Note: Descriptions are shown in the official language in which they were submitted.
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
10
HETEROCYCLIC COMPOUNDS AND
METHODS OF MAKING AND US1NG THEREOF
FIELD OF THE INVENTION
The present invention relates to compounds, pharmaceutical compositions, and
methods of making and use thereof.
2o BACKGROUND OF THE INVENTION
Glycated proteins and advanced glycation end products (AGE) contribute to
cellular
damage, for example, diabetic tissue injury. This can occur by at least by two
major
mechanisms: modulation of cellular functions through interactions with
specific cell surface
receptors, and alteration of the extracellular matrix leading to the formation
of protein cross-
links. Studies suggest that glycated protein and AGE interactions with cells
promote
inflammatory processes and oxidative cellular injury. AGE increases
lipoprotein
oxidisability and atherogenicity. Further, AGE binding to matrix proteins
induces synthesis
of IL-l, TNFa, VCAM-1, Heme oxygenase, insulin like growth factor, IL-6 and
activates
NF-?B. Diseases for which glycated protein and AGE accumulation is a suspected
3o etiological factor include, but are not limited to, vascular complications
of diabetes,
microangiopathies, renal insufficiency, and Alzheimer's disease.
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The exact mechanism by which high plasma glucose causes microvascular damage,
as seen in diabetes, are not completely understood. One potential mechanism by
which
hyperglycemia can be lil~lced to microangiopathies is through the process of
non-enzymatic
glycation of critical proteins. Non-enzymatic glycation of critical proteins
is discussed in
Nonenzymatic glycosylation and the pathogenesis of diabetic complications,
Ann. Intern.
Med., 1984(101)527-537; Advanced glycation end products up-regulate gene
expression
found in diabetic glomerular diseas, Proc. Natl. Acad. Sci. U S A., 1994
(91)9436-40;
Expression of advanced glycation end products and their cellular receptor RAGE
in diabetic
nephropathy and nondiabetic renal disease, J. Am. Soc. Nephrol., 2000 (11)1656-
66; and
l0 Activation of receptor for advanced glycation end products: a mechanism for
chronic
vascular dysfunction in diabetic vasculopathy and atherosclerosis., Circ.
Res., 1999 (84)489-
97).
Non-enzymatic glycation, i.e., the linking of proteins with glucose, leads to
the
formation of glycated proteins. The first step in this glycation pathway
involves the non-
enzymatic condensation of glucose with free amino groups in the protein,
primarily the
epsilon-amino groups of lysine residues, forming the .Amadori adducts. These
early
glycation products can undergo further reactions such as rearrangements,
dehydration, and
condensations to form irreversible advanced glycation end products (AGE).
These are a
highly reactive group of molecules whose interaction with specific receptors
on the cell-
surface that may lead to pathogenic outcomes. Accumulation of glycated
proteins have been
demonstrated in the basement membrane of patients with diabetes and are
thought to be
involved in the development of diabetic nephropathy and retinopathy. See
Immunohistochemical localization of glycated protein in diabetic rat kidney.,
Diabetes Res.
Clin, Pract., 1990(8)215-9; and Role of Amadori-modified nonenzymatically
glycated serum
proteins in the pathogenesis of diabetic nephropathy., J. Arn. Soc. Nephrol.,
1996(7)183-90.
See Inhibitors of AGE formation, such as aminoguanidine, have been shown to
block the
formation of AGE and prevent development of diabetes complications, including
diabetic
retinopathy (Aminoguanidine prevents diabetes- induced arterial wall protein
cross-linking,
Science, 1986(232)1629-1632; Prevention of cardiovascular and renal pathology
of aging by
the advanced glycation inhibitor aminoguanidine., Proc. Natl. Acad. Sci. U S
A.,
1996(93)3902-7; and Potential benefit of inhibitors of advanced glycation end
products in the
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
progression of type II diabetes: a study with aminoguanidine in C57BLI~sJ
diabetic mice.,
Metabolism, 1998(47)1477-80.
One characterized AGE receptor is RAGE, receptor for AGE. See Activation of
receptor for advanced glycation end products: a mechanism for chronic vascular
dysfunction
in diabetic vasculopathy and atherosclerosis., Circ. Res. 1999(84)489-97; and
Roles of the
AGE-RAGE system in vascular injury in diabetes., Ann. NY Acad. Sci. 2000
(902)163-70;
discussion 170-2. Several in vitro and in vivo studies demonstrate that
blocking RAGE
either by antibodies or by adding a soluble form of the receptor inhibits
diabetic vasculopathy
including diabetic atherosclerosis. See Receptor-mediated endothelial cell
dysfunction in
to diabetic vasculopathy. Soluble receptor for advanced glycation end products
blocks
hyperpermeability in diabetic rats., J. Clin. Invest., 1996(97)238-43;
Advanced glycation end
products interacting with their endothelial receptor induce expression of
vascular cell
adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice.
A potential
mechanism for the accelerated vasculopathy of diabetes., J. Clin. Invest.,
1995(96)1395-403;
and Suppression of accelerated diabetic atherosclerosis by the soluble
receptor for advanced
glycation endproducts., Nat. Med. 1998(4)1025-31. Other than AGE, RAGE appears
to
mediate the binding of several other ligands that are involved in normal
physiology as well as
pathology. See Blockade of RAGE-amphoterin signalling suppresses tumour growth
and
metastases., Nature, 2000(405)354-60; RAGE mediates a novel proinflammatory
axis: a
central cell surface receptor for 5100/calgranulin polypeptides., Cell.,
1999(97)889-901; and
Amyloid-beta peptide-receptor for advanced glycation end product interaction
elicits
neuronal expression of macrophage-colony stimulating factor: a proinflammatory
pathway in
Alzheimer disease., Proc. Natl. Acad. Sci., USA., 1997(94)5296-301. Thus,
merely
blocking RAGE might have other unintended consequences. Moreover, since
blocking
RAGE could lead to accumulation of AGE in circulation, the long-team effects
of blocking
RAGE are unknown and may be more harmful than the pathology sought to be
treated.
One useful method to block AGE effects would be to develop inhibitors that
block
AGE induced signaling. See Activation of the receptor for advanced glycation
end products
triggers a p21(ras)-dependent mitogen-activated protein kinase pathway
regulated by oxidant
3o stress., J. Biol. Chem., 1997(272)17810-4; and Cell activation by glycated
proteins.; AGE
receptors, receptor recognition factors and functional classification of
AGEs., Cell. Mol.
Biol.(Noisy-le-grand), 1998(44)1013-23. However, the sequence of these
signaling events
3
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
leading to inflammation is not clear. Accordingly, what is needed are
compounds that can
block AGE-induced activities, particularly AGE-induced inflammation, or more
particularly,
AGE-induced signaling events.
Other chronic conditions for which adequate and effective therapies do not
exist are
treatments of antiproliferative disorders. Smooth muscle cell (SMC)
hyperplasia is an
important factor in the development of atherosclerosis and also is responsible
for the
significant number of failure rates following vascular procedures such as
angioplasty and
coronary artery bypass surgery. See, The comparative pathobiology of
atherosclerosis and
restenosis. Am. J. Cardiol. 86:6H-11H (2000); and Restenosis: a challenge for
pharmacology.
Trends Pharmacol Sci. 21:274-9. In the normal vessel, SMC are quiescent, but
they
proliferate when damage to the endothelium occurs. Naturally occurring growth
modulators,
many of which are derived from the endothelium, tightly control SMC
proliferation in vivo.
Abnormal vascular smooth muscles cell (VSMC) proliferation may contribute to
the
pathogenesis of vascular occlusive lesions, including atherosclerosis, vessel
re-narrowing
after successful angioplasty (restenosis), and graft atherosclerosis after
coronary
transplantation. VSMC is discussed in The comparative pathobiology of
atherosclerosis and
restenosis. Am. J. Cardiol. 86:6H-11H; and Smooth muscle migration in
atherosclerosis and
restenosis. J Clin Invest. 100:587-9. Many humans and animals have limited
lifespans and
lifestyles because of such conditions. Currently there are no known effective
pharmacological treatments available that control these occlusive pathologies,
particularly
restenosis.
Percutaneous coronary artery intervention (PTCA) procedures are the most
common
in-patient hospital procedure in the United States. According to the American
Heart
Association, about one-third of the patients that undergo balloon angioplasty
have restenosis
of the widened segment within approximately six months. It may be necessary to
perform
another angioplasty or coronary artery bypass surgery on restenosed arteries.
A key feature
of restenosis is an injury response that results in activation of an
inflammatory cascade and
remodeling of the cells both inside and outside the carotid artery wall. This
includes
excessive growth of connective tissue and smooth muscle into the lumen of the
artery known
3o as neointimal hyperplasia. Currently there are no effective pharmacological
treatments
available that control the pathogenesis of vascular occlusive lesions, such
as, but not limited
to, arteriosclerosis, atherosclerosis, restenosis, and graft atherosclerosis
after coronary
4
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
transplantation. Identification of effective therapeutics with minimal side
effects will restore
quality of life without requiring additional surgical procedures such as
coronary artery bypass
surgery.
Smooth muscle cell (SMG) hyperplasia is a major event in the development of
atherosclerosis and also may contribute to failure rates following vascular
procedures such as
angioplasty and coronary artery bypass surgery. In the normal vessel, SMC are
quiescent,
but they proliferate when damage to the endothelium occurs. Naturally
occurring growth
modulators, many of which are derived from the endothelium, tightly control
SMC
proliferation in vivo. Accordingly, there is a need for methods and
compositions for the
to alteration of gene expression in arterial wall cells to inhibit thrombosis
and SMC
proliferation. In particular, what is needed are methods and compositions that
inhibit SMC
proliferation and related intimal hyperplasia.
U.S. Patent No. 6,028,088 is directed to specific thiazolidinedione compounds,
which
are described as antiproliferative, anti-inflammatory and antiinfective
agents. According to
the disclosure, these specific compounds are used in the treatment of certain
endocrine
diseases, malignant, and non-malignant proliferative diseases, and
cardiovascular disorders.
Thus, there is a need for treatments of vascular occlusive pathologic
conditions, and
particularly, restenosis. Since occurrence is frequent, the currently
available treatments are
costly and the conditions are refractory to many pharmacological therapies.
The mechanisms
involved in the control of vascular conditions related to SMC function are not
clear and no
conventional preventive therapy against SMC activation is available.
Accordingly, methods
and compositions for treatment and prevention of vascular occlusive conditions
are needed.
In particular, methods and compositions to prevent and treat restenosis
following treatments
of vascular tissues are needed. The present invention is directed to
overcoming these and
other deficiencies in the art.
SUMMARY OF THE INVENTION
The present invention is related to compounds of formula (I), and to methods
and/or
compositions comprising compounds that are effective in modulating
inflammatory
3o responses, such as those resulting from AGE and glycated protein
accumulation. The present
invention also is directed to methods and/or compositions comprising compounds
that are
5
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
effective in modulating smooth muscle cell proliferation and the diseases or
conditions
related thereto.
The present invention provides compounds and compositions that inhibit
inflammatory responses, particularly those resulting from AGE and glycated
protein
accumulation. Further, the present invention provides compounds and
compositions that
inhibit smooth muscle cell proliferation, which may be mediated by pro-
inflammatory
cytokines lilce IL-6, IL-l, TNF-a, MCP-l, or by inducing the expression of
perlecan, a
heparin sulfate proteoglycan (HSPG).
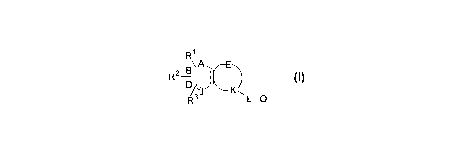
R~
Ra B\ A ' ~~ O
R ;l~ Kw
L-Q
l0 The present invention provides novel compounds of formula (I), their
pharmaceutically acceptable salts, and pharmaceutical compositions containing
one or more
of such compounds, optionally in combination with other active ingredients.
The present invention also provides a process for preparing compounds of the
formula (I) as defined above, their salts, and pharmaceutically acceptable
compositions
thereof.
The present invention also provides novel compounds of formula (II), their
pharmaceutically acceptable salts, and pharmaceutical compositions containing
one or more
of such compounds, optionally in combination with other active ingredients.
Y1
1
B\,A E R4 F~NR
R - ; II
Y-G_Z-Ar-=~~Y2 ( )
R
X
2o The present invention also provides a process for preparing compounds of
the
formula (II) as defined above, their salts, and pharmaceutically acceptable
compositions
thereof.
The present invention also provides novel compounds of formula (III),
including but
not limited to, their pharmaceutically acceptable salts and pharmaceutical
compositions
containing them, or their mixtures, or in combination with other active
ingredients.
6
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
E R4
R~ ~: . ~ ~ o_G_Z cIIn
~3
The present invention also provides a process for preparing compounds of the
formula (III) as defined above, their salts, and pharmaceutically acceptable
compositions
thereof.
The present invention also provides novel compounds of formula (IV), their
pharmaceutically acceptable salts, and pharmaceutical compositions cor~taining
one or more
of such compounds, optionally in combination with other active ingredients.
Y~
F'
D~~ 1I E ~ R4 s N R (I~
vJ _ I 2 I~-G-Z_ArY~
R ~ R5
X
The present invention also provides a process for preparing compounds of the
1o formula (IV) as defined above, their salts, and pharmaceutically acceptable
compositions
thereof.
The present invention provides novel compounds of formula (V), their
pharmaceutically acceptable salts, and pharmaceutical compositions containing
one or more
of such compounds, optionally in combination with other active ingredients.
RBA 1 ~E~R4 X4 s~p? W Ar
R2 B ~ _ _
K ~-CCFi2~ v C X~CH2~~C-O-R
i~
Ra ~' X~ Xs
The present invention also provides a process for preparing compounds of the
formula (V) as defined above, their pharmaceutically acceptable salts, and
their
pharmaceutically acceptable compositions.
According to one aspect of the present invention, a method of using a compound
of
formula (I) comprises treatment and/or prophylaxis of inflammatory conditions,
such as those
mediated by AGE or glycated protein accumulation. Such inflammatory conditions
include
7
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
diabetic vascular complications, including diabetic retinopathy,
mieroangiopathies, renal
insufficiency and Alzheimer's disease.
According to another aspect of the present invention, a method of inhibiting
smooth
muscle cell proliferation comprises administering an effective amount of a
compound
contemplated hereby. The present invention also provides methods for
inhibiting an
inflammatory response, including inflammatory responses in endothelial cells,
comprising
administering an effective amount of a compound contemplated hereby. The
present
invention also provides methods for inliibiting thrombosis comprising
administering an
effective amount of a compound contemplated hereby.
l0 The present invention also provides a method for treating or preventing
organ
transplant vasculopathy in a subject comprising the step of administering a
therapeutically
effective amount of a compound contemplated hereby. The transplanted organ may
include,
but is not limited to, liver, kidney, heart, lung, pancreas, pancreatic
islets, and skin. Such a
method may further comprise the step of administering a therapeutically
effective amount of
an immunosuppressive agent. The immunosuppressive agent may include, but is
not limited
to, CellCept, Gengraf, Micrhogam, Neoral, Orthoclone OKT3, Prograf, Rapamune,
Sandimmune, Thymoglobulin, and Zenapax.
The present invention also provides a method for treating or preventing
restenosis in a
subject comprising administering a therapeutically effective amount of a
compound
contemplated hereby. The present invention also provides a method for treating
or
preventing atherosclerosis in a subject comprising administering a
therapeutically effective
amount of a compound contemplated hereby.
The present invention also provides a method for treating disease mediated by
inflammation in a subject comprising the step of administering a
therapeutically effective
amount of a compound contemplated hereby. More specifically, the disease
mediated by
inflammation may be an autoimmune disease. In this regard, the autoimmune
disease may be
alopecia areata, ankylosing spondylitis, antiphospholipid syndrome, autoimmune
Addison's
disease, autoimmune hemolytic anemia, autoimmune hepatitis, Behcet's disease,
bullous
pemphigoid, cardiomyopathy, celiac sprue-dermatitis, chronic fatigue immune
dysfunction
syndrome (CFIDS), chronic inflammatory demyelinating polyneuropathy, Churg-
Strauss
syndrome, cicatricial pemphigoid, CREST syndrome, cold agglutinin disease,
Crohn's
disease, discoid lupus, essential mixed cryoglobulinemia, fibromyalgia-
fibromyositis,
8
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Graves' disease, Guillain-Barre, Hashimoto's thyroiditis, idiopathic pulmonary
fibrosis,
idiopathic thrombocytopenia purpura (ITP), IgA nephropathy, insulin dependent
diabetes,
juvenile arthritis, lichen planus, meniere's disease, mixed connective tissue
disease, multiple
sclerosis, myasthenia gravis, pemphigus vulgaris, pernicious anemia,
polyarteritis nodosa,
polychondritis, polyglandular syndromes, polymyalgia rheumatica, polymyositis
and
dermatomyositis, primary agammaglobulinemia, primary biliary cirrhosis,
psoriasis,
Raynaud's phenomenon, Reiter's syndrome, rheumatic fever, rheumatoid
arthritis,
sarcoidosis, scleroderma, Sjogren's syndrome, stiff man syndrome, systemic
lupus
erythematosus, Takayasu arteritis, temporal arteritis/giant cell arteritis,
ulcerative colitis,
uveitis, vasculitis, vitiligo, and Wegener's granulomatosis.
The present invention further provides a method for treating or preventing
cancer in a
subject comprising administering a therapeutically effective amount of a
compound
contemplated hereby. Moreover, the present invention provides a method for
treating or
preventing metastases in a subject comprising administering a therapeutically
effective
amount of a compound contemplated hereby to the subject.
Still another aspect of the present invention provides the methods, by using
compound of formula (I), which also comprises treatment and/or prophylaxis of
proliferative
conditions, particularly for inhibition of proliferation of smooth muscle
cells, comprising
administration of compositions comprising compounds of formula (I). In
accordance with
the present invention, uses of such compositions comprise prevention and
treatment of
vascular occlusive conditions including atherosclerosis and restenosis.
Still another aspect of the present invention provides the methods for the
treatment
and/or prophylaxis of diseases mediated by inflammatory conditions and
cellular
proliferative conditions, by using the compound of formula (I).
Still yet another aspect of the present invention provides treatment and/or
prophylaxis
of a disease or disorder mediated by cell adhesion molecules like VCAM-l,
where the
diseases are inflammatory disorders selected from rheumatoid arthritis,
osteoarthrites,
asthama, dermatitis, psoriasis, organ transplantation or allograft rejection,
autoimmune
diabetes or multiple sclerosis; a cardiovascular disease selected from
athresclerosis,
restenosis, coronary artery disease, angina, small artery disease, diabetes
mellitus, diabetic
nepropathy or diabetic retinopathy and one of the cell adhesion molecules is
VCAM-1.
9
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Still another aspect of the present invention provides treatment and/or
prophylaxis of
of a disease by delivering the compounds) of formula (I) at the site of the
disease by using a
compounds) of formula (I) coated stems.
The present invention further provides pharmaceutical compositions containing
compounds of the general formula (I), their salts, or any mixture thereof in
combination with
a suitable carrier, solvent, diluent, or medium typically employed in
preparing such
compositions.
Still further, the present invention provides various compounds and
compositions that
each may be administered by a route that is oral, parenteral, subcutaneous,
intramuscular,
to intravenous, intrarticular, intrabronchial, intraabdominal, intracapsular,
intracartilaginous,
intracavitary, intracelial, intracelebellar, intracerebroventricular,
intracolic, intracervical,
intragastric, intrahepatic, intramyocardial, intraosteal, intrapelvic,
intrapericardiac,
intraperitoneal, intrapleural, intraprostatic, intrapulmonary, intrarectal,
intrarenal, intraretinal,
intraspinal, intrasynovial, intrathoracic, intrauterine, intravesical, bolus,
vaginal, rectal,
buccal, sublingual, intranasal, or transdermal.
The compositions of the present invention also may include formulations of the
compounds disclosed, which may be suitable for oral, rectal, ophthalmic,
(including
intravitreal or intracameral) nasal, topical (including buccal and
sublingual), vaginal or
parenteral (including subcutaneous, intramuscular, intravenous, intradermal,
intratracheal,
2o and epidural) administration. The formulations may conveniently be
presented in unit
dosage form and may be prepared by conventional pharmaceutical techniques.
Such
techniques include the step of bringing into association the active ingredient
and the
pharmaceutical carner(s) or excipient(s). In general, the formulations are
prepared by
uniformly and intimately bringing into associate the active ingredient with
liquid carriers or
finely divided solid carriers or both, and then, if necessary, shaping the
product.
Still yet another aspect of the present invention provides novel
intermediates, a
process for their preparation and use of the intermediates in processes for
preparation of
compound of formula (I), their salts, and pharmaceutically acceptable
compositions thereof.
so DEFINITIONS
It is to be understood that this invention is not limited to the particular
methodology,
protocols, cell lines, constructs, and reagents described herein and as such
may vary. It is
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
also to be understood that the terminology used herein is for the purpose of
describing
particular aspects only, and is not intended to limit the scope of the present
invention which
will be limited only by the appended claims.
As used herein and in the appended claims, the singular forms "a", "an", and
"the"
include plural reference unless the context clearly indicates otherwise. Thus,
for example,
reference to a "compound" is a reference to one or more such compounds and
includes
equivalents thereof known to those skilled in the art, and so forth.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood to one of ordinary skill in the art at the time
this invention
1 o was made.
All publications and patents mentioned herein are incorporated herein by
reference
for the purpose of describing and disclosing, for example, the constructs and
methodologies
that are described in the publications, which might be used in connection with
the presently
described invention. The publications discussed above and throughout the text
are provided
solely for their disclosure prior to the filing date of the present
application. Nothing herein is
to be construed as an admission that the inventors are not entitled to
antedate such disclosure
by virtue of prior invention.
As used herein, the term "compound" includes both the singular and the plural,
and
includes any single entity or combined entities that have activity that can be
measured in the
assays of the present invention and combinations, fragments, analogs or
derivatives of such
entities.
The term "glycated protein", as used herein, includes proteins linleed to
glucose,
either enzymatically or non-enzymatically, primarily by condensation of free
epsilon-amino
groups in the protein with glucose, forming Amadori adducts. Furthermore,
glycated protein,
as used herein, includes not only proteins containing these initial glycation
products, but also
glycation products resulting from further reactions such as rearrangements,
dehydration, and
condensations that form irreversible advanced glycation end products (AGE). It
should be
understood that any agent that causes the cells or components of the assay to
respond in a
measurable manner is contemplated by the present invention. Enhanced formation
and
accumulation of glycated proteins and AGE are thought to play a major role in
the
pathogenesis of diabetic complications, and atherosclerosis, leading to the
development of a
range of diabetic complications including nephropathy, retinopathy and
neuropathy. There is
11
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
ample in vivo evidence that suggests that diabetes-related complications can
be reduced by
(1) preventing glycation of proteins, (2) by breaking the cross-links in
glycated proteins (The
cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced
glycation end-
product accumulation., Diabetologia., 2000(43)660-4) (or (3) by blocking
glycated protein
interaction with receptors. Despite the importance of AGE in the pathogenesis
of diabetic
microangiopathies, there are no currently available medications known to block
AGE
formation.
The term "phenylamine" refers to a primary or secondary b enzeneamine, more
commonly known as aniline. The amino group on the aniline may be optionally
substituted
to with hydrogen, alkyl (C~-C~Z, straight chain or branched), cycloallcyl (C~-
Clo), or optionally
substituted aryl groups. The phenyl ring of this aniline derivative may be
optionally
substituted with one or more functional groups, or a combination of functional
groups such
as alkyl, alkenyl, alkynyl, phenyl, benzyl, halo, cyano, nitre, hydroxy,
thioxy, alkoxy,
aryloxy, haloalkyloxy, alkylthio, arylthio, amino, alkyl amino, aryl amino,
acyl, carboxyl,
amide, sulfonamide, sulfonyl, sulfate, sulfonic acid, morpholino, piperazinyl,
pyridyl,
thienyl, furanyl, pyrroyl, pyrazoyl, phosphate, phosphonic acid, or
phosphonate. If
applicable, these groups can be represented in protected or unprotected forms
used in
standard organic synthesis.
The term "naphthylamine" refers to a primary or secondary a- or 13-
naphthylamine.
The ring substructure in the naphthylamine may be optionally substituted with
one or a
combination of functional groups such as allcyl, alkenyl, alkynyl, phenyl,
benzyl, halo, cyano,
nitre, hydroxy, thioxy, alkoxy, aryloxy, haloalkyloxy, alkylthio, arylthio,
amino, alkyl amino,
aryl amino, acyl, carboxyl, amide, sulfonamide, sulfonyl, sulfate, sulfon.ic
acid, morpholino,
thiomorpholino, piperazinyl, pyridyl, thienyl, furanyl, pyrroyl, p5rrazoyl,
phosphate,
phosphonic acid, or phosphonate. These groups can be represented in protected
or
unprotected forms used in standard organic synthesis.
The term "naphthylalkyl amine" refers to a primary or secondary a- and 13-
naphthylallcyl amine (for example, 2-a-naphthylethyl amine). The tern?
"benzalkyl amine"
refers to a primary or secondary benzylalkyl amine (for example, phenylcthyl
amine). These
aryl alkyl substructures or compounds can be optically active or optically
inactive. The aryl
(ring) substructures of the naphthylalkyl and benzalkyl amines can be
optionally subsituted
with one or a combination of functional groups, such as alkyl, alkenyl,
alkynyl, phenyl,
12
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
benzyl, halo, cyano, nitro, hydroxy, tluoxy, alkoxy, aryloxy, haloallcyloxy,
alkylthio, arylthio,
amino, alkyl amino, aryl amino, acyl, carbolyl, amido, sulfonamido, sulfonyl,
sulfate,
sulfonic acid, morpholino, piperazinyl, pyridyl, thienyl, furanyl, pyrroyl,
pyrazoyl,
phosphate, phosphoric acid, or phosphonate. If applicable these groups can be
represented in
protected or unprotected forms used in standard organic synthesis.
The term "quinolinyl amine" refers to primary or secondary quinolyl amines.
These
amines can be in optically active or inactive forms. The aryl (ring)
substructure of the
quinolyl amine may be be optionally substituted with one a combination of
functional
groups such as alkyl, allcenyl, alkynyl, phenyl, benzyl, halo, cyano, nitro,
hydroxy, thioxy,
l0 allcoxy, aryloxy, haloalkyloxy, alkylthio, arylthio, amino, alkyl amino,
aryl amino, aryl,
carboxyl, amido, sulfonamido, sulfonyl, sulfate, sulfonic acid, morpholino,
thiomorpholino,
piperazinyl, pyridyl, thienyl, furanyl, pyrroyl, pyrazoyl, phosphate,
phosphoric acid, or
phosphonate. These groups can be represented in protected or unprotected forms
used in
standard organic synthesis.
The term "heteroaryl amines" refers to pyrroles, pyrazoles, imidazoles, and
indoles.
The aryl (ring) substructure of the heteroaryl amine may be optionally
substituted with one or
a combination of functional groups such as alkyl, alkenyl, alkynyl, phenyl,
benzyl, halo,
cyano, nitro, hydroxy, thioxy, alkoxy, aryloxy, haloalkyloxy, alkylthio,
arylthio, amino, allcyl
amino, aryl amino, acyl, carboxyl, amido, sulfonamido, sulfonyl, sulfate,
sulfonic acid,
morpholino, thiomorpholino, piperazinyl, phosphate, phosphoric acid, or
phosphonate.
These groups can be represented in protected or unprotected forms used in
standard organic
synthesis.
The ternz "polynucleotide" refers generally to polymeric forms of nucleotides
of any
length, either ribonucleotides or deoxynucleotides. Thus, this term includes,
but is not
limited to, single-stranded, double-stranded, or mufti-stranded DNA or RNA.
Polynucleotides may further comprise genomic DNA, cDNA, or DNA-RNA hybrids.
Moreover, the polynucleotides of the present invention may be synthetically
produced.
Polynucleotides may comprise chemically modified, biochemically modified, or
derivatized nucleotides. For example, a polynucleotide may comprise, in part,
modified
nucleotides such as methylated nucleotides or nucleotide analogs.
Polynucleotides also may
comprise sugars, caps, nucleotide branches, and linking groups such as
fluororibose and
thioate. In addition, the sequence of nucleotides may be interrupted by non-
nucleotide
13
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
components. Furthermore, a polynucleotide may be modified after polymerization
to
facilitate its attachment to other polynucleotides, proteins, metal ions,
labelsng components,
or a solid support.
The backbone of the polynucleotide may comprise modified or optionally
substituted
sugar and/or phosphate groups. Alternatively, the backbone of the
polynucleotide may
comprise a polymer of synthetic subunits such as phosphoramidites and thus may
be an
oligodeoxynucleoside phosphoramidate or a mixed phosphoramidate-phosphodiester
oligomer. See Peyrottes et al., Nuct,. ACIDS IZES. (1996) 24:1841-1848, and
Chaturvedi et
al., NucL. Acms RES. (1996) 24:2318-2323.
The term "homology", as used herein, refers to a degree of complementarity.
There
may be partial homology or complete homology (i.e., identity). A partially
complementary
sequence is one that at least partially inhibits an identical sequence from
hybridizing to a
target polynucleotide; it is referred to using the functional term
"substantially homologous".
The inhibition of hybridization of the completely complementary sequence to
the target
sequence may be examined using a hybridization assay (Southern or Northern
blot, solution
hybridization) under conditions of low stringency. A substantially homologous
sequence or
probe will compete for and inhibit the binding (i.e., the hybridization) of a
completely
homologous sequence or probe to the target sequence under conditions of low
stringency.
This is not to say that conditions of low stringency are such that non-
specific binding is
2o permitted; low stringency conditions require that the binding of two
sequences to one another
be a specific (i.e., selective) interaction. The absence of non-specific
binding may be tested
by the use of a second target sequence which lacks even a partial degree of
complementarity
(for example, less than about 30% identity); in the absence of non-specific
binding, the probe
will not hybridize to the second non-complementary target sequence.
The term "gene" refers to a polynucleotide sequence that comprises coding
sequences
necessary for the production of a polypeptide or precursor, and also may
include expression
control sequences. The polypeptide can be encoded by a full length coding
sequence or by
any portion of the coding sequence. The gene may be derived in whole or in
part from any
source known to those of ordinary skill in the art including a plant, a
fungus, an animal, a
3o bacterial genome or episome, eukaryotic, nuclear or plasmid DNA, cDNA,
viral DNA, or
chemically synthesized DNA. A gene may constitute an uninterrupted coding
sequence or it
may include one or more introns, bound by the appropriate splice junctions.
Moreover, a
14
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
gene may contain one or more modifications in either the coding or the
untranslated regions
that could affect certain properties of the polynucleotide or polypeptide~
such as the
biological activity or the chemical structure of the expression product, the
rate of expression,
or the manner of expression control. Such modifications include, but are not
limited to,
mutations, insertions, deletions, and substitutions of one or more
nucleotides. In this regard,
such modified genes may be referred to as "variants" of the "native" gene
(discussed below).
"Gene expression" refers to the process by which a polynucleotide sequence
undergoes successful transcription and translation such that detectable levels
of the
nucleotide sequence are expressed.
l0 The term "gene expression profile" refers to a group of genes representing
a
particular cell or tissue type (for example, neuron, coronary artery
endothelium, or disease
tissue) in any activation state. In one aspect, a gene expression profile is
generated from cells
exposed to a compound of the present invention. This profile may be compared
to a gene
expression profile generated from the same type of cell prior to treatment
with a compound
of the present invention. Furtherniore, a series of gene expression profiles
may be generated
from cells treated with a compound of the present invention, specifically, at
different doses or
a time-course to assess the effects of the compound. A gene expression profile
also is known
as a gene expression signature.
The term "differential expression" refers to both quantitative as well as
qualitative
2o differences in the temporal and tissue expression patterns of a gene. For
example, a
differentially expressed gene may have its expression activated or completely
inactivated in
normal versus disease conditions. Such a qualitatively regulated gene may
exhibit an
expression pattern within a given tissue or cell type that is detectable in
either control or
disease conditions, but is not detectable in both. "Differentially expressed
polynucleotide",
as used herein, refers to a polynucleotide sequence that uniquely identifies a
differentially
expressed gene so that detection of the differentially expressed
polynucleotide in a sample is
correlated with the presence of a differentially expressed gene in a sample.
Similarly, a differentially expressed protein may have its expression
activated or
completely inactivated in normal versus disease conditions. Such a
qualitatively regulated
3o protein may exhibit an expression pattern within a given tissue or cell
type that is detectable
in either control or disease conditions, but is not detectable in both. A
"differentially
expressed protein", as used herein, refers to an amino acid sequence that
uniquely identifies a
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
differentially expressed protein so that detection of the differentially
expressed protein in a
sample is correlated with the presence of a differentially expressed protein
in a sample.
"Cell type" as used herein, refers to a cell from a given source (for example,
tissue or
organ), a cell in a given state of differentiation, or a cell associated with
a given pathology or
genetic makeup.
The term "polypeptide" refers to a polymeric form of amino acids of any
length,
which may include translated, untranslated, chemically modified, biochemically
modified
and derivatized amino acids. A polypeptide may be naturally occurring,
recombinant, or
synthetic, or any combination of these.
Moreover, the term "polypeptide" as used herein, refers to proteins,
polypeptides, and
peptides of any size, structure, or function. For example, a polypeptide may
comprise a
string of amino acids held together by peptide bonds. A polypeptide may
alternatively
comprise a Iong chain of amino acids held together by peptide bonds. Moreover,
a
polypeptide also may comprise a fragment of a naturally occurring protein or
peptide. A
polypeptide may be a single molecule or may be a mufti-molecular complex. In
addition,
such polypeptides may have modified peptide backbones as well.
The term "polypeptide" further comprises immunologically tagged proteins and
fusion proteins, including, but not limited to, fusion proteins with a
heterologous amino acid
sequence, fusion proteins with heterologous and homologous leader sequences,
and fusion
proteins with or without N-terminal methionine residues.
The term "protein expression" refers to the process by which a polynucleotide
sequence undergoes successful transcription and translation such that
detectable levels of the
amino acid sequence or protein are expressed.
The term "protein expression profile" refers to a group of proteins
representing a
particular cell or tissue type (for example, neuron, coronary artery
endothelium, or disease
tissue). In one aspect, a protein expression profile is generated from cells
exposed to a
compound of the present invention. This profile may be compared to a proteizi
expression
profile generated from the same type of cell prior to treatment with a
compound of the
present invention. Furthermore, a series of protein expression profiles may be
generated
from cells treated with a compound of the present invention, specifically, at
different doses or
a time-course to assess the effects of the compound. A protein expression
profile also is
known as a "protein expression signature".
16
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
As used herein, a "biomolecule" includes polynucleotides and polypeptides.
Moreover, a "biomolecular sequence", as used herein, is a term that refers to
all or a portion
of a polynucleotide sequence. A biomolecular sequence also may refer to all or
a portion of a
polypeptide sequence.
A "host cell" as used herein, refers to a microorganism, a prokaryotic cell, a
eukaryotic cell or cell line cultured as a unicellular entity that may be, or
has been, used as a
recipient for a recombinant vector or other transfer of polynucleotides, and
includes the
progeny of the original cell that has been transfected. It is understood that
the progeny of a
single cell may not necessarily be completely identical in morphology or in
genomic or total
l0 DNA complement as the original parent due to natural, accidental, or
deliberate mutation.
In the context of biomolecule, for example, Perlecan, the term "functional
equivalent"
refers to a protein or polynucleotide molecule that possesses functional or
structural
characteristics that are substantially similar to all or part of the native
Perlecan protein or
native Perlecan-encoding polynucleotides. A functional equivalent of a native
Perlecan
protein may contain modifications depending on the necessity of such
modifications for a
specific structure or the performance of a specific function. The term
"functional equivalent"
is intended to include the "fragments", "mutants", "derivatives", "alleles",
"hybrids",
"variants", "analogs", or "chemical derivatives", of native Perlecan.
In the context of immunoglobulins, the term "functional equivalent" refers to
2o immunoglobulin molecules that exhibit immunological binding properties that
are
substantially similar to the parent immunoglobulin. As used herein, the term
"immunological
binding properties" refers to non-covalent interactions of the type which
occur between an
immunoglobulin molecule and an antigen for which the immunoglobulin is
specific. Indeed,
a functional equivalent of a monoclonal antibody immunoglobulin, for example,
may snhibit
the binding of the parent monoclonal antibody to its antigen. A functional
equivalent may
comprise F(af)Z fragments, Flab) molecules, Fv fragments, single chain
fragment variable
displayed on phage (scFv), single domain antibodies, chimeric antibodies, or
the like so long
as the immunoglobulin exhibits the characteristics of the parent
immunoglobulin.
As used herein, the term "isolated" refers to a polynucleotide, a polypeptide,
an
antibody, or a host cell that is in an environment different from that in
which the
polynucleotide, the polypeptide, the antibody, or the host cell naturally
occurs. An isolated
polynucleotide, polypeptide, antibody, or host cell is generally substantially
purified.
17
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
As used herein, the term "substantially purified" refers to a compound that is
removed
from its natural environment and is at least about 60% free, at least about
65% free, at least
about 70% free, at Ieast about 75% free, at least about 80% free, at Ieast
about 83% free, at
least about 85% free, at least about 88% free, at least about 90% free, at
least about 91% free,
at least about 92% free, at least about 93% free, at least about 94% free, at
least about 95%
free, at least about 96% free, at least about 97% free, at least about 98%
free, at least about
99% free, at least about 99.9% free, or at least about 99.99% free from other
components
with which it is naturally associated. For example, a composition containing A
is
"substantially free of B when at least about 85% by weight of the total A+B in
the
composition is A. Alternatively, A comprises at least about 90% by weight of
the total of
A+B in the composition, further still, at least about 95% or even 99% by
weight.
"Diagnosis" as used herein, generally includes a determination of a subject's
susceptibility to a disease or disorder, a determination as to whether a
subject is presently
affected by a disease or disorder, a prognosis of a subject affected by a
disease or disorder
(for example, identification of pre-metastatic or metastatic cancerous states,
stages of cancer,
or responsiveness of cancer to therapy), and therametrics (for example,
monitoring a
subject's condition to provide information as to the effect or efficacy of
therapy)_
The term "biological sample" encompasses a variety of sample types obtained
from
an organism which may be used in a diagnostic, monitoring, or other assay_ The
term
encompasses blood and other liquid samples of biological origin, solid tissue
samples such as
a biopsy specimen, or tissue cultures or cells derived therefrom and the
progeny thereof. The
term specifically encompasses a clinical sample, and further includes cells in
cell culture, cell
supernatants, cell lysates, serum, plasma, urine, amniotic fluid, biological
fluids, and tissue
samples. The term also encompasses samples that have been manipulated in any
way after
procurement such as treatment with reagents, solubilization, or enrichment for
certain
components.
The terms "individual", "subject", "host", and "patient" refer to any
mammalian
subject for whom diagnosis, treatment, or therapy is desired. The individual,
subject, host, or
patient is optionally a human. Other subjects may include, but are not limited
to, cattle,
3o horses, dogs, cats, guinea pigs, rabbits, rats, primates, and mice.
The teens "treatment", "treating", "treat", are used herein to refer generally
to
obtaining a desired pharmacological andlor physiologic effect. The effect may
be
I8
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
prophylactic in that it may completely or partially prevent a disease or
symptom thereof
andlor may be therapeutic in that it rnay partially or completely stabilize or
cure a disease
and/or adverse effect attributable to the disease. "Treatment" as used herein
covers any
treatment of a disease in a mammal, particularly a human, and includes: (a)
preventing the
disease or symptom from occurring in a subject which may be predisposed to the
disease or
symptom, but has not yet been diagnosed as having it; (b) inhibiting the
disease symptom,
i.e., arresting its development; or (c) relieving the disease symptom, i.e.,
causing regression
of the disease or symptom.
The expression "therapeutically effective amount" refers to an amount of, for
example, a compound contemplated hereby, that is effective for preventing,
ameliorating,
treating, or delaying the onset of a disease or condition.
A "prophylactically effective amount" refers to an amount of, for example, a
compound contemplated hereby that is effective for preventing a disease or
condition.
A "liposome" is a small vesicle composed of various types of lipids,
phospholipids
and/or surfactant, which is useful for delivery of a drug to a mammal. The
compounds of the
present invention may be delivered by a liposome. The components of the
liposome are
commonly arranged in a bilayer formation, similar to the lipid arrangement of
biological
membranes.
"Hybridization", broadly defined, refers to any process by which a
polynucleotide
2o sequence binds to a complementary sequence through base pairing.
Hybridization conditior~s
can be defined by, for example, the concentrations of salt or formamide in the
prehybridization and hybridization solutions, or by the hybridization
temperature, and are
well known in the art. Hybridization can occur under various conditions
stringency.
Hybridization also may refer to the binding of a protein-capture agent to a
target protein
under certain conditions, such as normal physiological conditions.
As understood herein, the term "activation" refers to any alteration of a
signaling
pathway or biological response including, for example, increases above basal
levels,
restoration to basal levels from an inhibited state, and stimulation of the
pathway above basal
levels.
3o The term "biological activity" refers to the biological behavior and
effects of a
protein or peptide. The biological activity of a protein may be affected at
the cellular level
and the molecular level. For example, the biological activity of a protein may
be affected by
19
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
changes at the molecular level. For example, an antisense oligonucleotide may
prevent
translation of a particular mRNA, thereby inhibiting the biological activity
of the protein
encoded by the mRNA. In addition, an antibody may bind to a particular protein
and inhibit
that protein's biological activity.
The term "oligonucleotide" as used herein refers to a polynucleotide sequence
comprising, for example, from about 10 nucleotides (nt) to about 1000 nt.
Oligonucleotides
for use in the present invention are, for example, from about 15 nt to about
150 nt, or from
about 150 nt to about 1000 nt in length. The ohigonucleotide may be a
naturally occurring
oligonucleotide or a synthetic ohigonucleotide. Ohigonucheotides may be
prepared by the
phosphoramidite method (Beaucage and Carruthers, TETRAHEDRON LETT. (1981)
22:1859-
1862), or by the triester method (Matteucci et ah., J. Atn. CHEM. SoC. (1981)
103:3185), or by
other chemical methods known in the art.
The term "microarray" refers generally to the type of genes or proteins
represented on
a microarray by oligonucleotides (polynucleotide sequences) or protein-binding
agents, and
where the type of genes or proteins represented on the microarray is dependent
on the
intended purpose of the microarray (for example, to monitor expression of
human genes or
proteins). The oligonucheotides or protein-binding agents on a given
microarray may
correspond to the same type, category, or group of genes or proteins. Genes or
proteins may
be considered to be of the same type if they share some common characteristics
such as
species of origin (for example, human, mouse, rat); disease state (for
example, cancer);
function (for example, protein kinases, tumor suppressors); same biological
process (for
example, apoptosis, signal transduction, cell cycle regulation, proliferation,
differentiation).
For example, one microarray type may be a "cancer mieroarray" in which each of
the
microarray oligonucleotides or protein-binding agents correspond to a gene or
protein
associated with a cancer. An "epithelial microarray" may be a microarray of
oligonucheotides or protein-binding agents corresponding to unique epithelial
genes or
proteins. Similarly, a "cell cycle microarray" may be an microarray type in
which the
oligonucleotides or protein-binding agents correspond to unique genes or
proteins associated
with the cell cycle.
The term "detectable" refers to a polynucheotide expression pattern which is
detectable via the standard techniques of pohymerase chain reaction (PCR),
reverse
transcriptase-(RT) PCR, differential display, and Northern analyses, which are
well known to
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
those of skill in the art. Similarly, polypeptide expression patterns may be
"detected" via
standard techniques including immunoassays such as Western blots.
A "target gene" refers to a polynucleotide, often derived from a biological
sample, to
which an oligonucleotide probe is designed specifically to hybridize. It is
either the presence
s or absence of the target polynucleotide that is to be detected, or the
amount of the target
polynucleotide that is to be quantified. The target polynucleotide has a
sequence that is
complementary to the polynucleotide sequence of the corresponding probe
directed to the
target. The target polynucleotide also may refer to the specific subsequence
of a larger
polynucleotide to which the probe is directed or to the overall sequence (for
example, gene or
to mRNA) whose expression levels it is desired to detect.
A "target protein" refers to a polypeptide, often derived from a biological
sample, to
which a protein-capture agent specifically hybridizes or binds. It is either
the presence or
absence of the target protein that is to be detected, or the amount of the
target protein that is
to be quantified. The target protein has a structure that is recognized by the
corresponding
15 protein-capture agent directed to the target. The target protein or amino
acid also may refer
to the specific substructure of a larger protein to which the protein-capture
agent is directed
or to the overall structure (for example, gene or mRNA) whose expression
levels it is desired
to detect.
The term "complementary" refers to the topological compatibility or matching
2o together of the interacting surfaces of a probe molecule and its target.
The target and its
probe can be described as complementary, and furthermore, the contact surface
characteristics are complementary to each other. Hybridization or base pairing
between
nucleotides or nucleic acids, such as, for example, between the two strands of
a double-
stranded DNA molecule or between an oligonucleotide probe and a target are
25 complementary.
The term "background" refers to non-specific binding or other interactions
between,
for example, polynucleotides, polypeptides, small molecules and polypeptides,
or small
molecules and polynucleotides. "Background" also may refer to the non-specific
binding or
other interactions in the context of assays including immunoassays.
3o In the context of microarrays, the term "background" refers to
hybridization signals
resulting from non-specific binding, or other interactions, between the
labeled target
polynucleotides and components of the oligonucleotide microarray (for example,
the
21
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
oligonucleotide probes, control probes, the microarray support) or between
target proteins
and the protein-binding agents of a protein microarray. Background signals
also may be
produced by intrinsic fluorescence of the microarray components themselves. A
single
background signal may be calculated for the entire microarray, or a different
background
signal may be calculated for each target polynucleotide or target protein. The
background
may be calculated as the average hybridization signal intensity, or where a
different
background signal is calculated for each target gene or target protein.
Alternatively,
background may be calculated as the average hybridization signal intensity
produced by
hybridization to probes that are not complementary to any sequence found in
the sample (for
to example, probes directed to polynucleotides of the opposite sense or to
genes not found in the
sample such as bacterial genes where the sample is mammalian polynucleotides).
The
background also can be calculated as the average signal intensity produced by
regions of the
microarray which lack any probes or protein-binding agents at all.
A "small molecule" refers to a compound or molecular complex, either
synthetic,
naturally derived, or partially synthetic, composed of carbon, hydrogen,
oxygen, and
nitrogen, that also may contain other elements, and that may have a molecular
weight of less
than about 15,000, less than about 14,000, less than about 13,000, less than
about 12,000,
less than about 11,000, less than about 10,000, less than about 9,000, less
than about 8,000,
less than about 7,000, less than about 6,000, less than about 5,000, less than
about 4,000, less
than about 3,000, less than about 2,000, less than about 1,000, less than
about 900, less than
about 800, less than about 700, less than about 600, less than about 500, less
than about 400,
less than about 300, less than about 200, or less than about 100.
The term "fusion protein" refers to a protein composed of two or more
polypeptides
that, although typically not joined in their native state, are joined by their
respective amino
and carboxyl termini through a peptide linkage to form a single continuous
polypeptide. It is
understood that the two or more polypeptide components can either be directly
joined or
indirectly joined through a peptide linker/spacer.
The term "normal physiological conditions" means conditions that are typical
inside a
living organism or a cell. Although some organs or organisms provide extreme
conditions,
3o the infra-organismal and infra-cellular environment normally varies around
pH 7 (i.e., from
pH 6.5 to pH 7.5), contains water as the predominant solvent, and exists at a
temperature
22
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
above 0°C and below 50°C. The concentration of various salts
depends on the organ,
organism, cell, or cellular compartment used as a reference.
The term "cluster" refers to a group of clones or biomolecular sequences
related to
one another by sequence homology. In one example, clusters are formed based
upon a
specified degree of homology and/or overlap (for example, stringency).
"Clustering" may be
performed with the sequence data. For instance, a biomolecular sequence
thought to be
associated with a particular molecular or biological activity in one tissue
might be compared
against another library or database of sequences. This type of search is
useful to Look for
homologous, and presumably functionally related, sequences in other tissues or
samples, and
to may be used to streamline the methods of the present invention in that
clustering may be used'
within one or more of the databases to cluster biomolecular sequences prior to
performing a
method of the invention. The sequences showing sufficient homology with the
representative sequence are considered part of a "cluster". Such "sufficient"
homology may
vary within the needs of one skilled in the art.
As used herein, the term "internal database" refers to a database maintained
within a
local computer network. It contains, for example, biomolecular sequences
associated with a
project. It also may contain information associated with sequences including,
but not limited
to, a library in which a given sequence is found and descriptive information
about a likely
gene associated with the sequence. The internal database is optionally
maintained as a
2o private database behind a firewall within an enterprise network. However,
the present
invention contemplates an internal database that is available to the public.
The internal
database may include sequence data generated by the same enterprise that
maintains the
database, and also may include sequence data obtained from external sources.
The term "external database", as understood herein, refers to a database
located
outside all internal databases. Typically, an enterprise network differing
from the enterprise
network maintaining the internal database will maintain an external database.
The external
database may be used, for example, to provide some descriptive information on
biomolecular
sequences stored in the internal database. For example, the external database
may be
GenBank and associated databases maintained by the National Center for
Biotechnology
3o Information (NCBI), which is part of the National Library of Medicine.
23
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to compounds of general formula (I), its
analogs,
tautomeric forms, regioisomers, stereoisomers, polymorphs, pharmaceutically
acceptable
salts and pharmaceutically acceptable solvates thereof. Further, the present
invention is
directed to pharmaceutical compositions comprising compounds of general
formula (I), its
analogs, tautomeric forms, regioisomers, stereoisomers, polymorphs,
pharmaceutically
acceptable salts and pharmaceutically acceptable solvates thereof, either
individually or in
any combination thereof Still further, the present invention is directed to
methods of use of
compounds of general formula (I), its analogs, tautomeric forms, regioisomers,
stereoisomers, polymorphs, pharmaceutically acceptable salts and
pharmaceutically
acceptable solvates thereof, either individually or in any combination
thereof. Even further,
the present invention is directed to methods of making compounds of general
formula (I), its
analogs, tautomeric forms, regioisomers, stereoisomers, polymorphs,
pharmaceutically
acceptable salts and pharmaceutically acceptable solvates thereof.
Compounds of General Formula (I)
The present invention is related to compounds of formula (IJ, and to methods
and/or
compositions comprising compounds that are effective in modulating
inflammatory
responses, such as those resulting from AGE and glycated protein accumulation.
The present
2o invention also is directed to methods and/or compositions comprising
compounds that are
effective in modulating smooth muscle cell proliferation and the diseases or
conditions
related thereto.
According to one aspect of the present invention, various compounds of general
formula (I)
R~
R2 B\.A ~ ~~
D~JJ Kw
R3 L-Q
its tautomeric forms, its stereoisomers, its polymorphs, its pharmaceutically
acceptable salts,
and its pharmaceutically acceptable solvates are provided. According to this
aspect,
24
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
R~ B ~
D,';J ~Kw
R3
C is
R~ R~
R2 B~A 1 E R4 B~A E R4
p, J ~ ~-Q (la) R p J ~ ~-Q (Ib)
~3
X . or X
-Y-G-C-
In this and other aspects, L is -Y-G=Z-Ar- ~ X~ , or -(CHZ)t -, and Q is
Y~
X4 S(O}W-Ar
~NR
-(CHa)v C-X-(CH2}p
Y2 X~ ~C O R
5 X
R or 3
R1, R'', and R3 independently are hydrogen, a hydroxy group, a halogen, a
nitro group,
a carboxy group, a carbamoyl group, an optionally substituted amino group, an
alkyl group, a
cycloalkyl group, an alkoxy group, a cycloalkoxy group, an alkenyl group, a
cycloalkenyl
group, an alkoxyalkyl group, an alkenyloxy group, a cycloalkenyloxy group, an
acyl group,
an acyloxy group, an aryl group, an aryloxy group, an aroyl group, an aroyloxy
group, an
aralkyl group, an aralkoxy group, a heterocyclyl group, a heteroaryl group, a
heteroarallcyl
group, a heteroaryloxy group, a heteroaralkoxy group, an alkoxycarbonyl group,
an
aryloxycarbonyl group, a heteroarylcarbonyl group, an alkylsulfonyl group, an
arylsulfonyl
group, a heteroarylsulfonyl group, an aralkylsulfinyl group, an allcylsulfmyl
group, an
arylsulfinyl group, a heteroarylsul~nyl group, an aralkylsul~nyl group, an
alkylthio group, an
arylthio group, a heteroarylthio group, an aralkylthio group, an aryloxyalkyl
group,
carboxylic acid or a derivative thereof, or sulfonic acid or a derivative
thereof, wherein any
two of RI, R2, and R3 in combination optionally form a 5-member or 6-member
saturated
cyclic ring having from 1 to 3 heteroatoms, wherein the heteroatoms are O, S,
or N. The
cyclic ring formed by any two of R', R2, or R3 may be oxlanyl, 1,3-dioalanyl,
or 1,4-
dioxalanyl.
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R4 1S hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, an optionally substituted amino group, an alkyl group, a
cycloalkyl group,
an alkoxy group, a cycloalkoxy group, an alkenyl group, a cycloalleenyl group,
an
alkoxyalkyl group, an alkenyloxy group, a cycloalkenyloxy group, an acyl
group, an acyloxy
group, an aryl group, an aryloxy group, an aroyl group, an aroyloxy group, an
aralkyl group,
an aralkenyl group, an aralkynyl group, an aralkoxy group, a heterocyclyl
group, a
heterocyclenyl group, a heteroaryl group, a heteroaralkyl group, a
heteroaryloxy group, a
heteroaralkoxy group, an alkoxycarbonyl group, an aryloxycarbonyl group, an
aralkoxycarbonyl group, a heteroarylcarbonyl group, an allcylsulfonyl group,
an arylsulfonyl
group, a heteroarylsulfonyl group, an alkylsulfmyl group, an arylsulfinyl
group, an
arallcylsulfinyl group, a heteroarylsulfmyl group, an aralkylsulfinyl group,
an alkylthio group,
an arylthio group, a heteroarylthio group, an aralkylthio group, an
aryloxyalkyl group, an
aralkoxyalkyl group, a fused heteroarylcycloalleyl group, a fused
heteroarylcycloallcenyl
group, a fused heteroarylheterocyclenyl group, carboxylic acid or a derivative
thereof, or
sulfonic acid or a derivative thereof.
Any of RI, RZ, R3, and R4 independently optionally are substituted with
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
group, an alkyl group, a cycloalkyl group, an alkoxy group, a haloalkoxy
group, a cycloalkyl
group, an aryl group, a benzyloxy group, an acyl group, an acyloxy group, an
aroyl group, an
allcoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl group, a
heterocyclyl group,
an aralkyl group, an alkylsulfonyl group, an alkylsulfmyl group, an
arylsulfonyl group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piper~inyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alleoxy group, or an aryl group.
According to this and other aspects, A, B, D, and J independently are O, S, N,
>CH,
or (CHZ)"; '----' is an optional chemical bond; E is O, S, or -NR; K is N, C,
or CH; Y and Z
independently are O, -NR, fCH2~", or S(=O)"; G is -(CH~)S , -(CHZ)S CH=CH-
(CHZ)S-, or -
26
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
(CHZ)S C=C-(CHZ)S ; X, X~, XZ, X3, and X4 independently are O, S, or -NR; F is
O, S, or -
NR; Yl and YZ independently are O or S; n, w, a independently are an integer
from 0-2; p, t,
m, s, v independently are an integer from 0-5, and 'Ar' is a substituted or
unsubstituted
phenyl or a substituted or unsubstituted naphthyl group.
R and RS independently are hydrogen, potassium, sodium, a hydroxy group, a
halogen, a nitro group, an optionally substituted amino group, an alkyl group,
an alkoxy
group, an alkenyl group, an alkoxyalkyl group, a cycloalkenyloxy group, an
acyl group, an
aryl group, an aralkyl group, a heterocyclyl group, or a heteroaryl group.
The groups provided above are as follows:
'Halogen' is fluorine, chlorine, bromine, or iodine;
'Alkyl' group is a linear or branched (CI-Clo)alkyl group. Exemplary alkyl
groups
include methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, t-butyl, n-
pentyl, iso pentyl,
hexyl, heptyl, octyl.
'Cycloalkyl' group is a (C3-C~) cycloalkyl group which may be mon or
polycyclic.
Exemplary cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl.
'Allcoxy' is (C~-C~o)alkyl-O-, wherein the (C~-C~o)alkyl group is as defined
above.
Exemplary alkyl groups include methoxy, ethoxy, propoxy, butoxy, iso-propoxy.
'Cycloalkoxy' is a (C3-C6)cycloalkoxy group. Exemplary cycloalkoxy groups
include cyclopropoxy, cyclobutoxy, cyclopentoxy, cyclohexoxy.
'Alkenyl' is a (CZ-C6)alkenyl group. Exemplary alkenyl groups include ethenyl,
propenyl, butenyl, pentenyl, hexenyl.
'Cycloalkenyl' is (C3-C~)cycloalkenyl group. Exemplary cycloalkenyl groups
include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl.
'Alkoxyalkyl' is a (Cl-C6)alkoxy(CI-Clo)alkyl group, where alkoxy and alkyl
groups
are as defined above. Exemplary alkoxyalkyl groups include methoxymethyl,
methoxyethyl,
methoxypropyl, ethoxymethyl, ethoxyethyl.
'Allcenyloxy' is (C~-C6)alkenyl-O-, where the (C2-C6)allcenyl group is as
defined
above. Exemplary alkenyl groups include ethenyloxy, propenyloxy, butenyloxy,
pentenyloxy, hexenyloxy.
'Cycloalkenyloxy' is a (C3-C~)cycloalkenyl-O-, where the (C3-C~)cycloalkenyl
group
is as defined above. Exemplary cycloalkenyloxy groups include cycloethenyloxy,
cyclopropenyloxy, cyclobutenyloxy, cyclopentenyloxy.
27
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
'Acyl' is H-CO- or (C~-Cio)alkyl-CO-, where (Cl-C1o)alkyl group is as defined
above. Exemplary aeyl groups include acetyl, propionyl.
'Acyloxy' is (C~-C6)acyl-O-, where acyl group is as defined above. Exemplary
acyloxy groups include acetyloxy, propionyloxy.
'Aryl' is monocylic or polycyclic ring system of about 5 to 14 carbon atoms.
Exemplary groups include phenyl, naphthyl.
'Aryloxy' is an aryl-O- group, where the aryl group is as defined above.
Exemplary
aryloxy groups include phenoxy, naphthyloxy.
'Aroyl' is the aryl-CO- group, wherein the aryl group is as defined above.
Exemplary
1o aroyl groups include benzoyl, 1-naphthoyl.
'Aroyloxy' is the amyl-O- group, wherein the aroyl group is as defined above.
Exemplary aroyloxy groups include benzoyloxy, 1-naphthoyloxy.
'Aralkyl' is the aryl-(C1-Clo)alkyl group, wherein aryl and (C~-C~o)alkyl
groups are as
defined above. Exemplary arallcyl groups include benzyl, 2-phenylethyl.
'Aralkenyl' is aryl-(C~-C6)alkenyl group, wherein aryl and (C~-C6)allcenyl
groups are
as defined above.
'Aralkynyl' is aryl-(C~-C6)alkynyl group, wherein the aryl and group is as
defined
above.
'Aralkoxy' is arallcyl-O- group, wherein the aralkyl group as defined above.
Exemplary aralkoxy groups include benzyloxy, 2-phenethyloxy.
'Heterocyclyl' is a non-aromatic saturated monocyclic or polycyclic ring
system of
about 5 to about 10 carbon atoms, having at least one hetero atom selected
from O, S or N.
Exemplary heterocyclyl groups include aziridinyl, pyrrolidinyl, piperidinyl,
piperazinyl,
morpholinyl, thiomorpholinyl, thiazolidinyl, 1,3-dioxolanyl, 1,4-dioxanyl.
'Heterocyclenyl' is a non-aromatic monocyclie or polycyclic hydrocarbon ring
system of about 5 to 10 carbon atoms, having at least one hetero atom selected
from O, S or
N and one double bond. Exemplary heterocyclenyl groups include 1,2,3,4-
tetrahydropyrimidine, 1,2-dihydropyridyl, 1,4-dihydropyridyl, 1,2,3,6-
tetrahydropyridine,
1,4,5,6-tetrahych-opyrimidine, 2-pyrrolinyl, 3-pyrrolinyl, 2-imidazolinyl, 2-
pyrazolinyl.
'Heteroaryl' is an aromatic monocyclic or polycyclic ring system of about 5 to
about
10 carbon atoms, having at least one heteroatom selected from O, S or N.
Exemplary
heteroaryl groups include as pyrazinyl, isothiazolyl, oxazolyl, pyrazolyl,
pyrrolyl,
28
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
pyridazinyl, thienopyrimidyl, furyl, indolyl, isoindolyl, 1,3-benzodioxole,
1,3-benzoxathiole,
quinazolinyl, pyridyl, thiophenyl.
'Heteroaralkyl' is a heteroaryl-(C1-C~o)alkyl group, wherein the heteroaryl
and (CI
C~o)alkyl groups are as defined above. Exemplary heteroaralkyl groups include
thienylmethyl, pyridylinethyl, imidazolylmethyl.
'Heteroaryloxy' is heteroaryl-O-, wherein the heteroaryl group is as defined
above.
Exemplary heteroaryloxy groups include pyrazinyloxy, isothiazolyloxy,
oxazolyloxy,
pyrazolyloxy, phthalazinyloxy, indolyloxy, quinazolinyloxy, pyridyloxy,
thienyloxy.
'Heteroaralkoxy' is heteroaralkyl-O-, wherein the heteroaralkyl group is as
defined
above. Exemplary heteroaralkoxy groups include thienylmethyloxy,
pyridylmethyloxy.
'Alkylcarbonyl' or 'acyl' is (C1-Cjo)alkyl-CO-, wherein the (Cl-C~o)alkyl
group is as
defined above. Exemplary alkylcarbonyl groups include methylcarbonyl,
ethylcarbonyl,
propylcarbonyl.
'Alkoxycarbonyl' is (CI-C~o)alkyl-O-CO-, wherein the (Cl-Clo)alkyl group is as
defined above. Exemplary alkoxycarbonyl groups include methoxycarbonyl,
ethoxycarbonyl, t-butoxycarbonyl.
'Arylcarbonyl' or 'aroyl' is aryl-CO-, wherein the aryl group is as defined
above.
Exemplary arylcarbonyl groups include phenylcarbonyl, naphthylcarbonyl.
'Aryloxycarbonyl' is aryl-O-CO-, wherein the aryl group is as defined above.
Exemplary aryloxycarbonyl groups include phenoxycarbonyl, naphthyloxycarbonyl.
'Aralkoxycarbonyl' is aryl-(Cl-C6)alkoxy-CO-, where aryl and (C~-C6)alkoxy are
as
defined above. Exemplary aralkoxycarbonyl groups include benzyloxycarbonyl, 2-
phenethyloxycarbonyl.
'Heteroarylcarbonyl' is heteroaryl-CO-, wherein heteroaryl is as defined
above.
Exemplary heteroarylcarbonyl groups include pyrazinylcarbonyl,
isothiazolylcarbonyl,
oxazolylcarbonyl, pyrazolylcarbonyl, pyrrolylcarbonyl, pyridazinylcarbonyl,
indolylcarbonyl.
'Alkylsulfonyl' is (C~-CIO)alkyl-SO2-, wherein the (C~-Clo)alkyl group is as
defined
above. Exemplary alkylsulfonyl groups include methylsulfonyl, ethylsulfonyl,
3o propylsulfonyl.
'Arylsulfonyl' is aryl-S02-, wherein the aryl group is as defined above.
Exemplary
arylsulfonyl groups include benzenesulfonyl.
29
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
'Heteroarylsulfonyl' is heteroaryl-S02-, wherein heteroaryl is as defined
above.
Exemplary heteroarylsulfonyl groups include pyrazinylsulfonyl,
isothiazolylsulfonyl,
oxazolylsulfonyl, pyrazolylsulfonyl, pyrrolylsulfonyl, pyridazinylsulfonyl,
phthalazinylsulfonyl, quinazolinylsulfonyl, pyridylsulfonyl, thienylsulfonyl.
'Alkylsul~nyl' is (CI-C~o)alkyl-SO-, where (C~-CIO)alkyl is as defined above.
Exemplary alkylsulfinyl groups include methylsulfinyl, ethylsul~nyl,
propylsulfinyl.
'Arylsul~nyl' is aryl-SO-, wherein the aryl group is as defined above.
Exemplary
arylsulfonyl groups include phenylsulfmyl.
'Heteroarylsulfmyl' is heteroaryl-SO-, wherein heteroaryl is as defined above.
Exemplary heteroarylsulfmyl groups include pyrazinylsulfinyl,
isothiazolylsul~nyl,
oxazolylsul~nyl, pyrazolylsulfinyl, pyrrolylsulfinyl, pyridazinylsulfinyl,
phthalazinylsul~nyl,
quinazolinylsulfinyl, pyridylsul~nyl, and thienylsul~nyl.
'Aralkylsulfinyl' is aryl-(C~-C~n)alkyl-SO- group, where in aryl and (C~-
Clo)alkyl
groups are as defined above. Exemplary aralkylsulfmyl groups include
benzylsulfinyl, ~-
phenethylsul~nyl.
'Alkylthio' is (CI-C~o)alkyl-S-, wherein (Cl-C~o)alkyl is as defined above.
Exemplary alkylthio groups include methylthio, ethylthio, and propylthio.
'Arylthio' is aryl-S-, wherein aryl group is as defined above. Exemplary
arylthio
groups include phenylthio groups.
'Heteroarylthio' is heteroaryl-S-, wherein heteroaryl is as defined above.
Exemplary
heteroarylthio groups include pyrazinylthio, isothiazolylthio, oxazolylthio,
pyrazolylthio,
pyrrolylthio, pyridazinylthio, phthalazinylthio, quinazolinylthio,
pyridylthio, and thienylthio.
'Aralkylthio' is aryl-(Cl-C~o)alkyl-S- group, wherein aryl and (CI-C~o)alkyl
groups
are as defined above. Exemplary aralkylthio groups include benzylthio, and 2-
phenethylthio.
'Aryloxyalkyl' is aryl-O-(Cl-CIO)alkyl, where aryl and (CI-CIO)alkyl groups
are as
defined above. Exemplary aryloxyalkyl groups include phenoxymethyl,
phenoxyethyl, and
phenoxypropyl.
'Aralkoxyalkyl' is aryl-(CI-Glo)alkyl-O--(CI-C~o)alkyl, where (C~-Cio)alkyl
and aryl
groups are as defined above. Exemplary aralkoxyalkyl groups include
benzyloxyrnethyl,
benzyloxyethyl, and benzyloxypropyl.
'Fused heteroarylcycloalkyl' is fused heteroaryl and cyclo(C3-C6)alkyl,
wherein
heteroaryl and cyclo(C3-C6)alkyl groups are as defined herein. Exemplary fused
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
heteroarylcycloalkyl groups include 5,6,7,8-tetrahydroquinolinyl, and 5,6,7,8-
tetrahydroisoquinolyl.
'Fused heteroarylcycloalkenyl' is fused heteroaryl and cyclo(C3-C6)alkenyl,
wherein
heteroaryl and cyclo(C3-C6)alkenyl groups are as defined herein. Exemplary
fused
heteroarylcycloalkenyl groups include 5,6-dihydroquinolyl, 5,6-
dihydroisoquinolyl, 5,6-
dihydroquinoxalinyl.
'Fused heteroarylheterocyclenyl' is fused heteroaryl and heterocyclenyl,
wherein
heteroaryl and heterocyclenyl groups are as defined herein. Exemplary fused
heteroarylheterocyclenyl groups include 7,8-dihydro[1,7]naphthyridinyl, 1,2-
to dihydro[2,7]naphthyridinyl.
'Carboxylic acid or its derivatives' may be amides or esters. Exemplary
carboxylic
acid groups include CONH~, CONHMe, CONMe2, CONHEt, CONEt~, CONHPh, COOCH3,
COOC~HS or COOC3H~.
'Sulfonic acid or its derivatives' may be amides or esters. Exemplary sulfonic
acid
groups include S02NH~, SO~NHMe, SOZNMeZ, S02NHCF3, COOCH3, COOCZHS, or
COOC3H~.
As used herein:
Ra is hydrogen, a hydroxy group, a halogen, a vitro group, or an optionally
substituted
amino group;
2o Rb is an allcyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl
group;
R° is a cycloalkenyloxy group, an acyl group, an aryl group, an aralkyl
group, a
heterocyclyl group, or a heteroaryl group;
Rya is hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, or an optionally substituted amino group, an alkyl group, a
cycloalkyl
group, an alkoxy group, a cycloalkoxy group, an alkenyl group, a cycloalkenyl
group, an
alkoxyalkyl group, an alkenyloxy group, or a cycloalkenyloxy group;
Rlb is an acyl group, an acyloxy group, an aryl group, an aryloxy group, an
aroyl
group, an aroyloxy group, an arallcyl group, an aralkoxy group, a heterocyclyl
group, a
heteroaryl group, a heteroarallryl group, a heteroaryloxy group, a
heteroarallcoxy group, an
allcoxycarbonyl group, an aryloxycarbonyl group, or a heteroarylcarbonyl
group;
RIB is an alkylsulfonyl group, an arylsulfonyl group, a heteroarylsulfonyl
group, an
arallcylsulfinyl group, an allcylsulfmyl group, an arylsulfmyl group, a
heteroarylsulfinyl
31
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an aralkylsulfmyl group, an alkylthio group, an arylthio group, a
heteroarylthio group,
an aralkylthio group, an aryloxyalkyl group, carboxylic acid or a derivative
thereof, or
sulfonic acid or a derivative thereof;
RZa is hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, or an optionally substituted amino group, an alkyl group, a
cycloallcyl
group, an alkoxy group, a cycloalkoxy group, an alkenyl group, a cycloalkenyl
group, an
alkoxyalkyl group, an alkenyloxy group, or a cycloalkenyloxy group;
RZb is an acyl group, an acyloxy group, an aryl group, an aryloxy group, an
amyl
group, an aroyloxy group, an aralkyl group, an aralkoxy group, a heterocyclyl
group, a
l0 heteroaryl group, a heteroarallcyl group, a heteroaryloxy group, a
heteroaralkoxy group, an
alkoxycarbonyl group, an aryloxycarbonyl group, or a heteroarylcarbonyl group;
RZ° is an alkylsulfonyl group, an arylsulfonyl group, a
heteroarylsulfonyl group, an
aralkylsulfinyl group, an alkylsulfinyl group, an arylsulfinyl group, a
heteroarylsulfinyl
group, an arallcylsulfinyl group, an allcylthio group, an arylthio group, a
heteroarylthio group,
an arallcylthio group, an aryloxyalkyl group, carboxylic acid or a derivative
thereof, or
sulfonic acid or a derivative thereof;
R3a is hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, or an optionally substituted amino group, an alkyl group, a
cycloalkyl
group, an alkoxy group, a cycloalkoxy group, an alkenyl group, a cycloalkenyl
group, an
alkoxyalkyl group, an alkenyloxy group, or a cycloalkenyloxy group;
R3b is an acyl group, an acyloxy group, an aryl group, an aryloxy group, an
amyl
group, an aroyloxy group, an aralkyl group, an arallcoxy group, a heterocyclyl
group, a
heteroaryl group, a heteroaralkyl group, a heteroaryloxy group, a
heteroaralkoxy group, an
allcoxycarbonyl group, an aryloxycarbonyl group, or a heteroarylcarbonyl
group;
R3° is an allcylsulfonyl group, an arylsulfonyl group, a
heteroarylsulfonyl group, an
arallcylsulfinyl group, an allcylsul~nyl group, an arylsulfmyl group, a
heteroarylsulfinyl
group, an aralkylsulfmyl group, an alkylthio group, an arylthio group, a
heteroarylthio group,
an aralkylthio group, an aryloxyalkyl group, carboxylic acid or a derivative
thereof, or
sulfonic acid or a derivative thereof;
3o R4a is hydrogen, a hydroxy group, a halogen, a vitro group, or an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an alkoxy group,
a cycloallcoxy
3?
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl group, an
alkenyloxy group, or
a cycloalkenyloxy group;
R4b is an acyl group, an acyloxy group, an aryl group, an aryloxy group, aroyl
group
or an aroyloxy group, an aralkyl group, an aralkenyl group, an aralkynyl
group, an aralkoxy
group, a heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroarallcyl
group, a heteroaryloxy group, or a heteroaralkoxy group;
R4° is an allcoxycarbonyl group, an aryloxycarbonyl group, an
aralkoxycarbonyl
group, a heteroarylcarbonyl group, an allcylsulfonyl group, an arylsulfonyl
group, a
heteroarylsulfonyl group, an alkylsulfmyl group, an arylsulfinyl group, or an
aralkylsulfinyl
group, an alkylthio group, an arylthio group, a heteroarylthio group, an
aralkylthio group, a
fused heteroarylcycloalkyl group, a fused heteroarylcycloalkenyl group, a
fused
heteroarylheterocyclenyl group, carboxylic acid or a derivative thereof, or
sulfonic acid or a
derivative thereof;
Rsa is hydrogen, a hydroxy group, a halogen, a vitro group, or an optionally
substituted amino group;
RSb is an alkyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl
group;
R5~ is a cycloalkenyloxy group, an acyl group, an aryl group, an aralkyl
group, a
heterocyclyl group, or a heteroaryl group;
R'a is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
2o amino group, a hydroxy group, an allcoxy group, a carboxy group, a cyano
group, an oxo(O=)
group, or a thio(S=) group;
R'b is an alkyl group, a cycloalkyl group, an allcoxy group, a haloalkoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, a
heterocyclyl group, or an aralkyl group;
R'° is an alkylsulfonyl group, an alkylsulfinyl group, an arylsulfonyl
group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group;
R"a is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an allcoxy group, a carboxy group, a cyano
group, an oxo(O=)
group, or a thio(S=) group;
33
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R"b is an alkyl group, a cycloalkyl group, an aIkoxy group, a haloalkoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
amyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, a
heterocyclyl group, or an arallcyl group;
R"° is an alkylsulfonyl group, an alkylsul~nyl group, an arylsulfonyl
group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group.
R9~ is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
1o group, or a thio(S=) group;
R96 is an alkyl group, a cycloalkyl group, an alkoxy group, a haloalkoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an arallcyl group;
R9' is an alkylsulfonyl group, an alkylsul~nyl group, an arylsulfonyl group,
an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an allcyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alkoxy group, or an aryl group;
Rloa is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
R'°b is an alk 1 ou a c cloa 1 rou an alkox ou a haloalkox rou a
Y ~' P~ Y ~Y g p~ Y ~ p~ Y g p~
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, a
heterocyclyl group, or an arallcyl group;
Rl°° is an alkylsulfonyl group, an alkylsulfmyl group, an
arylsulfonyl group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
arallcylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
34
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
halogen, a hydroxyl group, a vitro group, an amino group, an alleyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alkoxy group, or an aryl group.
RI la is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
Rl~b is an alkyl group, a cycloalkyl group, an allcoxy group, a haloalkoxy
group, a
l0 cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an
acyloxy group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an aralkyl group;
Rn~ is an alkylsulfonyl group, an alkylsulfinyl group, an arylsulfonyl group,
an
arylsulfinyl group, an allcylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
allcyl group, an
allcoxy group, or an aryl group;
Ri2a is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(~=)
group, or a thio(S=) group;
Ri2b is an allcyl group, a cycloalleyl group, an alkoxy group, a haloalkoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an aralkyl group;
R12~ is an alkylsulfonyl group, an alkylsulfmyl group, an arylsulfonyl group,
an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
allcyl group, an
alkoxy group, or an aryl group;
Rl3a is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
R~3b is an alkyl group, a cycloalkyl group, an alkoxy group, a haloalkoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
amyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an aralkyl group;
RI3c is an alkylsulfonyl group, an alkylsulfmyl group, an arylsulfonyl group,
an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
arallcylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
is combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alkoxy group, or an aryl group;
Riaa is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
R~4b is an alkyl group, a cycloalkyl group, an allcoxy group, a haloallcoxy
group, a
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
amyl group, an allcoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an aralkyl group;
Rl~° is an alkylsulfonyl group, an alkylsulf'myl group, an arylsulfonyl
group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
3o combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
36
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alkoxy group, or an aryl group;
R2oa is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
Rz°b is an alk 1 ou a c cloal 1 rou an alkox rou a haloalkox rou a
Y bn' p~ Y kY g p~ Y g p~ Y g p
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
amyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, or a
heterocyclyl group, an aralkyl group;
to Rz°° is an alkylsulfonyl group, an alkylsul~nyl group, an
arylsulfonyl group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyI group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alleyl group, an
alkoxy group, or an aryl group;
Rzla is hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted
amino group, a hydroxy group, an alkoxy group, a carboxy group, a cyano group,
an oxo(O=)
group, or a thio(S=) group;
Rzib is an al 1 rou a c cloallc 1 ou an alkox ou a haloalkox rou a
kY g p~ Y Y ~ p~ Y ~' p~ Y g p~
cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an acyloxy
group, an
aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl
group, a
heterocyclyl group, or an aralkyl group;
Rz~~ is an alkylsulfonyl group, an alkylsulfmyl group, an arylsulfonyl group,
an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, which is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
alkoxy group, or an aryl group.
37
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Ga is -(CHZ)S-, where s is an integer from 0-5;
Gb is -(CHz)S CH=GH-(CHZ)S-, where s is an integer from 0-5;
G~ is -(CHZ)S-C=C-(CH2)S , where s is an integer from 0-5;
Za is O; Zb is NR; Z~ is f CHZ~" or S(=O)", where a is an integer from 0-2;
Ea is O; Eb is S; E° is NR;
pa is 0-1; pb is 2-3; p~ is 4-5;
va is 0-1; vb is 2-3; v~ is 4-5;
Wa 1S 0; Wb 1S l; W~ 1S ~;
Xa is O; Xb is S; and X~ is -NR.
to According to another aspect of the present invention, various compounds of
general
formula (I) having general formula (II)
Y1
1
B\A E R4 F~NR
R Dr ~ ~ I Y-G-Z-Ar=~Ya ~I)
R3 r R
X
its tautomeric forms, its stereoisomers, its polymorphs, its pharmaceutically
acceptable salts,
and its pharmaceutically acceptable solvates are provided. Except as otherwise
provided
15 herein, all symbols are as defined above in connection with formula (I).
According to another aspect of the present invention, various compounds of
general
formula (I) having general formula (III)
R1
4
R~~:, ~ E ~ o_~_ cnn
Rs
O
its tautomeric forms, its stereoisomers, its polymorphs, its pharmaceutically
acceptable salts,
2o and its pharmaceutically acceptable solvates are provided. Except as
otherwise provided, all
symbols are as defined above in connection with formula (I).
R' and R" independently are hydrogen, a halogen, a nitro group, an amino
group, a
mono- or di- substituted amino group, a hydroxy group, an alkoxy group, a
carboxy group, a
cyano group, an oxo(O=) group, a thio(S=) group, an alkyl group, a cycloallryl
group, an
3~
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
alkoxy group, a haloalkoxy group, a cycloalkyl group, an aryl group, a
benzyloxy group, an
acyl group, an acyloxy group, an amyl group, an alkoxycarbonyl group, an
aryloxycarbonyl
group, a heteroaryl group, a heterocyclyl group, an aralkyl group, an
alkylsulfonyl group, an
alkylsulfinyl group, an arylsulfonyl group, an arylsulfinyl group, an
alkylthio group, an
arylthio group, a heteroarylthio group, an aralkylthio group, or a
heterocyclyl sulfonyl group.
In one aspect of the present invention, R', R2, R3, and R5 are as defined
above; R4 is
an optionally substituted aryl group, and in some instances, is a phenyl group
optionally
substituted with a halogen, an alkoxy group, or both; E is O or -NR, where R
is as defined
above; G is -(CH2)S or -(CH2)S CH=CH-(CH2)S ; s is an integer from 1-3; and R'
and R"
1o are as defined above, and in some instances, independently are hydrogen, a
halogen, a nitro
group, an amino group, a mono- or di- substituted amino group, a hydroxy
group, an alkoxy
group, an alkyl group, a cycloalkyl group, an alkoxy group, an aryl group, or
an acyl group.
Numerous compounds having the general formula (III) are contemplated by the
present invention. Various configurations of such compounds provided herein
are also
encompassed by this invention, as provided below.
R'
O
R RQ R~ NR r~ E R4 R
R ~ I I O_G_Z O R ~. ~ ~ O-G-Z~~ / ~ R
R5 R3
O R" (1); O R~~ .R5 O (2)0
R~
R~ ~NR Rz E Ra R,
w
R R O I I
R2~ I I O-G Z ~ R R ~ O-G-Z ~ g O
R3 I / O R.. R5 NR
R~~ (3)~ °
R~
Rz Ra R' ~ / E R4
o-G_~ ~ o R ~ ~ ~ o-G_z ~ S~O
R O R.. R5-.. N R O R5__.. NR
(5)o O
R~ O Ra
E R4 R~ S~ a E R
R I I °-G ~~NR RZ ~ I I o
R3 O R..~RS O o R,. R5°.. NR
(7)o O (g)o
39
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R' R'
Ra Ra R, Ra R,
o_G_z~~~ s~o w ~ ~ o-G-z~~~ s~o
O R, R5°.. N a O R~~ RS..._ NR
O (9)p O
/ Ra R. ~ E Ra R.
O-G Z \ J S O Ra ~ O-G Z \ J S O
Ra O O
R~~ Rs NR R.. Rs-._. NR
o (11); o (la)o
E R4
i
R~ \ I I O-G_Z~ ~ O
Ra O Ry _ N
~R~~--(,~~s
and o (13);
where all symbols are as defined above in connection with formula (I). Thus,
by way of
example, and not by way of limitation, the present invention contemplates the
following
exemplary compounds:
R' R'
O R4 ~ ~ r~ S R4
R R3 I I O G Z J S O R 3 ~ ~ O G Z
O R" 5..._ O R~RS.... NR
o (14); o (15);
R~
i a R, ~ a R.
R ~ I N I o-G-Z ~~ s o Ra ~, ~ E ~ Oy CHa)5 ~-CI J S o
R3 ..
O
O R.. Rs-___ NR R,. Rs -.. N R
o (16); o (17);
R~
E R4
R L ~ I I ~(CH2)s'-CH=CI-F(CH2)s Z~~ S O
O R R5____
N,
/ R
o (18);
R~
E R4
Rz r ~
oUoH2)s o=o-(cH~>s z~
3
R5- ..
~R
0 (19);
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
i
R~ E Ra R E R R
R ~ I I o-G_~I', S O R~ I I O_O_N ~ S O
I" 5.... R O l 5.... NR
3
R~ R
p (20); o (21);
' ' a R.
R ~ Ra _ ~ R
p-G-(CHZ)u'~ i ~O ~'' ~3 I I O_G-S(-O)u~ J ~O
O R .... NR O R RS..__ NR
p (22); p (23);
MeO~~ E Ra R Me0 / E Ra
Me~~ I I O G Z ~ S O ~ ( I O G Z ~ ._ S O
O R.. 5-_ . NR OMe O R R5 NR
p (24); o (25);
Me0 / O Ra R
\ I I O-G z~~J
OMe O " R5'... NR
p (26); (27);
Me
/ OMe , OMe
Me0 o O \ I R Me0 p \ I R'
a
i ~_
OMe~r~
S~O \ ~ ~ O-G-Z~II~ S~ I O
OMe O R"R5 NR OMe O R~.~NR
p (28); and R p (29);
where all symbols are as defined above in connection with formula (I).
By way of further example, the present invention contemplates various
compounds
having the following general formula:
(30),
where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, R5, R, R~, and E of
formula
(30) are selected to produce various compounds of formula (30-1) through
formula (30-243)
as follows:
41
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
FormulaR R R R E
30-1 Ra R a R'a R"a Ea
30-2 Rb Rsa R'a R"a Ea
30-3 R Rsa R'a R"a Ea
30-4 Ra Rsb R'a R"a Ea
30-5 Rb Rsb R,a R"a Ea
30-6 R~ RSb R'a R"a Ea
30-7 Ra R5~ R'a R"a Ea
30-8 Rb RS R'a R"a Ea
30-9 R R5~ R'a R"a Ea
30-10 Ra Rsa R'b R"a Ea
30-11 Rb Rsa R'b R"a Ea
30-12 R RSa R'b R"a Ea
30-13 Ra RSU 12~b R"a E,a
30-14 Rb R5b R,b R"a Ea
30-15 R Rib Rrb R"a Ea
30-16 Ra RS R'b R"a Ea
30-17 Rb R5~ Rrb R"a Ea
30-18 R R5~ R,b R"a Ea
30-19 Ra Rsa R'~ R"a Ea
30-20 RU RSa R' R"a Ea
30-21 R RSa R'~ R"a Ea
30-22 Ra RSb R'~ R"a Ea
30-23 Rb Rsb R' R"a Ea
30-24 R~ RSb R' R"a Ea
30-25 Ra R5 R' R"a Ea
30-26 Rb R5~ R' R"a Ea
30-27 R~ R5~ R' R"a Ea
30-28 Ra Rsa R'a R"b Ea
30-29 Rb RSa R'a Rb Ea
30-30 R R5a R'a Rr,b Ea
30-31 Ra RSb R'a R"b Ea
30-32 Rb Rsb R'a R"b Ea
30-33 R RSb R'a R"b E,a
30-34 Ra RS R'a R"b Ea
30-35 Rb R5~ R'a R"b Ea
30-36 R~ R5~ R'a R"b Ea
30-37 Ra Rsa R'b R"b Ea
30-38 Rb R5a R~b R"b Ea
30-39 R~ Rsa Rrb Rb Ea
30-40 Ra Rsb R'b R"b Ea
30-41 Rb RSb R'b R"b Ea
30-42 R R5b R'b R"b Ea
30-43 R~ RS R'b R"b Ea
30-44 Rb R5~ R,b R"b Ea
30-45 R~ R5~ R'b R"b Ea
42
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30-46Ra RSa R'c Rrrb Ea
30-47Rb RSa R' Rrrb Ea
30-48R Rsa Rrc Rrrb Ea
30-49Ra Rsb Rrc Rrrb Ea
30-50Rb Rsb Rrc Rrrb Ea
30-51R RSb R'c R"b Ea
30-52Ra RSc R'c R"b Ea
30-53Rb Rsc R'c R"b Ea
30-54Rc Rsc R'c R"b Ea
30-55Ra RSa R'a R"c Ea
30-56Rb Rsa R'a R" Ea
30-57Rc Rsa R'a R" Ea
30-58Ra Rsb R'a R" Ea
30-59Rb Rsb R'a R"c Ea
30-60R Rsb R'a R" Ea
30-61Ra RSc R'a R" Ea
30-62Rb Rsc R'a R" Ea
30-63R Rsc R'a R"c Ea
30-64Ra Rsa Rrb Rrrc Ea
30-65Rb RSa Rrb R" Ea
30-66R R5a Rrb Rrrc Ea
30-67Ra Rsb Rrb Rrrc Ea
30-68Rb Rsb R'b R" Ea
30-69R RSb R'b R" Ea
30-70Ra Rsc R~b Rrrc Ea
30-71Rb RSc Rrb Rnc Ea
30-72Rc Rsc Rrb Rrrc Ea
30-73Ra RSa R'c R"c Ea
30-74Rb R5a R'c R" Ea
30-75Rc R5a R'c R" Ea
30-76Ra Rsb R'c R" Ea
30-77Rb Rsb R'c R" Ea
30-78R RSb R'c R"c Ea
30-79Ra RS' R'c R" Ea
30-80Rb Rsc R'c R"c Ea
30-81R~ R5c R' R"c Ea
30-82Ra Rsa R'a Rrra Eb
30-83Rb R5a Rra Rrra Eb
30-84Rc Rsa R'a Rrra Eb
30-85Ra RSb R'a Rrra Eb
30-86Rb R5b R'a Rrra Eb
30-87Rc RSb R'a R"a Eb
30-88Ra Rsc R'a R"a Eb
30-89Rb RSc R'a R"a Eb
30-90Rc Rsc R'a R"a Eb
30-91Ra RSa R'b R"a Eb
30-92Rb R5a R'b Rrra Eb
43
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30-93 R Rsa Rrb Rrra Eb
30-94 Ra Rsb Rrb Rrra Eb
30-95 Rb Rsb Rrb Rrra Eb
30-96 R Rsb R'b Rrra Eb
30-97 Ra R5 R,b R"a Eb
30-98 Rb Rsc Rrb Rrra Eb
30-99 Rc Rsc R'b R"a Eb
30-100Ra Rsa R'c R"a Eb
30-101Rb Rsa R'c R"a Eb
30-102Rc Rsa R'c R"a Eb
30-103Ra Rsb R' R"a Eb
30-104Rb Rsb R'c R"a Eb
30-105Rc Rsb R'c R"a Eb
30-106Ra Rsc R'c R"a Eb
30-107Rb Rsc R'c R"a Eb
30-108Rc Rsc R'c R"a Eb
30-109Ra Rsa R'a Rrrb Eb
30-110Rb Rsa R'a Rrrb Eb
30-111Rc Rsa R'a R"b Eb
30-112Ra Rsb R'a Rrrb Eb
30-113Rb Rsb R'a R"b Eb
30-lI4Rc Rsb Rra Rrrb Eb
30-115Ra Rsc R'a R"b Eb
30-116Rb Rsc Rra Rrrb Eb
30-117Rc Rsc R'a Rrrb Eb
30-118Ra Rsa R'b Rrrb Eb
30-119Rb Rsa R'b Rrrb Eb
30-120Rc Rsa R'b Rrrb Eb
30-121Ra Rsb Rrb Rrrb Eb
30-122Rb Rsb R'b Rrrb Eb
30-123Rc Rsb R'b R"b Eb
30-124Ra Rsc R'b R"b Eb
30-125Rb Rsc R'b R"b Eb
30-126Rc Rsc Rrb Rrrb Eb
30-127Ra Rsa R'c R"b Eb
30-128Rb Rsa Rrc R,rb Eb
30-129Rc Rsa R'c R"b Eb
30-130Ra Rsb R'c R"b Eb
30-131Rb Rsb R'c R"b Eb
30-132R Rsb R' R"b Eb
30-133Ra Rsc R'c R"b Eb
30-134Rb Rsc R'c R"b Eb
30-135R Rsc R'c R"b Eb
30-136Ra Rsa R'a R"c Eb
30-137Rb Rsa R'a R"c Eb
30-138Rc Rsa R'a R"c Eb
30-139Ra Rsb R'a R" Eb
44
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30-140Rb Rsb R'a R"c Eb
30-141Rc RSb R'a R" Eb
30-142Ra Rsc R'a R"c Eb
30-143Rb Rsc R'a R"c Eb
30-144R Rsc R'a R"c Eb
30-145Ra Rsa R'b R"c Eb
30-146Rb Rsa R'b R"c Eb
30-147Rc Rsa R'b R"c Eb
30-148Ra Rsb R'b R"c Eb
30-149Rb Rsb R'b R"c Eb
30-150R Rsb R'b R" Eb
30-151Ra Rsc R'b R" Eb
30-152Rb Rsc R'b R"c Eb
30-153R Rsc R'b R"c Eb
30-154Ra Rsa R'c R"c Eb
30-155Rb Rsa R'c R" Eb
30-156R Rsa R'c R"c Eb
30-157Ra Rsb R'c R"c Eb
30-158Rb Rsb R'c R"c Eb
30-159R Rsb R'c R"c Eb
30-160Ra Rsc R'c R"c Eb
30-16IRb Rsc R'c R" Eb
30-162R Rsc R'c R"c Eb
30-163Ra Rsa R,a R"a Ec
30-164Rb Rsa R'a R"a Ec
30-165R Rsa R'a R"a Ec
30-166Ra Rsb R'a R"a E
30-167Rb Rsb R'a R"a E
30-168R Rsb R'a R"a Ec
30-169Ra Rsc R'a R"a Ec
30-170Rb Rsc R'a R"a E
30-171Rc Rs R'a R"a Ec
30-I72Ra Rsa R,b R"a Eo
30-173Rb Rsa R'b R"a E
30-174Rc Rsa R'b R"a EC
30-175Ra Rsb R'b R"a Ec
30-176Rb Rsb R'b R"a E
30-177Rc Rsb R'b R"a Ec
30-178Ra Rso R,b R"a Ec
30-179Rb Rsc R'b R"a E
30-180Rc Rsc R'b R"a Ec
30-181Ra Rsa R'c R"a Ec
30-182Rb Rsa R'c R"a Ec
30-1$3Rc Rsa R'c R"a EC
30-184Ra Rsb R'c R"a E
30-185Rb Rsb R'c R"a E
30-186R Rsb R'c R"a Ec
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30-187Ra Rsc R'c R"a Ec
30-188Rb Rsc R' R"a Ec
30-189Rc Rsc R'c R"a Ec
30-190Ra Rsa R'a R"b Ec
30-191Rb Rsa R'a R"b Ec
30-192Rc Rsa R'a R"b Ec
30-193Ra Rsb R,a Rrrb Ec
30-194Rb Rsb R'a R"b Ec
30-195Rc Rsb R'a R"b Ec
30-196Ra Rsc R'a Rb Ec
30-197Rb Rsc R'a R"b Ec
30-198Rc Rsc R'a R"b Ec
30-199Ra Rsa R'b R"b Ec
30-200Rb Rsa R'b R"b Ec
30-201Rc Rsa R'b R"b Ec
30-202Ra Rsb R'b R"b Ec
30-203Rb Rsb R'b R"b Ec
30-204Rc Rsb R'b R"b Ec
30-205Ra Rsc R'b R"b Ec
30-206Rb Rsc R'b R"b EC
30-207Rc Rsc R'b R"b E
30-208Ra Rsa R'c R"b Ec
30-209Rb Rsa R'c R"b Ec
30-210Rc Rsa R'c R"b Ec
30-211Ra Rsb R'c R"b Ec
30-212Rb Rsb R'c R"b Ec
30-213Rc Rsb R'c R"b Ec
30-214Ra Rsc R'c R"b Ec
30-215Rb Rsc R'c R"b Ec
30-216Rc Rs R'c R"b Ec
30-217Ra Rsa R,a R"c Ec
30-218Rb Rsa R'a R"c Ec
30-219Rc Rsa R'a R"c Ec
30-220Ra Rsb R'a R"c Ec
30-221Rb Rsb R'a R"c EC
30-222Rc Rsb R'a R"c Ec
30-223Ra Rsc R'a R"c Ec
30-224Rb Rsc R'a R"c Ec
30-225Rc Rsc R'a R" Ec
30-226Ra Rsa R'b R"c Ec
30-227Rb Rsa R,b R"c Ec
30-228R Rsa R'b R"c Ec
30-229Ra Rsb R'b R"c Ec
30-230Rb Rsb R'b R"c Ec
30-231R Rsb R'b R"~ Ec
30-232Ra Rsc R'b R"c Ec
30-233Rb Rsc R'b R" Ec
46
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30-234R Rsc R'b R"c Ec
30-235Ra RSa R'c R"c Ec
30-236Rb Rsa R'c R"c Ec
30-237R R5a R' R"c Ec
30-23$Ra RSb R'c R"c Ec
30-239Rb RSb R'c R"c Ec
30-240R Rsb R'c R"c E
30-241Ra Rsc R'c R" Ec
30-242Rb RSc R'c R"c Ec
30-243Rc RSc R'c R"c Ec
where all symbols are as defined above.
In one aspect of formula (30) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, or an optionally substituted amino group; RS
is hydrogen, a
hydroxy group, a halogen, a vitro group, an optionally substituted amino
group, an allcyl
group, an alkoxy group, an alkenyl group, or an alkoxyalkyl group; R' and R"
independently
are hydrogen, a halogen, a vitro group, an amino group, a mono- or di-
substituted amino
group, an allcyl group, a cycloalkyl group, an alkoxy group, a haloallcoxy
group, a cycloallcyl
group, an aryl group, or a benzyloxy group; and E is O, S, or NH.
l0 In another aspect of formula (30) of the present invention, R is hydrogen,
an alkyl
group, potassium, or sodium; RS is hydrogen or an alkyl group; and all other
symbols are as
defined above in connection with formula (I);
In another aspect of formula (30) of the present invention, E is O, S, or NH;
R' and R?
independently are -H, -Cl, -Br, or -CH3; RS is -H, -CH3, or -CHZCHZCH3; and R
is -H, I~, or
Na.
Examples of compounds of formula (30) include, but are not limited to:
OMe OMe
/ OMe / OMe
Me0 ~ O W I Me0 ~ O \ I
O I ~ H O
OMe O ~O I / /S NH OMe O O N ~ / / NH
O O
/ OMe O NH / OMe O~NH
Me0 ~ O ~ I OMe / S~O Me0 ~ O ~ I OMe S O
/ I O~O I / I ~O
QMe O I / OMe O ~ I
> >
47
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
OMe / OMe
Me0 O
Me0 \ O \ OMe OMe O I ~ OMe
~~O
'O w ~ / 'ms's \ ''t
OMe O O Me0 I / /S H OMe O O I / / NH
O ~ O
/ OMe
OMe
Me0 \ O \ ~ Me0 I \ O I \ OMe O
OMe ~ / ~O
O O ' / / NH OMe O O CI I / /S NH
O
CH3 O
> >
/ OMe
OMe
Me0 O \ I Me0 ~ O \ I
OMe O I ~ OMe O
' O~O I \ S~ O~ ~ \ 5 H
OMe O CI / / NH- OMe O B / /
O O
> >
/ OMe , OMe
Me0 I \ O I \ I OMe O Me0 \ O ~ I OMe O
O \ / O O S
OMe O O I / /S NNa OMe O ~ I s i N~
o ; and o
The present invention also contemplates various compounds of general formula
(III)
having the formula:
R~
\ ~ O R4
R"
o~/E ~~ s~
R~ O ~/ / NH
R Me O
(31),
where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, RI, RZ, R4, E, R', and
R" of
to formula (31) are selected to produce various compounds of formula (31-1) to
(31-729) as
follows:
Formula R R R E R' R"
31-1 R a R a R4a Ea R'a R"a
31-2 Rib RZa R4a Ea R,a R"a
31-3 Rl R2a R4a Ea Ria Ra
3I-4 Rya R2b R4a Ea Ria R..a
31-5 R'b R2b R4a Ea R,a Rr,a
31-6 R~ Rzb R4a Ea R,a R"a
31-7 Rya R2c R4a Ea R,a R"a
48
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-8 R'b Rzc R4a Ea R,a R"a
31-9 R' Rzc R4a Ea R,a R"a
31-10Ria R2a R4b Ea R,a R"a
31-IlRlb Rza R4b Ea R,a R"a
31-12Rlc Rza R4b Ea R,a R"a
31-13Rla Rzb R4b Ea R,a R"a
31-14Rib Rzb R4b Ea R,a R"a
31-15Rlc Rzb R4b Ea R,a R"a
31-16Rla Rzc R4b Ea Ria R..a
3I-17Rlb R2c R4b Ea R,a R..a
31-18Rl Rzc R4b Ea R'a R.,a
31-19Rya Rza R4c Ea R,a R"a
31-20RIb Rza R4c Ea R,a R"a
31-21Roc R2a R4c Ea R,a R"a
31-22Rya R2b R4c Ea R,a R"a
31-23Rlb Rzb R4c Ea R,a R..a
31-24Rlc Rzb R4c Ea Rra R"a
31-25Rla Rzc R4c Ea R~a R"a
31-26Rib Rzc R4c Ea R,a R"a
31-27Roc Rzc R'~c Ea R,a R"a
31-28Rya Rza R4a Eb R,a R"a
31-29Rlb R2a R4a Eb R,a R"a
31-30RIc Rza R4a Eb R,a R"a
31-3IRya R2b R4a Eb R,a R"a
31-32Rib Rzb R4a Eb R,a R"a
31-33Roc Rzb R'~a Eb R'a R"a
31-34Rya Rzc R4a Eb R,a R"a
31-35Rlb Rzc R4a Eb R,a R"a
31-36Rlc Rzc R4a Eb R'a R.,a
31-37Rya Rza R4b Eb R,a R"a
31-38Rlb R2a R4b Eb R,a R"a
31-39Ric Rza R4b Eb R,a R"a
31-40Rya Rzb R4b Eb Ria Rna
31-41Rlb Rzb R4b Eb Ria R..a
31-42Roc Rzb R4b Eb R,a Rna
31-43Rla Rzc R4b Eb R,a R"a
31-44Rlb Rzc R4b Eb R,a R"a
31-45Rlc R2c R4b Eb R,a Raa
31-46Rya Rza R4c Eb R,a R"a
31-47Rlb Rza R4c Eb R,a R"a
31-48Rlc Rza R4c Eb R,a R"a
31-49RIa Rzb R4c Eb R,a R"a
31-50RIb Rzb R4c Eb R,a Rna
31-51Rlc Rzb R4c Eb R,a R"a
31-52Ria Rzc R4c Eb R,a R"a
31-53Rlb R2c R4c Eb R,a R"a
31-54R'c Rz R4c Eb R'a R"a
49
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-55 Rya Rza R4a Ec R,a R"a
31-56 RIb R2a R4a Ec R,a Rr,a
3I-57 R~ Rza R4a Ec R,a R"a
31-58 Rya R2b R4a Ec R,a R"a
31-59 Rlb R2b R4a Ec R,a R"a
31-60 Roc R2b R4a Ec Rra R"a
31-61 Rya R2c R4a Ec R,a R"a
31-62 Rlb R2c R4a Ec R,a R"a
31-63 Rlc R2c R4a Ec R,a R"a
31-64 Rya R2a R4b Ec R,a Rr,a
31-65 RIb R2a R4b Ec R,a R"a
31-66 Roc Rza R4b Ec R,a R"a
31-67 Rya R2b R4b Ec R,a Rrra
31-68 R'b Rzb R4b Ec R,a R"a
31-69 R~ Rzb Rib Ec R,a R"a
31-70 Rya R2c R~b Ec R,a R,ra
31-71 Rlb R2c R4b Ec R,a R"a
31-72 Rlc R2c R4b Ec R,a R"a
31-73 Rya R2a R4c Ec R,a R"a
31-74 Rib Rza R4c Ec R,a R"a
31-75 Rl R2a R4c Ec R,a R"a
31-76 Rya R2b R4c Ec R,a R"a
31-77 RIb Rzb R4c Ec R,a R"a
31-78 Rl R2b R4c Ec R,a R"a
31-79 Rla Rzc R4c Ec R,a R"a
31-80 Rib Rzc Roc Ec R,a R"a
31-81 Roc Rzc R4c Ec R,a R"a
31-82 RI R2a R4a Ea R,b R"a
a
31-83 Rib R2a R4a Ea R,b R,ra
31-84 R~ R2a R4a Ea Rrb R"a
31-85 RIa Rzb R4a Ea R,b R,ra
31-86 RIb R2b R4a Ea Rrb R"a
31-87 R~ Rzb Raa Ea Rrb Rr,a
31-88 Rla Rzc R4a Ea Rrb R"a
31-89 Rlb R2c R4a Ea R~b R"a
31-90 Rlc R2c R4a Ea R,b R"a
31-91 RIa R2a R4b Ea R,b R"a
31-92 Rib R2a R4b Ea R,b Rr,a
31-93 Rlc Rza R4b Ea R,b R"a
31-94 Rla R2b Rab Ea R,b Rr,a
31-95 Rib R2b R4b Ea Rrb R"a
31-96 Rlc R2b R4b Ea R,b R"a
31-97 Rya R2c R4b Ea Rrb R"a
31-98 RIb R2c R4b Ea R,b Rr,a
31-99 RIc R2c R4b Ea R,b R"a
31-100Rya R2a R4c Ea R,b Rr,a
31-101Rlb Rza R4c Ea R,b R"a
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-102Ric Rza R4c Ea R,b R"a
31-103Rya Rzb R4c Ea R,b R"a
31-104Rib R2b R4o E,a R,b R"a
31-105Ric R2b R4c E,a R,b R'ra
31-106Ria R2c R4c E,a Rrb R"a
31-I07Rib Rzc R'ic Ea R~b R"a
31-108Ric Rzc R4c Ea R,b R'ra
31-109Ria Rza R4a Eb R,b R,ra
31-110Rib Rza R4a Eb Rrb R"a
31-111Ric R2a R4a E,b R,b Rr,a
31-112Ria R2b R4a Eb R,b Rr,a
~
31-113Rib R2b R4a E,b R,b R"a
31-114Ric R2b R4a E,b R,b R"a
31-115Ria R2c R4a Eb R'b R"a
31-116Rib R2c R4a Eb R,b R"a
31-117Ric R2c R4a Eb R,b R"a
31-118Ria Rza R4b Eb R,b Rr,a
31-119Rib Rza R4b E,b R,b R,ra
31-120Ric R2a R'ib $b R~b R"a
31-121Ria Rzb R4b Eb R'b R"a
3I-122Rib R2b R4b Eb R,b R"a
31-123Roc R2b Rab Eb R,b R"a
31-124Ria Rzc R4b E,b R,b R"a
31-125Rib R2c R4b E,b R,b R'ra
31-126Ric R2c Rab Eb R'b R"a
31-127Ria Rza R4 gb Rrb Rrra
31-128Rib Rza R4c E,b R,b R"a
31-129Ric R2a R4c Eb R,b R"a
31-130Ria R2b R4c Eb R,b R"a
31-131Rib R2b R4c Eb R,b R"a
31-132Ric R2b R4c Eb R,b R"a
31-133Ria R2c R4c E,b R,b R"a
31-134Rib R2c R4c Eb R,b R"a
31-135Ric R2c R~tc Eb R'b R"a
31-136Ria R2a R4a E,c R,b R"a
31-137Rib R2a R4a Ec R,b R"a
31-138Ric R2a R4a Ec R,b R"a
31-139Ria R2b R4a $ R,b R"a
31-140Rib Rzb R4a E,c R,b R"a
31-141Ric R2b R4a Ec Rrb Rr,a
31-142Ria R2c R4a Ec R,b R"a
31-143Rib R2c R4a E,c R,b Rna
31-144Roc Rzc R4a Ec R,b R"a
31-145Ria R2a R4b Ec R,b R"a
3I-146Rib R2a R4b E R,b R"a
31-147Ric Rza R4b Ec R,b R"a
31-148Ria R2b R4b E,c R,b R"a
51
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-149Rlb Rzb R4b Ec R,b R"a
31-150RIc Rzb R4b Ec Rrb R"a
31-151Rya R2c R4b Ec R,b R"a
31-152Rib Rzc R4b Ec R,b R"a
31-153Roc Rzc R4b Ec R,b R"a
31-154Rya Rza R4c Ec Rrb R"a
31-155Rlb Rza R4c Ec R,b R"a
31-156Roc Rza R4c Ec Rrb R"a
31-157RIa Rzb R4c Ec R,b R"a
31-158Rlb Rzb R4c Ec R,b R"a
31-159Rlc Rzb R4c Ec Rrb Rrra
31-160RIa Rzc R4c Ec R,b R"a
31-161Rib Rzc R4c Ec R~b Rr,a
31-162R~ R2c R4c Ec Rrb R"a
31-163Rya Rza R4a Ea R,c R,ra
31-164Rib Rza R4a Ea R,c R"a
31-165Rlc Rza R4a Ea R,c R"a
31-166Rya Rzb R4a Ea R,c R,ra
31-167R'b Rzb R4a Ea R,c R"a
31-168Roc Rzb R4a Ea R,c Rr,a
31-169Rya Rzc R4a Ea R,c R"a
31-170Rib Rzc R4a Ea R,c R"a
31-171Rlc Rzc R4a Ea R,c R"a
31-172Rla Rza R4b Ea R,c R"a
31-173Rlb Rza R4b Ea R,c R"a
31-174Rlc R2a R4b Ea R,c R"a
31-175Ria R2b R~tb Ea R,c R,ra
31-176Rlb Rzb Rib Ea R,c R"a
31-177Ric Rzb R4b Ea R,c R"a
31-178R1a Rzc R4b Ea R,c R"a
31-179Rlb Rzc R4b Ea R,c R,ra
31-180Roc Rzc R4b Ea R,c R"a
31-181Rya Rza R4c Ea R,c R"a
31-182Rlb Rza R4c Ea R,c R"a
31-183R~ Rza R4c Ea R,c R,ra
31-184Rya Rzb R4c Ea R,c R"a
31-185R'b Rzb R'~ Ea R'c R"a
31-186Rlc Rzb R4c Ea R,c R"a
31-187Ria Rzc R4c Ea R,c R"a
31-188Rib Rzc R4c Ea R,c R,ra
31-189R~ Rzc R4c Ea R,c R"a
31-190Rya Rza R4a Eb R,c R"a
31-191Rib Rza R4a Eb R,c R"a
31-192Rlc Rza R4a Eb R,c R"a
31-193Rla R2b R4a Eb Rrc R"a
31-194Rib Rzb R4a Eb R,c R"a
31-195RIc Rzb R'~a Eb R'c R,ra
52
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-196Ria R2c R4a E,b R,c R"a
31-197Rib R2c R4a E,b R,c R"a
31-198Ric R2c R4a Eb R,c R"a
31-199Ria R2a R4b Eb R,c R"a
31-200Rib R2a R4b Eb R,c R"a
31-201Ric Rza R'~b Eb R~c Rna
31-202Ria R2b R4b E,b R,c R"a
31-203Rib R2b R4b E,b R,c R"a
31-204Ric Rzb R4b E,b R,c R"a
31-205Ria R2c R4b Eb R,c R"a
31-206Rib R2c R4b Eb R,c R"a
31-207Ric R2c R4b ~,b R,c R"a
31-208Ria R2a R4c E,b R,c R"a
31-209Rib R2a R4c Eb R,c R"a
31-210Ric R2a R4c E,b R,c R"a
31-211Ria Rzb Rac Eb R,c R"a
31-2I2Rib Rzb R4c Eb R,c R"a
31-213Ric Rzb R4c Eb R,c R"a
31-214Ria Rzc R4c Eb R'c R"a
31-215Rib Rzc R4c Eb R,c R"a
31-216Ric R2c R'ic Eb R'c R"a
31-217Ria R2a R4a E,c R,c R"a
31-218Rib R2a R4a Ec R,c R"a
31-219Ric R2a R4a Ec R,c R"a
31-220Ria R2b R4a E,c R,c R"a
31-221Rib R2b R4a Ec R,c R"a
31-222Ric R2b R4a E,c R,c R"a
31-223Ria R2c R4a Ec R,c R"a
31-224Rib R2c R4a E,c R,c R"a
31-225Ric R2c R4a Ec R,c R"a
31-226Ria R2a R4b E,c R,c R"a
31-227Rib R2a R4b E,c R,c R"a
31-228Ric R2a R4b Ec R,c R"a
31-229Ria Rzb R4b ~-,c R,o R"a
31-230Rib R2b R4b E,c R,c R"a
31-231Ric R2b R4b Ec R,c R"a
.
31-232Ria R2o Rib Ec R,c R"a
31-233Rib Rzc R4b Ec R,c R"a
31-234Ric R2c R4b E,c R,c R"a
31-235Ria Rza R4c E,c R,c R"a
31-236Rib R2a R4c Ec R,c R"a
31-237Ric Rza R4c E,c R,c R"a
31-238Ria R2b R4c $ R,c R"a
31-239Rib R2b R4c ~-,c Rrc R"a
31-240Ric R2b R4c E,c R,c R"a
31-241Ria R2c R4c E,c R,c R"a
31-242Rib R2c R4c Ec R,c R"a
53
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-243Ric Rzc R4c E R~ R~~a
31-244Ria R2a R4a Ea R,a R"b
31-245Rib Rza R4a Ea R,a R"b
31-246Ric Rza R4a Ea R,a R"b
31-247Ria R2b R4a Ea R,a R"b
31-248Rib R2b R4a Ea R,a R"b
31-249RI R2b R4a Ea Rra R"b
31-250Ria R2c R4a Ea R,a R"b
31-251Rib R2c R4a Ea R~a R"b
31-252Ric R2c R4a Ea R,a R"b
31-253Ria R2a R4b Ea R,a R"b
31-254Rib R2a R4b Ea R,a R"b
31-255Ric Rza R4b Ea R,a R"b
31-256Ria R2b R4b Ea R,a R"b
31-257Rib R2b R4b Ea R,a R"b
31-258Ric R2b R4b Ea R~a R"b
31-259Ria Rzc R4b Ea R,a R"b
31-260Rib Rzc R4b Ea R,a R"b
31-261Ric R2c R4b Ea R,a R"b
31-262Ria Rza R4c Ea R,a R"b
31-263Rib Rza R4c Ea R,a R"b
31-264Ric R2a R4c Ea R,a R"b
31-265Ria Rzb R4c Ea R,a R"b
31-266Rib R2b R4c Ea R,a R"b
31-267Ric Rzb R4c Ea R,a R"b
31-268Ria Rzc R4c Ea R,a R"b
31-269Rib Rzc R4c Ea R,a R"b
31-270RI R2c R4c Ea R,a R"b
31-271Ri R2a R4a Eb R,a R"b
a
31-272Rib R2a R4a Eb R,a R"b
31-273Ric R2a R4a Eb R,a R"b
31-274RI Rzb R4a Eb R,a R"b
a
31-275Rib Rzb R4a Eb R,a R"b
31-276RI Rzb R4a Eb R,a R"b
c
31-277RI R2c R4a Eb R,a R"b
a
31-278Rib R2c R4a Eb R,a R"b
31-279Ric R2c R4a E,b R,a R"b
31-280Ria R2a R4b Eb R,a R"b
31-281Rib Rza R4b Eb R,a R"b
31-282Ric R2a R4b Eb R,a R"b
31-283Ria R2b R4b Eb R,a R"b
31-284Rib R2b R4b Eb R,a R"b
31-285Ric R2b R4b Eb R,a R"b
31-286Ria R2c R4b Eb R,a R"b
31-287Rib Rzc Rab Eb R,a R"b
31-288Ric R2c R4b E,b R,a R"b
31-289Ria R2a R4c Eb R,a R"b
54
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-290Rib RZa R4c Eb R,a Rrrb
31-291RI Rza R4c Eb R,a R"b
c
31-292RI R2b R4c Eb R,a R"b
a
31-293Rib R2b R4c Eb R,a R,rb
31-294Ri RZb R4c Eb R,a R,rb
c
31-295RI RZc R4c Eb R,a Rr,b
a
31-296Rib R2c R4c Eb R,a R"b
31-297Ric RZc Rac Eb R,a R"b
31-298Ria R2a R4a Ec R,a R"b
31-299Rib Rza R4a Ec R,a R"b
31-300RI RZa Ra Ec R,a Rrrb
31-301Ri R2b R4a Ec R,a Rrrb
a
31-302Rib R2b R4a Ec R,a R"b
31-303RI R2b R4a Ec R,a R"b
31-304Ria R2c R4a Ec R,a R,rb
31-305Rib RZc R'~a Ec R,a Rr,b
31-306Ric R2c R4a Ec R,a R"b
31-307RI R2a R4b Ec R,a R"b
a
31-308Rib R2a R4b Ec R,a R"b
31-309RI R2a Rib Ec R,a Rnb
31-310RI RZb R4b Ec R,a R,rb
a
31-311RIb R'-b R4b Ec R,a R"b
31-312Ric RZb R4b Ec R,a R"b
31-313Ria RZc R4b Ec R,a R"b
31-314Rib R''c R4b E,c R,a R"b
31-315Ric R2c R4b Ec R,a R"b
31-316Ria R2a R4c Ec R,a R"b
31-317Rib R'a R4c Ec R,a R"b
31-318Ric R2a R4c Ec R,a R"b
31-319Ri R2b R4c Ec Rra R"b
a
31-320Rib R''b R'~c Ec R,a R"b
31-321Ric RZb R4c Ec Rra R,rb
31-322RI RZc R4c Ec R,a Rr,b
a
31-323Rib RZc R4c Ec R'a R"b
31-324Ric R2c R4c Ec R,a R"b
31-325RI R2a R4a Ea R,b R"b
a
31-326Rib R2a Raa Ea R,b R"b
31-327Ric R2a R4a Ea R,b R"b
31-328Ria R2b R4a Ea R,b R"b
31-329Rib R2b R4a Ea R,b R"b
31-330Ric R2b R4a Ea R,b R"b
31-331Ria R''c R4a Ea R,b R"b
31-332Rib R2c Raa Ea Rrb R"b
31-333Ric R2c R4a Ea R,b Rr,b
31-334Ria R2a R4b Ea R,b R,rb
31-335Rib R''a R4b Ea Rrb R"b
31-336Ric R''a R4b Ea R,b R"b
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-337Rya R2b R4b Ea R,b Rr,b
31-338Rib R2b R4b R,a R,b R,rb
31-339Rlc Rzb R4b E,a R,b R"b
31-340Rya Rzc R4b E,a R,b R,rb
31-341Rib Rzc R4b E,a R,b R,rb
31-342Roc R2c R4b E,a R,b Rrrb
31-343Rla R2a R4c E,a R,b Rrrb
31-344Rlb R2a R4c Ea R,b Rrrb
31-345Roc Rza R4c Ea R,b R"b
31-346Rla R2b R4c Ea R,b R"b
31-347Rib Rzb R4c E,a R,b R,rb
31-348R~ R2b R4c E,a R,b R"b
31-349Ria R2c R4c Ea R,b R,rb
31-350Rib Rzc R4c $a R,b R"b
31-351Rlc Rzc R4c E,a R,b R"b
31-352Rla Rza R4a E,b R,b Rr,b
31-353Rib R2a R4a E,b R,b R"b
31-354Rlc R2a R4a E,b R,b R"b
31-355Rla Rzb R4a E,b Rrb R"b
31-356Rib R2b R4a Eb R,b R"b
31-357R~ R2b R4a Eb R,b R"b
31-358Rla R2c R4a Eb R,b R"b
31-359Rlb R2c R4a $b R,b R"b
31-360Rlc R2c R4a $b R,b R"b
31-361Rla R2a R4b E,b R,b R"b
31-362Rlb Rza Rdb Eb Rrb R"b
31-363Rlc R2a R4b E,b Rrb R"b
31-364Rla Rzb R4b Eb R,b R"b
31-365Rlb Rzb Rab Eb R,b R"b
31-366Rlc Rzb R4b Eb R'b R"b
31-367Rya R2c R4b Eb R,b R"b
31-368Rlb R2c R4b Eb R,b R"b
31-369Rlc R2c R4b Eb R,b R"b
31-370Rya R2a R4c Eb R,b R"b
31-371Rlb R2a R4 Eb R,b R"b
31-372Rlc R2a Rdc Eb R,b R"b
31-373Rya R2b R4c Eb R,b R"b
31-374RIb Rzb Rac Eb R,b R"b
31-375R' R2b Rac Eb R,b R"b
31-376Rya Rzc R4c Eb R,b R"b
31-377Rlb Rzc R4c $b Rrb R"b
31-378Rlc R2c R4c Eb R,b R"b
31-379Rya R2a R4a Ec R,b R"b
31-380Rlb R2a R4a E,c R,b R"b
31-381Rlc R2a R4a E,c Rrb R"b
31-382R'a Rzb R4a E R'b R"b
31-383Rib R2b R4a E,c R,b R"b
56
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-384R~ R2b R4a Ec R,b Rrrb
31-385RIa R2c R4a Ec R,b R,rb
31-386Rib R2c R4a Ec R,b R,rb
31-387Rlc R2c R4a Ec R,b R"b
31-388Rya R2a R4b Ec R,b R"b
31-389Rlb R2a R4b Ec Rrb R"b
31-390Rlc R2a R4b Ec R,b Rr,b
31-391Rla R2b R4b Ec Rrb Rrrb
31-392Rlb Rzb R4b Ec R,b R"b
31-393Rlc R2b R4b Ec R,b R,rb
31-394Rla R2c R4b Ec R,b R"b
31-395Rlb R2c Rib Ec R,b Rrrb
31-396Rlc R2c R4b Ec R,b R"b
31-397Rya R2a R4c Ec R,b R"b
31-398Rib R2a Rdc Ec R,b R"b
31-399Rtc R2a R4c Ec Rrb Rr,b
31-400Rla R2b R4c Ec R,b Rr,b
31-401Rlb R2b R4c E R~b R,rb
31-402Rlc R2b R4c Ec R,b R"b
31-403Rla R2c R4c Ec R,b R"b
31-404Rib R2c R4c Ec R,b R"rb
31-405RIc R2c R4c E R'b R"b
31-406Rla R2a R4a Ea R,c Rr,b
31-407Rlb R2a R4a Ea R,c R"b
31-408Rlc R2a R4a Ea Rrc R"b
31-409Rya R2b R4a Ea R,c R"b
31-410Rlb R2b R4a Ea R,c R"b
31-411R~ R2b R4a Ea R,c Rrrb
31-412Rla R2c R4a Ea Rrc R"b
31-413RIb R2c R4a Ea R,c R"b
31-414Rlc Rzc R4a Ea Rrc Rr,b
31-415Rya R2a R4b Ea R,c Rr,b
31-416Rib R2a R4b Ea R,c R"b
31-417RIc R2a R4b Ea Rrc Rr,b
31-418Rya R2b R4b Ea R,c R"b
31-419Rlb R2b R4b Ea R,c R"b
31-420Roc R2b R4b Ea R,c R"b
31-421Rya R2c R4b Ea R,c R"b
31-422RIb R2c R4b Ea R,c R"b
31-423R~ R2c R4b Ea R,c Rrrb
31-424Rla R2a R4c Ea R,c Rr,b
31-425Rib R2a R4c Ea R,c Rrrb
31-426RIc R2a R4c Ea Rrc R"b
31-427Rla R2b R4c Ea R,c R"b
31-428Rib R2b R4c Ea R,c R"b
31-429Rlc R2b R4c Ea Rrc R"b
31-430Rla R2c R4c Ea R,c R"b
57
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-431Rib Rzc R4c Ea R,c R,rb
31-432Ric Rzc R'ic Ea R'c R,rb
31-433Ria Rza R4a Eb R,c R"b
31-434Rib Rza Rya Eb R'c R"b
31-435Ric Rza R4a Eb R,c Rr,b
31-436Ria Rzb R4a Eb R,c R"b
31-437Rib Rzb R4a Eb R,c R"b
31-438Ric Rzb R4a Eb Rrc R"b
31-439Ria Rzc R4a Eb R,c R"b
31-440Rib Rzc R4a Eb R,c R"b
31-441Ric Rzc R'la Eb R,c R"b
31-442Ria Rza R4b Eb R,c R"b
31-443Rib Rza R4b Eb Rrc R"b
31-444Ric Rza R4b Eb R,c R"b
31-445Ria Rzb R4b Eb R,c R"b
31-446Rib Rzb Rab Eb R,c R"b
31-447Ric Rzb Rab Eb R,c R"b
31-448Ria Rzc R4b Eb R,o R"b
31-449Rib Rzc Rab Eb R,c R"b
31-450Ric Rzc R4b Eb R'c R"b
31-451Ria Rza R4c Eb R,o R"b
31-452Rib Rza R4c Eb R,c Rr,b
31-453~ Ric Rza R4 E,b Rrc R"b
31-454Ria Rzb R4c Eb R,c R,rb
31-455Rib Rzb R4c Eb R'c R"b
31-456Ric Rzb R4c Eb R,c R"b
31-457Ria Rz R4c Eb Rrc R"b
31-458Rib Rzc Rac Eb R,c R"b
31-459Ric Rzc R4c Eb R' R"b
31-460Ria Rza R4a Ec R,c R"b
31-461Rib Rza R4a Ec R,c R"b
31-462Ric Rza R4a Ec Rrc R"b
31-463Ria Rzb R4a Ec R,c R"b
31-464Rib Rzb R4a Ec R,c R"b
31-465Ric Rzb R4a Ec R,c R"b
31-466Ria Rzc R4a Ec R,c R"b
31-467Rib Rz R4a Ec R,c R"b
31-468Ric Rzc R4a Ec R,c R"b
31-469Ria Rza R4b Ec R,c Rr,b
31-470Rib Rza R4b Ec R,c R"b
31-471Ric Rza R4b Ec R,c R,rb
31-472Ria Rzb R4b Eo R,c Rr,b
31-473Rib Rzb Rab Ec R'c R"b
31-474Ric Rzb R4b Ec R, R"b
31-475Ria Rzc R4b Ec R,c Rr,b
31-476Rib Rzc R4b Ec R,c R"b
31-477Ric Rzc R4b Ec R,c R"b
58
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-478R'a Rza R4c Ec R,c Rrrb
31-479R"' Rza R4c Ec R,c R"b
31-480Roc RZa R4~ Ec R,o R"b
31-481R'a Rzb R4o Ec R,c R"b
31-482R'b Rzb R4c Ec R,c Rrrb
31-483R'c Rzb Rdc Ec R,c R"b
31-484R'a Rzc Rdc Ec R,c R"b
31-485R'b Rzc Rac Ec R,~ R"b
31-486R'c Rzc Rac Ec R,c R"b
31-487R'a Rza R4a Ea R,a R"c
31-488R'b Rza R4a Ea R,a R"c
31-489Ric Rza R4a Ea Rra R"c
31-490Rya Rzb R4a Ea R,a R"c
31-491Rib R''b R4a Ea R'a R"c
31-492R' R2b R4a E, R, R"c
c a a
31-493R'a Rzc R4a Ea R,a R"c
31-494Rib Rzc R4a E,a R,a R"c
31-495R~ Rzc R4a Ea R'a R"c
31-496Rya Rza R4b Ea R,a R"c
31-497R'b R2a R4b Ea R,a Rr,c
31-498R'c Rza R4b Ea R,a R"c
31-499R'a Rzb R4b Ea Rra R"c
31-500R'b RZb R4b Ea R,a R"c
31-501R'c RZb R4b Ea R,a Rrrc
31-502R'a Rzc R4b Ea R,a R"c
31-503Rlb Rzc R''b Ea R,a R"c
31-504RIc Rzc R4b Ea R,a R"c
31-505R'a Rza R4c Ea Rra R"
31-506R"' Rza R4c Ea R,a R"c
31-507R'c Rza R4c Ea R,a R"c
31-508Rya Rzb R4c Ea R'a R"c
31-509R'b Rzb R4c Ea R,a R"c
31-510Rlc Rzb R4c Ea R,a R"c
31-511R'a Rzc R4c Ea R,a R"c
31-512R'b Rzc R4c Ea R,a R"c
31-513R'c Rzc R4c Ea R,a R"c
31-514R'a Rza R4a Eb R,a R,rc
31-515R"' Rza R4a Eb R,a R"c
31-516R~ Rza R4a Eb R,a R"c
31-517R'a Rzb R4a Eb R,a R"c
31-S R'b Rzb R4a Eb Rra R"c
I
8
31-519R'c R2b R4a Eb R,a R"c
31-520R'a Rzc R4a Eb R,a R"c
31-521R'b Rzc R4a Eb R,a R,rc
31-522R'c Rzc R4a Eb R,a R"c
31-523R'a Rza R4b Eb R,a R"c
31-524R'b Rza R4b Eb Rra R"
59
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-525Ric Rza R4b E,b R,a R"c
31-526Ria Rzi' R4b Eb R,a R"c
31-527Rib Rzb R4b Eb R,a R"c
31-528Ric R2b R4b Eb R,a R"c
31-529Ria Rzc R4b Eb R,a R"c
31-530Rib Rzc R4b Eb R,a R"c
31-53IRic Rzc R4b E,b R,a R"
31-532Ria Rza R4c Eb R,a R"c
31-533Rib Rza R4c Eb R,a R"c
31-534Ric Rza R4c Eb R,a R"c
31-535Ria Rzb R4c Eb R,a R"c
31-536Rib R2b R4c Eb R,a R"c
31-537Ric Rzb R4c Eb R,a R"c
31-538Ria Rzc R4c Eb R,a R"c
31-539Rib Rzc R4c ~,b R,a R"c
31-540Ric Rzc R4c E,b R,a R"c
31-541Ria Rza R4a E,c R,a R"c
31-542Rib Rza R4a Ec R,a R"c
31-543Ric Rza Rda Ec R,a R"c
31-544Ria Rzb R4a Ec R,a R"c
31-545Rib Rzb Rya Ec R,a R"c
31-546Ric Rzb R4a Ec R,a R"c
31-547Ria R2c R4a E,c R,a R"c
31-548Rib Rzc R4a Ec R,a R"
31-549Ric R2c R4a Ec R,a R"c
31-550Ria Rza Rib E,c R,a R"c
31-551Rib Rza R4b Ec R,a R"c
31-552Ric R2a R4b Ec R,a R"c
31-553Ria R2b R4b Eo R,a R"c
31-554Rib R2b R4b Ec R,a R"c
31-555Ric R2b R4b Ec R,a R"c
31-556Ria Rzc R4b E,c R,a R"c
31-557Rib Rzc R4b Ec R,a R"c
31-558Ric Rzc R4b E,c R,a R"c
31-559Ria R2a R4c E,c R,a R"c
31-560Rib Rza R4c B R,a R"c
31-561Ric R2a R4c E,c R,a R"c
31-562Ria Rzb R4c Ec R,a R"c
31-563Rib Rzb R4o E,c R,a R"c
31-564Ric Rzb Rac Ec R,a R"c
31-565Ria Rzc R4c Ec R,a R"c
31-566Rib Rzc R4c E,c R,a R"c
31-567Ric Rzc R4c Ec R,a R"c
31-568Ria Rza R4a Ea R,b R"c
31-569Rib Rza R4a Ea R,b R"c
31-570Ric Rza R4a Ea R,b R"c
31-571Ria Rzb R4a Ea R,b R"c
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-572Rlb Rzb R4a E,a R,b R"c
31-573RIc Rzb R4a Ea R,b R"c
31-574Ria R2c R4a Ea R,b R"c
31-575Rlb R2c R4a E,a R,b R"c
31-576Rlc RZc R4a Ea R,b R,rc
31-577RIa R2a R4b E,a R,b R"c
31-578Rlb R2a R4b Ea R,b R"c
31-579RIc Rza R4b Ea R,b R"c
31-580Rla R2b R4b Ea R,b R"c
31-581Rib R2b R4b E,a R,b R,rc
31-582Rlc RZb R4b Ea R,b R,rc
31-583Rla R2c R4b E,a R,b Rrrc
31-584Rlb RZc Rab Ea R,b Rr,c
31-585Rlc R2c R4b Ea R,b R,rc
31-586Rla R?a R4c Ea R,b R"c
31-587Rib RZa R4c Ea R,b R"c
31-588Rlc Rza Rac Ea Rrb Rr,c
31-589Rla Rzb R4c Ea R,b R"c
31-590Rlb Rzb R4c Ea R,b R"
31-591Rlc RZb R4c Ea R,b R"c
31-592Rya Rzc R4c E,a R,b Rr,c
31-593Rlb R2c R4c Ea R,b R"c
31-594Roc RZc R4c E,a R,b R"c
3I-595Rla R2a R4a Eb R,b R"c
31-596Rlb R2a R4a E,b Rrb R,rc
31-597Rlc R2a R4a E,b R,b Rrrc
31-598Ria R2b R4a Eb R,b R"c
31-599Rib R'b R4a E,b R,b Rr,c
31-600Rlc R2b R4a $b Rrb R"c
31-601Rla R2c R4a Eb R,b R"c
31-602Rlb R2c R4a E,b R,b R"c
31-603Roc R2c R4a Eb R'b R"
31-604Rya RZa R4b Eb R,b R"c
31-605Rlb R2a R4b E,b R,b R"c
31-606Roc R2a R4b Eb R,b R"c
31-607R'a R2b R4b E,b R,b Rrrc
31-608Rlb Rzb R4b Eb R,b R"
31-609Roc RZb R4b Eb R,b R"c
31-610Rya R2c R4b Eb Rrb R,rc
31-611Rib Rzc R4b Eb R,b R"c
31-612Rlc Rzc R4b Eb R,b Rr,c
31-613Rja R2a R4c Eb R,b R"c
31-614Rjb RZa R4c Eb Rrb R"c
31-615Rlc R2a R4c E,b R,b R"c
31-616RIa R2b R4c E,b R,b R"c
31-617Rib RZb R4c Eb R,b Rr,c
31-618Roc RZb R4c Eb R,b R"c
61
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-619R'a Rzc Rdc Eb R,b R"c
31-620R'b Rzc R4c Eb Rrb R"c
31-621Ric Rzc R4c Eb R'b R"
31-622R'a Rza R4a Ec R,b R,rc
31-623R'b Rza R4a Ec R,b R"c
31-624RIc Rza R4a Ec R,b R"c
3I-625R'a Rzb R4a Ec R,b R"c
31-626R'b R2b R4a Ec Rrb R"c
31-627Rlc Rzb R4a Ec R,b Rr,c
31-628R'a Rzc R4a Ec R,b Rr,c
31-629R'b Rzc R4a E R,b R"c
31-630RIc Rzc R4a Ec R,b Rr,c
31-631R'a Rza R4b Ec R,b R"c
31-632R'b Rza Rab Ec R,b R,rc
31-633R'c Rza R4b Ec R,b R"c
31-634R'a Rzb R4b Ec R,b R,rc
31-635R'b Rzb R4b Ec R'b R"c
31-636RIc Rzb R4b Ec R,b Rrrc
3I-637R'a Rzc R4b Ec R,b Rr,c
31-638R'b Rzc Rab Ec Rrb R"c
31-639R'c Rzc R4b Ec R'b R"c
31-640R'a Rza R4c Ec R,b R"c
31-641R'b Rza R4c Ec R,b R"c
31-642R'c Rza R4c Ec R,b R"c
31-643R'a Rzb R'~c Ec R,b R"c
31-644Rib Rzb R4c Ec R'b R"
31-645R'c Rzb R4c Ec R'b R"
31-646Rta Rzc R4c Ec R'b R"
31-647R'b Rzc R4c Ec R'b R"c
31-648Rlc Rzc R4c E R'b R"c
31-649R'a Rza R4a Ea R,c R"c
31-650R"' Rza R4a Ea R,c R"c
31-651Ric Rza R4a Ea R,c R"c
31-652Ria Rzb R4a Ea R,c R"c
31-653Rib Rzb R4a Ea R,c R"c
31-654R'c Rzb R4a Ea R' Rr'c
31-655R'a Rzc R4a Ea R'c R"c
31-656Rib Rzc R4a Ea R'c Rrrc
31-657R'c Rzc R4a Ea Rrc R"
31-658Ria Rza R4b Ea R,c R"c
31-659R'b Rza R4b Ea R,c R"c
31-660R' Rza R4b Ea R,c R"c
31-661R'a Rzb R4b Ea R' R"c
31-662R'b Rzb R4b Ea Rrc Rrrc
31-663R'c Rzb R4b Ea R,c R"c
31-664R'a Rzc R4b Ea R,c R"c
31-665R'b Rzc R4b Ea Rrc R"c
62
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-666R'c Rzc R4b Ea R,c R"c
31-667R'a Rza R4c Ea R,c R"c
31-668R'b Rza R''c Ea R'c R"c
31-669R'c Rza R4c Ea R,c R"c
31-670R'a Rzb R4c Ea R,c R"c
31-671R'b Rzb R4o Ea R,c R"c
31-672R'c Rzb R4c Ea R,c R"c
31-673R'a Rzc R4c Ea R,c R"c
31-674R'b R2c R4c Ea R,c R"c
31-675R'c Rzc R4c Ea R,c R"c
31-676R'a Rza R4a Eb R,c R"c
31-677R'b Rza R4a Eb R,c R"c
31-678R'c Rza R4a Eb R,c R"c
31-679R'a Rzb R4a Eb R,c R"c
31-680R'b Rzb R4a Eb R,c R"c
31-681R'c Rzb R4a Eb R,c R"c
31-682R'a Rzc R4a Eb R,c R"c
31-683R'b Rzc R4a Eb R,c R"c
31-684R'c Rzc R4a Eb R,c R"
31-685R'a Rza R4b E.,'' R,c R"c
31-686R"' Rza R4b Eb R,c R"c
31-687R'c Rza R4b Eb R'c R"c
31-688R'a Rzb R4b Eb R,c R"c
31-689R'b Rzb Rab Eb R,c R"c
31-690R'c Rzb Rab Eb R' R"c
31-691R'a Rzc R4b Eb R'c Rr,c
31-692R'b Rzc R4b Eb R,c R"c
31-693R'c Rzc R4b Eb R'c R"
31-694R'a Rza R4c Eb R,c R"c
31-695R'b Rza R4c Eb R,c R"c
31-696R'c Rza R4c Eb R'c R"
31-697R'a Rzb R4c Eb R,c R"c
31-698R'b Rzb R4c Eb R,c R"c
31-699R' Rzb R4c Eb R' R"c
31-700R'a Rzc R4c Eb R,c R"c
31-701R'b Rzc R4c Eb R,c R"
31-702R'c Rzc R4c Eb R,c R"c
31-703R'a Rza R4a Ec R,c R"c
31-704R'b Rza R4a Eo R,o R"c
31-705R'c Rza R4a Ec R,c R"c
31-706R'a Rzb R4a Ec R,c R"c
31-707R'b Rzb R4a Ec R,c R"c
31-708R'c Rzb R4a EC R,' R"c
31-709R'a Rzc R4a Ec R,c R"c
31-710R"' Rzo R4a Ec R,e R"c
31-711R'c Rzc R4a Ec R,c R"c
31-712R'a Rza R4b Ec R,c R"c
63
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
31-713R'b Rza R4b Ec R,c R"c
31-714R'c Rza R4b Ec R,c R"c
31-715R'a Rzb R4b Ec R,c R"c
31-716R'b Rib Rab Ec R'c R"
31-717R'c Rz'' R4b Ec R, R"
31-718R'a Rzc R4b Ec R,c R"c
31-719Rlb Rzc R4b Ec R'c R"c
31-720RIc Rzc R4b Ec R'c R"
31-721R'a Rza R4c E,c R,c R"c
31-722Rlb Rza R4c Ec R'c R"c
31-723Rlc Rza Rac Ec R,c Rac
31-724Rla Rzb R''c Ec R,c R"c
31-725R'b Rzb R'~ E R'c R"c
31-726R'c Rzb R4c Ec R,c R"c
31-727Rla Rzc R4c Ec R,c R"c
31-728R'b Rzc R4c Ec R'c R"c
31-729Rlc Rzc R4c E R'c R"c
where all symbols are as defined above.
In one aspect of formula (31) of the present invention, Rl and Rz
independently are
hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, or
an optionally substituted amino group, an alkyl group, a cycloalkyl group, an
alkoxy group, a
cycloalkoxy group, an allcenyl group, a cycloalkenyl group, an alkoxyalkyl
group, an
alkenyloxy group, or a cycloalkenyloxy group; R4 is hydrogen, a hydroxy group,
a halogen, a
vitro group, or an optionally substituted amino group, an alkyl group, a
cycloalkyl group, an
alkoxy group, a cycloallcoxy group, an alkenyl group, a cycloalkenyl group, an
alkoxyalkyl
group, an alkenyloxy group, a cycloalkenyloxy group; an acyl group, an acyloxy
group, an
aryl group, an aryloxy group, aroyl group or an aroyloxy group, an aralkyl
group, an
aralkenyl group, an aralkynyl group, an aralkoxy group, a heterocyclyl group,
a
heterocyclenyl group, a heteroaryl group, a heteroarallcyl group, a
heteroaryloxy group, or a
heteroaralkoxy group; R' and R" independently are hydrogen, a halogen, a vitro
group, an
amino group, a mono- or di- substituted amino group, a hydroxy group, an
alkoxy group, a
carboxy group, a cyano group, an oxo(O=) group, a thio(S=) group, an allcyl
group, a
cycloalkyl group, an alkoxy group, a haloallcoxy group, a cycloalkyl group, an
aryl group, a
benzyloxy group, an acyl group, an acyloxy group, an aroyl group, an
allcoxycarbonyl group,
an aryloxycarbonyl group, a heteroaryl group, a heterocyclyl group, or an
aralkyl group; and
2o all other symbols are as defined above in connection with formula (I).
64
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In another aspect of formula (31) of the present invention, RI is hydrogen, a
hydroxy
group, a halogen, a vitro group, a carboxy group, a carbamoyl group, or an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an alkoxy group;
R2 is
hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, or
an optionally substituted amino group, an alkyl group, a cycloalkyl group, an
allcoxy group;
R4 is a substituted or unsubstituted aryl group, R' is hydrogen, a halogen, or
an alkyl group;
and R" is hydrogen, a halogen, or an alkyl group; and all other symbols are as
defined above
in connection with formula (I).
In yet another aspect of formula (31) of the present= invention, R' is
hydrogen or an
allcoxy group; RZ is hydrogen or an alkoxy group; R4 is a substituted or
unsubstituted aryl
group, R' is hydrogen, a halogen, or an alkyl group; R" is hydrogen, a
halogen, or an alkyl
group; and E is O, S, or NH.
In still another aspect of formula (31) of the present invention, RI is -H or -
OCH3; R2
is -H or -OCH3; R4 is a substituted aryl group, R' is -H, -Cl, -Br, or -CH3;
and R" is -H, -Gl, -
Br, or -CH3; and E is O, S, or NH.
The present invention further contemplates various compounds of general
formula
(III) having the general formula:
O R4
R.. O
O~ E ~~ S
O ~/ / ~1 H
R~ Me
(32),
where all symbols are as defined above in connection with formula (I).
2o According to various aspects the present invention, R4, R', and R" of
formula (32) are
selected to produce various compounds of formula (32-1) through formula (32-
27) as
follows:
Formula R'' R' R"
32-1 R4a R'a R"a
32-2 Rb R'a R"~
32-3 R4~ R'a R"a
32-4 R4a R~ Rna
32-5 R4b R'b Ri,a
32-6 R4~ R~b Rna
32-7 R4a R' R"a
32-8 R4b R'~ R"~
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
32-9 R'~ R'~ R"a
32-10 R4a R'a Rr.b
32-11 R4b R'a R"b
32-12 R4~ R'a R'b
32-13 R4a R'b R'b
32-14 R4b R'b R"b
32-15 R4c R'b Rr'b
32-16 Ra R'c R"b
32-17 R4b R'c Rr'b
32-18 R4~ R'c R"b
32-19 R4a Rra Rr.c
32-20 R4b R~a Rr.c
32-21 R4 R'a R"c
32-22 R4a R'b R"c
32-23 R4b R'b R"c
32-24 R4 R'b R"c
32-25 R4a R' Rr.c
32-26 R4b R'c R.c
32-27 R4~ R' R"
where all symbols are as defined above.
In one aspect of the present invention, R~ is hydrogen, a hydroxy group, a
halogen, a
vitro group, an optionally substituted amino group, an alkyl group, a
cycloalkyl group, an
alkoxy group, or a cycloalkoxy group; R' is hydrogen, a halogen, a vitro
group, an amino
group, a mono- or di- substituted amino group, a hydroxy group, an alkoxy
group, a carboxy
group, a cyano group, an oxo(O=) group, a thio(S=) group, an alkyl group, a
cycloalkyl
group, an alkoxy group, a haloalkoxy group, a cycloalkyl group, an aryl group,
or a
benzyloxy group; R" is hydrogen, a halogen, a vitro group, an amino group, a
mono- or di-
l0 substituted amino group, a hydroxy group, an alkoxy group, a carboxy group,
a cyano group,
an oxo(O=) group, a thio(S=) group, an alkyl group, a cycloalkyl group, an
alkoxy group, a
haloalkoxy group, a cycloalkyl group, an aryl group, or a benzyloxy group; and
all other
symbols are as defined above in connection with formula (I).
In another aspect of the present invention, Rø is an alkenyl group, a
cycloalkenyl
group, an alkoxyalkyl group, an allcenyloxy group, a cycloalkenyloxy group, an
acyl group,
an acyloxy group, an aryl group, an aryloxy group, an ar~yl group, an aroyloxy
group, an
aralkyl group, an aralkenyl group, an aralkynyl group, or an aralkoxy group;
R' is hydrogen,
a halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
66
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an alkyl group, a cycloalkyl group, an alkoxy group, a haloalkoxy
group, a cycloalkyl
group, an aryl group, or a benzyloxy group; R" is hydrogen, a halogen, a vitro
group, an
amino group, a mono- or di- substituted amino group, a hydroxy group, an
alkoxy group, a
carboxy group, a cyano group, an oxo(O=) group, a thio(S=) group, an alkyl
group, a
cycloalkyl group, an allcoxy group, a haloalkoxy group, a cycloalkyl group, an
aryl group, or
a benzyloxy group; and all other symbols are as defined above in connection
with formula
(I).
In one aspect of the present invention, E is O or -NR; Rø is ~ ~ / optionally
N S
substituted with an alkyl group or an alkoxy group, ~ ~ \ , or '~ ~ ~ ; and R'
and R?
to are defined as above. Examples of such compounds include, but are not
limited to:
OMe O\LNH
W O I Ra R.. O \ O \ I Sl O
F~~~'~O~O ( ~ S NH ~ I OMe I
O ~/ / F O~ I \
R~ Me O . O /
OMe OCH3 O
/ I a ~NH
O \ I S O
O ~~// ~ ~
F O ~ I / /S~ H F
O , O \
/ OCH3
S
I
O \ H O \ O O
F I / O I O~N I / / ~ H F I / O I O~\r0 I \ S~ H
/ s
0 0
> ;
O s F
S ~ ~-NH O \ I
O w S w a O
\ ~O I I
I I F / o~°O \ sue(
F / O~O \ O ( / / NH
O I / . o
67
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
N g
O ~ I ~ O ~
O ~ ~ H O
~O W / ~N \
O O I ~ /S NH F O O ~ / / NH
o ;and
The present invention still further contemplates various compounds having the
general formula:
O Ra
~~O
I O~O ~ S
O I , / NH
O
(33),
where R4 is as defined above in connection with formula (I).
In one aspect of formula (33) of the present invention, R4 is hydrogen, a
hydroxy
group, a halogen, a vitro group, or an optionally substituted amino group, an
alkyl group, a
cycloallcyl group, an alkoxy group, a cycloalkoxy group, an alkenyl group, a
cycloalkenyl
group, an alkoxyalkyl group, an alkenyloxy group, or a cycloalkenyloxy group.
1o In another aspect of formula (33) of the present invention, R4 is an acyl
group, an
acyloxy group, an aryl group, an aryloxy group, amyl group or an aroyloxy
group, an aralkyl
group, an aralkenyl group, an aralkynyl group, an aralkoxy group, a
heterocyclyl group, a
heterocyclenyl group, a heteroaryl group, a heteroaralkyl group, a
heteroaryloxy group, or a
heteroaralkoxy group.
In yet another aspect of formula (33) of the present invention, R4 is an
alkoxycarbonyl group, an aryloxycarbonyl group, an aralkoxycarbonyl group, a
heteroarylcarbonyl group, an alkylsulfonyl group, an arylsulfonyl group, a
heteroarylsulfonyl
group, an alkylsulfinyl group, an arylsulfinyl group, or an aralkylsu~finyl
group, an alkylthio
group, an arylthio group, a heteroaryIthio group, an aralkylthio group, a
fused
2o heteroarylcycloalkyl group, a fused heteroarylcycloalIcenyl group, a fused
heteroarylheterocyclenyl group, carboxylic acid or a derivative thereof, or
sulfonic acid or a
derivative thereof.
68
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In still another aspect of formula (33) of the present invention, R4 is
O
SMe
O , or . Examples of such compounds include, but are not limited
to:
s
I
0 0 ~ 0 0
O~° I ~ S~ H I s I O~° I ~ S~ ~
o s i o s i
o ; o ; arid
The present invention further still contemplates various compounds having the
general formula:
(34),
where all symbols are as defined above in connection with formula (I).
where R''° and RZ~ independently are hydrogen, a halogen, a nitro
group, an amino
group, a mono- or di- substituted amino group, a hydroxy group, an allcoxy
group, a carboxy
group, a cyano group, an oxo(O=) group, a thio(S=) group, an allcyl group, a
cycloalkyl
group, an allcoxy group, a haloallcoxy group, a cycloalkyl group, an aryl
group, a benzyloxy
group, an acyl group, an acyloxy group, an aroyl group, an allcoxycarbonyl
group, an
aryloxycarbonyl group, a heteroaryl group, a heterocyclyl group, an aralkyl
group, an
alkylsulfonyl group, an allcylsulfinyl group, an arylsulfonyl group, an
arylsulfinyl group, an
alleylthio group, an arylthio group, a heteroarylthio group, an aralkylthio
group, or a
heterocyclyl sulfonyl group, which is optionally substituted with a halogen, a
hydroxyl
69
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, a vitro group, an amino group, an allcyloxy group, or any combination
thereof, and
wherein the heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or a piperzinyl group, wherein the substituent on the
heterocyclyl
group is a halogen, a vitro group, an amino group, an alkyl group, an alkoxy
group, or an aryl
group; and all other symbols are as defined above.
According to various aspects of the present invention, R, R5, R2°, Rzl,
R' and R" of
formula (34) are selected to produce various compounds of formula (34-1)
through formula
(34-729) as follows:
FormulaR RS R' R R' R"
34-1 Ra RSa R a R a R'a R"a
34-2 Rb RSa Rzoa Rz~a R,a R"a
34-3 Rc Rsa Rzoa R2~a R,a R"a
34-4 Ra Rsb R2oa R2~a R,a R"a
34-5 Rb Rsb Rzoa Rzla R,a R"a
34-6 Rc RSU R2oa Rzla R,a R"a
34-7 Ra Rsc R2oa R2la R,a R"a
34-8 Rb R5c Rzoa R2ta R,a R"a
34-9 Rc Rsc R2oa R2la R,a R"a
34-10 Ra Rsa R2ob R2la R,a R"a
34-11 Rb Rsa R2ob R2~a R,a R"a
34-12 Rc Rsa R2ob Rua R,a R"a
34-13 Ra Rsb Rzob Rz~a R,a R"a
34-14 Rb R5b R2ob R2~a R,a R"a
34-15 Rc Rsb Rzob Rzta R,a R"a
34-16 Ra RSc R2ob R2~a R,a R"a
34-17 Rb Rsc R2ob R2la R,a R"a
34-18 Rc R5c Rzob Rz~a R,a R"a
34-19 Ra Rsa Rzoc Rz~a R,a R"a
34-20 Rb Rsa R2oc R2la R,a R"a
34-21 Rc RSa Rzoc R2la R,a R"a
34-22 Ra Rsb R2oc R2la R,a R"a
34-23 Rb Rsb R2oc R2ta R,a R"a
34-24 Rc Rsb R2oc R2ta R,a R"a
34-25 Ra R5c Rzoc Rzla R'a R"a
34-26 Rb Rsc R2oc R2la R,a R"a
34-27 Rc R5c R2oc R2~a R,a R"a
34-28 Ra Rsa R2oa R2lb R,a R"a
34-29 Rb Rsa Rzoa Rz~b R,a R"a
34-30 Rc R5a R2oa R2~b R,a R"a
34-31 Ra RSb R2oa R2l R,a R"a
34-32 Rb Rsb R2oa R21 R,a R"a
34-33 Rc Rsb R2oa R2lb R,a R"a
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-34Ra RSc R2oa R2lb R,a R"a
34-35Rb Rsc R2oa R2lb R,a R"a
34-36Rc RSc Rzoa Rub R,a R"a
34-37Ra Rsa Rzob Rub R,a R"a
34-38Rb Rsa Rzob Rub R,a R"a
34-39Rc Rsa Rzob R2lb R,a R"a
34-40Ra Rsb R2ob R2lb R,a R"a
34-41Rb Rsb Rzob Rub R,a R"a
34-42Rc Rsb Rzob R2lb R,a R"a
34-43Ra Rsc R2ob Rub R,a R"a
34-44Rb RSc Rzob R2lb R,a R"a
34-45Rc R$c R2ob Rub R,a R"a
34-46Ra Rsa Rzoc Rub R,a R"a
34-47Rb Rsa R2oc R2lb R,a R"a
34-48Rc Rsa R2oc Rub R,a R"a
34-49Ra RSb R20c R2lb R,a R"a
34-SORb RSb Rzoc Rzlb R,a R"a
34-51Rc RSb R2oc R2lb R,a R"a
34-52Ra RSc R2oc R2lb R,a R"a
34-53Rb Rsc Rzoc R2lb R,a R"a
34-54Rc R5 R2oc R2lb R,a R"a
34-55Ra Rsa R2oa R2lc R,a R"a
34-56Rb RSa R2oa R2lc R,a R"a
34-57Rc R5a R2oa Ruc R,a R"a
34-58Ra R5b R2oa R2lc R,a R"a
34-59Rb Rsb Rzoa Ruc R,a R"a
34-60R RSb Rzoa Ruc R,a R"a
34-61Ra Rsc R20a Rzlc R,a R"a
34-62Rb RSc R2oa Rzlc R,a R"a
34-63Rc Rsc R2oa Rzlc R,a R"a
34-64Ra Rsa R2ob R2lc R,a R"a
34-65Rb Rsa Rzob R2lc R,a R"a
34-66Rc RSa R2ob Ruc R,a R"a
34-67Ra Rsb R2ob R2lc R,a R"a
34-68Rb Rsb R2ob R2lc R,a R"a
34-69Rc Rsb R2ob Rzlc R,a R"a
34-70Ra RSc R2ob R2lc R,a R"a
34-71Rb Rsc R2ob Ruc R,a R"a
34-72Rc Rsc Rzob Ruc R,a R"a
34-73Ra Rsa R2oc Ruc R,a R"a
34-74Rb Rsa R2oc Ruc R,a R"a
34-75Rc R5a R2oc Ruc R,a R"a
34-76Ra Rsb Rzoc Rzlc R,a R"a
34-77Rb Rsb R2oc Rzlc R,a R"a
34-78Rc Rsb Rzoc Ruc R,a R"a
34-79Ra RSc R2oc R2lc R,a R"a
34-80Rb RSc R2oc R2lc R,a R"a
71
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-81 Rc Rsc R2oc R2lc R,a R"a
34-82 Ra Rsa Rzoa R2la R,b R"a
34-83 Rb Rsa R2oa R2la R,b R"a
34-84 Rc Rsa R2oa Rzla R,b R"a
34-85 Ra Rsb R2oa R2la R,b R"a
34-86 Rb Rsb R2oa R2la R,b R"a
34-87 Rc Rsb R2oa R2la Rrb R"a
34-88 Ra Rsc R2oa R2la Rrb R"a
34-89 Rb Rsc Rzoa R2la Rrb Rr,a
34-90 R Rsc R2oa R2la R,b R"a
34-91 Ra Rsa R2ob Rua R,b R"a
34-92 Rb Rsa R2ob Rua R,b Rrra
34-93 Rc Rsa R2ob R2la R,b R"a
34-94 Ra Rsb R2ob Rua R,b R,ra
34-95 Rb Rsb R2ob Rua R,b Rr,a
34-96 Rc Rsb R2ob Rua R,b R"a
34-97 Ra Rsc R2ob Rua Rrb R"a
34-98 Rb Rsc R2ob R2la R,b R,ra
34-99 Rc Rsc R2ob Rua R,b R,ra
34-100Ra Rsa R2oc Rzla R,b R"a
34-101Rb Rsa R2oc Rua R,b R"a
34-102Rc Rsa Rzoc R2la R,b R"a
34-103Ra Rsb R2oc Ru R,b R"a
a
34-104Rb Rsb Rzoc R2l R,b R"a
a
34-105Rc Rsb R2oc Rua R,b R"a
34-106Ra Rsc R2oc Rua R,b R"a
34-107Rb Rsc R2oc Rua R,b R"a
34-108Rc Rsc Rzoc Rua R,b R"a
34-109Ra Rsa Rzoa Rub R,b R"a
34-110Rb Rsa Rzoa R2Ib R,b Rr,a
34-111Rc Rsa Rzoa Rub R,b R"a
34-112Ra Rsb R2oa Rzlb R,b R,ra
34-113Rb Rsb R20a Rub R,b R"a
34-114Rc Rsb R2oa R2lb R,b R"a
34-115Ra Rsc R2oa Rub R,b R"a
34-116Rb Rsc R2oa R2lb R,b R"a
34-117Rc Rsc R2oa R2lb Rrb Rr,a
34-lI8Ra Rsa Rzob R2lb R,b R"a
34-119Rb Rsa R2ob Rzlb R,b R"a
34-120Rc Rsa R2ob R2lb R,b R"a
34-121Ra Rsb R2ob Rub R,b Rr,a
34-122Rb Rsb Rzob Rub R,b R"a
34-123Rc Rsb R2ob Rub R,b R"a
34-124Ra Rsc R2ob Rub R,b R"a
34-125Rb Rsc R2ob Rub R,b R"a
34-126R Rsc Rzob Rzlb R,b R"a
34-127Ra Rsa R2oc R2lb R,b R"a
72
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-128Rb Rsa R2oc R2lb R,b R"a
34-129Rc RSa R2oc R2lb R,b R"a
34-130Ra RSb Rzoc Rzlb R,b R"a
34-131Rb RSb Rzoc R2lb R,b R"a
34-132Rc Rsb R2oc R2lb R,b R"a
34-133Ra Rsc R2oc Rzlb R,b R"a
34-134Rb RSc R2oc R2lb R,b R"a
34-I35Rc RSc R2oc Rzlb R,b R"a
34-136Ra Rsa R2oa R2sc R,b R"a
34-137Rb Rsa R2oa R2lc R,b R"a
34-138Rc R5a R2oa R2lc R,b R"a
34-139Ra R5b R2oa R2ic R,b R"a
34-140Rb Rsb R2oa R2lc R,b R"a
34-141R RSb Rzoa R2lc R,b R"a
34-142Ra Rsc Rzoa R2lc R,b R"a
34-143Rb Rsc R2oa R2lc R,b R"a
34-144Rc Rsc Rzoa Rzlc R,b R"a
34-145Ra Rsa Rzob Rzlc R,b R"a
34-146Rb RSa Rzob R2lc R,b R"a
34-147R Rsa Rzob R2lc R,b R"a
34-148Ra Rsb Rzob Rzlc R,b R"a
34-149Rb Rsb R2ob R2lc R,b R"a
34-150Rc RSb R2ob R2lc R,b R"a
34-151Ra Rsc R2ob R2lc R,b R"a
34-152Rb RSc R2ob R2lc R,b R"a
34-153Rc Rsc Rzob Rzlc R,b R"a
34-154Ra Rsa R2oc Rzlc R,b R"a
34-155Rb RSa Rzoc Ruc R,b R"a
34-156Rc Rsa R2oc R2lc R,b R"a
34-157Ra RSb R2oc R2lc R,b R"a
34-158Rb Rsb Rzoc R2lc R,b R"a
34-159Rc Rsb R2oc R2lc R,b R"a
34-160Ra RSc R2oc Rzlc R,b R"a
34-161Rb Rsc R2oc Rzlc R,b R"a
34-162Rc RSc R2oc R2l R,b R"a
c
34-163Ra Rsa R2oa R2la R,c R"a
34-164Rb RSa Rzoa Rzla R,c R"a
34-I65Rc RSa Rzoa R2la R,c R"a
34-166Ra Rsb R2oa Rzla R,c R"a
34-167Rb RSb Rzoa R2la R,c R"a
34-I68Rc Rsb R2oa Rua R,c R"a
34-169Ra RSc Rzoa R2la R,c R"a
34-170Rb R5c R2oa R2la R,c R"a
34-171Rc Rsc Rzoa R2la R,c R"a
34-I72Ra Rsa R2ob R2la R,c R"a
34-I73Rb RSa R2ob R2la R,c R"a
34-174Rc Rsa R2ob Rzla R,c R"a
73
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-175Ra RSb R2ob Rua R,c R"a
34-176Rb R5b R2ob Rua R,c R"a
34-177Rc R5b Rzob Rua R,c R"a
34-178Ra Rsc Rzob Rua R,c R"a
34-179Rb Rsc R2ob Rz~a R,c R"a
34-180R RSc R2ob Rzta R,c R"a
34-181Ra RSa R2oc Rzta R,c R"a
34-182Rb Rsa R2oc R2ta R,c R"a
34-183Rc Rsa R2oc Rua R,c R"a
34-184Ra R~' R2oc Rzta R,c R"a
34-185Rb RSb R2oc R2la R,c R"a
34-186Rc Rsb R2oc Rua R,c R"a
34-187Ra RSc Rzoc Rua R,c R"a
34-188Rb Rsc Rzoc Rua R,c R"a
34-189Rc Rsc Rzoc Rua R,c R"a
34-190Ra Rsa R2oa R2lb R,c R"a
34-191Rb Rsa Rzoa Rub R,c R"a
34-192Rc RSa R2oa Rub R,c R"a
34-193Ra Rsb R2oa Rub R,c R"a
34-194Rb Rsb R2oa Rub R,c R"a
34-195Rc Rsb R2oa Rub R,c R"a
34-196Ra Rsc Rzoa Rub R,c R"a
34-197Rb Rsc R2oa R2~b R,c R"a
34-198R RSc Rzoa Rub R,c R"a
34-199Ra RSa Rzob R2lb R,c R"a
34-200Rb Rsa R2ob R2tb R,c R"a
34-201Rc RSa Rzob R2~b R,c R"a
34-202Ra Rsb R2ob Rz~b R,c R"a
34-203Rb R~' R2ob Rub R,c R"a
34-204Rc RSb R2ob Rub R,c R"a
34-205Ra Rsc R2ob R2lb R,c R"a
34-206Rb Rsc Rzob R2lb R,c R"a
34-207Rc Rsc Rzob Rub R,c R"a
34-208Ra RSa R2oc Rub R,c R"a
34-209Rb Rsa R2oo Rub R,c R"a
34-210RC RSa R2oc Rub R,c R"a
34-211Ra Rsb R2oc Rub R,c R"a
34-212Rb Rsb R2oc Rzlb R,c R"a
34-213Rc RSb R2oc R2lb R,c R"a
34-214Ra Rsc R2oc Rub R,c R"a
34-215Rb Rsc R2oc Rub R,c R"a
34-216Rc Rsc Rzoc R2~b R,c R"a
34-217Ra Rsa R2oa R2lc R,c R"a
34-218Rb Rsa Rzoa Ruc R,c R"a
34-219R RSa R2oa Ruc R,c R"a
34-220Ra Rsb R2oa R2lc R,c R"a
34-221Rb RSb R2oa R2lc R,c R"a
74
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-222Rc Rsb Rzoa R2lc R,c R"a
34-223Ra RSc R2oa R2lc R,c R"a
34-224Rb RSc R2oa Ruc R,c R"a
34-225Rc Rsc Rzoa R2lc R,c R"a
34-226Ra Rsa R2ob Rzlc Rrc R"a
34-227Rb RSa Rzob R2lc Rrc R"a
34-228Rc Rsa Rzob R2lc Rrc Rrra
34-229Ra RSb R2ob R2lc R,c R"a
34-230Rb Rsb R2ob R2lc R,c R"a
34-231Rc RSb R2ob R2lc R,c R,ra
34-232Ra Rsc R2ob R2lc R,c R"a
34-233Rb RSc Rzob R2lc R,c R"a
34-234Rc RSc R2ob Rzlc R,c R"a
34-235Ra Rsa R2oc Rzlc R,c R"a
34-236Rb Rsa Rzoc R2lc R,c R"a
34-237Rc RSa Rzoc Rzlc Rrc Rr,a
34-238Ra Rsb R2oc Rzlc Rrc R,ra
34-239Rb Rsb R2oc Rzlc Rrc Rr,a
34-240Rc Rsb Rzoc R2l R,c R"a
c
34-241Ra R5c R2oc R2lc R,c R"a
34-242Rb RSc R2oc Rzlc R,c R"a
34-243Rc Rsc Rzoc R2lc R,c R"a
34-244Ra Rsa R2oa R2la R,a R"b
34-245Rb Rsa Rzoa R2la R,a R"b
34-246Rc Rsa R2oa R2la R,a R"b
34-247Ra Rsb R2oa R2la Rra Rrrb
34-248Rb Rsb Rzoa Rzla Rra Rrrb
34-249Rc Rsb R2oa Rzla R,a Rrrb
34-250Ra RSc Rzoa R2la Rra Rr,b
34-251Rb Rsc R2oa R2la R,a R"b
34-252Rc Rsc R2oa R2la R,a R"b
34-253Ra Rsa R2ob R2la Rra R"b
34-254Rb Rsa Rzob Rzla R,a R"b
34-255Rc Rsa R2ob Rzla R,a Rr,b
34-256Ra RSb R2ob Rzla Rra Rr,b
34-257Rb RSb Rzob R2la R,a R"b
34-258Rc Rsb R2ob Rzla R,a R"b
34-259Ra Rsc Rzob R2la R,a R"b
34-260Rb Rsc R2ob Rzla R,a R"b
34-261Rc RSc R2ob R2la Rra R"b
34-262Ra Rsa R2oc Rzla R,a R"b
34-263Rb Rsa Rzoc R2la R,a R,rb
34-264Rc RSa R2oc Rzla R,a R"b
34-265Ra Rsb Rzoc R2la R,a R"b
34-266Rb Rsb R2oc R2la R,a R"b
34-267Rc Rsb Rzoc Rzla R,a R"b
34-268Ra RSc R2oc Rzla R,a R"b
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-269Rb Rsc R2oc R2la R,a R"b
34-270Rc Rsc R2oc R2~a R,a Rr,b
34-271Ra Rsa Rzoa R2~b Rra Rrrb
34-272Rb Rsa Rzoa R2jb Rra Rrrb
34-273Rc Rsa Rzoa R2lb R,a R"b
34-274Ra Rsb R2oa R2lb R,a R"b
34-275Rb Rsb R2oa R2lb R,a R,rb
34-276Rc Rsb R2oa R2lb R,a Rr,b
34-277Ra Rsc R2oa R2lb R,a R"b
34-278Rb Rsc Rzoa Rz~b R,a R,rb
34-279Rc Rsc Rzoa R2lb R,a Rr,b
34-280Ra Rsa R2ob R2lb R,a R,rb
34-281Rb Rsa Rzob R2lb R,a R"b
34-282Rc Rsa Rzob R2lb R,a Rrrb
34-283Ra Rsb Rzob R2~b R,a R"b
34-284Rb Rsb Rzob Rub R,a R"b
34-285Rc Rsb R2ob Rzib Rra R"b
34-286Ra Rsc R2ob Rzlb R,a R"b
34-287Rb Rsc R2ob Rz~b R,a R"b
34-288Rc Rsc R2ob R2lb R,a R"b
34-289Ra Rsa R2oc R2lb R,a R"b
34-290Rb Rsa Rzoc R2lb R,a Rr,b
34-291Rc Rsa R2oc R2lb R,a R"b
34-292Ra Rsb Rzoc R2lb R,a R,rb
34-293Rb Rsb R2oc R2~b R,a R"b
34-294R Rsb R2oc R2lb R,a R"b
34-295Ra Rsc R2oc Rzlb R,a R"b
34-296Rb Rsc Rzoc Rz~b Rra R"b
34-297Rc Rsc Rzoc R2lb Rra Rr,b
34-298Ra Rsa Rzoa R2lc R,a Rrrb
34-299Rb Rsa Rzoa R2~c Rra Rr,b
34-300R Rsa R2oa R''' R,a Rr,b
34-301Ra Rsb Rzoa R2tc R,a R"b
34-302Rb Rsb R2oa Rzic R,a Rrrb
34-303Rc Rsb R2oa R2~c R,a R"b
34-304Ra Rsc Rzo~ Rzlc R,a R,rb
34-305Rb Rsc R2oa Rzlc R,a Rrrb
34-306Rc Rsc R2oa R2lc R,a R"b
34-307Ra Rsa R2ob R2~c R,a R"b
34-308Rb Rsa R2ob Rz~c R,a Rrrb
34-309Rc Rsa R2ob R2~c R,a R,rb
34-310Ra Rsb R2ob R2lc R,a R"b
34-311Rb Rsb Rzob R2~c R,a R"b
34-312Rc Rsb R2ob R2~c Rra R"b
34-313Ra Rsc Rzob R2n R,a R"b
34-314Rb Rsc Rzob RZic R,a R"b
34-315Rc Rsc Rzob R2ic R,a R"b
76
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-316Ra R5a R2oc R2lc Rra R"b
34-317Rb Rsa R2oc Rzlc Rra R"b
34-318Rc Rsa R2oc Rzlc R,a R"b
34-319Ra Rsb R2oc Rzlc R,a R"b
34-320Rb R5b R2oc R2lo R,a R"b
34-321Rc Rsb R2oc R2lc R,a R"b
34-322Ra RSc Rzoc R2lc Rra R"b
34-323Rb Rsc Rzoc R2lc R,a R"b
34-324Rc RSc Rzoc R2lc R,a R"b
34-325Ra Rsa R2oa R2la R,b R"b
34-326Rb Rsa Rzoa R2la R,b Rr,b
34-327Rc RSa Rzoa R2la R,b R"b
34-328Ra Rsb R2oa Rua R,b R"b
34-329Rb Rsb R2oa R2la R,b R"b
34-330Rc Rsb R2oa R2la Rrb R"b
34-331Ra RSc Rzoa R2la Rrb R"b
34-332Rb R5c R2oa Rzla R,b R,rb
34-333R R5c R2oa Rzla R,b R"b
34-334Ra Rsa R2ob R2la R,b R"b
34-335Rb Rsa R2ob R2la R,b R,rb
34-336Rc RSa R2ob Rzla R,b R,rb
34-337Ra Rsb Rzob Rzla Rrb R"b
34-338Rb Rsb R2ob R2la R,b R"b
34-339R Rsb Rzob R2la R,b R"b
34-340Ra Rsc R2ob Rua R,b R"b
34-341Rb RSc Rzob Rzla R,b Rrrb
34-342Rc RSc Rzob Rzla R,b R"b
34-343Ra RSa R2oc R2la R,b Rr,b
34-344Rb Rsa Rzoc Rzla R,b R"b
34-345Rc Rsa R2oc R2la R,b R"b
34-346Ra Rsb R2oc Rua R,b R"b
34-347Rb Rsb R2oc Rzla R,b R,rb
34-348Rc RSb R2~c Rzla R,b R"b
34-349Ra Rsc Rzoc R2la R,b R"b
34-350Rb Rsc R2oc Rzla R,b R"b
34-351Rc Rsc R2oc R2la R,b R"b
34-352Ra Rsa R2oa R2lb R,b R"b
34-353Rb Rsa R2oa R2lb Rrb Rr,b
34-354R RSa R2oa Rzlb R,b R"b
34-355Ra RSb R2oa R2lb R,b R"b
34-356Rb RSb R2oa Rzlb R,b R"b
34-357Rc Rsb R2oa R2lb R,b R"b
34-358Ra R5c Rzoa R2lb R,b R"b
34-359Rb Rsc Rzoa R2lb R,b R"b
34-360Rc RSc Rzoa R2lb R,b R"b
34-361Ra RSa R2ob Rzlb Rrb R,rb
34-362Rb R5a Rzob Rub R,b R"b
77
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-363Rc Rsa R2ob R2~b R,b Rrrb
34-364Ra Rsb R2ob R2lb R,b R"b
34-365Rb R5b Rzob R2~b R,b R"b
34-366Rc Rsb Rzob Rz~b R,b R"b
34-367Ra R5c R2ob R2~b R,b R"b
34-368Rb Rsc R20b R2lb R,b R"b
34-369Rc Rsc R20b R2lb R,b R"b
34-370Ra Rsa Rzoc R2lb Rrb R,rb
34-371Rb Rsa Rzoc R2ib R,b R"b
34-372Rc Rsa Rzoc R2~b R,b R"b
34-373Ra R5b R2oc R2~b R,b R"b
34-374Rb Rsb Rzoc Rz~b R,b R"b
34-375Rc Rsb Rzoc R2lb R,b R"b
34-376Ra Rsc R2oc R2lb Rrb R"b
34-377Rb R5c Rzoc R2lb R,b Rrrb
34-378Rc R5c R2oc R2ib R,b R"b
34-379Ra Rsa R2oa R2tc R,b Rrrb
34-380Rb Rsa R2oa R2lc R,b R"b
34-381Rc Rsa R2oa Rzlc R,b R"b
34-382Ra Rsb Rzoa Ruc R,b R"b
34-383Rb R5b R2oa Rztc R,b R,rb
34-384Rc Rsb R2oa Rzic Rrb R,rb
34-385Ra R5c Rzoa R2m Rrb Rr,b
34-386Rb Rsc R2oa R2lc R,b Rr,b
34-387R Rsc Rzoa Rzic R,b R"b
34-388Ra Rsa RZOb Rzic R,b R"b
34-389Rb Rsa Rzob Rzic R,b R"b
34-390Rc Rsa Rzob R2tc R,b Rr,b
34-391Ra Rsb Rzob R2~c R,b R"b
34-392Rb Rsb R2ob R2lc R,b R"b
34-393Rc R5b Rzob Rzlc R,b R"b
34-394Ra Rsc Rzob Rzlc R,b Rr,b
34-395Rb R5c Rzob Rz~c R,b R"b
34-396Rc R5c Rzob Rzm R,b R"b
34-397Ra R5a Rzoc R2~c R,b R"b
34-398Rb R5a R2oc R2lc R,b R"b
34-399Rc R5a R2oc R2~c R,b R"b
34-400Ra R5b Rzoc R2m R,b Rr,b
34-401Rb R5b Rzoc Rzic R,b R"b
34-402R Rsb R2oc Rzic R,b R"b
34-403Ra Rsc R2oc R2~c R,b Rr,b
34-404Rb Rsc R2oc Rzn R,b R"b
34-405Rc Rsc Rzc Rzm R,b R"b
34-406Ra R5a R2oa Rz~a R,c R"b
34-407Rb Rsa R2oa Rzla R,c R"b
34-408Rc Rsa R2oa Rz~a R,c R"b
34-409Ra R5b Rzoa Rz~a R,c R"b
78
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-410Rb Rsb Rzoa Rz~a R,c R"b
34-411Rc Rsb Rzoa Rzia R,c R"b
34-422Ra Rsc R2oa R2~a R,c R"b
34-413Rb Rsc R2oa R2la R,c R"b
34-414Rc Rsc R2oa R2~a R,c R"b
34-4I5Ra Rsa Rzob R2la R,c R"b
34-416Rb Rsa Rzob R2ta R,c R"b
34-417R Rsa Rzob R2la R,c R"b
34-418Ra Rsb Rzob R2ta R,c R,rb
34-419Rb Rsb R2ob R2~a R,c R"b
34-420Rc Rsb R2ob R2~a R,c R,rb
34-421Ra Rsc R2ob R2~a Rrc R,rb
34-422Rb Rsc R2ob R2~a R,c R"b
34-423Rc Rsc R2ob R2~a R,c R"b
34-424Ra Rsa R2oc R2la R,c R"b
34-425Rb Rsa R2oc R2la R,c R"b
34-426Rc Rsa Rzoc R2~a R,c R,rb
34-427Ra Rsb R2oc Rzla R,c R"b
34-428Rb Rsb R2oc Rzla R,c R"b
34-429Rc Rsb Rzoc R2~a R,c R"b
34-430Ra Rsc R2oc R2~a R,c R"b
34-431Rb Rsc Rzoc Rzla R,c R"b
34-432Rc Rsc Rzoc Rua R,c R"b
34-433Ra Rsa R2oa Rzib R,c R,rb
34-434Rb Rsa R2oa R2tb Rrc R"b
34-435Rc Rsa R2oa R2~b R,c R"b
34-436Ra Rsb R2oa R2ib R,c R"b
34-437Rb Rsb R2oa R2~b R,c R"b
34-438Rc Rsb R2oa R2~b Rrc R"b
34-439Ra Rsc R2oa R2~b R,c Rr,b
34-440Rb Rsc R2oa Rzlb R,c R"b
34-441Rc Rsc R2oa R2lb R,c R"b
34-442Ra Rsa R2ob R2lb R,c R"b
34-443Rb Rsa R2ob Rzlb R,c R"b
34-444Rc Rsa RZOb R2lb R,c R"b
34-445Ra Rsb Rzob R2ib R,c R"b
34-446Rb Rsb R2ob R2~b R,c R"b
34-447Rc Rsb Rzob R2lb R,c R"b
34-448Ra Rsc Rzob Rz~b R,c R"b
34-449Rb Rsc R20b R2lb R,c R"b
34-450Rc Rsc R20b R2~b R,c R"b
34-451Ra Rsa R2oc Rz~b Rrc R"b
34-452Rb Rsa R2oc R2~b Rrc R"b
34-453R Rsa R2oc R2lb R,c R"b
34-454Ra Rsb R2oc R2~b R,c Rr,b
34-455Rb Rsb Rzoc RZlb R,c R"b
34-456Rc Rsb R2oc Rz~b R,c R"b
79
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-457Ra RSc R2oc Rzlb R,c Rr,b
34-458Rb R5c Rzoc Rzlb R,c R,rb
34-459Rc Rsc R2oc Rzlb R,c R"b
34-460Ra RSa R2oa R2lc R,c R"b
34-461Rb RSa Rzoa R2lc R,c R"b
34-462Rc Rsa R2oa R2lc R,c Rr,b
34-463Ra RSb R2oa R2lc R,c R"b
34-464Rb RSb R2oa R2lc R,c Rr,b
34-465Rc Rsb R2oa R2lc R,c Rr,b
34-466Ra R5c R2oa Rzlc Rrc R"b
34-467Rb Rsc R2oa R2lc Rrc R"b
34-468Rc RSc R2oa Rzlc Rrc R"b
34-469Ra R5a Rzob R2lc R,c R"b
34-470Rb Rsa Rzob R2lc R,c R"b
34-471Rc RSa R2ob R2lc R,c R"b
34-472Ra Rsb R2ob R2lc R,c R"b
34-473Rb Rsb Rzob R2lc R,c R"b
34-474Rc Rsb Rzob R2sc R,c R"b
34-475Ra R5c Rzob R2lc R,c Rr,b
34-476Rb Rsc R2ob R2lc Rrc R"b
34-477Rc Rsc Rzob R2lc R,c R"b
34-478Ra RSa R2oc R2lc R,c R"b
34-479Rb Rsa R2oc R2lc R,c R"b
34-480Rc RSa R2oc Rzlc R,c R"b
34-481Ra RSb R2oc R2lc R,c R"b
34-482Rb RSb R2oc R2sc R,c R"b
34-483R R5b R2oc Ruc R,c R"b
34-484Ra Rsc Rzoc R2lc R,c Rr,b
34-485Rb Rsc Rzoc Rzlc R,c R"b
34-486R Rsc R2oc Rzlc R,c Rr,b
34-487Ra Rsa R2oa R2la R,a R"c
34-488Rb Rsa R2oa R2la Rra R"c
34-489Rc Rsa R2oa R2la R,a R"c
34-490Ra Rsb R2oa R2la R,a R,rc
34-491Rb RSb R2oa R2la R,a R,rc
34-492Rc RSb Rzoa R2la R,a R"c
34-493Ra RSc R2oa R2la R,a R"c
34-494Rb Rsc R2oa R2la R,a R"c
34-495Rc Rsc R2oa R2la R,a R,rc
34-496Ra Rsa R2ob R2la Rra R"c
34-497Rb Rsa R2ob R2la R,a R"c
34-498Rc Rsa R2ob R2la Rra Rr,c
34-499Ra Rsb R2ob R2la R,a R"c
34-500Rb Rsb R2ob R2la R,a R,rc
34-501Rc RSb R2ob Rzla R,a R"c
34-502Ra Rsc R2ob R2la R,a R,rc
34-503Rb Rsc R2ob Rzla R,a R"c
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-504Rc Rsc R2ob R2la R,a R"c
34-505Ra Rsa R2oc R2la R,a R"c
34-506Rb Rsa R2oc Rua Rra R"c
34-507Rc Rsa R2oc Rua R,a R"c
34-508Ra Rsb R2oc Rua R,a R,rc
34-509Rb Rsb R2oc Rua R,a R,rc
34-510Rc Rsb Rzoc R2la R,a R"c
34-511Ra Rsc Rzoc R2la R,a R"c
34-512Rb Rsc R2oc R2la R,a R,rc
34-513Rc Rsc R2oc R2la R,a R"c
34-514Ra Rsa R2oa R2ib R,a R"c
34-515Rb Rsa R2oa R2lb R,a R"c
34-516Rc Rsa Rzoa Rub R,a R"c
34-517Ra Rsb Rzoa Rub R,a R"c
34-518Rb Rsb Rzoa Rub R,a R"c
34-519R Rsb Rzoa Rub R,a R,rc
34-520Ra Rsc R2oa Rub R,a Rirc
34-521Rb Rsc R2oa Rzlb R,a R"c
34-522Rc Rsc Rzoa R2lb R,a R"c
34-523Ra Rsa R2ob Rzlb R,a R"c
34-524Rb Rsa R2ob R2lb R,a R"c
34-525Rc Rsa Rzob Rub R,a R"c
34-526Ra Rsb Rzob R2lb R,a R"c
34-527Rb Rsb R2ob R2lb R,a Rr,c
34-528Rc Rsb Rzob Rub R,a R"c
34-529Ra Rsc R2ob Rzlb Rra R,rc
34-530Rb Rsc R2ob Rzlb R,a R"c
34-531Rc Rsc R2ob Rub R,a Rr,c
34-532Ra Rsa Rzoc Rub R,a R"c
34-533Rb Rsa Rzoc R2lb R,a R,rc
34-534Rc Rsa R2oc R2lb R,a R,rc
34-535Ra Rsb R2oc Rub R,a R"c
34-536Rb Rsb R2oc Rzib R,a R"c
34-537Rc Rsb R2oc Rzlb R,a R"c
34-538Ra Rsc R2oc Rzlb Rra R"c
34-539Rb Rsc Rzoc R2lb R,a Rr,c
34-540Rc Rsc R2oc Rzlb R,a R"c
34-541Ra Rsa R2oa R2lc R,a R"c
34-542Rb Rsa R2oa R2lc R,a R"c
34-543Rc Rsa R2oa Ruc R,a R"c
34-544Ra Rsb R2oa Ruc R,a R"c
34-545Rb Rsb Rzoa R2lc R,a Rr,c
34-546Rc Rsb R2oa Ruc R,a R"c
34-547Ra Rsc R2oa Ruc R,a Rr,c
34-548Rb Rsc R2oa R2lc R,a R"c
34-549Rc Rsc R2oa Ruc Rra R"c
34-550Ra Rsa Rzob R2lc R,a R"c
81
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-551Rb Rsa R2ob R2lc R,a R,rc
34-552Rc Rsa R2ob R,2m R,a R"c
34-553Ra Rsb R2ob R2~c R,a R"c
34-554Rb Rsb Rzob R2~c R,a Rrrc
34-555Rc Rsb R2ob R2tc R,a R"c
34-556Ra Rsc R2ob Ruc R,a Rrrc
34-557Rb Rsc R2ob R2~c R,a R"c
34-558R Rsc R2ob R2~o R,a R"c
34-559Ra Rsa R2oc Rzlc R,a R"c
34-560Rb Rsa Rzoc R2lc R,a Rr,c
34-561Rc Rsa Rzoc R2lc R,a R"c
34-562Ra Rsb R2oc R2tc R,a R"c
34-563Rb Rsb R2oc R2lc R,a R"c
34-564Rc Rsb Rzoc Ruc R,a R"c
34-565Ra Rsc Rzoc R2~c R,a R"c
34-566Rb Rsc R2oc R2m R,a R"c
34-567Rc Rsc R2oc Rz~c R,a R"c
34-568Ra R5a R2oa R2~a R,b R"c
34-569Rb Rsa Rzoa R2~a R,b R"c
34-570R Rsa R2oa Rzla R,b R"c
34-571Ra Rsb Rzoa R2la R,b R"c
34-572Rb Rsb R2oa R2la R,b R,rc
34-573Rc R5b R2oa R2~a R,b R"c
34-574Ra Rsc R2oa R2~a R,b R"c
34-575Rb Rsc R2oa Rz~a R,b R"c
34-576Rc Rsc R2oa R2la R,b R"c
34-577Ra Rsa R2ob R,2~a R,b R"c
34-578Rb Rsa Rzob R2ta R,b R"c
34-579Rc Rsa R2ob R2~a R,b R"c
34-580Ra Rsb Rzob R2la R,b R"c
34-581Rb R5b R2ob Rz~a R,b R"c
34-582Rc Rsb R2ob Rz~a R,b R"c
34-583Ra Rsc R2ob R2la R,b R"c
34-584Rb Rsc R2ob Rz~a R,b R"c
34-585Rc Rsc Rzob R2~a R,b R"c
34-586Ra Rsa R2oc R2ta R,b R"c
34-587Rb Rsa R2oc R2~a R,b R"c
34-588Rc Rsa R2oc R2~a R,b R"c
34-589Ra Rsb R2oc Rz~a R,b R"c
34-590Rb Rsb R2oc R2~a R,b R"c
34-591Rc Rsb Rzoc R2la R,b R"c
34-592Ra Rsc R2oc R2~a R,b R"c
34-593Rb Rsc R2oc R2~a R,b R,rc
34-594Rc Rsc R2oc R2~a R,b R"c
34-595Ra Rsa R2oa Rz~b Rrb R"c
34-596Rb Rsa Rzoa R2lb R,b R"c
34-597Rc Rsa R2oa R2~b R,b Rr,c
82
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-598Ra Rsb Rzoa R2~b R,b R,rc
34-599Rb Rsb R2oa Rub R,b R,rc
34-600Rc Rsb Rzoa Rub R,b Rr,c
34-601Ra Rsc Rzoa R2lb R,b R"c
34-602Rb Rsc R2oa Rub R,b R"c
34-603Rc Rsc R2oa Rub R,b R"c
34-604Ra Rsa Rzob Rub R,b R"c
34-605Rb Rsa Rzob Rub R,b R"c
34-606Rc Rsa R2ob Rub R,b Rr,c
34-607Ra Rsb R2ob Rub Rrb Rr,c
34-608Rb Rsb R2ob R2lb Rrb R"c
34-609Rc Rsb R2ob Rub R,b R"c
34-610Ra Rsc R2ob R2lb R,b R"c
34-611Rb Rsc Rzob Rz~b R,b R"c
34-612Rc Rsc Rzob Rzib R,b R"c
34-6I3Ra Rsa R2oc Rub R,b R"c
34-614Rb Rsa Rzoc R2lb R,b R"c
34-615Rc Rsa R2oc Rub Rrb Rr,c
34-616Ra Rsb Rzoc Rub Rrb R"c
34-617Rb Rsb Rzoc R2lb R,b R"c
34-618Rc Rsb Rzoc Rub R,b R"c
34-619Ra Rsc R2oc Rub R,b R"c
34-620Rb Rsc Rzoc R2Ib R,b R"c
34-621Rc Rsc R2c Rz~b R,b R"c
34-622Ra Rsa R2oa Rz~c Rrb R"c
34-623Rb Rsa Rzoa R2lc Rrb R"c
34-624Rc Rsa Rzoa R2~c R,b Rr,c
34-625Ra Rsb R2oa R2~o R,b R,rc
34-626Rb Rsb Rzoa Rzic R,b R"c
34-627Rc Rsb Rzoa Ruc R,b R"c
34-628Ra RSc Rzoa Ruc R,b R,rc
34-629Rb Rs Rzoa R2~c R,b R"c
34-630Rc RsC R2oa Ruc R,b R"c
34-631Ra Rsa Rzob R2lc R,b R"c
34-632Rb Rsa Rzob R2lc R,b R"c
34-633Rc Rsa R2ob Rzlc R,b R"c
34-634Ra Rsb Rzob Ruc R,b R"c
34-635Rb Rsb R2ob Ruc R,b Rr,c
34-636Rc Rsb R20b R2lc R,b R"c
34-637Ra Rsc R2ob Ruc R,b R"c
34-638Rb Rsc R20b Rzlc R,b R"c
34-639Rc Rsc Rzob Rzm R,b R"c
34-640Ra Rsa Rzoc Ruc R,b R"c
34-641Rb Rsa R2oc Rzic R,b R"c
34-642Rc Rsa Rzoc Ru R,b R"c
c
34-643Ra Rsb R2oc R2lc R,b Rr,c
34-644Rb Rsb R2oc Ruc R,b R"c
83
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-645Rc Rsb R2oc R2lc R,b R"
34-646Ra R5c R2oc R2lc R,b R"c
34-647Rb Rsc Rzoc Rzlc R,b R"c
34-648R Rsc Rzoc Rzlc R'b R"c
34-649Ra Rsa R2oa Rzla R,c R"c
34-650Rb Rsa R2oa R2la R,c R"c
34-65IRc Rsa R20a R2la Rrc Rrrc
34-652Ra Rsb R2oa R2la Rrc R,rc
34-653Rb Rsb Rzoa R2la R,c R'rc
34-654R Rsb R2oa R2la R,c R"c
34-655Ra Rsc R2oa R2la R~c R"c
34-656Rb Rsc R2oa R2la R,c R,rc
34-657Rc R$c R2oa Rzla R,c R"c
34-6~8Ra Rsa R2ob Rzla R,c R"c
34-659Rb Rsa R2ob R2la Rrc Rrrc
34-660Rc Rsa R2ob Rzla R,c R"c
34-661Ra Rsb R2ob R2la R,c R"c
34-662Rb Rsb R2ob R2la R,c R,rc
34-663R Rsb R2ob R2la R,c R"c
34-664Ra Rsc Rzob R2la Rn R"c
34-665Rb Rsc R2ob Rua Rrc Rr,c
34-666Rc Rsc R2ob Rzla R'c R'rc
34-667Ra Rsa Rzoc R2la R,c R"c
34-668Rb Rsa R2oc R2la R,c R"c
34-669Rc Rsa Rzoc Rzla Rrc Rr,c
34-670Ra Rsb R2oc Rzla Rrc R"c
34-671Rb Rsb R2oc Rzla R,c R"c
34-672Rc Rsb R2oc R2la R,c R"c
34-673Ra R5c R2oc Rua R,c R"c
34-674Rb R5c R2oc R2la R,c R"c
34-675R Rsc Rzoc R2la R,c R"c
34-676Ra Rsa R2oa Rzlb R,c R"
34-677Rb Rsa R2oa R2lb R,c Rr,c
34-678Rc Rsa Rzoa Rzlb R'c Rr,c
34-679Ra Rsb R2oa R2lb R,c R"c
34-680Rb Rsb R2oa R2lb R,c R"c
34-681Rc Rsb R2oa R2lb R,c R"c
34-682Ra Rsc R2oa R2lb Rrc R,rc
34-683Rb Rsc R2oa R2lb R,c R"c
34-684R Rsc R2oa R2lb R,c R,rc
34-685Ra Rsa R2ob R2lb R,c R"c
34-686Rb Rsa Rzob R2lb R,c R"c
34-687Rc RSa R2~b Rub R,c R"c
34-688Ra Rsb R2ob Rzlb Rrc R"c
34-689Rb Rsb R2ob R2lb R,c R"c
34-690Rc Rsb Rzob R2lb R,c R"c
34-691Ra Rsc R2ob Rzlb R'c Rr,c
84
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
34-692Rb R5c Rzob R2~b R,c R"c
34-693Rc Rsc R2ob R2~b R,c R"c
34-694Ra R5a R2oc Rz~b R,c R"c
34-695Rb Rsa Rzoc Rz~b R,c R"c
34-696Rc Rsa Rzoc Rub R,c R"c
34-697Ra R5b R2oc Rzlb R,c R"c
34-698Rb R5b R2oc Rz~b R,c R"c
34-699Rc R5b R2oc Rzib R,c R"c
34-700Ra Rsc R2oc R2~b R,c R"c
34-701Rb Rsc Rzc Rzib R,c R"c
34-702Rc Rsc Rzc R2~b R, R"
34-703Ra R5a Rzoa R2n R,c R"c
34-704Rb Rsa R2oa R2~c R,c R"c
34-705Rc R5a R2oa R2lc R,c R"c
34-706Ra R5b Rzoa R2lc R,c R"c
34-707Rb Rsb R2oa R2m R,c R"c
34-708R R5b R2oa R2~c R,c R"c
34-709Ra R5c R2oa R2lc R,c R"c
34-710Rb R5c Rzoa R2~c R,c R"c
34-711Rc R5c Rzoa Rztc R,c R"c
34-712Ra Rsa R2ob Ruc R,c R"c
34-713Rb Rsa R2ob R2lc R,c R"c
34-714R Rsa R2ob R2lc R,c R"c
34-715Ra R5b Rzob R2m R,c R"c
34-716Rb R5b R2ob Rzm R,c R"
34-717Rc R5b Rzob R2lc R,c R"c
34-718Ra Rsc Rzob Rz~c R,c R"c
34-719Rb Rsc Rzob Ruc Rrc R"
34-720R R5c Rzob Rz~c R,c R"c
34-721Ra Rsa Rzoc R2lc R,c R"c
34-722Rb R5a R2oc R2lc R,c R"c
34-723Rc R5a Rzoc Rz~c R,c R"c
34-724Ra Rsb Rzoc R2lc R,o R"c
34-725Rb Rsb Rzoc Rzo R,c R"c
34-726Rc R5b R2oc R2lc R,c R"c
34-727Ra R5c R2oc R2lc R,c R"c
34-728Rb Rsc Rzoc R2ic R,c R"c
34-729Rc Rsc R2c Rzm R, R"c
where all symbols are as defined above.
In one aspect of formula (34) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a nitro group, an optionally substituted amino group, an
alkyl group, an
alkoxy group, an alkenyl group, or an alkoxyalkyl group; R5 is hydrogen, a
hydroxy group, a
halogen, a nitro group, an optionally substituted amino group, an alkyl group,
an alkoxy
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an alkenyl group, or an alkoxyalkyl group; R' and R? independently are
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group; a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
group; an alkyl group, a cycloallcyl group, an allcoxy group, a haloalkoxy
group, a cycloallcyl
group, an aryl group, or a benzyloxy group; and RZ° and R21
independently are hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
group; an alkyl group, a cycloallcyl group, an alkoxy group, a haloalkoxy
group, a cycloalkyl
group, an aryl group, a benzyloxy group, an acyl group, an acyloxy group, an
aroyl group, an
alkoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl group, a
heterocyclyl group,
or an aralkyl group.
In another aspect of formula (34) of the present invention, R is hydrogen or
an alkyl
group; RS is hydrogen or an alkyl group; R' and R? independently are hydrogen
or a halogen;
RZ° is hydrogen or a halogen; and R2' is hydrogen or a halogen.
In yet another aspect of formula (34) of the present invention, R is -H, CH3,
or
CHZCH3; R5 is -H or CH3; R' and R? independently are -H, -F, or -Cl;
RZ° is -H, -F, -Cl, or
-Br; and R21 is -H, CH3, or -F. Exemplary compounds include, but are not
limited to:
0
S o HaC S
° / I \ ~° / \
° NH /
N o
N W Hsc / \
CH3 I i
F ~ CH3
o i ~ S ° i ~ ~ S'~°
0 ~
o W I ,~NH I w I °~o ~ o/'NH
N I F o / N ~ CI
CH ( ~ CH3
F ~ F
S o
0 0
( o/~NH I ~ I oho
I / N w F
N
CH I / CH3
3 CI _ F
86
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
S S
o / I w ~ o / I w
0 0
I oho ~ o NH I ~ I °~o ~ o NH
/ N W / N ~ F
I / F . CH3 I / F .
> >
o S
~o o / I ~ S
0
I o I °~H ~ NH ~ °~ ~ NH
o I I o 0
/ N I ~ / N I ~ F F
CH3 s F . CH3 / F
Me
O o ~ S Ne ~ I
O
O~O ~ I O NH I / I O O
I I O ~ I / /S~ H
N
CH3 I /
F ~ O
O O~ ~ I \ NH O NH O
I I O O
N
CH3 I / Br
Me
O O I
I ~O I I I ~O S O
N I W O S O I / / ~ H
HsC ~F HN--
H3~ \\o; and o
The present invention also contemplates various compounds having the general
formula:
R
R~' (35),
1o where all symbols are as defined above in connection with formula (I).
~7
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
where Rz° and Rz' independently are hydrogen, a halogen, a nitro group,
an amino
group, a mono- or di- substituted amino group, a hydroxy group, an alkoxy
group, a carboxy
group, a cyano group, an oxo(O=) group, a thio(S=) group, an alkyl group, a
cycloalkyl
group, an alkoxy group, a haloalkoxy group, a cycloalkyl group, an aryl group,
a benzyloxy
s group, an acyl group, an acyloxy group, an amyl group, an alkoxycarbonyl
group, an
aryloxycarbonyl group, a heteroaryl group, a heterocyclyl group, an aralkyl
group, an
alkylsulfonyl group, an alkylsulfinyl group, an arylsulfonyl group, an
arylsulfinyl group, an
alkylthio group, an aryltl>so group, a heteroarylthio group, an aralkylthio
group, or a
heterocyclyl sulfonyl group, which is optionally substituted with a halogen, a
hydroxyl
group, a vitro group, an amino group, an alkyloxy group, or any combination
thereof, and
wherein the heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or a piperzinyl group, wherein the substituent on the
heterocyclyl
group is a halogen, a vitro group, an amino group, an alkyl group, an alkoxy
group, or an aryl
group; and R' is defined above.
According to some variations of the present invention, R', Rz°, and Rz'
of formula
(35) are selected to produce various compounds of fornmla (35-1) to formula
(35-27) as
follows:
Formula R Rz R '
35-1 R'a R' a R'a
35-2 R"' Rzoa R2la
35-3 jtl~ Rzoa R2~a
35-4 Rla R2ob Rzia
35-5 R'b Rzob Rzta
35-6 Rl~ Rzob Rz~a
35-7 Rla Rzoc R2~a
35-8 Rlb Rzoc Rua
35-9 RI R2oo R2la
35-10 RIa R2oa R2lb
35-11 R'b Rza R2~b
35-12 Rl Rzoa R2~b
35-13 Rya Rzob Rz~b
35-14 Rib Rzb Rz~b
35-15 R'~ Rzb Rzlb
35-16 R'a Rz 8216
35-17 Rlb Rz Rzib
35-18 R'~ Rz~ Rz'b
35-19 R'a Rzoa RZ'
35-20 R"' Rza Rz'
35-21 R'~ Rzoa Rzlc
88
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
35-22 Rya RZOb R2'°
35-23 Rlb Rzob RZ>c
35-24 Roc R20b R2lc
35-25 Rla R2oc R2n
35-26 Rlb Rzoc Rzm
35-27 Rlc R2°c R2m
where all symbols are as defined above.
In one aspect of formula (35) of the present invention, R' is hydrogen, a
hydroxy
group, a halogen, a vitro group, a carboxy group, a carbamoyl group, an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an allcoxy group,
a cycloalkoxy
group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl group, an
alkenyloxy group, or
a cycloalkenyloxy group; RZ° is hydrogen, a halogen, a vitro group, an
amino group, a mono-
or di- substituted amino group, a hydroxy group, an alkoxy group, a carboxy
group, a cyano
group, an oxo(O=) group, or a thio(S=) group; and RZ' is hydrogen, a halogen,
a vitro group,
an amino group, a mono- or di- substituted amino group, a hydroxy group, an
alkoxy group, a
carboxy group, a cyano group, an oxo(O=) group, or a thio(S=) group.
In another aspect of formula (35) of of the present invention, R' is a
halogen, R2o is
hydrogen or a halogen, and R''I is hydrogen or a halogen.
In yet another aspect of of formula (35) of the present invention, R' is Cl or
F, RZ° is -
H or -F, and R2' is -F.
Exemplary compounds of formula (35) include, but are not limited to:
CHg / F
F \ N \_
o ~~O
I \ I oho \ I o NH I / I O~O I \ S~ H
CI a tJ I ~ C
CH3 / F ; CHg C
/ \ S
S o I o
o / I \ ~o F \ °w/\o \ o NH
I \ I °~o \ o NH I ~- N I \ Cl
F N ~ F CH I
CH3 I a F . 3 F F
S9
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
o S ° / I ~ S~o
0
o~° ~ I ° NH I \ ' °~° ~ ° NH
CI / N I a F . CI / N I y F CI
CH3 / F CH3 / F
> >
/ I ~ S~o
F I ~ I °~° ~ ° NH
/ N w
CH3 I /
and F
The present invention further contemplates various compounds having the
general
formula:
(36),
where all symbols are as defined above in connection with formula (I).
According to some variations of the present invention, R, R4, R' and R" of
formula
(36) are selected to produce compounds of formula (36-1) through formula (36-
81) as
follows:
Formula R R" R' R" Formula R R R' R"
36-1 Ra R"a R'a R"a 36-42 R R R' R"
36-2 Rb R4a R'a R"a 36-43 Ra R4 Rib Rrrb
36-3 R~ R4a R'a R"a 36-44 Rb R4~ R'b Rrrb
36-4 Ra R4b R'a R"a 36-45 R~ R~ R'b Rrrb
36-5 Rb R4b R'a R"a 36-46 Ra R4a R' R"b
36-6 R R4b R'a R"a 36-47 Rb R4a R' R"b
36-7 Ra R4 R'a R"a 36-48 R~ R4a R' R"b
36-8 Rb R4~ R'a R"a 36-49 Ra R4b R'~ R.b
36-9 R~ R4~ R'a R"a 36-50 Rb R4b R'~ R"b
36-10 Ra R4a R'b R"a 36-51 R R4b R'~ Rrrb
36-11 Rb R4a Rrb Rna 36-52 Ra R4~ R' Rnb
36-12 R R4a R'b R"a 36-53 RU R4 R'~ R"b
36-13 Ra R'~bRrb Rrra 36-54 R R4~ R'o R~rb
36-14 Rb R4b Rrb Rrra 36-55 R~ R4a R'a R"~
36-15 Ro R4b R'b R"a 36-56 Rb R4a R'~ R"
36-16 Ra R4 R'b R"a 36-57 R R4a R'a R"~
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
36-17Rb R4c R'b Rr'a 36-58 Ra R4b R'a R"
36-18Rc R4c R'b R"a 36-59 Rb R4b R'a R"c
36-19Ra R4a R'c R"a 36-60 Rc R'~bR'a R"c
36-20Rb R4a R'c Rna 36-61 Ra R4c R'a R~~c
36-21Rc R4a R'c R"a 36-62 Rb R4c R'a R"c
36-22Ra R4b R'c R"a 36-63 Rc R4c R'a R"c
36-23Rb R4b R'c R"a 36-64 Ra Rya R'b R"c
36-24Rc R4b R'c R"a 36-65 Rb R4a R'b R"c
36-25Ra R4c R'c R"a 36-66 R R4a R'b R"c
36-26Rb R4c R'c R"a 36-67 Ra Rib R'b R"c
36-27Rc R4c R'c R"a 36-68 Rb Rib R'b R"c
36-28Ra R4a R'a Rib 36-69 Rc R4b R'b R'~c
36-29Rb R4a R'a R"b 36-70 Ra R4c R'b R"c
36-30Rc R4a Rra R~rb 36-71 Rb R4c R'b R"c
36-31Ra R4b R'a R"b 36-72 Rc R4c Rrb Rirc
36-32Rb R4b R'a R"b 36-73 Ra R4a R'c R"c
36-33Rc R4b R'a R"b 36-74 Rb R4a R'c R"c
36-34Ra R4c R'a R"b 36-75 Rc R4a R'c R"c
36-35Rb R4c R'a R"b 36-76 Ra R4b R'c R"c
36-36R R4c R'a R"b 36-77 Rb R4b R'c R"c
36-37Ra R4a R'b R"b 36-78 R R4b R'c R"
36-38Rb R4a R'b R"b 36-79 Ra R4c Rc R"c
36-39Rc R4a R'b R"b 36-80 Rb R4c Rc Rr'c
36-40Ra R4b R'b Rb 36_81 Rc R4c Rc R"c
36-41Rb R4b R'b R"b
where all symbols are as defined above.
In one aspect of formula (36) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, an optionally substituted amino group, an
alkyl group, an
allcoxy group, an alkenyl group, or an alkoxyalkyl group; R4 is an alkenyl
group, a
cycloallcenyl group, an alkoxyalkyl group, an alkenyloxy group, a
cycloalkenyloxy group, an
acyl group or an acyloxy group, an aryl group, an aryloxy group, an amyl
group, an aroyloxy
group, an arallcyl group, an aralkenyl group, an arallc~myl group, an aralkoxy
group, a
heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroaralkyl group, a
to heteroaryloxy group, or a heteroaralkoxy group; and R' and R" independently
are hydrogen,
a halogen, a vitro group, an amino group, a mono- or di- substituted amino
group; a hydroxy
group, an allcoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
group; an alkyl group, a cycloalkyl group, an allcoxy group, a haloalkoxy
group, a cycloalkyl
group, an aryl group, or a benzyloxy group.
91
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In another aspect of formula (36) of the present invention, R is hydrogen or
an alkyl
group; R4 is a cycloalkenyl group, a cycloalkenyloxy group, an acyl group or
an acyloxy
group, an aryl group, an aryloxy group, an aroyl group, an aroyloxy group, an
aralkyl group,
an aralkenyl group, an arallcynyl group, an aralkoxy group, a heterocyclyl
group, a
heterocyclenyl group, a heteroaryl group, a heteroaralkyl group, a
heteroaryloxy group, or a
heteroaralkoxy group; and R' and R" independently are hydrogen or a halogen.
In yet another aspect of formula (36) of the present invention, R is -H or
CH3; R4 is a
halogen substituted aryl group; and R' and R" independently are -H or -Cl; and
alI other
symbols are as defined above in connection with formula (I).
Examples of compounds of formula (36) include, but are not limited to:
°
I ~ I ono I ~
N
CH3 I , I
o S
HN-
o~
> >
O
0
N ~ ~ o NH
and CH3 ( / F
The present invention also contemplates various compounds having the general
formula:
O H
I \ ~ O~N ~ \
E R4
O S
HN-
i s O (37),
where all symbols are as defined above in connection with formula (I).
According to some variations of the present invention, E, R', and R4 of
formula (37)
are selected to produce compounds of formula (37-1) through formula (37-27):
Formula E R' R''
37-1 Ea R a R a
37-2 Eb Ria R4a
37-3 E~ R~ a R4a
92
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
37-4 Ea R R''a
37-5 Eb RIb R4a
37-6 Ec Rjb Rya
37-7 Ea Rlc Rya
37-8 Eb Roc R4a
37-9 E Ric R4a
37-10 Ea Rya R4b
37-11 Eb Ria R4b
37-12 Ec Ria R4b
37-13 Ea Rib R4b
37-14 Eb Rib R4b
37-15 Ec Rjb R4b
37-16 Ea RIc R4b
37-17 Eb R~ R4b
37-18 Ec Roc R4b
37-19 Ea Rya R4
37-20 Eb Rla R4c
37-21 Ec Rya R4c
37-22 Ea Rib R4c
37-23 Eb Rlb R4c
37-24 E RIb R4c
37-25 Ea Rlc R4c
37-26 Eb Roc R4c
37-27 Ec Roc Rac
where all symbols are as defined above.
In one aspect of formula (37) of the present invention, Rl is hydrogen, a
hydroxy
group, a halogen, a nitro group, a carboxy group, a carbamoyl group, an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an alkoxy group,
a cycloalkoxy
group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl group, an
alkenyloxy group, or
a cycloalkenyloxy group; R4 is an alkenyl group, a cycloalkenyl group, an
alkoxyalkyl group,
an alkenyloxy group, a cycloalkenyloxy group, an acyl group or an acyloxy
group, an aryl
group, an aryloxy group, an amyl group, an aroyloxy group, an aralkyl group,
an aralkenyl
i0 group, an arallcynyl group, an arallcoxy group, a heterocyclyl group, a
heterocyclenyl group, a
heteroaryl group, a heteroaralkyl group, a heteroaryloxy group, or a
heteroaralkoxy group;
and all other symbols are as defined above in connection with formula (I).
In another aspect of formula (37) of the present invention, R' is hydrogen, a
hydroxy
group, a halogen, a nitro group, a carboxy group, a carbamoyl group, an
optionally
substituted amino group, or an alkyl group; R4 is a cycloallcenyl group, a
cycloalkenyloxy
group, an acyl group or an acyloxy group, an aryl group, an aryloxy group, an
aroyl group, an
93
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
aroyloxy group, an aralkyl group, an arallcenyl group, an arallcynyl group, an
aralkoxy group,
a heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroaralkyl group, a
heteroaryloxy group, or a heteroarallcoxy group; and all other symbols are as
defined above
in connection with formula (I).
In yet another aspect of formula (37) of the present invention, R' is
hydrogen, a
halogen, or an alkoxy group; R4 is a cycloalkenyl group, a cycloalkenyloxy
group, an acyl
group or an acyloxy group, an aryl group, an aryloxy group, an aroyl group, an
aroyloxy
group, an aralkyl group, an aralkenyl group, an aralkynyl group, an aralkoxy
group, a
heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroaralkyl group, a
heteroaryloxy group, or a heteroaralkoxy group; and all other symbols are as
defined above
in connection with formula (I).
In still another aspect of formula (37) of the present invention, R' is
hydrogen, a
R~~
halogen, or an allcoxy group; E is ~ or -NR; and R4 is ~R23 or ~ ~ , where Rzz
and R23 independently are hydrogen, a halogen, a vitro group, an amino group,
a mono- or di-
substituted amino group, a hydroxy group, an alkoxy group, a carboxy group, a
cyano group,
an oxo(O=) group, a thio(S=) group, an alkyl group, a cycloallcyl group, an
alkoxy group, a
haloalkoxy group, a cycloalkyl group, an aryl group, a benzyloxy group, an
acyl group, an
acyloxy group, an amyl group, an alkoxycarbonyl group, an aryloxycarbonyl
group, a
heteroaryl group, a heterocyclyl group, an aralkyl group, an alkylsulfonyl
group, an
alkylsulfinyl group, an arylsulfonyl group, an arylsulfinyl group, an
alkylthio group, an
arylthio group, a heteroarylthio group, an aralkylthio group, or a
heterocyclyl sulfonyl group,
which is optionally substituted with a halogen, a hydroxyl group, a vitro
group, an amino
group, an alkyloxy group, or any combination thereof, and wherein the
heterocycle group is
optionally a substituted morpholinyl group, a thiomorpholinyl group, or a
piperzinyl group,
wherein the substituent on the heterocyclyl group is a halogen, a vitro group,
an amino group,
an alkyl group, an alkoxy group, or an aryl group; and all other symbols are
as defined above
in connection with formula (I).
In still another aspect of formula (37) of the present invention, R' is
hydrogen or a
halogen; E is O or NMe; R4 is a substituted aryl group or a heterocycyl group;
R22 is
94
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
hydrogen or an alkoxy group; R22 is hydrogen or an alkoxy group; and all other
symbols are
as defined above in connection with formula (I).
In yet a further aspect of formula (37) of the present invention, Rl is -H, -
F, or MeO;
R22
S
E is O or NMe; R4 is ~RZ3 or ~ ~ , where R2z is -H or OMe; and R23 is -F or
OMe.
An exemplary compound includes, but is not limited to:
OMe
Me0 ~ O ~ I OMe O
O ~ S NH
OMe O
O
According to another aspect of the present invention, various compounds of
general
formula (I) having general formula (IV)
Y~
Ew R4 F~NR
~-G-z_ArY~ I
( ~?
R I R5
its tautomeric forms, its stereoisomers, its polymorphs, its pharnaceutically
acceptable salts,
and its pharmaceutically acceptable solvates are provided. Except as otherwise
provided
herein, all symbols are as defined above in connection with formula (I).
A multitude of compounds having the general formula (IV) are contemplated by
the
present invention. Examples of such compounds include, but are not limited to:
Y1 v1
R1 44
D~~ 11 CYR4 F NR Due/ SYR F NR
~~-R~K-G---Z-Ar~Y2 ~~-RZI K-G---Z-ArY2
(38);
Y1 Y1
D~R1 N~ R4 F~NR R E R4 F~NR
0
K G--__Z-Ar YZ ~~.i K-G---Z-Ar Y~
R Rs R2 Rs
(40); X (41);
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Y~ Y1
S~R1I EYR4 F~NR NCR I EYR4 F~NR
y_R~K-G-Z-Ar~y~ ~~_I~K' -G-Z-Ar--~y ~
R5 (42); R~ IXI RS (43)~
y~
R
R E\ Ra F~NR ~' EYRa F NR
HC~J-~~~ _Z-Arv~Y2 DC- K-G-Z-Ar yz
Rz
R5 (44); RZ ~ R5 (45)~
Y~ Y~
R~ R~ //
E~ R4 F NR ' E~ Ra F~NR
~S_~~K G_-_Z-Ar yZ ~N.I~f~~--G_-_Z-Ar yz
R X RS (46); R2 X R5 (47)~
Y~ Y~
D~I1I EYR4 F NR D~I1I E~Ra F~NR
C. II K-G_Z_Ar~Y2 C.~K-G-Z_Ar~Yz
H~ R5 H ~ R5
R~ ~ (48); RZ X (49)~
Y~ Y~
D~''I EYRa F~NR D~I,I EYRa F~NR
~~_~K' - G (CH2)u'Ar~Y2 ~~-I~KI - G-'-S(=O)~ Ar-~y2
R ~X R (SO); R~ ICI R (Sl)i
Y'
R EYRa F~NR
oG/~ I~
~~ R~ K-(CHZ)5-Z-Ar--~yZ
R (52)~
Y~
1 EYR4 F~NR
2I K-(CH2)s CH=CH-(CH~)s Ar--'~yZ
(S3);
Y~
1 E~ R4 F~NR
~CH2)s C =C-(CH2)S'-Ar~Yz
R I R
(54);
96
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R1 4 ~1 R1 4 ~i
E~R
I EYR ~NR p~I' ~ ' ,NR
~0-~K-G=Z-Ar Yz ~~-I~K-G=Z-Ar~~'~Yz
R IOI R5 (55); Rz ISI R5
Y1
R1 /Y/1
~I EYR4 ~F NR ~~~j E~R4 O~NR
~J_~K-G=Z-Ar-C' "Yz Oy-I~K-G=z-Ar-~YZ
R [S~ ~R5 (S7); Rz jXj R5 (5g);
Y1 1
1
R1 R
4
RN NR
p,; ~ I E~R4 S NR D~; I I Ew R
vJ-I~ K-G=Z-Ar-~Yz ~J-I~ K G=Z-Ar~Yz
R f~I RS (59); R X R5 (60);
O S
1 EYR4 F~NR pTI1 E~R4 F~NR
vJ-I II K-G=Z_Ar~Yz vJ-I II K-G-Z_Ar~Y2
R X R5 (61); RZ X RS
Y1 Y1
R1 R1
E\ R F NR DPI I EYR4 F~NR
~J-I~K G=Z-Ar~O vJ-~K-G=Z-Ar
\ S
RZ X R5 (63); Rz X R5 (64);
Y1
R1 Y1
1 /'
E~R4 ~F~NR ~I E~ R4 F~NR
~J-~~ N G=Z-Ar--C' "Yz O~~-I I N (CHz)s-Z-Ar~Yz
Rz ERs R~ Rs
(65);
Y1 Y1
D~I1I EYR4 F NR OTI I E R F~NR
~-~N-(CHz)s O-Ar~Yz ~-~N-(CHz)s-S-Ar~Yz
R ~X RS (67); Rz IX' SRS (6g);
yi O
I EYR4 R ~F~(NR p~I 1I E~R4 ~F~NR
~~-I~N-(CHz)s-N-Ar~yz ~~-~N-(CHz)s-O-Ar-C' "O
R X ~R5 (69); Rz IoI ~R5
0 0
DPI i~ E~R4 F NR D/i I 1I EYRQ R F~NR
~J-~N-(CHz)s S-Ar~ ~J-~N-(CHz)s N-Ar~O
II IIO
R X RS (71); Rz O R5
97
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R1 4 R1 4
E R O
~I EYR ~NR p~I I Y 1 ,NR
~~-I~ IN-(CHz)s O-Ar~ \\O ~~-~N-(CHz)s S-Ar
R ~O R5 (73); Rz X R5
O O
R~ //
i
~I YR R O NR p~I ~I EYRq S~NR
~~-I~N-(CHz)s-N-Ar~O y_~N-(CHz)s-0-Ar-~O
R ~o R5 (75); RZ [~~ RS
0 0
D RI ( EYR S NR ~I ~ EYR R S~NR
~~-~N-(CHz)s S-Ar~O ~~-I~N-(CHz)s N-Ar~O
R X RS (77); Rz O R5 (7g);
R, 4 R, q
TI EYR \ ,NR ~~ EYR ~t'~R
D~~-I~N- (CHz)z Z-Ar~Yz D~~-I~ IN- (CHz)z O-Ar O
R 'XI R5 (79); Rz ~ R5 (g~);
O
R~ O
4 /,
Ew R O NR R E Rq O~NR
,~I '~'~ Y R
~~~~N-(CHz)2 S-Ar~O Oy-I~N-(CHz)z N-Ar~O
~x R5 (81), RZ ~X R5 (8a)~
JO/ /O/
O R~I E~Rq ~S~(NR p~I~1j E~Rq S~NR
~~-~N-(CHz)z-O-Ar~O ~~-I~N-(CHz)z S-Ar~O
R (X~ R5 (g3); Rz (X~ R5 (g4);
~e1
// 1
Due! 1' E~ Rq S~NR ~' EYRq F~NR
~~-~N (CHz)z N-Ar~O D~~-I~'N-(CHz)s Z-Ar~Yz
RZ ~X( R5 (85); and R2 X R5 (86), where all
symbols are as defined above in connection with formula (I).
Thus, for example, the present invention encompasses various compounds of
general
to compound (IV) having the formula:
R~
N~ R4
N' N G=
N
R O
(8~)~
98
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
where all symbols are as defined above in connection with formula (I). It
should be
understood that while various configurations are provided herein, other
configurations are
contemplated by the present invention. Thus, compounds having the general
formula:
0
R~ S~NR R~ G
N R4 ~' NYR4 S~NR
/ a _ 5 O / _
N'N I N G=Z \ ~ R N'N I N-G=Z
R
R ~ (gg); R
R1
N\ R4
1 /
R N\ Ra ~ N'N ~ N c-z \ /
r _ ~(
N'N ( N G=Z \ ~ S~NR R O Rs ~,
O a
~O
R R5 ~ (90); ~ R (91);
R
O~N O
R1 S R5
N~R4
NN~N G-Z \
and R O (92);
where all symbols are as defined above in connection with formula (I), are
also contemplated
hereby.
According to some variations of the present invention, R, R', R~ , G, and Z of
formulae (88), (89), (90), (91), (92) are selected to produce compounds of
formulae (88-1),
(89-1), (90-1), (91-1), and (92-1) through formulae (88-729), (89-729), (90-
729), (91-729),
and (92-729) as follows:
Formulae R R R R5 G 2
88-1 89-1 90-1 91-192-1 Ra R R''aR Ga Za
a a
88-2 89-2 90-2 91-292-2 Rb Rla R4a Rsa Ga ~a
88-3 89-3 90-3 91-392-3 R~ Rla R4a R5a Ga za
88-4 89-4 90-4 91-492-4 Ra Rib R4a Rsa Ga za
88-5 89-5 90-5 91-592-5 Rb Rlb R4a R5a Ga ~a
88-6 89-6 90-6 91-692-6 R~ Rib R4a RSa Ga Za
88-7 89-7 90-7 91-792-7 Ra RI~ R4a R5a Ga Za
88-8 89-8 90-8 91-892-8 Rb RI R4a R5a Ga Za
88-9 89-9 90-9 91-992-9 R~ R~~ R4a Rsa Ga Za
99
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-1089-10 90-10 91-10 92-10 Ra Ria R4b Rsa Ga Za
88-1189-11 90-11 91-11 92-11 Rb Ria R4b Rsa Ga Za
88-1289-12 90-12 91-12 92-12 Rc Ria R4b Rsa Ga Za
88-1389-13 90-13 91-13 92-13 Ra Rib R4b Rsa Ga Za
88-1489-14 90-14 91-14 92-14 Rb Rib R4b Rsa Ga Za
88-1589-15 90-15 91-15 92-15 R Rib R4b Rsa Ga Za
88-1689-16 90-16 91-16 92-16 Ra Ric R4b Rsa Ga Za
88-1789-17 90-17 91-17 92-17 Rb Ric R'ibRsa Ga Za
88-1889-18 90-18 91-18 92-18 Rc Ric R4b R5a Ga Za
88-1989-19 90-19 91-19 92-19 Ra Ria R4c Rsa Ga Za
88-2089-20 90-20 91-20 92-20 Rb Ria R4c Rsa Ga Za
88-2189-21 90-21 91-21 92-21 Rc Ria R4c Rsa Ga Za
88-2289-22 90-22 91-22 92-22 Ra Rib R4c Rsa Ga Za
88-2389-23 90-23 91-23 92-23 Rb Rib R4c Rsa Ga Za
88-2489-24 90-24 91-24 92-24 Rc Rib R4c Rsa Ga Za
88-2589-25 90-25 91-25 92-25 Ra Ric R4c Rsa Ga Za
88-2689-26 90-26 91-26 92-26 Rb Ric R4c Rsa Ga Za
88-2789-27 90-27 91-27 92-27 R Ric R4c Rsa Ga Za
88-2889-28 90-28 91-28 92-28 Ra Ria R4a Rsb Ga Za
88-2989-29 90-29 91-29 92-29 Rb Ria R4a Rsb Ga Za
88-3089-30 90-30 91-30 92-30 R Ria R4a Rsb Ga Za
88-3189-31 90-31 91-31 92-31 Ra Rib R4a Rsb Ga Za
88-3289-32 90-32 91-32 92-32 Rb Rib R4a Rsb Ga Za
88-3389-33 90-33 91-33 92-33 Rc Rib R4a Rsb Ga Za
88-3489-34 90-34 91-34 92-34 Ra Ric R4a Rsb Ga Za
88-3589-35 90-35 91-35 92-35 Rb Ric R4a Rsb Ga Za
88-3689-36 90-36 91-36 92-36 R Ric R4a Rsb Ga Za
88-3789-37 90-37 91-37 92-37 Ra Ria R4b Rsb Ga Za
88-38$9-38 90-38 91-38 92-38 Rb Ria R4b Rsb Ga Za
88-3989-39 90-39 91-39 92-39 R Ria R4b Rsb Ga Za
88-4089-40 90-40 91-40 92-40 Ra Rib R4b Rsb Ga Za
88-4189-41 90-41 91-41 92-41 Rb Rib R4b Rsb Ga Za
88-4289-42 90-42 91-42 92-42 Rc Rib R4b Rsb Ga Za
88-4389-43 90-43 91-43 92-43 Ra Ric R4b Rsb Ga Za
88-4489-44 90-44 91-44 92-44 Rb Ric R4b Rsb Ga Za
88-4589-45 90-45 91-45 92-45 Rc Ric R4b Rsb Ga Za
88-4689-46 90-46 91-46 92-46 Ra Ria R4c Rsb Ga Za
88-4789-47 90-47 91-47 92-47 Rb Ria R4c Rsb Ga Za
88-4889-48 90-48 91-48 92-48 Rc Ria R'icRsb Ga Za
88-4989-49 90-49 91-49 92-49 Ra Rib R4c Rsb Ga Za
88-5089-50 90-50 91-50 92-50 Rb Rib R4c Rsb Ga Za
88-5189-51 90-51 91-51 92-51 Rc Rib R4c Rsb Ga Za
88-5289-52 90-52 91-52 92-52 Ra Ric R4c Rsb Ga Za
88-5389-53 90-53 91-53 92-53 Rb Ric R4c Rsb Ga Za
88-5489-54 90-54 91-54 92-54 Rc Ric R4c Rsb Ga Za
88-5589-55 90-55 91-55 92-55 Ra Ria R4a Rsc Ga Za
88-5689-56 90-56 91-56 92-56 Rb Ria R4a Rsc Ga Za
100
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-5789-5790-57 91-57 92-57 R Ria R4a R5c Ga Za
88-5889-5890-58 91-58 92-58 Ra Rib R4a R5c Ga Za
88-5989-5990-59 91-59 92-59 Rb Rib R4a Rsc Ga Za
88-6089-6090-60 91-60 92-60 Rc Rib R4a Rsc Ga Za
$8-6189-6190-61 91-61 92-61 Ra Ric R4a Rsc Ga Za
88-6289-6290-62 91-62 92-62 Rb Ric Rya R5 Ga Za
88-6389-6390-63 91-63 92-63 Rc Ric R'iaRsc Ga Za
88-6489-6490-64 91-64 92-64 Ra Ria R4b Rsc ~a Za
88-6589-6590-65 91-65 92-65 Rb Ria R4b Rsc Ga Za
88-6689-6690-66 91-66 92-66 Rc Ria R'ibRsc Ga Za
88-67$9-6790-67 91-67 92-67 Ra Rib R4b Rsc Ga Za
88-6889-6890-68 91-68 92-68 Rb Rib R4b Rsc ~a Za
88-6989-6990-69 91-69 92-69 Rc Rib R4b Rsc Ga Za
88-7089-7090-70 91-70 92-70 Ra Ric R4b Rsc ~a Za
88-7189-7190-71 91-71 92-71 Rb Ric R4b Rsc Ga Za
88-7289-7290-72 91-72 92-72 R Ric R'ibRsc Ga Za
88-7389-7390-73 91-73 92-73 Ra Ria R4c Rsc Ga Za
$8-74$9-7490-74 91-74 92-74 Rb Ria R4c Rsc Ga Za
88-7589-7590-75 91-75 92-75 R Ria R'icRsc Ga Za
88-7689-7690-76 91-76 92-76 Ra Rib R4c R5 Ga Za
88-77$9-7790-77 91-77 92-77 Rb Rib R4c Rsc Ga Za
88-7889-7890-78 91-78 92-78 R Rib R4c Rsc ~a Za
88-7989-7990-79 91-79 92-79 Ra Ric R4c Rsc Ga Za
88-8089-8090-80 91-80 92-80 Rb Ric R4c Rsc Ga Za
88-8189-8190-81 91-81 92-81 Rc Ric R'icRsc Ga Za
88-8289-8290-82 91-82 92-82 Ra Ria R4a Rsa Gb Za
88-8389-8390-83 91-83 92-83 Rb Ria R4a Rsa Gb Za
88-8489-8490-84 91-84 92-84 Rc Ria R4a Rsa Gb Za
$8-8589-8590-85 91-85 92-85 Ra Rib R4a Rsa Gb Za
88-8689-8690-86 91-86 92-86 Rb Rib R4a Rsa Gb Za
88-8789-8790-87 91-87 92-87 Rc Rib R4a Rsa Gb Za
88-8889-8890-88 91-88 92-88 Ra Ric R4a Rsa ~b Za
88-8989-8990-89 91-$9 92-89 Rb Ric R4a Rsa Gb Za
88-9089-9090-90 91-90 92-90 Rc Ric R4a Rsa Gb Za
88-9189-9190-91 91-91 92-91 Ra Ria R4b Rsa Gb Za
88-9289-9290-92 91-92 92-92 Rb Ria R4b Rsa Gb Za
88-9389-9390-93 91-93 92-93 Rc Ria R4b Rsa ~b Za
88-9489-9490-94 91-94 92-94 Ra Rib R4b Rsa Gb Za
88-9589-9590-95 91-95 92-95 Rb Rib R4b Rsa Gb Za
88-9689-9690-96 91-96 92-96 Rc Rib R4b Rsa ~b Za
88-9789-9790-97 91-97 92-97 Ra Ric R4b Rsa Gb Za
88-9889-9890-98 91-98 92-98 Rb Ric R4b Rsa Gb Za
88-9989-9990-99 91-99 92-99 Rc Ric R4b Rsa Gb Za
88-10089-10090-10091-10092-100Ra Ria R'icRsa Gb Za
88-10189-10190-10191-10192-101Rb Ria R4c Rsa Gb Za
88-10289-10290-10291-10292-102Rc Ria R4c Rsa Gb Za
88-10389-10390-10391-10392-103Ra Rib R4c Rsa Gb Za
101
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-10489-10490-10491-10492-104Rb Rib R4c Rsa Gb Za
88-10589-10590-10591-10592-105R Rib R'icRsa Gb za
88-10689-10690-10691-10692-106Ra Ric R4c R5a Gb za
88-10789-10790-10791-10792-107Rb Ric Rdc R5a Gb ~a
88-10889-10890-1089I-10892-108Rc Ric R4c R5a Gb Za
88-10989-10990-10991-10992-109Ra Ria R4a R5b Gb za
88-11089-11090-11091-11092-110Rb Ria R4a Rsb Gb ~a
88-111.89-11190-11191-11192-111Rc Ria R4a RSb Gb Za
88-112$9-11290-11291-11292-112Ra Rib R4a R5b Gb ~a
88-11389-11390-11391-11392-113Rb Rib R4a R5b Gb za
88-11489-11490-11491-11492-114R Rib R4a R5b Gb ~a
88-11589-11590-11591-11592-115Ra Ric R4a Rsb Gb za
88-11689-11690-11691-11692-116Rb Ric R'iaR5b ~b za
88-11789-11790-11791-11792-117Rc Ric R4a R5b Gb za
88-11889-11890-11891-11892-118Ra Ria R4b R5b Gb za
88-11989-11990-11991-11992-I19Rb Ria R4b Rsb Gb za
88-12089-12090-12091-12092-120R Ria R4b R5b ~b za
88-12189-12190-12191-12192-121Ra Rib R4b R5b Gb za
88-12289-I2290-12291-12292-122Rb Rib R4b R5b Gb za
88-12389-12390-12391-12392-123Rc Rib R4b Rsb (ib Za
88-12489-12490-12491-12492-124Ra Ric R4b R5b Gb za
88-12589-12590-12591-12592-125Rb Ric R4b Rsb Gb ~a
88-12689-12690-1269I-12692-126Rc RI~ R4b Rsb ~b ~a
88-12789-12790-12791-12792-127Ra Ria R4c R5b Gb ~a
88-12889-12890-12891-12892-128Rb Ria R4c Rsb Gb Za
88-12989-12990-12991-12992-129R Ria R4c R5b Gb za
88-130$9-13090-13091-13092-130Ra Rib R4c R5b ~b Za
88-13189-13190-13191-13192-131Rb Rib R4c R5b Gb ~a
88-13289-13290-13291-13292-132R Rib R4c R5b Gb Za
88-13389-13390-13391-13392-133Ra Ric R4c Rsb Gb ~a
88-13489-13490-13491-13492-134Rb Ric R4c R5b Gb Za
88-13589-13590-13591-I3592-135R Ric R4c Rsb Gb Za
88-136$9-13690-13691-13692-136Ra Ria R4a R5c Gb za
88-13789-13790-13791-13792-137Rb Ria R4a Rsc Gb ~a
88-13889-13890-13891-13892-138Rc Ria R4a R5c Gb Za
88-13989-13990-13991-13992-139Ra Rib R4a R5c Gb Za
8$-14089-14090-14091-14092-140Rb Rib R4a R5c Gb Za
88-14189-14190-14191-14192-141Rc Rib R4a R5c Gb Za
88-14289-14290-14291-14292-142Ra Ric R4a Rsc ~b Za
88-14389-14390-14391-14392-143Rb Ric R4a R5c Gb za
88-14489-14490-14491-14492-144R Ric R4a R5c Gb Za
88-14S89-14590-14591-14592-145Ra Ria Rdb R5c Gb za
88-14689-14690-14691-14692-146Rb Ria R4b Rsc Gb za
88-14789-14790-14791-14792-147Rc Ria R4b R5c Gb ~a
88-14889-14890-14891-14892-148Ra Rib R4b R5c Gb Za
88-14989-14990-14991-14992-149Rb Rib R4b R5c Gb Za
88-15089-15090-15091-15092-150Rc Rib R4b R5c Gb za
102
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-15189-15190-1519I-15I92-151Ra R1c R4b Rsc Gb za
88-15289-15290-15291-15292-152Rb Rlc R'~bRsc ~b za
88-15389-15390-15391-I5392-153R Rlc R4b R5c Gb Za
8$-15489-15490-1549I-15492-154Ra Rya R4c Rsc ~b Za
88-15589-15590-15591-15592-I55Rb R1a R4c R5c ~b Za
88-15689-15690-15691-15692-156R Ria R4c Rsc Gb za
88-15789-15790-15791-15792-157Ra R1b R4c Rsc Gb za
88-I5889-15890-15891-15892-158Rb Rib R4c Rsc Gb Za
88-I5989-15990-15991-15992-159Rc Rib R4c R5c Gb ~a
88-16089-16090-16091-16092-160Ra R~ R4c R5c Gb za
88-I6189-16190-16191-I6192-161Rb Roc R'~cRsc Gb Za
88-16289-16290-16291-16292-162Rc Rlc R'~cRsc Gb za
88-16389-16390-16391-16392-163Ra R1a R4a R5a Gc Za
88-16489-16490-16491-I6492-164Rb R1a R4a RSa Gc Za
88-16589-16590-I6591-16592-165Rc Rya R4a R5a Gc ~a
88-16689-16690-16691-16692-166Ra Rib jZ~aRsa Gc za
88-16789-16790-I6791-16792-167Rb Rlb R4a R5a Gc ~a
88-16889-16890-16891-16892-168R Rlb R4a R5a Gc Za
88-16989-16990-16991-I6992-169Ra Roc R4a R5a Gc za
88-17089-17090-17091-17092-170Rb Roc Ra Rsa G Za
88-17189-17190-17191-17I92-171R Rlc R4a Rsa ~c Za
88-17289-17290-17291-17292-172Ra Rla R4b R5a Gc ~a
88-17389-17390-17391-17392-173Rb R1a R4b R5a Gc Za
88-I7489-17490-17491-17492-174Rc Rla R4b R5a Gc Za
88-17589-17590-17591-17592-175Ra Rib R4b Rsa Gc za
88-17689-17690-17691-17692-176Rb Rlb R4b R5a Gc Za
88-17789-17790-17791-17792-177R RIb R4b Rsa Gc ~a
88-17889-17890-17891-17892-178Ra Roc R4b Rsa Gc Za
88-17989-17990-1799I-17992-I79Rb Rlc R4b R5a Gc za
88-18089-18090-18091-18092-180Rc RIc R4b Rsa Gc Za
88-18189-18190-18191-18192-18IRa Rya R4c Rsa Gc za
88-I8289-18290-18291-18292-182Rb Rya R4c Rsa ~c za
88-18389-18390-18391-18392-183Rc Rla R4c Rsa Gc ~a
88-18489-18490-18491-18492-184Ra R1b R4c Rsa Gc Za
88-18589-18590-18591-18592-185Rb Rlb R4c R5a Gc ~a
88-18689-18690-18691-18692-186Rc Rib R4c R5a Gc ~a
88-18789-18790-18791-18792-187Ra Roc R'~cRsa ~a Za
88-18889-18890-I8891-18892-188Rb RIc Roc Rsa Gc Za
88-18989-18990-18991-18992-189R Ric R4c Rsa Gc Za
88-19089-19090-19091-19092-190Ra Rya R4a Rsb Gc ~a
88-19189-19190-19191-19192-191Rb Rya R4a Rsb ~c za
88-19289-19290-19291-19292-I92R RIa R4a R5b Gc Za
88-19389-19390-19391-19392-193Ra Rlb R4a R5b Gc Za
88-194$9-19490-19491-19492-194Rb Rib R4a Rsb ~c ~a
88-19589-19590-19591-I9592-195R Rlb R4a R5b Gc za
88-19689-I9690-19691-19692-196Ra R~ R4a Rsb Gc Za
88-19789-19790-19791-19792-197Rb Rlc R4a R5b Gc Za
103
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-19$89-19890-19891-19892-198Rc Ric R4a Rsb Gc Za
88-19989-19990-19991-19992-199Ra Ria R4b Rsb Gc Za
88-20089-20090-20091-20092-200Rb Ria R4b R5b Gc za
88-20189-20190-20191-20192-201Rc Ria R4b R5b Gc za
88-20289-20290-20291-20292-202Ra Rib R4b Rsb Gc Za
88-20389-20390-20391-20392-203Rb Rib R4b Rsb Gc Za
88-20489-20490-20491-20492-204R Rib Rdb Rsb Gc Za
88-20589-20590-20591-20592-205Ra Ric R4b Rsb Gc Za
88-20689-20690-20691-20692-206Rb Ric R'ibRsb Gc Za
88-20789-20790-20791-20792-207Rc Ric R4b R5b G za
88-20889-20890-20891-20892-208Ra Ria R'~ Rsb ~c za
88-20989-20990-20991-20992-209Rb Ria R'~cRsb ~c za
88-21089-2I090-21091-21092-210Rc Ria R4c Rsb Gc Za
88-21189-21190-21191-21192-211Ra Rib R4c Rsb Gc Za
88-21289-21290-21291-21292-212Rb Rib R4c Rsb ~c Za
88-21389-21390-21391-21392-213Rc Rib R4c Rsb Gc za
88-21489-2I490-21491-21492-214Ra Ric R4c Rsi'Gc 2a
88-21589-21590-21591-21592-215Rb Ric R'icRsb Gc za
88-21689-21690-21691-21692-216R Ric R4c R5b ~c Za
88-21789-21790-21791-21792-217Ra Ria R'iaRsc G Za
88-21889-21890-21891-21892-218Rb Ria R'iaRsc G Za
88-21989-21990-21991-21992-219Rc Ria R4a R5c Gc za
8$-22089-22090-22091-22092-220Ra Rib R4a RSc Gc Za
88-22189-22190-22191-22192-221Rb Rib R'iaR5c ~c ~a
88-22289-22290-22291-22292-222Rc Rib R'iaRsc G Za
88-22389-22390-22391-22392-223Ra Ric R4a Rsc Gc za
88-22489-22490-22491-22492-224Rb Ric R4a R5c Gc za
88-22589-22590-22591-22592-225R Ric R4a Rsc ~c ~a
88-22689-22690-22691-22692-226Ra Ria R'ibR5c Gc Za
88-22789-22790-22791-22792-227Rb Ria R'ibRsc Gc Za
88-22889-22890-22891-22892-228Rc Ria R4b R5c ~c Za
88-22989-22990-22991-22992-229Ra Rib R4b RSc Gc za
88-23089-23090-23091-23092-230Rb Rib R4b Rsc Gc Za
88-23189-23190-23191-23192-231Rc Rib jZ'~bR5c Gc Za
88-23289-23290-23291-23292-232Ra Ric R4b Rsc ~c za
88-23389-23390-23391-23392-233Rb Ric R4b Rsc Gc Za
88-23489-23490-23491-23492-234R Ric R4b Rsc Gc za
88-23589-23590-23591-23592-235Ra Ria R4c Rsc ~c za
88-23689-23690-23691-23692-236Rb Ria R'icR5c Gc Za
88-23789-23790-23791-23792-237R Ria R4c Rsc ~c za
88-238$9-23890-23891-23892-238Ra Rib R4c R5c Gc Za
88-23989-23990-23991-23992-239Rb Rib R4c R5c ~c Za
88-24089-24090-24091-24092-240R Rib R4c Rsc Gc Za
88-24189-24190-24191-24192-241Ra Ric R4c R5c Gc za
88-24289-24290-24291-24292-242Rb Ric R4c Rsc ~c ~a
88-24389-24390-24391-24392-243R Ric R'~cRsc Gc Za
88 89-24490-2449I-24492-244Ra Ria R'iaR5a Ga zb
244
104
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
$8-24589-24590-24591-24592-245Rb Rta R4a Rsa Ga zb
88-246$9-24690-24691-24692-246R Rya R4a Rsa Ga zb
88-24789-24790-24791-24792-247Ra RIb R4a Rsa Ga zb
88-24889-24890-24891-24$92-248Rb Rib R4a Rsa Ga Zb
88-24989-24990-24991-24992-249Rc Rlb R4a Rsa Ga zb
88-25089-25090-25091-25092-250Ra Roc R4a Rsa Ga Zb
88-25189-25190-25191-25192-251Rb Rlc R4a Rsa Ga zb
88-25289-25290-25291-25292-252R Rlc R4a Rsa Ga Zb
88-25389-25390-25391-25392-253Ra R1a R4b Rsa Ga ~b
88-25489-25490-25491-25492-254Rb Rla R4b Rsa Ga ~b
88-25589-25590-25591-25592-255R Rya R4b Rsa Ga zb
88-25689-25690-25691-25692-256Ra Rlb R4b Rsa Ga zb
88-25789-25790-25791-25792-257Rb Rib R4b Rsa Ga Zb
88-25889-25890-25891-25892-258Rc Rib R4b Rsa Ga ~b
88-25989-25990-25991-25992-259Ra Roc R'~bRsa Ga ~b
88-26089-26090-26091-26092-260Rb R1c R4b Rsa Ga ~b
88-26189-26190-26191-26I92-261Rc Ric R4b Rsa Ga Zb
88-26289-26290-26291-26292-262Ra Rya R4c Rsa Ga zb
88-26389-26390-26391-26392-263Rb R1a R4c Rsa Ga zb
88-26489-26490-26491-26492-264Rc Rla R'~cRsa Ga Zb
88-26589-26590-26591-26592-265Ra Rib R4c Rsa Ga ~b
88-26689-26690-26691-26692-266Rb Rib R4c Rsa Ga Zb
88-26789-26790-26791-26792-267R RIb R4c Rsa Ga ~b
88-26889-26890-26891-26892-268Ra R1c R4c Rsa Ga zb
88-26989-26990-26991-26992-269Rb Rlc R4c Rsa Ga ~b
88-27089-27090-27091-27092-270Rc Roc Roc Rsa Ga zb
88-27189-27190-27191-27192-271Ra R1a R4a Rsb Ga zb
88-27289-27290-27291-27292-272Rb Rya R4a Rsb Ga zb
88-27389-27390-27391-27392-273Rc Rla R4a Rsb Ga Zb
88-27489-27490-27491-27492-274Ra Rib R4a Rsb Ga zb
88-27589-27590-27591-27592-275Rb Rlb R4a Rsb Ga ~b
88-27689-27690-27691-27692-276R Rlb R4a Rsb Ga zb
$8-27789-27790-27791-27792-277Ra Rlc R4a Rsb Ga zb
88-27889-27890-27891-27892-278Rb Rlc R4a Rsb Ga zb
88-27989-27990-27991-27992-279Rc R~ R4a Rsb Ga ~b
88-28089-28090-28091-28092-280Ra Rya R4b Rsb Ga zb
88-28189-28190-28191-28192-281Rb Rya R4b Rsb Ga ~b
8$-28289-28290-28291-28292-282Rc Rla R4b Rsb Ga zb
88-28389-28390-28391-28392-283Ra Rib R4b Rsb Ga Zb
88-28489-28490-28491-28492-284Rb Rlb R4b Rsb Ga Zb
88-28589-28590-28591-28592-285Rc Rlb R4b Rsb Ga Zb
88-28689-28690-28691-28692-286Ra R1c R4b Rsb Ga zb
88-28789-28790-28791-28792-287Rb Rlc R4b Rsb Ga Zb
88-28889-28890-28891-28892-288Rc RIc R4b Rsb Ga zb
88-28989-28990-28991-28992-289Ra Rla R4c Rsb Ga Zb
88-29089-29090-29091-29092-290Rb Rya R4c Rsb Ga zb
88-29189-29190-29191-29192-291Rc Rya Rc Rsb Ga Zb
105
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-29289-29290-29291-29292-292Ra Rib R4c R5b Ga zb
88-29389-29390-29391-29392-293Rb Rib R4c R5b Ga zb
88-29489-29490-29491-29492-294Rc Rib R4c R5b Ga Zb
88-29589-29590-29591-29592-295Ra Ric R4c R5b Ga Zb
88-29689-29690-29691-29692-296Rb Roc R4c Rsb Ga Zb
88-29789-29790-29791-29792-297R Ric R4c Rsb Ga zb
88-29889-29890-29891-29892-298Ra Ria R4a Rsc Ga zb
88-29989-29990-29991-29992-299Rb Rla R4a Rsc Ga zb
88-30089-30090-30091-30092-300Rc Ria R4a Rsc Ga zb
88-30189-30190-30191-30192-301Ra Rib R4a R5c Ga zb
88-30289-30290-30291-30292-302Rb Rib R4a R5c Ga ~b
88-30389-30390-30391-30392-303R Rib R4a R5c Ga Zb
88-30489-30490-30491-30492-304Ra Ric R4a R5c Ga Zb
88-30589-30590-30591-30592-305Rb Ric R4a R5c ~a zb
88-30689-30690-30691-30692-306Rc Ric R4a R5c Ga zb
88-30789-30790-30791-30792-307Ra Ria R'~bRsc Ga Zb
88-30889-30890-30891-30892-308Rb Ria R4b R5c ~a Zb
88-30989-30990-30991-30992-309Rc Ria R4b Rsc Ga Zb
88-31089-31090-31091-31092-310Ra Rib R4b R5c Ga ~b
88-31189-31190-31191-31192-311Rb Rib R4b R5c Ga Zb
88-31289-31290-31291-31292-312Rc Rib R4b R5c ~a ~b
88-31389-31390-31391-31392-313Ra Ric R4b Rsc Ga zb
88-31489-31490-31491-31492-314Rb R~ R4b Rsc Ga zb
88-31589-31590-31591-31592-315Rc Ric R4b R5c ~a Zb
88-31689-31690-31691-31692-316Ra Ria R4c Rsc Ga ~b
88-31789-3I790-31791-31792-317Rb Ria R4c Rsc ~a zb
88-31889-31890-31891-31892-318Rc Ria R4c Rsc Ga zb
88-31989-31990-31991-31992-319Ra Rib R4c R5c Ga Zb
88-32089-32090-32091-32092-320Rb Rib R4c Rsc Ga zb
88-32189-32190-32191-32192-321Rc Rib R4c Rsc ~a zb
88-32289-32290-32291-32292-322Ra Ric R4c Rsc Ga zb
88-32389-32390-32391-32392-323Rb Ric R4c Rsc Ga Zb
88-32489-32490-32491-32492-324R Ric R4c R5c Ga zb
88-32589-32590-32591-32592-325Ra Ria R4a R5a Gb Zb
88-32689-32690-32691-32692-326Rb Ria R4a Rsa ~b Zb
88-32789-32790-32791-32792-327R Ria R4a RSa Gb zb
88-32889-32890-32891-32892-328Ra Rib R4a RSa Gb Zb
$8-32989-32990-32991-32992-329Rb Rib R4a R5a ~b zb
88-33089-33090-33091-33092-330Rc Rib R4a R5a Gb Zb
88-33189-33190-33191-33192-331Ra Ric R4a Rsa ~b Zb
88-33289-33290-33291-33292-332Rb Ric R4a R5a Gb Zb
88-33389-33390-33391-33392-333Rc Ric R4a R5a Gb Zb
88-33489-33490-33491-33492-334Ra Ria R4b R5a ~b Zb
88-33589-33590-33591-33592-335Rb Ria R4b Rsa Gb zb
88-33689-33690-33691-33692-336Rc Ria R4b R5a Gb zb
88-33789-33790-33791-33792-337Ra Rib R4b R5a Gb zb
88-33889-33890-33891-33892-338Rb Rib R4b Rsa Gb Zb
106
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
8$-33989-33990-33991-33992-339 R Rib R4b R5a ~b Zb
88-34089-34090-34091-34092-340 Ra Ric Rib Rsa Gb Zb
88-34189-34190-34191-34192-341 Rb Ric R4b Rsa Gb Zb
88-34289-34290-34291-34292-342 R Ric R4b R5a Gb Zb
88-34389-34390-34391-34392-343 Ra Ria R4c R5a Gb zb
88-34489-34490-34491-34492-344 Rb Ria R4c R5a Gb Zb
88-34589-34590-34591-34592-345 Rc Ria R4c Rsa ~b zb
88-346.89-34690-34691-34692-346 Ra Rib R4c R5a ~b zb
88-34789-34790-34791-34792-347 Rb Rib R4c R5a Gb Zb
88-34889-34890-34891-34892-348 Rc Rib R4c R5a Gb zb
88-34989-34990-34991-34992-349 Ra Ric R4c Rsa Gb zb
88-35089-35090-35091-35092-350 Rb Ric R4c R5a ~b zb
88-35189-35190-35191-35192-351 Rc Ric R4c R5a Gb Zb
88-35289-35290-35291-35292-352 Ra Ria R4a R5b Gb zb
88-35389-35390-35391-35392-353 Rb Ria R4a R5b Gb zb
88-35489-35490-35491-35492-354 R Ria R'iaR5b Gb Zb
88-35589-35590-35591-35592-355 Ra Rib R4a R5b Gb Zb
88-35689-35690-35691-35692-356 Rb Rib R4a Rsb Gb zb
88-35789-35790-35791-35792-357 R Rib R4a Rsb ~b zb
88-35889-35890-35891-35892-358 Ra Ric R4a R5b Gb Zb
88-35989-35990-35991-35992-359 Rb Ric R4a R5b Gb ~b
88-36089-36090-36091-36092-360 R Ric R4a Rsb Gb zb
88-36189-36190-36191-36192-361 Ra Ria R4b R5b ~b Zb
88-36289-36290-36291-36292-362 Rb Ria R4b R5b Gb zb
88-36389-36390-36391-36392-363 Rc Ria R4b R5b ~b Zb
88-36489-36490-36491-36492-364 Ra Rib R4b R5b Gb ~b
88-36589-36590-36591-36592-365 Rb Rib R4b Rsb Gb zb
88-36689-36690-36691-36692-366 Rc Rib R4b Rsb ~b Zb
88-36789-36790-36791-36792-367 Ra Ric Rab Rsb Gb zb
88-36889-36890-36891-36892-368 Rb Ric R4b R5b Gb zb
88-36989-36990-36991-36992-369 Rc Ric R4b Rsi'Gb Zb
88-37089-37090-37091-37092-370 Ra Ria R4c R5b Gb zb
88-37189-37190-37191-37192-371 Rb Ria R4c R5b Gb Zb
88-37289-37290-37291-37292-372 Rc Ria R4c R5b Gb zb
88-37389-37390-37391-37392-373 Ra Rib R4c R5b Gb zb
88-37489-37490-37491-37492-374 Rb Rib Roc R5b Gb zb
88-37589-37590-37591-37592-375 Rc Rib R4c Rsb Gb Zb
$8-37689-37690-37691-37692-376 Ra Ric R4c Rsb Gb Zb
88-37789-37790-37791-37792-377 Rb Ric R4c R5b Gb Zb
88-37889-37890-37891-37892-378 Rc Ric R4c R5b Gb zb
88-37989-37990-37991-37992-379 Ra Ria R4a Rsc Gb Zb
88-38089-38090-38091-38092-380 Rb Ria R4a R5c Gb ~b
88-38189-38190-38191-38192-381 Rc Ria R4a Rsc ~b zb
88-38289-38290-38291-38292-382 Ra Rib R4a R5c Gb Zb
88-38389-38390-38391-38392-383 Rb Rib R4a R5c Gb zb
88-38489-38490-38491-38492-384 Rc Rib R'iaRsc Gb Zb
88-38589-38590-38591-38592-385 Ra Ric R4a RSc Gb Zb
107
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-38689-38690-38691-38692-386Rb Roc Raa Rsc Gb zb
88-38789-38790-38791-38792-387Rc R~ Raa Rsc Gb Zb
88-38889-38890-38891-38892-388Ra Rla Rab R5c Gb Zb
88-38989-38990-38991-38992-389Rb RIa Rab R5c Gb Zb
88-39089-39090-39091-39092-390Rc Rla Rab Rsc Gb zb
88-391$9-39190-39191-39I92-391Ra Rlb Rab Rsc Gb zb
88-39289-39290-39291-39292-392Rb Rlb Rab Rsc Gb Zb
88-39389-39390-39391-39392-393R Rib Rab Rsc Gb zb
88-39489-39490-39491-39492-394Ra Rlc Rab R5c Gb zb
88-39589-39590-39591-39592-395Rb Rlc Rab Rsc Gb zb
88-39689-39690-39691-39692-396R Rlc Rab Rsc Gb zb
88-39789-39790-39791-39792-397Ra Rla Rac R5c Gb Zb
88-39889-39890-39891-39892-39$Rb Rya Rac Rsc Gb Zb
88-39989-39990-39991-39992-399Rc Rya Rac Rsc Gb Zb
88-40089-40090-4009I-40092-400Ra RIb Rac R5c Gb zb
88-40189-40190-40191-40192-401Rb Rlb Rac Rsc Gb 2b
88-40289-40290-40291-40292-402R Rib Rac Rsc Gb Zb
$8-40389-40390-40391-40392-403Ra Roc Rac Rsc Gb Zb
88-40489-40490-40491-40492-404Rb R' Rac Rsc Gb Zb
88-40589-40590-40591-40592-405Rc Rlc Rac Rsc Gb ~b
88-40689-40690-40691-40692-406Ra Rya Raa Rsa Gc Zb
88-40789-40790-40791-40792-407Rb Rya Raa Rsa Gc Zb
88-40889-40890-40891-40892-408R Rla Raa R5a Gc Zb
88-40989-40990-40991-40992-409Ra Rlb Raa R5a Gc zb
88-41089-41090-41091-41092-410Rb Rlb Raa Rsa Gc Zb
88-41189-41190-41191-41192-411Rc Rib Raa Rsa Gc ~b
88-41289-41290-41291-41292-412Ra Rlc Raa Rsa Gc ~b
88-41389-41390-41391-41392-413Rb Rlc Raa Rsa Gc Zb
88-41489-41490-41491-41492-414Rc Rlc Raa Rsa Gc Zb
88-41589-41590-41591-41592-415Ra Rya Rab Rsa Gc zb
88-41689-41690-41691-41692-416Rb Rla Rab R5a Gc zb
88-41789-41790-41791-41792-417R Rla Rab R5a Gc Zb
88-41889-41890-41891-41892-418Ra Rib Rab R5a Gc zb
88-41989-41990-41991-41992-419Rb Rjb Rab Rsa ~c Zb
88-42089-42090-42091-42092-420Rc Rlb Rab R5a Gc ~b
88-42189-42190-42191-42192-421Ra Roc Rab RSa Gc zb
88-42289-42290-42291-42292-422Rb Roc Rab Rsa Gc zb
88-42389-42390-42391-42392-423R R~ Rab R5a Gc Zb
88-42489-42490-42491-42492-424Ra Rla Rac Rsa Gc zb
88-42589-42590-42591-42592-425Rb Rla Rac R5a Gc ~b
88-42689-42690-42691-42692-426Rc Rya Rac Rsa Gc Zb
88-42789-42790-42791-42792-427Ra R1b Rac RSa Gc zb
88-42889-42890-42891-42892-428Rb Rlb Rac Rsa Gc Zb
88-42989-42990-42991-42992-429R Rib jZa Rsa Gc Zb
88-43089-43090-43091-43092-430Ra Roc Rac Rsa ~c zb
8$-43189-43190-43191-43192-431Rb R~ Rac Rsa
88-43289-43290-43291-43292-432Rc Rlc RaC R5a Gc Zb
108
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-43389-43390-43391-43392-433Ra lZ~a R4a R5b Gc Zb
88-43489-43490-43491-43492-434Rb Rya R4a R5b Gc Zb
88-43589-43590-43591-43592-435R Rla Ra R5b Gc zb
88-43689-43690-43691-43692-436Ra R1b R4a R5b Gc Zb
88-43789-43790-43791-43792-437Rb RIb R4a R5b Gc Zb
88-43889-43890-43891-43892-438Rc Rlb R4a RSb Gc Zb
88-43989-43990-43991-43992-439Ra Rlc R4a R5b Gc zb
88-44089-44090-44091-44092-440Rb Rlc R'~aR5b Gc Zb
88-44189-44190-44191-44192-441Rc RIc R4a R5b Gc zb
88-44289-44290-44291-44292-442Ra RIa R4b RSb Gc zb
88-44389-44390-44391-44392-443Rb Rla R4b Rsb Gc Zb
88-44489-44490-44491-44492-444Rc Rya R4b Rsb Gc zb
88-44589-44590-44591-44592-445Ra Rtb R4b R5b Gc Zb
88-44689-44690-44691-44692-446Rb Rib Rab Rsb Gc Zb
88-44789-44790-44791-44792-447Rc Rlb Rib Rsb G Zb
88-44889-44890-4489I-44892-448Ra Rlc R4b Rsb Gc Zb
88-44989-44990-44991-44992-449Rb Roc R4b Rsb Gc zb
88-45089-45090-45091-45092-450Rc Roc R4b Rsb G zb
8$-45189-45190-45191-45192-451Ra Rya R4c Rsb ~c Zb
88-45289-45290-45291-45292-452Rb Rla R4c R5b Gc zb
88-45389-45390-45391-45392-453R Rla R4c R5b Gc ~b
88-45489-45490-45491-45492-454Ra Rib R4c Rsb ~c Zb
88-45589-45590-4559I-45592-455Rb RIb Rac Rsb Gc Zb
88-45689-45690-45691-45692-456R Rlb R4c Rsb Gc Zb
88-45789-45790-45791-45792-457Ra Rlc R4c R5b Gc Zb
88-45889-45890-45891-45892-458Rb Rlc R4 Rsb Gc Zb
88-45989-45990-45991-45992-459Rc Roc R4c Rsb Gc Zb
88-46089-46090-46091-46092-460Ra Rya R4a R5c Gc Zb
88-46189-46190-46191-46192-461Rb Rya R4a Rsc G Zb
88-46289-46290-46291-46292-462Rc Rla R4a R5c Gc Zb
88-46389-46390-46391-46392-463Ra Rib R4a R5c Gc Zb
88-46489-46490-46491-46492-464Rb Rib R4a R5c Gc Zb
88-46589-46590-46591-46592-465R Rib R4a Rsc Gc Zb
88-46689-46690-46691-46692-466Ra Roc R4a R5c Gc zb
88-46789-46790-46791-46792-467Rb Roc R4a Rsc G Zb
88-46889-46890-46891-46892-468R Roc R4a Rsc Gc Zb
88-46989-46990-46991-46992-469Ra Rla R4b R5c Gc zb
88-47089-47090-47091-47092-470Rb RIa R4b Rsc Gc Zb
88-47189-47190-47191-47192-471R Rla jZ4bR5c Gc Zb
88-47289-47290-47291-47292-472Ra Rib R4b Rsc ~c zb
88-47389-47390-47391-47392-473Rb RIb R'~bRsc Gc Zb
88-47489-47490-47491-47492-474Rc Rib R4b Rsc G Zb
88-47589-47590-47591-47592-475Ra Roc R4b Rsc Gc ~b
88-47689-47690-47691-47692-476Rb R' R4b Rsc G Zb
88-47789-47790-47791-47792-477Rc Rlc R4b Rsc Gc Zb
88-47889-47890-47891-47892-478Ra Rya R4c R5c Gc zb
88-47989-47990-47991-47992-479Rb Rya R4c Rsc Gc zb
109
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-48089-48090-48091-48092-480 R Rya R4c R5c Gc zb
88-48189-48190-48191-48192-481 Ra RIb R4c R5c Gc zb
88-48289-48290-48291-48292-482 Rb Rib R4c Rsc Gc Zb
88-48389-48390-48391-48392-483 R Rlb R4c Rsc G Zb
88-48489-48490-4$491-48492-484 Ra RIc R4c RSc Gc zb
88-48S89-48S90-48S91-48592-485 Rb R~ R4c Rsc Gc Zb
88-48689-48690-48691-48692-486 R Roc R4c Rsc G Zb
88-48789-48790-48791-48792-487 Ra Rla R4a R5a ~a ~c
88-48889-48890-48891-48892-488 Rb Rja R4a R5a Ga Zc
88-48989-48990-48991-48992-489 R Rya R4a R5a Ga zc
88-49089-49090-49091-49092-490 Ra Rjb R'~aRsa Ga Zc
88-49189-49190-49191-49I92-491 Rb Rlb R'~aRsa Ga Zc
88-49289-49290-49291-49292-492 Rc RIb R4a R5a Ga zc
88-49389-49390-49391-49392-493 Ra Rlc R4a R5a Ga ~c
88-49489-49490-49491-49492-494 Rb Rlc Ra R5a ~a ~c
88-49589-49S90-49S91-49S92-495 Rc R~ R4a Rsa Ga zc
88-49689-49690-49691-49692-496 Ra Rla R4b R5a Ga Zc
88-49789-49790-49791-49792-497 Rb Rja R4b R5a Ga ~c
88-49889-49890-49891-49$92-498 R Rla R4b RSa Ga 2c
88-49989-49990-49991-49992-499 Ra Rib R4b R5a Ga zc
88-50089-S0090-S0091-50092-500 Rb RIb R4b R5a Ga Zc
88-50189-S0190-50191-50192-501 Rc Rjb R4b R5a Ga zc
88-50289-S0290-50291-S0292-S02 Ra Rlc jZ'~bRsa Ga Zc
88-S0389-50390-50391-S0392-S03 Rb Roc R4b R5a Ga zc
88-S0489-S0490-S0491-50492-S04 R Rlc R4b R5a ~a Zc
88-50589-50590-50591-SOS92-SOS Ra Rya R4c R5a Ga zc
88-50689-S0690-S0691-50692-S06 Rb R1a R4c Rsa Ga ~c
88-50789-50790-S0791-S0792-507 R Rya R4c R5a Ga zc
88-S0889-S0890-S0891-50892-508 Ra RIb R'~cRsa Ga zc
88-50989-50990-S0991-50992-S09 Rb Rlb R4c R5a Ga Zc
88-S1089-51090-51091-51092-S10 Rc R1b R4c Rsa ~a ~c
88-S1189-51190-51191-51192-511 Ra Rlc R4c Rsa Ga ~c
$8-S1289-51290-51291-S1292-S12 Rb RIc R4c R5a ~a Zc
88-S 89-S 90-51391-51392-S Rc R~ R'~cRsa ~a Zc
13 13 13
88-S 89-51490-S 91-S 92-514 Ra Rl R4a R5b Ga Zc
14 14 14 a
88-S 89-S 90-51591-51592-515 Rb RIa R4a R5b Ga 2c
1 1
S S
88-S1689-51690-51691-S1692-516 Rc Rya R4a Rsb Ga Zc
88-51789-51790-51791-51792-S17 Ra Rlb R4a Rsb Ga ~c
88-S1889-51890-51891-S1892-S18 Rb RIb R4a Rsb Ga ~c
88-51989-S1990-51991-51992-S19 Rc Rlb R4a Rsb Ga ~c
88-S2089-52090-52091-S2092-S20 Ra Rlc R4a Rsb ~a Zc
88-52189-52190-52191-52192-S21 Rb Roc R'~aRsb ~a zc
88-52289-52290-52291-52292-S22 R Rlc R4a R5b Ga Zc
88-S2389-S2390-52391-S2392-S23 Ra Rla R4b RSb Ga Zc
88-S2489-52490-52491-52492-S24 Rb Ria R4b R5b Ga Zc
88-S2S89-S2590-52S91-52592-S25 R Rla R4b R5b Ga Zc
88-S2689-52690-S2691-S2692-S26 Ra R1b R4b R5b Ga Zc
110
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-52789-52790-52791-52792-527Rb Rib Rb Rsb
88-52889-52890-52891-52892-528Rc Rib R4b Rsb Ga Zc
88-52989-52990-52991-52992-529Ra R~ R4b Rsb Ga ~c
88-53089-53090-53091-53092-530Rb Rlc R4b Rsb Ga Zc
88-53189-53190-53191-53192-531R Rlc R4b Rsb Ga zc
88-53289-53290-53291-53292-532Ra Rta R4c R56 ~a ~c
88-53389-53390-53391-53392-533Rb Rla R4c Rsb Ga Zc
88-53489-53490-53491-53492-534Rc Rta R4c Rsb Ga zc
88-53589-53590-5359I-53592-535Ra Rib R4c Rsb Ga zc
88-53689-53690-53691-53692-536Rb Rlb R4c Rsb Ga Zc
88-53789-53790-53791-53792-537Rc Rlb R4c Rsb Ga zc
88-53889-53890-53891-53892-538Ra Roc R4c Rsb ~a zc
88-53989-53990-53991-53992-539Rb RIc R4c Rsb Ga zc
88-54089-54090-54091-54092-540Rc Rlc R4c Rsb Ga Zc
88-54189-54190-54191-54192-541Ra Rla R'~aRsc ~a Zc
88-54289-54290-54291-54292-542Rb Rya R4a Rsc Ga ~c
88-54389-54390-54391-54392-543R Rya R4a Rsc Ga Zc
88-54489-54490-54491-54492-544Ra Rib R4a Rsc Ga zc
88-54589-54590-54591-54592-545Rb Rlb R4a Rsc Ga zc
88-54689-54690-54691-54692-546Rc Rib R4a Rsc Ga zc
88-54789-54790-54791-54792-547Ra Rlc R4a Rsc Ga ~c
88-54889-54890-54891-54892-548Rb Rlc R4a Rsc Ga Zc
88-54989-54990-54991-54992-549Rc RIc R4a Rsc Ga zc
88-55089-55090-55091-55092-550Ra Rya R4b R5c Ga Zc
88-55189-55190-55191-55192-551Rb Rya R4b Rsc Ga Zc
88-55289-55290-55291-55292-552R Rla R4b Rsc Ga zc
88-55389-55390-55391-55392-553Ra Rib R4b Rsc Ga Zc
88-55489-55490-55491-55492-554Rb Rlb R'~bRsc Ga Zc
88-55589-55590-55591-55592-555Rc Rib R4b Rsc Ga Zc
88-55689-55690-55691-55692-556Ra Ric R4b Rsc Ga ~c
88-55789-55790-55791-55792-557Rb Roc R4b Rsc Ga Zc
88-55889-55890-55891-55892-558Rc R~c R4b Rsc ~a zc
8$-55989-55990-55991-55992-559Ra RIa R4c Rsc Ga Zc
88-56089-56090-56091-56092-560Rb R1a R4c Rsc Ga Zc
88-56189-56190-56191-56192-561Rc Rla R4c Rsc Ga Zc
88-56289-56290-56291-56292-562Ra Rlb R4c Rsc Ga zc
88-56389-56390-56391-56392-563Rb Rlb R4c Rsc Ga Zc
88-56489-56490-56491-56492-564Rc Rlb R4c Rsc Ga Zc
88-56589-56590-56591-56592-565Ra Roc R4c Rsc ~a Zc
88-56689-56690-56691-56692-566Rb Roc R4c Rsc ~a zc
88-56789-56790-56791-56792-567Rc R~c R4c Rsc ~a zc
88-56889-56890-56891-56892-568Ra Rla R4a Rsa Gb Zc
88-56989-56990-56991-56992-569Rb R1a R4a Rsa Gb Zc
88-57089-57090-57091-57092-570Rc Rla R4a Rsa ~b zc
88-57189-57190-57191-57192-571Ra Rib R4a Rsa Gb Zc
88-57289-57290-57291-57292-572Rb Rib R4a Rsa Gb zc
88-57389-57390-57391-57392-573Rc Rib R4a Rsa Gb zc
111
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-57489-57490-57491-57492-574Ra R~ R4a Rsa Gb zc
88-57589-57590-57591-57592-575Rb Roc R4a Rsa Gb zc
88-57689-57690-57691-57692-576Rc Rlc R4a Rsa Gb zc
88-57789-57790-57791-57792-577Ra RIa R4b Rsa Gb zc
88-57$89-57890-57891-57892-57$Rb RIa R4b Rsa Gb zc
88-57989-57990-57991-57992-579Rc Rya R4b Rsa Gb Zc
88-58089-58090-58091-58092-580Ra Rlb R4b Rsa Gb zc
88-58189-58190-58191-58192-581Rb RIb R4b Rsa Gb zc
88-58289-58290-58291-58292-582RC RIb R4b Rsa Gb zc
88-583$9-58390-58391-58392-5$3Ra R1c R4b Rsa Gb zc
88-58489-58490-58491-58492-584Rb RIc R4b Rsa Gb Zc
88-58589-58590-58591-58592-585Rc RIc R4b Rsa Gb zc
88-58689-58690-58691-58692-586Ra Rya R4c Rsa Gb Zc
88-58789-58790-58791-58792-587Rb Rya R4c Rsa Gb Zc
88-58$89-58890-58891-58892-588Rc Rya R4c Rsa Gb zc
88-58989-58990-58991-58992-589Ra jyb R4c Rsa Gb zc
88-59089-59090-59091-59092-590Rb Rlb R4c Rsa Gb zc
$8-59189-59190-59191-59192-591Rc Rib R4c Rsa Gb zc
88-59289-59290-59291-59292-592Ra Roc R4c Rsa Gb zc
88-59389-59390-59391-59392-593Rb Roc R4c Rsa Gb zc
88-59489-59490-59491-59492-594R R' Roc Rsa Gb Zc
88-59589-59590-59591-59592-595Ra Rla R4a Rsb Gb Zc
88-59689-59690-59691-59692-596Rb Rla R4a Rsb Gb Zc
88-59789-59790-59791-59792-597Rc Ria R4a Rsb Gb zc
88-59889-59890-59891-59892-598Ra RIb R4a Rsb Gb zc
88-59989-59990-59991-59992-599Rb Rtb R4a R5b Gb Zc
88-60089-60090-60091-60092-600R Rlb R4a Rsb Gb zc
$8-60189-60190-60191-60192-601Ra Rlc R4a Rsb Gb zc
88-60289-60290-60291-60292-602Rb Roc R4a R~' Gb Zc
88-60389-60390-60391-60392-603Rc RIc R4a Rsb Gb zc
88-60489-60490-60491-60492-604Ra Rya R4b Rsb Gb zc
88-60589-60590-60591-60592-605Rb R1a R4b Rsb Gb zc
8$-60689-60690-60691-60692-606R Rla R4b Rsb Gb Zc
88-60789-60790-60791-60792-607Ra Rjb R4b Rsb Gb zc
88-60889-60890-60891-60892-608Rb Rlb Rab Rsb Gb Zc
88-60989-60990-60991-60992-609Rc Rlb Rb Rsb Gb Zc
88-610$9-61090-61091-61092-610Ra Roc R4b Rsb Gb zc
88-61189-61190-61191-61192-611Rb Rlc Rab Rsb Gb Zc
88-61289-61290-61291-61292-612R RIc R4b Rsb Gb Zc
8$-61389-61390-61391-61392-613Ra Rya R4c Rsb Gb zc
88-61489-61490-61491-61492-614Rb Ria R4c Rsb Gb Zc
88-61589-61590-61591-61592-615R Rya R4c Rsb Gb zc
88-61689-61690-61691-61692-616Ra Rib R4c Rsb Gb Zc
88-61789-61790-61791-61792-617Rb Rib R'~cRsb Gb Zc
8$-61889-61890-61891-61892-618R Rib R4c Rsb Gb Zc
88-61989-61990-61991-61992-619Ra Rtc R4c Rsb Gb zc
88-62089-62090-62091-62092-620Rb Rlc R4c Rsb Gb zc
112
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-62189-62190-62191-62192-621Rc Rlc Rc Rsb Gb Zc
88-62289-62290-62291-62292-622Ra Rla Rda Rsc Gb Zc
88-62389-62390-62391-62392-623Rb RIa R4a R5c ~b Zc
88-62489-62490-62491-62492-624Rc Rya R4a R5c Gb zc
88-62589-62590-62591-62592-625Ra Rlb R'~aR5c Gb zc
88-62689-62690-62691-62692-626Rb Rib R'~aR5c ~b zc
88-62789-62790-62791-62792-627R Rlb R'~aRsc Gb Zc
88-62889-628.90-62891-62892-628Ra Rlc R4a R5c Gb zc
88-62989-62990-62991-62992-629Rb R~ R4a Rsc Gb Zc
88-63089-63090-63091-63092-630R Roc R4a RSc Gb zc
88-63189-63190-63191-63I92-631Ra Rya R4b Rsc Gb zc
88-63289-63290-63291-63292-632Rb Rla R4b R5c ~b Zc
88-63389-63390-63391-63392-633R Rta R4b Rsc Gb Zc
88-63489-63490-63491-63492-634Ra R1b R4b Rsc Gb zc
88-63589-63590-63591-63592-635Rb Rlb Rib Rsc Gb Z
88-63689-63690-63691-63692-636Rc Rlb Rab R5c Gb zc
88-63789-63790-63791-63792-637Ra Roc R4b R5c Gb zc
88-63889-63890-63891-63892-638Rb R1c R4b Rsc Gb zc
88-63989-63990-63991-63992-639Rc Rlc R4b Rsc Gb zc
88-64089-64090-64091-64092-640Ra Rla R4c R5c Gb 2c
88-64189-64190-64191-64192-641Rb Rya R4c R5c Gb zc
$8-64289-64290-64291-64292-642Rc Rya R4c Rsc Gb
88-64389-64390-64391-64392-643Ra Rib R4c R5c Gb Zc
88-64489-64490-64491-64492-644Rb Rib Rc Rsc Gb Zc
88-64589-64590-64591-64592-645Rc Rlb Rc Rsc Gb Z
88-64689-64690-64691-64692-646Ra Roc R4c R5c ~b Zc
88-64789-64790-64791-64792-647Rb Rlc R4c Rsc Gb Zc
88-64889-64890-64891-64892-648Rc Rlc R4c Rsc Gb Z
88-64989-64990-64991-64992-649Ra RIa R4a R5a ~c zc
88-65089-65090-65091-65092-650Rb Rla R4a R5a Gc zc
88-65189-65190-65191-65192-651Rc RIa R4a Rsa Gc Zc
88-65289-65290-65291-65292-652Ra Rlb R4a R5a Gc zc
88-65389-65390-65391-65392-653Rb RIb R4a R5a Gc Zc
88-65489-65490-65491-65492-654Rc Rlb R4a R5a ~c Zc
88-65589-65590-65591-65592-655Ra RIc R4a R5a Gc Zc
88-65689-65690-65691-65692-656Rb Rlc R4a R5a Gc Zc
88-65789-65790-65791-65792-657R R1c R4a RSa Gc zc
88-65889-65890-65891-65892-658Ra Rla R4b R5a ~c Zc
88-65989-65990-65991-65992-659Rb Rya R4b Rsa Gc Zc
88-66089-66090-66091-66092-660Rc Rla R4b R5a Gc Zc
88-66189-66190-66191-66192-661Ra RIb R4b Rsa Gc zc
88-66289-66290-66291-66292-662Rb Rtb R4b R5a Gc Zc
88-66389-66390-66391-66392-663Rc Rlb R4b Rsa Gc Zc
88-66489-66490-66491-66492-664Ra Rlc R4b R5a Gc zc
88-66589-66590-66591-66592-665Rb Rlc R4b R5a Gc 2c
88-66689-66690-66691-66692-666R R1c R4b R5a Gc zc
88-66789-66790-66791-66792-667Ra Rla R4c Rsa Gc Zc
113
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-66889-66890-66891-66892-668Rb Ria R4c R5a Gc zc
88-66989-66990-66991-66992-669R Ria R4c Rsa Gc zc
88-67089-67090-67091-67092-670Ra Rib R4c Rsa Gc Zc
88-67189-67I90-67191-67192-671Rb Rib R4c Rsa ~c zc
$8-67289-67290-67291-67292-672Rc Rib R4c Rsa Gc ~c
88-67389-67390-67391-67392-673Ra Ric R4c R5a Gc ~c
88-67489-67490-67491-67492-674Rb Ric R4c R5a Gc zc
88-67589-67590-67591-67592-675Rc Ric R'icRSa Gc zc
88-67689-67690-67691-67692-676Ra Ria R4a R5b Gc ~c
88-67789-67790-67791-67792-677Rb Ria R4a R5b Gc zc
88-67889-67890-67891-67892-678Rc Ria R4a R5b Gc zc
88-67989-67990-67991-67992-679Ra Rib R4a R5b Gc ~c
88-68089-68090-68091-68092-680Rb Rib R4a R5b Gc zc
88-68189-68190-68191-68192-681Rc Rib R4a R5b Gc 2c
88-68289-68290-68291-68292-682Ra Ric R'laR5b Gc zc
8$-68389-68390-68391-68392-683Rb Ric R4a R5b Gc zc
88-68489-68490-68491-68492-684Rc Ric R4a R5b ~c zc
88-68589-68590-68591-68592-685Ra Ria R4b Rsb Gc 2c
88-68689-68690-68691-68692-686Rb Ria R4b Rsb ~c zc
88-68789-68790-68791-68792-687R Ria R4b Rsb Gc ~c
88-68889-68890-68891-68892-6$8Ra Rib R4b R5b Gc ~c
88-68989-68990-68991-68992-689Rb Rib Rb Rsb Gc Zc
88-69089-69090-69091-69092-690R Rib Rab Rsb G Z
88-69189-69190-69191-69192-691Ra Ric R4b Rsb Gc zc
88-69289-69290-69291-69292-692Rb Ric R4b Rsb G' Z
88-69389-69390-69391-69392-693Rc Ric R4b Rsb Ci Z
88-69489-69490-69491-69492-694Ra Ria R4c R5b Gc zc
88-69589-69590-69591-69592-695Rb Ria R4c R5b ~c zc
88-69689-69690-69691-69692-696Rc Ria R4c RSb Gc zc
88-69789-69790-69791-69792-697Ra Rib R4c R5b Gc zc
88-69889-69890-69891-69892-698Rb Rib R'icRsb G Z
88-69989-69990-69991-69992-699Rc Rib R4c Rsb G Z
88-70089-70090-70091-70092-700Ra Ric R4c Rsb Gc 2c
88-70189-70190-70191-70192-701Rb Ric R4c Rsb G Z
88-70289-70290-70291-70292-702Rc Ric R4c Rsb G Z
88-70389-70390-70391-70392-703Ra Ria R4a Rsc Gc zc
88-70489-70490-70491-70492-704Rb Ria R4a R5c Gc zc
88-70589-70590-70591-70592-705R Ria R4a R5c Gc zc
88-70689-70690-70691-70692-706Ra Rib R4a R5c Gc zc
88-70789-70790-70791-70792-707Rb Rib R4a Rsc Gc zc
88-70889-70890-70891-70892-708Rc Rib R4a Rsc Gc Zc
88-70989-70990-70991-70992-709Ra Ric R'laRsc ~c zc
88-71089-71090-71091-71092-710Rb Ric R4a Rsc Gc zc
88-71189-71190-71191-71192-71IR Ric R4a Rsc Gc Zc
88-71289-71290-71291-71292-712Ra Ria R4b Rsc Gc zc
88-71389-71390-71391-71392-713Rb Ria R4b Rsc Gc zc
88-71489-71490-71491-71492-714Rc Ria R4b RSc Gc zc
114
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
88-71589-71590-71591-71592-715Ra Rib Rab Rsc Gc zc
88-71689-71690-71691-71692-716Rb Rlb Rab Rsc Gc Zc
88-71789-71790-71791-71792-717Rc Rlb Rab Rsc G Zc
8$-71889-71890-71891-71892-718Ra R~ RaU Rsc G Z
88-71989-71990-71991-71992-719Rb Roc Rab Rsc Gc Zc
88-72089-72090-72091-72092-720Rc Rlc Rab Rsc G Zc
88-72189-72190-72191-72192-721Ra Rla Rac R5c Gc Zc
88-72289-72290-72291-72292-722Rb Rya Rac Rsc Gc Zc
88-72389-72390-72391-72392-723Rc RIa Rac RSc Gc zc
88-72489-72490-72491-72492-724Ra Rlb Rac Rsc Gc zc
88-72589-72590-72591-72S92-725Rb Rjb Rac Rsc Gc Z
88-72689-72690-72691-72692-726Rc Rlb Rac Rsc Gc Zc
88-72789-72790-7279I-72792-727Ra Roc Rac Rs Gc zc
88-72889-72890-72891-72892-728Rb Roc Rac Rsc Gc Zc
88-72989-72990-72991-72992-729R Roc Rac Rsc Gc Z
where all symbols are as defined above.
In one aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is -H or CH3, and all other symbols are as defined above in
connection with
formula (I).
In another aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is H or GH3, Rs is -H, and all other symbols are as defined above
in connection
with formula (1).
In another aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
to invention, R is -H or CH3; Rs is CH3; and all other symbols are as defined
above in
connection with formula (I).
In still another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3; G is -(CH?)S , where s is an integer from 0-5; and
alI other
symbols are as defined above in connection with formula (I).
In a further aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is -H or -CH3; Rs is -H; G is -(CHZ)S , where s is an integer
from 0-5; and all
other symbols are as defined above in connection with formula (I).
In a further aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is -H or CH3; Rs is CH3; G is -(CHZ)S , where s is an integer
from 0-5; and all
other symbols are as defined above in connection with formula (I).
115
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In a further aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is -H or CH3, Z is -NR; and all other symbols are as defined
above in connection
with formula (I).
In a further aspect of any of formulae (88), (89), (90), (91), and (92) of the
present
invention, R is -H or CH3, RS is -H or CH3; Z is -NR; and all other symbols
are as defined
above in connection with formula (I).
In a still further aspect of any of formulae (88), (89), (90), (91), and (92)
of the
present invention, R is -H or CH3, G is -(CHZ)S , where s is an integer from 0-
5; Z is -NR;
and all other symbols are as defined above in connection with formula (I).
l0 In a still further aspect of any of formulae (88), (89), (90), (9I), and
(92) of the
present invention, R is -H or CH3; RS is -H; G is -(CH2)S , where s is an
integer from 0-5; Z
is -NR; and all other symbols are as defined above in connection with formula
(I).
In a yet further aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3, Z is O; and all other symbols are as defined above
in connection
with formula (I).
In a yet further aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3; R5 is CH3; G is -(CHZ)S , where s is an integer
from 0-5; Z is -NR;
and all other symbols are as defined above in connection with formula (I).
In still another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3, RS is -H or CH3, Z is O, and all other symbols are
as defined
above in connection with formula (I).
In still another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3; G is -(CH2)S , where s is an integer from 0-5; Z is
O; and all other
symbols are as defined above in connection with formula (I).
In still another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3, G is -(CH?)S , where s is an integer from 0-5; Z is
O; and all other
symbols are as defined above in connection with formula (I).
In still another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3; RS is -H; G is -(CHz)S , where s is an integer from
0-5; Z is O; and
3o all other symbols are as defined above in connection with formula (I).
116
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In yet another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, R is -H or CH3; Rs is CH3; G is -(CH2)S-, where s is an integer
from 0-5; Z is O;
and all other symbols are as defined above in connection with formula (I).
In yet another aspect of any of formulae (88), (89), (90), (91), and (92) of
the present
invention, Rø is a substituted or unsubstituted aryl group; and all other
symbols are as defined
above in connection with formula (I).
The present invention also encompasses various compounds of general formula
(IV)
having a formula:
Ra
N- G=
R O
(93),
NY
N
N
to where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, R4, Rs, G, and Z of
formula
(93) are selected to produce compounds of formula (93-1) through (93-243) as
follows:
Formula R R Rs G Z
93-1 Ra R a Rsa Ga Za
93-2 Rb R4a Rsa Ga Za
93-3 R~ R4a Rsa Ga Za
93-4 Ra R4b Rsa Ga Za
93-5 Rb R4b Rsa Ga Za
93-6 R~ R4U Rsa Ga Za
93-7 Ra IZ4 Rsa Ga Za
93-8 Rb R4 Rsa Ga Za
93-9 R R4~ Rsa Ga Za
93-10 Ra R4a Rsb Ga Za
93-11 Rb R'~a Rsb Ga Za
93-12 R~ R4a Rsb Ga Za
93-13 Ra R4b Rsb Ga Za
93-14 Rb R'~b Rsb Ga Za
93-15 R R4b Rsb Ga Za
93-16 Ra R'~ Rsb Ga Za
93-17 Rb R4 Rsb Ga Za
93-18 R~ R4 Rsb Ga Za
93-19 Ra R4a Rs~ Ga Za
93-20 Rb Raa IZs~ Ga Za
93-21 R R'~~ Rs~ Ga Za
117
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
93-22Ra R4b Rsc Ga Za
93-23Rb R4b Rsc Ga za
93-24R R4b Rsc Ga Za
93-25Ra R4c Rsc Ga Za
93-26Rb R4c Rsc ~a za
93-27Rc R4c Rsc Ga Za
93-28Ra Rda Rsa Gb Za
93-29Rb R4a Rsa Gb Za
93-30Rc R4a Rsa Gb za
93-31Ra R4b Rsa Gb za
93-32Rb R4b R5a Gb za
93-33Rc R4b Rsa Gb Za
93-34Ra R4c Rsa Gb Za
93-35Rb R4c Rsa Gb Za
93-36Rc R4c Rsa Gb za
93-37Ra R4a Rsb ~b Za
93-38Rb R4a Rsb ~b za
93-39Rc R4a Rsb ~b Za
93-40Ra R4b Rsb Gb za
93-41Rb 1Z'~b Rsb ~b za
93-42Rc R4b Rsb ~b za
93-43Ra R4c Rsb ~b za
93-44Rb R4c Rsb Gb za
93-45Rc R4c Rsb Gb Za
93-46Ra R4a Rsc Gb za
93-47Rb R4a Rsc ~b za
93-48Rc R4a Rsc Gb za
93-49Ra R4b Rsc ~b za
93-50Rb R4b Rsc Gb za
93-51R R4b Rsc Gb ~a
93-52Ra R4c Rsc Gb ~a
93-53Rb R4c Rsc ~b ~a
93-54Rc R4c Rsc Gb za
93-SSRa R4a R5a ~c ~a
93-56Rb R4a Rsa Gc za
93-57Rc R4a Rsa Gc Za
93-58Ra R4b Rsa ~c 2a
93-59Rb R4b Rsa Gc za
93-60Rc R4b Rsa Gc Za
93-61Ra R4c Rsa ~c za
93-62Rb R4c Rsa ~c za
93-63Rc R4c Rsa Gc Za
93-64Ra R4a Rsb Gc Za
93-65Rb R4a Rsb Gc za
93-66R R4a Rsb ~c ~a
93-67Ra R4b Rsb ~c za
93-68Rb R4b Rsb Gc za
118
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
93-69 Rc R4b Rsb Gc za
93-70 Ra R4c Rsb Gc za
93-71 Rb R4C Rsb Gc 2a
93-72 Rc R4c Rsb ~c za
93-73 Ra Rya R5c Gc Za
93-74 Rb R4a Rsc Gc za
93-75 Rc R4a Rsc ~c za
93-76 Ra R4b Rsc Gc za
.
93-77 Rb R4b Rsc Gc Za
93-78 Rc R4b Rsc Gc za
93-79 Ra R4c Rsc Gc za
93-80 Rb R4c Rsc Gc Za
93-81 Rc R4 Rsc Gc ~a
93-82 Ra R4a Rsa Ga zb
93-83 Rb R4a Rsa Ga ~b
93-84 Rc R4a Rsa Ga zb
93-85 Ra R4b Rsa Ga zb
93-86 Rb R4b Rsa Ga
93-87 R R4b Rsa Ga zb
93-88 Ra R4c Rsa ~a ~b
93-89 Rb R4c Rsa Ga 2b
93-90 R R4c Rsa Ga zb
93-91 Ra R4a Rsb Ga ~b
93-92 Rb Rda Rsb Ga ~b
93-93 R R4a Rsb (sa Zb
93-94 Ra R4b Rsb Ga zb
93-95 Rb R4b Rsb Ga zb
93-96 Rc R4b Rsb Ga Zb
93-97 Ra R'~c Rsb Ga zb
93-98 Rb R4c Rsb Ga zb
93-99 Rc R4c Rsb Ga zb
93-100Ra R4a Rsc Ga
93-101Rb R'~a Rsc Ga Zb
93-102R R4a Rsc ~a
93-103Ra R4b Rsc Ga zb
93-104Rb R4b Rsc Ga Zb
93-105Rc R4b Rsc Ga Zb
93-106Ra R4c Rs Ga ~b
93-107Rb R4c Rsc Ga Zb
93-108Rc R'~ Rsc Ga Zb
93-109Ra R4a Rsa Gb Zb
93-110Rb R4a Rsa Gb zb
93-111Rc R4a Rsa Gb zb
93-112Ra R4b Rsa Gb zb
93-113Rb R4b Rsa Gb zb
93-114R R4b Rsa Gb Zb
93-115Ra R4c Rsa Gb zb
119
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
93-116Rb R4c Rsa Gb Zb
93-117Rc R4c Rsa Gb Zb
93-118Ra R4a Rsb Gb zb
93-119Rb R4a Rsb Gb zb
93-120Rc R4a R5b Gb zb
93-121Ra R4b Rsb Gb ~b
_
93-122Rb R'~b Rsb Gb Zb
93-123R R4b Rsb Gb zb
93-124Ra R4c Rsb Gb zb
93-125Rb R4c Rsb Gb ~b
93-126R R4c Rsb Gb zb
93-127Ra R4a Rsc Gb zb
93-128Rb R4a Rsc Gb Zb
93-129R R4a R5c ~b ~b
93-130Ra R4b Rsc Gb zb
93-131Rb R4b Rsc Gb Zb
93-132R R4b Rsc Gb Zb
93-133Ra R4c Rsc Gb Zb
93-134Rb R4c Rsc Gb zb
93-135Rc R4c Rsc Gb Zb
93-136Ra R4a Rsa Gc Zb
93-137Rb R4a Rsa Gc zb
93-138Rc R4a Rsa ~c Zb
93-139Ra R4b Rsa ~c zb
93-140Rb R4b Rsa ~c ~b
93-141Rc R'~b Rsa Gc Zb
93-142Ra R4c Rsa ~c zb
93-143Rb R4c R5a Gc ~b
93-144R R4c Rsa Gc zb
93-145Ra R4a Rsb ~c zb
93-146Rb R4a Rsb ~c ~b
93-147R R4a Rsb Gc Zb
93-148Ra R4b Rsb Gc zb
93-149Rb R4b Rsb Gc Zb
93-150Rc Rib Rsb Gc Zb
93-151Ra R4c Rsb Gc Zb
93-152Rb R4c Rsb Gc Zb
93-153Rc R4c Rsb G Zb
93-154Ra Ra Rsc Gc Zb
93-155Rb R4a R5c Gc Zb
93-156Rc R'~a Rsc Gc Zb
93-157Ra R4b Rsc Gc ~b
93-158Rb R4b Rsc Gc Zb
93-159Rc R4b Rsc GC Zb
93-160Ra R4c Rsc G Zb
93-161Rb R4c Rsc Gc Zb
93-162Rc R4c Rsc Gc Zb
120
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
93-163Ra R4a Rsa Ga Zc
93-164Rb R4a Rsa Ga zc
93-165R R4a Rsa Ga zc
93-166Ra R4b Rsa Ga zc
93-167Rb R4b Rsa Ga Zc
93-168Rc R4b Rsa Ga zc
93-169Ra R4c Rsa ~a zc
93-170Rb R4c Rsa Ga Zc
93-171Rc R4c Rsa Ga Zc
93-172Ra R4a Rsb Ga Zc
93-173Rb R4a Rsb Ga zc
93-174Rc R4a Rsb ~a zc
93-175Ra R4b Rsb ~a zc
93-176Rb R4b Rsb ~a zc
93-177R R4b Rsb ~a zc
93-178Ra R4 Rsb Ga Zc
93-179Rb R4c Rsb Ga Zc
93-180R R4c Rsb Va zc
93-181Ra R4a Rso Ga Zc
93-182Rb R4a Rsc Ga zc
93-183Rc R4a R5c Ga zc
93-184Ra R'~b Rsc Ga Z
93-185Rb R4b Rsc ~a Zc
93-186Rc R4b R5c Ga ~c
93-187Ra R4c Rsc ~a Zc
93-188Rb R4c Rsc Ga ~c
93-189Rc R4c Rsc Ga Zc
93-190Ra R4a Rsa Gb ~c
93-191Rb R4a Rsa Gb ~c
93-192Rc R4a Rsa Gb ~c
93-193Ra R4b R5a ~b Zc
93-194Rb R4b Rsa ~b ~c
93-195Rc R4b Rsa Gb zc
93-196Ra R4 Rsa Gb Zc
93-197Rb R4c Rsa Gb Zc
93-198R R4c Rsa Gb zc
93-199Ra R4a Rsb Gb zc
93-200Rb R4a R5b Gb Zc
93-201Rc R4a Rsb Gb Z
93-202Ra R4b Rsb Gb zc
93-203Rb R4b Rsb Gb Zc
93-204Rc R'~b Rsb Gb zc
93-205Ra R4c Rsb Gb Zc
93-206Rb R4c Rsb Gb Zc
93-207Rc R4c Rsb Gb Z
93-208Ra R4a Rsc Gb Zc
93-209Rb R4a Rsc Gb Zc
121
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
93-210Rc R'~a Rsc ~b zc
93-211Ra R4b Rsc Gb Zc
93-212Rb R4b Rsc Gb Z
93-213R R4b Rsc Gb Z
93-214Ra R4c Rsc Gb zc
93-215Rb R4c Rs Gb Zc
93-216Rc Roc Rsc Gb Zc
93-217Ra R4a Rsa Gc zc
93-218Rb R4a Rsa Gc zo
93-219R R4a Rsa Gc zc
93-220Ra R4b Rsa Gc Zc
93-221Rb R4b R5a Gc Zc
93-222Rc R4b Rsa Gc zc
93-223Ra R4c Rsa Gc zc
93-224Rb R4c Rsa Gc Zc
93-225Rc R4c Rsa G Zc
93-226Ra R4a Rsb ~c zc
93-227Rb R4a Rsb Gc zc
93-228Rc R4a Rsb ~c zc
93-229Ra R4 Rsb Gc zc
93-230Rv R4b Rsb Gc Zc
93-231R R'~b Rsb Gc Zc
93-232Ra R'~ Rsb Gc Zc
93-233Rb R4c Rsb (1c ~c
93-234R Roc Rsb G Zc
93-235Ra R4a Rsc Gc ~o
93-236Rb R4a Rsc ~c zc
93-237Rc R4a Rsc Gc ~c
93-238Ra R4b Rsc Gc Zc
93-239Rb R4b Rsc Gc Zc
93-240Rc R4b Rsc G Z
93-241Ra R4c Rsc Gc ~o
93-242Rb R4c Rsc Gc Zc
93-243R R4c Rsc Gc Zc
where all symbols are as defined above.
In one aspect of any of formula (93) of the present invention, R is -H or CH3,
and all
other symbols are as defined above in connection with formula (I).
In another aspect of any of formula (93) of the present invention, R4 is a
substituted
or unsubstituted aryl group; and all other symbols are as defined above in
connection with
formula (I).
In another aspect of any of formula (93) of the present invention, RS is -H or
CH3, and
all other symbols are as defined above in connection with formula (I).
122
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In yet another aspect of any of formula (93) of the present invention, G is -
(CH2)s-,
where s is an integer from 0-5; and all other symbols are as defined above in
connection with
formula (I).
In yet another aspect of formula (93) of the present invention, R is hydrogen,
a
hydroxy group, a halogen, a vitro group, an optionally substituted amino
group, an alkyl
group, an alkoxy group, an alkenyl group, or an alkoxyalkyl group; R4 is an
alkenyl group, a
cycloallcenyl group, an alkoxyalkyl group, an alkenyloxy group, a
cycloalkenyloxy group, an
acyl group or an acyloxy group, an aryl group, an aryloxy group, an aroyl
group, an aroyloxy
group, an aralkyl group, an aralkenyl group, an aralkynyl group, an aralkoxy
group, a
l0 heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroaralkyl group, a
heteroaryloxy group, or a heteroaralkoxy group; RS is hydrogen, a hydroxy
group, a halogen,
a vitro group, an optionally substituted amino group, an alkyl group, an
allcoxy group, an
alkenyl group, or an alkoxyalkyl group; and all other symbols are as defined
above in
connection with formula (I).
In still another aspect of formula (93) of the present invention, R is
hydrogen, a
hydroxy group, a halogen, a vitro group, a carboxy group, a carbamoyl group,
an optionally
substituted amino group, or an alkyl group; R4 is a cycloalkenyl group, a
cycloalkenyloxy
group, an acyl group or an acyloxy group, an aryl group, an aryloxy group, an
aroyl group, an
aroyloxy group, an arallcyl group, an aralkenyl group, an arallcynyl group, an
aralkoxy group,
a heterocyclyl group, a heterocyclenyl group, a heteroaryl group, a
heteroaralkyl group, a
heteroaryloxy group, or a heteroaralkoxy group; RS is hydrogen, a hydroxy
group, a halogen,
an alkyl group, or an alkoxy group; and all other symbols are as defined above
in connection
with formula (I).
In yet another aspect of formula (93) of the present invention, R is hydrogen
or an
alkyl group; R4 is a substituted or unsubstituted aryl group; G is f CH2~2,
(CH~33, or (CH2~4; Z
is O, S, or NH; and RS is hydrogen or an alkyl group.
In still another aspect of formula (93) of the present invention, R is -H or
CH3; R4 is a
substituted or unsubstituted aryl group; G is f CHZj~, (CH2)3, or f CHZ)4; Z
is O, S, or NH; and
RS is -H or CH3.
The present invention also encompasses various compounds of general formula
(IV)
as follows:
123
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
0
Rio O Rio ~--NR
R9 ~~ ' NR RW~ S O
I ° N ~
Nr I N~ w Ns I w ; Rs
~N~N G=Z ~ / N~N G O ~
tv~e ~o (94); tv~e IoI (95);
0
Rio °\~ R~o ~L--NR
RwGI ~N O R~~~I S O
I , N I
Nr ~(I N ~ Rs N/ ~ ~ ~ R5
N~N G-S ~ / N_ 1fN C'
Me [°~ (96); and Me IoI (97);
where R9 and RI° independently are hydrogen, a halogen, a nitro group,
an amino group, a
mono- or di- substituted amino group, a hydroxy group, an alkoxy group, a
carboxy group, a
cyano group, an oxo(O=) group, a thio(S=) group, an alkyl group, a cycloalkyl
group, an
alkoxy group, a haloalkoxy group, a cycloalkyl group, an aryl group, a
benzyloxy group, an
acyl group, an acyloxy group, an amyl group, an alkoxycarbonyl group, an
aryloxycarbonyl
group, a heteroaryl group, a heterocyclyl group, an arallcyl group, an
alkylsulfonyl group, an
to alkylsulfinyl group, an arylsulfonyl group, an arylsulfinyl group, an
alkylthio group, an
arylthio group, a heteroarylthio group, an aralkylthio group, or a
heterocyclyl sulfonyl group,
which is optionally substituted with a halogen, a hydroxyl group, a nitro
group, an amino
group, an alkyloxy group, or any combination thereof, and wherein the
heterocycle group is
optionally a substituted morpholinyl group, a thiomorpholinyl group, or a
piperzinyl group,
wherein the substituent on the heterocyclyl group is a halogen, a nitro group,
an amino group,
an alkyl group, an alkoxy group, or an aryl group; and all other symbols are
as defined above
in connection with formula (I).
According to various aspects of the present invention, R, R5, G, Z, R9, and
Rl° of any
of formulae (94), (95), (96), and (97) are selected to produce compounds of
formulae (94-1),
2o (95-1), (96-1), and (97-1) through formulae (94-729), (95-729), (96-729),
and (97-729) as
follows:
124
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Formulae R RS R R G Z
94-1 95-1 96-1 97-1 Ra RSa R RI Ga Za
a a
94-2 95-2 96-2 97-2 Rb RSa R9a RlOa Ga Za
94-3 95-3 96-3 97-3 R Rsa R9a RlOa Ga za
94-4 95-4 96-4 97-4 Ra RSb R9a RlOa Ga za
94-5 95-5 96-5 97-5 Rb RSb R9a Rioa Ga Za
94-6 95-6 96-6 97-6 Rc RSb R9a Rloa Ga Za
94-7 95-7 96-7 97-7 Ra RSc R9a RlOa Ga ~a
94-8 95-8 96-8 97-$ Rb Rsc R9a RlOa Ga ~a
94-9 95-9 96-9 97-9 R R5c R9a RIOa Ga za
94-109S-10 96-10 97-10 Ra RSa R9b Rloa Ga Za
94-1195-11 96-11 97-11 Rb RSa R9b RIOa Ga ~a
94-1295-12 96-12 97-12 R RSa R9b RIOa Ga Za
94-1395-13 96-13 97-13 Ra RSb R9b RlOa Ga ~a
94-1495-14 96-14 97-14 Rb RSb R9b RlOa Ga Za
94-1595-15 96-15 97-15 Rc Rsb R9b RIOa Ga Za
94-1695-16 96-16 97-16 Ra Rsc R9b RlOa Ga za
94-1795-17 96-17 97-17 Rb RSc R9b RlOa Ga ~a
94-1895-18 96-18 97-18 R R5c R9b RlOa Ga Za
94-1995-19 96-19 97-19 Ra RSa R9c RlOa Ga ~a
94-2095-20 96-20 97-20 Rb R5a R9c RlOa Ga Za
94-2195-21 96-21 97-21 R R5a R9c RlOa Ga Za
94-2295-22 96-22 97-22 Ra RSb R9c RIOa Ga Za
94-2395-23 96-23 97-23 Rb RSb R9c RlOa Ga ~a
94-2495-24 96-24 97-24 Rc RSb R9c RIOa Ga Za
94-2595-25 96-25 97-25 Ra RSc R9c RlOa Ga Za
94-2695-26 96-26 97-26 Rb RSc R9c RlOa Ga Za
94-2795-27 96-27 97-27 R RSc R9c RlOa Ga za
94-2895-28 96-28 97-28 Ra RSa R9a RlOb Ga za
94-2995-29 96-29 97-29 Rb RSa R9a Rlob Ga za
94-3095-30 96-30 97-30 Rc Rsa R9a RlOb Ga ~a
94-3195-31 96-31 97-31 Ra R5b R9a RlOb Ga Za
94-3295-32 96-32 97-32 Rb RSb R9a RIOb Ga za
94-3395-33 96-33 97-33 Rc Rsb R9a RlOb Ga Za
94-3495-34 96-34 97-34 Ra RSc R9a RlOb Ga za
94-3595-35 96-35 97-35 Rb R5c R9a RlOb Ga Za
94-3695-36 96-36 97-36 RC RSc R9a RlOb Ga Za
94-3795-37 96-37 97-37 Ra RSa R9b RlOb Ga Za
94-3895-38 96-38 97-38 Rb RSa R9b RlOb Ga Za
94-3995-39 96-39 97-39 Rc Rsa R9b RlOb Ga za
94-4095-40 96-40 97-40 Ra RSb R9b Riob Ga Za
94-4I95-41 96-41 97-41 Rb R5b R9b Rrob Ga Za
94-4295-42 96-42 97-42 Rc Rsb R9b RlOb Ga za
94-4395-43 96-43 97-43 Ra Rsc R9b RlOb Ga Za
94-4495-44 96-44 97-44 Rb Rsc R9b RIOb Ga Za
94-4595-45 96-45 97-45 Rc RSc R9b RIOb Ga Za
.
125
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-4695-46 96-46 97-46 Ra R5a R9c RlOb Ga za
94-4795-47 96-47 97-47 Rb RSa R9c RlOb Ga za
94-4895-48 96-48 97-48 Rc Rsa R9c RIOb Ga Za
94-4995-49 96-49 97-49 Ra Rsb R9c RlOb Ga Za
94-5095-50 96-50 97-50 Rb R5b R9c RlOb Ga Za
94-5195-51 96-51 97-51 Rc RSb R9c RlOb Ga Za
94-5295-52 96-52 97-52 Ra R5c R9c RIOb Ga za
94-5395-53 96-53 97-53 Rb Rsc R9c RlOb Ga za
94-5495-54 96-54 97-54 Rc RSc Rgc RlOb Ga za
94-5595-55 96-55 97-55 Ra RSa R9a RlOc Ga Za
94-5695-56 96-56 97-56 Rb RSa R9a RlOc Ga za
94-5795-57 96-57 97-57 Rc R5a R9a RlOc Ga ~a
94-5895-58 96-58 97-58 Ra R5b R9a RlOc Ga ~a
94-5995-59 96-59 97-59 Rb RSb R9a RlOc Ga za
94-6095-60 96-60 97-60 Rc R5b R9a RlOc Ga Za
94-6195-6I 96-61 97-61 Ra R5c R9a RlOc Ga za
94-6295-62 96-62 97-62 Rb RSc R9a RIOc Ga za
94-6395-63 96-63 97-63 R Rsc R9a RIOc Ga za
94-6495-64 96-64 97-64 Ra R5a R9b RlOc Ga ~a
94-6595-65 96-65 97-65 Rb RSa R9b RIOc Ga za
94-6695-66 96-66 97-66 Rc Rya R9b RlOc ~a za
94-6795-67 96-67 97-67 Ra R5b R9b RIOc Ga Za
94-6895-68 96-68 97-68 Rb R5b R9b RIOc Ga Za
94-6995-69 96-69 97-69 Rc R5b R9b RlOc Ga za
94-7095-70 96-70 97-70 Ra Rsc R9b RlOc Ga za
94-7I95-71 96-71 97-71 Rb Rsc R9b RIOc Ga ~a
94-7295-72 96-72 97-72 R Rsc R9b Rloc ~a za
94-7395-73 96-73 97-73 Ra R5a R9c RlOc ~a Za
94-7495-74 96-74 97-74 Rb RSa R9c RlOc ~a ~a
94-7595-75 96-75 97-75 Rc RSa R9c RlOc Ga Za
94-7695-76 96-76 97-76 Ra Rsb R9c RlOc Ga za
94-7795-77 96-77 97-77 Rb RSb R9c RlOc ~a za
94-7895-78 96-78 97-78 Rc R5b R9c RlOc Ga za
94-7995-79 96-79 97-79 Ra RSc R9c RIOc Ga Za
94-8095-80 96-80 97-80 Rb R5c R9c RlOc Ga Za
94-8195-81 96-81 97-81 R Rsc R9c RlOc Ga Za
94-8295-82 96-82 97-82 Ra Rsa R9a RlOa Gb Za
94-8395-83 96-83 97-83 Rb R5a R9a Rioa Gb Za
94-8495-84 96-84 97-84 Rc RSa R9a R~oa Gb za
94-8595-85 96-85 97-85 Ra R5b R9a RlOa Gb Za
94-8695-86 96-86 97-86 Rb Rsb R9a RIOa Gb Za
94-8795-87 96-87 97-87 R RSb R9a RlOa Gb Za
94-8895-88 96-88 97-8$ Ra RSc R9a RIOa Gb Za
94-8995-89 96-89 97-89 Rb RSc R9a RiOa Gb Za
94-9095-90 96-90 97-90 Rc RSc R9a RlOa Gb za
94-9195-91 96-91 97-91 Ra R5a R9b RlOa ~b Za
94-9295-92 96-92 97-92 Rb R5a R9b RlOa Gb Za
126
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-9395-93 96-93 97-93 R Rsa R9b RlOa Gb Za
94-9495-94 96-94 97-94 Ra RSb R9b RIOa Gb Za
94-9595-95 96-95 97-95 Rb RSb R9b RlOa Gb za
94-9695-96 96-96 97-96 Rc R5b R9b RlOa Gb za
94-9795-97 96-97 97-97 Ra Rsc R9b RIOa Gb Za
94-9895-98 96-98 97-98 Rb RSc R9b RIOa Gb ~a
94-9995-99 96-99 97-99 Rc R5c R9b Rloa Gb za
94-10095-10096-10097-100Ra RSa R9c RIOa Gb za
94-10195-10196-10197-101Rb Rsa R9c RlOa Gb za
94-10295-10296-10297-102Rc Rsa R9c RiOa Gb za
94-10395-10396-10397-103Ra RSb R9c RlOa Gb ~a
94-10495-10496-10497-104Rb RSb R9c RlOa ~b Za
94-10595-10596-10597-105Rc Rsb R9c RIOa Gb za
94-10695-10696-10697-106Ra Rsc R9c RlOa ~b ~a
94-10795-10796-10797-107Rb Rsc R9c RlOa ~b ~a
94-10895-10896-10897-108R RSc R9c RlOa Gb ~a
94-10995-10996-10997-109Ra RSa R9a RIOb Gb Za
94-11095-11096-11097-110Rb Rsa R9a RlOb Gb za
94-11195-11196-11197-111Rc RSa R9a RlOb Gb Za
94-11295-11296-11297-112Ra Rsb R9a RlOb Gb za
94-11395-11396-11397-113Rb Rsb R9a RlOb ~b ~a
94-11495-11496-11497-114Rc R5b R9a RlOb Gb za
94-11595-11596-11597-115Ra Rsc R9a RIOb Gb Za
94-11695-11696-11697-116Rb Rsc R9a RlOb Gb Za
94-11795-11796-11797-117Rc R5c R9a RlOb Gb ~a
94-11895-11896-11897-118Ra RSa R9b RIOb Gb ~a
94-11995-11996-11997-119Rb RSa R9b Rlob Gb za
94-12095-12096-12097-120R Rsa R9b RlOb Gb ~a
94-12195-12196-12197-121Ra Rsb R9b RlOb Gb Za
94-12295-12296-12297-122Rb RSb R9b RIOb ~b ~a
94-12395-12396-12397-123Rc RSb R9b RIOb ~b za
94-12495-12496-12497-124Ra RSc R9b RIOb Gb Za
94-12595-12596-12597-125Rb Rsc R9b RlOb Gb Za
94-12695-12696-12697-126Rc RSc R9b RlOb Gb za
94-12795-12796-12797-127Ra R5a R9c Rlob Gb Za
94-12895-12896-12897-128Rb Rsa R9c RlOb Gb Za
94-12995-12996-12997-129R Rsa R9c RlOb Gb Za
94-13095-13096-13097-130Ra RSb R9c RlOb Gb Za
94-13195-13196-13197-131Rb Rsb R9c RIOb Gb Za
94-13295-13296-13297-132R R5b R9c Rlob ~b Za
94-13395-13396-13397-133Ra Rsc R9c RlOb Gb Za
94-13495-13496-13497-134Rb Rsc R9c RlOb Gb za
94-13595-13596-13597-135Rc Rsc R9c RlOb Gb Za
94-13695-13696-13697-136Ra RSa R9a RlOc Gb Za
94-13795-I3796-13797-137Rb Rsa R9a RlOc Gb Za
94-13895-13896-13897-138Rc Rsa R9a RlOc Gb za
94-13995-13996-13997-139Ra RSb R9a RlOc Gb Za
127
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-14095-14096-14097-140Rb RSb R9a RlOc Gb za
94-14195-14196-14197-141Rc RSb R9a RlOc Gb Za
94-14295-14296-14297-142Ra R5c R9a RIOc Gb Za
94-14395-14396-14397-143Rb RSc R9a RlOc Gb ~a
94-14495-14496-14497-144R Rsc R9a RlOc Gb Za
94-14595-14596-14597-145Ra RSa R9b RlOc Gb za
94-14695-14696-14697-146Rb RSa R9b RlOc Gb Za
94-14795-14796-I4797-147R Rsa R9b RlOc ~b za
94-14895-14896-14897-148Ra RSb R9b RIOc Gb Za
94-I4995-I4996-14997-I49Rb RSb R9b RlOc Gb Za
94-15095-15096-15097-150Rc RSb R9b Rloc Gb za
94-15195-15196-ISI97-151Ra RSc R9b RlOc Gb za
94-15295-15296-15297-152Rb RSc R9b RIOc Gb za
94-15395-I5396-15397-153R RSc R9b RlOc Gb za
94-I5495-15496-15497-I54Ra RSa R9c Rloc Gb za
94-15595-I5596-I5597-I55Rb RSa R9c RIOc ~b za
94-15695-15696-15697-156Rc Rsa R9c RlOc Gb za
94-15795-15796-15797-157Ra RSb R9c RlOc Gb za
94-15895-I5896-15897-158Rb RSb R9c RlOc ~b za
94-15995-15996-15997-159Rc Rsb R9c RIOc Gb za
94-I6095-16096-16097-160Ra RSc R9c RlOc Gb za
94-16195-I6196-16197-I61Rb Rsc R9c RIOc Gb Za
94-16295-16296-16297-I62RC Rsc R9c RIOc Gb ~a
94-I6395-16396-16397-163Ra RSa R9a RiOa ~c Za
94-16495-16496-16497-164Rb RSa R9a RlOa Gc ~a
94-16595-16596-16597-I65Rc RSa R9a RIOa Gc za
94-16695-16696-16697-166Ra Rib R9a RIOa Gc za
94-I6795-16796-16797-167Rb Rsb R9a RlOa Gc Za
94-16895-16896-16897-168Rc RSb R9a Rloa Gc ~a
94-16995-16996-16997-I69Ra R5c R9a RIOa ~c za
94-17095-17096-17097-170Rb Rsc R9a RIOa Gc za
94-17195-17196-17197-I71R Rsc R9a RlOa Gc Za
94-17295-17296-17297-172Ra Rsa R9b RIOa Gc Za
94-17395-17396-17397-173Rb RSa R9b RIOa ~c Za
94-17495-17496-17497-174R Rsa R9b RlOa Gc Za
94-17595-I7596-17597-175Ra RSb R9b RlOa ~c Za
94-17695-17696-17697-176Rb Rsb R9b RlOa Gc ~a
94-17795-17796-17797-177R Rsb R9b RIOa Gc Za
94-17895-17896-17897-178Ra RSc R9b RlOa Gc za
94-17995-17996-17997-179Rb R5c R9b RlOa ~c Za
94-18095-18096-18097-180Rc RSc R9b RIOa Gc Za
94-18195-18196-18197-181Ra RSa R9c RlOa Gc za
94-18295-18296-18297-182Rb RSa R9c RlOa Gc Za
94-18395-18396-18397-183Rc RSa R9c RlOa Gc Za
94-18495-18496-18497-184Ra Rsb R9c RIOa ~c Za
94-18595-18596-18597-185Rb Rsb R9c R~oa Gc za
94-18695-18696-18697-186Rc Rsb R9c RlOa Gc Za
128
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-18795-18796-18797-187Ra RSc R9c RlOa Gc za
94-18895-18896-18897-188Rb RSc R9c RlOa Gc Za
94-18995-18996-18997-189Rc RSc R9c RlOa Gc za
94-19095-19096-19097-190Ra Rsa R9a RlOb Gc Za
94-19195-19196-19197-191Rb RSa R9a RlOb Gc za
94-19295-19296-19297-192Rc RSa Rga RlOb Gc Za
94-19395-19396-19397-193Ra R5b R9a RlOb Gc Za
94-19495-19496-19497-194Rb R5b R9a RIOb Gc Za
94-19595-19596-19597-195Rc Rsb R9a RIOb Gc za
94-19695-19696-19697-196Ra Rsc R9a RlOb Gc Za
94-19795-19796-19797-197Rb Rsc R9a RlOb Gc za
94-19895-19896-19897-198R Rsc R9a RlOb Gc Za
94-19995-19996-19997-199Ra RSa R9b RIOb Gc Za
94-20095-20096-20097-200Rb RSa R9b RlOb Gc za
94-20195-20196-20197-201R Rsa R9b RlOb Gc za
94-20295-20296-20297-202Ra R56 R9b RIOb ~c za
94-20395-20396-20397-203Rb RSb R9b RlOb Gc ~a
94-20495-20496-20497-204Rc R56 Rib RIOb Gc Za
94-20595-20596-20597-205Ra RSc R9b RIOb Gc Za
94-20695-20696-20697-206Rb Rsc R96 RlOb ~c za
94-20795-20796-20797-207Rc Rsc R9b RiOb Gc za
94-20895-20896-20897-208Ra R5a R9c RIOb Gc Za
94-20995-20996-20997-209Rb RSa R9c RlOb Gc za
94-21095-21096-21097-210Rc Rsa R9c RlOb Gc Za
94-21195-21196-21197-211Ra Rsb R9c RIOb ~c ~a
94-21295-21296-21297-212Rb R56 R9c RIOb Gc ~a
94-21395-21396-21397-213Rc RSb ROc RIOb ~c za
94-21495-21496-21497-214Ra RSc R9c RlOb ~c Za
94-21595-21596-21597-215Rb R5c R9c RIOb ~c Za
94-21695-21696-21697-216Rc R5c R9c RlOb Gc za
94-21795-21796-21797-217Ra RSa R9a RIOc Gc Za
94-21895-21896-21897-218Rb RSa R9a RIOc Gc Za
94-21995-21996-21997-219Rc RSa R9a RIOc ~c za
94-22095-22096-22097-220Ra RSb R9a RlOc ~c Za
94-22195-22196-22197-221Rb RSb R9a RlOc Gc za
94-22295-22296-22297-222Rc R56 R9a RIOc Gc Za
94-22395-22396-22397-223Ra R5c R9a RIOc Gc Za
94-22495-22496-22497-224Rb R5c R9a RlOc Gc Za
94-22595-22596-22597-225R Rsc R9a RIOc Gc za
94-22695-22696-22697-226Ra RSa R9b RlOc Gc Za
94-22795-22796-22797-227Rb RSa R9b RlOc Gc Za
94-22895-22896-22897-228Rc RSa R9b RlOc Gc Za
94-22995-22996-22997-229Ra RSb R9b RIOc Gc za
94-23095-23096-23097-230Rb R56 R9b RlOc Gc Za
94-23195-23196-23197-231R R5b R9b RlOc Gc Za
94-23295-23296-23297-232Ra Rsc R96 RlOc Gc Za
94-23395-23396-23397-233Rb RSc R9b Rtoc Gc Za
129
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-23495-23496-23497-234Rc RSc Rgb Rloc Gc 2a
94-23595-23596-23597-235Ra RSa R9c RlOc Gc Za
94-23695-23696-23697-236Rb R5a R9c RlOc Gc za
94-23795-23796-23797-237R RSa R9c RlOc Gc za
94-23895-23896-23897-238Ra R5b R9c RlOc ~c Za
94-23995-23996-23997-239Rb RSb R9c Rtoc Gc Za
94-24095-24096-24097-240R R~' R9c RlOc Gc za
94-24195-24196-24197-241Ra R5c R9c RlOc Gc za
94-24295-24296-24297-242Rb R5c R9c RlOc Gc za
94-24395-24396-24397-243Rc Rsc R9c RlOc Gc ~a
94-24495-24496-24497-244Ra RSa R9a RIOa Ga zb
94-24595-24596-24597-245Rb Rsa R9a Rloa Ga zb
94-24695-24696-24697-246Rc RSa R9a RlOa Ga zb
94-24795-24796-24797-247Ra R56 R9a RlOa Ga zb
94-24895-24896-24897-248Rb R56 R9a RlOa Ga Zb
94-24995-24996-24997-249Rc R5b R9a Rloa Ga Zb
94-25095-25096-25097-250Ra R5c Rga RlOa Ga ~b
94-25195-25196-25197-251Rb Rsc R9a RlOa Ga zb
94-25295-25296-25297-252Rc Rsc R9a RlOa Ga ~b
94-25395-25396-25397-253Ra R5a R9b RIOa ~a ~b
94-25495-25496-25497-254Rb R5a R9b RIOa Ga zb
94-25595-25596-25597-255R Rsa R9b RIOa Ga Zb
94-25695-25696-25697-256Ra R5b R9b RlOa Ga Zb
94-25795-25796-25797-257Rb RSb R9b RlOa Ga zb
94-25895-25$96-25897-258Rc RSb R9b RlOa Ga zb
94-25995-25996-25997-259Ra R5c R9b RlOa Ga zb
94-26095-26096-26097-260Rb RSc R96 RIOa Ga Zb
94-26195-26196-26197-261R Rsc R9b RlOa Ga Zb
94-26295-26296-26297-262Ra R5a R9c RlOa ~a ~b
94-26395-26396-26397-263Rb R5a R9c RlOa Ga ~b
94-26495-26496-26497-264Rc Rsa R9c RIDa Ga Zb
94-26595-26596-26597-265Ra R5b R9c Rloa Ga zb
94-26695-26696-26697-266Rb RSb R9c RlOa Ga zb
94-26795-26796-26797-267R Rsb R9c RlOa ~a zb
94-26895-26896-26897-268Ra Rsc R9c RlOa ~a zb
94-26995-26996-26997-269Rb R5c R9c RlOa ~a ~b
94-27095-27096-27097-270R Rsc R9c RlOa ~a ~b
94-27195-27196-27197-271Ra RSa R9a RIOb Ga Zb
94-27295-27296-27297-272Rb R5a R9a 8106 Ga zb
94-27395-27396-27397-273Rc Rsa R9a RlOb Ga ~b
94-27495-27496-27497-274Ra R56 R9a RlOb Ga Zb
94-27595-27596-27597-275Rb R5b R9a RIOb Ga ~b
94-27695-27696-27697-276Rc R5b R9a RIOb Ga zb
94-27795-27796-27797-277Ra Rsc R9a Rlob ~a ~b
94-27895-27896-27897-278Rb RSc R9a RIOb Ga zb
94-27995-27996-27997-279R Rsc R9a RlOb Ga ~b
94-28095-28096-28097-280Ra RSa R9b RlOb Ga Zb
130
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-28195-28196-28197-281Rb RSa R9b RlOb Ga Zb
94-28295-28296-28297-282Rc RSa R9b RlOb Ga Zb
94-28395-28396-28397-283Ra RSb R9b RlOb Ga Zb
94-28495-28496-28497-284Rb R~' Rgb RIOb Ga zb
94-28595-28596-28597-285RC R5b R9b RlOb Ga ~b
94-28695-28696-28697-286Ra RSc R9b Rlob Ga zb
94-28795-28796-28797-287Rb RSc R9b RlOb ~a zb
94-28895-28896-28897-288Rc RSc R9b RlOb ~a zb
94-28995-28996-28997-289Ra RSa jZ~ RlOb Ga zb
94-29095-29096-29097-290Rb R5a Rgc R~Ob Ga zb
94-29195-29196-29197-291Rc R5a R9c RIOb Ga zb
94-29295-29296-29297-292Ra RSb R9c RIOb Ga zb
94-29395-29396-29397-293Rb Rsb R9c RIOb Ga zb
94-29495-29496-29497-294R RSb R9c RlOb Ga zb
94-29595-29596-29597-295Ra Rsc R9c RlOb Ga zb
94-29695-29696-29697-296Rb Rsc R9c RlOb Ga zb
94-29795-29796-29797-297R Rsc R9c RlOb Ga Zb
94-29895-29896-29897-298Ra RSa R9a RlOc Ga ~b
94-29995-29996-29997-299Rb Rsa R9a RlOc Ga ~b
94-30095-30096-30097-300Rc RSa R9a RlOc Ga Zb
94-30195-30196-30197-301Ra R5b R9a RlOc Ga ~b
94-30295-30296-30297-302Rb RSb R9a RIOc Ga ~b
94-30395-30396-30397-303Rc RSb R9a RlOc ~a ~b
94-30495-30496-30497-304Ra R5c R9a RlOc Ga Zb
94-30595-30596-30597-305Rb RSc R9a RlOc Ga ~b
94-30695-30696-30697-306Rc Rsc R9a RlOc Ga Zb
94-30795-30796-30797-307Ra Rsa R9b RIOc Ga ~b
94-30895-30896-30897-308Rb RSa R9b RIOc Ga ~b
94-30995-30996-30997-309Rc Rsa R9b RlOc Ga zb
94-31095-31096-31097-310Ra Rsb R9b RlOc Ga Zb
94-31195-31196-31197-311Rb RSb R9b RIOc Ga zb
94-31295-31296-31297-312Rc Rsb R9b RlOc ~a ~b
94-31395-31396-31397-313Ra Rsc R9b RlOc ~a Zb
94-31495-31496-31497-314Rb Rsc R9b RIOc Ga Zb
94-31595-31596-31597-315Rc Rsc R9b RIOc Ga zb
94-31695-31696-31697-316Ra RSa R9c RIOc ~a zb
94-31795-31796-31797-317Rb Rsa R9c RlOc Ga ~b
94-31895-31896-31897-318Rc RSa R9c RIOc ~a Zb
94-31995-31996-31997-319Ra RSb R9c RIOc Ga ~b
94-32095-32096-32097-320Rb R5b R9c RlOc Ga ~b
94-32195-32196-32197-321Rc RSb Rgc RlOc Ga zb
94-32295-32296-32297-322Ra Rsc R9c RlOc Ga ~b
94-32395-32396-32397-323Rb RSc R9c RlOc Ga Zb
94-32495-32496-32497-324R RSc R9c RlOc Ga ~b
94-32595-32596-32597-325Ra RSa R9a RlOa ~b Zb
94-32695-32696-32697-326Rb R5a R9a RlOa Gb zb
94-32795-32796-32797-327R Rsa R9a RlOa Gb zb
131
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-32895-32896-32897-328Ra RSb R9a RlOa Gb zb
94-32995-32996-32997-329Rb Rsb R9a RlOa Gb zb
94-33095-33096-33097-330Rc RSb R9a RIOa Gb zb
94-33195-33196-33197-331Ra Rsc R9a RIOa Gb zb
94-33295-33296-33297-332Rb RSc R9a RIOa Gb Zb
94-33395-33396-33397-333Rc RSc R9a RlOa Gb zb
94-33495-33496-33497-334Ra R5a R9b Rloa Gb Zb
94-33595-33596-33597-335Rb Rsa R9b RIOa Gb Zb
94-33695-33696-33697-336Rc Rsa R9b RIOa Gb zb
94-33795-33796-33797-337Ra RSb R9b RlOa ~b Zb
94-33895-33896-33897-338Rb RSb R9b RlOa Gb zb
94-33995-33996-33997-339Rc RSb R9b RIOa Gb zb
94-34095-34096-34097-340Ra RSc R9b RiOa Gb Zb
94-34195-34196-34197-341Rb RSc R9b RlOa Gb ~b
94-34295-34296-34297-342Rc RSc R9b RIOa Gb ~b
94-34395-34396-34397-343Ra RSa R9c RiOa Gb zb
94-34495-34496-34497-344Rb R5a R9c RIOa Gb zb
94-34595-34596-34597-345Rc Rsa R9c RIOa Gb Zb
94-34695-34696-34697-346Ra Rsb R9c RlOa ~b Zb
94-34795-34796-34797-347Rb RSb R9c RIOa Gb Zb
94-34895-34896-34897-348Rc RSb R9c RlOa ~b Zb
94-34995-34996-34997-349Ra Rsc R9c RlOa ~b zb
94-35095-35096-35097-350Rb RSc R9c RiOa Gb Zb
94-35195-35196-35197-351Rc R5c R9c RlOa Gb Zb
94-35295-35296-35297-352Ra R5a R9a RIOb Gb zb
94-35395-35396-35397-353Rb Rsa R9a RiOb Gb Zb
94-35495-35496-35497-354Rc R5a R9a RlOb Gb Zb
94-35595-35596-35597-355Ra RSb R9a RlOb Gb ~b
94-35695-35696-35697-356Rb R5b R9a RiOb Gb Zb
94-35795-35796-35797-357Rc RSb R9a RlOb Gb Zb
94-35895-35896-35897-358Ra Rsc R9a Riob Gb zb
94-35995-35996-35997-359Rb Rsc R9a RlOb Gb zb
94-36095-36096-36097-360R RSc R9a RIOb Gb Zb
94-36195-36196-36197-361Ra RSa R9b RlOb ~b zb
94-36295-36296-36297-362Rb Rsa R9b RlOb ~b Zb
94-36395-36396-36397-363Rc Rsa R9b RlOb Gb Zb
94-36495-36496-36497-364Ra Rsb Rib RlOb Gb Zb
94-36595-36596-36597-365Rb Rsb R9b RIOb ~b zb
94-36695-36696-36697-366R RSb R9b Rlob Gb Zb
94-36795-36796-36797-367Ra RSc R9b RIOb Gb ~b
94-36895-36896-36897-368Rb R5c R9b RlOb Gb Zb
94-36995-36996-36997-369R RSc R9b Riob Gb ~b
94-37095-37096-37097-370Ra Rsa R9c RlOb Gb Zb
94-37195-37196-37197-371Rb RSa R9c RlOb Gb zb
94-37295-37296-37297-372R R5a R9c RiOb Gb Zb
94-37395-37396-37397-373Ra Rsb R9c RIOb Gb Zb
94-37495-37496-37497-374Rb Rsb R9c RIOb Gb Zb
132
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-37595-37596-37597-375Rc RSb R9c RiOb Gb Zb
94-37695-37696-37697-376Ra Rsc R9c RIOb Gb Zb
94-37795-37796-37797-377Rb Rsc R9c Rlob Gb zb
94-37895-37896-37897-378Rc RSc R9c RlOb Gb Zb
94-37995-37996-37997-379Ra RSa R9a RlOc Gb ~b
94-38095-38096-38097-380Rb Rsa R9a RlOc Gb zb
94-38195-38196-38197-381Rc R5a R9a RlOc Gb Zb
94-38295-38296-38297-382Ra RSb Rya RIOc Gb zb
94-38395-38396-38397-383Rb RSb R9a RlOc Gb zb
94-38495-38496-38497-384Rc RSb R9a R~o Gb zb
94-38595-38596-38597-385Ra RSc R9a RIOc Gb zb
94-38695-38696-38697-386Rb Rsc Rga RlOc ~b Zb
94-38795-38796-38797-387R RSc R9a RlOc Gb Zb
94-38895-38896-38897-388Ra Rsa R9b RlOc Gb zb
94-38995-38996-38997-389Rb RSa R9b RlOc ~b zb
94-39095-39096-39097-390Rc RSa R9b RIOc Gb zb
94-39195-39196-39I97-391Ra R5b R9b RIOc Gb Zb
94-39295-39296-39297-392Rb RSb R9b Rloc Gb Zb
94-39395-39396-39397-393Rc RSb R9b RlOc Gb Zb
94-39495-39496-39497-394Ra R5c Rob RIOc Gb ~b
94-3959S-39596-39597-395Rb Rsc R9b RIOc Gb zb
94-39695-39696-39697-396Rc Rsc R9b Rloc Gb Zb
94-39795-39796-39797-397Ra Rsa R9c RIOc Gb ~b
94-39895-39896-39897-398Rb RSa R9c Rloc Gb zb
94-39995-39996-39997-399R R5a R9c RlOc Gb zb
94-40095-40096-40097-400Ra RSb R9c RlOc Gb Zb
94-40195-40196-40197-401Rb RSb R9c R~o Gb Zb
94-40295-40296-40297-402Rc RSb R9c RlOc Gb zb
94-40395-40396-40397-403Ra Rsc R9c RlOc Gb ~b
94-40495-40496-40497-404Rb RSc R9c RlOc ~b Zb
94-40595-40596-40597-405Rc RSc R9c RlOc Gb zb
94-40695-40696-40697-406Ra RSa R9a RlOa ~c Zb
94-40795-40796-40797-407Rb RSa R9a RlOa ~c zb
94-40895-40896-40897-408R RSa R9a RlOa Gc Zb
94-40995-40996-40997-409Ra RSb R9a RIOa Gc Zb
94-41095-41096-41097-410Rb RSb R9a , Gc Zb
RlOa
94-41195-41196-41197-411Rc Rsb R9a RlOa Gc zb
94-41295-41296-41297-412Ra Rsc R9a R~oa Gc zb
94-4I395-41396-41397-413Rb R5c R9a RlOa Gc zb
94-41495-41496-41497-414Rc R5c R9a RlOa Gc Zb
94-41595-41596-41597-415Ra Rsa R9b RlOa Gc Zb
94-41695-41696-41697-416Rb RSa R9b RiOa Gc zb
94-41795-41796-41797-417R RSa R9b RlOa Gc ~b
94-4I895-41896-41897-418Ra Rsb R9b R~oa Gc Zb
94-41995-41996-41997-419Rb RSb R9b RIOa Gc zb
94-42095-42096-42097-420RC RSb R9b RIOa Gc Zb
94-42195-42196-42197-421Ra RSc R9b RlOa Gc Zb
133
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-42295-42296-42297-422Rb RSc Rib RlOa Gc zb
94-42395-42396-42397-423Rc RSc R9b RlOa Gc zb
94-42495-42496-42497-424Ra Rsa R~ RlOa Gc zb
94-42595-42596-42597-425Rb R5a R9c RlOa Gc zb
94-42695-42696-42697-426R Rsa R9c RlOa ~c zb
94-42795-42796-42797-427Ra Rsb R9c RIOa Gc Zb
94-42895-42896-42897-428Rb RSb R9c Rtoa Gc zb
94-42995-42996-42997-429Rc Rsb R9c RIOa Gc zb
94-43095-43096-43097-430Ra RSc R9c RlOa Gc zb
94-43195-43196-43197-431Rb RSc R9c Rloa Gc Zb
94-43295-43296-43297-432Rc Rsc R9c RlOa Gc Zb
94-43395-43396-43397-433Ra RSa R9a RlOb Gc zb
94-43495-43496-43497-434Rb RSa Rya RlOb Gc ~b
94-43595-43596-43597-435Rc RSa R9a RlOb Gc zb
94-43695-43696-43697-436Ra R5b R9a RIOb ~c zb
94-43795-43796-43797-437Rb R5b R9a RIOb ~c ~b
94-43895-43896-43897-438Rc R5b R9a RlOb Gc Zb
94-43995-43996-43997-439Ra Rsc R9a RlOb ~c ~b
94-44095-44096-44097-440Rb RSc R9a RlOb Gc Zb
94-44195-44196-44197-441Rc Rsc R9a RIOb Gc zb
94-44295-44296-44297-442Ra RSa R9b RlOb Gc Zb
94-44395-44396-44397-443Rb RSa R9b RlOb Gc ~b
94-44495-44496-44497-444Rc Rsa R9b RIOb ~c ~b
94-44595-44596-44597-445Ra RSb R9b RIOb Gc Zb
94-44695-44696-44697-446Rb R5b R9b RlOb Gc zb
94-44795-44796-44797-447R RSb R9b RlOb Gc Zb
94-44895-44896-44897-448Ra RSc R9b Rob G~ Zb
94-44995-44996-44997-449Rb Rsc R9b RIOb ~o Zb
94-45095-45096-45097-450Rc RSc Rgb RlOb ~c ~b
94-45195-45196-45197-45IRa RSa R9c RlOb Gc zb
94-45295-45296-45297-452Rb RSa R9c RlOb Gc zb
94-45395-45396-45397-453Rc Rsa R9c RlOb Gc Zb
94-45495-45496-45497-454Ra R5b R9c RlOb Gc zb
94-45595-45596-45597-455Rb R5b R9c RI06 Gc zb
94-45695-45696-45697-456R R5b R9c RlOb Gc zb
94-45795-45796-45797-457Ra Rsc R9c Rlob Gc zb
94-45895-45896-45897-458Rb RSc R9c RlOb Gc zb
94-45995-45996-45997-459R RSc R9c Rlob ~c ~b
94-46095-46096-46097-460Ra R5a R9a RIOc ~c Zb
94-46195-46196-46197-461Rb Rsa R9a RlOc Gc zb
94-46295-46296-46297-462R Rsa R9a RlOc ~c zb
94-46395-46396-46397-463Ra Rsb R9a Rloc ~c Zb
94-46495-46496-46497-464Rb RSb R9a RIOc Gc zb
94-46595-46596-46597-465Rc Rsb R9a RIOc Gc Zb
94-46695-46696-46697-466Ra RSc R9a RlOc Gc Zb
94-46795-46796-46797-467Rb Rsc R9a RlOc Gc Zb
94-46895-46896-46897-468R Rsc R9a RlOc Gc zb
134
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-46995-46996-46997-469Ra RSa R9b RlOc Gc zb
94-47095-47096-47097-470Rb RSa R9b RlOc Gc Zb
94-47195-47196-47197-471R RSa R9b RIOc Gc Zb
94-47295-47296-47297-472Ra RSb R9b RIOc ~c zb
94-47395-47396-47397-473Rb RSb R9b RlOc ~c Zb
94-47495-47496-47497-474R RSb R9b RIOc Gc zb
94-47595-47596-47597-475Ra Rsc R9b Rloc Gc zb
94-47695-47696-47697-476Rb RSc R9b RlOc Gc zb
94-47795-47796-47797-477R Rsc R9b RlOc Gc zb
94-47895-47896-47897-478Ra Rsa R9c RlOc Gc Zb
94-47995-47996-47997-479Rb RSa R9c RIOc Gc Zb
94-48095-48096-48097-480Rc R5a R9c R30c Gc ~b
94-48195-48196-48197-481Ra Rsb R9c RIOc Gc ~b
94-48295-48296-48297-482Rb Rsb R9c RIOc ~c zb
94-48395-48396-48397-483Rc RSb R9c RlOc ~c ~b
94-48495-48496-48497-484Ra R5c R9c RIOc ~c zb
94-48595-48596-48597-485Rb Rsc R9c RlOc Gc zb
94-48695-48696-48697-486Rc Rsc R9c RlOc Gc Zb
94-48795-48796-48797-487Ra RSa R9a RlOa ~a zc
94-48895-48896-48897-488Rb RSa R9a RlOa Ga Zc
94-48995-48996-48997-489Rc Rsa R9a RlOa Ga ~c
94-49095-49096-49097-490Ra RSb R9a RlOa Ga zc
94-49195-49196-49197-491Rb RSb R9a RlOa Ga Zc
94-49295-49296-49297-492Rc RSb R9a RIOa ~a zc
94-49395-49396-49397-493Ra RSc R9a RlOa Ga ~c
94-49495-49496-49497-494Rb Rsc R9a RlOa ~a zc
94-49595-49596-49597-495Rc Rsc R9a RlOa Ga Zc
94-49695-49696-49697-496Ra RSa R9b RlOa Ga Zc
94-49795-49796-49797-497Rb Rsa R9b RlOa Ga zc
94-49895-49896-49897-498R Rsa R9b RlOa ~a Zc
94-49995-49996-49997-499Ra RSb R9b RlOa Ga ~c
94-50095-50096-50097-500Rb R5b R9b RlOa ~a ~c
94-50195-50196-50197-501Rc RSb R9b Rloa Ga 2c
94-50295-50296-50297-502Ra RSc R9b RlOa Ga zc
94-50395-50396-50397-503Rb Rsc R9b Rloa Ga Zc
94-50495-50496-50497-504Rc R5c R9b RIOa Ga Zc
94-50595-50596-50597-505Ra RSa R9c RlOa Ga ~c
94-50695-50696-50697-506Rb RSa R9c RIOa Ga zc
94-50795-50796-50797-507R Rsa R9c RlOa Ga zc
94-50895-50896-50897-508Ra RSb R9c RIOa Ga Zc
94-50995-50996-50997-509Rb RSb R9c RlOa Ga zc
94-51095-51096-51097-510R RSb R9c RIOa Ga zc
94-51195-51196-51197-511Ra RSc R9c RIOa Ga zc
94-51295-51296-51297-512Rb RSc R9c RlOa Ga ~c
94-51395-51396-51397-513R Rsc R9c RIOa Ga zc
94-51495-51496-51497-514Ra R5a R9a RIOb Ga zc
94-51595-51596-51597-515Rb RSa R9a RlOb Ga Zc
135
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-51695-51696-51697-516Rc Rsa R9a RlOb Ga zc
94-51795-51796-51797-517Ra R5b R9a RIOb Ga Zc
94-51895-51896-51897-518Rb RSb R9a RIOb Ga zc
94-51995-51996-51997-519R Rsb R9a RlOb Ga Zc
94-52095-52096-52097-520Ra Rsc R9a RlOb Ga Zc
94-52195-52196-52197-521Rb RSc R9a RlOb Ga zc
94-52295-52296-52297-522R Rsc R9a RlOb ~a ~c
94-52395-52396-52397-523Ra RSa R9b RlOb Ga zc
94-52495-52496-52497-524Rb RSa R9b RIOb ~a ~c
94-52595-52596-52597-525R Rsa R9b RIOb Ga Zc
94-52695-52696-52697-526Ra R5b R9b RiOb Ga Zc
94-52795-52796-52797-527Rb R5b R9b RlOb Ga ~c
94-52895-52896-52897-52$Rc Rsb R9b RlOb Ga Zc
94-52995-52996-52997-529Ra RSc R9b RIOb Ga Zc
94-53095-53096-53097-530Rb RSc R9b RIOb Ga Zc
94-53195-53196-53197-531Rc Rsc R9b RIOb Ga Zc
94-53295-53296-53297-532Ra R5a R9c RlOb ~a zc
94-53395-53396-53397-533Rb R5a R9c RiOb Ga zc
94-53495-53496-53497-534Rc RSa R9c RlOb ~a zc
94-53595-53596-53597-535Ra RSb R9c RIOb Ga zc
94-53695-53696-53697-536Rb Rsb R~ RIOb Ga ~c
94-53795-53796-53797-537Rc RSb jZ~ RlOb ~a zc
94-53895-53896-53897-538Ra RSc R9c RlOb Ga zc
94-53995-53996-53997-539Rb Rsc R9 RIOb Ga zc
94-54095-54096-54097-540Rc Rsc R9c RlOb Ga ~c
94-54195-54196-54197-541Ra R5a R9a Rloc Ga ~c
94-54295-54296-54297-542Rb RSa R9a RlOc Ga zc
94-54395-54396-54397-543R RSa R9a RlOc ~a Zc
94-54495-54496-54497-544Ra Rsb R9a RlOc ~a ~c
94-54595-54596-54597-545Rb RSb R9a Rtoc Ga Zc
94-54695-54696-54697-546Rc R5b R9a RlOc Ga Zc
94-54795-54796-54797-547Ra R5c R9a RlOc Ga zc
94-54895-54896-54897-548Rb RSc R9a RIOc Ga Zc
94-54995-54996-54997-549Rc R5c R9a RlOc Ga Zc
94-55095-55096-55097-550Ra R5a R9b RlOc ~a Zc
94-55195-55196-55197-551Rb Rsa R9b RlOc ~a Zc
94-55295-55296-55297-552Rc RSa R9b RIOc Ga Zc
94-55395-55396-55397-553Ra R~' R9b RIOc Ga ~c
94-55495-55496-55497-554Rb RSb Rgb RlOc Ga Zc
94-55595-55596-55597-555R Rsb R9b RlOc Ga ~c
94-55695-55696-55697-556Ra Rsc R9b Rloc Ga Zc
94-55795-55796-55797-557Rb Rsc R9b RlOc Ga Zc
94-55895-55896-55897-558R Rsc R9b RlOc Ga Zc
94-55995-55996-55997-559Ra R5a R9c RIOc Ga zc
94-56095-56096-56097-560Rb RSa R9c RlOc Ga zc
94-56195-56196-56197-561Rc R5a R9c RlOc Ga Zc
94-56295-56296-56297-562Ra RSb R9c Rloc Ga Zc
136
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-56395-56396-56397-563Rb RSb R9c RlOc Ga zc
94-56495-56496-56497-564Rc RSb R9c RlOc ~a zc
94-56595-56596-56597-565Ra RSc R9c RlOc Ga Zc
94-56695-56696-56697-566Rb RSc R9c Rloc Ga zc
94-56795-56796-56797-567Rc RSc R9c RlOc Ga Zc
94-56895-56896-56897-568Ra RSa R9a RlOa Gb zc
94-56995-56996-56997-569Rb RSa R9a RiOa Gb zc
94-57095-57096-57097-570Rc RSa R9a RlOa ~b zc
.
94-57195-57196-57197-571Ra RSb R9a RIOa Gb zc
94-57295-57296-57297-572Rb Rsb R9a RIOa ~b Zc
94-57395-57396-57397-573R Rsb R9a RIOa Gb zc
94-57495-57496-57497-574Ra R5c R9a RlOa ~b Zc
94-57595-57596-57597-575Rb RSc R9a RIOa Gb Zc
94-57695-57696-57697-576Rc Rsc R9a RIOa Gb Zc
94-57795-57796-57797-577Ra RSa R9b RIOa ~b Zc
94-57895-57896-57897-578Rb Rsa R9b RlOa Gb zc
94-57995-57996-57997-579Rc RSa R9b RlOa Gb zc
94-58095-58096-58097-580Ra Rsb R9b RlOa Gb zc
94-58195-58196-58197-581Rb Rsb R9b RlOa Gb zc
94-58295-58296-58297-582Rc Rsb R9b RlOa Gb zc
94-58395-58396-58397-583Ra Rsc R9b RlOa Gb Zc
94-58495-58496-58497-584Rb R5c R9b RlOa Gb Zc
94-58595-58596-58597-585Rc Rsc R9b RIOa Gb zc
94-58695-58696-58697-586Ra R5a R9c RlOa Gb 2c
94-58795-58796-58797-587Rb RSa R9c RlOa Gb ~c
94-58895-58896-58897-588R Rsa R9c Rloa Gb Zc
94-58995-58996-58997-589Ra RSb R9c RIOa ~b Zc
94-59095-59096-59097-590Rb R5b R9c RIOa ~b Zc
94-59195-59196-59197-591Rc RSb R9c RlOa ~b Zc
94-59295-59296-59297-592Ra RSc R9c RIOa Gb Zc
94-59395-59396-59397-593Rb RSc R9c RIOa Gb Zc
94-59495-59496-59497-594Rc RSc R9c RlOa Gb Zc
94-59595-59596-59597-595Ra Rsa R9a RlOb Gb zc
94-59695-59696-59697-596Rb Rsa R9a RlOb Gb Zc
94-59795-59796-59797-597Rc Rsa R9a Rlob Ob Zc
94-59895-59896-59897-598Ra RSb R9a RlOb Gb Zc
94-59995-59996-59997-599Rb RSb R9a RlOb Gb zc
94-60095-60096-60097-600Rc Rsb R9a RlOb Gb zc
94-60195-60196-60197-601Ra RSc R9a RIOb Gb Zc
94-60295-60296-60297-602Rb Rsc R9a RlOb Gb Zc
94-60395-60396-60397-603Rc Rsc R9a RlOb ~b Zc
94-60495-60496-60497-604Ra RSa R9b RlOb Gb Zc
94-60595-60596-60597-605Rb RSa R9b Rlob Gb zc
94-60695-60696-60697-606Rc R5a R9b RlOb Gb Zc
94-60795-60796-60797-607Ra Rsb R9b Rlob Gb Zc
94-60895-60896-60897-608Rb R5b R9b RlOb Gb Zc
94-60995-60996-60997-609R Rsb R9b RiOb Gb Zc
137
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-61095-61096-61097-610Ra Rsc R9b RIOb Gb zc
94-61195-61196-61197-611Rb RSc R9b RlOb ~b zc
94-61295-61296-61297-612Rc Rsc R9b RIOb Gb Zc
94-61395-61396-61397-613Ra RSa R9c RIOb Gb Zc
94-61495-61496-61497-614Rb R5a R9c RlOb Gb zc
94-61595-61596-61597-615Rc RSa R9c RlOb Gb zc
94-61695-61696-61697-616Ra R5b R9c RlOb Gb ~c
94-61795-61796-61797-617Rb RSb R9c RlOb Gb zc
94-61895-61896-61897-618Rc RSb R9c Rlob Gb zc
94-61995-61996-61997-619Ra RSc R9c RIOb Gb Zc
94-62095-62096-62097-620Rb RSc R9c RlOb Gb Zc
94-62195-62196-62197-621Rc RSc R9c RlOb Gb zc
94-62295-62296-62297-622Ra R5a R9a RlOc Gb zc
94-62395-62396-62397-623Rb Rsa R9a RlOc Gb Zc
94-62495-62496-62497-624Rc RSa R9a RlOc Gb Zc
94-62595-62596-62597-625Ra RSb R9a RIOc Gb Zc
94-62695-62696-62697-626Rb R5b R9a RIOc Gb ~c
94-62795-62796-62797-627R R5b R9a R~Oc Gb Zc
94-62895-62896-62897-628Ra RSc R9a RlOc ~b Zc
94-62995-62996-62997-629Rb Rsc R9a RlOc Gb zc
94-63095-63096-63097-630R RSc h'~a RiOc Gb 2c
94-63195-63196-63197-631Ra RSa R9b RlOc Gb zc
94-63295-63296-63297-632Rb Rsa R9b RIOc Gb Zc
94-63395-63396-63397-633R R5a R9b RIOc Gb Zc
94-63495-63496-63497-634Ra RSb R9b RlOc Gb Zc
94-63595-63596-63597-635Rb Rsb R9b RlOc Gb Zc
94-63695-63696-63697-636Rc Rsb R9b RlOc Gb zc
94-63795-63796-63797-637Ra Rsc R9b RlOc Gb ~c
94-63895-63896-63897-638Rb Rsc R9b RIOc Gb Zc
94-63995-63996-63997-639R Rsc R9b RIOc Gb Zc
94-64095-64096-64097-640Ra R5a R9c RlOc ~b Zc
94-64195-64196-64197-641Rb R5a R9c RlOc Gb ~c
94-64295-64296-64297-642Rc RSa R9c RlOc Gb Zc
94-64395-64396-64397-643Ra R5b R9c RIOc ~b Zc
94-64495-64496-64497-644Rb Rsb R9c Rloc Gb zc
94-64595-64596-64597-645Rc RSb R9c R1 Gb Zc
oc
94-64695-64696-64697-646Ra Rsc R9c RlOc Gb zc
94-64795-64796-64797-647Rb RSc R9c Rloc Gb Zc
94-64895-64896-64897-648Rc Rsc R9c RlOc Gb Zc
94-64995-64996-64997-649Ra R5a R9a RIOa Gc Zc
94-65095-65096-65097-650Rb RSa R9a R~oa Gc zc
94-65195-65196-65197-651R Rsa R9a RiOa Gc zc
94-65295-65296-65297-652Ra R5b R9a RlOa Gc zc
94-65395-65396-65397-653Rb RSb R9a RlOa Gc zc
94-65495-65496-65497-654R R5b R9a RlOa Gc zc
94-65595-65596-65597-655Ra RSc R9a RlOa Gc Zc
94-65695-65696-65697-656Rb RSc R9a RlOa Gc ~c
138
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-65795-65796-65797-657Rc RSc R9a RIOa ~c zc
94-65895-65896-65897-658Ra R5a R9b RIOa Gc Zc
94-65995-65996-65997-659Rb RSa R9b RlOa Gc Zc
94-66095-66096-66097-660Rc RSa R9b RlOa Gc zc
94-66195-66196-66197-661Ra RSb R9b RIOa Gc Zc
94-66295-66296-66297-662Rb Rsb R9b RlOa Gc Zc
94-66395-66396-66397-663Rc Rsb R9b Rloa ~c zc
94-66495-66496-66497-664Ra RSc R9b RlOa ~c zc
94-66595-66596-66597-665Rb RSc R9b RlOa ~c zc
94-66695-66696-66697-666Rc Rsc R9b RlOa Gc Zc
94-66795-66796-66797-667Ra R5a R9c RlOa Gc Zc
94-66895-66896-66897-668Rb R5a R9c Rloa Gc Zc
94-66995-66996-66997-669R Rsa R9c RlOa Gc zc
94-67095-67096-67097-670Ra RSb R9c Rloa Gc zc
94-67195-67196-67197-671Rb Rsb R9c RIOa Gc Zc
94-67295-67296-67297-672Rc RSb R9c RlOa Gc Zc
94-67395-67396-67397-673Ra R5c R9c RIOa Gc Zc
94-67495-67496-67497-674Rb Rsc R9c RlOa Gc zc
94-67595-67596-67597-675R RSc R9c RlOa Gc Zc
94-67695-67696-67697-676Ra RSa R9a RlOb ~c zc
94-67795-67796-67797-677Rb RSa Rya RIOb Gc Zc
94-67895-67896-67897-678Rc Rsa R9a RIOb Gc ~c
94-67995-67996-67997-679Ra RSb R9a RIOb Gc zc
94-68095-68096-68097-680Rb R5b R9a RIOb Gc ~c
94-68195-68196-68197-681Rc RSb R9a RlOb Gc zc
94-68295-68296-68297-682Ra RSc R9a RlOb ~c zc
94-68395-68396-68397-683Rb R5c R9a RIOb Gc zc
94-68495-68496-68497-684R R5c R9a RIOb Gc 2c
94-68595-68596-68597-685Ra RSa R9b RiOb Gc Zc
94-68695-68696-68697-686Rb RSa R9b RlOb Gc ~c
94-68795-68796-68797-687Rc Rsa R9b RIOb ~c ~c
94-68895-68896-68897-688Ra RSb R9b RlOb Gc zc
94-68995-68996-68997-689Rb RSb R9b RIOb Gc zc
94-69095-69096-69097-690Rc Rsb R9b RlOb Gc zc
94-69195-69196-69197-691Ra RSc R9b RlOb Gc zc
94-69295-69296-69297-692Rb RSc R9b RIOb Gc zc
94-69395-69396-69397-693Rc RSc Rgb Rlob Gc zc
94-69495-69496-69497-694Ra Rsa R9c RIOb Gc Zc
94-69595-69596-69597-695Rb RSa R9c RIOb Gc zc
94-69695-69696-69697-696Rc Rsa R9c RlOb Gc ~c
94-69795-69796-69797-697Ra R5b R9c RIOb Gc Zc
94-G9895-69896-69897-698Rb RSb R9c Blob Gc Zc
94-69995-69996-69997-699R Rsb R9c RIOb Gc zc
94-70095-70096-70097-700Ra RSc R9c RlOb Gc ~c
94-70195-70196-70197-701Rb R5c R9c RIOb ~c zc
94-70295-70296-70297-702R RSc R9c RIOb Gc Zc
94-70395-70396-70397-703Ra RSa R9a RlOc Gc Zc
139
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
94-70495-70496-70497-704Rb R5a R9a RIOc Gc zc
94-70595-70596-70597-705R Rsa R9a RlOc Gc zc
94-70695-70696-70697-706Ra R55 R9a RlOc Gc zc
94-70795-70796-70797-707Rb R5b R9a RIOc Gc Zc
94-70895-70896-70897-708Rc RSb Rya R~o Gc Z
94-70995-70996-70997-709Ra Rsc R9a RlOc Gc Zc
94-71095-71096-71097-710Rb Rsc R9a RIOc Gc Zc
94-71195-71196-71197-711Rc Rsc R9a RIOc Gc zc
94-71295-71296-71297-712Ra Rsa R9b RIOc Gc Zc
94-71395-71396-71397-713Rb Rsa R9b RlOc Gc Zc
94-71495-71496-71497-714R Rsa R9~'RIOc Gc Zc
94-71595-71596-71597-715Ra RSb R9b RIOc Gc ~o
94-71695-71696-71697-716Rb Rsb R9b Rloc Gc zc
94-71795-71796-71797-717Rc Rsb R9b Bloc Gc Z
94-71895-71896-71897-718Ra Rsc R9b RlOc Gc zc
94-71995-71996-71997-7I9Rb R5c Rsb Rloc Gc zc
94-72095-72096-72097-720Rc RSc R9b RlOc Gc zc
94-72195-72196-72197-721Ra R5a R9c RIOc Gc Zc
94-72295-72296-72297-722Rb RSa R9c RlOc Gc Zc
94-72395-72396-72397-723Rc RSa R9c RIOc Gc zc
94-72495-72496-72497-724Ra RSb R9c RlOc Gc Zc
94-72595-72596-72597-725Rb Rsb R9c Rloc Gc 2c
94-72695-72696-72697-726R R5b R9c Rloc Gc Zc
94-72795-72796-72797-727Ra R5c R9c RIOc Gc Zc
94-72895-72896-72897-728Rb RSc R9c RIOc Gc zc
94-72995-72996-72997-729Rc RSc R9c RlOc Gc zc
where all symbols are as defined above.
In one aspect of any of formulae (94), (95), (96), and (97) of the present
invention, R
is hydrogen, a hydroxy group, a halogen, a vitro group, an optionally
substituted amino
group, an alkyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl
group; RS is
hydrogen, a hydroxy group, a halogen, a vitro group, an optionally substituted
amino group,
an alkyl group, an alkoxy group, an alkenyl group, or an allcoxyalkyl group;
R9 and R'°
independently are hydrogen, a halogen, a vitro group, an amino group, a mono-
or di-
substituted amino group, a hydroxy group, an alkoxy group, a carboxy group, a
cyano group,
to an oxo(O=) group, or a thio(S=) group; G is f CHZ)z, fCH2)3, or f CHZ)4;
and all other symbols
are as defined above in connection with formula (I).
In another aspect of any of formulae (94), (95), (96), and (97) of the present
invention, R is hydrogen, a hydroxy group, a halogen, a vitro group, an
optionally substituted
amilio group, an alkyl group, an alkoxy group, an alkenyl group, or an
alkoxyalkyl group; RS
140
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
is hydrogen, a hydroxy group, a halogen, a vitro group, an optionally
substituted amino
group, an alkyl group, an allcoxy group, an allcenyl group, or an alkoxyalkyl
group; R9 and
Rl° independently are an alkyl group, a cycloalkyl group, an allcoxy
group, a haloalleoxy
group, a cycloalkyl group, an aryl group, a benzyloxy group, an acyl group, an
acyloxy
group, an aroyl group, an alkoxycarbanyl group, an aryloxycarbonyl group, a
heteroaryl
group, a heterocyclyl group, or an aralkyl group; and G is f CH232, f CHZ)3,
or ~CH2)~; and all
other symbols are as defined as above in connection with formula (I).
In yet another aspect of of formulae (94), (95), (96), and (97) the present
invention, R
is hydrogen, a hydroxy group, a halogen, a vitro group, an optionally
substituted amino
group, an alkyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl
group; RS is
hydrogen, a hydroxy group, a halogen, a vitro group, an optionally substituted
amino group,
an alkyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl group; R~
and R' °
independently are an alkylsulfonyl group, an alkylsulfinyl group, an
arylsulfonyl group, an
arylsulfinyl group, an alkylthio group, an arylthio group, a heteroarylthio
group, an
aralkylthio group, or a heterocyclyl sulfonyl group, wluch is optionally
substituted with a
halogen, a hydroxyl group, a vitro group, an amino group, an alkyloxy group,
or any
combination thereof, and wherein the heterocycle group is optionally a
substituted
morpholinyl group, a thiomorpholinyl group, or a piperzinyl group, wherein the
substituent
on the heterocyclyl group is a halogen, a vitro group, an amino group, an
alkyl group, an
allcoxy group, or an aryl group; and G is ~CHZ)Z, ECH2~3, or fCH2)~; and all
other symbols are
as defined as above in connection with formula (I).
In still another aspect of any of formulae (94), (95), (96), and (97) of the
present
invention, R is hydrogen or an alkyl group; R5 is hydrogen or an alkyl group;
R9 is hydrogen,
O
-~-S-N~ 'Me
an alkoxy group, or O ; Rl° is hydrogen or an alkoxy group; and G is f
CHZ)2,
~CHZ)3, or f CHZ)4.
In still another aspect of any of formulae (94), (95), (96), and (97) of the
present
O
-~-S-N~ N'Me
invention, R is -H or Me; RS is -H or Me; R9 is -H, -OMe, or O ; R'° is
-H, -
OMe, or -OEt; and G is f CHz32, fCH~j~, or f CHZj4.
141
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The present invention further encompasses various compounds of general formula
(IV) as follows:
OMe O O
R~ / OMe ~NR R~ /~OMe ~NR
I S O
Nw w _ I s0 ~~ Nw w I _ . s
R
N~N~N G-Z ~ ~ R N~N~N G-Z
Me [°J (98); Me I°I (
°
R~ /OEt ~NR R~
'I °
R5 N~ I
N,N I N-G-Z ~ ~ ,N~
Me ° (100); and Me (101);
where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, R', R5, G, and Z of
any of
formulae (98), (99), (100), and (101) are selected to produce compounds of
formulae (98-1),
(99-1), (100-1), and (101-1) through formulae (98-243), (99-243), (100-243),
and (101-243)
as follows:
Formulae R R R5 G Z
98-1 99-1 100-1 101-1 Ra R Rsa Ga Za
a
98-2 99-2 100-2 101-2 Rb Rta Rsa Ga za
98-3 99-3 100-3 101-3 R Rla Rsa Ga Za
98-4 99-4 100-4 101-4 Ra RIb Rsa Ga Za
98-5 99-5 100-5 101-5 Rb R1b Rsa Ga Za
98-6 99-6 100-6 101-6 R RIb Rsa Ga Za
98-7 99-7 100-7 101-7 Ra R~~ Rsa Ga Za
98-8 99-8 100-8 101-8 Rb Rl~ Rsa Ga Za
98-9 99-9 100-9 101-9 R RI Rsa Ga Za
98-10 99-10 100-10 101-10 Ra Rta Rsb Ga Za
98-11 99-11 100-11 141-11 Rb Rla R56 Ga Za
98-12 99-12 100-12 101-12 R Rla Rsb Ga Za
98-13 99-13 100-13 101-13 Ra Rlb Rsb Ga Za
98-14 99-14 100-14 101-14 Rb Rtb R56 Ga Za
98-15 99-15 100-15 101-15 R~ Rtb R56 Ga Za
98-16 99-16 100-16 101-16 Ra Rt R56 Ga Za
98-17 99-17 100-17 101-17 Rb Rl~ Rsb Ga Za
98-18 99-18 100-18 101-18 RC R~~ R56 Ga Za
98-19 99-19 100-19 101-19 Ra Rta Rsc Ga Za
98-20 99-20 100-20 101-20 Rb Rta Rsc Ga Za
142
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
98-2199-21 100-21 101-21 R Ria Rsc Ga Za
98-2299-22 100-22 101-22 Ra Rib Rsc Ga Za
9$-2399-23 100-23 101-23 Rb Rib R5c Ga za
98-2499-24 100-24 101-24 Rc Rib Rsc ~a za
98-2599-25 100-25 101-25 Ra Ric Rsc Ga ~a
98-2699-26 100-26 101-26 Rb Ric R5c Ga ~a
98-2799-27 100-27 101-27 R Ric Rsc Ga za
98-2899-28 100-28 101-28 Ra Ria Rsa ~b za
98-2999-29 100-29 101-29 Rb Ria Rsa Gb za
98-3099-30 100-30 101-30 Rc Ria Rsa Gb za
98-3199-31 100-31 101-31 Ra Rib Rsa Gb ~a
98-3299-32 100-32 101-32 Rb Rib Rsa Gb za
98-3399-33 100-33 101-33 Rc Rib Rsa ~b 2a
98-3499-34 100-34 101-34 Ra Ric Rsa Gb za
98-3599-35 100-35 101-35 Rb Ric Rsa ~b za
98-3699-36 100-36 101-36 R Ric Rsa Gb za
98-3799-37 100-37 101-37 Ra Ria Rsb ~b za
98-3899-38 100-38 101-38 Rb Ria Rsb Gb za
98-3999-39 100-39 101-39 Rc Ria Rsb Gb za
98-4099-40 100-40 101-40 Ra Rib R5b ~b ~a
9$-4199-41 100-41 101-41 Rb Rib Rsb Gb za
98-4299-42 100-42 101-42 Rc Rib Rsb Gb za
98-4399-43 100-43 101-43 Ra Ric Rsb Gb za
98-4499-44 100-44 101-44 Rb Ric Rsb Gb za
98-4599-45 100-45 101-45 Rc Ric Rsb Gb za
98-4699-46 100-46 101-46 Ra Ria Rsc Gb za
98-4799-47 100-47 101-47 Rb Rla Rsc ~b za
98-4899-48 100-48 101-48 Rc Ria Rsc ~b za
98-4999-49 100-49 101-49 Ra Rib Rsc Gb za
98-5099-50 100-50 101-50 Rb Rib Rsc Gb za
98-5199-51 100-51 101-51 Rc Rib Rsc ~b za
98-5299-52 100-52 101-52 Ra Ric Rsc Gb za
98-5399-53 100-53 101-53 Rb Ric Rsc (ib 2a
98-5499-54 100-54 101-54 Rc Roc Rsc ~b ~a
98-5599-55 100-55 101-55 Ra Rya R5a ~c Za
98-5699-56 100-56 101-56 Rb Ria R5a Gc 2a
98-5799-57 100-57 101-57 Rc Ria Rsa ~c 2a
98-5899-58 100-58 101-58 Ra Rib Rsa Gc ~a
98-5999-59 100-59 101-59 Rb Rib Rsa Gc Za
98-6099-60 100-60 101-60 Rc Rib Rsa ~c za
98-6199-61 100-61 101-6I Ra Ric Rsa Gc za
98-6299-62 100-62 101-62 Rb Ric Rsa ~ za
98-6399-63 100-63 101-63 Rc Roc Rsa Gc ~a
98-6499-64 100-64 101-64 Ra Rya Rsb Gc Za
98-6599-65 100-65 101-65 Rb Rya Rsb Gc Za
98-6699-66 100-66 101-66 R Rya Rsb ~c ~a
98-6799-67 100-67 101-67 Ra Rib Rsb ~c Za
143
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
98-6899-68 100-68 101-68 Rb R1b Rsb Gc 2a
98-6999-69 100-69 101-69 Rc RIb Rsb Gc ~a
98-7099-70 100-70 101-70 Ra Rlc Rsb Gc 2a
98-7199-71 100-71 10I-71 Rb Rlc Rsb Gc Za
98-7299-72 100-72 101-72 R RIc Rsb Gc Za
98-7399-73 100-73 101-73 Ra Rla Rsc Gc Za
98-7499-74 100-74 101-74 Rb R1a Rsc Gc Za
98-7599-75 100-75 101-75 Rc Rla Rsc Gc Za
98-7699-76 100-76 101-76 Ra Rib Rsc Gc Za
98-7799-77 100-77 101-77 Rb Rib Rsc G Za
98-7899-78 100-78 101-78 Rc Rib Rsc Gc Za
98-7999-79 100-79 101-79 Ra RIc Rsc Gc Za
98-8099-80 100-80 101-80 Rb R~ Rsc Gc ~a
98-8199-81 100-81 101-81 R Rlc Rsc Gc 2a
98-8299-82 100-82 101-82 Ra R1a Rsa Ga zb
98-8399-83 100-83 l0I-83 Rb Rla Rsa Ga Zb
98-8499-84 100-84 101-84 R Rya Rsa Ga 2b
98-8599-85 I00-85 101-$5 Ra Rlb Rsa Ga 2b
98-8699-86 100-86 101-86 Rb Rib Rsa Ga ~b
98-8799-87 100-87 101-87 R RIb Rsa Ga Zb
98-8899-88 100-88 101-88 Ra Rlc Rsa Ga Zb
9$-8999-89 100-89 101-89 Rb Roc Rsa Ga Zb
98-9099-90 100-90 101-90 R Rjc Rsa Ga ~b
98-9199-91 100-91 101-91 Ra R1a Rsb Ga Zb
98-9299-92 100-92 101-92 Rb Rla Rsb Ga ~b
98-9399-93 100-93 101-93 Rc Rla Rsb Ga ~b
98-9499-94 100-94 101-94 Ra Rlb Rsb Ga ~b
98-9599-95 100-95 101-95 Rb Rib Rsb Ga zb
98-9699-96 100-96 101-96 Rc RIb Rsb Ga Zb
98-9799-97 100-97 101-97 Ra Rlc Rsb Ga ~b
98-9899-98 100-98 101-98 Rb Rlc Rsb Ga Zb
98-9999-99 100-99 101-99 Rc Rlc Rsb Ga 2b
98-10099-100100-100101-100Ra Rla Rsc Ga Zb
98-10199-101100-101101-101Rb R1a Rsc Ga zb
98-10299-102100-102101-102R Rla Rsc Ga zb
98-10399-103100-103101-103Ra RIb Rsc Ga ~b
98-10499-104100-104101-104Rb RIb Rsc Ga zb
98-10599-105100-105101-105Rc Rlb Rsc Ga zb
98-10699-106100-106101-106Ra Rlc Rsc Ga 2b
98-10799-107100-107101-107Rb RIc R5c Ga ~b
98-10899-108100-108101-108R Rlc Rsc Ga Zb
98-10999-109100-109101-109Ra Rla Rsa Gb Zb
98-11099-110100-I10101-110Rb R1a Rsa Gb Zb
98-11199-111100-111101-111R Rla Rsa Gb Zb
98-11299-112100-112101-112Ra R1b Rsa Gb ~b
98-11399-113100-113101-113Rb Rlb Rsa Gb Zb
98-11499-114100-114101-114R RIb Rsa Gb 2b
144
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
98-11599-115 100-115101-115Ra Ric Rsa Gb Zb
98-lI699-116 100-116101-I16Rb Ric Rsa Gb Zb
98-11799-117 100-117101-117R Ric Rsa Gb Zb
98-11899-118 I00-II8I0I-118Ra Ria Rsb Gb Zb
9$-11999-119 100-119IOI-119Rb Ria Rsb Gb Zb
98-12099-120 100-120101-120R Ria Rsb Gb Zb
98-12199-121 100-121101-12IRa Rib Rsb Gb Zb
98-I2299-122 100-122101-122Rb Rib Rsb Gb Zb
98-12399-123 100-123101-123R Rib Rsb Gb Zb
98-12499-124 100-124l0l-124Ra Ric Rsb Gb Zb
98-12599-I25 100-I25101-I25Rb Ric Rsb Gb Zb
98-12699-126 100-I26101-126R Ric Rsb Gb Zb
98-I2799-127 100-127101-I27Ra Ria Rsc Gb Zb
98-12899-128 100-128101-I28Rb Ria Rsc Gb Zb
98-12999-129 100-129101-129Rc Ria Rsc Gb Zb
98-13099-130 100-130101-130Ra Rib Rsc Gb Zb
98-13199-131 100-131101-131Rb Rib Rsc Gb Zb
98-13299-132 100-132101-132R Rib Rsc Gb Zb
98-13399-133 100-133101-I33Ra Ric Rsc Gb Zb
98-13499-134 100-134101-134Rb Ric Rsc Gb Zb
98-13599-135 100-135101-135R Ric Rsc Gb Zb
98-13699-136 100-136101-136Ra Ria Rsa Gc Zb
98-13799-I37 100-137101-137Rb Ria Rsa Gc Zb
98-13899-138 100-138101-I38R Ria Rsa Gc Zb
98-13999-139 100-139101-139Ra Rib Rsa Gc Zb
98-14099-140 100-140101-140Rb Rib Rsa Gc Zb
98-14199-141 I00-141101-14IRc Rib Rsa Gc Zb
98-14299-142 100-142101-142Ra Ric Rsa Gc Zb
98-14399-143 100-143101-143Rb Ric Rsa Gc Zb
98-14499-144 100-144101-144R Ric Rsa Gc Zb
98-14599-145 100-145101-145Ra Ria Rsb G Zb
98-14699-146 100-146101-146Rb Ria Rsb Gc Zb
98-14799-147 100-147101-147Rc Ria Rsb Gc Zb
98-14899-148 I00-148101-148Ra Rib Rsb Gc Zb
98-14999-149 I00-149101-149Rb Rib Rsb Gc Zb
98-15099-150 100-150101-150R Rib Rsb Gc Zb
98-15199-151 100-151101-151Ra Ric Rsb Gc Zb
98-15299-I52 100-152101-152Rb R' Rsb G Zb
98-15399-153 100-153101-153Rc Ric Rsb G Zb
98-15499-154 100-154101-154Ra Ria Rsc Gc Zb
98-I5599-155 100-155I01-155Rb Ria Rsc Gc Zb
98-15699-156 100-156101-156Rc Ria Rsc Gc Zb
98-15799-157 100-157101-157Ra Rib Rsc Gc Zb
98-15899-158 100-158101-158Rb Rib Rsc G Zb
98-15999-I59 100-159101-159R Rib Rsc Gc Zb
98-16099-160 100-160101-160Ra Ric Rsc Gc Zb
98-16199-161 100-161101-161Rb Ric Rsc Gc Zb
145
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
98-16299-162 100-162101-162R Roc Rsc G Zb
98-I6399-163 100-163101-I63Ra R1a Rsa Ga Zc
98-I6499-I64 100-164101-164Rb R1a Rsa Ga Zc
98-16599-165 100-165101-165R Rla Rsa Ga Zc
98-16699-166 100-166101-166Ra R1b Rsa Ga Zc
98-16799-167 100-I67l0l-167Rb Rlb Rsa Ga Zc
98-16899-168 100-168101-16$Rc RIb Rsa Ga Zc
98-16999-169 100-169I01-169Ra Rlc Rsa Ga Zc
98-17099-170 100-170101-170Rb RIc Rsa Ga Zc
98-17199-171 100-17Il0l-171Rc Rlc Rsa Ga Zc
98-17299-I72 100-I72101-I72Ra Rla Rsb Ga Zc
98-17399-173 100-173101-173Rb Rla Rsb Ga Zc
98-17499-174 I00-174101-174R Rya Rsb Ga Zc
98-17599-175 100-175101-175Ra R1b Rsb Ga Zc
98-17699-I76 100-I76101-176Rb Rlb Rsb Ga Zc
98-I7799-177 100-177101-177Rc Rlb Rsb Ga Zc
98-17899-178 100-178101-178Ra R1c Rsb Ga Zc
98-17999-179 100-179101-179Rb Ric Rsb Ga Zc
98-18099-I80 100-180101-180Rc Roc Rsb Ga Zc
98-18199-I81 100-181101-181Ra Rya R5 Ga Zc
98-18299-182 100-182101-182Rb R1a Rsc Ga Zc
98-18399-I$3 100-183I01-183Rc Rla Rsc Ga Zc
98-18499-184 100-I84101-I84Ra RIb Rsc Ga Zc
98-18599-185 100-185101-I85Rb RIb Rsc Ga Zc
98-18699-I86 100-186101-186R RIb Rsc Ga Zc
98-18799-187 100-187101-187Ra Rlc Rsc Ga Zc
98-18899-188 100-188101-188Rb Rlc Rsc Ga Zc
98-18999-189 100-189101-189Rc Roc Rsc Ga Zc
98-19099-I90 100-190101-I90Ra Rla R5a Gb Zc
98-19199-19I 100-191101-191Rb R1a Rsa Gb Zc
98-I9299-192 100-192101-192Rc Rla Rsa Gb Zc
98-19399-193 100-I93101-193Ra Rlb Rsa Gb Zc
98-19499-194 100-194101-I94Rb j'yb Rsa Gb Zc
98-19599-195 100-195101-195Rc Rlb Rsa Gb Zc
98-19699-196 100-I96101-196Ra R1c Rsa Gb Zc
98-19799-197 100-197101-197Rb R~ Rsa Gb Zc
98-I9899-198 100-198101-198Rc RIc Rsa Gb Zc
98-19999-199 100-199101-199Ra R1a Rsb Gb Zc
98-20099-200 100-200101-200Rb Rla Rsb Gb Zc
98-20199-201 100-201101-201Rc Rya Rsb Gb Zc
98-20299-202 100-202IOI-202Ra Rib R'~' Gb Z
98-20399-203 100-203101-203Rb Rib Rsb Gb Z
98-20499-204 I00-204101-204R Rib Rsb Gb Zc
98-20599-205 I00-205101-205Ra RI Rsb Gb Zc
98-20699-206 100-206101-206Rb Roc Rsb Gb Zc
98-20799-207 100-207101-207R Rlc Rsb Gb Z
98-20899-208 100-208101-208Ra Rya Rsc Gb Zc
146
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
98-20999-209 I00-209101-209 Rb RIa Rsc Gb Z
98-21099-210 100-210101-210 Rc Rla Rsc Gb Zc
98-21199-211 100-211101-211 Ra R1b Rsc Gb Zc
98-21299-212 100-212101-212 Rb Rlb Rsc Gb Z
98-21399-213 100-213101-213 Rc Rib Rsc Gb Z
98-21499-214 100-214101-214 Ra Rlc Rsc Gb Zc
98-21599-215 100-215101-215 Rb Rlc Rsc Gb Z
98-21699-216 100-216101-216 R Rlc Rsc Gb Z
98-21799-217 100-217101-217 Ra Rya Rsa Gc Zc
98-21899-218 100-218101-218 Rb RIa Rsa Gc Zc
98-21999-219 100-219101-219 Rc Rla Rsa Gc Zc
98-22099-220 100-220101-220 Ra Rlb Rsa Gc Zc
98-22199-221 100-221101-221 Rb Rib Rsa G Zc
98-22299-222 100-222101-222 R RIb Rsa G Zc
98-22399-223 100-223101-223 Ra Rlc Rsa Gc Zc
98-22499-224 100-224101-224 Rb Ric Rsa Gc Zc
98-22599-225 100-225101-225 Rc Roc Rsa G Zc
98-22699-226 100-226101-226 Ra RIa Rsb Gc Zc
98-22799-227 100-227I01-227 Rb Rla Rsb Gc Zc
98-22899-228 100-228101-228 R Rla Rsb Gc Zc
98-22999-229 100-229101-229 Ra Rib Rsb G Zc
98-23099-230 100-230101-230 Rb Rib Rsb Gc Z
98-23199-231 100-231101-231 Rc RIb Rsb Gc Zc
98-23299-232 100-232101-232 Ra Rlc Rsb Gc Zc
98-23399-233 100-233101-233 Rb RIc Rsb Gc Zc
98-23499-234 100-234101-234 R Rlc Rsb Gc Zc
98-23599-235 100-235101-235 Ra RIa Rsc Gc Zc
98-23699-236 100-236101-236 Rb Rla Rsc Gc Zc
98-23799-237 100-237141-237 Rc Rla Rsc Gc Zc
98-23899-238 100-238101-238 Ra Rib Rsc Gc Z
98-23999-239 100-239101-239 Rb Rlb Rsc Gc Z
98-24099-240 100-240101-240 Rc RIb Rsc Gc Zc
98-24199-241 100-241101-241 Ra RIc Rsc Gc Zc
98-24299-242 100-242101-242 Rb RIc Rsc Gc Z
98-24399-243 100-243101-243 R Roc Rsc Gc Z
where all symbols are as defined above.
In one aspect of any of formulae (98), (99), (100), and (101) of the present
invention,
R is hydrogen, a hydroxy group, a halogen, a nitro group, an optionally
substituted amino
group, an allcyl group, an alkoxy group, an alkenyl group, or an alkoxyalkyl
group; RI is
hydrogen, a hydroxy group, a halogen, a nitro group, a carboxy group, a
carbamoyl group, or
an optionally substituted amino group, an allcyl group, a cycloalkyl group, an
alkoxy group, a
cycloalkoxy group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl
group, an
147
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
allcenyloxy group, or a cycloalkenyloxy group; RS is is hydrogen, a hydroxy
group, a
halogen, a vitro group, a carboxy group, a carbamoyl group, or an optionally
substituted
amino group, an alkyl group, a cycloalkyl group, an alleoxy group, a
cycloalkoxy group, an
alkenyl group, a cycloalkenyl group, an allcoxyalkyl group, an allcenyloxy
group, or a
cycloalkenyloxy group; and all other symbols are as defined above in
connection with
formula (I).
In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, G is -(CH2)S , where s is an integer from 0-5; and all other
symbols are as defined
above in connection with formula (I).
to In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, G is -(CHZ)S CH=CH-(CH~)S-, where s is an integer from 0-5; and all
other
symbols are as defined above in connection with formula (I).
In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, G is -(CH2)5 C=C-(CHZ)S , where s is an integer from 0-5; and all
other symbols
are as defined above in cormection with formula (I).
In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, Z is O, and all other symbols are as defined above in connection
with formula (I).
In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, Z is NR, and all other symbols are as defined above in connection
with formula
(I).
In another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, Z is fCH~)" or S(=O)", where a is an integer from 0-2; and all
other symbols are as
defined above in connection with formula (I).
In still another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, E is O, and all other symbols are as defined above in connection
with formula (I).
In still another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, E is S, and all other symbols are as defined above in connection
with formula (I).
In still another aspect of any of formulae (98), (99), (100), and (101) of the
present
invention, E is NR, and all other symbols are as defined above in connection
with formula
(I).
Examples of compounds having general formula (IV) include, but are not limited
to:
148
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
d
/~N
s o I ~ o N~o
"~o
OCHa
\ OCHa
N/ N~O \ O NH
O I / / _ ~O
N_N~
> >
~NiCHa
OEt
~~ / N
I '\o
N\ \
O N\
N~ II N V ' ~ ~O
O
O ~ ~ ~ S
and
According to another aspect of the present invention, various compounds of
general
formula (I) having general formula (V)
1
Rz B\A ~E~R4 X4 g~C~ W Ar
K L-~CH~~ v -C-X~CH~~ ~C-O-R
X~ X
3
149
CA 02540460 2006-03-10
WO 2005/042712 , PCT/US2004/035939
its tautomeric forms, its stereoisomers, its polymorphs, its pharmaceutically
acceptable salts,
and its pharmaceutically acceptable solvates are provided. Except as otherwise
provided
herein, all symbols are as defined above in connection with formula (I).
Examples of compounds having the general formula (V' contemplated by the
present
invention include, but are not limited to:
R~
4
R~ B .p I ~E~R X4 g(C) W Ar
D~ ~ J K-L-(CHa) v -C-X-(CH2) p \C-O-R
Rs ~ XZ X
(102);
R~
4
R2 B ;S~~E~R X4 S(p) W Ar
I ~/
p/~~ \ 'K-L-(CH2)v-~-X-(CH2)p \Ii-C-R
R ~X ~ Xs
(103);
R~
4
R~ B N i vE\ /R XQ S(C) w Ar
D: ~~ ~ ~K(-L-(GH2)v -C-X-(CH2) p ~C-O-R
R~ ~ IX2 IX
(104);
R~
R~ B ;C ~E~R4 X4 S(p) W Ar
p: ~~ ~ K-L-(CH2) v -C-X-(CH~)~C-O-R
R~ ~ X2 X
(105);
R~
R~ HB ~~cH2~%E~R4 ~X4 S(O),"r Ar
K-L-(CH2)v- i-X-(CH2)p \C-C-R
Rs ~ Xz X
3 (106);
R~
4
R~ p A~~E~R X4 S(0) w Ar
D~~~s' ~K-~--(CH2)v-IX-X-(CH2)p \Ii-O-R
R/ IXI ~ X3 (107);
150
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R1
4
R~ S A ~ ~E~R X4 S(C) w Ar
D/~~ ~K-~ (CHa)v-X X-(CH~)~C-O-R
R IX' ~ Xs
(108);
R1
RZ N A I ~E~R4 X4 S(C) W Ar
D: ~ ~ TK-~-(CHa)v-C-X-(CH2)~C_0_R
/ ~ II
Rs X X~ II
X3 (109);
R1
4
RZ HC A~'E~R X4 S(C) w Ar
~J I ~./
D~~~~ ~K-L-(CH2)v-~ -X-(CH2)~I~-C_R
R IXI 2 Xs (110)i
R~
~A ~E~R4 g(p) W Ar
R2 cHzC>~ ~ I Xa
DS~3: ~K-~-(CHZ)v-X X-(CH2 p--CII C R
R IXI 2 X3
(111);
R~
a _
R2 B p'~~E~R X4 g(C) w Ar
o~,~: ~~~-~-(~H2)v-~ -X-(~H2)~~-o_R
IXI X~
X3 (112);
R1
Ra g \A i ~E~R4 X4 S(C) w Ar
I _ _
S/ J~ ~~-~ (CH2)v-i X (CH2)P I~ C R
R/ X ~ X3 (113);
R1
R2 g~A~~E~R4 X4 S(C) w Ar
HC~ J~' ~I'K-~'-(CH2)v-C-X-(CH~)~C_C_R
Rs IXI X~ II
X3 (114);
15I
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
\ 4
R~ g \A /E~R X4 S(O) w Ar
~H2C)n / 3 ~ K-L-(CH2) v -C-X-(CHI) p~C-O-R
Rs ~ X2 X
(115);
R~
Ra
~A 1 /E\ / X4 SAO) W Ar
R2 B ,~
I _ _ _
\ 'K-L-(CH2)v (~ X (CHOP ~~ O R
R/a ~X X2 X
(116);
R~
4 X4 g(O) W Ar
~E~R
g~A
~
~
~ _
R i (CFi2)-p-C
~ I -X -O-f2
D~~S~
K-L-(CH2)v-
~ i
/ I
I
R 2 X3
X (117);
R1
4 X4 g(O) W Ar
sE~R
g~A
T
2
~
I -~-X-(CH~)p C_O-R
R
~
D/NJ
K-L (CHa)v
~
I
I
R ~ X3 (118);
X
R~
\ 4 X4 S(O) w Ar
g~A
~E~R'
2
~ ~
R K-L-(CH~)v-C-X--O-R
~ I (CH~)~
p~C~ \ '
R H ~ i
X X3 (119);
R~
4 X4 g(O) W Ar
~E~R
g~A
2 ' II
~ -C-X -(CH2)-p-
R -O-R
K-L-(CH2)v
i
X lI (12U)i
X3
R~
a X4 g(O) W Ar
~O~R
g~A
1
2
~
R -X
~ X-(CHa)~
I -O-R
p/ ~ ~
K-L-(CHI)
v
~ i
R X ~ Xs
(121);
152
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
g~A X4 S(p) w Ar
~~~R4
a
~ ~/
R X-(CH2)~
D/~: -p-R
K-~ (CHZ)v -
~ i
I X
I
R Z X
X (122);
R~
R Ra
X4 g(p) W Ar
g~A ~N~
2
R I _ ~
D,/~ ~ K-L-(CH~)v -C-X (CH2) p 'C-p-R
/ ~
R X~ IX
(123);
R~
4 X4 g(p) W Ar
g~A
~E~R
2
~ -C-X-(CH2)~C-O-R
R
D~ ~~
N-L-(CHZ)
v
~
R X 2 X3 (124);
R1
~E~R4 X4 S(p) w Ar
g~'4
R~ -C-X-(CHa)p \C-O-R
i
IC-L-(CH~)v
D/~~
~
R X 2 X3 (125);
R~
4 X4 g(p) W Ar
g~A
oE~R
R~ CH-L-(CHz) - i -X-(CH2) p \C-p-R
~ v
D/ ~:
~
I
I
R3 X~ IX3 (126)
X
R~
~A ~E~R 4 g(p) W Ar
B ~ ~ X
2I
R (CH2)v C-X-(CHZ) p ~C-p-R
p/~~
K-~-G-Z-Ar-
\
~
R
X X3 (127);
R~
~A ,E /g(p) W Ar
Ra X
B
~
R2
~
~. ~/
4
(CH2)v
IX-X-(CH~)~C-p-R
D~~~:
~K-Y-G-~
~ ~
R/ I
I X
x Xa (128);
153
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
4 ~g(O) W Ar
~A
,E
R
R2D:
,
~
~
(CH~)t-(CHa)
v
C-X-(CHI)
p
a
O
R
~
~ X
~
R ~ I ( (129);
X3
R~
4 g(O) W Ar
,E
R X
B
~
R2~ 4
~~~~~ (CH2)V
\ X (CH2) p~C-O-R
/K-L-
~ O
R Xs
X (130);
R~
4 og(O) W Ar
~A
,E
R
R2D: (CH2)v C-X-(CH2) p a
~ I
~ C-O-R
~
L-
~
J
~ S
R X3 (131);
R~
4 ~g(O) W Ar
/E~R
X
R2- ~
~ a
I X-(CH2) p \C-O_R
p~ (CH~)v C
J~
\
/K-L-
~ R
R X3 (132);
X
R~
B X4 S(O) W Ar
A
/E~Ra
R~~ \ /K-L -(CH~)v
p~~J~~ i-X-(CH~)p C_O-R
~ I
R3 X~ O (133);
X
R~
4 g(O) W Ar
,E
R X
B
~
Rz~ 4
p~ (CH~)v yX-(CH2)~C-O_R
J~
~K-L-
R 2 S (134);
X
R~
4 X
B S(O) W Ar
A
~E~R
R~~ 4
~~~Je' -(L'H2)V C-X-(CH~)~C-p-R
~K-~-
/
I
I
R X2 NI R (135)
3
X
154
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R1
R~ B A~~E~Ra O~S(O) W Ar
D/~~ \/K-L-(CH~)v I I X (CH2) p~C-O-R
R3 fXI X2 X
(136);
R1
R2 B A ~E~Ra S~S(O),N Ar
K-L-(CHa)v C-X-(CH~)~C-O-R
R/ ~ x2 I I
X3 (137);
R1
4
R2 B A~~E~R RN~g(O) W Ar
K-L-(CH2)v C-X-(CH2)p \C-O-R
R/s ~ ?C~ X
(138);
R1 _ / R11
B A of Ra X~S(O) w \ ,R12
R2~ Ja
p/~~: ~K-L-(CH~)v iI-X (CH2p-\C-O-R
Rs IXI X2 II
X3 (139); and
R11
R1 a
B A~~E R X~S(O)w \ ~
R2 D:~ ~ ~ L-(CH~)v C-X-(CH2)-~a \R1~
R~.1 ~ X~ C-O-R
X3 (140),
where all symbols are as defined above in connection with formula (I).
The present invention contemplates various compounds of general formula (V)
having the formula:
Rts
H HN o~~Rta
CH2) ~ ~O~R
H2)p
0 0 (141),
155
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
where RI1, Ri2, RI3, and R14 independently are hydrogen, a halogen, a vitro
group, an amino
group, a mono- or di- substituted amino group, a hydroxy group, an alkoxy
group, a carboxy
group, a cyano group, an oxo(O=) group, a thio(S=) group, an alkyl group, a
cycloalkyl
group, an alkoxy group, a haloalkoxy group, a cycloalkyl group, an aryl group,
a benzyloxy
group, an aryl group, an acyloxy group, an amyl group, an allcoxycarbonyl
group, an
aryloxycarbonyl group, a heteroaryl group, a heterocyclyl group, an aralkyl
group, an
alkylsulfonyl group, an alkylsulf'myl group, an arylsulfonyl group, an
arylsulfmyl group, an
alkylthio group, an arylthio group, a heteroarylthio group, an aralkylthio
group, or a
heterocyclyl sulfonyl group, which is optionally substituted with a halogen, a
hydroxyl
l0 group, a vitro group, an amino group, an alkyloxy group, or any combination
thereof, and
wherein the heterocycle group is optionally a substituted morpholinyl group, a
thiomoipholinyl group, or a piperzinyl group, wherein the substituent on the
heterocyclyl
group is a halogen, a vitro group, an amino group, an alkyl group, an allcoxy
group, or an aryl
group; and all other symbols are defined as above in connection with formula
(I).
In one aspect of formula (141) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, or an optionally substituted amino group; and
all other
symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, R is an alkyl
group, an
alkoxy group, an alkenyl group, or an alkoxyalkyl group; and all other symbols
are as
defined in connection with formula (I).
In another aspect of formula (141) of the present invention, Rl is hydrogen, a
hydroxy
group, a halogen, a vitro group, a carboxy group, a carbamoyl group, or an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an allcoxy group,
a cycloalkoxy
group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl group, an
alkenyloxy group, or
a cycloalkenyloxy group; and all other symbols are as defined in connection
with formula (I).
In another aspect of formula (141) of the present invention, R'' is hydrogen,
a hydroxy
group, a halogen, a vitro group, a carboxy group, a carbamoyl group, or an
optionally
substituted amino group, an alkyl group, a cycloalkyl group, an alkoxy group,
a cycloalkoxy
group, an allcenyl group, a cycloallcenyl group, an alkoxyallcyl group, an
alkenyloxy group, or
a cycloalkenyloxy group; and all other symbols are as defined in connection
with formula (I}.
In yet another aspect of formula (141) of the present invention, R' ~ is
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
156
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an allcoxy group, a carboxy group, a cyano group, an oxo(O=) group, or
a thio(S=)
group; and all other symbols are as defined in connection with formula (I).
In still another aspect of formula (141) of the present invention, RIl is an
alkylsulfonyl group, an alkylsulfmyl group, an arylsulfonyl group, an
arylsulfmyl group, an
alkylthio group, an arylthio group, a heteroarylthio group, an aralkylthio
group, or a
heterocyclyl sulfonyl group, which is optionally substituted with a halogen, a
hydroxyl
group, a vitro group, an amino group, an alkyloxy group, or any combination
thereof, and
wherein the heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or a piperzinyl group, wherein the substituent on the
heterocyclyl
l0 group is a halogen, a vitro group, an amino group, an alkyl group, an
alkoxy group, or an aryl
group; and all other symbols are as defined in connection with formula (I).
In yet another aspect of formula (141) of the present invention, R12 is
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, or a
thio(S=)
is group; and all other symbols are as defined in connection with formula (I).
In still another aspect of formula (141) of the present invention, R'2 is an
alkylsulfonyl group, an alkylsulfmyl group, an arylsulfonyl group, an
arylsulfmyl group, an
alkylthio group, an arylthio group, a heteroarylthio group, an aralkylthio
group, or a
heterocyclyl sulfonyl group, which is optionally substituted with a halogen, a
hydroxyl
20 group, a vitro group, an amino group, an alkyloxy group, or any combination
thereof, and
wherein the heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or a piperzinyl group, wherein the substiiuent on the
heterocyclyl
group is a halogen, a vitro group, an amino group, an allcyl group, an alkoxy
group, or an aryl
group; and all other symbols are as defined in connection with formula (I).
25 In a further aspect of fomnula (141) of the present invention, R13 is
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, or a
thio(S=)
group; and all other symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, R13 is an
alleylsulfonyl
30 group, an alkylsulfinyl group, an arylsulfonyl group, an arylsulfinyl
group, an alkylthio
group, an arylthio group, a heteroarylthio group, an arallcylthio group, or a
heterocyclyl
sulfonyl group, which is optionally substituted with a halogen, a hydroxyl
group, a vitro
157
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
group, an amino group, an alkyloxy group, or any combination thereof, and
wherein the
heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or
a piperzinyl group, wherein the substituent on the heterocyclyl group is a
halogen, a vitro
group, an amino group, an alkyl group, an alkoxy group, or an aryl group; and
all other
s symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, R14 is hydrogen,
a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, or a
thio(S=)
group; and all other symbols are as defined in connection with formula (I).
In yet another aspect of formula (141) of the present invention, RI4 is an
alkylsulfonyl
group, an alkylsulfinyl group, an arylsulfonyl group, an arylsulfinyl group,
an alkylthio
group, an arylthio group, a heteroarylthio group, an aralkylthio group, or a
heterocyclyl
sulfonyl group, which is optionally substituted with a halogen, a hydroxyl
group, a vitro
group, an amino group, an alkyloxy group, or any combination thereof, and
wherein the
heterocycle group is optionally a substituted morpholinyl group, a
thiomorpholinyl group, or
a piperzinyl group, wherein the substituent on the heterocyclyl group is a
halogen, a vitro
group, an amino group, an alkyl group, an alkoxy group, or an aryl group; and
all other
symbols are as defined in connection with formula (I).
In yet another aspect of formula (141) of the present invention, R is hydrogen
or an
2o alkyl group, and all other symbols are as defined in connection with
formula (I).
In another aspect of formula (141) of the present invention, R is -H, CH3, or
CZHS,
and all other symbols are as defined in connection with formula (I).
In still another aspect of formula (141) of the present invention, RI is
hydrogen or an
alkyl group, and all other symbols are as defined in connection with formula
(I).
In yet another aspect of formula (141) of the present invention, R' is -H,
CH3, or
C2H5, and all other symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, RZ is hydrogen or
an alleyl
group, and all other symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, RZ is -H, CH3, or
CZHS,
3o and all other symbols are as defined in connection with formula (I).
158
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In yet another aspect of formula (141) of the present invention, Rll is
hydrogen, a
halogen, an alkoxy group, or an alkylthio group; and all other symbols are as
defined in
connection with formula (I).
In another aspect of formula (141) of the present invention, R' 1 is -H, -Cl, -
OGH3, or -
SCH3, and all other symbols are as defined in connection with formula (I).
In a further aspect of formula (141) of the present invention, Rlz is
hydrogen, a
halogen, an alkoxy group, or an alkylthio group; and all other symbols are as
defined in
connection with formula (I).
In a another aspect of formula (141) of the present invention, R~Z is H, Cl, -
OCH3, or
to -SCH3, and all other symbols are as defined in connection with formula (I).
In a further aspect of formula (141) of the present invention, RI3 is
hydrogen, a
halogen, or an allcyl group, and all other symbols are as defined in
connection with formula
(I).
In a still further aspect of formula (141) of the present invention, RI3 is -
H, -F, or
CH3, and all other symbols are as defined in connection with formula (I).
In yet another aspect of formula (141) of the present invention, R'4 is
hydrogen, a
halogen, or an alkyl group, and all other symbols are as defined in connection
with formula
(I).
In a further aspect of formula (141) of the present invention, R14 is -H, -F,
or -CH3,
and all other symbols are as defined in connection with formula (I).
In another aspect of formula (141) of the present invention, RI and R2
independently
are hydrogen, a hydroxy group, a halogen, a nitro group, a carboxy group, a
carbamoyl
group, an optionally substituted amino group, an alkyl group, a cycloalkyl
group, an alkoxy
group, a cycloalkoxy group, an alleenyl group, a cycloalkenyl group, an
alkoxyallcyl group,
an alkenyloxy group, or a cycloalkenyloxy group; RII, R'2, R'3, and R14
independently are
hydrogen, a halogen, a nitro group, an amino group, a mono- or di- substituted
amino group,
a hydroxy group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=)
group, or a
thio(S=) group, an alkyl group, or a cycloalkyl group, an alkoxy group; and
all other symbols
are as defined as above in connection with formula (I).
In another aspect of formula (141) of the present invention, Ri and R2
independently
are hydrogen, a hydroxy group, a halogen, an allcoxy group; RI', RIZ, R13, and
Rla
159
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
independently are hydrogen, a halogen, a hydroxy group, an alkoxy group; and
all other
symbols are as defined as above in coimection with formula (I).
In yet another aspect of formula (141) of the present invention, RI and Rz
independently are -H or -OCH3; Rl1 is -Cl, -OCH3, or -SCH3; RIZ is -Cl, -OCH3,
or -H; R is
H, CH3, or CZHS; R13 is F or CH3; RI4 1S F or CH3; v is 0 or 1; and all other
symbols are as
defined as above in connection with formula (I).
The present invention also contemplates various compounds of general formula
(V)
as follows:
0 0
1
R~~ I I N.H O
Rz/~ NR ~OR
SO H
(142),
to where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, Rl, and R2 of
formula (142)
are selected to produce various compounds of formula (142-1) through formula
(142-27) as
follows:
Formula R R R
142-1 Ra Rla R a
142-2 Rb Rla Rza
142-3 R~ Rla Rza
142-4 Ra Rlb Rza
142-5 Rb Rib Rza
142-6 R~ Rlb Rza
142-7 Ra Rl~ Rza
142-8 Rb RI Rza
142-9 R Rl~ Rza
142-10 Ra Rla Rzb
142-11 Rb Rla Rzb
142-12 R Rla Rzb
142-13 Ra Rlb Rzb
142-14 Rb Rlb Rzb
142-15 R Rib Rzb
142-16 Ra RI Rzv
142-17 Rb R'~ Rzb
142-18 R Rl~ Rzb
142-19 Ra Rl~ Rz
160
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
142-20Rb Rla Rzc
142-21R Rla Rz
142-22Ra Rib Rzc
142-23RU Rib Rzc
142-24R RIb Rzc
142-25Ra Rlc Rzc
142-26Rb R' Rzc
142-27R R~ Rz
where all symbols are as defined above.
In one aspect of formula (142) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, an optionally substituted amino group, alkyl
group, an
alkoxy group, an allcenyl group, or an alkoxyalkyl group; and Rl and Rz
independently are
hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, an
optionally substituted amino group, an alkyl group, a cycloalkyl group, an
alkoxy group, a
cycloallcoxy group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl
group, an
alkenyloxy group, or a cycloalkenyloxy group.
l0 In another aspect of formula (142) of the present invention, R' and Rz are
independently a halogen or an alkyl group; and R is hydrogen, an allcyl
NOz _
group, ~ ~ \ , or ~~ .
In still another aspect of formula ( 142) of the present invention, R' is -
OCH3 or -F; Rz
NOz _
is -OCH3 or -Cl; R is -H or CZHS, ~ / \ , or ~~ .
~s The present invention also contemplates various compounds of general
formula (~
as follows:
R~ N (CHZ)v NH~(CH2)P COzR
~\ ~ /
1 ~ ~'
RZ ~%/ NCO \ I O NH
O=S=O
O
/I
R1g\/~R14 (143)
where all symbols are as defined above in connection with formula (I).
In one aspect of formula (143) of the present invention, Rl and Rz
independently are
20 hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, an
optionally substituted amino group, an alkyl group, a cycloalkyl group, an
alkoxy group, a
161
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
cycloalkoxy group, an allcenyl group, a cycloalkenyl group, an allcoxyallcyl
group, an
alkenyloxy group, or a cycloalkenyloxy group; RI3 and R'4 independently are
hydrogen, a
halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, a
thio(S=)
group, an alkyl group, a cycloalkyl group, an allcoxy group, a haloalkoxy
group, a cycloalkyl
group, an aryl group, a benzyloxy group, an acyl group, an acyloxy group, an
aroyl group, an
allcoxycarbonyl group, an aryloxycarbonyl group, a heteroaryl group, or a
heterocyclyl
group, an aralkyl group; and all other symbols are as defined in connection
with formula (I).
In another aspect of formula (143) of the present invention, RI and R2
independently
are an acyl group, an acyloxy group, an aryl group, an aryloxy group, an aroyl
group, an
aroyloxy group, an aralkyl group, an aralkoxy group; a heterocyclyl group, a
heteroaryl
group, a heteroaralkyl group, a heteroaryloxy group, a heteroaralkoxy group,
an
alkoxycarbonyl group, an aryloxycarbonyl group, or a heteroarylcarbonyl group;
R13 and R14
independently are hydrogen, a halogen, a vitro group, an amino group, a mono-
or di-
substituted amino group, a hydroxy group, an alkoxy group, a carboxy group, a
cyano group,
an oxo(O=) group, a thio(S=) group, an alkyl group, a cycloalkyl group, an
allcoxy group, a
haloalkoxy group, a cycloalkyl group, an aryl group, a benzyloxy group, an
aryl group, an
acyloxy group, an aroyl group, an alkoxycarbonyl group, an aryloxycarbonyl
group, a
heteroaryl group, or a heterocyclyl group, an aralleyl group; and all other
symbols are as
defined in connection with formula (I).
In another aspect of formula (143) of the present invention, RI and RZ
independently
are hydrogen, an alkyl group, or an alkoxy group; R13 and RI4 independently
are hydrogen, a
halogen, an alkyl group, or an alkoxy group; and all other symbols are as
defined in
connection with formula (I).
In yet another aspect of formula (143) of the present invention, R' and RZ are
-H or -
OCH3; RI3 is CH3 or -F; RI4 is -H or -F; and all other symbols are as defined
in connection
with formula (I).
The present invention also contemplates various compounds of general formula
(V)
having the formula:
162
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
~(CH2)p CO~R
R ~\ N\ / (CH2)~~NH
RZ ~%/ \IN~O \ ~ ~O NH
O=S=O
O
cH3 (144),
where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, R', Rz, v, and p of
formula
(144) are selected to produce various compounds of formula (144-1} through
(144-243) as
follows:
FormulaR R R v p
144-1 Ra Ria R a va pa
144-2 Rb Rla Rza va pa
144-3 R Rla Rza va pa
.
144-4 Ra Rlb Rza va pa
144-5 Rb Rlb Rza va pa
144-6 R Rlv Rza va pa
144-7 Ra Rl Rza va pa
144-8 Rb RI R''a va pa
144-9 R~ Rl~ Rza va pa
144-10 Ra Rla Rzb va pa
144-11 Rb Rla Rzb va pa
144-12 R~ Rla Rzt, va pa
144-13 Ra Rtb Rzb va pa
144-14 Rb Rlb Rzb va pa
144-15 R Rlb R2b va pa
I44-16 Ra RI~ Rzb va pa
144-17 Rb RI~ Rzb va pa
144-18 R Rl~ Rzb va pa
144-19 Ra R~ a Rz~ va pa
144-20 Rb R~ a Rz va pa
144-21 R Rla Rz va pa
144-22 Ra Rlb Rzo va pa
144-23 Rb Rlb R2~ va pa
144-24 R~ Rib Rz va pa
144-25 Ra Rye Rz~ va pa
144-26 Rb Rl~ R2~ va pa
144-27 R~ R1 Rz~ va pa
144-28 Ra Rla Rza vb pa
144-29 Rb R1a Rza vb pa
144-30 R~ Rya Rza vb pa
144-31 Ra RIb Rza vb pa
163
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
144-32Rb Rib Rza vb pa
144-33R Rib Rza vb pa
I44-34Ra Ri Rza vb pa
144-35Rb Ric Rza vb pa
144-36R Ric Rza vb pa
144-37Ra Ria Rzb vb pa
144-38Rb Ria Rzb vb pa
144-39Rc Ria Rzb vb pa
144-40Ra Rib Rzb vb pa
144-41Rb Rib Rzb vb pa
144-42Rc Rib Rzb vb pa
144-43Ra Ric Rzb vb pa
I44-44Rb Ri c Rzb vb pa
144-45Rc Ric Rzb vb pa
144-46Ra Ri a Rzc vb pa
144-47Rb Ria Rzc vb pa
144-48R Ria Rzc vb pa
144-49Ra Rib Rzc vb pa
144-50Rb Rib Rzc vb pa
144-51Rc Rib Rzc vb pa
144-52Ra RI c Rzc vb pa
144-53Rb Ric Rzc vb pa
144-54Rc Ri Rz vb pa
144-55Ra Ria Rza vc pa
144-56Rb Ria Rza vc pa
144-57R Ria Rza vc pa
144-58Ra Rib Rza vc pa
144-59Rb Rib Rza vc pa
144-60R Rib Rza v pa
144-61Ra Ri c Rza vc pa
144-62Rb Ric Rza vc pa
144-63Rc Ri c Rza vc pa
144-64Ra Ria Rzb vc pa
144-65Rb Ria Rzb v pa
144-66Rc Ria R2b vc pa
144-67Ra Rib R2b vc pa
144-68Rb Rib Rzb vc pa
144-69Rc Rib Rzb v pa
144-70Ra Ric Rzb vc pa
144-71Rb Ric R2b vc pa
144-72R Ri Rzb vc pa
144-73Ra Ria Rzc vc pa
144-74Rb Ria Rzc vc pa
144-75Rc Ria Rzc vc pa
144-76Ra Rib Rzc vc pa
144-77Rb Rib Rzc vc pa
144-78Rc Rib Rzc v pa
164
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
144-79Ra R'c Rzc vc pa
144-80Rb Rlc Rzc v pa
144-81Rc Rlc Rzc vc pa
144-82Ra R' Rza va pb
a
144-83Rb R'a Rza va pb
144-84Rc R' Rza va pb
a z b
'b
144-85Ra R R va p
" a b
z
144-86Rb R a va p
' R b
" z
144-87R R R Va p
' a
144-88Ra Roc Rza va pb
144-89Rb R'c Rza va pb
144-90R R' Rza va pb
c
144-91Ra R' Rzb va pb
a b
144-92Rb R' Rzb va p
a
144-93R R'a Rzb va pb
144-94Ra R'b Rzb va pb
144-95Rb R'b Rzb va pb
144-96Rc Rtb Rzb va pb
144-97Ra Roc Rzb pa pb
144-98Rb Rlc Rzb va pb
144-99Rc Rlc Rzb va pb
144-100Ra R'a Rzc va pb
' b
144-101Rb R Rzc va p
a
144-102Rc R'a Rzc va pb
b z b
144-103Ra R' R va p
c
144-104Rb R'b Rzc va pb
144-105Rc Rib Rzc Va pb
144-106Ra Rlc Rzc Va pb
b
144-107Rb Ric Rzc va p
144-108R R'c Rzc va b
p
b
144-109Ra R'a Rza vb p
144-110Rb R'a Rza vb pb
144-111R R'a Rza vb pb
144-112Ra R'b Rza vb pb
144-113Rb R'b Rza Vb pb
b b
144-114R R'b Rza v p
144-115Ra R'c Rza vb pb
144-116Rb Rlc Rza Vb b
p
b
144-117Rc Rlc Rza vb p
144-118Ra R'a Rzb ~b pb
144-119Rb Rla Rzb Vb pb
144-120R R'a Rzb vb pb
144-121Ra R'b Rzb vb pb
b b b b
144-122Rb R' Rz v p
b b b b
144-123R R' Rz V p
144-124Ra R'c Rzb vb pb
144-125Rb R'c Rzb vb pb
165
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
144-126Rc Ric Rzb Vb b
p
i z b
144-127Ra R R v pb
a c
144-128Rb Ria Rzc Vb b
P
i z b
144-129R R c v b
a R P
ib
144-130Ra R Rzc vb pb
144-131Rb Rib Rzc vb pb
144-132Rc Rib Rzc vb pb
144-133Ra Ric Rzc vb b
P
b i z b
144-134R R R v pb
c c
144-I35Rc Ric Rzc vb b
P
144-136Ra Ria Rza vc b
P
144-137Rb Ria Rza vc pb
144-138R Ria R2a vc pb
144-139Ra Rib Rza vc b
P
b ib z
144-140R R a vc b
R P
ib
144-141Rc R Rza vc pb
144-142Ra Ric Rza vc pb
144-I43Rb Ric Rza vc pb
144-144Rc Ric Rza vc b
P
144-145Ra Ria Rzb v b
P
144-146Rb Ria Rzb Vc b
P
144-147Rc Ria Rzb vc b
p
ib zb
144-148Ra R R vc b
P
144-149Rb Rib Rzb vc pb
144-150R Rib Rzb vc b
P
i zb
144-151Ra c R vc pb
R
144-152Rb Ric R2b vc pb
144-153R Ric Rzb vc pb
144-154Ra Ria Rzc vc pb
144-155Rb Ria Rzc vc pb
144-156R Ria Rzc ~c pb
144-157Ra Rib Rzc vc b
P
144-I58Rb Rib Rzc vc pb
144-159R Rib Rzc v pb
144-160Ra Ric Rzc vc b
h
b i
144-161R R Rz vc pb
c
144-162R Ric Rzc v pb
144-163Ra Ria Rza va pc
144-164Rb Ria Rza va pc
144-165Rc Ria Rza va pc
144-166Ra Rib Rza va pc
144-167Rb Rib Rza va pc
144-168R Rib Rza va pc
144-169Ra Ric Rza va pc
144-170Rb Ric Rza va pc
144-171R Ric Rza va pc
144-172Ra Ria Rzb va pc
166
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
144-I73Rb Rla Rzb va pc
144-174R Rya Rzb va ~c
144-175Ra Rlb Rzb va pc
144-176Rb R'b Rzb ',a pc
144-177R Rlb Rzb Va pc
144-178Ra R'c Rzb va pc
144-179Rb Rlc Rzb va ~o
144-180R Rlc Rzb va pc
144-181Ra R'a Rzc va pc
144-182Rb Rla Rzc va pc
144-183Rc Rla Rzc va pc
144-184Ra R"' Rzc va pc
144-185Rb Rlb Rzc va pc
144-186Rc Rlb Rzc va pc
144-187Ra Rlc Rzc va ~c
144-188Rb R'c Rzc va pc
144-189Rc R'c Rzc va pc
144-190Ra Rla Rza Vb pc
144-191Rb Rla Rza Vb pc
144-192R Rya Rza vb ~c
144-193Ra R"' Rza vb pc
144-194Rb R"' Rza vb pc
144-195R Rlb Rza vb pc
144-196Ra Rlc Rza vb pc
144-197Rb Rlc R2a vb pc
144-198Rc Rlc Rza vb pc
144-199Ra Rta Rzb vb pc
144-200Rb R'a Rzb vb pc
144-201Rc Rla Rzb Vb pc
144-202Ra Rlb Rzb vb ~c
144-203Rb Rlb Rzb vb pc
144-204R Rlb Rzb vb p
144-205Ra R' Rzb vb pc
144-206Rb R'c Rzb vb p
144-207Rc R' Rzb vb pc
c
I44-208Ra jZla R2c ~b pc
144-209Rb Rl Rzc vb pc
a
144-210Rc R' Rzc Vb pc
a
144-211Ra R"' Rzc Vb pc
144-212Rb R'b Rzc vb pc
144-213Rc R'b Rzc vb pc
144-214Ra R' Rz vb pc
144-215Rb R' Rzc vb pc
144-216R R'c Rzc vb pc
144-217Ra R' Rza uc pc
a
144-218Rb R' Rza vc pc
a
144-219Rc R' Rza vc pc
a
167
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
144-220 Ra Rlb R2a vc pc
144-22I Rb Rib R2a vc pc
144-222 R RIb R2a vc pc
144-223 Ra RIc RZa vc pc
144-224 Rb RIc R2a vc pc
144-225 R Rtc R2a ~ pc
144-226 Ra Rja R2b vc pc
144-227 Rb RI R2b vc pc
a
144-228 Rc Rla R2b vc pc
144-229 Ra RIb R2b vc pc
144-230 Rb RIb R2b vc pc
144-231 Rc Rib R2b vc pc
144-232 Ra Rlc R2b v pc
144-233 Rb Rlc R2b vc pc
144-234 Rc RI R2b vc pc
144-235 Ra Ria R?c vc pc
144-236 Rb Ria R2c vc pc
144-237 Rc Rla RZc vc pc
144-238 Ra Rlb R2c vc pc
144-239 Rb RIb Rzc vc p
144-240 Rc Rlb RZc v p
144-241 Ra Rlc R2c vc pc
144-242 Rb RIc R'' vc pc
144-243 R~ RI R2c vc pc
c
where all symbols are as defined above.
In one aspect of formula (144) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, an optionally substituted amino group, an
allcyl group, an
alkoxy group, an allcenyl group, or an alkoxyalkyl group; Rl and RZ
independently are
hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, an
optionally substituted amino group, an alkyl group, a cycloalleyl group, an
alkoxy group, a
cycloalkoxy group, an alkenyl group, a cycloalkenyl group, an alkoxyalkyl
group, an
allcenyloxy group, or a cycloallcenyloxy group; and all other symbols are as
defined in
to connection with formula (I).
In another aspect of formula (144) of the present invention, Rl and RZ are
independently hydrogen, an alkyl group, or an alkoxy group; and all other
symbols are as
defined in cormection with formula (I).
In yet another aspect of formula (144) of the present invention, RI and R2 are
-OCH3;
and all other symbols are as defined W connection with formula (I).
168
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The present invention further contemplates various compounds of general
formula
(V) having the formula:
R~~
~~/R1z
/ O
O W O~ N. H O
I ~ O ~OR
Ii
Ra~~ Ov,N
z / I SO .H
(145),
where all symbols are as defined above in connection with formula (I).
According to various aspects of the present invention, R, RI, R2, RI', and R12
of
formula (145 are selected to provide various compounds of formula (145-1)
through formula
(145-243) as follows:
Formula R Rl R' R' R12
145-1 Ra Rla Rza R a RI2a
145-2 Rb RI a R2a RI 1 RI2a
a
145-3 R~ RI a R2a Rt t Rt2a
a
145-4 Ra Rlb R2a Rlla Rl2a
145-5 Rb RIb R2a Rna Rl2a
145-6 R~ Rlv Rza R~~a Rl2a
145-7 Ra RI~ R2a Rna Rt2a
145-8 Rb Rl~ R2a Rna Rl2a
145-9 R~ Rl~ R2a Rlla Rlza
145-10 Ra Rla R2b Rna R~za
145-11 Rb RIa R2t Rna R~2a
145-12 R~ Rl a R2b R> > Rl2a
a
145-13 Ra R1b R2b Rlla R~2a
145-14 Rv Rib R2b Rlla R,~2a
145-15 R~ Rib Rzb R~~a R~2a
145-16 Ra R~~ R2b Rlla Rl2a
145-17 Rb Rm R2b Rna R~2a
145-18 R~ R'~ R2b Rtm Rl2a
145-19 Ra RIa R2c Rna Rt2a
145-?0 Rb Rla Rzc Rna Rt2a
145-21 R R~ a Rz RI t R~
a 2a
145-22 Ra R~ b R2~ R> > Rl2a
a
145-23 Rb Rlb Rzc Rna Rt2a
145-24 R~ Rib R2~ Rtla R~2a
145-25 Ra RI Rzc Rna R~2a
145-26 Rb RI Rzc R> > Rl2a
a
169
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
145-27R Rl Rzc Rlla Rl2a
145-28Ra Rla Rza Rllb Rl2a
145-29Rb Rla R2a Rllb Rl2a
145-30R Rla R2a Rllb Rl2a
I45-31Ra Rlb R2a Rllb Rl2a
145-32Rb Rlb Rza Rl Rl2a
ib
145-33Rc Rlb Rza Rllb Rl2a
145-34Ra Rlc R2a Rltb Rlza
145-35Rb Rlc R2a Rllb Rlza
145-36Rc Rlc R2a Rllb Rl2a
145-37Ra Rla R2b Rllb Rl2a
145-38Rb Rla Rzb Rllb Rl2a
145-39R Rla R2b Rllb Rl2a
145-40Ra Rlb Rzb Rilb RI2a
145-4IRb Rlb Rzb Rltb Rl2a
145-42Rc Rlb R2b Rllb Rl2a
145-43Ra Rlc R2b Rllb Rl2a
145-44Rb Rlc R2b Rllb Rl2a
145-45R Rl R2b Rllb Rl2a
145-46Ra Rla R2c Rllb Rl2a
145-47Rb Rla R2c Rllb Rl2a
145-48R Rla Rzc Rllb Rl2a
145-49Ra Rlb R2c Rllb Rlza
145-50Rb Rlb Rzc Rilb Rl2a
145-51Rc Rlb R2c Rllb Rl2a
145-52Ra Rl R2c Rllb Rl2a
145-53Rb Rlc R2c Rllb Rl2a
145-54Rc Rlc R2c Rllb Rl2a
145-55Ra Rla R2a Rilc Rl2a
145-56Rb Rla R2a Rllo Rl2a
145-57Rc Rla Rza Rllc Rlza
145-58Ra Rlb R2a Rllc Rl2a
145-59Rb Rlb R2a Rllc Rl2a
145-60R Rlb R2a Rllc Rlza
145-61Ra Rlc R2a Rllc Rl2a
145-62Rb Rlc Rza Rllc Rl2a
145-63Rc Rlc R2a Rllc Rl2a
145-64Ra Rla Rzb Rllc Rl2a
145-65Rb Rla R2b Rllc Rlza
145-66Rc Rla Rzb Rllc Rl2a
145-67Ra Rlb Rzb Rllc Rl2a
145-68Rb Rlb R2b Rllc Rl2a
145-69Rc Rlb R2b Rllc Rl2a
145-70Ra Rl Rzb Rl Rl2a
1
145-71Rb Rlc R2b Rlic Rlza
145-72Rc Rlc Rzb Rllc Rl2a
145-73Ra Rl R2o Rl Rl2a
a l
c
170
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
I45-74Rb Rla R2c Rllc Rl2a
145-75Rc Rla R2c Rllc Rl2a
I45-76Ra Rlb R2c Rllc Rl2a
145-77Rb Rlb R2c Rllc Rt2a
145-78R Rlb R2c Rllc Rl2a
145-79Ra Rlc R2c Rilc Rl2a
145-80Rb Rl R2c Rl t Rl2a
c .
145-81R Rlc R2c Rllc Rl2a
145-82Ra Rla R2a Rlla Rl2b
145-83Rb Rla R2a Rlla Rl2b
145-84Rc Rla R2a Rlla Rl2b
145-85Ra Rlb R2a Rlla Rl2b
145-86Rb Rlb R2a Rlla Rl2b
145-87R Rlb Rza Rlia Rl2b
145-88Ra Rlc R2a Rlla Rl2b
145-89Rb Rlc Rza Rlla RI2b
145-90Rc Rl R2a Rl l Rl2b
c a
145-91Ra Rl R2b Rl l Rt
a a 2b
145-92Rb Rl R2b Rl l Rl2b
a a
145-93Rc Rl R2b RI l Rl2b
a a
145-94Ra Rlb R2b Rlla Rl2b
145-95Rb Rlb R2b Rlla Rl2b
145-96R Rlb R2b Rlla Rl2b
145-97Ra Rlc R2b Rlla Rl2b
145-98Rb Rl R2b Rl l Rl2b
a
145-99Rc Rl R2b Rl l Rl2b
c a
I45-100Ra Rla Rzc Rlla Rl2b
145-101Rb Rla Rzc Rlla Rl2b
145-102Rc Rla R2c Rlla Rl2b
145-103Ra Rlb R2c Rlta Rlzb
145-104Rb Rlb R2c Rlla Rl2b
145-105Rc Rlb R2c Rlla RI2b
145-106Ra Rlc Rzc Rlla Rl2b
145-107Rb Rlc R2c Rlla Rl2b
145-108Rc Rl Rzc Rl l Rl2b
a
145-109Ra Rla R2a Rllb Rl2b
145-110Rb Rla R2a Rllb Rl2b
145-111Rc Rla Rza Rllb Rlzb
145-112Ra Rlb R2a Rllb Rl2b
145-113Rb Rlb R2a Rllb Rl2b
145-114Rc Rlb R2a Rllb Rl2b
145-115Ra Rl R2a Rlib Rl2b
145-116Rb Rlc R2a Rllb Rl2b
145-117R Rlc R2a Rllb Rlzb
145-118Ra Rla R2b Rllb Rl2b
145-119Rb Rla Rzb Rllb Rl2b
145-120R Rla Rzb Rllb Rl2b
171
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
145-121Ra Rib R2b Ri 1b Ri2b
145-122Rb Rib Rzb Riib Ri2b
145-123R Rlb R2b Rllb Rl2b
145-124Ra Rlc Rzb Riib Rlzb
145-125Rb Ric Rzb Riib Rizb
145-126Rc Ric R2b Rlib Rizb
145-127Ra Ria R2c Rlib Rizb
145-128Rb Ria R2c Riib Rizb
145-129Rc Ria R2c Riib Ri2b
145-130Ra Rib Rzc Rlib Ri2b
145-131Rb Rib Rzc Rilb Rl2b
145-132Rc Rib Rzc Rnb Ri2b
145-133Ra Ric R2c Riib Rl2b
145-134Rb Ric R2c Riib Ri2b
145-135Rc Ric R2c Ri ib Rizb
145-136Ra ~la R2a Riic Ri2b
145-137Rb Ria R2a Rilc Rl2b
145-138R Ria R2a Rilc Rl2b
145-139Ra Rib R2a Rilc Ri2b
145-140Rb Rib R2a Rllc Rl2b
145-141Rc Rib R2a Rlic Ri2b
145-142Ra Ric R2a Rlic Ri2b
145-143Rb Rlc R2a Riic Rlzb
145-144Rc Ric Rza Riic Ri2b
145-145Ra Rla R2b Riic Rl2b
145-146Rb Ria R2b Rllc Rl2b
145-147R Ria R2b Riic Rlzb
145-148Ra Rib R2b Rllc Ri2b
145-149Rb Rlb Rzb Riic Ri2b
145-150R Rib R2b Rlic Ri2b
145-151Ra Rlc Rzb Riic Ri2b
145-152Rb Ric R2b Rilc Ri2b
145-153R Ric R2b Rllc RI2b
145-154Ra Ria R2c Riic Rl2b
145-155Rb Ria R2c Riic Rl2b
145-156R Ria R2c Rllc Ri2b
145-157Ra Rib R2c Rllc Rl2b
145-158Rb Rib Rzc Rllc RI2b
145-159R Rib R2c Riic Ri2b
145-160Ra Ric R2c Rllc Ri2b
145-161Rb Ric R2c Rllc Ri2b
145-162R Rlc Rzc Riic Rizb
145-163Ra Ria R2a Rila Rl2c
145-164Rb Rla R2a Rlia Ri2c
145-165R Ria R2a Rila Rl2c
145-166Ra Rib R2a Rlla Rl2c
145-167Rb Rib R2a Rila Ri2c
172
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
145-168R R"' R2a R"a Rl2c
145-169Ra R'c R2a Rlla Rt2c
145-170Rb RIc Rza Rtta Rl2c
145-171Rc Rlc Rza Rna Rl2c
I45-172Ra Rla R2b Rna Rt2c
145-173Rb RIa R2b Rlla Rlzc
I45-174Rc Rla R2b Rlla Rl2c
145-175Ra R1b R2b Rlla Rtzc
145-176Rb Rib R2b Rlla Rl2c
145-177R R"' R2b Ra Rl2c
145-178Ra RIc R2b Rtta Rl2c
145-179Rb Rlc R2b Rna Ri2c
145-180R Rlc R2b Rna Rl2c
145-181Ra R'a Rzc R"a R'2c
145-182Rb R'a R2c R"a R'2c
I45-183R RIa R2c Rlta Rl2c
I45-184Ra Rlb R2c Rna Rl2c
145-185Rb R"' R2c R"a R'2c
145-186R R"' R2c Rla Rl2c
I45-187Ra Rtc R2c Rlta Ri2c
145-188Rb R'c R2c Rna Rt2c
I45-189R Rlc Rzc Rlta Rl2c
145-190Ra Rta Rza Rllb Rl2c
145-191Rb R'a R2a Rnb Rlzc
145-192R Rla R2a Rllb Rl2c
145-193Ra R"' Rza R"'' R'2c
145-194Rb R'b R2a R"'' Rl2c
145-195R R"' R2a R> R~2o
>b
145-196Ra Rtc R2a Rllb Rlzc
145-197Rb R'c R2a Rb R~2c
145-198R Rlc R2a Rllb Rlzc
145-199Ra RIa R2b Rnb Rl2c
145-200Rb Rla Rzb Rllb Rl2c
145-201R Rla R2b Rnb Rl2c
145-202Ra R"' R2b R"b R'2c
145-203Rb R'b Rzb R"b Rizc
145-204R R'b R2b R"b Rlzc
I45-205Ra R'c Rzb Rnb Rlzc
I45-206Rb Rlc R2b Rllb Rl2c
145-207Rc R'c Rzb R"b R'2c
I45-208Ra RIa R2c Rnb Rlzc
145-209Rb Rla R2c Rnb Rl2c
145-210R R'a R2c R1b Rt2c
145-211Ra Rib Rzc Rt Rlzc
tb
I45-212Rb R'b R2c Rtlb Rl2c
145-213R R'b R2c R"b R'2c
I45-214Ra R'c R2c Rllb Rl2c
173
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
I45-215Rb Rlc Rzc Rub RI2c
145-216R RIc Rzc Rnb Rl2c
145-217Ra RI a R2a Rn c Rt2c
145-218Rb RI a R2a R11 Rl2c
c
145-219R RIa R2a Rllc Rl2c
145-220Ra Rib R2a Rnc Rl2c
145-221Rb Rib Rza RI~c R~2c
145-222R Rib Rza Rllc R~2c
145-223Ra Rtc Rza Rilc R~2c
145-224Rb Rlc R2a Rnc Rl2c
145-225Rc Rlc Rza Rnc R~2o
145-226Ra Rla R2b R" Rl2c
145-227Rb RIa R2b Rllc Rl2c
145-228Rc Rta Rzb Rnc Rl2c
145-229Ra Rib R2b Rm Rl2c
145-230Rb Rib Rzb RI~c R~z
145-231R Rib Rzb Rnc R~zc
145-232Ra Rlc Rzb Rnc R~2c
145-233Rb Rlc R2b R~~ R~2c
145-234R Rlc Rzb Rnc R~zc
145-235Ra Rya Rzc Rl~ Rl2c
145-236Rb Rla Rzc Rt~o Rl2c
145-237R Rya Rzc Rnc R~2c
145-238Ra Rib Rzc RIIc Rizc
145-239Rb Rlb Rzc R~Ic R~z
145-240R Rlb R2c Rn RI2c
145-241Ra Rlc Rzc Rnc Rl2c
145-242Rb Rl Rzc Ro Rl2c
145-243Rc R' Rzc R11 Rlzc
where all symbols are as defined above.
In one aspect of formula (145) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, an optionally substituted amino group, an
allcyl group, an
allcoxy group, ari alkenyl group, or an alkoxyallcyl group; Rl and R2
independently are
hydrogen, a hydroxy group, a halogen, a vitro group, a carboxy group, a
carbamoyl group, an
optionally substituted amino group, an alkyl group, a cycloalkyl group, an
allcoxy group, a
cycloallcoxy group, an allcenyl group, a cycloalkenyl group, an alkoxyalkyl
group, an
alkenyloxy group, or a cycloallcenyloxy group; and R' 1 and RIZ independently
are hydrogen,
l0 a halogen, a vitro group, an amino group, a mono- or di- substituted amino
group, a hydroxy
group, an alkoxy group, a carboxy group, a cyano group, an oxo(O=) group, or a
thio(S=)
group, an alkyl group, or a cycloalkyl group, an allcoxy group.
174
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In another aspect of formula (145) of the present invention, R is hydrogen or
an alkyl
group; RI and RZ independently are hydrogen, a hydroxy group, a halogen, an
alkoxy group;
and R'1 and R~Z independently are hydrogen, a halogen, a hydroxy group, or an
alkoxy
group.
In yet another aspect of formula (145) of the present invention, R is -H or
CZHS; Rl
and RZ are -OCH3; and Rl ~ and R12 independently are -H, -F, or CH3.
The present invention still further contemplates various compounds of general
formula (V) having the formula:
(146),
where R is as defined above in connection with formula (I).
In one aspect of formula (146) of the present invention, R is hydrogen, a
hydroxy
group, a halogen, a vitro group, or an optionally substituted amino group.
In another aspect of formula (146) of the present invention, R is an alkyl
group, an
allcoxy group, an alkenyl group, or an alkoxyallcyl group.
In yet another aspect of formula (146) of the present invention, R is a
cycloalkenyloxy group, an acyl group, an aryl group, an arallcyl group, a
heterocyclyl group,
or a heteroaryl group.
In still another aspect of formula (146) of the present invention, R is -H or
an alkyl
group.
In still another aspect of formula (146) of the present invention, R is -H or
CzHs.
Additional examples of compounds having general formula (V) include, but are
not
limited to:
00
Me0 ~ N' / NH~COzC2H5
C-O O \ II
H' I ~' I O CH' _ Me0 I / NCO \ I O tJH
~C O O-S-O
hl,C O O HN j~ \ ~ ~ /
O ~ ~ N~O~Cht~ \ I
CH3
> >
175
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
> ;
0
~N,H
N~ / ~ ~C02C2Hs
MeO
%I ~~N~O HN ~O
5. H Me0
\ I O . O ~ ~ / F
F
OCH3
/ OCH3
OOHS
ocH, ~ N\
I \ ° I \ I o ' ~ ~ N,N ~ N~N~COOC2H5
.~ \
O O~O I / N H~OC2H5 / O O NH ~ ~ ~ CH3
II ii
0 0 - O
> >
ci s cl ~ ~ CHs
\ ° \ I o / I
I O~O I \ I-I HN,S \
0 ~N~OCZHS
O [OI ~ N02
SCH3
\ O \ I O / I
I / I Oi\r0 I \ H HN SO \
O ~N~OC2H5
> >
F / F
OS ~
H HN. .O
N~OC~HS ~ I s H
~O
> ;
176
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
O O
s SCH3 F ~H
~N O
o w ~
I ono ~ o o CI ' N OH
O ( / N~OCzHS
H w ,N.
HN. ~ S H
~ ; and
OCH3
/ OCH3
H3C0 ~ O W I
O~O I ~ O O
OCH30 ~ [j
N~OC2H5
H
HN. .O
~S I
O
It is contemplated that any compound shown or described herein, including
compounds of the various formulae shown or described above, may be provided as
a
pharmaceutically acceptable salt. Pharmaceutically acceptable salts forming
part of this
invention include salts derived from inorganic bases such as Li, Na, K, Ca,
Mg, Fe, Cu, Zn,
Mn; salts of organic bases such as N,N'-diacetylethylenediamine, betaine,
caffeine, 2-
diethylaminoethanol, 2-dimethylaminoethanol, N-ethyhnorpholine, N-
ethylpiperidine,
to glucamine, glucosamine, hydrabamine, isopropylamine, methylglucamine,
morpholine,
piperazine, piperidine, procaine, purines, theobromine, triethylamine,
trimethylamine,
tripropylamine, tromethamine, diethanolamine, meglumine, ethylenediamine, N,N'-
diphenylethylenediamine, N,N'-dibenzylethylenediamine, N-benzyl
phenylethylamine,
choline, choline hydroxide, dicyclohexylamine, metformin, benzylamine,
phenylethylamine,
dialkylamine, trialkylamine, thiamine, aminopyrimidine, aminopyridine, purine,
or
spermidine; chiral bases like alkylphenylamine, glycinol, or phenyl glycinol;
salts of natural
amino acids such as glycine, alanine, valine, leucine, isoleucine, norleucine,
tyrosine, cystine,
cysteine, methionine, proline, hydroxy proline, histidine, ornithine, lysine,
arginine, serine,
threonine, phenylalanine; unnatural amino acids such as D-isomers or
substituted amino
2o acids; guanidine, substituted guanidine wherein the substituents are
selected from nitro,
amino, allcyl, alkenyl, or alkynyl; ammonium or substituted ammonium salts and
aluminum
salts. Salts may include acid addition salts where appropriate which are,
sulphates, nitrates,
177
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
phosphates, perchlorates, borates, hydrohalides, acetates, tartrates,
maleates, citrates,
succinates, palmoates, methanesulphonates, benzoates, salicylates,
hydroxynaphthoates,
benzenesulfonates, ascorbates, glycerophosphates, ketoglutarates.
Pharmaceutically
acceptable solvates may be hydrates or may comprise other solvents of
crystallization such as
alcohols.
Processes for Preparing the Compounds
The compounds of the present invention can be prepared according to the
following
processes. However, it should be understood that other processes having other
process
l0 conditions may be used to form the compounds of the present invention.
PROCESS 1
According to one aspect of the present invention, a process for preparing a
compound
of general formula (II)
Y1
1
z BMA E R4 F-'~/NR
- ~ I
Y-G =~-Ar~Y2
~~ ~'R
where R' is attached to B; R~ is attached to J; R3 is -H; A, B, D and J
independently are -CH;
Rl and Rz independently are an alkoxy group or an aralkoxy group; R4 is a
phenyl group
optionally substituted with an alkoxy group or an aralkoxy group at the third
position and/or
fourth position respectively, X and E are each O, G is -(CHz)S ; ~CHz)S CH=CH-
(CHz)S , or
2o f CHz)S CH=CH-(CHz)S , where s is an integer from 0-5; F is O, S or -NR; Y
and Z
independently are O, -NR, f CHz~", or S(=O)", where n is an integer from 0-2;
Yl and Yz
independently are O or S; R and RS independently are hydrogen, a hydroxy
group, a halogen,
a vitro group, an optionally substituted amino group, an allcyl group, an
alkoxy group, an
alkenyl group, an alkoxyalkyl group, a cycloalkenyloxy group, an acyl group,
an aryl group,
an aralkyl group, a heterocyclyl group or a heteroaryl group; and 'Ar' is an
optionally
substituted phenyl group or an optionally substituted naphthyl group is
provided.
The process comprises first alkylating the Rutin hydrate of formula (IIa)
178
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
R2 B\ A E R4
p. , ; ~ (IIa)
R J~ Y.Rut
X
where 'Rut' is rutinose; RI is attached to B; R~ is attached to J; R3 is H; A,
B, D and J
independently are -CH, Rl and RZ independently are a hydroxy group; R4 is a
phenyl group
optionally substituted with a hydroxy group at the third andlor fourth
positions; X, Y, and E
are O; and '----' is an optional chemical bond;
to a compound of formula (IIa), where RI is attached to B; RZ is attached to
J; R3 is H;
A, B, D and J independently are -CH; Rl and RZ independently are an allcoxy
group or an
aryloxy group; R4 is a phenyl group optionally substituted with an alkoxy
group or an
aralkoxy group at the third and fourth positions; X, Y, and E are O; and all
other symbols are
as defined above.
The alkylation is carried out using an alkyl halide alkylating or aralkylating
agent.
Examples of agents that may be suitable include MeI, EtI, EtBr, n-PrI, n-PrBr,
i-PrBr, i-PrI,
n-BuCl, or s-BuBr; a dialkylsulphate such as dimethylsulphate or
diethylsulphate; or an
aralkyl halide such as benzyl halide. The reaction may be carried out in the
presence of an
alkali, for example, sodiumhydride (NaH), potassiumhydride (KH), potassium
tertiary
butoxide (t-BuOK), potassium acetate (KOAc), sodium acetate (NaOAc), n-butyl
lithium (n-
BuLi), sec-butyl lithium (s-BuLi), tert butyl lithium (t-BuLi), lithium
diisopropyl amide
(LDA), sodium carbonate (Na2C03), potassium carbonate (K2C03), sodium
bicarbonate
(NaHC03), potaasium bicarbonate (KHC03), sodium hydroxide (NaOH), potassium
hydroxide (KOH), or any mixture thereof. The solvent used is, for example,
dimethylformamide (DMF), dimethylsulfoxide (DMSO), hexametaphosphoric acid
(HMPA),
1,4-dioxane, acetone, dimethyl ether, diethyl ether, tetrahydrofuran (THF), or
any mixture
thereof.
According to one aspect of the invention, the reaction temperature may be from
about
-30°C to about 250°C, for example, from about 30°C to
about 100°C. The duration of the
reaction may be from about 0.5 hours to about 100 hours, for example, from
about 20 hours
to about ~0 hours. The reaction may be carned out under an inert atmosphere
of, for
example, nitrogen (NZ), argon (Ar), or helium (He).
Next, the compound of formula (IIa) is hydrolysed to a compound of formula
(IIb)
179
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
R2 B~ A ' E R4
(IIb)
R~'J ~ YH
3
X
where Rl is attached to B; R2 is attached to J; R3 is H; A, B, D and J
independently are -CH;
R' and RZ independently are an alkoxy group or an aralkoxy group; R4 is a
phenyl group
optionally substituted with an alkoxy group or an aralkoxy group at the third
and fourth
positions; X, Y, and E are O; and all other symbols are as defined above.
The hydrolysis is optionally carried out using an inorganic acid, such as
hydrochloric
acid (HCl), sulfuric acid (H2S04), or a mixture thereof with water. The
reaction temperature
may be maintained at from about -30°C to about 250°C, for
example, from about 50°C to
about 150°C. The duration of the reaction may be from about 0.5 hours
to about 100 hours,
l0 for example, from about 1 hour to about 50 hours.
Next, the compound of formula (IIb) is reacted with a compound of formula
(IIc),
O,
~'--Ar-Z-G-Hal (IIc)
R5
where 'Hal' is a halogen; 'Ar', G, Z and RS are as defined above; and '----'
is an optional
chemical bond, to obtain a compound of formula (IId)
R~
R~ B\ A E. R4 O
Dr ~ % I Y-G-Z-Ar' ( 5 (IId
R3 R )
where R' is attached to B; R2 is attached to J; R3 is H; A, B, D, and J
independently are -CH;
RI and R2 independently are an allcoxy group or an aralkoxy group; R4 is a
phenyl group
optionally substituted with an alkoxy group or an aralkoxy group at the third
and fourth
positions; and all other symbols are as defined above.
2o This reaction is carried out in the presence of a base, for example, NaH,
I~H, KOtBu,
KOAc, NaOAc, NaOEt, KOEt, n-BuLi, s-BuLi, t-BuLi, LDA, Na2C03, I~ZC03, NaHC03,
I~HG03, NaOH, or KOH. The reaction is optionally carried out in the presence
of a solvent,
for example, DMF, DMSO, HMPA, 1,4-dioxane, acetone, dimethyl ether, diethyl
ether,
THF, or any mixture thereof. The reaction temperature may be maintained at
from about -
30°C to 150°C, for example, from about 30°C to about
100°C. The duration of the reaction
180
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
may be from about 1 hour to about 50 hours, for example, from about 2 hours to
about 25
hours. The reaction may be carried out under an inert atmosphere of N2, Ar, or
He.
Lastly, the compound of formula (IId) is condensed with a compound of formula
(IIe),
H
't ~ 2
Y .~ Y (IIe)
F
where F, YI, and YZ are as defined above, to obtain a compound of formula (II)
Y~
1
2 B.\,A E R4 F~NR
R - ~ I -~ (II)
D. . ~ Y-G-Z-Ar Y2
R5
X
where all symbols are as defined above.
The condensation may be carned out using a base, for example, Et3N,
diethylamine,
1o diisopropylethyl amine, diisopropyl amine, DBU, piperidine, or any mixture
thereof. The
reaction may be carried out in the presence of an acid, for example, benzoic
acid, formic
acid, acetic acid, or any mixture thereof. The reaction may be carried out in
the presence of a
solvent, for example, benzene, toluene, xylene, ethanol, i-propanol, bytanol,
DMF, DMSO,
1,4-dioxane, or any mixture thereof. The reaction may be maintained at a
temperature of
is from about 30°C to about 300°C, for example, from about
50°C to about 200°C. The
duration of the reaction may be from about 10 hours to about 150 hours, for
example, from
about 20 hours to about 80 hours. The reaction may be carried out under an
inert atmosphere
of NZ, Ar, or He.
2o PROCESS 2
According to another aspect of the present invention, a process for preparing
a
compound of formula (II) is provided. All symbols are as defined above, except
that X and E
are O.
Y~
B A E R4 F~NR
I
R D. ~~ I Y-G-Z-Ar=~Y2
R
X
181
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
First, a compound of formula (IIf)
R~
EH
R~ B
3
R 3'
where X and E are O; and all other symbols are as defined above, is acylated
to a compound
of formula (IIg)
R~
Ra B\'A Y~ E~ (IIg)
Dj~~ X
R3
The acylation may be carried out by using an acylating agent such as, for
example,
acetic anhydride. The reaction is optionally carried out in the presence of a
base such as, for
example, Na2CO3, K~C03, NaHC03, KHC03, NaOH, I~OH, or any mixture thereof. The
reaction may be maintained at a temperature of from about -30°C to
about 150°C, for
l0 example, from about 10°C to about 50°C. The duration of the
reaction may be from about 10
minutes to about 5 hours, for ea~ample, from about 20 minutes to about 2
hours.
The compound of formula (IIg) is then rearranged to a compound of formula
(IIh}
R~
R2 B\A EH (I )
Ih
R~J
3
X
where X and E are O; and all other symbols are as defined above. This reaction
is optionally
carned out in the presence of a solvent, for example, DCM, CHC13, 1,2-
dichloroethane,
carbon tetrachloride, carbon disulfide, nitrobenzene, 1,2-dichlorobenzene, or
any mixture
thereof. The reaction may be carried out in the presence of a Lewis acid, such
as alumizuum
chloride (A1C13), zinc chloride (ZnCl2), or tin chloride (SnCI~,.), or in the
presence of UV
light. The reaction temperature may be maintained at from about 50°C to
about 300°C, for
2o example, from about 80°C to about 200°C. The duration of the
reaction may be from about
10 minutes to about 50 hours, for example, from about 20 minutes to about 10
hours. The
reaction may be carried out under anhydrous reaction conditions.
The compound of formula (IIh) is then condensed to a compound of formula (IIi)
182
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
R~
R~ B\ A E H R4
(Bi)
D~ '
~J
R3
where X and E represent O and all other symbols are as defined above. The
reaction is
carried out in the presence of a base, for example, NaZC03, K2C03, NaHC03,
KHCO3,
NaOH, KOH, or any mixture thereof. The reaction temperature may be maintained
at from
s about -30°C to about 50°C, for example, from about 0°C
to about 20°C. The duration of the
reaction may be from about 2 hours to about 50 hours, for example, from about
5 hours to
about 20 hours.
The compound of formula (IIi) then undergoes a cyclization reaction to form a
compound of formula (IIb)
R~
R~ B\ A E R4
(IIb)
RJ'J ~ YH
3
where all symbols are as defined above. This reaction is carried out using a
base, for
example, Na2C03, K~C03, NaHC03, KHC03, NaOH, KOH, or any mixture thereof. The
reaction temperature may be maintained at from about -30°C to about
50°C, for example,
from about -5°C to about 30 °C. The duration of the reaction may
be from about 0.5 hours to
about 10 hours, for example, from about 0.2 hours to about 5 hours.
The compound of formula (IIb) is then reacted with a compound of formula (IIc)
~Ar -Z-G-Hal (IIc)
R5
where 'Hal' is a halogen; and all other symbols are as defined above, to
obtain a compound
of formula (IId)
R~
R2 B\ A E R4 O
D~~~ ~ Y-G =-Z-Ar-~~ 5 (IId
R3 R )
where all symbols are as defined above.
The compound of formula (IId) is then reacted with a compound of formula (IIe)
183
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
H
Y1~~Y2 (IIe)
F
where F is O, S, or -NR; YI and YZ independently are O or S, to obtain a
compound of
formula (II)
Y1
1
2 B\,A E R'~ F~NR
R D. ~J I Y-G-Z-Ar-~~,2 (II)
R
X
where E and X are O; and all other symbols are as defined above.
The conversion of compound of formula (ITb) to compound of formula (II) is
carned
out as provided in Process 1.
PROCESS 3
l0 According to another aspect of the present invention, a process for
preparing a
compound of formula (II) is provided,
Y1
1
2 B\A E R'~ F~NR
R D~ ~ ~ [ Y-G-Z-Ar-=~~Y2 (II)
R3 .I R
X
where X is O, E is -NR, and all other symbols are as defined above.
First a compound of formula (IIj)
R1
\A NHR
R2 B ~~ ~ ~IJ)
D~':J- 'COOMe
Rs
where all symbols are as defined above, is converted to a compound of formula
(IIk),
R1
R2 B.\,A NHR (Illz)
D~ :1 ~COOH
R3
where all symbols are as defined above. This reaction may be carried out using
a base, for
example, Na2C03, KZC03, NaHC03, KHCO3, NaOH, KOH, or any mixture thereof. The
184
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
reaction may be carried out in the presence of a solvent, for example,
benzene, toluene,
xylene, methanol, ethanol, i-propanol, butanol, DMF, DMSO, 1,4-dioxane, or any
mixture
thereof. The reaction temperature may be maintained at from about -30°C
to about 150 °C,
for example, from about 20°C to about 80°C. The duration of the
reaction may be from about
0.5 hours to about 20 hours, for example, from about 2 hours to about 10
hours.
The compound of formula (IIIc) is then reacted with a compound of formula
(IIm)
Ra
~Hal (IIm)
O
where 'Hal' is a halogen, and R4 is as defined above, to obtain a compound of
formula (IIn)
R~
B\A NHR
R ; (IIn)
~~;J- _COO R~
R3
where all symbols are as defined above.
This reaction may occur in the presence of a brominating agent, for example,
bromine, bromine water, N-bromosuccinamide, copper bromide, or any mixture
thereof. The
solvent is acetic acid, propanoic acid, butanoic acid, pentanoic acid,
hexanoic acid,
dichlromethane (DCM), chloroform (CHCl3), 1,2-dichloroethane, carbon
tetrachloride,
methanol, ethanol, propanol, butanol, or any mixture thereof. The reaction may
be carried
out in the presence of catalytic amount of hydrobromic acid. The reaction
temperature may
be from about -10°C to about 150°C, for example, from about
0°C to about 40 °C. The
duration of the reaction may be from about 1 hour to about 72 hours, for
example, from about
1 hour to about 20 hours.
Alternatively, the reaction may be carried out in the presence of a solvent,
for
example, acetonitrile, DMF, DMSO, DCM, CHCl3a 1,2-dichloroethane, carbon
tetrachloride,
methanol, ethanol, propanol, butanol, HMPA, 1,4-dioxane, acetone, dimethyl
ether, diethyl
ether, THF, water, or any mixture thereof. The reaction may be carned out in
the presence of
a base, for example, NaH, KH, KOtBu, KOAc, NaOAc, n-BuLi, s-BuLi, t-BuLi, LDA,
Na2C03, K2CO3, NaHCO3, KHC03, NaOH, KOH, an amine base such as Et3N, diethyl
amine, diisopropylethyl amine, diisopropyl amine, DBU, or any mixture thereof.
The
reaction temperature may be from about -78°C to about 150°G, for
example, from about -
185
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
30°C to 40°C. The duration of the reaction may vary from about
10 minutes to about 72
hours, for example, from about 30 minutes to about 15 hours. The reaction may
be carried
out under an inert atmosphere maintained by N2, Ar, or He.
The compound of formula (IIn) is then converted to a compound of formula (IIb)
R1
R2 B\ A E R4
(IIb)
R3J ~ YH
X
where X is O, E is -NR, and all other symbols are as defined above. This
reaction may be
carned out using polyphosphoric acid. Optionally, the reaction may be carried
out in the
presence of a solvent, for example, acetonitrile, DMSO, 1,4-dioxane, THF,
water, or any
mixture thereof. The reaction temperature may be from about 0°C to
300°C, for example,
to from about 50°C to about 180°C. The duration of the reaction
may be from about 10 minutes
to about 72 hours, for example, from about 2 to I5 hours. The reaction may be
carried out
under an inert atmosphere maintained by NZ, Ar, or He.
The compound of formula (IIb) is then reacted with a compound of formula (IIc)
~'-Ar-Z---G--Hal (IIc)
R5
where all symbols are as defined above, to obtain a compound of formula (IId)
R1
B~AER4
R= ; O
Y-G-Z-Ar~R5 (IId)
R X
where E is -NR, and all other symbols are as defined above.
The compound of formula (Ird) is then reacted with a compound of formula (IIe)
H
Y1~~Y2 ~Ie)
F
2o where all symbols are as defined above, to obtain a compound of formula
(II)
Y1
1
B\,A E R4 F ~NR
R D. ~: I Y-G =Z-Ar-=~Yz
R
X
186
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
where X is O, E is NR, and all other symbols are as defined above.
PROCESS 4
According to another aspect of the present invention, a process for preparing
a
compound of formula (IV) is provided. The process comprises the following:
0
i 'I
/'/ EHz Ri E~Ra EYR
~ ~_~Iz KHz + R4COC1 ~p ~R~ ~ ~ ~z~ K'H
R z KHz R
X IVb X X IVd
IVa IVc
0
~Ar -Z-G-Hal
Rs
IIC
i
R CYRa
O
O~~RzI K~G~Z-Ar-~(Rs
X IVe
H
Yi~~Yz
F
/Y/t
t
E~Ra F~NH
O~~_~z~ K~G~Z-Ar--CR5 'yz
R
X (IV)
where all symbols are as defined above.
The conversion of a compound of formula (IVa) to a compound of formula of
(IVc)
may be carned out using an appropriate acylchloride of formula IVb in the
presence of a
to base, for example, Na?C03, KZCO3, NaHCO3, KHC03, NaOH, KOH, triethyl amine,
diisopropylethylamine, or any mixture thereof. The reaction may be carried out
in a solvent,
for example, benzene, toluene, xylene, DMF, DMSO, 1,4-dioxane,
dichloromethane, CHC13,
1,2-dichloroethane, carbon tetrachloride, or any mixture thereof. The reaction
temperature
may be maintained at from about -30°C to about 150°C, for
example, from about 20°C to
about 80°C. The duration of the reaction may be from about 6 hours to
about 72 hours, for
example, from about 2 hours to about 24 hours. The reaction may be carned out
under an
inert atmosphere of N?, Ar, or He.
The conversion of a compound of formula (IVc) to a compound of (IVd) may be
carried out using a base, for example, NaH, KH, KOtBu, KOAc, NaOAc, NaOEt,
KOEt, n-
2o BULi, s-BULi, t-BULi, LDA, NaZC03, KZC03, NaHC03, KHC03, NaOH, KOH, or any
mixture thereof. The reaction may be carned out in a solvent, for example,
benzene, toluene,
187
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
xylene, methanol, ethanol, i-propanol, t-butanol, or any mixture thereof. The
reaction
temperature may be maintained at from about -70° C to about 250°
C, for example, from
about -10° C to about 150 °C. The duration of reaction may be
from about 5 hours to about
150 hours, for example, from about 20 to about 100 hours. The reaction may be
carried out
under an inert atmosphere of N2, Ar, or He.
The conversion of compound of formula (IVd) to a compound of formula (IV) may
be carned out as provided in Process 1.
PROCESS 5
l0 According to another aspect of the present invention, a process for
preparing a
compound of formula (Va) is provided. The process comprises:
R~A~~ E RQ O RBA E R4
+ ~'-Ar-Z= G-Hal ~ B~ ~~ ~ ~ O
0~3J YH R5 D~J~ Y-G-Z-Ar~ s
R X
X
R (Ilc) R (Ild) W here R5
(IIb) represents alkoxy,
cycloalkenyloxy
XQ S(O)W Ar
H2N_(CH2)P\C-O-F2 B A~ E R4
(Ilp) X3 D/ J~~ ~ Y-G-Z-A O
RZ R3X R5
W here RS
R1A E Rq
B~~ "~ I I O 4~S(O),"-Ar (Iid) represents hydroxy
Y-G =Z-Ar--~ X group
NH-(CHa)p ~C-O-R
Xa
(V8)
The conversion of a compound of formula (IIb) to a compound of formula (IId)
may
1s be carried out as provided in Process 1.. The conversion of the compound of
formula (IId) to
a compound of formula (Va) may be carned out by reacting the compound of
formula (IId)
with compound of formula (IIp) in the presence of a reagent, for example, EDCI
or CDI, and
a solvent, for example, DMF, chloroform, dichloromethane, dimethylacetamide,
tetrahydrofuran, dioxane, ether, or any mixture thereof. The temperature of
the reaction may
20 be maintained at from about 10°C to about 60°C, for example,
from about 20°C to about
35°C. The duration of the reaction may be from about 5 hours to about
12 hours, for
example, from about 10 hours to about 12 hours. The reaction may be carried
out in a
nitrogen atmosphere.
188
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
PROCESS 6
According to another aspect of the present invention, a process for preparing
a
compound of formula (Vb) is provided
B A, N~
D/~ ~ I N-Y-G-Z-Ar--~O ~ S(O)W-Ar
R2 R3X NH-(CH2)OC-O-R
X3
(Vb)
The process comprises:
B A, COOH RBA N
it
D! ~ ~ ~ r
R J NH2 D~~ ° NH
R3
X
VIa VIb
The conversion of a compound of formula (VIa) to a compound of formula (VIb)
is
carried out in the presence of formamide in a nitrogen atmosphere. The
temperature of the
reaction may be maintained at from about 10°C to about 70°C, for
example, 25°C to about
l0 45°C. The duration of the reaction may be from about 1 hour to about
9 hours, for example,
from about 2 to about 4 hours.
The conversion of the compound of formula (VIb) to a compound of formula (Vb)
is
carried out as provided in Process 5.
It should be understood that in any of the reactions presented herein, any
reactive
group on the substrate molecule may be protected according to conventional
chemical
practice. Suitable protecting groups include, for example,
tertiarybutyldimethylsilyl,
methoxymethyl, triphenyl methyl, benzyloxycarbonyl, or tetrahydropyran (THP)
to protect a
hydroxyl or phenolic hydroxy group; N-tert-butoxycarbonyl (N-Boc), N-
benzyloxycarbonyl
(N-Cbz), N-9-fluorenyl methoxy carbonyl (-N-FMOC), benzophenoneimine, or
propargyloxy carbonyl (POC) to protect an amino or anilino group; acetal
protection for an
aldehyde; and ketal protection for a ketone. The methods of formation and
removal of such
protecting groups are those conventional methods appropriate to the molecule
being
protected.
The enantiomers of compound of formula (II) may be prepared by using reactants
in
their single enantiomeric form in the process wherever applicable or by
conducting the
189
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
reaction in the presence of reagents or catalysts in their single enantiomeric
form. The single
enantiomers also may be prepared by resolving the racemic mixture by
conventional
methods.
The stereoisomers of the compounds of the present invention may be prepared by
using reactants in their single enantiomeric form in the process, by
conducting the reaction in
the presence of reagents or catalysts in their single enantiomer form, or by
resolving the
mixture of stereoisomers by conventional methods. Some of the methods include
using
microbial resolution, resolving the diastereomeric salts formed with chiral
acids such as
mandelic acid, camphorsulfonic acid, tartaric acid, or lactic acid, wherever
applicable, or
chiral bases such as brucine, cinchona allcaloids and their derivatives.
Commonly used
methods are compiled by JAQUES, ENANTIOMERS, RACEMATES AND RESOLUTION (I981).
Where appropriate, the compounds of formula (I) may be resolved by: treating
with chiral
amines, aminoacids, aminoalcohols derived from aminoacids; employing
conventional
reaction conditions to convert the acid into an amide; separating the
diastereomers by
i5 fractional crystallization or chromatography; and preparing the
stereoisomers of compound
of formula (I) by hydrolyzing the pure diastereomeric amide.
The stereoisomers of the present invention also may include E and Z isomers or
their
mixtures in various rations.
Formulations and Pharmaceutical Compositions
The present invention provides compounds of general formula (I),
pharmaceutical
compositions comprising one or more compound of general formula (I), their
salts, or their
pharmaceutically acceptable compositions, in combination with pharmaceutically
acceptable
carriers and diluents.
The pharmaceutical compositions of the present invention may be used for the
treatment of bacterial infections. They also may be used for the tl-eatment of
bacterial
infections associated with multi-drug resistance. The pharmaceutical
compositions of the
present invention also may be used to modulate inflammatory responses,
particularly those
resulting from AGE and glycated protein accumulation. The pharmaceutical
compositions of
the present invention also may be used to modulate smooth muscle cell
proliferation and the
diseases or conditions related thereto. The compositions provided herein also
may be used to
treat vascular occlusive conditions, such as stenosis, restenosis and
atherosclerosis; diseases
190
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
mediated by inflammation, such as autoimrnune diseases; and hyperproliferative
diseases,
such as cancer.
A. Pharmaceutically Acceptable Salts
The compositions of the present invention optionally include one or more salts
of the
compounds of the present invention contained therein. Such salts are commonly
referred to
as non-toxic, "pharmaceutically acceptable salts". Other salts, however, may
be useful in the
preparation of the compounds of the present invention or of their
pharmaceutically
acceptable salts. Suitable pharmaceutically acceptable salts of the compounds
of the present
invention include acid addition salts that may, for example, be formed by
mixing a solution
of the compound with a solution of a pharmaceutically acceptable acid, for
example,
hydrochloric acid, sulfuric acid, fumaric acid, malefic acid, succinic acid,
acetic acid, benzoic
acid, citric acid, tartaric acid, carbonic acid, phosphoric acid, or any
mixture thereof.
Furthermore, where the compounds of the invention carry an acidic moiety,
suitable
pharmaceutically acceptable salts thereof may include alkali metal salts, for
example, sodium
or potassium salts; alkaline earth salts, for example, calcium or magnesium
salts; salts
formed with suitable organic ligands, for example, quaternary ammonium salts;
or any
mixture thereof.
Examples of pharmaceutically acceptable salts include, but are not limited to,
acetate,
benzenesulfonate, benzoate, bicarbonate, bisulfate, bitartrate, borate,
bromide, calcium
edetate, camsylate, carbonate, chloride, clavulanate, citrate,
dihydrochloride, edetate,
edisylate, estolate, esylate, fumarate, gluceptate, gluconate, glutamate,
glycollylarsanilate,
hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate,
iodide,
isothionate, lactate, lactobionate, laurate, malate, maleate, mandelate,
mesylate,
methylbromide, methylnitrate, metlrylsulfate~ mutate, napsylate, nitrate, N-
methylglucamine
ammonium salt, oleate, pamoate (embonate), palmitate, pantothenate,
phosphate/diphosphate, polygalacturonate, salicylate, stearate, sulfate,
subacetate, succinate,
tannate, tartrate, teoclate, tosylate, triethiodide, and valerate.
B. Alternative Forms of the Compounds
Where the compounds of the present invention have at least one chiral center,
they
may accordingly exist as enantiomers. Where the compounds possess two or more
chiral
centers, they additionally may exist as diastereomers. Where compounds of the
present
invention have geometrical isomers, it is to be understood that all such
isomers and mixtures
191
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
thereof are encompassed within the scope of the present invention.
Furthermore, some of the
crystalline forms for the compounds may exist as polymorphs and are
contemplated hereby.
In addition, some of the compounds may form solvates with water (i.e.,
hydrates) or common
organic solvents, and such solvates are also intended to be encompassed
hereby. Where the
processes for the preparation of the compounds according to the invention give
rise to
mixture of stereoisomers, these isomers may be separated by conventional
techniques such as
preparative chromatography.
Moreover, the compounds of the present invention may be prepared in racemic
form.
Alternatively, individual enantiomers may be prepared either by
enantiospecific synthesis or
to by resolution. The compounds may be resolved into their component
enantiomers by
standard techniques, such as the formation ~f diastereomeric pairs by salt
formation with an
optically active acid, for example, (+)di-p-toluoyl-d-tartaric acid and/or (+)-
di-p-toluoyl-1-
tartaric acid, followed by fractional crystallization and regeneration of the
free base. The
compounds also may be resolved by formation of diastereomeric esters or
amides, followed
by chromatographic separation and removal of the chiral auxiliary.
Alternatively, the
compounds may be resolved using a chiral HPLC column.
The compounds of the present invention optionally are formulated and
administered
as a prodrug. In general, prodrugs comprise functional derivatives of the
claimed compounds
that are capable of being enzymatically activated or converted into the more
active parent
form. Thus, in the treatment methods of the present invention, the term
"administering"
encompasses the treatment of the various disorders described with the compound
specifically
disclosed or with a compound that may not be specifically disclosed, but that
converts to the
specii~ied compound in vivo after administration to the patient. Conventional
procedures for
the selection and preparation of suitable prodrug derivatives are described,
for example, in
DESIGN OF PRODRUGS (1985); Wihnan, 14 gIOCHEM. SOC. TRANS. 375-82 (1986);
STELLA ET
AL., Prodrugs: A Chemical Approach to Targeted Drug Delivery in DIRECTED DRUG
DELIVERY 247-67 (1985), each of which is incorporated by reference herein in
its entirety.
The prodrugs of present invention include, but are not limited to, phosphate-
containing prodrugs, thiophosphate-containing prodrugs, sulfate-containing
prodrugs,
3o peptide-containing prodrugs, D-amino acid-modified prodrugs, glycosylated
prodrugs, I3-
lactam-containing prodrugs, optionally substituted phenoxyacetamide-containing
prodrugs or
192
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
optionally substituted phenylacetamide-containing prodrugs, 5-fluorocytosine,
and other 5-
fluorouridine prodrugs that may be converted into the more active drug.
Enzymes that may be used in the methods and compositions of the present
invention
include, but are not limited to, alkaline phosphatase for converting phosphate-
containing
prodrugs into free drugs; arylsulfatase for converting sulfate containing
prodrugs into free
drugs; cytosine deaminase for converting non-toxic S-fluorocytosine into the
anti-cancer
drug, 5-fluorouracil; proteases, such as serratia protease, thermolysin,
subtilisin,
carboxypeptidases, and cathepsins, such as cathepsins B and L, for converting
peptide-
containing prodrugs into free drugs; D-alanylcarboxypeptidases for converting
prodrugs that
l0 contain D-amino acid substituents; carbohydrate cleaving enzymes such as 13-
galactosidase
and neuraminidase for converting glycosylated prodr~gs into free drugs; J3-
lactamase for
converting drugs derivatized with 13-lactams into free drugs; and penicillin
amidases, such as
penicillin V amidase or penicillin G amidase, for converting drugs derivatized
at their amine
nitrogens with phenoxyacetyl or phenylacetyl groups, respectively, into free
drugs.
Alternatively, antibodies with enzymatic activity, also known in the art as
"abzymes",
may be used to convert the prodrugs of the present invention into free active
drugs. See for-
exanzple, Massey, 328 NATURE 457-48 (1987).
C. Pharmaceutical Auxiliaries
In addition to the compounds contemplated hereby, the pharmaceutical
compositions
2o of the present invention optionally comprise at least one suitable
auxiliary or carrier such as,
but not limited to, a diluent, binder, stabilizer, buffer, salt, lipophilic
solvent, preservative,
adjuvant, or any combination thereof. Pharmaceutically acceptable auxiliaries
typically are
used. Examples and methods of preparing such sterile solutions are described
in, for
example, 1ZEMINGTON'S PHARMACEUTICAL SCIENCES (Gennaro, Ed., 18th Edition,
Mack
Publishing Co. (1990)), incorporated by reference herein in its entirety.
Pharmaceutically
acceptable carriers routinely are selected to be suitable for the mode of
administration,
solubility, and/or stability of the compound.
Pharmaceutical excipients and additives useful in the present invention
include, but
are not limited to, proteins, peptides, amino acids, lipids, and carbohydrates
(for example,
sugars, including monosaccharides, di-, tri-, tetra-, and oligosaccharides;
derivatized sugars
such as alditols, aldonic acids, esterified sugars; and polysaccharides),
which can be present
singly or in combination, comprising alone or in combination 1-99.99% by
weight or
193
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
volume. Exemplary protein excipients include serum albumin, such as human
serum
albumin (HSA), recombinant human albumin (rHA), gelatin, casein, or any
combination
thereof. Representative amino acid components, which also can function in a
buffering
capacity, include alanine, glycine, arginine, betaine, histidine, glutamic
acid, aspartic acid,
cysteine, lysine, leucine, isoleucine, valine, methionine, phenylalanine, and
aspartame..
Carbohydrate excipients suitable for use in the present invention include, for
example, monosaccharides such as fructose, maltose, galactose, glucose, D-
mannose,
sorbose; disaccharides, such as lactose, sucrose, trehalose, cellobiose;
polysaccharides, such
as raffinose, melezitose, maltodextrins, dextrans, starches; and alditols,
such as mannitol,
l0 xylitol, maltitol, lactitol, xylitol sorbitol (glucitol), myoinositol.
The pharmaceutical compositions comprising the compounds of the present
invention
also can include a buffer or a pH adjusting agent. Typically, the buffer is a
salt prepared
from an organic acid or base. Exemplary buffers include organic acid salts,
such as salts of
citric acid, ascorbic acid, gluconic acid, carbonic acid, tartaric acid,
succinic acid, acetic acid,
or phthalic acid; Tris; tromethamine hydrochloride; phosphate buffers; or any
combination
thereof.
Additionally, pharmaceutical compositions of the invention optionally include
polymeric excipients/additives, such as polyvinylpyrrolidones, ficolls (a
polymeric sugar),
dextrates (for example, cyclodextrins, such as 2-hydroxypropyl-(3-
cyclodextrin),
polyethylene glycols, flavoring agents, anti-microbial agents, sweeteners,
antioxidants, anti-
static agents, surfactants (for example, polysorbates such as "TWEEN 20" and
"TWEEN
SO"), lipids (for example, phospholipids, fatty acids), steroids (for example,
cholesterol),
chelating agents (for example, EDTA), and any combination thereof. Exemplary
pharmaceutical excipients and/or additives are described in REM1NGTON: THE
SCIENCE &
PRACTICE OF PHARMACY (19th ed., Williams 8z Williams (1995)) and PHYSICIAN'S
DESK
REFERENCE (52nd ed., Medical Economics (1998)), each of which is incorporated
herein by
reference in its entirety.
1. Pharmaceutical Compositions for Oral Administration
For oral administration in the form of a tablet or capsule, a compound may be
3o combined with an oral, non-toxic pharmaceutically acceptable inert carrier
such as ethanol,
glycerol, water, or any mixture thereof. Moreover, suitable binders,
lubricants, disintegrating
agents, and coloring agents also may be incorporated into the mixture.
Suitable binders
194
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
include, without limitation, starch; gelatin; natural sugars such as glucose
or beta-lactose;
corn sweeteners; natural and synthetic gums such as acacia, tragacanth, or
sodium alginate,
carboxymethylcellulose; polyethylene glycol; waxes; or any combination
thereof. Lubricants
used in these dosage forms include, without limitation, sodium oleate, sodium
stearate,
magnesium stearate, sodium benzoate, sodium acetate, sodium chloride, or any
combination
thereof. Disintegrators include, without limitation, starch, methyl cellulose,
agar, bentonite,
xanthan gum, or any combination thereof.
Formulations of the present invention suitable for oral administration may be
presented as discrete units such as capsules, cachets, or tablets, each
containing a
to predetermined amount of the active ingredient; as a powder Qr granules; as
a solution or a
suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in-water
liquid
emulsion or a water-in-oil emulsion and as a bolus.
A tablet may be made by compression or molding, ~ptionally with one or more
auxiliary ingredients. Compressed tablets typically are prepared by
compressing, in a
suitable machine, the active ingredient in a free-flowing form such as a
powder or granules
optionally mixed with a binder, lubricant, inert diluent, preservative,
surface active or
dispersing agent. Molded tablets typically are made by molding, in a suitable
machine, a
mixture of the powdered compound moistened with an inert liquid diluent. The
tablets
optionally are coated or scored and may be formulated to provide a slow or
controlled release
of the active ingredient therein.
The compositions of the present invention optionally are incorporated into a
biodegradable polymer, thereby allowing for sustained release of the compound.
The
polymer is implanted in the vicinity of where drug delivery is desired, for
example, at the site
of restenosis. Such biodegradable polymers are described, for example, in Brem
et al., 74 J.
NEUROSURG. 441-46 (1991). Suitable examples of sustained-release compositions
include
semipermeable matrices of solid hydrophobic polymers containing a compound of
the
present invention, which matrices are foamed into shaped articles, for
example, films, or
microcapsules. Examples of sustained-release matrices include polyesters,
hydrogels (for
example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)),
polylactides (U.S.
3o Patent No. 3,773,919, incorporated by reference herein in its entirety),
copolymers of L-
glutamic acid and y ethyl-L-glutamate, non-degradable ethylene-vinyl acetate,
degradable
lactic acid-glycolic acid copolymers such as the LUPRON DEPOTS (Tap
Pharmaceuticals,
195
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Inc., Chicago, IL) (injectable microspheres composed of lactic acid glycolic
acid copolymer
and leuprolide acetate), and poly-D-(-)-3-hydroxybutyric acid.
2. Pharmaceutical Compositions for Parenteral Administration
As used herein, "parenteral" includes subcutaneous injections, intravenous,
intramuscular, intraperitoneal injections, or infusion techniques.
Formulations suitable for
parenteral administration include aqueous and non-aqueous sterile injection
solutions that
may contain anti-oxidants, buffers, bacteriostats, and solutes that render the
formulation
isotonic with the blood of the intended recipient, and aqueous and non-aqueous
sterile
suspensions that optionally include suspending agents and thickening agents.
The
formulations may be presented in unit-dose or mufti-dose containers, for
example, sealed
ampules and vials, and may be stored in a freeze-dried (lyophilized) condition
requiring only
the addition of the sterile liquid carrier, for example, water for injections,
immediately prior
to use. Extemporaneous injection solutions and suspensions may be prepared
from sterile
powders, granules, and tablets, such as those described above.
For parenteral administration, sterile suspensions and solutions are desired.
Isotonic
preparations which generally contain suitable preservatives are employed when
intravenous
administration is desired. The pharmaceutical compositions may be administered
parenterally via injection of a formulation consisting of the active
ingredient dissolved in an
inert liquid carrier. Acceptable liquid carriers include, for example,
vegetable oils such as
peanut oil, cotton seed oil, sesame oil, and organic solvents such as solketal
and glycerol
formal. The formulations may be prepared by dissolving or suspending the
active ingredient
in the liquid Garner such that the final formulation contains from about
0.005% to 30% by
weight of the active ingredient, for example, a compound of the present
invention.
3. Pharmaceutical Compositions for Other Routes of Administration
Formulations suitable for topical administration in the mouth include lozenges
comprising the ingredients in a flavored basis or medium, usually sucrose and
acacia or
tragacanth; pastilles comprising the active ingredient in an inert basis or
medium such as
gelatin and glycerin, or sucrose and acacia; and mouthwashes comprising the
compound to
be administered in a suitable liquid carrier. The liquid forms rnay include
suitably flavored
suspending or dispersing agents, such as the synthetic and natural gums, for
example,
tragacanth, acacia, and methyl-cellulose.
196
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Formulations for rectal administration may be presented as a suppository with
a
suitable base comprising, for example, cocoa butter or a salicylate.
Formulations suitable for vaginal administration may be presented as
pessaries,
tamports, creams, gels, pastes, foams, or spray formulations comprising the
active ingredient
s and an appropriate carrier.
The compounds also may be entrapped in microcapsules prepared, for example,
by coacervation techniques or by interfacial polymerization, for example,
hydroxymethylcellulose or gelatin-microcapsules and poly(methylmethacylate)
microcapsules, respectively, in colloidal drug delivery systems (for example,
liposomes,
albumin microspheres, microemulsions, nano-particles and nanocapsules) or in
macroemulsions. REMINGTON'S PHARMACEUTICAL SCIENCES (A. Osol ed., 16th ed.
(1980)),
incorporated by reference herein in its entirety.
The compounds contemplated hereby optionally are formulated as liposomes.
Liposomes may be prepared by any suitable method, such as those described in
U.S. Patent
is Nos. 5,013,556; 4,485,045; 4,544,545; WO 97/38731; Epstein et al., 82
PI20C. NATL. ACRD.
SCI. USA 3688 (1985); and Hwang et al., 77 PROC. NATL. ACAD. SCI. USA 4030
(1980),
each of which is incorporated by reference herein in its entirety. The
compounds of the
present invention also can be administered in the form of liposome delivery
systems, such as
small unilamellar vesicles, large unilamellar vesicles, and multilamellar
vesicles. Liposomes
2o can be formed from a variety of phospholipids, such as cholesterol,
stearylamine, or
phophatidylcholines.
Compounds of the present invention also may be delivered by the use of
monoclonal
antibodies as individual carriers to which the compound molecules are coupled.
The
compounds of the present invention also may be coupled with soluble polymers
as targetable
2s drug carriers. Such polymers can include polyvinylpyrrolidone, pyran
copolymer,
polyhydroxypropylmethacrylamidephenol, polyhydroxyethylaspartamidephenol, or
polyethyl-eneoxidepolylysine optionally substituted with palmitoyl residue.
D. Pharmaceutically Acceptable Preservatives
The present invention provides stable formulations, preserved solutions and
3o formulations containing a preservative, and mufti-use preserved
formulations suitable for
pharmaceutical or veterinary use, comprising at least one compound
contemplated hereby in
a pharmaceutically acceptable formulation. Preserved formulations contain at
least one
197
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
known preservative comprising at least one of phenol, m-cresol, p-cresol, o-
cresol,
chlorocresol, benzyl alcohol, phenylmercuric nitrite, phenoxyethanol,
formaldehyde,
chlorobutanol, magnesium chloride (for example, hexahydrate), alkylparaben
(methyl, ethyl,
propyl, butyl), benzalkonium chloride, benzethonium chloride, sodium
dehydroacetate and
thimerosal, or any mixture thereof, in an aqueous diluent. Any suitable
concentration or
mixture can be used, such as 0.001-5%, or any range or value therein
including, but not
limited to, 0.001, 0.003, 0.005, 0.009, 0.01, 0.02, 0.03, 0.05, 0.09, 0.1,
0.2, 0.3, 0.4., 0.5, 0.6,
0.7, 0.8, 0.9, 1.0, l.l, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1,
2.2, 2. 3, 2.4, 2.5, 2.6, 2.7,
2.8, 2.9, 3.0, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4.0, 4.3, 4.5,
4.6, 4.7, 4.8, 4.9. Non-
limiting examples include, no preservative, 0.1-2% m-cresol (for example, 0.1,
0.2, 0.3. 0.4,
0.5, 0.9, 1.0%), 0.1-3% benzyl alcohol (for example, 0.5, 0.9, 1.1., 1 _5,
1.9, 2.0, 2.5%),
0.001-0.5% thimerosal (for example, 0.005, 0.01), 0.001-2.0% phenol <for
example, 0.05,
0.25, 0.28, 0.5, 0.9, 1.0%), and 0.0005-1.0% alkylparaben(s) (for example,
0.00075, 0.0009,
0.001, 0.002, 0.005, 0.0075, 0.009, 0.01, 0.02, 0.05, 0.075, 0.09, 0.1, 0.2,
0.3, 0.5, 0.75, 0.9,
1.0%).
Other excipients, for example, isotonicity agents, buffers, antioxidants,
preservative
enhancers, optionally are added to the diluent. An isotonicity agent, such as
glycerin, is
commonly used at known concentrations. A physiologically tolerated buffer is
typically
added to provide improved pH control. The formulations can cover a wide range
of pHs,
such as from about pH 4 to about pH 10, specifically, a range from about pH 5
to about pH 9,
more specifically, a range of about 6.0 to about 8Ø According to one aspect
of the present
invention, the formulations of the present invention have pH between about 6.8
and about
7.8. Suitable buffers include phosphate buffers, for example, sodi~un
phosphate and
phosphate buffered saline (PBS).
Other additives, such as a pharmaceutically acceptable solubilizers like Tween
20
(polyoxyethylene (20) sorbitan monolaurate), Tween 40 (polyoxyethylene (20)
sorbitan
monopalmitate), Tween 80 (polyoxyethylene (20) sorbitan monooleate), Pluronic
F68
(polyoxyethylene polyoxypropylene block copolymers), and PEG (polyethylene
glycol) or
non-ionic surfactants such as polysorbate 20 or 80 or poloxamer 184 or 188,
Pluronic~
polyls, other block co-polymers, and chelators such as EDTA and EGTA is
optionally added
to the pharmaceutical compositions to reduce aggregation. These additives are
particularly
useful if a pump or plastic container is used to administer the pharmaceutical
composition.
198
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The presence of pharmaceutically acceptable surfactant mitigates the
propensity for the
composition to aggregate.
During any of the processes of preparing of the compounds of the present
invention,
it may be necessary and/or desirable to protect sensitive or reactive groups
on any of the
molecules concerned. This may be achieved by means of conventional protecting
groups,
such as those described in PROTECTIVE GROUPS IN ORGANIC CHEMISTRY (1973); and
GREENE
AND WUTS, PROTECTIVE GROUPS IN ORGANIC SYNTHESIS (1991), each of which is
incorporated by reference herein in its entirety. The protecting groups may be
removed at a
convenient subsequent stage using methods known from the art.
E. Combination Therapy
In addition, co-administration or sequential administration of the compounds
of the
present invention and other therapeutic agents may be desirable, such as
chemotherapeutic
agents, immunosuppressive agents, cytokines, cytotoxic agents, nucleolytic
compounds,
radioactive isotopes, receptors, and pro-drug activating enzymes, which may be
naturally
occurring or produced by recombinant methods. The combined administration
includes co-
administration, using separate formulations or a single pharmaceutical
formulation, and
consecutive administration in either order, where there is a time period while
both (or all)
active therapeutic agents simultaneously exert their biological activities.
The compounds of this invention optionally are administered in combination
with an
antirheumatic (for example, methotrexate, auranofin, aurothioglucose,
azathioprine,
etanercept, gold sodium thiomalate, hydroxychloroquine sulfate, leflunomide,
sulfasalzine), a
muscle relaxant, a narcotic, a non-steroid anti-inflammatory drug (NSAID), an
analgesic, an
anesthetic, a sedative, a local anesthetic, a neuromuscular blocker, an anti-
cancer, an
antimicrobial (for example, aminoglycoside, an antifungal, an antiparasitic,
an antiviral, a
carbapenem, cephalosporin, a flurorquinolone, a macrolide, a penicillin, a
sulfonamide, a
tetracycline, another antimicrobial), an anti-psoriatic, a corticosteriod, an
anabolic steroid, a
diabetes-related agent, a mineral, a nutritional, a thyroid agent, a vitamin,
a calcium-related
hormone, an antidiarrheal, an anti-tussive, an anti-emetic, an anti-ulcer, a
laxative, an
anticoagulant, an erythropieitin (for example, epoetin alpha), a filgrastim
(for example, G-
CSF, Neupogen), a sargramostim (GM-CSF, Leukine), an immunization, an
immunoglobulin, an immunosuppressive (for example, basiliximab, cyclosporine,
daclizumab), a growth hormone, a hormone replacement drug, an estrogen
receptor
199
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
modulator, a mydriatic, a cycloplegic, an alkylating agent, an anti-
metabolite, a mitotic
inhibitor, a radiopharmaceutical, an anti-depressant, anti-manic agent, an
anti-psychotic, an
anxiolytic, a hypnotic, a sympathomimetic, a stimulant, donepezil, tacrine, an
asthma
medication, a beta agonist, an inhaled steroid, a leukotriene inhibitor, a
methylxanthine, a
cromolyn, an epinephrine or analog thereof, dornase alpha (Pulmozyme), a
cytokine, or any
combination thereof.
Such anti-cancer or antimicrobial compounds also can include toxin molecules
that
are associated, bound, co-formulated, co-administered, or sequentially
administered, in either
order, with at least one of the compounds of the present invention. The term
"toxin" includes
to both endotoxins and exotoxins produced by any naturally occurring, mutant,
or recombinant
bacteria or viruses that may cause any pathological condition in humans and
other mammals,
including toxin shock, which can result in death. The toxin optionally can act
to kill
selectively the pathologic cell or tissue. The pathologic cell can be a cancer
or other cell.
Such toxins can be, but are not limited to, purified or recombinant toxin or
toxir~ fragment
comprising at least one functional cytotoxic domain of toxin, for example,
selected from at
least one of ricin, diphtheria toxin, a venom toxin, or a bacterial toxin.
Such toxins may
include, but are not limited to, enterotoxigenic E. coli heat-labile
enterotoxin (LT), heat-
stable enterotoxin (ST), Shigella cytotoxin, Aeromonas enterotoxins, toxic
shock syndrome
toxin-1 (TSST-1), Staphylococcal enterotoxin A (SEA), B (SEB), or C (SEC),
Streptococcal
enterotoxins. Such bacteria include, but are not limited to, strains of a
species of
enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (for example,
strains of serotype
0157:H7), Staphylococcus species (for example, Staphylococcus aureus,
Staphylococcus
pyogenes), Shigella species (for example, Shigella dysenteriae, Shigella
flexneri, Shigella
boydii, and Shigella sonnei), Salmonella species (for example, Salmonella
typhi, Salmonella
cholera-suis, Salmonella enteritidis), Clostridium species (for example,
Clostridium
per&ingens, Clostridium dificile, Clostridium botulinum), Camphlobacter
species (for
example, Camphlobacter jejuni, Camphlobacter fetus), Heliobacter species, (for
example,
Heliobacter pylori), Aeromonas species (for example, Aeromonas sobria,
Aeromonas
hydrophila, Aeromonas caviae), Pleisomonas shigelloides, Yersina
enterocolitica, Vibrios
species (for example, Vibrios cholerae, Vibrios parahemolyticus), Klebsiella
species,
Pseudomonas aeruginosa, and Streptococci. See, for example, Stein, ed.,
INTERNAL
MEDICINE 1-13 (3rd ed. Little, Brown and Co., Boston) (1990); EVANS ET AL.,
BACTERIAL
200
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
INFECTIONS OF HUMANS: EPIDEMIOLOGY AND CONTROL 239-254 (2d. ed. Plenum Medical
Book Co., New York) (1991); MANDELL ET AL., PRINCIPLES AND PRACTICE OF
INFECTIOUS
DISEASES (3d. ed. Churchill Livingstone) (1990); BERKOW ET AL., THE MERCK
MANUAL
(16th ed. Merck and Go.) (1992); Wood et al., 76 FEMS MICROBIOLOGY IMMUNOLOGY
121-
s 134 (1991); Marrack et al., 248 SCIENCE 705-711 (1990), each of which is
incorporated by
reference in its entirety.
The compound of the present invention is optionally administered in
combination
with at least one immunosuppressive agent for use in, for example, treating or
preventing a
vascular occlusive condition, such as transplant vasculopathy. Suitable
imrnunosuppressive
1o agents include, but are not limited to, CellCept (Roche Labs.), Gengraf
(Abbott Labs., Inc.),
Micrhogam (Ortho-Clinical), Neoral (Novartis), Orthoclone OKT3 (Ortho-
Biotech), Prograf
(Fujisawa), Rapamune (Wyeth-Ayerst), Sandimmune (Novartis), Thymoglobulin
(SangStat),
Zenapax (Roche), or any combination thereof.
The therapeutic agent may be administered simultaneously or sequentially, in
either
is order and at various times with a compound of the present invention that
comprises a
chemotherapeutic agent. A "chemotherapeutic agent" is a compound useful in the
treatment
of cancer. Examples of chemotherapeutic agents include alkylating agents such
as thiotepa
and cyclosphosphamide; alkyl sulfonates such as busulfan, improsulfan and
piposulfan;
aziridines such as benzodopa, carboquone, meturedopa, and uredopa;
ethylenimines and
2o methylamelamines including altretamine, triethylenemelamine,
trietylenephosphoramide,
triethylenethiophosphaoramide and trimethylolomelamine; nitrogen mustards such
as
chlorambucil, chlornaphazine, cholophosphamide, estramustine, ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembiehin,
phenesterine, prednimustine, trofosfamide, uracil mustard; nitroureas such as
cannustine,
25 chlorozotocin, fotemustine, lomustine, nimustine, ranimustine; antibiotics
such as
aclacinomysins, actinomycin, authramycin, azaserine, bleomycins, cactinomycin,
calicheamicin, carabicin, carminomycin, carzinophilin, chromoinycins,
dactinomycin,
daunorubicin, detorubicin, 6-diazo-5-oxo-L-norleucine, doxorubicin,
epirubicin, esorubicin,
idambicin, marcellomycin, mitomycins, mycophenolic acid, nogalamycin,
olivomycins,
30 peplomycin, potfiromycin, puromycin, quelamycin, rodorubicin,
streptonigrin, streptozocin,
tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as
methotrexate and 5-
fluorouracil (5-FI.J); folic acid analogues such as denopterin, methotrexate,
pteropterin,
201
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
trimetrexate; purine analogs such as fludarabine, 6-mercaptopurine,
thiamiprine, thioguanine;
pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine, carmofur,
cytarabine,
dideoxyuridine, doxifluridine, enocitabine, floxuridine, 5-FU; androgens such
as calusterone,
dromostanolone propionate, epitiostanol, rnepitiostane, testolactone; anti-
adrenals such as
aminoglutethimide, mitotane, trilostane; folic acid replenisher such as
frolinic acid;
aceglatone; aldophosphamide glycoside; aminolevulinic acid; amsacrine;
bestrabucil;
bisantrene; edatraxate; defofamine; demecolcine; diaziquone; elfornithine;
elliptinium
acetate; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidamine;
mitoguazone;
mitoxantrone; mopidamol; nitracrine; pentostatin; phenamet; pirarubicin;
podophyllinic acid;
2-ethylhydrazide; procarbazine; PSK~; razoxane; sizofrran; spirogermanium;
tenuazonic
acid; triaziquone; 2, 2',2"-trichlorotriethylamine; urethan; vindesine;
dacarbazine;
mannomustine; mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside
("Ara-C");
cyclophosphamide; thiotepa; taxoids, for example, paclitaxel (TAXOL~, Bristol-
Myers
Squibb Oncology, Princeton, NJ) and doxetaxel (TAXOTERE~, Rhone-Poulenc Rorer,
Antony, France); chlorambucil; gemcitabine; 6-thioguanine; mercaptopurine;
methotrexate;
platinum analogs such as cisplatin and carboplatin; vinblastine; platinum;
etoposide (VP-16);
ifosfamide; mitomycin C; mitoxantrone; vincristine; vinorelbine; navelbine;
novantrone;
teniposide; daunomycin; aminopterin; xeloda; ibandronate; CPT-11;
topoisomerase inhibitor
RFS 2000; difluoromethylornithine (I~MFO); retinoic acid; esperamicins;
capecitabine; and
2o pharmaceutically acceptable salts, acids, or derivatives of any of the
above. Also included in
this definition are anti-hormonal agents that act to regulate or inhibit
hormone action on
tumors such as anti-estrogens including for example tamoxifen, raloxifene,
aromatase
inhibiting 4(5)-imidazoles, 4 hydroxytamoxifen, trioxifene, lceoxifene,
onapristone, and
toremifene (Fareston); and anti-androgens such as flutamide, nilutamide,
bicalutamide,
leuprolide, and goserelin; and pharmaceutically acceptable salts, acids or
derivatives of any
of the above.
The therapeutic agent may comprise a cytolcine. The term "cytokine" is a
generic
term for proteins released by one cell population which act on another cell as
intercellular
mediators. As used herein, the term "cytokine" includes proteins from natural
sources or
from recombinant cell culture and biologically active equivalents of the
native sequence
cytokines. Examples of such cytokines are lymphokines, monokines, and
traditional
polypeptide hormones. Included among the cytokines are growth hormones such as
human
202
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
growth hormone, N-methionyl human growth hormone, and bovine growth hormone;
parathyroid hormone; thyroxine; insulin; proiiisulin; relaxin; prorelaxin;
glycoproteW
hormones such as follicle stimulating hormone (FSH), thyroid stimulating
hormone (TSIi~,
and luteinizing hormone (LH); hepatic growth factor; fibroblast growth factor;
prolactin;
placental lactogen; tumor necrosis factor-a and -13; mullerian-inhibiting
substance; mouse
gonadotropin-associated peptide; inhibin; activin; vascular endothelial growth
factor;
integrin; thrombopoietin (TPO); nerve growth factors such as NGF-13; platelet
growth factor;
transforming growth factors (TGFs) such as TGF-a and TGF-13; insulin-like
growth factor-I
and -II; erythropoietin (EPO); osteoinductive factors; interferons such as
interferon-a, -13 and
-?; colony stimulating factors (CSFs) such as macrophage-CSF (M-CSF);
granulocyte-
macrophage-CSF (GM-CSF); and granulocyte-CSF (GCSF); interleukins (ILs) such
as IL-l,
IL-la, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-11, IL-12, IL-15; a
tumor necrosis
factor such as TNF-a or TNF-13; and other polypeptide factors including LIF
and kit ligar~d
(KL).
The compounds of the present invention may be administered in combination with
an
anti-inflammatory agent including, but not limited to, adrenocortical steroids
(cortisol,
cortisone, fludrocortisone, prednisone, prednisolone, 6a-methylprednisolone,
triamcinolone,
betamethasone, and dexamethasone), non-steroidal agents (salicylic acid
derivatives, i.e_,
aspirin; para-aminophenol derivatives, i.e., acetominophen; indole and indene
acetic acids
(indomethacin, sulindac, and etodalac), heteroaryl acetic acids (tolmetin,
diclofenac, and
lcetoroIac), arylpropionic acids (ibuprofen and derivatives), anthranilic
acids (mefenamic
acid, and meclofenamic acid), enolic acids (piroxicam, tenoxicam,
phenylbutazone, and
oxyphenthatrazone}, nabumetone, gold compounds (auranof'm, aurothioglucose,
gold sodium
thiomalate). Commercially available nonsteroidal anti-inflammatory drugs
include, but are
not limited to, Anaprox (Roche Labs.), Arthrotec (Searle), Cataflam
(Novartis), Celebrex
(Pfizer), , Clinoril (Merck), Dolobid (Merck), Feldene (Pfizer), Indocin
(Merck), Lodine
(Wyeth-Ayerst), Mobic (Boehringer Ingelheim), Motrin (McNeil Consumer),
Naprosyn
(Roche Labs.), Orudis (Wyeth-Ayerst), Oruvail (Wyeth-Ayerst), Ponstel (First
Horizon,
Relafen (GlaxoSmithKline), Tolectin (Ortho-McNeil), Toradol (Roche Labs.,
Inc.), Vioxx
(Merck), Voltaren (Novartis), Advair (GlaxoSmithKline}, Flovent
(GlaxoSmithKline~,
Pulrnicort (AstranZeneca), and Vanceril (Schering), Asacol (Procter & Gamble),
Colazal
(Salix), Dipentum (Pharmacia & Upjohn), and Rowasa (Solvay).
203
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The compounds of the present invention may be admistered in combination with
an
antirheumatic agent. Commercially available antirheumatic agents include, but
are not
limited to, Anaprox (Roche Labs.), Arava (Aventic), Arthrotec (Searle),
Azulfidine
(Pharmacia & Upjohn), Gataflam (Novartis), Celebrex (Pfizer), Gelestone
(Schering),
s Cuprimine (Merck), Enbrel (Immunex), Feldene (Pfizer), Gengraf (Abbott),
Indocin (Merck),
Lodine (Wyeth-Ayerst), Naprosyn (Roche Labs.), Neoral (Novartis), Pediapred
(Celltech),
Prednisone (Roxanne), Remicade (Centocor), Solu-Medrol (Pharmacia & Upjohn),
Triliate
(Purdue Frederick), and Voltaren (Novartis).
Moreover, the compounds of the present invention may be used in combination
with
to any cardiovascular agent including, but not limited to, adrenergic blockers
such as Cardura
(Pfizer), Dibenzyline (WellSpring), Hytrin (Abbott), Minipress (Pfizer), and
Minizide
(Pfizer); adrenergic stimulants such as Aldoclor (Merck), Aldomet (Merck),
Aldoril (Merck),
Catapres (Boehringer Ingelheim), Clorpres (Bertek), and Tenex (Robins);
alpha/beta
adrenergic blockers such as Coreg (GlaxoSmithI~line), and Normodyne
(Schering);
15 angiotensin converting enzyme inhibitors, such as Accupril (Parke-Davis),
Aceon (Solway),
Altace (Monarch), Captopril (Mylan), Enalaprilat (Baxter Anesthesia), Lotensin
(Novartis),
Mavik (Abbott), Monopril (Bristol-Myers Squibb), Prinivil (Merck), Univasc
(Schwarz),
Vaotec (Merck), and Zestril (AstraZeneca); angiotenisin converting enzyme
inhibitors such
as Lexxel (AstraZeneca), Lotrel (Novartis), Tarka (Abbott), Accuretic (Parke-
Davis),
20 Lotensin (Novartis), Prinzide (Merck), Uniretic (Schwarz), Vaeretic
(Merck), and Zestoretic
(AstraZeneca); angiotensin II receptor antagonists such as Atacand
(AstraZeneca), Avapro
(Briston-Myers Squibb), Cozaar (Merck), Diovan (Novartis), Micardis
(Boehringer
Ingelheim), and Teveten (Unimed); antiarrhythmics (Groups I-IV), antilipemic
agents such
as bile acid sequestrants, fibric acid derivatives, HMG-CoA reductase
inhibitors, and
25 nicotinic acid; Beta adrenergic blocking agents; calcium channel blockers;
inotropic agents;
vasodilators including coronoary vasodilators, natriuretic peptides, and
peripheral
vasodilators; and vasopressors.
According to one aspect of the present invention, the therapeutic agent
comprises a
small molecule toxin, including maytansine, calicheamicin, trichothene, and CC
1065.
30 According to another aspect of the present invention, the therapeutic agent
comprises one
more calicheamicin molecules. Members of the calicheamicin family of
antibiotics are
capable of producing double-stranded DNA breaks at sub-picomolar
concentrations.
204
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Structured analogues of calicheamicin are also known. See Hinman et al., 53
CANCER
RESEARCH 3336-42 (1993); Lode et al., 58 CANCER RESEARCH 2925-28 (1998),
incorporated
herein by reference in its entirety.
The therapeutic agent may comprise one or more enzymatically active toxins and
fragments thereof. Examples of such toxins include nonbinding active fragments
of
diphtheria toxin, diphtheria A chain, exotoxin A chain (from Pseudomonas
aeruginosa), ricin
A chain, abrin A chain, modeccin A chain, alpha-sarcin, dianthin proteins,
Phytolaca
americana proteins (PAPI, PAPAII, and PAP-S), momordica charantia inhibitor,
curcin,
crotin sapaonaria officinalis inhibitor, gelonin, mitogellin, restrictoein,
phenomvcin,
enomycin and the tricothecenes. See, for example, WO 93/21232, incorporated
herein by
reference in its entirety.
The present invention further contemplates therapeutic agents that have
nucleolytic
activity such as a ribonuclease and a deoxyribonuclease. In addition, a
variety of radioactive
isotopes are available for the production of radioconjugated binding partners.
Examples
include Y9°, Atzzz, Ret86, Re186, Sm~53, Bizlz, psz and radioactive
isotopes of Lu.
The compound of the present invention may be conjugated to a receptor, such as
streptavidin, for utilization in tumor pretargeting. Briefly, the compound-
receptor conjugate
is administered to the patient and unbound conjugate is removed from
circulation with a
clearing agent. A ligand, such as biotin, which is conjugated to a cytotoxic
agent, is then
2o administered.
1. Timing of Administration
According to one aspect of the present invention, a compound described herein
is
administered before a second therapeutic agent. The administration of a
compound may
occur anytime from several minutes to several hours before the administration
of the second
therapeutic agent. The compound may alternatively be administered anytime from
several
hours to several days, possibly several weeks, and up to several months before
the second
therapeutic agent.
More specifically, a compound of the present invention may be administered at
least
about 1 minute, at least about minutes, at least about mW utes, at least about
minutes, at
3o least about minutes, at least about 2 minutes, at least about 3 minutes, at
least about 4
minutes, at least about 5 minutes, at least about 6 minutes, at least about 7
minutes, at least
about 8 minutes, at least about 9 minutes, at least about 10 minutes, at least
about 11 minutes,
205
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
at least about 12 minutes, at least about 13 minutes, at least about 14
minutes, at least about
15 minutes, at least about 16 minutes, at least about 17 minutes, at least
about 18 minutes, at
least about 19 minutes, at least about 20 minutes, at least about 21 minutes,
at least about 22
minutes, at least about 23 minutes, at least about 24 minutes, at least about
25 minutes, at
least about 26 minutes, at least about 27 minutes, at least about 28 minutes,
at least about 29
minutes, at least about 30 minutes, at least about 31 minutes, at least about
32 minutes, at
least about 33 minutes, at least about 34 minutes, at least about 35 minutes,
at least about 36
minutes, at least about 37 minutes, at least about 38 minutes, at least about
39 minutes, at
least about 40 minutes, at least about 41 minutes, at least about 42 minutes,
at least about 43
minutes, at least about 44 minutes, at least about 45 minutes, at least about
46 minutes, at
least about 47 minutes, at least about 48 minutes, at least about 49 minutes,
at least about 50
minutes, at least about 51 minutes, at least about 52 minutes, at least about
53 minutes, at
least about 54 minutes, at least about 55 minutes, at least about 56 minutes,
at least about 57
minutes, at least about 58 minutes, at least about 59 minutes, or at least
about 60 minutes
before the second therapeutic agent.
Furthermore, a compound of the present invention may be administered at least
about
1 hour, at least about 2 hours, at least about 3 hours, at least about 4
hours, at least about 5
hours, at least about 6 hours, at least about 7 hours, at least about 8 hours,
at least about 9
hours, at least about 10 hours, at least about 11 hours, at least about 12
hours, at least about
13 hours, at least about 14 hours, at least about 15 hours, at least about 16
hours, at least
about 17 hours, at least about 18 hours, at least about 19 hours, at least
about 20 hours, at
least about 21 hours, at least about 22 hours, at least about 23 hours, or at
least about 24
hours before the second therapeutic agent.
Moreover, a compound of the present invention may be administered at least
about
1 day, at least about 2 days, at least about 3 days, at least about 4 days, at
least about 5 days,
at least about 6 days, at least about 7 days, at least about 8 days, at least
about 9 days, at least
about 10 days, at least about 11 days, at least about 12 days, at least about
13 days, at least
about 14 days, at least about 15 days, at least about 16 days, at least about
17 days, at least
about 18 days, at least about 19 days, at least about 20 days, at least about
21 days, at least
about 22 days, at least about 23 days, at least about 24 days, at least about
25 days, at least
about 26 days, at least about 27 days, at least about 28 days, at least about
29 days, at least
206
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
about 30 days or at least about 31 days before the administration of the
second
therapeutic agent.
A compound of the present invention may be administered at least about 1 week,
at
least about 2 weeks, at least about 3 weeks, at least about 4 weelcs, at least
about 5 weeks, at
s least about 6 weeks, at least about 7 weeks, at Least about 8 weeks, at
least about 9 weeks, at
least about 10 weeks, at least about 11 weeks, at least about 12 weeks, at
least about 13
weeks, at least about 14 weeks, at least about 15 weeks, at least about 16
weeks, at least
about 17 weeks, at least about 18 weeks, at least about 19 weeks, or at least
about 20 weeks
before the second therapeutic agent.
1 o Further, a compound of the present invention may be administered at least
about one
month, at Least about two months, at least about three months, at least about
four months, at
least about five months, at least about six months, at least about seven
months, at least about
eight months, at least about nine months, at least about ten months, at least
about eleven
months, or at least about twelve months before the second therapeutic agent.
15 According to another aspect of the present invention, a compound of the
present
invention is administered after the therapeutic agent. The administration of a
compound may
occur anytime from several minutes to several hours after the administration
of the
therapeutic agent. A compound may alternatively be administered anytime from
several
hours to several days, possibly several weeks, and even up to several months
after the second
20 therapeutic agent.
More specifically, a compound of the present invention may be administered at
least
about 1 minute, at least about minutes, at least about minutes, at least about
minutes, at
least about minutes, at least about 2 minutes, at least about 3 minutes, at
least about 4
minutes, at least about 5 minutes, at least about 6 minutes, at least about 7
minutes, at least
25 about 8 minutes, at least about 9 minutes, at least about 10 minutes, at
least about 11 minutes,
at least about 12 minutes, at least about 13 minutes, at least about 14
minutes, at least about
15 minutes, at least about 16 minutes, at least about 17 minutes, at least
about 18 minutes, at
least about 19 minutes, at Least about 20 minutes, at least about 21 minutes,
at least about 22
minutes, at least about 23 minutes, at least about 24 minutes, at least about
25 minutes, at
30 least about 26 minutes, at least about 27 minutes, at least about 28
minutes, at least about 29
minutes, at least about 30 minutes, at least about 31 minutes, at least about
32 minutes, at
least about 33 minutes, at least about 34 minutes, at Least about 35 minutes,
at least about 36
207
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
minutes, at least about 37 minutes, at least about 38 minutes, at Ieast about
39 minutes, at
least about 40 minutes, at least about 41 minutes, at least about 42 minutes,
at least about 43
minutes, at least about 44 minutes, at least about 45 minutes, at least about
46 minutes, at
least about 47 minutes, at least about 48 minutes, at least about 49 minutes,
at least about 50
s minutes, at least about 51 minutes, at least about 52 minutes, at least
about 53 minutes, at
least about 54 minutes, at least about 55 minutes, at least about 56 minutes,
at least about 57
minutes, at least about 58 minutes, at least about 59 minutes, or at least
about 60 minutes
after the second therapeutic agent.
More specifically, a compound of the present invention may be administered at
least
about 1 hour, at least about 2 hours, at least about 3 hours, at least about 4
hours, at least
about 5 hours, at least about 6 hours, at least about 7 hours, at least about
8 hours, at least
about 9 hours, at least about 10 hours, at least about 11 hours, at least
about 12 hours, at least
about 13 hours, at least about 14 hours, at least about 15 hours, at least
about 16 hours, at
least about 17 hours, at least about 18 hours, at least about 19 hours, at
least about 20 hours,
at least about 21 hours, at least about 22 hours, at least about 23 hours, or
at least about 24
hours after the second therapeutic agent.
Moreover, a compound of the present invention may be administered at least
about
1 day, at least about 2 days, at least about 3 days, at least about 4 days, at
least about 5 days,
at least about 6 days, at least about 7 days, at least about 8 days, at least
about 9 days, at least
about 10 days, at least about 11 days, at least about 12 days, at least about
13 days, at least
about 14 days, at least about 15 days, at least about 16 days, at least about
17 days, at least
about 18 days, at least about 19 days, at least about 20 days, at least about
21 days, at least
about 22 days, at least about 23 days, at least about 24 days, at least about
25 days, at least
about 26 days, at least about 27 days, at least about 28 days, . at least
about 29 days, at least
about 30 days or at least about 31 days after the administration of the second
therapeutic agent.
A compound of the present invention may be administered at least about 1 week,
at
least about 2 weeks, at least about 3 weeks, at least about 4 weeks, at least
about 5 weeks, at
least about 6 weeks, at least about 7 weeks, at least about 8 weeks, at least
about 9 weeks, at
least about 10 weeks, at least about 11 weeks, at least about 12 weeks, at
least about 13
weeks, at least about 14 weeks, at least about 15 weeks, at least about 16
weeks, at least
208
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
about 17 weeks, at least about 18 weeks, at least about 19 weeks, or at least
about 20 weeks
after the second therapeutic agent.
Further, a compound of the present invention may be administered at least
about one
month, at least about two months, at least about three months, at least about
four months, at
least about five months, at least about six months, at least about seven
months, at least about
eight months, at least about nine months, at least about ten months, at least
about eleven
months, or at least about twelve months after the second therapeutic agent.
The compound of formula (I) also may be administered in conjunction with other
medications used in the treatments of cardiovascular diseases, including
platelets aggregation
to inhibitors such as aspirin, antithrombotic agents such as coumadin, calcium
channel blockers
such as dilteazem and nefidipine, angiotension converting enzyme (ACE)
inhibitors such as
captopril and enalopril and 13 Mockers such as propanalol. The compound also
can be
administered in combination with non steroid antiinflamatory agents such as
ibuprofen,
indomethacin, sulindac, or COX II inhibitors such as rofecoxib or celecoxib. A
therapeutic
amount of the compound of formula (I) also can be administered with a
carticosteroid. They
also can be administered in combination with a TNF-a modulating agent for
example
etanercept or infliximab. A therapeutic amount of the compound of formula (I)
also can be
administered also can be administered with HMGCoA reductose inhibitors, PPAR-?
agonists,
HDL elevators or retinoids.
Methods of Administration
The compounds of the present invention may be administered by any suitable
means,
including, but not limited to, parenteral, subcutaneous, intramuscular,
intravenous,
intrarticular, intrabronchial, intraabdominal, intracapsular,
intracartilaginous, intracavitary,
intracelial, intracelebellar, intracerebroventricular, intracolic,
intracervical, intragastric,
intrahepatic, intramyocardial, intraosteal, intrapelvic, intrapericardiac,
intraperitoneal,
intrapleural, intraprostatic, intrapulmonary, intrarectal, intrarenal,
intraretinal, intraspinal,
intrasynovial, intrathoracic, intrauterine, intravesical, bolus, vaginal,
rectal, buccal,
sublingual, intranasal, or transdemnal means.
3o A. Pulmonary/Nasal Administration
There are a several desirable features of an inhalation device for
administering a
compound of the present invention. For example, delivery by the inhalation
device is
209
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
advantageously reliable, reproducible, and accurate. For pulmonary
administration, at least
one pharmaceutical composition is delivered in a particle size effective for
reaching the
lower airways of the lung or sinuses. The inhalation device optionally
delivers small dry
particles, typically less than about 10 pm, for example, about 1-5 Vim, for
good respirability.
The pharmaceutical composition of the present invention can be delivered by
any
suitable inhalation or nasal device. Devices capable of depositing aerosolized
formulations
in the sinus cavity or alveoli of a patient include, but are not limited to,
metered dose
inhalers, nebulizers, dry powder generators, and sprayers. Other devices
suitable for
directing pulmonary or nasal administration are also known in the art.
to All such devices may be used for the administration of a pharmaceutical
composition
in an aerosol. Such aerosols may comprise either solutions (both aqueous and
non aqueous)
or solid particles. Metered dose inhalers like the Ventolin~ metered dose
inhaler, typically
use a propellent gas and require actuation during inspiration. See, for
example, WO
98/35888; WO 94/16970. Dry powder inhalers like Turbuhaler~ (Astray,
Rotahaler~
(Glaxo), Diskus° (Glaxo), Spiros inhaler (Dura), devices marketed by
Inhale Therapeutics,
and the Spinhaler~ powder W baler (Fisons), use breath-actuation of a mixed
powder. See
U.S. Patent Nos. 5,458,135; 4,668,218; WO 97/25086; WO 94/08552; WO 94106498;
and EP
0 237 507, each of which is incorporated by reference herein in its entirety.
Nebulizers, for
example, AERx~, Aradigm, the Ultravent~ nebulizer (Mallinckrodt), and the
Acorn II~
nebulizer (Marquest Medical Products) produce aerosols from solutions, while
metered dose
inhalers, and dry powder inhalers generate small particle aerosols. These
specific examples
of commercially available inhalation devices are intended to be a
representative of specific
devices suitable for the practice of the invention, and are not intended as
limiting the scope of
the invention.
Where the earner is a solid, formulations suitable for nasal administration
include a
coarse powder having a particle size, for example, from about 20 to 500
microns that is
administered in the manner in which snuff is administered, i.e., by rapid
inhalation through
the nasal passage from a container of the powder held close up to the nose.
Where the earner
is a liquid, suitable formulations for administration as, for example, a nasal
spray or as nasal
drops, include aqueous or oily solutions of the active ingredient.
1. Administration as a Spray
210
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
A spray comprising a pharmaceutical composition of the present invention can
be
produced by forcing a suspension or solution of a compound contemplated hereby
through a
nozzle under pressure. The nozzle size and configuration, the applied
pressure, and the
liquid feed rate are chosen to achieve the desired output and particle size.
An electrospray
s can be produced, for example, by an electric field in connection with a
capillary or nozzle
feed. Typically, particles of at least one compound delivered by a sprayer
have a particle size
less than about 20 ~.m, less than about 19 Win, less than about 18 Nm, less
than about 17 ~,rn,
less than about 16 N,m, less than about 15 Vim, less than about 14 Nrn, less
than about 13 Nrn,
less than about 12 Etm, less than about 11 urn, less than about 10 fun, less
than about 9 Eun,
to less than about 8 Vim, less than about 7 ~.un, less than about 6 ~.m, less
than about 5 qm, less
than about 4 Vim, less than about 3 Vim, less than about 2 Nm, less than about
1 qm.
Pharmaceutical compositions according to the present invention suitable for
use with
a sprayer typically include a compound contemplated hereby in an aqueous
solution at a
concentration of about 0.1 mg to about 100 mg of a compound contemplated
hereby per mL
is of solution or mg/gm, or any range or value therein including, but not
hnited to, 0.1, 0.2., 0.3,
0.4, 0.5, 0.6, 0.7, 0.8, 0.9, l, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21,
22, 23, 24, 25, 26, 27, 28, 29, 30, 40, 45, 50, 60, 70, 80, 90 or 100 mg/mL or
mg/gm. The
pharmaceutical composition can include agents such as an excipient, a buffer,
an isotonicity
agent, a preservative, a surfactant, and, for example, zinc. The
pharmaceutical composition
20 also can include an excipient or agent for stabilization of the compound,
such as a buffer, a
reducing agent, a bulk protein, or a carbohydrate. Bulk proteins useful in
pharmaceutical
compositions suitable for use in a sprayer include albumin, protamine, or the
like. Typical
carbohydrates useful in pharmaceutical compositions include sucrose, mannitol,
lactose,
trehalose, glucose, or the like. The pharmaceutical composition also can
include a surfactant,
2s which can reduce or prevent surface-induced aggregation of the
pharmaceutical composition
caused by atomization of the solution in forming an aerosol. Various
conventional
surfactants can be employed such as polyoxyethylene fatty acid esters and
alcohols, and
polyoxyethylene sorbitol fatty acid esters. Amounts will generally range
between 0.001 and
10% by weight of the formulation. Suitable surfactants include, but are not
limited to,
3o polyoxyethylene sorbitan monooleate, polysorbate 80, polysorbate 20, or the
like. Additional
agents known in the art also can be included in the pharmaceutical
composition.
211
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
2. Administration by a Nebulizer
A pharmaceutical composition of the present invention can be administered by a
nebulizer such as a jet nebulizer or an ultrasonic nebulizer. Typically, in a
jet nebulizer, a
compressed air source is used to create a high-velocity air jet through an
orifice. As the gas
expands beyond the nozzle, a low-pressure region is created, which draws a
solution of
composition protein through a capillary tube connected to a liquid reservoir.
The liquid
stream from the capillary tube is sheared into unstable filaments and droplets
as it exits the
tube, creating the aerosol. A range of configurations, flow rates, and baffle
types can be
employed to achieve the desired performance characteristics from a given jet
nebulizer. In
to an ultrasonic nebulizer, high-frequency electrical energy is used to create
vibrational,
mechanical energy, typically employing a piezoelectric transducer. This energy
is
transmitted to the formulation of composition protein either directly or
through a coupling
fluid, creating an aerosol including the composition protein. Advantageously,
particles of the
pharnlaceutical composition delivered by a nebulizer have a particle size less
than about 20
Vim, less than about 19 E.im, less than about 18 Eam, less than about 17 Eun,
less than about 16
Eim, less than about 15 Eun, less than about 14 Eim, less than about 13 Etm,
less than about I2
E.un, less than about 11 Eim, less than about 10 E.im, less than about 9 Eim,
less than about 8
E.un, less than about 7 qm, less than about 6 E rn, less than about 5 Eam,
less than about 4 E.vm,
less than about 3 E~m, less than about 2 E~m, less than about 1 E~m.
2o Pharmaceutical compositions comprising a compound of the present invention
suitable for use with a nebulizer, either jet or ultrasonic, typically include
a concentration of
about 0.1 mg to about 100 mg of a compound contemplated hereby per mL of
solution or
mg/gm, or any range or value therein including, but not limited to, 0.1, 0.2.,
0.3, 0.4, 0.5, 0.6,
0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18,
19, 20, 21, 22, 23, 24,
25, 26, 27, 28, 29, 30, 40, 45, 50, 60, 70, 80, 90 or 100 mg/mL or mg/gm. The
pharmaceutical composition can include agents such as an excipient, a buffer,
an isotonicity
agent, a preservative, a surfactant, and, for example, zinc. The
pharmaceutical composition
also can include an excipient or agent for stabilization of the compound such
as a buffer, a
reducing agent, a bulk protein, or a carbohydrate. Bulk proteins useful in
pharmaceutical
3o compositions suitable for use in a sprayer include albumin, protamine, or
the like. Typical
carbohydrates useful in pharmaceutical compositions include sucrose, mannitol,
lactose,
trehalose, glucose, or any combination thereof. The pharmaceutical composition
also can
212
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
include a surfactant, which can reduce or prevent surface-induced aggregation
of the
pharniaceutical composition caused by atomization of the solution in forming
an aerosol.
Various conventional surfactants can be employed such as polyoxyethylene fatty
acid esters
and alcohols, and polyoxyethylene sorbitol fatty acid esters. Amounts will
generally range
s between 0.001 and 10% by weight of the formulation. Suitable surfactants for
purposes of
this invention are polyoxyethylene sorbitan monooleate, polysorbate 80,
polysorbate 20, or
the like. Additional agents known in the art also can be included in the
pharmaceutical
composition.
3. Administration by a Metered Dose Inhaler
l0 In a metered dose inhaler (MDI), a propellant, a compound of the present
invention,
and any excipients or other additives are contained in a cannister as a
mixture including a
liquefied, compressed gas. Actuation of the metering valve releases the
mixture as an
aerosol, typically containing particles in the size range of less than about
20 pm, less than
about 19 Vim, less than about 18 Vim, less than about 17 Nrn, less than about
16 Vim, less than
1s about 15 Nm, less than about 14 Nxn, less than about 13 Nrn, less than
about 12 Vim, less than
about 11 pm, less than about 10 Vim, less than about 9 Vim, less than about 8
~xn, less than
about 7 Nrn, less than about 6 Vim, less than about 5 ~,m, less than about 4
Nm, less than about
3 Vim, less than about 2 l.un, less than about 1 Eun.
The desired aerosol particle size can be obtained by employing a formulation
of a
20 compound of the present invention produced by various methods known to
those of skill in
the art including, but not limited to, jet-milling, spray drying, critical
point condensation.
Suitable metered dose inhalers include those manufactured by 3M or Glaxo and
employing a
hydrofluorocarbon propellant.
Pharmaceutical compositions for use with a metered-dose inhaler device will
2s generally include a finely divided powder containing a compound
contemplated hereby as a
suspension in a non-aqueous medium, for example, suspended in a propellant
with the aid of
a surfactant. The propellant can be any conventional material employed for
this purpose
such as chlorofluorocarbon, a hydrochlorofluorocarbon, a hydrofluorocarbon, or
a
hydrocarbon including trichlorofluoromethane, dichlorodifluoromethane,
3o dichlorotetrafluoroethanol and 1,1,1,2-tetrafluoroethane, HFA-134a
(hydrofluroalkane-134a),
HFA-227 (hydrofluroalkane-227), or the like. The surfactant can be chosen to
stabilize the
compound of the present invention as a suspension in the propellant, to
protect the active
213
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
agent against chemical degradation. Suitable surfactants include sorbitan
trioleate, soya
lecithin, oleic acid, or the like. In some cases solution aerosols are formed
using solvents
such as ethanol. One of ordinary skill in the art will recognize that the
methods of the
present invention can be achieved by pulmonary administration of a compound
contemplated
hereby via devices not described herein.
B. Mucosal Administration
For absorption through mucosal surfaces, the compositions and methods of the
present invention for administering a compound contemplated hereby include an
emulsion
comprising a plurality of submicron particles, a mucoadhesive macromolecule, a
bioactive
peptide, and an aqueous continuous phase, which promotes absorption through
mucosal
surfaces by achieving mucoadhesion of the emulsion particles. See, for
exazyzple, U.S. Patent
No. 5,514,670. Mucous surfaces suitable for application of the compositions of
the present
invention can include corneal, conjunctival, buccal, sublingual, nasal,
vaginal, pulmonary,
abdominal, intestinal, and rectal routes of administration. Pharmaceutical
compositions for
vaginal or rectal administration, such as suppositories, can contain as
excipients, for example,
polyalkyleneglycols, vaseline, cocoa butter. Pharmaceutical compositions for
intranasal
administration can be solid and contain excipients, for example, lactose or
can be aqueous or
oily solutions of nasal drops. For buccal administration, excipients include
sugars, calcium
stearate, magnesium stearate, pregelinatined starch. See, for example, U.S.
Patent No.
5,849,695.
C. Transdermal Administration
The pharmaceutical compositions of the present invention may be adminstered
via
transdermal routes using forms of transdermal skin patches. For transdermal
administration,
a compound of the present invention is encapsulated in a delivery device such
as a liposome
or polymeric nanoparticle, microparticle, microcapsule, or microsphere
(referred to
collectively as "microparticles" unless otherwise stated). Any suitable
delivery device may
be used, for example, microparticles made of synthetic polymers, such as
polyhydroxy acids,
for example, polylactic acid, polyglycolic acid and copolymers thereof,
polyorthoesters,
polyanhydrides, and polyphosphazenes, and natural polymers such as collagen,
polyamino
3o acids, albumin and other proteins, alginate and other polysaccharides, and
any combination
thereof. See, foz~ example, U.S. Patent No. 5,814,599, incorporated by
reference herein in its
214
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
entirety. To be administered in the form of a transdermal delivery system, the
dosage
administration may be continuous rather than intermittent throughout the
dosage regimen.
Formulations suitable for topical administration to the skin may be presented
as
ointments, creams, gels, and pastes comprising the ingredient to be
administered in a
pharmaceutical acceptable carrier. According to one aspect of the present
invention, a
transdermal patch is used as a topical delivery system.
Topical compositions may be admixed with a variety of Garner materials
including,
for example, alcohols, aloe vera gel, allantoin, glycerine, vitamin A and E
oils, mineral oil,
PPG2 myristyl propionate, or any mixture thereof, to form, for example,
alcoholic solutions,
to topical cleansers, cleansing creams, skin gels, skin lotions, and shampoos
in cream or gel
formulations. Examples of such carriers and methods of formulation may be
found in
1ZEMINGTON'S PHARMACEUTICAL SCIENCES (1990), incorporated by reference herein
in its
entirety. Pharnaceutical formulations may contain from about 0.005% to about
10% by
weight of the active ingredient, for example, from about 0.01% to 5% by weight
of the active
ingredient.
D. Prolonged Administration
It may be desirable to deliver the compounds of the present invention to the
subject
over prolonged periods of time, for example, for periods of one week to one
year for a single
adminstration. Certain medical devices may be employed to provide a continuous
2o intermittent or on demand dosing of a patient. The devices may include a
pump or diffusion
apparatus, or any other device containing a reservoir of drug and optionally
diagnostic or
monitoring components to regulate the delivery of the drug. Various slow-
release, depot, or
implant dosage forms can be utilized. For example, a dosage form can contain a
pharnaceutically acceptable non-toxic salt of compound contemplated hereby
that has a low
degree of solubility in body fluids, for example, (a) an acid addition salt
with a polybasic acid
such as phosphoric acid, sulfuric acid, citric acid, tartaric acid, tannic
acid, pamoic acid,
alginic acid, polyglutamic acid, naphthalene mono- or di-sulfonic acids,
polygalacturonic
acid, or any mixture thereof; {b) a salt with a polyvalent metal cation such
as zinc, calcium,
bismuth, barium, magnesium, aluminum, copper, cobalt, nickel, cadmium, or any
mixture
thereof, or with an organic cation formed from for example, N,N'-dibenzyl-
ethylenediamine
or ethylenediamine; or (c) combinations of (a) and (b), for example, a zinc
tannate salt.
Additionally, the compounds of the present invention or a relatively insoluble
salt, such as
215
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
those just described, can be formulated in a gel, for example, an aluminum
monostearate gel
with, for example, sesame oil, suitable for injection. Exemplary salts
include, but are not
limited to, zinc salts, zinc tannate salts, pamoate salts, and any mixture
thereof. Another type
of slow-release depot formulation for injection may contain the compound or
salt dispersed
or encapsulated in a slow degrading, non-toxic, non-antigenic polymer such as
a polylactic
acid/polyglycolic acid polymer, for example, as described in U.S. Patent No.
3,773,919. The
compounds or relatively insoluble salts thereof also can be formulated in
cholesterol matrix
silastic pellets, particularly for use in animals. Additional slow-release,
depot, or implant
formulations, for example, gas or liquid liposomes are described in, for
example, U.S. Patent
NO. 5,770,222; SUSTAINED AND CONTROLLED RELEASE DRUG DELIVERY SYSTEMS (1978),
incorporated by reference herein in its entirety.
Dosage Determination
In general, the compounds contemplated hereby may be used alone or in concert
with
other therapeutic agents at appropriate dosages to obtain optimal efficacy
while minimizing
any potential toxicity. The dosage regimen utilizing a compound of the present
invention
may be selected in accordance with a variety of factors including type,
species, age, weight,
sex, medical condition of the patient; the severity of the condition to be
treated; the route of
administration; the renal and hepatic function of the patient; and the
particular compound
employed. A physician or veterinarian of ordinary skill can readily determine
and prescribe
the effective amount of the drug required to prevent, counter, or arrest the
progress of the
condition.
Optimal precision in achieving concentrations of drug within the range that
yields
maximum efficacy with minimal toxicity may require a regimen based. on the
kinetics of the
compound's availability to a target site(s). Distribution, equilibrium, and
elimination of a
drug may be considered when determiliing the optimal concentration for a
treatment regimen.
The dosages of a compound contemplated hereby may be adjusted when combined to
achieve desired effects. On the other hand, dosages of these various
therapeutic agents may
be independently optimized and combined to achieve a synergistic result
wherein the
pathology is reduced more than it would be if either agent were used alone.
In particular, toxicity and therapeutic efficacy of a compound contemplated
hereby
may be determined by standard pharmaceutical procedures in cell cultures or
experimental
216
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
animals, for example, for determining the LDSO (the dose lethal to 50% of the
population) and
the EDSO (the dose therapeutically effective in 50% of the population). The
dose ratio
between toxic and therapeutic effect is the therapeutic index and it may be
expressed as the
ratio LDSO/EDSO. Compounds exhibiting large therapeutic indices typically are
used.
Although compounds that exhibit toxic side effects may be used, care should be
taken to
design a delivery system that targets such compounds to the site of affected
tissue in order to
minimize potential damage to uninfected cells and, thereby, reduce side
effects. Generally,
the compounds of the present invention may be administered in a manner that
maximizes
efficacy and minimizes toxicity.
Data obtained from cell culture assays and animal studies may be used in
formulating
a range of dosages for use in humans. The dosages of such compounds are
generally within a
range of circulating concentrations that include the EDSO with little or no
toxicity. The
dosage may vary within this range depending upon the dosage form employed and
the route
of administration utilized. For any compound used in the methods of the
invention, the
therapeutically effective dose may be estimated initially from cell culture
assays. A dose
may be formulated in animal models to achieve a circulating plasma
concentration range that
includes the ICSO (the concentration of the test compound that achieves a half
maximal
inhibition of symptoms) as determined in cell culture. Such information may be
used to
determine accurately useful doses in humans. Levels in plasma may be measured,
for
example, by high performance liquid chromatography.
Moreover, the dosage administration of the pharmaceutical compositions of the
present invention may be optimized using a pharmacokinetic/pharmacodynamic
modeling
system. For example, one or more dosage regimens may be chosen and a
pharmacokinetic/pharmacodynamic model may be used to, determine the
pharmacokinetic/pharmacodynamic profile of one or more dosage regimens. Next,
one of
the dosage regimens for administration may be selected which achieves the
desired
pharmacokinetic/pharmacodynamic response based on the particular
pharmacokinetic/pharmacodynamic profile. See WO 00/67776, incorporated herein
by
reference in its entirety.
3o Methods are known in the art for determining effective doses for
therapeutic and
prophylactic purposes for the disclosed pharmaceutical compositions or the
disclosed drug
combinations, whether or not formulated in the same composition. For
therapeutic purposes,
217
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
the term "jointly effective amount", as used herein, means that amount of each
active
compound or pharmaceutical agent, alone or in combination, that elicits the
biological or
medicinal response in a tissue system, animal or human that is being sought by
a researcher,
veterinarian, medical doctor, or other clinician, which includes alleviation
of the symptoms
of the disease or disorder being treated. For prophylactic purposes (i.e.,
inhibiting the onset
or progression of a disorder), the term "jointly effective amount" refers to
that amount of
each active compound or pharmaceutical agent, alone or in combination, that
inhibits in a
subject the onset or progression of a disorder as being sought by a
researcher, veterinarian,
medical doctor or other clinician. Thus, the present invention provides
combinations of two
l0 or more therapeutic agents wherein, for example, (a) each therapeutic agent
is administered
in an independently therapeutically or prophylactically effective amount; (b)
at least one
therapeutic agent in the combination is administered in an amount that is sub-
therapeutic or
subprophylactic if administered alone, but is therapeutic or prophylactic when
administered
in combination with the second or additional therapeutic agents according to
the invention; or
(c) both therapeutic agents are administered in an amount that is
subtherapeutic or sub-
prophylactic if administered alone, but are therapeutic or prophylactic when
administered
together. Combinations of three or more therapeutic agents are analogously
possible.
Methods of combination therapy W clude coadministration of a single
formulation containing
all active agents; essentially contemporaneous administration of more than one
formulation;
and administration of two or more active agents separately formulated.
Dosages
The pharmaceutical compositions of the present invention may be administered
in a
single daily dose, or the total daily dosage may be administered in divided
doses of two,
three, or four times daily. In the case of oral administration, the daily
dosage of the
compositions may be varied over a wide range from about 0.0001 to about 1,000
mg per
patient, per day. The range may more particularly be from about 0.001 mg/kg to
10 mg/kg of
body weight per day, about 0.1-100 mg, about 1.0-50 mg or about 1.0-20 mg per
day for
adults (at about 60 kg).
For oral administration, the pharmaceutical compositions may be provided in a
form
of scored or unscored tablets containing about 0.01, 0.05, 0.1, 0.5, 1.0, 2.5,
5.0, 10.0, 15.0,
218
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
25.0, 50.0, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, or 700
mg of the
active ingredient for the symptomatic adjustment of the dosage for the patient
to be treated.
In the case of injections, it is usually convenient to give by an intravenous
route in
an amount of about 0.01-30 mg, about 0.1-20 mg or about 0.1-10 mg per day to
adults
(at about 60 kg). In the case of other animals, the dose calculated for 60 kg
may be
administered as well.
The daily dosage of the pharmaceutical compositions may be varied over a wide
range from about 5 to about 1000 mg per adult human per day. For oral
administration, the
pharmaceutical compositions optionally are provided in the form of tablets
containing, 5.0,
l0 10.0, 15.0, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, or
700 milligrams of
the active ingredient for the symptomatic adjustment of the dosage to the
patient to be
treated. An effective amount of the drug typically is provided at a dosage
level of from about
0.1 mg/kg to about 20 mg/kg of body weight per day. According to one aspect of
the present
invention, the dosage level is from about 0.2 mg/kg to about 10 mg/kg of body
weight per
day. According to another aspect of the present invention, the dosage level is
from about 0.5
mg/kg to about 10 mg/kg of body weight per day. The compounds may be
administered on a
regimen of about 1 to about 10 times per day.
Doses of a compound of the present invention optionally can include 0.1, 0.2,
0.3,
0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21,
22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40,
41, 42, 43, 44, 45, 46,
47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 62, 63, 64, 65, 66,
67, 68, 69, 70, 71, 72,
73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91,
92, 93, 94, 95, 96, 97,
98, 99 and/or 100-500 mg/kg/administration or any range, value or fraction
thereof, or to
achieve a serum concentration of 0.1, 0.5, 0.9, 1.0, 1.1, 1.2, 1.5, 1.9, 2.0,
2..5, 2.9, 3.0, 3.5,
3.9, 4.0, 4.5, 4.9, 5.0, 5.5, 5.9, 6.0, 6.5, 6.9, 7.0, 7.5, 7.9, 8.0, 8.5,
8.9, 9.0, 9.5, 9.9, 10, 10.5,
10.9, 11, 11.5, 11.9, 20, 12.5, 12.9, 13.0, 13.5, 13.9, 14.0, 14.5, 4.9, 5.0,
5.5., 5.9, 6.0, 6.5,
6.9, 7.0, 7.5, 7.9, 8.0, 8.5, 8.9, 9.0, 9.5, 9.9, 10, 10.5, 10.9, 11, 11.5,
11.9, 12, 12.5, 12.9, 13.0,
13.5, 13.9, 14, 14.5, 15, 15.5, 15.9, 16, 16.5, 16.9, 17, 17.5, 17.9, 18,
18.5, 18.9, 19, 19.5,
19.9, 20, 20.5, 20.9, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 35, 40, 45, 50,
55, 60, 65, 70, 75,
80, 85, 90, 96, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, 1500, 2000,
2500, 3000,
3500, 4000, 4500, and/or 5000 ~g/mL serum concentration per single or multiple
administration or any range, value or fraction thereof.
219
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
As a non-limiting example, treatment of humans or animals can be provided as a
one-
time or periodic dosage of a compound of the present invention 0.1 to 100
mg/kg such as 0.5,
0.9, 1.0, l.l, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20, 21, 22, 23, 24,
25, 26, 27, 28, 29, 30, 40, 45, 50, 60, 70, 80, 90 or 100 mglleg, per day, on
at least one of day
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 l, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21,
22, 23, 24, 25, 26, 27, 28,
29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, or 40, or alternatively or
additionally, at Least one of
week l, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20,
21, 22, 23, 24, 25, 26,
27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45,
46, 47, 48, 49, 50, Sl,
or 52, or alternatively or additionally, at least one of 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 1 l, 12, 13, 14,
l0 15, 16, 17, 18, 19, or 20 years, or any combination thereof, using single,
infusion or
repeated doses.
Specifically, the pharmaceutical compositions of the present invention may be
administered at least once a week over the course of several weeks. According
to one aspect
of the present invention, the pharmaceutical compositions are administered at
least once a
week over several weeks to several months. According to another aspect of the
present
invention, the pharmaceutical compositions are administered once a week over
four to eight
weeks. According to yet another aspect of the present invention, the
pharmaceutical
compositions are administered once a week over four weeks.
More specifically, the pharmaceutical compositions may be administered at
least once
a day for about 2 days, at least once a day for about 3 days, at least once a
day for about 4
days, at least once a day for about 5 days, at least once a day for about 6
days, at least once a
day for about 7 days, at least once a day for about 8 days, at least once a
day for about 9
days, at least once a day for about 10 days, at least once a day for about 11
days, at least once
a day for about 12 days, at least once a day for about 13 days, at least once
a day for about 14
days, at least once a day for about 15 days, at least once a day for about 16
days, at Least once
a day for about 17 days, at least once a day for about 18 days, at least once
a day for about 19
days, at least once a day for about 20 days, at least once a day for about 21
days, at least once
a day for about 22 days, at least once a day for about 23 days, at least once
a day for about 24
days, at least once a day for about 25 days, at least once a day for about 26
days, at least once
3o a day for about 27 days, at least once a day for about 28 days, at least
once a day for about 29
days, at least once a day for about 30 days, or at least once a day for about
31 days.
220
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Alternatively, the pharmaceutical compositions may be administered about once
every day, about once every 2 days, about once every 3 days, about once every
4 days, about
once every 5 days, about once every 6 days, about once every 7 days, about
once every 8
days, about once every 9 days, about once every 10 days, about once every 11
days, about
once every 12 days, about once every 13 days, about once every 14 days, about
once every
days, about once every 16 days, about once every 17 days, about once every 18
days,
about once every 19 days, about once every 20 days, about once every 21 days,
about once
every 22 days, about once every 23 days, about once every 24 days, about once
every 25
days, about once every 26 days, about once every 27 days, about once every 28
days, about
10 once every 29 days, about once every 30 days, or about once every 31 days.
The pharmaceutical compositions of the present invention may alternatively be
administered about once every week, about once every 2 weeks, about once every
3 weeks,
about once every 4 weeks, about once every 5 weeks, about once every 6 weeks,
about once
every 7 weeks, about once every 8 weeks, about once every 9 weeks, about once
every 10
15 weeks, about once every 11 weeks, about once every 12 weeks, about once
every 13 weeks,
about once every 14 weeks, about once every 15 weeks, about once every 16
weeks, about
once every 17 weeks, about once every 18 weeks, about once every 19 weeks,
about once
every 20 weeks.
Alternatively, the pharmaceutical compositions of the present invention may be
2o administered about once every month, about once every 2 months, about once
every 3
months, about once every 4 months, about once every 5 months, about once every
6 months,
about once every 7 months, about once every 8 months, about once every 9
months, about
once every 10 months, about once every 11 months, or about once every 12
months.
Alternatively, the pharmaceutical compositions may be administered at least
once a
week for about 2 weeks, at least once a week for about 3 weeks, at least once
a week for
about 4 weeks, at least once a week for about 5 weeks, at least once a week
for about 6
weeks, at least once a week for about 7 weeks, at least once a week for about
8 weeks, at
least once a week for about 9 weeks, at least once a week for about 10 weeks,
at least once a
week for about 11 weeks, at least once a week for about 12 weeks, at Ieast
once a week for
about 13 weeks, at least once a week for about 14 weeks, at least once a week
for about 15
weeks, at least once a week for about 16 weeks, at least once a week for about
17 weeks, at
221
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
least once a week for about 18 weeks, at least once a week for about 19 weeks,
or at least
once a week for about 20 weeks.
Alternatively the pharmaceutical compositions may be administered at least
once a
week for about 1 month, at least once a week for about 2 months, at least once
a week for
about 3 months, at least once a week for about 4 months, at least once a week
for about 5
months, at least once a week for about 6 months, at least once a week for
about 7 months, at
least once a week for about 8 months, at least once a week for about 9 months,
at least once a
week for about 10 months, at least once a week for about 11 months, or at
least once a week
for about 12 months.
Methods of Using the Compounds
A. Heparan Sulfate Proteoglycan Modulation
The present invention comprises methods and compositions comprising the
identification of compounds for the treatment and prevention of vascular,
particularly
cardiovascular diseases. More specifically, the present invention relates to
methods and
compositions for the treatment and prevention of smooth muscle cell
proliferation, such as
"anti-proliferative" compounds that effect synthesis of proteoglycans. Methods
for screening
for compounds or molecules that induce HSPG synthesis comprise the addition of
such
compounds to assays and measuring HSPG synthesis including, but not limited
to, the
2o production of Syndecan, Glypican, and Perlecan. Methods for measuring the
induction of
Perlecan synthesis are also contemplated hereby. Although some aspects of the
present
invention are described with respect to Perlecan, it is important to note that
the compositions,
methods, and assays described herein are equally applicable in the context of
other HSPGs
including Syndecan and Glypican. HSPG production is important in regulating
SMC
proliferation and the methods and compositions described herein provide for
high throughput
screening of molecules that induce HSPG production and regulate SMC
proliferation.
Additionally, the present invention comprises methods and compositions for
gene
therapy, comprising administering compositions comprising nucleic acids that
effect the
synthesis or expression of HSPG, particularly Perlecan. For example, vectors
comprising
nucleic acids coding for Perlecan or active fragments of Perlecan are provided
to cells, for
example, circulatory tissue cells such as, for example, endothelial cells.
Such vectors are
222
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
known to those skilled in the art and can be administered in formulations that
enhance the
uptake of the vector by the cells.
The present invention also comprises methods and compositions for inducing the
synthesis or expression of HSPGs, including, but not limited to HSPGs such as
Syndecan,
Glypican and Perlecan, and also comprises induction and synthesis of active
fragments of
HSPGs, for example, active fragments of Perlecan. As used herein, when an HSPG
is
referred to, the entire molecule or fragments are included therein. For
example, Perlecan
refers to the entire Perlecan molecule or fragments thereof. Fragments of
Perlecan may have
the same or different effects on cells. All of these fragments and activities
are contemplated
to in the present invention.
A major extracellular HSPG in the blood vessel matrix is Perlecan, a protein
originally identified in basement membrane. It interacts with extracellular
matrix proteins,
growth factors and receptors. Perlecan also is present in basement membranes
other than
blood vessels and in other extracellular matrix structures. It consists of a
core protein of
Mr.~ 450,000 kDa to which three HS chains of Mr~70 kDa are attached to one end
of the
molecule. Perlecan core protein has a complex functional organization
consisting of five
consecutive domains with homologies to molecules involved in control of cell
proliferation,
lipoprotein binding and cell adhesion. The N-terminal domain I (aa ~l-195)
contains
attachment sites for HS chains. Domain II comprises four repeats homologous to
the ligand-
2o binding portion of the LDL receptor. Domain III has homology to domains IVa
and IVb of
laminin and is thought to mediate cell attachment.
SMC hyperplasia is a major event in the development of atherosclerosis and
also is
responsible for the significant number of failure rates following vascular
procedures such as
angioplasty and coronary artery bypass surgery, particularly due to
restenosis. Proliferation
of arterial wall SMC in response to local injury is a major feature of many
vascular
proliferative disorders. While not wishing to be bound by theory, it is
generally thought that
the endothelium regulates the growth of the underlying SMC. In the normal
vessel, SMC are
quiescent, but they proliferate when damage to the endothelium occurs.
Naturally occurnng
growth modulators, many of which are derived from the endothelium, tightly
control SMC
3o proliferation in vivo.
Though not wishing to be bound by any particular mechanism, it is believed
that
extracellular HSPGs mediate quiescence in SMCs. In serum-starved quiescent
SMC,
223
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Perlecan synthesis is induced. For example, Perlecan inhibits DNA synthesis
and SMC
proliferation, and blocking Perlecan results in stimulation of DNA synthesis
even in the
absence of serum and growth factors. Induction of Perlecan and other HSPGs is
an important
event for the inhibition of SMC growth. Known antiproliferative agents fail to
inhibit SMC
proliferation when the effects of Perlecan are blocked. Thus, the present
invention comprises
methods and compositions for mediating Perlecan and other HSPG synthesis,
expression and
amounts are taught for the maintenance of SMC in a quiescent state. Such
methods and
compositions of the present invention also comprise treatment and prevention
of vascular
diseases, more specifically, pathologies related to SMC proliferation. In
particular, such
l0 pathologies include atherosclerosis and restenosis.
The present invention also comprises methods and compositions for the
treatment and
prevention of vascular occlusive conditions including, but not limited to,
neointimal
hyperplasia, restenosis, transplant vasculopathy, cardiac allograft
vasculopathy,
atherosclerosis, and arteriosclerosis. Such methods and compositions comprise
methods for
inhibition of smooth muscle cell (SMC) growth and proliferation, and for
induction of
quiescence in smooth muscle cells. The present invention further comprise
methods and
compositions for inducing HSPG synthesis and expression including, but not
limited to, the
induction of HSPGs such as Syndecan, Glypican and Perlecan, for example,
Perlecan
synthesis and gene expression.
Neointimal hyperplasia is commonly seen after various forms of vascular injury
and a
major component of the vein graft's response to harvest and surgical
implantation into high-
pressure arterial circulation. In neointimal hyperplasia, smooth muscle cells
in the middle
layer of the vessel wall become activated, divide, proliferate, and migrate
into the inner layer.
The resulting abnormal neointimal cells express pro-inflammatory molecules,
including
cytokines, chemolcines, and adhesion molecules that further trigger a cascade
of events that
lead to occlusive neointimal disease and eventually graft failure.
Proliferation of SMC in response to local injury is a major feature of
vascular
proliferative disorders such as atherosclerosis and restenosis after
angioplasty. Though not
wishing to be bound to any particular theory, it is generally believed that
the endothelium
regulates the growth of the underlying SMC. In normal vessels, SMC are
quiescent, but they
proliferate when damage to the endothelium occurs. The endothelium, in
addition to
producing a variety of growth factors, also generates key growth inhibitors.
HSPGs are
224
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
components of vascular cell membranes and extracellular matrix that are
believed to control
a variety of vascular functions including functioning as a barrier against
cationic molecules
and macromolecules, protecting the main structural component of the basement
membrane,
type IV collagen, from proteolytic attack, binding cytokines and growth
factors including,
but not limited to, basic fibroblast growth factor (bFGF), vascular
endothelial growth factor
(VEGF), hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), and
transforming growth factor 13 (TGF-J3), functioning as storage for these
cytokines, regulating
mesodermal cell fate, positioning of the heart, acting in vasculogenesis and
angiogenesis
after ischemic injury, effecting interactions of cells with adhesive proteins
and blood vessels,
inducing proliferation of smooth muscle cells during atherogenesis, acting to
increase cell
spreading, inhibiting chemotaxis, and effecting the metabolism of lipoproteins
and
nonthrombogenic characteristics of endothelial cells. Additionally, it is
believed that the
HSPGs have different functions in different locations. For example, while cell
surface
HSPGs function as co-receptors for growth factors and support cell growth,
extracellular
HSPG can inlubit cell growth.
Although it is currently believed that endothelial HSPGs inhibit SMC
proliferation, it
is not known whether SMC synthesize antiproliferative HSPGs that act as
autocrine
inhibitors. Not wishing to be bound by any particular mechanism, it is
currently believed
that HSPGs inhibit DNA synthesis and SMC proliferation, and blocking HSPGs
results in
stimulation of DNA synthesis even in the absence of serum and growth factors.
Indeed,
known antiproliferative agents fail to inhibit SMC proliferation when the
effects of HSPGs
are blocked.
Examples of HSPGs include Syndecan, Glypican, and Perlecan, which are
generated
within the cardiovascular system. Vascular SMCs express Syndecans l, 2 and 4,
Glypican-1
and Perlecan. The regulation of HSPG expression in these cells, however, is
not known.
Cell growth stimulators such as platelet derived growth factor (PDGF),
thrombin, serum,
oxidized low density lipoproteins (LDL) and lysolecithin have been shown to
decrease
HSPG, and in particular, to decrease Perlecan. In contrast, cellular
antiproliferative agents,
TGF-(3, apolipoprotein E and heparin stimulate HSPGs.
The present invention comprises methods and compositions for the treatment and
prevention of smooth muscle cell proliferation, including vascular occlusive
pathologies.
Such methods comprise administering compositions comprising therapeutic agents
capable
225
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
of inhibiting SMC proliferation. Administration of such therapeutic agents
that are effective
in inhibiting SMC proliferation, such as the aforementioned thizolidinedione
compositions,
are administered to humans and animals suspected of having or who have, for
example,
vasculopathy or who have undergone angioplasty or other procedures damaging to
the
endothelium. Effective amounts are administered to such humans and animals in
dosages
that are safe and effective. Routes of administration include, but are not
limited to,
intravenous, subcutaneous, transdermal, nasal, and inhalation therapies. Such
therapeutic
agents may be used in conjunction with other therapeutic agents or altered
patient activities,
such as changes in exercise or diet.
The compounds of the present invention are also useful in the treatment or
prophylaxis of at least one cardiovascular disease in a cell, tissue, organ,
animal, or patient
including, but not limited to, cardiac stun syndrome, myocardial infarction,
congestive heart
failure, stroke, ischemic stroke, hemorrhage, arteriosclerosis,
atherosclerosis, restenosis,
diabetic ateriosclerotic disease, hypertension, arterial hypertension,
renovascuIar
hypertension, syncope, shock, syphilis of the cardiovascular system, heart
failure, cor
pulmonale, primary pulmonary hypertension, cardiac arrhythmias, atrial ectopic
beats, atrial
flutter, atrial fibrillation (sustained or paroxysmal), post perfusion
syndrome,
cardiopulmonary bypass inflammation response, chaotic or multifocal atrial
tachycardia,
regular narrow QRS tachycardia, specific arrythmias, ventricular fibrillation,
His bundle
arrythmias, atrioventricular block, bundle branch block, myocardial ischemic
disorders,
coronary artery disease, angina pectoris, myocardial infarction,
cardiomyopathy, dilated
congestive cardiomyopathy, restrictive cardiomyopathy, valvular heart
diseases, endocarditis,
pericardial disease, cardiac tumors, aordic and peripheral aneuryisms, aortic
dissection,
inflammation of the aorta, occulsion of the abdominal aorta and its branches,
peripheral
vascular disorders, occulsive arterial disorders, peripheral atherlosclerotic
disease,
thromboangitis obliterans, functional peripheral arterial disorders, Raynaud's
phenomenon
and disease, acrocyanosis, erythromelalgia, venous diseases, venous
thrombosis, varicose
veins, arteriovenous fistula, lymphederma, lipedema, unstable angina,
reperFusion injury,
post pump syndrome, ischemia-reperfusion injury, and dyslipidemia. Such a
method
optionally comprises administering an effective amount of a composition or
pharmaceutical
composition comprising at least one compound to a cell, tissue, organ, animal,
or patient in
need of such modulation, treatment, or therapy.
226
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
1. Assessing HSPG Activity
The present invention comprises methods and compositions for determining
therapeutic agents that are capable of effecting SMC proliferation. Such
assays are taught
herein and can be used as assays to determine agents that affect the amount or
activity of
HSPGs, for example, Perlecan, in such assays. For example, in one assay,
Perlecan is
induced in cells by certain inducers, and the response is measured. Potential
therapeutic
agents are then added to a replicate assay and the effect on Perlecan
induction is determined.
Using such methods and compositions, therapeutic agents are determined that
can either
inhibit Perlecan, elevate induction of Perlecan, or that have no effect at
all. Such therapeutic
l0 agents can then be used in animals with SMC proliferation pathologies.
The present invention also comprises compositions comprising the compounds
identified by the methods as having a desired activity. The compositions have
utility in
treatment of cells, tissues, or whole organisms. Such compositions are
formulated for use in
methods of administration in an effective amount for treatment of conditions
such as
biological conditions including, but not limited to, vascular occlusive
lesions including
atherosclerosis, transplant vasculopathy, cardiac allograft vasculopathy,
restenosis, and graft
atherosclerosis after coronary transplantation. The compositions may comprise
other
compounds including compounds with activities and pharmaceutical adjuncts that
are needed
for administration of the compound or compounds with the desired activity. The
2o compositions may additionally be administered exclusively or in conjunction
with other
pharmaceutical compositions and surgical methods for treating smooth muscle
cell
proliferation and vascular occlusive diseases, including, but not limited to,
before, during and
after PTCA procedures.
In the assays of the present invention, the compound initially has unknown
activity,
effect, or effects. The activity of the compound is unknown, in that the
compound's effects
in the assays of the present invention are not yet determined. The compound
may have many
other known activities, and may be a compound that has other therapeutic uses.
Any agent
that causes the cells or components of the assay to respond in a measurable
manner is
contemplated by the present invention.
3o The present invention comprises methods and compositions for measuring the
activity
of unknown compounds. Such methods comprise assays for specific activity of
biological
components involved in a known cellular response. The assays provide a
measurable
227
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
response in which the activity of the unknown compounds is determined. This
response can
be measured by methods known to those skilled in the art, for example, in an
ELISA. One
aspect of the present invention comprises measurement of the effects of
compounds on SMC
proliferation in response to an HSPG-inducing agent.
s According to one aspect of the present invention, a compound suspected of
effecting
HSPG synthesis is added to cells in an assay. The response of the cells can be
measured by
determining levels of HSPG synthesis measured by methods known to those
skilled in the art
and compared to the amount of HSPG synthesis in untreated cells. The compound
may have
a stimulating effect, an inhibitory effect, a stabilizing effect, or no effect
at all.
l0 According to another aspect of the present invention, a composition
suspected of
effecting SMC proliferation is added to smooth muscle cells in growth medium
or serum-free
medium. The change in cell proliferation can be measured by methods known to
those
skilled in the art and compared to the proliferation of cells which are not
treated with the
compound. The composition may have a stimulating effect, an inhibitory effect,
a stabilizing
15 effect, or no effect at all.
Compositions with HSPG stimulating effects, particularly Perhecan stimulating
effects, are usefuh as anti-proliferative therapeutics, specifically,
inhibiting SMC proliferation
and thus, treating vascular occlusive conditions. These selective activators
of, for example,
Perlecan include small organic molecules, peptides, peptoids, or
polynucleotides that act
20 directly upon Perlecan to modulate the biological activity or to increase
the biological
stability of the protein. In addition, the selective activators of Perlecan
can increase the
biosynthesis of Perlecan by increasing the transcription of the Perlecan gene,
increasing the
biological stability of the Perlecan mIRNA or increasing the translation of
Perlecan mIRNA
into protein. Furthermore, the selective activators of Perhecan can block or
decrease the
2s effects of agents or proteins that inhibit the activity of Perlecan.
The present invention also comprises methods and compositions for assays that
may
be used to identify such selective activators or inhibitors of Perlecan. These
assays readily
determine the activators that up-regulate and the inhibitors that down-
regulate the amount of
Perlecan and its biological activity. In general, such assays include, but are
not limited to,
3o promoter-based assays to identify compounds that affect Perlecan and assays
for Perlecan
biological activity in recombinant, partially purified protein, or lysates
from cells expressing
Perlecan in the presence or absence of compounds of interest. Measurements of
Perlecan
228
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
include biological activity assays and quantitation of Perlecan protein, using
ELISA or
Western blot determinations, or quantitation of Perlecan RNA using RT-PCR, or
Northern
blots.
Both indirect and direct methods of measurement of changes in Perlecan are
contemplated by the present invention. The assay methods contemplated hereby
rely on
indirect measurement of Perlecan through measurement of determinants of
Perlecan activity
or expression.
Additionally, direct determination of the change in the amount of Perlecan
protein
can be done using other immunological methods, such as Western blots,
densitometric
l0 measurements or ELISA methods. Alternatively, the direct determination of
the change in
the amount of Perlecan mRNA can be accomplished using RT-PCR or Northern
analysis
methods which are known to one skilled in the art. Measurements are also
directly made
using lysates of cells, and purified or partially purified Perlecan protein
that is either a
recombinant or natural form of the protein. The means for the measurement of
biological
activity are known to those skilled in the art.
Another method of identifying and determining compounds that affect Perlecan
comprises identifying compounds that interact with the promoter regions of the
Perlecan
gene, or interact and effect proteins that interact with the promoter region,
and are important
in the transcriptional regulation of Perlecan expression. In general, the
method comprises a
vector comprising regulatory sequences of the Perlecan gene and an indicator
region
controlled by the regulatory sequences, such as an enzyme, in a promoter-
reporter construct.
The protein product of the indicator region is referred to herein as a
reporter enzyme or
reporter protein. The regulatory region of the sequence of Perlecan comprises
a range of
nucleotides from approximately -4000 to +2000, where the transcription
initiation site is -I-l,
for example, from -2500 to +1200, for example, from -1500 to +800 relative to
the
transcription initiation site.
Cells are transfected with the vector and then treated with compounds of
interest. For
example, the transfected cells are treated with a compound suspected of
effecting the
transcription of Perlecan and the level of activity of the Perlecan regulatory
sequences are
compared to the level of activity in cells that were not treated with the
compound. The level
of activity of the Perlecan regulatory sequences are determined by measuring
the amount of
the reporter protein or determining the activity of the reporter enzyme
controlled by the
229
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
regulatory sequences. An increase in the amount of the reporter protein or the
reporter
enzyme activity shows a stimulatory effect on Perlecan, by positively
affecting the promoter,
whereas a decrease in the amount or the reporter protein or the reporter
enzyme activity
shows a negative effect on the promoter and thus, on Perlecan.
Additionally, the present invention comprises methods and compositions for
identifying selective inhibitors of Perlecan protein or biological activity.
These selective
inhibitors of Perlecan are small organic molecules, peptides, peptoids, or
polynucleotides that
act directly upon Perlecan or the promoter region of Perlecan to modulate
expression or to
decrease the biological stability of the protein. In addition, the selective
inhibitors of
Perlecan can decrease the biosynthesis of Perlecan by decreasing the
transcription of the
Perlecan gene, decreasing the biological stability of the Perlecan mRNA or
decreasing the
translation of Perlecan mRNA into protein. Furthermore, the selective
inhibitors of Perlecan
can block or decrease the effects of agents or proteins that increase the
activity of Perlecan.
Table 1 presents exemplary that have been shown to induce HSPG.
Table 1.
S. No Compound Fold induced at 10
M
1 2.9
° ~ I ~ S'~ o
I ~ I °~.° ~ ° NH
N
CH3
F
2 2.8
~ I ~ S~o
( °~° ~ ° NH
N
CH3 I
CI
3 1.181
° ~ I ~ S~o
I °~° ~ ° NH
N W F
CH3
F
230
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
4 , F 1.66
Me
N ~ I F
~O
I / I O~O ~ s
O I ~ s NH
CI
O
2.6
° / I ~ S~o
I o~H \ ° NH
N
CH3 (
F
B. Heparanase Modulation
HSPGs are important components of the subendothelial extracellular matrix and
the
basement membrane of blood vessels. Rosenberg et al., 99 J. CLIN. INVEST. 2062-
70
5 (1997). Basement membranes are continuous sheets of extracellular matrix
composed of
collagenous and noncollagenous proteins and proteoglycans that separate
parenchyma) cells
from underlying interstitial connective tissue. They have characteristic
permeabilities and
play a role in maintaining tissue architecture.
In addition to HSPGs, the basal lamina consists predominantly of a complex
network
to of adhesion proteins, fibronectin, laminin, collagen and vitronectin. Wight
et al., 6 CURB.
OPIN. LIPIDOL. 326-334 (I995). Heparan sulfate (HS) is an important structural
component
of the basal lamina. Each of the adhesion proteins interacts with HS side
chains of HSPGs
within the matrix. Thus, HSPGs function as a barrier to the extravasation of
metastatic and
inflammatory cells. ~ Cleavage of HS by the endoglycosidase Heparanase
produced by
metastatic tumor cells and inflammatory cells destroys the filtering
properties of the lamina.
In addition, the degradation of the HS may assist in the disassembly of the
extracellular
matrix and thereby facilitate cell migration by allowing blood home cells to
escape into the
bloodstream. Vlodavsky et al., 12 INVASION METASTASIS 112-127 (1992).
Heparanase activity has been described in a number of tissues and cell types
2o including liver, placenta, platelets, fibroblasts, neutrophils, activated T
and B-lymphocytes,
monocytes, and endothelial cells (7-16). Nakajima et al., (31) CANCER LETT.
277-283
(1986); Nakajima et al., 36 J. CELL. BIOCIIEM. 157-167 (1988); Ricoveri et
al., 46 CANCER
231
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
IZES. 3855-3861 (1986); Gallagher et al., 250 BIOCHBM. J. 719-726 (1988);
Dempsey et al.,
GLYCOBIOLOG1' 467 (2000); Goshen et aL, 2 MoL. HUM. IZEPROD. 679 (1996);
Parish et
al., 76 IM1VILINOL CELL BIOL. 104-113 (1998); Gilat et al., 181 J. ExP. MED.
1929-1934
(1995); Graham, et al., 39 BIOCHEM. MoL. BIOL. INT. 56371 (1996); Pillarisetti
et al., 270
5 J.BIOL.CHEM. 29760-29765 (1995).
There is increasing interest in heparan sulfate compounds and their related
enzymes
due to a possible relationship between changes in normal activity and tumor
invasiveness and
tumor metastatic activity. An important process in tissue invasion by blood-
borne tumor
cells and white cells involves their passage through the vascular endothelial
cell layer and
1o subsequent degradation of the underlying basal lamina or basement membranes
and
extracellular matrix with a battery of secreted proteases and glycosidases.
Nakajinia et al.,
220 SCIENCE 611-613 (1983); Vlodavsky et a1.,12 INVASION METASTASIS 112-127
(1992).
Heparanase activity was shown to correlate with the metastatic potential of
animal
and human tumor cell lines. Nakajima et al., 31 CANCER LETT. 277-283 (1986);
Nakajima et
al., 212 PROD CLIN BIOL 1ZES. 113-122 (1986); Freeman et al., 325 BIOCHEM. J.
229-237
(1997); Vlodavsky et al., 5 NAT. MED. 793-802 (1999); Hulett et al., 5 NAT
MED. 803-809
(1999). It also is known to regulate growth factor activity. Many growth
factors remain
bound to heparan sulfate in storage form and are disassociated by Heparanase
during
angiogenesis, improving the survival rate of cancer cells.
Serum Heparanase levels in rats were higher by more than an order of magnitude
after injection of the rats with highly metastatic mammary adenocarcinoma
cells. In
addition, Heparanase activity in the sera of rats bearing MTLn3 tumors
correlated well with
the extent of the metastases. Moreover, serum/urine Heparanase activity in
cancer patients
was shown to be 2-4 fold increased in particular where tissue metastases were
present.
Because the cleavage of HS appears to be essential for the passage of
metastatic tumor cells
and leukocytes through basement membranes, studies of Heparanase inhibitors
provides the
potential of developing a novel and highly selective class of anti-metastatic
and anti-
inflammatory drugs.
Thus, the present invention further relates to compounds that modulate
Heparanase
3o activity. Such compounds are useful in treating and/or preventing cancer
including, but not
limited to, malignant and non-malignant cell growth, leukemia, acute leukemia,
acute
lymphoblastic leukemia (ALL), B-cell, T-cell or FAB ALL, acute myeloid
leukemia (AML),
232
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
chromic myelocytic leukemia (CML), chronic lymphocytic leukemia (CLL), hairy
cell
leukemia, myelodyplastic syndrome (MDS), a lymphoma, Hodgkin's disease, a
malignant
lymphoma, non-hodgkin's lymphoma, Burkitt's lymphoma, multiple myeloma,
Kaposi's
sarcoma, colorectal carcinoma, pancreatic carcinoma, nasopharyngeal carcinoma,
malignant
histiocytosis, paraneoplastic syndrome/hypercalcemia of malignancy, solid
tumors,
adenocarcinomas, sarcomas, malignant melanoma, hemangioma, metastatic disease,
cancer
related bone resorption, cancer related bone pain,.
According to another aspect of the present invention, the compounds
contemplated
hereby are useful in modulating heparanase activity as a means for treating
and preventing
autoimmune diseases.
By way of background, in the normal course of resolution of a disease in an
infected
tissue, local resting immune effector cells in the body become activated after
recognizing
antigens of the infecting organism as foreign. Upon activation these effector
cells in the
body synthesize and secrete signaling molecules (chemokines, lymphokines and
cytokines),
which attract additional immune effector cells to the site of infection, where
they are also
activated. Once activated, these immune effector cells become capable of
exiting the
vasculature and entering the infected tissue where they begin to attract and
destroy the
infectious agent and the infected tissue. This process continues until the
infection is
eradicated.
2o Occasionally, however, the immune system malfunctions or overreacts to the
initial
insult, wluch can lead to the initiation of debilitating and life threatening
chronic and acute
diseases. This can occur when (1) the immune system mistakenly identifies a
cell surface
molecule on normal tissue as a foreign molecule, (2) the synthesis arid
secretion of
chernokines, cytokines, and lymphokines is not shut down after the eradication
of the disease,
or (3) the immune system overreacts to the apparent infection and destroys
vast quantities of
surrounding normal tissue.
In normal activity, the activated effector cells attract other effector cells
to the blood
vessels near the infection. To be "effective" these activated cells must leave
the blood
vessels and enter the infected tissue. The process of exiting the circulation
and entering the
3o inflamed tissue involves two distinct steps. First, the immune effector
cells must bind to the
luminal/apical surface of the blood vessel walls. This is accomplished through
the
233
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
interaction of adhesion molecules on the immune effector cells with their
locally upregulated
cognate receptors on the endothelial cells lining the vasculature near the
site of infection.
Second, after binding to the apical surface and before entering the inflamed
tissue, the
immune effector cells must breach the basement membrane (BM) and extracellular
matrix
(ECM) that surround the basal portion of the blood vessels and give the
vessels their shape
and strength. The BM and ECM consists of structural proteins embedded in a
fiber
meshwork consisting mainly of complex carbohydrate containing structures
(glycosaminoglycans), of which the main constituent is heparin sulfate
proteoglycan (HSPG).
In order to breach this barrier the immune effector cell must weaken or
destroy it, which is
to accomplished through the local secretion of proteases and heparanase(s).
Thus, the inhibition of heparanase using the compounds of the present
invention finds
utitlity in treating arthritis and other autoimmune diseases. More
specifically, the compounds
of the present invention are useful in the treatment or prophylaxis of at
least one
autoimmune-related disease in a cell, tissue, organ, animal, or patient
including, but not
limited to, rheumatoid arthritis, juvenile rheumatoid arthritis, systemic
onset juvenile
rheumatoid arthritis, psoriatic arthritis, ankylosing spondilitis, gastric
ulcer, seronegative
arthropathies, osteoarthritis, inflammatory bowel disease, ulcerative colitis,
systemic lupus
erythematosis, antiphospholipid syndrome, iridocyclitis/uveitis/optic
neuritis, idiopathic
pulmonary fibrosis, systemic vasculitis/wegener's granulomatosis, sarcoidosis,
orchitis/vasectomy reversal procedures, allergic/atopic diseases, asthma,
allergic rhinitis,
eczema, allergic contact dermatitis, allergic conjunctivitis, hypersensitivity
pneumonitis,
transplants, organ transplant rejection, graft-versus-host disease, systemic
inflammatory
response syndrome, sepsis syndrome, gram positive sepsis, gram negative
sepsis, culture
negative sepsis, fungal sepsis, neutropenic fever, urosepsis, meningococcemia,
trauma/hemorrhage, burns, ionizing radiation exposure, acute pancreatitis,
adult respiratory
distress syndrome, rheumatoid arthritis, alcohol-induced hepatitis, chronic
inflammatory
pathologies, Crolm's pathology, sickle cell anemia, diabetes, nephrosis,
atopic diseases,
hypersensitity reactions, allergic rhinitis, hay fever, perennial rhinitis,
conjunctivitis,
endometriosis, asthma, urticaria, systemic anaphalaxis, dermatitis, pernicious
anemia,
hemolytic disesease, thrombocytopenia, graft rejection of any organ or tissue,
kidney
translplant rejection, heart transplant rejection, liver transplant rejection,
pancreas transplant
rejection, lung transplant rejection, bone marrow transplant (BMT) rejection,
skin allograft
234
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
rejection, cartilage transplant rejection, bone graft rejection, small bowel
transplant rejection,
fetal thymus implant rejection, parathyroid transplant rejection, xenograft
rejection of any
organ or tissue, allograft rejection, anti-receptor hypersensitivity
reactions, Graves disease,
Raynoud's disease, type B insulin-resistant diabetes, asthma, myasthenia
gravis, -meditated
cytotoxicity, type III hypersensitivity reactions, POEMS syndrome
(polyneuropathy,
organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes
syndrome),
polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin
changes
syndrome, anti-phospholipid syndrome, pemphigus, scleroderma, mixed connective
tissue
disease, idiopathic Addison's disease, diabetes mellitus, chronic active
hepatitis, vitiligo,
vasculitis, post-MI cardiotomy syndrome, type IV hypersensitivity , contact
dermatitis,
hypersensitivity pneumonitis, allograft rejection, granulomas due to
intracellular organisms,
drug sensitivity, metabolic/idiopathic, Wilson's disease, hemachromatosis,
alpha-1-
antitrypsin deficiency, diabetic retinopathy, hashimoto's thyroiditis,
osteoporosis,
hypothalamic-pituitary-adrenal axis evaluation, primary biliary cirrhosis,
thyroiditis,
encephalomyelitis, cachexia, cystic fibrosis, neonatal chronic lung disease,
chronic
obstructive pulmonary disease (COPD), familial hematophagocytic
lymphohistiocytosis,
dermatologic conditions, psoriasis, alopecia, nephrotic syndrome, nephritis,
glomerular
nephritis, acute renal failure, hemodialysis, uremia, toxicity, preeclampsia,
ankylosing
spondylitis, Behcet's disease, bullous pemphigoid, cardiomyopathy, celiac
sprue-dermatitis,
chronic fatigue immune dysfunction syndrome (CFIDS), chronic inflammatory
demyelinating polyneuropathy, Churg-Strauss syndrome, cicatricial pemphigoid,
CREST
syndrome, cold agglutinin disease, discoid lupus, essential mixed
cryoglobulinemia,
fibromyalgia-fibromyositis, Graves' disease, Guillain-Barre, Hashimoto's
thyroiditis,
idiopathic thrombocytopenia purpura (ITP), IgA nephropathy, insulin dependent
diabetes,
juvenile arthritis, lichen planus, meniere's disease, multiple sclerosis,
pemphigus vulgaris,
polyarteritis nodosa, Cogan's syndrome, polychondritis, polyglandular
syndromes,
polymyalgia rheumatica, polymyositis arid dermatomyositis, primary
agammaglobulinemia,
Raynaud's phenomenon, Reiter's syndrome, rheumatic fever, Sjogren's syndrome,
stiff man
syndrome, Talcayasu arteritis, temporal arteritis/giant cell arteritis,
Wegener's
3o granulomatosis; okt3 therapy, anti-cd3 therapy, cytokine therapy,
chemotherapy, radiation
therapy (for example, including but not limited toasthenia, anemia, cachexia),
chronic
salicylate intoxication,.
235
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
1. Heparanase Assays
The present invention further relates to methods for assaying Heparanase
activity. In
this regard, the effect of the compounds of the present invention may be
evaluated using such
assays. Future candidate compounds also useful in the treatment methods of the
present
invention also may be evaluated using the assays discussed herein.
Furthermore, the present
invention also contemplates compositions and methods for assays measuring any
glycosidase
activity including, but not limited to, any enzymes with glycosaminoglycan-
degrading
activity, chondroitinase, heparan sulfate endoglycosidase, heparan sulfate
exoglycosidase,
polysaccharide lyases, keratanase, hyaluronidase, glucanase, amylase, and
other glycosidases
i 0 and enzymes.
Thus, in one aspect, the present invention comprises compositions and methods
for
the measurement of cellular and enzymatic activities. Such assays can be used
to measure
such activities, both qualitatively and quantitatively. Moreover, the assays
described herein
for determining the presence of such activities may be used in methods for
diagnosing
metastases, metastatic potential and inflammatory states. In addition, the
assays of the
present invention also can be used to screen for compounds that alter, either
stimulate or
inhibit, such cellular and enzymatic activities.
Existing Heparanase assays require preparation of the radiolabeled substrate
and
separation of degraded products from the uncleaved substrate. See Goshen et
al., 2 Moi,.
2o Hunt. IZEPROD. 679-84 (1996); Nakajiina et al., 31 CANCER LETT. 277-83
(1986). Other
Heparanase assays require the biosynthetic radiolabeling of matrix-associated
HSPG and the
detection of HS chain degradation by gel-filtration analysis of radiolabeled
material released
from the matrix. Vlodaslcy et al., 12 INVASION METASTASIS 112-27 ( 1992).
Solid-phase Heparanase assays have also been developed where chemically and
biosynthetically radiolabeled heparin and HS chains were attached to a solid
support, with
release of radiolabel from the solid support being a measure of enzyme
activity. Assays
using such procedures are taught in U.S. Patent No. 4,859,581, which is
entirely expressly
herein incorporated by reference.
Previous studies have also radiolabeled both heparin and HS by iodination at
naturally occurring glucosamine residues or by N-acetylation of the partially
de-N-sulfated
substrate. Such procedures require the use of radioactive iodine, which is a
powerful ?
emitter and therefore extremely hazardous. For example, one sensitive
radioactive assay for
236
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Heparanase requires affinity chromatography of the Heparanase-cleaved products
on
columns of histidine-rich glycoprotein Sepharose. Freeman and Parish, 325
BIOCHEM. J.
229-37 (1997).
There are also some non-radioactive assays available for Heparanase. One assay
for
Heparanase involves measuring the optical density (at 230 nm) of unsaturated
uronic acids
formed during degradation of heparin. A color-based assay for measuring
Heparanase
activity utilizes heparin's ability to interfere with color development during
the interaction of
protein with the dye Coomassie brilliant blue. Kahn and Newman, 196 ANAL.
BIOCHEM.
373-76 (1991).
In another Heparanase assay, a composition comprising biotin-HS is mixed with
a
biological sample such as a tumor sample, bodily fluid, or other fluid
suspected of having
Heparanase activity, to form a reaction mixture. Tlus sample may be pretreated
to remove
contaminating or reactive substances such as endogenous biotin. After
incubation, an aliquot
or portion of the reaction mixture is removed and placed in a biotin-binding
plate.
After washing with buffers, a Streptavidin-enzyme conjugate is added to the
biotin-binding
plate. Reagents for the enzyme are added to form a detectable color product.
For example,
a decrease in color formation, from a known standard, indicates there was
Heparanase
activity in the sample. The biotin-binding plate comprises any means for
binding biotin, for
example, to a solid surface. See WO 02/23197, which is entirely expressly
incorporated
2o herein by reference.
In general, a method for measuring Heparanase activity comprises attaching one
of a
binding partner to a substrate for the enzyme to be measured. Incubation with
a sample
comprising the enzyme to be measured allows for activity by the enzyme to be
measured in a
reaction mixture. A portion or the whole reaction mixture, depending on the
amount needed,
is then mixed with the complementary binding partner, so that the binding
partners are bound
together. This is the first binding reaction. After incubating to allow for
binding, washings
are performed. A complementary binding partner, complementary to the first
binding partner
attached to the substrate, is added. This complementary binding partner may or
may not be
the same as the first complementary binding partner. This is the second
binding reaction.
The complementary binding partner in the second binding reaction is labeled in
a manner that
is detectable. For example, the complementary binding partner is labeled with
an enzyme
that causes a detectable color change when the appropriate reaction conditions
exist.
237
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Some methods comprise the use of binding partners including, but not limited
to,
biotin and Streptavidin. Other ways of binding one of the binding partners
such as biotin,
can be used at either biotin-binding step, either binding biotin to the plate
or in detection of
the available biotins. The number of biotins, or other binding partner, that
are available for
the second binding is the quantitative result of the assay. "Complementary
binding partner"
means one of the pair of the binding partners, such as biotin and Streptavidin
or an antibody
and its antigen. The biotin is the complementary binding partner of
Streptavidin;
Streptavidin is the complementary binding partner of biotin. An antibody that
specifically
binds biotin also is a complementary binding partner of biotin.
l0 In the above method, the labeled binding partner, i.e., the enzyme labeled-
streptavidin, can be labeled with any detectable marker including but not
limited to, enzymes,
dyes, chemiluminescence, and other methods known in the art. One such method
comprises
labeling with an enzyme that produces a color change in its substrate that is
detectable. This
method is safe, easy, and effective and can be used in both qualitative and
quantitative
methods.
Using the above methods, the amount of enzyme activity in a sample can be
determined. Also, the above methods can be used to determine compounds that
can inhibit
enzyme activity. For example, a composition comprising the candidate compound
is added
to a known amount of Heparanase either before or during the incubation of the
Heparanase
and its substrate-binding parh~er. If the compound alters the activity of the
Heparanase, the
assay methods of the present invention will show a change in the amount of
detectable label.
Such assays are used for high throughput determination of the activity of
candidate
compounds. See WO 02/23197, which is entirely expressly incorporated herein by
reference.
C. Inflammation Modulation
The present invention is directed to methods and compositions comprising
compounds or molecules that have specific biological effects and are useful as
therapeutic
agents. In particular, the present invention is directed to methods and
compositions
comprising compounds or molecules that are effective in effecting
inflammation. More
particularly, the present invention is directed to methods and compositions
comprising
3o compounds or molecules that are effective in inhibiting inflammation caused
by the
accumulation or presence of glycated proteins or AGE. The present invention
also provides
compositions for and methods of treatment of biological conditions including,
but not limited
238
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
to, vascular complications of type I and type II diabetic-induced
vasculopathies, other
vasculopathies, microangiopathies, renal insufficiency, Alzheimer's syndrome,
and
inflammation-induced diseases such as atherosclerosis.
The present invention has utility in inhibiting inflammation or cell
activation by
glycated proteins or AGE. Pharmacological inhibition of AGE-induced cell
activation
provides the basis for therapeutic intervention in many diseases, most notably
in diabetic
complications and Alzheimer's disease. Therapeutic approaches for inhibition
of AGE-
induced inflammation include, but are not limited to, blocking the glycation
of proteins,
blocking AGE interactions with receptors and blocking AGE-induced signaling or
signaling-
l0 associated inflammatory responses.
For example, a method of the present invention is to block AGE effects by
inhibiting
AGE induced signaling. The sequence of these signaling events leading to
inflammation is
not clear, but inhibition of these signaling events leads to reduced or no
inflammatory results.
Compounds that block AGE-induced up-regulation of inflammatory molecules were
determined using screening assays. The present invention comprises methods and
compositions comprising compounds or molecules such as the thizolidinedione
compounds
provided herein.
Other aspects of the present invention comprise methods and compositions
comprising compounds that block glycated protein-induced inflammation. Further
aspects of
the present invention comprise thizolidinedione compounds that are capable of
inhibiting
AGE effects. Still further aspects of the present invention employ
compositions comprising
the compounds of the formulae contemplated hereby that block glycated protein-
induced
inflammation.
Enhanced formation and accumulation of glycated proteins and AGE are thought
to
play a major role in the pathogenesis of diabetic complications, and
atherosclerosis, leading
to the development of a range of diabetic complications including nephropathy,
retinopathy,
and neuropathy. There is ample in vivo evidence that suggests that diabetes-
related
complications can be reduced by 1) preventing glycation of proteins, 2) by
breaking the
cross-links in glycated proteins, or 3) by blocking glycated protein
interaction with receptors.
3o Despite the importance of AGE in the pathogenesis of diabetic
microangiopathies, there are
no currently available medications known to block AGE formation.
239
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Aminoguanidine, which prevents AGE formation, is actively pursued as a therapy
for
diabetic vasculopathy. However it is not clear whether this drug would affect
normal glucose
metabolism or glycosylation of proteins. Moreover, some studies show that
although
aminoguanidine reduces AGE formation, it did not inhibit glomerular basement
thickness in
diabetic rats nor improved endothelial function. See, for example, Birrell et
al., 43
DIABETOLOGIA 110-16 (2000); Wada et al., 42 DIABETOLOGIA 743-47 (1999); Soulis
et al.,
50 KIDNEY INT. 627-34 ( 1996).
In addition to the AGE formation inhibitors, AGE cross-link breakers are also
actively pursued as a therapy for vasculopathy. N-Phenacylthiazolium bromide
(PTB) is a
to prototype AGE cross-link breaker that reacts with and cleaves covalent AGE-
derived protein
cross-links. Although PTB reduced AGE accumulation, it did not prevent
vascular
permeability. Cooper et al., 43 DIABETOLOGIA 660-64 (2000); ~turai et al.,
49(8)
METABOLISM 996-1000 (2000).
Inhibition of reactions with receptors of AGE is an alternative approach to
treatment
of related pathologies. RAGE, a known receptor for AGE, is a possible
therapeutic target.
Blocking RAGE also inhibited AGE-induced inflammation. However, because of the
multiple functions of RAGE and possible long teen side effects of accumulated
AGE in
plasma, this method is not currently pursued in humans. Using the methods and
compositions of the present invention, more speciEc inhibitory compounds can
be used for
treatments.
Endothelium is the target organ of damage in diabetes. See Laight et al., 15
DIABETES METAB. RES. REV. 274-82 (1999); Stehouwer et al., 34 CARDIOVASC. 55-
68
(1997). Up-regulation of molecules involved in endothelial inflammation, such
as IL-6 and
monocyte chemoattractant protein-1 (MCP-1) leads to endothelial dysfunction
and
vasculopathy. See Stehouwer et al., 34 CARDIOVASC. 55-68 (1997); Libby, 247 J.
INTERN.
MED. 349-58 (2000); Van Lente, 293 GLII~IICA. CHIMICA. ACTA. 31-52 (2000).
An overall approach to the understanding and treatment of diabetes and its
complications is to interfere in the regulation of genes, such as those
leading to the
production of cytokines, and to inhibit AGE-induced inflammation.
The effectiveness of the compounds of the present invention in inhibiting
glycated
protein- and AGE-induced inflammation can be determined using the assays
described herein
and in U.S. Provisional Patent Application Serial No. 60/259,306, which is
incorporated by
240
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
reference in herein its entirety. Such assays comprise measurement of the
specific activity of
biological components involved in a known cellular response. The assays
provide a
measurable response in which the activity of the compounds is determined. One
aspect of
the present invention comprises measurement of the effects of compounds on an
inflammatory response by cells to the presence of a stimulating agent. Yet
another aspect of
the present invention includes an assay comprising endothelial cells that are
stimulated by the
addition of a glycated protein, the stimulating agent. The endothelial cells
respond by
producing specific cytokines. The amount of cytokines produced is determined
by
measurement protocols known to those skilled in the art. The compounds of the
present
to invention are then added to the assay and the production of cytokines is
measured. From the
comparison of the assay without the compound with the assay with the compound,
the
biological effect of the compound can be determined. The compound may have an
inhibitory
effect, a stimulatory effect, or no effect at all. Compounds for treatment of
inflammation
include those that have an inhibitory effect.
Assays comprise endothelial cells that are stimulated in an inflammatory
response by
the presence of the glycated protein, glycated human serum albumin. Such
endothelial cells
produce cytokines. A method in accordance with the present invention comprises
measurement of the amount of the cytokine IL-6, and another aspect of the
present invention
comprises measurement of the amount of the cytokine MCP-1. Preferably,
although not
required, the amount of cytokine produced is determined using immunological
methods, such
as ELISA assays. The methods of the present invention are not limited by the
type of assay
used to measure the amount of cytokine produced, and any methods known to
those skilled in
the art and later developed can be used to measure the amount of cytokines
produced in
response to the stimulating agent and to the compound having unknown activity.
IL-6 is a pro-inflammatory cytokine that is known to play a key role izi the
pathogenesis of diabetes and atherosclerosis. See Horii et al., 39 KIDNEY INT.
SUPPL. 71-5
(1993); Huber et al., 19 ARTERIOSCLER THROMB. VASC. BIOL. 2364-67 (1999);
Shikano et
al., 85 NEPHRON 81-5 (2000); Picleup et al., 8(67) LIFE SCI. 291-300 (2000).
IL-6 also
promotes the growth of renal mesangial cells thus contributing to nephropathy.
See Kado et
3o al., 36 AcTa. DIABETOL. 67-72 (1999). The serum IL-6 level in diabetic
subjects was
significantly higher than in normal healthy controls (3.48 +/- 3.29 pg/mL vs
0.784 +/- 0.90
241
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
pg/mL, mean +/- SD). In addition the urinary IL-6 level is a good indicator of
diabetic
nephropathy. Serum IL-6 is useful in the evaluation of atherosclerosis and
nephropathy.
MCP-l, another pro-inflammatory cytokine is found highly expressed in human
atherosclerotic lesions and postulated to play a central in monocyte
recruitment into the
s arterial wall and developing lesions. See Libby, 247 J. INTERN. MED. 349-58
(2000).
Recent results show that MCP-1 also is a key pathogenic molecule in diabetic
nephropathy.
See Eitner et al., 51 KIDNEY INT. 69-78 (1997); Banba et al. 58 KIDNEY INT.
684-90 (2000).
Glycated albumin stimulates endothelial production of IL-6 and MCP-1. The
effects
of glycated albumin on IL-6 production are comparable to that of TNFa, a known
inducer of
l0 IL-6. Because of the well established role of these cytokines in vascular
diseases, screening
for compounds that block AGE-induction of these cytokines provides a novel
approach for
identifying therapeutic agents that block AGE-induced inflammation in vivo.
Once the baseline response to the stimulating agent for the production of
cytokines by
the endothelial cells is established, thus comprising the control levels for
the screening assay,
15 the methods comprise addition of compounds having unknown activities. The
effect of the
compound on the baseline response is determined by comparing the amount of
cytokine
produced in the presence of the stimulating agent and the amount of cytokine
produced in the
presence of the stimulating agent and the compound of the present invention.
In one method,
compounds that have inhibitory effects on the inflammation of the cells in the
presence of
20 glycated albumin are then used as therapeutic agents. One or more compounds
may be added
to the screening assay. Combinations or mixtures of compounds can be added.
Different
amounts and formulations of the compounds are added to determine the effects
on the
screening assay. The screening assay also may be used to determine stimulatory
compounds
or compounds that have no effects in the assay.
25 Table 2 presents examples that have inhibited Proinflammatory cytokines IL-
6 and
MCP-1.
Table 2.
S. Compound 1'roinflammat% of Concentration
No or c inhibitionin
tokine M
1 IL-6 50 4.64
NH
N
~
CH3
F
242
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
2 S MCP-1 50 7.9
0 o I ~ ~o
I \ I °~° \ ° NH
o N
cH3 I o
F
3 S IL-6 49 5
0
I w ° I °~° ~ I °~NH
o N
CH3 I o
CI
4 p o I ~ S~p MCP-1 44 5
I I O~O \ O NH
o N
CH3 I o
Br
MCP-1 50 4.4
° o I ~ S~ o
I ~ I °~° ~ ° NH
N W F
CH3 I ~ F
6 o I F MCP-1 61 5
Ne
Iw I F
o O~O ~ S
O I o / NH
CI
O
'1 MCP-1 SO 6.5
° o I ~ S'~ o
I ~ I °~° ~ ° NH
I o F
S MCP-1 50 7
° o ~ ~ S'~ o
°~NH
I o N I I w F F
CH3 o F
243
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The present invention also comprises compositions comprising the compounds
identified by the methods as having a desired activity. The compositions have
utility in
treatment of cells, tissues, or whole organisms. Such compositions are
formulated for
administration in an effective amount for treatment of conditions such as
biological
conditions including, but not limited to, vascular complications of type I and
type II diabetic
induced vasculopathies, other vasculopathies, microangiopathies, renal
insufficiency,
Alzheimer's syndrome, and inflammation-induced diseases such as
atherosclerosis. The
compositions may comprise pharmacutical adjuncts that are needed for
administration of the
compound or compounds with the desired activity.
to Moreover, the compounds of the present invention are useful in the
treatment or
prophylaxis of at least one autoimmune-related disease in a cell, tissue,
organ, animal, or
patient including, but not limited to, rheumatoid arthritis, juvenile
rheumatoid arthritis,
systemic onset juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing
spondilitis, gastric
ulcer, seronegative arthropathies, osteoarthritis, inflammatory bowel disease,
ulcerative
colitis, systemic lupus erythematosis, antiphospholipid syndrome,
iridocyclitis/uveitis/optic
neuritis, idiopathic pulmonary fibrosis, systemic vasculitis/wegener's
granulomatosis,
sarcoidosis, orchitis/vasectomy reversal procedures, allergic/atopic diseases,
asthma, allergic
rhinitis, eczema, allergic contact dermatitis, allergic conjunctivitis,
hypersensitivity
pneumonitis, transplants, organ transplant rejection, graft-versus-host
disease, systemic
inflammatory response syndrome, sepsis syndrome, gram positive sepsis, gram
negative
sepsis, culture negative sepsis, fungal sepsis, neutropenic fever, urosepsis,
meningococcemia,
traumalhemorrhage, burns, ionizing radiation exposure, acute pancreatitis,
adult respiratory
distress syndrome, rheumatoid arthritis, alcohol-induced hepatitis, chronic
inflammatory
pathologies, Crohn's pathology, sickle cell anemia, diabetes, nephrosis,
atopic diseases,
hypersensitity reactions, allergic rhinitis, hay fever, perennial rhinitis,
conjunctivitis,
endometriosis, asthma, urticaria, systemic anaphalaxis, dermatitis, pernicious
anemia,
hemolytic disesease, thrombocytopenia, graft rejection of any organ or tissue,
kidney
translplant rejection, heart transplant rejection, liver transplant rejection,
pancreas transplant
rejection, lung transplant rejection, bone marrow transplant (BMT) rejection,
skin allograft
3o rejection, cartilage transplant rejection, bone graft rejection, small
bowel transplant rejection,
fetal thymus implant rejection, parathyroid transplant rejection, xenograft
rejection of any
organ or tissue, allograft rejection, anti-receptor hypersensitivity
reactions, Graves disease,
244
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Raynoud's disease, type B insulin-resistant diabetes, asthma, myasthenia
gravis, -meditated
cytotoxicity, type III hypersensitivity reactions, POEMS syndrome
(polyneuropathy,
organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes
syndrome),
polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin
changes
syndrome, anti-phospholipid syndrome, pemphigus, scleroderma, mixed connective
tissue
disease, idiopathic Addison's disease, diabetes mellitus, chronic active
hepatitis, vitiligo,
vasculitis, post-MI cardiotomy syndrome, type IV hypersensitivity , contact
dernlatitis,
hypersensitivity pneumonitis, allograft rejection, granulomas due to
intracellular organisms,
drug sensitivity, metaboIic/idiopathic, Wilson's disease, hemachromatosis,
alpha-1-
1 o antitrypsin deEciency, diabetic retinopathy, hashimoto's thyroiditis,
osteoporosis,
hypothalamic-pituitary-adrenal axis evaluation, primary biliary cirrhosis,
thyroiditis,
encephalomyelitis, cachexia, cystic fibrosis, neonatal chronic lung disease,
chronic
obstructive pulmonary disease (COPD), familial hematophagocytic
lymphohistiocytosis,
dermatologic conditions, psoriasis, alopecia, nephrotic syndrome, nephritis,
glomerular
is nephritis, acute renal failure, hemodialysis, uremia, toxicity,
preeclampsia, ankylosing
spondylitis, Behcet's disease, bullous pemphigoid, cardiomyopathy, celiac
sprue-dermatitis,
chronic fatigue immune dysfunction syndrome (CFIDS), chronic inflammatory
demyelinating polyneuropathy, Churg-Strauss syndrome, cicatricial pemphigoid,
CREST
syndrome, cold agglutinin disease, discoid lupus, essential mixed
cryoglobulinemia,
2o fibromyalgia-fibromyositis, Graves' disease, Guillain-Barre, Hashimoto's
thyroiditis,
idiopathic thrombocytopenia purpura (ITP), IgA nephropathy, insulin dependent
diabetes,
juvenile arthritis, lichen planus, meniere's disease, multiple sclerosis,
pemphigus vulgaris,
polyarteritis nodosa, Cogan's syndrome, polychondritis, polyglandular
syndromes,
polymyalgia rheumatica, polymyositis and dermatomyositis, primary
agammaglobulinemia,
25 Raynaud's phenomenon, Reiter's syndrome, rheumatic fever, Sjogren's
syndrome, stiff man
syndrome, Takayasu arteritis, temporal arteritis/giant cell arteritis,
Wegener's
granulomatosis; okt3 therapy, anti-cd3 therapy, cytokine therapy,
chemotherapy, radiation
therapy (for example, including but not limited toasthenia, anemia, cachexia),
chronic
salicylate intoxication,. See, for- example, The Merck Manual, 12th-17th
Editions, Merck &
3o Company, Rahway, NJ (1972, 1977, 1982, 1987, 1992, 1999); Pharmacotherapy
Handbook,
Wells et aL, eds., Second Edition, Appleton and Lange, Stamford, Conn. (1998,
2000).
D. Hyperproliferative Diseases
245
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Several of the compounds of the present invention have cytotoxic activity and,
thus,
are also useful in the treatment or prophylaxis of at least one
hyperproliferative disease in a
cell, tissue, organ, animal, or patient including, but not limited to,
malignant and non-
malignant cell growth, leukemia, acute leukemia, acute lymphoblastic leukemia
(ALL), B-
cell, T-cell or FAB ALL, acute myeloid leukemia (AML), chromic myelocytic
leukemia
(CML), chronic lymphocytic leukemia (CLL), hairy cell leukemia, myelodyplastic
syndrome
(MDS), a lymphoma, HodgkW 's disease, a malignamt lymphoma, non-hodgkin's
lymphoma,
Burkitt's lymphoma, multiple myeloma, Kaposi's sarcoma, colorectal carcinoma,
pancreatic
carcinoma, nasopharyngeal carcinoma, malignant histiocytosis, paraneoplastic
io syndrome/hypercalcemia of malignancy, solid tumors, adenocarcinomas,
sarcomas,
malignant melanoma, hemangioma, metastatic disease, cancer related bone
resorption, cancer
related bone pain, or any combination thereof.
Drug-Coated Medical Devices
The compounds of the present invention may be used alone or in combination
with
other agents along with delivery devices to effectively prevent and treat
vascular disease, and
in particular, vascular disease caused by injury and/or by transplantation.
Various medical
treatment devices utilized in the treatment of vascular disease may ultimately
induce further
complications. For example, balloon angioplasty is a procedure utilized to
increase blood
flow through an artery and is the predominant treatment for coronary vessel
stenosis. As
stated above, however, the procedure typically causes a certain degree of
damage to the
vessel wall, thereby potentially exacerbating the problem at a point later in
time. Although
other procedures and diseases may cause similar injury, exemplary compounds of
the present
invention will be described with respect to the treatment of restenosis and
related
complications following percutaneous transluminal coronary angioplasty and
other similar
arterial/venous procedures, including the joining of arteries, veins and other
fluid carrying
conduits in other organs or sites of the body, such as the liver, lung,
bladder, kidney, brain,
prostate, neck and legs.
The local delivery of a compound of the present invention and, optionally,
other
3o therapeutic agents, from a stmt prevents vessel recoil and remodeling
through the scaffolding
action of the stmt. In addition, drug-coated stems can prevent multiple
components of
neointimal hyperplasia or restenosis as well as a reduce inflammation and
thrombosis. Local
246
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
administration of a compound of the present invention and other therapeutic
agents to stented
coronary arteries also may have additional therapeutic benefit. For example,
higher tissue
concentrations of the compounds of the present invention and other therapeutic
agents may
be achieved utilizing local delivery rather than systemic administration. In
addition, reduced
systemic toxicity may be achieved utilizing local delivery rather than
systemic administration
while maintaining higher tissue concentrations. In utilizing local delivery
from a stmt rather
than systemic administration, a single procedure may suffice with better
patient compliance.
An additional benefit of combination therapeutic agent and/or compound therapy
may be to
reduce the dose of each of the therapeutic agents, thereby limiting their
toxicity, while still
to achieving a reduction in restenosis, inflammation and thrombosis. Local
stmt-based therapy
is therefore a means of improving the therapeutic ratio (efficacy/toxicity) of
anti-restenosis,
anti-inflammatory, and anti-thrombotic therapeutic agents.
Although exemplary compounds of the present invention are described herein
with
respect to the treatment of restenosis and other related complications, it is
important to note
that the local delivery of a compound of the present invention, alone or as
part of a
therapeutic agent combination, may be utilized to treat a wide variety of
conditions utilizing
any number of medical devices, or to enhance the function and/or life of the
device. For
example, intraocular lenses, placed to restore vision after cataract surgery
is often
compromised by the formation of a secondary cataract. The latter is often a
result of cellular
overgrowth on the lens surface and can be potentially minimized by combining a
drug or
drugs with the device. Other medical devices that often fail due to tissue in-
growth or
accumulation of proteinaceous material in, on and around the device, such as
shunts for
hydrocephalus, dialysis grafts, colostomy bag attachment devices, ear drainage
tubes, leads
for pace makers and implantable defibrillators also can benefit from the
device-drug/drug
combination approach. Other surgical devices, sutures, staples, anastornosis
devices,
vertebral disks, bone pins, suture anchors, hemostatic barriers, clamps,
screws, plates, clips,
vascular implants, tissue adhesives and sealants, tissue scaffolds, various
types of dressings,
bone substitutes, intraluminal devices, and vascular supports could also
provide enhanced
patient benefit using this drug-device combination approach. Any type of
medical device
may be coated in some fashion with a compound of the present invention, alone
or as part of
a therapeutic agent combination that enhances treatment over the singular use
of the device
or therapeutic agent.
247
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
In addition to various medical devices, the coatings may be used to deliver a
compound of the present invention in combination with other therapeutic agents
including
antiproliferative/antimitotic agents including natural products such as vinca
alkaloids (for
example, vinblastine, vincristine, and vinorelbine), paclitaxel,
epidipodophyllotoxins (for
example, etoposide, teniposide), antibiotics (dactinomycin (actinomycin D)
daunorubicin,
doxorubicin and idarubicin), anthracyclines, mitoxantrone, bleomycins,
plicamycin
(mithramycin) and mitomycin, enzymes (L-asparaginase which systemically
metabolizes L-
asparagine and deprives cells which do not have the capacity to synthesize
their own
asparagine); antiplatelet agents such as G(GP) IIb/IIIa inhibitors and
vitronectin receptor
antagonists; antiproliferative/antimitotic alkylating agents such as nitrogen
mustards
(mechlorethamine, cyclophosphamide and analogs, melphalan, chlorambucil),
ethylenimines
and methylinelamines (hexamethylmelamine and thiotepa), alkyl sulfonates-
busulfan,
nirtosoureas (carmustine (BGNU) and analogs, streptozocin), trazenes-
dacarbazinine (DTIC);
antiproliferative/antimitotic antimetabolites such as folic acid analogs
(methotrexate),
pyrimidine analogs (fluorouracil, floxuridine, and cytarabine), purine analogs
and related
inhibitors (mercaptopurine, thioguanine, pentostatin and 2-
chlorodeoxyadenosine
(cladribine)); platinum coordiilation complexes (cisplatin, carboplatin),
procarbazine,
hydroxyurea, mitotane, aminoglutethimide; hormones (e.g. estrogen);
anticoagulants
(heparin, synthetic heparin salts and other inhibitors of thrombin);
fibrinolytic agents (such as
tissue plasminogen activator, streptokinase and urokinase), aspirin,
dipyridamole, ticlopidine,
clopidogrel, abciximab; antimigratory; antisecretory (breveldin); anti-
inflammatory agents
such as adrenocortical steroids (cortisol, cortisone, fludrocortisone,
prednisone, prednisolone,
6a-methylprednisolone, triamcinolone, betamethasone, and dexamethasone), non-
steroidal
agents (salicylic acid derivatives, i.e., aspirin; para-aminophenol
derivatives, i.e.,
acetominophen; indole and indene acetic acids (indomethacin, sulindac, and
etodalac),
heteroaryl acetic acids (tolinetin, diclofenac, and ketorolac), arylpropionic
acids (ibuprofen
and derivatives), anthranilic acids (mefenamic acid, and meclofenamic acid),
enolic acids
(piroxicam, tenoxicam, phenylbutazone, and oxyphenthatrazone), nabumetone,
gold
compounds (auranofin, aurothioglucose, gold sodium thiomalate);
immunosuppressives
(Cyclosporine, tacrolimus (FK-506), sirolimus (rapamycin), azathioprine,
mycophenolate
mofetil); angiogenic agents: vascular endothelial growth factor (VEGF),
fibroblast growth
factor (FGF); angiotensin receptor blockers; nitric oxide donors; anti-sense
oligionucleotides
248
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
and combinations thereof; cell cycle inhibitors, mTOR inhibitors, and growth
factor signal
transduction kinase inhibitors.
Although any number of stems may be utilized in accordance with the present
invention, for simplicity, a limited number of stems will be described herein.
The skilled
artisan will recognize that any number of stems may be utilized in connection
with the
present invention. In addition, as stated above, other medical devices may be
utilized.
A stmt is commonly used as a tubular structure left inside the lumen of a duct
to
relieve an obstruction. Typically, stems are inserted into the lumen in a non-
expanded form
and are then expanded autonomously, or with the aid of a second device in
situ. A common
1o method of expansion occurs through the use of a catheter-mounted,
angioplasty balloon that
is inflated within the stenosed vessel or body passageway in order to shear
and disrupt the
obstructions associated with the wall components of the vessel and to obtain
an enlarged
lumen.
A stmt may resemble an expandable cylinder and may comprise a fenestrated
structure for placement in a blood vessel, duct or Iumen to hold the vessel,
duct or lumen
open, more particularly for protecting a segment of artery from restenosis
after angioplasty.
The scent may be expanded circumferentially and maintained in an expanded
configuration
that is circumferentially or radially rigid. The stmt may be axially flexible
and when flexed
at a band, for example, the stmt avoids any externally protruding component
parts.
2o The stmt may be fabricated utilizing any number of methods. For example,
the stent
may be fabricated from a hollow or formed stainless steel tube that may be
machined using
lasers, electric discharge milling, chemical etching or other means. The stmt
is inserted into
the body and placed at the desired site in an unexpanded form. Expansion may
be effected in
a blood vessel by a balloon catheter, where the final diameter of the stmt is
a function of the
diameter of the balloon catheter used. It should be appreciated that a stmt in
accordance
with the present invention may be embodied in a shape-memory material
including, for
example, an appropriate alloy of nickel and titanium or stainless steel.
Structures formed from stainless steel may be made self expanding by
configuring
the stainless steel in a predetermined manner, for example, by twisting it
into a braided
3o configuration. After the stmt has been formed it may be compressed to
occupy a space
sufficiently small as to permit its insertion in a blood vessel or other
tissue by insertion
means, wherein the insertion means include a suitable catheter, or flexible
rod. Upon
249
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
emerging from the catheter, the stmt may be configured to expand into the
desired
configuration where the expansion is automatic or triggered by a change in
pressure,
temperature, or electrical stimulation.
Furthermore, a stmt may be modified to comprise one or more reservoirs. Each
of
the reservoirs may be opened or closed as desired. These reservoirs may be
specifically
designed to hold the therapeutic agent/therapeutic agent combination to be
delivered.
Regardless of the design of the stmt, it is preferable to have the therapeutic
agent/therapeutic agent combination dosage applied with enough specificity and
a sufficient
concentration to provide an effective dosage in the affected area. In this
regard, the reservoir
size in the bands is sized to apply adequately the therapeutic
agent/therapeutic agent
combination dosage at the desired location and in the desired amount.
Alternatively, the entire inner and outer surface of the stmt may be coated
with
therapeutic agent/therapeutic agent combination in therapeutic dosage amounts.
The coating
techniques may vary depending on the therapeutic agent/therapeutic agent
combination.
Also, the coating techniques may vary depending on the material comprising the
stmt or
other intraluminal medical device.
One or more compounds of the present invention and, in some instances, other
therapeutic agents as a combination, may be incorporated onto or affixed to
the stmt in a
number of ways. For example, the compound may be directly incorporated into a
polymeric
2o matrix and sprayed onto the outer surface of the stmt. The compound elutes
from the
polymeric matrix over time and enters the surrounding tissue. The compound
typically
remains on the stmt for at least three days up to approximately six months,
for example,
between seven and thirty days.
Any number of non-erodible polymers may be utilized in conjunction with the
compound. According to one aspect of the present invention, the polymeric
matrix
comprises two layers. The base layer comprises a solution of polyethylene-
covinylacetate)
and polybutylmethacrylate. The compound is incorporated into this base layer.
The outer
layer comprises only polybutylmethacrylate and acts as a diffusion barrier to
prevent the
compound from eluting too quickly. The thickness of the outer layer or topcoat
determines
the rate at which the compound elutes from the matrix. Essentially, the
compound elutes
from the matrix by diffusion through the polymer matrix. Polymers are
permeable, thereby
allowing solids, liquids and gases to escape therefrom. The total thickness of
the polymeric
250
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
matrix is from about one micron to about twenty microns or greater. It is
important to note
that primer layers and metal surface treatments may be utilized before the
polymeric matrix
is affixed to the medical device. For example, acid cleaning, alkaline (base)
cleaning,
salinization and parylene deposition may be used as part of the overall
process described
above.
The polyethylene-co-vinylacetate), polybutylinethacrylate and compound
solution
may be incorporated into or onto the stmt in a number of ways. For example,
the solution
may be sprayed onto the stmt or the stmt may be dipped into the solution. The
solution may
be sprayed onto the stmt and then allowed to dry. The solution may be
electrically charged
to to one polarity and the stmt electrically charged to the opposite polarity.
In this manner, the
solution and stmt will be attracted to one another. In using this type of
spraying process,
waste may be reduced and more precise control over the thickness of the coat
may be
achieved. Other methods include spin coating and plasma polymerization.
Drug-coated stems are manufactured by a number of companies including Johnson
&
Johnson, Inc. (New Brunswick, NJ), Guidant Corp. (Santa Clara, CA), Medtronic,
Inc.
(Minneapolis, MN), Cook Group Incorporated (Bloomington, IN), Abbott Labs.,
Inc.
(Abbott Park, IL), and Boston Scientific Corp. (Natick, MA). See for example,
U.S. Patent
No. 6,273, 913; U.S. Patent Application No. 20020051730; WO 02/26271; and WO
02/26I 39, each expressly entirely incorporated herein by reference.
Expression Profiles and Microarray Methods of Use
The present invention contemplates a variety of microarrays that may be used
to
study and monitor gene expression in response to treatment with the compounds
of the
present invention. For example, the microarrays of the present invention may
be derived
from, or representative of, for example, a specific organism or cell type,
including human
microarrays, vascular microarrays, inflammation microarrays, cancer
microarrays, apoptosis
microarrays, oncogene and tumor suppressor microarrays, cell-cell interaction
microarrays,
cytokine and cytokine receptor microarrays, blood microarrays, cell cycle
microarrays,
neuroarrays, mouse microarrays, and rat microarrays, or combinations thereof.
The
3o microarrays may represent diseases including cardiovascular diseases,
vasculopathic
conditions, inflammatory diseases, autoimmune diseases, neurological diseases,
251
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
irmnunologicah diseases, various cancers, infectious diseases, endocrine
disorders, and
genetic diseases.
Alternatively, the microarrays useful in assessing the efficacy of the
compounds of
the present invention may represent a particular tissue type including, but
not limited to,
heart, liver, prostate, lung, nerve, muscle, or connective tissue; for
example, coronary artery
endothelium, umbilical artery endothelium, umbilical vein endothelium, aortic
endothelium,
dermal microvascuhar endothelium, pulmonary artery endothelium, myometrium
microvascular endothelium, keratinocyte epithelium, bronchial epithelium,
mammary
epithelium, prostate epithelium, renal cortical epithelium, renal proximal
tubule epithelium,
small airway epithelium, renal epithelium, umbilical artery smooth muscle,
neonatal dermal
fibroblast, pulmonary artery smooth muscle, dermal ~broblast, neural
progenitor cells,
skeletal muscle, astrocytes, aortic smooth muscle, mesangial cells, coronary
artery smooth
muscle, bronchial smooth muscle, uterine smooth muscle, lung fibroblast,
osteoblasts,
prostate stromal cells, or combinations thereof.
The present invention further contemplates microarrays comprising a gene
expression
profile comprising one or more polynucleotide sequences including
complementary and
homologous sequences, wherein said gene expression profile is generated from a
cell type
treated with a compound of the present invention and is selected from the
group comprising
coronary artery endothelium, umbilical artery endothelium, umbilical vein
endothelium,
aortic endothelium, dermal microvascular endothelium, pulmonary artery
endothelium,
myometrium microvascular endothelium, keratinocyte epithelium, bronchial
epithelium,
mammary epithelium, prostate epithelium, renal cortical epithelium, renal
proximal tubule
epithelium, small airway epithelium, renal epithelium, umbilical artery smooth
muscle,
neonatal dermal fibrobhast, pulmonary artery smooth muscle, dermal hbroblast,
neural
progenitor cells, skeletal muscle, astrocytes, aortic smooth muscle, mesangial
cells, coronary
artery smooth muscle, bronchial smooth muscle, uterine smooth muscle, lung
fibroblast,
osteobhasts, and prostate stromal cells.
The present invention contemplates microarrays comprising one or more protein-
binding agents, wherein a protein expression profile is generated from a cell
type treated with
3o a compound of the present invention and is selected from the group
comprising coronary
artery endothelium, umbilical artery endothelium, umbilical vein endothelium,
aortic
endothelium, dermal microvascuhar endothelium, pulmonary artery endothelium,
252
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
myometrium microvascular endothelium, keratinocyte epithelium, bronchial
epithelium,
mammary epithelium, prostate epithelium, renal cortical epithelium, renal
proximal tubule
epithelium, small airway epithelium, renal epithelium, umbilical artery smooth
muscle,
neonatal dermal fibroblast, pulmonary artery smooth muscle, dermal fibroblast,
neural
progenitor cells, skeletal muscle, astrocytes, aortic smooth muscle, mesangial
cells, coronary
artery smooth muscle, bronchial smooth muscle, uterine smooth muscle, lung
fibroblast,
osteoblasts, and prostate stromal cells.
More specifically, the present invention contemplates methods for the
reproducible
measurement and assessment of the expression of specific mRNAs or proteins in,
for
to example, a specific set of cells. One method combines and utilizes the
techniques of laser
capture microdissection, T7-based RNA amplification, production of cDNA from
amplified
RNA, and DNA microarrays containing immobilized DNA molecules for a wide
variety of
specific genes, including HSPGs such as Perlecan, to produce a profile of gene
expression
analysis for very small numbers of specific cells. The desired cells are
individually identified
and attached to a substrate by the laser capture technique, and the captured
cells are then
separated from the remaining cells. RNA is then extracted from the captured
cells and
amplified about one million-fold using the T7-based amplification technique,
and cDNA may
be prepared from the amplified RNA. A wide variety of specific DNA molecules
are
prepared that hybridize with specific polynucleotides of the microarray, and
the DNA
2o molecules are immobilized on a suitable substrate. The cDNA made from the
captured cells
is applied to the microarray under conditions that allow hybridization of the
cDNA to the
immobilized DNA on the microarray. The expression profile of the captured
cells is
obtained from the analysis of the hybridization results using the amplified
RNA or cDNA
made from the amplified RNA of the captured cells, and the specific
immobilized DNA
molecules on the microarray. The hybridization results demonstrate, for
example, which
genes of those represented on the microarray as probes are hybridized to cDNA
from the
captured cells, and/or the amount of specific gene expression. The
hybridization results
represent the gene expression profile of the captured cells. The gene
expression profile of the
captured cells can be used to compare the gene expression profile of a
different set of
3o captured cells. For example, gene expression profiles may be generated from
cells treated
(and not treated) with a compound of the present invention. The similarities
and differences
provide useful information for determining the differences between the same
cell type under
253
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
different conditions, more specifically, the change in gene expression in
response to
treatment with a compound of the present invention.
The techniques used for gene expression analysis are likewise applicable in
the
contea~t of protein expression profiles. Total protein may be isolated from a
cell sample and
hybridized to a microarray comprising a plurality of protein-binding agents,
which may
include antibodies, receptor proteins, small molecules,. Using any of several
assays known
in the art, hybridization may be detected and analyzed as described above. In
the case of
fluorescent detection, algorithms may be used to extract a protein expression
profile
representative of the particular cell type. In this regard, the change in
protein expression in
l0 response to treatment of cells with a compound of the present invention may
be evaluated.
Thus, in one aspect, the present invention relates to at least one microarray
corresponding to a population of genes isolated from a particular tissue or
cell type is used to
detect changes in gene transcription levels that result from exposing the
selected tissue or
cells to a candidate drug such as a compound of the present invention. A
biological sample
derived from an organism, or an established cell line, may be exposed to the
candidate drug
in vivo or ex vivo. Thereafter, the gene transcripts, primarily mRNA, of the
tissue or cells
are isolated by methods well-known in the art. SAMBROOK ET AL., MOLECULAR
CLONING: A
LAB. MANUAL (2001). The isolated transcripts are then contacted with a
microarray under
conditions where the transcripts hybridize with a corresponding probe to form
hybridization
pairs. Thus, the microarray provides a model of the transcriptional
responsiveness following
exposure to a particular drug candidate. A hybridization signal may then be
detected at each
hybridization pair to obtain a gene expression profile.
Gene and/or protein expression profiles and microarrays also may be used to
identify
activating or non-activating compounds of a particular gene such as Perlecan
or other HSPG.
Compounds that increase transcription rates or stimulate, maintain, or
stabilize the activity of
a protein are considered activating, and compounds that decrease rates or
inhibit the activity
of a protein are non-activating. Moreover, the biological effects of a
compound may be
reflected in the biological state of a cell. This state is characterized by
the cellular
constituents. One aspect of the biological state of a cell is its
transcriptional state. The
transcriptional state of a cell includes the identities and amounts of the
constituent RNA
species, especially mRNAs, in the cell under a given set of conditions. Thus,
the gene
expression profiles, microarrays, and algorithms discussed herein may be used
to analyze and
254
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
characterize the transcriptional state of a given cell or tissue following
exposure to an
activating or non-activating compound, specifically, a compound of the present
invention.
Microarray techniques and methods for analyzing results are well known in the
art.
See U.S. Patent Nos. 6,263,287; 6,239,209; 6,218,122; 6,197,599; 6,156,501;
5,874,219;
5,837,832; 5,700,637; 5,445,934; U.S. Patent Application Nos. 200110014461 Al;
2001/0039016 Al; 2001/0034023 Al; WO 01/94946; and WO 01/77668. See also, Haab
et
al., 2 GENOME BIOLOGY 1-12 (2001); Brown et al., 97 PROC. NATL. ACRD. SCI. USA
262-7
(2000); Getz et al., 97 PROC. NATL. ACAD. SCI. USA 12079-84 (2000); Harrington
et al., 3
CURRENT OPINION MICROBIOL 285-91 (2000); Hotter et al., 97 PROC. NATL. ACRD.
SCI. USA
l0 8409-14 (2000); MacBeath et al., 289 SCIENCE 1760-63 (2000); Duggan et al.,
21 NATURE
GENET 10-14 (1999); Lipshutz et al., 21 NATURE GENET 5-9 (1999); Eisen et al.,
95 PROC.
NATL. ACAD. SCI. USA 14863-68 (1998); Ermolaeva et al., 20 NATURE GENET. 19-23
(1998); Hacia et al., 26 NUCLEIC ACIDS lZES. 3865-66 (1998); Lockhart et al.,
NUCLEIC ACIDS
SYMP. SER. 11-12 (1998); Schena et aL, 16 TRENDS BIOTECHNOL. 301-6 (1998);
Shalom 46
PATROL. BIOL. 107-9 (1998); Welford et al., 26 NUCLEIC ACID IZES. 3059-65
(1998);
Blanchard et al., 11 BIOSENSORS BIOELECTRONICS 687-90 (1996); Lockhart et al.,
14
NATURE BIOTECHNOL. 1675-80 (1996); Schena et al., 93 PROC. NATL. ACRD. SCI.
USA
10614-19 (1996); Tomayo et al., 96 PROC. NATL. ACRD. SCI. USA 2907-12 (1996);
Schena
et al., 270 SCIENCE 467-70 (1995).
Database Creation Database Access and Associated Methods of Use
The present invention comprises a variety of methods including methods for
providing diagnostics and predictors relating to biomolecules including HSPGS,
particularly,
Perlecan. The present invention further comprises methods of providing
diagnostics and
predictors relating to the efficacy of the compounds of the present invention.
The present
invention still further contemplates methods of providing expression profile
databases, and
methods for producing such databases, for normal and diseased tissues.
The expression profile database may be an internal database designed to
include
annotation information about the expression profiles generated to assess the
effect of the
3o compounds of the present invention and through other sources and methods.
Such
information may include, for example, the databases in which a given
biomolecule was
found, patient information associated with the expression profile, including
age, cancer or
255
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
tumor type or progression, information related to a compound of the present
invention such
as dosage and administration information, descriptive information about
related cI~NAs
associated with the sequence, tissue or cell source, sequence data obtained
from external
soaxces, expression profiles for a given gene and the related disease state or
course of
disease, for example whether the expression profile relates to or signii'ies a
particular disease
state, and preparation methods. The expression profiles may be based on
protein and/or
polynucleotide microarray data obtained from publicly available or proprietary
sources. The
database may be divided into two sections: one for storing the sequences and
related
expression profiles and the other for storing the associated information. This
database may
l0 be maintained as a private database with a firewall within the central
computer facility.
However, this invention is not so limited and the expression profile database
may be made
available to the public.
The database may be a network system comiecting the network server with
clients.
The network may be any one of a number of conventional network systems,
including a local
area network (LAN) or a wide area network (WAN), as is known in the art (for
example,
Ethernet). The server may include software to access database information for
processing
user requests, and to provide an interface for serving information to client
machines. The
server may support the World Wide Web and maintain a website and Web browser
for client
use _ Client/server environments, database servers, and networks are well
documented in the
2o technical, trade, and patent literature.
Through the Web browser, clients may construct search requests for retrieving
data
from, for example, a microarray database and an expression profile database.
For example,
the user may "point and click" to user interface elements such as buttons,
pull down menus,
and scroll bars. The client requests may be transmitted to a Web application
that formats
them to produce a query that may be used to gather information from the system
database,
based, for example, on microarray or expression data obtained by the client,
and/or other
phenotypic or genotypic information. Specifically, the client may submit
expression data
based on microarray expression profiles obtained from a patient treated with a
compound of
the present invention and use the system to obtain a diagnosis based on that
information
based on a comparison by the system of the client expression data with the
expression data
contained in the database. By way of example, the system compares the
expression profiles
submitted by the client with expression profiles contained in the database and
then provides
256
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
the client with diagnostic information based on the best match of the client
expression
profiles with the database profiles. Thus, in one aspect, the comparison of
expression
profiles aids the clinician in determining the effectiveness of treatment with
a compound of
the present invention. Based on such a comparison, the clinician may alter or
adjust the
treatment regimen.
In addition, the website may provide hypertext links to public databases such
as
GenBank and associated databases maintained by the National Center for
Biotechnology
Information (NCBI), part of the National Library of Medicine as well as, any
links providing
relevant information for gene expression analysis, genetic disorders, and
scientific literature.
to Information including, but not limited to, identifiers, identifier types,
biomolecular
sequences, common cluster identifiers (GenBank, Unigene, Incyte template
identifiers, and
so forth) and species names associated with each gene, is contemplated.
The present invention also provides a system for accessing and comparing
bioinformation, specifically expression profiles and other information which
is useful in the
context of the compositions and methods of the present invention. The computer
system may
comprise a computer processor, suitable memory that is operatively coupled to
the computer
processor, and a computer process stored in the memory that executes in the
computer
processor and which comprises a means for matching an expression profile of a
biomolecular
sequence from a patient with expression profile and sequence identification
information of
2o biomolecular sequences in a database. More specifically, the computer
system is used to
match an expression profile generated from a biological sample treated with a
compound of
the present invention with expression profile and other information in a
database.
Furthermore, the system for accessing and comparing information contained in
biomolecular databases comprises a computer program comprising computer code
providing
an algorithm for matching an expression profile generated from a patient, for
example,
treated with a compound of the present invention, with expression profile and
sequence
identification information of biomolecular sequences in a biomolecular
database.
The present invention contemplates, for example, the use of a Graphical User
Interface ("GUI") for the access of expression profile information stored in a
biomolecular
3o database. The GUI may be composed of two frames. A first frame may contain
a selectable
list of biomolecular databases accessible by the user. When a biomolecular
database is
selected in the first frame, a second frame may display information resulting
from the pair-
257
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
wise comparison of the expression profile database with the client-supplied
expression
profile as described above, along with any other phenotypic or genotypic
information.
The second frame of the GUI may contain a listing of biomolecular sequence
expression information and profiles contained in the selected database.
Furthermore, the
second frame may allow the user to select a subset, including all of the
biomolecular
sequences, and to perform an operation on the list of biomolecular sequences.
The user may
select the subset of biomolecular sequences by selecting a selection box
associated with each
biomolecular sequence. The operations that may be performed include, but are
not limited
to, downloading all listed biomolecular sequences to a database spreadsheet
with
classification information, saving the selected subset of biomolecular
sequences to a user file,
downloading all listed biomolecular sequences to a database spreadsheet
without
classification information, and displaying classification information on a
selected subset of
biomolecular sequences.
If the user chooses to display classification information on a selected subset
of
biomolecular sequences, a second GUI may be presented to the user. The second
GUI may
contain a listing of one or more external databases used to create the
expression profile
databases as described above. Furthermore, for each external database, the GUI
may display
a list of one or more fields associated with each external database. The GUI
may allow the
user to select or deselect each of the one or more fields displayed in the
second GUI. The
GUI also may allow the user to select or deselect each of the one or more
external databases.
The methods of the present application father relate to the commercial and
other uses
of the compositions and methodologies of the present invention. In one aspect,
the methods
include the marketing, sale, or licensing of the compositions and
methodologies of the
present invention in the context of providing consumers, i.e., patients,
medical practitioners,
medical service providers, researchers, and pharmaceutical distributors and
manufacturers,
with expression profile databases including, in particular, databases produced
in accordance
with the use of the compounds of the present invention.
The methods of the present invention include establishing a distribution
system for
distributing the pharmaceutical compositions of the present invention for
sale, and may
optionally include establishing a sales group for marketing the pharmaceutical
composition.
The present invention provides a method of conducting target discovery
comprising
identifying, by one or more of the above drug discovery methods, a test
compound, as
258
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
described above, which modulates the level of expression of a gene or the
activity of a gene
product such as Perlecan; conducting therapeutic profiling of agents
identified, or further
analogs thereof, for efficacy and toxicity in animals; and optionally
formulating a
pharmaceutical composition including one or more of the agents identified as
having an
acceptable therapeutic profile; and optionally licensing or selling, the
rights for further drug
development of said identified agents.
The present invention is further illustrated by the following preparations and
examples, which are not to be construed in any way as imposing limitations
upon the scope
thereof. It will be clear to one of skill in the art that various other
modifications,
i0 embodiments, and equivalents thereof exist that do not depart from the
spirit of the present
invention and/or the scope of the appended claims.
PREPARATION 1
1-[4-(2-Bromo ethoxy) phenyl]-1-ethanone
O
\ / O~s Br
is
A mixture of 4-hydroxyacetophenone (20 g, 147 mmol) and potassium carbonate
(81
g, 588 mrnol) was placed into 2 L round bottomed flask and acetone (1 L) was
added. To
this reaction mixture dibromoethane (38 mL) was added in one portion, and then
the reaction
mixture was allowed to reflux for 36 hours, under nitrogen atmosphere. The
reaction mixture
20 was cooled to room temp, and filtered off, residue was washed with acetone
(2 ~ 100 mL),
and the filtrates were combined and concentrated under reduced pressure. The
crude was
chromatographed over silica gel by using 10-15 % ethyl acetate / pet. ether (2
L), affording
the title compound 7 g (20 %) as a white solid. Mp. 58-61°C.
IR: ? max (fir, cm 1): 1678, 1603;
25 1H NMR (200 MHz, CDC13): d 7.93 (d, J= 8.87 Hz, 2H), 6.93 (d, J= 8.87 Hz,
2H),
4.35 (t, J= 6.18 Hz, 2H), 3.67 (t, J= 6.28 Hz, 2H), 2.55 (s, 3H);
Mass (CI method, I-butane): 245 (MH+, 100), 243 (M'-, 100).
259
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
PREPARATION 2
2-(3,4-Dimethoxyphenyl)-3-hydroxy-5,7-dimethoxy-4H 4-chromenone
OMe
OMe O
Step (i):
3-[(6-O-(deoxy-a-L-manopyranosyl)-13-glucopyranosyl)oxy)-2-(3,4-
dimethoxyphenyl)-5,7-
dimethoxy-4H-1-benzopyran-4-one
OMe O
A mixture of Rutin hydrate (1) (80 g, 120.5 mmol) and potassium carbonate (320
g,
2319 mmol) was placed into a 2 L three neck round bottom flask, fitted with a
reflux
condenser with nitrogen atmosphere and dropping funnel and acetone (1.5 L) was
added. To
this reaction mixture dimethyl sulfate (160 mL) was added dropwise. The
reaction mixture
was refluxed at 60°C for 68 hours. Then the reaction mixture was cooled
to 25°C and the
solid separated was filtered. The residue was washed with acetone (1 L)
followed by
methanol (500 mL), filtrates were combined and concentrated under reduced
pressure
affording the title compound (80 g, 91 %), as a yellow gummy solid.
Step (ii):
2-(3,4-dimethoxyphenyl)-3-hydroxy-5,7-dimethoxy-4H 4-chromenone
The compound obtained in step (i) (80 g, 110 mmol) was placed in a 2 L single
neck
round bottom flask and hydrochloric acid (20 %, 1 L) was added at 25
°C. The reaction
2o mixture was allowed to reflux at 100°C for 2 hours and then cooled
to 25 °G. The solid that
separated was filtered, washed with isopropanol (200 mL) and dried under
vacuum to
affording the title compound (27.5g, 70%) as a pale yellow solid. Mp. 192-
194°C.
IR: ? max (fir, em ~): 3279, 2925, 1609, 1516;
260
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
1H NMR (200 MHz, CDC13): d 7.83-7.79 (m, 2H), 7.00 (d, J= 9.14 Hz, 1H), 6.56
(d, J
= 1.88 Hz, 1H), 6.36 (s, 1H), 3.99 (s, 6H), 3.96 (s, 3H), 3.93 (s, 3I~;
Mass (CI method, I-butane): 359 (M+, 100).
s PREPARATION 3
1-(4- { 2-[2-(3,4-Dimethoxyphenyl)-5,7-dimethoxy-4-oxo-4H-3-chromenyloxy]
ethoxy}
phenyl)-1-ethanone
M
O
A mixture of compound obtained in Preparation 2 (25 g, 69.6 mmol), a compound
io obtained in Preparation 1 (21.5 g, 88.4 mmol) and potassium carbonate (77
g, 557 mmol)
was placed in a I L round bottomed flask and DMF (400 mL) was added to the
reaction
mixture. The reaction mixture was heated to 80°C with stirring for 3
hours under a nitrogen
atmosphere. The reaction mixture was cooled to 25°C and poured slowly
into ice-cold water
(1 L). The separated solid was filtered and washed with water (2 X 500 mL). It
was
is triturated with methanol and filtered to afford the title compound (31.5 g,
87 %), as a pale
brown solid, after drying under vacuum. Mp.143-I44 °C.
IR: ? maX (KBr, cW 1): 1668, 1624, 1600;
1H NMR (200 MHz, CDC13): d 7.87 (d, J= 8.79 Hz, 2H), 7.71-7.67 (m, 2H), 6.87-
6.76 (m, 3H), 6.51 (s, 1H), 6.36 (s, 1H), 4.47 (d, J= 4.40 Hz, 2H), 4.29 (t,
J= 4.40 Hz,
20 2H), 3.97 (s, 3H), 3.90 (s, 9H), 2.54 (s, 3H);
Mass (CI method, I-butane): 521 (MH+, 30), 385 (100).
261
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
PREPARATION 4
4-Fluorophenylacetate
o~
F
4-Fluorophenol (20 g, 178.5 mmol) was placed into single neck 1 L round
bottomed
flask to which sodium hydroxide solution (12 g in 100 mL water) was added. The
reaction
mixture was stirred for 5-10 min at 25°C and crushed ice (50 g) was
added to it followed by
acetic anhydride (30 mL). The reaction mixture was stirred for 15 min at the
same
temperature and water (300 mL) followed by hydrochloric acid (6 N, 60 mL) was
added to it.
The mixture was extracted with chloroform (3 ~ 100 mL), combined extracts were
dried over
sodium sulphate and concentrated under reduced pressure to afford the title
compound (26 g,
95 %) as a white solid.
IR: ? ",aX (I~Br, cm 0:1764;
IH NMR (200MHz, CDCI~): 7.05 (s, 2H), 7.01 (s, 2H), 2.27 (s, 3H);
Mass (CI method, I-butane): 155(M+', 100).
PREPARATION 5
(2-Hydroxy-4-fluoro phenyl)-1-ethanone
OH
FI/
O
A mixture of 4-fluorophenylacetate obtained in Preparation 4 (25 g, 223 mmol),
aluminium chloride (89 g, 670 mmol), was placed into 1 L single neck round
bottom flask,
fitted with an air condenser and calcium chloride guard tube. The reaction
mixture was
slowly heated to 120-125°C over 30 minutes, and then to 165°C
(generation of HCl gas was
observed). The mixture was stirred at the same temperature for 30 min and then
cooled to
room temp. Water (500 mL) was added to it followed by 6 N HCl (150 mL). The
mixture
was extracted with chloroform (3 ~ 200mL), combined organic layers were dried
over
sodium sulfate and concentrated under reduced pressure to afford the title
compound (21 g,
84 %) as a white solid.
IR: ? maX (KBr, crri I): 3442, 1650;
262
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
1H NMR (200MHz, CDC13): 11.98 (s, H, D20 exchangeable), 7.43-7.37 (m, 1H),
7.27-7.17 (m, 1H), 6.98-6.9I (m, 1H), 2.62 (s, 3H);
Mass (CI method, I-butane): 155 (M+1, 47).
s PREPARATION 6
1-(5-Fluoro-2-hydroxyphenyl)-3-(4-methoxyphenyl)-2-propen-1-one
OMe
OH
F
O
To a mixture of (2-hydroxy-4-fluoro phenyl)-1-ethanone (3 g, 19.7 mmol)
obtained in
Preparation s, and 4-fluorobenzaldehyde (4.37 g, 19.7 mmol) in methanol was
slowly added
to sodium hydroxide solution at 0 °C, under NZ atm. The reaction
mixture was allowed to stir
for 10 hours at 0-10 °C. Water (100 mL) was added to it followed by 6 N
HCl (ls mL).
Solid separated was filtered off and dried under vacuum to afford 3 g (41 %)
of the title
compound as a yellow solid.
IR: ? maX (KBr, crri 1): 3s00, 1642;
15 1H NMR (200MHz, CDCl3): 12.6 (s, 1H, D20 exchangeable), 7.92 (d, J=15.3 Hz,
2H), 7.76-7.SS (m, 3H), 7.41 (d, J=15.3 Hz, 2H), 7.0-6.94 (m, 2H), 3.87 (s,
3H);
Mass (CI method, I-butane): 272 (M+, 100%).
PREPARATION 7
20 6-Fluoro-2-(4-rnethoxy phenyl)- 3-hydroxy - 4H 4-chromenone
OMe
O
F OH
O
The chalcon product (3.0 g, 11 mmol), obtained in Preparation 6, was dissolved
in
methanol (30 mL) and cooled to 0 °C. To this mixture was added sodium
hydroxide solution
(20 mL, 20%) and then the reaction mixture was stirred at the same temperature
for s-10
2s min. Hydrogen peroxide was added to this mixture and stirring continued at
0-10°C for 1
hours. Water (100 mL) was added to it followed by 6 N HCl (30 mL). Separated
solid was
263
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
filtered off and dried under vacuum to afford 1.0 g (32 %) of the title
compound as a yellow
solid.
IR: ? max (I~Br, crri I):3261, 1602, 1559;
IH NMR (200MHz, CDC13): X8.23 (d, J=9.13 Hz, 2H), 7.90-7.85 (m, 1H),7.63-7.56
(m, 1H), 7.48 -7.38 (m, 1H), 7.06 (d, J=9.13 Hz, 2H), 3,91 (s, 3H);
Mass (CI method, I-butane):287(M+l, 100%}.
PREPARATION 8
1-(4-{2-[6-Fluoro-2-(4-methoxyphenyl)-4-oxo-4H 3-chromenyloxy} phenyl)-1-
ethanone
s OMe
O
O W
O
O
A mixture of the product obtained in Preparation 7 (0.3 g, 1.04 mmol), a
compound
obtained in Preparation 1 (0.25 g, 1.04 mmol) and potassium carbonate (0.86 g,
6.2 mmol)
was placed in 1 L round bottomed flask and DMF (15 mL) was added to the
mixture. The
mixture was heated to 80°C with stirring for 3 hours under a nitrogen
atmosphere. The
reaction mixture yeas cooled to 25°C and poured slowly into ice-cold
water (1 L). The solid
that separated was filtered and washed with water (2 ~ 500 mL). It was
triturated with
methanol and filtered to afford the title compound (0.4 g, 85 %), as a pale
brown solid, after
drying under vacuum.
2o PREPARATION 9
N-Methyl anthranilic acid
~~OOH
NHMe
To a solution of methyl-N-methyl anthranilate (20 g, 121 mmol) in methanol
(100
mL), placed in a 250 mL single neck round bottomed flask, was added a solution
of NaOH
(9.69 g, 242 mmol) in 25 mL of water at 0-10 °C. The reaction mixture
was heated to 50°C
for 6 hours and then cooled to room temperature. Methanol was removed
completely from
264
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
the reaction mixture and water (100 mL) was added to it. The mixture was
washed with
ether (3 ~ 50 mL) and the aqueous layer was acidified (pH ~ 5-6) with ice cold
2 N HCI. The
solid that separated was filtered, washed with water (2 ~ 50 mL) and dried
under vacuum to
afford the title compound 17.0 g (93 %) as a white color solid.mp-178-180
°C.
IH NMR (200MHz, CDCl3): 87.99 (dd, 1H, J = 1.34 Hz), 7.46-7.25 (m, 1H), 6.70-
6.58 (m, 2H), 2.93 (s, 3H);
Mass (CI method): 152 (M+1, 100%).
PREPARATION 10
2-Bromo-1-(4-methylphenyl)-1-ethanone
O
Br
Me
To a stirring solution of 20 g (150 mmol) of 4-methylacetophenone in 100 mL of
glacial acetic acid was added catalytic amount of HBr (0.5 mL) followed by
21.40g (134
mmol) of bromine dissolved in acetic acid (30 mL) dropwise at 10-15°C.
The reaction
mixture was stirred at 25-35°C for 5 hrs, then poured into water (100
mL). The solid that
separated was filtered to give the required product (20 g, 65%).
IH NMR (200MHz, CDCl3):87.88(d, J=8.3Hz, 2H), 7.29(d, BHz, 2H), 4.42(s, 2H),
2.41(s, 3H).
2o PREPARATION 11
2-(4-Methyl phenyl)-2-oxo ethyl -2-methylaminobenzoate
Me
COO
O
NHMe
To a solution of N-methyl anthranilic acid (lO.Og, 66 mmol), obtained in
Preparation
9, in 100 mL of dimethyl formamide, placed in a 250 mL single neck round
bottomed flask
was added a solution of KOH (3.89g, 69 mmol) in 10 mL of water and the mixture
was
stirred for 45 min at 25-35°C. The mixture was cooled to 10°C,
and the bromoketone (16.9 g,
79 mmol), obtained in Preparation 10, was added to it. The reaction mixture
was stirred for
265
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
hours at room temperature and then poured in ice water (500 mL). The solid
that
separated out was filtered, washed with water (2 x 100 mL) and dried under
vacuum to
afford the title compound (11.0 g, 58 %) as a white color solid. Mp: 96-
98°C.
IR (KBr, cm 1): 3382, 1684, 1674;
s 1H NMR (200MHz, DMSO-d6): 8 7.91-7.87 (m, 3H), 7.47-7.34 (m, 3H), 6.75-6.57
(m, 2H), 5.62 (s, 2H), 3.32 (s, NH), 2.83 (d, J=4.3 Hz, 3H), 2.38 (s, 3H};
Mass (CI method): 284 (M+l, 100%).
PREPARATION 12
10 3-Hydroxy-1-methyl-2-(4-methylphenyl)-1,4-dihydro-4-quinolinone
Me
Me
w N w I
I/ I
OH
O
Polyphosphoric acid (PPA, 80 g) was heated to 140°C under nitrogen
atmosphere in a
250 mL single neck round bottom flask. 2-(4-Methyl phenyl)-2-oxo ethyl -2-
methylamW obenzoate (10 g, 35 mmol) obtained in Preparation 11 was added in
small
is portions and the mixture was stirred at 140 °C for 6 hours. The
mixture was cooled to 25-
35°C and ice cooled water was added to the mixture and stirred for 30
min. Solids that
separated were filtered, washed with water and dried under vacuum to afford
the title
compound (6.0 g, 73 %) as brown solid. Mp. 216-218°C.
IR (KBr, cm I): 3433, 1598;
2o IH NMR (200MHz, DMSO-d6): 8 8.44 (d, J=8.3 Hz, 1H), 8.06-7.91 (m, 2H), 7.75-
7.61 (m, 1H), 7.48-7.35 (m, 4H), 5.21 (bs, OH), 3.70 (s, 3H), 2.43 (s, 3H);
Mass (CI method): 266 (M+1, 100%).
PREPARATION 13
25 4-(2-Bromoethoxy)benzaldehyde
~\
\ / o~Br
A mixture of 4-hydroxybenzaldehyde (10.0 g, 82 mrnol) and potassium carbonate
(46
g, 326 mmol) was placed into 2L round bottom flask, and DMF (150 mL) was
added. The
266
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
mixture was stirred for 45 min. and dibromoethane (46 g) was added in one
portion, then the
reaction mixture was allowed to stir at 25-35°C for 96 hrs under a
nitrogen atmosphere. The
reaction mixture was cooled to 25-35°C and then poured into water (500
mL). The mixture
was extracted with EtOAc (3 x 100 mL), combined organic layers were dried over
anhydrous
Na2S04 and concentrated under reduced pressure. The crude product was purified
by column
chromatography over silicagel by using 10-15% ethyl acetate / pet. ether to
afford the title
compound (8.50 g, 45 %) as a white solid.
IR(KBr, c~ i):3439, 1682, 1602, 1577;
1H NMR (200MHz, CDCl3):~9.88(s, 1H), 7.86(d, J=8.8Hz ,2H), 7.03(d, J=8.8Hz
,2H), 4.40(t, 2H, J=6.2Hz), 3.69(t, J=5.9Hz ,2H);
Mass(CI method):231(M+2, 231, 100%).
PREPARATION 14
1-(3-f2-[1-Methyl-2-(4-methylphenyl)-4-oxo-1,4-dihydro-3-quinoliniloxyJethoxy)
phenyl)-
1 s 1-ethanone
O
A mixture of hydroxy compound obtained in Preparation 12 (3.0 g, 11 mmol),
bromoketo compound obtained in Preparation 1 (2.43 g, 10 mmol) and potassium
carbonate
(6.24 g, 45 mmol) was placed in a 1 L round bottomed flask and DMF (30 mL) was
added.
2o The mixture was heated to 80°C with stirring and held at this
temperature for 12 hours under
a nitrogen atmosphere. The mixture was cooled to 25°C and poured slowly
into ice-cold
water (1 L). The solid that separated was filtered and washed with water (2 ~
500 mL). It
was triturated with methanol and ftltered to afford the title compound (2.8 g,
64 %), as a pale
brown solid, after drying under vacuum.
2s 'H NMR (200MHz, CDCl3): b 8.60 (d, J=7.8Hz, 1H), 7.74-7.21 (m, lOH), 6.93
(d,
J=8.3Hz, 1H), 4.37 (t, J=4.4Hz,2H), 4.02 (t, J=4.9Hz, 2H), 3.52 (s, 3H), 2.56
(s, 3H),
2.37 (s, 3H).
Mass (CI method): 428 (M+l, 428, 100%).
267
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
PREPARATION 15
4-(2-Bromo-ethoxy)-benzoic acid ethyl ester
GO2Et
Br~O
Step (i)
To a solution of 4-hydroxybenzoic acid (15g, 108.6 mmol) in ethanol (200 mL)
was
added SOC12 (16 mL, 217.4 mmol) at 0 °C under anhydrous condition. The
mixture was
heated to reflux for 7 hours with stirnng. After completion of the reaction,
the mixture was
concentrated under vacuum and the residue was neutralized by using aqueous
NaHCO3
to solution until the pH reached 7Ø The solid separated was filtered, washed
with water (2 x
50 mL), and dried under vacuum to afford the desired compound in 89% yield (
16 g).
Step (ii)
A mixture of 4-hydroxybenzoic ester (5 g, 30.12. mmol) and anhydrous I~ZC03
(4.62
g, 33.51 inmol) in acetone .(50 mL) was stirred at 50 °C for 30 min.
under Nitrogen
atmosphere. 1,2-Dibromoethane (34 g, 180.7 mmol) was added to the mixture at
the same
temperature, and stirring continued for 6 hrs. The mixture was filtered and
the residue was
washed with acetone (2 x 25 mL). The filtrates were collected, combined and
concentrated.
The residue was purified by crystallization from hexane to give the desired
product in 96
yield (6.0 g).
PREPARATION 16
4-{2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H chromen-3-yloxy~
-ethoxy}-benzoic acid ethyl ester
o~
268
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
A mixture of 2-(3,4-dimethoxyphenyl)-3-hydroxy-5,7-dimethoxy-4H 4-chromenone
(4 g, 11.17 mmol) obtained in Preparation 2, 4-(2-Bromo-ethoxy)-benzoic acid
ethyl ester
(3.66 g, 13.40 mmol) obtained in Preparation 15 and KZC03 (4.62 g, 33.51 mmol)
in DMF
(20 mL) was stirred at 80°C for 9 hrs under Nitrogen atmosphere. The
mixture was poured
into water (60 mL) and stirred for 30 min. The separated solid was filtered,
washed with
water (2 x 20 mL) and dried under vacuum to give the desired product in 68 %
yield (4.2 g).
PREPARATION 17
4-~2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-chromen-3-yloxy]
to -ethoxy}-benzoic acid
H3C
OH
To a solution of 4- f 2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-
chromen-
3-yloxy]-ethoxy}-benzoic acid ethyl ester (4g, 7.27 mmol) obtained in
Preparation 16, in a
mixture of methanol (40 mL) and dioxane (40 mL) was added a solution of KOH
(2.0 g,
36.36 rnmol) in water (10 mL) at 25-35°C and the mixture was stirred at
60°C for 6 hrs.
Then solvent was removed from the mixture under vacuum and the residue was
acidified
with cold HCI. The solid separated was filtered, washed with cold water (2 x 3
mL) and
dried under vacuum. The crude product was purified further by crystallization
from ethanol
to give the desired acid in 84 % yield (3.2 g).
PREPARATION 18
2-(Toluene-4-sulfonylamino)-succinamic acid
O _
O HN~S ~ ~ CHs
O
H2N OH
O
269
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
To a stirred solution of L-Aspergine (15 g, 100 mmol), NaOH (4.4 g, 110 mmol)
in a
mixture of water (75 mL) and dioxane (75 mL) was added p-toluenesulfonyl
chloride (20.9 g,
110 mmol) at 0°C. After stirring for I min. additional quantity of NaOH
(4.4g, 110 mmol)
in water (75 mL) was added to the reaction mixture at the same temperature.
Stirnng
continued for 1 hr. and then dioxane was removed from the mixture under low
vacuum. The
residue was washed with ethylacetate (2 x 30 mL), aqueous layers collected,
combined, and
acidified with conc. HCl very slowly with stirring at 0°C. The solid
separated was filtered
and washed with cold water (2 x 30 mL) to afford the desired product in 59%
yield (17 g).
mp: 198-200°C.
to
PREPARATION I9
3-Amino-2-(toluene-4-sulfonylamino)propionic acid ethyl ester
Step (i):
3-Amino-2-(toluene-4-sulfonylamino)-propionic acid
O _
NN~S
O
HZN~OH
0O
To a cold (0°C) and stirring solution of NaOH (1.95 g, 48.95 mmol) in
water (8.7
mL) was added bromine (0.36 mL, 6.99 mmol) slowly and drop wise. After 5 min.
a cold
solution of Preparation 18 (2.0 g, 6.99 mmol) and NaOH (0.55 g) in water (6.4
mL) was
added in one portion. The solution was stirred for 20 min. at 0 °C and
then for 30 min. at
90°C. The mixture was cooled to 0 °C and the pH was adjusted to
7.0 by slow addition of
cons. HCI. The solid separated was filtered, washed with cold EtOAc (2 x 25
mL) and dried
under vacuum to afford the desired compound in 61 % yield (1.1 g). mp 225-
226°C.
Step (ii)
3-Amino-2-(toluene-4-sulfonylamino)-propionic acid ethyl ester
270
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
O _
HN~ ISI ~ ~ CHs
O
HaN~O~CH3
O
To a cold (0°C) and stirring solution of the compound (2 g, 7.75 mmol),
obtained in
step (i), in ethanol (20 mL) was added SOCl2 (1.25 mL, 17.05 mmol) under
anhydrous
condition. The mixture was heated to reflux for 12 hrs with stirring. After
completion of the
reaction, the mixture was concentrated under vacuum to afford the
hydrochloride salt of title
compound in 90% yield (2.0 g). This was used for the next step without further
purification.
PREPARATION 20
4-(3,4-Dimethoxyphenylcarboxamido)-1-methyl-3-propyl-1H-5-pyrazolecarboxamide
N- O
-N
'H
O NH2 ~ OMe
OMe
A mixture of 4-amino-1-methyl-3-propyl-1H-5-pyrazolecarboxmide (19.57g, 107.5
mmol} and triethylamine (54.4g, 134.38 mmol) in dichloromethane (300 mL) were
taken in a
1 liter 3 neck round bottom flask fitted with a nitrogen balloon, pressure
equalizing addition
funnel and a septum. To the mixture was added a solution of 3,4-dimethoxy-1-
benzenecarbonylchloride (21.5g, 107.5mmol) in dichloromethane (100mL) at
0°C through a
pressure equalizing addition funnel over a period of 0.5 hours under nitrogen
atmosphere.
The reaction temperature was raised to 25°C after addition and the
contents were stirred for
another 12 hours. Dichloromethane was removed from the reaction mixture under
reduced
pressure and the solid obtained was washed with cold water (2 x 150 mL),
filtered and dried
2o under vacuum to get the title compound 33g, (89 %) as a white solid. Mp:
176-178°C.
IR: v maX (KBr, crri 1): 3370, 3243, 2960, 16$2, 1631;
IH NMR (200 MHz, CDCl3): 8 7.81 (s, 1H), 7.49 (d, J = 6.45Hz, 2H), 6.94 (d, J
=
~.86Hz, 1H), 3.99-3.96 (m, 9H), 2.53 (t, J = 7.22Hz, 2H), 1.68-1.57 (m, 2H),
0.92 (t,
J = 7.SlHz, 3H);
271
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Mass (CI method, I-butane): 347(MH+, 100).
PREPARATION 21
5-(3,4-Dimethoxyphenyl)-1-methyl-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-
d]pyrimidin-7-
one
O
N NH
N \ I ~ OMe
~N
OMe
4-(3,4-dimethoxyphenylcarboxamido)-1-methyl-3-propyl-1 H-5-
pyrazolecarboxamide, obtained in Preparation 20 (17g, 49.13 mmol) in tert-
butanol (350 mL)
was taken in a one liter single neck round bottom flask fitted with a reflux
condenser and to it
potassium tertiary butoxide (16.55g, 147.38 mmol) was added carefully and the
contents
were refluxed for 63 hours under nitrogen atmosphere. The reaction mixture was
cooled to
25-35°C and tent-butanol was completely removed under vacuum. To the
residue cold water
(200 mL) was added followed by addition of dilute hydrochloric acid (3N) under
stirring
until the pH was constant at 7. The solid formed was filtered off and dried
under vacuum to
afford the title compound 13g (81%) as a white solid. Mp: 210-212°C.
IR: v maX : (KBr, cm ~): 3438, 3204, 1670;
IH NMR (200 MHz, DMSO-d6): 8 12.3 (bs, DZO exchangeable, 1H), 7.73 (m, 2H),
7.08 (d, J = 8.32 Hz, 1H), 4.15 (s, 3H), 3.86 (s, 3H), 3.83 (s, 3H), 2.81 (t,
J = 7.25 Hz,
2H), 1.83-1.72 (m, 2H), 0.96 (t, J = 7.24 Hz, 3H);
Mass (CI method, i-butane): 329(M+~, 100).
PREPARATION 22
1-[4-(2-Bromoethylamino) phenyl]-1-ethanone
0
i
N s~ Br
H
To a suspension of 60% NaH (5.93g, 247.08 mmol) in DMF (80 mL) taken in a one
liter 2 neck round bottom flask fitted with a pressure equalizing addition
funnel and a septum
272
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
was added a solution of p-aminoacetophenone (208, 148.1 mmol) in DMF (60 mL)
in drops
through the pressure equalizing addition funnel under nitrogen atmosphere at
0°C and the
contents were stirred for 2 hours at 25°C. Then to the stirred solution
was added 1,2-
dibromoethane (97.488, 518.5 mmol) in drops and the contents were further
stirred for
another 18 hours at 90°C. The reaction mixture was cooled to 25-
35°C and was carefully
added to cold water (650 mL) while stirring. The organics were extracted with
ethylacetate
(3 x 200 mL) and combined organics were washed with water (2 x 100 mL)
followed by a
brine wash. The separated organics were dried over Na2S04 and concentrated
under reduced
pressure. The crude was chromatographed over silicagel by using 15-20% ethyl
acetate / pet.
l0 ether (3 Lit), affording the title compound S.lg (14 %) as a pale yellow
solid.
Mp: 92-94°C.
IR: v max : (I~Br, ciri 1): 3360, 2927, 1650;
'H NMR (200 MHz, CDCI3): ~ 7.84 (d, J = 8.89 Hz, 2H), 7.48 (d, J = 8.58 Hz,
2H),
3.68-3.52 (m, 4H), 2.51 (s, 3H);
Mass (CI method, I-butane): 244(M+2, 10), 162 (100).
PREPARATION 23
1-(4-{2-[5-(3,4-Dimethoxyphenyl)-1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-
pyrazolo[4,3-
d]pyrimidin-6-yl]ethylamino}phenyl)-1-ethanone
\ O H
N ~N
N\ ~ i ~ \
N
\ ~ O
OMe
OMe
A mixture of 5-(3,4-dimethoxyphenyl)-1-methyl-3-propyl-6,7-dihydro-1H-
pyrazolo[4,3-d]pyrimidin-7-one obtained in Preparation 21 ( 2g, 6.09 mmol), 1-
[4-(2-
bromoethylamino) phenyl]-1-ethanone obtained in Preparation 22 (1.558, 6.405
mmol) and
potassium carbonate (4.2138, 3.5 mmol) were taken in 100 mL round bottom flask
and DMF
(20 mL) was added to this. The reaction mixture was stirred at 25°C for
16 hours under
nitrogen atmosphere. The reaction mixture was slowly poured into ice-cold
water (100 mL).
The solid separated was filtered, washed with water (2 x 5 mL) and dried under
vacuum to
afford the title compound 2.6 g (87%), as a pale yellow solid. Mp: 182-
184°C.
273
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
IR: v max (KBr, cW ~): 3381, 2927, 1660, 1599;
IH NMR (200 MHz, DMSO-d6): 8 7.93 (d, J = 5.68 Hz, 2H), 7.71 (d, J = 8.31 Hz,
2H), 7.03 (d, J = 8.79 Hz, 1H), 6.91 (s, 1H), 6.69 (d, J = 8.79 Hz, 2H), 4.84
(m, 2H),
4.10 (s, 3I~, 3.84 (s, 3H), 3.83 (s, 3H), 3.72 (t, J = 4.5 Hz, 2H), 2.91 (t, J
= 7.33 Hz,
2H}, 2.40 (s, 3H), 1.89-1.78 (m, 2H), 0.93 (t, J = 7.32 Hz, 3H);
Mass (CI method, I-butane): 490 (M+1, 100).
PREPARATION 24
6,7-Dimethoxyquinazolin-4(3H)-one
O
Me0 I ~ ~ H
Me0 ~ N
A mixture of 2-amino-4,5-dimethoxybenzoic acid (29.6g, 0.15 mol) and formamide
(0.6 mol, 24 mL) was stirred vigorously under nitrogen atmosphere. The mixture
was heated
to 145°C for 4 hours. After completion the reaction mixture was cooled
and water (120 mL)
was added. The solid was filtered, washed with cold water (2 x 20 mL) followed
by hexane
(2 x 20 mL) to give l2.Sg of the desired product in 40% yield. Mp. 295-
296°C (lit 296-
297°C).
~HNMR (DMSO-d6, 200 MHz) 12.0 (bs, D20 exchangeable, lI~, 7.97 (s, 1H), 7.44
(s, 1H), 7.10 (s, 1H), 3.88 (s, 3H), 3.86 (s, 3H).
Reference: LeMahieu, R. A.; Carson, M.; Nason, W. C.; Parrish, D. R.; Welton,
A.
2o F.; Baruth, H. W.; Yaremko, B. J. Med. Chem. 1983, 26, 420.
EXAMPLE 1
5-[-1-(4-~2-[2-(3,4-Dimethoxy phenyl)-5,7-dimethoxy-4-oxo-4h-3-
chromenyloxy]ethoxy}
phenyl)ethylidene]-1,3-thiazolane-2,4-dione
274
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
A mixture of compound (31 g, 59.6 mmol), obtained in Preparation 3,
thiazolidene-
1,3-dione (40 g, 341 mrnol), benzoic acid (14.5 g, 118.8 rmnol) and piperidine
(10.1 g, 118.8
mmol) were placed into 1 L single neck round bottomed flask, to this toluene
(600 mL) was
added. The round bottomed flask was fitted with dean stark apparatus, which
was connected
to a reflux condenser. The reaction mixture was heated to reflux for 48 hours
under nitrogen
atmosphere. The reaction mixture was cooled to 25°C and was allowed to
pass through a
silica geI column. The product was eluted by using 0.5-1 % MeOH / CHCl3 (5 L)
to afford
the title compound, 22 g (60 %) as off white solid. Mp: 205-206°G.
IR: ? "~~ (KBr, cm ~): 3220, 1735, 1698, 1627, 1604;
IH NMR (200 MHz, CDCl3): d 9.07 (bs, 1H, exchangeable with DSO), 7.76-7.69 (m,
2H), 7.26 (d, J = 8.30 Hz, 2H), 6.90 (d, J = 8.79 Hz, 1H), 6.81 (d, J = 8.79
Hz, 2H),
6.52 (s, 1H), 6.37 (s, 1H), 4.47 (t, J= 4.40 Hz, 2H), 4.29 (t, J= 4.40 Hz,
2H), 3.97 (s,
3H), 3.93 (s, 3H), 3.91 (s, 6H), 2.69 (s, 3H);
Mass (ES method): 619 (M'~, 100).
EXAMPLE 2
Example 2 was prepared according to the methodology provided in Example 1.
/ OMe O N~H
Me0 O \ I d'O
OMe / ~S
O
OMe O /
1H NMR (200 MHz, CDCl3): d 12 (s, DSO exchangable), 7.68-7.44 (m, 4H), 7.10-
6.84 (m,
4H), 6.51 (s, 1H), 4.37-4.33 (m, 4H), 3.90 (s, 3H), 3.85 (s, 3H), 3.76 (s,
3H), 3.71 (s, 3H),
2.50 (s, 3H). Mp: 120-124°C.
EXAMPLE 3
Example 3 was prepared according to the methodology provided in Example 1.
OMe O
/ ~-NFi
MeO I \ O ~ \ I OMe S I O
/ O~O /
OMe O \ I
275
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
1H NMR (200 MHz, CDCl3): d 8.65 (s, D20 exchangeable), 7.75-7.71 (m, 2H), 7.28-
7.23 (m,
2H), 6.90-6.79 (m, 2I~, 6.70 (s, 1H), 6.53 (s, 1H), 6.37 (s, 1H), 4.49 (s,
2H), 4.25 (s, 2H),
3.97 (s, 3H), 3.91 (s, 9H), 2.68 (s, 3H). Mp: 210-214°G.
EXAMPLE 4
Example 4 was piepared according to the methodology provided in Example 1.
OMe
Me0 ~ O ~ I OMe OMe
~~O
I O~O ~ S
OMe O Me0 I ~ ~ NH
O
'H NMR (200 MHz, CDCl3): d 9.18 (s, D20 exchangeable, 1H), 7.92 (s, 1H), 7.91-
7.81 (m,
1H), 7.69 (s, 1H), 6.95 (d, J=8.79 Hz, 1H), 6.65 (s, 2H), 6.54 (s, 1H), 6.34
(s, 1H), 4.42 (s,
l0 4H), 3.95 (s, 6H), 3.93 (s, 3H), 3.91 (s, 3H), 3.80 (s, 6H). Mp: 207-
210°C.
EXAMPLE 5
Example 5 was prepared according to the methodology provided in Example 1.
OMe
Me0 ~ O ~ I pMe
~~~O~sS ~ S
OI Me IOI ( , / NH
O
IH NMR (200 MHz, CDCI3}: d 7.79 (d, J=8.36 Hz, 2H), 7.66 (s, 2H), 7.27 (d,
J=9.7 Hz, 2H),
6.94 (d, J=8.9 Hz, 1H), 6-5 (s, 1H), 6.36 (s, 1H), 4.22 (t, J=6.74 Hz, 2H),
3.96 (s, 3H), 3.95
(s, 3H), 3.93 (s, 3H), 3.90 (s, 3H), 3.39 (t, J= 6.74 Hz, 2H}, 2.54 (s, 3H).
Mp: 138-142°C.
EXAMPLE 6
Example 6 was prepared according to the methodology provided in Example 1.
,_O
~!/~S
OMe O I / / NH
T
CH3 O
OMe
Me0 ~ O ~ I OMe
~~~O~O
276
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
IH NMR (200 MHz, CDC13): d 7.72-7.67 (d, J=10.78, 3H), 6.86 (d, J=8.36 Hz,
1H), 6.64-
6.52 (m, 3H), 6.37 (s, 1H), 4.47 (s, 2H), 4.27 (s, 2H), 3.98 (s, 3H), 3.91 (s,
9H), 2.53 (s, 3H),
2.5I (s, 3H). Mp: 126-130°C.
EXAMPLE 7
Example 7 was prepared according to the methodology provided in Example 1.
OMe
s OMe
Me0 \ O \
H
'~O~N \ S
OMe O I / / NH
O
IH NMR (200 MHz, CDCl3): d 8.31 (s, D20 exchangeable, 1H), 7.73 (d, J=8.3 Hz,
1H), 7.64
(s, 1H), 7.36 (d, J=10.2 Hz, 2H), 7.28 (m, 2H), 7.01 (d, J=8.79 Hz, 1H), 6.58
(s, 1H), 6.43 (s,
io 1H), 4.2 (s, 2H), 4.0 (s, 3H), 3.97 (s, 3H), 3.95 (s, 3H), 3.93 (s, 3H),
3.56 (s, 2H), 2.7 (s, 3H).
Mp: 192-195°C.
EXAMPLE 8
Example 8 was prepared according to the methodology provided in Example 1.
1H NMR (200 MHz, CDC13): d 8.17 (s, D20 exchangeable, 1H), 7.76 (d, J=8.3 Hz,
IH), 7.68
(s, 1H), 7.28 (s, 1H), 7.11 (m, 1H), 6.98-6.89 (m, 2H), 6.52 (s, 1H), 6.37 (s,
1H), 4.48 (bs,
2H), 4.4I (bs, ZH), 3.97 (s, 3H), 3.91 (s, 9H), 3.13 (t, J= 7.3 Hz, 2H), 1.6-
1.4 (m, 2H), 0.92
(t, J=7.3 Hz, 3H). Mp: 204-208°C.
277
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 9
Example 9 was prepared according to the methodology provided in Example 1.
/ OMe
Me0 \ O \
OMe O
~O°\r0 \ S
OMe O C~ I / /
b
IH NMR (200 MHz, CDCI3): d 8.27 (s, D20 exchangeable, 1H), 7.76 (d, J=8.53 Hz,
1H),
s 7.67 (s, 1H), 7.34 (s, 1H), 7.17 (m, 1H), 6.97-6.89 (m, 2H), 6.53 (s, 1H),
6.37 (s, 1H), 4.5 (s,
2H), 4.4 (s, 2H), 3.98 (s, 3H), 3.92 (s, 9H), 2.68 (s, 3H). Mp: 230-
233°C.
EXAMPLE 10
Example 10 was prepared according to the methodology provided in Example 1.
/ OMe
Me0 \ O \
OMe O
~O O I S~ H
OMe O B~ / /
p
IH NMR (200 MHz, CDC13): d 8.25 (s, D20 exchangeable, 1H), 7.75 (d, J=6.74 Hz,
1H),
7.66 (s, 1H), 7.51 (s, 1H), 7.21 (s, 1H), 6.90 (d, J=8.4 Hz, 2H), 6.52 (s,
1H), 6.37 (s, 1H), 4.5
(bs, 2H), 4.39 (bs, 2H), 3.98 (s, 3H), 3.91 (s, 6H), 3.90 (s 3H), 2.69 (s,
3H).
Mp: 235-236 °C.
is
EXAMPLE 11
Example 11 was prepared according to the methodology provided in Example 1.
/ OMe
Me0 \ O \ I
~OMe O
O \ S
OMe O I / / NNa
O
IH NMR (200 MHz, CDCl3): d 7.74 (d, J = 8.3 Hz, 1H), 7.69 (s, 1H), 7.24 (d, J
= 7.9 Hz,
2H), 7.00 (d, J = 8.0 Hz, 1H), 6.83 (d, J = 6.10 Hz, 2 H), 6.76 (s, 1H), 6.5
(s, 1H), 4.33 (s,
2H), 4.2 (s, 2H), 3.90 (s, 3H), 3 .85 (s, 3H), 3.81 (s, 3H), 3.77 (s, 3H),
2.53 (s, 3H). Mp: 225-
228°C.
278
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 12
Example 12 was prepared according to the methodology provided in Example 1.
/ OMe
MeO ~ O
OMe O
I O~rO ~ S
OMe O I / /
b
'H NMR (200 MHz, CDC13): d 7.76-7.69 (m, 2H), 7.3 (d, J = 8.3 Hz, 2H), 7.00
(d, J = 8.3
Hz, 1H), 6.87-6.84 (m, 3H), 6.50 (s, 1H), 4.33 (s, 2H), 4.22 (s, 2H), 3.90 (s,
3H), 3.85 (s,
3H), 3.81 (s, 3H), 3.77 (s, 3H), 2.51 (s, 3H). Mp: 195-198°C.
EXAMPLE 13
5-[-1-(4- f 2-[6-Fluoro-2-(4-methoxyphenyl)-4-oxo-4H-3-
chromenyloxy]ethoxy}phenyl)
lo. efliylidene]-1,3-thiazolane-2,4-dione
A mixture of compound obtained in Preparation 8 (0.35 g, 0.72 mmol),
thiazolidene-
1,3-dione (0.54 g, 4.68 mmol), benzoic acid (0.19 g, 1.56 mmol) and piperidine
(0.13 g, 1.56
is mol) were placed into a 50 mL single neck round bottomed flask, to this
toluene (15 mL) was
added. The round bottomed flask was fitted with Dean-Starlc apparatus, which
was
connected to a reflux condenser. The reaction mixture was heated to reflux for
48 hours
under nitrogen atmosphere. The reaction mixture was cooled to 25°C and
was allowed to
pass through a silica gel column. The product was eluted by using 0.5-1 % MeQH
/ CHCl3
20 (5 L) to afford the title compound, 0.32 g (75 %) as off white solid. Mp:
210-212 °C.
IH NMR (200 MI3z, CDC13): d 12.2 (s, D20 exchangeable, H), 8.14 (d, J=8.87 Hz,
2H), 7.91-7.77 (m, 1H), 7.71 (d, J= 8.6 Hz, 2H), 7.36 (d, J=8.59 Hz, 2H), 7.02
(d, J=
9.14 Hz, 2H), 6.92 (d, J= 8.59 Hz, 2H), 4.44 (s, 2H), 4.24 (s, 2H), 3.81 (s,
3H), 2.5 (s,
3H).
279
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 14
This compound was prepared according to the procedure provided in Example 13.
OCH3 O
/ ~-NH
O \ I S O
I
F / O~O
o \I
IH NMR (200 MHz, CDC13): d 12.2 (s, DZO exchangeable, 1H), 8.14 (d, J=8.87 Hz,
2H),
s 7.91-7.784 (m, 1H), 7.75 (d, J= 8.6 Hz, 2H), 7.38-7.34 (d, J=8.59 Hz, 2H),
7.02 (d, J= 9.14
Hz, 2H), 6.92 (d, J= 8.59 Hz, 2H), 4.44 (s, 2H), 4.24 (s, 2H), 3.81 (s, 3H),
2.5 (s, 3H). Mp:
227-230°C.
EXAMPLE 15
l0 This compound was prepared according to the procedure provided in Example
13.
/ OCH3
\ O \
H O
s ~N \
F O O ~ / / ~ H
O
IH NMR (200 MHz, CDCl3): d 12.21 (s, DSO exchangeable, 1H), 8.1 (m, 2H), 7.85-
7.74 (m,
3H), 7.23 (m, 2H), 7.02 (d, J=7.79 Hz, 2H), 6.62 (d, J= 8.06 Hz, 2H), 6.44 (s,
DSO
exchangeable, 1H), 4.14 (s, 2H), 3.82 (s, 3H), 3.35 (s, 2H), 2.5 (s, 3H).
15 Mp:182-185°C.
EXAMPLE 16
This compound was prepared according to the procedure provided in Example 13.
s
o \
0
I ~ I ~o \ ~/C/
O O I a es~ H
O
20 IH NMR (200 MHz, CDCl3): d 12.2 (s, DZO exchangeable, 1H), 8.13 (d, J=3.23
Hz, 1H),
7.99 (d, J=5.1 Hz 1H), 7.71 (m, 1H), 7.73-7.69 (d, J=8.05 Hz, 2H), 7.38 (d,
J=8.6 Hz, 2H),
7.31 (m, 1H), 7.00 (d, J=8.85 Hz, 2H), 4.62 (s, 2H), 4.43 (s, 2H), 2.5 (s,
3H). Mp: 238-240
°C.
280
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 17
This compound was prepared according to the procedure provided in Example 13.
0
S ~ ~-NH
O \ S O
F / O~O \
O I
1H NMR (200 MHz, CDC13): d 12.31 (s, D20 exchangeable, 1H), 8.13 (d, J=3.8 Hz,
1H),
7.96 (d, J=5.1 Hz, 1H), 7.85-7.77 (m, 1H), 7.71 (d, J=8.33 Hz, 2H), 7.4-7.3
(m, 2H), 6.96 (d,
J=8.87 Hz, 2H), 6.93-6.92 (m, 1H), 4.62 (s, 2H), 4.4 (s, 2H), 2.5 (s, 3H). Mp:
2I8-220 °C.
EXAMPLE 18
This compound was prepared according to the procedure provided in Example 13.
s
0
H o
s ~N ~/C/
F O o ' s /s~ H
O
'H NMR (200 MHz, CDCl3): d 12.1 (s, D20 exchangeable, 1H), 8.08 (d, J=2.95 Hz,
1H),
7.99 (d, J=4.83 Hz 2H), 7.91-7.78 (m, IH), 7.72 (d, J=8.06 Hz, 2H), 7.3-7.23
(m, 2H), 6.65
(d, J=8.59 Hz, 2H), 6.56 (m, DZO exchangeable, 1H), 4.33 (t, J=5.36 Hz, 2H),
3.59 (t, J=5.64
Hz, 2H), 2.62 (s, 3H). Mp: 218-219 °C.
EXAMPLE 19
This compound was prepared according to the procedure provided in Example 13.
/ F
\ O
O
~O J~
F O O I / /S
O
1H NMR (200 MHz, CDCl3): d 8.21-8.14 (m, 1H), 7.87-7.85 (m, 1H), 7.77 (d,
J=8.3 Hz, 2H),
7.36-7.26 (m, 4H), 6.88 (d, J=8.8 Hz, 3H), 4.46 (s, 2H), 4.21 (s, 2H), 2.5 (s,
3H). Mp: 262-
265 °C.
281
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 20
This compound was prepared according to flee procedure provided in Example 13.
N
O \
°
~O \ J.J~~
O ° I / /S~ H
1H NMR (200 MHz, CDCl3): d 12.27 (s, DSO exchangeable, 1H), 9.35 (s, 1H), 8.67
(d,
5 J=4.57 Hz, 1H), 8.48 (d, J=8.33 Hz, 1H), 7.97-7.91 (m, 1H), 7.80 (d, J= 8.3
Hz, 2H), 7.56
7.50 (m, 1H), 7.35 (d, J=8.3 Hz, 2H), 6.89 (d, J=8.6 Hz 2H), 4.53 (s, 2H),
4.23 (s, 2H), 2.5 (s,
3H). Mp: 251-254 °C.
EXAMPLE 21
l0 This compound was prepared according to the procedure provided in Example
13.
i
0
0
ono \ s
O I ~ s ~ H
O
'H NMR (200 MHz, CDC13): d 10.4 (bs, D20 exchangeable, 1H), 8.06-8.00 (m, 1H),
7.78-
7.65 (m, 4H), 7.48-7.40 (m, 2H), 7.14 (d, J=7.79 Hz, 2H), 7.01 (d, J= 8.86 Hz,
1H) 6.84 (d,
J=8.06 Hz, 2H), 6.04 (s, 2H), 4.44 (s, 2H), 4.18 (s, 2H). Mp: 198
200°C.
EXAMPLE 22
This compound was prepared according to the procedure provided in Example 13.
s
o \
r ono \ s
O I s ~ NH
O
IH NMR (200 MHz, CDC13): d 10.43 (bs, D20 exchangeable, 1H), 8.15-8.07 (m,
2H), 7.96
(d, J=4.88 Hz, 1H) 7.84-7.73 (m, 2H), 7.53-7.46 (m, 1H), 7.32-7.28 (m, 1H),
7.19-7.15 (d,
J=8.3 Hz, 2H), 6.92 (d, J=8.79 Hz, 2H), 4.62 (m, 2H), 4.32 (m, 2H). Mp: 166-
168°C.
282
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 23
This compound was prepared according to the procedure provided in Example 13.
/ s~
0
0
I~o~°° ~ s
O I / / ~ H
O
IH NMR (200 MHz, CDCl3): d 8.12 (bs, D20 exch), 8.08-8.04 (m, 3H), 7.83-7.74
(m, 3H),
7.51 (d, J=8.3 Hz, 2H), 7.29 (d, J=8.3 Hz, 2H), 6.97 (d, J=8.89 Hz, 2H), 4.46
(m, 2H), 4.24
(m, 2H), 2.48 (s, 3H). Mp: 220-222°C.
EXAMPLE 24
5-[ 1-(4- {2-[ 1-Methyl-4-oxo-2-(4-methylphenyl)-1,4-dihydro-3-quinolinyloxyJ
ethoxy)
l0 phenyl}methylideneJ-1,3-thiazolane-2,4-dione
,O
O ~ , os NH
O
Me
Ne
W
~O
A mixture of compound obtained irr Preparation 14 (150 mg, 0.36 mmol),
thiazolidene-1,3-dione (64 mg, 0.54 mmol), benzoic acid (88 mg, 0.72 mmol) and
piperidine
(61 mg, 0.72 mmol) were placed into 1 L single neck round bottomed flask, to
this toluene
i5 (600 mL) was added. The round bottomed flask was fitted with dean stark
apparatus, which
was connected to a reflux condenser. The reaction mixture was heated to reflux
for 48 hours
under nitrogen atmosphere. The reaction mixture was cooled to 25°C and
was allowed to
pass through a silica gel column. The product was eluted by using 0.5-1 % MeOH
/ CHCl3
(5 L) to afford the title compound, 100 mg (54 %) as brown solid. Mp: 250-252
°C.
2o IH NMR (200 MHz, CDCl3): d 12.5 (s, NH), 8.33 (d, 1H, J=8.2 Hz), 7.78 (m,
2H),
7.52-7.22 (m, 8H), 6.88 (d, 2H, J=8.3 Hz), 4.23 (s, 2H), 3.99 (s, 2H), 3.47
(s, 3H),
2.34 (s, 3H).
283
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 25
This compound was prepared according to the procedure provided in Example 24.
o / ~ \ S
0
°\/~o \ o NH
N
CHs
1H NMR (200 MHz, CDCl3): d 12.5 (s, NH), 8.34 (d, 1H, J=7.8 Hz), 7.78-7.47 (m,
11H),
s 6.90 (d, 2H, J=8.4 Hz), 4.23 (s, 2H), 3.99 (s, 2H), 3.47 (s, 3H). Mp: 250-
252 °C.
EXAMPLE 26
This compound was prepared according to the procedure provided in Example 24.
CHs
° ~ I \ S~o
\ o NH
N
CHs I /
F
io 1H NMR (200 MHz, CDCl3): d 12.22 (s, NH), 8.34 (d, 2H, J=8.1 Hz), 7.8 (s,
2H), 7.53-7.26
(m, 6H), 6.82 (d, 2H, J=8.3 Hz), 4.25 (s, 2H), 3.97 (s, 2H), 3.48 (s, 3H),
2.63 (s, 3H). Mp:
196-198°C.
EXAMPLE 27
This compound was prepared according to the procedure provided in Example 24.
CHs
~ ~ \ S~o
\ I °~o \ o NH
N \
CHs
is CHs
1H NMR (200 MHz, CDCl3): d 12.25 (bs, NH), 8.34 (d, 1H, J=7.8 Hz), 7.78 (d,
2H, J=2.9
Hz), 7.45-7.28 (m, 7H), 6.80 (d, 2H, J=8.8 Hz), 4.23 (s, 2H), 3.94 (s, 2H),
3.48 (s, 3H), 2.64
(s, 3H), 2.35 (s, 3H). Mp: 228-232°C.
284
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 28
This compound was prepared according to the procedure provided in Example 24.
,,o
o H3C -'~S
\ - N
- ~ °~\ - II
N / o
o \
H3C ~ \
CH3
IH NMR (200 MHz, CDC13): d 12.3 (NH, 1H), 8.33 (d, 1H, J=8.2 Hz), 7.77 (d, 2H,
J=3.2
s Hz), 7.46-7.27 (m, 6H), 6.95 (d, 1H, J=7.5 Hz), 6.78 (d, 2H, J=8.8 Hz), 4.22
(s, 2H), 3.92 (s,
2H), 3.46 (s, 3H), 2.62 (s, 3H), 2.33 (s, 3H). Mp: 210-212 °C.
EXAMPLE 29
3-(4- {2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-chromen-3 -yloxy]-
ethoxy} -
benzoylamino)-2-(toluene-4-sulfonylamino~-propionic acid ethyl ester
0 0
To a solution of 4-~2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-
chromen-3-yloxy]-ethoxy}-benzoic acid obtained in Preparation 17 (3 g, 5.74
mmol), 3-
Amino-2-(toluene-4-sulfonylamino)-propionic acid ethyl ester obtained in
Preparation 19
(2.22 g, 6.89 mmol) in DMF (20 mL) was added EDCI (1.64 g, 8.61 mmol), HOBt (1
g, 7.46
mmol) and N-methyl morpholine (2.0 g, 20.09 rnmol) at 25-35°C under
Nitrogen
atmosphere. The mixture was stirred at the same temperature for 12 hrs. After
completion of
the reaction the mixture was poured into water (60 mL) and stirred for 30 min.
The separated
solid was Bltered, washed with water (2 x 20 mL) and dried under vacuum. The
crude
product was purified further by crystallization from ethanol to give the
desired product in 51
yield (2.3 g).
285
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
~HNMR (CDCl3, 200 MHz) 7.73-7.65 (m, SH), 7.25 (d, J = 8.8Hz, 2H), 6.88-6.73
(rn,
4H), 6.52(d, J = 2Hz, 1H), 6.36 (d, J = 2Hz, 1H), 5.91 (d, J = 7.8Hz, D20
exchangeable, 1H), 4.47 (m, 2H), 4.23 (m, 2H), 4.10-3.91 (m, 16H), 3.8 (m,
1H), 2.37
(s, 3H), 1 _14 (t, J = 7.3Hz, 3H).
EXAMPLE 30
This compound was prepared according to the procedure provided in Example 29.
c
HI NMR (CDCl3, 200 MHz) 10.06 (m, DZO exchangeable, 1H), 8.82(s, 1H), 8.17-
8.06 (m,
to 2H), 7.74 (d, J = 8.3Hz, 2H), 7.27-7.19 (m, 3H), 6.24 (d, J = 7.8Hz, DZQ
exchangeable, 1H),
4.15-4.01 (m, 3H), 3.84-3.70 (m, 2H), 3.77-3.51 (m, 1H), 2.31 (s, 3H), 1.41
(d, J = 6.3Hz,
1H), 1.25-1.13 (m, SH).
EXAMPLE 31
This compound was prepared according to the procedure provided in Example 29.
N~H O
~OC~HS
~~ .N.
I SO H
Hl NMR (CDC13, 200 MHz) 9.30 (bs, DSO exchangeable, 1H), 7.76 (d, J = 8.lHz,
2H), 7.60
(d, J = 6Hz, 1H), 7.47 (s, 1H), 7.21 (d, J = 8.lHz, 2H), 6.99 (d, J = 7.SHz,
1H), 6. 76 (d, J =
9.OHz, DZO exchangeable, 1H), 6.53 (s, 1H), 6.41 (s, 1H), 4.17 (m, 2H), 4.02-
3.86 (m, 15H),
3.46-3.39 (m, 2H), 2.35 (s, 3H), 1.06 (t, J = 7.OHz, 3H).
286
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 32
This compound was prepared according to the procedure provided in Example 29.
o, ~ I
H HN.SO
N~OC2H5
- ~O
Hl NMR (DMSO-d6, 200 MHz), 8.32 (d, J = 8.8Hz, 2H), 8.10 (d, J = B.OHz, 1H),
7.80-7.50
(m, 7H), 7.28 (d, J = 7.8Hz, 2H), 7.03 (d, J = 8.60Hz, l I~, 6.85 (d, J =
8.3Hz, 2H), 4.43 (m,
2H), 4.37-4.26 (m, 2H), 4.07-4.03 (rn, 2H), 3.79 (m, 8H), 3.47(m, 1H), 2.30(s,
3H), 0.95 (t, J
= 7.3Hz, 3H).
EXAMPLE 33
to This compound was prepared according to the procedure provided in Example
29.
ci ~ ci
I
o, a 1
I O~O ~ H HN~So
O I i N~OCZHS
O _ [~O
Hl NMR (DMSO-d6, 200 MHz), 8.32 (d, J = 8.OHz, 2H), 8.17 (d, J = 8.OHz, 1H),
7.83 (d, J =
7.OHz, 1H), 7.69-7.47 (m, 7H), 7.28 (d, J = 8.OHz, 2H), 6.73 (d, J = 8.9Hz,
2H), 4.39 (bs,
2H), 4.07 (bs, 2H), 3.82-3.75 (m, 2H), 3.34 (m, 3H), 2.30 (s, 3H), 0.95 (t, J
= 7.OHz, 3H).
EXAMPLE 34
This compound was prepared according to the procedure provided in Example 29.
a SCH3
o ~I o ~I
.~
I / I O~O ~ HN.S.
O I / N~OCzHg
O _ ~O
2o Hl NMR (CDCl3, 200 MHz), 8.25 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 8.6 Hz,
2H), 7.73 -
7.65(m, 5H), 7.52 (d, J = 7.9 Hz, 1H), 7.40 (t, J = 7.OHz, 1H), 7.24- 7.18 (m,
4H), 6.74 (d, J
= 8.6 Hz, 2H), 6.64 (m, D20 exchangeable, 1H), 5.69 (d, J = 7.3 Hz, D20
exchangeable, 1H),
287
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
4.52 (m, 2H), 4.22 (m, 2H), 4.10 - 3.99 (m, 3H), 3.95 - 3.85 (m, 1F3', 3.69 -
3.59 (m, 1H),
2.48 (s, 3H), 2.37 (s, 3H), 1.13 (t, J = 7.0Hz, 3H).
EXAMPLE 35
This compound was prepared according to the procedure provided in Example 29.
OCH3
OCH3
H3C0 ~ O ~ ~ OF , ~ F
r I O~O I ~ H H ,SO w
OCH30 ~N~OCZHS
O - [~O
Hl NMR (CDCl3, 200 MHz), 7.87 - 7.84 (m, 1H), 7.70 - 7.64 (m, 3H), 6.93 - 6.72
(m, SH),
6.53 (d, J = 3.0 Hz, 1H), 6.37 - 6.29 (m, 2H), 4.46 (m, 2H), 4.23 (m, 2H),
4.17 - 4.06 (m,
2H), 3.96 - 3.74 (m, 15 H), 1.18 (t, J = 7.OHz, 3H).
to
EXAMPLE 36
This compound was prepared according to the procedure provided in Example 29.
HI NMR (CDCl3, 200 MHz), 8.25 (d, J = 7.81 Hz, 1H), 8.13 (d, J = 8.8 Hz, 2H),
7.70 -
7.67(m, 3H), 7.54 (d, J = 8.3 Hz, 1H), 7.41 (t, J = 7.3 Hz, 1H), 7.29 - 7.24
(m, 4H), 7.14 (d,
J = 8.3 Hz, 2H), 6.77 (d, J = 8.3 Hz, 2H), 6.01 (m, 1H, D20 exchangeable),
5.65 (d, J = 8.4
Hz, 1H, D20 exchangeable), 4.52 - 4.51 (m, 2H), 4.21 (m, 2H), 4.02- 3.92 (m,
3H), 3.59 -
3.40 (m, 4H), 2.51 (s, 3H), 2.40 (s, 3H), 1.06 (t, J = 7.3Hz, 3H).
2o EXAMPLE 37
This compound was prepared according to the procedure provided in Example 29.
288
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Hl NMR (CDC13, 200 MHz) 7.75-7.66 (m, 3H), 7.29 (m, 2H), 7.09 (d, J = 8.3 Hz,
2H), 6.91
(d, J = 9.3 Hz, 2H), 6.72 (d, J = 8.3 Hz, 2H), 6.52 (s, 1H), 6.36 (s, 1H),
5.99 (m, D20
exchangeable, 1H), 5.62 (d, J = 7.7 Hz, D20 exchangeable, 1H), 4.46 (m, 2H),
4.20 (m, 2H),
4.14-3.87 (m, 16H), 3.49 (m, 3H), 2.40 (s, 3H), 1.10 (t, J = 7.3Hz, 3H).
EXAMPLE 3 8
This compound was prepared according to the procedure provided in Example 29.
Me0 \ N1 / NH~COzC2Fis
Me0 I / IN~p \ I O I~JH
O=S=O
O
/I
CH3
Hl NMR (CDC13, 200 MHz) 7.67-7.59 (m, 3H), 7.26 - 7.23 (m, 3H), 7.15 - 7.10
(m, 3H),
io 6.84 (d, J =8.6 Hz, 2H), 6.01 (bs, D20 exchangeable, 1H), 5.78 (bs, D20
exchangeable, 1H),
4.39 -4.30 (m, 4H), 3.98 - 3.88 (m, lOH), 3.60 - 3.36 (m, 3H), 2.38 (s, 3H),
1.06 (t, J =
7.OHz, 3H).
EXAMPLE 39
This compound was prepared according to the procedure provided in Example 29.
0
Me0\~/Nl ~N~COaC2H5
MeO~II ''''/°~~~''' INFO ['\~I H NIH
O O-S=O
/
CH3
Hl NMR (CDC13, 200 MHz) 8.30 (s, DZO exchangeable, 1H), 8.26 (s, 1H), 7.69 -
7.57 (m,
4H), 7.47 (s, 1H), 7.26 (d, J =8.0 Hz, 2H), 7.14 (s, 1H), 6.98 (d, J = 8.6 Hz,
2H), 4.36 (bs,
4H), 4.02 (m, 1H), 3.92-3.87 (m, 9H), 3.33 (m, 1H), 2.27 (s, 3H), 0.93 (t, J =
7.3Hz, 3H).
EXAMPLE 40
This compound was prepared according to the procedure provided in Example 29.
0
NH
Me0 w N1 \ 1 ~COZCzHs
~ NCO HN~S
Me0 O 0
~F
F
289
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
Hi NMR (CDCl3, 200 MHz) 8.13 (s, 1H), 7.89 - 7.78 (m, 1H), 7.69 (d, J = 8.0
Hz, 2H), 7.60
(s, 1H), 7.12 (s, 1H), 6.96 - 6.74 (m, 4H), 6.35 (d, J = 8.1 Hz, 1H), 4.41-
4.31 (rn, 4H), 4.21
- 4.17 (rn, 1H), 4.13 - 4.02 (m, 2H), 3.99 (s, 6H), 3.86 - 3.84 (m, 1H), 3.78 -
3 _ 68 (m, 1H),
1.15 (t, J = 7.3Hz, 3H).
EXAMPLE 41
This compound was prepared according to the procedure provided in Example 29 _
OCH3
/ OCH3
N
N~~\
N N~N~COOCaHs
O O NH.~ CH
3
H' NMR NMR (CDCl3, 200MHz): d 8.09(d, 2H, J=8.8Hz), 7.58(d, 2H, J=8.3I3z),
7.26(m,
3H), 6.98(d, 2H, J=8.3Hz), 5.18(s, 2 H), 4.77(s, 2H), 4.30(s, 3H), 4.03(s,
3H), 3.95(s, 3H),
3.55-3.40(m, 1H), 3.07-2.95(q, 2H, J=7.3Hz),2.37(s, 3H), 1.98-1.87(q, 2H,J= 7_
8Hz), 1.13-
1.03(m, 6H).
EXAMPLE 42
This compound was prepared according to the procedure provided in Example 29_
HO
N.S ~ / CHa
O
)OGzHS
Hl NMR (CDCl3, 200MHz):d 12.0(bs, 1H), 8.43(m, 3H), 7.9(m, 1H), 7.75(m, 2H),
7.55-
7.10(m, SH), 6.05(s, 1H), 5.39(s, 1H), 4.2(m, 2H), 4.0-3.6(m, 8H), 2.4(s, 3H),
1.3-0.9(m,
3H).
290
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 43
3-(4-{2-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-chromen-3-yloxy]-
ethoxy] -
benzoylamino)-2-(toluene-4-sulfonylamino)-propionic acid
O-CH3
H3C-O \ O \ I
O-CH3
OS ~ ~ CH'
H3C-O O ~ _ HN~II
O ~ ~ N~OH
O '' ~O
To a solution of ethyl ester of 3-(4-{2-[2-(3,4-Dimethoxy-phenyl)-5,7-
dimethoxy-4-
oxo-4H-chromen-3-yloxy]-ethoxy]-benzoylamino)-2-(toluene-4-sulfonylamino)-
propionic
acid (500 mg, 0.73 mmol) obtained in example 29 in a mixture of ethanol (10
mL) and
dioxane (10 mL) was added a solution of KZC03 (300 mg, 2.19 mmol) in water (5
mL) at 25-
35°C and .the mixture was stirred at the same temperature for 24 hrs.
Then solvent was
removed from the mixture under vacuum and the residue was acidified with cold
HCI. The
solid separated was filtered, washed with cold water (2 x 5 mL) and dried
under vacuum to
give the desired acid in 52 % yield (250 rng).
jHNMR (DMSO-d6, 200 MHz) 12.9 (bs, D20 exchangeable, 1H), 8.32 (s, D20
exchangeable, 1H}, 8.13 (d, J = 8.3Hz, 1H), 7.72-7.63 (m, 4H), 7.32 (d, J =
7.3Hz,
2H), 7.14 (d, J = 8Hz, 1H), 6.86 (s, 1H}, 6.52 (s, 1H), 4.26 (s, 2H), 3.90-
3.82 (m,
15H), 2.33 (s, 3H).
EXAMPLE 44
This compound was prepared according to the procedure provided in Example 43.
_H
H' NMR (DMSO-d6, 200 MHz) d 12.9 (bs, D20 exchangeable, 1H), 8.32 (s, D20
exchangeable, 1H), 8.13 (d, J = 8.3Hz, 1H), 7.72-7.63 (m, 4H), 7.32 (d, J =
7.3Hz, 2H), 7.14
(d, J = 8Hz, 1H), 6.86 (s, 1H), 6.52 (s, 1H), 4.26 (s, 2H), 3.90-3.82 (m,
15H), 2.33 (s, 3H).
291
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 45
This compound was prepared according to the procedure provided in Example 43.
0 0
I I N.H o
CI N Y -OH
~~ . IN
/ I SO .H
Hl NMR (DMSO-d6, 200 MHz): d 9.72 (bs, DZO exchangeable, 1H), 8.59 (s, 1H),
8.37(d, J
= 6.2 Hz, 1H), 8.14 (d, J = 9.4Hz, 1H), 7.60 (d, J = BHz, 2H), 7.19 (d, J =
8.OHz, 2H), 3.76-
3.43 (m, 4H), 2.49 (s, 3H), 1.31 (d, J = 6.OHz, 2H), 1.11 (s, 2H).
EXAMPLE 46
5-[(E,Z)-1-(4-{2-[5-(3,4-Dimethoxyphenyl)-1-methyl-7-oxo-3-propyl-6,7-dihydro-
1H-
pyrazolo[4,3-d]pyrimidin-6-yl]ethylamino)phenyl) ethylidene]1,3-thiazolane-2,4-
dione
N.N~
N~O
N
HN ~ \ \ O
Me0
S~NH
Me0
O
A mixture of 1-(4-{2-[5-(3,4-dimethoxyphenyl)-1-methyl-7-oxo-3-propyl-6,7-
dihydro-1H-pyrazolo[4,3-d]pyrimidin-6-yl]ethylamino)phenyl)-1-ethanone
obtained in
Preparation 23 (2.4g, 4.91 mmol), thiazolidene-2,4-dione (2.87g, 24.54 mmol),
benzoic acid
(1.20g, 9.81 mmol) and piperidine (0.848, 9.81 mmol) was taken into 100 mL
single neck
round bottom flask, to this toluene (60 mL) was added. The round bottomed
flask (RBF) was
fitted with dean stark, which was connected to a reflux condenser. The
reaction mixture was
heated to reflux for 35 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and stirred for an hour. The solid product formed was filtered
off. The pure product
was obtained by triturating the solid with isopropanol (5 mL), filtered off
and dried under
vacuum to afford the title compound as a pale green solid (l.Slg, 2.56 mmol).
Mp: 215-218
°C.
IR: V m~ (KBr, cm ~): 3380, 2956, 1679;
292
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
'H NMR (200 MHz, DMSO-d6): ~ 12.1(bs, DZO exchangeable, 1H), 7.95 (d, J = 6.98
Hz, 2H), 7.22 (d, J = 8.6 Hz, 2H), 7.03 (d, J = 7.86 Hz, 1H), 6.70 (d, J =
8.59 Hz, 2H),
6.53 (bs, D20 exchangeable, 1H), 4.82 (m, 2H), 4.07 (s, 3H), 3.84 (s, 3H),
3.81 (s,
3H), 3.67 (m, 2H), 2.90 (t, J = 7.25 Hz, 2H), 2.59 (s, 3H), 1.88-1.77 (m, 2H),
0.95 (t,
J = 7.25 Hz, 3H);
Mass (ESMS): 589 (MH+, 100), Purity = 94.5 %.
EXAMPLE 47
This compound was prepared according to the procedure provided in Example 43.
HI NMR: d 12.5(bs, D20 exchangeable, 1H), 8.40-8.42 (m, 2H), 7.75 (s, 1H),
7.59-7.50 (m,
SH), 7.19 (d, J = 8.3Hz, 2H), 5.07 (m, 2H), 4.62 (m, 2H), 4.10 (s, 3H), 2.95
(t, J = 7.32Hz,
2H), 1.86-1.83 (m, 2H), 0.97 (t, J = 7.32Hz, 3H)
EXAMPLE 48
This compound was prepared according to the procedure provided in Example 46.
H~ NMR: d 12.4 (bs, D20 exchangeable, 1H), 8.36 (m, 2H), 7.48-7.31 (m, 7H),
4.94 (m, 2H),
4.05 (s, 3H), 3.64 (m, 2H), 2.91 (m, 2H), 2.60 (s, 3H), 1.85-1.82 (m, 2H),
0.98 (t, 3 = 6.84Hz,
3H)
293
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 49
This compound was prepared according to the procedure provided in Example 46.
H,CO
Hl NMR: d 12.28 (bs, D20 exchangeable, 1H), 7.93 (m, 2H), 7.49-7.29 (m, 4H),
7.04 (d, J =
8.86Hz, 1H), 4.93 (m, 2H), 4.04 (s, 3H), 3.83 (s, 3H), 3.82 (s, 3H), 3.64 (m,
2H), 2.90 (t, J =
7.52Hz, 2H), 2.59 (s, 3H), 1.89-1.78 (m, 2H), 0.96 (t, J = 7.25Hz, 3H).
EXAMPLE 50
This compound was prepared according to the procedure provided in Example 46.
to
HI NMR: d 7.95-7.90 (m, 2H), 7.67 (s, 1H), 7.44-7.33 (m, 3H), 6.95 (d, J =
8.63Hz, 2H),
4.65- 4.59 (m, 2H), 4.33-4.21(m, 7H), 3.03 (m, 4H), 2.80 (t, J = 7.25Hz, 2H),
2.53-2.34 (m,
4H), 2.23 (s, 3H), 1.79-1.69 (m, 2H), 1.30 (t, J = 6.99Hz, 3H), 0.95 (t, J =
7.25Hz, 3H).
EXAMPLE 51
This compound was prepared according to the procedure provided in Example 46.
N ~ ~N~
O
N_N\
294
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
H' NMR: d 7.34-7.16 (m, 7H), 6.97-6.88 (m, 2H), 4.42 (s, 2H), 4.37-4.22 (m,
4H), 4.18 (s,
3H), 2.84 (t, J = 7.33Hz, 2H), 2.63 (s, 3H), 1.83-1.72 (m, 2H), 0.93 (t, J =
7.32Hz, 3H).
EXAMPLE 52
This compound was prepared according to the procedure provided in Example 46.
N-N~
Hl NMR: d 12.5 (bs, D20 exchangeable, 1H) 7.98 (d, J = 7.53Hz, 2H), 7.72 (s,
1H), 7.51 (d,
J = 8.59Hz, 2H), 7.08-7.01 (m, 3H), 4.76 (m, 2H), 4.18 (m, 2H), 4.1 (s, 3H),
3.85 (s, 3H),
3.81 (s, 3H), 2.91 (t, J = 6.99Hz, 2H), 2.08-2.04 (m, 4H), 1.89-1.78 (m, 2H),
0.97 (t, J =
7.25Hz, 3H).
EXAMPLE 53
This compound was prepared according to the procedure provided in Example 46.
~o
s
NH
~ O
O
N N
N~ I N / OCH3
OCH3
Hl NMR: d 12.52 (bs, DZO exchangeable, 1H), 8.01-7.96 (m, 2H), 7.72 (s, 1H),
7.3 (d, J =
8.64Hz, 2H), 7.12-7.02 (m, 3H), 4.85-4.8 (m, 2H), 4.4-4.32 (m, 2H), 4.13 (s,
3H), 3.85 (s,
3H), 3.82 (s, 3H), 2.91 (t, J = 7.25Hz, 2H), 2.5-2.39 (m, 2H), 1.86-1.82 (m,
2H), 0.97 (t, J =
7.26Hz, 3H).
'o EXAMPLE 54
This compound was prepared according to the procedure provided in Example 46.
295
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
OCH3
OCH3
i
N ~ N~O I w O NH
O i i Ss~O
N-N,
HI NMR: d 12.25 (bs, D20 exchangeable, 1H), 8.01-7.96 (m, 2H), 7.42-6.97 (m,
SH), 5.02
(m, 2H), 4.56 (m, 2H), 4.07 (s, 3H), 3.86 (s, 3H), 3.83 (s, 3H), 2.91 (t, J =
7.25, 2H), 3.36 (s,
3H), 1.89-1.78 (m, 2H), 0.97 (t, J = 7.25Hz, 3H).
s
EXAMPLE 55
This compound was prepared according to the procedure provided in Example 46.
0
I ~ ~
NH
O
O /O
J\
N N
N ~ I ~ , OCH3
N
I\ OCH3
H' NMR: d 8.52 (bs, D20 exchangeable, 1H), 8.07 (d, J = 6.72Hz, 2H), 7.41-7.33
(m, 2H),
l0 6.97-6.92 (m, 3H), 5.07 (m, 2H), 4.49-4.48 (m, 2H), 4.19 (s, 3H), 4.02 (s,
3H), 3.96 (s, 3H),
3.03 (t, J = 7.52Hz, 2H), 2.69 (s, 3H), 1.98-1.86 (m, 2H), 1.04 (t, J =
7.25Hz, 3H).
EXAMPLE 56
5-[1-(3-Fluoro-4-{2-[2-(4-fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-
15 3-yloxy]-ethoxy~ -phenyl)-ethylidene]-thiazolidine-2,4-dione
° / I ~ S~o
W ~ °~° ~ °~NH
N \ F
i
CH3
F
A mixture of compound 3-[2-(4-Acetyl-2-fluoro-phenoxy)-ethoxy]-2-(4-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.45 g, 1.0 mmol), 2,4-thiazolidenedione
(0.703 g,
296
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
6.01 mmol), benzoic acid (225 mg, 1.84 mmol), and piperidine (150 mg, 1.76
mmol) was
taken into a single neck round bottom flask, to this toluene (50 mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 1
MeOH-CHC13 to afford the title compound 209 mg (38%) as white solid.
1H NMR (200 MHz, DMSO-d6): d 12.31 (bs, D20 exchangeable, NH), 8.32 (d, J =
8.0 Hz, 1H), 7.79 (d, J = 3.2 Hz, 2H), 7.50-6.99 (m, 8H), 4.27 (s, 2H), 4.06
(s, 2H),
3.47 (s, 3H), 2.64 (s, 3H).
1o Mp: 220-222 °C
EXAMPLE 57
5-[ 1-(3-Ghloro-4- {2-[2-(2-fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-
3-yloxy]
ethoxy}-phenyl)-ethylidene]-thiazolidine-2,4-dione
0
A mixture of compovmd 3-[2-(4-Acetyl-2-chloro-phenoxy)-ethoxy]-2-(2-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (400 mg, 0.86 mmol), 2,4-thiazolidenedione
(504 mg,
4.3 mmol), benzoic acid (250 mg, 2.04 mmol), and piperidine (160 mg, 1.88
mmol) was
taken into 50 mL single neck round bottom flask, to this toluene (50 mL) was
added. The
2o RBF was fitted with dean stark, which is connected to reflux condenser. The
reaction
mixture was heated to reflux for 48 hrs under nitrogen atmosphere. The
reaction mixture was
cooled to 25°C and concentrated under vacuum. The residue was purified
by column
chromatography followed by washing with ether to afford the title compound 200
mg (41 %)
as off white solid.
2s 1H NMR (200 MHz, DMSO-d6): d12.39 (s, NH], 8.43 (d, J=8.0 Hz, 1H), 7.89 (d,
J=3.0 Hz, 2H), 7.54 (s, 4H), 7.41-7.27 (m, 3H), 7.12 (d, J=8.6Hz, 1H), 4.41
(s, 2H),
4.16 (s, 2H), 3.59 (s, 3H), 2.71 (s, 3H).
Mp: 220-222 °C
297
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 58
5-[ 1-(4-{2-[2-(4-Fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethoxy]-
phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I w S~°
I ~ I °~° ~ o NH
/ N
CH3 I /
F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-2-(4-fluoro-phenyl)-1-
methyl-1H-quinolin-4-one (39 g, 9 mmol), 2,4-thiazolidenedione (63.5 g, 54
mmol), benzoic
acid (22.2 g, 18.1 mmol), and piperidine (16 g, 18.79 mmol) was taken into a
single neck
round bottom flask, to this toluene (500 mL) was added. The RBF was fitted
with dean stark,
to which is connected to reflux condenser. The reaction mixture was heated to
reflux for 48 hrs
under nitrogen atmosphere. The reaction mixture was cooled to 25°C and
concentrated under
vacuum. The residue was purified by column chromatography using 0-1% MeOH-
CHC13 to
afford the title compound 24 g (50%) as light brown solid.
1H NMR (200 MHz, DMSO-d6): d12.09 (bs,D20 exchangeble, 1H), 8.34 (d, J=7.8Hz,
1H), 7.80 (d, J=3.4Hz, 2H), 7.53-7.22 (m, 7H), 6.82(d, J=8.2Hz, 2H), 4.23(s,
2H),
3.96(s, 2H), 3.48(s, 3H), 2.64 (s, 3H).
Mp: 228-230°C
EXAMPLE 59
5-[1-(3-{2-[2-(3,4-Dimethoxy-phenyl)-6-fluoro-4-oxo-4H-chromen-3-yloxy]-
ethoxy}-
phenyl)-ethylidene]-thiazolidine-2,4-dione
OMe O
-NH
I
O I \ OMe S ( O
F / O~O
O I /
A mixture of compound 3-[2-(3-Acetyl-phenoxy)-ethoxy]-2-(3,4-dimethoxy-phenyl)-
6-fluoro-chromen-4-one (0.5 g, 1.04 mmol), 2,4-thiazolidenedione (0.79 g, 6.6
mmol),
benzoic acid (0.27 g, 2.2 mmol), and piperidine (0.19 g, 2.23 mmol) was taken
into a single
298
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
neck round bottom flask, to this toluene (35 mL) was added. The RBF was fitted
with dean
stark, which is connected to reflux condenser. The reaction mixture was heated
to reflux for
48 hrs under nitrogen atmosphere. The reaction mixture was cooled to 25
°C, stirred for 6h at
room temperature and filtered. The solid was triturated with i-PrOH (20 mL)
and filtered to
s afford the title compound 0.38 g (63%) as off white solid.
IH NMR (200 MHz, DMSO-d6): d 12.88 (bs, DSO exchangeable, 1H), 7.89-7.71 (m,
SH), 7.28 (s, 1H), 7.06 (d, J = 8.6 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H), 6.87
(d, J = 8.32
Hz, 1H), 6.75 (s, 1H), 4.43 (s, 2H), 4.20 (s, 2H), 3.76 (s, 3H), 2.6 (s, 3H).
Mp: 228-230 °C
to
EXAMPLE 60
5-[1-(4- f 2-[2-(4-Chloro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethoxy] -phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I ~ S~ o
°' NH
N
CH3
CI
15 A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-2-(4-chloro-phenyl)-1-
methyl-1H-quinolin-4-one (1.19 g, 2.45 mmol), 2,4-thiazolidenedione (1.72 g,
14.70 mmol),
benzoic acid (0.59 g, 4.83 mmol), and piperidine (0.415 g, 4.82 mrnol) was
taken into a
single neck round bottom flask, to this toluene (100 mL) was added. The RBF
was fitted
with dean stark, which is connected to reflux condenser. The reaction mixture
was heated to
2o reflux for 72 hrs under a nitrogen atmosphere. The reaction mixture was
cooled to 25°C and
concentrated under vacuum. The residue was purified by column chromatography
using 6%
MeOH-CHC13 to afford the title compound 0.73 g (30%) as light brown solid.
1H NMR (200 MHz, DMSO-d6): 8.32 (d, J = 7.5 Hz, 1H), 7.79 (s, 2H), 7.97-7.32
(m,
7H), 6.79 (d, J = 8.5 Hz, 2H), 4.24 (s, 2H), 3.96 (s, 2H), 3.47 (s, 3H), 2.69
(s, 3H).
2s Mp: 238-240 °C
299
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 61
5-[ 1-(3-{2-[2-(3,4-Difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-
yloxy]-ethoxy}
phenyl)-ethylidene]-thiazolidine-2,4-dione
A mixture of compound 3-[2-(3-Acetyl-phenoxy)-ethoxy]-2-(3,4-difluoro-phenyl)-
1-
methyl-1H-quinolin-4-one (1.0 g, 2.22 mmol), 2,4-thiazolidenedione (1.56 g,
13.36 mmol),
benzoic acid (325 mg, 2.67 mmol), and piperidine (325 mg, 3.82 mmol) was taken
into a
single neck round bottom flask, to this toluene (30 mL) was added. The RBF was
fitted with
dean stark, which is connected to reflux condenser. The reaction mixture was
heated to
reflux for 72 hrs undei nitrogen atmosphere. The reaction mixture was cooled
to 25°C and
concentrated. The residue was purified by column chromatography to afford the
title
compound 488 mg (40 %) as light brown solid.
'H NMR (200 MHz, DMSO-d6}: d 12.31 (NH, 1H), 8.32 (d, 1H, J=7.SHz), 7.81 (s,
2H), 7.62-7.30 (m, SH), 6.95 (d, 1H, J = 7.8 Hz), 6.77 (d, 2H, J = 9.7Hz),
4.26 (s,
is 2H), 3.95 (s, 2H), 3.49 (s, 3H), 2.64 (s, 3H).
Mp: 236-240 °C
EXAMPLE 62
5-[I-(4- f 2-[1-Ethyl-2-(4-fluoro-phenyl)-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethoxy}-
phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I ~ S~o
° NH
F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-1-ethyl-2-(4-fluoro-
phenyl)-1H-quinolin-4-one (0.6 g, 1.35 mmol), 2,4-thiazolidenedione (0.946 g,
8.08 mmol),
benzoic acid (200 mg, 1.64 mmol), and piperidine (200 mg, 2.35 mmol) was taken
into a
300
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
single neck round bottom flask, to this toluene (30 mL) was added. The RBF was
fitted with
dean stark, which is connected to reflex condenser. The reaction mixture was
heated to
reflex for 72 hrs under nitrogen atmosphere. The reaction mixture was cooled
to 25°C and
concentrated. The residue was purified by column chromatography using MeOH-
CHCl3 to
afford the title compound 333 mg (45 %) as light brown solid.
IH NMR (200 MHz, DMSO-db~: d 12.09((bs, 1H, d20 exchangeble), 8.35(d,
J=7.8Hz, 1H}, 7.87-7.77 (m, 2H), 7.55-7.23 (m, 7H), 6.83 (d, J=8.7Hz, 2H),
4.23(s,
2H), 4.02-3.98(m, 4H), 2.64(s, 3H), 1.16(t, J=6.8Hz, 3H).
Mp: 214-216 °C
EXAMPLE 63
5-[1-(4- f 2-[2-(3,4-Difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-
yloxy]-ethoxy}-
phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I ~ S~o
° NH
N W F
CH3
F
is A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-2-(3,4-difluoro-
phenyl)-1-
methyl-1H-quinolin-4-one (1.75 g, 3.89 mmol), 2,4-thiazolidenedione (2.74 g,
23.38 mmol),
benzoic acid (475 mg, 3.89 mmol), and piperidine (331 mg, 3.89 mmol) was taken
into a
single neck round bottom flask, to this toluene (30 mL) was added. The RBF was
fitted with
dean stark, which is connected to reflex condenser. The reaction mixture was
heated to
reflex for 72 hrs under nitrogen atmosphere. The reaction mixture was cooled
to 25°C and
concentrated. The residue was purified by column chromatography using MeOH-
CHCl3 to
afford the title compound 830 mg (39 %) as light brown solid.
'H NMR (200 MHz, DMSO-d~: d 12.24 (bs, NH, DZO exchangeable), 8.32 (d, J =
8.0 Hz, 1H), 7.81 (s, 2H), 7.66-7.32 (m, 6H), 6.80 (d, 3 = 8.6 Hz, 2H), 4.25
(s, 2H),
3.97 (s, 2H), 3.49 (s, 3H), 2.64 (s, 3H).
Mp: 248-250°C
301
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 64
5-[1-(4-{2-[7-Chloro-2-(4-fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-
yloxy]-
ethoxy}-phenyl)-ethylidene]-thiazolidine-2,4-dione
° / I ~ S~o
°~° ~ ° NH
CI
CH/3\ iI ~ F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-7-chloro-2-(4-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.4 g, 0.85 mmol), 2,4-thiazolidenedione
(0.502 g,
4.29 mmol), benzoic acid (190 mg, 1.55 mmol), and piperidine (145 mg, 1.70
mmol) was
taken into a single neck round bottom flaslc, to this toluene (50 mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
l0 heated to reflux for 48 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and filtered. The solid was treated with i-PrOH under reflux for 2
hours and then
filtered. The solid was washed with hexane and purified by column
chromatography to
afford the title compound 200 mg (41 %) as white solid.
'H NMR (200 MHz, DMSO-d6~: d 12.10 (bs, D20 exchangeble, 1H), 8.32 (d, J =
7.SHz, 1H), 7.89 (s, 1H), 7.48-7.27 (m, 7H), 6.82 (d, J = 8.0 Hz, 2H), 4.23
(s, 2H),
3.95 (s, 2H), 3.45 (s, 3H), 2.63 (s, 3H).
Mp: 292-296°C
EXAMPLE 65
5-[1-(4-{2-[2-(4-Fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethylamino}-phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ ~ \ S~o
° NH
N
CH3
F
A mixture of compound 3-[2-(4-Acetyl-phenylamino)-ethoxy]-2-(4-fluoro-phenyl)-
1-
methyl-1H-quinolin-4-one (0.4 g, 0.93 mmol), 2,4-thiazolidenedione (0.65 g,
5.6 mmol),
302
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
benzoic acid (225 mg, 1.84 mmol), and piperidine (180 mg, 2.11 mmol) was taken
into a
single neck round bottom flask, to this toluene (100 mL) was added. The RBF
was fitted
with dean stark, which is connected to reflux condenser. The reaction mixture
was heated to
reflux for 48 hrs under nitrogen atmosphere. The reaction mixture was cooled
to 25°C and
concentrated. The residue was purified by column chromatography using 0-2%
MeOH-
CHG13 to afford the title compound 200 mg (41 %) as yellow solid.
1H NMR (200 MHz, DMSO-db7: d 8.61 (d, J = 8.3 Hz, 2H), 7.77 (t, J = 8.2 Hz,
2H),
7.56-7.19 (m, 6H), 6.56 (d, J = 8.3 Hz, 2H), 5.99 (bs, NH), 3.94-3.92 (m, 2H),
3.53-
3.41 (m, 4H), 3.18 (s, 2H), 2.68 (s, 3H).
l0 Mp: 130-132 °C
EXAMPLE 66
5-(4- f 2-[2-(4-Fluoro phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethoxy}-
benzylidene)-thiazolidine-2,4-dione
o ° I ~ Sao
I ~ I ono ~ o NH
° N
CH3 I
A mixture of compound 4- f 2-[2-(4-Fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-yloxy]-ethoxy}-benzaldehyde (0.3 g, 0.719 mmol), 2,4-
thiazolidenedione (0.168
g, 1.43 mmol), benzoic acid (30 mg, 0.24 mmol), and piperidine (30 mg, 0.35
mmol) was
taken into a single neck round bottom flask, to this toluene (50 mL) was
added. The RBF
2o was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 12 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
50°C and filtered. The residue was washed with hot MeOH and dried under
vacuum to
afford the title compound 200 mg (54%) as brown solid.
1H NMR (200 MHz, DMSO-dc~: d 12.5 (s, NH, D20 exchangeble), 8.35 (d, J=8.lHz,
1H), 7.81 (d, J = 3.2 Hz, 2H), 7.72 (s, 1H), 7.51-7.47 (m, SH), 7.29-7.25 (m,
2 H),
6.88 (d, J = 8.6 Hz, 2H), 4.20 (s, 2H}, 3.99 (s, 2H), 3.50 (s, 3H).
Mp: 225-228 °C
303
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 67
5-(4- {2-[2-(4-Bromo-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
ethoxy} -
s A mixture of compound 4-{2-[2-(4-Bromo-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-yloxy]-ethoxy]-benzaldehyde (0.125 g, 0.25 mmol), 2,4-
thiazolidenedione (0.178
g, 1.5 mmol), benzoic acid (63 mg, 0.51 mmol), and piperidine (45 mg, 0.52
mmol) was
taken into a single neck rowed bottom flask, to this toluene (50 mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
to heated to reflux for 12 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The solid separated was filtered, washed with
diethyl ether and dried
under vacuum to afford the title compound 80 mg (53%) as light brown solid.
1H NMR (200 MHz, DMSO-cl~: d 8.35 (d, J = 8.8 Hz, 1H), 7.87-7.39 (m, 11H),
6.89
(d, J = 8.7 Hz, 2H), 4.24 (bs, 2H), 4.01 (bs, 4H), 1.16 (t, J = 6.8Hz, 3H).
1s Mp: 151-I54 °C
EXAMPLE 68
5-[ 1-(4-{2-[2-(5-Fluoro-2-methyl-phenyl)-1-methyl-4-oxo-I,4-dihydro-quinolin-
3-yloxy]
ethoxy} -phenyl)-ethylidene]-thiazolidine-2,4-dione
S
O
NH
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-2-(5-fluoro-2-methyl-
phenyl)-1-methyl-1H-quinolin-4-one (0.25 g, 0.56 mmol), 2,4-thiazolidenedione
(0.394 g,
3.37 mmol), benzoic acid (142 mg, 1.16 mmol), and piperidine (100 mg, 1.17
mmol) was
taken into a single neck round bottom flask, to this toluene (50 mL) was
added. The RBF
304
benzylidene)-thiazolidine-2,4-dione
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 48 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 3-20%
EtOAc-CHCl3 to afford the title compound 80 mg (26%) as brown solid.
'H NMR (200 MHz, DMSO-dc~: d 12.30 (s, D20 exchangeble, 1H), 8.35 (d, J=7.8Hz,
1H), 7.80 (s, 2H), 7.47-7.16 (m, SH), 6.96 (d, J = 7.8 Hz, 1H), 6.79 (d, J =
9.1 Hz,
2H), 4.31-4.26 (m, 2H), 3.95 (m, 2H), 3.52 (s, 3H), 2.62 (s, 3H), 2.21 (s,
3H).
Mp: I92-296 °C
io EXAMPLE 69
5-[ 1-(3-{2-[2-(4-Fluoro-2-methyl-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-
3-yloxy]
ethoxy}-phenyl)-ethylideneJ-thiazolidine-2,4-dione
A mixture of compound 3-[2-(3-Acetyl-phenoxy)-ethoxy]-2-(4-fluoro-2-methyl-
is phenyl)-1-methyl-IH-quinolin-4-one (0.25 g, 0.56 mmol), 2,4-
thiazolidenedione (0.394 g,
3.37 mmol), benzoic acid (150 mg, 1.22 mmol), and piperidine (100 mg, 1.17
mmol) was
taken into a single neck round bottom flask, to this toluene (50 mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 48 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
20 25°C and concentrated. The residue was purified by column
chromatography using 3-20%
EtOAc-CHCl3 to afford the title compound 120 mg (39%) as brown solid.
'H NMR (200 MHz, DMSO-dG): d 11.22 (bs, 1H), 8.59 (d, J = 7.2 Hz, 1H), 7.76-
6.63
(m, lOH), 4.45-4.26 (m, 2H), 4.0-3.98 (m, 2H), 3.58 (s, 3H), 2.67 (s, 3H),
2.I5 (s,
3H).
2s Mp: 225-226 °C
305
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 70
5-[ I-(4-~3-[2-(4-Fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
propoxy}-
phenyl)-ethylidene]-thiazolidine-2,4-dione
0
w I ono I w
N ~ s
CH3 I
F o ~S
HN--
0
A mixture of compound 3-[3-(4-Acetyl-phenoxy)-propoxy]-2-(4-fluoro-phenyl)-1-
methyl-1H-quinolin-4-one (0.40 g, 0.898 mmol), 2,4-thiazolidenedione (0.631 g,
5.39
mmol), benzoic acid (225 mg, 1.82 mmol), and piperidine (175 mg, 2.05 mmol)
was taken
into a single neck round bottom flask, to this toluene (30 mL) was added. The
RBF was
fitted with dean stark, which is connected to reflux condenser. The reaction
mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by colunm
chromatography using 1
MeOH-CHCl3 to afford the title compound 130 mg (27 %) as white solid.
IH NMR (200 MHz, DMSO-d~: d 12.27 (bs, NH, DSO exchangeable), 8.30 (d, J =
7.8 Hz, 1H), 7.81 (s, 2H), 7.55-7.3 (m, 7H), 6.88 (d, J = 7.8 Hz, 2H), 4.01
(s, 2H),
3.62 (s, 2H), 3.49 (s, 3H), 2.68 (s, 3H), 1.82 (s, 2H).
Mp: 262-264 °C
EXAMPLE 71
5-[ 1-(3- { 3-[2-(4-Fluro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-yloxy]-
propoxy} -
phenyl)-ethylidene]-thiazolidine-2,4-dione
0
I w I oho I w w S~o
N ~ ~ o NH
CH3 I
F
A mixture of compound 3-[3-(3-Acetyl-phenoxy)-propoxy]-2-(4-fluoro-phenyl)-1-
methyl-1H-quinolin-4-one (0.45 g, 1.011 mmol), 2,4-thiazolidenedione (0.710 g,
6.06
mmol), benzoic acid (300 mg, 2.46 mmol), and piperidine (300 mg, 3.52 mmol)
was taken
306
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
into a single neck round bottom flask, to this toluene (50 mL) was added. The
RBF was
fitted with dean stark, which is connected to reflux condenser. The reaction
mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 0.3%
MeOH-CHC13 to afford the title compound 187 mg (34 %) as light brown solid.
IH NMR (200 MHz, DMSO-d~: d 12.29 (NH, 1H), 8.31 (d, 1H, J = 7.8 Hz), 7.78 (s,
2H), 7.56-7.22 (m, 6H), 6.97 (d, 1H, J = 7.SHz), 6.80 (d, 2H, J = 6.7 Hz),
3.99 (s,
2H), 3.58-3.55 (m, 2H), 3.46 (s, 3H), 2.66 (s, 3H), 1.79 (s, 2H).
Mp: 168-170 °C
EXAMPLE 72
5-[ 1-(3-Chloro-4-{3-[2-(4-fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-
3-yloxy)-
propoxy) -phenyl)-ethylidene]-thiazolidine-2,4-dione
0
~ I ono I ~
N \ CI
cH3 I~~ I
S
H N-
0
A mixture of compound 3-[3-(4-Acetyl-2-chloro-phenoxy)-propoxy]-2-(4-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.40 g, 0.86 nnnol), 2,4-thiazolidenedione
(0.603 g,
5.16 mmol), benzoic acid (150 mg, 1.23 mmol), and piperidine (300 mg, 3.52
mmol) was
taken into a single neck round bottom flask, to this toluene (SO mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 2%
MeOH-CHC13 to afford the title compound 140 mg (30 %) as light brown solid.
'H NMR (200 MHz, DMSO-d~: d 12.31 (NH, 1H), 8.32 (d, 1H, J = 7.8Hz), 7.80 (d,
2H, J = 2.9 Hz), 7.48-7.13 (m, 7H), 7.02 (d, 1 H, J = 8.3 Hz), 4.29 (s, 2I~,
4.07 (s,
2H), 3.47 (s, 3H), 2.64 (s, 3H), 1.82 (s, 2H).
Mp: 232-234 °C
307
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 73
5-[ 1-(4- f 2-[7-Fluoro-2-(4-fluoro-phenyl)-I-methyl-4-oxo-1,4-dihydro-
quinolin-3-yloxy]-
ethoxy} -phenyl)-ethylidene]-thiazolidine-2,4-dione
CH3 / F
F ~ N w I
I ~ I O'~O .~ s
O I / / NH
CH3 O
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-7-fluoro-2-(4-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.40 g, 0.89 mmol), 2,4-thiazolidenedione
(0.521 g,
4.45 mmol), benzoic acid (200 mg, 1.63 mmol), and piperidine (150 mg, 1.76
mmol) was
taken into a single neck round bottom flask, to this toluene (50 mL) was
added. The RBF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 48 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 0.5-I
MeOH-CHCI3 to afford the title compound 100 mg (21 %) as brown solid.
1H NMR (200 MHz, DMSO-d~: d 12.24 (bs, 1H), 8.39 (t, J = 7.30 Hz, 1H), 7.67
(d,
J = 11.8 Hz, 1H), 7.52 (t, J = 8.30 Hz, 2H), 7.36 (t, J = 8.80 Hz, SH), 6.82
(d, J = 8.8
is Hz, 2H), 4.22 (s, 2H), 3.95 (s, 2H), 3.33 (s, 3H), 2.63 (s, 3H).
Mp: 276-278 °C
EXAMPLE 74
5-[ 1-(4-{2-[2-(3,4-Difluoro-phenyl)-7-fluoro-1-methyl-4-oxo-1,4-dihydro-
quinolin-
3-yloxy]-ethoxy} -phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I ~ S'~o
° NH
F I / N I ~ F
CH3 I /
F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-2-(3,4-difluoro-phenyl)-
7-
fluoro-1-methyl-IH-quinolin-4-one (0.40 g, 0.85 mmol), 2,4-thiazolidenedione
(0.50 g, 4.28
mmol), benzoic acid (190 mg, 1.55 mmol), and piperidine (145 mg, 1.70 mmol)
was taken
308
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
into a single neck round bottom flask, to this toluene (50 mL) was added. The
RBF was
fitted with dean stark, which is connected to reflux condenser. The reaction
mixture was
heated to reflux for 48 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
50 °G, filtered and washed with hot toluene. The solid was treated with
toluene under reflux
for 10 hours, filtered, washed with hot MeOH and dried to afford the title
compound 125 mg
(26%) as white solid.
1H NMR (200 MHz, DMSO-d~: d 12.23 (s, 1H), 8.39 (t, J = 8.6 Hz, 1H), 7.68-7.32
(m, 7H), 6.8I (d, J = 8.6 Hz, 2H), 4.25 (s, 2H), 3.97 (s, 2H), 3.43 (s, 3H),
2.64 (s,
3H).
Mp: 270-272 °C
EXAMPLE 75
5-[ 1-(4- ~2-[2-(3,4-Difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-quinolin-3-
yloxy]-ethoxy}
3-fluoro phenyl)-ethylidene]-thiazolidine-2,4-dione
\ S~ o
°~o ~ o NH
N ( W F F
CH3 I ~ F
A mixture of compound 3-[2-(4-Acetyl-2-fluoro-phenoxy)-ethoxy]-2-(3,4-difluoro-
phenyl)-I-methyl-1H-quinolin-4-one (0.40 g, 0.856 mmol), 2,4-thiazolidenedione
(0.701 g,
5.99 mmol), benzoic acid (200 mg, 1.64 mmol), and piperidine (200 mg, 2.35
mmol} was
taken into a single neck round bottom flask, to this toluene (30 mL) was
added. The RBF
2o was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 1-2%
MeOH-CHCl3 to afford the title compound 170 mg (35 %) as light brown solid.
1H NMR (200 MHz, DMSO-d~: d 12.29 (NH, 1H), 8.32 (d, 1H, J = 7.8 Hz), 7.80 (d,
2H, J = 2.9 Hz), 7.62-6.99 (m, 7H), 4.28 (s, 2H), 4.07 (s, 2H), 3.48 (s, 3H),
2.64 (s,
3H).
Mp: 204-206 °C
309
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 76
5-[1-(4-{2-[7-Chloro-2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-
yloxy]-ethoxy]-phenyl)-ethylidene]-thiazolidine-2,4-dione
° ~ I ~ S~o
o~° W ° NH
CI ° N' \ F
CH~3\ iI\~/
F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-7-chloro-2-(3,4-difluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.60 g, 1.2 mmol), 2,4-thiazolidenedione
(0.872 g, 7.0
mmol), benzoic acid (151 mg, 1.2 mmol), and piperidine (105 mg, 1.2 mmol) was
taken into
a single neck round bottom flask, to this toluene (20 mL) was added. The RBF
was fitted
with dean stark, which is connected to reflux condenser. The reaction mixture
was heated to
reflux for 48 hrs under nitrogen atmosphere. The reaction mixture was cooled
to 25°C and
concentrated. The residue was purified by column chromatography using MeOH-
CHCl3 to
afford the title compound 150 mg (21 %) as white solid.
1H NMR (200 MHz, DMSO-db~: d 12.21 (bs, D2O exchangeable, NH), 8.30 (d, J =
8.8 Hz, 1H), 7.89 (s, 1H), 7.62-7.30 (m, 6H), 6.79 (d, J = 8.8 Hz, 2H), 4.24
(s, 2H),
3.94 (s, 2H), 3.45 (s, 3H), 2.62 (s, 3H).
Mp: 296-298 °C
EXAMPLE 77
5-[1-(3-Chloro-4-{2-[7-chloro-2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-1,4-
dihydro-quinolin-
3-yloxy]-ethoxy}-phenyl)-ethylidene]-thiazolidine-2,4-dione
=o
CI
A mixture of compound 3-[2-(4-Acetyl-2-chloro-phenoxy)-ethoxy]-7-chloro-2-(3,4-
difluoro-phenyl)-1-methyl-1H-quinolin-4-one (0.50 g, 0.96 mmol), 2,4-
thiazolidenedione
(0.677 g, 5.0 mmol), benzoic acid (117 mg, 0.96 mmol), and piperidine (95 mg,
0.96 mmol)
310
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
was taken into a single neck round bottom flask, to this toluene (20 mL) was
added. The
RBF was fitted with dean stark, which is connected to reflux condenser. The
reaction
mixture was heated to reflux for 48 hrs under nitrogen atmosphere. The
reaction mixture was
cooled to 25°C and concentrated. The residue was purified by column
chromatography using
MeOH-CHCl3 to afford the title compound 120 mg (20%) as white solid.
1H NMR (200 MHz, DMSO-d~: d 12.25 (bs, D20 exchangeable, NH), 8.26 (d, J =
8.3 Hz, 1H), 7.86 (s, 1H), 7.46-7.19 (m, 6H), 6.99 (d, J = 8.8 Hz, IH), 4.20
(s, 2H),
4.02 (s, ZH), 3.39 (s, 3H), 2.57 (s, 3H).
Mp: 140-142 °C
EXAMPLE 78
5-[ 1-(4- {2-[ 6-Fluoro-2-(4-fluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-yloxy]
ethoxy}-phenyl)-ethylidene]-thiazolidine-2,4-dione
° / I ~ S~o
F I ~ I °~° ~ ° NH
N
CH3
F
A mixture of compound 3-[2-(4-Acetyl-phenoxy)-ethoxy]-6-fluoro-2-(4-fluoro-
phenyl)-1-methyl-1H-quinolin-4-one (0.4 g, 0.82 mmol), 2,4-thiazolidenedione
(0.581 g,
4.96 mmol), benzoic acid (650 mg, 5.32 mmol), and piperidine (500 mg, 5.87
mmol) was
taken into a single neck round bottom flask, to this toluene (30 mL) was
added. The RSF
was fitted with dean stark, which is connected to reflux condenser. The
reaction mixture was
heated to reflux for 72 hrs under nitrogen atmosphere. The reaction mixture
was cooled to
25°C and concentrated. The residue was purified by column
chromatography using 0.5-1
MeOH-CHCl3 to afford the title compound 80 mg (16 %) as light brown solid.
'H NMR (400 MHz, DMSO-d6): d12.27 (bs, D20 exchangeable, NH), 8.35 (dd, J =
8.9, 7.0 Hz, 1H), 7.64 (d, J = 12 Hz, 1H), 7.55-7.47 (m, 3H), 7.39-7.23 (rn,
3H), 7.04
(d, J = 8.6 Hz, 1H), 4.28-4.26 (m, 2H), 4.07-4.06 (m, 2H), 3.42 (s, 3H), 2.63
(s, 3H).
Mp: 245-248 °C
311
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLE 79
5-[1-(3-Chloro-4-~2-[2-(3,4-difluoro-phenyl)-6-fluoro-1-methyl-4-oxo-1,4-
dihydro-quinolin-
3-yloxy]-ethoxy}-phenyl)-ethylidene]-thiazolidine-2,4-dione
~ I ~ S~o
F ~ °~o ~ o NH
N I ~ Cl
CH3
F
F
A mixture of compound 3-[2-(4-Acetyl-2-chloro-phenoxy)-ethoxy]-2-(3,4-difluoro-
phenyl)-6-fluoro-1-methyl-1H-quinolin-4-one (1.4 g, 2.70 mmol), 2,4-
thiazolidenedione
(1.96 g, 16.7 mmol), benzoic acid (600 mg, 4.91 mmol), and piperidine (470 mg,
5.51 mmol)
was taken into a single neck round bottom flask, to this toluene (30 mL) was
added. The
RBF was fitted with dean stark, which is connected to reflux condenser. The
reaction
l0 mixture was heated to reflux for 72 hrs under nitrogen afimosphere. The
reaction mixture was
cooled to 25°C and concentrated. The residue was purified by column
chromatography using
0.5-1 % MeOH-CHCl3 to afford the title compound 40 mg (8 %) as light brown
solid.
IH NMR (400 MHz, DMSO): d12.27 (bs, D20 exchangeable, NH), 8.40-8.33 (m,
1H), 7.64 (d, J =12.0 Hz, 1H), 7.55-7.47 (m, 3H), 7.39-7.23 (m, 3H), 7.04 (d,
J = 8.6
Hz, 1H), 4.30 (m, 2H), 4.08 (m, 2H), 3.42 (s, 3H), 2.63 (s, 3H).
Mp: 215-218 °C
EXAMPLE 80
5-[1-(3-Chloro-4- f 2-[2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-
yloxy]-ethoxy} -phenyl)-ethylidene]-thiazolidine-2,4-dione
r
'NH
A mixture of compound 3-[2-(4-Acetyl-2-chloro-phenoxy)-ethoxy]-2-(3,4-difluoro-
phenyl)-1-methyl-1H-quinolin-4-one (97 g, 200 mmol), thiazolidine-2,4-dione
(141 g, 1200
312
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
mmol), benzoic acid (44 g, 361 mmol) and piperidine (35 g, 4I 1.7 mmol) were
taken a single
neck round bottomed flask, to this toluene (1000 mL) was added. The round-
bottomed flask
was fitted with dean stark apparatus, which was connected to a reflux
condenser. The
reaction mixture was heated to reflux for 48 hours under a nitrogen
atmosphere. The reaction
mixture was cooled to 25°C and was allowed to pass through a silica gel
column. The
product was eluted by using 0.3-0.9 % MeOH / CHC13 to afford the title
compound, 37 g
(32%) as off white solid.
IH NMR (200 MHz, CDCI3) ~ 12.30 (s, 1H), 8.32 (m, 1H), 7.77 (m, 2H), 7.52
(ddd, J
= 8.0, 2.0, 0.8 Hz, 1 H), 7.48 (d, J = 2.0 Hz, 1 H), 7.45 (m, 1 H), 7.25 (m, 1
H), 7.3 5
(dd, J = 10.0, 2.4 Hz, 1 H), 7.31 (dd, J = 8.4, 2.4 Hz, 1 H), 7.04 (d, J = 8.4
Hz, 1 H),
4.31 (dd, J= 3.4, 6.8 Hz, 2H), 4.09 (dd, J= 3.4, 6.8 Hz, 2H), 3.47 (s, 3H),
2.63 (s,
3H). Mp: 212-214 °C
EXAMPLE 81
5-[1-(4-{3-[2-(3,4-Dimethoxy-phenyl)-5,7-dimethoxy-4-oxo-4H-chromen-3-yloxy]-
propoxy] -phenyl)-ethylidene]-thiazolidine-2,4-dione
/ OMe
Me0 I ~ O I ~ ( OMe
O~O ~ S NH
OMe O
O
A mixture of compound 3-[3-(4-Acetyl-phenoxy)-propoxy]-2-(3,4-dimethoxy
phenyl)-7-ethyl-5-methoxy-chromen-4-one (0.30 g, 0.562 mmol), 2,4-
thiazolidenedione (330
mg, 2.82 mmol), benzoic acid (132 mg, 1.08 mmol), and piperidine (96 mg, 1.13
mmol) was
taken into 50 mL single neck round bottom flask, to this toluene (15 mL) was
added. The
RBF was fitted with dean stark, which is connected to reflux condenser. The
reaction
mixture was heated to reflux for 48 hrs under a nitrogen atmosphere. The
reaction mixture
was cooled to 25°C and was filtered. The solid was dried to afford the
title compound 189
mg (34%) as white solid.
'H NMR (200 MHz, CDCl3): d 8.38 (s, 1H), 7.68-7.65 (m, 2H), 7.3-7.25 (m, 2H),
6.91-6.85 (m, 3H), 6.51 (s, 1H), 6.37 (s, 1H), 4.25-4.13 (m, 4H), 3.97-3.91
(m, 12H),
2.7 (s, 1H), 2.25-2.19 (m, 2H)
Mp: 198-200 °C
313
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
EXAMPLES 82-91
The following compounds are readily prepared by one of skill in the art using
the
processes set forth above:
Exam Com ound
le
82 5-[1-(3-Chloro-4-{2-[2-(3,4-difluoro-phenyl)-4-oxo-4H-
chromen-3-yloxy]-ethoxy} -phenyl)-ethylidene]-thiazolidine-
2,4-dione
83 5-[1-(3-Chloro-4-{3-[2-(3,4-difluoro-phenyl)-4-oxo-4H-
chromen-3-yl]-propoxy] -phenyl)-ethylidene]-thiazolidine-2,4-
dione
84 5-[1-(3-Chloro-4-{3-[2-(3,4-difluoro-phenyl)-4-oxo-4H-
chromen-3- yloxy]-propyl}-phenyl)-ethylidene]-thiazolidine-
2,4-dione
85 5-[1-(3-Chloro-4-{3-[2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-
1,4-dihydro-quinolin-3-yl]-propoxy]-phenyl)-ethylidene]-
thiazolidine-2,4-dione
86 5-[1-(3-Chloro-4-{3-[2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-
1,4-dihydro-quinolin-3-yloxy]-propyl}
-phenyl)-ethylidene]-
thiazolidine-2,4-dione
87 5-[1-(3-Chloro-4-{2-[5-(3,4-difluoro-phenyl)-1,3-dimethyl-7-
oxo-1,7-dihydro-pyrazolo[4,3-d]pyrimidin-6-yl]-ethoxy}-
phenyl)-ethylidene]-thiazolidine-2,4-dione
88 5-[1-(3-Chloro-4-{3-[5-(3,4-difluoro-phenyl)-1,3-dimethyl-7-
oxo-1,7-dihydro-pyrazolo[4,3-d]pyrimidin-6-yl]-propoxy}-
phenyl)-eth lidene]-thiazolidine-2,4-dione
89 3-[2-(3-Chloro-4-{2-[2-(3,4-difluoro-phenyl)-4-oxo-4H-
chromen-3-yloxy]-ethoxy]-phenyl)-acetylamino]-2-(toluene-
4-sulfonylamino)-propionic acid ethyl
ester
90 3-[2-(3-Chloro-4-{2-[2-(3,4-difluoro-phenyl)-1-methyl-4-oxo-
1,4-dihydro-quinolin-3-yloxy]-ethoxy}-phenyl)-acetylamino]-
2-(toluene-4-sulfonylamino)- ro ionic
acid ethyl ester
91 3-(4-{2-[2-(3,4-Difluoro-phenyl)-1-methyl-4-oxo-1,4-dihydro-
quinolin-3-yloxy]-ethoxy] -benzoylamino)-2-(toluene-4-
sulfonylamino)-pro ionic acid ethyl ester
Similarly, other starting materials and intermediates are prepared by the
application or
adaptation of known methods, for example methods as described in the reference
examples
or their obvious chemical equivalents (Ref (i) J. HET. CHEM., 1999(36)141;
(ii) For
preparation of bromolcetone see (a) J. MED. CHEM. 1996(39), 2939-2952; (b) J.
HET.
CHEM., 1972(9) 887; (b) INDIAN 3. CHEM. SECT., 1990(29) 77; (c) TETRAHEDRON
LETT.,
1997(38) 3581; (d) CHEM. PHARM. BULL. 1992(40)1170).
314
CA 02540460 2006-03-10
WO 2005/042712 PCT/US2004/035939
The pharmaceutically acceptable salts are prepared by reacting the compounds
of
formula (I) wherever applicable with 1 to 4 equivalents of a base, for
example, sodium
hydroxide, sodium methoxide, sodium hydride, potassium t-butoxide, calcium
hydroxide,
magnesium hydroxide, or any mixture thereof, in the presence of a solvent, for
example,
ether, THF, methanol, t-butanol, dioxane, isopropanol, ethanol, or any mixture
thereof.
Organic bases, for example, lysine, arginine, diethanolamiiie, choline,
tromethamine,
guanidine, or any derivative or mixture thereof, also may be used.
Alternatively, acid
addition salts wherever applicable are prepared by treatment with acids, for
example,
hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric
acid, p-
l0 toluenesulphonic acid, methanesulfonic acid, acetic acid, citric acid,
malefic acid salicylic
acid, hydroxynaphthoic acid, ascorbic acid, palinitic acid, succinic acid,
benzoic acid,
benzenesulfonic acid, tartaric acid, or any mixture thereof, in the presence
of a solvent, for
example, ethyl acetate, ether, alcohols, acetone, THF, dioxane, or any mixture
thereof. The
salts of amino acid groups and other groups may be prepared by reacting the
compounds of
formula (I) with the respective groups in the presence of a solvent, for
example, alcohols and
ketones, or any mixture thereof.
Various polymorphs of a compound of general formula (I) according to the
present
invention may be prepared by crystallization of compound of formula (I) under
different
conditions, for example, by using different solvents or their mixtures for
recrystallization; by
performing crystallizations at different temperatures; or by using various
modes of cooling,
ranging from very fast to very slow cooling during crystallizations. Heating
or melting the
compound followed by gradual or fast cooling also may obtain polymorphs. The
presence of
polymorphs may be determined by solid probe NMR spectroscopy, IR spectroscopy,
differential scanning calorimetry, powder X-ray diffraction or such other
techniques.
Pharmaceutically acceptable solvates of compound of formula (I) forming part
of this
invention may be prepared by conventional mefliods such as dissolving the
compounds of
formula (I) in the presence of a solvent, for example, water, methanol,
ethanol etc., for
example, water and recrystallizing by using different crystallization
techniques.
The regioisomers of a compound of formula (I) may be prepared by modifying the
reaction conditions, for example, by using reagents, for example, acid to base
or base to acid,
or by reaction with free base hydrazine instead of its salt with diketone. The
molar
proportion also can change the regioisomer formation.
315