Note: Descriptions are shown in the official language in which they were submitted.
CA 02540567 2006-03-21
TRI-PEEL PACK
FOR DISPENSING MEDICANTS
FIELD OF THE INVENTION
The present invention relates to a tamper-evident package and more
particularly,
relates to a tamper-evident package adapted to contain unit does capsules,
tablets or like
products in a patient compliant form having single or multiple folded data
layers for a
1 week, 2 week, 28 day 30 day pack or B.I.D. dosage regimen, etc.
BACKGROUND OF THE INVENTION
The art is replete with tamper-evident packaging wherein a unit article is
packaged in
a manner such that subsequent to the packaging, access cannot be obtained to
the article
without leaving a telltale trace.
One of the most common fields in which tamper-evident packaging is employed is
in
the pharmaceutical field, although products other than pharmaceuticals have
also been
packaged in such a manner.
As aforementioned, it is desirable to provide for a child resistant and tamper-
evident
packaging that will give a clear indication when the contents of the packaging
have been
tampered with. However, at the same time, the package must be sufficiently
easy for the
average senior to open. Still further, it is desirable that the package be
child resistant, i.e.
that a child would have a certain degree of difficulty in obtaining access to
one product in
the multiple packaging.
In the art, a conventional-type package which is utilized, is a laminate which
comprises a blister layer having capsule receiving pockets and a foil layer
over the back of
the blister pack. The foil material is peelable such that when the peelable
blister bubble
-1-
CA 02540567 2006-03-21
forming the pocket is peeled off, the capsule or other item in the blister
pocket will be
exposed for use.
In the art, a second conventional-tupe package which is utilized is a laminate
which
comprises a blister layer having capsule receiving pockets and a foil layer
over the back of
the blister pack. The foil material is peelable, such that when the lidding
material over the
blister bubble forming the pocket is removed, the capsule is exposed and
removed for use.
While this type of arrangement has been found to be suitable, such a package
is not child
resistant and is not as patient compliant.
SUMMARY OF THE INVENTION
It is therefore an object of the present invention to provide a tamper-
resistant package
which, while providing reasonable, easy access to the end user, has much
greater child
resistant features. A patient compliant dosage regimen package and an
information data
layer for various information depending on the product to be packaged such as
information
concerning dosage and administration, directions for use, pharmaceutical
information,
adverse reactions and clinical pharmacology, indication and clinical use,
contraindications,
warnings and precautions, all part of the inner data layer or part of the
extended inner and
outer extended data layer.
According to the present invention, such a package comprises an outer back
CR/SF
layer, and front layer, which are extended to form a creased fold over
compliance. The
extended inner back layer is glued to the intermediate laminate (foil/paper).
This composite
is then sealed to a blister layer with at least one capsule-receiving pocket
formed therein, a
peelable foil/paper adhered to the blister layer with the foil/paper overlying
said pocket, and
at least two apertures formed in the laminate. Two tab members formed in the
back layer,
-2-
CA 02540567 2006-03-21
two removable panels formed in the back layer, the two removable panels, one
overlying the
tab member and at least one of the capsule-receiving pockets and the other can
only be
accessed when the first tab member is removed and which covers the leading
edge of the
peelable lidding material and is facing the lead edge of the first tab member,
the outer back
layer being formed of a material which, when a force is applied to remove the
removable
panel, the material will partially delaminate to leave a portion thereof
adhering to the
foil/paper only towards the back end of the cavity, which allows the peelable
lidding
material to be removed enough for the product to be exposed and easily
dispensed, when the
second tab member is lifted and perforation torn up to a crease.
The delamination is a pattern delamination, whereby the front part of the
cavity
provides for a unsealed, non-delaminated senior friendly area to bend the
packaging to
remove the tab member overlaying the cavity where the delamination occurs
towards the
centre and back of the blister cavity, but still makes it impossible to gain
access to the lead
area of the peelable lidding material, unless the second tab member is removed
to expose the
leading edge of the peelable lidding. This sequence of three child resistant
procedures
ensures that a child will not be able to gain access to the product, but is
sufficiently
accessible for a senior to gain access to a product.
In greater detail, the laminate layer is comprised of a suitable blister-
packaging layer
in having at least one capsule-receiving pocket therein. Conventionally, a
plurality of such
capsule-receiving pockets will be provided for dispensation of unit doses of
pharmaceuticals. As is known in the art, this layer may comprise a normally
rectangular
continuous blister sheet of a flexible clear plastic film having a plurality
of capsule-
receiving pockets therein. Normally, this blister sheet is made of a clear
flexible film, which
-3-
CA 02540567 2006-03-21
cannot be easily ruptured, such a film being a vinyl thermoplastic film
normally about 12
mils in thickness.
The backing sheet or peelable lidding film layer is also well-known in the
art; this
peelable lidding layer is co-extensive with the blister sheet and covers the
capsule-receiving
pockets so as to close the pockets and the capsules or products contained
therein. A
conventional material utilized is an aluminum foil approximately 1 mil in
thickness and
40 lbs of paper per ream is glued to the foil. The pre-lacquered foil is
secured to the blister
sheet by normal heat seal means. The back layer is a 12 mil (up to 18 mil)
partial
delaminating paperboard is glued to the paper side of the foil, which, if an
extended data
panel is required, is extended and double-creased to form a single spine with
an extended
data layer panel, which is normally folded over the original data layer panel.
The back of
the extended part, when folded, becomes the inside front of the package to
which the
pharmacist's label can be adhered to. The patient compliance packaging is
produced by
printing, in register, the top of the top data with the proper dosage regimen
and or arrows to
indicate the dosage sequence and week or weeks of treatment.
The main purpose of this invention is to design a dispensing sequence which is
very
difficult for a child to learn and do to dispense even only one very toxic
tablet, while at the
same time making.
The present invention contemplates the use of at least two-aperture cut
through the
peelable sheet and the blister sheet for reasons, which will become apparent
hereinafter.
The number of apertures will depend on the number of unit doses or pockets; in
one
embodiment, each pocket is at least partially separated from an adjacent
pocket by two
apertures, again for reasons will become apparent hereinafter.
-4-
CA 02540567 2006-03-21
The laminate is glued to an outer back layer; the outer back layer being
designated as
that layer which lies adjacent to the peelable film in register with the
blister cavities forming
capsule-receiving pockets. The extension of the back layer becomes the spines
and fron
panel of the data layer.
In particular, the outer back layer is adapted to adhere to the peelable film
with a
strength sufficient that the first outer back layer cannot readily be peeled
from the film, but
rather a patterned delamination of the outer back layer will occur and second
outer back
layer must be lifted and torn back to the crease to expose the lead tip of the
peelable lidding.
The tab member may be of any conventional shape, but in a preferred aspect of
the
invention, it is of a rectangular configuration for reasons to be discussed
hereinbelow. The
outer back layer has formed there at least two removable panels. The two
panels are defined
by a plurality of die cuts and perforations in a conventional manner. These
panels are co-
extensive with the portion of the outer back layer, which overlies at least
one of the capsules
receiving pockets.
In operation of the first embodiment, the first removable panel is removed to
allow
one to lift and tear the second panel back to the crease, which exposes the
lead edge of the
peelable foil/paper layer of the laminate. The individual product may then be
removed in a
conventional manner by peeling the foil/paper off the laminate and exposing
the product to
be removed.
The first tab member, as will be discussed in the preferred embodiments,
requires a
tearing force applied thereto before it can readily be detached to remove the
first removable
panel and when the first tab is removed, the child still cannot peel the
lidding foil because
the peelable lidding foil lead is not exposed, therefore the lidding cannot be
removed. A
-5-
CA 02540567 2006-03-21
second tab must be torn back to expose the leading edge of the lidding foil.
This provides a
further safeguard against a small child accidentally gaining access to the
contents of the
package, while allowing a senior to easily grasp the tab of the removable
panel and remove
it.
Naturally, the package can take many different forms and styles such as
calendar
packs, etc., as shown the following figures, using various indicia and the
likes as is
conventional in the art.
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus generally described the invention, reference will be made to the
accompanying drawings illustrating embodiment thereof, in which:
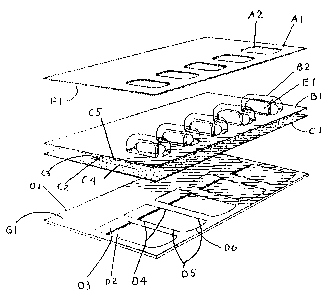
Figure 1 is a top view of the Tri-Peel pack showing the data layer panel A1,
the
diecut apertures of the area where the product in the blister protrudes
through the diecut
apertures A2, the vacuum formed blister sheet B 1, the blister cavity B2, the
product B3, the
laminated 40 lbs. of tissue paper/1 mil foil C 1, the position of the diecut
in the paper/foil
laminate C2, the outer CR panel D 1, the position of the first pull tab D2,
the position of the
second pull tab D3;
Figure 2 is a top view of the back panel layer D1, which is sealed or glued to
the
paper side of the foil C 1 and the foil sealed to the blister B 1 with the
blister blued or sealed
to the inside of the top data layer A I. The design also shows the unsealed
area of the lead
area of the first tab D2 being peeled. This first tab D2 is completely
removed. When this
first tab D2 is removed, it exposes the two cut lines C2 on the peelable
paper/foil C 1, but the
unsealed lead area C3 of the peelable paper/foil is not exposed and therefore
cannot be
accessed and removed;
-6-
CA 02540567 2006-03-21
Figure 3 is a top view of the back panel layer D 1, which is sealed or glued
to the
paper side of the foil C 1 and the foil sealed to the blister with the blister
B 1 glued or sealed
to the inside of the top data layer A1. The design also shows first tab D2
completely peeled
off and shows the second tab D3 being torn back and partially peeled off. This
second tab
D3 can be completely removed or can be folded back to expose the lead edge C3
of the
peelable paper/foil C I. The unsealed lead area C3 of the peelable paper/foil
along with the
side cuts C2 in the paper/foil is now exposed and therefore can be accessed
and removed;
Figure 4 is a top view of the back panel layer D1, which is sealed or glued to
the
paper side of the foil C 1 and the foil sealed to the blister B 1 with the
blister blued or sealed
to the inside of the top data layer A I. The design also shows first tab D2
completely peeled
off and shows the second tab D3 completely peeled off exposing the diecut lead
edge C3 of
the peelable paper/foil C3 as well as exposes the two cut lines C2 on the
peelable
paper/foil C 1 is now exposed and therefore can be accessed and removed;
Figure 5 is a top view of the back panel layer D1, which is sealed or glued to
the
paper side of the foil C 1 and the foil sealed to the blister B 1 with the
blister glued or sealed
to the inside of the top data layer A1. The design also shows first tab D2
completely peeled
off and shows the second tab D3 completely peeled off exposing the diecut lead
edge C3 of
the peelable paper/foil C 1. The unsealed lead area of the peelable paper/foil
C 1 is now
exposed. The design also shows the lead area C3 of the unsealed area of the
foil C 1 lifted
and partially removed;
Figure 6 is a top view of the back panel layer D1, which is sealed or glued to
the
paper side of the foil C 1 and the foil sealed to the blister B 1 with the
blister glued or sealed
to the inside of the top data layer A I. The design also shows first tab D2
completely peeled
-7-
CA 02540567 2006-03-21
off and shows the second tab D3 completely peeled off exposing the diecut lead
edge of the
peelable paper/foil C 1 as well as exposes the two cut lines C2 on the
peelable paper/foil C 1.
The unsealed lead area of the peelable paper/foil C 1 is now exposed. The
design also shows
the lead area C3 of the unsealed area of the paper/foil C 1 lifted and
partially removed
exposing 60% of the capsule E1 in the cavity B2;
Figure 7 is a top view of the back panel layer D 1, which is sealed or glued
to the
paper side of the foil c I and the foil sealed to the blister B 1 with the
blister glued or sealed
to the inside of the top data layer A I. The design also shows first tab D2
completely peeled
off and shows the second tab D3 completely removed as well as shows the
peelable
paper/foil layer C4 completely removed exposing the whole capsule E1 in the
cavity B2.
The capsule E 1 can now be taken.
The above packages provide tamper-evident child resistant/senior friendly,
patient
compliant advantages while at the same time, is easily openable. The features
of the method
of diecutting, the first panel D2 and second panel D3 as well as the
diecutting of the
peelable paper/foil C2 and C4 prevent access by the young child to the package
but at the
same time, is senior use effective.
It will be understood that the above described embodiments are for purposes of
illustration only and changes and modifications may be made thereto without
departing from
the spirit and scope of the invention.
-8-