Note: Descriptions are shown in the official language in which they were submitted.
CA 02536679 2006-02-23
POWDERY PREPARATIONS CONTAINING 2,4,6-TRIANILINO-P-(CARBO-2'-
ETHYLHEXYL-1'-OXI)-1,3,5-TRIAZIN E
The invention relates to powdered preparations comprising 2,4,6-trianilino-p-
(carbo-2'-
ethylhexyl-1'-oxy)-1,3,5-triazine, to their preparation and to the use thereof
as
photostable photoprotective agents.
The quality and life span of many organic materials, for example plastics and
coating
materials, but also pharmaceutical and cosmetic preparations, can be adversely
affected by the action of light, in particular by UV rays. These losses in
quality
frequently become evident in the case of plastics and coating materials from
yellowing,
discoloration, cracking or embrittlement of the material. In the case of
pharmaceutical
and cosmetic preparations, the effect of UV rays can lead to the degradation
of the
active ingredients present in the formulations.
The harmful effect of the ultraviolet part of solar radiation on the skin or
hair, which in
the widest sense are also an organic material, is likewise a problem which is
increasing
in importance. While rays having a wavelength of less than 290 nm (the UVC
region)
are absorbed by the ozone layer in the earth's atmosphere, rays in the range
between
290 nm and 320 nm, the UVB region, cause an erythema, simple sunburn or even
burns of varying severity on the skin.
A maximum for the erythema activity of sunlight is given as the relatively
narrow range
around 308 nm.
Numerous compounds are known for protecting against UVB radiation; these are,
inter
alia, triazine derivatives; derivatives of 3-benzylidenecamphor, of 4-
aminobenzoic acid,
of cinnamic acid, of salicylic acid, of benzophenone and of 2-
phenylbenzimidazole.
It is also important to have available filter substances for the range between
about
320 nm and about 400 nm, the UVA region, since its rays can cause reactions in
cases
of photosensitive skin. It has been proven that UVA radiation leads to damage
of the
elastic and collagenous fibers of the connective tissue, leading to premature
aging of
the skin, and that it is to be regarded as a cause of numerous phototoxic and
photoallergic reactions. The harmful effect of UVB radiation can be
intensified by UVA
radiation.
To protact against UVA rays, derivatives of dibenzoylmethane are used, the
photostability of which, however, is inadequate (Int. J. Cosm. Science 10, 53
(1988)).
However, UV radiation can also lead to photochemical reactions, in which case
the
photochemical reaction products then intervene in the skin's metabolism.
rr 54t38Z
CA 02536679 2006-02-23
2
Such photochemical reaction products are mainly free-radical compounds, for
example
hydroxyl radicals. Undefined free-radical photo products formed in the skin
itself can
also trigger uncontrolled secondary reactions as a result of their high
reactivity.
However, singlet oxygen, a non-radical excited state of the oxygen molecule,
can also
arise during UV irradiation, as can short-lived epoxides and many others.
Singlet
oxygen, for example, differs from normal triplet oxygen (free-radical ground
state) by
virtue of its increased reactivity. However, activated, reactive (free-
radical) triplet states
of the oxygen molecule also exist.
Furthermore, UV radiation is a form of ionizing radiation. There is therefore
the risk that
ionic species will also form during UV exposure, which then for their part are
able to
intervene oxidatively in the biochemical processes.
One applications-relevant disadvantage of many UV filters is their poor
solubility in
water and/or in natural and synthetic oils, for example in silicone oils and
in fatty acid
triglycerides, as a result of which their use, for example in cosmetic
formulations, is
often restricted.
A further disadvantage associated with the application of some photoprotective
agents
is the appearance of skin irritations and allergies resulting from too high a
skin
permeability.
Numerous methods have already been published for improving the formulation
properties of insoluble or only sparingly soluble UV absorbers.
For example, GB-A-2 303 549 describes a grinding process for the preparation
of
micronized insoluble organic UV absorbers in the presence of alkyl
polyglycosides. The
resulting micronizates can be incorporated into cosmetic photoprotective
preparations.
GB-A-2 286 774 likewise describes a grinding process for the micronization of
insoluble organic UV absorbers.
EP-A-1 127 567 describes aqueous dispersions of sparingly water-soluble or
water-
insoluble organic UV filter substances and dry powders produced therefrom,
wherein
they comprise at least one sparingly water-soluble or water-insoluble organic
UV filter
substance as colloidally disperse phase in amorphous of partially amorphous
form. The
use of the protective colloids specified in this specification - in particular
gelatin or
casein or caseinate - leads to powdered products whose solubility in cold
water is
unsatisfactory. In addition, gelatin and casein in cosmetic formulations can
cause skin
allergies.
It was then an object of the present invention to provide a method of
producing triazine-
containing photoprotective agent formulations which offer effective protection
for
organic material, in particular for the human skin and/or human hair, against
UV rays,
PF 54882
CA 02536679 2006-02-23
3
which are well tolerated by the skin and which can be incorporated easily both
into
lipophilic and also in particular into aqueous systems.
This object was achieved by a method of producing powdered preparations
comprising
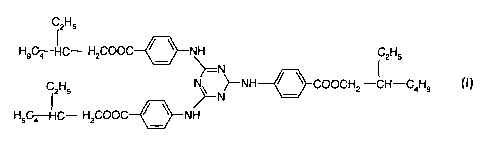
2,4,6-trianilino-p-(carbo-2'-ethylhexyl-1'-oxy)-1,3,5-triazine of the formula
I
IzHs
H9C4 HC- HzCOOC / ~ NH CzHs
~--N
CzHS N~ ~-NH~COOCHz -CH-C4H9
N
N
H9C4 HC- H2COOC~ H
by
a) dispersing the triazine I in an aqueous molecularly disperse or colloidally
disperse solution of a protective colloid and
b) converting the dispersion obtained into a dry powder by removing the water
and,
if appropriate, additionally used solvents, and drying,
wherein the protective colloid used in process step a) is modified starch.
2,4,6-Trianilino-p-(carbo-2'-ethylhexyl-1 '-oxy)-1,3,5-triazine of the formula
I is
marketed by BASF Aktiengesellschaft under the trade name Uvinul~ T150 as a UVB
filter. Uvinul~ T150 is notable, inter alia, for good UV absorption properties
with an
exceptionally high absorbance coefficient > 1500 at 314 nm.
For the purposes of the present invention, the term aqueous dispersions is
understood
as meaning both aqueous suspensions and emulsions. Preferred aqueous
suspensions which may be mentioned are those in which the disperse phase
comprises the triazine I as nanoparticulate particles.
For the purposes of the present invention, the term modified starch preferably
comprises esters of starch with organic acids, e.g. with acetic acid and
higher fatty
acids (C6-Czs), and with succinic acid, adipic acid and citric acid. The
starch can be
obtained here, inter alia, from corn, potatoes or wheat. A particularly
preferred modified
starch is octenyl succinate starch, which is marketed under the trade name
HiCap~ by
National Starch or EmCap~ by Cerestar.
PF 54882
CA 02536679 2006-02-23
4
A preferred variant of the method according to the invention is one in which
the
dispersion in stage a) comprises the following steps:
a,) dissolving the triazine I in one or more water-miscible organic solvents)
or in a
mixture of water and one or more water-miscible organic solvents) or
a2) dissolving the triazine I in one or more water-immiscible organic
solvents) and
a3) mixing the solution obtained after a,) or a2) with an aqueous molecularly
disperse
or colloidally disperse solution of modified starch, where the hydrophobic
phase
of the triazine I is formed as nanodisperse phase.
Depending on the type of solvent used, the nanodisperse phase in step a3) may
be
solid nanoparticles [suspension; obtainable by combining a,) and a3)] or
nanodroplets
[emulsion; obtainable by combining a2) and a3)].
The water-miscible solvents used in stage a,) are primarily water-miscible,
thermally
stable, volatile solvents comprising only carbon, hydrogen and oxygen, such as
alcohols, ethers, esters, ketones and acetals. It is expedient to use those
solvents
which are at least 10°I° water-miscible, have a boiling point
below 200°C, preferably
below 100°C, and/or have fewer than 10 carbons. Particular preference
is given to
methanol, ethanol, n-propanol, isopropanol, 1,2-butanediol 1-methyl ether, 1,2-
propanediol 1-n-propyl ether, tetrahydrafuran or acetone or mixtures thereof,
and very
particular preference is given to using isopropanol or acetone.
For the purposes of the present invention, the term "a water-immiscible
organic
solvent" is an organic solvent with a solubility in water at atmospheric
pressure of less
than 10%. Suitable possible solvents here are, inter alia, hafogenated
aliphatic
hydrocarbons, such as, for example, methylene chloride, chloroform or carbon
tetrachloride, carboxylic acid esters, such as diethyl carbonate, ethyl
formate, methyl,
ethyl or isopropyl acetate, and ethers, such as methyl tert-butyl ether.
Preferred water-
immiscible organic solvents are the following compounds from the group
consisting of
dimethyl carbonate, propylene carbonate, ethyl formate, ethyl acetate,
isopropyl
acetate and methyl tert-butyl ether.
The dry powder in process step b) can be produced here, inter alia, by spray-
drying,
spray-cooling, freeze-drying, and by drying in a fluidized bed, convection
drying or
contact drying, it also being possible to carry out the drying in the presence
of a coating
material (powdering agent). Suitable coating agents are, inter alia, corn
starch, silica
+0 and also tricalcium phosphate.
PF 54882
CA 02536679 2006-02-23
During the lyophilization of the nanoparticles according to the invention,
cryoprotective
substances such as, for example, trehalose or polyvinylpyrrolidones, can be
added to
the nanoparticles according to the invention.
5 Particular preference is given to an embodiment of the method according to
the
invention in which
a,) the triazine I is dissolved in acetone or isopropanol or a mixture of
water and
acetone or water and isopropanol at temperatures in the range from 50 to
240°C,
a3) the solution obtained is mixed with an aqueous molecularly disperse or
colloidally disperse solution of modified starch, in particular octenyf
succinate
starch, at temperatures in the range from 25 to 120°C and
b) the suspension formed is spray-dried after removing the organic solvent.
The abovementioned dry powders are advantageously produced by dissolving the
triazine of the formula I in acetone or isopropanol or a mixture of water and
acetone or
water and isopropanol at temperatures in the range from 50°C to
240°C, in particular
100°C to 200°C, particularly preferably in the range from
105°C to 180°C.
To produce the molecularly disperse solution rapidly, the application of
increased
pressure, e.g. in the range from 20 bar to 80 200 bar, preferably 30 to 100
bar, may be
advantageous.
The molecularly disperse solution obtained in this way is then mixed directly
with the, if
appropriate cooled, aqueous molecularly disperse or colloidally disperse
solution of the
modified starch, in particular octenyl succinate starch, in such a way that a
mixing
temperature of about 25°C to 120°C, preferably 40°C to
80°C, particularly preferably
45°C to 70°C, is established.
In so doing, the solvent component is converted into the aqueous phase and the
hydrophobic phase of the triazine is formed as nanodisperse phase.
The mixing in step a3) can be carried out by initially introducing the
solution comprising
triazine, and metering in the aqueous solution of modified starch, or vice
versa, or
preferably by metering in both solutions simultaneously and continuously into
a mixing
chamber.
With regard to a more detailed description of the method and apparatus
relating to the
abovementioned dispersion, reference is made at this point to EP-B-0 065 193.
PF 54882
CA 02536679 2006-02-23
s
To increase the mechanical stability of the end product, in some cases it may
be
advantageous to add a further plasticizer to the colloid, such as sugars or
sugar
alcohols, e.g. sucrose, glucose, glucose syrup, dextrin, inverted sugar,
sorbitol,
mannitol or glycerol.
To increase the stability of the active ingredient against oxidative
degradation, it may
likewise be expedient to add stabilizers such as a-tocopherol, t-
butylhydroxytoluene, t-
butylhydroxyanisofe, ascorbic acid or ethoxyquin. They can either be added to
the
aqueous phase or to the solvent phase, although they are preferably dissolved
together
with the triazine 1 in the solvent phase.
In addition, the photoprotective agent formulations can comprise low molecular
weight
surface-active compounds (emulsifiers) in a concentration of from 0.01 to 70%
by
weight, preferably 0.1 to 50% by weight, particularly preferably 0.5 to 20% by
weight,
based on the dry mass of the photoprotective agent formulation. Suitable as
such are
primarily amphiphilic compounds or mixtures of such compounds. In principle,
all
surfactants with an HLB value of from 5 to 20 are suitable. Suitable
corresponding
surface-active substances are, for example: esters of long-chain fatty acids
with
ascorbic acid, mono- and diglycerides of fatty acids and oxymethylation
products
thereof, esters of monofatty acid glycerides with acetic acrd, citric acid,
lactic acid or
diacetyltartaric acid, polyglycerol fatty acid esters, such as, for example,
the
monostearate of triglycerol, sorbitan fatty acid esters, propylene glycol
fatty acid esters
and lecithin. Preference is given to using ascorbyl palmitate.
To increase the stability of the active ingredient against microbial
degradation, it may
be expedient to add preservatives to the preparation, such as, for example,
methyl 4-
hydroxybenzoate, propyl 4-hydroxybenzoate, sorbic acid or benzoic acid or
salts
thereof.
According to the invention, dry powders can thus be obtained which no longer
lose
their properties obtained in the primary dispersion. This means that the
amorphous or
partially crystalline character of the UV filter substance is retained. It is
also a property
according to the invention that these powders, upon redispersion, have the
same
particle size distribution which they had as primary dispersion with a
deviation of 20%,
preferably < 15°!°.
A further preferred embodiment of the abovementioned method is one in which
the
suspension prepared in process step a) is ground before being converted into a
dry
powder.
PF 54882
CA 02536679 2006-02-23
7
The grinding method is preferably carried out by suspending the triazine I in
crystalline
form in an aqueous molecularly disperse or colloidally disperse solution of
modified
starch, and comminuting to the desired particle size by grinding.
The grinding can be carried out here in a manner known per se, e.g. using a
ball mill.
Depending on the type of mill used, grinding is carried out until the
particles have an
average particle size, determined via Fraunhofer diffraction, D[4.3] of from
0.01 to
100 Nm, preferably from 0.02 to 50 Nm, particularly preferably 0.05 to 20 Nm,
very
particularly preferably 0.05 to 5 Nm, in particular 0.1 to 1 Nm. The term
D[4.3] refers to
the volume-weighted average diameter (see handbook for Malvern Mastersizer S,
Malvern Instruments Ltd., UK).
By heating the aqueous suspension after the grinding process to a temperature
above
the melting point of the triazine I and then spray-drying the "melt emulsion",
it is
possible to increase the amorphous fraction of the triazine in the resulting
dry powder.
Details regarding the grinding of active ingredients in aqueous protective
colloid
solutions are given in EP-B-0 498 824 and EP-B-0 684 973.
The invention also provides triazine-comprising powdered preparations
obtainable by
the abovementioned methods.
The novel photoprotective agent formulations are notable for the fact that
they
comprise the triazine of the formula I, the amorphous fraction of which is in
the range
greater than 10%, preferably greater than 30%, particularly preferably in the
range from
50 to 100%, very particularly preferably in the range from 75 to 99%. The
degree of
crystallinity of the triazine I can be determined here, for example, by X-ray
diffraction
measurements.
The content of triazine of the formula I in the photoprotective agent
formulations
according to the invention is in the range from 0.1 to 70% by weight,
preferably in the
range from 2 to 40% by weight, particularly preferably in the range from 3 to
30% by
weight, very particularly preferably in the range from 5 to 25% by weight,
based on the
dry mass of the formulations.
The average particle size D[4.3] of the nanoparticulate particles in the
aqueous
dispersion is, depending on the formulation method, in the range from 0.01 to
100 Nm,
preferably in the range from 0.02 to 50 Nm, particularly preferably in the
range from
0.05 to 20 Nm, very particularly preferably in the range from 0.05 to 5 Nm, in
particular
0.1 to 1 Nm.
Whereas ground UV filter substances, when incorporated into skin creams, have
an
increased propensity for particle size growth, which can lead to a
deterioration of the
PF 54882
CA 02536679 2006-02-23
8
sun protection factor and to an unpleasant feel on the skin, the dry powders
according
to the invention do not have such tendencies on account of their matrix and
protective
colloid structure.
The formulations according to the invention - dispersions and dry powders
prepared
therefrom - are highly suitable for stabilizing organic material inter alia
against the
effect of light, oxygen and heat. They are added to the organic material to be
stabilized
in a concentration of from 0.01 to 10°!o by weight, preferably 0.01 to
5% by weight,
particularly preferably from 0.02 to 2°I° by weight, based on
the organic material,
before, during or after its preparation.
Organic material is understood as meaning, for example, photographic recording
materials, in particular photographic emulsions or precursors for plastics and
surface
coatings, but in particular plastics and surface coatings themselves.
Organic material, however, also means cosmetic preparations, such as, for
example,
creams, lotions, gels, lipsticks.
The present invention further relates to organic material stabilized against
the action of
light, oxygen and heat, in particular plastics and surface coatings,
comprising 0.01 to
10% by weight, preferably 0.01 to 5°!° by weight, particularly
preferably from 0.02 to 2%
by weight, based on the total amount of the organic material, of the triazine
I in the form
of the formulations according to the invention.
For mixing the formulations according to the invention primarily with
plastics, it is
possible to use all known devices and methods for mixing stabilizing agents or
other
additives into polymers.
The organic material stabilized by the formulations according to the invention
can, if
appropriate, comprise further additives, e.g. antioxidants, light stabilizing
agents, metal
deactivators, antistatic agents, flame retardants, pigments and fillers.
Antioxidants and light stabilizers which can be added in addition to the
formulations
according to the invention are, for example, compounds based on sterically
hindered
phenols or costabilizers comprising sulfur or phosphorus.
Examples of such phenolic antioxidants are 2,6-di-tert-butyl-4-methylphenol, n-
octadecyl-(3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate, 1,1,3-tris(2-
methyl-4-
hydroxy-5-tert-butylphenyl)butane, 1,3,5-trimethyl-2,4,6-tris(3,5-di-tert-
butyl-4-
hydroxybenzyl)-benzene, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)
isocyanurate,
1,3,5-tris[(3-(3,5-di-tert-butyl-4- hydroxyphenyl)propionylethyl]
isocyanurate, 1,3,5-
tris(2,6-dimethyl-3-hydroxy-4-tert-butylbenzyl) isocyanurate and
pentaerythritol tetrakis-
[(3-(3,5-di-tert-butyl- 4-hydroxyphenyl) propionate].
Examples of suitable phosphorus-comprising antioxidants are tris(nonylphenyl)
phosphate, distearylpentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl)
phosphate,
PF 54882
CA 02536679 2006-02-23
9
tris(2-tert-butyl-4-methylphenyl) phosphate, bas(2,4-di-tert-
butylphenyl)pentaerythritol
diphosphite and tetrakis(2,4-di-tert-butylphenyl) 4,4'-biphenylenediphosphite.
Examples of sulfur-comprising antioxidants are dilauryl thiodipropionate,
dimyristyl
thiodipropionate, distearyl thiodipropionate, pentaerythritol tetrakis(a-
laurylthiopropionate) and pentaerythritol tetrakis-((3-hexylthiopropionate).
Other antioxidants and light stabilizers which can be used together with the
formulations according to the invention are, for example, 2-(2'-hydroxyphenyl)-
benzotriazoles, 2-hydroxybenzophenones, aryl esters of hydroxybenzoic acids, a-
cyanocinnamic acid derivatives, benzimidazolecarboxanilides, nickel compounds
or
oxalanilides.
Particularly good stabilization is achieved when at feast one light stabilizer
from the
compound class of sterically hindered amines is also added in the usual
concentration
to the formulations according to the invention.
Examples of suitable sterically hindered amines are: bas(2,2,6,6-
tetramethylpiperidyl)
sebacate, bas(1,2,2,6,6-pentamethylpiperidyl) sebacate, the condensation
product of 1-
hydroxyethyl-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensation product of N,N'-di(2,2,6,6-
tetramethylpiperidyl)hexamethylenediamine
and 4-tert-octylamino-2,6-dichloro-1,3,5-triazine, tris(2,2,6,6-
tetramethylpiperidyl)
nitrilotriacetate, tetrakis(2,2,6,6-tetramethyl-4-piperidyl) 1,2,3,4-
butanetetracarboxylate,
1,1'-(1,2-ethanediyl)-bas(3,3,5,5-tetramethylpiperazinone), the condensation
products of
4-amino-2,2,6,6-tetramethylpiperidines and tetramethylolacetylenediureas.
Examples of plastics which can be stabilized by the compounds I according to
the
invention and may be mentioned are:
polymers of mono- and diolefins, such as, for example, low density or high
density
polyethylene, polypropylene, linear poly-1-butene, polyisoprene,
polybutadiene, and
copolymers of mono- or diolefins or mixtures of said polymers;
copolymers of mono- or diofefins with other vinyl monomers, such as, for
example,
ethylene/alkyl acrylate copolymers, ethylene/alkyl methacrylate copolymers,
ethylene/vinyl acetate copolymers or ethylene/acrylic acid copolymers;
polystyrene and copolymers of styrene or -a-methylstyrene with dienes and/or
acrylic
derivatives, such as, for example, styrene/butadiene, styrene/acrylonitrile
(SAN),
styrene/ethyl methacrylate, styrene/butadiene/ethyl acrylate,
styrene/acryfonitrilelmethacrylate, acrylonitrile/butadienelstyrene (ABS) or
methyl
methacrylate/butadiene/styrene (MBS);
halogen-containing polymers, such as, for example, polyvinyl chloride,
polyvinyl
fluoride, polyvinylidene fluoride and copolymers thereof;
PF 54882
CA 02536679 2006-02-23
polymers derived fram a,f3-unsaturated acids and derivatives thereof, such as
polyacrylates, polymethacrylates, polyacrylamides and polyacrylonitriles;
polymers derived from unsaturated alcohols and amines or acyl derivatives or
acetals
5 thereof, e.g. polyvinyl alcohol and polyvinyl acetate;
polyurethanes, polyamides, polyureas, polyesters, polycarbonates,
polysulfonates,
polyether sulfones and polyether ketones.
10 Furthermore, the formulations according to the invention can be used to
stabilize
aqueous emulsion paints and surface coatings, e.g. industrial finishes. Of
these,
particular attention is drawn to baking finishes, and, in turn, of these,
automotive
finishes, preferably two-coat finishes.
The formulations can be added in solid or liquid form to the surface coating.
Their good
solubility in surface coating systems is of particular advantage here.
Even in the case of the use as stabilizers in surface coatings, it is possible
also to use
the additional additives already listed, in particular antioxidants and light
stabilizers.
The photoprotective agent formulations according to the invention are also
very
particularly preferably suitable as photostable UV filters in cosmetic and
dermatological
preparations for protecting human skin or human hair from solar rays and also
from
artificial light which has high UV contents, alone or together with compounds
which
absorb in the UV region and are known for cosmetic or pharmaceutical
preparations.
Thus, in the widest sense, the term organic materials also means human skin
and
human hair. The cosmetic and pharmaceutical preparations as such are of course
also
stabilized at the same time in order to remain effective for as long as
possible.
Accordingly, the present invention also relates to cosmetic and pharmaceutical
preparations comprising photoprotective agents for protecting human skin or
human
hair from UV light in the range from 280 to 400 nm, which comprise, as
photostable UV
filters and in a cosmetically or pharmaceutically suitable carrier, effective
amounts of a
formulation of the triazine I in amorphous or partially amorphous form - alone
or
together with compounds which absorb in the UV-A and UV-B region and are known
per se for cosmetic and pharmaceutical preparations - the formulations being
aqueous
dispersions according to the invention mentioned in the introduction or the
dry powders
prepared therefrom.
The amount of triazine I in the form of the formulations according to the
invention which
is used in the cosmetic and pharmaceutical preparations is in the range from
0.05 to
20% by weight, preferably 0.1 to 10% by weight, particularly preferably in the
range
from 1 to 7°1° by weight, based on the total amount of the
cosmetic and pharmaceutical
preparation.
PF 54882
CA 02536679 2006-02-23
11
The cosmetic and pharmaceutical preparations comprising photoprotective agents
are
generally based on a carrier which comprises at least one oil phase.
Preparations
based solely on aqueous components are, however, also possible. Accordingly,
suitable preparations are oils, oil-in-water and water-in-oil emulsions,
creams and
pastes, lip-protection stick compositions or grease-free gels.
Suitable emulsions are inter alia also ONV macroemulsions, O/W microemulsions
or
O1V11/O emulsions containing triazine of the formula I present in dispersed
form, the
emulsions being obtainable by phase inversion technology, as in DE-A-197 26
121.
Customary cosmetic auxiliaries which may be suitable as additives are, for
example,
coemulsifiers, fats and waxes, stabilizers, thickeners, biogenic active
ingredients, film
formers, fragrances, dyes, pearlizing agents, preservatives, pigments,
electrolytes (e.g.
magnesium sulfate) and pH regulators. Suitable coemulsifiers are, preferably,
known
W/O and also O/1N emulsifiers, such as, for example, polyglycerol esters,
sorbitan
esters or partially esterified glycerides. Typical examples of fats are
glycerides; waxes
which may be mentioned are inter alia beeswax, paraffin wax or
microcrystalline
waxes, if appropriate in combination with hydrophilic waxes. Stabilizers which
may be
used are metal salts of fatty acids, such as, for example, magnesium, aluminum
and/or
zinc stearate. Examples of suitable thickeners are crosslinked polyacrylic
acids and
derivatives thereof, polysaccharides, in particular xanthan gum, guar guar,
agar agar,
alginates and tyloses, carboxymethylcellulose and hydroxyethylcellulose, and
also fatty
alcohols, monoglycerides and fatty acids, polycrylates, polyvinyl alcohol and
polyvinylpyrrolidone. The term biogenic active ingredients means, for example,
plant
extracts, protein hydrolyzates and vitamin complexes. Customary film formers
are, for
example, hydrocolloids, such as chitosan, microcrystalline chitosan or
quaternary
chitosan, polyvinylpyrrolidone, vinylpyrrolidone/vinyl acetate copolymers,
polymers of
the acrylic acid series, quaternary cellulose derivatives and similar
compounds.
Examples of suitable preservatives are formaldehyde solution, p-
hydroxybenzoate or
sorbic acid. Examples of suitable pearlizing agents are glycol distearic
esters, such as
ethylene glycol distearate, but also fatty acids and fatty acid monoglycol
esters. Dyes
which may be used are the substances suitable and approved for cosmetic
purposes,
as listed, for example, in the publication "Kosmetische Farbemittel" [Cosmetic
Colorants] from the Farbstoffkommission der Deutschen Forschungsgemeinschaft
[Dyes Commission of the German Research Council], published by Verlag Chemie,
Weinheim, 1984. These dyes are usually used in a concentration of from 0.001
to 0.1
by weight, based on the total mixture.
An additional content of antioxidants is generally preferred. Thus, favorable
antioxidants which can be used ace all antioxidants which are suitable or
customary for
cosmetic and/or dermatological applications.
The antioxidants are advantageously chosen from the group consisting of amino
acids
(e.g. glycine, histidine, tyrosine, tryptophan) and derivatives thereof,
imidazoles (e.g.
urocanic acid) and derivatives thereof, peptides such as D,L-carnosine, D-
carnosine,
L-carnosine and derivatives thereof (e.g. anserine), carotenoids, carotene
(e.g. (3-
PF 54882
CA 02536679 2006-02-23
12
carotene, lycopene) and derivatives thereof, chlorogenic acid and derivatives
thereof,
lipoic acid and derivatives thereof (e.g. dihydrolipoic acid),
aurothioglucose,
propylthiouracil and other thiols (e.g. thiorodoxin, glutathione, cysteine,
cystine,
cystamine and the glycosyl, N-acetyl, methyl, ethyl, propyl, amyl, butyl and
lauryl,
palmitoyl, oleyl, y-linoleyl, cholesteryl and glyceryl esters thereof) and
salts thereof,
dilauryl thiodipropionate, distearyl thiodipropionate, thiodipropionic acid
and derivatives
thereof (esters, ethers, peptides, lipids, nucleotides, nucleosides and salts)
and
sulfoximine compounds (e.g. buthionine sulfoximines, homocysteine
sulfoximines,
buthionine sulfones, yenta-, hexa-, heptathionine sulfoximine) in very low
tolerated
doses (e.g. pmol to Nmollkg), also (metal) cheiating agents (e.g. a-
hydroxyfatty acids,
palmitic acid, phytic acid, lactoferrin), a-hydroxy acids (e.g. citric acid,
lactic acid, malic
acid), humic acid, bile acid, bile extracts, bilirubin, biliverdin, EDTA and
derivatives
thereof, unsaturated fatty acids and derivatives thereof (e.g. y-linolenic
acid, linoleic
acid, oleic acid), folic acid and derivatives thereof, ubiquinone and
ubiquinol and
derivatives thereof, vitamin C and derivatives thereof (e.g. ascorbyl
palmitate, Mg
ascorbyl phosphate, ascorbyl acetate), tocopherol and derivatives (e.g.
vitamin E
acetate, tocotrienol}, vitamin A and derivatives (vitamin A palmitate) and
coniferyl
benzoate of benzoin resin, rutic acid and derivatives thereof, a-
glycosyfrutin, ferulic
acid, furfurylideneglucitol, carnosine, butylhydroxytoluene,
butylhydroxyanisole,
nordihydroguaiac resin acid, nordihydroguaiaretic acid,
trihydroxybutyrophenone, uric
acid and derivatives thereof, mannose and derivatives thereof, zinc and
derivatives
thereof (e.g. ZnO, ZnS04), selenium and derivatives thereof (e.g.
selenomethionine),
stilbenes and derivatives thereof (e.g. stilbene oxide, trans-stilbene oxide}.
The amount of the abovementioned antioxidants (one or more compounds) in the
preparations is preferably 0.001 to 30% by weight, particularly preferably
0.05 to 20°t°
by weight, in particular 1 to 10% by weight, based on the total weight of the
preparation.
If vitamin E and/or derivatives thereof are the antioxidant or antioxidants,
it is
advantageous to choose the respective concentration thereof from the range
0.001 to
10% by weight, based on the total weight of the formulation.
If vitamin A and/or derivatives thereof or carotenoids are the antioxidant or
antioxidants, it is advantageous to choose the respective concentration
thereof from
the range 0.001 to 10% by weight, based on the total weight of the
formulation.
Customary oil components in cosmetics are, for example, silicone oils,
paraffin oil,
glyceryl stearate, isopropyl myristate, diisopropyl adipate, cetylstearyl 2-
ethylhexanoate, hydrogenated polyisobutene, vaseline, capryliclcapric
triglycerides,
microcrystalline wax, lanolin and stearic acid.
The total proportion of auxiliaries and additives can be 1 to 80% by weight,
preferably 6
to 40% by weight, and the nonaqueous proportion ("active substance") can be 20
to
80% by weight, preferably 30 to 70% by weight, based on the compositions. The
compositions can be prepared in a manner known per se, i.e. for example by
hot, cold,
PF 54882
CA 02536679 2006-02-23
13
hot-hotlcold or PIT emulsification. This is a purely mechanical process, and
no
chemical reaction takes place.
Such sunscreen preparations can accordingly be in liquid, paste or solid form,
for
example as water-in-oil creams, oil-in-Water creams and lotions, aerosol foam
creams,
gels, oils, marking pencils, powders, sprays or alcohol-aqueous lotions.
Finally, it is possible additionally to use further substances known per se
which absorb
in the UV region, provided they are stable in the overall system of the
combination of
UV filters to be used according to the invention.
The majority of photoprotective agents in the cosmetic and pharmaceutical
preparations used to protect the human epidermis consists of compounds which
absorb UV fight in the UV-B region, i.e. in the range from 280 to 320 nm. For
example,
the proportion of the UV-A absorbers to be used according to the invention is
10 to
90% by weight, preferably 20 to 50% by weight, based on the total amount of UV-
B and
UV-A absorbing substances.
Suitable UV filter substances which are used in combination with the
formulations to be
used according to the invention are any UV-A and UV-B filter substances.
Examples
which may be mentioned are:
No Substance CAS No.(=acid)
1 4-Aminobenzoic acid 150-13-0
2 3-(4'-Trimethylammonium)benzylidenebornan-2-one52793-97-2
I methylsulfate
3 3,3,5-Trimethylcyclohexyl salicylate(homosalate)118-56-9
4 2-Hydroxy-4-methoxybenzophenone(oxybenzone) 131-57-7
5 2-Phenylbenzimidazole-5-sulfonic acid and 27503-81-7
its potassium,
sodium and triethanolamine salts
6 3,3'-(1,4-Phenylenedimethine}-bis(7,7-dimethyl-2-90457-82-2
oxobicyclo[2.2.1]heptane-1-methanesulfonic
acid) and its salts
7 Polyethoxyethyl4-bis(polyethoxy)aminobenzoate113010-52-9
8 2-Ethylhexyl4-dimethylaminobenzoate 21245-02-3
9 2-Ethylhexyl salicylate 118-60-5
10 2-Isoamyl4-methoxycinnamate 71617-10-2
11 2-Ethylhexyl 4-methoxycinnamate 5466-77-3
12 2-Hydroxy-4-methoxybenzophenone-5-sulfonic 4065-45-6
acid
(sulisobenzone) and the sodium salt
13 3-(4'-Sulfobenzylidene)bornan-2-one and salts58030-58-6
14 3-Benzylidenebornan-2-one 16087-24-8
15 1-(4'-Isopropylphenyl~--3-phenylpropane-1,3--dione63260-25-9
16 4-Isopropylbenzyl salicylate 94134-93-7
17 3-Imidazol--~-ylacrylic acid and its ethyl ~ 104-98-3
ester
PF 54882
CA 02536679 2006-02-23
14
18 Ethyl2-cyano-3,3-diphenylacrylate 5232-99-5
19 2'-Ethylhexyl2-cyano-3,3-~iiphenylacrylate 6197-30-4
20 Menthyl o-aminobenzoate or:5-methyl-2-(1-methylethyl}-2-134-09-8
aminobenzoate
21 Glyceryl p-aminobenzoate or:1-glyceryl 4-aminobenzoate136-44-7
22 2,2'-Dihydroxy-4-methoxybenzophenone (dioxybenzone)131-53-3
23 2-Hydroxy-d-methoxy-4-methylbenzophenone (mexenone)1641-17-4
24 Triethanolamine salicylate 2174-16-5
25 Dimethoxyphenylglyoxalic acid or sodium 3,4- 4732-70-1
dimethoxyphenylglyoxalate
26 3-(4'-Sulfobenzylidene)bornan-2-one and its 56039-58-8
salts
27 4-tert-Butyi-4'-methoxydibenzoylmethane 70356-09-1
28 2,2',4,4'-Tetrahydroxybenzophenone 131-55-5
29 2,2'-Methylenebis[6(2H-benzotriazol-2-y1~4-(1,1,3,3-103597-45-1
tetramethylbutyl)phenolJ
30 2,2'-(1,4-Phenylene)-bis-1 H-benzimidazofe-4,6-disulfonic180898-37-7
acid, Na salt
31 2,4-bis(4-(2-Ethylhexyloxy}-2-hydroxy]phenyl-6-(4-187393-00-6
methoxyphenyl~-(1,3,5--triazine
32 3-(4-Methylbenzylidene)camphor 36861-47-9
33 Polyethoxyethyl4-bis(polyethoxy)paraaminobenzoate113010-52-9
34 2,4-Dihydroxybenzophenone 131-56-6
35 2,2'-Dihydroxy-4,4'-dimethoxybenzophenone-5,5'-disodium3121-60-6
sulfonate
Polymeric or polymer-bonded filter substances can also be used according to
the
invention.
The cosmetic and dermatological preparations according to the invention can
additionally advantageously comprise inorganic pigments based on metal oxides
andlor other metal compounds which are insoluble or sparingly soluble in
water, for
example the oxides of titanium (Ti02), zinc (Zn0), iron (e.g. Fe203),
zirconium (Zr02),
silicon (Si02), manganese (e.g. Mn0), aluminum (A1203), cerium (e.g. Ce203),
mixed
oxides of the corresponding metals, and mixtures of such oxides. Particular
preference
is given to pigments based on Ti02 and ZnO.
The inorganic pigments may be present here in hydrophobic form, i.e. surface-
treated
to repel water. This surface treatment may involve providing the pigments with
a thin
hydrophobic layer by a method known per se, as described in DE-A-33 14 742.
To protect human hair from UV rays, the photoprotective agent formulations
according
to the invention can be incorporated into shampoos, lotions, gels, hairsprays,
hair
colorants, aerosol foam creams or emulsions in concentrations of from 0.1 to
10% by
weight, preferably 1 to 7% by weight. The respective formulations can inter
alia be
used for washing, coloring and for styling hair.
PF 54882
CA 02536679 2006-02-23
The formulations to be used according to the invention are usually notable for
a
particularly high absorbance in the UV-A radiation region with a sharp band
structure.
Moreover, they are readily soluble in cosmetic oils and can easily be
incorporated into
cosmetic formulations. The emulsions prepared with the formulations are
particularly
5 notable for their high stability, the formulations I themselves are notable
for their high
photostability, and the preparations prepared therewith are notable for their
pleasant
feel on the skin.
The UV fitter action of the formulations according to the invention can also
be utilized
10 for stabilizing active ingredients and auxiliaries in cosmetic and
pharmaceutical
formulations.
The preparations according to the invention are notable for particularly high
absorbance in the UV-B radiation region with a sharp band structure and high
light
15 protection factors.
In particular, the high sun protection factor of the preparations which was
measured
even at low concentrations of triazine compound I was surprising.
In addition, the preparations according to the invention have the advantage
over other
triazine-containing formulations of improved dispersibility in cold water.
The examples below serve to illustrate the present invention without limiting
it.
Example 1
Preparation of a Uvinul~ T150-containing dry powder having an active
ingredient
content of 20% by weight
a) Preparation of the aqueous dispersion
A molecularly disperse solution of 5 g of Uvinul~ T150 in 95 g of acetone was
passed at 150°C to a mixing chamber, where it was mixed with 1300 g of
a 30°C
hot aqueous solution of HiCap~ (15 g!1). The entire process was carried out
with
a pressure limit of 40 bar in order to prevent evaporation of the solvent.
After
mixing, a colloidally disperse Uvinul~ T150 dispersion with a white cloudy
color
was obtained.
Fraunhofer diffraction was used to determine the average volume distribution
as
D (4.3) = 0.50 ~m with a fines content of the distribution of 98.5% < 1.22 pm.
b) Preparation of a Uvinul~ T150-containing aqueous dry powder
PF 54882
CA 02536679 2006-02-23
16
Spray-drying the dispersion resulted in a dry powder having an active
ingredient
content of 20.56% by weight of Uvinul~ T150 (content determination by means of
UV/VIS spectroscopy). The dry powder could be redispersed in demineralized
water again to form a white cloudy dispersion (hydrosol).
Fraunhofer diffraction was used to determine the average volume distribution
in
the redispersion as D (4.3) = 0.65 pm with a fines content of the distribution
of
97.1 °l° < 1.22 pm.
Example 2
Preparation of a Uvinul~ T150-containing dry powder having an active
ingredient
content of 20% by weight
a) Preparation of the aqueous dispersion
A molecularly disperse solution of 10 g of Uvinul~ T150 in 90 g of
isopropanol/water (9:1, vlv) was passed at 150°C to a mixing chamber,
where it
was mixed with 1000 g of a 30°C hot aqueous solution of HiCap~ (30
g/1). The
entire process was carried out with a pressure limit of 40 bar in order to
prevent
evaporation of the solvent. After mixing, a colloidally disperse Uvinul~ T150
dispersion with a white cloudy color was obtained.
Fraunhofer diffraction was used to determine the average volume distribution
as
D (4.3) = 0.50 ~m with a fines content of the distribution of 98.5% < 1.22
Vim.
b) Preparation of a Uvinul~ T150-containing aqueous dry powder
Spray-drying the dispersion resulted in a dry powder having an active
ingredient
content of 20.56% by weight of Uvinul~ T150 (content determination by means of
UVNIS spectroscopy). The dry powder could be redispersed in demineralized
water again to form a white cloudy dispersion (hydrosol).
Fraunhofer diffraction was used to determine the average volume distribution
in
the redispersion as D (4.3) = 0.65 ~m with a fines content of the distribution
of
97.1 % < 1.22 pm.
Example 3
Preparation of a Uvinul~ T150-containing dry powder having an active
ingredient
content of 15°!° by weight
PF 54882
CA 02536679 2006-02-23
17
a) Preparation of the aqueous dispersion
25 g of Uvinul~ T150 were dissolved in 216 g of isopropanol at room
temperature to give a molecularly disperse solution. To precipitate out the
Uvinul~ T150 in colloidally disperse form, the solution was passed at
240°C to a
mixing chamber, where it was mixed with an aqueous solution of 45 g of HiCap
in 1455 ml of demineralized water. The entire process was carried out with a
pressure limit of 40 bar in order to prevent evaporation of the solvent. After
mixing, a colloidally disperse Uvinul~ T150 dispersion with a white cloudy
color
was obtained.
Fraunhofer diffraction was used to determine the average volume distribution
as
D (4.3) = 0.52 Nm with a fines content of the distribution of 98.5% < 1.22 Nm.
b) Preparation of a Uvinul~ T150-containing aqueous dry powder
Spray-drying the dispersion resulted in a dry powder having an active
ingredient
content of 15.86% by weight of Uvinul~ T150 (content determination by means
of UVNIS spectroscopy). The dry powder could be redispersed in demineralized
water again to form a white cloudy dispersion (hydrosol).
Fraunhofer diffraction was used to determine the average volume distribution
in
the redispersion as D (4.3) = 1.91 Nm with a fines content of the distribution
of
84.67% < 1.22 Nm.
Preparations
Example 4: Lip care composition
Mass content (% by weight)
ad 100 Eucerinum anhydricum
10.00 Glycerol
10.00 Titanium dioxide, micronized
5.00 Uvinul~ T150 dry powder from Example
1
8.00 Octyl methoxycinnamate
5.00 Zinc oxide
4.00 Castor oil
4.00 Pentaerythritil stearatelcaprate/caprylate
adipate
3.00 Glyceryl stearate SE
2.00 Beeswax
2.00 Microcrystafline wax
PF 54882
CA 02536679 2006-02-23
18
2.00 Quaternium-18 bentonite
1.50 PEG-.45/dodecyl glycol copolymer
Example 5: Composition for sunblock containing micropigments
Mass content (% by weight)
ad 100 Water
10.00 Octy1 methoxycinnamate
6.00 PEG-7-Hydrogenated castor oil
6.00 Titanium dioxide, micronized
5.00 Uvinul~ T150 dry powder from
Example 1
5.00 Mineral oil
5.00 Isoamyl p-methoxycinnamate
5.00 Propylene glycol
3.00 Jojoba oil
3.00 4-Methylbenzylidenecamphor
2.00 PEG-45/dodecyl glycol copolymer
1.00 Dimethicone
0.50 PEG-40 hydrogenated castor
oil
0.50 Tocopheryl acetate
0.50 Phenoxyethanol
0.20 EDTA
Example 6: Grease-free gel
Mass content (% by weight)
ad 100 Water
8.00 Octyl methoxycinnamate
7.00 Titanium dioxide, micronized
5.00 Uvinul~ T150 dry powder from Example
1
5.00 Glycerol .
5.00 PEG-25 PABA
1.00 4-Methylbenzylidenecamphor
0.40 Acrylates C,o-C3o alkyl acrylate
crosspolymer
0.30 Imidazolidinylurea
0.25 Hydroxyethylcellulose
0.25 Sodium methylparaben
0.20 Disodium EDTA
0.15 Fragrance
0.15 Sodium propylparaben
PF 54882
CA 02536679 2006-02-23
19
0.10 Sodium hydroxide
Example 7: Suncream (SPF 20)
Mass content (% by weight)
ad 100 Water
8.00 Octyf methoxycinnamate
8.00 Titanium dioxide, micronized
6.00 PEG-7-Hydrogenated castor oil
5.00 Uvinul~ T150 dry powder from
Example 1
6.00 Mineral oil
5.00 Isopropyl palmitate
0.30 Imidazolidinylurea
3.00 Jojoba oil
2.00 PEG-45/Dodecyl glycol copolymer
1.00 4-Methylbenzylidenecamphor
0.60 Magnesium stearate
0.50 Tocopheryl acetate
0.25 Methylparaben
0.20 Disodium EDTA
0.15 Propylparaben
Example 8: Water-resistant suncream
Mass content (% by weight)
ad 100 Water
8.00 Octyl methoxycinnamate
5.00 PEG-7-Hydrogenated castor oil
5.00 Propylene glycol
4.00 Isopropyl palmitate
4.00 Caprylic/capric triglyceride
5.00 Uvinul~ T150 dry powder from
Example 1
4.00 Glycerol
3.00 Jojoba oil
2.00 4-Methylbenzylidenecamphor
2.00 Titanium dioxide, micronized
1.50 PEG-45/dodecyl glycol copolymer
1.50 Dimethicone
0.70 Magnesium sulfate
0.50 Magnesium stearate
PF 54882
CA 02536679 2006-02-23
0.15 Fragrance
Example 9: Sun milk (SPF 6)
5 Mass content (% by weight)
ad 100 Water
10.00 Mineral oil
6.00 PEG-7-Hydrogenated castor oil
10 5.00 Isopropyl palmitate
3.50 Octyl methoxycinnamate
5.00 Uvinul~ T150 dry powder from
Example 1
3.00 Caprylic/capric triglyceride
3.00 Jojoba oil
15 2.00 PEG-45ldodecyl glycol copolymer
0.70 Magnesium sulfate
0.60 Magnesium stearate
0.50 Tocopheryl acetate
3.00 Glycerol
20 0.25 Methylparaben
0.15 Propylparaben
0.05 Tocopherol
Example 10: Day lotion with UV protection
Mass content (°!° by weight)
ad 100 Water
2.00 Cetearyl alcohol
1.00 Glycerol monostearate
2.00 Vaseline
7.50 Octyl methoxycinnamate
4.00 Octyl salicylate
3.00 Uvinul~ T150 dry powder from
Example 1
1.50 4-tert-Butyl-4'-methoxydibenzoylmethane
0.50 Dimethicone
5.00 Propylene glycol
0.20 EDTA
0.20 Carbomer
5.00 C,z--C,5 Alkyl benzoate
0.27 Triethanolamine
1.00 Tocopheryl acetate
PF 54882
CA 02536679 2006-02-23
21
q.s. Fragrance
Example 11: Day cream with UV protection
Mass content (% by weight}
ad 100 Water
2.00 Cetearyl alcohol
2.00 Cetyl alcohol
1.00 Glycerol monostearate
2.00 Vaseline
7.50 Octyl methoxycinnamate
4.00 Octyl salicylate
3.00 Uvinul~ T150 dry powder from
Example 1
1.50 4-tert-Butyf-4'-methoxydibenzoylmethane
4.00 Propylene glycol
0.20 EDTA
0.20 Carbomer
0.20 Xanthan
0.20 C,o-C3o Alkyl acrylate crosspolymer
5.00 C,2-C,5 Alkyl benzoate
0.54 Triethanolamine
1.00 Tocopheryl acetate
q.s. Fragrance
q.s. Preservative
Example 12: Liquid make up
Mass content (% by weight)
ad 100 Water
2.00 Cetearyl alcohol
2.00 Ceteareth 25
6.00 Glycerol monostearate
1.00 Cetyl alcohol
8.00 Paraffin oil
7.00 Cetearyl octanoate
0.2 Dimethicone
3.00 Propylene glycol
1.00 Panthenol
3.00 Uvinul~ T150 dry powder from
Example 1
1.50 4-tent-Butyl-4'-methoxydibenzoylmethane
PF 54882
CA 02536679 2006-02-23
22
3.50 Octyl methoxycinnamate
0.1 Bisabolol
5.70 Titanium dioxide
1.10 Iron oxide
q.s. Fragrance
Example 13: Hair gel with sun protection
Mass content (°l° by weight)
ad 100 Water
1.20 Carbomer
0.50 Hydroxyethylcellulose
4.00 Triethanolamine
0.70 PEG-40 Hydrogenated castor
oil
1.50 Uvinul~ T150 dry powder from
Example 1
0.70 4-tert-Butyl-4'-methoxydibenzoylmethane
2.80 Octyl methoxycinnamate
5.00 Propylene glycol
0.01 EDTA
q.s. Fragrance
q.s. Sicovit Patent Blue 85 E 131