Note: Descriptions are shown in the official language in which they were submitted.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
Attorney Docket No.: 62446-PCT(51955)
Express Mail Label No. EV517916041US
TREATMENT OF INFLAMMATORY DISORDERS OF THE
EPITHELIUM WITH LOW DOSE 2,3-BENZODIAZEPINES
Cross-Reference to Related Applications
The present application is a continuation-in-part of international
application PCT/LTS03/38643 as filed on December 3, 2003 which application
claims priority to U.S. patent application No. 10/309,573 as filed on December
3, 2002. The present application claims further benefit from U.S. patent
application No. 10/727,940 as filed on December 3, 2003. The disclosures of
the PCT/LJS03/38643 and U.S. patent application Nos. 10/309,573 and
10/727,940 are each incorporated herein by reference.
Field of the Invention
The present invention relates to methods of treatment for inflammatory
disorders, particularly disorders of epithelial tissue such as that of the
skin or
gastrointestinal tract.
Background of the Invention
I. Leukotriene B4 (LTB4).
Leukotriene B4 is produced by leukocytes, particularly macrophages and
monocytes, upon activation by immune complexes, phagocytosis or other
stimuli. ~ In this process, membrane phospholipids are broken down by
phospholipase AZ to release arachidonic acid, which is further metabolized via
one of two pathways. The first is via cycloxygenases to produce
prostaglandins.
The second is via lipoxygenases to form leukotriene A4 (LTA4). LTA4 is
converted to LTB4 or LTC4. LTB4 is a potent chemotactic agent that stimulates
neutrophil and macrophage migration (chemotaxis) to sites of inflammation.
The structure of LTB4 is shown below.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
The known pathophysiological responses of LTB4 include: induction of
potent neutrophil chemotactic activity, promotion of adhesion of
polymorphonuclear leukocytes (PMN's) to vasculature, increase in vascular
permeability, stimulation of the release of lysosomal enzymes, by PMN's. The
pro-inflammatory action of LTB4 has been demonstrated in vivo, wherein
topical LTB4 on human skin promotes the infiltration of PMN's and other
inflammatory cells. Intradermal injection of LTB4 induces accumulation of
neutrophils at the injection site. Intravenous injection of LTB4 causes rapid
but
transient neutropenia (Kingsbury et al., J. Med. Chem., 1993, 36, 3308-3320;
and references cited therein).
In addition, the presence of physiologically relevant LTB4 concentration
at inflammatory sites has been associated with, for example, disease states
such
as psoriasis, asthma and active gout; in colonic mucosa associated ' with
inflammatory bowel disease; in synovial fluid from patients with active
rheumatoid arthritis (R.A); and in reperfusion injury. All of these
observations
together support the involvement of LTB4 in human inflammatory disease
(Kingsbury et al, and Griffeths et al., Proc. Natl. Acad. Sci.Vol. 92, pp517-
521,
Jan. 1995; and references cited therein.).
LTB4 is believed to interact with two sub-groups of receptor: a high-
affinity receptor and a low-affinity receptor. Research indicates that the
high-
affinity receptor mediates chemotaxis and that the low-affinity receptor
mediates LTB4-induced secretory and oxidase-activation responses (Yokomizo
et al. 2000). Some LTB4 antagonists are observed to antagonize all LTB4
mediated activity. Other LTB4 antagonists modulate only the activity
associated
with one but not the other sub-population of LTB4 receptors.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
I. LTB4 Antagonists.
Compounds, which act as antagonists of LTB4 include, for example:
structural analogs of LTB4 such as LTB4-dimethyl amide and 20-CF3-LTB4;
SM-9064; U-75302; Ly-223982; SC-41930; ONO 4057 (Prostaglandins,
44(4):261-275, 1992); RG-14893; (E)-3-[2-[6-[3-(3-butoxyphenyl)-3-
hydroxypropenyl]pyridin-2-yl]-1-hydroxyethyl]benzoate-benzoic acid, lithium
salt (Kingsbury J. Med. Chem., 1993, 36, 3308-3320, and references cited
therein); the natural product Leucettamine A and a structural analog, 1-methyl-
2-amino-4-[[4'-[4"-(hydroxybutyl)phenyl]methyl]-5-(phenyl-methyl)imidazole
(Boehm et al, J. Med. Chem., 1993, 36, 22, 3333-3340); a series of pyridine-2-
acrylic acids (I~ingsbury et al., J. Med. Chem., 1993, 36, 22, 3321-3332); SC-
45694 (Tsai et al, J. Pharm. Exp. Tlaef ., 268, 3, 1493-1498); a series of
essential
fatty acids (Yagaloff et al., Prostaglandiyas, Leukotrienes and Essential
Fatty
Acids (1995), 52, 293-297); and FPL 55712 and FPL 55231 (Cheng et al., J.
Pha~m. Exp. Tlae~., 236(1), 1985). The structures of these compounds show
many similarities to the structure of LTB4.
III. 2,3-Benzodiazepines.
Certain 2,3-benzodiazepines have been explored extensively for their
potent CNS modulating activity. Compounds such as tofisopam (Grandaxin~),
girisopam, and norisopam have demonstrated substantial anxiolytic and
antipsychotic activity.
Tofisopam has been shown in humans to have an activity profile that is
significantly different from that of widely used 1,4-benzodiazepine (BZ)
anxiolytics such as diazepam (Valium~) and chlordiazepepoxide (LibriumOO ).
The 1,4-benzodiazepine, in addition to having sedative-hypnotic activity, also
possess muscle relaxant and anticonvulsant properties which, though
therapeutically useful in some disease states, are nonetheless potentially
untoward side effects. Thus, the 1,4-benzodiazepines, though safe when
administered alone, may be dangerous in combination with other CNS drugs
including alcohol.
CA 02548038 2006-05-31
WO 2005/056017 , PCT/US2004/040403
-4-
Tofisopam, in contrast, is a non-sedative anxiolytic that has no
appreciable sedative, muscle relaxant or anticonvulsant properties (Horvath et
al., Progress in Neurobiology, 60 (2000), 309-342). In clinical studies,
tofisopam improved rather than impaired psychomotor performance and showed
no interaction with ethanol (Id.). These observations comport with data that
show that tofisopam does not interact with central BZ receptors and binds only
weakly to peripheral BZ receptors. Additional studies have shown that
tofisopam enhances mitogen-induced lymphocyte proliferation and IL-2
production in vitro.
Other 2,3-benzodiazepines that are structurally similar to tofisopam have
been investigated and shown to have varying activity profiles. For example,
GYI~I-52466 and GYKI-53655 (structures shown below) act as noncompetitive
glutamate antagonists at the AMPA (oc-amino-3-hydroxy-5-methyl-4-
isoxazolepropionic acid) site, and have demonstrated neuroprotective, muscle
relaxant and anticonvulsant activity (Id.). Another group of 2,3-
benzodiazepines that have been investigated are represented by the compound
GYI~I-5295, and show activity as selective dopamine uptake inhibitors with
potential use in antidepressant and anti-Parkinsonism therapy.
Tofisopam (structure shown below); with the atom numbering system
indicated) is a racemic mixture of (R)- and (S~- enantiomers. This is due to
the
asymmetric carbon, i.e., a carbon with four different groups attached, at the
5
position of the benzodiazepine ring.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
The molecular structure and conformational properties of tofisopam have
been determined by NMR, CD and X-ray crystallography (Visy et al., Chirality
1:271-275 (1989)). The 2,3-diazepine ring exists as two different conformers.
The major tofisopam conformers, (+)R and (-)S, contain a 5-ethyl group in a
quasi-equatorial position. The 5-ethyl group is positioned quasi-axially in
the
minor conformers, (-)R and (+)S. Thus, racemic tofisopam can exist as four
molecular species, i.e., two enanti~omers, each of which exists as two
conformations. The sign of the optical rotation is reversed upon inversion of
the
diazepine ring from one conformer to the other. In crystal form, tofisopam
exists only as the major conformations, with dextrorotatory tofisopam being of
the (R) absolute configuration. (Toth et al., J. Heterocyclic Chem., 20:709-
713
(1983); Fogassy et al., Bioorganic Heterocycles, Van der Plas, H.C., Otvos, L,
Simongi, M., eds. Budapest Amsterdam: Akademia; Kiado-Elsevier, 229:233
(1984)).
Differential binding of the (+) and (-) conformations of tofisopam has
been reported in binding studies with human albumin (Simongi et al. Biochem.
Pharm., 32(12), 1917-1920, 1983). The two (+/-) conformers have also been
reported as existing in equilibrium (Zsila et al., Journal of Liquid
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-6-
Claromatog~aphy & Related Technologies, 22(5), 713-719, 1999; and references
therein).
The optically pure (R)-enantiomer of tofisopam (R)-1-(3,4-
dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-2,3-benzodiazepine) has
been isolated and shown to possess the nonsedative anxiolytic activity of the
racemic mixture. See US Patent 6,00,736; the entire disclosure of which is
incorporated herein by reference.
IV. Inflammatory Disorders.
Frequently, inflammatory disorders occur at or near an epithelium, such
as that of the skin, the cornea, or the gastrointestinal lining.
A. Inflammatory Bowel Disease
Crohn's disease (CD) and ulcerative colitis (UC), and, to a lesser extent,
indeterminate colitis and infectious colitis, are collectively referred to as
inflammatory bowel disease IBD. Inflammatory bowel diseases are chronic
recurrent inflammatory diseases of unclear etiology, affecting the small
intestine
and colon. IBD can involve either or both. the small and large bowel. These
disorders fall into the category of "idiopathic" IBD because the etiology for
them is unknown.
Pathologic findings are generally not specific, although they may suggest
a particular form of IBD. "Active" IBD is characterized by acute inflammation.
"Chronic" IBD is characterized by architectural changes of crypt distortion
and
scarring. The term "crypt" refers to a deep pit that protrudes down into the
connective tissue surrounding the small intestine. Crypt abscesses (active IBD
characterized by the presence of neutrophils in crypt lumens) can occur in
many
forms of IBD, not just UC. Under normal conditions the epithelium at the base
of the crypt is the site of stem cell proliferation and the differentiated
cells move
upwards and are shed 3-5 days later at the tips of the villi. This normal
process,
necessary for proper bowel function, is interrupted by IBD.
UC involves the colon as a diffuse mucosal disease with distal
predominance. The rectum is virtually always involved, and additional portions
of colon may be involved extending proximally from the rectum in a continuous
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
_'7_
pattern. Most often UC occurs in young people 15 to 40 years of age. UC
occurs only in the inner lining of the colon (large intestine) or rectum. When
it
is localized in the rectum, it is called "proctitis".
CD is a chronic inflammatory disease that has periods of remission (time
when person feels well) and relapse (when a person feels ill). CD is an
inflammation and ulceration process that occurs in the deep layers of the
intestinal wall. The most common areas affected are the lower part of the
small ,
intestine, called the ileum, and the first part of the colon. This type of CD
is
called ileocolitis. CD can infrequently affect any part of the upper
gastrointestinal tract. Aphthous ulcers, which are similar to cold sores, are
common. Ulcers can also occur in the esophagus, stomach and duodenum.
Therapy for IBD has historically included administration of
corticosteroids. However drawbacks of long term corticosteroid therapy include
masking (or induction) of intestinal perforation, osteonecrosis and metabolic
1 S bone disease. Additional problems relate to development of corticosteroid
dependency (Habnauer, New England Journal ofMedicine, 334(13), p 841-848,
1996). Aminosalicylates such as sulfasalazine and mesalamine have been used
to treat mild or moderately active UC and CD, and to maintain remission (Id at
843). Immunomodulatory drugs such as azathioprine and mercaptopurine have
been used in long term treatment for patients with IBD. Common complications
with both of these drugs include pancreatitis, which occurs with an incidence
of
3-15% of patients, and bone marrow suppression, which requires regular
monitoring. More potent immunosuppressive drugs such as cyclosporine and
methotrexate have been employed, but toxicity of these drugs limits their use
to
specific situations of refractory disease states. Other therapeutic approaches
include antibiotic therapy and nutritional therapy. Often, therapy involves a
combination of the above-described drug therapies in addition to surgical
resection of the bowel.
There is no cure for IBD. Ultimately, the chronic and progressive nature
of IBD demands a long-term treatment that maximizes the local
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
antiinflammatory effect while minimizing the global systemic effect on the
immune system.
Chronic inflammatory disorders such as CD typically demonstrate
periods of remission between intervals when the inflammatory is active and
requires acute treatment. This is an example of a circumstance wherein it is
known beforehand that an individual will develop, or is likely to develop an
inflammatory disorder.
Irritable bowel syndrome (IBS) is a disorder of the bowel which is
distinct from IBD. IBS affects at least 10% to 20% of adults in the U.S. IBS
is
the most common disorder diagnosed by gastroenterologists and one of the top
ten most frequently diagnosed conditions among U.S. physicians.
IBS is classified as a "functional gastrointestinal disorder," which means
there is a disturbance in bowel function. IBS is not a considered a disease,
but
rather a syndrome, i. e., a group of symptoms. The symptoms typically include
chronic abdominal pain/discomfort, and irregular bowel function, e.g.,
diarrhea,
constipation, or alternating diarrhea and constipation.
Unlike IBD, IBS does not cause inflammation. IBS sufferers show no
sign of disease or abnormalities on examination of the colon. Thus, though IBD
and IBS share some similar symptoms, particularly cramping and diarrhea, the
underlying disease process is quite different. IBD involves inflammation or
destruction of the bowel wall, which can lead to deep ulcerations and
narrowing
of the intestines. IBS is a disorder of the gastrointestinal (GI) tract for
which no
apparent cause can be found. An individual can simultaneously have both IBS
and an inflammatory disorder such as IBD. When this occurs, imprecise
diagnosis may lead to inadequate medical intervention.
B. Inflammatory Skin Disorders:
1. Psoriasis.
Another chronic inflammatory condition believed to be mediated by
LTB4 is psoriasis. Psoriasis is a chronic, recurrent, papulosquamous plaque on
areas of trauma such as the elbow, lcnee or scalp, though it may appear
elsewhere on the slcin. Psoriasis may coexist with lupus erythematosis in some
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
_y_
individuals. Current treatments include topical administration of psoralens.
"Psoralens" refers to a group of substances found in many different plants;
especially psoralea co~ylifolia. Psoralens interact with nucleic acids and are
also used as research tools. Psoriasis is also treated by long-wave
ultraviolet
radiation. Neither treatment cures or prevents recurrence of psoriasis
symptoms.
2. Atopic Dermatitis/Eczema.
Atopic dermatitis is a chronic disease that affects the skin. In atopic
dermatitis, the skin becomes extremely itchy. Scratching leads to redness,
swelling, cracking, "weeping" clear fluid, and finally, crusting and scaling.
In
most cases, there are periods of exacerbations followed by periods of
remissions. Although it is difficult to identify exactly how many people are
affected by atopic dermatitis, an estimated 20% of infants and young children
experience symptoms of the disease. Approximately 60% of these infants
continue to have one or more symptoms of atopic dermatitis in adulthood.
Thus, more than 15 million people in the United States have symptoms of the
disease
3. Contact Dermatitis.
Contact dermatitis is a reaction that occurs when the skin comes into
contact with an allergen, i.e., a substance to which the body is allergic.
Allergens, though harmless to most individuals, cause an allergic reaction in
individuals having a congenital or acquired hypersensitivity to the specific
allergen.
C. Rheumatoid Arthritis (RA).
Another chronic inflammatory disorder believed to be mediated by LTB4
is RA, which is an autoimmune disease of the joints. RA is characterized by
the
following criteria 1-7, wherein criteria 1-4 are present for more than 6
weeks:
(1) morning stiffness in and around joints lasting at least one hour before
maximum improvement; (2) soft tissue swelling (arthritis) of three or more
joints observed by a physician; (3) swelling (arthritis) of the proximal
interphalangeal, metacarpal phalangeal, or wrist joints; (4) symmetric
swelling;
(5) rheumatoid nodules, i.e., a granulomatous lesion characterized by central
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-1 U-
necrosis encircled by a palisade of monocytes and an exterior mantle of
lymphocytic infiltrate. These lesions present as subcutaneous nodules,
especially at pressure points such as the elbow in individuals with RA or
other
rheumatoid disorders; (6) presence of rheumatoid factors, i.e., an
autoantibody
in the serum of individuals with RA; and (7) roentgenographic erosions, i.e.,
joint lesions visible on an X-ray.
RA is a chronic disorder for which there is no known cure. The major
goals of treatment of RA are to reduce pain and discomfort, prevent
deformities
and loss of joint function, and maintain a productive and active life.
Inflammation must be suppressed and mechanical and structural abnormalities
corrected or compensated by assistive devices. Treatment options include
reduction of joint stress, physical and occupational therapy, drug therapy,
and
surgical intervention.
There are three general classes of drugs commonly used in the treatment
of RA: non-steroidal anti-inflammatory agents (NSAID's), corticosteroids, and
remittive agents or disease modifying anti-rheumatic drugs (DMARD's).
NSAID's and corticosteroids have a short onset of action while DMARD's can
take several weeks or months to demonstrate a clinical effect. DMARD's
include leflunomide (AravaTM), etanercept (EnbrelTM), infliximab
(RemicadeTM), antimalarials, methotrexate, gold salts, sulfasalazine, d-
penicillamine, cyclosporin A, ~ cyclophosphamide and azathioprine. Because
cartilage damage and bony erosions frequently occur within the first two
years,
rheumatologists now move more aggressively to a DMARD agent.
Treatment of RA by chronic administration of a corticosteroid involves
the same side effect profile as discussed regarding IBD above. Chronic
administration of NSAID's also produces side effects. The most common
toxicity of NSAID's is gastrointestinal disturbance. Because prostaglandins
play
a role in the regulation of renal blood flow and maintenance of glomerular
filtration, NSAID's can impair renal function in certain patients. Weight gain
and cushingoid appearance is a frequent problem and source of patient
complaints. Recent studies have raised concern over the increased
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-11-
cardiovascular risk and accelerated osteoporosis associated with low dose
prednisone particularly at doses above 10 mg daily.
D. Gout.
Gout is another inflammatory disorder believed to be mediated by LTB4.
Gout is characterized by a disturbance of uric-acid metabolism occurnng
chiefly
in males. Gout is characterized by painful inflammation of the joints,
especially
of the feet and hands, and arthritic attacks resulting from elevated levels of
uric
acid in the blood and the deposition of orate crystals around the joints. The
condition can become chronic and result in deformity.
Gout can present another circumstance wherein it is known beforehand
that an individual will or is likely to develop an inflammatory disorder. In
the
instance of patients undergoing radiotherapy or chemotherapy, the individual
may experience a dramatic rise in serum uric acid levels associated with lysis
of
the tumor mass. Such large increases in uric acid can deposit orate crystals
in
synovial fluid of joints thereby causing the inflammatory disorder, gout. When
such a rise in serum uric acid levels is known to be likely, prophylaxis with
an
LTB4 antagonist can act to prevent the inflammatory condition of gout.
E. Radiation-induced Gastrointestinal Inflammation.
Radiation-induced gastrointestinal inflammation is another
inflammatory disorder believed to be mediated by LTB4. Radiation works by
damaging cancer cells, but unfortunately can damage non-diseased tissue as
well, causing a typical inflammatory reaction in response. Therapeutic
radiation
is thus generally applied to a defined area of the subj ect's body which
contains
abnormal proliferative tissue in order to maximize the dose absorbed by the
abnormal tissue and minimize the dose absorbed by the nearby normal tissue.
However, it is difficult (if not impossible) to selectively administer
therapeutic
ionizingxadiation to the abnormal tissue. Thus, normal tissue proximate to the
abnormal tissue is also exposed to potentially damaging doses of ionizing
radiation throughout the course of treatment. Moreover, some treatments that
require exposure of the subject's entire body to the radiation, in a procedure
called "total body irradiation", or "TBL" The efficacy of radiotherapeutic
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-1G-
techniques in destroying abnormal proliferative cells is therefore necessarily
balanced by the associated cytotoxic effects on nearby normal cells.
After or during a course of radiotherapy, LTB4-mediated inflammatory
processes may be triggered, causing damage to the bowel, and leading to
sloughing of the cells of the inner lining of the GI tract. Radiation-induced
gastrointestinal inflammation can present another circumstance wherein it is
known beforehand that an individual will or is likely to develop an
inflammatory disorder. In the instance of patients undergoing radiotherapy,
the
inflammation, damage and sloughing of the gastrointestinal tract is a
predictable
side effect of the radiotherapy.
F. Mucositis.
Mucositis involves ulcerative breakdown of mucosal epithelial tissue,
and is literally defined as inflammation of the mucous membrane. The
pathophysiology of mucositis in response to toxic insults to the mucosa by
chemotherapy or by ionizing radiation is complex and involves a cascade of
interactions among cells, cytokines and the oral microflora. The underlying
premise for susceptibility of the mucosa of the oropharynx and
gastrointestinal
tract to chemotherapy or radiation damage is related to rapid epithelial stem
cell
turnover. Mucositis may be characterized by the following phases:
1. Early inflammatory phase characterized by release of
inflammatory cytokines in response to local tissue damage caused
by cytotoxic agent(s);
2. Epithelial phase characterized by death of basal cells,
which hinders re-population of the epithelium. This inability to
regenerate leads to atrophy followed by ulceration. The ulceration
represents loss of an important anatomic barrier at a site of local
microflora;
3. Infection phase characterized by local invasion of
microflora that results in an inflammatory response to the local
infection. The inflammation results in additional local tissue
damage and possibly erosive ulceration; and
4. Healing phase characterized by resolution of the
infection and regeneration of epithelium.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-13-
Oral mucositis produces the following clinical symptoms and signs
resulting from cellular damage: 1) sensation of dryness; 2) asymptomatic
redness and erythema; 3) solitary white elevated desquamative patches which
are painful upon pressure contact; and 4) large, painful, contiguous
pseudomembranous lesions associated with dysphagia and decreased oral
intake. These spontaneously painful lesions histopathologically show loss of
epithelial cells to the basement membrane, which exposes the connective tissue
stroma with its associated innervation.
As with oral mucosa, gastrointestinal mucosal damage results from
disturbance of cellular mitosis that leads to reduction in the turnover rate
of the
basal cells of the intestinal crypts. The symptoms and signs of
gastrointestinal
mucositis include tenesmus (painful ineffectual straining at stool), pain,
bleeding, diarrhea, telangectasia (neovascularization), and progression to
ulceration. Early signs of diarrhea include increased stool frequency, loose
or
watery stool, food aversion, increased bowel sounds, abdominal pain, and some
loss of skin turgor indicative of dehydration. When the diarrhea is severe it
may
be associated with mucosal ulceration, bleeding,, intestinal perforation and
proctitis. Stool examination may reveal occult blood and fecal leukocytes.
G. Necrotizing Enterocolitis.
Necrotizing enterocolitis is an inflammatory disease of unknown
etiology that afflicts between 1-5% of all infants admitted to neonatal
intensive
care units, most of whom are premature infants. Signs and symptoms include
abdominal distention, gastrointestinal hemorrhage, and feeding intolerance.
The
disease most often involves the ileum and colon, and is characterized by loss
of
epithelium and submucosal edema, ulcerations, and, in severe cases, transmural
necrosis.
H. Aphthous Ulcers (Oral).
Although the cause of aphthous ulcers remains unknown, many
physicians believe they are caused by autoimmune phenomena, which cause the
destruction of discrete areas of the oral mucosa which leads to oral
ulceration.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-14-
Among the cytokines present in these active areas of ulceration, TNF-a appears
to play a predominant role.
I. Gingivitis/Periodontitis.
Adult periodontitis is strongly associated with infection by
Porphyromonas gingivalis. Proteolytic enzymes, which are produced in large
quantity by this bacteria, are considered as important pathogenic agents. The
increased production and flow of gingival crevicular fluid (GCF) is an
important
change in gingival tissues during periodontal infection, correlating with
clinical
indices of gingival inflammation. Salivary protein and albumin concentrations
of individuals with periodontitis, which are an indication of plasma leakage
due
to vascular permeability enhancement (VPE), are significantly increased
compared to healthy subjects. The production of GCF appears dependent on
VPE induced at periodontitis sites, presumably involving proteinase(s) of P.
gingivalis in their generation.
J. Esophagitis.
The most common cause of esophagitis is the chronic reflux of
hydrochloric acid from the stomach due to inefficiency of the cardiac
sphincter
of the stomach. The chronic presence of acid in the lower esophagus leads to
damage of the esophageal mucosa. In the most severe form, a syndrome called
Barrett's esophagus can develop which often leads to esophageal cancer. Other
causes of esophagitis include parenteral chemotherapy and ionizing radiation,
associated with radiation therapy for cancer in the thoracic cavity.
K. Pharyngitis.
Pharyngitis is defined as an infection or irntation of the pharynx and/or
tonsils. The etiology is usually infectious, with 40-60% of cases being of
viral
origin and 5-40% of cases being of bacterial origin. Other causes include
allergy, trauma, toxins, and neoplasia. It has been estimated that children in
the
US experience more than five upper respiratory infections (IJRIs) per year and
an average of one streptococcal infection every four years. The occurrence in
adults is about one half that rate. The most significant bacterial agent
causing
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-15-
pharyngitis in both adults and children is GABHS infection (Streptococcus
pyogenes), and the most significant viruses are rhinovirus and adenovirus.
L. Ocular Diseases.
1. Retinitis.
Inflammation of the light sensitive retina, retinitis, can occur due to a
variety of viral, bacterial or autoimmune etiologies. The end result is
destruction of the retina and loss of sight.
2. Uveitis.
Inflammation of the anterior portion of the eye and/or its associated
structures, the iris and cornea occurs with a relatively high frequency in
patients
with autoimmune disorders.
3. Conjunctivitis.
Conjunctivitis is an inflammation of the conjunctivae, which are the
mucous membranes covering the white of the eyes and the inner side of the
eyelids. There are five major types of conjunctivitis. (1) Bacterial
conjunctivitis is an infection caused by bacteria. (2) Viral conjunctivitis
may be
caused by a virus called'adenovirus'. (3) Chlamydial conjunctivitis is caused
by
an organism called Chlamydia trachomatis. (4) Allergic conjunctivitis is
common in people who have other signs of allergic disease such as hay fever,
asthma and eczema. (5) Reactive conjunctivitis (chemical or irritant
conjunctivitis) is caused by a chemical irntant, e.g., chemicals in swimming
pools, smoke or solvent fumes.
M. Peptic Ulcer Disease.
Inhibition of gastric acid secretion with Ha-receptor antagonists and,
more recently, Mockers of H+, K+ -ATPase (also known as the proton pump) has
been the mainstay of therapy for peptic ulcer disease. The pathophysiology of
peptic ulcers remains obscure. An appreciation of the complexity of the
physiology of the gastric mucosa has led to a hypothesis that peptic ulcers
are
the result of an imbalance in the relative importance of aggressive (acid,
pepsin)
and protective (mucus, bicarbonate, blood flow, prostaglandins, etc.) factors.
Infection of the mucosa of the human gastric antrum with the bacterium
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-16-
Helicobacter pylori has been widely accepted as the cause of chronic, active,
type B gastritis. Further, this form of gastritis has been linked directly to
peptic
ulcer disease by studies showing that eradication of H. pylori reverses this
gastritis and prevents duodenal ulcer relapse.
V. Agents Useful in Treatment of Inflammatory Disorders.
Numerous chemical entities have been investigated for biological
activity as antiinflammatory agents. Particular classes of compounds which
have been investigated include aminosalicylates, corticosteroids,
antimetabolites, immunosuppressants, tumor necrosis factor alpha (TNF-a)
inhibitors, inhibitors of leukotriene synthesis e.g., 5-lipoxygenase (5-LO)
inhibitors, and leukotriene antagonists.
Exemplary compounds of interest that have been shown to possess'
activity in models of inflammatory disorders are listed in Table 1 along with
putative mechanisms of action and the indications for which they have been
investigated.
Table 1
Agent Indication or Mechanism of action
potential
indication
ETH615 dermatitis 5-LO inhibitor
(4-[2-quinolylmethoxy]-N-[3- Inhibits inflammation-induced
flurobenzyl]-phenyl- chemotaxis
aminomethyl-4-benzoic
acid
15-HETE (15-hydroxyeicosa-psoriasis Inhibits LTB4 synthesis
tetraenoic acid) Inhibits LTB4-induced
chemotaxis
Leflunomide (Arava)asthma (marketed)Inhibits pyrimidine
synthesis
Inhibits LTBd release
Inhibits inflammation-induced
chemotaxis
Inhibits proliferation
of epidermal
keratinocytes
Linetastine 5-LO inhibitor
Lonapalene (RS psoriasis 5-LO inhibitor
43179)
R-68,151 psoriasis 5-LO inhibitor
MK 886 asthma Inhibits 5-LO activation
protein
3-[1-(p-Chloro-benzyl)-5-
(isopropyl)-3-tert-butylthio-
indol-2-yl]-2,
2- dimethyl-
propanoic acid
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-17-
Agent Indication or Mechanism of action
potential
indication
Zileuton (Zyflo) asthma (marketed)5-LO inhibitor
SC41930 psoriasis, IBD,LTB4 receptor antagonist
RA
7-[3-(4-acetyl-3-methoxy-2-
propylphenoxy)propoxy]-8-
propylchromane-2-carboxylic
acid
SC50605 LTB4 receptor antagonist
SC53228 psoriasis, UC, LTB~ receptor antagonist
((+)-(S)-7-[3-{2-Cyclo-dermatitis
propylmethyl}-3-methoxy-4-
{(methylamino)carbonyl}
phenoxy]propoxy)-3,4-
dihydro-8-propyl-2H-1-
benzopyran-2-propanoic
acid
SC52798 Inflammation, LTB4 receptor antagonist
Psoriasis, UC
CGS-25019C asthma, bronchitis,LTB4 receptor antagonist
RA
4-(5-[4-{Aminoiminomethyl}
phenoxy]-pentoxy)-3-
methoxy-N,N-bis(1-metlryl-
ethyl)-benzamide-(Z)-2-
butenedioate
ONO-4057 psoriasis, UC, LTB4 receptor antagonist
Behcet's
5-{3-[(SE)-6-(4-methoxy-
phenyl)hex-5-enyloxy]-2-(2-
carboxyethyl)phenoxy}penta
noic acid
SB-201993 psoriasis, RA LTB4 receptor antagonist
SB-209247 psoriasis, eczemaLTB4 receptor antagonist
(E-3-(6-[{(2,6-Dichloro-
phenyl)-thin }-methyl]-3-[2-
phenyl-ethoxy]-2-pyridinyl)-
2-propenoic acid
VML295 (LY293111) psoriasis, asthma,LTBd receptor antagonist
UC,
(2-[2-Propyl-3-{2-ethyl-4-(4-~~ mBa~ation
fluoro-phenyl)-5-hydroxy-
phenyl}propoxy]phenoxy)be
nzoic acid
CP-105696 RA, asthma, LTB4 receptor antagonist
rejection
1-{(3S,4R)-4-hydroxy-3-[(4-
phenylphenyl)-methyl]-
chroman-7-yl}cyclo-pentane-
carboxylic acid
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-18-
Agent Indication or Mechanism of action
potential
indication
CP-195543 R.A, inflammationLTB4 receptor antagonist
(+)-2-(3-Benzyl-4-hydroxy-
chroman-7-yl)-4-trifluoro-
methylbenzoic acid
BIIL 284 cystic fibrosis,LTB4 receptor antagonist
asthma
U-75302 asthma LTB4 receptor antagonist
6-[6-((SZ,1 E)-3-hydroxy-
undeca-1,5-dienyl)-2-
pyridyl]-hexane-1,5-diol
LY 255283 asthma LTB4 receptor antagonist
VM 301 wound healing, LTB4 receptor antagonist
dermatitis,
inflammation
ZK 158252 psoriasis LTB4 receptor antagonist
New antiinflammatory agents are needed which are useful in the
treatment of inflammatory disorders such as IBD, RA, gout, psoriasis and
radiation-induced gastrointestinal inflammation. In particular, agents are
needed
that are appropriate for chronic long-term use in treatment. In addition,
agents
are needed that are useful in the prevention of inflammatory disorders,
particularly LTB4-mediated inflammatory disorders that occur secondary to
observable events such as ionizing radiation therapy.
Summary of the Invention
In one embodiment of the invention there is provided a method of
treatment or prevention of inflammatory disorders in an individual,
particularly,
inflammatory disorders affecting epithelial tissues, in particular, IBD,
including
CD and, UC, psoriasis; rheumatoid arthritis; gout and radiation-induced
gastrointestinal inflammation.
The method comprises administering to the individual an effective
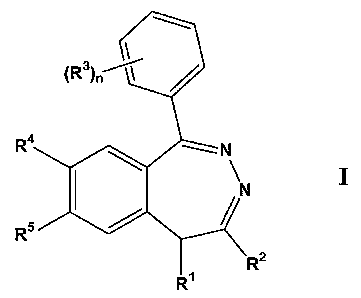
amount of at least one compound according to formula I:
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-1 y-
0 4
R
I
R5
wherein:
Rl is -(Cl-C~)hydrocarbyl or -(C2-C6)heteroalkyl;
R2 is selected from the group consisting of H, and -(Cl-C~)hydro-
caxbyl, wherein Rl and R2 may combine to form a carbocyclic or heterocyclic 5-
or 6-membered ring;
R3 is independently selected from the group consisting of -O(CI-
C6)alkyl, -OH, -O-acyl, -SH, -S(Cl-C3)alkyl, NH2, -NH(Ci-C6)alkyl, -N((C1-
C6)alkyl)2, -NH-acyl, -N02 and halogen;
n is 1, 2 or 3;
R4 and RS are independently selected from the group consisting of
-O(Cl-C6)alkyl, -OH, O-acyl, -SH, -S(C1-C3)alkyl, NHZ, NH-acyl and
halogen, wherein R4 and RS may combine to form a 5, 6 or 7-membered
heterocyclic ring; ora pharmaceutically-acceptable salt of such a compound,
wherein the compound is administered at a dose of less than about 50 mg/day.
Preferably the compound is administered at a dose of less than about 25
mg/day,
more preferably less than aboutl0 mg/day and even more preferably at a dose of
less than about 1 mg/day. Preferred liquid formulations are administered at a
dose of less than about 10 mg/ml and more preferably at a dose of less than
about 1 mg/ml. According to one embodiment of the invention, there is
provided a method of treatment of inflammatory disorders of epithelial tissue,
wherein said inflammatory disorder is a slcin disorder. In another embodiment,
the inflammatory disorder of the epithelial tissue is a gastrointestinal
disorder.
According to another embodiment of the invention, the administered compounds
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-20-
according to formula I comprise a racemic mixture of compounds with respect
to the absolute conformation at the 5-position of the benzodiazepine ring.
According to a preferred embodiment of the invention, the administered
compounds according to formula I comprise an (R)-enantiomer, substantially
free of the corresponding (S~-enantiomer of the same compound, with respect to
the absolute conformation at the 5-position of the benzodiazepine ring,
Preferably, the administered compound of formula I comprising an (R)-
enantiomer, or a pharmaceutically-acceptable salt of such a compound,
comprises 85% or more by weight of the (R)-enantiomer. More preferably, the
administered compound of formula I comprising an (R)-enantiomer, or a
pharmaceutically-acceptable salt of such a compound, comprises 90% or more
by weight of the (R)-enantiomer. Even more preferably, the administered
compound of formula I comprising an (R)-enantiomer, or a pharmaceutically-
acceptable salt of such a compound, comprises 95% or more by weight of the
(R)-enantiomer. Most preferably, the administered compound, comprising an
(R)-enantiomer of formula I, or a pharmaceutically-acceptable salt of such a
compound, comprises 99% or more by weight of the (R)-enantiomer.
According to one embodiment of the invention:
Rl is -(Cl-C6)alkyl;
R2 is selected from the group consisting of H and -(C1-C6)alkyl;
R3 is independently selected from the group consisting of -O(C1-
' C6)alkyl, -O-acyl and -OH;
n is 1, 2 or 3; and
R4 and RS are independently selected from -O(Cl-C6)alkyl, -O-acyl and
-OH, wherein, R4 and RS may combine to form a 5, 6 or 7-membered
heterocyclic ring; or a pharmaceutically-acceptable salt of such a compound.
According to a preferred embodiment of the invention:
Rl is -CHaCH3;
RZ is -(Cl-C~)allcyl;
R3, R4 and RS are independently selected from the group consisting of
-OH and -OCH3; and
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-21-
n is 1, 2 or 3;
or a pharmaceutically-acceptable salt of such a compound.
According to a further preferred embodiment of the invention:
Rl is -CH2CH3;
Ra is -CH3;
R3, R4 and RS are independently selected from the group consisting of
-OH and -OCH3; and
n is 1, 2 or 3; or a pharmaceutically-acceptable salt of such a compound.
According to a further preferred embodiment of the invention:
Rl is -CH2CH3;
R2 is -CH3;,
R3, R4 and RS are independently selected from the group consisting of
-OH and -OCH3; and
n is 2 ; or a pharmaceutically-acceptable salt of such a compound.
According to a further preferred embodiment of the invention:
Rl is -CH2CH3;
R2 is -CH3;
R3, R4 and RS are independently selected from the group consisting of
-OH and -OCH3;
n is 2; and wherein R3 comprises substituents at the 3- and 4- positions
of the phenyl ring; or a pharmaceutically-acceptable salt of such a compound.
Preferred racemic compounds according to formula I, for administration,
are selected from the group consisting of
1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7, 8-dimethoxy-SH-2, 3-
benzodiazepine;
1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-methoxy-SH-
2,3-benzodiazepine;
1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-
2,3-benzodiazepine;
1-(3-methoxy-4-hydroxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-
2,3-benzodiazepine;
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-22-
1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-methoxy-8-hydroxy-SH-
2,3-benzodiazepine;
1-(3-methoxy-4-hydroxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-
methoxy-SH-2,3-benzodiazepine;
1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-
methoxy-SH-2,3-benzodiazepine; and
pharmaceutically-acceptable salts of such compounds.
More preferably, the compound according to formula I, for
administration is 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-
2,3-benzodiazepine; or
a pharmaceutically-acceptable salt thereof.
Preferred compounds comprising (R)-enantiomers according to formula
I, for administration, are selected from the group consisting of
(R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7, 8-dimethoxy-5 H-2,3-
benzodiazepine;
(R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-methoxy-
SH-2,3-benzodiazepine;
(R)-1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-
SH-2,3-benzodiazepine;
(R)-1-(3-methoxy-4-hydroxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-
SH-2,3-benzodiazepine;
(R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-methoxy-8-hydroxy-
SH-2,3-benzodiazepine;
(R)-1-(3-methoxy-4-hydroxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-
methoxy-SH-2,3-benzodiazepine; and
(R)-1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-
methoxy-SH-2,3-benzodiazepine;
substantially free of the corresponding (~-enantiomers,
and pharmaceutically-acceptable salts of such compounds.
Most preferably, the compound according to formula I, for
administration is (R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-23-
SH-2,3-benzodiazepine, substantially free of the corresponding (S~-enantiomer;
or a pharmaceutically-acceptable salt thereof.
The compound, (R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8
dimethoxy-SH-2,3-benzodiazepine, the (R)-enantiomer of tofisopam, is shown
in the structure diagram below.
(R)-tofisopam
According to another aspect of the invention, the aforesaid compounds
are used in the preparation of medicaments for treating or preventing an
inflammatory disorder.
According to another embodiment of the invention there is provided a
method of treatment or prevention of inflammatory disorders in an individual,
particularly, inflammatory disorders of the epithelium comprising
administering
to the individual an effective amount of at least one compound according to
formula I as defined herein in combination with at least one additional
therapeutic agent. Preferably the additional therapeutic agent is selected.
from
the _ group consisting of aminosalicylates, corticosteroids, antimetabolites,
immunosuppressants, TNF-a inhibitors, 5-LO inhibitors and leulcotriene
antagonists, wherein the leukotriene antagonist is not a compound of formula
I.
The invention also encompasses a method of addressing one or a
combination of inflammatory disorders of epithelial tissue in a mammal in
which an unexpectedly low amount of the compound can be used. More
specifically, it has been found that it is possible to achieve good anti-
inflammatory activity when the compound is contacted directly on the shin
(i.e.,
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-24-
adminstered topically). Preferred administration routes generally include
contacting the epithelium with a formulation in which the compound is present
in an amount well below about 100 mg/ml, for example, about 50 mg/ml or well
below 10 mg/ml, for example 1 mg/ml or O.lmg/ml. For skin or for the cornea,
a preferred formulation is a topical formulation.
It is believed that the compounds of Formula I are particularly useful to
address one or a combination of gastrointestinal disorders in a mammal such as
a human patient. Thus according to one embodiment, the invention features a
method for preventing, treating, reducing the severity of, or delaying onset
of a
gastrointestinal disorder that includes administering to the patient an
effective
amount of the compound. The compound can be used alone as the sole active
agent or in combination with one, a few or several other agents including
other
compounds according to Formula I. Preferred administration routes include
those generally well suited for gastrointestinal delivery such as intracolonic
delivery (eg an enema or suppository) and other delivery methods described
below.
Definitions
The term "inflammation" or inflammatory response" refers to a defense
reaction of living tissue to injury. The response serves to contain and to
repair
the injury. Multiple chemical mediators of inflammation derived from either
plasma or cells have been observed. Compounds produced in the metabolism of
arachidonic acid, such as prostaglandins and leukotrienes, also affect
inflammation, leukotrienes mediating essentially every aspect of acute
inflammation.
An "inflammatory disorder mediated by LTB4" or a "LTB4-mediated
disorder", refers to a disorder resulting from an inflammatory response
wherein
LTB4 mediation is implicated as a factor by observation of LTB4 presence at
the
site of the inflammation.
An "inflammatory disorder of an epithelial tissue" or "of an epithelium"
refers to an inflammatory disorder in which one or more epithelial tissues or
tissues adjacent to the epithelial layer are affected. Exemplary epithelial
tissues
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-25-
include the epidermal layer of the skin, the cornea epithelium of the eye, and
the
epithelia associated with the mucosal linings of the respiratory, alimentary,
gastrointestinal and urinary tracts.
The term "receptor" refers to a molecular structure or site on the surface
or interior of a cell that binds with substances such as hormones, antigens,
drugs, or neurotransmitters. An "agonist" at a receptor refers to a drug or
other
chemical that can bind to a receptor to produce the physiologic reaction that
is
typical of a naturally occurring substance. An "antagonist" refers to a
chemical
substance that acts at a receptor to produce a physiologic reaction that is
other
than the action produced by the natural, endogenous receptor-binding entity or
natural ligand. Such antagonist activity may occur when a drug or chemical
substance binds the receptor at a much lower concentration than the natural
ligand, and thereby displaces the natural ligand and prevents or reduces the
amount of receptor binding to the natural ligand.
The term "LTB4 antagonist" means a chemical substance that
competitively binds to the LTB4 receptor such that (a) the binding of the
natural
ligand (LTB4) is inhibited by occupation of the LTB4 receptor by the LTB4
antagonist, and (b) the LTB4 antagonist bound to the LTB4 receptor does not
generate the same physiological response produced by native LTB4 bound to the
LTB4 receptor.
The terin "aryl" means a radical of the general formula -C(=O)-R,
wherein R is hydrogen, hydrocarbyl, amino, alkylamino, dialkylamino
hydroxy or alkoxy." Examples include for example, acetyl (-C(=O)CH3),
propionyl (-C(=O)CHZCH3), benzoyl (-C(=O)C6H5), phenylacetyl
(-C(=O)CH2C6H5), carboethoxy (-C02CH2CH3), and dimethylcarbamoyl
(-C(=O)N(CH3)z). When the R group in the acetyl radical is alkoxy, alkyl
amino or dialkyl amino, the alkyl portion is preferably (C1-C6)alkyl, more
preferably (C1-C3)alkyl. When the R is hydrocarbyl, it is preferably (C1
C~)hydrocarbyl. When R is hydrocarbyl, it is preferably alkyl, more preferably
(C1-CG)alkyl.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-26-
The term "alkyl", by itself or as part of another substituent means, unless
otherwise stated, a straight, branched or cyclic chain hydrocarbon radical,
including di- and mufti-radicals, having the number of carbon atoms designated
(i.e. Cl-C6 means one to six carbons). Alkyl groups include straight chain,
branched chain or cyclic groups, with straight being preferred. Examples
include: methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl,
pentyl,
neopentyl, hexyl, cyclohexyl and cyclopropylmethyl. (C1-C6)alkyl is preferred.
Most preferred is (C1-C3)alkyl, particularly ethyl, methyl and isopropyl.
The term "alkoxy" employed alone or in combination with other terms
means, unless otherwise stated, an alkyl group having the designated number of
carbon atoms, as defined above, connected to the rest of the molecule via an
oxygen atom, such as, for example, methoxy, ethoxy, 1-propoxy, 2-propoxy
(isopropoxy) and the higher homologs and isomers. Preferred are (C1-
C6)alkoxy. More preferred is (C1-C3)alkoxy, particularly ethoxy and methoxy.
The term "amine" or "amino" refers to radicals of the general formula
-NRR', wherein R and R' are independently selected from hydrogen or a
hydrocarbyl radical, or wherein R and R' combined form a heterocycle.
Examples of amino groups include: NH2, methyl amino, diethyl amino,
anilino, benzyl amino, piperidinyl, piperazinyl and indolinyl. Preferred
hydrocarbyl radicals are (C1-C~)hydrocarbyl radicals. Preferred are
hydrocarbyl
radicals that are alkyl radicals. More preferred are (Cl-C6)alkyl.
The term "aromatic" refers to a carbocycle or heterocycle having one or
more polyunsaturated rings having aromatic character (4n + 2) delocalized ~
(pi) electrons).
The term "aryl" employed alone or in combination with other terms,
means, unless otherwise stated, a carbocyclic aromatic system containing one
or
more rings (typically one, two or three rings) wherein such rings may be
attached together in a pendent manner, such as a biphenyl, or may be fused,
such as naphthalene. Examples include phenyl; anthracyl; and naphthyl.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-27-
The term "hydrocarbyl" refers to any moiety comprising only hydrogen
and carbon atoms. This definition includes for example alkyl, alkenyl,
alkynyl,
aryl and benzyl groups. Preferred are (Cl-C~)hydrocarbyl.
The term "heteroalkyl" by itself or in combination with another term
means, unless otherwise stated, a stable straight or branched chain radical
consisting of the stated number of carbon atoms and one or two heteroatoms
selected from the group consisting of O, N, and S. Nitrogen and sulfur atoms
may be optionally oxidized to the N-oxide and sulfoxide or sulfone,
respectively. In addition, a nitrogen heteroatom may be optionally
quaternized.
The heteroatom(s) may be placed at any position of the heteroalkyl group,
including between the rest of the heteroalkyl group and the fragment to which
it
is attached, as well as attached to the most distal carbon atom in the
heteroalkyl
group. Preferred are (C2-C6)heteroalkyl. More preferred are (C2-
C4)heteroalkyl.
Examples include: -O-CH2-CH2-CH3, -CHZ-CH2CH2-OH, -CH2-CH2-NH-CH3,
-CH2-C(=O)-CH3, -CH2-N=N-CHz-CH3, -CH2-S-CH2-CH3, -CH2CH2-S(=O)-
CH3 and -CHZ-CH2-NH-S02-CH3. Up to two heteroatoms may be consecutive,
such as, for example, -CH2-NH-OCH3, or -CH2-CH2-S-S-CH3. More preferred
are heteroalkyl groups containing one or two oxygen atoms.
When two groups may "combine to form a carbocyclic or heterocyclic 5-
or 6-membered ring," a carbocyclic ring is preferably saturated. Preferred
heterocyclic rings are saturated rings containing one or two heteroatoms
selected
from N, O and S. Heterocyclic rings annulated to the benzodiazepine seven-
membered ring in this way include, for example, furan, dihydrofuran,
tetrahydrofuran, pyran, dihydropyran, tetrahydropyran, thiophene,
dihydrothiophene, tetrahydrothiophene, pyrrole, dihydropyrrole, pyrrolidine,
pyridine, dihydropyridine, tetrahydropyridine and piperidine.
When two groups may "combine to form a 5-, 6- or 7-membered
heterocyclic ring," preferred heterocyclic rings are 5- or 6-membered rings
containing one or two heteroatoms selected from N, O and S. More preferred
are heterocyclic rings containing one heteroatom selected from N, O and S.
Heterocyclic rings annulated to the benzodiazepine phenyl ring in this way
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-28-
include, for example, furan, dihydrofuran, dioxane, dioxolane, pyran,
dihydropyran, tetrahydropyran, thiophene, dihydrothiophene, pyridine,
dihydropyridine, tetrahydropyridine, piperidine, pyrrole, dihydropyrrole,
imidazole, dihydroimidazole, thiazole, dihydrothiazole, oxazole, and
dihydrooxazole.
The term "substituted" means that an atom or group of atoms has
replaced hydrogen as the substituent attached to another group. For aryl and
heteroaryl groups, the term "substituted" refers to any level of substitution,
namely mono-, di-, tri-, tetra-, or penta-substitution, where such
substitution is
permitted. The substituents are independently selected, and substitution may
be
at any chemically accessible position.
The phrase "optically active" refers to a property whereby a material
rotates the plane of plane-polarized light. A compound that is optically
active is
nonsuperimposable on its mirror image. The property of nonsuperimposablity
of an object on its mirror image is called chirality.
The property of "chirality" in a molecule may arise from any structural
feature that makes the molecule nonsuperimposable on its mirror image. The
most common structural feature producing chirality is an asymmetric carbon
atom, i.e., a carbon atom having four nonequivalent groups attached thereto.
The term "enantiomer" refers to each of the two nonsuperimposable
isomers of a pure compound that is optically active. Single enantiomers are
designated according to the Cahn-Ingold-PYelog system, a set of priority rules
that rank the four groups attached to an asymmetric carbon. See March,
Advanced Organic Chemistry, 4th Ed., (1992), p. 109. Once the priority ranking
' 25 of the four groups is determined, the molecule is oriented so that the
lowest
ranking group is pointed away from the viewer. Then, if the descending rank
order of the other groups proceeds clockwise, the molecule is designated R and
if the descending rank of the other groups proceeds counterclockwise, the
molecule is designated S. In the example below, the Cahn-hagold-Prelog
ranlcing sequence id A > B > C > D. The lowest ranlcing atom, D is oriented
away from the viewer.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-29-
A A
.",~wv D ...,,~wv D
C B B C
R configuration S configuration
The term "racemate" or the phrase "racemic mixture" refers to a 50-50
mixture of the (R)- and (S~-enantiomers of a compound such that the mixture
does not rotate plane-polarized light.).
The term "substantially isolated", or "substantially free" of the other
enantiomer or the term "resolved" or the phrase "substantially free" of the
corresponding (S~-enantiomer, when used to refer to an optically active
compound of formula I, means the (R)- and (~-enantiomers of the compound
have been separated such that the composition is 80% or more by weight a
single enantiomer.
Thus, , by "(R)-2,3-benzodiazepine substantially free of the
enantiomer" is meant a 2,3-benzodiazepine compound that comprises 80% or
more by weight of its (R)-enantiomer and likewise contains 20% or less of its
(S~-enantiomer ~as a contaminant, by weight.
The term "effective amount" when used to describe therapy to an
individual suffering from an inflammatory disorder, particularly a LTB4-
mediated inflammatory disorder, refers to the amount of a compound of formula
I, or of a combination of a compound of formula I with one or more additional
agents, e.g., aminosalicylates, corticosteroids, antimetabolites,
immunosuppressants, TNF-a inhibitors, inhibitors of leukotriene synthesis, or
leukotriene antagonists, that inhibits the inflammatory process. The
inhibition
of the inflammatory process results in a therapeutically useful and selective
reduction in the symptoms of inflammation when administered to a patient
suffering from a disorder which manifests chronic or acute inflammation,
particularly inflammation associated with physiologically relevant
concentrations of LTB4. An effective amount of a compound of formula I, or of
a combination of a compound of formula I with one or more additional agents,
e.g., aminosalicylates, corticosteroids, antimetabolites, immunosuppressants,
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-30-
TNF-a inhibitors, inhibitors of leukotriene synthesis, or leukotriene
antagonists,
for the prevention of an inflammatory disorder, particularly a LTB4-mediated
inflammatory disorder, is an amount which prevents or delays the onset of
symptoms of an inflammatory disorder in an individual during a time interval
coinciding with an increased risk of the inflammatory disorder. The term
"individual" or "subject", includes human beings and non-human animals. With
respect to the disclosed methods of treating an inflammatory disorders,
particularly LTB~-mediated inflammatory disorders, these terms refer, unless
the
context indicates otherwise, to an organism that is afflicted with such an
inflammatory disorder.
With respect to disclosed methods of preventing inflammatory disorders,
particularly, preventing LTB4-mediated inflammatory disorders, this term
refers
unless the context indicates otherwise, to an organism that is likely to be
afflicted with such an inflammatory disorder. The selection of an individual
likely to incur such an inflammatory disorder may take into account the
presence of inflammatory conditions that historically are known to have a high
incidence of recurrence, such as, for example, IBD. The likelihood of
incurring
such an inflammatory disorder may also be due to tissue insult that is known
beforehand, such as a surgical procedure. The future inflammatory disorder
may also result from a secondary effect of an initial tissue insult. An
example of
this is inflammation due to gout caused by elevated uric acid levels that
occur
secondary to lysis of a tumor mass following administration of cytotoxic
chemotherapy or therapeutic radiation treatment. The term "prevention" in this
context also includes a delay in the onset of inflammation or the symptoms
thereof or a prolongation of periods of remission in an individual who
experiences recun-ing inflammatory disorders.
Description of the Figures
Fig. 1 is a plot of data gathered in a competitive binding study of the
displacement of [3H]LTB4 by (R)-tofisopam from LTB4 receptors. ICSO and K;
values for (R)-tofisopam displacement of [3H]LTB4 were generated.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-31-
Fig. 2 is a plot of data gathered in a competitive binding study of the
displacement of [3H]LTB4 by racemic tofisopam from LTB4 receptors. ICso and
K; values for racemic tofisopam displacement of [3H]LTB4 were generated.
Fig. 3 is a plot of data gathered in a competitive binding study of the
displacement of [3H]LTB4 by (S~-tofisopam from LTB4 receptors. ICSO and K;
values for (S~-tofisopam displacement of [3H]LTB4 were generated.
Detailed Description of the Invention
According to the present invention, 2,3-benzodiazepines of formula I,
and pharmaceutically acceptable salts thereof, are antagonists of leukotriene
B4.
The compounds are useful in methods of treatment or prevention of
inflammatory disorders, particularly, inflammatory disorders mediated by
leukotriene B4.
Inflammatory disorders believed to be treatable or preventable by
methods of the invention include, for example: inflammatory bowel diseases
(e.g., CD, UC, indeterminate colitis, and infectious colitis); RA; gout;
mucositis
(e.g., oral mucositis, gastrointestinal mucositis, nasal mucositis, and
proctitis);
necrotizing enterocolitis; inflammatory skin disorders (e.g., psoriasis,
atopic
dermatitis, and contact hypersensitivity); aphthous ulcers; pharyngitis;
esophagitis; peptic ulcers; gingivitis; periodontitis; ocular diseases (e.g.,
conjunctivitis, retinitis, and uveitis); and radiation-induced
gastrointestinal
inflammation.
In addition, compounds of formula I are believed useful in the treatment
of the above inflammatory disorders in an individual who is also suffering
from
IBS.
Preparation of 2,3-Benzodiazepines of Formula I.
The (R)-2,3-benzodiazepines of formula I useful in the present invention
may be prepared by one of several methods. These methods generally follow
the synthetic strategies and procedures used in the synthesis of racemic 2,3-
benzodiazepines of formula I, such as tofisopam and tofisopam analogs. See
U.S. Patent Nos. 3,736,315 and 4,423,044 (tofisopam syntheses) and Horvath et
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
al., P~og~ess in Neurobiology 60(2000) p.309-342 and references cited therein
(preparation of tofisopam and analogs thereof), the entire disclosures of
which
are incorporated herein by reference. In the synthesis methods that follow,
the
products of the chemical syntheses are racemic mixtures of (R)- and (S)-2,3-
benzodiazepines of formula I. These racemic mixtures may be subsequently
separated using known methods of resolution to produce the (R)-2,3-
benzodiazepines of formula I substantially free of the (~-enantiomers. By an
"(R)-2,3-benzodiazepine" is meant a 2,3-benzodiazepine that has an (R)
absolute
conformation by virtue of a substitution at the 5-position of the
benzodiazepine
ring to give a resolvable chiral carbon at the 5-position. By an "(R)-2,3-
benzodiazepine substantially free of the (.S)-enantiomer" or "an (R)-
enantiomer
of a compound of formula I substantially free of the corresponding (.S~-
enantiomer" is meant a compound that comprises 80% or more by weight of the
desired (R)-enantiomer and likewise contains 20% or less of the (.S~-
enantiomer
as a contaminant, by weight. Preferably, compounds used in methods of the
present invention have a composition that is 85% by weight or greater of the
(R)-enantiomer, and 15% by weight, or less, of the (~-enantiomer. More
preferably, compounds used in methods of the present invention have a
composition that is 90% by weight or greater of the (R)-enantiomer and 10% by
weight, or less, of the (~-enantiomer. More preferably, compounds used in
methods of the present invention have a composition that is 95% by weight or
greater of the (R)-enantiomer and 5% by weight, or less, of the (S~-
enantiomer.
Most preferably, compounds used in methods of the present invention have a
composition that is 99% by weight or greater of the (R)-enantiomer and 1 % by
weight, or less, of the (~-enantiomer.
Racemic 2,3-benzodiazepines of formula I may be synthesized, as shown
in Scheme 1, from the corresponding 2-benzopyrilium salt H by reaction with
hydrazine hydrate, wherein X- is a counterion such as, for example
perchlorate:
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-3J-
Q1
R'
hydrazine hydrate
R
Scheme 1
Accordingly, hydrazine hydrate (98%, approximately 3 equivalents
based on the 2-benzopyrylium salt) is added dropwise to a stirred solution of
the
2-benzopyrylium salt H in glacial acetic acid (approximately 1mL/3mmol of 2-
benzopyrylium salt). During this operation, the solution is maintained at an
elevated temperature, preferably, 80-100°C. The solution is then
maintained a
higher elevated temperature, preferably 95-100°C, for about one hour.
Then the
reaction mixture is diluted with 2% aqueous sodium hydroxide solution
(approximately 3 equivalents based on the 2-benzopyrylium salt) and cooled.
The product 2,3-benzodiazepine separates as a solid and is removed by
filtration, washed with water and dried. The crude product may be purified by
taking it up in a polar aprotic solvent such as dimethylformamide (DMF) at an
elevated temperature, preferably 100-130°C, and decolorizing the
solution with
activated carbon. The carbon is removed by filtration and the filtered
solution is
diluted with water. The purified product precipitates out of the solution and
is
collected by filtration.
See Korosi et al., US Patent 4,322,346, the entire disclosure of which is
incorporated herein by reference, disclosing three .variations of the reaction
protocol for preparing a substituted 2,3-benzodiazepine from the precursor
benzopyrilium salt.
Retrosynthetically, the intermediate benzopyrilium salt, H, may be
prepared from one of several starting materials. According to one such method,
illustrated in Scheme 2, intermediate H is prepared from the corresponding
aryl
ethanol derivative D via the isochroman intermediate F.
Another variation for preparing 2,3-benzodiazepines is illustrated in
Scheme 3 and 4 (Examples 2 and 3). The synthesis there proceeds from
intermediate G without isolation of the intermediate benzopyrilium salt H.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-34-
2-Benzopyrylium salts H may be synthesized from intermediate 2-
substituted phenyl ethanol derivatives D through isochroman intermediate F,
wherein X- is a counterion such as, for example, perchlorate:
R~ R1
O HO
R5 R5 Rs \ Rz
w
\ OEt ~ \ ~Rz
RZCHaMgx a / Ra ~ / C
R ~ R pTsOH
(X = Cl, Br, I) B ~ 1. BH
3
R~ 2. HzOa
Rs \ Rz ' CHO R~
'HCl \ R5 \ Rz
Ra ~ /
F i~~3 4 ~ / OH
~R )n R D
Cr03
HZSO4
S°l'
perchloric acid
Scheme 2
Accordingly, a substituted benzoic acid ester, A is dissolved in a suitable
solvent, preferably ether and cooled to 0°C. Two equivalents of a
suitable
Grignard reagent are added dropwise and the reaction is allowed to warm to
room temperature and monitored for disappearance of starting material. When
the reaction is complete, it may be quenched with a proton source such as
acetic
acid. Volatiles are removed ira vacuo, and the product B is used for the next
step
without purification.
The a,a-substituted benzyl alcohol B is taken up in a high boiling
solvent such as toluene and a catalytic amount of para-toluene sulfonic acid
(p-
TsOH). The mixture is warmed to reflux and may be monitored for
disappearance of starting materials. When the reaction is complete, the
volatiles
are removed in vacuo and the cnide product C is purified by column
chromatography.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-35-
The substituted styrene C is hydroxylated under anti-Markovnikov
conditions to give intermediate phenylethyl alcohol D. A solution of D, and of
a
suitably substituted benzaldehyde E (1.2 eq) are added to anhydrous dioxane.
The resulting solution is then saturated with gaseous HCl and warmed,
preferably to reflux temperature for about one hour. The mixture is then
cooled
to room temperature, poured into water, basified (preferably with aqueous
sodium hydroxide) and extracted with an organic solvent (preferably ethyl
acetate). The extract is dried, filtered and concentrated under vacuum. The
resulting residue is purified, preferably by crystallization, to yield F.
To a stirred, cooled, (preferably to 0-5°C) solution of F (2g) in
acetone
(30mL), is added dropwise a solution of chromium trioxide (2g) in 35% sulfuric
acid (20mL). The latter solution is added at a rate such that the reaction
temperature remains below 5° C. After the addition is complete, the
reaction
mixture is allowed to rise to room temperature and is stirred at room
temperature for two hours. The reaction mixture is then poured into water and
extracted with an organic solvent, preferably ethyl acetate. The organic
extract
is washed with water and then with ice-cold 10% aqueous sodium hydroxide.
The aqueous alkaline fraction is then acidified, preferably with dilute
aqueous
hydrochloric acid, and extracted with an organic solvent, preferably,
chloroform. The chloroform extract is dried, filtered and concentrated under
vacuum to give G. The crude residue may further be purified by column
chromatography.
The 2-a-acyl hydrocaxbylbenzophenone G (Sg) is dissolved in glacial
acetic acid (15 mL). To this mixture is added 60% perchloric acid (7.5 mL).
The resulting mixture is warmed to 100°C (steam bath) for three
minutes. The
mixture is allowed to cool to room temperature. Crystallization of the crude
product may begin spontaneously at this point or may be induced by addition of
ether or ethyl acetate. The product 2-benzopyrylium salt H is removed by
filtration and purified by recrystallization, preferably from ethanol or
glacial
acetic acid/ethyl acetate.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-3 6-
A similar synthetic sequence for preparation of 2,3-benzodiazepines is
disclosed in US Patent 3,736,315, the entire disclosure of which is
incorporated
herein by reference. Synthetic strategies for preparation of 2,3-
benzodiazepines
are also disclosed in Horvath et al., Progress in Neurobiology 60(2000) p309-
342 and references cited therein; the entire disclosures of which are
incorporated
herein by reference.
Alternative methods for preparation of intermediate H start with an aryl
acetonide or indanone starting material. See Kunnetsov, E.V., and Dorofeenko,
G.N., Z1Z. Org. Khim., 6, 578-581. and M. Vajda, Acta Chem. Acad. Sci. Hung.,
40, p.295-307, 1964, respectively, the entire disclosures of which are
incorporated herein by reference.
To synthesize a 2,3-benzodiazepine derivative of formula I having an
amine substituent, the starting aromatic amine components must be protected
with a protecting group or otherwise rendered unreactive in order for the
amine
to be rendered stable to the reaction conditions employed in the reaction
schemes shown or referenced above. A means of circumventing the need for a
protecting group may be to use a starting material containing an aromatic
vitro
groups) in place of the desired aromatic amino group(s). The vitro group
performs the same function as an amine protecting group in this synthesis and
it
may, following the synthesis steps that are incompatible with an amine
substituent, be then reduced to an amine. Reduction of the aromatic vitro
group
can be done, for example, via catalytic hydrogenation. Catalytic hydrogenation
provides the capability to selectively reduce the aromatic vitro group without
reducing the olefin or other functionality in the intermediate. This synthetic
strategy is disclosed in US Patent 4,614,740, wherein racemic 2,3-
benzodiazepines were prepared with amino groups at a position corresponding
the R3 of formula I of the present invention. The entire disclosure of US
4,614,740 is incorporated herein by reference.
Resolution of (R)-2,3-Benzodiazepines of Formula I.
The synthetic procedures shown (or referenced) above produce racemic
mixtures of 2,3-benzodiazepines of formula I that are useful in methods of the
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-J / _
present invention. In order to prepare the preferred (R)-2,3-benzodiazepines
of
formula I that are useful in methods of the present invention, the racemic
mixture must be resolved.
Racemic 2,3-benzodiazepines of formula I may, for example, be
converted to the (~-dibenzoyltartaric acid salt, which is a diastereomeric
mixture of SS and RS configurations. The pair of diastereomers (R,~ and (S,~
possess different properties, e.g., differential solubilities, that allow for
the use
of conventional separation methods. Fractional crystallization of
diastereomeric
salts from a suitable solvent is one such separation method. This resolution
has
been successfully applied to the resolution of racemic tofisopam. See
Hungarian Patent 178516 and also Toth et al., J. Heterocyclic. Chen2., 20:09-
713 (1983), the entire disclosures of which are incorporated herein by
reference.
Alternatively, racemic-2,3-benzodiazepines of formula I may be
derivatized via, for example, acylation of an aryl hydroxy moiety, with a
chiral
acylating reagent, e.g., (,S~-mandelic acid. The resulting ester, has a second
chiral center, and thus exists as a diastereomeric pair separable using
conventional methods such as crystallization or chromatography. Following the
separation, the chiral moiety with which the racemic 2,3-benzodiazepine is
derivatized, may be removed.
Racemic 2,3-benzodiazepines of formula I may be separated without
diastereomer formation by differential absorption on a chiral stationary phase
of
a chromatography column, particularly a preparative HPLC column. Chiral
HPLC columns are commercially available with a variety of packing materials
to suit a broad range of separation applications. Exemplary stationary phases
suitable for resolving the racemic 2,3-benzodiazepines of formula I include:
(i) macrocyclic glycopeptides, such as silica-bonded vancomycin which
contains 18 chiral centers surrounding three pockets or cavities;
(ii) chiral a,l-acid glycoprotein;
(iii) human serum albumin; and
(iv) cellobiohydrolase (CBH).
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-3 8-
Chiral al-acid glycoprotein is a highly stable protein immobilized onto
spherical silica particles that tolerates high concentrations of organic
solvents,
high and low pH, and high temperatures. Human serum albumin, though
especially suited for the resolution of weak and strong acids, zwitterionic
and
nonprotolytic compounds, has been used to resolve basic compounds. CBH is a
very stable enzyme which has been immobilized onto spherical silica particles
and is preferentially used for the separation of enantiomers of basic drugs
from
many compound classes.
The resolution of tofisopam by chiral chromatography using macrocyclic
glycopeptide as a stationary phase on a Chirobiotic VT"" column (ASTEAC,
Whippany, NJ) is disclosed in US Patent 6,080,736. Fitos et al. (J.
Chromatog~., 709 265 (1995)), discloses another method for resolving racemic
tofisopam by chiral chromatography using a chiral al-acid glycoprotein as a
stationary phase on a CHIRAL-AGPT"" column (ChromTech, Cheshire, UI~).
The latter method separates the (R)- and (S~- enantiomers and also resolves
the
two conformers (discussed below) of each enantiomer. These chromatographic
methods, may be used generally to separate racemic 2,3-benzodiazepines of
formula I into individual (R)- and (S~-enantiomers. The Chirobiotic VT""
column
is available in a semi-preparative size as employed for the above separation
SOOmm x lOmm). The stationary phase of the Chirobiotic VT"" column is
commercially available in bulk for packing of preparative chromatography
columns with larger sample capacity.
(R)- and (~-enantiomers of 2,3-benzodiazepines may also exist in two
stable conformations that may be assumed by the benzodiazepine ring, as
generally depicted below:
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-3 9-
R(+)-isomer R(-)-isomer
S(-)-isomer S(+)-isomer
The present invention includes methods as described herein that use any
and all observable conformations of compounds of formula I, preferably
compounds having the (R)-absolute configuration at carbon 5 of the
benzodiazepine ring, which are biologically active in treatment or prevention
of
inflammatory disorders, particularly inflammatory disorders mediated by LTB4..
It will be understood that compounds of formula I useful in the methods
of the present invention may contain one or more chiral centers in addition to
chiral center at the 5-position of the benzodiazepine ring of compounds of
formula I. Such compounds may exist in, and may be isolated as pure
enantiomeric or diastereomeric forms or as racemic mixtures. The present
invention therefore includes methods that use any possible enantiomers,
diastereomers, racemates or mixtures thereof of formula I (dictated by a
chiral
center other than the 5-position of the benzodiazepine ring) which are
biologically active in the treatment or prevention of inflammatory disease
states,
particularly inflammatory disease states mediated by LTB4.
Salts of Compounds of Formula I.
The compounds used in the methods of the present invention may take
the form of pharmaceutically-acceptable salts. The term "salts", embraces
salts
commonly used to form alkali metal salts and to form addition salts of free
acids
or free bases. The term "pharmaceutically-acceptable salt" refers to salts
that
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-40-
possess toxicity profiles within a range so as to have utility in
pharmaceutical
applications. Pharmaceutically unacceptable salts may nonetheless possess
properties such as high crystallinity, which have utility in the practice of
the
present invention, such as for example utility in a synthetic process or in
the
process of resolving enantiomers from a racemic mixture. Suitable
pharmaceutically-acceptable acid addition salts may be prepared from an
inorganic acid or from an organic acid. Examples of such inorganic acids are
hydrochloric, hydrobromic, hydroiodic, nitric, carbonic, sulfuric and
phosphoric
acid. Appropriate organic acids may be selected from aliphatic,
cycloaliphatic,
aromatic, araliphatic, heterocyclic, carboxylic and sulfonic classes of
organic
acids, example of which are formic, acetic, propionic, succinic, glycolic,
gluconic, lactic, malic, tartaric, citric, ascorbic, glucuronic, malefic,
fumaric,
pyruvic, aspartic, glutamic, benzoic, anthranilic, mesylic, salicyclic,
salicyclic,
4-hydroxybenzoic, phenylacetic, mandelic, embonic (pamoic), methanesulfonic,
ethanesulfonic, benzenesulfonic, pantothenic, 2-hydroxyethanesulfonic,
toluenesulfonic, sulfanilic, cyclohexylaminosulfonic, stearic, algenic, beta-
hydroxybutyric, salicyclic, galactaric and galacturonic acid.
Suitable pharmaceutically acceptable base addition salts of compounds
of formula I useful in methods of the invention include for example, metallic
salts made from calcium, magnesium, potassium, sodium and zinc or organic
salts made from N,N'-dibenzylethylenediamine, chloroprocaine, choline,
diethanolamine, ethylenediarnine, meglumine (N methylglucamine) and
procaine. All of these salts may be prepared by conventional means from the
corresponding compound of formula I by reacting, for example, the appropriate
acid or base with the compound of formula I.
Administration of Compounds of Formula I.
The compounds useful in methods of the invention may be administered
to individuals (mammals, including animals and humans) afflicted with
inflammatory disorders, particularly individuals afflicted with LTB4-mediated
inflammatory disorders.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-41-
For prophylactic administration, the compounds useful in the practice of
methods of the invention should be administered far enough in advance of a
known event that increases the chance of an inflammatory disorder,
particularly
an inflammatory disorder mediated by LTB4, such that the compound is able to
reach the site of action in sufficient concentration to exert an effect. The
pharmacokinetics of specific compounds may be determined by means known in
the art and tissue levels of a compound in a particular individual may be
determined by conventional analyses.
One or more compounds useful in the practice of the present inventions
may be administered simultaneously, or different compounds useful in the
practice of the present invention may be administered at different times
during
treatment or prevention therapy.
In addition, compounds of formula I may be administered for treatment
' of inflammatory disorders, in combination with one or more additional
therapeutic agents. Such additional ~ agents include aminosalicylates,
corticosteroids, antimetabolites, immunosuppressants, TNF-oc inhibitors,
inhibitors of leukotriene synthesis, and leukotriene antagonists; wherein when
the additional therapeutic agents are leukotriene antagonist, they are other
than
compounds of formula I.
Aminosalicylates believed useful in combination with compounds of
formula I in methods of the invention include, for example, sulfasalazine and
mesalamine.
Corticosteroids believed useful in combination with compounds of
formula I in methods of the invention include, for example, prednisone and '
budesonide.
Antimetabolites believed useful in combination with compounds of
formula I in methods of the invention include, for example, azathioprine.
Immunosuppressants believed useful in combination with compounds of
formula I in methods of the invention include, for example, cyclosporine and
tacrolimus.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-42-
TNF-oc inhibitors believed useful in combination with compounds of
formula I in methods of the invention include, for example, infliximab,
etanercept, and adalimumab.
Inhibitors of leukotriene synthesis believed useful in combination with
compounds of formula I in methods of the invention include, for example, 5-LO
inhibitors, e.g., ETH615, linetastine, lonapalene (RS43179), MK 886, and
zileuton. Other inhibitors of leukotriene synthesis believed useful in
combination with compounds of formula I include, for example, 15-HETE and
leflunomide.
Leukotriene antagonists useful in combination with compounds of
formula I in methods of the invention include, for example, SC41930, SC53228,
CGS-25019C, ONO-4057, SB-202247, VML295 (LY293111), CP-105696, CP-
195543, and U-75302.
Routes of Administration.
The compounds useful in methods of the invention may be administered
for therapeutic effect by any route, for example enteral (e.g., oral, rectal,
intranasal, etc.) and parenteral achninistration. Parenteral administration
includes, for example, intravenous, intramuscular, intraarterial,
intraperitoneal,
intravaginal, intravesical (e.g., into the bladder), intradernal, topical or
subcutaneous administration. Also contemplated within the scope of the
invention is the instillation of drug in the body of the patient in a
controlled
formulation, with systemic or local release of the drug to occur at a later
time.
For anti-inflammatory use, the drug may be localized in a depot for controlled
release to the circulation, or controlled release to a local site of
inflammation.
As discussed, the invention can be used, for instance, to prevent or treat
an inflammatory skin disorder such as psoriasis, atopic dermatitis, and
contact
hypersensitivity. Other indications of interest include dry skin (sometimes
called
"winter itch"). In these invention examples, topical application of at least
one of
the compounds of Formula I (e:g., one, two or three) will be often be
indicated
for some indications.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-43-
Topical formulations in accord with the invention typically include a
topical vehicle suitable for administration to the subject (particularly a
mammal
such as a human patient) in an amount suitable to reduce, inhibit or eliminate
existing or potential skin irritation or inflammation. More specific
formulations
for topical use preferably less than about 100mg/ml of one or more of the
compounds provided above as Formula I, more preferably between from about
O.OOlmg/ml to about 50 mg/ml, even more preferably between from about 0.01
mglml to about 10 mg/ml with about 0.1 mglml to about 5 mg/ml being suitable
for many applications. Thus, the administered dose may be less than about 100
mg/day, more preferably less than about 50 mg/day, even more preferably less
than about 10 mg or 5 mg/day and yet more preferably less than about 1
mg/day.
Optimal topical concentrations of one or more of the compounds of
Formula I can sometimes be adjusted (generally decreased) if one or more other
anti-inflammatory component is included in the formulation. In particular, it
is
contemplated that lower (eg., one-half less) amounts of compound may be used,
while still maintaining comparable levels of anti-inflammation activity on the
skin, by further including an approximately equal concentration of, for
example
a steroid or non-steroidal anti-inflammatory agent.
In embodiments in which it is desirable to topically administer a salt of
one or more of the compounds according to Formula I, a variety of suitable
salts
can be employed such as those mentioned above. Appropriate formulations
generally suited for topical use include such vehicles (or vehicle components)
as
water; organic solvents such as alcohols (particularly lower alcohols readily
capable of evaporating from the skin such as ethanol), glycols (such as
glycerin), aliphatic alcohols (such as lanolin); mixtures of water and organic
solvents (such as water and alcohol), and mixtures of organic solvents such as
alcohol and glycerin (optionally also with water); lipid-based materials such
as
fatty acids, acylglycerols (including oils, such as mineral oil, and fats of
natural
or synthetic origin), phosphoglycerides, sphingolipids and waxes; protein-
based
materials such as collagen and gelatin; silicone-based materials (both non-
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-44-
volatile and volatile) such as cyclomethicone, demethiconol and dimethicone
copolyol (Dow Corning); hydrocarbon-based materials such as petrolatum and
squalane; anionic, cationic and amphoteric surfactants and soaps; sustained-
release vehicles such as microsponges and polymer matrices; stabilizing and
suspending agents; emulsifying agents; and other vehicles and vehicle
components that are suitable for administration to the skin, as well as
mixtures
of topical vehicle components as identified above or otherwise known to the
art.
The vehicle may further include components adapted to improve the stability or
effectiveness of the applied formulation, such as preservatives, antioxidants,
skin penetration enhancers, sustained release materials, and the like.
Examples
of such vehicles and vehicle components are well known in the art and are
described in such reference works as Martindale--The Extra Pharmacopoeia
(Pharmaceutical Press, London 1993) and Martin (ed.), Remington's
Pharmaceutical Sciences.
The choice of a suitable vehicle will depend on the particular physical
form and mode of delivery that the formulation is to achieve. Examples of
suitable forms include liquids (including dissolved forms of the cations of
the
invention as well as suspensions, emulsions and the like); solids and
semisolids
such as gels, foams, pastes, creams, ointments, "sticks" (as in lipsticks or
underarm deodorant sticks), powders and the like; formulations containing
liposomes or other delivery vesicles; rectal or vaginal suppositories, creams,
foams, gels or ointments; and other forms. Typical modes of delivery for use
as
an anti-inflammatory for the skin include application using the fingers;
application using a physical applicator such as a cloth, tissue, swab, stick
or
brush (as achieved for example by soaking the applicator with the formulation
just prior to application, or by applying or adhering a prepared applicator
already containing the formulation--such as a treated or premoistened bandage
or patch, wipe, washcloth or stick--to the skin); spraying (including mist,
aerosol
or foam spraying); dropper application (as for example with ear or eye drops);
sprinkling (as with a suitable powder form of the formulation); and soaping.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-45-
Other "soothing" ingredients for optional use with the topical
applications of the invention include, but are not limited to glycerin, aloe
vera,
chamomile, cola nitida extract, green tea extract, tea tree oil, licorice
extract,
allantoin, urea, caffeine or other xanthines, and glycyrrhizic acid and its
derivatives may also be beneficially used with the invention to help reduce or
block unwanted inflammation of the skin.
As also discussed, it is an object of the present invention to address one
or a combination of gastrointestinal disorders in a mammal such as a human
patient by administering an effective amount the compound of Formula I. A
variety of particular administration routes can be employed such as those
known
to target the alimentary canal, stomach, small intestine or colon. Of
particular
interest is the delivery of the compounds intracolonically, for example by
suppository or enema.
See, for instance, Groning R., et al., Drug Dev. Ind Pharm,117:527-39
(1984); Sheth, P. R., DrugDev. Ind. Pharm. 10:313-39 (1983); Chien, Y. W.,
Drug Dev. Ind. Pharm 9:1291-330 (1983); Desai, S. and Bolton, S., Plaarm. Res.
10: 1321-5 (1993)); Banakar (Amer. Pharm. 27: 39-48 (1987); (Cargill, R., et
al., Pharm. Res 5:533-536 (1988); Cargill, R., et al., Pharm. Res. 5:506-509
(1989); (Ritschel, W. A., Angewante Biopharmazie, Stuttgart (1973), pp. 396-
402; Agyilirah, G. A., et al., Polymers for E~r.teric Coatifag Applications in
Polymers for Controlled Drug Delivery, Tarcha, P. J. ed., CRC Press, (1991)
Boca Raton, pp. 39-66); Magersohn, M., Modern Pharmaceutics, Marcel
Dekker, New York (1979), pp. 23-85); Ritschel, W. A., Meth Find Ex. Clin.
Plaarmacol 13(5):313-336 (1991); Ritschel, W. A. Angewndte Biopharmazio,
Stuttgart Wissensec. Verlag (1973), pp 396-402; Agyilirah, G. A. and Banker,
G. S., Polymers for Enteric Coating Applications, ibid, pp.39-66).
See also U.S. Pat. Nos. 5,525,634; 4,627,850; 4,904,474; 6,531,152;
W097/25979 (disclosing other gastrointestinal tract application routes).
In one invention embodiment, any of the delivery devices disclosed in
U.S. Pat. No. 6,531,152 can be used to localize release of a desired agent in
the
gastrointestinal tract of the animal. Preferably, such a device includes a
core
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-46-
that includes at least one of the compounds of Formula I. The compounds can
be used as the sole active agent or as already discussed can be combined with
one or more other agents to address the gastrointestinal indication. In this
embodiment, nearly any suitable amount of the compound can be used.
However, for many uses, the core diameter can range from 1 mm to 15 mm with
a coating level ranging from 2 to 50 mg/cm2 , for instance.
In general, formulations for targeting a compound of Formula I to the
gastrointestinal tract typically contain preferably less than about 100mg/ml
of
one or more of the compounds provided above as Formula I, more preferably
between from about 0.001mg/ml to about 50 mg/ml, even more preferably
between from about 0.01 mg/ml to about 10 mg/ml with about 0.1 mg/ml to
about 5 mg/ml being suitable for many applications. Thus, the administered
dose may be less than 100 mg/day, more preferably less 50 mg/day, even more
preferably less than 10 mg or 5 mg/day, and yet more preferably less than 1
mg/day of a compound of Formula I.
Pharmaceutical Compositions.
The methods of the present invention may comprise administering 2,3-
benzodiazepines, preferably (R)-2,3-benzodiazepines~ in the form of a
pharmaceutical composition, in combination with a pharmaceutically acceptable
Garner. The active ingredient in such formulations may comprise from 0.1 to
99.99 weight percent. By "pharmaceutically acceptable carrier" is meant any
Garner, diluent or excipient which is compatible with the other ingredients of
the
formulation and to deleterious to the recipient.
The active agent is preferably administered with a pharmaceutically
acceptable Garner selected on the basis of the selected route of
administration
and standard pharmaceutical practice. The active agent may be formulated into
dosage forms according to standard practices in the field of pharmaceutical
preparations. See Alphonso Gennaro, ed., Renzizzgton's ~ Plzar~tnaceutical
Sciences, 18th Ed., (1990) Mack Publishing Co., Easton, PA. Suitable dosage
forms may comprise, for example, tablets, capsules, solutions, parenteral
solutions, troches, suppositories, or suspensions.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-47-
For parenteral administration, the active agent may be mixed with a
suitable carrier or diluent such as water, an oil (particularly a vegetable
oil),
ethanol, saline solution, aqueous dextrose (glucose) and related sugar
solutions,
glycerol, or a glycol such as propylene glycol or polyethylene glycol.
Solutions
for parenteral administration preferably contain a water-soluble salt of the
active
agent. Stabilizing agents, antioxidizing agents and preservatives may also be
added. Suitable antioxidizing agents include sulfite, ascorbic acid, citric
acid
and its salts, and sodium EDTA. Suitable preservatives include benzalkonium
chloride, methyl- or propyl-paraben, and chlorbutanol. The composition for
parenteral administration may take the form of an aqueous or nonaqueous
solution, dispersion, suspension or emulsion.
For oral administration, the active agent may be combined with one or
more solid inactive ingredients for the preparation of tablets, capsules,
pills,
powders, granules or other suitable oral dosage forms. For example, the active
agent may be combined with at least one excipient such as fillers, binders,
humectants, disintegrating agents, solution retarders, absorption
accelerators,
wetting agents absorbents or lubricating agents. According to one tablet
embodiment, the active agent may be combined with carboxymethylcellulose
calcium, magnesium stearate, mannitol and starch, and then formed into tablets
by conventional tableting methods.
The compositions of the present invention can also be formulated so as
to provide slow or controlled-release of the active ingredient therein. In
general,
a controlled-release preparation is a composition capable of releasing the
active
ingredient at the required rate to maintain constant pharmacological activity
for
a desirable period of time. Such dosage forms can provide a supply of a drug
to
the body during a predetermined period of time and thus maintain drug levels
in
the therapeutic range for longer periods of time than other non-controlled
formulations.
For example, U.S. Patent No. 5,674,533 discloses controlled-release
compositions in liquid dosage forms for the administration of moguisteine, a
potent peripheral antitussive. U.S. Patent No. 5,059,595 describes the
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-4~-
controlled-release of active agents by the use of a gastro-resistant tablet
for the
therapy of organic mental disturbances. U.S. Patent No. 5, 591,767 discloses a
liquid reservoir transdermal patch for the controlled administration of
ketorolac,
a non-steroidal anti-inflammatory agent with potent analgesic properties. U.S.
Patent No. 5,120,548 discloses a controlled-release drug delivery device
comprised of swellable polymers. U.S. Patent No. 5,073,543 discloses
controlled-release formulations containing a trophic factor entrapped by a
ganglioside-liposome vehicle. U.S. Patent No. 5,639,476 discloses a stable
solid
controlled-release ~ formulation having a coating derived from an aqueous
dispersion of a hydrophobic acrylic polymer. U.S. Patent No. 6,531,152
discloses, for instance, an immediate release gastrointestinal drug delivery
system in which release of drugs in the gastrointestinal tract can be
controlled in
a location- and time-dependent manner. The patents cited above are
incorporated herein by reference in their entirety.
Biodegradable microparticles can be used in the controlled-release
formulations of this invention. For example, U.S. Patent No. 5,354,566
discloses a controlled-release powder that contains the active ingredient.
U.S.
Patent No. 5,733,566 describes the use of polymeric microparticles that
release
antiparasitic compositions. These patents are incorporated herein by
reference.
The controlled-release of the active ingredient can be stimulated by
various inducers, for example pH, temperature, enzymes, water, or other
physiological conditions or compounds. Various mechanisms of drug release
exist. For example, in one embodiment, the controlled-release component can
swell and form porous openings large enough to release the active ingredient
after administration to a patient. The term "controlled-release component" in
the context of the present invention is defined herein as a compound or
compounds, such as polymers, polymer matrices, gels, permeable membranes,
liposomes and/or rnicrospheres, that facilitate the controlled-release of the
active
ingredient (e.g., (R)=tofisopam or a pharmaceutically-acceptable salt thereof)
in
the pharmaceutical composition. In another embodiment, the controlled-release
component is biodegradable, induced by exposure to the aqueous environment,
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-4y-
pH, temperature, or enzymes in the body. In another embodiment, sol-gels can
be used, wherein the active ingredient is incorporated into a sol-gel matrix
that
is a solid at room temperature. This matrix is implanted into a patient,
preferably a mammal, having a body temperature high enough to induce gel
formation of the sol-gel matrix, thereby releasing the active ingredient into
the
patient.
The practice of the invention is illustrated by the following non-limiting
examples.
Examples
Example 1: Synthesis of 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl=7-hydroxy-
8-methoxy-SH-2,3-benzodiazepine.
4.41 g (lOmmol) of 1-(3,4-dimethoxyphenyl)-3-methyl-4-ethyl-6,7-
dimethoxyisobenzopyrilium chloride hydrochloride is dissolved in methanol
(35mL) at a temperature of 40°C. After cooling to 20-25°C,
hydrazine hydrate
(0.75g, l5mmol, dissolved in SmL methanol) is added. The reaction is
monitored by HPLC and when complete, is evaporated to dryness. The residue
is triturated with cold water (3mL), filtered and dried to yield the crude
(R,S)-1-
(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-methoxy-SH-2,3-benzo-
diazepine which is subsequently triturated with hot ethyl acetate to yield the
pure product.
Example 2: Resolution of 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7
hydroxy-8-methoxy-SH-2,3-benzodiazepine to yield (R)-1-(3,4-dimethoxy
phenyl)-4-methyl-5-ethyl-7-hydroxy-8-methoxy-SH-2,3-benzodiazepine.
(R,S)-1-(3,4-Dimethoxyphenyl)-4-methyl-5-ethyl-7-hydroxy-8-methoxy-
SH-2,3-benzodiazepine (43mg, dissolved in acetonitrile) is injected onto a
Chirobiotic V column (ASTEAC, Whippany, NJ) Elution of the racemate with
methyl-ter-t-butyl ether/acetonitrile 90/10 (v/v), at 40mL/minute, is
monitored at
310nm, 2mm path.
The R(+) enantiomer is the first compound to elute, and is collected and
dried. The R(-), S(+), S(-) enantiomers, and some residual R(+) enantiorner
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-50-
coelute and are collected in subsequent fractions. Approximately 20% of the
R(+) isomer is converted to the R(-) isomer if left in the eluent for 24
hours. A
stable 80/20 equilibrium (R(+) to R(-)) is observed between the conformers in
the eluent solution.
Example 3: Synthesis of racemic-1-(3-hydroxy-4-methoxyphenyl)-4-methyl-
5-ethyl-7,8-dimethoxy-SH-2,3-benzodiazepine
Racemic-1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7, 8-
dimethoxy-SH-2,3-benzodiazepine was synthesized according to the route of
Scheme 3.
CH3
HO
H3C0 \ COOH EtOH H3CO ~ \ COOEt EtMgI _ H3C0
CH3
H3C0 ~ H SO ~ H CO
2 4 HgCO 3
p-TsOH
HO CHO H3C
H3C0 \ ~ CH3
H3CO 1. BH3 v
2. HZOZ H CO
3
BnBr
H3C OH
Bn0 \ CHO
H3C0 \ CH3 HC1
H3C0 /
H3C0
OCH3
1.
2. HCi \ nu F
Scheme 3
A. Esterification of 3,4-dimethoxybenzoic acid to yield ethyl-3,4-
dimethoxybenzoate([3943-77-9]).
Cr03
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-51-
A solution of 200g of 3,4-dimethoxybenzoic acid and 35g of
concentrated sulfuric acid in 600mL of absolute ethanol was heated at reflux
overnight. The mixture was concentrated and the residue poured into water.
Methylene chloride was added and the solution washed successively with water,
dilute sodium bicarbonate and water, then dried and concentrated. The residue
was recrystallized from acetone/hexane.
B. Addition of ethyl magnesium iodide to ethyl-3,4-dimethoxybenzoate acid to
yield 3-(3,4-dimethoxyphenyl)pentan-3-ol.
A solution of 4.8mL of iodoethane in 20mL of ether was added dropwise
to a suspension of l.Sg of magnesium turnings in lOmL of ether. After SmL of
the iodoethane solution had been added, a few grains of iodine were added and
the mixture was heated to induce formation of the Grignard reagent. The
remaining iodoethane solution was then added. After the Grignard formation
was complete, a solution of Sg of ethyl 3,4-dimethoxybenzoate in ether was
added and the mixture was allowed to stir at room temperature overnight. The
reaction was quenched by addition of saturated ammonium chloride. The
mixture was extracted with ether. The combined ether extracts were dried and
concentrated to an oily residue. Yield: Sg.
C. Elimination of H20 from 3-(3,4-dimethoxyphenyl)pentan-3-of to yield 4-
(( 1 Z)-1-ethylprop-1-enyl)-1,2-dimethoxybenzene.
A solution of Sg of crude 3-(3,4-dimethoxyphenyl)pentan-3-of and 0.25g
of p-tolenesulfonic acid in 80mL of benzene was heated at reflux for lhr with
azeotropic removal of water. The mixture was then filtered through a pad of
sodium bicarbonate and the filtrate concentrated. The residue was purified by
distillation under reduced pressure. Yield: 2.9g.
D. Addition of Ha0 to 4-((1Z)-1-ethylprop-1-enyl)-1,2-dimethoxybenzene to
yield 3-(3,4-dimethoxyphenyl)pentan-2-ol.
To a solution of 26g of 4-((1Z)-1-ethylprop-1-enyl)-1,2
dimethoxybenzene in tetrahydrofuran (THF) at 0°C was added 189mL of a
1.OM solution of borane-THF complex in THF. The mixture was stirred for 3hr
at 0°C, then 35.6mL of 50% hydrogen peroxide was added, with
simultaneous
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-52-
addition of SM sodium hydroxide to maintain the mixture at pH 8. The mixture
was extracted with ether. The combined ether extracts were dried and
concentrated.
E. Benzylation of 3-hydroxy-4-methoxybenzaldehyde to yield 4-methoxy-3-
(phenylmethoxy)benzaldehyde ([6346-OS-0]).
A solution of 100g of 3-hydroxy-4-methoxybenzaldehyde and 135g of
benzyl bromide in SOOmL of acetone containing a suspension of 137g of
potassium carbonate was heated at reflux overnight. The mixture was filtered,
the filtrate concentrated and the residue recrystallized from toluene/hexane.
Yield:65g.
F. Reaction of 3-(3,4-dimethoxyphenyl)pentan-2-of with 4-methoxy-3-(phenyl-
methoxy)benzaldehyde to yield 4-(4-ethyl-6,7-dimethoxy-3-methyliso-
chromanyl)-1-methoxy-2-(phenylmethoxy)benzene.
A solution of 14g of 4-methoxy-3-(phenylmethoxy)benzaldehyde and
15g of 3-(3,4-dimethoxyphenyl)pentan-2-of in 0.3L of dioxane was saturated
with hydrogen chloride gas. The mixture was heated at reflux for 3hr,
saturated
again with hydrogen chloride gas and allowed to stir at room temperature
overnight. It was then poured into water, basified with dilute sodium
hydroxide
and extracted with methylene chloride. The combined methylene chloride
extracts were dried and concentrated.
G. Ring-opening of 4-(4-ethyl-6,7-dimethoxy-3-methyliso-chromanyl)-1-
methoxy-2-(phenylinethoxy)benzene to yield 3-(4,5-dimethoxy-2- f [4-methoxy-
3-(phenylmethoxy)phenyl]carbonyl]phenyl)pentan-2-one.
To a solution of 30g of crude 4-(4-ethyl-6,7-dimethoxy-3-methyliso-
chromanyl)-1-methoxy-2-(phenylmethoxy)benzene in 450mL of acetone at
5°C
was added a solution of 30g of chromic oxide in 300mL of 35% sulfuric acid.
The mixture was stirred at room temperature for 2hr, neutralized by adding
cold
10% sodium hydroxide and concentrated to remove acetone. Then, water was
added and the mixture was extracted with methylene chloride. The combined
methylene chloride extracts were dried and concentrated. The residue was
purified by column chromatography on silica gel. Yield: l Og.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-53-
H. Debenzylation of 3-(4,5-dimethoxy-2- f [4-methoxy-3-(phenylmethoxy)-
phenyl]carbonyl~phenyl)pentan-2-one to yield 3-{2-[(3-hydroxy-4-methoxy-
phenyl)carbonyl]-4,5-dimethoxyphenyl~pentan-2-one.
A solution of l Og of 3-(4,5-dimethoxy-2- f [4-methoxy-3
(phenyhnethoxy)-phenyl]carbonyl~phenyl)pentan-2-one in methylene chloride
containing a suspension of 0.9g of 10% palladium on carbon was hydrogenated
at 80psi for lhr. The mixture was filtered through diatomaceous earth and the
filtrate concentrated. Yield: 6.Sg.
I. Annulation of 3- f 2-[(3-hydroxy-4-methoxyphenyl)carbonyl]-4,5-
dimethoxyphenyl~pentan-2-one by reaction with hydrazine to yield 1-(3-
hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-2,3-
benzodiazepine.
A solution of 6.Sg of 3-{2-[(3-hydroxy-4-methoxyphenyl)carbonyl]-4,5-
dimethoxyphenyl)pentan-2-one and 2.2mL of hydrazine in 130mL of ethanol
was heated at reflux for O.Shr. After allowing the solution to cool to room
temperature, it was saturated with HCl gas. The mixture was then concentrated
to a volume of about SmL, basified with concentrated ammonium hydroxide,
and extracted with methylene chloride. The combined methylene chloride
extracts were dried and concentrated, and the residue recrystallized from
ethyl
acetate/hexane. Yield: 0.97g
The product 1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7,8-
dimethoxy-SH-2,3-benzodiazepine was analyzed by HPLC, elemental analysis,
GC/MS, proton NMR and differential scanning calorimetry (DSC). The data
are as follows:
Purity: 99.29% by HPLC (% area). Column: Betasil Phenyl 4.6 x 150mm.
Mobile Phase: Acetonitrile::0.O1M Phosphate Buffer (70::30). Flow Rate:
O.SmL/min. Wavelength:254nm.
GC-MS; M/e = 358; with the fragmentation pattern matching the proposed
structure.
DSC: Temperature program 100°C to 300°C at 5°C/min,
indicated molar purity
= 99.75% and melting point of 158.6°C.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-54-
Elemental analysis (calculated/analysis): %C - 68.09/68.08; %H - 6.61/6.57;
N - 7.53/7.35. Calculated values include 0.02 equivalents of ethyl acetate and
0.09 equivalents of residual water.
NMR (DCCl3) (performed on GE QE 300): 1.08ppm (t, 3H); 1.99 (s, 3H); 2.11
(m, 2H); 2.75 (m, 1H); 3.75 (s, 3H); 3.93 (s, 3H); 3.97 (s, 3H); 6.46 (bs,
1H);
6.72 (s, 1H); 6.86 (m, 2H); 7.18 (d, 1H); 7.48 (s, 1H).
Example 4: Synthesis of 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7
methoxy-8-hydroxy-5H-2,3-benzodiazepine
Racemic 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-methoxy-8-
hydroxy-5H-2,3-benzodiazepine was synthesized according to the route of
Scheme 4.
H3C0 ~ ~ COOH EtOH HsCO ~ ~ COOEt B~Br H3C0 ~ COOEt
HZS04 ' /~~/ Bn0
HO HO
EtMgI
H3C OH HaC HO
H3C0 ~ CH3 1. BH3 HaCO ~ ~ CH3 p_TsOH H3C0 ~ ~ CH3s
Bn0 ~ / ~ Bn0 ~ / ~ Bn0 /
H3C 1. NHZNHz
2. HCl
Cr03
HZS04
OCH3
5~,.."...,. -.
OCH3
Hz
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-55-
A. Esterification of 3-methoxy-4-hydroxybenzoic acid to yield ethyl-3-
methoxy-4-hydroxybenzoate.
A solution of 100g of 3-methoxy-4-hydroxybenzoic acid and 17g of
concentrated sulfuric acid in 300mL of absolute ethanol was heated at reflux
overnight. The mixture was concentrated and the residue poured into water.
Methylene chloride was added and the solution washed successively with water,
dilute sodium bicarbonate and water, then dried and concentrated. Yield: 118g
B. Benzylation of ethyl-3-methoxy-4-hydroxybenzoate to yield ethyl-3-
methoxy-4-benzyloxybenzoate.
A solution of 118g of ethyl-3-methoxy-4-hydroxybenzoate and 86mL of
benzyl bromide in 600mL of acetone containing a suspension of 124g of
potassium carbonate was heated at reflux overnight. The mixture was filtered,
the filtrate concentrated and the residue recrystallized from acetone.
C. Addition of ethyl magnesium iodide to ethyl-3-methoxy-4-
benzyloxybenzoate to yield 3-(3-methoxy-4-benzyloxyphenyl)pentan-3-ol.
Iodoethane (112mL) was added dropwise to a suspension of 35g of
magnesium turnings in 160mL of ether. After the formation of ethyl magnesium
iodide was complete, a solution of 142g of ethyl 3-methoxy-4-
benzyloxybenzoate in ether was added and the mixture was allowed to stir at
room temperature for 3 days. The reaction was quenched by addition of
saturated ammonium chloride. The layers were separated and the ether layer was
dried and concentrated to an oily residue. Yield: 1 l Og.
D. Elimination of H20 from 3-(3-methoxy-4-benzyloxyphenyl)pentan-3-of to
yield 4-((1Z)-1-ethylprop-1-enyl)-1-benzyloxy-2-methoxybenzene.
A solution of 110g of crude 3-(3-methoxy-4-benzyloxyphenyl)pentan-3-
ol and 7g of p-tolenesulfonic acid in 2L of benzene was heated at reflux for
4hr
with azeotropic removal of water. The mixture was then filtered through a pad
of sodium bicarbonate and the filtrate concentrated. The residue was purified
by
column chromatography on neutral alumina.
E. Addition of HZO to 4-((1Z)-1-ethylprop-1-enyl)-1-benzyloxy-2-
methoxybenzene to yield 3-(3-methoxy-4-benzyloxyphenyl)pentan-2-ol.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-56-
To a solution of 96g of 4-((1Z)-1-ethylprop-1-enyl)-1-benzyloxy-2-
methoxybenzene in THF at 0°C was added S l OmL of a 1.OM solution of
borane-
THF complex in THF. The mixture was stirred for 3hr at 0°C, then
204mL of
25% hydrogen peroxide was added. The mixture was adjusted to pH 8 by
addition of SM sodium hydroxide and extracted with ether. The combined ether
extracts were dried and concentrated. Yield: 1028.
F. Reaction of 3-(3-methoxy-4-benzyloxyphenyl)pentan-2-of with 3,4-
dimethoxybenzaldehyde to yield 4-(4-ethyl-6-methoxy-'7-benzyloxy-3-
methyliso-chromanyl)-1,2-dimethoxybenzene.
A solution of 46g of 3,4-dimethoxybenzaldehyde and 100g of crude 3-
(3-methoxy-4-benzyloxyphenyl)pentan-2-of in 0.3L of dioxane was saturated
with hydrogen chloride gas. The mixture was heated at reflux for 3hr, then
poured into water, basified with dilute sodium hydroxide and extracted with
methylene chloride. The combined methylene chloride extracts were dried and
concentrated.
G. Ring-opening of 4-(4-ethyl-6-methoxy-7-benzyloxy-3-methyliso-
chromanyl)-1,2-dimethoxybenzene to yield 3-(4-benzyloxy-5-methoxy-2- f [3,4-
dimethoxyphenyl]carbonyl}phenyl)pentan-2-one.
To a solution of SOg of crude 4-(4-ethyl-6-methoxy-7-benzyloxy-3-
methyliso-chromanyl)-1,2-dimethoxybenzene in acetone at 5°C was added a
solution of SOg of chromic oxide in SOOmL of 35% sulfuric acid. The mixture
was stirred at room temperature for 2hr, neutralized by adding cold 10% sodium
hydroxide and concentrated to remove acetone. Water was added and the
mixture extracted with methylene chloride. The combined methylene chloride
extracts were dried and concentrated. The residue was purified by column
chromatography on silica gel. Yield: 18g.
H. Debenzylation of 3-(4-benzyloxy-5-methoxy-2-{[3,4-dimethoxy-
phenyl]carbonyl}phenyl)pentan-2-one to yield 3-{2-[(3,4-dimethoxy-
phenyl)carbonyl]-4-hydroxy-5-methoxyphenyl)pentan-2-one.
A solution of 18g of 3-(4-benzyloxy-5-methoxy-2- f [3,4-dimethoxy-
phenyl]carbonyl}phenyl)pentan-2-one in methylene chloride containing a
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
suspension of 2g of 10% palladium on carbon was hydrogenated at 80psi for
lhr. The mixture was filtered through diatomaceous earth and the filtrate
concentrated. Yield: 15g.
I. Annulation of 3-{2-[(3,4-dimethoxy-phenyl)carbonyl]-4-hydroxy-5
methoxyphenyl}pentan-2-one by reaction with hydrazine to yield 1-(3,4
dimethoxyphenyl)-4-methyl-5-ethyl-7-methoxy-8-hydroxy-SH-2,3
benzodiazepine.
A solution of 14g of 3-~2-[(3,4-dimethoxy-phenyl)carbonyl]-4-hydroxy-
5-methoxyphenyl)pentan-2-one and 4.7mL of hydrazine in 280mL of ethanol
was heated at reflux for O.Shr. After allowing the solution to cool to room
temperature, it was saturated with HCl gas. The mixture was then concentrated
to a volume of about SmL, basified with concentrated ammonium hydroxide,
and extracted with methylene chloride. The combined methylene chloride
extracts were dried and concentrated, and the residue recrystallized from
ethyl
acetatelhexane. Yield: l.Sg.
The product 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7-methoxy-8-
hydroxy-SH-2,3-benzodiazepine was analyzed by HPLC, elemental analysis,
GC/MS, proton NMR and differential scanning calorimetry (DSC). The data
are as follows:
Purity: 98.36% by HPLC (% area). Column: Betasil Phenyl 4.6 x 150mm.
Mobile Phase: Acetonitrile::0.O1M Phosphate Buffer (70::30). Flow Rate:
O.SmL/min. Wavelength:254nm.
GC-MS; M/e = 358; with the fragmentation pattern matching the proposed
structure.
Differential scanning calorimetry (DSC): Temperature program 100°C
to 300°C
at 5°C/min, indicated molar purity = 99.14% and melting point of
146.2°C.
Elemental analysis (calculated/analysis): %C - 68.14/68.12; %H - 6.63/6.63;
N - 7.43/7.20. The calculated values include O.1M of residual ethyl acetate.
NMR (DCC13) (performed on GE QE 300): 1.08ppm (t, 3H); 1.96 (s, 3H); 2.10
(m, 2H); 2.77 (m, 1H); 3.91 (s, 3H); 3.93 (s, 3H); 3.98 (s, 3H); 5.73 (bs,
1H);
6.70 (s, 1H); 6.80 (d, 1H); 6.95 (s, 1H); 7.00 (d, 1H); 7.58 (s, 1H).
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-J O-
Example 5: Resolution of 1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl
7,8-dimethoxy-SH-2,3-benzodiazepine
- The enantiomers of racemic-1-(3-hydroxy-4-methoxyphenyl)-4-methyl
5-ethyl-7,8-dimethoxy-SH-2,3-benzodiazepine are resolved by chiral
chromatography as follows.
Racemic-1-(3-hydroxy-4-methoxyphenyl)-4-methyl-5-ethyl-7, 8-
dimethoxy-SH-2,3-benzodiazepine is loaded onto a semipreparative (SOOmm x
lOmm) Chirobiotic V column (ASTEC, Whippany, NJ). Elution of the
enantiomeric mixture with methyl-tent-butyl ether/ acetonitrile (90/10 V/V),
at a
flow rate of 40mL/min, is monitored at 310nm. Fraction size is 10-20 mL and
fractions are subjected to analytical chromatography using the same solvent
composition on an analytical (150 x 4.6mm) Chirobiotic V column. The
fractions containing each isolated enantiomer are processed by removing the
elution solvent in vacuo.
Example 6: LTB4 Binding Assay.
The LTB4 receptor binding activity of racemic tofisopam and
enantiomerically pure (R)- and (~S~-tofisopam was determined via the guinea
pig
spleen membrane assay of Cheng et al., J. Phaf~naacol. Exp. Tlaef-., 236(1),
126-
132, 1986.
Reactions were carried out in a phosphate buffer (pH 7.4) containing
NaCI, MgCl2, EDTA, and bacitracin. The reaction volume of 150~,L containing
l.Omg/mL of the Guinea pig spleen membrane preparation and 1nM [3H]LTB4,
with or without a competitor, was incubated at 0-4°C for 2 hours.
Competitors
included 2,3-benzodiazepines, and LTB4 as a ~ control. The reaction was
terminated by rapid vacuum filtration onto glass fiber filters. The filter was
washed with cold 'buffer, dried and placed in a scintillation vial.
Radioactivity
trapped onto the filters was determined and compared to control values in
order
to ascertain any interactions of the test compound with the LTB4 binding site.
Data gathered in the binding experiments for test compounds and standards are
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-J y-
shown graphically in Fig. 1, Fig. 2 and Fig. 3, and summarized in Table 2
below.
Table 2: Summary of [3H]LTB4 binding data for (R)-,
(S~- and racemic-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-
7,8-dimethoxv-SH-2,3-benzodiazenine
Test Substance g~ (~
LTB4 0.0002-0.0009
(S~-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-76.0
dimethoxy-SH-2,3-benzodiazepine
Racemic-1-(3,4-dimethoxyphenyl)-4-methyl-5-4.52
ethyl-7,8-dimethoxy-SH-2,3-benzodiazepine
(R)-1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-0.444
dimethoxy-SH-2,3-benzodiazepine
The binding data shows that binding of racemic 1-(3,4-dimethoxy-
phenyl)-4-methyl-5-ethyl-7,8-dimethoxy-SH-2,3-benzodiazepine to the LTB4
receptor is primarily due to the (R)-enantiomer, which binds with a K; greater
than 150x that of the (~-enantiomer.
Example 7: Effect of Tofisopam in a Rabbit Model of LTB4-induced Dermal
Inflammation.
A. Test Animals and Test Compounds.
Ten female New Zealand White Rabbits were assigned to dose groups as
summarized in Table 3 below.
Table 3: Test groups for the Rabbit model of LTB4-
induced dermal inflammatinn_
Test Animal Test article Dose
Group #
(mg~g~
~)
1 151-152 Vehicle p
2 251-252 (~-tohsopam 60
3 351-352 (R)-tohsopam 60
4 451-452 (R,f)-tohsopam60
5 0.5
551-552 he vehicle
dexamethasone was first
Test
compounds
were
prepared
as
follows.
T
prepared by dissolving 100mg of hydroxypropylrnethylcellulose 2910
(HPMC)(Sigma Chemical, St. Louis, MO) in SOmL of 0.9% saline to yield a
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-60-
concentration of 2% HPMC. The three test articles, (R)-, (f)- and racemic
tofisopam, were formulated by adding 1 g of each test article to l OmL of
vehicle.
B. Dosing and Intradermal Challenge.
On Day 0, the rabbits were sedated with ketamine/xylazine (35/5 mg/kg,
s.c.) and an area of approximately 8x14 cm on the back was closely and
carefully clipped to expose the skin, but not inflame or otherwise damage the
epithelium. A grid of ten squares each approximately 2.5 x 2.5 cm was drawn
on each animal's back using an indelible marker. The animals were then dosed
intraperitoneally with the appropriate test article corresponding to the
group.
Thirty minutes after dosing with test or control article, the animals were
challenged intradermally with the LTB4 or LTB4-aminopropamide (LTB4-AP, a
synthetic LTB4 agonist) in the appropriate site as shown in Table 4. Each
inj ection site was marked with an indelible marker in order to identify the
exact
location of the intradermal inj ection site. Sixty minutes after challenge,
the
animals were treated again with tofisopam or control articles. The animals
were
sacrificed 4 hrs. after challenge, and the intradermal injection sites were
excised,
fixed in 10% neutral buffered formalin (NBF), and submitted for
histopathology. H&E-stained sections were read by a board-certified veterinary
pathologist using light microscopy.
Table 4: Intradermal infection site grid for test animals.
Ante rior
0 0
500 500
Left 1000 1000 Right
2000 2000
2000 2000
Post erior
C. Necropsy.
All animals were sacrificed 4 hr after challenge. The intradermal
challenge sites were excised, placed on unique cardboard squares, fixed in 10%
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-61-
NBF, and submitted for histopathology. The skin sections included the
epidermis and the subcutis, down to the back musculature.
D. Histopathology.
The marked skin sections were fixed for at least 48 hours on small
cardboard squares. At gross trimming, three levels were cut through marked
skin region in order to assure that the injection sites is brought into the
plane of
section. The skins were gross trimmed, processed by dehydration, embedded in
paraffin, sectioned at 3-5 Vim, and stained with hematoxylin and eosin.
The tissues were evaluated histopathologically via light microscopy by a
board-certified veterinary pathologist. Initially, the identity of the slides
was
masked in order to perform the initial evaluation and rank the slides. The
slides
were then unmasked and there was a careful assessment of the lesions and
careful comparison of the tofisopam-treated sites to the vehicle and positive
control tissues. The lesions were graded based upon the degree of inflammation
and the degree of edema. The severity of the inflammation and edema was
keyed as follows: 0=normal; T=trace; 1=minimal; 2=mild; 3=moderate;
4=marked.
E. Histopathological Findings.
The histopathology findings are summarized in Table 5 below. Both
LTB4 and LTB4-ap produced focal skin wheals characterized by edema of the
subdermis and a brisk, dose-dependent influx of neutrophils. The inflammation
was composed of numerous neutrophils attached and marginated in small
vessels, perivascular inflammatory cuffs, and scattered throughout the dermis.
Additionally, some inflammation and edema was present in the superficial
dermis. The administration of (R)-tofisopam resulted in a meaningful reduction
in the severity of the inflammatory cell infiltration and less edema. There
were
no meaningful reduction in the inflammation and edema in the vehicle-treated,
the (~-tofisopam treated, the racemic tofisopam treated, or the dexamethasone-
treated animals.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-62-
Table 5: Summary of histologic findings in rabbits injected intradermally
with LTS4 or LTE-ap and treated with (R)-, S' -, or racemic tofisopam,
vehicle or dexamethasone.
Test animal/LT AgentDose Test compoundInflammationEdema
injection (ng) score score
site
151/0-L Vehicle0 vehicle T T
151/0-R Vehicle0 vehicle 1 1
151/500-L LTB4 500 vehicle 1 1
151/500-R LTB-ap 500 vehicle 1 2
151/1000-L LTB4 1000 vehicle 3 2
151/1000-R LTB-ap 1000 vehicle 3+ 2
151/2000-L LTB4 2000 vehicle 3 2
151/2000-R LTB-ap 2000 vehicle 3 2
151/2000-L LTB4 2000 vehicle 3 2
151/2000-R LTB-ap 2000 vehicle 3 2
152/0-L Vehicle0 vehicle 1 1
152/0-R Vehicle0 vehicle 1 1
152/500-L LTB4 500 vehicle 2 2
152/500-R LTB-ap 500 vehicle 2 3
152/1000-L LTB4 1000 vehicle 3 4
152/1000-R LTB-ap 1000 vehicle 3 4
152/2000-L LTB4 2000 vehicle 3 4
152/2000-R LTB-ap 2000 vehicle 2 4
152/2000-L LTB4 2000 vehicle 2 2
152/2000-R LTB-ap 2000 vehicle 2 2
251/0-L Vehicle0 (S~-tofisopamT T
251/0-R Vehicle0 (s~-tofisopam1 1
251/500-L LTB4 500 (S7-tofisopam1 2
251/500-R LTB-ap 500 (S~-tofisopam1 2
251/1000-L LTB4 1000 (S~-tofisopam1 3
~
251/1000-R LTB-ap 1000 (S~-tofisopam1 3
251/2000-L LTB4 2000 (S~-tofisopam3 2
251/2000-R LTB-ap 2000 (S~-tofisopam2 2
251/2000-L LTB4 2000 (~-tofisopam2 2
251/2000-R LTB-ap 2000 (S~-tofisopam2 1
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
Test animal/LT AgentDose Test compoundInflammationEdema
injection (ng) score score
site
252/0-L Vehicle 0 (~-tofisopam1 1
252/0-R Vehicle 0 (S~-tofisopam1 1
252/500-L LTB4 500 (S~-tofisopam2 2
252/500-R LTB-ap 500 (S~-tofisopam3-~-
252/1000-L LTB4 1000 (S~-tofisopam3 ~.. 4
252/1000-R LTB-ap 1000 (S~-tofisopam3-.~-
252/2000-L LTB4 2000 (S~-tofisopam3-~- q,
252/2000-R LTB-ap 2000 (S~-tofisopam3-~- q,
252/2000-L LTB4 2000 (S~-tofisopam2 2
252/2000-R LTB-ap 2000 (S~-tofisopam2 2
351/0-L Vehicle 0 (R)-tofisopam0 0
351/0-R Vehicle 0 (R)-tofisopam1 1
351/500-L LTB4 500 (R)-tofisopam1 1
351/500-R LTB-ap 500 (R)-tofisopam1 1
351/1000-L LTB4 1000 (R)-tofisopam1 2
351/1000-R LTB-ap 1000 (R)-tofisopam1 2
351/2000-L LTB4 2000 (R)-tofisopam1-2 2
351/2000-R LTB-ap 2000 (R)-tofisopam1 2
351/2000-L LTB4 2000 (R)-tofisopam1 2
35112000-R LTB-ap 2000 (R)-tofisopam1 2
352/0-L Vehicle 0 (R)-tofisopam1 2
352/0-R Vehicle 0 (R)-tofisopam1 2
352/500-L LTB4 500 (R)-tofisopam1 3
352/500-R LTB-ap 500 (R)-tofisopam1 3
352/1000-L LTB4 1000 (R)-tofisopam2 3
352/1000-R LTB-ap 1000 (R)-tofisopam2 3
352/2000-L LTB4 2000 (R)-tofisopam1 3
352/2000-R LTB-ap 2000 (R)-tofisopam2 3
352/2000-L LTB4 2000 (R)-tofisopam1 2
352/2000-R LTB-ap 2000 (R)-tofisopam1 1
451/0-L Vehicle 0 (R,,S~-tofisopam1 2
451/0-R Vehicle 0 (R,,S~-tofisopam1 2
451/500-L LTB4 S00 (R,,S~-tofisopam2 2
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-64-
Test animal/LT AgentDose Test compoundInflammationEdema
injection (ng) score score
site
451/500-R LTB-ap 500 (R,S~-tofisopam2 2
451/1000-L LTB4 1000 (R,,S~-tofisopam3 3
451/1000-R LTB-ap 1000 (R,S)-tofisopam3 3
451/2000-L LTB4 2000 (R,S~-tofisopam2 2
451/2000-R LTB-ap 2000 (R,S~-tofisopam2 2
451/2000-L LTB4 2000 (R,~-tofisopam1 1
451/2000-R LTB-ap 2000 (R~~-tofisopam1 1
452/0-L Vehicle0 (R,S~-tofisopam1 1
45210-R Vehicle0 (R,S~-tofisopam1 2
452/500-L LTB4 500 (R,S~-tofisopam2 3
452/500-R LTB-ap 500 (R,S~-tofisopam2 3
452/1000-L LTB4 1000 (R~~-tofisopam2 3
452/1000-R LTB-ap 1000 (R,~-tofisopam3 3
452/2000-L LTB4 2000 (R,~-tofisopam3 3
452/2000-R LTB-ap 2000 (R~S~-tofisopam2 3
452/2000-L LTB4 2000 (R,S~-tofisopam3 2
452/2000-R LTB-ap 2000 (R,S~-tofisoparii2 2
551/0-L Vehicle0 dexamethasone1 0
551/0-R Vehicle0 dexamethasone1 0
551/500-L LTB4 500 dexamethasone3 3
551/500-R LTB-ap 500 dexamethasone2 3
551/1000-L LTB4 1000 dexamethasone3 3
551/1000-R LTB-ap 1000 dexamethasone2 3
551/2000-L LTB4 2000 dexamethasone2 3
551/2000-R LTB-ap 2000 dexamethasone2 2
551/2000-L LTB4 2000 dexamethasone1 1
551/2000-R LTB-ap 2000 dexamethasone1 1
552/0-L Vehicle0 dexamethasone0 1
552/0-R Vehicle0 dexamethasone1 1
552/500-L LTB4 500 dexamethasone2 2
552/500-R LTB-ap 500 dexamethasone3 2
552/1000-L LTB4 1000 dexamethasone3 2
552/1000-R LTB-ap 1000 dexamethasone2 3
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-65-
Test animal/LT AgentDose Test compoundInflammationEdema
injection (ng) score score
site
55212000-L LTB4 2000 dexamethasone1+ 3
552/2000-R LTB-ap 2000 dexamethasone2 3
552/2000-L LTB4 2000 dexamethasone1 2
552/2000-R LTB-ap 2000 dexamethasone2 1
Example 8: Dextran Sulfate Sodium Induced Colitis: Mouse Model of IBD.
In this model of colitis, an acute inflammation of the colon was produced
by administration of dextran sulfate sodium (DSS) as a 5% solution in tap
water.
This colitis was characterized by lustological events and an influx of
neutrophils, macrophages and mediators of inflammation similar to those
observed with human inflammatory bowel diseases. Several drugs known to be
of useful for treating IBD, such as corticosteroids and 5-ASA, have been shown
to have activity in this model. The following study was conducted in
accordance with protocols of Okayasu et al., Gastroenterology, 98:694-702,
1990.
One hundred ten test animals (female, 6 weelc old Swiss Webster mice,
18-30g) were divided into ten groups, selected to eliminate any statistical
differences in mean group weight.
Each animal was dosed daily (IP) with either a test substance or vehicle,
starting on Day 0. Beginning on Day l, acute colon inflammation was induced
by the administration ad libitum in drinking water of dextran sulfate sodium
(DSS) as a 5% solution in tap water (lOmL/mouse/day for 5-6 days), with no
other fluid source for animals in the DSS arm of the study. Filtered tap water
was available ad libiturn except for animals receiving 5% DSS as the sole
source
of fluid. After four days, signs of acute disease occurred with the loss of
weight,
diarrhea and bloody stools. Histological changes included initial shortening
of
the crypts, then areas of separation of the crypts and the muscularis naucosae
in
the absence of destructive inflammatory filtrate. After five days,
pathological
changes became confluent with the appearance of erosions and early
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-66-
hyperplastic epithelium. Inflammation scores were high with neutrophils,
lymphocytes, and plasma cells in the lamina propria but sparing the
epithelium.
Test compounds were administered intraperitoneally (IP). Test
compounds given during this period were evaluated for prophylactic activity
and
test compounds given after the disease state was established were evaluated
for
therapeutic activity. Ten test animals were assigned to each of ten dose
groups
listed in Table 6, below.
Table 6: Dose group assignments for the DSS-Induced Colitis: Mouse
Model of IBD.
Group Test substance DSS or control
1 Vehicle IP daily + tap water
2 Vehicle Il' daily + DSS 5% in tap
water
3 Racemic tofisopam 64mg/kg + DSS 5% in tap
IP daily water
4 Racemic tofisopam 32mg/kg + DSS 5% in tap
11' daily water
5 Racemic tofisopam l6mg/kg + DSS 5% in tap
1P daily water
6 (R)-tofisopam 64mg/kg lP + DSS 5% in tap
daily water
7 (R)-tofisopam 32mg/kg IP + DSS 5% in tap
daily water
8 (R)-to~sopam l6mg/kg lP + DSS 5%. in tap
daily water
9 (,S~-tofisoparn 64mglkg + DSS 5% in tap
IP daily water
10 (,S~-tofisopam 32mg/kg IP + DSS 5% in tap
daily water
11 (S~-tofisopam l6mglkg IP + DSS 5% in tap
daily water
Test animals were weighed daily from Day 0 to Day 8, or until
completion of the study. The total duration of the study with DSS arm of the
study was varied depending on the time progress of colitis. The condition of
the
test animals and consistency of stools was noted.
At the conclusion of the study, test animals were euthanized (COZ), a
midline incision was made and a stool sample was obtained. The sample was
placed on a slide and tested for occult blood (Quic-CultT"", Laboratory
Diagnostics Co., Morganville, NJ). Occult blood was determined by placing
two drops of the reagent onto the sample and observing any color change.
Occult blood presence was graded using a scoring protocol assigning a score of
0 for no color; 1 for a very light blue color (+/-) forming in > 30 seconds; 2
for a
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-67-
blue color developing in 30 seconds or more (+); 3 for a change in color
occurring in less than 30 seconds (++); and 4 for gross blood observable on
the
slide. The colon was gently stretched and the length from the colon-cecal
junction to the end of the distal rectum was measured to the nearest O.lcm. A
Disease Activity Index (DAl~ was determined using the criteria summarized in
Table 7 below:
Table 7: Scoring criteria for determination of Disease
Activity Index (DAI) in the DSS-Induced Colitis: Mouse
Model of 1<BD.
Score Weight loss Stool consistencyBlood in
(%) feces
0 0 or gain Normal Negative
1 1-4.9 Soft +/-
2 5.0-9.9 Mixed soft +
and
diarrhea
3 10-15 Diarrhea ++
4 >15 bloody diarrheagross blood
The scores for each test animal were added and then divided by three to
give a DAI score for each animal. The data for the eleven groups is summarized
in Tables 8, 9 and 10 below.
Table 8: Disease Activity Index for test animals in DSS-induced colitis
study.
Test compound n Mean Mean
DAI SEM DAI* SEM
0.1 % CMC + water 10 0.07 0.040.00 0.00
0.1 % CMC + DSS 10 2.83 0.243.25 0.24
Racemic tofisopam 64mg/kg 10 2.87 0.313.25 0.26
Il' daily
Racemic tofisopam 32mg/kg 10 2.50 0.302.85 0.24
IP daily
Racemic tofisopam l6mg/kg 9 2.59 0.272.61 0.30
Il' daily
(R)-tofisopam 64mg/lcg IP 10 1.77 0.2812.20 0.261
daily
(R)-tofisopam 32mglkg IP 10 2.37 0.302.75 0.26
daily
(R)-tofisopam l6mg/kg IP 9 2.33 0.402.61 0.37
daily
(~-tofisopam 64mg/kg IP daily9 2.93 0.223.17 0.19
(~-tofisopam 32mg/kg IP daily10 2.77 0.263.15 0.22
(S~-tofisopam l6mg/lcg IP 10 2.37 0.242.85 0.18
daily
~ - Significant difference from vehicle + DSS control - Two-tailed T-test, p <
0.05
DAI - Disease Activity Index; DAI* - DAI without weight loss parameter.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-6S-
Table 9: Colon Length assessment for test animals in DSS-induced colitis
study.
Test compound n Mean colon % of Colon
length normalshortening
CM SEM lengthinhib.
0.1 % CMC + water 10 12.5 0.11 100 --
0.1% CMC + DSS 10 7.9 0.18 63.5 --
Racemic tofisopam 64mg/kg10 8.2 0.18 65.5
IP daily
Racemic tofisopam 32mg/kg10 8.3 0.11 66.7 9
IP daily
Racemic tofisopam l6mg/kg10 8.2 0.32 65.4 7
IP daily
(R)-tofisopam 64mg/kg 10 9.8 0.35 78.5 411
IP daily
(R)-tofisopam 32mg/kg 10 8.9 0.41 71.3 22
IP daily
(R)-tofisopam l6mg/kg 9 9.0 0.28 72.4 24
IP daily
(b~-tofisopam 64mg/kg 9 8.2 0.20 65.4 7
IP daily
(,5~-tofisopam 32mg/kg 10 8.1 0.19 64.9 4
IP daily
(S~-tofisopam l6mg/kg 10 8.4 0.19 67.3 11
IP daily
1- Significant difference from vehicle + DSS control - p < 0.05 One way ANOVA
and Tukey-Kramer Multiple Comparisons Test.
Table 10: % Weight change for test animals in DSS-induced colitis study.
Mean ht
weight change
(g) SEM
and
%
weig
Test CompoundDay Day % Day % Day
0 8 9 10
change change change
0.1 % CMC 26.10.426.50.6+1.51.826.80.6+2.71.426.50.5+1.61.3
+ water
0.1 % CMC 2G.10.525.80.6-1.21.325.00.6-4.30.924.10.6-7.71.6
+ DSS
Racemic 26.00.425.50.7-2.01.824.50.8-5.82.323.50.9-9.63.0
tofisopam
64mg/kg
IP daily
Racemic 26.10.425.60.5-1.81.524.70.6i-5.32.024.00.7-8.02.4
tofisopam
32mg/kg
IP daily
Racemic 25.90.425.10.6-3.11.524.00.8-7.42.522.51.0
tofisopam
l6mg/kg 13.33.1
IP daily
(R)-tofisopam26.20.52G.20.5+0.11.925.40.5-2.91.925.40:5-2.82.5
64mg/kg
IP daily
(R)-tofisopam25.00.42G.20.4+0.81.G25.50.6-1.92.124.60.6-4.42.1
32mg/kg
IP daily
(R)-tofisopam26.10.526.10.60.01.425.2O.G-3.31.824.10.8-7.53.0
l6mg/kg .
IP daily
(~-tofisopam25.90.425.6O.G-1.21.524.10.5-G.91.823.20.6
64mg/kg 10.41.7
IP daily
(~-tofisopam25.90.425.10.6-3.02.324.60.6-4.92.423.20.9-
32mg/kg 10.43.2
IP daily
(~-tofisopam26.00.4ZG.O0.50.01.525.60.5-1.51.524.GO.G-5.41.G
lGmg/kg
IP daily
1- Significant difference from Vehicle + DSS group - p < 0.05 - One way ANOVA
and Tukey-Kramer Multiple Comparisons Test. Statistical Analysis Incomplete.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-6H-
The data show that at a dose of 64mg per kg, (R)-tofisopam provided
significant protection from the LTB4-mediated inflammatory responses to DSS-
induced colitis. This assessment was based on colon length assessment and an
overall Disease Activity Index (DAI) which incorporates scores for indices of
colitis progression that include weight loss, stool consistency and amount of
detected blood in feces.
I Example 9: Dexiran Sulfate Sodium Induced Colitis- Effects of Low-Dosage
R Tofisopam.
This study was conducted using a mouse model of inflammatory bowel disease
as described by Okayasu et al (Gastr~oente~olo~ 98:694-702, 1990) and modified
by
Mutfihy et al. (Digest. Dis. Sci. 38:1722-1734,1993). In brief, the method
involves feeding
5% dextran sulfate sodium (DSS) in drinking water for 5-6 days to mice. After
four days,
sighs of acute disease occur with the loss of weight, diarrhea and bloody
stools.
Histological changes include initial shortening of the crypts, then areas of
separation of
the crypts and the muscularis mucosae in the absence of destructive
inflammatory
infiltrate. After five days, pathological changes become confluent with the
appearance of
erosions and early hyperplastic epithelium. Inflammation Scores are high with
neutrophils, lymphocytes, and plasma cells in the lamina propria but sparing
the
epithelium. Agents given during this period are tested for potential
prophylactic activity.
Those given after the disease is established are used to evaluate therapeutic
activity.
A. Experimental Design and Analysis.
Female CVF (Swiss derived) mice were obtained from Hilltop Labs
(Scottdale, PA). The animals were grouped housed (5 mice/cage) in plastic
cages
with water absorbent bedding. The animal room was temperature controlled and
maintained on a 12-hour light/dark cycle. Following an acclimation period of
13 days,
60 healthy female mice were selected for the study. The test animals were
distributed (10
mice/group) into each one of the six test groups as shown below.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-7U-
GROUP PRODUCT m DOSE
NUMBER LEVEL
(mg IC/0.2
ml
1 Vehicle (0.5% CMC in distilled 0
water) no
DSS
2 Vehicle (0.5% CMC in distilled 0
water) with
DSS
3 Mesalamine (5-ASA) 4
4 R-Tofisopam + 5% DSS 3.2
R-Tofisopam + 5% DSS 1.6
6 R-Tofiso am + 5% DSS 0.8
IC - intracolonic
The test compounds were suspended in a 0.5% w/w solution of
carboxymethylcellulose (CMC) in distilled water. Each group of animals
received
5 the appropriate dose of the test compound starting on Day 0, as described
above, by
intracolonic (IC) administration using a ball tipped dosing needle and syringe
at a
daily dose volume of 0.2 ml/mouse/day. Groups 2 through 6 were given a 5% w/w
solution of dexatran sulfate sodium (DSS) in distilled water substituted for
their
normal water supply from Day 1 through the day before necropsy. Group 1
animals
received water bottles containing water without DSS. No other source of fluids
was
available. Bottles were refilled daily with the appropriate volume of fresh
DSS solution
or water.
All mice were weighed on Day 0 and then daily from Days 5 through 9 and
observed for signs of gross toxicity and behavioral changes, consistency of
stool and
presence of gross blood during the study. On Day 9, all surviving animals were
euthanized by C02 inhalation and necropsied. Following euthanasia, a stool
sample
was obtained from the colon of each animal and was tested for occult blood
(Quik-
Cult Laboratory Diagnostics Co., Morganville, NJ). The colons were then
removed
and the length from the colo-cecal junction to the end of the distal rectum
was
measured. The whole colon was collected from each animal and preserved in 10%
formalin.
For each group, the disease activety index (DAI) was determined by
evaluating changes in weight, Hemoccult positivity or gross bleeding, and
stool
consistency using the following system.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-~/ 1-
Criteria for Scoring Disease Activity Index (DAI)*
ScoreWeight Loss Stool Blood in Feces
(%)
0 0 or gain Normal Negative
1 1-4.9 Soft +/-
2 5.0-9.9 Mixed (soft & +
diarrhea)
3 10-15 Diarrhea ++
4 >15 Bloody diarrhea Gross blood
*DAI= (combined score of weight loss, stool consistency and bleeding)/3
# Normal stools = well-formed pellets; diarrhea = liquid stools that stick to
the
anus.
Data was analyzed using analysis of variance (single factor). Statistical
significance between test and control groups was established at a probability
of
p <0.05.
B. Results and Discussion.
In this study DSS produced a typical degree of colonic inflammation,
colonic shortening and loss of body weight. Two of ten mice died in the
control
group. Mice were sacrificed on the ninth day. Results of the study are
tabulated
in Table 11A-C and Table 12 below.
The results show that R-Tofisopam produced a significant reduction in
DAINWT (DAI without the weight factor) but not DAI at dosages of 3.2, 1.6 and
0.8 mg administered IC daily (Table 11A).
Referring to Table 11B, all doses of R-Tofisopam produced a
statistically significant inhibition of colonic shortening by DSS, with
maximal
activity at 1.6 mg.
There was no statistically significant difference in weight loss (percent
weight change) compared to DSS + Vehicle controls (Table 11C). Collectively
the data show that low doses (in the range of about 0.5-5.0 mg per day) of R-
Tofisopam are effective to significantly reduce the disease activity index
(without
the weight factor) and inhibit colonic shortening in a mouse model of DSS-
induced colitis when R-tofisopam is applied at or near the gastrointestinal
lining.
Mesalamine (5-ASA) is the active component of sulfasalazine. In contrast
to results with R-Tofisopam, 4 mg/mouse of mesalamine administered by the
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-72-
intracolonic route did not produce a statistically significant decrease in DAI
or
DAINWT (Table 11A), or protection from DSS-induced loss of body weight
(Table 11 C). However, mesalamine did produce a significant protection from
DSS-induced colonic shortening (Table 11B).
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
Table 11: Effect of R-Tofisopam or Mesalamine in DSS-Induced Colitis in
Mice.
A: Disease Activity Index
Compound Dose Mean Mean
IC N DAI~SEM DAINWT~SEM
m dail
0.5% CMC + --- 10 0.10 ~ 0.07**0.05 ~ 0.05**
Water
0.5% CMC + --- 8 2.54 + 0.24 3.06 ~ 0.22
DSS
Mesalamiue 4 10 2.33 + 0.16 2.40 + 0.19
+ DSS
R-Tofiso am+ 3.2 10 2.00 + 020 2.10 ~ 0.16**
DSS
R-Tofiso am+DSS1.6 10 1.83 ~ 0.13 1.95 ~ 0.23**
R-Tofiso am+DSS0.8 8 1.96 ~ 0.31 2.00 ~ 0.23**
Statistical evaluation ANOVA one-way.
Statistically significant difference from vehicle + DSS control: *p<0.05; **
p<0.01
Dunnett Multiple Comparison Test - Control group = Vehicle + DSS
DAI - Disease Activity Index
DAINWT - Disease Activity Index without weight loss parameter
B: Colon Length
Compound Dose Mean Colon Percent % Inh.
IC N Length of Colonic
CM ~ SEM normal lengthLength
mg shortening
dail
0.5% CMC + --- 10 12.5 ~ 0.14**100 ---
Water
0.5% CMC + --- 8 7.4 ~ 0.24 59.7 ---
DSS
Mesalamine+DSS4 10 9.1 ~ 0.30** 72.7 32
R-Tofiso am+DSS3.2 10 9.1 + 0.22** 73.1 33
R-Tofiso am+ 1.6 10 9.6 + 0.22** 77.1 43
DSS
R-Tofisopam+DSS0.8 8 9.4+0.26** 75.8 40
Statistical evaluation ANOVA one-way.
Statistically significant difference from vehicle + DSS control: *p<0.05; **
p<0.01
Dunnett Multiple Comparison Test - Control group = Vehicle + DSS
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-74-
Table 11 (coot.): Effect of R Tofisopam or Mesalamine in DSS-Induced
Colitis in Mice.
C: Percent Weight Change from Pre-DSS Starting
Mean Wei~ht_( rams) and Weight Change SEM
CompoundDose Dav Day % Day % Day
0 7 8 9
.
m IC chap than than
a a a
0.5%CMC+-- 28.Sf0.229.00.3+1.8f1.229.20.3+2.51.129.2f0.4+2.Sf
Water
** 13
0.5%CMC+--- 27.40.227.Of0.6-1.32.326.8f0.6-2.2f2.525.80.8-5.92.9
DSS
Mesalamine4 27.3 26.9 -1.4 27.2 -0.3 24.7 -9.4
+ ~ ~ ~ 1.1 ~ ~ ~ 0.5 ~
0.3 0.3 0.4 1.0 2.1
ASS
R-Tofsopam3.2 28.810.228.9 +0.3 27.8 -3.5 26.810.6-6.9
+ ~ ~ 1.5 ~ ~ f
0.5 0.5 1.6 2.0
DSS
R-Tofisopam1.6 27.9 27.9 0.0 27.110.62.9 26.2 -6.112.0
+ f f ~ 1.9 + ~ 0.6
0.3 0.6 1.9
DSS
R-Tofisopam0.8 28.4 27.4 -3.5 26.6 -0.911.427.0 -4.812.3
+ ~ f ~ 2.0 f ~ ~ 0.7
0.4 0.6 0.6
~
DSS
Statistical evaluation ANOVA one-way.
Statistically significant difference from vehicle + DSS control: *p<0.05;
*~ p<0.01
Dunnett Multiple Comparison Test - Control group = Vehicle + DSS
Body weight data for individual mice are tabulated in Table 12. Table 12
also indicates that no deaths occurred in the groups receiving the 3.2 or 1.6
mg
daily dose of R-Tofisopam; however two deaths occurred in the low dose group
(0.8 mg). No deaths occurred in the Mesalamine group.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
Table 12. Body weights in DSS-Induced Colitis Model
Group 1: Vehicle + Tap Water
dal Bodyweight
( )
No. Sex Day Day Day Day Day Day
0 5 6 7 8 9
5991 F 29 26 26 28 28 27
5992 F 28 30 30 30 30 30
5993 F 28 26 26 27 2g 28
5994 F 29 2g 28 29 30 30
5995 F 29 2g 28 30 30 30
5996 F 28 30 30 30 30 30
5997' F 2g 29 29 29 29 30
5998 F 29 29 30 30 30 30
5999 F 28 29 29 29 29 29
6000 F 28 28 2g 28 28 28
Group 2: Vehicle + DSS
Animal Bodyweight(g)
Sex
No. Da Da Da 6 Da Day Day
0 5 7 8 9
6001 F 27 27 27 27 28 27
6002 F 27 25 25 23 21
6003 F 27 27 26 27 26 25
6004 F 27 28 28 29 28 26
6005 F 28 27 26 24 23 21
6006 F 27 28 27 27 26 25
6007 F 28 29 29 29 28 28
6008 F 28 27 26 26 27 27
6009 F 27 28 27
6010 F 27 28 27 27 28 27
' Animal #6002 was found dead on Day 9 (B.W. 0.20g).
ZAnimal #6009 was found dead on Day 7 (B.W. 0.25g).
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-76-
Table 12 (coot.): Bodyweights
Group3: Mesalamine(5-ASA) (4m~ ICS
fg)
No. ~x DayO Days Day6 Day7 Day8 Day9
6011 F 28 30 28 29 29 26
6012 F 28 28 26 27 28 24
6013 F 28 26 25 26 27
6014 F 29 30 28 28 28 75
6015 F 28 28 26 27 27 23
6016 F 27 26 26 26 27 26
6017 F 27 28 28 27 28 27
6018 F 26 26 26 26 26 24
6019 F 26 26 26 26 25 23
6020 F 26 28 27 27 27 a~
(soup 4: R Tofbsopam (3.2 mglC~
t
~
No. DayO Days Day6 Day7 Day8 Day9
6021 F 28 28 28 29 28 28
6022 F 29 30 31 31 30 29
6023 F 29 30 29 31 31 30
6024 F 29 28 28 28 27 26
6025 F 29 30 29 30 2g 27
6026 F 29 28 27 28 27 25
6027 F 29 31 30 29 27 2
5
6028 F 28 28 27 27 26 ,
26
6029 F 28 27 26 26 26 24
6030 F 30 31 30 30 28 28
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
_77_
Table 12 (cont.): Bodyweights
Group 5: R-Tofisopam (1.6 mg IC)
AnimalSex Bodyweight
No. (g)
Da
6
Da
7
Da
8
Da
9
6031 F 27 29 28 29 28 28
6032 F 29 29 28 28 28 27
6033 F 28 29 29 29 29 28
6034 F 28 28 27 28 27 27
6035 F 27 28 28 28 27 26
6036 F 27 27 25 24 23 22
6037 F 29 30 29 31 30 28
-
6038 F 28 28 27 28 27
6039 F 29 27 27 27 26 25
6040 F 27 27 26 27 26 25
Group 6: R Tofisopam (0.8 mg IC)
Animal Bod
ei
ht
No. Sex Day Day Day Day Day Day
0 5 6 7 8 9
6041 F 27 27 26 26 26 25
6042 F 29 ' - - - -
6043 F 28 30 29 29 29 28
6044 F 29 30 30 30 30 29
6045 F 27 28 27 28 28 26
6046 F 29 28 28 31 31 30
6047 F 29 27 27 29 29 28
6048 F 28 24 25 27 27 25
6049 F 29 24 24
6050 F 30 26 27 29 2~ 25
'Animal #6042 was found dead on Day 5 (B.W. 23 g).
Z Animal #6049 was found dead on Day 7 (B.W. 24 g).
Example 10: Effects of R-Tofisopam on Arachidonic Acid -Induced
Inflammation Model in Mouse Ear.
In this study the anti-inflammatory effects of R-tofisospam were tested
in a model of acute inflammation of the mouse ear produced by administration
of arachidonic acid Several dnigs have been shown to have anti-edematous
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
_%S_
effects in this model. The following study was conducted in accordance with
the protocols of Burchart et al., P~ostaglandins Leukot. Essent. Fatty Acids
1997
Apr; 56(4):301-306; PYOStaglahdirZS Leukot. Essent. Fatty Acids 1999
Jan;60(1):5-11.
A. Test Animals and Test Compounds.
Forty Swiss-Webster female mice 8-9 weeks of age, ranging in weight
from 20-24g, were group housed and acclimated for six days in a controlled
environment (temperature 18-26°C; relative humidity 30-70%; 12 hours
artificial light and 12 hours dark) in compliance with the National Research
Council "Guide for the Care and Use of Laboratory Animals." Temperature and
humidity were monitored daily. All animals had access to drinking water and
Certified Rodent Diet (TEKLAD) ad libitum and were monitored at least once
daily for any abnormalities or for the development of infectious disease.
Test compounds were prepared as follows. A solution of arachidonic
acid (AA; Cat. No. A-9673, Sigma Chemical, St. Louis, MO) was prepared at
room temperature at a concentration of 200 mg/ml in EtOH vehicle (Pharmco,
Lot 0110264), transferred to an amber glass screw top vial and stored until
use
at approximately -20°C. Test article R-tofisopam (Vela Pharmaceuticals,
Lawrenceville NJ) was stored in powder form at room temperature, protected
from light. Just prior to use, test article solutions were prepared in EtOH
vehicle
at the following concentrations: 1.0, 0.1, and 0.01 mg/ml. All dose
preparations
were protected from light.
Following acclimation, mice were weighed, placed in individual
housing, and identified by color coding. Only mice considered suitable for use
were placed in the study. Mice were selected based upon body weight and
apparent good health. Mice were assigned to dose groups as summarized in
Table 13 below.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-/7-
Table 13. Test Groups for the Mouse Ear Model of Arachidonic Acid-
Induced Inflammation.
Group Number Topical Absorption Topical
of Treatment 1 Time Treatment Sacrifice
Animals (outer 2
surface of (inner
surface)
Female each ear Ri ht Left Time
Ear Ear
1 10 Vehicles 30 minutes Ethanol AAb 30 minutes
10 ~,l 10 ~,l 10 ~.l
2 10 R-tofisopam 30 minutes Ethanol AA 30 minutes
0.01 mg/ml 10 pl 10 ~,1
3 10 R-tofisopam 30 minutes Ethanol AA 30 minutes
0.1 mg/ml ~ 10 ~,1 10 pl
4 10 R-tofisopam 30 minutes Ethanol AA 30 minutes
1.0 mg/ml 10 ~.l 10 pl
A-__7_:~___
n
< » u.v>uuvmtt~ a.~.lu etL G 111/ 1 V ~..1,1
B. Experimental Design and Method of Analysis.
The vehicle or test article was administered topically to the outer surface of
both
the right and left ear in a volume of 10 ~,l/ear. Following a 30 minute (~5
minutes) absorption period, a 10 ~.1 volume of AA/EtOH prepared as described
above was administered topically to the inner surface of the ear. Thirty
minutes
(~5 minutes) after the administration of AA/EtOH, the animals were euthanized
by CO2 asphyxiation without exsanguination. Ears were measured for thickness
with a Mitutoyo micrometer and edema was calculated by subtracting the
thickness measurement (in mm) of the control ear (right) from that of the test
ear
(left). Mean edema was calculated and compared using an ANOVA followed
by a Tukey HSD Multiple Comparison Test (p<0.05)- Systat, v.9.01.
C. Results.
All animals appeared normal during the course of the study. Mean
animal data from the edema studies are presented in Table 14 and individual
data are presented in Table 15. Referring to Tables 14 and 15, it is seen that
the
topical administration of R-tofisopam at 0.01, 0. l and 1.0 mg/ml with a 30
minute absorption time produced 30%, 26% and 45% reductions in edema,
respectively, when compared to the vehicle control group. The 45% reduction
was statistically significant (p<0,05) and was considered biologically
relevant,
whereas the lower dose concentrations showed only slight effects of similar
magnitude.
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-uU-
b
0
A \ ~ ~ N ~n
~
C~
H
.,.,
O
O O O
O O p O
-I-I -I-~ -I-I +I
v
W W N O O O
~' O
y O O O
O
y
~ N
'~"' O
O O O
C/~
., v~
O
W U U U O U O
O O O
a
N N N N
H
t~
, i~.
O ~ O O O .-, O
,-.a ,-a ,-,
O ~O ~O ~O
~' W W W W
~
H
N
O
O ,s' U
~ ~ ~ O
U
r1
~
~ ,~ ~~ ,O ~ N O
d O
'~
S
~I~ U O O ''~ O 'd
.O ~ -; "'~
C
C
iU
n O , ~ ~ "'_' cct N
E~ ~ 4~ tF
~ ~- ~
~
~
,., ~ O O ~ ,
,~ O ~ O
y
O
~ ~' ~ ~ i
V ~ " ~
~ ~
Q
~ ~ '
'~" ~"~ , y -
y .S Qi a
. , r-
n U
O s p ~.'-' O
p
~
E-I O .-i
M
O ~
O 'r O
O
r~"~ N
O
~~C ~ O O 1
. O O ~ 4
~
W ~ a~ N b
s~
z~ w ~
~ ~
w
~, ~
o~
_
H '~ N M
N
~
~
_
~
W ~ cry
U ~F
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
-t51-
Table 15. Effect of R-Tofisopam on Arachidonic Acid-Induced
Inflammation in the Mouse Ear: Tntlivirlnal Animal n.,+..
itui0..
Group Mouse Topical Topical Measurements Edema
Number Number Treatment '~ millimeters (L-R)
1 Treatment
(outer (inner
surface surface)
of each
ear)
Right Left Riglit Left (mm)
Ear Ear Ear Ear
1 1 Vehicle Ethanol AA 0.305 0.393 0.088
2 10 ~l 10 ~,l 2 mg/100.275 0.394 0.119
~N,l
0.305 0.420 0.115
4 Absorption 0.336 0.539 0.203
Time 0.319 0.456 0.137
6 30 minutes 0.285 0.418 0.133
7 0.296 0.426 0.130
8 0.300 0.395 0.095
0.293 0.436 0.143
10 0.289 0.409 0.120
2 11 R-tofisopamEthanol AA 0.296 0.359 0.063
12 0.01 mg%ml10 ~1 2 mg/100.300 0.406 0.106.
wl
13 10 ~,l 0.276 0.407 0.131
14 0.325 0.355 0.030
15 Absorption 0.350 0.426 0.076
16 Time 0.353 0.437 0.084
17 30 minutes 0.271 0.418 0.147
l
~~t
18 0.302 0.472 0.170
19 0.286 0.351 0.065
20 0.334 0.360 0.026
~r h~ 1 _
E
a is a - tOH
CA 02548038 2006-05-31
WO 2005/056017 PCT/US2004/040403
82=
Table 15, cont'd.
Group Mouse Topical Topical Measurements Edema
Number Number Treatment Treatment millimeters (L-R)
1 (inner
(outer surfacesurface)
of each
ear)
Right Left Wight Left (mm)
Ear Ear Ear Ear
3 21 R-tofisopamEthanol AA 0.256 0.399 0.143
22 0.1 mg/ml 10 ~.l 2 mg/10 0.257 0.345 0.088
~,1
23 10 ~,l 0.285 0.326 0.041
24 0.276 0.313 0.037
25 Absorption 0.324 0.450 0.126
26 Time 0.339 0.490 0.151
27 ~30 minutes 0.283 0.371 0.088
2g 0.295 0.468 0.173
-
29 - ~ 0.294 0.386 0:092
-
30 0.285 0.294 0.009
4 31 R-tofisopamEthanol AA 0.280 0.329 0.049
32 1.0 mg/ml 10 ~1 2 mg/10 0.300 0.325 0.025
pl
~
~33 10 ~.1 ~ 0.280 0.374 0.094
34 0.282 0.417 0.135
35 Absorption 0.320 0.350 0.030
36 Time 0.309 0.397 0.088
37 30 minutes 0.273 0.359 0.086
3g 0.281 0.342 0.061
39 0.265 0.345 0.080
40 0.291 0.346 0.055
The data show that topical administration of R-tofisopam at 1.0 mg/ml
produced a biologically relevant and statistically significant (p<0.05)
reduction
in mouse ear inflammation induced by arachidonic acid.
All references cited herein are incorporated by reference. The present
invention may be embodied in other specific forms without departing from the
spirit or essential attributes thereof and, accordingly, reference should be
made
to the appended claims, rather than to the foregoing specification, as
indication
the scope of the invention.