Note: Descriptions are shown in the official language in which they were submitted.
CA 02552179 2006-07-06
Physical and mechanical properties of pea starch edible films containing
beeswax
emulsions
BACKGROUND OF THE INVENTION
Starch is one of the most abundant, inexpensive and commonly used natural
polysaccharides (Guilbert and Gontard 2005; Narayan 1994), and in both its
native
and modified forms, has been playing important roles in the food industry.
Starches
and their derivatives have been used to modify physical properties of food
products,
which include texture, viscosity, gel-formation, adhesion, binding, moisture
retention,
product homogeneity and film-formation (Thomas and Atwell 1997; Liu 2005). The
use of polysaccharides as film and coating materials has grown extensively in
recent
years because of their edibility, low permeability to oxygen and contribution
to quality
preservation (Mark and others 1966; Roth and Mehltretter 1970; Lourdin and
others
1995; Paiviainen and others 2001; Forssell and others 2002; Han and Gennadios
2005). Edible films and coatings are generally prepared using edible
biopolymers
such as polysaccharides, proteins and lipids, and food-grade additives
(Gennadios
and others 1997; Han and Gennadios 2005). Especially, starch is an attractive
raw
material for edible packaging because of its renewability, biodegradability
and low
cost compared with protein (Guilbert 2000; Guilbert and Gontard 2005; Liu
2005).
Starch is normally a mixture of amylose and amylopectin polymers. Most
starches, such as those from wheat, corn and potato, contain about 25% amylose
and
75% amylopectin (Haase 1993; BeMiller and Whistler 1996). However, some legume
starches including those of peas are characterized by a high-amylose content
(24-
65%, varies with species) (Hoover and Sosulski 1991). High-amylose starch is a
very
useful film-forming material because it normally improves mechanical strength
including tensile strength and gas barrier properties (Wolff and others 1951;
Lourdin
and others 1995; Pawiainen and others 2001). This is probably due to the
higher
degree of crystallinity of amylose-rich region after dehydration (Garcia and
others
2000; Liu and Han 2005).
CA 02552179 2006-07-06
2
Pea is an important grain legume, as an animal feed and human food, which is'
cultivated in many regions of the world and ranks fourth in terrns of world
production
of food legumes behind soybean, peanuts and dry beans (FAO 2000). Pea starch
is
mainly used in industrial applications, but not much in food applications.
Therefore, it
is economically important to explore possible avenues for improving the
functional
properties of pea starch for it to be successfully utilized in the food
industry.
The efficiency and functional properties of edible film and coating materials
are highly dependent on the inherent characteristics of fiim-forming materials
including a variety of polysaccharides and their derivatives (Liu 2005;
Lacroix and Le
Tien 2005). Films from polysaccharides possess excellent oxygen barrier
properties
due to their tightly packed and ordered structure through intermolecular
hydrogen
bonds (Fang and Hanna 2000; Diaz-Sobac and others 2001), but their barrier
properties against water vapor is poor due to their hydrophilicity (Guilbert
1986;
Kester and Fennema 1986; Gennadios and others 1994; Lacroix and Le Tien 2005).
Many functions of edible films and coatings are similar to those of synthetic
packaging
films; however, edible film and coating materials must be chosen according to
the
specific food application, the types of food products, and the major
mechanisms of
quaiity deterioration (Petersen and others 1999; Guilbert 2002; Guilbert and
Gontard
2005).
Edible films composed of polysaccharides have suitable mechanical and
optical properties, but are highly sensitive to moisture and are poor water
vapor
barrier materials (Perez-Gago and Krochta 2005). An approach to improving
water
vapor barrier properties of the films is to produce a composite film by adding
hydrophobic components such as lipid and wax materials, which results in bi-
layer or
emulsion films (Qebeaufort and others 1993). Significant variables for
modifying
moisture resistance of emulsion composite films, which have been studied, were
lipid
types, location, volume fraction, polymorphic phase, and drying conditions
(Krochta
1997; Perez-Gago and Krochta 2005). Emulsion composite films require oniy a
single
casting process for film-formation without any further process of lamination;
however
their physical and mechanical properties are highly dependent on lipid
content, lipid
CA 02552179 2006-07-06
3
particle size and visco-eiasticity of the lipid (Debeaufort and others 1993;
Perez-Gago'
and Krochta 2005).
Emulsion of lipids in starch films can modify the surface properties of the
films
to be more hydrophobic, which can affect the wettability, water resistance,
grease
resistance, static discharge, surface energy and ink printabiiity.
Herein we identify physical and chemical effects of the addition of lipid
emulsions Into high-amylose pea starch for the purpose of specific
modification of the
chemical structure-related properties of pea starch films.
SUMMARY OF THE INVENTION
According to the invention, there is provided an edible film cornprising a
high amylose starch and an edible, hydrophobic lipid.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1. Microscopic image of pea starch films with 0% beeswax (A
and B), 20% beeswax (C and D), and 40% beeswax (E and F). A, C and E are with
4x
magnification, and B, D and F are with 10x magnification. Scale bars show 100
m.
Figure 2. Diameter and shape factor distributions of beeswax particles in
pea starch films.
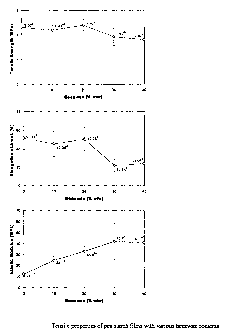
Figure 3. Tensile properties of pea starch films with various beeswax
contents.
Figure 4. Water vapour permeability (g mm m2 h" kPa-') and oxygen
penmeability (cc pm m"2 d" kPa'') of pea starch films with verious beeswax
contents.
Figure 5. DSC thermogrems of pea starch and pea starch fiims. native
pea starch (A), pea starch film without glycerol and beeswax (B), pea starch
film with
glycerol and without beeswax (C), and pea starch film with glycerol and 40%
beeswax
(D, E).
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Unless defined othennrise, all technical and scientific terms used herein
CA 02552179 2006-07-06
4
have the same meaning as commonly understood by one of ordinary skill in the
art to'
which the invention belongs. Although any methods and materials similar or
equivalent to those described herein can be used in the practice or testing of
the
present invention, the preferred methods and materials are now described. All
publications mentioned hereunder are incorporated herein by reference.
The invention comprises a combination of two materials: (1) starch and (2)
lipids. Preferably, the starch contains a certain amount of amylose.
Conventional 1ow
amylose starches (for example corn starch, wheat starch, tapioca starch, rice
starch,
potato starch, cassava starch and other starches) make a partially or fully
soluble
films which are not good moisture baniers. High-amylose starches from high
amylose
rice, high amylose oorn or high amylose peas possess stronger barrier
properties
against moisture and water than conventional starches due to the amylose
component of starch can increase crystallinity of gelatinized starch films.
Starch
containing more than 25% of amylose (i.e., less than 75% amylopectin) can form
quite
good moisture barrier films. Increasing percentage of amylose in starch will
create
better moisture barrier as well as stronger films with higher tensile strength
and elastic
modulus. However, starch containing more than 40% of amylose requires high
pressure chambers (or containers) in order to gelatinize the high amylose
starch.
Starch with more than 40% starch has i#s gelatinization temperature over 100C,
and
to achieve this temperature for starch dispersion in water a high pressure
container is
required. Starch with lower than 40% amylose has its gelatinization
temperature
below 100C and the boiling of starch dispersion will gelatinize the starch.
Accordingly,
in some embodiments, the starch has an amylose content of at least 25%. In
other
embodiments, the starch has an amylose content between 25-40%.
The second material of this invention are hydrophobic lipids such as waxes,
paraffins or Shellac. Any edible lipids can be used for the hydrophobic
materials in
starch films; however, specifically in this invention, solid lipids are
suggested to be
used. At room temperature (around 20 - 25C) or the temperature of end product
use
(such as the refrigeration temperature), this hydrophobic material should not
be liquid
oil. Liquid oil are leached out (or sweated out) from the starch film if they
are used as
CA 02552179 2006-07-06
rJ
hydrophobic lipid materials. The solid lipids can be melted at the temperature
used for
the gelatinized of the starch solution, and then solidified during cooling and
drying
following homogenization in the starch solution. Preferably, the solid lipid
is selected
from the group consisting of beeswax, carnauba wax, (and other natural waxes),
parafFn wax (or other petroleum based edible waxes known in the art), animal
fats
(i.e., tallow, lard or butter-fat), Shellac and other edible lipids. The
addition of
hydrophobic materials in hydrophilic polymeric materials is generally
attempted to
increase the water resistance of the hydrophilic films. When hydrophilic
starch films
contain hydrophobic solid lipid particles, for example, beeswax although any
suitable
wax from various sources, for example, paraffins and Shellac as well as other
discussed above, the tensile strength, elongation-at-break, water vapor
permeability
and oxygen permeability did not change significantly until the beeswax content
reaches more 20 - 30% of pea starch. However, above 30% of beeswax content,
the
tensile strength, elongation-at-break, and water vapor permeability decreased,
Elastic
modulus has been increased when the film contains beeswax. The oxygen
permeability was increased at 40% of beeswax content in the film. DSC results
identified the existence of amylose-lipid complex endothermic peak of the
starch films
containing 40% beeswax. Overall the addition of beeswax in pea starch films
alters
the mechanical, physical and thermal properties of the starch films when the
films
contain very high concentration of beeswax. The addition of beeswax under the
concentration range of 30% was not effective to increase the water resistance
of the
hydrophilic starch films.
The addition of more wax may increase the resistance of water-related
properties of starch films. However, an extreme amount of wax in starch will
reduce
the advantageous properties of starch. Starch is acting as the structural
polymer of
the film matrix. Therefore, a high conoentration of wax in starch without
damaging the
structural properties of the starch polymer matrix is the most desirable
product.
Microstructure
CA 02552179 2006-07-06
6
All film samples were observed before using a microscope. They were'
homogeneous and translucent. Figure 1 shows the increase in particle numbers
when
beeswax content increased (Fig. 1A, C and E). Beeswax particles are
distributed in
the continuous starch matrix. Particle size and shape v+ri11 affect the
allowable amount
of wax in the starch, as well as the physical and rnechanical properties of
starch films.
Smaller particle size is beneficial for the minimization of the interference
of wax with
physical and mechanical properties of starch film, Also smaller particle size
(lower
than 1000 nm) will create new application area of this invention in the
nanotechnology
applications. Figure 1 B is the picture of very thin part of pea starch film
that is not a
usual part of the film, therefore, can not be compared with other pictures in
Fig. 1.
Figure 1 B shows many filament structures which are connecting together. The
filament structure of Fig. 1 B is composed mainly of amylose extracted from
starch
granules during heat gelatinization prooess. Liu and Han (2005) reported the
fibrous
structure formation during drying of the amylose solution resulting in
dendrite crystals.
This fibrous structure was also observed from Fig. I B.
Table 1 listed film thickness and particle size. Film thickness was increased
after the addition of beeswax compared to 0% wax film; however, from 10% to
40% of
beeswax concentration, the film thickness remained the same. With fairly large
standard deviation, the average emulsion size did not show any difFerence at
10%
significance interval. However, the coefficient of variation increased from
58.1%,
59.1%, 66.8% to 66.9% for 10%, 20%, 30% and 40% beeswax, respectively.
Increase
in coefficient of variation means more heterogeneity (lower homogeneity) in
the film
structure. This heterogeneity will increase variability of film
characteristics of end
products. Low concentration of beeswax could generate more homogeneous
distribution (i.e., smaller coefficient of variation) of emulsion particle
size. Total 250 -
350 particles were identified from one frame of image. Figure 2A shows the
distnbutions of particle size and indicates that the peak height at 4 - 5 m
is
decreasing as the beeswax concentration increased, which supports the increase
in
the coefficient of variation. From Fig. 2B, the major peak of the emulsion
shape factor
was shifted from 0.9, for 10% beeswax, to 0.3 - 0.4 for 20%, 30% and 40%
beeswax
CA 02552179 2006-07-06
7
concentration, indicating the spherical shape of beeswax emulsion particles at
low
concentration of beeswax changed to rod shape of emulsion particles when the
beeswax concentration increased. The spherical shape will minimize the effect
of the
particle on the starch film properties because the sphere takes the smallest
volume
per weight of wax. However, rod shape particles would be beneficial to the
moisture
vapor or other hydrophilic barrier properties because they will increase the
tortuosity
of the hydrophilic migrant through the starch film. Increasing beeswax
concentration
may raise the shearing effect of the high speed homogenizer to the particles
and
create more rod shape when the beeswax was hardened during cooling process. To
obtain the more homogeneous spheres the termination time of the homogenizer
operation and the temperature of film-forming solution should be controlled.
The
homogenization process should be complete before the beeswax emulsions start
to
be solidified.
Tensile properties
Figure 3 shows that the addition of beeswax emulsions in starch films
decreased tensile strength and elongation, and increased elastic modulus of
the
starch films. This implies that the addition of beeswax reduces the ductility
of starch
films. If the final product requires more flexibility and elongation of starch
film with high
level of wax content, more plasticizer may be required. But up to 40% of
beeswax
content the starch film still possesses very applicable mechanical properties.
At 30%
and 40% of beeswax, the starch films possessed statistically significant
difference in
tensile strength, elongation and elastic modulus as compared to those of 0%,
10%
and 20% of beeswax contents. However, the magnitude of the difference was less
than 3 times. Compared to other tensile properties, tensile strength was
reduced least
significantly by the addition of beeswax. This implies that the main material
to
maintain the strength is the starch matrix and beeswax emulsion particles were
dispersed in the starch matrix without interfering with the tensile stress
distribution in
the film structure at the concentration below 20%. Elongation-at-break did not
show
any difference at the beeswax level ranging from 0% to 20% and started to
decrease
CA 02552179 2006-07-06
8
at 30% of beeswax, The maximum content of beeswax emulsions was 20% which did
not create any reduction in tensile strength or elongation. However, the
elastic
modulus of the starch films responded difFen;ntly.. Elastic modulus increased
linearly
as the beeswax content increased from 0% to 30% which is the concentration
range
showing the insignificance of tensile strength and elongation from 0% to 20%.
Maximum stress and elongation-at-break were not changed when the beeswax
content Increased from 0% to 20%. However, the elastic modulus which has been
obtained from the initial slope of the stress-strain curve, increased. Figure
3 did not
show any data of yield stress of starch film. However, when starch film
contained
beeswax, the stress-strain profile showed unambiguous yield stress and after-
yield
stress deformation, which is the characteristics of heterogeneous elastic
materials.
The addition of beeswax up to 20 - 30% made the starch film liittle more stiff
and
glassy, but did not affect the maximum stress and strain at break (i.e.,
breaking force).
The starch film becomes a little more stiff but the breaking strength is the
same as for
starch-only film.
Gas barrier properties
Figure 4 shows the changes in permeabilities of water vapor and oxygen
through beeswax emulsion films. When beeswax concentration increased, the
water
vapor permeabiiity (WVP) decreased slightly but significantly at high
concentration of
beeswax. The films contained 30 and 40% of beeswax have lower WVP than those
of
0, 10 and 20%. Beeswax particles are absolutely water resistant oompaned to
starch
matrix. Therefore, this slight difference indicates that the film still has
many spaces
and channels enough to allow the penetration of water vapor through the starch
matrix, Due to the large portion of starch matrix in the film compared to the
portion of
beeswax particies, the WVP changed very little after the incorporation of
beeswax.
More addition of beeswax may decrease WVP much more significantly. However,
the
extra amount of beeswax could decrease tensile strength and elongation of
starch
films, and would create inferior mechanical properties of the film.
CA 02552179 2006-07-06
9
Oxygen permeability (OP) did not change until the starch films contain 30% of
beeswax. However 40% of beeswax addition in the film increased OP
significantly.
Since the permeation mechanism of gas consists of diffusion and absorption,
this
result may be explained by two theoretical hypotheses of the mechanisms.
Hypothesis 1(Diffusion): Oxygen molecules diffuse through hydrophobic beeswax
channels. Oxygen molecules are water soluble as well as oil soluble. The
starch film
did not contain free water that could be utilized as an oxygen diffusion
passage.
Furthermore, the starch polymer contains many hydroxyl groups which can
interact
with diffusing oxygen molecules. Oxygen may penetrate through beeswax/starch
interfaces which are connected together between beeswax particles providing
oxygen
penetration channels when the film contains high wax content. High
concentration of
beeswax in starch films increases the possibility of the contact of beeswax
particles
and oxygen penetration passages, which resulted in larger value of
diffusivity.
Hypothesis 2 (Absorption): Increased amount of beeswax in the starch films
exposes
more beeswax on the surFace of starch film and consequently decreases the
surface
energy (i.e., increase the hydrophobicity) of starch films. This lower surface
energy
with higher hydrophobicity of the film surface may accelerate the absorption
of oxygen
from atmosphere and increase the solubility of oxygen. Since permeability
consists of
diffusivity and solubility, the significantly increased solubility could
increase the
permeability of oxygen. Both theories explain the significant increase of OP
at 40% of
beeswax concentration and no change in OP at the low concentration ranging
from 0
to 30%.
Differential scanning calorimetry
Native pea starch showed traditional gelation endothermic peak (Fig. 5A). Pea
starch film made of 3% suspension after gelatinization and dehydration showed
two
peaks (Fig. 5B). The range of temperature of the first peak corresponds with
the
gelation endothermic peak of crystalline mefting of starch molecule. The
second peak
is the range of 143 to 155 C, which corresponds with endothermic peak of
enzyme-
resistant starch (Gruchala and Pomeranz 1993; Sievert and Wursch 1993). A
CA 02552179 2006-07-06
previous study suggested that the enzyme-resistant starch could be formed by
the
retrogradation of starch, especially high amylose content starch, which
resulted from
water involved food processing such as cooking, :baking and autoclaving
(Gidley and
others 1995; Gruchala and Pomeranz 1993). After starch gelatinization, a-
glucan
5 chains reform double helices and eventually readign the crystalline
structure which is
called retrogradation (Miles and others 1985). Pea starch film without
glycerol should
be formed through enzyme-resistant starch formation. In detail, starch
moiecule
involved in recrystallization or retrogradation, and then enzyme-resistant
starch
formed by retrograded starch during the drying process of the film formation.
10 Pea starch films treated with glycerol showed a similar endothermic peak in
the range of geiation temperature. However, the aH increased from 4.8 to 8.2
(Jlg) as
oompared to the pea starch film without the treatment of glycerol (Fig. 5C).
Additionally, there is no second peak after the gelation peak. It should be
suggested
that glyceroi, as an ingredient of film, affects the crystalline structure of
film, prevents
the formation of enzyme-resistant starch, and increases AH. Glycerol may
position
between the hydroxyl groups of starch, interfere with the hydrogen bonds
between the
hydroxyl groups, and inhibit the formation of retrogradation of starch.
Pea starch film treated with glycerol and 40 % beeswax showed the first
geiatson endothermic peak and showed the second peak in the range from 120 to
130
C (Fig. 5D and E) or 95 - 140 C (Table 2), which corresponds with amylose-
lipid
complexes (Biliaderis and others 1985; Ward and others 1994). This suggests
that
beeswax plays a role in the formation of amylose-lipid complexes. Since added
lipids
can form complexes with amylose, less amount of amylose can be used to form
enzyme-resistant double helices (Eeriingen and others 1994).
The endotherm of amylose-lipid complex with 40% beeswax showed different
onset, peak, completion temperature, and AH in each DSC running. This could be
the
result of the amylose-lipid complex in film fomning a heterogeneous structure
due to
physical reorganization of beeswax in matrices of starch-beeswax during the
storage
time. The physical reorganization of polysaccharide matrices speciaily
involved in
CA 02552179 2006-07-06
11
amylose-lipid complexes was also observed In previous study (Tester and Debori
20Q0).
The 10, 20, and 30% of beeswax do not show amylose-lipid complexes, which
indicates a certain amount of beeswax is required to start formation of
amylose-lipid
complexes. The melting endotherm of beeswax (65 C) was overlapped with the
starch crystalline melting area; it results in higher enthalpy change at 50 -
80 C as
the amount of beeswax is increased. In some embodiments, especially with fine
spherical shape and size of beeswax, the starch film may contain more than 60%
of
beeswax in the starch polymer matrix. In this case starch polymer wili works
as a glue
(adhesive) material between beeswax particles and make them in one piece. This
will
have a structure similar to that of MDF board containing adhesive, wood chips
and
saw dust.
The observed values such as thermal properties, oxygen permeability, tensile
properties and others were significantly changed in pea starch film with 40%
beeswax
in both studies of DSC and mechanical properties. DSC study clearly
demonstrates
the change of thermal properties of pea starch films following the addition of
glycerol
and beeswax.
Materials
Commercial starch of Canadian yellow field peas (Pisum sativum L. Miranda)
produced by wet milling process, which contains 35 - 40% amylose was supplied
by
Nutri-Pea Ltd. (Portage-La-Prairie, Manitoba, Canada).. Starch consists of
amylose
and amylopectin. Common starch contains low amylose (i.e., high amylopectin).
Low
amylose starch produces a less water resistant film, while high amylose starch
produces a highly water resistant starch. If amylose is higher than 40%, the
gelatinization temperature is generally over 100 C. Therefore, the
gelatinization
process requires high pressure chamber to increase the boiling point of water
over
100 C. Pea starch has a gelatinization temperature bellow 90 C. So simple
boiling of
pea starch dispersion can gelatinize the starch. Increasing the amylose
content
produces a more resistant starch structure after gelatinization and
retrogradation. The
resistant starch is not water soluble, and possesses high water resistance.
Glycerol
CA 02552179 2006-07-06
12
(Sigma Chemical Co. Ltd., St. Louis, MO) was used as a plasticizer. Beeswax
(refined, melting temperature = 65 C) was also purchased from Sigma Chemical
Co.
Glycerol and beeswax are the most common. plasticizer and lipid components,
respectively, for the edible emulsion composite film studies, therefore, the
results of
experiments could easily be compared with those of other researchers.
Film preparation
Aqueous dispersion of 3% (w/w) pea starch (PS) was prepared with de-ionized
water. Glycerol (Gly) was added in the starch dispersion at the mass ratio of
60/40 of
PS/Gly. Beeswax was added in the starch dispersion at the 10, 20, 30 and 40%
(w/w
of PS). The PS dispersion with beeswax was heated and held at boiling
temperature
for 15 min with continuous stirring for complete gelatinization of starch and
melt of
beeswax. As will be apparent to one of skill in the art, instead of boiling
with water,
other heat prooess with water can gelatinize starch. Such processes may
include
extrusion, microwave heating and steaming cook. After boiling, the gelatinized
starch
dispersion with molten beeswax was blended by a high-speed homogenizer
(Fischer
Scientific Ltd., Nepean, ON, Canada) at 20,000 rpm for 15 min. The
homogenization
process was conducted under vacuum to avoid coagulation of beeswax and
eliminate
air bubbles in the final films. The vacuum helps eliminate air bubbles in the
beeswax-
starch dispersion. If there is no serious effect of air bubbles on the end
product
properties, vacuum is not essential. Temperature of the gelatinized starch
solution
(i.e,,, film-forming solution) after homogenization was 75 - 80 C. In
generai, the
homogenization temperature should be above the melting temperature of lipid
(beeswax).
About 15 g of film-forming solution was cast onto a polystyrene petri dish (10
cm in diameter) which was placed on a levelled flat surface. After the
solution was
allowed to be dried at room temperature for at least 48 h, the films were
peeled off
from the petri dishes and their thicknesses were measured using a digital
micrometer
at 5 different random positions of the films.
CA 02552179 2006-07-06
13
Image analysis of beeswax particles
Test films were placed onto microscope slides and observed using an inverted
phase contrast microscope (Nikon Diaphot TMD,_Kanagawa, Japan) equipped with a
TV camera (Panasonic WV-1550, Matsushita Electric Industrial Co., Ltd., Osaka,
Japan). The transmitted light source in the microscope was a tungsten halogen
bulb
(12V/50W). An NC810 (blue) color-correction filter (Nikon) was used in order
to
reduce the spectral bandwidth of the source to the range of 430 - 530 nm. The
DL
(dark low) series of objectives (4x, lOx or 100x magnification) were used to
produce
positive contrast in specimens having a significant difference in refractive
index from
the surrounding medium. A 2.5x relay lens was placed in the tube adapter to
connect
the TV camera to the microscope. The image of a test film was recorded by a
personal computer equipped with a video capture card. The image resolution was
set
at 640x480 pixels. The images were recorded to 8-bit greyscale images in
bitmap
PCX format. An image analysis program (SigmaScan Pro 5.0, Statistical
Solutions,
Saugus, MA) was used to measure the dimensions of beeswax particles in the
images, which were scaled using a stage objective micrometer (1/100 mm per
unit
scale). From the particle dimension analysis, the diameter and shape factor
(SF) of
particles were determined. Particle shape factor is a dimensionless constant
defined
as:
SF = 4~
p
where, A and p are area and perimeter of the particle, respectively. A perfect
circle,
therefore, has a shape factor of 1, and a rod shape has near zero.
Water vapor permeability (WVP) and oxygen permeability (OP)
WVP was determined by the procedure of Choi and Han (2001) which had
been modified from McHugh and others (1993). Briefly, 10 mL distilled water
was
taken into a flat-bottom acrylic cup with a wide rim. The cup was covered with
a test
film, which was then sealed with a seal ring and silicon sealant (High Vacuum
Grease,
Dow Corning, Midland, MI). The whole assembly was then kept inside a closed
CA 02552179 2006-07-06
14
chamber with a fan, a digital RH-meter and anhydrous calcium suifate (W.A:
Hammond Drierite Co., Xenia, OH) at 25 C temperature. The weight changes and
RH inside the chamber were monitored after every hour. Once the steady state
of
weight loss was achieved, the water vapor transmission rate (WVrR in g n72
h'') was
calculated. The WVP was determined from multiplying the WVTR with the
thickness
of the film and divided by the RH difference between inside the acrylic cup
and the
chamber. The RH inside the cup was calculated by the procedure of McHugh and
others (1993).
Oxygen permeability (OP) of the films was determined using an oxygen
transmission rate test machine (OxTran 2/20, Mocon, Minneapoiis, MN). After a
film
was placed in a cell and then flushed with nitrogen at 23 C and 50% relative
humidity
for 'I h, oxygen flow was intnaduced on one side of the films and the oxygen
transmission rate (OTR) was measured. Oxygen permeability (OP) in [cc m m'2
d"l
kPa') was calculated from the mean OTR multiplied by the film thickness (pm)
and
divided by the oxygen gradient in the cell of the testing machine (1 atm).
Tensile test
Film specimens (1 cm wide and 8 cm long) were made from the films. They
were condidoned in a controlled relative humidity chamber for 48 h at 50% RH.
Tensile strength (TS) was determined from a stress-strain curve using a
texture
analyzing instrument (Texture Analyser, TA-XT2, Texture Technologies, Corp.,
Scarsdale, NY) based on the procedure outlined in ASTM method D882-91 (ASTM,
1991). The initiai grip distance and crosshead speed were 5 cm and 100 mm/min,
respectively. TS was calculated by dividing the peak load by the cross
sectional area
of film (thickness of film x 1 cm) of the initial film. Elongation (E) was
calculated by the
percentiie of the change in the length of specimen to the original distanc:e
between the
grips (5 cm). Elastic modulus (EM) was calculated from the initiai slope of
the stress-
strain curve. An average of five or six replicates was obtained with a
standard
deviation.
CA 02552179 2006-07-06
Differential scanning calorimetry (DSC)
Thermal properties of native pea starch and pea starch films were analyzed
wi#h a Perkin-Elmer DSC-7 (Norwalk, CT) equipped with an intracooler and
Thermal
5 Analysis Controller TAC 7/DX (Perkin-Elmer). Samples (approx. 20 mg each,
db)
were weighed into stainless steel pans (Perkin-Elmer) designed to withstand
high
pressures and suppress the volatilization of solvent. The distilled water was
added
double the weight of sample into the pan using a microsyringe. The stainless
steel
pan was sealed with an 0-ring, and allowed to reach equilibrium of moisture
for
10 ovemight. The DSC instrument was calibrated with an indium, and an empty
DSC pan
was used for a reference pan. The heating rate was programmed by holding at -
20 C
for 1 min, followed by ramping the temperature range of -20 C to 180 C at a
rate of
C/min, and holding at -180 C for I min. Measurements were made at least in
duplicate for one treatment.
Statistical analysis
Mean values of each treatment were compared using a least significant
difference (LSD) test. The LSD value was calculated by following equation,
where Se
is pooled standard deviation of all treatment. The mean difference values
bigger than
LSD value were identified manually as significant differences. The confidence
level of
t value was 95% and total number of samples (n) was 7- 9 for each treatment.
L,SD - rU 12S,2
n
While the preferred embodiments of the invention have been described above,
it will be recognized and understood that various modifications may be made
therein,
and the appended claims are intended to cover all such modifications which may
fall
within the spirit and scope of the invention.
CA 02552179 2006-07-06
16
REFERENCES
ASTM. 1991. Standard test method for tensile properties of thin plastic
sheeting.
D882 In: Annual Book of American Society for Testing Methods 313-321.
Philadelphia, PA.
BeMiller JN, Whistler RL. 1996. Carbohydrates. In: Fennema OR, editor. Food
chemistry. 3rd ed. New York: Marcel Dekker. p 157-223.
Biliaderis CG, Page CM, Slade L, Sirett RR. 1985. Thermal-behavior of amylose-
lipid
complexes. Carbohydr Polym 5(5): 367-389.
Choi WS, Han JH. 2001. Physical and mechanical properties of pea-protein-based
edible films. J Food Sci 66: 319-322.
Debeaufort F, Martin-Polo M, Voilley A. 1993. Polarity homogeneity and
structure
affect water vapor permeability of model edible films. J Food Sci 58: 426-434.
Diaz-Sobac R, Beristain CI, Vernon-Carter EJ. 2001. Water vapor permeability
of an
emulsion coating of maltodextrin and surfactants. J Food Proc Pres 25: 25-35.
Eerlingen RC, Cillen G, Delcour JA. 1994. Enzyme-resistant starch. 4. Effect
of
endogenous lipids and added sodium dodecyl-sulfate on formation of resistant
starch.
Cereal Chem 71(2): 170-177.
Fang Q, Hanna MA. 2000. Water adsorption characteristics and abrasion
resistance
of starch-based foams. Trans ASAE 43: 89-94.
CA 02552179 2006-07-06
17
FAO (Food and Agricufture Organization of the United Nations). 2000 FAOSTAT
statistics database - Agriculture, Rome, Italy. h://a s.fao.or / i-bin/n h
db.al?sub-set=aariculture.
Forssell P, Lahtinen R, Myllsrinen P. 2002. Oxygen permeability of amylose and
amylopectin films. Carbohydr Polym 47: 125-129.
Garcia MA, Martino MN, Zaritzky NZ. 2000. Microstructural characterization of
plasticized starch-based films. Starch 52: 118-124.
Gennadios A, Hanna MA, Kurth LB. 1997. Application of edible coatings on
meats,
poultry and seafoods: a review. Lebensm Wiss u Technol 30(4): 337-350.
Gennadios A, McHugh TH, Weller CL, Krochta JM. 1994. Edible coatings and films
based on proteins. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M, editors.
Edible
coatings and films to improve food quality. Lancaster, PA: Technomic
Publishing Co.,
Inc, p 201-277.
Gidley MJ, Cooke D, Drake AH, Hoffmann RA, Russell AL, Greenwell P. 1995.
Molecular order and structure in enzyme-resistant retrograded starch.
Carbohydr
Polym 28(1): 23-31.
Gruchala L, Pomeranz Y. 1993. Enzyme-resistant starch - Studies using
differential
scanning calorimetry. Cereal Chem 70(2): 163-170.
Guilbert S. 1986. Technology and application of edible protective films. In:
Mathlouthi
M, editor. Food packaging and preservation: Theory and practice. London, UK:
Elsevier Applied Science Publishing Co. p 371-394.
Guilbert S. 2000. Edible films and coatings and biodegradable packaging.
Packaging
of milk products. Bull IDF 346: 10-16.
CA 02552179 2006-07-06
18
Guilbert S. 2002. Edible and biodegradable coating/film systems. In: Han JH,
editor,-
Active food packaging. Winnipeg, Canada: SCI Publication and Communication
Services. p 4-10.
Guilbert S, Gontard N. 2005. Agro-polymers for edible and biodegradable films:
review of agriculturai polymeric materials, physical and mechanical
characteristics. In:
Han JH, editor. Innovations in Food Packaging. Oxford, UK: Elsevier Academic
Press.
p 263-276.
Haasse NU. 1993. A rapid test procedure for estimating the amylose content of
pea
starch. Plant Breeding 111: 325-329.
Han, JH, Gennadios A. 2005. Edible films and coatings: a review. In: Han JH,
editor.
Innovations in Food Packaging. Oxford, UK: Elsevier Academic Press. p 239-262.
Hoover R, Sosulski FW. 1991. Composition, structure, functionality and
chemical
modification of legume starches. Can J Physiol Pharm 69: 79-92.
Kester JJ, Fennema O. 1986. Edible films and coatings: A review. Food Technol
40(12):47-59.
Krochta JM. 1997. Edible protein films and coatings. In: S. Damodaran and A.
Paraf,
editors, Food Proteins and Their Applications. New York, NY: Marcel Dekker. p
529-
549.
lacroix M, Le Tien C. 2005. Edible films and coatings from non-starch
polysaccharides. In: Han JH, editor. Innovations in Food Packaging. Oxford,
UK:
Elsevier Academic Press. p 338-361.
Liu Z. 2005. Edible films and coatings from starches. In: Han JH, editor.
Innovations in
Food Packaging. Oxford, UK: Elsevier Academic Press. p 318-337.
CA 02552179 2006-07-06
19
Liu Z, Han JH. 2005. Film forming character9stics of starches. J Food Sci
70(1): E31-
36.
Lourdin D, Deila Valle G, Colonna P. 1995. Influence of amylose content on
starch
films and foams. Carbohydr Polym 27: 261-270.
Mark AM, Roth W6, Mehitretter CL, Rist CE. 1966. Oxygen permeability of
amylomaize starch films. Food Technol 20:75-77.
McHugh TH, Avena-Bustillos R, Krochta JM. 1993. Hydrophilic edible films
modified
procedure for water vapor permeability and explanations of thickness effects.
J Food
Sci 58: 899-903.
Miles MJ, Morris VJ, Orford PD, Ring SG. 1985. The roles of amylose and
amyiopectin in the geiation and retrogradation of starch. Carbohydr Res
135(2): 271-
281.
Narayan R. 1994. Polymeric materials from agricultural feedstocks, In: Fishman
ML,
Friedman RB, Huang SJ, editors. Polymers from agricultural coproducts.
Washington,
DC: American Chemical Society. p 2-28.
Paiviainen P, Heingm8ki J, Myilgrinen P, l.ahtinen R, Yliruusi J. Forssell P.
2001.
Com starches as film formers in aqueous-based film coating. Pharm Develop
Techonol 6: 353-361.
Perez-Gago MB, Krochta JM. 2005. Emulsion and bi-layer edible films. In: Han
JH,
editor. Innovations in Food Packaging. Oxford, UK: Elsevier Academic Press. p
384-
402.
Petersen K, Nielsen PV, Bertelsen G. 1999. Potential of biobased materials for
food
packaging. Trends Food Sci Technol 10: 52-68.
CA 02552179 2006-07-06
Roth WB, Mehitretter CL. 1970. Films from mixture of viscose and alkali high-
amylose
com starch. J Appi Polym Sci 14: 1387-1389.
Sievert D, Wursch P. 1993. Thermal-behavior of potato amylose and enzyme-
resistant starch from maize. Cereal Chem 70(3): 333-338,
5 Tester RF, Debon SJJ. 2000. Annealing of starch - a review. Int J Biol
Macromol
27(1): 1-12.
Ward KEJ, Hoseney RC, Seib PA. 1994. Retrogradation of amylopectin from maize
and wheat starches. Cereal Chem 71(2): 150-155.
Thomas DJ, Atwell WA. 1997. Starches. Eagan Press, St Paul, MN.
10 Wolff IA, Davis HA, Cluskey JE, Gundrum LJ, Rist CE. 1951. Preparation of
films from
amylose. Ind Eng Chem 43: 915-919.
CA 02552179 2006-07-06
21
Table 1. Physical properties of pea starch films containing beeswax emulsions
Beeswax (w/w starc Thickness ( m) Average emulsion si
h) ze (tim dia
0% 85.46* f 6.530) 10% 114.02b t 11,081 5.9a ( 3.43)
20% 109.75d 11.630 6.5a 3.84
30% 108.75 + 10.713) 7.98 5.28
40% 117.53" 9.844) 7,80 5.08
Values in parentheses are standard deviations. Different superscript letters
indicate
significant differences of means after the least significant difference test
at 10%.
CA 02552179 2006-07-06
ZZ
p - - -
~fn ~~.{ M cD N
7 C 00 ch trp
E
(D W ~ ~ r r
x
ul LO
u) o co
.0 x = co ao CY Vi
c4
~ N
g a r~ ~ ch
~ N ~ * C7
N N
E a ~ r r r
Lo r~
tO N N
qr
v-
t7 IT tl7 ci L'i7 N q
O O, O r r
fl +I +1 +1 +1 +I +I
~ W Cr3 O 07 O N O N
K1 (C r 'm cO tD (O
fn tD (~ fYi N N 1~ It
p "' v U7 G) f70 N N 1o
~ ~'-' ~'I +1 +I ~ ~1 iNl ii
+_~ S CO NUl) Oqc N
C6 N N 0
0 N CE N IA A u l
~ ~ ~i ~I i +1 ii i 03
Q1 qa r p> (N P= Cf W c_
2 ~~ tom m m m
V 2
~CR 0 Op r U7 e- C~ V
E lV O N r+1
H +1 +i ~
t c r' 1- tfl 9'i tD ps =-
I-- O~ ~ou ~u
cV c -0
>
p
H ~ fD m 0 00
,@
Ul') E
aaii