Note: Descriptions are shown in the official language in which they were submitted.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 1 -
METHODS FOR PRODUCING PULP AND TREATING BLACK LIQUOR
FIELD OF THE INVENTION
The present invention relates to a method for producing pulp from
graminaceous fibres and also to a method of treatment of black liquor that may
be a
by-product of said pulp production method or may have arisen otherwise e.g.
Kraft
black liquor or a mixture of soda black liquor with Kraft black liquor.
1o BACKGROUND TO THE INVENTION
The Kraft process
The principal component of wood is long straight translucent cellulose fibres
based on chains of glucose molecules that make up about 42 wt% of softwoods
and
45 wt% of hardwoods. Hemicelluloses form a further component of wood and are
short, branched chains of glucose and other sugar molecules that are
relatively
soluble in water and are removed during the pulping process. The cellulose
fibres are
held together by lignin which is a three-dimensional phenolic polymer network
that
2o holds the cellulose fibres together and imparts rigidity. Lignin comprises
about
28wt% of softwood and about 20wt% hardwood. It is selectively removed during
chemical pulping and subsequent bleaching without significantly degrading the
cellulose fibres. Extractives account for about 3wt % of softwoods and about
5wt
of hardwoods. They include plant hormones, resin and fatty acids.
The Kraft or sulphate process is preferred for the chemical pulping of wood
because it can deal effectively with the resin component of many woods. It
uses
sodium hydroxide as the main cooking chemical and sodium sulphide as catalyst,
and
it gives stronger final pulp than the soda process, which employs sodium
hydroxide
3o alone. Anthraquinone is often used as an auxiliary catalyst in both the
Kraft and soda
processes. In the Kraft process, chips are cooked in a digester under heat and
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 2 -
pressure with "white liquor" (in this case aqueous sodium hydroxide and sodium
sulphide) to dissolve the lignin selectively. After 2 to 4 hours, the cooked
mixture of
pulp, spent pulping chemicals and wood waste is discharged from the digester.
The
resulting pulp is separated form a mixture of pulping chemicals and waste
referred to
as "black liquor".
The treatment chemicals (sodium sulphide and sodium hydroxide) are then
regenerated from the black liquor by a process whose main piece of equipment
is a
so-called Tomlinson furnace. Black liquor at about 65% dry solids content is
sprayed
1o into the furnace. During their descent, the black liquor droplets lose the
remaining
water by evaporation and the solids undergo pyrolysis to form a char bed at
the
bottom of the furnace. The char bed burns under reducing conditions at a
temperature
of ?SO°-1050°C. and the recovered chemicals, mainly NaZC03 and
Na2S, are drained
from the furnace as a smelt which is dissolved in water to produce so-called
green
liquor, the precursor of the white~liquor. The gases generated during
pyrolysis and
burning of the char are fully combusted at a higher location in the furnace.
Flue gases
must be thoroughly scrubbed to remove mercaptans that form under the process
conditions. The furnace is provided with a suitable heat exchanger to recover
heat
from the hot combustion gases for steam and electricity generation.
Although useful recovery of chemicals and energy can be achieved in
commercial operation, the use of a Tomlinson furnace presents ~ a number of
problems. For example, inadvertent contact between water or dilute black
liquor and
the inorganic smelt may result in an explosion. Also, high char bed
temperatures lead
to increasing emission of sodium salts and excessive fouling of the steam
pipes in the
upper part of the furnace. Furthermore, the technology currently used to treat
black
liquor effluent is, depending on local economic conditions, only viable on a
scale of
not less than 60,000 tonnes of pulp production per annum, which may be
compared
with the typical scale of a modem wood pulp mill which is over 360,000 tonnes
of
pulp production per annum. Treatment of straw and other grarnininaceous
materials
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 3 -
is, of course, on a much smaller scale inter olio because long-distance
transport of
bulky agricultural residues such as straw is uneconomic.
Fluidised bed recovery in the Kraft process
To solve these problems, and also to reduce capital investment and increase
the energy efficiency of the recovery operation, a number of Kraft recovery
process
have been described in which the smelt-water explosion hazard is eliminated
and the
emission of sodium salts reduced by maintaining the inorganic chemicals in'
solid
1 o rather than molten form.
This principle was disclosed in US-A-3309262 (Copeland et al) which
discloses a process for treating black liquor in a reaction vessel containing
a fLuidized
bed of solid particles consisting substantially entirely of residual inorganic
materials
derived from the black liquor. The process comprises:
(a) concentrating the black liquor by evaporation to a solids content of 20-45
wt%, said liquor having a combustible content sufficient to support autogenous
combustion;
(b) spraying the concentrated black liquor into free space above the bed so
that substantial evaporation is achieved within said free space, and the
remaining
further concentrated atomised black liquor flows into the fluidised bed;
(c) maintaining fluidity of the bed by introducing at a speed of 30-150 cm/sec
(1-5 ft/sec) oxygen-containing fluidizing gas in an amount sufficient to
effect
complete elimination of organic material as off gas by substantially total
autogenous
combustion within the fluidised bed;
(d) maintaining the bed at a non-smelting temperature below the eutectic
temperature of the residual chemical mixture within the bed but in a
temperature
range of about 540 - 982°C to form gaseous combustion products above
the bed and
agglomerates within the bed from the residual inorganic materials of the black
liquor
3o that are of sufficient weight to prevent their entrainrnent in the
fluidizing gas;
(e) discharging the agglomerates from the fluidized bed; and
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 4 -
(f) discharging the off gas from above the bed.
For sodium-based waste liquor the maximum recommended bed temperature
is 760°C (although the inventors are aware that this value was exceeded
in practical
operation). Introduction of the black liquor as a mixture of coarse and fme
droplets is
recommended in order to combine rapid evaporation of water, an efficient
scrubbing
action that reduces dust loading, and promotion and control of agglomeration
of the
bed particles. Oxidising conditions within the reactor are maintained to
prevent the
formation of hydrogen sulphide gas, and conversion of organic material into
l0 combustible gas is not disclosed. The end products are Na2C03 and Na2S04
which
have to be subjected to recaustication to regenerate white liquor. Although it
has been
reported in the patent literature that there have been attempts to
commercialise the
Copeland process, the inventors are aware that it is prone to severe bed
agglomeration, especially when treating black liquor of relatively low
calorific value
from the cooking of straw, and that the process has since fallen iota disuse
for lack of
technical and commercial viability. The experience of the inventors is that
simple
fluidized beds of the kind disclosed by Copeland are subject to unacceptable
agglomeration, which makes operation impractical for anything beyond a short
start-
up period.
US-A-3523864 (Osterman) discloses a recovery process for Kraft black liquor
based on a reaction vessel having lower, intermediate and upper fluidized beds
disposed one above another and each formed by pellets of CaO. The lower bed
operates at 704-760°C (1300-1400°F) and contains solid reaction
products in which
Na2SOa becomes reduced to NaaS. The intermediate bed is at 648-704°C
(1200-
1300°F) and is fed with black liquor and preheated air in an amount of
about 30% of
that required for complete combustion to produce Na2C03 and NaaS04 which
become deposited on the surface of the Ca0 pellets together with combustion
gases
and organics. The upper bed receives recycled CaC03 which becomes calcined to
regenerate Ca0 and provide the material far the fluidised beds which descends
progressively from upper to lower beds. Overhead combustion gases are partly
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 5 -
recycled as fluidising gas and after cyclone treatment are partly fed to a
steam
generator. Again all three beds are of the simple bubbling type, and the
intermediate
bed is subject to unacceptable agglomeration for the reasons already given.
There are two further reasons for the absence of commercial utilization of
these low-temperature fluidized bed processes: firstly the relatively high
temperature
required for fast and complete conversion of Na2S04 to Na~S and secondly the
ease
of formation of H2S when NaaS is contacted with combustion gases below the
melting point of the inorganic salts. So, while high temperatures favour the
to reduction, the above alternative processes require a relatively low
temperature just
below the melting point of the inorganic salt mixture. The consequence is that
in fluid
bed processes operating in the reducing mode, most of the Na2S formed is
rapidly
converted to HZS (and some COS) according to the overall reaction
Na2S + CO~ + Ha0 -->. Na~C03 + HAS
resulting in a low recovery of solid Na2S.
For the sake of completeness, there should be mentioned US-A-4011129
(Tomlinson) which discloses a method for increasing the chemical recovery
capacity
of a Kraft recovery furnace by injecting solid pellets of sodium sulphate and
sodium
2o carbonate directly onto the char bed in the reducing zone of the furnace
while
maintaining the temperature and reducing atmosphere in that zone, thereby
forming a
smelt containing sodium sulphide and sodium carbonate from the injected
pellets.
These pellets may be produced from a further quantity of black liquor in an
auxiliary
incinerator such as a fluid bed combustion unit, which permits recovery
capacity to
be increased without needing the construction of a further recovery furnace.
Production of non-wood cellulosic pulp
The use of agricultural residues from graminaceous annual crops could
provide a solution to many problems of concern to the pulp and paper industry
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 6 -
including fibre supply, farmers' concerns over the cost and availability of
disposal
alternatives, and consumer concerns over limited forest resources.
Broadly defined, graminaceous crop residues are the materials left over after
annual agricultural crops have been harvested for their primary or intended
purpose.
They include cereal straws, such as wheat, rice, barley and oats; seed grass
straws
such as flax and rye; the crushed stalks of sugar cane known as bagasse;
sorghum and
corn stalks; and other agricultural residues e.g. cotton linters, the short
fibres
adhering to cotton seed after cotton ginning In countries where there is
little or no
to supply of wood, pulp from straw and bagasse is being used in high
proportions for
paper-making - up to 90% for high quality printing and writing paper. For
example,
in China, over 85% of papermaking pulp has been reported to come from non-wood
raw materials, predominantly straw. In India, approximately 55% of papermaking
pulp has been reported to come from non-wood sources with about half from
agricultural-residues. As legislation increasingly prohibits the burning of
agricultural
wastes, there is new incentive to develop alternative uses for this resource.
With
proper soil management, farmers can supply small scale pulping mills with a
continuous source of fibre while sustaining crop production.
Agricultural residues such as wheat and rice straw contain cellulose and can
be a good raw material for papermaking. As previously mentioned, these raw
materials are bulky, so that transportation casts and logistics mean that they
are best
pulped locally and therefore on a relatively small scale of around 10-100
tonnes of
pulp production per day. Pulp mills generate black liquor effluent that if
discharged
2s to watercourses causes severe pollution. Lack of economically viable
technology to
deal with black liquor effluent under 60,000 tonnes per annum of production
has
meant that many existing small pulp mills have been forced to close to stop
pollution
of watercourses. This lack of suitable technology has also prevented the
establishment of new small pulp mills, in particular new mills that might have
used
3o agricultural residues. The subsequent lack of demand for small pulp mills
has meant
that little research and development of small pulp mill technology has been
carried
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
_7_
out. Consequently small pulp mill technology and straw pulping in particular
has not
advanced as far as large-scale wood pulping technology.
Straw can be pulped by chemical processes and by a combination mechanical
and chemical process (chemi-mechanical pulping). For the cooking of non-wood
raw
material sodium hydroxide alone is recommended as the active chemical because
most non-wood fibre does not contain sticky resins and the sodium sulphide
catalyst
is unnecessary. For this reason the major part of chemical pulp production
from this
class of raw materials is performed with a process called the soda process in
which
to raw material is heated together with a highly alkaline caoking liquor
containing
sodium hydroxide to a temperature of 140-170°C under pressure. Under
these
conditions, the main portion of lignin dissolves. Sodium hydroxide can be
recovered
from the resulting black liquor and organic substances in the black liquor can
be
used as fuel for energy generation. Contrary to the Draft process, in which
recovery
requires reduction of sulphate to sulphide, black liquor from the soda process
can be
burnt even under highly oxidising conditions. Chemical recovery therefore
involves
evaporating the black liquor to a suitable content of dry matter and burning
the
evaporated liquor by means of excess oxygen. The inorganic combustion residue,
consisting mainly of sodium carbonate, is dissolved in water and re-
causticised with
burnt lime to regenerate sodium hydroxide, which is recycled. In a variant,
slaked
lime Ca(OH)2 has been used in admixture with NaOH as active chemicals in white
liquor because it also serves as a digesting agent and is of lower cost.
However, a
process for recycling NaOH/Ca(OH)2 black liquors has not been described and
such
black liquors have in the past merely been discharged untreated.
Silica in pulp from graminaceous starting materials
The relatively high content of silica in straw and other non-wood cellulosic
agricultural products presents difficulties for chemical recovery. Wheat straw
contains 4~10 w% silica as small crystals embedded in the straw. Rice straw
has an
even higher silica content, 9-14 wt%. Other cereals such as barley, oat and
rye straw
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
_ g _
have 1 - 6 wt% silica. Wood on the other hand has a silica content of less
than 1
wt%. In the soda process as applied to straw pulping, mast of the silica in
the straw
reacts with the sodium hydroxide to form water-soluble sodium silicate, which
remains in the black liquor in addition to lignin and other organic compounds.
Black
liquor of high silica content gives rise to scaling (coating equipment with a
glass like
substance) especially in an evaporative process. A modified wood based
recovery
system may be used if the silica content of the cereal straw is less than 5 -
6 wt%, but
at higher capital and operating costs. However, for products of higher silica
content,
especially for rice straw, there has been up to now no process that is
technically and
to commercially viable.
Our WO 03/014467 (the disclosure of which is incorporated herein by
reference) discloses a method for treating raw elongate material suitable for
use in a
paper making plant comprising:
extracting contrary material from the raw material;
crushing the raw material from which contrary material has been removed to
remove nodes;
splitting the crushed raw material lengthways;
supplying the split raw material to a co-rotating screw conveyor divided into
a
plurality of zones and processing said material in said conveyor to produce
pulp and a
black liquor effluent;
supplying treatment material to at least one zone;
controlling the temperature and/or pressure of at least one zone; and
spraying concentrated black liquor into a processing vessel in the form of a
fluidised bed reactor for treatment of said black liquor, said processing
vessel being
part of treatment material and energy recovery means. The alkali supplied to
the co-
rotating screw conveyor to bring about pulping may include sodium hydroxide
and
additionally calcium hydroxide, which has the effect of precipitating silica
onto the
cellulosic fibres and preventing silica from entering the black liquor as
calcium
silicate.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
_ g _
WO 03/014467 further describes a process for treating black liquor in which
black liquor effluent arising from the pulping process is collected in a
digestion
liquor storage tank and concentrated to 30-70% solids using a standard
evaporator
designed for concentration purposes. If the black liquor effluent has a solids
concentration of 30% or above it may be treated directly in the processing
vessel
eliminating the evaporation step. The concentrated black liquor is moved to a
reactor
vessel at a temperature in excess of 90°C using a pipe or an enclosed
twin- sc rew
transport system. The enclosed transport system is used to minimise the loss
of
organic components through vaporisation. A temperature in excess of
90°C is
to required to decrease the viscosity of the black liquor so that it will
transport without
resistance. The black liquor is treated in a toroidal fluidised bed reactor
vessel.
Although a limit of 650°C is specified for the upper temperature of the
fluidised bed,
in practice a maximum temperature of 610°C has only ever been used.
This is
because at temperatures above 600°C, volatilisation of the inorganic
alkali metal
species present in the black liquor (e.g. Na and K) has been demonstrated to
occur in
other processes. When these species are in the vapour phase, additional
process
equipment is required to recover them at increased overall cost.
SU1VIMARY OF THE INVENTION
The advantage of a higher reaction temperature is the enhanced rate of
reaction for black liquor processing and hence throughput may be increased,
whilst
maintaining output quality. We have now discovered that a temperature of above
650°C can be used in the above mentioned black liquor recovery process.
Recent
experiments have shown that the loss of inorganic species from the black
liquor when
heated to between 650 and 700°C or even 725°C in the fluidised
bed, was minimal,
i.e. the losses were not economically significant, and hence no additional
equipment
was required to ensure their recovery.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 10 -
According to the invention there is provided a method for treating black
liquor, which comprises heating the black liquor at a temperature above
650°C in a
reactor containing an alkaline earth metal oxide, e.g. calcium oxide.
In the reactor the black liquor may react with the alkaline earth metal oxide
to
form a mixture of sodium hydroxide and sodium carbonate and alkaline earth
metal
carbonate and a volatile gas and liquid component which contains a combustible
component and can be used as a fuel as in conventional treatment processes,
e.g. a
boiler.
l0
In an alternative aspect, the invention provides a method of treating
graminaceous materials which reduces or overcomes the problems associated with
a
high silica content in the resulting black liquor.
The invention further provides a method of converting graminaceous raw
material to pulp for paper or board, said method comprising:
digesting said raw material with a white liquor based on sodium hydroxide
and further comprising calcium hydroxide in an amount effective to
substantially
convert silica of said raw material to calcium silicate;
2o recovering pulp and black liquor substantially free of soluble silicate;
heating the black liquor in a fluidized bed reactor containing calcium oxide
for catalysing conversion of organic content of said black liquor to gas and
for
providing recovered solids including sodium values of said white liquor and
calcium
oxide; and
regenerating said white liquor using said recovered solids.
Under some conditions, there is a risk that significant quantities of silicate
may pass into black liquor streams during the pulp washing process. However,
the
inclusion of calcium hydroxide in alkali liquor used for pulping causes the
silicate to
3o pass into the black liquor as calcium silicate in preference to, or to the
substantial
exclusion of, sodium silicate. Calcium silicate is significantly less likely
than sodium
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 11 -
silicate to give rise to downstream processing problems. In a further aspect,
the
invention relates to the use of calcium hydroxide as an additive in the soda,
process
digestion of a graminaceous starting material to form pulp for inhibiting
scaling
during black liquor concentration and recovery when processing black liquor at
least
partly from pulp washing.
DESCRIPTION OF THE DRAWINGS
The invention will now be described in greater detail, by way of example,
i o with reference to the drawings in which:
FIG. 1 is an overall block diagram of a process for making pulp from wheat
straw according to the invention;
FIG. 2 is a schematic view of a roller arrangement for use in a raw material
pre-treatment process forming part of the pulp manufacturing process of Fig.
1;
FIG. 3 is a schematic view of the construction of a self cleaning pin roller
that
may be used in the roller arrangement of Fig. 2;
FIG. 4 is a schematic view of a possible embodiment of a co-rotating twin
screw conveyor that may be used for converting straw to pulp in the process of
Fig.
1; and
FIG. 5 is a block diagram of a preferred black liquor effluent treatment
apparatus that may be used in the process of Fig. 1.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Overview of the treatment of wheat or rice straw
The present process will be described, by way of illustration with reference
to
the treatment of wheat straw, which is usually chopped before pulping and
which
contains nodes within the stem that usually remain intact if straw is chopped
before
pulping. This is a serious drawback in the production of quality paper pulp
with the
resulting poor quality paper being produced. A method is therefore preferably
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 12 -
employed which crushes the nodes, opens out the straw stem lengthways in a
gentle
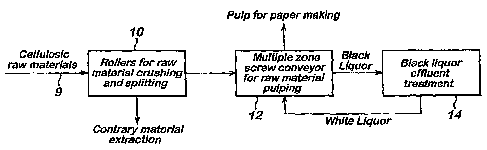
fashion and feeds the raw material into a digester in a positive, metered and
continuous process. The straw to be treated passes from a delivery conveyor 9
to a
straw pre-treatment station 10 where the stems are crushed between rollers,
contrary
material is extracted, and the stems are split longitudinally. The conditioned
straw is
then supplied to a digesting or pulping station 12 where it is subjected to
mechanical
work in the presence of aqueous alkali (white liquor) whilst subject to
elevated
temperatures and pressures. The resulting black liquor is then passed to an
effluent
treatment station 14 where it is heat treated to provide solids that can form
a so-called
1o green liquor That liquor in turn is contacted with lime derived from a
CaC03 feed
and regenerated to white liquor for recycling to the pulping station 12. OfF
gas
recovered from heat-treating the black liquor can be used for generating steam
and
for process heat. A solids bleed of CaC03 sludge is removed to avoid excessive
build-up of trace metals in the white liquor.
Pre-treatment
Where straw is the raw material from which pulp is to be made, it may take
the form of chopped straw, straw that has been subjected to a longitudinal
splitting or
2o shredding operation, or straw that has been both longitudinally split
and/or shredded
and chopped.
Referring now to FIG. 2, according to a preferred pre-treatment method, after
the bales of straw have been opened the straw is passed along a conveyor belt
101
where dust, heavy items such as stones and other contraries such as plastic
string are
removed. The straw is then passed into a feed hopper 103 which feeds the straw
into
to an arrangement of knurled rollers 105 and 107 which crush the nodes in the
straw
stem and rollers with pins which open the straw stem out lengthways in a
gentle
fashion. Thus straw is fed between first and second counter-rotating knurled
crushing
rollers 105 and 107 to crush the straw nodes. The crushed material then passes
through two counter-rotating intermediate rollers 109 and 111 that prevent any
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 13 -
contrary materials from damaging the rollers below. The straw then passes
through
two more rollers 113 and 115, this time rotating in the same direction. These
latter
rollers are provided with pins that open and shred the straw lengthways and
act in co-
operation with a feed shoe.
The action of this system leaves the straw as shortened and opened
outlshredded material without nodes. This will facilitate quicker chemical and
steam
penetration and so faster and more uniform pulping, whilst treating the fibres
gently
so preserving their length. This results in the production of an improved
quality of
to pulp including a very significant reduction in visible "shiners" in the
paper sheet, due
to dispersion of parenchyma cells, improved drainage, a higher tensile and
tear
strength, a higher pulp yield and a reduced demand for pulping chemicals.
The treated straw then drops from the pinned rollers 113 and 11 S into feed
hopper 117 leading to either a conveyor or blower system (not shown) that
feeds the
treated straw into a live bottom bin for buffer storage of the prepared
material prior to
pulping. The above discussed pinned and knurled or fluted roller opening and
feeding system is specifically designed for straw but, with minor
modifications, could
be used for any other suitable raw materials including flax, hemp, bagasse and
wood
2o chips or sawdust.
The pinned rollers can also be constructed to be self cleaning when used with
longer fibered cellulosic raw materials such as hemp and flax. This is to
prevent the
material wrapping around the rollers and fouling the apparatus. A schematic of
the
functioning part of a pin roller is shown in FIG. 3. The pin roller 120 has an
outer
surface having a large number of radially extending pins 122. This is used
with a
matching perforated or woven belt 124 on which the material 126 being treated
is
carried. The pins 122 pick up the material 126 and as the belt 124 leaves the
pins, it
takes off the material keeping the pin roller 120 free from tangled fibres.
The above process has been tested and developed with straw, flax and hemp
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 14 -
through pilot scale laboratory trials. Furthermore, the above-described
pinning
treatment for raw materials can be used with wood if the logs are flaked
rather than
chipped, as has been demonstrated using pinned wood wool instead of chips.
This
method of raw material preparation is particularly useful if the wood has a
short fibre
because the combination of flaking and pinning protects fibre length.
Digestion of graminaceous and other cellulosic starting materials
Although chopped straw can be used as a feed, the preferred feed for the
1o digestion stage of the present process is straw or other graminaceous plant
stem
material that has been longitudinally split and/or shredded. In such material
the
white liquor used for digestion makes easy contact with the graminaceous plant
stem
material and with any remaining node material, dissolving silica therein and
digesting
lignin and other alkali-sensitive materials.
For the cooking of nonwood raw material, sodium hydroxide alone is
required as an active chemical, and for this reason the major part of chemical
pulp
production from these raw materials is performed with the process called the
soda
process. In that process the raw material is heated together with an alkaline
cooking
liquor containing sodium hydroxide to a temperature in the range from 140 to
170°C
or in some instances up to 180°C under pressure. The cooking liquor
should have a
high alkaline concentration. Under these conditions, the main portion of
lignin will
be dissolved from the raw material; however, also the main portion of any
silicon in
the raw material will react with the sodium hydroxide, forming water-soluble
sodium
silicate. Thus the black liquor produced in cooking will contain silicate ions
in
addition to lignin and other organic compounds. The preferred white liquor
used is
therefore of the soda type (i.e. no sodium sulphide) and additionally contains
calcium
hydroxide in an amount effective to precipitate the silica. Either calcium
hydroxide is
present in the white liquor with which the graminaceous feed is treated, or
calcium
3o hydroxide is added shortly after the sodium hydroxide and in either case
the amount
of calcium hydroxide should be suff cient to precipitate silica onto the straw
fibres as
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 15 -
calcium silicate to reduce the soluble silicate content of the resulting black
liquor. An
effective amount of calcium hydroxide should be present to convert
substantially all
the silica or a desired portion thereof into insoluble silicate, much of which
precipitates on the straw fibres, before extraction of black liquor from
partially or
s completely digested straw is initiated.
The straw or other non-wood cellulosic material may be digested using a
continuous digester, e.g. a single tube or multi-tube screw-fed digester, for
example a
Pandia digester available from Lenzing Technik GmbH & Co KG. The use of fast
i o cooking of the plant fibre in a horizontal tube continuous digester with
screw feeder
is reviewed in Atchison, J.E., Rapid Cooking Horizontal Tube Continuous
Digester
with Screw Feeder - Novv the World Standard for Pulping Non-Wood Plant Fibers,
1990 Pulping Conference Proceedings. It is explained that this technology was
developed initially for the pulping of bagasse, but is also applicable to
other forms of
15 non-wood plant material including wheat straw and rice straw. It is stated
to permit a
cooking time of only 10-15 minutes for bagasse,~ straw and most other non-wood
plant fibres, in contrast, to earlier cooking methods using rotary batch
digesters,
which required cooking cycles of four hours or more. In the experience of the
present
inventors, this performance is over-stated, and it is impossible to produce a
semi-
2o chemical pulp in less than 20 minutes unless uneconomic quantities of
cooking
chemicals are used. The use of a screw feed permits the density of the feed
material
to be increased, and thereby the processing capacity of the digester, and also
promotes continuous mixing of the white liquor and feed material. Again, to
the
inventors' knowledge, the digester normally comprises 2, 3 or 4 series
connected
25 tubes each of diameter about 1 metre, so that the installation is of
considerable size
and capital cost.
Because of the rapid absorbency of straw or bagasse when these materials are
subjected to pressure and elevated temperature in a horizontal tube digester,
3o especially after the pinning operation described above, pulping starts
immediately
and proceeds rapidly. The white liquor may be of the soda or Kraft type, and
for
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 16 -
straw or bagasse pulping typically 12-14 wt°,/o.Na~H or 6-7wt% Na~H and
6-7 wt%
Na2S are used based on the weight of the dry straw, with pulping temperatures
of 170
- 180°C, pulping pressures of 7 - 9 bar and cooking times stated to be
10-15 minutes
in order to produce a chemical pulp, and 3-5 minutes for a semi-chemical pulp.
As
explained above these figures are in the inventars' experience not obtainable
in
practice.
A preferred pulping process for bagasse or straw (including rice straw) uses a
horizontal tubular digester in which straw transport is by a conveyor based on
a co-
lo rotating and intermeshing double screw system. Such a digester can be made
physically small for its processing capacity and consequently carries a lower
capital
cost than competing technalogies. A significant advantage is that pulping can
be
conducted with low amounts of water, permitting black liquor to be produced at
higher concentration and reducing or removing the need for subsequent black
liquor
evaporation. Shredded and/or chopped straw is continuously fed from storage
into the
barrel of the digester where white liquor and steam are injected through ports
in the
barrel or instead of steam injection electrical heating external to the barrel
is
provided. As the intermeshing twin screws rotate, the straw and black liquor
are
intermixed and the straw is worked mechanically and digested. The twin screw
used
2o is co-rotating, which reduces the mechanical treatment given to the fibres
and thus
minimises fibre damage to the fibres. In a twin screw arrangement transport
along the
barrel is primarily by the intermeshing flights of the two screws, whereas in
a single
screw conveyor transport is because the material is trapped between the
advancing
screw flights and the static barrel wall. The single screw arrangement is
therefore less
efficient because of friction and it can give rise to slippage (pressure in
the barrel
causes the straw to slip between the screw and the barrel wall) and surging.
The
material in the twin screw digester barrel with co-rotating screws travels in
a figure-
of eight shaped path and thus takes a longer route than if the screws were
counter
rotating which gives better mixing of the straw and black liquor. The state of
the art
3o in relation to twin screw digesters far making paper pulp is shown in US-A-
4088528
(Berger et al) and US-A-4214947 (Eerger), the disclosures of which are
incorporated
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 17 -
herein by reference. It will be noted that both of these references are
concerned with
the treatment of wood chips rather than graminaceous material.
Trials have confirmed that it is possible to produce using a single digester
of
s the twin screw type to convert pinned shredded straw of length about 25mm
into a
chemical or semi-chemical pulp having a kappa number in the range 30-70 and
useful
e.g, for use in box or carton manufacture, corrugated packaging or the like.
In a series
of experiments, straw was fed into the inlet of a 40 mm diameter twin-screw
digester,
the pitch of the screw varying along its length to define five treatment zones
of
to temperature ranging from 90°C at the inlet to 165°C in the
penultimate zone. Alkali
was added using a metering pump, with sufficient water to achieve the
indicated
liquid to solids digestion ratio. A temperature in the downstream cooking
zones of
the digester of not less than 165°C was found preferable in order to
achieve a well-
disintegrated pulp. It was concluded that it is possible to achieve Kappa
numbers in
15 the low forties from a relatively short 40mm diameter twin screw, in less
than one
minute and with caustic additions below 10%, which represents a quality
consistent
with a semi-chemical pulp. In a full-scale machine it is envisaged that the
cooking
temperature would be 170°C which is usual for making semi-chemical pulp
using a
continuous screw digester.
Run No. 1 2 3 4 5 6 7
Temperature165165 165165 165 165 165
Screw Speed120120 120120 120 120 50
Straw Feed6 3.756 6 6.3 6 3.75
Rate
Liquid
to fibre
ratio ( 1.62.5 1.61.6 1.5 3.2 3.36
to 1)
Caustic
to fibre
ratio % 9.415 9.49.4 9 9.4 8.3
Kappa No. 45.845.843.743.939.960.5 423
It is predicted that using a commercial scale twin-screw extruder, it may be
possible to achieve fully digested chemical pulp suitable for printing or
writing
papers without an additional cooking stage with a retention time of e.g. about
1
minute and a cooking pressure of 7 bar. Alternatively the twin screw extruder
may be
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 18 -
used to produce semi-chemical pulp in a first stage, which pulp may be
converted
into chemical pulp in a second cooking stage e.g. with 30 minutes additional
cooking
at a pressure of 7 bar before pulp de-watering.
An embodiment of a twin-screw co-rotating digester 131 appears in Fig 4.
Graminaceous raw material (straw, flax, hemp, bagasse), wood chips or any
other
cellulosic raw material from buffer storage can be pulped. To this end the raw
material is drawn into the digester 131 in which the screw profiles are
specially
designed with two outer sections 133 and 134 having flights going in a first
direction
to while in a middle section 135 the flight direction is reversed. The flights
of the
conveyor screws are manufactured from hardened steel with a deep cut flight to
improve the size of the region where raw material is positively conveyed as
explained
above and are specially designed to minimize fibre damage. This particular
design
results in a reduced energy demand, which means that a smaller drive shaft and
gearbox can be used, which also reduces capital cost. The design of the screw
profile
and the reduced drive shaft size also allows throughput of raw material to be
increased by an anticipated 400% over conventional co-rotating twin screws.
As can be seen schematically in FIG. 4, one embodiment of the conveyor has
2o a first zone 137 to which the raw material is fed through a feed hopper
139. The
flights of the conveyor screws in zone 1 are designed to be as open as
possible in
order to accept the material into the unit. In a second zone 141 cooking
liquor which
is preferably a soda, process white liquor not containing significant sulphide
but
containing Ca(OIT)2 can be added through an inlet 143 and steam can be
introduced
into the second zone of the digester barrel through inlet 145. The length of
the region
141 and the dwell time of the raw material in that region vary depending upon
the
nature of the material, but are sufficient for a digestion of significant
quantities of
lignin located in regions of the starting material that are readily accessible
to the
pulping liquor (easy lignin) and dissolving other readily soluble material.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 19 -
As explained above, it is desirable to convert silica present in straw or
other
graminaceous starting materials into insoluble silicate, much of which becomes
precipitated onto the cellulosic fibres when pulping straw to prevent harmful
amounts
of silica from entering the black liquor effluent as soluble silicates which
can give
rise to scaling of evaporators or other parts of the downstream chemical
recovery
system. For that purpose when pulping straw or other graminaceous material
calcium
hydroxide can be added in the second zone 141 at a rate of 4% to dry raw
material
(straw) with 8% sodium hydroxide. In general, there is used about one part by
weight
of calcium hydroxide for two parts by weight of sodium hydroxide. This method
is
1o applicable in any alkaline based pulping system and has the efFect of re-
precipitating
sodium silicate onto the cellulosic fibres as calcium silicate some, most or
substantially all of which can remain in situ on those fibres (depending upon
the
subsequent pulp treatment conditions) when they have separated as pulp and
during
subsequent washing, bleaching and conversion into paper. In a mixed alkali
system in
which both NaOH and Ca(OH)a are present, reaction to form insoluble CaSi03 is
highly favoured over the competitive reaction to form soluble NazSi03, so that
the
silica is retained on the fibres as calcium silicate or enters the black
liquor as
insoluble calcium silicate. In consequence there is no significant difference
as regards
soluble silica content between the black liquor from graminaceous materials
treated
2o with NaOH/Ca(OH)Z and the black liquor from wood pulping, which is
amendable to
treatment by known methods. The precipitated calcium silicate, once formed, is
not
prone to re-dissolve under the conditions encountered in subsequent pulp
processing
operations including bleaching, de-watering and paper or board manufacture
none of
which employ low pH values (pH < 4), and it simply provides an innocuous part
of
the ash content of the pulp, being chemically similar to Wollastonite which
can be
used as a filler in paper-making and also being similar to china clay which is
a
complex silicate. The only difFerence, so far as subsequent processing is
concerned is
that the pulp from graminaceous materials may have an ash content of e.g. 3-4%
whereas the ash content of wood pulp is usually about 1%. It will be noted
that the
3o effluent treatment station 14 described below which gasifies the black
liquor using a
fluidized bed of Ca0 naturally produces a white liquor containing Ca(OH)2 as
well as
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 20 -
regenerated Na~H, this black liquor both digesting the feed and precipitating
calcium
silicate on the cellulosic fibres so that it does not enter the black liquor
in harmful
quantities.
The partially digested raw material passes to a third zone 147 where the
conveyor screws have a reversed flight region 135 which acts as a braking zone
for
the advancing raw material which forms a plug of material being processed,
with a
high pressure zone thus being generated upstream of the plug. In this zone the
barrel
wall has a perforated region 149 through which some of the cooking liquor
becomes
squeezed out. The action of the white liquor on the raw material is to rapidly
solubilize all or much of the readily accessible lignin content of the raw
material
which dissolves with soluble hemicellulose and other dissolvable organic
solids. In
the third region a portion of the white liquor which may typically correspond
to about
half of the white liquor initially added exits the region 149 as a black
liquor stream of
high solids content, typically about 30 wt% solids. Removal of the easy
lignin,
soluble hemicellulose and other soluble organic materials in this black liquor
stream
means that these materials are no longer present to impede alkali attack on
lignin
remaining in the partly digested raw material in subsequent zones, assists the
later
stages of digestion, and also provides a solids-rich black liquor stream that
contributes to recovery of a final combined black liquor stream of relatively
high
solids content. A remainder of the barrel and conveyor screws define a fourth
zone
151 and a fifth zone 132 leading to an outlet 153 for pulp. In Zone 4
temperature and
pressure are increased as indicated so that digestion continues through
residual white
liquor on the pulp which also serves to lubricate the partly digested starting
material
as it progresses along the digester barrel. In Zone 5 temperature and pressure
are
reduced in preparation for the material leaving the twin-screw digester. The
material
travels through the twin-screw unit in between 2-3 minutes. The screw speed
may be
around 200 rpm. It will be appreciated that while the twin-screw digester
shown is set
up with four/five zones, any number of zones, suitably from 3 upwards can be
used
and for whatever treatment regime is required.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 21 -
The digester is suitably of modular construction which facilitates making
changes to both screw and barrel configurations. This should be a cost-
effective way
to make use of one standard twin-screw unit to process many different types of
cellulosic raw materials and/or to produce different grades of pulp simply by
changing the screw and barrel configurations. Machine speeds of between 50-500
rpm may be used. A speed of 50-250 rpm has been used in practice. The speed
needs
to be adjusted for the raw material used and the pulp quality required. The
twin-
screw digester can be built in such a way that chemicals and liquids can be
injected
and liquids or steam can be vented or removed in each zone, which is a
standard
1 o feature of twin-screw extruders. It has further been realized that a
sophisticated
gearbox and drive of the type conventionally used in twin-screw extruders is
not
necessary to suitably pulp fibres. A simple gearbox and drive can be used,
reducing
the capital cost and energy consumption. It is anticipated that the pulping
system will
consume less than half the energy of a conventional twin screw used for this
purpose.
In another embodiment of the digester (not shown), the feed zone into the
conveyor
screws is enlarged compared to the other zones to allow the raw material to be
fed
freely into said zone to increase the throughput of the conveyer. As the raw
material
moves forward into the treatment zone and the first and second pressure zones,
the
area within the co-rotating twin screw conveyor may be continually decreased
which
2o has the effect of continually increasing the pressure within the zones.
Using a co-rotating twin screw with a barrel size of 100 millimeters, the co-
rotating intermeshing-twin screw extruder is set up with five zones as
described
below.
Zone 1 Z 3 4 5
Type Straw intakeTreatment Initial DigestionDigestionldischarge
liquor
steam/alkalirecovery
T C. 65 100 130 150 130
3o P (bar)~ 0 0 2-3 4-5 2-3
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 22 -
The pulp exiting from the twin screw may have approximately 50% moisture
content and would be expected to have a Kappa Number of 30-40. This is an
unbleached chemical pulp ready for bleaching using standard methods and
suitable
for printing and writing papers. A semi-chemical pulp with a higher kappa
number
suitable for use in fluted packaging can also be produced if required. The
result is a
function of the rpm and the flight design or time spent in the twin-screw
extruder
together with the pressure, temperature and amount of pulping chemicals used.
It is
expected that kappa-numbers as low as 20 may be achievable using a single
screw.
1o If production of a full chemical pulp is not achieved in the twin-screw
digester 131 as expected, it may then be necessary for the pulp to be further
digested
e.g. in a further twin-screw digester or in a single screw digester of the
kind disclosed
by Atchison above e.g. using steam at 1-2 bar pressure (120°) for a
further 20-40
minutes, and optionally adding further white liquor. Atchison discloses the
use of a
two horizontal tube digesters disposed one above another with the discharge
outlet of
the upper digester feeding partly digested material into the inlet of the
second
digester. In practice, in the prior art, it has often been necessary to use
three such
digesters arranged in series. However, the twin-screw digester disclosed above
produces signif cant breakdown of the graminaceous or other starting material,
and it
is expected that only a single further twin-or single-screw digester stage
will be
needed to achieve a chemical pulp ready for bleaching so that installation
size and
capital cost may be reduced. A Kappa Number of 14-20 may be achieved after
this
further digestion.
Pulp from the twin-screw digester or from a subsequent further digestion
stage is then washed for further recovery of black liquor before further
treatment e.g.
bleaching in the case of a chemical pulp. Washing is normally a multi-stage
operation
in which e.g. the pulp is successively contacted with wash liquid and passed
through
a plurality of dewatering stages arranged in series e.g. 2-4 such stages with
3 stages
being usual. Some of the calcium silicate on the fbres may become dispersed in
the
wash liquor, and at the end of the multi-stage washing operation under come
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 23 -
conditions e.g. about 50% of the calcium silicate may become re-dispersed into
the
wash liquor. Re-dispersion of calcium silicate may be inhibited by
incorporation of a
flocculating agent e.g. polyacrylamide into the wash liquor. However, under
the
conditions used in evaporation of the black liquor, any re-dispersed calcium
silicate is
significantly less prone to form harmful deposits than sodium silicate.
Furthermore,
calcium silicate is relatively water-insoluble and high melting, Wollastonite
melting
at 1540°C, well above the bed temperatures for fluidized bed
gasification of black
liquor and well above the melting temperature of sodium silicate. Under the
process
conditions contemplated herein, it is expected that any calcium silicate in
the black
liquor will remain as discrete particles, and will not either volatilise or
promote
agglomentation of the fluidized bed used in the recovery process.
Each dewatering stage may take place in a screw-type press in which an
elongated rotating screw fits within a foraminous sleeve that in turn is
contained
within a housing forming means for collecting wash liquor that has passed
through
the sleeve, pulp being advanced longitudinally of the screen by the rotating
screw and
being subjected to a squeezing action e.g. with the cross-sectional area of
the channel
defined by the screw thread or the spacing between adjacent screw threads
diminishing from the inlet end of the screen towards the outlet, so that the
pulp
2o collected becomes compressed and liquid is progressively squeezed from the
pulp.
US-A-6792850, US-A-6393728, US-A-6736054 and US-A-3256808 illustrate some
of the common features of this type of press. Wash liquid normally progresses
in the
opposite sense to the pulp so that if there are first, second and third screw
presses
connected in series, water is supplied to a mixing tank for washing the pulp
to be fed
to the third screw press, recovered wash liquor from the third press is
supplied to a
further mixing tank to wash the pulp to be fed to the second screw press, and
recovered wash liquor from the second screw press fed to a mixing tank for
washing
the chemical pulp supplied to the first screw press, recovered liquor from the
first
screw press which may by now have a solids concentration of above 10 wt% e.g.
3o about 12-15 wt% providing a relatively concentrated black liquor stream
which can
be combined with the still more concentrated black liquor stream at 149 and
passed
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 24 -
for alkali recovery. Some, or even a major part of the silica that has become
converted into calcium silicate will find its way into the black liquor.
However, the
calcium silicate will not tend to dissolve under the recovery process
conditions
employed and in contrast to sodium silicate which is soluble will give rise to
no or to
reduced harmful glassy deposits when the black liquor is concentrated by
evaporation. It will be appreciated that in-line mixers may be used instead of
mixing
tanks, and that other known ways of dewatering pulp may be used in place of
screw
presses. Screw presses are relatively small compared to drum washers that
could also
be used, and are preferable for the smaller-scale operations involved in
pulping of
to graminaceous materials.
Black liquor recovery
The present invention provides a treatment process to recover organic and
is inorganic chemicals and energy from black liquor effluent arising from the
pulping of
cellulosic raw materials to make paper. It is specifically intended to be used
with the
above described pulping process but could be used alone to treat black liquor
from
other pulping processes. It is designed to be economically viable at small
throughputs.
Owing to the absence of sulphides, the black liquor may be treated to
volatilise the organic component thereof in a fluidized bed reactor under
oxidizing
conditions in the presence of a stochiometric amount of oxygen or oxygen-
containing
gas such as air. Preferably, however, the black liquor is gasified (partially
oxidized)
to a synthesis gas having as components zhter alia CO 2, CO, HaO, and H2
usually
together with methane and, Ca+ components under pyrolysing or partial
oxidizing
conditions in the presence of a sub-stochiometric amount of oxygen or free-
oxygen-
containing gas. Such gas may be a mixture of steam and combustion gas from a
natural gas boiler, or a mixture of steam and combustion gas from a boiler
supplied
with cleaned recycled synthesis gas supplemented with natural gas as required.
The
gases act as fluidising medium for the bed, and the bed material consists of
or
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 25 -
comprises CaO which catalyses the gasification process and also the
gasification of
any char which may form as by-product within the bed. In such a process, the
amount
of oxygen in the gas mixture supplied to the bed should be sufficient to
support
partial oxidation and maintain bed temperature, but insufficient to convert
the sodium
s and/or calcium hydroxide content of the black liquor entirely to carbonate,
it being
believed possible to conduct the reaction so that at least some of the NaOH
remains
as such in the bed. Thus the oxygen content of the fluidizing gas may be <5%
oxygen
and usually about 1.5-2% oxygen, giving an oxygen content in the off gas above
the
bed of < 1 %, typically about 0.8%, all by volume.
Thermal degradation of the organic matter in black liquor begins above
200°C
producing water vapor, CO~, CO, H2, light hydrocarbons, tar and in the case of
Kraft
and other sulfur containing liquors, light sulfurous compounds (e.g.
mercaptans). By
600°C devolatilisation is essentially complete with the char residue
containing fixed
carbon, same hydrogen and most of the inorganic matter. Char composition can
vary
widely and depends upon both processing conditions (e.g. temperature) and fuel
characteristics. Straw black liquor has a lower calorific value than wood
black
liquor, which should be taken into account when designing the fluidized bed
reactor.
2o A series of non-isothermal experiments was conducted using temperature
ramp thermogravimetric analysis (TGA). This technique allows rapid measurement
of the temperature decomposition profile of a material and the subsequent
determination of its thermal decomposition kinetics. For the straw black
liquor,
heated under Nz at a rate of 20°Clmin, five separate peaks were
identified: (a) 25 to
2s 105°C loss of moisture, (b) 105 to 250°C main volatiles peak,
(c) 300 to 350°C
smaller volatiles peak, (d) 425 to 500°C smaller volatiles peak and (e)
650°C
devolatilisation of inorganic species (e.g. Na and K). It was not possible to
identify
which organic components of the liquor were associated with specific peaks
from
these tests although it is likely the three main organic components, lignin,
30 hemicellulose and carboxylic acids can be attributed to the three main
volatiles peaks.
These tests also indicate that the operating temperature of the industrial
reactor
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 26 -
should preferably not exceed 750°C in order to allow the majority of
pulping
chemicals (i.e. Na and K) to be recovered in the preferred solid form,
reaction
temperatures of e.g. 675-725°G being preferred, e.g. 675-700°C.
Batch fluidized bed experiments using a conventional or bubbling fluidized
bed of silica sand of 0.1 m internal diameter were conducted to determine
typical off
gas compositions and yields of char, off gas and tars from black liquor
pyrolysis,
gasification and combustion experiments. Bed mixing and agglomeration tendency
was also investigated. Black liquor concentrated to 29% and 45% solids was fed
to
1o the bed operating at 500 - 700°C with U/U~= 4 (i.e. vigorously
fluidized), supplied
with mixtures of Na and O~. Analysis of the off gas showed typical
compositions of
the main product gases ranged between 0-5% for Ha, 7 -12.5% for CH4, 7.5-15%
for
CO, 55-89% for COa and 0-8% for CZ+ species. Bed agglomeration was severe in
all
cases despite the high U/CT,~ and typically resulted in the experiment being
terminated in less than 20 minutes, due to loss of fluidization. Gas yields
were
typically very low (around 9% at 550°C increasing to 25% at
700°C). Together the
low gas yields and gas compositions (i.e, high [C02] and low [CO] and [H2])
suggest
that the steam reforming reactions of the char were not occurring as they
should
under gasifying conditions. This is most likely due to the poor gas-solid
contact
occurring in an agglomerated fluidized bed. The increase in gas yield with
temperature is important however and suggests an industrial reactor (which is
designed to have a significantly higher tolerance for bed agglomeration)
should be
run as close to the melting point of K and Na as possible in order to maximize
the
production of synthesis gas. For pyrolysis experiments (with N2 only), when a
condenser was added to the off gas line, the yield of tar (the condensable
fraction)
was measured to be 30 and 38% at 550 and 700 °C respectively. Ghar
yields were
45% at 550°C decreasing to 39% at 600°C and 31% at 700°C,
again suggesting
temperature is an important process variable.
A spouted fluidized bed difFers from a bubbling ffuidized bed in that it is
designed with a central jet that farces material from the base of the bed
along the
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 27 -
central axis before it is allowed to settle back down along the walls of the
vessel. The
bed is typically comically shaped rather than cylindrical to assist with the
bulk
circulation movement. It may exhibit greater bed stability and fewer
tendencies to
agglomerate than a bubbling fluidized bed.
The reactor is preferably a toroidal fluidized bed reactor; such reactors are
described in US Patents 4479920 and 4559719 (the disclosures of which are
incorporated herein by reference) and are available from Torftech Limited of
Reading, UK (www.torftech.com). Such a reactor is intended to overcome the
1o problems of conventional fluidised bed reactors as regards control of
temperature and
rate of heat transfer within the bed resulting from the random nature of
lateral
movement of the bed particles in a bed that remains essentially static and is
fluidised
by a vertical flow of gas/air mixture. Both a bubbling fluidized bed and a
spouting
fluidized bed suffer from these problems. In the experience, of the inventors,
agglomeration occurs when there are hot spots in the bed. A toroidal fluidised
bed
minimises this risk and the active Ca0 bed will also help.
The solution proposed by Torftech and which is followed according to a
preferred aspect of the invention, as applied to the treatment of black
liquor,
comprises
providing a reactor having a processing region provided with a mass of
particulate material consisting of or containing calcium oxide;
supplying heated fluidizing gas to the processing region so as to generate a
swirling flow of fluid within said processing region, the fluid of said
swirling flow of
fluid causing the particulate material to assume a compact band and circulate
about
an axis of said processing region in a turbulent manner, the fluidizing gas
including
oxygen for at least partially combusting organic material in the black liquor;
feeding the black liquor into said compact band of particulate material and
treating the black liquor in said bed so as to gasify organic materials in
said black
liquor;
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 28 -
recovering organic material from said black liquor as off gas from said bed;
and
recovering inorganic material from said black liquor as solids from said bed.
It is believed that each particle travels to and fro inside the processing
region
along the full periphery of the compact band so that uniform processing
conditions
may be obtained. The motions of the particles within the particulate mass are
determined by the combined effects of the fluid flow, gravity, and the
centrifugal
forces created by the swirling of the fluid, and the result is a thorough and
continuous
to muting of these particles and matter to be processed, on the supply of such
matter
into the band of panicles. Consequently, a very efficient processing operation
may be
achieved using only a shallow band of particles. Furthermore, the process gas
stream
impacts on and minimises the insulating microscopic gas layer around each
particle.
As a result, the heat and mass transfer rate is greater than in other types of
reactor,
which should permit faster and more effective processing.
Tests have been carried out using a semi-industrial scale reactor of the above
toroidal fluidized bed type. Where the black liquor to be treated is converted
into
solids form, the solids were fed through a two-stage screw feeder (one dosing
screw,
one feeding screw). Where the black liquor to be treated was in the form of a
liquid,
it was fed using a centifugal pump either through the top of the reactor or
through a
two-phase nozzle (supplied with Ar orN2) directly above and perpendicular to
the
bed surface. The ofF gas and some solid material exited through the top of the
reactor
and passed through a length of duct into a cyclone and venturi scrubber before
being
passed to an afterburner chamber operating at 850°C. Bed solids were
removed from
the bed through a central discharge oririce. Solid and gas samples were taken
from
different locations around the plant. The reactor chamber was of a high
temperature
ceramic (not strictly required for this work) and was of 400 mm at the base
rising to
500 n~rn at the top and was of height 850mm. The distributor through which
3o fluidizing gas entered the reactor chamber from beneath the fluidized bed
comprised
a number of parallel plates aligned at an angle, between which plates the
fluidizing
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 29 -
gases passed. The free surface area of the distributor was 30%. The effect of
the
distributor was to impart a severe swirling motion within the bed, which
caused high
local gas velocities and high turbulence without blowing particles out of the
top of
the reactor. The nominal fluidizing velocity used for all experiments was 10
m/s,
which adequately supported a calcium oxide bed of mean particle size 1.5 mm.
Two different processing modes were considered for black liquor treatment in
these experiments: (a) preparation of a premix containing black liquor and
calcium
oxide in a specified ratio and (b) direct spraying of black liquor (of various
solids
1o contents) onto a resident bed of quicklime in the reactor. Operationally,
feeding a
dried solid to the toroidal fluidized bed is the simplest alternative, but
there are
engineering issues that prevent this from being preferred. The reaction
product with
Ca0 is a plastic mass with thermoplastic properties (even using a substantial
excess
of lime) that requires further heating to achieve complete dryness and then
cooling to
achieve solidification before it can be crushed and fed through a gas-tight
seal (e.g. a
screw feeder or rotary valve) into the reactor. This is expensive and
inconvenient.
Furthermore, equilibrium modeling shows that an excess of CaO prevents the
formation of hydrocarbons (particularly CH4) by reaction to form CaC03. The
alternative is to pump the black liquor directly onto a resident bed of Ca0
(mixed
with inert material; either silica sand or alumina) inside the gasifier using
a
conventional black liquor spray nozzle. Swelling of black liquor as it reaches
200°C
is a difficulty at the semi-industrial scale, but is not expected to be a
problem with the
larger equipment used on the industrial scale.
The bed solids containing the inorganic components from the black liquor, as
well as the calcium from the catalyst, exit the bed via a central discharge
weir where
they can be dissolved to give a mixture of Na:'-, Ca2+, CO32' and (OH)' ions
in solution
according to the equation:
Ca0 + H20 + Na2C03 --> 2NaOH + CaC03.
3o This solution is then treated as described above.
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 30 -
The processing temperature used in these experiments ranged between S50
and 725°C. In general an increase in temperature increased the rate of
conversion of
the black liquor to reaction products. The majority of the inorganic content
of the
black liquor could be recovered in solid form at higher temperatures than
previously
believed possible. Typical recoveries were in excess of 90% where experimental
data
was measured within 95% confidence limits.
The influence of temperature upon the off gas quality was indiscernible. The
black liquor solids concentration also did not have a discernable influence
upon either
to sodium recovery or ofF gas composition but as expected the ofF gas
contained more
water when the more dilute liquors (16% solids) were processed. It is expected
that
the optimum solids content for feeding to the black liquor process will be
dictated by
upstream processing conditions and economic constraints, i.e. the solids
content
produced by the pulping process and an analysis of the cost of evaporating
water in
the toroidal fluidized bed reactor compared with doing so in advance in a
standard
multi effect evaporator.
As regards oxygen concentration in the fluidizing gas, early experiments with
5 - 20% [02] in the produced off gas mixtures of N2, CO2 and H2O as expected
under
2o these oxidizing conditions. Inorganic species recovery, i.e. Na, C and K,
was good.
Bed and cyclone underflow samples ranged from 39 - 60wt% Ca and 2.5 - l3wt%
Na. Experiments conducted with < 2% [02] produced gas mixtures containing
predominantly Na, C02, H20 and hydrocarbons, H2 or CO being difficult to
detect
chromatographically as they were obscured by the nitrogen peak.
Equilibrium modeling has shown that Ca0 is important for the gasification
reaction. It also prevents solid carbon from forming (i.e. char and tars) and
forms
complexes with sulfur (specifically CaS), although this may been seen as very
much
a beneficial side reaction. It is preferably added in the preferred amount
CaO:DS (dry
3o solids) of 0.2:1 to 0.4:1, most preferably about 0.35:1. At a ratio of
1.2:1 it lowers the
amount of hydrocarbons formed considerably by tying up carbon as CaC03
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 31 -
Thus, in a preferred embodiment, black liquor of solids content 10-40% e.g.
15-30% may be supplied directly to a toroidal fluidized bed reactor containing
either
calcium oxide alone or calcium oxide and an inert material, and supplied with
steam
and combustion gas from a burner supplied with recycled synthesis gas from the
reactor supplemented with natural gas as required. An evaporation plant and a
calciner to recover Ca0 from CaC03 are desirable, and a small quantity (about
10%)
of the CaC03 stream will need to go to waste to prevent build-up of heavy
elements
which are naturally drawn up form the soil by the plants. This material could
however be sent for local re-processing and used to make bricks or in cement
works.
Referring to FIG. 5, a preferred embodiment of the effluent treatment process
will now be described.
Black liquor effluent arising from the pulping process is collected in a
digestion liquor storage tank 301 and concentrated to 30-70% solids using a
standard
evaporator 302 designed for concentration purposes. If the black liquor
effluent
comes from the co-rotating twin-screw conveyor at a solids concentration of
30% or
above it may be treated directly in the processing vessel eliminating the
evaporation
step. The concentrated black liquor is moved to a reactor vessel 304 at a
temperature
2o in excess of 90°C. using an enclosed twin-screw transport system
303. The enclosed
transport system is used to minimize the loss of organic components through
vaporization. A temperature in excess of 90°C. is required to decrease
the viscosity of
the black liquor so that it becomes easy to transport. The black liquor is
treated in the
reactor vessel 304 in either of two methods.
In a first method, the black liquor is introduced into a toroidal fluidized
bed
reactor 304 by spraying the concentrated liquor into the chamber of the
reactor in
which a bed of fluidized material is supported. The material may be an earth
oxide
such as lime at a ratio of 0.3:1 of lime to black liquor dry solids. The mean
particle
3o size of the earth oxide may be between 1 and 4 mm. As previously explained,
the
reactor may operate under staichiometric or sub-stoichiometric conditions. In
a
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 32 -
second method, black liquor effluent is pre-mixed in the twin screw conveyor
303
with an earth oxide such as lime (CaC)) in the ratio e.g. 0.3:1 lime to black
liquor dry
solids to convert the black liquor a granular friable material which may then
be screw
fed into a toroidal fluidized bed reactor 304. Again when the black liquor is
converted to a dry solid before it is supplied to the bed, the reactor may
operate under
stoichiometric or sub-stoichiometric conditions. In a variation of both
methods, the
ratio of earth oxide, e.g. lime to black liquor dry solids may be in the range
0.2 to
1.3:1 lime to black liquor dry solids. The earth oxide may be supplied by a
standard
calciner 308. In both cases the chamber of the toroidal fluidized bed reactor
304 is
1o maintained within the temperature range 300 to ?50°C and preferably
650-?50°.
where the necessary chemical reaction takes place in the space of seconds. In
a
further possible embodiment of the process a portion of the solids within the
toroidal
fluidized bed reactor 304 may be recycled via the screw feeder 303 back to the
reactor 304.
The black liquor is converted by a chemical reaction to:
(1) Sodium hydroxide and sodium carbonate and lime within the fluidized
bed reactor 304. The bed will overflow through a central discharge point and
the
overflowing material is then dissolved in a dissolving tank 305 to recover
sodium
hydroxide as green liquor in the traditional manner known as re-
causticisation. The
green liquor is then filtered using a known filter 306 to form a calcium
carbonate
sludge and white liquor (containing sodium hydroxide and calcium hydroxide)
for re-
use in the pulping process. However, in a variation of the process, if the
temperature
is carefully controlled, re-causticisation can take place in the reactor. In
this case,
sodium carbonate is not formed and the sodium hydroxide can be recovered
without
the use ofthe dissolving tank (306).
(2.) A gas and liquids with a combustible component which can be utilized
for energy production. The gas is collected to power a boiler 309 that will
produce
energy and steam for use in the pulp mill process line. In a further possible
embodiment of the process the gas containing combustible components may be
recycled to the f(uidized bed reactor to provide heat for the chemical
recovery
CA 02544426 2006-05-O1
WO 2005/045126 PCT/GB2004/050023
- 33 -
reaction.
The calcium carbonate sludge may be dried to remove some water and sent to
a second calciner reactor 308, which may be a toroidal fluidized bed reactor.
This
reactor may operate at a temperature of around '1100°C where calcium
carbonate
CaC03 is converted back to calcium oxide Ga0 for re-use in the black liquor
effluent
chemical recovery process. Approximately 10% of the fluidized bed material
generated may need to be removed from the process continuously in order to
prevent
the build up of heavy metals and other materials in the process. If required,
black
liquor effluent below 30% solids can also be processed using this method (and
has
been tested). However energy consumption is greater and so this is not
preferred.
It will be appreciated that various changes may be made to the embodiment
described above without departing from the invention. For example, the black
liquor
treated in the fluidized bed could be a Kraft liquor or a mixture of the
sodalCa(OH)2
black liquor and Kraft liquor or black liquor from the soda/anthraquinone
process.