Note: Descriptions are shown in the official language in which they were submitted.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
CALCITONIN GENE RELATED PEPTIDE RECEPTOR ANTAGONISTS
Field of the Invention
The present invention relates to novel small molecule antagonists of
calcitonin gene-related peptide receptors ("CGRP-receptor"), pharmaceutical
compositions comprising them, methods for identifying them, methods of
treatment
using them and their use in therapy for treatment of neurogenic vasodilation,
neurogenic inflammation, migraine, cluster headache and other headaches,
thermal
injury, circulatory shock, flushing associated with menopause, airway
inflammatory
diseases, such as asthma and chronic obstructive pulmonary disease (COPD), and
other conditions the treatment of which can be effected by the antagonism of
CGRP-
receptors.
Background of the Invention
Calcitonin gene-related peptide (CGRP) is a naturally occurring 37-amino-
acid peptide first identified in 1982 (Amara, S. G. et al, Science 1982, 298,
240-244).
Two forms of the peptide are expressed (aCGRP and (3CGRP) which differ by one
and three amino acids in rats and humans, respectively. The peptide is widely
distributed in both the peripheral (PNS) and central nervous system (CNS),
principally localized in sensory afferent and central neurons, and displays a
number
of biological effects, including vasodilation.
When released from the cell, CGRP binds to specific cell surface G protein-
coupled receptors and exerts its biological action predominantly by activation
of
intracellular adenylate cyclase (Poyner, D. R. et al, BrJPharmacol 1992, 105,
441-7;
Van Valen, F. et al, Neurosci Lett 1990,119, 195-8.). Two classes of CGRP
receptors, CGRP1 and CGRP2, have been proposed based on the antagonist
properties
of the peptide fragment CGRP(8-37) and the ability of linear analogues of CGRP
to
activate CGRPZ receptors (Juaneda, C. et al. TIPS 2000, 21, 432-438). However,
there is lack of molecular evidence for the CGRP2 receptor (Brain, S. D. et
al, TIPS
2002, 23, 51-53). The CGRP~ receptor has three components: (i) a 7
transmembrane
calcitonin receptor-like receptor (CRLR); (ii) the single transmembrane
receptor
activity modifying protein type one (RAMP 1 ); and (iii) the intracellular
receptor
component protein (RCP) (Evans B. N. et al., JBiol Chem. 2000, 275, 31438-43).
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
RAMP 1 is required for transport of CRLR to the plasma membrane and for ligand
binding to the CGRP-receptor (McLatchie, L. M. et al, Nature 1998, 393, 333-
339).
RCP is required for signal transduction (Evans B. N. et al., JBiol Chem. 2000,
275,
31438-43). There are known species-specific differences in binding of small
molecule antagonists to the CGRP-receptor with typically greater affinity seen
for
antagonism of the human receptor than for other species (Brain, S. D. et al,
TIPS
2002, 23, 51-53). The amino acid sequence of RAMP1 determines the species
selectivity, in particular, the amino acid residue Trp74 is responsible for
the
phenotype of the human receptor (Mallee et al. JBiol Chem 2002, 277, 14294-8).
Inhibitors at the receptor level to CGRP are postulated to be useful in
pathophysiologic conditions where excessive CGRP receptor activation has
occurred.
Some of these include neurogenic vasodilation, neurogenic inflammation,
migraine,
cluster headache and other headaches, thermal injury, circulatory shock,
menopausal
flushing, and asthma. CGRP receptor activation has been implicated in the
pathogenesis of migraine headache (Edvinsson L. CNS Drugs 2001;15(10):745-53;
Williamson, D. J. Microsc. Res. Tech. 2001, 53, 167-178.; Grant, A. D. Brit.
J.
Pharmacol. 2002, 135, 356-362.). Serum levels of CGRP are elevated during
migraine (Goadsby PJ, et al. Ann Neurol 1990;28:183-7) and treatment with anti-
migraine drugs returns CGRP levels to normal coincident with alleviation of
headache (Gallai V. et al. Cephalalgia 1995;15: 384-90). Migraineurs exhibit
elevated basal CGRP levels compared to controls (Ashina M, et al., Pain.
2000;86(1-
2):133-8.2000). Intravenous CGRP infusion produces lasting headache in
migraineurs (Lassen LH, et al. Cephalalgia. 2002 Feb;22(1):54-61). Preclinical
studies in dog and rat report that systemic CGRP blockade with the peptide
antagonist CGRP(8-37) does not alter resting systemic hemodynamics nor
regional
blood flow (Shen, Y-T. et al, JPharmacol Exp Ther 2001, 298, 551-8). Thus,
CGRP-
receptor antagonists may present a novel treatment for migraine that avoids
the
cardiovascular liabilities of active vasoconstriction associated with non-
selective 5-
HTiBUD agonists, 'triptans' (e.g., sumatriptan).
There are various in vivo migraine models known in the literature (see
De Vries, P. et al, EurJPharmacol 1999, 375, 61-74). Some electrically
stimulate
the trigeminal ganglion and measure dilation of the intracranial vessels which
they
innervate (e.g., Williamson et al. Cephalalgia 1997 17:518-24). Since facial
arteries
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
are also innervated by the trigeminal nerve, other models study changes in
facial
blood flow induced by electrical trigeminal activation (e.g., Escott et al.
Brain Res
1995 669:93). Alternatively, other peripheral nerves (e.g., saphenous) and
vascular
beds (e.g., abdominal blood flow) are also studied (e.g., Escott et al. Br
JPharmacol
1993 110, 772-6;). All models have been shown to be blocked by pretreatment
with
the peptide antagonist CGPR(8-37) a peptide fragment that is absent the 1s'
seven
residues, or by a small molecule CGRP-receptor antagonist. In some instances,
exogenous CGRP has been used as a stimulus. However, these models are all
invasive terminal procedures, and none have shown the clinically important
abortive
effect of reversing an established increase in artery dilation or increased
blood flow
using post-treatment of a CGRP-receptor antagonist. Williamson et al.
Cephalalgia
1997 17:518-24, and Williamson et al. Cephalalgia. 1997 17:525-31: used inter
alia
i.v. CGRP as a stimulus to increase intracranial dural artery diameter in
sodium
pentobarb anesthetized rats employing a terminal 'intravital' procedure that
involved
drilling to thin the skull and the creation of a closed cranial window to
visualize dural
arteries. The effect was blocked by pretreatment with i.v. CGRP(8-37). Escott
et al.
Brain Res 1995 669:93; inter alia drilled into the rat skull and used brain
electrodes
to electrically stimulate the trigeminal ganglion and measured laser Doppler
facial
blood flow in a terminal procedure in sodium pentobarb anesthetized rats
involving
neuromuscular blockade, tracheal intubation and artificial ventilation. The
effect was
blocked by pretreatment with CGRP(8-37). Escott et al. Br JPharmacol 1993 110,
772-6; inter alia used intradermal (i.d.) CGRP as the stimulus to increase
blood flow
in rat abdominal skin of sodium pentobarb anesthetized animals outfitted with
cannulated jugular veins for anesthetic and drug delivery. The effect was
blocked by
pretreatment with i.v. CGRP(8-37). Chu et al. Neurosci Lett 2001 310, 169-72
used
inter alia i.d. CGRP as the stimulus in rats and measured laser Doppler
changes in
blood flow in the skin of the back in a terminal method using sodium pentobarb
anesthetized and tracheal cannulated animals; and showed pretreatment blockade
by
continuous release of CGRP(8-37) from subcutaneously (s.c.) implanted osmotic
pumps. Hall et al BrJPharmacol 1995 114, 592-7 and Hall et al BrJPharmacol
1999 i26, 280-4 inter alia used topical CGRP to increase hamster cheek pouch
arteriole diameter, and i.d. CGRP to increase blood flow in rat dorsal skin of
sodium
pentobarb anesthetized animals outfitted with cannulated jugular veins for
anesthetic
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
and drug delivery. The effect was blocked by pretreatment with i.v. CGRP(8-
37).
Doods et al. Br J Pharmacol. 2000 Feb; 129(3):420-3 inter alia drilled into
the skull
of the marmoset (new world monkey) and used brain electrodes to produce
electrical
stimulation of the trigeminal ganglion and measured facial blood flow in an
invasive
terminal procedure involving neuromuscular blockade and artificial ventilation
of
sodium pentobarbital anesthetized primates. Increase in flow was blocked by
pre-
treatment of a small molecule CGRP antagonist. See also WO 03/272252 Isolated
DNA Molecules Encoding Humanized Calcitonin Gene-Related Peptide Receptor,
Related Non-Human Transgenic Animals and Assay Methods. Thus the method of
the present invention procedure being inter alia a non-invasive survival model
in
primates measuring exogenous CGRP-induced changes in facial blood flow and
demonstrating pre- and post-treatment effects of peptide and small molecule
CGRP
antagonists in spontaneously breathing isoflurane anesthetized marmosets who
recover from the procedure offers significant advantages.
A number of non-peptidic, small molecule CGRP-receptor antagonists have
been recently reported. WO 97/09046 and equivalents disclose inter alia
quinine and
quinidine related compounds which are ligands, in particular antagonists, of
CGRP-
receptor. WO 98/09630 and WO 98/56779 and equivalents disclose inter alia
variously substituted, nitrobenzamide compounds as CGRP-receptor antagonists.
WO
01/32649, WO 01/49676, and WO 01/32648 and equivalents disclose inter alia a
series of 4-oxobutanamides and related cyclopropane derivatives as CGRP-
receptor
antagonists. WO 00/18764, WO 98111128 and WO 00/55154 and equivalents
disclose inter alia benzimidazolinyl piperidines as antagonists to CGRP-
receptor.
Unrelated to CGRP, a series of somatostatin antagonists have been disclosed in
WO
99/52875 and WO 01/25228 and equivalents. See also U.S. 6,344,449, U.S.
6,313,097, U.S. 6,521,609, U.S. 6,552,043, US 20030181462, US20030191068 and
WO 03/076432 and related applications. Thus, novel CGRP-receptor antagonists
effective for the treatment of neurogenic inflammation, migraine and other
disorders
would be greatly advantageous.
Summar~of the Invention
Thus according to a first embodiment of the first aspect of the present
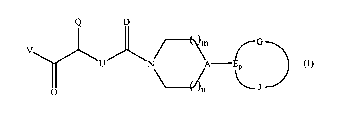
invention are provided compounds of Formula (I)
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
Q D
G
\'V
U N A ~ (I)
~J
and pharmaceutically acceptable salts and solvates thereof
wherein
V is -N(Rl)(RZ) or OR4;
R4 is H, C1_6alkyl, C»haloalkyl or (Cl~alkylene)o_1R4~
R4~ is C3_~cycloalkyl, phenyl, adamantyl, quinuclidyl,
azabicyclo[2.2.1]heptyl, azetidinyl, tetrahydrofuranyl, furanyl,
dioxolanyl, thienyl, tetrahydrothienyl, pyrrolyl, pyrrolinyl,
pyrrolidinyl, imidazolyl, imidazolinyl, imidazolidinyl,
pyrazolyl, pyrazolinyl, pyrazolidinyl, oxazolyl, isoxazolyl,
thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl,
pyranyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl,
triazinyl, piperidinyl, piperazinyl, morpholino, thiomorpholino
or dioxolanyl; and
R4~ is optionally substituted with 1 or 2 of the same or different
substituents selected from the group consisting of halo,
cyano, C~.~alkyl, Cl~haloalkyl, Cl~alkoxy, hydroxy,
amino, C3_~cycloalkyl, C1_3alkylamino, C1_
3dialkylamino, (C,_3alkyl)o_ZUreido, phenyl and benzyl;
and
R4~ optionally contains 1 or 2 carbonyls wherein the carbon
atom of said carbonyl is a member of the ring structure
of R4~;
R1 and Rz are each independently L', wherein L1 is selected from the
group consisting of H, C~_6alkyl, C2_6alkenyl, CZ_6alkynyl, -C1_
6alkylene-amino(CI_3alkyl)2, C3_~cycloalkyl, phenyl, azetidinyl,
adamantyl, tetrahydrofuranyl, furanyl, dioxolanyl, thienyl,
tetrahydrothienyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
6
pyrazolinyl, pyrazolidinyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyranyl,
pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazinyl,
piperidinyl, piperazinyl, morpholino, thiomorpholino and
dioxolanyl; and
R1 and RZ are each optionally and independently substituted
with 1 or 2 of the same or different substituents
selected from the group consisting of halo, cyano, C1_
4alkyl, Cl~haloalkyl, Cl~alkoxy, hydroxy, amino, C3_
~cycloalkyl, Cl_3alkylamino, C1_3dialkylamino, (C1_
3alkyl)o_2ureido, phenyl and benzyl;
R1 and R2 optionally and independently contain 1 or 2
carbonyls wherein the carbon atom of said carbonyl is a
member of the heterocycles comprising R1 and RZ;
wherein Ll is optionally and independently interrupted from
the nitrogen to which it is attached by L2, wherein LZ is
independently C1_3alkylene or C~_3alkylidene; or
Rl and R2 together with the nitrogen to which they are attached form
X,
wherein X is azetidinyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolinyl, imidazolidinyl, pyrazolinyl,
pyrazolidinyl, azepinyl, diazepinyl, piperazinyl,
piperidinyl, morpholino or thiomorpholino;
wherein X is optionally substituted with Y, wherein Y
is dioxolanyl, C1_9alkyl, CZ_9alkenyl, C2_
9alkynyl, C,~alkylamino, Cl~dialkylamino, C~_
4alkoxy, C3_7cycloalkyl, phenyl, azetidinyl,
furanyl, thienyl, pyrrolyl, pyrrolinyl,
pyrrolidinyl, pyrrolidinonyl, imidazolyl,
imidazolinyl, imidazolidinyl, imidazolidinonyl,
pyrazolyl, pyrazolinyl, pyrazolidinyl, azepinyl,
diazepinyl, pyridyl, pyrimidinyl,
dihydrobenzimidazolonyl, piperazinyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
7
piperidinyl, morpholino, benzothiazolyl,
benzisothiazolyl or thiomorpholino;
and wherein X and Y are
optionally interrupted with Z, wherein Z
is NHC(O)O-, NHC(O)NH-,
NC(O)NH2, -NH-, -C 1 _
3alkylene-, -C~_3alkylene-, -C1_
3alkenylene-NHC(O)O-C1_
3alkylene-; and
optionally and independently substituted
with 1 or 2 of the same or
different substituents selected
from the group consisting of Cl_
4alkyl, amino, C1_3alkylamino,
-Ci-6alkylene-amino(C1_3alkyl)2,
(C~_3alkyl)o_ZUreido, phenyl and
benzyl;
X and Y optionally and independently
contain 1 or 2 carbonyls wherein
the carbon atom of said carbonyl
is a member of the heterocycles
comprising X and Y;
provided that if X is substituted with Y, and if
X and Y are not interrupted with Z, then
X and Y optionally share one carbon
atom and together form a
spirocyclic moiety;
QisQ'orQ";
wherein
Q' is (SY)sR3; and
Q" is NH(Sy)SR3, NHC(O)(S'')SR3, NHC(O)O(S'')SR3,
NHC(O)NH(Sy)SR3, O(SY)SR3, (S'')SNHR3,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
8
(S'')SNHC(O)R3, (S'')SNHC(O)OR3, (SY)SNHC(O)NHR3
or (SY)SOR3;
wherein SY is C,_3alkylene or C,_3alkylidene and s is 0
or 1;
U is CHz or NH;
provided that if Q is Q", then U is CHZ;
R3 1S R3a Or R3b
wherein
R3a 1S
(i) a heterocycle having two fused rings with 5 to 7
members in each of said rings, said heterocycle
containing one to five of the same or different
heteroatoms selected from the group consisting of O, N
and S and said heterocycle optionally containing 1 or 2
carbonyls wherein the carbon atom of said carbonyl is a
member of said fused rings;
(ii) a 4 to 6 membered heterocycle containing one to three
of the same or different heteroatoms selected from the
group consisting of O, N and S, optionally containing
1 to 2 carbonyls, wherein the carbon atom of said
carbonyl is a member of said 4 to 6 membered
heterocycle;
(iii) C3_~cycloalkyl;
(iv) carbazolyl, fluorenyl, phenyl, -O-phenyl, -O-C1_
4alklylene-phenyl, or napthyl; or
(v) C1_galkyl, CZ_~alkenyl, -C(O)R3', CHC(O)O-R3',
CH(CH3)C(O)O-R3',-C(O)O-R3' or CZ_7alkynyl; and
wherein R3a is optionally substituted with 1 to 3 of the same or
different substituents selected from the group
consisting of benzyl, phenyl, -O-phenyl, -O-C~_
3alkylenephenyl, -C~_3alkylene-OC(O)-phenyl, cyano,
amino, nitro, halo, C,_6alkyl, C,_3mono-bi-tri-haloalkyl,
C1_3mono-bi-tri-haloalkyloxy, (C1_3alkyl)~_Zamine,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
9
-OR3~, -C(O)R3', -C(O)O-R3', -O-C(O)R3', -N(R3')2,
-C(O)N(R3~)z~ -N(R3~)C(O)(R3~)2~ -N(R3')C(O)N(R3~)z~
-N(R3')C(O)OR3', -O-C(O)N(R3')z, -N(R3')S02R3',
-S02N(R3')2 and -SOZR3';
R3'is H or -C1_6alkyl;
provided that if R3a is , -C(O)R3', CHC(O)O-R3',
CH(CH3)C(O)O-R3'or -C(O)O-R3', then said
-C(O)R3', CHC(O)O-R3', CH(CH3)C(O)O-R3'or
-C(O)O-R3' are unsubstituted;
R3b is R3a but is not phenyl, 1-naphthyl, 2-naphthyl, 1,2,3,4-
tetrahydro-1-naphthyl, 1H-indol-3-yl, 1-methyl-1H-
indol-3-yl, 1-formyl-1H-indol-3-yl, 1-(1,1-
dimethylethoxycarbonyl)-1H-indol-3-yl, 4-imidazolyl,
1-methyl-4-imidazolyl, 2-thienyl, 3-thienyl, thiazolyl,
1H-indazol-3-yl, 1-methyl-1H-indazol-3-yl,
benzo[b]fur-3-yl, benzo[b]thien-3-yl, pyridinyl,
quinolinyl or isoquinolinyl; optionally substituted in
the carbon skeleton with mono-, di- or trisubstituted by
fluorine, chlorine or bromine atoms or by branched or
unbranched alkyl groups, C3_g -cycloalkyl groups,
phenylalkyl groups, alkenyl, alkoxy, phenyl,
phenylalkoxy; trifluoromethyl, alkoxycarbonylalkyl,
carboxyalkyl, alkoxycarbonyl, carboxy,
dialkylaminoalkyl, dialkylaminoalkoxy, hydroxy, nitro,
amino, acetylamino, propionylamino, benzoyl,
benzoylamino, benzoylmethylamino,
methylsulphonyloxy, aminocarbonyl,
alkylaminocarbonyl, dialkylaminocarbonyl, alkanoyl,
cyano, tetrazolyl, phenyl, pyridinyl, thiazolyl, furyl,
trifluoromethoxy, trifluoromethylthio,
trifluoromethylsulphinyl- or trifluoromethylsulphonyl
groups;
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
wherein said substituents may be the same or different
and the above-mentioned benzoyl,
benzoylamino- and benzoylmethylamino groups
may in turn additionally be substituted in the
phenyl moiety by a fluorine, chlorine or
bromine atom, or by an alkyl, trifluoromethyl,
amino or acetylamino group;
D is O, NCN or NSOZC1_3alkyl;
A is C, N or CH;
10 m and n are independently 0, 1 or 2;
provided that
if m and n are 0, then A is not N;
if m is 2, then n is not 2; or
if n is 2, then m is not 2;
E is N, CH or C;
pis0orl;
if p is l, then G, J and E together form A" or Ay'
A" is a fused heterocycle having two fused rings with 5 to 7
members in each of said rings, said heterocycle
containing one to four of the same or different
heteroatoms selected from the group consisting of O, N
and S; and
optionally containing 1 or 2 carbonyls wherein the
carbon atom of said carbonyl is a member of
said fused heterocycle;
AY is a 4 to 6 membered heterocycle containing one to three
heteroatoms selected from the group consisting of O, N
and S; and
optionally containing 1 to 2 carbonyls, wherein the
carbon atom of said carbonyl is a member of
said 4 to 6 membered heterocycle;
wherein A" and Ay are optionally substituted with C1_
4alkyl, C,~alkoxy, C»haloalkyl, cyano, C3_
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
11
7cycloalkyl, phenyl, halophenyl, halo, furanyl,
pyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl,
imidazolinyl, imidazolidinyl, pyrazolyl,
pyrazolinyl, pyrazolidinyl, pyridyl, pyrimidinyl,
piperidinyl, piperazinyl or morpholino; or
if p is 0 such that G and J are each attached to A, then A is C, and G, J
and A together form a spirocyclic ring system with said rings
of said system containing A and wherein G, J and A together
are GJA' or GJA' ;
wherein
GJA' is A" or AY; and
GJA" is A" or Ay;
provided that
A" is not a 1,3-diaza-fused heterocycle;
and
AY is not a 1,3-diaza-heterocycle;
and further provided that
if Q is Q", then R3 is R3a; and
if Q is Q', then
R3 is R3b ; or
R3 is R3a, p is 0 and G, J and A together form GJA".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and R3 is R3b.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', R3 is R3a and p is 0 such that G, J and A
together
form GJA".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and Q' is (SY)SR3 and s is 0.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
12
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and Q' is (S'')SR3, Sy is C1_3alkylene and s
is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and Q' is (S'')SR3, SY is C1_3alkylidene and
s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and U is CH2.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', Q' is (SY)S R3 , s is 0 and U is CH2.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', Q' is (SY)S R3 , Sy is C1_3alkylene, s is 1
and U is
CH2.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', Q' is (SY)S R3, SY is C1_3alkylidene, s is
land U is
CHZ.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q' and U is NH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', Q' is (SY)S R3 , s is 0 and U is NH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q', Q' is (SY)S R3 , Sy is C1_3alkylene, s is
land U is
NH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
13
present invention wherein Q is Q', Q' is (SY)S R3 , SY is C~_~alkylidene, s is
land U is
NH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NH(S'')SR3.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NH(S'')SR3 and s is 0.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NH(SY)SR3, SY is C~_3alkylene and
s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NH(SY)SR3, SY is Cl_3alkylidene
and s is
1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)(SY)SR3.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)(Sy)SR3 and s is 0.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)(SY)SR3, SY is C~_3alkylene
and s
is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)(Sy)SR3, Sy is
C~_3alkylidene and
s is 1.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
14
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)O(Sy)SR3.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)O(SY)SR3 and s is 0.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)O(SY)SR3, SY is C1_3alkylene
and
s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)O(SY)SR3, SY is
C1_3alkylidene
and s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)NH(SY)SR3.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)NH(SY)SR3 and s is 0.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)NH(SY)SR3, SY is
C~_3alkylene
and s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and Q" is NHC(O)NH(SY)SR3, SY is
C~_3alkylidene
and s is 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is OR4.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is ORS and R4 is C~_6alkyl.
According to another embodiment of the first aspect of the present invention
5 are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R1)(RZ).
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein
10 V is -N(R~)(RZ) or OR4;
R4 is H, C1_6alkyl, Cl.~haloalkyl, (C»alkylene)o_lRa~
R4~ is C3-~cycloalkyl, phenyl, adamantyl, quinuclidyl,
azabicyclo[2.2.1 ]heptyl, azetidinyl, tetrahydrofuranyl, furanyl,
dioxolanyl, thienyl, tetrahydrothienyl, pyrrolyl, pyrrolinyl,
15 pyrrolidinyl, imidazolyl, imidazolinyl, imidazolidinyl,
pyrazolyl, pyrazolinyl, pyrazolidinyl, oxazolyl, isoxazolyl,
thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl,
pyranyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl,
triazinyl, piperidinyl, piperazinyl, morpholino, thiomorpholino
or dioxolanyl; and
R4~ is optionally substituted with 1 or 2 of the same or different
substituents selected from the group consisting of halo,
cyano, C 1 alkyl, C 1 ~haloalkyl, C 1 ~alkoxy, hydroxy,
amino, C3_~cycloalkyl, C1_3alkylamino, C1_
3dialkylamino, (C1_3alkyl)o_2ureido, phenyl and benzyl;
R4~ optionally contains 1 or 2 carbonyls wherein the carbon
atom of said carbonyl is a member of the ring structure
of R4~;
Rl and RZ are each independently L1, wherein Ll is selected from the
group consisting of H, C,_6alkyl, -C1_6alkylene-amino(CI_
3alkyl)Z, C3_~cycloalkyl, phenyl, adamantyl, azetidinyl,
tetrahydrofuranyl, furanyl, dioxolanyl, thienyl,
tetrahydrothienyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
16
imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl,
pyrazolinyl, pyrazolidinyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyranyl,
pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazinyl,
piperidinyl, piperazinyl, morpholino, thiomorpholino and
dioxolanyl; and
Rl and R2 are each optionally and independently substituted
with 1 or 2 of the same or different substituents
selected from the group consisting of halo, cyano, C1_
4alkyl, C,~haloalkyl, Cmalkoxy, hydroxy, amino, C3_
~cycloalkyl, C1_3alkylamino, Cz_3dialkylamino, (C1_
3alkyl)o_ZUreido, phenyl and benzyl;
R' and R2 optionally and independently contain 1 or 2
carbonyls wherein the carbon atom of said carbonyl is a
member of the heterocycles comprising Rl and R2;
wherein L1 is optionally interrupted from the nitrogen to which
it is attached by L2, wherein L2 is C~_3alkylene; or
Rl and RZ together with the nitrogen to which they are attached form
X,
wherein X is azetidinyl, pyrrolinyl, pyrrolidinyl, imidazolinyl,
imidazolidinyl, pyrazolinyl, pyrazolidinyl, azepinyl,
diazepinyl, piperazinyl, piperidinyl, morpholino or
thiomorpholino;
wherein X is optionally substituted with Y, wherein Y
is dioxolanyl, CI-aalkyl, Cl~alkylamino, C~_
4dialkylamino, Cl.~alkoxy, C3_~cycloalkyl,
phenyl, azetidinyl, pyrrolyl, pyrrolinyl,
pyrrolidinyl, pyrrolidinonyl, imidazolyl,
imidazolinyl, imidazolidinyl, imidazolidinonyl,
pyrazolyl, pyrazolinyl, pyrazolidinyl, azepinyl,
diazepinyl, pyridyl, pyrimidinyl,
dihydrobenzimidazolonyl, piperazinyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
17
piperidinyl, morpholino, benzothiazolyl,
benzisothiazolyl or thiomorpholino;
and wherein X and Y are
optionally interrupted with Z, wherein Z
is -NHC(O)O-, NHC(O)NH-,
NC(O)NH2, -NH-, -C1_
3alkylene-, -C~_3alkylene-
NHC(O)O-C1_3alkylene-; and
optionally and independently substituted
with 1 or 2 of the same or
different substituents selected
from the group consisting of C~_
4alkyl, amino, C1_3alkylamino,
-C 1 _6alkylene-amino(C, _3alkyl)Z,
(Ci-3alkyl)o_zureido, phenyl and
benzyl;
X and Y optionally and independently
contain 1 or 2 carbonyls wherein
the carbon atom of said carbonyl
is a member of the heterocycles
comprising X and Y;
provided that if X is substituted with Y, and if
X and Y are not interrupted with Z, then
X and Y optionally share one carbon
atom and together form a
spirocyclic moiety.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R4 is H, C1_6alkyl, Cl~haloalkyl or
(Cl~alkylene)o_1R4~; Ra
is C3_~cycloalkyl, phenyl, adamantyl, quinuclidyl, azabicyclo[2.2.1 ~heptyl,
azetidinyl,
tetrahydrofuranyl, furanyl, dioxolanyl, thienyl, tetrahydrothienyl, pyrrolyl,
pyrrolinyl,
pyrrolidinyl, imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl,
pyrazolinyl,
pyrazolidinyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl,
thiadiazolyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
18
triazolyl, pyranyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazinyl,
piperidinyl,
piperazinyl, morpholino, thiomorpholino or dioxolanyl; and R4~ is optionally
substituted with 1 or 2 of the same or different substituents selected from
the group
consisting of halo, cyano, C,~,alkyl, Cl~haloalkyl, C»alkoxy, hydroxy, amino,
C3_
~cycloalkyl, Ci-3alkylamino, C1_3dialkylamino, (C,_3alkyl)o_2ureido, phenyl
and
benzyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R4 is H, C1_6alkyl, C»haloalkyl or
(C»alkylene)o_1R4~; R4'
is C3_~cycloalkyl, phenyl, adamantyl, quinuclidyl, azabicyclo[2.2.1]heptyl,
azetidinyl,
tetrahydrofuranyl, furanyl, dioxolanyl, thienyl, tetrahydrothienyl, pyrrolyl,
pyrrolinyl,
pyrrolidinyl, imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl,
pyrazolinyl,
pyrazolidinyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl,
thiadiazolyl,
triazolyl, pyranyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazinyl,
piperidinyl,
piperazinyl, morpholino, thiomorpholino or dioxolanyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R4 is H, C1_6alkyl or (C»alkylene)o_1R4~; R4~ is C3_
~cycloalkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R~)(RZ) and
Rl and RZ are each independently L', wherein L' is selected from the
group consisting of H, C~_6alkyl, -C1_6alkylene-amino(C1_
3alkyl)2, C3_~cycloalkyl, phenyl, azetidinyl, adamantyl,
tetrahydrofuranyl, furanyl, dioxolanyl, thienyl,
tetrahydrothienyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl,
pyrazolinyl, pyrazolidinyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyranyl,
pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazinyl,
piperidinyl, piperazinyl, morpholino, thiomorpholino and
dioxolanyl; or
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
19
R' and RZ together with the nitrogen to which they are attached form
X,
wherein X is azetidinyl, pyrrolinyl, pyrrolidinyl, imidazolinyl,
imidazolidinyl, pyrazolinyl, pyrazolidinyl, azepinyl,
diazepinyl, piperazinyl, piperidinyl, morpholino or
thiomorpholino;
wherein X is substituted with Y, wherein Y is
dioxolanyl, C»alkyl, Cl~alkoxy, C3_
~cycloalkyl, phenyl, azetidinyl, pyrrolyl,
pyrrolinyl, pyrrolidinyl, pyrrolidinonyl,
imidazolyl, imidazolinyl, imidazolidinyl,
imidazolidinonyl, pyrazolyl, pyrazolinyl,
pyrazolidinyl, azepinyl, diazepinyl, pyridyl,
pyrimidinyl, dihydrobenzimidazolonyl,
piperazinyl, piperidinyl, morpholino,
benzothiazolyl, benzisothiazolyl or
thiomorpholino;
and wherein X and Y optionally share one
carbon atom and together form a
spirocyclic moiety.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(Rl)(RZ) and
R' and RZ are each independently L', wherein L' is selected from the
group consisting of H, C1_6alkyl, or
R1 and RZ together with the nitrogen to which they are attached form
X,
wherein X is piperidinyl or morpholino;
wherein X is substituted with Y, wherein Y is
dioxolanyl, C»alkyl or piperidinyl;
and wherein X and Y optionally share one
carbon atom and together form a
spirocyclic moiety.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(Rl)(RZ) and wherein R' and RZ are each
independently L1, wherein L1 is selected from the group consisting of H,
C~_6alkyl.
5 According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(RI)(RZ) and wherein
Rl and RZ together with the nitrogen to which they are attached form
X,
10 wherein X is piperidinyl or morpholino;
wherein X is substituted with Y, wherein Y is
dioxolanyl, Cl~alkyl or piperidinyl;
and wherein X and Y optionally share one
carbon atom and together form a
15 spirocyclic moiety.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R1)(RZ) and wherein
R1 and RZ together with the nitrogen to which they are attached form
20 X,
wherein X is piperidinyl;
wherein X is substituted with Y, wherein Y is
piperidinyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R1)(RZ) and wherein
R' and RZ together with the nitrogen to which they are attached form
X,
wherein X is morpholino;
wherein X is substituted with Y, wherein Y is Cl~alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R1)(RZ) and wherein
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
21
R1 and RZ together with the nitrogen to which they are attached form
X,
wherein X is piperidinyl;
wherein X is substituted with Y, wherein Y is Cl~alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein V is -N(R1)(RZ) and wherein
Rl and R2 together with the nitrogen to which they are attached form
X,
wherein X is piperidinyl;
wherein X is substituted with Y, wherein Y is
dioxolanyl;
and wherein X and Y share one carbon atom and
together form a spirocyclic moiety.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein X and Y are not interrupted with Z.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein X and Y are not interrupted with Z; and X and Y
share one
carbon atom and together form a spirocyclic moiety
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3b.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is a heterocycle having two fused rings with 5
to 7
members in each of said rings, said heterocycle containing one to five of the
same or
different heteroatoms selected from the group consisting of O, N and S.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
22
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is a heterocycle having two fused rings with 5
to 7
members in each of said rings, said heterocycle containing one to five of the
same or
different heteroatoms selected from the group consisting of O, N and S and
said
heterocycle optionally containing 1 or 2 carbonyls wherein the carbon atom of
said
carbonyl is a member of said fused rings.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is a heterocycle having two fused rings with 5
to 7
members in each of said rings, said heterocycle containing one to five of the
same or
different heteroatoms selected from the group consisting of O, N and S and
said
heterocycle optionally containing 1 or 2 carbonyls wherein the carbon atom of
said
carbonyl is a member of said fused rings; wherein R3a is optionally
substituted with 1
to 3 of the same or different substituents selected from the group consisting
of
benzyl, phenyl, -O-phenyl, -O-C1_3alkylphenyl, -C~_3alkylene-OC(O)-phenyl,
cyano,
amino, nitro, halo, C1_3mono-bi-tri-haloalkyl, C~_3mono-bi-tri-haloalkyloxy,
C1_
6alkoxy, (C1_3alkyl),_zamine, -OR3', -C(O)R3', -C(O)O-R3', -O-C(O)R3', -
N(R3')z,
-C(O)N(R3')z, -N(R3')C(O)(R3~)z~ -N(R3~)C(O)N(R3~)z~ -N(R3')C(O)OR3', -O_
C(O)N(R3')z, -N(R3')SOZR3', -SOZN(R3')z and -SOZR3'; R3'is H or -C1_6alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is a 4 to 6 membered heterocycle containing one
to
three of the same or different heteroatoms selected from the group consisting
of O, N
and S.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is a 4 to 6 membered heterocycle containing one
to
three of the same or different heteroatoms selected from the group consisting
of O, N
and S, optionally containing 1 to 2 carbonyls, wherein the carbon atom of said
carbonyl is a member of said 4 to 6 membered heterocycle.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
23
present invention wherein R3a is a 4 to 6 membered heterocycle containing one
to
three of the same or different heteroatoms selected from the group consisting
of O, N
and S, optionally containing 1 to 2 carbonyls, wherein the carbon atom of said
carbonyl is a member of said 4 to 6 membered heterocycle; wherein R3a is
optionally
substituted with 1 to 3 of the same or different substituents selected from
the group
consisting ofbenzyl, phenyl, -O-phenyl, -O-C1_3alkylphenyl, -C~_3alkylene-
OC(O)-
phenyl, cyano, amino, vitro, halo, C~_3mono-bi-tri-haloalkyl, C~_3mono-bi-tri-
haloalkyloxy, Cl_6alkoxy, (CI_3alkyl)I_zamine, -OR3', -C(O)R3', -C(O)O-R3', -O-
C(O)R3'~ -N(R3')2~ -C(O)N(R3')2~ -N(R3')C(O)(R3')2~ -N(R3')C(O)N(R3~)2~
-N(R3')C(O)OR3', -O-C(O)N(R3')z, -N(R3')S02R3', -SOZN(R3')z and -SOZR3';
R3'is H or -Cl_6alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is C3_~cycloalkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is C3_~cycloalkyl; wherein R3a is optionally
substituted
with 1 to 3 of the same or different substituents selected from the group
consisting of
benzyl, phenyl, -O-phenyl, -O-C1_3alkylphenyl, -C1_3alkylene-OC(O)-phenyl,
cyano,
amino, vitro, halo, C1_3mono-bi-tri-haloalkyl, Cl_3mono-bi-tri-haloalkyloxy,
C1_
6alkoxy, (C1_3alkyl)1_zamine, -OR3~,-C(O)R3', -C(O)O-R3', -O-C(O)R3', -
N(R3')z,
-C(O)N(R3')z, -N(R3')C(O)(R3')z, -N(R3')C(O)N(R3')z, -N(R3')C(O)OR3', -O_
C(O)N(R3')z, -N(R3')SOZR3', -SOZN(R3')z and -SOzR3'; R3'is H or -C1_6alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is carbazolyl, fluorenyl, phenyl, -O-phenyl, -O-
C~_
4alklylene-phenyl, or napthyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3a is carbazolyl, fluorenyl, phenyl, -O-phenyl, -O-
C~_
4alklylene-phenyl, or napthyl; wherein R3a is optionally substituted with 1 to
3 of the
same or different substituents selected from the group consisting of benzyl,
phenyl, -
O-phenyl, -O-Cl_3alkylphenyl, -Cl_3alkylene-OC(O)-phenyl, cyano, amino, vitro,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
24
halo, Cl_3mono-bi-tri-haloalkyl, C~_3mono-bi-tri-haloalkyloxy, C,_6alkoxy,
(Ci_
3alkyl)~_Zamine, -OR3', -C(O)R3', -C(O)O-R3', -O-C(O)R3', -N(R3')2, -
C(O)N(R3')2,
-N(R3')C(O)(R3')z, -N(R3')C(O)N(R3')2, -N(R3')C(O)OR3', -O-C(O)N(R3')~,
-N(R3')SOZR3', -SOZN(R3')2 and -SOZR3'; R3'is H or -C1_6alkyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3ais C1_8alkyl, CZ_~alkenyl, -C(O)R3', -C(O)O-R3'
or CZ_
~alkynyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3ais C1_galkyl, CZ_~alkenyl, -C(O)R3', -C(O)O-R3'
or CZ_
~alkynyl; wherein R3a is optionally substituted with 1 to 3 of the same or
different
substituents selected from the group consisting of benzyl, phenyl, -O-phenyl, -
O-C1_
3alkylphenyl, -C~_3alkylene-OC(O)-phenyl, cyano, amino, nitro, halo, C1_3mono-
bi-
tri-haloalkyl, C1_3mono-bi-tri-haloalkyloxy, C~_balkoxy, (C~_3alkyl)~_Zamine, -
OR3~,
-C(O)R3', -C(O)O-R3', -O-C(O)R3', -N(R3')z, -C(O)N(R3')2, -N(R3')C(O)(R3')2,
-N(R3')C(O)N(R3~)z, -N(R3~)C(O)OR3', -O-C(O)N(R3')2, -N(R3')S02R3',
-SOZN(R3')2 and -S02R3'; R3'is H or -C1_6alkyl; provided that if R3a is -
C(O)R3',
CHC(O)O-R3', CH(CH3)C(O)O-R3'or -C(O)O-R3', then said -C(O)R3', CHC(O)O-
R3', CH(CH3)C(O)O-R3'or -C(O)O-R3' are unsubstituted.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a and R3a is phenyl, hydroxyphenyl,
azetidinyl,
napthyl, C~_6alkyl, CZ_6alkenyl, CZ_balkynl, dihydroquinolinonyl,
hydroquinolinonyl,
quinolinyl, dihydroisoquinolinonyl, hydroisoquinolinonyl, isoquinolinyl,
dihydroquinazolinonyl, hydroquinazolinonyl, quinazolinyl,
dihydroquinoxalinonyl,
hydroquinoxalinonyl, quinoxalinyl, benzimidazolyl, indazolyl,
dihydrobenzimidazolonyl, hydrobenzimidazolonyl, benzimidazolinyl, dihydro-
benzthiazolonyl, hydrobenzthiazolonyl, benzthiazolyl, dihydrobenzoxazolyl,
benzotriazolyl, dihydrobenzothiophenonyl, hydrobenzothiophenonyl,
benzothienyl,
dihydrobenzofuranonyl, hydrobenzofuranonyl, benzofuranyl, benzdioxolanyl,
dihydroindolonyl, hydroindolonyl, indolyl, indolizinyl, isoindolyl, indolinyl,
indazolyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, furanyl, thienyl, pyrrolyl,
pyrrolinyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
pyrrolidinyl, imidazolyl, imidazolinyl, imidazolidinyl, pyridyl, purinyl,
carbazolyl,
pyrimidinyl, piperidinyl, triazolopyrimidinyl, tetrahydropyrazolopyridinyl,
piperazinyl or morpholino; optionally substituted as provided in the first
embodiment
of the first aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a arid R3a is phenyl, napthyl, indazolyl,
benzimidazolinyl, dihydrobenzoxazolyl, benzotriazolyl, benzothienyl,
benzdioxolanyl, dihydroindolonyl, indolyl, furanyl, thienyl, pyridyl, purinyl,
10 carbazolyl, piperidinyl, triazolopyrimidinyl, tetrahydropyrazolopyridinyl;
optionally
substituted as provided in the first embodiment of the first aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a and R3a is dihydro-benzthiazolonyl,
15 hydrobenzthiazolonyl, benzthiazolyl, dihydrobenzothiophenonyl,
hydrobenzothiophenonyl, benzothienyl, dihydrobenzofuranonyl,
hydrobenzofuranonyl, benzofuranyl, dihydroindolonyl, hydroindolonyl, indolyl,
indolizinyl, isoindolyl, indolinyl or indazolyl; optionally substituted as
provided in
the first embodiment of the first aspect.
20 According to another embodiment of the first aspect of the present
invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a arid R3a is dihydrobenzoxazolyl,
benzotriazolyl,
indolyl, halonitrophenyl, halopyrimidine, halopurinyl, C,_3alkyl-
nitroaminopyrimidine, triazolopyrimidinyl, pyridyl, indazolyl, phenyl or
25 benzdioxolanyl; optionally substituted as provided in the first embodiment
of the first
aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3a and R3a is naphthyl, phenyl-O-phenyl, or
thienyl;
optionally substituted as provided in the first embodiment of the first
aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3b.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
26
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3b and R3b 1S
1 H-Indol-5-yl
HN
TY
/
~5
1 H-Indazol-5-yl
HN-N
Ty
/
~5
,~"., .
1 H-Benzotriazol-5-yl
HN-N
TY / vN
~5
1,3-Dihydro-indol-2-on-5-yl
0
HN
TY
~5
'"''
3 H-B enzooxazol-2-on-6-yl
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
27
O
HN-
Tv O
~6
1,3-Dihydro-benzoimidazol-2-on-5-yl
0
HN
TY NH
~5
1-Methyl-1,3-dihydro-benzoimidazol-2-on-6-yl
0
HN
Tv N -'
~6
3,4-Dihydro-1 H-quinolin-2-on-6-yl
0
HN
TY
6/
1~
1,4-Dihydro-benzo [d] [ 1, 3 ] oxazin-2-on-6-yl
0
HN "O
TY
6/
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
28
3,4-Dihydro-1 H-quinazolin-2-on-6-yl
0
HN_ -NH
Ty
6/
3-Methyl-3,4-dihydro-1 H-quinazolin-2-on-6-yl
0
HN~N~
Ty
6/
; or
4H-Benzo[1,4]oxazin-3-on-7-yl
0
HN' l
TY ~ IO
wherein Ty is H, C»alkyl, F, C1, Br or nitrite.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 is R3b and R3b is azetidinyl, C1_6alkyl,
C2_6alkenyl, C2_
6alkynl, dihydroquinolinonyl, hydroquinolinonyl, dihydroisoquinolinonyl,
hydroisoquinolinonyl, dihydroquinazolinonyl, hydroquinazolinonyl,
quinazolinyl,
dihydroquinoxalinonyl, hydroquinoxalinonyl, quinoxalinyl, benzimidazolyl, 1 H-
indazol-5-yl, dihydrobenzimidazolonyl, hydrobenzimidazolonyl,
benzimidazolinyl,
dihydro-benzthiazolonyl, hydrobenzthiazolonyl, benzthiazolyl,
dihydrobenzothiophenonyl, hydrobenzothiophenonyl, dihydrobenzofuranonyl,
hydrobenzofuranonyl, benzdioxolanyl, dihydrobenzoxazolyl, benzotriazolyl,
dihydroindolonyl, hydroindolonyl, indolizinyl, isoindolyl, indolinyl,
pyrazolyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
29
pyrazolinyl, pyrazolidinyl, furanyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolinyl,
imidazolidinyl, purinyl, carbazolyl, pyrimidinyl, piperidinyl, piperazinyl or
morpholino; optionally substituted as provided in the first embodiment of the
first
aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 1S1Z3b and lZ3b 1S dihydrobenzimidazolonyl,
hydrobenzimidazolonyl, benzimidazolinyl, dihydro-benzthiazolonyl,
hydrobenzthiazolonyl, benzthiazolyl, dihydrobenzothiophenonyl,
hydrobenzothiophenonyl, dihydrobenzofuranonyl, hydrobenzofuranonyl, 1H-
indazol-5-yl, benzdioxolanyl, dihydrobenzoxazolyl, benzotriazolyl,
dihydroindolonyl, hydroindolonyl, indolizinyl, isoindolyl, indolinyl,
pyrazolyl,
pyrazolinyl, pyrazolidinyl, furanyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolinyl,
imidazolidinyl, purinyl, carbazolyl, pyrimidinyl, piperidinyl, piperazinyl or
1 S morpholino; optionally substituted as provided in the first embodiment of
the first
aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 isR3b and R3b is azetidinyl, C1_6alkyl,
C2_6alkenyl, CZ_
6alkynl, dihydroquinolinonyl, hydroquinolinonyl, dihydroisoquinolinonyl,
hydroisoquinolinonyl, dihydroquinazolinonyl, hydroquinazolinonyl,
quinazolinyl,
dihydroquinoxalinonyl, hydroquinoxalinonyl, quinoxalinyl, benzimidazolyl, 1H-
indazol-5-yl, dihydrobenzimidazolonyl, hydrobenzimidazolonyl,
benzimidazolinyl,
dihydro-benzthiazolonyl, hydrobenzthiazolonyl, benzthiazolyl,
dihydrobenzothiophenonyl, hydrobenzothiophenonyl, dihydrobenzofuranonyl,
hydrobenzofuranonyl, benzdioxolanyl, dihydrobenzoxazolyl, benzotriazolyl,
purinyl,
carbazolyl, pyrimidinyl, piperidinyl, piperazinyl or morpholino; optionally
substituted as provided in the first embodiment of the first aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein R3 isR3b and R3b is azetidinyl, C ~ _6alkyl,
CZ_balkenyl, CZ_
6alkynl, dihydroquinolinonyl, hydroquinolinonyl, dihydroisoquinolinonyl,
hydroisoquinolinonyl, dihydroquinazolinonyl, hydroquinazolinonyl,
quinazolinyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
dihydroquinoxalinonyl, hydroquinoxalinonyl, quinoxalinyl, benzimidazolyl,
benzdioxolanyl, dihydrobenzoxazolyl, benzotriazolyl, dihydroindolonyl,
hydroindolonyl, 1 H-indazol-5-yl, indolizinyl, isoindolyl, indolinyl,
pyrazolyl,
pyrazolinyl, pyrazolidinyl, furanyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
imidazolinyl,
5 imidazolidinyl, purinyl, carbazolyl, pyrimidinyl, piperidinyl, piperazinyl
or
morpholino; optionally substituted as provided in the first embodiment of the
first
aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
10 present invention wherein R3 is R3b and R3b is benzdioxolanyl,
dihydrobenzoxazolyl,
benzotriazolyl, purinyl, carbazolyl; optionally substituted as provided in the
first
embodiment of the first aspect.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
15 present invention wherein R3 is R3b and R36 is dihydrobenzoxazolyl,
benzotriazolyl,
indolyl, halonitrophenyl, halopyrimidinyl, halopurinyl, Cl_3alkyl-
nitroaminopyrimidinyl, triazolopyrimidinyl, pyridyl, 1H-indazol-5-yl, phenyl
or
benzdioxolanyl.
According to another embodiment of the first aspect of the present invention
20 are provided compounds according to the first embodiment of the first
aspect of the
present invention wherein Q is Q' and wherein said compounds have an absolute
configuration of R.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
25 present invention wherein Q is Q' and wherein said compounds have an
absolute
configuration of S.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Q is Q" and wherein said compounds have an absolute
30 configuration of R.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
31
present invention wherein Q is Q" and wherein said compounds have an absolute
configuration of S.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein m and n are each 1.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein D is O.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A is C.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A is CH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A is N.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein E is N.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein E is CH.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein E is C.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein said compounds exhibit as described herein a CGRP
Binding ICSO of less than 10 nM.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
32
present invention wherein said compounds exhibit as described herein a CGRP
Binding ICSO of less than 100 nM.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein said compounds exhibit as described herein a CGRP
Binding ICSQ of less than 1000 nM.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 1; and G, J and E together form A" or AY.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 1; and G, J and E together form A".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 1; and G, J and E together form AY.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A" is a fused heterocycle having two fused rings
with 5 to
7 members in each of said rings, said heterocycle containing one to four of
the same
or different heteroatoms selected from the group consisting of O, N and S; and
optionally containing 1 or 2 carbonyls wherein the carbon atom of said
carbonyl is a
member of said fused heterocycle.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A" is a fused heterocycle having two fused rings
with 5 to
7 members in each of said rings, said heterocycle containing one to four of
the same
or different heteroatoms selected from the group consisting of O, N and S.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A" is a fused heterocycle having two fused rings
with 5 to
7 members in each of said rings, said heterocycle containing one to four of
the same
or different heteroatoms selected from the group consisting of O, N and S and
wherein A" is substituted with phenyl.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
33
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein A" is a fused heterocycle described herein.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Ay is a 4 to 6 membered heterocycle containing one
to
three heteroatoms selected from the group consisting of O, N and S; and
optionally
containing 1 to 2 carbonyls, wherein the carbon atom of said carbonyl is a
member of
said 4 to 6 membered heterocycle.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein AY is a 4 to 6 membered heterocycle containing one
to
three heteroatoms selected from the group consisting of O, N and S.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Ay is a 4 to 6 membered heterocycle containing one
to
three heteroatoms selected from the group consisting of O, N and S; and
optionally
containing 1 to 2 carbonyls, wherein the carbon atom of said carbonyl is a
member of
said 4 to 6 membered heterocycle; and wherein AY is substituted with phenyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein Ay is a 4 to 6 membered heterocycle described
herein.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA' or GJA".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA'.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
34
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA' and GJA' is A".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA' and GJA' is AY.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA" and GJA" is A".
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are GJA" and GJA" is AY.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydroquinoxalinonyl,
dihydrobenzoxazinyl, hydrobenzoxazinyl, dihydrobenzoxazinonyl,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
dihydrobenzimidazolonyl, dihydrobenzimidazolyl, dihydro-benzthiazolonyl,
dihydrobenzthiazolyl, dihydrobenzothiophenonyl, dihydrobenzofuranonyl,
dihydroindolonyl, indolinyl, pyrazolinyl, pyrazolidinyl, pyrrolinyl,
pyrrolidinyl,
imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl and morpholino; wherein
said
5 heterocycle is optionally substituted with C,~alkyl, Cl~alkoxy,
Cl~haloalkyl, cyano,
C3_~cycloalkyl, phenyl, halophenyl, furanyl, pyrrolyl, pyrrolinyl,
pyrrolidinyl,
imidazolyl, imidazolinyl, imidazolidinyl, pyrazolyl, pyrazolinyl,
pyrazolidinyl,
pyridyl, pyrimidinyl, piperidinyl, piperazinyl or morpholino.
According to another embodiment of the first aspect of the present invention
10 are provided compounds according to the first embodiment of the first
aspect of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
15 dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydroquinoxalinonyl,
dihydrobenzoxazinyl, hydrobenzoxazinyl, dihydrobenzoxazinonyl,
dihydrobenzimidazolonyl, dihydrobenzimidazolyl, dihydro-benzthiazolonyl,
dihydrobenzthiazolyl, dihydrobenzothiophenonyl, dihydrobenzofuranonyl,
dihydroindolonyl, indolinyl, pyrazolinyl, pyrazolidinyl, pyrrolinyl,
pyrrolidinyl,
20 imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl and morpholino;
wherein said
heterocycle is optionally substituted with Cl~alkyl, Cl~alkoxy, Cl~haloalkyl,
cyano,
C3_~cycloalkyl, phenyl, halophenyl, furanyl, pyrazolyl, pyrazolinyl,
pyrazolidinyl,
pyridyl, pyrimidinyl, piperidinyl, piperazinyl or morpholino.
According to another embodiment of the first aspect of the present invention
25 are provided compounds according to the first embodiment of the first
aspect of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
30 dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydrobenzofuranonyl,
dihydroindolonyl, indolinyl, pyrazolinyl, pyrazolidinyl, pyrrolinyl,
pyrrolidinyl,
imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl and morpholino; wherein
said
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
36
heterocycle is optionally substituted with Cl~alkyl, C»alkoxy, Cl~haloalkyl,
cyano,
C3_~cycloalkyl, phenyl, halophenyl, piperazinyl or morpholino.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydroquinoxalinonyl,
dihydrobenzoxazinyl, hydrobenzoxazinyl, dihydrobenzoxazinonyl,
dihydrobenzimidazolonyl, dihydrobenzimidazolyl, dihydro-benzthiazolonyl,
dihydrobenzthiazolyl, dihydrobenzothiophenonyl, dihydrobenzofuranonyl,
dihydroindolonyl, indolinyl, pyrazolinyl, pyrazolidinyl, pyrrolinyl,
pyrrolidinyl,
imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl and morpholino.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydroquinoxalinonyl,
dihydrobenzoxazinyl, hydrobenzoxazinyl and dihydrobenzoxazinonyl.
According to another embodiment of the first aspect of the present invention
are provided compounds according to the first embodiment of the first aspect
of the
present invention wherein p is 0 such that G and J are each attached to A,
then G, J
and A together form a spirocyclic ring system with said rings of said system
containing A and wherein G, J and A together are form a heterocycle selected
from
the group consisting of imidazolinonyl, imidazolidinonyl, dihydroquinolinonyl,
dihydroisoquinolinonyl, dihydroquinazolinonyl, dihydroquinoxalinonyl and
dihydrobenzoxazinyl.
According to various embodiments of a second aspect of the present invention
are provided pharmaceutical compositions comprising compounds of Formula (I)
as
defined herein.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
37
According to various embodiments of a third aspect of the present invention
are provided methods of treating inflammation (particularly neurogenic
inflammation), headache (particularly migraine), pain, thermal injury,
circulatory
shock, diabetes, Reynaud's syndrome, peripheral arterial insufficiency,
subarachnoid/
cranial hemorrhage, tumor growth, flushing associated with menopause and other
conditions the treatment of which can be effected by the antagonism of the
CGRP
receptor by the administration of pharmaceutical compositions comprising
compounds of Formula (I) as defined herein.
According to various embodiments of a fourth aspect of the present invention
are uses of the compounds of the present invention selected from the group
consisting
of (a) immune regulation in gut mucosa (b) protective effect against cardiac
anaphylactic injury (c) stimulating or preventing interleukin-lb(IL-lb)-
stimulation of
bone resorption (d) modulating expression of NK1 receptors in spinal neurons
and
(e) airway inflammatory diseases and chronic obstructive pulmonary disease
including asthma. See (a) Calcitonin Receptor-Like Receptor Is Expressed on
Gastrointestinal Immune Cells. Hagner, Stefanie; Knauer, Jens; Haberberger,
Rainer;
Goeke, Burkhard; Voigt, Karlheinz; McGregor, Gerard Patrick. Institute of
Physiology, Philipps University, Marburg, Germany. Digestion (2002), 66(4),
197-
203; (b) Protective effects of calcitonin gene-related peptide-mediated
evodiamine on
guinea-pig cardiac anaphylaxis. Rang, Wei-Qing; Du, Yan-Hua; Hu, Chang-Ping; ,
Ye, Feng; Tan, Gui-Shan; Deng, Han-Wu; Li, Yuan-Jian. School of Pharmaceutical
Sciences, Department of Pharmacology, Central South University, Xiang-Ya Road
88, Changsha, Hunan, Naunyn-Schmiedeberg's Archives of Pharmacology (2003),
367(3), 306-311; (c) The experimental study on the effect calcitonin gene-
related
peptide on bone resorption mediated by interleukin-1. Liars, Kai; Du,
Jingyuan; Rao,
Zhenyu; Luo, Huaican. Department of Orthopedics, Xiehe Hospital, Tongji
Medical
College, Huazhong University of Science and Technology, Wuhan, Peop. Rep.
China. Journal of Tongji Medical University (2001 ), 21 (4), 304-307, (d)
Calcitonin
gene-related Peptide regulates expression of neurokininl receptors by rat
spinal
neurons. Seybold VS, McCarson KE, Mermelstein PG, Groth RD, Abrahams LG.
J. Neurosci. 2003 23 (5): 1816-1824. epartment of Neuroscience, University of
Minnesota, Minneapolis, Minnesota 55455, and Department of Pharmacology,
Toxicology, and Therapeutics, University of Kansas Medical Center, Kansas
City,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
38
Kansas 66160 (e) Attenuation of antigen-induced airway hyperresponsiveness W
CGRP-deficient mice. Aoki-Nagase, Tomoko; Nagase, Takahide; Oh-Hashi, Yoshio;
Shindo, Takayuki; Kurihara, Yukiko; Yamaguchi, Yasuhiro; Yamamoto, Hiroshi;
Tomita, Tetsuji; Ohga, Eijiro; Nagai, Ryozo; Kurihara, Hiroki; Ouchi,
Yasuyoshi.
Department of Geriatric Medicine, Graduate School of Medicine, University of
Tokyo, Tokyo, Japan. American Journal of Physiology (2002), 283(S,Pt. 1), L963-
L970; (~ Calcitonin gene-related peptide as inflammatory mediator. Springer,
Jochen; Geppetti, Pierangelo; Fischer, Axel; Groneberg, David A. Charite
Campus-
Virchow, Department of Pediatric Pneumology and Immunology, Division of
Allergy
Research, Humboldt-University Berlin, Berlin, Germany. Pulinonary Pharmacology
& Therapeutics (2003), 16(3), 121-130; and (g) Pharmacological targets for the
inhibition of neurogenic inflammation. Helyes, Zsuzsanna; Pinter, Erika;
Nemeth,
Jozsef; Szolcsanyi, Janos. Department of Pharmacology and Pharmacotherapy,
Faculty of Medicine, University of Pecs, Pecs, Hung. Current Medicinal
Chemistry:
Anti-Inflammatory & Anti-Allergy Agents (2003), 2(2), 191-218 all incorporated
by
reference herein.
According to various embodiments of a fifth aspect of the present invention
are provided combinations of the compounds of the present invention with one
or
more agents selected from the group consisting of COX-2 inhibitors, NSAIDS,
aspirin, acetaminophen, triptans, ergotamine and caffeine for the treatment of
migraine.
According to a sixth aspect of the present invention are provided in vivo non-
terminal methods of identifying anti-migraine compounds.
According to the first embodiment of the sixth aspect of the present invention
is provided an in vivo non-terminal method of identifying anti-migraine
compounds
comprising administering a CGRP-receptor agonist to a mammal in an amount
capable of inducing an increase in blood flow, followed by administering a
test
compound in an amount capable of reversing said CGRP-induced increase in blood
flow, wherein said mammal is a transgenic mammal with humanized RAMP 1 having
Trp74 or a mammal endogenously expressing RAMP1 having Trp74.
According to another embodiment of the sixth aspect of the present invention
is provided an in vivo non-terminal method of identifying anti-migraine
compounds
comprising administering to a mammal a test compound prior to the delivery of
a
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
39
CGRP-receptor agonist wherein said CGRP-receptor agonist is administered in an
amount capable of inducing an increase in blood flow and wherein said test
compound is administered in an amount capable of suppressing said CGRP-induced
increase in blood flow, wherein said mammal is a transgenic mammal with
humanized RAMP1 having Trp74 or a mammal endogenously expressing RAMP1
having Trp74.
According to another embodiment of the sixth aspect of the present invention
is provided an in vivo non-terminal method of identifying anti-migraine
compounds
comprising administering to a mammal a CGRP-receptor agonist in an amount
capable of inducing an increase in peripheral artery diameter, followed by
administering a test compound in an amount capable of reversing said CGRP-
induced
increase in peripheral artery diameter, wherein said mammal is a transgenic
mammal
with humanized RAMP 1 having Trp74 or a mammal endogenously expressing
RAMP1 having Trp74.
According to another embodiment of the sixth aspect of the present invention
is provided an in vivo non-terminal method of identifying anti-migraine
compounds
comprising administering to a mammal a test compound prior to the delivery of
a
CGRP-receptor agonist wherein said CGRP-receptor agonist is administered in an
amount capable of inducing an increase in peripheral artery diameter and
wherein
said test compound is administered in an amount capable of suppressing said
CGRP-
induced increase in peripheral artery diameter, wherein said mammal is a
transgenic
mammal with humanized RAMP1 having Trp74 or a mammal endogenously
expressing RAMP 1 having Trp74.
According to other embodiments of the sixth aspect of the present invention
are provided in vivo non-terminal methods of identifying anti-migraine
compounds
as described herein wherein said blood flow is facial blood flow.
According to other embodiments of the sixth aspect of the present invention
are provided in vivo non-terminal methods of identifying anti-migraine
compounds
as described herein wherein said mammal endogenously expressing RAMP 1 having
Trp74 is a non-human primate.
According to other embodiments of the sixth aspect of the present invention
are provided in vivo non-terminal methods of identifying anti-migraine
compounds
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
as described herein wherein said mammal endogenously expressing RAMP 1 having
Trp74 is man.
According to other embodiments of the sixth aspect of the present invention
are provided in vivo non-terminal methods of identifying anti-migraine
compounds
as described herein wherein said mammal endogenously expressing RAMP 1 having
Trp74 is a non-human primate and said non-human primate is a marmoset.
According to other embodiments of the sixth aspect of the present invention
are provided in vivo non-terminal methods of identifying anti-migraine
compounds
as described herein wherein said anti-migraine compounds are CGRP-receptor
10 antagonists.
Other embodiments of the present invention may comprise a suitable
combination of two or more of the embodiments and/or aspects disclosed herein.
Yet other embodiments of the present invention may comprise a suitable
subset of an embodiment and/or aspect disclosed herein.
15 Still yet other embodiments and aspects of the invention will be apparent
according to the description provided below.
Brief Description of the Fi ogres
Figure 1. Schild Analysis.
20 Dose response of CGRP stimulated cAMP production in the absence (filled
squares)
and presence (all others) of increasing concentrations (left-to-right) of the
CGRP
antagonist Example 2. Inset is Schild plot of log dose ratio minus 1 (Y-axis)
against
log concentration of the antagonist Example 2 (X-axis): Slope = 0.94, Kh =
0.16 nM.
25 Figure 2. Direct Validation of Facial Blood Flow as Surrogate for
Intracranial Artery
Dilation in the Rat.
Intravenous delivery of i.v. haCGRP induces comparable percent increases (100-
120% of baseline) in rat middle meningeal artery diameter and rat facial blood
flow
(left and right striped bars, respectively). Pretreatment with the peptide
antagonist
30 CGRP(8-37) produces a 50% inhibition of subsequent i.v. haCGRP
administration
for both measures (filled bars). Intracranial artery diameter and facial blood
flow
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
41
were measured concurrently in each animal (n = 5 rats). Data are mean ~ sem *
p <
0.05, ** p < 0.01 vs corresponding haCGRP alone.
Figure 3. Dose-Response for haCGRP in Non-Human-Primate Laser Doppler Facial
Blood Flow.
Delivery of haCGRP induces dose-dependent increase in laser Doppler facial
blood
flow in non-human primates (e.g., common marmoset). Animals (n= 6) received
increasing doses of haCGRP at 30 min intervals. Data are peak % change from
baseline ~ sem, with each animal serving as its own control.
Figure 4. Inhibitition of CGRP-Induced Changes in Non-Human Primate Facial
Blood Flow.
The novel CGRP antagonist Example 2 (filled bars) delivered prior to haCGRP
(striped bar), dose-dependently inhibits the CGRP-induced increase in laser
Doppler
facial blood flow. Vehicle (open bar) was without effect. Data are mean ~ sem
(n =
5-6 primates per group). *p < 0.05 compared CGRP alone.
Figure 5. Effect of CGRP Antagonist on Non-Human Primate Blood Pressure.
In contrast to the dose-dependent inhibition of primate facial blood flow (see
Figure
4.), Example 2 has negligible effect on blood pressure (parallel studies in
separate
animals, n=6). Animals received repeat doses of Example 2 at 20 min intervals.
BP
data are mean t sem over 20 min period measured by arm cuff
Detailed Description of the Invention
The description of the invention herein should be construed in congruity with
the laws and principals of chemical bonding. For example, it may be necessary
to
remove a hydrogen atom in order accommodate a substitutent at any given
location.
As used herein, "heterocyclic" or "heterocycle" includes cyclic moieties
containing one or more heteroatoms, (e.g., O, N or S) said heterocycles
include those
that are aromatic and those that are not, i.e., "alicyclic", unless otherwise
specified.
As used herein, the term "fused bicyclic system" when describing for example
a 5.6-fused bicyclic system containing 1 to 4 nitrogen atoms includes aromatic
and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
42
alicyclic systems, e.g. indolizine, indole, isoindole, 3H-indole, indoline,
indazole or
benzimidazole.
If a substitutent is named generically, then any and all species of that genus
comprise that aspect of the invention. For example, a substituent generically
named
as "pyrrolonyl" (the radical of "pyrrolone", a pyrrole having a carbonyl)
includes
pyrrol-2-onyls wherein the carbonyl is adjacent to the nitrogen and pyrrol-3-
onyls
wherein the carbonyl and nitrogen have an intervening methylene.
Similarly, the present invention comprises that a substituent may be attached
at any and all suitable points of attachement on said substituent unless
otherwise
specified.
However, it is also understood that the compounds encompassed by the
present invention are those that are chemically stable, i.e., heteroalicyclic
substituents
of the present invention should not be attached in such a way that a
heteroatom in
said heteroalicyclic substituent is alpha to a point of attachment wherein
said point of
attachment is also a heteroatom.
An embodiment or aspect which depends from another embodiment or aspect,
will describe only the variables having values or provisos that differ from
the
embodiment or aspect from which it depends. If for example a dependent
embodiment only addresses RZ, then the variables and provisos not related to
RZ
should reflect that of the embodiment from which it depends.
If a variable is quantified with a value of zero, then a bond attaching said
variable should no longer be represented.
As used herein, "alkylene" means a divalent alkane, i.e., an alkane having two
hydrogen atoms removed from said alkane (said hydrogen removed from two
different carbon atoms when said alkane contains more than one carbon atom),
e.g.,
-CHZCHZCHZ- .
As used herein, "alkylidene" means an alkane having two hydrogen atoms
removed from one carbon atom in said alkane, e.g. ,
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
43
It should be understood that the alternating double bond designations in the
six-membered ring of the 5,6-membered fused structure represented in Formula
(I)
are relative and represent the delocalized ~ orbital electrons of said ring.
As used herein, "aryl" or "ar-" includes phenyl or napthyl.
As used herein, "heterocyclic" or "heterocyclo" includes both heteroaryl and
heteroalicyclic.
As used herein, "halo" or "halogen" includes fluoro, chloro, bromo and iodo
and further means one or more of the same or different halogens may be
substituted
on a respective moiety.
Unless specificied otherwise, acyclic hydrocarbons such as alkyl, alkoxy,
alkenyl and alkynyl may be branched or straight chained.
It is to be understood that the present invention may include any and all
possible stereoisomers, geometric isomers, diastereoisomers, enantiomers,
anomers
and optical isomers, unless a particular description specifies otherwise.
As used herein, "Trp74", means that the 74th residue in RAMP 1 is tryptophan
(Mallee et al. JBiol Chem 2002, 277, 14294-8) incorporated by reference
herein.
As used herein "anti-migraine compound" includes any compound, peptide or
peptide fragment (modified or unmodified) capable of reversing or attenuating
CGRP-receptor mediated vasodilation, (e.g., CGRP-receptor antagonists).
As used herein "test compound" includes any compound, peptide or peptide
fragment (modified or unmodified) being tested to determine if it is capable
of
reversing or attenuating CGRP-receptor mediated vasodilation, (e.g., putative
CGRP-
receptor antagonists).
As used herein, "CGRP-receptor agonist" includes any compound, peptide or
peptide fragment (modified or unmodified) capable of inducing CGRP-receptor
mediated vasodilation particularly by example aCGRP or (3CGRP; other members
of
the calcitonin family, e.g, adrenomedullin; N-terminal CGRP fragments, e.g,
CGRP(1-12) CGRP(1-15) and CGRP(1-22); C-terminal amide (NH2) versions of
CGRP e.g., CGRP(1-8+NH2), CGRP(1-13+NH2) or CGRP(1-14+NH2); and non-
naturally occurring CGRP analogues e.g., [Alai 1/~(CH2NH)Cys2]hCGRP which
contains a pseudopeptide bond between Alal and Cyst. See Maggi CA, Rovero P,
Giuliani S, Evangelista S, Regoli D, Meli A. Biological activity of N-terminal
fragments of calcitonin gene-related peptide. Eur J Pharmacol. 1990 Apr
10;179(1-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
44
2):217-9; Qing X, Wimalawansa SJ, Keith IM. Specific N-terminal CGRP fragments
mitigate chronic hypoxic pulmonary hypertension in rats. Regul Pept. 2003 Jan
31;110(2):93-9; and Dennis T, Fournier A, St Pierre S, Quirion R. Structure-
activity
profile of calcitonin gene-related peptide in peripheral and brain tissues.
Evidence for
receptor multiplicity. J Pharmacol Exp Ther. 1989 Nov;251 (2):718-25
incorporated
by reference herein.
The compounds of this invention may exist in the form of pharmaceutically
acceptable salts. Such salts may include addition salts with inorganic acids
such as,
for example, hydrochloric acid and sulfuric acid; and with organic acids such
as, for
example, acetic acid, citric acid, methanesulfonic acid, toluenesulfonic acid,
tartaric
acid and malefic acid. Further, in case the compounds of this invention
contain an
acidic group, the acidic group may exist in the form of alkali metal salts
such as, for
example, a potassium salt and a sodium salt; alkaline earth metal salts such
as, for
example, a magnesium salt and a calcium salt; and salts with organic bases
such as a
triethylammonium salt and an arginine salt. In the case of a sublingual
formulation a
saccharin salt or maleate salt may be of particular benefit. The compounds of
the
present invention may be hydrated or non-hydrated.
The compounds of this invention can be administered in such oral dosage
forms as tablets, capsules (each of which includes sustained release or timed
release
formulations), pills, powders, granules, elixirs, tinctures, suspensions,
syrups and
emulsions. The compounds of this invention may also be administered
intravenously, intraperitoneally, subcutaneously, or intramuscularly, all
using dosage
forms well known to those skilled in the pharmaceutical arts. The compounds
can be
administered alone, but generally will be administered with a pharmaceutical
carrier
selected upon the basis of the chosen route of administration and standard
pharmaceutical practice. Compounds of this invention can also be administered
in
intranasal form by topical use of suitable intranasal vehicles, or by
transdermal
routes, using transdermal skin patches. When compounds of this invention are
administered transdermally the dosage will be continuous throughout the dosage
regimen.
While dosing from 0.01 mg/kg to 30 mg/kg is envisaged for compounds of
the present invention, the dosage and dosage regimen and scheduling of a
compounds
of the present invention must in each case be carefully adjusted, utilizing
sound
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
professional judgment and considering the age, weight and condition of the
recipient,
the route of administration and the nature and extent of the disease
condition. In
accordance with good clinical practice, it is preferred to administer the
instant
compounds at a concentration level which will produce effective beneficial
effects
5 without causing any harmful or untoward side effects.
S'mthesis
Compounds of the present invention may be synthesized according to the
general schemas provided below. Variables provided in the schema below are
10 defined in accordance with the description of compounds of the above
Formula
unless otherwise specified. The compounds of the present invention may be
prepared
according to Scheme 1 or Scheme 2. It may also be possible to use variations
of said
schemes to prepare the compounds of the present inventions, said variations
known
to those of ordinary skill in the art.
Scheme 1. Synthesis of Formula I Compounds
Q R~ Q
HO~N.PG R~ N~N.PG
O H O H
II I~
RZ Q D R2 Q
~ m
WN N- \ ~ - ' R1~N NH2
R
p H ~--~/~ ~. J O
1 IV
The synthesis described in Scheme 1 begins with a compound of Formula II,
which is an amino acid with a protected amino terminus. Common amino
protecting
groups (PG) include BOC, CBZ, and FMOC and their addition and removal are well
known in the field. The carboxylic acid moiety of a Formula II compound is
coupled
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
46
with an amine of formula HNR~RZ using standard peptide coupling reagents to
form
an amide of Formula III. The amino protecting group is removed resulting in a
Formula IV compound. This compound is then coupled with an amine of Formula V
(see below) in a mixed urea or urea isostere reaction, generating a Formula I
compound. Mixed urea formation is conveniently carried using phosgene,
disuccinimidyl carbonate, carbonyl diimidazole or other equivalents. Formation
of
urea isosteres, such as cyanoguanidines and sulfonylguanidines, are known in
the
literature.
Scheme 2. Synthesis of Formula I Compounds
Q Q D
MeO~ Me0
~z ~ N
O O H ~-~, ~J
V
VI
R\ Q D Q D
N
RW N~ ~ ~ HO N
O H ~--~, ~ J O H ~ ~ J
I
The synthesis described by Scheme 2 begins with. a compound of Formula V,
which is an amino acid with a protected carboxylate terminus. The protection
is
generally a methlyl ester, but other protecting groups such as ethyl, t-butyl,
and
benzyl esters may also be used. The Formula V compound is coupled with an
amine
of Formula VIII (see below) in a mixed urea or urea isostere reaction, as
above, to
generate a Formula VI compound. The Formula VI compound is converted to a free
acid compound of Formula VII which is then coupled with an amine of Formula
HNR1R2 to generate a Formula I compound.
Scheme 3. Synthesis of Formula I Compounds
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
47
HO IJ u
Q N~ ~ - Ra~O Q N~ ~ -
~H ~ ~~ ~H ~ ~~
~n ~ J O ~n
VII
The synthesis described by Scheme 3 begins with a compound of Formula VII
from Scheme 2. The Formula V compound is coupled with an alcohol, R4-OH. Such
ester-forming reactions are well known in the art and can be carried out, for
example,
with carbodiimide coupling agents such as N,N-dicyclohexylcarbodiimide. In
addition, it is often advantageous, especially for esters of secondary and
tertiary
alcohols, to include additives that accelerate acylations such as 4-
dimethylaminopyridne.
Preparation of HNR1R2 and Formula VIII amines
Formula VIII and HNR1R2 amines are commercially available, made by
literature methods or described herein.
m
- VIII
~n ~ J
Preparation of Formula II and Formula V amino acids
Q R3\Q
HO N,PG II Me0 V
H ~NHZ
O O
Formula II and Formula V amino acids may be commercially available or
made as described in Scheme 4
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
48
Scheme 4. Synthesis of Formula II and Formula V Compounds
R3
R3-CHO R40 I N. PG
OMe
IX O~P-OMe O H
R40 N ~ PG XI
O H
X
R3 R3
HO N. PG R40 PG
~N~
O H O H
II XII
R3
R40
NHZ
O
V
The synthesis described in Scheme 4 begins with an aldehyde of Formula IX,
S which is reacted with a glycine phosphonate of Formula X in a Wadsworth-
Emmons
coupling reaction. The compound of Formula X is deprotonated with a base such
as
diazabicycloundecene or tetramethylguanidine or other organic or inorganic
bases
well known in the art. The double bond of the resulting Formula XI compound is
reduced to give compounds of Formula XII. Reduction can be carried out to give
either a racemate or by use of a stereoselective catalyst to give either
enantiomer of
Formula XII. Such reductions can result from transfer hydrogenation from
hydrogen
donors such as formic acid or cyclohexadiene, or hydrogenation using gaseous
hydrogen, both in the presence of a suitable catalyst. Compounds of Formula II
are
prepared by acid or base hydrolysis of the ester. Compounds of Formula V are
prepared by removal of the protecting group (PG) using methods well known in
the
art.
Other amino acid derivatives of Formula XII may be prepared as shown in
Scheme S.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
49
Scheme 5. Synthesis of Formula XII
R3H R3
4
R O N. PG XIV R40 N, PG
O H O H
XIII
XII
Where, for the purposes of Scheme 5, compounds of Formula XIV are
nucleophilic compounds such as amines or alcohols that are able to participate
in a
Michael Reaction with a compound of Formula XIII as shown.
Other compounds of Formula I may be prepared according to Scheme 6 or
Scheme 7. It may also be possible to use variations of said schemes to prepare
the
compounds of the present inventions, said variations known to those of
ordinary skill
in the art.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
Scheme 6. Synthesis of Formula I Compounds
3 ~ 3 3
R ECHO R ~COOH~ R ~COOH R O
IX XV Bn ~ ~O
O
XVI
RZ O
R3 O R~.I'1H R3 O R~~O
Rz -- '~ '~-
\N~O~ HO O~ Bn N O
R~' O O '-O
X~ XV1II
XVII
~~m G
~A_E~
~ 3
1/n ~ R
R3 O VIII RZ O ~--~", ~C',
R2~ N N A-E
R~ N O OH R~ O ~ n
XX
The synthesis described in Scheme 6 begins with commercially available or
synthesized aldehydes. The two-carbon homologation and double-bond reduction
5 which are well-known in the literature and lead to compounds of Formula XV.
Some
Formula XV compounds are also commercially available and others may be
prepared
by other methods well known in the art. Preparation of compounds of Formula
XVI
and XVII are known in the literature as substrates and products of the Evans
chiral
asymmetric synthesis. Hydrolysis leads to compounds of Formula XVIII. As with
10 compounds of Formula VII in Scheme 2, these carboxylic acids can react with
amines of formula RIRZNH to afford compounds of Formula XIX using well known
amide coupling protocols. Hydrolysis of the tert-butyl ester leads to
compounds of
Formula XX, which can be further coupled with compounds of Formula VIII to
afford Formula I compounds.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
51
Scheme 7. Synthesis of Formula I Compounds
R3 R3
O O
R yH0 O OOH ~ O OH
IX O~ OMe OMe
OMe XXI XXII
OMe
~m G
HN A-
l/ n
V1II
R3 R3
HO O N/ 'xA-I p - Me0 O N~A-
Q ~n Q 1/n
XXN XXIII
R2 ~R4 OH
R,NH
R2 Rs R3
\N O ~--~r" R4 O ~--~m G
R~' N A-~ ~O N A-E=p
O \/ n ~ O 1/ n
Scheme 7 also starts with commercially available or synthesized aldehydes.
These are reacted with dimethyl succinate in the presence of bases to give
compounds of Formula XXI. The double bond of the Formula XXI compound is
reduced to give compounds of Formula XXII. Reduction can be carried out to
give
either a racemate or by use of a stereoselective catalyst to give either
enantiomer of
Formula XXII. Such reductions can result from transfer hydrogenation from
hydrogen donors such as formic acid or cyclohexadiene, or hydrogenation using
gaseous hydrogen, both in the presence of a suitable catalyst. Amide coupling
with
amines of Formula VIII lead to compounds of Formula XXIII using well known
amide synthesis protocols. Hydrolysis of methyl ester leads to Formula XXIV
compounds, which are further coupleded with various amines or alcohols to give
1 S amides of Formula I and esters of Formula I, respectively.
Compounds of Formula I may also be prepared according to Scheme 8.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
52
Scheme 8. Synthesis of Formula I Compounds
O~NH O
O~ NH O '~ I O
O ~N~ A-
OH O tln
O XXV
O RZ ~O
RZO~NH O rlm G ~.NH HO~NH O ~m
/~C~R
~N-~N A-~ ~N~ A-
~ O ~.I
R O XXVII '/n ~ '/n
XXVI
R3
R2 NHZ O ~m R\ \NH O ~m G
R~,N~N/ lxp,-~ R~,N~N/ l~A-f=p
O l/ n ~ O l! n
XXVIII
The synthesis described in Scheme 8 begins with a commercially available N-
tert-butyloxycarbonyl-L-aspartic acid benzyl ester. Differently protected
aspartic acid
derivatives may also be used for synthetic convenience. The beta carboxyl
group is
coupled with amines of Formula VIII using standard peptide coupling protocols.
The
alpha-carboxyl protecting group of the Formula XXV compound is removed by
hydrogenolysis giving compounds of Formula XXVI. These are further coupled
with
amines of the formula HNR~RZ to give compounds of formula XXVII. The amino
protecting group is removed by treatment with strong acids such as
trifluoroacetic
acid or hydrogen chloride in organic solvents. The resulting compounds of
Formula
XXVIII are then reacted with a variety of electrophilic reagents to generate
Formula I
compounds. For example, they can be coupled with halo-aromatic compounds using
known methods involving heating at various temperatures or by catalysis with
transition metals such as palladium or copper, either in stoichiometric
amounts or as
catalysts. They can also react with various aldehydes or ketones under
reductive
alkylation conditions, well described in the art. They can also react with
isocyanates,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
53
acyl chlorides, or carbamoyl chlorides to generate urea, amide or carbamate
derivatives, respectively. It is understood that the sequence of the
modifications
described above can be changed depending on the selection of protecting groups
and
the order of their removal.
Compounds of Formula I may also be prepared according to Scheme 9.
Scheme 9. Synthesis of Formula I Compounds
O
O O + R3 R3 ~.N CIZn~O~ R3~NH O
~H I O I O
OEt NHZ
OEt OEt
XXIX XXX
~m G
HN~ A-E
R ~ R3 ~
3~NH O lln ~ NH O
~--~m ~ V 11I OOH
Et0 O
OEt
n
XXXII XXXI
R2 Rs
R3~NH O ~m RNH RZ ~NH O ~m
HON/ 1~A-~ ' N-~N/ 'xA-E
O ~n ~ R~ O
XXXII1
l~
The synthesis described in Scheme 9 begins with an imine of Formula XXIX,
prepared by condensation of ethyl glyoxalate and amines of formula R3-NH2.
These
are reacted with 2-tert-butoxy-2-oxoethylzinc chloride to give compounds of
Formula XXX. Treatment wit strong acids removes the tert-butyl ester
protecting
I 5 group to give free acids of Formula XXXI which are coupled to amines of
Formula
VIII to yield compounds of Formula XXXII. The ethyl ester is hydrolyzed with a
metal hydroxide salt or aqueous base to give free alpha-acids of Formula
XXXIII.
These, in turn are coupled with amines of the formula HNR1R2 to give compounds
of
formula I.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
54
Ureidoamide Intermediates and Examples
General. 'H- and 13C-NMR spectra were run on a Bruker 500 or 300 MHz
instrument and chemical shifts were reported in ppm (8) with reference to
tetramethylsilane (8 = 0.0). All evaporations were carried out under reduced
pressure. Unless otherwise stated, LC/MS analyses were carried out on a
Shimadzu
instrument using a YMC C 18 column (3 x 50 mm) employing a 2 min linear
gradient
of 0% to100% solvent B in A in a 3 min run. For LC/MS and for Shimadzu
Preparative HPLC system, Solvent A-was:10% methanol/90% water/0.1
trifluoroacetic acid, and solvent B was 90% methanol/10% water/0.1%
trifluoroacetic
acid with a LJV detector set at 220 nm.
1-Benzyl-2',3'-dihydro-2'-oxospiro-[piperidine-4,4'(1'H)-quinazoline
0
HN' 'NH
N
Polyphosphoric acid (113 g ) was heated to 100-110°C and stirred
while 1-
benzyl-piperidin-4-one (9.27 ml, 50 mmol) was added. Immediately afterwards,
phenyl urea (9.55 g, 70. mmol) was added in portions small enough to avoid
excessive foaming. The mixture was heated at 150-160°C overnight. Water
(200
mL) was then added slowly to the mixture which had been allowed to cool to 100-
110°C (at lower temperatures the mixture becomes too viscous to stir).
The resulting
solution was neutralized with l ON NaOH to ca. pH 8, and then extracted wth
chloroform. The organic phase was dried over magnesium sulfate and then
concentrated to give the crude product which was purified by flash column
chromatography on silica gel (6:4 ethyl acetate/hexanes) to give the desired
product
(9.0 g, 58% ). Mass spec.: 308.25 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
2', 3'-dihydro-2'-ox ospiro-[piperidine-4,4'( 1'H)-quinazoline
o
HN"NH
HN J
To a solution of 1-benzyl-2',3'-dihydro-2'-oxospiro-[piperidine-4,4'(1'H)-
quinazoline (1.00 g) in degassed methanol ( 50 ml ) and 6N hydrochloric acid
(2.0
5 ml) was added 10% palladized charcoal (150 mg ). The mixture was shaken on a
Parr apparatus under an atmosphere of hydrogen at 60 psi overnight. LC/MS
showed
incomplete reaction. More 10% palladized charcoal (200 mg) was added, and the
mixture was shaken for 2 more days. At that point, all starting material was
consumed. The mixture was filtered and the filtrate concentrated to give 531
mg of
10 the desired compound (64%). Mass spec.: 218.12 (MH)+.
4-Amino-4-cyano-piperidine-1-carboxylic acid tert-butyl ester
NC NHZ
NJ
o~o~
To a well stirred solution of 4-oxo-piperidine-1-carboxylic acid tert-butyl
ester
15 (9.0 g, 45.3 mmol) in methanol was added ammonium chloride (2.66 g, 49.8
mmol)
at room temperature and stirred for 1 h. Sodium cyanide (2.44 g, 49.8 mmol)
was
added and stirring was continued for additional 16 h. The reaction mixture was
quenched with 5% aqueous sodium hydrogencarbonate (50 mL), diluted with water,
and the methanol removed by rotary evaporation. The cyanoamine was extracted
with
20 methylene chloride (3x 100 mL), dried over sodium sulfate, and the solvents
evaporated to give the desired compound as an oil in 91% yield. 'H-NMR (300
MHz,
CDC13): S 3.95 - 3.90 (m, 1 H), 3.80 - 3.71 (m, 1 H), 3.42 - 3.06 (m, 2 H),
2.04 - 1.94
(m, 1 H), 1.71 - 1.50 (m, 3 H). Mass spec.: 226 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
56
2-Phenyl-1,3,8-triaza-spiro[4.5]dec-1-en-4-one, hydrochloride
HN
0 1N
NJ
H
To a solution of 4-amino-4-cyano-piperidine-1-carboxylic acid tert-butyl ester
(1.0 g, 4.44 mmol) in methylene chloride (30 mL) was added triethylamine (1.24
mL,
8.88 mol), followed by benzoyl chloride (936 mg, 6.66 mmol). After 30 min, 4-
(dimethylamino)pyridine (40 mg, 0.33 mmol) was added and stirring continued
for
additional 12 h. The reaction mixture was then quenched with 1 M sodium
hydroxide
(10 mL), diluted with ethyl acetate (100 mL), and separated. The organic layer
was
washed sequentially with 1M sodium hydroxide (40 mL), aqueous sodium
hydrogencarbonate (50 mL), and brine (50 mL) then dried over sodium sulfate.
The
desired product, 4-benzoylamino-4-cyano-piperidine-1-carboxylic acid tert-
butyl
ester was obtained in 90% yield through crystallization using 30% ethyl
acetate in
hexane as a solvent.
To a solution of 4-benzoylamino-4-cyano-piperidine-1-carboxylic acid tert-
butyl ester (1.3 g, 4 mmol) in ethanol (10 mL) was added 6M sodium hydroxide
(1.5
mL) followed by 30% hydrogen peroxide. The reaction mixture was then refluxed
for
3 h. The reaction mixture was then diluted with water (30 mL), and the ethanol
removed. The residue was diluted with ethyl acetate (100 mL). The organic
phase
was washed with brine (30 mL) and dried over sodium sulfate. The desired
product,
4-oxo-2-phenyl-1,3,8-triaza-spiro[4.5]dec-1-ene-8-carboxylic acid tert-butyl
ester
was obtained in 80% yield through crystallization from 30% ethyl acetate in
hexane.
The tert-butyl ester was then dissolved in methylene chloride (5 mL) and a
saturated
solution of hydrogen chloride in dioxane (25 mL)was added. After 2 h, the
solvent
was removed to give 2-phenyl-1,3,8-triaza-spiro[4.5]dec-1-en-4-one,
hydrochloride
as white powder in 95% yield. 'H-NMR (500 MHz, CD30D): 8 8.23 - 8.21 (m, 2 H),
7.96 - 7.92 (m, 1 H), 7.79 - 7.76 (m, 2 H), 3.68 - 3.64 (m, 3 H), 3.31 - 3.30
(m, 1 H),
2.47 - 2.44 (m, 4 H). Mass spec.: 230 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
57
5-Formyl-indazole-1-carboxylic acid tent-butyl ester
O
~H
N
N
O~O
A methylene chloride (2 mL) solution of di-tert-butyldicarbonate (388 mg,
1.78 mmol) was added dropwise at room temperature to a solution of 1H indazole-
5-
carbaldehyde (273 mg, 1.87 mmol), 4-dimethylaminopyridine (114 mg, 0.94 mmol),
and triethylamine (0.26 mL, 1.87 mmol) in methylene chloride (10 mL). The
resulting bright yellow solution was stirred at room temperature for 16 h.
Solvents
were removed iu vacuo and the residue was subjected to flash chromatography
with
silica gel (25 g) and ethyl acetate/hexanes (1:1) containing 1% triethylamine
as eluent
to afford the title compound as a brownish yellow liquid (414 mg, 90%). ' H-
NMR
(CDC13, 500 MHz) S 10.08 (s, 1H), 8.38 (s, 1H), 8.34 (s, 1H), 8.25 (d, J= 8.5
Hz,
1 H), 8.04 (d, J = 8.8 Hz, 1 H), 1.71 (s, 9H). ' 3CNMR (CDC13, 125 MHz) S
191.8,
149.0, 142.5, 140.6, 133.0, 128.3, 126.4, 125.8, 115.3, 85.7, 27.8.
5-(2-Benzyloxycarbonylamino-2-methoxycarbonyl-vinyl)-indazole-1-carboxylic
acid
tert-butyl ester
N_
O N
OC
HN
O-~ O
/ \ O
H3
A solution of N-(benzyloxycarbonyl)-oc-phosphonoglycine trimethyl ester
(5.50 g, 16.6 mmol) and tetramethylguanidine (1.99 mL, 15.9 mmol) in anhydrous
tetrahydrofuran (50 mL) was stirred at -78°C for 20 min. To this was
added a
solution of 5-formyl-indazole-1-carboxylic acid tent-butyl ester (3.72 g, 15.1
mmol)
in tetrahydrofuran (25 mL) slowly via syringe over 10 min. The reaction
mixture
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
58
was stirred at -78°C for 4 h and then allowed to warm to room
temperature overnight.
The solvent was evaporated and the resulting residue subjected to flash column
chromatography on silica gel (1:2 ethyl acetatelhexane) giving the title
compound as
a white foam (5.77 g, 85%). 1H-NMR (CDCl3, 500 MHz) 8 8.09 (d, J = 9.0 Hz,
1 H), 8.08 (s, 1 H), 7.84 (s, 1 H), 7.67 (d, J = 9.0 Hz, 1 H), 7.47 (s, 1 H),
7.30 (br s, SH),
6.43 (br s, 1H), 5.09 (s, 2H), 3.84 (s, 3H), 1.72 (s, 9H). Mass spec.: 452
(MH)+.
(t)-5-(2-Amino-2-methoxycarbonyl-ethyl)-indazole-1-carboxylic acid tent-butyl
ester
N_
O N
OCH3
HZN
O
A mixture of 5-(2-benzyloxycarbonylamino-2-methoxycarbonyl-vinyl)-
indazole-1-carboxylic acid tert-butyl ester (524 mg, 1.16 mmol) and 10%
palladium
on carbon (60 mg) in methanol (20 mL) was shaken for 4.5 h under 50 psi
hydrogen
gas using a Pan hydrogenator. The reaction mixture was evacuated and purged
with
nitrogen. Then, the reaction mixture was filtered through a pad of celite and
the pad
was rinsed with several portions of methanol. The methanol filtrate was
evaporated
to give the title compound (351 mg, 95%). 1H-NMR (CDC13, 500 MHz) b 8.12-8.10
(m, 2H), 7.55 (br s, 1H), 7.37 (dd, J = 8.9, 1.5 Hz, 1H), 3.77-3.75 (m, 1H),
3.70 (s,
3H), 3.19 (dd, J = 13.7, 5.5 Hz, 1 H), 2.99 (dd, J = 13.7, 8.0 Hz, 1 H), 1.72
(s, 9H).
Mass spec.: 320 (MH)+.
(t)-S-(2-Methoxycarbonyl-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-3-yl)-
piperidine
1-carbonyl]-amino}-ethyl)-indazole-1-carboxylic acid tert-butyl ester
N
O~ N ~ ~ O
IO ~ N~N O
HsCO H N~NH
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
59
A solution of 5-(2-amino-2-methoxycarbonyl-ethyl)-indazole-1-carboxylic
acid tent-butyl ester (307 mg, 0.96 mmol), N,N disuccinimidyl carbonate (246
mg,
0.961 mmol), and N,N diisopropylethylamine (0.67 mL, 3.84 mmol) in methylene
chloride was stirred for 30 min at room temperature. 3-piperidin-4-yl-3,4-
dihydro-
1H quinazolin-2-one (238 mg, 1.03 mmol) was added and the reaction mixture was
stirred at room temperature for 16 h. The solvent was evaporated and the
residue
subjected to flash chromatography using methylene
chloride/methanol/triethylamine
(93:5:2) as eluent, giving the product (259 mg, 47%). 'H-NMR (CDC13, 300 MHz)
8
8.13-8.10 (m, 2H), 7.48 (br s, 1 H), 7.31 (dd, J = 8.8, 1.6 Hz, 1 H), 7.16 (t,
J = 8.0 Hz,
1 H), 7.05 (d, J = 7.0 Hz, 1 H), 6.94 (t, J = 7.7 Hz, 1 H), 6.82 (s, 1 H),
6.66 (d, J = 8.0
Hz, 1H), 4.98 (d, J = 7.7 Hz, 1H), 4.87-4.81 (m, 1H), 4.58-4.49 (m, 1H), 4.26
(s, 2H),
4.05-3.97 (m, 2H), 3.74-3.67 (m, 4H), 3.29-3.23 (m, 2H), 2.93-2.84 (m, 2H),
1.76-
1.62 (m, 1H), 1.70 (s, 9H), 1.48-1.42 (m, 1H). Mass spec.: 577 (MH)+.
2-Trimethylsilanyl-ethanesulfonyl chloride
~Si
~S02C1
Sulfuryl chloride (43 ml, 539 mmol) was added in 3 min to a clear solution of
triphenylphosphine (129 g, 490 mmol) in methylene chloride (200 mL) at
0°C in a
flame-dried three-neck round bottom flask. After stirring at 0°C for 5
min, the ice-
water bath was removed and sodium 2-trimethylsilylethanesulfonate (50 g, 245
mmol) was added in portions over 10 min. The resulting white suspension was
stirred at room temperature for 16 h, then it was filtered through a pad of
celite. The
filtrate was concentrated to ca 50 mL, ethyl acetate/hexanes (1:3, 1000 mL)
and celite
(40 g) were added. The mixture was stirred at room temperature for 15 min and
filtered through a pad of celite. Solvents were removed in vacuo and the
residue was
loaded onto a pre-wetted column with silica gel (300 mL) using 1:3 ethyl
acetate/hexanes as the eluent. Solvents were removed and the title compound
was
obtained as a light tan liquid (41.9g, 85%). If not used immediately, the
final product
should be stored under nitrogen in the freezer or refrigerator to minimize
decomposition. 'H-NMR (CDC13, 500 MHz) b 3.61-3.57 (m, 2H), 1.32-1.27 (m, 2H),
0.10 (s, 9H).
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indole-5-carboxylic acid ethyl ester
C02Et
~/
N
O S
O'
Si
~\
A solution of 1H-indole-5-carboxylic acid ethyl ester (10.31g, 58.8 mmol) in
5 dimethylformamide (50 mL) was added dropwise at 0°C to a mixture of
sodium
hydride (1.83g, 76.4 mmol) in dimethylformamide (150 mL). The resulting
mixture
was stirred at 0°C for 30 min, then a solution of 2-trimethylsilanyl-
ethanesulfonyl
chloride (17.7 g, 88.2 mmol) in dimethylformamide (100mL) was added slowly at
0°C to the above mixture. After 2 h, sat. aqueous ammonium chloride
(200 mL) was
10 added, and the mixture was extracted with ethyl acetate (300 mL). After
separation,
the aqueous layer was extracted with ethyl acetate (2 x 1 SO mL). The combined
organic layers were washed with brine (3 x 150 mL), and dried over anhydrous
sodium sulfate. Solvents were removed in vacuo and the residue was subjected
to
flash chromatography on silica gel using 1:1.5 methylene chloride/hexanes as
eluent
15 to afford the title compound as a white solid (15.8 g, 79%). 'H-NMR (CDC13,
500
MHz) 8 8.36 (d, J = 1.5 Hz, 1 H), 8.03 (dd, J = 9.0, 2.0 Hz, 1 H), 7.92 (d, J
= 8.5 Hz,
1 H), 7.50 (d, J = 3.5 Hz, 1 H), 6.75 (d, J = 3.5 Hz, 1 H), 3.94 (s, 3 H),
3.21- 3.18 (m,
2H), 0.84 - 0.80 (m, 2H), -0.06 (s, 9H). '3C-NMR (CDC13, 125 MHz) b 167.3,
137.7, 130.3, 128.3, 125.9, 125.5, 124.0, 112.8, 108.3, 52.2, S 1.2, 10.1, -
2.1. Mass
20 spec. 354.12 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
61
Similarly prepared:
1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indazole-5-carboxylic acid ethyl
ester
C02Et
N~
~N
O~~
~~S
O
Si
~\
1H-NMR (CDC13, 500 MHz) 8 8.51 (s, 1H), 8.34 (s, 1H), 8.21 (dd, J = 8.9, 1.5
Hz,
1H), 8.12 (d, J = 9.2 Hz, 1H), 3.96 (s, 3H), 3.42 - 3.39 (m, 2H), 0.86 - 0.82
(m, 2H),
-0.02 (s, 9H). 13C-NMR (CDC13, 125 MHz) S 166.4, 143.1, 141.2, 130.1, 126.5,
125.0, 124.2, 112.9, 52.5, 51.3, 9.8, -2.1. Mass spec. 355.13 (MH)+.
[1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indol-5-yl]-methanol
~oH
N
O~,
/S
O
Si
A solution of diisobutylaluminum hydride (82.9 mL, 1M in toluene, 82.9
mmol) was added slowly at 0°C to the solution of 1-(2-trimethylsilanyl-
ethanesulfonyl)-1H indole-5-carboxylic acid ethyl ester (8.81g, 25.9 mmol) in
toluene (200mL). After it was stirred at 0°C for 45 min, the reaction
was quenched
by the addition of methanol (26mL), pulverized sodium sulfate decahydrate (194
g)
and celite (26 mL). The mixture was warmed up to room temperature in 1 h and
filtered through a pad of celite. Solvents were removed in vacuo to afford the
title
compound as a very viscous liquid, which solidified upon cooling. A white
solid
(8.08 g, 100% yield). 'H-NMR (CDC13, 500 MHz) 8 7.87 (d, J = 8.5 Hz, 1H), 7.62
(s, 1 H), 7.44 (d, J = 3.7 Hz, 1 H), 7.35 (dd, J = 8.6, 1.5 Hz, 1 H), 6.66 (d,
J = 3.7 Hz,
1 H), 4.79 (s, 2H), 3.18 - 3.14 (m, 2H), 1.73 (s, 1 H), 0.85 - 0.82 (m, 2H), -
0.06 (s,
9H). Mass spec. 312.14 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
62
[1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-methanol
r
N
~N
O~~
/S
O
Si
~\
A solution of 1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazole-5-carboxylic
acid ethyl ester (azeotropically dried with toluene (2x), 5.77g, 16.9 mmol) in
tetrahydrofuran (50 mL) was added at 0°C to a mixture of lithium
borohydride
(3.68g, 169 mmol) in tetrahydrofuran (100 mL). The mixture was warmed up to
room temperature and stirred for 14h. It was cooled to 0°C and lithium
borohydride
(3.5g) was added. The mixture was warmed up to room temperature and stirred
for
14h. It was re-cooled to 0°C and sat. aqueous ammonium chloride (25 mL)
was
added slowly. The resulted white suspension was filtered through a pad of
celite,
solvents were removed and the residue was subjected to flash chromatography
using
ethyl acetate/hexanes (1:1.5) with 1% triethylamine to afford the title
compound as a
white solid (3.8g, 72%). 'H-NMR (CD30D, 500 MHz) S 8.41 (s, 1H), 8.04 (d, J =
8.5 Hz, 1 H), 7.85 (s, 1 H), 7.61 (dd, J = 8.5, 1.2 Hz, 1 H), 4.76 (s, 2H),
3.49 - 3.46 (m,
2H), 0.76 - 0.72 (m, 2H), -0.03 (s, 9H);'3C-NMR (CD30D, 125 MHz) 8 141.2,
140.9, 138.3, 129.2, 125.8, 119.6, 112.7, 63.8, 50.8, 9.9, -3.2. Mass spec.
313.12
(MH)+.
1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indole-5-carbaldehyde
0
~H
N
OS
O'
Si
~ \
A solution of [1-(2-trimethylsilanyl-ethanesulfonyl)-1H indol-S-yl]-methanol
(2.1 g, 6.74 mmol) in methylene chloride (30 mL) was added at 0°C to a
mixture of
activated manganese dioxide (22g, azeotropically dried with toluene (2x)) and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
63
methylene chloride (70 mL) in a 500 mL round bottom flask. The reaction
mixture
was stirred at 0°C for 30 min and filtered through a pad of celite.
Solvents were
removed in vacuo to afford the title compound as a white solid (1.8g, 80%). ~H-
NMR (CDCl3, 500 MHz) 8 10.06 (s, 1 H), 8.15 (s, 1 H), 8.01 (d, J = 8.6 Hz, 1
H), 7.87
(dd, J = 8.6, 1.5 Hz, 1 H), 7.54 (d, J = 3.4 Hz, 1 H), 6. 80 (d, J = 3.6 Hz, 1
H), 3.24 -
3.20 (m, 2H), 0.86 - 0.82 (m, 2H), -0.06 (s, 9H). '3C-NMR (CDC13, 125 MHz) 8
191.9, 138.5, 132.3, 130.7, 128.8, 125.3, 125.1, 1134.6, 108.4, 51.4, 10.2, -
2.1. Mass
spec. 310.12 (MH)+.
Similarly prepared:
1-(2-Trimethylsilanyl-ethanesulfonyl)-1H indazole-5-carbaldehyde
0
~H
N
~N
O~,
~~S
O
Si
Mass spec. 311.10 (MH)+.
2-Benzyloxycarbonylamino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indol-5-
yl~-
acrylic acid methyl ester
H
0
N~O
H3C0
O
N
O~~
/S
O
Si
1,1,3,3-Tetramethylguanidine (0.68 mL, 5.43 mmol) was added at room
temperature to a solution of N-(benzyloxycarbonyl)-oc-phophonoglycine
trimethyl
ester (1.88g, 5.69 mmol) in tetrahydrofuran (40 mL). The mixture was stirred
at
room temperature for 15 min and cooled to -78°C, and a solution of 1-(2-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
64
trimethylsilanyl-ethanesulfonyl)-1H indole-5-carbaldehyde (1.6g, 5.17 mmol) in
tetrahydrofuran (1 S mL) was added slowly. The resulting reaction mixture was
stirred at -78°C for 2h and then warmed to room temperature in 3h.
Solvents were
removed in vacuo and the residue was subjected to flash chromatography on
silica
gel using methylene chloride/hexanes (1:1.5) with 1% triethylamine as eluent
to
afford the title compound as a 92:8 Z/E mixture (determined by integration of
C02CH3, for Z isomer at 3.79 ppm, and E isomer at 3.65 ppm). For the Z isomer:
'H-NMR (CD3CN, 500 MHz) b 7.96 (s, 1H), 7.91 (d, J = 8.5 Hz, 1H), 7.66 (d, J =
8.5
Hz, 1H), 7.56 (d, J = 3.7 Hz, 1H), 7.51 (s, 1H), 7.43 - 7.35 (m, 5H), 7.67 (d,
J = 3.7
Hz, 1 H), 5.16 (s, 2H), 3.79 ('s, 3H), 3.42 - 3.38 (m, 2H), 0.87 - 0.83 (m,
2H), -0.04
(s, 9H). Mass spec. 515.20 (MH)+.
Similarly prepared:
2-Benzyloxycarbonylamino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-
yl]-acrylic acid methyl ester
H
N' /O
H3C0
O
N~
~N
O~~
~~S
O
Si
~\
Flash chromatography on silica gel using methylene chloride containing 1
triethylamine as eluent afforded the title compound as a 95:5 Z/E mixture
(determined by the integration of -CH=C(COZMe)(NHCBz), 3.72g, 92%). For the Z
isomer: ' H-NMR (CD3CN, 500 MHz) 8 8.39 (s, 1 H), 8.12 (s, 1 H), 8.03 (d, J =
8.8
Hz, 1 H), 7.84 (dd, J = 8.8, 1.2 Hz, 1 H), 7.51 (s, 1 H), 7.43 - 7.35 (m, 5H),
5.14 (s,
2H), 3.81 (s, 3H), 3.51 - 3.47 (m, 2H), 0.83 - 0.79 (m, 2H), -0.02 (s, 9H).
Mass
spec. 516.18 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
(t)-2-Amino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indol-5-yl]-propionic
acid
methyl ester
H
N' /O
H3C ~0
O
N
O~~
~S
O
Si
~\
To a flame dried 500 mL of round bottom flask was added 2-
5 benzyloxycarbonylamino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indol-5-
yl]-
acrylic acid methyl ester (2.24h, 4.36 mmol), methanol (100 mL) and 10%
palladium
on charcoal (0.52g). The mixture was degassed and purged with hydrogen five
times.
It was stirred at room temperature for 1h and filtered through a pad of
celite.
Solvents were removed and the residue was subjected to flash chromatography
using
10 ethyl acetate/hexanes (1:1 and 2:1) containing 1% triethylamine to afford
the tile
compound as a colorless viscous liquid (1.27g, 76%), which solidified upon
cooling.
1H-NMR (CD3CN, 500 MHz) 8 7.82 (d, J = 8.2 Hz, 1H), 7.51 - 7.49 (m, 2H), 7.22
(dd, J = 8.6, 1.5 Hz, 1 H), 6.72 (d, J = 3.7 Hz, 1 H), 3.70 (dd, J = 7.3, 6.1
Hz, 1 H), 3.65
(s, 3H), 3.38 - 3.34 (m, 2H), 3.08 (dd, J =13.4, 5.8 Hz, 1H), 2.95 (dd, J
=13.4, 7.3
15 Hz, 1 H), 0.82 - 0.79 (m, 2H), -0.05 (s, 9H). 13C-NMR (CDCl3, 125 MHz) 8
176.0,
134.4, 133.4, 131.1, 127.9, 126.4, 122.4, 113.1, 107.7, 56.6, 51.7, 50.8,
41.3, 10.1, -
2.7. Mass spec. 383.16 (MH)+.
Similarly prepared:
20 (~)-2-Amino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-
propionic
acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
66
O
H
HgCO Nu0 \
\ I IO
N/
~N
O~~
~S
O
Si
~\
' H-NMR (CD3CN, 500 MHz) ~ 8.34 (s, 1 H), 7.98 (d, J = 8.6 Hz, 1 H), 7.69 (s,
1 H),
7.46 (dd, J = 8.6, 1.5 Hz, 1 H), 3.71 (dd, J = 7.3, 5.8 Hz, 1 H), 3.65 (s,
3H), 3.48 - 3.44
(m, 2H), 3.12 (dd, J = 13.7, 5.8 Hz, 1H), 2.97 (dd, J = 13.7, 7.6 Hz, 1H),
0.83 - 0.79
(m, 2H), -0.02 (s, 9H). '3C-NMR (CDC13, 125 MHz) 8 175.9, 141.1, 140.5, 134.6,
131.5, 126.0, 122.2, 112.7, 56.4, 51.8, 51.1, 40.9, 9.8, -2.6. Mass spec.
384.15
(MH)+.
(R)-2-Benzyloxycarbonylamino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-yl]-propionic acid methyl ester
N-
N
O
/ S.O w
,Sip HN OCH3
~O
O
In a glove bag that was subjected to 3 vacuum/nitrogen purge cycles, an
AIRFREE~ (Schlenk) reaction flask equipped with stir bar was charged with (-)-
1,2-
bis((2R,5R)-2,5-diethylphospholano)benzene(cyclooctadiene) rhodium (I)
trifluoromethylsulfonate (123 mg, 0.17 mmol, 5 mol%), sealed with a rubber
septum,
and removed from the glove bag. The 2-benzyloxycarbonylamino-3-[1-(2-
trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-acrylic acid methyl ester
(1.75 g,
3.40 mmol) was weighed into a second AIRFREE~ (Schlenk) reaction flask
equipped
with stir bar and sealed with a rubber septum. After 3 vacuum/nitrogen purge
cycles,
it was dissolved in a mixture of anhydrous methanol (75 mL ) and anhydrous
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
67
methylene chloride (15 mL). Both solvents were deoxygenated prior to addition
by
sparging with nitrogen for at least 1 h. Once in solution, the mixture was
again
subjected to 3 vacuum/nitrogen purge cycles. The dehydroamino acid solution
was
introduced into the AIRFREE° (Schlenk) reaction flask containing the
catalyst via
cannula. The reaction mixture was subjected to 5 vacuum/hyrogen purge cycles
before opening the flask to 1 atm. of hydrogen (balloon). After 16 h, the
reaction
mixture was purged with 3 vacuum/nitrogen purge cycles . The solvent was
evaporated and the residue was subjected to column chromatography (gradient
1:4
ethyl acetate/hexanes to 1:2 ethyl acetate/hexanes) to give 1.5 g (85%) of the
title
compound as a white solid with 98.4% ee as determined by HPLC analysis using a
Chirocel OD column with 80% hexane/20% ethanol as eluent (retention times:
13.9
min for title compound and 11.2 min for S-enantiomer). 'H-NMR (CDC13, 300
MHz) S 8.17 (s, 1 H), 7.98 (d, J = 8.8 Hz, 1 H), 7.47 (s, 1 H), 7.35-7.25 (m,
6H), 5.29-
5.24 (m, 1H), 5.08 (dd, J= 19.0, 12.1 Hz, 2H), 4.73-4.67 (m, 1H), 3.73 (s,
3H), 3.38-
3.32 (m, 2H), 3.29 (dd, J = 14.2, 5.6 Hz, 1 H), 3.19 (dd, J =13.9, 5.6 Hz, 1
H), 0.91-
0.85 (m, 2H), -0.02 (s, 9H). Mass spec.: 518 (MH)+.
(R)-2-Amino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-
propionic
acid methyl ester
N-
O~ ~N
O
~S~~O ~
~Si~ H2N OCH3
A mixture of (R)-2-Benzyloxycarbonylamino-3-[1-(2-trimethylsilanyl-
ethanesulfonyl)-1H indazol-5-yl]-propionic acid methyl ester (1.24 g, 2.40
mmol)
and 10% palladium on carbon (124 mg) in methanol (50 mL) was agitated for 2 h
under 50 psi hydrogen using a Parr hydrogenator. The reaction mixture was
purged
with 3 vacuum/nitrogen purge cycles. The reaction mixture was then filtered
through
a pad of celite and the pad was rinsed with several portions of methanol. The
methanol filtrate was evaporated to give 879 mg (96%) of the title compound as
a
sticky gum. ' H-NMR (CDC13, 300 MHz) S 8.21 (s, 1 H), 8.02 (d, J = 8.8 Hz, 1
H),
7.59 (s, 1 H), 7.38 (d, J = 8.8 Hz, 1H), 3.72 (s, 3H), 3.38-3.32 (m, 2H), 3.21
(dd, J =
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
68
13.9, 5.1 Hz, 1H), 2.98 (dd, J =13.9, 7.9 Hz, 1H), 0.91-0.85 (m, 2H), -0.02
(s, 9H).
Mass spec.: 384 (MH)+
7-Methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H indazole-5-carbaldehyde
I
Sip
i .N O
H ~ ~ N!S
11
O
O
To a suspension of 7-methylindazole 5-aldehyde (3.0 g, 18.7 mmol) in
methylene chloride (150 mL) was added triethylamine (7.83 mL, 56.2 mL, 3
equiv)
followed by dropwise addition of neat 2-trimethylsilanyl-ethanesulfonyl
chloride
(5.60 g, 28.1 mmol, 1.5 equiv). The mixture gradually became homogeneous and
was allowed to stir at room temperature for 16 h. The solution was
concentrated to a
minimum amount of methylene chloride and then subjected to flash column
chromatography on silica gel (1:4 ethyl acetate/hexanes) to give 4.7 g (77%)
of the
product as a pale yellow solid. 'H-NMR (CDCl3, 300 MHz) 8 9.98 (s, 1H), 8.77
(s,
1H), 8.09 (s, 1H), 7.64 (s, 1H), 3.64-3.58 (m, 2H), 2.65 (s, 3H), 0.88-0.82
(m, 2H),
0.01 (s, 9H).
2-Benzyloxycarbonylamino-3-[7-methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H
indazol-5-yl]-acrylic acid methyl ester
O
v
OCH3
~Si HN ~O
o
0
To a solution of N-(benzyloxycarbonyl)-oc-phosphonoglycine trimethyl ester
(4.93 g, 14.9 mmol, 1.1 equiv) in anhydrous tetrahydrofuran (75 mL) was added
tetramethylguanidine (1.78 mL, 1.05 equiv). The mixture was stirred at room
temperature under nitrogen for 5 min and was then cooled to -78°C.
After stirring for
15 min at -78°C, a solution of 7-methyl-2-(2-trimethylsilanyl-
ethanesulfonyl)-2~-I-
indazole-5-carbaldehyde in tetrahydrofuran (25 mL) was added. The reaction
mixture was allowed to slowly warm to room temperature overnight. Although the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
69
reaction was incomplete, the solvent was evaporated. The resulting residue was
dissolved in ethyl acetate and washed with 1 M sulfuric acid. The organic
layer was
separated, dried over magnesium sulfate, filtered and evaporated. Flash column
chromatography (1:4 ethyl acetate/hexanes) gave 2.66 g (37 %) of the product
as
white glass foam. 1H-NMR (CDCl3, 300 MHz) S 8.48 (s, 1H), 7.62 (s, 1H), 7.38-
7.25 (m, 7H), 6.48 (bs, 1H), 5.10 (s, 2H), 3.83 (s, 3H), 3.58-3.52 (m, 2H),
2.51 (s,
3H), 0.89-0.83 (m, 2H), 0.02 (s, 9H). Mass spec.: 530 (MH)+.
(R)-2-Benzyloxycarbonylamino-3-[7-methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-
2H indazol-5-yl]-propionic acid methyl ester
1
Si,
O
i ~ N O
w / ~ ,
O NH N~S~
0~.,,~~~/ ~. WO
home
In a glove bag that was subjected to 3 vacuum/nitrogen purge cycles, an
AIRFREE° (Schlenk) reaction flask equipped with stir bar was charged
with (-)-1,2-
bis((2R,SR)-2,5-diethylphospholano)benzene(cyclooctadiene) rhodium (I)
trifluoromethylsulfonate (259 mg, 0.36 mmol, 9 mol-%), sealed with a rubber
septum, and removed from the glove bag. The 2-benzyloxycarbonylamino-3-[7-
methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H indazol-5-yl]-acrylic acid
methyl
ester (2.03 g, 3.83 mmol) was weighed into a second AIRFREE° (Schlenk)
reaction
flask equipped with stir bar and sealed with a rubber septum. After 3
vacuum/nitrogen purge cycles, it was dissolved in anhydrous methanol (80 mL,
deoxygenated prior to addition by sparging with nitrogen for at least 1 h).
Once in
solution, it was again subjected to 3 vacuum/nitrogen purge cycles. The
dehydroamino acid solution was transferred via cannula to the AIRFREE°
(Schlenk)
reaction flask containing the catalyst. The reaction mixture was purged with 5
vacuum/hydrogen purge cycles before opening the flask to a balloon of hydrogen
(1
atm). After 2.5 h, the reaction mixture was purged with 3 vacuum/nitrogen
purge
cycles. The solvent was evaporated and the residue was subjected to column
chromatography (gradient 1:4 ethyl acetate/hexanes to 1:2 ethyl
acetate/hexanes) to
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
give 1.4 g (68%; ee = 99.2%) of the title compound as a white solid. I H-NMR
(CDC13, 300 MHz) S 8.43 (s, 1 H), 7.34 (s, 5H), 7.19 (s, 1 H), 6.87 (s, 1 H),
5.24 (d, J =
8.1 Hz, 1 H), 5.08 (dd, J = 18.3, 12.1 Hz, 2H), 4.67 (dd, J = 13.9, 6.2 Hz, 1
H), 3.73 (s,
3H), 3.57 -3.51 (m, 2H), 3.16 (dd, J = 14.0, 5.9 Hz, 1H). 3.06 (dd, J = 13.9,
6.6 Hz,
5 1 H), 2.55 (s, 3H), 0.89-0.83 (m, 2H), 0.01 (s, 9H). 13C-NMR (CDCl3, 75 MHz)
8
172.0, 155.7, 151.7, 136.2, 132.2, 129.8, 129.5, 128.6, 128.4, 128.2, 125.1,
121.1,
118.1, 67.1, 54.7, 52.5, 51.1, 38.6, 17.1, 9.7, -2Ø Mass spec.: 532 (MH)+.
(R)-2-Amino-3-[7-methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H indazol-5-yl]-
10 propionic acid methyl ester
0
14
i O N- ~ i; OMe
~O
2
2-Benzyloxycarbonylamino-3-[7-methyl-2-(2-trimethylsilanyl-
ethanesulfonyl)-2H indazol-5-yl]-propionic acid methyl ester, (1.35 g, 2.54
mmol)
and 10% palladium on carbon (135 mg) in methanol (40 mL) were agitated for 3.0
h
15 under 55 psi hydrogen using a Parr apparatus. The reaction mixture was
purged with
3 vacuum/nitrogen purge cycles. The reaction mixture was then filtered through
a
pad of celite and the pad was rinsed with several portions of methanol. The
methanol filtrate was evaporated to give the title compound (1.01 g,
quantitative
yield) as a sticky gum. 1H-NMR (CDC13, 300 MHz) b 8.45 (s, 1H), 7.29 (s, 1H),
20 6.97 (s, 1H), 3.79-3.73 (m, 1H), 3.73 (s, 3H), 3.56-3.50 (m, 2H), 5.12 (dd,
J = 13.5,
5.12 Hz, 1H), 4.85 (dd, J= 13.5, 8.1 Hz, 1H), 2.58 (s, 3H), 0.87-0.81 (m, 2H),
0.01
(s, 9H).'3C-NMR (CDC13, 75 MHz) 8175.5, 151.8, 133.7, 129.9, 129.4, 125.0,
121.3, 117.9, 55.5, 52.1, 51.1, 41.4, 17.1, 9.8, -2.1. Mass spec.: 398 (MH)+.
25 (R)-3-[7-Methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H indazol-5-yl]-2-
{[4-(2-
oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carbonyl]-amino)-
propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
71
s'/
HN~ N ~ S
O N,N ~O
N
~/-NH
O
==J''~~O
OCH3
A mixture of 2-amino-3-[7-methyl-2-(2-trimethylsilanyl-ethanesulfonyl)-2H
indazol-5-yl]-propionic acid methyl ester (500 mg, 1.26 mmol), N,N
diisopropylethylamine (0.66 mL, 3.77 mmol) and disuccinimidylcarbonate (322
mg,
1.26 mmol) were stirred together in methylene chloride (20 mL) for 30 min at
room
temperature. Then, 3-piperidin-4-yl-3,4-dihydro-1H quinazolin-2-one (444 mg,
1.35
mmol) was added and the reaction mixture was allowed to stir overnight at room
temperature. The solvent was evaporated and the residue was subjected to flash
column chromatography (1:4 acetone/ethyl acetate) to give 490 mg (60 % yield)
of
the title compound as a white solid. 1H-NMR (CDC13, 300 MHz) 8 8.47 (s, 1H),
7.23
(s, 1 H), 7.19-7.14 (m, 1 H), 7.04 (d, J = 7.3 Hz, 1 H), 6.97-6.93 (m, 2H),
6.77 (s, 1 H),
6.65 (d, J = 7.7 Hz, 1 H), 4.99 (d, J = 7.3 Hz, 1 H), 4.81 (dd, J = 13.5, 6.2
Hz, 1 H),
4.58-4.46 (m, 1H), 4.27 (s, 2H), 4.10-3.98 (m, 2H), 3.73 (s, 2H), 3.57-3.51
(m, 2H),
3.14-3.11 (m, 2H), 2.95-2.83 (m, 2H), 2.58 (s, 3H), 1.77-1.65 (m, 4H), 0.92-
0.84 (m,
2H), -0.01 (s, 9H). Mass spec.: 655 (MH)+.
Similarly prepared:
(~)-2- f [4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-
amino]-3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indol-5-yl]-propionic acid methyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
72
H
O\/N
O 'N~
H
N NJ
H3C0
O
N
~Si~S O
1H-NMR (CD30D, 500 MHz) 8 7.85 (d, J = 8.2 Hz, 1H), 7.55 (s, 1H), 7.51 (d, J =
3.7
Hz, 1 H), 7.27 (dd, J = 8.6, 1.5 Hz, 1 H), 7.16 (t, J = 7.6 Hz, 1 H), 7.10 (d,
J = 7.6
Hz, l H), 6.95 (t, J = 7.6 Hz, 1 H), 6.79 (d, J = 8.0 Hz, 1 H), 6.73 (d, J =
3.7 Hz, 1 H),
S 4.44 - 4.38 (m, 1H), 4.26 (s, 2H), 4.13 - 4.08 (m, 2H), 3.73 (s, 3H), 3.34 -
3.29 (m,
4H), 3.13 (dd, J = 13.5, 9.4 Hz, 1H), 2.89 - 2.79 (m, 2H), 1.76 -1.70 (m, 1H),
1.63 -
1.59 (m, 3H), 0.76 - 0.72 (m, 2H), -0.07 (s, 9H); Mass spec.: 640.40 (MH)+.
(R)-2-{[4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carbonyl]-amino]-
3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-propionic acid methyl
ester
O
w II~,~ OCH3
N/ r / HN
N 1N
,O
O;S
N
NH
,gi'
f
A solution of (R)-2-Amino-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-5-yl)-propionic acid methyl ester (764 mg, 1.99 mmol), N,N
diisopropylethylamine (1.10 mL, 5.97 mmol) and disuccinimidylcarbonate (509
mg,
1.99 mmol) in methylene chloride (20 mL) was stirred for 40 min at room
temperature. Then, 3-piperidin-4-yl-3,4-dihydro-1H quinazolin-2-one (70% pure,
703 mg, 2.13 mmol) was added and the reaction mixture was allowed to stir
overnight at room temperature. The solvent was evaporated in vacuo and the
residue
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
73
was subjected to flash column chromatography (1:4 acetonelethyl acetate) to
give
1.15 g (90 %) of the title compound. ' H-NMR (CDC13, 300 MHz) 8 8.21 (s, 1 H),
8.01 (d, J = 8.5 Hz, 1 H), 7.53 (s, 1 H), 7.32 (d, J = 8.5 Hz, 1 H), 7.16 (t,
J = 7.8 Hz,
1 H), 7.06 (d, J = 7.6 Hz, 1 H), 6.95 (d, J = 7.6 Hz, 1 H)1, 6.76 (s, 1 H),
6.65 (d, J = 7.9
Hz, 1 H), 5.01 (d, J = 7.6 Hz, 1 H), 4. 84 (dd, J = 13 .1, 6.0 Hz, 1 H), 4.5 6-
4.49 (m, 1 H),
4.28 (s, 2H), 4.13-3.98 (m, 2H), 3.73 (s, 3H), 3.39-3.35 (m, 2H), 3.28 (dd, J
= 14.0,
6.1 Hz, 1H), 3.24 (dd, J = 13.7, 5.8 Hz, 1H), 2.94-2.87 (m, 2H), 1.75-1.67 (m,
4H),
0.91-0.87 (m, 2H), -0.02 (s, 9H). Mass spec.: 641 (MH)+.
Similarly prepared:
(~)-2- { [4-(2-Oxo-2, 3-dihydro-benzoimidazol-1-yl)-piperidine-1-carbonyl] -
amino } -3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indol-5-yl]-propionic acid methyl
ester
O~NH
N) ~
O~N
~NH
C02Me
N
O~~S
ii
O
Sip
~\
'H-NMR (CD3CN, 500 MHz) 8 9.78 (s, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.56 (s,
1H),
7.49 (d, J = 3.7 Hz, 1H), 7.28 (dd, J = 8.5, 1.5 Hz, 1H), 7.10 - 7.08 (m, 1H),
7.05 -
7.03 (m, 1 H), 6.99 - 6.97 (m, 2H), 6.70 (d, J = 3.7 Hz, 1 H), 5.91 (d J = 7.9
Hz, 1 H),
4.66 (q, J = 8.2 Hz, 1H), 4.45 - 4.39 (m, 1H), 4.14 (br s, 1h), 3.68 (s, 3H),
3.36 - 3.32
(m, 2H), 3.27 (dd, J = 14.0, 5.5 Hz, 1 H), 3.18 (dd, J = 13.7, 8.5 Hz, 1 H),
2.90 - 2.84
(m, 2H), 2.55 (br s, 1H), 2.36 - 2.21 (m, 2H), 1.74 - 1.70 (m, 2H), 0.82 -
0.78 (m,
2H), -0.09 (s, 9H). Mass spec. 626.26 (MH)+.
(~)-2- { [4-(2-Oxo-2, 3-dihydro-benzoimidazol-1-yl)-piperidine-1-carbonyl]-
amino } -3
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-propionic acid methyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
74
O~NH
Nl ~
O~N
~N'H
N~
~N / C02Me
O~ 8
ii
O
Si
~\
1H-NMR (CD3CN, 500 MHz) 8 9.61 (br s, 1H), 8.35 (s, 1H), 8.00 (d, J = 8.5 Hz,
1 H), 7.74 (s, 1 H), 7.51 (dd, J = 8.8, 1.5 Hz, 1 H), 7.10 - 7.06 (m, 1 H),
7.05 - 7.02 (m,
1 H), 7.00 - 6.97 (m, 2H), 5.90n(d, J = 7.9 Hz, 1 H), 4.67 4.62 (m, 1 H), 4.42
-4.36 (m,
1 H), 4.13 - 4.07 (br s, 1 H), 3.68 (s, 3H), 3.45 - 3.42 (m, 2H), 3.30 (dd, J
= 14.0, 5.8
Hz, 1H), 3.20 (dd, J = 13.7, 8.8Hz, 1H), 2.89 - 2.84 (m, 2H), 2.52 (br s, 1H),
2.33-
2.23 (m, 2H), 1.72 - 1.69 (m, 2H), 0.80 - 0.76 (m, 2H), -0.07 (s, 9H). Mass
spec.
627.25 (MH)+.
(~)-2-{[4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-
3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indol-5-yl]-propionic acid methyl
ester
H
O\ /N
~N ~ /
O H
N N
H3C0
O
i O
~Si~SO
'H-NMR (CD30D, 500 MHz) 8 7.85 (d, J = 8.2 Hz, 1H), 7.55 (s, 1H), 7.51 (d, J =
3.7
Hz, 1 H), 7.27 (dd, J = 8.6, 1.5 Hz, 1 H), 7.16 (t, J = 7.6 Hz, 1 H), 7.10 (d,
J = 7.6
Hz, l H), 6.95 (t, J = 7.6 Hz, 1 H), 6.79 (d, J = 8.0 Hz, 1 H), 6.73 (d, J =
3.7 Hz, 1 H),
4.44 - 4.38 (m, 1 H), 4.26 (s, 2H), 4.13 - 4.08 (m, 2H), 3.73 (s, 3H), 3.34 -
3.29 (m,
4H), 3.13 (dd, J = 13.5, 9.4 Hz, 1 H), 2.89 - 2.79 (m, 2H), 1.76 -1.70 (m, 1
H), 1.63 -
1.59 (m, 3H), 0.76 - 0.72 (m, 2H), -0.07 (s, 9H). Mass spec. 640.40 (MH) +.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
(~)-2- { [4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-
amino }-3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indazol-5-yl]-propionic acid methyl
ester
H
O\ /N
~N ~ /
O H
N N
H3C0
O
i N
N
~~O
jSi~SO
5 ' H-NMR (CD30D, 500 MHz) S 8.39 (d, J = 0.5 Hz, 1 H), 8.02 (d, J = 8.5 Hz, 1
H),
7.75 (s, 1 H), 7.52 (dd, J = 8.5, 1.5 Hz, 1 H), 7.14 - 7.10 (m, 2H), 6.94 (t,
J = 7.5 Hz,
1 H), 6.78 (d, J = 7.5 Hz, 1 H), 4.63 - 4.60 (m, 1 h), 4.43 - 4.37 (m, 1 H),
4.27 (s, 2H),
4.11 (br s, 1H), 4.08 (br s, 1H), 3.71 (s, 3H), 3.47 - 3.43 (m, 2H), 3.37 -
3.33 (m,
1 H), 3.18 (dd, j = 13.5, 10.0 Hz, 1 H), 2.87 - 2.79 (m, 2H), 1.73 - 1.59 (m,
4H), 0.80
10 - 0.75 (m, 2H), -0.05 (s, 9H);'3C-NMR (CD30D, 125 MHz) 8 173.7, 155.5,
158.1,
141.0, 140.6, 137.2, 134.4, 131.3, 128.2, 126.1, 125.8, 122.2, 121.9, 118.3,
113.4,
112.6, 55.9, 52.1, 51.7, 50.8, 48.9, 48.6, 48.4, 48.2, 48.0, 47.9, 47.7, 47.5,
43.8, 43.7,
43.1, 37.2, 28.5, 9.8, -3.2. Mass spec.: 641.40 (MH)+.
15 Example 1
(~)-3-(1H Indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-3-yl)-
piperidine-1-
carbonyl]-amino}-propionic acid
N
HN / ~ O
~N O
N ~
HO--~H N' \NH
O
/I
A solution of 5-(2-methoxycarbonyl-2-{[4-(2-oxo-1,4-dihydro-2H
20 quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-ethyl)-indazole-1-carboxylic
acid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
76
tent-butyl ester (168 mg, 0.29 mmol) was dissolved in tetrahydrofuran (5 mL)
in
methanol (5 mL) was cooled to 0°C. A solution of lithium hydroxide
monohydrate
(49 mg, 2.04 mmol) in water (5 mL) was added. The reaction mixture was stirred
at
0°C for 6 h and then placed in the freezer for a further 16 h. The
solvents were
S removed in vacuo and the residue dissolved in water (15 mL). The pH of the
aqueous solution was adjusted to ca. 1 with 1N hydrochloric acid. The
resulting
white solid precipitated was collected by filtration. The solid was dried
under
vacuum to give the title compound (108 mg, 80%). 'H-NMR (DMSO-d6, 300 MHz)
b 12.94 (bs, 1 H), 9.19 (s, 1 H), 8.01 (s, 1 H), 7.61 (s, 1 H), 7.46 (d, J =
8.4 Hz, 1 H),
7.28 (dd, J = 8.5 , 1.5 Hz, 1 H), 7.13-7.06 (m, 2H), 6.86 (t, J = 7.0 Hz, 1
H), 6.76-6.72
(m, 2H), 4.32-4.24 (m, 2H), 4.09-4.02 (m, 4H), 3.17-2.97 (m, 2H), 2.72-2.59
(m,
2H), 1.57-1.35 (m, 4H). IR (KBr, cm') 3424, 2963, 2930, 1660, 1628, 1505,
1474,
1446, 753. Mass spec.: 463 (MH)+.
(R)-2-~[4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-
3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-propionic acid
O
\g; ~'S .O
/ ~N-N
H
N~N~N ,y
~ O ~OH
N' \O O
H
A solution of (R)-2-{[4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-
1-carbonyl]-amino}-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-
propionic acid methyl ester (775 mg, 1.21 mmol) in tetrahydrofuran (9 mL) and
methanol (3 mL) was cooled to 0°C. A solution of lithium hydroxide
monohydrate
(115 mg, 4.84 mmol) in water (3 mL) was added. The reaction mixture was
stirred at
0°C for 2 h and then placed in the freezer at -15°C for 16 h.
While cooling the
reaction mixture with an ice bath, the pH was increased to ca. 7 by addition
of 1N
hydrochloric acid (3.8 mL). Organic solvents were removed under vacuum. The
resulting aqueous solution was extracted with ethyl acetate after additon of
more 1N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
77
hydrochloric acid (0.5 mL). The combined extracts were dried over magnesium
sulfate, filtered and evaporated to give 684 mg (90%) of the title compound as
a
white solid. 1H-NMR (DMSO-d6, 300 MHz) 8 9.21 (s, 1H), 8.58 (s, 1H), 7.90 (d,
J =
8.4 Hz, 1 H), 7.78 (s, 1 H), 7.56 (d, J = 8.1 Hz, 1 H), 7.13-7.09 (m, 2H),
6.88-6.83 (m,
1H), 6.76-6.74 (m, 2H), 4.33-4.27 (m, 2H), 4.18 (s, 2H), 4.09-3.96 (m, 3H),
3.57-
3.51 (m, 2H), 3.25-3.04 (m, 2H), 2.74-2.60 (m, 2H), 1.54-1.43 (m, 4H), 0.70-
0.64 (m,
2H), -0.08 (s, 9H). Mass spec.: 627 (MH)+.
Similarly prepared:
(~)-2-{[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carbonyl]-amino}-
3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indol-5-yl]-propionic acid
O~NH
Nl
O~ N J
''~N'H
N I / OzH
O~~ ~
,S
O
Si
~\
Mass spec. 612.25 (MH)+.
(~)-2-{[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carbonyl]-amino}-
3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H indazol-5-yl]-propionic acid
O~ N H
Nl ~
o~N~
NH
N~
'N / C02H
O~~S
ii
O
Si
~\
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
78
Mass spec. 613.26 (MH)+.
(~)-2- { [4-(2-Oxo-1,4-dihydro-2 H-quinazolin-3-yl)-piperidine-1-carbonyl] -
amino } -3-
[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-indazol-5-yl]-propionic acid
H
O\/N
O 'N~
H
HO N N J
O
,N
N
',O
~Si~SO
'H-NMR (CD3CN, 500 MHz) 8 8.37 (s, 1H), 8.08 (s, 1H), 8.01 (d, J = 8.5 Hz,
1H),
7.77 (s, 1 H), 7.53 (dd, J = 8.5, 1.5 Hz, 1 H), 7.19 (t, J = 7.3 Hz, 1 H),
7.14 (d, J = 7.3
Hz, 1 H), 6.98 (td, j = 7.6, 1.2 Hz, 1 H), 6.79 (d, j = 8.0 Hz, 1 H), 6.28 (br
s, 3 H), 4.54
- 4.49 (m, 1 H), 4.37 - 4.32 (m, 1 H), 4.30 (s, 2H), 3.98 - 3.92 (m, 2H), 3.45
- 3.41
(m, 2H), 3.37 (dd, j = 14.0, 4.9 Hz, 1 H), 3.20 (dd, J = 14.0, 9.7 Hz, 1 H),
2.84 - 2.77
(m, 2H), 1.65 -1.57 (m, 4H), 0.79 - 0.76 (m, 2H), -0.05 (s, 9H). Mass spec.:
627.30
(MH)+.
(R)-4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carboxylic acid {2-
[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-ylmethyl]-ethyl } -amide
OII YN
N~ ~ 1~~,~N~
,N~ HN ~O
O_- ~_
S_O
lSi~ N NH
i
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
79
To a solution of (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-yl]-propionic acid (554 mg, 0.88 mmol) and N,N diisopropylethylamine (0.62
mL,
3.54 mmol) in methylene chloride (20 mL) was added a solution of 4-
piperidinopiperidine (164 mg, 0.97 mmol) and PyBOP~ (460 mg, 0.88 mmol) in
methylene chloride (15 mL). The reaction mixture was stirred for 16 h at room
temperature. It was then concentrated to approximately 2 mL and subjected to
flash
column chromatography using methylene chloride/methanolJtriethylamine (94:5:1
) as
eluent to give 599 mg (87%) of the title compound as a white solid. 1H-NMR
(CD3CN, 300 MHz) ~ 8.37 (s, 0.5H), 8.36 (s, 0.5H), 8.02-7.96 (m, 1H), 7.74 (s,
0.5H), 7.71 (s, 0.5H), 7.55-7.46 (m, 1H), 7.21-7.12 (m, 2H), 6.97-6.92 (m,
1H), 6.79
(d, J = 8.1 Hz, 1 H), 5.71 (t, J = 8.1 Hz, 1 H), 5.00 (dd, J = 15.0, 8.1 Hz, 1
H), 4.63-
4.51 (m, 1H), 4.39-4.29 (m, 1H), 4.29 (s, 2H), 4.10-3.96 (m, 3H), 3.46-3.40
(m, 2H),
2.92-2.70 (m, 8H), 2.58-2.37 (m, 5H), 1.74-1.40 (m, 13H), 0.80-0.74 (m, 2H), -
0.04
(s, 9H). Mass spec.: 778 (MH)+.
Similarly prepared:
(t)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid {2-
[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indol-5-
ylmethyl]-ethyl}-amide
0
\ N~ N
N ~ H N ~ ~/ ~~//O
O, ~
S;O N
TMS
N~O
NH
'H-NMR (CD3CN, 500 MHz) b 9.42 (br s, 1H), 7.80 (d, J = 8.5 Hz, 0.6 H), 7.78
(d, J
= 8.2 Hz, 0.4 H), 7.50 (s, 1 H), 7.43 (t, J = 3.0 Hz, 1 H), 7.27 (d, J = 8.5
Hz, 0.6 H),
?.23 (d, J = 8.5 Hz, 0.4 H), 7.10 - 7.07 (m, 1 H), 7.02 - 6.95 (m, 3H), 6.69
(s, 0.4 H),
6.68 (s, 0.6 H), 5.88 (d, J = 8.5 Hz, 0.6 H), 5.85 (d, J = 8.4 Hz, 0.4 H),
5.04 - 4.98
(m, 1 H), 4.49 (s, 0.4 H), 4.46 (s, 0.6 H), 4.36 - 4.30 (m, 1 H), 4.11 - 4.07
(m, 1 H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
3.97 - 3.91 (m, 1 H), 3.31 - 3.28 (m, 2H), 3.11 - 3.05 (m, 6 H), 2.87 - 2.80
(m, 2H),
2.43 - 2.07 (m, 8 H), 1.78 - 1.74 (m, 4H), 1.71 - 1.65 (m, 2H), 1.46 - 1.40
(m, 2H),
1.37 - 1.31 (m, 2H), 0.80 - 74 (m, 2H), -0.10 (s, 9H). LC/MS: tR = 2.47 min,
762.37
(MH)*.
5
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid f 2-
[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-ylinethyl]-ethyl} -amide
0
N~ N
N / ~ ~/ ~~//T
N / HN' /O
O;S;O '~N
TMS
N~O
~ NH
10 IH-NMR (CD3CN, 500 MHz) ~ 9.67 (s, 1H), 8.32 (s, 1H), 7.96 (d, J = 8.7 Hz,
0.55
H), 7.93 (d, J = 8.6 Hz, 0.45 H), 7.70 (s, 1H), 7.51 (d, J = 8.6 Hz, 0.55 H),
7.47 (d, J
= 8.8 Hz, 0.45 H), 7.08 - 7.05 (m, 1 H), 7.03 - 6.99 (m, 1 H), 6.98 - 6.94 (m,
2H),
6.01 (d, J = 7.9 Hz, 0.45 H), 5.96 (d, J = 7.9 Hz, 0.55 h), 5.05 - 5.00 (m,
1H), 4.49 -
4.46 (m, 1 H), 4.35 - 4.29 (m, 1 H), 4.10 - 4.05 (m, 1 H), 4.00 - 3.93 (m, 1
H), 3.40 -
15 3.36 (m, 2H), 3.17 - 3.30 (m, 6H), 2.91 - 2.71 (m, 2H), 2.52 - 2.13 (m,
8H), 1.76 9br
s, 4H), 1.69 -1.65 (m, 2H), 1.44 -1.41 (m, 2H), 1.34 - 1.30 (m, 2H), 0.77 -
0.71 (m,
2H), -0.08 (s, 9H). LC/MS: tR = 2.35 min, 763.35 (MH)+.
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid f 2-
20 [1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H-
indol-5-
ylmethyl]-ethyl}-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
81
H
O\/N
~N I /
O H
N N N
O
'N
..
O =S ~/" S~
ii
O
'H-NMR (CD3CN, 500 MHz) S 8.17 (s, 0.6H), 8.16 (s, 0.4H), 7.84 (d, J = 8.5 Hz,
0.6
H), 7.81 (d, J = 8.5 Hz, 0.4 H), 7.54 (s, 0.4 H), 7.53 (s, 0.6H), 7.48 (t, J =
4.1 Hz,
1 H), 7.31 (dd, J = 8.5, 1.5 Hz, 0.6 H), 7.28 (dd, J = 8.5, 1.5 Hz, 0.4 H),
7.18 (t, j = 7.4
Hz, 1 H), 7.09 - 7.06 (m, 1 H), 6.93 (t, J = 7.3 Hz, 1 H), 6.83 (d, J = 7.9
Hz, 1 H), 6.72
(d, J = 3.6 Hz, 1 H), 6.09 (d, J = 8.2 Hz, 1 H), 5.05 - 4.99 (m, 1 H), 4.53 -
4.50 (m,
1H), 4.40 - 4.34 (m, 1H), 4.26 (s, 1.2H)< 4.24 (s, 0.8H), 3.99 - 3.94 (m, 1H),
3.35 -
3.30 (m, 2H), 3.15 - 3.07 (m, 3H), 3.08 - 3.03 (m, 1H), 2.81 - 2.73 (m, 3H),
2.55 -
2.37 (m, 6H), 2.21 - 2.16 (m, 1H), 2.13 - 2.08 (m, 1H), 1.69 - 1.57 (m, 4H),
1.51 -
1.45 (m, 4H), 1.41 - 1.35 (m, 4H), 0.83 - 0.74 (m, 2H), -0.06 (s, 9H). Mass
spec.:
776.44 (MH)+.
(t)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid {2-
[ 1,4']bipiperidinyl-1'-yl-2-oxo-1-[ 1-(2-trimethylsilanyl-ethanesulfonyl)-1 H-
indazol-
5-ylinethyl]-ethyl}-amide
H
O~ N
O N
H
N N NJ
O
'N
,N
N /
O~S~"Si
O
Purified by silica gel chromatography using methylene
chloride:methanol/triethylamine (90:10:0.5) as eluent. 1H-NMR (CD3CN, 500 MHz)
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
82
8 8.36 (s, 1 H), 8.04 (s, I H), 8.01 (d, J = 8.8 Hz, 0.6H), 7.97 (dd, J = 8.8
Hz, 0.4 H),
7.74 (s, 1 H), 7.54 (dd, J = 8.5, 1.5 Hz, 0.6 H), 7.51 (dd, J = 8.5, 1.5 Hz,
0.4 H), 7.18
(t, J = 7.4 Hz, 1 H), 7.11 (t, J = 7.3 Hz, 1 H), 6.94 (t, J = 7.3 Hz, 1 H),
6.83 (d, J = 7.9
Hz, 1 H), 6.05 (d, J = 8.5 Hz, 0.4 H), 6.02 (d, J = 8.5 Hz, 0.6 H), 5.06 -
5.01 (m, 1 H),
4.52 - 4.50 (m, 1 H), 4.39 - 4.34 (m, 1 H), 4.27 (s, 1.2 H), 4.25 (s, 0.8 H),
4.00 - 3.97
(m, 2H), 3.45 - 3.40 (m, 2H), 3.20 - 3.08 (m, 2H), 2.81 - 2.74 (m, 2H), 2.56 -
2.39
(m, 8H), 2.27 - 2.24 (m, 1 H), 2.20 - 2.16 (m, 1 H), 1.68 -1.57 (m, 4H), 1.52 -
1.45
(m, 4H), 1.41 -1.34 (m, 4H), 1.06 -1.01 (m, 1 H), 0.80 -0.75 (m, 2H), -0.07
(s, 9H).
Mass spec.: 777.40 (MH)+.
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid f 2-
(4-
isobutyl-piperazin-1-yl)-2-oxo-1-[ 1-(2-trimethylsilanyl-ethanesulfonyl)-1 H-
indol-S-
ylmethyl]-ethyl}-amide
O~NH
NI
O\/N
~NH
N~ Oj\N
O l
~N~
O
Si-
/\
1H-NMR (CD3CN, 500 MHz) 8 9.75 (s, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.54 (s,
1H),
7.48 (d, J = 3.6 Hz, 1 H), 7.28 (d, J = 8.5 Hz, 1 H), 7.12 - 7.09 (m, 1 H),
7.04 - 7.02
(m, 1 H), 7.00 - 6.97 (m, 2H), 6.72 (d, J = 3.7 Hz, 1 H), 5.97 (d, J = 8.2 Hz,
1 H), 5.01
(dd, J = 14.6, 7.2 Hz, 1 H), 4.40 - 4.34 (m, 1 H), 4.15 - 4.08 (m, 2H), 3.58 -
3.54 (m,
1H), 3.50 - 3.45 (m, 2H), 3.39 - 3.35 (m, 1H), 3.36 - 3.32 (m, 2H), 3.14 -
3.10 (m,
8H), 2.89 - 2.83 (m, 2H), 2.34 - 2.23 (m, 4H), 2.17 - 2.13 (m, 1 H), 0.85 (d,
J = 6.7
Hz, 6H), 0.83 - 0.80 (m, 2H), -0.06 (s, 9H). Mass spec.: 736.40 (MH)+.
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-I-carboxylic acid {2-
(1,4-
dioxa-8-aza-spiro[4.5]dec-8-yl)-2-oxo-1-[ 1-(2-trimethylsilanyl-
ethanesulfonyl)-1 H-
indol-5-ylmethyl]-ethyl}-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
83
O~NH
N ~
O\/N
'N~ H
I / ~
N~ 0%\N
O~g O
OJ
S. '
'H-NMR (CD3CN, 500 MHz) b 9.27 (s, 1H), 7.82 (d, J = 8.5 Hz, 1H), 7.55 (s,
1H),
7.48 (d, J = 3.6 Hz, 1 H), 7.28 (dd, J = 8.5, 1.5 Hz, 1 H), 7.13 - 7.10 (m, 1
H), 7.06 -
7.03 (m, 1 H), 7.01 - 6.98 (m, 2H), 6.72 (d, J = 3.6 Hz, 1 H), 5.95 (d, J =
8.0 Hz, 1 H),
5.05 (dd, J = 15.0, 7.3 Hz, 1 H), 4.41 - 4.34 (m, 1 H), 4.14 - 4.08 (m, 2H),
3.90 - 3.86
(m, 3H), 3.68 - 3.64 (m, 1H), 3.60 - 3.56 (m, 2H), 3.45 - 3.40 (m, 1H), 3.35 -
3.31
(m, 2H), 3.15 (dd, J = 13.4, 7.1 Hz, 1 H), 3.05 (dd, J = 13.4, 7.0 Hz, 1 H),
2.89 - 2.83
(m, 2H), 2.34 - 2.19 (m, 3H), 1.73 - 1.70 (m, 2H), 1.64 - 1.56 (m, 2H), 1.53 -
1.49
(m, 1H), 1.29 - 1.26 (m, 1H), 0.84 - 0.80 (m, 2H), -0.05 (s, 9H). Mass spec.:
737.37
(MH)+.
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid {2-
(4-
isobutyl-piperazin-1-yl)-2-oxo-1-[1-(2-trimethylsilanyl-ethanesulfonyl)-1 H-
indazol-
5-ylmethyl]-ethyl } -amide
O~ NH
N
O\/N
'N~ H
N
N~ O~N
O.~ '
~N~
Si'
' H-NMR (CD3CN, 500 MHz) 8 9.84 (s, 1 H), 8.37 (s, 1 H), 7.98 (d, J = 8.5 Hz,
1 H),
7.74 (s, 1 H), 7.52 (dd, J = 8.8, 1.5 Hz, 1 H), 7.11 - 7.09 (m, 1 H), 7.06 -
7.03 (m, 1 H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
84
7.02 - 6.98 (m, 2H), 5.97 (d, J = 8.2 Hz, 1H), 5.02 (dd,J = 14.3, 7.3 hz, 1H),
4.39
4.33 (m, 1H), 4.14 - 4.07 (m, 2H), 3.53 - 3.50 (m, 3H), 3.46 - 3.42 (m, 2H),
3.45
3.39 (m, 1H), 3.20 - 3.06 (m, SH), 2.89 - 2.83 (m, 2H), 2.30 - 2.27 (m, 4H),
2.21
2.17 (m, 1 H), 1.74 - 1.70 (m, 3H), 0.86 (d, J = 6.7 Hz, 6H), 0.81 - 0.77 (m,
2H),
0.04 (s, 9H). Mass spec.: 737.40 (MH)+.
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid f2-
(1,4-
dioxa-8-aza-spiro[4.5]dec-8-yl)-2-oxo-1-[ 1-(2-trimethylsilanyl-
ethanesulfonyl)-1 H-
indazo l-5-ylinethyl]-ethyl ) -amide
O~NH
NI ~
O\ /N
'N~H
N/
O N
O~~S O
°~ of
S;-
'H-NMR (CD3CN, 500 MHz) 8 9.34 (s, 1H), 8.36 (s, 1H), 7.97 (d, J = 8.5 Hz,
1H),
7.74 (s, 1 H), 7.52 (dd, J = 8.5, 1.5 Hz, 1 H), 7.11 - 7.08 (m, 1 H), 7.06 -
7.03 (m, 1 H),
7.02 - 6.98 (m, 2H), 5.98 (d, J = 8.2 Hz, 1H), 5.06 (dd, J = 14.6, 7.3 Hz,
1H), 4.39 -
4.32 (m, 1 H), 4.13 - 4.03 (m, 2H), 3.92 - 3.88 (m, 2H), 3.71 - 3.66 (m, 1 H),
3.63 -
3.53 (m, 2H), 3.48 - 3.45 (m, 1 H), 3.44 - 3.40 (m, 2H), 3.19 (dd, j = 13.4,
6.5
Hz, l H), 3.08 (dd, J = 13.7, 7.3 Hz, 1 H), 2.85 (t, J = 12.8 Hz, 2H), 2.32 -
2.20 (m,
4H), 1.73 - 1.70 (m, 2H), 1.67 - 1.51 (m, 3H), 1.38 - 1.33 (m, 1H), 0.81 -
0.77 (m,
2H), -0.04 (s, 9H). Mass spec.: 738.32 (MH)+.
Example 2
(R)-4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(1H indazol-5-ylmethyl)-2-oxo-ethyl)-amide ( )
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
O
N
N ~ ~ lli, N
'N / H N ~O
'~~'H
N
O
N~NH
A solution of (R)-4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-
carboxylic acid {2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-
ethanesulfonyl)-1H indazol-5-ylmethyl]-ethyl]-amide (568 mg, 0.73 mmol) and
5 cesium fluoride (1.l l g, 7.31 mmol) was heated at 80°C in
acetonitrile (50 mL) for
4.5 h. The reaction mixture was concentrated and the residue was subjected to
flash
column chromatography (methylene chloride/methanol/triethylamine, 94:5:1 ) to
give
280 mg (63% yield) of the title compound as a white solid with 98.2% ee as
determined by HPLC analysis using a Chirocel OD column with 20% B (A =
ethanol,
10 B = 0.05% diethylamine in hexanes) as eluent (Retention times: 9.51 min for
title
compound and 15.9 min for S-enantiomer). 'H-NMR (CD30D, 500 MHz) 8 8.04 (s,
0.75H), 8.03 (s, 0.25H), 7.67 (s, 0.75H), 7.65 (s, 0.25H), 7.56 (d, J = 8.5
Hz, 0.75H),
7.51 (d, J = 8.5 Hz, 0.25H), 7.41 (d, J = 8.5 Hz, 0.75H), 7.31 (d, J = 8.5 Hz,
0.25H),
7.19-7.12 (m, 2H), 6.97-6.94 (m, 1 H), 6.80 (d, J = 7.9 Hz, 1 H), 5.08-5.05
(m, 1 H),
15 4.60-4.53 (m, 1H), 4.48-4.40 (m, 1H), 4.37 (s, 1.5H), 4.26 (s, 0.5H), 4.24-
4.14 (m,
2H), 4.06-3.97 (m, 1 H), 3.15 (d, J = 7.9 Hz, 1.5H), 3.12-3.05 (m, 0.5H), 2.94-
2.86
(m, 3H), 2.57-2.51 (m, 1.5H), 2.47-2.42 (m, 1H), 2.37-2.33 (m, 0.75H), 2.03-
2.02 (m,
1.5H), 1.87-1.75 (m, 3.75H), 1.73-1.68 (m, 2H), 1.67-1.54 (m, 3H), 1.53-1.44
(m,
4H), 1.43-1.34 (m, 2H), 1.30-1.26 (m, 1H), 0.83-0.77 (m, 0.75H),
20 -0.16 to -0.24 (m, 0.75H). Mass spec.: 613 (MH)+.
Similarly prepared:
Example 3
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [2-
25 [1,4']bipiperidinyl-1'-yl-1-(1H indol-5-ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
86
N ~NH
O~N~ O
NH
N ( ~ O~ N
H
N
'H-NMR (DMSO-d6, 500 MHz) ~ 10.99 (s, 0.6 H), 10.96 (s, 0.4 H), 10.85 (s, 1H),
7.41 (s, 0.4H), 7.36 (s, 0.6H), 7.33 (d, J = 8.0 Hz, 0.6H), 7.29 - 7.26 (m, 1
H), 7.16 -
7.14 (m, 1 H), 7.10 (d, J = 7.6 Hz, 0.4 H), 7.02 - 6.96 (m, 4H), 6.81 (br s, 1
H), 6.37 -
6.35 (m, 1H), 4.86 (q, J = 8.0 Hz, 0.6 H), 4.80 (q, J = 7.5 Hz, 0.4 H), 4.45
(br s, 1H),
4.38 - 4.32 (m, 1 H), 4.21 - 4.16 (m, 1 H), 3.98 (br s, 1 H), 3.18 (d, J = 5.2
Hz, 0.6H),
3.04 - 2.92 (m, 2.4 H), 2.82 - 2.74 (m, 4H), 2.37 - 2.33 (m, 2H), 2.25 - 2.08
(m,
4H), 2.04 - 1.90 (m, 2H), 1.47 - 1.24 (m, l OH), 0.75 - 0.71 (m, 1 H). LC/MS:
tR =
1.90 min, 598.42 (MH)+.
Example 4
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(1H indazol-5-ylmethyl)-2-oxo-ethyl]-amide
O~ NH
N ~
O~N
~N'H
N
N ~ ~ O~ N
H
N
1H-NMR (DMSO-d6, 500 MHz) S 10.70 (s, 1H), 8.22 (d, J = 8.2 Hz, 0.6H), 8.11
(s,
0.4H), 8.00 (s, 0.6H), 7.89 (d, J = 9.1 Hz, 0.4 H), 7.62 - 7.57 (m, 1 H), 7.50
- 7.43 (m,
1 H), 7.30 - 7.26 (m, 1 H), 7.14 - 7.08 (m, 1 H), 6.99 - 6.95 (m, 2H), 6.85
(br s, 1 H),
4.89 - 4.80 (m, 1H), 4.45 - 4.31 (m, 2H), 4.18 - 4.00 (m, 2H), 3.26 - 3.16 (m,
1H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
87
3.09 - 2.96 (m, 2H), 2.82 - 2.73 (m, 4H), 2.38 - 2.34 (m, 2H), 2.24 - 2.08 (m,
4H),
2.03 - 1.88 (m, 2H), 1.47 - 1.22 (m, 10H), 0.90 - 0.84 (m, 1H). LC/MS: tR =
1.73
min, 599.32 (MH)+.
Example 5
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid (2-
[ 1,4']bipiperidinyl-1'-yl-1-( 1 H-indol-5-ylmethyl)-2-oxo-ethyl]-amide
H
O\ /N
~N ~ /
O H
N N
~N
O
~N
N
H
A mixture of 4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-
carboxylic acid {2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-trimethylsilanyl-
ethanesulfonyl)-1H-indol-5-ylmethyl]-ethyl-amide (52 mg, 0.067 mmol), cesium
fluoride (51 mg, 0.33 mmol) in acetonitrile (5 mL) was heated at 80°C
for 4h. The
solvents were removed in vacuo and the residue was subjected to chromatography
on
silica gel using methylene chloride/methanol/triethylamine (93:5:2) as eluent
to
afford the title compound as a white solid (70% yield). 'H-NMR (CD3CN, 500
MHz) 8 9.30 (s, 1 H), 7.48 (s, 1 H), 7.42 (s, 1 H), 7.39 (d, J = 8.2 Hz,
0.6H), 7.36 (d, J
= 8.2 Hz, 0.4 H), 7.24 - 7.21 (m, 1 H), 7.19 (t, J = 7.9 Hz, 1 H), 7.12 - 7.09
(m, 1 H),
7.06 (d, J = 8.2 Hz, 0.6 H), 7.02 (d, J = 8.2 Hz, 0.4 H), 6.95 (t, J = 7.4 Hz,
1, 4.04 -
3.93 (m, 1 H), 3.07 - 3.02 (m, 1.6H), 2.95 (dd, J = 13.7, 7.1 Hz, 0.4 H), 2.85
- 2.72
(m, 3H), 2.56 - 2.37 (m, 3H), 2.42 - 2.37 (m, 1 H), 1.99 - 1.95 (m, 7H), 1.76 -
1.51
(m, 8H), 1.45 -1.40 (m, 3H). LC/MS: tR = 1.91 min, 612.44 (MH)+.
Example 6
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(1H-indazol-5-ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
88
H
O\ /N
~N ~ /
O H
N N
~N
O
~N
,N
N
H
Purified by silica gel chromatography using methylene
chloride:methanolariethylamine (93:5:2) as eluent to afford the title compound
as a
white solid (90% yield). 'H-NMR (CD30D, 500 MHz) ~ 8.04 (s, 0.7 H), 8.02 (s,
0.3
H), 7.67 (s, 0.7 H), 7.65 (s, 0.3H), 7.56 (d, J = 8.5 Hz, 0.7 H), 7.51 (d, J =
8.5 Hz, 0.3
H), 7.40 (d, J = 8.5 Hz, 0.7 H), 7.33 (d, J = 8.5 Hz, 0.3 H), 7.19 - 7.12 (m,
2H), 6.97
- 6.94 (m, 1 H), 6.80 (d, J = 8.0 Hz, 1 H), 5.08 - 5.05 (m, 1 H), 4.59 - 4.54
(m, 1 H),
4.48 - 4.42 (m, 1 H), 4.37 (s, 1 H), 4.27 - 4.20 (m, 2H), 4.04 (d, J = 13.4
Hz, 0.3 H),
3.99 (d, J = 13.4 Hz, 0.7 H), 3.19 - 3.08 (m, 2H), 2.94 - 2.86 (m, 3H), 2.57
(br s,
2H), 2.51 - 2.36 (m, 2H), 2.07 - 2.05 (m 1 H), 1.90 -1.31 (m, 16 H). LC/MS: tR
=
1.85 min, 613.44 (MH)+. The (R)-enantiomer, whose discrete synthesis is
described
above (Example 1 ), was obtained by chiral separation of the racemate by
employing
the following conditions: Chiracel OD prep column, 50 x 500 mm, 20 um; A =
EtOH, B= 0.05%diethylamine/hexane; 20%B @ 65 mUmin for 45 min; retention
1 S times: 20.5 min for R and 32.8 min for S enantiomers.
Example 7
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [1-
(1H-
indol-5-ylmethyl)-2-(4-isobutyl-piperazin-1-yl)-2-oxo-ethyl]-amide
~a"b
NH
H
~ N
LC/MS: tR = 2.05 min, 572.31 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
89
Example 8
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [2-
(1,4-
dioxa-8-aza-spiro[4.5]dec-8-yl)-1-( 1 H-indol-5-ylmethyl)-2-oxo-ethyl]-amide
O~ NH
N ~
O~N
~N'H
N ~ / O~ N
H O
of
LC/MS: tR = 2.35 min, 573.26 (MH)+.
Example 9
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [1-
(1H-
indazol-5-ylmethyl)-2-(4-isobutyl-piperazin-1-yl)-2-oxo-ethyl]-amide
0
~NH
N
O\ /N J
~NH
N
N~ O~N
H ~
~N~
LCIMS: tR = 1.86 min, 573.28 (MH)+.
Example 10
(~)-4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [2-
(1,4-
dioxa-8-aza-spiro[4.5]dec-8-yl)-1-(1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
O~ NH
N ~
O~N
~N'H
N
N ~ ~ O~ N
H O
of
LC/MS: tR = 2.18 min, 574.23 (MH)+.
Example 11
(~)-4-(2-Oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
(1,4-
dioxa-8-aza-spiro[4.5]dec-8-yl)-1-(1H indazol-5-ylmethyl)-2-oxo-ethyl]-amide
N
i
HN
_ O
O H~N
N 1O
N
NH
O O
To a solution of the 3-(1H indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H
quinazolin-3-yl)-piperidine-1-carbonyl]-amino]-propionic acid (95 mg, 0.21
mmol)
10 and N,N diisopropylethylamine (0.14 mL, 0.82 mmol) in dimethylformamide (5
mL)
was added a solution of 1,4-dioxa-8-azaspiro[4,5]decane (32 mg, 0.23 mmol) and
PyBOP° (107 mg, 0.21 mmol) in methylene chloride (5 mL). The reaction
mixture
was stirred for 16 h at room temperature. All solvent was removed using high
vacuum. The residue was subjected to flash column chromatography using
15 methylene chloride/methanol/ triethylamine (93:5:2) to give the title
compound as a
white solid (67 mg, 56% yield). 1H-NMR (CDC13, 500 MHz) S 10.52 (s, 1H), 7.97
(s, 1 H), 7.54 (s, 1 H), 7.37 (d, J = 8.6 Hz, 1 H), 7.20 (d, J = 10.7 Hz, 1
H), 7.16 (t, J =
7.2 Hz, 1 H), 7.04 (d, J = 7.6 Hz, 1 H), 7.01 (s, 1 H), 6.94 (t, J = 8.6 Hz, 1
H), 6.67 (d, J
= 7.6 Hz, 1 H), 5.64 (d, J = 7.9 Hz, 1 H), 5.16 (dd, J = 15.0, 6.7 Hz, 1 H),
4.56-4.49 (m,
20 1H), 4.25 (s, 2H), 4.11 (br t, J = 15.6 Hz, 2H), 3.92-3.84 (m, 4H), 3.73-
3.69 (m, 1H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
91
3.60-3.56 (m, 1 H), 3.48-3.43 (m, 1 H), 3.22-3.17 (m, 1 H), 3.11 (d, J = 6.7
Hz, 2H),
2.90-2.85 (m, 2H), 2.68-2.60 (m, 4H), 1.67-1.61 (m, 2H), 1.54-1.49 (m, 2H).
Mass
spec.: 588 (MH)+.
4-Bromo-2,6-dimethylphenyldiazo-t-butyl sulfide
~S
N~ N
Br
4-Bromo-2,6-dimethylaniline (20.00 g, 100 mmol) was ground to a powder
with a mortar and pestle and then suspended in 24% hydrochloric acid (41 mL).
The
stirred mixture was cooled to -20°C and treated with sodium nitrite
(7.24 g, 1.05
equiv) in water (16 mL), dropwise over 40 min while the temperature was
maintained
below -5°C. After a further 30 min at -5°C to -20°C, the
mixture was buffered to ca.
pH S with solid sodium acetate. This mixture (kept at ca. -10°C) was
added in
portions to a stirred solution of t-butyl thiol (11.3 mL, 1 equiv) in ethanol
(100 mL)
at 0°C over ca. 10 min. Following addition, the mixture was stirred at
0°C for 30 min
and then crushed ice (ca. 150 mL) was added. The mixture was stored in the
refrigerator overnight. The resulting light-brown solid was collected by
filtration,
washed with water, and dried under high vacuum for several h.
(26.90 g, 89%). The compound appeared to be stable as a solid but underwent
significant decomposition when recrystallization from ethanol was attempted.
'H-
NMR (CDCl3, 500 MHz) 8 1.58 (9H, s), 1.99 (6H, s), 7.21 (2H, s). Mass spec.:
303.05 (MH)+.
5-Bromo-7-methylindazole
HN-N
Br
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
92
Into a flame-dried round bottom flask, 4-bromo-2,6-dimethylphenyldiazo-t-
butyl sulfide (12.50 g, 41.5 mmol) and potassium t-butoxide (46.56 g, 10
equiv) were
combined. A stir bar was added and the mixture placed under nitrogen. To this
was
added dry DMSO (120 mL). The mixture was stirred vigorously overnight at rt.
The
reaction mixture was then carefully poured into a mixture of crushed ice (400
mL)
and 10% hydrochloric acid (200 mL). The resulting suspension was left to stand
at
4°C overnight and the solid was collected by filtration and washed with
water. The
crude solid was dissolved in 5:1 methylene chlorideJmethanol and the solution
dried
over magnesium sulfate and evaporated to give the product as an off white
solid
(7.60 g, 87%). 'H-NMR (CDCl3/CD30D, 500 MHz) 8 2.51 (3H, s), 7.22 (1H, s),
7.69 ( 1 H, s), 7.94 ( 1 H, s). Mass spec.: 211.03 (MH)+.
7-methylindazole-5-carboxaldehyde
HN-N
H ~O
5-Bromo-7-methylindazole (6.10 g, 28.9 mmol) and sodium hydride (60% in
mineral oil, 1.27 g, 1.1 equiv) were weighed into a flame-dried round-bottom
flask
containing a magnetic stir bar. Under a nitrogen atmosphere at room
temperature,
dry tetrahydrofuran (30 mL) was added. The mixture was stirred at room
temperature for 15 min, during which time it became homogeneous. The stirred
mixture was cooled to -70°C and a solution of sec-butyllithium in
cyclohexane
(1.4M, 45 mL, 2.2 equiv) was added over several minutes. After 1 h at -
70°C,
dimethylformamide (10 mL) was added over several minutes. The mixture was
allowed to warm to room temperature and was stirred overnight. It was then
cooled
to 0°C and carefully treated with 1N hydrochloric acid (60 mL). After a
few minutes,
solid sodium bicarbonate was added to basify the mixture to pH 9-10. The
layers
were separated and the aqueous phase washed twice with ethyl acetate. The
combined organic phases were extracted with 0.8M sodium hydrogen sulfate (3 x
125
mL). The combined aqueous phases were washed with ethyl acetate (100 mL) and
then the pH was adjusted to ca. 10 with solid sodium hydroxide. The resulting
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
93
suspension was extracted with ethyl acetate (3 x 150 mL). The combined organic
phases were washed with brine, dried (magnesium sulfate) and evaporated to
give the
product as a light-tan solid (3.01 g, 65%). 'H-NMR (CDC13, S00 MHz) 8 2.63
(3H,
s), 7.73 ( 1 H, s), 8.12 ( 1 H, s), 8.25 ( 1 H, s), 10.03 ( 1 H, s). Mass
spec.: 161.06 (MH)+.
2-Benzyloxycarbonylamino-3-(7-methyl-1H-indazol-5-yl)-acrylic acid methyl
ester
HN-N
/ I
\ /
H
\. N~O \
I IO
H3C0 O
A stirred solution of N-benzyloxycarbonyl-a-phosphonoglycine trimethyl
ester (5.51 g, 1.2 equiv.) in tetrahydrofuran (30 mL) at room temperature was
treated
with tetramethylguanidine (1.91 mL, 1.1 equiv). After 10 min, 7-methylindazole-
5-
carboxaldehyde (2.22 g, 13.86 mmol) in tetrahydrofuran (20 mL) was added.
Disappearance of starting material was monitored by TLC and LC/MS. After 5
days
at room temperature, the solvent was evaporated and the residue dissolved in
ethyl
acetate. The solution was washed with 2% phosphoric acid and brine, dried
(magnesium sulfate) and evaporated. The residue was purified by flash
chromatography on silica gel, eluting with 1) 1:l and 2) 2:1 ethyl
acetatelhexane, to
give the product as a colorless foam (4.93 g, 97%). 1H-NMR (CDC13, S00 MHz) 8
2.43 (3H, s), 3.80 (3H, s), 5.12 (2H, s), 6.66 (1H, s), 7.28 (5H, brs), 7.33
(1H, s), 7.47
(1H, s), 7.74 (1H, s), 7.96 (1H, s). Mass spec.: 366.16 (MH)+.
(~)-2-Amino-3-(7-methyl-1H-indazol-5-yl)-propionic acid methyl ester
HN-N
/I
NHZ
~O ~O
A solution of 2-benzyloxycarbonylamino-3-(7-methyl-1H-indazol-5-yl)-
acrylic acid methyl ester (4.93 g, 13.49 mmol) in methanol (125 mL) was
degassed
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
94
by bubbling nitrogen through it for 30 min and then 10% palladium on charcoal
(0.6
g) was carefully added. The mixture was hydrogenated at 40 psi in a Parr
shaker
apparatus overnight. The catalyst was removed by filtration through a pad of
celite
and the filtrate was concentrated in vacuo to give the product as a colorless
foam
(3.62 g, quant.). 'H-NMR (CD30D, 500 MHz) b 2.45 (3H, s), 2.99 (1H, Abq), 3.22
(1H, Abq), 3.74 (3H, s), 3.89 (1H, m), 6.91 (1H, s), 7.31 (1H, s), 7.73 (1H,
s). Mass
spec.: 234.11 (MH)+.
Example 12
(~)-3-(7-Methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino)-propionic acid methyl ester
HN-N H
/ ~ O\ /N \
\ ~ ~N' ~ /
H
N NJ
~O O
A stirred solution of (~)-2-amino-3-(7-methyl-1H-indazol-5-yl)-propionic
acid methyl ester (162.9 mg, 0.698 mmol) in methylene chloride (3 mL) at room
temperature was treated with carbonyl diimidazole (113.2 mg, 1 equiv). After
1.5 h
at room temperature, 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one (161.5
mg, 1
equiv.) was added. The mixture was stirred at room temperature overnight. A
white
precipitate had formed that was shown to be the desired product. The solvent
was
evaporated and the residue triturated with methylene chloride. The product was
collected by filtration, washed with methylene chloride and dried in vacuo to
give a
white solid (241.5 mg, 71%). Some product remained in the mother liquors. 1H-
NMR (dimethylformamide-d~, 500 MHz) S 1.75 (4H, m), 2.78 (3H, s), 2.7-3.1 (4H,
m), 3.35 (2H, m), 3.86 (3H, s), 4.44 (2H, s), 4.57 (1H, m), 4.72 (1H, m), 7.11
(3H,
m), 7.31 (1H, s), 7.34 (2H, m), 7.72 (1H, s), 9.34 (1H, s). Mass spec.: 491.13
(MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
Similarly prepared:
Example 13
3-(7-Methyl-1 H-indazol-5-yl)-2-[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-(1
H)-
quinazoline)carbonyl amino]-propionic acid methyl ester
N-NH
O
I / HN"NH
H
N~N \
\O O 'I0
1H-NMR (DMSO-d6) b 1.59 (4H, m), 2.46 (3H, s), 3.00 -3.08 ( 4H, m), 3.6 ( 3H,
s),
3.78-3.81 (2H, m), 4.30-4.32 (1H, m),6.78-6.88 (4H, m), 7.03 (1H, s), 7.10
(IH,
m),7.13 (1H, s),7.41 ( lH,s), 7.96 (1H, s), 9.12 (1H, s). Mass spec.: 477.11
(MH)+.
10 Example 14
3-(7-Methyl-1 H-indazol-5-yl)-2-( 1,2-dihydro-2-oxospiro-4H-3,1-dihydro-
benzoxazine-4'4-piperidine-carbonylamino)-propionic acid methyl ester
N-NH
O
O~NH
H
N~N \
I IO
H3C0 O
Mass spec.: 478.15 (MH)+.
3-(7-Methyl-1 H-indazol-5-yl)-2 { 3',4'-dihydro-2'-oxospiro-(piperidine-4,4'-(
1 H)-
quinolinecarbonyl amino}-propionic acid methyl ester
N-NH
O
NH
H
N~N \ I
I'O
H3C0 O
'H-NMR (DMSO-d6) 8 1.42-1.56 (4H, m), 2.47 (3H, s), 2.50-2.54 (1H, d), 2.60-
2.64
(1H, d), 2.98-3.06 4H, m), 3.60 (3H, s) 3.80 (2H, m), 4.30 (1H, m), 6.86 (2H,
d), 6.95
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
96
(2H, m), 7.15 (1H, m ), 7.40 (1H, s), 7.95 (1H, s), 8.32 (1H, s), 10.14 (1H,
s), 13.05
( 1 H, s). Mass spec.: 476.17 (MH)+.
3-(7-Methyl-1 H-indazol-5-yl)-2-[2'-phenyl-1',3',8'-triaza-spiro(4',5')deo-1-
ene-8-
carbonyl amino]-propionic acid methyl ester
HN-N
O
H NH
N~N~N
H3C0 O O
1H-NMR (DMSO-db) 8 1.50 (2H, m), 1.68 (2H, m), 2.46 (3H, s was overlapped with
DMSO), 3.05 (2H, m), 3.30 (2H, m), 3.60 (3H, s), 3.86 (2H, m), 4.28 (1H, m),
6.98
( 1 H, d), 7.04 ( 1 H, s), 7.40 ( 1 H, s), 7.5 8 (2H, m), 7. 65 ( 1 H, m),
8.00 ( 1 H, s), 8.04 (2H,
m). Mass spec.: 489.15 (MH)+.
Example 15
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid
HN-N H
\ O\ /N \
\ ~ ~N' ~ /
H
N NJ
O
Ho 0
A suspension of (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
(240.0 mg, 0.489 mmol) in 1:1 tetrahydrofuran/methanol (20 mL) at room
temperature was treated with a solution of lithium hydroxide (140.5 mg, 7
equiv) in
water (10 mL). Within 1 min, the mixture became homogeneous and it was left to
stand at 4°C overnight. The solvents were evaporated at ca. 30°C
and the pH was
adjusted to ca. 1 with 1N hydrochloric acid. The resulting white suspension
was
stored at 4°C for several hours and the product was collected by
filtration, washed
with a small amount of water, and dried in vacuo (169.0 mg, 73%). Solid sodium
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
97
chloride was added to the filtrate resulting in precipitation of more product
(5.2 mg,
total yield 75%). 'H-NMR (CD30D, 500 MHz) 8 1.2-1.7 (4H, m), 2.58 (3H, s), 2.5-
3.2 (4H, m), 3.3 5 (2H, m), 4. I 5 (2H, m), 4.3 6 ( I H, m), 4.60 ( 1 H, m),
6.79 ( I H, d),
6.96 ( 1 H, t), 7.18 (3H, m), 7.49 ( 1 H, s), 8.00 ( I H, s). Mass spec.:
477.13 (MH)+.
Similarly prepared:
3-(7-Methyl-1 H-indazol-5-yl)-2-[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-(I
H)-
quinazolinecarbonyl amino]-propionic acid
N-NH
O
HN"NH
I\
H /
N~N \ I
I IO
HO O
1H-NMR (DMSO-d6) S 1.58 (4H, m), 2.46 (3H, s), 3.00-3.23 (3H, m), 3.78-3.91
(3H,
m), 3.88 (2H, m) 4.28 (1H, s), 6.70 (1H, d), 6.75-6.85 ( 3H, m), 7.04 (1H, d),
7.11
( 1 H, m) 7.18 ( 1 H, s), 7.96 ( 1 H, s), 13.02 ( 1 H, m). Mass spec.: 463.09
(MH)+.
3-(7-Methyl-1 H-indazol-5-yl)-2-( 1,2-dihydro-2-oxospiro-4H-3,1-dihydro-
I 5 benzoxazine-4'4-piperidine-carbonylamino)-propionic acid methyl ester
N-NH
O
/ O~NH
H /
N~N \ I
O
HO O
1H-NMR (DMSO-d6) 8 1.63-1.98 (4H, m), 2.46 (3H, s, 7-Me was overlapped with
DMSO), 2.98-3.32 (4H, m), 3.90 (2H, m), 4.28 (1H, m), 6.78 (1H, d ), 6.87 (2H,
m),
6.96 ( 1 H, m), 7.05 ( I H, s), 7.24 ( 1 H, m), 7.41 ( 1 H, s), 7.96 ( 1 H,
s), I 0.22 ( 1 H, s)
12.42 (1H, br. ) 13.02 (1H, m). Mass spec.: 464.07 (MH)+.
3-(7-Methyl-1 H-indazol-5-yl)-2 { 3',4'-dihydro-2'-oxospiro-(piperidine-4,4'-(
1 H)-
quinoline- carbonyl amino}-propionic acid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
98
N-NH
O
NH
H
N~N \ I
'1O
HO O
'H-NMR (DMSO-d6) S 1.39-1.45 (2H, m), 1.53-1.56 (2H, m), 2.46 (3H, s), 2.50-
2.54
(1H, d), 2.60-2.63 (1H, d), 2.88-3.00(3H, m), 3.09-3.11 (1H, m), 3.78-3.81
(2H, m),
4.27 ( 1'H, m), 6.69-6.70 ( 1 H, d), 6.86-6.87 ( 1 H, d), 6.93-6.94 ( 1 H,
m)6.99-7.00 ( 1 H,
m), 7.05 ( 1 H, m), 7.41 ( 1 H, s), 7.95 ( 1 H, s), 10.13 ( 1 H, s), 12.50 ( 1
H, m), 13.03 ( 1 H,
m). Mass spec.: 462 (MH)+.
3-(7-Methyl-1 H-indazol-5-yl)-2-[2'-phenyl-1',3',8'-triaza-spiro(4',5')deo-1-
ene-8-
carbonyl amino]-propionic acid
HN-N
O
I
H NH
N~NWN I \
HO O O
'H-NMR (DMSO-d6) 8 1.36 (2H, m), 1.63 (2H, m), 2.46 (3H, s was overlapped with
DMSO), 2.98-3.03 (2H.m), 3.09-3.11 (2H, m), 3.86 (2H, m), 4.21 (1H, m), 6.69
(1H,
m), 7.04 (1H, s), 7.40 (1H, s), 7.52-7.58 (3H, m), 7.99 (3H, m), 11.55 (1H,
m), 13.00
(1H, m). Mass spec.: 475.08 (MH)+.
Example 16
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-methyl-1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
99
HN'N H
/ O N
N ~ /
H
N NJ
O
~N O
~N
A stirred solution of (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid (65.7
mg, 0.138 mmol) in 2:1 dimethylformamide/methylene chloride (1.5 mL) at
0°C was
treated with 4-(1-piperidyl)-piperidne (46.5 mg, 2 equiv),
diisopropylethylamine
(0.048 mL, 2 equiv) and PyBOP° (75.5 mg, 1.05 equiv). The ice bath was
allowed to
melt and the mixture was stirred at room temperature overnight. The solvents
were
removed under high vacuum and the residue was purified by flash chromatography
on silica gel, eluting with 18:1 methylene chloride/methanol containing 1
triethylamine, to give the product as a pale-yellow solid (80.4 mg, 93%). IH-
NMR
(CD30D, 500 MHz) 8 -0.28 (1H, m), 0.75 (1H, m), 1.2-2.0 (12H, m), 2.08 (2H,
m),
2.4-2.5 (3H, m), 2.59 (3H, s), 2.68 (2H, m), 2.90 (4H, m), 3.08 (4H, m), 3.9-
5.1 (4H,
several m), 6.81 ( 1 H, d), 6.96 ( 1 H, t), 7.16 (3 H, m), 7.49 ( 1 H, s),
8.03 ( 1 H, s). Mass
spec.: 627.29 (MH)+.
Similarly prepared:
Example 17
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(7-
methyl-1 H-indazol-5-ylmethyl)-2-oxo-2-piperidin-1-yl-ethyl]-amide
HN-N H
/ \ O~ N
N ~ /
H
N NJ
O
~N O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
100
'H-NMR (CD30D, 500 MHz) 8 0.87 (1H, m), 1.33 (1H, m), 1.47 (2H, m), 1.80 (6H,
m), 2.57 (3H, s), 2.89 (2H, m), 3.06 (2H, m), 3.18 (4H, m), 3.40 (2H, m), 3.61
(1 H,
m), 4.16 ( 1 H, m), 4.28 ( 1 H, Abq), 4.43 ( 1 H, m), 5.02 ( 1 H, m), 6.51 ( 1
H, d), 6.79
( 1 H, d), 6.96 ( 1 H, t), 7.11 ( 1 H, d), 7.15 ( 1 H, t), 7.48 ( 1 H, s),
8.01 ( 1 H, s). Mass
spec.: 544.24 (MH)+.
Example 18
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
dimethylcarbamoyl-2-(7-methyl-1 H-indazol-5-yl)-ethyl]-amide
HN-N H
/ ~ O\ /N \
\ ~ ~N' ~ /
H
N NJ
~N O O
'H-NMR (CD30D, 500 MHz) 8 1.12 (2H, d), 1.64 (2H, m), 2.57 (3H, s), 2.74 (1H,
m), 2.87 (3H, s), 2.89 (3H, s), 2.86 (2H, m), 3.07 (2H, m), 3.20 (1H, m), 4.17
(1H,
m), 4.25 ( 1 H, Abp, 4.43 ( 1 H, m), 4.97 ( 1 H, m), 6.79 ( 1 H, d), 6.95 ( 1
H, t), 7.0-7.4
(3H, m), 7.48 (1H, d), 8.01 (1H, s). Mass spec.: 504.15 (MH)+.
Example 19
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(7-
methyl-1 H-indazol-5-ylmethyl)-2-(4-methyl-piperazin-1-yl)-2-oxo-ethyl]-amide
HN-N H
/ ~ O\ /N \
\ ~ ~N' ~ /
H
N NJ
~N O O
/N J
'H-NMR (CD30D, 500 MHz) 8 1.30 (2H, m), 1.66 (2H, m), 1.78 (1H, m), 1.90 (1H,
m), 2.00 (3H, s), 2.19 (1H, m), 2.35 (1H, m), 2.58 (3H, s), 2.88 (2H, m), 3.09
(2H, d),
3.10-3.45 (3H, m), 3.66 (1H, m), 4.19 (2H, d), 4.20 (2H, s), 4.43 (1H, m),
4.98 (1H,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
101
t), 6.80 ( 1 H, d), 6.9 5 ( 1 H, t), 7.11 (2H, m), 7.16 ( 1 H, t), 7.47 ( 1 H,
s), 8.02 ( 1 H, s).
Mass spec.: 559.23 (MH)+.
Example 20
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(7-
methyl-1 H-indazol-5-ylmethyl)-2-oxo-2-pyrrolidin-1-yl-ethyl ]-amide
HN-N H
~ O\ /N \
\ ~ ~N' ~ /
H
N NJ
~N O O
'H-NMR (CD30D, 500 MHz) & 1.40-1.90 (5H, m), 2.02 (3H, brs), 2.57 (3H, s),
2.86
(1H, m), 2.89 (2H, q), 3.09 (2H, m), 3.16 (1H, m), 3.25 (2H, m), 3.40 (1H, m),
3.56
( 1 H, m), 4.17 (2H, d), 4.27 (2H, s), 4.40 ( 1 H, m), 4. 69 ( 1 H, t), 6. 80
( 1 H, d), 6.95 ( 1 H,
t), 7.10 (1H, s), 7.16 (1H, m), 7.48 (1H, s), 7.53 (1H, m), 8.01 (1H, s). Mass
spec.:
530.19 (MH)~.
Example 21
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(7-
methyl-1 H-indazol-5-ylmethyl)-2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
amide
HN-N H
/ ~ O~ N \
N ~ /
H
N NJ
~N O O
\ NJ
N,J
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
102
'H-NMR (CD30D, 500 MHz) 8 1.38 (1H, t), 1.68 (2H, m), 1.81 (1H, m), 2.30 (1H,
m), 2.53 (3H, s), 2.95 (4H, m), 3.13 (2H, d), 3.22 (1H, m), 3.35-3.65 (4H, m),
3.79
( 1 H, m), 4.18 (2H, d), 4.31 (2H, s), 4.42 ( 1 H, m), 4.99 ( 1 H, t), 6.64
(2H, d), 6.80 ( 1 H,
d), 6.89 ( 1 H, m), 6.96 ( 1 H, t), 7.14 (3 H, m), 7.51 ( 1 H, s), 7.99 ( 1 H,
s), 8.10 (2H, d),
8.16 (1H, m). Mass spec.: 622.26 (MH)+.
Example 22
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(7-
methyl-1 H-indazol-5-ylmethyl)-2-oxo-2-(4-pyridin-2-yl-piperazin-1-yl)-ethyl]-
amide
HN-N H
/ ~ O~ N \
\ ~ N ~ /
H
N NJ
~N O O
N NJ
/
H-NMR (CD30D, S00 MHz) b 1.27 ( 1 H, m), 1.38 ( 1 H, m), 1.67 (2H, m), 1.84 (
1 H,
m), 2.54 (3H, s), 2.65 (1H, m), 2.88 (2H, m), 3.15 (4H, m), 3.35 (1H, m), 3.58
(3H,
m), 3.77 ( 1 H, m), 4.18 (2H, d), 4.30 (2H, s), 4.42 ( 1 H, m), 5.01 ( 1 H,
t), 6.62 ( 1 H, d),
6.70 ( 1 H, t), 6. 80 ( 1 H, d), 6.95 ( 1 H, t), 7.10 3 H, m), 7.5 0 ( 1 H,
s), 7. 54 ( 1 H, t), 7.99
(1H, s), 8.05 (1H, 7). Mass spec.: 622.25 (MH)+.
Example 23
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1,4-bipiperidin]-1-yl-2-oxoethyl]-
2',3'-
dihydro-2'-oxospiro-[piperidine-4,4'-( 1 H)-quinazoline]-1-carboxamide
HN-N
O
HN' \NH
H
N~N
IOI
~N O
~N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
103
'H-NMR (DMSO-db, 500 MHz) 8 1.2-1.73 (14H, m), 2.46 ( 3H, s), 2.75-3.24 (12H,
m), 3.87 (2H, m), 4.45 (1H, m), 4.78-4.85 (1H, m), 6.80 (IH,m), 6.86 (1H, m),
7.05
1 H, m), 7.12 ( 1 H, m), 7.21 ( 1 H, m), 7.27 (2H, m), 7.98 ( 1 H, m), 9.23 (
1 H, m). Mass
spec.: 613.25 (MH)+
Example 24
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-( I -piperidinyl)-2-oxoethyl]-2',3'-
dihydro-
2'-oxospiro-[piperidine-4,4'-(1 H)-quinazoline]-1-carboxamide
HN-N
\ O
HN' \NH
H
N~N
IOI
~N O
'H-NMR (CD30D, 500 MHz) 8 0.87 ( 1H, m), 1.28-1.47 (5H, m), 1.74-1.85 (4H, m),
2.53 (3H, s), 3.02-3.38 (8H, m), 3.92 (2H, m), 5.02 (1H, m), 6.82 (1H, d),
6.99 (1H,
d), 7.04-7.09 (2H, m), 7.17 (1H, m), 7.32 (2H, s), 7.45 (1H, s), 7.96 (1H, s).
Mass
spec.: 530.17 (MH)+.
Example 25
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1,4-bipiperidin]-I-yl-2-oxoethyl]-
1',2'-
dihydro-2'--oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
HN-N
/ I O
O
H ~NH
N~N
N O IO
GN
'H-(DMSO-db, 500 MHz) 8 1.88 (14H, m), 2.64 (3H, s), 2.78 (l2H,m), 4.0 (2H,
m),
4.4 ( I H, m),4.85 ( 1 H, m), 6.80-6.8 8 (2H, m), 7.03 (2H, m), 7.11 ( 1 H,
m), 7.23 ( 1 H,
m), 7.36 (2H, m), 7.97 (1H, m). Mass spec.: 614.73 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
104
Example 26
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-(1-piperidinyl)-2-oxoethyl]-1',2'-
dihydro-
2'--oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
HN-N
O
O
H ~NH
N~N J i
N O IO \
G
1H-NMR (DMSO-d6, 500 MHz) 8 1.15-1.91 (10H, m), 2.47 (3H, s), 2.95-3.05 (6H,
m) 3.40 ( 4H, m) 3.95 (2H, d), 4.81 ( 1 H, m), 6.81 ( 1 H, d), 6.88 ( 1 H, d),
6.94 ( 1 H, m),
6.99 (1H, m), 7.04 (1H, s), 7.24 (1H, m), 7.37 (1H, s), 7.96 (1H, s). Mass
spec.:
531.23 (MH)+.
Example 27
(~)-[ 1-Dimethylcarbamoyl-2-(7-methyl-1 H-indazol-5-yl)-ethyl]-1',2'-dihydro-
2'-
oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
HN-N
O
O~NH
H
N~N
~N O IOI \
1
1H-NMR (DMSO-d6, 500 MHz) b 1.68-1.88 (4H, m), 2.47 (3H, m), 2.79 (6H, s),
2.89-3.04 (4H, m), 3.96 (2H, d), 4.75 (1H, m), 6.81 (1H, d), 6.88 (1H, m),
6.93 (1H,
m), 6.98 (1H, m), 7.05 (1H, s), 7.24 (1H, m), 7.43 (1H, s), 7.97 (1H, m), 8.32
(1H, s).
Mass spec.: 491.14 (MH)+.
Example 28
(~)-[ 1-(2-adamantyl-carbamoyl)-2-(7-methyl-1 H-indazol-5-yl)-ethyl]-1',2'-
dihydro-
2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
105
HN-N
O
H O~NH
N~N J
II I
HN O O \
'H-NMR (DMSO-d6, 500 MHz) 8 1.40-1.95 (15H, m), 2.46 (3H, m), 2.89-3.07 (4H,
m), 3.81 ( 1 H, m), 3.90 (2H, m), 4.48 ( 1 H, m), 6.74 (2H, m), 6.86 ( 1 H,
d), 6.97 ( 1 H,
m), 7.11 ( 1 H, s), 7.24 ( 1 H, m), 7. 3 6 ( 1 H, s), 7.44 ( 1 H, s), 7.96 ( 1
H, s). Mass Spec.:
597.27 (MH)+.
Example 29
(~)-1',2'-Dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine-1-
carboxylic acid
[ 1-(7-methyl-1 H-indazol-5-ylinethyl)-2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-
amide
HN-N
O
O
H ~NH
N~N J
N O IOI \ I
NJ
Nr
LC/MS: tR = 1.56 min, 609.14 (MH)+.
Example 30
(~)-1',2'-Dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine-1-
carboxylic acid
{2-(7-methyl-1 H-indazol-5-yl)-1-[(pyridin-4-ylmethyl)-carbamoyl]-ethyl }-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
106
HN-N
O
\ I
O~NH
H
N~N
N O O \
Nr\~H
LC/MS: tR = 1.49 min, 553.12 (MH)+.
Example 31
(~)-1-(7-Methyl-1H-indazol-5-ylmethyl)-2-[1,4-bipiperidin]-1-yl-2-
oxoethyl]3',4'-
dihydro-2'-oxospiro-[piperidine-4,4'-( 1 H)-quinoline]-1-carboxamide
HN-N
O
H NH
N~N J
N 0 0 \
GN
IH-NMR (DMSO-d6, 500 MHz) 8 1.20-2.00 ( 14H, m), 2.46 (3H, s), 2.38-3.03 (12H,
m), 3.87 (2H, m), 4.34 (1H, m), 4.76-4.87 (1H, m), 6.65 (1H, m), 6.82-7.64
(3H, m),
7.13-7.23 (2H, m), 7.36 (3h, m), 7.96 (1H, s). Mass spec.: 612.32 (MH)+.
Example 32
(~)- 1-(7-Methyl-1H-indazol-5-ylmethyl)-2-[1-piperidinyl]-2-oxoethyl]3',4'-
dihydro-
2'-oxospiro-[piperidine-4,4'-(1 H)-quinoline]-1-carboxamide
HN-N
\
O
H NH
N~N J
N O O \
G
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
107
'H-NMR (DMSO-d6, 500 MHz) 81.10-1.68 (10H, m), 2.46 (3H, s), 2.50-2.60 (2H,
m), 2.82-2.97 (4H, m), 3.39 (2H, m), 3.85 (2H, m), 4.80 (1H, m), 6.68 (1H, m),
6.87
( 1 H, d), 6.94 ( 1 H, m), 7.03 ( 1 H, s), 7.06 ( 1 H, m), 7.15 ( 1 H, m), 7.3
7 ( 1 H, s), 7.40
(1H, s), 7.96 (1H, s). Mass spec.: 529.25 (MH)+.
Example 33
(~)-[1-Dimethylcarbmoyl-2-(7-methyl-1H-indazol-5-yl)-ethy]1-3',4'-dihydro -2'-
oxospiro- [piperidine-4,4'-(1H)-quinoline]-1-carboxamide
HN-N
O
H NH
N~N J
~N O
1H-NMR (DMSO-d6, 500 MHz) 8 1.43 (2H, m), 1.56 (2H, m), 2.46 (3H, s), 2.56
(2H,
m), 2.79 (3H, s), 2.90 (5H, m), 3.84 (2H, m), 4.73 1H, m), 6.69 (1H, d), 2.69
(1H, d),
6.94 ( 1 H, m), 7.05 (2H, m), 7.14 ( 1 H, m), 7.3 7 ( 1 H, s), 7.42 ( 1 H, s),
7.96 ( 1 H, s).
Mass spec.: 489.2 (MH)+.
Example 34
(~)-4-Oxo-2-phenyl-1,3,8-triaza-spiro[4,SJdec-1-ene-8-carboxylic acid{1-(7-
methyl-
1H-indazol-5- y1 methyl)-2-[1,4]bipiperidinyl-1'-yl-2-oxo-ethyl}-amide
HN-N
O
H NH
N~N~N ~ \
N O O
GN
'H-NMR (DMSO-d6, 500 MHz) 8 1.34-2.00 (14H, m), 2.48 (3H, s overlapped with
DMSO), 2.70-3.30 (12H, m), 3.90 ( 2H, m), 4.40 (1H, m), 4.82 (1H, m), 6.82
(1H,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
108
m), 7.04 (1H, s), 7.37 (2H, m), 7.56 (3H, m), 7.98 (3H, m). Mass spec.: 625.29
(MH)+.
Example 35
(~)-4-Oxo-2-phenyl-1,3,8-triaza-spiro[4,5]dec-1-ene-8-carboxylic acid{1-(7-
methyl-
1H-indazol-5- y1 methyl)-2-[1-piperidinylyl]-2-oxo-ethyl}-amide
HN-N
O
H NH
N~N~N
N O ~O
G
'H-NMR (DMSO-d6, 500 MHz) 8 1.10-1.62 (6H, m), 1.73 (4H, m), 2.48 (3H, s),
3.00 (6H, m), 3.39 (2H, m), 3.93 (2H, m), 4.82 (1H, m), 6.78 (1H, m), 7.05
(1H, s),
7.37 (2H, s), 7.40 (1H, s), 7.53 (2H, m), 7.98 (2H, m). Mass spec.: 543.26
(MH)+.
Example 36
(~)-4-Oxo-2-phenyl-1,3,8-triaza-spiro[4,5]dec-1-ene-8-carboxylic acid[1-
dimethylcarbamoyl -2-(7-methyl-1H-indazol-5- yl)-ethyl]amide
HN-N
O
NH
H
N N N
~N O O
'H-NMR (DMSO-d6, 500 MHz) 8 1.28-1.61 (4H, m), 2.78 (4H, m), 2.90 (6H, m),
3.94 (2H, m), 4.74 (1H, m), 6.77 (1H, m), 7.05 (1H, s), 7.37 (4H, s), 7.42
(1H, s),
7.52 (2H, m), 7.98 (2H, m). Mass spec.: 502.21 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
109
Example 37
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-I-carboxylic acid {1-(1H-
indazol-5-ylmethyl)-2-oxo-2-[4-(2-oxo-I ,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-
yl]-ethyl } -amide
HN-N H
/ ~ O~ N \
\ ~ N ~ /
H
N NJ
O
~N O
/ ~ ~N
\ N ~O
LC/MS: tR = 1.51 min, 674 (MH)-'
Example 38
4-(3-( 1 H-Indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-
carbonyl]-amino}-propionyl)-piperazine-1-carboxylic acid benzyl ester
HN-N H
/ ~ O~ N \
\ ~ N ~ /
H
N NJ
/ ~ ~N O O
\ O~N J
'~'~O
LC/MS: tR= 1.74 min, 665 (MH)+.
Example 39
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-(1H-
indazol-S-ylmethyl)-2-oxo-2-piperazin-1-yl-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
110
HN-N H
/ O N \
\ ~ N ~ /
H
N NJ
~N O O
HIND
To a degassed solution of 4-(3-(1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionyl)-piperazine-1-
carboxylic acid benzyl ester ( 280 mg, 0.42 mmol ) in methanol (50 ml) was
added
10% palladized charcoal (50 mg). The mixture was shaken in a Parr apparatus
under
an atmosphere of hydrogen at 50 psi for 3 h. The mixture was filtered through
celite.
The filtrate was concentrated under reduced pressure to give the desired
product in
91% yield. LC/MS: tR = 1.22 min, 531 (MH)~.
Example 40a
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid {1-(1H-
indazol-5-ylmethyl)-2-[4-(2-methyl-butyl)-piperazin-1-yl]-2-oxo-ethyl}-amide
HN-N H
/ \ 01 /N \
\ ~ ~N ~ /
H
N NJ
~N O O
~N J
A stirred solution of 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-
carboxylic acid [1-(1H-indazol-5-ylmethyl)-2-oxo-2-piperazin-1-yl-ethyl]-amide
(100 mg, 0.188 mmol) in methanol (25 mL) was treated with 2-methyl-
butyraldehyde
(0.03 ml, 0.376 mmol). After 1h at room temperature, sodium
triacetoxyborohydride
(80 mg, 0.316 mmol) was added. The mixture was allowed to stir overnight. The
solution was filtered through an SCX cartridge. The cartridge was eluted first
with
methanol and then with a 1 M solution of ammonia in methanol. The solvent was
removed in vacuo to give the desired product in 50% yield. LC/MS: tR = 1.31
min,
601 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
111
General Experimental Procedure for the Preparation of Examples 40b-40k.
The appropriate aldehyde (0.04 mmol) was added to a solution of Example 39
piperazine (0.02 mmol) in methanol (2.0 mL) and the resulting solution was
shaken
at room temperature for 1 h. Sodium triacetoxyborohydride (0.2 mmol) was then
added and the solution allowed stir overnight at room temperature. The
solution was
then filtered through a SCX cartridge and the cartridge washed with methanol
and an
ammonia/methanol solution. The ammonia/methanol solution was concentrated in
vacuo and the crude products were purified by preparative HPLC to the afford
the
products listed in Table 1.
Table 1. Examples 40b-40k.
HPLC
Mass spec
Example No. Structure Retention
(MH)
time (min)
HN-N H
O~N
\I ~H' I/
40b N ~ ~ 2.62 629
~N O O
Me N J
~"~e
HN-N H
O_" N
\I NN I/
40c H ~ H~ 1.41 587
~N O O
Me N J
~-r~
HH-N H
O-" N
\I ~N I/
40d N~N~ 1.27 573
~N O ~O
Me N J
W
HN-N H
O-" N
\I NN I/
40e N 1.74 611
~
oy'J o0
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
112
HPLC
Mass spec
Example No. Structure Retention
(MH)
time (min)
HN-N H
O_" N
~
\~
40f N ~/ 1.89 643
N
N~
~
E~t ~ N O 0
Me~N
M ~'a
HN-N H
O-" N
\~ ~N ~/
40g NON 1.48 610
y'] o0
N
N
H
HN-N H
O'" N
\~ ~N ~/
40h N 2.19 614
N~
~
~N O 0
MepN~N J
HN-N H
O-" N
\~ ~N ~/
40i N~N~ 2.36 629
Et ~N O'IO
N ,
-~ J
~P.
HN-N H
O N
\ ~ ~ ~ /
40j N 1.66 647
~
~N O O
Ph~N J
HN-N H
O~N
~
'
N
40k ~ / 2.61 545
\ ~
N~
N
~
~N 0 O
.NJ
Me
Example 41a
3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid cyclohexyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
113
HN-N H
/ ~ O\ /N
~N' ~ /
H
N NJ
O
O O
To a stirred solution of (~) -2-amino-3-(7-methyl- 1 H-indazol-5-yl)-propinic
acid (20 mg, 0.042 mmoles), 4-(dimethylamino)pyridine (2.5 mg, 0.02 mmoles),
and
1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (33 mg, 0.17
mmoles) in methylene chloride (2 mL) and dimethylforamide (1 mL), was added
cyclohexanol (13.3 ~L, 0.126 mmoles). The reaction mixture was stirred at 50-
55°C
for 4 h. The solvent was removed under reduced pressure, the the residue
purified by
preparative TLC on silica gel (9:1 chlorofornn/methanol) to give the desired
product
as white solid (9.4 mg, 40%). 1H-NMR (CD30D, 500 MHz) 81.32-1.87 (14H, m),
2.57 (3H, s), 2.86 (2H, m), 3.11-3.26 (2H, m), 4.13-4.22 (3H, m), 4.46 (1H,
m), 4.55
(1H, m), 4.80 (1H, m), 6.79 (1H, d), 6.97 (1H, m), 7.08-7.18 ( 2H, m), 7.35 (
1H, s),
7.47 (1H, s), 8.01-8.02 (1H, m). Mass spec.: 559.22 (MH)+.
Similarly prepared:
Example 41b
3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-benzyl-piperidin-4-yl ester
HN-N H
~ O\ /N
~N ~ /
H
N N NJ
/
O O
LC/MS: tR = 1.76 min, 650.30 (MH)k.
Example 41 c
3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl-piperidin-4-yl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
114
HN-N H
/ \ O~N \
\ ~ N ~ /
H
~N N N J
O O
LC/MS: tR = 1.59 min, 574.27 (MH)+.
Example 41 d
3-(7-Methyl-1 H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 4-phenyl-cyclohexyl ester
HN-N H
O\ /N \
~N ~ /
~N~
~O
LC/MS: tR = 2.69 min, 635.29 (MH)+.
Example 41 a
3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid (R)-1-pyridin-4-yl-ethyl ester
HN-N H
/ \ O\ /N \
\ ~ ~N' ~ /
H
N NJ
O O O
N /
LC/MS: tR = 1.66 min, 582.22 (MH)+.
Example 41 f
3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid (S)-1-pyridin-4-yl-ethyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
115
HN-N H
O\ /N \
~N ~ /
H
N NJ
\ ,,,,0 0 0
N,J
LC/MS: tR = 1.65 min, 582.23 (MH)+.
4-Bromo-2-chloro-6-methylphenyldiazo-t-butyl sulfide
~S
N~ N
CI
Br
4-Bromo-2-chloro-6-methylaniline (4.0 g, 18.3 mmol) was suspended in 24%
hydrochloric acid (5 mL). The stirred mixture was cooled to -20°C and
treated with
sodium nitrite (1.32 g, 1.05 equiv.) in water (2 mL), dropwise over 10 min
while the
temperature was maintained below -5°C. After a further 30 min at -
5°C to -20°C, the
mixture was buffered to ca. pH 5 with solid sodium acetate. This mixture (kept
at ca.
-10°C) was added in portions to a stirred solution of t-butyl thiol
(2.06 mL, 1 equiv.)
in ethanol (18.5 mL) at 0°C over ca. 10 min. Following addition, the
mixture was
stirred at 0°C for 30 min and then crushed ice (ca. 50 mL) was added.
The mixture
was stored in the refrigerator overnight. The resulting light-brown solid was
collected by filtration, washed with water, and dried under high vacuum for
several
hours (4.60 g, 78%). Mass spec.: 323.03 (MH)+.
5-Bromo-7-chloroindazole
HN-N
CI
Br
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
116
Into a flame-dried round bottom flask, 4-bromo-2; chloro-6-
methylphenyldiazo-t-butyl sulfide (4.60 g, 14.4 mmol) and potassium t-butoxide
(16.1 g, 10 equiv) were combined. A stir bar was added and the mixture placed
under nitrogen. To this was added dry DMSO (50 mL). The mixture was stirred
vigorously for 10 min at room temperature. The reaction mixture was then
carefully
poured into a mixture of crushed ice (150 mL) and 10% hydrochloric acid (74
mL).
The resulting suspension was left to stand at 4°C overnight and the
solid was
collected by filtration and washed with water. The solid was collected and
dried in
vacuo to give 2.86 g (86%) as a beige solid. 1H-NMR (CDC13, 500 MHz) 8 7.52
(d,
J=1.5, 1H), 7.82 (d, J=1.5, 1H), 8.08 (s, 1H). Mass spec.: 230.90 (MH)+.
7-Chloroindazole-S-carboxaldehyde
HN-N
CI
H O
5-Bromo-7-chloroindazole (2.0 g, 8.7 mmol) and sodium hydride (221 mg,
1.1 equiv) were weighed into a flame-dried round-bottom flask containing a
magnetic
stir bar. Under a nitrogen atmosphere at room temperature, dry tetrahydrofuran
(30
mL) was added. The mixture was stirred at room temperature for 15 min, during
which time it became homogeneous. The stirred mixture was cooled to -
78°C and a
solution of tent-butyllithium in pentane (1.7 M, 10.5 mL, 2.0 equiv) was added
over
several minutes. After 30 min at -78°C, the reaction was gradually
warmed to to -
50°C, kept there for 15 min, and recooled to -78°C.
Dimethylformamide (2.8 mL)
was slowly added and the mixture allowed to warm to -50°C. The solution
was
quickly transferred to a separatory funnel containing diethyl ether and water.
The
aqueous was made acidic by the addition of 1 M potassium hydrogen sulfate and
neutralized by the addition of sodium bicarbonate. The aqueous was extracted
with
diethyl ether (3x) which was washed with water, then brine, dried over
magnesium
sulfate, and concentrated to give 1.7g (100%) of nearly pure material. An
analytically pure sample was obtained by recrystallization from hot methanol.
'H-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
117
NMR (CDC13, 500 MHz) 8 7.97 (s, 1 H), 8.20 (s, 1 H), 8.30 (s, 1 H), 10.02 (s,
1 H).
Mass spec.: 181.09 (MH)+.
2-Benzyloxycarbonylamino-3-(7-chloro-1 H-indazol-5-yl)-acrylic acid methyl
ester
HN-N
CI \
/ I
\ /
H
\ N ' /O \
~0 0 0
A stirred suspension of potassium tert-butoxide (375 mg, 1.2 equiv.) in
methylene chloride (20 mL) was cooled to -20°C and treated with a
solution of N-
benzyloxycarbonyl-a-phosphonoglycine trimethyl ester (1.11 g, 1.2 equiv.) in
methylene chloride (5 mL). After 10 min, 7-chloroindazole-S-carboxaldehyde
(0.50
g, 2.79 mmol) in methylene chloride (5 mL) was added. The reaction was allowed
to
gradually warm to room temperature and was stirred for 3 days. The reaction
was
poured into a separatory funnel containing water and diethyl ether. The
aqueous was
extracted with diethyl ether (3x) which was washed with brine, dried over
magnesium sulfate, and concentrated. Column chromatography gave 0.40 g (37%)
of
product along with 0.20 g (40%) of starting material. 1H-NMR (CDC13, 500 MHz)
8
3.64 (s, 3H), 5.11 (s, 2H), 6.44 (bs, 1H), 7.30 (bs, SH), 7.43 (s, 1H), 7.62
(s, 1H),
7.80 (s, 1H), 8.07 (s, 1H). Mass spec.: 386.16 (MH)+.
(~)_2-Amino-3-(7-chloro-1H-indazol-5-yl)-propionic acid methyl ester
HN-N
CI /
\I
NHy
A solution of 2-benzyloxycarbonylamino-3-(7-chloro-1 H-indazol-5-yl)-
acrylic acid methyl ester (300 mg, 0.78 mmol) in methanol (10 mL) was treated
with
trifluoroacetic acid (0.2 mL), flushed with nitrogen, and treated with 10%
palladium
on charcoal (30 mg). The flask was flushed with hydrogen and allowed to stir
under
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
118
an atmosphere of hydrogen. After 4 days, all starting material had been
consumed.
The reaction was flushed with nitrogen, filtered through celite, and
concentrated.
Column chromatography gave 78 mg (40%). 'H-NMR (CDC13, 500 MHz) 8 1.31
(bs, 3H), 2.95 (dd, J=13.7, 7.9, 1H), 3.18 (dd, J=13.7, 5.2, 1H), 3.48 (s,
3H), 3.78
(dd, J=7.9, 5.2, 1 H), 7.23 (s, 1 H), 7.46 (s, 1 H), 8.00 (s, 1 H). Mass
spec.: 254.06
(MH)+.
Example 42
(~)-3-(7-Chloro-1H-indazol-S-yl)-2- f [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino-propionic acid methyl ester
HN-N
CI / ~ O\ /N \
\ ~ ~N ~ /
H
N NJ
O
H3C0 O
A stirred solution of (~)-2-amino-3-(7-chloro-1 H-indazol-5-yl)-propionic acid
methyl ester (78 mg, 0.31 mmol) in tetrahydrofuran (2 mL) at 0°C was
treated with
carbonyl diimidazole (50 mg, 1 equiv). The reaction was stirred for 5min,
warmed to
room temperature, stirred 10 min, and treated with 3-piperidin-4-yl-3,4-
dihydro-1H-
quinazolin-2-one (78 mg, 1.1 equiv). The mixture was stirred at room
temperature
overnight. The solvent was evaporated and the residue purified by column
chromatography to give 148 mg (94%) as a white powder. IH-NMR (DMSO-d6, 500
MHz) 8 1.46 (m, 4H), 2.55-2.80 (m, 2H), 3.05 (dd, J=13.7, 10.7, 1H), 3.15 (m,
1H),
3.62 (s, 3H), 4.04 (d, J=13.4, 2H), 4.11 (s, 2H), 4.22-4.39 (m, 2H), 6.76 (d,
J=7.9,
1 H), 6.87 (dd, J=7.3, 7.3, 1 H), 6.90 (d, J=8.2, 1 H), 7.08 (d, J=7.6, 1 H),
7.12 (dd,
J=7.6, 7.6, 1 H), 7.40 (s, 1 H), 7.60 (s, 1 H), 8.15 (s, 1 H), 9.18 (s, 1 H),
13.48 (s, 1 H).
Mass spec.: 511.18 (MH)+.
Example 43
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-chloro-1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
119
HN-N H
CI / ~ O\ /N
\ ~ ~N' ~ /
H
N NJ
O
~N O
~N
A suspension of (~)-3-(7-chloro-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
(15
mg, 0.029 mmol) in 1:1 tetrahydrofuran /methanol (1 mL) at room temperature
was
treated with a solution of lithium hydroxide (3.0 mg, 2.5 equiv) in water
(0.25 mL),
and the resulting solution was stirred for 1.5 h. The solution was cooled to
0°C,
treated with aqueous 1 M potassium hydrogen sulfate (60 ~,L, 2.0 equiv), and
concentrated to give the crude acid which was immediately used without
purification.
The crude acid was dissolved in dimethylformamide (0.3 mL) and sequentially
treated with methylene chloride (0.15 mL), 4-piperidyl-piperidine (10.1 mg, 2
equiv),
diisopropylethylamine (10 ~L, 2 equiv), and PyBOP° (16.5 mg, 1.1
equiv). The
solution was stirred 30 min and concentrated. The product was purified by
column
chromatography to give 14.7 mg (77%, 2 steps). 1H-NMR (CDC13, 500 MHz) S
1.30-1.60 (m, 8H), 1.65-1.88 (m, SH), 2.14 (m, 1H), 2.23 (m, 1H), 2.30-2.70
(m, 7H),
2.80-3.20 (m, SH), 3.94 (d, J=13.4, 13.1, 1H), 4.10-4.30 (m, 4H), 4.55 (m,
1H), 4.62
(dd, J=13.1, 12.8, 1 H), 5.19 (m, 1 H), 5.91 (dd, J=30.2, 22.3, 1 H), 6.70 (d,
J=7.6,
1 H), 6.92 (dd, J=7.6, 7.3, 1 H), 7.01 (dd, J, 7.9, 7.6, 1 H), 7.13 (s, 0.4H),
7.15 (dd,
J=7.9, 7.6, 1 H), 7.24 (s, 0.6H), 7.33 (s, 0.4H), 7.43 (s, 0.6H), 7.49 (bs, 1
H), 7.91 (s,
0.4H), 7.95 (s, 0.6H), 11.25 (bd, J=50.7, 1 H). Mass spec.: 647.37 (MH)+.
4-Bromo-2-ethyl-6-methyl-phenylamine
NHZ
Br
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
120
2-Ethyl-6-methyl-phenylamine (14 mL, 100 mmol) was dissolved in
concentrated hydrochloric acid (30 mL) and water (220 mL) and cooled to
0°C. To
this was added bromine (5.1 mL, 1 equiv.) dropwise. There was rapid formation
of a
white precipitate. The precipitate was filtered and washed with diethyl ether.
The
precipitate was suspended in water and neutralized with aqueous potassium
carbonate. An oil formed which was extracted into diethyl ether. The ethereal
was
dried over potassium carbonate, filtered, and concentrated to give 7.0 g (33%)
as a
purple oil which was used without purification. Mass spec.: 214.01 (MH)+.
4-Bromo-2-ethyl-6-methylphenyldiazo-t-butyl sulfide
/ \s
i
N
'' N
Br
4-Bromo-2-ethyl-6-methylaniline (7.0 g, 33 mmol) was suspended in 7.8 M
hydrochloric acid (30 mL). The stirred mixture was cooled to -20°C and
treated with
sodium nitrite (2.27 g, 1.05 equiv.) in water (5 mL), dropwise over 10 min
while the
temperature was maintained below -5°C. After a further 30 min at -
5°C to -20°C, the
mixture was buffered to ca. pH 5 with solid sodium acetate. This mixture (kept
at ca.
-10°C) was added in portions to a stirred solution of t-butyl thiol
(3.7 mL, 1 equiv.) in
ethanol (50 mL) at 0°C over ca. 10 min. Following addition, the mixture
was stirred
at 0°C for 30 min and then crushed ice (ca. 50 mL) was added. The
mixture was
stored in the refrigerator for 2 h. The resulting light-brown solid was
collected by
filtration, washed with water, and dried under high vacuum for several hours
(9.47 g,
92%). Mass spec.: 315.05 (MH)+.
5-Bromo-7-ethyl-1 H-indazole
HN-N
Br
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
121
To a stirred solution of potassium t-butoxide (33.6 g, 10 equiv.) in DMSO
(200 mL) was added a solution of 4-bromo-2-ethyl-6-methylphenyldiazo-t-butyl
sulfide (9.4 g, 30 mmol) in DMSO (100 mL) via cannula. The mixture was stirred
vigorously for 1 h. The reaction mixture was then carefully poured into a
mixture of
crushed ice (500 mL), concentrated hydrochloric acid (25 mL), and water (100
mL).
The resulting precipitate was filtered, washed with water, dissolved in
methanol, and
concentrated to give 5.7 g (85%) as a tan solid. 1H-NMR (CDC13, 500 MHz) 8
1.39
(t, J=7.6, 3H), 2.92 (q, J=7.6, 2H), 7.30 (s, 1 H), 7.75 (s, 1 H), 8.04 (s, 1
H). Mass
spec.: 225.00 (MH)+.
7-Ethyl-1 H-indazole-5-carbaldehyde
HN-N
H ~O
5-Bromo-7-ethyl-1H-indazole (2.0 g, 8.9 mmol) and sodium hydride (226 mg,
1.1 equiv.) were weighed into a flame-dried round-bottom flask containing a
magnetic stir bar. Under a nitrogen atmosphere at room temperature, dry
tetrahydrofuran (60 mL) was added. The mixture was stirred at room temperature
for
15 min. The stirred mixture was cooled to -78°C and a solution of tert-
butyllithium
in pentane (1.7 M, 10.5 mL, 2.0 equiv.) was added over several minutes. After
15
min at -78°C, the reaction was gradually warmed to to -50°C, and
recooled to -78°C.
Dimethylformamide (2.8 mL) was slowly added and the mixture allowed to warm to
-50°C. The solution was quickly transferred to a stirred solution of
water 300 mL
and 1 M potassium hydrogen sulfate (25 mL). The resulting suspension was
extracted with diethyl ether, washed with water, then brine, dried over
magnesium
sulfate, and concentrated. Column chromatography gave 160 mg (10%) as a white
solid. 'H-NMR (CD30D, 500 MHz) S 1.38 (t, J=7.6, 3H), 2.98 (q, J=7.6, 2H),
7.71
(s, 1 H), 8.22 (s, 1 H), 8.24 (s, 1 H), 9.96 (s, 1 H). Mass spec.: 175.08
(MH)+.
2-Benzyloxycarbonylamino-3-(7-ethyl-1H-indazol-5-yl)-acrylic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
122
HN-N
\ /
H
\ N "O \
~O
To a stirred solution of N-benzyloxycarbonyl-a,-phosphonoglycine trimethyl
ester (0.61 g, 2.0 equiv.) and 7-ethyl-1H-indazole-5-carbaldehyde (160 mg,
0.92
mmol) in tetrahydrofuran (5 mL) at 0°C was added tetramethylguanidine
(0.22 mL,
1.9 equiv.). The reaction was allowed to slowly warm to room temperature
overnight. The reaction was concentrated, dissolved in diethyl ether, washed
with
water, then brine, dried (magnesium sulfate), and concentrated. The residue
was
purified by column chromatography to give 333 mg (95%) as an oil. 'H-NMR
(CDC13, 500 MHz) 81.33 (t, J=7.6, 3H), 2.86 (q, J=7.3, 2H), 3.83 (s, 3H), 5.11
(s,
2H), 6.39 (bs, 1 H), 7.29 (bs, SH), 7.43 (s, 1 H), 7.50 (s, 1 H), 7.78 (s, 1
H), 8.04 (s,
1H). Mass spec.: 380.17 (MH)+.
(~)-2-Amino-3-(7-ethyl-1H-indazol-5-yl)-propionic acid methyl ester
HN-N
N H2
~O ~O
To a solution of 2-benzyloxycarbonylamino-3-(7-ethyl-1H-indazol-5-yl)-
acrylic acid methyl ester (330 mg, 0.78 mmol) in methanol (5 mL) under
nitrogen
was added palladium on charcoal (10%, 33 mg). The flask was flushed with
hydrogen and allowed to stir under an atmosphere of hydrogen overnight. The
reaction was flushed with nitrogen, filtered through celite, and concentrated
to give
210 mg (98%) which was used without purification. 'H-NMR (CDC13, 500 MHz) 8
1.34 (t, J=7.6, 3H), 2.85 (q, J=7.6, 2H), 2.96 (dd, J=13.7, 7.6, 1H), 3.19
(dd, J=13.7,
8.6, 1 H), 3.48 (s, 2H), 3.73 (s, 3H), 3.80 (dd, J=7.6, 5.2, 1 H), 6.99 (s, 1
H), 7.38 (s,
1 H), 7.97 (s, 1 H). Mass spec.: 248.1 S (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
123
Example 44
(~)-3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino]-propionic acid methyl ester
HN-N
\ H
\ I O\ 'N \,
I
H
N NJ
H3C0 O O
A stirred solution of (~)-2-amino-3-(7-ethyl-1H-indazol-5-yl)-propionic acid
methyl ester ( 100 mg, 0.41 mmol) in tetrahydrofuran (2 mL) at 0°C was
treated with
carbonyl diimidazole (66 mg, 1 equiv). The reaction was stirred for 5 min,
warmed
to room temperature, stirred for 15 min, and then treated with 3-piperidin-4-
yl-3,4-
dihydro-1H-quinazolin-2-one (103 mg, 1.1 equiv). The mixture was stirred at
room
temperature overnight. The solvent was evaporated and the residue purified by
column chromatography to give 188 mg (92%) as a white solid. 1H-NMR (CDCl3,
500 MHz) 8 1.36 (t, J=7.6, 3H), 1.69 (m, 4H), 2.86 (m, 2H), 2.90 (q, J=7.6,
2H),
3.22 (dd, J=5.5, 4.9, 2H), 3.75 (s, 3H), 4.03 (dd, J=44.0, 13.7, 2H), 4.26 (s,
2H), 4.51
(m, 1 H), 4.84 (m, 1 H), 5.02 (m, 1 H), 6.70 (d, J=7.9, 1 H), 6.90-7.05 (m,
4H), 7.16
(dd, J=7.6, 7.6, 1 H), 7.34 (s, 1 H), 8.03 (s, 1 H). Mass spec.: 505.29 (MH)+.
Example 45
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-ethyl-1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
HN-N H
O\ /N \
H
N N
O
~N O
-N
To a solution of (~)-3-(7-ethyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino-propionic acid methyl ester
(15
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
124
mg, 0.03 mmol) in methanol (0.6 mL) was added a solution of lithium hydroxide
monohydrate (3.0 mg, 2.5 equiv) in water (0.1 mL), and the resulting solution
was
stirred for 6 h. The solution was cooled to 0°C, treated with aqueous 1
M potassium
hydrogen sulfate (60 ~L, 2.0 equiv), and concentrated to give the crude acid
which
was immediately used without purification. The crude acid was dissolved in
dimethylformamide (0.4 mL), cooled to 0°C, and sequentially treated
with methylene
chloride (0.2 mL), 4-piperidyl-piperidine (11 mg, 2.2 equiv),
diisopropylethylamine
(12 ~L, 2.3 equiv.), and PyBOP° (19 mg, 1.2 equiv). The solution was
stirred for 15
min at 0°C, warmed to room temperature, stirred 1.5 h, and
concentrated. The
product was purified by column chromatography to give 14.5 mg (76%, 2 steps).
1H-
NMR (CDC13, 500 MHz) 8 1.28-1.48 (m, 10H), 1.52 (m, 2H), 1.60-1.82 (m, 6H),
1.95 (m, 1.4H), 2.06 (m, 1.6H), 2.20-2.50 (m, 5H), 2.77-2.93 (m, 5H), 2.96-
3.17 (m,
2H), 3.76 (d, J=13.4, 0.4H), 3.86 (d, J=13.7, 0.6H), 4.10-4.20 (m, 2H), 4.26
(s, 2H),
4.57 (m, 2H), 5.10-5.24 (m, 1H), 5.67 (d, J=8.2, 0.6H), 5.74 (d, J=7.9, 0.4H),
6.67
(d, J=7.9, 1 H), 5.67 (d, J=8.2, 0.6H), 5.74 (d, J=7.9, 0.4H), 6.67 (d, J=7.9,
1 H), 6.93
(dd, J=7.6, 7.3, 1 H), 6.96 (s, 0.4H), 7.03 (dd, J=7.0, 6.7, 1 H), 7.09 (m,
1.6H), 7.15
(dd, J=7.0, 6.7, 1H), 7.31 (s, 0.4H), 7.38 (s, 0.6H), 7.94 (s, 0.4H), 7.95 (s,
0.6H).
Mass spec.: 641.50 (MH)+.
(3,4-Dinitro-phenyl)-methanol
02N ~ OH
02N
Borane-tetrahydrofuran complex (1M in tetrahydrofuran, 800 mL, 800 mmol)
was added at -20°C over 45 min to a solution of 3,4-dinitrobenzoic acid
(93.5 g, 441
mmol) in tetrahydrofuran (300 mL). The resulting mixture was stirred at -
20°C for 1
h and then warmed to room temperature and stirred overnight. It was quenched
by
the addition of 32 mL of 1:1 acetic acid/water. Solvents were removed in vacuo
and
the residue was poured into an ice-cold 1000 mL of sat. sodium bicarbonate
with
vigorous stirring over 15 min. The mixture was extracted with ethyl acetate (3
x 500
mL). The combined organic layers were washed with sat. sodium bicarbonate,
brine
and dried over sodium sulfate. After filtration, solvents were removed to
afford the
title compound as a light yellow solid (100%). 1H-NMR (CDCl3, 500 MHz) 8
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
125
7.91(d, J = 8.0 Hz, 1H), 7.89 (s, 1H), 7.71 (dd, J = 8.5, 1.0 Hz, 1H), 4.87
(s, 2H), 2.30
(s, 1 H).
3,4-Dinitro-benzaldehyde
OZN ~ CHO
OzN
A solution of (3,4-dinitro-phenyl)-methanol (95.3g, 481 mmol) in methylene
chloride (500 mL) was added all at once to a suspension of pyridinium
chlorochromate (156 g, 722 mmol) in methylene chloride (900 mL). The mixture
was stirred at room temperature for 1.5 h and then ether (1500 mL) was added.
The
supernatant was decanted from the resulting black gum, and the insoluble
residue was
washed thoroughly with methylene chloride (3 x 250 mL). The combined organic
solution were filtered through a pad of florisil to afford a light bright
yellow clear
solution. Solvents were removed in vacuo and the residue was purified by
silica gel
chromatography using methylene chloride as eluent to afford the title compound
as a
yellow solid (71 %). ' H-NMR (CDCl3, 300 MHz) 8 8.45 (d, J = 1.5 Hz, 1 H),
8.28
(dd, J = 8.1, 1.5 Hz, 1H), 8.07 (d, J = 8.1 Hz, 1H). 13CNMR (CD30D, 125 MHz) 8
187.7, 139.2, 134.2, 126.2, 125.7.
2-Benzyloxycarbonylamino-3-(3,4-dinitro-phenyl)-acrylic acid methyl ester
OZN ~ ~ C02Me
/ NHCbz
ozN
1,1,3,3-Tetramethylguanidine (41.2 mL, 329 mmol) was added at room
temperature to a solution of N-(benzyloxycarbonyl)-alpha-phophonoglycine
trimethyl
ester (114.1g, 344 mmol) in tetrahydrofuran (800 mL). The mixture was stirred
at
room temperature for 15 min and cooled to -78°C. A solution 3,4-dinitro-
benzaldehyde (61.4 g, 313 mmol) in tetrahydrofuran (200 mL) was slowly added
via
cannula. The resulting mixture was stirred at -78°C for 2h and then
allowed to warm
to room temperature overnight. Solvents were removed in vacuo, and the yellow
residue was dissolved in 4.5 L of ethyl acetate. The solution was washed with
1.5 L
of 1N sulfuric acid, water twice, brine and dried over sodium sulfate. After
filtration,
solvents were removed in vacuo and the residue was crystallized from ethyl
acetate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
126
(20 g crude product1100 mL of ethyl acetate). The yellow crystals were
collected and
further purified by chromatography on silica gel using methylene chloride as
eluent.
The title compound was obtained a s yellow crystals (77%). 'H-NMR (CDC13, 500
MHz) 8 7.85 (d, J = 1.5 Hz, 1 H), 7.74 (d, J = 8.0 Hz, 1 H), 7.62 (dd, J =
8.5, 1.5 Hz,
1 H), '7.35 - 7.34 (m, 3H), 7.34 (br s, 2H), 7.23 (s, 1 H), 6.95 (br s, I H),
5.07 (s, 2H),
3.90 (s, 3H).
Similarly prepared:
2-Benzyloxycarbonylamino-3-(3-hydroxy-4-vitro-phenyl)-acrylic acid methyl
ester
HO ~ ~ COyMe
NHCbz
o2N
IH-NMR (CDC13, 500 MHz) 8 7.93 (d, J = 9.0 Hz, 1H), 7.32 (br s, sH), 7.28 (br
s,
2H), 7.17 (s, 1 H), 7.16 (d, J = 2.0 Hz, 1 H), 7.01 (dd, J = 9.0, 2.0 Hz, 1
H), 6.74 (br s,
1H), 5.06 (s, 2H), 3.86 (s, 3H).
(R)-2-Benzyloxycarbonylamino-3-(3,4-dinitro-phenyl)-propionic acid methyl
ester
H
02N ~ COyMe
NHCbz
OZN
An oven-dried 500 mL Shlenck flask was put into a glove-bag filled with
nitrogen. After the glove-bag was evacuated and filled with nitrogen (3x), the
flask
was sealed and taken out of the glove-bag and weighed. It was put back into
the
glove-bag and evacuated and filled with nitrogen (3x), then it was charged
with (-)-
1,2-bis((2R,SR)-2,5-diethylphospholano)benzene(cyclooctadienene)rhodium (I)
trifluoromethanesulfonate. The flask was sealed and taken out of the glove-bag
and
weighed (784 mg, 1.08 mmol). 2-Benzyloxycarbonylamino-3-(3,4-dinitro-phenyl)-
acrylic acid methyl ester (8.72 g, 21.7 mmol) was added to another 500 mL of
Schlenck flask and was evacuated and filled with nitrogen (3x). Methylene
chloride
(350 mL, degassed with nitrogen for 2 h) was added and the resulting solution
was
transferred to the catalyst flask via cannula. The flask was purged and filled
with
hydrogen (4x) and the mixture was stirred at room temperature for 4h. The
solvents
were removed in vacuo and the residue was purified by silica gel
chromatography
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
127
using ethyl acetate/hexanes (1:1) as eluent to afford the title compound as a
light tan
gummy solid (99% yield and 99.2% ee determined by HPLC analysis using the
following conditions: Chiralpak AD column (4.6 x 250 mm, 10 um; A = ethanol, B
= hexane; 40% B @ 1.0 mL/min for 14 min; retention times: 10.9 min for R
enatiomer and 6.9 min for S enatiomer). 1H-NMR (CDC13, 500 MHz) 8 7.80 (d, J =
8.0 Hz, 1H), 7.63 (s, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.38 - 7.31 (m, 5H), 5.37
(d, J =
6.0 Hz, 1H), 5.13 - 5.05 (m, 2H), 4.68 (d, J = 6.0 Hz, 1H), 3.71 (s, 3H), 3.36
(dd, J =
13.5, 5.0 Hz, 1 H), 3.17 (dd, J = 13.5, 6.0 Hz, 1 H).
Similarly prepared:
(R)-2-Benzyloxycarbonylamino-3-(3-hydroxy-4-nitro-phenyl)-propionic acid
methyl
ester
H
HO ~ C02Me
NHCbz
02N
1H-NMR (CDC13, 500 MHz) 8 7.97 (d, J = 9.0 Hz, 1H), 7.36 - 7.30 (m, 5H), 6.90
(s,
1 H), 6.71 (d, J = 8.5 Hz, 1 H), 5.29 (d, j = 7.0 Hz, 1 H), 5.11 (d, J = 12.5
Hz, 1 H), 5 .07
(d, J = 12.0 Hz, 1 H), 4.68 (dd, j = 13.0, 6.0 Hz, 1 H), 3.74 (s, 3H), 3.20
(dd, j = 13.5,
5.0 Hz, 1 H), 3.05 (dd, J = 13.5, 6.0 Hz, 1 H).
(R)-2-Benzyloxycarbonylamino-3-(3,4-diamino-phenyl)-propionic acid methyl
ester
H
HyN ~ COZMe
NHCbz
H2N
Solid ammonium formate (2.27 g, 36 mmol) was added in small portions at
0°C to a methanol (50 mL, degassed with nitrogen for 2 h) suspension of
(R)-2-
benzyloxycarbonylamino-3-(3,4-dinitro-phenyl)-propionic acid methyl ester
(1.45 g,
3.6 mmol) and zinc powder (1.41 g, 21.6 mmol). The resulting mixture was
stirred at
room temperature overnight. The solvents were removed in vacuo and then
toluene
(30 mL, degassed) and ethyl acetate (30 mL, degassed) were added, followed by
acetic acid (3 mL). The mixture was further diluted until all organic solids
dissolved,
then it was washed with water, brine and dried over sodium sulfate. After
filtration,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
128
solvents were removed in vacuo to afford the title compound containing 1
equivalent
of acetic acid as a reddish gummy solid (85%). Mass Spec.: 344.18 (MH)+.
(R)-2-Benzyloxycarbonylamino-3-(2-methyl-1 H-benzoimidazol-5-yl)-propionic
acid
methyl ester
H
N ~ C02Me
Me-~~
N / NHCbz
H
A solution of (R)-2-benzyloxycarbonylamino-3-(3,4-diamino-phenyl)-
propionic acid methyl ester-acetic acid (640 mg) in acetic acid (8 mL) was
heated at
130°C for 4h. The mixture was then poured into water and cooled to
0°C. The pH
was adjusted to 8 by gradual addition of solid sodium bicarbonate. The mixture
was
then extracted with ethyl acetate (3 x 100 mL), and the combined organic
layers were
washed with water, brine and dried over sodium sulfate. After filtration,
solvents
were removed to afford the title compound as a brownish foamy solid (95%). 'H-
NMR (CDC13, 500 MHz) 8 7.39 (d, J = 8.5 Hz, 1H), 7.35 (s, 1H), 7.26 - 7.22 (m,
5 H), 7.06 (d, J = 8.0 Hz, 1 H), 5.03 (d, J = 12.5 Hz, 1 H), 4.99 (d, J = 13.0
hz, 1 H),
4.51 (dd, J = 8.5, 5.5 Hz, 1 H), 3.70 (s, 3H), 3.27 (dd, J = 13.5, 5.0 Hz, 1
H), 3.03 (dd,
J= 14.0, 9.0 Hz, 1H), 2.55 (s, 3H). Mass spec.: 368.19 (MH)+.
(R)-2-Benzyloxycarbonylamino-3-[2-methyl-3-(2-trimethylsilanyl-ethanesulfonyl)-
3H-benzoimidazol-S-yl]-propionic acid methyl ester
and
(R)-2-Benzyloxycarbonylamino-3-[2-methyl-1-(2-trimethylsilanyl-ethanesulfonyl)-
1 H-benzoimidazol-5-yl]-propionic acid methyl ester
H H COZMe
SNS COZMe N
Me--~ ~ NHCbz
I
Me N ~ / NHCbz N
SES
To a suspension of (R)-2-benzyloxycarbonylamino-3-(2-methyl-1H-
benzoimidazol-5-yl)-propionic acid methyl ester (533 mg, 1.96 mmol), and
sodium
carbonate in acetonitrile (20 mL) was added neat 2-trimethylsilanyl-
ethanesulfonyl
chloride all at once. The mixture was stirred at room temperature overnight.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
129
Solvents were removed and the residue was purified by chromatography on silica
gel
using ethyl acetate/hexanes (1:2) as eluent to afford the title compound as a
waxy
solid (1:1 mixture of N1 and N3 isomers, 66°I°). 'H-NMR (CDC13,
500 MHz) S 7.68
(d, J = 8.5 hz, 0.5H), 7.55 (d, J = 8.5 Hz, 0.5 H), 7.53 (s, 0.5H), 7.41 (s,
0.5 H), 7.34 -
7.29 (m, 5H), 7.06 - 7.04 (m, 1 H), 5.22 (d, J = 8.0 Hz, 0.5 H), 5.17 (d, J =
7.5 Hz, 0.5
H), 5.11 - 5.07 (m, 2H), 4.72 - 4.69 (m, 1 H), 3.75 (s, 1.5 H), 3.72 (s, 1.5
H), 3.24 -
3.17 (m, 2H), 2.79 (s, 3H), 0.92 - 0.83 (m, 2H), -0.02 (s, 4.5 H), -0.05 (s,
4.5H).
Mass spec.: 532.26 (MH)+.
(R)-2-Amino-3-[2-methyl-1-(2-trimethylsilanyl-ethanesulfonyl)-1H-benzoimidazol-
5-yl]-propionic acid methyl ester
and
(R)-2-Amino-3-[2-methyl-3-(2-trimethylsilanyl-ethanesulfonyl)-3H-benzoimidazol-
5-yl]-propionic acid methyl ester
H
N ~ C02Me SES H
Me--<~ ~ T N ~ COyMe
NHy
SES Me~N ~ / NHy
A methanol (50 mL) suspension of (R)-2-benzyloxycarbonylamino-3-[2-
methyl-3-(2-trimethylsilanyl-ethanesulfonyl)-3H-benzoimidazol-5-yl]-propionic
acid
methyl ester and (R)-2-Benzyloxycarbonylamino-3-[2-methyl-1-(2-
trimethylsilanyl-
ethanesulfonyl)-1H-benzoimidazol-5-yl]-propionic acid methyl ester (1:1
mixture,
600 mg), and 10% palladium on charcoal (180 mg) was agitated on a Parr
apparatus
overnight under 40 psi of hydrogen at room temperature. After replacing the
hydrogen atmosphere with nitrogen, the mixture was filtered through a pad of
celite.
Solvents were removed in vacuo to afford the title compound as a tan solid
(80%).
'H-NMR (CD30D, 500 MHz) 8 7.81 (d, J = 8.5, 0.5 Hz, 0.5 H), 7.70 (s, 0.5 H),
7.58
(d, J = 8.5 Hz, 0.5 H), 7.49 (s, 0.5 H), 7.25 (d, J = 9.0 Hz, 1H), 3.89 (dd, J
=14.0, 6.5
Hz, 1 H), 3.75 (s, 1.5 H), 3.72 (s, 1.5 H), 3.55 - 3.51 (m, 2H), 3.18 (d, J =
6.0 Hz,
1 H), 3.22 - 3.18 (m, 0.5 H), 3.14 - 3.09 (m, 0.5 H), 2.81 (s, 1.5 H), 2.80
(s, 1.5 H),
0.92 - 0.88 (m, 2H), 0.02 (s, 4.5 H), 0.01 (s, 4.5 H); '3CNMR (CD30D, 125 MHz)
8
174.3, 174.1, 153.5, 153.3, 141.7, 140.6, 133.9, 133.82, 133.78, 132.7, 126.5,
126.3,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
130
119.7, 119.0, 114.1, 113.4, 55.6, 51.8, 51.7, 51.6, 40.2, 39.8, 15.83, 15.77,
9.9, -3.07,
-3.11. Mass spec.: 398.20 (MH)+.
(R)-3-[2-Methyl-1-(2-trimethylsilanyl-ethanesulfonyl)-1 H-benzoimidazol-5-yl]-
2-
{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-
propionic acid methyl ester
and
(R)-3-[2-Methyl-3-(2-trimethylsilanyl-ethanesulfonyl)-3H-benzoimidazol-5-yl]-2-
{ [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino } -
propionic acid methyl ester
H SES H
N ~ C02Me N ~ COZMe
Me--<~N ~ / HN O Me~N ~ I
/ HN' /O
SE '~S
O\ /N O\ 'N
H~N' H~'N
Prepared as described above for (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-
3-yl)-piperidine-1-carbonylJ-amino } -3-[ 1-(2-trimethylsilanyl-
ethanesulfonyl)-1 H
indazol-5-yl]-propionic acid methyl ester. Purified by silica gel
chromatography
using ethyl acetate with 1 % triethylamine as eluent to afford the title
compound as an
off white solid (87%). 'H-NMR (CD30D, 500 MHz) S 7.82 (d, J = 8.5 Hz, 0.5 H),
7.80 (s, 0.5 H), 7.59 (d, J = 8.0 Hz, 0.5 H), 7.55 (s, 0.5 H), 7.33 - 7.30 (m,
1H), 7.16
(t, J = 8.0 Hz, 1 H), 7.12 (t, J = 7.5 Hz, 1 H), 6.95 (t, J = 7.5 Hz, 1 H),
6.79 (d, J = 7.5
Hz, 1 H), 4.60 - 4.55 (m, 1 H), 4.45 - 4.40 (m, 1 H), 4.29 - 4.27 (m, 2H),
4.15 - 4.10
(m, 2H), 3.77 (s, 1.5 H), 3.74 (s, 1.5 H), 3.56 - 3.51 (m, 2H), 3.35 - 3.31
(m, 2H),
3.21 - 3.15 (m, 1 H), 2.91 - 2.80 (m, 2H), 2.78 (s, 1.5 H), 2.77 (s, 1.5 H),
1.76 - 1.73
(m, 1 H), 1.66 -1.61 (m, 2H), 0.92 - 0.87 (m, 2H), 0.009 (s, 4.5 H), -0.007
(s, 4.5 H).
i3CNMR (CD30D, 125 MHz) 173.8, 173.7, 158.2, 158.1, 155.6, 153.4, 153.2,
141.6,
140.3, 137.2, 135.3, 135.1, 133.7, 132.5, 128.2, 126.4, 126.3, 125.7, 122.13,
122.10,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
131
119.6, 118.8, 118.4, 114.0, 113.4, 113.2, 57.3, 56.2, 51.9, 51.7, 51.5, 43.8,
43.7, 42.9,
37.6, 37.2, 28.4, 17.4, 15.7, 15.6, 9.9, -3.1, -3.2. Mass spec.: 655.36 (MH)+.
(R)-3-(2-Methyl-1 H-benzoimidazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-
quinazolin-
3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
N ~ COZH
Me-<~
N / HN\ /O
'~H
N
O\ /N
H~N'
The 1:1 mixture of (R)-3-[2-Methyl-1-(2-trimethylsilanyl-ethanesulfonyl)-
1 H-benzoimidazol-5 -yl]-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-
carbonyl]-amino}-propionic acid methyl ester and (R)-3-[2-Methyl-3-(2-
trimethylsilanyl-ethanesulfonyl)-3H-benzoimidazol-5-yl]-2-{[4-(2- oxo-1,4-
dihydro-
2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
was
treated as described above for (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-yl]-propionic acid. The hydrolysis conditions (lithium hydroxide/methanol-
tetrahydrofuran-water (1:1:1) at -15 °C overnight were used. The title
compound
was obtained as a white solid (25%). Mass spec.: 477.24 (MH) +.
Example 46
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(2-methyl-1H-benzoimidazol-5-ylmethyl)-2-oxo-
ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
132
O
N ~ ~~\\~N~N~
~r
N / HN~O
'~H
N
N~O
'N~H
Prepared as described above for (R)-4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-piperidine-1-carboxylic acid {2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-
trimethylsilanyl-ethanesulfonyl)-1H indazol-5-ylmethyl]-ethyl-amide. Purified
by
silica gel chromatography using methylene chloride:methanolariethylamine
(93:5:2)
as eluent to give a white solid. This was dissolved in ethyl acetate (60 mL)
and
washed with a 1:1 sat. sodium bicarbonate/brine twice and dried over sodium
sulfate.
After filtration, solvents were removed to afford the title compound as a
white solid
(11% yield). LC/MS: tR = 1.59 min, 627.34 (MH)+.
(R)-3-(4-Amino-3-hydroxy-phenyl)-2-benzyloxycarbonylamino-propionic acid
methyl ester hydochloride
H
HO ~ COZMe
I / NHCbz
HyN
HCI
Powdered iron (3.7 g, 66.4 mmol) and ammonium chloride (5.9 g, 111 mmol)
1 S were added at 0°C to a solution of (R)-2-benzyloxycarbonylamino-3-
(3-hydroxy-4-
nitro-phenyl)-propionic acid methyl ester (2.07 g, 5.53 mmol) in degassed 1:1
methanol/water (400 mL). The resulting mixture was stirred at room temperature
for
48 h. Trifluoroacetic acid (7 mL) was added, and the mixture was swirled until
it was
a clear dark red solution containing a suspension of unreacted iron powder.
The
mixture was filtered and the filtrate was concentrated in vacuo. The residue
was
extracted with ethyl acetate (2 x 150 mL), the combined organic layers were
washed
with brine and dried over sodium sulfate. After filtration, hydrochloric acid
(4.2 mL,
4M in dioxane) was added. Solvents were removed in vacuo, and the title
compound
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
133
was obtained as a tan foamy solid (80%). 'H-NMR (CD30D, 500 MHz) ~ 7.34 -
7.28 (m, 5H), 7.20 (d, J = 8.0 hz, 1 H), 6.88 (s, 1 H), 6.78 (d, J = 7.5 Hz, 1
H), 5.05 -
5.00 (m, 2H), 4.42 (dd, J = 8.5, 5.0 Hz, 1H), 3.70 (s, 3H), 3.65 (s, 1H), 3.33
(br s,
2H), 3.11 (dd, J = 14.0, 5.0 hz, 1 H), 2.90 (dd, J = 13.5, 9.0 Hz, 1 H).
'3CNMR
(CD30D, 125 MHz) 172.5, 157.4, 151.2, 140.2, 137.0, 128.5, 128.0, 127.7,
123.8,
120.9, 117.0, 116.9, 67.2, 55.7, 52.0, 37.2. Mass spec.: 345.20 (MH)+.
(R)-2-Benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic
acid methyl ester
H
O ~ COyMe
1
N / NHCbz
H
A methylene chloride (15 mL) solution of carbonyl diimidazole (498 mg, 3.07
mmol) was added at 0°C to a solution of (R)-3-(4-amino-3-hydroxy-
phenyl)-2-
benzyloxycarbonylamino-propionic acid methyl ester (1.17g, 3.07 mmol),
diisopropylethylamine (1.60 mL, 9.21 mmol), and methylene chloride (85 mL).
The
mixture was stirred at 0°C for 4 h. The solvents were removed in vacuo
and the
residue was purified by silica gel chromatography using ethyl acetate/hexanes
as
eluent to afford the title compound as a white solid (51%). 'H-NMR (CDC13, 500
MHz) b 9.07 (s, 1 H), 7.37 - 7.29 (m, 5H), 6.96 (s, 1 H), 6.90 (d, J = 8.0 Hz,
1 H), 6.87
(d, J = 8.0 Hz, 1 H), 5.36 (d, J = 8.0 Hz, 1 H), 5.11 (d, J = 12.0 Hz, 1 H),
5.07 (d, J =
12.5 Hz, 1 H), 4.65 (dd, J = 13.5, 5.5 Hz, 1 H), 3.74 (s, 3H), 3.17 (dd, J
=14.0, 5.5 Hz,
1 H), 3.07 (dd, J = 14.0, 6.0 Hz, 1 H). ' 3CNMR (CDC13, 125 MHz) b 171.9,
155.7,
155.5, 144.1, 136.2, 130.8, 128.6, 128.42, 128.38, 128.2, 125.1, 111.1, 109.8,
67.2,
55.1, 52.6, 38.3. Mass spec.: 371.18 (MH)+.
(R)-2-Amino-3-(2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid methyl ester
H
O ~ COyMe
I / NH2
N
H
A solution of (R)-2-benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester (310 mg) in 4.4% formic acid in
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
134
methanol ( 20 ml, freshly prepared in degassed methanol) was added via cannula
to a
suspension of 10% palladium on charcoal in 4.4% formic acid in methanol ( 20
ml,
freshly prepared in degassed methanol). The resulting mixture was stirred at
room
temperature for 4 h. After filtration through a pad of celite, the solvents
were
removed in vacuo giving a tan solid. The solid was dissolved in a mixture of
ethyl
acetate (50 mL), toluene (10 mL) and ethanol (40 ml), and solid sodium
bicarbonate
(3.1 g) was added. The mixture was stirred at room temperature for 2h and
filtered.
Solvents were removed in vacuo to afford the title compound. 'H-NMR (CD30D,
500 MHz) 8 8.41 (br s, 2H), 7.17 (s, 1H), 7.09 (br s, 2H), 4.32 (s, 1H), 3.83
(s, 3H),
3.33 (s, 1H), 3.30 (s, 1H), 3.22 (s, 1H). Mass spec.: 237.20 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- f [4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
H
O ~ COZMe
N / HN\ /O
'~H
N
O\ /N
H~N
Prepared as described above for (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-
3-yl)-piperidine-1-carbonyl]-amino}-3-[ 1-(2-trimethylsilanyl-ethanesulfonyl)-
1H
indazol-5-yl]-propionic acid methyl ester. Purified by silica gel
chromatography
using methylene chloride:methanolariethylamine (93:5:2) as eluent to afford
the title
compound as a white solid (33%). ' H-NMR (CD30D, 500 MHz) S 7.17 - 7.13 (m,
3H), 7.08 (d, J = 7.9 hz, 1 H), 7.03 (d, J = 8.0 Hz, 1 H), 6.95 (t, J = 7.0
Hz, 1 H), 6.79
(d, J = 8.0 Hz, 1 H), 4.55 - 4.51 (m, 1 H), 4.44 - 4.41 (m, 1 H), 4.33 (s,
2H), 4.14 -
4.10 (m,2H), 3.?4 (s, 3H), 3.33 (br s, 2H), 3.23 (dd, j =13.7, 5.2 Hz, 1H),
3.03 (dd, J
= 14.0, 9.7 Hz, 1H), 2.92 - 2.82 (m, 2H), 1.79 -1.63 (m, 4H). 13CNMR (CD30D,
125 MHz) 173.8, 158.2, 156.2, 155.6, 144.4, 137.1, 132.7, 129.3, 128.2, 125.7,
125.0, 122.2, 118.4, 113.4, 110.6, 109.6, 56.2, 52.0, 51.7, 43.8, 42.9, 37.3,
28.4.
Mass spec.: 494.30 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
135
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O \ COyH
O~ I / HN O
N
H
N
O\/N
H~N'
\I
Prepared as described above for (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-
3-yl)-piperidine-1-carbonyl]-amino}-3-[ 1-(2-trimethylsilanyl-ethanesulfonyl)-
1H
indazol-5-yl]-propionic acid. The hydrolysis conditions (lithium
hydroxide/methanol-tetrahydrofuran-water (1:1:1) at -1 S°C overnight
were used.
The title compound was obtained as a white solid (95%). Mass spec.: 480.30
(MH)+.
Example 47
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-2-oxo-1-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
ethyl]-
amide
O ~N
~L N- /
O \
O~ I / HN O
N
H
N
N ~O
\ 'N~H
1$
Prepared as described above for (R)-4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-piperidine-1-carboxylic acid {2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
136
trimethylsilanyl-ethanesulfonyl)-1H indazol-5-ylmethyl]-ethyl}-amide. The
crude
product was purified by silica gel chromatography using methylene
chloride:methanolariethylamine (93:5:2) as eluent to give a white solid. This
was
dissolved in ethyl acetate (60 mL) and washed with a 1:1 sat. sodium
bicarbonate/brine twice and dried over sodium sulfate. After filtration,
solvents were
removed to afford the title compound as a white solid (70%). 'H-NMR (CD30D,
500
MHz) S 7.20 - 7.14 (m 4H), 7.08 (d, J = 9.0 Hz, 1 H), 6.96 (td, J = 7.5, 1.0
Hz, 1 H),
6.79 (d, J = 8.0 Hz, 1H), 4.99 - 4.94 (m, 1H), 4.61 - 4.58 (m, 1H), 4.47 -
4.43 (m,
1 H), 4.39 (s, 1 H), 4.23 - 4.16 (m, 2H), 4.08 - 4.04 (m, 1 H), 3.06 - 2.88
(m, SH), 2.74
- 2.69 (m, 2H), 2.59 - 2.52 (m, 2H), 2.41 - 2.33 (m, 2H), 1.96 - 1.89 (m, 1
H), 1.88
1.47 (m, 16H). LC/MS: tR = 1.86 min, 630.31 (MH)+.
(R)-3-(1H-Benzotriazol-5-yl)-2-benzyloxycarbonylamino-propionic acid methyl
ester
H
N ~ C02Me
N
'N / NHCbz
H
To a solution of (R)-2-benzyloxycarbonylamino-3-(3,4-diamino-phenyl)-
propionic acid methyl ester mono acetate (2.68 g, 6.65 mmol) in acetic acid
(30 mL)
and water (40 mL), at room temperature was added a solution of sodium nitrite
(0.46
g, 6.65 mmol) in water (8 mL), dropwise over several minutes. The resulting
mixture
was stirred at room temperature for 20 min, then cooled to 0°C,
concentrated
ammonium hydroxide was added to adjust pH to 11. The mixture was extracted
with
ethyl acetate twice in the presence of solid sodium chloride, and the organic
layers
were dried over sodium sulfate. After filtration, solvents were removed in
vacuo and
the residue was purified by chromatography on silica gel using ethyl
acetate/hexanes
(6:4) as eluent to afford the title compound as a tan solid (94% yield). 'H-
NMR
(CD30D, 500 MHz) 8 7.75 (d, J = 8.5 Hz, 1H), 7.58 (s, 1H), 7.31- 7.25 (m, 5H),
7.18 (d, J = 8.5 Hz, 1 H), 5.39 (d, J = 8.0 Hz, 1 H), 5.10 (d, J = 12.0 Hz, 1
H), 5.05 (d, J
= 12.0 Hz, 1 H), 4.74 (dd, j = 13.5, 6.0 Hz, 1 H), 3.73 (s, 3H), 3.34 (dd, J =
14.0, 5.5
Hz, l H), 3.22 (dd, J = 13.5, 6.0 Hz, 1 H). ' 3CNMR (CD30D, 125 MHz) 8172.1,
156.0, 136.1, 128.6, 128.3, 128.1, 67.2, 55.2, 52.7, 38.5. Mass spec. 355.18
(MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
137
(R)-2-Amino-3-(1H-benzotriazol-5-yl)-propionic acid methyl ester
H
N N ~ C02Me
/ NH2
N
H
Prepared as described above for (R)-2-amino-3-(2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. 'H-NMR (CD30D, 500 MHz) 8 8.38
(br s, 2H), 7.89 (d, J = 7.5 Hz, 1 H), 7.81 (s, 1 H), 7.40 (d, J = 7.5 Hz, 1
H), 4.44 (s,
1 H), 3.81 (s, 3H), 3.48 - 3.45 (m, 1 H), 3.40 - 3.37 (m, 1 H), 3.33 (br s, 1
H).
'3CNMR (CD30D, 125 MHz) 8 169.8, 139.4, 138.9, 133.0, 127.6, 115.52, 115.47,
54.3, 52.6, 36.7. Mass spec. 221.15 (MH)+.
Example 48
(R)-3 -( 1 H-B enzotriazol-5 -yl)-2- { [4-(2-oxo-1,4-dihydro-2 H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid methyl ester
H
N ~ COyMe
N~ ~ / HN O
N
H
N
N' /O
'N~H
Prepared as described above for (R)-2-{[4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-3-[1-(2-trimethylsilanyl-ethanesulfonyl)-1H
indazol-
5-yl]-propionic acid methyl ester except that carbonyl diimidazole was used in
place
of N,N disuccinimidyl carbonate (DSC). IH-NMR (CD30D, 300 MHz) 8 7.82 (d, J =
8.4 Hz, 1 H), 7.24 (s, 1 H), ?.39 (dd, J = 8.7, 1.2 Hz, 1 H), 7.15 - 7.08 (m,
2H), 6.94
(td, J = 7.5, 0.9 Hz, 1 H), 6.75 (d, J = 7.8 Hz, l H), 4.67 - 4.60 (m, 1 H),
4.39 - 4.31 (m,
1H), 4.15 (s, 2H), 4.08 - 4.03 (m, 2H), 3.72 (s, 3H), 3.38 (dd, J =13.9, 5.5
Hz, 1H),
3.32 - 3.29 (m, 1 H), 3.17 (dd, J = 13.9, 10.3 Hz, 1 H), 2.87 - 2.71 (m, 2H),
1.64 -
1.48 (m, 4H). Mass spec. 478.30 (MH)+.
Example 49
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
138
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(1H-
benzotriazol-5-ylmethyl)-2-[ 1,4']bipiperidinyl-1'-yl-2-oxo-ethyl]-amide
0
H N~ N
NN
'N / HN\ /O
'~H
N
N' /O
'N~H
I/
Prepared as described above for (R)-4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-piperidine-1-carboxylic acid {2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-
trimethylsilanyl-ethanesulfonyl)-1H indazol-5-ylmethyl]-ethyl}-amide. Purified
by
silica gel chromatography using methylene chloride/methanol/triethylamine
(93:5:2)
as eluent. IH-NMR (CD30D, 500 MHz) 8 7.83 d, J = 8.2 Hz, 0.75H), 7.79 (d, J =
8.5
Hz, 0.25H), 7.71 (s, 0.25H), 7.69 (s, 0.75H), 7.33 (d, J = 9.2 Hz, 1H), 7.16 -
7.12 (m,
2H), 6.96 - 6.91 (m, 1H), 6.78 (d, J = 8.0 Hz, 0.75H), 6.77 (d, J = 8.0 Hz,
0.25H),
5.07 - 5.03 (m, 1 H), 4.58 - 4.55 (m, 1 H), 4.45 - 4.40 (m, 1 H), 4.34 (s,
1.25H), 4.24
(s, 0.75H), 4.20 - 4.05 (m, 2.25H), 4.00 - 3.96 (m, 0.75H), 3.24 - 3.09 (m,
2H), 2.91
- 2.78 (m, 4H), 2.64 - 2.61 (m, 2H), 2.56 - 2.42 (m, 2H), 2.15 - 2.10 (m,
1.25H),
2.02 - 1.98 (m, 1.75H), 1.95 - 1.90 (m, 1 H), 1.68 - 1.60 (m, 8H), 1.54 - 1.46
(m,
6H). LC/MS: tR =1.86 min, 614.28 (MH)+.
(R)-2-Benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-1 H-indol-5-yl)-propionic
acid
methyl ester
H
C02Me
O
N / NHCbz
H
PyHBr3 (1.28 g, 4.02 mmol) was added in small portions over 30 min to a
solution of (R)-2-benzyloxycarbonylamino-3-(1H indol-5-yl)-propionic acid
methyl
ester (0.47 g, 1.34 mmol) in t-butanol (10 mL) while the reaction temperature
was
maintained between 25-30°C. The resulting mixture was stirred at room
temperature
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
139
for 3.5 h. The solvent was removed in vacuo, and the residue was extracted
with
ethyl acetate (2x). The combined organic phases were washed with brine and
dried
over sodium sulfate. After filtration, solvents were removed and the residue
was
azeotropically dried with anhydrous ethanol. The residue was dissolved in
glacial
acetic acid (10 mL) and zinc powder (0.88 g, 13.4 mmol) was added. The mixture
was stirred at room temperature overnight. After the acetic acid was removed
in
vacuo, the residue was purified by flash chromatography on silica gel using
ethyl
acetate/hexanes [(1:3) first and then (3:2)] as eluent to afford the title
compound as a
white solid (41% for 2 steps). 'H-NMR (CDCl3, 500 MHz) S 8.03 (s, 1H), 7.36 -
7.31 (m, 5H), 6.94 (s, 1H), 6.91 (d, J = 8.0 Hz, IH), 6.73 (d, J = 7.5 Hz,
1H), 5.26 (d,
J = 8.0 Hz, 1 H), 5.11 (d, J = 12.0 Hz, 1 H), 5.05 (d, j = 12.5 Hz, 1 H), 4.61
(dd, J =
13.5, 6.0 hz, 1 H), 3.72 (s, 3H), 3.45 (s, 2H), 3.10 (dd, J = 14.0, 5.5 Hz, 1
H), 3.00 (dd,
J = 14.0, 6.0 Hz, 1H). ~3CNMR (CDC13, 125 MHz) $ 177.7, 172.2, 155.7, 141.7,
136.3, 129.8, 128.9, 128.6, 128.3, 128.2, 125.8, 125.6, 109.8, 67.1, 55.1,
52.5, 38.0,
36. Mass spec. 369.20 (MH)+.
(R)-2-Amino-3-(2-oxo-2,3-dihydro-1H-indol-5-yl)-propionic acid methyl ester
H
C02Me
O
N / NH2
H
Prepared as described above for (R)-2-amino-3-(2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. 1H-NMR (CD30D, 500 MHz) b 8.48
(br s, 2H), 7.16 (s, 1 H), 7.10 (s, 1 H), 6.89 (s, 1 H), 4.21 (s, 1 H), 3.81
(s, 3 H), 3.54 (s,
1 H), 3.33 (s, 2H), 3.20 (s, 1 H), 3.12 (s, 1 H). 13CNMR (CD30D, 125 MHz)
8178.9,
170.7, 143.3, 129.0, 128.6, 126.9, 125.6, 110.0, 57.3, 54.6, 52.3, 37Ø Mass
spec.
235.30 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-1 H-indol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-
quinazolin-
3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
140
H
C02Me
O
N~ HN O
H
N
N' /O
'N~H
A solution of phosgene in toluene (2M, 0.158 mL, 0.30 mmol) was added to
a vigorously stirred mixture of (R)-2-amino-3-(2-oxo-2,3-dihydro-1H indol-5-
yl)-
propionic acid methyl ester (?0 mg, 0.25 mmol) in methylene chloride (15 mL)
and
saturated sodium bicarbonate (?.5 mL). After the mixture was stirred at room
temperature for 30 min, 3-piperidin-4-yl-3,4-dihydro-1H quinazolin-2-one (58
mg,
0.25 mmol) was added. The resulting mixture was stirred at room temperature
for
1.5 h, diluted with ethyl acetate, and washed with 0.25 N hydrochloric acid
that had
been saturated with solid sodium chloride. The organic layers were dried over
sodium sulfate. After filtration, solvents were removed to afford the title
compound
as a tan viscous oil. LC/MS: tR = 2.01 min, 492.10 (MH)+.
Example 50
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
(1,4']bipiperidinyl-1'-yl-2-oxo-1-(2-oxo-2,3-dihydro-1H-indol-5-ylmethyl)-
ethyl]-
amide
o
H N~ N
O
N / HN' /O
'~H
N
N' /O
'N~H
/
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
141
Prepared as described above for (R)-4-(2-oxo-1,4-dihydro-2H quinazolin-3-
yl)-piperidine-1-carboxylic acid f2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-[1-(2-
trimethylsilanyl-ethanesulfonyl)-1H indazol-5-ylmethyl]-ethyl]-amide. Purified
by
flash chromatography on silica gel using methylene
chloride/methanol/triethylamine
(93:5:2) as eluent. 'H-NMR (CD30D, 500 MHz) 8 7.20 - 7.09 (m, 4H), 6.97 (t, J
=
7.3 Hz, 1H), 6.88 (d, J = 7.9 Hz, 0.65 H), 6.84 (d, J = 7.6 Hz, 0.35 H), 6.80
(d, J = 7.7
Hz, 1 H), 5.51 (s, 0.65 H), 5.23 (s, 0.35 H), 4.99 - 4.95 (m, 0.65 H), 4.92 -
4.88 (m,
0.35 H), 4.60 - 4.56 (m, 1.65 H), 4.46 - 4.41 (m, 1.35 H), 4.39 (s, 1.3 H),
4.36 (s, 0.7
H), 4.24 - 4.17 (m, 2H), 4.05 - 4.02 (m, 1 H), 3.65 - 3.61 (m, 2H), 3.52 -
3.47 (m,
1 H), 3.20 - 3.16 (m, 1 H), 3.00 - 2.88 (m, 2H), 2.70 - 2.64 (m, 2H), 2.53 -
2.46 (m,
2H), 2.40 - 2.34 (m, 2H), 1.94 -1.46 (m, 1 SH), 1.39 -1.36 (m, 2H). LC/MS: tR
=
1.83 min, 628.40 (MH)+.
2-(Di-tert-butoxycarbonylamino)-acrylic acid methyl ester
0 0
Boc2N pCH + Boc2N pCH
3 3
1 S OBOC
To a solution of 2-tert-butoxycarbonylamino-3-hydroxy-propionic acid
methyl ester (10.0 g, 39 mmol) and di-tert-butyl-dicarbonate (21.8 g, 2.6
equiv.) in
acetonitrile (40 mL) was added 4-dimethylaminopyridine (0.48 g, 0.1 equiv) at
room
temperature. The solution was stirred overnight and concentrated. The residue
was
dissolved in diethyl ether, washed sequentially with 1 M potassium hydrogen
sulfate
(2x), saturated sodium bicarbonate, brine, dried over magnesium sulfate, and
concentrated to give 15.6 g (quant.) as an oil. Inspection of the'H NMR showed
a
mixture of the title compound and 2-(di-tert-butoxycarbonylamino)-3-tert-
butoxycarbonyloxy-propionic acid methyl ester. As it was later found that both
react
with secondary amines to give the same products, the mixture was used without
separation. 2-(Di-tert-butoxycarbonylamino)-acrylic acid methyl ester:'H-NMR
(CDCl3) b 1.45 (s, 18H), 3.78 (s, 3H), 5.63 (s, 1H), 6.33 (s, 1H). Mass spec.:
324.14
(M+Na)+. 2-(Di-tert-butoxycarbonylamino)-3-tert-butoxycarbonyloxy-propionic
acid methyl ester: 'H-NMR (CDC13, 500 MHz) 8 1.46 (s, 9H), 1.49 (s, 18H), 3.72
(s,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
142
3H), 4.42 (dd, J=11.6, 9.2, 1H), 4.75 (dd, J=11.3, 4.6, 1H), 5.30 (dd, J=9.2,
4.6, 1H).
Mass spec.: 442.21 (M+Na)+.
(~)-3-(4-B enzyloxy-2-oxo-2 H-pyridin-1-yl)-2-(di-tert-butoxycarbonylamino)-
propionic acid methyl ester
0
Boc2N
~OCHg
p / p ~ \
To a solution of 2-(di-tert-butoxycarbonylamino)-acrylic acid methyl ester
(900 mg, 3.0 mmol), and 4-benzyloxy-1 H-pyridin-2-one (630 mg, 1.03 equiv) in
acetonitrile (2.5 mL) was added cesium carbonate (100 mg, 0.10 equiv). The
resulting suspension was heated to 80°C via microwave for 2 h. The
reaction was
concentrated, dissolved in water, and extracted with methylene chloride (3x).
The
combined organic phases were washed with brine, dried over magnesium sulfate,
and
concentrated to give 1.47 g (97%) which was used without purification. Mass
spec.:
503.56 (MH)+.
(t)-4-Benzyloxy-1-[3-[ 1,4']bipiperidinyl-1'-yl-2-(di-tert-
butoxycarbonylamino)-3-
oxo-propyl]-1 H-pyridin-2-one
O N
N!
(Boc)yN
N
~~O
O
To a stirred solution of 3-(4-benzyloxy-2-oxo-2H-pyridin-1-yl)-2-(di-tert-
butoxycarbonylamino)-propionic acid methyl ester (1.47 g, 2.9 mmol) in
methanol
(17 mL) was added a solution of lithium hydroxide monohydrate (0.50 g, 4
equiv) in
water (2.85 mL). The reaction mixture was stirred for 3 h at room temperature,
cooled to 0°C, treated with concentrated hydrochloric acid (0.99 mL),
and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
143
concentrated to afford the crude acid, half of which was taken on in the
following
step. The crude acid was dissolved in methylene chloride (6 mL), cooled to
0°C and
treated sequentially with 4-piperidyl-piperidine (0.25 g, 1 equiv),
triethylamine (0.31
mL, 2.5 equiv), and bis-2-oxo-3-oxazolidinyl)phoshinic chloride (0.38 g, 1
equiv).
The reaction was allowed to warm to room temperature and stirred overnight.
The
reaction was concentrated, and purified by Prep HPLC to afford 489 mg (52%, 2
steps). Mass spec.: 639.41 (MH)+.
Example 51 '
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(4-
benzyloxy-2-oxo-2H pyridin-1-ylmethyl)-2-[1,4']bipiperidinyl-1'-yl-2-oxo-
ethyl]-
amide
H
/ N' /O
'N~ O
I H
N"N
N
O I''~~//I~~ N
N
O
O
To a stirred solution of 4-benzyloxy-1-[3-[1,4']bipiperidinyl-1'-yl-2-(di-tert-
butoxycarbonylamino)-3-oxo-propyl]-1H-pyridin-2-one in methylene chloride (3
mL) was added trifluoroacetic acid (1 mL) at 0°C. After 2 h, the
reaction was
concentrated to afford the crude amine (151 mg, 97%) as its trifluoroacetic
acid salt
[Mass spec.: 439.61 (MH)+] which was split into two portions, using half in
the
following procedure. To a solution of the crude amine (75 mg, 0.11 mmol) and
diisopropylethylamine (80 ~.L, 4 equiv) in methylene chloride (3 mL) at
0°C was
added carbonyl diimidazole (29 mg, 1.6 equiv, in 2 portions). After stirring
for 10
min, the solution was treated with 3-piperidin-4-yl-3,4-dihydro-1 H-quinazolin-
2-
acetic acid (40 mg, 1.15 equiv). The reaction was warmed to room temperature
and
stirred overnight. The reaction was concentrated and purified by prep TLC to
give
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
144
40.8 mg (53%). 'H-NMR (CD30D, 500 MHz) 81.25-1.56 (m, 4H), 1.56-1.84 (m,
9H), 1.90-2.08 (m, 2H), 2.60-2.95 (m, 8H), 3.11 (dd, J=24.1, 12.8, 1H), 3.89
(ddd,
J=22.0, 13.2, 9.2, 1H), 4.10 (dd, J=14.3, 14.1, 2H), 4.27-4.54 (m, SH), 4.60
(bd,
J=11.9, 1H), 5.08 (dd, J=13.2, 12.2, 2H), 5.26 (ddd, J=9.4, 9.4, 4.8, 1H),
6.05 (dd,
J=13.7, 2.7, 1 H), 6.16 (m, 1 H), 6.77 (d, J=8.0, 1 H), 6.84 (ddd, J=7.6, 7.6,
2.1, 1 H),
7.04 (d, J=7.6, 1 H), 7.12 (dd, J=7.6, 7.4, 1 H), 7.28-7.43 (m, SH), 7.48 (d,
J=7.6,
1 H). Mass spec.: 696.85 (MH)+.
Example 52
(~)-4-(2-OXO-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(4-hydroxy-2-oxo-2H-pyridin-1-ylmethyl)-2-oxo-
ethyl]-
amide
H
/ N' /O
'N~ O
I H
N"N
N
I~IO
N N
O
OH
A stirred solution of 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-
carboxylic acid [1-(4-benzyloxy-2-oxo-2H-pyridin-1-ylmethyl)-2-
[1,4']bipiperidiny1-
1'-yl-2-oxo-ethyl]-amide (29 mg) and 10% palladium on charcoal (5 mg) in
methanol
(1 mL) was placed under an atmosphere of hydrogen. After 1 h at room
temperature,
the reaction was flushed with nitrogen, filtered through celite, and
concentrated to
give the product. IH-NMR (CD30D, 500 MHz) b 1.40-1.85 (m, 12H), 2.04 (dd,
J=27.4, 17.0, 2H), 2.66 (dd, J=21.1, 11.0, 1 H), 2.80-3.19 (m, 8H), 3.95 (ddd,
J=49.8, 12.5, 7.9, 1H), 4.07-4.28 (m, 3H), 4.34 (bs, 2H), 4.36-4.59 (m, 2H),
4.63 (bd,
J=12.8, 1 H), 5.20 (m, 1 H), 5.75 (dd, J=7.3, 2.1, 1 H), 5.97 (dd, J=8.9, 7.6,
1 H), 6.78
(d, J=7.6, 1H), 6.93 (dd, J=7.6, 7.3, 1H), 7.08-7.18 (m, 2H), 7.33 (dd,
J=18.3, 11.0,
1 H). Mass spec.: 606.32 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
145
(~)-2-(Di-tert-butoxycarbonylamino)-3-(4-hydroxy-piperidin-1-yl)-propionic
acid
methyl ester
0
Boc2N
home
N
~OH
To a solution of 2-(di-tert-butoxycarbonylamino)-acrylic acid methyl ester
(1.0 g, 3.0 mmol) in acetonitrile (10 mL) was added piperidin-4-of (0.33 g,
1.1
equiv). A gentle stream of nitrogen was placed over the reaction while it
stirred
overnight. The crude oil which resulted was dissolved in ethyl acetate, washed
with
water, then brine, dried over magnesium sulfate, and concentrated to give 1.38
g
(quant.) as an oil which was used without purification. Mass spec.: 403.42
(MH)+.
(t)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(di-tert-butoxycarbonylamino)-3-(4-hydroxy-
p iperidin-1-yl)-propan-1-one
o
BocyN
N
N " 'N
HO
To a solution of 2-(di-tert-butoxycarbonylamino)-3-(4-hydroxy-piperidin-1-
yl)-propionic acid methyl ester (1.0 g, 2.5 mmol) in methanol (6 mL) was added
a
solution of lithium hydroxide monohydrate (400 mg, 3.9 equiv) in water (1 mL).
The
reaction was stirred 6 h, cooled to 0°C, neutralized with concentrated
hydrochloric
acid, and concentrated. The crude acid was used without purification. The
crude
acid was suspended in methylene chloride (25 mL), treated with a few drops of
methanol to aid in dissolving the acid, and cooled to 0°C. The
resulting suspension
was treated sequentially with 4-piperidyl-piperidine (0.53 g, 1.25 equiv),
triethylamine (0.70 mL, 2. equiv), and bis-2-oxo-3-oxazolidinyl)phoshinic
chloride
(0.80 g, 1.25 equiv). The reaction was allowed to warm to room temperature
overnight. The reaction was concentrated and then purified by Prep HPLC to
afford
310 mg (23%, 2 steps). Mass spec.: 539.49 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
146
(~)-2-Amino-1-[ 1,4']bipiperidinyl-1'-yl-3-(4-hydroxy-piperidin-1-yl)-propan-1-
one
0
HyN
N
N v 'N
HO
To a solution of 1-[1,4']bipiperidinyl-1'-yl-2-(di-tert-butoxycarbonylamino)-
3-(4-hydroxy-piperidin-1-yl)-propan-1-one (310 mg, 0.58 mmol) in methylene
chloride (5 mL) at 0°C was added trifluoroacetic acid (2.0 mL). The ice
bath was
removed and the reaction stirred for 30 min. The reaction was concentrated to
afford
the product as its trifluoroacetic acid salt (400 mg, quant.) which was used
without
purification. Mass spec.: 339.46 (MH)+.
(~)- [2-[1,4']Bipiperidinyl-1'-yl-1-(4-hydroxy-piperidin-1-ylmethyl)-2-oxo-
ethyl]-
carbamic acid tert-butyl ester
0
BocHN
N
N ~N
HO
To a solution 2-amino-1-[1,4']bipiperidinyl-1'-yl-3-(4-hydroxy-piperidin-1-
yl)-propan-1-one (trifluoroacetic acid salt, 300 mg, 0.58 nunol) and
diisopropylethylamine (0.30 mL, 4 equiv) in tetrahydrofuran (5 mL) was added
di-
tert-butyl-dicarbonate (128 mg, 1 equiv). The resulting solution was stirred
at room
temperature for 1 h, and concentrated. The residue was dissolved in ethyl
acetate,
washed with water, then brine, dried over magnesium sulfate, and concentrated
to
afford to 248 mg (98%) which was used without purification. Mass spec.: 439.65
(MH)+.
(~)- [2-[1,4']Bipiperidinyl-1'-yl-2-oxo-1-(4-oxo-piperidin-1-ylmethyl)-ethyl]-
carbamic acid tert-butyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
147
O
BocHN
N
N v 'N
O
To a solution of 1-[1,4']bipiperidinyl-1'-yl-2-(di-tert-butoxycarbonylamino)-3-
(4-
hydroxy-piperidin-1-yl)-propan-1-one (200 mg, 0.37 mmol) in methylene chloride
(4
mL) was added Dess-Martin periodinane (316 mg, 2 equiv) in two portions. After
1
h, the reaction was quenched by the addition of saturated sodium bicarbonate,
and
extracted into methylene chloride (3x). The combined organic phases were
washed
with brine, dried over magnesium sulfate, and concentrated to give 187 mg
(94%)
which was used without purification. Mass spec.: 437.63 (MH)+.
(~)-1-(2-Amino-3-[1,4']bipiperidinyl-1'-yl-3-oxo-propyl)-piperidin-4-one
0
HyN
N
N v _N
O
To a solution of [2-[1,4']bipiperidinyl-1'-yl-2-oxo-1-(4-oxo-piperidin-1-
ylmethyl)-ethyl]-carbamic acid tert-butyl ester (100 mg, 0.23 mmol) in
methylene
chloride (5 mL) at 0°C was added trifluoroacetic acid. The ice bath was
removed,
stirring continued for 1 h, and the reaction concentrated to give 150 mg (96%)
as its
trifluoroacetic acid salt which was used without purification. Mass spec.:
337.64
(MH)+.
Example 53
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(4-hydroxy-piperidin-1-ylmethyl)-2-oxo-ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
148
H
N ~O
'N~ O
I H
N' /N
N
O N
N
-OH
To a solution of 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-
carboxylic acid [2-[1,4']bipiperidinyl-1'-yl-1-(4-hydroxy-piperidin- 1-
ylmethyl)-2-
oxo-ethyl]-amide (3 trifluoroacetic acid salt, 200 mg, 0.39 mmol) in methylene
chloride (5 mL) at 0°C was added diisopropylethylamine (0.27 mL, 3.9
equiv), and
carbonyl diimidazole (63 mg, 1 equiv). After stirring for 15 min, the solution
was
treated with 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one (acetic acid
salt, 142
mg, 1.25 equiv). The solution was warmed to room temperature and stirred
overnight. The reaction was concentrated and purified by Prep TLC to give 130
mg
(56%) as an oil. LC/MS: tR = 1.17 min, 596.44 (MH)+.
3-Dimethylaminomethylene-4-oxo-piperidine-1-carboxylic acid tert-butyl ester
0
~O~ N
~O
MeZN
4-Oxo-piperidine-1-carboxylic acid tert-butyl ester (10 g, 50 mmol) was
dissolved in dimethyl formamide dimethylacetal (50 mL) and heated to reflux
for
1.25 h. The solution was cooled, concentrated, and purified by flash
chromatography
to give 2.55 g (19%). Mass spec.: 255.16 (MH)+.
1,4,6,7-Tetrahydro-pyrazolo[4,3-c]pyridine-5-carboxylic acid tert-butyl ester
0
~O ~ N
~ ~NH
-N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
149
To a solution of 3-dimethylaminomethylene-4-oxo-piperidine-1-carboxylic
acid tert-butyl ester (2.55 g, 10 mmol) in methanol (50 mL) was added
hydrazine
hydrate (0.61 mL, 1.25 equiv). The solution was heated to reflex, immediately
allowed to cool to room temperature, and concentrated to give 1.4 g (63%)
which was
used without purification. Mass spec.: 224.11 (MH)+.
4, 5, 6, 7-Tetrahydro-1 H-pyrazo l o [4, 3-c]pyri dine
HN
~ ~NH
-N
1,4,6,7-Tetrahydro-pyrazolo[4,3-c]pyridine-5-carboxylic acid tert-butyl ester
(0.70 g, 3.1 mmol) was dissolved in trifluoroacetic acid (10 mL) at
0°C, stirred for 1
h, and was concentrated. The residue was dissolved in ethanol and treated with
concentrated hydrochloric acid (1 mL). The bis-hydrochloride salt precipitated
out as
a white solid which was filtered to give 510 mg (83%). The free base was
prepared
as needed by dissolving the salt in water, loading it onto an SCX column,
flushing
with methanol, and then eluting with 2 M ammonia in methanol.
(~)-2-(Di-tert-butoxycarbonylamino)-3-( 1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-
yl)-propionic acid methyl ester
0
BocZN
home
N
~ ~NH
1N
To a solution of4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine (160 mg) in
2.5 mL methanol was added 2-(di-tert-butoxycarbonylamino)-acrylic acid methyl
ester (400 mg). The reaction was concentrated to approximately 1.5 mL by
application of a gentle stream of nitrogen. The solution was stirred at room
temperature overnight. The reaction was concentrated , dissolved in ethyl
acetate,
washed with brine, dried over magnesium sulfate, and concentrated. The
resulting
residue was pure enough to use without purification. 'H-NMR (CDCl3, 500 MHz) 8
1.44 (s, 9H), 2.73 (m, 3H), 2.91 (m, 1H), 3.06 (dd, J=13.4, 8.6, 1H), 3.22
(dd,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
150
J=13.4, 8.2, 1H), 3.54 (d, J=13.4, 1H), 3.63 (d, J=13.4, 1H), 3.71 (s, 3H),
5.11 (dd,
J=8.5, 5.2, 1 H), 7.25 (s, 1 H). Mass spec.: 425.23 (MH)+.
(~)-2-Amino-3-(1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-propionic acid
methyl ester
0
HzN
'OMe
N
~ ~NH
-N
To a solution of 2-(di-tert-butoxycarbonylamino)-3-(1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl)-propionic acid methyl ester (0.55 g, 1 equiv.) in
methylene chloride (5 mL, 0°C) was added trifluoroacetic acid (1.5 mL).
The ice
bath was removed and stirring continued for 2 h. The solution was
concentrated,
redissolved in methanol, and passed onto a column of strong canon exchange
resin.
After flushing with methanol, the product was removed from the column by
eluting
with 2 M ammonia in methanol to afford the product as its free base (275 mg,
95%).
1H-NMR (CDC13, 500 MHz) 8 2.71 (dd, J=12.8, 8.6, 1 H), 2.74-2.91 (m, 6H), 3.48
(s,
2H), 3.54 (d, J=13.4, 1H), 3.62 (d, J=13.4, 1H), 3.69 (dd, J=8.2, 4.9, 1H),
3.73 (s,
3H), 7.27 (s, 1H). Mass spec.: 225.16 (MH)+.
3,3-Dimethyl-4-oxo-piperidine-1-carboxylic acid tent-butyl ester
o
~o~N
0
To a solution of 4-oxo-piperidine-1-carboxylic acid tert-butyl ester (16 g, 80
mmol) in tetrahydrofuran (400 mL) at 0°C was added sodium hydride (4.1
g, 2.1
equiv) in 4 portions. To this was added iodomethane (12.5 mL, 2.5 equiv)
dropwise.
The reaction was allowed to gradually warm to room temperature and stirred
overnight. The reaction was concentrated, dissolved in diethyl ether, washed
with
brine, dried over magnesium sulfate, and concentrated. The product was
crystallized
from hot pentane (2X) to give 5.9 g (32%). 1H-NMR (CDCl3, 500 MHz) 8 1.09 (s,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
151
6H), 1.47 (s, 9H), 2.47 (dd, J=6.4, 6.4, 2H), 3.41 (m, 2H), 3.70 (m, 2H). Mass
spec.:
250.12 (M+Na)+.
5-Dimethylaminomethylene-3,3-dimethyl-4-oxo-piperidine-1-carboxylic acid tert-
butyl ester
0
~O~ N
~O
MeyN
3,3-Dimethyl-4-oxo-piperidine-1-carboxylic acid tert-butyl ester (5 g, 22
mmol) was dissolved in dimethyl formamide dimethylacetal (25 mL) and heated at
reflux for 2 h. The reaction mixture was then heated to 130°C for 1 h
via microwave,
and concentrated to give 6.43 g (quart.) as an oil which used without
purification.
'H-NMR (CDC13, 500 MHz) 8 1.07 (s, 6H), 1.45 (s, 9H), 3.06 (s, 6H), 3.37 (m,
2H),
4.57 (m, 2H), 7.41 (bs, 1 H).
7,7-Dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridine-5-carboxylic acid tert-
butyl
ester
0
~o~ N
~ ~NH
-N
To a solution of 5-dimethylaminomethylene-3,3-dimethyl-4-oxo-piperidine-1-
carboxylic acid tert-butyl ester (6.35 g, 22 mmol) in methanol (15 mL) was
added
hydrazine hydrate (1.2 mL, 1.1 equiv). The solution was stirred at room
temperature
overnight and concentrated to give 5.3 g (94%) which was used without
purification.
Mass spec.: 252.19 (MH)+.
7,7-Dimethyl-4,5,6,7-tetrahydro-1 H-pyrazolo[4,3-c]pyridine
HN
~ ~NH
-N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
152
To a solution of 7,7-dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridine-5-
carboxylic acid tert-butyl ester (5.3 g, 21 mmol) in methylene chloride (10
mL) at
0°C was added trifluoroacetic acid (5 mL). The reaction was allowed to
warm to
room temperature, stirred 1 S min, and treated with additional trifluoroacetic
acid (5
mL). After 1 h, the reaction was concentrated, dissolved in ethanol (10 mL),
cooled
to 0°C, treated with concentrated hydrochloric acid (3 mL), and
concentrated. The
resulting solid was triturated with ethanol, and filtered to give 3.02 g (64%)
as its bis-
hydrochloride salt. The free base was prepared as needed by dissolving the
salt in
water, loading it onto an SCX column, flushing with methanol, and then eluting
with
2M ammonia in methanol. 'H-NMR (D20, 500 MHz) 8 1.49 (s, 6H), 3.46 (s, 2H),
4.39 (s, 2H), 7.86 (s, 1 H). Mass spec.: 152.14 (MH)+.
(~)-2-(Di-tert-butoxycarbonylamino)- 3-(7,7-dimethyl-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl)-propionic acid methyl ester
0
BocyN
home
N
~ ~NH
N
To a solution of 7,7-dimethyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine
(160 mg) in methanol (3 mL) was added 2-(di-tert-butoxycarbonylamino)-acrylic
acid methyl ester (331 mg). A gentle stream of nitrogen was applied and the
reaction
stirred overnight. In the morning, the volume was greatly reduced. The last
traces of
solvent were removed under high vacuum to give 490mg (quant.) which was used
without purification. 'H-NMR (CDC13, 500 MHz) ~ 1.24 (s, 3H), 1.26 (s, 3H),
1.38
(s, 18H), 2.33 (d, J=11.3, 1H), 2.57 (d, J=11.3, 1H), 3.09 (dd, J=13.1, 5.5,
1H), 3.15
(dd, J=13.4, 9.5, 1H), 3.35 (d, J=12.8, 1H), 3.57 (d, J=12.8, 1H), 3.68 (s,
3H), 5.13
(dd, J=9.5, 3.7, 1H), 7.16 (s, 1H). Mass spec.: 453.30 (MH)+.
(~)-2-Amino-3-(7,7-dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-
propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
153
O
HyN
home
N
~ ~NH
-N
To a solution of 2-(di-tert-butoxycarbonylamino)- 3-(7,7-dimethyl-1,4,6,7-
tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-propionic acid methyl ester (0.49 g, 1
equiv)
in methylene chloride (5 mL, 0°C) was added trifluoroacetic acid (1.5
mL). The ice
bath was removed and stirring continued for 2 h. The solution was
concentrated,
redissolved in methanol, and loaded onto a column of strong cation exchange
resin.
After flushing with methanol, the product was removed from the column by
eluting
with 2M ammonia in methanol to afford the product as its free base (250 mg,
94%).
1H-NMR (CDCI3, 500 MHz) 8 1.27 (s, 3H), 1.28 (s, 3H), 2.41 (d, J=11.3, IH),
2.50
(d, J=11.3, I H), 2.69 (dd, J=12.5, 7.9, 1 H), 2.82 (dd, J=12.5, 5.2, 1 H),
3.45 (d,
J=12.8, 1H), 3.52 (d, J=12.8, 1H), 3.67 (m, 1H), 3.69 (s, 3H), 7.19 (s, 1H).
Mass
spec.: 253.16 (MH)+.
(~)-2- { [4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-
amino } -3-
(1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-propionic acid methyl ester
H
N ~O
\ ~ '~N
H O
N~N
home
O
N
~ ~NH
-N
To a solution of 2-amino-3-(1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-
propionic acid methyl ester (260 mg, 1 equiv) in methylene chloride (2 mL,
0°C) was
added carbonyl diimidazole (188 mg, I equiv). After 15 min, 3-piperidin-4-yl-
3,4-
dihydro-IH-quinazolin-2-one (295 mg, 1.1 equiv) was added in one portion. The
ice
bath was removed and stirring continued overnight. The reaction was
concentrated
and purified by column chromatography to give 118 mg (21%). 'H-NMR (CDC13,
500 MHz) 8 1.60-I .80 (m, 4H), 2.70-3.05 (m, 8H), 3.45 (s, 2H), 3.56 (d,
J=13.4,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
154
1H), 3.62 (d, J=13.4, 1H), 3.75 (s, 3H), 4.02 (d, J=13.1, 1H), 4.10 (d,
J=12.5, 1H),
4.24 (s, 2H), 4.45-4.57 (m, 2H), 5.79 (bs, 1 H), 6.68 (d, J=7.94, 1 H), 6.90
(dd, J=7.3,
7.3, 1 H), 7.00 (d, J=7.3, 1 H), 7.13 (dd, J=7.6, 7.3, 1 H), 7.25 (s, 1 H),
7.82 (s, 1 H).
Mass spec.: 482.27 (MH)+.
Example 54
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-2-oxo-1-( 1,4, 6,7-tetrahydro-pyrazolo [4, 3-c]
pyridin-5-
ylmethyl)-ethyl]-amide
H
/ N' /O
'~N
H O
N~N
N
I~IO
N N
i~
NH
N
To a solution of 2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-
carbonyl]-amino}-3-(1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-propionic
acid
methyl ester (16 mg, 1 equiv) in methanol (0.6 mL) was added lithium hydroxide
monohydrate (3 mg, 2.2 equiv) in water (0.1 mL) and stirred for 4 h at room
temperature. The solution was cooled to 0°C, treated with aqueous 1 M
potassium
hydrogen sulfate (60 p.1, 1.8 equiv), and concentrated to give the crude acid
which
was immediately used without purification. The crude acid was dissolved in
dimethylformamide (0.3 mL) and sequentially treated with methylene chloride
(0.15
mL), 4-piperidyl-piperidine (11 mg, 2 equiv), diisopropylethylamine (12 p.L, 2
equiv), and PyBOP° (19 mg, 1.1 equiv). The solution was stirred 30 min
and
concentrated. The product was purified by column chromatography to give 17.6
mg
(85%, 2 steps). 'H-NMR (CDC13, 500 MHz) S 1.30-1.60 (m, 9H), 1.62-1.78 (m,
5H),
1.81 (bd, J=11.0, 2H), 2.23-2.49 (m, 6H), 2.55-3.10 (m, 11H), 3.59 (d, J=7.3,
2H),
4.00-4.20 (m, 3H), 4.23 (s, 2H), 4.50 (m, 1 H), 4.63 (m, 1 H), 5.03 (m, 1 H),
5.71 (d,
J=7.3, 1 H), 6.67 (d, J=7.9, 1 H), 6.91 (dd, J=7.6, 7.3, 1 H), 7.02 (dd,
J=7.9, 7.3, 1 H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
155
7.14 (dd, J=7.6, 7.6, 1 H), 7.24 (s, 1 H), 7.39 (s, 1 H), 10.76 (bs, 1 H).
Mass spec.:
618.34 (MH)+.
Example SS
(~)-3-(7,7-Dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-yl)-2-{[4-(2-
oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
methyl ester
H
/ N\ /O
'N~ O
I H
N~N
home
O
N
~ ~NH
-N
To a solution of 2-amino-3-(7,7-dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl)-propionic acid methyl ester (250 mg, 1 equiv) in
tetrahydrofuran (4
mL, 0°C) was added carbonyl diimidazole (162 mg, 1 equiv). After 5 min,
the ice
bath was removed and the reaction stirred at room temperature for 30 min. To
this
was added 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one (250 mg, 1.1 equiv)
in
one portion, and the reaction stirred overnight. The reaction was concentrated
and
purified by column chromatography to give 228 mg (45%). 'H-NMR (CDC13, 500
MHz) 81.30 (s, 3H), 1.31 (s, 3H), 1:60-1.80 (m, 4H), 2.43 (d, J=11.6, 1H),
2.53 (d,
J=11.3, 1H), 2.80-2.95 (m, 4H), 3.51 (dd, J=20.4, 13.1, 2H), 3.74 (s, 3H),
4.00 (d,
J=13.7, 1H), 4.10 (d, J=12.2, 1H), 4.25 (dd, J=16.2, 14.4, 2H), 4.86 (m, 2H),
6.66
(d, J=7.6, 1 H), 6.92 (dd, J=7.6, 7.3, 1 H), 7.02 (d, J=7.3, 1 H), 7.14 (dd,
J=7.6, 7.6,
1 H), 7.24 (s, 1 H). Mass spec.: 510.27 (MH)+.
Example 56
(t)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7,7-dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-
ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
156
H
/ N' /O
\ I '~N
H O
N~N
'N l
I''~~//I\\O
N / I N N
~N
H
To a solution of 3-(7,7-dimethyl-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridin-5-
yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-
amino } -
propionic acid methyl ester (20 mg, 1.0 equiv) in methanol (0.6 mL) was added
lithium hydroxide monohydrate (4 mg, 2.2 equiv) in water (0.1 mL) and stirred
for 4
h at room temperature. The solution was cooled to 0°C, treated with
aqueous 1 M
potassium hydrogen sulfate (75 ~1, 1.8 equiv), and concentrated to give the
crude
acid which was immediately used without purification. The crude acid was
dissolved
in dimethylformamide (0.3 mL) and sequentially treated with methylene chloride
(0.15 mL), 4-piperidyl-piperidine (13 mg, 2 equiv), diisopropylethylamine (14
~L, 2
equiv), and PyBOP~ (22 mg, 1.1 equiv). The solution was stirred 1.5 h and
concentrated. The product was purified by column chromatography to give a
product
which was tainted with HOBT. The HOBT was removed by passing the product
through a plug of basic alumina, eluting with 10% methanol in methylene
chloride.
Concentration gave 18.3 mg (72%, 2 steps). 'H-NMR (CDCl3, 500 MHz) 8 1.25-1.32
(m, 6H), 1.40 (m, 4H), 1.54 (m, SH), 1.65 (m, 4H), 1.83 (m, 2H), 2.30-2.56 (m,
8H),
2.81 (m, 4H), 3.04 (dt, J=57.1, 12.2, 1H), 3.43-3.60 (m, 2H), 4.00-4.17 (m,
2H),
4.18-4.26 (m, 3H), 4.49 (m, 1 H), 4.62 (m, 1 H), 5.03 (m, 1 H), 5.80 (dd,
J=16.8, 9.8,
1 H), 6.69 (d, J=7.9, 1 H), 6.90 (dd, J=7.3, 7.3, 1 H), 6.99 (dd, J=7.6, 7.3,
1 H), 7.13
(dd, J=7.6, 7.6, 1 H), 7.19 (s, 1 H), 7.66 (bd, J=12.8, 1 H). Mass spec.:
646.43 (MH)+.
2-Benzyloxycarbonylamino-3-(6-methoxy-pyridin-3-yl)-acrylic acid methyl ester
COZMe
CBZHN \
I \
N /
OMe
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
157
To a suspension of potassium tert-butoxide (1.23 g, 1.5 equiv) in methylene
chloride (70 mL, -20°C) was added a solution of N-benzyloxycarbonyl-a-
phosphonoglycine trimethyl ester (3.63 g, 1.5 equiv) in methylene chloride (15
mL).
The resulting solution was stirred 5 min and treated with the 6-methoxy-
pyridine-3-
carbaldehyde (1.0 g, 7.3 mmol) in methylene chloride (15 mL). After stirring
for 1.5
h, the reaction was warmed to 0°C and stirred 1 h. The reaction was
quickly poured
into a separatory funnel containing ethyl acetate and water. Brine was added
to aid in
separation of the layers. The aqueous was extracted with ethyl acetate (3x)
which
were in turn washed with brine, dried over magnesium sulfate, and concentrated
to
give 2.63 g (quant.) which was used without purification. Mass spec.: 343.08
(MH)+.
(~)-2-Amino-3-(6-methoxy-pyridin-3-yl)-propionic acid methyl ester
C02Me
HyN
N /
OMe
A flask containing 2-benzyloxycarbonylamino-3-(6-methoxy-pyridin-3-yl)-
acrylic acid methyl ester (620 mg), palladium on charcoal (10%, 100 mg), ethyl
acetate (10 mL) and methanol (20 mL) was flushed with nitrogen, then hydrogen,
before finally affixing a balloon of hydrogen. The reaction was allowed to
stir
overnight. The flask was flushed with nitrogen, filtered through celite, and
concentrated to give 390 mg (quant.) which was used without purification. Mass
spec.: 211.11 (MH)~.
(~)-3-(6-Methoxy-pyridin-3-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino-propionic acid methyl ester
H
N' /O
'~N
I H
N ~ N C02Me
O
\N home
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
158
To a solution of 2-amino-3-(6-methoxy-pyridin-3-yl)-propionic acid methyl
ester (130 mg) and diisopropylethylamine (0.3 mL) in methylene chloride (2 mL,
0°C) was added N,N'-disuccinimidyl carbonate (158 mg). After 30 min, 3-
piperidin-
4-yl-3,4-dihydro-1H-quinazolin-2-one (120 mg) in methylene chloride (1 mL) was
added via canula. The reaction was warmed to room temperature and stirred
overnight. The reaction was concentrated and purified by prep HPLC to give
160mg
(55%). Mass spec.: 468.19 (MH)+.
Example 57
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(6-methoxy-pyridin-3-ylmethyl)-2-oxo-ethyl]-amide
H
/ N' /O
'~N
1 H °
° N
N~N N
N OMe
To a solution of 3-(6-methoxy-pyridin-3-yl)-2- f [4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
(160
mg) in methanol (6 mL) was added a solution of lithium hydroxide monohydrate
(29
mg) in water (1 mL). The reaction was stirred at room temperature for 4 h and
cooled to 0°C. The reaction was treated with 1N hydrochloric acid (0.6
mL),
concentrated. The residue obtained was dissolved in methylene chloride (5 mL),
and
treated sequentially with 4-piperidyl-piperidine (75 mg), triethylamine (0.14
mL),
and bis-2-oxo-3-oxazolidinyl)phoshinic chloride (104 mg). The reaction was
stirred
overnight, concentrated, and purified by prep HPLC to give 94 mg (45%). LC/MS:
tR
=1.86 min, 604.51 (MH)+.
2-Benzyloxycarbonylamino-3-(2-methoxy-pyrimidin-5-yl)-acrylic acid methyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
159
C02Me
CBZHN
N~N
OMe
To a suspension of potassium t-butoxide (1.23 g) in methylene chloride (70
mL, -30°C) was added a solution of N-benzyloxycarbonyl-a-
phosphonoglycine
trimethyl ester (3.63 g) in methylene chloride (15 mL). The resulting solution
was
S stirred 5 min and treated with the 2-methoxy-pyrimidine-5-carbaldehyde ( 1.0
g) in
methylene chloride (15 mL). After stirring for 1.5 h, the reaction was warmed
to 0°C
and stirred 1 h. The reaction was quickly poured into a sep funnel containing
ethyl
acetate and water. Brine was added to aid in separation of the layers. The
aqueous
was extracted with ethyl acetate (3X) which were in turn washed with brine,
dried
over magnesium sulfate, and concentrated. The crude product was recrystallized
from hot methanol to give 1.4g of pure material. Mass spec.: 344.10 (MH)+.
(~)-2-Amino-3-(2-methoxy-pyrimidin-5-yl)-propionic acid methyl ester
COyMe
H2N
N' //N
home
A flask containing amino ester (700 mg), palladium on charcoal (10%, 100
mg) and methanol (20 mL) was flushed with nitrogen, then hydrogen, before
finally
affixing a balloon of hydrogen. The reaction was allowed to stir overnight.
The flask
was flushed with nitrogen, filtered through celite, and concentrated to give
379 mg
(88%) which was used without purification. Mass spec.: 212.08 (MH)+.
(~)-3-(2-Methoxy-pyrimidin-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
160
H
N' /O
'IAN
I H
N ~ N COZMe
O
~ 'N
\N- 'OMe
To a solution of 2-Amino-3-(2-methoxy-pyrimidin-5-yl)-propionic acid
methyl ester (125 mg) and diisopropylethylamine (0.3 mL) in methylene chloride
(2
mL, 0°C) was added N,N'-disuccinimidyl carbonate (155 mg). After 30
min, 3-
piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one (120 mg) in methylene chloride
(2
mL) was added via canula. The reaction was warmed to room temperature and
stirred overnight. The reaction was concentrated and purified by prep HPLC to
give
99mg (36%). Mass spec.: 469.10 (MH)+.
Example 58
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(2-methoxy-pyrimidin-5-ylmethyl)-2-oxo-ethyl]-
amide
H
N' /O
'N~ O
I H
N " N N
O N
~ 'N
\N- 'OMe
To a solution of 3-(2-methoxy-pyrimidin-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester (99
mg)
in methanol (6 mL) was added a solution of lithium hydroxide monohydrate (18
mg)
in water (1 mL). The reaction was stirred at room temperature for 4 h and
cooled to
0°C. The reaction was treated with 1N hydrochloric acid (0.4 mL),
concentrated.
The residue obtained was dissolved in methylene chloride (3 mL), and treated
sequentially with 4-piperidyl-piperidine (50 mg), triethylamine (88 ~L), and
bis-2-
oxo-3-oxazolidinyl)phoshinic chloride (71 mg). The reaction was stirred
overnight,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
161
concentrated, and purified by prep HPLC to give 103 mg (45%). LClMS: tR = 1.23
min, 605.54 (MH)+.
2-Benzyloxy-5-bromo-pyridine
Br / \ o \ /
A suspension of 2,5-dibromopyridine (2.0 g, 8.4 mmol), dibenzo-18-crown-6
(0.14 g, .05 equiv), benzyl alcohol (1.1 mL, 1.3 equiv), and potassium
hydroxide (1.1
g, 2.4 equiv) in toluene (30 mL) were heated at reflux for 3 h in an apparatus
fitted
with a Dean-Stark trap. The suspension was cooled, concentrated, suspended in
water, and extracted into methylene chloride. The combined organic phases were
washed with water, then brine, dried over magnesium sulfate, and concentrated
to
give 1.9 g (85%) which was used without purification. Mass spec.: 264.25
(MH)+.
6-Benzyloxy-pyridine-3-carbaldehyde
N- O
O
H
To a solution of 2-benzyloxy-5-bromo-pyridine (1.64 g, 6.2 mmol) in
tetrahydrofuran (25 mL, -78°C) was added n-butyllithium (2.5 M in
hexane, 2.61 mL,
1.05 equiv). After 1 h at -78°C, dimethylformamide (0.97 mL, 2 equiv)
was added
and the mixture stirred for 30 min. The reaction was quickly poured into a
stirred
solution of 5% aqueous sodium bicarbonate (50 mL) and extracted with diethyl
ether
(3x). The ethereal was washed with brine, dried over magnesium sulfate, and
concentrated to give 1.16 g (quant.) which was used without purification. Mass
spec.: 186.34 (MH)+.
2-Benzyloxycarbonylamino-3-(6-benzyloxy-pyridin-3-yl)-acrylic acid methyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
162
O
~O
HN
COZMe
N-
O \
To a stirred suspension of potassium tert-butoxide (0.440 g, 1.7 equiv) in
methylene chloride (25 mL) at -20°C was added N-benzyloxycarbonyl-a-
phosphonoglycine trimethyl ester (1.3 g, 1.7 equiv) in methylene chloride (5
mL).
The resulting solution was stirred for 5 min and treated with the 6-benzyloxy-
pyridine-3-carbaldehyde (0.49 g, 2.28 mmol) in methylene chloride (5 mL). The
reaction was stirred at -20°C for 1 h, allowed to gradually warm to
0°C, and poured
into a separatory funnel containing water and diethyl ether. The reaction was
extracted with diethyl ether (2x), washed with brine, dried over magnesium
sulfate,
and concentrated to give 0.98 g (quant.) as an oil which was used without
purification. Mass spec.: 419.32 (MH)+.
(~)-2-Benzyloxycarbonylamino-3-(6-benzyloxy-pyridin-3-yl)-propionic acid
methyl
ester
0
0
HN
C02Me
N-
O \
IS
A flask was charged with 2-benzyloxycarbonylamino-3-(6-benzyloxy-
pyridin-3-yl)-acrylic acid methyl ester (0.50 g, 1.2 mmol), Wilkinson's
catalyst (200
mg, 0.2 equiv), methanol (5 mL), and toluene (3 mL). The flask was flushed
with
nitrogen, then hydrogen, heated to 35°C, and allowed to stir under an
atmosphere of
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
163
hydrogen for 4 days. The reaction was flushed with nitrogen, diluted with
methanol,
filtered, and concentrated to afford the crude product which was purified by
column
chromatography to give 145 mg (29%).
(~)-2-Amino-3-(6-benzyloxy-pyridin-3-yl)-propionic acid methyl ester
HZN
COyMe
N-
O \
To a stirred solution of 2-benzyloxycarbonylamino-3-(6-benzyloxy-pyridin-3-
yl)-propionic acid methyl ester (130 mg, 0.31 mmol) in methylene chloride (5
mL,
0°C) was added trimethylsilyl iodide (44 ~L, 1.0 equiv). The ice bath
was removed
and stirring continued for 1 h. Reaction was poured into saturated sodium
bicarbonate, extracted with ethyl acetate (3x), washed with brine, dried over
magnesium sulfate, and concentrated to give 8lmg (91%) which was used without
purification. Mass spec.: 287.37 (MH)+.
(~)-3-(6-Benzyloxy-pyridin-3-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid methyl ester
H
/ N, i0
\ I ''N
1 H °
N\/N
~OCHg
O
I
\N ~O \
I
To a stirred solution of 2-amino-3-(6-benzyloxy-pyridin-3-yl)-propionic acid
methyl ester (60 mg, 0.21 mmol) in methylene chloride (1 mL, 0°C) was
added
carbonyl diimidazole (34 mg, 1.0 equiv.). After 15 min, a solution of 3-
piperidin-4-
yl-3,4-dihydro-1H-quinazolin-2-one (58 mg, 1.2 equiv.) in methylene chloride
(0.5
mL) was added via canula. The ice bath was removed and stirring continued
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
164
overnight. The reaction was concentrated and purified by column chromatography
to
give 59 mg (52%). Mass spec.: 544.49 (MH)+.
Example 59
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
(6
benzyloxy-pyridin-3-ylmethyl)-2-[ 1,4']bipiperidinyl-1'-yl-2-oxo-ethyl]-amide
H
/ N' /O
'~N
l H °
° N
N~N N
~ i
To a stirred solution of 3-(6-benzyloxy-pyridin-3-yl)-2- f [4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
methyl
ester (59 mg, 0.11 mmol) in methanol (3 mL) was added a solution of lithium
hydroxide monohydrate (9.1 mg, 2 equiv) in water (0.5 mL). The reaction was
stirred 2 h at room temperature, cooled to 0°C, quenched by addition of
1N
hydrochloric acid (0.15 mL), and concentrated. The crude product was used
without
purification. The crude acid was dissolved in methylene chloride (2 mL,
0°C), and
treated sequentially with 4-piperidino-piperidine (34 mg, 1.8 equiv),
triethylamine
(35 ~.L, 2.3 equiv.), and bis-2-oxo-3-oxazolidinyl)phoshinic chloride (34 mg,
1.2
equiv). The ice bath was removed and the reaction allowed to stir overnight.
The
reaction was concentrated and purified by Prep TLC to give 30.3 mg (41 %).
LC/MS:
tR = 1.49 min, 680.29 (MH)+.
Example 60
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4'] b ip iperidinyl-1'-yl-2-oxo-1-(6-oxo-1,6-dihydro-pyridin-3-ylmethyl)-
ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
165
H
/ N' /O
'~N
H O
N I I N N
O N
N O
H
A flask was charged with 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carboxylic acid [1-(6-benzyloxy-pyridin-3-ylmethyl)-2-
[1,4']bipiperidinyl-1'-yl-2-oxo-ethyl]-amide (27 mg, 0.04 mmol), palladium on
charcoal (10%, 4 mg), and methanol (1 mL). The flask was flushed with
nitrogen,
then hydrogen, and allowed to stir under an atmosphere of hydrogen overnight.
The
flask was flushed with nitrogen, and the reaction filtered through celite to
give 22.1
mg (94%). LC/MS: tR = 0.93 min, 590.32 (MH)+.
Piperidine-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl ester
0 0~
N
o~o
To a solution of ethyl isonipecotate (5.00 g, 0.032 mol) and triethylamine
(4.9
mL, 0.035 mmol) in dichloromethane (25 mL) at 0°C was slowly added a
solution of
di-tert-butyldicarbonate (7.2 g, 0.033 mol) in dichloromethane (25 mL). The
reaction
mixture was stirred at room temperature overnight, then washed with potassium
hydrogen sulfate three times and with brine once. The organic extract was
dried over
anhydrous sodium sulfate, filtered and concentrated in vacuum to give the
desired
product (8.23 g, 100%) as colorless oil. 'H NMR (C6D6, 500 MHz) ~ 3.88 (q, J=
7.5
Hz, 2H), 2.52 (m, 1H), 1.60-1.48 (m, 8H), 1.42 (s, 9H), 0.92 (t, 3H). Mass
spec.:
280.44 (M+Na)+.
4-(2-Nitro-benzyl)-piperidine-1,4-dicarboxylic acid 1-tent-butyl ester 4-ethyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
166
O NOz
~o
J
N
O"O
To a solution of piperidine-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl
ester (8.23 g, 0.032 mol) in tetrahydrofuran (85 mL) was slowly added a
solution of
sodium bis(trimethylsilyl)amide (44 mL, 0.044 mol). After the resulting
mixture had
been stirred at -78°C for 1 h, a solution of 2-nitrobenzyl bromide
(8.21 g, 0.038 mol)
was added. The reaction mixture was allowed to warm up to room temperature and
was stirred overnight. It was then concentrated and the residue was
partitioned
between water and ethyl acetate. The organic extract was washed with brine,
dried
over anhydrous magnesium sulfate, filtered, and concentrated under vacuum. The
final product was purified from the complex reaction mixture by way of column
chromatography on silica gel (eluent - hexanes - ethyl acetate 4:1 ) to give
the desired
product (1.61 g, 13%) as brown oil. Mass spec.: 415.38 (M+Na)+.
4-(2-Amino-benzyl)-piperidine-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl
ester
O NHz
J
N
O"O
A mixture of 4-(2-nitro-benzyl)-piperidine-1,4-dicarboxylic acid 1-tert-butyl
ester 4-ethyl ester (1.61 g, 4.102 mmol) and 10% palladium on charcoal (0.10
g) in
ethanol (190 mL) was hydrogenated at 50 psi overnight. The resultant mixture
was
filtered through a plug of celite, and the filtrate concentrated under vacuum
to
provide the desired product (1.29 g, 99%) as colorless oil. Mass spec.: 363.45
(MH)+.
4-(2-Amino-benzyl)-piperidine-4-carboxylic acid ethyl ester hydrochloride
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
167
O NHz
~o
J a
N
H - HCI
To a solution of 4-(2-amino-benzyl)-piperidine-1,4-dicarboxylic acid 1-tert-
butyl ester 4-ethyl ester (1.29 g, 4.102 mmol) in dichloromethane (15 mL) was
added
a 4.0M solution of hydrogen chloride in dioxane (5 mL). The resulting solution
was
stirred at room temperature overnight. The concentration of the solution under
vacuum provided the title compound (1.23 g, 100%) as white solid, which was
used
in the next step without purification. Mass spec.: 263.40 (MH)+.
3,4-Benzo-2,9-diazaspiro[5.5]undeca-1-one
H
N
O
HN
A solution of 4-(2-amino-benzyl)-piperidine-4-carboxylic acid ethyl ester
hydrochloride (1.23 g, 4.102 mmol) was dissolved in methanol and the resulting
solution was stirred at room temperature overnight. The solution was diluted
by half
with water and passed through a short plug of the hydroxide form of AG~ 1-X2
ion-
exchange resin (100-200 mesh), eluting with 50% aqueous methanol. The
evaporation of the collected fractions gave the desired product (0.89 g, 100%)
as
white solid. 1H-NMR (CD30D, 500 MHz) 8 7.23 (m, 2H), 7.05 (d, J= 7.5 Hz, 1H),
6.89 (d, J= 8.0 Hz, 1H), 3.46-3.41 (m, 2H), 3.34-3.30 (m, 2H), 2.14-2.09 (m,
2H),
1.73-1.67 (m, 4H). Mass spec.: 217.46 (MH)+.
(R)-2-Amino-3-benzo[b]thiophen-3-yl-1-[ 1,4']bipiperidinyl-1'-yl-propan-1-one,
dihydrochloride
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
168
S
CN--( N NHy
~f O
To a well stirred solution of 3-benzo[b]thiophen-3-yl-(2R)-2-tert-
butoxycarbonylamino-propionic acid (1.0 g, 3.1 mmol) in methylene chloride (30
mL) at room temp was added 4-piperidinopiperidine (573 mg, 3.4 mmol),
triethylamine (1.3 mL, 9.3 mmol) followed by 3-(diethoxyphosphoryloxy)-1,2,3-
benzotriazin-4(3H)-one (1.02 g, 3.4 mmol). After 3 h, the reaction mixture was
treated with aqueous sodium hydrogencarbonate (15 mL), brine (20 mL) and dried
(sodium sulfate). The crude mixture was purified by flash chromatography using
5%
methanol in methylene chloride to give (1R)-1-benzo[b]thiophen-3-ylmethyl-2-
~[1,4']bipiperidinyl-1'-yl-2-oxo-ethyl)-carbamic acid tert-butylester in 82%
yield.
( 1 R)-1-Benzo[b]thiophen-3-ylmethyl-2-[ 1,4']bipiperidinyl-1'-yl-2-oxo-ethyl)-
carbamic acid tert-butylester (1.2 g, 2.54 mmol) in methylene chloride (5 mL)
was
added to a saturated solution of hydrogen chloride in dioxane (20 mL) and
stirred for
2 h. The solvents were removed to give (2R)-2-amino-3-benzo[b]thiophen-3-yl-1-
[1,4']bipiperidinyl-1'-yl-propan-1-one,dihydrochloride in 98% yield. 1H-NMR
(500
MHz, CD30D): 8 7.98 - 7.88 (m, 2 H), 7.55 - 7.40 (m, 3 H), 4.85 - 4.83 (m, 1
H),
3.66 - 2.68 (m, 9 H), 1.92 - 1.44 (m, 12 H). Mass spec.: 372 (MH)+.
Example 61
(R)-1-Oxo-3,4-benzo-2,9-diaza-spiro [5.5] undec-3-ene-9-carboxylic acid (1-
benzo
[b] thiophen-3-ylmethyl-2-[1,4'] bipiperidinyl-1'-yl-2-oxo-ethyl)-amide
N _
O
S ~ ~NH
~, y- N~.-~r~ r~
i ~ O NH
0
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
169
To a solution of 2-amino-3-benzo[b]thiophen-3-yl-1-[1,4'] bipiperidinyl-1'-yl-
propan-1-one (50.0 mg, 0.135 mmol) in 1,2-dichloroethane (1.5 mL) were added
N,N'-disuccinimidyl carbonate (34.6 mg, 0.135 mmol) and diisopropylethyl amine
(0.09 mL, 0.500 mmol). The resulting solution was stirred for 1 h, at which
point 3,4-
benzo-2,9-diazaspiro[5.5]undeca-1-one (30.4 mg, 0.140 mmol) was added. The
reaction mixture was stirred at room temperature overnight and concentrated.
The
purification was achieved by way of reversed-phase preparative HPLC to give
the
desired product (75.5 mg, 77%) as brown oil.'H-NMR (CD30D, S00 MHz) 8 7.92-
7.85 (m, 2H), 7.44-7.34 (m, 3H), 7.21-7.16~(m, 2H), 7.00 (t, J= 7.0 Hz, 1H),
6.86 (t,
J = 8.5 Hz, 1 H), 5.15-5.02 (m, 1 H), 4.72-4.45 (m, 1 H), 3.95-3.20 (m, 8H),
3.18-2.92
(m, 4H), 2.92-2.75 (m, 2H), 2.75-2.63 (m, 1 H), 2.40-2.30 (m, 1 H), 2.08-1.64
(m,
8H), 1.58-1.20 (m, 6H). Mass spec.: 614.37 (MH)+.
Example 62
N-[(1R)-1-(Benzo[b]thien-3-ylmethyl)-2-[1,4-bipiperidin]-1-yl-2-oxoethyl]-
3',4'-
dihydro-2-oxospiro-[piperidine-4,4'( 1 H)-quinoline]-1-carboxamide
_ o
S
NH
N NJ \
O
~N O
~N
Prepared as described for (R)-1-oxo-3,4-benzo-2,9-diaza-spiro [5.5] undec-3-
ene-9-carboxylic acid (1-benzo [b] thiophen-3-ylmethyl-2-[1,4'] bipiperidinyl-
1'-yl-
2-oxo-ethyl)-amide from 3',4'-dihydro-2-oxospiro-[piperidine-4,4'(1H)-
quinoline
(M.S. Chambers, et al., J. Med. Chem., 1992, 35, 2033-2039; WO-94/13696). 1H-
NMR (CDCl3, 500 MHz) 8 -0.35 (1H, m), 0.79 (1H, m), 1.2-2.1 (12H, m), 2.22
(5H,
m), 2.38 (2H, m), 2.74 (2H, ABq), 3.19 (3H, m), 3.33 (2H, m), 3.65 (1H, d),
3.80
( 1 H, m), 3.93 ( 1 H, t), 4.49 ( 1 H, d), 5.31 ( 1 H, t), 5.96 ( 1 H, t),
6.89 ( 1 H, d), 7.05 ( 1 H,
2 S t), 7.18 ( 1 H, d), 7.26 ( 1 H, m), 7. 3 3 ( 1 H, m), 7.40 ( 1 H, m), 7.7
8 ( 1 H, m), 7.96 ( 1 H,
Abq), 9.01 (1H, brs), 9.17 (1H, brs). Mass spec.: 614.36 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
170
Example 63
N-[( 1 R)-1-(Benzo[b]thien-3-ylmethyl)-2-[ 1,4-bipiperidin]-1-yl-2-oxoethyl]-
2',3'-
dihydro-1-oxospiro-[piperidine-4,4'( 1 H)-isoquinoline]-1-carboxamide
H
S ~ N O
" J '~
N\ /N
O
~N O
'N
Prepared as described for (R)-1-oxo-3,4-benzo-2,9-diaza-spiro [5.5] undec-3-
ene-9-
carboxylic acid (1 benzo [b] thiophen-3-ylmethyl-2-[1,4'] bipiperidinyl-1'-yl-
2~xo-ethyl)-amide from 2',3'-dihydro-1-oxospiro-[piperidine-4,4'(1H)-
isoquinoline (M.S. Chambers, et al., J. Med. Chem., 1992, 35, 2033-2039;
WO-94/13696). 1H-NMR (CDCl3, 500 MHz) 8 0.01 (1H, m), 0.78 (1H, m),
1.1-2.0 (12H, m), 2.15-2.30 (5H, m), 2.74 (1H, t), 3.0-3.6 (9H), 3.89(2H, m),
4.46 (1H, d), 5.29 (1H, m), 5.62 (1H, d), 6.47 (1H, brs), 7.38 (5H, m), 7.51
( 1 H, m), 7.77 ( 1 H, m), 7.85 ( 1 H, m), 8.11 ( 1 H, d). Mass spec.: 614.42
(MH)+.
Example 64
N-[(1 R)-1-(Benzo[b]thien-3-ylmethyl)-2-[ 1,4'-bipiperidin]-1'-yl-2-oxoethyl]-
1,2-
dihydro-2-oxospiro-[4H-3,1-benzoxazine-4,4'-piperidine]-1'-carboxamide
1
S O
O
/~ II NH
CN--( N
N~N
O H
Prepared as described for (R)-1-oxo-3,4-benzo-2,9-diaza-spiro [5.5] undec-3-
ene-9-carboxylic acid (1-benzo [b] thiophen-3-ylmethyl-2-[1,4'] bipiperidinyl-
1'-yl-
2-oxo-ethyl)-amide from 1,2-dihydro-2-oxospiro-[4H-3,1-benzoxazine-4,4'-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
171
piperidine (prepared as described in Takai, et al.; Chem. Pharm. Bull. 1985,
33,
1129-1139) to give the title compound (76%). Mass spec.: 616 (MH)+. Rf= 1.42.
Succinate Intermediates and Examples
3-Benzo[b]thiophen-3-yl-acrylic acid
S
/ \ ~ cooH
A suspension of 1-benzothiophene-3-carbaldehyde (4.9 g, 0.03 mol), malonic
acid (6.6 g, 0.06 mol) and piperidine ( 1 mL) in 100 mL anhydrous pyridine was
heated at 110°C overnight. The reaction mixture was cooled to room
temperature
and the solvent was removed in vacuo. The residue was taken up in 100 mL of
water
and 1 N hydrochloric acid was added to adjust the pH of this solution to ca.
3. The
suspension was filtered and the yellow solid was collected, washed with water
(3 x
50 mL) and concentrated in vacuo to give the indicated product with 95% purity
(5.65g, 91 %).
3-Benzo[b]thiophen-3-yl-propionic acid
S
/ \ cooH
A suspension of 3-benzo[b]thiophen-3-yl-acrylic acid: (5.6 g, 0.027 mol) and
10% PdJC (600 mg) in 1:1 methanol/ethyl acetate (50 mL) was hydrogenated in a
Parr apparatus at 50 psi overnight. The mixture was filtered and concentrated
to give
the crude product without further purification (ca.100% conversion). Mass
spec.:
205(MH)-.
3-(3-Benzo[b]thiophen-3-yl-propionyl)-4(R)-benzyl-oxazolidin-2-one
S
O
/
Bnv N~O
~O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
172
To a solution of 3-benzo[b]thiophen-3-yl-propionic acid (2.1 g, 0.010 mol),
triethylamine (4.12 g, 0.040 mol) in anhydrous tetrahydrofuran (100 mL) at
0°C was
added pivaloyl chloride (1.38 mL, 0.011 mol). After stirring for 1.5 h at
0°C, lithium
chloride (0.475 g, 0.011 mol) and (R)-4-benzyl-2-oxazolidinoe (1.988 g, 0.011
mol)
were added. The reaction mixture was allowed to warm up to room temperature
and
stirred overnight. Then the mixture was washed with water (3 x 150 mL). The
organic layer was separated, dried, and evaporated to give the crude product.
The
title product was obtained as a brown oil (90%) by flash chromatography on
silica gel
eluting with 100% methylene chloride . This compound was used immediately in
the
following procedure.
3(S)-Benzo[b]thiophen-3-ylmethyl-4-(4-benzyl-2-oxo-oxazolidin-3-yl)-4-oxo
butyric
acid tert-butyl ester
O
O
Bn~~O
To a solution of 3-(3-benzo[b]thiophen-3-yl-propionyl)-4-benzyl-oxazolidin-
2-one (3.35 g, 9.18 mmol) in 100 mL anhydrous tetrahydrofuran at -78°C
was added
lithium diisopropyl amide in tetrahydrofuran (6.1 mL, 11.01 mmol) and the
reaction
mixture was stirred for 30 min Following addition of t-butyl bromoacetate
(1.62
mL, 11.01 mmol) at -78°C, the mixture was stirred overnight while it
was allowed to
warm to room temperature. The solvent was evaporated and the residue diluted
with
ethyl acetate. The organic layer was washed with water (3 x 100 mL), dried,
filtered,
and concentrated to give the crude product. The title product was obtained by
filtration through a pad of silica, eluting with methylene chloride (49 %).
2(S)-Benzo[b]thiophen-3-ylmethyl-succinic acid, 4-tent-butyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
173
S
HO O
O
To a stirred solution of 3-benzo[b]thiophen-3-ylinethyl-4-(4-benzyl-2-oxo-
oxazolidin-3-yl)-4-oxobutyric acid tent-butyl ester (2.15 g, 4.49 mmol) in
tetrahydrofuran (50 mL) and water (30 mL) at 0°C was added 30 % aqueous
hydrogen peroxide (1 mL) followed by lithium hydroxide (0.2155 g, 8.98 mmol).
The reaction mixture was stirred overnight. Tetrahydrofuran was removed in
vacuo
and the resulting solution was acidified with 10% citric acid, and extracted
with ethyl
acetate (3 x 50 mL). The organic layer was washed with sodium bisulfate
solution,
dried and concentrated to give the title product.
3(S)-Benzo[b]thiophen-3-ylmethyl-4-[1,4']bipiperidinyl-1'-yl-4-oxo-butyric
acid tert-
butyl ester
N O'
v
A solution of 2-benzo(b]thiophen-3-ylmethyl-succinic acid 4-tert-butyl ester
(1.8420 g, 5.76 mmol), piperidylpiperidine (1.2240 g, 7.28 mmol) and
triethylamine
(0.7353 g, 7.28 mmol) in 100 mL methylene chloride was treated with 3
(diethoxyphosphoryloxy)-1,2,3-benzotriain-4(3H)-one (DEPBT, 1.8953 g, 6.34
mmol). The mixture was stirred overnight and then washed with water (3 x 40
mL).
The organic layer was dried, filtered, and concentrated in vacuo to give the
crude
product. This was further purified by flash chromatography on silica gel,
eluting
with 0-10% 2 M ammonia in methanol/methylene chloride, to give the desired
product. This product was carried on without further purification.
3(S)-Benzo[b]thiophen-3-ylmethyl-4-[1,4']bipiperidinyl-1'-yl-4-oxo-butyric
acid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
174
OH
V
A solution of 3-benzo[b]thiophen-3-ylmethyl-4-[1,4']bipiperidinyl-1'-yl-4-
oxo-butyric acid tent-butyl ester in 15 mL methylene chloride was treated with
trifluoroacetic acid (3 mL) and the reaction mixture was stirred overnight at
room
temperature. The solvent was evaporated to give the corresponding
trifluoroacetate
salt of the title product (99%).
Example 65
1-[ 1,4']Bipiperidinyl-1'-yl-2-(3(S)-Benzo[b]thiophen-3-ylmethyl)-4-[ 1',2'-
dihydro-2'-
oxospiro-[4H-3',l-benzoxazine-4,4'-piperidinyl]-butane-1,4-dione
s o
N NH
O
O O
N
N
A solution of 3-benzo[b]thiophen-3-ylmethyl-4-[1,4')bipiperidinyl-1'-yl-4-
oxo-butyric acid (25.0 mg, 0.060 mmol), 1,2-dihydro-2-oxospiro-4H-3,1-dihydro-
benzoxazine-4'4-piperidine (15.7 mg, 0.072 mmol) and triethylamine (7.3 mg,
0.072
mmol) in 5 mL methylene chloride at room temperature was treated with 3-
(diethoxyphosphoryloxy)-1,2,3-benzotriain-4(3H)-one (DEPBT, 21.5 mg, 0.072
mmol). The solution was stirred overnight and then washed with water (3 x 5
mL).
The organic layer was dried, concentrated, and the crude product was purified
by
flash chromatography on silica gel, eluting with 0-10% 2M ammonia in
methanol/methylene chloride, to give the desired product in 60% yield. LC/MS:
tR=1.34 min, 615.45 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
175
2-(7-Methyl-1H-indazol-5-ylmethylene)-succinic acid 1-methyl ester
OH
N/ I / O
'N O OCH3
H
To a mixture of 7-methyl indazole aldehyde (0.2619 g , 1.64 mmol) and
DBE-4 dibasic ester (dimethyl succinate) (0.32 mL, 2.45 mmol) in t-butanol (20
mL)
was added potassium t-butoxide (0.4036 g, 3.60 mmol). The reaction mixture was
heated at 50°C for 2h under nitrogen. After a further 16h at room
temperature, the
solvent was removed in vacuo and the residue was taken up in water (100 mL)
and
extracted with ethyl acetate (3 x 50 mL). The aqueous layer was acidified with
1 N
hydrochloric acid to pH 3 ~ 4 and extracted with ethyl acetate (3 x 50 mL).
The
combined ethyl acetate solution was dried and concentrated in vacuo to give
the
crude product as a yellow solid (99%, cis/trans isomer approximately 40:60).
The
crude mixture was carried to next step without further purification. Mass
spec.: 275
(MH)+.
(~)-2-(7-Methyl-1H-indazol-5-ylmethyl)-succinic acid 1-methyl ester
OH
N/
'N ~ O"OCH
H 3
A suspension of 2-(7-methyl-1H-indazol-5-ylmethylene)-succinic acid 1-
methyl ester (0.4440 g, 1.62 mmol) and 10% Pd/C (0.04 g) in ethyl acetate (15
mL)
and methanol (5 mL) was hydrogenated in a Parr apparatus overnight at 50 psi.
The
reaction mixture was filtered through a pad of celite and the filtrate
evaporated to
give the desired product as a yellow solid (100%). Mass spec.: 277 (MH)+.
Example 66
(t)-2-(7-Methyl-1H-indazol-5-ylmethyl)-4-oxo-4-[1',2'-dihydro-2'-oxospiro-[4H-
3',1-
benzoxazine-4,4'-piperidinyl]-butyric acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
176
OCH3
N ~ I \ s0
,N / O
H
N
H O
A solution of 2-(7-methyl-IH-indazol-5-ylmethyl)-succinic acid 1-methyl
ester (0.2253 g, 0.82 mmol), 1,2-dihydro-2-oxospiro-4H-3,1-dihydro-benzoxazine-
4'4-piperidine (0.1938 g, 0.89 mmol) and triethylamine (0.099 g, 0.98 mmol) in
methylene chloride (15 mL) was treated with 3-(diethoxyphosphoryloxy)-1,2,3-
benzotriain-4(3H)-one (DEPBT, 0.2685 g, 0.90 mmol). The mixture was stirred
overnight and then washed with water (3 x 5 mL). The organic layer was dried,
and
concentrated in vacuo. The residue was purified by flash chromatography on
silica
gel, eluting with 0-10% 2M ammonia in methanol/methylene chloride, to afford
the
desired product (53%). LC/MS: tR=1.40 min, 477.28 (MH)+.
Similarly prepared:
Example 67
(~)-2-(7-Methyl-1 H-indazol-S-ylmethyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-I-yl)-butyric acid methyl ester
H
O\/N \
'N~ I /
NJ
N ~ I \ ~ 10
,N ~ O OCH3
H
'H-NMR (400 MHz, CDC13) 8 8.02 (1H, s), 7.98(1H, m), 7,90 (1H, m), 7.35 - 6.89
(4H, m), 6.72 ( 1 H, m), 4.71 ( 1 H, m), 4.57 ( 1 H, m), 4.27 ( 1 H, s), 4.22
( 1 H, m), 3.85
(1H, m), 3.65 (3H, m), 3.30 (1H, m), 3.1 I(2H, m), 2.83 (2H, m), 2.81 - 2.54
(4H, m),
2.35 (1H, m), 1.7 3 - 1.67 (4H, m). Mass spec.: 490.32 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
177
(~)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-4-oxo-4-[ 1',2'-dihydro-2'-oxospiro-
[4H-3',1-
benzoxazine-4,4'-piperidinyl)-butyric acid
OH
N / ~ \ w0
.N / O
H
N
O
N
H O
A solution of 2-(7-methyl-1 H-indazol-5-ylmethyl)-4-oxo-4-[ 1',2'-dihydro-2'-
oxospiro-[4H-3',l-benzoxazine-4,4'-piperidinyl]-butyric acid methyl ester
(0.1911 g,
0.40 mmol) and lithium hydroxide (19.3 mg, 0.80 mmol) in tetrahydrofuran (10
mL)
and water (8 mL) was stirred overnight at room temperature. The reaction
mixture
was acidified with 1 N hydrochloric acid to ca. pH 1 and concentrated to
remove
tetrahydrofuran in vacuo to afford a white solid precipitate which was
collected by
filtration. The solid was washed twice with small amounts of water and dried
in
vacuo overnight (100%). Mass spec.: 477 (MH)+.
Example 68
(~)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(7-methyl-1 H-indazol-5-ylmethyl)-4-[ 1',2'-
dihydro-
2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butane-1,4-dione
0
O" NH
O NJ
N
'N / O N
H
A solution of 2-(7-methyl-1 H-indazol-5-ylmethyl)-4-oxo-4-[ 1',2'-dihydro-2'-
oxospiro-[4H-3',l-benzoxazine-4,4'-piperidinyl]-butyric acid (0.020 g, 0.04
mmol),
piperidylpiperidine (0.0087 g, 0.05 mmol) and triethylamine (0.09 g, 0.08
mmol) in
methylene chloride (S mL) at room temperature was treated with 3-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
178
(diethoxyphosphoryloxy)-1,2,3-benzotriain-4(3H)-one (DEPBT, 0.0155 g, 0.05
mmol). The mixture was stirred overnight and then washed with water (3 x 5
mL).
The organic layer was dried and the solvents were removed in vacuo. The crude
product was purified by preparative TLC on silica gel (10% 2 M ammonium
hydroxide/methanol in methylene chloride) to give the desired product (36%).
LC/MS: tR=1.18 min, 613.47 (MH)+.
Similarly prepared:
Example 69
(~)-1-[1,4']Bipiperidinyl-1'-yl-2-(7-methyl-1H-indazol-5-ylmethyl)-4-[4-(2-oxo-
1,4-
dihydro-2 H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
H
O\/N
'N~ ( /
N
N 1
1 H-NMR (400 MHz, CDC13) b 7.99 ( 1 H, m), 7.62 ( 1 H, m), 7.3 8 ( 1 H, m),
7.14( 1 H,
m), 7.04 - 6.90 (3H, m), 6.70 (2H, d, J=8.0 Hz), 4.70-4.58 (3H, m), 4.24 (2H,
m),
4.00 (2H, m), 3.70 ( 1 H, m), 3.18 - 2.72 (5H, m), 2.64 - 2.22 (8H, m), 2.18 -
0.82
(17H, m). Mass spec.: 626.34 (MH)+.
Example 70
(~)-1-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-2-(7-methyl-1H-indazol-5- ylmethyl)-
4-
[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
179
H
O\/N \
'N~ I /
O N
N
'N / O N
H O
of
1H-NMR (400 MHz, CDC13) 8 8.06 (1H, s), 7.75 (1H, m), 7.36 (1H, m), 7.14 (1H,
m), 7.01 - 6.79 (3H, m), 6.70 (1H, m), 4.70 - 4.49 (2H, m), 4.23 (2H, m), 3.98
(1H,
m), 3.87 (3H, m), 3.65 - 3.44 (4H, m), 3.26 (1H, m), 3.10 - 2.88 (3H, m), 2.75
(1H,
m), 2.51 (3H, s), 2.35 ( 1 H, m), 2.00 ( 1 H, m), 1.70 - 1.00 (9H, m). Mass
spec.:
601.38 (MH)+.
Example 71
(~)-1-(1,4-Dioxa-8-aza-spiro[4.S]dec-8-yl)-2-(7-methyl-1H-indazol-5- ylmethyl)-
4-
[1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butane-1,4-
dione
O
O~NH
N
J
N ~
,N / O"N
H O
OJ
'H-NMR (400 MHz, CDC13) 8 9.27 (1H, m), 8.00 (1H, s), 7.37 (1H, m), 7.23 (1H,
m), 7.10 - 6.99 (3H, m), 6.87 (1H, m), 4.54 (1H, m), 3.97 - 3.50 (10H, m),
3.30 (1H,
m), 3.16 - 2.76 (4H, m), 2.53 (3H, s), 2.35 (1H, m), 2.20 - 1.00 (9H, m). Mass
spec.:
588.36 (MH)+.
Example 72
(~)-N,N Dimethyl-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butyramide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
180
H
O\/N
'N I /
O N
N
I~
'N / ~ i
O N
H
LC/MS: tR=1.36 min, 525.35 (M+Na)+.
Example 73
(~)-1-(2, 6-Dimethyl-morpholin-4-yl)-2-(7-methyl-1 H-indazol-5-ylmethyl)-4-[4-
(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
H
O\/N
'N I /
N
N, I _
,N / O' _ N
H
O
LCJMS: tR=1.41 min, 573.39 (MH)+.
Example 74
(+)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-1-(4-methyl-piperidin-1-yl)-4-[4-(2-
oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
H
O\/N
'N I /
O NJ
i
N
'N / O N
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
181
IH-NMR (400 MHz, CDC13) 8 8.06 (1H, b), 7.60 - 6.73 (7H, m), 4.71 (1H, m),
4.54
(2H, m), 4.26 (2H, m), 4.05 - 3.89 (2H, m), 3.65 (1H, m), 3.09 - 2.81 (4H, m),
2.61
(3H, s), 2.41 (2H, m), 1.76 - 0.51 (15H, m). Mass spec.: 557.38 (MH)+.
Example 75
(~)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-1-morpho lin-4-yl-4-[4-(2-oxo-1,4-
dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
H
O\/N
'N~ I /
O N
N/
,N / O N
H ~O
LC/MS: tR=1.32 min, 545.42 (MH)+.
Example 76
(~)-N,N Dimethyl-2-(7-methyl-1 H-indazol-5-ylmethyl)-4-oxo-4-[ 1',2'-dihydro-
2'-
oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butyramide
O
O~NH
NJ y
N ~
'N / O~ N ~
H
LC/MS: tR=1.27 min, 512.30 (M+Na)+.
Example 77
(~)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-1-(piperidin-1-yl)-4-[ 1',2'-dihydro-
2'-
oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
182
O
O~NH
O NJ
i
N
'N / O N
H
'H-NMR (400 MHz, CDC13) 8 9.26 - 9.01 (1H, m), 8.09 (1H, s), 7.42 - 6.89 (7H,
m),
4.56 (1H, m), 3.84 (1H, m), 3.65 (3H, m), 3.30 (2H, m), 3.05 (3H, m), 2.81
(1H, m),
2.60 (3H, s), 2.39 (1H, m), 2.09 (2H, m), 1.85 (1H, m), 1.43 - 0.79 (9H, m).
Mass
spec.: 530.34 (MH)+.
Example 78
(~)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-
yl)-piperidin-1-yl]-1-piperidin-1-yl-butane-1,4-dione
H
O\/N
~N I /
O N
N/
'N / O N
H
' H-NMR (400 MHz, CDC13) 8 8.02 ( 1 H, s), 7.82 ( 1 H, m), 7.3 7 ( 1 H, m),
7.14 ( 1 H,
m), 7.04 - 6.90 (3 H, m), 6. 73 ( 1 H, d, J = 8.0 Hz), 4. 69 ( 1 H, m), 4. 5 6
( 1 H, m), 4.24
(2H, d, J = 7.2 Hz), 4.02 (1H, m), 3.65 (2H, m), 3.33 (3H, m), 3.07 (3H, m),
2.78
(1H, m), 2.55 (3H, s), 2.36 (1H, m), 1.80 - 1.50 (4H, m), 1.43 (4H, b), 1.26
(2H, b),
1 S 0.81 (2H, b). Mass spec.: 543.40 (MH)+.
Example 79
(~)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(1 H-indazol-5-ylmethyl)-4-[ 1',2'-dihydro-
2'-
oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
183
O
O~NH
O NJ
'W
N
'N ~ O N
H
N 1
1H-NMR (400 MHz, CDC13) 8 8.86 (1H, m), 7.98 (1H, s), 7.54 - 6.85 (7H, m),
4.73 -
4.48 (3H, m), 3.96 - .80 (3H, m), 3.73 - 3.58 (3H, m), 3.17 - 2.78 (5H, m),
2.55 - 2.24
(5H, m), 2.02 - 1.79 (6H, m), 1.70 - 0.79 (7H, m). Mass spec.: 599.31 (M+Na)+.
Example 80
(~)-1-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-2-(1H-indazol-S- ylinethyl)-4-
[1',2'-
dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butane-1,4-dione
O
O" NH
O NJ
'W
N
'N ~ O N
H
O
LC/MS: tR=1.25 min, 574.25 (MH)+.
Example 81
(~)-1-( 1,4-Dioxa-8-aza-spiro[4.5] dec-8-yl)-2-( 1 H-indazol-5-ylmethyl)-4-[4-
(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
184
H
O~ N
''N~ I
N J
N~N
H O N
O
~J
LC/MS: tR=1.34 min, 587.38 (MH)+.
Example 82
(~)-2-( 1 H-Indazol-5 -ylmethyl)-N,N-dimethyl-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazol in-3-yl)-piperidin-1-yl J-butyramide
H
O\/N
'N I /
O N
NN
H O N
LC/MS: tR=1.28 min, 489.33 (MH)+.
Example 83
(~)-5- f2-([1,4'JBipiperidinyl-1'-carbonyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyl}-indazole-1-carboxylic acid tent-butyl
ester
O\/N
'N I /
O N
N/
'N / O N
O ~O N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
185
LCIMS: tR=1.47 min, 742.55 (M+Na)+
Example 84
(~)-2-(7-Methyl-1 H-indazol-5-ylmethyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-N-prop-2-ynyl-butyramide
O\/NH \
'N~
O N
N ~
'N / O' -NH
H
LC/MS: tR=1.33 min, 535.32 (M+Na)+.
As~artate Intermediates and Exam 1-~l-.es
(L)-2-tent-Butoxycarbonylamino-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-butyric acid benzyl ester
/ O' -NH O
\ ~ O N O
o N- 'NH
To a stirred solution of N tent-butyloxycarbonyl-L-aspartic acid-alpha-benzyl
ester (1.4 g, 4.33 mmol) and 3,4-dihydro-3-(4-piperidinyl-2(1H)-quinazolinone
{1.26
g, 4.33 mmol) in methylene chloride (12 mL) was added 3-
(diethoxyphosphoryloxy)-
1,2,3-benzotriain-4(3H)-one (DEPBT, 1.425 g, 4.76 mmol) in one portion
followed
by dropwise addition of triethylamine (0.724 mL, 5.20 mmol). The resulting
suspension gradually became homogeneous with stirring and was stirred at room
temperature overnight (15 h). The mixture was diluted with methylene chloride
and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
186
washed with sodium hydroxide (0.5 N) and water. The layers were separated and
the
organic layer was dried with sodium sulfate, and concentrated in vacuo to give
a light
yellow foam. The crude product was purified by flash column chromatography
(10%
methanol in methylene chloride) to give a colorless oil. Mass spec.: 559
(M+Na)+.
(L)-2-tert-Butoxycarbonylamino-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-butyric acid
/ 'o
O~NH O
HO
~~~ N O
O
N~NH
To a solution of 2-tert-butoxycarbonylamino-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butyric acid benzyl ester (1.48 g, 2.76
mmol) in
ethyl acetate/methanol (16 mL, 1:1) in a Parr bottle was added 10% palladized
charcoal (150 mg) in one portion. Hydrogenation was carried out with a Parr
apparatus at 52 psi for 1 h. TLC (10% methanol in methylene chloride)
indicated a
quantitative conversion. The mixture was filtered and concentrated in vacuo to
afford
a glassy colorless solid (1.14 g, 93%).
Example 85
(L)- f 1-([1,4']Bipiperidinyl-1'-carbonyl)-3-oxo-3-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-propyl}-carbamic acid tert-butyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
187
~O
O' -NH O
O
'~~ N O
N
N~NH
N \
To a stirred solution of 2-tert-butoxycarbonylamino-4-oxo-4-(4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butyric acid (1.14 g, 2.55 mmol)
and 4-
piperidinyl-piperidine (525 mg, 2.81 mmol) in methylene chloride (20 mL) was
added 3-(diethoxyphosphoryloxy)-1,2,3-benzotriain-4(3F~-one (DEPBT, 840 mg,
2.81 mmol) in one portion followed by dropwise addition of triethylamine
(0.427
mL, 3.06 mmol). The resulted mixture was stirred at room temperature overnight
(15
h). The mixture was diluted with methylene chloride and washed with sodium
hydroxide (0.5 N) solution and water. The layers were separated and the
organic
layer was dried with sodium sulfate and concentrated in vacuo to give a light
yellow
foam. The crude product was purified by flash column chromatography (10% (1M
ammonia in methanol) in methylene chloride) to give a colorless foam (1.08 g,
71%).
'H-NMR (400 MHz, CDC13) S 8.86 - 8.55 (1H, br), 7.05 (1H, br), 6.93 (1H, br),
6.82
( 1 H, br), 6.72 ( 1 H, d, J = 7.6 Hz), 6.10 - 5.68 ( 1 H, br), 5.20 ( 1 H,
m), 54.70 - 4.40
(2H, br), 4.20 (2H, br), 4.01 - 3.82 (2H, br.), 3.10 - 2.88 (3H, br), 2.99
(3H, br), 2.53
(6H, br), 1.90 - 1.10 (23H, m). Mass spec.: 597 (MH)+.
(L)-2-Amino-1-[ 1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-
yl)-piperidin-1-yl]-butane-1,4-dione
NH2 O
O
'~~ N O
N N~NH
N \
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
188
To a stirred solution of {1-([1,4']bipiperidinyl-1'-carbonyl)-3-oxo-3-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-propyl}-carbamic acid tert-
butyl ester (1.05 g, 1.76 mmol) in methylene chloride (12 mL) was added
trifluroacetic acid (2 mL). The mixture was stirred at room temperature until
complete conversion (monitored by LCMS, ca. 15 h). The mixture was then
diluted
with water and sodium hydroxide (1.5 g) was slowly added with stirring. The
layers
were separated and the aqueous layer was extracted with methylene chloride.
The
combined organic layers were dried over sodium sulfate and concentrated in
vacuo to
give a light yellow foam (860 mg, 98%). Mass spec.: 497 (MH)+.
Example 86
(L)-1-[ 1,4'] B ip iperidinyl-1'-yl-2-( 1 H-indol-5-ylamino)-4-[4-(2-oxo-1,4-
dihydro-2 H-
quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
HN
NH O
O
~~~ N O
N
N~NH
/
N
To a solution of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (52 mg, 0.105 mmol) and N
tert-BOC-5-bromo-indole (prepared as described in Tetrahedron 2000, pp 8473-
8482) (31 mg, 0.105 mmol) in tetrahydrofuran (1 mL) in a 5 mL drum vial was
added
2-dicyclohexylphosphino-2'-(N, N dimethylamino)-biphenyl (4.1 mg, 0.0105
mmol),
Pd2(dba)3 (4.8 mg, 0.005 mmol), and cesium carbonate (54.6 mg, 0.168 mmol)
under
nitrogen. The vial was sealed with a teflon~-lined cap. The deep orange-
colored
reaction mixture was heated at 80°C with stirring. The reaction was
continued at
80°C overnight. Conversion reached approximately 50% after 17 h. The
solvent was
removed in vacuo and the residue dissolved in methylene chloride and filtered.
The
desired product was purified by prep arative TLC (10% methanol in methylene
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
189
chloride) to afford the tent-butyloxycarbonyl-protected product (11 mg, 15%).
Mass
spec.: 712 (MH)+. This intermediate (11 mg) was dissolved in 3 mL methylene
chloride and treated with trifluoroacetic acid (1.5 mL). The colorless
solution turned
to a tan color and was stirred at room temperature for 1.5 h. The mixture was
concentrated in vacuo and dried under high vacuum to a give tan powder (15 mg,
100%). Mass spec.: 612 (MH)+.
Example 87
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(5-chloro-2-nitro-phenylamino)-4-[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
NOy
CI \ NH O
O
~~~ N O
N
N~NH
N
U
To a stirred solution of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (33.7 mg, 0.068
mmol) and 4-chloro-1,2-dinitrobenzene (16.8 mg, 0.075 mmol) in ethanol (0.5
mL)
was added a saturated sodium bicarbonate solution (4 drops). The mixture was
stirred at room temperature for 70 h to approximately 60% conversion. The
product
was purified by preparative HPLC to give a yellow solid (17.7 mg, 40%). Mass
spec.: 652 (MH)+.
Example 88
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(6-chloro-pyrimidin-4-ylamino)-4-[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
190
N~N
CI ~ I NH O
O
~~~ N O
N
N~NH
N
A mixture of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (22.3 mg, 0.045 mmol) and 4,
6-
dichloropyrimidine (16 mg, 0.095 mmol) in 2-propanol (0.5 mL) in a
microwavable
S vial was heated at 130°C under microwave irradiation for 40 min.
LCIMS indicated
90% conversion. The solvent was removed in vacuo and the residue was
partitioned
between methylene chloride and 1 N sodium hydroxide solution. The organic
layer
was separated, dried over sodium sulfate, and concentrated in vacuo. The
residue was
purified by flash column chromatography (10 % (1N ammonia in methanol) in
methylene chloride) to afford a white solid (23 mg, 84%). 1H-NMR (400 MHz,
CDC13) 8 8.36 (1 H, d, J = 12.8 Hz), 8.04 - 7.81 (1H, 2s), 7.14 (1H, t, J =
7.6 Hz),
7.10 - 6.80 (2H, m), 6.74 (1H, t, J = 8.2 Hz), 6.52 - 6.42 (1H, m), 5.90 -
5.50 (1H,
br), 4.85 - 4.40 (3H, m), 4.40 - 4.05 (3H, m), 4.05 - 3.82 (1 H, m), 3.20 -
3.00 (2H,
m), 3.00 - 2.68 (2H, m), 2.68 - 2.30 (8H, m), 2.05 - 1.90 (2H, m), 1.90 - 0.70
(12H,
m). Mass spec.: 609 (MH)+.
Similarly prepared:
Example 89
(L)-1-[ 1,4'] B ipiperidinyl-1'-yl-2-(2-chloro-9H-purin-6-ylamino)-4-[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
191
HN
N
CI N NH O
O
~~~~ N O
N N~NH
N
LC/M5: tR = 1.10 min, 649 (MH)+.
Example 90
(L)-2-(4-Amino-6-methyl-5 -nitro-pyrimidin-2-ylamino)-1-[ 1,4']bipiperidinyl-
1'-yl-4-
[4-(2-oxo- I ,4-dihydro-2 H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
NH2
OZN ~ N
Me ~N~NH O
O
'~'~ N O
N N~NH
N
LC/MS: tR = 1.12 min, 649 (MH)+.
Example 91
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(4,5-diamino-6-methyl-pyrimidin-2-ylamino)-
4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
192
NHZ
H2N ~ N
Me \N- -NH O
O
'~~ N O
N N~NH
N
U
To a solution of 2-(4-amino-6-methyl-5-nitro-pyrimidin-2-ylamino)-1-
[ 1,4']b ipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-quinazo lin-3-yl)-
piperidin-1-yl] -
butane-1,4-dione in 2:1 methanol/ethyl acetate (6 mL) in a Parr bottle was
added 10%
palladized charcoal (60 mg). The mixture was shaken under a hydrogen
atmosphere
at 55 psi for 20 h. The mixture was filtered through celite and the filtrate
was
concentrated in vacuo to afford a colorless solid (41.2 mg, 49.2% for two
steps) .
LC/MS: tR = 0.86 min, 619 (MH)+.
Example 92
(L)-1-[1,4']Bipiperidinyl-1'-yl-2-(7-methyl-1 H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-
ylamino)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-
1,4-
dione
N=N
HN
~ ~N
Me \N~NH O
O
~~~ N O
N
N~NH
N
U
To a stirred solution of 1-[1,4']bipiperidinyl-1'-yl-2-(4,5-diamino-6-methyl-
pyrimidin-2-ylamino)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
yl]-
butane-1,4-dione (10.6 mg, 0.0125 mmol) in acetic acid (1.5 mL) was added
sodium
nitrite (24 mg) followed by a few drops of water. The resulting light yellow
solution
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
193
was stirred at room temperature for 6 h. The reaction mixture was diluted with
water
and methanol and purified by preparative HPLC to afford a colorless oil/solid
(3.0
mg, 28%) . LC/MS: tR = 1.07 min, 630 (MH)+.
General procedure for the synthesis of Examples 93-95:
A mixture of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (0.014 mmol), one of a
series of
aldehydes (0.07 mmol, 5 equiv) and solid anhydrous magnesium sulfate (0.031
mmol, 2.2 equiv) in 1,2-dichloroethane (3.0 mL) was treated with a catalytic
amount
of acetic acid and was shaken overnight. Sodium cyanoborohydride ( 0.07 mmol,
5
eq) was then added in one portion and the suspension was again shaken
overnight.
Purification was carried out either by filtration through an SCX cartridge or
by
preparative HPLC.
Example 93
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-((2'-pyridyl)-methyl-amino)-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
I~
N /
NH O
O
'~~ N O
N N- -NH
/
N \
LC/MS: tR = 0.87 min, 588 (MH)+.
Example 94
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-((5'-indazolyl)-methyl-amino)-4-[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
194
HN-N
NH O
O
~~~ N O
N
N NH
/
N
U
LC/MS: tR = 0.92 min, 626 (MH)+.
Example 95
(L)-1-[ 1,4'] B ipiperidinyl-1'-yl-2-((3'-methyl-phenyll)-methyl-amino)-4-[4-
(2-ox o-
1,4-dihydro-2 H-quinazolin-3-yl)-piperidin-1-yl] -butane-1,4-dione
/
NH O
O
~~~ N O
N
N NH
/
N
U
LC/MS: tR = 1.08 min, 600 (MH)+.
Example 96
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-2-(pyrimidin-4-ylamino)-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
195
N~N
-NH O
O
~~~ N O
N ~
N- 'NH
N
U
To a solution of 1-[1,4']bipiperidinyl-1'-yl-2-(6-chloro-pyrimidin-4-ylamino)-
4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
(21
mg) was dissolved in 4 mL ethyl acetate/methanol (1:1) in a Parr bottle was
added
10% palladized charcoal (10 mg). Hydrogenation was carried out on a Parr
apparatus
at 55 psi overnight. The degassed mixture was then filtered and concentrated
in
vacuo. The residue was purified by preparative HPLC to afford a yellow solid
(12.4
mg, 45%). Mass spec.: 575 (MH)+.
Example 97
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(4-hydroxy-cyclohexylamino)-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl ]-butane-1,4-dione
HO
~NH O
O
~~~ N O
N
N~NH
N
U
To a stirred mixture of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-(4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (47.9 mg, 0.096
mmol) and 4-hydroxy-cyclohexanone (Synthesis reported in Can. .~ Chem. 1994,
72,
1699 - 1704) (11 mg, 0.096 mmol) in methanol (1.0 mL) was added excess zinc
chloride followed by sodium cyanoborohydride (5 equiv). The suspension was
stirred at room temperature for 6 days. The methanol was removed in vacuo and
the
residue partitioned between methylene chloride and 1 N sodium hydroxide. The
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
196
aqueous layer was extracted with methylene chloride (3x). The combined
methylene
chloride solution was passed through a celite cartridge and concentrated in
vacuo.
The residue was purified by preparative TLC (10% (1N ammonia in methanol) in
methylene chloride) to afford the desired product as a white solid (15.3 mg,
27%).
Mass spec.: 595 (MH)+.
Example 98
(L)-1-[ 1,4']Bipiperidinyl-1'-yl-2-[( 1 H-imidazol-4-ylmethyl)-amino]-4-[4-(2-
oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
~NH
N
NH O
O
'~~ N O
N N~NH
N
To a stirred solution of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (20.6 mg, 0.0415
mmol) and 4-imidazlecarboxyaldehyde (4 mg, 0.0415 mmol) in methylene chloride
(1.0 mL) was added sodium cyanoborohydride (8.8 mg, 0.0415 mmol) in one
portion.
The suspension was stirred at room temperature for 2 days and then partitioned
between methylene chloride and 1N sodium hydroxide. The layers were separated
and the aqueous layer was extracted with methylene chloride. The combined
organic
layers were dried over sodium sulfate, and concentrated in vacuo. The residue
was
purified by preparative TLC (10% (1N ammonia in methanol) in methylene
chloride)
to afford the desired product as a colorless oil that solidified upon standing
(6.1 mg,
26%). IH-NMR (400 MHz, CDCl3) 8 7.61 (1H, d, J = 4.8 Hz), 7.16 (1H, t, J = 7.6
Hz), 7.10 - 6.85 (3H, m), 6.67 (1 H, d, J = 8.0 Hz), 4.85 - 4.63 (2H, m), 4.63
- 4.40
(1 H, m), 4.40 - 3.65 (7H, m), 3.25 - 2.40 (1 OH, m), 2.15 - 0.70 (18H, m).
Mass
spec.: 577 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
197
Example 99
(L)-N- { 1-([ 1,4'] Bipiperidinyl-1'-carbonyl)-3-oxo-3-[4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidin-1-yl]-propyl}-4-methoxy-benzamide
OCH3
O ~NH O
O~~N O
IN
N~NH
N
U
To a stirred mixture of 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione (91.5 mg, 0.184
mmol) andp-anisoyl chloride (34.6 mg, 0.203 mmol) in methylene chloride was
added two drops of triethylamine (35 ~,L). The light yellow solution was
stirred at
room temperature for 2.5 h to achieve complete conversion. The reaction
mixture
was washed with sodium hydroxide (1N) and the aqueous layer was then extracted
with methylene chloride. The combined organic layers were passed through a
celite
cartridge and concentrated in vacuo to give a glassy solid. The crude product
was
purified by flash column chromatography (10% (1N ammonia in methanol) in
methylene chloride) to give a glassy solid (92.8 mg, 80%). 'H-NMR (400 MHz,
CDCl3) 8 8.55 - 8.47 (1H, d), 8.10 - 7.78 (3H, m), 7.09 (1H, t, J = 7.4 Hz),
6.96 -
6.74 (4H, m), 5.62 - 5.44 ( 1 H, br), 4.75 - 4.40 (3H, m), 4.40 - 4.05 (3H,
m), 4.05 -
3.82 (1 H, br), 3.76 (3H, s), 3.18 - 2.88 (3H, m), 2.88 - 2.70 (1 H, m), 2.70 -
2.30
(8H, m), 2.05 - 1.19 (14H, m). Mass spec.: 631 (MH)+.
Example 100
(L)-N- { 1-([ 1,4']Bipiperidinyl-1'-carbonyl)-3-oxo-3-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-propyl } -4-hydroxy-benzamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
198
OH
O' ~NH O
O ~~~ N O
N
N~NH
/
N
A stirred solution of N { 1-([1,4']bipiperidinyl-1'-carbonyl)-3-oxo-3-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-propyl }-4-methoxy-
benzamide
solution in methylene chloride (69 mg) was treated with boron tribromide (1M
in
methylene chloride, 0.6 mL), dropwise at room temperature. The resulting
suspension was stirred at room temperature for 7 h and then the reaction was
quenched with excess triethylamine followed by methanol. The solvents were
removed in vacuo and the residue was dissolved in methanol and purified by
preparative HPLC. LC/MS: tR = 1.03 min, 617 (MH)~.
Example 101
(L)-1H-Pyrazole-3-carboxylic acid {1-([1,4']bipiperidinyl-1'-carbonyl)-3-oxo-3-
[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-propyl]-amide
/ NH
,N
O' ~NH O
O ~~~ N O
N
N~NH
/
N
U
To a stirred solution of pyrrazole-3-carboxylic acid (4 mg, 0.036 mmol) and
2-amino-1-[ 1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidin-1-y1J-butane-1,4-dione (13 mg, 0.026 mmol) in methylene chloride (1
mL)
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
199
was added 3-(diethoxyphosphoryloxy)-1,2,3-benzotriain-4(3H)-one (DEPBT, 8.6
mg, 0.036 mmol) in one portion followed by one drop of triethylamine. The
resulting
mixture was stirred at room temperature overnight (15 h). The mixture was then
partitioned between sodium hydroxide (0.5 N) and methylene chloride. The
layers
were separated and the aqueous layer was extracted with methylene chloride
(3x).
LCMS indicated that the product was remained in the aqueous layer. The product
was purified by preparative HPLC to give a yellow oil (17.2 mg, 94%). Mass
spec.:
591 (MH)+.
General procedure for the synthesis of Examples 102-134:
,R
X
O' -NH O
O=~~N O
N N~NH
N
U
The starting amine, 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione, was dispersed in
a 96-
well mini-reactor (ca. 10 mg each) in 1 mL dichloroethane. Individual acyl
chlorides
(ca. 2 equiv.) were added followed by a resin-bound solid-phase piperidine
base (4
equiv). The block was shaken overnight. Approximately 4 equivalents of tris-
amine
resin was added to each well and the mini-reactor was shaken for another S h.
The
reaction mixtures were filtered, and purified by either preparative HPLC or
filtration
through an SCX cartridge or both. HPLC retention times and mass spectral data
for
each example are listed in Table 2.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
200
Table 2. Amides and Carbamates
HPLC MS
Example Structure tR ~m~) ~M+)
Chiral
~N
\~N O
O
102 \H ~ ~ 1.84 637.38
O N O
HN N
Chiral
N /
O N
103 o H 1.39 565.45
/~N
/I HN
G 'IN
0
Chiral
N
104 N 1.89 641.46
O H O
N ~ O
N--( ,N
N
O H H"'
Chiral
F
O
105 HN O-' H 1.?3 619.42
~N
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
201
HPLC MS
Example Structure
tR (min) (M+)
Chiral
106 1.62 615.41
~p H O
H -
N O H
O
Chiral
N
~N O
F
H ;, F
107 ~H I ~ ~F 2.25 737.37
~N O
/ICI F F
~ F
N- 'O
H
Chiral
~N
\~N O
O
H,,
CI
108 \H ~ I 2.12 669.3
/~N O
HN N I~/I CI
Chiral
109 1.59 675.46
o ~ o _
H
H
O O O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
202
HPLC MS
Example Structure
tR (min) (M+)
/ Chiral
N' /NH
O N~ ?I IfO
110 ~ H 1.62 601.43
~N
/ICI HN O
N
Chiral
N
~N O
Ho.
CI
111 H ~ ~ 2.09 669.33
O ~N O ~ CI
HN_ 'N
O Chiral
/ \N N O O'1 N N
~H
NH
112 0 1.91 665.36
0
/ \
ci
Chiral
O
113 H o\\ 1.68 646.37
N N O O N / \
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
203
HPLC MS
Example Structure
tR (min) (M+)
Chiral
HN~N
1I N O
O ~ H O
N
114 N 1.66 645.4
O NH
~O
of
cnira~
N
H O
H N
115 N o 2.14 690.45
O HN~O
~N
/ \ ~ \
cnirai
N
0
116 o H"" N S 1.59 607.39
N o \ /
N
N~O
H
O a Chiral
O N
H
117 N~ 1.59 621.4
HN O
S
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
204
HPLC MS
Example S tructure
tR (min) (M+)
/ cnirai
N~NH
O N~ ?~~fO
I18 o H 2.01 735.43
~N /
HN 0 \
N
~O O
Chirai
HN~N
't~O~f ~N O
O
119 H N I.92 b79.32
O NH
N
Sr
Chiral
I20 N i.22 537.4
,.O H O
~J~JH
l \ ~N O L H
O
Chiral
F~ \ ~
F F O
I21 HN O~~ 2.03 685.4
0
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
205
HPLC MS
Example Structure
tR (min) (M+)
Chiral
N
~N O
H"
122 H ~ ( F 1.79 637.38
O ~N O
HN~N/I~I
Chiral
N
123 o H o 1.84 669.3
H
N ~ O
N~N ' H CI
O
CI
O\ 'N \ Chiral
v ~I'.
~N O O N /
O
N
124 ''~H o 1.53 636.35
~N O
GN
Chiral
~N O I
~N O
H~,,
H S
O
125 2.04 691.35
N
HN
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
206
HPLC MS
Example Structure tR (min) (M+)
Chiral
N
O
126 "~ ~ ° 1.89 657.35
N~N O
N
O H
CI / Chiral
N
O NH
127 °~~~~ H~N 1.86 649.39
0
N
N_ v
\ I ~o
cniral
N~NH
O N~ 1~ ~fO
O H
128 N 1.67 691.42
HN O
N
~O ~
/O
Chiral
CI \
O
129 HN O~H 1.84 635.38
N
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
207
HPLC MS
Example Structure
tR (min) (M+)
Chiral
130 N 1.69 617.42
\O H O
H
O
~N O H
O
Chiral
~ N
O"NH
n "'' N
131 ' 1.74 635.38
0
N
N_ v
I NCO
H
Chiral
132 N 1.84 631.44
~O H O
N
O
N O H O
Br Chiral
~I
O
~ N
O"NH
N
133 '' 1.94 695.28
0
N
N_ v
w I ~o
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
208
HPLC MS
Example Structure
tR (min) (M+)
Chiral
134 1.7 647.41
o ,~ o _
0
r v ~ ~'
0 0
General procedure for the synthesis of Examples 135-200:
R
HN
O"NH O
0,~~ N O
N
N~NH
N
U
The starting amine, 2-amino-1-[1,4']bipiperidinyl-1'-yl-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione, was dispersed in
a 96-
well mini-reactor (ca. 10 mg in each well) in dichloroethane (1 mL).
Individual
isocyanates (ca. 2 equiv) were added to individual wells. The block was shaken
for 2
days. Approximately 4 equivalent of tris-amine resin was added to each well
and the
mini-reactor was shaken for another two days. The reaction mixtures were
filtered,
and individual product was purified by either preparative HPLC or filtration
through
an SCX cartridge or both. HPLC retention times and mass spectral data for each
example are listed in Table 3.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
209
Table 3. Ureas
HPL
MS
Example Structure C tR
(MH)+
(min)
Chiral
N
135 N 1.43 665.84
O H O
N-
~O
O H
N
H
Chiral
N
N
O H O
136 N o 1.56 707.88
N~N O H
H
O
Chiral
IV
137 a ,,o H o 1.39 665.84
0
N~N O H \
N
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
210
HPL
MS
Example Structure C tR
(mm) ~MH~+
Chiral
O O
H \ N\~ N
N ii~~"
H H
138 0 1.3 643.83
N
N
O
H
Chiral
N
139 N
H .p H O 1.44 657.86
0
N~ . H \
O
H
CI / Chiral
NH
O "NH N
140 ~~~'''~~ N
H ~ 1.42 650.22
0
~N O
/ I ~N
H- 'O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
211
HPL
MS
Example Structure C tR
(min) (MH)+
Chiral
N
,
N
141 o H o 1.26 629.81
V
H~ O
/ ~ --~~ H \
Chiral
N
142 1.41 643.83
~O H O
N
O
N_-~~ H \
O N
H
Chiral
N
143 1.24 615.78
\O H O
v
O
N~N O H
N
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
212
HPL
MS
Example Structure C tR
~MH)+
(min)
Chiral
N
144 N 1.53 691.88
H O
N
O
N~N O I H
N
H
Chiral
HN"N O\ /NH
145 I~~I N ~IN'H 1.21 629.81
01 I 'H
O N
N.
Chiral
N
N
146 H //c H c 1.52 707.88
N~ O
N -~
O ', H \
H~O
Chiral
147 N 1.19 657.82
O H O O
H
N O
N~N O H
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
213
HPL
MS
Example Structure C tR +
(MH)
(min)
Chirai
~N
~~N O
H ~O
1.44 684.67
14g HN ~~N O
HN O N NH
ci
ci
Chiral
N
N
149 H ,,O H O 1.3 645.81
N~j~/, O
~N O H
H
O
Chiral
N
1 ~a N 1.24 645.81
H ,.O H O
N O O
N~N
N
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
214
HPL
MS
Example Structure C tR
(MH)+
(min)
Chiral
N
IV
151 o H o 1.33 643.83
H //
v
/~ O
N--( ,N O H
H
Ci Chiral
F
F
F
NH
152 0 1.56 718.22
H
ii~~~.
N--~ N '--J N " HO N~N
H
O
F F Chiral
F
HN~N 01 /NH
153 Io' N~.,,,,, ~IN'H 1.55 683.78
O H
O N,
,N~
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
215
HPL
MS
Example Structure C tR
(MH)+
(min)
Chiral
N
N O
O
H
154 ~ N~ ~ 1.37 655.84
O NH N NH
H ,.,.
H
Chiral
N
155 H ° n ° 1.27 675.83
N O
N~N ' H \
° H ~ ~ O
O
O,' Chiral
N
HN
156 ° 1.26 651.76
HN
F
F
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
216
HPL
MS
Example Structure C tR
(mm) ~MH~+
Chiral
N
157 o H o 1.39 643.83
H //
N~ O
N~N O H
H
Chiral
N
158 N 1.43 643.83
~O H O
H
~O
N~N
// O H N
H
CI Chiral
CI
NH
159 0 1.57 684.67
NH
N N--~ H~ N
N-~ O O
H~O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
217
HPL
MS
Example Structure C tR
(mm) ~MH~+
F F Chiral
F ~~
NH
~ N
O' -NH
160 ",,.~~~ N 1.46 683.78
H
O O
~N
~N
N' 'O
H
O,' Chiral
N OH~~n N
HN
161 0 1.48 684.67
HN
CI
CI
Chiral
162 1.5 657.86
~O H O
H
O
'._/N , H
O N
H
O,, Chiral
N /~
HN
163 0 1.14 651.76
HN F
F
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
218
HPL
MS
Example Structure C tR
~MH~+
CI Chiral
CI
164 0 1.34 685.66
NH
N N~ H~ N
N~ O O
H \\
O
Chiral
N
165 o H o 1.26 675.83
H
N O O
N
O H
H
O-
Chiral
O
N N HO N
H \\
O NH
166 O 1.28 701.87
H H
O O
Chiral
i
HN~N O\ /NH
167 ~o~~ N~.~,", ~?IN'H 1.52 718.22
10t H
O N'
N, l
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
219
HPL
MS
Example Structure C tR
(MH)+
(min)
F Chiral
/ F
-F
HN"N O\ /NH
168 Imo \~N ",,, ~?IN'H 1.35 669.75
~H~
O N
~~//\\N~
Chiral
N
i
N'
169 O N~N~NH 1.24 649.86
O~NH H ~' O
NH
S
Chiral
N N HO
H \\
O H
170 °~H 1.11 639.8
0
0
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
220
HPL
MS
Example Structure C tR +
(MH)
(min)
O~ Chira!
N
171 H ~0 1.31 633.77
H
F
O', H Chiral
N O nii N
172 H o 1.34 650.22
H
CI
O\~~ Chiral
° o
N
173 H o 1.47 684.67
iiN
CI ~ \ CI
Chiral
174 o H ° 1.27 675.83
0 0-
N 1_.JN I H \
O H
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
221
HPL
MS
Example Structure C tR
~MH~+
Chiral
N
N
O
175 N~O H o 1.34 659.83
N~N O H \
H
O
/ Br Chiral
HN"N O~NH
176 1I° ~N "~~ ~T~N'H 1.41 694.68
~H~
O N
N
\ / F Chiral
\~
HN~IV O\ /NH
1~~O~f \~N ~.,~, ~IN'H
177 ~ 1.28 633.77
O H
O N
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
222
1-IPL
MS
Example Structure C tR
(MH)+
(min)
Ci Chirai
HN"N O~NH
178 Imo ~N .,,,~t ~?~N'H 1.39 650.22
~H~
O N
N
Br / Chiral
N
O NH
179 "''~~ 1.42 694.68
0 0
/~N
N O
H
F / Chiral
N
O NH
180 "''~~ 1.26 633.77
0 0
//~N
N O
H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
223
HPL
MS
Example Structure C tR
(m~) ~MH~+
Chiral
N
181 N 1.19 645.81
o ~ o
,,o
O H N
H
Chiral
N
N
182 H o H o 1.34 687.84
0
\ N~N H~ O
O N
H O
O~N Chiral
~?~' \
N
O N
H H
183 /\iN~N 1.08 581.76
~~~'H
O
O N
~N~
Chiral
0 0 ~b
N
184 H o 1.31 651.76
HN
F ~ ~ F
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
224
HPL
MS
Example Structure C tR
(mm) ~MH~+
Chiral
N
N
185 o H o 1.39 643.83
0
/ \ o I~
H \ /
Chiral
CI / \
186 0 1.33 664.25
NH
\ j m".
N N--~ H~N N
O O
O
CI ~ Chiral
~ of
HN~N O\ 'NH
18 7 1~O N '% ~IN'H
1.41 680.25
O H
O N
~N~
O H Chiral
O O ~---N
N H"n N
HN
188 ~0 1.48 718.22
HN
F
F
F
CI
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
225
HPL
MS
Example Structure C tR
(MH)+
(min)
Chiral
N
189 N 1.28 659.83
o ~_ o
H O
V
N~N O H
H
Chiral
190 1.41 643.83
o ~ o
H //
v
O
N ~/
O H
H
CI / Chiral
\
N
O NH
N
191 °''~' H 1.41 664.25
0 0
IN
\
N O
H
/ CI Chiral
/ \
HN"N O\ /NH
1IO \~N ~~.., ~IN'H
192 ~ 1.41 664.25
O H
O N
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
226
HPL
MS
Example Structure C tR
(mm) ~MH~+
CI Chiral
F /
~I
N
O NH
193 ,~,,.,~- N~ 1.41 668.21
0 0
/~N
/ I ,N
N- 'O
H
Br / Chiral
'N
O N Ir~H
194 ° H N 1.45 78.71
O O
~N
/~~
N O
H
F ~ Chiral
/
HN' /N O\ /NH
195 ICI N~"°., ~IN'H 1.39 647.8
O H
O N
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
227
HPL
MS
Example Structure C tR
(MH)+
(min)
Chiral
196 1.27 673.82
n ° o
0
' H \
O N
H
Chiral
N
N
197 o H o
/ 0 1.45 691.88
~N O H
H
O\ 'N Chiral
N /
O N
O
198 I 1N HN H p 1.26 643.83
GN
H~H
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
228
HPL
MS
Example Structure C tR
(mm) (MH)+
cnira~
N
H
O\'N \
N NN
199 ~ N 1.45 693.89
O~IVH O
~1~N'H
/ \
\ /
O H Chiral
O O ~N
HN
200 ~0 1.4 699.78
H
F °
F 'F
2-(1H-Indazol-5-ylamino)-succinic acid 4-tert-butyl ester 1-ethyl ester
N_
HN
NH O
O
OEt
To a solution/suspension of 5-aminoindazole (1.01 g, 7.6 mmol) in
tetrahydrofuran (20 mL) was added ethyl glyoxlate solution (ca. 50% in
toluene, 1.7
mL, 1.1 equiv) in one portion followed by magnesium sulfate (4.6 g). The
mixture
was stirred at room temperature overnight (23 h) and then filtered and
concentrated in
vacuo. The resulting crude imine intermediate (1.3 g, 6 mmol) was dried by
azeotroping with anhydrous benzene and further dried under high vacuum. The
residue was then dissolved in tetrahydrofuran (20 mL) and cooled at
0°C. A solution
of 2-tert-butoxy-2-oxoethylzinc chloride (0.5 M in ether, 24 mL, 2 equiv) was
then
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
229
slowly added. After stirring at 0°C for 1 h, the mixture was stored at
4°C overnight.
The mixture was then diluted with ethyl acetate and quenched with half
saturated
ammonium chloride solution along with a minimum amount of 0.5 N HCl to
dissolve
the precipitated solids. The layers were separated and the aqueous layer was
extracted with ethyl acetate. The combined organic layers were washed with
water
and saturated sodium bicarbonate solution. The.organic layer was dried over
sodium
sulfate and concentrated in vacuo. The crude product was purified by flash
column
chromatography on silica gel, eluting with 10% methanol in methylene chloride,
to
afford the desired product (1.3 g, 65%) as a tan oil. 'H-NMR (400 MHz, CDCl3)
b
7.89 (1H, s), 7.40 - 7.27 (1H, m), 6.98 - 6.77 (2H, m), 4.42 - 4.35 (1H, m),
4.30 -
4.12 (3H, m), 2.80 (2H, d, J = 4.4 Hz), 1.43 (9H, s), 1.27 - 1.17 (4H, m).
Mass spec.:
356.24 (M+Na)+, 278.23 (M - 'Bu)+, tR = 1.287 min.
2-(1H-Indazol-5-ylamino)-succinic acid 1-ethyl ester
N_
HN
NH O
O ~ ~
~~~OH
OEt
A stirred solution of 2-(1H-indazol-5-ylamino)-succinic acid 4-tert-butyl
ester
1-ethyl ester (123.6 mg, 0.37 mmol) in methylene chloride (2 mL) and
trifluoroacetic
acid (0.5 mL) was stirred at room temperature overnight. The reaction mixture
was
then diluted with ethyl acetate and washed with saturated ammonium chloride
solution, water and brine. The organic layer was dried and concentrated to
give a
dark green oil: LC/MS: tR = 0.643 min, 278.19 (MH)+.
2-(1 H-Indazol-5-ylamino)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butyric acid ethyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
230
N_
HN
NH O
O
~~~ N O
OEt
N~NH
To a stirred solution of 2-(1 H-indazol-5-ylamino)-succinic acid 1-ethyl ester
(84 mg, 0.215 mmol) in methylene chloride (1mL) was added the amine (99 mg,
0.429 mmol, 2 equiv) followed by DEPBT (128 mg, 0.43 mmol, 2 equiv.) and
triethylamine (70 p,L, 0.47 mmol, 2.2 equiv). The mixture was stirred
overnight and
then diluted with ethyl acetate and washed with half saturated ammonium
chloride
solution, water and brine. The organic layer was dried and concentrated to a
tan oil.
The crude product was purified by flash column chromatography on silica gel,
eluting with 10% methanol in methylene chloride, to give the desired product
(36.2
mg, 34.5% for two steps) as a reddish oil. 1H-NMR (400 MHz, CDCl3) 8 7.90 (2H,
d, J = 4.4 Hz), 7.33 (1H, d, J = 8.4 Hz), 7.20 - 7.14 (1H, m), 7.00 - 6.80
(4H, m),
6.70 (1 H, t, J = 6.8 Hz), 4.58 - 4.48 (1 H, m), 4.65 - 4.40 (2H, m), 4.34 -
4.05 (3H,
m), 4.02 - 3.82 (1 H, m), 3.20 - 2.99 (2H, m), 2.99 - 2.84 (1 H, m), 2.70 -
2.52 (1 H,
m),1.80- 1.50 (5H, m), 1.35 - 1.12 (5H, m). LC/MS: tR = 1.130 min, 491.37
(MH)+.
2-( 1 H-Indazol-5-ylamino)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butyric acid
N_
HN
\ NH O
O
~~~ N O
OH
N~NH
To a solution of the ethyl ester (34 mg, 0.069 mmol) in tetrahydrofuran (0.3
mL) was added lithium hydroxide in water(1M, 280 ~L, 4 equiv) and the mixture
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
231
was stirred at room temperature for 17 h. The solution was dried under a
stream of
nitrogen. To the residue was added 0.2 mL tettrahydrofuran and 0.2 mL
anhydrous
benzene and the suspension was blown dry again with a stream of nitrogen.
LC/MS:
tR = 0.900 min, 463.30 (MH)+.
Example 201
(~)-1-[ 1,4']Bipiperidinyl-1'-yl-2-( 1 H-indazol-5-ylamino)-4-[4-(2-oxo-1,4-
dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
N_
HN
/
NH O
O
~~~ N O
N
N~NH
/
N
U
To a solution of 2-(1H-indazol-5-ylamino)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyric acid ethyl ester (0.069 mmol) in
dimethylformamide (0.5 mL) in a capped drum vial was added
piperidinylpiperidine
(14.3 mg, 0.076 mmol, 1.1 equiv), DEPBT (22.8 mg, 1.1 equiv) and triethylamine
(8
drops, ca. 160 ~L). The mixture was stirred at room temperature overnight. The
final product was purified by preparative HPLC to afford the desired product
(15 mg,
26% for two steps) as a tan solid. LC/MS: tR = 0.917 min, 613.54 (MH)+.
Additional Examples
(1-Benzyl-piperidin-4-yl)-(2-nitro-benzyl)-amine
NOZ ~ N
N /
H
2-Nitrobenzaldehyde (1 g, 6.61 mmol) and 4-amino-1-benzylpiperidine (1.35
mL, 6.61 mmol) were combined in ethanol (20 mL). The resulting suspension was
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
232
stirred at room temperature for 20 min before a solution of sodium borohydride
(0.25
g, 6.61 mmol) in ethanol (5 mL) was added dropwise over 10 min. After the
addition
was complete, the reaction was stirred for 1 h, cooled to 0°C and
concentrated
ammonium chloride was added to the reaction mixture until no bubbling was
observed. The solvents were evaporated in vacuo and the resultant crude
mixture
was dissolved in water (10 mL) and methylene chloride (10 mL). The layers were
separated and the organic layer washed with water (2x) and brine (2x), dried
over
sodium sulfate, filtered, and concentrated to afford 1.5 g (70%) of the
desired
product. LC/MS: tR = 0.7 min, 326.18 (MH)+.
(2-Amino-benzyl)-(1-benzyl-piperidin-4-yl)-amine
NHZ ~ N
N /
H
( 1-Benzyl-piperidin-4-yl)-(2-nitro-benzyl)-amine ( 1.2 g, 3.7 mmol) and zinc
dust (1 g, excess) were combined in 75% aqueous acetic acid (16 mL) and
stirred at
60°C for 2 h. After cooling to room temperature, the solvents were
removed in vacuo
and the resultant crude dissolved in water (10 mL), followed by addition of
ammonium hydroxide until pH 3 was attained. The solution was extracted with
methylene chloride (3x). The organic layers were pooled together washed with
water
(2x), brine (2x), dried over sodium sulfate, filtered, and concentrated to
afford 0.8 g
(73%) of the desired product. 1H-NMR (CD30D) 8 2.50 (m, 2H), 3.20 (m, 2H),
3.49
(dd, J=7.0, 7.3, 1H), 3.62 (m, 4H), 4.20 (s, 2H), 4.36 (s, 2H), 7.04 (m, 2H),
7.32 (dd,
J=7.3, 7.6, 1H), 7.41 (d, J=7.9, 1H), 7.50 (m, SH). Mass spec.: 296.40 (MH)+.
3-( 1-Benzyl-piperidin-4-yl)-3,4-dihydro-1 H-benzo[ 1,2,6]thiadiazine-2,2-
dioxide
~N
O~ ~O
HN~S~N /
I~
/
A solution of (2-amino-benzyl)-(1-benzyl-piperidin-4-yl)-amine (1.0 g, 3.39
mmol) and sulfamide (0.64 g, 6.78 mmol) in pyridine was heated at reflux for
14 h.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
233
After cooling to room temperature, the solvent was evaporated and the crude
product
dissolved in water. After being adjusted to pH 9 with 6N sodium hydroxide, the
resulting mixture was extracted with methylene chloride (2x). The extracts
were
washed with water (2x), dried over sodium sulfate, filtered, and concentrated
to
afford an oily residue which was dissolved in ethyl acetate (4 mL). This
solution was
mixed with 4N HCl in 1,4-dioxane (2 mL) followed by addition of diethyl ether
until
precipitation of product occurred. The desired product was obtained by
filtration to
afford 0.7 g (53%). LC/MS: tR = 0.96 min, 358.16 (MH)+.
3-Piperidin-4-yl-3,4-dihydro-1H-benzo[1,2,6]thiadiazine-2,2-dioxide
NH
O~ ~O
HN~S~N
3-( 1-Benzyl-piperidin-4-yl)-3,4-dihydro-1 H-benzo[ 1,2,6]thiadiazine-2,2-
dioxide (0.46 g, 1.29 mmol) in methanol (10 mL) was flushed with nitrogen, and
treated with palladium on charcoal (10%, 46 mg). The flask was flushed with
hydrogen and allowed to stir under an atmosphere of hydrogen overnight. The
reaction was flushed with nitrogen, filtered through celite, and concentrated.
Column
chromatography gave 0.26 g (75%) of the desired material. 1H-NMR (CD30D) 8
1.53-1.61 (m, 2H), 1.80 (m, 2H), 2.55 (m, 2H), 2.95-3.05 (m, 2H), 3.30 (m,
2H),
3.70 (m, 2H), 4.65 (s, 2H), 6.70 (d, J=7.9, 1 H), 7.40 (dd, J=8.2, 6.7, 1 H),
7.10 (m,
2H). Mass spec.: 268.10 (MH)+.
6-Bromo-3-piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one
H
N' /O
NN
Br
NH
3-Piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one (0.2 g, 0.87 mmol) was
dissolved in acetic acid (2 mL). To this solution was added a solution of
bromine
(1.8 mL, 35.14 mmol) in acetic acid (0.5 mL) dropwise over 5 min. After
stirring for
at room temperature for 1 h, the reaction mixture was diluted with methylene
chloride, washed with water (2x), brine (2x), dried over sodium sulfate,
filtered, and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
234
concentrated to afford 0.16 g (59%) which was used immediately without further
purification. LC/MS: tR = 0.91 min, 310.15 (MH)+.
2-Oxo-3-piperidin-4-yl-1,2, 3,4-tetrahydro-quinazoline-6-carbonitrile
H N
CN
O/~ N I /
H
6-Bromo-3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one (0.16 g, 0.52 mmol),
zinc cyanide (37 mg, 0.31 mmol) and tetrakis(triphenylphosphine)palladium(0)
(60
mg, 0.05 mmol) were combined in dimethylformamide (4 mL). The reaction flask
was connected to high vacuum and degassed (3x) by a freeze-thawing method,
before
being heated at 90°C with stirring under nitrogen for 1 h. After
cooling to room
temperature, the solution was evaporated in vacuo and the crude mixture
purified by
preparative HPLC to afford 50 mg (38%) of the desired nitrite. 'H-NMR (CD30D)
b
1.99 (m, 2H), 2.08-2.23 (m, 2H), 3.15 (m, 2H), 3.50 (bs, 1 H), 3.55 (bs, 1 H),
4.40 (m,
1H), 4.47 (s, 2H), 6.93 (d, J 8.1, 1H), 4.10 (m, 2H). Mass spec.: 257.13
(MH)+.
N (1-Benzyl-piperidin-4-yl)-2-(2-nitro-phenyl)-acetamide
N02
H
N /
o N ~I
(2-Nitro-phenyl)-acetic acid (2.0 g, 11.04 mmol), 4-amino-1-benzylpiperidine
(2.25 mL, 10.03 mmol), 1-hydroxybenzotriazole (1.49 g, 11.04 mmol) and 1-(3-
dimethylaminopropyl)-3-ethyl carbodiimide (2.3 g, 12.03 mmol) were combined in
ethyl acetate (25 mL). To this solution was added triethylamine (4.2 mL. 30.1
mmol)
and the reaction mixture stirred at 40°C for 2 h. After cooling to room
temperature,
the mixture was diluted with ethyl acetate and washed with water (2x), S%
sodium
bicarbonate, brine (2x), dried over sodium sulfate, and concentrated to afford
3.5 g
(98%) of the desired product. LC/MS: tR = 1.24 min, 354.30 (MH)+.
[2-(2-Amino-phenyl)-ethyl]-( 1-benzyl-piperidin-4-yl)-amine
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
235
NH2
H
N
N
Into a flame dried flask, N (1-benzyl-piperidin-4-yl)-2-(2-nitro-phenyl)-
acetamide (3.2 g, 9.06 mmol) and lithium aluminium hydride (1.0 g, 18.12 mmol)
were combined. 1,4-Dioxane (15 mL) was added and the mixture slowly brought to
reflux over 1 h and stirred at reflux for 16 h. The reaction mixture was
cooled to 0°C
and excess lithium aluminium hydride destroyed by dropwise addition of
methanol,
followed by careful addition of 20% potassium hydroxide. The aluminum salts
were
filtered, the filtrate concentrated and used as is for the next reaction.
3-(1-Benzyl-piperidin-4-yl)1,3,4,5-tetrahydro-benzo[d][1,3]diazepin-2-one
H , _O
~(\/N
N,
N
A stirred solution of [2-(2-amino-phenyl)-ethyl]-(1-benzyl-piperidin-4-yl)-
amine (0.44 g, 1.42 mmol) in tetrahydrofuran (5 mL) at 0°C was treated
with
carbonyl diimidazole (0.23 g, 1.42 mmol). The reaction was stirred for 30 min
at 0°C
and at reflux for 1 h. After cooling to room temperature, the solvent was
evaporated
and the residue purified by column to afford 100 mg (21 %) of the desired
product.
LC/MS: tR = 1.29 min, 336.34 (MH)+.
3-Piperidin-4-yl-1,3,4,5-tetrahydro-benzo[d][1,3]diazepin-2-one
H
-l~(N
N~
.NH
3-(1-Benzyl-piperidin-4-yl)1,3,4,5-tetrahydro-benzo[d][1,3]diazepin-2-one (100
mg,
0.3 mmol) in methanol (5 mL) was flushed with nitrogen, and treated with
palladium
on charcoal (10%, 10 mg). The flask was flushed with hydrogen and allowed to
stir
under an atmosphere of hydrogen overnight. The reaction was flushed with
nitrogen,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
236
filtered through celite, and concentrated. Column chromatography gave 50 mg
(68%) of the desired material. LC/MS: tR = 1.07 min, 246.26 (MH)+.
3-[(1-Benzyl-piperidin-4-yl-amino)-methyl]-4-nitro-phenol
N02 ~ N
N ~ i
H
OH
5-Hydroxy-2-nitro-benzaldehyde (5 g, 29.9 mmol) and 4-amino-1-
benzylpiperidine (5.6 mL, 29.9 mmol) were combined in ethanol (30 mL). The
resulting suspension was stirred at room temperature for 20 min before a
solution of
sodium borohydride (1.13 g, 29.9 mmol) in ethanol (10 mL) was added dropwise
over 10 min. After the addition was complete, the reaction was stirred at room
temperature for 1 h, cooled to 0°C and concentrated ammonium chloride
added to the
reaction mixture until no bubbling was observed. The solvents were evaporated
in
vacuo and the resultant crude mixture was dissolved in water (30 mL) and
methylene
chloride (40 mL). The layers were separated and the organic layer washed with
water (2x), brine (2x), dried over sodium sulfate, filtered, and concentrated
to afford
5.8 g (57%) of the desired product. LC/MS: tR = 0.95 min, 342.27 (MH)+.
4-Amino-3-[( 1-benzyl-piperidin-4-yl-amino)-methyl]-phenol
NHZ N
i
OH
(1-Benzyl-piperidin-4-yl)-(2-nitro-benzyl)-amine (0.25 g, 0.7 mmol) and zinc
dust (0.2 g, excess) were combined in 75% aqueous acetic acid (8 mL) and
stirred at
60°C for 2 h. After cooling to room temperature, the solvents were
removed in vacuo
and the resultant crude mixture dissolved in water (10 mL), followed by
addition of
ammonium hydroxide until pH 3 was attained. The solution was extracted with
methylene chloride (3x). The organic layers were pooled together, washed with
water (2x), brine (2x), dried over sodium sulfate, filtered, and concentrated
to afford
0.18 g (79%) of the desired product.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
237
3-( 1-Benzyl-piperidin-4-yl)-6-hydroxy-3,4-dihydro-1 H-quinazolin-2-one
H
\ N' /O
/ NN
HO
N
/
A stirred solution of 4-amino-3-[(1-benzyl-piperidin-4-yl-amino)-methyl]-
phenol (0.16 g, 0.51 mmol) in tetrahydrofuran (3 mL) at 0°C was treated
with
carbonyl diimidazole (52 mg, 0.51 mmol). The reaction was stirred for 30 min
at
0°C and at reflex for 1 h. After cooling to room temperature, the
solvent was
evaporated and the residue purified by column to afford 100 mg (57%) of the
desired
product. LC/MS: tR = 1.09 min, 338.28 (MH)+.
6-Hydroxy-3 -piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one
H
\ N\ /O
NN
HO
NH
3-(1-Benzyl-piperidin-4-yl)-6-hydroxy-3,4-dihydro-1H-quinazolin-2-one (100 mg,
0.3 mmol) in methanol (5 mL) was flushed with nitrogen, and treated with
palladium
on charcoal (10%, 10 mg). The flask was flushed with hydrogen and allowed to
stir
under an atmosphere of hydrogen overnight. The reaction was flushed with
nitrogen,
filtered through celite, and concentrated. Column chromatography gave 60 mg
(81 %) of the desired material. LC/MS: tR = 0.75 min, 248.22 (MH)+.
N (1-Benzyl-piperidin-4-yl)-2-methoxy-6-nitro-benzamide
NOZ O -N
~N i
H
/ O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
238
2-Methoxy-6-nitro-benzoic acid (2.0 g, 10.1 mmol), 4-amino-1-
benzylpiperidine (1.9 mL, 10.1 mmol), 1-hydroxybenzotriazole (1.43 g, 10.5
mmol)
and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide (1.9 g, 10.1 mmol) were
combined in ethyl acetate (25 mL). To this solution was added triethylamine
(4.2
mL. 30.3 mmol) and the reaction mixture stirred at 40°C for 2 h. After
cooling to
room temperature, the mixture was diluted with ethyl acetate and washed with
water
(2x), 5% sodium bicarbonate, brine (2x), dried over sodium sulfate, and
concentrated
to afford 3.2 g (86%) of the desired product. LC/MS: tR = 1.10 min, 370.28
(MH)+.
(2-Amino-6-methoxy-benzyl)-(1-benzyl-piperidin-4-yl)-amine
NHZ ~ N
~N i
H
O
Into a flame dried flask, N (1-benzyl-piperidin-4-yl)-2-methoxy-6-nitro-
benzamide (1.0 g, 2.8 mmol) and lithium aluminium hydride (0.31 g, 8.45 mmol)
were combined. To the mixture was added anhydrous 1,4-dioxane (15 mL). The
mixture was slowly brought to reflux over 1 h and stirred at reflux for 16 h.
The
reaction mixture was cooled to 0°C and excess lithium aluminium hydride
destroyed
by dropwise addition of methanol, followed by careful addition of 20%
potassium
hydroxide. The aluminum salts were filtered, the filtrate concentrated and
used as is
for the next reaction.
3-(1-Benzyl-piperidin-4-yl)-8-methoxy-3,4-dihydro-1 H-quinazolin-2-one
0
H
\ N' /O
/ NN
N
/
A stirred solution of (2-amino-6-methoxy-benzyl)-(1-benzyl-piperidin-4-yl)-
amine (0.2 g, 0.62 mmol) in tetrahydrofuran (3 mL) at 0°C was treated
with carbonyl
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
239
diimidazole (99 mg, 0.62 mmol). The reaction was stirred for 30 min at
0°C and at
reflex for 1 h. After cooling to room temperature, the solvent was evaporated
and the
residue purified by column to afford 150 mg (68%) of the desired product.
LC/MS:
tR = 1.41 min, 352.30 (MH)+.
8-Methoxy-3-piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one
0
H
N' /O
/ NN
NH
3-(1-Benzyl-piperidin-4-yl)-8-methoxy-3,4-dihydro-1H-quinazolin-2-one (100 mg,
0.28 mmol) in methanol (5 mL) was flushed with nitrogen, and treated with
palladium on charcoal (10%, 10 mg). The flask was flushed with hydrogen and
allowed to stir under an atmosphere of hydrogen overnight. The reaction was
flushed
with nitrogen, filtered through celite, and concentrated. Column
chromatography
gave 68 mg (93%) of the desired material. LC/MS: tR = 1.11 min, 262.23 (MH)+.
N (1-Benzyl-piperidin-4-yl)-2-chloro-6-nitro-benzamide
NOZ O ~ N
N /
H
/ CI
2-Chloro-6-nitro-benzoic acid (1.2 g, 5.97 mmol), 4-amino-1-
benzylpiperidine (1.l mL, 5.97 mmol), 1-hydroxybenzotriazole (0.84 g, 1.05
equiv)
and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide (1.1 g, 1.05 equiv) were
combined in ethyl acetate (20 mL). To this solution was added triethylamine
(2.5
mL. 3.0 equiv) and the reaction mixture stirred at 40°C for 2 h. After
cooling to
room temperature, the mixture was diluted with ethyl acetate and washed with
water
(2x), 5% sodium bicarbonate, brine (2x), dried over sodium sulfate, and
concentrated
to afford 1.9 g (85%) of the desired product.
(2-Amino-6-chloro-benzyl)-(1-benzyl-piperidin-4-yl)-amine
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
240
NHZ ~ N
\ N /
H
CI
Into a flame dried flask, N (1-benzyl-piperidin-4-yl)-2-chloro-6-nitro-
benzamide ( 1.67 g, 4.47 mmol) and lithium aluminium hydride (0.51 g, 13.43
mmol)
were combined. To this was added anhydrous 1,4-dioxane ( 15 mL). The mixture
was slowly brought to reflux and stirred for 16 h. The reaction mixture was
cooled to
0°C and excess lithium aluminium hydride destroyed by dropwise addition
of
methanol, followed by careful addition of 20% potassium hydroxide. The
aluminum
salts were filtered, the filtrate concentrated and used as is for the next
reaction.
3-(1-Benzyl-piperidin-4-yl)-8-chloro-3,4-dihydro-1H-quinazolin-2-one
CI
H
\ N' /O
I / NN
N
/
A stirred solution of (2-amino-6-chloro-benzyl)-(1-benzyl-piperidin-4-yl)-
amine (0.66 g, 2.0 mmol) in tetrahydrofuran (8 mL) at 0°C was treated
with carbonyl
diimidazole (0.36 g, 2.05 mmol). The reaction was stirred for 30 min at
0°C and at
reflex for 1 h. After cooling to room temperature, the solvent was evaporated
and the
residue purified by column to afford 0.58 g (82%) of the desired product.
LC/MS: tR
= 1.40 min, 356.25 (MH)+.
2-Chloro-3-piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one
c1
H
\ N' /O
I / NN
2o NH
3-(1-Benzyl-piperidin-4-yl)-8-chloro-3,4-dihydro-1H-quinazolin-2-one (0.17 g,
0.48
mmol) in methanol (10 mL) was flushed with nitrogen, and treated with
palladium on
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
241
charcoal (10%, 17 mg). Trifluoroacetic acid (0.2 mL) was added and the mixture
flushed with nitrogen then allowed to stir under an atmosphere of hydrogen
overnight. The reaction was flushed with nitrogen, filtered through celite,
and
concentrated. Column chromatography gave 100 mg (79%) of the desired material.
LC/MS: tR = 0.99 min, 266.08 (MH)+.
5-Bromo-1 H-indole-3-carbonitrile
CN
Br \
t,
N
H
A mixture of 5-bromo-indole-3-carboxaldehyde (5 g, 22.3 mmol),
diammonium hydrogen phosphate (15.6 g, 31.8 mmol) in 1-nitropropane (66 mL)
and acetic acid (22 mL) were heated at reflux for 16 h. After cooling to room
temperature, the solvents were removed under reduced pressure and water added
to
the dark residue. After a short while, 5-bromo-1H-indole -3-carbonitrile
precipitated rapidly. The solid was filtered, washed severally with water and
dried
for several hours to afford 4.3 g (86%) of the desired product. 'H-NMR (CD30D)
b
7.40 (m, 2H), 7.77 (s, 1 H), 7.97 (s, 1 H). Mass spec.: 222.95 (MH)+.
5-Formyl-1 H-indole-3-carbonitrile
CN
H ~ \
N
H
5-Bromo-1H-indole -3-carbonitrile (4.25 g, 19.23 mmol) and sodium hydride
(0.51 g, 21.2 mmol) were weighed into a flame-dried round-bottom flask
containing a
magnetic stir bar. Under a nitrogen atmosphere at room temperature, dry
tetrahydrofuran (24 mL) was added. The mixture was stirred at room temperature
for
15 min, during which time it became homogeneous. The stirred mixture was
cooled
to -78°C and a solution of sec-butyllithium in cyclohexane (1.4M, 30.2
mL, 2.2
equiv) was added over several minutes. After 1 h at -78°C,
dimethylformamide (6.0
mL) was slowly added and the mixture allowed to warm to room temperature
overnight. The solution was cooled to 0°C and carefully treated with 1
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
242
hydrochloric acid (45 mL). After a few minutes, solid sodium bicarbonate was
added
until a pH of 9-10 was attained. The two layers were separated and the aqueous
phase washed twice with ethyl acetate. The combined organic layers were washed
with water (2x), brine (2x), dried over sodium sulfate, and concentrated.
Column
chromatography gave 2.4 g (72%) of pure material. LC/MS: tR = 0.99 min, 171.07
(MH)+.
2-Benzyloxycarbonylamino-3-(3-cyano-1H-indol-5-yl)-acrylic acid methyl ester
w
0 0~0
NH
CN
N
H
A stirred solution of N benzyloxycarbonyl-a-phosphonoglycine trimethyl
ester (1.68 g, 5.1 mmol) in tetrahydrofuran (10 mL) at room temperature was
treated
with tetramethylguanidine (0.6 mL, 1.1 equiv). After 10 min, 5-formyl-1H-
indole-3-
carbonitrile (0.72 g, 4.24 mmol) was added. After stirring at room temperature
for 3
days, the solvent was evaporated and the residue washed with water (2x), brine
(2x),
dried over sodium sulfate, and concentrated. Column chromatography gave 1.3 g
(82%) of pure material. LC/MS: tR = 1.43 min, 376.22 (MH)+.
(~)-2-Amino-3-(3-cyano-1 H-indol-S-yl)-propionic acid methyl ester
0
NHZ
CN
N
H
2-Benzyloxycarbonylamino-3-(3-cyano-1H-indol-5-yl)-acrylic acid methyl
ester (0.5 g, 1.3 mmol) in methanol (8 mL) was flushed with nitrogen, and
treated
with palladium on charcoal (10%, 50 mg). The flask was flushed with hydrogen
and
allowed to stir under an atmosphere of hydrogen overnight. The reaction was
flushed
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
243
with nitrogen, filtered through celite, and concentrated. Column
chromatography
gave 0.3 g (92%) of the desired material. LC/MS: tR = 0.80 min, 244.20 (MH)+.
Example 202
(~)-3-(3-Cyano-1H-indol-5-yl)-2- f [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid methyl ester
O~ N
O N
O N~ i
O NH
CN
/ N
H
A stirred solution of 2-amino-3-(3-cyano-1H-indol-5-yl)-propionic acid
methyl ester (25 mg, 0.11 mmol) in tetrahydrofuran (3 mL) at 0°C was
treated with
carbonyl diimidazole (17.5 mg, 1.1 equiv.). The reaction was stirred for S min
at
0°C, warmed to room temperature, stirred 10 min, and treated with 3-
piperidin-4-yl-
3,4-dihydro-1H-quinazolin-2-one (25 mg, 1.1 equiv.). The mixture was stirred
at
room temperature overnight. The solvent was evaporated and the residue
purified by
column chromatography to give 40 mg (75%) as a white powder. LC/MS: tR = 1.37
min, 501.33 (MH)+.
Example 203
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(3-cyano-1 H-indol-5-yl-methyl)-2-oxo-ethyl]-
amide
H
O\'N
~N' ~ /
O H
N" N J
N
O
N
HN ~ CN
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
244
A solution of 3-(3-cyano-1H-indol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino]-propionic acid methyl ester (15
mg,
0.03 mmol) in methanol (0.6 mL) at room temperature was treated with a
solution of
lithium hydroxide monohydrate (3.0 mg, 2.5 equiv.) in water (0.1 mL). The
solution
was stirred at room temperature for 2 h. The solution was cooled to
0°C, and treated
with aqueous 1M potassium hydrogen sulfate (60 ~,L, 2.0 equiv.), and the
solvents
evaporated to give the crude acid which was used immediately without
purification.
The crude acid was dissolved in dimethylformamide (0.4 mL) cooled to
0°C, and
sequentially treated with methylene chloride (0.2 mL), 4-piperidyl-piperidine
(11 mg,
2.2 equiv), N,N diisopropylethylamine (12 pL, 2.3 equiv) and PyBop (19 mg, 1.2
equiv). The solution was stirred for 1 S min at 0°C, warmed to room
temperature,
stirred 1.5 h, and concentrated. The product was purified by column
chromatography
to give 10.1 mg (52% 2 steps). 1H-NMR (CD30D) 8 1.60-2.10 (m, 14H), 2.53 (d,
J 13.0, 1 H), 2.58 (d, J 12.2, 1 H), 2.65-3.00 (m, 7H), 3.12 (d, J 7.0, 1 H),
3.17 (d,
J 7.0, 1 H), 3.84 (s, 1 H), 3.46 (bs, 1 H), 4.08-4.86 (m, SH), 4.70 (m, 1 H),
5.02 (dd,
J=8.2, 6.7, 1 H), 6.79 (d, J 7.6, 1 H), 6.9(m, 1 H), 7.10 (dd, J--7.3, 7.9, 1
H), 9.18 (s,
1 H), 7.15 (dd, J--7.3, 7.6, 1 H), 7.30 (m, 1 H), 7.50 (m, 1 H), 8.00 (s, 1
H). Mass spec.:
647.41 (MH)+.
3-(4-Bromo-2-methyl-phenylamino)-2-methyl-acrylic acid ethyl ester
0
~o~
I I
N~NH
Br
To a solution of 4-bromo-2-methyl aniline (7.0 g, 37.8 mmol) in acetonitrile
(80 mL) was added, sequentially, concentrated hydrochloric acid (15 mL), water
(40
mL) and a solution of sodium nitrite (2.74 g, 39.7 mmol) in water (40 mL)
under ice
cooling to give the diazonium salt. The solution was transferred dropwise to a
solution of 50% potassium hydroxide (16 mL) and ethyl-2-methyl acetoacetate
(5.38
mL, 38 mmol) in ethanol (50 mL) at 0°C. After the addition was
complete, the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
245
reaction mixture was poured into ice-water (150 mL) and extracted with ethyl
acetate. The organic layer was washed with brine (2x), dried over sodium
sulfate,
filtered, and concentrated to give 7.5 g (66%) of the title compound which was
used
immediately without purification. 1H-NMR (CD30D) 8 1.80 (t, J 7.0, 3H), 2.13
(s,
3H), 2.29 (s, 3H), 4.26 (dd, J 5.8, 7.0, 1 H), 4.30 (dd, J 5.8, 7.0, 1 H),
7.26 (m, 2H),
7.43 (m, 1 H). Mass spec.: 323.07 (MNa)+.
5-Bromo-7-methyl-1H-indole-2-carboxylic acid ethyl ester
Br ~ O
I, ~
_N O
H
A solution ofp-toluenesulfonic acid monohydrate (4.26 g, 75 mmol) in
toluene (80 mL) was heated at reflux under a dean-stark water separator for
1.5 h. To
this solution was added a solution of 5-bromo-7-methyl-1H-indole-2-carboxylic
acid
ethyl ester (7.5 g, 25.0 mmol) in toluene (40 mL) and the reaction mixture
heated at
reflux for 5 h. After cooling to room temperature, the reaction mixture was
poured in
to ice-water (120 mL) and extracted twice with ethyl acetate. The organic
layers
were pooled together and washed with sodium bicarbonate (2x), brine (2x),
dried
over sodium sulfate, filtered, and concentrated. Column chromatography on
silica
gel afforded 5.5 g (78%) of the title compound. 'H-NMR (CD30D) S 1.35 (t, J
7.0,
3H), 2.52 (s, 3H), 4.36 (q, J 7.0, 2H), 7.13 (s, 1H), 7.14 (s, 1H), 7.70 (s,
1H), 11.90
(s, 1 H). Mass spec.: 284.09 (MH)+.
5-Bromo-7-methyl-1 H-indole
Br
'N
H
5-Bromo-7-methyl-1H-indole-2-carboxylic acid ethyl ester (5.3 g, 18.7
mmol) was added to a potassium hydroxide solution in 1:1 water/ethanol mixture
(20
mL) and heated at reflux for 12 h. After cooling to room temperature, the
solvents
were removed in vacuo and the resultant residue transferred to a 6N
hydrochloric
acid solution (20 mL). The white precipitate that formed was filtered, washed
severally with water, and dried for several hours. The crude solid was
dissolved in
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
246
quinoline (14 mL) and heated at reflex for 4 h. After cooling to room
temperature,
the crude mixture was poured into a mixture of ice water ( 100 mL) and
concentrated
hydrochloric acid (16 mL), extracted with ethyl acetate (2x), brine (2x),
dried over
sodium sulfate, and concentrated. Attempts to recrystallize the desired
product from
isopropanol resulted in significant decomposition. The title compound was
obtained
by flash chromatography on silica get to afford 1.5 g (38%, 2 steps). LC/MS:
tR =
1.72 min, 210.05 (MH)+.
5-Bromo-7-methyl-1 H-indole-3-carboxaldehyde
H
O
Br
~N
5-Bromo-7-methyl-1H-indole (1.2 g, 5.71 mmol) was dissolved in acetonitrile
(6 mL) and transferred slowly to a solution of bromomethylene dimethyl
ammonium
bromide (1.36 g, 6.28 mmol) in acetonitrile (9 mL) at-10°C to
0°C. The reaction
was stirred at 0°C for 2 h and at room temperature for 30 min. The
solvents were
evaporated and the crude mixture dissolved in water and stirred at 50°C
for 4 h.
After cooling to room temperature, the crude mixture was extracted with ethyl
acetate
(2x). The organic layers were pooled together and washed with brine (2x),
dried over
magnesium sulfate, filtered, and concentrated. Purification by flash
chromatography
on silica gel afforded 0.7 g (52%, 2 steps) of the desired compound. 1H-NMR
(CD30D) 8 2.50 (s, 3H), 7.24 (s, 1 H), 8.34 (m, 1 H), 9.93 (s, 1H). Mass
spec.: 238.05
(MH)+
5-Bromo-7-methyl-1 H-indole-3-carbonitrile
CN
Br
'N
H
A mixture of 5-bromo-indole-3-carboxaldehyde (0.7 g, 2.94 mmol),
diammonium hydrogen phosphate (2.05 g, 15.5 mmol) in 1-nitropropane (9 mL) and
acetic acid (3 mL) were heated at reflex for 16 h. After cooling to room
temperature,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
247
the solvents were removed under reduced pressure and water added to the dark
residue. After a short while, 5-bromo-1H-indole-3-carbonitrile precipitated
rapidly,
was filtered, washed severally with water and dried for several hours to
afford 0.6 g
(87%) of the desired nitrile. LC/MS: tR = 1.71 min, 235.01 (MH)+.
5-Formyl-7-methyl-1 H-indole-3-carbonitrile
CN
H ~ \
/ ,
N
H
5-Bromo-7-methyl-1H-indole -3-carbonitrile (0.58 g, 2.46 mmol) and sodium
hydride (68 mg, 2.7 mmol) were weighed into a flame-dried round-bottom flask
containing a magnetic stir bar. Under a nitrogen atmosphere at room
temperature,
dry tetrahydrofuran (9 mL) was added. The mixture was stirred at room
temperature
for 15 min, during which time it became homogeneous. The stirred mixture was
cooled to -78°C and a solution of sec-butyllithium in cyclohexane
(1.4M, 3.8 mL,
2.2 equiv) was added over several minutes. After 1 h at -78°C,
dimethylformamide
(0.9 mL) was slowly added and the mixture allowed to warm to room temperature
overnight. The solution was cooled to 0°C and carefizlly treated with
1N
hydrochloric acid. After a few minutes, solid sodium bicarbonate was added
until a
pH of 9-10 was attained. The two layers were separated and the aqueous phase
washed twice with ethyl acetate. The combined organic layers were washed with
water (2x), brine (2x), dried over sodium sulfate, and concentrated. Column
chromatography gave 60 mg (14%) of desired product and 0.4 g of unreacted
starting material. LC/MS: tR = 1.21 min, 185.10 (MH)+.
2-Benzyloxycarbonylamino-3-(3-cyano-7-methyl-1H-indol-5-yl)-acrylic acid
methyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
248
O O~ O
A stirred solution of N benzyloxycarbonyl-a-phosphonoglycine trimethyl
ester (180 mg, 0.54 mmol) in tetrahydrofuran (3 mL) at room temperature was
treated
with tetramethylguanidine (40 ~L, 1.1 equiv). After 10 min, 5-formyl-7-methyl-
1H-
indole-3-carbonitrile (50 mg, 0.27 mmol) was added. After stirring at room
temperature for 3 days, the solvent was evaporated and the residue washed with
water
(2x), brine (2x), dried over sodium sulfate, and concentrated. Column
chromatography gave 100 mg (95%) of pure material. LC/MS: tR = 1.59 min,
390.24 (MH)+.
(~)-2-Amino-3-(3-cyano-7-methyl-1H-indol-5-yl)-propionic acid methyl ester
0
2-Benzyloxycarbonylamino-3-(3-cyano-7-methyl-1H-indol-5-yl)-acrylic acid
methyl
ester (0.1 g, 0.26 mmol) in methanol (2.5 mL) was flushed with nitrogen, and
treated
with palladium on charcoal (10%, 10 mg). The flask was flushed with hydrogen
and
allowed to stir under an atmosphere of hydrogen overnight. The reaction was
flushed
with nitrogen, filtered through celite, and concentrated. Column
chromatography
gave 60 mg (90%) of the desired material. LC/MS: tR = 0.93 min, 258.22 (MH)+.
Example 204
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
249
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1 (3-cyano-7-methyl-1 H-indol-5-yl-methyl)-2-oxo-
ethyl]-
amide
HN \
CN O N
/ ~ /
N
N J
I IO
~N O
~N
Prepared as describe above for Example 203: 'H-NMR (CD30D) 8 1.55-2.10 (m,
16H), 2.50 (s, 3H), 2.80-3.20 (m, 9H), 4.10-4.40 (m, 7H), 4.90 (m, 3H), 6.72
(d,
J 7.9, 1 H), 6.93 (dd, J 8.5, 8.5, 1 H), 7.40 (s, 1 H), 7.88(s, 1 H), 7.90 (s,
1 H), 7.99 (s,
1 H). Mass spec.: 651.57 (MH)+.
4-Bromo-2-isopropyl-6-methyl-phenylamine
NH2
Br
2-Isopropyl-6-methyl-phenylamine (5 g, 33.5 mmol) was dissolved in acetic
acid (20 mL). To this solution was added a solution of bromine (1.8 mL, 35.14
mmol) in acetic acid (5 mL) dropwise over 10 min. After stirring for 1 h at
room
temperature, the reaction mixture was diluted with methylene chloride, washed
with
water (2x), saturated sodium thiosulfate (2x), saturated sodium bicarbonate
(2x), and
brine. The organic phase was dried over sodium sulfate, filtered, and
concentrated.
Purification by flash chromatography on silica gel afforded 7.6 g
(quantitative) of the
desired product. LC/MS: tR = 1.37 min, 230.07 (MH)+.
4-Bromo-2-isopropyl-6-methyl-phenyldiazo-t-butyl sulfide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
250
S
I
,N
N
Br
4-Bromo-2-isopropyl-6-methyl-phenylamine (7.6 g, 33.5 mmol) was
suspended in 24% hydrochloric acid (15 mL). The stirred mixture was cooled to -
20°C and treated with sodium nitrite (2.4 g, 1.05 equiv.) in water (5
mL), dropwise
over 30 min while the temperature was maintained below -5°C. After a
further 30
min at -5°C to -20°C, the mixture was buffered to ca. pH 5 with
solid sodium acetate.
This mixture (kept at ca. -10°C) was added in portions to a stirred
solution of t-butyl
thiol (3.77 mL, 1.0 equiv.) in ethanol (25 mL) at 0°C over ca. 30 min.
Following
addition, the mixture was stirred at 0°C for 30 min and then crushed
ice (ca. 50 mL)
was added. The resulting light-brown solid was collected by filtration, washed
with
water, and dried under high vacuum for several hours to afford 7.9 g (72%) of
the
desired product. 'H-NMR (CDC13) 8 1.15 (t, J 6.7, 3H), 1.58 (s, 9H), 2.00 (s,
3H),
2.54 (m, 1 H), 7.20 (s, 1 H), 7.28 (s, 1 H). Mass spec.: 331.08 (MNa)+.
5-Bromo-7-isopropyl-1H-indazole
HN~N
Br
A flame-dried round bottom flask was charged with 4-bromo-2,6-
diethylphenyldiazo-t-butyl sulfide (7.94 g, 24 mmol) and potassium t-butoxide
(27 g,
10 equiv). A stir bar was added and the mixture placed under nitrogen. To this
was
added dry dimethylsulfoxide (70 mL). The mixture was stirred vigorously
overnight
at room temperature. The reaction mixture was carefully poured into a mixture
of
crushed ice (250 mL) and 10% hydrochloric acid (120 mL). The resulting
suspension was collected by filtration and washed severally with water. The
solid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
251
was collected and dried in vacuo to give 4.2 g (74%) of the desired product.
LC/MS:
tR = 1.73 min, 241.06 (MH)+.
7-Isopropyl-1 H-indazole-5-carbaldehyde
HN-N
H ~O
5-Bromo-7-isopropyl-1H-indazole (3.1 g, 12.1 mmol) and sodium hydride
(0.34 g, 1.1 equiv.) were weighed into a flame-dried round-bottom flask
containing a
magnetic stir bar. Under a nitrogen atmosphere at room temperature, dry
tetrahydrofuran (18 mL) was added. The mixture was stirred at room temperature
for
15 min, during which time it became homogeneous. The stirred mixture was
cooled
to -78°C and a solution of sec-butyllithium in cyclohexane (1.4M, 20
mL, 2.2 equiv.)
was added over several minutes. After 1 h at -78°C, dimethylformamide
(3.0 mL)
was slowly added and the mixture allowed to warm to room temperature
overnight.
The solution was cooled to 0°C and carefully treated with 1 N
hydrochloric acid (35
mL). After a few minutes, solid sodium bicarbonate was added until a pH of 9-
10
was attained. The two layers were separated and the aqueous phase washed twice
with ethyl acetate. The combined organic layers were washed with water (2x),
brine
(2x), dried over sodium sulfate, and concentrated. Column chromatography gave
2.1
g (92%) of pure material. LC/MS: tR = 1.1 S min, 189.12 (MH)+.
2-Benzyloxycarbonylamino-3-(7-isopropyl-1 H-indazol-5-yl)acrylic acid methyl
ester
HN~N
/
N"O
~O O ~O
A stirred solution of N benzyloxycarbonyl-a-phosphonoglycine trimethyl
ester (0.39 g, 1.2 equiv) in tetrahydrofuran (5 mL) at room temperature was
treated
with tetramethylguanidine (0.16 mL, 1.1 equiv.). After 10 min, 7-isopropyl-1H-
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
252
indazole-5-carbaldehyde (0.2 g, 1.06 mmol) was added. After stirring at room
temperature for 3 days, the solvent was evaporated and the residue purified by
flash
chromatography on silica gel to give 0.35 g (84%) of product. LC/MS: tR = 1.61
min, 394.16 (MH)+.
(~)-2-Amino-3-(7-isopropyl-1H-indazol-5-yl)propionic acid methyl ester
HN~N
NHZ
\O \O
A solution of 2-benzyloxycarbonylamino-3-(7-isopropyl-1H-indazol-5-
yl)acrylic acid methyl ester (0.35 g, 0.89 mmol) in methanol (7 mL) was
flushed with
nitrogen, and treated with palladium on charcoal (10%, 35 mg). The flask was
flushed with hydrogen and allowed to stir under an atmosphere of hydrogen
overnight. The reaction was flushed with nitrogen, filtered through celite,
and
concentrated. Column chromatography gave 0.21 g (90%) of the desired material.
Example 205
(~)-3-(7-Isopropyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-
piperidine-1-carbonyl]-amino]-propionic acid methyl ester
HN~N .
\ H
O\/N
/ NN ~ /
N\ 'NJ
\O O ~O
Prepared as described above for 3-(3-cyano-1H-indol-5-yl)-2-{[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino]-propionic acid
methyl
ester. LC/MS: tR = 1.49 min, 519.35 (MH)+.
Example 206
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
253
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1 (7-isopropyl-1 H-indazol-5-yl-methyl)-2-oxo-
ethyl]-amide
HN~N
\ H
\ O~ N ~ \
N /
N\ 'NJ
~O
N O
-N
Prepared as described above for Example 203 from 3-(7-isopropyl-1H-indazol-5-
yl)-
2- f [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-
propionic acid methyl ester: ' H-NMR (CD30D) 8 1.45 (m, 6H), 1.60-2.05 (m,
14H),
2.20-2.50 (m, 4H), 2.73 (d, J 13.7, 1 H), 2.90 (m, 4H), 4.05 (d, J 14.0, 1 H),
4.20 (m,
2H), 4.35 (s, 1H), 4.65 (dd, J 12.2, 14.3, 1H), 4.95 (m, 2H), 6.79 (d, J=7.9,
1H),
6.92 (dd, J 7.6, 6.1, 1 H), 7.13 (m, 1 H), 7.80 (s, 1 H), 7.45 (s, 1 H), 8.05
(s, 1 H). Mass
spec.: 655.40 (MH)+.
4-Bromo-2,6-diethylphenyldiazo-t-butyl sulfide
~s
i
N.. N
Br
4-Bromo-2,6-diethylaniline (6.3 g, 27.6 mmol) was suspended in 24%
hydrochloric acid (15 mL). The stirred mixture was cooled to -20°C and
treated with
sodium nitrite (2.0 g, 1.05 equiv.) in water (S mL), dropwise over 30 min
while the
temperature was maintained below -5°C. After a further 30 min at -
5°C to -20°C, the
mixture was buffered to ca. pH 5 with solid sodium acetate. This mixture (kept
at ca.
-10°C) was added in portions to a stirred solution of t-butyl thiol
(3.15 mL, 1.0
equiv.) in ethanol (25 mL) at 0°C over ca. 30 min. Following addition,
the mixture
was stirred at 0°C for 30 min and then crushed ice (ca. 50 mL) was
added. The
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
254
resulting light-brown solid was collected by filtration, washed with water,
and dried
under high vacuum for several hours to afford 6.0 g (66%) of the desired
product.
'H-NMR (CDC13) b 1.15 (t, J=7.6, 6H), 1.50 (s, 9H), 2.27 (m, 4H), 7.21 (s,
2H).
Mass spec.: 331.08 (MH)+.
5-Bromo-7-ethyl-3-methylindazole
HN-N
Br
A flame-dried round bottom flask was charged with 4-bromo-2,6-
diethylphenyldiazo-t-butyl sulfide (4.0 g, 12.1 mmol) and potassium t-butoxide
(13.2
g, 10 equiv). A stir bar was added and the mixture placed under nitrogen. To
this
was added dry dimethylsulfoxide (35 mL). The mixture was stirred vigorously
overnight at room temperature. The reaction mixture was carefully poured into
a
mixture of crushed ice (130 mL) and 10% hydrochloric acid (60 mL). The
resulting
suspension was collected by filtration and washed severally with water. The
solid
was collected and dried in vacuo to give 2.85 g (98%) as a beige solid. 'H-NMR
(CD30D) 8 1.32 (t,.l=7.6, 3H), 2.50 (s, 3H), 2.88 (m, 2H), 7.25 (s, 1H), 7.68
(s, 1H).
Mass spec.: 239.26 (MH)+.
7-Ethyl-3-methylindazole-5-carboxaldehyde
HN~ N
~ H ~o
5-Bromo-7-ethyl-3-methylindazole (2.85 g, 11.9 mmol) and sodium hydride
(0.31 g, 1.1 equiv.) were weighed into a flame-dried round-bottom flask
containing a
magnetic stir bar. Under a nitrogen atmosphere at room temperature, dry
tetrahydrofuran ( 15 mL) was added. The mixture was stirred at room
temperature for
1 S min, during which time it became homogeneous. The stirred mixture was
cooled
to -78°C and a solution of tert-butyllithium in pentane (1.4M, 18.7 mL,
2.0 equiv)
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
255
was added over several minutes. After 1 h at -78°C, dimethylformamide
(2.8 mL)
was slowly added and the mixture allowed to warm to room temperature
overnight.
The solution was cooled to 0°C and carefully treated with 1N
hydrochloric acid (30
mL). After a few minutes, solid sodium bicarbonate was added until a pH of 9-
10
was attained. The two layers were separated and the aqueous phase washed twice
with ethyl acetate. The combined organic layers were washed with water (2x),
brine
(2x), dried over sodium sulfate, and concentrated. Column chromatography gave
1.5
g (67%) of pure material. LC/MS: tR = 1.15 min, 189.12 (MH)+.
2-Benzyloxycarbonylamino-3-(7-ethyl-3-methyl-1 H-indazol-5-yl)-acrylic acid
methyl ester
HN-N
A stirred solution of N benzyloxycarbonyl-oc-phosphonoglycine trimethyl
ester (3.17 g, 9.57 mmol, 1.2 equiv.) in tetrahydrofuran (15 mL) at room
temperature
was treated with tetramethylguanidine (1.1 mL, 1.1 equiv.). After 10 min, 7-
ethyl-3-
methylindazole-5-carboxaldehyde (1.5 g, 7.98 mmol) was added. After stirring
at
room temperature for 3 days, the solvent was evaporated and the residue
purified by
flash chromatography on silica gel to give 2.5 g (80%) of product. LC/MS: tR =
1.61 min, 394.16 (MH)+.
(~)-2-Amino-3-(7-ethyl-3 methyl-1H-indazol-5-yl)-propionic acid methyl ester
HN~N
2-Benzyloxycarbonylamino-3-(7-ethyl-3-methyl-1H-indazol-5-yl)-acrylic acid
methyl ester (1.0 g, 2.54 mmol) in methanol (15 mL) was flushed with nitrogen,
and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
256
treated with palladium on charcoal ( 10%, 100 mg). The flask was flushed with
hydrogen and allowed to stir under an atmosphere of hydrogen overnight. The
reaction was flushed with nitrogen, filtered through celite, and concentrated.
Column
chromatography gave 0.6 g (91 %) of the desired material. 'H-NMR (CD30D) ~
1.32
(m, 3H), 2.50 (s, 3H), 2.88 (dd, J=7.3, 7.6, 1H), 2.89 (dd, J=7.6, 7.6, 1H),
3.02 (dd,
J 6.4, 7.0, 1 H), 3 .11 (dd, J 7.6, 5. 8, 1 H), 3 .3 5 (s, 1 H), 3. 65 (m, 3
H), 7.00 (s, 1 H),
7.33 (s, 1H). Mass spec.: 262.24 (MH)+.
Example 207
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4' ] bipiperidinyl-1 '-yl-1 (7-ethyl-1 H-indazol-S-yl-methyl)-2-oxo-ethyl
]-amide
HN-N H
\ O~ N ~ \
/ N
H
N NJ
O
~N O
~N
Prepared as described above for Example 203 from (~)-2-amino-3-(7-ethyl-3
methyl-
1H-indazol-5-yl)-propionic acid methyl ester. 'H-NMR (CD30D) 8 1.35 (m, 3H),
1.85-2.20 (m, 4H), 2.50 (s, 1H), 2.70 (m, 2H), 2.85 (s, 3H), 2.88-3.25 (m,
7H), 3.35
(s, 1 H), 3.47 (dd, J=7.3, 7.3, 1 H), 4.00-4.40 (m, 7H), 4.70 (m, 1 H), 5.00
(m, 3H),
6.79 (d, J=7.6, 1 H), 6.93 (dd, J=7.3, 7.3, 1 H), 7.10 (m, 1 H), 7.15 (dd, J
7.3, 7.6,
1H), 7.45 (m, 1H). Mass spec.: 655.50 (MH)+.
Example 208
(~)-4-(2,2-Dioxo-1,4-dihydro-2H-2~,6-benzo[ 1,2,6]thiadiazin-3-yl)-piperidine-
1-
carboxylic acid [2-[1,4']bipiperidinyl-1'-yl-1(7-methyl-1H-indazol-5-yl-
methyl)-2-
oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
257
HN~N
\ O H
\ ~S. N I \
O I
N
N"N J
~O
~N O
~N
Prepared as described above for Example 203 from 3-piperidin-4-yl-3,4-dihydro-
1H-
benzo[1,2,6]thiadiazine-2,2-dioxide: 'H-NMR (CD30D) 8 1.20-2.10 (m, 12H), 2.20-
2.60 (m, 6H), 2.90 (m, 6H), 3.78-4.11 (m, 4H), 4.60 (s, 3H), 4.90 (m, 1H),
6.70 (d,
J=8.1, 1 H), 6.79 (dd, J=7.67, 7.3, 1 H), 7.44 (s, 1 H), 7.10 (m, 1 H), 7.13
(m, 3 H), 8.03
(s, 1H). Mass spec.: 663.60 (MH)+.
Example 209
(~)-4-(2,2-Dioxo-1,4-dihydro-2H-2~,6-benzo[ 1,2,6]thiadiazin-3-yl)-piperidine-
1-
carboxylic acid [2-[1,4']bipiperidinyl-1'-yl-1(7-ethyl-3-methyl-1H-indazol-5-
yl-
methyl)-2-oxo-ethyl]-amide
HN~N
\ O H
\ ~S, N I \
O I
N
N" N J
~O
N O
~N
Prepared as described above for Example 203 from 3-piperidin-4-yl-3,4-dihydro-
1H
benzo[1,2,6]thiadiazine-2,2-dioxide: 1H-NMR (CD30D) S 1.35 (m, 3H), 1.42-2.05
(m, 10H), 2.40 (m, 3H), 2.55 (s, 3H), 2.67-3.12 (m, 7H), 3.85 (m, 1H), 3.97
(s, 1H),
4.03 (m, 3 H), 4.65 (m, 4H), 4.95 (dd, J=4.9, 5.8, 1 H), 6.73 (d, J 7.9, 1 H),
6.98 (dd,
J=7.3, 6.4, 1 H), 7.20 (m, 2H), 7.88 (s, 1 H). Mass spec.: 691.51 (MH)+.
Example 210
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
258
(~)-2-[4-(6-Cyano-2-oxo-1,4-dihydro-2H-qinazolin-3-yl)-piperidine-1-carbonyl]-
amino]-3-(7-methyl-1H-indazol-5-yl)-propionic acid methyl ester
H
N\ /O
NC \ ~ 'N~ O~
N~N~O
O
NH
-N
Prepared as described above for 3-(3-cyano-1H-indol-5-yl)-2-{[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
methyl
ester: LC/MS: tR = 1.34 min, 516.40 (MH)+.
Example 211
(t)-4-(6-Cyano-2-oxo-1,4-dihydro-2H-qinazolin-3-yl)-piperidine-1-carboxylic
acid
{2-[1,4']bipiperidinyl-1'-yl-1-(7-methyl-1H-indazol-5-yl methyl)-2-oxo-ethyl}-
amide
N
H
N' /O
NC \ N N
I H
N" N
'O
O
NH
~N
Prepared as described above for Example 203 from 2-oxo-3-piperidin-4-yl-
1,2,3,4-
tetrahydro-quinazoline-6-carbonitrile: 'H-NMR (CD30D) 8 1.80 (m, 12H), 2.4
0(m,
4H), 2.60 (s, 3H), 2.70-3.20 (m, 10H), 4.00-4.30 (m, 6H), 5.00 (m, 1H), S.SO
(s, 2H),
6.90 (d, J 7.8, 1 H), 7.21 (s, 1 H), 7.50 (m, 4H), 8.05 (s, 1 H). Mass spec.:
652.64
(MH)+.
Example 212
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
259
(~)-4-(2-Oxo-1,2,4,5-tetrahydro-benzo[d][1,3]diazepin-3-yl-1-carboxylic acid
}2-
[1,4']bipiperidinyl-1'-yl-1-(7-methyl-1H-indazol-5-yl methyl)-2-oxo-ethyl}-
amide
HN' N i
N_ 'NH
N ~ ~O
O
-N O
-N
Prepared as described above for Example 203 from 3-piperidin-4-yl-1,3,4,5-
tetrahydro-benzo[d][1,3]diazepin-2-one: 'H-NMR (CD30D) b 1.40-2.00 (m, 12H),
2.30-2.60 (m, 8H), 2.70-3.20 (m, 10H), 3.70 (m, 2H), 3.60 (d, J 9.5, 1H), 4.00-
4.30
(m, 4H), 4.70 (m, 1 H), 5.00 (m, 1 H), 6.90 (m, 2H), 7.10 (m, 3H), 7.20 (s, 1
H), 7.50
(s, 1 H), 8.05 (s, 1 H). Mass spec.: 652.64 (MH)+.
Example 213
(~)-4-(6-Hydroxy-2-oxo-1,4-dihydro-2H-qinazolin-3-yl)-piperidine-1-carboxylic
acid
f2-[1,4']bipiperidinyl-1'-yl-1-(7-methyl-1H-indazol-5-yl methyl)-2-oxo-ethyl}-
amide
HN~N OH
i
~N
N~ N' / ~ NH
II O
O
-N O
-N
Prepared as described above for Example 203 from 6-hydroxy-3 -piperidin-4-yl-
3,4-
dihydro-1H-quinazolin-2-one: LC/MS: tR = 1.24 min, 643.62 (MH)+.
Example 214
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
260
(~)-4-(8-Methoxy-2-oxo-1,4-dihydro-2H-qinazolin-3-yl)-piperidine-1-carboxylic
acid {2-[1,4']bipiperidinyl-1'-yl-1-(7-methyl-1H-indazol-5-yl methyl)-2-oxo-
ethyl}-
amide
HN~N
/
H
N N~ N~ NH O
//O
O
N O
-N
Prepared as described above for Example 203 from 8-methoxy-3-piperidin-4-yl-
3,4-
dihydro-1H-quinazolin-2-one: 'H-NMR (CD30D) 8 1.40-2.00 (m, 12H), 2.40 (m,
2H), 2.50 (s, 3H), 2.80 (m, 3H), 3.00-3.20 (m, 3H), 3.50 (m, 2H), 4.00-4.60
(m, 6H),
5.00 (m, 2H), 6.70 (dd, J 8.5, 10.1, 1H), 6.85 (m, 2H), 7.10 (m, 1H), 7.20 (s,
1H),
7.47 (s, 1H). Mass spec.: 657.41 (MH)+.
Example 215
(~)-4-(8-Chloro-2-oxo-1,4-dihydro-2H-qinazolin-3-yl)-piperidine-1-carboxylic
acid
{2-[1,4']bipiperidinyl-1'-yl-1-(7-methyl-1H-indazol-S-yl methyl)-2-oxo-ethyl}-
amide
HN~N CI
\ H
O\ 'N
/ ~N' ~ /
N" N J
~O
N O
~N
Prepared as described above for Example 203 from 2-chloro-3-piperidin-4-yl-3,4-
dihydro-1H-quinazolin-2-one: 'H-NMR (CD30D) 8 1.40-2.00 (m, 14H), 2.30-
2.60(m, 8H), 2.80 (m, 4H), 3.50 (m, 3H), 3.98 (s, 1H), 4.10 (m, 4H), 4.40 (m,
2H),
4.60 (m, 1 H), 4.95 (m, 1 H), 6.95 (dd, J 7.9, 7.9, 1 H), 7.10 (m, 1 H), 7.26
(dd, J 6.7,
7.6, 1 H), 7.47 (m, 1 H), 8.04 (s, 1 H). Mass spec.: 661.27 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
261
Example 216
(t)-N (3-(7-Ethyl-3-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-
yl)propan-2-yl)-2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-quinazoline)-1-
carboxamide
HN-N
\ O
I HN- 'NH
N NJ \
O
-N O
-N
Prepared as described above for Example 203: LC/MS: tR = 1.51 min, 641.63
(MH)+.
Example 217
(~)-N (3-(7-Ethyl-3-methyllH-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-
yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-b enzo [d) [ 1, 3
) oxazine)-
1-carboxamide
HN-N
\ O
I O" NH
N NJ \
O
~N O
~N
Prepared as described above for Example 203: LC/MS: tR = 1.48 min, 642.61
(MH)+.
tent-Butyl 2-fluorophenylcarbamate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
262
~ NHBoc
F
To a solution of di-tert-butyldicarbonate (45.2 g, 207 mmol, 1.0 equiv.) in
tetrahydrofuran (210 mL) at room temperature was added 2-fluoroaniline (20.0
mL,
207 mmol). The reaction was heated to reflux and held there for 6h. It was
cooled,
concentrated, dissolved in pentane, washed with 5% citric acid, then 1 M
potassium
bisulfate (2x), then water, then 20% potassium hydroxide, then brine, dried
over
magnesium sulfate, and concentrated to give 48.0 g (quant.) as an amber oil
which
was used without purification. 'H-NMR (CDCl3, 500 MHz) 8 1.52 (s, 9H), 6.68
(bs,
1H), 6.85-7.20 (m, 3H), 8.07 (dd, J=8.1, 8.1, 1H). Mass spec.: 234.18 (MNa)+.
2-(tert-Butoxycarbonylamino)-3-fluoro-benzoic acid
C02H
NHBoc
F
To a solution of tent-butyl 2-fluorophenylcarbamate (44.0 g, 208 mmol) in
tetrahydrofuran (660 mL) at -78°C was added tent-butyllithium in
pentane (1.7 M,
306 mL, 2:5 equivx) dropwise. After addition was complete, the reaction was
stirred
at -78°C for 30 min. The solution was allowed to gradually reach -
20°C before being
recooled to -78°C and transferred via canula to a slurry of carbon
dioxide (excess)
and tetrahydrofuran (500 mL). The solution was allowed to slowly warm to room
temperature. The reaction mixture was concentrated to remove most of the
tetrahydrofuran, and poured into a sep funnel containing water and diethyl
ether. The
layers were separated, and the aqueous extracted with diethyl ether twice
more. The
ethereals were discarded. The aqueous was acidified with 5% citric acid,
extracted
with diethyl ether (3x). The ethereal was dried over magnesium sulfate, and
concentrated to give a light yellow solid which was recrystallized from hot
toluene to
give 37.1 g (70%) as a faint yellow solid. 1H-NMR (CDCl3, 500 MHz) b 1.50 (s,
9H), 6.25 (bs, 1 H), 7.18 (ddd, J=7.9, 7.9, 4.9, 1 H), 7.33 (dd, J=9.5, 9.2, 1
H), 7.79 (d,
J=7.9, 1 H), 7.94 (s, 1 H). Mass spec.: 278.21 (MNa)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
263
tert-Butyl 2-(1-benzylpiperidin-4-ylcarbamoyl)-6-fluorophenylcarbamate
BnN 1 O NHBoc
F
To a solution of 2-(tent-butoxycarbonylamino)-3-fluoro-benzoic acid (37.1 g,
145 mmol), 4-amino-1-benzylpiperidine (35.6 mL, 1.20 equiv.), 1-
hydroxybenzotriazole (21.6 g, 1.1 equiv.), and triethylamine (44.1 g, 3.0
equiv.)
in ethyl acetate (450 mL) was added 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (30.7 g, 1.1 equiv.) in one portion.
Initially,
everything went into solution, but a precipitate formed very rapidly. The
reaction
was fitted with a reflux condenser and heated at reflux for 5 h. The reaction
was
diluted with ethyl acetate, washed with water (2x), then 1N sodium hydroxide
(2x),
then brine, dried over magnesium sulfate, and concentrated to give 67.0 g
(quant.) as
a white solid which was used without purification. 'H-NMR (CDC13, 500 MHz) 8
1.48 (s, 9H), 1.55 (m, 2H), 1.99 (bd, J=11.0, 2H), 2.17 (dd, J=11.0, 11.0,
2H), 2.84
(bd, J=11.3, 2H), 3.51 (s, 2H), 3.94 (m, 1 H), 6.13 (bd, J=7.6, 1 H), 7.10-
7.28 (m,
4H), 7.31 (m, 4H), 7.59 (s, 1 H). Mass spec.: 428.41 (MH)+.
2-Amino-N ( 1-benzylpiperidin-4-yl)-3-fluorobenzamide
BnN 1 O NHZ
F
H
To a solution of tent-butyl 2-(1-benzylpiperidin-4-ylcarbamoyl)-6-
fluorophenylcarbamate (67.0 g, 157 mmol) in dichloromethane (700 mL) at
0°C was
added trifluoroacetic acid (100 mL). The ice bath was removed and the reaction
stirred at room temperature overnight. The reaction was concentrated and
partitioned
between ethyl acetate and saturated sodium bicarbonate. The aqueous was
extracted
with ethyl acetate (2x), which were washed with water (3x), then brine, dried
over
magnesium sulfate, and concentrated to give 47.6 g (93%) as a white solid
which was
used without purification. Mass spec.: 328.33 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
264
N (2-Amino-3-fluorobenzyl)-1-benzylpiperidin-4-amine
BnN 1 NHZ
F
To a refluxing suspension of lithium aluminum hydride (16.1 g, 424 mmol,
3.50 equiv.) in dioxane (800 mL) was added a solution of 2-amino-N (1-
benzylpiperidin-4-yl)-3-fluorobenzamide (39.7 g, 121 mmol) in dioxane (250 mL)
at
such a rate that gas evolution was limited to a safe flow. Upon completion of
the
addition, the resulting suspension was heated at reflux for 4 h. The reaction
was
cooled to 0°C, and quenched by the cautious addition of 20% potassium
hydroxide.
Upon formation of a white, filterable precipitate, the solid was filtered
through a
course glass sintered funnel, and the eluent concentrated to give 36.3 g (96%)
as a
light yellow oil which was used without purification. Mass spec.: 314.29
(MH)+.
3-( 1-Benzylpiperidin-4-yl)-8-fluoro-3,4-dihydroquinazolin-2( 1 H)-one
H F
O'\/ N
~N I /
BnN
To a solution ofN (2-amino-3-fluorobenzyl)-1-benzylpiperidin-4-amine (36.3
g, 116 mmol) in tetrahydrofuran (600 mL) at room temperature was added
carbonyl
diimidazole (20.7 g, 1.10 equiv.) in one portion. The reaction was stirred at
room
temperature for 3 h, heated at reflux for 30 min, and concentrated. The
resulting
solid was dissolved in 1:1 diethyl ether/ethyl acetate, washed with water
(3x), then
brine, dried over magnesium sulfate, and concentrated to give the crude
product as a
wet, yellow solid. The solid was triturated with diethyl ether and filtered to
give 30.0
g (76%) as a white powder. 'H-NMR (CDCl3, 500 MHz) b 1.68 (m, 2H), 1.86 (dddd,
J=11.9, 11.9, 11.9, 3.4, 2H), 2.14 (dd, J=11.6, 10.1, 2H), 2.98 (d, J=11.6,
2H), 3.51
(s, 2H), 4.34-4.44 (m, 3H), 6.71 (bs, 1H), 6.79-6.89 (m, 2H), 6.94 (dd, J=9.2,
9.2,
1H), 7.21-7.34 (m, SH). Mass spec.: 340.30 (MH)+.
8-Fluoro-3,4-dihydro-3-(piperidin-4-yl)quinazolin-2( 1 H)-one
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
265
H F
O~ N
N
HN
A 250 mL flask was charged with 3-(1-benzylpiperidin-4-yl)-8-fluoro-3,4-
dihydroquinazolin-2(1H)-one (1.40 g, 4.12 mmol) and methanol (25.0 mL). The
suspension was heated with a heat gun to aid in dissolution. The flask was
flushed
with nitrogen, treated with palladium on charcoal (141 mg, 0.032 equiv.),
flushed
with nitrogen, then hydrogen, and vigorously stirred under an atmosphere of
hydrogen overnight. The reaction was flushed with nitrogen, filtered through
celite,
and concentrated to give 0.99g (97%) as a white solid which was used without
purification. 'H-NMR (CDC13, 500 MHz) 8 1.71 (m, 4H), 2.75 (m, 2H), 3.16 (m,
2H), 4.38 (s, 2H), 4.46 (m, 1 H), 6.77 (bs, 1 H), 6.81-6.89 (m, 2H), 6.95 (m,
1 H).
Mass spec.: 250.22 (MH)+.
3-( 1-Benzylpiperidin-4-yl)-8-fluoroquinazoline-2,4( 1 H,3H)-dione
H F
O\'N
'N~
BnN J O
To a solution of 2-amino-N (1-benzylpiperidin-4-yl)-3-fluorobenzamide (750
mg, 2.29 mmol) in dichloromethane (30.0 mL) at 0°C was added
triphosgene (227
mg, 0.33 equiv.) as a solution in dichloromethane (5 mL). The ice bath was
removed
and the reaction heated at reflux for 6h. The reaction was concentrated,
dissolved in
ethyl acetate, washed with saturated sodium bicarbonate, then water, then
brine, dried
over magnesium sulfate, and concentrated to give 700 mg of a white solid. The
crude
product was purified by flash chromatography to give 205 mg (25%) as a white
solid.
Mass spec.: 354.13 (MH)+.
8-Fluoro-3-(piperidin-4-yl)quinazoline-2,4(1 H,3H)-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
266
H F
O\/ N \
~N
HN J O
A flask containing a solution of 3-(1-benzylpiperidin-4-yl)-8-
fluoroquinazoline-2,4(1H,3H)-dione (75.0 mg, 0.21 mmol) and palladium on
charcoal (8.00 mg, 0.035 equiv) in methanol (3.00 mL) was flushed first with
nitrogen, then hydrogen. The reaction was stirred under an atmosphere of
hydrogen
overnight. The reaction was flushed with nitrogen, filtered through celite,
and
concentrated to give 53 mg (95%) as a white solid which was used without
purification. Mass spec.: 264.25 (MH)+.
8'-Fluoro-2',3'-dihydro-2'-oxospiro-(1-phenylmethylpiperidine)-4,4'-
quinazoline
O
HN~ NH
F
BnN
A 500 mL 3-neck flask was charged with polyphosphoric acid (110 mL) and
fitted with an overhead stirrer, a nitrogen inlet, and a bubbler. The flask
was flushed
with nitrogen and heated to 105°C in an oil bath. To this was added 1-
benzyl-4-
piperidone (21.0 mL, 115 mmol). To this was added N (2-Fluorophenyl)urea (21.3
g,
1.2 equiv) in many small portions over 2h. The reaction was heated to
160°C with
vigorous stirring. After 2h, the reaction was quenched by pouring over crushed
ice
and neutralizing with 20% potassium hydroxide. The reaction mixture was
extracted
with dichloromethane, washed with water, then brine, dried over magnesium
sulfate,
and concentrated. The whole lot was purified by preparative HPLC 0130
injections)
to give a much purer product. The product was repurified by flash
chromatography
to give a solid which was triturated with diethyl ether and filtered to give
275 mg
(0.7%) as a white solid. 'H-NMR (CDC13, 500 MHz) 8 1.91 (dd, J=13.7, 2.1, 2H),
2.10 (ddd, J=13.1, 13.1, 4.3, 2H), 2.27 (ddd, J=12.5, 12.5, 2.1, 2H), 2.86 (m,
2H),
3.57 (s, 2H), 5.40 (bs, 1H), 6.90 (bs, 1H), 6.90-7.05 (m, 3H), 7.27 (m, 1H),
7.32 (m,
4H). Mass spec.: 326.13 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
267
8'-Fluoro-2',3'-dihydro-2'-oxospiro-piperidine-4,4'-quinazoline
O
HN~NH
/ F
HN
To a solution of 8'-fluoro-2',3'-dihydro-2'-oxospiro-(1-
phenylmethylpiperidine)-4,4'-quinazoline (250 mg, 0.77 mmol) in methanol (4
mL)
and dichloromethane (4 mL) was added palladium on charcoal (30.0 mg, 0.037
equiv.). The reaction was flushed with hydrogen, and stirred under an
atmosphere of
hydrogen overnight. The balloon was removed, the reaction flushed with
nitrogen,
filtered through celite, washed with additional methanol, and concentrated to
give
158 mg (87%) as a white solid which was used without purification. 1H-NMR
(CDC13/CD30D, S00 MHz) 81.87 (d, J=12.8, 2H), 2.15 (ddd, J=14.0, 14.0, 5.5,
2H),
3.10 (m, 4H), 6.84 (m, 2H), 6.93 (d, J=7.0, 1H). Mass spec.: 236.11 (MH)+.
Example 218
(~)-N (3-(7-Ethyl-1H-indazol-5-yl)-1-(6,7-dihydro-1H-pyrazolo[4,3-c]pyridin-
5(4H)-
yl)-1-oxopropan-2-yl)-4-( 1,2-dihydro-2-oxoquinazolin-3(4H)-yl)piperidine-1-
carboxamide
N~NH H
\ O~ N \
/ N I /
N II NJ
O
N/~N O
~1~JN
H
Prepared as described above for Example 203: ' H-NMR (CD30D, 500 MHz) b 1.24
(m, 2H), 1.55-2.07 (m, SH), 2.57 (m, 1H), 2.82 (m, 4H), 3.08 (m, 2H), 3.30 (m,
3H),
3.35 (m, SH), 3.48 (m, 3H), 3.65 (m, 1H), 4.14 (m, 2H), 4.27 (m, 2H), 4.33-
4.57 (m,
2H), 5.06 (dd, J=6.7, 6.7, 1 H), 5.22 (d, J=1.8, 2H), 6.78 (d, J=7.6, 1 H),
6.93 (m,
1H), 7.00-7.18 (m, 3.5 H), 7.37 (d, J=9.8, 1H), 7.46 (s, O.SH), 7.91 (dd,
J=10.1, 1.8,
1H). Mass spec.: 596.43 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
268
Example 219
(~)-N (3-(7-Ethyl-1H-indazol-5-yl)-1-(6,7-dihydro-7,7-dimethyl-1H-pyrazolo[4,3-
c]pyridin-5(4H)-yl)-1-oxopropan-2-yl)-4-( 1,2-dihydro-2-oxoquinazolin-3(4H)-
yl)piperidine-1-carboxamide
N~NH H
\ O~ N I \
N /
N~NJ
N/ I N O O
~N
H
Prepared as described above for Example 203: 'H-NMR (CD30D, 500 MHz) 8 1.11
(m, 3H), 1.50-1.80 (m, 4H), 2.87 (m, 4H), 3.10 (m, 2H), 3.32 (m, 9H), 3.48 (m,
4H),
4.00-4.45 (m, 6H), 5.05-5.25 (m, 2H), 6.77 (d, J=6.1, 1 H), 6.93 (m, 1 H),
7.13 (m,
3H), 7.30-7.60 (m, 2H), 7.95 (m, 1H). Mass spec.: 624.49 (MH)+.
Example 220
(~)-Methyl 2-(4-(8-fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)piperidine-1-
carboxamido)-3-(7-methyl-1 H-indazol-5-yl)propanoate
N~NH H F
O~ N
N /
N\ /NJ
Me0 O ~O
Prepared as described above for 3-(3-cyano-1H-indol-5-yl)-2-{[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
methyl
ester: IH-NMR (CDC13, 500 MHz) 8 1.53-1.68 (m, 4H), 2.48 (s, 3H), 2.82 (m,
2H),
3.05 (m, 6H), 3.09 (dd, JAB=13.7, 6.1, 1H), 3.14 (dd, JAB=14.0, 6.1, 1H), 3.35
(bs,
1H), 3.68 (s, 3H), 3.88-4.02 (m, 2H), 4.22 (d, JAB=15.6, 1H), 4.25 (d,
JAB=15.3, 1H),
4.44 (m, 1 H), 4.71 (dd, J=6.1, 6.1, 1 H), 6.78 (d, J=7.3, 1 H), 6.84 (ddd,
J=7.6, 7.6,
4.9, 1 H), 6.88-6.95 (m, 2H), 7.28 (s, 1 H), 7.91 (s, 1 H). Mass spec.: 509.25
(MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
269
Example 221
(~)-4-(8-Fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)-N (3-(7-methyl-1H-
indazol-
5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-
carboxamide
N~NH H F
O\/N
I/ NN
N' /N J
~O
~N O
-N
Prepared as described above for Example 203: 1H-NMR (CD30D, 500 MHz) 8 -0.25
(m, 1 H), 0.82 (m, 1 H), 1.25-2.10 (m, 13H), 2.20-2.63 (m, 6H), 2.68-2.98 (m,
4H),
3.00-3.22 (m, 3H), 3.31 (m, 2H), 3.44 (bs, 1 H), 4.00-4.50 (m, 6H), 4.64 (m, 1
H), 4.96
(m, 1H), 6.85-7.05 (m, 3H), 7.08 (s, 0.4H), 7.20 (s, 0.6H), 7.46 (d, J=7.0,
1H), 7.99
(s, 0.4H), 8.05 (d, J=2.4, 0.6H). Mass spec.: 645.58 (MH)+.
Example 222
(t)-4-(8-Fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)-N (3-(7-methyl-1H-
indazol-
5-yl)-1-oxo-1-(4-phenylpiperazin-1-yl)propan-2-yl)piperidine-1-carboxamide
N~NH H F
O\/N
/ NN I /
N\/NJ
~N O O
NJ
Prepared as described above for Example 203: ' H-NMR (CDC13, 500 MHz) 8 1.73
(m, 4H), 2.49 (m, 4H), 2.80-3.26 (m, 7H), 3.43 (m, 2H), 3.65-3.95 (m, 3H),
4.14 (dd,
J=21.7, 14.3, 2H), 4.32 (s, 2H), 4.51 (m, 1 H), 5.15 (dd, J=7.9, 6.4, 1 H),
5.90 (bs,
1 H), 6.80 (d, J=7.3, 1 H), 6.83-7.01 (m, 4H), 7.06 (dd, J=7.6, 7.3, 1 H),
7.10 (s, 1 H),
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
270 _ .
7.26-7.33 (m, 2H), 7.44 (s, 1H), 7.87 (s, 1H), 8.06 (s, 1H). Mass spec.:
639.36
(MH)+.
Example 223
(~)-4-(8-Fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)-N (1-(4-(4-
fluorophenyl)piperazin-1-yl)-3-(7-methyl-1 H-indazol-5-yl)-1-oxopropan-2-
yl)piperidine-1-carboxamide
N~NH H F
O\' N
NN
N II NJ
~N O O
NJ
i/
F
Prepared as described above for Example 203: 'H-NMR (CDC13, 500 MHz) b 1.73
(m, 4H), 2.26 (dd, J=7.9, 7.6, 1H), 2.49 (s, 3H), 2.75-3.05 (m, 4H), 3.09 (m,
2H),
3.19-3.45 (m, 3H), 3.63 (m, 1H), 3.78 (m, 2H), 4.13 (dd, J=16.5, 15.3, 2H),
4.32 (s,
2H), 4.50 (m, 1 H), 5.15 (dd, J=8.2, 6.1, 1 H), 5.85 (bs, 1 H), 6.70-6.84 (m,
3H), 6.85-
7.02 (m, 5H), 7.09 (s, 1 H), 7.43 (s, 1 H), 7.78 (s, 1 H), 8.06 (s, 1 H). Mass
spec.:
657.35 (MH)+.
Example 224
(~)-4-(8-Fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)-N (1-(4-(2-
fluorophenyl)piperazin-1-yl)-3-(7-methyl-1 H-indazol-5-yl)-1-oxopropan-2-
yl)piperidine-1-carboxamide
N~NH H F
O\'N
~ / 'N~ ~ /
N II NJ
~N O O
NJ
/
F
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
271
Prepared as described above for Example 203: 1H-NMR (CDC13, 500 MHz) 8 1.62-
1.78 (m, 4H), 2.24 (dd, J=7.9, 8.2, 1 H), 2.50 (s, 3H), 2.70-2.85 (m, 2H),
2.85-2.96
(m, 2H), 2.00 (m, 1H), 3.08 (dd, JAB=13.1, 8.6, 1H), 3.12 (m, 1H), 3.30 (m,
1H), 3.57
(m, 1H), 3.73 (m, 2H), 4.13 (dd, J=19.8, 15.0, 2H), 4.33 (s, 2H), 4.53 (m,
1H), 5.18
(dd, J=8.2, 5.8, 1 H), 5.82 (bs, 1 H), 6.58 (dd, J=8.2, 8.2, 1 H), 6.81 (d,
J=7.6, 1 H),
6.85-7.05 (m, SH), 7.09 (s, 1 H), 7.44 (s, 1 H), 7.58 (s, 1 H), 8.05 (s, 1 H).
Mass spec.:
657.37 (MH)+.
Example 225
(~)-4-(8-Fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)-N (3-(7-methyl-1H-
indazol
5-yl)-1-oxo-1-(4-o-tolylpiperazin-1-yl)propan-2-yl)piperidine-1-carboxamide
N~NH H F
\ O~ N I \
N
N\ /N J
~N O O
\ NJ
Prepared as described above for Example 203: 1H-NMR (CDC13, 500 MHz) 8 1.60-
1.79 (m, 4H), 2.03 (dd, J=8.5, 8.2, 1H), 2.22 (s, 3H), 2.49 (s, 3H), 2.54 (dd,
J=8.6,
8.5, 1 H), 2.65 (m, 1 H), 2.81 (m, 1 H), 2.85-2.97 (m, 2H), 3.05-3.22 (m, 3H),
3.38 (m,
1H), 3.50-3.65 (m, 2H), 3.83 (m, 1H), 4.15 (dd, J=15.9, 15.3, 2H), 4.31 (s,
2H), 4.53
(m, 1 H), 5.19 (dd, J=7.9, 5.8, 1 H), 5.84 (bs, 1 H), 6.54 (d, J=7.6, 1 H),
6.81 (d, J=7.6,
1 H), 6.89 (ddd, J=7.6, 7.6, 5.2, 1 H), 6.96 (m, 2H), 7.00-7.23 (m, 4H), 7.39
(s, 1 H),
7.43 (s, 1H), 8.04 (s, 1H). Mass spec.: 653.38 (MH)+.
Example 226
(t)-Methyl 2-(4-(8-fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)piperidine-1-
carboxamido)-3-(7-ethyl-3-methyl-1 H-indazol-5-yl)propanoate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
272
N~NH H F
\ O~N I \
N
N II NJ
Me0 O O
Prepared as described above for 3-(3-cyano-1H-indol-S-yl)-2-{[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
methyl
ester: 1H-NMR (CD30D, 500 MHz) 8 1.33 (m, 3H), 1.39-1.72 (m, 4H), 2.70-2.95
(m, 3H), 3.06 (m, 1H), 3.25 (m, 1H), 3.70 (m, 3H), 3.95-4.30 (m 4H), 4.38 (m,
1H),
4.57 (m, 1H), 6.80-7.05 (m, 3H), 7.08 (s, 1H), 7.38 (s, 1H). Mass spec.:
537.47
(MH)+.
Example 227
(~)-N (3-(7-Ethyl-3-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-
yl)propan-2-yl)-4-(8-fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-yl)piperidine-1-
carboxamide
N1NH F
\ O~ N \
/ N
N' /NJ
N O ~~
N
Prepared as described above for Example 203: 'H-NMR (CD30D, 500 MHz) 8 -0.36
(m, 1H), 0.70 (m, 1H), 1.21 (bd, J=11.9, 1H), 1.28-2.00 (m, 19H), 2.31 (dd,
J=11.6,
11.3, 1 H), 2.40 (dd, J=13.1, 11.6, 1 H), 2.79-3.16 (m, 7H), 3.72 (m, 1 H),
3.85-4.03
(m, 1H), 4.10-4.48 (m, SH), 4.53 (bd, J=11.0, 1H), 5.05 (m, 1H), 6.85-7.03 (m,
3H),
7.08 (s, 0.2H), 7.18 (s, 0.8H), 7.37 (s, 1H). Mass spec.: 673.42 (MH)+.
Example 228
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
273
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-yl)propan-2-yl)-4-(8-fluoro-1,2-dihydro-2-oxoquinazolin-3(4H)-
yl)piperidine-1-carboxamide
O
-NH H F
O I \ O~N I \
N
H
N NJ
O
~N O
~N
Prepared as described above for Example 203: 1H-NMR (CD30D, 500 MHz) 8 0.71
(m, 1H), 1.26 (m, 1H), 1.40-2.15 (m, 13H), 2.50-3.29 (m, 9H), 3.32-3.64 (m,
3H),
4.14 (d, JAB=12.8, 1H), 4.17 (d, JAB=11.6, 1H), 4.32-4.45 (m, 3H), 4.68 (bd,
J=13.4,
1 H), 4.92 (m, 1 H), 6.87-7.22 (m, 6H). Mass spec.: 648.47 (MH)+.
Example 229
(t)-N (3-(7-Methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-
yl)propan-2-yl)-8'-fluoro-2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-
quinazoline)-1-
carboxamide
N~NH O
I\
HN~NH
F
N\/NJ \
N O ~O
~N
Prepared as described above for Example 203: 'H-NMR (CD30D, 500 MHz) 8 -0.23
(m, 1H), 0.85 (m, 1H), 1.20-2.10 (m, 22H), 2.25-2.55 (m, 7H), 2.58 (s, 3H),
2.74 (d,
J=11.3, 1H), 2.94 (dd, J=12.5, 12.2, 2H), 3.00-3.20 (m, 5H), 3.40-3.65 (m,
2H),
3.80-4.15 (m, 4H), 4.55-4.73 (m, 2H), 4.96 (dd, J=7.9, 7.6, 1H), 5.01 (dd,
J=10.4,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
274
5.8, 1 H), 6.65-7.15 (m, SH), 7.21 (s, 1 H), 7.47 (s, 1 H), 7.96 (m, 1 H),
8.04 (s, 1 H).
Mass spec.: 631.29 (MH)+.
Example 230
(t)-4-(8-Fluoro-1,2-dihydro-2,4-dioxoquinazolin-3(4H)-yl)-N (3-(7-methyl-1H-
indazol-5-yl)-1-oxo-1-(4-(p iperidin-1-yl)piperidin-1-yl)propan-2-yl)p
iperidine-1-
carboxamide
N~NH H F
O\'N
/ NN /
N\/NJ O
N O ~O
-N
Prepared as described above for Example 203: 'H-NMR (CD30D, 500 MHz) S -0.26
(m, 1 H), 0.81 (m, 1 H), 1.20-2.10 (m, 11 H), 2.20-2.80 (m, 9H), 2.90 (m, 3H),
3.10 (m,
3 H), 3.34 (m, 1 H), 3.44 (m, 1 H), 4.06 (bd, J=13.4, 1 H), 4.17 (d, JAB=15.9,
1 H), 4.22
(d, JAB=13.1, 1H), 4.64 (dd, J=24.4, 13.1, 1H), 4.91-5.13 (m, 2H), 7.00-7.25
(m, 2H),
7.44 (m, 2H), 7.81 (m, 1 H), 7.92-8.08 (m, 1 H). Mass spec.: 659.59 (MH)+.
(R)-Methyl 2-amino-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-yl)propanoate
F F N
~/
'N / NHZ
H
A mixture of (R)-2-benzyloxycarbonylamino-3-(3,4-diamino-phenyl)-propionic
acid
methyl ester (500 mg, 1.20 mmol) and trifluoroacetic acid (6 mL) was heated at
80°C
for 16 h. The reaction mixture was poured into ice water (75 mL), neutralized
to pH
7 with aqueous saturated sodium bicarbonate, and extracted with ethyl acetate
(2 x
250 mL). The organic extracts were dried over sodium sulfate, filtered and
evaporated to give the title compound as the trifluoroacetic acid salt (459
mg, 84
yield). 'H-NMR (CDC13, 300 MHz) 8 7.37 (bs, 1 H) 7.35 (bs, 1 H), 7.17 (d, J =
8.4
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
275
Hz, 1H), 4.70 (s, 2 H), 3.85 (dd, J = 8.4, 4.8 Hz, 1H), 3.77 (s, 3H), 3.30
(dd, J = 13.9,
4.8 Hz, 1 H), 2.97 (dd, J = 13.5, 8.4 Hz, 1 H). Mass spec.: 288 (MH)+.
(R)-Methyl2-(4-(1,2-dihydro-2-oxoquinazolin-3(41~-yl)piperidine-1-carboxamido)-
3-(2-(trifluoromethyl)-1H benzo[d]imidazol-S-yl)propanoate
F F N ~ ~i~. ~O O
F N / HN ~-NH
H ~-N~ N
O
A solution of the amino ester (R)-methyl 2-amino-3-(2-(trifluoromethyl)-1H
benzo[d]imidazol-5-yl)propanoate (230 mg, 0.51 mmol), diisopropylethylamine
(262
mg, 2.03 mmol), and disuccinimidyl carbonate (129 mg, 0.51 mmol) in a mixture
of
methylene chloride/dimethylformamide (15:1 ratio) was stirred at room
temperature
for 30 min. To the solution was added 4-(2-keto-1-benzimidazolinyl)piperidine
and
the reaction mixture was allowed to stir at room temperature for 16 h. The
reaction
mixture was filtered to remove any solids and was then purified by flash
column
chromatography (95:3:2 methylene chloride/methanol/triethylamine) to give the
title
compound (215 mg, 77 % yield) as a tan solid. 1H-NMR (CDCl3, 300 MHz) S 7.67
(d, J = 8.4 Hz, 1 H), 7.39 (s, 1 H), 7.21-7.16 (m, 1 H), 7.05-6.94 (m, 3H),
6.70-6.68 (m,
2H), S.11 (d, J = 7.3 Hz, 1 H), 4.78 (dd, J = 12.1, 5.5 Hz, 1 H), 4.42 (d, J =
4.4 Hz,
2H), 4.29 (d, J = 12.1 Hz, 1 H), 3.82-3.72 (m, 2H), 3.74 (s, 3H), 3.44 (dd, J
= 13.9, S.5
Hz, 1H), 3.22 (dd, J = 13.9, 5.5 Hz), 2.95-2.83 (m, 3H), 2.18-2.03 (m, 2H),
1.79-1.68
(m, 2H). Mass spec.: 545 (MH)+,
(R)-2-(4-( 1,2-dihydro-2-oxoquinazolin-3(4I~-yl)piperidine-1-carboxamido)-3-(2-
(trifluoromethyl)-1H benzo[d]imidazol-5-yl)propanoic acid
OH
F F N ~ ~~'~~O O
F / HN ~NH
H ~N~N
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
276
To a solution of the ester (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoate (220 mg, 0.40 mmol) in tetrahydrofuran and methanol ( 1:1
mixture, 20
mL) at 0°C was added lithium hydroxide (36 mg, 1.51 mmol) in water (10
mL). The
mixture was stirred at 0°C for 2 h and then stored at -15°C for
16 h. The organic
solvents were evaporated. The aqueous solution was extracted with ethyl
acetate
while adjusting the pH to 4 with 1N HCl (3 mL). The organic extracts were
dried
over sodium sulfate, filtered, and evaporated to give the title compound (176
mg,
82% yield). LC/MS: tR = 2.01 min, 531 (MH)+.
Example 231
N ((R)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-yl)-1-oxo-1-(4-(piperidin-
1-
yl)piperidin-1-yl)propan-2-yl)-4-( 1,2-dihydro-2-oxoquinazolin-3(41-
yl)piperidine-
1-carboxamide
F F
~F
HN \ N
NJ
O HN
~N
N //O
N~O
NH
To a stirred solution of the acid (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(41~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid (33 mg, 0.06 mmol) and diisopropylethylamine (33 mg, 0.25
mmol) in methylene chloride (2 mL) was added a solution of PyBOP (33 mg, 0.06
mmol) and 4-piperidinopiperidine (12 mg, 0.07 mmol) in methylene chloride
(1mL).
The reaction mixture was stirred at room temperature for 16 h and was
subjected to
preparative thin layer chromatography for purification (1:10 2M ammonia in
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
277
methanol/methylene chloride) to give the title compound (4.6 mg, 12% yield).
1H-
NMR (CD30D, 500 MHz) 8 7.73-7.71 (m, 1 H), 7.62 (bs, 1 H), 7.39-7.36 (m, 1 H),
7.19-7.11 (m, 2H), 6.96 (t, J = 7.2 Hz, 1 H), 6.81 (d, J = 7.9 Hz, 1 H), 5.06-
5.02 (m,
1 H), 4.67-4.58 (m, 1 H), 4.49-4.40 (m, 1 H), 4.38 (s, 1 H), 4.33 (bs, 1 H),
4.25-4.16 (m,
2H), 4.10-4.03 (m, 1H), 3.22-3.14 (m, 3H), 3.04-2.87 (m, 4H), 2.79-2.71 (m,
1H),
2.58-2.48 (m, 1H), 2.44-2.33 (m, 1H), 2.31-2.22 (m, 1H), 2.04-1.92 (m, 1H),
1.86-
1.43 (m, 11 H), 1.33-1.29 (m, 1 H), 0.94-0.84 (m, 1 H), -0.04- -0.12(m, 1 H).
LC/MS:
tR = 1.97 min, 681 (MH)+.
Example 232
N ((R)-1-(dimethylcarbamoyl)-2-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)ethyl)-4-(1,2-dihydro-2-oxoquinazolin-3(41~-yl)piperidine-1-carboxamide
F F
~F
HN \ N
HN \
Oy
IN O
N~O
NH
Prepared as described above for Example 231. IH-NMR (CD30D, 300 MHz) 8 7.69-
7.56 (m, 2H), 7.34 (d, J = 7.7 Hz, 1 H), 7.17-7.08 (m, 2H), 6.92 (t, J = 7.7
Hz, 1 H),
6.77 (d, J = 8.4 Hz, 1 H), 6.56 (d, J = 7.7 Hz, 1 H), 5.02-4.97 (m, 1 H), 4.46-
4.35 (m,
1 H), 4.29 (s, 2H), 4.1 S (d, J = 12.8 Hz, 1 H), 3.26-3.11 (m, SH), 2.87 (s,
6H), 1.86-
1.68 (m, 2H), 1.66-1.59 (m, 2H). LC/MS: tR = 2.37 min, 558 (MH)+.
Benzyl (R)-1-(methoxycarbonyl)-2-(2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-
yl)ethylcarbamate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
278
~O
H /i..
~(N ~ O
O \ ~ / HN
N
H O
O
To a dilute solution of (R)-2-benzyloxycarbonylamino-3-(3,4-diamino-phenyl)-
propionic acid methyl ester (600 mg, 1.44 mmol) in tetrahydrofuran (125 mL)
was
added triethylamine (320 mg, 3.17 mmol) followed by 1,1'-carbonyldiimidazole
(280
mg, 1.73 mmol). The reaction mixture was stirred at room temperature for 16 h
and
then filtered to remove solid. The filtrate was evaporated and subjected to
flash
column chromatography (1:12 methanol/methylene chloride) to give the title
compound (313 mg, 59% yield). 1H-NMR (CD30D, 300 MHz) b 7.28-7.21 (m, SH),
6.94-6.83 (m, 3H), 5.06-4.95 (m, 2H), 4.46-4.41 (m, 1 H), 3.68 (s, 3H), 3.17-
3.11 (m,
1 H), 2.95-2.88 (m, 1 H). LC/MS: tR = 2.11 min, 370 (MH)+.
(R)-methyl 2-amino-3-(2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-yl)propanoate
0
H /i..
N ~ O
O
N / NHZ
H
Benzyl (R)-1-(methoxycarbonyl)-2-(2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-
yl)ethylcarbamate (265 mg, 0.72 mmol) and 10% palladium on carbon (30 mg) in
methanol (15 mL) were agitated for 1.5 h under 50 psi hydrogen using a Parr
apparatus. The reaction mixture was purged with 3 vacuum/nitrogen purge
cycles.
The reaction mixture was then filtered through a pad of Celite~ and the pad
was
rinsed with several portions of methanol. The methanol filtrate was evaporated
to
give the title compound (168 mg, quantitative yield). 'H-NMR (CD30D, 300 MHz)
S 6.97 (d, J = 8.1 Hz, 1 H), 6.8 7 (s, 1 H), 6.86 (d, J = 8.2 Hz, 1 H), 3.71-
3.64 (m, 1 H),
3.67 (s, 3H), 3.04-2.89 (m, 2H). LC/MS: tR = 0.87 min, 236 (MH)+.
Example 233
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
279
(R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-yl)piperidine-1-
carboxamido)-
3-(2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-yl)propanoate
H
N~O
O
NH
N~NH
N O~'nl
H ~O ~\'O
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. 'H-NMR (CD30D, 300 MHz) 8 7.16-7.08 (m, 2H), 6.98-6.90 (m,
4H), 6.76 (d, J = 8.1 Hz, 1 H), 4.52-4.47 (m, 1 H), 4.39-4.35 (m, 1 H), 4.27
(s, 2H),
4.13-4.05 (m, 2H), 3.70 (s, 3H), 3.21-3.14 (m, 1H), 3.04-2.96 (m, 1H), 2.89-
2.74 (m,
2H), 1.78-1.59 (m, 4H). LC/MS: tR = 1.77 min, 493 (MH)+.
(R)-2-(4-( 1,2-dihydro-2-oxoquinazolin-3 (41~-yl)piperidine-1-carboxamido)-3-
(2,3-
dihydro-2-oxo-1H benzo[d]imidazol-6-yl)propanoic acid
O H
N~O
N~N~NH / ~ NH
H~ 0~,~// w
_~lO
OH
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[a'~imidazol-5-
yl)propanoic acid. 'H-NMR (CD30D, 300 MHz) 8 7.16-7.09 (m, 2H), 6.99-6.90 (m,
4H), 6.76 (d, J = 7.3 Hz, 1 H), 4.53-4.48 (m, 1 H), 4.28 (s, 2H), 4.13-4.03
(m, 2H),
3.07-2.97 (m, 1H), 2.89-2.77 (m, 2H), 1.79-1.60 (m, 4H), 1.28-1.21 (m, 1H).
LC/MS: tR = 1.83 min, 479 (MH)+.
Example 234
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
280
N ((R)-3-(2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-yl)-1-oxo-1(4-piperidin-1-
yl)piperidine-1-yl)propan-2-yl)-4-( 1,2-dihydro-2-oxoquinazolin-3(41-
yl)piperidine-
1-carboxamide
O H
r II N~O
N~N~NH ~ ~ NH
H~ O~.I'' w
O
N
N
Prepared as described above for Example 231. 'H-NMR (CD30D, 300 MHz) 8 7.17-
7.10 (m, 2H), 7.01 (s, 1H), 6.95-6.90 (m, 3H), 6.78 (d, J = 8.1 Hz, 1H), 4.98-
4.93 (m,
1H), 4.62-4.55 (m, 1H), 4.41-4.33 (m, 2H), 4.20-4.16 (m, 2H), 4.04-3.96 (m,
1H),
3.05-2.85 (m, 7H), 2.71-2.57 (m, 1H), 2.53-2.32 (m, 1H), 1.86-1.76 (m, 2H),
1.70-
1.61 (m, 8H), 1.50-1.41 (m, 2H), 1.03-0.89 (m, 1H), 0.10- - 0.02 (m, 1H). Mass
spec.: 629.22 (MH)+
Example 235
N ((R)-1-(dimethylcarbamoyl)-2-(2,3-dihydro-2-oxo-1H benzo[dJimidazol-6-
yl)ethyl)-4-( 1,2-dihydro-2-oxoquinazolin-3(4F~-yl)piperidine-1-carboxamide
O H
N~O
N~N~NH / ~ NH
H \\ O~.~i~
O
~N1
Prepared as described above for Example 231. LC/MS: tR = 1.96 min, 506 (MH)+.
(R)-Methyl 2-[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-( 1I~-
quinazoline)carbonylamino)-3-2,3-dihydro-2-oxo-1H benzo[c~imidazol-6-
yl)propanoate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
281
H O
N
0
N~ NH
~~~il
HN' /NH O
O-
O
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(4I~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[dJimidazol-
5-
yl)propanoate. 'H-NMR (DMSO-d6, 500 MHz) S 10.54 (s, 1H), 10.50 (s, 1H), 9.22
(s, 1 H), 7.21 (s, 1 H), 7.13-7.10 (m, 1 H), 6.96-6.79 (m, 7H), 4.29-4.25 (m,
1 H), 3.82-
3.78 (m, 2H), 3.60 (s, 3H), 3.32-3.23 (m, 1 H), 3.16-3.14 (m, 1 H), 3.00-2.90
(m, 2H),
2.08 (s, 1H), 1.67-1.55 (m, 4H). LC/MS: tR= 1.62 min, 479 (MH)+.
(R)-2-[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-( 11~-
quinazoline)carbonylamino]-
3-2,3-dihydro-2-oxo-1H benzo[d]imidazol-6-yl)propanoic acid
HN~O
INH
O
N/ 'NH
~.~il
O
HN~NH OH
[1O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
282
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. ' H-NMR (CD30D, 300 MHz) 8 7.19-7.14 (m, 1 H), 7.05-6.95
(m,
SH), 6.81 (d, J = 7.7 Hz, 1 H), 5.04-4.90 (m, 1 H), 4.57-4.52 (m, 1 H), 3.96-
3.84 (m,
2H), 3.24-3.14 (m, 2H), 3.07-2.95 (m, 1H), 1.94-1.73 (m, 4H). LC/MS: tR = 1.67
min, 465 (MH)+.
Example 236
N ((R)-3-(2,3-dihydro-2-oxo-1H benzo[dJimidazol-6-yl)-1-oxo-1(4-piperidin-1-
yl)piperidine-1-yl)propan-2-yl)-4-(2',3'-dihydro-2'-oxospiro(piperidine-4,4'-
(ll~-
quinazoline)carboxamide
0
HN~ NH
~N' /
H N N' J
O
N O
~NH
~ / N'~
O
H
Prepared as described above for Example 231. LC/MS: tR = 1.55 min, 615 (MH)+.
4-Acetamido-3-methylbenzoic acid
0
NH
HO 'O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
283
To a suspension of 4-amino-3-methylbenzoic acid (60 g, 0.40 mol) in methylene
chloride (800 mL) was added triethylamine (121 g, 1.19 mol). The solution
became
clear. Then, acetic anhydride (81 g, 0.79 mol) was added and the reaction
mixture
was stirred for 60 h at room temperature. The solvent was evaporated. The
residue
was diluted with water (400 mL) and extracted with ethyl acetate (3 x 600 mL).
The
combined organic extracts were dried over magnesium sulfate, filtered and
evaporated to give the title compound as a tan solid (43 g, 56% yield). 'H-NMR
(d6-
DMSO, 300 MHz) 8 9.36 (s, 1H), 7.77 (s, 1H), 7.10 (s, 2H), 2.27 (s, 3H), 2.10
(s,
3H). LC/MS: tR = 1.22 min, 194 (MH)+.
4-Acetamido-3-methyl-5-nitrobenzoic acid
0
~NH
N02
HO O
To a solution of 60% nitric acid in sulfuric acid (410 mL) was added 4-
acetamido-3-
methylbenzoic acid (43 g, 0.22 mol) in small portions over 40 min while
cooling with
an ice bath. After addition of all amide was complete, the reaction mixture
was
stirred for 1 h at 0°C and then very slowly poured over 1500 mL of ice.
The yellow
solid was collected by filtration and washed with ice cold water to give the
title
compound (38 g, 72% yield). 'H-NMR (CD30D, 300 MHz) 8 8.29 (s, 1H), 8.18 (s,
1H), 2.39 (s, 3H), 2.16 (s, 3H). Mass spec.: 237 (MH)+.
4-Amino-3-methyl-5-nitrobenzoic acid
NH2
NOZ
(/
HO O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
284
A suspension of 4-acetamido-3-methyl-5-nitrobenzoic acid (38 g, 0.16 mol) in
3N
hydrochloric acid (800 mL) was heated at reflex for 8 h and then stirred at
room
temperature for 8 h. The yellow solid was collected by filtration and
transferred to a
2 L flask with a mixture of methylene chloride and methanol. The solvent was
evaporated under high vacuum to give the title compound (23 g, 74% yield). 'H-
NMR (DMSO-d6, 300 MHz) 8 12.79 (bs, 1 H), 8.46 (s, 1 H), 7.79 (s, 1 H), 7.61
(s,
2H), 2.34 (s, 3H). '3C-NMR (d6-DMSO, 75 MHz) 8 166.0, 147.0, 135.1, 130.0,
126.4, 125.9, 116.7, 17.9. LC/MS: tR = 1.23 min, 195 (MH)-.
3-Methyl-4,5-dinitrobenzoic acid
NOZ
NOZ
HO O
To a suspension of 4-amino-3-methyl-5-nitrobenzoic acid (5.0 g, 25.5 mmol) in
trifluoroacetic acid (200 mL) was added hydrogen peroxide (50 wt-% , 15 mL).
The
reaction mixture was heated at 50°C for 2 h and the solution eventually
went from a
dark orange clear solution to a pale yellow clear solution. The reaction
mixture was
slowly poured into ice water (800 mL). The solid was collected by filtration
and
dried under vacuum to give the title compound as an off white solid (4.0 g,
70%
yield). 'H-NMR (CD30D, 300 MHz) 8 8.59 (s, 1H), 8.40 (s, 1H), 2.45 (s, 3H).
'3C-
NMR (CD30D, 75 MHz) ~ 165.8, 147.4, 142.0, 139.3, 134.8, 134.6, 125.4, 17.2.
Mass spec.: 225.14 (MH)-.
(3-Methyl-4,5-dinitrophenyl)methanol
N02
N02
HO
A solution of 3-methyl-4,5-dinitrobenzoic acid (4.0 g, 17.7 mmol) in
tetrahydrofuran
(200 mL) was cooled to -70°C with a dry ice/acetone bath. To this
solution was
added borane-tetrahydrofuran (1M in tetrahydrofuran, 35.4 mL). The reaction
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
285
mixture was allowed to slowly warm to room temperature and was stirred for 16
h.
The reaction was incomplete, was again cooled to -50°C and additional
borane-
tetrahydrofuran (1M in tetrahydrofuran, 35.4 mL) was added. Again, the
reaction
mixture was allowed to slowly warm to room temperature overnight. The reaction
was quenched with a mixture of acetic acid and water (1:1, 30 mL) while
cooling at
0°C. After stirring for 30 min, all organic solvent was evaporated and
the aqueous
material was neutralized by pouring into ice cold saturated sodium bicarbonate
(350
mL) in small portions. The aqueous layer was extracted with ethyl acetate. The
extracts were washed with brine, dried over magnesium sulfate, filtered and
evaporated. The residue was subjected to flash column chromatography (1:2
hexanes/ethyl acetate) to give the title compound (3.2 g, 86% yield). 1H-NMR
(CDCl3, 300 MHz) 8 8.00(s, 1H), 7.62 (s, 1H), 4.82 (s, 2H), 2.41 (s, 3H). 13C-
NMR
(CDC13, 75 MHz) 8 144.5, 143.3, 140.9, 134.3, 132.9, 120.8, 63.0, 17.4.
3-Methyl-4,5-dinitrobenzaldehyde
NOZ
N02
O H
In a flame-dried flask, manganese (IV) oxide (36.0 g, 414 mmol) was
azeotropically
dried with toluene. Then, a solution of (3-methyl-4,5-dinitrophenyl)methanol
(3.2 g,
15 mmol) in chloroform (100 mL) was transferred to the flask containing the
manganese dioxide. The reaction mixture was heated at 50°C with
stirring for 3 h.
Upon completion of the reaction, the reaction mixture was filtered through a
pad of
Celite~ to remove manganese dioxide and the Celite was washed with chloroform
several time. The filtrate was evaporated to give the title compound (1.4 g,
44%
yield). ' H-NMR (CDC13, 300 MHz) 8 10.09 (s, 1 H), 8.51 (s, 1 H), 8.16 (s, 1
H), 2.51
(s, 3H).
Benzyl (Z)-1-(methoxycarbonyl)-2-(3-methyl-4,5-dinitrophenyl)vinylcarbamate
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
286
\O
o2N ~ \ \ ~O
H
O2N
O
O
To a solution of N (benzyloxycarbonyl)-a-phophonoglycine trimethyl ester (2.4
g,
7.3 mmol) in tetrahydrofuran (40 mL) at -78°C was added 1,1,3,3-
tetramethylguanidine (729 mg, 6.33 mmol) and the mixture was stirred for 1 h
at -
78°C. To this mixture was added a solution of 3-methyl-4,5-
dinitrobenzaldehyde
(1.4 g, 6.7 mmol) in tetrahydrofuran (15 mL). The reaction mixture was allowed
to
slowly warm to room temperature and was then stirred for 16 h at room
temperature.
The solvent was evaporated and the residue subjected to flash column
chromatography (gradient, 1:2 to 1:1 ethyl acetatelhexanes). The product was
then
recrystallized from ethyl acetate/hexanes (1:l) to give the title compound
(1.7 g, 62%
yield). 'H-NMR (CDC13, 300 MHz) b 8.01 (s, 1H), 7.55 (s, 1H), 7.33-7.22 (m,
6H),
6.94 (bs, 1H), 5.06 (s, 2H), 3.89 (s, 3H), 2.29 (s, 3H). 13C-NMR (CDCl3, 75
MHz) 8
164.7, 152.5, 143.1, 140.6, 137.7, 137.0, 135.3, 132.5, 128.8, 128.7, 128.6,
127.1,
123.5, 123.2, 68.3, 53.5, 17.4. Mass spec.: 414.20 (MH)-.
Benzyl (R)-1-(methoxycarbonyl)-2-(3-methyl-4,5-dinitrophenyl)ethylcarbamate
O2N \ /i,. ~O
/ HN
02N ~ ~O
O
In a glove bag that was subjected to 3 vacuum/nitrogen purge cycles, an
AIRFREE°
(Schlenk) reaction flask equipped with stir bar was charged with (-)-1,2-
bis((2R,SR)-
2,5-diethylphospholano)benzene(cyclooctadiene) rhodium (I)
trifluoromethylsulfonate (125 g, 0.173 mmol, 4 mol%), sealed with a rubber
septum,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
287
and removed from the glove bag. The benzyl (Z)-1-(methoxycarbonyl)-2-(3-methyl-
4,5-dinitrophenyl)vinylcarbamate (1.65 g, 3.97 mmol) was weighed into a second
AIRFREE~ (Schlenk) reaction flask equipped with stir bar and sealed with a
rubber
septum. After 3 vacuum/nitrogen purge cycles, it was dissolved in a mixture of
anhydrous methylene chloride (40 mL). The solvent was deoxygenated prior to
addition by sparging with nitrogen for at least 1 h. Once in solution, the
mixture was
again subjected to 3 vacuum/nitrogen purge cycles. The dehydroamino acid
solution
was introduced into the AIRFREE~ (Schlenk) reaction flask containing the
catalyst
via cannula. The reaction mixture was subjected to 5 vacuum/hyrogen purge
cycles
before opening the flask to 1 atmosphere of hydrogen. After 16 h, the reaction
mixture was purged with 3 vacuum/nitrogen purge cycles. The solvent was
evaporated and the residue was subjected to column chromatography (1:1 ethyl
acetate/hexanes) to give the title compound (1.58 g, 95%). IH-NMR (CDC13, 300
MHz) 8 7.75 (s, 1H), 7.39-7.33 (m, 6H), 5.37 (d, J = 7.0 Hz, 1H), 5.15-5.04
(m, 2H),
4.70-4.46 (m, 1 H), 3.77 (s, 3H), 3.30 (dd, J = 13.9, 5.5 Hz, 1 H), 3.12 (dd,
J = 13.9,
6.2 Hz, 1H), 2.33 (s, 3H). LC/MS: tR = 2.71 min, 418 (MH)+.
Benzyl (R)-1-(methoxycarbonyl)-2-(3,4-diamino-5-methylphenyl)ethylcarbamate
HZN \ /i..~0
/ HN
H2N ~ ~ O
O
Solid ammonium formate (755 mg, 11.9 mmol) was added in small portions at
0°C to
suspension of benzyl (R)-1-(methoxycarbonyl)-2-(3-methyl-4,5-
dinitrophenyl)ethylcarbamate (500 mg, 1.20 mmol) and zinc powder (470 mg, 7.19
mmol) in methanol (20 mL, degassed with nitrogen for 2 h). The resulting
mixture
was stirred at room temperature for 60 h. Reaction was incomplete. The
reaction
mixture was again cooled to 0°C and additional zinc powder (470 mg,
7.19 mmol)
was added. The reaction was stirred for 4 h at which time the reaction was
complete.
The reaction mixture was filtered to remove zinc. The filtrate was evaporated.
A
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
288
mixture of toluene and ethyl acetate (1:1) were added, followed by acetic acid
(2
mL). The mixture was further diluted until all organic solids dissolved, then
it was
washed with water, brine, dried over sodium sulfate, and evaporated. The
residue
was then redissolved in ethyl acetate and 4N hydrogen chloride in dioxane (4
mL)
S was added. The solvent was evaporated to give the title compound as the
dihydrochloride salt (515 mg, quantitative yield). 'H-NMR (CD30D, 300 MHz) 8
7.35-7.30 (m, SH), 6.94-6.93 (m, 2H), 5.03 (s, 2H), 4.42-4.37 (m, 1H), 3.70
(s, 3H),
3.09-3.03 (m, 1H), 2.87-2.79 (m, 1H), 2.25 (s, 3H). LC/MS: tR = 1.79 min, 358
(MH)+.
Benzyl (R)-1-(methoxycarbonyl)-2-(7-methyl-1H benzo[d][1,2,3]triazol-5-
yl)ethylcarbamate
N ~ ~~,. ~O
N~ ~ / HN
H
N ~ /_O
O
To a solution of benzyl (R)-1-(methoxycarbonyl)-2-(3,4-diamino-5-
methylphenyl)ethylcarbamate ( 250 mg, 0.58 mmol) in acetic acid (6 mL) and
water
(10 mL) was added a solution of sodium nitrite (40 mg, 0.58 mmol) in water (1
mL),
dropwise over several minutes at room temperature. The resulting mixture was
stirred at room temperature for 30 min, then cooled to 0°C. A mixture
of
ammonium hydroxide and water (1:1, 15 mL) was added to adjust pH to 11. The
mixture was extracted with ethyl acetate twice. The organic layers washed with
brine
and dried over sodium sulfate. After filtration, solvents were removed in
vacuo and
the residue was purified by flash column chromatography (1:1 ethyl
acetate/hexanes)
on silica gel to afford the title compound as a tan solid (155 mg, 72% yield).
~H-
NMR (CDCl3, 300 MHz) 8 7.34 (s, 1 H), 7.32-7.28 (m, 6H), 6.93 (s, 1 H), 5.40
(d, J =
8.1 Hz, 1H), 5.13-5.02 (m, 2H), 4.76-4.69 (m, 1H), 3.73 (s, 3H), 3.28 (dd, J =
13.9,
5.5 Hz, 1 H), 3.16 (dd, J = 13.9, 6.2 Hz, 1 H), 2.64 (s, 3H). LC/MS: tR = 2.30
min,
369 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
289
(R)-Methyl 2-amino-3-(7-methyl-1H benzo[d][1,2,3]triazol-5-yl)propanoate
fi'''r 'O
NN II
~N / NHz
H
Benzyl (R)-1-(methoxycarbonyl)-2-(7-methyl-1H benzo[d][1,2,3]triazol-5-
yl)ethylcarbamate (146 mg, 0.40 mmol) was dissolved in 12 mL of a solution of
4.4% formic acid in methanol. The reaction flask containing this solution was
equipped with a magnetic stirbar and then flushed with nitrogen over several
minutes.
To the solution was added palladium on carbon (10%, 200 mg) and the reaction
was
stirred for 16 h at room temperature under nitrogen atmosphere. The reaction
mixture was filtered through a pad of Celite~ washing the pad several times
with
methanol. The filtrate was evaporated to give the title compound (quantitative
yield).
'H-NMR (CDC13, 300 MHz) S 8.40 (bs, 1H), 7.55 (s, 1H), 7.14 (s, 1H), 4.29-4.24
(m, 1H), 3.78 (s, 3H), 3.39-3.19 (m, 2H), 2.69 (s, 3H). LC/MS: tR= 1.18 min,
235
(MH)+.
(R)-methyl 2-(4-( 1,2-dihydro-2-oxoquinazolin-3 (4I~-yl )piperidine-1-
carboxamido)-
3-(7-methyl-1H benzo[d][1,2,3]triazol-S-yl)propanoate
''r 'O
~N I / INH
H
O N~ O
N' _ NH
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. LC/MS: tR = 2.17 min, 492 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
290
(R)-2-(4-( 1,2-dihydro-2,4-dioxoquinazolin-3 (4I~-yl)piperidine-1-carboxamido)-
3-(7-
methyl-1H benzo[d][1,2,3]triazol-5-yl)propanoic acid
OH
N ~ /i.. ~O
N' I / NH
H
O
N ~ O~ N
N' -NH
OiT
I~1\
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(41~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. LC/MS: tR = 2.11 min, 492 (MH)+.
Example 237
4-(1,2-dihydro-2,4-dioxoquinazolin-3(41-yl)-N ((R)-3-(7-methyl-1H
benzo[d][1,2,3]triazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-
2-
yl)piperidine-1-carboxamide
N
NJ
/~~' ~O
NN
'N
H
O N O
N' -NH
O /'\ J
Prepared as described above for Example 231. 1H-NMR (CD30D, 300 MHz) 8 8.01
(d, J = 8.1 Hz, 1 H), 7.63 (t, J = 7.5 Hz, 1 H), 7.28-7.11 (m, 4H), 5.06-5.00
(m, 1 H),
4.70-4.60 (m, 1 H), 4.31-4.17 (m, 2H), 3.50-3.44 (m, 1 H), 3.20-2.82 (m, 7H),
2.75-
2.47 (m, 6H), 2.12-2.02 (m, 2H), 1.93-1.67 (m, 11H), 1.37-1.28 (m, 2H, 0.97-
0.79
(m, 2H), 0.23-0.09 (m, 1 H). Mass spec.: 642 (MH)+,
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
291
(R)-2-Benzyloxycarbonylamino-3-(4-chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester
O ~ COzCH3
O
N / NHCbz
H
CI
A mixture of (R)-2-benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-
yl)-propionic acid methyl ester (373 mg, 1.01 mmol), N-chlorosuccinimide (168
mg,
1.26 mmol), silica gel (EM Scientific, 230 - 400 mesh, 3.73 g) in
dichloroethane (20
mL) was heated at 90°C for 16 h. After cooling down to room
temperature, the
solvents were removed in vacuo. The residue was subjected to silica gel
chromatography using ethyl acetate/hexanes (1:2) as eluent to afford the title
compound (40 mg, 9.8%), also 2-benzyloxycarbonylamino-3-(5-chloro-2-oxo-2,3-
dihydro-benzooxazol-6-yl)-propionic acid methyl ester (78 mg, 19%). The
structure
was confirmed by 2D NMR and by comparison with that of 2-
benzyloxycarbonylamino-3-(5-chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester prepared by the reaction shown below. 1H-NMR
(CD3COCD3, 500 MHz) 8 7.37 - 7.27 (m, 5H), 7.18 (d, J = 1.0 Hz, 1 H), 7.16 (s,
1 H),
6.76 (d, J = 8.5 hz, 1 H), 5.06 (d, J =12.5 Hz, 1 H), 5.02 (d, J = 12.5 Hz, 1
H), 4.55 -
4.51 (m, 1 H), 3.72 (s, 3H), 3.26 (dd, J = 14.0, 5.0 Hz, 1 H), 3.04 (dd, J =
14.0, 9.5 Hz,
1H);'3C-NMR (CD3COCD3, 125 MHz) 8 172.2, 156.4, 154.0, 144.8, 137.6, 133.3,
128.7, 128.2, 128.0, 127.9, 125.0, 66.3, 55.9, 52.0, 37.3; Mass spec. 405
(MH+).
(R)-2-Benzyloxycarbonylamino-3-(5-chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester
O ~ COyCHg
O~ ~ -
N / CI NHCbz
H
N-Chlorosuccinimide (315 mg, 2.36 mmol) was added to a solution of (R)-2-
benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl ester (700 mg, 1.89 mmol) in acetic acid (50 mL) at room temperature.
The
mixture was heated at 100°C for 16h. After it was cooled down to room
temperature,
solvents were removed in vacuo. The residue was subjected to silica gel
chromatography using ethyl acetate/hexanes (4:6) then (l :l) as eluent to
afford the
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
292
title compound as an off yellow solid (242 mg, 32%). The structure of the
product
was confirmed by 2D NMR. 'H-NMR (CD3COCD3, 500 MHz) 8 10.47 (s, 1H), 7.36
- 7.28 (m, 6H), 7.20 (s, 1 H), 6.80 (d, J = 8.5 Hz, 1 H), 5.05 (d, J = 12.5
Hz, 1 H), 5.00
(d, J = 12.5 Hz, 1 H), 4.65 - 4.60 (m, 1 H), 3.73 (s, 3H), 3.43 (dd, J = 14.0,
5.0 Hz,
1H), 3.08 (dd, J = 14.0, 10.5 Hz, 1H); '3C-NMR (CD3COCD3, 125 MHz) 8 172.2,
156.5, 154.5, 143.1, 137.5, 130.8, 129.0, 128.9, 128.7, 128.2, 128.0, 112.8,
110.9,
66.3, 54.3, 52.1, 35.8; Mass spec. 405 (MH+).
(R)-2-Benzyloxycarbonylamino-3-(4-bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester
COyCH3
O I -
N / NHCbz
H
Br
A mixture of (R)-2-benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-
yl)-propionic acid methyl ester (418 mg, 1.13 mmol), N-bromosuccinimide (221
mg,
1.24 mmol), silica gel (EM Scientific, 230 - 400 mesh, 2.51 g) and methylene
chloride (70 mL) was stirred at room temperature for 16 h. Solvents were
removed in
vacuo and the residue was subjected to silica gel chromatography using ethyl
acetate/hexanes (2:3) as eluent to afford the title compound. 'H-NMR
(CD3COCD3,
500 MHz) 8 10.71 (s, 1 H), 7.35 - 7.28 (m, 6H), 7.21 (s, 1 H), 6.75 (d, J =
7.5 Hz, 1 H),
5.06 (d, 12.5 Hz, 1 H), 5.02 (d, J = 12.5 Hz, 1 H), 4.56 - 4.51 (m, 1 H), 3.73
(s, 3H),
3.26 (dd, J = 14.0, 5.0 Hz, 1 H), 3.03 (dd, J = 14.0, 10.0 Hz, 1 H); ' 3C-NMR
(CD3COCD3, 125 MHz) 8 172.2, 156.4, 153.8, 144.4, 137.6, 133.7, 129.8, 128.7,
128.2, 128.0, 127.8, 110.1, 100.9, 66.3, 55.9, 52.0, 37.3; Mass spec. 448.03
(MH+).
(R)-2-Benzyloxycarbonylamino-3-(5-bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester
p ~ COyCH3
O
N / Br NHCbz
H
A mixture of (R)-2-benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-
yl)-propionic acid methyl ester (1.07 g, 2.89 mmol), N-bromosuccinimide (643
mg,
3.61 mmol), and acetic acid (150 mL) was heated at 105°C for 14 h.
After cooling to
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
293
room temperature, the solvents were removed in vacuo. The residue was
subjected to
silica gel chromatography using ethyl acetate/hexanes (2:3), then (1:1) as
eluent to
afford the title compound (446 mg, 34%). The structure of the title compound
was
confirmed by 2D NMR. 'H-NMR (CD3COCD3, 500 MHz) 8 10.46 (s, 1H), 7.36 -
7.28 (m, 7H), 6.82 (d, J = 8.5 Hz, 1 H), 5.05 (d, J = 12.5 Hz, 1 H), 5.00 (d,
J = 12.5
Hz, 1 H), 4.67 - 4.62 (m, 1 H), 3.73 (s, 3H), 3.43 (dd, J = 14.0, 5.0 Hz, 1
H), 3.10 (dd,
J = 14.0, 10.5 Hz, 1H); '3C-NMR (CD3COCD3, 125 MHz) S 172.2, 156.4, 154.2,
143.7, 137.6, 131.1, 130.6, 128.7, 128.2, 128.0, 118.2, 113.9, 112.9, 66.2,
54.3, 52.1,
38.3; Mass spec. 448.03 (MH+).
(R)-2-Benzyloxycarbonylamino-3-(4-iodo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-
propionic acid methyl ester
COZCH3
N / NHCbz
H
1
A mixture of (R)-2-benzyloxycarbonylamino-3-(2-oxo-2,3-dihydro-benzooxazol-6-
yl)-propionic acid methyl ester (324 mg, 0.87 mmol), I(PyH)ZBF4 (409 mg, 1.08
mmol), silica gel (EM Scientific, 230 - 400 mesh, 3.24 g) and dichloroethane
(20
mL) was heated at 90°C for 6 h. After cooling to room temperature the
solvents were
removed in vacuo. The residue was subjected to silica gel chromatography using
ethyl acetate/hexanes (1:2) as eluent to afford the title compound (175 mg,
40%).
The structure of the title compound was confirmed by 2D NMR. 'H-NMR
(CD3COCD3, 500 MHz) 8 10.47 (s, 1 H), 7.46 (s, 1 H), 7.37 - 7.29 (m, 5H), 7.22
(s,
1 H), 6.74 (d, J = 8.5 Hz, 1 H), 5.07 (d, J = 12.5 Hz, 1 H), 5.02 (d, J = 12.5
Hz, 1 H),
4.54 - 4.49 (m, 1 H), 3.72 (s, 3H), 3,23 (dd, J = 14.0, 5.0 Hz, 1 H), 3.01
(dd, J = 14.0,
9.5 Hz, 1H);'3C-NMR (CD3COCD3, 125 MHz) 8 172.2, 156.4, 153.4, 143.3, 137.6,
134.1, 133.64, 133.60, 128.7, 128.2, 128.0, 110.7, 71.1, 66.3, 56.0, 52.0,
37.1; Mass
spec. 496.01 (MH+).
(R)-2-Amino-3-(5-bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
294
O ~ COZCH3
O
N / Br NH2
H
Trimethylsilyliodide (73 mL, 0.73 mmol) was added to a solution of
azotropically
dried (R)-2-benzyloxycarbonylamino-3-(5-bromo-2-oxo-2,3-dihydro-benzooxazol-6-
yl)-propionic acid methyl ester (146 mg, 0.33 mmol) in acetonitrile (10 mL) at
room
temperature, and the resulting mixture was stirred at room temperature for 2
h.
Triethylamine (0.12 mL) was added and the mixture was stirred at room
temperature
for 15 min. The solvents were removed in vacuo, and the residue was extracted
with
ethyl acetate. The combined organics were washed with sodium bicarbonate and
brine, dried over sodium sulfate and filtered. Solvents were removed and the
residue
was used directly in the next step. Mass spec. 315.10 (MH)+.
(R)-2-Amino-3-(4-bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl ester
O ~ C02Me
O
N / NHy
H
Br
Prepared as described above for (R)-2-amino-3-(5-bromo-2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. Mass spec. 315.06 (MH)+.
(R)-2-Amino-3-(5-chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl ester
O ~ COZCH3
N / CI NHz
H
Prepared as described above for (R)-2-amino-3-(5-bromo-2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. Mass spec. 271.10 (MH)+.
(R)-2-Amino-3-(4-chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
295
_ COyCH3
N / NHy
H
CI
Prepared as described above for (R)-2-amino-3-(5-bromo-2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. Mass spec. 271.16 (MH)+.
(R)-2-Amino-3-(4-iodo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-propionic acid
methyl
ester:
C02CH3
N / NHy
H
I
Prepared as described above for (R)-2-Amino-3-(5-bromo-2-oxo-2,3-dihydro-
benzooxazol-6-yl)-propionic acid methyl ester. Mass spec. 363.04 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [2,4-dihydro-2'-oxospiro-
(piperidine-4,4'-1H-benzo[d][1,3]oxazine)-1-carbonyl]-amino-propionic acid
methyl
ester
\~~~C02Me
o~ I t
N / HN' /O
'~H
N
/ ~ ~O
N ~O
H
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(4I~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. Mass spec. 481.20 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [2,4-dihydro-2'-oxospiro-
(piperidine-4,4'-1H-quinazoline)-1-carbonyl]-amino-propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
296
O ~ \~~~C02Me
o~ I t
N / HN' /O
'~H
N
I ~NH
N_ 'O
H
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[a~imidazol-
5-
yl)propanoate. Mass spec. 480.24 (MH)+.
(R)-3-(4-Chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
H
O ~ COyMe
O~ I
N / HN' /O
'~H
CI N
O\'N
H~N'
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. Mass spec. 528.16 (MH)+.
(R)-3-(5-Chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino)-propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
297
H
O ~ C02Me
o~ I t
N / HN' /O
H C '~I
N
O~ N
H'~N' /
I
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-I-carboxamido)-3-(2-(trifluoromethyl)-IH benzo[d]imidazol-
5-
yl)propanoate. Mass spec. 528.20 (MH)+.
(R)-3-(4-Bromo-2-oxo-2, 3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo- I ,4-
dihydro-2H-
quinazolin-3-yl)-piperidine-I-carbonyl]-amino}-propionic acid methyl ester
H
O ~ C02Me
O~ I
N / HN' /O
'~H
Br N
O~ N
H'~N' /
I
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(4X)-yl)piperidine-I-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. Mass spec. 572.20 (MH)+.
(R)-3-(5-Bromo-2-oxo-2, 3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo- I ,4-
dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonylJ-amino}-propionic acid methyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
298
H
O ~ COZMe
O~ I / HN O
N Br
H
N
O' /N
H~N'
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(4I~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
5-
yl)propanoate. Mass spec. 572.15 (MH)+.
(R)-3-(4-Iodo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid methyl ester
H
O ~ COyMe
O~ I / HN O
N
H
I N
O~ N
H'~N'
I
Prepared as described above for (R)-methyl 2-(4-(1,2-dihydro-2-oxoquinazolin-
3(41~-yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-
S-
yl)propanoate. Mass spec. 620.20 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [2,4-dihydro-2'-oxospiro-
(piperidine-4,4'-1H-benzo[d][1,3]oxazine)-1-carbonyl]-amino}-propionic acid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
299
O ~ \~yCOyH
O~ I / HN O
N
H
N
I ~O
N~O
H
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 467.18 (MH)+.
(R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [2,4-dihydro-2'-oxospiro-
(piperidine-4,4'-1H-quinazoline)-1-carbonyl]-amino}-propionic acid
O ~ \~~~C02H
O~ I / HN O
N
H N
I ~NH
N~O
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(41~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 466.20 (MH)+.
(R)-3-(4-Chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O ~ COZH
v~
N / HN'/O
'~H
CI N
O' /N
H~N'
\ I
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
300
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 514.20 (MH)+.
(R)-3-(5-Chloro-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2-{[4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O ~ COyH
O
N / HN\ /O
H C '~I
N
O\ /N
H~N'
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(41-~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 514.24 (MH)+.
(R)-3-(4-Bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O ~ COZH
o~
N / HN\ /O
'~H
Br N
O\ /N
H~N'
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 558.30 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
301
(R)-3-(5-Bromo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O ~ C02H
I I
N / HN\ /O
H B '~~
N
O\ /N
H~N'
~I
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(41~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 558.25 (MH)+.
(R)-3-(4-Iodo-2-oxo-2,3-dihydro-benzooxazol-6-yl)-2- { [4-(2-oxo-1,4-dihydro-
2H-
quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid
H
O ~ COZH
o~ I I
N / HN' /O
'~H
I N
O\ /N
H~N'
~ I
Prepared as described above for (R)-2-(4-(1,2-dihydro-2-oxoquinazolin-3(4I~-
yl)piperidine-1-carboxamido)-3-(2-(trifluoromethyl)-1H benzo[d]imidazol-5-
yl)propanoic acid. Mass spec. 606.10 (MH)+.
Example 238
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
(4-
cyclohexyl-piperazin-1-yl)-2-oxo-1-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
302
O ~N~
~L N
O \
O~ I
N / HN\ /O
'~H
N
N' /O
\ 'N~H
I~
Prepared as described above for Example 231. LC/MS: tR = 1.80 min, 630.37
(MH)+.
Example 239
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
(4-
isopropyl-piperazin-1-yl)-2-oxo-1-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
ethyl]-amide
O N N
L
O \
o~
N / HN' /O
'~H
N
N' /O
\ 'N~H
Prepared as described above for Example 231. LC/MS: tR = 1.71 min, 590.34
(MH)+.
Example 240
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-
benzo[d][1,3]oxazine)-1-carboxamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
303
O ~N
~~ N' /
O \
O
N / HN\ /O
'~H
N
/ ~ ~O
\ N ~O
H
Prepared as described above for Example 231. LC/MS: tR = 1.64 min, 617.34
(MH)+.
Example 241
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(cyclohex-1-
yl)piperazin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-
benzo[d] [ 1,3]oxazine)-1-carboxamide
O ~N'O
~L N
O \
O
N / HN' /O
'~H
N
/ ~ ~O
\ N ~O
H
Prepared as described above for Example 231. LC/MS: tR = 1.69 min, 617.35
(MH)+.
Example 242
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(prop-2-
1 S yl)piperazin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1
H-
benzo[d] [ 1,3] oxazine)-1-carboxamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
304
O~N N
O \
O~ I / HN O
N
H
N
/ I ~O
\ N ~O
H
Prepared as described above for Example 231. LC/MS: tR = 1.57 min, 577.32
(MH)+.
Example 243
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(piperidin-1-
yl)piperidin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-
quinazoline)-1-carboxamide
O ~N
O \\~L N ' /
I\ ~1
N / HN\ /O
'~H
N
/ I ,~
\ N O
H
Prepared as described above for Example 231. LC/MS: tR = 1.74 min, 616.37
(MH)+.
Example 244
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(cyclohex-1-
yl)piperazin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-
1 S quinazoline)-1-carboxamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
305
O ~N~
~L N~
o \
o~
N / HN' /O
'~H
N
I ~NH
\ N- 'O
H
Prepared as above.- LC/MS: tR = 1.79 min, 616.36 (MH)+.
Example 245
(R)-N ((R)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-yl)-1-oxo-1-(4-(prop-2-
yl)piperazin-1-yl)propan-2-yl)-2,4-dihydro-2'-oxospiro-(piperidine-4,4'-1 H-
quinazoline)-1-carboxamide
O ~N~
L
O \
O~ I ~ r
N / HN' /O
'~H
N
~NH
\ N ~O
H
Prepared as described above for Example 231. LC/MS: tR = 1.67 min, 576.34
(MH)+.
Example 246
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(4-chloro-2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-2-
oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
306
O N
O ~L N
O
N / HN' /O
'~H
CI N
N' /O
'N~H
Prepared as described above for Example 231. LC/MS: tR = 1.91 min, 664.35
(MH)+.
Example 247
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(5-chloro-2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-2-
oxo-ethyl]-amide
O ~N
~L N ' /
O
O
N / CHN\ /O
'~H
N
N' /O
'N~H
Prepared as described above for Example 231. LC/MS: tR = 1.91 min, 664.34
(MH)+.
Example 248
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(4-bromo-2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
2-
oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
307
O N
O ~y N
O
N / HN' /O
'~H
Br N
N' /O
'N~H
Prepared as described above for Example 231. LC/MS: tR = 1.96 min, 708.31
(MH)+.
Example 249
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(5-bromo-2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-2-
oxo-ethyl]-amide
O ~N
~L N' /
O
O
N / BHN\ /O
'~H
N
N' /O
'N~H
Prepared as described above for Example 231. LC/MS: tR = 1.96 min, 708.31
(MH)+.
Example 250
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(4-iodo-2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
2-
oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
308
O ~N
~L N' /
O \
O~ I / HN O
N
H
I N
N' /O
\ 'N~H
I/
Prepared as described above for Example 231. LC/MS: tR = 1.97 min, 756.36
(MH+).
Example 251
(~)-N (1-Benzyl-2-hydroxy-ethyl)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-
[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butyramide
0
O_ -NH
/ \ N J \
N, I i i
N / ~ O
H O NH
~OH
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.38 min, 596 (MH)+.
Example 252
(~)-N (1-Benzyl-2-hydroxy-ethyl)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-[4-
(2-oxo-1,4-dihydro-2 H-quinazolin-3-yl)-piperidin-1-yl]-butyramide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
309
H
O\ /N
~N ~ /
N
N / ~ / O~ O
N ~ NH
H
OH
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.50 min, 609 (MH)+. 'H NMR (400 MHz,
CD30D) 8 7.90 ( 1 H, s), 7.64-7.84 ( 1 H, m), 6.71-7.42 ( 11 H, m), 4.5 8 ( 1
H, m), 3.82-
4.50 (6H, m), 2.21-3.52 (13H, m), 1.42-1.87 (4H, m).
Example 253
(~)-Phenyl-acetic acid N'-{2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-[4-(2-
oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butyryl ] -hydrazide
H
O\ /N
~N ~ /
N J
N\
N / ~ O
H O NH O
HN
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.43 min, 630 (M+Na)+.
Example 254
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
310
(t)-1-[ 1,4'] B ipiperidinyl-1'-yl-4-[4-(8-fluoro-2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-
piperidin-1-yl]-2-(7-methyl-1 H-indazol-5-ylmethyl)-butane-1,4-dione
H F
O\ /N
~N ~ /
N
N/
N / O~ O
H N
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.18 min, 644 (MH)+. 'H NMR (400
MHz, CDC13) 8 8.00 (1H, s), 6.82-7.40 (6H, m), 4.48-4.70 (3H, m), 4.31 (2H,
s),
3.85-4.11 (2H, m), 3.65 (1H, m), 2.70-3.16 (5H, m), 2.53 (3H, s), 0.72-2.52 (
23H,
m).
Example 255
(~)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(7-methyl-1 H-indazol-5-ylmethyl)-4-[2',3'-
dihydro-
2'-oxospiro-(piperidine-4,4'-quinazoline]-butane-1,4-dione
0
HN- -NH
NJ ~~
N~ ( / O~ O
N
H ~ N
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.09 min, 612 (MH)+.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
311
2-Oxo-2,3-dihydro-benzooxazole-6-carbaldehyde
0
-o
HN
CHO
A solution of 6-bromo-3H-benzooxazol-2-one (0.9236g , 4.31 micromoles) in
anhydrous tetrahydrofuran (25 mL) and dimethylformamide (3 mL) under nitrogen
was cooled to -78°C before addition of n-butyllithium (2.5M in hexane)
(3.8 mL, 2.2
equiv). After stirring for 10 min at -78°C, 24 mL of sec-butyllithium
(1.4 M in
cyclohexane, 8 equiv) was added. The reaction was stirred while slowly warming
to
~0°C. When this temperature was reached, the reaction was quenched by
addition
of methanol. The reaction mixture was concentrated in vacuo and water was
added.
The aqueous layer was acidified with 1N HCl (ca. pH 5) and extracted with
ethyl
acetate (3 x 50 mL), dried over sodium sulfate, filtered and concentrated to
give the
product, 0.6402 g (91%). MS (ESI) 164 (MH)+. 'H NMR (400 MHz, DMSO-d6) 8
9.90 ( 1 H, s), 7.79 ( 1 H, d, J=8.0 Hz), 7.74 ( 1 H, s), 7.28 ( 1 H, d, J=8.0
Hz).
3-(2-Oxo-2,3-dihydro-benzooxazol-6-ylmethylene)-pentanedioic acid monomethyl
ester
0
HN
Prepared as described above for 2-(7-methyl-1H-indazol-5-ylmethylene)-succinic
acid 1-methyl ester (1.4 g, 90% yield). MS (ESI) 300 (M+Na)+.
(~)-3-(2-Oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-pentanedioic acid monomethyl
ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
312
O
v
HN
Prepared as described above for (~)-2-(7-methyl-1H-indazol-5-ylmethyl)-
succinic
acid 1-methyl ester (1.4 g, 99% yield). MS (ESI) 302 (M+Na)+.
(~)-4-Oxo-2-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-ylJ-butyric acid methyl ester
H
O\ /N
~N ~ /
O ~ N
0
N / O O O
H
Prepared as described above for (~)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-
[1',2'-dihydro-2'-oxospiro-(4H-3',1-benzoxazine-4,4'-piperidinyl]-butyric acid
methyl
ester. MS (ESI) 493 (MH)+.
(~)-4-Oxo-2-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-4-[2',3'-dihydro-2'-
oxospiro-(piperidine-4,4'-quinazoline)]-butyric acid methyl ester
0
HN"NH
o ~ NJ ~~
N / O O O
H
Prepared as described above for (t)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-
( 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butyric
acid methyl
ester. MS (ESI) 479 (MH)+.
(~)-4-Oxo-2-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-4-(2',3'-dihydro-2'-
oxospiro-(piperidine-4,4'-quinazoline)]-butyric acid
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
313
O
HN"NH
o \ NJ ~~
N / O' -OHO
Prepared as described above for (~)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-
[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butyric
acid. MS
(ESI) 465 (MH)+.
(~)-4-Oxo-2-(2-oxo-2, 3 -dihydro-benzooxazol-6-ylmethyl)-4-[4-(2-oxo-1,4-
dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butyric acid
O\ /N \
~N ~ /
O \ N
O
N / O' -OH
H
Prepared as described above for (~)-2-(7-methyl-1H-indazol-5-ylmethyl)-4-oxo-4-
[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidinyl]-butyric
acid. MS
(ESI) 479 (MH)+.
Example 256
(~)-1-(4-Cyclohexyl-piperazin-1-yl)-2-(2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-
4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
O\ 'N \
~N ~ /
O \ N
O ~ ~ / O~ O
N N
H
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
314
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.10 min, 629 (MH)+.
Example 257
(t)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butane-1,4-dione
H
O\ /N
~N ~ /
O
O
N
H
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.08 min, 629 (MH)+. 'H NMR (400
MHz, CDC13) S 9.89 (1H, s), 8.28 (1H, d, J=11.2 Hz),6.90-7.25 (5H, m), 6.75
(1H, d,
J=8.0 Hz), 4.40-4.79 (3H, m), 4.35 (2H, s), 2.27-3.98 (19H ,m), 1.46-2.10 (9H,
m),
1.36 (1H, m), 1.08 (1H, m), 0.12 (1H, m).
Example 258
(~)-1-[ 1,4']Bipiperidinyl-1'-yl-2-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
4-
[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-quinazoline)]-butane-1,4-dione
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
315
O
HN"NH
o ~ NJ ~ ~
o~ o
N N
H
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.02 min, 615 (MH)+.
Example 259
(~)-1-(4-cyclohexyl-piperazin-1-yl)-2-(2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-
4-[2',3'-dihydro-2'-oxospiro-(piperidine-4,4'-quinazoline)]-butane-1,4-dione
0
HN"NH
o ~ NJ ~~
,o
N N
H
Prepared as described above for (~)-1-[1,4']bipiperidinyl-1'-yl-2-(7-methyl-1H-
indazol-5-ylmethyl)-4-[ 1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-
piperidinyl]-butane-1,4-dione. LC/MS: tR=1.04 min, 615 (MH)+.
Example 260
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
(4-
cyclohexyl-piperazin-1-yl)-1-(7-methyl-1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
316
HN-N H
/ ~ O~ N \
\ ~ N ~ /
H
N NJ
~N O O
NJ
Prepared as described above for Example 16. IH-NMR (CD30D, 500 MHz) 8 0.81
( 1 H, in), 0. 89 ( 1 H, m), 1.02 ( 1 H, m), 1.1-2.0 ( 12H, m), 2.23 ( 1 H,
d), 2.47 ( 1 H, d),
2.61 (3H, s), 2.90 (4H, t), 3.08 (4H, m), 3.2-3.5 (4H, m), 3.82 (1 H, m), 4.14
(2H, d),
4.29 (2H, s), 4.40 (1H, t), 6.80 (1H, d), 6.95 (1H, t), 7.12 (3H, m), 7.47
(1H, s), 8.01
(1H, s). Mass spec.: 627.47 (MH)+.
Example 261
(~)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[4-(4-
fluoro-phenyl)-piperazin-1-yl]-1-(7-methyl-1H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
HN-N H
/ ~ O~N \
\ ~ N ~ /
H
N NJ
~N O O
N J
/
F
Prepared as described above for Example 16. LC/MS: tR=2.34 min, 621.42 (MH)+.
Example 262
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino]-propionic acid tert-butyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
317
N-NH
/ H
\ O~ N \
/ N I /
H
N NJ
O O O
A solution of (~)-3-(7-methyl-1H-indazol-5-yl)- 2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]- amino}-propionic acid (50 mg, 0.105
mmol)
and dicyclohexylcarbodiimide (25 mg, 0.12 mmol) in dimethylformamide was
stirred
for 30 min at room temperature, and then pentafluorophenol (26 mg, 1.3 mmol)
was
added. Stirring was continued at room temperature overnight, and then the
solvent
was removed, the residue was dried under high vacuum for 4 h. The crude
pentafluorophenyl ester was used without further purification in the next
step.
To a solution of tert-butyl alcohol (10 equiv.) in tetrahydrofuran at -
78°C under
nitrogen was added 1.4M sec-butyllithium in cyclohexane (10 equiv.). After 10-
15
min, a solution of pentafluorophenol ester (1 equiv.) in tetrahydrofuran was
added.
The reaction mixture was stirred at room temperature overnight. The solvents
were
removed in vacuo, and the residue was purified by preparative-HPLC to give the
desired compound. 'H-NMR(CD30D) 8 1.40 (s, 9H ) 1.56 (m, 4H ), 2.54 (s, 3H)
2.85(m,2H)3.OS(m,lH)3.19(m,lH)4.14(m,4H)4.44(m,2H)6.76 (d,J=7.68
Hz, 1 H ) 6.93 ( t, J=7.5 Hz, 1 H ) 7.10 (m, 3 H ) 7.14 (s, 1 H ) 7.97 (s, 1 H
). LC/MS
tR=2.19 min, 533.36 (MH)+.
Example 263
(~)-3-(7-Methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl cyclohexyl ester
N-NH
/ H
\ O~ N \
/ N I /
H
N NJ
O
O O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
318
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2- f [4-(2-
oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid tent-
butyl
ester. LC/MS: tR=2.47 min, 574.39 (MH)+.
Example 264
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-aza-bicyclo[2.2.2]oct-3-yl
ester
N-NH H
O~ N
/ '~N' ~ /
H
N NJ
O
O O
('N
To a solution of (~)-3-(7-methyl-1H-indazol-5-yl)- 2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidine-1-carbonyl]- amino}-propionic acid (50 mg, 0.105
mmol), EDCI (100 mg), and 4-dimethylaminopyridine (0.2 equiv.) in
dimethylformamide was added aza-bicyclo[2.2.2]oct-3-yl alcohol (0.525 mmol, 5
equiv.). The mixture was stirred at room temperature overnight. The solvent
was
removed in vacuo and the residue was dissolved in ethyl acetate, washed with
brine,
dried over magnesium sulfate, and purified by preparative HPLC to yield the
desired
compound. LC1MS: tR=1.62 min, 586.41 (MH)+.
Example 265
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid piperidin-4-yl ester
N-NH
H
O~ N
'~N ~ /
/N
H ~I
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
319
Prepared as described above for (~)-3-(7-methyl-1H-indazol-S-yl)-2-{[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid 1-aza-
bicyclo[2.2.2]oct-3-yl ester. LC/MS: tR=1.58 min, 560.37 (MH)+.
Example 266
(~)-4-(3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-
piperidine-1-carbonyl]-amino}-propionyloxy)-piperidine-1-carboxylic acid tert-
butyl
ester
N-NH
H
O\'N \
O ~N' I /
~ N
~O~ ~
O
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid 1-aza-
bicyclo[2.2.2]oct-3-yl ester. LC/MS: tR=2.38 min, 660.42 (MH)+.
Example 267
(~)-3-(7-Methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl ester
N-NH
/ H
\ O~ N \
N~ / N I /
H
N N
N
I~~//I\\O O O
Prepared as described above for (~)-3-(7-methyl-1 H-indazol-S-yl)-2- { [4-(2-
oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid 1-aza-
bicyclo[2.2.2]oct-3-yl ester. LC/MS: tR=1.67 min, 637.43 (MH)+.
Example 268
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
320
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino-propionic acid 1-diethylamino-1-methyl-ethyl
ester
N-NH
/ H
\ O~N \
I / N I /
H
N NJ
~N~O O O
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino-propionic acid 1-aza-
bicyclo[2.2.2]oct-3-yl ester. LC/MS: tR=1.66 min, 590.44 (MH)+.
Example 269
(t)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1,1-dimethyl-2-phenyl-ethyl ester
N-NH
/ H
\ O~ N \
/ N I /
H
N NJ
O
O O
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid t-
butyl
ester. LC/MS: tR=2.52 min, 609.46 (MH)+.
Example 270
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1,1-dimethyl-3-phenyl- propyl
ester
N-NH
/ H
\ O~ N \
/ N I /
H
N NJ
I \ O O O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
321
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2- f [4-(2-
oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonylJ-amino}-propionic acid t-
butyl
ester. LC/MS: tR=2.61 min, 623.48 (MH)+.
Example 271
(~)-3-(7-Methyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2 H-quinazolin-3-
yl)-
piperidine-1-carbonyl]-amino}-propionic acid ethyl ester
N-NH
/ H
\ O N \
H
N NJ
~O O
Prepared as described above for (~)-3-(7-methyl-1H-indazol-5-yl)-2-{[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino}-propionic acid t-
butyl
ester. LC/MS: tR=1.98 min, 505.32 (MH)+.
Example 272
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1-pyridin-4-yl-methylJ-2-oxoethyl]
-2', 3'-
dihydro-2'-oxospiro-[piperidine-4,4'-(1H)-quinazoline]-1-carboxamide
N-NH
/ O
HN- -NH
H
N' /N
O
~N O
H
N
Prepared as described above for Example 16. LC/MS: tR=1.49 min, 553.12 (MH)+.
Example 273
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1-pyridin-4-yl-piperazinyl]-2-
oxoethyl]-
2',3'-dihydro-2'-oxospiro-[piperidine-4,4'-(1 H)-quinazoline]-1-carboxamide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
322 _ .... ..
N-NH
/ O
HN' -NH
H
N"N
~N O O
NJ
N
Prepared as described above for Example 16. LC/MS: tR=1.56 min, 608.18 (MH)+.
Example 274
(~)-1-(7-Methyl-1H-indazol-5-ylmethyl)-2-[(2-dimethylamino-ethyl-ethyl
carbamoyl)-2-oxoethyl]-2',3'-dihydro-2'-oxospiro-[piperidine-4,4'-( 1 H)-
quinazoline]-
1-carboxamide
N-NH
O
HN' -NH
H
N"N
\N~N O O
Prepared as described above for Example 16. LC/MS: tR=1.58 min, 561.20 (MH)+.
Example 275
(~)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1-pyridin-4-yl-piperazinyl]-2-
oxoethyl]-
1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
N-NH
O
O' -NH
H
N' /N
~N O O
NJ
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
323
Prepared as described above for Example 16. LC/MS: tR=1.56 min, 609.14 (MH)+.
Example 276
(t)-1-(7-Methyl-1 H-indazol-S-ylmethyl)-2-[ 1-pyridin-2-yl-piperazinyl]-2-
oxoethyl]-
1',2'-dihydro-2'-oxospiro-[4H-3',1-benzoxazine-4,4'-piperidine]-1-carboxamide
N-NH
/ O
O_ -NH
H /
N' /N
~N O O
NJ
/N
Prepared as described above for Example 16. LC/MS: tR=1.57 min, 609.17 (MH)+.
Example 277
(R)-4-(2-Oxo-1,4-dihydro-2H-quinazolin-3yl)-piperidine-ld-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-methyl-1 H-indazol-5-ylmethyl)-2-oxo-
ethyl]amide
HN-N H
/ O N \
\ ~ N ~ /
H
N NJ
O
-N O
~N
Prepared as described above for Example 16. 'H-NMR (CD30D, 500 MHz) 8 -0.27
(1H, m), 0.75 (1H, m), 1.1-2.0 (12H, m), 2.10 (2H, m), 2.4-2.5 (3H, m), 2.57
(3H, s),
2.68 (2H, m), 2.92 (4H, m), 3.10 (4H, m), 3.9-5.1 (4H, several m), 6.82 ( 1 H,
d), 6.96
(1H, t), 7.18 (3H, m), 7.50 (1H, s), 8.05 (1H, s). LC/MS: tR=1.68 min, 627.42
(MH)+.
Example 278
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
324
(R)-1-(7-Methyl-1 H-indazol-5-ylmethyl)-2-[ 1,4-bipiperidin]-1-yl-2-oxoethyl]-
2',3'-
dihydro-2'-oxospiro-[piperidine-4,4'-( 1 H)-quinazoline]-1-carboxamide
N-NH
O
/ HN- -NH
H /
N' /N
~[O
~N O
Prepared as descr ibed above for Example 16. LC/MS: tR=1.63 min, 613.36 (MH)+.
Among other compounds envisaged within the present invention and capable
of being made according to the description provided herein or those methods
known
to those skilled in the art include the following prophetic examples:
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-bromo-1 H-indazol-5-ylmethyl)-2-oxo-ethyl]-
amide
HN-N H
Br / ~ O\ 'N \
\I ~N I/
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-oxo-1-
(2-
oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-
amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
325
O
HN-~ H
/ O O N
H
N NJ
~N O O
N J
N,J
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-oxo-1-
(2-
oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-2-piperidin-1-yl-ethyl]-amide
0
HN-~ H
/ O O N
N ~ /
H
N NJ
O
~N O
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-(4-
methyl-piperazin-1-yl)-2-oxo-1-(2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-
ethyl]-
amide
0
HN-~ H
/ O O N
N ~ /
H
N NJ
~N O O
iNJ
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(4-methyl-2-oxo-2,3-dihydro-benzooxazol-6-
ylmethyl)-2-
oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
326
O
HN~ H
/ O O N
\ ~ N ~ /
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-(4-
methyl-2-oxo-2,3-dihydro-benzooxazol-6-ylinethyl)-2-oxo-2-piperidin-1-yl-
ethyl]-
amide
0
HN-
H
/ o O N \
\ ~ N ~ /
H
N NJ
O
~N O
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-(4-
chloro-
2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-2-oxo-2-piperidin-1-yl-ethyl]-amide
0
HN-~ H
CI O O N
\ ~ N ~ /
H
N NJ
0
~N O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
327
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-
dimethylcarbamoyl-2-(4-methyl-2-oxo-2,3-dihydro-benzooxazol-6-yl)-ethyl]-amide
0
HN~ H
/ O O N \
\ ~ N ~ /
H
N NJ
\N O O
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-(4-
chloro-
2-oxo-2,3-dihydro-benzooxazol-6-yl)-1-dimethylcarbamoyl-ethyl]-amide
0
HN~ H
CI O O N
\ ~ N ~ /
H
N NJ
\N O O
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-(4-
methyl-2-oxo-2,3-dihydro-benzooxazol-6-ylmethyl)-2-oxo-2-(4-pyridin-4-yl-
piperazin-1-yl)-ethyl]-amide
0
HN~ H
/ O. O N \
\ ~ N ~ /
H
N NJ
~N O O
\ NJ
N,J
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
328
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [1-(4-
chloro-
2-oxo-2, 3-dihydro-b enzooxazol-6-ylinethyl)-2-oxo-2-(4-pyridin-4-yl-piperazin-
1-yl)-
ethyl]-amide
0
HN~ H
CI O O N
\ ~ N ~ /
H
N NJ
~N O O
\ NJ
N,J
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(4-ethyl-2-oxo-2, 3-dihydro-benzooxazol-6-
ylmethyl)-2-
oxo-ethyl]-amide
0
HN~ H
O O N
\ ~ N ~ /
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-methyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
329
O
HN~ H
/ NH O N \
\ ~ N ~ /
H
N NJ
O
-N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[1,4']bipiperidinyl-1'-yl-1-(7-chloro-2-oxo-2,3-dihydro-1 H-benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
0
HN~ H
CI NH O N
\ ~ N ~ /
H
N NJ
O
-N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-ethyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
0
HN-~ H
/ NH O N \
\ ~ N ~ /
H
N NJ
O
~N O
~N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
330
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(3-methyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
0
HN~ H
N~ O N
\ ~ N ~ /
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4'] b ipiperidinyl-1'-yl-1-(3, 7-dimethyl-2-oxo-2, 3-dihydro-1 H-
benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
0
HN-~ H
/ N' O N \
\ ~ N ~ /
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-chloro-3-methyl-2-oxo-2,3-dihydro-1 H-
benzoimidazol-
5-ylmethyl)-2-oxo-ethyl]-amide
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
331
O
HN~ H
CI N-- O N
\ ~ N ~ /
H
N NJ
O
~N O
~N
4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-
[ 1,4']bipiperidinyl-1'-yl-1-(7-ethyl-3-methyl-2-oxo-2, 3 -dihydro-1 H-
benzoimidazol-5-
ylmethyl)-2-oxo-ethyl]-amide
0
HN~ H
N ~- O N
\ ~ N ~ /
H
N NJ
O
~N O
~N
3-(7-Methyl-1 H-indazol-5-yl)-2-,{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid isopropyl ester
O NH
HN~ HN
/~ N
N ~N-
O
3-(7-Chloro-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid isopropyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
332
O NH
HN-~ HN
N ~N-
O
3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid isopropyl ester
O IH
HN~ HN
N--~N-
O
3-(7-Chloro-1 H-indazo l-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid tert-butyl ester
c1
O ~ ~ NH
HN~ /~ HN
N~N-~ O
O O
3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid tert-butyl ester
O ~ ~ NH
HN-~ HN
~N
N~N~ O
O O
3-(7-Chloro-1 H-indazol-S-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid cyclohexyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
333 .... .. . .. ..... ..... ...... .
O NH
HN-~ HN
N
N~N-
O
U
3-(7-Ethyl-1 H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid cyclohexyl ester
O ~ ~ NH
HN~ HN
/~ ~N
N~N~ O
O O
3-(7-Chloro-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl-piperidin-4-yl ester
O ~ ~ NH
HN~ HN
~N
N ~N -~ O
O O
N
3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl-piperidin-4-yl ester
O ~ ~ NH
HN-~ HN
/~ ~N
N~N~ O
O O
N
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
334
3-(7-Chloro-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl-cyclohexyl ester
c1
O NH
HN-~ HN
~ N
N~N
O
3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 1-methyl-cyclohexyl ester
O ~ ~ NH
HN~ HN
~N
N~N~ O
O O
3-(7-Chloro-1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 4-phenyl-cyclohexyl ester
0 0 /~
O ~N~N
HN/ ~ ~ NH ~/ ~NH
O
CI
3-(7-Ethyl-1 H-indazol-5-yl)-2- { [4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidine-1-carbonyl]-amino}-propionic acid 4-phenyl-cyclohexyl ester
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
335
O O
O ~N~N
HN / ~ ~ NH ~/ ~--NH
O
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
336
CGRP Binding Assay
Tissue Culture. SK-N-MC cells were grown at 37 °C in 5% COz as a
monolayer in
medium consisting of MEM with Earle's salts and L-glutamine (Gibco)
supplemented with 10% fetal bovine serum (Gibco).
Cell Pellets. The cells were rinsed twice with phosphate-buffered saline (155
mM
NaCI, 3.3 mM NazHP04, 1.1 mM KH2P04, pH 7.4), and incubated for 5-10 min. at 4
°C in hypotonic lysis buffer consisting of 10 mM Tris (pH 7.4) and 5 mM
EDTA.
The cells were transferred from plates to polypropylene tubes (16 x 100 mm)
and
homogenized using a polytron. Homogenates were centrifuged at 32,000 x g for
30
min. The pellets were resuspended in cold hypotonic lysis buffer with 0.1
mammalian protease inhibitor cocktail (Sigma) and assayed for protein
concentration.
The SK-N-MC homogenate was then aliquoted and stored at -80 °C until
needed.
Radioligand Binding Assay. The compounds of invention were solubilized and
carried through serial dilutions using 100% DMSO. Aliquots from the compound
serial dilutions were further diluted 25 fold into assay buffer (50 mM Tris-Cl
pH 7.5,
5 mM MgClz, 0.005% Triton X-100) and transferred (volume 50 p1) into 96 well
assay plates. ~lzsl]_CGRP (Amersham Biosciences) was diluted to 60 pM in assay
buffer and a volume of 50 p.1 was added to each well. SK-N-MC pellets were
thawed, diluted in assay buffer with fresh 0.1% mammalian protease inhibitor
cocktail (Sigma), and homogenized again. SK-N-MC homogenate (5 ~.g/well) was
added in a volume of 100 p1. The assay plates were then incubated at room
temperature for 2 h. Assays were stopped by addition of excess cold wash
buffer (20
mM Tris-Cl pH 7.5, 0.1% BSA) immediately followed by filtration over glass
fiber
filters (Whatman GF/B) previously soaked in 0. 5% PEI. Non-specific binding
was
defined with 1 pM beta-CGRP. Protein bound radioactivity was determined using
a
gamma or scintillation counter. The ICso was defined as the concentration of a
compound of invention required to displace 50% of radioligand binding.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
337
In the table below, results are denoted as follows: A <_ l OnM; l OnM < B 5
100 nM;
100 nM < C <_ 1000 nM; D > 1000 nM.
Table 4. CGRP Binding, cAMP Function and Ex Vivo Human Cerebral Artery Data
CGRP binding' cAMP Function' Cerebral Artery'
Example
# ICSO W) ICso W) ECso W)
1 C
2 A A A
3 B B B
4 B B
5 A A
6 A A A
7 C C
8 C C
9 B B
C B
11 B B
12 B C
13 C
14 D
C C
16 A A A
17 A A A
18 A B A
19 A A A
A A A
21 A A A
22 A A
23 A A A
24 B B
~ A ~ A ~ A
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
338
CGRP binding' cAMP Function' Cerebral Artery'
Example
# ICso W) ICso W) ECso W)
26 B B
27 B C
28 C
29 A
30 B
31 A A
32 C
33 C
34 A A
35 B B
36 B B
37 A B
38 B B
39 C C
40a A A
40b B
40c D
40d C
40e D
40f D
40g D
40h D
40i B
40j D
40k D
41a B
41b A
41c A
41d B
41e A
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
339
CGRP binding' cAMP Function' Cerebral Arterys
Example
# ICso (nM) ICso (~) ECso (~)
41f B
42 C
43 A A A
44 C
45 A
46 B B
47 A A A
48 D
49 A
50 A
51 D
52 D
53 D
54 B C A
55 C
56 A A
57 C
58 D
59 C
60 C
61 C C
62 B B
63 C C
64 B * B
65 A B B
66 C
67 B C B
68 A A A
69 A A A
70 A A A
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
340
CGRP bindings cAMP Function' Cerebral Artery'
Example
# ICso W) ICso W) ECso W)
71 A A A
72 A A A
73 B B
74 A A A
75 A B
76 B B A
77 B B
78 A A
79 B C
80 C
81 B C
82 B C
83 B C
84 B B
85 C
86 C B C
87 B B
88 C B
89 C B
90 B
91 C
92 B C
93 C
94 C C
95 C
96 D
97 D
98 D
99 D D
100 C
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
341
CGRP binding' cAMP Function' Cerebral Artery'
Example
# ICso W) ICso W) ECso W)
101 D
102 C
103 C
104 C
105 C
106 C
107 C
108 C
109 C
110 C
111 C
112 C
113 C
114 C
115 C
116 C
117 C
118 C
119 C
120 C
121 C
122 C
123 B
124 C
125 C
126 C
127 C
128 C
129 C
130 C
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
342
CGRP binding' cAMP Functionz Cerebral Artery
Example
# ICso (~) ICso (~) ECso (~)
131 C
132 C
133 C
134 C
135 C
136 C
137 C
138 C
139 C
140 B * *
141 C
142 C
143 C
144 C
145 C
146 B
147 C
148 B
149 B
150 B
151 C
152 C
153 C
154 C
155 C
156 C
157 C
158 B
159 B
160 C
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
343
CGRP binding' cAMP Functions Cerebral Arter
Example
# ICso (~I) ICSO (nNI) ECSO (~I)
161 B
162 C
163 C
164 C
165 C
166 C
167 C
168 C
169 C
170 C
171 B
172 B
173 C
174 C
175 C
176 B
177 B
178 B
179 C
180 C
181 C
182 C
183 C
184 B
185 C
186 C
187 C
188 C
189 C
190 C
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
344
CGRP bindings cAMP Function' Cerebral Artery]
Example
# ICso (~) ICso (~) ECso (~)
191 C
192 C
193 B
194 C
195 C
196 B
197 C
198 C
199 B
200 B
201 C
Cyclic AMP Assay
Functional Antagonism. Antagonism of the compounds of invention was determined
by measuring the formation of cyclic AMP (adenosine 3'S'-cyclic monophosphate)
in
SK-N-MC cells that endogenously express the human CGRP receptor. CGRP
receptor complex is coupled with Gs protein and CGRP binding to this complex
leads
to the cyclic AMP production via Gs - dependent activation of an adenylate
cyclase
(Juaneda C et al., TIPS, 2000; 21:432-438; incorporated by reference herein).
Consequently, CGRP receptor antagonists inhibit CGRP - induced cyclic AMP
formation in SK-N-MC cells (Doods H et al., Br J Pharmaco1,2000; 129(3):420-
423)
incorporated by reference herein). For cyclic AMP measurements SK-N-MC cells
were incubated with 0.3 nM CGRP alone or in the presence of various
concentrations
of the compounds of invention for 30 min at room temperature. Compounds of
invention were pre-incubated with SK-N-MC cells for 15 min before the addition
of
CGRP to allow receptor occupancy (Edvinsson et al., Eur J Pharmacol, 2001,
415:39-
44; incorporated by reference herein). Cyclic AMP was extracted using the
lysis
reagent and its concentration was determined by radioimmunoassay using RPA559
cAMP SPA Direct Screening Assay Kit (Amersham Pharmacia Biotech). ICSO values
were calculated using Excel fit. The tested compounds of invention were
determined
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
345
to be antagonists as they exhibited a dose - dependent inhibition of the CGRP -
induced cyclic AMP production. See Table 3 for summary of results.
Schild Analysis. Schild analysis can be used to characterize the nature of
antagonism
of the compounds of invention. The dose response of CGRP stimulated cAMP
production was generated either with CGRP alone, or in the presence of various
concentrations of compounds of invention. The antagonist dose is plotted as X
against the dose ratio (defined as IC50 of agonist with the presence of the
compounds
divided by the IC50 of the agonist alone) minus 1 as Y. Linear regression was
then
performed with both X and Y axis log-transformed. A slope that does not differ
significantly from unity (1) indicates competitive antagonism. Kb is the
dissociation
constant of the antagonist.
Table 5. Schild Ana~sis
Example Kb(nM) slope
#
2 0.16 0.94
3 55 0.96
5 3 0.92
6 0.36 0.93
16 1.3
17 1.1 0.92
18 1 0.8
21 0.018 0.89
43 0.018 1.2
45 1.4
47 0.1 0.93
69 0.016 1
70 0.71
71 2 0.87
See Figure 1. Schild Analysis.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
346
Ex Vivo Human Cerebral Artery Assay
Rationale and Overview. To provided direct evidence of the ability for novel
compounds to reverse CGRP-induced dilation in human cerebral vessels, an ex
vivo
assay was designed. Briefly, isolated vessel rings were mounted in a tissue
bath
where vessels were pre-contracted with potassium chloride (KCl ) and fully
dilated
with hCGRP, then this relaxation was reversed by the cumulative addition of
CGRP-
receptor antagonists (complete details follow).
Tissue Samples. Autopsy samples of human arteries were obtained from vendors
(ABS Inc. or NDRI). All vessels were transported on ice-cold HEPES buffer
(composition in mM: NaCI 130, KCl 4, KH2P04 1.2, MgS04 1.2, CaCl2 1.8,
Glucose 6, NaHC03 4, HEPES 10, EDTA 0.025). Upon receipt, the vessels were
placed in cold Kreb's buffer (composition in mM: NaCI 118.4, KCl 4.7, KH2P04
1.2, MgS04 1.2, CaCl2 1.8, Glucose 10.1, NaHC03 25) saturated with carbogen
(5%
C02 and 95 % oxygen).
Isolated Tissue Baths. The vessels were cleaned of connective tissues and cut
into
cylindrical segments of 4-5mm in length. The vessels were then mounted in
tissue
baths between two stainless steel hooks; one of which is fixed and the other
of which
was connected to a force displacement transducer. The vessel tension was
continuously recorded using a data acquisition system (Powerlab,
ADInstruments,
Mountain View, CA) connected to the transducer. The tissue baths containing
Krebs
buffer and mounted vessels were temperature (37 °C) and pH (7.4)
controlled with
continuous bubbling of carbogen. The artery segments were allowed to
equilibrate for
about 30-45 min until a stable resting tone was achieved. Prior to the assay,
vessels
were primed (conditioned) with 100 mM KCl and subsequently washed. The vessels
were pre-contracted with 10 mM KCl and fully dilated with 1 nM hCGRP.
Concentration-response curves to CGRP-receptor antagonists were performed by
the
cumulative addition of drugs in half log units in fully dilated vessels. At
each
concentration, the effects of the drugs were expressed as % reversal of CGRP-
induced relaxation in each vessel. The actual assay and data analysis were
performed
for each vessel individually, fitting the concentration-response data to a
four
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
347
parameter logistic function by non-linear regression analysis, to estimate the
EC50
values. A summary of results is provided in Table 3.
Non-Terminal Method for Assessing In Vivo Efficacy of Small Molecule CGRP-
receptor antagonists in Mammals
Overview. Blocking cerebral artery dilation induced by calcitonin gene-related
peptide (CGRP) has been proposed as a treatment for migraine headache,
however,
novel small molecule CGRP-receptor antagonists have shown species-specific
differences with relatively poor activity in rodents (Mallee et al. J Biol
Chem 2002
277:14294) requiring new models for assessment of in vivo efficacy. Non-human
primates (e.g., marmosets) are the only animals known to have human-like CGRP
receptor pharmacology conferred by the presence of the specific amino acid
residue
(Trp74) in their RAMP1 sequence which is responsible for the phenotype of the
human receptor (Mallee et al. J Biol Chem 2002 277:14294). Since current
migraine
models primarily use rats (Escott et al. Brain Res 1995 669:93; Williamson et
al.
Cephalalgia 1997 17:525), or are invasive, terminal procedures in primates
(Doods et
al. Br J Pharmacol 2000 129:420), a novel non-invasive, survival model in non-
human primates for in vivo efficacy assessment of CGRP-receptor antagonists as
in
the present invention is a significant contribution. While it is known that
trigeminal
activation increases both cerebral (Goadsby & Edvinsson, 1993) and facial
blood
flow (Doods et al., 2000), demonstration of a direct relationship between
facial blood
flow and cerebral artery dilation conducted in the same animals was not known.
Therefore, before initiating studies in non-human primates, laser Doppler
measurement of facial blood flow was directly validated in the rat as a
surrogate for
cerebral artery dilation in terminal studies that measured both cerebral
artery
diameter and changes in facial blood flow in the same animals (see Figure 2.
Direct
Validation of Facial Blood Flow as Surrogate for Cerebral Artery Dilation in
the
Rat). In both measures, comparable increases were induced by i.v. CGRP and
blocked by the peptide antagonist ha,CGRP(8-37). Next, the method of i.v. CGRP-
induced changes in facial blood flow was validated as a recovery model in
isoflurane
anesthetized rats using haCGRP(8-37). The survival method was then established
in
non-human primates and a dose-response study characterizing i.v. CGRP activity
was
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
348
completed (see Figure 3. Dose-Response for haCGRP in Non-Human-Primate Laser
Doppler Facial Blood Flow). Peptide and small-molecule CGRP-receptor
antagonists
were used to validate the non-human primate model. Pre-treatment with small
molecule antagonists or haCGRP(8-37) dose-dependently inhibited i.v. CGRP-
stimulated increases in primate facial blood flow (see Figure 4. Inhibitition
of CGRP-
Induced Changes in Non-Human Primate Facial Blood Flow), without altering
blood
pressure (see Figure 5. Effect of CGRP Antagonist on Non-Human Primate Blood
Pressure). Post-treatment of antagonists also reversed CGRP-induced increases
in
facial blood flow (not shown). This survival model provides a novel, non-
invasive
recovery procedure for evaluating prophylactic and abortive effects of CGRP-
receptor antagonists in non-human primates, or in transgenic animals with
humanized
RAMPl (Trp74) which have similar CGRP receptor pharmacology, as a surrogate
marker for activity in cerebral vessel diameter.
Animals. Adult male and female common marmosets (Callithrix jacchus) purchased
from Harlan and weighing 350-550 g served as subjects. Other mammals
endogenously expressing RAMP 1 having Trp 74 or transgenic mammals with
humanized RAMP1 having Trp 74 can also be employed in the method described
herein.
Anesthesia & Surgical Preparation. Animals are anesthetized by isoflurane
inhalation in an induction chamber (4-5% rapid induction, maintained with 1-
2.5%;
Solomon et al., 1999). Anesthesia is maintained by delivering a constant
supply of
air:oxygen (50:50) and isoflurane via face mask, or by intubation and
ventilation
(with blood gas monitoring). Body temperature is maintained at 38 ~ 0.5
°C by
placement on an automated temperature controlled surface with rectal probe. A
small
area of fur (approx. 1.5 cm square) is removed from one or both sides of the
face by
application of a depilatory cream and/or shaving. Surgical areas are clipped
and
prepared with betadine. An i.v. line is placed in any accessible vein for the
administration of test compounds and CGRP-receptor agonist and, if needed,
withdrawal of blood samples (max 2.5 ml, 10%) for blood gas monitoring and
content analysis. A solution of 5% dextrose is administered i.v. in order to
maintain
blood sugar levels. Anesthesia depth is monitored by measuring blood pressure
and
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
349
heart rate using a non-invasive arm cuff method and a pulse oximeter,
respectively.
Guanethidine 5-10 mg/kg i.v., supplemented with 5 mg/kg i.v. as needed, may be
given to stabilize the peak flux in facial blood flow seen with repeated
stimulation-
induced changes in blood flow (Escott et al., 1999; incorporated by reference
herein).
Microvascular blood flow is monitored by attaching a self adhesive laser
Doppler
flow probe to the facial skin.
Compound Administration Test compounds may be administered i.v. (0.01-5
ml/kg),
i.m. (0.01-0.5 ml/kg), s.c. (0.01-5 ml/kg) or p.o. (0.1-10 ml/kg) (Diehl et
al., 2001;
incorporated by reference herein). CGRP-receptor agonists may be delivered
i.v.
(0.01-5 ml/kg), i.d. (10-100 ul/site) or s.c. (10-100 ul/site).
Laser Doppler Flowmetry A control increase in facial blood flow is induced by
administration of a vasodilator, such as CGRP (0.05-100 ug/kg i.v.) or 2-20
pmol/site i.d) or adrenomedullin (ADM, 0.05-5 mg/kg i.v. or 10-100 pmol/site
i.d.).
Test compound or vehicle is administered either before (pre-treatment) or
after (post-
treatment) subsequent repeat administration of the vasodilating agent,
providing the
ability to assess prophylactic or therapeutic actions. Blood pressure is
monitored
continuously to ensure adequate depth of anesthesia, and anesthetic is
adjusted to
maintain stable levels that match pre-treatment values. During collection of
laser
Doppler flowmetry data, isoflurane may be reduced to 0.25-0.75% as previous
electrophysiologic studies in marmosets found that recordings were sensitive
to
isoflurane concentration (Solomon, 1999; incorporated by reference herein). To
reduce the number of animals used, the effect of test compound on i.v.
vasodilator-
induced changes in blood flow may be repeated up to 6 times in a single
session.
Recovery Animals are returned to the transport cage which is placed on a
temperature controlled surface to keep the animals warm until fully awake and
ambulatory. Animals may be tested again after 7-14 days rest, and may be
tested
repeatedly at 7-14 day intervals depending on the health of the animal.
See Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal
JM,
van de Vorstenbosch C. A good practice guide to the administration of
substances
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
350
and removal of blood, including routes and volumes. J Appl Toxicol. 2001 Jan-
Feb;21 ( 1 ):15-23; Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel
W,
Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small
molecule CGRP-receptor antagonist. Br J Pharmacol. 2000 Feb;129(3):420-3;
Edvinsson L. Calcitonin gene-related peptide (CGRP) and the pathophysiology of
headache: therapeutic implications. CNS Drugs 2001;15(10):745-53; Escott KJ,
Beattie DT, Connor HE, Brain SD. Trigeminal ganglion stimulation increases
facial
skin blood flow in the rat: a major role for calcitonin gene-related peptide.
Brain
Res. 1995 Jan 9;669(1):93-9; Goadsby PJ, Edvinsson L. The trigeminovascular
system and migraine: studies characterizing cerebrovascular and neuropeptide
changes seen in humans and cats. Ann Neurol. 1993 Jan;33(1):48-56; Lassen LH,
Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olsen J. CGRP may play a
causative role in migraine. Cephalalgia, 2002, 22, 54-61; Mallee JJ, Salvatore
CA,
LeBourdelles B, Oliver KR, Longmore J, Koblan KS, Kane SA. RAMP1 determines
the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem.
2002 Feb 14 [epub ahead of print]; Solomon SG, White AJ, Martin PR. Temporal
contrast sensitivity in the lateral geniculate nucleus of a New World monkey,
the
marmoset Callithrix jacchus. J Physiol. 1999 Jun 1 S;S 17 ( Pt 3):907-17; all
incorporated by reference herein.
Departures from Other Migraine Models. This invention represents a novel
migraine
model and is remarkably distinct from other migraine models. Some of the
distinguishing characteristics of the method of the present invention include:
(i) the
only survival model of migraine in any species; (ii) the only model to
demonstrate
the abortive (post-treatment) effects of CGRP antagonists on active induced
increases
in blood flow; (iii) the only demonstration of a direct relationship between
facial
blood flow and intracranial artery dilation carried out in the same animals;
(iv) the
only model to use non-invasive surgical techniques, and does not require
catheter
placement, intubation, or neuromuscular blockade; (v) the only primate model
to use
exogenous CGRP as the stimulus and demonstrate pretreatment blockade by CGRP
antagonism and post-treatment reversal by CGRP antagonism; (vi) the only
migraine
model to use isoflurane anesthesia in spontaneously breathing animals. The
models
described in Williamson et al., Sumatriptan inhibits neurogenic vasodilation
of dural
blood vessels in the anaesthetized rat-intravital microscope studies.
Cephalalgia.
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
351
1997 Jun;l7(4):525-31; Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL.
Intravital microscope studies on the effects of neurokinin agonists and
calcitonin
gene-related peptide on dural vessel diameter in the anaesthetized rat.
Cephalalgia.
1997 Jun;17(4):518-24; Escott KJ et al., Trigeminal ganglion stimulation
increases
facial skin blood flow in the rat: a major role for calcitonin gene-related
peptide.
Brain Res. 1995 Jan 9;669(1):93-9; Chu DQ et al., The calcitonin gene-related
peptide (CGRP) antagonist CGRP(8-37) blocks vasodilatation in inflamed rat
skin:
involvement of adrenomedullin in addition to CGRP. Neurosci Lett. 2001 Sep
14;310(2-3):169-72; Escott KJ, Brain SD. Effect of a calcitonin gene-related
peptide
antagonist (CGRPB-37) on skin vasodilatation and oedema induced by stimulation
of
the rat saphenous nerve. Br J Pharmacol. 1993 Oct;110(2):772-6; Hall JM, Siney
L,
Lippton H, Hyman A, Kang-Chang J, Brain SD. Interaction of human
adrenomedullin 13-52 with calcitonin gene-related peptide receptors in the
microvasculature of the rat and hamster. Br J Pharmacol. 1995 Feb;114(3):592-
7;
Hall JM, Brain SD. Interaction of amylin with calcitonin gene-related peptide
receptors in the microvasculature of the hamster cheek pouch in vivo. Br J
Pharmacol. 1999 Jan;126( 1 ):280-4; and Doods H, Hallermayer G, Wu D,
Entzeroth
M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the
first selective small molecule CGRP-receptor antagonist. Br J Pharmacol. 2000
Feb; 129(3):420-3 fail to possess the remarkable features of the method of the
present
invention.
In the table below, results are denoted as follows: W _< 25%; 25% < X <_ 50%;
50% <
Y < 75%; Z > 75%.
Table 6. Inhibition of CGRP-Induced Increase in Laser Doppler Facial Blood
Flow
in the Non-Human Primate (e.g., Common Marmoset)
CA 02549330 2006-06-02
WO 2005/065779 PCT/US2003/038799
352
Non-Human
Primate
(% Inhibition)
of CGRP-induced
(10
ug/kg,
iv) increase
in laser
Doppler
facial
blood
flow
Example 0.01 0.03 mg/kg,0.1 mg/kg,0.3 mg/kg,1 mg/kg,
# mg/kg, iv iv iv iv
iv
2 W X X Y Z
6 Z
16 ~ Y
69 Y Z
haCGRP Z
(8_37)
W <_
25%;
25%
< X
<_ 50%;
50%
< Y
<_ 75%;
Z >
75%.
See Figure 5. Effect of CGRP Antagonist on Non-Human Primate Blood Pressure.