages are cut from the web, such as
with a bucket
accumulator or a linear accumulator.
In the preferred embodiment, a stream of products is packaged and biolagically
treated
by the system. The system has an index conveyor with a filling station and a
packaging station.
Discrete packages are filled with the stream of products, sealed and moved on
an index
conveyor. The system moves the discrete packages an irradiation chamber at a
steady rate on a
continuous speed conveyor. A controller matches the cyclical rate of the index
conveyor with
the steady rate of the continuous speed conveyor and uses a buffer to
transition from the cyclical
rate to the steady rate.
Accordingly, fl1e present invention provides a means for irradiation
processing of goods
through the integi°ation of an electron beam ou X-ray irradiation
device with a form-FI1 and seal
type packaging system. This provides a means to allow the delivery of this
irradiation energy to
individually packaged items or devices in a single layer multiple array
formats, while these
items are being conveyed by the packing system. The electron beam or X-ray
energy has the
ability to sufficiently penetrate the packaging material and the product being
processed while
delivering an irradiation dose sufficient to kill microorganisms and sterilize
the material or treat
harmful pathogens in the case of food.
In the case of sterilization of a medical disposable product such as a
syringe, it is
important to deliver a sufficient radiation dose to kill the contaminating
bacteria while the dose
absorbed in the body of the syringe is minimized. This is important because
the sterilizir.~g
energy may also degrade the mechanical properties of the plastics used in the
body of the
syringe, tJluS providing a minimum dose to these materials preserves the
mechanical integrity of
the device. Additionally, by minimizing the delivered dose we conserve energy
and the pov:~er
_$_
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
requirements for the process are reduced.
Fuuher features and advantages of the present invention, as well as the
structure and
operation of various embodiments of the present invention, are described in
detail below with
reference to the accompanying drawings.
Brief Descni~tioiz of tlae Drawi~tgs
The accompanying drawings, which are incorporated in and form a part of the
specification, illustrate the embodiments of the present invention and
together with the
description, serve to explain the principles of the invention. In the
drawings:
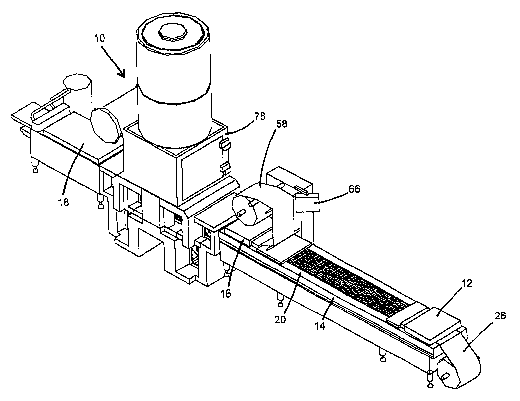
Figure 1 illustrates m isometric view of a first embodiment of the present
invention with
a roller accumulator buffer;
Figure 2 illustrates a detailed view of the roller accumulator buffer;
Figure 3 illustrates a side view of the first embodiment;
Fipre 4 illustrates the path shielding in a side view ofthe roller accumulator
buffer;
Figure 5 illustrates a side view of the a second embodiment of the present
invention with
a bucket accumulator buffer;
Figure 6 illustrates a detailed view of the bucket accumulator buffer;
Figure 7A illustrates a side view of a third embodiment of the present
invention;
Figure 7B illustrates a plan view of a linear accumulator buffer;
Figure 8A illustrates an isometric view of the first embodiment with all
shielding;
Figure 8B illustrates a plm view of a roller accumulator buffer;
Figure 9 illustrates a schematic of the controller system; and
Figure 10 illustrates a magazine dispenser for the packaging system.
_6_
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
Detailed Desc~iptioit of the P~efe~recl Enabotlinzents
Referring to the accompanying drawings in which like reference numbers
indicate like
elements, Figure 1 illustrates the packaging machine 10 of the present
invention. Machine 10
comprises a container fonnilib station 12, a product loading station 14, a
sealing station 16, a
separating station 18 and a conveyor 20. It should he appreciated that while
the present
invention is described with reference to a packaging machine that can draw a
vacuum, it is not
necessarily limited to only those in-line form-fill-seal packaging machines
with this vacuum
capability. The present invention can be used with any type of in-line form-
fill-seal packaging
machine, including those which use a magazine to load packages onto a
conveyor.
Additionally, the packaging may be evacuated or be partially filled with an
inert gas, air or some
other medium or material, possibly even a liquid.
Conveyor 20 moves incrementally at spaced intervals along the length of the
machine
10. Preferably, conveyor 20 includes a track having compartments 22 of a size
to receive the
bottom surface of the containers and an electric motor 24 or other prime mover
for moving the
compartments 22 along their path of travel. It is understood that other
conveying means may
prove worleable to marshal the containers through the stations and are
therefore within the scope
of the invention.
The machine 10 includes a lower film web supply roll 26 disposed at one end of
machine 10. The lower flm web 28 dispensed from roll 26 comprises a
thermoformable and
heat sealable packaging material of a type well lazown to those skilled in the
an. Roll 26 is
preferably rotatably mounted on an axle 30 disposed in a horizontal plane.
The container forming station 12 includes a plurality of molds 32 generally in
the shape
of the product container. A heating element 34 is positioned above molds 32
and overlies the
lower web film 28, which is indexed by conveyor 20 into the forming station
12. Mold 32 is
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
defined within container forming die 36 which underlies the heating element 34
and the lower
film web 28. A duct 38 is disposed within the forming die 36 and connects mold
32 to a
vacuum system 40. An optional vacuum system 40 is adapted to create negative
pressure,
thereby removing air from the mold 32, and positive pressure, thereby filling
mold 32 with
pressurized air. Station 12 forms a unit 42 comprising a plurality of packages
or containers 44.
The unit 42 normally comprises two containers 44 but may include more than
two. The
containers 44 include sidewalk 46, a bottom 48 and a fiat outwardly projecting
rim 50. It is
understood, however, that any configuration of container 44 may be operable
with the-present
invention.
Product 52 is introduced into the containers 44 at the product loading station
14. Product
52 may be loaded by mechanical means, such as shown in Figure 5, or manually
as product 52
characteristics reduire. It suffices that any loading means suitable to
introduce product 52 into
containers 44 is contemplated by the present invention.
Gantainers 44 holding product 52 are vacuum sealed at the sealing station 16.
Sealing
station 16 is adapted to simultaneously seal at least two units 42 of
containers 44. Sealing
station 16 includes a sealing die 54 underlying a thermosealing element 56 and
an upper film
supply roll 58 holding upper film web 59. Die 54 has at least one evacuation
duct 60 connected
to vacuum system 40 or, in the alternative, to a separate vacuum system (not
shown).
The sealed containers 44 of the unit 42 are detached into individual
containers at the
separating station 18. At the separating station unit 42 is separated into
individual containers 44
by a mechanical knife (not shown) or other suitable separating means.
The control circuit of machine 10 is shown in Figure 9 in schematic form and
is
represented by the numeral 62. In its most simplified form, the control
circuit 62 includes
startlstop switch 64, a controller 66 and appropriate leads 68 between the
controller 66 and the
_g_
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
conveyor motor 24 and start/stop switch 64. Control leads 68 also extend from
the controller 66
to container forming station 12, product loading station 14, vacuum system 40,
sealing station
16 and separating station 18. It is to be understood that the controller 66
may be a hard-wired
logic circuit, a microprocessor or other equivalent means for activating and
deactivating the
stations described above. Preferably, controller 6G C0111pr15eS a
1111CrOprOC2SSOr which has been
preprogrammed to index conveyor 20 along spaced intervals and to activate and
deactivate the
various stations at predetermined times.
In operation, conveyor 20 is activated by controller 66 indexing lower film
web 28 from
right to left when viewing Figure 5. As web 28 passes the container forming
station 12, the
controller 66 will momentarily stop the conveyor 20. Container forming die 36
is then raised
under the heating element 34 thereby creating a seal between the heating
element 34, the lower
web 28 and the die 36. Pressurized air or other gas is forced into the die 36
through duct 38 by
vacuum system 40 causing the lower film web 28 to cotltact the heating element
34. Once the
lower film web 28 has been heated to become sufficiently formable, vacuum is
applied to the
die 36 through duct 38 causing the web 28 to be drawn into the product
container molds 32
wherein web 28 is conformed into the shape of the molds 32. Die 36 then lowers
from the
heating element 34 and the formed unit 42 of containers 44 is indexed by the
conveyor 20 to the
product loading station 14.
At the product loading station 14, product is introduced into the individual
containers 44
of the unit 42 by mechanical or manual means after every index of conveyor 20.
Once loaded,
the unit 42 of containers 44 is indexed by the conveyor 20 to the sealing
station 16.
After every other index of the conveyor 20, controller 66 activates the
sealing station 14.
It is to be understood, however, that where sealing die 54 is adapted to
vacuum seal more than
two units 42 of containers 44, controller 66 would activate sealing station 14
only after the
-9-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
corresponding number of indices. For example, if die 54 is adapted to vacuum
seal four units 42
of containers 44, controller wauld activate the sealing station 16 after every
fourth index of
conveyor20.
Upon activation, die 54 is raised to the thennosealing element 56 thereby
creating an
airtight seal between the sealing die 54, the upper film web 59 and the
thermosealing element
56. In closed position, an evacuation chamber 50 is formed by the sealing die
54. Vacuum
system 40 is activated to evacuate air from chamber 50 through evacuation
ducts 60, thereby
creating negative pressure within the chamber 50. When the desired degree of
evacuation is
reached, the thermosealing element 56 is lowered onto the upper film web 59
depressing the
web 59 onto the rims 50 of containers 44. The thermosealing element 56
hermetically seals the
web 59 to the rims 50. When the seal is complete, thermosealing element 56 is
raised from the
sealed containers 44 and the sealing die 54 is vented through evacuation ducts
60. Sealing die
54 is then lowered. The controller 66 then causes the sealed containers 44 to
be advanced by the
conveyor 20 to the separating station 18 where units 42 are separated into
individual containers
44.
If circumstances require, inert gas may be back-flushed into the chamber 50
during the
sealing phase. More particularly, modified atmosphere may be injected into the
chamber 50
after it has been evacuated but before the thermosealing element 56 has been
applied to the
upper web 59. The effect of back-flushing is to fill the containers 44 with a
gas that does not
present the problems of contamination and spoilage associated in ambient air.
Baclc-flushing is
also advantageous to prevent the container 44 from crushing or compressing
delicate product
once the sealing die 54 is vented.
An alternative embodiment of the present invention is illustrated in FIG. 10
and is
designated by the numeral 110. In this embodiment, machine 110 utilizes a
different form of
-10-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
container forming station 112. The containers 144 of machine I 10 are
preformed and preferably
stored in an upright magazine 170 above the conveyor 120. The preformed
containers 144 are
dispensed, either individually or in mufti-container units, onto the conveyor
120 in response to
controller 66. Once dispensed onto conveyor 120, containers 144 are filled
with product,
vacuum sealed and separated in substantially the same manner as described in
connection with
the preferred embodiment.
US Pat. No. 5,685,130 describes a queuing operation in which a steady flow of
incoming packages is buffered for the index conveyor. In the present
invention, a controller
matches the cyclical rate of the index conveyor for packaging operations with
the steady rate of
the continuous speed conveyor for biological treatment/irradiation operations
and uses a buffer
to transition from the cyclical rate to the steady rate. The present system
has an index conveyor
with a filling station and a packaging station. Discrete packages are filled
with the stream of
products, sealed and moved on an index conveyor. The system moves the discrete
packages an
irradiation chamber at a steady rate on a continuous speed conveyor. A
controller matches the
cyclical rate of the index conveyor with the steady rate of the continuous
speed conveyor and
uses a buffer to transition from the cyclical rate to the steady rate.
The object of this invention is to provide a means to irradiation treat
materials or
products while they are still captive in the cycle of the form fill and seal
packaging process. h~
order to accomplish this new configuration design for the integrated
accelerator and radiation
shielding needed to be realized. Additional to this a very important aspect of
the product
transport method needed to be realized.
This last requirement specifically refers to the standard material movement in
a form till
and seal packaging system which moves or indexes the product in an
intermittent motion. This
intennitent motion allows for both the "form" of the receptor or tray, the
"fill" of the product
-11-
CA 02536297 2006-02-20
WO 2005/019033 PCT/US2004/027186
into that formed tray and eventually the cutting of the package array into
individual item size at
the end of the process. Contrary to this the requirements for the uniform
delivery of the electron
beam or the X-ray energy to such a material requires that the product move or
is conveyed in a
very smooth and CO17t111L10uS IllOtloll, at a speed which is proportional to
the electron or photon
fILIeIICe 1n Older t0 deliver the appropriate dose t0 the lllaterlal. This
requirement UI11f01111 dOSe 1S
lalown and accepted in the industry and referred to in many patents today.
In a form fill and seal packaging system the materials and products are
typically
conveyed in a linear fashion in one plane. This flat plane makes it very easy
to load and package
the products since gravity is working with you. This configuration also holds
the product
horizontal until they are cut into individual packages and exit the system.
One aspect of this new
invention is in the design of a roller to allow the "bending" of the packaged
product line to pass
this material into and out of the radiation shielding enclosure. Since this
type of packaging
system provides a configuration in a linear array with repeatable spacing
between product rows
the packaged product line could only be "bent" in these spaces. This length of
the package that
cannot be bent is determined by the product loaded into the package itself.
The bending of the
packaged product is accomplished on a hollow roller that has essentially only
supporting
structures to hold the product on the packaging material between where the
product is loaded.
This bending then allows the product to be conveyed in a circuitous path
allowing product entry
to the shield and limiting radiation leakage from the shield.
Another aspect of this new invention is a method for the translation of this
intermittent
motion of the form fill and seal packaging system into a continuous motion
reduired in the
electron or x-ray dose delivery. This is accomplished by the design of roller
or accumulator
devices placed in the line which dampen and effectively remove the
intermittent motion of the
product web. This accumulatol/loller slides freely on a shaft which can be
tensioned by
-12-
CA 02536297 2006-02-20
mechanical means or simply provide tension through gravity. This
accumulator/roller is
positioned between the intermittent control of the packaging system and the
continuous speed
underbeam system. This accumulator, along with a variable speed drive
mechanism which
positively controls the speed of the web while it is subjected to the
irradiation energy, effectively
accomplishes this product speed translation from intermittent to continuous.
The packaging
system continues to operate with the intermittent motion that is required for
its process while the
material is then smoothly conveyed for the irradiation process. This
accoumulator/ro(ler device
may also be used after the material has been irradiation processed to
translate this smooth
motion back to intermittent if this is required subsequently in the process.
The accumulator device can utilize a variety of formats, including a bucket
accumulator,
a roller accumulator, a linear accumulator, and a slackening trough. An
embodiment utilizing a
roller accumulator is shown in Figures 1-4, 8A and 8B. Another embodiment
using a bucket
accumulator is illustrated in Figures 5-6. Another embodiment with a linear
accumulator is
shown in Figures 7A and 7B.
Detailed drawing describing this accumulator device and the drive mechanism
for the
"underbeam" speed control is seen in the drawings included. This section of
the product
transport system can be installed in the product line before the "cut" of the
product into
individual packages. This then allows a means for positively moving a single
layer of many
products through an electron beam or x-ray irradiation system easily,
effectively and provides a
2 0 means to control the speed, thus the irradiation dose that the product
receives.
As illustrated in the figures, the buffer between the index conveyor and the
steady rate
conveyor of the unit includes an entry region 80 and an exit region 82 at
either end of the
accumulator. The buffer receives packages at a cyclical rate at the entry
region 80. In a
preferred embodiment, an irradiation chamber 84 lies within the buffer region.
The buffer
AMENDIvIENTS UNDER ARTICLE 34
REPLACEIvIENT SI~ET FOR PAGE I3
CA 02536297 2006-02-20
moves the packages through the irradiation chamber 84 at a steady rate. The
irradiation
chamber 84 includes a beam generator or accelerator 78 (described in more
detail below), a
beam distributor, a target region, a beam generator shield, a target region
shield, and a
serpentine shield 86. The serpentine shield 86 surrounds the circuitous path
through the buffer
region. This circuitous path includes an entry point and an exit point.
Between the entry and
exit point of the path, there is a portion of the path 74 within the
irradiation chamber 84 that is
substantially straight. This portion 74 of the circuitous path forms the
target region of the
chamber. Surrounding the substantially straight target region 74, the path may
have arcuate
portions that lead to and from the target region.
The shielding for the irradiation chamber may further include exit and entry
shields,
such as shutter shield. In this embodiment, the shutter shields open to permit
the entry of an
untreated package or set of packages into the target region and to permit a
treated package or set
of packages to exit the chamber through the exit region.
The separation station 18 (described in more detail above) may be located in
the buffer
region preceding the irradiation chamber 84. In such an embodiment, the formed
unit 42 is
broken into discrete packages 44 prior to passing through the irradiation
chamber 84.
Another method for accumulation of the material may simply be accomplished by
providing a series of rollers which allow the material to "hang" in the
radiation shielding
labyrinth entrance in sufficient length to bend around the shielding material.
2 0 The conveyance of the product in this for also allows for easier movement
of the product
or material into and out of the irradiation shield or chamber. Since one of
the primary design
issues with these type system is to allow for product entrance and exit from
the shield while at
the same time effectively trapping or reducing the x-ray energy leaking from
the shield. The
conveyance of products in this type of low profile format minimizes the x-ray
leakage and
AMENDMENTS UNDER ARTICLE 34
REPLACEMENT SHEET FOR PAGE 14
CA 02536297 2006-02-20
therefore allows for the shielding to be designed in a more compact and
economical manner.
In this process it is important to be able to correlate the dose delivered to
a particular
product for the purpose of process certification. This provides the user with
the means of
tracability for processing food stuffs, medical product sterilization and
overall process control.
The integration of these two processing systems (irradiation and packing)
which includes the
parameters from the irradiation system and the tracking of individual products
in the packaging
system permits strict control requirements such as those in product sterility
processing. This
provides a means for process control and validation of the product coming off
the packaging
system for individual products that is not done today in irradiation
processing. By providing
specific signals for beam intensity, beam scanning distribution, energy
stability ete, to the
packaging system PLC this tracability of sterility can easily be accomplished.
h-radiation processing of materials, specifically electron beam irradiation
processing of
materials, which are done in the format detailed above are typically described
as having been
"single sided processed". This term, as it is used in the industry, refers to
the fact the electron
beam is delivered to the product from one direction only and the product has
not been "flipped
over" or treated from the opposite side with electrons, i.e., "single sided
processing". The
limitation in this style of one sided treatment is that the energy of the
electron must then be
sufficiently high to penetrate the product being treated to effectively treat
the "whole" product
from this single side delivery of electrons. This requirement for the higher
energy accelerator
2 0 means that more radiation shielding must be used to limit the x-ray
leakage which increases the
overall system cost. One embodiment of this invention is to utilize a high
energy, low power
accelerator to treat the irradiated product in the manner described above with
sufficient energy
to treat the whole product.
When material and product densities are sufficiently low the energy of the
accelerator
AMENDMENTS UNDER ARTICLE 34
REPLACEMENT SI-SET FOR PAGE 15
CA 02536297 2006-02-20
may be reduced to a minimum level to allow for reduced size, radiation
shielding and power
requirements.
One might imagine that if there was a means for delivering the electrons from
a single
side in a manner to more effectively distribute the electron energy in the
product and not require
S the typical increase in the system energy that a more cost effective and
smaller system could be
produced. One means for accomplishing this is to provide reflector plates in
the beam chamber
around the exit of the scanner which are positioned to deflect a portion of
the electron beam and
low energy scatter electrons into the material. These deflected or reflected
electrons enter the
material from wide angles and provide dose increases around the edges of the
product being
treated. This effect can improve the dose distribution and irradiation process
uniformity.
Radiation dosimetry is the typical method for qualifying whether a particular
product
has been properly treated. One unique concept for this type of system, where
the label for each
package is individually printed after the package has been sealed and before
it has been
irradiated, is to use radiation sensitive inks for a portion of the label.
This radiation sensitive ink
1 S will change color during the irradiation process and be a clear indicator
that the package has
been treated. This may or may not be utilized in the quality control process
for assurance of
product treatment.
An example of one type of accelerator that may be used in this application is
the DC,
Dynamitron system described here, works on a similar principle as a television
tube. Free
2 0 electrons are generated by heating a filament which is part of the
electron gun assembly. A high
voltage of the correct polarity draws the electrons away from the gun and
accelerates them
through the vacuum tube. The electrons gain energy and velocity as they are
accelerated in the
vacuum tube. As the beam of electrons passes from this acceleration or beam
tube, they travel
down a vacuum beam line and may pass through the scan magnet or other types of
beam
AMENDtvIENTS UNDER ARTICLE 34
REPLACE1VIENT SI~ET FOR PAGE I6
CA 02536297 2006-02-20
distribution devices which are known in the ar>~ This magnet, and its
oscillating magnetic field,
SWeepS the beam back and forth across the scan window. At the scan window the
electrons pass
from the vacuum chamber into the air where they are delivered directly to the
product or to an x-
ray convertor to provide photons for x-ray processing
Electron beam system for this type of process range from low voltages in the
100's of
kV range up to accelerators that deliver electrons to 10 or more mega volts.
Electrons
accelerated to an energy of 5 MeV are traveling at approximately
99.6°!0 of the speed of light, or
nearly 300,000 km/sec, when they enter the product. The amount of beam
current, which
partially determines the processing rate is measure in mirco or millamperes.s
It is interesting to
note that I mA of beam current represents about 6 million billion electron
particles every
second.
Where the objective of the electrons generated in a television is to create a
picture, a
Dynamitron bundles electrons into a 3 to 5 cm diameter beam to irradiate
materials. The
enormous number of electrons and the high acceleration voltage produces rapid
reactions by
operating directly on the molecules within the product. This produces an
efficiency that is
outstanding when compared with other methods such as heat, light, and chemical
reagents.
There are many different type of accelerators available to provide this type
of processing
capability, to one skilled in the art of electron beam system this would be
understood. The
Dynamitron described here is but one example, but other types of AC
accelerators such as
2 0 multiple and single cavity Linac's, single cavity mufti-pass Rhodotrons or
DC ICT's (Insulated
Core Transformers) or single gap DC accelerators may be used.
In view of the foregoing, it will be seen that the several advantages of the
invention are
achieved and attained.
The embodiments were chosen and described in order to best explain the
principles of
AMENDMENTS UNDER ARTICLE 34
REPLACEMENT SHEET FOR PAGE 17
CA 02536297 2006-02-20
the invention and its practical application to thereby enable ofihers skilled
in the art to best
utilize the invention in various embodiments and with various modifications as
are suited to the
particular use contemplated.
As various modifications could be made in the constructions and methods herein
described and illustrated without departing from rte scope of the invention,
it is intended that all
matter contained in the foregoing description or shown in the accompanying
drawings shall be
interpreted as illustrative rather than limiting. For example, while the
present invention
discusses the details of an electron beam device for the sterilization
process, other forms of
electromagnetic sterilization may be used. Also, while an active beam scanner
is described, it
will also be appreciated that other devices to distribute the beam in the
target region can be
used, such as a diffuser. Thus, the breadth and scope of the present invention
should not be
limited by any of the above-described exemplary embodiments, but should be
defined only in
accordance with the following claims appended hereto and their equivalents.
AMENDMENTS UNDER ARTICLE 34
REPLACEMENT S(n:ET FOR PAGE 18