Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02555063 2006-08-21
Xylanase gene sequences from the genomic DNA of unpurified
rumen microorganisms
FIELD OF THE INVENTION
The present invention relates to the fields of molecular biology and microbial
biodiversity. In particular, the invention relates to a gene encoding xylanase
obtained
from unisolated strains of the rumen microorganisms. The xylanase can be used
as an
enzyme, which is thermo-tolerable and highly specific for xylans with high
activity.
BACKGROUND OF THE INVENTION
Xylan is a major component of hemicellulose found predominantly in plant cell
walls. Endo-xylanases (E.C. 3.2.1.8) are able to randomly hydrolyze the beta
(1-4)
glycosidic bonds between xylose residues making up the backbone of xylans. The
xylanase enable plant structural polysaccharide to be hydrolyzed, and these
products
can be exploited as a rich source of carbon and energy for the growth of
herbivores and
microorganisms.
The plant cell wall consists largely of polysaccharides and contains lesser
amounts
of lignin and protein. The major polysaccharide components of plant cell walls
are
cellulose, hemicellulose, and pectin (Saha 2003). Fibrils of cellulose
embedded in a
matrix of pectin, hemicellulose (comprising various .beta.-xylan polymers),
phenolic
esters and protein produce a protective structure resistant to dehydration and
penetration by phytopathogens through mechanical and enzymatic mechanisms. It
represents a rich source of an important renewable resource utilized by the
pulp and
paper, lumber, food, and biofuel industries(Beg, Kapoor et al. 2001; Lachke
2002;
Saha 2003; Bajpai 2004).
1
1 - I. 1 d I
CA 02555063 2006-08-21
The plant structural polysaccharides provide an important protection for plant
and
useful applications for human, but these components also hinder men from much
utilization of plant products. For example, cereals are a major component of
diets fed
to mono-gastric animals, the endosperm cell wall of cereals containing non-
starch
polysaccharide (NSP)(Engberg, Hedemann et al. 2004). The animals do not
synthesize
the enzymes capable of degrading these structural polysaccharides(e.g.
hemicellulose),
and as a result, these undigested NSP can often be problematic for mono-
gastric
animals being fed such a diet, causing intestinal disturbances, typified by
sticky
droppings and poor growth in young animals. It has been demonstrated
previously that
the anti-nutritive effects of NSP are related to their propensity to form high
molecular-weight viscous aggregates in the gastrointestinal tract(Choct and
Annison
1992). The problems and bad effects of hemicellulose also can be found in pulp
making, pulp and juice production(Beg, Kapoor et al. 2001).
Hemicellulose, the second most prevalent polysaccharide in many plant cell
walls
is composed mainly of xyloglucan or xylan polymers. Xylans have a backbone
structure of .beta.(1-4)-linked xylose residues. The structure of xylan is
complicated by
the attachment of various side chains (e.g., acetic acid, arabinose, coumaric
acid,
ferulic acid, glucuronic acid, 4-O-methylglucuronic acid) to the xylose
residues (Saha
2003). The strands of hemicellulose are hydrogen bonded to cellulose fibrils
to form a
strong interconnected lattice. Cell wall composition varies with plant
species, tissue
type, growth conditions, and age.
Degradation of the plant cell wall is complicated by the structure of
polysaccharides. Cellulose is a linear glucose polymer of /3(1-4)-linkage and
requires
the synergistic hydrolysis of endoglucanase, and cellobiohydrolase and
2
i
CA 02555063 2006-08-21
beta-glucosidase for complete degradation. In comparison, xylan is the most
common
in hemicellulosic polysaccharides. Xylan is a major polysaccharide comprising
a
backbone of xylose residues linked by 0-1,4-glycosidic bonds. The main chain
of
xylan is composed of 13-xylopyranose residues but highly substituted in its
side chain,
thus, xylan requires more and different enzymes, for complete degradation. An
endoxylanase randomly cleaves the xylan backbone into xylooligosaccharides
which
are subsequently degraded to xylose by a xylosidase. Ferulic and p-coumaric
acid
crosslinks are degraded by feruloyl and p-coumaryl esterases. Substituents of
xylan
backbone are cleaved from the xylan backbone with arabinofuranidase,
acetylxylan
esterase and c-glucuronidase(Castanares 1992; Christov and Prior 1993; Saha
2003).
Although various enzymes are necessary to the complete degradation,
liquefaction of
hemicellulose requires only the shortening of the xylan polymers.
Consequently, this
objective may be achieved by the production of xylooligosaccharides through
the
hydrolysis reaction of an endoxylanase(Beg, Kapoor et al. 2001).
Numerous applications of xylanases have been developed for many purposes. For
instance, xylanases was used in biopulping to remove xylan impurities from
cellulose
pulps or to produce pulps with different characteristics. This green process
is able to
reduce the amount of chemical bleacher (chlorine) and the energy needed for
refining
pulp(Bajpai 2004). Xylanases can be the feeding enzyme, to improve the
digestibility
of cereal by poultry and swine fed on cereals with high arabinoxylan
content(Beg,
Kapoor et al. 2001; Bruyer, Giec et al. 2001; Cowieson, Hruby et al. 2005).
Xylanases
can be used in bioconversion involving the hydrolysis of xylan to
xylooligosaccharides
may not only serve as prebiotics for bifidobacteria(Howard, Gordon et al.
1995) but
also provide an alternative and healthy sweetener for diabetics and
portlies(Campbell,
3
CA 02555063 2006-08-21
Fahey et al. 1997). Further, xylanases are useful in the retting of flax
fibers, the
clarification of fruit juices, the preparation of dextrans for use as food
thickeners and
the production of fluids and juices from plant materials(Beg, Kapoor et al.
2001).
Because of the important and potential applications of xylanases in
industries, an
important aspect of xylanase research is to obtain high activity and
specification of
xylanases. Consequently several bacteria and fungi have been selected for the
sources
of xylanase. Among xylanolytic microorganisms, rumen fungi are able to degrade
the
most-resistant plant cell-wall polymers, thus, the rumen fungal population
represents a
rich and underutilized source of novel enzymes with tremendous potential for
industrial and agricultural applications. Those cellulases and xylanase
produced by
these fungi are among the most-active fibrolytic enzymes described to date,
and many
cellulase and xylanase genes have been cloned from specific strains such as
Orpinomyces PC-2 (Li, Chen et al. 1997) and Neocallimastix frontalis SK(Huang,
Huang et al. 2005). The recombinant products of the xylanase genes were
presented
highly active and specific activity of endoxylanase when expressed in E. coli.
In view of the foregoing, there remains a need for low cost xylanases having
biochemical characteristics well suited for use in biobleaching, baking,
animal feeding
supplements, and xylooligosaccharide production. These previous xylanase genes
usually obtained from the specific strain from rumen by molecular biology
based
specific technologies such PCR amplification, cDNA library construction and
screening. Thus, the isolation of microbes from rumen would become one of the
limitations to future successes at attempting to isolate novel genes and to
comprehend
the fibrolytic systems from rumen ecosystem. Accordingly, it is of great
importance to
obtain genes encoding xylan-degrading enzymes from novel sources. To the best
of
4
I
CA 02555063 2006-08-21
our knowledge, however, it is estimated that more than 90% of the total
microbial
population can not be isolated by currently known methods. In order to
overcome such
a problem and avoid complicated microbe-isolated protocols, the present
invention
provides a method directly obtain mixed genomic DNA from unpurified ruminal
microbes as a gene source without isolating the microorganisms.
SUMMARY OF THE INVENTION
The fact has been proved that rumen is a rich source of microorganism which
produce xylanases having biochemical characteristics desirable for industrial
application such as animal feed supplementation and biobleaching. The rumen
microorganism may be bacteria or fungi, as used herein, the rumen
microorganisms
particularly refer to the rumen fungi which have been identified as providing
specific
active xylanases capable of catalyzing the hydrolysis of backbone of xylose
residues
linked by Q-1,4-glycosidic bonds. To make full use of the aforesaid
characteristic in
many ways, the primary object of the present invention is to provide an
isolated and
purified nucleic acid comprising a DNA sequence of SEQ ID NO: 1 or a portion,
a
fragment, a variant or a complementary strand thereof.
Another object of the present invention is to provide an isolated and purified
protein comprising an amino acid sequence of SEQ ID NO:2 or a portion, a
fragment,
or a variant thereof.
Yet another object of the present invention is to provide a host cell
transformed
with a DNA fragment comprising the DNA sequence of SEQ ID NO:1, which encodes
a xylanase.
Yet another object of the present invention is to provide a method for
isolating a
xylanase gene from unisolated and mixed strains of rumen microorganisms and
the
5
CA 02555063 2006-08-21
method overcomes the obstacle in obtaining a xylanase gene from the mixed
genomic
DNA.
Yet another object of the present invention is to provide a method for
degrading the
/3-1,4-glycosidic bonds of xylans by contacting the xylanase and the degrading
products can be exploited as a rich source of carbon and energy for the growth
of plants
and microorganisms..
A further object of the present invention is to provide a composition for
hydrolyzing the /3-1,4-glycosidic bonds of xylans. The composition can be used
as
feed additives.
To achieve the aforesaid objects, the present invention provides an isolated
and
purified nucleic acid comprising a DNA sequence of SEQ ID NO: 1; or a portion,
a
fragment, a variant or a complementary strand thereof.
Preferably, the nucleic acid is xylanse gene, and further, the DNA sequence of
SEQ
ID NO: 1; or a portion, a fragment, a variant or a complementary strand
thereof
encoding a xylanase having an amino acid sequence of SEQ ID NO:2.
The present invention also provides an isolated and purified protein
comprising an
amino acid sequence of SEQ ID NO:2 or a portion, a fragment or a variant
thereof.
Preferably, the amino acid sequence is SEQ ID NO:2, which is xylanase. The
xylanse is an enzyme with thermo-tolerate and highly specific for xylans.
Furthermore, the present invention provides a host cell transformed with a DNA
fragment encoding a xylanase, wherein the DNA fragment sequence is SEQ ID NO:
1.
Preferably, the host cell is animal cell, plant cell, fungi cell, protozoan
cell,
prokaryotic host cell or virus. The xylanase encoded by the DNA fragment of
SEQ
ID. NO:1 comprises a amino acid sequence of SEQ ID NO: 2.
Moreover, the present invention provides a method for isolating a xylanase
gene
from unisolated and mixed strains of rumen microorganisms which directly
obtain
6
CA 02555063 2010-04-21
mixed genomic DNA from unpurified ruminal microbes as a gene source without
isolating
or identifying the species of fungi, and the method can overcome the
limitations of current
known methods of isolating novel genes and to comprehend the fibrolytic
systems from
rumen ecosystem. It comprises the steps of. (a) obtaining rumen samples of
unisolated and
mixed strains of rumen microorganisms; (b) suspending said rumen samples in
extraction
buffer; (c) incubating said rumen samples and then adding proteinase K for
incubating
again; (d) extracting twice with phenol and twice with phenol-chloroform; (e)
precipitating
DNA with ethanol, and resuspending resulting DNA pellets in TE buffer to
obtain
extracted genomic DNA samples; (f) using said extracted genomic DNA samples as
PCR
template for amplification of DNA fragments; and (g) screening the xylanase
gene from
said amplified DNA fragments.
The present invention yet provides a method for degrading the xylan-containing
structure, comprises hydrolyzing the (3-1,4- glycosidic bonds of xylans by
contacting the
protein of an isolated and purified protein which comprises an amino acid
sequence of
SEQ ID NO: 2 or a portion, a fragment or a variant thereof.
Preferably, the protein is xylanase with the characters of then-no-tolerate
and highly
specific for xylans.
Additionally, the present invention provides a composition for hydrolyzing the
(3-1,4- glycosidic bonds of xylans, comprising a xylanase containing SEQ ID
NO: 2.
7
CA 02555063 2010-04-21
Preferably, the composition is used as food additives and the composition can
further comprise proteases, alpha-amylase, cellulose, beta-glucanase or a
mixture thereof.
In another aspect of the invention, there is provided an isolated and purified
nucleic
acid, comprising a DNA sequence of SEQ ID NO: 1.
In another aspect of the invention, there is provided an isolated and purified
protein, comprising the amino acid sequence of SEQ ID NO: 2.
In another aspect of the invention, there is provided a host cell transformed
with a
DNA fragment encoding a xylanase, wherein said DNA fragment comprises the DNA
sequence of SEQ ID NO: 1.
To sum up, the present invention discloses a novel xylanase gene sequence, its
encoded novel xylanase, and a method of hydrolyzing the (3-1,4- glycosidic
bonds of
xylans disclosed herein are useful in numerous applications such as animal
feed
7a
CA 02555063 2006-08-21
supplements, biobleacing or biofuel industries, etc. Hence, the present
invention
essentially provides the excellent way for implementation of green industry
including
cleaner production, resource recovery, and renewable energy.
BRIEF DESCRIPTION OF THE DRAWINGS
The above objects and advantages of the present invention will become more
apparent with reference to the appended drawings wherein:
Figure 1 shows the mixed genomic DNA of rumen microorganisms. The genomic
DNA of unisolated microorganisms was extracted by phenol-chloroform methods,
separated on an agarose gel and visualized after ethidium bromide staining.
Figure 2 shows PCR results of the xylanase gene amplification. Lane 1, DNA
marker
(1 kb ladder). Lane 2-4, first PCR products amplified by using xynF4 and xynR2
primers. Lane 5-6, secondly amplified PCR fragments extended from BamHI-xynF4
and Notl-xynR2 primers.
Figure 3 exhibits nucleotide and deduced amino acid sequence of xynR8 from
mixed
genomic DNA of rumen fungi. The forward and reverse primers for PCR
amplification
are underlined. The putative region and conserved residues of glycol hydrolase
family
11 are showed in bold type and double underline, respectively. The reiterated
sequence
RTTT is boxed.
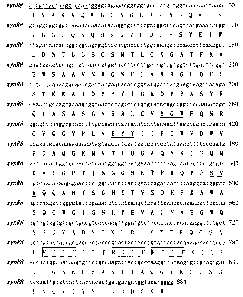
Figure 4 exhibits alignment of the deduced amino acid of xynR8 and xylanase
genes of
known rumen fungi. Amino acid residus with an identical match (*) and those
with
different degrees of conservation (: or.) are indicated. The reiterated
sequences (RTTT)
8
I
CA 02555063 2006-08-21
of linker are showed in bold type. Dockerin domains (partial) of xylanase are
boxed. Gaps (dashes) were introduced to maximize the regions of sequence
alignment.
The reference sequences shown in this figure are Orpinomyces sp. PC-2 xylanase
A
(xynA, U57819), Neocallimastix patriciarum W-1 xylanase W1-4(xynwl-4NP,
AY133992) and N. frontalis SK xylanase skl-15 (xynskl-15, AY134032).
Figure 5 shows Western blotting using anti-His tag antibody. The IPTG-induced
E. coli
BL21(DE3) broth and cells were divided into 4 fractions. Each fraction was
detected
by Western blotting using the monoclonal anti-His tag antibody. Lane 1,
extracellular
broth. Lane2, Periplasmic space. Lane 3, cytoplasm extract. Lane 4, insoluble
precipitate.
Figure 6 shows SDS-PAGE analysis of XYNR8. The intercellular extract of
pET21 aR8 transformed E. coli was purified by CM and Ni-NTA chromatography.
Lane 1, protein standard. Lane 2, intercellular extract. Lane3, CM-column
purified
products. Lane 4. Ni-NTA column purified XYNR8
Figure 7 illustrates the temperature optima for xylanase (XYNR8) activities.
The optimal reaction temperature is 50 degree C.
Figure 8 illustrates the thermostability of XYNR8. XYNR8 showed a broad range
of
thermostaility when hydrolyzed oat spelt xylan.
Figure 9 shows TLC analysis of the hydrolysis products released from oat spelt
xylan
by xylanase from E. coli BL21(DE3). Lane 1, Xylooligosaccharide standard. Lane
2,
the hydrolysis products of xylan.
9
1
CA 02555063 2006-08-21
DETAILED DESCRIPTION OF THE INVENTION
The present invention is described as follows. The diagrams accompanying the
descriptions below are not presented in actual proportion; they are used only
for
illustration of the equipment setup of the present invention.
The present invention relates to a method for isolating a xylanase gene,
denoted
xynR8, from unisolated and mixed strains of rumen microorganisms, comprising
the
steps of : (a) obtaining rumen samples of unisolated and mixed strains of
rumen
icroorganisms; (b)suspending said rumen samples in extraction buffer;
(c)incubating
said rumen samples and then adding proteinase K for incubating again;
(d)extracting
twice with phenol and twice with phenol-chloroform; (e)precipitating DNA with
ethanol, and resuspending the resulting DNA pellet in TE buffer to obtain
extracted
genomic DNA samples; (f) using said extracted genomic DNA samples as PCR
template for amplification of DNA fragments; and (g) screening the xylanase
gene
from said amplified DNA fragments.
The aforesaid method of isolating the xynR8 gene encoding a xylanase which
features the use of the mixed genomic DNA without isolating and identifying
process,
and it obtains a novel gene fragment showing about 20% divergence in DNA
sequence
from ones using of prior arts in the same field. Besides, it expresses
outstandingly high
enzyme activity in E. coli ^ Table 1, as below example 7 ^ .
Furthermore, xynR8 gene encoding a xylanase of the present invention operably
is
linked to control sequences capable of directing expression of the xylanase in
a
suitable host cell. As used herein "host cell" includes animal, plant, fungi,
protozoan,
prokaryotic host cells and virus. For example, the host cell, which includes
eubacteria
and archaebacteria, can be transformed with a DNA encoding a xylanase of the
present
CA 02555063 2006-08-21
invention so that the gene modified prokaryotes is capable of expressing the
xylanase.
The fungi can follow the same protocol and express the xylanase, as used
herein,
"fungi" includes filamentous and yeast form fungi.
In another preferred embodiment of the present invention, the method for
degrading the xylan-containing structure, comprises hydrolyzing the f-1,4-
glycosidic
bonds of xylans by contacting the protein comprising an amino acid sequence of
SEQ
ID NO:2 or a portion, a fragment or a variant thereof. The hydrolyzing enzyme
is
namely xylanase which degrading the fl-1,4- glycosidic bonds between xylose
residues making up the backbone of xylans being a major plant structure
polysaccharides. Additionally, hemicellulose, the second most prevalent
polysaccharide in plant cell wall, is also hydrolyzed by the same mechanism.
And then,
it can promote the development of relative applications of xylanase in
industry, such as
pulp making, lumber, food and biofuel.
Accordingly, the invention extends to novel feed compositions and feed
additives
containing a xylanase of the present invention. Such feed compositions and
supplements may also contain other enzymes, such as, proteases, alpha-amylase,
cellulase, and beta-glucanase. The xylanase may be added directly to an
untreated,
pelletized, or otherwise processed feedstuff or it may be provided separately
from the
feedstuff in, for instance, powder, a pill, a gel formulation, a liquid
formulation, or in
drinking water. The invention extends to feed inoculant preparations
comprising
lyophilized microorganisms which express xylanases of the present invention
under
normal growing conditions. With respect to these feed inoculant preparations,
"normal
growing conditions" mean culture conditions prior to harvesting and
lyophilization of
the microorganisms. The microorganisms express xylanases during growth of the
microbial cultures in large-scale fermenters. The activity of xylanase in the
11
1
CA 02555063 2006-08-21
microorganisms is preserved by lyophilization of the harvested microbial
concentrates
containing the xylanase.
In conclusion, xylanases of the present invention are useful in a wide variety
of
applications involving the hydrolysis of xylan. Such applications include use
in animal
feed supplements, biobleaching and xylooligosaccharide production. Xylanases
of the
present invention may also be used to convert the hemicellulose of plant to
biofuels(i.e.,
alcohol). The xylan content of certain feedstuffs such as cerels decreases
their value as
protein sources for fish, monogastric animals, young ruminants and infants
because the
xylan decreases the bioavailability of nutrients by circumventing structural
polysaccharides, and limiting amino acids and proteins. Treatment of such
feedstuffs
with the xylanase of the present invention will reduce their xylan content by
xylanase
mediated hydrolysis, rendering the feedstuffs more suitable for use as protein
sources
and providing xylooligosaccharides for intestinal probiotics.It is to be
understood that
the following examples of the present invention should not be based to
restrict the
invention, and that all equivalent modifications and variations made without
departing
from the intent and import of the following descriptions of the examples
should be
included in the following claims.
EXAMPLES:
Example 1 : Rumen Sample Preparation and DNA Extraction
Rumen content from a water buffalo was sampled through a cannula, fragmented
by a blender and squeezed through 2 layers of cheesecloth, following which 0.5
ml of
filtrate was syringed into a Hungate tube (125 X 16 mm, Bellco Glass)
containing 5 ml
enrichment medium. For the suppression of bacterial growth, 1.2% (w/v)
penicillin-G;
0.265% (w/v) streptomycin and 0.06% (w/v) chloramphenicol were used. The
12
CA 02555063 2006-08-21
enrichment method as reported by Chen et al. was followed throughout(Chen,
Hseu et
al. 2003). The tubes containing medium and rumen fluid were incubated at 39 C
for 1
day, and the biomass was collected by centrifuge (4 C, 6000 rpm, 30 min). All
of the
samples used for DNA extraction had been frozen in liquid nitrogen, ground to
a fine
powder with a mortar and pestle, and then stored at -20 T.
The protocols for DNA extraction was based upon phenol-chloroform extraction.
Rumen samples were resuspended in extraction buffer (25 mM Tris-HC1, pH 8.0;
10
mM EDTA; 50 mM glucose; 0.5% (w/v) Sodium dodecyl sulfate (SDS)) and incubated
at 37 C for 1 h. Proteinase K (0.1 mg/mL) was added, and the mixture was
incubated
for 1 h at 55 C, extracted twice with phenol and twice with phenol-
chloroform. The
DNA was precipitated with ethanol, and the resulting DNA pellet resuspended in
TE
(10 mM Tris, pH 8.0; 1 mM EDTA) buffer. The extracted genomic DNA samples were
examined by agarose electrophoresis (Figure 1) and stored at -20 C prior to
use.
Example 2: Amplification of the Xylanase Gene from Rumen Microbial Genomic
DNA
The PCR reaction adopted herein was used for the amplification of xylanase
genes
obtained from the mixed genomic DNA samples extracted from unpurified rumen
microorganism cultures. Two primers, xynF4
(5'-ACTGTTGCTAAGGCCCAATG-3')(SEQ ID NO:3) and xynR2 (5'-
CCCCATTTACCATCGTCATCAGTG-3') ( SEQ ID NO:4), were designed based
upon the rumen fungal xylanase sequences. The reaction conditions of PCR are
as
follows: under 94° C. for 2 minutes, and then successively repeating
the
following four conditions for 35 times: (1) under 94° C. for 45 seconds
13
I I I
CA 02555063 2006-08-21
(denature DNA), (2) under 45° C. for 45 seconds, (3) under 72°
C. for 90
seconds, (4) under 72° C. for 10 minutes. The amplified products were
examined by agarose gel electrophoresis (Figure 2). The diluted PCR product so
produced was subsequently amplified again using BamHI-xynF4
(5'-CGGGATCCCGTTAACTGTTGCTAAGGCCCAATG-3') (SEQ ID NO:5) and
NotI-xynR2 primers (5'-ATTTGCGGCCGCTTTACCCCATTTACCATCGTCA-3')
(SEQ ID NO:6) and an appropriate PCR process. BamHI and NotI restriction sites
were incorporated into xynF4 and xynR2, respectively, in order to facilitate
the cloning
of the xylanase gene to the pGEX4T-1 (Amersham-Pharmacia, Piscatway, NJ)
expression vector for subsequent screening purposes. The reaction conditions
of
secondary PCR are as follows: under 94° C. for 1 minutes, and then
successively repeating the following four conditions for 35 times: (1) under
94°
C. for 30 seconds (denature DNA), (2) under 52° C. for 45 seconds, (3)
under
72° C. for 90 seconds, (4) under 72° C. for 10 minutes. The PCR
fragments were also analyzed on an agarose gel (Figure 2).
Example 3: Screening of the Xylanase Gene from Amplified DNA Fragments
The xynlanase gene enriched pool was constructed by ligating the BamHI- and
Notl-digested (New England Biolabs, Beverly, MA) PCR products into the pGEX4T-
1
vector. The ligation mixture was used to transform E. coli DH5a (Invitrogen,
Carlsbad,
CA) by electroporation (Sambrook and Russell 2001). The electroporated cells
were
spread on Luria-Bertani (LB) agar (Difco, Detroit, MI) containing 0.2% xylan
(Oat
spelt, Sigma, St. Louis, MO). Subsequent to overnight incubation at 37 degree.
C, the
transformants were transferred to another LB plates and screened by Congo-red
14
1 1 11 CA 02555063 2006-08-21
staining (Teather and Wood 1982). Those colonies surrounded by clear zone
indicated
a level of xylanase activity of the clones. The resultant plasmids (pGEX4T-1
R8) were
purified and the sequence of the xylanase gene (xynR8) inserts was determined
by
automatic sequencing (MDBio Inc. Taipei). The nucleotide sequence of xynR8 has
been deposited with GenBank (Accession No. AY941119)..
Example 4: Nucleotide Sequence and Structural Analyses of the xvnR8 Gene
The computer program Bioedit was used to analyze and align the xylanase
sequences. Sequence analysis of the inserts in the plasmids obtained from the
clones
revealed that pGEX4T- 1 R8 contained The total length of the xylanase insert
(xynR8)
was 884 bp (Figure 3). The deduced amino acid sequence of xynR8 was
significantly
similar to those of several anaerobic fungal xylanases belonging to family 11
glycosyl
hydrolases (Figure 4) (Henrissat and Romeu 1995; Henrissat and Bairoch 1996).
xynR8 exhibited an amino acid sequence highly similar to that of xynA of
Orpinomyces sp. PC-2 (Accession No. U57819), xynwl-4 of N. patriciarum
(Accession No. AY133992) and xynskl-15 of N. frontalis (Accession No.
AY134032).
The xynR8 gene revealed amino acid identities of 95.9%, 89.1% and 88.8% when
compared, respectively, with xynA, xynwl-4 andxynskl-15.
Example 5: Overexpression of the Rumen Microbial Xylanase Gene (xynR8)
Isolation and characterization of xynR8 from uncultured rumen microbes enables
the large scale production of Xylanase R8 in any of a number of prokaryotic
(e.g., E.
coli, lactic acid bacteria and B. subtilis) or eukaryotic (e.g., fungal--
Pichia,
Saccharomyces, Aspergillus, Trichoderma; plant--Brassica, Zea, Solanum; or
CA 02555063 2010-04-21
animal--poultry, swine or fish) expression systems using known methods.
Example 6: Cloning of the xynR8 in an Escherichia coli-specific Expression
Construct
To obtain abundant xylanase, the xynR8 was fused with T7 promoter for
efficient
expression in E. coli. A number of E. coli expression vectors based on the T7
promoters
are commercially available. The xylanase gene (xynR8) was subcloned into pET21
a
(Novagen Inc.) and generated pET21 aR8. Strain suitable for high levels of
protein
expression, such as BL21 (DE3), is employed. Positive clones are further
characterized by
nucleotide sequence analysis. The resultant plasmids were transformed into E.
coli
BL21(DE3) to express recombinant proteins. All recombinant proteins had the
His6-tag at
each protein C-termini.
Example 7: The Expression and Purification of Recombinant XYNR8
For the xylanase production in E. coli, the positive clone was grown in 500 ml
LB
to an OD600 of 0.6-0.9 before 0.5 mM of IPTG(isopropyl-thio-(3-D-galactopyrano
side)
was added for the induction. After 3.5 h of induction at 37 degree C, the
cells were
harvested by centrifugation (4000g, 10 min) for recombinant protein
purification.
The expressed XynR8 can be extracted by sonication of the E. coli cells.
Protein
inclusions of XynR8 can be harvested by centrifugation. The xylanase activity
of prepared
cell extracts was assayed by DNS methods. One unit of xylanase activity was
defined as
one mol of reducing sugar equivalents released per minute.
Fusion protein purification was performed using ion-exchange (CM-Sepharose,TM
16
I 1. 1 I
CA 02555063 2006-08-21
Amersham Bioscience) and nickel affinity (Ni-NTA-agarose, Qiagen) columns. The
recombinant protein was dialyzed with citrate buffer (50mM, pH6) to remove
imidazol.
Protein concentrations were determined using a Micro BCA Protein Assay Reagent
Kit
(Pierce Biotechnology, Rockford, IL). Figure 6 and table 1 illustrate show the
results
of purification steps, and a 34KDa product was obtained after a Ni-NTA
affinity
chromatography. The product was also confirmed by Western blotting (Figure 5).
Table 2 summarizes the purification steps of XYNR8. XYNR8 had the highest
specific
activity (23244.85 U/mg) against oat spelt xylan.
Table 1. Purification of XYN from xynR8 transformed E. coll.
Volume Total Protein Specific Purification Recovery
Purification step (mL) Activity (mg) activity Fold (%)
(U) (U/mg)
Crude extract 22.5 108037.74 42.07 2568.27 1 100
CM-Sepharose column 9.5 76639.69 5.26 14568.65 5.7 71
Ni-NTA column 9.5 75510.80 3.25 23244.85 9.1 70
Table2. Substrate specificity of XYNR8
Substrate(2%) Relative activity(%)
Oat spelt xylan 27.36
Soluble oat spelt xylan 100
Birchwood xylan 67.18
Beechwood xylan 38.80
Xylooligosaccharides(X2,X3) 1.83
17
CA 02555063 2006-08-21
Cellulose 0
Avicel 0
Carbonxymethyl cellulose 0
Starch 0
Cellobiose 0
Example 8: Biochemical Characteristics of the XYNR8
The biochemical characteristics of the recombinant xylanase were determined
using purified xylanase. Xylanase activity was determined by measuring the
amount of
reducing sugars released from substrates according to the method of DNS method
(Miller 1959). The temperature optimum for the purified xylanase activity was
55° C. (figure 7), and the enzyme was thermostable when treated in
30-55° C. for 10 minutes (figure 8). These suggest that the enzyme is
able to
acclimatize various applications of industries. The Vmax and Km for oat spelt
xylan
hydrolysis were 1.1 mM/min and 11.1 mg/ml, respectively, and the value of Kcat
for oat
spelt xylan hydrolysis was 38943.2 sec-1. The XYNR8 showed high specific to
the
xylan substrates as the above-mentioned table 2. XYNR8 hydrolyzed oat spelt
xylan,
birchwood xylan and birch wood xylan well, and it gave the highest specific
activity
against soluble xylan.
XYNR8 against different polysaccharide substrates were also examined. The
enzyme were inactive against starch, cellulobiose, CMC(carboxyl methyl
cellulose)
and avicel.
Example 9: Xylan hydrolysis by the recombinant xylanase XynR8
18
I 11 11,1111, -
CA 02555063 2006-08-21
The hydrolysis products released from oat spelt xylan by XynR8 were analyzed
using TLC (Thin layer chromatography), the results being presented in Figure
9. The
principal products of xylan hydrolysis of oat spelt xylan were xylobiose and
xylotriose,
which indicated that the recombinant xylanase was an endoxylanase, and the
pattern of
such hydrolysis classified the xylanase R8 as being endoenzyme b-1,4 xylan
xylanohydrolase (EC 3.2.1.8) (Huang, Huang et al. 2005) This result also
suggests
XYNR8 has the ability to hydrolyze the xylan of hemicellulose.
Other Embodiments
It is to be understood that the foregoing description of the present invention
should not
be based to restrict the invention, and that all equivalent modifications and
variations
made without departing from the intent and import of the foregoing description
should
be included in the following claim.
19
I
CA 02555063 2006-08-21
References
Bajpai, P. (2004). "Biological bleaching of chemical pulps." Crit Rev
Biotechnol 24(1):
1-58.
Beg, Q. K., M. Kapoor, et al. (2001). "Microbial xylanases and their
industrial
applications: a review." Appl Microbiol Biotechnol 56(3-4): 326-38.
Bruyer, D., R. Giec, et al. (2001). "Effect of a bacterial xylanase and/or
antibiotic
growth promoter on zootechnical performances of piglets fed arabinoxylan rich
diets." Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 66(3b): 467-8.
Campbell, J. M., G. C. Fahey, Jr., et al. (1997). "Selected indigestible
oligosaccharides
affect large bowel mass, cecal and fecal short-chain fatty acids, pH and
microflora in
rats." J Nutr 127(1): 130-6.
Castanares, A. (1992). "Hemicellulose and Hemicellulases, M. P. Coughlan and
G.
Hazelwood, Eds." Portland Press, Cambridge,: 85-102.
Chen, Y. C., R. S. Hseu, et al. (2003). "The genetic similarity of different
generations
of Neocallimastix frontalis SK." FEMS Microbiol Lett 221(2): 227-3 1.
Choct, M. and G. Annison (1992). "Anti-nutritive effect of wheat pentosans in
broiler
chickens: roles of viscosity and gut microflora." Br. Poult. Sci. 33(821?34).
Christov, L. P. and B. A. Prior (1993). "Esterases of xylan-degrading
microorganisms:
production, properties, and significance." Enzyme Microb Technol 15(6): 460-
75.
I I I I
CA 02555063 2006-08-21
Cowieson, A. J., M. Hruby, et al. (2005). "The effect of conditioning
temperature and
exogenous xylanase addition on the viscosity of wheat-based diets and the
performance of broiler chickens." Br Poult Sci 46(6): 717-24.
Engberg, R. M., M. S. Hedemann, et al. (2004). "Influence of whole wheat and
xylanase on broiler performance and microbial composition and activity in the
digestive tract." Poult Sci 83(6): 925-38.
Henrissat, B. and A. Bairoch (1996). "Updating the sequence-based
classification of
glycosyl hydrolases." Biochem J 316 (Pt 2): 695-6.
Henrissat, B. and A. Romeu (1995). "Families, superfamilies and subfamilies of
glycosyl hydrolases." Biochem J 311 (Pt 1): 350-1.
Howard, M. D., D. T. Gordon, et al. (1995). "Dietary fructooligosaccharide,
xylooligosaccharide and gum arabic have variable effects on cecal and colonic
microbiota and epithelial cell proliferation in mice and rats." J Nutr
125(10): 2604-9.
Huang, Y. H., C. T. Huang, et al. (2005). "Effects of dockerin domains on
Neocallimastixfrontalis xylanases." FEMS Microbiol Lett 243(2): 455-60.
Lachke, A. (2002). "Biofuel from D-xylose - the Second Most Abundant Sugar."
Resonance 50-58).
Li, X. L., H. Chen, et al. (1997). "Monocentric and polycentric anaerobic
fungi
produce structurally related cellulases and xylanases." Appl Environ Microbiol
63(2): 628-35.
Miller, G. L. (1959). "Use of dinitrosalicylic acid reagent for determination
of reducing
sugar." Anal Chem 31(426-428).
Saha, B. C. (2003). "Hemicellulose bioconversion." J Ind Microbiol Biotechnol
30(5):
279-91.
21
CA 02555063 2006-08-21
Sambrook, J. and D. W. Russell (2001). "Molecular cloning: a laboratory
manual." 3rd
ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Teather, R. M. and P. J. Wood (1982). "Use of Congo red-polysaccharide
interactions
in enumeration and characterization of cellulolytic bacteria from the bovine
rumen."
Appl Environ Microbiol 43(4): 777-80.
22
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.