Note: Descriptions are shown in the official language in which they were submitted.
CA 02543816 1998-01-16
ePTFE GRAFT STENT COMPOSITE DEVICE
FIELD OF INVENTION
The present invention relates generally to tubular implantable prosthetic
devices such
as vascular grafts and other endoprostheses. More particularly, the present
invention relates
to a graft formed of porous expanded polytetrafluoroethylene (ePTFE) which
supports a
stent in an ePTFE composite graft-stent device.
BACI{GROUND OF THE INVENTION
Intraluminal devices such as grafts and stents are known for treating
stenosis,
stricture, aneurysms and the like. These devices may be implanted either
transluminally in
a minimally invasive procedure or may be surgically implanted.
Such intraluminal devices provide a technique for expanding a constricted
vessel or
for maintaining an open passageway through a Nessel. One common technique used
to hold
open a blocked or constricted vessel such as a blood vessel is to employ a
vascular stent.
Stents are implantable intraluminal devices typically formed of wire.which may
be radially
expanded to hold open constricted vessels. Thus, wire stents are useful to
prevent restenosis
of a dilated vessel or to eliminate the danger of reocclusion of the vessel.
In addition, wire
stents can also be used to reinforce various lumen in danger of collapse.
However, stents
are not generally designed as conduits or bypass devices.
Intraluminal or endoprosthetic grafts, however, aro designed as bypass devices
which allow fluid flow therethrough. Often, these devices are percutaneously
implanted
within the vascular system to reinforce collapsing, partialYy occluded,
weakened or
CA 02543816 1998-01-16
abnormally dilated localized sections of, e.g., a blood vessel. Grafts may
also be surgically
implanted by anastomosis to replace a badly damaged portion of vessel.
Vascular grafts may be manufactured from a variety of bio-compatible
materials.
For example, it is well known to use extruded tubes of polytetrafluoroethylene
(PTFE) as
vascular grafts. PTFE is particularly suitable as it exhibits superior
biocompatibility.
PTFE tubes may be used as vascular grafts in the replacement or repair of
blood vessels
because PTFE exhibits low thrombogenicity. Further, expanded PTFE (ePTFE)
tubes have
a microporous structure which allows natural tissue ingrowth and cell
endothelialization
once implanted into the vascular system. This contributes to long term healing
and graft
patency.
Grafts formed of ePTFE have a fibrous state which is defined by interspaced
nodes
inteiconnected by elongated fibrils. The spaces between the node surfaces that
are spanned
by the fibrils are defined as the internodal distance (IND). The art is
replete with examples
of vascular grafts made of microporous ePTFE tubes useful as vascular grafts.
The porosity
of an ePTFE vascular graft is controlled by varying the IND of the microporous
structure of
. ~ ,.
the tube. An increase in the IND within a given structure results in enhanced
tissue
ingrowth, as well as, cell endothelialization aldng the inner surface thereof.
Increasing the
porosity of the tubular structure, however, reduces the ability of the graft
to retain a suture'
placed therein during implantation and tends to exhibit low axial tear
strength. In order to,;
' strike an effective balance between porosity and radial strength, multi-
layer ePTFE tubes
have been developed. The porosity of these, multilayered tubes vary as between
the outer
and inner layers to achieve a composite structure having sufficient porosity
for tissue
ingrowth and cell endothelialization while still retaining sufficient radial
strength.
It is known in the art to use stents in combination with vascular grafts and
other
2
CA 02543816 1998-01-16
endoprostheses. Stents may be positioned at one or both ends of a graft to
support the graft
within a portion of the vessel. Thus positioned, the stents help fix the graft
to the vessel
wall. In addition, stents serve to keep the lumen open and to anchor the graft
.in place. A
single stent may also be employed in combination with a graft to allow the
graft to "float"
downstream toward the affected vessel. Once properly positioned, the single
stent is
expanded to anchor the graft in place.
--~ Several techniques for securing one or more stents to a graft are known.
For
example, hooks or barbs extending from the stent have been used for securing
stents to a
graft. Alternatively, a stent may be sutured to a graft. Each of these
techniques requires
either specialized stent attachment means or secondary procedures to secure
the stents to the
graft.
Traditional stents have various shapes and sizes depending upon their intended
function. For example, structures which have previously been used as stents
include coiled
stainless steel springs, helically wound coiled springs manufactured from an
expandable
,
heat-sensitive material, expanding stainless steel stents formed of stainless
steel wire in a
"zig-zag" pattern, cage-like devices made from~n4lleable metal, and flexible
tubes having a
~
plurality of separate expandable ring-like scaffold members which permit
radial expansion
of a graft. Each of these devices is designed to be radially compressible and
expandable so
that it will easily pass through a blood vessel in a collapsed state and can
be radially,
expanded to an implantable size after the target area of the vessel has been
reached. Radial
expansion and contraction of each of these causes associated longitudinal
expansion and
contraction of the stent.
Such expandable stents may be supported betweenthe layers of a multi-layer
tubular
graft. The expandable stent would anchor and support the multi-layer tube
within the
3
CA 02543816 1998-01-16
lumen. Upon radial expansion, the stent would hold the graft outwardly against
the inner
wall of the lumen. One example of such a graft-stent combination is shown in
United States Patent No.
5,123,917 issued to Lee et al. A stent-graft combination shown therein
includes a plurality
of separate scaffold members (stents) mounted between an inner tube and an
outer tube
forming the multi-layer graft. In one embodiment of this invention, the
scaffold members
are free floating within an intermediate pocket formed by the inner and outer
tubes. In
another embodiment, the scaffold members are adhesively affixed to the outer
surface of the
inner tube. In yet another embodiment of this invention, the inner and outer
tubes are
adhered to each other in such a manner that separaw pockets are formed in
which individual
scaffold members are placed within each pocket.
ln each of these different embodiments of the '917 patent, radial expansion of
the
scaffold member causes a change in the longitudinal expanse thereof. Thus, a
drawback to
the device shown in the '917 patent is that the net length of the scaffold
member increases
as-the graft contracts. Accordingly, this increase in the net length of the
scaffold member
increases the stress forces on the graft as well as. tends to delaminate the
layers. Thus, these
stress forces increase the likelihood that the inner tube will become
separated from the outer
tube and/or that the graft willtear upon expansion of the scaffold members. /
Accordingly, it would be desirable to provide an improved intraluminal device,
=in
particular, an ePTFE graft-stent composite device with improved radial
strength that allows
for the deployment of a stent and graft simultaneously with the stent already
permanently
positioned on the graft such that additional stress is not placed on the graft
by the stent upon
expansion.
4
CA 02543816 1998-01-16
SUMMARY OF THE INVENTION
In accordance with the present invention, an improved composite graft-stent
device
is provided. More particularly, the present invention is formed from two non
thrombogenic
tubes which are laminated or fused together with one or more stents secured
therebetween.
This composite device is then expanded to place it in intimate contact with
the inner surface
of the lumen in which it is positioned.
The composite device is preferably an implantable intraluminal device with a
first
porous tube that has two opposed ends, an interior luminal surface and an
exterior surface.
The composite device also contains a second porous tube which is disposed
concentrically
about the exterior surface of the first tube and is secured to the exterior
surface of the first
tube. A radially expandable member is disposed about the exterior surface of
the first tube
and is longitudinally immobilized between the first and second tubes when they
are secured.
In the present invention, the second tube is secured to the first tube by
fusion or by
lamination.
The radially expandable member between the two tubes includes a longitudinal
expanse. As.used herein, the term "longitudinal expanse" means the width of
the radially
expandable member as measured along the axis of the tube. In the present
invention, when
the member is expanded, there is no distortion along the longitudinal expanse
of the
member, e.g., the width remains constant as the member is expanded. The member
is
preferably an expandable stent.
The expandable stent of the invention includes an elongated element with a
first end
and a second opposed end. In one embodiment of the invention, the elongated
element is
formed in a generally circular configuration with the first end adjacent to
and overlapping
5
CA 02543816 1998-01-16
the second opposed end. The stent is expandable through the relative movement
of the first
end with respect to the second opposed end.
In another embodiment of the invention, the end extent of the first end of the
elongated element has a plurality of second end engagement means .for engaging
the distal
end of the second end to provide finite adjustability to the stent.
In yet another embodiment of the present invention, the radially expandable
stent
includes an elongate element having a first end and a second opposed end. This
elongate
element is formed in a generally circular configuration with the first and
second ends in
axial alignment to each other. In this embodiment of the invention, the second
end has an
elongate open-ended channel wherein radial expansion is achieved by movement
of the first
end out of the open-ended channel of the second end.
The implantable intraluminal device of the present invention is preferably
fabricated
out of a biocompatible metal. Most preferably, the implantable intraluminal
device is
stainless steel, platinum, gold, nitinol, tantalum and alloys thereof.
. The fn-st and second tubes of the present invention are preferably-
fabricated out of a
bio-compatible material. Most preferably, the first and second tubes are
fabricated out of
expanded polytetrafluoroethylene (eFTFE).
In the present invention, a stent may be disposed about the exterior surface
of the -'
first tube adjacent to either of the first or second ends. Alternatively, a
stent.may be
disposed about the exterior surface of the first tube at both ends. In yet
another
embodiment, a plurality of stents may be disposed about the exterior surface
of the fist tube
and longitudinally spaced between stents located at the i''irst and second
ends of the device.
In the present invention, the device may be expanded by an inflation force.
Preferably, the inflation force is supplied by inflating a balloon catheter.
6
CA 02543816 1998-01-16
The process of the present invention hereby incorporates by reference all of
the
limitations described above for the intraluminal implantable device. By way of
summary, in
the process of the invention an implantable intraluminal device is provided
which includes a
first luminal porous tube having first and second ends, an interior luminal
surface and an
exterior surface. One or more radially expandable members is/are then radially
disposed
about the exterior surface of the first tube. A second porous tube is then
concentrically
positioned over the first tube and the radially expandable member(s). The
first tube is then
secured to the second tube so that one or more expandable members is/are
immobilized
along the longitudinal axis of the first and second tubes.
BRIEF DESCRIPTION OF THE DRAWINGS
- The present invention can be further understood with reference to the
following
description in conjunction with the appended drawings, wherein like elements
are provided
with the same reference numbers. In the drawings:
FIGURE 1 is a longitudinal cross-section of the composite graft-stent of the
present
invention. -
FIGURE 2 is a front view of the "key-ring" type stent employed in the
composite
graft-stent of Figure 1.
FIGURE 3 is a front view of another embodiment of a stent of the composite
graft-
stent of Figure 1.
FIGURE 4 is a front view, partially in section of a still further embod'unent
of a
stent of the composite graft-stent of Figure 1.
7
CA 02543816 1998-01-16
DETAILED DESCRIPTION OF TEE PREFERRED EMBODIMENT
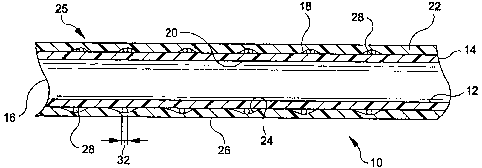
Now turning to Fig. 1, a longitudinal cross-section of the preferred
embodiment of
the graft-stent composite device 10 is shown. This device 10 includes a
multilayer graft 25
which is formed of inner and outer tubes 12 and 22 that are preferably formed
of
expandable polytetrafluoroethylene (ePTFE). Although it is preferred that
tubes 12 and 22
be made of ePTFE, any appropriate bio-compatible material, such as porous
polyurethane,
is also contemplated. Other potential materials for this application include
DACRON, a
proline mesh or the like. Ideally, the material should be inert and should not
promote a
significant amount of scar formation.
Graft 25 has first and second opposed ends 14 and 16, respectively. Tube 12
includes an exterior surface 18 and an interior luminal surface 20. Tube 22
has an interior
surface 24 and an exterior vascular surface 26. Tube 22 is disposed
concentrically over the
exterior surface 18 of tube 12 to form the multilayer graft 25.
A plurality of longitudinally spaced stents 28 are disposed between the
exterior
surface 18 of tube 12 and the interior surface 24 of tube 22. As will be
described
hereinbelow, each stent 28 is of the type which may be radially expanded.
Stents 28 are
longitudinally immobilized between tubes 12 and 22 when they are secured to
each other.
The stents 28 are positioned at spaced locations along the multi-layer graft
25 in numbers
which may be selected based on use and application of the device 10.
Fig. 1 shows tubes 12 and 221aminated together to form graft 25 with stents 28
disposed therebetween. Although Fig. 1 shows tubes 12 and 221aminated
together, any
appropriate method of securement, such as fusion, is contcmplated. The
lamination of tubes
12 and 22 causes stents 28 to be immobilized along the longitudinal axis of
the multi-layer
graft 25.
8
CA 02543816 1998-01-16
Now turning to Fig. 2, a preferred embodiment of stent 28 is shown. Stent 28
may
be formed from a wire 30 which is wound in the shape of a simple circle
generally
described as a"key-ring." The circular wire 30 includes a first end 34
adjacent to and
overlapping a second opposed end 36. The wire 30 is radially expandable by
movement of
first end 34 and second end 36 in opposing directions relative to each other
as indicated by
arrow A. Radial expansion is accomplished by, for example, the expansion of a
balloon
catheter exerting radial pressure on wire 30. This radial expansion is
achieved without a
change in the longitudinal expanse 32 of the wire 30 which is shown in Figure
1. While
balloon expansion is described, it is also contemplated that stent 28 may be
of the self-
expanding variety.
Now with reference to Fig. 3, a further embodiment of the stent of the present
invention is shown. Stent 28' may be formed from a wire employing a simple
"ratchet"
design. The wire 30' is formed in a circular configuration and includes a
first end 34'
adjacent to and overlapping its second opposed end 36'. Several ratchet-like
teeth 38' are
located at an end extent 40' of the first end 34' : The other end 36' includes
a pawl 41' at a
distal end 42. thereof for adjustable engagement with teeth 38'. Upon relative
movement of
opposed ends 34' and 36', teeth 38' are engaged by pawl 41' to provide
adjustable
interlocking therebetween. This allows the diameter, of the circular stent 28'
to be
adjustably expanded in incremental fashion, to set the diameter thereof at
discrete
increments.
Now with reference to Fig. 4, an additional embodiment of a stent is shown.
Stent
28" is a wire stent employing telescoping-ends. The wire 30" is formed in a
circular
configuration and includes a first end 34" that is positioned in general axial
alignment with a
second opposed end 36". A distal portion 34a" of first end 34" telescopes into
an elongate
9
CA 02543816 1998-01-16
open-ended channe144" formed at opposed end 36". The diameter of the stent 28"
is
contracted and expanded by movement of first end 34" into and out of open-
ended channel
44".
The various embodiments of each of the stents 28 described herein are
preferably
manufactured out of a bio-compatible metal. Most preferably, the bio-
compatible metal is
stainless steel, platinum, gold, nitinol, tantalum and alloys thereof.
One or more such stents 28 may be disposed between tubes 12 and 22. For
example, in one embodiment a single stent 28 is disposed about one end of tube
12. In an
alternative embodiment of the invention, two stents 28 are disposed about each
end of tube
12. In yet another embodiment of the invention, several stents 28 are disposed
about the
exterior surface 18 of tube 12 and are longitudinally spaced therealong
between the two
ends 14 and 16 of tube 12.
While the preferred embodiments of the invention are shown and described
below,
other embodiments that fall within the scope of the disclosure and appended
claims are also
contemplated. -
The invention being thus described, it will be obvious that the same may be
varied in
many ways. Such variations are not to be. regarded as a departure from the
spirit and scope
of the invention and all such modifications are intended to be included within
the scope of
the following claims.