Note: Descriptions are shown in the official language in which they were submitted.
CA 02543629 2006-04-25
WO 2005/047275 1 PCT/EP2004/011695
2-Phenylbenzofuran derivatives, a process for preparing them, and their
use.
A, large number of antibiotics are employed therapeutically for treating
infectious diseases caused by bacteria. However, the pathogens are
becoming increasingly resistant to the drugs employed; there is even the
threat of serious danger arising due to what are termed muitiresistant
organisms, which have not merely become resistant to single antibiotic
groups, such as ~3-lactam antibiotics, glycopeptides or macrolides, but in
fact carry several resistances simultaneously. There even exist pathogens
which have become resistant to all the antibiotics which are commercially
available. It is no longer possible to treat infectious diseases which are
caused by these organisms. For this reason, there is a great need for new
compositions which can be used against resistant organisms. While many
thousand antibiotics have been described in the literature, most of them are
too toxic to be used as drugs.
The cell walls of Gram-positive and Gram-negative bacteria consist for the
most part of peptidoglycan (murein), which, as what is termed the murein
sacculus, encloses the cell completely and lends its mechanical stability as
well as helping to determine its morphological form. Peptidoglycan is a
macromolecule which is composed of an alternating sequence of the 1,4-~3-
glycosidically linked amino sugars N-acetylglucosamine and N-
acetylmuramic acid. Crosslinkings by way of short peptide bridges provide
the sugar chains with a high degree of stability. The biosynthesis of
peptidoglycan is catalyzed by a number of enzymes which are dissolved in
cytoplasm or else membrane-bound. Many of these enzymes are specific
to bacteria and represent ideal points of attack in the search for new
antibiotics.
The bifunctional bacterial N-acetylglucosamine-1-phosphate uridyl-
transferase (GImU) catalyzes the formation of UDP-N-acetylglucosamine
from glucosamine-1-phosphate in a two-step reaction. UDP-N-acetyl-
glucosamine is a fundamental building block in bacterial cell wall
biosynthesis and inhibiting its formation is therefore a promising route for
finding novel antibacterial therapeutic agents (Sulzenbacher et al., J. Biol.
Chem. 2001, 276 (15), 11844-11851 ).
CA 02543629 2006-04-25
2
There have already been reports of compounds from cultures of Aspergillus
flavipes, such as flavipin (Raistrick et al., Biochem. J. 1956, 63, 395),
which
has been described as being an inhibitor of the respiratory chain, and the
phytotoxin flavipucine (Findlay et al., J.C.S. Perkin I 1972, 2071) or the
compound F-90558 (Kuraya et al., JP 20012862292 A2), which have been
described as being antineoplastic agents. Pyrimidin-2-ylamine-substituted
phenylbenzofurans are described, for example, in Burri et al.,
WO 02/10156.
It has now been found, surprisingly, that the strain Aspergillus flavipes
ST003878 (DSM 15290) is able to form novel antibiotics which are effective
inhibitors of GImU and are suitable for use as model structures for
developing additional agents possessing antibacterial activity.
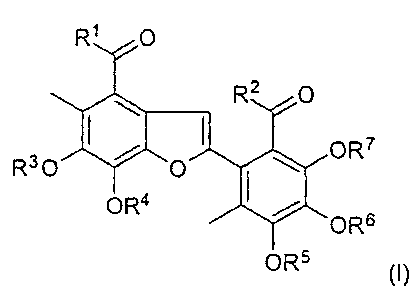
The invention relates to a compound of the formula (I)
R2 O
OR7
R O ~ ~0
ORS ~ ~ s
Y ~OR
ORs (1)
where
R~ and R2 are, independently of each other, H, OH or -O-(C~-C6)-alkyl,
and
R~, R4, R5, R6 and R~ are, independently of each other, H, -(C~-Cg)-alkyl
or-C(O)-(C~-Cg)-alkyl,
or a physiologically tolerated salt of a compound of the formula (I).
(C~-Cg)-Alkyl denotes a straight-chain or branched hydrocarbon group
having 6 carbon atoms, for example methyl (Me), ethyl, n-propyl, isopropyl,
tert-butyl or n-hexyl, preferably methyl.
CA 02543629 2006-04-25
3
Preference is given to R~ and R2 in the compound of the formula (I) being
H.
Preference is furthermore given to R3, R4, R5, R6 and R7 in the compound
of the formula (I) being, independently of each other, H or methyl.
Particular preference is given to R~ and R2 in the compound of the formula
(I) being H and to R3, R4, R5, R6 and R~ being, independently of each
other, H or methyl.
In addition, the invention relates to a compound of the formula (I) which is
characterized by a compound of the formula (1l)
H, ~O
,~ H O
OH
HO ~ ~O ~ ~
OH ~ OH,
OH ~~~)~
a compound of the formula (III)
H, , o
H O
OH
HO ~ ~O
OH
home
OH X111),
CA 02543629 2006-04-25
4
a compound of the formula (IV)
H_ ,o
H O
' OH
Ho ~ 'o
OMe ~ pH
OMe
and a compound of the formula (~
H_ ,o
H O
HO I ~ Q I I ~ OH
OMe
OMe
OMe
The present invention furthermore relates to all obvious chemical
equivalents of the compounds of the formula (I) according to the invention.
These equivalents are compounds which exhibit a minor chemical
difference, and consequently have the same effect, or are converted, under
mild conditions, into the compounds according to the invention. Said
equivalents also include, for example, salts, reduction products, oxidation
products, esters, ethers, acetals or amides of the compounds of the
formula (I) as well as equivalents which the skilled person can prepare
using standard methods, and, in addition, all optical antipodes and
diastereomers, and all stereoisomeric forms.
CA 02543629 2006-04-25
Physiologically tolerated salts of compounds of the formula (I) are
understood as being both their organic salts and their inorganic salts as are
described in Remington's Pharmaceutical Sciences (17th Edition, page
1418 (1985)). Because of their physical and chemical stability, and their
5 solubility, sodium salts, potassium salts, calcium salts and ammonium salts
are preferred, inter alia, for acid groups; salts of hydrochloric acid,
sulfuric
acid and phosphoric acid, or of carboxylic acids or sulfonic acids, such as
acetic acid, citric acid, benzoic acid, malefic acid, fumaric acid, tartaric
acid
and p-toluenesulfonic acid, are preferred, inter alia, for basic groups.
The compounds of the formula (I) according to the invention are unrelated
to conventional antibiotics such as the ~3-lactams (penicillins and
cephalosporins), aminoglycosides (streptomycin), macrolides
(erythromycin), quinolones (ciprofloxacin), sulfonamides and glycopeptides
(vancomycin).
The invention furthermore relates to a process for preparing a compound of
the formula (I), which comprises
1. fermenting the microorganism Aspergillus flavipes ST003878 (DSM
15290), or one of its variants andlor mutants, in a culture medium
until one or more compounds of the formula (I) accrues in the culture
medium, and
2. isolating a compound of the formula (I) from the culture medium, and
3. where appropriate derivatizing the compound of the formula (I)
and/or converting it into a physiologically tolerated salt.
The invention preferably relates to a process for preparing a compound of
the formula (I) wherein, in step 1, the compound of the formula ((l) is
formed during the fermentation, in step 2, the compound of the formula (II)
is isolated and, in step 3, it is derivatized, where appropriate, to give a
compound of the formula (I} and/or converted into a physiologically
tolerated salt.
The process comprises culturing Aspergillus flavipes ST003878 (DSM
15290) under aerobic conditions in a culture medium which contains a
carbon source, a nitrogen source, inorganic salts and, where appropriate,
trace elements. In this culture medium, Aspergillus flavipes ST003878
(DSM 15290) forms a mixture of compounds of the formula (I). The
CA 02543629 2006-04-25
6
quantitative proportion of one or more of the compounds according to the
invention can be varied in dependence on the composition of the culture
medium. In addition, the composition of the medium can be used to drive
the synthesis of individual compounds.
Suitable carbon sources for the fermentation are assimilible carbohydrates
and sugar alcohols, such as glucose, lactose, sucrose, glycerol, starch or
D-mannitol, and also carbohydrate-containing natural products, such as
malt extract and yeast extract. Examples of nitrogen-containing nutrients
are amino acids; peptides and proteins and also their breakdown products,
for example casein, peptones or tryptones; meat extracts; yeast extracts;
gluten; ground seeds, for example from maize, wheat, oats, beans,
soybean or the cotton plant; distillation residues from alcohol production;
meat meals; yeast extracts; ammonium salts; nitrates. Preference is given
to the nitrogen source being one or more peptides which have been
obtained synthetically or biosynthetically. Examples of inorganic salts are
chlorides, carbonates, sulfates or phosphates of the alkali metals or
alkaline earth metals, iron, zinc, cobalt and manganese. Examples of trace
elements are cobalt and manganese.
The culture medium preferably contains glucose, starch, rolled oats and/or
glycerol.
The compounds according to the invention are particularly preferably
formed in a nutrient solution which contains from about 0.05 to to
5°!°,
preferably from 2 to 3%, of potato starch and from about 0.05 to
3°!°,
preferably from 0.05 to 1 %, of yeast extract. The values in percent are in
each case based on the weight of the total nutrient solution.
The microorganism is cultured aerobically, i.e., for example, submerged
while being shaken or stirred in shaking flasks or fermenters, or on solid
medium, where appropriate while introducing air or oxygen. The
microorganism can be cultured in a temperature range of from about 15 to
35°C, preferably at from about 20 to 30°C, in particular at
25°C. The pH
range should be between 3 and 10, preferably between 4.5 and 6.5. In
general, the microorganism is cultured under these conditions over a period
of from 2 to 30 days, preferably of from 72 to 360 hours. The organism is
advantageously cultured in several steps, i.e. one or more preliminary
CA 02543629 2006-04-25
7
cultures are initially prepared in a liquid nutrient medium, with these
preliminary cultures then being inoculated into the actual production
medium, i.e. the main culture, for example in a volume ratio of 1:10 to
1:100. The preliminary culture is obtained, for example, by inoculating the
mycelium into a nutrient solution and allowing it to grow for from about 72
to 360 hours, preferably from 96 to 240 hours. The mycelium can be
obtained, for example, by allowing the strain to grow for from about 1 to 20
days, preferably from 6 to 10 days, on a solid or liquid nutrient substrate,
for example yeast malt agar, rolled oats agar or potato dextrose agar.
The course of the fermentation, and the formation of the compounds of the
formula (I) according to the invention, can be monitored in accordance with
the methods which are known to the skilled person, for example by means
of detecting biological activity in bioassays or by means of chromatographic
methods, such as thin layer chromatography (TLC) or high performance
liquid chromatography (HPLC).
The fungus Aspergillus flavipes ST003878 (DSM 15290) is able, for
example, to form compounds of the formula (I) by means of a surface
culture or stationary culture on solid nutrient substrates. Solid nutrient
substrates are prepared by, for example, adding agar or gelatin to aqueous
nutrient media. However, it is also possible to obtain the compound of the
formula (I) by means of fermenting the fungus Aspergillus flavipes
ST003878 (DSM 15290) while submerged, i.e. in an aqueous suspension,
with the compound (I) usually being located in the culture cell mass. It is
therefore expedient to separate off the fermentation solution by means of
filtration or centrifugation. The filtrate is extracted using an absorption
resin
as the solid phase. The mycelium and the surface culture are expediently
extracted with an organic solvent which is miscible, or partially miscible,
with water, for example a (C~-C4)-alcohol, preferably methanol or
2-propanol.
While the extractions can be carried out over a wide pH range, it is
expedient to carry them out in a neutral or weakly acid medium, preferably
between pH 3 and pH 7, where appropriate in the added presence of a
reducing agent. The extracts can, for example, be concentrated and dried
in vacuo.
CA 02543629 2006-04-25
8
One method of purifying the compounds according to the invention is that
of solution distribution between a stationary and a mobile phase, in a
manner known per se, for example by means of chromatography on
adsorption resins, for example on Diaion~ HP-20 (Mitsubishi Casei Corp.,
Tokyo), on Amberlite~ XAD 7 (Rohm and Haas, USA) or Amberchrom~
CG, (Toso Haas, Philadelphia, USA). Numerous reverse, phase supports,
for example RPg and RP~g, as are well known within the context of high
pressure liquid chromatography (HPLC), are also suitable.
Another possibility for purifying the antibiotic according to the invention is
that of using what are termed normal-phase chromatography supports, for
example silica gel or AI203, or others, in a manner known per se.
An alternative isolation method is that of using molecular sieves, such as
Fractogel~ TSK HW-40 (Merck, Germany) or Sephadex~ G-25 (Amersham
Biosciences), in a manner known per se. In addition to this, it is also
possible to use crystallization to isolate biflavipin from enriched material.
Organic solvents and their mixtures, either anhydrous or with water added,
are, for example, suitable for this purpose. An additional method for
isolating and purifying the antibiotics according to the invention consists in
using anion exchangers, preferably in a pH range from 4 to 10, and cation
exchangers, preferably in a pH range of from 2 to 5. The use of buffer
solutions to which portions of organic solvents have been added is
particularly suitable for this purpose.
An isolate of the microorganism strain Aspergillus tlavipes ST003878 was
deposited, in accordance with the rules of the Budapest Treaty, in the
Deutsche Sammlung von Mikroorganismen and Zellkulturen [German
collection of microorganisms and cell cultures] GmbH (DSMZ),
Mascheroder Weg 1 B, 38124 Brunswick, Germany, under the number
DSM 15290.
The fungus Aspergiilus flavipes ST003878 (DSM 15290) has a white
substrate mycelium. During sporulation, the color of the mycelium changes
to yellow to brown. Cultures on Czapek agar medium which ace about 10
days of age form large amounts of yellow to brownish exudates. The
conidia are colorless and round, having a diameter of 2-3 Nm The primary
CA 02543629 2006-04-25
9
and secondary sterigmata are of similar length (5-6 Nm) and are brown in
color.
Instead of the strain Rspergillus flavipes ST003878 (DSM 15290), it is also
possible to use one of its mutants and/or variants which is able to produce
one of the compounds of the formula (I) according to the invention.
A mutant is a microorganism in which one or more genes in the genome
have been modified, with the gene or the genes which is/are responsible, in
the case of the present invention, for the ability of the organism to produce
one or more of the compounds of the formula (I) remaining functional and
inheritable.
These mutants can be generated, in a manner known per se, using
physical means, for example irradiation, as with ultraviolet rays or x-rays,
or
using chemical mutagens, such as ethyl methanesulfonate (EMS);
2-hydroxy-4-methoxybenzophenone (MOB) or N-methyl-N'-nitro-N-
nitrosoguanidine (MNNG), or as described by Brock et al. in "Biology of
Microorganisms, Prentice Hall, pages 238-247 (1984).
A variant is a phenotype of the microorganism. Microorganisms have the
ability to adapt to their environment and therefore exhibit pronounced
physiological flexibility. In the case of phenotypic adaptation, alf the cells
of
the microorganism are involved, with the nature of the change not being
genetically conditioned and being reversible under altered circumstances
(H. Stolp, Microbial ecology: organism, habitats, activities. Cambridge
University Press, Cambridge, GB, page 180, 1988).
Screening for mutants and/or variants which synthesize one or more of the
compounds according to the invention is effected in accordance with the
following scheme:
- lyophilization of the fermentation medium;
- extraction of the lyophilizate with an organic solvent
- extraction of a compound from the culture filtrate using solid phases
- analysis by means of HPLC or TLC or by means of testing the
biological activity.
CA 02543629 2006-04-25
The above-described fermentation conditions apply both for Aspergillus
flavipes ST003878 (DSM 15290) and for mutants andlor variants thereof.
Derivatizations are carried out as follows: hydroxyl groups of the compound
5 of the formula (I) (R3 to R7 are, independently of each other, H) can be
esterified using an activated acid, preferably an acid chloride CI-C(O)-(C~-
Cg)-alkyl (R3 to R7 are, independently of each other, -C(O)-(C~-Cg)-alkyl).
In addition, hydroxyl groups can be etherified by, for example, reaction with
a (C~-Cg)-alkyl halide in acid medium or by reaction with a trimethylsilyl-
10 diazo-(C~-Cg)-alkyl compound, preferably with trimethylsilyldiazomethane.
A phenylic aldehyde function (R~ and/or R2 is H) is, for example, oxidized
to an acid function using manganese oxide or using Jones reagent (sulfuric
chromium(VI) oxide). The acid function which is formed in this connection
can be esterified by means of 4-dimethylaminopyridine (4-DMAP) under
Steglich conditions or with a (C~-Cg)-alcohol in acid medium. Said
derivatizations are examples of methods which are known per se and are
described, inter alia, in J. March, Advanced Organic Chemistry, John Wiley
& Sons, 4th Edition, 1992. In order to carry out the reactions selectively, it
may be advantageous to introduce protecting groups, in a manner known
per se, prior to the reaction. The protecting groups are eliminated after the
reaction and the reaction product is then purified.
The compounds according to the invention are powerful inhibitors of
bacterial N-acetylglucosamine-1-phosphate uridyltransferase (GImU); they
are therefore suitable for the treatment and/or prophylaxis of diseases
which are caused by bacterial infections or mycoses.
The inhibition of the GImU can be determined in a biochemical test using
the following method:
The assay was carried out in a 384-plate format. The reaction volume was
p1 per test. The reaction mixture contained 10 NI of test substance and
15 NI of substrate solution consisting of acetyICoA and D-glucosamine-1-
phosphate. The enzyme reaction was started by adding 15 NI of GImU
35 enzyme solution. After 60 minutes of incubation at 30°C, the
reaction was
stopped by adding 10 NI of developer reagent (DTNB dissolved in
guanidine). The plates were then centrifuged (1 min, 1000 rpm) and stored
for a further 45 minutes at room temperature before the absorption was
CA 02543629 2006-04-25
11
read at 405 nm in a photometer. In order to be able to calculate the
inhibitory activity of the test compounds, positive controls (signal with
GImU) and negative controls (signal without GImU) were included on each
plate. In order to exclude falsely positive samples, which could be caused
by nonspecific substance effects (e.g. color), blank plates, which contained
the substances but no GImU, were included in parallel with the test plates.
After correction for the blanks, the inhibitory activities of the substances
were calculated in accordance with the following formula:
Inhibition (%) = 100 x [1-(OD405 nm substance/OD405 nm positive control)]
An inhibition constant, ICSp, of 1 NM was determined for the inhibitory effect
of the compound of formula (II) on the enzyme N-acetylglucosamine-1-
phosphate uridyltransferase (GImU).
The invention therefore furthermore relates to the use of a compound of the
formula (I), preferably of a compound of the formula (1l), or of a
physiologically tolerated salt thereof, for producing a pharmaceutical in
human or animal medicine, in particular for producing a pharmaceutical for
the treatment and/or prophylaxis of bacterial infectious diseases and/or
mycoses.
In addition, the present invention relates to a pharmaceutical having a
content of at least one compound of the formula (I) according to the
invention, preferably having a content of the compound of the formula (II).
Said pharmaceutical is produced by mixing at least one compound of the
formula (I) with one or more pharmacologically suitable auxiliary
substances or carrier substances and bringing the mixture into a form
suitable for administration.
While the pharmaceuticals according to the invention can be administered
orally or parenteraliy, it is also in principle possible to use them rectally.
Examples of suitable solid or liquid galenic preparation forms are granules,
powders, tablets, sugar-coated tablets, (micro)capsules, suppositories,
syrups, emulsions, suspensions, aerosols, drops or injectable solutions in
ampoule form, and also preparations giving a protracted release of active
compound, in the production of which use is customarily made of
CA 02543629 2006-04-25
12
pharmacologically suitable carrier substances or auxiliary substances, such
as disintegrants, binders, coating agents, swelling agents, gliders,
lubricants, flavorings, sweeteners or solubilizers, for example magnesium
carbonate, titanium dioxide, lactose, mannitol and other sugars, talc,
lactalbumin, gelatin, starch, vitamins, cellulose and its derivatives, animal
or vegetable oils, polyethylene glycols and solvents, such as water,
alcohols, glycerol and polyhydric alcohols.
Where appropriate, the dosage units for oral administration can be
microencapsulated in order to delay the release, or extend it over a longer
period, as, for example, by means of coating or embedding the active
compound, in particle form, in suitable polymers, waxes or the like.
0.1-1000, preferably 0.2-100 mg/kg of body weight islare administered as
an expedient dose. These quantities are expediently administered in
dosage units which at least contain the effective daily quantity of the
compounds according to the invention, e.g. 30-3000, preferably 50-
1000 mg.
The daily dose to be administered depends on the body weight, age, sex
and condition of the mammal. However, higher and lower daily doses may
sometimes also be appropriate. The daily dose can be administered both
by means of a once-only administration in the form of a single dosage unit,
or in several smaller dosage units, and by means of the multiple
administration of subdivided doses at predetermined intervals.
The following examples are intended to clarify the invention without limiting
the scope of the invention in any way.
Percentage values relate to the weight. Mixing ratios in the case of liquids
relate to volume unless otherwise indicated.
Example 1: Preparing a glycerol culture of Aspergillus flavipes ST003878
(DSM 15290)
30 ml of nutrient solution (malt extract, 2.0°I°, yeast extract,
0.2%, glucose,
1.0%, (NH4)2HP04, 0.05%, pH 6.0) were inoculated, in a sterile 100 ml
Erlenmeyer flask, with the strain Aspergillus flavipes ST003878 (DSM
CA 02543629 2006-04-25
13
15290) and incubated for 6 days, at 25°C and 140 rpm, on a rotating
shaker. 1.5 ml of this culture were subsequently diluted with 2.5 ml of 80%
glycerol and stored at -135°C.
Example 2: Preparing a preliminary culture of Aspergillus flavipes
ST003878 (DSM 15290) in an Erlenmeyer flask.
100 ml of nutrient solution (malt extract, 2.0%, yeast extract, 0.2°l0,
glucose,
1.0%, (NH4)2HP04, 0.05%, pH 6.0) are inoculated, in a sterile 300 ml
Erlenmeyer flask, with the strain Aspergillus flavipes ST003878 (DSM
15290) and incubated for 4 days, at 25°C and 140 rpm, on a rotating
shaker. 2 ml of this preliminary culture are then inoculated for the purpose
of preparing the main cultures.
Example 3: Fermenting Aspergillus flavipes ST003878 (DSM 15290) in an
Erlenmeyer flask
In each case 100 ml of nutrient solution (potato dextrose broth, 2.4%, yeast
extract, 0.2%, pH 5.2) are inoculated, in a sterile 300 ml Erlenmeyer flask,
with the strain Aspergillus flavipes ST003878 (DSM 15290) and incubated
for 11 days, at 25°C and 140 rpm, on a rotating shaker. 300 ml of this
preliminary culture are then inoculated for the purpose of preparing the
main cultures.
Example 4: Fermenting Aspergillus flavipes ST003878 (DSM 15290) in a
fermenter
A 10 I fermenter is operated under the following conditions:
Nutrient medium: 24 g of potato dextrose broth/I
2 g of yeast extractJl
pH 5.2 (prior to sterilization)
Incubation period: 166 hours
Incubation temperature: 28°C
Stirrer speed: 180 rpm
Aeration: 16 L min ~
CA 02543629 2006-04-25
14
Foam formation was suppressed by repeatedly adding an ethanolic
solution of polyol. The production maximum was reached after approx. 160
hours.
Example 5: Isolating the compound of the formula (II)
25 liters of culture solution, obtained as described in example 4, contained
90 mg of the compound of the formula (II). The culture broth was filtered
and the mycelium (1.65 kg) was extracted with 10 liters of 2-propanol. The
clear alcoholic phase was concentrated down in vacuo to 2 I and, after 1 g
of ascorbic acid had been added as antioxidant, loaded onto a column of
785 ml in volume which was filled with the adsorption resin MCI Gel
CHP20P. Column dimensions: width x height: 10 cm x 25 cm. The column
was eluted with a solvent gradient of from 5% acetonitrile in water to 100%
acetonitrile and a column throughput of 15 liters per hour. Fractions which
each contained 2.5 liters were collected. Fractions 7 to 9, which contained
the compound of the formula (II), were checked by HPLC analyses, pooled
and concentrated in vacuo. The pH of the solution, which had to a large
extent been freed from the isopropanol, was also adjusted to 4.2 and once
again loaded onto an MCI Gel~ CHP20P column (490 ml, column
dimensions: 5 cm x 25 cm). The elution was effected using a gradient of
from 0.01 % ammonium formate (pH 4.2) to 100°l° acetonitrile.
With the
column throughput being 40 ml per minute, fractions containing 200 ml
were taken. Fractions 12 and 13 contained the enriched compound of the
formula (II). They were combined, concentrated in vacuo and, for the
purpose of further chromatographic purification, separated on a Luna 10N
C18 phenomenex~ column (column dimensions: 21 mm x 250 mm).
0.025% trifluoroacetic acid and from 0% to 100% acetonitrile were used as
the eluent, with the flow-through rate being 25 ml/minute. The fraction size
was 50 ml. Fraction 14 contained the greatly enriched compound of the
formula (II); it was slightly concentrated in vacuo and left to stand at
+4°C.
The lemon-yellow compound of the formula (II) crystallized out and was
filtered off, dried and bottled under argon (20 mg).
Example 6: High pressure liquid chromatography (HPLC) of the compound
of the formula (II)
Column: YMC-PRO 18~, 120°A, 250-4.4, with precolumn,
CA 02543629 2006-04-25
Mobile phase: A: 5% acetonitrife in 0.02°l° trifluoroacetic
acid, 2
minutes,
B: 100% acetonitrile,
Gradient: from A to B in 18 minutes.
5 Flow rate: 1 ml per minute,
Detection by UV absorption at 210 nm.
The compound of the formula (II) was found to have a retention time of
13.9 minutes.
CA 02543629 2006-04-25
16
Example 7: Characterizing the compound of the formula (II)
A molar mass (M - H} of 357.0673 was found by means of electron spray
ionization mass spectrometry (ESI-MS} in the negative mode while a
molecular weight (M + H} of 359.0811 was found in the positive mode,
corresponding to the empirical formula C~gH~40g and a molecular weight
of 358.31.
The physicochemical and spectroscopic properties of the compound (II)
can be summarized as follows:
Appearance: lemon-yellow crystalline substance which is soluble in
medium-polar and polar organic solvents. Stable in neutral and mildly acid
medium but unstable in strongly acidic and alkaline solutions and under the
influence of oxygen.
Empirical formula: C~gH~40g
Molecular weight: 358.31
UV maxima: 220, 245, 307 and 365 nm in water/acetonitrile (1:1) at pH 2.
Table 1: Chemical shifts of the compound (II) in DMSO at 300 K.
H C
2 - 151.57
3 7.47 108.94
3a - 122.83
4 - 117.21
5 - 127.54
5-Me 2.58 11.03
6 - 140.91
6-OH broad -
7 - 136.42
7-OH broad -
7a - 142.35
8 10.42 190.2
broad)
9 - 124.84
10 - 112.61
11 - 150.17
11-OH 12.1 (broad)-
CA 02543629 2006-04-25
17
12 - 132.68
12-OH broad -
13 - 151.52
13-OH broad -
14 - 118.72
14-Me 2.01 12.66
15 9.48 194.74
Example 8: Methylating the compound of the formula (II)
12 mg of the compound of the formula (II), obtained as described in
example 5, were dissolved in 3 ml of methanol after which 1 ml of
(trimethylsilyl)diazomethane [2 M solution in hexane, Aldrich] was added.
The reaction mixture was left to stand at room temperature and the course
of the reaction was monitored by HPLC. After the compound of the formula
(II) employed had been completely reacted, the reaction was terminated by
adding water and concentrating in vacuo. The resulting methylation
products were purified chromatographically on a Luna 5N RP18
phenomenex~ column using a gradient containing 0.02% trifluoroacetic
acid and acetonitrile. After the pure fractions had been freeze-dried, the
following were obtained by means of HPLC (conditions as in example 5):
2 mg of compound of the formula (III), monomethyl ether, retention time,
15.4 minutes,
2.7 mg of compound of the formula (I~, dimethyl ether, retention time, 16.9
minutes,
1.9 mg of compound of the formula (~, trimethyl ether, retention time, 19.4
minutes.
Example 9: Characterizing the compound of the formula (Ill)
In ESI-MS, the compound of the formula (III) gave a molecular peak of
373.0 in the positive mode (M + H) and of 371.0 in the negative mode (M -
H), corresponding to a MW = 372 and an empirical formula of C~gH~gOg.
CA 02543629 2006-04-25
18
8 i
5/ 4 3a 3 15
1 I ~ 10 OH
HO 6 ~7a O 14 ~ \ 11
OH
112'
OH
Table 2: Chemical shifts of the compound (III) in DMSO at 300 K.
H C
2 - 150.95
3 7.51 109.16
3a - 122.65
4 - 117.28
- 127.75
5-Me 2.58 11.06
6 - 141.03
6-OH broad -
7 - 136.47
7-OH broad -
7a - 142.45
8 10.42 190.24
9 - 129.11
- 112.98
11 - 154.87
11-OH 12.22 -
12 - 134.76
12-OMe 3.83 60.28
13 - 155.68
13-OH broad -
14 - 119.05
14-Me 2.01 12.66
9.49 194.73
5
Example 10: Characterizing the compound of the formula (I~
CA 02543629 2006-04-25
19
In ESI-MS, the compound of the formula (I~ gave a molecular peak of
387.1 in the positive mode (M + H) and of 385.0 in the negative mode,
corresponding to a MW = 386 and an empirical formula of C2pH~gOg.
CA 02543629 2006-04-25
Table 3: Chemical shifts of the compound (I~ in DMSO at 300 K.
H C
2 - 151.51
3 7.51 108.72
3a - 122.93
4 - 119.83
5 - 127.10
5-Me 2.58 10.86
6 - 143.62
6-OH 9.21 -
7 - 137.18
7-OMe 4.15 60.44
7a - 143.74
8 10.48 190.88
9 - 122.97
10 - 115.84
11 - 150.54
11-OH 11.70 -
12 - 139.24
12-OH 9.68 -
13 - 151.69
13-OMe 3.87 59.90
14 - 123.98
14-Me 2.08 12.80
15 ~ 9.72 ~ 195.45
Example 11: Characterizing the compound of the formula (~
5 In ESI-MS, the compound of the formula (~ gave a molecular peak of
401.1 in the positive mode (M + H) and of 399.0 in the negative mode (M -
H), corresponding to MW = 400 and an empirical formula of C2~H2pOg.
CA 02543629 2006-04-25
21
Table 4: Chemical shifts of the compound (~ in DMSO at 300 K.
H "C
2 - 150.90
3 7.56 108.95
3a - 122.73
4 - 119.91
- 127.28
5-Me 2.58 10.85
6 - 143.74
6-OH 9.25 -
7 - 137.18
7-OMe 4.14 60.45
7a - 143.82
8 10.48 190.88
9 - 127.83
- 116.20
11 - 155.63
11-OH 11.82 -
12 - 140.70
12-OMe 3.88 60.53
13 - 156.89
13-OMe 3.98 60.74
14 - 123.77
14-Me 2.06 12.85
~ 9.73 ~ 195.00