Note: Descriptions are shown in the official language in which they were submitted.
CA 02551163 2006-07-17
DEMANDES OU BREVETS 'VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATI:ONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME OF _2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
Methods for Treating Inflammatory, Autoimimune or Bone Resorption Diseases
BACKGROUND OF THE INVENTION
1. TECHNICAL FIELD
This invention relates to compositions and methods of treating an
inflammatory,
autoimmune or bone resorption disease by inhibiting plexin-A1-DAP 12
interaction,
plexin-A 1-Trem-2 interaction or DAP 12-Trem-2 interaction.
2. BACKGROUND INFORMATION
Semaphorins and their receptors play diverse roles in axon guidance,
organogenesis, vascularization/angiogenesis, oncogenesis, and regulation of
immune responses 1- 11. The primary receptors for semaphorins are members of
the
plexin family Z 12-14. In particular, plexin-Al, with ligand-binding
neuropilins,
transduces repulsive axon guidance signals for soluble class III semaphorins
15
whereas plexin-Al plays multiple roles in chick cardiogenesis as a receptor
for a
transmembrane semaphorin Sema6D independent of neuropilins 16. Additionally,
plexin-Al has been implicated in dendritic cell (I)C) functions in the immune
system 17. However, the role of plexin-Al in vivo and its important roles in
immune
responses and in bone homeostasis up until the present invention have been
unclear.
BRIEF SUMMARY OF THE INVENTION
It is therefore an object of the invention to provide a method of treating an
inflammatory, autoimmune or bone resorption disease, by administering to a
patient a
composition which inhibits plexin-A1-DAP12 interaction.
It is a further object of the invention to provide a method of treating an
inflammatory,
autoimmune or bone resorption disease, by administering to a patient a
composition
which inhibits plexin-Al-Trem-2 interaction.
-1-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
It is yet another object of the invention to provide a method of treating an
inflammatory,
autoimmune or bone resorption disease, by admir.iistering to a patient a
composition
which inhibits DAP 12-Trem-2 interaction.
It is yet still another object of the invention to provide a method or kit to
identify a
compound that controls interaction of plexin-A 1 with DAP 12 activity in a
cell,
comprising: (1) contacting a cell with a putative regulatory compound, wherein
the cell
includes a plexin-A1 protein and a DAP12 protein; and (2) assessing the
ability of the
putative regulatory compound to inhibit the interaction of plexin-A1 with DAP
12.
It is yet still another object of the invention to pravide a composition that
controls
interaction of plexin-Al with DAP12 activity in a cell wherein the composition
is
therapeutically useful in treating an inflammatory, autoimmune or bone
resorption
disease.
BRIEF DESCRIPTION OF THE FIGURES
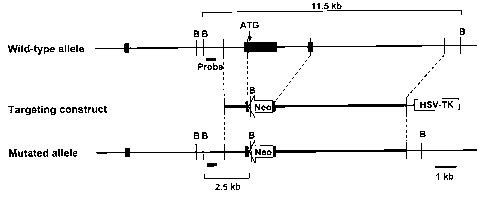
Figure 1: Generation of plexin-A1-/- mice. (a) Disruption of the plexin-Al
gene. The
gene structure of wild-type plexin-A1 allele (top), plexin-Al-targeting
construct
(middle) and the resulting plexin-Al mutant allele (bottom) are shown. Filled
boxes
denote exons. The 3.0-kb fragment containing the initiation codon and the
coding
sequence of the sema-domain was replaced with Neo. The HSV-tk gene was
appended
to allow for selection against random integration. B, BamHI. (b) Southern blot
analysis.
To assess the genotype of wild-type (+/+), heterozygous (+/-), and homozygous
(-/-)
mutant mice, tail. DNA was digested with BamH][, electrophoresed, and
hybridized with
the radio-labelled probe that is shown by a gray box in (a). The 11.5-kb
fragment
represents the wild-type allele, and the 2.5-kb fragment depicts the targeted
allele. (c)
Northern blot analysis. RNA prepared from the brain of wild-type (+/+) or
plexin-Al-/-
(-/-) mice was electrophoresed, and hybridized with radio-labelled probes. (d)
RT-PCR
analysis. cDNAs derived from the brain, heart and spleen of wild-type (+/+) or
plexin-A1-/- (-/-) mice were used for RT-PCR to determine plexin-A1
expression.
-2-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
Figure 2: There are no apparent differences between wild-type (+/+) and plexin-
A1-/-
(-/-) embryos (E12). Whole-mount staining of wild-type (+/+) or plexin-A1-/- (-
/-) E12
embryos using anti-neurofilament antibodies (2H:3) as described previously
(Giger et al,
Neuron 25:29, 2000). IV, trochlear nerve; V, trigeminal nerve; Vop, opthalmic
branch
of the trigeminal nerve; Vmax, maxillary branch of trigeminal nerve; VII,
facial nerve;
VIII, vestibulocochlear nerve; X, vague nerve.
Figure 3: Expression profile of plexin-Al. Expression of plexin-Al transcripts
was
determined by RT-PCR using multiple mouse tissue panel cDNAs (Clontech). G3PDH-
transcripts were used as controls.
Figure 4: Normal development of lymphocytes in plexin-A1-/- mice. Cells were
prepared from the thymus and spleen of wild-type (+/+) or plexin-A1-/- (-/-)
mice,
stained with various antibodies and analysed by flow cytometry.
Figure 5: Normal B-cell proliferative responses in plexin-A1-/- mice. Small
resting
B-cells prepared from wild-type (open circles) or plexin-A1-/- mice (closed
circles)
were cultured for 72 h with various concentrations of the indicated factors.
[3H]-
thymidine was added for the last 14 h. Data are the mean S.D.
Figure 6: Normal CD4+ T-cell proliferative responses in plexin-Al-/- mice.
CD4+
T-cells purified from wild-type (open circles) or plexin-A1-/- mice (closed
circles) were
cultured with various concentrations of immobilized anti-CD3 in the absence
(a) or
presence (b) of anti-CD28 (10 g ml-1) for 48 h. [3H]-thymidine was added for
the last
14 h. Data are the mean S.D.
Figure 7: Normal osteoblast functions in plexin-.A1-/- mice. (a, b) Comparable
expression of osteocalcin (a) and BSP (b) in plexin-Al-/- osteoblasts. The
levels of
serum osteocalcin were determined by ELISA. Expression of transcripts of bone
sialoprotein (BSP) in wild-type (+/+) or plexin-A. 1 -/- (-/-) osteoblasts was
determined
by real time monitoring quantitative PCR analysis. G3PDH-transcripts were used
as
internal controls. Data are the mean S.D. (c) A typical calcein experiment is
shown.
-3-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
Mineral apposition rate (MAR) and bone formation rate (BFR) of wild-type (+/+)
and
plexin-A1-/- (-/-) mice are shown. Data are from 3 mice s.e.m.
Figure 8: Expression profiles of Sema6D transcripts in T-cells and
osteoclasts. (a)
cDNA was prepared from unstimulated- or anti-CD3 stimulated-T-cells.
Expression of
transcripts of Sema6D was determined by real time monitoring quantitative PCR
analysis. G3PDH-transcripts were used as internal controls. (b) cDNA was
prepared
from resting T-cells (-), Thl (IL-12 plus anti-IL-4)- and Th2 (IL-4 plus
anti-IL-12)-polarized cells. Expression of transcripts of Sema6D and G3PDH in
these
cells was determined by PCR using their specific primers. cDNA was also
prepared
from osteoclasts induced by M-CSF plus RANKL (in vitro) or from femurs and
tibiae
of 13-day-old mice (primary). Expression of transcripts of plexin-Al and G3PDH
were
determined by PCR using their specific primers.
Figure 9: Control soluble Sema4A proteins exert no effects on DCs and
osteoclasts.
(a) Recombinant Sema4A does not bind to DCs. Wild-type BMDCs were cultured
with
anti-CD40 mAb for 24 h and stained with biotinylated recombinant Sema4A (thick
lines) or biotinylated human IgGI (dotted lines) plus streptavidin-APC. (b)
Sema4A
does not induce IL-12 production by DCs. BMDCs from wild-type mice were
cultured
with recombinant Sema4A, Sema6D-Fc, anti-CD40 mAbs or LPS for 72 h and
production of IL-12p40 was measured by ELISA. Data are the mean-+S.D. (c)
Sema4A
has no effects on osteoclast development. Bone marrow cells from wild-type
mice were
cultured with M-CSF (10 ng ml-1) and Sema4A-Fc (10 gg ml-1) for 2 days, and
further
cultured with M-CSF (10 ng ml-1) and a suboptiinal dose of RANKL (5 ng ml-1)
for 3
days. The number of TRAP-positive cells was measured.
Figure 10: Reduced but substantial Sema6D binding observed in plexin-A1-/-
preosteoclasts. Osteoclast precursor cells from wild-type (+/+) or plexin-Al-/-
(-/-)
littermates were stained with biotinylated Sema6D-Fc (thick lines) or
biotinylated
human IgGI (dotted lines) plus streptavidin-APC'.
Figure 11: Expression profiles of plexin-A subfamily members in DCs. cDNA was
-4-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
prepared from BMDCs stimulated with anti-CD40. Expression of transcripts of
plexin-Al, -A2, -A3 -A4 and G3PDH were determined by PCR using their specific
primers. As controls, 10 ng of cDNAs of plexin-A1, -A2, -A3 and -A4 were
amplified
by PCR by their specific primers.
Figure 12: Fluorescence resonance energy transfer (FRET) between Plexin-A1 and
Trem-2. The binding of PlexinAl (deleted in its cytoplasmic region)-CFP to
Trem-2-YFP or YFP-pm (control) was analyzed in COS7 cells by intermolecular
FRET.
COS7 cells were transfected with expression plasmids and imaged for YFP, FRET,
and
CFP, which were used to represent FRETC in the IMD mode. Eight colors (red to
blue)
represent FRET efficiency, whereby the intensity of each color indicates the
mean
intensity CFP. The scale bar shows 10 m. pCAGGS-YFP-pm encoded a YFP fused to
the carboxy-terminus of K-Ras4B protein (a.a. 169-188).
(Intermolecular FRET analysis) COS7 cells expressing the fluorescent probes
were
imaged and the data were processed as described previously (a). In brief,
fluorescent
images were acquired sequentially through YFP (excitation, 510/23 nm;
emission,
560/15 nm), CFP (excitation, 420/20 nm; emission, 480/20 nm), and FRET
(excitation,
420/20 nm; emission, 535/35 nm) filter channels. Fluorescence through the FRET
filter
set consisted of a FRET-component ("corrected" FRET, FRETC) and non-FRET
components, spectral bleedthrough and cross-excitation. The non-FRE'T
components
were subtracted as previously described (b). For our experimental conditions,
we used
the following equation:
FRETC = FRET -(0.34 x CFP) -(0.10 x YFP)
After the calculation of FRETC, statistical analysis was performed with
Microsoft
Excel. a. Terai,K. & Matsuda,M. Ras binding opens c-Raf to expose the docking
site for
mitogen-activated protein kinase kinase. EMBO Rep. 6, 251-255 (2005).
b. Sorkin,A., McClure,M., Huang,F. & Carter,R. Interaction of EGF receptor and
grb2 in living cells visualized by fluorescence resonance energy transfer
(FRET)
microscopy. Curr. Biol. 10, 1395-1398 (2000).
Figure 13: Plexin-A1-/- mice were resistant to EAE induced by immunization
with a
MOG-peptide. (a) 6-8-wk-old wild-type (n=8) and plexin-A1-/- (n=8) mice were
-5-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
immunized with 100 g of MOG 35-55 in CFA slubcutaneously. 100 ng of pertussis
toxin was injected intravenously on the day of immunization and 2 d later. The
mice
were clinically scored daily: 0, no disease; 1, limp tail; 2, hind limb
weakness; 3, hind
limb paralysis; 4, hind and forelimb paralysis; 5, moribund state. Mean
clinical score
was calculated by averaging the scores of all mice, including animals that did
not
develop EAE. (b) The spinal cord of plexin-A1-/- mice were not infiltrated
with
inflammatory mononucletic cells. Spinal cords were removed and fixed in 10%
formalin. Paraffin-embedded sections were stained with hematoxylin-eosin for
light
microscopy.
(c) Impaired T-cell priming in plexin-A1-/- mice. Wild-type (open circles) and
plexin-A1-/- mice (closed circles) were immunized with 100 g of MOG 35-55 in
CFA
into the hind footpad. Seven days after priming, cells prepared from the
draining lymph
nodes were re-stimulated with various concentrations of MOG 35-55.
Figure 14: Impaired calcium oscillation in plexini-A1-/- cells. Calcium
signalling in
plexin-A 1-/- osteoclast precursor cells stimulateci with M-CSF and RANKL.
Osteoclast
precursor cells from wild-type (+/+) or plexin-A1-/- (-/-) mice were incubated
with
RANKL in the presence of M-CSF for 24 h and subjected to calcium measurement
as
previously described 25.
Figure 15: Activation of Rac induced by Sema6l) is not affected by the absence
of
DAP 12. Wild-type (+/+) or DAP 12-/- (-/-) BMDCs were stimulated with Sema6D-
Fc or
control IgG for 30 min. Cell lysates were incubated with PAK-1-GST-agarose or
Protein-G sepharose plus anti-Rac mAb, and blotted with anti-Rac mAb.
Figure 16: (a-b) FITC in plexin-A1-/- mice in comparison to those seen in wild-
type
littermates and (c) expression levels of co-stimulatory molecules, including
CD40,
CD80, CD86 and MHC class II, between wild-type and plexin-A1-/- DCs.
Figure 17: (a) plexin-A1-/- DCs stimulated allogeneic T-cells compared to wild-
type
DCs. (b) cell proliferation of CD4+ T-cells from plexin-A1-/- mice or wild-
type
littermates cultured with allogeneic wild-type DCs. (c) ability of plexin-A1-/-
DCs to
-6-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
stimulate antigen-specific T cells in vitro. (d) Proliferative responses and
cytokine
production by CD4+ T-cells in plexin-A1-/- mice.
Figure 18: (a-b) bone mass from three-dimensional microstructural analyses
using
high-resolution microcomputed tomography. (c) bone morphometric analyses. (d)
sections of plexin-A1-/- long bones showing increased trabecular mass compared
to
wild-type bones. (e) plexin-Al-/- osteoblasts promoted the formation of wild-
type
osteoclasts to the same extent as wild-type osteoblasts. (f) bone morphometric
analysis
showed normal ratios of osteoblast surface to bonie surface.
Figure 19: (a) histological bone morphometric arialyses using TRAP-staining
showed that plexin-Al-/- mice showing decreased osteoclast numbers and lower
ratios
of osteoclast surface to bone surface. (b) plexin-Al-/- mice displayed a
decrease in
deoxypirydinoline (Dpyd) and collagen type I fragments. (c) in vitro induction
of
osteoclasts 18,19 was reduced in the absence of plexin-A1. (d) plexin-A1 was
predominantly expressed in freshly isolated osteoclasts.
Figure 20: (a-c) incubation of DCs with recombi:nant soluble Sema6D 16 induced
IL-12
production and the up-regulation of MHC class I][-expression. (d) soluble
recombinant
Sema6D promoted substantial osteoclast differentiation in vitro.
Figure 21: (a) screen of several candidate molecules with putative
functions in both DCs and osteoclasts for association with plexin-A1. (b and
c)
association of plexin-Al with DAP12 in the presence of Trem-2. (d and e) cell
expression of these plexin-A1 with DAP12.
Figure 22: (a-b) 'loss of function' experiment to determine if RNAi against
Trem-2
reduced the stimulatory activities of Sema6D on DCs (Fig. 22b). (c) DAP12-/-
DCs
exhibited considerably reduced responses to Sema6D. (d) RAW264.7 cells
expressing
plexin-Al, Trem-2 and DAP12 were stimulated with recombinant soluble Sema6D
protein, tyrosine phosphorylation of DAP 12 was observed.
-7-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
DETAILED DESCRIPTION OF THE INVENTION
In a first generic embodiment, there is provided a method of treating an
inflammatory,
autoimmune or bone resorption disease, by administering to a patient a
composition
which inhibits plexin-A 1-DAP 12 interaction.
The present inventors generated plexin-Al-deficient (plexin-A1-/-) mice and
identified
its important roles not only in immune responses but also in bone homeostasis.
Furthermore, we show that plexin-A1 associates with the triggering receptor
expressed
on myeloid cells-2 (Trem-2), linking semaphorin=-signaling to the immuno-
receptor
tyrosine-based activation motif (ITAM)-bearing adaptor protein, DAP 12. Thus,
these
findings reveal an unexpected role for plexin-Al and present a novel signaling
mechanism for exerting pleiotropic functions of semaphorins.
In order to better understand the role of plexin-A 1 in vivo, the present
inventors
generated mice deficient in the plexin-Al gene by homologus recombination by
gene
targeting (see Fig. 1), and we confirmed the successful deletion of plexin-A1
by both
Northern blotting and reverse transcription polynierase chain reaction (RT-
PCR) (see
Fig. 1). Mice were born with the expected Mendelian ratios from intercrosses
of
heterozygous mutants, and the resulting plexin-A 1-/- mice were fertile.
Apparent
abnormalities were not observed by gross macroscopic or histological
examination of
the embryos (E11.5) and the brain, kidney, lung, heart, liver, and spleen in 4-
week-old
mice, all tissues in which plexin-Al-transcripts are expressed (see Fig. 2 and
3).
These observations strongly suggest the existence of functional redundancy in
the above
tissues among the plexin family members during embryonic development. However,
mutant mice had functional defects in the immune system as well as morphologic
abnormalities in the skeletal tissues. Therefore, we investigated the
biological functions
of plexin-A1 further with a focus on the immune and skeletal tissues as
described
below.
Lymphocyte development appeared to be normal in plexin-Al-/- mice. We did
not observe any differences in the expression of cell surface phenotype
markers,
-8-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
numbers and ratios of T-cells, B-cells, macrophages and Dendritic Cells (DCs)
in the
spleen and thymus between wild-type and plexin-=A1-/- mice (see Fig. 4).
Plexin-Al is highly expressed in DCs 17, and we examined the influence of
plexin-Al-deficiency on DC functions. FITC-dextran-uptake by DCs and the
appearance of fluorescent DCs in the draining lyr.nph nodes after skin
painting with
FITC in plexin-A1-/- mice were comparable to those seen in wild-type
littermates (Fig.
16a and 16b). In addition, no significant differences were seen in the
expression levels
of co-stimulatory molecules, including CD40, CD80, CD86 and MHC class II,
between
wild-type and plexin-A1-/- DCs (Fig. 16c). However, plexin-Al-/- DCs poorly
stimulated allogeneic T-cells compared to wild-type DCs (Fig. 17a). In
contrast, when
CD4+ T-cells from plexin-Al-/- mice or wild-type littermates were cultured
with
allogeneic wild-type DCs, no differences in cell proliferation were observed
(Fig. 17b),
suggesting an important role for DC-expressed plexin-Al in stimulating
allogeneic T-
cells. In addition, the ability of plexin-A1-/- DCs to stimulate antigen-
specific T cells in
vitro was also impaired (Fig. 17c). These observations are consistent with the
work of
Wong et al. using RNAi-targeting plexin-AI 17; they showed that RNAi-mediated
knock-down of plexin-AI in DCs results in a substantial reduction in T-cell
stimulation.
Thus, the expression of plexin-A1 by DCs appeai-s essential for normal T-cell
stimulation. We next examined the generation of antigen (Ag)-specific T-cells
in
immunized plexin-A1-/- mice. CD4+ T-cells were prepared from the draining
lymph
nodes of immunized wild-type or plexin-A1-/- mice and Ag-specific T-cell
responses
were examined in vitro.
Proliferative responses and cytokine production by CD4+ T-cells were
considerably
reduced in plexin-Al-/- mice (Fig. 17d), demonstrating an important role for
plexin-A1
in generating Ag-specific T-cells. In contrast, there were no differences in
the in vitro
responses of B- and T-cells to mitogenic stimulation between wild-type and
plexin-Al-
/- mice (see Fig. 5 and 6), consistent with low levels of plexin-Al-expression
in B- and
T-cells.
In the course of isolating bone marrow cells frorr.i plexin-A1-/- mice, we
observed reduced cellularity (by 25 5%) in the long bones of plexin-A1-/-
animals
-9-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
compared to wild-type littermates. In contrast, cell numbers and lymphocyte
populations in the lymphoid organs were similar between mutant and control
mice as
described (see, Fig. 4). This role of plexin-A1 and it's function in bone
homeostasis and
bone resorption diseases was therefore investigated.
Three-dimensional microstructural analyses using high-resolution microcomputed
tomography revealed that plexin-Al-deficiency unexpectedly resulted in
increased bone
mass (Fig. 18a and 18b), which we confirmed using bone morphometric analyses
(Fig.
18c). Sections of plexin-A1-/- long bones had increased trabecular mass
compared to
wild-type bones (Fig. 18d), indicating the development of osteopetrosis in
plexin-Al-/-
mice. The increased bone mass in plexin-A1-/- mice could be a consequence of
increased osteoblast function, decreased osteoclast function, or both. To
elucidate the
cellular mechanism of the observed osteopetrosis in plexin-Al-/- mice, we
examined the
development and functions of osteoblasts and osteoclasts. Plexin-A1-/-
osteoblasts
promoted the formation of wild-type osteoclasts to the same extent as wild-
type
osteoblasts (Fig. 18e) and plexin-A1-/- osteoblasts isolated from calvarias
had no
obvious functional differences in the secretion of osteoclastogenic factors,
including M-
CSF and soluble receptor activator of NF-KB ligand (RANKL), and in vitro
calcification
(data not shown). There were no differences in the levels of osteoblast
markers between
wild-type and plexin-A1-/- mice (see Fig. 7). In addition, bone morphometric
analysis
showed normal ratios of osteoblast surface to bor.ie surface (Fig. 18f).
Calcein labelling
also showed normal osteoblast activity in vivo (see Fig. 7).
Collectively, the loss of plexin-A1 had no apparent influence on osteoblast
development
and function. In contrast, histological bone morpllometric analyses using TRAP-
staining
showed that plexin-Al-/- mice had considerably decreased osteoclast numbers
and
lower ratios of osteoclast surface to bone surface (Fig. 19a). In addition,
plexin-Al-/-
mice displayed a decrease in deoxypirydinoline (Dpyd) and collagen type I
fragments
(Fig. 19b), both of which are markers of osteoclast activity and bone
resorption,
indicating reduced in vivo bone turnover by ostoclasts. Consistent with this,
the in vitro
induction of osteoclasts 1 8'19 was reduced in the albsence of plexin-Al (Fig.
19c). The
number of TRAP-positive cells varied between individual mutant mice, however,
and
-10-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
some mutant mice (-40%) exhibited a normal number of TRAP-positive cells in
vitro.
The expression of all plexin-A members was seen in in vitro induced
osteoclasts, while
plexin-Al was predominantly expressed in freshly isolated osteoclasts (Fig.
19d). It is
possible that this variability might be due to the compensatory mechanisms
involving
other plexin-A family members.
Plexin-A1 is clearly involved in the generation of'immune responses and
skeletal homeostasis, but the ligands responsible for these effects were
unclear. In the
nervous system, plexin-A1 associates with neuropilins, functioning as a signal
transducing receptor component for class III semaphorins such as Sema3A
2,15,20
However, recombinant Sema3A neither promoteci IL- 12 production or co-
stimulatory
molecule expression on DCs, nor enhanced osteoclastogenesis in vitro (data not
shown).
Conversely, we previously identified plexin-Al as a receptor for Sema6D during
chick
cardiac development 16. In the immune system, Sema6D is highly expressed in T-
cells
(see Fig. 8), implying a role for Sema6D-plexin-A1 interactions in T-cell-DC
cell-cell
contacts. We thus examined the effects of soluble recombinant Sema6D on DC
function. Incubation of DCs with recombinant soluble Sema6D 16 induced IL-12
production and the up-regulation of MHC class II-expression (Fig. 20a-20c),
while such
effects were not observed in control recombinant Sema4A proteins (see Fig. 9).
In
addition, Sema6D is expressed on osteoclasts (see Fig. 8), and soluble
recombinant
Sema6D promoted substantial osteoclast differentiation in vitro (Fig. 20d).
Collectively,
these results strongly suggest that plexin-A1 is a functional receptor for
Sema6D in both
the immune and skeletal tissues as well as during; chick cardiac development.
Consistent
with this hypothesis, when plexin-A1-/- DCs were incubated with Sema6D, Sema6D
binding, IL-12 production, and MHC class II up-regulation were all
considerably
reduced (Fig. 20a-20c). However, the residual Sema6D-binding was observed in
plexin-A1-/- osteoclast precursors induced in viti=o (see Fig. 10), thereby
recombinant
soluble Sema6D still promoted the in vitro induction of osteoclasts in the
absence of
plexin-Al (data not shown). As previously described 16, Sema6D can weakly bind
other
plexin-A subfamily members such as plexin-A4. The different responsiveness to
Sema6D between DCs and osteoclasts in plexin-A1-/- mice is likely due to the
-11-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
expression of other plexin-A subfamily members in in vitro induced osteoclasts
(Fig.
19d and see Fig. 11).
A common mechanism may underlie plexin-Al-function in the immune system
and skeletal tissue. Alternatively, these functions may be unrelated. It is
noteworthy that
plexins utilize different co-receptors to exert a vairiety of biological
effects 2'16'21
Indeed, plexin-A1 forms a receptor complex with receptor-type tyrosine kinases
such as
vascular endothelial growth factor receptor 2 (VEGFR2) or Off-track in a
region-specific manner during chick cardiac morphogenesis. However, VEGFR2 and
Off-track expression was not detected in DCs (data not shown). Therefore,
plexin-A1
may associate with additional novel co-receptors to exert the functions
described here.
To better understand the mechanisms by which p:lexin-A1 affects both the
immune
system and bone homeostasis, we screened several candidate molecules with
putative
functions in both DCs and osteoclasts for association with plexin-Al. Using
such an
approach, we found that Trem-2 associated with plexin-A1 (Fig. 21a and Fig.
12).
Therefore in a second generic embodiment, the irivention also provides a
method of
treating an inflammatory, autoimmune or bone resorption disease, by
administering to a
patient a composition which inhibits plexin-Al-Trem-2 interaction.
Trem-2 forms a receptor complex with DAP 12, an ITAM-bearing activating
adaptor
protein, via a positively-charged amino acid in its transmembrane domain 22'23
Interestingly, Trem-2 and DAP12 play critical roles not only in the
development of
immune responses but also in bone homeostasis by regulating osteoclast
development
24,25 In COS7 cells transfected with plexin-Al, T'rem-2 and DAP12, we observed
the
association of plexin-Al with DAP 12 in the presence of Trem-2 (Fig. 21b and
21 c), and
this was also confirmed in cells stably expressing these proteins and DCs
(Fig. 21 d and
21 e). To determine the structural requirements for the association of plexin-
AI and
Trem-2, we co-transfected constructs encoding T'rem-2 with a series of N-
terminal
truncation mutants of plexin-A1. As shown in Fig. 4f, the association of
plexin-A1 with
Trem-2 was still detected even in the absence of the Sema and
-12-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
plexin/semaphorin/integrin (PSI) domains of plexin-Al, although the
association was
considerably reduced. The association, however, was completely abolished by
deletion
of the plexin-Al TIG domain. It thus appears that the plexin-A1-TIG domain is
minimally required for the interaction of plexin-A,l with Trem-2.
In yet another embodiment of the invention there is provided a method of
treating an
inflammatory, autoimmune or bone resorption disease, by administering to a
patient a
composition which inhibits DAP 12-Trem-2 interaction.
In order to determine the role of Trem-2 and DAI' 12 in semaphorin-mediated
signals,
we performed a'loss of function' experiment. RNAi against Trem-2considerably
reduced the stimulatory activities of Sema6D on DCs (Fig. 22b). Similarly, DAP
12-/-
DCs exhibited considerably reduced responses to Sema6D (Fig. 22c). When
RAW264.7
cells expressing plexin-A 1, Trem-2 and DAP 12 vvere stimulated with
recombinant
soluble Sema6D protein, tyrosine phosphorylation of DAP12 was observed (Fig.
22d).
Collectively, these results strongly suggest that DAP 12 and Trem-2 are
functional
receptor components for Sema6D.
Plexin-Al is expressed in a broad range of tissues from embryos to adults (see
Fig. 3),
and a role for plexin-A1 in axon guidance and cairdiac morphogenesis during
development has been suggested 15'16 However, our present study has revealed
that the
developmental or functional defects of plexin-A1-/- mice are primarily
restricted to the
immune and skeletal tissues. Our failure to detect: defects in the nervous and
cardiovascular systems may be due to compensatory mechanisms by other plexin
family
members. In addition, there is a possibility that the mutant mice may have
subtle defects
that were overlooked in our gross macroscopic and histological analyses. More
detailed
examination of the mutant mice is required to answer these questions. Either
Ag-uptake
or expression levels of costimulatory molecules on plexin-A1-/- DCs were
comparable
to those on wild-type DCs (Fig. 16a-16c), indicating that plexin-A1 is not
involved in
the development of DCs. However, the allogeneic and Ag-specific T-cell
stimulatory
activities of DCs were impaired in plexin-A1-/- naice (Fig. 17a and 17c),
suggesting that
the deficiency of plexin-Al on DCs is primarily i-esponsible for the impaired
activity of
DCs to stimulate T-cells. In this context, the redu ced stimulatory activities
of plexin-
-13-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
A1-/- DCs could explain the defective T-cell prirning in plexin-A1-/- mice. Of
note,
Sema6D is abundantly expressed on T-cells but is down-regulated during T
helper cell
(Th) differentiation (see Fig. 8), suggesting the involvement of Sema6D-plexin-
A1
interactions in relatively early phases of immune :responses through T-cell-DC
contacts.
However, at the moment, we can not exclude the possible involvement of Sema6D-
plexin-Al interactions in effector phases of immuine responses.
The expression of Sema6D was detected not only in in vitro induced osteoclasts
but also in freshly isolated osteoclasts (see Fig. 8). However, it is
noteworthy that,
although Sema6D is a transmembrane-type semaphorin, it has been demonstrated
that a
soluble form of Sema6D is cleaved from the cell surface 16. Thus, Sema6D
appears to
act in osteoclastogenesis in an autocrine manner. In this regard, it is
possible that
Sema6D can function in both autocrine and paracrine manners during
osteoclastogenesis. However, it remains unclear if the effects of Sema6D are
different depending on the autocrine versus paracrine stimulation. Also,
further studies
will be required to know whether the biological activity of the transmembrane-
type
Smea6D is functionally different from that of soluble Sema6D.
One embodiment of the present invention relates to a method to identify, and a
kit for
identifying a compound that controls interaction of plexin-A1 with DAP12
activity in a
cell, comprising: (1) contacting a cell with a putative regulatory compound,
wherein the
cell includes a plexin-A1 protein and a DAP12 protein; and (2) assessing the
ability of
the putative regulatory compound to inhibit the interaction of plexin-A1 with
DAP 12.
The assessment step preferably comprises either i) determining the cytokine
production
as described herein-below, ii) in vitro osteoclastogenesis performed as
described
previously 24'25 and methods known in the art.
Another embodiment of the present invention relates to a method to identify a
compound that controls interaction of plexin-AI with Trem-2 activity in a
cell,
comprising: (1) contacting a cell with a putative regulatory compound, wherein
the cell
includes a plexin-Al protein and a Trem-2 protein; and (2) assessing the
ability of the
putative regulatory compound to inhibit the interaction of plexin-Al with Trem-
2. The
assessment step preferably comprises either i) determining the cytokine
production as
-14-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
described herein-below, ii) in vitro osteoclastogeriesis performed as
described
previously 24'25 and methods known in the art.
Yet another embodiment of the present invention relates to a method to
identify a
compound that controls interaction of DAP 12 with Trem-2 activity in a cell,
comprising: (1) contacting a cell with a putative regulatory compound, wherein
the cell
includes a DAP 12 protein and a Trem-2 protein; and (2) assessing the ability
of the
putative regulatory compound to inhibit the interaction of DAP 12 with Trem-2.
The
assessment step preferably comprises either i) determining the cytokine
production as
described herein-below, ii) in vitro osteoclastogenesis performed as described
previously 24'25 and methods known in the art.
The term "regulate" refers to controlling the activity of a molecule and/or
biological
function, such as enhancing or diminishing such activity or function.
The term "patient" includes both human and non-human mammals.
The terms "treating" or "treatment" mean the treatment of a disease-state in a
patient,
and include:
(i) preventing the disease-state from occurring in a patient, in particular,
when such
patient is genetically or otherwise predisposed to the disease-state but has
not yet
been diagnosed as having it;
(ii) inhibiting or ameliorating the disease-state in a patient, i.e.,
arresting or slowing
its development; or
(iii) relieving the disease-state in a patient, i.e., causing regression or
cure of the
disease-state.
Yet another embodiment of the present invention relates to an antibody or
antibody
binding site which binds plexin-A1, Trem-2 or DAP12 or fragments thereof.
3o Embodiments of the present invention further include polyclonal and
monoclonal
antibodies. Preferred embodiments of the present: invention include a
monoclonal
antibody such an anti-plexin-Al monoclonal antibody. The above antibody or
antibody
-15-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
binding site which binds plexin-A 1, Trem-2 or DAP 12 inhibits binding of
plexin-A1 to
DAP 12 or Trem-2, or Trem-2 binding to DAP 12.
Yet another embodiment of the present invention relates to a biotherapeutic
composition
comprising plexin-A1 protein, Trem-2 protein or DAP12 protein or fragments
thereof,
wherein the biotherapeutic is useful for treating an inflammatory, autoimmune
or bone
resorption disease.
The term "composition" as referred to herein include a putative compound, or a
substantially pure protein selected from plexin-A1, Trem-2 or DAP12 or
fragments
thereof, an antibody or antibody binding site which binds plexin-A1, Trem-2 or
DAP 12
or fragments thereof, to an expression vector encoding plexin-A1, Trem-2 or
DAP12 or
fragments thereof, a fusion protein comprising plexin-A 1, Trem-2 or DAP 12 or
fragments thereof. In the antibody binding site ernbodiments, the antibody
binding site
may be: specifically immunoreactive with a mature protein selected from the
group
consisting of the plexin-A1, Trem-2 or DAP12; raised against a purified or
recombinantly produced human or mouse plexin-A1, Trem-2 or DAP12; in a
monoclonal antibody, Fab, or F(ab)2; immunoreactive with denatured antigen; or
in a
labeled antibody. In certain embodiments; the antibody binding site is
detected in a
biological sample by a method of: contacting a binding agent having an
affinity for
plexin-A1, Trem-2 or DAP12 with the biological sample; incubating the binding
agent
with the biological sample to form a binding agent: plexin-A1, Trem-2 or DAP12
protein complex; and detecting the complex. In a preferred embodiment, the
biological
sample is human, and the binding agent is an antibody.
Putative compounds as referred to herein include, for example, compounds that
are
products of rational drug design, natural products and compounds having
partially
defined signal transduction regulatory properties. A putative compound can be
a
protein-based compound, a carbohydrate-based compound, a lipid-based compound,
a
nucleic acid-based compound, a natural organic compound, a synthetically
derived
organic compound, an anti-idiotypic antibody and/or catalytic antibody, or
fragments
thereof A putative regulatory compound can be obtained, for example, from
libraries of
-16-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
natural or synthetic compounds, in particular from chemical or combinatorial
libraries
(i.e., libraries of compounds that differ in sequence or size but that have
the same
building blocks; see for example, U.S. Pat. Nos. 5,010,175 and 5,266,684 of
Rutter and
Santi) or by rational drug design. In a preferred embodiment, such a compound
has a
molecular mass of less than 1000 daltons.
In a rational drug design procedure, the three-dimensional structure of a
compound,
such as a signal transduction molecule can be analyzed by, for example,
nuclear
magnetic resonance (NMR) or x-ray crystallography. This three-dimensional
structure
can then be used to predict structures of potential compounds, such as
putative
regulatory compounds by, for example, computer modelling. The predicted
compound
structure can then be produced by, for example, chemical synthesis,
recombinant DNA
technology, or by isolating a mimetope from a natural source (e.g., plants,
animals,
bacteria and fungi). Potential regulatory compouncis can also be identified
using SELEX
technology as described in, for example, PCT Publication Nos. WO 91/19813; WO
92/02536 and WO 93/03172.
In particular, a naturally-occurring intracellular signal transduction
molecule can be
modified based on an analysis of its structure and function to form a suitable
regulatory
compound. For example, a compound capable of regulating the plexin-A1-TIG
domain
can comprise a compound having similar structure to the amino acid residues in
this
domain. Such a compound can comprise a peptide, a polypeptide or a small
organic
molecule.
Putative regulatory compounds can also include rnolecules designed to
interfere with
plexin-A 1. For example, mutants of plexin-A1 can be created that interfere
with the
coupling of the protein with Trem-2 and or DAP 1:2. Putative regulatory
compounds can
include agonists and antagonists of plexin-Al, Trem-2 or DAP12. Such agonists
and
antagonists can be selected based on the structure of a naturally-occurring
ligand to
these proteins.
-17-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boelhringer Ingelheim International GmbH
The technology for producing monoclonal antiboclies is well known. In general,
an
immortal cell line (typically myeloma cells) is fused to lymphocytes
(typically
splenocytes) from a mammal immunized with whole cells expressing a given
antigen,
e.g., plexin-Al, and the culture supernatants of the resulting hybridoma cells
are
screened for antibodies against the antigen. See, generally, Kohler et at.,
1975, Nature
265: 295-497, "Continuous Cultures of Fused Cells Secreting Antibody of
Predefined
Specificity".
Immunization may be accomplished using standai-d procedures. The unit dose and
immunization regimen depend on the species of niammal immunized, its immune
status,
the body weight of the mammal, etc. Typically, the immunized mammals are bled
and
the serum from each blood sample is assayed for particular antibodies using
appropriate
screening assays. For example, anti-integrin antibodies may be identified by
immunoprecipitation of 1251-labeled cell lysates from integrin-expressing
cells.
Antibodies, including for example, anti-plexin-Al antibodies, may also be
identified by
flow cytometry, e.g., by measuring fluorescent staining of antibody-expressing
cells
incubated with an antibody believed to recognize plexin-Al molecules. The
lymphocytes used in the production of hybridoma cells typically are isolated
from
immunized mammals whose sera have already tested positive for the presence of
anti-
plexin-Al antibodies using such screening assays.
Typically, the immortal cell line (e.g., a myeloma cell line) is derived from
the same
mammalian species as the lymphocytes. Preferred immortal cell lines are mouse
myeloma cell lines that are sensitive to culture medium containing
hypoxanthine,
arninopterin and thymidine ("HAT medium"). Typically, HAT-sensitive mouse
myeloma cells are fused to mouse splenocytes using 1500 molecular weight
polyethylene glycol ("PEG 1500"). Hybridoma cells resulting from the fusion
are then
selected using HAT medium, which kills unfusecl and unproductively fused
myeloma
cells (unfused splenocytes die after several days because they are not
transformed).
Hybridomas producing a desired antibody are deltected by screening the
hybridoma
culture supernatants. For example, hybridomas prepared to produce anti- plexin-
Al
-18-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
antibodies may be screened by testing the hybridoma culture supernatant for
secreted
antibodies having the ability to bind to a recombinant plexin-Al-expressing
cell line.
To produce antibody homologs which are within the scope of the invention,
including
for example, anti- plexin-Al antibody homologs, that are intact
immunoglobulins,
hybridoma cells that tested positive in such screening assays were cultured in
a nutrient
medium under conditions and for a time sufficien't to allow the hybridoma
cells to
secrete the monoclonal antibodies into the culture medium. Tissue culture
techniques
and culture media suitable for hybridoma cells are well known. The conditioned
hybridoma culture supernatant may be collected and the anti- plexin-A1
antibodies
optionally further purified by well-known methods.
Alternatively, the desired antibody may be produced by injecting the hybridoma
cells
into the peritoneal cavity of an unimmunized mouse. The hybridoma cells
proliferate in
the peritoneal cavity, secreting the antibody which accumulates as ascites
fluid. The
antibody may be harvested by withdrawing the ascites fluid from the peritoneal
cavity
with a syringe.
Fully human monoclonal antibody homologs against, for example plexin-A 1, are
2o another preferred binding agent which may block. antigens in the method of
the
invention. In their intact form these may be prepared using in vitro-primed
human
splenocytes, as described by Boerner et al., 1991, J. Immunol. 147:86-95,
"Production
of Antigen-specific Human Monoclonal Antibodies from In Vitro-Primed Human
Splenocytes".
Alternatively, they may be prepared by repertoire cloning as described by
Persson et al.,
1991, Proc. Nat. Acad. Sci. USA 88: 2432-2436, "Generation of diverse high-
affinity
human monoclonal antibodies by repertoire cloning" and Huang and Stollar,
1991, J.
Immunol. Methods 141: 227-236, "Construction of representative immunoglobulin
variable region CDNA libraries from human peripheral blood lymphocytes without
in
vitro stimulation". U.S. Pat. No. 5,798,230 (Aug. 25, 1998, "Process for the
preparation
of human monoclonal antibodies and their use") describes preparation of human
-19-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
monoclonal antibodies from human B cells. Accoirding to this process, human
antibody-
producing B cells are immortalized by infection with an Epstein-Barr virus, or
a
derivative thereof, that expresses Epstein-Barr virus nuclear antigen 2
(EBNA2).
EBNA2 function, which is required for immortalization, is subsequently shut
off, which
results in an increase in antibody production.
In yet another method for producing fully human antibodies, U.S. Pat. No.
5,789,650
(Aug. 4, 1998, "Transgenic non-human animals for producing heterologous
antibodies")
describes transgenic non-human animals capable of producing heterologous
antibodies
1o and transgenic non-human animals having inactivated endogenous
immunoglobulin
genes. Endogenous immunoglobulin genes are suppressed by antisense
polynucleotides
and/or by antiserum directed against endogenous immunoglobulins. Heterologous
antibodies are encoded by immunoglobulin genes not normally found in the
genome of
that species of non-human animal. One or more transgenes containing sequences
of
unrearranged heterologous human immunoglobulin heavy chains are introduced
into a
non-human animal thereby forming a transgenic animal capable of functionally
rearranging transgenic immunoglobulin sequences and producing a repertoire of
antibodies of various isotypes encoded by human immunoglobulin genes. Such
heterologous human antibodies are produced in B-cells which are thereafter
immortalized, e.g., by fusing with an immortalizing cell line such as a
myeloma or by
manipulating such B-cells by other techniques to perpetuate a cell line
capable of
producing a monoclonal heterologous, fully human antibody homolog.
The conditions under which the cell of the present invention is contacted with
a putative
regulatory compound, such as by mixing, are conditions in which the cell can
exhibit
plexin-Al, Trem-2 or DAP 12 activity if essentially no other regulatory
compounds are
present that would interfere with such activity. Achieving such conditions is
within the
skill in the art, and includes an effective medium in which the cell can be
cultured such
that the cell can exhibit plexin-A1, Trem-2 or DAP12 activity. For example,
for a
mammalian cell, effective media are typically aqueous media comprising RPMI
1640
medium containing 10% fetal calf serum.
-20-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
Cells of the present invention can be cultured in a variety of containers
including, but
not limited to, tissue culture flasks, test tubes, microtiter dishes, and
petri plates.
Culturing is carried out at a temperature, pH and carbon dioxide content
appropriate for
the cell. Such culturing conditions are also within 'the skill in the art. For
example, for
Ramos cells, culturing can be carried out at 37 C, in a 5% CO2 environment.
Acceptable protocols to contact a cell with a putative regulatory compound in
an
effective manner include the number of cells per container contacted, the
concentration
of putative regulatory compound(s) administered to a cell, the incubation time
of the
putative regulatory compound with the cell, the concentration of ligand and/or
intracellular initiator molecules administered to a cell, and the incubation
time of the
ligand and/or intracellular initiator molecule with the cell. Determination of
such
protocols can be accomplished by those skilled in the art based on variables
such as the
size of the container, the volume of liquid in the container, the type of cell
being tested
and the chemical composition of the putative regulatory compound (i.e., size,
charge
etc.) being tested.
In one embodiment of the method of the present invention, a suitable number of
cells
are added to a 96-well tissue culture dish in culture medium. A preferred
number of
cells includes a number of cells that enables one to detect a change in plexin-
Al, Trem-
2 or DAP 12 activity using a detection method of the present invention
(described in
detail below). A more preferred number of cells includes between about 1 and 1
x 106
cells per well of a 96-well tissue culture dish. Following addition of the
cells to the
tissue culture dish, the cells can be preincubated at 37 C, 5% CO2 for between
about 0
to about 24 hours.
A suitable amount of putative regulatory compound(s) suspended in culture
medium is
added to the cells that is sufficient to regulate the activity of a plexin-A1,
Trem-2 or
DAP 12 protein in a cell such that the regulation is detectable using a
detection method
of the present invention. A preferred amount of putative regulatory
compound(s)
comprises between about I nM to about 10 mM of putative regulatory compound(s)
per
well of a 96-well plate. The cells are allowed to incubate for a suitable
length of time to
-21
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boetiringer Ingelheim International GmbH
allow the putative regulatory compound to enter a cell and interact with
plexin-A 1,
Trem-2 or DAP 12 protein. A preferred incubation time is between about 1
minute to
about 48 hours.
In another embodiment of the method of the present invention, cells suitable
for use in
the present invention are stimulated with a stimulatory molecules capable of
binding to
plexin-A 1, Trem-2 or DAP 12 protein of the present invention to initiate a
signal
transduction pathway and create a cellular response. Preferably, cells are
stimulated
with a stimulatory molecule following contact of a putative regulatory
compound with a
cell. Suitable stimulatory molecules can include, for example, antibodies that
bind
specifically to plexin-A1, Trem-2 or DAP12 protein. A suitable amount of
stimulatory
molecule to add to a cell depends upon factors such as the type of liganci
used (e.g.,
monomeric or multimeric; permeability, etc.) and the abundance of plexin-A1,
Trem-2
or DAP12 protein. Preferably, between about 1.0 nM and about 1 mM of ligand is
added to a cell.
The method of the present invention include determining if a composition is
capable of
regulating plexin-A1, Trem-2 or DAP12 protein activation. Such methods include
assays described in detail in the Examples section. The method of the present
invention
can further include the step of performing a toxicity test to determine the
toxicity of the
composition.
Another aspect of the present invention includes a kit to identify
compositions capable
of regulating plexin-A 1, Trem-2 or DAP 12 protein activity in a cell. Such a
kit includes:
(1) a cell comprising plexin-Al, Trem-2 or DAP1:2 protein; and (2) a means for
detecting regulation of either the plexin-A 1, Trem-2 or DAP 12 protein. Such
a means
for detecting the regulation of plexin-A1, Trem-2 or DAP12 protein include
methods
and reagents known to those of skill in the art, for example, plexin-Al
protein activity
can be detected using, for example, activation assays described herein-below.
Means for
detecting the regulation of plexin-A1, Trem-2 or DAP12 protein also include
methods
and reagents known to those of skill in the art. Suitable cells for use with a
kit of the
-22
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
present invention include cells described in detail herein. A preferred cell
for use with a
kit includes a human cell.
METHODS OF THERAPEUTIC USE
It has been found for the first time by the present inventors that plexin-A 1
can associate
with DAP 12, in both the development of normal immune responses and bone
homeostasis.
ITAM-mediated signaling through DAP12 has been previously shown to be an
important co-stimulatory signal not only for the proper development of immune
responses but also for osteoclast differentiation 24"26. As reported in DAP 12-
/- mice 26
plexin-A1-/- mice displayed impaired generation of Ag-specific T-cells, in
which they
were resistant to the development of experimenta:l autoimmune
encephalomyelitis
(EAE) (see Fig. 13). Also in the skeletal tissues, the defects in the DAP 12
gene as well
as Trem-2 gene are known to result in impaired differentiation of osteoclasts
24 ,25,27,28
Thus, the association of plexin-A1 with DAP121i.kely provides co-stimulatory
signals to
both DCs and osteoclasts. In support of this, calcium signaling was affected
in plexin-
A1-/- cells (see Fig. 12,14) as it is the case for DAP12-/- cells 25. The
invention
therefore provides a method of treating an inflammatory, autoimmune or bone
resorption disease, by administering to a patient a composition which inhibits
plexin-
A 1-DAP 12 interaction.
The present inventors have also shown that Trem-2 acts as a bridge for the
plexin-A 1-
DAP12 association. The invention therefore also provides a method of treating
an
inflammatory, autoimmune or bone resorption disease, by administering to a
patient a
composition which inhibits plexin-A 1-Trem-2 interaction.
The present inventors have also demonstrated that that DAP 12 and Trem-2 are
functional receptor components for Sema6D. The invention therefore also
provides a
method of treating an inflammatory, autoimmune or bone resorption disease, by
administering to a patient a composition which iiihibits DAP 12-Trem-2
interaction.
-23-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boeliringer Ingelheim International GmbH
A composition which would block the interaction of plexin-A1 with DAP12,
plexin-A1-
Trem-2, or DAPI2-Trem-2 would block inflammatory cytokine production from
cells.
The inhibition of cytokine production is an attractive means for preventing
and treating
a variety of cytokine mediated diseases or conditions associated with excess
cytokine
production, e.g., diseases and pathological conditions involving
inflamnlation,
autoimmune responses or bone resorption. Thus, the compositions are useful for
the
treatment of diseases and conditions including the following:
osteoarthritis, atherosclerosis, contact dermatitis, bone resorption diseases
including
osteoporosis, reperfusion injury, asthma, multiple sclerosis, Guillain-Barre
syndrome,
Crohn's disease, ulcerative colitis, psoriasis, graft versus host disease,
systemic lupus
erythematosus and insulin-dependent diabetes mellitus, rheumatoid arthritis,
toxic shock
syndrome, Alzheimer's disease, diabetes, inflamnlatory bowel diseases, acute
and
chronic pain as well as symptoms of inflammation and cardiovascular disease,
stroke,
myocardial infarction, alone or following thrombolytic therapy, thermal
injury, adult
respiratory distress syndrome (ARDS), multiple organ injury secondary to
trauma, acute
glomerulonephritis, dermatoses with acute inflammatory components, acute
purulent
meningitis or other central nervous system disorders, syndromes associated
with
hemodialysis, leukopherisis, granulocyte transfusion associated syndromes, and
necrotizing entrerocolitis, complications including restenosis following
percutaneous
transluminal coronary angioplasty, traumatic arthritis, sepsis, chronic
obstructive
pulmonary disease and congestive heart failure. Said composition may also be
useful for
anticoagulant or fibrinolytic therapy (and the diseases or conditions related
to such
therapy).
Anti-cytokine activity can be demonstrated by using methods known in the art.
See for
example Branger et al., (2002) Jlmmunol. 168: 4070-4077, and the 46 references
cited
therein.
A composition according to the invention will also be useful for treating
oncological
diseases. These diseases include but are not limited to solid tumors, such as
cancers of
the breast, respiratory tract, brain, reproductive organs, digestive tract,
urinary tract, eye,
-24-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
liver, skin, head and neck, thyroid, parathyroid ancl their distant
metastases. Those
disorders also include lymphomas, sarcomas, and leukemias.
Examples of breast cancer include, but are not limited to invasive ducta]
carcinoma,
invasive lobular carcinoma, ductal carcinoma in situ, and lobular carcinoma in
situ.
Examples of cancers of the respiratory tract include, but are not limited to
small-cell and
non-small-cell lung carcinoma, as well as bronchial adenoma and
pleuropulmonary
blastoma and mesothelioma.
Examples of brain cancers include, but are not limited to brain stem, optic
and
hypophtalmic glioma, cerebella and cerebral astrocytoma, medulloblastoma,
ependymoma, as well as pituitary,neuroectodermal and pineal tumor.
Examples of peripheral nervous system tumors include, but are not limited to
neuroblastoma, ganglioneuroblastoma, and peripheral nerve sheath tumors.
Examples of tumors of the endocrine and exocrine system include, but are not
limited to
thyroid carcinoma, adrenocortical carcinoma, pheochromocytoma, and carcinoid
tumors.
Tumors of the male reproductive organs include, but are not limited to
prostate and
testicular cancer.
Tumors of the female reproductive organs include, but are not limited to
endometrial,
cervical, ovarian, vaginal, and vulvar cancer, as well as sarcoma of the
uterus.
Tumors of the digestive tract include, but are not limited to anal, colon,
colorectal,
esophageal, gallblader, gastric, pancreatic, rectal, small-intestine, and
salivary gland
cancers.
Tumors of the urinary tract include, but are not limited to bladder, penile,
kidney, renal
pelvis, ureter, and urethral cancers.
Eye cancers include, but are not limited to intraocular melanoma and
retinoblastoma.
-25-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boelhringer Ingelheim International GmbH
Examples of liver cancers include, but are not limited to hepatocellular
carcinoma (liver
cell carcinomas with or without fibrolamellar variant), hepatoblastoma,
cholangiocarcinoma (intrahepatic bile duct carcinoma), and mixed
hepatocellular
cholangiocarcinoma.
Skin cancers include, but are not limited to squam.ous cell carcinoma,
Kaposi's sarcoma,
malignant melanoma, Merkel cell skin cancer, and non-melanoma skin cancer.
Head-and-neck cancers include, but are not limited to laryngeal/
hypopharyngeal/nasopharyngeal/oropharyngeal cancer, and lip and oral cavity
cancer.
Lymphomas include, but are not limited to AIDS-related lymphoma, non-
Hodgkin's
lymphoma, Hodgkins lymphoma, cutaneous T-cell lymphoma, and lymphoma of the
central nervous system.
Sarcomas include, but are not limited to sarcoma of the soft tissue,
osteosarcoma,
Ewings sarcoma, malignant fibrous histiocytoma, lymphosarcoma, angiosarcoma,
and
rhabdomyosarcoma. Leukemias include, but are rLot limited to acute myeloid
leukemia,
acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic
myelogenous
leukemia, and hairy cell leukemia.
Plasma cell dyscrasias include, but are not limiteci to multiple myeloma, and
Waldenstrom's macroglobulinemia.
These disorders have been well characterized in rnan, but also exist with a
similar
etiology in other mammals, and can be treated by pharmaceutical compositions
of the
present invention.
For therapeutic use, the compositions may be adrninistered in any conventional
dosage
form in any conventional manner. Routes of administration include, but are not
limited
to, intravenously, intramuscularly, subcutaneously, intrasynovially, by
infusion,
sublingually, transdermally, orally, topically or by inhalation. The preferred
modes of
administration are oral and intravenous.
-26-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
The compositions may be administered alone or in combination with adjuvants
that
enhance stability of the inhibitors, facilitate admiriistration of
pharmaceutical
compositions containing them in certain embodirnents, provide increased
dissolution or
dispersion, increase inhibitory activity, provide adjunct therapy, and the
like, including
other active ingredients. Advantageously, such combination therapies utilize
lower
dosages of the conventional therapeutics, thus avoiding possible toxicity and
adverse
side effects incurred when those agents are used as monotherapies. The above
described compositions may be physically combined with the conventional
therapeutics
or other adjuvants into a single pharmaceutical composition. Advantageously,
the
compositions may then be administered together iin a single dosage forrn. In
some
embodiments, the pharmaceutical compositions comprising such combinations of
compositions contain at least about 5%, but more preferably at least about
20%, of a
composition (w/w) or a combination thereof. The optimum percentage (w/w) of a
composition of the invention may vary and is wit:hin the purview of those
skilled in the
art. Alternatively, the compositions may be administered separately (either
serially or in
parallel). Separate dosing allows for greater flexibility in the dosing
regime.
As mentioned above, dosage forms of the compositions described herein include
pharmaceutically acceptable carriers and adjuvants known to those of ordinary
skill in
the art. These carriers and adjuvants include, for example, ion exchangers,
alumina,
aluminum stearate, lecithin, serum proteins, buffer substances, water, salts
or
electrolytes and cellulose-based substances. Pref'erred dosage forms include,
tablet,
capsule, caplet, liquid, solution, suspension, emulsion, lozenges, syrup,
reconstitutable
powder, granule, suppository and transdermal patch. Methods for preparing such
dosage forms are known (see, for example, H.C. Ansel and N.G. Popovish,
Pharmaceutical Dosage Forms and Drug Delivery Systems, 5th ed., Lea and
Febiger
(1990)). Dosage levels and requirements are well-recognized in the art and may
be
selected by those of ordinary skill in the art from available methods and
techniques
suitable for a particular patient. In some embodiments, dosage levels range
from about
1-1000 mg/dose for a 70 kg patient. Although one dose per day maybe
sufficient, up to
5 doses per day may be given. For oral doses, up to 2000 mg/day may be
required. As
the skilled artisan will appreciate, lower or higher doses may be required
depending on
-27-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
particular factors. For instance, specific dosage and treatment regimens will
depend on
factors such as the patient's general health profile, the severity and course
of the
patient's disorder or disposition thereto, and the judgment of the treating
physician.
-28-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
EXPERIMENTAL METHODS
Mice
To construct the plexin-Al targeting vector, a 3-kb fragment containing the
second exon
with the initiation codon and third exon with the coding sequence of the sema-
domain
was replaced with the neo resistance cassette, and the Herpes simplex virus
thymidine
kinase (HSV-tk) gene was inserted for selection against random integration.
The
linearized targeting plasmid DNA was transfected into ES cells by
electroporation.
After double selection with G418 and gancyclovir, 96 resistant clones were
screened for
homologous recombination of the plexin-Al targeted allele by PCR and Southern
blot
analysis as described below. Two clones with hornologous recombination were
identified and isolated. ES cells from the two independent plexin-Al mutant
clones
were injected separately into blastocysts from C5'7BL/6 mice. The blastocysts
were
transferred to pseudopregnant ICR foster mothers and chimeric males were then
backcrossed to C57BL/6 or BALB/c females. Heterozygous mice were mated to
produce homozygotes. For immunological analysis, heterozygous male mice were
backcrossed to C57BL/6 or BALB/c females for five generations. Gerrnline
transmission and the genotype of plexin-Al-targeted allele were further
assayed by
Southern blot and PCR analysis. PCR was carrieci out with 35 cycles at 94 C
for 30s,
60 C for 30s, 72 C for 60s. The following oligonucleotide primers were used to
identify
the rearranged plexin-Al locus. Primer 1(5'-AG(:ACCACACTCACACCCTCTTT-3')
was complementary to genomic DNA that was located in the 3'-untranslated
region.
Primer 2 (5'-TCCTTGATTTTCTCCTTGATGGCC-3') was complementary to
sequences at the 3'-terminus of the second exon. Primer 3
(5'-TCCCTGTCAGAGAAAACCTGGTTT-3') was complementary to genomic DNA
that was located in the untranslated region in the third exon. For Southern
blot analysis,
genomic DNA from the tails was digested with BamHI and subjected to agarose
gel
electrophoresis. DNA was transferred onto nylon blotting membranes (Hybond N;
Amersham Pharmacia), according to the manufacturer's protocol. Filters were
hybridized with radio-labelled probes overnight. Filters were then washed in
0.1xSSC,
0.1 % SDS at 65 C for one hour before autoradiography. For RT-PCR analysis,
RNA
was isolated from the brain, heart and spleen usirig RNeasy kits (Qiagen) and
treated
with DNase I (Invitrogen) to eliminate genomic DNA. cDNA was synthesized using
a
-29-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
SuperScript II cDNA synthesis kit (Invitrogen) and RT-PCR was performed with
35
cycles at 94 C for 30s, 60 C for 30s, 72 C for 30s using the primers
(5'-ACATCTACTATGTGTACAGTTTCC-3') and
(5'-AAAAACCACGGTGCGGCCTTGGGTA-3'). For northern blot analysis, total RNA
isolated from the brain was subjected to formalde:hyde-containing gel
electrophoresis
and transferred onto the blotting membrane and hybridized with radio-labelled
probes
overnight. Mice deficient in DAP12 were described previously 24. OT-2 Tg mice
were
kindly provided by Dr. William R. Heath 30. Mice were maintained in a specific
pathogen-free environment. All experimental procedures were consistent with
our
institutional guidelines.
In vitro assay
Splenic DCs were isolated from the spleen using MACS (Miltenyi Biotech). The
resulting purity was >95% in each experiment. Bone marrow-derived I)Cs (BMDCs)
were generated from bone marrow progenitors using GM-CSF. For FITC-dextran
uptake, BMDCs were stained with allophycocyanin (APC)-conjugated anti-CD11c
and
incubated with pre-warmed medium containing 2 mg ml-1 FITC-dextran for 10 min
at
37 C. After washing 3 times with chilled mediurn, internalised FITC-dextran
was
measured by FACS. For MLRs, irradiated (3000 rad) splenic DCs were cultured
with
allogeneic CD4+ T-cells (5x104 cells/well) for 48 h. To measure cell
proliferation, cells
were pulsed with 2 Ci of [3H] thymidine for the last 14 h of the culture
period.
In vivo T-cell responses For T-cell priming, mice were immunized with 100 g
of KLH
in CFA into the hind footpads 7,8. Five days afteir immunization, CD4+ T-cells
isolated
from the draining lymph nodes were stimulated vvith various concentrations of
KLH for
72 h. For proliferation assays, cells were pulsed vvith 2 Ci [3H] thymidine
for the last
14 h. Cytokine production in the culture supernatants was measured by Bio-Plex
suspension array system.
Osteoclast and osteoblast cultures
In vitro osteoclastogenesis was performed as described previously 24'25 In
brief, bone
marrow progenitor cells derived from wild-type- or plexin-A1-/- mice were
cultured
with M-CSF (10 ng ml-1) in a-MEM containing 10% FCS at 5x105 cells ml-1. At
day
-30-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
2, cells were harvested and further cultured for 3(iays with M-CSF (10 ng ml-
1) and
RANKL (10 ng ml-1) at 5x104 cells ml-1 in flat-bottomed 96-well plates. The
resulting
cells were fixed and stained with tartrate-resistant acid phosphatase using a
TRAP-
staining kit (Takara, Japan). Primary osteoblasts vvere isolated from neonatal
mouse
calvaria after sequential digestion with 0.1 % collaigenase and 0.2% dispase.
In co-
culture experiments, calvarial osteoblasts and stromal cells were co-cultured
with
nonadherent bone marrow cells in medium supplemented with 10 nM 1,25(OH)2-
vitamin D3 and 1 Mm prostaglandin E2.
Analysis of bone phenotype
Histological, histomorphmetric and microradiographic examinations were
performed
using essentially the same method as described pr=eviously 25. Statistical
analysis was
performed using Student's t-test (*p<0.05; **p<0.01; ***p<0.001).
Establishment of stable transfectants
Stable plexin-Al- , Trem-2-, and DAP12-expressing 293T cell transfectants were
established by introducing Flag-tagged plexin-Al, V5-tagged Trem-2, and myc-
tagged
DAP 12 expression constructs with pMC 1 neo vector by Lipofectamine
(Invitrogen)
according to the manufacturer's protocol. Transfe:ctants expressing Flag-
tagged
plexin-A1, V5-tagged Trem-2 and myc-tagged DAP 12 were selected in the
presence of
G418 and screened by anti-Flag mAb (M2, Sigma), anti-V5 mAb (Invitrogen) and
anti-myc antibodies (9B11, Cell Signaling Technology) and cloned.
RNAi
Four siRNA sequences specific for mouse Trem-2
(5'-CCACGGTGCTGCAGGGCAT-3', 5'-TGA(--CAAGATGCTGGAGAT-3',
5'-CGGAATGGGAGCACAGTCA -3' and 5'-GCACAGTCATCGCAGATGA-3'),
were selected (Dharmacon). All siRNA sequences were synthesized and annealed
by the
manufacturer (Dharmacon). Transfection was performed using RNAiFect (QIAGEN)
according to the manufacturer's protocol. Briefly, DCs were washed and plated
in
24-well plates in complete RPMI 1640. siRNA were incubated with RNAiFect
reagent
in complete RPMI 1640 at room temperature for 10 min and then added to the DC
-31-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
culture. After 48 h of incubation, the resulting cells were harvested, washed
and used
for subsequent experiments. Transfection efficiencies were determined using
fluorescein-labelled non-silencing RNA (40 to 50%).
Immunoprecipitation
Mouse antisera against mouse plexin-A 1 were obtained by immunizing plexin-A 1-
/-
mice with soluble plexin-Al protein in CFA and used for immunoblotting. Rabbit
antisera against mouse plexin-Al were used for immunoprecipitation. Wild-type
or
DAP12-/- BMDCs were stimulated with anti-CD40 mAb for 24 h. Cells were
solubilized in buffer containing 1% Digitonin, 10 mM Tris-Cl, 150 mM NaCI, 0.5
mM
PMSF, 5 g ml-1 aprotinin, 5 g ml-1 leupeptin, and protease inhibitor
cocktail
(Nakarai, Japan). Cell lysates were incubated with protein A-sepharose plus
anti-plexin-
A1 for 3 h at 4 C. After washing five times with lysis buffer,
immunoprecipitates were
separated by SDS-PAGE and immunoblotted with anti-DAP12 25. Whole cell lysates
were immunoblotted with anti-plexin-A I.
Phosphorylation of DAP 12
RAW264.7 cells were co-transfected with Flag-tagged plexin-Al, V5-tagged Trem-
2
and myc-tagged DAP 12 expression constructs by Lipofectamine (Invitrogen)
according
to the manufacturer's protocol and incubated for 24 h. Cells were stimulated
with 15 g
ml-1 Sema6D-Fc after 6 h of serum starvation. At various time points, cells
were
solubilized in buffer containing 1% Nonidet-P40, 10 mM Tris-Cl, 150 mM NaCl, 1
mM
EDTA,10 mM Na3VO4, 0.5 mM PMSF, 5 g ml-1 aprotinin, 5 g ml-1 leupeptin, and
protease inhibitor cocktail (Roche). Cell lysates were incubated with protein
G-agarose
plus anti-myc mAb for 3 h at 4 C. After washing five times with lysis buffer,
immunoprecipitates were separated by SDS-PAGE and immunoblotted with
anti-phosphotyrosine (4G10, Upstate Biotechnology) or anti-myc Abs.
-32-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim Intemational GmbH
References
1. Tessier-Lavigne, M. & Goodman, S. C. The molecular biology of axon
guidance.
Science 274, 1123-1133 (1996).
2. Pasterkamp, R. J. & Kolodkin, A. L. Semaphorin junction: making tracks
toward
neural connectivity. Curr Opin Neurobiol 13, 79-89 (2003).
3. Sekido, Y. et al. Human semaphorins A(V) and IV reside in the 3p21.3 small
cell
lung cancer deletion region and demonstrate distinct expression patterns.
Proc Natl Acad Sci U S A 93, 4120-5 (1996).
4. Gu, C. et al. Neuropilin-1 conveys semaphorin ,and VEGF signaling (luring
neural
and cardiovascular development. Dev Cell 5, 45-57 (2003).
5. Toyofuku, T. et al. Guidance of myocardial patterning in cardiac
development by
Sema6D reverse signalling. Nat Cell Biol 6, 1204==11 (2004).
6. Kumanogoh, A. et al. Identification of CD72 as a lymphocyte receptor for
theclass IV
semaphorin CD 100: a novel mechanism for regulating B cell signaling. Immunity
13,
621-31. (2000).
7. Shi, W. et al. The class IV semaphorin CD 100 plays nonredundant roles in
the
immune system: defective B and T cell activation in CD100-deficient
mice.Immunity
13, 633-42. (2000).
8. Kumanogoh, A. et al. Class IV semaphorin Sema4A enhances T-cell activation
and
interacts with Tim-2. Nature 419, 629-33 (2002).
9. Kumanogoh, A. et al. Nonredundant Roles of Sema4A in the Immune System:
Defective T Cell Priming and Thl/Th2 Regulation in Sema4A-Deficient
Mice.Immunity 22, 305-16 (2005).
10. Kikutani, H. & Kumanogoh, A. Semaphorins in interactions between T cells
andantigen-presenting cells. Nat Rev Immunol 3, 159-67 (2003).
11. Elhabazi, A., Marie-Cardine, A., Chabbert-de Ponnat, I., Bensussan, A. &
Boumsell,
L. Structure and function of the immune semaphoring CD100/SEMA4D. Crit Rev
Immunol 23, 65-81 (2003).
12. Tamagnone, L. & Comoglio, P. M. Signalling by semaphorin receptors: cell
guidance and beyond. Trends Cell Biology 10, 377-383 (2000).
13. Granziero, L. et al. CD 100/Plexin-B 1 interactions sustain proliferation
and survival
of normal and leukemic CD5+ B lymphocytes. Blood 101, 1962-9 (2003).
-33-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boe:hringer Ingelheim International GmbH
14. Walzer, T., Galibert, L., Comeau, M. R. & De Smedt, T. Plexin C1
engagement on
mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton
rearrangement and inhibits integrin-mediated adhesion and chemokine-induced
migration. J Immunol 174, 51-9 (2005).
15. Takahashi, T. et al. Plexin-neuropilin-1 complexes form functional
semaphorin-3A
receptors. Ce1199, 59-69. (1999).
16. Toyofuku, T. et al. Dual roles of Sema6D in cardiac morphogenesis through
region-
specific association of its receptor, Plexin-Al, with off-track and vascular
endothelial
growth factor receptor type 2. Genes Dev 18, 435-47 (2004).
17. Wong, A. W. et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell
interactions. Nat Immunol 4, 891-8 (2003).
18. Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates
osteoclastdifferentiation and activation. Ce1193, 165-76 (1998).
19. Theill, L. E., Boyle, W. J. & Penninger, J. M. RANK-L and RANK: T cells,
bone
loss, and mammalian evolution. Annu Rev Immunol 20, 795-823 (2002).
20. Takahashi, T. & Strittmatter, S. M. Plexinal autoinhibition by the plexin
sema
domain. Neuron 29, 429-39. (2001).
21. Giordano, S. et al. The semaphorin 4D receptor controls invasive growth
bycoupling
with Met. Nat Cell Biol 4, 720-4 (2002).
22. Lanier, L. L. & Bakker, A. B. The ITAM-bearing transmembrane adaptor DAP
12 in
lymphoid and myeloid cell function. Immunol Today 21, 611-4 (2000).
23. Colonna, M. TREMs in the immune system and beyond. Nat Rev Immuno13,445-
53 (2003).
24. Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic
degeneration in DAP12-deficient mice. J Clin Irivest 111, 323-32 (2003).
25. Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate
with
RANKL for bone homeostasis. Nature 428, 758-63 (2004).
26. Bakker, A. B. et al. DAP 12-deficient mice fail to develop autoimmunity
due to
impaired antigen priming. Immunity 13, 345-53 (2000).
27. Paloneva, J. et al. Loss-of-function mutatioris in TYROBP (DAP 12) result
in a
presenile dementia with bone cysts. Nat Genet 25, 357-61 (2000).
-34-
CA 02551163 2006-07-17
P02-0244/CA University of Osaka
Boehringer Ingelheim International GmbH
28. Paloneva, J. et al. Mutations in two genes encoding different subunits of
a receptor
signaling complex result in an identical disease plienotype. Am J Hum Genet
71, 656-62
(2002).
29. Turner, L. J., Nicholls, S. & Hall, A. The activity of the plexin-Al
receptor is
regulated by Rac. J Biol Chem 279, 33199-205 (2004).
30. Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. Defective TCR
expression in transgenic mice constructed using cDNA-based alpha- and
21 beta-chain genes under the control of heterologous regulatory elements.
Immunol Cell Bio176, 34-40 (1998).
-35-
CA 02551163 2006-07-17
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME il~ OF ,2
NOTE: For additional volumes please contact the Canadian Patent Office.