Note: Descriptions are shown in the official language in which they were submitted.
CA 02553908 1996-06-04
30517-137D
Microbicidal compositions
-1-
Tliis application is a divisional application of copend,ing application
2,221,759, filed
June 4, 7 996.
The present invention relates to novel crop-protecting mixtures of active
ingredients
having a synergistically increased microbicidal action and compzising at least
two active
components, and to methods of using such mixtures in crop protection, in
particular for
controlling and preventing the incidence of diseases.
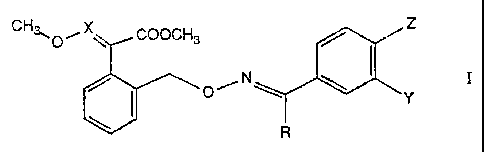
Component I is a compound of the formula I
Z
CH~O~ ~ COOCH3
'~ ~ O'~ N \
'.,Y
R
in which:
X is CFI or N;
R is 'C..~'~i3 or cyclopropyl;
Y is. H, F, Cl, Br, CF3, CF30, propargyloxy;
Z iS H, F. Cl, CF3, CF30; OI
Y' and Z togethez are a methylenedioxy, a (difluoromethylene)dioxy, an
ethylenedioxy, a
(trifluotoethylene)dioxy or a benzo group.
These compounds have been described in EP-A-4.03 618, EP-A-4.60 575, WO
92/18494
and other publications.
Component II is a compound selected from the group consisting of
II A) methyl N-(2-methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate ("metalaxyl' ;
GB-1 500 581), in particular its R enantiomer,
II B) methyl N-(2-methoxyacetyl)-N-(2,6-xylyl)-D-alaninate ("R-metalaxyl";
GB-1 500 58I),
II C) methyl N-(2-furoyl}-N-(2,6-xylyl)-DL-alaninate ("furalaxyl' ; GB-1448
810),
II D) methyl N-phenylacetyl-N-2,6-xyIyl-DL-alaninate ("benalaxyl' ; DE-29 03
612),
CA 02553908 1996-06-04
-2-
II E) (~)-a-(2-chloro-N-2,6-xylylacetamido)-Y-butyrolactone ("ofurace"; USP. 4
141 989),
II F) 2-methoxy-N-(2-oxo-1,3-oxazolidin-3-yl)-acet-2',6'-xylidide ("oxadixyl";
GB-P. Z
058 059),
II G) 1-(2-cyano-2-methoxyiminoacetyl)-3-ethylurea, ("cymoxanil"; US 3 957
847) and
II H) manganese ethylenebis(dithiocarbamate) polymer zinc complex ("mancozeb'
; USP 2
974 156).
The invention furthermore relates to mixtures in which in component I of the
formula I:
X is CH or N;
R is CH3 or cyclopropyl;
Y is H, F, Cl, Br, CF3, CF30, propargyloxy;
2 is H, F, Cl; or
Y and Z together are a methylenedioxy, a (difluoromethylene)dioxy, an
ethylenedioxy, a
(trifluoroethylene)aioxy or a benzo group; and in which component II is a
compound
selected from the group consisting of
II A) methyl N-(2-methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate ("metalaxyl"), in
particular
its R enantiomer,
II B) methyl'N-(2-methoxyacetyl)-N-(2,6-xylyl}-D-alaninate ("R-metalaxyl"),
II C) methyl N-(2-furoyl)-N-(2,6-xylyl)-DL-alaninate ("furalaxyl"),
II D) methyl N-phenylacetyl-N-2,6-xylyl-DL-alaninate ("benalaxyl"),
II E) (~)-a-(2-chloro-N-2,6-xylylacetamido)-y-butyrolactone ("ofurace"),
II F) 2-methoxy-N-(2-oxo-1,3-oxazolidin-3-yl)-acet-2',6'-xylidide
("oxadixyl"), and
II G) 1-(2-cyano-2-methoxyiminoacetyl)-3-ethylurea ("cymoxanil").
Surprisingly, it has emerged that in the prevention and control of plant
diseases the
mixtures according to the invention, of components I and II, not only exert a
mutually
complementary action against a variety of target pathogens or a purely
additive action
CA 02553908 1996-06-04
- J -
against the same pathogens, but that they display a pronounced,
synergistically increased
action.
Advantageous mixing ratios of the two active ingredients are I:II = 25:1 to
1:20,
preferably I:L( = 20:1 to 1:10 and 12: l to 1:8.
Particularly advantageous mixing ratios are
I:IIA=10:1to1:10
I:IIB=l0:lto1:8
I:IIC=6:1 to 1:8
I:UD=8:1to1:4
I:ITE = l0: l to l:6
I:IIF = 10:1 to 1:8
I:IIG=10:1to1:5
I:IIH = 1:30 to 1:1
Preferred two-component mixtures are those in which component I is selected
from the
group consisting of the following compounds:
(01) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl]acrylate;
(OZ) methyl 3-methoxy-2-[a- { [(a-cyclopropyl-3,4-
(difluoromethylenedioxy)benzyl)-
imino]oxy }-o-tolyl]acrylate;
(03) methyl2-[a-{[(a-methyl-3-bromobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(04) methyl 2-[a-{[(a-cyclopropyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime;
(OS) methyl 3-methoxy-2-[a-{[(a-methyl-3-propargyloxybenzyl)irnino]oxy}-
o-tolyl]acrylate;
(06) methyl 2-[a-{((a-methyl-3,4-(difluoromethylenedioxy)benzyl)imino]-oxy}-
o-tolyl]glyoxylate O-methyloxime;
(07) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-3,4-
methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(08) methyl 2-[a-{[(a-methyl-3-propargyloxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(09) methyl 2-[a-{[(a-cyclopropyl-4-fluorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(10) methyl 2-[a-([(a-methyl-4-fluoro-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]
CA 02553908 1996-06-04
-4-
glyoxylate O-methyloxime;
(11) methyl 3-methoxy-2-[a-([(1-((3-naphthyl)ethyl)imino]oxy}-o-
tolyl]acrylate;
(12) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(13) methyl 2-[a-{[(a-methyl-3-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(I4) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-ethylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(15) methyl2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(16) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-3-chlorobenzyl)imino]oxy}-
o-tolyl] acrylate;
(I7) methyl 2-[a-{[(a-cyclopropyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl] acrylate;
(19) methyl 2-[a-{[(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(20) methyl 2-[a-{ [(a-cyclopropyl-3,4-
(difluoromethylenedioxy)benzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime;
(Z1) methyl 2-[a-{[(a-methyl-3,4-ethylenedioxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methylaxime;
(22) methyl 3-methoxy-2-[a-{[(1-(2,3-dihydro-2,2,3-trifluoro-
1,4-benzodioxan-6-yI)ethyl)imino] oxy } -o-tolyl] acrylate;
(23) 2-[a-{[(1-(2,3-dihydro-2,2,3-trifluoro-1,4-benzodioxan-6-
yl)ethyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime;
(24) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl]acrylate;
(25) methyl 2-[a-{[(a-methyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(26) methyl 3-methoxy-2-[a-{[(a-methyl-4-chlorobenzyl)imino]oxy}-o-
tolyl]acrylate;
(27) methyl 3-methoxy-2-[a-{[(a-methyl-3-bromobenzyl)imino]oxy}-o-
tolyl]acrylate;
(28) methyl 2-[a-{[(1-({i-naphthyl)ethyl)imino]ox;y}-o-tolyl]glyoxylate O-
methyloxime;
(29) methyl 3-methoxy-2-[a- { [(a-methyl-3,4-(difl,uoromethylenedioxy)benzyl)-
irnino]oxy }-o-tolyl]acrylate;
(30) methyl 3-methoxy-2-[a- { [(a-cyclopropyl-4-fluorobenzyl)imino]oxy }-
o-tolyl]acrylate;
CA 02553908 1996-06-04
-5-
(31) methyl 3-methoxy-2-[a-{[(a-methyl-4-fluoro-3-
trifluoromethylbenzyl)imino]oxy}-
o-tolyl]acrylate;
(32) methyl 2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(33) methyl 2-[a-([(a-cyclopropyl-3-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(34) methyl 3-methoxy-2-[a-{[(a-methyl-3-chlorobenzyl)imino]oxy}-o-
tolyI]acrylate;
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(36) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imina]oxy}-
o-tolyl]acrylate;
(37) methyl 2-[a-{[(a-cyclopi-opyl-4-trifluoromethylbenzyl)imino]oxy}-o-
talyl]glyoxylate
O-methyloxime;
(38) methyl 2-[a-([(a-cyclopropyl-4-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime.
The following are particularly preferred amongst these:
(25) methyl 2-[a-{[(a-methyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime; ,
(24) methyl 3-methoxy-2-(a-{((a-methyl-3-trifluaromethylbenzyl)imino]oxy}-
o-tolyl] acrylate;
(32) methyl 2-(a-{[(a-methyl-3-trifluoramethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(12) methyl 3-methoxy-2-[a-{((a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-o-
tolyl]-
acrylate;
(09) methyl2-[a-{[(a-cyclopropyI-4-fluorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(26) methyl 3-methoxy-2-[a-([(a-methyl-4-chlorobenzyl)imino]oxy}-o-
tolyl]acrylate;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy)-
o-talyl]acrylate;
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethyIbenzyI)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(15) methyl 2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(36) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-
o-tolyl] acrylate;
CA 02553908 1996-06-04
-(-
(37) methyl2-[a-{[(a-cyclopropyl-4-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(38) methyl2-[a-[[(a-cyclopropyl-4-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime.
Particularly advantageous mixtures result when a component of the formula I is
employed
with either metalaxyl as component II, but in particular with the enantiomeric
R-metalaxyl
II B in at least 85 % pure form (remainder S-enantiomer).
Other preferred mixtures are those in which component II is cymoxanil or
mancozeb.
Also advantageous are mixtures of components I and II which comprise mancozeb
as an
additional, third component.
The active ingredient mixtures I+II according to the invention have highly
advantageous
properties protecting plants against the incidence of disease. Using the
present active
ingredient mixtures, the microorganisms which are found on plants or parts of
plants
(fruits, flowers, foliage, stalks, tubers, 'roots) of a variety of crops of
useful plants can be
contained or destroyed, and even parts of plants which grow at a later point
in time remain
unharmed by such microorganisms. They can also be used as seed-dressing
materials for
the treatment of plant propagation material, in particular seed (fruits,
tubers, kernels) and
nursery plants (for example rice) for protecting them against fungal
infections and against
soil-borne phytopathogenic fungi. The active ingredient mixtures according to
the
invention are distinguished by the fact that they are well tolerated by plants
and by being
environmentally friendly.
As a result of the presence of typical preparations for controlling Oomycetes
(for example
Phytophthora, Peronospora, Bremia, Pythium, Plasmopara) as component II, the
mixtures
according to the invention are distinguished by a high efficacy against these
pests.
However, the active ingredient mixtures are furthermore active against the
phytopathogenic fungi which belong to the following classes: Ascomycetes (for
example
Venturia, Podosphaera, Erysiphe, Monilinia, Mycosphaerella, Uncinula);
Basidiomycetes
(for example the species Hemileia, Rhizoctonia, Puccinia); and Fungi
imperfecti (for
example Botrytis, Helminthosporium, Rhynchosporium, Fusarium, Septoria,
Cercospora,
Alternaria, Pyricularia and, in particular, Pseudocercosporella
herpotrichoides).
Examples of plant species which are suitable as target crops for the
indications disclosed
CA 02553908 1996-06-04
7_
here are, within the scope of the present invention, the following: cereals
(wheat, barley,
rye, oats, rice, sorghum and related species); beet (sugar and fodder beet);
pomaceous
fruit, stone fruit, soft fruit (apples, pears, plums, peaches, almonds,
cherries, strawberries,
raspberries and blackberries); leguminous plants (beans, lentils, peas, soya);
oil crops
(oilseed rape, mustard, poppies, olives, sunflowers, coconut, castor, cocoa,
groundnuts);
cucurbits (pumpkins, cucumbers, melons); fibre plants (cotton, flax, hemp,
jute); citrus
fruit (oranges, lemons, grapefruit, tangerines); vegetables (spinach, lettuce,
asparagus,
cabbages, carrots, onions, tomatoes, potatoes, bell peppers); Lauraceae
(avocado,
Cinnamonum, camphor) or plants such as maize, tobacco, nuts, coffee, sugar
cane, tea,
grapevines, hops, Musaceae and Iatex plants, and also ornamentals (flowers,
shrubs,
deciduous trees and coniferous trees such as conifers). This enumeration is
not limiting.
The active ingredient mixtures according to the invention are particularly
advantageous
far use in crops which are endangered by Oomycetes, i.e. those which are
damaged by
various species of downy mildew. These crops include grapevines, potatoes,
tobacco,
vegetables (for example tomatoes, courgettes, cucumbers, avocados) and fruit
such as
citrus fruit; hops, sugar beet, bananas, maize, ornamental lawn (turf) and
others.
Propagation material (such as seed) of leguminous plants (peas, beans,
lentils), of maize,
sorghum and sunflowers can furthermore be protected with the active ingredient
mixtures,
in particular against attack by Peronosporaceae. In addition, however, these
mixtures can
also be employed advantageously in other crops, especially in cereals such as
wheat and
barley, as already mentioned above.
The mixtures of the active ingredients of the formulae I and II are usually
used in the form
of combinations. The active ingredients of the formulae I and II can be
applied to the az~a
or plant to be treated either simultaneously, or one after the other on the
same day, if
desired together with other carriers, surfactants or other application-
enhancing additives
conventionally used in the art of formulation. Suitable carriers and additives
can be solid
or liquid and are those substances which are expedient in the art of
formulation, for
example natural or regenerated mineral substances, solvents, dispersants,
wetting agents,
adhesives, thickeners, binders and fertilizers.
A preferred method of applying an active ingredient mixture which comprises in
each case
at Ieast one of these active ingredients I and II is application to the aerial
parts of the
plants, especially the foliage (foliar application). Number and rates of
application depend
on the biological and climatic environment of the pathogen. Alternatively, the
active
ingredient can reach the plant via the soil or the water through the root
system (systemic
CA 02553908 1996-06-04
_g_
action), by drenching the locus of the plant with a liquid preparation (for
example in rice
growing) or by incorporating the substances into the soil in solid form, for
example in the
form of granules (soil application). The compounds of the formulae I and II
can also be
applied to seed kernels for the purposes of seed treatment (coating), either
by soaking the
tubers or kernels in succession with a liquid preparation of an active
ingredient or by
coating them with a pre-combined moist or dry preparation. In addition, other
types of
application to plants are possible in specific cases, for example the specific
treatment of
buds or the fruiting heads. The compounds of the combination are employed as
pure active
ingredients or, preferably, together with the auxiliaries conventionally used
in the art of
formulation, and they are therefore processed in a known manner to give, for
example,
emulsion concentrates, spreadable pastes, directly sprayable or dilutable
solutions, dilute
emulsions, wettable powders, soluble powders, dusts, granules, or by means of
encapsulations, for example in polymers. The application methods, such as
spraying,
atomizing, dusting, scattering, brushing or pouring, and the nature of the
compositions, are
selected to suit the intended aims and the prevailing circumstances.
Advantageous rates of
application of the active ingredient mixture are generally 20 g to 1000 g of
a.i./ha, in
particular 50 g to 800 g of a.i./ha, particularly preferably 100 g to 700 g of
a.i.fia. The
rates of application for seed treatment are 0.5 g-800 g, preferably 5 g-100 g,
of a.i. per
100 kg of seed.
The formulations are prepared in a known manner, for example by intimately
mixing
and/or grinding the active ingredients with extenders, for example with
solvents, solid
carriers, and, if appropriate, surface-active compounds (surfactants).
The following are possible solvents: aromatic hydrocarbons, preferably the
fractions Cg to
C12, for example xylene mixtures or substituted naphthalenes, phthalic esters
such as
dibutyl phthalate or dioctyl phthalate, aliphatic hydrocarbons such as
cyclohexane or
paraffins, alcohols and glycols and their ethers and esters such as ethanol,
ethylene glycol,
ethylene glycol monomethyl ether or ethylene glycol monoethyl ether, ketones
such as
cyclohexanone, strongly polar solvents such as N-methyl-2-pyrrolidone,
dimethyl
sulfoxide or dimethylformamide, and epoxidized yr unepoxidized vegetable oils,
such as
epoxidized coconut oil or soya oil; or water.
Solid carriers which are generally used, for example for dusts and dispersible
powders, are
ground natural minerals, such as calcite, talc, kaolin, montmorillonite or
attapulgite. To
improve the physical properties, it is also possible to add highly disperse
silica yr highly
disperse absorptive polymers. Possible particulate, adsorptive carriers for
granules are the
CA 02553908 1996-06-04
-9-
porous types, for example pumice, brick, grit, sepiolite or bentonite, or non-
sorptive
carrier materials, for example calcite or sand. In addition, a large number of
pregranulated
materials of inorganic or organic nature can be used such as, in particular,
dolomite or
comminuted plant residues.
Depending on the nature of the active ingredients of the formulae I and II to
be
formulated, surface-active compounds which are suitable are non-ionic,
cationic and/or
anionic surfactants which have good emulsifying, dispersing and wetting
properties.
Surfactants are also to be understood as meaning mixtures of surfactants.
Particularly advantageous adjuvants which enhance application are furthermore
natural or
synthetic phospholipids from the series of the cephalins and lecithins, for
example
phosphatidylethanolamine, phosphatidylserine, phosphatidylglycerine,
lysolecithin.
As a rule, the agrochemical preparations comprise 0.1 to 99 %, in particular
0.1 to 95 %,
of active ingredients of the formulae I and II, 99.9 to 1 %, in particular
99.9 to 5 %, of a
solid or liquid additive and 0 to 25 %, in particular 0.1 to 25 %, of a
surfactant.
While concentrated compositions are more preferred as commercial products, the
end user
tends to use dilute compositions.
Such compositions are part of the present invention.
The examples which follow are intended to illustrate the invention, "active
ingredient"
being understood as meaning a mixture of compound I and compound II (in
particular
metalaxyl II A, preferably R-metalaxyl II B) in a specific mixing ratio.
Formulation examgles
Wettablepowders a) b) c)
Active ingredient [I:II=4:1 (a),
1:4(b), 3:2(c)) 25 % 50 % 75 %
Sodium lignosulfonate S % 5 % -
Sodium lauryl sulfate 3 % -
Sodium diisobutylnaphthalenesulfonate - 6 % 10
Octylphenol polyethylene glycol ether - 2 % -
(7-8 mol of ethylene oxide)
CA 02553908 1996-06-04
-10-
Highly disperse silica 5 % 10 % 10 %
Kaolin 62 % 27 % -
The active ingredient is mixed thoroughly with the additives and the mixture
is ground in
a suitable mill. This gives wettable powders which can be diluted with water
to give
suspensions of any desired concentration.
Emulsion concentrate
Active ingredient (I:II=3:7) 10 %
Octylphenolpolyethylene glycol ether 3 %
(4-5 mol of ethylene oxide)
Calcium dodecylbenzenesulfonate 3 %
Castor oil polyglycol ether 4 %
(35 mol of ethylene oxide)
Cyclohexanone 30
Xylene mixture 50 %
Emulsions of any desired dilution which can be employed in crop protection can
be
prepared from this concentrate by diluting it with water.
Dusts a) b) c)
Active ingredient [I:II=2:3(a);
5:1 (b) and 1:1 (c)] 5 % 6 % 4
Talc 95 % - -
Kaolin - 94 % -
Rock meal - - 96 %
Ready-to-use dusts are obtained by mixing the active ingredient with the
carrier and
grinding the mixture in a suitable mill. Such powders can also be used for dry
seed-dressing.
Extruder granules
Active ingredient (I:II=4:11) 15 %
Sodium Iignosulfonate 2 %
Carboxymethylcellulose 1 %
Kaolin 82 %
CA 02553908 1996-06-04
-11-
The active ingredient is mixed with the additives, and the mixture is ground
and moistened
with water. This mixture is extruded and subsequently dried in a stream of
air.
Coated Qranules
Active ingredient (I:II=7:1) 8 %
Polyethylene glycol (MW 200) 3
Kaolin 89 %
(MW = molecular weight)
In a mixer, the finely ground active ingredient is applied uniformly to the
kaolin which has
been moistened with polyethylene glycol. In this manner, dust-free coated
granules are
obtained.
Suspension concentrate
Active ingredient (I:II=3:1 ) 40 %
Propylene glycol 10 %
Nonylphenol polyethylene glycol ether
(15 mol of ethylene oxide) 6 %
Sodium Iignosulfonate IO %
Carboxymethylcellulose I %
Silicone oil
(in the form of a 75 % aqueous emulsion) 1 %
Water 32
The finely-ground active ingredient is mixed intimately with the additives.
This gives a
suspension concentrate from which suspensions of any desired dilution can be
prepared by
diluting it with water. Such dilurions can be used for treating live plants
and plant
propagation material by spraying, pouring or immersing, thus protecting them
against
microbial attack.
Biological examples
A synergistic effect is present when the activity of the active ingredient
combination
exceeds the total of the activities of the individual components.
The activity E to be expected for a given active ingredient combination obeys
the
so-called COLBY formula and can be calculated as follows (COLBY, S.R.
"Calculating
CA 02553908 1996-06-04
-12-
synergistic and antagonistic responses of herbicide combinations", Weeds, Vol.
15, pages
20-22; 1967):
if
ppm = milligrams of active ingredient (= a.i.) per litre of spray mixture
X = % activity caused by active ingredient I when p ppm of active ingredient
are applied
Y = % activity caused by active ingredient II when q ppm of active ingredient
is applied
E = expected activity of the active ingredients I+II when p+q ppm of active
ingredient is
applied (additive effect),
then Colby's formula reads: E = X + Y - X ' Y
100
If the actually observed activity (O) exceeds the expected activity (E), then
the activity of
the combination is superadditive, i.e. a synergistic effect is present. O/E =
synergism
factor (SF).
In the examples which follow, the disease level of the untreated plants is
equated to
100 %, which corresponds to an activity of 0 %.
B-1: Activity against Puccinia recondite in wheat
a) Residual-protective action
6 days after sowing, wheat plants are sprayed to drip point with an aqueous
spray mixture
prepared with a wettable powder of the active ingredient mixture (0.02 % of
active
ingredient) and, 24 hours later, infected with a uredo spoor suspension of the
fungus. After
an incubation time of 48 hours (conditions: 95 to 100 per cent relative
atmospheric
humidity at 20°), the plants are placed in a greenhouse at 22°.
12 days after the infection,
the fungus infestation is assessed.
b) Systemic action
An aqueous spray mixture is prepared with a wettable powder of the active
ingredient
mixture (0.006 % of active ingredient based on the soil volume) is poured next
to wheat
plants 5 days after sowing. Care is taken that the spray mixture does not come
into contact
wi h aerial parts of the plants. 48 hours later, the plants are infected with
a uredo spore
suspension of the fungus. After an incubation time of 48 hours (conditions: 95
to 100 per
cent relative atmospheric humidity at 20°), the plants are placed in a
greenhouse at 22°.
12 days after the infection, the fungus infestation is assessed.
A good synergistic effect is shown in particular by active ingredient mixtures
in which
component I is
(29) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-(difluoromethylenedioxy)benzyl)-
CA 02553908 1996-06-04
-13-
imino]oxy }-o-tolyl]acrylate;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)-
imino]oxy }-o-tolyl]acrylate;
(09) methyl 2-[a-{[(a-cyclopropyl-4-fluorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(32) methyl2-[a-([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(15) methyl 2-[a-([(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(35) methyl 2-[a-([(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime; or
(6) methyl 2-[a-{[(a-methyl-3,4-(difluoromethylenedioxy)benzyl) imino)oxy}-
o-tolyl]glyoxylate O-methyloxime.
Example B-2: Activity against~Plasmopara viticola in grapevines .
Grapevine seedlings at the 4-to-5-leaf stage are sprayed to drip point with an
aqueous
spray mixture prepared with a wettable powder of the active ingredient mixture
(0.02 % of
active ingredient) and, 24 hours later, infected with a sporangia suspension
of the fungus.
The fungus infestation is assessed 6 days after infection, during which time a
relative
atmospheric humidity of 95 to 100 % and a temperature of 20° are
maintained.
A marked synergistic effect is shown, in particular, by active ingredient
mixtures in which
component I is
(12) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(3Z) methyl 2-[a-([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(15) methyl 2-[a-([(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(6) methyl 2-[a-{[(a-methyl-3,4-(difluoromethylenedioxy)benzyI) imino]oxy}-
o-tolyl]glyoxylate O-methyloxime or
(35) methyl 2-[a-( [(a-methyl-3-trifluoromethylbenzyl)imino]oxy }-o-
tolyl]glyoxylate
O-methyloxime;
and in which component II is ofurace (II E), oxadixyl (II ~, or in particular
metalaxyl
(lI A) or R-metalaxyl (II B).
CA 02553908 1996-06-04
- 14-
Example B-3: Residual protective action against Venturia inaegualis in apples
Apple cuttings with fresh shoots 10 to 20 cm in length are sprayed to drip
point with an
aqueous spray mixture prepared with a wettable powder of the active ingredient
mixture
(0.02 % of active ingredient) and, 24 hours later, infected with a conidia
suspension of the
fungus. The plants are incubated for 5 days at a relative atmosphezic humidity
of 90 to
100 % and placed in a greenhouse at 20 to 24° far a further 10 days.
The fungus
infestation is assessed 12 days after the infection.
The active ingredient mixtures according to the invention have a markedly
increased
activity.
Example B-4: Activity against Erysiphe ~raminis in barley
Residual-protective action
Barley plants approximately 8 cm high are sprayed to drip point with an
aqueous spray
mixture prepared with a wettable powder of the active ingredient (0.02 % of
active
ingredient) and, 3 to 4 hours later, dusted with conidia of the fungus. The
infected plants
are placed in a greenhouse at 22°. The fungus infestation is assessed
12 days after the
infection.
Systemic action
An aqueous spray mixture prepared with a wettable powder of the active
ingredient
(0.002 % of active ingredient based on the soil volume) is poured next to
barley plants
approximately 8 cm high. Care is taken that the spray mixture does not come
into contact
with the aerial parts of the plants. 48 hours later, the plants are dusted
with conidia of the .
fungus. The infected plants are placed in a greenhouse at 22°. The
fungus infestation is
assessed 12 days after the infection.
A good synergistic effect is shown, in particular, by active ingredient
mixtures in which
component (n is
(6) methyl2-[a-{[(a-methyl-3,4-(difluoromethylenedioxy)benzyl) imino]oxy}-
o-tolyl]glyoxylate O-methyloxime;
(09) methyl 2-[a-([(a-cyclopropyl-4-fluorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(32) methyl 2-[a-([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(15) methyl 2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a-([(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
CA 02553908 1996-06-04
-15-
o-tolyl]acrylate;
(25) methyl 2-[a-{[(a- methyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]axy}-o-
tolyl]glyoxylate
O-methyloxime;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl] acrylate;
(36) methyl 3-methoxy-2-[a- { [(a-cyclopropyl-4-chlorobenzyl)imino]oxy }-o-
tolyl]acrylate
or
(37) methyl 2-[a-{[(a-cyclopropyl-4-trifluoromethylbenzyl)imino)oxy}-o-
tolyl]glyoxylate
O-methyloxime.
Example B-5: Activity against Phytophthora infestans in tomatoes
a) Curative action
Tomato plants cv. "Roter Gnom" are grown for three weeks and then sprayed with
a
zoospore suspension of the fungus and incuhated in a cabin at 18 to 20°
and saturated
atmospheric humidity. The humidification is interrupted after 24 hours. After
the plants
have dried, they are sprayed with a mixture which comprises the active
ingredient
formulated as a wettable powder at a concentration of 200 ppm. After the spray
coating
has dried, the plants are returned to the humid chamber for 4 days. Number and
size of the
typical foliar lesions which have appeared after this time are used as a scale
for assessing
the efficacy of the test substances.
b) Preventive-systemic action
The active ingredient which is formulated as a wettable powder is introduced,
at a
concentration of 60 ppm (relative to the soil volume), onto the soil surface
of
three-week-old tomato plants cv. "Roter Gnom" in pots. After an interval of
three days,
the underside of the leaves is sprayed with a zoospore suspension of
Phytophthora
infestans. They are then kept for 5 days in a spray cabinet at 18 to
20°C and saturated
atmospheric humidity. After this time, typical foliar lesions appear whose
number and size
are used for assessing the efficacy of the test substances.
A good synergistic effect is shown, in particular, by active ingredient
mixtures in which
component I is
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(32) methyl 2-[a-{ [(a-methyl-3-trifluoromethoxybenzyl)imino]oxy )-o-
tolyl]glyoxylate
O-methyloxime;
CA 02553908 1996-06-04
- 16-
(12) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(15) methyl 2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl] acrylate;
(36) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-
o-tolyl]acrylate;
and component II is oxadixyl (II F), ofurace (II E), or in particular
metalaxyl (II A) or
R-metalaxyl (II B).
Example B-6: Residual protective action against Cercospora arachidicola in
groundnuts
Groundnut plants 10 to 15 cm high are sprayed to drip point with an aqueous
spray
mixture (0.02 % of active ingredient) and, 48 hours later, infected with a
conidia
suspension of the fungus. The plants are incubated for 72 hours at 21°
and high
atmospheric humidity and subsequently placed in a greenhouse until the typical
foliar
lesions appear. The activity of the~active ingredient is assessed 12 days
after the infection
on the basis of number and size of the foliar lesions.
A markedly increased activity is shown, in particular, by active ingredient
mixtures in
which component I is
(32) methyl 2-[a-{ [(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl]acrylate;
(15) methyl 2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate; or
(36) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-
o-tolyl]acrylate;
(38) methyl 2-[a-{[(a-cyclopropyl-4-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime or
(26) methyl 3-methoxy-2-[a-{[(a-methyl-4-chlorobenzyl)imino]oxy}-o-
tolyl]acrylate.
Example B-7: Activit~a~ainst Pyricularia oryzae in rice
a) Residual-protective action
Rice plants are grown for two weeks and then sprayed to drip point with an
aqueous spray
CA 02553908 1996-06-04
-17-
mixture (0.02 % of active ingredient) and, 48 hours later, infected with a
conidia
suspension of the fungus. The fungus infestation is assessed 5 days after
infection, during
which a relative atmospheric humidity of 95 to 100 % and a temperature of
22° are
maintained.
b)Systemic action
An aqueous spray mixture (0.006 % of active ingredient based on the soil
volume) is
poured next to two-week-old rice plants. Care is taken that the spray mixture
does not
come into contact with aerial parts of the plants. The pots are then filled
with water to
such an extent that the bottom-most portions of the rice plant stalks are
submerged. After
96 hours, the plants are infected with conidia suspension of the fungus and
kept for 5 days
at a relative atmospheric humidity of 9~ to 100 % and a temperature of
24°C.
Active ingredient mixtures according to the invention show an increased
activity against
Pyricularia.
Example B-8: Activity against Botrytis cinerea on apple fruits. Residual-
yrotective action
Artificially damaged apples are treated by applying a spray mixture (0.02 % of
active
ingredient) dropwise to the site of damage. The treated fruits are
subsequently inoculated
with a spore suspension of the fungus and incubated for one week at high
atmospheric
humidity and approximately 20°C. The fungicidal activity of the test
substance is deduced
from the number of the sites of damage where rotting has been observed.
A markedly increased activity is shown in particular by active ingredient
mixtums in
which component I is
(09) methyl2-[a-([(a-cyclopropyl-4-fluorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(32) methyl 2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(35) methyl2-[a-([(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a- ( [(a-methyl-3-trifluoromethylbenzyl)imino]oxy j-
o-tolyl]acrylate;
(15) methyl2-[a-([(a-cyclopropyl-4-chlorobenzyl)imino]oxyj-o-tolyl]glyoxylate
O-methyloxime;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate; or
(36) methyl 3-methoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-
o-tolyl] acrylate;
CA 02553908 1996-06-04
- 1g -
and in which component II is furalaxyl (II C), metalaxyl (II A) or R-metalaxyl
(II B).
Example B-9: Activity against Helminthosporium eramineum
Wheat kernels are contaminated with a spore suspension of the fungus and
allowed to dry.
The contaminated kernels are dressed with a suspension of the test substance
(600 ppm of
active ingredient based on the seed weight). After two days, the kernels are
arranged in
suitable agar dishes and, after a further four days, the development of fungal
colonies
around the kernels is assessed. Number and size of the fungal colonies are
used for
assessing the test substance. Active ingredient mixtures according to the
invention show a
markedly increased activity.
Example B-10: Activity against Fusarium nivale in rve
Rye cv. Tetrahell which has been infected naturally with Fusarium nivale is
dressed with
the test fungicide in a roller mixer, the following concentrations being used:
20 or 6 ppm
of a.i. (based on the seed weight). The infected and treated rye is sown in
the open in
October in plots of 3 m length and 6 seed rows, using a seed drill. 3
replications per
concentration. Until the disease level is evaluated, 'the experimental field
is subjected to
the field conditions of normal crop management (preferably in a region with
unbroken
snow cover during the winter months). To assess the phytotoxicity, seedling
emergence is
scored in autumn and plant density/tillering in spring.
. To assess the activity of the active ingredient, the percentage of Fusarium-
infected plants
is evaluated early in the year, immediately after the snows have melted.
A good synergistic effect is shown, in particular,.by active ingredient
mixtures in which
component I is
(26) methyl 3-methoxy-2-[a- { [(a-methyl-4-chlorobenzyl)imino]oxy }-o-
tolyl]acrylate;
(32) methyl 2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(18) methyl 3-methoxy-2-[a-.([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(6) methyl2-[a-{[(a-methyl-3,4-(difluoromethylenedioxy)benzyl) imino]oxy}-
o-tolyl]glyoxylate O-methyloxime;
(38) methyl 2-[a-{[(a-cyclopropyl-4-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]glyoxylate O-methyloxime.
Example B-11: Activity against Septoria nodorum in wheat
Wheat plants in the 3-Ieaf stage are sprayed with a spray mixture (60 ppm of
a.i.) prepared
with a wettable powder of the active ingredient. After 24 hours, the treated
plants are
CA 02553908 1996-06-04
-I9-
infected with a conidia suspension of the fungus. The plants are subsequently
incubated
for 2 days at a relative atmospheric humidity of 90-100 % and placed for a
further 10 days
in a greenhouse at 20-24°C. 13 days after the infection, the fungus
infestation is assessed.
A markedly increased activity is shown, in particular, by active ingredient
mixtures in
which component I is
(32) methyl 2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(25) methyl 2-[a-{ [(a-methyl-4-chlorobenzyl)imino]oxy}-o-tolyl]glyoxylate
O-methyloxime;
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl] acrylate;
(36) methyl 3-rnethoxy-2-[a-{[(a-cyclopropyl-4-chlorobenzyl)imino]oxy}-
o-tolyl]acrylate.
Example B-12: Activity against Phytophthora in potato plants
a) Residual-protective action
2-3 week old potato plants (Bintje variety) are grown for 3 weeks and then
sprayed with a
spray mixture (0.02 % of active ingredient) prepared with a wettable powder of
the active
ingredient. After 24 hours, the treated plants are infected with a sporangia
suspension of
the fungus. The fungus infestation is assessed after the infected plants have
been
incubated for 5 days at a relative atmospheric humidity of 90-100 % and
20°C.
b) Systemic action
A spray mixture (0.002 % of active ingredient based on the soil volume)
prepared with a
wettable powder of the active ingredient is poured next to 2-3 week old potato
plants
(Bintje variety) which have been grown for 3 weeka. Care is taken that the
spray mixture
does not come into contact with the aerial parts of the plants. After 48
hours, the treated
plants are infected with a sporangia suspension of the fungus. Fungus
infestation is
assessed after the infected plants have been incubated for 5 days at a
relative atmospheric
humidity of 90-100 % and 20°C.
A good synergistic effect is shown, in particular, by active ingredient
mixtures in which
component I is
(32) methyl 2-[a-([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
(6) methyl 2-[a-{ [(a-methyl-3,4-(difluoromethylenedioxy)benzyl) imino]oxy}-
CA 02553908 1996-06-04
-20-
o-tolyl]glyoxylate O-methyloxime.
(15) methyl2-[a-([(a-cyclopropyl-4-chlorobenzyl;)imino)oxy}-o-toiyl]glyoxylate
O-methyloxime;
(24) methyl 3-methoxy-2-[a- ( [(a-methyl-3-trifluoromethylbenzyl)iminoJoxy }-
o-tolylJacrylate;
(12) methyl 3-methoxy-2-[a-{[(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(18) methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate; or
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime;
in particular in a combination with metalaxyl (11 A), benalaxyl (II D) or R-
metalaxyl
(II B).
Example B-13: Activity against Pythium debaryanum in sugar beet (Beta
vulgaris)
a) Activity following soil application
The fungus is grown on sterile oat kernels and added to a mixture of soil and
sand. This
infected soil is filled into flower pots and sugar beet seeds are sown.
Immediately after
sowing, the test preparations, formulated as wettable powders, are poured over
the soil in
the form of an aqueous suspension. The pots are then placed in the greenhouse
for
2-3 weeks at 20-24°C. The soil is constantly kept uniformly moist by
gently spraying with
water. When evaluating the tests, the emergence of the sugar beet plants and
the
proportion of healthy and diseased plants are determined.
b) Activity following seed dressing
The fungus is grown on sterile oat kernels and added to a mixture of soil and
sand. This
infected soil is filled into flower pots and sugar beet seeds which had been
treated with the
test preparations formulated as seed-dressing powders. The pots in which the
seeds had
been sown were placed in the greenhouse for 2-3 weeks at 20-24°C. The
soil is kept
uniformly moist by gently spraying with water. When evaluating tests, the
emergence of
the sugar beet plants and the proportion of healthy and diseased plants are
determined.
The plants treated with the mixtures according to the invention are healthy in
appearance,
while the few untreated plants which emerge look unhealthy. Preferred active
ingredient
components I are
(12) methyl 3-methoxy-2-[a-([(a-methyl-3,4-methylenedioxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(15) methyl 2-[a-([(a-cyclopropyl-4-chlorobenzyl)iminoJoxy}-o-tolyl]glyoxylate
CA 02553908 1996-06-04
-21 -
O-methyloxime;
(18) methyl 3-methoxy-2-[a-([(a-methyl-3-trifluoromethoxybenzyl)imino]oxy}-
o-tolyl]acrylate;
(24) methyl 3-methoxy-2-[a-([(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-
o-tolyl]acrylate;
(29) methyl 3-methoxy-2-[a-([(a-methyl-3,4-(difluoromethylenedioxy)benzyl)-
imino] oxy } -o-tolyl]acrylate;
(35) methyl 2-[a-{[(a-methyl-3-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime and
(37) methyl 2-[a-{[(a-cyclopropyl-4-trifluoromethylbenzyl)imino]oxy}-o-
tolyl]glyoxylate
O-methyloxime.
Preferred active ingredient components II are benalaxyl (II D), ofurace (II
E), oxadixyl
(II F~, but preferably metalaxyl (II A) and R-metalaxyl (II B).