Note: Descriptions are shown in the official language in which they were submitted.
CA 02549916 2011-12-21
1
Transdermal Delivery System of Hormones without Penetration Enhancers
Field of invention
The present invention relates to the field of pharmaceutical formulation
techniques. The
invention provides a low dosage pharmaceutical composition for transdermal
delivery of at
least one hormone, preferably a progestin, such as a gestodene, and optionally
an
estrogen, so as to achieve plasma concentration profiles effective in
inhibiting ovulation in
a woman.
Background
Transdermal delivery of estrogens and progestins for providing contraception
is a known
concept (Sltruk-Ware, Transdermal application of steroid hormones for
contraception, 3
Steroid Blochem Moiecul Biol, Volume 53, p 247-251). However, estrogens and
progestins
do generally poorly penetrate the skin, for which reason it is common, in
transdermal
systems, to Incorporate agents with skin penetrating enhancing effect.
The following documents describe a number of transdermal systems with a
progestin and
an estrogen present in the adhesive layer and wherein the need of penetration
enhancers
Is emphasised:
WO 92/07590 describes compositions with penetration enhancers for the
transdermal
delivery of Gestodene and an estrogen so as to achieve maximal plasma levels
of
Gestodene of about 0.9 ng/ml.
WO 97/397443 relates to a transdermal system containing between 30 to 60% of
penetration enhancers so as to deliver a contraceptively effective amount of
an estrogen
and a progestin.
WO 01/37770 relates to transdermal systems containing between 10 to 60 % of
penetration enhancers for delivery of Ethinylestradiol and Levonorgestrel in
contraceptively
effective amounts.
US 5,512,292 is directed to compositions comprising a contraceptIvely
effective amount of
Gestodene and an estrogen, such as Ethinylestradiol, together with a suitable
permeation
enhancer. The amount of the estrogen co-delivered is kept at a constant and
contraceptive
effective rate while the amount of Gestogen co-delivered varies depending on
the phase of
the menstrual cycle.
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
2
In US 5,376,377 comparable studies between transdermal systems with and
without
penetration enhancers are shown. The studies include an adhesive layer made of
ethylene
vinyl acrylate and as the active ingredient, Gestodene and an estrogen
(ethinylestradiol).The study results indicate the need of incorporation
penetration
enhancers in the adhesive layer so as to achieve contraceptive effective
amounts. Maximal
plasma levels of about 0.8 ng/m1 were achieved.
Finally, WO 90/04397 also discloses examples of compositions for transdermal
delivery of
gestodene, optionally in combination with an estrogen, such as
Ethinylestradiol, wherein
the composition further may comprise a penetration enhancer, such as 1,2-
propandiol or
isopropylmyristat. As adhesive layer is mentioned a number of various
polymers. Examples
on polar polymers (polyacrylates and silicones) in combination with a
penetration enhancer
are specifically disclosed. The resulting plasma levels of Gestodene at steady
state
conditions were approx. 250 to 337 pg/ml.
In addition to penetration enhancers, it is also suggested to add solubilising
agents or the
like to the drug-containing layer in order to increase the amount of dissolved
drug or to
add agents inhibiting the crystallisation of the drug in the layer.
For example, in US 6,521,250 is disclosed an adhesive layer that comprises a
mixture of
styrene-isoprene block copolymer and a hydrogenated resin acid or its
derivatives, the
amount of the resin being of 55-92%. Such an adhesive layer seems suitable for
the
transdermal delivery of estradiol in combination with a progestin in that such
systems have
a proper adhesive contact with the skin for long-term application and prevent
crystallisation of the hormones.
US 5,904,931 relates to ITS systems containing in the drug-containing layer a
steroid
(such as Gestodene) and dimethyl isosorbide. The latter enhances the
solubility of the
steroid in the drug-containing layer. The concentration of the Gestodene in
the drug-
containing layer may vary from 1-40 % by weight of the layer and the drug-
containing
layer may consist of adhesives such as polyacrylates, silicones, styrene-
butadiene co-
polymers and polyisobutylenes. Especially, polar polymers, such as
polyacrylates, are
suitable.
DE 199 06 152 relates to a transdermal drug delivery system in which Gestodene
is
embedded in a polar polymer, such as polyvinylpyrrolidone, methylcellulose,
ethylcellulose,
and hydroxypropylcellulose, previous to being added to an adhesive polymer,
such as
polyisobutylene. Thus, this transdermal drug delivery system is a twophase
system and
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
3
the drug-containing layer is not transparent because of the content of the
polar polymers,
which upon exposure to water will result in the presence of milky-white spots.
The amount
of Gestodene in the drug-containing layer is 5.1 % by weight of the drug-
containing layer.
In WO 02/45701 it is emphasised that the addition of a rosin ester in an
amount up to
25% by weight to an adhesive layer may sufficiently suppress crystal formation
of active
agents such as for example hormones. The adhesive layer may include all non-
toxic
natural and synthetic polymers known and which are suitable for use in
transdermal
systems, for example polyacrylates, polysiloxanes, polyisobutylenes, styrene
block
copolymers and the like. Particularly, polyacrylates are emphasised. The US
system is
suitable for steroids (Gestodene), which may be incorporated in the adhesive
layer in an
amount substantially at or near or even above the saturation with respect to
their
concentration in the carrier composition rather than substantially at
subsaturation.
Preferably, the amount of the steroid is from about 0.1% to about 6% by
weight, based on
the dry weight of the total carrier composition.
Unfortunately, penetration enhancers may adversely affect the skin such as
irritating the
skin, which to some extend renders transdermal systems unacceptable for the
user.
Additionally, it is generally known that penetration enhancers may negatively
affect the
stability of active substances rendering long-term storage problematic.
Furthermore, it is
also recognised that the viscosity is reduced by incorporation of penetration
enhancers
resulting in the risk of formation of dark rings along the edges of the patch.
Therefore, there is a need for transdermal systems without the above-mentioned
drawbacks, such as transdermal systems that do not require penetration
enhancers for
achieving therapeutically effective amounts of a steroidal hormone, such as
Gestodene.
Gestodene is a known orally active synthetic progestin with a progesterone-
like profile of
activity (see, U.S. Pat. No. 4,081,537). It is used as an oral contraceptive
in combination
with certain estrogens.
Summary of the invention
This invention relates to compositions suitably formulated for transdermal
delivery of
hormones, so that contraceptively effective levels are achieved without the
need of
incorporating penetration enhancers in the hormone-containing adhesive layer.
The actual
hormones are preferably steroidal hormones, such as progestins, such as a
Gestodene,
which optionally may be used in combination with an estrogen. Unlike the
general
knowledge in the art, the present inventors have provided transdermal systems
comprising
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
4
a limited number of ingredients. For example penetration enhancers and or
permeation
enhancers are not required in order to achieve high release rate and
therapeutically
effective plasma levels.
The present inventors have found that the selection of a drug-containing layer
that has a
solubility for the steroidal hormone (e.g. Gestodene) is critical for the
successful
achievement of therapeutically effective levels of a hormone in the blood.
Example 2
herein shows the comparison of two Gestodene-containing layers with respect to
their
release rate of Gestodene. It is clearly demonstrated that a drug-containing
layer of a
polar polymer (polyacrylate) requires a concentration of Gestodene of 3.9 % by
weight of
this layer to achieve the desired high release rate. Surprisingly, and in
contradiction to
what has previously been known, the same high release rate can be achieved
with a
concentration of 1.9 % by weight of Gestodene in a drug-containing layer
comprising a less
polar polymer, such as polyisobutylene, even without the use of a penetration
enhancer. Also
in-vivo studies have revealed that drug-containing layers containing the more
non-polar type of
polymer such as the polyisobutylene in preference to the polyacrylates are
better in terms of
achieving high plasma AUC (Example 4)
Thus, the present inventors have found, unlike what could be expected, that
the use of drug-
containing layers preferably containing a apolar polymer, such as
polyisobutylenes, and
characterised by having a limited solubility with respect to Gestodene of no
more than 3% by
weight have a high release rate of Gestodene despite the actual load of
Gestodene in the drug-
containing layer is low. This is clearly an advantage over the previously
known TT'S systems in
terms of decreasing the risk of skin irritation, decreasing the exposure of
hormone to the user
and to the environment.
A further advantage is that the drug-containing layer is a monophasic system
and contains the
drug evenly distributed throughout the layer. That is to say that the drug-
containing layer is
homogenous. In absence of polar polymers or other polar additives with
tendency to absorb
water or to retain water, the drug-containing layer is transparent. Thus, a
transparent
composition, wherein the skin can be visually inspected through the drug
delivery system,
has been provided by the present invention. Transparency of a transdernnal
drug delivery
system is a clear advantage for the user because non-transparent systems are
visible and
thus indicate sickness, which is not the intention with a contraceptive patch.
Accordingly, a first aspect of the invention relates to compositions for
transdermal delivery
of a steroidal hormone, preferably a progestin, such as Gestodene or a
derivative thereof
(ester thereof), and optionally an estrogen. The composition comprises a drug-
containing
layer comprising said hormone and one or more pharmaceutically acceptable
excipients or
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
carriers, and the drug-containing layer having a solubility for said hormones
(such as
Gestodene) of no more than 3% by weight of the drug-containing layer.
In a particular aspect thereof, the invention relates to a composition
comprising a
5 progestin, such as Gestodene or a derivative thereof, and a polymer in an
amount of from
about 15 to 99 % by weight of the drug-containing layer, the polymer being
selected from
the group consisting of hydrocarbon polymers, polysiloxanes, poly-acrylates
and mixtures
thereof that form a drug-containing layer having a solubility for Gestodene of
no more than
3% by weight of the drug-containing layer.
In another particular aspect thereof, the invention relates to compositions
comprising a
drug-containing layer consisting essentially of a progestin such as Gestodene
or a
derivative thereof, a polymer in an amount of from about 15 to 99 13/0 by
weight of the
drug-containing layer, a tackifier, such as a rosin ester in an amount of up
to 85% by
weight, such as in an amount in the range of 1-85% of the drug-containing
layer, and
optionally an estrogen.
In a still particular aspect, the invention relates to a composition for
transdermal delivery
comprising a drug-containing layer that comprises:
i) a progestin, preferably a Gestodene or derivative thereof, such as an ester
thereof; and
ii) a polymer selected from the group consisting of polyisobutylenes,
polybutenes,
polyisoprenes, polystyrenes, styrene isoprene styrene block polymers, styrene
butadiene
styrene block polymers and mixtures thereof.
A still particular aspect of the invention relates to a transparent
composition for
transdermal delivery, the composition comprises a drug-containing layer that
comprises a
progestin, preferably a Gestodene or derivative thereof (such as an ester
thereof) and the drug-
containing has a solubility for Gestodene of no more than 3% by weight of the
drug-
containing layer.
It has surprisingly been found that relatively high plasma levels of Gestodene
can be
maintained for a prolonged period of time by administering a Gestodene
formulated in a
composition of the invention. The plasma profile and plasma levels of
Gestodene, as
resulting from the administration of a Gestodene and optionally an estrogen,
are effective
in inhibiting the ovulation In a woman.
Therefore compositions of the invention may be used for inhibiting ovulation
or alternatively for
the treatment of endometriosis, pre-menstrual syndrome, climacteric disorders,
prevention
of osteoporosis, regulating the menstrual cycle or stabilising the menstrual
cycle.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
6
Thus, in still further aspects, the invention relates to the use of a
composition as described
herein optionally in combination with an estrogen, for inhibition of ovulation
in a female.
When the composition is singly administered, then a plasma concentration-time
curve of
Gestodene is achieved characterised by having plasma levels of Gestodene in a
concentration of at least 1.0 ng/ml, as determined at steady state conditions.
In
association herewith, the invention relates to a method for inhibiting
ovulation in a female,
such as a woman. The method comprises administering topically to the skin or
mucosa an
effective amount of Gestodene or a derivative thereof, optionally in
combination with an
estrogen, so as upon singly administering said Gestodene, then a plasma
concentration-
time curve of Gestodene is achieved characterised by having plasma levels of
Gestodene in
a concentration of at least 1.0 ng/ml, as determined at steady state
conditions.
Finally, the invention relates to a kit comprising 1 to 11 dosage units, such
as 9 or 3
dosage units, dependent on the length of the treatment period, formulated in a
form for
transdermal delivery of Gestodene or a derivative thereof, such in a form of a
composition
as described herein. The dosage form comprising a drug-containing layer
comprising
Gestodene and one or more pharmaceutically acceptable excipients or carriers,
wherein'
the drug-containing layer has a solubility with respect to Gestodene of no
more than 3%
by weight of the drug-containing layer.
Detailed description
The invention provides compositions for transdermal delivery of hormones
(transdermal
therapeutic system) that upon topical application to the skin or a to a mucosa
results in
therapeutically effective amounts, such as contraceptively effective amounts
of the
hormones, although skin penetration enhancers are not necessarily incorporated
in the
drug-containing layer.
As used herein, the term "topical" or "topically" denotes the direct contact
of the
composition with a surface area of a mammal including skin and mucosa.
The term "mucosa" means any surface membrane on a mammal, which is not skin,
such
as a surface present in the buccal cavity, vagina, rectum, nose or eye. Thus,
the mucosa
may be buccal, vaginal, rectal, nasal or ophthalmic mucosa.
The compositions of the invention may be designed in several various
application forms
provided that the composition comprises a drug-containing layer, which is
adapted to be
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
7
placed near to or in direct contact with skin or mucosa upon topically
administering the
composition.
Therefore, in a preferred application form, the composition, e.g. the
transdermal
therapeutic system, consists essentially of
a) a backing layer;
b) at least one drug-containing layer comprising said hormone or a mixture of
said
hormones and one or more pharmaceutically acceptable ingredients; and
c) optionally a removable release liner or protective layer
Preferably, the backing layer, the drug-containing layer and the removable
release liner (or
protective layer) are transparent, which means that the skin is visible.
In case the drug-containing layer fails to exhibit sufficient self-tackiness
to the skin or
mucosa, it may be provided with an additional layer of a pressure-sensitive
adhesive layer
or with a pressure-sensitive adhesive edge or ring so as to ensure adherence
of the
composition to the skin over the whole application period. The pressure-
sensitive adhesive
layer may be located between the drug-containing layer and the skin and the
adhesive ring
may be located around or at the edge of the drug-containing layer. Optionally,
the
composition may also additionally comprise one or more membranes or adhesive
layers.
For example, a membrane for controlling the release of the hormones can be
located
between the drug-containing layer and the pressure sensitive layer or between
the drug-
containing layer and the skin.
The size of the drug-containing layer is selected from a variety of reasonable
sizes. As
used herein, a reasonable size is meant to be a surface area of from about 5
to 20 cm2,
preferably of from about 7 to 15 cm2, most preferably of from about 8 to 12
cm2, such as
10 cm2. Notably, the surface area is the area that is in contact with or in
close proximity to
the skin or mucosa.
According to the invention it is found that a drug-containing layer with
minimised solubility
for the hormone provides sufficient skin penetration of a steroidal hormone.
In the present
invention, the drug-containing layer is characterised by defining Its
solubility for
Gestodene. Notably, the skin penetration rate suffices without the need of
incorporation of
a skin penetration enhancer. For example, it is surprisingly found that the
skin penetration
of a steroidal hormone of the invention results in therapeutically effective
amounts of the
steroidal hormone in the circulating blood, such as contraceptively effective
amounts of the
hormone.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
8
Accordingly, the invention relates in a first aspect to a composition for
transdermal
delivery of a steroidal hormone, such as a progestin, e.g. Gestodene or a
derivative
thereof. The composition comprises a drug-containing layer and one or more
pharmaceutically acceptable excipients or carriers and the drug-containing
layer has a
solubility for Gestodene of no more than 3% by weight of the drug-containing
layer. The
composition may optionally comprise an estrogen.
In some embodiments thereof, the solubility of the drug-containing layer for
Gestodene is
no more than 2.5 A) by weight of the drug-containing layer, preferably no
more than 2.0
%, such as no more than 1.8%. In principle, the solubility could be very low,
but it is
considered that the critical level with respect to the lowest level of said
solubility is about
0.1%, such as about 0.2%, 0.3%, 0.5%, 0.7%, 0.8%, 0.9% or 1% by weight of
Gestodene in the drug-containing layer. It is generally considered that the
solubility of the
drug-containing layer for Gestodene is in the range between about 0.1% and 3%,
such as,
about 0.2% and3%, 0.40/0 and30/0, 0.5% and30/0, 0.8% and30/0 or 1% and30/0. In
further
embodiments thereof, the upper limit in the range is not 3 /o by weight but
lower, such as
2.5% 2.2% or 2.0%.
As used herein, the term "a gestodene" is denoted to mean Gestodene (130-Ethyl-
17a-
ethyny1-1713-hydroxy-4,15-gonadien-3-one), a derivative thereof or a mixture
thereof,
such as mixtures of the derivatives or a mixture of Gestodene and a
derivative. The
derivative may be a derivative of the 1713-hydroxy group, such as ether,
ester, acetal or a
pharmaceutically acceptable salt thereof. For example an ester with 2 to 12
carbon atoms
in the acyl radical, including alkanoates with 2 to 8 carbon atoms in the
alkanoyl radical.
In preferably embodiments of the invention, an ester of Gestodene is Gestodene
propionate, Gestodene valerate, and/or Gestodene capronate, which aredescribed
In US
5,858,394.
As mentioned, the inventors have found that a suitable drug-containing layer
of the
invention is one that has solubility for the steroidal hormone (e.g.
Gestodene) of no more
than 3% by weight of the drug-containing layer.
The phrase " a solubility fora steroidal hormone, such as " a solubility
forGestodene, of no
more than 3% by weight of the drug-containing layer" is meant to characterise
the
quantity of the steroidal hormone, such as Gestodene that can dissolve in a
particular
drug-containing layer yielding a visually clear solution. The term (a
solubility for) should
not be understood as meaning the actual concentration of hormone, such as
Gestodene, in
the drug-containing layer. As described below, the total concentration of
hormone in the
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
9
drug-containing layer may be above or below 3% by weight of the drug-
containing layer.
Furthermore, the total concentration of hormone in the drug-containing layer
may give rise
to a drug-containing layer comprising the hormone at saturated or sub-
saturated levels.
In order to select drug-containing layers having the specified solubility for
a hormone, such
as Gestodene, the following test may be carried out in order to determine
whether the
hormone is completely dissolved and the resulting drug-containing layer is
visually clear.
One test method is to determine whether the hormone is completely dissolved in
that solid
particles cannot be detected visually or by using a microscope having a
magnification of
25X.
Another method is to actually determine the the solubility of the drug-
containing layer for
the hormone by the following method. The method is based on the determination
of the
release rate constants of the hormone from a drug-containing layer comprising
the
hormone on completely dissolved form and from a drug-containing layer with the
hormone
partially dissolved.
The method includes the following steps:
Firstly, identical drug-containing layers, but with various, such as
increasing, amounts of
the hormone, are to be manufactured. As a rule, at least 3 of the various drug-
containing
layers should contain the hormone in a form completely dissolved in the drug-
containing
layer and at least 3 drug-containing layers should contain the hormone as
partially
dissolved, e.g. with the hormone in the form of solid particles.
As concerning the manufacturing process, the following steps can be followed.
Any other
method known in the art to manufacture adhesive layers may also be
appropriate:
- The drug substance is dissolved or suspended in an appropriate solvent.
- The polymer, optionally the tackifier and other excipients are dissolved in
an
appropriate solvent.
- The two solutions are combined while stirring, so as to obtain a
homogeneous mixture.
- The mixture is coated onto a release liner in an appropriate thickness
and dried under
heat to evaporate the solvents.
- The resulting laminate is covered with the backing foil.
Secondly, the release rate of the drug from the drug-containing layer is
tested using the
equipment and test conditions given in the relevant section of the European
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
Pharmacopoeia (Ph.Eur. 2.9.4. Dissolution test for transdermal patches) or the
United
States Pharmacopoeia (USP 26, <724> DRUG RELEASE, apparatus 5 (Paddle over
Disk).
- A sample with a defined area is cut from the drug-containing layer.
- After removal of the release liner the sample is placed in a vessel (as
described in the
5 Pharmacopoeia mentioned above) which is previously filled with an
appropriate
dissolution media equilibrated at 32 "C 0.5 C. The drug-containing layer
should be in
contact with the dissolution media. Samples of the dissolution media are taken
at pre-
defined time intervals and the amount of hormone dissolved in the dissolution
media is
measured using HPLC or other appropriate quantification methods. The
dissolution
10 media can be selected from those ensuring sink conditions. For example,
suitable
dissolution media are aqueous solutions containing up to 30% by weight of
organic
solvents, such as ethanol, isopropanol and dioxane.
Thirdly, the release rate of hormones from the two types of drug-containing
layers is
determined as follows:
- the amount of hormone released per area unit of each of the drug-
containing layer is
calculated from the dissolution data mentioned above and the specific area of
the drug-
containing layer.
- the release rate constant for each drug-containing layer is determined as
the slope
obtained by the linear regression analysis of the at last three data points on
the amount
of hormone per area unit versus the square root of time.
- The release-rate constant for each of the drug-containing layers is
plotted against the
concentration of hormone in the drug-containing layer. Linear regression
analysis is
then carried out separately for the drug-containing layers with completely
dissolved
hormone and for drug-containing layers with partially dissolved hormone, i.e.
with solid
particles of hormone present. The two regression lines will cross each other
at a point
The concentration of the hormone that can be read at the cross point of the
two
regression lines denotes the "solubility" of the hormone in the drug-
containing layers.
The term "drug-containing layer" is meant to denote that part of the
transdermal
composition or system wherein the steroidal hormone is present. The drug-
containing layer
can be in semi-solid or solid form and comprises the hormone formulated
directly in the
layer. The hormone of the invention may be dispersed, partly dispersed, partly
dissolved or
fully dissolved in it dependent on the concentration and the physico-chemical
properties of
the hormone. The drug-containing layer is not meant to be in the form of a gel
or a liquid.
Intentionally, the drug-containing layer is meant to be in direct contact with
the skin or
mucosa. However, in some embodiments, there is an additional layer, a so-
called no-drug-
containing layer, located between the drug-containing layer and the skin or
mucosa.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
11
As said, compositions according to the invention do not necessarily comprise a
skin
penetration enhancer. Thus, in some embodiments of the invention, the drug-
containing
layer exclude the presence of a skin penetration rate enhancer, which means
that the
drug-containing layer consists essentially of ingredients not including a skin
penetration
rate enhancer. This means that for example less than 2%, such as less than 1%,
preferably less than 0.5%, such as less than 0.2%, such as less than 0.1% by
weight of
the drug-containing layer is composed of a skin penetration enhancer.
According to the present invention, the selection of the proper drug-
containing layer
effects the skin penetration. The proper drug-containing layer is preferably
made of a
polymer or mixtures of polymers. The polymers may have adhesive properties or
may be
without noticeable adhesive properties. In some embodiments, the drug-
containing layer is
a so-called pressure sensitive adhesive layer.
In principle any mixture of polymers resulting in the said solubility for
Gestodene may be
applied. Thus, in one embodiment of the invention the drug-containing layer
comprises at
least one polymer that may have adhesive properties or not. Typically, such
polymers are
biologically acceptable lipophilic polymers of the types of a hydrocarbon
polymer, a
polysiloxane, a poly-acrylate, or mixtures thereof. Preferably, the polymers
may be
selected from those hydrocarbon polymers, polysiloxanes and/or poly-acrylates
that form a
drug-containing layer having a solubility forGestodene, of no more than 3 /o
by weight of
the drug-containing layer.
The amount of the polymer is to some extent a critical parameter. The proper
amount may
depend On the actual type of polymer and the steroidal hormone in use. In
general, the
amount of the polymer is at least 1% by weight of the drug-containing layer,
such as at
least 5%, 10%, 15% or 20%. However, preferably, the polymer is present in the
drug-
containing layer in an amount of at least 25%, 30%, 35%, 40%, 45%, 50%, 55%,
60%,
65%, 70%, 75% or at least 80% by weight of the drug-containing layer. In other
words,
the polymer can be used in an amount of from about 1 to 99 % by weight of the
drug-
containing layer, such as from about 5 to 99%, 10 to 99 %, 15 to 99% or 20 to
99 Gk.
Preferably, the polymer is present in the drug-containing layer in an amount
ranging from
about 15 to 99%, such as from about 15 to 90%, 15 to 85% or 15 to 80%, such as
from
about 20 to 85%, 20 to 75%, such as from about 25 to 85 %, 25 to 75% by weight
of the
drug-containing layer.
This being said, a particular aspect of the invention is directed to a
composition for
transdermal delivery of a steroidal hormone, such as a Gestodene or a
derivative thereof,
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
12
and optionally an estrogen, the composition comprising a drug-containing layer
that
comprises
- at least one steroidal hormone, such as a Gestodene or a derivative thereof
and one or
more pharmaceutically acceptable ingredients or carriers;
- a polymer or a mixture of polymers, preferably in an amount of from about 15
to 99 %
by weight of the drug-containing layer,
wherein the polymer is being selected from the group consisting of hydrocarbon
polymers,
polysiloxanes, poly-acrylates and mixtures thereof that form a drug-containing
layer having a
solubility with respect to said steroidal hormone of no more than 3% by weight
of the
drug-containing layer.
As mentioned, it has been found that adhesive layers comprising the more non-
polar types
of polymers, such as the hydrocarbon polymers, have been shown to be superior
to the
polar type polymers, such as the polyacrylates (Example 2). Therefore in a
preferred
embodiment of the invention, the drug-containing layer comprises as the
polymer a
hydrocarbon polymer, which preferably may include polyisobutylenes,
polybutenes,
polyisoprenes, polystyrenes, styrene isoprene styrene block polymers, styrene
butadiene
styrene block polymers and/or mixtures thereof.
As mentioned above, in some embodiments of the invention, the drug-containing
layer is
adhesive. Preferentially, the polymer of the drug-containing layer has
suitable adhesive
properties so that no further sticky agent, such as a tackifier is required.
Whenever it is
considered necessary to improve the adhesive strength of the drug-containing
layer, the
layer further comprises a tackifier.
The term" tackifier" denotes an agent that improves the adhesive strength of
the adhesive
layer to the skin or mucosa.
Examples of tackifiers are selected from hydrocarbon resins, rosin resins and
terpene
resins. Examples of hydrocarbon resins are commercially available under the
tradename
Escorez from ExxonMobil; Regalite , Piccotac and Picco from Eastman or
Indopol
from BP. Examples of rosin esters suitable for transdermal systems according
to the
present invention include esters of hydrogenated wood rosin e.g.
pentaerythritol ester of
hydrogenated wood rosin, esters of partially hydrogenated wood rosin e.g.
pentaerythritol
esters of partially hydrogenated wood rosin, esters of wood rosin, esters of
modified wood
rosin, esters of partially dimerized rosin, esters of tall oil rosin, esters
of dimerized rosin,
and similar rosins, and combinations and mixtures thereof. Such rosin esters
are
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
13
commercially available under the tradenames Foral , Foralyn , Pentalyn ,
Permalyn
and Staybelite .
In a preferred embodiment of the invention, the drug-containing layer
comprises a tackifier
in the form of a a rosin ester such as pentaerythritol ester.
It is generally considered that the tackifier can be present in any suitable
amount as long
as the said critical solubility of the steroidal hormone in the drug-
containing layer is not
affected noticeable. Thus, a tackifier may be present in the drug-containing
layer in an
amount of from about 0.1 to 95 0/0, such as 0.5% to 95%, such as 1% to 95% by
weight
of the drug-containing layer. That is to say that the tackifier can be present
in an amount
of from about 1% to 85%, 1 to 75 %, 1 to 65 %, 1 to 55 %, 1 to 50 %, 1 to 45
%, 1 to 40
% or more preferably of about 1 to 35 % such as preferably 1 to 30 %, more
preferably 1
to 25%. Obviously, the amount of tackifier in the drug-containing layer may be
critical to
the solubility of the steroidal hormone in the drug-containing layer. Thus, in
still other
embodiments of the invention, the tackifier is present in the drug-containing
layer in an
amount of up to 35%, such as of up to 30%. More preferably, the amount of
tackifier is up
to 25%, such as up to 20% or 15%, more preferably of up to 10%, 7%, 5% by
weight of
the drug-containing layer.
It follows that a further particular aspect of the invention relates to a
composition for
transdermal delivery of Gestodene or an ester thereof, and optionally an
estrogen, the
composition comprising a drug-containing layer consisting essentially of:
- at least one steroidal hormone, such as a gestodene, and one or more
pharmaceutically
acceptable ingredient(s) and/or carrier(s) ;
- a polymer or a mixture of polymers, preferably in an amount of from about 15
to 99 %
by weight of the drug-containing layer, the polymer is preferebly selected
from the group
consisting of hydrocarbon polymers, polysiloxanes, poly-acrylates and mixtures
thereof, most
preferably hydrocarbon polymers (polyisobutylenes, polybutenes, polyisoprenes,
polystyrenes,
styrene isoprene styrene block polymers, styrene butadiene styrene block
polymers and/or
mixtures thereof). As mentioned the drug-containing layer has a solubility for
Gestodene as
mentioned above;and
- a tackifier in an amount of up to 85% by weight of the drug-containing
layer; and
- optionally an estrogen.
As being said, the drug-containing layer is to be composed of ingredients
forming a layer
wherein the solubility criterion for Gestodene is met. Preferably, this
criterion is met when
the drug-containing layer is mainly composed of the hormones of the invention
together
with a polymer or a mixture of polymers and optionally a tackifier.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
14
In some embodiments, it is found that a suitable polymer of the invention is
lipophilic and
essentially without any presence of free hydrophilic groups. Thus, any
suitable
hydrocarbon polymer, polysiloxane or poly-acrylate is selected from those
polymers with a
limited amount of functional groups in the side chains, such as free
hydrophilic functional
groups, such as carboxylic-, ester-, hydroxy-, amino-, amide, halogen- or
sulfo- groups.
Therefore, in some embodiments, the polymer essentially excludes poly-
acrylates, of which
some comprise free carboxylic and/or hydroxyl groups.
In preferred embodiments, the polymer is a hydrocarbon polymer, a poly-
siloxane or a
mixture of the two types of polymers. As mentioned, the hydrocarbon type
polymers are
of the non-polar type polymers essentially without free hydrophilic functional
groups, such
as carboxylic-, ester-, hydroxy-, amino-, amide, halogen- or sulfo- groups in
the side
chains. Therefore, in a most preferred embodiment, the polymer is a
hydrocarbon polymer.
A plethora of hydrocarbon polymers exist of both high molecular weight and low
molecular
weight or mixtures thereof.. Typically, the hydrocarbon polymers are in the
form of
polyisobutylenes, polybutenes, polyisoprenes, polystyrenes, styrene isoprene
styrene block
polymers, styrene butadiene styrene block polymers or mixtures thereof. The
molecular
weight of high molecular weight polymers (at least with respect to
polyisobutylene) is
usually within the range of 500,000 to 2,000,000 Da, whereas that of low
molecular weight
is of range from about 20,000 to 100,000 Da. Typically, such polymers comprise
a mixture
of high molecular weight polymers and low molecular weight polymers wherein
the amount
of the low molecular weight polyisobutylene in the total mixture is at least
50%.
In preferred embodiments, the hydrocarbon polymer is a polyisobutylene,
polybutene,
polyisoprene, most preferably polyisobutylene.
In some embodiments of the invention the polymer excludes isoprene co-
polymers.
The poly-siloxanes are typically high molecular weight polydimethylsiloxanes
with free
silanol groups or endcapped silanol groups (Bio-PSA ).
Typical examples of poly-acrylates, which as mentioned above may be excluded
or used in
minimal amounts in some embodiments, include polymers selected from
homopolymers of
acrylic esters, copolymers of two or more types of acrylic ester units or
copolymers of
acrylic esters or other functional monomers. Acrylic esters include, but are
not limited to,
butyl methacrylate, pentyl methacrylate, hexyl methacrylate, heptyl
methacrylate, octy
methacrylate, nonyl methacrylate, decyl methacrylate. For example
ethylene/ethylacrylate
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
copolymers, polymethacrylate polymers and polysiloxane-polymethacrylate
copolymers. As
mentioned, functional monomers containing a hydrophilic functional group may
be
excluded as a suitable polymer, such as hydroxyethyl methacrylate,
hydroxypropyl
methacrylate, etc. and monomers containing an amide group such as
methacrylate,
5 dimethylnnethacrylamide.
Moreover, for the same reason polymers such as polyalkylenes (polyethylene,
polypropylene, ethylene/propylene copolymers, chlorinated polyethylene,
polytetrafluoroethylene), polyacetates (ethylene/vinyl acetate copolymers,
vinyl chloride-
10 vinyl acetate copolymer), polyvinylenes (polyvinylidene chloride, ethylene-
vinyl alcohol
copolymer, ethylene-vinyloxyethanol copolymer, polyvinylpyrrolidone)
polycarbonates,
cellulose polymers (methyl or ethyl cellulose derivatives, hydroxypropyl
methyl cellulose
and cellulose esters) may not be appropriate polymers according to the
invention and are
to be excluded or at least used in a restricted amount in some embodiments of
the
15 invention.. The exclusion does not however prevent the use of such polymers
in other
layers or parts of the composition.
Furthermore, in some embodiments, the drug-containing layer does not comprise,
or at
least only comprise a restricted amount of, a hydrophilic polymer, such as
crystallisation
inhibitors like polyvinylpyrrolidone, cellulose polymers, such as methyl or
ethyl cellulose
derivatives or hydroxypropyl methyl cellulose, or mixtures thereof.
Also In some embodiments a solubiliser, such as dimethyl-isosorbide, is not
present in the
drug-containing layer of at least only present in a restricted amount.
By the term "restricted amount" is meant that the polymer or solubillser in
question is
present in a concentration in the drug-containing layer of less than 10%, such
as less than
8, 5, 3, 2, 1, 0.5 or 0.2% by weight of the layer.
As mentioned, it has been possible to provide sufficient skin penetration of a
steroidal
hormone without incorporating a skin penetration enhancer and/or permeation
enhancer.
That Is to say that in interesting embodiments of the invention, a skin
penetration
enhancer and/or permeation enhancer is excluded in the drug-containing layer
or present
in the drug-containing layer in a restricted amount, this amount being less
than 5%, such
as less than 4%, 3%, 2%, 1%, 0.5%, or 0.2% by weight of the drug-containing
layer.
The terms "skin penetration enhancers" and "permeation enhancers" are in the
present
invention meant to be interchangeable terms and denotes compounds, which
provide
enhanced skin penetration/permeation to the active drugs when it is
administered together
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
16
with the drugs to the skin of a user. Penetration/permeation enhancers in
transdermal
formulations will change the thermodynamic activity of the drug in the drug-
containing
layer, and thereby lead to a positive or negative "push" effect. In addition,
some
penetration/permeation enhancers may conceivably penetrate into the highly
ordered
intercellular lipid structure of the stratum corneum and reduce its resistance
by increasing
lipid acyl chain mobility, thus providing a "pull" effect.
Any skin penetrating/permeation enhancing effect of a substance may be
recognised upon
testing identical formulations with and without penetration enhancer, for
example by using
nude mouse skin or the like. The skilled person knows such test methods.
Typical penetration/permeation enhancers are included in the group of compound
listed
below:
- Alcohols, such as monohydric alcohols having about 2 to 10 carbon atoms,
such as
ethyl, isopropyl, butyl, pentyl, octanyl decanyl and/or benzyl alcohols;
dihydric alcohols
such as 1,2-propanediol polyhydric alcohols such as glycerin, sorbitol and/or
polyethylene glycol; saturated and unsaturated fatty alcohols with 8-18 carbon
atoms,
such as capryl-, decyl-, lauryl-; 2-lauryl-, myristyl-, cetyl-, stearyl-,
oleyl-, linoeyl- and
linolenylalcohol.
- Fatty acids, such as saturated or unsaturated fatty acids that may include 8-
18 carbon
atoms, for example, lauric acid, myristic acid, stearic acid, oleic acid,
linoleic acid,
linolenacid and palmitic acid, triacetin, ascorbic acid, panthenol, butylated
hydroxytoluene, tocopherol, tocopherol acetate, tocopheryl linoleate. Other
fatty acids
include but are not limited to valerian acid, capronic acid, capryl acid,
pelargon acid,
caprin acid, isovalerian acid, neopentan acid, neoheptan acid and/or
isostearin acid.
- Esters such as aliphatic esters ethyl acetate, lower (Cl - C4) alkyl ester
of lactic acid,
fatty acid esters of the general formula CH3 --(CH2)n --COOR, wherein n is a
number
from 8 to 18 and R is an alkyl residue of maximally 6 carbon atoms, such as
fatty acid
esters for example, those of lauric acid, myristic acid, stearic acid and
palmitic acid,
e.g., the methyl esters, ethyl esters, propyl esters, isopropyl esters, butyl
esters, sec-
butyl esters, isobutyl esters of these acids, or dicarboxylic acid diesters of
the general
formula R'OCO(CH2)m COOR', wherein m is a number from 4 to 8 and R' in each
case
means an alkyl residue of maximally 6 carbon atoms, such as propyl oleate,
decyl
oleate, isopropyl palmitate, glycol palmitate, glycol laurate, dodecyl
myristate, isopropyl
myristate and glycol stearate, suitable dicarboxylic acid diesters are, for
example, the
diisopropyl adipate, diisobutyl adipate and dlisopropyl sebacate.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
17
- Ethers, such as polyethylene glycol ethers of aliphatic alcohols (such as
cetyl, lauryl,
leyl and stearyl) including polyoxyethylene (4) lauryl ether, polyoxyethylene
(2) ()leyl
ether and polyoxyethylene (10) oleyl ether.
- Alkanes, such as alkanes with chain lengths of 6 to 17 carbon atoms.
- Amides such as dimethyl acetamide, dimethyl formamide, dimethyl
lauramide, dimethyl
laurylamide and/or fatty acid amides and theirs derivatives.
- Amides, such as amides with long aliphatic chains, or aromatic amides, urea
and urea
derivatives such as cyclic urea, dodecyl-urea, dlphenyl-urea and /or
allantoin.
- Amino acids.
- Amino acetates, such as derivatives of amino acetates such as dodecyl-N,N-
dimethylaminoacetate and dodecy1-2-methyl-2-(N,N-methylaminoacetate), decy1-2-
(N,N-dimethylamino)-propionat, decy1-2-(N,N-dimethylamino)-butyrat, octy1-2-
(N,N-
dimethylamino)-propionat, and /or docecyl-(N,N-dimethylaminophenylacetate.
- Azone derivatives such as 1-dodecylazacycloheptane-2-one derivatives,
azacycloalkanone derivatives and/or hexamethylentaura mid derivatives.
Cyclodextrins such as alpha, beta, and gamma cyclodextrins.
- Glycerides, such as Monoglycerides, including glycerol monooleate, glycerol
monolaurate and glycerol monollnoleate, polyethylene glycol-3-lauramide (PEG
LR),
polyethylene glycol monolaurate (PGML) glycerol monooleate (GMO), glycerol
monolinoleate and/or glycerol monolaurate (GML).
- Glycols such as ethylene glycol, diethylene glycol, or propylene glycol
dipropylene glycol
and/or trimethylene glycol.
- Oils, such as mineral, vegetable, animal and fish fats and oils such as
cotton seed, corn,
safflower, olive and castor oils, squalene, and/or lanolin.
- Polyols such as propylen glycol.
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
18
- Pyrrolidones such as 2-pyrrolidone, N-methyl- 2-pyrrolidone, dodecyl-
pyrrolidone, 2-
pyrrolidon-5-carboxylicacid, N-hexyl-, N-lauryl-, 4-carboxy-, 4-carboxycarbon
derivatives, 3-hydroxy-N-methyl-2-pyrrolidon, N-farnesy1-2-pyrrolidon, N-
(2(decylthio)ethyl)-2-pyrrolidone and/or N-(2-hydroxyethyl)-2-pyrrolidone.
- Sulfoxides such as sulfoxide derivatives such as methyloctyl-sulfoxide,
dimethyl-
sulfoxide (DMSO), hexylmethyl-sulfoxide (hexyl-MSO) and/or decylmethyl-
sulfoxide
(decyl-MSO).
- Surface active agents such as cationic surfactants like
cetyltrimethylannmonium
bromide, octadecyltrimethylammoniumchloride, cetylpyridiniumchloride and/or
equivalent cationic compounds, anionic surfactants such as sulphate salts
which include
but are not limited to compounds such as sodium lauryl sulphate and/or sodium
dodecyl
sulphate, and non-ionic surfactants such as esters of sorbitol and sorbitol
anhydride
which include but are not limited to polysorbate, sorbitan-monopalmiate and/or
sorbitan-polyoleat.
- Terpenes, ketones and oxides.
In addition to the steroidal hormone, such as a progestin, the one or more
polymers, the
one or more tackifiers and the optional estrogen, the drug-containing layer or
other parts ,
of the composition also contain stabilisers, dyes, pigments, inert fillers,
anti-ageing agents,
anti-oxidants, elastomers, thermoplastics and other conventional components of
transdermal compositions that are known in the art. Preferably, the
composition, or at
least the drug-containing layer, does not comprise or does only comprise in a
restricted
amount (less than 1%, 0.8%, 0.5%, 0.2% or 0.1 /0 by weight og the drug-
containing
layer) poiyvinylpyrrolidone, methylcellulose, ethyl cellulose, hydroxypropyl
methylcellu lose,
and/or dimethyl-isosorbide.
It should be understood that compositions of the inventions are transparent or
at least in
very interesting embodiments transparent, which means that the skin can be
visually
inspected through the drug delivery system. That is to say that the drug-
containing layer is
a monophasic system in which the drug (here progestin) is completely dissolved
in the
drug-containing layer. The property as a monophasic system may be identified
by
mechanical stretching of the drug-containing layer by using a test method as
described
below.
The drug-containing layer is further characterised by being homogenous. The
term
"homogeneous" is used to describe a monophasic system, wherein the matrix is
composed
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
19
of one polymer phase. These systems can be distinguished from multiphasic
systems,
which are composed of at least two polymer phases. In most cases multiphasic
systems
can be detected visually by their opaque appearance. The opaque appearance is
caused by
the light diffraction due to differences in the diffraction index of the
polymer phases. Other
methods to detect monophaslc systems are microscopic or rheological methods or
by
mechanical streching thin polymer films. During mechanical stretching the thin
polymer
film composed of multiphasic systems turns opaque, as determined visually.
Thus, in summary it should be understood that interesting embodiments of the
invention
include:
a drug-containing layer that comprises:
i) a progestin such as Gestodene or an ester thereof; and
ii) a polymer selected from the group consisting of polyisobutylenes,
polybutenes,
polyisoprenes, polystyrenes, styrene isoprene styrene block polymers, styrene
butadiene
styrene block polymers and mixtures thereof.
In further interesting embodiments thereof the drug-containing layer is
characterised by the
following parameters, which may be present as a single parameter or as mixture
of parameters;
= The drug-containing layer has a solubility for Gestodene of no more than 3%
by weight
of the drug-containing layer;
= The drug-containing layer excludes dimethylisosorbide or contains an
amount of
dimethylisosorbide of less than 0.5% by weight of the layer;
= The drug-containing layer excludes polyvInylpyrrolidone,methylcellulose,
ethylcellulose
and/or hydroxypropylcellulose or contains an amount of less than 2% of
polyvinylpyrrolidone,methylcellulose, ethylcellu lose and/or
hydroxypropylcellulose by
weight of the layer;
= The drug-containing layer contains the progestin (Gestodene or an ester
thereof)
completely dissolved in the layer;
= The drug-containing layer comprises the Gestodene or an ester thereof in an
amount of 0.5-
3% by weight of the drug-containing layer;
= The drug-containing layer Is transparent;
= The drug-containing layer is homogenous;
= The drug-containing layer is monophasic;
= The drug-containing layer excludes a skin penetration enhancer or contains
an amount of
less than 2% by weight of the layer;
= The drug-containing layer comprises said polymer in an amount of from
about 15 to 99
% by weight of the layer;
= The drug-containing layer comprises a tackifier, such as a rosin ester,
in an amount of
up to 85% by weight of the drug-containing layer;
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
As mentioned above, the compositions of the invention are characterised by
delivering an
effective contraceptive amount of a steroidal hormone, such as a progestin,
such as a
gestodene, optionally in combination with an estrogen. In the embodiment,
wherein the
5 progestin is Gestodene or a derivative thereof the composition may be
characterised by
providing an in-vitro nude mouse skin permeation rate of Gestodene and/or a
derivative
thereof of at least 25 pg/cm2*24h. In other terms, the composition may be
characterised
by having a drug-containing layer delivering Gestodene and/or a derivative
thereof in an
amount of from about 40 to 70 pg per day.
Not only Gestodene or a derivative thereof may be used as a drug in the
composition of
the invention. Other progestins may be included in the drug-containing layer
together with
Gestodene or in place of a gestodene, such as dienogest, drospirenone,
levornorgestrel,
cyproteronacetate, tetrahydrodienogest, norethisterone, norethisteronacetate,
desogestrel,
3-keto-desorgestrel, norgestimate, lynestrenol, medroxy-progesteroneacetate,
norgestrel,
norethisteroneenanthate, trimegestone or alpha and beta-progesterone receptor
ligands.
As Mentioned, the composition is effective in inhibiting ovulation. In some
instances, the
composition further comprises an estrogen. The estrogen may be incorporated
together
with the progestin in the same drug-containing layer or be incorporated in a
separate
drug-containing layer free of the progestin.
The term "estrogen" includes both the natural 17p-estradiol and the semi-
synthetic
estrogen derivatives such as esters of natural estrogen and 17-alkylated
estrogens. Semi-
synthetic esters of natural estrogen include for example estradio1-17-0-
enanthate,
estradio1-17-p-valerate, estradio1-17-0-benzoate, estradlo1-17-0-undecanoate,
estradio1-
16,17-hemisuccinate or estradio1-17-0-cypionate. Examples on 17-alkylated
estrogens are
Ethinylestradiol, Ethinylestradiol-3-isopropylsulphonate, quinestrol,
mestranol or methyl
estradiol. The term "estrogen" may also include a non-steroidal compound
having estrogen
activity, such as diethylstilbestrol, dienestrol, clomifen, chlorotrianesene
or cyclofenil.
In a preferred embodiment, the estrogen is Ethinylestradiol.
To achieve the therapeutically effective amount of hormone in the blood, the
actual
concentration of the drug in the drug-containing layer may be adjusted.
Generally
speaking, the drug-containing layer should contain some hormone in excess of
the amount
of hormone to be absorbed for achieving the therapeutically effective amount
of the
hormone. Normally, this excess is small, such as the amount of hormone is less
than 10
times the desired/required amount of hormone, preferably less than 5 fold,
such as less
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
21
than 2 fold. For example, it is also considered important to limit the amount
of hormone so
as to reduce the overall exposure of hormone to the user. Appropriate
concentrations of a
steroidal hormone, such as a progestine, such as Gestodene or a derivative
thereof, in the
drug-containing layer is therefore from about 0.5 to 10% by weight of the drug-
containing
layer. In still more preferred embodiments, the concentration of said hormone
is about 0.5
to 10%, such as from about 0.75 to 5%. "As mentioned, the total concentration
of
hormone, such as Gestodene may result in drug-containing layers comprising the
hormone
in saturated or sub-saturated levels. In a very interesting embodiment, the
concentration
of a steroidal hormone, such as Gestodene or a derivative thereof, in the drug-
containing
layer is from about 1 to 3%, such as 1 to 2%.
Likewise, in some embodiments of the invention that further comprise an
estrogen, the
estrogen is present in the drug-containing layer in an amount of from about
0.5 to 10% by
weight of the adhesive layer, preferably of from about 0.75 to 5%, more
preferably of from
about 1 to 3%, such as 1 to 2%.
Moreover, the said progestin, such as Gestodene or a derivative thereof is in
a mass ratio
to said estrogen in the range of from about 4 to 0.5, preferably 2 to 0.5 such
as 1:1.
It has surprisingly been found that relatively high plasma levels of Gestodene
can be
maintained for a prolonged period of time by administering Gestodene or a
derivative
thereof formulated in a composition of the invention. Also surprisingly, it is
found that the
plasma profile and plasma levels of Gestodene, as resulting from the
administration of
Gestodene or a derivative thereof and optionally an estrogen, are effective in
inhibiting the
ovulation in a woman.
Therefore, further aspects of the invention relate to the use of a composition
of the
invention , optionally in combination with an estrogen, for the inhibition of
ovulation in a
female, such as a woman. When the medicament is singly administered, then a
plasma
concentration-time curve of Gestodene is achieved characterised by having
plasma levels
of Gestodene in a concentration of at least 1.0 ng/ml, as determined at steady
state
conditions. As follows, an aspect of the invention relates to a method for
inhibiting
ovulation in a female, such as a woman, comprising administering topically to
skin or
mucosa an effective amount of Gestodene or a derivative thereof, optionally in
combination with an estrogen, so as upon singly administering said gestodene,
then a
plasma concentration-time curve of Gestodene is achieved characterised by
having plasma
levels of Gestodene in a concentration of at least 1.0 ng/ml, as determined at
steady state
conditions.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
22
Alternatively, the uses and methods of the invention are for the treatment of
other
symptoms, disorders or symptoms which are normally treated by administering a
progestin, such as Gestodene or a derivative thereof or a combination of a
progestin and
an estrogen.
Therefore, it should in general be understood that in some embodiments of the
invention,
the uses and methods are for the treatment of endometriosis, pre-menstrual
syndrome,
climacteric disorders, regulating menstrual cycle and/or stabilising menstrual
cycle.
For example, administering a progestin without concurrent therapy with an
estrogen can
treat irregular bleeding and abnormal bleeding. As used herein, the term
"irregular
bleeding" characterises any uterine bleeding, outside the regular monthly
menstrual
periods of non-pregnant women. Uterine bleeding are irregular if menstrual
cycles or
menstrual periods are too short, too long, too frequent, too infrequent, or
occur at
irregular intervals which falls outside the regular 26-30 days menstrual
cycle. The
menstrual period is classified as too long when being delayed with 15 to 50
days or more
to the expected onset of said bleeding. The term "abnormal bleeding"
characterises heavy
bleeding typically soaking through enough sanitary protection products to
require changing
more than every one or two hours, having a period that lasts over seven days.
Abnormal
bleeding does not include bleeding in women who have already reached
menopause,
abnormal uterine bleeding due to side effects of estrogen replacement therapy,
abnormal
bleeding as a symptom of uterine cancer, as a result of a consequence of
abnormal blood
clotting normally, an inherited bleeding disorder or because of a medical
illness that affects
levels of blood platelets.
In other embodiments, the progestin is administered in combination with an
estrogen,
such as by administering a medicament of the invention comprising the
combination of a
progestin, such as Gestodene or a derivative thereof, and an estrogen for the
treatment of
climacteric disorders, such as symptoms and diseases associated with meno-
pause, such
as hot flushes, sweating attacks, palpitations, sleep disorders, mood changes,
nervousness, anxiety, poor memory, loss of confidence, loss of libido, poor
concentration,
diminished energy, diminished drive, irritability, urogenital atrophy, atrophy
of the breasts,
cardiovascular disease, changes in hair distribution, thickness of hair,
changes in skin
condition and/or osteoporosis. Most notably the treatment is directed to hot
flushes,
sweating attacks, palpitations, sleep disorders, mood changes, nervousness,
anxiety,
urogenital atrophy, atrophy of the breasts or for the prevention or management
of
osteoporosis.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
23
In connection with treatment of climacteric disorders, the estrogen may be
selected from
natural estrogens, such as estradiol and esters thereof, such as estradiol
valerate,
estradiol benzoate. Furthermore, natural estrogens include estrone, estriol,
estriol
succinate and conjugated estrogens, including conjugated equine estrogens such
as
estrone sulfate, 17(3-estradiol sulfate, 17a-estradiol sulfate, equilin
sulfate, 170-
dihydroequilin sulfate, 17a-dihydroequilin sulfate, equilenin sulfate, 1713-
dihydroequilenin
sulfate and17a-dihydroequilenin sulfate or mixtures thereof.
In some embodiments of the invention, the plasma concentration-time curve of
Gestodene
at steady state conditions is characterised by having plasma levels of
Gestodene in a
concentration of at least 1.5 ng/ml, such as at least 2.0 ng/ml or at least
2.5 ng/ml. In
other interesting embodiments, the plasma concentration-time curve of
Gestodene at
steady state conditions is characterised by having plasma levels of Gestodene
in the range
of 1 to 8 ng/ml, preferably in the range of 1.5 to 6 ng/ml following the first
6 days after
single administration of a composition of Gestodene or a derivative thereof,
preferably in
the form of a composition of the invention.
In still further embodiments, the plasma concentration-time curve of Gestodene
at steady
state conditions is characterised by having maximal plasma levels of Gestodene
in the
period of 18 to 60 hours following single administration of the medicament
and/or having
plasma levels of Gestodene at steady state conditions in the period of 5 to 7
days following
single administration of the medicament in the order of at least 50% of the
maximal
plasma levels of Gestodene obtained during the first 18 to 60 hours following
administration.
The gestodene is preferably administered repeatedly in cycles of 28 days such
that within
each cycle of 28 days, the gestodene/composition is administered with an
interval of 7
days in a period of 21 days (3 weeks) followed by no administration of
Gestodene or a
derivative thereof for 7 days (one week). That is to say that the
gestodene/composition is
administered on day 1, 8 and 15 within each cycle of 28 days. Preferably, said
day 1 may
be the day of the start of menstruation, or any other suitable day, such as
the first,
second, third, fourth, fifth or sixth day following the day of start of
menstruation. In
another embodiment, the gestodene, optionally in combination with an estrogen,
is
administered repeatedly in cycles of 12 weeks such that within each cycle of
12 days, the
gestodene/composition is administered with an interval of 7 days in a
continuous period of
11 weeks followed by no administration of Gestodene or a derivative thereof
for 7 days
(one week).
CA 02549916 2006-06-08
WO 2005/058287
PCT/1112004/052752
24
To improve the contraceptive efficacy and security, an estrogen may be
administered
concomitantly with the gestodene. The estrogen may be selected from the
estrogens
mentioned above.
As may be understood, the uses and methods of the invention include the
application of
Gestodene or a derivative thereof, which may be in the form of a composition
as defined
herein. Thus, the term "medicament" includes a composition as defined herein.
Furthermore, the term "medicament" is meant to include a kit of the invention.
In a still further aspect, the invention relates to a kit comprising 1 to 11
dosage units
intended for a treatment period of 12 weeks formulated in a form for
transdermal delivery
of a progestin, such as of Gestodene or a derivative thereof, said dosage
units comprise a
drug-containing layer comprising gestodene and one or more pharmaceutically
acceptable
excipients or carriers and the drug-containing layer has a solubility with
respect to said
gestodene of no more than 3% by weight of the drug-containing layer. The
dosage unit
may comprise a composition as described herein. It is to be understood that in
one
embodiment thereof, 11 dosage units are administered continuously once weekly
during a
period of 11 weeks followed by one week of no administration of a dosage unit
or
administration of a placebo.
In other embodiments, the kit is intended for a treatment period of 12 weeks,
but the kit
comprises 1 to 9 dosage units. In one embodiment, 3-dosage units are
administered
weekly for a period of 3 weeks, followed by one week of no administration of a
dosage unit
or administration of a placebo. That is to say that the kit is intended for a
treatment period
of 4 weeks and the kit comprises 1-3 dosage units.
The dose of the progestin, such as of Gestodene or a derivative thereof in
each dosage unit
corresponds to a dose selected from a 6-day dose, 7-day dose, 8 day-dose, 14-
day dose
or 21-day dose. In one embodiment thereof, each dosage unit comprises
Gestodene or a
derivative thereof in a dose from about 0.5 to 5 mg, preferably 1 to 3 mg,
more preferably
1.5 to 2.5 mg.
It is further to be understood that the kit may further comprise an estrogen
as mentioned
above. The estrogen may be combined together with the progestin, such as
Gestodene or
a derivative thereof, in the same dosage unit or provided in separate dosage
units. The kit
may for example further comprises 1 to 30 dosage units comprising an estrogen
and no
gestodene. The estrogen may be in a dosage form formulated for transdermal
delivery,
vaginal delivery or the like. Alternatively, the estrogen may be in the form
of dosage units
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
formulated for peroral delivery of an estrogen, such as in the form of a
tablet, pill, capsule,
powder, paste or granules.
The compositions of the invention may be fabricated using procedures known in
the art.
5 One example is included herein.
Figures
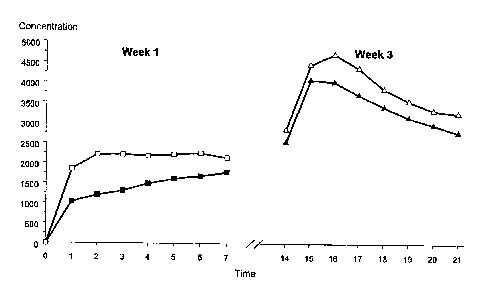
Figure 1. Mean serum levels of Gestodene (GSD) during two treatment cycles,
week 1 and
week 3, respectively. The legends are as follows:
= cycle 1, week 1
cycle 2, week 1
= cycle 1, week 2
A cycle 2, week 2
Examples
Example 1
Manufacturing of a patch
A composition of the invention is prepared as follows.
In a first step 380 g of Gestodene, and optionally 180 g of Ethinylestradiol,
is dissolved in
an appropriate solvent, such as 16,8 kg of dioxane. In a second step, about 57
kg of a
mixture of polyisobutylene and rosin ester in heptane (Arcare MA 24A) is
weighed off.
The hormone solution of the first step is transferred under stirring to the
polymer solution
and stirring is continued until a homogeneous solution is achieved. The thus
obtained
drug-containing solution is coated onto a release liner (such as FL 2000 75pm
PET 1S; Fa
Loparex) and dried under appropriate conditions. The dried drug-containing
layer is then
laminated with a backing foil/layer, such as Cotran , 9720, 3M. The thus
obtained
laminate is divided into patches of the size of 10 cm2 and the resulting patch
has the
following composition:
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
26
Gestodene: 1.9 mg
Optionally Ethinylestradiol: 0.9 mg
Polymer 97,2 mg
(in the form of polyisobutylene in
combination with a tackifier e.g
MA-24A )
Release liner: 10 cm2
Backing layer: 10 crn2
In another example, the resulting patch is similar to the above-mentioned but
the amount
of Ethinylestradiol is 0.6 mg.
In still another example, the resulting patch is similar to the above-
mentioned but the
adhesive is Durotak , 10711, which is composed of a hydrocarbon polymer.
Example 2
Skin penetration rates of compositions with various polymers.
Six compositions (A-F) were manufactured with acrylate-vinyiacetate polymers
as the
polymer in the drug-containing layer. Furthermore, composition G was made
using
polyisobutylene as the polymer in the drug-containing layer. The manufacturing
was
performed according to the process as described in Example 1. None of the
compositions
comprises a skin penetration enhancer.
Each of the compositions were tested in the in-vitro mouse skin permeation
test. The test
is performed using skin preparations of nude mouse skin (HsdCpb: NMRI-nu)
available
from Harlan Bloservice for Science GmbH, Walsrode, Germany. The test
formulation is
attached on the outside of a skin specimen. Both are placed into the
permeation cell with
the inside In contact with the receptor media. HEPES buffered aqueous solution
is used as
receptor media. Sodium azide is added to prevent microbial growth. The
receptor solution
is maintained at 32 C. Samples are taken from the receptor solution at defined
time
intervals and the concentration of Gestodene (GSD) and Ethinylestradiol (EE)
in the
receptor media were analysed by HPLC. The flux rate was then calculated as the
amount of
drug released per area and time unit [pg/cm2*24111 using the calculated
amounts of active
substances.
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
27
Results on skin penetration rate in vitro:
Table 1.
Formulation A
GSD (% w/w) 1.9 3.9 2.1 4.2 1.9 3.8 1.9
EE (0/0 w/w) 1.0 2.0 1.1 2.1 1.0 1.9 0.9
Polymer Acrylate Acrylate Acrylate Acrylate Acrylate Acrylate PIB
e.g.
MA-24A
vinylace vinylace vinylace vinylace vinylace vinylace
tate e.g. tate e.g. tate e.g. tate e.g. tate e.g. tate e.g.
Durotak Durotak Durotak Durotak Durotak Durotak
387- 387- 87-2097 87-2097
387- 2825 2825
387- 2051
2051
GSD 14.8 32.8 12.6 -14.3 13.5 16.7 30.9
permeation
ug/cm2*24h
EE permeation 2.6 6.5 1.5 2.8 1.3 1.9 8.1
ug/cm2*24h
The results indicate that the permeation rate of Gestodene as well as of
Ethinylestradiol
from composition G (polyisobutylene) is superior to that from compositions A-
F.
Example 3
Contraceptive effect and pharmacokinetic profile
The effect on ovulation inhibition, serum drug concentrations and safety of a
patch of the
Invention was investigated in a selected population of women. The study design
was based
on the requirements of the EMEA guideline for clinical studies with
contraceptive steroids
(Commitee for Proprietary Medicinal Products, CPMP/EWP 519/98).
Study design
The study has three phases; a pre-treatment phase Including two washout cycles
and one
additional cycle to ensure that the selected women were ovulatory. Finally,
the second
phase is a treatment phase of two cycles, which was followed by a third phase
consisting
of one-cycle post-treatment phase.
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
28
Women enrolled in the study were required to be healthy, non-pregnant, non-
smoking,
non-lactating female volunteers aged between 18 and 35 years, with a normal
body mass
index of 18-26 kg/m2and a normal menstrual cycle length such as 28 days 4
days. Only
women with light skin were included so that application sites could be easily
and uniformly
assessed.
The test patch is a patch comprising a drug-containing layer of 0.9 mg
Ethinylestradiol and
1.0 mg Gestodene and Arcare MA-24A , the drug containing layer has a size of
10 cm2.
Arcare MA-24A is a polyisobutylene based adhesive from Adhesive Research.
During the study, blood was drawn for the determination of endogenous
hormones, such
as estradiol, progesterone, follicle stimulating hormone, sex hormone binding
proteine,
Ethinylestradiol, Gestodene. Transvaginal ultrasound examination were carried
out to
evaluate development of ovarian follicle-like structures. Patch adhesion, skin
reactions at
the application site and the woman's general health status was assessed.
Vaginal bleeding
was also evaluated.
During the pre-treatment cycle normal and spontaneous ovulation was
established by
assessment of serum progesterone values in that only women with an ovulatory
pre-cycle
and progesterone serum levels higher than 5 nmo1/1 were admitted to the
treatment
phase.
The treatment phase includes a period of two menstrual cycles. The first
treatment in the
first cycle began one day after the volunteers started menstruating in this
cycle by
application of a patch. A total of three test patches were applied in
intervals of 7 days
between, such as application at days 1, 8 and 15 of the first cycle - each at
different
application sites- during each treatment cycle. Each patch was worn for 7
days, then
replaced with a new patch to complete a total of 21 days of continuous use.
This was
followed by a 7-day treatment-free interval before the next treatment period
started with a
total of three patches applied, each applied with an interval of 7 days. If
the patches were
lost or became more than 40% detached, a new patch was applied.
Patches were applied to the clean, dry intact and preferably hairless skin of
the lower
abdomen, below the navel, starting with the right side in the first treatment
cycle, then
alternating sides.
Determination of pharmacodynamic variables
The primary pharmacodynamic variable is the proportion of women with ovulation
inhibited. In accordance with the so-called Hoogland method, ovulation
requires follicular
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
29
growth beyond 13 mm and subsequent ovarian rupture, plus a serum progesterone
concentration > 5nmol/Ithat concurs with the follicle rupture. As follows, the
ovulation is
said to be inhibited when the follicle is below 13 mm and the serum
progesterone
concentration is below 5nmo1/1 at the time of follicle rupture.
Determination of pharmacokinetic variables
Pharmacokinetic parameters such as area under the drug concentration time
curve during
the patch-wearing period AUC(0-168h)r Cmaxi tmaxr and accumulation factors
within each cycle,
such as determined by the AUC(0-168h) of 3rd patch/AUC(0-168h) of 1st patch
within cycle 1 or
2, or accumulation factors between two cycles, such as AUC(0-168h) of 3rd
patch in cycle 2/
AUC(0-1681,) of 3rd patch in cycle 1. The AUC was calculated according to the
linear
trapezoidal rule.
Serum concentrations of the estrogen and progestin, including Ethinylestradiol
and
Gestodene, were determined throughout the study to assess the pharmacokinetic
characteristics of the patch. The sampling points were at the day 18 of the
last pre-
treatment cycle and days 1, 2, 3, 4, 5, 6, 7, 8, 15, 16, 17, 18, 19, 20, 21
and 22 of cycle 1
as well as days 1 (before application of new patch), 2, 3, 4, 5, 6, 7, 8, 15,
16, 17, 18, 19,
20, 21 and 22 of cycle 2.
The concentrations of Ethinylestradiol and Gestodene were determined by
conventional
methods known in the art. Specifically, the concentration of Ethinylestradiol
was
determined by gas chromatography using mass spectrometry in the chemical
ionisation
mode as the detection method following the extraction of Ethinylestradiol from
acidified
serum and consecutive derivatization. Gestodene concentrations were determined
by
radioimmunioassay using a rabbit antiserum and 31-I-labelled Gestodene. After
incubation
and centrifugation, the resultant precipitate was redissolved with NaOH. The
assay has a
lower limit of quantification of approx. 250 pg/ml.
Results
The primary pharmacodynamic variable was the proportion of women with
ovulation
inhibition.
Ovarian activity was suppressed effectively, i.e.;
No Ovarian Activity: cycle 1: 78%, cycle 2: 56%.
Potential Activity: cycle 1: 15%, cycle 2: 22%.
Non-active FLS: cycle 1: 4%, cycle 2: none;
Active FLS: cycle 1: 4%, cycle 2: 22%
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
No cases of ovulation were found throughout the study. Ovulation inhibition,
defined as a
Hoogland score lower than 6 (ovulation), was sufficient for all volunteers in
the per
protocol data set throughout the study.
5 Progesterone concentrations were adequately suppressed below 2.5 nmo1/1 in
each of the
treatment cycles. Mean estradiol levels in the blood was under 20 pg/ml on all
days on
which a patch was applied.
Pharmacokinetic results
In all predose samples, Ethinylestradiol (EE) and Gestodene (GSD) serum
concentrations
were below the limit of quantitation (LOQ: 10pg/m1 for EE, 250pg/m1 for GSD).
After
administering the study medication, EE and GSD serum concentrations were
quantifiable
for at least 168 hours in all subjects. See results in Table 2 and 3 as well
as in figure 1.
Table 2. Mean pharmacokinetic parameters of ethinyl estradiol (EE)
Pharmacokinetic Cycle 1 Cycle 2
Unit ______________
parameter Week 1 Week 3 Week 1 Week 3
Cmax pg/ml 45.6 50.4 45.2 48.0
Tmax h 48 24 48 48
AUC(0-168h) ng x h
5.3 6.1 5.1 5.8
/ml
Table 3. Mean pharmacokinetic parameters of gestodene (GSD)
Pharmacokinetic Un it Cycle 1 Cycle 2
parameter Week 1 Week 3 Week 1 Week 3
Cmax
pg/ml 1564 3896 2219 4416
Tmax
144 48 96 48
AUC(0-168h) ng x h
194 524 302 598
/m1
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
31
Example 4
Comparison of polyisobutylene adhesive and acrylic formulation in human.
A randomized, cross-over study to determine the average daily delivery of
Ethinylestradiol
(EE) and gestodene (GSD) from three different transdermal patch formulations
(A-C) in
healthy postmenopausal volunteers following single administration was carried
out. The
aim was also to compare the transdermal application with that of intravenous
injection.
The test formulations were as follows:
Formulation A (Polyisobutylene)
No. Name of ingredient Amount per 10 cmi patch Remark
1 Ethinylestradiol 0.95 mg
2 Gestodene 1.9 mg
3 Polyisobutylene Adhesive 97.15 mg MA-24A
Formulation B (Polyisobutylene, 70% of Formulation A)
No. Name of ingredient Amount per 10 cmi patch Remark
1 Ethinylestradiol 0.67 mg
2 Gestodene 1.33 mg
3 Polyisobutylene Adhesive 98.00 mg MA-24A
Formulation C (Acrylate)
No. Name of ingredient Amount per 10 cmz patch Remark
1 Ethinylestradiol 0.67 mg
2 Gestodene 1.33 mg
3 Acrylic Adhesive 73.5 mg Gelva 7883
4 Isopropyl myristate 5.0 mg Permeability
enhancer
5 Copovidone 15.0 mg Crystalization inhibitor
(Kollidon VA64, BASF,
Germany)
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
32
The study was carried out according to the following parameters:
The patches were administered by single transdermal administration with a 7-
day wearing
period per test. One week of washout was carried out after removal of patch at
each test
treatment. A dose of 60pg EE and 75pg of GSD was administered intravenously
once.
Blood sampling for kinetic measurements was at 72 hours after intravenous
administration
and during a period of 12 days after transdermal administration as described
in Example 3.
Determination of blood levels of GSD and EE were carried out according to
Example 3.
Results:
The maximum concentrations of gestodene are given in the following table
indicating the
difference of the polyisobutylene formulations A and B and the acrylic
formulation. The
figure shows the time course of the gestodene mean serum levels.
Parameter Formulation A Formulation B Formulation C
Cmax [1:19/ml]: 2082 1995 1277
tmax [h]: 168 144 156
AUC (0-7d): 243 257 155
[h*ng/m1..]
The results indicate that the drug delivery of Formulation C, which included a
drug-
containing layer of an acrylate in combination with a penetration enhancer, is
significantly
lower than the drug delivery from Formulations A and B.
CA 02549916 2006-06-08
WO 2005/058287 PCT/1B2004/052752
33
Example 5
Drug-containing layers containing 1.9 mg of Gestodene and 0.9 mg of
Ethinylestradiol and
composed of different mixtures of polymers are shown (A to M).
Polymer Polymer A
BCDE F G
Brand name Chemical name
Foral 85E Pentaerythritol ester of 20.0
hydrogenated rosin
Foral 105E Glycerol ester of hydrogenated 20.0 30.0
rosin
Oppanol B10 N / Polyisobutylene (MW about 57.2 57.2 67.2 55.2
SFN 40.000 Daltons)
Oppanol B11 Polyisobutylene (MW about 62.2
SFN 49.000)
Oppanol B12 Polyisobutylene (MW about 62.2 62.2
SFN 55.000 dalton)
Oppanol B30 Polyisobutylene (MW about
SFN 200.000 dalton)
Oppanol B100 Polyisobutylene (MW about 20.0
20.0 12.0 12.0 20.0 12.0 15.0
1.000.000 Dalton)
Oppanol B150 Polyisobutylene (MW about
2.600.000 Dalton)
Indopol H300 Synthetic Polybutene (MW 10.0
about 1.300 Dalton)
Indopol I-11900 Synthetic Polybutene (MW 23.0
about 2.500 Dalton)
Indopol H2100 Synthetic Polybutene (MW 23.0
about 2.500 Dalton)
Escorez 5300 cyclic aliphatic petroleum 20.0
hydrocarbon resin
Staybelite Ester Ester of Hydrogenated Resin
3E
Staybelite Ester glycerol ester of partially
5E JQ hydrogenated rosin
Kraton D1161NU styrene-isoprene-styrene block
polymer
CA 02549916 2006-06-08
WO 2005/058287
PCT/1B2004/052752
34
Polymer Polymer H I I K L
Brand name Chemical name
Foral 85E Pentaerythritol ester of 10.0
hydrogenated rosin
Forel 105E Glycerol ester of hydrogenated 10.0 65.0
rosin
Oppanol B10 N / Polyisobutylene (MW about 67.2 67.2 75.7
75.7
SFN 40.000 Daltons)
=
Oppanol B11 Polyisobutylene (MW about
SFN 49.000)
Oppanol B12 Polyisobutylene (MW about 57.2
SFN 55.000 dalton)
Oppanol B30 Polyisobutylene (MW about 10.0 10.0
SFN 200.000 dalton)
Oppanol B100 Polyisobutylene (MW about 10.0 10.0 15.0
1.000.000 Dalton)
Oppanol B150 Polyisobutylene (MW about 10.8 10.8
2.600.000 Dalton)
Indopol H300 Synthetic Polybutene (MW
about 1.300 Dalton)
Indopol H1900 Synthetic Polybutene (MW
about 2.500 Dalton)
Indopol H2100 Synthetic Polybutene (MW
about 2.500 Dalton)
Escorez 5300 cyclic aliphatic petroleum
hydrocarbon resin
Staybelite Ester Ester of Hydrogenated Resin 10.8
3E
Staybelite Ester glycerol ester of partially 25.0 10.8
5E JQ hydrogenated rosin
Kraton D1161NU styrene-isoprene-styrene block 32.2
polymer