Note: Descriptions are shown in the official language in which they were submitted.
CA 02544482 2006-04-21
CORROSION AND WEAR RESISTANT ALLOY
DESCRIPTION OF THE INVENTION
Field of the Invention
[001] The invention relates to powder metallurgy corrosion and wear
resistant tool steel alloy article, manufactured by hot isostatic compaction
of
nitrogen atomized, prealloyed high-chromium, high-vanadium, high-niobium
powder particles. The alloy of the article of the invention is characterized
by
very high wear and corrosion resistance, making it in particular useful as a
material from which to make components for advanced bearing designs as
well as machinery parts exposed to severe abrasive wear and corrosion
conditions such as those, among many others, in the plastic injection
molding industry and the food industry.
Background of the Invention
[002] To perform satisfactorily, the alloys that are used in a number
of demanding applications-such as screws and barrels in the plastic
injection molding industry, for example-must be resistant to wear and
corrosion attack. The trend in the industry is to keep increasing processing
parameters (e.g., temperature and pressure), which in turn imposes ever-
increasing demands on the alloys and their ability to successfully withstand
corrosion attack and wear of the materials being processed. In addition, the
corrosiveness and abrasiveness of those materials are constantly
increasing.
[003] The wear resistance of tool steels depends on the amount, the
type, and the size distribution of primary carbides, as well as the overall
hardness. The main function of primary alloy carbides, due to their very
high hardness, is to provide wear resistance. Of all types of primary
CA 02544482 2006-04-21
2
carbides commonly found in tool steels, V-rich and V-Nb-rich MC primary
carbides possess the highest hardness.
[004] The corrosion resistance of tool steels depends primarily on
the amount of "free" chromium in the matrix, i.e., the amount of chromium
that is not "tied up" into carbides. For good corrosion resistance, through-
hardening tool steel must contain at least about 12 wt. % "free" chromium in
the martensitic matrix after heat treatment. However, corrosion and wear
resistant tool steels must also contain a relatively high level of carbon for
heat treatment response. As chromium has a high affinity for carbon with
which it forms chromium-rich carbides, a corrosion and wear resistant tool
steel must contain excess chromium.
[005] The corrosion resistance of tool steels is further improved by
the presence of molybdenum in the martensitic matrix. Some tool steels
that contain about 10 wt. % "free" chromium in the martensitic matrix are
corrosion resistant because they also contain a sufficient amount of "free"
molybdenum. An example is Crucible 154 CM grade, which is based on the
Fe-1.05C-14Cr-4Mo system.
[006] In order to withstand the stresses imposed during operation,
the tool steel must also possess sufficient mechanical properties, such as
hardness, bend fracture strength, and toughness. In addition, the tool steel
must possess sufficient hot workability, as well as machinability and
grindability, to ensure that parts with the required shape and dimensions can
be manufactured. In general, the higher the volume fraction of primary
carbides, the higher the wear resistance of the tool steel, and the lower its
toughness and hot workability.
[007] The corrosion and wear resistant martensitic tool steels
currently used include grades such as CPM S90V, M390, Elmax, Anval 10V-
12, HTM X235, for example. Despite the fact that the overall chromium
content of some of these alloys is as high as 20 wt. % (e.g., M390), the
corrosion resistance is not necessarily as high as one might expect.
CA 02544482 2006-04-21
3
Depending on the overall chemical composition and the heat treatment
parameters, a large amount of chromium, which is a strong carbide former,
is pulled out of the matrix and tied up into chromium-rich carbides. This tied
up chromium does not contribute toward the corrosion resistance.
[008] One of the practices that has been used to improve the
combination of resistance to corrosion and wear, as exemplified by CPM
S90V, is to add vanadium. This alloying addition forms hard vanadium-rich
MC primary carbides and ties up a part of the carbon. Due to the fact that
the affinity of vanadium toward carbon is higher than that of chromium, the
presence of vanadium in tool steels decreases the amount of chromium-rich
primary carbides, all other conditions being equal (i.e., the overall chromium
and carbon content, the heat treatment parameters, for example). In the
alloy of the invention, in addition to vanadium, niobium is used as well in
order to further increase the amount of MC primary carbides, and in turn
decrease the amount of chromium-rich primary carbides, due to the fact that
niobium has even a higher affinity toward carbon than vanadium.
[009] A primary object of the invention is to provide wear and
corrosion resistant, high chromium, high vanadium, high niobium, powder
metallurgy tool steel article with significantly improved corrosion and wear
resistance.
SUMMARY OF THE INVENTION
[010] It has been discovered that the improved balance between
wear resistance, the corrosion resistance, and the hardness of the high-
chromium, high-vanadium, powder metallurgy martensitic stainless steel
article of the invention is affected by adding niobium. The alloy article of
the
invention possesses a unique combination of corrosion and wear properties
that are achieved by balancing its overall chemical composition as well as
selecting an appropriate heat treatment.
CA 02544482 2006-04-21
4
[011] It has been discovered that the addition of niobium decreases
the solubility of chromium in V-Nb-rich MC primary carbides, which in turn
increases the amount of "free" chromium in the martensitic matrix.
According to thermodynamic calculations, the carbon sublattice of the V-Nb-
rich MC primary carbides that precipitate in the alloy of the invention has
less vacancies compared to the carbon sublattice of the comparable V-rich
MC primary carbides: (V, Nb)Co.83 versus VCo,~9.
[012] It has been discovered that the presence of niobium in the alloy
of the invention also lowers the amount of chromium that dissolves in MC
primary carbides. This in turn increases the amount of "free" chromium in
the matrix, which further improves the corrosion resistance.
[013] The major alloying elements used in the alloy of the invention
(chromium, molybdenum, vanadium, and niobium) are ferrite stabilizers.
High amounts of these ferrite stabilizers can lead to the presence of ferrite
in
the heat-treated microstructure. It has been discovered, however, that the
presence of about 2 wt. % cobalt in the alloying system of the invention is a
necessary and sufficient measure to eliminate ferrite in the heat-treated
microstructure.
[014] Finally, in order to obtain a desired combination of wear and
corrosion resistance, along with good mechanical properties, such as bend
fracture strength, toughness, and grindability, it is necessary to control
closely the atomization process (to obtain fine spherical powder) and the hot
isostatic parameters of the prealloyed powders as is well-known in the art.
The alloy of the invention is to be preferably hot isostatically pressed at
the
temperature of 2150°F (~25°F) and the pressure of at least 14.5
ksi.
[015] In accordance with the invention, there is provided a corrosion
and wear resistant article produced by hot isostatic composition of nitrogen
gas atomized prealloyed powder particles within the following composition
limits, in weight percent, carbon, 2.0 to 3.5, preferably 2.7 to 3.0; silicon
1.0
CA 02544482 2006-04-21
5
max.; chromium 12.0 to 16.0, preferably 13.5 to 14.5; molybdenum 2.0 to
5.0 preferably 3.0 to 4.0; vanadium 6.0 to 11.0, preferably 8.5 to 9.5;
niobium 2.0 to 6.0, preferably 3.0 to 4.0; cobalt 1.5 to 5.0, preferably 2.0
to
3.0; nitrogen 0.05 to 0.30, preferably 0.10 to 0.20; and balance iron and
incidental impurities.
[016] Preferably, carbon is balanced with chromium, molybdenum,
vanadium, and nitrogen in accordance with
Cmin = 0.4 + 0.099x(%Cr - 11 ) + 0.063x%Mo + 0.177x%V + 0.13x%Nb
- 0.85x%N
Cmax = 0.6 + 0.099x(%Cr - 11 ) + 0.063x%Mo + 0.177x%V + 0.13x%Nb
- 0.85x%N
CA 02544482 2006-04-21
6
BRIEF DESCRIPTION OF THE DRAWINGS
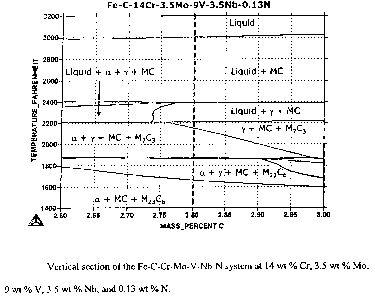
[017] Figure 1 shows a vertical section of the Fe-C-Cr-Mo-V-Nb-N
system at 14 wt % Cr, 3.5 wt % Mo, 9 wt % V, 3.5 wt % Nb, and
0.13 wt % N;
[018] Figure 2 is a vertical section of the Fe-C-Cr-Mo-V-Nb-Co-N
system at 14 wt % Cr, 3.5 wt % Mo, 9 wt % V, 3.5 wt % Nb, 2 wt % Co, and
0.13 wt % N;
[019] Figure 3 shows the etched microstructure (magnification of
1500X) of the alloy of the invention (04-099) hardened from 2150°F in
oil
and tempered at 975°F for 2h+2h+2h; and
[020] Figure 4 shows the etched microstructure (magnification of
1500X) of the hardened alloy (04-100) with no cobalt present.
CA 02544482 2006-04-21
7
DESCRIPTION OF THE EMBODIMENTS
Chemical Compositions Tested
[021] Table 1 gives the chemical compositions that were examined
experimentally and that led to the alloy of the article of the invention that
achieves an improved combination of corrosion and wear resistant
properties. The reported alloys 03-192 through 04-099 are alloys in
accordance with the invention.
[022j All examined compositions were prepared using the Crucible
Particle Metallurgy (CPM) technology. Prealloyed tool steel grades of the
various reported chemical compositions were melted in a nitrogen
atmosphere, atomized by nitrogen gas, and hot-isostatically-pressed (HIP)
at the temperature of 2150°F and the pressure of 14.5 ksi for four
hours.
[023] With respect to the various alloying elements in the wear and
corrosion resistant tool steel are concerned, the following applies.
[024] Carbon is present in an amount of at least 2.0 %, while the
maximum content of carbon may amount to 3.5 %, and preferably in the
range of 2.7-3.0 %. It is important to carefully control the amount of carbon
in order to obtain a desired combination of corrosion and wear resistance,
as well as to avoid forming either ferrite or unduly large amounts of retained
austenite during heat treatment. The carbon in the articles of the invention
may preferably be balanced with the chromium, molybdenum, vanadium,
and nitrogen contents of the alloy of the invention according to the following
formulae:
C°,i" = 0.4 + 0.099x(%Cr - 11 ) + 0.063x%MO + 0.177x%V + 0.13x%Nb
- 0.85x%N
CmaX = 0.6 + 0.099x(%Cr - 11 ) + 0.063x%MO + 0.177x%V + 0.13x%Nb
- 0.85x%N
CA 02544482 2006-04-21
8
[025] Nitrogen is present in an amount of 0.05-0.30 %, and
preferably in the range of 0.10-0.20 %. The effects of nitrogen in the alloy
of
the invention are rather similar to those of carbon. In tool steels, where
carbon is always present, nitrogen forms carbonitrides with vanadium,
niobium, tungsten, and molybdenum. Unlike carbon, nitrogen improves the
corrosion resistance of the alloy of the invention when dissolved in the
martensitic matrix.
[026] Silicon may be present in an amount of up to 1 %, and
preferably up to 0.5 %. Silicon functions to deoxidize the prealloyed
materials during the melting phase of the gas-atomization process. In
addition, silicon improves the tempering response. Excessive amounts of
silicon are undesirable, however, as it decreases toughness and promotes
the formation of ferrite in the microstructure.
[027] Manganese may be present in an amount of up to 1 %, and
preferably up to 0.5%. Manganese functions to control the negative effects
of sulfur on hot workability. This is achieved through the precipitation of
manganese sulfide. In addition, manganese improves hardenability and
increases the solubility of nitrogen in the liquid prealloyed materials during
the melting phase of the gas-atomization process. Excessive amounts of
manganese are undesirable, however, as it can lead to the formation of
unduly large amounts of retained austenite during the heat treatment.
[028] Chromium is present in an amount of 12.0-16.0 %, and
preferably in the range of 13.5-14.5 %. The main purpose of chromium is to
increase the corrosion resistance, and, to a lesser degree, to increase
hardenability and secondary-hardening response.
[029] Molybdenum is present in an amount of 2.0-5.0 %, and
preferably in the range of 3.0-4.0 %. Like chromium, molybdenum increases
the corrosion resistance, hardenability, and secondary-hardening response
of the alloy of the invention. Excessive amounts of molybdenum, however,
reduce hot workability.
CA 02544482 2006-04-21
9
[030] Vanadium is present in an amount of 6.0-11.0 %, and
preferably in the range of 8.5-9.5 %. Vanadium is critically important for
increasing wear resistance. This is achieved through the formation of
vanadium-rich MC type primary carbonitrides.
[031] Niobium is present in an amount of 2.0-6.0 %, and preferably
in the range of 3.0-4.0 %. Every percent of niobium is equivalent to the
amount of vanadium calculated as follows:
%V=(50.9/92.9)x%Nb
where 50.9 and 92.9 are atomic weights of vanadium and niobium,
respectively. Niobium and vanadium are equivalent elements when it comes
to the wear resistance. However, these two elements do not have the same
effect on the corrosion resistance. The presence of niobium decreases the
solubility of chromium in MC primary carbides, i.e., niobium-vanadium-rich MC
primary carbides contain a smaller amount of chromium compared to
vanadium-rich MC primary carbides. This in turn increases the amount of
"free" chromium in the matrix, which in turn increases the corrosion
resistance.
[032] To illustrate the effect of niobium on the alloy of the invention,
Thermo-Calc software, coupled with TCFE3 steel thermodynamic database,
was used to model two alloys that have the equivalent amount of vanadium;
one with niobium (Fe-2.8C-14Cr-3.5Mo-9V-3.5Nb-2Co-0.13N) and the other
one without niobium (Fe-2.8C-14Cr-3.5Mo-11 V-2Co-0.13N). The two alloys
have the same vanadium equivalency (11% V). Thermodynamic
calculations were performed for the following two austenitization
temperatures: 2050°F and 2150°F. The results are given in Tables
2 and 3.
The amount of "free" chromium in the matrix is higher in the alloy that
contains niobium. Based on thermodynamic calculations, it has been
discovered that the presence of niobium decreases the solubility of
CA 02544482 2006-04-21
10
chromium in MC primary carbides (see Table 3), which in turn results in a
higher level of "free" chromium in the matrix.
[033j Cobalt is present in an amount of 1.5-5.0 %, and preferably in
the range of 2.0-3.0 % in order to prevent the undesirable presence of ferrite
(a) in the heat-treated microstructure of the alloy of the invention.
CA 02544482 2006-04-21
11
[034] Table 1. Chemical compositions that were experimentally
examined as well as modeled with Thermo-Calc software.
Alloy C Cr Mo W V Nb Co N
02-
1.64 16.89 2.85 2.78 - 3.66 5.25 0.206
354
02- 1.77 16.85 2.85 2 - 66 5 0
78 3 23 207
355 . . . .
02-
1.88 16.87 2.86 2.79 - 3.66 5.23 0.205
356
02- 1.g0 17.00 2.91 2 - 68 34 0
69 3 5 183
357 . . . .
02
2.14 17.05 2.92 2.69 - 3.68 5.34 0.182
358
02-
2,33 17.08 2.92 2.70 - 3.68 5.35 0.182
359
03 2.61 14.23 3.02 - 8 08 1 0
10 3 95 157
192 . . . .
03- 2.66 14.23 3.02 - 8 3 1 0
10 08 95 157
193 . . . .
03- 2.71 14.23 3.02 - 8 3 1 0
10 08 95 157
194 . . . .
03- 2,g1 14.23 3.02 - 8 3 1 0
10 08 95 157
195 . . . .
03- 2.49 14.20 2.97 - 7 3 1 0
78 13 99 115
199 . . . .
03- 2,59 14.20 2.97 - 7 13 1 0
78 3 99 115
200 . . . .
03- 2.64 14.20 2.97 - 7 3 1 0
78 13 99 115
201 . . . .
04-
2,76 13.76 3.49 - 8.98 3.50 1.96 0.127
098 ~
04- 2,g3 13.76 3.49 - 8 51 1 0
99 3 96 134
099 . . . .
04- 2,68 13.89 3.35 - 9 3 - 0
03 42 125
100 . . .
[035] Table 2. Chemical composition of austenitic matrix at 2050°F
and 2150°F.
Chemical
Allo F Composition
of
Austenitic
Matrix
[wt.
%]
y [
]
C Cr Mo V Nb Co N Fe
CA 02544482 2006-04-21
12
9V 0.42 13.392.45 1.19 0.008 2.48 0.0042bal.
3.5Nb
2050
11 V- 0.43 12.552.29 1.43 - 2.46 0.0024bal.
ONb
V 0.55 13.952.60 1.45 0.012 2.45 0.0062bal.
~
3.5N
b
2150
11 V- 0.56 13.082.46 1.75 - 2.42 0.0038bal.
ONb
[036] Table 3. Chemical composition of MC primary carbides at
2050°F and 2150°F.
Chemical
Composition
of
MC
Primary
Carbides
[at.
%]
Alloy [
F]
C Cr Mo V Nb Co N Fe
9V 9
43.1 5.12 3.62 36.41 9.12 0.0028 2.19 0.35
3.5Nb
2050
11 V- 41.95 7.44 3.84 43.84 - 0.0036 2.18 0.75
ONb
V 43 5.86 3.33 35.91 9.09 0.0039 2.16 0.49
15
3.5Nb .
2150
11 V- 41.82 8.44 3.49 43.06 - 0.0049 2.15 1.05
ONb
[037] Table 4. Heat treatment response of alloys hardened from
2150°F in oil and tempered for 2h+2h+2h.
Bar Tempering
Temperature
No. 500 750 975 1000 1025 1050 1100 1200
04-098 59.4 59.7 62.5 60.7 59.7 58.3 53.1 46.7
04-099 60.1 60.7 63.5 61.4 60.7 58.6 53.3 47.4
04-100 49.3 51.8 54.2 51.9 50.8 49.0 47.0 40.2
S90V 58.5 60.5 60.5 I
CA 02544482 2006-04-21
13
[038] Table 5. Pin-abrasion wear resistance of alloys.
Austenitization Hardness Pin
Alloy Temperature Temper [HRC] Abrasion
500F 59.5 49.5 mg
04-098 F 62.5 33.7 mg
2150
975F
500F 60.0 45.4 mg
04-099 2150
F 63.5 29.4 mg
975F
500F 49.5 65.0 mg
i
04-100 2150
F 54.0 49.1 mg
975F
500F 59.0 52.0 mg
CPM S90V F 61.5 37.3 mg
2150
975F
500F 57.0 70.0 mg
Elmax 1975
F
975F
500F 58.0 62.0 mg
M390 F
2100
975F
500F
X235 F
2100 59.5 52.5
986F/1022F
[039] Table 6. Calculated matrix chemical compositions of corrosion
and wear resistant tool steels.
Chemical PRE
Composition
of
Austenitic
Matrix
[wt.
%]
Alloy [
F] C Cr Mo V W Nb Co N
440C 1900 0.43 11.570.06 - - - - 0.06512.81
10V- 2100 0.49 11.220.82 1.69 - - - 0.00313.97
12
S90V 2100 0.54 12.330.75 1.71 - - - 0.00214.84
Elmax 2100 0.57 12.7 0.92 1.17 - - - 0.02116.08
S30V 2000 0.46 10.921.71 1.03 - - - 0.00516.65
X235 2100 0.52 13.970.91 1.15 - 0.01 - 0.01317.17
M390 2100 0.52 13.790.93 1.31 0.55- - 0.02518.16
MPL-1 2100 0.54 12.642.37 1.67 - - - 0.00420.52
S 11 2100 0.48 13.662.53 1.31 - 0.01 2.47 0.00522.09
OV
CA 02544482 2006-04-21
14
[040] Table 7. Pitting potentials (Ep;t) in 1 %NaCI aqueous solution.
Ep;t
All PRE [mV]
vs.
SCE
oy 500F 750F 975F 1025F
440C 12.81-140 -249 -355 -321
Anva110V-1213.979 38 -180 -138
CPM S90V 14.8459 -17 -176 -183
Elmax 16.08213 243 -211 -216
CPM S30V 16.6579 -2 -240 -236
X235 17.1797 138 -164 -282
M390 18.16160 -121 -170 -179
MPL-1 20.52-72 15 -94 -100
04-099 22.09403 272 -17 -71
Microstructure
[041] Figure 3 shows the microstructure of an alloy of the invention
(alloy number 04-099). The alloy was hardened from 2150°F in oil and
tempered at 975°F for 2h+2h+2h. After etching with Vilella's reagent
for 90
seconds, the total volume of primary carbides was measured to be 21.7
percent, the standard deviation being 0.7 percent.
[042] During the designing stage, the thermodynamics calculations
performed on the Fe-2.8C-14Cr-3.5Mo-9V-3.5Nb-0.13N alloy indicated the
presence of ferrite (a) when the alloy is austenitized at a temperature that
is
below 2156°F (see Figure 1 ). The y+MC+M~C3 field needed to be
expanded, or, in other words, the line that divides the y+MC+M~C3 field and
the oc+y+ MC+M~C3 field needed to be shifted toward the left-hand side of
the diagram in order to prevent the presence of ferrite in the heat-treated
microstructure.
CA 02544482 2006-04-21
15
[043] Additional thermodynamic calculations indicated that the
addition of about 2 wt % of cobalt would sufficiently extend the y+ MC+M~C3
field, eliminating the possibility of the ferrite presence in the hardened
condition (see Figure 2).
[044] The first set of compositions examined experimentally was
centered around the Fe-C-17Cr-2.5Mo-2.5W-3.5Nb-5Co-0.2N system
(alloys 02-354 through 02-359; see Table 1 ). The problem with this alloying
system was retained austenite that was difficult to transform into martensite
even after sub-zero treatments.
[045] The second set of compositions examined experimentally was
centered around the Fe-C-14Cr-3Mo-8V-3Nb-2Co-N system (alloys 03-192
through 03-195 and 03-199 through 03-201 ). The levels of carbon balance
tested ranged from -0.20 to +0.20, and were calculated using the following
formula:
Cbal = %C - [0.4 + 0.099x(%Cr - 11 ) + 0.063x%Mo + 0.177x%V +
0.13x%Nb - 0.85x%N]
[046] It is a well established fact that the amount of carbon present in
the steel has the most profound effect on the properties of any corrosion and
wear resistant tool steel grade. The amount of carbon has a direct effect on
the hardness, the wear resistance, and the corrosion resistance of wear and
corrosion resistant tool steel. For a given chemical composition of the steel,
the carbon balances were targeted to be close to zero (~0.2%).
[047] The alloys that are based on the Fe-C-14Cr-3Mo-8V-3Nb-2Co-
N system exhibited better hardness response, better corrosion resistance,
and marginally better wear characteristics when compared to other
corrosion and wear resistant martensitic tool steels.
[048] In order to examine whether the wear and corrosion resistance
of the second set of compositions could be further improved, an additional
set of compositions centered around the Fe-2.8C-14Cr-3.5Mo-9V-3.5Nb-
CA 02544482 2006-04-21
16
2Co-0.13N system was manufactured and experimentally examined (alloys
04-098 through 04-100). The tests showed that the alloys of the third set
exhibited better heat treat response (see Table 4) and better wear
characteristics (see Table 5) compared to CPM S90V. The alloy of the
invention also has better corrosion resistance (see Table 6) compared to
other widely used corrosion and wear resistant tool steels (see Table 6).
Influence of Cobalt on the Microstructure
[049] An alloy (04-100) was prepared specifically to demonstrate the
influence of cobalt and the necessity to use it in the alloy of the invention.
Both thermodynamic calculations and experimental results clearly indicated
that the Fe-2.8C-14Cr-3.5Mo-9V-3.5Nb-0.13N system has to contain at least
1.5 wt. pct. Co, if ferrite is to be eliminated from the heat treated
microstructure. The major alloying elements in the alloy of the invention
(chromium, molybdenum, vanadium, and niobium) are all ferrite forming
elements. The presence of ferrite, as well as a poor heat treat response,
was indeed observed in the alloy that contained no cobalt (04-100).
[050] As predicted by thermodynamic calculations, the matrix of the
heat treated alloy that contains no cobalt (alloy number 04-100) has some
ferrite present (see Figure 4), which resulted in poor heat-treat response for
the alloy (less than 54 HRC). The other two alloys of the third set that
contain about 2 wt. pct. of cobalt (04-098 and 04-099) developed desired
heat-treated responses (62.5 HRC and 63.5 HRC, respectively) as well as
microstructures that consist of V-Nb-rich MC and Cr-rich M~C3 primary
carbides in the matrix of tempered martensite.
Corrosion Resistance
[051] Pitting Resistance Equivalent Number: The pitting
resistance equivalent number (PRE) is useful for evaluating the resistance
CA 02544482 2006-04-21
17
of an austenitic stainless steels to pitting and crevice corrosion. The PRE is
calculated using the following equation:
Cr + 3.3(Mo + 0.5W) +16N
[052] Generally, the PRE is calculated using the bulk chemical
composition. However, the alloys disclosed herein contain high amounts of
primary carbides that deplete the matrix of some of the necessary elements
needed for corrosion resistance. Therefore, the PRE of these alloys was
calculated using an estimated matrix composition as determined by Thermo-
Calc software (see Table 6). The alloys are listed by increasing PRE
values.
[053] Based on the matrix composition, the invention alloy (04-099)
has the highest PRE even though it does not have the highest matrix
chromium content. The PRE of this alloy (04-099) is even higher than those
alloys with higher bulk chromium contents such as MPL-1, X235, M390 and
Elmax. Since the matrix chromium content of these alloys is similar, the
high PRE of the invention alloy is due to its high contents of chromium and
molybdenum in the matrix. This is because 30-47.5% of the chromium in the
high chromium alloys is used in the formation of the primary carbides in
these materials. Only about 2.5% of the chromium in the invention alloy is
used in the formation of the primary carbides thereby keeping most of the
chromium in the matrix to aide in corrosion resistance. More chromium is
present in the matrix in the invention alloy due to the presence of niobium
and vanadium which preferentially form more stable MC type carbides
compared to the M~C3 type (chromium rich) carbides.
(054] Corrosion Tests: Potentiodynamic tests were used to
evaluate the pitting resistance of the invention alloy and of commercially
available wear and corrosion resistant alloys. The tests were conducted in
an aqueous solution containing 1 % NaCI. The tests were conducted by
varying the potential from -0.8V vs. SCE (saturated calomel reference
electrode) to at most 0.5V at a scan rate of 0.2mV/sec. Two graphite rods
CA 02544482 2006-04-21
18
were used as the counter electrodes. The test solution was purged with
nitrogen gas for at least 20 minutes before testing each specimen. The
pitting resistance of the alloys is defined by the pitting potential (Ep;t)
obtained from a potentiodynamic curve. The more positive the pitting
potential, the more resistant the alloy is to pitting. Prior to each test, the
specimen was ground down by 600 grit paper. The specimen was then
washed and dried with alcohol.
[055] Depending on the application, the wear and corrosion resistant
alloys are given different heat treatments. If corrosion resistance is of
utmost concern, the alloy is typically tempered at or below 750°F,
which
allows more of the chromium to stay in the matrix by minimizing the
precipitation of secondary carbides. If hardness and wear resistance is the
primary concern, then the alloys are typically tempered at 950°F and
above
to allow for secondary hardening effects to take place. Therefore, each alloy
was tempered at 500°F, 750°F, 975°F and 1025°F.
[056] Corrosion Resistance Results: The pitting potential (Ep;t ) for
each alloy at each tempering temperature is given in Table 7. The results
show that the invention alloy (04-099) with the highest PRE also has the
highest resistance to pitting at all tempering temperatures. The Ep;t for the
invention alloy is almost 50% higher that that of the next closest alloy,
Elmax, at a tempering temperature of 500°F. In general, the alloys
with 18-
20% bulk chromium content, i.e., Elmax, M390 and X235, have mediocre
pitting resistance compared to the invention alloy at all tempering
temperatures. The alloy with the highest bulk chromium content actually
has one of the lowest pitting potentials at the low tempering temperatures.
These results indicate that the total chromium content is not an indicator of
how resistant the material is to corrosion.
[057] The matrix compositions of X235 and the alloy of the invention
are similar. However, the pitting resistance of these two alloys is
CA 02544482 2006-04-21
19
significantly different. This difference in pitting resistance is attributed
to the
higher molybdenum content of the invention alloy. The cobalt in the
invention alloy is not expected to significantly affect the pitting resistance
of
the alloy of the invention.
Heat Treatment Response
[058] When compared with CPM S90V, the alloys of the invention
(04-098 and 04-099) offer better heat-treatment response-approximately
1.5-2.0 HRC higher for the same heat treatment. The heat-treatment
responses of the alloys of the invention and CPM S90V are given in Table 4.
Abrasive Wear Resistance
[059] All the pin-abrasion wear resistance test specimens were
austenitized at 2150°F for 10 minutes, quenched in oil, and then
tempered
at either 500°F (for maximum corrosion resistance) or 975°F (for
maximum
secondary-hardening response) for 2h+2h+2h. The results are given in
Table 5. The pin-abrasion wear resistance of other corrosion and wear
resistant martensitic tool steels is included as well for comparison purposes.
[060] All element amounts are reported in weight percent.