Note: Descriptions are shown in the official language in which they were submitted.
CA 02545452 2006-05-10
WO 2005/047240 PCT/EP2004/012394
1
Ortho-substituted pentafluoride sulfanyl-benzenes, method for the
production thereof and the use thereof in the form of valuable synthesis
intermediate stages
The chemistry of pentafluorosulfanyl derivatives has gained importance in
the last few years, especially since novel preparation processes have been
found (Tetrahedron 56 (2000) 3399; Organic Letters 4(17) (2002) 3013).
However, to date only very few compounds are known which bear
substituents other than hydrogen and fluorine on a phenyl ring in the ortho-
position to the pentafluorosulfanyl group. The only known synthetic route
(Journal of Fluorine Chemistry 112 (2001) 287) uses expensive reagents
such as AgF2 and is afflicted with poor yields. The authors account for this
by the large bulk of the pentafluorosulfanyl group which generally makes
ortho-substitution very difficult. This opinion is also shared by other
authors
(J. Am. Chem. Soc. 84 (1962) 3064). It is therefore surprising that it is
possible to electrophilically substitute in the ortho-position to the
pentafluorosulfanyl group. In this way, novel ortho-substituted
pentafluorosulfanylbenzenes are obtained which constitute valuable
intermediates, for example for preparing medicaments, diagnostic aids,
liquid crystals, polymers, pesticides, herbicides, fungicides, nematicides,
parasiticides, insecticides, acaricides and arthropodicides.
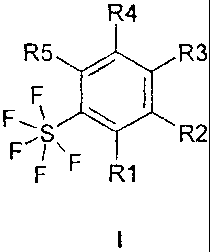
The invention relates to pentafluorosulfanylbenzenes of the formula I
R4
R5 / R3
F
Fs, \ R2
F F R1
where
R1 is Cl, Br, I, -CN, -SO2R6, NO2, alkoxy having 1, 2, 3 or 4 carbon
atoms, NR7R8, -O-(CH2)b-(CF2)c-CF3, -(SOd)e-(CH2)f-(CF2)g-CF3,
alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or cycloalkyl having 3, 4,
5, 6, 7 or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may
be replaced by fluorine atoms;
R6 is OH, F, Cl, Br, I or alkyl having 1, 2, 3 or 4 carbon
CA 02545452 2007-09-05
2
atoms;
R7 and R8
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms or -CH2-CF3;
b and c
are each independently zero or 1;
d is zero, 1 or 2;
e is zero or 1;
f is zero, 1, 2, 3 or 4;
g is zero or 1;
or
R1 is -(CH2)h-phenyl or -0-phenyl,
in which the phenyl radicals are unsubstituted or substituted by 1, 2
or 3 radicals selected from the group consisting of F, Cl, Br,
I, -Oj-(CH2)k-CF3, alkoxy having 1, 2, 3 or 4 carbon atoms, alkyl
having 1, 2, 3 or 4 carbon atoms and -SO2CH3;
j is zero or 1;
k is zero, 1, 2 or 3;
h is zero, 1, 2, 3 or 4;
or
R1 is -(CH2)1-heteroaryl
which is unsubstituted or substituted by 1, 2 or 3 radicals selected
from the group consisting of F, Cl, Br, I, -Om-(CH2)n-CF3, alkoxy
having 1, 2, 3 or 4 carbon atoms, alkyl having 1, 2, 3 or 4 carbon
atoms and -SO2CH3;
m is zero or 1;
n is zero, 1, 2 or 3;
1 is zero, 1, 2, 3 or 4;
R2 and R4
are each independently hydrogen, F, Cl, Br, I, -CN,
NR9R1O, -OR11, -SR12, -COR13, -SOgCH3, -(SOr)s-(CH2)t-
(CF2)u-CF3, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7 or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen
atoms may be replaced by fluorine atoms;
R9 and R10
are each independently alkyl having 1, 2, 3 or 4 carbon
atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl having 1, 2,
3 or 4 carbon atoms, alkylsulfonyl having 1, 2, 3 or
CA 02545452 2006-05-10
3
4 carbon atoms;
or
R9 and R10, together with the nitrogen atom bearing them,
form a heterocycle of the formula III:
/X :c
N
Y
III
X and Y
are each independently CO or SO2;
R11 and R12
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl
having 1, 2, 3 or 4 carbon atoms, alkylsulfonyl having
1, 2, 3 or 4 carbon atoms;
R13 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms;
gand r
are each independently 1 or 2;
s is zero or 1 -1
t is zero, 1, 2, 3 or 4;
u is zero or 1;
v is zero, 1, 2, 3 or 4;
w is zero or 1 -,
R3 is hydrogen, F, Cl, Br, I, -CN, -NO2, -COR14, -SO2CH3, alkyl having
1, 2, 3, 4, 5 or 6 carbon atoms, alkoxy having 1, 2, 3 or 4 carbon
atoms, -Ox-(CH2)y-CF3,
R14 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms,
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms
or -Oaa-(CH2)bb-CF3;
x is zero or1;
y is zero, 1, 2 or 3;
as is zero or 1;
bb is zero, 1, 2 or 3;
R5 is hydrogen, F, Cl, Br, I, -CN, -SO2CH3, alkoxy having 1, 2, 3 or
4 carbon atoms, NR15R16, -O-(CH2)ee-(CF2)f:-CF3, -(SOgg)hh-
(CH2)jj-(CF2)kk-CF3, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or
CA 02545452 2006-05-10
4
cycloalkyl having 3, 4, 5, 6, 7 or 8 carbon atoms in which 1, 2, 3 or 4
hydrogen atoms may be replaced by fluorine atoms;
R15 and R16
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms or -CH2-CF3;
eeand ff
are each independently zero or 1;
gg is zero, 1 or 2;
hh is zero or1;
jj is zero, 1, 2, 3 or 4;
kk is zero or1;
or
R5 is -(CH2)11-phenyl or -0-phenyl,
in which the phenyl radicals are unsubstituted or substituted by 1, 2
or 3 radicals selected from the group consisting of F, Cl, Br,
I, -Omm-(CH2)nn-CF3, alkoxy having 1, 2, 3 or 4 carbon atoms, alkyl
having 1, 2, 3 or 4 carbon atoms and -SO2CH3;
mm is zero or 1;
nn is zero, 1, 2 or 3;
II is zero, 1, 2, 3 or 4;
or
R5 is -(CH2)00-heteroaryl
which is unsubstituted or substituted by 1, 2 or 3 radicals selected
from the group consisting of F, Cl, Br, I, -Opp-(CH2)rr-CF3, alkoxy
having 1, 2, 3 or 4 carbon atoms, alkyl having 1, 2, 3 or 4 carbon
atoms and -SO2CH3;
pp is zero or 1;
rr is zero, 1, 2 or 3;
00 is zero, 1, 2, 3 or 4;
and salts thereof;
excluding compounds of the formula I in which R2 and R4 are each Cl and
R3 is F or Cl,
excluding compounds of the formula I in which one of the R2 and R4
substituents is Cl and the other of the R2 and R4 substituents is CN and R3
is Cl and
excluding compounds of the formula I in which R1 is NO2 and the other
substituents are each hydrogen.
CA 02545452 2007-09-05
Preference is given to compounds of the formula I in which:
R1 is Cl, Br, I, -CN, -S02R6, NO2, alkoxy having 1, 2, 3 or 4 carbon
atoms, NR7R8, -0-(CH2)b-(CF2)c-CF3, -(SOd)e-(CH2)f-(CF2)g-CF3,
alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or cycloalkyl having 3, 4,
5 5, 6, 7 or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may
be replaced by fluorine atoms;
R6 is OH, F, Cl, Br, I or alkyl having 1, 2, 3 or 4 carbon
atoms;
R7 and R8
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms or -CH2-CF3;
b and c
are each independently zero or 1;
d is zero, 1 or 2;
e is zero or 1;
f is zero, 1, 2, 3 or 4;
g is zero or1;
or
R1 is -(CH2)h-phenyl or -0-phenyl,
in which the phenyl radicals are unsubstituted or substituted by 1, 2
or 3 radicals selected from the group consisting of F, Cl, Br,
I, -Oj-(CH2)k-CF3, alkoxy having 1, 2, 3 or 4 carbon atoms, alkyl
having 1, 2, 3 or 4 carbon atoms and -SO2CH3;
j is zero or 1;
k is zero, 1, 2 or 3;
h is zero, 1, 2, 3 or 4;
or
R1 is -(CH2)1-heteroaryl
which is unsubstituted or substituted by 1, 2 or 3 radicals selected
from the group consisting of F, Cl, Br, I, -Om-(CH2)n-CF3, alkoxy
having 1, 2, 3 or 4 carbon atoms, alkyl having 1, 2, 3 or 4 carbon
atoms and -SO2CH3;
m is zero or 1;
n is zero, 1, 2 or 3;
I is zero, 1, 2, 3 or 4;
R2 and R4
are each independently hydrogen, F, Cl, Br, I, -CN,
NR9R1O, -OR11, -SR12, -COR13, -(SOr)s-(CH2)t-(CF2)u-CF3, alkyl
CA 02545452 2006-05-10
6
having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl having 3, 4, 5, 6, 7
or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may be
replaced by fluorine atoms;
R9 and R10
are each independently alkyl having 1, 2, 3 or 4 carbon
atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl having 1, 2,
3 or 4 carbon atoms, alkylsulfonyl having 1, 2, 3 or
4 carbon atoms;
or
R9 and R10, together with the nitrogen atom bearing them,
form a heterocycle of the formula III:
X
N\ I
Y
III
X and Y
are each independently CO or S02;
R11 and R12
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl
having 1, 2, 3 or 4 carbon atoms, alkylsulfonyl having
1, 2, 3 or 4 carbon atoms;
R13 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms;
s is zero;
t and u
are each independently zero or 1;
v and w
are each independently zero or 1;
R3 is hydrogen, F, Cl, Br, I, -CN, -NO2, -COR14, -SO2CH3, alkyl having
1, 2, 3, 4, 5 or 6 carbon atoms, alkoxy having 1, 2, 3 or 4 carbon
atoms, -Ox-(CH2)y-CF3,
R14 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms,
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms
or -Oaa-(CH2)bb-CF3;
x is zero or1;
y is zero, 1, 2 or 3;
CA 02545452 2007-09-05
7
as is zero or 1;
bb is zero, 1, 2 or 3;
R5 is hydrogen or F;
and salts thereof;
excluding compounds of the formula I in which R2 and R4 are each Cl and
R3 is F or Cl,
excluding compounds of the formula I in which one of the R2 and R4
substituents is Cl and the other of the R2 and R4 substituents is CN and R3
is CI and
excluding compounds of the formula I in which R1 is NO2 and the other
substituents are each hydrogen.
Particular preference is given to compounds of the formula I in which:
R1 is Cl, Br, 1, -S02R6 or N02;
R6 is OH, F, Cl, Br, I or alkyl having 1, 2, 3 or 4 carbon
atoms;
R2 and R4
are each independently hydrogen, F, Cl, Br, I, -CN,
NR9R10, -OR11, -SR12, -COR13, -(SOr)s-(CH2)t-(CF2)u-CF3, alkyl
having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl having 3, 4, 5, 6, 7
or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may be
replaced by fluorine atoms;
R9 and R10
are each independently alkyl having 1, 2, 3 or 4 carbon
atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl having 1, 2,
3 or 4 carbon atoms, alkylsulfonyl having 1, 2, 3 or
4 carbon atoms;
or
R9 and R10, together with the nitrogen atom bearing them,
form a heterocycle of the formula Ilia:
O
N
0 Ilia
R11 and R12
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl
CA 02545452 2006-05-10
8
having 1, 2, 3 or 4 carbon atoms, alkylsulfonyl having
1, 2, 3 or 4 carbon atoms;
R13 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms;
s is zero;
t and u
are each independently zero or 1;
v and w
are each independently zero or 1;
R3 is hydrogen, F, Cl, Br, I, -CN, -NO2, -COR14, -SO2CH3, alkyl having
1, 2, 3, 4, 5 or 6 carbon atoms, alkoxy having 1, 2, 3 or 4 carbon
atoms, -Ox-(CH2)y-CF3,
R14 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms,
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms
or -Oaa-(CH2)bb-CF3;
x is zero or1;
y is zero, 1, 2 or 3;
as is zero oil;
bb is zero, 1, 2 or 3;
R5 is hydrogen or F;
and salts thereof;
excluding compounds of the formula I in which R2 and R4 are each Cl and
R3 is F or Cl,
excluding compounds of the formula I in which one of the R2 and R4
substituents is Cl and the other of the R2 and R4 substituents is CN and R3
is Cl and
excluding compounds of the formula I in which R1 is NO2 and the other
substituents are each hydrogen.
In one embodiment, preference is given to compounds of the formula I in
which:
R1 is Cl, Br, I, -CN, -S02R6, NO2, alkoxy having 1, 2, 3 or 4 carbon
atoms, NR7R8, -Oa-(CH2)b-(CF2)c-CF3, -(SOd)e-(CH2)f (CF2)g-CF3,
alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or cycloalkyl having 3, 4,
5, 6, 7 or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may
be replaced by fluorine atoms;
R6 is OH, F, Cl, Br, I or alkyl having 1, 2, 3 or 4 carbon
atoms;
CA 02545452 2006-05-10
9
R7 and R8
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms or -CH2-CF3;
a, b and c
are each independently zero or 1;
d is zero, 1 or 2;
e is zero or 1;
f is zero, 1, 2, 3 or 4;
g is zero or 1
or
R1 is -(CH2)h-phenyl or -0-phenyl,
in which the phenyl radicals are unsubstituted or substituted by 1, 2
or 3 radicals selected from the group consisting of F, Cl, Br,
I, -Oj-(CH2)k-CF3, alkoxy having 1, 2, 3 or 4 carbon atoms, alkyl
having 1, 2, 3 or 4 carbon atoms and -SO2CH3;
j is zero or 1;
k is zero, 1, 2 or 3;
h is zero, 1, 2, 3 or 4;
or
R1 is -(CH2)1-heteroaryl,
which is unsubstituted or substituted by 1, 2 or 3 radicals selected
from the group consisting of F, Cl, Br, I, -Om-(CH2)n-CF3, alkoxy
having 1, 2, 3 or 4 carbon atoms, alkyl having 1, 2, 3 or 4 carbon
atoms and -SO2CH3;
m is zero or 1;
n is zero, 1, 2, or 3;
is zero, 1, 2, 3 or 4;
R2 and R4
are each independently hydrogen, F, Cl, Br, I, -CN, -(CH2)o-(CF2)p-
CF3, NR9R1O, -OR11, -SR12, -COR13, -SOqCH3, -(SOr)s-(CH2)t-
(CF2)u-CF3, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7 or 8 carbon atoms, in which 1, 2, 3 or 4 hydrogen
atoms may be replaced by fluorine atoms;
R9, R10, R11 and R12
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl
having 1, 2, 3 or 4 carbon atoms, alkylsulfonyl having
1, 2, 3 or 4 carbon atoms;
CA 02545452 2006-05-10
R13 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms;
o and p
are each independently zero or 1
5 g and r
are each independently zero, 1 or 2;
s is zero or 1;
t is zero, 1, 2, 3 or 4;
u is zero or1;
10 vandw
are each independently zero or 1;
R3 is hydrogen, F, Cl, Br, I, -CN, -COR14, -SO2CH3, alkyl having 1, 2,
3, 4, 5 or 6 carbon atoms, alkoxy having 1, 2, 3 or 4 carbon
atoms, -Ox-(CH2)y-CF3,
R14 is OH, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms,
alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms
or -Oaa-(CH2)bb-CF3;
x is zero or1;
y is zero, 1, 2 or 3;
as is zero or 1;
bb is zero, 1, 2 or 3;
R5 is hydrogen, F, Cl, Br, I, -CN, -SO2CH3, alkoxy having 1, 2, 3 or 4
carbon atoms, NR15R16, -Odd-(CH2)ee-(CF2)ff-
CF3, -(SOgg)hh-(CH2)jj-(CF2)kk-CF3, alkyl having 1, 2, 3, 4, 5 or 6
carbon atoms or cycloalkyl having 3, 4, 5, 6, 7 or 8 carbon atoms, in
which 1, 2, 3 or 4 hydrogen atoms may be replaced by fluorine
atoms;
R15 and R16
are each independently hydrogen, alkyl having 1, 2, 3
or 4 carbon atoms or -CH2-CF3;
dd, ee and if
are each independently zero or 1;
gg is zero, 1 or 2;
hh is zero or 1 -,
jj is zero, 1, 2, 3 or 4;
kk is zero or 1;
or
R5 is -(CH2)11-phenyl or -0-phenyl,
CA 02545452 2006-05-10
11
in which the phenyl radicals are unsubstituted or substituted by 1, 2
or 3 radicals selected from the group consisting of F, Cl, Br,
I, -Omm-(CH2)nn-CF3, alkoxy having 1, 2, 3 or 4 carbon atoms, alkyl
having 1, 2, 3 or 4 carbon atoms and -SO2CH3;
mm is zero or 1;
nn is zero, 1, 2 or 3;
II is zero, 1, 2, 3 or 4;
or
R5 is -(CH2)00-heteroaryl,
which is unsubstituted or substituted by 1, 2 or 3 radicals selected
from the group consisting of F, Cl, Br, I, -Opp-(CH2)rr-CF3, alkoxy
having 1, 2, 3 or 4 carbon atoms, alkyl having 1, 2, 3 or 4 carbon
atoms and -SO2CH3;
pp is zero or 1;
rr is zero, 1, 2, or 3;
00 is zero, 1, 2, 3, or 4;
and the salts thereof;
excluding the compound of the formula I in which R1 and R4 are each NH2
and R2, R3 and R5 are each hydrogen, and
excluding compounds of the formula I in which R2 and R4 are each Cl and
R3 is F or Cl, and
excluding compounds of the formula I in which one of the R2 and R4
substituents is Cl and the other of the R4 and R2 substituents is CN and R3
is Cl.
In one embodiment, preference is given to compounds of the formula I in
which R1 is described by Cl, Br, I, -S02R6 where R6 is OH, F, Cl, Br, I or
alkyl having 1, 2, 3 or 4 carbon atoms, or -NO2; particular preference is
given to compounds of the formula I in which R1 is described by Cl, Br,
I, -S02R6 where R6 is OH or Cl, or -NO2; very particular preference is
given to compounds of the formula I in which R1 is described by Cl or N02,
in particular by NO2. In a further embodiment, particular preference is given
to compounds of the formula I in which R1 is described by Cl, Br, I, -SO2R6
where R6 is OH or CI.
In a further embodiment, preference is given to compounds of the formula I
in which R2 and R4 are each independently described by hydrogen, F, Cl,
Br, I, -CN, -(SOr)s-(CH2)t-(CF2)u-CF3 where s is zero and t and u are each
CA 02545452 2006-05-10
12
independently zero or 1, or by -NR9R1O, -OR11, -SR12, -COR13, alkyl
having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl having 3, 4, 5, 6, 7 or
8 carbon atoms, in which 1, 2, 3 or 4 hydrogen atoms may be replaced by
fluorine atoms, where R9 and R10 are each independently alkyl having 1,
2, 3 or 4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl having 1, 2, 3 or
4 carbon atoms or alkylsulfonyl having 1, 2, 3 or 4 carbon atoms, where v
and w are each independently zero or 1, or R9 and R10, together with the
nitrogen atom which bears them, form a heterocycle of the formula III-
N/X \
\
'
Y
/
Ill
where X and Y are each independently described by CO or SO2,
R11 and R12 are each independently hydrogen, alkyl having 1, 2, 3 or
4 carbon atoms, -(CH2)v-(CF2)w-CF3, alkylcarbonyl having 1, 2, 3 or
4 carbon atoms or alkylsulfonyl having 1, 2, 3 or 4 carbon atoms, where v
and w are each independently zero or 1, and where R13 is OH, alkyl
having 1, 2, 3, 4, 5 or 6 carbon atoms or alkoxy having 1, 2, 3, 4, 5 or
6 carbon atoms; particular preference is given to compounds in which R2
and R4 are each independently described by hydrogen, NR9R1O and
COR13, where R9 and R10 are each independently alkylcarbonyl having 1,
2, 3 or 4 carbon atoms, in particular methylcarbonyl, or R9 and R10,
together with the nitrogen atom which bears them, form a heterocycle of
the formula III,
where X and Y are each independently described by CO or SO2;
in particular, R9 and R10, together with the nitrogen atom which bears
them, may form a heterocycle of the formula Ilia:
O
N
O Ilia
and where R13 is alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms, in
particular methoxy.
In a further embodiment, one of the R2 and R4 radicals in the compounds
of the formula I is described by hydrogen.
CA 02545452 2006-05-10
13
In a further embodiment, preference is given to compounds of the formula I
in which R3 is described by hydrogen, F, Cl, Br, I, -CN or -COR14 where
R14 is OH or alkoxy having 1, 2, 3, 4, 5 or 6 carbon atoms, in particular
methoxy; particular preference is given to compounds of the formula I in
which R3 is described by hydrogen, CN or COOCH3.
In a further embodiment, preference is given to compounds of the formula I
in which R5 is described by hydrogen or F; particular preference is given to
compounds of the formula I in which R5 is described by hydrogen.
Radicals which occur more than once may be the same or different and
each independently have the definitions specified.
When the substituents R1 to R5 contain one or more centers of asymmetry,
they may each independently have either the S or the R configuration. The
compounds may be in the form of optical isomers, of diastereomers, of
racemates or of mixtures thereof in all ratios.
The present invention encompasses all tautomeric forms of the compounds
of the formula I.
Alkyl radicals may be straight-chain or branched. This also applies if they
bear substituents or occur as substituents of other radicals, for example in
fluoroalkyl radicals or alkoxy radicals. Examples of alkyl radicals are
methyl, ethyl, n-propyl, isopropyl (= 1-methylethyl), n-butyl, isobutyl
(= 2-methylpropyl), sec-butyl (= 1-methyipropyl), tert-butyl
(= 1,1-dimethylethyl), n-pentyl, isopentyl, tert-pentyl, neopentyl and hexyl.
Preferred alkyl radicals are methyl, ethyl, n-propyl and isopropyl. One or
more, for example 1, 2, 3, 4 or 5, hydrogen atoms in alkyl radicals may be
replaced by fluorine atoms. Examples of such fluoroalkyl radicals are
trifluoromethyl, 2,2,2-trifluoroethyl and pentafluoroethyl. Substituted alkyl
radicals may be substituted in any positions.
Examples of cycloalkyl radicals are cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl or cyclooctyl. In cycloalkyl radicals, one or more,
for
example 1, 2, 3 or 4, hydrogen atoms may be replaced by fluorine atoms.
Substituted cycloalkyl radicals may be substituted in any positions.
CA 02545452 2006-05-10
14
Phenyl radicals may be unsubstituted or be mono- or polysubstituted, for
example mono-, di- or trisubstituted, by identical or different radicals. When
a phenyl radical is substituted, it preferably has one or two identical or
different substituents. This likewise applies to substituted phenyl radicals
in
groups such as, for example, phenylalkyl or phenyloxy. In monosubstituted
phenyl radicals, the substituent may be in the 2-position, 3-position or
4-position. Disubstituted phenyl may be substituted in the 2,3-position,
2,4-position, 2,5-position, 2,6-position, 3,4-position or 3,5-position. The
substituents in trisubstituted phenyl radicals may be in the 2,3,4-position,
2,3,5-position, 2,4,5-position, 2,4,6-position, 2,3,6-position or 3,4,5-
position.
Heteroaryl radicals are aromatic ring compounds in which one or more ring
atoms are oxygen atoms, sulfur atoms or nitrogen atoms, for example 1, 2
or 3 nitrogen atoms, 1 or 2 oxygen atoms, 1 or 2 sulfur atoms or a
combination of different heteroatoms. The heteroaryl radicals may be
attached via all positions, for example via the 1-position, 2-position,
3-position, 4-position, 5-position, 6-position, 7-position or 8-position.
Heteroaryl radicals may be unsubstituted or be mono- or polysubstituted,
for example mono-, di- or trisubstituted, by identical or different radicals.
This applies likewise to heteroaryl radicals, for example in the
heteroarylalkyl radical. Examples of heteroaryl are furanyl, thienyl,
pyrrolyl,
imidazolyl, pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, indolyl,
indazolyl,
quinolyl, isoquinolyl, phthalazinyl, quinoxalinyl, quinazolinyl and
cinnolinyl.
Heteroaryl radicals are in particular 2- or 3-thienyl, 2- or 3-furyl, 1-, 2-
or
3-pyrrolyl, 1-, 2-, 4- or 5-imidazolyl, 1-, 3-, 4- or 5-pyrazolyl, 1,2,3-
triazol-
1-, -4- or -5-yl, 1,2,4-triazol-1-, -3- or -5-yl, 1- or 5-tetrazolyl, 2-, 4-
or
5-oxazolyl, 3-, 4- or 5-isoxazolyl, 1,2,3-oxadiazol-4- or -5-yl,
1,2,4-oxadiazol-3- or -5-yl, 1,3,4-oxadiazol-2-yl or -5-yl, 2-, 4- or 5-
thiazolyl,
3-, 4- or 5-isothiazolyl, 1,3,4-thiadiazol-2- or -5-yl, 1,2,4-thiadiazol-3-
or -5-yl, 1,2,3-thiadiazol-4- or -5-yl, 2-, 3- or 4-pyridyl, 2-, 4-, 5- or
6-pyrimidinyl, 3- or 4-pyridazinyl, 2- or 3-pyrazinyl, 1-, 2-, 3-, 4-, 5-, 6-
or 7-
indolyl, 1-, 2-, 4- or 5-benzimidazolyl, 1-, 3-, 4-, 5-, 6- or 7-indazolyl, 2-
, 3-,
4-, 5-, 6-, 7- or 8-quinolyl, 1-, 3-, 4-, 5-, 6-, 7- or 8-isoquinolyl, 2-, 4-,
5-, 6-,
7- or 8-quinazolinyl, 3-, 4-, 5-, 6-, 7- or 8-cinnolinyl, 2-, 3-, 5-, 6-, 7-
or
8-quinoxalinyl, 1-, 4-, 5-, 6-, 7- or 8-phthalazinyl. Also included are the
corresponding N-oxides of these compounds, for example 1-oxy-2-, -3-
or -4-pyridyl.
CA 02545452 2006-05-10
Particularly preferred heteroaromatic radicals are 2- or 3-thienyl, 2- or
3-furyl, 1-, 2- or 3-pyrrolyl, 1-, 2-, 4- or 5-imidazolyl, 2-, 3-, 4-, 5-, 6-,
7- or
8-quinolyl, 1-, 3-, 4- or 5-pyrazolyl, 2-, 3- or 4-pyridyl, 2- or 3-pyrazinyl,
2-,
5 4-, 5- or 6-pyrimidinyl and 3- or 4-pyridazinyl.
The invention further relates to a process for preparing the compounds of
the formula I or the salts thereof, which comprises converting compounds
of the formula II by electrophilic aromatic substitution to compounds of the
10 formula I
R4 R4
R5 R3 R5 R3
F ~ F~l
FIS\ R2 F~SF R2
F F F R1
II I
where R1 to R5 are each as defined above.
In the preparation of the compounds of the formula I, the procedure is to
15 carry out an electrophilic aromatic substitution, preferably a
halogenation,
chlorosulfonation or nitration.
In one embodiment, halogenation (R1 = Cl, Br or I) is affected as described
in R.C. Larock, Comprehensive Organic Transformations: A Guide to
Functional Group Preparations, VCH Publishers, New York, Weinheim,
1999, pages 619-628 and in the literature cited therein. The chlorination is
effected, for example, with NCIS in an inert solvent, for example
isopropanol, CHCI3, CH2CI2 or EA at a temperature between -30 C and
100 C, preferably between 40 C and the boiling point of the solvent.
In another embodiment, sulfonation or chlorosulfonation (R1 = S02R6
where R6 is OH or CI) is effected as described in March's Advanced
Organic Chemistry 5th edition 2001, pages 702-703 and in the literature
cited therein.
In another embodiment, nitration (R1 = NO2) is effected as described, for
example, in Houben-Weyl, Methoden der organischen Chemie, 4th edition,
Organo-Stickstoff-Verbindungen IV, part 1, Georg Thieme Verlag Stuttgart
1992, pages 262-341 and in the literature cited therein. Compounds of the
formula II where R3 = COOH are nitrated, for example, with a mixture of
CA 02545452 2006-05-10
16
90% HNO3 and 96% H2SO4 at a temperature between -40 C and 80 C,
preferably between 0 C and 40 C.
From the compounds of the formula I where R1 = NO2, it is possible to
prepare the corresponding anilines (R1 = NH2) as described in
R.C. Larock, Comprehensive Organic Transformations: A Guide to
Functional Group Preparations, VCH Publishers, New York, Weinheim,
1999, 821-828 and the literature cited therein. From these anilines, it is
possible to synthesize, via the diazonium salts by methods known to those
skilled in the art, as described, for example, in Houben-Weyl, Methoden der
organischen Chemie, 4th edition, Organo-Stickstoff-Verbindungen I, part 2,
Georg Thieme Verlag Stuttgart 1990, pages 1060-1136 and in the
references cited therein, the compounds of the formula I with further
definitions of R1.
The starting compounds of the formulae II are commercially available or
can be prepared by processes similar to those described in the literature
and/or known to those skilled in the art.
In the starting compounds, functional groups may also be present in
protected form or in the form of precursors, and then be converted to the
desired groups in the compounds of the formula I prepared by the process
described above. Appropriate protecting group techniques are known to
those skilled in the art.
The workup and, if desired, the purification of the products and/or
intermediates is effected by conventional methods such as extraction,
chromatography or crystallization and conventional dryings.
Also claimed are the compounds of the formula I and/or the salts thereof for
use as a synthetic intermediate, in particular for use as a synthetic
intermediate for preparing medicaments, diagnostic aids, liquid crystals,
polymers, pesticides, herbicides, fungicides, nematicides, parasiticides,
insecticides, acaricides and arthropodicides.
Examples of the various possible uses of pentafluorosulfanyl derivatives
are described in the following publications: WO 9421606, WO 03093228
(insectides, acaricides); DE 19711953, GB 2276379 (herbicides);
CA 02545452 2006-05-10
17
DE 10124480, DE 10353658, Angew. Chem. 1999, 111, 2174, Angew.
Chem. 2000, 112, 4384 (liquid crystals); WO 03097591, DE 10353202
(medicaments, diagnostic aids); US 5220070, US 5302692 (polymers);
WO 03093228, WO 9625401 (pesticides); GB 2276381, GB 2276380
(fungicides), US 5637607 (nematicides), WO 9947139 (parasiticides),
US 6531501, WO 9516676 (arthropodicides).
The compounds of the formula I can be isolated in the form of their salts.
These are obtained by the conventional methods by reaction with acids or
bases. Useful acid addition salts are, for example, halides, especially
hydrochlorides, hydrobromides, lactates, sulfates, citrates, tartrates,
acetates, phosphates, methylsulfonates, benzenesulfonates,
p-toluenesulfonates, adipates, fumarates, gluconates, glutamates,
glycerol phosphates, maleates, benzoates, oxalates and pamoates and
trifluoroacetates, and in the case of the preparation of active ingredients
preferably pharmaceutically acceptable salts. If the compounds contain an
acidic group, they can form salts with bases, for example alkali metal salts,
preferably sodium or potassium salts, or ammonium salts, for example as
salts with ammonia or organic amines or amino acids. They may also be in
the form of a zwitterion.
List of abbreviations:
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
N~
N
DIP diisopropyl ether
DIPEA diisopropylethylamine
DME 1,2-dimethoxyethane
DMF N,N-dimethylformamide
EA ethyl acetate (EtOAc)
eq. equivalent
HEP n-heptane
HOAc acetic acid
MeOH methanol
mp melting point
MTB tent-butyl methyl ether
NCIS N-chlorosuccinimide
CA 02545452 2006-05-10
18
dppf 1,1'bis(diphenylphosphino)ferrocene
RT room temperature
THE tetrahydrofuran
Example 1 2-Methyl-5-nitro-4-pentafluorosulfuranylbenzoic acid
F
F` S, F
F--I
_F
O
O.N+ Y
I I
OH
a) 4-Aminophenylsulfur pentafluoride
FSS \
NH2
A solution of tin(II) chloride (1465 g, 7.73 mol) in concentrated (32 percent)
aqueous HCI solution was heated with stirring to 80 C and then, with ice
cooling, 4-nitrophenylsulfur pentafluoride (584 g, 2.344 mol) was introduced
in 8 portions within 1 h. The internal temperature was kept below 100 C.
Subsequently, the mixture was stirred at an internal temperature of 85 C
for 1.5 h and then allowed to cool to 45 C within a further hour. A mixture of
ice (12 kg), NaOH (2 kg) and dichloromethane (1.5 1) was prepared and
added to the reaction mixture with vigorous stirring. The phases were
separated, the aqueous phase was extracted 3 times with 1 1 each time of
dichloromethane, and the combined organic phases were dried over
Na2SO4 and concentrated under reduced pressure. 510 g of
4-aminophenylsulfur pentafluoride were obtained as a bright yellow
crystalline powder, m.p. 63-65 C (lit.: 57-59 C)
b) 4-Amino-3-bromophenylsulfur pentafluoride
F5S \ Br
i~
NH 2
4-Aminophenylsulfur pentafluoride (510 g, 2.327 mol) was dissolved in
dichloromethane (7 I), the solution was cooled to 5 C and, while stirring,
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (326 g, 1.14 mol) was
CA 02545452 2006-05-10
19
introduced in several portions with ice cooling such that the internal
temperature was kept at 3-8 C (about 1 h). Subsequently, the mixture was
stirred without external cooling for 1 h and allowed to warm to room
temperature. The mixture was filtered through a bed of silica gel (volume
about 1 1) and washed with dichloromethane (5.5 I), and the filtrate was
concentrated under reduced pressure. About 700 g of a red-brown
crystalline mass were obtained and were dissolved in n-heptane (600 ml) at
60 C and then crystallized in a refrigerator at 4 C. Filtration with suction
gave 590 g (85%) of 4-amino-3-bromophenylsulfur pentafluoride as
brownish crystals, m.p. 59-59.5 C.
c) 4-Amino-3-methylphenylsulfur pentafluoride
F5S CH3
NH2
A mixture of Cs2CO3 (794 g, 2.7 mol), dimethoxyethane (2 I), water
(300 ml) and trimethylboroxine (50 percent solution in THE, 225 g, 0.9 mol)
was heated to 70 C, PdC12 (dppf) = CH2CI2 (37 g, 45 mmol) was added,
and a solution of 4-amino-3-bromophenylsulfur pentafluoride (270 g,
0.9 mol) in dimethoxyethane (400 ml) was added dropwise within 2 h while
the reaction mixture was heated to reflux. It was subsequently heated to
reflux for a further 3 h and then cooled to room temperature, diluted with
MTB (500 ml), filtered through a silica gel column (14 x 7 cm, 70-200 pm)
and washed with MTB (2500 ml). The filtrate was concentrated under
reduced pressure. 490 g of a black, semicrystalline mass were obtained
and were subjected to a steam distillation. A total of 5.5 I of condensate
was collected, from which the crystals of the product separated out. The
condensate was extracted 3 times with MTB, and the combined organic
phases were dried over Na2SO4 and concentrated under reduced
pressure. 4-Amino-3-methylphenylsulfur pentafluoride (181 g, 76%) was
obtained as colorless crystals, m.p. 65-66 C.
d) 4-Bromo-3-methylphenylsulfur pentafluoride
F5S a CH3
Br
A mixture of tert-butyl nitrite (90 percent, 37 ml, 280 mmol) and CuBr2
CA 02545452 2006-05-10
(35.8 g, 160 mmol) in acetonitrile (260 ml) was cooled to 5 C and, while
stirring and cooling with ice, a solution of 4-amino-3-methylphenylsulfur
pentafluoride (30.9 g, 132.5 mmol) in MTB (140 ml) was added dropwise at
5-8 C within 1 h. Evolution of nitrogen started after about 2 min. The
5 mixture was then allowed to warm with stirring to room temperature within
1 h, a mixture of ice (250 g), 26 percent aqueous NH3 solution (50 ml) and
MTB (250 ml) was added, and the mixture was stirred for 10 min. The
phases were separated, the aqueous phase was extracted 3 times with
MTB (150 ml each time), and the combined organic phases were shaken
10 once with 400 ml of water. Drying with Na2SO4 and evaporation of the
organic phase gave 39 g of 4-bromo-3-methylphenyisulfur pentafluoride as
a red-brown oil which was contaminated with 8 mol% 4,5-dibromo-
3-methylphenylsulfur pentafluoride, but was used further without further
purification. Yield 89% based on a purity of 90%.
e) 4-Cyano-3-methylphenyisulfur pentafluoride
F5S CH3
CN
A mixture of 4-bromo-3-methylphenyisulfur pentafluoride (136.4 g, purity
80%, 0.367 mol), Zn(CN)2 (72.8 g, 0.62 mol) and Zn dust (7.2 g, 0.11 mol)
in dimethylacetamide (900 ml) and water (40 ml) was initially charged with
nitrogen sparging, heated to 125 C with stirring, and PdCI2(dppf) = CH2CI2
(32.7 g, 40 mmol) was added. After stirring at 125 C for one hour,
PdC12(dppf) = CH2CI2 (16.3 g, 20 mmol) and Zn dust (3.6 g, 55 mmol) was
again added, and stirring was continued at 125 C for 2 h. Subsequently,
the mixture was cooled to room temperature, diluted with n-heptane
(400 ml) and stirred vigorously with addition of 5 N aqueous NH4CI solution
(250 ml) and water (450 ml) for 15 min. The mixture was filtered with
suction through a layer of kieselguhr, the phases were separated, and the
aqueous phase was extracted twice with n-heptane (200 ml). The
combined organic phases were shaken with water (450 ml), dried over
MgSO4 and concentrated under reduced pressure. The resulting black
residue was dissolved in 200 ml of n-heptane, filtered and again
concentrated under reduced pressure. 78 g of a dark brown liquid were
obtained and were purified by chromatography on a silica gel column
(7 x 55 cm, 60-200 m, 4:1 to 3:2 n-heptane/dichloromethane). The first
CA 02545452 2006-05-10
21
fraction obtained was 6.5 g of 4-bromo-3-methylphenylsulfur pentafluoride
(reactant) as yellowish liquid, and then 71.1 g (80%) of 4-cyano-
3-methylphenylsulfur pentafluoride as a pale yellow oil.
f) 2-Methyl-4-pentafluorosulfuranylbenzoic acid
F5S CH3
CO2H
A mixture of 4-cyano-3-methylphenylsulfur pentafluoride (41.2 g, 169.4 g),
NaOH (20.4 g, 510 mmol) and water (60 ml) in ethylene glycol (160 ml)
was heated to 130 C and stirred at this temperature for 4 h. It was then
cooled to room temperature and diluted with MTB (150 ml) and water
(250 ml), and the mixture was filtered with suction. The phases of the
filtrate were separated, and the aqueous phase was acidified with
concentrated aqueous HCI solution, and the precipitated solid was filtered
off with suction. 41.1 g (93%) of 2-methyl-4-pentafluorosulfuranylbenzoic
acid were obtained as colorless crystals, m.p. 138-139 C.
g) 2-Methyl-5-nitro-4-pentafluorosulfuranylbenzoic acid
6.0 g of 2-methyl-4-pentafluorosulfuranylbenzoic acid were dissolved in
60 ml of a 90% aqueous HNO3 solution and, at RT, 6 ml of a 96% H2SO4
were added dropwise. The mixture was left to stand at RT for 28 h, then
poured onto 300 g of ice, 300 ml of water were added, the mixture was
stirred for 1 h and then the product was filtered off. The pale yellow solid
was dried in air to give 6.5 g, m.p. 218-220 C.
Rf (DIP/2%HOAc) = 0.27 MS (ES-): 306
Example 2: Methyl 3-amino-4-chloro-5-pentafluorosulfanylbenzoate
F O
F\\ ,F
S
F'/
I O
F
CI
NH2
CA 02545452 2006-05-10
22
and methyl 5-amino-2-chloro-3-pentafluorosulfanylbenzoate
F CI O
F\S,F
F'/ O
F I
NH2
a) 3-Pentafluorosulfanylbenzoic acid
F OH
FBI ,F
F--/ O
F
13.00 g of (3-iodophenyl)sulfur pentafluoride (Tetrahedron 56, (2000) 3399)
and 6.15 g of methyl iodide were dissolved in 200 ml of diethyl ether
(anhydrous) and the solution was added dropwise to 2.87 g of
magnesium/20 ml of diethyl ether. The reaction mixture was stirred at reflux
for one hour, then cooled to -10 C and sparged under atmospheric
pressure with CO2. The mixture was stirred at RT for 16 hours, then the
reaction mixture was adjusted to pH 3-4 using dilute aqueous HCI solution
and extracted 3 times with 200 ml each time of EA. Drying was effected
over MgSO4 and the solvent was removed under reduced pressure. 7.20 g
of a colorless, amorphous powder were obtained.
Rf (DIP)/2%HOAc) = 0.51 MS (DCI): 249
b) 3-Nitro-5-pentafluorosulfanylbenzoic acid
F OH
FJF
FMS O
F
NO2
4.0 g of 3-pentafluorosulfanylbenzoic acid were dissolved at RT in 50 ml of
100% HNO3 and 10 ml of H2SO4 were added with ice cooling. The mixture
was stirred at RT for 6 days, then poured onto 200 g of ice and stirred for a
further hour, and finally the product was filtered off with suction. 4.4 g of
bright yellow crystals were obtained, m.p. 140 C.
MS (ES-): 292
CA 02545452 2006-05-10
23
c) Methyl 3-nitro-5-pentafluorosulfanylbenzoate
F O
F\\ ~F
FMS O
F
NO2
4.4 g of 3-nitro-5-pentafluorosulfanylbenzoic acid were dissolved in 100 ml
of MeOH and 5.4 ml of SOC12 were added dropwise at RT. The mixture
was boiled to reflux for 5 h, the volatile constituents were removed under
reduced pressure and the residue was coevaporated once with 100 ml of
toluene. The residue was chromatographed on silica gel using 1:8 EA/HEP
and 4.2 g of a colorless oil were obtained.
Rf (EA/HEP 1:8) = 0.18 MS (DCI): 308
d) Methyl 3-amino-5-pentafluorosulfanylbenzoate
F O
F ,F
F /S O
F
NH2
3.0 g of methyl 3-nitro-5-pentafluorosulfanylbenzoate were dissolved in
50 ml of MeOH and 5 ml of HOAc and 200 mg of Pd/C (10%) were added.
The mixture was hydrogenated under a standard pressure of hydrogen
atmosphere for 20 h, then hydrogenation was effected under 6 bar of
hydrogen for a further 2 days. The catalyst was filtered off and the solvent
removed under reduced pressure to obtain 2.5 g of an amorphous solid.
Rf (DIP) = 0.48 MS (DCI): 278
e) Methyl 3-amino-4-chloro-5-pentafluorosulfanylbenzoate and methyl
5-amino-2-chloro-3-pentafluorosulfanylbenzoate
2.2 g of methyl 3-amino-5-pentafluorosulfanylbenzoate were dissolved in
20 ml of isopropanol and 1.1 g of NCIS were added at 60 C. The solution
was boiled to reflux for 2 h, then allowed to cool to RT. 10 ml of a saturated
aqueous Na2SO3 solution and 100 ml of a saturated aqueous Na2CO3
CA 02545452 2006-05-10
24
solution were then added and extraction was effected 3 times with 150 ml
each time of EA. Drying was effected over MgSO4, the solvent was
removed under reduced pressure and the residue was chromatographed
on silica gel using 1:6 EA/HEP. 508 mg of methyl 3-amino-4-chloro-
5-pentafluorosulfanylbenzoate and 94 mg of methyl 5-amino-2-chloro-
3-pentafluorosulfanylbenzoate as well as 1.39 g of methyl 3-amino-
2-chloro-5-pentafluorosulfanylbenzoate were obtained; each as colorless
oils.
Rf (EA/HEP 1:6) = 0.26: methyl 3-amino-2-chloro-5-pentafluorosulfanyl-
benzoate
Rf (EA/HEP 1:6) = 0.15: methyl 3-amino-4-chloro-5-pentafluorosulfanyl-
benzoate
Rf (EA/HEP 1:6) = 0.26: methyl 5-amino-2-chloro-5-pentafluorosulfanyl-
benzoate
MS (ES+): each 352 (M+CH3C=N)
Example 3: 2-Chloro-3-pentafluorosulfanylaniline and 4-chloro-3-penta-
fluorosulfanylaniline
F CI F
I F \ F
F'S/ NH2 FMS` NH2
F F a
CI
8.00 g of 3-pentafluorosulfanylaniline (Tetrahedron 56, (2000) 3399) were
dissolved in 200 ml of isopropanol and 4.87 g of NCIS were added in
portions at 60 C (within 30 minutes). The mixture was stirred at 60 C for a
further 20 minutes, then boiled under reflux for 2 h. The reaction mixture
was allowed to cool to RT and half of the solvent was removed under
reduced pressure. 300 ml of a semisaturated aqueous NaHCO3 solution
and 50 ml of a saturated aqueous Na2SO3 solution were then added and
extraction was effected 3 times with 100 ml each time of CH2CI2. Drying
was effected over MgSO4, the solvent was removed under reduced
pressure and the residue was chromatographed on silica gel using 1:4
EA/HEP. 2.02 g of 2-chloro-3-pentafluorosulfanylaniline and 1.10 g of
4-chloro-3-pentafluorosulfanylaniline as well as 2.73 g of 2-chloro-
5-pentafl uorosulfanylaniline were obtained.
Rf (EA/HEP 1:4) = 0.31: 2-chloro-5-pentafluorosulfanylaniline
Rf (EA/HEP 1:4) = 0.18: 2-chloro-3-pentafluorosulfanylaniline
Rf (EA/HEP 1:4) = 0.11: 4-chloro-3-pentafluorosulfanylaniline
CA 02545452 2006-05-10
MS (DCI): each 253
Example 4: 2-(4-Nitro-3-pentafluorosulfanylphenyl)isoindole-l,3-dione and
2-(2-nitro-5-pentafluorosulfanylphenyl)isoindole-1, 3-dione
_ O
O OWN+O
N
N
O
O
N+
II ,F S,F
O FF FF/ \11 FF
5 F F
a) 2-(3-Pentafluorosulfanylphenyl)isoindole-1,3-dione:
O
N
/ O
~-F
F11S~F
F
FF
15 g (68.44 mmol) of 3-pentafluorosulfanylphenylamine was suspended
with 10.14 g (68.44 mmol) of phthalic anhydride in 40 ml of a acetic acid
10 and boiled under reflux for 2 h. The cool reaction mixture was admixed with
400 ml of water, heated in an ultrasound bath for 30 min and filtered. The
residue was washed with water and subsequently with a little ethanol and
dried under reduced pressure. 2-(3-Pentafluorosulfanylphenyl)isoindole-
1,3-dione was obtained with a melting point of 188-190 C.
b) 2-(4-Nitro-3-pentafluorosulfanylphenyl)isoindole-1,3-dione and 2-(2-nitro-
5-pentafluorosulfanylphenyl)isoindole-1,3-dione
1 g (2.863 mmol) of 2-(3-pentafluorosulfanylphenyl)isoindole-1,3-dione was
dissolved at 0 C in 3.29 ml of concentrated nitric acid, and the mixture was
stirred at 0 C for 2 h. Afterward, the mixture was left to stand at room
temperature overnight. The reaction solution was added to 50 g of ice-
water and the mixture was stirred for 1 h; then the precipitate was filtered
off with suction, washed with water, dried and purified chromatographically
CA 02545452 2006-05-10
26
on silica gel using toluene as the eluent. 2-(4-Nitro-3-pentafluorosulfanyl-
phenyl)isoindole-1,3-dione having a melting point of 200-203 C and
2-(2-nitro-5-pentafluorosulfanylphenyl)isoindole-1,3-dione having a melting
point of 175-177 C were obtained in a ratio of 1:2.
Example 5: 2-(4-Amino-3-pentafluorosulfanylphenyl)isoindole-1,3-dione
0
N
O
H2N
I~F
F~'SF
FF
1.94 g (4.92 mmol) of 2-(4-nitro-3-pentafluorosulfanylphenyl)isoindole-
1,3-dione (prepared in example 4) were dissolved in 20 ml of methanol,
admixed with 53mg of 10% palladium on activated carbon and
hydrogenated at room temperature at a hydrogen pressure of 5 bar. On
completion of reaction, the catalyst was filtered off and the filtrate
concentrated. The residue was stirred in a mixture of dichloromethane and
n-heptane, filtered with suction and dried under reduced pressure.
2-(4-amino-3-pentafluorosulfanylphenyl)isoindole-1,3-dione having a
melting point of 176-178 C was obtained.
When the above-described reaction was terminated prematurely,
2-(4-hydroxyamino-3-pentafluorosulfanylphenyl)isoindole-1,3-dione
0
N
HO,
N 0
H F
FFFF
having a melting point (with decomposition) of 171-173 C was obtained.
CA 02545452 2006-05-10
27
Example 6: 4-(1,3-Dioxo-1,3-dihydroisoindole-2-yi)-2-pentafluorosulfanyl-
benzonitrile:
0
N
O
N S_ F
F~SF
FF
0.46 ml (8.24 mmol) of semiconcentrated sulfuric acid was slowly added
dropwise at 0 C to a solution of 1g (2.74 mmol) of 2-(4-amino-
3-pentafluorosulfanylphenyl)isoindole-1,3-dione (prepared in example 5) in
acetic acid. The mixture was stirred at 0 C for 10 min; then a solution of
189.4 mg of sodium nitrite in 2 ml of water was slowly added dropwise with
stirring, and the resulting solution was stirred at 0 C for 30 min. This
solution was finally added dropwise to a solution, cooled to 0 C, of 246 mg
(2.74 mmol) of copper(l) cyanide and 536 mg (8.23 mmol) of potassium
cyanide in 5 ml of water. The reaction mixture was stirred at 0 C for 30 min
and afterward at room temperature for another 3 h. After the end of the
reaction, the mixture was added to water and the aqueous phase extracted
twice with ethyl acetate. The organic phase was dried over magnesium
sulfate and filtered, the filtrate was concentrated and the residue purified
chromatographically on silica gel first with toluene and then with 20/1
toluene/ethyl acetate. 4-(1,3-Dioxo-1,3-dihydroisoindol-2-yl)-2-pentafluoro-
sulfanylbenzonitrile was obtained. 1 H NMR (500 MHz; dg-dmso: 6 [ppm] _
8.4 (m, 2H); 8.1-7.95 (m, 5H).
Example 7: 4-Amino-2-pentafluorosulfanylbenzonitrile and ethyl N-(4-cyano-
3-pentafluorosulfanylphenyl)phthalamate
0
0
O
NH2 NH
N~ -F N~ _F
F/ F FF
FF FF
CA 02545452 2006-05-10
28
610 mg (1.63 mmol) of 4-(1,3-dioxo-1,3-dihydroisoindol-2-yl)-2-penta-
fluorosulfanylbenzonitrile (prepared in example 7) were dissolved in 30 ml
of ethanol and admixed with 100 mg (1.956 mmol) of hydrazine hydrate
(100%). The mixture was stirred at room temperature overnight. Afterward,
the reaction mixture was concentrated under reduced pressure and the
residue was purified by chromatography (preparative HPLC; Purospher
STAR RP-18e (10 pm); eluent: 5/95 -* 95/5 [45 min.] acetonitrile/water
(0.5% trifluoroacetic acid)). 4-Amino-2-pentafluorosulfanylbenzonitrile
(1 H NMR (500 MHz; d6-dmso) 6 [ppm] = 7.65 (s, 1 H); 7.2 (s, 1 H; 6.8 (m,
3H)) and N-(4-cyano-3-pentafluorosulfanylphenyl)phthalamate (1 H NMR
(500 MHz; d6-dmso) 6 [ppm] = 11.3 (s, 1 H); 8.6 (s, 1 H); 8.2 (d, 1 H); 8.1
(d,
1 H); 7.95 (d, 1 H); 7.75 (m, 1 H); 7.7 (m, 2H); 4.2 (q, 2H); 1.15 (t, 3H))
were
obtained.
Example 8: N-(4-Nitro-3-pentafluorosulfanylphenyl)acetamide and N-(2,4-
dinitro-5-pentafluorosulfanylphenyl)acetamide ":"r F F/F O F,F O~
FMS NH F,S NH
F F
O=N / O, N+ / N+-.O
II II I -
U O 0
1.0 g of N-(3-pentafluorosulfanylphenyl)acetamide (preparation as in
Tetrahedron 56, (2000) 3399) was dissolved in portions at 0-3 C in 10 ml of
90% HNO3. The mixture was stirred at 0 C for 15 minutes, then poured
onto 100 g of ice and extracted 3 times with 100 ml each time of EA. Drying
was effected over MgSO4, the solvent was removed under reduced
pressure and the residue was chromatographed on silica gel using DIP.
195 mg of N-(4-nitro-3-pentafluorosulfanylphenyl)acetamide and 280 mg of
N-(2,4-dinitro-5-pentafluorosulfanylphenyl)acetamide as well as 645 mg of
N-(2-nitro-5-pentafluorosulfanylphenyl)acetamide were obtained.
Rf (DIP) = 0.41: N-(2-nitro-5-pentafluorosulfanylphenyl)acetamide
MS (El): 306
Rf (DIP) = 0.18: N-(2,4-dinitro-5-pentafluorosulfanylphenyl)acetamide
MS (El): 351
Rf (DIP) = 0.11: N-(4-nitro-3-pentafluorosulfanylphenyl)acetamide
MS (El): 306
CA 02545452 2006-05-10
29
Example 9: N-(4-Nitro-3-pentafluorosulfanylphenyl)acetamide
F F 0
S NH
F~ ~
:,a F
O,N+
I I
O
20.00 g of N-(3-pentafluorosulfanylphenyl)acetamide (preparation as in
Tetrahedron 56, (2000) 3399) were dissolved in portions at from -35 C
to -40 C in 100 ml of 90% HNO3. The mixture was stirred at -40 C for
minutes, then poured onto 1 kg of ice and stirred at RT for 1 h. The
product was then filtered, washed with water and dried under reduced
pressure. Chromatography on silica gel using DIP afforded 3.61 g of
N-(4-nitro-3-pentafluorosulfanylphenyl)acetamide as well as 17.00 g of
10 N-(2-nitro-5-pentafluorosulfanylphenyl)acetamide.
Rf (DIP) = 0.41: N-(2-nitro-5-pentafluorosulfanylphenyl)acetamide
MS (El): 306
Rf (DIP) = 0.11: N-(4-nitro-3-pentafluorosulfanylphenyl)acetamide
MS (El): 306
Example 10: 1,3-Dibromo-2-methoxy-4-nitro-5-pentafluorosulfanylbenzene
F 01N1-0
FjS,F Br
F--/
F
O
Br
a) 4-Pentafluorosulfanylphenol
F
F\S,F
F'I
F
~aOH
40.00 g of 4-pentafluorosulfanylaniline were suspended in 500 ml of a 35%
aqueous H2SO4 solution and a solution of 13.85 g of NaNO2 in 30 ml of
water was added dropwise at 0 C over a period of 10 minutes.
Subsequently, the mixture was stirred at 0 C for 35 minutes, then a
solution, at 0 C, of 171.10 g of Cu(N03)2 in 200 ml of water was poured in
CA 02545452 2006-05-10
and, directly thereafter, 26.11 g of Cu20 were added in portions. The
mixture is stirred at RT for a further 2 hours, then extraction is effected
3 times with 200 ml each time of CH2CI2. Drying was effected over MgSO4
and the solvent was removed under reduced pressure. 38.00 g of a pale
5 yellow oil were obtained which was used further without purification.
b) 4-Methoxypentafluorosulfanylbenzene
F
Fj,F
F'S \
F
O
5.00 g of 4-pentafluorosulfanylphenol were dissolved in 50.00 g of dimethyl
10 carbonate and 3.46 g of DBU were added. The mixture was boiled under
reflux for 10 hours, then allowed to cool and diluted with 200 ml of EA.
Subsequently, the mixture was washed twice with 100 ml each time of a
5% aqueous HCl solution, then with 100 ml of a 5% aqueous NaOH
solution. Drying was effected over MgSO4 and the solvent was removed
15 under reduced pressure. Chromatography on silica gel using 1:1 DIP/HEP
afforded 2.2g of a colorless oil.
Rf (DIP/HEP 1:1)= 0.52
c) 2,6-Dibromo-4-pentafluorosulfanylphenol
F
F-S,F Br
F I
OH
20 Br
3.34 g of 4-methoxypentafluorosulfanylbenze were dissolved in 200 ml of
CHCI3 and 0.46 g of FeBr2 were added. At RT, 6.84 g of bromine were
then added dropwise and the mixture was stirred at RT for 4 days.
Subsequently, a further 400 mg of FeBr2 were added and the mixture was
25 stirred at RT for a further 23 hours. The reaction mixture was then poured
cautiously onto 100 ml of a saturated aqueous Na2SO3 solution and
extracted 3 times with 50 ml each time of CH2CI2. Drying was effected over
MgSO4 and the solvent was removed under reduced pressure.
Chromatography on silica gel using DIP afforded 3.00 g of an amorphous
CA 02545452 2006-05-10
31
solid.
Rf (DIP) = 0.22
d) 1,3-Dibromo-2-methoxy-5-pentafluorosulfanylbenzene
F
F -S~F Br
F I
O
Br
450 mg of 2,6-dibromo-4-pentafluorosulfanylphenol, 329 mg of K2CO3 and
186 mg of CH3I were stirred at RT in 5 ml of anhydrous DMF for 24 hours.
Subsequently, the reaction mixture was poured onto 100 ml of EA and
extracted 3 times with 30 ml each time of water. Drying was effected over
MgSO4 and the solvent was removed under reduced pressure to obtain
500 mg of a colorless oil.
Rf (DIP/HEP 1:1) = 0.51 MS (El): 392
e) 1,3-Dibromo-2-methoxy-4-nitro-5-pentafluorosulfanylbenzene
630 mg of 1,3-dibromo-2-methoxy-5-pentafluorosulfanylbenzene were
stirred in 2 ml of a 90% aqueous HNO3 solution at 0 C for 1 hour.
Subsequently, the mixture was stirred at RT for 20 minutes and then
poured onto 50 g of ice. An aqueous Na2CO3 solution was used to adjust
to pH = 6 and extraction was effected three times with 50 ml each of EA.
Drying was effected over Na2SO4 and the solvent was removed under
reduced pressure. Chromatography on silica gel using 1:3 DIP/HEP
afforded 260 mg of a pale yellow oil.
Rf (DIP/HEP 1:3) = 0.40
Example 11: 1-Bromo-3-chloro-2-methoxy-4-nitro-5-pentafluorosulfanyl-
benzene and 3-bromo-1-chloro-2-methoxy-4-nitro-5-pentafluorosulfanyl-
benzene
F 0~' N+.O F 0"N+.0
F F
FS~ Cl FMS/ Br
F F
--f 1-1 0 '1~1 0
Br CI
CA 02545452 2006-05-10
32
a) 2-Chloro-4-pentafluorosulfanylphenol
5.00 g of 4-pentafluorosulfanylphenol (prepared in example 11 a) were
dissolved in 100 ml of acetic acid and a chlorine gas stream was passed
through at 0 C for 10 minutes. This warmed the solution to 30 C which was
subsequently stirred at RT for a further 90 minutes. Argon was used to
drive the chlorine out of the solution and the solvent was subsequently
removed under reduced pressure. 5.50 g of a pale yellow oil were obtained.
Rf (DIP) = 0.23
b) 2-Chloro-1-methoxy-4-pentafluorosulfanylbenzene
F
F;S,F CI
F
O
1
5.50 g of 2-chloro-4-pentafluorosulfanylphenol, 7.89 g of K2CO3 and 4.05 g
of CH3I were stirred at RT in 30 ml of anhydrous DMF for 2 hours and left
to stand at RT for 2 days. The mixture was then diluted with 300 ml of EA
and washed 3 times with 100 ml each time of water. Drying was effected
with Na2SO4 and the solvent was removed under reduced pressure to
obtain 5.40 g of a pale yellow oil.
Rf (DIP) = 0.68
c) 2-Bromo-6-chioro-4-pentafluorosulfanylphenol
F
F -S~F CI
F f /
OH
Br
5.30 g of 2-chloro-1-methoxy-4-pentafluorosulfanylbenzene were dissolved
in 150 ml of CHCI3 and admixed with 4.73 g of bromine and 638 mg of
FeBr2. The mixture was stirred at RT for 18 hours, then admixed with a
further 200 mg of FeBr2, stirred at RT for 6 hours and then admixed with a
further 300 mg of FeBr2, stirred at RT for 2 hours and left to stand at RT for
18 hours. The reaction mixture was then poured onto 300 ml of a saturated
aqueous Na2SO3 solution and extracted with 300 ml of CH2CI2. The
organic phase was then washed with 100 ml of water and dried over
Na2SO4, and the solvent was removed under reduced pressure. 4.20 g of
CA 02545452 2006-05-10
33
a colorless oil were obtained which was reacted further without purification.
d) 1-Bromo-3-chloro-2-methoxy-5-pentafluorosulfanylbenzene
F
F
FMS/ CI
F
1? O
Br
4.20 g of 2-bromo-6-chloro-4-pentafluorosulfanylphenol were stirred
together with 3.48 g of K2CO3 and 2.68 g of CH3I in 50 ml of anhydrous
DMF at RT for 24 hours. The solvent was then removed under reduced
pressure and subsequently taken up with 100 ml each of water and EA.
The phases were left to separate and extraction was then effected twice
more with 100 ml each time of EA. Drying was effected over Na2SO4 and
the solvent was removed under reduced pressure. Chromatography on
silica gel using 1:1 DIP/HEP afforded 3.44 g of a colorless viscous liquid.
Rf (DIP/HEP 1:1) = 0.53 MS (El): 346
e) 1-Bromo-3-chloro-2-methoxy-4-nitro-5-pentafluorosulfanylbenzene and
3-bromo-1-chloro-2-methoxy-4-nitro-5-pentafluorosulfanylbenzene
3.40 g of 1-bromo-3-chloro-2-methoxy-5-pentafluorosulfanylbenzene were
added dropwise at from 0 C to 5 C to 40 ml of a 90% aqueous HNO3
solution. The mixture was stirred at 0 C for 60 minutes, then stirred at RT
for 90 minutes. Subsequently, the reaction mixture was poured onto 200 g
of ice and extracted 3 times with 200 ml each time of EA. Drying was
effected over Na2SO4 and the solvent was removed under reduced
pressure. Chromatography on silica gel using 1:3 DIP/HEP afforded 2.00 g
of a pale yellow oil.
MS (EI): 391
Example 12: 2-Chloro-4-nitro-5-pentafluorosulfanylaniline
F
` F
F'S/ N H 2
F
O\N4 / Cl
I I
0
CA 02545452 2006-05-10
34
a) 2.60 g of 2-chloro-5-pentafluorosulfanylaniline (example 3) were added
dropwise at 0 C to 30 ml of 100% HNO3. The mixture was stirred at 0 C for
1 hour, then poured onto 100 g of ice and adjusted to pH = 7 using
saturated aqueous NaHCO3 solution. Extraction was then effected 3 times
using 100 ml each time of EA, then drying was effected over MgSO4. The
solvent was removed under reduced pressure to obtain 2.50 g of a pale
yellow oil.
Rf(EA)=0.13