Note: Descriptions are shown in the official language in which they were submitted.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
1
PROTECTIVE COATING FOR AUTOMOTIVE TRIM PIECES AND
METHOD OF MAKING THE SAME
Microfiche Appendix
(0001] Not Applicable.
Field of the Invention
[0002] The present invention generally relates to the field of protective
coatings and
in particular to protective coatings for aluminum or aluminum alloy surfaces.
Background of the Invention
[0003] Anodically produced oxide layers are frequently applied to aluminum
surfaces
to protect the aluminum or aluminum alloys against weathering and/or
corrosion. A
number of processes, such as anodizing, are known for the application of
anodic oxide
layers to aluminum. These oxide layers protect the aluminum surface from the
effects
of weather and other corroding media. In addition, anodic oxide layers also
provide a
harder surface thereby giving the aluminum an increased resistance to wear.
[0004] Anodizing is an electrochemical conversion process wherein a coating of
aluminum oxide is grown from aluminum on a surface of an aluminum article. The
anodizing of aluminum is performed by making the aluminum article the anode or
positive end of an electrical circuit within an acid electrolyte in which the
aluminum
article is immersed. By passing an electrical current through the acid
electrolyte from
the cathode, an aluminum oxide layer develops on the surface of the aluminum
article.
This aluminum oxide layer can be formed so that it has a porous quality. The
aluminum oxide layer can be dyed in a variety of colors. The coating thickness
and
surface characteristics are controlled to meet the desired specifications of
the final
product.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
[0005] Another method of providing protection from weathering is by
electrodeposition coating. Electrodeposition coating is widely used for primer
coating
of cars, household electric appliances, and industrial machines.
Electrodeposition
coating has a good efficiency in application and a high corrosion resistance
and can
also minimize risk of environmental contamination because water is employed as
a
solvent.
[0006] Cationic electrodeposition provides excellent corrosion-protective
coatings for
automobiles and for appliances while diminishing the usage of volatile organic
solvents required for painting. It has been used as an undercoating method of
articles
to be coated which are large and complicated in shape and are required to have
high
rust prevention, such as car bodies. Cationic electrodeposition is capable of
coating
intricately shaped articles in an automated and continuous manner. As compared
with
other coating methods, the technology is highly efficient in coating
consumption,
hence economical, and has come into wide use as an industrial coating method.
[0007] Cationic electrodeposition of water-soluble compositions utilizes a
direct
current to cause positively charged electrolytes in an aqueous medium to form
a
coating on the cathode. The electrolytes are usually polymers, e.g. acrylic
and/or
epoxy polymers. The positively charged polymers are dispersed in an aqueous
medium such that upon applying a voltage between an article having a
conductive
surface serving as a cathode and a counter-electrode both of which are in
contact with
the aqueous medium, the positively charged polymer migrates to the conductive
surface. There the polymer loses its charge, becomes insoluble and forms an
insulating film on the conductive surface. As the deposition progresses, the
conductive surface becomes insulated which advantageously allows uniform
coating
over even remote areas, i.e. interior or recessed areas.
[0008] Furthermore, cathodic electrodeposition provides excellent coatings for
corrosion protection of automobiles and appliances while diminishing the usage
of
volatile organic solvents required for painting. Where metals are
electrocoated,
pretreatment is required to obtain optimal corrosion resistance for the
coating. A
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
chrome rinse is usually used as a pretreatment. However, chromium or chromium
compounds have a high toxicity and hence it is desirable to provide an
electrocoating
method that does not require a chrome pretreatment.
[0009] The present invention is directed to improving the appearance of
anodized
aluminum and protecting it from corrosion, stains and weathering. It had
previously
been proposed to protect anodized aluminum by means of a clear coating, but
the
performance of prior art coatings has not been as good as desired,
particularly with
regard to adhesion. It is believed that anodizing tends to seal the surface
irregularities
of an aluminum surface, thereby making it more difficult for a subsequently
applied
coating to adhere. As a result, prior art clear coatings on anodized aluminum
have
typically failed in relatively short periods of time by chipping and peeling,
which not
only leaves the anodized layer exposed to corrosion, but also produces an
unattractive
appearance.
[0010] Coatings based on a variety of film-forming resin systems are generally
considered appropriate for coating aluminum surfaces, including alkyds,
polyesters,
silicone-polyesters, thermoplastic acrylics, thermosetting acrylics, and
fluoropolymers. When used on anodized aluminum surfaces, however, most of
these
conventional coating compositions do not exhibit the desired degree of
adhesion to
the anodized surface. A variety of additives for promoting adhesion to an
anodized
aluminum surface are available commercially.
[0011] There are two types of electrocoating compositions that are based on
epoxy
resins and acrylic resins. However, it has been found that epoxy-based
electrocoats
start to peel and discolor after a relatively small period of time, e.g. about
six months.
Furthermore, epoxy resins are not UV stable and hence they are not suited for
exterior
applications, such as on automotive trim pieces and in particular on class "A"
surfaces.
[0012] Another problem with the application of an electrocoat over top of
anodized
aluminum occurs during the thermal curing step of the electrocoat. The high
curing
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
temperatures crack or craze anodic oxide coatings on aluminum because the
thermal
expansion of these oxide coatings is significantly lower than that of the
underlying
aluminum base metal. Craze lines extending through the anodic oxide film
substantially detract from the protective and esthetic value of the anodic
coating.
[0013] U.S. Patent No. 3,798,143 to Rolles et al. provides a method for an
electrophoretic deposition of acrylic copolymers or interpolymers from aqueous
colloidal dispersions on aluminum or anodized aluminum. This overcoat is
applied
directly on the anodized aluminum surface to seal the pores and to avoid
additional
steps) of sealing the porous surface by means of a nickel seal or boiling in
hot water.
Furthermore, Rolles et al. eliminate the thermal curing step to avoid cracks
or crazing
of the anodic oxide film. This is done by employing an aqueous colloidal
dispersion
comprising an interpolymer of methyl methacrylate and an acid selected.from
the
group of acrylic acid, methacrylic acid, malefic acid, and itaconic acid, and
thereafter
coalescing the resulting coating on the anodized aluminum. The coalescing is
achieved by heating the coating at relatively low temperatures not exceeding
200°F.
If a coalescing agent is employed, such as xylol or butyl Cellosolve, the
coalescing
may be accomplished at room temperature, or about 65-90°F. Thus, Rolles
et al.
employ temperatures to coalesce the resin films that are below those at which
extensive crazing of anodic coatings occurs. However, the employed coalescing
agents are carcinogens and hence not environmentally friendly.
[0014] Furthermore, it is desirable to provide a coating with improved
corrosion
resistance and weathering resistance/LJV stability. In accordance with the
present
invention a thermosetting resin is electrodeposited over an anodized aluminum
or
aluminum alloy surface which requires relatively high temperatures for the
thermal
curing step of the electrocoat.
[0015] Hence it is further desirable to provide a method of coating over
anodized
aluminum or aluminum alloys that will maintain a substantially continuous
underlying
anodized layer, without cracks or crazing, during thermal curing of the
thermosetting
resin.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
[0016] It is desirable to provide an electrolytic coating to an anodized
aluminum
surface.
Summary of the Invention
[0017] In accordance with one aspect of the invention there is provided, a
method of
providing a protective coating on a surface of an aluminum article comprising
the
steps of providing an anodized coating on the surface of the aluminum article,
sealing
pores of the anodized coating, electrocoating the anodized coating of the
aluminum
article with a thermosetting cationic acrylic resin, and curing the
thermosetting
cationic acrylic resin, wherein the anodized coating has a sufficient softness
such that
a curing of the thermosetting cationic acrylic resin does not cause a
formation of
fractures in the anodized coating.
[0018] In accordance with another aspect of the invention, the anodized
coating is
provided at a temperature between substantially about 20 to 30°C, at a
voltage of
substantially about 10 to 15V, and at an electrolyte concentration of
substantially
about 10 to 15% by volume. If desired, the electrolyte is sulfuric acid.
[0019] In accordance with a further aspect of the invention, the thermosetting
cationic
acrylic resin is a clear resin.
[0020] In accordance with another aspect of the invention, the thermosetting
cationic
acrylic resin comprises polyurethane.
[0021] In accordance with yet another aspect of the invention, the
thermosetting
cationic acrylic resin comprises a UV stabilizer. The step of curing is
performed at a
temperature of substantially about 163-177°C for substantially about 20
to 50 minutes.
[0022] In another aspect of the invention, the method further comprises the
step of
coloring the aluminum article after providing the anodized coating and prior
to sealing
pores of the anodized coating. The coloring step is an electrolytic coloring
step using
a metal salt as a colorant, such as cobalt sulfate and tin sulfate.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
[0023] In accordance with the invention, there is further provided, a method
of
providing a protective coating for automotive trim pieces comprising the
following
steps anodizing the aluminum article at a temperature between substantially
about 20
to 30°C, at a voltage of substantially about 10 to 15V, and at an
electrolyte
concentration of substantially about 10 to 15% by volume for providing a layer
of
aluminum oxide, said layer of aluminum oxide is a porous layer, sealing the
porous
layer of aluminum oxide for providing a sealed layer of aluminum oxide,
electrocoating the sealed layer of aluminum oxide with a thermosetting
cationic
acrylic resin for providing an electrocoat, and curing the thermosetting
cationic
acrylic resin.
[0024] In accordance with another aspect of the invention, there is provided,
an
automotive trim piece having a protective coating, said protective coating
comprising
an aluminum or aluminum alloy base metal, a layer of aluminum oxide provided
over
top said aluminum or aluminum alloy base metal, and an electrocoat layer
provided
over top of said layer of aluminum oxide, said electrocoat layer comprising a
clear
thermosetting cationic acrylic resin, said layer of aluminum oxide having a
softness so
as to avoid fractures in said layer of aluminum oxide when the electrocoat
layer is
provided over top the layer of aluminum oxide.
[0025] In accordance with another embodiment of the invention, the automotive
trim
piece further comprises an electrolytic colorant inlthe layer of aluminum
oxide.
[0026] Advantageously, the present invention provides a protective coating for
a
variety of aluminum articles, and in particular protection from weathering/UV
light
and corrosion. The protective coating in accordance with the invention is
advantageously applied on exterior automotive trim pieces. The protective
coating of
the present invention has a good adhesion and does not fracture upon a thermal
curing
of an electrocoat layer that is applied over top of an anodized layer.
[0027] The electrodeposition step of the present invention provides a method
that
requires a lower usage of volatile organic solvents and hence it is more
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
environmentally friendly. In addition, the method of providing a protective
coating
on automotive trim pieces obviates a chrome pretreatment step.
Brief Description of the Drawings
[0028] Exemplary embodiments of the invention will now be described in
conjunction with the following drawings wherein like numerals represent like
elements, and wherein:
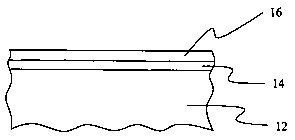
[0029] Fig. 1 presents a schematic drawing of an aluminum article having a
protective
coating in accordance with the present invention;
[0030] Fig. 2 shows a flow chart of the various cleaning and pretreating
steps;
[0031] Fig. 3 shows a flow chart of the steps for a clear anodizing process;
[0032] Fig. 4 shows a flow chart of the steps for a color anodizing process;
[0033] Fig. 5 shows a flow chart of the electrocoating steps; and
[0034] Fig. 6 shows a flow chart of the steps for an alternative clear
anodizing
process wherein the anodized aluminum article is faintly colored.
Detailed Description of the Preferred Embodiments
[0035] The present invention provides a protective coating for an aluminum
article
and a method of making the same. The term aluminum as used herein is intended
to
include both aluminum and aluminum alloys. Advantageously, the protective
coating
in accordance with the present invention imparts corrosion and weathering
protection
to the coated aluminum article including ultra-violet (UV) stability.
Initially, an
aluminum article 12 is anodized to provide a layer of aluminum oxide 14 and
subsequently it is provided with an electrocoat 16 by means of
electrodeposition as
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
shown in Fig. 1. The method of providing the protective coating is explained
hereinafter in further detail in conjunction with Figs. 2 to 6.
[0036] The anodizing step is typically performed by subjecting the aluminum
article
to anodic oxidation by passing a DC electric current through an acidic
electrolyte
solution, for example containing sulfuric acid (H2S04), and between the
aluminum
article arranged as the anode and a cathode arranged as the counter-electrode,
advantageously after degreasing. The electrolytic oxidation of the aluminum
surface
produces a protective oxide coating. The anodic coating consists of hydrated
aluminum oxide which imparts resistance to corrosion and abrasion. The
aluminum
oxide coating is generally transparent but it may be colored.
[0037] Because anodic coatings are normally transparent, any modifications or
improvements of the surface of the aluminum article to be anodized carry
through the
anodizing process. As a result, many process lines for anodizing alumina
articles
include pretreatment steps.
[0038] The pretreatment operations and the anodizing step are performed in a
series
of tanks and the aluminum article is moved from tank to tank. The aluminum
article
is rinsed thoroughly after each operation so as to avoid contamination and
interference
during the next processing step.
[0039] A typical process line for the anodizing step in accordance with the
present
invention includes cleaning steps, pretreating steps, an anodizing step, a
coloring step
(if desired), and sealing steps.
[0040] Turning now to Fig. 2, a flow chart is presented showing the various,
cleaning
and pretreating steps. The aluminum article is loaded 100 on a rack and then
passed
to a first tank where an alkali cleaning step 102 is performed. The aluminum
article is
then rinsed 104 and subsequently an acid cleaning step 106 and a rinsing step
108 are
performed. These cleaners remove, for example, fabrication oils, fats, greases
and
buffing compounds.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
[0041] Following the cleaning steps, a pretreatment step is performed to
improve the
surface of the aluminum article prior to the anodizing step. In accordance
with the
present invention a bright dip step 110 is used as a pretreatment step. The
bright dip
process takes place in a bath of mixed acids to impart a bright, shiny finish
by
chemically polishing the surface of the aluminum article so as to level
microscopic
peaks and valleys on the surface. The bright dip step is usually preceded by
an acid
cleaning, as indicated by reference numeral 106, to remove an oxide layer from
the
surface of the aluminum article so as to provide a uniform surface finish.
Bright dip
anodizing enhances the glossy appearance of aluminum for exterior
ornamentation
applications on class A surfaces. It achieves a highly electro polish finish
similar to
the quality of plating. In contrast to plating, bright dip is a chemical
process that
brightens aluminum and does not leave deposits on the surface of the part.
After the
aluminum article is bright dipped, it can be anodized clear or dyed to a
variety of
colors.
(0042] Following the bright dip 110, the aluminum article undergoes a drag out
rinse
112 as a quick bright dip rinse followed by a clean rinse 114. After the
bright dip 110
and rinsing steps 112 and 114, the aluminum article is dipped into a
deoxidizer
solution 116 to remove any residues of alloying agents or oxides. Following
the
deoxidizing step 116, the aluminum article is first rinsed 118 and then spray
rinsed
120. Subsequently, the anodizing step is performed.
[0043] The anodizing step is the step that produces the actual alumina
coating. It is
performed in an electrolytic cell using sulfuric acid as the electrolyte. The
aluminum
article is made the anode (positive electrode). When a direct current (DC) is
passed
through the electrolytic cell, water is decomposed to form oxygen on the
surface of
the aluminum article. The oxygen reacts with the aluminum to form a porous
layer of
aluminum oxide. The thickness of this aluminum oxide coating is determined by
the
electrical current and the length of time it is applied.
[0044] In accordance with the present invention, a clear or color anodizing
step is
performed, depending on whether the anodized aluminum article is to be colored
or to
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
remain clear. If a coloring of the anodized aluminum article is to be
performed, the
anodizing step is performed for a longer period of time so as to grow a
thicker film
that is suitable for coloring. In accordance with the invention, the anodizing
step is
typically performed between about 8 to 24 minutes.
5 [0045] In accordance with a further embodiment of the invention, the alumina
film
produced on the surface of the aluminum article needs to have a certain degree
of
softness so that this anodic oxide coating on the aluminum article maintains
continuity
and does not crack or craze when the subsequently applied electrocoat is cured
at
elevated temperatures. The softness of the alumina film is affected by the
temperature
10 of the anodizing bath, the voltage used during the anodizing step and the
concentration of the electrolyte. During conventional sulfuric acid anodizing,
the
article to be anodized is immersed in a solution at a temperature of about 18-
20°C
with an electrolyte concentration of about 15-18% by weight and anodized at a
voltage of about 18 V DC. In accordance with the present invention, it was
found that
the alumina film formed during the anodizing step has a sufficient softness
when the
anodizing step is performed between about 20-30°C at a voltage of 10-
15V with an
electrolyte concentration of 10-15% by volume. In other words, the anodizing
step is
performed at a higher temperature and a lower voltage in comparison to
conventional
anodizing. This produces an anodic oxide film having a greater density with
more
cell pores per area. Generally, the use of low acid concentrations and
temperature
favor a less porous, harder coating with the opposite favoring a more porous,
softer
coating. Sulfuric acid anodizing provides good corrosion protection and
resistance to
wear. In addition, it provides the possibility to color the anodized aluminum
with a
wide variety of colorants.
[0046] If desired, a commercial liquid additive to prevent burn marks at a
contact
point is added to the electrolyte bath at a concentration of 1-1.5% by volume.
Such
contact points occur for example where the racks contact the parts. This
allows for an
anodization at a higher temperature and improves a uniformity of the produced
film
thickness. The anodized part/electrolyte is much more forgiven for temperature
stratification.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
11
[0047] Hard coat anodizing on the other hand usually produces a very thick and
hard
anodic coating. This is accomplished with a bath similar to the sulfuric
anodizing
process but with the solution temperature reduced to about 0°C to slow
the dissolution
rate. A higher voltage of about 50 V DC is applied to enable the coating to
continue
to build after the insulation value of the coating starts slowing down the
coating
formation. The lower solution temperature and the higher voltage produce a
dense
crystal with small pore size. Such coatings, for example, would not have the
degree
of softness that is needed to ensure a continuity of the anodic oxide coating
during a
thermal curing of the subsequently applied electrocoat.
[0048] Soft, anodizing is the most effective type of anodizing with respect to
ornamental applications because it is available for the various color
treatments
together with corrosion protection. However, soft anodizing is often not a
desirable
type of anodizing due to the fact that it scratches easily when compared to
conventional or hard anodizing.
[0049]. Thus, in accordance with the invention the anodized layer should have
a
degree of softness/flexibility so as to maintain a continuity during a thermal
curing of
a subsequently applied electrocoat. This means that the anodic aluminum oxide
coating does not fracture during the thermal curing step of an electrocoat
applied over
top of the anodic oxide coating.
[0050] Turning now to Fig. 3, a flow chart is presented showing the steps for
a clear
anodizing process. The aluminum article is anodized 200 and then rinsed three
times
with water 202, 204, 206. After the actual anodizing and rinsing steps are
performed,
the micro pores in the freshly anodized alumina coating are sealed or closed
to
provide a smooth, corrosion resistant surface. In accordance with the
invention, a
Nickel Pre-Seal 208 is performed first, followed by 1a nickel rinse 210. Then,
a hot
water seal 212 is performed, followed by rinsing steps 214, 216, and 218. The
sealing
steps convert the amorphous aluminum oxide to a more stable crystalline
hydrate
form to close off the pores in the anodized coating on the surface of the
aluminum
article and hence sealing it.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
12
[0051] Fig. 4 shows a flow chart presenting the steps for a color anodizing
process.
Again, the aluminum article is first anodized 300 followed by rinsing steps
302, 304,
and 306. However, the aluminum article undergoes a coloring process before the
freshly anodized pores in the alumina coating are sealed. In accordance with
the
invention, an electrolytic coloring step 308 is performed by immersing the
aluminum
article after the anodizing step in a bath containing an inorganic metal salt.
A current
is applied to deposit the metal salt in the pores of the freshly anodized
alumina
coating. The resulting color depends on the metal used. Commonly used metal
salts
include cobalt sulfate, tin sulfate, nickel salts, and copper salts. The
coloring step 308
takes advantage of the fact that the freshly anodized alumina coating is
porous and
hence capable of absorbing the colorants. In accordance with the invention,
the
deposited metal salt is conductive. This ensures a conductivity of the
anodized
coating which facilitates the further application of an electrocoat. The
colored
aluminum article is then rinsed in steps 310 and 312. Subsequently, the pores
of the
freshly anodized and colored alumina coating are sealed, as explained
heretofore in
conjunction with Fig. 3, by a nickel pre-seal 314, followed by a nickel rinse
316,
followed by a hot water seal 318, followed by rinsing steps 320, 322, and 324.
The
sealing steps also seal the colorant in the coating thus preventing a loss of
the colorant
and/or an absorption of unwanted stains later on. Further, as explained
heretofore, a
sealed coating also has a higher corrosion resistance.
[0052] Having regard to Fig. 5, a flow chart representing the electrocoating
steps is
presented. At first, the previously anodized aluminum article undergoes an
electrocoating step 400 wherein paint particles are deposited on the anodized
aluminum article to form an even and continuous film over the surface of the
anodized aluminum article. In accordance with the present invention a water-
soluble
thermosetting clear cationic acrylic electrocoating composition is used
containing
about 80-90% deionized water and about 10-20% paint solids. By the term
"clear" is
meant that the electrocoating is free of noticeable haze and yellowing
following
thermal treatment. Furthermore, the electrocoating material includes a UV-
stabilizer
to provide protection from weathering. The function of the LTV light
stabilizer is to
protect the long-term degradation from all forms of wavelength of light.
Today,
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
13
stabilizer packages utilizing a combination of UV absorbers and hindered amine
light
stabilizers (HALS) are state of the art for the protection of coatings. UV
absorbers
(UVA) filter out harmful I1V light and HALS act as radical scavengers. The
four
most important UVA classes are hydroxy-benzophenones, oxanilides,
hydroxyphenyl-
benzotriazoles (BTZ) and hydroxyphenyl-s-triazines (HPT). HALS are based on a
tetra-methyl-piperidine structure. After the electrodeposition of the
electrocoat on the
anodized aluminum article is finished, two ultrafiltrate rinsing steps 402 and
404 are
performed to remove excess paint solids and provide a smoother finish
appearance.
The use of ultrafilters produces a permeate for rinsing and allows for a
recovery of the
excess paint solids. The rinsing steps are followed by a curing step 406 in an
oven to
cross-link and cure the electrocoat film over the anodized aluminum article.
In doing
so, the electrocoat film is baked at 325-350°F (163-177°C) for
20 to 50 minutes.
Then, the electrocoated anodized aluminum article is allowed to cool and
transferred
408 to a customer or assembly line.
[0053] In accordance with yet a further embodiment of the invention, the
aluminum
article is faintly colored using a blue or black dye. Fig. 6 shows a flow
chart of the
steps for such an alternative clear anodizing process wherein the anodized
aluminum
article is faintly colored. After an anodizing step 600 and rinsing steps 602,
the
freshly anodized aluminum article is immersed in a solution containing the
dissolved
dye to perform a dye coloring step 604. The porous alumina coating absorbs the
dye.
The intensity of the color is dependent on the dye concentration, immersion
time, and
temperature. In accordance with this embodiment of the invention, the dye
concentration is relatively small yielding a blue undertone so as to create a
chrome-
like appearance of the anodized aluminum article. Following the dye coloring
step
604, the aluminum article is rinsed 606 and the pores are sealed by means of a
nickel
pre-seal 608 and nickel rinse 610 followed by a hot water seal 612 and rinsing
steps
614 as described heretofore.
[0054] The following examples are provided to further illustrate the process
of the
present invention, but these are not to be regarded as limiting.
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
14
Example I
[0055] An aluminum article was subjected to pretreatment steps in which it was
degreased and cleaned by dipping it for 12 minutes in a commercially available
alkali
cleaner (pH=9) heated to 50~5°C; rinsed with water for 6 minutes at
ambient; cleaned
by dipping it for 3 minutes into a commercially available acid cleaner (based
on
phosphoric acid) heated to 45~5°C; rinsed with water for 3 minutes at
ambient; bright
dipped by immersing the aluminum article for 3.5 minutes in a commercially
available bright dip solution (based on phosphoric acid) heated to
99~3°C to polish
the aluminum article; drag out rinsed for 3 minutes with water heated to
30~5°C;
rinsed with water for 3 minutes at ambient; deoxidized by dipping the article
for 3
minutes into a commercially available deoxidizes solution (acid) at ambient to
remove
leftovers from the bright dip step; rinsed with water for 3 minutes at
ambient; and
spray rinsed with water for 3 minutes at ambient. If desired, the water used
in the
various rinsing steps is distilled or deionized water. The pretreated aluminum
article
was then subjected to clear anodization using a 12-13 volume % sulfuric acid
electrolyte operated with a DC 13V source at a current density of 15-17
amps/ft.2 at a
bath temperature of 23°C. However, the current density is dependent on
the surface
are of the aluminum article to be coated and as the surface area increases so
does the
current density (amperage). The aluminum article was thus anodized for 9
minutes.
The anodized layer has a thickness of about 0.3-0.4 mils or 7.5-10 microns.
[0056] The described conditions provide a freshly anodized alumina coating on
the
surface of the article that has a sufficient degree. of softness so that the
thermal curing
of a subsequently applied electrocoat over top of the alumina coating does not
craze
or fracture the alumina coating, maintaining a continuous layer. Thereafter,
the
aluminum article was rinsed three times with water for 6 minutes at ambient.
The
aluminum article was then subjected to a nickel pre-seal for a period of 3
minutes
using a nickel acetate solution and then rinsed with deionized water. The
nickel pre-
seal acts as a catalyst for the subsequently applied hot water seal by
subjecting the
aluminum article for 6 minutes to boiling deionized water. Thereafter, the
aluminum
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
article was washed three times for a period of 6 minutes by means of reverse
osmosis
rinse.
[0057] The aluminum article was then placed in an electrocoat tank containing
a
thermosetting clear cationic acrylic electrocoat composition and subjected to
5 electrodeposition for 3 minutes at 50-125 V at a temperature of
26~2°C and a pH of
5.0~0.2. The conductivity was 500-650 microSiemens for a total dissolved
solids
contents of 10-15%. The employed electrocoating material is Powercron~ 935C,
an
electrocoating composition commercially available from PPG Industries, Inc.
Typically, the electrocoating composition includes approximately 80-90%
deionized
10 water, 0-5% solvents, 1-10% pigments, 10-15% resins. The electrocoating
composition contains an acrylic polyurethane modified with an ultraviolet (UV)
stabilizer to provide UV/weathering resistance in addition to corrosion
resistance.
[0058] After electrocoating, the aluminum article was subjected to two
ultrafiltrate
rinse cycles for a period of 3 minutes per cycle. Ultrafiltration produces a
permeate
15 for rinsing and allows for a recycling of the excess resin. The aluminum
article was
then baked in an oven for 48 minutes at 350 F (177°C) to crosslink and
cure the
acrylic electrocoat. Usually, 20 to 50 minutes are required for the thermal
curing of
the electrocoat with a minimum of 20 minutes. Thereafter, the anodized and
electrocoated aluminum article is allowed to cool and can be transferred to a
customer. The anodized layer maintains a continuous surface without cracks
being
formed or crazing occurring even after the electrocoat is baked at the curing
temperatures of the thermosetting resin.
[0059] The present invention provides excellent coatings on the protected
aluminum
surfaces having good adhesion and wear resistance as well as strong UV light
and
corrosion resistance against alkali solutions, acid solutions, saline
solutions, and the
like.
Example II
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
16
[0060] The aluminum article was cleaned and pretreated as described in example
I.
The pretreated aluminum article was then subjected to color anodization using
a 12-13
volume % sulfuric acid electrolyte operated with a DC 13V source at a current
density
of 15-17 amps/ft.2 at a bath temperature of 23°C. However, in
accordance with an
embodiment of the invention, the aluminum article was color anodized, i.e. the
aluminum article was anodized for a longer period of time as described in
example I,
viz. for a period of 12 minutes. The longer anodization time provides a
thicker
alumina coating with deeper pores on the surface of the aluminum article for
the
following coloring step. Thereafter, the alumina article was washed by rinsing
it three
times with water for 6 minutes at ambient followed by electrolytic coloring
using
cobalt sulfate at a concentration of 110-120 g/1. The cobalt coloring was
performed
for 20~2 minutes at a voltage of 16~2 V AC and at a temperature of
22~2°C. During
the cobalt coloring step, cobalt is electrolytically deposited into the pores
of the
freshly anodized coating yielding a black appearance of the aluminum article.
The
thus anodized and black colored aluminum article was washed twice by rinsing
with
water for 6 minutes at ambient. Thereafter, the pores of the freshly anodized
coating
on the aluminum article were sealed (nickel pre-seal and a hot water seal) and
electrocoated as described in example I.
Example III
[0061] The aluminum article was anodized for 24 minutes during the clear and
color
anodization steps.
Example IV
[0062] The aluminum article was treated as described in example I. Prior to
the
electrocoating step, however, the anodized aluminum article was colored for 2-
3
minutes at a bath temperature of about 40°C using a small amount of a
black metal
complex of an azo dye, and then rinsed four times with deionized water for
about 6
minutes followed by the sealing steps as described in example I. The
concentration of
the dye was 0.068 g/1 ~ 0.002 g/1. Using such a small amount of colorant
yields an
CA 02536765 2006-O1-30
WO 2005/014894 PCT/CA2003/001176
17
anodized aluminum article of clear appearance having a blue undertone with a
generally clear silvery look, thus creating a chrome-like appearance.
[0063] The above described embodiments of the invention are intended to be
examples of the present invention and numerous modifications, variations, and
adaptations may be made to the particular embodiments of the invention without
departing from the scope of the invention, which is defined in the claims.