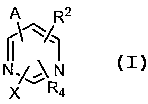Note: Descriptions are shown in the official language in which they were submitted.
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
PYRIMIDINE COMPOUNDS
FOR THE TREATMENT OF INFLAMMATION
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of U.S. Provisional
Application Serial No. 60/513,770, filed October 23, 2003, the contents of
which are hereby incorporated by reference in their entirety.
FIELD OF THE INVENTION
[0002] This invention generally relates to anti-inflammatory
pharmaceutical agents and specifically relates to pyrimidine compounds as
inhibitors of IKK-2, an IKB kinase. The invention is further related to
compositions comprising such compounds, and methods for treating cancer,
inflammation, and inflammation-associated disorders such as arthritis.
BACKGROUND OF THE INVENTION
[0003] Rheumatoid arthritis is a common inflammatory disease
affecting approximately 1 % of the population. The disease is characterized by
multiple painful swollen joints that severely limit the patient's daily
function, and
can progress to the destruction of the affected joints. A common treatment for
rheumatoid arthritis is anti-inflammatory steroids. Steroids are clinically
very
effective, but are limited in their use because of multiple severe side-
effects.
Thus, a need exists for an anti- rheumatoid arthritis treatment that offers
the
potency of steroids without the associated toxicity. One of the mechanisms by
which steroids exert their broad spectrum anti-inflammatory action is by
inhibiting the activation of the transcription factor NF-~cB. NF-KB plays a
prominent role in immune and inflammatory responses by regulating the
transcription of many early, inducible genes in a variety of cells including
inflammatory enzymes such as COX-2 and iNOS. NF-KB is sequestered in an
inactive form in the cytoplasm by a member of the IKB family of inhibitory
1
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
proteins, and this prevents gene transcription of these responsive genes in
the
nucleus. Stimulation of cells leads to the phosphorylation, ubiquination and
degradation of IKB thereby releasing NF-KB to the nucleus for activation of
gene transcription. Chronic activation of NF-KB has been demonstrated in
vascular endothelium and synovial lining cells from patients with RA. Recently
the I~eB kinases (IKK-1 and IKK-2), which phosphorylate IKB and thereby
initiate
its degradation, have been cloned and initially characterized; these kinases
appear to represent the critical, common denominator in the activation of NF-
KB since antisense or dominant-negative IKK constructs block NF-~cB nuclear
translocation and inhibit NF-KB linked reported genes. Therefore, IKK-1 and/or
IKK-2 represent novel and powerful targets for drug development.
[0004] It has been reported that selective IKK-2 inhibitors could be
useful for the treatment of inflammatory diseases. See, e.g., Karin et al.,
Nat.
Revs. 3, 17-26, 2004.
SUMMARY OF THE INVENTION
[0005] This invention provides for, in part, IKK-2-inhibiting
compounds of Formula I:
p, R2
NIv~N
[0006] X R4 I
[0007] wherein X is aryl substituted by R'a, R'b, R'°, R'd, and R'e;
[0008] wherein A is selected from the group consisting of cycloalkyl,
cycloalkenyl, aryl, heterocycloalkyl, heterocycloalkenyl, and heteroaryl,
wherein
A is optionally substituted by one or more substituents independently selected
from the group consisting of R3;
[0009] wherein R'a, R'b, R'°, R'd, R'e, and R3 are independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
alkyl, haloalkyl, hydroxyalkyl, alkylsulfinyl, alkylsulfonyl, alkoxycarbonyl,
2
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
haloalkoxy, aryl, alkenyl, heterocycloalkyl, heterocycloalkenyl, heteroaryl,
acylamino, -OR'°, -SR'a, -S02N(R'a)R'b, -NR8aR8b,-NRBaCORB°, -
NReaCO(OR8°),
-NReaS02R9a, -NRBaSO~N(R9a)R9b, -NR~aCON(R9a)R9b, -CORBa, -CO~R'a, and
-CON(R'a)R'b, wherein said aryl, heterocycloalkyl, heterocycloalkenyl,
heteroaryl, or alkenyl may be substituted with one or more substituents
selected from the group consisting of RBa;
[0010] wherein R~ is -NR"aR"b;
[0011] wherein R4 is selected from the group consisting of cyano,
-C02R5a, and -CH~ORSa, CONR5aR5b;
[0012] wherein Rsa, RSb, and R6 are independently selected from the
group consisting of hydrido, hydroxyl, alkoxy, alkyl, haloalkyl, aryl, and
heteroaryl;
[0013] wherein R'a and R'b are independently selected from the group
consisting of hydrido, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl, haloalkyl, aralkylamino, alkylaminoalkyl, N,N-
dialkylaminoacyl, alkyl, alkenyl, alkynyl, and heteroaralkyl;
[0014] wherein R8a and R8b are independently selected from the group
consisting of hydrido, alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl, cycloalkyl, haloalkyl, aralkylamino, amino, aminoalkyl,
aminoacyl, and heteroaralkyl, wherein said alkyl, aryl, heteroaryl,
aminoalkyl, or
aralkyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, alkyl, alkylthio,
alkylsulfinyl, alkylsulfonyl, N-alkylamino, alkylsulfonamido, aminoalkyl,
alkylaminoalkyl, alkoxy, halo, acyloxy, haloalkyl, haloalkoxy, acyl,
hydroxyalkoxy, dialkylaminoacyl, thioalkyl, aminoacyloxy, alkyldioxy,
hydroxyalkyl, N-alkylamino, alkoxycarbonyl, alkoxyalkyl, alkenylamino,
alkynylamino, alkenyl, alkynyl, N,N-dialkylaminoalkoxy, heterocycloalkyl,
heterocycloalkenyl, and heteroaryl, wherein said heterocycloalkyl,
3
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
heterocycloalkenyl, or heteroaryl substituents may be substituted with a
substituent selected from the group consisting of alkyl, N-alkylamino,
aminoalkyl, hydroxyalkyl, and alkylaminoalkyl;
[0015] wherein R8° is selected from the group consisting of hydrido,
nitro, azido, alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl,
cycloalkyl, haloalkyl, aralkylamino, amino, aminoalkyl, aminoacyl, and
heteroaralkyl, wherein said alkyl, aryl, heteroaryl, aminoalkyl, or aralkyl
may be
substituted with one or more substituents selected from the group consisting
of
oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy, nitro, azido,
benzyloxy, thiocyanate, isothiocyanate, alkyl, alkylthio, alkylsulfinyl,
alkylsulfonyl, N-alkylamino, alkylsulfonamido, aminoalkyl, alkylaminoalkyl,
alkoxy, halo, acyloxy, haloalkyl, haloalkoxy, acyl, hydroxyalkoxy,
dialkylaminoacyl, thioalkyl, aminoacyloxy, alkyldioxy, hydroxyalkyl, N-
alkylamino, alkoxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, N,N-dialkylaminoalkoxy, heterocycloalkyl, heterocycloalkenyl, and
heteroaryl, wherein said heterocycloalkyl, heterocycloalkenyl, or heteroaryl
substituents may be substituted with a substituent selected from the group
consisting of alkyl, N-alkylamino, aminoalkyl, hydroxyalkyl, and
alkylaminoalkyl;
[0016] wherein R9a and R9b are independently selected from the group
consisting of hydrido, alkyl, heteroaryl, heterocycloalkyl,
heterocycloalkenyl,
haloalkyl, aralkylamino, heteroaralkyl, aryl, and aralkyl, wherein said aryl,
heterocycloalkyl, heterocycloalkenyl, heteroaryl, or aralkyl moieties may be
substituted with one or more radicals selected from the group consisting of
alkyl, alkoxy, halo, haloalkyl, cyano, haloalkoxy, acyl, carboxyl, hydroxy,
hydroxyalkoxy, phenoxy, benzyloxy, N,N-dialkylaminoalkoxy, heteroaryl,
heterocycloalkyl, and heterocycloalkenyl;
[0017] wherein R'° is selected from the group consisting of hydrido,
aryl, heteroaryl, alkyl, haloalkyl, alkenyl, alkynyl,~hydroxyalkyl,
aminoalkyl,
4
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
alkylaminoalkyl, alkoxyalkyl, heterocycloalkyl, heteroaryl, and
heterocycloalkenyl;
[0018] wherein R"a and R"b are independently selected from the
group consisting of hydrido, aryl, heteroaryl, alkyl, haloalkyl, alkenyl,
alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocycloalkyl,
heteroaryl, and heterocycloalkenyl;
[0019] wherein Ra and R4 may form a 4- to 6-membered heterocyclic
ring having 1 to 3 heteroatoms selected from the group consisting of S, SO,
SO2, O, N, and NR6;
[0020] wherein R'a and R'b may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, S02, O, N, and NRBa; and
[0021] wherein R9a and R9bmay be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, S02, O, N, and NRea;
[0022] or a pharmaceutically acceptable salt thereof.
[0023] The instant invention is also directed to pharmaceutical
compositions comprising a compound of Formula I or a pharmaceutically-
acceptable salt thereof, as defined above, and a pharmaceutically acceptable
carrier, diluent, or adjuvant.
[0024] The instant invention is also directed to a method of treating
or preventing inflammation or an inflammation-associated disorder, the method
comprising administering a compound of Formula I or a pharmaceutically
acceptable salt thereof to a subject in need of such treatment or susceptible
to
such inflammation or inflammation-associated disorder.
[0025] Other objects of the invention will be in part apparent and in
part pointed out hereinafter.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
5
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0026] In accordance with the present invention, Applicants have
discovered a class of IKK-2-inhibiting compounds of Formula I:
p Rz
N w~ N
[0027] X R4
[0028] wherein X is aryl substituted by R'a, R'b, R'~, R'd, and R'e;
[0029] wherein A is selected from the group consisting of cycloalkyl,
cycloalkenyl, aryl, heterocycloalkyl, heterocycloalkenyl, and heteroaryl,
wherein
A is optionally substituted by one or more substituents independently selected
from the group consisting of R3;
[0030] wherein R'a, R'b, R'°, R'd, R'e, and R3 are independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
alkyl, haloalkyl, hydroxyalkyl, alkylsulfinyl, alkylsulfonyl, alkoxycarbonyl,
haloalkoxy, aryl, alkenyl, heterocycloalkyl, heterocycloalkenyl, heteroaryl,
acylamino, -OR'°, -SR'a, -S02N(R'a)R'b, -NR8aR8b,-NRBaCORB°, -
NR8aC0(OR8°),
-NReaS02R9a, -NR$aSO2N(R9a)R9b, -NReaCON(R9a)R9b, -CORBa, -COaR'a, and
-CON(R'a)R'b, wherein said aryl, heterocycloalkyl, heterocycloalkenyl,
heteroaryl, or alkenyl may be substituted with one or more substituents
selected from the group consisting of RBa;
[0031] wherein R~ is -NR"aRl1b;
[0032] wherein R4 is selected from the group consisting of cyano,
-C02R5a, and -CH~ORSa, CONR5aR5b;
[0033] wherein RSa, R5b' and R6 are independently selected from the
group consisting of hydrido, hydroxyl, alkoxy, alkyl, haloalkyl, aryl, and
heteroaryl;
[0034] wherein R'a and R'b are independently selected from the group
consisting of hydrido, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl, haloalkyl, aralkylamino, alkylaminoalkyl, N,N-
dialkylaminoacyl, alkyl, alkenyl, alkynyl, and heteroaralkyl;
6
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0035] wherein Rea and Rab are independently selected from the group
consisting of hydrido, alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl, cycloalkyl, haloalkyl, aralkylamino, amino, aminoalkyl,
aminoacyl, and heteroaralkyl, wherein said alkyl, aryl, heteroaryl,
aminoalkyl, or
aralkyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, alkyl, alkylthio,
alkylsulfinyl, alkylsulfonyl, N-alkylamino, alkylsulfonamido, aminoalkyl,
alkylaminoalkyl, alkoxy, halo, acyloxy, haloalkyl, haloalkoxy, acyl,
hydroxyalkoxy, dialkylaminoacyl, thioalkyl, aminoacyloxy, alkyldioxy,
hydroxyalkyl, N-alkylamino, alkoxycarbonyl, alkoxyalkyl, alkenylamino,
alkynylamino, alkenyl, alkynyl, N,N-dialkylaminoalkoxy, heterocycloalkyl,
heterocycloalkenyl, and heteroaryl, wherein said heterocycloalkyl,
heterocycloalkenyl, or heteroaryl substituents may be substituted with a
substituent selected from the group consisting of alkyl, N-alkylamino,
aminoalkyl, hydroxyalkyl, and alkylaminoalkyl;
[0036] wherein R8° is selected from the group consisting of hydrido,
nitro, azido, alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl,
cycloalkyl, haloalkyl, aralkylamino, amino, aminoalkyl, aminoacyl, and
heteroaralkyl, wherein said alkyl, aryl, heteroaryl, aminoalkyl, or aralkyl
may be
substituted with one or more substituents selected from the group consisting
of
oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy, nitro, azido,
benzyloxy, thiocyanate, isothiocyanate, alkyl, alkylthio, alkylsulfinyl,
alkylsulfonyl, N-alkylamino, alkylsulfonamido, aminoalkyl, alkylaminoalkyl,
alkoxy, halo, acyloxy, haloalkyl, haloalkoxy, acyl, hydroxyalkoxy,
dialkylaminoacyl, thioalkyl, aminoacyloxy, alkyldioxy, hydroxyalkyl, N-
alkylamino, alkoxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, N,N-dialkylaminoalkoxy, heterocycloalkyl, heterocycloalkenyl, and
' heteroaryl, wherein said heterocycloalkyl, heterocycloalkenyl, or heteroaryl
7
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
substituents may be substituted with a substituent selected from the group
consisting of alkyl, N-alkylamino, aminoalkyl, hydroxyalkyl, and
alkylaminoalkyl;
[0037) wherein R9a and R9b are independently selected from the group
consisting of hydrido, alkyl, heteroaryl, heterocycloalkyl,
heterocycloalkenyl,
haloalkyl, aralkylamino, heteroaralkyl, aryl, and aralkyl, wherein said aryl,
heterocycloalkyl, heterocycloalkenyl, heteroaryl, or aralkyl moieties may be
substituted with one or more radicals selected from the group consisting of
alkyl, alkoxy, halo, haloalkyl, cyano, haloalkoxy, acyl, carboxyl, hydroxy,
hydroxyalkoxy, phenoxy, benzyloxy, N,N-dialkylaminoalkoxy, heteroaryl,
heterocycloalkyl, and heterocycloalkenyl;
[0038] wherein R'° is selected from the group consisting of hydrido,
aryl, heteroaryl, alkyl, haloalkyl, alkenyl, alkynyl, hydroxyalkyl,
aminoalkyl,
alkylaminoalkyl, alkoxyalkyl, heterocycloalkyl, heteroaryl, and
heterocycloalkenyl;
[0039] wherein R"a and R"b are independently selected from the
group consisting of hydrido, aryl, heteroaryl, alkyl, haloalkyl, alkenyl,
alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocycloalkyl,
heteroaryl, and heterocycloalkenyl;
[0040) wherein R~ and R4 may form a 4- to 6-membered heterocyclic
ring having 1 to 3 heteroatoms selected from the group consisting of S, SO,
SO~, O, N, and NR6;
[004'1] wherein R'a and R'bmay be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, SOz, O, N, and NRBa; and
[0042] wherein R9a and R9b may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, S02, O, N, and NRea;
[0043] or a pharmaceutically acceptable salt thereof.
8
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0044] Compounds of Formula I may be useful for treating, among
other things, inflammation in a subject, such as, as an analgesic in the
treatment of pain and headaches, or as an antipyretic for the treatment of
fever. For example, compounds of the present invention may be useful to treat
arthritis, including but not limited to rheumatoid arthritis,
spondyloarthropathies,
gouty arthritis, osteoarthritis, systemic lupus erythematosus, juvenile
arthritis,
acute rheumatic arthritis, enteropathic arthritis, neuropathic arthritis,
psoriatic
arthritis, and pyogenic arthritis.
[0045] Compounds of the invention may be further useful in the
treatment of frailty, asthma, chronic obstructive pulmonary disease (COPD),
bronchitis, menstrual cramps (e.g., dysmenorrhea), premature labor,
tendinitis,
bursitis, dermatological conditions such as psoriasis, eczema, burns, sunburn,
dermatitis, pancreatitis, hepatitis, and from post-operative inflammation
including from ophthalmic surgery such as cataract surgery and refractive
surgery. Compounds of the invention also would be useful to treat
gastrointestinal conditions such as inflammatory bowel disease, Crohn's
disease, gastritis, irritable bowel syndrome and ulcerative colitis. Compounds
of the invention would be useful for the prevention or treatment of cancer,
such
as colorectal cancer, and cancer of the breast, lung, prostate, bladder,
cervix
and skin, as well as treatment of cancer stem cells. Compounds of the
invention would be useful in treating inflammation and tissue damage in such
diseases as vascular diseases, migraine headaches, periarteritis nodosa,
thyroiditis, aplastic anemia, Hodgkin's disease, sclerodoma, rheumatic fever,
type I diabetes, neuromuscular junction disease including myasthenia gravis,
white matter disease including multiple sclerosis, sarcoidosis, nephrotic
syndrome, Behcet's syndrome, polymyositis, gingivitis, nephritis,
hypersensitivity, swelling occurring after injury, myocardial ischemia, and
the
like.
9
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0046] The compounds would also be useful in the treatment of
pulmonary inflammation, such as that associated with viral infections and
cystic
fibrosis. The compounds would also be useful for the treatment of certain
central nervous system disorders, such as cortical dementias including
Alzheimer's disease, and central nervous system damage resulting from
stroke, ischemia and trauma. The compounds of the invention are useful as
anti-inflammatory agents, such as for the treatment of arthritis, with the
additional benefit of having significantly less harmful side effects. These
compounds would also be useful in the treatment of allergic rhinitis,
respiratory
distress syndrome, and atherosclerosis. The compounds would also be useful
in the treatment of pain, but not limited to postoperative pain, dental pain,
muscular pain, and pain resulting from cancer. The compounds would be
useful for the prevention of dementias, such as Alzheimer's disease.
[0047] Besides being useful for human treatment, these compounds
are also useful for veterinary treatment of companion animals, exotic animals
and farm animals, including mammals, rodents, and the like. More preferred
animals include horses, dogs, and cats.
[0048] The present compounds may also be used in co-therapies,
partially or completely, in place of other conventional antiinflammatory
therapies, such as together with steroids, NSAIDs, COX-2 selective inhibitors,
5-lipoxygenase inhibitors, LTB4 antagonists and LTA4 hydrolase inhibitors.
[0049] Other conditions in which the compounds of the present
invention may provide an advantage include cardiovascular ischemia, diabetes
(type I or type II), congestive heart failure, myocarditis, atherosclerosis,
migraine, glaucoma, aortic aneurysm, reflux esophagitis, diarrhea, irritable
bowel syndrome, cystic fibrosis, emphysema, asthma, bronchiectasis,
hyperalgesia (allodynia), and cerebral ischemia (both focal ischemia,
thrombotic stroke and global ischemia (for example, secondary to cardiac
arrest).
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0050] The compounds of the present invention may also be useful in
the treatment of pain including somatogenic (either nociceptive or
neuropathic),
both acute and chronic. A compound of the present invention could be used in
any situation including neuropathic pain that a common NSAID or opioid
analgesic would traditionally be administered.
[0051] Conjunctive treatment of a compound of the present invention
with an antineoplastic agent may produce a beneficial effect or alternatively
reduce the toxic side effects associated with chemotherapy by reducing the
therapeutic dose of the side effect-causing agent needed for therapeutic
efficacy or by directly reducing symptoms of toxic side effects caused by the
side effect-causing agent. A compound of the present invention may further be
useful as an adjunct to radiation therapy to reduce side effects or enhance
efficacy. In the present invention, another agent which can be combined
therapeutically with a compound of the present invention includes any
therapeutic agent which is capable of inhibiting the enzyme cyclooxygenase-2
("COX-2"). Preferably such COX-2 inhibiting agents inhibit COX-2 selectively
relative to the enzyme cyclooxygenase-1 ("COX-1 "). Such a COX-2 inhibitor is
known as a "COX-2 selective inhibitor". More preferably, a compound of the
present invention can be therapeutically combined with a COX-2 selective
inhibitor wherein the COX-2 selective inhibitor selectively inhibits COX-2 at
a
ratio of at least 10:1 relative to inhibition of COX-1, more preferably at
least
30:1, and still more preferably at least 50:1 in an in vitro test. COX-2
selective
inhibitors useful in therapeutic combination with the compounds of the present
invention include celecoxib, valdecoxib, deracoxib, etoricoxib, rofecoxib, ABT-
963 (2-(3,4-difluorophenyl)-4-(3-hydroxy-3-methyl-1-butoxy)-5-[4-
(methylsulfonyl)phenyl-3(2H)-pyridazinone; described in PCT Publication No.
WO 00/24719), or meloxicam. A compound of the present invention can also
be advantageously used in therapeutic combination with a prodrug of a COX-2
selective inhibitor, for example parecoxib.
11
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0052] Another chemotherapeutic agent which may be useful in
combination with a compound of the present invention can be selected, for
example, from the following non-comprehensive and non-limiting list: Alpha-
difluoromethylornithine (DFMO), 5-FU-fibrinogen, acanthifolic acid,
aminothiadiazole, brequinar sodium, carmofur, Ciba-Geigy CGP-30694,
cyclopentyl cytosine, cytarabine phosphate stearate, cytarabine conjugates,
Lilly DATHF, Merrill Dow DDFC, dezaguanine, dideoxycytidine,
dideoxyguanosine, didox, Yoshitomi DMDC, doxifluridine, Wellcome EHNA,
Merck & Co. EX-015, fazarabine, floxuridine, fludarabine phosphate, 5-
fluorouracil, N-(2'-furanidyl)-5-fluorouracil, Daiichi Seiyaku FO-152,
isopropyl
pyrrolizine, Lilly LY-188011, Lilly LY-264618, methobenzaprim, methotrexate,
Wellcome MOPES, norspermidine, NCI NSC-127716, NCI NSC-264880, NCI
NSC-39661, NCI NSC-612567, Warner-Lambert PALA, pentostatin, piritrexim,
plicamycin, Asahi Chemical PL-AC, Takeda TAC-788, thioguanine, tiazofurin,
Erbamont TIF, trimetrexate, tyrosine kinase inhibitors, tyrosine protein
kinase
inhibitors, Taiho UFT, uricytin, Shionogi 254-S, aldo-phosphamide analogues,
altretamine, anaxirone, Boehringer Mannheim BBR-2207, bestrabucil,
budotitane, Wakunaga CA-102, carboplatin, carmustine, Chinoin-139, Chinoin-
153, chlorambucil, cisplatin, cyclophosphamide, American Cyanamid CL-
286558, Sanofi CY-233, cyplatate, Degussa D-19-384, Sumimoto
DACHP(Myr)2, diphenylspiromustine, diplatinum cytostatic, Erba distamycin
derivatives, Chugai DWA-21148, ITI E09, ,elmustine, Erbamont FCE-24517,
estramustine phosphate sodium, fotemustine, Unimed G-6-M, Chinoin GYKI-
17230, hepsul-fam, ifosfamide, iproplatin, lomustine, mafosfamide, mitolactol,
Nippon Kayaku NK-121, NCI NSC-264395, NCI NSC-342215, oxaliplatin,
Upjohn PCNU, prednimustine, Proter PTT-119, ranimustine, semustine,
SmithKline SK&F-101772, Yakult Honsha SN-22, spiromus-tine, Tanabe
Seiyaku TA-077, tauromustine, temozolomide, teroxirone, tetraplatin,
trimelamol, Taiho 4181-A, aclarubicin, actinomycin D, actinoplanone, Erbamont
12
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
ADR-456, aeroplysinin derivative, Ajinomoto AN-201-II, Ajinomoto AN-3,
Nippon Soda anisomycins, anthracycline, azino-mycin-A, bisucaberin, Bristol-
Myers BL-6859, Bristol-Myers BMY-25067, Bristol-Myers BMY-25551, Bristol-
Myers BMY-26605, Bristol-Myers BMY-27557, Bristol-Myers BMY-28438,
bleomycin sulfate, bryostatin-1, Taiho C-1027, calichemycin, chromoximycin,
dactinomycin, daunorubicin, Kyowa Hakko DC-102, Kyowa Hakko DC-79,
Kyowa Hakko DC-88A, Kyowa Hakko DC89-A1, Kyowa Hakko DC92-B,
ditrisarubicin B, Shionogi DOB-41, doxorubicin, doxorubicin-fibrinogen,
elsamicin-A, epirubicin, erbstatin, esorubicin, esperamicin-A1, esperamicin-
Alb,
Erbamont FCE-21954, Fujisawa FK-973, fostriecin, Fujisawa FR-900482,
glidobactin, gregatin-A, grincamycin, herbimycin, idarubicin, illudins,
kazusamycin, kesarirhodins, Kyowa Hakko KM-5539, Kirin Brewery KRN-8602,
Kyowa Hakko KT-5432, Kyowa Hakko KT-5594, Kyowa Hakko KT-6149,
American Cyanamid LL-D49194, Meiji Seika ME 2303, menogaril, mitomycin,
mitoxantrone, SmithKline M-TAG, neoenactin, Nippon Kayaku NK-313, Nippon
Kayaku NKT-01, SRI International NSC-357704, oxalysine, oxaunomycin,
peplomycin, pilatin, pirarubicin, porothramycin, pyrindamycin A, Tobishi RA-I,
rapamycin, rhizoxin, rodorubicin, sibanomicin, siwenmycin, Sumitomo SM-
5887, Snow Brand SN-706, Snow Brand SN-07, sorangicin-A, sparsomycin,
SS Pharmaceutical SS-21020, SS Pharmaceutical SS-7313B, SS
Pharmaceutical SS-9816B, steffimycin B, Taiho 4181-2, talisomycin, Takeda
TAN-868A, terpentecin, thrazine, tricrozarin A, Upjohn U-73975, Kyowa Hakko
UCN-10028A, Fujisawa WF-3405, Yoshitomi Y-25024 zorubicin, alpha-
carotene, alpha-difluoromethyl-arginine, acitretin, Biotec AD-5, Kyorin AHC-
52,
alstonine, amonafide, amphethinile, amsacrine, Angiostat, ankinomycin, anti-
neoplaston A10, antineoplaston A2, antineoplaston A3, antineoplaston A5,
antineoplaston AS2-1, Henkel APD, aphidicolin glycinate, asparaginase,
Avarol, baccharin, batracylin, benfluron, benzotript, Ipsen-Beaufour BIM-
23015,
bisantrene, Bristo-Myers BMY-40481, Vestar boron-10, bromofosfamide,
13
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Wellcome BW-502, Wellcome BW-773, caracemide, carmethizole
hydrochloride, Ajinomoto CDAF, chlorsulfaquinoxalone, Chemex CHX-2053,
Chemex CHX-100, Warner-Lambert CI-921, Warner-Lambert CI-937, Warner-
Lambert CI-941, Warner-Lambert CI-958, clanfenur, claviridenone, ICN
compound 1259, ICN compound 4711, Contracan, Yakult Honsha CPT-11,
crisnatol, curaderm, cytochalasin B, cytarabine, cytocytin, Merz D-609, DABIS
maleate, dacarbazine, datelliptinium, didemnin-B, dihaematoporphyrin ether,
dihydrolenperone, dinaline, distamycin, Toyo Pharmar DM-341, Toyo Pharmar
DM-75, Daiichi Seiyaku DN-9693, elliprabin, elliptinium acetate, Tsumura
EPMTC, ergotamine, etoposide, etretinate, fenretinide, Fujisawa FR-57704,
gallium nitrate, genkwadaphnin, Chugai GLA-43, Glaxo GR-63178, grifolan
NMF-5N, hexadecylphosphocholine, Green Cross HO-221, homoharringtonine,
hydroxyurea, BTG ICRF-187, ilmofosine, isoglutamine, isotretinoin, Otsuka JI-
36, Ramot K-477, Otsuak K-76COONa, Kureha Chemical K-AM, MECT Corp
KI-8110, American Cyanamid L-623, leukoregulin, lonidamine, Lundbeck LU-
23-112, Lilly LY-186641, NCI (US) MAP, marycin, Merrel Dow MDL-27048,
Medco MEDR-340, merbarone, merocyanine derivatives, methylanilinoacridine,
Molecular Genetics MGI-136, minactivin, mitonafide, mitoquidone, mopidamol,
motretinide, Zenyaku Kogyo MST-16, N-(retinoyl)amino acids, Nisshin Flour
Milling N-021, N-acylated-dehydroalanines, nafazatrom, Taisho NCU-190,
nocodazole derivative, Normosang, NCI NSC-145813, NCI NSC-361456, NCI
NSC-604782, NCI NSC-95580, octreotide, Ono ONO-112, oquizanocine, Akzo
Org-10172, pancratistatin, pazelliptine, Warner-Lambent PD-111707, Warner-
Lambert PD-115934, Warner-Lambent PD-131141, Pierre Fabre PE-1001,
ICRT peptide D, piroxantrone, polyhaematoporphyrin, polypreic acid, Efamol
porphyrin, probimane, procarbazine, proglumide, Invitron protease nexin I,
Tobishi RA-700, razoxane, Sapporo Breweries RBS, restrictin-P, retelliptine,
retinoic acid, Rhone-Poulenc RP-49532, Rhone-Poulenc RP-56976,
SmithKline SK&F-104864, Sumitomo SM-108, Kuraray SMANCS, SeaPharm
14
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
SP-10094, spatol, spirocyclopropane derivatives, spirogermanium, Unimed, SS
Pharmaceutical SS-554, strypoldinone, Stypoldione, Suntory SUN 0237,
Suntory SUN 2071, superoxide dismutase, Toyama T-506, Toyama T-680,
taxol, Teijin TEI-0303, teniposide, thaliblastine, Eastman Kodak TJB-29,
tocotrienol, Topostin, Teijin TT-82, Kyowa Hakko UCN-01, Kyowa Hakko UCN-
1028, ukrain, Eastman Kodak USB-006, vinblastine sulfate, vincristine,
vindesine, vinestramide, vinorelbine, vintriptol, vinzolidine, withanolides,
Yamanouchi YM-534, uroguanylin, combretastatin, dolastatin, idarubicin,
epirubicin, estramustine, cyclophosphamide, 9-amino-2-(S)-camptothecin,
topotecan, irinotecan (Camptosar), exemestane, decapeptyl (tryptorelin), or an
omega-3 fatty acid.
[0053] Examples of radioprotective agents which may be used in a
combination therapy with the compounds of this invention include AD-5,
adchnon, amifostine analogues, detox, dimesna, I-102, MM-159, N-acylated-
dehydroalanines, TGF-Genentech, tiprotimod, amifostine, WR-151327, FUT-
187, ketoprofen transdermal, nabumetone, superoxide dismutase (Chiron) and
superoxide dismutase Enzon.
[0054] The compounds of the present invention may also be useful in
treatment or prevention of angiogenesis-related disorders or conditions, for
example, tumor growth, metastasis, macular degeneration, and
atherosclerosis.
[0055] In a further embodiment, the present invention also provides
therapeutic combinations for the treatment or prevention of ophthalmic
disorders or conditions such as glaucoma. For example the present inventive
compounds advantageously may be used in therapeutic combination with a
drug which reduces the intraocular pressure of patients afflicted with
glaucoma.
Such intraocular pressure-reducing drugs include without limitation
latanoprost,
travoprost, bimatoprost, or unoprostol. The therapeutic combination of a
compound of the present invention plus an intraocular pressure-reducing drug
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
may be useful because each is believed to achieve its effects by affecting a
different mechanism.
[0056] In another combination of the present invention, the present
inventive compounds can be used in therapeutic combination with an
antihyperlipidemic or cholesterol-lowering drug such as a benzothiepine or a
benzothiazepine antihyperlipidemic drug. Examples of benzothiepine
antihyperlipidemic drugs useful in the present inventive therapeutic
combination can be found in U.S. Patent No. 5,994,391, herein incorporated by
reference. Some benzothiazepine antihyperlipidemic drugs are described in
PCT Publication No. WO 93/16055. Alternatively, the antihyperlipidemic or
cholesterol-lowering drug useful in combination with a compound of the present
invention can be an HMG Co-A reductase inhibitor. Examples of HMG Co-A
reductase inhibitors useful in the present therapeutic combination include,
individually, benfluorex, fluvastatin, lovastatin, pravastatin, simvastatin,
atorvastatin, cerivastatin, bervastatin, ZD-9720 (described in PCT Publication
No. WO 97/06802), ZD-4522 (CAS No. 147098-20-2 for the calcium salt; CAS
No. 147098-18-8 for the sodium salt; described in European Patent No. EP
521471 ), BMS 180431 (CAS No. 129829-03-4), or NK-104 (CAS No. 141750-
63-2). The therapeutic combination of a compound of the present invention
plus an antihyperlipidemic or cholesterol-lowering drug may be useful, for
example, in reducing the risk of formation of atherosclerotic lesions in blood
vessels. For example, atherosclerotic lesions often initiate at inflamed sites
in
blood vessels. It is established that antihyperlipidemic or cholesterol-
lowering
drug reduce risk of formation of atherosclerotic lesions by lowering lipid
levels
in blood. Without limiting the invention to a single mechanism of action, it
is
believed that one way the compounds of the present combination may work in
concert to provide improved control of atherosclerotic lesions by, for
example,
reducing inflammation of the blood vessels in concert with lowering blood
lipid
levels.
16
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
(0057] In another embodiment of the invention, the present
compounds can be used in combination with other compounds or therapies for
the treatment of central nervous conditions or disorders such as migraine. For
example, the present compounds can be used in therapeutic combination with
caffeine, a 5-HT-1 B/1 D agonist (for example, a triptan such as sumatriptan,
naratriptan, zolmitriptan, rizatriptan, almotriptan, or frovatriptan), a
dopamine
D4 antagonist (e.g., sonepiprazole), aspirin, acetaminophen, ibuprofen,
indomethacin, naproxen sodium, isometheptene, dichloralphenazone,
butalbital, an ergot alkaloid (e.g., ergotamine, dihydroergotamine,
bromocriptine, ergonovine, or methyl ergonovine), a tricyclic antidepressant
(e.g., amitriptyline or nortriptyline), a serotonergic antagonist (e.g.,
methysergide or cyproheptadine), a beta-andrenergic antagonist (e.g.,
propranolol, timolol, atenolol, nadolol, or metprolol), or a monoamine oxidase
inhibitor (e.g., phenylzine or isocarboxazid).
[0058] The present invention includes compounds that selectively
inhibit IKK-2 over other kinases. Such other kinases include, but are not
limited
to, Abl(h), Abl(T3151), Abl(T3151), AMPK, Aurora-A, BTK, CaMKII, CaMKIV,
CDK1/cyclinB, CDK2, CDK2/cyclin A, CDK2/cyclinE, CHK1, CHK2, CK1,
CK1 (y), CK1 ~, CK2, c-RAF(h), CSK, cSRC(h), DYRK1 a, ERK2, Fyn, GSK3f3,
IGF-1R, IKK1, IKKi, IKK2(h), JNK/SAPK1c, JNK1, JNK1a1(h), JNK2,
JNK2a2(h), JNK3, Lck, MAPK1 (h), MAPK2(h), MAPK2/ERK2, MAPKAP-K1 a,
MAPKAP-K2, MEK1, MK-2, MK-3, MKK1, MKK4, MKK6, MKK7, MKK7(~(h),
MNK, MRSK2/APKAPkIb, MSK, MSK1, NEK2a, NEK6, p38 alpha, p38 beta,
p38 delta, p38 gamma, p70 S6K, PAK2, PDGFR(3, PDK1, PHK, PKA, PKB~ph,
PKC~, PKCa, PKCy, PKCb, PKCc, PRAK, ROCK-II, Rsk1, Rsk2, RSKB,
SAPK2a/p38, SAPK2b, SAPK2blp38f32, SAPK3, SAPK3/p38g, SAPK4,
SAPK4/p38d, SGK, TBK-1, and ZAP-70. The compounds may have an IKK-2
ICSO of less than about 10 pM, preferably less than about 1 pM, and have a
selectivity ratio of IKK-2 inhibition over IKK-1 inhibition of at least 50, or
at least
17
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
100. The compounds may have an IKK-1 IC5° of greater than 10 pM, or
greater
than 100 pM.
[0059] In one preferred embodiment, the compound of Formula I is a
compound wherein X is CS_,2 aryl substituted by R'a, R'b, R'°, R'd, and
R'e;
[0060] wherein A is selected from the group consisting of C3_,2
cycloalkyl, C3_,~ cycloalkenyl, C5_,2 aryl, 5- to 12-membered
heterocycloalkyl, 5-
to 12-membered heterocycloalkenyl, and 5- to 12-membered heteroaryl,
wherein A is optionally substituted by one or more substituents independently
selected from the group consisting of R3;
[0061] wherein R'a, R'b, R'°, R'd, R'e, and R3 are independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
C,_6
alkyl, C,_6 haloalkyl, C,_6 hydroxyalkyl, C,_6 alkylsulfinyl, C,_s
alkylsulfonyl, C~_,
alkoxycarbonyl, C,_6 haloalkoxy, C5_,2 aryl, Ca_6 alkenyl, 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, 5- to 12-membered
heteroaryl, C~_,° acylamino, -OR'°, -SR'a, -SO~N(R'a)R'b, -
NR$aRBb,-NRBaCORB°,
-NR8aC0(OR8°), -NRBaSOaR9a, -NRBaSOzN(R9a)R9b, -NReaCON(R9a)R9b, -
CORBa,
-CO~R'a, and -CON(R'a)R'b, wherein said aryl, heterocycloalkyl,
heterocycloalkenyl, heteroaryl, or alkenyl moiety may be substituted with one
or more substituents selected from the group consisting of Rea;
[0062] wherein R4 is selected from the group consisting of cyano,
-COZRS~, and -CH~ORSa, CONR5aR5b;
[0063] wherein RSa, R5b' and R6 are independently selected from the
group consisting of hydrido, hydroxyl, C,_6 alkoxy, C,_6 alkyl, C,_s
haloalkyl, C5_,2
aryl, and 5- to 12-membered heteroaryl;
[0064] wherein R'a and R'b are independently selected from the group
consisting of hydrido, C5_,2 aryl, 5- to 12-membered heteroaryl, C4_,e
aralkyl, 3- to
12-membered heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C,_6
haloalkyl, C4_,8 aralkylamino, C2_,2 alkylaminoalkyl, N-N-di(C,_6
alkyl)amino(C,_6
alkyl), C,_6 alkyl, C~_6 alkenyl, C2_6 alkynyl, and 4- to 18-membered
heteroaralkyl;
18
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0065] wherein R8a and R8b are independently selected from the group
consisting of hydrido, C,_6 alkyl, C5_,~ aryl, 5- to 12-membered heteroaryl,
C4_,e
aralkyl, 3- to 12-membered heterocycloalkyl, 3- to 12-membered
heterocycloalkenyl, C3_,2 cycloalkyl, C,_6 haloalkyl, C4_,8 aralkylamino,
amino, C,_s
aminoalkyl, C2_,o aminoacyl, and 4- to 18-membered heteroaralkyl, wherein said
alkyl, aryl, heteroaryl, aminoalkyl, or aralkyl may be substituted with one or
more substituents selected from the group consisting of oxy, formyl, cyano,
carboxyl, hydroxy, sulfamyl, phenoxy, nitro, azido, benzyloxy, thiocyanate,
isothiocyanate, C,_6 alkyl, C,_s alkylthio, C,_s alkylsulfinyl, C,_6
alkylsulfonyl, N-(C,_6
alkyl)amino, C,_6 alkylsulfonamido, C,_6 aminoalkyl, C2_,Z alkylaminoalkyl,
C,_s
alkoxy, halo, Ca_,o acyloxy, C,_6 haloalkyl, C,_6 haloalkoxy, Ca_,o acyl, C,_6
hydroxyalkoxy, N,N-di(C,_6 alkyl)amino(Ca_,o acyl), C,_6 thioalkyl, Cz_,o
aminoacyloxy, C,_6 alkyldioxy, C,_6 hydroxyalkyl, N-(C,_6 alkyl)amino, C2_,
alkoxycarbonyl, C~_,Z alkoxyalkyl, C~_s alkenylamino, C2_6 alkynylamino, C2_6
alkenyl, C2_6 alkynyl, N,N-di(C,_6 alkyl)amino(C,_6 alkoxy), 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, and 5- to 12-
membered heteroaryl, wherein said 3- to 12-membered heterocycloalkyl, 3- to
12-membered heterocycloalkenyl, or 5- to 12-membered heteroaryl moiety may
be substituted with a substituent selected from the group consisting of C,_6
alkyl, N-(C,_6 alkyl)amino, C,_6 aminoalkyl, C,_6 hydroxyalkyl, and CZ_,2
alkylaminoalkyl;
[0066] wherein Re° is selected from the group consisting of hydrido,
nitro, azido, C,_6 alkyl, C5_,~ aryl, 5- to 12-membered heteroaryl, C4_,e
aralkyl, 3-
to 12-membered heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C3_,2
cycloalkyl, C,_6 haloalkyl, C4_,8 aralkylamino, amino, C,_6 aminoalkyl, C~_,o
aminoacyl, and 4- to 18-membered heteroaralkyl, wherein said alkyl, aryl,
heteroaryl, aminoalkyl, or aralkyl may be substituted with one or more
substituents selected from the group consisting of oxy, formyl, cyano,
carboxyl,
hydroxy, sulfamyl, phenoxy, nitro, azido, benzyloxy, thiocyanate,
19
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
isothiocyanate, C,_6 alkyl, C,_6 alkylthio, C,_6 alkylsulfinyl, C,_6
alkylsulfonyl, N-(C,_s
alkyl)amino, C,_6 alkylsulfonamido, C,_6 aminoalkyl, C2_,2 alkylaminoalkyl,
C,_6
alkoxy, halo, C~_,° acyloxy, C,_6 haloalkyl, C,_6 haloalkoxy,
C~_,° acyl, C,_6
hydroxyalkoxy, N,N-di(C,_6 alkyl)amino(Ca_,o acyl), C,_6 thioalkyl, C2_,0
aminoacyloxy, C,_6 alkyldioxy, C,_s hydroxyalkyl, N-(C,_6 alkyl)amino, C2_,
alkoxycarbonyl, C2_,2 alkoxyalkyl, C2_6 alkenylamino, C2_s alkynylamino, C2_s
alkenyl, Ca_s alkynyl, N,N-di(C,_6 alkyl)amino(C,_6 alkoxy), 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, and 5- to 12-
membered heteroaryl, wherein said 3- to 12-membered heterocycloalkyl, 3- to
12-membered heterocycloalkenyl, or 5- to 12-membered heteroaryl moiety may
be substituted with a substituent selected from the group consisting of C,_s
alkyl, N-(C,_6 alkyl)amino, C,_6 aminoalkyl, C,_6 hydroxyalkyl, and C2_,2
alkylaminoalkyl;
[0067] wherein R9a and R9b are independently selected from the group
consisting of hydrido, C,_6 alkyl, 5- to 12-membered heteroaryl, 3- to 12-
membered heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C,_6
haloalkyl, C4_,8 aralkylamino, 4- to 18-membered heteroaralkyl, C5_,2 aryl,
and
C4_,8 aralkyl, wherein said aryl, heterocycloalkyl, heterocycloalkenyl,
heteroaryl,
or aralkyl moiety may be substituted with one or more radicals selected from
the group consisting of C,_6 alkyl, C,_6 alkoxy, halo, C,_6 haloalkyl, cyano,
C,_6
haloalkoxy, C~_,° acyl, carboxyl, hydroxy, C,_6 hydroxyalkoxy, phenoxy,
benzyloxy, N,N-di(C,_6 alkyl)amino(C,_6 alkoxy), 5- to 12-membered heteroaryl,
3- to 12-membered heterocycloalkyl, and 3- to 12-membered
heterocycloalkenyl;
[0068] wherein R'° is selected from the group consisting of hydrido,
CS_,2 aryl, 5- to 12-membered heteroaryl, C,_6 alkyl, C,_6 haloalkyl, CZ_6
alkenyl,
CZ_6 alkynyl, C,_6 hydroxyalkyl, C,_6 aminoalkyl, C~_,~ alkylaminoalkyl, C~_,2
alkoxyalkyl, 3- to 12-membered heterocycloalkyl, 5- to 12-membered
heteroaryl, and 3- to 12-membered heterocycloalkenyl;
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0069] wherein R"a and R"b are independently selected from the
group consisting of hydrido, C5_,a aryl, 5- to 12-membered heteroaryl, C,_6
alkyl,
C,_6 haloalkyl, CZ_s alkenyl, C~_6 alkynyl, C,_6 hydroxyalkyl, C,_6
aminoalkyl, Cz_,2
alkylaminoalkyl, Ci_6 alkoxy, Ca_,~ alkoxyalkyl, 3- to 12-membered
heterocycloalkyl, 5- to 12-membered heteroaryl, and 3- to 12-membered
heterocycloalkenyl;
[0070] wherein RZ and R4 may form a 4- to 6-membered heterocyclic
ring having 1 to 3 heteroatoms selected from the group consisting of S, SO,
SOz, O, N, and NR6;
[0071] wherein R'a and R'b may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, SO2, O, N, and NRBa; and
[0072] wherein R9a and Rgb may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, S02, O, N, and NRBa;
[0073] or a pharmaceutically acceptable salt thereof.
[0074] In one particularly preferred embodiment, the compound of
Formula I is a compound wherein X is selected from the group consisting of
phenyl, biphenyl, naphthyl, and indenyl, wherein X is substituted by R'a, R'b,
R'°, R1d, and R'e;
[0075] wherein A is selected from the group consisting of cyclopentyl,
cyclohexyl, cycloheptyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, phenyl,
piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl,
isoindolyl, dihydroindolyl, dihydropyridinyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, and pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, wherein A is optionally substituted by one or more
substituents independently selected from the group consisting of R3;
21
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0076] wherein R'a, Rlb, R'~, R'd, R'e, and R3 are independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
methyl, ethyl, propyl, butyl, pentyl, hexyl, chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylsulfinyl, ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl,
ethylsulfonyl, propylsulfonyl, butylsulfonyl, methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, butoxycarbonyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, phenyl,
biphenyl, naphthyl, indenyl, ethenyl, propenyl, butenyl, pentenyl,
piperidinyl,
pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl,
isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl,
methylcarbonylamino, ethylcarbonylamino, propylcarbonylamino,
butylcarbonylamino, pentylcarbonylamino, hexylcarbonylamino,
phenylcarbonylamino, benzylcarbonylamino, -OR'°, -SR'a, -SOzN(R'a)R'b,
-NR8aR8b,-NRBaCORB°, -NR8aC0(OR8°), -NR8aSO2R9a, -
NReaS02N(R9a)R9b,
-NReaCON(R9a)R9b, -CORBa, -CO~R'a, and -CON(R'a)R'b, wherein said phenyl,
biphenyl, naphthyl, indenyl, piperidinyl, pyrrolidinyl, pyrazolidinyl,
imidazolidinyl,
isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, ethenyl,
propenyl,
butenyl, or pentenyl may be substituted with one or more substituents selected
from the group consisting of Rea;
22
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0077] wherein R4 is selected from the group consisting of cyano,
-COZRSa, and -CH~ORSa, CONR5aR5b;
[0078] wherein RSa, R5b, and R6 are independently selected from the
group consisting of hydrido, hydroxyl, methoxy, ethoxy, propoxy, butoxy,
methyl, ethyl, propyl, butyl, pentyl, hexyl, chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, phenyl,
biphenyl,
naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl, isoquinolinyl,
quinolinyl,
thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, and
isoindoledionyl;
[0079] wherein R'a and R'b are independently selected from the group
consisting of hydrido, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, benzyl,
phenylethyl,
piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl,
isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl,
dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl,
dihydrooxazolyl, chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl,
difluoromethyl, trifluoromethyl, benzylamino, phenylethylamino,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, propylaminohexyl, N,N-dimethylaminomethyl, N,N-
dimethylaminoethyl, N-methyl-N-ethylaminomethyl, N-methyl-N-
ethylaminoethyl, N-methyl-N-propylaminomethyl, N-methyl-N-propylaminoethyl,
N,N-diethylaminomethyl, N,N-diethylaminoethyl, N-ethyl-N-propylaminomethyl,
N-ethyl-N-propylaminoethyl, N,N-dipropylaminomethyl, N,N-dipropylaminoethyl,
methyl, ethyl, propyl, butyl, pentyl, hexyl, ethenyl, propenyl, butenyl,
pentenyl,
ethynyl, propynyl, butynyl, pentynyl, pyridinylmethyl, pyridinylethyl,
23
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, and isoindoledionylethyl;
[0080] wherein R8a and R8b are independently selected from the group
consisting of hydrido, methyl, ethyl, propyl, butyl, pentyl, hexyl, phenyl,
biphenyl, naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl,
quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl,
oxazolyl,
isoindoledionyl, benzyl, phenylethyl, piperidinyl, pyrrolidinyl,
pyrazolidinyl,
imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl,
isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl, difluoromethyl, trifluoromethyl, benzylamino, phenylethylamino,
amino, aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl,
aminohexyl, aminomethylcarbonyl, aminoethylcarbonyl, aminopropylcarbonyl,
aminobutylcarbonyl, aminopentylcarbonyl, aminohexylcarbonyl,
aminophenylcarbonyl, aminobenzylcarbonyl, pyridinylmethyl, pyridinylethyl,
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, and isoindoledionylethyl, wherein said methyl, ethyl,
propyl, butyl, pentyl, hexyl, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, aminomethyl,
24
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl, benzyl, or
phenylethyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, methyl, ethyl, propyl,
butyl,
pentyl, hexyl, methylthio, ethylthio, propylthio, butylthio, methylsulfinyl,
ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl, ethylsulfonyl,
propylsulfonyl, butylsulfonyl, N-methylamino, N-ethylamino, N-propylamino,
methylsulfonamido, ethylsulfonamido, propylsulfonamido, butylsulfonamido,
aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, propylaminohexyl, methoxy, ethoxy, propoxy, butoxy, chloro,
fluoro, bromo, iodo, methylcarbonyloxy, ethylcarbonyloxy, propylcarbonyloxy,
butylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy, phenylcarbonyloxy,
benzylcarbonyloxy, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
methylcarbonyl, ethylcarbonyl, propylcarbonyl, butylcarbonyl, pentylcarbonyl,
hexylcarbonyl, phenylcarbonyl, benzylcarbonyl, hydroxymethoxy,
hydroxyethoxy, hydroxypropoxy, hydroxybutoxy, N,N-
dimethylaminomethylcarbonyl, N,N-dimethylaminoethylcarbonyl, N,N-
dimethylaminophenylcarbonyl, N-methyl-N-ethylaminomethylcarbonyl, N-
methyl-N-ethylaminoethylcarbonyl, N-methyl-N-ethylaminophenylcarbonyl, N-
methyl-N-propylaminomethylcarbonyl, N-methyl-N-propylaminoethylcarbonyl,
N-methyl-N-propylaminophenylcarbonyl, N,N-diethylaminomethylcarbonyl, N,N-
diethylaminoethylcarbonyl, N,N-diethylaminophenylcarbonyl, N-ethyl-N-
propylaminomethylcarbonyl, N-ethyl-N-propylaminoethylcarbonyl, N-ethyl-N-
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
propylaminophenylcarbonyl, N,N-dipropylaminomethylcarbonyl, N,N-
dipropylaminoethylcarbonyl, N,N-dipropylaminophenylcarbonyl, thiomethyl,
thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl,
aminomethylcarbonyloxy,
aminoethylcarbonyloxy, aminopropylcarbonyloxy, aminobutylcarbonyloxy,
aminopentylcarbonyloxy, aminohexylcarbonyloxy, aminophenylcarbonyloxy,
aminobenzylcarbonyloxy, methyldioxy, ethyldioxy, propyldioxy, butyldioxy,
pentyldioxy, hexyldioxy, hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl, hydroxypentyl, hydroxyhexyl, N-methylamino, N-ethylamino, N-
propylamino, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl,
ethoxyethyl, ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl,
butoxymethyl, butoxyethyl, butoxypropyl, ethenylamino, propenylamino,
butenylamino, pentenylamino, ethynylamino, propynylamino, butynylamino,
pentynylamino, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, N,N-dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, piperidinyl, pyrrolidinyl,
pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, and isoindoledionyl,
wherein
said piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl,
26
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, or isoindoledionyl may be substituted with a substituent selected
from
the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl, N-
methylamino, N-ethylamino, N-propylamino, aminomethyl, aminoethyl,
aminopropyl, aminobutyl, aminopentyl, aminohexyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, and propylaminohexyl;
[0081] wherein Re~ is selected from the group consisting of hydrido,
nitro, azido, methyl, ethyl, propyl, butyl, pentyl, hexyl, phenyl, biphenyl,
naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl, isoquinolinyl,
quinolinyl,
thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
isoindoledionyl,
benzyl, phenylethyl, piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl,
isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl, difluoromethyl, trifluoromethyl, benzylamino, phenylethylamino,
amino, aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl,
aminohexyl, aminomethylcarbonyl, aminoethylcarbonyl, aminopropylcarbonyl,
aminobutylcarbonyl, aminopentylcarbonyl, aminohexylcarbonyl,
aminophenylcarbonyl, aminobenzylcarbonyl, pyridinylmethyl, pyridinylethyl,
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
27
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, and isoindoledionylethyl, wherein said methyl, ethyl,
propyl, butyl, pentyl, hexyl, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyf, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyf, furyl,
pyrazolyl, imidazofyl, isoxazolyl, oxazolyl, isoindoledionyl, aminomethyl,
aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl, benzyl, or
phenylethyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, methyl, ethyl, propyl,
butyl,
pentyl, hexyl, methylthio, ethylthio, propylthio, butylthio, methylsulfinyl,
ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl, ethylsulfonyl,
propylsulfanyl, butylsulfonyl, N-methylamino, N-ethylamino, N-propylamino,
methylsulfonamido, ethylsulfonamido, propylsulfonamido, butylsulfonamido,
aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, propylaminohexyl, methoxy, ethoxy, propoxy, butoxy, chloro,
fluoro, bromo, iodo, methylcarbonyloxy, ethylcarbonyloxy, propylcarbonyloxy,
butylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy, phenylcarbonyloxy,
benzylcarbonyloxy, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
methylcarbonyl, ethylcarbonyl, propylcarbonyl, butylcarbonyl, pentylcarbonyl,
hexylcarbonyl, phenylcarbonyl, benzylcarbonyl, hydroxymethoxy,
hydroxyethoxy, hydroxypropoxy, hydroxybutoxy, N,N-
dimethylaminomethylcarbonyl, N,N-dimethylaminoethylcarbonyl, N,N-
dimethylaminophenylcarbonyl, N-methyl-N-ethylaminomethylcarbonyl, N-
28
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
methyl-N-ethylaminoethylcarbonyl, N-methyl-N-ethylaminophenylcarbonyl, N-
methyl-N-propylaminomethylcarbonyl, N-methyl-N-propylaminoethylcarbonyl,
N-methyl-N-propylaminophenylcarbonyl, N,N-diethylaminomethylcarbonyl, N,N-
diethylaminoethylcarbonyl, N,N-diethylaminophenylcarbonyl, N-ethyl-N-
propylaminomethylcarbonyl, N-ethyl-N-propylaminoethylcarbonyl, N-ethyl-N-
propylaminophenylcarbonyl, N,N-dipropylaminomethylcarbonyl, N,N-
dipropylaminoethylcarbonyl, N,N-dipropylaminophenylcarbonyl, thiomethyl,
thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl,
aminomethylcarbonyloxy,
aminoethylcarbonyloxy, aminopropylcarbonyloxy, aminobutylcarbonyloxy,
aminopentylcarbonyloxy, aminohexylcarbonyloxy, aminophenylcarbonyfoxy,
aminobenzylcarbonyloxy, methyldioxy, ethyldioxy, propyldioxy, butyldioxy,
pentyldioxy, hexyldioxy, hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl, hydroxypentyl, hydroxyhexyl, N-methylamino, N-ethylamino, N-
propylamino, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl,
ethoxyethyl, ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl,
butoxymethyl, butoxyethyl, butoxypropyl, ethenylamino, propenylamino,
butenylamino, pentenylamino, ethynylamino, propynylamino, butynylamino,
pentynylamino, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, N,N-dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, piperidinyl, pyrrolidinyl,
pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
29
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, and isoindoledionyl,
wherein
said piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, or isoindoledionyl may be substituted with a substituent selected
from
the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl, N-
methylamino, N-ethylamino, N-propylamino, aminomethyl, aminoethyl,
aminopropyl, aminobutyl, aminopentyl, aminohexyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, and propylaminohexyl;
[0082] wherein R9a and R9b are independently selected from the group
consisting of hydrido, methyl, ethyl, propyl, butyl, pentyl, hexyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, piperidinyl,
pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl,
isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, benzylamino, phenylethylamino, pyridinylmethyl,
pyridinylethyl,
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, isoindoledionylethyl, phenyl, biphenyl, naphthyl,
indenyl,
benzyl, and phenylethyl, wherein said phenyl, biphenyl, naphthyl, indenyl,
piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl,
isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl,
dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl,
dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl, isoquinolinyl,
quinolinyl,
thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
isoindoledionyl,
benzyl, or phenylethyl may be substituted with one or more radicals selected
from the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl,
methoxy,
ethoxy, propoxy, butoxy, chloro, fluoro, bromo, iodo, chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl,
cyano, chloromethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy,
difluoromethoxy, trifluoromethoxy, methylcarbonyl, ethylcarbonyl,
propylcarbonyl, butylcarbonyl, pentylcarbonyl, hexylcarbonyl, phenylcarbonyl,
benzylcarbonyl, carboxyl, hydroxy, hydroxymethoxy, hydroxyethoxy,
hydroxypropoxy, hydroxybutoxy, phenoxy, benzyloxy, N,N-
dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, pyridinyl, benzothiophenyl,
indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl,
imidazolyl,
isoxazolyl, oxazolyl, isoindoledionyl, piperidinyl, pyrrolidinyl,
pyrazolidinyl,
imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl,
isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, and dihydrooxazolyl;
31
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[0083] wherein R'° is selected from the group consisting of hydrido,
phenyl, biphenyl, naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, methyl, ethyl, propyl, butyl, pentyl, hexyl,
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl,
hydroxypentyl, hydroxyhexyl, aminomethyl, aminoethyl, aminopropyl,
aminobutyl, aminopentyl, aminohexyl, methylaminomethyl, ethylaminomethyl,
propylaminomethyl, methylaminoethyl, ethylaminoethyl, propylaminoethyl,
methylaminopropyl, ethylaminopropyl, propylaminopropyl, methylaminobutyl,
ethylaminobutyl, propylaminobutyl, methylaminopentyl, ethylaminopentyl,
propylaminopentyl, methylaminohexyl, ethylaminohexyl, propylaminohexyl,
methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl, ethoxyethyl,
ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl, butoxymethyl,
butoxyethyl, butoxypropyl, piperidinyl, pyrrolidinyl, pyrazolidinyl,
imidazolidinyl,
isoxazolidinyl, oxazolidinyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl,
quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl,
oxazolyl,
isoindoledionyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, and dihydrooxazolyl;
[0084] wherein R"a and R"b are independently selected from the
group consisting of hydrido, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, methyl, ethyl,
propyl,
butyl, pentyl, hexyl, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl, ethen~yl, propenyl, butenyl, pentenyl,
ethynyl,
propynyl, butynyl, pentynyl, hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl, hydroxypentyl, hydroxyhexyl, aminomethyl, aminoethyl,
32
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
aminopropyl, aminobutyl, aminopentyl, aminohexyl, methylaminomethyl,
ethylaminomethyl, propylaminomethyl, methylaminoethyl, ethylaminoethyl,
propylaminoethyl, methylaminopropyl, ethylaminopropyl, propylaminopropyl,
methylaminobutyl, ethylaminobutyl, propylaminobutyl, methylaminopentyl,
ethylaminopentyl, propylaminopentyl, methylaminohexyl, ethylaminohexyl,
propylaminohexyl, methoxy, ethoxy, propoxy, butoxy, methoxymethyl,
methoxyethyl, methoxypropyl, ethoxymethyl, ethoxyethyl, ethoxypropyl,
propoxymethyl, propoxyethyl, propoxypropyl, butoxymethyl, butoxyethyl,
butoxypropyl, piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl,
isoxazolidinyl, oxazolidinyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl,
quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl,
oxazolyl,
isoindoledionyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl!
dihydroisoxazolyl, and dihydrooxazolyl;
[0085] wherein R2 and R4 may form a 4- to 6-membered heterocyclic
ring having 1 to 3 heteroatoms selected from the group consisting of S, SO,
SOa, O, N, and NR6;
[0086] wherein R'a and R'b may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, SO~, O, N, and NRBa; and
[0087] wherein R9a and R9b may be taken together to form a 3- to 7-
membered heterocyclic moiety having 1 to 3 heteroatoms selected from the
group consisting of S, SO, SOz, O, N, and NRBa;
[0088] or a pharmaceutically acceptable salt thereof.
[0089] In a particularly preferred embodiment, the compound of
Formula I is a compound of Formula II:
33
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Ra
A~R2
IY \IY~
N ,N
R1a R1e
R1b ~ R1d
[0090] R'° II
[0091] wherein A, R'a, R'b, R'°, R'd, R'e, R~, and Ra are as defined
above for Formula I; or a pharmaceutically acceptable salt thereof.
[0092] In a particularly preferred embodiment, the compound of
Formula I is a compound of Formula III:
H
N
CN
R3
R~
N ~N
R1a ~ OH
R1b ~ ~ R1d
[0093] R'° I I I
[0094] wherein R'a, R'b, R'°, R'd, and R3 are independently selected
from the group consisting of hydrido, cyano, hydroxyl, vitro, halo, alkyl,
haloalkyl, hydroxyalkyl, alkylsulfinyl, alkylsulfonyl, alkoxycarbonyl,
haloalkoxy,
aryl, alkenyl, heterocycloalkyl, heterocycloalkenyl, heteroaryl, acylamino, -
OR'°,
-SR', -SOzNHR', -NHRBa,-NReaCORe°, -NRBaCO(OR8°), -NRBaSO~R9,
-NR8aS02NHR9, -NRBaCONHR9, -CORBa, -C02R', and -CONHR', wherein said
aryl, heterocycloalkyl, heterocycloalkenyl, heteroaryl, or alkenyl may be
substituted with one or more substituents selected from the group consisting
of
Rea;
[0095] wherein R~ is -NHR";
(0096] wherein R' is selected from the group consisting of hydrido,
aryl, heteroaryl, aralkyl, heterocycloalkyl, heterocycloalkenyl, haloalkyl,
34
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
aralkylamino, alkylaminoalkyl, N,N-dialkylaminoacyl, alkyl, alkenyl, alkynyl,
and
heteroaralkyl;
[0097] wherein Rea is selected from the group consisting of hydrido,
alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl, heterocycloalkenyl,
cycloalkyl,
haloalkyl, aralkylamino, amino, aminoalkyl, aminoacyl, and heteroaralkyl,
wherein said alkyl, aryl, heteroaryl, aminoalkyl, or aralkyl may be
substituted
with one or more substituents selected from the group consisting of oxy,
formyl,
cyano, carboxyl, hydroxy, sulfamyl, phenoxy, nitro, azido, benzyloxy,
thiocyanate, isothiocyanate, alkyl, alkylthio, alkylsulfinyl, alkylsulfonyl, N-
alkylamino, alkylsulfonamido, aminoalkyl, alkylaminoalkyl, alkoxy, halo,
acyloxy, haloalkyl, haloalkoxy, acyl, hydroxyalkoxy, dialkylaminoacyl,
thioalkyl,
aminoacyloxy, alkyldioxy, hydroxyalkyl, N-alkylamino, alkoxycarbonyl,
alkoxyalkyl, alkenylamino, alkynylamino, alkenyl, alkynyl, N,N-
dialkylaminoalkoxy, heterocycloalkyl, heterocycloalkenyl, and heteroaryl,
wherein said heterocycloalkyl, heterocycloalkenyl, or heteroaryl substituents
may be substituted with a substituent selected from the group consisting of
alkyl, N-alkylamino, aminoalkyl, hydroxyalkyl, and alkylaminoalkyl;
[0098] wherein Reb is selected from the group consisting of hydrido,
nitro, azido, alkyl, aryl, heteroaryl, aralkyl, heterocycloalkyl,
heterocycloalkenyl,
cycloalkyl, haloalkyl, aralkylamino, amino, aminoalkyl, aminoacyl, and
heteroaralkyl, wherein said alkyl, aryl, heteroaryl, aminoalkyl, or aralkyl
may be
substituted with one or more substituents selected from the group consisting
of
oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy, nitro, azido,
benzyloxy, thiocyanate, isothiocyanate, alkyl, alkylthio, alkylsulfinyl,
alkylsulfonyl, N-alkylamino, alkylsulfonamido, aminoalkyl, alkylaminoalkyl,
alkoxy, halo, acyloxy, haloalkyl, haloalkoxy, acyl, hydroxyalkoxy,
dialkylaminoacyl, thioalkyl, aminoacyloxy, alkyldioxy, hydroxyalkyl, N-
alkylamino, alkoxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, N,N-dialkylaminoalkoxy, heterocycloalkyl, heterocycloalkenyl, and
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
heteroaryl, wherein said heterocycloalkyl, heterocycloalkenyl, or heteroaryl
substituents may be substituted with a substituent selected from the group
consisting of alkyl, N-alkylamino, aminoalkyl, hydroxyalkyl, and
alkylaminoalkyl;
[0099] wherein R9 is selected from the group consisting of hydrido,
alkyl, heteroaryl, heterocycloalkyl, heterocycloalkenyl, haloalkyl,
aralkylamino,
heteroaralkyl, aryl, and aralkyl, wherein said aryl, heterocycloalkyl,
heterocycloalkenyl, heteroaryl, or aralkyl moieties may be substituted with
one
or more radicals selected from the group consisting of alkyl, alkoxy, halo,
haloalkyl, cyano, haloalkoxy, acyl, carboxyl, hydroxy, hydroxyalkoxy, phenoxy,
benzyloxy, N,N-dialkylaminoalkoxy, heteroaryl, heterocycloalkyl, and
heterocycloalkenyl; and
[00100] wherein R'° is selected from the group consisting of hydrido,
aryl, heteroaryl, alkyl, haloalkyl, alkenyl, alkynyl, hydroxyalkyl,
aminoalkyl,
alkylaminoalkyl, alkoxyalkyl, heterocycloalkyl, heteroaryl, and
heterocycloalkenyl;
[00101] wherein R" is selected from the group consisting of hydrido,
aryl, heteroaryl, alkyl, haloalkyl, alkenyl, alkynyl, hydroxyalkyl,
aminoalkyl,
alkylaminoalkyl, alkoxy, alkoxyalkyl, heterocycloalkyl, heteroaryl, and
heterocycloalkenyl;
[00102] or a pharmaceutically acceptable salt thereof.
[00103] In one preferred embodiment, the compound of Formula III is
a compound wherein R'a, R'b, R'°, R'a, and R3 are independently
selected from
the group consisting of hydrido, cyano, hydroxyl, nitro, halo, C,_6 alkyl,
C,_6
haloalkyl, C,_6 hydroxyalkyl, C,_6 alkylsulfinyl, C,_6 alkylsulfonyl, C2_,
alkoxycarbonyl, C,_6 haloalkoxy, C5_,~ aryl, CZ_s alkenyl, 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, 5- to 12-membered
heteroaryl, C~_,° acylamino, -OR'°, -SR', -SOzNHR', -NHRea,-
NRBaCORBb,
-NRBaCO(ORBb), -NReaS02R9, -NRBaSOZNHR9, -NRBaCONHR9, -CORBa, -CO~R',
and -CONHR', wherein said aryl, heterocycloalkyl, heterocycloalkenyl,
36
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
heteroaryl, or alkenyl moiety may be substituted with one or more substituents
selected from the group consisting of Rea;
[00104] wherein R' is selected from the group consisting of hydrido,
C5_,~ aryl, 5- to 12-membered heteroaryl, C4_,8 aralkyl, 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C,_6 haloalkyl, C4_,8
aralkylamino, C~_,2 alkylaminoalkyl, N-N-di(C,_6 alkyl)amino(C,_6 alkyl), C,_6
alkyl,
C~_6 alkenyl, C2_6 alkynyl, and 4- to 18-membered heteroaralkyl;
(00105] wherein R8a is selected from the group consisting of hydrido,
C,_6 alkyl, C5_,2 aryl, 5- to 12-membered heteroaryl, C4_,$ aralkyl, 3- to 12-
membered heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C3_,~
cycloalkyl, C,_6 haloalkyl, C4_,8 aralkylamino, amino, C,_6 aminoalkyl, C~_,o
aminoacyl, and 4- to 18-membered heteroaralkyl, wherein said alkyl, aryl,
heteroaryl, aminoalkyl, or aralkyl may be substituted with one or more
substituents selected from the group consisting of oxy, formyl, cyano,
carboxyl,
hydroxy, sulfamyl, phenoxy, nitro, azido, benzyloxy, thiocyanate,
isothiocyanate, C,_6 alkyl, C,_6 alkylthio, C,_s alkylsulfinyl, C,_6
alkylsulfonyl, N-(C,_6
alkyl)amino, C,_6 alkylsulfonamido, C,_6 aminoalkyl, C~_,2 alkylaminoalkyl,
C,_6
alkoxy, halo, CZ_,o acyloxy, C,_6 haloalkyl, C,_6 haloalkoxy, CZ_,o acyl, C,_6
hydroxyalkoxy, N,N-di(C,_6 alkyl)amino(C2_,o acyl), C,_6 thioalkyl, Cz_,o
aminoacyloxy, C,_6 alkyldioxy, C,_6 hydroxyalkyl, N-(C,_6 alkyl)amino, CZ_,
alkoxycarbonyl, C2_,2 alkoxyalkyl, C~_6 alkenylamino, Ca_s alkynylamino, CZ_6
alkenyl, C2_6 alkynyl, N,N-di(C,_6 alkyl)amino(C,_6 alkoxy), 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, and 5- to 12-
membered heteroaryl, wherein said 3- to 12-membered heterocycloalkyl, 3- to
12-membered heterocycloalkenyl, or 5- to 12-membered heteroaryl moiety may
be substituted with a substituent selected from the group consisting of C,_6
alkyl, N-(C,_6 alkyl)amino, C,_6 aminoalkyl, C,_6 hydroxyalkyl, and Cz_,~
alkylaminoalkyl;
37
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00106] wherein R8b is selected from the group consisting of hydrido,
nitro, azido, C,_6 alkyl, C5_,z aryl, 5- to 12-membered heteroaryl, C4_,8
aralkyl, 3-
to 12-membered heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, C3_,2
cycloalkyl, C,_6 haloalkyl, C4_,e aralkylamino, amino, C,_6 aminoalkyl, CZ_,o
aminoacyl, and 4- to 18-membered heteroaralkyl, wherein said alkyl, aryl,
heteroaryl, aminoalkyl, or aralkyl may be substituted with one or more
substituents selected from the group consisting of oxy, formyl, cyano,
carboxyl,
hydroxy, sulfamyl, phenoxy, nitro, azido, benzyloxy, thiocyanate,
isothiocyanate, C,_6 alkyl, C,_6 alkylthio, C,_6 alkylsulfinyl, C,_6
alkylsulfonyl, N-(C,_6
alkyl)amino, C,_6 alkylsulfonamido, C,_6 aminoalkyl, C~_,2 alkylaminoalkyl,
C,_6
alkoxy, halo, C2_,o acyloxy, C,_6 haloalkyl, C,_6 haloalkoxy, Ca_,o acyl, C,_6
hydroxyalkoxy, N,N-di(C,_6 alkyl)amino(C~_,o acyl), C,_6 thioalkyl, C2_,0
aminoacyloxy, C,_6 alkyldioxy, C,_6 hydroxyalkyl, N-(C,_6 alkyl)amino, C2_,
alkoxycarbonyl, C2_,2 alkoxyalkyl, Cz_s alkenylamino, C2_6 alkynylamino, CZ_6
alkenyl, C~_6 alkynyl, N,N-di(C,_6 alkyl)amino(C,_6 alkoxy), 3- to 12-membered
heterocycloalkyl, 3- to 12-membered heterocycloalkenyl, and 5- to 12-
membered heteroaryl, wherein said 3- to 12-membered heterocycloalkyl, 3- to
12-membered heterocycloalkenyl, or 5- to 12-membered heteroaryl moiety may
be substituted with a substituent selected from the group consisting of C,_6
alkyl, N-(C,_6 alkyl)amino, C,_6 aminoalkyl, C,_6 hydroxyalkyl, and C~_,2
alkylaminoalkyl;
[00107] wherein R9 is selected from the group consisting of hydrido,
C,_6 alkyl, 5- to 12-membered heteroaryl, 3- to 12-membered heterocycloalkyl,
3- to 12-membered heterocycloalkenyl, C,_6 haloalkyl, C4_,8 aralkylamino, 4-
to
18-membered heteroaralkyl, C5_,a aryl, and C4_,8 aralkyl, wherein said aryl,
heterocycloalkyl, heterocycloalkenyl, heteroaryl, or aralkyl moiety may be
substituted with one or more radicals selected from the group consisting of
C,_6
alkyl, C,_6 alkoxy, halo, C,_6 haloalkyl, cyano, C,_6 haloalkoxy, C2_10 acyl,
carboxyl,
hydroxy, C,_6 hydroxyalkoxy, phenoxy, benzyloxy, N,N-di(C,_6 alkyl)amino(C,_6
38
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
alkoxy), 5- to 12-membered heteroaryl, 3- to 12-membered heterocycloalkyl,
and 3- to 12-membered heterocycloalkenyl;
[00108] wherein R'° is selected from the group consisting of hydrido,
~5-12 ar'Yls 5- to 12-membered heteroaryl, C,_6 alkyl, C,_6 haloalkyl, C2_6
alkenyl,
Ca_6 alkynyl, C,_s hydroxyalkyl, C,_s aminoalkyl, C~_,2 alkylaminoalkyl, C2_,2
alkoxyalkyl, 3- to 12-membered heterocycloalkyl, 5- to 12-membered
heteroaryl, and 3- to 12-membered heterocycloalkenyl;
[00109] wherein R" is selected from the group consisting of hydrido,
C5_,2 aryl, 5- to 12-membered heteroaryl, C,_6 alkyl, C,_6 haloalkyl, C~_s
alkenyl,
C2_6 alkynyl, C,_6 hydroxyalkyl, C,_6 aminoalkyl, CZ_,2 alkylaminoalkyl, C,_6
alkoxy,
CZ_,~ alkoxyalkyl, 3- to 12-membered heterocycloalkyl, 5- to 12-membered
heteroaryl, and 3- to 12-membered heterocycloalkenyl;
[00110] or a pharmaceutically acceptable salt thereof.
[00111] In one particularly preferred embodiment, the compound of
Formula III is a compound wherein R'a, R'b, R'', R1d, and R3 are independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
methyl, ethyl, propyl, butyl, pentyl, hexyl, chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylsulfinyl, ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl,
ethylsulfonyl, propylsulfonyl, butylsulfonyl, methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, butoxycarbonyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, phenyl,
biphenyl, naphthyl, indenyl, ethenyl, propenyl, butenyl, pentenyl,
piperidinyl,
pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl,
isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl,
39
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
methylcarbonylamino, ethylcarbonylamino, propylcarbonylamino,
butylcarbonylamino, pentylcarbonylamino, hexylcarbonylamino,
phenylcarbonylamino, benzylcarbonylamino, -OR'°, -SR', -SO~NHR', -
NHRBa,
-NReaCORBb, -NR8aC0(ORBb), -NR$aSO~R9, -NReaSOaNHR9, -NReaCONHR9,
-CORBa, -COzR', and -CONHR', wherein said phenyl, biphenyl, naphthyl,
indenyl, piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl,
isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, ethenyl, propenyl, butenyl, or pentenyl may be
substituted with one or more substituents selected from the group consisting
of
Rea.
(00112] wherein R' is selected from the group consisting of hydrido,
phenyl, biphenyl, naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isQindoledionyl, benzyl, phenylethyl, piperidinyl, pyrrolidinyl,
pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, benzylamino, phenylethylamino, methylaminomethyl,
ethylaminomethyl, propylaminomethyl, methylaminoethyl, ethylaminoethyl,
propylaminoethyl, methylaminopropyl, ethylaminopropyl, propylaminopropyl,
methylaminobutyl, ethylaminobutyl, propylaminobutyl, methylaminopentyl,
ethylaminopentyl, propylaminopentyl, methylaminohexyl, ethylaminohexyl,
propylaminohexyl, N,N-dimethylaminomethyl, N,N-dimethylaminoethyl, N-
methyl-N-ethylaminomethyl, N-methyl-N-ethylaminoethyl, N-methyl-N-
propylaminomethyl, N-methyl-N-propylaminoethyl, N,N-diethylaminomethyl,
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
N,N-diethylaminoethyl, N-ethyl-N-propylaminomethyl, N-ethyl-N-
propylaminoethyl, N,N-dipropylaminomethyl, N,N-dipropylaminoethyl, methyl,
ethyl, propyl, butyl, pentyl, hexyl, ethenyl, propenyl, butenyl, pentenyl,
ethynyl,
propynyl, butynyl, pentynyl, pyridinylmethyl, pyridinylethyl,
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, and isoindoledionylethyl;
[00113] wherein R$a is selected from the group consisting of hydrido,
methyl, ethyl, propyl, butyl, pentyl, hexyl, phenyl, biphenyl, naphthyl,
indenyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, benzyl,
phenylethyl, piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl,
isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl,
difluoromethyl, trifluoromethyl, benzylamino, phenylethylamino, amino,
aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl,
aminomethylcarbonyl, aminoethylcarbonyl, aminopropylcarbonyl,
aminobutylcarbonyl, aminopentylcarbonyl, aminohexylcarbonyl,
aminophenylcarbonyl, aminobenzylcarbonyl, pyridinylmethyl, pyridinylethyl,
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
41
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
isoindoledionylmethyl, and isoindoledionylethyl, wherein said methyl, ethyl,
propyl, butyl, pentyl, hexyl, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, aminomethyl,
aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl, benzyl, or
phenylethyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, methyl, ethyl, propyl,
butyl,
pentyl, hexyl, methylthio, ethylthio, propylthio, butylthio, methylsulfinyl,
ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl, ethylsulfonyl,
propylsulfonyl, butylsulfonyl, N-methylamino, N-ethylamino, N-propylamino,
methylsulfonamido, ethylsulfonamido, propylsulfonamido, butylsulfonamido,
aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, propylaminohexyl, methoxy, ethoxy, propoxy, butoxy, chloro,
fluoro, bromo, iodo, methylcarbonyloxy, ethylcarbonyloxy, propylcarbonyloxy,
butylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy, phenylcarbonyloxy,
benzylcarbonyloxy, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
methylcarbonyl, ethylcarbonyl, propylcarbonyl, butylcarbonyl, pentylcarbonyl,
hexylcarbonyl, phenylcarbonyl, benzylcarbonyl, hydroxymethoxy,
hydroxyethoxy, hydroxypropoxy, hydroxybutoxy, N,N-
dimethylaminomethylcarbonyl, N,N-dimethylaminoethylcarbonyl, N,N-
dimethylaminophenylcarbonyl, N-methyl-N-ethylaminomethylcarbonyl, N-
methyl-N-ethylaminoethylcarbonyl, N-methyl-N-ethylaminophenylcarbonyl, N-
42
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
methyl-N-propylaminomethylcarbonyl, N-methyl-N-propylaminoethylcarbonyl,
N-methyl-N-propylaminophenylcarbonyl, N,N-diethylaminomethylcarbonyl, N,N-
diethylaminoethylcarbonyl, N,N-diethylaminophenylcarbonyl, N-ethyl-N-
propylaminomethylcarbonyl, N-ethyl-N-propylaminoethylcarbonyl, N-ethyl-N-
propylaminophenylcarbonyl, N,N-dipropylaminomethylcarbonyl, N,N-
dipropylaminoethylcarbonyl, N,N-dipropylaminophenylcarbonyl, thiomethyl,
thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl,
aminomethylcarbonyloxy,
aminoethylcarbonyloxy, aminopropylcarbonyloxy, aminobutylcarbonyloxy,
aminopentylcarbonyloxy, aminohexylcarbonyloxy, aminophenylcarbonyloxy,
aminobenzylcarbonyloxy, methyldioxy, ethyldioxy, propyldioxy, butyldioxy,
pentyldioxy, hexyldioxy, .hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl, hydroxypentyl, hydroxyhexyl, N-methylamino, N-ethylamino, N-
propylamino, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl,
ethoxyethyl, ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl,
butoxymethyl, butoxyethyl, butoxypropyl, ethenylamino, propenylamino,
butenylamino, pentenylamino, ethynylamino, propynylamino, butynylamino,
pentynylamino, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, N,N-dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, piperidinyl, pyrrolidinyl,
pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, and isoindoledionyl,
wherein
43
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
said piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, or isoindoledionyl may be substituted with a substituent selected
from
the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl, N-
methylamino, N-ethylamino, N-propylamino, aminomethyl, aminoethyl,
aminopropyl, aminobutyl, aminopentyl, aminohexyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, and propylaminohexyl;
[00114] wherein R8b is selected from the group consisting of hydrido,
nitro, azido, methyl, ethyl, propyl, butyl, pentyl, hexyl, phenyl, biphenyl,
naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl, isoquinolinyl,
quinolinyl,
thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
isoindoledionyl,
benzyl, phenylethyl, piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl,
isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl, difluoromethyl, trifluoromethyl, benzylamino, phenylethylamino,
amino, aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl,
aminohexyl, aminomethylcarbonyl, aminoethylcarbonyl, aminopropylcarbonyl,
aminobutylcarbonyl, aminopentylcarbonyl, aminohexylcarbonyl,
aminophenylcarbonyl, aminobenzylcarbonyl, pyridinylmethyl, pyridinylethyl,
44
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, and isoindoledionylethyl, wherein said methyl, ethyl,
propyl, butyl, pentyl, hexyl, phenyl, biphenyl, naphthyl, indenyl, pyridinyl,
benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl,
pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl, aminomethyl,
aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl, benzyl, or
phenylethyl may be substituted with one or more substituents selected from the
group consisting of oxy, formyl, cyano, carboxyl, hydroxy, sulfamyl, phenoxy,
nitro, azido, benzyloxy, thiocyanate, isothiocyanate, methyl, ethyl, propyl,
butyl,
pentyl, hexyl, methylthio, ethylthio, propylthio, butylthio, methylsulfinyl,
ethylsulfinyl, propylsulfinyl, butylsulfinyl, methylsulfonyl, ethylsulfonyl,
propylsulfonyl, butylsulfonyl, N-methylamino, N-ethylamino, N-propylamino,
methylsulfonamido, ethylsulfonamido, propylsulfonamido, butylsulfonamido,
aminomethyl, aminoethyl, aminopropyl, aminobutyl, aminopentyl, aminohexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, propylaminohexyl, methoxy, ethoxy, propoxy, butoxy, chloro,
fluoro, bromo, iodo, methylcarbonyloxy, ethylcarbonyloxy, propylcarbonyloxy,
butylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy, phenylcarbonyloxy,
benzylcarbonyloxy, chloromethyl, dichloromethyl, trichloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
methylcarbonyl, ethylcarbonyl, propylcarbonyl, butylcarbonyl, pentylcarbonyl,
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
hexylcarbonyl, phenylcarbonyl, benzylcarbonyl, hydroxymethoxy,
hydroxyethoxy, hydroxypropoxy, hydroxybutoxy, N,N-
dimethylaminomethylcarbonyl, N,N-dimethylaminoethylcarbonyl, N,N-
dimethylaminophenylcarbonyl, N-methyl-N-ethylaminomethylcarbonyl, N-
methyl-N-ethylaminoethylcarbonyl, N-methyl-N-ethylaminophenylcarbonyl, N-
methyl-N-propylaminomethylcarbonyl, N-methyl-N-propylaminoethylcarbonyl,
N-methyl-N-propylaminophenylcarbonyl, N,N-diethylaminomethylcarbonyl, N,N-
diethylaminoethylcarbonyl, N,N-diethylaminophenylcarbonyl, N-ethyl-N-
propylaminomethylcarbonyl, N-ethyl-N-propylaminoethylcarbonyl, N-ethyl-N-
propylaminophenylcarbonyl, N,N-dipropylaminomethylcarbonyl, N,N-
dipropylaminoethylcarbonyl, N,N-dipropylaminophenylcarbonyl, thiomethyl,
thioethyl, thiopropyl, thiobutyl, thiopentyl, thiohexyl,
aminomethylcarbonyloxy,
aminoethylcarbonyloxy, aminopropylcarbonyloxy, aminobutylcarbonyloxy,
aminopentylcarbonyloxy, aminohexylcarbonyloxy, aminophenylcarbonyloxy,
aminobenzylcarbonyloxy, methyldioxy, ethyldioxy, propyldioxy, butyldioxy,
pentyldioxy, hexyldioxy, hydroxymethyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl, hydroxypentyl, hydroxyhexyl, N-methylamino, N-ethylamino, N-
propylamino, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl,
ethoxyethyl, ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl,
butoxymethyl, butoxyethyl, butoxypropyl, ethenylamino, propenylamino,
butenylamino, pentenylamino, ethynylamino, propynylamino, butynylamino,
pentynylamino, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, N,N-dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, piperidinyl, pyrrolidinyl,
46
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
pyrazolidinyl, imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, and isoindoledionyl,
wherein
said piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, or isoindoledionyl may be substituted with a substituent selected
from
the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl, N-
methylamino, N-ethylamino, N-propylamino, aminomethyl, aminoethyl,
aminopropyl, aminobutyl, aminopentyl, aminohexyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
methylaminomethyl, ethylaminomethyl, propylaminomethyl, methylaminoethyl,
ethylaminoethyl, propylaminoethyl, methylaminopropyl, ethylaminopropyl,
propylaminopropyl, methylaminobutyl, ethylaminobutyl, propylaminobutyl,
methylaminopentyl, ethylaminopentyl, propylaminopentyl, methylaminohexyl,
ethylaminohexyl, and propylaminohexyl;
[00115] wherein R9 is selected from the group consisting of hydrido,
methyl, ethyl, propyl, butyl, pentyl, hexyl, pyridinyl, benzothiophenyl,
indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, piperidinyl, pyrrolidinyl, pyrazolidinyl,
imidazolidinyl,
isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl, isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, dihydrooxazolyl, chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl,
benzylamino, phenylethylamino, pyridinylmethyl, pyridinylethyl,
47
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
benzothiophenylmethyl, benzothiophenylethyl, indolylmethyl, indolylethyl,
isoquinolinylmethyl, isoquinolinylethyl, quinolinylmethyl, quinolinylethyl,
thienylmethyl, thienylethyl, pyrrolylmethyl, pyrrolylethyl, furylmethyl,
furylethyl,
pyrazolylmethyl, pyrazolylethyl, imidazolylmethyl, imidazolylethyl,
isoxazolylmethyl, .isoxazolylethyl, oxazolylmethyl, oxazolylethyl,
isoindoledionylmethyl, isoindoledionylethyl, phenyl, biphenyl, naphthyl,
indenyl,
benzyl, and phenylethyl, wherein said phenyl, biphenyl, naphthyl, indenyl,
piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl,
isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl,
dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl,
dihydrooxazolyl, pyridinyl, benzothiophenyl, indolyl, isoquinolinyl,
quinolinyl,
thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
isoindoledionyl,
benzyl, or phenylethyl may be substituted with one or more radicals selected
from the group consisting of methyl, ethyl, propyl, butyl, pentyl, hexyl,
methoxy,
ethoxy, propoxy, butoxy, chloro, fluoro, bromo, iodo, chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl,
cyano, chloromethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy,
difluoromethoxy, trifluoromethoxy, methylcarbonyl, ethylcarbonyl,
propylcarbonyl, butylcarbonyl, pentylcarbonyl, hexylcarbonyl, phenylcarbonyl,
benzylcarbonyl, carboxyl, hydroxy, h.ydroxymethoxy, hydroxyethoxy,
hydroxypropoxy, hydroxybutoxy, phenoxy, benzyloxy, N,N-
dimethylaminomethoxy, N,N-dimethylaminoethoxy, N-methyl-N-
ethylaminomethoxy, N-methyl-N-ethylaminoethoxy, N-methyl-N-
propylaminomethoxy, N-methyl-N-propylaminoethoxy, N,N-
diethylaminomethoxy, N,N-diethylaminoethoxy, N-ethyl-N-
propylaminomethoxy, N-ethyl-N-propylaminoethoxy, N,N-
dipropylaminomethoxy, N,N-dipropylaminoethoxy, pyridinyl, benzothiophenyl,
indolyl, isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl,
imidazolyl,
isoxazolyl, oxazolyl, isoindoledionyl, piperidinyl, pyrrolidinyl,
pyrazolidinyl,
48
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
imidazolidinyl, isoxazolidinyl, oxazolidinyl, isoindolyl, dihydroindolyl,
isoindoline,
dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl,
dihydroimidazolyl, dihydroisoxazolyl, and dihydrooxazolyl;
[00116] wherein R'° is selected from the group consisting of hydrido,
phenyl, biphenyl, naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, methyl, ethyl, propyl, butyl, pentyl, hexyl,
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl,
hydroxypentyl, hydroxyhexyl, aminomethyl, aminoethyl, aminopropyl,
aminobutyl, aminopentyl, aminohexyl, methylaminomethyl, ethylaminomethyl,
propylaminomethyl, methylaminoethyl, ethylaminoethyl, propylaminoethyl,
methylaminopropyl, ethylaminopropyl, propylaminopropyl, methylaminobutyl,
ethylaminobutyl, propylaminobutyl, methylaminopentyl, ethylaminopentyl,
propylaminopentyl, methylaminohexyl, ethylaminohexyl, propylaminohexyl,
methoxymethyl, methoxyethyl, methoxypropyl, ethoxymethyl, ethoxyethyl,
ethoxypropyl, propoxymethyl, propoxyethyl, propoxypropyl, butoxymethyl,
butoxyethyl, butoxypropyl, piperidinyl, pyrrolidinyl, pyrazolidinyl,
imidazolidinyl,
isoxazolidinyl, oxazolidinyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl,
quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl, isoxazolyl,
oxazolyl,
isoindoledionyl, isoindolyl, dihydroindolyl, isoindoline, dihydrothiophenyl,
dihydropyrrolyl, dihydrofuryl, dihydropyrazolyl, dihydroimidazolyl,
dihydroisoxazolyl, and dihydrooxazolyl;
[00117] wherein R" is selected from the group consisting of hydrido,
phenyl, biphenyl, naphthyl, indenyl, pyridinyl, benzothiophenyl, indolyl,
isoquinolinyl, quinolinyl, thienyl, pyrrolyl, furyl, pyrazolyl, imidazolyl,
isoxazolyl,
oxazolyl, isoindoledionyl, methyl, ethyl, propyl, butyl, pentyl, hexyl,
chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
49
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
trifluoromethyl, ethenyl, propenyl, butenyl, pentenyl, ethynyl, propynyl,
butynyl,
pentynyl, hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl,
hydroxypentyl, hydroxyhexyl, aminomethyl, aminoethyl, aminopropyl,
aminobutyl, aminopentyl, aminohexyl, methylaminomethyl, ethylaminomethyl,
propylaminomethyl, methylaminoethyl, ethylaminoethyl, propylaminoethyl,
methylaminopropyl, ethylaminopropyl, propylaminopropyl, methylaminobutyl,
ethylaminobutyl, propylaminobutyl, methylaminopentyl, ethylaminopentyl,
propylaminopentyl, methylaminohexyl, ethylaminohexyl, propylaminohexyl,
methoxy, ethoxy, propoxy, butoxy, methoxymethyl, methoxyethyl,
methoxypropyl, ethoxymethyl, ethoxyethyl, ethoxypropyl, propoxymethyl,
propoxyethyl, propoxypropyl, butoxymethyl, butoxyethyl, butoxypropyl,
piperidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, isoxazolidinyl,
oxazolidinyl,
pyridinyl, benzothiophenyl, indolyl, isoquinolinyl, quinolinyl, thienyl,
pyrrolyl,
furyl, pyrazolyl, imidazolyl, isoxazolyl, oxazolyl, isoindoledionyl,
isoindolyl,
dihydroindolyl, isoindoline, dihydrothiophenyl, dihydropyrrolyl, dihydrofuryl,
dihydropyrazolyl, dihydroimidazolyl, dihydroisoxazolyl, and dihydrooxazolyl;
[00118] or a pharmaceutically acceptable salt thereof.
[00119] In another particularly preferred embodiment, the compound
of Formula I is a compound of Formula IV:
H
N
CN
NH2
N rN
R1a ~ OH ,
R1b ~ ~ R1d
[00120] R1° IV
[00121] wherein Rla, Rlb, R'°, and R1d are independently selected from
the group consisting of hydrido, cyano, hydroxyl, nitro, halo, alkyl,
haloalkyl,
hydroxyalkyl, haloalkoxy, -OR1°, -NHRB,-NHCORe, -NHCO(ORe), -NHCONHR9,
-CORB, -CO~R', and -CONHR'; and
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00122] wherein R', Re, R9, and R'° are independently selected from
the group consisting of hydrido, haloalkyl, alkyl, cycloalkyl,
cycloalkylalkyl, and
alkenyl;
[00123] or a pharmaceutically acceptable salt thereof.
[00124] In one preferred embodiment, the compound of Formula IV is
a compound wherein R'a, R'b, R'°, and R'd are independently selected
from the
group consisting of hydrido, cyano, hydroxyl, nitro, halo, C,_6 alkyl, C,_s
haloalkyl, C,_6 hydroxyalkyl, C,_6 haloalkoxy, -OR'°, -NHRB,-NHCORB,
-NHCO(ORe), -NHCONHR9, -CORB, -COaR', and -CONHR'; and
[00125] wherein R', R8, R9, and R'° are independently selected from
the group consisting of hydrido, C,_6 haloalkyl, C,_6 alkyl, C3_,~ cycloalkyl,
C4_,8
cycloalkylalkyl, and C2_6 alkenyl;
[00126] or a pharmaceutically acceptable salt thereof.
[00127] In one particularly preferred embodiment, the compound of
Formula IV is a compound wherein R'a, R'b, R'°, and R'd are
independently
selected from the group consisting of hydrido, cyano, hydroxyl, nitro, halo,
methyl, ethyl, propyl, butyl, pentyl, hexyl, chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl, hydroxypentyl, hydroxyhexyl,
chloromethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy,
difluoromethoxy, trifluoromethoxy, -OR'°, -NHRB,-NHCORB, -NHCO(OR8),
-NHCONHR9, -CORe, -C02R', and -CONHR'; and
[00128] wherein R', R8, R9, and R'° are independently selected from
the group consisting of hydrido, chloromethyl, dichloromethyl,
trichloromethyl,
fluoromethyl, difluoromethyl, trifluoromethyl, methyl, ethyl, propyl, butyl,
pentyl,
hexyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl,
cyclopropylethyl, cyclobutylmethyl, cyclobutylethyl, cyclopentylmethyl,
cyclopentylethyl, cyclohexylmethyl, cyclohexylethyl, and ethenyl, propenyl,
butenyl, and pentenyl;
51
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00129] or a pharmaceutically acceptable salt thereof.
[00130] In a particularly preferred embodiment, the compound of
Formula I is selected from the group of compounds consisting of the
compounds shown in Table I below:
Table I
Example Name Structure
1 4-amino-2-(2,6-dihydroxyphenyl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride cN
NHa
I
N ,N
HCI
HO / OH
3 4-amino-2-(5-chloro-2-hydroxyphenyl)-6-piperidin-3- cN
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHZ
I I
N iN
OH
ci ~ HCI
4 4-amino-2-(2-hydroxyphenyl)-6-piperidin-3- cN
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NH2
I I
N ,N
OH
HCI
5 4-amino-2-(3,5-dichloro-2,6-dihydroxyphenyl)-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHS
I I
N ,N
HO / OH
HCI
ci ~ ci
6 4-amino-2-(2-hydroxy-6-methoxyphenyl)-6-piperidin- cN
3-ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHZ
I I
N ,N
HO / ~ O~~H3
HCI
52
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Example Name Structure
9 4-amino-2-(2,5-dihydroxypheny!)-6-plperidin-3- cN
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHS
N /N
OH
Ho NCI
4-amino-2-(2-fluoro-6-hydroxyphenyl)-6-piperidin-3- cN
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHS
N ~N
F / OH
HCI
11 4-amino-2-[2-hydroxy-5-(trifluoromethyl)phenyl]-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile hydrochloride HN ~ NH2
N ,N
/ OH
F3C HCI
12 4-amino-2-[2-hydroxy-4-(trifluoromethyl)phenyl]-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHz
N ,N
/ OH
HCI
CF3
23 4-amino-2-(2-hydroxy-6-propylphenyl)-6-piperidin-3- NH
ylpyrimidine-5-carbonitrile
OH N ~ ON
I N NH2
H3C
53
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Example Name Structure
24 4-amino-2-(2-hydroxy-6-isobutylphenyl)-6-piperidin-3- NH
ylpyrimidine-5-carbonitrile
OH N ~ CN
I N~ NHz
H3C CH3
25 4-amino-2-[2-hydroxy-6-(3-methylbutyl)phenyl]-6- NH
piperidin-3-ylpyrimidine-5-carbonitrile
OH N ~ CN
I N NHa
/
CH3
CH3
29 4-amino-2-(5-bromo-2-hydroxyphenyl)-6-piperidin-3- NH
ylpyrimidine-5-carbonitrile
CN
OH N
I N NH2
Br
30 4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(piperidin-3- NH
yl)pyrimidine-5-carbonitrile
OH N ~ CN
I N/ NHZ
/
CI
[00131] In another preferred embodiment, the compound of Formula I
is selected from the group of compounds consisting of the compounds shown
iri Table II below:
Table II
Name Structure
54
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
~N
HN N ~ ~ F
HO
4-amino-2-(2-hydroxy-5-iodophenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
'N
HN N
HO
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
~N
HN N ~ ~ CH3
HO
4-amino-2-(3-fluoro-2,6-dihydroxyphenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
~~N OH
HN N ~ ~ F
HO
4-amino-2-(2,6-dihydroxy-3-iodophenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
~~ N OH
HN N
HO
4-amino-2-(2,6-dihydroxy-3-methylphenyl)-6-(piperidin-3- NHz
yl)pyrimidine-5-carbonitrile Nc
~~N OH
i ~ CH3
HN~ 'N /
HO
4-amino-2-(3-(trifluoromethyl)-2,6-dihydroxyphenyl)-6- NHz
(piperidin-3-yl)pyrimidine-5-carbonitrile Nc
N OH
HN N ~ ~ CF3
HO
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(3,5-difluoro-2,6-dihydroxyphenyl)-6-(piperidin- NH
3-yl)pyrimidine-5-carbonitrile
OH N \ CN
F \ ( N NHa
I/
'OH
F
4-amino-2-(2,6-dihydroxy-3,5-diiodophenyl)-6-(piperidin-
~NH
3-yl)pyrimidine-5-carbonitrile
OH N \ CN
I \ I N NHa
I/
'OH
I
4-amino-2-(2,6-dihydroxy-3,5-dimethylphenyl)-6- NH
(piperidin-3-yl)pyrimidine-5-carbonitrile
OH N \ CN
H3C \ I N NHZ
I /
'OH
CH3
2-(3,5-bis(trifluoromethyl)-2,6-dihydroxyphenyl)-4-amino- NH
6-(piperidin-3-yl)pyrimidine-5-carbonitrile
CN
OH N \
F3C \ I N NH2
I/
'OH
CF3
4-amino-6-cyclohexyl-2-(2-hydroxyphenyl)pyrimidine-5- NH2
carbonitrile Nc
I ~~N OH
.N I /
4-amino-6-(3-aminocyclohexyl)-2-(2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
H2N I N \
I /
56
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-aminocyclohexyl)-2-(2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
~\
HzN /
4-amino-2-(2-hydroxyphenyl)-6-(3- NHz
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
CH3 ~ ~ N OH
HN N \
/
4-amino-2-(2-hydroxyphenyl)-6-(4- NC NH2
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile
HN ~ ~N
i
H3C N- OH
4-amino-6-(3-(dimethylamino)cyclohexyl)-2-(2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
CH3 ~ ~ N OH
HaC~ N N ~ \
4-amino-6-(4-(dimethylamino)cyclohexyl)-2-(2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
-N
H3C~N ( /
I
CH3
4-amino-6-(3-(aminomethyl)cyclohexyl)-2-(2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
NH2 ~ ~ N OH
4-amino-6-(4-(aminomethyl)cyclohexyl)-2-(2- NC NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
N
HaN N- OH
4-amino-2-(2-hydroxyphenyl)-6-(3- NHz
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile H3c~NH N~ ~ N off
57
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(2-hydroxyphenyl)-6-(4- NC NHa
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile /
N
H3C-NH N- OH
/
4-amino-6-cyclohexyl-2-(2,5-dihydroxyphenyl)pyrimidine-
5-carbonitrile
CN
N \
HO \ I N NHZ
/
OH
4-amino-6-(3-aminocyclohexyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
HO \ I N~ NHa
/
OH
4-amino-6-(4-aminocyclohexyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile
\ CN
N
HO \ I N NH2
/
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3-
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile NH
HO CH3
l ~ cN
N-
OH NHS
4-amino-2-(2,5-dihydroxyphenyl)-6-(4- NHz
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
N OH
-N
HaC~N ~ /
H
OH
58
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(dimethylamino)cyclohexyl)-2-(2,5- NH2
dihydroxyphenyl)pyrimidine-5-carbonitrile cH Nc N off
w
I 3
HsC~N N I \
OH
4-amino-6-(4-(dimethylamino)cyclohexyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~~ N OH
'N
HaC~N I /
I
CH3 OH
4-amino-6-(3-(aminomethyl)cyclohexyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
HzN ~ ~N I
OH
4-amino-6-(4-(aminomethyl)cyclohexyl)-2-(2,5- NH2
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
~N
HzN I /
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3-
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile
HO HN-CH3
~ cN
N-
OH NHz
4-amino-2-(2,5-dihydroxyphenyl)-6-(4- NH2
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile Nc
I ~ N off
~N
H C' N I /
3
OH
59
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-cyclohexyl-2-(5-fluoro-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
N .~ CN
F \ I N NH2
OH
4-amino-6-(3-aminocyclohexyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F \ I N NHZ
OH
4-amino-6-(4-aminocyclohexyl)-2-(5-fluoro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F \ I N NHz
/
OH
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3- NH2
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
N OH
H3C/ N N
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4- NH2
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
~~N OH
~N
HsCwN ~ /
H
F
4-amino-6-(3-(dimethylamino)cyclohexyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile NC
CH3 ~ ~~N OH
,N i
H3C ~ N
F
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-(dimethylamino)cyclohexyl)-2-(5-fluoro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
~N
H3CwN ~ /
I
CH3 F
4-amino-6-(3-(aminomethyi)cyclohexyl)-2-(5-fluoro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~N ON
HEN N
F
4-amino-6-(4-(aminomethyl)cyciohexyl)-2-(5-fluoro-2- NHz
hydroxypheny!)pyrimidine-5-carbonitrile Nc
N OH
~N
HaN ~ /
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3-
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitriie
F HN-CH3
CN
N-
OH NH2
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4- NHz
((methyiamino)methyl)cyciohexyl)pyrimidine-5-carbonitriie Nc
N OH
H
N
H3C~ /
F
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-
cyclohexylpyrimidine-5-carbonitrile
CN
N \
CI \ I i
~N NH2
/ OH
61
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclohexyl)-2-(5-chloro-2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
CI \ I N NH2
OH
4-amino-6-(4-aminocyclohexyl)-2-(5-chloro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
CI \ I N NHS
/
OH
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NH2
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
N OH
HaC~N ~ N \
/
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NH2
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
N OH
~\
H3CwN /
H
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NH2
(dimethylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
i H3 ~ ~ N OH
HsC~N N ~ \
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NH2
(dimethylamino)cyciohexyl)pyrimidine-5-carbonitrile N~
~~ N OH
~N
HaCwN ~ /
I
CH3 CI
62
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(aminomethyl)cyclohexyl)-2-(5-chloro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
_/ \
H2N ~ N
CI
4-amino-6-(4-(aminomethyl)cyclohexyl)-2-(5-chloro-2- ° NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~N OH
/ \
~N
HaN
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3-
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile
CI HN-CH3
CN
N-
OH NHz
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NH2
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile Nc
~~ N OH
/
~N
~N ~ /
H3C
CI
4-amino-6-cyclohexyl-2-(2-hydroxy-5-
methylphenyl)pyrimidine-5-carbonitrile
N \ CN
N3C \ I N NH2
/
OH
4-amino-6-(3-aminocyclohexyl)-2-(2-hydroxy-5- NH2
methylphenyl)pyrimidine-5-carbonitrile
CN
N
H3C \ I N NHZ
/
OH
63
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-aminocyclohexyl)-2-(2-hydroxy-5- NH2
methylphenyl)pyrimidine-5-carbonitrile
N \ CN
H3C \ ~ N NHz
I /
OH
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3- NHa
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
~~N OH
H3C~N
I \
CH3
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(4- NHa
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
~N
H3C~N I /
H
CH3
4-amino-6-(3-(dimethylamino)cyclohexyl)-2-(2-hydroxy-5- NH2
methylphenyl)pyrimidine-5-carbonitrile ~H N~ ~ N OH
3
N I ~ \
H3C~ I
CH3
4-amino-6-(4-(dimethylamino)cyclohexyl)-2-(2-hydroxy-5- NH2
methylphenyl)pyrimidine-5-carbonitrile Nc
I 'N OH
'N
H3C~N _ I /
I
CH3 CH3
4-amino-6-(3-(aminomethyl)cyclohexyl)-2-(2-hydroxy-5- NHa
methylphenyl)pyrimidine-5-carbonitrile Nc
I ~~ N OH
H2N ~ ~N I
CH3
64
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-(aminomethyl)cyclohexyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile Nc
~~N OH
~N
HZN ~ /
CH3
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile
H3C HN-CH3
CN
N-
OH NHZ
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(4- NHz
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile Nc
N OH
-N
H C~ N ~ /
3
CH3
4-amino-6-cyclohexyl-2-(5-(trifluoromethyl)-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
C \ I N NHS
/
OH
4-amino-6-(3-aminocyclohexyl)-2-(5-(trifluoromethyl)-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F3C \ I N NHZ
/
OH
4-amino-6-(4-aminocyclohexyl)-2-(5-(trifluoromethyl)-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
\ CN
N
C \ I N NHS
/
OH
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3-
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile . NH
F3C CH3
CN
N-
OH NHS
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4- NHS
(methylamino)cyclohexyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
~N
H3C~N I /
H
CF3
4-amino-6-(3-(dimethylamino)cyclohexyl)-2-(5- NH2
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile ~H N~ ~ N off
3
HaC~ N N I \
CF3
4-amino-6-(4-(dimethylamino)cyclohexyl)-2-(5- NH2
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
~N
H3C~N I /
I
CH3 CF3
4-amino-6-(3-(aminomethyl)cyclohexyl)-2-(5- NH2
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
HZN ~ 'N
CF3
4-amino-6-(4-(aminomethyl)cyclohexyl)-2-(5- NHS
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
~N
HEN I /
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile F c HN-cH
3 3
~ CN
N-
OH NHS
66
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4- NH2
((methylamino)methyl)cyclohexyl)pyrimidine-5-carbonitrile Nc
I ~~N OH
~N
H C~ N I /
3
CF3
4-amino-6-cyclopentyl-2-(2-hydroxyphenyl)pyrimidine-5- NHa
carbonitrile Nc
I ~ N OH
N
4-amino-6-(3-aminocyclopentyl)-2-(2- ~ NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
HzN N
4-amino-2-(2-hydroxyphenyl)-6-(3- NHa
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
~~N OH
HaC I N \
HN I
4-amino-6-(3-(dimethylamino)cyclopentyl)-2-(2- NHa
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
HaC I i \
~N
H3C I /
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~N OH
H2N I ~ \
'N
4-amino-2-(2-hydroxyphenyl)-6-(3- NH2
((methylamino)methyl)cyclopentyl)pyrimidine-5- ~H N~ N OH
w
carbonitrile HN 3 I
N
4-amino-6-cyclopentyl-2-(2,5-dihydroxyphenyl)pyrimidine-
5-carbonitrile
\ CN
N
HO \ I N NHZ
I /
OH
67
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclopentyl)-2-(2,5- NHZ
dihydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
HO \ I N NHZ
/
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3- NH2
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
~~ N OH
HN N
H3C ~ /
OH
4-amino-6-(3-(dimethylamino)cyclopentyl)-2-(2,5- NH2
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~N OH
HsC ~ i \
~N
H3C ~ /
OH
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(2,5- NHa
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~ N OH
N
HEN ~ /
OH
4-amino-2-(2,5-dihydroxyphenyi)-6-(3- NH2
((methylamino)methyl)cyclopentyl)pyrimidine-5- Nc
carbonitrile I ~ N off
N
HaC-NH ~ /
OH
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-
cyclopentylpyrimidine-5-carbonitrile
CN
N \
CI \ I N NHS
OH
68
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclopentyl)-2-(5-chloro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
CI \ I N NH2
/
OH
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- H
N
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile ~cH3
cl
~ cN
N-
OH NH2
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NHz
(dimethylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
N OH
HsC ~ N \
N
H3C ~ /
CI
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(5-chloro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~ N OH
N
HaN ~ /
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NHz
((methylamino)methyl)cyclopentyl)pyrimidine-5- Nc
carbonitrile ~ ~~N OH
N
Hs~-NH ~ /
GI
4-amino-6-cyclopentyl-2-(5-fluoro-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
\ I N NH2
OH
69
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclopentyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
F \
~N NHS
OH
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3- NHz
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
~~N OH
HN N
HsC
F
4-amino-6-(3-(dimethylamino)cyclopentyl)-2-(5-fluoro-2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile NC
~~ N OH
HsC ~ i \
~N
H3C ~ /
F
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(5-fluoro-2- NHz
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
N
HZN ~ /
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3- NH2
((methylamino)methyl)cyclopentyl)pyrimidine-5- Nc
carbonitrile I ~ N OH
N
HsC~NH ~ /
F
4-amino-6-cyclopentyl-2-(2-hydroxy-5-
methylphenyl)pyrimidine-5-carbonitrile
\ CN
N
H3C \ I N NH2
/
OH
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclopentyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile
N \ CN
H3C \ I N NH2
/
OH
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3- NH2
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
N OH
HN N
H3C ~ /
CH3
4-amino-6-(3-(dimethylamino)cyclopentyl)-2-(2-hydroxy-5- NHa
methylphenyl)pyrimidine-5-carbonitrile Nc
N OH
HaG ~ i \
~N
H3C ~ /
CH3
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile Nc
~N OH
i \
N
HZN ~ /
CH3
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3- NH2
((methylamino)methyl)cyclopentyl)pyrimidine-5- Nc
carbonitrile I ~~N OH
N
HsC-NH ~ /
CH3
4-amino-6-cyclopentyl-2-(5-(trifluoromethyl)-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F3C \ I N NH2
/
OH
71
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocyclopentyl)-2-(5-(trifluoromethyl)-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
F3C \ I N NHZ
/
OH
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3- NH2
(methylamino)cyclopentyl)pyrimidine-5-carbonitrile Nc
N OH
HN N
H3C ~ /
CF3
4-amino-6-(3-(dimethylamino)cyclopentyl)-2-(5- NHz
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
N OH
HsC ~ i \
N ~N
H3C ~ /
CF3
4-amino-6-(3-(aminomethyl)cyclopentyl)-2-(5- NH2
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~~ N OH
N
H2N ~ /
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3- NHz
((methylamino)methyl)cyclopentyl)pyrimidine-5- Nc
carbonitrile ~ ~~N OH
N
HaC~NH ~ /
CF3
4-amino-6-cycloheptyl-2-(5-(trifluoromethyl)-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
F3C \ I N NHz
OH
72
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocycloheptyl)-2-(5-(trifluoromethyl)-2-NHS
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F3C \ I N NHa
I
/
OH
4-amino-6-(4-aminocycloheptyl)-2-(5-(trifluoromethyl)-2-NH2
hydroxyphenyi)pyrimidine-5-carbonitrileNc
~~
H
I
N O
\
I
HEN /
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3-NHS
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
~ N OH
H
I
HaC_N i
~N I \
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
~ N OH
I
H3C N \
HN I /
CF3
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(5-NHz
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc
~ N OH
N
I
,
HaC
N I \
CF3
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(5-NH2
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrileNc
I ~ N OH
\
H3C\ N
I
N /
H3C
CF3
73
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(5- NHz
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~~ N OH
H2N
~N I \
CF3
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(5- NHz
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
I ~ N OH
HzN N I \
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3- NHz
((methylamino)methyl)cycloheptyl)pyrimidine-5- Nc ~ N off
carbonitrile H CN I \
_i
N I
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4- NHz
((methylamino)methyl)cycloheptyl)pyrimidine-5- Nc
~~ N OH
carbonitrile cH3
I \
HN N I
CF3
4-amino-6-cycloheptyl-2-(2,5-dihydroxyphenyl)pyrimidine-
5-carbonitrile
\ CN
N
HO \ I N NH2
I /
OH
4-amino-6-(3-aminocycloheptyl)-2-(2,5- NH
z
dihydroxyphenyl)pyrimidine-5-carbonitrile
\ CN
N
HO \ I N NHZ
I/
OH
74
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-aminocycloheptyl)-2-(2,5-NHS
dihydroxyphenyl)pyrimidine-5-carbonitrileNc
~ N OH
I
~N
HEN I /
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
OH
~
H
N
I
HaC._N
i
~N I \
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(4-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
~
OH
I
N
H3C N \
HN I /
OH
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(2,5-NH2
dihydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc ~
off
N
H3C~N
~N I \
OH
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(2,5-NH2
dihydroxyphenyl)pyrimidine-5-carbonitrileNc
~~
H
I
N O
\
H3C\ N
I
N /
i
H3C
OH
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(2,5-NH2
dihydroxyphenyl)pyrimidine-5-carbonitrileNc
~~
N OH
I
HEN
~N I \
OH
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(2,5-NH2
dihydroxyphenyl)pyrimidine-5-carbonitrileNo
~N
H
I
O
H2N N I \
OH
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(2,5-dihydroxyphenyl)-6-(3-NH2
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~ off
carbonitrile I
N
HN
/
~N \
HsC I
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(4-NHz
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~ N off
carbonitrile I
CH3
HN N I \
OH
4-amino-6-cycloheptyl-2-(5-fluoro-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
F \ I N NHz
I
/
OH
4-amino-6-(3-aminocycloheptyl)-2-(5-fluoro-2-NH
z
hydroxyphenyl)pyrimidine-5-carbonitrile
cN
N \
F \ I N NHS
I
/
OH
4-amino-6-(4-aminocycloheptyl)-2-(5-fluoro-2-NHz
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
N
HaN /
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3-NHz
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNo
'N OH
H
I
HsC.N i
~N I \
a
F
76
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc ~ N off
\
H3C~
HN /
F
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(5-fluoro-2-NHS
hydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc ~ N OH
HsC~N I
~N I \
F
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(5-fluoro-2-NHZ
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
H3C\ N
\
I
N /
H3C
F
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(5-fluoro-2-NHa
hydroxyphenyl)pyrimidine-5-carbonitrileNc
'N OH
H2N
~N I \
F
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(5-fluoro-2-NHz
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
HEN N
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3-NH2
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~ N off
carbonitrile I
N
i
N I \
H3
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4-NH2
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
w N off
carbonitrile I
cH3
HN N
F
77
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-cycloheptyl-2-(5-chloro-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
CI \ I N NHa
I
/
OH
4-amino-6-(3-aminocycloheptyl)-2-(5-chloro-2-NH
2
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
CI \ I N NHS
I
/
OH
4-amino-6-(4-aminocycloheptyl)-2-(5-chloro-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
,N
HEN /
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3-NHZ
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
' OH
H
N
I
H3C~ N
~N I \
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4-NHa
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
~ OH
I
N
H3C N \
HN I /
CI
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(5-chloro-2-NHa
hydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc
' H
N O
I
I
H3C~N
N
CI
78
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(5-chloro-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
~
I ~ OH
N
HsC Ni \
\
N I /
H3C
CI
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(5-chloro-2-NHz
hydroxyphenyl)pyrimidine-5-carbonitrileNc
~ N off
I
HzN
~N I \
CI
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(5-chloro-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
~ N OH
I
H2N N I \
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3-NHz
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~~ OH
carbonitrile N
I
HN
C ~N \
H
3
/
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4-NHz
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~ ff
~
carbonitrile N o
I
CH3
HN N I \
CI
4-amino-6-cycloheptyl-2-(5-methyl-2-
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
H3C \ I N NHz
I
/
OH
79
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminocycloheptyl)-2-(5-methyl-2-NH
a
hydroxyphenyl)pyrimidine-5-carbonitrile
N \ CN
n
H3C
\ I N NH2
I
/
OH
4-amino-6-(4-aminocycloheptyl)-2-(5-methyl-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
N
HEN /
CH3
4-amino-2-(5-methyl-2-hydroxyphenyl)-6-(3-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
'N OH
H I
H3C~N
~N I \
CH3
4-amino-2-(5-methyl-2-hydroxyphenyl)-6-(4-NH2
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
~~
OH
I
N
H3~, N
\
I
HN /
CH3
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(5-methyl-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc
'N OH
I
H3C
wN I \
CH3
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(5-methyl-2-NHz
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
H3C\ N
\
I
N /
H3C
CH3
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(5-methyl-2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
'N OH
HZN
~ \
N I
CH3
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(5-methyl-2-NHa
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
H2N N I \
CH3
4-amino-2-(5-methyl-2-hydroxyphenyl)-6-(3-NHa
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
~~N off
carbonitrile I
/ \
N
'N
H C I
CH3
4-amino-2-(5-methyl-2-hydroxyphenyl)-6-(4-NH2
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc off
~ N
carbonitrile I
cH3
HN N
CH3
4-amino-6-cycloheptyl-2-(2-hydroxyphenyl)pyrimidine-5-
carbonitrile
\ CN
N
N NHS
I
/
OH
4-amino-6-(3-aminocycloheptyl)-2-(2- NH
2
hydroxyphenyl)pyrimidine-5-carbonitrile
\ CN
N
N NH2
I
/
OH
81
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-aminocycloheptyl)-2-(2- NHZ
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
~N
HEN /
4-amino-2-(2-hydroxyphenyl)-6-(3- NHZ
(methylamino)cycloheptyl)pyrimidine-5-carb~nitrileNc
'N OH
H
H3C~N I
~ \
'N
4-amino-2-(2-hydroxyphenyl)-6-(4- NHa
(methylamino)cycloheptyl)pyrimidine-5-carbonitrileNc
'N OH
H3C N I \
HN /
4-amino-6-(3-(dimethylamino)cycloheptyl)-2-(2-NHS
hydroxyphenyl)pyrimidine-5-carbonitrilecH3 Nc ~ N off
C~N I
H
a
'N
4-amino-6-(4-(dimethylamino)cycloheptyl)-2-(2-NHZ
hydroxyphenyl)pyrimidine-5-carbonitrileNc ~ N OH
I
\
H3C N
I
N /
H3C
4-amino-6-(3-(aminomethyl)cycloheptyl)-2-(2-NH2
hydroxyphenyl)pyrimidine-5-carbonitrileNc
'N OH
HZN
~N
4-amino-6-(4-(aminomethyl)cycloheptyl)-2-(2-NHS
hydroxyphenyl)pyrimidine-5-carbonitrileNc
I 'N OH
HEN N I \
4-amino-2-(2-hydroxyphenyl)-6-(3- NHS
((methylamino)methyl)cycloheptyl)pyrimidine-5-Nc
w N off
carbonitrile I
N
N I \
H C
3
/
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-methyl-2-hydroxyphenyl)-6-(4- NHS
((methylamino)methyl)cycloheptyl)pyrimidine-5- Nc
N OH
carbonitrile cH3
HN N
4-amino-6-phenyl-2-(2-hydroxyphenyl)pyrimidine-5- NH2
carbonitrile Nc
N OH
\ ~ N ~ /
4-amino-6-(3-aminophenyl)-2-(2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
~N OH
HaN / ~ N \
\ ~ ~ /
4-amino-6-(4-aminophenyl)-2-(2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
\ ~ N ~ /
H2N
4-amino-2-(2-hydroxyphenyl)-6-(3- NHS
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
CH3 ~ 'N OH
I
HN
4-amino-2-(2-hydroxyphenyl)-6-(4- NC NHz
(methylamino)phenyl)pyrimidine-5-carbonitrile -
HN \ ~ ~ \N
H3C N- OH
4-amino-6-(3-(dimethylamino)phenyl)-2-(2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile ~H N~ ~ N OH
3
H3C~ N / N ~ \
/
4-amino-6-(4-(dimethylamino)phenyl)-2-(2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
/ N \
H3C\N
I
CH3
83
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(aminomethyl)phenyl)-2-(2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile NH NC w N off
\ ~ N ~ /
4-amino-6-(4-(aminomethyl)phenyl)-2-(2- NC NHZ
hydroxyphenyl)pyrimidine-5-carbonitrile
N
HaN N- OH
4-amino-2-(2-hydroxyphenyl)-6-(3- NHS
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile H3c~NH NC w N off
N ~ /
4-amino-2-(2-hydroxyphenyl)-6-(4- NC NHz
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile -
N
H3C-NH \ / N- OH
4-amino-6-phenyl-2-(2,5-dihydroxyphenyl)pyrimidine-5-
carbonitrile
\
\ CN
N
HO \ I N NH2
/
OH
4-amino-6-(3-aminophenyl)-2-(2,5- / NH2
dihydroxyphenyl)pyrimidine-5-carbonitrile
\
\ CN
N
HO \ I N NH2
/
OH
84
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-aminophenyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile
/I
\
N \ CN
HO \ I N NHZ
I/
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3-
(methylamino)phenyl)pyrimidine-5-carbonitrile H~ \ / N cHs
/ \ i \ cN
N-
OH NHS
4-amino-2-(2,5-dihydroxyphenyl)-6-(4- NHz
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
I 'N OH
N \
H3~,N \ I I /
H
OH
4-amino-6-(3-(dimethylamino)phenyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
CH3 I 'N OH
HsC~ N / I N I \
\ /
OH
4-amino-6-(4-(dimethylamino)phenyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
I 'N OH
/ N \
H3C\ N \ I
OH
CH3
4-amino-6-(3-(aminomethyl)phenyl)-2-(2,5- NHz
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
HzN \ I N I /
OH
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-(aminomethyl)phenyl)-2-(2,5- NH2
dihydroxyphenyl)pyrimidine-5-carbonitrile Nc ~ N off
I
/ N \
HZN \I I/
OH
4-amino-2-(2,5-dihydroxyphenyl)-6-(3- off
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile
\ I ~ I / NCH
3
OH N w CN
NHp
4-amino-2-(2,5-dihydroxyphenyl)-6-(4- off
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile
H
\ I ~ I / N ~CH
3
OH N ~ CN
NHS
4-amino-6-phenyl-2-(5-fluoro-2-hydroxyphenyl)pyrimidine- / I
5-carbonitrile \
\ CN
N
F \ I N NHZ
I/
OH
4-amino-6-(3-aminophenyl)-2-(5-fluoro-2- / NHa
hydroxyphenyl)pyrimidine-5-carbonitrile I
\
CN
N \
\ I N NH2
I/
OH
4-amino-6-(4-aminophenyl)-2-(5-fluoro-2- NHa
hydroxyphenyl)pyrimidine-5-carbonitrile
/I
\ CN
N
\ I N NHZ
I/
OH
86
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3- NH2
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
~N OH
HaC~ N / ~ N ~ \
\ /
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4- NHS
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
'N OH
/ N \
H3C\N \
H
F
4-amino-6-(3-(dimethylamino)phenyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile ~H N~ ~ N off
3
H3~~N / N ~ \
\ ~ /
F
4-amino-6-(4-(dimethylamino)phenyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
N \
H3~\N
I
CH3 F
4-amino-6-(3-(aminomethyl)phenyl)-2-(5-fluoro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
H2N \ ~ N
F
4-amino-6-(4-(aminomethyl)phenyl)-2-(5-fluoro-2- NHS
hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
/ N \
HEN \ ~ I /
F
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(3-
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile F N-cH
3
cN
N-
OH NHS
~7
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure -.
4-amino-2-(5-fluoro-2-hydroxyphenyl)-6-(4- NH2
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile Nc
~~ N OH
/ N \
\ ~ ~ /
H3C
F
4-amino-2-(5-chloro-2-hydroxyphenyl)-6- /
phenylpyrimidine-5-carbonitrile \
\ CN
N
CI \ I N NH2
/
OH
4-amino-6-(3-aminophenyl)-2-(5-chloro-2- / NH2
hydroxyphenyl)pyrimidine-5-carbonitrile
CN
N \
CI \ I N NHS
OH
4-amino-6-(4-aminophenyl)-2-(5-chloro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile
/
\ CN
N
CI \ I N NH2
OH
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NHS
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
'N OH
HsC~ N / ~ N
\ /
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NHZ
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
'N OH
H3C~N \ ~ 'N
H
CI
8$
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3- NH2
(dimethylamino)phenyl)pyrimidine-5-carbonitrile ~H N~ w N off
3
H3C~ N / I N I \
\ /
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NH2
(dimethylamino)phenyl)pyrimidine-5-carbonitrile Nc
I 'N OH
HsC~N \ I N I /
I CI
CH3
4-amino-6-(3-(aminomethyl)phenyl)-2-(5-chloro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc ~ N off
I
HzN \ I N I /
CI
4-amino-6-(4-(aminomethyl)phenyl)-2-(5-chloro-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile Nc ~ N OH
I
N \
H2N \ I I /
CI
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(3-
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile ~I ~ ~ HN-cH3
CN
N-
OH NHz
4-amino-2-(5-chloro-2-hydroxyphenyl)-6-(4- NHz
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile Nc I w N off
N \
,N \ I I /
H3C
CI
89
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-phenyl-2-(2-hydroxy-5- /
methylphenyl)pyrimidine-5-carbonitrile
N \ CN
H3C \ I N NH2
/
OH
4-amino-6-(3-aminophenyl)-2-(2-hydroxy-5- / NH2
methylphenyl)pyrimidine-5-carbonitrile
\
CN
N \
H3C \ I N NHa
OH
4-amino-6-(4-aminophenyl)-2-(2-hydroxy-5- NH2
methylphenyl)pyrimidine-5-carbonitrile
/
\
\ CN
N
H3C \ I N NHS
/
OH
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3- NHS
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
'N OH
HaC~N / ~ N ~ \
\ /
CH3
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(4- NHZ
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
'N OH
N \
H3C\N
H -
CH3
4-amino-6-(3-(dimethylamino)phenyl)-2-(2-hydroxy-5- NHZ
methylphenyl)pyrimidine-5-carbonitrile CH N~ ~ N OH
3
HsC~ N / ~ N ~ \
\ /
CH3
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(4-(dimethylamino)phenyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile Nc
N OH
/ N \
H3C\N \
I
CH3 CH3
4-amino-6-(3-(aminomethyl)phenyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile Nc
~N OH
H2N \ ~ N
CH3
4-amino-6-(4-(aminomethyl)phenyl)-2-(2-hydroxy-5- NHz
methylphenyl)pyrimidine-5-carbonitrile Nc
'N OH
/ N \
HzN
CH3
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(3- -
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile H c ~ ~ HN-cH3
3
~ cN
N-
OH NH2
4-amino-2-(2-hydroxy-5-methylphenyl)-6-(4- NHz
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile Nc I ~ N off
/ N \
~N \ ~ ~ /
H3C
CH3
4-amino-6-phenyl-2-(5-(trifluoromethyl)-2- /
hydroxyphenyl)pyrimidine-5-carbonitrile
\ CN
N
C \ I N NHS
/
OH
91
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-aminophenyl)-2-(5-(trifluoromethyl)-2- / NH2
hydroxyphenyl)pyrimidine-5-carbonitrile I
\
N ~ CN
F3C \ I N NHZ
I/
OH
4-amino-6-(4-aminophenyl)-2-(5-(trifluoromethyl)-2- NH2
hydroxyphenyl)pyrimidine-5-carbonitrile /
\I
CN
N \
F3C \ I N NHa
I/
OH
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3-
(methylamino)phenyl)pyrimidine-5-carbonitrile NH
F3C CH3
~ cN
N-
OH NHS
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4- NHz
(methylamino)phenyl)pyrimidine-5-carbonitrile Nc
I 'N OH
/I N I\
H3C~N \ /
H
CF3
4-amino-6-(3-(dimethylamino)phenyl)-2-(5- NHZ
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile cH3 Nc ~ N off
~N / I N \
H3C \ I I /
CF3
4-amino-6-(4-(dimethylamino)phenyl)-2-(5- NHa
(trifluoromethyl)-2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc I w N off
N \
H3C\N \ I I /
CH3 CF3
92
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Name Structure
4-amino-6-(3-(aminomethyl)phenyl)-2-(5-(trifluoromethyl)- NH2
2-hydroxyphenyl)pyrimidine-5-carbonitrile Nc
'N OH
HEN \ I 'N I /
CF3
4-amino-6-(4-(aminomethyl)phenyl)-2-(5-(trifluoromethyl)- NHa
2-hydroxyphenyl)pyrimidine-5-carbonitrile N~ ~ N OH
I \
H2N \ I 'N I /
CF3
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(3
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile
F3C HN-CH3
CN
N
OH NHZ
4-amino-2-(5-(trifluoromethyl)-2-hydroxyphenyl)-6-(4- NH2
((methylamino)methyl)phenyl)pyrimidine-5-carbonitrile Nc I ~ N off
N \
H CAN \ I I /
3
CF3
DEFINITIONS
[00132] The term "hydrido" denotes a single hydrogen atom (H). This
hydrido radical may be attached, for example, to an oxygen atom to form a
hydroxyl radical or two hydrido radicals may be attached to a carbon atom to
form a methylene (-CHI ) radical.
[00133] The term "halo" denotes halogen atoms such as fluorine,
chlorine, bromine, or iodine.
[00134] The term "carbonyl", whether used alone or with other terms
such as "alkylcarbonyl", denotes -(C=O)-.
[00135] The terms "carboxy" or "carboxyl", whether used alone or with
other terms, such as "carboxyalkyl", denotes -COaH.
93
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00136] The term "sulfonyl," whether used alone or linked to other
terms such as alkylsulfonyl, denotes the divalent radical -S02 .
[00137] The term "amido" when used by itself or with other terms such
as "amidoalkyl", "N-monoalkylamido", "N-monoarylamido", "N,N-dialkylamido",
"N-alkyl-N-arylamido", "N-alkyl-N-hydroxyamido" and "N-alkyl-N-
hydroxyamidoalkyl", embraces a carbonyl radical substituted with an amino
radical.
[0013] The terms "N-alkylamido" and "N,N-dialkylamido" denote
amido groups which have been substituted with one alkyl radical and with two
alkyl radicals, respectively.
[00139] The terms "N-monoarylamido" and "N-alkyl-N-arylamido"
denote amido radicals substituted, respectively, with one aryl radical, and
one
alkyl and one aryl radical.
[00140] The term "N-alkyl-N-hydroxyamido" embraces amido radicals
substituted with a hydroxyl radical and with an alkyl radical.
[00141] The terms "sulfamyl" or "sulfonamidyl" denotes a sulfonyl
radical substituted with an amino radical, forming a sulfonamide (-SO~NH2).
The amino radical may be substituted with alkyl and/or aryl moieties to form,
e.g., "N-alkylsulfamyl", "N-arylsulfamyl", "N,N-dialkylsulfamyl," and "N-alkyl-
N-
arylsulfamyl" radicals.
[00142] The term "amidino" denotes a -C(=NH)NHZ radical.
[00143] The term "cyanoamidino" denotes a -C(=N-CN)NHz radical.
[00144] The term "alkyl," used alone or within other terms such as
"haloalkyl" and "alkylsulfonyl," embraces linear or branched radicals having
one to about twenty carbon atoms. More preferred are "lower alkyl" radicals
having one to about eight carbon atoms. Examples of alkyl radicals include
methyl, ethyl, propyl (including n-propyl and isopropyl), butyl (including n-
butyl,
isobutyl, sec-butyl, and t-butyl), pentyl (including n-pentyl and isoamyl),
hexyl,
octyl and the like.
94
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
j00145] The term "cycloalkyl" embraces radicals having three to ten
carbon atoms, and includes monocyclic, bicyclic, and tricyclic radicals.
Examples of cycloalkyl radicals include cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, decahydronaphthyl, octahydroindyl,
octahydropentalene, bicyclo[1.1.0]butyl, bicyclo[2.1.0]pentyl,
bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl, and
bicyclo[4.2.2]decyl.
[00146] The term "alkylcarbonyl" embraces radicals having a carbonyl
radical substituted with an alkyl radical. An example of an alkylcarbonyl
radical
is acetyl.
[00147] The term "alkylthio" embraces radicals containing a linear or
branched alkyl radical, of one to ten carbon atoms, attached to a divalent
sulfur
atom. An example of an alkylthio radical is methylthio (CH3S-)
[00148] The term "alkylsulfinyl" embraces radicals containing a linear
or branched alkyl radical, of one to ten carbon atoms, attached to a divalent
-S(=O)- radical. An example of an alkyisuifinyl radical is methylsulfinyl
(CH3S(=O)-).
[00149] The term "alkylsulfonyl" embraces alkyl radicals as defined
above attached to a divalent sulfonyl radical, -SOZ .
[00150] The term "amidoalkyl" embraces alkyl radicals substituted with
amido radicals.
[00151] The term "N-alkyl-N-hydroxyamidoalkyl" embraces alkyl
radicals substituted with an N-alkyl-N-hydroxyamido radical.
[00152] The term "aminoalkyl" embraces alkyl radicals substituted with
amino radicals.
[00153] The term "carboxyalkyl" embraces radicals having a carboxyl
moiety attached to an alkyl radical.
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00154] The term "haloalkyl" embraces radicals wherein any one or
more of the alkyl carbon atoms is substituted wifih halo as defined above.
Specifically embraced are monohaloalkyl, dihaloalkyl, and polyhaloalkyl
radicals. A monohaloalkyl radical, for one example, may have a bromo, chloro,
or a fluoro atom within the radical. Dihaloalkyl radicals may have two of the
same halo atoms or a combination of different halo radicals; polyhaloalkyl
radicals may have more than two of the same halo atoms or a combination of
different halo radicals.
[00155] The term "hydroxyalkyl" embraces linear or branched alkyl
radicals having one to about ten carbon atoms, any of which may be
substituted with one or more hydroxyl radicals.
[00156] The terms "N-alkylamino" and "N, N-dialkylamino" denote
amino groups which have been substituted with one alkyl radical and with two
alkyl radicals, respectively.
[00157] The term "alkoxy" embraces linear or branched oxy-containing
alkyl radicals having one to about ten carbon atoms. Examples of "alkoxy"
radicals include methoxy and butoxy.
[00158] The term "alkoxyalkyl" embraces linear or branched alkyl
radicals having one to about ten carbon atoms substituted by one or more
alkoxy radicals each having one to about ten carbon atoms.
[00159] "Alkoxy" or "alkoxyalkyl" radicals may be further substituted
with one or more halo atoms, such as fluoro, chloro, or bromo, to provide
"haloalkoxy" or "haloalkoxyalkyl" radicals.
[00160] The term "alkoxycarbonyl" means a radical containing an
alkoxy radical, as defined above, attached via an oxygen atom to a carbonyl
radical. Examples of such alkoxycarbonyl radicals include methoxycarbonyl
and t-butoxycarbonyl.
[00161] The term "alkoxycarbonylalkyl" embraces radicals having
alkoxycarbonyl moiety, as defined above substituted to an alkyl radical.
96
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Examples of such alkoxycarbonylalkyl radicals include methoxycarbonylethyl
(-(CH2)a(O=)COCH3) and t-butoxycarbonylethyl (-(CH~)a(O=)COC(CH3)3).
[00162] The term "alkylaminoalkyl" embraces aminoalky) radicals
wherein the nitrogen atom is substituted with an alkyl radical.
[00163] The term "alkylcarbonylalkyl" denotes an alkyl radical
substituted with an "alkylcarbonyl" radical.
[00164] The term "alkenyl," used alone or within other terms such as
"haloalkenyl," embraces unsaturated linear or branched radicals having two to
about twenty carbon atoms and containing at least one carbon-carbon double
bond. Examples of alkenyl radicals include ethenyl, propenyl butenyl,
pentenyl,
and the like.
[00165] The term "cycloalkenyl" embraces unsaturated radicals having
three to ten carbon atoms and containing at least one carbon-carbon double
bond, and includes monocyclic, bicyclic, and tricyclic radicals. Examples of
cycloalkenyl radicals include cyclopropenyl, cyclobutenyl, cyclopentenyl,
cyclohexenyl, cycloheptenyl, decahydronaphthenyl, hexahydroindenyl,
hexahydropentalenyl, bicyclo[2.1.0]pentenyl, bicyclo[1.1.1]pentenyl,
bicyclo[2.1.1]hexenyl, bicyclo[2.2.1]heptenyl, bicyclo[3.1.1]heptenyl,
bicyclo[3.2.1]octenyl, bicyclo[2.2.2]octenyl, and bicyclo[4.2.2]decenyl.
[00166] The term "alkynyl," used alone or within other terms such as
"haloalkynyl," embraces unsaturated linear or branched radicals having two to
about twenty carbon atoms and containing at least one carbon-carbon triple
bond. Examples of alkynyl radicals include ethynyl, propynyl butynyl,
pentynyl,
and the like.
[00167] The term "aryl", alone or in combination, means a carbocyclic
aromatic system containing one, two, or three rings wherein at least one of
the
rings is aromatic, and wherein such rings may be attached together in a
pendant manner or may be fused. Examples of aryl radicals include phenyl,
naphthyl, tetrahydronapthyl, indyl, and biphenyl. Aryl moieties, alone or in
97
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
combination, may be optionally substituted by one or more substituents
selected from the group consisting of amino, halo, cyano, hydroxyl, alkyl,
alkoxy, and carboxyl.
[00168] The term "aralkyl" embraces aryl-substituted alkyl radicals
such as benzyl, diphenylmethyl, triphenylmethyl, phenethyl, and diphenethyl.
[00169] The term "arylsulfonyl" embraces aryl radicals as defined
above attached to a sulfonyl radical.
[00170] The term "acyl," whether used alone or within a term such as
"acylamino," denotes a radical provided by the residue after removal of
hydroxyl from an organic acid.
[00171] The term "acylamino" embraces an amino radical substituted
with an acyl group. An examples of an "acylamino" radical is acetylamino
(CH3C(=O)NH-)
[00172] The term "heterocyclic" or "heterocycle" means a saturated or
unsaturated mono- or multi-ring carbocyclic system wherein one or more
carbon atoms in the system are replaced by nitrogen, sulfur, phosphorous,
and/or oxygen. The term "heterocyclic" embraces "heteroaryl" groups, which
means a carbocyclic aromatic system containing one, two, or three rings
wherein at least one of the rings is aromatic, wherein such rings may be
attached together in a pendant manner or may be fused, and wherein one or
more carbon atoms in the system are replaced by nitrogen, sulfur,
phosphorous, and/or oxygen. "Heterocyclic" includes, for example, the
following structures:
z' z3
Z3 O I2
[00173] ~~z2 or z'z~ z
[00174] wherein Z, Z', Z2, and Z3 are independently carbon, sulfur,
phosphorous, oxygen, or nitrogen, with the proviso that one of Z, Z', Z~, or
Z3 is
other than carbon, but is not oxygen or sulfur when attached to another Z atom
by a double bond or when attached to another oxygen or sulfur atom.
98
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Furthermore, the optional substituents are understood to be attached to Z, Z',
Z2, or Z3 only when each is carbon. For example, the term "heterocyclyl"
embraces each of the following groups, although this listing is not meant to
limit
the definition to these groups only: furanyl; thienyl; pyrrolyl; 2-
isopyrrolyl; 3-
isopyrrolyl; pyrazolyl; 2-isoimidazolyl; 1,2,3-triazolyl; 1,2,4-triazolyl; 1,2-
dithiolyl;
1,3-dithiolyl; 1,2,3-oxathiolyl; isoxazolyl; oxazolyl; thiazolyl;
isothiazolyl; 1,2,3-
oxadiazolyl; 1,2,4-oxadiazolyl; 1,2,5-oxadiazolyl; 1,3,4-oxadiazolyl; 1,2,3,4-
oxatriazolyl; 1,2,3,5-oxatriazolyl; 1,2,3-dioxazolyl; 1,2,4-dioxazolyl; 1,3,2-
dioxazolyl; 1,3,4-dioxazolyl; 1,2,5-oxathiazolyl; 1,3-oxathiolyl; 1,2-pyranyl;
1,4-
pyranyl; 1,2-pyranonyl; 1,4-pyranonyl; 1,2-dioxinyl; 1,3-dioxinyl; pyridyl;
pyridazyl; pyrimidyl; pyrazinyl; piperazyl; 1,3,5-triazinyl; 1,2,4-triazinyl;
1,2,3-
triazinyl; 1,2,4-oxazinyl; 1,3,2-oxazinyl; 1,3,6-oxazinyl; 1,2,6-oxazinyl; 1,4-
oxazinyl; o-isoxazinyl; p-isoxazinyl; 1,2,5-oxathiazinyl; 1,4-oxazinyl; o-
isoxazinyl; p-isoxazinyl; 1,2,5-oxathiainzyl; 1,2,6-oxathiainzyl; 1,4,2-
oxadiainzyl; 1,3,5,2-oxadiainzyl; morpholino; azepinyl; oxepinyl; thiepinyl;
1,2,4-diazepinyl; benzofuranyl; isobenzofuranyl; benzothiofuranyl;
isobenzothiofuranyl; indolyl; indoleninyl; 2-isobenzazolyl; 1,5-pyrindinyl;
pyrano[3,4-b]pyrrolyl; isoindazolyl; indoxazinyl; benzoxazolyl; anthranilyl;
1,2-
benzopyranyl; quinolyi; isoquinolyl; cinnolyi; quinazolyl; naphthyridyl;
pyrido[3,4-b]pyridyl; pyrido[3,2-b]pyridyl; pyrido[4,3-b]pyridyl; 1,3,2-
benzoxazyl;
1,4,2-benzoxazyl; 2,1,3-benzoxazyl; 3,1,4-benzoxazyl; 1,2-benzoisoxazyl; 1,4-
benzoisoxazyl; carbazolyl; xanthenyl; acridinyl; purinyl; thiazolidyl;
piperidyl;
pyrrolidyl; 1,2-dihydroazinyl; 1,4-dihydroazinyl; 1,2,3,6-tetrahydro-1,3-
diazinyl;
perhydro-1,4-diazinyl; 1,2-thiapyranyl; and 1,4-thiapyranyl. Heterocyclic
moieties, alone or in combination, may be optionally substituted by one or
more
substituents selected from the group consisting of amino, halo, cyano,
hydroxyl, alkyl, alkoxy, and carboxyl.
[00175] The term "heteroaryl" also embraces radicals where
heterocyclic radicals are fused with aryl radicals as defined herein. Examples
99
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
of such fused bicyclic radicals include benzofuran, benzothiophene, and the
like.
[00176] The term "heterocycloalkyl" embraces heterocyclic-substituted
alkyl radicals such as pyridylmethyl and thienylmethyl.
[00177] The terms benzyl and phenylmethyl are interchangeable.
[00178] The phrases "combination therapy", "co-administration",
"administration with", or "co-therapy", in defining the use of a selective IKK-
2
inhibitory agent in combination with another therapeutic agent such as another
analgesic agent, is intended to embrace administration of each agent in a
sequential manner in a regimen that may provide beneficial effects of the drug
combination, and is intended as well to embrace co-administration of these
agents in a substantially simultaneous manner, such as in a single capsule or
dosage device having a fixed ratio of these active agents or in multiple,
separate capsules or dosage devices for each agent, where the separate
capsules or dosage devices can be taken together contemporaneously, or
taken within a period of time sufficient to receive a beneficial effect from
both of
the constituent agents of the combination.
[00179] The term "subject" for purposes of treatment includes any
human or animal subject who is in need of the prevention of, or who has pain,
inflammation and/or any one of the known inflammation-associated disorders.
The subject is typically a human subject.
[00180] The phrase "therapeutic combination" as used herein refers to
the combination of two or more therapeutic compounds and, optionally, one or
more pharmaceutically acceptable carrier used to provide dosage forms that
produce a beneficial effect of each therapeutic compound in the subject at the
desired time, whether the therapeutic compounds are administered
substantially simultaneously, or sequentially.
[00181] The phrase "therapeutically effective" as used herein refers to
an amount of a therapeutic compound, or amounts of combined therapeutic
100
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
compounds in combination therapy. The amount or combined amounts achieve
one or more of the goals of preventing, inhibiting, reducing or eliminating
the
inflammation or inflammation-related disease or condition. A "therapeutically-
effective" amount of each agent in a combination therapy is expected to be
less than an amount used in treatment using agent by itself, thus while
avoiding adverse side effects typically associated with alternative therapies,
namely higher dose monotherapy of each agent by itself.
[00182] The terms "treating" or "to treat" means to alleviate symptoms,
eliminate the causation either on a temporary or permanent basis, or to
prevent
or slow the appearance of symptoms in a subject. The term "treatment"
includes alleviation, elimination of causation of or prevention of pain and/or
inflammation associated with, but not limited to, any of the diseases or
disorders described above.
[00183] Pharmaceutically acceptable salts of the compounds of
Formula I include the acid addition and base salts thereof.
[00184] Suitable acid addition salts are formed from acids that form
non-toxic salts. Examples include the acetate, aspartate, benzoate, besylate,
bicarbonate/carbonate, bisulphate/sulphate, borate, camsylate, citrate,
edisylate, esylate, formate, fumarate, gluceptate, gluconate, glucuronate,
hexafluorophosphate, hibenzate, hydrochloride/chloride,
hydrobromide/bromide, hydroiodide/iodide, isethionate, lactate, malate,
maleate, malonate, mesylate, methylsulphate, naphthylate, 2-napsylate,
nicotinate, nitrafie, orotate, oxalate, palmitate, pamoate, phosphate/hydrogen
phosphate/dihydrogen phosphate, saccharate, stearate, succinate, tartrate,
tosylate and trifluoroacetate salts.
(00185] Suitable base salts are formed from bases which form non-
toxic salts. Examples include the aluminium, arginine, benzathine, calcium,
choline, diethylamine, diolamine, glycine, lysine, magnesium, meglumine,
olamine, potassium, sodium, tromethamine and zinc salts.
101
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00186] Hemisalts of acids and bases may also be formed, for
example, hemisulphate and hemicalcium salts.
[00187] Pharmaceutically acceptable salts of compounds of Formula I
may be prepared by one or more of three methods: (i) by reacting fihe
compound of Formula I with the desired acid or base; (ii) by removing an acid-
or base-labile protecting group from a suitable precursor of the compound of
Formula I or by ring-opening a suitable cyclic precursor, for example, a
lactone
or lactam, using the desired acid or base; or (iii) by converting one salt of
the
compound of Formula I to another by reaction with an appropriate acid or base
or by means of a suitable ion exchange column. All three reactions are
typically
carried out in solution. The resulting salt may precipitate out and be
collected
by filtration or may be recovered by evaporation of the solvent. The degree of
ionization in the resulting salt may vary from completely ionized to almost
non-
ionized.
[00188] The compounds of the invention may exist in both unsolvated
and solvated forms. The term "solvate" is used herein to describe a molecular
complex comprising the compound of the invention and a stoichiometric
amount of one or more pharmaceutically acceptable solvent molecules, for
example, ethanol. The term "hydrate" is employed when said solvent is water.
[00189] Included within the scope of the invention are complexes such
as clathrates, drug-host inclusion complexes wherein, in contrast to the
aforementioned solvates, the drug and host are present in stoichiometric or
non-stoichiometric amounts. Also included are complexes of the drug
containing two or more organic and/or inorganic components which may be in
stoichiometric or non-stoichiometric amounts. The resulting complexes may be
ionized, partially ionized, or non-ionized. For a review of such complexes,
see
Haleblian, J. Pharm. Sci., 64(8), 1269-1288 (1975).
102
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00190] Hereinafter all references to compounds of Formula I include
references to salts, solvates and complexes thereof and to solvates and
complexes of salts thereof.
[00191] The compounds of the invention include compounds of
Formula I as hereinbefore defined, including all polymorphs and crystal habits
thereof, prodrugs and isomers thereof (including optical, geometric and
tautomeric isomers) as hereinafter defined and isotopically-labeled compounds
of Formula I.
[00192] As indicated, so-called prodrugs of the compounds of Formula
I are also within the scope of the invention. The term "prodrug" refers to a
compound that is a drug precursor which, following administration to a subject
and subsequent absorption, is converted to an active species in vivo via some
process, such as a metabolic process. Other products from the conversion
process are easily disposed of by the body. The more preferred prodrugs are
those involving a conversion process that produces products that are generally
accepted as safe.
[00193] Prodrugs in accordance with the invention can, for example,
be produced by replacing appropriate functionalities present in the compounds
of Formula I with certain moieties known to those skilled in the art as "pro-
moieties."
[00194] Some examples of prodrugs in accordance with the invention
include: (i) where the compound of Formula I contains a carboxylic acid
functionality (-C02H), an ester thereof, for example, a compound wherein the
hydrogen of the carboxylic acid functionality of the compound of Formula I is
replaced by C,-C8 alkyl; (ii) where the compound of Formula I contains an
alcohol functionality (-OH), an ether thereof, for example, a compound wherein
the hydrogen of the alcohol functionality of the compound of Formula I is
replaced by C,-C6 alkanoyloxymethyl; and (iii) where the compound of Formula
I contains a primary or secondary amino functionality (-NHZ or -NHR where R ~
103
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
H), an amide thereof, for example, a compound wherein, as the case may be,
one or both hydrogens of the amino functionality of the compound of Formula I
is/are replaced by C,-C,o alkanoyl.
[00'195] Further examples of replacement groups in accordance with
the foregoing examples and examples of other prodrug types may be found in
the aforementioned references.
[00196] Moreover, certain compounds of Formula I may themselves
act as prodrugs of other compounds of Formula I.
[00197] Also included within the scope of the invention are metabolites
of compounds of Formula I, that is, compounds formed in vivo upon
administration of the drug. Some examples of metabolites in accordance with
the invention include: (i) where the compound of Formula I contains a methyl
group, an hydroxymethyl derivative thereof (-CH3 -~ -CH20H); (ii) where the
compound of Formula I contains an alkoxy group, an hydroxy derivative thereof
(-OR -~ -OH); (iii) where the compound of Formula I contains a tertiary amino
group, a secondary amino derivative thereof (-NRaRb ~ -NHRa or -NHRb); (iv)
where the compound of Formula I contains a secondary amino group, a
primary derivative thereof (-NHR -~ -NH2); (v) where the compound of Formula
I contains a phenyl moiety, a phenol derivative thereof (-Ph ~ -PhOH); and
(vi)
where the compound of Formula I contains an amide group, a carboxylic acid
derivative thereof (-CONHa -~ -COOH).
[00198] Compounds of Formula I containing one or more asymmetric
carbon atoms can exist as two or more stereoisomers. Where a compound of
Formula I contains an alkenyl or alkenylene group, geometric cis/trans (or
Z/E)
isomers are possible. Where structural isomers are interconvertible via a low
energy barrier, tautomeric isomerism ("tautomerism") can occur. This can take
the form of proton tautomerism in compounds of Formula I containing, for
example, an imino, keto, or oxime group, or so-called valence tautomerism in
104
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
compounds which contain an aromatic moiety. It follows that a single
compound may exhibit more than one type of isomerism.
[00199] Included within the scope of the present invention are all
stereoisomers, geometric isomers and tautomeric forms of the compounds of
Formula I, including compounds exhibiting more than one type of isomerism,
and mixtures of one or more thereof. Also included are acid addition or base
salts wherein the counterion is optically active, for example, d-lactate or I-
lysine, or racemic, for example, dl-tartrate or dl-arginine.
[00200] Cis/trans isomers may be separated by conventional
techniques well known to those skilled in the art, for example, chromatography
and fractional crystallization.
[00201] Conventional techniques for the preparation/isolation of
individual enantiomers include chiral synthesis from a suitable optically pure
precursor or resolution of the racemate (or the racemate of a salt or
derivative)
using, for example, chiral high pressure liquid chromatography (chiral HPLC).
[00202] Alternatively, the racemate (or a racemic precursor) may be
reacted with a suitable optically active compound, for example, an alcohol,
or,
in the case where the compound of Formula I contains an acidic or basic
moiety, a base or acid such as 1-phenylethylamine or tartaric acid. The
resulting diastereomeric mixture may be separated by chromatography and/or
fractional crystallization and one or both of the diastereoisomers converted
to
the corresponding pure enantiomer(s) by means well known to a skilled
person.
[00203] Chiral compounds of the invention (and chiral precursors
thereof) may be obtained in enantiomerically-enriched form using
chromatography, typically HPLC, on an asymmetric resin with a mobile phase
consisting of a hydrocarbon, typically heptane or hexane, containing from 0 to
50% by volume of isopropanol, typically from 2 to 20%, and from 0 to 5% by
105
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
volume of an alkylamine, typically 0.1 % diethylamine. Concentration of the
eluate affords the enriched mixture.
[00204] Stereoisomeric conglomerates may be separated by
conventional techniques known to those skilled in the art.
[00205] The present invention includes all pharmaceutically
acceptable isotopically-labeled compounds of Formula I wherein one or more
atoms are replaced by atoms having the same atomic number, but an atomic
mass or mass number different from the atomic mass or mass number which
predominates in nature.
[00206] Examples of isotopes suitable for inclusion in the compounds
of the invention include isotopes of hydrogen, such as ~H and 3H, carbon, such
as'1C,'3C and'4C, chlorine, such as 36C1, fluorine, such as'8F, iodine, such
as
'231 and'ZSI, nitrogen, such as'3N and'SN, oxygen, such as 15~, 1~p and 180,
phosphorus, such as 3~P, and sulphur, such as 35S.
[00207] Certain isotopically-labeled compounds of Formula I, for
example, those incorporating a radioactive isotope, are useful in drug and/or
substrate tissue distribution studies. The radioactive isotopes tritium (3H)
and
'4C are particularly useful for this purpose in view of their ease of
incorporation
and ready means of detection.
[0020] Substitution with heavier isotopes such as deuterium (aH) may
afford certain therapeutic advantages resulting from greater metabolic
stability,
for example, increased in vivo half-life or reduced dosage requirements, and
hence may be preferred in some circumstances.
[00209] Substitution with positron-emitting isotopes, such as "C,'8F,
'S0 and'3N, can be useful in Positron Emission Topography (PET) studies for
examining substrate receptor occupancy.
[00210] Isotopically-labeled compounds of Formula I can generally be
prepared by conventional techniques known to those skilled in the art or by
processes analogous to those described in the accompanying Examples using
106
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
an appropriate isotopically-labeled reagent in place of the non-labeled
reagent
previously employed.
[00211] Pharmaceutically acceptable solvates in accordance with the
invention include those wherein the solvent of crystallization may be
isotopically substituted, e.g. DSO, dfi acetone, or ds DMSO.
[00212] Compounds of the invention intended for pharmaceutical use
may be administered as crystalline or amorphous products. They may be
obtained, for example, as solid plugs, powders, or films by methods such as
precipitation, crystallization, freeze drying, spray drying, or evaporative
drying.
Microwave or radio frequency drying may be used for this purpose.
[00213] Generally, the compounds of the invention may be
administered as a formulation in association with one or more pharmaceutically
acceptable excipients. The term "excipient" is used herein to describe any
ingredient other than the compounds) of the invention. The choice of excipient
will to a large extent depend on factors such as the particular mode of
administration, the effect of the excipient on solubility and stability, and
the
nature of the dosage form.
[00214] The compounds of the invention may be administered alone
or in combination with one or more other compounds of the invention or in
combination with one or more other drugs (or as any combination thereof). For
example, compounds of Formula I may be used in co-therapies, partially or
completely, in place of other conventional antiinflammatory therapies, such as
together with other IKK-2 inhibitors, steroids, NSAIDs, COX-2 selective
inhibitors, matrix metalloproteinase inhibitors, 5-lipoxygenase inhibitors,
LTB4
antagonists and LTA4 hydrolase inhibitors.
[00215] Pharmaceutical compositions suitable for the delivery of
compounds of the present invention and methods for their preparation will be
readily apparent to those skilled in the art.
107
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00216] The compounds of the invention may be administered orally.
Oral administration may involve swallowing, so that the compound enters the
gastrointestinal tract, or buccal or sublingual administration may be employed
by which the compound enters the blood stream directly from the mouth.
[00217] Formulations suitable for oral administration include solid
formulations such as tablets, capsules containing particulates, liquids, or
powders, lozenges (including liquid-filled), chews, multi- and nano-
particulates,
gels, solid solution, liposome, films, ovules, sprays and liquid formulations.
[00218] Liquid formulations include suspensions, solutions, syrups
and elixirs. Such formulations may be employed as fillers in soft or hard
capsules and typically comprise a carrier, for example, water, ethanol,
polyethylene glycol, propylene glycol, methylcellulose, or a suitable oil, and
one
or more emulsifying agents andlor suspending agents. Liquid formulations may
also be prepared by the reconstitution of a solid, for example, from a sachet.
[00219] The compounds of the invention may also be used in fast-
dissolving, fast-disintegrating dosage forms such as those described in Liang
and Chen, Expert Opinion in Therapeutic Patents, 11(6), 981-986 (2001).
[00220] For tablet dosage forms, depending on dose, the drug may
make up from 1 to 80 wt.% of the dosage form, more typically from 5 to
60 wt.% of the dosage form. In addition to the drug, tablets generally contain
a
disintegrant. Examples of disintegrants include sodium starch glycolate,
sodium carboxymethyl cellulose, calcium carboxymethyl cellulose,
croscarmellose sodium, crospovidone, polyvinylpyrrolidone, methyl cellulose,
microcrystalline cellulose, lower alkyl-substituted hydroxypropyl cellulose,
starch, pregelatinised starch and sodium alginate. Generally, the disintegrant
will comprise from 1 to 25 wt.%, preferably from 5 to 20 wt.% of the dosage
form.
[00221] Binders are generally used to impart cohesive qualities to a
tablet formulation. Suitable binders include microcrystalline cellulose,
gelatin,
108
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
sugars, polyethylene glycol, natural and synthetic gums, polyvinylpyrrolidone,
pregelatinised starch, hydroxypropyl cellulose and hydroxypropyl
methylcellulose. Tablets may also contain diluents, such as lactose
(monohydrate, spray-dried monohydrate, anhydrous and the like), mannitol,
xylitol, dextrose, sucrose, sorbitol, microcrystalline cellulose, starch and
dibasic
calcium phosphate dehydrate.
[00222] Tablets may also optionally comprise surface active agents,
such as sodium lauryl sulfate and polysorbate 80, and glidants such as silicon
dioxide and talc. When present, surface active agents may comprise from 0.2
to 5 wt.% of the tablet, and glidants may comprise from 0.2 to 1 wt.% of the
tablet.
[00223] Tablets also generally contain lubricants such as magnesium
stearate, calcium stearate, zinc stearate, sodium stearyl fumarate, and
mixtures of magnesium stearate with sodium lauryl sulphate. Lubricants
generally comprise from 0.25 to 10 wt.%, preferably from 0.5 to 3 wt.% of the
tablet.
[00224] Other possible ingredients include anti-oxidants, colorants,
flavoring agents, preservatives and taste-masking agents.
[00225] Exemplary tablets contain up to about 80% drug, from about
10 to about 90 wt.% binder, from about 0 to about 85 wt.% diluent, from about
2 to about 10 wt.% disintegrant, and from about 0.25 to about 10 wt.%
lubricant.
[00226] Tablet blends may be compressed directly or by roller to form
tablets. Tablet blends or portions of blends may alternatively be wet-, dry-,
or
melt-granulated, melt congealed, or extruded before tabletting. The final
formulation may comprise one or more layers and may be coated or uncoated;
it may even be encapsulated.
[00227] Consumable oral films for human or veterinary use are
typically pliable water-soluble or water-swellable thin film dosage forms
which
109
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
may be rapidly dissolving or mucoadhesive and typically comprise a compound
of Formula I, a film-forming polymer, a binder, a solvent, a humectant, a
plasticiser, a stabilizer or emulsifier, a viscosity-modifying agent and a
solvent.
Some components of the formulation may perform more than one function.
[00228] The compound of Formula I may be water-soluble or
insoluble. A water-soluble compound typically comprises from 1 to 80 wt.%,
more typically from 20 to 50 wt.%, of the solutes. Less soluble compounds may
comprise a greater proportion of the composition, typically up to 88 wt.% of
the
solutes. Alternatively, the compound of Formula I may be in the form of
multiparticulate beads.
[00229] The film-forming polymer may be selected from natural
polysaccharides, proteins, or synthetic hydrocolloids and is typically present
in
the range 0.01 to 99 wt.%, more typically in the range 30 to 80 wt.%.
[00230] Other possible ingredients include anti-oxidants, colorants,
flavorings and flavor enhancers, preservatives, salivary stimulating agents,
cooling agents, co-solvents (including oils), emollients, bulking agents, anti-
foaming agents, surfactants and taste-masking agents.
[00231] Films in accordance with the invention are typically prepared
by evaporative drying of thin aqueous films coated onto a peelable backing
support or paper. This may be done in a drying oven or tunnel, typically a
combined coater dryer, or by freeze-drying or vacuuming.
[00232] Solid formulations for oral administration may be formulated to
be immediate and/or modified release. Modified release formulations include
delayed-, sustained-, pulsed-, controlled-, targeted- and programmed-release.
[00233] Suitable modified release formulations for the purposes of the
invention are described in U.S. Patent No. 6,106,864. Details of other
suitable
release technologies such as high energy dispersions and osmotic and coated
particles are to be found in Verma et al., Pharmaceutical Technology On-line,
110
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
25(2), 1-14 (2001 ). The use of chewing gum to achieve controlled release is
described in PCT Publication No. WO 00/35298.
[00234] The compounds of the invention may also be administered
directly into the blood stream, into muscle, or into an internal organ.
Suitable
means for parenteral administration include intravenous, intraarterial,
intraperitoneal, intrathecal, intraventricular, intraurethral, intrasternal,
intracranial, intramuscular and subcutaneous. Suitable devices for parenteral
administration include needle (including microneedle) injectors, needle-free
injectors and infusion techniques.
[00235] Parenteral formulations are typically aqueous solutions which
may contain excipients such as salts, carbohydrates and buffering agents
(preferably to a pH of from 3 to 9), but, for some applications, they may be
more suitably formulated as a sterile non-aqueous solution or as a dried form
to be used in conjunction with a suitable vehicle such as sterile, pyrogen-
free
water.
[00236] The preparation of parenteral formulations under sterile
conditions, for example, by lyophilization, may readily be accomplished using
standard pharmaceutical techniques well known to those skilled in the art.
[00237] The solubility of compounds of Formula I used in the
preparation of parenteral solutions may be increased by the use of appropriate
formulation techniques, such as the incorporation of solubility-enhancing
agents.
[00238] Formulations for parenteral administration may be formulated
to be immediate and/or modified release. Modified release formulations include
delayed-, sustained-, pulsed-, controlled-, targeted- and programmed-release.
Thus compounds of the invention may be formulated as a solid, semi-solid, or
thixotropic liquid for administration as an implanted depot providing modified
release of the active compound. Examples of such formulations include drug-
coated stents and poly(dl-lactic-coglycolic)acid (PGLA) microspheres.
111
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00239] The compounds of the invention may also be administered
topically to the skin or mucosa, that is, dermally or transdermally. Typical
formulations for this purpose include gels, hydrogels, lotions, solutions,
creams, ointments, dusting powders, dressings, foams, films, skin patches,
wafers, implants, sponges, fibers, bandages and microemulsions. Liposomes
may also be used. Typical carriers include alcohol, water, mineral oil, liquid
petrolatum, white petrolatum, glycerin, polyethylene glycol and propylene
glycol. Penetration enhancers may be incorporated; see, e.g., Finnin and
Morgan, J Pharm Sci, 88(10), 955-958 (1999).
(00240] Other means of topical administration include delivery by
electroporation, iontophoresis, phonophoresis, sonophoresis and microneedle
or needle-free (e.g. PowderjectT"", BiojectT"", etc.) injection.
[00241] Formulations for topical administration may be formulated to
be immediate andlor modified release. Modified release formulations include
delayed-, sustained-, pulsed-, controlled-, targeted- and programmed-release.
[00242] The compounds of the invention can also be administered
intranasally or by inhalation, typically in the form of a dry powder (either
alone,
as a mixture, for example, in a dry blend with lactose, or as a mixed
component
particle, for example, mixed with phospholipids, such as phosphatidylcholine)
from a dry powder inhaler or as an aerosol spray from a pressurized container,
pump, spray, atomizer (preferably an atomizer using electrohydrodynamics to
produce a fine mist), or nebulizer, with or without the use of a suitable
propellant, such as 1,1,1,2-tetrafluoroethane or 1,1,1,2,3,3,3-
heptafluoropropane. For intranasal use, the powder may comprise a
bioadhesive agent, for example, chitosan or cyclodextrin.
[00243] The pressurized container, pump, spray, atomizer, or
nebulizer contains a solution or suspension of the compounds) of the
invention comprising, for example, ethanol, aqueous ethanol, or a suitable
alternative agent for dispersing, solubilizing, or extending release of the
active,
112
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
a propellants) as solvent and an optional surfactant, such as sorbitan
trioleate,
oleic acid, or an oligolactic acid.
[00244] Prior to use in a dry powder or suspension formulation, the
drug product is micronized to a size suitable for delivery by inhalation
(typically
less than 5 pm). This may be achieved by any appropriate comminuting
method, such as spiral jet milling, fluid bed jet milling, supercritical fluid
processing to form nanoparticles, high pressure homogenization, or spray
drying.
[00245] Capsules (made, for example, from gelatin or
hydroxypropylmethylcellulose), blisters and cartridges for use in an inhaler
or
insufflator may be formulated to contain a powder mix of the compound of the
invention, a suitable powder base such as lactose or starch and a performance
modifier such as I-leucine, mannitol, or magnesium stearate. The lactose may
be anhydrous or in the form of the monohydrate, preferably the latter. Other
suitable excipients include dextran, glucose, maltose, sorbitol, xylitol,
fructose,
sucrose and trehalose.
[00246] A suitable solution formulation for use in an atomizer using
electrohydrodynamics to produce a fine mist may contain from 1 pg to 20 mg of
the compound of the invention per actuation and the actuation volume may
vary from 1 to 100 pL. A typical formulation may comprise a compound of
Formula I, propylene glycol, sterile water, ethanol and sodium chloride.
Alternative solvents which may be used instead of propylene glycol include
glycerol and polyethylene glycol.
[00247] Suitable flavors, such as menthol and levomenthol, or
sweeteners, such as saccharin or saccharin sodium, may be added to those
formulations of the invention intended for inhaled/intranasal administration.
[00248] Formulations for inhaledlintranasal administration may be
formulated to be immediate and/or modified release using, for example, PGLA.
113
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Modified release formulations include delayed-, sustained-, pulsed-,
controlled-
targeted- and programmed-release.
[00249] In the case of dry powder inhalers and aerosols, the dosage
unit is determined by means of a valve which delivers a metered amount. Units
in accordance with the invention are typically arranged to administer a
metered
dose or "puff" containing from 20 to 1000 pg of the compound of Formula I.
The overall daily dose will typically be in the range 100 pg to 10 mg which
may
be administered in a single dose or, more usually, as divided doses throughout
the day, for Step 2, 3, 4 or 8 times, giving for example, 1, 2 or 3 doses each
time.
[00250] The compounds of the invention may be administered rectally
or vaginally, for example, in the form of a suppository, pessary, or enema.
Cocoa butter is a traditional suppository base, but various alternatives may
be
used as appropriate.
[00251] Formulations for rectal/vaginal administration may be
formulated to be immediate and/or modified release. Modified release
formulations include delayed-, sustained-, pulsed-, controlled-, targeted- and
programmed-release.
[00252] The compounds of the invention may also be administered
directly to the eye or ear, typically in the form of drops of a micronized
suspension or solution in isotonic, pH-adjusted, sterile saline. Other
formulations suitable for ocular and aural administration include ointments,
biodegradable (e.g., absorbable gel sponges, collagen) and non-biodegradable
(e.g., silicone) implants, wafers, lenses and particulate or vesicular
systems,
such as niosomes or liposomes. A polymer such as crossed-linked polyacrylic
acid, polyvinylalcohol, hyaluronic acid, a cellulosic polymer, for example,
hydroxypropylmethylcellulose, hydroxyethylcellulose, or methyl cellulose, or a
heteropolysaccharide polymer, for example, gelan gum, may be incorporated
114
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
together with a preservative, such as benzalkonium chloride. Such formulations
may also be delivered by iontophoresis.
[00253] Formulations for ocular/aural administration may be
formulated to be immediate and/or modified release. Modified release
formulations include delayed-, sustained-, pulsed-, controlled-, targeted- or
programmed-release.
[00254] The compounds of the invention may be combined with
soluble macromolecular entities, such as cyclodextrin and suitable derivatives
thereof or polyethylene glycol-containing polymers, in order to improve their
solubility, dissolution rate, taste-masking, bioavailability and/or stability
for use
in any of the aforementioned modes of administration.
[00255] Drug-cyclodextrin complexes, for example, are found to be
generally useful for most dosage forms and administration routes. Both
inclusion and non-inclusion complexes may be used. As an alternative to direct
complexation with the drug, the cyclodextrin may be used as an auxiliary
additive, i.e., as a carrier, diluent, or solubilizer. Most commonly used for
these
purposes are alpha-, beta- and gamma-cyclodextrins, such as those described
in PCT Publication No. WO 98/55148.
[00256] Inasmuch as it may desirable to administer a combination of
active compounds, for example, for the purpose of treating a particular
disease
or condition, it is within the scope of the present invention that two or more
pharmaceutical compositions, at least one of which contains a compound in
accordance with the invention, may conveniently be combined in the form of a
kit suitable for coadministration of the compositions.
[00257] Such kits comprises two or more separate pharmaceutical
compositions, at least one of which contains a compound of Formula I in
accordance with the invention, and means for separately retaining said
compositions, such as a container, divided bottle, or divided foil packet. An
115
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
example of such a kit is the familiar blister pack used for the packaging of
tablets, capsules and the like.
[00255] Such kits are particularly suitable for administering different
dosage forms, for example, oral and parenteral, for administering the separate
compositions at different dosage intervals, or for titrating the separate
compositions against one another. To assist compliance, the kit typically
comprises directions for administration and may be provided with a so-called
memory aid.
[00259] The amount of therapeutically active compounds that are
administered and the dosage regimen for treating a disease condition with the
compounds and/or compositions of this invention depends on a variety of
factors, including the age, weight, sex and medical condition of the subject,
the
severity of the inflammation or inflammation related disorder, the route and
frequency of administration, and the particular compound employed, and thus
may vary widely. The pharmaceutical compositions may contain active
ingredients in the range of about 0.1 to 1000 mg, preferably in the range of
about 7.0 to 350 mg. A daily dose of about 0.01 to 100 mg/kg body weight,
preferably between about 0.1 and about 50 mg/kg body weight and most
preferably between about 0.5 to 30 mg/kg body weight, may be appropriate.
The daily dose can be administered in one to four doses per day. In the case
of
skin conditions, it may be preferable to apply a topical preparation of
compounds of this invention to the affected area two to four times a day.
[00260] It will be understood, however, that the specific dose level for
any particular patient will depend upon a variety of factors including the
activity
of the specific compound employed, the age, body weight, general health, sex,
diet, time of administration, route of administration, and rate of excretion,
drug
combination and the severity of the particular disease undergoing therapy.
[00261] These dosages are based on an average human subject
having a weight of about 60 to 70 kg. The physician will readily be able to
116
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
determine doses for subjects whose weight falls outside this range, such as
infants and the elderly.
[00262] For the avoidance of doubt, references herein to "treatment"
include references to curative, palliative and prophylactic treatment.
[00263] "DMF" is N,N-dimethylformamide.
[00264] "DMSO" is dimethylsulfoxide.
[00265] "ESI" is electrospray ionization Mass spectrometry.
[00266] "NMR" is nuclear magnetic resonance.
[00267] "Ph" is phenyl.
[00268] "EtOAc" is ethyl acetate.
[00269] "Boc" is t-butoxycarbonyl.
[00270] "dppf" is bis(diphenylphosphino)ferrocene.
REACTION SCHEMES
[00271] The compounds of the invention can be synthesized
according to the following procedures of Scheme I and II, wherein the R
substituents are as defined for Formula I and II, except where further noted.
SCHEMEI
Synthesis of tent-butyl 3-(2,2-dicyano-1-methoxyvinyl)piperidine-1-carboxylate
~cocp2 ~ ~hz~~No
Boc'N~CO~H cat. DMF/tol Boc'N v _COCI NaH, THF
0°C, 2h 5°C to RT
CN
CN Me2S04 ~CN
NaHC03 Boc IIN
Boc'N~CN OMe
dioxane/water
O reflux, 2.5 h
117
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
SCHEME II
Synthesis of substituted pyrimidines
O NH2 O NHS Me0 NH
OH BnCI, KZC03 OBn BFaOMe OBn
\ \
Rl~i / R1~~ / Rlli /
CN
HZN NH Boc'N / CN Boc r
NH3 (g) OMe
OBn
NaOMe/EtOH
R
CN
Boc' 2 HN ~ NHZ
HCI N ~ N
HZ, Pd/C
EA / OH
R
EXAMPLES
[00272] Example 1: 4-amino-2-(2,6-dihydroxyphenyl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride
H
N
CN
\ NHZ
N /N
HCI
HO / OH
[00273]
[00274] Step 1: Preparation of 2,6-bis(benzyloxy)benzamide
HEN O
O O \
[00275]
118
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00276] To a room temperature solution of 2,6-dibenzyloxybenzonitrile
(50 g, 160 mmol) in 120 mL benzyl alcohol was added 32 g of KOH and 20 mL
HaO. The resulting suspension was placed in a 130°C oil bath for 18
h. The
benzyl alcohol was then removed on a rotary evaporator, and the resulting
solid was slurried in 700 mL H20. The solid was collected and suspended in
boiling diethyl ether to yield the desired product as a white solid. LC/MS
m/z=334.1 (m+1 ).
[00277] Step 2: Preparation of methyl 2,6-
bis(benzyloxy)benzenecarboximidoate
HN O~CH3
~O O
[00278]
[00279] To a room temperature solution of 2,6-
bis(benzyloxy)benzamide (Step 1, 24 g, 72 mmol) in 160 mL methylene
chloride was added trimethyloxonium tetrafluoroborate (12.8 g, 86.4 mmol) and
the resulting suspension was stirred for 4 h. The reaction was then filtered
and
concentrated to give a light brown semi-solid, which was used without further
purification. LC/MS mlz=348.2 (m+1 ).
[00280] Step 3: Preparation of 2,6-
bis(benzyloxy)benzenecarboximidamide hydrochloride
HN NHS
0 0
[00281] Hci
[00282] To a solution of methyl 2,6-
bis(benzyloxy)benzenecarboximidoate (Step 2, 23 g, 66 mmol) in 300 mL
ethanol, was added 300 mL of condensed ammonia. The solution was sealed
and heated to 80°C at 300 psi. After 40 h the reaction was cooled,
filtered, and
then concentrated. The resulting solid was suspended in 200 mL of 4N
hydrogen chloride in 1,4-dioxane. After 1 h the suspension was concentrated
119
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
and the resulting solids were purified by reverse phase HPLC to yield the
desired compound as the hydrochloride salt. LC/MS m/z=333.1 (m+1 ).
[00283] St_ ep 4: Preparation of tent-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate
NC
CN
[00284] B~cN O-CH3
[00285] To a 0°C solution of Boc-piperidine carboxylic acid (100.88 g,
0.440 mol) in toluene (1 L) was added oxalylchloride (55.85 g, 0.440 mol) and
a few drops of DMF. The mixture was stirred for 2 h. CH2CI2 (100 mL) was
added and the mixture was clarified by filtration. The filtrate was
concentrated
under reduced pressure to yield the acid chloride as a brown oil. NaH (14.7 g,
0.613 mol) was added to a 0°C solution of malononitrile (20.3 g, 0.307
mol) in
THF (300 mL). Then the acid chloride (75.9 g) in THF (75 mL) was added. The
reaction was stirred at room temperature for 1 h. Then dimethylsulfate (46.4
g,
0.368 mol) was added and the mixture was refluxed for 2.5 h After cooling to
room temperature, ether (500 mL) was added and then washed with water (2 x
250 mL). The reaction mixture was dried over MgS04 and concentrated in
vacuo to yield the crude product. The material was purified by flash column
chromatography to yield the title product.
[00286] Step 5: Preparation of tert-butyl 3-~6-amino-2-[2,6-
bis(benzyloxy)phenyl]-5-cyanopyrimidin-4-yl)piperidine-1-carboxylate
B0c
N CN
\ NHS
/ N ~N /
O O \
\
[00287]
[00288] To a solution of tent-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate (Step 4, 3.5 g, 12 mmol) and 2,6-
120
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
bis(benzyloxy)benzenecarboximidamide hydrochloride (Step 3, 4.4 g, 12 mmol)
in 35 mL absolute ethanol, was added sodium methoxide (25% w/w solution in
methanol, 6.2 mL). The solution was then heated to reflux for 3 h, then
concentrated to remove the ethanol. The resulting crude material was
partitioned between 100 mL ethyl acetate and 30 mL H20. The organic layer
was dried over MgS04 and concentrated. The resulting solid was triturated with
500 mL diethyl ether, and the solids were then collected by filtration and the
filtrate concentrated to give the desired compound as a yellow solid of good
purity. LC/MS m/z=592.2 (m+1 ).
[00289 Step 6: Preparation of tert-butyl 3-[&-amino-5-cyano-2-(2,6-
dihydroxyphenyl)pyrimidin-4-yl]piperidine-1-carboxylate
NHS
NC
~ ~N OH
BocN \N I
[00290] Ho
[00291 To a solution of tert-butyl 3-{6-amino-2-[2,6-
bis(benzyloxy)phenyl]-5-cyanopyrimidin-4- yl)piperidine-1-carboxylate (Step 5,
2.9 g, 5 mmol) in 25 mL ethyl acetate and 5 mL glacial acetic acid was added
800 mg of 10% Palladium on carbon. The reaction was then blanketed with a
hydrogen balloon and stirred for 18 h. The reaction was vented and filtered
through celite. Then filtrate was concentrated and the resulting crude solid
was
crystallized from boiling ethanol to give the desired compound. LC/MS
m/z=412.1 (m+1 ).
[00292] Step 7: Preparation of 4-amino-2-(2,6-dihydroxyphenyl)-6-
piperidin-3-ylpyrimidine-5-carbonitrile hydrochloride
NHa
NC / N OH
HN \N I
HO ~ /
[00293] Hci
121
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00294] Tert-butyl3-[6-amino-5-cyano-2-(2,6-
dihydroxyphenyl)pyrimidin-4-yl]piperidine-1-carboxylate (Step 6, 100 mg, 0.4
mmol) was stirred in 3 mL of 4N hydrogen chloride in 1,4-dioxane for 1 h. The
mixture was then concentrated and suspended in a boiling 1:1:1 mixture (1 mL
each) of 1 N hydrochloric acid, acetonitrile, and methanol. After 15 min the
suspension was filtered to give the desired yellow compound as the
hydrochloride salt. LC/MS m/z=312.2 (m+1 ).
[00295] Examples 2-12 were prepared in a similar manner.
Example Name and structure Name and structure Mass
Spec.
3 4-amino-2-(5-chloro-2-hydroxyphenyl)-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile
hydrochloride HN \ NHS
I
N iN
OH
\
ci HCI
4 4-amino-2-(2-hydroxyphenyl)-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile
hydrochloride HN \ NHS
I
N ,N
OH
HCI
5 4-amino-2-(3,5-dichloro-2,6- cN
dihydroxyphenyl)-6-piperidin-3- HN \ NH2
ylpyrimidine-5-carbonitrile hydrochloride I
N iN
HO / OH
ci \ ci HCI
122
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Example Name and structure Name and structure Mass
Spec.
6 4-amino-2-(2-hydroxy-6- cN
methoxyphenyl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHz
I
N iN
HO / O~
CH3
HCI
7 4-amino-2-[2-(cyclopropylmethoxy)-6- cN
hydroxyphenyl]-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride HN ~ NHS
I
N iN
HO / O
HCI
8 4-amino-2-(2-hydroxy-6- cN
isobutoxyphenyl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride HN I ~ NHz
N /N
CH3
HO / O~OH3
HCI
9 4-amino-2-(2,5-dihydroxyphenyl)-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile
hydrochloride HN ~ NH2
I
N rN
OH
Hcl
HO
4-amino-2-(2-fluoro-6-hydroxyphenyl)-6- cN
piperidin-3-ylpyrimidine-5-carbonitrile
hydrochloride HN ~ NHS
I
N /N
F / OH
HCI
11 4-amino-2-[2-hydroxy-5- cN
(trifluoromethyl)phenyl]-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride HN I ~ NHS
N iN
OH
F3~ HCI
123
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Example Name and structure Name and structure Mass
Spec.
12 4-amino-2-[2-hydroxy-4- cN
(trifluoromethyl)phenyl]-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride HN I ~ NHZ
N ,N
OH
HCI
CF3
[00296] For the remaining examples, unless otherwise noted,'H NMR
spectra were obtained on a Bruker AV 500 or a Bruker AV-300 spectrometer.
Spectra are given in ppm (~) and coupling constants, J, are reported in Hertz.
Tetramethylsilane was used as an internal standard for proton spectra and the
solvent peak was used as the reference peak for carbon spectra. Mass spectra
were obtained on a Perkin Elmer Sciex 100 atmospheric pressure chemical
ionization (APCI) mass spectrometer, or a Finnigan LCQ Duo LCMS ion trap
electrospray ionization mass spectrometer. Thin-layer chromatography (TLC)
was performed using Analtech silica gel plates and visualized by ultraviolet
(UV) light. HPLC analyses were obtained using a Phenomenex Luna C18(2)
column (150 ~ 4.6 mm) with UV detection at 254 nm, using aqueous TFA in
acetonitrile as the eluent on a Varian Prostar HPLC. Purification using
preparative HPLC was performed using a Phenomenex Luna C18(2) column
(250 ~ 21.2 mm', 10~.) on a Varian Prostar, with UV detection at 254 nm and a
concentrated aqueous solution of NH40H in acetonitrile as the eluent. Medium
pressure column chromatographies were performed on a Biotage Horizon
instrument using Biotage cartridge Si Flash 12+M, Flash 25+M or Flash 40+M,
with UV detection at 254 nm. Flash chromatography column purifications were
performed using silica gel 60, 230-400 mesh (E. Merck). Elemental analyses
were performed by Quantitative Technologies, Inc. (Whitehouse, NJ). All
reactions were carried out under nitrogen unless specified otherwise.
124
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00297] Example 13: tent-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate
soy
N
CN
CN
,O
[00298] H3c
[00299] Synthesis was done according to a procedure published in J.
Org. Chem. 43, 3631-3632 (1978). To an ice-cold solution of 1-(tert-
butoxycarbonyl)-3-piperidine carboxylic acid (10.0 g, 43.6 mmol),
malononitrile
(2.40 g, 36.35 mmol) and diethylcyanophosphonate (7.4 mL, 43.6 mmol) in
THF (100 mL) was added triethylamine (16.2 mL, 116.3 mmol). The resulting
solution was stirred at 0°C for 2 h, then at room temperature
overnight. The
reaction was quenched by adding an aqueous solution of 1 N HCI (50 mL)
followed by a dilution with CHZCIZ (100 mL). The aqueous phase was extracted
with CHZCh (1 x 100 mL). The combined organic extracts were washed with a
saturated aqueous solution of NaHC03 (2 x 50 mL). The later aqueous phase
was back-extracted with CH2C12 (1 x 100 mL). The combined organic extracts
were dried (Na~S04) and concentrated to dryness under reduced pressure. The
residue (~36 mmol) was dissolved in a mixture of 1,4-dioxane (109 mL) and
water (9.0 mL). To this solution was added NaHC03 (12.2 g, 145.2 mmol) and
dimethylsulfate (11.2 mL, 117.7 mmol). The resulting mixture was heated to
reflux for 45 min. To the cooled reaction mixture was added water (198 mL).
The solution thus obtained was extracted with CH2CIZ (3 x 200 mL). The
combined organic extracts were dried (Na2S04) and concentrated under
reduced pressure. Purification by flash chromatography (eluents, 2:1 to 1:1
hexanes/EtOAc) gave the title compound as a yellow oil.
[00300] Example 14: tert-butyl 2-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate
125
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
o~CH3
Boc
N ~ CN
[00301 ] cN
[00302] Prepared according to the procedure of Example 13.'H NMR
(500 MHz, CDC13) ~ 4.92 (br s, 1 H), 4.36 (s, 3H), 3.81 (br s, 1 H), 3.15 (br
s,
1 H), 1.98-1.84 (m, 2H), 1.68-1.54 (m, 4H), 1.47 (s, 9H).
[00303] Example 15: pyridine-2-carboximidamide
HN NH2
~~ N
(00304]
[00305] To a slurry of NH4C1 (4.62 g, 86.4 mmol) in toluene (34 mL) at
0°C was added dropwise a solution of AI(CH3)3 (7.6 mL, 79.7 mmol) in
toluene
(32 mL). The reaction mixture was warmed to room temperature and stirred for
2 h prior to the addition of a solution of 2-cyanopyridine (5.0 g, 48.0 mmol)
in
toluene (15.0 mL). The resulting solution was heated to 80°C for 20 h.
The
cooled reaction mixture was poured into a slurry of silica gel (24.0 g) in
CHCI3
(80 mL), followed by vigorous stirring for 10 min. The silica gel was filtered
off
and the cake was rinsed in turn with methanol and an aqueous solution of 2N
NaOH (100 mL). The combined filtrates were extracted with CHC13 (3 x 300
mL), then concentrated to dryness under reduced pressure, diluted with a small
amount of methanol, and treated with a 2N HCI solution in methanol. The
precipitate that formed was isolated by filtration and dried in a vacuum oven
to
afford the title compound as an off-white HCI salt.'H NMR (500 MHz, DMSO-
ds) b 9.66 (s, 2H), 9.52 (s, 2H), 8.85-8.81 (m, 1 H), 8.37 (d, J = 7.9 Hz, 1
H),
8.17 (td, J = 1.8, 1.6 Hz, 1 H), 7.80 (ddd, J = 7.7, 4.7, 0.9 Hz, 1 H).
[00306] Example 16: 2-hydroxybenzenecarboximidamide
126
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
HN NHS
OH
[00307]
[00308] St_ ep 1: Preparation of 2-benzyloxy-benzonitrile
CN
O ~
[00309]
[00310] To a slurry of 2-cyanophenol (25.0 g, 209.9 mmol), K~C03
(46.4 g, 335.8 mmol) in acetone (1.05 L) was added benzyl bromide (30.0 mL,
252.2 mmol) and Nal (2.0 g, 13.4 mmol). The reaction mixture was stirred for
24 h at room temperature, then filtered. The filtrate was concentrated to
dryness under reduced pressure. Purification by flash column chromatography
(eluent, hexanes to 95:5 hexanes/EtOAc to 95:0.5:0.5 to 8:1.5:0.5
CH2C1~/hexanes/EtOAc) gave the desired product as a white solid.'H NMR
(500 MHz, CDC13) ~ 7.56 (dd, J = 7.8, 1.6 Hz, 1 H), 7.51-7.43 (m, 3H), 7.41-
7.35
(m, 2H), 7.34-7.30 (m, 1 H), 7.00-6.96 (m, 2H), 5.20 (s, 2H).
[00311] Step 2: Preparation of 2-hydroxybenzenecarboximidamide
HN NHz
OH
[00312]
[00313] To a slurry of NH4C1 (20.2 g, 377.6 mmol) in toluene (147 mL)
at 0°C was added dropwise a solution of AI(CH3)3 (33.4 mL, 348.6 mmol)
in
toluene (174 mL). The reaction mixture was warmed to room temperature and
stirred for 2 h prior to the addition of a solution of 2-benzyloxy-
benzonitrile
(Step 1, 42.0 g, 210.0 mmol) in toluene (80.0 mL). The resulting solution was
heated to 80°C for 20 h. The supernatant was discarded, and the solid
residue
at the bottom of the flask was suspended in a mixture of 2N aqueous solution
of NaOH and MeOH, sonicated and filtered. That operation was repeated. The
combined filtrates were extracted with CHC13, then with a mixture of
127
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
CHC13/isopropanol (80/20). The combined extracts were concentrated to
dryness under reduced pressure. The final cake was suspended three times in
a mixture of CHC13 and isopropanol (80/20), stirred overnight and then
filtered.
The combined filtrates were concentrated to dryness under reduced pressure.
The residues were combined, dissolved in a minimum amount of MeOH, and
treated with a 2N solution of HCI in MeOH. The precipitate that had formed was
isolated by filtration and dried to afford the title compound as an off-white
solid
HCI salt.'H NMR (500 MHz, DMSO-ds) b 11.21 (s, 1H), 9.13 (s, 2H), 9.06 (s,
2H), 7.55 (dd, J = 7.8, 1.6 Hz, 1 H), 7.49-7.43 (m, 1 H), 7.16 (dd, J = 8.2,
0.7 Hz,
1 H), 6.98-6.93 (m, 1 H).
[00314] Example 17: tert-butyl 2-[amino(imino)methyl]benzoate
HN NH2
Boc
[00315]
[00316] Into a solution of 2-benzyloxy-benzonitrile (Example 4, Step
1, 10.0 g, 47.8 mmol) in EtOH (200 mL) at -78°C was bubbled HCI gas for
2 h
during which time the reaction mixture temperature rose to -50°C. The
reaction
mixture was warmed to 0°C and stirred overnight while warming to room
temperature. The reaction mixture was diluted with EtOH (100.0 mL) and
cooled to -78°C. Ammonia gas was then bubbled into the reaction
solution for
1.5 h. The white slurry thus obtained was warmed to 0°C and ammonia
bubbling was maintained overnight. The slurry was filtered and the cake was
rinsed in turn with MeOH and EtOH. The filtrate was concentrated and the
residue was diluted with CH2C12 (200 mL) and 2 N aqueous solution of NaOH
(50 mL). The aqueous phase was extracted with CH~Ch (200 mL), CHC13 (200
mL) and with a 3/1 mixture of CHC13 and isopropanol (300 mL). The combined
extracts were dried (Na~S04) and concentrated under reduced pressure to give
some unidentified impurities. The aqueous phase was concentrated to dryness
128
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
under reduced pressure to afford a white solid, which was triturated with a
6/1
mixture of CH2CIz/MeOH and filtered. The cake was rinsed with the same
solvent mixture. The filtrates were concentrated to dryness under reduced
pressure to give a white solid, which was dissolved in MeOH and purified by
flash column chromatography (eluent, 95/4.5/0.5 to 90/9/1 to 80/18/2
CH~CIZ/MeOH/conc'd NH40H) to give the title compound as an off-white solid.
'H NMR (300 MHz, DMSO-ds) b 7.52-7.45 (m, 3H), 7.42-7.30 (m, 4H), 7.16 (d,
J = 7.9 Hz, 1 H), 6.99 (dt, J = 7.4, 0.7 Hz, 1 H), 5.15 (s, 2H).
[00317] Example 18: 4-amino-6-(3-methoxyphenyl)-2-pyridin-2-
ylpyrimidine-5-carbonitrile
NHS
N \ CN
I
N~ I N \ O
[00318]
[00319] Step 1: Preparation of 2-(methoxy(3-
methoxyphenyl)methylene)malononitrile
OCH3
CN
CN
[00320] ocH3
[00321] To a solution of malononitrile (1.94 g, 29.3 mmol) in THF (30
mL) at 0°C was added sodium hydride (60% in mineral oil, 2.34 g, 58.6
mmol);
the reaction mixture was stirred for 30 min. 3-Methoxybenzoyl chloride (5.00
g,
29.3 mmol) was added at 0°C and the solution was slowly warmed to room
temperature and stirred for 1 h. Dimethyl sulfate (7.39 g, 58.6 mmol) was then
added and the reaction mixture was refluxed for 2 h. The reaction mixture was
diluted with diethyl ether (40 mL) and washed with water (15 mL). The aqueous
phase was then washed with diethyl ether (3 x 30 mL). The combined organic
phase was dried (Na2S04) and concentrated. Purification by flash column
129
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
chromatography (eluent, 3:1 HexaneslEtOAc; the mixture was loaded onto the
column as a solution in CH2C12) gave the product as a clear oil.'H NMR (300
MHz, CDC13) b 7.48 (t, J = 8.0 Hz, 1 H), 7.14 (dd, J = 8.1, 2.1 Hz, 1 H), 7.04
(d, J
= 7.5 Hz, 1 H), 6.96 (s, 1 H), 3.93 (s, 3H), 3.87 (s, 3H).
[00322] Step 2: Preparation of 4-amino-6-(3-methoxyphenyl)-2-pyridin-
2-ylpyrimidine-5-carbonitrile
NH2
N ~ CN
I I
N\ N ~ O
[00323] I ~ ~ (structure from ChemLink)
[00324] To a suspension of pyridine-2-carboximidamide (Example 15,
307 mg, 1.95 mmol) and the product obtained in Step 1 (500 mg, 2.33 mmol) in
ethanol (20 mL) was added sodium methoxide (421 mg, 7.80 mmol) and stirred
for 15 min at room temperature. The reaction mixture was then filtered and the
remaining solid was washed with a solution of 9:1 ethanoU water. Purification
by preparative HPLC yielded the desired product, a portion of which was then
dissolved in DMF and filtered. To the filtrate was added water and the title
compound precipitated from solution as a white solid. mp 231-235°C.'H
NMR
(500 MHz, DMSO-ds): ~ 8.74 (d, J = 4.0 Hz, 1 H), 8.55-7.65 (br s, 2H), 8.40
(d, J
= 8.0 Hz, 1 H), 7.98 (dt, J = 3.9, 1.8 Hz, 1 H), 7.57-7.49 (m, 4H), 7.20-7.18
(m,
1 H), 3.85 (s, 3H). ESI MS m/z 304 [M+H]+. Anal. Calcd for C"H,3N50: C, 67.32;
H, 4.32; N, 23.09. Found: C, 67.24; H, 4.12; N, 22.82.
[00325] Example 19: 4-amino-2-(2-hydroxyphenyl)-6-(3-
methoxyphenyl)pyrimidine-5-carbonitrile
NHz
OH NI ~ CN
I
N ~ O
[00326]
130
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00327] Prepared as a yellow solid following a procedure similar to
that described in Example 18, Step 2, using 2-
hydroxybenzenecarboximidamide (Example 16, 256 mg, 1.95 mmol) and the
material obtained in Example 18, Step 1 (500 mg, 2.33 mmol). mp 240-
244°C.
'H NMR (500 MHz, DMSO-ds): 5 13.22 (s, 1 H), 8.63 (br s, 1 H), 8.37 (dd, J =
8.0, 2.0 Hz, 1 H), 8.01 (br s, 1 H), 7.55-7.44 (m, 4H), 7.23-7.20 (m, 1 H),
6.97-
6.93 (m, 2H), 3.86 (s, 3H). ESI MS m/z 319 [M+H]+. Anal. Calcd for C,8H,4N4O2:
C, 67.92; H, 4.43; N, 17.60. Found: C, 67.56; H, 4.27; N, 17.25.
[00328] Example 20: 4-amino-6-piperidin-2-yl-2-pyridin-2-ylpyrimidine-
5-carbonitrile
NH2
CN
N ~
N I / N
\N
[00329]
[00330] Step 1: Preparation of tert-butyl 2-(5-cyano-6-piperidin-2-yl-2-
pyridin-2-ylpyrimidin-4-yl)piperidine-1-carboxylate
N~
[00331 ]
[00332] To a solution of pyridine-2-carboximidamide (Example 15,
300 mg, 1.90 mmol) and tert-butyl 2-(2,2-dicyano-1-methoxyvinyl)piperidine-1-
carboxylate (Example 14, 664 mg, 2.28 mmol) in ethanol (28 mL) was added
sodium methoxide (411 mg, 7.60 mmol) and stirred at reflux for 3 h. The
reaction mixture was then concentrated, filtered through silica gel eluting
with
80:19:1 CHZCh/MeOH/concd NH40H. The filtrate was concentrated, diluted
with water (15 mL) and extracted with CH2C12 (4 x 40 mL). The combined
131
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
organic phase was dried (Na2S04) and concentrated. Purification by flash
column chromatography (eluent, 95:4.5:0.5 CH~CI~/MeOH/concd NH40H; the
mixture was loaded onto the column as a solution in CHZCh) gave the product
as a white solid. 'H NMR (500 MHz, DMSO-d6) b 8.73 (d, J = 3.5 Hz, 1 H), 8.10
(br s, 2H), 8.28 (d, J = 8.0 Hz, 1 H), 7.99 (dt, J = 7.6, 1.5 Hz, 1 H), 7.55-
7.53 (m,
1 H), 5.20 (s, 1 H), 3.90 (dd, J = 10.5, 2.5 Hz, 1 H), 3.62 (dt, J = 12.5, 3.0
Hz,
1 H), 2.05 (br s, 1 H), 1.95 (br s, 1 H), 1.75 (br s, 1 H), 1.57-1.53 (m, 1
H), 1.50-
1.38 (m, 2H), 1.25 (s, 9H).
[00333] Step 2: Preparation of 4-amino-6-piperidin-2-yl-2-pyridin-2-
ylpyrimidine-5-carbonitrile
NH2
CN
N
N I i N
N
[00334]
[00335] The product obtained in Step 1 (300 mg, 0.78 mmol) was
dissolved in 4 N HCI (dioxane, 4 mL) and stirred for 15 min at room
temperature, during which the desired product precipitated from solution. The
reaction mixture was diluted with MeOH (2 mL), filtered and washed with ether
to give the title compound as a white solid. mp 235-250°C dec.'H NMR
(300
MHz, DMSO-ds) ~ 9.74-9.71 (m, 1 H), 9.49 (br s, 1 H), 8.94 (d, J = 4.8 Hz, 1
H),
8.83 (br s, 1 H), 8.70 (d, J = 7.8 Hz, 1 H), 8.49-8.37 (m, 2H), 7.99 (s, 1 H),
4.60
(t, J = 9.6 Hz, 1 H), 3.44-3.37 (m, 1 H), 3.22-3.19 (m, 1 H), 2.14 (d, J = 9.6
Hz,
1 H), 1.89-1.68 (m, 5H). ESI MS m/z 280 [M+H]+. Anal. Calcd for C,5H,6N6 ~
2HC1
~ H20: C, 48.53; H, 5.43; N, 22.64. Found: C, 48.58; H, 5.25; N, 22.39.
[00336] Example 21: 4-amino-2-pyridin-2-yl-6-pyrrolidin-2-ylpyrimidine-
5-carbonitrile
132
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NH2
CN
N
N I ~ N
[00337]
[00338] Step 1: Preparation of 1-(tert-butoxycarbonyl)proline
Boc OH
[00339] °
[00340] To a suspension of proline (10.0 g, 86.9 mmol) in DMF (200
mL) was added di-tert-butyl dicarbonate (37.98 g, 174 mmol) followed by
triethylamine (17.6 g, 174 mmol). The reaction mixture was stirred overnight
at
room temperature. The solution was diluted with water (200 mL) and extracted
first with EtOAc (2 x 400 mL) followed by CHZCh (3 x 400 mL). The combined
organic phase was dried (Na2S04) and concentrated. The crude product was
carried forth to the next step.'H NMR (300 MHz, CDC13) ~ 4.37-4.22 (m, 1 H),
3.57-3.39 (m, 2H), 2.70-1.89 (m, 4H), 1.48-1.40 (m, 9H).
[00341] Step 2: Preparation of tert-butyl 2-(dicyanoacetyl)pyrrolidine-
1-carboxylate
Boc °
N
CN
[00342] Nc
[00343] To a solution of the product obtained in Step 1 (~43 mmol) in
THF (100 mL) at room temperature was added malononitrile (2.39 g, 36.2
mmol) and diethylcyanophosphonate (7.60 g, 43.5 mmol). The reaction
solution was then cooled to 0°C prior to the addition of triethylamine
(11.7 g,
116 mmol). The solution was stirred for 2 h at 0°C and at room
temperature
overnight. The reaction mixture was diluted with an aqueous solution of 1 N
HCI (33 mL) and extracted with CHZCIZ (4 x 60 mL). The combined organic
phase was dried (Na2S04) and concentrated.'H NMR (300 MHz, CDC13) b
4.70-4.66 (m, 1 H), 3.47-3.41 (m, 2H), 2.31-2.24 (m, 1 H), 1.91-1.81 (m, 4H),
1.43 (s, 9H).
133
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00344] Step 3: Preparation of tert-butyl 2-(2,2-dicyano-1-
methoxyvinyl)pyrrolidine-1-carboxylate
H3C
O
\ CN
[00345] Nc
[00346] To a mixture of the product obtained in Step 2 (~43 mmol) in
1,4-dioxane (120 mL) and water (10 mL) was added sodium bicarbonate
(14.62 g, 174 mmol) and dimethyl sulfate (19.20 g, 152 mmol). The reaction
mixture was then refluxed for 2 h, diluted with water (10 mL) and extracted
with
CH~CIz (3 x 100 mL). The combined organic phase was dried (Na2S04) and
concentrated. Purification by flash column chromatography (eluent, 2:1
Hexanes/EtOAc; the mixture was loaded onto the column as a solution in
CHzCh) gave the product (4.84 g, 40°l° over three steps) as a
1:1 mixture of
rotamers in the form of an orange solid. 'H NMR (500 MHz, CDC13) b 4.76 (dd,
J = 8.4, 5.5 Hz, 1 H), 4.72 (dd, J = 8.3, 5.8 Hz, 1 H), 4.34 (s, 3H), 4.29 (s,
3H),
3.61-3.56 (m, 1 H), 3.50-3.42 (m, 3H), 2.49-2.44 (m, 2H), 1.99-1.89 (m, 6H),
1.48 (s, 9H), 1.47 (s, 9H).
[00347] St_ ep 4: Preparation of tert-butyl 2-(6-amino-5-cyano-2-
pyridin-2-ylpyrimidin-4-yl)pyrrolidine-1-carboxylate
s°~N
cN
N~ I
~N NHS
[00348] ~ N
[00349] The above compound was prepared in 62% yield as a tan
solid following a procedure similar to that described in Example 18, Step 2,
using pyridine-2-carboximidamide (Example 15, 300 mg, 1.90 mmol) and the
material obtained in Step 3 (633 mg, 2.28 mmol).'H NMR (500 MHz, DMSO-
ds): ~ 8.72 (d, J = 4.5 Hz, 1 H), 8.39-7.68 (br s, 2H), 8.30-8.28 (m, 1 H),
7.97 (dt,
J = 7.8, 1.7 Hz, 1 H), 7.54-7.52 (m, 1 H), 4.87-4.84 (m, 1 H), 3.60-3.55 (m, 1
H),
134
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
3.53-3.49 (m, 1 H), 2.41-2.38 (m, 1 H), 2.06-2.00 (m, 1 H), 1.94-1.86 (m, 2H),
1.37 (s, 3H), 1.12 (s, 6H).
[00350] Step 5: Preparation of 4-amino-2-pyridin-2-yl-6-pyrrolidin-2-
ylpyrimidine-5-carbonitrile
NHS
CN
N
N I ~ N
[00351 ]
[00352] Prepared as a white solid following a procedure similar to that
described in Example 20, Step 2, using the material obtained in Step 4 (300
mg, 0.823 mmol). mp 240-245°C dec.'H NMR (500 MHz, DMSO-ds) b 10.39
(br s, 1 H), 9.52 (br s, 1 H), 8.97 (s, 1 H), 8.45 (br s, 1 H), 8.69 (d, J =
7.5, 1 H),
8.58 (s, 1 H), 8.41 (br s, 1 H), 8.07 (s, 1 H), 4.96 (q, J = 7.1 Hz, 1 H),
3.62-3.57
(m, 1 H), 3.40 (m, 1 H), 2.58-2.54 (m, 1 H), 2.09-1.96 (m, 3H). ESI MS m/z 267
[M+H]+. Anal. Calcd for C,4H,4N6 ~ 2HC1 ~ 0.25H20: C, 48.92; H, 4.84; N,
24.45.
Found: C, 49.23; H, 4.65; N, 24.15.
[00353] Example 22: tert-butyl 3-[6-amino-5-cyano-2-(2-hydroxy-6-
propylphenyl)pyrimidin-4-yl]piperidine-1-carboxylate
NH2
OH N ~ CN
IN
J
B0c
~CH3
[00354]
[00355] Step 1: Preparation of 2-[(4-methylbenzyl)oxy]-6-
propylbenzonitrile
CN
~O CH3
a
[00356]
135
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00357] To a suspension of 2-fluoro-6-[(4-
methylbenzyl)oxy]benzonitrile (5.0 g, 20.7 mmol) and MnCl2 (2.60 g, 20.7
mmol) in THF (50 mL) was added n-PrMgCI (2.0 M in ether, 31 mL, 62 mmol)
slowly at 0°C. The solid gradually dissolved to give a brown solution.
The
reaction was allowed to warm up to room temperature and was stirred for 3 h,
then slowly poured into an ice-cooled NH4C1 solution (200 mL). The product
was extracted with ethyl acetate (3 x 150 mL). The combined organic phase
was washed with brine (150 mL), dried (Na~S04) and concentrated under
vacuum. The residue was purified by flash column chromatography (1:19 to 1:1
ethyl acetate/hexanes) to provide the desired product as a viscous oil.'H NMR
(300 MHz, CDC13): b 7.38-7.33 (m, 3H), 7.20 (d, J = 7.8 Hz, 2H), 6.86 (d, J =
7.6 Hz, 1 H), 6.82 (d, J = 8.4 Hz, 1 H), 5.15 (s, 2H), 2.78 (t, J = 7.5 Hz,
2H), 2.35
(s, 3H), 1.74-1.66 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H).
[00358] Step 2: Preparation of 2-[(4-methylbenzyl)oxy]-6-
propylbenzonitrile
H3C / O NHS
O CH3
v
[00359]
[00360] To a solution of 2-[(4-methylbenzyl)oxy]-6-propylbenzonitrile
(Step 1, 1.0 g, 3.8 mmol) in benzyl alcohol (2.6 mL) was added KOH (0.52 g,
9.4 mmol) and H20 (0.35 mL, 19.4 mmol). The reaction mixture and heated to
120°C for 3 h. The cooled reaction mixture was diluted with ethyl
acetate (100
mL) and washed with water (200 mL). The aqueous layer was extracted with
ethyl acetate (100 mL). The combined organic extracts were washed with brine
(150 mL), dried (Na~S04) and concentrated under reduced pressure. The
residue was purified by flash column chromatography (1:19 to 1:0 ethyl
acetate/hexanes) to provide the desired compound as a white solid.'H NMR
(300 MHz, CDC13): 5 7.31-7.20 (m, 3H), 7.18 (d, J = 7.8 Hz, 2H), 6.87 (d, J =
7.6 Hz, 1 H), 6.83 (d, J = 8.2 Hz, 1 H), 5.78 (s, 2H), 5.05 (s, 2H), 2.68 (t,
J = 7.7
136
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
Hz, 2H), 2.34 (s, 3H), 1.69-1.59 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). ESI MS m/z
284 [M+H]+.
[00361] Step 3: Preparation of 2-hydroxy-6-propylbenzamide
O NH2
HO ~ CH3
[00362]
[00363] To a solution of the material obtained in Step 2 (1.0 g, 3.5
mmol), in a mixture of ethanol/ethyl acetate (40 mL/20 mL) was added Pd/C
(0.5 g). The reaction mixture was agitated on a Parr hydrogenator at 40 psi
for
14 h at room temperature. The mixture was filtered through a short pad of
diatomaceous earth. The filtrate was concentrated to give the product as a
white solid. 'H NMR (500 MHz, acetone-ds): 5 9.4 (s, 1 H), 7.14 (t, J = 7.8
Hz,
1 H), 6.99 (br s, 2H), 6.74-6.71 (m, 2H), 2.68 (t, J = 7.1 Hz, 2H), 1.66-1.59
(m,
2H), 0.92 (t, J = 7.3 Hz, 3H). ESI MS m/z 180 [M+H]+.
[00364] Step 4: Preparation of 2-hydroxy-6-propylbenzamidine
HN NH2
HO ~ CH3
[00365]
[00366] To a suspension of the material obtained in Step 3 (0.59 g 3.3
mmol) in CHC13 (15 mL) was added trimethyloxonium tetrafluoroborate (0.59 g,
3.95 mmol) at room temperature. The mixture was stirred at room temperature
for 14 h. The solvent was removed and the residue was transferred into a
sealed tube with methanol (2 mL). Ammonia (7.0 N in MeOH, 8 mL) was added
and the reaction mixture was heated to 80°C for 14 h. The reaction
mixture
was then concentrated to a smaller volume under reduced pressure. The
residue was diluted with ethyl acetate (100 mL) and washed with a saturated
aqueous solution of NaHCO3 (100 mL). The aqueous layer was extracted with
ethyl acetate (50 mL). The combined organic extracts were washed with brine
(150 mL), dried (Na2S04) and concentrated under vacuum. The residue was
137
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
purified by flash column chromatography (eluent, 90:9:1 CHZCIZ/MeOH/concd
NH40H to 9:1 MeOH/concd NH40H) to provide the product as a white solid.'H
NMR (300 MHz, CD3OD-d4): ~ 7.26 (t, J = 7.9 Hz, 1 H), 6.82-6.76 (m, 2H), 2.63
(t, J = 7.7 Hz, 2H), 1.76-1.63 (m, 2H), 0.99 (t, J = 7.2 Hz, 3H). ESI MS m/z
179
[M+H]+.
[00367] Step 5: Preparation of tert-butyl 3-[6-amino-5-cyano-2-(2-
hydroxy-6-propylphenyl)pyrimidin-4-yl]piperidine-1-carboxylate
~NBoc
OH N ~ CN
I N
J
N
Boc
~CH3
[00368]
[00369] To a suspension of the material from Step 4 (127 mg, 0.71
mmol) and tert-butyl 3-(2,2-dicyano-1-methoxyvinyl)piperidine-1-carboxylate
(Example 13, 250 mg, 0.86 mmol) in anhydrous ethanol was added sodium
methoxide powder (154 mg, 2.85 mmol) at room temperature. The reaction
mixture was then refluxed for 1 h. The reaction was cooled to room
temperature and poured into a saturated aqueous solution of NH4C1 (100 mL).
The product was extracted with ethyl acetate (2 x 100 mL). The organics were
combined, washed with brine, dried (NaZS04) and concentrated under vacuum.
The material was purified by flash column chromatography (eluent, 90:9:1
CH~CIa/MeOH/concd NH40H). A second purification by Biotage HPFC (17:83 to
28:72 ethyl acetate/hexanes) gave the title compound as a yellow solid. mp
192-193°C.'H NMR (300 MHz, CDC13) 8 12.70 (s, 1 H), 7.26 (t, J = 6.3
Hz, 1 H),
6.86-6.78 (m, 2H), 5.67 (s, 2H), 3.14-3.03 (m, 4H), 2.15-1.56 (m, 8H), 1.48
(s,
9H), 0.95 (t, J = 7.2 Hz, 3H). ESI MS m/z 438 [M+H]+. Anal. Calcd for
C24H31N502' C, 65.88; H, 7.14; N, 16.01. Found: C, 65.94; H, 7.24; N, 15.79.
138
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00370] Example 23: 4-amino-2-(2-hydroxy-6-propylphenyl)-6-
piperidin-3-ylpyrimidine-5-carbonitrile
NHS
NC
N ~CH3
HN N ~ \
[00371 ] Ho
[00372] To tart-butyl 3-[6-amino-5-cyano-2-(2-hydroxy-6-
propylphenyl)pyrimidin-4-yl]piperidine-1-carboxylate (Example 22, 0.50 g, 1.1
mmol) in a round bottom flask was added a solution of 4N HCI in 1,4-dioxane
(5 mL) at room temperature. The reaction mixture was stirred for 1.5 h at room
temperature. The solvent was removed at room temperature under vacuum.
Trituration with a mixture of methanol/ether provided the title compound as a
light yellow solid. mp 150°C dec. 'H NMR (300 MHz, DMSO-d6): ~ 9.17-
8.90
(m, 2H), 7.94 (br s, 2H), 7.13 (t, J = 7.8 Hz, 1 H), 6.76-6.69 (m, 2H), 3.65-
3.14
(m, 4H), 3.00-2.80 (m, 1 H), 2.53-2.49 (m, 2H), 2.05-1.50 (m, 4H), 1.43-1.36
(m,
2H), 0.78 (t, J = 7.3 Hz, 3H). ESI MS m/z 338 [M+H]+. Anal. Calcd for
C,9H23NSO
~ HCI ~ H20: C, 58.23; H, 6.69; N, 17.87. Found: C, 58.37; H, 6.52; N, 17.56.
[00373] Example 24: 4-amino-2-(2-hydroxy-6-isobutylphenyl)-6-
piperidin-3-ylpyrimidine-5-carbonitrile
NH2 CH3
NC
wN ~CH3
HN~ -N
[00374] ICI Ho
[00375] Step 1: Preparation of 2-isobutyl-6-[(4-
methylbenzyl)oxy]benzonitrile
H3C ~ ~ CN
~O CH3
00376
[ ]
139
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00377] Prepared as a colorless oil following a procedure similar to
that described in Example 22, Step 1, using 2-fluoro-6-[(4-
methylbenzyl)oxy]benzonitrile (5.0 g, 18.9 mmol) and isobutyl magnesium
chloride (3.8 g, 13.6 mmol).'H NMR (300 MHz, CDC13): b 7.37-7.31 (m, 3H),
7.20 (d, J = 7.9 Hz, 2H), 6.83-6.80 (m, 2H), 5.18 (s, 2H), 2.68 (d, J = 7.3
Hz,
2H), 2.35 (s, 3H), 2.02-1.95 (m, 1 H), 0.95 (d, J = 7.2 Hz, 6H).
[00378] Step 2: Preparation of 2-isobutyl-6-[(4-
methylbenzyl)oxy]benzamide
H3C / O NH2
O CH3
[00379] I i cH3
[00380] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 2, starting with 2-isobutyl-6-[(4-
methylbenzyl)oxy]benzonitrile (Step 1, 3.8 g, 13.6 mmol).'H NMR (500 MHz,
CDC13): ~ 7.30 (d, J = 7.9 Hz, 2H), 7.21 (t, J = 8.0 Hz, 1 H), 7.17 (d, J =
7.9 Hz,
2H), 6.83-6.81 (m, 2H), 5.74 (s, 2H), 5.05 (s, 2H), 2.61 (d, J = 7.3 Hz, 2H),
2.35
(s, 3H), 2.01-1.90 (m, 1 H), 0.91 (d, J = 6.6 Hz, 6H). ESI MS m/z 298 [M+H]+.
[00381] Step 3: Preparation of 2-hydroxy-6-isobutylbenzamide
O NHS
HO \ CH3
[00382] I ~ cH3
[00383] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 3, starting with 2-isobutyl-6-[(4-
methylbenzyl)oxy]benzamide (Step 2, 0.76 g, 2.6 mmol). ESI MS m/z 194
[M+H]+.
[00384] St_ ep 4: Preparation of 2-hydroxy-6-isobutylbenzamidine
HN NHS
HO \ CH3
[00385] I ~ cH3
140
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00386] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 4, starting with 2-hydroxy-6-isobutylbenzamide
(Step 3, 0.50 g, 26 mmol).'H NMR (300 MHz, CD30D-d4): b 7.26 (t, J= 7.9 Hz,
1 H), 6.81-6.75 (m, 2H), 2.54 (d, J = 7.3 Hz, 2H), 2.03-1.91 (m, 1 H), 0.95
(d, J =
6.5 Hz, 6H). ESI MS m/z 193 [M+H]+.
[00387] Step 5: Preparation of tert-butyl 3-[6-amino-5-cyano-2-(2-
hydroxy-6-isobutylphenyl)pyrimidin-4-yl]piperidine-1-carboxylate
NH2 CH3
NC
wN ~CH3
BocN N
[00388] Ho
[00389] Prepared as a light yellow solid following a procedure similar
to that described in Example 22, Step 5, starting with 2-hydroxy-6-
isobutylbenzamidine (Step 4, 0.20, 1.06 mmol) and tert-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate (Example 13, 0.43 g, 1.5 mmol). ESI
MS m/z 452 [M+H]+.
[00390] Step 6: Preparation of 4-amino-2-(2-hydroxy-6-
isobutylphenyl)-6-piperidin-3-ylpyrimidine-5-carbonitrile
NHZ CH3
NC
~~N ~CH3
HN N
[00391 ] Ho
[00392] Prepared as a light yellow solid following a procedure similar
to Example 23, starting with tert-butyl 3-[6-amino-5-cyano-2-(2-hydroxy-6-
isobutylphenyl)pyrimidin-4-yl]piperidine-1-carboxylate (Step 5, 0.23 g, 0.50
mmol).'H NMR (300 MHz, CD3OD-d4): ~ 9.08-8.80 (m, 2H), 8.0 (br s, 2H), 7.14
(t, J = 7.8 Hz, 1 H), 6.76 (d, J = 7.7 Hz, 1 H), 6.67 (d, J = 7.4 Hz, 1 H),
3.40-3.15
(m, 4H), 3.12-2.89 (m, 1 H), 2.46-2.42 (m, 2H), 2.05-1.50 (m, 5H), 0.72 (d, J
=
141
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
6.5 Hz, 6H). ESI MS m/z 352 [M+H]+. Anal. Calcd for C~oH25N50 ~ 2HCI ~ HaO:
C, 54.30; H, 6.61; N, 15.83. Found: C, 54.61; H, 6.45; N, 15.68.
[00393] Example 25: 4-amino-2-[2-hydroxy-6-(3-methylbutyl)phenyl]-6-
piperidin-3-ylpyrimidine-5-carbonitrile
[00394]
[00395] Step 1: Preparation of 2-[(4-methylbenzyl)oxy]-6-(4-
methylpentyl)benzonitrile
CN
\~\/O CH3
\
[00396]
[00397] Prepared as a colorless oil following a procedure similar to
that described in Example 22, Step 1, using 2-fluoro-6-[(4-
methylbenzyl)oxy]benzonitrile (5.0 g, 20.7 mmol) and 3-methylbutyl
magnesium bromide (2.0 M in ether, 31 mL, 62 mmol).'H NMR (500 MHz,
CDC13): b 7.36-7.33 (m, 3H), 7.25 (d, J = 8.3 Hz, 2H), 6.86 (d, J = 7.6 Hz, 1
H),
6.80 (d, J = 8.4 Hz, 1 H), 5.05 (s, 2H), 2.81-2.78 (m, 2H), 2.35 (s, 3H), 1.66-
1.60 (m, 1 H), 1.56-1.51 (m, 2H), 0.96 (d, J = 6.6 Hz, 6H).
[00398] Step 2: Preparation of 2-[(4-methylbenzyl)oxy]-6-(4-
methylpentyl)benzamide
H3C / O NH2
O CH3
[00399]
[00400] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 2, starting with 2-[(4-methylbenzyl)oxy]-6-(4-
methylpentyl)benzonitrile (Step 1, 4.8 g, 16.4 mmol).'H NMR (500 MHz,
142
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
acetone-ds): b 7.38 (d, J = 7.9 Hz, 2H), 7.21-7.16 (m, 3H), 6.96-6.88 (m, 2H),
6.84-6.82 (d, J = 7.6 Hz, 1 H), 6.66 (br s, 1 H), 5.06 (s, 2H), 2.77-2.64 (m,
2H),
2.31 (s, 3H), 1.59-1.51 (m, 3H), 0.92 (d, J = 6.5 Hz, 6H). ESI MS m/z 312
[M+H]+.
[00401] Step 3: Preparation of 2-hydroxy-6-(4-
methylpentyl)benzamide
O NH2
HO ~ CH3
[00402] I ~ cH3
[00403] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 3, starting with 2-[(4-methylbenzyl)oxy]-6-(4-
methylpentyl)benzamide (Step 2, 2.8 g, 9.0 mmol).'H NMR (500 MHz,
acetone-ds): b 9.41 (s, 1 H), 7.18-7.10 (m, 1 H), 7.02 (br s, 2H), 6.74-6.70
(m,
2H), 2.84-2.78 (m, 2H), 1.58-1.48 (m, 3H), 0.92 (d, J = 6.5 Hz, 6H). ESI MS
m/z 208 [M+H]+.
[00404] Step 4: Preparation of 2-hydroxy-6-(4-
methylpentyl)benzenecarboximidamide
HN NH2
HO ~ CH3
[00405] I ~ cH3
[00406] Prepared as a white solid following a procedure similar to that
described in Example 22, Step 4, starting with 2-hydroxy-6-(4-
methylpentyl)benzamide (Step 3, 1.8 g, 9.2 mmol). ESI MS m/z 207 [M+H]+.
[00407] Step 5: Preparation of tert-butyl 3-{6-amino-5-cyano-2-[2-
hydroxy-6-(4-methylpentyl)phenyl]pyrimidin-4-yl~piperidine-1-carboxylate
[00408]
143
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
(00409] Prepared as a light yellow solid following a procedure similar
to that described in Example 22, Step 5, starting with 2-hydroxy-6-(4-
methylpentyl)benzenecarboximidamide (Step 4, 0.48 g, 2.3 mmol) and tert-
butyl 3-(2,2-dicyano-1-methoxyvinyl)piperidine-1-carboxylate (Example 13,
1.36 g, 4.7 mmol). ESI MS m/z 466 [M+H]+.
[00410] Step 6: Preparation of 4-amino-2-[2-hydroxy-6-(3-
methylbutyl)phenyl]-6-piperidin-3~-ylpyrimidine-5-carbonitrile
[00411 ]
[00412] Prepared as a light yellow solid following a procedure similar
to Example 23, starting with tert-butyl 3-{6-amino-5-cyano-2-[2-hydroxy-6-(4-
methylpentyl)phenyl]pyrimidin-4-yl}piperidine-1-carboxylate (Step 5, 0.38 g,
0.82 mmol).'H NMR (300 MHz, DMSO-ds): S 9.08-8.80 (m, 2H), 8.0 (br s, 2H),
7.13 (t, J = 7.8 Hz, 1 H), 6.76 (d, J = 7.7 Hz, 1 H), 6.67 (d, J = 7.4 Hz, 1
H), 3.40-
3.15 (m, 4H), 3.12-2.89 (m, 1 H), 2.46-2.42 (m, 2H), 2.05-1.50 (m, 5H), 0.72
(d,
J = 6.5 Hz, 6H). ESI MS m/z 366 [M+H]+. Anal. Calcd for C~,H~5N50 ~ HCI
0.75H20: C, 60.71; H, 7.16; N, 16.86. Found: C, 60.91; H, 6.91; N, 16.80.
[00413] Example 26: 4-amino-6-piperidin-3-yl-2-pyridin-2-ylpyrimidine-
5-carbonitrile
NH2
NC
~N
N
HN, Y ~N
[00414]
[00415] Step 1: Preparation of tert-butyl 3-(6-amino-5-cyano-2-pyridin-
2-ylpyrimidin-4-yl)piperidine-1-carboxylate
144
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NH2
NC
~N
N
BocN~ ~ N
[00416]
[00417] Prepared as a light yellow solid following a procedure similar
to that described in Example 22, Step 5, starting with pyridine-2-
carboximidamide (Example 15, 0.5 g, 3.2 mmol) and tent-butyl 3-(2,2-dicyano-
1-methoxyvinyl)piperidine-1-carboxylate (Example 13, 1.0 g, 3.4 mmol). ESI
MS m/z 381 [M+H]+
[00418] Step 2: Preparation of 4-amino-6-piperidin-3-yl-2-pyridin-2-
ylpyrimidine-5-carbonitrile
NHS
NC
~N
N
HN, Y ~N
[00419]
[00420] Prepared as a white solid (0.54 g, 1.92 mmol) following a
procedure similar to Example 23, starting with tert-butyl 3-(6-amino-5-cyano-2-
pyridin-2-ylpyrimidin-4-yl)piperidine-1-carboxylate (Step 1, 0.80 g, 2.1
mmol).
'H NMR (500 MHz, DMSO-ds): ~ 9.42-9.40 (m, 1 H), 9.01-8.99 (m, 1 H), 8.89-
8.88 (m, 1 H), 8.51-8.50.(m, 1 H), 8.46-6.44 (m, 1 H), 7.88-7.87 (m, 1 H),
3.59-
3.28 (m, 4H), 3.04-2.98 (m, 1 H), 1.98-1.84 (m, 4H). ESI MS m/z 281 [M+H]+.
Anal. Calcd for C,SH,6N6 ~ 2HC1 ~ 2H20: C, 46.28; H, 5.70; N, 21.59. Found: C,
46.25; H, 5.35; N, 21.61.
[00421] Example 27: 4-amino-6-piperidin-3-yl-2-pyrazin-2-ylpyrimidine-
5-carbonitrile
NHz
NC
~N
HN N ~ N
[00422] N
145
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00423] Step 1: Preparation of tart-butyl 3-(6-amino-5-cyano-2-
pyrazin-2-ylpyrimidin-4-yl)piperidine-1-carboxylate
~NSOc
N ~ CN
N' \Y 'N NH2
[00424] ~ IN
[00425] Prepared as a light yellow solid following a procedure similar
to that described in Example 22, Step 5, starting with commercially available
pyrazine-2-carboxamidine (0.13 g, 0.82 mmol) and tart-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate (Example 13, 0.20 g, 0.69 mmol). ESI
MS m/z 382 [M+H]+.
[00426] Step 2: Preparation of 4-amino-6-piperidin-3-yl-2-pyrazin-2-
ylpyrimidine-5-carbonitrile
NHS
NC
~N
H N N ~ N,
[00427] JN
[00428] Prepared as a light yellow solid following a procedure similar
to Example 23, starting with tart-butyl 3-(6-amino-5-cyano-2-pyrazin-2-
ylpyrimidin-4-yl)piperidine-1-carboxylate (Step 1, 0.2 g, 0.52 mmol). 'H NMR
(300 MHz, DMSO-d6): b 9.54 (m, 1 H), 9.23-9.20 (m, 1 H), 8.93-8.81 (m, 3H),
8.70-7.70 (m, 2H), 3.41-3.23 (m, 4H), 3.01-2.97(m, 1H), 2.05-1.75 (m, 4H). ESI
MS m/z 282 [M+H]+. Anal. Calcd for C,5H,6N6 ~ HCI ~ 0.25H~0: C, 52.18; H,
5.16;
N, 30.42. Found: C, 52.16; H, 4.99; N, 30.18.
[00429] Example 28: 4-amino-2-(6-oxo-1,6-dihydropyridin-2-yl)-6-
piperidin-3-ylpyrimidine-5-carbonitrile
146
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NH2
NC
~N
N O
HN~~N
[00430]
[00431] Step 1: Preparation of 6-bromo-2-methoxy-1,2-dihydropyridine
,CHs
Br N O
[00432] H
[00433] To a solution of 2,6-dibromopyridine (5.0 g, 21.1 mmol) in
MeOH was added NaOMe (30% in MeOH, 4 mL, 21.1 mmol). The solution was
refluxed for 14 h, then concentrated to dryness under reduced pressure. The
residue was diluted with ethyl acetate (200 mL) and washed with Hz0 (200 mL)
and brine (200 mL). The extracts were dried (Na2S04) and concentrated to
provide a colorless oil. ESI MS m/z 188 [M+H]+. The crude product was used
as is in the next reaction without purification.
[00434] Step 2: Preparation of 1,6-dihydro-6-methoxypyridine-2-
carbonitrile
NC O~CH3
[00435]
[00436] To a solution of 6-bromo-2-methoxy-1,2-dihydropyridine (Step
1, 4.0 g, 21.2 mmol) in DMF (40 mL) was added CuCN (2.3 g, 25.4 mmol) at
room temperature. The reaction mixture was heated to 150°C overnight.
The
reaction was poured into a solution of ethylenediamine in Ha0 (10%, 220 mL).
The mixture was vigorously shaken. Ethyl acetate (2 x 100 mL) was used to
extract the product. The organic extracts were combined, washed with brine,
dried (Na~S04) and concentrated. Purification by flash column chromatography
(eluent, 1:5 ethyl/hexanes) provided the product as a white solid.'H NMR (300
MHz, CDC13): b 7.68-7.63 (m, 1 H), 7.31-7.26 (m, 1 H), 6.98-6.95 (m, 1 H),
3.96
(s, 3H). ESI MS m/z 135 [M+H]+.
[00437] Step 3: Preparation of 1,6-dihydro-6-oxopyridine-2-carbonitrile
147
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NC N O
[0043] H
[00439] To a solution of 1,6-dihydro-6-methoxypyridine-2-carbonitrile
(Step 2, 1.0 g, 7.46 mmol) in acetonitrile (27 mL) cooled to 0°C was
added Nal
(1.79 g, 11.9 mmol), TMSCI (1.53 mL, 11.9 mmol) and HZO (53 pL, 2.93 mmol).
The mixture was then heated at 65°C for 60 h. The cooled reaction
mixture
was poured into 10% NaHS03 solution (100 mL). The product was extracted
with ethyl acetate (2 x 100 mL). The organic extracts were combined, washed
with brine, dried (NazS04), and concentrated. The residue was purified by
flash
column chromatography (19:1 CH2C12/MeOH) to provide a white solid.'H NMR
(300 MHz, DMSO-dfi): ~ 11.81 (s, 1 H), 7.84-7.78 (m, 1 H), 7.45 (d, J = 7.0
Hz,
1 H), 6.97 (d, J = 8.6 Hz, 1 H). ESI MS m/z 121 [M+H]+.
[00440] Step 4: Preparation of 1,6-dihydro-6-oxopyridine-2-
carboxamidine
H2N ~
N- 'O
I H
[00441] NH
[00442] To 1,6-dihydro-6-oxopyridine-2-carbonitrile (Step 3, 0.9 g,
6.71 mmol) cooled to 0°C was added LiN(TMS)2 in THF (1.OM, 8.05 mL) in
one
portion. The reaction was allowed to warm up to room temperature and was
stirred for 14 h. The reaction mixture was added to a solution of 2.ON HCI in
ether slowly to give a white viscous precipitate. Ether was evaporated and the
residual aqueous solution was basified to pH 11 using 2.ON NaOH. The
product was extracted with ethyl acetate (3 x 100 mL). The organic extracts
were combined, washed with brine, dried (Na2S04) and concentrated to provide
the product as a yellow solid.'H NMR (300 MHz, CDC13): ~ 7.56-7.48 (m, 2H),
6.70-6.65 (m, 2H), 3.78 (s, 3H). ESI MS mlz 152 [M+H]+.
[00443] Step 5: Preparation of 4-amino-2-(6-oxo-1,6-dihydropyridin-2-
yl)-6-piperidin-3-ylpyrimidine-5-carbonitrile
148
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NHz
NC ~
N
N O
HN~ ~N
[00444]
[00445] To a solution of 1,6-dihydro-6-oxopyridine-2-carboxamidine
(Step 4, 0.33 g, 2.4 mmol) in DMF (4 mL) was added NaH (60% in mineral oil,
0.23 g, 9.6 mmol), and the reaction mixture was stirred for 10 min at room
temperature. To the resulting mixture was added tert-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate (Example 13, 0.25 g, 0.86 mmol) in
DMF (2 mL) and the resulting mixture was stirred for 1 h at room temperature.
The reaction mixture was poured into a saturated aqueous solution of NH4C1
(100 mL) and the product was extracted with ethyl acetate (2 x 100 mL). The
organic extracts were combined, washed with brine (100 mL), dried (Na~S04)
and concentrated. The material was purified by Biotage HPFC (98.5:1.5 to 95:5
CH2C1~/MeOH) to provide the product as a yellow solid: ESI MS m/z 397
[M+H]+. To the yellow solid in a round bottom flask was added 4N HCI in 1,4-
dioxane (5 mL). The mixture was stirred for 45 min at room temperature. The
solvent was removed under vacuum and the residue was triturated with
MeOH/ether to provide the title compound as a light yellow solid.'H NMR (300
MHz, CD30D-d4): ~ 7.86-7.83 (m, 1 H), 7.67-7.65 (m, 1 H), 6.87-6.84 (m, 1 H),
3.64-3.31 (m, 4H), 3.27-3.12 (m, 1 H), 2.14-1.94 (m, 4H). ESI MS m/z 297
[M+H]+. Anal. Calcd for C,SH,sN60 ~ 2HC1 ~ H20: C, 46.52; H, 5.21; N, 21.70.
Found: C, 46.73; H, 5.28; N, 21.55.
[00446] Example 29: 4-amino-2-(5-bromo-2-hydroxyphenyl)-6-
piperidin-3-ylpjrrimidine-5-carbonitrile
NHS
NC
~N
HN N ~ ~ Br
[00447] Ho
149
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00448] Step 1: Preparation of 5-bromo-2-hydroxybenzamidine
Br
H2N
[00449] NH OH
[00450] To a suspension of NH4C1 (0.54 g, 10.1 mmol) in toluene
cooled to 0°C was added AI(CH3)3 (2.0 M in toluene, 5.1 mL, 10.2 mmol).
After
stirring at 0°C for 15 min, the solution was added to a round bottom
flask
charged with 5-bromo-2-hydroxybenzonitrile (0.50 g, 2.52 mmol). The reaction
mixture was heated to 80°C for 14 h. The reaction was cooled to room
temperature and poured into a slurry of silica gel (20 g) in CHC13 (100 mL).
The
slurry was stirred for 10 min at room temperature, then filtered and washed
with a solvent mixture (20% MeOH in CH2C12) and MeOH until no product
eluted out. The filtrate was concentrated to give a light yellow solid.'H NMR
(300 MHz, DMSO-d6): ~ 10.30 (br s, 1 H), 7.76 (d, J = 2.5 Hz, 1 H), 7.32-7.21
(m, 4H), 6.61 (d, J = 9.0 Hz, 1 H). ESI MS m/z 214 [M]+.
[00451] Step 2: Preparation of 4-amino-2-(5-bromo-2-hydroxyphenyl)-
6-piperidin-3-ylpyrimidine-5-carbonitrile
NHZ
NC
'N
HN N ~ ~ Br
[00452] Ho
[00453] To a solution of 5-bromo-2-hydroxybenzamidine (Step 1, 0.29
g, 1.38 mmol) in DMF was added NaH (60% in mineral oil, 4 equiv.), and the
reaction mixture was stirred for 10 min at room temperature. To the resulting
mixture was added tert-butyl 3-(2,2-dicyano-1-methoxyvinyl)piperidine-1-
carboxylate (Example 13, 0.2 g, 0.69 mmol) in DMF and the resulting mixture
was stirred for 1 h at room temperature. The reaction mixture was poured into
a saturated aqueous solution of NH4C1 and the product was extracted with ethyl
acetate. The organic extracts were combined, washed with brine, dried
150
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
(Na~S04) and concentrated. The material was purified by Biotage HPFC. To the
yellow solid in a round bottom flask was added 4 N HCI in 1,4-dioxane (5 mL).
The mixture was stirred for 45 min at room temperature. The solvent was
removed under vacuum and the residue was triturated with MeOH/ether to
provide the title compound as a light yellow solid.'H NMR (300 MHz, DMSO-
dfi): b 12.95 (s, 1 H), 9.09 (br s, 1 H), 8.85-8.71 (m, 2H), 8.43 (s, 1 H),
8.14 (br s,
1 H), 7.60 (d, J = 8.8 Hz, 1 H), 6.95 (d, J = 8.8 Hz, 1 H), 3.42-3.25 (m, 4H),
3.05-
3.02 (m, 1 H), 2.03-1.70 (m, 4H). ESI MS m/z 374 [M+H]+. Anal. Calcd for
C,6H,6BrN60 ~ HCI ~ 0.25H20: C, 46.28; H, 4.25; N, 16.87. Found: C, 46.48; H,
4.02; N, 16.66.
[00454] Example 30: 4-amino-2-(5-chloro-2-hydroxyphenyl)-6-
(piperidin-3-yl)pyrimidine-5-carbonitrile
NHS
NC
~N
i ~ CI
HN~ -N
[00455 ICI Ho
[00456] Step 1: Preparation of 5-chloro-2-hydroxybenzamidine
HEN NH
OH
[00457] cl
[00458] Prepared as a light yellow solid following a procedure similar
to that described in Example 29, Step 1, starting with 5-chloro-2-
hydroxybenzonitrile (0.5 g, 3.3 mmol). ESI MS m/z 171 [M+H]+.
[00459] Step 2: Preparation of 4-amino-2-(5-chloro-2-hydroxyphenyl)-
6-(piperidin-3-yl)pyrimidine-5-carbonitrile
151
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NHz
NC
N
HN N ~ ~ CI
[00460] Ho
[00461] Prepared as a light yellow solid following a procedure similar
to that described in Example 28, Step 5, starting with 5-chloro-2-
hydroxybenzamidine (Step 1, 0.5 g, 2.9 mmol), but purified by preparative
HPLC to give the product as the 0.75 trifluoroacetate salt.'H NMR (300 MHz,
DMSO-d6): S 12.92 (s, 1 H), 9.05 (m, 1 H), 8.85-8.71 (m, 2H), 8.31 (s, 1 H),
8.15
(br s, 1 H), 7.47 (d, J = 8.8 Hz, 1 H), 7.01 (d, J = 8.8 Hz, 1 H), 3.50-3.25
(m, 4H),
3.10-2.97 (m, 1 H), 2.03-1.70 (m, 4H). ESI MS m/z 330 [M+H]+. Anal. Calcd for
C,6H,6CIN60 ~ 0.75CF3C02H ~ 1.25H20: C, 48.01; H, 4.43; N, 16.00. Found: C,
48.19; H, 4.25; N, 15.64.
[00462] Example 31: 4-amino-2-(1 H-indol-7-yl)-6-(piperidin-3-
yl)pyrimidine-5-carbonitrile
NHS
NC
~~N HN \
HN N
[00463]
[00464] Step 1: Preparation of 1 H-indole-7-carbonitrile
CN
NH
[00465]
[00466] Prepared as a white solid following a procedure similar to that
described in Example 28, Step 2, starting with the 7-bromoindole (1.0 g, 5.1
mmol).'H NMR (500 MHz, DMSO-ds): ~ 11.99 (br s, 1 H), 7.93 (d, J = 7.8 Hz,
1 H), 7.59 (d, J = 7.7 Hz, 1 H), 7.50-7.49 (m, 1 H), 7.15 (t, J = 7.7 Hz, 1
H), 6.63-
6.62 (m, 1 H). ESI MS m/z 143 [M+H]+.
[00467] Step 2: Preparation of 1 H-indole-7-carboxamidine
152
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
NH2
~NH
NH
[00468]
[00469] Prepared as a white solid following a procedure similar to that
described in Example 29, Step 1, starting with 1H-indole-7-carbonitrile (Step
1,
0.40 g, 2.8 mmol). 'H NMR (300 MHz, MeOD-d4) 8 7.92 (d, J = 7.9 Hz, 1 H),
7.48-7.43 (m, 2H), 7.21 (t, J = 7.7 Hz, 1 H), 6.65 (d, J = 3.1 Hz, 1 H); ESI
MS
m/z 160 [M+H]+.
[00470] Step 3: Preparation of 4-amino-2-(1 H-indol-7-yl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile
NHZ
NC
~~N HN \
HN N
[00471 ] /
[00472] The above compound was prepared in 9% yield as a brown
solid (0.020 g, 0.063 mmol) following a procedure similar to that described in
Example 29, Step 5, starting with 1 H-indole-7-carboxamidine (Step 2, 0.22 g,
1.4 mmol) and tert-butyl 3-(2,2-dicyano-1-methoxyvinyl)piperidine-1-
carboxylate (Example 13, 0.20 g, 0.69 mmol).'H NMR (500 MHz, DMSO-ds): b
11.58 (s, 1 H), 8.91 (br s, 1 H), 8.67 (br s, 1 H), 8.39-8.26 (m, 2H), 7.88-
7.72 (m,
2H), 7.50-7.49 (m, 1 H), 7.17 (t, J = 7.6 Hz, 1 H), 6.60-6.59 (m, 1 H), 3.49-
3.26
(m, 4H), 3.05-3.02 (m, 1 H), 2.04-1.77 (m, 4H). ESI MS m/z 319 [M+H]+.
[00473] Example 32: 4-amino-2-chloro-6-(piperidin-3-yl)pyrimidine-5-
carbonitrile hydrochloride
~NH
NI ~ CN
HCI
[00474] CI' _N NH2
153
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00475] Step 1: Preparation of 3-cyanamino-2-cyano-3-(1-t-
butoxycarbonylpiperidyl)-propenenitrile, sodium salt
eo~
N
CN
~ CN
N ' Na+
[00476] 'cN
[00477] Metallic sodium (0.316 g, 13.74 mmol) was dissolved in EtOH
(20 mL) prior to the portionwise addition of Cyanamid (0.58 g, 13.73 mmol).
The
mixture was stirred for 30 min at room temperature, after which time the
reaction mixture turned into a white slurry. Tert-butyl 3-(2,2-dicyano-1-
methoxyvinyl)piperidine-1-carboxylate (Example 13, 4.0 g, 13.7 mmol) was
added to the reaction mixture and the resulting mixture was stirred for 1 h.
The
reaction mixture was concentrated to dryness under reduced pressure and the
residue was triturated with CHC13 to afford a white solid (3.12 g), which was
used without further purification in Step 2.
[00478] Step 2: Preparation of 4-amino-2-chloro-6-(piperidin-3-
yl)pyrimidine-5-carbonitrile hydrochloride
~NH
NI ~ CN
HCI
i
[00479] cI~N NH2
[00480] To a concentrated solution of HCI (13 mL), the solid obtained
in Step 1 was added portionwise over 24 min while sporadically cooling the
reaction mixture with an ice bath. The mixture thus obtained was stirred at
room temperature for 1 h, then diluted with water (100 mL). The precipitate
that
formed was isolated by filtration. The cake was rinsed with water and dried to
afford the title compound as a white solid. mp >300°C.'H NMR (500 MHz,
DMSO-ds): ~ 9.29-9.24 (m, 1 H), 9.01-8.93 (m, 1 H), 8.63 (br s, 1 H), 8.18 (br
s,
1 H), 3.38-3.30 (m, 2H), 3.28-3.23 (m, 1 H), 3.14-3.01 (m, 1 H), 2.97-2.76 (m,
1 H), 1.96-1.84 (m, 2H), 1.84-1.72 (m, 1 H), 1.67-1.57 (m, 1 H). ESI MS m/z
238
154
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[M+H]+. Anal. Calcd for C,oH,zNs ' HCI: C, 43.81; H, 4.78; N, 25.55. Found: C,
43.62; H, 4.68; N, 25.19.
[00481] Example 33: 1-(tert-butoxycarbonyl)indolin-2-yl-2-boronic acid
OH
I
B~OH
/ \ NH
[00482)
[00483] Step 1: Preparation of tert-butyl 1 H-indole-1-carboxylate
/ \ ~ Boc
[00484]
[00485] To a solution of indole (3.00 g, 25.6 mmol) in CH3CN (18 mL)
was added di-tert-butyl-dicarbonate (6.15 g, 28.2 mmol) and a catalytic amount
of DMAP. The solution was stirred overnight at room temperature. The reaction
mixture was diluted with cold 1 N HCI (30 mL) and extracted with Et~Ac (3 x
30 mL). The organic phase was dried (NaaS04) and concentrated under
reduced pressure. The crude product was carried forth to the next step.
[00486] Step 2: Preparation of 1-(tert-butoxycarbonyl)indolin-2-yl-2-
boronic acid
OH
I
B~OH
/ \ NBoc
[00487]
[00488] To an ice-cold solution of tert-butyl 1 H-indole-1-carboxylate
(Step 1, ~25 mmol) and triisopropyl borate (7.22 g, 38.4 mmol) in THF (32 mL)
was added a solution of 2.0 M LDA in THF (16 mL, 32.0 mmol). The reaction
mixture was stirred at 0°C for 1 h, after which the reaction was
quenched with
aqueous 2N HCI (30 mL) and extracted with CH~C12 (40 mL). The organic
phase was dried (NazS04) and concentrated under reduced pressure.
Recrystallization of the residue in 1:1 CH3CN/H~O (60 mL) gave the product as
155
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
a white solid. 'H NMR (300 MHz, CD30D) b 8.10 (d, J = 7.7 Hz, 1 H), 7.54 (d, J
= 6.3 Hz, 1 H), 7.28-7.17 (m, 2H), 6.63 (s, 1 H), 1.68 (s, 9H).
[00489] Example 34: 4-amino-2-(1 H-indol-2-yl)-6-piperidin-3-
ylpyrimidine-5-carbonitrile hydrochloride
H
[00490]
[00491] Step 1: Preparation of tert-butyl 3-(6-amino-2-chloro-5-
cyanopyrimidin-4-yl)piperidine-1-carboxylate
~NSo~
N ~ CN
i
[0049] CI~N NH2
[00493] To a slurry of 4-amino-2-chloro-6-(piperidin-3-yl)pyrimidine-5-
carbonitrile hydrochloride (Example 32, 0.50 g, 2.10 mmol) in DMF (10 mL)
was added triethylamine (0.9 mL, 6.29 mmol) and di-tert-butyl dicarbonate
(1.15 g, 5.27 mmol). The reaction mixture was stirred at room temperature
overnight, then diluted with EtOAc (100 mL). The solution thus obtained was
washed with water (3 x 10 mL), dried (Na2S04) and concentrated to dryness
under reduced pressure. The residue was triturated with MeOH to afford the
first batch of the desired product as a white solid. The filtrate was
concentrated
to dryness under reduced pressure. Purification by flash column
chromatography (eluent, 1:1 CH2Clz/hexanes to 1:1:1 CH~C12/hexanes/EtOAc)
gave a second batch of the desired product as a white solid:'H NMR (500
MHz, DMSO-ds) 5 8.53 (br s, 1 H), 8.03 (br s, 1 H), 3.98 (br s, 1 H), 3.91 (d,
J =
12.9 Hz, 1 H), 3.05-2.76 (m, 4H), 1.93-1.61 (m, 3H), 1.40 (s, 9H).
156
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00494] Step 2: Preparation of tart-butyl 2-{4-amino-6-[1-(tert-
butoxycarbonyl)piperidin-3-yl]-5-cyanopyrimidin-2-yl}indoline-1-carboxylate
soot
[00495]
[00496] A dry flask was loaded with tart-butyl 3-(6-amino-2-chloro-5-
cyanopyrimidin-4-yl)piperidine-1-carboxylate (Step 1, 0.20 g, 0.59 mmol) and
1-(tart-butoxycarbonyl)indolin-2-yl-2-boronic acid (Example 33, 0.30 g, 0.89
mmol). Then was added toluene (7.0 mL) and a 2M aqueous solution of
NaHC03 (2.5 mL). The mixture thus obtained was blanketed with argon and
sonicated. PdCl2dppf (0.039 g, 0.044 mmol) was added and the reaction
mixture was heated to 70°C overnight. The cooled reaction mixture was
diluted
with water (5.0 mL) and the resulting solution was extracted with CH2Clz (3 x
30
mL). The combined organic extracts were concentrated to dryness under
reduced pressure. Purification by flash column chromatography (eluent, 1:1
CHZCIz/hexanes to 1:1:1 CH~CI~/hexanes/EtOAc) gave the desired product as a
white solid. 'H NMR (300 MHz, DMSO-ds) b 7.99 (d, J = 8.2 Hz, 1 H), 7.70 (d, J
= 7.6 Hz, 1 H), 7.46-7.38 (m, 1 H), 7.33-7.25 (m, 1 H), 7.12 (s, 1 H), 4.12-
3.91 (m,
2H), 3.53-3.45 (m, 1 H), 3.12-2.86 (m, 2H), 2.83-2.65 (m, 1 H), 1.98-1.85 (m,
1 H), 1.83-167 (m, 2H), 1.41 (s, 9H), 1.40 (s, 9H).
[00497] Step 3: Preparation of 4-amino-2-(2,3-dihydro-1 H-indol-2-yl)-
6-piperidin-3-ylpyrimidine-5-carbonitrile
NH2 HCI
NC
i N
HN~ ~N
[00498]
[00499] To a solution of tart-butyl 2-{4-amino-6-[1-(tert-
butoxycarbonyl)piperidin-3-yl]-5-cyanopyrimidin-2-yl}indoline-1-carboxylate
157
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
(Step 2, 0.20 g, 0.38 mmol) in MeOH (4.0 mL) was added dropwise a solution
of 4N HCI in 1,4-dioxane (3.0 mL). The reaction solution was stirred for 4 h
at
room temperature, then concentrated to dryness under reduced pressure. The
residue was triturated with MeOH to afford the title HCI salt as a yellow
solid.
'H NMR (300 MHz, DMSO-ds) 5 11.62 (s, 1 H), 9.35-9.29 (m, 1 H), 8.93-8.87 (m,
1 H), 7.85 (br s, 1 H), 7.64 (d, J = 7.9 Hz, 1 H), 7.55 (d, J = 8.20 Hz, 1 H),
7.30-
7.19 (m, 2H), 7.05 (t, J = 7.2 Hz, 1 H), 3.57-3.29 (m, 4H), 3.02-2.82 (m, 1
H),
1.99-1.78 (m, 4H). ESI MS m/z 319 [M+H]+. Anal. Calcd for C,8H,8N6 ~ 1.5HC1
1.5H~0: C, 54.04; H, 5.67; N, 21.01. Found: C, 54.23; H, 5.39; N, 20.64.
[00500] Step 40: IKK-2 ICSO determination
[00501] Materials
[00502] SAM2 TM 96 Biotin capture plates were from Promega. Anti
FLAG affinity resin, FLAG-peptide, NP-40 (Nonidet P-40), BSA, ATP, ADP,
AMP, LPS (E. coli serotype 0111:B4), and dithiothreitol were obtained from
Sigma Chemicals. Antibodies specific for NEMO (IKK-y) (FL-419), IKK-1 (H-
744), IKK-2(H-470) and IKBa(C-21 ) were purchased from Santa Cruz
Biotechnology. Ni-NTA resin was purchased from Qiagen. Peptides were
purchased from American Peptide Company. Protease inhibitor cocktail tablets
were from Boehringer Mannheim. Sephacryl S-300 column was from
Pharmacia LKB Biotechnology. Centriprep-10 concentrators with a molecular
weight cutoff of 10 kDa and membranes with molecular weight cut-off of 30
kDa were obtained from Amicon. [Y'-33P] ATP (2500 Ci/mmol) and [~('-32P] ATP
(6000 Ci/mmol) were purchased from Amersham. The other reagents used
were of the highest grade commercially available.
[00503] Cloning and Expression
[00504] cDNAs of human IKK-1 and IKK-2 were amplified by reverse
transcriptase-polymerise chain reaction from human placental RNA
(Clonetech). hIKK-1 was subcloned into pFastBac HTa (Life Technologies) and
158
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
expressed as N-terminal His6-tagged fusion protein. The hIKK-2 cDNA was
amplified using a reverse oligonucleotide primer which incorporated the
peptide
sequence for a FLAG-epitope tag at the C-terminus of the IKK-2 coding region
(DYKDDDDKD). The hIKK-2:FLAG cDNA was subcloned into the baculovirus
vector pFastBac. The rhIKK-2 (S177S, E177E) mutant was constructed in the
same vector used for wild type rhIKK-2 using a QuikChangeT"" mutagenesis kit
(Stratagene). Viral stocks of each construct were used to infect insect cells
grown in 40L suspension culture. The cells were lysed at a time that maximal
expression and rhIKK activity were demonstrated. Cell lysates were stored at -
80°C until purification of the recombinant proteins was undertaken as
described below.
[00505] EnzLrme Isolation
[00506] All purification procedures were carried out at 4°C unless
otherwise noted. Buffers used are: buffer A: 20 mM Tris-HCI, pH 7.6,
containing 50 mM NaCI, 20 mM NaF, 20 mM ~i-Glycerophosphate, 500 uM
sodium orthovanadate, 2.5 mM metabisulfite, 5 mM benzamidine, 1 mM EDTA,
0.5 mM EGTA, 10% glycerol, 1 mM DTT, 1X CompIeteT"" protease inhibitors;
buffer B: same as buffer A, except 150 mM NaCI, and buffer C: same as buffer
A, except 500 mM NaCI.
[00507] Isolation of rhIKK-1 homodimer
[00508] Cells from an 8-liter fermentation of baculovirus-expressed
IKK-1 tagged with His peptide were centrifuged and the cell pellet (MOI 0.1,
I=72 h was re-suspended in 100 mL of buffer C. The cells were microfluidized
and centrifuged at 100,000 X g for 45 min. The supernatant was collected,
imidazole added to the final concentration of 10 mM and incubated with 25 mL
of Ni-NTA resin for 2 h. The suspension was poured into a 25 mL column and
washed with 250 mL of buffer C and then with 125 mL of 50 mM imidazole in
buffer C. rhIKK-1 homodimer was eluted using 300 mM imidazole in buffer C.
BSA and NP-40 were added to the enzyme fractions to the final concentration
159
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
of 0.1 %. The enzyme was dialyzed against buffer B, aliquoted and stored at -
80°C.
[00509] Isolation of rhIKK-2 homodimer
[00510] A 10-liter culture of baculovirus-expressing IKK-2 tagged with
FLAG peptide was centrifuged and the cell pellet (MOI=0.1 and I=72 h) was re-
suspended in buffer A. These cells were microfluidized, and centrifuged at
100,000 X g for 45 min. Supernatant was passed over a G-25 column
equilibrated with Buffer A. Protein peak was collected and incubated with anti-
FLAG affinity resin on a rotator overnight in buffer B. The resin was washed
in
batch with 10-15 bed volumes of buffer C. Washed resin was poured into a
column and rhIKK-2 homodimer was eluted using 5 bed volumes of buffer B
containing FLAG peptide. 5 mM DTT, 0.1 % NP-40 and BSA (concentrated to
0.1 % in final amount) was added to the eluted enzyme before concentrating in
using an Amicon membrane with a molecular weight cut-off of 30 kDa. Enzyme
was aliquoted and stored at -80°C.
[00511] Isolation of rhIKK-1/IKK-2 heterodimer
[00512] The heterodimer enzyme was produced by coinfection in a
baculovirus system (FLAG IKK-2/IKK-1 His; MOI=0.1 and I=72 h). Infected
cells were centrifuged and the cell pellet (10.0 g) was suspended in 50 mL of
buffer A. The protein suspension was microfluidized and centrifuged at
100,000 X g for 45 min. Imidazole was added to the supernatant to a final
concentration of 10 mM. The protein was allowed to bind 25 mL of Ni-NTA
resin by mixing for 2 h. The protein-resin slurry was poured into a 25 mL
column and washed with~250 mL of buffer A containing 10 mM imidazole
followed by 125 mL of buffer A containing 50 mM imidazole. Buffer A,
containing 300 mM imidazole, was then used to elute the protein. A 75 mL pool
was collected and NP-40 was added to a final concentration of 0.1 %. The
protein solution was then dialyzed against buffer B. The dialyzed heterodimer
enzyme was then allowed to bind to 25 mL of anti-FLAG M2 agarose affinity
160
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
gel overnight with constant mixing. The protein-resin slurry was then
centrifuged for 5 min at 2,000 rpm. The supernatant was collected and the
resin re-suspended in 100 mL of buffer C containing 0.1 % NP-40. The resin
was washed with 375 mL of buffer C containing 0.1 % NP-40. The protein-resin
was poured into a 25 mL column and the enzyme eluted using buffer B
containing FLAG peptide. Enzyme fractions (100 mL) were collected and
concentrated to 20 mL using an Amicon membrane with molecular weight cut-
off of 30 kDa. Bovine serum albumin was added to the concentrated enzyme to
final concentration of 0.1 %. The enzyme was then aliquoted and stored at -
80°C.
[00513] Cell Culture
[00514] The wild type (wt) human pre-B cell line, 70Z/3, and its
mutant, 1.3E2, were generously provided by Dr. Carol Sibley. Wt 70Z/3 and
1.3E2 cells were grown in RPMI 1640 (Gibco) supplemented with 7 % defined
bovine serum (Hyclone) and 50 pM 2-mercaptoethanol. Human monocytic
leukemia THP-1 cells, obtained from ATCC, were cultured in RPMI 1640
supplemented with 10% defined bovine serum, 10 mM HEPES, 1.0 mM
sodium pyruvate and 50 pM 2-mercaptoethanol. For experiments, cells were
plated in 6 well plates at 1x106 cells/mL in fresh media. Pre-B cells were
stimulated by the addition of 10 pg/mL LPS for varying lengths of time ranging
from 0-4 h. THP-1 cells were stimulated by the addition of 1 pg/mL LPS for 45
minutes. Cells were pelleted, washed with cold 50 mM sodium phosphate
buffer, pH 7.4 containing 0.15 M NaCI and lysed at 4°C in 20 mM Hepes
buffer,
pH 7.6 containing 50 mM NaCI, 1 mM EDTA, 1 mM EGTA, 1 mM sodium
orthovanadate, 10 mM (3-glycerophosphate, 1 mM NaF, 1 mM PMSF, 1 mM
DTT and 0.5 % NP40 (lysis buffer). The cytosolic fractions obtained following
centrifugation at 10,000 ?C g were stored at -80°C until used.
. [00515] Immunoprecipitation and Western Blotting
161
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
[00516] SF9 cells paste containing rhIKKs were centrifuged (100,000
X g, 10 min) to remove debris. rhIKKs were immunoprecipitated (100 pg of cell
paste) from the cell supernatant using 3 pg of anti-NEMO antibody (FL-419),
followed by coupling to protein A sepharose beads. rhIKKs were also
immunoprecipitated from affinity chromatography purified protein preparations
(1 pg) using anti-FLAG, anti-His or anti-NEMO antibodies (1-4 pg) followed by
protein A sepharose coupling. The native, human IKK complex was
immunoprecipitated from THP-1 cell homogenates (300 pg/condition) using the
anti-NEMO antibody. Immune complexes were pelleted and washed 3 times
with 1 mL cold lysis buffer. Immunoprecipitated rhIKKs were chromatographed
by SDS-PAGE (8% Tris-glycine) and transferred to nitrocellulose membranes
(Novex) and detected by chemiluminescense (SuperSignal) using specific anti-
IKK antibodies (IKK-2 H-470, IKK-1 H-744). Native IKK-2, IKBa, and NEMO
proteins from cytosolic lysates (20-80 pg) were separated by SDS-PAGE and
visualized by chemiluminescense using specific antibodies.
[00517] Phosphatase Treatment
[00518] Immunoprecipitated rhIKKs were washed 2 times in 50 mM
Tris-HCI, pH 8.2 containing 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF and 2 mM
MnCh and resuspended in 50 pL. Phosphatase (APPase, 1000 U) was pre-
diluted in the same buffer and added to the IKK samples. Following incubation
at room temperature for 30 minutes with intermittent mixing, cold lysis buffer
was added to the tubes to stop the reaction. After several washes, 10 % of the
beads were removed for Western analysis, and the remaining material was
pelleted and resuspended in 100 pL of the buffer used for the in vitro kinase
assay.
[00519] IKK-1 SAM Enzyme Assay
[00520] IKK-1 kinase activity was measured using a biotinylated I~eBa
peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-Asp-Ser32 Gly-
Leu-Asp-Ser36 Met-Lys-Asp-Glu-Glu), a SAM2 T"" 96 Biotin capture plate and a
162
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
vacuum system. The standard reaction mixture contained 5 pM biotinylated
IKBa peptide, 1 pM [y 33 P] ATP (about 1 X 105 cpm), 1 mM DTT, 50 mM KCI,
2 mM MgCla, 2 mM MnCla, 10 mM NaF, 25 mM Hepes buffer, pH. 7.6 and
enzyme solution (1-10 pL) in a final volume of 50 pL. After incubation at
25°C
for 30 min, 25 pL of the reaction mixture was withdrawn and added to a SAM2
TM 96 Biotin capture 96-well plate. Each well was then washed successively
with 800 pL 2 M NaCI, 1.2 mL of NaCI containing 1 % H3P04, 400 pL HaO, and
200 pL 95% ethanol. The plate was allowed to dry in a hood at 25°C for
1 h
and then 25 pL of scintillation fluid (Microscint 20) was added to each well.
Incorporation of [y 33P] ATP was measured using a Top-Count NXT (Packard).
Under each assay condition, the degree of phosphorylation of I~cBa peptide
substrate was linear with time and concentration for all purified enzymes.
Results from the biotinylated peptide assay were confirmed by SDS-PAGE
analysis of kinase reaction utilizing a GST-I~cBa,_54and [y 3~P] ATP. The
resulting radiolabeled substrate was quantitated by Phosphoimager (Molecular
Dynamics). An ion exchange resin assay was also employed using [y-33P] ATP
and GST-I~cBa,_54 fusion protein as the substrates. Each assay system yielded
consistent results in regard to Km and specific activities for each of the
purified
kinase isoforms. One unit of enzyme activity was defined as the amount
required to catalyze the transfer of 1 nmole of phosphate from ATP to I~cBa
peptide per min. Specific activity was expressed as units per mg of protein.
For
experiments related to Km determination of purified enzymes, various
concentrations of ATP or IKBa peptide were used in the assay at either a fixed
IKBa or ATP concentration. For IKBa peptide Km, assays were carried out with
0.1 pg of enzyme, 5 pM ATP and IKBa peptide from 0.5 to 20pM. For ATP Km,
assays were carried out with 0.1 pg of enzyme, 10 pM IKBa peptide and ATP
from 0.1 to 10pM. For Km determination of rhIKK-1 homodimer, due to its low
activity and higher Km for IKBa peptide, rhIKK-1 homodimer (0.3 pg) was
assayed with 125 pM IKBa peptide and a 5-fold higher specific activity of ATP
163
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
(from 0.1 to 10 pM) for ATP Km experiments and a 5-fold higher specific
activity
of 5 pM ATP and IKBa peptide (from 5 to 200 pM) for I~cBa peptide Km
experiments.
[00521] IKK heterodimer Resin Enzyme Assay
[00522] IKK heterodimer kinase activity was measured using a
biotinylated IKBa peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-
Asp-Ser3~ Gly-Leu-Asp-Ser36-Met-Lys-Asp-Glu-Glu) (American Peptide Co.). 20
pL of the standard reaction mixture contained 5 pM biotinylated I~eBa peptide,
0.1 pCi/reaction [y_33P] ATP (Amersham) (about 1 X 105 cpm), 1 pM ATP
(Sigma), 1 mM DTT (Sigma), 2 mM MgCla (Sigma), 2 mM MnCh (Sigma),
10 mM NaF (Sigma), 25 mM Hepes (Sigma) buffer, pH 7.6 and 20 pL enzyme
solution and 10 ~I inhibitor in a final volume of 50 pL. After incubation at
25°C
for 30 min, 150 pL resin (Dowex anion-exchange resin AG1X8 200-400 mesh)
in 900 mM formate, pH 3.0 was added to each well to stop the reaction. Resin
was allowed to settle for 1 h and 50 pL of supernatant was removed to a
Micolite-2 flat bottom plate (Dynex). 150 pL of scintillation fluid
(Microscint 40)
(Packard) was added to each well. Incorporation of [y-33P] ATP was measured
using a Top-Count NXT (Packard).
[00523] IKK-2 Resin Enzyme Assay
[00524] IKK-2 kinase activity was measured using a biotinylated IKBa
peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-Asp-Ser32 Gly-
Leu-Asp-Ser3s Met-Lys-Asp-Glu-Glu) (American Peptide Co.). 20 pL of the
standard reaction mixture contained 5 pM biotinylated IKBa peptide, 0.1
pCi/reaction [y 33P] ATP (Amersham) (about 1 X 105 cpm), 1 pM ATP (Sigma),
1 mM DTT (Sigma), 2 mM MgClz(Sigma), 2 mM MnClz(Sigma), 10 mM NaF
(Sigma), 25 mM Hepes (Sigma) buffer, pH 7.6 and 20 pL enzyme solution and
10 pL inhibitor in a final volume of 50 pL. After incubation at 25°C
for 30 min,
150 pL resin (Dowex anion-exchange resin AG1X8 200-400 mesh) in 900 mM
formate, pH 3.0 was added to each well to stop the reaction. Resin was
164
CA 02542514 2006-04-11
WO 2005/040133 PCT/IB2004/003314
allowed to settle for 1 h and 50 pL of supernatant was removed to a Micolite-2
flat bottom plate (Dynex). 150 pL of scintillation fluid (Microscint 40)
(Packard)
was added to each well. Incorporation of [y 33P~ qTP was measured using a
Top-Count NXT (Packard).
[00525] IKK-2 ICSO values obtained from the assay described above
are shown in the table below.
Example IKK-2 ICSO Example IKK-2 ICSO
No. (NM) No. (NM)
1 0.438 21 > 20
3 0.701 22 > 20
4 0.633 23 7.52
5 3.97 24 13.7
6 4.78 25 3.05
7 >20 26 > 20
8 24.4 27 > 20
9 0.316 28 > 20
1.52 29 0.439
11 3.03 30 0.324
12 16.7 31 > 20
18 > 20 32 > 20
19 > 20 34 > 20
> 20
165