Note: Descriptions are shown in the official language in which they were submitted.
CA 02547679 2002-10-16
Flue Gas Desulfurization Apparatus, Flue Gas Desulfurization
System, and Method for Operating Flue Gas Desulfurization
Apparatus
This application is a division of Canadian Application
Serial Number 2,438,355, which is the national phase
application of PCT International Application PCT/JP02/10739,
filed October 16, 2002.
Technical Field
The present invention relates to a flue gas
desulfurization apparatus and system for removing sulfur
oxides (SOX) contained in a discharge gas generated by a
boiler, a gas turbine, an engine, an incinerator, or a
similar facility combusting a fuel such as coal or heavy oil;
and to a method for operating the flue gas desulfurization
apparatus.
The present invention also relates to a desulfurization
method for removing sulfur oxides (SOX) contained in a
discharge c~as .
Background Art
Sulfur oxides (SOX) such as sulfur dioxide are contained
in discharge gases generated by thermal power stations;
plants such as chemical-production plants, metal-processing
plants, sintering plants, and paper-making plants; and gas
turbines, engines, incinerators, and similar facilities
provided with a boiler employing a fuel such as coal or heavy
oil. Thus, a flue gas desulfurization apparatus is employed
in order to remove SOX contained in discharge gases. The flue
gas desulfurization apparatus removes SOX contained in a
1
CA 02547679 2002-10-16
discharge gas, by causing SOX to be adsorbed by a porous
carbon material such as activated carbon fiber, oxidizing a
sulfur component by oxygen contained in the discharge gas in
the presence of the porous carbon material serving as a
catalyst, and absorbing the oxidation product in water, to
thereby form sulfuric acid, which is removed from the porous
carbon material.
Among conventional flue gas desulfurization apparatuses,
some contain a catalyst unit formed of plate-like activated
carbon fiber sheets and corrugated activated carbon fiber
sheets, which are alternatingly juxtaposed. In such
apparatuses, water is added dropwise to activated carbon
fiber contained in the catalyst unit, and a discharge gas is
caused to pass through conduits provided between the sheets,
whereby a sulfur component is removed in the form of sulfuric
acid. In order to enhance discharge gas purifying
performance (desulfurization efficiency), water must be added
to activated carbon fiber so that uniform water distribution
is attained. In addition, in order to prevent an increase in
the size of an auxiliary facility for supplying water, a
minimum required amount of water must be evenly added to the
activated carbon fiber.
In one method (known as a lime-gypsum method) for
removing sulfur oxides by employment of a flue gas
desulfurization apparatus, a sulfur component contained in a
discharge gas is collected in gypsum form by use of limestone
slurry or slaked lime slurry serving as an absorbent. In an
2
CA 02547679 2002-10-16
alternative method called the dry adsorption method,
activated carbon is used in dry format.
The aforementioned conventional lime-gypsum method
includes spraying of limestone slurry or slaked lime slurry
into a discharge gas, whereby humidifying and cooling of the
discharge gas and absorption of SOx are performed
simultaneously. Accordingly, a large amount of slurry must
be circulated, thereby requiring power and a large amount of
water for circulating the slurry. In addition, since the
thus-formed gypsum is in slurry form, an apparatus for
separating water from the slurry so as to collect gypsum is
required. Thus, when the lime-gypsum method is employed,
dimensions and complexity of the desulfurization facility
unavoidably increase.
The dry adsorption method requires a large amount of
heat for releasing an adsorbed sulfur component from
activated carbon through heating. In addition, there arise
problems such as disposal of the formed dilute sulfuric acid
and loss of the employed adsorbent. Therefore, demand has
arisen for a desulfurization apparatus which can produce
sulfuric acid during desulfurization without requiring an
absorbent for sulfur oxides or a large desulfurization
facility.
In this connection, there has been proposed an
apparatus for removing SOx contained in a discharge gas in
which SOx contained in the discharge gas is adsorbed by a
porous carbon material such as activated carbon fiber; a
3
CA 02547679 2002-10-16
sulfur component is oxidized by oxygen contained in the
discharge gas in the presence of the porous carbon material
serving as a catalyst; and the oxidation product is absorbed
in water, to thereby form sulfuric acid, which is removed
from the porous carbon material (see Japanese Patent
Application Laid-Open (kokai) No. 11-347350).
The above conventional flue gas desulfurization
apparatus employing activated carbon fiber includes an
activated carbon fiber board, disposed in an adsorption tower,
for adsorbing SOx contained in a discharge gas. In the
desulfurization apparatus, a discharge gas is fed from the
bottom of the tower, and SOz is oxidized on the surface of
the activated carbon fiber, to thereby form 503. The thus-
formed S03 is reacted with supplied water, to thereby form
sulfuric acid ( HzSO-0 ) .
A considerable amount of discharge gas is generated
from a boiler combusting a fuel such as coal or heavy oil.
Such a large amount of discharge gas must be treated
continuously to thereby enhance desulfurization efficiency.
In order to perform continuous operation, it is essential
that a large adsorption tower be employed. However, there is
desired an adsorption tower which attains higher
desulfurizati.on efficiency of activated carbon fiber with a
desulfurization system of small size.
In order to effectively attain catalytic action,
catalytic reaction conditions must be optimized, and S03
produced through oxidation of S02 contained in a discharge
4
CA 02547679 2002-10-16
gas must be effectively removed by use of water. In addition,
in order to avoid increase in size of an auxiliary water-
supply facility, water must be distributed uniformly in the
catalyst through addition of a minimum required amount of
water.
When industrial water or similar water is supplied to
the catalyst, the total cost of the system increases.
Therefore, improvement in efficiency of the system is
required.
In this connection, utilization of waste water produced
from the system or similar water is a conceivable approach
for solving the problem. However, when this approach is
actually employed, catalyst activity problematically lowers.
Since water is needed for catalytic action, the
catalyst must maintain an appropriate water content_ When of
the catalyst is not sufficiently wet, the catalytic action
cannot fully be attained, which is problematic. Particularly
at the time of starting the desulfurization apparatus, poor
wetting conditions are problematic.
When the aforementioned plants are stopped, water for
humidifying-cooling and additional water are fed to a
desulfurization apparatus, since hot airflow is continuously
fed to the apparatus even after the boilers are stopped. In
this case, since a discharge gas containing sulfur oxides is
not fed to the desulfurization apparatus, concentration of
the formed sulfuric acid gradually lowers. When sulfuric
acid having a concentration below a certain level is used to
CA 02547679 2002-10-16
form gypsum, separation and collection of the product become
difficult, which is problematic. Thus, conventionally, such
low-concentration sulfuric acid which cannot be used to
produce gypsum must be treated as industrial waste, which is
also problematic.
In the case where dilute sulfuric acid is produced
without producing gypsum, when the concentration of dilute
sulfuric acid is excessively low, the size of the
concentration facility must be increased, thereby
problematically elevating cost of the sulfuric acid
production facility.
The present invention has been accomplished under the
above-described circumstances. Thus, an object of the
present invention is to provide a flue gas desulfurization
apparatus comprising a catalyst unit formed of at least one
activated carbon fiber board allowing uniform water
distribution therein.
The present invention has been accomplished under the
above-described circumstances. Thus, another object of the
present invention is to provide a desulfurization method for
removing suli=ur oxides (SOX) by evenly adding water to an
activated carbon fiber board.
The present invention has been accomplished under the
above-described circumstances_ Thus, still another object of
the present invention is to provide a desulfurization method
which for removing sulfur oxides (SOX) by evenly adding a
minimum required amount of water to an activated carbon fiber
6
CA 02547679 2002-10-16
board.
The present invention has been accomplished under the
above-described circumstances. Thus, still another object of
the present invention is to provide a flue gas
desulfurization apparatus which discharges no industrial
waste and which attains high efficiency.
The present invention has been accomplished under the
above-described circumstances. Thus, still another object of
the present invention is to provide a flue gas
desulfurization apparatus which requires no absorbent for
sulfur oxides, can be operated without a large
desulfurization facility, and can produce high-concentration
sulfuric acid during desulfurization; i.e., to provide a flue
gas desulfurization apparatus which can reduce the amount of
supplied water and which attains uniform water distribution.
The present invention has been accomplished under the
above-described circumstances. Thus, still another object of
the present invention is to provide a flue gas
desulfurization apparatus which can perform desulfurization
reaction at high efficiency by use of activated carbon fiber;
which provides a simple desulfurization system; and which
attains high efficiency and small size.
The present invention has been accomplished under the
above-described circumstances. Thus, still another object of
the present invention is to provide a flue gas
desulfurization apparatus which assures high overall
efficiency of a desulfurization system and which maintains
7
CA 02547679 2002-10-16
desulfurization performance over a long period of time.
Disclosure of the Invention
The present invention provides a flue gas
desulfurization apparatus including a catalyst unit formed of
at least one activated carbon fiber board and water-supply
means for supplying, to the catalyst unit, water for forming
sulfuric acid, the catalyst unit being provided in the
apparatus in the form of a tower through which a discharge
gas containing sulfur oxide passes, the water-supply means
being provided above the catalyst unit or in an upper section
of the unit and in the apparatus in the form of a tower,
characterized in that the activated carbon fiber board
provided in the catalyst unit is formed by alternatingly
juxtaposing one or more plate-like activated carbon fiber
sheets and one or more corrugated activated carbon fiber
sheets so as to provide vertically extending conduits, and
the water-supply means comprises permeation means for
supplying water to an upper section of the active carbon
fiber board by the mediation of a capillary member.
With thc: above structure, when water is supplied, by
the mediation of the permeation member, to the plate-like
activated carbon fiber sheets and the corrugated activated
carbon fiber sheets, the water is allowed to be present
evenly over t:he entirety of the activated carbon fiber board
of the catalyst unit.
Preferably, the capillary member of the permeation
8
CA 02547679 2002-10-16
means is made of fabric.
In this case, cost can be reduced.
Preferably, the capillary member of the permeation
means assumes the form of cord.
In this case, cost can be reduced.
The present invention also provides a flue gas
desulfurization apparatus including a catalyst unit formed of
at least one activated carbon fiber board, and water-supply
means for supplying, to the catalyst unit, water for forming
sulfuric acid, the catalyst unit being provided in the
apparatus in the form of a tower through which a discharge
gas containing sulfur oxide passes, the water-supply means
being provided above the catalyst unit or in an upper section
of the unit and in the apparatus in the form of a tower,
characterized in that the activated carbon fiber board
provided in the catalyst unit is formed by alternatingly
juxtaposing one or more plate-like activated carbon fiber
sheets and one or more corrugated activated carbon fiber
sheets so as to provide vertically extending conduits, and
the water-supply means is spray means for directly spraying
water in mist form to an upper portion of a surface of the
activated carbon fiber board.
With the above structure, when water is supplied, by
means of the spray means, directly to the plate-like
activated carbon fiber sheets and the corrugated activated
carbon fiber sheets, the water is allowed to be present
evenly over the entirety of the activated carbon fiber board
9
CA 02547679 2002-10-16
of the catalyst unit.
Preferably, the catalyst unit is formed of a plurality
of activated carbon fiber boards which are vertically
disposed, and the activated carbon fiber boards are linked
via capillary means.
With the above structure, when two stages of activated
carbon fiber boards are vertically disposed for reducing the
size of the apparatus or other reasons, and water is supplied,
by the mediation of the capillary member, to the plate-like
activated carbon fiber sheets and the corrugated activated
carbon fiber sheets of each disposed activated carbon fiber
board, without being affected by characteristics of the
discharge gas such as flow rate. Thus, water is allowed to
be present evenly over the entirety of the activated carbon
fiber boards of a catalyst unit.
The present invention provides a desulfurization method
including causing a discharge gas containing sulfur oxides to
pass through a catalyst unit formed of at least one activated
carbon fiber board and supplying water for forming sulfuric
acid, characterized by comprising supplying water to the
catalyst unit through a capillary phenomenon.
In this case, the desulfurization method attains
removal of sulfur oxides by means of the activated carbon
fiber board in which water is uniformly dispersed.
The present invention also provides a flue gas
desulfurization apparatus including a catalyst unit formed of
at least one activated carbon fiber board, and water-supply
CA 02547679 2002-10-16
means for supplying, to the catalyst unit, water for forming
sulfuric acid, the catalyst unit being provided in the
apparatus in the form of a tower through which a discharge
gas containing sulfur oxide passes, the water-supply means
being provided in the apparatus in the form of a tower,
characterized by comprising humidifying-cooling means for
cooling and humidifying the discharge gas outside or inside
the apparatus in the form of a tower and a liquid-feed line
for feeding, to the humidifying-cooling means, dilute
sulfuric acid which has been collected in the apparatus in
the form of <3 tower and has a low concentration equal to or
lower than a predetermined sulfuric acid concentration.
Preferably, the dilute sulfuric acid having a low
concentration equal to or lower than a predetermined sulfuric
acid concentration has a concentration of 0.50 or lower.
Preferably, the dilute sulfuric acid having a low
concentration equal to or lower than a predetermined sulfuric
acid concentration is collected after desulfurization is
stopped.
Preferably, the desulfurization apparatus further
includes an inlet for introducing the discharge gas
containing sulfur oxide into a lower section of the apparatus
in the form of a tower; an outlet for discharging the
discharge gas in an upper section of the apparatus; and an
apparatus for supplying water for producing sulfuric acid
disposed above the catalyst unit and in the tower.
The present invention provides a flue gas
11
CA 02547679 2002-10-16
desulfurization system characterized by comprising any one of
the aforementioned flue gas desulfurization apparatuses; a
gypsum reaction tank for yielding gypsum slurry by reacting
dilute sulfuric acid fed from the flue gas desulfurization
apparatus with lime slurry; and a dewatering apparatus for
separating water from gypsum slurry produced in the gypsum
reaction tank, to thereby yield gypsum.
The present invention also provides a flue gas
desulfurization system characterized by comprising any one of
the aforementioned flue gas desulfurization apparatuses; and
a condensation tank for condensing dilute sulfuric acid
produced by means of the desulfurization apparatus.
Preferably, the discharge gas is discharged from a
boiler, a gas turbine, an engine, or any of a variety of
incinerators, and the flue gas desulfurization system further
comprises soot-removing means for removing soot contained in
the discharge gas.
The present invention provides a method for operating a
flue gas desulfurization apparatus including starting,
stopping, and restarting the flue gas desulfurization
apparatus containing a catalyst unit formed of at least one
activated carbon fiber board, and water-supply means for
supplying, to the catalyst unit, water for forming sulfuric
acid, the catalyst unit being provided in the apparatus in
the form of a tower through which a discharge gas containing
sulfur oxide passes, the water-supply means being provided in
the apparatus in the form of a tower, characterized by
12
CA 02547679 2002-10-16
comprising performing humidifying-cooling and cooling through
addition of water until the discharge gas is cooled to
approximately 70°C; collecting low-concentration dilute
sulfuric acid; and upon restarting of the apparatus,
supplying the low-concentration dilute sulfuric acid instead
of water for humidifying-cooling employed in humidifying-
cooling means or supplying the low-concentration dilute
sulfuric acid instead of water.
Preferably, the method for operating the flue gas
desulfurization apparatus comprises supplying, upon
restarting of the apparatus, the low-concentration dilute
sulfuric acid instead of water for humidifying-cooling
employed in humidifying-cooling means or supplying the low-
concentration dilute sulfuric acid instead of water; and
collecting dilute sulfuric acid having a concentration equal
to or higher than a predetermined concentration, to thereby
obtain a sulfuric acid product.
Alternatively, preferably, the method for operating the
flue gas desulfurization apparatus comprises supplying, upon
restarting of the apparatus, the low-concentration dilute
sulfuric acid instead of water for humidifying-cooling
employed in humidifying-cooling means or supplying the low-
concentration dilute sulfuric acid instead of water;
collecting dilute sulfuric acid having a concentration equal
to or higher than a predetermined concentration; and reacting
the resultant dilute sulfuric acid with lime slurry, to
thereby obtain gypsum.
13
CA 02547679 2002-10-16
Conventionally, dilute sulfuric acid produced in a
desulfurization apparatus and having a concentration which
decreases during a halt of a plant has been treated as
industrial waste. However, according to the above methods,
such dilute sulfuric acid can be employed as cooling liquid
for a humidifying-cooling apparatus, whereby waste treatment
is omitted. In addition, the concentration of the dilute
sulfuric acid is elevated by spraying the sulfuric acid as
humidifying-cooling liquid into a discharge gas and
subjecting the same to desulfurization again in the
desulfurization tower. The resultant concentration-elevated
sulfuric acid is reacted with lime slurry, to thereby produce
a good gypsum product.
The present invention also provides a flue gas
desulfurization apparatus including a catalyst unit formed of
at least one activated carbon fiber board, and water-supply
means for supplying, to the catalyst unit, water for forming
sulfuric acid, the catalyst unit being provided in the
apparatus in the form of a tower through which a discharge
gas containing sulfur oxide passes, the water-supply means
being provided in the apparatus in the form of a tower,
characterized by comprising, in the apparatus in the form of
a tower, a humidifying tank for humidifying a catalyst, the
tank containing the catalyst unit therein.
Preferably, a plurality of stages of the catalyst units
are stacked by means of a ~>upporting apparatus.
Preferably, the desulfurization apparatus further
14
CA 02547679 2002-10-16
comprises an inlet for introducing the discharge gas
containing sulfur oxide into a lower section of the apparatus
in the form of a tower; an outlet for discharging the
discharge gas in an upper section of the apparatus; and an
apparatus for supplying water for producing sulfuric acid
disposed above the catalyst unit.
The present invention provides a flue gas
desulfurization system characterized by comprising any one of
the aforementioned flue gas desulfurization apparatuses; a
gypsum reaction tank for yielding gypsum slurry by reacting
dilute sulfuric acid fed from the flue gas desulfurization
apparatus with lime slurry; and a dewatering apparatus for
separating water from gypsum produced in the gypsum reaction
tank.
The present invention also provides a flue gas
desulfurization system characterized by comprising any one of
the aforementioned flue gas desulfurization apparatuses; and
a condensation tank for condensing dilute sulfuric acid
produced by means of the desulfurization apparatus.
Preferably, the discharge gas is discharged from a
boiler, a gas turbine, an engine, or any of a variety of
incinerators, and the flue gas desulfurization system further
comprises soot-removing means for removing soot contained in
the discharge gas.
The present invention provides a method for starting a
flue gas desulfurization apparatus containing a catalyst unit
formed of at least one activated carbon fiber board, and
CA 02547679 2002-10-16
water-supply means for supplying, to the catalyst unit, water
for forming sulfuric acid, the catalyst unit being provided
in the apparatus in the form of a tower through which a
discharge gas containing sulfur oxide passes, the water-
supply means being provided in the apparatus in the form of a
tower, characterized by comprising humidifying the catalyst
unit in advance, placing in the apparatus in the form of a
tower the catalyst which has been humidified, and
subsequently starting the apparatus.
The present invention provides a method for starting a
flue gas desulfurization apparatus containing a catalyst unit
formed of at least one activated carbon fiber board, and
water-supply means for supplying, to the catalyst unit, water
for forming sulfuric acid, the catalyst unit being provided
in the apparatus in the form of a tower through which a
discharge gas containing sulfur oxide passes, the water-
supply means being provided in the apparatus in the form of a
tower, characterized by comprising freezing the catalyst unit
in advance, placing in the apparatus in the form of a tower
the catalyst which has been frozen, and subsequently starting
the apparatus.
The present invention provides a method for starting
the aforementioned flue gas desulfurization apparatus
characterized by comprising supplying steam or water to the
humidifying tank containing the catalyst unit, to thereby
humidify the catalyst unit, and subsequently starting the
apparatus.
16
CA 02547679 2002-10-16
Preferably, the humidified catalyst unit retains water
in an amount of at least twice the weight of the unit.
With the above structure, favorable humidifying
conditions in the desulfurization apparatus can be
effectively attained upon starting the apparatus. Thus,
activated carbon fiber contained in the catalyst unit can be
sufficiently humidified, whereby effective catalytic activity
at an initial stage is provided and deterioration of the
catalyst during subsequent operation is prevented.
The present invention provides a flue gas
desulfurization apparatus characterized by comprising a
plurality of catalyst stages provided in the apparatus in the
form of a tower through which a discharge gas containing
sulfur oxide passes, and water-supplying means for supplying
water for producing sulfuric acid to the catalyst stages,
each catalyst stages being formed of at least one activated
carbon fiber board, the water-supply means being provided
above the uppermost catalyst stage and in the apparatus in
the form of a tower.
With the above structure, In this case, water droplets
are caused to dispersed, during falling between catalyst
stages, to thereby attain substantially uniform water
distribution. Thus, there can be provided a flue gas
desulfurization apparatus containing catalyst means formed of
at least one activated carbon fiber board allowing a uniform
water distribution.
The present invention provides a flue gas
17
CA 02547679 2002-10-16
desulfurization apparatus characterized by comprising a
plurality of catalyst stages provided in the apparatus in the
form of a tower through which a discharge gas containing
sulfur oxide passes, and water-supplying means for supplying
water for producing sulfuric acid to each catalyst stage, the
catalyst stages being formed of at least one activated carbon
fiber board, the water-supply means being provided above each
catalyst stage and in the apparatus in the form of a tower.
With the above structure, a required amount of water
can be supplied to a portion where water is needed, to
thereby attain uniform water distribution. Thus, through
addition of a minimum required amount of water, there can be
provided a flue gas desulfurization apparatus containing
catalyst means formed of at least one activated carbon fiber
board allowing a uniform water distribution.
Preferably, the flue gas desulfurization apparatus
further comprises oxygen concentration detection means for
detecting oxygen concentration of a discharge gas passing
through the apparatus in the form of a tower; sulfur oxide
concentration detection means for detecting sulfur oxide
concentration on a gas-outlet side of each catalyst stage and
on a gas-inlet side of the catalyst stage on the uppermost
stream side, 'the catalyst stages being provided in the
apparatus in the form of a tower; and control means for
controlling supply conditions of water supplied from each
water-supply means on the basis of detected information
provided from the oxygen concentration detection means and
18
CA 02547679 2002-10-16
the sulfur oxide concentration detection means.
With the above structure, an optimum amount of water
can be distributed to each catalyst means in accordance with
the sulfur oxide concentration and the oxygen concentration.
Thus, a required amount of water can be supplied to required
catalyst means, and efficiency for removal of sulfur oxides
can be maintained at a high level through addition of a
minimum required amount of water.
Preferably, the control means includes means for
reducing the amount of water supplied from each water-supply
means in accordance with an increase in oxygen concentration
as detected by the oxygen concentration detection means and
for increasing the amount of water supplied from each water-
supply means in accordance with an increase in sulfur oxide
concentration as detected by the sulfur oxide concentration
detection means.
In this case, water can be supplied in an optimum
amount for maintaining excellent efficiency for removal of
sulfur oxides .
Preferably, the control means includes means for
storing a predetermined value of sulfur oxide concentration
on an outlet side of each catalyst stage, and for controlling
supply conditions of water supplied from each water-supply
means on the basis of comparison of the stored predetermined
value with information detected by the sulfur oxide
concentration detection means, to thereby maintain the sulfur
oxide concentration on an outlet side of each catalyst stage
19
CA 02547679 2002-10-16
at the predetermined value.
In this case, a required amount of water can be
supplied to a target catalyst stage through addition of a
minimum required amount of water while sulfur oxide
concentration is maintained at a predetermined level. Thus,
excellent efficiency for removal of sulfur oxides can be
maintained through addition of a minimum required amount of
water.
The present invention also provides a flue gas
desulfurization apparatus including a catalyst unit formed of
at least one activated carbon fiber board, and water-supply
means for supplying, to the catalyst unit, water for forming
sulfuric acid, the catalyst unit being provided in the
apparatus in the form of a tower through which a discharge
gas containing sulfur oxide passes, the water-supply means
being provided in the apparatus in the form of a tower,
characterized by comprising a humidifying-cooling apparatus
for humidifying and cooling the discharge gas for feeding to
the desulfurization apparatus in the form of a tower, and in
that a supernatant obtained from gypsum slurry is supplied,
as water for humidifying-cooling, to the humidifying-cooling
apparatus.
Preferably, the supernatant is obtained from a gypsum
settling tank.
Preferably, the supernatant is separated from the
gypsum settling tank by means of a stationary tank, a
cyclator, a filter, or a combination thereof.
CA 02547679 2002-10-16
Preferably, the desulfurization apparatus includes a
cooling tank for cooling the supernatant obtained from the
gypsum settling tank.
Preferably, the desulfurization apparatus includes a
salt-out tank for salting-out a salt component contained in
the supernatant obtained from the gypsum settling tank.
Preferably, the cooling temperature during humidifying-
cooling is 40 to 60°C.
Preferably, the mist contained in the discharge gas
which has been humidified and cooled has a particle size of
50 to 150 Eun.
Preferably, the desulfurization apparatus further
comprises an inlet for introducing the discharge gas
containing sulfur oxide into a lower section of the apparatus
in the form of a tower; an outlet for discharging the
discharge gas in an upper section of the apparatus; an
apparatus for supplying water for producing sulfuric acid
disposed above the catalyst unit; and a humidifying-cooling
apparatus for humidifying and cooling the discharge gas for
feeding to the apparatus in the form of a tower.
Preferably, the humidifying-cooling apparatus is
provided on the upstream side of the apparatus in the form of
a tower.
Preferably, the humidifying-cooling apparatus is
provided on the upstream side of the catalyst unit contained
in the apparatus in the form of a tower.
The present invention provides a flue gas
21
CA 02547679 2002-10-16
desulfurization system characterized by comprising the
aforementioned flue gas desulfurization apparatus; a gypsum
reaction tank for depositing gypsum by feeding lime slurry to
dilute sulfuric acid discharged from the flue gas
desulfurization apparatus; a stationary tank for settling the
gypsum; and a dewatering apparatus for separating water from
gypsum slurry, to thereby yield gypsum.
Accordingly, the amount of water supplied from the
outside of the apparatus for use in humidifying-cooling can
be reduced, since a humidifying-cooling apparatus for cooling
and humidifying the discharge gas for feeding to the
desulfurization apparatus in the form of a tower is provided
in the flue gas desulfurization apparatus of the present
invention including a catalyst unit formed of at least one
activated carbon fiber board, and water-supply means for
supplying, to the catalyst unit, water for forming sulfuric
acid, the catalyst unit being provided in the apparatus in
the form of a. tower through which a discharge gas containing
sulfur oxide passes, the water-supply means being provided in
the apparatus in the form of a tower.
In addition, a re-processed supernatant obtained
through stationary settling of a supernatant obtained from
gypsum slurry is used as water to be supplied to the
humidifying-cooling apparatus, so that adhesion of gypsum or
a similar substance on activated carbon fiber constituting
the catalyst unit can be prevented. Thus, desulfurization
performance can be maintained for a long period of time
22
CA 02547679 2002-10-16
without variation or deterioration of desulfurization
efficiency.
Brief Description of the Drawings
FIG. 1 is a system configuration of a discharge gas
process system employing the flue gas desulfurization
apparatus according to a first embodiment of the present
invention. FIG. 2 is a system configuration of a discharge
gas process system according to another embodiment of the
present invention. FIG. 3 is an elevation showing an
essential portion of an activated carbon fiber board for
constituting a catalyst unit. FIG. 4 is a perspective view
showing a portion of an upper section of an activated carbon
fiber board. FIG. 5 is a cross-sectional view showing an
activated carbon fiber board. FIG. 6 is a cross-sectional
view showing activated carbon fiber boards according to other
embodiments. FIG. 7 is a cross-sectional view showing an
activated carbon fiber sheet. FIG. 8 is an elevation showing
an essential portion of an activated carbon fiber board
equipped with a capillary member according to another
embodiment. FIG. 9 is an elevation showing an essential
portion of activated carbon fiber boards equipped with a
capillary member according to another embodiment. FIG. 10 is
a schematic diagram of a discharge gas process system
(production of sulfuric acid) employing the flue gas
desulfurization apparatus according to a second embodiment.
FIG. 11 is a schematic diagram of a discharge gas process
23
CA 02547679 2002-10-16
system (production of gypsum) according to another embodiment.
FIG. 12 is a structural diagram of a flue gas desulfurization
apparatus according to a second embodiment. FIG. 13 is a
perspective view of an activated carbon fiber board. FIG. 14
is a flow chart showing a procedure of stopping supply of
discharge gas. FIG. 15 is a structural diagram of a flue gas
desulfurization apparatus according to a third embodiment.
FIG. 16 is a structural diagram of a flue gas desulfurization
apparatus according to a fourth embodiment. FIG. 17 is a
perspective view of a catalyst unit. FIG. 18 shows an
elevation and a plan view of catalyst units. FIG. 19 is a
structural diagram of a flue gas desulfurization apparatus
according to a fifth embodiment. FIG. 20 is a structural
diagram of a flue gas desulfurization apparatus according to
a sixth embodiment. FIG. 21 is a perspective view of an
activated carbon fiber board according to another embodiment.
FIG. 22 is a system configuration of a discharge gas process
system employing the flue gas desulfurization apparatus
according to a seventh embodiment of the present invention.
FIG. 23 is a schematic diagram of a desulfurization tower.
FIG. 24 is a block diagram showing control means. FIG. 25 is
a graph showing the relationship between the stage of
catalyst and sulfur oxide concentration or amount of water.
FIG. 26 is a graph showing the relationship between amount of
water and sulfur dioxide concentration. FIG. 27 is a graph
showing the relationship between amount of water and oxygen
concentration. FIG. 28 is a graph showing a time-elapsed
24
CA 02547679 2002-10-16
change in sulfur oxide concentration. FIG. 29 is a graph
showing a time-elapsed change in percent opening of valves
for regulating water amount. FIG. 30 is a schematic diagram
of a discharge gas process system (production of gypsum)
employing the flue gas desulfurization apparatus according to
an eighth embodiment of the present invention. FIG. 31 is a
schematic view showing the flue gas desulfurization apparatus.
Modes for Carrying Out the Invention
The present invention will next be described in more
detail with reference to the attached drawings.
With reference to FIG. 1, a discharge gas process
system employing the flue gas desulfurization apparatus
according to a first embodiment of the present invention will
be described.
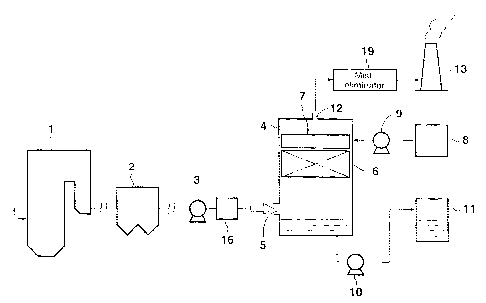
As shown in FIG. 1, a boiler 1; for example, a boiler
for generating steam for driving a steam turbine (not
illustrated) of a thermal power plant, combusts fuel f (e. g.,
coal or heavy oil) in its furnace. A discharge gas generated
from the boiler 1 contains sulfur oxides (SOx). The
discharge gas undergoes a NOx removal process by means of an
NOX removal unit (not illustrated), is cooled by means of a
gas heater, and subsequently undergoes a soot removal process
by means of a soot collector 2.
The soot-removed discharge gas is fed, by means of a
feed pump 3, to a humidifying-cooling apparatus 16, where
water (including dilute sulfuric acid) is added, to thereby
CA 02547679 2002-10-16
yield a discharge gas in saturated vapor form. The thus-
humidified discharge gas may contain mist. The discharge gas
in saturated vapor form produced in the humidifying-cooling
apparatus 16 is fed to a desulfurization tower 4
(desulfurization apparatus in the form of a tower) via an
inlet 5 provided in a lower section of the tower. The
desulfurization tower 4 contains therein a catalyst unit 6
formed of at least one activated carbon fiber board, and
water for producing sulfuric acid is supplied to the catalyst
unit 6 via a capillary member 7 provided above the catalyst
unit. Water is supplied to the capillary member 7 from a
water tank 8 by use of a pump 9. Water-supply means includes
the capillary member 7, the water tank 8, and the pump 9.
The discharge gas is introduced from the lower section
of the tower and caused to pass through the catalyst unit 6
onto which water has been supplied, whereby SOX contained in
the discharge gas is removed through reaction. The discharge
gas which has passed through the catalyst unit 6 is
discharged from an outlet 12, and mist contained in the
discharged gas is removed by a mist-eliminator 19, whereby
generation of white smoke is suppressed. The thus-treated
discharge gas is released to the air through a smokestack 13.
'rhe mist-eliminator 19 may be omitted.
On a surface of the activated carbon fiber board
contained in the catalyst layer 6, desulfurization proceeds
in accordance with, for example, a following reaction
mechanism which includes:
26
CA 02547679 2002-10-16
(1) adsorption of sulfur dioxide (SO2) by the activated
carbon fiber board contained in the catalyst layer 6;
(2) oxidation of the adsorbed sulfur dioxide (SOZ) with
oxygen (OZ) (may be supplied separately) contained in the
discharge gas, to thereby form sulfur trioxide (S03);
(3) dissolution of the resultant sulfur trioxide (S03) in
water (HZO), to thereby form sulfuric acid (HZSOQ); and
( 4 ) release of the resultant sulfuric acid ( HzS09 ) from the
activated carbon fiber board.
The overall reaction is expressed as follows.
SOZ + 1 / 202 + Hz0 --~ HZS04
The thus-released sulfuric acid (HZSOq) is dilute
sulfuric acid and is discharged into a sulfuric acid tank 11
via a discharge pump 10. As described above, desulfurization
of the discharge gas is performed by causing, by means of the
catalyst unit 6 for oxidation, absorption of sulfur dioxide
(SOz) contained in the discharge gas, reacting the oxidation
product with water (H20), to thereby form sulfuric acid
(HZSOQ), and releasing the sulfuric acid from the catalyst
unit.
With reference to FIG. 2, a discharge gas process
system according to another embodiment of the present
invention will be described. Herein, the same structural
members as employed in the discharge gas process system shown
in FIG. 1 are denoted by the same reference numerals, and
repeated descriptions thereof are omitted.
According to the discharge gas process system shown in
27
CA 02547679 2002-10-16
FIG. 2, sulfur oxides contained in a discharge gas are
removed by means of a desulfurization apparatus, whereby
sulfuric acid is formed, and lime slurry is fed to the
resultant sulfuric acid, to thereby produce gypsum.
As shown in FIG. 2, the system includes a gypsum
reaction tank 52 for storing dilute sulfuric acid fed from a
desulfurization tower 4 via a discharge pump 10 and for
depositing gypsum by reaction with supplied lime slurry 51.
In addition, a settling tank (thickener) 53 is also provided
for settling gypsum deposited in the gypsum reaction tank 52.
Gypsum slurry 54 formed in the settling tank (thickener) 53
is transferred to a dewatering apparatus 56, where water is
removed from the gypsum slurry, to thereby yield gypsum 55.
In the discharge gas process system shown in FIG. 1,
sulfuric acid obtained through desulfurization is used as a
sulfuric acid product. However, in the discharge gas process
system shown in FIG. 2, lime slurry 51 is fed to the produced
sulfuric acid, thereby forming gypsum slurry 54, followed by
dehydration, to thereby yield a gypsum product 55.
The structure of the activated carbon fiber board
contained in the catalyst unit 6 will be described with
reference to FIGs. 3 to 7.
An activated carbon fiber board 20 is formed by
alternatingly juxtaposing plate-like activated carbon fiber
sheets 21 and corrugated (continuous V-shaped waves)
activated carbon fiber sheets 22. Spaces extending straight
and provided between two sheets serve as conduits 15, with
28
CA 02547679 2002-10-16
the conduits 15 extending vertically. The plate-like
activated carbon fiber sheets 21 and the corrugated activated
carbon fiber sheets 22 are formed by mixing cotton-form
activated carbon fiber (e. g., pitch-derived or phenol-derived
carbon fiber) with a binder and forming the mixture into a
sheet. In the case where the corrugated activated carbon
fiber sheet 22 is formed, the sheet is worked by use of a
corrugator. Subsequently, the thus-formed sheets are heated
in a non-oxidizing atmosphere (e. g., nitrogen) at high
temperature (e. g., 600°C to 1,200°C), to thereby yield
activated carbon fiber sheets for use in desulfurization.
Briefly, a highly hydrophobic surface of activated carbon
fiber is provided through heat treatment, so as to readily
adsorb sulfur dioxide (SOS) and rapidly release formed
sulfuric acid (HzS04) from activated carbon fiber.
No particular limitation is imposed on the type of
activated carbon fiber employed in the present invention, so
long as the activated carbon fiber exerts the aforementioned
catalytic action. Examples include pitch-derived activated
carbon fiber, polyacryloni.trile activated carbon fiber,
phenol-derived activated carbon fiber, and cellulose-derived
activated carbon fiber.
Production of the activated carbon fiber board will
next be described in detail.
Phenol-derived activated carbon fiber (Kuractive-20,
product of Ku:raray Chemical Co., Ltd.) is fired at 900 to
1,200°C in an nitrogen atmosphere for one hour.
29
CA 02547679 2002-10-16
Polyacrylonitrile activated carbon fiber (FX-600, product of
Toho Rayon Co., Ltd.) is fired at 900 to 1,200°C in an
nitrogen atmosphere for one hour.
The thus-heat-treated plate-like activated carbon fiber
sheets 21 and corrugated activated carbon fiber sheets 22 are
alternatingly juxtaposed, and the Beak of each corrugated
activated carbon fiber sheet 22 is joined to the plate-like
activated carbon fiber sheet 21 through melt adhesion of the
binder, to thereby produce a carbon fiber board module of
predetermined size. Since the peak of each corrugated
activated carbon fiber sheet 22 is joined to the plate-like
activated carbon fiber sheet 21 through melt adhesion of the
binder, no additional adhesive, such as an organic substance,
is used. Thus, adverse effect of the adhesive on
desulfurization reaction is eliminated, and reliability of
joining i.s enhanced, thereby eliminating the effect of
pressure loss.
In one mode, four modules of the activated carbon fiber
board 20 are juxtaposed such that the conduits 15 are
disposed vertically, to thereby yield one unit. Two units
are stacked, and the stacked units are placed and immobilized
in a casing. Briefly, a plurality of activated carbon fiber
boards 20 are disposed and stacked in a vertical direction,
to thereby provide the catalyst unit 6. Thus, the size of
each activated carbon fiber board 20 can be reduced, thereby
facilitating assembly of the catalyst unit.
As shown in FIG. 4, the pitch p between plate-like
CA 02547679 2002-10-16
activated carbon fiber sheets 21 is predetermined to be, for
example, approximately 4 mm, and the width h of each
protruded portion of the corrugated activated carbon fiber
board 22 is predetermined to be, for example, approximately
mm. From a position above the activated carbon fiber
boards, water is sprayed thereonto in the form of droplets
approximately 200 Eun in size, and a discharge gas is
introduced from a position below the activated carbon fiber
boards. Water which has passed through the activated carbon
fiber boards 20 f ails, in the form of droplets of about some
mm in size, to the bottom of the desulfurization tower 4.
The discharge gas passes through comparatively narrow
conduits 15 provided by alternatingly juxtaposing the plate-
like activated carbon fiber sheets 21 and corrugated
activated carbon fiber sheets 22. Thus, an increase in
pressure loss can be suppressed.
S03 formed through oxidation of SOZ on the surface of
activated carbon fiber is transformed into sulfuric acid by
water, and the sulfuric acid is discharged. When the amount
of water is insufficient, discharge of sulfuric acid cannot
be attained and subsequent oxidation of SOZ is insufficient,
whereas when the amount of water is excessive, the yielded
sulfuric acid is diluted. Furthermore, when the amount of
water further increases; for example, in the case where the
activated carbon fiber is covered with a thin layer or a wall
of water which covers active sites of the activated carbon
fiber, such activated carbon fiber loses catalytic action of
31
CA 02547679 2002-10-16
oxidizing SOZ, thereby failing to attain desulfurization or
deteriorating desulfurization efficiency.
Therefore, the amount of water supplied when a
discharge gas comes into contact with the activated carbon
fiber boards 20 contained in the catalyst unit 6 is
predetermined such that water is sprayed thereonto in the
form of droplets of approximately 200 ~m in size from a
position above the activated carbon fiber boards 20, and such
that water which has passed through the activated carbon
fiber boards 20 falls, in the form of droplets of about some
mm in size, to the bottom of the desulfurization tower 4.
Accordingly, water falls intermittently in the form of
spherical droplets, although falling conditions depend on the
conditions of the discharge gas. Thus, water can be supplied
to the surface of activated carbon fiber in a sufficient, yet
not excessive amount, and sulfuric acid can be released at
high efficiency. As a result, desulfurization of a discharge
gas can be effectively performed .
As shown in FIG. 6(A), the corrugated activated carbon
fiber sheet 31 may be formed so as to have a continuous U-
shape pattern, and a plurality of the corrugated activated
carbon fiber sheets 31 and plate-like activated carbon fiber
sheets 21 are alternatingly juxtaposed such that the U-shape
patterns are oriented in the same direction. Alternatively,
as shown in FIG. 6(B), a plurality of the corrugated
activated carbon fiber sheets 31 and plate-like activated
carbon fiber sheets 21 are alternatingly juxtaposed such that
32
CA 02547679 2002-10-16
the orientation of the U-shape pattern is alternatingly
disposed. Alternatively, as shown in FIG. 6(C), minute
raised/dented patterns 32 may be provided in the surface of
the corrugated activated carbon fiber sheets 31.
As illustrated in FIG. 7, a plate-like activated carbon
fiber sheet 21 and corrugated activated carbon fiber sheets
22 and 31 are produced by tightly attaching a fired carbon
sheet 35 on each side of a core material 34, to thereby form
a laminated board. The core material 34 may be omitted.
With reference to FTGs. 3 and 4, the structure of the
capillary member 7 for supplying water to the activated
carbon fiber board 20 will next be described.
As shown in FIGs. 3 and 4, water reservoirs 25 are
provided in the vicinity of the activated carbon fiber board
20 so as to receive water fed from a water tank 8 via a pump
9. A cord 26 serving as an element of a capillary member is
provided from each water reservoir 25 to an upper section of
a plate-like activated carbon fiber sheet 21 and that of a
corrugated activated carbon fiber sheet 22, so as to supply
water through a capillary phenomenon. Water reserved in each
reservoir 25 is supplied directly to the plate-like activated
carbon fiber sheet 21 and the corrugated activated carbon
fiber sheet 2?. by permeating the cord 26. Thus, water
permeates all the surfaces of carbon sheets facing the
conduits 15 so as to attain uniform water distribution.
The cord 26 may be provided around a portion along the
upper edge face (upper ends of the conduits 15) of the
33
CA 02547679 2002-10-16
activated carbon fiber board 20.
In this case, water can be supplied, by the mediation
of the cord 26, to a plate-like activated carbon fiber sheet
21 and a corrugated activated carbon fiber sheet 22
regardless of the characteristics of the discharge gas, such
as flow rate. Thus, water can be supplied to the entirety of
the activated carbon fiber board 20 so as to attain uniform
water distribution, and the catalyst unit 6 formed of the
activated carbon fiber board 20 can be provided. Use of the
cord 26 can suppress cost.
As a practical matter, water reservoirs 25 and the
cords 26 are provided at positions in the catalyst unit 6
such that pressure loss of the discharge gas is prevented.
Regarding the capillary member, an upper section itself
of a plate-like activated carbon fiber sheet 21 and that of a
corrugated activated carbon fiber sheet 22 may serve as the
capillary member, when water is supplied directly, by spray
means such as a sprinkler or a pipe-form shower, to the upper
section of a plate-like activated carbon fiber sheet 21 and
that of a corrugated activated carbon fiber sheet 22. When
the activated carbon fiber boards 20 are built in a frame
body, the frame body itself may serve as a pipe-form shower.
When water is supplied, by means of a sprinkler or a pipe-
form shower, to the upper section of a plate-like activated
carbon fiber sheet 21 and that of a corrugated activated
carbon fiber sheet 22, smooth water supply can be attained
through provision of a baffle plate or a similar member so as
34
CA 02547679 2002-10-16
to prevent scattering of water due to flow of the discharge
gas coming from a lower section.
With reference to FIG. 8, a capillary member according
to another embodiment of the present invention will be
described. FIG. 8 is an elevation showing an essential
portion of an activated carbon fiber board equipped with a
capillary member according to another embodiment. Herein,
the same structural members as shown in FIG. 3 are denoted by
the same reference numerals, and repeated descriptions
thereof are omitted.
As shown in FIG. 8, a spray nozzle 28 is provided in
the vicinity of the activated carbon fiber board 20, and
water is fed from a water tank 8 and a pump 9 to the nozzle.
Fabric 29 in the form of, for example, a slip serving as an
structural element of a capillary member is provided under
the spray nozzle 28. A first edge of the fabric member 29 is
connected to an upper section of a plate-like activated
carbon fiber sheet 21 and that of a corrugated activated
carbon fiber sheet 22. Water is sprayed from the nozzle 28
onto the fabric member 29, and is supplied directly to the
plate-like activated carbon fiber sheet 21 and the corrugated
activated carbon fiber sheet 22 by permeating the fabric
member 29. Thus, water permeates the entirety of carbon
fiber sheets facing the conduits 15 attaining uniform water
distribution.
The fabric member 29 may be provided around a portion
along the upper edge face (upper ends of the conduits 15) of
CA 02547679 2002-10-16
the activated carbon fiber board 20.
In this case, water is supplied, by the mediation of
the fabric 29, to a plate-like activated carbon fiber sheet
21 and a corrugated activated carbon fiber sheet 22 of each
disposed carbon fiber board, without being affected by
characteristics of the discharge gas such as flow rate. Thus,
water can be supplied to the entirety of the activated carbon
fiber boards 20 so as to attain uniform water distribution,
and a catalyst unit 6 formed of the activated carbon fiber
boards 20 can be provided. Use of the fabric 29 can reduce
the cost.
With reference to FIG. 9, a capillary member according
to another embodiment of the present invention will be
described. FIG. 9 is an elevation showing an essential
portion of an activated carbon fiber board equipped with a
capillary member according to another embodiment. Herein,
the same structural members as employed in FIG. 3 are denoted
by the same reference numerals, and repeated descriptions
thereof are omitted.
FIG. 9 shows a catalyst unit according to another
embodiment in which two stages of modules of the activated
carbon fiber board 20 are stacked. A catalyst unit 6 is
formed of the upper and lower activated carbon fiber boards
20, which are linked by cords 26 serving as constitutional
elements of the capillary member which water permeates.
Water reserved in each water reservoir 25 is supplied
directly to the upper activated carbon fiber boards 20 by
36
CA 02547679 2002-10-16
permeating the cords 26. Water droplets which have been
passed through the upper activated carbon fiber boards 20 are
supplied directly to, by permeating the cords 26, a plate-
like activated carbon fiber sheet 21 and a corrugated
activated carbon fiber sheet 22 which constitute the lower
activated carbon fiber boards 20. Thus, in the lower
activated carbon fiber boards 20, water permeates the
entirety of carbon fiber sheets facing the conduits 15 so as
to attain uniform water distribution.
In this case, even when two stages of activated carbon
fiber boards 20 are provided, water can be supplied, by the
mediation of the cords 26, to the plate-like activated carbon
fiber sheets 21 and the corrugated activated carbon fiber
sheets 22 provided in the upper and lower activated carbon
fiber boards 20, without being affected by characteristics of
the discharge gas such as flow rate. Thus, water can be
supplied to the entirety of the upper and lower activated
carbon fiber boards 20 so as to attain uniform water
distribution, and the catalyst unit 6 formed of the activated
carbon fiber boards 20 can be provided.
The upper and lower activated carbon fiber boards 20
may be linked by the fabric member 29 shown in FIG. 8. Water
can be supplied to the upper activated carbon fiber board 20
by the mediation of the fabric member 29. Alternatively,
water may be supplied directly, by spray means such as a
sprinkler or a pipe-form shower, to the upper section of a
plate-like activated carbon fiber sheet 21 and that of a
37
CA 02547679 2002-10-16
corrugated activated carbon fiber sheet 22.
Accordingly, the aforementioned flue gas
desulfurization apparatus can be provided with a catalyst
unit 6 containing an activated carbon fiber board 20 allowing
uniform water distribution. In addition, the aforementioned
desulfurization method attains removal of sulfur oxides (SOx)
by means of an activated carbon fiber board 20 allowing a
uniform water distribution.
With reference to FIG. 10, a discharge gas process
system according to a second embodiment of the present
invention will be described. Herein, the same structural
members as employed in the discharge gas process system shown
in FIG. 1 are denoted by the same reference numerals. In the
discharge gas process system shown in FIG. 10, the capillary
member 7 contained in the discharge gas process system shown
in FIG. 1 is replaced by a water-supplying nozzle 60.
According to the discharge gas process system shown in
FIG. 10, sulfur oxides contained in a discharge gas are
removed by means of a desulfurization apparatus, to thereby
form sulfuric acid. As shown in FIG. 10, the desulfurization
apparatus includes a boiler 1 for driving a steam turbine so
as to generate steam; a soot collector 2 for removing soot
contained in a discharge gas 100 generated by the boiler 1; a
feed fan 3 for feeding the soot-removed discharge gas into a
desulfurization tower 4; a humidifying-cooling apparatus 16
for cooling and humidifying the discharge gas 100 before
feeding to the desulfurization tower 4 (or in the towerj; the
38
CA 02547679 2002-10-16
desulfurization tower 4, including a catalyst unit 6, for
introducing the discharge gas 100 from an inlet 5 provided in
a lower section of the sidewall of the tower and for feeding
water through a water-supplying nozzle 60 provided above the
catalyst unit 6, to thereby effect desulfurization in which
SOX contained in the discharge gas is converted to dilute
sulfuric acid (HzS04); a chimney 13 for discharging, to the
outside, a purified (desulfurized) discharge gas released
from an outlet 12 of the top of the tower; and a sulfuric
acid tank 11 for storing dilute sulfuric acid fed from the
desulfurization tower 4 by means of a discharge pump 10. An
optional mist-eliminator 19 may be inserted in the line for
discharging a purified (desulfurized) discharge gas generated
from the desulfurization tower, to thereby separate water
contained in the discharge gas.
Herein, the boiler l; for example, a boiler for
generating steam for driving a steam turbine (not
illustrated) of a thermal power plant, combusts fuel f (e. g.,
coal or heavy oil) in its .furnace. A discharge gas generated
from the boiler 1 contains sulfur oxides (SOX). The
discharge gas undergoes a NOX removal process by means of an
NOX removal unit (not illustrated), is cooled by means of an
air pre-heater, and subsequently undergoes a soot removal
process by means of a soot collector 2.
The thus-soot-removed discharge gas 100 is fed from the
inlet 5 provided in a lower section of the sidewall of the
desulfurization tower 4 to the desulfurization tower 4 by
39
CA 02547679 2002-10-16
means of a feed fan 3. The desulfurization tower 4 includes
the catalyst unit 6 formed of an activated carbon fiber board,
and water for producing sulfuric acid is supplied from a
water-supplying nozzle 60 to the catalyst unit 6. When the
discharge gas is fed from the bottom of the catalyst unit and
caused to pass through the catalyst unit 6 to which water is
supplied from the above nozzle, SOX contained in the
discharge gas 100 can be removed through chemical reaction.
The discharge gas which has passed through the catalyst unit
6 is discharged from an outlet 12, and is released to air
through a chimney 13.
The aforementioned catalyst unit 6 contains a catalyst
comprising a plurality of activated carbon fiber boards. On
a surface of each activated carbon fiber board,
desulfurization reaction occurs (see reaction mechanism shown
in the description in relation to FIG. 1).
The thus-released sulfuric acid (HZS04) is dilute
sulfuric acid and discharged into a sulfuric acid tank 11 via
a discharge pump 10. As described above, desulfurization of
the discharge gas is performed by causing sulfur dioxide
(SOZ) contained in the discharge gas 100 to be adsorbed by
the activated carbon fiber boards contained in the catalyst
unit 6 for oxidation, reacting the oxidation product with
water (H20), to thereby form sulfuric acid (HZSOQ), and
releasing the sulfuric acid from the catalyst unit.
With reference to FIG. 11, a discharge gas process
system according to another embodiment of the present
CA 02547679 2002-10-16
invention will be described. Herein, the same structural
members as employed in the discharge gas process system shown
in FIG. 2 are denoted by the same reference numerals. In the
discharge gas process system shown in FIG. 11, the capillary
member 7 contained in the discharge gas process system shown
in FIG. 2 is replaced by a water-supplying nozzle 60.
According to the discharge gas process system shown in FIG.
11, sulfur oxides contained in a discharge gas are removed by
means of a desulfurization apparatus, to thereby form
sulfuric acid, and lime slurry is fed to the resultant
sulfuric acid, to thereby produce gypsum.
As shown in FIG. 11, the desulfurization apparatus
includes a boiler 1 for driving a steam turbine so as to
generate steam; a soot collector 2 for removing soot
contained in a discharge gas 100 generated by the boiler 1; a
feed fan 3 for feeding the soot-removed discharge gas into a
desulfurization tower 4; a humidifying-cooling apparatus 16
for cooling and humidifying the discharge gas 100 in the
desulfurization tower or before feeding to the
desulfurization tower; the desulfurization tower 4, including
a catalyst unit, for introducing the discharge gas 100 from
an inlet 5 provided in a lower section of the sidewall of the
tower and for feeding water through a water-supplying nozzle
60 provided above the catalyst unit 6, to thereby effect
desulfurization in which SOX contained in the discharge gas
is converted to dilute sulfuric acid (HzS04); a chimney 13
for discharging, to the outside, a purified (desulfurized)
41
CA 02547679 2002-10-16
discharge gas released from an outlet 12 of the top of the
tower; a gypsum reaction tank 52 for storing dilute sulfuric
acid (HZS04) fed from the desulfurization tower 4 via a
discharge pump 10 and for depositing gypsum by reaction with
supplied lime slurry 51; a settling tank (thickener) 53 for
settling gypsum , and a dewatering apparatus 56 for removing
water as waste water (filtrated liquid) 57 from gypsum slurry
54, to thereby yield gypsum 55.
In the discharge gas process system shown in FIG. 10,
sulfuric acid obtained through desulfurization is used as a
sulfuric acid product. However, in the discharge gas process
system shown in FIG. 11, lime slurry is fed to the produced
sulfuric acid, thereby forming gypsum slurry, followed by
dehydration, to thereby yield a gypsum product.
The same flue gas desulfurization apparatus according
to the second embodiment is employed in the systems shown in
FIGS. ZO and 11. Thus, the structure of the flue gas
desulfurization apparatus will be described with reference to
FIG. 12.
As shown in FIG. 12, the flue gas desulfurization
apparatus includes an inlet 5 for introducing a discharge gas
100 containing sulfur oxides in the sidewall (or lower
section) of the apparatus in the form of a tower; in an upper
portion, an outlet 12 for discharging the discharge gas 100;
and a water-supplying nozzle 60 disposed above a catalyst
unit 6 which is provided in the desulfurization tower 4 and
which supplies water for producing sulfuric acid to the
42
CA 02547679 2002-10-16
catalyst unit formed of an activated carbon fiber board.
In a lower section of the desulfurization tower
4provided is a sulfuric acid reservoir 40, where dilute
sulfuric acid 41 collected by the catalyst unit 6 is stored.
FIG. 13 shows the structure of the catalyst unit 6. FIG.
13 is a perspective view showing the catalyst unit, which
corresponds to that shown in the aforementioned FIG. 4.
As shown in FIG. 13, the activated carbon fiber board
20 which forms a unit catalyst in the catalyst unit 6 is
formed by alternatingly juxtaposing one or more plate-like
activated carbon fiber sheets 21 and one or more corrugated
activated carbon fiber sheets 22. Spaces extending
straightly and provided between two sheets serve as conduits
15, with the conduits 15 being vertically extending. The
plate-like activated carbon fiber sheets 21 and the
corrugated activated carbon fiber sheets 22 are formed into
sheets. The corrugated activated carbon fiber sheet 22 is
formed by use of a corrugator or similar means.
Alternatively, these activated carbon fiber sheets may be
formed into a honeycomb shape so as to enable passage of the
discharge gas in parallel to the sheets.
From the water-supplying nozzle 60, water is sprayed
thereto, and the discharge gas 100 is introduced from a
position below the activated carbon fiber boards 20. Water
which has passed through the activated carbon fiber boards 20
falls, in the form of droplets of about some mm in size, to
the bottom of the tower. The discharge gas 100 passes
43
CA 02547679 2002-10-16
through conduits 15 provided by alternatingly juxtaposing the
plate-like activated carbon fiber sheets 21 and corrugated
activated carbon fiber sheets 22. Thus, increase of pressure
loss can be suppressed.
The thus-released sulfuric acid (H2S04) is dilute
sulfuric acid 41 and discharged into a sulfuric acid tank 11
via a discharge pump 10. As described above, desulfurization
of the discharge gas is performed by causing sulfur dioxide
(S02) contained in the discharge gas 100 to be adsorbed by
the activated carbon fiber boards contained in the catalyst
unit 6 for oxidation, reacting the oxidation product with
water ( Hz0 ) , to thereby form sulfuric acid ( HZSOQ ) , and
releasing the sulfuric acid from the catalyst unit.
The aforementioned sulfuric acid reservoir 40 includes
a sulfuric acid concentration meter 42, which measures the
sulfuric acid concentration inside the reservoir.
When the aforementioned plants are stopped, water for
humidifying-cooling 16a and additional water 8a are fed to
the desulfurization apparatus, since hot airflow is
continuously fed to the apparatus. In this case, since a
discharge gas 100 containing sulfur oxides is not fed to the
desulfurization apparatus, concentration of the formed
sulfuric acid gradually lowers.
When the sulfuric acid concentration is decreased below
a certain level (0.5~ or less), as measured by means of the
sulfuric acid concentration meter 42, gypsum is formed but a
product of the formed gypsum cannot be obtained. Thus,
44
CA 02547679 2002-10-16
discharge of. such dilute sulfuric acid to the outside is
stopped, and dilute sulfuric acid is stored in the sulfuric
acid reservoir 40.
Subsequently, upon starting the plants, the stored low-
concentration sulfuric acid is fed to the humidifying-cooling
apparatus 16 through a liquid-feed line 44 by means of a
liquid-feed pump 45 inserted in the line, and is employed as
a liquid to be sprayed for humidifying and cooling.
When the low-concentration sulfuric acid is employed as
a liquid to be sprayed for humidifying and cooling, the
amount of SOX originating from sulfuric acid increases.
However, the increase in SOX concentration is small as
compared with the SOX concentration in a discharge gas at
restarting of the desulfurization apparatus. Thus, use of
the low-concentration sulfuric acid prevents increase in
process load of the desulfurization tower 4.
In this case, since the low-concentration sulfuric acid
produced during a halt of the plants is fed back to the
humidifying-cooling apparatus 16 and is used in
desulfurization again, the sulfuric acid concentration in the
sulfuric acid reservoir 40 gradually increases. When the
concentration is elevated to a level higher than a
predetermined concentration enabling to produce gypsum, the
destination of discharge of dilute sulfuric acid is switched
from the humidifying-cooling apparatus 16 to a sulfuric acid
tank 11.
The procedure will be described with reference to FIG.
CA 02547679 2002-10-16
14.
As shown in FIG. 14, passage of the discharge gas 100
is stopped, when the plants are stopped (S-11).
In this case, supplying of water from the water-
supplying nozzle 60 is continued (S-12).
Through continuous supplying of water, wet conditions
of activated carbon fiber placed in the catalyst unit 6 is
maintained (S-13).
The catalyst is washed by water supplied from the
water-supplying nozzle 60, and the liquid obtained through
washing is dilute sulfuric acid. The dilute sulfuric acid is
stored in the sulfuric acid reservoir 40 (S-14).
Upon restarting of the plants, the low-concentration
dilute sulfuric acid is fed to the humidifying-cooling
apparatus 16 and employed as a liquid 16a for the humidifying
and cooling the discharge gas (S-15).
As described above, the low-concentration sulfuric acid
which is produced during a halt and starting of the
desulfurization apparatus is effectively utilized, to thereby
enhance desulfurization efficiency. In addition, sulfuric
acid present on a surface of activated carbon fiber contained
in the catalyst unit is washed, to thereby eliminate
poisoning of the catalyst by sulfuric acid, leading to
prevention of decrease in catalytic activity.
The present invention has been described by way of the
above embodiment in which low-concentration sulfuric acid is
employed as a liquid for humidifying and cooling. However,
46
CA 02547679 2002-10-16
the embodiment should not be construed as limiting the
invention thereto. The low-concentration sulfuric acid may
be used as a liquid 8a to be supplied by the water-supplying
nozzle 60 provided above the catalyst unit 6.
FIG. 15 is a general structural diagram of a flue gas
desulfurization apparatus according to a third embodiment.
With reference to FIG. 15, the desulfurization
apparatus of the embodiment includes the desulfurization
apparatus shown in FIG. 12, wherein a sulfuric acid
concentration meter 42 inserted in a sulfuric acid discharge
line 46; a shift valve 47 for switching lines in accordance
with the sulfuric acid concentration as measured by means of
the sulfuric acid concentration meter 42; a low-concentration
sulfuric acid tank 48 for temporarily storing sulfuric acid
for humidifying-cooling fed by switching the valve 47; and a
sulfuric acid tank 11 are provided.
According to the desulfurization apparatus, when the
sulfuric acid concentration decreases to a low level (10 or
less, or 0.50 or less) due to presence of small amounts of
sulfur oxides during desulfurization and is regarded as a
level which cannot produce gypsum, feeding of sulfuric acid
to the sulfuric acid tank 10 is stopped. Then, feeding of
sulfuric acid is switched to the low-concentration sulfuric
acid tank 48 for humidifying-cooling, to thereby prevent
decrease in sulfuric acid concentration in the sulfuric acid
tank 10.
47
CA 02547679 2002-10-16
As described above, low-concentration sulfuric acid
produced during a halt of the desulfurization apparatus is
employed as a liquid for humidifying and cooling upon
restarting of the apparatus. Thus, dilute sulfuric acid is
not required to undergo industrial waste treatment.
With reference to FIG. 16, the flue gas desulfurization
apparatus according to a fourth embodiment will be described.
The discharge gas process system including the flue gas
desulfurization apparatus according to a fourth embodiment is
identical to those shown in FIG. 10 or 11. Thus, the flue
gas desulfurization apparatus shown in FIG. 16 employs the
catalyst unit 6 employed in the discharge gas process system
as shown in FIG. 10 or 11. Herein, the same members as
employed in the flue gas desulfurization apparatus shown in
FIG. 12 are denoted by the same reference numerals. The
activated carbon fiber boards employed in the catalyst unit 6
are described with reference to FIG. 13.
As shown in FIG. 16, the desulfurization apparatus
includes an inlet 5 for introducing a discharge gas 100
containing sulfur oxides in the sidewall (or lower section)
of the apparatus in the form of a tower; an outlet 12 for
discharging the discharge gas 100; and a water-supplying
nozzle 60 disposed above a catalyst unit 6 which is provided
in the desulfurization tower 4 and which supplies water for
producing sulfuric acid to the catalyst unit formed of an
activated carbon fiber board.
48
CA 02547679 2002-10-16
As shown in FIG. 13, the activated carbon fiber board
20 which forms a unit catalyst in the catalyst unit 6 is
formed by alternatingly juxtaposing one or more plate-like
activated carbon fiber sheets 21 and one or more corrugated
activated carbon fiber sheets 22. Spaces extending
straightly and provided between two sheets serve as conduits
15, with the conduits 15 being vertically extending. The
plate-like activated carbon fiber sheets 21 and the
corrugated activated carbon fiber sheets 22 are formed into
sheets. The corrugated activated carbon fiber sheet 22 is
formed by use of a corrugator or similar means.
Alternatively, these activated carbon fiber sheets may
be formed into a honeycomb shape so as to enable passage of
the discharge gas in parallel to the sheets.
From the water-supplying nozzle 60, water is sprayed
thereto, and the discharge gas 100 is introduced from a
position below the activated carbon fiber boards 20. Water
which has passed through the activated carbon fiber boards 20
falls, in the form of droplets of about some mm in size, to
the bottom of the tower. The discharge gas 100 passes
through comparatively narrow conduits 15 provided by
alternatingly juxtaposing the plate-like carbon fiber sheets
21 and corrugated carbon fiber sheets 22. Thus, increase of
pressure loss can be suppressed.
The procedure of provision of the catalyst unit 6 in
the above desulfurization tower 4 will next be described with
reference to FIGS. 17 and 18.
49
CA 02547679 2002-10-16
As shown in FIG. 17, a frame body 71 is filled with
juxtaposed activated carbon fiber boards 20, to thereby
provide a catalyst unit 72 (e.g., height: 0.5 m to 4 m).
Then, the catalyst unit 72 is immersed in a wetting
bath (not illustrated) separately provided outside the
desulfurization apparatus, to thereby wet the catalyst unit.
In this case, wetting conditions are preferably controlled
such that the amount of water is twice or more the self
weight of the catalyst (i.e., the catalyst's own weight).
For example, when the net weight of the catalyst containing a
plurality of activated carbon fiber boards 20 is 40 to 50 kg,
the catalyst is preferably impregnated with water in an
amount of at least 80 to at least 100 kg.
After the catalyst has been wetted, the catalyst unit
72 is placed in the desulfurization tower 4 by use of lifting
means such as a crane or similar means.
Specifically, when a large amount of discharge gas is
processed by a desulfurization apparatus of a capacity of
1,000 m3 (height: 10 m, area 100 m2), 2,000 units of catalyst
units (1 m x 1 m x 0.4 m) are required. Thus, a large amount
of water is needed to wet the catalyst. If the water is
supplied in a non-stop sprinkling manner, an enormously large
amount of water must be used, which is not preferred from the
economical viewpoint. In a manner employed in the embodiment
of the present invention, catalyst units are individually
wetted, followed by placing in the desulfurization tower.
Thus, the desulfurization apparatus can be started
CA 02547679 2002-10-16
immediately, leading to enhancement of efficiency.
FIG. 18 shows an assembled catalyst unit in which one
module includes four catalyst units 72, two modules are
stacked, and the stacked units are placed in a casing 73.
FIGs. 18(A) and 18(B) show an elevation and a plan view of
catalyst units, respectively.
In this case, catalyst units 72 A to 72 D are
individually wetted, and successively placed by use of a
crane into a casing 73 which has been provided in advance in
the apparatus in the form of tower.
The catalyst module may be stacked a plurality of times
(e.g., 3 to 5).
Alternatively, when additional wetted catalyst units
are placed in the desulfurization tower, the entirety of the
catalyst units are wetted and frozen. The thus-frozen
catalyst units are placed in the tower by use of a crane.
The above method of placement of the units in a frozen
state is an effective method, when the wetting bath cannot be
placed in the vicinity of the desulfurization tower 4. In
other words, when the catalyst units must be transported with
a long distance to the desulfurization tower 4, water
contained in the catalyst units 72 which have been wetted in
advance is lost during transportation. Thus, in order to
start a flue gas desulfurization apparatus employing such
catalyst units, additional supplying of water for wetting
must be performed after placement of the catalyst units in
the desulfurization tower 4_ In contrast, even when the
51
CA 02547679 2002-10-16
frozen catalyst units are vibrated during transportation,
water is not lost. Thus, the desulfurization apparatus can
be started immediately after placement of the catalyst units
in the desulfurization tower 4, leading to enhancement of
efficiency.
The amount of water (water/humidified discharge gas)
contained in the above discharge gas coming into contact with
the catalyst units in the desulfurization tower 4 of the flue
gas desulfurization apparatus of the present invention is
saturated vapor amount plus 0.5 to 10, preferably saturated
vapor amount plus 1.0 to 1.5 vol.o. The saturated vapor
amount is, for example, 12.2 vol.~ (50°C).
The saturated vapor amount is 7.3 vol.% at 40°C or 19.7
vol.o at 60°C. When the amount of water is below the above
saturated vapor amount, the aforementioned release of
sulfuric acid during desulfurization cannot effectively be
attained.
Accordingly, wetting the catalyst units before starting
of the apparatus facilitates operation of the apparatus at a
water content equal to or higher than the saturated vapor
amount. In other words, if desulfurization is started
without wetting before starting the apparatus, activated
carbon fiber fails to attain uniform water distribution
(presence of wetted portions and non-wetted portions),
failing to perform effective desulfurization.
The cooling temperature during humidifying-cooling is
appropriately determined on the basis of the relationship
52
CA 02547679 2002-10-16
between temperature and water content of the discharge gas.
During desulfurization, the cooling temperature is preferably,
for example, 40 to 60°C for the following reason. When the
cooling temperature is higher than 60°C, the amount of
vaporized water increases, thereby increasing the amount of
water to be supplied, resulting in increase in process cost.
A cooling temperature lower than 40°C cannot substantially be
attained, when a discharge gas is cooled through a typical
humidifying-cooling method.
When the discharge gas 100 having a saturated vapor
amount which has been attained by humidifying-cooling by
means of the humidifying-cooling apparatus 16 is fed into the
desulfurization tower 4 and comes into contact with the
catalyst units contained in the catalyst units 6, release of
S03 formed on the catalyst surface through oxidation of SOZ
smoothly proceeds by controlling the water content of the
discharge gas to be saturated vapor amount plus 0.5 to 10
(preferably, saturated vapor amount plus 1.0 to 1.5 vol.o).
Thus, sulfuric acid does not remain on the surface of
activated carbon fiber, and active sites are effectively
utilized, thereby increasing desulfurization efficiency.
As described above, sufficiently wet conditions of the
catalyst units are essentially attained upon starting of the
flue gas desulfurization apparatus. Thus, after the catalyst
units have been wetted through a variety of methods, the
apparatus is started, thereby efficiently performing
desulfurization.
53
CA 02547679 2002-10-16
Next, the case in which the catalyst is wetted by
placing a wetting tank in the desulfurization tower will be
described.
FIG. 19 is a system configuration of a flue gas
desulfurization apparatus according to a fifth embodiment.
As shown in FIG. 19, the flue gas desulfurization
apparatus includes an inlet 5 for introducing a discharge gas
100 containing sulfur oxides in the sidewall (or lower
section) of the apparatus in the form of a tower; an outlet
12 for discharging the discharge gas 100; and a water-
supplying nozzle 60 disposed above a catalyst unit 6 which is
provided in the desulfurization tower 4 and which supplies
water for producing sulfuric acid to the catalyst unit formed
of an activated carbon fiber board. The catalyst unit 6 is
disposed inside a catalyst wetting tank 61, and the catalyst
wetting tank 61 is equipped with a wetting water supply line
63 for supplying wetting water 62 and a circulation line 64
for circulating wetting water 62 serving as circulation water.
Each of the upper and lower faces of the catalyst
wetting tank 61, a discharge-gas-inlet hole and a discharge-
gas-outlet hole (not illustrated) are provided such that
these holes can be arbitrarily opened and shut. Upon
desulfurization, the aforementioned holes remain open.
According to the apparatus, the catalyst unit 6 is
placed in the catalyst wetting tank 61 before starting the
flue gas desulfurization apparatus. Subsequently, wetting
water 62 is supplied from the wetting water supply line 63,
54
CA 02547679 2002-10-16
to thereby wet the catalyst. Wetting conditions may be
judged by means of detection means (not illustrated) such as
a sensor.
A filter layer 65 may be inserted in the circulation
line 64 for circulating wetting water 62 so as to prevent
migration of undesired objects during circulation.
As described above, activated carbon fiber contained in
the catalyst unit can be fully wetted during starting of the
flue gas desulfurization apparatus by placing the catalyst
unit 6 in the catalyst wetting tank 61. Thus, effective
catalytic activity at an initial stage can be attained, and
deterioration of the catalyst can be prevented during
subsequent operation.
Next, the case in which the catalyst is wetted in the
desulfurization apparatus by monolithically placing a
plurality of wetting tanks inside the desulfurization tower
will be described.
FIG. 20 .is a system configuration of a flue gas
desulfurization apparatus according to a sixth embodiment.
As shown in FIG. 20, the flue gas desulfurization
apparatus according to the embodiment effectively wets a
plurality of catalyst units 6.
As shown in FIG. 20, the flue gas desulfurization
apparatus according to the embodiment includes a plurality of
catalyst wetting tanks 61 (four tanks in this embodiment).
Catalyst wetting charnbers 61A to 61D contain catalyst units 6
CA 02547679 2002-10-16
A to 6D, respectively. Wetting water 62 is sequentially
transferred from catalyst wetting chambers 61A to 61D.
Specifically, the catalyst unit 6 is placed in each of
catalyst wetting chambers 61A to 61D, and wetting water 62 is
supplied from the outside to the chambers. At first, the
catalyst wetting chamber 61A is filled with water. After the
catalyst units have been immersed in water for a
predetermined period of time, wetting water 62 is transferred
to the catalyst wetting chamber 61B, to thereby fill the
chamber fully with water. The procedure is sequentially
performed.
In this case, even when a plurality of catalyst units 6
are placed, the amount of wetting water is saved to the
amount of one chamber, leading to reduction of the amount of
water used. In addition, a load on the desulfurization tower
4 during water supply can be mitigated.
Next, an embodiment of the invention in which water-
retaining ability is imparted to a plate-like activated
carbon fiber sheet forming the activated carbon fiber board
serving as a member of the catalyst unit 6 will be described.
According to the aforementioned embodiments, water is
supplied from the outside to the catalyst unit 6, and the
catalyst unit is wetted by water permeating the catalyst.
~iowever, according to the present embodiment, the activated
carbon fiber board is imparted with self-wetting ability.
As shown in FIG. 21, the activated carbon fiber board
200 according to the embodiment is formed of plate-like
56
CA 02547679 2002-10-16
activated carbon fiber sheets 201 and corrugated activated
carbon fiber sheets 22. In each plate-like activated carbon
fiber sheet 201, a water-retaining layer 202 is
monolithically formed. The water-retaining layer 202, which
is made of fiber having high water absorbability, can
effectively supply water which is fed to the catalyst unit to
the corrugated activated carbon fiber sheets 22. In this
case, wetting conditions are attained by water retained
inside the activated carbon fiber board.
The activated carbon fiber board 200 can be adapted to
any one of the aforementioned embodiments. Particularly when
the board is used in combination with the catalyst wetting
tanks 61 as shown in FIGS. 19 and 20, wetting effect
increases.
With reference to FIG. 22, a discharge gas process
system according to a seventh embodiment of the present
invention will be described. In the discharge gas process
system shown in FIG. 22, the catalyst unit 6 and the
capillary member 7 contained in the discharge gas process
system shown in FIG. 1 are replaced by catalyst means 75 and
water-supply means 76. Herein, the same structural members
as employed in the discharge gas process system shown in FIG.
1 are denoted by the same reference numerals.
As shown in FIG. 22, a boiler 1 for generating steam
for driving a steam turbine (not illustrated) of a thermal
power plant, combusts fuel f (e.g., coal or heavy oil) in its
57
CA 02547679 2002-10-16
furnace. A discharge gas generated from the boiler 1
contains sulfur oxides (SOX). The discharge gas undergoes a
NOX removal process by means of an NOX removal unit (not
illustrated), is cooled by means of a gas heater, and
subsequently undergoes a soot removal process by means of a
soot collector 2.
The thus-soot-removed discharge gas is fed, by means of
a feed pump 3, from the inlet 5 provided in a lower section
of the desulfurization tower 4 to the desulfurization tower.
The desulfurization tower 4 includes the catalyst means 75 in
which a plurality of catalyst stages formed of an activated
carbon fiber board, and water for producing sulfuric acid is
supplied from water-supply means 76 to the catalyst means 75.
When the discharge gas is fed from the bottom of the catalyst
means 75 and caused to pass through the catalyst means 75 to
which water is supplied from the above nozzle, SOX contained
in the discharge gas can be removed through chemical reaction.
The discharge gas which has passed through the catalyst means
75 is discharged from an outlet 12, and is released to air
through a chimney 13.
The catalyst means 75 contains a catalyst comprising an
activated carbon fiber board. On a surface of the activated
carbon fiber board, desulfurization reaction occurs (see
reaction mechanism shown in the description in relation to
FIG. 1).
The thus-released sulfuric acid (HzS04) is dilute
sulfuric acid and discharged into a sulfuric acid tank 11 via
58
CA 02547679 2002-10-16
a discharge pump 10. As described above, desulfurization of
the discharge gas is performed by causing sulfur dioxide
(SOz) contained in the discharge gas to be adsorbed by the
activated carbon fiber boards contained in the catalyst means
75 for oxidation, reacting the oxidation product with water
( HZO ) , to thereby form sulfuric acid ( HZSOQ ) , and releasing
the sulfuric acid from the catalyst unit.
The discharge gas process system as shown in FIG. 22
may include a mist-eliminator 19 shown as in FIG. 1.
The structure of the catalyst means 75 provided in the
desulfurization tower 4 will now be described with reference
to FIG. 23.
The catalyst means 75 provided in the desulfurization
tower 4 includes three catalyst stages 119 (height of each
stage: 2 m to 4 m), each incorporating the activated carbon
fiber board 20 (see FIGs. 3 through 5). The number of the
stages of the catalyst stages 119 provided in the catalyst
means 75 is not limited to three, and may be two, or four or
more. Water-supplying nozzles 107 (water supply means 76)
are provided above the respective catalyst stages 119. Water
for forming sulfuric acid is supplied from each of the water-
supplying nozzles 107 onto the corresponding catalyst stage
119. Water is supplied from a water tank 8 via a pump 9 and
feed lines 118 to the water-supplying nozzles 107.
A first valve 135 is provided on the feed line 118 of
the water-supplying nozzle 107 from which water is supplied
to the bottom (upstream) catalyst stage 119; a second valve
59
CA 02547679 2002-10-16
136 is provided on the feed line 118 of the water-supplying
nozzle 107 from which water is supplied to the middle
catalyst stage 119; and a third valve 13? is provided on the
feed line 118 of the water-supplying nozzle 107 from which
water is supplied to the top catalyst stage 119.
Opening/closure operation of the first valve 135, the second
valve 136, and the third valve 137 is controlled by the
control means 125, to thereby regulate the amount of water
supplied to each of the catalyst stages 119. The first valve
135, the second valve 136, and the third valve 137 may be
operated manually.
02 analysis means (oxygen concentration detection means)
126 for detecting the concentration of oxygen (Oz) in the
desulfurization tower 4 is provided in the vicinity of the
inlet 5 of the desulfurization tower 4. Detection data from
the OZ analysis means 126 are input to the control means 125.
The concentration of OZ in the desulfurization tower is on
the order of some percent. Therefore, the OZ analysis means
126 may be provided in the vicinity of the outlet 12 or any
other portion of the desulfurization tower. Even when the OZ
analysis means 126 is provided on an arbitrary position of
the desulfurization tower, data obtained by the analysis
means can be employed as Oz concentration data at the
catalyst means 75. The concentration of Oz in the
desulfurization tower 4 may be calculated on the basis of the
combustion conditions (e.g., fuel/air ratio) in the boiler 1.
The desulfurization tower 4 includes SOZ analysis means
CA 02547679 2002-10-16
(sulfur oxide concentration detection means) 127 for
detecting sulfur oxide (SOZ) concentration on a gas-outlet
side of each of the catalyst stages 119 and on a gas-inlet
side of the upstream catalyst stage 119. By means of the SO2
analysis means 127, the SOZ concentration on the gas-outlet
side of the bottom catalyst stage 119 is detected as a first
concentration; the SOZ concentration on the gas-outlet side
of the middle catalyst stage 119 is detected as a second
concentration; and the SOZ concentration on the gas-outlet
side of the top catalyst stage 119 is detected as a third
concentration. Detection data from the SOZ analysis means
127 are input to the control means 125. The SOz analysis
means 127 may be provided at any position of the
desulfurization tower at which S02 concentration is to be
detected.
The structure of the control means 125 will now be
described with reference to a block diagram shown in FIG. 24.
The control means 125 includes first command means 128
for outputting an opening/closure command to the first valve
135; second command means 129 for outputting an
opening/closure command to the second valve 136; and third
command means 130 for outputting an opening/closure command
to the third valve 137. Data of the first concentration from
the SOZ sensor 127 and data from the Oz sensor 126 are input
to the first command means 128; data of the second
concentration from the SOZ sensor 127 and data from the Oz
sensor 126 are input to the second command means 129; and
61
CA 02547679 2002-10-16
data of the third concentration from the SOZ analysis means
127 and data from the OZ analysis means 126 are input to the
third command means 130.
Predetermined values corresponding to the SOZ
concentrations on the gas-outlet sides of the catalyst stages
119 are stored in the aforementioned command means;
specifically, a first predetermined value is stored in the
first command means 128, a second predetermined value is
stored in the second command means 129, and a third
predetermined value is stored in the third command means 130.
The first concentration is compared with the first
predetermined value in the first command means 128; the
second concentration is compared with the second
predetermined value in the second command means 129; and the
third concentration is compared with the third predetermined
value in the third command means 130. On the basis of the
results of the above comparisons and the Oz concentration
data from the OZ analysis means 126, openingJclosure
operation of the first, second, and third valves 135, 136,
and 137 is controlled, to thereby regulate the amount of
water fed to the desulfurization tower such that the first,
second, and third concentrations are regulated to the first,
second, and third predetermined values, respectively,
Conditions for supplying water to the catalyst stages
119 will now be described.
Data regarding the relation between SOz concentration
(ppm) and water amount (1/min) under a certain fixed Oz
62
CA 02547679 2002-10-16
concentration are stored in a matrix form in the control
means 125. As shown in FIG. 26, the amount of water supplied
to the catalyst stages is increased in accordance with an
increase in SOZ concentration. Also, data regarding the
relation between OZ concentration (%) and water amount
(1/min) under a certain fixed SOZ concentration are stored in
a matrix form in the control means 125. As shown in FIG. 27,
the amount of water supplied to the catalyst stages is
reduced in accordance with an increase in OZ concentration.
The control means 125 employs an SOZ concentration function
and an Oz concentration function, to thereby calculate SO~
and Oz concentrations. On the basis of the thus-calculated
results, the control means 125 outputs percent-opening
regulation commands to the first, second, and third valves
135, 136, and 137.
When the concentration of S02 contained in a discharge
gas introduced through the inlet 5 is A ppm (e. g., 400 ppm),
and the concentration of 02 in the desulfurization tower 4 is
Bo (e. g., 2 to 3~), the first predetermined value, the second
predetermined value, and the third predetermined value are
determined as C ppm (e. g., 150 ppm), D ppm (e. g., 30 ppm),
and E ppm (e.g., 4 ppm), respectively. Subsequently, as
shown in FIG. 25, the amounts of water supplied to the
catalyst stages 119 are regulated such that the SOZ
concentrations on the gas-outlet sides of the bottom catalyst
stage 119, the middle catalyst stage 119, and the top
catalyst stage 119 are regulated to C ppm, D ppm, and E ppm,
63
CA 02547679 2002-10-16
respectively. Specifically, the amount of water supplied to
the bottom catalyst stage 119 is regulated to F 1/min (e. g.,
150 1/min); the amount of water supplied to the middle
catalyst stage 119 is regulated to G 1/min (e. g., 50 1/min);
and the amount of water supplied to the top catalyst stage
119 is regulated to H 1/min (e. g., 10 1/min).
As shown in FIG. 28, the SOZ concentration detected on
the gas-outlet side of each of the catalyst stages 119
gradually increases with passage of time. The first command
means 128 compares the SOL concentration detected at the
bottom catalyst stage with the first predetermined value, to
thereby output an opening/disclosure command to the first
valve 135 such that the detected SOZ concentration approaches
the first predetermined value. The second command means 129
compares the SOz concentration detected at the middle
catalyst stage with the second predetermined value, to
thereby output an opening/disclosure command to the second
valve 136 such that the detected SOz concentration approaches
the second predetermined value. The third command means 130
compares the SOz concentration detected at the top catalyst
stage with the third predetermined value, to thereby output
an opening/disclosure command to the third valve 137 such
that the detected SOz concentration approaches the third
predetermined value. Therefore, as shown in FIG. 29, for
each of the first, second, and third valves 135, 136, and 137,
percent opening increases in accordance with an increase in
SO2 concentration, whereby the amount of water supplied to
64
CA 02547679 2002-10-16
the respective catalyst stages 119 increases.
In the aforementioned flue gas processing apparatus
including the catalyst means 75, three catalyst stages 119
are provided, and an appropriate amount of water is supplied
from each water-supplying nozzle 107 to each catalyst stage
119 by a command of the control means 125. Thus, added water
can be uniformly distributed, and constant SOZ removal
efficiency can be attained. In addition, since an optimum
amount of water is supplied to each catalyst stage 119 in
accordance with the S02 concentration, a required amount of
water can be supplied to the target catalyst stage 119,
thereby maintaining excellent SOZ removal efficiency by a
minimum required amount of water. Furthermore, since a
required amount of water is provided, deterioration of each
catalyst stage 119 due to dryness of sulfuric acid formed on
the catalyst stage 119 can be prevented. When water is not
supplied to the top catalyst stage 119, the catalyst stage
119 exerts a finishing effect on removal of SOZ and can serve
as a mist-catcher.
In the aforementioned embodiment, three catalyst stages
119 are provided, and water is supplied to each catalyst 119
from each water-supplying nozzle 107. By supplying of water
to the top catalyst stage 119 from at least the nozzle
provided thereabove, droplets of water falling between the
catalyst stages 119 are scattered. Thus, added water can be
distributed almost uniformly. In this case, a member for
dispersing water may be inserted as water-dispersion means
CA 02547679 2002-10-16
between catalyst stages 119. However, pressure loss must be
prevented.
Although, in the description in relation to the flue
gas processing apparatuses of the aforementioned embodiments,
dilute sulfuric acid is discharged to the sulfuric acid tank
11, dilute sulfuric acid may also be discharged to a gypsum
deposition tank (see FIG. 2).
With reference to FIG. 30, a discharge gas process
system including a flue gas desulfurization apparatus
according to an eighth embodiment of the present invention
will be described. The discharge gas process system shown in
FIG. 30 includes the discharge gas process system shown in
FIG. 2 and a stationary tank to which a supernatant obtained
in a settling tank 53 is fed. Herein, the same members as
employed in the discharge gas process system shown in FIG. 2
are denoted by the same reference numerals.
As similar to the discharge gas process system shown in
FIG. 2, in the discharge gas process system shown in FIG. 30,
sulfur oxides contained in a discharge gas are removed by
means of a desulfurization apparatus, to thereby form
sulfuric acid, and lime slurry is fed to the resultant
sulfuric acid, to thereby produce gypsum.
As shown in FIG. 30, the desulfurization apparatus
according to the present embodiment includes a boiler 1 for
generating steam for driving a steam turbine; a soot
collector 2 for removing soot contained in a discharge gas
100 generated by the boiler l; a feed fan 3 for feeding the
66
CA 02547679 2002-10-16
soot-removed discharge gas into a desulfurization tower 4; a
humidifying-cooling apparatus 16 for cooling and humidifying
the discharge gas 100 in the desulfurization tower or before
feeding to the desulfurization tower; the desulfurization
tower 4, including a catalyst unit 6, for introducing the
discharge gas 100 from an inlet 5 provided in a lower section
of the sidewall of the tower and for feeding water through a
water-supplying nozzle provided above the catalyst unit 6, to
thereby effect desulfurization in which SOx contained in the
discharge gas is converted to dilute sulfuric acid; a chimney
13 for discharging, to the outside, a purified (desulfurized)
discharge gas released from an outlet 12 of the top of the
tower; a gypsum reaction tank 52 for storing dilute sulfuric
acid fed from the desulfurization tower 4 via a discharge
pump 10 and for depositing gypsum by reaction with supplied
lime slurry 51; a settling tank (thickener) 53 for settling
gypsum; a dewatering apparatus 56 for removing water as waste
water (filtrated liquid) 57 from gypsum slurry 54, to thereby
yield gypsum 55; a stationary tank 162 for allowing a
supernatant 160 obtained in the settling tank 53 to stand, to
thereby yield a sediment 161; and a liquid-feed pump 165 for
feeding a supernatant 163 obtained in the stationary tank 162
to the humidifying-cooling apparatus 16 as humidifying-
cooling water 164.
The amount of gypsum slurry in the gypsum reaction tank
52 is controlled to 7 to 8 wt.~, and that in the settling
tank 53 is controlled to approximately 20 wt.~.
67
CA 02547679 2002-10-16
An optional mist-eliminator 19 may be inserted in the
line for discharging a purified (desulfurized) discharge gas
generated from the desulfurization tower 4, to thereby
separate water contained in the discharge gas.
The discharge gas process system employs the
supernatant 160 obtained in the gypsum reaction tank 52 by
adding lime slurry 51 to dilute sulfuric acid produced from
the desulfurization tower 4 serving as the desulfurization
apparatus. Thus, special water such as industrial water is
not required to be used as water for humidifying-cooling,
thereby attaining an effective system which reduces the
amount of waste water.
In addition, gypsum slurry and salt components (e. g.,
Ca, Na, and K) remaining in the supernatant 160 are removed
as a sediment 161 through provision of the stationary tank
162. Thus, problematic deposition of gypsum and salt
components on the catalyst can be prevented. As a result,
deterioration of desulfuirzation efficiency due to pressure
loss and deterioration of catalytic activity for promoting
desulfurization are prevented, attaining effective
desulfurization.
In the present embodiment, the sediment 161 is removed
by the stationary tank 162. However, the present invention
is not limited to this embodiment. For example, gypsum
particles remaining in the supernatant 160 in the gypsum
settling tank can be separated through separation means such
as a cyclator or a filter. These separation means may be
68
CA 02547679 2002-10-16
employed singly or in combination.
In addition, a cooling tank for cooling the supernatant
160 in the gypsum settling tank may be provided. Through
provision of the cooling tank, dissolved salt components can
be precipitated, thereby further reducing the impurity
content of the supernatant.
Furthermore, a salting-out tank for salting out salt
components contained in the supernatant 160 may be provided.
In the salting-out tank, salt components contained in the
supernatant is intentionally removed on the basis of a
chemical technique, which is in turn different from the
aforementioned physical processes for separating impurities.
The salting-out tank may be used singly or in combination
with the above-described physical separation means.
The discharged water 57 obtained from the dewatering
apparatus 56 may be fed to the stationary tank 162, to
thereby yield a supernatant 163again, and the supernatant may
be employed as water for use in the humidifying-cooling
apparatus 16.
The aforementioned boiler 1 for generating steam for
driving a steam turbine (not illustrated) of a thermal power
plant, combusts fuel f (e.g., coal or heavy oil) in its
furnace. A discharge gas generated from the boiler 1
contains sulfur oxides (SOx). The discharge gas undergoes a
NOx removal process by means of an NOX removal unit (not
illustrated), is cooled by means of a gas heater, and
subsequently undergoes a soot removal process by means of a
69
CA 02547679 2002-10-16
soot collector 2.
The thus-soot-removed discharge gas 100 is fed, by
means of a feed fan 3, from the inlet 5 provided in the side
wall in a lower section of the desulfurization tower 4 to the
desulfurization tower. The desulfurization tower 4 includes
the catalyst unit 6 formed of an activated carbon fiber board,
and water for producing sulfuric acid is supplied from a
water-supplying nozzle 60 to the catalyst unit 6. When the
discharge gas is fed from the bottom of the catalyst unit and
caused to pass through the catalyst unit 6, to which water is
supplied from the above nozzle, SOX contained in the
discharge gas 100 can be removed through chemical reaction.
The discharge gas which has passed through the catalyst unit
6 is discharged from an outlet 12, and is released to air
through a chimney 13.
The catalyst unit 6 contains a catalyst comprising a
plurality of activated carbon fiber boards. On a surface of
each activated carbon fiber board, desulfurization reaction
occurs (see reaction mechanism shown in the description in
relation to FIG. 1).
The sulfuric acid (HZSOQ) removed through the process is
discharged as dilute sulfuric acid into the gypsum reaction
tank 52 via a discharge pump 10. As described above,
desulfurization of the discharge gas is performed by causing
sulfur dioxide (SOz) contained in the discharge gas 100 to be
adsorbed by the activated carbon fiber boards contained in
the catalyst unit 6 for oxidation, reacting the oxidation
CA 02547679 2002-10-16
product with water (H20), to thereby form sulfuric acid
(HZSOQ), and releasing the sulfuric acid from the catalyst
unit.
With reference to FIG. 31, the structure of flue gas
desulfurization apparatus will be described. FIG. 31 is a
schematic view showing the flue gas desulfurization apparatus.
As shown in FIG. 31, the flue gas desulfurization
apparatus includes an inlet 5 for introducing a discharge gas
100 containing sulfur oxides in the sidewall (or lower
section) of the apparatus in the form of a tower; an outlet
12 for discharging the discharge gas 100; and a water-
supplying nozzle 60 disposed above a catalyst unit 6 which is
provided in the desulfurization tower 4 and which supplies
water for producing sulfuric acid to the catalyst unit formed
of an activated carbon fiber board 20. In a lower section of
the tower, a distributor 242 having a dispersion holes 241
for distributing the supplied discharge gas 100 is provided.
The activated carbon fiber board 20 which forms a unit
catalyst in the catalyst unit 6 is formed by alternatingly
juxtaposing on or more plate-like activated carbon fiber
sheets 21 and one or more corrugated activated carbon fiber
sheets 22. Spaces extending straightly and provided between
two sheets serve as conduits 15, with the conduits 15 being
vertically extending. The plate-like activated carbon fiber
sheets 21 and the corrugated activated carbon fiber sheets 22
are formed into sheets. The corrugated activated carbon
fiber sheet 22 is formed by use of a corrugator or similar
71
CA 02547679 2002-10-16
means.
Alternatively, these activated carbon fiber sheets may
be formed into a honeycomb shape so as to enable passage of
the discharge gas in parallel to the sheets.
From the water-supplying nozzle 60, water is sprayed
thereto, and the discharge gas 100 is introduced from a
position below the activated carbon fiber boards. Water
which has passed through the activated carbon fiber boards 20
falls, in the form of droplets of about some mm in size, to
the bottom of the tower. The discharge gas 100 passes
through comparatively narrow conduits 15 provided by
alternatingly juxtaposing the plate-like carbon fiber sheets
21 and corrugated carbon fiber sheets 22. Thus, increase of
pressure loss can be suppressed.
In order to place a catalyst unit in the
desulfurization tower, a frame body (not illustrated) is
filled with juxtaposed activated carbon fiber boards 20, to
thereby provide a catalyst unit (e.g., height: 0.5 m to 4 m).
The catalyst unit is placed in the desulfurization tower 4 by
use of lifting means such as a crane.
The amount of water (water/humidified discharge gas)
contained in the above discharge gas coming into contact with
the catalyst units in the desulfurization tower 4 of the
discharge gas desulfurization apparatus of the present
invention is saturated vapor amount plus 0.5 to 10,
preferably saturated vapor amount plus 1.0 to 1.5 vol.~. The
saturated vapor amount is, for example, 12.2 vol.$ (50°C).
72
CA 02547679 2002-10-16
The saturated vapor amount is 7.3 vol.o at 40°C or 19.7
vol.% at 60°C. When the amount of water is below the above
saturated vapor amount, the aforementioned release of
sulfuric acid during desulfurization cannot effectively be
attained.
The cooling temperature during humidifying-cooling is
appropriately determined on the basis of the relationship
between temperature and water content of the discharge gas.
For example, the cooling temperature is preferably 40 to 60°C
for the following reason. When the cooling temperature is
higher than 60°C, the amount of vaporization increases,
thereby increasing the amount of water to be supplied,
resulting in increase in cost. A cooling temperature lower
than 40°C cannot be attained, when a discharge gas is cooled
through a typical humidifying-cooling method.
When the discharge gas 100 having a saturated vapor
amount which has been attained by humidifying-cooling by
means of the humidifying-cooling apparatus 10 is fed into the
desulfurization tower 4 and comes into contact with the
catalyst contained in the catalyst units 6, release of S03
formed on the catalyst surface through oxidation of SOZ
smoothly proceeds by controlling the water content of the
discharge gas to be saturated vapor amount plus 0.5 to 10
(preferably, saturated vapor amount plus 1.0 to 1.5 vol.~).
Thus, sulfuric acid does not remain on the surface of
activated carbon fiber, and active sites are effectively
utilized, thereby increasing desulfurization efficiency.
73
CA 02547679 2002-10-16
In the apparatus shown in FIG. 31, the discharge gas
100 is supplied from a lower section of the tower. In this
case, mist (a form of water) possibly resides in a lower
section of the tower. Thus, the amount of water supplied
from the water-supplying nozzle 60 must be slightly increased
such that the water content of discharge gas is saturated
vapor amount plus 0.5 to 10 (preferably, saturated vapor
amount plus 1.0 to 1.5 vol.%).
In contrast to the structure of FIG. 31, when the
discharge gas 100 is supplied downward from an upper section
of the tower, mist is also fed to the catalyst unit 6. Thus,
the amount of supplied water might be decreased as compared
with the structure of FIG. 31.
In either case, it is important for the discharge gas
to have a water content equal to or higher than saturated
vapor amount. Particularly, when the water content is
controlled to saturated vapor amount plus 1.0 to 1.5 vol.o,
whereby water can be supplied at remarkably high efficiency
to the surface of activated carbon fiber serving as a
catalyst. More specifically, only the saturated vapor amount
is insufficient for forming sulfuric acid from
S03--bxidation product of SOZ and releasing sulfuric acid
with water. When the water content is in excess of saturated
vapor amount plus 1.5 vol.~, excessive water further dilutes
dilute sulfuric acid and the amount of water required
increases, which is disadvantageous. When the water content
further increases, active sites of the surface of activated
74
CA 02547679 2002-10-16
carbon fiber are covered with water, thereby failing to
attain catalytic action. Thus, desulfurization efficiency
decreases.
The correlation between saturated vapor and vapor mist
has not been elucidated. When S03 formed through oxidation
of SO2 on the surface of activated carbon fiber is
transformed into sulfuric acid by water and the sulfuric acid
is discharged, insufficient amount of water inhibits
discharge of sulfuric acid, and subsequent oxidation of SOz
becomes insufficient. When the amount of water is excessive,
the yielded sulfuric acid is diluted. Furthermore, when the
amount of water further increases, for example in the case in
which the activated carbon fiber is covered with a thin layer
or wall of water which covers active sites of the activated
carbon fiber, such activated carbon fiber loses catalytic
action of oxidizing SO2, failing to attain desulfuirzation or
deteriorating desulfurization efficiency.
Thus, according to the present invention, the amount of
water (water/humidified discharge gas) contained in a
discharge gas coming into contact with a catalyst unit is
controlled to saturated vapor amount plus 1.0 to 1.5 vol.o,
water falls intermittently as spherical droplets, and water
can be supplied to the surface of activated carbon fiber in a
sufficient, not excessive amount, and sulfuric acid can be
released at high efficiency. As a result, desulfurization of
a discharge gas can be effectively performed.
The particle size of droplets of cooling water supplied
CA 02547679 2002-10-16
from the water-supplying nozzle 60 serving as the water-
supply means is preferably controlled to 300 to 1,000 Eun.
This is because when the particle size is falling outside
(excess) the above range, water cannot effectively be
supplied to the surface of activated carbon fiber and release
of sulfuric acid does not proceed sufficiently, which is not
preferred. Particularly when a discharge gas is supplied
from a lower section of the tower, water mist flies from the
water-supplying nozzle, failing to attain favorable water
supply. In contrast, when the particle size of droplets
excessively increases, water forms a wall on the activated
carbon fiber. In this case, active sites covered with water
unfavorably stop desulfurization, although sulfuric acid can
be released.
The amount of water fed from the aforementioned water-
supply means is preferably 5 to 50 ml/m3, wherein the passage
rate of the discharge gas 100 fed into the tower is 0.5 to 5
m/s.
The particle size of mist formed in the discharge gas
which has been humidified and cooled by the humidifying-
cooling apparatus 16 is preferably controlled to 50 to 150 Vim.
This is because when the particle size is less than 50 Eun,
water contained in the discharge gas rapidly vaporize before
feeding to the desulfurization tower , which is not preferred,
whereas when the particle size is in excess of 150 Vim, water
is adhered inside the piping, which is not preferred.
In order to capture the aforementioned mist, a mist-
76
CA 02547679 2002-10-16
catcher (not illustrated) may be provided. The mist-catcher
suppresses incorporation of water to the desulfurization
tower, thereby preventing dilution of sulfuric acid formed
through desulfurization by means of the catalyst unit.
The discharge gas 100 which has been humidified and
cooled in the aforementioned manner has a passage rate to the
tower of 0.5 to 5 m/s, preferably 1 to 3 m/s. This is
because when the passage rate is in excess of 5 m/s, pressure
loss increases, whereas when the passage rate is less than
0.5 m/s, the apparatus requires wide area for desulfurization,
both cases being disadvantageous.
As shown in FIG. 30, the apparatus according to the
embodiment employs the supernatant 160 obtained in the
settling tank 53 upon formation of gypsum slurry as
humidifying-cooling water fed to the aforementioned
humidifying-cooling apparatus 16, to thereby effectively
utilize water.
In order to utilize the supernatant, the supernatant
160 obtained during settling gypsum slurry 54 in the settling
tank 53 is subjected to settling again in the stationary tank
162. After separation of the settled gypsum slurry 54, the
resultant supernatant 163 is utilized as water for
humidifying-cooling 164.
In this case, residual matter remaining in the
supernatant obtained by separating gypsum from gypsum slurry
54 is settled, and the resultant supernatant is utilized as
water for humidifying-cooling that is fed, by means of the
77
CA 02547679 2002-10-16
liquid-feed pump 165, to the humidifying-cooling apparatus 16.
Thus, deposition of gypsum or a similar substance on
activated carbon fiber constituting the catalyst unit 6 is
prevented. As a result, desulfurization performance can be
maintained for a long period of time without deteriorating
desulfurization efficiency.
Industrial Applicability
As described hereinabove, the present invention
provides a flue gas desulfurization apparatus for removing
sulfur oxides (SOX) by evenly adding a minimum required
amount of water to an activated carbon fiber board, the
method being capable of reducing the amount of water required
for removing sulfur oxides (SOX).
The present invention also provides a flue gas
desulfurization apparatus which discharges no industrial
waste and which attains high efficiency, and a flue gas
desulfurization apparatus which requires no absorbent for
sulfur oxides, can be operated without a large
desulfurization facility, and can produce high-concentration
sulfuric acid during desulfurization; i.e., a flue gas
desulfurization apparatus which can reduce the amount of
supplied water and which allows uniform water distribution in
catalyst means.
The present invention also provides a flue gas
desulfurization apparatus which can perform desulfurization
reaction at high efficiency by use of activated carbon fiber;
78
CA 02547679 2002-10-16
which provides a simple desulfurization system; and which
attains high efficiency and small size, and a flue gas
desulfurization apparatus which assures high overall
efficiency of a desulfurization system and which maintains
desulfurization performance over a long period of time.
79