Note: Descriptions are shown in the official language in which they were submitted.
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
WASTE CONVERSION PROCESS
FIELD OF USE
The present invention relates to a process for converting organic waste
materials into a
carbon-rich char material, more particularly, preparing a synthetic coal of
superior quality.
BACKGROUND OF THE INVENTION
The disposal of solid organic waste materials has been traditionally handled
by landfilling.
However, landfilling has become less of a solution to waste disposal and more
of a means of
storing waste until an effective means of disposal or utilization can be
developed. The desire
to reduce the amount of waste volume landfilled, and to avoid some of the
issues associated
with less than perfect waste containment in landfills, has led to programs for
recycling,
composting and incineration of organic waste materials. Each program brings
some benefit,
but does not represent a full solution for the waste problem. Effective
recycling requires
economic justification, and most components of the waste stream do not have
sufficient
economic value to offset their cost of separation and recovery. Composting is
effective on
some parts of the waste stream, but the majority of the waste is not amenable
to compost
production. Incineration converts the organic fraction of the waste stream to
heat energy
which can be used to generate process steam or electricity. However, the high
moisture
content, variability of composition and physical characteristics of organic
waste materials
have made incineration systems expensive, inefficient, high maintenance, and
unpopular with
the general public. What is needed is a system that can alter the chemical and
physical
characteristics of organic waste in such a way as to make its handling,
storage, and utilization
fully compatible with existing uses, technology and infrastructure. By far the
most likely and
logical end user for the calorific value of the waste stream is the fuel
industry.
Many have looked to pyrolysis as a means of chemically altering and improving
the
characteristics of urban waste. Heat is added to the waste materials in an
oxygen-free
environment, breaking the organic matter into a slate of products ranging from
mineral
1
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
matter to carbon-rich charcoal to oil mixtures to non-condensible gases and
water vapor.
Some have sought to focus on the oil byproducts, looking to the motor fuel
industry as a
potential market. However, waste pyrolysis oils are not readily compatible
with petroleum-
based liquid fuels, and therefore require extensive and expensive upgrading to
achieve that
compati bi lity.
Others have modified the process to increase production of fuel gases.
However, the
pyrolysis gases are not compatible with today's natural gas pipeline systems,
and must be used
on-site. Still others have sought to drive the pyrolysis process to its
extreme, yielding a small
quantity of concentrated solid carbon. These processes are often characterized
by high
energy consumption (low thermal efficiency), high reaction temperature, low
product yield,
long processing time and batch processing. This avenue also requires expensive
upgrading, as
the char from most pyrolysis processes using urban waste does not have the
porosity, surface
area and high chemical reactivity desired by the activated carbon market.
Other pyrolysis processes differ in the means by which reaction heat is
conveyed to the waste
materials, the source of that waste heat, the means by which solids are
conveyed within and
from the reactor, and the pyrolysis processing conditions themselves. Early
processes used
the partial combustion of the solid material to produce high temperature gases
that directly
contacted the fresh waste material. However, these processes have little
temperature
control, and produce a wide spectrum of byproducts ranging from tars and heavy
oils to light
combustible gases, alt diluted by the products of partial combustion. While
the transfer of
heat to the feed material is efficient, the handling of the byproducts is
often difficult.
Most pyrolysis processes recognize the desirability of avoiding the heating of
the waste by
direct contact with hot combustion gases, and have developed a wide range of
indirect
heating schemes. A few have involved the circulation of hot inert solids from
a combustion
reactor to the pyrolysis reactor, but most rely on the conduction of heat from
combustion
products to the waste solids through a heating surface or reactor wall. These
designs suffer
from several limitations. (1 ) Pyrolysis heating occurs for material in direct
contact with the
heating surface, but is much less effective for the remainder of the material,
(2) waste must
2
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
be well stirred in order that all waste have sufficient contact with the
heating surface, (3) for
heat to flow the wall surface must be significantly higher in temperature than
the waste
material, making the wall surface a target for high temperature corrosion, (4)
reactor designs
in which the waste containment is interior to the combustion gas containment
must be
heavily insulated to avoid loss of valuable heat through the exterior walls
rather than through
the heating surface to the waste material, (5) the temperature of the
combustion gases
leaving the reactor is always higher than the waste temperature and may be
higher than the
maximum reaction temperature, resulting in tow thermal efficiency unless some
mechanism is
provided for utilization of that heat, (6) high temperature wall surfaces may
be prone to
overheating of the mineral matter in urban waste, resulting in the production
of sticky
deposits on wall surfaces, similar to those produced in cement, lime and
taconite kilns, (7)
systems where only a portion of the waste is subject to heating at one time
often result in
end products that see a wide range of variability in the amount of pyrolysis
that has been
achieved, with some material overcooked and other material relatively raw, and
(8) these
systems are limited by the effectiveness and availability of heating surface.
A review of the
prior art discloses:
U.S. Patent No. 6,558,644 (Berman) describes a process for preparing activated
carbon from
urban waste. The waste is first stored to remove foreign materials and the
size of the waste
particles is reduced. The waste is dried under anaerobic conditions at a
temperature range
of 100 to 150°C and partially pyrolyzed in a rotating, externally
heated drum, at a
temperature of between 140 and 400°C. The product is granulated using
an extruder/mixer
and the granules are carbonized under anaerobic conditions at a temperature in
the range of
140 to 500°C. The carbonized granules are activated in the presence of
steam and
combustion gases of between 750 and 900 ° C. Finally, the activated
granules are purified by
rinsing in an aqueous hydrochloric acid solution, and subsequently drying the
activated
carbon.
U.S. Patent No. 5,194,069 (Someus) discloses a method and an apparatus for the
refinement
of organic material. Converting and processing organic material is achieved
with or without
organic and inorganic additions. The base material uses animal or plant waste
material, i.e.
3
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
slaughter-house waste and forest industry waste. A slowly rotating waste
container inside a
furnace is used. Fuel for heating the cylindrical container is a mix of
pyrolysis gases and
purchased oil or natural gas. The reaction temperature is at 1650°F,
and reduces the char to
little more than carbon. The method and apparatus produces carbon
powder/granulate as
fuel, charcoal for grilling/smoking, activated carbon, or additives for steel
production.
U.s. Patent No. 5,017,269 (Loomans et. al.) and U.S_ Patent No. 4,908,104
(Loomans et al.)
disclose a method of continuously carbonizing a mixture of primarily organic
waste material
to a high BTU char product. A stream of comminuted municipal waste material
with a
substantial organic material content is fed to one end of a mixer barrel. The
material is
compressed to form a barrel-filling mass functioning as a first vapor block,
and the work
energy required to compress the waste material and squeeze out entrapped air
is used to
raise the temperature of the material adiabatically. Air and any steam created
are vented.
The material downstream from the first vapor block is decompressed in a second
vent region.
The material is recompressed in the absence of ai r to form another vapor
block, while
exclusively utilizing the work energy required to compress it to raise the
temperature of the
material adiabatically to a volatile-releasing temperature in the neighborhood
of 400 to
600°F, and to carbonize the material. The volatiles are vented, and the
product is
discharged as a dry, particulate char. The term "adiabatic heating" suggests
that there is no
heat transfer into or out of the reaction chamber_ Loomans states that this
adiabatic
compression heating process is applicable for waste materials that have been
predried to a
moisture content in the range of 8 to 9 percent, and which have a plastic
content in the range
of 4 to 8 percent.
U.S. Patent No. 4,098,649 (Redker) discloses an apparatus and method of
converting organic
material such as that separated from municipal and industrial waste into
useful products by
using a form of an extruder in a continuous destructive distillation process,
and in which the
material being processed is compressed in the extruder in the absence of air,
and is heated to
carefully controlled temperatures in separate zones to extract different
products from each
of the zones. External electric resistive heating elements are used to apply
heat to the
outside wall of the reactor, such heat being conducted through the wall
surface. Heat
4
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
transfer is limited to the wall surface contacting the heating elements, and
the solids must be
in contact with it to be heated.
U.S. Patent No. 3,787,292 (Keappler) describes a process and apparatus for the
pyrolysis of
solid wastes including a retort defining a plurality of interior temperature
zones, a
combustion tube disposed through the retort, a means for rotating the retort
about the
combustion tube, a waste infeed, a residue outlet, and at least one fluid
exhaust
communicating with the interior of the retort. The comhmt;nn tnhP ;r
r,r,~;r;~nA~ ~~".,n +h"
center of what is essentially a rotary kiln, and is heated to 1500°F.
Heat transfer is limited to
the surface of the tube, and the solids must be in contact with it to be
heated.
The conversion process of the present invention focusses instead on the
maximizing of end
product uniformity, weight and energy yield, while minimizing reaction
temperature, energy
input and processing time. It utilizes a high capacity, continuous reactor to
produce large
quantities of synthetic coal consistent with the end use fuel market it is
intended to serve.
It is an object of the present invention to provide an improved pyrolytic
process for organic
waste materials.
It is a further object of the invention to introduce an improved process for
the preparation of
a high quality synthetic coal of uniform composition from urban waste.
It is a further object of the invention to more efficiently pyrolyze organic
waste materials, to
maximize the utilization of energy imparted to the reactor for pyrolysis.
It is a further object of the invention to introduce an effective means of
recovering byproduct
energy from the pyrolysis process, and returning it to the reactor for reuse.
It is a further object of the invention to introduce a pyrolysis system
capable of continuous
operation with high processing capacity, readily amenable to commercial scale
large volume
applications.
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
Other objects of the invention will become apparent as the description
proceeds.
SUMMARY OF THE INVENTION
The process of the present invention addresses each of these objectives.
According to the
present invention, the organic fraction of waste is converted to a high
quality synthetic coal
via an improved pyrolytic process. The synthetic coal has a high calorific
value, low moisture
content, low sulfur content, and burning characteristics similar to naturally
occurring high
volatile bituminous coals.
The invention is directed to a process for the preparation of synthetic coal
from organic
waste comprising the steps of:
a) Sorting the waste to remove foreign materials, such as metals, glass,
ceramics;
b) Reducing the size of the waste particles;
c) Pyrolysing the waste at a temperature in the range of 450 to 600°F
to produce a
granulated synthetic coal having a moisture content of 3~ or less;
d) Collecting and cooling the granulated synthetic coal;
e) Collecting and utilizing the byproduct oils and gases to produce mechanical
energy to be
imported into the pyrolysis reactor to accomplish the in-situ heating of the
organic
waste.
This process improvement is further optimized such that the extent of
pyrolysis is adjustable
by configuration of the mixing and conveying elements within the reactor,
allowing for the
optimization of yields between the desired synthetic coal and the remaining
pyrolysis
byproducts. In this process, using urban waste, the yield is set to
approximately balance the
energy content of the byproduct materials with the mechanical energy input
needs of the
reactor, including driver and other efficiency losses, minimizing the need for
external fuel or
other energy sources.
6
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
This process improvement is further optimized to include a system for
utilization of the
byproduct gases and other heat sources emanating from the reactor, as energy
sources to
create the mechanical work input necessary to drive the pyrolysis reactions,
in a highly
efficient total system.
As used herein, the following definitions are used. In-situ means that the
heat is generated
within the waste itself. Organic waste includes various types of waste
produced in the urban
and industrial environments. Such waste includes domestic waste, commercial
waste, and
industrial waste. Domestic waste includes waste produced in an average normal
household
which comprises food waste, paper products and packaging, biomass, leather,
rubber,
textiles, plastic products, wood, glass and metal. Commercial waste is the
waste produced
by the commercial sector. Much of the commercial waste is generated by food
establishments, markets, grocery stores and the like. Foreign metals means
materials that
cannot be pyrolyzed and may interfere with the process, such as metal, glass,
stones and
ceramics. Unless otherwise specified, all percentages are by weight, and all
ratios between
various process components are also by weight.
Those skilled in the art will readily recognize and and be able to utilize the
teachings herein
to manufacture char, charcoal, synthetic coal and intermediate char-containing
materials.
For purposes of discussion in this specification, the endproduct is a
synthetic coal.
For a more complete understanding of the waste conversion process of the
present invention,
reference is made to the following detailed description and accompanying
drawings in which
the presently preferred embodiment of the invention is shown by way of
example. As the
invention may be embodied in many forms without departing from the spirit of
essential
characteristics thereof, it is expressly understood that the drawings are for
purposes of
illustration and description only, and are not intended as a definition of the
limits of the
invention.
7
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
BRIEF DESCRIPTION OF THE DRAWINGS
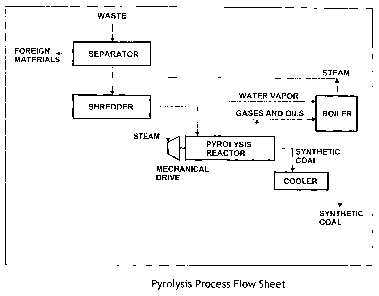
FIGURE 1 is a block diagram of the preferred embodiment of the process for
preparing
synthetic coal from organic waste of the present invention.
FIGURE 2 is a schematic diagram of the pyrolysis reactor and mechanical drive
used in the
waste conversion process for preparing synthetic coal from organic waste of
FIGURE 1.
FIGURE 3 is a comparison of the compositions of synthetic coal products
resulting from the
pyrolysis of five different Refuse Derived Fuel (RDF) sources.
FIGURE 4 is a comparison of the compositions of synthetic coal products
resulting from the
pyrolysis of a wide range of waste sources, including Refuse Derived Fuel,
Industrial Waste,
composting plant residues and automobile shredder residues.
FIGURE 5 is a comparison of the heating values of synthetic coals produced
from a wide range
of waste sources, plotted as a function of the moisture and mineral matter
content of each
feedstock.
DETAILED DESCRIPTION OF THE INVENTION
The conversion process of the present invention takes advantage of in-situ
heating, resulting
from the conversion of mechanical work to heat through the shear forces of
viscous mixing.
The heat so produced is used to drive the chemical reactions of pyrolysis.
Mixing paddles
within the reactor promote intense mixing of the waste matter, with the heat
release being
developed by interaction of the paddles with the waste and by interaction
between waste
particles themselves. As a result, there is (1 ) no performance penalty due to
limited contact
with heating surfaces, (2) uniformity of heating throughout the waste
material, resulting in a
product of uniform quality, (3) the ability to rapidly impart large quantities
of heat to the
waste material in a short period of time, resulting in a high capacity
reactor, (4) the ability to
achieve high levels of pyrolysis at a lower reactor temperature due to the in-
situ method of
8
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
heat addition, making reactor materials selection simpler, (5) avoidance of
the exterior wall
heat loss problem inherent in reactor designs that use a high temperature
external
combustion chamber to heat waste contained in an internal reaction chamber,
and (6)
avoidance of combustion product heat losses to the stack in reactors that rely
on transfer of
heat from combustion products to the waste materials. Because heat is produced
uniformly
throughout the mixing volume of the reactor, scaling of reactor size becomes a
function of
reactor volume rather than heating surface area _ Capacity of the reactor then
varies
approximately with the cube of the diameter rather than the square, as is the
case with
heated surface systems, resulting in a much greater economy of scale.
Referring now to FIGURE 1, the preferred embodiment of the system of the
present invention
for converting organic waste materials into synthetic coal product, comprises
a separator, a
shredder, a mechanical drive unit, a reactor, a boiler, and a cooler.
The separator sorts waste material suitable for pyrolysis from waste material
not suitable for
pyrolysis. The waste is then shredded to a particle size of about 5 cm in
diameter. The
shredded waste material is directly transferred to the mechanical feeder at
the drive end of
the pyrolysis reactor. The drive unit converts energy generated from the
byproduct oils and
gases in the conversion process into mechanical work necessary to drive
pyrolytic conversion
in the reactor. The mechanical drive unit is commercially available from
Murray
Turbomachinery Corp. of Burlington, Iowa.
The reactor has an exfiruder design, using a plurality of co-rotating mixers.
The mixers impart
mechanical work to the waste material as it is moves through the reactor. The
reactor
includes both a drying zone and at least one pyrolytic zone, and preferably
two mixing zones.
The waste material is heated in the drying zone and moisture is removed and
exhausted
through a first reactor vent. The pyrolytic zones are positioned downstream of
the drying
zone. Pyrolysis occurs in the following mixing zones, with a region following
each zone which
allows for the release of pyrolysis oils and gases through vents atop the
reaction vessel. The
boiler uses byproduct oils, gases, and/or vapors from the reactor to recover
energy for
9
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
recycling through the reaction process. The boiler uses vortex burners that
are commercially
available from T-Thermal of Blue Bell, Pennsylvania.
The waste material is compacted within the drying and pyrolytic zones to form
a mass of
moving treated material filling the cross section of the reactor and
functioning as a barrier to
contain vapors within these zones. Mixing mechanisms disposed within the
drying and
pyrolytic zones heat the treated material while extending the residence time
of mixing. The
reactor downstream of an oil vent includes a combination of forwarding and
reversing mixing
elements to move the treated material to a reactor discharge port.
The remaining solids are conveyed to the discharge end of the mixing reactor,
where they are
then delivered to an enclosed contact cooler, having a water cooled conveying
screw. This
cooling system reduces the temperature of the synthetic coal product in an
oxygen-free
environment, without direct contact with process water, stopping the pyrolysis
reaction while
preventing the possibility of combustion of the hot synthetic coal. The
synthetic coal cooler is
commercially available from Metso Minerals, Inc. of Milwaukee, Wisconsin.
Referring now to FIGURE 2, the reactor is a high intensity mixer extruder
design,
commercially available from B&tP Process Equipment Company of Saginaw,
Michigan. The
reactor uses multiple co-rotating mixing augurs of overlapping design to
transport the waste
through the vessel and to impart the mechanical work to the waste material.
Three mixing
zones along the reactor volume deliver the work energy to the waste material
within the
reactor to accomplish drying and pyrolysis. Within these zones, the material
is compacted to
form a mass of moving material filling the cross section of the reactor and
functioning as a
barrier to contain vapors within each zone.
Each zone includes some or all of the following screw elements in combination:
forwarding
screw sections to transport solids into the mixing zone; sequential mixing
paddles arranged in
a forwarding helical array to shred, heat and compact the solids into a plug
that fills the cross
section of the mixing chamber; an array of sequential mixing paddles arranged
at 90° to
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
impart mechanical mixing energy to the solids, but without forwarding or
compaction;
sequential mixing paddles arranged in a reversing helical array to both heat
and retard the
flow of solids, to extend the residence time of mixing; and reversing screw
sections, to
further extend the residence time of mixing and to assist in keeping the cross
section of the
mxing zone filled with solids. The configuration of screw elements varies from
zone to zone,
and with the type of feedstock being processed. The final section of the
reactor, downstream
of the third vent, contains a combination of forwarding and reversing
conveying screw
elements, to move material to the discharge end of the reactor while at the
same time
creating a barrier to contain vapors within the reactor.
The first zone heats the waste material to the range of 220 to 260° F,
to remove moisture.
The water vapor so produced is removed through a vent at the top of the
reactor,
immediately following the first mixing zone, where compaction is relieved and
the solid
material is transported past the vent without filling the reactor cross
section, so as to allow
separation of solids and vapors. Pyrolysis occurs in the following two mixing
zones, with a
region following each zone which allows for the release of pyrolysis oils and
gases through
vents atop the reaction vessel. The material temperature in the pyrolysis zone
reaches from
between 450 and 600° F. Drying and pyrolysis combined take
approximately 90 seconds. The
finished synthetic coal is transported by the mixing augurs to the discharge
port at the end of
the reactor vessel, where its temperature is reduced using a water-cooled
screw conveyor.
The byproduct oils produced by this low ternperature process are relatively
light and free
flowing, even at room temperature, contrasting them with much heavier, viscous
oils
produced by high temperature pyrolysis processes and gasification processes.
The oils
resulting from pyrolysis of the organic waste are typically low in sulfur, low
in ash, and have a
heating value in the range of 10,000 - 12,000 Btus/pound.
In the preferred embodiment of this process, byproduct oils and gases from the
two reactor
pyrolysis zone vents are conveyed in vapor phase, without cooling, directly
into a refractory-
lined combustion chamber, where they serve as the primary fuel source. Water
vapor from
the reactor drying zone vent is combined with combustion air entering this
same chamber. A
11
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
small amount of pilot fuel is used to start the byproduct oil and gas ignition
process and to
balance the energy needs of the pyrolysis process, as required. For urban
waste, however,
the process is optimized such that the energy content of the vent gases and
oils closely
approximates that needed to drive the pyrolysis reactor, when all process and
drive
inefficiencies are accounted for. In the alternative, the energy content of a
portion of the
char produced in the reactor is converted to mechanical work for the purpose
of driving
pyrolytic conversion in the reactor.
The combustor operates at a temperature in excess of 2700°F, for a
residence time in excess
of 1 second, to ensure thorough combustion of the hydrocarbons. Combustion
products enter
a boiler system optimized to maximize the energy recoverable from the
superheated steam,
while minimizing the risk of high temperature acid gas corrosion. The steam is
directed to a
condensing steam turbine mechanical drive which delivers the mechanical energy
to the
pyrolysis reactor. In the alternative, the steam turbine may drive an electric
generator,
which provides electric power for use by an electric motor drive on the
pyrolysis reactor. Any
surplus power thus generated can be used by conveyors, shredders and other
waste
separation equipment within the plant.
The synthetic coal obtained from urban waste by the process of the present
invention has a
calorific value of approximately 9,000-10,500 Btus/lb, a moisture content of
2~ or less, and a
sulfur content of approximately 0.2~. The material is granular, and may be
directly burned
or blended with natural coal for use in boilers and other combustion systems.
The following example is illustrative of a preferred embodiment of the
invention, with
reference to FIGURE 1, which is a block diagram of the process. The following
example is not
to be construed as limiting, it being understood that a skilled person may
carry out many
obvious variations to the process.
12
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
EXAMPLE 1
Initially, 2000 pounds of urban waste are sorted to remove foreign material
(FIGURE 1 ), and
shredded to produce approximately 1500 pounds of organic matter equivalent to
Refuse
Derived Fuel (RDF). The shredded waste material is fed by metered conveyor to
the feed
port of the pyrolysis reactor (see FIGURE 2), where it is conveyed and
compacted by the
internal reactor augurs, which deliver it to the first mixing zone. Here
intense mixing
converts mechanical work into direct in-situ heating of the waste materials
through shear
forces within the viscous material. During the short period where the waste is
maintained
within the first mixing zone, the temperature of the waste is increased to
approximately
260°F, liberating moisture in the form of water vapor. The waste leaves
the mixing zone,
passing into an area without compaction, which permits the vapors and solids
to separate,
with the water vapor leaving the reactor from a vent on its top surface, at a
temperature of
approximately 260 ° F.
The waste is again compacted, and transported to the first of two pyrolysis
zones. Again
intense mixing by elements of the augurs convert mechanical work into in-situ
viscous
heating, raising the temperature of the waste materials to approximately 450
to 500°F. After
approximately 30 seconds, the partially pyrolized waste has been conveyed
through the
second mixing zone, where it again enters a zone without compaction. Here
pyrolysis
byproduct oils and gases are liberated from the solids and leave the reactor
in vapor phase
through a second vent port on the top.
A third stage of compaction and mixing occurs, raising the temperature of the
waste
materials to approximately 500 to 600°F, more fully pyrolyzing the
waste materials. After
approximately 30 seconds of in-situ heating and pyrolysis, the material is
conveyed to a final
uncompacted de-gassing zone, in which byproduct oil and gas vapors are
liberated. A third
vent port on the top of the reactor allows for the removal of these byproduct
materials. The
remaining solids are conveyed to the discharge end of the mixing reactor,
where they are
then delivered to an enclosed contact cooler, having a water cooled conveying
screw. This
cooling system reduces the temperature of the synthetic coal product in an
oxygen-free
13
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
environment, without direct contact with process water, stopping the pyrolysis
reaction while
preventing the possibility of combustion of the hot synthetic coal. Since the
cooling water
does not contact the char directly, a potential waste water stream is
eliminated. In the
preferred embodiment of this process, heat recovered from the cooling system
is utilized for
boiler feedwater heating.
The solid product of pyrolysis, weighing about 830 pounds, is similar in
appearance to
granular coal, has a moisture content of less than 29, a calorific value of
approximately 9,500
Btuslpound, and a sulfur content of approximately 0.29. It is easily
ignitable, and exhibits a
burning profile similar to high volatile bituminous coat. The material may be
transported,
stored, pulverized and burned in a manner similar to natural coal, and may
also be blended
with natural coal for boiler and other combustion applications.
EXAMPLE 2
An industrial waste of approximately equal parts of cardboard, waste wood and
mixed
plastics, was shredded and fed to the reactor. The solid product of pyrolysis,
approximately
54.8 percent by weight of the initial feedstock, was granular in nature, has a
moisture
content of approximately 1.19, a calorific value of approximately 11,470
Btus/lb, and a sulfur
content of approximately 0.069. The oils produced from this feedstock exhibit
a specific
gravity of 1.12, and a viscosity of 5.1 centipoise at 60°F, roughly
comparable to kerosene.
EXAMPLE 3
A mixture of composting plant reject materials, including sand, grit, broken
glass, as well as
cardboard containers, mixed plastics, leather goods, soiled diapers and other
waste
materials, was shredded and fed to the reactor. The solid product of
pyrolysis, approximately
64 percent by weight of the initial feedstock, is granular in nature, has a
moisture content of
0.69, a calorific value of approximaterly 8,150 Btus/pound, and a sulfur
content of
approximaterly 0.2~. The oils produced from this feedstock are free flowing at
room
temperature, and exhibit a moisture-free calorific value of approximately
11,650 Btus/pound.
14
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
EXAMPLE 4
A sample of commercial automotive shredder residue was sorted to remove tramp
metal,
shredded and fed to the reactor. The solid product of pyrolysis, approximately
80.6 percent
by weight of the initial feedstock, is granular in nature, nas a moisture
content of 0.54, a
calorific value of approximately 6,650 Btus/pound, and a sulfur content of
0.2~.
Because of the uniform heating and intense mixing that are achieved in this
process, the char
produced is very uniform in composition, and feedstocks of similar origin
produce very similar
char products. FIGURE 3 plots the ultimate analysis (weight percent Carbon,
Hydrogen,
Oxygen, Nitrogen, Sulfur, Moisture and Ash) and higher heating value of chars
produced from
various commercial Refuse Derived Fuels (RDF). The samples show remarkable
similarity in
composition and heating value.
The in-situ viscous heating method allows for successful operation on a much
wider range of
waste materials than are suitable for many other processes. These waste
materials include
industrial wastes, compost stabilate, automobile shredder residue, tire chips,
waste water
treatment sludge, and other organic waste sources. Synthetic coal products
have been
successfully made with feedstocks ranging from 4 to 56 percent moisture, 2 to
57~ ash, and
from zero to about 30~ plastics. Again the remarkable uniformity of
composition of the
synthetic coal produced by this process is seen, when coal products from a
wide range of
waste sources are compared. FIGURE 4 plots the elemental components and higher
heating
value of synthetic coals produced from five different RDF samples, one mixed
industrial
waste, three composting plant waste mixtures and three automobile shredder
residue
sources. When expressed on a moisture- and ash-free basis, the compositions of
these
synthetic coals appear very similar.
The consistancy of residence time, temperature and intense mixing inherent in
this process
allows for fairly accurate prediction of the fuel qualities for a given
synthetic coal, if the
mineral matter and moisture content of the waste sample is known. FIGURE 5
illustrates the
higher heating value observed in of a number of actual char products, as
plotted against their
CA 02543320 2006-04-21
WO 2005/049530 PCT/US2004/038447
feedstock moisture and ash (mineral matter) content. As an example, U.S,
refuse derived
fuel having a moisture content of 21~ and an ash content of 12~ by weight
produces a
synthetic coal having a higher heating value of approximately 9,500 Btus/lb. A
very high
moisture municipal waste from a Mediterranean source produced a coal of
roughly
comparable heating value, while a dry, low-ash industrial waste high in
plastics content
produced a coat of superior heating value. Low grade wastes such as automobile
shredder
residue produced synthetic coal of lesser quality.
Throughout there are various patents referenced by patent number and inventor.
The
disclosures of these patents in their entireties are hereby incorporated by
reference into this
specification in order to more fully describe the state-of-the-art.
It is evident that many alternatives, modifications, and variations of the
waste conversion
process of the present invention will be apparent to those skilled in the art
in light of the
disclosure herein. It is intended that the metes and bounds of the present
invention be
determined by the appended claims rather than by the language of the above
specification,
and that all such alternatives, modifications, and variations which form a
conjointly
cooperative equivalent are intended to be included within the spirit and scope
of these
claims.
16