Note: Descriptions are shown in the official language in which they were submitted.
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
IMPROVED SILVER PLATING METHOD AND ARTICLES MADE THEREFROM
FIELD OF THE INVENTION
[0001] The present invention relates to an improved, electroless silver
plating method
particularly suitable for the production of articles having antimicrobial and
anti-static
properties.
BACKGROUND OF THE INVENTION
[0002] Metallization of organic substrates (e.g., polymeric materials) with
silver and
other noble metals is well known in the art. One such technique is described
in U.S. Patent
No. 3,877,965 to Broadbent et al., which describes metallizing nylon
substrates with silver
and is incorporated herein by reference. Articles metallized with silver have
found a wide
variety of uses due to the inherent antimicrobial and anti-static properties
of silver. For
example, silver plated nylon fibers are commonly woven into textile materials
which in turn
are used for consumer products (e.g., socks, wound dressings) and for
electromagnetic
interference (EMI) shielding applications for electronic equipment (e.g.,
cellular telephones,
computers).
[0003] However, current processes for metallizing substrates with silver do
have
certain disadvantages. For example, the process described in Patent No:
3,877,965 has been
found to exhibit several disadvantages. First, with most metallization
processes, the pre-
metallization steps require the tin salt (e.g., stannous or stannic chloride)
to be dissolved with
the aid of a water-soluble alcohol (e.g., a Cl-C4 alcohol). The use of an
alcohol results in
significant evaporation problems.
[0004] A more significant problem is the use of a surfactant (e.g., sodium
lauryl
sulfate) during the metallization process. For example, the surfactant can
lead to gelling of
-1-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
the plating bath if the bath temperature is too low. Likewise, the surfactant
can cause
significant foaming in the plating bath, which is difficult to remove after
metallization is
completed. Typically, foam generated during the metallization process ends up
on the
surface of the fibers. Once on the fiber surface, the foam becomes difficult
to rinse off
properly. This in turn potentially results in inhibiting silver ion release
and also present
adhesion issues due to the surface cracking when exposed to high temperatures.
Cracking
occurs as the contaminants (e.g., entrained air) are forced out under pressure
from beneath the
surface of the silver layer.
[0005] In addition, the use of a surfactant results in the color of the
deposited silver
being a very dark gray/silver (and sometimes even brown). This alternative
color of the
deposited silver lowers the aesthetic value of the product since consumers
normally expect
metallized silver to exhibit a bright metallic color.
[0006] However, one of the most significant problems associated with the use
of a
surfactant is the environmental impact and the associated costs of removing
the surfactant
from the waste effluent. Local sewer authorities, the Environmental Protection
Agency
(EPA) and other similar organizations now mandate very low discharge levels of
"Methylene
Blue Activated Substance" (i.e., MBAS). Surfactants such as sodium lauryl
sulfate fall
within the category of MBAS. In order to reduce MBAS to acceptable limits
(typically 5
parts per million (ppm) or lower), significant effort and expense are required
on waste
treatment which in turn increase productions costs and time. Typically,
removal of MBAS
requires the use activated charcoal chemistries along with high-grade
adsorbent (activated
charcoal) filters, which must be changed frequently due to clogging by
particulate. Likewise,
the amount of silver recovered from the waste is problematic because the
recovered silver is
in a low concentration as a percentage of the amount of sludge (e.g., axound
about 1 percent
by weight).
-2-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
[0007] In view of the disadvantages associated with surfactants, other
metallization
processes have been developed that use non-surfactant baths. Typically, these
processes
employ disodium ethylenediaminetetraacetic acid (EDTA) as a ligand instead of
a surfactant.
Representative patents relating to the use of disodium EDTA in silver plating
are U.S. Patent
Nos. 5,318,621 and 5,322,553, both assigned Applied Electroless Concepts.
Another
example is U.S. Patent No. 5,158,604 assigned to Monsanto Company. However,
this
technology is not without problems since high amounts of caustic soda must be
used to
dissolve and adjust pH of the plating bath.
SUMMARY OF THE INVENTION
[0008] The present invention advantageously provides an improved method for
plating am organic substrate with silver that avoids many of the disadvantages
associated with
prior silver plating methods. The method of the invention entails at least
three (3) steps
followed in sequence: (a) scouring; (b) pre-metallization; and (c) plating.
Organic substrates
to be plated can be in the form of fibers, a textile woven from fibers, or a
polymeric foam
(e.g., an open cell foam). In accordance with invention, the organic substrate
is first scoured
to prepare the surface for pre-metallization. Preferably, an aqueous cleaning
solution is used.
[0009] Once the organic substrate has been sufficiently cleaned, the scoured,
organic
substrate is contacted with an aqueous, pre-metallization solution including a
tin salt and an
inorganic acid. In one embodiment, pre-metallization solution omits a water-
soluble or
water-miscible solvent. In another embodiment, the pre-metallization solution
omits a
surfactant. Tin salts to be used include stannous chloride, stannic chloride,
and mixtures
thereof. Inorganic acids to be used include hydrochloric acid, sulfuric acid,
and mixtures
thereof.
-3-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
[00010] The pre-metallized, organic substrate is thereafter plated with
silver, which
comprisesOI) contacting the pre-metallized, organic substrate with an aqueous
Na4EDTA
solution;(ii) subsequently contacting the pre-metallized, organic substrate
with an additional
aqueous, silver salt solution to effect deposition of a silver oxide on the
organic substrate,
wherein the silver salt solution further includes a complexing agent; and
(iii) contacting the
organic substrate having the deposited silver oxide with a reducing agent
thereby effecting
formation of metallic silver on the organic substrate. Particularly preferred
silver salts and
complexing agents are silver nitrate and aqueous ammonia, respectively. A
preferred class of
reducing agents is reducing agents including an aldehyde functional group.
Representative
examples of reducing agents include formaldehyde, rochelle salts (sodium
potassium
tartrate), hydrazine, dextrose, triethanol amine, glyoxal, inverted sugar,
glucose, sodium
borohydride, dimethyl amineborane, hydrazine borane and mixtures thereof. In
another
embodiment, all of the solutions in the plating step preferably omit a
surfactant.
[00011] The present invention also provides articles prepared in accordance
with the
method of the invention. In one embodiment, the organic substrate further
includes at least
one layer of a non-noble metal disposed thereon, and is preferably disposed on
the plated
metallic silver layer. One particularly preferred non-noble metal is copper.
In accordance
with the invention, the metallic silver layer is least 5 percent by weight of
the article, with at
least 10 percent by weight being more preferred.
(00012] Advantageously, the method of the invention allows the use of
surfactants to
be omitted while increasing the recovery of silver from waste products. Thus,
environmental
concerns can be alleviated through the use of the invention as compared to
prior processes.
-4-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
BRIEF DESCRIPTION OF THE DRAWING
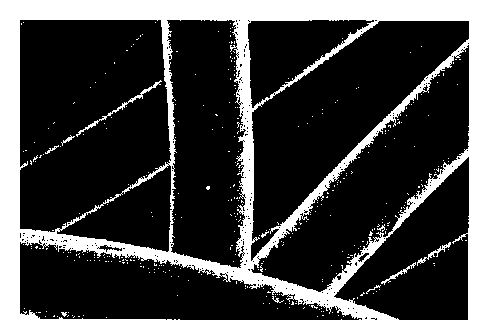
[00013] FIG. 1 is an electron micrograph at 960x magnification of nylon fibers
plated
with the silver using the method of the invention.
[00014] FIG. 2 is an electron micrograph at SOOOx magnification of nylon
fibers
plated with the silver using a prior art process.
DETAILED DESCRIPTION OF THE INVENTION
[00015] The present invention provides an improved method for plating an
organic
substrate with metallic silver while avoiding many of the disadvantages
associated with the
prior art. The method of the invention entails first scouring the organic
substrate to prepare
the surface for pre-metallization. Once the organic substrate has been
sufficiently scoured,
the organic substrate is contacted with an aqueous, pre-metallization solution
including a tin
salt and an inorganic acid. Plating is thereafter accomplished by contacting
the pre-
metallized, organic substrate with an aqueous Na~EDTA solution that in turn is
followed by
contacting the pre-metallized, organic substrate with an aqueous, silver salt
solution to effect
deposition of a silver oxide on the organic substrate. The silver salt
solution further includes
a known complexing agent. The deposited silver oxide is converted (i.e.,
reduced ) to
metallic silver by contacting the organic substrate with a reducing agent
thereby effecting
formation of metallic silver.
[00016] In accordance with the invention, organic substrates to be metallized
with
silver include any organic material capable of receiving a deposited metallic
layer. The
organic material can be synthetic or natural with synthetic (e.g., polymeric)
materials being
preferred. Examples of synthetic polymeric materials to be used include, but
are not limited
to, nylon, polyester, acrylic, rayon, and polyurethane. Examples of natural
materials include,
-5-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
but are not limited to, cellulose, and silk. The organic materials can be in
any physical form
capable of receiving the deposited metallic layer. For example, the organic
material can be in
the form of filaments, fabrics, staple, chopped fibers, micronized fiber,
foams, particulates
and filler materials. Preferably, the organic material is in the form of a
fiber or filament, or a
textile matrix made therefrom. If the organic material is in the form of a
foam, an open-cell
foam (i.e., has a three-dimensional interconnected network of cells) is
preferred to allow
metallization throughout.
[00017] The organic substrate is first prepared for pre-metallization by
scouring to
remove debris and/or to remove any coatings or film on the material that may
interfere with
metallization. Scouring is a technique well known in the art and thus does not
require much
discussion. Typically, the material is washed with an aqueous cleaning
solution that may or
may not contain a surfactant (e.g., a nonionic surfactant). In accordance with
the invention,
reference to "aqueous" means at least a majority of the medium is water with
the remaining
portion being a water-soluble or water-miscible organic solvent. The organic
material can
also be abraded using a scouring brush or equivalent device. In a preferred
embodiment,
scouring is accomplished with a high-speed water spray, which facilitates in-
line processing
and avoids the necessity of a scouring brush.
[00018] Once the organic substrate has been sufficiently scoured, the material
is
subjected to pre-metallization with an aqueous solution of a tin salt and an
inorganic acid. As
will be apparent to one skilled in the art, such a solution is often referred
to as a "sensitizing"
solution. However, unlike prior art "sensitizing" solutions, the pre-
metallization solution
preferably omits a surfactant and/or a water-soluble or water-miscible organic
solvent such as
a C1-C4 alcohol. Preferably, the tin salt is a halide such as stannous
chloride, stannic
chloride, or mixtures thereof. Examples of inorganic acids include, but are
not limited to,
-6-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
hydrochloric acid, sulfuric acid, and mixtures thereof.- In a more preferred
embodiment, the
tin salt is stannous chloride and the inorganic acid is hydrochloric acid.
Ranges of the two
components are set forth in Table 1:
Table 1
Tin Salt Inorganic Acid
( ams/liter) (% by Volume)
Preferred 1-30 1-20
More Preferred 2-25 3-18
O timal 4-20 6-15
[00019] Once the organic substrate has been pre-metallized, the organic
substrate is
preferably washed to remove excess salt and acid from the organic substrate
that can interfere
with subsequent metallization. For example, the organic substrate can be
washed with a
counter flow rinse with controlled water flow. This enables the removal of any
excess salts
and acids from the substrate material while leaving optimal amount of
activated sites on the
surface of the substrate. Preferred levels of water flow to wash the substrate
range from
about 25 to about 55 gallons per minute (gpm), with 30 to 50 gpm being more
preferred, and
35 to 45 gpm being more preferred.
[00020] In accordance with the present invention, metallization is
accomplished in
three (3) substeps. An aqueous tetrasodium ethylenediaminetetraacetic acid
(Na4EDTA) is
prepared into which the pre-metallized organic substrate is contacted
preferably by
immersing the substrate in the aqueous solution. The aqueous solution is
preferably prepared
using de-ionized (DI) water to avoid possible contamination. The DI water
should have a
resistance of about 0.4 to about 20 megaohms, with 0.8 to 10 megaohms being
preferred, and
3 to 7 megaohms being more preferred. The concentration of the aqueous Na4EDTA
solution
should range from about 5 to about 30 percent by weight (wt. %), with 10 to 25
wt. % being
preferred, and 10 to 20 wt. % being even more preferred. Preferably, the
Na4EDTA solution
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
omits a surfactant as is typically found in conventional silver plating
processes. Likewise, the
Na~EDTA solution also preferably omits caustic soda as typically found in
Na2EDTA
solutions. Advantageously, the use of Na~EDTA facilitates the deposition of
metallic silver
with a tighter grain structure, which in turn leads to a relatively smoother
surface as
evidenced by examination of silver-plated nylon fiber by electron microscopy.
Likewise,
Na~EDTA allows a surfactant to be omitted thus alleviating environmental
concerns
regarding levels of surfactant in the waste effluent.
[00021] An aqueous silver salt solution is also prepared for subsequent
contacting of
the organic substrate. Preferably, the organic substrate is contacted with the
silver salt
solution by adding the silver salt solution directly to the bath containing
the organic substrate
and the aqueous Na~EDTA solution. Thus, the organic substrate is
contemporaneously
immersed in both solutions, which is referred to,as the "metallization bath."
Alternatively,
the organic substrate can be removed from the Na~EDTA solution and
subsequently
immersed in the silver salt solution. One particularly preferred silver salt
is silver nitrate (i.e.,
AgN03). The silver salt solution additionally includes a complexing agent as
known in the
art, which form a complex ih situ with the dissolved silver salt. One
particularly preferred
complexing agent is aqueous or aqua ammonia (i.e., NH40H) which is commonly
used as a
complexing agent for silver nitrate. As with the Na4EDTA solution, the silver
salt solution
preferably omits a surfactant.
[00022] The silver salt solution is preferably prepared by first dissolving
the silver salt
in water. Once the silver salt has been dissolved, the complexing agent is
added to the
solution. A precipitate of a silver oxide can form and is re-dissolved through
the addition of
excess complexing agent. The addition of excess complexing agent is believed
to form a
complex of the silver salt and the complexing agent. For example, when silver
nitrate and
-g_
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
aqua ammonia are used, a precipitate of silver oxide forms in situ which is re-
dissolved upon
the addition of excess aqua ammonia to provide a metallization bath having a
light amber
color. Preferred initial weightlvolume ratios of silver salt (i.e., AgX) to
water (H20) and of
percent by volume of complexing agent are set forth in Table 2. Preferred
molar ratios of
silver salt to complexing agent in the final metallization bath (i.e., upon re-
dissolution of the
silver precipitate) are set forth in Table 3.
Table 2
AgX:H20 Complexing Agent
(wei t:volume ercent by volume
Preferred 0.25:2 to 1.75:2 17 to 38
More Preferred 0.5:2 to 1.5:2 20 to 35
Optimal 0.75:2 to 1.25:2 25 to 31
Table 3
Molar Ratio of Complexing A ent:AgX
Preferred 2.5:1 to 5.5:1
More Preferred 3:1 to 5:1
O timal 3.5:1 to 4.5:1
[00023] In accordance with the invention, immersion of the organic substrate
in the
metallization bath results in the deposition of silver oxide on the substrate
surface. As will be
apparent to one skilled in the art, deposition can be confirmed by a visual
inspection of the
substrate undergoing a change in color due to the deposited silver oxide. For
example, in the
case of AgN03:NH3 one will typically observe the substrate to develop a shade
of brown on
the surface which correlating to a silver oxide layer. Preferably, the organic
substrate is
immersed in the metallization bath prior to the addition of the reducing agent
for about 30
seconds, with 20 seconds being more preferred. The temperature of the
metallization bath is
not critical and can range from about 15 to about 45°C, with 20 to
30°C being more
preferred. These parameters can be easily determined by one skilled in the
art.
(00024] The organic substrate with the silver oxide thereon is subsequently
contacted
with a reducing agent to convert the silver oxide to metallic silver.
Preferably, contacting is
-9-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
accomplished by adding the reducing agent directly to the metallization bath.
Alternatively,
the organic substrate is removed from the metallization bath and is separately
contacted (e.g.,
immersed) with an aqueous solution of the reducing agent. Reducing agents to
be used in
accordance with the invention are well known in the art. Examples of reducing
agents to be
used include, but are not limited to, formaldehyde, rochelle salts (sodium
potassium tartrate),
hydrazine, dextrose, triethanol amine, glyoxal, inverted sugar, glucose,
sodium borohydride,
dimethyl amineborane, hydrazine borane. More preferred are reducing agents
containing an
aldehyde functional group such as formaldehyde. In the case where AgN03:NH3 is
used, the
addition of the reducing agent (e.g., formaldehyde) results in the silver
oxide layer on the
substrate changing color to a bright gold or gray-gold color as the silver
oxide is converted to
metallic silver. Preferably, the amount of reducing agent used ranges from
about 5 to about
40 percent by weight of substrate, with 6 to 25 percent by weight being more
preferred, and 8
to 22 percent by weight being even more preferred.
[00025] Once sufficient metallic silver has been converted, the organic
substrate is
removed from the metallization bath and washed. Preferably, the silver-plated
substrate is
immersed in hot water. The silver-plated substrate is then preferably immersed
in a weak
solution of sodium hydroxide, which brightens the silver plating to a light
gold color or a
light gray color. This indicates that a pure layer of silver deposited on the
substrate. The
article can be subjected to multiple rinse cycles to ensure the cleanliness of
product.
[00026] As will be apparent to one skilled in the art, the amount of metallic
silver
deposited on the organic substrate is a function of immersion time. In
accordance with the
invention, the time for complete deposition of the metallic silver layer will
be less than 4
hours. However, time periods for immersing the substrate in the various
solutions can easily
be altered depending on the amount of deposited silver desired. The amount of
silver
-10-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
deposited on the organic substrate can range from 0.1% to 15% by weight,
depending on the
specific characteristics desired for the final product. Preferably, the
deposited silver layer is
at least 5 percent by weight, with at least 10 percent by weight being more
preferred. The
actual amount of silver deposited on the substrate is easily calculated by a
simple titration
such as the Vollard process.
[00027] Unlike the prior art process of U.S. Patent No: 3,877,965, the
remaining
metallization bath is treated with ease. The pH of the metallization bath is
raised to
approximately 13 which results in the dissolved silver being. converted into a
colloidal form.
The pH is then lowered to around 2 typically with the help of an inorganic
acid (e.g., sulfuric
acid). The colloidal silver precipitates and settles down at the bottom of the
container after
several hours. The purity of the silver precipitated has been found to be up
to 50 percent by
weight.
[00028] As will be apparent to one skilled in the art, the adhesion of the
plated silver is
easily ascertained. One simple test for adhesion of the silver to the
substrate requires placing
a sample into an oven at 200°C for about 5 minutes and then boiling the
same sample for 1
hour in water. The resistance of the sample before and after heating and
boiling are
compared. A variation in resistance of no more than about 20 percent indicates
excellent
adhesion. In a more preferred embodiment, the variation of resistance is no
more than 10
percent.
[00029] In another embodiment of the invention, the silver-plated substrate is
additionally plated with a non-noble metal such as copper as described in U.S.
Patent No.
3,877,965. Copper is auto-catalytic on silver and thus can reduce itself
easily for form a
copper layer. Using such process up to 30% by weight of copper is deposited on
to the
-11-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
silver-plated substrate. Commercial plating solutions are available from
Atotech USA,
Enthone OMI, and MacDermid Corporation.
[00030] The following non-limiting examples illustrate the use of the method
of the
invention to plate organic substrates with metallic silver layers.
EXAMPLES
Example 1
[00031] A 30/10 knit sample of nylon weighing 25 grams was scoured to remove
any
contaminants. The knit sample was wrapped into a skein and scoured in counter
flow de-
ionized water. The sample was pre-metallized with a solution containing 1 % by
volume
HCL and 10 grams of anhydrous tin chloride (SnCl2) for about 2 minutes. A
silver salt
solution was prepared by dissolving 0.04 grams of silver nitrate (0.1 % silver
by weight
target) in de-ionized water. The silver salt was then complexed with 0.045 mL
of 27 % by
volume aqua ammonia. A tetrasodium EDTA solution was prepared by dissolving
0.002
grams Na4EDTA in 1 liter of de-ionized water. The skein was placed in the
reactor
containing the Na4EDTA solution and made to revolve. The silver salt solution
(i.e.,
complexed silver nitrate and ammonia) was added to the reactor slowly until
all contents
were emptied. This was followed by 0.016 mL formaldehyde. After three hours
the sample
was removed and subjected to hot water rinse. A 0.1 % by volume NaOH solution
(1 liter)
was prepared with a temperature of 70°C. The metallized skein was then
dipped into the
solution and rinsed thoroughly. The sample was subjected to Dow Corning
Corporate Test
Method 0923: organism - Staphylocococcus aureaus ATCC 7538; sample size - 0.75
grams;
results - percent reduction in colony >99.9%.
-12-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
Example 2
[00032] As in example l, a 30/10 knit sample of nylon weighing 25 grams was
scoured
to remove any contaminants. The knit sample was wrapped into a skein and
scoured in
counter flow de-ionized water. The sample was pre-metallized with a solution
containing 1
by volume HCL and 10 grams of anhydrous tin chloride (SnCla) for about 2
minutes. A
silver salt solution was prepared by dissolving 1.95 grams of silver nitrate
(5 % silver by
weight target) in de-ionized water. The silver salt was then complexed with
2.25 ml of 27
by volume aqua ammonia. A tetrasodium EDTA solution was prepared by dissolving
0.1
grams of NadEDTA in 1 liter of de-ionized water. Skein was placed in the
reactor containing
the Na4EDTA solution and made to revolve. The silver salt solution (i.e.,
complexed silver
nitrate and ammonia) was added to the reactor followed by 0.8 mL of
formaldehyde. After
three hours the sample was removed and subjected to hot water rinse. The
metallized skein
was rinsed in a NaOH solution as in Example 1. The sample was subjected to Dow
Corning
Corporate Test Method 0923: organism - Staphylocococcus aureaus ATCC ?538;
sample
size - 0.75 grams; results - percent reduction in colony >99.9%.
Example 3
[00033] A 25 grams sample of ripstop fabric was processed following the
procedure of
examples 1 and 2. The silver-plated sample was then subjected to Dow Corning
Corporate
Test Method 0923: organism - Staphylocococcus aureaus ATCC 7538; sample size -
0.75
grams; results - percent reduction in colony >99.9%.
-13-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
Example 4
[00034] A 25 gram sample of filler material including nano powders (i.e.,
powder
made from nylon and polyethylene) was processed following the procedure of
examples 1
and 2. The silver-plated sample was then subjected to Dow Corning Corporate
Test Method
0923: organism - Staphylocococcus aureaus ATCC 7538; sample size - 0.75 grams;
results -
percent reduction in colony >99.9%.
Example 5
[00035] A 30110 knit sample of nylon weighing 118 grams was scoured to remove
any
contaminants. The knit sample was wrapped into a skein and scoured in counter
flow de-
ionized water. The sample was pre-metallized with a solution containing 10 %
by volume
HCL and 100 grams of anhydrous tin chloride (SnCla) for about 2 minutes. A
silver salt
solution was prepared by dissolving 45 grams of silver nitrate (about 22 %
silver by weight
target) in de-ionized water. The silver salt was then complexed with 52 mL of
27 % by
volume aqua ammonia. A tetrasodium EDTA solution was prepared by dissolving
2.2 grams
Na4EDTA in 6 liters of de-ionized water. The skein was placed in the reactor
containing the
NaqEDTA solution and made to revolve. The silver salt solution (i.e.,
complexed silver
nitrate and ammonia) was added to the reactor slowly until all contents were
emptied. This
was followed by 18 mL of formaldehyde. After three hours the sample was
removed and
subjected to hot water rinse. A 0.1 % by volume NaOH solution (5 liters) was
prepared with
a temperature of 70°C. The metallized skein was then dipped into the
solution and rinsed
thoroughly. The color changed to light almost gold colored silver. The sample
was dried and
then sent for an adhesion check. The results were as follows: as is - 484 Ohms
(SO cm
-14-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
distance) using a Keithley 580 micro-ohmmeter; after heat - 345 Ohms; and
after boil - 365
f
Ohms.
Example 6
[00036] A sample obtained from the silver-plated materials from example 5 was
cut to
make a 1.5 gram sleeve. The sleeve was then placed in a beaker with 5 % by
volume sodium
chloride solution for a 24-hour period. The solution after the 24-hour period
was then tested
for silver ions using a Perkin Eliner Analyst 300. The same test was repeated
over a period
of 7 days. The release of ions was consistent each day at 0.5 ppm illustrating
the sustained
release of silver prepared in accordance with the invention.
Example 7
[00037] A sample obtained from the silver-plated materials from example 5 was
cut to
weigh 0.75 grams and subjected to Dow Corning Corporate Test Method 0923.
Organism
used was Staphylococcus aureus ATCC 6538. The sample reduced organism growth
by over
99.9%.
Example 8
[00038] A 210/34 knit nylon sample weighing 118 grams was cleaned. The sample
was wrapped into a skein and scoured with a counter flow of de-ionized water.
The skein
was pre-metallized in a solution of 10 % by volume HCL and 100 grams of
anhydrous tin
chloride (SnCl2) for 2 minutes. A silver salt solution was prepared by
dissolving 45 grams of
silver nitrate in de-ionized water. The silver salt was then complexed with 52
ml of 27 % by
volume aqua ammonia. A tetrasodium EDTA solution was prepared by dissolving
2.2
grams of Na~EDTA in 6 liters of de-ionized water. Skein was placed in the
reactor
-15-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
containing the Na4EDTA solution and made to revolve. The silver salt complex
was added to
the reactor and followed by 18 mL of formaldehyde. After three hours the
sample was
removed and subjected to hot water rinse. As in the previous examples, a 0.1%
by volume
NaOH solution was prepared and the metallized skein was dipped into the
solution. The
color changed from grey to a light alinost gold colored silver.
[00039] The silver-plated sample was then metallized with commercially
available
copper chemistry from Atotech USA. The metallization process was carried out
following
the instructions suggested by supplier. Completion of the deposition of copper
can be
visually determined when the bath changes color from a deep blue to colorless,
which
indicates a complete reduction of the metal.
[00040] A 10.6 grams sample of the silver-copper material was then cut and
placed in
a beaker filled with 2.1 grams of Rochelle salt (i.e., sodium potassium
tartrate) dissolved with
de-ionized water. A silver salt complex made up of 3.6 grams of silver nitrate
and 4.3 mL of
aqua ammonia was then poured into the sample under constant agitation. The
pink color of
silver-copper changed to a light brown at this time. A few drops of further
diluted aqua
ammonia were added drop wise into the bath with an ink dropper under constant
agitation.
The color of the sample then started to change to a dull white and eventually
a bright white
color. This step took about 35 minutes to complete. At that time sample is
removed from
bath and rinsed thoroughly with de-ionized water for 15 minutes. Silver was
calculated to
provide a 15% by weight gain. The resistance of the material after deposition
of each
metallic layer was also recorded; resistance with Silver - 80 Ohms/50 cm using
a Keithley
580 micro-ohmmeter; resistance with Silver-Copper - 25 Ohms/50 cm; and
resistance with
Silver-Copper-Silver -18 Ohms/50 cm.
-16-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
Example 9
[00041] A sample of the silver-copper-silver material from example 8 was cut
into a
1.5 gram sleeve. The sleeve was then placed in a beaker with 5 % by volume
sodium
chloride solution for a 24-hour period. The solution after a 24-hour period
was then tested for
silver and copper ions using a Perkin Elmer Analyst 300. The same test was
repeated over a
period of 7 days. The release of ions was consistent each day at 2 pprn of
silver and 3 ppm of
copper.
Example 10
[00042] A sample of the silver-copper-silver material from example 8 was cut
to
weight 0.75 grams and subjected to Dow Corning Corporate Test Method 0923.
Organism
used was Staphylococcus aureus ATCC 6538 and the material caused a reduction
of
organism growth by over 99.9%.
Example 11
[00043] A quenched foam sample weighing 11.8 grams was cleaned with non-ionic
surfactant Triton X-100 and rinsed thoroughly. A 83 % by weight sulfuric acid
solution was
prepared and the foam was dipped in the solution for 25 seconds to 45 seconds
to ensure
proper etching of the surface. Immediately the sample was rinsed with copious
amounts of
de-ionized water. The foam was pre-metallized with solution containing 12 % by
volume
HCL and 120 grams of anhydrous tin chloride (SnCla) for 2 minutes. The foam
sample was
then rinsed in counter flow de-ionized water. A tetrasodium EDTA solution was
prepared by
dissolving 0.22 grams of Na4EDTA was dissolved in 2 liters of de-ionized
water. The pre-
metallized foam was placed in reactor containing the Na4EDTA solution and made
to
-17-
CA 02539656 2006-03-20
WO 2004/027113 PCT/US2003/029293
revolve. A silver salt solution was prepared by dissolving 4.5 grams of silver
nitrate in de-
ionized water. The silver salt solution was then complexed with 5.2 mL of 27 %
by volume
aqua ammonia. The silver salt complex was added to the reactor and followed by
18 mL of
formaldehyde. After three hours the sample was removed and subjected to hot
water rinse.
The metallized foam was dipped into a NaOH solution as prepared in the
previous examples.
The color changed to a dull, white silver. Sample was dried and evaluated for
resistance. The
silver-plated foam exhibited a resistance of 0.5 Ohms/50 cm using a Keithley
580 micro-
ohmmeter.
Example 12
[00044] Samples of silver-plated fiber prepared in accordance with the
invention were
compared to silver-plated fibers prepared following the procedure set forth in
U.S. Patent No.
3,877,965. Samples of both samples of fiber were examined with a scanning
electron
microscope. A photomicrograph of the inventive fibers is found in FIG. 1,
while a
photomicrograph of the comparative fibers is found in FIG. 2. As can be
clearly seen from
the photomicrographs, the fibers plated with the inventive method exhibit a
smoother surface
as compared to fibers prepared with the prior art process. This is believed
due to the tighter
grain structure of the silver plating provided by the method of the present
invention.
-18-