Note: Descriptions are shown in the official language in which they were submitted.
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
AMIDINE DERIVATIVES FOR TREATING AMYLOIDOSIS
Related Apulications
This application is related to PCT patent application publication no. WO
2003/017,994.
Background of the Invention
Amyloidosis refers to a pathological condition characterized by the presence
of amyloid
fibrils. Amyloid is a generic term referring to a group of diverse but
specific protein deposits
(intracellular or extracellular) which are seen in a number of different
diseases. Though diverse
in their occurrence, all amyloid deposits have common morphologic properties,
stain with
specific dyes (e.g., Congo red), and have a characteristic red-green
birefringent~appearaiic~'in~ ~. ~~
polarized light after staining. They also share common ultrastructural
features and common
X-ray diffraction and infrared spectra.
Amyloid-related diseases can either be restricted to one organ or spread to
several organs.
The first instance is referred to as "localized amyloidosis" while the second
is referred to as
"systemic amyloidosis."
Some amyloidotic diseases can be idiopathic, but most of these diseases appear
as a
complication of a previously existing disorder. For example,primary
amyloidosis can appear
without any other pathology or can follow plasma cell dyscrasia or multiple
myeloma.
Secondary amyloidosis is usually seen associated with chronic infection (such
as
tuberculosis) or chronic inflammation (such as rheumatoid arthritis). A
familial form of
secondary amyloidosis is also seen in Familial Mediterranean Fever (FMF). This
familial type
of amyloidosis, as one of the other types of familial amyloidosis, is
genetically inherited and is
found in specific population groups. In both primary and secondary
amyloidosis, deposits are
found in several organs and are thus considered systemic amyloid diseases.
Another type of systemic amyloidosis is found in 'long-term hemodialysis
patients. In
each of these cases, a different amyloidogenic protein is involved in amyloid
deposition.
-1-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
"Localized amyloidoses" are those that tend to involve a single organ system.
Different
amyloids are also characterized by the type of protein present in the deposit.
For example,
neurodegenerative diseases such as scrapie, bovine spongiform encephalitis,
Creutzfeldt-Jakob
disease, and the like are characterized by the appearance and accumulation of
a protease-resistant
form of a prion protein (referred to as AScr or PrP-27) in the central nervous
system. Similarly,
Alzheimer's disease, another neurodegenerative disorder, is characterized by
neuritic plaques
and neurofibrillary tangles. In this case, the plaque and blood vessel amyloid
is formed by the
deposition of fibrillary A(3 amyloid protein. Other diseases such as adult-
onset diabetes (type II
diabetes) are characterized by the localized accumulation of amyloid in the
pancreas.
Once these amyloids have formed, there is no known, widely accepted therapy or
treatment which significantly dissolves amyloid deposits in situ or that
prevents further amyloid
deposition. There is also no widely known or accepted therapy or treatment
which prevents
amyloid deposition from occuring.
Each amyloidogenic protein has the ability to organize into (3-sheets and to
form
insoluble fibrils which may be deposited extracellularly or intracellularly.
Each amyloidogenic
protein, although different in amino acid sequence, has the same property of
forming fibrils and
binding to other elements such as proteoglycan, amyloid P and complement
component.
Moreover, each amyloidogenic protein has amino acid sequences which, although
different, will
show similarities, such as regions with the ability to bind to the
glycosaminoglycan (GAG)
portion of proteoglycan (referred to as the GAG binding site) as well as other
regions which will
promote (3-sheet formation.
In specific cases, amyloidotic fibrils, once deposited, can become toxic to
the
surrounding cells. For example, the A(3 fibrils organized as senile plaques
have been shown to
be associated with:dead neuronal cells and microgliosis in patients with
Alzheimer's disease.
When tested in vitf-o, oligomeric as well as fibrillar A(3 peptide was shown
to be capable of
triggering an activation process of microglia (brain macrophages), which would
explain the
presence of microgliosis and brain inflammation found in the brain of patients
with Alzheimer's
disease. '.Both oligomeric and fibrillar A(3 peptide can also induce neuronal
cell death in vitro.
In another type of amyloidosis seen in patients with type II diabetes, the
amyloidogenic
protein IAPP, when in its oligomeric form or when organized in fibrils, has
been shown to
induce (3-islet cell toxicity in vitro. Hence, appearance of IAPP fibrils in
the pancreas of type II
diabetic patients contributes to the loss of the (3 islet cells (Langerhans)
and organ dysfunction.
Recent findings indicate that oligomeric IAPP can also be toxic.
-2-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
People suffering from Alzheimer's disease develop a progressive dementia in
adulthood,
accompanied by three main structural changes in the brain: diffuse loss of
neurons in multiple
parts of the brain; accumulation of intracellular protein deposits termed
neurofibrillary tangles;
and accumulation of extracellular protein deposits termed amyloid or senile
plaques, surrounded
by misshapen nerve terminals (dystrophic neurites). A main constituent of
these amyloid
plaques is the amyloid-~i peptide (A(3), a 39-43 amino-acid protein that is
produced through
cleavage of the (3-amyloid precursor protein (APP).
Extensive research has been conducted on the relevance of A[3 deposits in AD
(D.J.Selkoe, Trends in Cell Biology 8, 447-53 (1998)). A(3 naturally arises
from the metabolic
processing of the amyloid precursor protein ("APP") in the endoplasmic
reticulum ("ER"), the
Golgi apparatus, or the endosomal-lysosomal pathway, and most is normally
secreted as a 40
("A(3i~o") or 42 ("Aj31~2") amino acid peptide (D.J.Selkoe, Azznu. Rev. Cell
Biol. 10, 373-403
(1994)). A role for A[3 as a primary cause for AD is supported by the presence
of extracellular
amyloid [3 peptide ("A[3") deposits in senile plaques of Alzheimer's disease
("AD"), the increased
production of A(3 in cells harboring mutant AD associated genes, e.g., amyloid
precursor protein,
presenilin I and presenilin II; the toxicity of extracellular fibrillar A(3 to
cells in culture (reviewed
by D.J.Selkoe, Trends izz Cell Biology 8, 447-453 (1998)); and the toxicity of
oligomeric non-
fibrillar A(3. See, e.g., F.Gervais, Europeafz Biopharmaceutical Review, 40-
42, Autumn 2001;
May, P.C., DDT, 6:459-462, 2001). Although symptomatic treatments exist for
Alzheimer's
disease, this disease cannot be prevented or cured at this time.
Summary of the Invention
The present invention relates to the use of amidine compounds in the treatment
of
amyloid-related diseases. In particular, the invention relates to a method of
treating or
preventing an amyloid-related disease in a subject comprising administering to
the subject a
therapeutic amount of an amidine compound. Among the compounds for use in the
invention are
those according to the following Formula, such that, when administered,
amyloid fibril
formation, neurodegeneration, or cellular toxicity is reduced or inhibited:
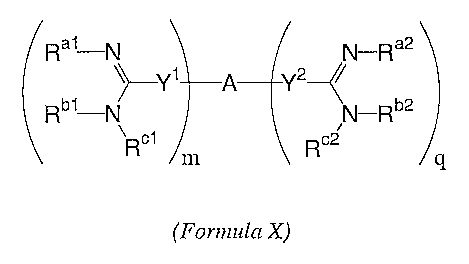
Rai-N N-Ra2
~~-Y1 A Y2~~
Rb1-N N-Rb2
~R~i R~2
m q
(Formula X)
-3-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
In one embodiment, the amidine compounds disclosed herein prevent or inhibit
amyloid
protein assembly into insoluble fibrils which, in vivo, are deposited in
various organs, or they
reverse or slow deposition in subjects already having deposits. In another
embodiment, the
amidine compound may also prevent the amyloid protein from binding or adhering
to a cell
surface and causing cell damage or toxicity. In yet another embodiment, the
compound may
block amyloid-induced cellular toxicity or microglial activation. In another
embodiment, the
amidine compound may block amyloid-induced neurotoxicity.
The amidine compounds of the invention may be administered therapeutically or
prophylactically to treat diseases associated with amyloid-(3 fibril
formation, aggregation or
deposition. The compounds of the invention may act to ameliorate the course of
an amyloid-~3
related disease using any of the following mechanisms (this list is meant to
be illustrative and not
limiting): slowing the rate of amyloid-(3 fibril formation or deposition;
lessening the degree of
~amyloid-(3 deposition; inhibiting, reducing, or preventing amyloid-(3 fibril
formation; inhibiting
neurodegeneration or cellular toxicity induced by amyloid-(3; inhibiting
amyloid-(3 induced
inflammation; or enhancing the clearance of amyloid-(3 from the brain. The
"amyloid-(3 disease"
(or "amyloid-(3 related disease," which terms as used herein are synonymous)
may be Mild
Cognitive Impairment; vascular dementia; Alzheimer's disease, including
sporadic
(non-hereditary) Alzheimer',s disease and familial (hereditary) Alzheimer's
disease; cerebral
amyloid angiopathy or hereditary cerebral hemorrhage; senile dementia; Down's
syndrome;
inclusion body myositis; or age-related macular degeneration.
Therapeutic compounds of the invention may be effective in controlling amyloid-
(3
deposition either following their entry into the brain (following penetration
of the blood brain
barrier) or from the periphery. When acting from the periphery, a compound may
alter the
equilibrium of A(3 between the brain and the plasma so as to favor the exit of
A(3 from the brain.
An increase in the exit of A(3 from the brain would result in a decrease in
A~3 brain concentration
and therefore favor a decrease in A(3 deposition. Alternatively, compounds
that penetrate the
brain could control deposition by acting directly on brain A(3, e.g., by
maintaining it in a non-
fibrillar form or favoring its clearance from the brain. These compounds could
also prevent A(3
in the brain from interacting with a cell surface and therefore prevent
neurotoxicity or
inflammation.
In another embodiment, the method is used to treat Alzheimer's disease (e.g.,
sporadic or
familial Alzheimer's disease).
Additionally, abnormal accumulation of APP and of amyloid-(3 protein in muscle
fibers
has been implicated in the pathology of sporadic inclusion body myositis (IBM)
(Askanas, et al.,
Proc. Natl. Acad. Sci. USA 93, 1314-1319 (1996); Askanas, et al., Current
Opinion i~a
Rheurncztology 7, 486-496 (1995)). Accordingly, the compounds of the invention
can be used
-4-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
prophylactically or therapeutically in the treatment of disorders in which
amyloid-beta protein is
abnormally deposited at non-neurological locations, such as treatment of IBM
by delivery of the
compounds to muscle fibers.
Additionally, it has been shown that A(3 is associated with abnormal
extracellular
deposits, known as drusen, that accumulate along the basal surface of the
retinal pigmented
epithelium in individuals with age-related macular degeneration ("AMD"). AMD
is a cause of
irreversible vision loss in older individuals. It is believed that A(3
deposition could be an
important component of the local inflammatory events that contribute to
atrophy of the retinal
pigmented epithelium, drusen biogenesis, and the pathogenesis of AMD (Johnson,
et al., Proc.
Natl. Acad. Sci. USA 99(18), 11830-5 (2002)).
The invention also pertains to new compounds and to pharmaceutical
compositions
comprising those compounds. The invention also pertains to pharmaceutical
compositions for
the treatment of amyloid-related diseases. In some embodiments, the
pharmaceutical
compositions comprise a compound as described herein that prevents or inhibits
amyloid-(3 fibril
formation, neurodegeneration, or cellular toxicity.
The present invention therefore relates to the use of amidine compounds in the
prevention
or treatment of amyloid-related diseases, including, inter alia, Alzheimer's
disease, Mild
Cognitive Impairment, cerebral amyloid angiopathy, inclusion body myositis,
Down's syndrome,
macular degeneration, and type II diabetes.
Detailed Description of the Invention
The present invention relates to the use of amidine compounds in the treatment
of
amyloid-related diseases. For example, the invention relates to a method of
treating or
preventing an amyloid-related disease in a subject (for example, a human)
comprising
administering to the subject a therapeutic amount of a compound as described
herein, such that
amyloid fibril formation or deposition, neurodegeneration, or cellular
toxicity is reduced or
inhibited. In another embodiment, the invention relates to a method of
treating or preventing an
amyloid-related disease in a subject (for example, a human) comprising
administering to the
subject a therapeutic amount of a compound as described herein, such that
cognitive function is
improved or stabilized or further deterioration in cognitive function is
prevented, slowed, or
stopped in patients with brain amyloidosis, e.g., Alzheimer's disease or
cerebral amyloid
angiopathy. For convenience, some definitions of terms referred to herein are
set forth below.
-5-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Arrzyloid-Related Diseases
AA (Reactive) Arnyloidosis
Generally, AA amyloidosis is a manifestation of a number of diseases that
provoke a
sustained acute phase response. Such diseases include chronic inflammatory
disorders, chronic
local or systemic microbial infections, and malignant neoplasms.
AA fibrils are generally composed of 8,000 Dalton fragments (AA peptide or
protein)
formed by proteolytic cleavage of serum amyloid A protein (ApoSAA), a
circulating
apolipoprotein which once secreted is complexed with HDL and which is
synthesized in
hepatocytes in response to such cytokines as II,-1, IL-6 and TNF. Deposition
can be widespread
in the body, with a preference for parenchymal organs. The spleen is usually a
deposition site,
and the kidneys may also be affected. Deposition is also common in the heart
and gastrointestinal
tract.
AA amyloid diseases include, but are not limited to inflammatory diseases,
such as
rheumatoid arthritis, juvenile chronic arthritis, ankylosing spondylitis,
psoriasis, psoriatic
arthropathy, Reiter's syndrome, Adult Still's disease, Behcet's syndrome, and
Crohn's disease.
AA deposits are also produced as a result of chronic microbial infections,
such as leprosy,
tuberculosis, bronchiectasis, decubitus ulcers, chronic pyelonephritis,
osteomyelitis, and
Whipple's disease. Certain malignant neoplasms can also result in AA fibril
amyloid deposits.
These include such conditions as Hodgkin's lymphoma, renal carcinoma,
carcinomas of gut,
lung and urogenital tract, basal cell carcinoma, and hairy cell leukemia.
AL Arnyloidoses
AL amyloid deposition is generally associated with almost any dyscrasia of the
B
lymphocyte lineage, ranging from malignancy of plasma cells (multiple myeloma)
to benign
monoclonal gammopathy. At times, the presence of amyloid deposits may be a
primary
indicator of the underlying dyscrasia.
Fibrils of AL amyloid deposits are composed of monoclonal immunoglobulin light
chains
or fragments thereof. More specifically, the fragments are derived from the N-
terminal region of
the light chain (kappa or lambda) and contain all or part of the variable (VL)
domain thereof.
Deposits generally occur in the mesenchymal tissues, causing peripheral and
autonomic
neuropathy, carpal tunnel syndrome, macroglossia, restrictive cardiomyopathy,
arthropathy of
large joints, immune dyscrasias, myelomas, as well as occult dyscrasias.
However, it should be
noted that almost any tissue, particularly visceral organs such as the heart,
may be involved.
-6-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Hereditary Syste»aic Amyloidoses
There are many forms of hereditary systemic amyloidoses. Although they are
relatively
rare conditions, adult onset of symptoms and their inheritance patterns
(usually autosomal
dominant) lead to persistence of such disorders in the general population.
Generally, the
syndromes are attributable to point mutations in the precursor protein leading
to production of
variant amyloidogenic peptides or proteins. Table 1 summarizes the fibril
composition of
exemplary forms of these disorders.
Table 1
Fibril Composition of Exemplary Amyloid-Related Diseases
Fibril Genetic ~ Clinical
Peptide/Protein Variant Syndrome
Transthyretin and Met30, many Familial amyloid polyneuropathy
fragments others (FAP), (Mainly peripheral
(ATTR) nerves)
Transthyretin and Thr45, A1a60, Cardiac involvement predominant
fragments Ser84, Met111, without neuropathy
(ATTR) I1e122
N-terminal fragment Arg26 Familial am loid
of y polyneuropathy
Apolipoprotein A1 (FAP), (mainly peripheral
(apoAI)
nerves)
N-terminal fragment Arg26, Arg50, Ostertag-type, non-neuropathic
of
Apoliproprotein A1 Arg 60, others (predominantly visceral
(AapoAI) involvement)
Lysozyme (Alys) Thr56, His67 Ostertag-type, non-neuropathic
(predominantly visceral
involvement)
Fibrogen ~/ chain Leu554, Val Cranial neuropathy with
fragment 526 lattic
corneal dystrophy
Gelsolin fragment Asn187, Tyr187 Cranial neuropathy with
(Agel) lattice
corneal dystrophy
Cystatin C fragment G1u68 Hereditary cerebral hemorrhage
' (cerebral amyloid angiopathy)
-
Icelandic type
~3-amyloid protein G1n693 Hereditary cerebral hemorrhage
(A(3)
derived from Amyloid (cerebral amyloid angiopathy)
-
Precursor Protein Dutch type
(APP)
(3-amyloid protein I1e717, Phe717,Familial Alzheimer's Disease
(A(3)
derived from Amyloid G1y717
Precursor Protein
(APP)
(3-amyloid protein Asn670, Leu671 Familial Dementia - probabl
(A(3) Y
derived from Amyloid Alzheimer's Disease
Precursor Protein
(APP)
-7_
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Table 1, continued
Fibril Genetic Clinical
Peptide/Protein Variant Syndrome
Prion Protein (PrP) Leu102, Va1167,Familial Creutzfeldt-Jakob
derived from Prp Asn178, Lys200 disease;
precursor protein Gerstmann-Straussler-Scheinker
51-91 insert syndrome (hereditary spongiform
encephalopathies, prion
diseases)
AA derived from Serum Familial Mediterranean fever,
amyloid A protein predominant renal involvement
(ApoSAA) (autosomal recessive)
AA derived from Serum Muckle-Well's syndrome,
amyloid A protein nephropathy, deafness,
(ApoSAA) urticaria, limb pain
Unknown Cardiomyopathy with persistent
atrial standstill
Unknown Cutaneous deposits (bullous,
papular, pustulodermal)
Lava aenvea from nan ~ z , repys mus. Amyozaosis. ttzstopatzzotogy, GJ(J J,
4U3-414 (1VOV 1'J'J4).
The data provided in Table 1 are exemplary and are not intended to limit the
scope of the
invention. For example, more than 40 separate point mutations in the
transthyretin gene have
been described, all of which give rise to clinically similar forms of familial
amyloid
polyneuropathy.
Transthyretin (TTR) is a 14 kiloDalton protein that is also sometimes referred
to as
prealbumin. It is produced by the liver and choroid plexus, and it functions
in transporting
thyroid hormones and vitamin A. At least 50 variant forms of the protein, each
characterized by
a single amino acid change, are responsible for various forms of familial
amyloid
polyneuropathy. For example, substitution of proline for leucine at position
55 results in a
particularly progressive form of neuropathy; substitution of methionine for
leucine at position
111 resulted in a severe cardiopathy in Danish patients.
Amyloid deposits isolated from heart tissue of patients with systemic
amyloidosis have
revealed that the deposits are composed of a heterogeneous mixture of TTR and
fragments
thereof, collectively referred to as ATTR, the full length sequences of which
have been
characterized. ATTR fibril components can be extracted from such plaques and
their structure
and sequence determined according to the methods known in the art (e.g.,
Gustavsson, A., et al.,
Laboratory Invest. 73: 703-708, 1995; Kametani, F., et al., Biochem. Biophys.
Res. Commun.
125: G22-628, 1984; Pras, M., et al., PNAS 80: 539-42, 1983).
_g_
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Persons having point mutations in the molecule apolipoprotein A1 (e.g., Gly-
~Arg26;
Trp~Arg50; Leu--~Arg60) exhibit a form of amyloidosis ("Ostertag type")
characterized by
deposits of the protein apolipoprotein AI or fragments thereof (AApoAI). These
patients have
low levels of high density lipoprotein (HDL) and present with a peripheral
neuropathy or renal
failure.
A mutation in the alpha chain of the enzyme lysozyme (e.g., Ile-~Thr56 or Asp-
~His57)
is the basis of another form of Ostertag-type non-neuropathic hereditary
amyloid reported in
English families. Here, fibrils of the mutant lysozyme protein (Alys) are
deposited, and patients
generally exhibit impaired renal function. This protein, unlike most of the
fibril-forming proteins
described herein, is usually present in whole (unfragmented) form (Benson,
M.D., et al. CIBA
Fdn. Symp. 199: 104-131, 1996).
Amyloid-(3 peptide ("A[3") is a 39-43 amino acid peptide derived by
proteolysis from a
large protein known as Beta Amyloid Precursor protein ("(3APP"). Mutations in
(3APP result in
familial forms of Alzheimer's disease, Down's syndrome or senile dementia,
characterized by
cerebral deposition of plaques composed of A(3 fibrils and other components,
which are
described in further detail below. Known mutations in APP associated with
Alzheimer's disease
occur proximate to the cleavage sites of ~3 or gamma-secretase, or within A(3.
For example,
position 717 is proximate to the site of gamma-secretase cleavage of APP in
its processing to A[3,
and positions 670/671 are proximate to the site of (3-secretase cleavage.
Mutations at any of
these residues may result in Alzheimer's disease, presumably by causing an
increase in the
amount of the 42/43 amino acid form of A(3 generated from APP. The structure
and sequence of
A(3 peptides of various lengths are well known in the art. Such peptides can
be made according
to methods known in the art, or extracted from the brain according to known
methods (e.g.,
Glenner and Wong, Biochem Bioplays. Res. Comm. 129: 885-890, 1984; Glenner and
Wong,
Biochem Biophys. Res. Comm. 122: 113 1-1135, 1984). In addition, various forms
of the
peptides are commercially available.
As used herein, the term "(3 amyloid" or "amyloid-(3" refer to amyloid (3
proteins or
peptides, amyloid (3 precursor proteins or peptides, intermediates, and
modifications and
fragments thereof, unless otherwise specifically indicated. In particular,
"A~3" refers to any
peptide produced by proteolytic processing of the APP gene product, especially
peptides which
are associated with amyloid pathologies, including A(31_3~, A(3mo, A(31-41,
Aal-a2, and A(31_43. For
convenience of nomenclature, "A(31~2" may be referred to herein as "A(3(1-42)
or simply as
"A(342" or "A(342" (and likewise for any other amyloid peptides discussed
herein). As used
herein, the terms "(3 amyloid," "amyloid-(3," and "A(3" are synonymous. Unless
otherwise
specified, the term "amyloid" refers to amyloidogenic proteins, peptides, or
fragments thereof
which can be soluble (e.g., monomeric or oligomeric) or insoluble (e.g.,
having fibrillary
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
structure or in amyloid plaque). See, e.g., MP Lambert, et al., Proc. Nat'l
Acad. Sci. USA 95,
6448-53 (1998).
According to certain aspects of the invention, amyloid-(3 is a peptide having
39-43
amino-acids, or amyloid-(3 is an amyloidogenic peptide produced from aAPP. The
amyloid-(3
diseases that are the subject of the present invention include age-related
cognitive decline, early
Alzheimer's disease as seen in Mild Cognitive Impairment ("MCI"), vascular
dementia, or
Alzheimer's disease ("AD"), which may be sporadic (non-hereditary) Alzheimer's
disease or
familial (hereditary) Alzheimer's disease. The amyloid-(3 disease may also be
cerebral amyloid
angiopathy ("CAA") or hereditary cerebral hemorrhage. The amyloid-(3 disease
may be senile
dementia, Down's syndrome, inclusion body myositis ("IBM"), or age-related
macular
degeneration ("ARMD")
Gelsolin is a calcium binding protein that binds to fragments and actin
filaments.
Mutations at position 187 (e.g., Asp~Asn; Asp~Tyr) of the protein result in a
form of
hereditary systemic amyloidosis, usually found in patients from Finland, as
well as persons of
Dutch or Japanese origin. In afflicted individuals, fibrils formed from
gelsolin fragments (Agel),
usually consist of amino acids 173-243 (68 kDa carboxyterminal fragment) and
are deposited in
blood vessels and basement membranes, resulting in corneal dystrophy and
cranial neuropathy
which progresses to peripheral neuropathy, dystrophic skin changes and
deposition in other
organs. (Kangas, H., et al., Human Mol. Genet. 5(9): 1237-1243, 1996).
Other mutated proteins, such as mutant alpha chain of fibrinogen (AfibA) and
mutant
cystatin C (Acys) also form fibrils and produce characteristic hereditary
disorders. AfibA fibrils
form deposits characteristic of a nonneuropathic hereditary amyloid with renal
disease; Acys
deposits are characteristic of a hereditary cerebral amyloid angiopathy
reported in Iceland
(Isselbacher, Harrison's Principles of Internal Medicine, McGraw-Hill, San
Francisco, 1995;
Benson, et al.). In at least some cases, patients with cerebral amyloid
angiopathy (CAA) have
been shown to have amyloid fibrils containing a non-mutant form of cystatin C
in conjunction
with amyloid beta protein (Nagai, A., et al., Molec. Chem. Neuropatl2ol. 33:
63-78, 1998).
Certain forms of prion disease are now considered to be heritable, accounting
for up to
15% of cases, which were previously thought to be predominantly infectious in
nature. (Baldwin,
et al., in Research Advances in Alzheimer's Disease arid Related Disorders,
John Wiley and
Sons, New York, 1995). In such prion disorders, patients develop plaques
composed of abnormal
isoforms of the normal prion protein (PrPs°).
A predominant mutant isoform, PrPs°, also referred to as AScr, differs
from the normal
cellular protein in its resistance to protease degradation, insolubility after
detergent extraction,
deposition in secondary lysosomes, post-translational synthesis, and high (3-
pleated sheet
-10-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
content. Genetic linkage has been established for at least five mutations
resulting in
Creutzfeldt-Jacob disease (CJD), Gerstmann-Straussler-Scheinker syndrome
(GSS), and fatal
familial insomnia (FFI). (Baldwin, supra) Methods for extracting fibril
peptides from scrapie
fibrils, determining sequences and making such peptides are known in the art
(e.g., Beekes, M.,
et al., J. Gen. Virol. 76: 2567-76, 1995).
For example, one form of GSS has been linked to a PrP mutation at codon 102,
while
telencephalic GSS segregates with a mutation at codon 117. Mutations at codons
198 and 217
result in a form of GSS in which neuritic plaques characteristic of
Alzheimer's disease contain
PrP instead of A(3 peptide. Certain forms of familial CJD have been associated
with mutations at
codons 200 and 210; mutations at codons 129 and 178 have been found in both
familial CJD and
FFI. (Baldwin, supra).
Senile Syste»aic Amyloidosis
Amyloid deposition, either systemic or focal, increases with age. For example,
fibrils of
wild type transthyretin (TTR) are commonly found in the heart tissue of
elderly individuals.
These may be asymptomatic, clinically silent, or may result in heart failure.
Asymptomatic
fibrillar focal deposits may also occur in the brain (A(3), corpora amylacea
of the prostate (A[32
microglobulin), joints and seminal vesicles.
Cerebral Amyloidosis
Local deposition of amyloid is common in the brain, particularly in elderly
individuals.
The most frequent type of amyloid in the brain is composed primarily of A(3
peptide fibrils,
resulting in dementia or sporadic (non-hereditary) Alzheimer's disease. In
fact, the incidence of
sporadic Alzheimer's disease greatly exceeds forms shown to be hereditary.
Fibril peptides
forming these plaques are very similar to those described above, with
reference to hereditary
forms of Alzheimer's disease ("AD")
Cerebral amyloid angiopathy ("CAA") refers to the specific deposition of
amyloid fibrils
in the walls of leptomingeal and cortical arteries, arterioles and in
capillaries and veins. It is
commonly associated with Alzheimer's disease, Down's syndrome and normal
aging, as well as
with a variety of familial conditions related to stroke or dementia (see
Frangione et al., Amyloid:
J. Protein Folding Disord. 8, Suppl. 1, 36-42 (2001)). CAA can occur
sporadically or be
hereditary. Multiple mutation sites in either A(3 or the APP gene have been
identified and are
clinically associated with either dementia or cerebral hemorrhage. Exemplary
CAA disorders
include, but are not limited to, hereditary cerebral hemorrhage with
amyloidosis of Icelandic type
(HCHWA-I); the Dutch variant of HCHWA (HCHWA-D; a mutation in A(3); the
Flemish
-11-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
mutation of A(3; the Arctic mutation of A(3; the Italian mutation of A(3; the
Iowa mutation of A(3;
familial British dementia; and familial Danish dementia.
Dialysis-Related Amyloadosis
Plaques composed of /32 microglobulin (A(32M) fibrils commonly develop in
patients
receiving long term hemodialysis or peritoneal dialysis. /32 microglobulin is
a 11.8 kiloDalton
polypeptide and is the light chain of Class I MHC antigens, which are present
on all nucleated
cells. Under normal circumstances, it is continuously shed from cell membranes
and is normally
filtered by the kidney. Failure of clearance, such as in the case of impaired
renal function, leads
to deposition in the carpal tunnel and other sites (primarily in collagen-rich
tissues of the joints).
Unlike other fibril proteins, A(32M molecules are generally present in
unfragmented form in the
fibrils. (Benson, supra).
Islet Amyloid Polypeptide azzd Diabetes
Islet hyalinosis (amyloid deposition) was first described over a century ago
as the
presence of fibrous protein aggregates in the pancreas of patients with severe
hyperglycemia .
(Opie, EL., JExp. Med. 5: 397-428, 1990). Today, islet amyloid, composed
predominantly of
islet amyloid polypeptide (IAPP), or amylin, is a characteristic
histopathological marker in over
90% of all cases of type II diabetes (also known as Non-Insulin Dependent
Diabetes, or
N117DM). These fibrillar accumulations result from the aggregation of the
islet amyloid
polypeptide (IAPP) or amylin, which is a 37 amino acid peptide, derived from a
larger precursor
peptide, called pro-IAPP.
IAPP co-localizes and is co-secreted with insulin in response to (3-cell
secretagogues.
This pathological feature is not associated with insulin-dependent (type I)
diabetes and is a
unifying characteristic for the heterogeneous clinical phenotypes diagnosed as
NIDDM (type II
diabetes).
Longitudinal studies in cats and immunocytochemical investigations in monkeys
have
shown that a progressive increase in islet amyloid is associated with a
dramatic decrease in the
population of insulin-secreting (3-cells and increased severity of the
disease. More recently,
transgenic studies have strengthened the relationship between IAPP plaque
formation and (3-cell
dysfunction, indicating that amyloid deposition is a principal factor in Type-
II diabetes.
IAPP has also been shown to induce (3-islet cell toxicity in vitro, indicating
that
appearance of IAPP fibrils in the pancreas of type II or type I diabetic
patients
(post-transplantation) could contribute to the loss of the (3 islet cells
(Langerhans) and organ
dysfunction. In patients with Type-II diabetes, the accumulation of pancreatic
IAPP leads to a
buildup of IAPP-amyloid as insoluble fibrous deposits which eventually replace
the
-12-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
insulin-producing (3 cells of the islet resulting in (3 cell depletion and
failure (Westermark, P.,
Grimelius, L., Acta Path. Microbiol. Scand., sect. A. 81: 291-300, 1973; de
Koning, EJP., et al.,
Diabetologia 36: 378-384, 1993; and Lorenzo, A., et al., Nature 368: 756-760,
1994).
Diseases caused by the death or malfunctioning of a particular type or types
of cells can
be treated by transplanting into the patient healthy cells of the relevant
type of cell. This
approach has been used for type I diabetes patients. Often pancreatic islet
cells are cultured in
vitro prior to transplantation to increase their numbers, to allow them to
recover after the
isolation procedure or to reduce their immunogenicity. However, in many
instances islet cell
transplantation is unsuccessful, due to death of the transplanted cells. One
reason for this poor
success rate is LAPP, which can form fibrils and become toxic to the cells in
vitro. In addition,
IAPP fibrils are likely to continue to grow after the cells are transplanted
and cause death or
dysfunction of the cells. This may occur even when the cells are from a
healthy donor and the
patient receiving the transplant does not have a disease that is characterized
by the presence of
fibrils. For example, compounds of the present invention may also~be used in
preparing tissues
or cells for transplantation according to the methods described in
International Patent
Application (PCT) number WO 01/03,680.
Hormone-Derived Amyloidoses
Endocrine organs may harbor amyloid deposits, particularly in aged
individuals.
Hormone-secreting tumors may also contain hormone-derived amyloid plaques, the
fibrils of
which are made up of polypeptide hormones such as calcitonin (medullary
carcinoma of the
thyroid), islet amyloid polypeptide (amylin; occurring in most patients with
type II diabetes), and
atrial natriuretic peptide (isolated atrial amyloidosis). Sequences and
structures of these proteins
are well known in the art.
Miscellaneous Anayloidoses
There are a variety of other forms of amyloid disease that are normally
manifest as
localized deposits of amyloid. In general, these diseases are probably the
result of the localized
production or lack of catabolism of specific fibril precursors or a
predisposition of a particular
tissue (such as the joint) for fibril deposition. Examples of such idiopathic
deposition include
nodular AL amyloid, cutaneous amyloid, endocrine amyloid, and tumor-related
amyloid.
The compounds of the invention may be administered therapeutically or
prophylactically
to treat diseases associated with amyloid-(3 fibril formation, aggregation or
deposition. The
compounds of the invention may act to ameliorate the course of an amyloid-(3
related disease
using any of the following mechanisms (this list is meant to be illustrative
and not limiting):
slowing the rate of amyloid-(3 fibril formation or deposition; lessening the
degree of amyloid-(3
-13-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
deposition; inhibiting, reducing, or preventing amyloid-(3 fibril formation;
inhibiting
neurodegeneration or cellular toxicity induced by amyloid-(3; inhibiting
amyloid-(3 induced
inflammation; or enhancing the clearance of amyloid-(3 from the brain.
Compounds of the invention may be effective in controlling amyloid-(3
deposition either
following their entry into the brain (following penetration of the blood brain
barrier) or from the
periphery. When acting from the periphery, a compound may alter the
equilibrium of A[3
between the brain and the plasma so as to favor the exit of A(3 from the
brain. An increase in the
exit of A~3 from the brain would result in a decrease in A(3 brain
concentration and therefore
favor a decrease in A(3 deposition. Alternatively, compounds that penetrate
the brain could
control deposition by acting directly on brain A(3, e.g., by maintaining it in
a non-fibrillar form
or favoring its clearance from the brain.
In an embodiment, the method is used to treat Alzheimer's disease (e.g.,
sporadic or
familial AD). The method can also be used prophylactically or therapeutically
to treat other
clinical occurrences of amyloid-(3 deposition, such as in Down's syndrome
individuals, patients
with Mild Cognitive Impairment, patients with cerebral amyloid angiopathy
("CAA"), or
hereditary cerebral hemorrhage.
Additionally, abnormal accumulation of APP and of amyloid-[3 protein in muscle
fibers
has been implicated in the pathology of sporadic inclusion body myositis (IBM)
(Askanas, V., et
al. (199G) Proc. Natl. Acad. Sci. USA 93: 1314-1319; Askanas, V. et al. (1995)
Current Opifaiora
in Rheumatology 7: 48G-49G). Accordingly, the compounds of the invention can
be used
prophylactically or therapeutically in the treatment of disorders in which
amyloid-beta protein is
abnormally deposited at non-neurological locations, such as treatment of IBM
by delivery of the
compounds to muscle fibers.
Additionally, it has been shown that A(3 is associated with abnormal
extracellular
deposits, known as drusen, that accumulate along the basal surface of the
retinal pigmented
epithelium in individuals with age-related macular degeneration ("AMD"). AMD
is a cause of
irreversible vision loss in older individuals. It is believed that A(3
deposition could be an
important component of the local inflammatory events that contribute to
atrophy of the retinal
pigmented epithelium, drusen biogenesis, and the pathogenesis of AMD (Johnson,
et al., Proc.
Natl. Acad. Sci. USA 99(18), 11830-5 (2002)).
The present invention therefore relates to the use of amidine compounds in the
prevention
or treatment of amyloid-related diseases, including, ifater- alia, Alzheimer's
disease, Mild
Cognitive Impairment, cerebral amyloid angiopathy, inclusion body myositis,
Down's syndrome,
and type II diabetes.
-14-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
In one embodiment, compounds of the invention have at least two amidine
moieties (for
example, arylamidines or benzamidines).
In one particular embodiment, the present invention relates to the novel use
of amidine
compounds in the prevention or treatment of amyloid-related diseases, such as
those disclosed in
U.S. Patent Nos. 5,428,051, 4,963,589, 5,202,320, 5,935,982, 5,521,189,
5,686,456, 5,627,184,
5,622,955, 5,606,058, 5,668,167, 5,667,975, 6,025,398, 6,214,883, 5,817,687,
5,792,782,
5,939,440, 6,017,941, 5,972,969, 6,046,226, 6,294,565 (B1), 6,156,779,
6,326,395, 6,008,247,
6,127,554, 6,172,104, 4,940,723, 5,594,138, 5,602,172, 5,206,236, 5,843,980,
4,933,347,
5,668,166, 5,817,686, 5,723,495, 4,619,942, 5,792,782, 5,639,755, 5,643,935,
and 5,578,631,
each of which are hereby incorporated herein by reference in their entirety.
Additional synthesis
protocols may be found in PCT Patent Application Publication No. WO
20031017,994. Still
further additional examples and synthesis protocols may be found in U.S.
Patent Application
Publication No. 2002/0161043, incorporated herein by reference.
In another embodiment, the invention relates to a method of treating or
preventing an
amyloid-related disease in a subject (for example, a human) comprising
administering to the
subject a therapeutic amount of a compound according to the following Formula,
such that
amyloid.fibril formation or deposition, neurodegeneration, or cellular
toxicity is reduced or
inhibited. In another embodiment, the invention relates to a method of
treating or preventing an
amyloid-related disease in a subject (for example, a human) comprising
administering to the
subject a therapeutic amount of a compound according to the following Formula,
such that
cognitive function is stabilized or further deterioration in cognitive
function is prevented, slowed,
or stopped in patients with brain amyloidosis, e.g., Alzheimer's disease or
cerebral amyloid
angiopathy:
Ra1-N N-Ra2
~~--Y1 A Y2--~~
Rb1-N N-Rb2
~Ro1 Rc2
m q
(Formula X)
wherein
each of Ral, Rbl, R~i, Ra2, Rb2~ and R°2 is independently a hydrogen, a
Z group, or Ral and
Rbl or Ra2 and Rb2 are both taken together along with the nitrogen atoms to
which
they are bound to form a ring structure;
each of Yl and Y2 is independently a direct bond or a linking moiety;
-15-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
m and q are each independently an integer selected from zero to five
inclusive, such that
1_<m+q<_5, or in another embodiment, 2<m+q<_5, or in another embodiment
1<_m+q<_10, or in another embodiment, 2<m+q<_10; and
A is a carrier moiety selected from substituted or unsubstituted aliphatic and
aromatic
groups, and combinations thereof; for example, such that the Yl and YZ
moieties are
bonded to an aromatic group.
The A group may be a divalent group (i.e., m+q=2) such as an alkylene group
(i.e.,
-(CHZ)k- and substituted analogs thereof (including groups in which a -CHZ-
moiety is substituted
by an oxygen atom), where k is 1 to 12 (for example, 6 to 9, or 7 to 9), an
alkenylene group (for
example, 2 to 12 carbon atoms, or 6 to 9 carbon atoms, including groups with
more than one
double bond), an alkynylene group (for example, ably 2 to 12 carbon atoms, or
6 to 9 carbon
atoms, including groups with more than one triple bond), an alkoxyalkylene
group, an
alkylaminoalkylene group, a thioalkoxyalkylene group, an arylenedialkylene
group, a
heteroarylenedialkylene group, an arylene group, a heteroarylene group, an
oligoethereal group
such as an oligo(alkyleneoxide) group, or an arylene-di(oligoalkyleneoxide)
group, each of
which may be substituted (with a Z group as defined below, e.g., a
hydroxyalkylene group) or
unsubstituted.
The A group also includes the corresponding moieties of the Formulae I - IV
herein as
well as those moieties exemplified in by the compounds (and amyloid-targeting
moieties) herein,
including the groups in Table 2.
In some exemplary aspects of the invention, the invention relates to a method
of treating
or preventing an amyloid-related disease in a subject (for example, a human)
comprising
administering to the subject a therapeutic amount of a compound according to
one of the
following Formulae, such that amyloid fibril formation or deposition,
neurodegeneration, or
cellular toxicity is reduced or inhibited. In another embodiment, the
invention relates to a
method of treating or preventing an amyloid-related disease in a subject (for
example, a human)
comprising administering to the subject a therapeutic amount of a compound
according to one of
the following Formulae, such that cognitive function is stabilized or further
deterioration in
cognitive function is prevented, slowed, or stopped in patients with brain
amyloidosis, e.g.,
Alzheimer's disease or cerebral amyloid angiopathy:
-16-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
~Rl~n
Ra1-N
1
Rb1- ~Y ~ ~ X~,M,
~Ro1
m q
(Formula 1)
Ra1-N
\/ Y1
Rbi-N ~ ~ ~X1_R1~
~R~1 ~ n
m
(Formula II)
Ra1
Rb1\ b2
f
1,M~X2
(Formula 111)
Ra1
Rb1\ Rb2
N
I
R
q
-17-
~ , h.
(Formula IV)
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Ra,
,Rb1\r ~b2
1
v F
q
(Formula IVb)
* Rci Rc2
R 1 N A-N R2
N~Ra1 N~Ra2
(Formula V)
wherein
each of Ral, Rby R~l, Raz, Rb2~ and R°2 is independently a hydrogen, a
Z group, or Ral and
Rbl or Ra2 and Rb2 are both taken together along with the nitrogen atoms to
which
they are bound to form a ring structure; ,
each of Yl and YZ is independently a direct bond or a joining moiety;
A is a carrier moiety selected from substituted or unsubstituted aliphatic and
aromatic
groups, and combinations thereof; for example, such that the Yl and YZ
moieties are
bonded to an aromatic group; -
each of Rl and Rz is independently a hydrogen or a Z group, or two adjacent or
proximate
Rl and RZ groups, along with the corresponding Xl and XZ groups, if present
(e.g., in
Formula II), taken together with the ring (e.g., phenyl ring) to which they
are bound
form a fused ring structure, e.g., an aromatic or heteroaromatic (e.g.,
benzofuran)
structure, or a cycloalkyl or heterocylic structure;
each of R3 and R4 is independently selected from the group consisting of
hydrogen,
substituted or unsubstituted straight or branched alkyl (for example, Cl-CS),
cycloalkyl (for example, C3-C8), carbocyclic, aryl (e.g., phenyl),
heterocyclic, and
heteroaryl;
each of Rl* and R2* is independently selected from the group consisting of
substituted or
unsubstituted straight or branched alkyl, cycloalkyl, heterocyclic, aryl
(including
phenyl), and heteroaryl;
-1&-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
each of Xl and XZ is independently a direct bond, or an oxygen, a NR' group
(where R' is
hydrogen (i.e., NH), a C1-CS alkyl, CZ-CS alkenyl, CZ-CS alkynyl, or aryl
group), a
sulfonamide group (i.e., NHSOZ or SO~NH), a carbonyl, amide (i.e., NHCO or
CONH), a Cl-CS alkylene group (e.g., -CHZ-), C2-CS alkenylene group (e.g., E
or Z
-CH=CH-), C2-C5 alkynylene group, or a sulfur atom, or combinations thereof
(e.g.,
-OCH~-, -CH20-, E or Z -OCH=CH- or -CH=CHO-);
M is a divalent group such as an alkylene group, i.e., -(CHz)k- and
substituted analogs
thereof (including groups in which a -CH2- moiety is substituted by an oxygen
atom),
where k is 1 to 12 (for example, 5 to 10, or 6 to 9, or even 7 to 8), an
alkenylene
group (for example, 2 to 12 carbon atoms, or 6 to 9 carbon atoms, including
groups
with more than one double bond), an alkynylene group (for example, 2 to 12
carbon
atoms, or 6 to 9 carbon atoms, including groups with more than one triple
bond), an
alkoxyalkylene group, an alkylaminoalkylene group, a thioalkoxyalkylene group,
an
arylenedialkylene group, an alkylenediarylene group, a heteroarylenedialkylene
group, an aryl'ene group, a heteroarylene group, an oligoethereal group such
as an
oligo(alkyleneoxide) group, or an arylene-di(oligoalkyleneoxide) group, each
of
which may be substituted (with, for example, a Z group as defined herein,
e.g., a
hydroxyalkylene group such as -(CH2)o_6(CHOH)(CH2)o_6 ; or other such
substituted
moieties, e.g., -(CH2)o-s(CHZ)(CHZ)o_~-, including -
(CH2)o_6(CHCOZalkyl)(CHZ)o_6-)
or unsubstituted;
Z is a substituted or unsubstituted moiety selected from straight or branched
alkyl (for
example, Cl-CS), cycloalkyl (for example, C3-C$), alkoxy (for example, Cl-C6),
thioalkyl (for example, Cl-C~), alkenyl (for example, CZ-C6), alkynyl (for
example,
Cz-C6), heterocyclic, carbocyclic, aryl (e.g., phenyl), aryloxy (e.g.,
phenoxy), aralkyl
(e.g., benzyl), aryloxyalkyl (e.g., phenyloxyalkyl), arylacetamidoyl,
alkylaryl,
heteroaralkyl, alkylcarbonyl and arylcarbonyl or other such acyl group,
heteroarylcarbonyl, or heteroaryl group, (CR'R")o3NR'R" (e.g., -NHZ),
(CR'R")o_3CN
(e.g., -CN), NOZ, halogen (e.g., F, Cl, Br, or I), (CR'R")o_3C(halogen)3
(e.g., -CF3),
(CR'R")o_3CH(halogen)Z, (CR'R")o_3CH2(halogen), (CR'R")o_3CONR'R",
(CR'R")o_3(CNH)NR'R", (CR'R")o_3S(O)1-zNR'R", (CR'R")o_3CHO,
(CR~R")o-s0(CR'R")o-3H~ (CR'R")o-sS(O)o-sR' (e.g., -S03H)
(CR'R")o-s0(CR'R")o-sH (e~g~, -CHzOCH3 and -OCH3), (CR'R")o-sS(CR'R")o_3H
(e.g., -SH and -SCH3), (CR'R")o_30H (e.g., -OH), (CR'R")o_3COR',
(CR'R")o_3(substituted or unsubstituted phenyl), (CR'R")o_3(C3-C$ cycloalkyl),
(CR'R")o_3COZR' (e.g., -C02H), or (CR'R")o_30R' group, or the side chain of
any
naturally occurring amino acid;
-19-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
m and q are each independently an integer selected from zero to five
inclusive;
in Formula I, m and q are each independently an integer selected from zero to
four
inclusive, and n and p are each independently an integer selected from zero to
four
inclusive, such that m+n<5 and p+q<_5, wherein either m or q is at least one;
for
example, m and q are one;
in Formula II, m is an integer selected from one to six inclusive, and n is an
integer
selected from zero to five inclusive, such that m+n<_6;
in Formula III, m, n, p, and q are each independently an integer selected from
zero to
three inclusive, m+n<-4, p+q_<4, and m+q>_1 (for example, m=q=1);
in Formula IV and IVb, m and n are each independently an integer selected from
zero to
three inclusive, p and q are each independently an integer selected from zero
to four
inclusive, m+n<_4, p+q<_5, and m+q>-1 (for example, m=q=1);
and pharmaceutically acceptable salts thereof.
In another embodiment, Z is a substituted or unsubstituted moiety selected
from straight
or branched alkyl (for example, Cl-C5), cycloalkyl (for example, C3-C$),
alkoxy (for example,
Cl-C6), thioalkyl (for example, Cl-C~), alkenyl (for example, C2-C6), alkynyl
(for example,
C2-C6), heterocyclic, carbocyclic, aryl (e.g., phenyl), aryloxy (e.g.,
phenoxy), aralkyl (e.g.,
benzyl), aryloxyalkyl (e.g., phenyloxyalkyl), arylacetamidoyl, alkylaryl,
heteroaralkyl,
alkylcarbonyl and arylcarbonyl or other such acyl group, heteroarylcarbonyl,
or heteroaryl group,
(CR'R")o_IONR'R" (e.g., -NHZ), (CR'R")o_IOCN (e.g., -CN), N02, halogen (e.g.,
F, Cl, Br, or I),
(CR'R")o_loC(halogen)3 (e.g., -CF3), (CR'R")o_IOCH(halogen)Z,
(CR'R")o_loCH2(halogen),
(CR'R")o-ioCONR'R", (CR'R")o-io(CNH)NR'R", (CR'R")o-ioS(O)i-aNR'R", (CR'R")o-
ioCHO,
(CR'R")o-io0(CR'R")o-loH~ (CR'R")o-ioS(O)o-3R' (e.g., -S03H)~ (CR'R")o-
ioO(CR'R")o-ioH (e.g.~
-CH20CH3 and -OCH3), (CR'R")o_loS(CR'R")o_3H (e.g., -SH and -SCH3),
(CR'R")o_IOOH (e.g.,
-OH), (CR'R")o_IOCOR', (CR'R")o_lo(substituted or unsubstituted phenyl),
(CR'R")o_lo(C3-Ca
cycloalkyl), (CR'R")o_loCO2R' (e.g., -C02H), or (CR'R")o_IOOR' group, or the
side chain of any
naturally occurring amino acid; wherein R' and R" are each independently
hydrogen, a Cl-C5
alkyl, C2-CS alkenyl, CZ-C5 alkynyl, or aryl group, or R' and R" taken
together are a benzylidene
group or a -(CH2)2O(CHZ)2- group.
The chemical structures herein are drawn according to the conventional
standards known
in the art. Thus, where an atom, such as a carbon atom, as drawn appears to
have an unsatisfied
valency, then that valency is assumed to be satisfied by a hydrogen atom even
though that
hydrogen atom is not necessarily explicitly drawn. The structures of some of
the compounds of
this invention include stereogenic carbon atoms. It is to be understood that
isomers arising from
such asymmetry (e.g., all enantiomers and diastereomers) are included within
the scope of this
invention unless indicated otherwise. That is, unless otherwise stipulated,
any chiral carbon
-20-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
center may be of either (R)- or (S)-stereochemistry. Such isomers can be
obtained in
substantially pure form by classical separation techniques and by
stereochemically-controlled
synthesis. Furthermore, alkenes can include either the E- or Z geometry, where
appropriate. In
addition, the compounds of the present invention may exist in unsolvated as
well as solvated
forms with acceptable solvents such as water, THF, ethanol, and the like. In
general, the
solvated forms are considered equivalent to the unsolvated forms for the
purposes of the present
invention. The compounds are typically synthetic and and may be isolated and
purified to
homogeneity.
In an alternate embodiment, the invention relates to novel compounds, and
novel methods
of their use as described herein, which are within the scope of the Formulae
disclosed herein, and
which are not disclosed in the above-referenced U.S. Patents and Patent
Applications.
The groups Ral, Rbl, R°l, Ra2, Rb2~ and R~2 in the above Formulae may
be a hydrogen, or a
substituted or unsubstituted Cl-C8 alkyl or Ci-C$ alkoxy group or a hydroxy
group. Example Ral
and Ra' groups are hydrogen, hydroxyl, alkyloxy groups (especially lower
alkyloxy groups, e.g.
methoxy), aryloxy, acyloxy, and aroyloxy (i.e., R-(C=O)-O-, wherein R is
aliphatic or aromatic).
The phrase "Ra and Rb both taken together along with the nitrogen atoms to
which they
are bound to form a ring structure" means that the two Ra and Rb groups are a
moiety which joins
the two nitrogen atoms in a heterocycle, such as the following ring
structures:
~/Zr
wherein r is an integer from zero to 4 inclusive,
N~/~~ r
/N
;~.~ ~Rc
, wherein r is an integer from zero to 2 inclusive,
~Zr
N
N
;~.~ ~Rc
wherein r is an integer from zero to 6 inclusive,
/ /Zr
~N~
or ~ \R~ , wherein r is an integer from zero to 4 inclusive.
-21 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
"Pharmaceutically acceptable" denotes compounds, materials, compositions, or
dosage
forms which are, within the scope of sound medical judgment, suitable for use
in contact with the
tissues of human beings and animals without excessive toxicity, irritation,
allergic response, or
other problem or complication, commensurate with a reasonable benefit/risk
ratio.
"Pharmaceutically acceptable salts" includes, for example, derivatives of
compounds
modified by making acid or base salts thereof, as described further below and
elsewhere in the
present application. Examples of pharmaceutically acceptable salts include
mineral or organic
acid salts of basic residues such as amines; and alkali or organic salts of
acidic residues such as
carboxylic acids. Pharmaceutically acceptable salts include the conventional
non-toxic salts or
the quaternary ammonium salts of the parent compound formed, for example, from
non-toxic
inorganic or organic acids. Such conventional non-toxic salts include those
derived from
inorganic acids such as hydrochloric, hydrobromic, sulfuric, sulfamic,
phosphoric, and nitric
acid; and the salts prepared from organic acids such as acetic, propionic,
succinic, glycolic,
stearic, lactic, malic, tartaric, citric, ascorbic, palmoic, malefic,
hydroxymaleic, phenylacetic,
glutamic, benzoic, salicylic, sulfanilic, 2-acetoxybenzoic, fumaric,
toluenesulfonic,
methanesulfonic, ethane disulfonic, oxalic, and isethionic acid.
Pharmaceutically acceptable
salts may be synthesized from the parent compound which contains a basic or
acidic moiety by
conventional chemical methods. Generally, such salts may be prepared by
reacting the free acid
or base forms of these compounds with a stoichiometric amount of the
appropriate base or acid
in water or in an organic solvent, or in a mixture of the two.
In another embodiment of the invention, for example, in compounds of Formula
II, Ral
and Rbl or Ra2 and Rb2 are both taken together along with the nitrogen atoms
to which they are
bound to form a ring structure which is a nonaromatic ring, or an alicyclic
ring, or a monocyclic
ring, or a non-fused ring.
In some embodiments of Formula II, e.g., Ral, Rby Roy Ra', Rb2, and R°2
may be a
hydrogen, or a substituted or unsubstituted Cl-C8 alkyl group, wherein the
alkyl substituent is
any member of the group Z defined above, but not an aryl (e.g., phenyl) or
alkyl group.
Likewise, in certain embodiments of Formula II, Rl is a moiety selected from
the Z group
defined above other than an substituted aryl (e.g., phenyl) or heteroaryl
group.
The groups Rl and RZ may be a hydrogen, a substituted or unsubstituted Cl-C$
alkyl
group, a substituted or unsubstituted CZ-C8 alkenyl group, a halogen
(particularly bromine), a
substituted or unsubstituted aryl or heteroaryl group, a substituted or
unsubstituted amino group,
a nitro group, or a substituted or unsubstituted Cl-C8 alkoxy group
(particularly methoxy).
-22-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Each Y group may be a direct bond, or a "linking moiety" (or "linking group")
which is a
group that is covalently bound to at least two other moieties and may be, for
example, a single
divalent atom or an oligomethylene group. A linking moiety which is a linear
chain of carbon
atoms may be optionally substituted or unsaturated.
A linking moiety is relatively small compared to the rest of the molecule, and
may be less
than about 250 molecular weight, and even less than about 75 molecular weight.
Example
linking moieties are -(CHZ)n (wherein n is 1, 2, or 3), -NR'- (where R' is
hydrogen, a Cl-C~
alkyl, C2-CS alkenyl, CZ-C5 alkynyl, or aryl group), -S-, -O-,-NH-CHZ-, and -
CH=CH- (both E
and Z configurations), or combinations thereof. The linking moiety may also be
(CR°RW)n,
CR°ORW(CR"RY)n, CR"SH(CRXRY)", CR"NRWR"(CRyRZ)n, (CR''RW)n0(CR"Ry)",
wherein each n
is independently either 0, 1, 2, or 3, and R°, RW, R", Ry, and RZ are
each independently hydrogen,
a substituted or unsubstituted Cl-C5 branched or straight chain alkyl or
alkoxy, C2-C5 branched
or straight chain alkenyl, aryloxycarbonyl, arylaminocarbonyl, arylalkyl,
acyl, aryl, or C3-C$ ring
group.
In certain embodiments, Yl and YZ are each independently selected from the
groups
consisting of a direct bond, substituted or unsubstituted Cl-C$ alkylene
groups, and -NH-.
"Inhibition" of amyloid deposition includes preventing or stopping of amyloid
formation,
e.g., fibrillogenesis, inhibiting or slowing down of further amyloid
deposition in a subject with
amyloidosis, e.g., already having amyloid deposits, and reducing or reversing
amyloid
fibrillogenesis or deposits in a subject with ongoing amyloidosis. Inhibition
of amyloid
deposition is determined relative to an untreated subject, or relative to the
treated subject prior to
treatment, or, e.g., determined by clinically measurable improvement in
pancreatic function in a
diabetic patient, or in the case of a patient with brain amyloidosis, e.g., an
Alzheimer's or
cerebral amyloid angiopathy patient, stabilization of cognitive function or
prevention of a further
decrease in cognitive function or prevention of recurrence of hemorrhagic
stroke due to CAA
(i.e., preventing, slowing, or stopping disease progression). Inhibition of
amyloid deposition
may also be monitored by determining in a subject the relative levels of
amyloid-(3 in the brain or
CSF as well as in the plasma, before and after treatment.
"Modulation" of amyloid deposition includes both inhibition, as defined above,
and
enhancement of amyloid deposition or fibril formation. The term "modulating"
is intended,
therefore, to encompass prevention or stopping of amyloid formation or
accumulation, inhibition
or slowing down of further amyloid aggregation in a subject with ongoing
amyloidosis, e.g.,
already having amyloid aggregates, and reducing or reversing of amyloid
aggregates in a subject
with ongoing amyloidosis; and enhancing amyloid deposition, e.g., increasing
the rate or amount
of amyloid deposition in vivo or in vitro. Amyloid-enhancing compounds may be
useful in
animal models of amyloidosis, for example, to make possible the development of
amyloid
- 23
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
deposits in animals in a shorter period of time or to increase amyloid
deposits over a selected
period of time. Amyloid-enhancing compounds may be useful in screening assays
for
compounds which inhibit amyloidosis i~a vivo, for example, in animal models,
cellular assays and
in vitro assays for amyloidosis. Such compounds may be used, for example, to
provide faster or
more sensitive assays for compounds. In some cases, amyloid enhancing
compounds may also
be administered for therapeutic purposes, e.g., to enhance the deposition of
amyloid in the lumen
rather than the wall of cerebral blood vessels to prevent CAA. Modulation of
amyloid
aggregation is determined relative to an untreated subject or relative to the
treated subject prior
to treatment.
The term "subject" includes living organisms in which amyloidosis can occur.
Examples
of subjects include humans, monkeys, cows, sheep, goats, dogs, cats, mice,
rats, and transgenic
species thereof. Administration of the compositions of the present invention
to a subject to be
treated can be carried out using known procedures, at dosages and for periods
of time effective to
modulate amyloid aggregation in the subject as further described herein. An
effective amount of
the therapeutic compound necessary to achieve a therapeutic effect may vary
according to factors
such as the amount of amyloid already deposited at the clinical site in the
subject, the age, sex,
and weight of the subject, and the ability of the therapeutic compound to
modulate amyloid
aggregation in the subject. Dosage regimens can be adjusted to provide the
optimum therapeutic
response. For example, several divided doses may be administered daily or the
dose may be .
proportionally reduced as indicated by the exigencies of the therapeutic
situation. The term
"modulating" is intended to encompass prevention or stopping of amyloid
formation or
accumulation, inhibition or slowing down of further amyloid aggregation in a
subject with
ongoing amyloidosis, e.g., already having amyloid aggregates, and reducing or
reversing of
amyloid aggregates in a subject with ongoing amyloidosis. Modulation of
amyloid aggregation
is determined relative to an untreated subject or relative to the treated
subject prior to treatment.
In an exemplary aspect of the invention, the subject is a human. For example,
the subject
may be a human over about 40 years old, or a human over about 50 years old, or
a human over
about 60 years old, or even a human over about 70 years old, or in some cases
a human over
about ~0 years old. The subject may be a female human, including a
postmenopausal female
human, who may be on hormone (estrogen) replacement therapy. The subject rnay
also be a
male human. When the subject is a human with Down's syndrome, the age may be
over about
20 years old; or in another embodiment, the age may be less than about 40
years old.
A subject may be a human at risk for Alzheimer's disease, e.g., being over the
age of 40
or having a predisposition for Alzheimer's disease. Alzheimer's disease
predisposing factors
identified or proposed in the scientific literature include, among others, a
genotype predisposing
a subject to Alzheimer's disease; environmental factors predisposing a subject
to Alzheimer's
-24-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
disease; past history of infection by viral and bacterial agents predisposing
a subject to
Alzheimer's disease; and vascular factors predisposing a subject to
Alzheimer's disease. A
subject may also have one or more risk factors for cardiovascular disease
(e.g., atherosclerosis of
the coronary arteries, angina pectoris, and myocardial infarction) or
cerebrovascular disease
(e.g., atherosclerosis of the intracranial or extracranial arteries, stroke,
syncope, and transient
ischemic attacks), such as hypercholesterolemia, hypertension, diabetes,
cigarette smoking,
familial or previous history of coronary artery disease, cerebrovascular
disease, and
cardiovascular disease. Hypercholesterolemia typically is defined as a serum
total cholesterol
concentration of greater than about 5.2 mmol/L (about 200 mg/dL).
Several genotypes are believed to predispose a subject to Alzheimer's disease.
These
include the genotypes such.as presenilin-1, presenilin-2, and amyloid
precursor protein (APP)
missense mutations associated with familial Alzheimer's disease, and a-2-
macroglobulin and
LRP-1 genotypes, which are thought to increase the risk of acquiring sporadic
(late-onset)
Alzheimer's disease. Evan Uden, et al., J. Neurosci. 22(21), 929-304 (2002);
J.J.Goto, et al.,
J. Mol. Neurosci. 19(1-2), 37-41 (2002). Another genetic risk factor for the
development of
Alzheimer's disease are variants of ApoE, the gene that encodes apolipoprotein
E (particularly
the apoE4 genotype), a constituent of the low-density lipoprotein particle. WJ
Sfirittmatter, et al.,
Arznu. .Rev. Neurosci. 19, 53-77 (1996). The ApoE4 allele has been shown to
influence the rate
of amyloid-(3 deposition as well as to favor trapping of amyloid-[3 in the
brain, which may
increase the levels of amyloid-(3 that can become fibrillar. The molecular
mechanisms by which
the various ApoE alleles alter the likelihood of developing Alzheimer's
disease are unknown,
however the role of ApoE in cholesterol metabolism is consistent with the
growing body of
evidence linking cholesterol metabolism to Alzheimer's disease. Environmental
factors have
been proposed as predisposing a subject to Alzheimer's disease, including
exposure to
aluminum, although the epidemiological evidence is ambiguous. In addition,
prior infection by
certain viral or bacterial agents may predispose a subject to Alzheimer's
disease, including the
herpes simplex virus and chlamydia pneumoniae. Finally, other predisposing
factors for
Alzheimer's disease can include risk factors for cardiovascular or
cerebrovascular disease,
including cigarette smoking, hypertension and diabetes. "At risk for
Alzheimer's disease" also
encompasses any other predisposing factors not listed above or as yet
identified and includes an
increased risk for Alzheimer's disease caused by head injury, medications,
diet, or lifestyle.
The methods of the present invention can be used for one or more of the
following: to
prevent, to treat Alzheimer's disease, or ameliorate symptoms of Alzheimer's
disease, to regulate
production of or levels of amyloid-(I (A(3) peptides. In one alternative
embodiment, the human
carries one or more mutations in the genes that encode (3-amyloid precursor
protein, presenilin-1
or presenilin-2. In another alternative embodiment, the human carries the
Apolipoprotein
-25-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
s4 gene. In another alternative embodiment, the human has a family history of
Alzheimer's
Disease or dementia illness. In another alternative embodiment, the human has
trisomy 21
(Down's Syndrome). In another alternative embodiment, the subject has a normal
or low serum
total blood cholesterol level. In another embodiment, the serum total blood
cholesterol level is
less than about 200 mg/dL, or less than about 180, and it can range from about
150 to about 200
mg/dL. In another embodiment, the total LDL cholesterol level is less than
about 100 mg/dL, or
less than about 90 mg/dL and can range from about 30 to about 100 mg/dL.
Methods of
measuring serum total blood cholesterol and total LDL cholesterol are well
known to those
skilled in the art and for example include those disclosed in WO 99/38498 at
p.l l, incorporated
'by reference herein. Methods of determining levels of other sterols in serum
are disclosed in H.
Gylling, et al., "Serum Sterols During Stanol Ester Feeding in a Mildly
Hypercholesterolemic
Population", J. Lipid ReS. 40: 593-600 (1999).
In another alternative embodiment, the subject has an elevated serum total
blood
cholesterol level. In another embodiment, the serum total cholesterol level is
at least about 200
mg/dL, or at least about 220 mg/dL .and can range from about 200 to about 1000
mg/dL. In
another alternative embodiment, the subject has an elevated total LDL
cholesterol level. In
' another embodiment, the total LDL cholesterol level is greater than about
100 mg/dL, or even
greater than about 110 mg/dL and can range from about 100 to about 1000 mg/dL.
In another alternative embodiment, the human is at least about 40 years of
age. In another
alternative embodiment, the human is at least about 60 years of age. In
another embodiment, the
human is at least about 70 years of age. In yet another embodiment, the human
is at least about
80 years of age. In one embodiment, the human is between about 60 and 100
years of age.
In still a further embodiment, the subject is shown to be at risk by a
diagnostic brain
imaging technique, for example, that measures brain activity, plaque
deposition, or brain
atrophy. For example, positron emission tomography ("PET") may be used to
measure brain
activity and plaque deposition, while magnetic resonance imaging ("MRI") may
be used to
measure the brain volume of a subject.
In another embodiment, the subject exhibits no symptoms of Alzheimer's
Disease. In
another embodiment, the subject is a human who is at least 40 years of age and
exhibits no
symptoms of Alzheimer's Disease. In another embodiment, the subject is a human
who is at least
years of age and exhibits one or more symptoms of Alzheimer's Disease.
-26-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
In another embodiment, the subject has mild cognitive impairment ("MCI"),
which is a
condition characterized by a state of mild but measurable impairment in
thinking skills, but is not
necessarily associated with the presence of dementia. MCI frequently, but not
necessarily,
precedes Alzheimer's disease. It is a diagnosis that has most often been
associated with mild
memory problems, but it can also be characterized by mild impairments in other
thinking skills,
such as language or planning skills. However, in general, an individual with
MCI will have
more significant memory lapses than would be expected for someone of their age
or educational
background. As the condition progresses, a physician may change the diagnosis
to Mild-to-
Moderate Cognitive Impairmentor to Alzheimer's disease, as is well understood
in the art.
By using the methods of the present invention, the levels of amyloid ~3
peptides in a
subject's brain or blood can be reduced from levels prior to treatment from
about 10 to about 100
percent, or even about 50 to about 100 percent. Alternatively, the levels of
amyloid-(3 peptides in
a subject's blood or plasma maybe increased from levels prior to treatment due
to, for example,
a "sink" effect (i.e., facilitating clearance of amyloid-(3 out of the brain).
In an alternative embodiment, the subject can have an elevated level of
amyloid A(3øo and
A(342 peptide in the blood and CSF prior to treatment, according to the
present methods, of
greater than about 10 pg/mL, or greater than about 20 pg/mL, or greater than
about 35 pg/mL, or
even greater than about 40 pg/mL. In another embodiment, the elevated level of
amyloid A(34a
peptide can range from about 30 pg/mL to about 200 pg/mL, or even to about 500
pg/mL. One
skilled in the art would understand that as Alzheimer's disease progresses,
the measurable levels
of amyloid-(3 peptide in the CSF may decrease slightly from elevated levels
present before onset
of the disease. This effect is attributed to increased deposition, i.e.,
trapping of A[3 peptide in the
brain instead of .normal clearance from the brain into the CSF.
in an alternative embodiment, the subject can have an elevated level of
amyloid A(3ao
peptide in the blood and CSF .prior to treatment, according to the present
methods, of greater than
about 5 pg A~34o/mL or greater than about 50 pg A(34o/mL, or greater than
about 400 pg/mL. In
another embodiment, the elevated level of amyloid A(34o peptide can range from
about 200
pg/mL to about 800 pg/mL, to even about 1000 pg/mL.
In another embodiment, the subject 'can have an elevated level of amyloid
A(342 peptide in
the CSF prior to treatment, according to the present methods, of greater than
about 5 pg/mL, or
greater than about 10 pg/mL, or greater than about 200 pg/mL, or greater than
about 500 pg/mL.
In another embodiment, the level of amyloid-(3 peptide can range from about 10
pg/rnL to about
1,000 pg/mL, or even about 100 ~pg/mL to about 1,000 pg/mL.
-27-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
In another embodiment, the subject can have an elevated level of amyloid A(34o
peptide in
the CSF prior to treatment according to the present methods of greater than
about 10 pg/mL, or
greater than about 50 pg/mL, or even greater than about 100 pg/mL. In another
embodiment, the
level of amyloid (3 peptide can range from about 10 pg/mL to about 1,000
pg/mL.
The amount of amyloid [3 peptide in the brain or blood of a subject can be
evaluated by
enzyme-linked immunosorbent assay ("ELISA") or quantitative immunoblotting
test methods or
by quantitative SELDI-TOF which are well known to those skilled in the art,
such as is disclosed
by Zhang, et al., J. Biol. Chefzz. 274, 8966-72 (1999) and Zhang, et al.,
Biochemistry 40, 5049-55
(2001). See also, A.K.Vehmas, et al., DNA Cell Biol. 20(11), 713-21 (2001),
P.Lewczuk, et al.,
Rapid Commuzz. Mass Spectronz. 17(12), 1291-96 (2003); B.M.Austen, et al., J.
Peptide Sci. 6,
459-69 (2000); and H.Davies, et al., BioTeclzzziques 27, 1258-62 (1999). These
tests are
performed on samples of the brain or blood which have been prepared in a
manner well known
to one skilled in the art. Another example of a useful method for measuring
levels of amyloid (3
peptides is by Europium immunoassay (EIA). See, e.g., WO 99/38498 at p.11.
In another embodiment, the amount of total ApoE in the bloodstream or brain of
a subject
can be reduced from levels prior to treatment by about 5 to about 75 percent,
or, in another
embodiment, by about 5 to about 50 percent. The amount of total ApoE can be
measured in a
manner well known to one skilled in the art, for example using an ELISA test
kit such as Apo-
Tek ApoE test kit that is available from Organon Teknica.
The methods of the invention may be applied as a therapy for a subject having
Alzheimer's disease or a dementia, or the methods of the invention may be
applied as a
prophylaxis against Alzheimer's disease or dementia for subject with such a
predisposition, as in
a subject, e.g., with a genomic mutation in the APP gene, the ApoE gene, or a
presenilin gene.
The subject may have'(or may be predisposed to developing or may be suspected
of having)
vascular dementia, or senile dementia, or Mild Cognitive Impairment. In
addition to
Alzheimer's disease, the subject may have another amyloid-(3 related disease
such as cerebral
amyloid angiopathy, or the subject may have amyloid deposits, especially
amyloid-(3 amyloid
deposits in the subject's brain.
The essential features of a dementia are multiple cognitive deficits that
include memory
impairment and at least one of the following: aphasia, apraxia, agnosia, or a
disturbance in
executive functioning (the ability to think abstractly and to plan, initiate,
sequence, monitor, and
stop complex behavior). The order of onset and relative prominence of the
cognitive disturbances
and associated symptoms vary with the specific type of dementia, as discussed
in the following.
-28-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Memory impairment is generally a prominent early symptom. Individuals with
dementia
have difficulty learning new material and may lose valuables, such as wallets
and keys, or forget
food cooking on the stove. In more severe dementia, individuals also forget
previously learned
material, including the names of loved ones. Individuals with dementia may
have difficulty with
spatial tasks, such as navigating around the house or in the immediate
neighborhood (where
difficulties with memory are unlikely to play a role). Poor judgment and poor
insight are
common as well. Individuals may exhibit little or no awareness of memory loss
or other
cognitive abnormalities. They may make unrealistic assessments of their
abilities and make plans
that are not congruent with their deficits and prognosis (e.g., planning to
start a new business).
They may underestimate the risks involved in activities (e.g., driving).
In order to make a diagnosis of dementia, the cognitive deficits must be
sufficiently
severe to cause impairment in occupational or social functioning and must
represent a decline
from a previous level of functioning. The nature and degree of impairment are
variable and often
depend on the particular,social setting of the individual. For example, Mild
Cognitive
Impairment may significantly impair an individual's ability to perform a
complex job but not a
less demanding one.
Cognitive or degenerative brain disorders are characterized clinically by
progressive loss
of memory, cognition, reasoning, judgment and emotional stability that
gradually leads to
profound mental deterioration and ultimately death. It is generally believed
that the disease
begins a number of years before it manifests itself in the mild cognitive
changes that are the early
signs of Alzheimer's disease. "Dementia of the Alzheimer's Type" begins
gradually, and is
usually diagnosed after other specific causes have been ruled out. Diagnostic
criteria for
Dementia of the Alzheimer's Type include the development of multiple cognitive
deficits
manifested by both memory impairment (anterograde or retrograde, i.e.,
impaired ability to learn
new information or to recall previously learned information); and one or more
of the following
cognitive disturbances: aphasia (language disturbance), apraxia (impaired
ability to carry out
motor activities despite intact motor function), agnosia (failure to recognize
or identify objects
despite intact sensory function), disturbance in executive functioning (i.e.,
planning, organizing,
sequencing, and abstracting); where these cognitive deficits each cause
significant impairment in
social or occupational functioning and represent a significant decline fiom a
previous level of
functioning. The course is characterized by gradual onset and continuing
cognitive decline, and
the cognitive deficits are not due to another condition that causes
progressive deficits in memory
and cognition (e.g., cerebrovascular disease, brain tumor, hypothyroidism,
vitamin B or folic
acid deficiency, niacin deficiency, hypercalcemia, neurosyphilis, HIV
infection, or chemical
exposure). The cognitive disturbance may be accompanied by a behavioral
disturbance, such as
wandering, aggression, or agitation, or a psychological disturbance, such as
depression or
-29-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
psychosis. See "Diagnostic and Statistical Manual of Mental Disorders," 4~'
Ed., Text Revision,
by American Psychiatric Association (2000). For example, the National
Institute of Neuro-
logical and Communicative Disorders and Stroke-Alzheimer's Disease and the
Alzheimer's
Disease and Related Disorders Association (NINCDS-ADRDA) criteria can be used
to diagnose
Alzheimer's Disease (McKhann et al., 1984, Neurology 34:939-944). The
patient's cognitive
function can be assessed by the Alzheimer's Disease Assessment Scale-cognitive
subscale
(ADAS-cog; Rosen, et al., 1984, Am. J. Psychiatry 141:1356-1364).
Alzheimer's Disease is the prototype of a cortical degenerative disease. A
major
component of the presenting symptoms is usually subjective complaints of
memory difficulty,
language impairment, dyspraxia, at which point diagnosis is primarily based on
exclusion of
other possible etiologies for dementia. No features of the physical
examination or laboratory
evaluation are pathognomonic for dementia of the Alzheimer's type. Some
studies have
apparently discriminated patients with dementia of the Alzheimer's type from
patients with
dementia of other etiologies and from normal controls by using techniques such
as EEG, MRI,
and SPECT, but these studies have been difficult to replicate consistently,
and at present, brain-
imaging studies are best used to exclude other identifiable causes.
A variety of diagnostic tests have been developed for Alzheimer's disease.
Clinical
criteria have been verified prospectively in autopsy studies and have been
found to be highly
specific although only moderately sensitive. Implementation of the criteria
requires extensive
evaluation, including an informant-based history; neurological examination,
neuropsychological
testing, and laboratory, and neuroimaging data. Alzheimer's disease is
characterized
pathologically by generalized atrophy of the cerebral cortex and by
neurofibrillary tangles,
neuritic (amyloid) plaques, and granulovacuolar degeneration. Although plaques
and tangles
may be detected in the brains of the elderly without Alzheimer's disease, they
are more
numerous in patients with dementia. Controversy remains whether brains with
plaques from
individuals without Alzheimer's diseasewere "normal variations" or early
pathological signs of
incipient disease.
"Treatment" of a subject includes the application or administration of a
composition of
the invention to a subject, or application or administration of a composition
of the invention to a
cell or tissue from a subject, who has a amyloid-(3 related disease or
condition, has a symptom
of such a disease or condition, or is at risk of (or susceptible to) such a
disease or condition, with
the purpose of curing, healing, alleviating, relieving, altering, remedying,
ameliorating,
improving, or affecting the disease or condition, the symptom of the disease
or condition, or the
risk of (or susceptibility to) the disease or condition. The term "treating"
refers to any indicia of
success in the treatment or amelioration of an injury, pathology or condition,
including any
objective or subjective parameter such as abatement; remission; diminishing of
symptoms or
-30-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
making the injury, pathology or condition more tolerable to the subject;
slowing in the rate of
degeneration or decline; making the final point of degeneration less
debilitating; improving a
subject's physical or mental well-being; or, in some situations, preventing
the onset of dementia.
The treatment or amelioration of symptoms can be based on objective or
subjective parameters;
including the results of a physical examination or a psychiatric evaluation.
For example, the
methods of the invention successfully treat a subject's dementia by slowing
the rate of or extent
of cognitive decline.
The term "alkyl" includes saturated aliphatic groups, including straight-chain
alkyl
groups (e.g., methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl,
nonyl, decyl, etc.),
branched-chain alkyl groups (isopropyl, tert-butyl, isobutyl, etc.),
cycloalkyl (alicyclic) groups
(cyclopropyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, etc.), alkyl
substituted cycloalkyl
groups, and cycloalkyl substituted alkyl groups. Unless otherwise specified,
the term alkyl
further includes alkyl groups, which can further include oxygen, nitrogen,
sulfur or phosphorous
atoms replacing one or more carbons of the hydrocarbon backbone.
In certain embodiments, a straight chain or branched chain alkyl has 6 or
fewer carbon
atoms in its backbone (e.g., Cl-C~ for straight chain, C3-C~ for branched
chain), or 4 or fewer.
Likewise, cycloalkyls may have from 3-8 carbon atoms in their ring structure,
or 5 or 6 carbons
in the ring structure. The term Cl-C6 includes alkyl groups containing 1 to 6
carbon atoms. An
"alkylene" group is a divalent moiety derived from the corresponding alkyl
group.
Moreover, unless otherwise specified the term alkyl includes both
"unsubstituted alkyls"
and "substituted alkyls," the latter of which refers to alkyl moieties having
substituents replacing
one or more hydrogens on one or more carbons of the hydrocarbon backbone. Such
substituents
can include, for example, alkenyl, alkynyl, halogen, hydroxyl,
alkylcarbonyloxy,
arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy, carboxylate,
alkylcarbonyl,
arylcarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl,
alkylthiocarbonyl, alkoxyl, phosphate, phosphonato, phosphinato, cyano, amino
(including alkyl
amino, dialkylamino, arylamino, diarylamino, and alkylarylamino), acylamino
(including
alkylcarbonylamino, arylcarbonylamino, carbamoyl and ureido), amidino, imino,
sulfhydryl,
alkylthio, arylthio, thiocarboxylate, sulfates, alkylsulfinyl, sulfonato,
sulfamoyl, sulfonamide,
nitre, trifluoromethyl, cyano, azido, heterocyclyl, alkylaryl, or an aromatic
or heteroaromatic
moiety. Cycloalkyls~may be further substituted, e.g., with the substituents
described above.
An "arylalkyl" moiety is an alkyl group substituted with an aryl (e.g.,
phenylmethyl (i.e.,
benzyl)). An "alkylaryl" moiety is an aryl group substituted with an alkyl
group (e.g.,
p-methylphenyl (i.e., p-tolyl)). The term "n-alkyl" means a straight chain
(i.e., unbranched)
unsubstituted alkyl group.
-31-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The term "alkenyl" includes unsaturated aliphatic groups analogous in length
and
possible substitution to the alkyls described above, but that contain at least
one double bond. For
example, the term "alkenyl" includes straight-chain alkenyl groups (e.g.,
ethylenyl, propenyl,
butenyl, pentenyl, hexenyl, heptenyl, octenyl, nonenyl, decenyl, etc.),
branched-chain alkenyl
groups, cycloalkenyl (alicyclic) groups (cyclobutenyl, cyclopentenyl,
cyclohexenyl,
cycloheptenyl, cyclooctenyl, etc.), alkyl or alkenyl substituted cycloalkenyl
groups, and
cycloalkyl or cycloalkenyl substituted alkenyl groups. The term alkenyl may
further include
alkenyl groups which include oxygen, nitrogen, sulfur or phosphorous atoms
replacing one or
more carbons of the hydrocarbon backbone.
In certain embodiments, a straight chain or branched chain alkenyl group has 6
or fewer
carbon atoms in its backbone (e.g., C2-C6 for straight chain, C3-C6 for
branched chain).
Likewise, cycloalkenyl groups may have from 3-8 carbon atoms in their ring
structure, or 5 or 6
carbons in the ring structure. The term CZ-C6 includes alkenyl groups
containing 2 to 6 carbon
atoms. An "alkenylene" group is a divalent moiety derived from the
corresponding alkenyl
group.
Moreover, unless otherwise specified the term alkenyl includes both
"unsubstituted
alkenyls" and "substituted alkenyls," the latter of which refers to alkenyl
moieties having
substituents replacing one or more hydrogens on one or more carbons of the
hydrocarbon
backbone. Such substituents can include, for example, alkyl groups, alkynyl
groups, halogens,
hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy,
carboxylate (and lower alkyl esters thereof), alkylcarbonyl, arylcarbonyl,
alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, alkylthiocarbonyl,
alkoxyl,
phosphate, phosphonato, phosphinato, cyano, amino (including alkyl amino,
dialkylamino,
arylamino, diarylamino, and alkylarylamino), acylamino (including
alkylcarbonylamino,
arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl,
alkylthio, arylthio,
thiocarboxylate, sulfates, alkylsulfinyl, sulfonato, sulfamoyl, sulfonamido,
nitro, trifluoromethyl,
cyano, azido, heterocyclyl, alkylaryl, or an aromatic or heteroaromatic
moiety.
The term "alkynyl" includes unsaturated aliphatic groups analogous in length
and
possible substitution to the alkyls described above, but which contain at
least one triple bond.
For example, the term "alkynyl" includes straight-chain alkynyl groups (e.g.,
ethynyl, propynyl,
butynyl, pentynyl, hexynyl, heptynyl, octynyl, nonynyl, decynyl, etc.),
branched-chain alkynyl
groups, and cycloalkyl or cycloalkenyl substituted alkynyl groups. Unless
specified otherwise,
the term alkynyl further includes alkynyl groups which include oxygen,
nitrogen, sulfur or
phosphorous atoms replacing one or more carbons of the hydrocarbon backbone.
In certain
embodiments, a straight chain or branched chain alkynyl group has 6 or fewer
carbon atoms in
its backbone (e.g., C2-C~ for straight chain, C3-C~ for branched chain). The
term C2-C~ includes
-32-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
alkynyl groups containing 2 to G carbon atoms. An "alkynylene" group is a
divalent moiety
derived from the corresponding alkynyl group.
Moreover, unless otherwise specified the term alkynyl includes both
"unsubstituted
alkynyls" and "substituted alkynyls," the latter of which refers to alkynyl
moieties having
substituents replacing one or more hydrogens on one or more carbons of the
hydrocarbon
backbone.
Such substituents can include, for example, alkyl groups, alkynyl groups,
halogens,
hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy,
carboxylate, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato, cyano,
amino (including alkyl amino, dialkylamino, arylamino, diarylamino, and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido), amidino,
imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl, sulfonato,
sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido, heterocyclyl,
alkylaryl, or an
aromatic or heteroaromatic moiety.
Unless the number of carbons is otherwise specified, "lower alkyl" as used
herein means
an alkyl group, as defined above, but having from one to five carbon atoms in
its backbone
structure. "Lower alkenyl" and "lower alkynyl" have chain lengths of, for
example, 2-5 carbon
atoms.
The term "acyl" refers to a carbonyl group that is attached through its carbon
atom to a
hydrogen (i.e., a formyl), an aliphatic group (e.g., acetyl), an aromatic
group (e.g., benzoyl), and
the like. The term "substituted acyl" includes acyl groups where one or more
of the hydrogen
atoms on one or more carbon atoms are replaced by, for example, an alkyl
group, alkynyl group,
halogen, hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy,
carboxylate, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato, cyano,
amino (including alkyl amino, dialkylamino, arylamino, diarylamino, and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido), arnidino,
imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl, sulfonato,
sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido, heterocyclyl,
alkylaryl, or an
aromatic or heteroaromatic moiety.
The term "acylamino" includes moieties wherein an amino moiety is bonded to an
acyl
group. For example, the acylamino group includes alkylcarbonylamino,
arylcarbonylamino,
carbamoyl and ureido groups.
-33-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The terms "alkoxyalkyl", "alkylaminoalkyl" and "thioalkoxyalkyl" include alkyl
groups,
as described above, which further include oxygen, nitrogen or sulfur atoms
replacing one or
more carbons of the hydrocarbon backbone.
The terms "alkoxy" or "alkyloxy" include substituted and unsubstituted alkyl,
alkenyl,
and alkynyl groups covalently linked to an oxygen atom. Examples of alkoxy
groups include
methoxy, ethoxy, isopropyloxy, propoxy, butoxy, and pentoxy groups. Examples
of substituted
alkoxy groups include halogenated alkoxy groups.
The alkoxy groups can be substituted with groups such as alkenyl, alkynyl,
halogen,
hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy,
carboxylate, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato, cyano,
amino (including alkyl amino, dialkylamino, arylamino, diarylamino, and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido), amidino,
imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl, sulfonato,
sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido, heterocyclyl,
alkylaryl, or an
aromatic or heteroaromatic moieties. Examples of halogen substituted alkoxy
groups include,
but are not limited to, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
chloromethoxy,
dichloromethoxy, trichloromethoxy, etc., as well as perhalogenated alkyloxy
groups.
The term "amine" or "amino" includes compounds or moieties in which a nitrogen
atom
is covalently bonded to at least one carbon or heteroatom. The term
"alkylamino" includes
groups wherein the nitrogen is bound to at least one alkyl group. The term
"dialkylamino"
includes groups wherein the nitrogen atom is bound to at least two alkyl
groups. The term
"arylamino" and "diarylamino" include groups wherein the nitrogen is bound to
at least one or
two aryl groups, respectively. The term "alkylarylamino" refers to an amino
group which is
bound to at least one alkyl group and at least one aryl group. The term
"alkaminoalkyl" refers to
an alkyl, alkenyl, or alkynyl group substituted with an alkylamino group.The
term "amide" or
"aminocarbonyl" includes compounds or moieties which contain a nitrogen atom
which is bound
to the carbon of a carbonyl or a thiocarbonyl group.
The term "carbonyl" or "carboxy" includes compounds and moieties which contain
a
carbon connected with a double bond to an oxygen atom. Examples of moieties
which contain a
carbonyl include aldehydes, ketones, carboxylic acids, amides, esters,
anhydrides, etc.
The term "ether" or "ethereal" includes compounds or moieties which contain an
oxygen
bonded to two carbon atoms. For example, an ether or ethereal group includes
"alkoxyalkyl"
which refers to an alkyl, alkenyl, or alkynyl group substituted with an alkoxy
group.
-34-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The term "hydroxy" or "hydroxyl" includes the groups -OH or -O- (with an
appropriate
counter ion).
The term "halogen" includes fluorine, bromine, chlorine, iodine, etc. The term
"perhalogenated" generally refers to a moiety wherein all hydrogens are
replaced by halogen
atoms.
Arylenedialkylene or arylenedialkyl groups include those groups which have an
arylene
group to which are bound two other alkylene groups, which may be the same or
different, and
which two alkylene groups are in tum bound to other moieties. Examples of
arylenedialkylene
or arylenedialkyl groups include the following:
Rh
R ~ R2)f ~ ~ ~ \ ~ h
h (CR2)f
~CR2)f / \ C ;R2)g (CR2)g -(CR
/ ~ 2)9
~(CR2)f
\
/ / (CR2)f \ \~CR2\ g
~CR~ s ~ /
~(CR2)f
\ \
CR
\\(2\g //
~CR2)f / / (CR2)g
and
wherein each R group is independently a hydrogen or is selected from the group
Z defined
above, and 1<-f<-8, 1<g<_8, 0<h<4.
Alkylenediarylene groups include groups which have an alkylene (or
cycloalkylene)
group to which are bound two other arylene groups, which may be the same or
different, and
which two alkylene groups are in turn bound to other moieties. Examples of
alkylenediarylene
groups include the following:
-35-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Rn
(CR2)f / \ (CR2)y \ ~ (C~ 2)g and
Rh -~ i
\ (Cg-CgCyClO
(CR2)f alkyl) \ ~ (C~R2)g
wherein each R group is independently a hydrogen or is selected from the group
Z defined
above, 1<_y<_10 (for example, 1<_y<_4), 1<_f<_8, 1<_g<_8, 0<_h<_4, and
0<_i<_4.
Heteroarylenedialkylene or heteroarylenedialkyl groups include those groups
which have
a heteroarylene group to which are bound two other alkylene groups, which may
be the same or
different, and which two alkylene groups are in turn bound to other moieties.
Examples of
heteroarylenedialkylene or heteroarylenedialkyl groups include the following:
15
n Ri
(~R2)f /. ~ ~ (~\ 2)g
,
wherein 0<h<3, and 0<i<3, and X = NR' (wherein R' is hydrogen, a Cl-C~ alkyl,
CZ-C5 alkenyl,
C2- -CS alkynyl, or aryl group), O, or S, 1<<f<_8, 1<_g<g,
Rh~ CR2)f Rh
R (CR2)f / \ (CR2)g
(C\ 2)9 or X \
wherein 0<h<2, and X = NR' (wherein R' is hydrogen, a Cl-CS alkyl, CZ-C5
alkenyl, C2-C5
alkynyl, -or aryl group), O, or S, 1<_f<_8, 1<_g<g,
Rh (CR2)f Rh\ \
\~~1 \
(C\R2)g (CR2)f N (CR2)9
N or ~ , wherein 0<1~3, 1<_f<_8, 1<g<_8,or
-3G-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Rh N
Rn~N~CR2)f \ w
~ ~R ~
\ 2~g ~CR2~f N OR2)g
N or
wherein 0<-h~2,
wherein each R group is independently a hydrogen or is selected from the group
Z defined
above, 1_<f_<8, 1<g<_8, and h and i are as indicated.
An arylene group is an aromatic group which is capable of being connected
covalently to
other substituents through at least two positions, including the following
examples:
Rh Rh
ih ~ ~ ~ I ~ ~ \ \
/ /
> > > >
\ \
\ \ ~ \ \ / /
/ / , / / , or
wherein each R group is independently a hydrogen or is selected from the group
Z defined
above, and 0<h<4; for~example:
OH
\
A heteroarylene group is a heteroaromatic group which is capable of being
connected
covalently to other substituents through at least two positions, including the
following examples:
h _~ i
X
-37-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein 0<h<3, and 0<_i<_3, and X = NR' (wherein R' is hydrogen, a CI-CS
alkyl, C2-CS alkenyl,
C2-CS alkynyl, or aryl group), O, or S,
Rn Rn
/
/w
X or
wherein 0_<h<2, and X = NR' (wherein R' is hydrogen, a C1-CS alkyl, C2-CS
alkenyl, C2-CS
alkynyl, or aryl group), O, or S,
R~\'~. Rn\ \
J
N or N , wherein 0<_h<_3, or
R~\N~ Rn\Nw
N or N
wherein 0<-h<-2,
wherein each R group is independently a hydrogen or is selected from the group
Z defined
above, and h and i areas indicated; for example, the following groups:
O N
\ ;N / N\ p \ O
i
N , , , ,
H3C CH3 CHs
N ~ ~ I ~ N~~N
CH3 ~ O ~ O , or
Likewise, the invention relates to the following heteroarylene groups
Rn
\ / i \ -I '
(CR2~f ~ )( ~ ~C\ 2~g
-38-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein X = NR' (wherein R' is hydrogen, a Cl-CS alkyl, C2-CS alkenyl, CZ-CS
alkynyl, or aryl
group), O, or S; 0_<f_<<8,'0_g<_8; and each R group is independently a
hydrogen or is selected from
the group Z defined above.
In general, the term "aryl" includes groups, including 5- and 6-membered
single-ring
aromatic groups that may include from zero to four heteroatoms, for example,
groups derived
from benzene, pyrrole, furan, thiophene, thiazole, isothiaozole, imidazole,
triazole, tetrazole,
pyrazole, oxazole, isooxazole, pyridine, pyrazine, pyridazine, and pyrimidine,
and the like.
Furthermore, the term "aryl" includes multicyclic aryl groups, e.g., groups
derived from
tricyclic, bicyclic, e.g., naphthalene, benzoxazole, benzodioxazole,
benzothiazole,
benzoimidazole, benzothiophene, methylenedioxyphenyl, quinoline, isoquinoline,
napthyridine,
indole, benzofuran, purine, benzofuran, deazapurine, or indolizine.
Those aryl groups having heteroatoms in the ring structure may also be
referred to as
"aryl heterocycles," "heterocycles," "heteroaryls" or "heteroaromatics".
An aromatic ring can be substituted at one or more ring positions with such
substituents
as described above, as for example, halogen, hydroxyl, alkyl (e.g. tolyl),
alkoxy,
alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy,
carboxylate,
alkylcarbonyl, alkylaminoacarbonyl, arylalkyl aminocarbonyl,
alkenylaminocarbonyl,
alkylcarbonyl, arylcarbonyl, arylalkylcarbonyl, alkenylcarbonyl,
alkoxycarbonyl,
aminocarbonyl, alkylthiocarbonyl, phosphate,;phosphonato, phosphinato, cyano,
amino
(including alkyl amino, dialkylamino, arylamino, diarylamino, and
alkylarylamino), acylamino
(including alkylcarbonylamino, arylcarbonylamino, carbamoyl and ureido),
amidino, imino,
sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl,'sulfonato, sulfamoyl,
sulfonamide, nitre, trifluoromethyl, cyano, azido, heterocyclyl, alkylaryl, or
an aromatic or
he'teroaromatic moiety.
Aryl groups can also be fused or bridged with alicyclic or heterocyclic rings
which are
not aromatic so as to form a polycycle (e.g., tetralin).
The term "heterocyclic" or "heterocycle" includes heteroaryls as well as any
ring formed
which incorporate a heteroatom or an atom which is not carbon. The ring may be
saturated or
unsaturated and may contain one or more double bonds. Examples of heterocyclic
groups
include pyridyl, furanyl, thiophenyl, morphelinyl, and indolyl groups. The
term "heteroatom"
includes atoms of any element other than carbon or hydrogen. Heteroatoms may
be nitrogen,
oxygen, sulfur and phosphorus.
-39-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
An "arylene" group is a divalent moiety derived from an aryl group.
Examples of heterocycles include, but are not limited to, acridinyl; azocinyl;
benzimidazolyl; benzofuranyl; benzothiofuranyl; benzothiophenyl; benzoxazolyl;
benzthiazolyl;
benztriazolyl; benztetrazolyl; benzisoxazolyl; benzisothiazolyl;
benzimidazolinyl; carbazolyl;
4aH-carbazolyl; carbolinyl; chromanyl; chromenyl; cinnolinyl;
decahydroquinolinyl; 2H,6H-
1,5,2-dithiazinyl; dihydrofuro[2,3-b]tetrahydrofuran; furanyl; furazanyl;
imidazolidinyl;
imidazolinyl; imidazolyl; 1H-indazolyl; indolenyl; indolinyl; indolizinyl;
indolyl; 3H-indolyl;
isobenzofuranyl; isochromanyl; isoindazolyl; isoindolinyl; isoindolyl;
isoquinolinyl; isothiazolyl;
isoxazolyl; methylenedioxyphenyl; morpholinyl; naphthyridinyl;
octahydroisoquinolinyl;
oxadiazolyl; 1,2,3-oxadiazolyl; 1,2,4-oxadiazolyl; 1,2,5-oxadiazolyl; 1,3,4-
oxadiazolyl;
oxazolidinyl; oxazolyl; oxazolidinyl; pyrimidinyl; phenanthridinyl;
phenanthrolinyl; phenazinyl;
phenothiazinyl; phenoxathiinyl; phenoxazinyl; phthalazinyl; piperazinyl;
piperidinyl;
piperidonyl; 4-piperidonyl; piperonyl; pteridinyl; purinyl; pyranyl;
pyrazinyl; pyrazolidinyl;
pyrazolinyl; pyrazolyl; pyridazinyl; pyridooxazole; pyridoimidazole;
pyridothiazole; pyridinyl;
pyridyl; pyrimidinyl; pyrrolidinyl; pyrrolinyl; 2H-pyrrolyl; pyrrolyl;
quinazolinyl; quinolinyl;
4H-quinolizinyl; quinoxalinyl; quinuclidinyl; tetrahydrofuranyl;
tetrahydroisoquinolinyl;
tetrahydroquinolinyl; tetrazolyl; 6H-1,2;5-thiadiazinyl; 1,2,3-thiadiazolyl;
1,2,4-thiadiazolyl;
1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; thianthrenyl; thiazolyl; thienyl;
thienothiazolyl;
thienooxazolyl; thienoimidazolyl; thiophenyl; triazinyl; 1,2,3-triazolyl;
1,2,4-triazolyl; 1,2,5-
triazolyl; 1,3,4-triazolyl; and xanthenyl. Heterocycles include, but are not
limited to, pyridinyl;
furanyl; thienyl; pyrrolyl; pyrazolyl; pyrrolidinyl; imidazolyl; indolyl;
benzimidazolyl; 1H-
indazolyl; oxazolidinyl; benzotriazolyl; benzisoxazolyl; oxindolyl;
benzoxazolinyl; and isatinoyl
groups. Also included are fused ring and spiro compounds containing, for
example, the above
heterocycles.
An oligoethereal group, such as an oligo(alkyleneoxide) group, includes
polyethyleneglycol (PEG) and short chain analogs thereof including -
[(CR2)SO]t(CR~)S-, wherein
1<t<_6 and 1_<s_<6, and each R group is independently a hydrogen or is
selected from the group Z
defined above.
An arylene-di(oligoalkyleneoxide) group is an aryl group which has two
oligoalkyleneoxide groups bound to it which in turn are bound to other
moieties, and include the
following examples:
L(CR2)S~lc(CR2)S ArY~-f(CR2)s~lt(CR2)s
-40-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein "Aryl" is an arylene moiety, 1<t<_6, 0<s<6, and each R group is
independently a
hydrogen or is selected from the group Z defined above. Example
arylene-di(oligoalkyleneoxide) groups include:
Rn
L(CR2)s0]t(CR2)s / \ L(CR2)s0]t(CR2)s
Rn
L~R2)s0]t(CR2)s
L(CR2)s0]t( \R2)s. or
Rn
\CR O] (CR )
L( 2)s t 2 s
L(~ R2)s0]t(CR2)s
wherein 1<-t<_6, 0<_s<_6, 0<_h<_4, and each R group is independently a
hydrogen or is selected from
the group Z defined above.
It will be understood that "substitution" or "substituted with" includes the
implicit
proviso that such substitution is in accordance with permitted valence of the
substituted atom and
the substituent, and that the substitution results in a stable compound, e.g.,
which does not
spontaneously undergo transformation such as by rearrangement, cyclization,
elimination, etc.
As used herein,, the term "substituted" is meant to include all permissible
substituents of organic
compounds. In a broad aspect, the permissible substituents include acyclic and
cyclic, branched
and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic
substituents of organic
compounds. The permissible substituents can be one or more and the same or
different for
appropriate organic compounds.
In some embodiments, a "substituent" may be, selected from the group
consisting of, for
example, halogeno, trifluoromethyl, nitro, cyano, Cl-C6 alkyl, CZ-C6 alkenyl,
CZ-C~ alkynyl,
Cl-C~ alkylcarbonyloxy, arylcarbonyloxy, Cl-C~ alkoxycarbonyloxy,
aryloxycarbonyloxy,
Cl-C6 alkylcarbonyl, Cl-C~ alkoxycarbonyl, Cl-C~ alkoxy, Cl-C~ alkylthio,
arylthio,
heterocyclyl, aralkyl, and aryl (including heteroaryl) groups.
-41 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
It will be understood that "substitution" or "substituted with" includes the
implicit
proviso that such substitution is in accordance with permitted valence of the
substituted atom and
the substituent, and that the substitution results in a stable compound, e.g.,
which does not
spontaneously undergo transformation such as by rearrangement, cyclization,
elimination, etc.
As used herein, the term "substituted" is meant to include all permissible
substituents of organic
compounds. In a broad aspect, the permissible substituents include acyclic and
cyclic, branched
and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic
substituents of organic
compounds. The permissible substituents can be one or more and the same or
different for
appropriate organic compounds.
In some embodiments, a "substituent" may be, selected from the group
consisting of, for
example, halogeno, trifluoromethyl, nitro, cyano, Cl-C6 alkyl, C~-C~ alkenyl,
CZ-C~ alkynyl,
Cl-C~ alkylcarbonyloxy, arylcarbonyloxy, Cl-C6 alkoxycarbonyloxy,
aryloxycarbonyloxy,
Cl-C~ alkylcarbonyl, Cl-C6 alkoxycarbonyl, Cl-C6 alkoxy, Cl-C~ alkylthio,
arylthio,
heterocyclyl, aralkyl, and aryl (including heteroaryl) groups.
In some embodiments, the term "substituted" means that the moiety has
substituents
placed on the moiety other than hydrogen which allow the molecule to perform
its intended
function. Examples of substituents include moieties selected from straight or
branched alkyl (for
example, C1-CS), cycloalkyl (for example, C3-C8), alkoxy (for example, Cl-C6),
thioalkyl (for
example, C1-C6), alkenyl (for example, C2-C~), alkynyl (for example, CZ-C6),
heterocyclic,
carbocyclic, aryl (e.g., phenyl), aryloxy (e.g., phenoxy), aralkyl (e.g.,
benzyl), aryloxyalkyl (e.g.,
phenyloxyalkyl), arylacetamidoyl, alkylaryl, heteroaralkyl, alkylcarbonyl and
arylcarbonyl or
other such acyl group, heteroarylcarbonyl, or heteroaryl group,
(CR'R")o_3NR'R" (e.g., -NHS),
(CR'R")o_3CN (e.g., -CN), NO2, halogen (e.g., F, Cl, Br, or I),
(CR'R")o_3C(halogen)3 (e.g.,
-CF3), (CR'R")o_3CH(halogen)2, (CR'R")o_3CH~(halogen), (CR'R")o_3CONR'R",
(CR'R")o_3(CNH)NR'R", (CR'R")o_3S(O)1_2NR'R", (CR'R")o_3CH0,
(CR'R")o_30(CR'R")o_3H,
(CR'R")o-3S(O)o-3R' (e.g., -S03H), (CR'R")o-s0(CR'R")o-sH (e~g~, -CHaOCH3 and -
OCH3),
(CR'R")o_3S(CR'R")o_3H (e.g., -SH and -SCH3), (CR'R")o_30H (e.g., -OH),
(CR'R")o_3COR',
(CR'R")o_3(substituted or unsubstituted phenyl), (CR'R")o_3(C3-C$ cycloalkyl),
(CR'R")o_3C02R'
(e.g., -C02H), or (CR'R")o_30R' group, or the side chain of any naturally
occurring amino acid;
wherein R' and R" are each independently hydrogen, a Cl-C5 alkyl, CZ-CS
alkenyl, C2-C5
alkynyl, or aryl group, or R' and R" taken together are a benzylidene group or
a -(CHZ)ZO(CH2)a-
group. For example, substitutions enhance the ability of the compounds of the
invention to
perform its intended function, e.g., inhibit formation of amyloid deposits. In
some embodiments,
a Z group may be a substituent as defined in this paragraph.
-42-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
In one example group of compounds of the invention, m=1 and that n=0, 1, or 2.
In
compounds of Formula I, for example, p=0, 1, or 2, and q=1. Molecules
according to Formula I
may be symmetric, thus Ral=Ra2, Rbi=Rb2~ R~i=R~z~ m=q, n=p, and Yl=Y2.
Likewise, Rl=R' and
Xl=XZ are examples of molecules of Formula I.
One group of compounds of the invention are those of Formula Ia:
~Ra2
N N
Rb2
N / / wN~
Rci \ ~ M \ ~ Rc2 i
(Formula la)
wherein M is
Rh1 Rh2
X
wherein, in a one aspect, Ral and Rbl together, or Ra2 and Rb2 together,
represent a C2 to C3
alkylene; R°1 and R°2 are H; Rhl is H; and Rhz is OCH3 or
O(C6H4)R, wherein R is H or
lower-alkyl, and X is O, NR' (wherein R' is hydrogen, a Cl-C5 alkyl, CZ-CS
alkenyl, CZ-C5
alkynyl, or aryl group), or S.
In another group of compounds of Formula Ia, Ral and Rbl together, or Ra2 and
Rbz
together, represent a CZ linear, saturated alkylene; R°1 and R°2
are -(Power alkyl)-OH; and Rhi and
Rh2 are each H. The "lower alkyl" group of R°1 and R°Z may
be ethylene.
In yet another group of compounds of Formula Ia, Ral and Rbl together, or Ra2
and Rb'
together, represent a C4 alkylene; R°1 and R°2 are H, lower
alkyl, cycloalkyl, aryl, hydroxyalkyl,
aminoalkyl or alkylaminoalkyl; Rhl and Rh2 are independently selected from the
group consisting
of H, lower alkyl, halogen, alkoxy, aryloxy, or arylalkoxy.
In still yet another group of compounds of Formula Ia, Ral, Ra2, Rbi and Rb2
are H; R°i
and R°Z are isopropyl or -(CH2)3N(CH3)2; and Rhl and Rh2 are H.
In a further group of compounds of Formula Ia, Ral and Rbl together, or Ra2
and Rba
together, represent a phenylene group which is optionally substituted with up
to three
-CONHRdNReRf groups where Rd is lower alkyl and Re and Rf are each
independently selected
from the group consisting of H or lower alkyl; and R~l, R~2, R~'i, and Rh2 are
H.
- 43 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
An example compound of Formula Ia has Rhl, Ri'z, Rby R°i, Rb2, and
R°2 being H, and Rai
and Ra2 groups being hydroxy or methoxy.
Another group of compounds are those of Formula Ib:
Ra1-N N-Ra2
/ /
b1 \~\ ~ ~~ b2
R -N M \ N-R
' c1 i
R R1 R2 R°2 (Forfraula Ib)
wherein M is
Rn1 Rn2
X
wherein X is O, NR' (wherein R' is hydrogen, a C1-CS alkyl, C2-CS alkenyl, C2-
C5 alkynyl, or
aryl group), or S; Rhl and Rh2 are each independently selected from the group
consisting of H,
loweralkyl, aryl, alkylaryl, aminoalkyl, aminoaryl, halogen, alkoxy, aryloxy,
or oxyarylalkyl; Rl
and R2 are each independently selected from the group consisting of H,
loweralkyl, alkoxy,
alkylaryl, aryl, aryloxy, aminoalkyl, aminoaryl, or halogen; and each Ral,
Ra2, Rby and Rb'' group
is independently selected from the group consisting of H, loweralkyl,
alkoxyalkyl, hydroxyalkyl,
aminoalkyl, alkylaminoalkyl, cycloalkyl, aryl, hydroxy, or alkylaryl; or Ral
and Rbl together, or
Ra2 and Rb2 together, represent C2-Clo alkyl, hydroxyalkyl, or alkylene; and
each R~l and R°z
group is independently H, hydroxy, loweralkyl, alkoxyalkyl, hydroxyalkyl,
aminoalkyl,
alkylamino, alkylaminoalkyl, cycloalkyl, hydroxycycloalkyl, alkoxycycloalkyl,
aryl, or alkylaryl.
Another group of compounds are those of Formula Ic:
R2
~ -Ra2
l~Mw 2 \ ~ -Rb2
X X ,
Rc2
(Formula Ic)
-44-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein M is
Rn1 Rn2
X
wherein X is S, O, or NR' (wherein R' is hydrogen, a Cl-CS alkyl, C2-CS
alkenyl, C2-CS alkynyl,
or aryl group); Rbi, Rbz, R°l, and R°Z are each independently
selected from the group consisting
of H, loweralkyl, alkoxy, alkoxyalkyl, cycloalkyl, aryl, hydroxyalkyl,
aminoalkyl or
alkylaminoalkyl; Rl and R' are H, lower alkyl, alkoxy, alkoxyalkyl,
hydroxyalkyl, cycloalkyl,
aryl, aminoalkyl, alkylaminoalkyl or halogen; Ral and R~ are -OY, or Ral and
Rbl together, or
Ra2 and Rb' together represent
R5 ~
i
wherein R5 is
YON
RcRbN/\
Y is H or lower alkyl; each of Xl and XZ are -(CH2)n-, where n is an integer
from 0 to 2; and Rhi
and Rh2 are each independently selected from the group consisting of H, lower
alkyl, halogen,
alkoxy, aryloxy, or oxyarylalkyl.
Yet another group of compounds are those of Formula Ic, wherein M is
-(CH2)n where n is an integer from 2 to 16 (or 2 to 12, or 2 to 10); each of
Xl and XZ is O, NH,
or S; Ral, Ra2, Rby and Rb2 are H; or Ral and Rbl together, or Ra2 and Rb2
together represent
-(CH2)m , wherein m is 2, 3, or 4; each of Rl and RZ are H, OCH3, NOZ or NH2;
R~i and R°Z are
H, CH3 or CHZCH3.In another embodiment, when Xl is O or S, both Rl and R~l
cannot be H; and
when XZ is O or S, both RZ and R°2 cannot be H.
- 45 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Another group of compounds are those of Formula Id:
Ra
Rb \ ~b2
(Formula Id)
wherein each Ral, Ra2, Rby and Rb2 are independently selected from the group
consisting of H,
loweralkyl, alkoxyalkyl, hydroxyalkyl, aminoalkyl, alkylaminoalkyl,
cycloalkyl, aryl, or
alkylaryl; or two Ral and Rbi together, or Raz and Rb2 together represent C~-
Clo alkylene; R°1 and
R°Z are independently H, hydroxy, loweralkyl, alkoxyalkyl,
hydroxyalkyl, aminoalkyl,
alkylaminoalkyl, cycloalkyl, aryl, or alkylaryl; and R' is H, loweralkyl,
alkoxyalkyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, cycloalkyl, aryl, or alkylaryl.
Another group of compounds are those of Formula Ie:
R1
Ra1-N
Rb1- ~~X1
(Formula Ie)
wherein M is an alkylene group (e.g., CZ to Cl~), and Xl and X2 are oxygen.
In another group of compounds of Formula Ie, Ral and Rbl together, or Ra2 and
Rbz
together, represent a C2 linear, saturated alkylene; R°1 and R°Z
are H.
Another group of compounds of the invention are those of Formula lla:
i
R1)
n
(For-rrrula IIa)
-4G-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein E is
Rai-N . N N
Rb1-
N N
c1 ' c1 ' c1
or R or
wherein Y1, Y', Z, and R1 are as defined above; n is 0 - 4; example YZ groups
are O, NH, S, a
substituted or unsubstituted methylene group, or a direct bond; Z may be a
hydrogen atom, or Z
may be alkyl, aryl, alkoxy, aryloxy, hydroxy, a substituted or unsubstituted
amino, nitro, sulfo, or
halogen group; Ral, Rby and R°1 are independently hydrogen, lower
alkyl, aromatic, hydroxyl, or
alkoxy; and B is a direct bond or a substituted or unsubstituted alkylene
group containing from 1
to 16 carbon atoms, or a biphenylene group, or a combination biphenylene-
alkylene group, the
group -[(CH2)n0]~,(CH2)n where m is 1 to 6 and n is 2 to 6, or a heterocyclic
group.
Compounds of Formula IIb are also within the invention:
NH
H2N ~ R
~UH2O
(Formula llb)
wherein n = 2, 3, 4, 5, 6, 7, 8, 9, or 10; and R = hydrogen, hydroxy, halogen,
phenyl, biphenyl,
naphthyl, alkoxy, ~carboxy, alkoxycarbonyl, aryloxycarbonyl, or aryloxy.
Another group of compounds are of Formula IIIa:
Ra1-N _ _ N_Ra2
b1_ \ ~ N ~ / _ b2
R N N R
'Rci N M N Rc2
1
H H (Formula Illa)
wherein M is
Rni Rn2
X
-47-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
wherein X is S, O, or NR' (wherein R' is hydrogen, a Cl-CS alkyl, C2-C5
alkenyl, C2-C5 alkynyl,
or aryl group); Ral, Ra2, Rby and Rb2 are each independently selected from the
group consisting
of H, lower alkyl, alkoxyalkyl, cycloalkyl, aryl, alkylaryl, hydroxyalkyl,
aminoalkyl, or
alkylaminoalkyl; or Rai and Rbl together, or Ra2 and Rb2 together represent a
CZ to Cl° alkyl,
hydroxyalkyl, or alkylene; or Ral and Rbl together, or Ra2 and Rb2 together
are:
~Rlo~ i
n
wherein n is a number from 1 to 3, and R1° is H or -CONHR11NR15Rls,
wherein R11 is lower
alkyl and Rls and Rl~ are each independently selected from the group
consisting of H and lower
alkyl; and R°1 and R°2 are H, hydroxy, lower alkyl, cycloalkyl,
aryl, alkylaryl, alkoxyalkyl,
hydroxycycloalkyl, alkoxycycloalkoxy, hydroxyalkyl, aminoalkyl or
alkylaminoalkyl; and Rhi
and Rh2 are each independently selected from the group consisting of H, lower
alkyl, halogen,
aryl, arylalkyl, aminoalkyl, aminoaryl, alkoxy, aryloxy, or oxyarylalkyl.
Yet another group of compounds are of Formula IIIb:
Ra1 ,Ra2
~N
Rb1\ ~ ~b2 ,
Rc1
N' \
H (Formula IIIb)
wherein each pair of Ral with Rbl and R~ with Rb2 together represent -(CHZ)m-
wherein m is
from two to four; R~l and R~2 are independently H or loweralkyl; and M, which
may be
substituted with a lower alkyl group, is selected from the group consisting of
-CH=CH-CH2-CH?-, -CHZ-CH=CH-CHZ-, and -CH=CH-CH=CH-.
- 48 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Another group of compounds are those of Formula IIIc:
Ra
Rb1\ ~b2
(Formula Illc)
wherein Rl and RZ are independently H or -CONHRSNR6R~, wherein RS is lower
alkyl, R~ and
R~ are each independently selected from the group consisting of H and lower
alkyl; Ral, Ra2, Rby
and Rb2 are independently selected from the group consisting of H, lower
alkyl, alkoxyalkyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, cycloalkyl, aryl, or alkylaryl, or
Ral and Rbl
together, or Ra2 and Rb2 together represent C2-Clo alkylene; R°l and
R°2 are independently H,
hydroxy, lower alkyl, alkoxyalkyl, hydroxyalkyl, aminoalkyl, alkylaminoalkyl,
cycloalkyl, aryl,
or alkylaryl; R~3 and R~ are independently H, hydroxy, loweralkyl,
alkoxyalkyl, hydroxyalkyl,
aminoalkyl, alkylaminoalkyl, cycloalkyl, aryl, or alkylaryl; and R' is H,
loweralkyl, alkoxyalkyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, cycloalkyl, aryl, alkylaryl, or
halogen.
In another embodiment, the present invention relates to pharmaceutical
compositions
comprising compounds according to any of the Formulae herein for the treatment
of an amyloid-
related disease. The invention also pertains to the use of compounds in the
manufacture of
pharmaceutical compositions, as well as methods of manufacturing such
pharmaceutical
compositions. Such pharmaceutical compositions may be useful in the treatment
or prevention
of an amyloid-related disease.
The compositions of the invention may be supplied in a solution with an
appropriate
solvent or in a solvent-free form (e.g., lyophilized). In another aspect of
the invention, the agents
and buffers necessary for carrying out the methods of the invention may be
packaged as a kit.
The kit may be commercially used according to the methods described herein and
may include
instructions for use in a method of the invention. Additional kit components
may include acids,
bases, buffering agents, inorganic salts, solvents, antioxidants,
preservatives, or metal chelators.
The additional kit components are present as pure compositions, or as aqueous
or organic
solutions that incorporate one or more additional kit components. Any or all
of the kit
components optionally further comprise buffers.
-49-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The compositions of the invention may be administered therapeutically or
prophylactically to treat diseases associated with amyloid-(3 fibril
formation, aggregation, or
deposition. The compositions of the invention may act to ameliorate the course
of an amyloid-(3
related disease by a variety of mechanisms. In one embodiment, the
pharmaceutical
compositions disclosed herein prevent or inhibit amyloid protein assembly into
insoluble fibrils
which, in vivo, are deposited in various organs, or it favors plaque clearance
or slows deposition
in subjects already having deposits. In another embodiment, the pharmaceutical
compositions
may also prevent the amyloid protein, in its soluble, oligomeric form or in
its fibrillar form, from
binding or adhering to a cell surface and causing cell damage or toxicity. In
yet another
embodiment, the pharmaceutical compositions may block amyloid toxicity. In
other
embodiments, the compound may act by slowing the rate of amyloid-(3 fibril
formation or
deposition. In yet another embodiment, the compound may lessen the degree of
amyloid-(3
deposition. Still other examples include inhibiting, reducing, or preventing
amyloid-(3 fibril
formation; inhibiting neurodegeneration or cellular toxicity induced by
amyloid-(3; inhibiting
amyloid-(3 induced inflammation; or enhancing the clearance of amyloid-(3 from
the brain brain
or enhancing its degradation rate in the brain or in the peripheral organs.
The compounds of the invention can be formulated to ensure proper distribution
in vivo.
For example, the blood-brain barrier (BBB) excludes many highly hydrophilic
compounds. To
ensure that the more hydrophilic therapeutic compounds of the invention cross
the BBB, they
can be formulated, for example, in liposomes. For methods of manufacturing
liposomes, see,
e.g., U.S. Patent Nos. 4,522,811; 5,374,548; and 5,399,331. The liposomes may
comprise one or
more moieties which are selectively transported into specific cells or organs
("targeting
moieties"), thus providing targeted drug delivery (see, e.g., V. V. Ranade
(1989) J. Clin.
Pharmacol. 29:685).
Exemplary targeting moieties include folate or biotin (see, e.g., U.S. Patent
No.
5,416,O1G to Low et al.); mannosides (Umezawa et al. (1988) Biochem. Biophys.
Res. Comrnun.
153:1038); antibodies (P. G. Bloeman et al. (1995) FEBS Lett. 357:140; M.
Owais et al. (.1995)
Antimicrob. Agents Chemother. 39:180); surfactant protein A receptor (Briscoe
et al. (1995)
Am. J. Physiol. 1233:134); gp120 (Schreier et al. (1994) J. Biol. Chem.
269:9090); see also K.
Keinanen; M. L. Laukkanen (1994) FEBS Lett. 346:123; J. J. Killion; I. J.
Fidler (1994)
Immunomethods 4:273. In an embodiment, the therapeutic compounds of the
invention are
formulated in liposomes; in another embodiment, the liposomes include a
targeting moiety.
-50-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
To ensure that compounds of the invention cross the BBB, they may be coupled
to a BBB
transport vector (for review of BBB transport vectors and mechanisms, see
Bickel, et cal., Adv.
Drug Delivery Reviews, vol. 46, pp. 247-279, 2001). Exemplary transport
vectors include
cationized albumin or the OX26 monoclonal antibody to the transfernn receptor;
these proteins
undergo absorptive-mediated and receptor-mediated transcytosis through the
BBB, respectively.
Examples of other BBB transport vectors that target receptor-mediated
transport systems
into the brain include factors such as insulin, insulin-like growth factors
(IGF-I, IGF-II),
angiotensin II, atrial and brain natriuretic peptide (ANP, BNP), interleukin I
(IL-1) and
transferrin. Monoclonal antibodies to the receptors which bind these factors
may also be used as
BBB transport vectors. BBB transport vectors targeting mechanisms for
absorptive-mediated
transcytosis include cationic moieties such as canonized LDL, albumin or
horseradish peroxidase
coupled with polylysine, canonized albumin or canonized immunoglobulins. Small
basic
oligopeptides such as the dynorphin analogue E-2078 and the ACTH analogue
ebiratide can also
cross the brain via absorptive-mediated transcytosis and are potential
transport vectors.
Other BBB transport vectors target systems for transporting nutrients into the
brain.
Examples of such BBB transport vectors include hexose moieties, e.g. glucose,
monocarboxylic
acids, e.g. lactic acid, neutral amino acids, e.g. phenylalanine, amines, e.g.
choline, basic amino
acids,:e.g. arginine, nucleosides, e.g. adenosine, purine bases, e.g. adenine,
and thyroid hormone,
e.g. triiodothyridine. Antibodies to the extracellular domain of nutrient
transporters can also be
used as transport vectors. Other possible vectors include angiotensin II and
ANP, which may be
involved in regulating BBB permeability.
In some cases, the bond linking the therapeutic compound to the transport
vector may be
cleaved following transport into the brain in order to liberate the
biologically active compound.
Exemplary linkers include disulfide bonds, ester-based linkages, thioether
linkages, amide bonds,
acid-labile linkages, and Schiff base linkages. Avidin/biotin linkers, in
which avidin is
covalently coupled to the BBB drug transport vector, may also be used. Avidin
itself may be a
drug transport vector.
To administer the therapeutic compound by other than parenteral
administration, it may
be necessary to coat the compound with, or co-administer the compound with, a
material to
prevent its inactivation. For example, the therapeutic compound may be
administered to a
subject in an appropriate carrier, for example, liposomes, or a diluent.
Pharmaceutically
acceptable diluents include saline and aqueous buffer solutions. Liposomes
include
water-in-oil-in-water CGF emulsions as well as conventional liposomes (Strejan
et al., (1984)
J. Neuroirnmuuol. 7:27).
-51-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The therapeutic compound may also be administered parenterally,
intraperitoneally,
intraspinally, or intracerebrally. Dispersions can be prepared in glycerol,
liquid polyethylene
glycols, and mixtures thereof and in oils. Under ordinary conditions of
storage and use, these
preparations may contain a preservative to prevent the growth of
microorganisms.
Pharmaceutical compositions suitable for injectable use include sterile
aqueous solutions
(where water soluble) or dispersions and sterile powders for the
extemporaneous preparation of
sterile injectable solutions or dispersion. In all cases, the composition must
be sterile and must
be fluid to the extent that easy syringability exists. It must be stable under
the conditions of
manufacture and storage and must be preserved against the contaminating action
of
microorganisms such as bacteria and fungi.
The vehicle can be a solvent or dispersion medium containing, for example,
water,
ethanol, polyol (for example, glycerol, propylene glycol, and liquid
polyethylene glycol, and the
like), suitable mixtures thereof, and vegetable oils. The proper fluidity can
be maintained, for
example, by the use of a coating such as lecithin, by the maintenance of the
required particle size
in the case of dispersion and by the use of surfactants. Prevention of the
action of
microorganisms can be achieved by various antibacterial and antifungal agents,
for example,
parabens, chlorobutanol, phenol, ascorbic acid, thimerosal, and the like. In
many cases, it will be
preferable to include isotonic agents, for example, sugars, sodium chloride,
or polyalcohols such
as rnannitol and sorbitol, in the composition. Prolonged absorption of the
injectable
compositions can be 'brought about by including in the composition an agent
which delays
absorption, for example, aluminum monostearate or gelatin.
Sterile injectable solutions can be prepared by incorporating the therapeutic
compound in
the required amount in an appropriate solvent with one or a combination of
ingredients
enumerated above, as required, followed by filtered sterilization. Generally,
dispersions are
prepared by incorporating the therapeutic compound into a sterile vehicle
which contains a basic
dispersion medium and the required other ingredients from those enumerated
above. In the case
of sterile powders for the preparation of sterile injectable solutions, the
methods of preparation
are vacuum drying and freeze-drying which yields a powder of the active
ingredient (i.e., the
therapeutic compound) plus any additional desired ingredient from a previously
sterile-filtered
solution thereof.
The therapeutic compound can be orally administered, for example, with an
inert diluent
or an assimilable edible carrier. The therapeutic compound and other
ingredients may also be
enclosed in a hard or soft shell gelatin capsule, compressed into tablets, or
incorporated directly
into the subject's diet. For oral therapeutic administration, the therapeutic
compound may be
incorporated with excipients and used in the form of ingestible tablets,
buccal tablets, troches,
capsules, elixirs, suspensions, syrups, wafers, and the like. The percentage
of the therapeutic
-52-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
compound in the compositions and preparations may, of course, be varied. The
amount of the
therapeutic compound in such therapeutically useful compositions is such that
a suitable dosage
will be obtained.
It is especially advantageous to formulate parenteral compositions in dosage
unit form for
ease of administration and uniformity of dosage. Dosage unit form as used
herein refers to
physically discrete units suited as unitary dosages for the subjects to be
treated; each unit
containing a predetermined quantity of therapeutic compound calculated to
produce the desired
therapeutic effect in association with the required pharmaceutical vehicle.
The specification for
the dosage unit forms of the invention are dictated by and directly dependent
on (a) the unique
characteristics of the therapeutic compound and the particular therapeutic
effect to be achieved,
and (b) the limitations inherent in the art of compounding such a therapeutic
compound for the
treatment of amyloid deposition in subjects.
The present invention therefore includes pharmaceutical formulations
comprising the
compounds of the Formulae described herein, including pharmaceutically
acceptable salts
thereof, in pharmaceutically acceptable carriers for aerosol, oral and
parenteral administration.
Also, the present invention includes such compounds, or salts thereof, which
have been
lyophilized and which may be reconstituted to form pharmaceutically acceptable
formulations
for administration, as by intravenous, intramuscular, or subcutaneous
injection. Administration
may also be intradermal or transdermal.
In accordance with the present invention, a compound of the Formulae described
herein,
and pharmaceutically acceptable salts thereof, may be administered orally or
through inhalation
as a solid, or may be administered intramuscularly or intravenously as a
solution, suspension or
emulsion. Alternatively, the compounds or salts may also be administered by
inhalation,
intravenously or intramuscularly as a liposomal suspension.
Pharmaceutical formulations are also provided which are suitable for
administration as an
aerosol, by inhalation. These formulations comprise a solution or suspension
of the desired
compound of any Formula herein, or a salt thereof, or a plurality of solid
particles of the
compound or salt. The desired formulation may be placed in a small chamber and
nebulized.
Nebulization may be accomplished by compressed air or by ultrasonic energy to
form a plurality
of liquid droplets or solid particles comprising the compounds or salts. The
liquid droplets or
solid particles should have a particle size in the range of about 0.5 to about
5 microns. The solid
particles can be obtained by processing the solid compound of any Formula
described herein, or
a salt thereof, in any appropriate manner known in the art, such as by
micronization. Most
preferably, the size of the solid particles or droplets will be from about 1
to about 2 microns. In
this respect, commercial nebulizers are available to achieve this purpose.
-53-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Preferably, when the pharmaceutical formulation suitable for administration as
an aerosol
is in the form of a liquid, the formulation will comprise a water-soluble
compound of any
Formula described herein, or a salt thereof, in a carrier which comprises
water. A surfactant may
be present which lowers the surface tension of the formulation sufficiently to
result in the
formation of droplets within the desired size range when subjected to
nebulization.
Active compounds are administered at a therapeutically effective dosage
sufficient to
inhibit amyloid deposition in a subject. A "therapeutically effective" dosage
preferably inhibits
amyloid deposition by at least about 20%, or by at least about 40%, or even by
at least about
60%, or by at least about 80% relative to untreated subjects. In the case of
an Alzheimer's
patient, a "therapeutically effective" dosage stabilizes cognitive function or
prevents a further
decrease in cognitive function (i.e., preventing, slowing, or stopping disease
progression). The
present invention accordingly provides therapeutic drugs. By "therapeutic" or
"drug" is meant
an agent having a beneficial ameliorative or prophylactic effect on a specific
disease or condition
in a living human or non-human animal.
Furthermore, active compounds are administered at a therapeutically effective
dosage
sufficient to decrease deposition in a subject of amyloid protein, e.g., A[340
or A[342. A
therapeutically effective dosage inhibits amyloid deposition by, for example,
at least about 15%,
or by at least about 40%, or even by at least 60%, or at least by about 80%
relative to untreated ,
subjects:
In another embodiment, active compounds are administered at a therapeutically
effective
dosage sufficient to increase or enhance amyloid protein levels, e.g., A(340
or A(342, in the
plasma or CSF of a subject. A therapeutically effective dosage increases the
concentration by,
for example, at least about 15%, or by at least about 40%, or even by at least
60%, or at least by
about 80% relative to untreated subjects.
In another embodiment, active compounds are administered at a therapeutically
effective
dosage sufficient to decrease or reduce amyloid protein levels, e.g., A[340 or
A[342, in the plasma
or CSF of a subject. A therapeutically effective dosage decreases the
concentration by, for
example, at least about 15%, or by at least about 40%, or even by at least
60%, or at least by
about 80% relative to untreated subjects.
In yet another embodiment, active compounds are administered at a
therapeutically
effective dosage sufficient to improve ADAS-cog (Alzheimer's Disease
Assessment Scale-
cognitive subscale) test scores by, e.g., at least about 1 point, at least
about 2 points, at least
about 3 points, at least about 4 points, at least about 5 points, at least
about 10 points, at least
about 12 points, at least about 15 points, or at least about 20 points
relative to untreated subjects.
(ADAS-cog; Rosen, et al., 1984, Arra. J. Psychiatry 141:1356-1364).
-54-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
The ability of a compound to inhibit amyloid deposition can be evaluated in an
animal
model system that may be predictive of efficacy in inhibiting amyloid
deposition in human
diseases, such as a transgenic mouse expressing human APP or other relevant
animal models
where A(3 deposition is seen. Likewise, the ability of a compound to prevent
or reduce cognitive
impairment in a model system may be indicative of efficacy in humans.
Alternatively, the ability
of a compound can be evaluated by examining the ability of the compound to
inhibit amyloid
fibril formation ifz vitro, e.g., using a fibrillogenesis assay including a
ThT, CD, or EM assay as
described in, e.g., WO 2003/017,994. Also the binding of a compound to amyloid
fibrils may be
measured using a MS assay as described herein.
The present invention is also related to prodrugs of the compounds of the
Formulae
disclosed herein. Prodrugs are compounds which are converted ifz vivo to
active forms (see, e.g.,
R.B. Silverman, 1992, "The Organic Chemistry of Drug Design and Drug Action,"
Academic
Press, Chp. 8). Prodrugs can be used to alter the biodistribution (e.g., to
allow compounds which
would not typically enter the reactive site of the protease) or the
pharmacokinetics for a
particular compound. For example, a carboxylic acid group, can be esterified,
e.g., with a methyl
group or an ethyl group to yield an ester. When the ester is administered to a
subject, the ester is
cleaved, enzymatically or non-enzymatically, reductively, oxidatively, or
hydrolytically, to
reveal the anionic group. An anionic group can be esterified with moieties
(e.g., acyloxymethyl
esters) which are cleaved to reveal an intermediate compound which
subsequently decomposes
to yield the active compound. The prodrug moieties may be metabolized izz vivo
by esterases or
by other mechanisms to carboxylic acids.
Examples of prodrugs and their uses are well known in the art (See, e.g.,
Berge et al.
(1977) "Pharmaceutical Salts", J. Plzarm. Sci. 66:1-19). The prodrugs can be
prepared izz situ
during the final isolation and purification of the compounds, or by separately
reacting the
purified compound in its free acid form with a suitable derivatizing agent.
Carboxylic acids can
be converted into esters via treatment with an alcohol in the presence of a
catalyst.
Examples of cleavable carboxylic acid prodrug moieties include substituted and
unsubstituted, branched or unbranched lower alkyl ester moieties, (e.g., ethyl
esters, propyl
esters, butyl esters, pentyl esters, cyclopentyl esters, hexyl esters,
cyclohexyl esters), lower
alkenyl esters, dilower alkyl-amino lower-alkyl esters (e.g.,
dimethylaminoethyl ester),
acylamino lower alkyl esters, acyloxy lower alkyl esters (e.g.,
pivaloyloxymethyl ester), aryl
esters (phenyl ester), aryl-lower alkyl esters (e.g., benzyl ester),
substituted (e.g., with methyl,
halo, or methoxy substituents) aryl and aryl-lower alkyl esters, amides, lower-
alkyl amides,
dilower alkyl amides, and hydroxy amides.
-55-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Certain embodiments of the present compounds can contain a basic functional
group,
such as amino or alkylamino, and are, thus, capable of forming
pharmaceutically acceptable salts
with pharmaceutically acceptable acids. The term "pharmaceutically acceptable
salts" in this
respect, refers to the relatively non-toxic, inorganic and organic acid
addition salts of compounds
of the present invention. These salts can be prepared in situ during the final
isolation and
purification of the compounds of the invention, or by separately reacting a
purified compound of
the invention in its free base form with a suitable organic or inorganic acid,
and isolating the salt
thus formed.
Representative salts include the hydrohalide (including hydrobromide and
hydrochloride), sulfate, bisulfate, phosphate, nitrate, acetate, valerate,
oleate, palmitate, stearate,
laurate, benzoate, lactate, phosphate, tosylate, citrate, maleate, fumarate,
succinate, tartrate,
napthylate, mesylate, glucoheptonate, lactobionate, 2-hydroxyethylsulfonate,
and
laurylsulphonate salts and the like. (See, e.g., Berge et al. (1977)
"Pharmaceutical Salts", J.
Pharm. Sci. 66:1-19).
15, ' In other cases, the compounds of the present invention may contain one
or more acidic
functional groups and, thus, are capable of forming pharmaceutically
acceptable salts with
pharmaceutically acceptable bases. The term "pharmaceutically acceptable
salts" in these
instances refers to the relatively non-toxic, inorganic and organic base
addition salts of
compounds of the present invention.
These salts can likewise be prepared in situ during the final isolation and
purification of
the compounds, or by separately reacting the purified compound in its free
acid form with a
suitable base, such as the hydroxide, carbonate or bicarbonate of a
pharmaceutically acceptable
metal cation, with ammonia, or with a pharmaceutically acceptable organic
primary, secondary
or tertiary amine. Representative alkali or alkaline earth salts include the
lithium, sodium,
potassium, calcium, magnesium, and aluminum salts and the like. Representative
organic
amines useful for the formation of base addition salts include ethylamine,
diethylamine,
ethylenediamine, ethanolamine, diethanolamine, piperazine and the like.
Some selected compounds of the present invention are illustrated in Table 2,
below.
Although particular salts are depicted (such as the hydrochloride), the free
base or non-salt form,
as well as other pharmaceutically acceptable salts are within the present
invention.
Those skilled in the art will recognize, or be able to ascertain using no more
than routine
experimentation, many equivalents to the specific embodiments and methods
described herein.
Such equivalents are intended to be encompassed by the scope of the following
claims. All
patents, patent applications, and literature references cited herein are
hereby expressly
-56-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
incorporated by reference in their entirety. This invention is further
illustrated by the following
examples, which should not be construed as limiting.
Examules
The synthesis of amidine compounds of the invention is described in U.S.
Patent Nos.
5,428,051, 4,963,589, 5,202,320, 5,935,982, 5,521,189, 5,686,456, 5,627,184,
5,622,955,
5,606,058, 5,668,167, 5,667,975, 6,025,398, 6,214,883, 5,817,687, 5,792,782,
5,939,440,
6,017,941, 5,972,969, 6,046,226, 6,294,565 (B1), 6,156,779, 6,326,395,
6,008,247, 6,127,554,
6,172,104, 4,940,723, 5,206,236, 5,843,980, 4,933,347, 5,668,166, 5,817,686,
5,723,495,
4,619,942, 5,792,782, 5,639,755, 5,643,935, 5,602,172, 5,594,138, and
5,578,631. Additional
synthesis protocols may be found in PCT Patent Application Publication No. WO
2003/017,994.
Many of the compounds may also be purchased from Sigma-Aldrich Co. (Milwaukee,
USA).
The compounds may also be synthesized according to art-recognized techniques.
In general, the compounds of the present invention may be prepared by the
methods
illustrated in the general reaction schemes as, for example, described below,
or by modifications
thereof, using readily available starting materials, reagents and conventional
synthesis ,
procedures. In these reactions, it is also possible to make use of variants
which are in themselves
known, but are not mentioned here. Functional and structural equivalents of
the compounds
described herein and which have the same general properties (e.g., functioning
as anti-amyloid
compounds), wherein one or more simple variations of substituents are made
which do not
adversely affect the essential nature or the utility of the compound.
The compounds of the present invention may be readily prepared in accordance
with the
synthesis schemes and protocols described herein, as illustrated in the
specific procedures
provided. However, those skilled in the art will recognize that other
synthetic pathways for
forming the compounds of this invention may be used, and that the following is
provided merely
by way of example, and is not limiting to the present invention. See, e.g.,
"Comprehensive
Organic Transformations" by R. Larock, VCH Publishers (1989). It will be
further recognized
that various protecting and deprotecting strategies will be employed that are
standard in the art
' (See, e.g., "Protective Groups in Organic Synthesis" by Greene and Wuts).
Those skilled in the
relevant arts will recognize that the selection of any particular protecting
group (e.g., amine and
carboxyl protecting groups) will depend on the stability of the protected
moiety with regards to
the subsequent reaction conditions and will understand the appropriate
selections.
-57-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Further illustrating the knowledge of those skilled in the art is the
following sampling of
the extensive chemical literature: "Chemistry of the Amino Acids" by J.P.
Greenstein and
M. Winitz, John Wiley & Sons, Inc., New York (1961); "Comprehensive Organic
Transformations" by R. Larock, VCH Publishers (1989); T.D. Ocain, et al., J.
Med. Chem. 31,
2193-99 (1988); E.M. Gordon, et al., J. Med. Chem. 31, 2199-10 (1988);
"Practice of Peptide
Synthesis" by M. Bodansky and A. Bodanszky, Springer-Verlag, New York (1984);
"Protective
Groups in Organic Synthesis" by T. Greene and P. Wuts (1991); "Asymmetric
Synthesis:
Construction of Chiral Molecules Using Amino Acids" by G.M. Coppola and H.F.
Schuster,
John Wiley & Sons, Inc., New York (1987); "The Chemical Synthesis of Peptides"
by J. Jones,
Oxford University Press, New York (1991); and "Introduction of Peptide
Chemistry" by
P.D. Bailey, John Wiley & Sons, Inc., New York (1992).
Test compounds were purchased from commercial sources or synthesized and
screened
by mass spectroscopy ("MS") assay, which gives data on the ability of
compounds to bind to an
amyloid protein.
Samples were prepared as aqueous solutions containing 20% ethanol, 200 ~,M of
a test
compound and 20 N.M of solubilized A(340. The pH value of each sample was
adjusted to 7.4
(~0.2) by addition of 0.1% aqueous sodium hydroxide. The solutions were then
analyzed by
electrospray ionization mass spectroscopy using a Waters ZQ 4000 mass
spectrometer. Samples
were introduced by direct infusion at a flow-rate of 25 p.L/min within 2 hr.
after sample
preparation. The source temperature was kept at 70 °C and the cone
voltage was 20 V for all the
analysis. Data were processed using Masslynx 3.5 software. The MS assay gives
data on the
ability of compounds to bind to A(3, whereas the ThT, EM and CD assays give
data on inhibition
of fibrillogenesis.
Some selected compounds of the present invention are presented in Table 2
below.
Although particular salts are depicted (such as the hydrochloride), the free
base and other
pharmaceutically acceptable salts, as well as the non-salt forms, are within
the present invention.
- 58 -
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Table 2
Structures and Activities of Some Compounds of the Invention in Soluble Af3
Assavs
Code MS
Structure and Name No. Assay
NH
CF3COZH
I \ ~NH ~NHz
145 +
Br
N-(6-aminoheptyl)-4-bromobenzamidine,
trifluoroacetic acid salt
NH CFgCOZH O
~NH HN ( \
/ 146 +
Br Br
4-bromo-N-{7-[(4-bromobenzimidoyl)-amino]-heptyl}-
benzamide, trifluoroacetic acid salt
\ O
~OH
HzN I / N.H
HON 2[CF3COzH] 147 -
N-hydroxy-4-(9-hydroxyaminononyloxy)-benzamidine,
trifluoroacetic acid salt
O O
I \ ~NH HN I \ '
Et0 / / NHz
148 +
O CF3COZH N H
N-[7-(4-carbamimidoylbenzoylamino)-heptyl]
terephthalamic acid ethyl ester, trifluoroacetic acid salt
O ~ NH
Et0 ~NHz
\ I NH HN \ I
v 149 +
CF3C0?H ~ O
N-[9-(4-carbamimidoyl-benzoylamino)-nonyl]
terephthalamic acid ethyl ester, trifluoroacetic acid salt
-59-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Table 2, Continued
Structure and Name Coda MS
No. Assay
NH ~ NH
\ ,H H I \
150 +
Br ~,[CF3COzH] Br
4,4'-(pentamethylenediamidino)di(p-bromobenzene),
trifluoroacetic acid salt
Br / I ~ / I Br
\ NH HN \ 151 +
NH NH 2[CF3C02H]
4,4'-(nonamethylenediamidino)di(p-bromobenzene),
trifluoroacetic acid salt .
H3C0 / / OCH3
\ I NH HN \ I
152 -
NH NH
2[CF3COzH]
4,4'-(nonamethylenediamidino)di(p-methoxybenzene),
trifluoroacetic acid salt
H3C0 / / OCH3
H
\ I NON \
N J NH CF3COZH 153 +
4-methoxy-N-{2-[2-(4-methoxyphenyl)-
4,5-dihydroimidazol-1-y1]-ethyl}-benzamidine,
trifluoroacetic acid salt
2[HCI] NH ~ NH
I\ ~N N I\
/ H H '\~ 154 +
Me0 OMe
4,4'-(pentamethylenediamidino)di(p-methoxybenzene),
hydrochloride salt
-GO-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Table 2, Continued
Structure and Name Coda MS
No. Assay
NH
/ ( ~NH2
O O ~ O
/ I H~N~H I ~ 155 -
H2N ~ / NH2
NH NH
2,2'-(p-amidinophenylcarboxamido)
N,N-diethyl-p-amidinobenzamide
O O
I / I / CF3C02H
N N NHS 156
H H
{4-[5-(p=aminophenoxy)-pentyloxy]-phenyl}-(4,5-dihydro
1H-imidazol-2-yl)-amine, trifluoroacetic acid salt
S~N NHS
H H
H2N / 2[CF3C02H] / NHZ 158 -
NH NH
4,4'-(nonamethylenedisulfamyl)di(amidinobenzene),
trifluoroacetic acid salt
NHZ
HN
HN ~ \ NHZ
O NH 159 nd
2[CF3C02H] HN
O
3,3'-(pentamethylenediaminocarbonyl)di(amidinobenzene),
trifluoroacetic acid salt
-61-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Table 2, Continued
Code MS
Structure and Name No. Assay
H2N HN
HN O NH / \ NHZ
2~CFgC02H] 160 nd
O NH
4,4'-(tetramethylenediaminocarbonyl)di(amidinobenzene),
trifluoroacetic acid salt
In each indicated assay, "+" = achve; "- = inactive; "nd" = not determined.
The present invention also relates to novel compounds and the synthesis
thereof.
Accordingly, the following examples are presented to illustrate how some of
those compounds
may be prepared.
General Aspects
Chemicals were purchased from Aldrich. The compounds were identified and
characterized according to methods well known in the art, and the chemical
structures depicted
herein are consistent with those findings. Analytical thin-layer
chromatography (TLC) was
performed on silica gel 60 F2s4 plastic-backed plates. Solvents were reagent
grade unless
otherwise specified. The 1H (500 MHz) and 13C (125 MHz) were recorded on a
Varian Inova
500. The chemical shifts are reported on the 8 scales in parts per million
(ppm). The infra-red
(IR) spectra were carried out on a Perkin-Elmer Spectra One spectrometer (neat
compound on
NaCI plate).
Preparation of the starting material for cofrzpourids #145, #146, #150, and #1
Sl
NH. HCI
CN
\ I HC1, EtOH
gr 1,4-dioxane gr \
A cold solution (0 °C) of 4-bromobenzonitrile (1 g, 5.5 mrnol) in
ethanol (25 mL) was
saturated with dry HCl(g). The mixture was then stirred at room temperature
for 18 hours, after
which FT-IR showed complete disappearance of the nitrite. The solution was
concentrated to
dryness, and further dried in vacuo. The ethyl 4-bromobenzimidate
hydrochloride thus obtained
was used in the synthesis of compounds 145, 146, 150, and 151.
-62-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Compoufzd #145 and 146
NH HCI NH CF3CO2H
CF3COpH
/ p~ / ~NH NHz
1) 1,7-diaminoheptane, EtOH
B 2) RP-Prep HPLC Br
145
CF3C02H NH + O
/ ~ ~NH HN ~~
B ~ ~ Br
146
A solution of 1,7-diaminoheptane (257 mg, 1.97 mmol) in ethanol (10 mL) was
added to
a suspension of the ethyl 4-bromobenzimidate hydrochloride (1.21 g, 4.57 mmol)
in ethanol (5
mL). The mixture was stirred at room temperature for 24h. The mixture was
concentrated to
dryness. The crude products were separated by preparative RP-HPLC (Vydac C18,
215 nm, 50
mL/min, '0 % to 90 % MeCN in HZO containing 0.1 % TFA) to give compound # 145
as a waxy
solid (29.2 mg, 0.054 mmol, 3% yield) and compound #146 as a waxy solid (18.7
mg,
0.031 mmol, 1.6 % yield).
Cofnpound #147
o
0
NC ~ I Br 1) Na2C03, NH20H.HC1 I OH
H20, EtOH, D H2 ~ ~H
2) Preparative HPLC
N
HO~
CF3C02H CF3C02H
A mixture of 9-(4-cyanophenoxy)heptylbromide (181 mg, 0.5 mmol), sodium
carbonate
(180 mg, 1.7 mmol) and hydroxylamine hydrochloride (280 mg, 4 mmol) in 80 %
ethanol (10
mL) was heated at reflux for 2 h. The mixture was cooled to room temperature.
A solid
precipitated and was removed by filtration. The filtrate was concentrated to
dryness under
reduced pressure. The crude product was purified by preparative RP-HPLC (Vydac
C18, 215
nm, 50 mL/min, 0 % to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized
to give the
title compound as a clear oil (48.8 mg, 0.091 mmol, 18 % yield).
-63-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Conzpound #14~
0
I
ci ~ o 0
H N'J// H N'
NC~
Et3N, ~ I H
DMF NC CN
Step 1: To a cold solution (0 °C) of 1,7-diaminopentane (248 mg, 1.9
mmol) and
triethylamine (0.65 mL, 4.6 mmol) in DMF (8 mL) was added 4-cyanobenzoyl
chloride (685 mg,
4.1 mmol). The mixture was stirred overnight at room temperature, and then
diluted with water.
The beige solid that precipitated was collected by filtration and dried in
vacuo, giving the
corresponding amide (0.64 g, 1.65 mmol, 87 % yield).
0 0 ~ o
N
H H - 1) HCI, EtOH Et0 I 'H H ~\ ~ '
2) (NHy)C03, EtOH ~ '~NHZ
3) Preparative HPL IIC
NC NC O CF3COZH NH
Step 2: A suspension of 1,7-bis-(4-cyanobenzamido)heptane (389 mg, 1 mmol) in
a
mixture of absolute ethanol (12 mL) and 1,4-dioxane (16 mL), was cooled to 0
°C, saturated with
dry HCI, and the resulting mixture was stirred for 4 days at room temperature.
The solvent was
evaporated under reduced pressure. A brownish solid was obtained. A mixture of
the solid and
ammonium carbonate (2 g, 20 mmol) in ethanol (25 mL) was stirred overnight at
room
temperature. Then, the mixture was filtered. The filtrate was concentrated to
dryness. The crude
product was purified by preparative RP-HPLC (Vydac C18, 215 nm, 50 mL/min, 0 %
to 90 %
MeCN in H20 containing 0.1 % TFA) and lyophilized to give the title compound
as a white solid
(79.9 mg, 0.141 mmol, 14 % yield).
Compoufzd #149
O
I ~C~+ ~ NC \ ~ ~ CN
NC I / N N
NH2 NH2 Et3N I H H I
DMF O O
-G4-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Step 1: To a cold solution (0 °C) of 1,9-diaminononane (296 mg, 1.9
mmol) and
triethylamine (0.65 mL, 4.6 mmol) in DMF (8 mL) was added 4-cyanobenzoyl
chloride (685 mg,
4.1 mmol). The mixture was stirred overnight at room temperature, and then
diluted with water.
The beige solid that precipitated was collected by filtration and dried ifz
vacuo, giving the
corresponding amide (0.64 g, 1.54 mmol, 81 % yield).
NC ~ ~ ~ CN O NH
N I Et ~ ~ NH2
/ I I /
I H H I 1) HCI, EtOH / N I /
O O 2) (NH4)C03, EtOH p H H p CF3C02H
3) Preparative HPLC
Step 2: A suspension of 1,9-bis-(4-cyanobenzamido)nonane (417 mg, 1 mmol), in
a
mixture of absolute ethanol (12 mL) and 1,4-dioxane (16 mL), was cooled to 0
°C, saturated with
dry HCI, and the resulting mixture was stirred for 4 days at room temperature.
The solvent was
evaporated under reduced pressure. A brownish solid was obtained. A mixture of
the solid and
ammonium carbonate (2 g, 20 mmol) in ethanol (25 inL) was stirred overnight at
room
temperature. Then, the mixture was filtered. The filtrate was concentrated to
dryness. The crude
product was purified by preparative RP-HPLC (Vydac C18, 215 nm, 50 mL/min, 0 %
to 90 %
MeCN in H20 containing 0.1 % TFA) and lyophilized to give the title compound
as a white solid
(96.5 mg, 0.132 mmol, 16 % yield).
Conzpoufzd #1 SO
NH HCI CF3COZH NH~ NH CF3COZH
/ O~ + H ~NH --> / \ /
2 1)Na2C03,EtOH I H H
2) Prep RP-HPLC B ~ ~ Br
Sodium carbonate (1.3 g) was loaded into a dry 25 mL round bottom flask. The
1,5-
diaminopentane (0.65 mmol, 0.077mL) was added, followed by the ethyl 4-
bromobenzimidate
hydrochloride (465 mg, 1.7 mmol) and finally the ethanol (10 mL). The mixture
was stirred
vigorously at room temperature for 22 hours then filtered to remove solids.
The solids were
washed with methanol (20 mL). The filtrate was concentrated to dryness under
reduced pressure.
The crude product was purified by preparative RP-HPLC (Vydac C18, 215 nm, 50
mL/min, 0 %
to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give the title
compound as a
white solid (246.6 mg, 0.355 mmol, 54 % yield).
-65-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Compomad #151
NH HCI
v
O~ Br I ~ ~ I ~ Br
Br ~ + NH NH ~ / NH HN
2 1)Na2C03, EtOH
2) Prep RP-HPLC NH NH
CF3C02H CF3CO~H
Sodium carbonate (1.3 g) was loaded into a dry 25 mL round bottom flask. The
1,9-
diaminononatane (0.68 mmol, 108 mg) was added, followed by ethyl 4-
bromobenzimidate
hydrochloride (500 mg, 1.89 mmol) and finally the ethanol (14 mL). The mixture
was stirred
vigorously at room temperature for 22 hours then filtered to remove solids.
The solids were
washed with methanol (20 mL). The filtrate was concentrated to dryness under
reduced pressure.
The crude product was purified by preparative RP-HPLC (Vydac C18, 215 nm, 50
mL/min, 0 %
to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give the title
compound as a
white solid(231.3 mg, 0.308 mmol, 47 % yield).
Preparation of the starting material for compouf2ds #152, #153, arad # 154
CN ~ NH HCI
HCI, EtOH ~ I O
Me0 ~ 1,4-dioxane
Me
A cold solution (0 °C) of 4-methoxybenzonitrile (1.29 g, 9.59 mmol) in
ethanol (30 mL)
was saturated with dry HChg). The mixture was then stirred at room temperature
for 2 days, after
which FT-IR showed complete disappearance of the nitrite. The solution was
concentrated to
dryness, and further dried ifz vacuo. The ethyl 4-methoxybenzimidate
hydrochloride thus
obtained was used in the synthesis of compounds 152, 153 and 154.
-GG-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Compound # 152
NH HCI
v
O~ CH30 ~ ~ OCH3
Me ~ NHz NH2 1)Na2C~ I / NH HN
2) Prep RP-HPLC NH NH
CF3C02H CF3CO2H
Sodium carbonate (1.50 g) was loaded into a dry 25 mL round bottom flask. The
1,7-
diaminoheptane (1 mmol, 159 mg) was added, followed by the ethyl 4-
methoxybenzimidate
hydrochloride (550 mg, 2.50 mmol) and finally the ethanol (10 mL). The mixture
was stirred
vigorously at room temperature for a day, and then solids were removed by
filtration. The solids
were washed with methanol (20 mL). The filtrate was concentrated to dryness
under reduced
pressure. The crude product was purified by preparative RP-HPLC (Vydac C18,
215 nm, 50
mL/min, 0 % to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give
the title
compound as a white solid (249.3 mg, 0.382 mmol, 38 % yield).
Cozzz»ouyzd # 153
NH HCI C
H2N~~NHz
Me ~ + H
1)Na2C03, EtOH
2) Prep RP-HPLC
Sodium carbonate (1.45 g) was loaded into a dry ~25 mL round bottom flask. The
diethylenetriamine (1 mmol, 0.090 mL) was added, followed by the ethyl 4-
methoxybenzimidate
hydrochloride (430 mg, 2.0 mmol) and finally the ethanol (10 mL). The mixture
was stirred
vigorously at room temperature for a day, and then solids were removed by
filtration. The solids
were washed with methanol (20 mL). The filtrate was concentrated to dryness
under reduced
pressure. The crude product was purified by preparative RP-HPLC (Vydac C18,
215 nm, 50
mLlmin, 0 % to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give
the title
compound as a white solid (177.4 mg, 0.306 mmol, 31 % yield).
-67-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
Compound # 154
NH HCI HCI NH NH HCI
O~ N"N
~ + Hz~NH2 -~ / I
Me0' J 1 ) Na2C03, EtOH ~ ~ H H
2) Prep RP-HPLC Me0 OMe
2) HCi~aq,~, acetone
Sodium carbonate (1.45 g) was loaded into a dry 25 mL round bottom flask. The
1,5-
diaminopentane (0.84 mmol, 0.10 mL) was added, followed by the ethyl 4-
methoxybenzimidate
hydrochloride (400 mg, 2.23 mmol) and finally the ethanol (10 mL). The mixture
was stirred
vigorously at room temperature for a day, and then solids were removed by
filtration. The solids
were washed with methanol (20 mL). The filtrate was concentrated to dryness
under reduced
pressure. The crude product was purified by preparative RP-HPLC (Vydac C18,
215 nm, 50
mL/min, 0 % to 90 % MeCN in HZO containing 0.1 % TFA). It still contained
traces of
impurities after two purifications by preparative HPLC. The Product was
recrystallized from 0.5
mL of 2 N HCl in acetone (4.5 mL). The crystals slowly formed at -20
°C. The crystals were
collected by filtration, rinsed with acetone and dried in vacuo. The title
compound was obtained
as a white solid (37.6 mg, 0.085 mmol, 10 % yield).
Compound # 1 SS
0
CN
CI HZN~~NHz
NC ~ H 1) ESN, DMF, 0 °C to r.t. O O
2) Water / ~~
NC ~ I H H ~ I CN
Step 1: To a cold solution (0 °C) of diethylenetriamine (0.110 mL, 1.0
mmol) and
triethylamine (0.41 mL, 3.0 mmol) in DMF (5.5 mL) was added 4-cyanobenzoyl
chloride (365
mg, 2.2 mmol). The mixture was stirred overnight for 4 hours, and then diluted
with water (40
mL). The aqueous layer was extracted with ethyl acetate (4 x 25 mL). The
combined organic
layers were washed with water (2 x 10 mL), brine (1 x 15 mL) then dried over
magnesium
sulfate. The solvent was evaporated under reduced pressure and the residue was
dried in-vacuo
to give the compound as a tan solid (296.7 mg, O.G05 mmol, 61 % yield).
-G8-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
\ CN
O O ~ ~ O
1) HCI, EtOH
NH CF3CO2H
NG \ \ CN 2) ~NH4)zC03, EtOH
3) Prep. RP-HPLC ~N~
CF3CO2H
CF3CO2H
Step 2: A suspension of the crude tris-nitrite (296.7 mg, 0.605 rnmol) in a
mixture of
absolute ethanol (10 mL) and 1,4-dioxane (5 mL), was cooled to 0 °C,
saturated with dry HCI,
and the resulting mixture was stirred for one day at room temperature. The
volume of the
solution was reduced to about 4 mL and ether was added. The crude amidate was
collected by
filtration and dried ifz vacuo. A mixture of the solid and ammonium carbonate
(1.20 g, 12.0
mmol) in ethanol (10 mL) was stirred overnight at room temperature. The
mixture was filtered,
then the filtrate was concentrated to dryness. The crude product was purified
by preparative RP-
HPLC (Vydac C18, 215 nm, 50 mL/min, 0 % to 90 % MeCN in H20 containing 0.1 %
TFA). A
white solid formed upon concentration of the preparative HPLC fractions. The
solid was
collected by filtration and dried in vacuo. The title compound was obtained as
a white solid
(129.1 mg, 0.146 mmol, 24 % yield).
Compouh.d #156
o-~cH2)s- cF3co2 ~ o-~cH~s-o
N
o \ I \ ~ No ~I \ I \
2 2 1) H2, Pt02, EtOH, EtOAc ~~ ~N~
2) 1,4-dioxane, NMM H 1 CF CO H
H a 2
C~- ,_
NH ~Br
The 1,5-bis(4-nitrophenoxy)pentane (176 mg, 0.508 mmol) was reduced under HZ
(55 psi) with platinum (IV) oxide (14 mg) in a mixture of ethanol (3 mL) and
ethyl acetate
(3 mL) for 4 hours. The mixture was filtered over Celite and the pad was
washed with methanol
(5 rnL}. The filtrate was concentrated to dryness under reduced pressure and
the residue was
dried ifz vacuo for 30 minutes. The residue was then dissolved in a mixture of
1,4-dioxane
(5 mL) and dichloromethane (1.5 mL). N-Methylmorpholine (0.13 mL, 1.13 mmol)
was added,
-69-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
followed by the 1-(4,5-dihydro-1H-imidazol-2-yl)-3,5-dimethyl-1H-pyrazole
hydrobromide
(270 mg, 1.08 mmol). The suspension was stirred for a day at room temperature.
Dichloromethane was evaporated and the mixture was heated by an oil bath at 60
°C for 2 days.
The reaction was monitored by mass spectrometry. The solids were removed by
filtration and
the filtrate was acidified with 2 N HCl (2 mL) then concentrated to dryness
under reduced
pressure. The crude product was purified by preparative RP-HPLC (Vydac C18,
215 nm,
50 mL/min, 0 % to 90 % MeCN in H20 containing 0.1 % TFA). The title compound
was
isolated as an oil (25.1 mg, 0.043 mmol, 8.5 % yield).
Cof~zpourzd # 158
o~ ~o
SCI O~ i0 O~ i0
+ H2N-(CH2)s-NHa -~ \ SAN-(CH2)s'N~S
NC ~ 1 ) Et3N, DMF I ~ / H H ~
0 °C to r.t. NC CN
2) Water
Step 1: To a cold solution (0 °C) of 1,9-diaminononane (480 mg, 3.0
mmol) and
triethylamine (2.05 mL, 14.5 mmol) in DMF (9.0 mL) was added 4-
cyanobenzenesulfonyl
chloride (1.25 g, 6.3 mmol). The mixture was stirred overnight for 4 hours,
and then diluted with
water (40 mL). The beige solid that precipitated was collected by filtration
and dried in vacuo,
giving the corresponding sulfonamide (1.28 g, 2.62 mmol, 87 % yield).
o~ ~o o~ ~o
o~ ~o o ~o
SAN-(CH~9 r>'S a ~ ~N-~cH~s-L~S s
NC I ~ H H ~ I CN t) HCI, EtOH HZ I / H H ~ I NHZ
2) NHdOA°, EtOH
3) Prep. RP-HPLC NH CF3COZH CF3COZH NH
Step 2: A suspension of 1,9-bis(4-cyanobenzesulfonamidoamido)nonane (318 mg,
0.651
mmol), in a mixture of absolute ethanol (10.0 mL) and 1,4-dioxane (4.0 mL),
was cooled to 0 °C,
saturated with dry HCI, and the resulting mixture was stirred 24 hours at room
temperature. The
solvent was evaporated under reduced pressure. A tan solid was obtained. A
mixture of the solid
and ammonium acetate (1.05 g, 13.6 mmol) in ethanol (10 mL) was stirred 24
hours at room
temperature. The mixture had turned to a gel. Addition of methanol (10 mL)
produced a solution.
The addition of concentrated HCl (1.5 mL) precipitated some of the ammonium
chloride. The
mixture was filtered, then filtrate was concentrated to dryness and dried ifz
vacuo. The crude
product was purified by preparative RP-HPLC (Vydac C18, 215 nm, 50 mL/min, 0 %
to 90 %
-70-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
MeCN in H20 containing 0.1 % TFA) and lyophilized to give the title compound
as a white solid
(205.2 mg, 0.273 mmol, 42 % yield).
Compound # 159
0 0 0
NC I NC CN
I + H2 NHz I I I
Et3N ~ H H
DMF
Step 1: To a cold solution (0 °C) of 1,5-diaminopentane (0.35 mL, 3
mmol) and
triethylamine (0.49 mL, 3.5 mmol) in DMF (6 mL) was added 3-cyanobenzoyl
chloride (550 mg,
3.3 mmol). The mixture was stirred overnight at room temperature, and then
diluted with water
(40 mL). The beige solid that precipitated was collected by filtration and
dried in vacuo, giving
the corresponding amide, 500 mg, 1.39 mmol, 90 % yield.
O O
NC / ~ N I / ~ CN
H C2i H2oN402 F ~ 1 ) HCI, EtOH
360.42 2) (NH4)2C03, EtOH
360.158626 3) Preparative HPLC
CF3C02H CF3C02H
NH O O NH
H2 ~~ ~ I I ~ ~ ~NH2
H H
C25H28F6N6~6
622.52
622.197452
Step 2: A suspension of 1,5-bis-(3-cyanobenzamido)pentane (480 mg, 1.33 mmol),
in a
mixture of absolute ethanol (lOmL) and 1,4-dioxane (10 mL), was cooled to 0
°C, saturated with
dry HCI, and the resulting mixture was stirred for a days at room temperature.
The solvent was
evaporated under reduced pressure. A brownish solid was obtained. A mixture of
the solid and
ammonium carbonate (2.55 g, 20 mmol) and 4A molecular sieves (175 mg) in
ethanol (20 mL)
was stirred overnight at room temperature. Then, the mixture was filtered, the
solids were rinsed
with methanol (2 x 10 mL). TFA (1.5 mL) was added and the filtrate was
concentrated to
dryness. The crude product was purified by preparative RP-HPLC (Vydac C18, 215
nm, 50
-71-
CA 02552094 2006-06-28
WO 2005/079780 PCT/IB2004/000617
mL/min, 0 % to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give
the title
compound as a white solid, 180.2 mg, 0.289 mmol, 25 % yield.
Compound # 160
O O H I \ CN
/ /
/ CI + Ha
NC \ I ~NHz Et3N NC \ H O
DMF
Step l: To a cold solution (0 °C) of 1,4-diaminobutane (0.3 mL, 3
mmol) and
triethylamine (0.49 mL, 3.5 mmol) in DMF (9 mL) was added 4-cyanobenzoyl
chloride (550 mg,
3.3 mmol). The mixture was stirred overnight at room temperature, and then
diluted with water
(20 mL). The beige solid that precipitated was collected by filtration and
dried in vacuo, giving
the corresponding amide, 0.48 g, 1.39 mmol, 90 % yield.
O H ~ ~ CN
N
1) HCI, EtOH
H O 2) (NH4)2C03, EtOH
NC ~ 3) Preparative HPLC
NH2
Step 2: A suspension of 1,4-bis-(4-cyanobenzamido)butane (460 mg, 1.33 mmol),
in a
mixture of absolute ethanol (lOmL) and 1,4-dioxane (10 mL), was cooled to 0
°C, saturated with
dry HCI, and the resulting mixture was stirred for a week at room temperature
with 3 saturation
with HCI.. The solvent was evaporated under reduced pressure. A brownish solid
was obtained.
A mixture of the solid and ammonium carbonate (2.55 g, 20 mmol) and 4~
molecular sieves
(175 mg) in ethanol (20 mL) was stirred overnight at room temperature. Then,
the mixture was
concentrated to dryness. The crude product was re-suspended in water and the
suspension
centrifuged. The supernatant was purified by preparative RP-HPLC (Vydac C18,
215 nm, 50
mL/min, 0 % to 90 % MeCN in H20 containing 0.1 % TFA) and lyophilized to give
the title
compound as a white solid, 191 mg, 0.307 mmol, 23 % yield.
-72-