Note: Descriptions are shown in the official language in which they were submitted.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
REAL-TIME ELECTRONIC CELL SENSING SYSTEM AND APPLICATIONS
FOR CYTOTOXICITY PROFILING AND COMPOUND ASSAYS
This application claims benefit of priority to U.S. Patent Application Number
10/987,732, entitled "Real time electronic cell sensing system and application
for cell
based assays" filed November 12, 2004; U. S. Provisional Application Number
60/542,927 filed February 9, 2004; U. S. Provisional Application 60/548,713,
filed
February 27, 2004; and U.S. Provisional Application Number 601614,601, filed
September 29, 2004; each of which are herein incorporated by reference in
their entirety.
This application incorporates by reference the following documents in their
entirety: U. S. Provisional Application Number 60/519,567, filed November 12,
2003;
U.S. Patent Application Number 10/705,447 filed November 10, 2003, entitled
"Impedance Based Devices and Methods for Use in Assays"; U. S. Provisional
Patent
Application 60/397,749, filed July 20, 2002; U. S. Provisional Patent
Application
60/435,400, filed December 20, 2002; U. S. Provisional Patent Application
60/469,572,
filed May 9, 2003; PCT application PCT/LJS03/22557, filed July 18, 2003, U.S.
Patent
Application Number 10/705,615, entitled "Impedance Based Apparatuses and
Methods
for Analyzing Cells and Particles", filed on November 10, 2003; and PCT Patent
Application PCT/LTS03/22537, filed on July 18, 2003.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
BACKGROiJND OF THE INVENTION
Technical Field
This invention relates to the field of cell-based assays. In particular, the
invention
provides impedance-based devices, apparatuses and systems for analyzing cells
and for
conducting cell-based assays.
Background Art
Bioelectronics is a progressing interdisciplinary research field that involves
the
integration of biomatereials with electronic devices. Bioelectronic methods
have been
used for analyzing cells and assaying biological molecules and cells. In one
type of
application, cells are cultured on microelectrodes and cell-electrode
impedance is
measured and determined to monitor cellular changes.
In PCT Application No. PCT/LJS03/22557, entitled "IMPEDANCE BASED
DEVICES AND METHODS FOR USE IN ASSAYS", filed on July 18, 2003, a device
for detecting cells and/or molecules on an electrode surface is disclosed. The
device
detects cells and/or molecules through measurement of impedance changes
resulting from
the attachment or binding of cells and/or molecules to the electrode surfaces.
A number
of embodiments of the device is disclosed, together with the apparatuses,
system for
using such devices to perform certain cell based assays.
In anticancer drug development, the study of the tune dependence of cytotoxic
and
cell proliferation inhibitory effect of a drug is an important element for
gaining
information to use in the development of clinical dosing strategies. In
particular, time
dependent IC50's are derived and different time dependent patterns for IC50's
are
observed (e.g., see Hassan SB, Jonsson E, Larsson R and Karlsson MO in J.
Pharrraacology and Expe~-imeratal Therapeutics, 2001, Vol. 299, No. 3, pp 1140-
1147;
Levasseur LM, Slocum HK, Rustum YM and Greco WR, in Cafacer Research, 1998,
vol.
58, pp 5749-5761.). Typically, these studies used end-point single-measurement
assays.
Each time point for a dose concentration of drug or compound applied to the
cultured
cells required a separate experiment. This limits the time resolution and the
number of
2
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
time points of such time-dependent cytotoxicity studies. Thus, new
technologies or
methods that can provide higher time resolution and permit measurements on
many time
points are needed.
The present invention further expands the inventions disclosed in PCT
Application No. PCT/US03/22557, entitled "IMPEDANCE BASED DEVICES AND
METHODS FOR USE IN ASSAYS", filed on July 18, 2003 and disclosed in United
States patent application No.101705,447, entitled "IMPEDANCE BASED DEVICES
AND METHODS FOR USE IN ASSAYS," Attorney Docket No. ACE-OOlOl.P.l.l-US,
filed on November 10, 2003. The invention provides a real time cell electronic
sensing
system for conducting cell-based assays based on measurement of cell-substrate
impedance and provides the method for using such a system to perform cell-
based assays.
3
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
SUMMARY OF THE INVENTION
In one aspect, the present invention is directed to a device for monitoring
cell-
substrate impedance, which device comprises: a) a nonconducting substrate; b)
two or
more electrode arrays fabricated on the substrate, where each of the two or
more
electrode arrays comprises two electrode structures; and c) at least two
connection pads,
each of which is located on an edge of the substrate. Each electrode array of
the device
has an approximately uniform electrode resistance distribution across the
entire array.
The substrate of the device has a surface suitable for cell attachment or
growth; where
cell attachment or growth on said substrate can result in a detectable change
in impedance
between or among the electrode structures within each electrode array. In
preferred
embodiments, each electrode array on the substrate of a device of the present
invention is
associated with a fluid-impermeable container.
In another aspect, the present invention is directed to a cell-substrate
impedance
measurement system comprising: a) at least one multiple-well device monitoring
cell-
substrate impedance, in which at least two of the multiple wells each comprise
an
electrode array at the bottom of the well; b) an impedance analyzer; c) a
device station
capable of engaging the one or more multiple-well devices and capable of
selecting and
electrically connecting electrode arrays within any of the multiple wells in
to the
impedance analyzer; and d) a software program to control the device station
and perform
data acquisition and data analysis on impedance values measured by the
impedance
analyzer. In preferred embodiments of this aspect of the present invention,
each electrode
array of the multiple-well device is individually addressed.
In yet another aspect, the present invention provides a method for monitoring
cell-
substrate impedance using a device of the present invention. The method
includes:
providing a multiple array device of the present invention; connecting said
multiple array
device to an impedance analyzer; depositing cells on at least one of the two
or more
arrays of the device; and monitoring cell-substrate impedance on one or more
arrays of
the device.
In yet another aspect, the present invention provides methods for calculating
a
Cell Change Index for quantifying and comparing cell-substrate impedance.
4
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In yet another aspect, the present invention provides methods for calculating
resistance of electrical traces connecting an array of a cell-substrate
monitoring device
with a connection pad. Such calculations of electrical trace resistance can be
used for
calculating Cell Index.
In yet another aspect, the present invention provides a method for monitoring
cell-
substrate impedance using a cell-substrate impedance measurement system of the
present
invention. The method includes: providing a cell-substrate impedance
measurement
system of the present invention, adding cells to at least one well of the
multiple-well
device that comprises an electrode array, and monitoring cell-substrate
impedance from
one or more of the wells that comprise cells. Impedance can be monitored at
regular or
irregular time intervals. In preferred embodiments, cell-substrate impedance
is monitored
in at least two wells of a multiple-well device.
In yet another aspect, the present invention provides a method for performing
real-time cell-based assays investigating the effects of one or more compound
on cells,
comprising: providing an above described cell-substrate impedance measurement
system;
introducing cells into at least one well of the system that comprises an
electrode array;
adding one or more compounds to one or more of the wells containing cells; and
monitoring cell-substrate impedance over the electrode array of the one or
more wells
before and after adding the one or more compounds. Preferably, cell-substrate
impedance
is monitored at regular or irregular time intervals after adding one or more
compounds to
the one or more of the wells containing cells. The time dependent impedance
change can
provide information about time dependent cell status before addition of the
compound
and about time dependent cell status under the interaction of the compound.
This
information can be used to determine the effect of a compound on the cells.
In yet another aspect, the present invention provides a method for performing
real-time cytotoxicity assays of at least one compound, comprising: providing
an above
described cell-substrate impedance measurement system; introducing cells into
one or
more wells of the system that comprise an electrode array; adding one or more
compounds to the one or more wells containing cells; and monitoring cell-
substrate
impedance of the one or more wells before and after adding the one or more
compounds,
wherein the time dependent impedance change provides information about time
5
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
dependent cytotoxicity of the compound or compounds. Preferably, cell-
substrate
impedance is monitored at regular or irregular time intervals after adding one
or more
compounds to the one or more of the wells containing cells. The time dependent
impedance change can provide information about any potential cytotoxic effects
of the
compound.
In one embodiment of the above methods, multiple wells with same cell types
are
used, wherein different concentrations of a compound are added to different
wells that
comprise cells. The method can monitor and quantitate time-dependent and
concentration-dependent cellular responses.
In yet another aspect, the present invention provides a method for analyzing
and
comparing time-dependent effects of a first compound and a second compound on
a cell
type, comprising: a) performing a real-time assay on a cell type with the
first compound
using the method described above; b) performing a real-time assay on said cell
type with
the second compound using the method described above; and c) comparing the
time-
dependent responses of the first compound and the second compound.
In one embodiment of this method, time-dependent cellular responses are
determined for a first compound at multiple dose concentrations. In another
embodiment,
time-dependent responses are determined for a second compound at multiple dose
concentrations. In yet another embodiment, time-dependent cellular responses
are
determined for both a first compound and a second compound at multiple dose
concentrations.
In yet another aspect, the present invention provides methods for cytotoxicity
profiling for a compound on multiple cell types, comprising: a) performing
real-time
cytotoxicity assays on different cell types with the compound using the method
described
above, and b) analyzing real-tune cytotoxic responses of different cell types
to the
compound to provide a cytotoxicity profile of the compound. In yet another
embodiment, the above methods are applied to perform cytotoxicity profiling of
multiple
compounds on multiple cell types.
6
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
BRIEF DESCIPTION OF THE DRAWINGS
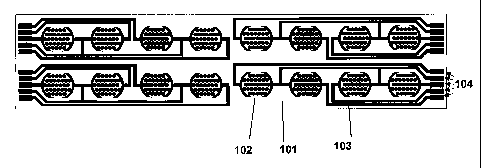
Figure 1 shows schematic drawings of one design of a cell-substrate impedance
measurement device of the present invention. A) depicts the substrate having
16 electrode
arrays (or 16 electrode structure units) that are arranged in a 2-row by 8-
column
configuration on a substrate. B) depicts a single electrode array of a device.
C) shows a
schematic drawing of an electrode array, illustrating the requirement of
approximately
uniform distribution of electrode resistance across the array.
Figure 2 shows real-time monitoring of proliferation of H460 cells seeded at
different
initial cell seeding numbers on a cell substrate impedance monitoring system
of the
presnet invention. The cell proliferation was continuously recorded every 15
minutes for
over 125 hours. The cell growth curves in the log scale show exponential cell
growth or
cells in the stationary phase.
Figure 3 shows real time monitoring of cell attachment and spreading of NIH3T3
cells
using a cell-substrate imepdnace monitoring system of the presnet invention.
The cells
were seeded onto devices coated with either poly- L-lysine or fibronectin. The
cell
attachment and cell spreading processes on the different coating surfaces were
monitored
every 3 minutes for over 3 hours in real time.
Figure 4 shows real-time monitoring of morphological changes in Cos-7 cells
uisng a
cell-substrate impedance monitoring system of the presnet invention. The cells
were
serum starved for 8 hours and stimulated with or without 50 ng/mL EGF. Changes
in cell
morphology were monitored at 3 min intervals for 2 hours and then 1 hour
interval for 14
hours. The initial jump in the signal in EGF-treated cells is due to membrane
ruffling and
actin dynamics in response to EGF. The arrow indicates the point of EGF
stimulation.
Figure 5 shows time-dependent cell index for H460 cells treated by anticancer
drug
paclitaxel. Different wells of cultured H460 cells were treated at their
exponential
growth phase with different concentrations of Paclitaxel. The dynamic response
of the
7
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
cells to different doses of paclitaxel was monitored in real time every 15
minutes for 50
hours after treatment using a cell-substrate impedance monitoring system of
the presnet
invention. For paclitaxel concentration between 67 nM and 500 nM, H460 cells
exhibited a gradual decrease in cell index initially after the compound
addition.
However, the cell index reached a minimum at a time dependent on the compound
concentration between about 15 hours and 20 hours after compound addition.
After that
point, the cell index exhibited a gradual increase in cell index. The cell
index for
compound concentration of 33 nM exhibited a near-constant value for time up to
about
15 hours after compound addition. After 15 hours following the compound
addition, the
cell index exhibited a gradual increase in cell index.
Figure 6 shows time-dependent cell index for H460 cells treated by anticancer
drug
AC101103. Different wells of cultured H460 cells were treated at their
exponential
growth phase with different concentrations of AC101103. The dynamic response
of the
cells to different doses of AC101103 was monitored in real time every 30
minutes for
about 20 hours on a cell substrate impedance monitoring system of the presnet
invention.
The time-dependent cell index in Figure 6 is significantly different from
those shown in
Figure 5. For compound concentrations at 3.125 ug/ml, 6.25 ug/ml and 12.5
ug/ml, the
cell index exhibited a near-constant value for about 5 hrs, about 15 hrs and >
20 hrs
respectively. For compound concentrations at 3.125 ug/ml and 6.25 uglml, the
cell index
started to increase after about 5 hrs and about 15 hrs following compound
addition. For
the compound concentration of 25 ug/ml, there was a gradual, yet slow decrease
in the
cell index after compound addition. For the compound concentration of 50
ug/ml, there
was an about 10 hr time period over which the cell index remained near-
constant, and
after that, the cell index decreased steadily.
Figure 7 shows dynamic drug response curves of A549 cells treated with
doxorubicin.
10,000 A549 cells were seeded in each well of a 16 X device. The cell
attachment and
cell growth were monitored on the cell-substrate impedance monitoring system
of the
present invention in real time before treatment. When the cells were in
exponential
growth phase, doxorubicin at different concentration was added to the cells.
Same
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
volume of a solvent used for dissolve the drug was served as vehicle control.
The time,
and drug dose dependent cell response to doxorubicin was recorded in real
time.
Figure 8 shows titration of NIH3T3 cells on the devices of the present
invention. The
indicated cell number of cells were seeded into microtiter devices fabricated
with
electronic sensor arrays shown in Figure 1B. The electronic sensor arrays were-
precoated with fibronectin. Two hours after seeding, the cell index number was
determined using a cell-substrate imepdnace monitoring system of the present
invention.
Figure 9A and B shows the responses of various cell types (listed in Table 1)
to
doxorubicin treatment as monitored using a cell-substrate imepdnace monitoring
system
of the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1B. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
with doxorubicin.
Figure 10A and B shows the responses of various cell types (listed in Table 1)
to
olomoucine treatment as monitored using a cell-substrate imepdnace monitoring
system
of the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1B. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
with olomoucine.
Figure 11A and 11B show the responses of various cell types (listed in Table
1) to
paclitaxel treatment as monitored using a cell-substrate imepchiace monitoring
system of
the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1B. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
with paclitaxel. -
9
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Figure 12A shows the response of MV522 cells to doxorubicin treatment as
monitored
using a cell-substrate imepdnace monitoring system of the presnet invention.
MV522
cells were seeded onto microtiter devices fabricated with electronic sensor
arrays shown
in Figure 1B and were treated with either DMSO or doxorubicin at the indicated
time and
concentration.
Figure 12B shows the characterization of the cell biological effect of
doxorubicin
treatment on MV522 cells. The cells were either processed for cell cycle
analysis using
FACS or treated with CFDA and Cy3-Annexin V to assess cell viability. In
addition, the
cells were fixed and stained with phalloidin to examine cell morphology. For
viability
and morphology, the cells were visualized and photographed using a
fluorescence
microscope equipped with CCD camera.
Figure 13A shows the response of A549 cells to olomoucine treatment as
monitored
using a cell-substrate imepdnace monitoring system of the presnet invention.
A549 cells
were seeded onto microtiter devices fabricated with electronic sensor arrays
shown in
Figure 1B and were treated with either DMSO or olomoucine at the indicated
time and
concentration.
Figure 13B shows the characterization of the cell biological effect of
olomoucine
treatment on MV522 cells. The cells were either processed for cell cycle
analysis using
FACS or treated with CFDA and Cy3-Annexin V to assess cell viability. In
addition, the
cells were fixed and stained with phalloidin to examine cell morphology. For
viability
and morphology, the cells were visualized and photographed using a
fluorescence
microscope equipped with CCD camera.
Figure 14A shows the response of A549 cells to paclitaxel treatment as
monitored using
a cell-substrate imepdnace monitoring system of the presnet invention. A549
cells were
seeded onto microtiter devices fabricated with electronic sensor arrays shown
in Figure
1B and were treated with either DMSO or paclitaxel at the indicated time and
concentration.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Figure 14B shows the characterization of the cell biological effect of
paclitaxel n
treatment on A549 cells. The cells were either processed for cell cycle
analysis using
FACE or treated with CFDA and Cy3-Annexin V to assess cell viability. In
addition, the
cells were fixed and stained with phalloidin to examine cell morphology. For
viability
and morphology, the cells were visualized and photographed using a
fluorescence
microscope equipped with CCD camera.
Figure 15. The time dependent IC values for each compound ( 15A: Doxorubicin;
15B:
Paclitaxel; 15C: Olomoucine; 15D: Tamoxifan) for the indicated cell lines as
estimated
at 5 hr intervals from the cell index curves obtained using a cell-substrate
imepdnace
monitoring system of the presnet invention .
Figure 16A shows the cell index curves of HT29 cells before and after
treatment with
various compounds. Also shown is an theoretical exponential increase of cell
index with
time (labeled as "Log-growth, model") and cells treated with DMSO vehicle
control
(labeled as "Control, HT29")
Figure 16B shows the derived cell change index (CCI) from the cell index
curves shown
in Figure 16A. Also shown is the "black-white shading codes" used for
different
responses based on the convention shown in Figure 16C.
Figure 16C shows the color-coding scheme used for representing the CCI curves.
If the
DT is the doubling time for the cells undergoing exponential growth in the
cell culture
media used, then CCI having different values relative to 0.7/DT indicates the
different
cell change status. If CCI » 0.7/DT, cell index increases faster than that
expected for
an exponential growth (or log growth) of the cells (such region n the CCI
curve is
represented as ~.. Rectangle). If CCI is about 0.7/DT, cell index increases in
the same
rate as that expected for an exponential growth of the cells (such region in
the CCI curve
is represented as .~r~;~ Rectangle). If CCI is more than zero but somewhat
smaller than
0.7/DT, then cell index increases in the rate slowed than that expected for an
exponential
11
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
growth (such region of the CCI curve is represented as ~'Y Rectangle). If CCI
is about
zero, then cell index shows a near constant value (such region of the CCI
curve is
represented as ~ Rectangle). If CCI is negative, then the cell index is
decreasing with
time, showing the cells losing attachment to the electrode surface or changing
their
morphology (such region of the curve is shown as ~~.~... Rectangle). If CCI is
very
negative, then the cell index decreases rapidly with time, showing that either
cells lose
attachment to the electrode surfaces quickly or cells change their morphology
very
quickly (such region of the CCI curve is represented as ~ Rectangle). The
transient,
quick noise in the CCI values are removed so that the whole CCI curve is
represented
after compound addition by one, two or three black/white-shaded rectangles.
Figure 17 shows the cell response profile of each cell line tested against the
indicated
chemotherapeutic agents. For each cell line and compound, the time-dependent
cell
change index (CCI) was calculated from their corresponding RT-CES responses at
an
IC50 concentration. (IC 50 is time dependent so that the IC50 concentration at
30h, or
the concentration closest to that, after drug addition is used). The specific
CCI curves as
related to specific cellular responses were coded according to the convention
described in
Figure 16C and displayed in groups of compounds with similar mechanism of
action.
DOX: doxorubicin; 5-F: 5-Fluorouracil; COL: Colcemid; TAXOL: paclitaxel; VIN:
vinblastin; GLOM: Olomoucine; ROS: Roscovitine; STAU: Staurosporine; TAMO:
Tamoxifan; RIFA: Rifampicin; ACEA-1: an ACEA test compound.
Figure 18. Dynamic monitoring of cell proliferation. H1080 fibrosarcoma cells,
H460
lung cancer cells, HepG2 hepatosarcoma cancer cells and NIH3T3 mouse
fibroblast cell
lines were seeded at a density of 2500 and 10,000 cells per well of ACEA 96x e-
Plate
device. The adhesion, spreading and proliferation of the cells were
dynamically
monitored every 30 minutes using a cell-substrate imepdnace monitoring system
of the
presnet invention.
12
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Figure 19. Correlation between cell-substrate impedance measurement (as shown
here,
Cell Index) and number of cells seeded and comparison of Cell Index with MTT.
(A)Increasing numbers of NIH3T3 ranging from 100 cells all the way up to
10,000 cells
were seeded in a device of the present invention and the cells were monitored
for 10
hours at which point the Cell Index was obtained. The Cell Index value was
plotted
against the corresponding number of cells. (B) The cells described in Figure
19A were
assayed by MTT assay at the end of the experiment and the optical density at
590 nm was
plotted against the number of cells seeded.
Figure 20. Dynamic monitoring of drug interaction with target cells using a
cell-
substrate imepdnace monitoring system of the presnet invention. (A) A549 cells
were
seeded in a device of present invention at a density of 10,000 cells per well
and the cells
were continuously monitored up to 24 hours at which point paclitaxel was added
at the
indicated final concentrations. (B) Annexin V staining of A549 cells treated
with DMSO
or 12.5 nM paclitaxel for 20 hours. The cells were observed with fluorescence
microscope and images were captured with an attached digital camera.
Figure 21. Dynamic monitoring of cell cycle arrest using a cell-substrate
imepdnace
monitoring system of the presnet invention. A549 cells were seeded in a device
of
present invention at 10,000 cells per well and continuously monitored using
the RT-CES.
The cells were treated with either (A) DMSO or 100 ~M Olomoucine (B) A549
cells
growing on tissue culture dishes for 20 hours were treated with DMSO or 100 ~M
Olomoucine. Cell cycle analysis was performed by flow cytometry.
Figure 22. Dynamic monitoring of cytotoxic compounds with target cells using a
cell-
substrate imepdnace monitoring system of the presnet invention. A549 cells
were seeded
in a device of the present inventoon and continuously monitored using the RT-
CES
system. The cells were treated with the indicated final concentrations of (A)
staurosporine, (B) vinblastine and (C) 5-flourouracil.
13
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
DETAILED DESCRIPTION OF THE INVENTION
A. Definitions
For clarity of disclosure, and not by way of limitation, the detailed
description of
the invention is divided into the subsections that follow.
Unless defined otherwise, all technical and scientific terms used herein have
the
same meaning as is commonly understood by one of ordinary skill in the art to
which this
invention belongs. All patents, applications, published applications and other
publications referred to herein are incorporated by reference in their
entirety. If a
definition set forth in this section is contrary to or otherwise inconsistent
with a definition
set forth in the patents, applications, published applications and other
publications that
are herein incorporated by reference, the definition set forth in this section
prevails over
the definition that is incorporated herein by reference.
As used herein, "a" or "an" means "at least one" or "one or more."
As used herein, "membrane" is a sheet of material.
As used herein, "biocompatible membrane" means a membrane that does not have
deleterious effects on cells, including the viability, attachment, spreading,
motility,
growth, or cell division.
When a suspension of viable, unimpaired, epithelial or endothelial cells is
added
to a vessel, a surface of the vessel "is suitable for cell attachment" when a
significant
percentage of the cells are adhering to the surface of the vessel within
twelve hours.
Preferably, at least 50% of the cells are adhering to the surface of the
vessel within
twelve hours. More preferably, a surface that is suitable for cell attachment
has surface
properties so that at least 70% of the cells are adhering to the surface
within twelve hours
of plating (i.e., adding cells to the vessel). Even more preferably, the
surface properties
of a surface that is suitable for cell attachment results in at least 90% of
the cells adhering
to the surface within twelve hours of plating. Most preferably, the surface
properties of a
surface that is suitable for cell attachment results in at least 90% of the
cells adhering to
the surface within eight, six, four, two hours of plating. To have desired
surface
properties for cell attachment, the surface may need to chemically-treated
(e.g. treatment
14
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
with an acid and/or with a base), and/or physically treated (e.g. treatment
with plasma),
and/or biochemically treated (e.g. coated with one or more molecules or
biomolecules
that promotes cell attachment). In the present invention, a biocompatible
surface (such as
a membrane) preferably is suitable for the attachment of cells of the type
that are to be
used in an assay that uses the biocompatible surface (e.g., membrane), and
most
preferably, allows the attachment of at least 90% of the cells that contact
the
biocompatible surface during the assay.
A "biomolecular coating" is a coating on a surface that comprises a molecule
that
is a naturally occurring biomolecule or biochemical, or a biochemical derived
from or
based on one or more naturally occurring biomolecules or biochemicals. For
example, a
biomolecular coating can comprise an extracellular matrix component (e.g.,
fibronectin,
collagens), or a derivative thereof, or can comprise a biochemical such as
polylysine or
polyornithine, which are polymeric molecules based on the naturally occurring
biochemicals lysine and ornithine. Polymeric molecules based on naturally
occurnng
biochemicals such as amino acids can use isomers or enantiomers of the
naturally-
occuring biochemicals.
An "extracellular matrix component" is a molecule that occurs in the
extracellular
matrix of an animal. It can be a component of an extracellular matrix from any
species
and from any tissue type. Nonlimiting examples of extracellular matrix
components
include laminins, collagens fibronectins, other glycoproteins, peptides,
glycosaminoglycans, proteoglycans, etc. Extracellular matrix components can
also
include growth factors.
An "electrode" is a stntcture having a high electrical conductivity, that is,
an
electrical conductivity much higher than the electrical conductivity of the
surrounding
materials.
As used herein, an "electrode structure" refers to a single electrode,
particularly
one with a complex structure (as, for example, a spiral electrode structure),
or a collection
of at least two electrode elements that are electrically connected together.
All the
electrode elements within an "electrode structure" are electrically connected.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
As used herein, "electrode element" refers to a single structural feature of
an
electrode structure, such as, for example, a fingerlike projection of an
interdigitated
electrode structure.
As used herein, an "electrode array" or "electrode structure unit" is two or
more
electrode structures that are constructed to have dimensions and spacing such
that they
can, when connected to a signal source, operate as a unit to generate an
electrical field in
the region of spaces around the electrode structures. Preferred electrode
structure units of
the present invention can measure impedance changes due to cell attachment to
an
electrode surface. Non-limiting examples of electrode structure units are
interdigitated
electrode structure units and concentric electrode structure units.
An "electrode bus" is a portion of an electrode that connects individual
electrode
elements or substructures. An electrode bus provides a common conduction path
from
individual electrode elements or individual electrode substructures to another
electrical
connection. In the devices of the present invention, an electrode bus can
contact each
electrode element of an electrode structure and provide an electrical
connection path to
electrical traces that lead to a connection pad.
"Electrode traces" or "electrically conductive traces" or "electrical traces",
are
electrically conductive paths that extend from electrodes or electrode
elements or
electrode structures toward one end or boundary of a device or apparatus for
connecting
the electrodes or electrode elements or electrode structures to an impedance
analyzer.
The end or boundary of a device may correspond to the comlection pads on the
device or
apparatus.
A "connection pad" is an area on an apparatus or a device of the present
invention
which is electrically connected to at least one electrode or all electrode
elements within at
least one electrode structure on an apparatus or a device and which can be
operatively
connected to external electrical circuits (e.g., an impedance measurement
circuit or a
signal source). The electrical connection between a connection pad and an
impedance
measurement circuit or a signal source can be direct or indirect, through any
appropriate
electrical conduction means such as leads or wires. Such electrical conduction
means
may also go through electrode or electrical conduction paths located on other
regions of
the apparatus or device.
16
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
"Interdigitated" means having proj ections coming one direction that interlace
with
projections coming from a different direction in the manner of the fingers of
folded hands
(with the caveat that interdigitated electrode elements preferably do not
contact one
another).
As used herein, a "high probability of contacting an electrode element" means
that, if a cell is randomly positioned within the sensor area of a device or
apparatus of the
present invention, the probability of a cell (or particle) contacting on an
electrode
element, calculated from the average diameter of a cell used on or in a device
or
apparatus of the present invention, the sizes of the electrode elements, and
the size of the
gaps between electrode elements, is greater than about 50%, more preferably
greater than
about 60%, yet more preferably greater than about 70%, and even more
preferably
greater than about 80%, greater than about 90%, or greater than about 95%.
As used herein, "at least two electrodes fabricated on said substrate" means
that
the at least two electrodes are fabricated or made or produced on the
substrate. The at
least two electrodes can be on the same side of the substrate or on the
different side of the
substrate. The substrate may have multiple layers, the at least two electrodes
can be
either on the same or on the different layers of the substrate.
As used herein, "at least two electrodes fabricated to a same side of said
substrate" means that the at least two electrodes are fabricated on the same
side of the
substrate.
As used herein, "at least two electrodes fabricated to a same plane of said
substrate" means that, if the nonconducting substrate has multiple layers, the
at least two
electrodes are fabricated to the same layer of the substrate.
As used herein, "said . . . electrodes [or electrode structures] have
substantially
the same surface area" means that the surface areas of the electrodes referred
to are not
substantially different from each other, so that the impedance change due to
cell
attachment or growth on any one of the electrodes (or electrode structures)
referred to
will contribute to the overall detectable change in impedance to a same or
similar degree
as the impedance change due to cell attachment or growth on any other of the
electrodes
(or electrode structures) referred to. In other words, where electrodes (or
electrode
structures) have substantially the same surface area, any one of the
electrodes can
17
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
contribute to overall change in impedance upon cell attachment or growth on
the
electrode. In most cases, the ratio of surface area between the largest
electrode and the
smallest electrode that have "substantially the same surface area" is less
than 10.
Preferably, the ratio of surface area between the largest electrode and the
smallest
electrode of an electrode array is less than 5, 4, 3, 2, 1.5, 1.2 or 1.1. More
preferably, the
at least two electrodes of an electrode structure have nearly identical or
identical surface
area.
As used herein, "said device has a surface suitable for cell attachment or
growth"
means that the electrode and/or non-electrode area of the apparatus has
appropriate
physical, chemical or biological properties such that cells of interest can
viably attach on
the surface and new cells can continue to attach, while the cell culture
grows, on the
surface of the apparatus. However, it is not necessary that the device, or the
surface
thereof, contain substances necessary for cell viability or growth. These
necessary
substances, e.g., nutrients or growth factors, can be supplied in a medium.
Preferably,'
when a suspension of viable, unimpaired, epithelial or endothelial cells is
added to the
"surface suitable for cell attachment" when at least 50% of the cells are
adhering to the
surface within twelve hours. More preferably, a surface that is suitable for
cell
attachment has surface properties so that at least 70% of the cells are
adhering to the
surface within twelve hours of plating (i.e., adding cells to the chamber or
well that
comprises the said device). Even more preferably, the surface properties of a
surface that
is suitable for cell attachment results in at least 90% of the cells adhering
to the surface
within twelve hours of plating. Most preferably, the surface properties of a
surface that is
suitable for cell attachment results in at least 90% of the cells adhering to
the surface
within eight, six, four, two hours of plating.
As used herein, "detectable change in impedance between or among said
electrodes" (or "detectable change in impedance between or among said
electrode
structures") means that the impedance between or among said electrodes (or
electrode
structures) would have a significant change that can be detected by an
impedance
analyzer or impedance measurement circuit when molecule binding reaction
occurs on
the electrode surfaces. The impedance change refers to the difference in
impedance
values when molecule binding reaction occurs on the electrode surface of the
apparatus
18
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
and when no molecular reaction occurs on the electrode surface. Alternatively,
the
impedance change refers to the difference in impedance values when cells are
attached to
the electrode surface and when cells are not attached to the electrode
surface, or when the
number, type, activity, adhesiveness, or morphology of cells attached to the
electrode-
s comprising surface of the apparatus changes. In most cases, the change in
impedance is
larger than 0.1 % to be detectable. Preferably, the detectable change in
impedance is
larger than 1 %, 2%, 5%, or 8%. More preferably, the detectable change in
impedance is
larger than 10%. Impedance between or among electrodes is typically a function
of the
frequency of the applied electric field for measurement. "Detectable change in
impedance between or among said electrodes" does not require the impedance
change at
all frequencies being detectable. "Detectable change in impedance between or
among
said electrodes" only requires a detectable change in impedance at any single
frequency
(or multiple frequencies). In addition, impedance has two components,
resistance and
reactance (reactance can be divided into two categories, capacitive reactance
and
inductive reactance). "Detectable change in impedance between or among said
electrodes" requires only that either one of resistance and reactance has a
detectable
change at any single frequency or multiple frequencies. In the present
application,
impedance is the electrical or electronic impedance. The method for the
measurement of
such impedance is achieved by, (1) applying a voltage between or among said
electrodes
at a given frequency (or multiple frequencies, or having specific voltage
waveform) and
monitoring the electrical current through said electrodes at the frequency (or
multiple
frequencies, or having specific waveform), dividing the voltage.amplitude
value by the
current amplitude value to derive the impedance value; (2) applying an
electric current of
a single frequency component (or multiple frequencies or having specific
current wave
form) through said electrodes and monitoring the voltage resulted between or
among said
electrodes at the frequency (or multiple frequencies, or having specific
waveform),
dividing the voltage amplitude value by the current amplitude value to derive
the
impedance value; (3) other methods that can measure or determine electric
impedance.
Note that in the description above of "dividing the voltage amplitude value by
the current
amplitude value to derive the impedance value", the "division" is done for the
values of
current amplitude and voltage amplitude at same frequencies. Measurement of
such
19
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
electric impedance is an electronic or electrical process that does not
involve the use of
any reagents.
As used herein, "said at least two electrodes have substantially different
surface
area" means that the surface areas of any electrodes are not similar to each
other so that
the impedance change due to cell attachment or growth on the larger electrode
will not
contribute to the overall detectable impedance to a same or similar degree as
the
impedance change due to cell attachment or growth on the smaller electrodes.
Preferably, any impedance change due to cell attachment or growth on the
larger
electrode is significantly smaller than the impedance change due to cell
attachment or
growth on the smaller electrode. Ordinarily, the ratio of surface area between
the largest
electrode and the smallest electrode is more than 10. Preferably, the ratio of
surface area
between the largest electrode and the smallest electrode is more than 20, 30,
40, 50 or
100.
As used herein, "multiple pairs of electrodes or electrode structures
spatially
arranged according to wells of a multi-well microplate" means that the
multiple pairs of
electrodes or electrode structures of a device or apparatus are spatially
arranged to match
the spatial configuration of wells of a mufti-well microplate so that, when
desirable, the
device can be inserted into, joined with, or attached to a multiwell plate
(for example, a
bottomless multiwell plate) such that multiple wells of the mufti-well
microplate will
comprise electrodes or electrode structures.
As used herein, "arranged in a row-column configuration" means that, in terms
of
electric connection, the position of an electrode, an electrode array or a
switching circuit
is identified by both a row position number and a column position number.
As used herein, "each well contains substantially same number . . . of cells"
means that the lowest number of cells in a well is at least 50% of the highest
number of
cells in a well. Preferably, the lowest number of cells in a well is at least
60%, 70%,
80%, 90%, 95% or 99% of the highest number of cells in a well. More
preferably, each
well contains an identical number of cells.
As used herein, "each well contains . . .same type of cells" means that, for
the
intended purpose, each well contains same type of cells; it is not necessary
that each well
contains exactly identical type of cells. For example, if the intended purpose
is that each
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
well contains mammalian cells, it is permissible if each well contains same
type of
mammalian cells, e.g., human cells, or different mammalian cells, e.g., human
cells as
well as other non-human mammalian cells such as mice, goat or monkey cells,
etc.
As used herein, "each well contains . . . serially different concentration of
a test
compound" means that each well contains a test compound with a serially
diluted
concentrations, e.g., an one-tenth serially diluted concentrations of 1 M, 0.1
M, 0.01 M,
etc.
As used herein, "dose-response curve" means the dependent relationship of
response of cells on the dose concentration of a test compound. The response
of cells can
be measured by many different parameters. For example, a test compound is
suspected to
have cytotoxicity and cause cell death. Then the response of cells can be
measured by
percentage of non-viable (or viable) cells after the cells are treated by the
test compound.
Plotting this percentage of non-viable (or viable) cells as a function of the
does
concentration of the test compound constructs a dose response curve. In the
present
application, the percentage of non-viable (or viable) cells can be expressed
in terms of
measured impedance values, or in terms of cell index derived from impedance
measurement, or in terms of cell change indexes. For example, for a give cell
type and
under specific cellular physiological condition (e.g., a particular cell
culture medium),
cell index can be shown to have a linear correlation or positive correlation
with the
number of viable cells in a well from which cell index was derived from the
impedance
measurement. Thus, in the present application, one can plot cell index as a
function of
the dose concentration of the test compound to construct a "dose-response
curve". Note
that, generally, cell index not only correlate with the number of viable cells
in the wells
but also relate to the cell morphology and cell attachment. Thus plotting cell
index
versus doss concentration provides information not only about number of cells
but also
about their physiological status (e.g. cell morphology and cell adhesion).
Furthermore,
an important advantage offered by the system and devices of the present
application is
that in a single experiment, one can obtain "dose-response curves" at multiple
time points
since the system allows for the continuous monitoring of cells and provides
impedance
measurement at many time points over a time range as short as a few minutes to
as long
as days or weeks.
21
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
As used herein, "the electrodes have, along the length of the microchannel, a
length that is substantially less than the largest single-dimension of a
particle to be
analyzed" means that the electrodes have, along the length of the
microchannel, a length
that is at least less than 90% of the largest single-dimension of a particle
to be analyzed.
Preferably, the electrodes have, along the length of the microchannel, a
length that is at
least less than 80%, 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5% of the largest
single-
dimension of a particle to be analyzed.
As used herein, "the microelectrodes span the entire height of the
microchannel"
means that the microelectrodes span at least 70% of the entire height of the
microchannel.
Preferably, microelectrodes span at least 80%, 90%, 95% of the entire height
of the
microchannel. More preferably, microelectrodes span at least 100% of the
entire height
of the microchannel.
As used herein, "an aperture having a pore size that equals to or is slightly
larger
than size of said particle" means that aperture has a pore size that at least
equals to the
particle size but less than 300% of the particle size. Here both pore size and
particle size
are measured in terms of single dimension value.
As used herein, "microelectrode strip or electrode strip" means that a non-
conducting substrate strip on which electrodes or electrode structure units
are fabricated
or incorporated. The non-limiting examples of the non-conducting substrate
strips
include polymer membrane, glass, plastic sheets, ceramics, insulator-on-
semiconductor,
fiber glass (like those for manufacturing printed-circuits-board). Electrode
structure units
having different geometries can be fabricated or made on the substrate strip
by any
suitable microfabrication, micromachining, or other methods. Non-limiting
examples of
electrode geometries include interdigitated electrodes, circle-on-line
electrodes, diamond-
on-line electrodes, castellated electrodes, or sinusoidal electrodes.
Characteristic
dimensions of these electrode geometries may vary from as small as less than 5
micron,
or less than 10 micron, to as large as over 200 micron, over 500 micron, over
1 mm. The
characteristic dimensions of the electrode geometries refer to the smallest
width of the
electrode elements, or smallest gaps between the adjacent electrode elements,
or size of a
repeating feature on the electrode geometries. The microelectrode strip can be
of any
geometry for the present invention. One exemplary geometry for the
microelectrode
22
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
strips is rectangular shape - having the width of the strip between less than
50 micron to
over 10 mm, and having the length of the strip between less than 60 micron to
over 15
mm. An exemplary geometry of the microelectrode strips may have a geometry
having a
width of 200 micron and a length of 20 mm. A single microelectrode strip may
have two
electrodes serving as a measurement unit, or multiple such two-electrodes
serving as
multiple measurement units, or a single electrode structure unit as a
measurement unit, or
multiple electrode structure units serving as multiple electrode structure
units. In one
exemplary embodiment, when multiple electrode structure units are fabricated
on a single
microelectrode strip, these electrode structure units are positioned along the
length
direction of the strip. The electrode structure units may be of squared-shape,
or
rectangular-shape, or circle shapes. Each of electrode structure units may
occupy size
from less than 50 micron by 50 micron, to larger than 2 mm x 2mm.
As used herein, "sample" refers to anything which may contain a moiety to be
isolated, manipulated, measured, quantified, detected or analyzed using
apparatuses,
microplates or methods in the present application. The sample may be a
biological
sample, such as a biological fluid or a biological tissue. Examples of
biological fluids
include suspension of cells in a medium such as cell culture medium, urine,
blood,
plasma, serum, saliva, semen, stool, sputum, cerebral spinal fluid, tears,
mucus, amniotic
fluid or the like. Biological tissues are aggregates of cells, usually of a
particular kind
together with their intercellular substance that form one of the structural
materials of a
human, animal, plant, bacterial, fungal or viral structure, including
connective,
epithelium, muscle and nerve tissues. Examples of biological tissues also
include organs,
tumors, lymph nodes, arteries and individual cell(s). The biological samples
may further
include cell suspensions, solutions containing biological molecules (e.g.
proteins,
enzymes, nucleic acids, carbohydrates, chemical molecules binding to
biological
molecules) .
As used herein, a "liquid (fluid) sample" refers to a sample that naturally
exists as
a liquid or fluid, e.g., a biological fluid. A "liquid sample" also refers to
a sample that
naturally exists in a non-liquid status, e.g., solid or gas, but is prepared
as a liquid, fluid,
solution or suspension containing the solid or gas sample material. For
example, a liquid
23
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
sample can encompass a liquid, fluid, solution or suspension containing a
biological
tissue.
A "test compound" is any compound whose activity or direct or indirect effect
or
effects on cells is investigated in any assay. A test compound can be any
compound,
including, but not limited to, a small molecule, a large molecule, a molecular
complex, an
organic molecule, an inorganic molecule, a biomolecule such as but not limited
to a lipid,
a steroid, a carbohydrate, a fatty acid, an amino acid, a peptide, a protein,
a nucleic acid,
or any combination of these. A test compound can be a synthetic compound, a
naturally
occurnng compound, a derivative of a naturally-occurnng compound, etc. The
structure
of a test compound can be known or unknown.
A "known compound" is a compound for which at least one activity is known. In
the present invention, a known compound preferably is a compound for which one
or
more direct or indirect effects on cells is known. Preferably, the structure
of a known
compound is known, but this need not be the case. Preferably, the mechanism of
action of
a known compound on cells is known, for example, the effect or effects of a
known
compound on cells can be, as nonlimiting examples, effects on cell viability,
cell
adhesion, apoptosis, cell differentiation, cell proliferation, cell
morphology, cell cycle,
IgE-mediated cell activation or stimulation, receptor-ligand binding, cell
number, cell
quality, cell cycling, etc.
An "impedance value" is the impedance measured for electrodes in a well with
or
without cell present. Impedance is generally a function of the frequency,
i.e., impedance
values depend on frequencies at which the measurement was conducted. For the
present
application, impedance value refers to impedance measured at either single
frequency or
multiple frequencies. Furthermore, impedance has two components, one
resistance
component and one reactance component. Impedance value in the present
application
refers to resistance component, or reactance component, or both resistance and
reactance
component. Thus, when "impedance value" was measured or monitored, we are
refernng
to that, resistance, or reactance, or both resistance and reactance were
measured or
monitored. In many embodiments of the methods of the present application,
impedance
values also refer to parameter values that are derived from raw, measured
impedance
24
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
data. For example, cell index, or normalized cell index, or delta cell index
could be used
to represent impedance values.
A "Cell Index" or "CI" is a parameter that can derived from measured impedance
values and that can be used to reflect the change in impedance values. There
are a
number of methods to derive or calculate Cell Index.
A "Normalized Cell Index" at a given time point is calculated by dividing the
Cell
Index at the time point by the Cell Index at a reference time point. Thus, the
Normalized
Cell Index is 1 at the reference time point.
A "delta cell index" at a given time point is calculated by subtracting the
cell
index at a standard time point from the cell index at the given time point.
Thus, the delta
cell index is the absolute change in the cell index from an initial time (the
standard time
point) to the measurement time.
A "Cell Change Index" or "CCI" is a parameter derived from Cell Index and
"CCI" at a time point is equal to the 1St order derive of the Cell Index with
respect to
time, divided by the Cell Index at the time point. In other words, CCI is
calculated as
CCI(t) = dCl(t)
CI (t) ~ dt
B. Devices and systems for monitoring cell-substrate impedance
Devices for Measuring Cell-Substrate Impedance
The present invention includes devices for measuring cell-substrate impedance
that comprise a nonconducting substrate; two or more electrode arrays
fabricated on the
substrate, where each of the two or more electrode arrays comprises two
electrode
structures; and at least two connection pads, each of which is located on an
edge of the
substrate. Each electrode array of the device has approximately uniform
electrode
resistance across the entire array. The substrate of the device has a surface
suitable for
cell attachment or growth; where cell attachment or growth on said substrate
can result in
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
a detectable change in impedance between or among the electrode structures
within each
electrode array.
An electrode array is two or more electrode structures that are constructed to
have
dimensions and spacing such that they can, when connected to a signal source,
operate as
a unit to generate an electrical field in the region of spaces around the
electrode
structures. An electrode structure refers to a single electrode, particularly
one with a
complex structure. (For example, an electrode structure can comprise two or
more
electrode elements that are electrically connected together.) In devices of
the present
invention, an electrode array comprises two electrode structures, each of
wluch comprises
multiple electrode elements, or substructures. In preferred embodiments of the
present
invention, the electrode structures of each of the two or more electrode
arrays of a device
have substantially the same surface area. In preferred embodiments of a device
of the
present invention, each of the two or more electrode arrays of a device
comprise two
electrode structures, and each electrode structure comprises multiple
electrode elements.
Each of the two electrode structures of an electrode array is connected to a
separate
connection pad that is located at the edge of the substrate.
Thus, in devices of the present invention, for each of the two or more
electrode
arrays of the device, the first of the two electrode structures is connected
to one of the
two or more connection pads, and the second of the two electrode structures is
connected
to another of the two or more connection pads. Preferably, each array of a
device is
individually addressed, meaning that the electrical traces and connection pads
of the
arrays are configured such that an array can be connected to an impedance
analyzer in
such a way that a measuring voltage can be applied across a single array at a
given time
by using switches (such as electronic switches).
Each electrode array of the device has an approximately uniform electrode
resistance distribution across the entire array. By "uniform resistance
distribution across
the array" is meant that when a measurement voltage is applied across the
electrode
structures of the array, the electrode resistance at any given location of the
array is
approximately equal to the electrode resistance at any other location on the
array.
Preferably, the electrode resistance at a first location on an array of the
device and the
electrode resistance at a second location on the same array does not differ by
more than
26
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
30%. More preferably, the electrode resistance at a first location on an array
of the device
and the electrode resistance at a second location on the same array does not
differ by
more than 15%. Even more preferably, the electrode resistance at a first
location on an
array of the device and a second location on the same array does not differ by
more than
5%. More preferably yet, the electrode resistance at a first location on an
array of the
device and a second location on the same array does not differ by more than
2%.
For a device of the present invention, preferred arrangements for the
electrode
elements, gaps between the electrodes and electrode buses in a given electrode
array are
used to allow all cells, no matter where they land and attach to the electrode
surfaces, to
contribute similarly to the total impedance change measured for the electrode
array. Thus,
it is desirable to have similar electric field strengths at any two locations
within any given
array of the device when a measurement voltage is applied to the electrode
array. At any
given location of the array, the field strength is related to the potential
difference between
the nearest point on a first electrode structure of the array and the nearest
point on a
second electrode structure of the array. It is therefore desirable to have
similar electric
potential drops across the electrode elements and across the electrode buses
of a given
array. Based on this requirement, it is preferred to have an approximately
uniform
electrode resistance distribution across the whole array where the electrode
resistance at a
location of interest is equal to the sum of the electrode resistance between
the nearest
point on a first electrode structure (that is the point on the first electrode
structure nearest
the location of interest) and a first connection pad connected to the first
electrode
structure and the electrode resistance between the nearest point on a second
electrode
structure (that is the point on the first electrode structure nearest the
location of interest)
and a second connection pad connected to the second electrode structure.
Devices of the present invention are designed such that the arrays of the
device
have an approximately uniform distribution across the whole array. This can be
achieved,
for example, by having electrode structures and electrode buses of particular
spacing and
dimensions (lengths, widths, thicknesses and geometrical shapes) such that the
resistance
at any single location on the array is approximately equal to the resistance
at any single
other location on the array. In most embodiments, the electrode elements (or
electrode
structures) of a given array will have even spacing and be of similar
thiclcnesses and
27
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
widths, the electrode buses of a given array will be of similar thicknesses
and widths, and
the electrode traces leading from a given array to a connection pad will be of
closely
similar thicknesses and widths. Thus, in these preferred embodiments, an array
is
designed such that the lengths and geometrical shapes of electrode elements or
structures,
the lengths and geometrical shapes of electrode traces, and the lengths and
geometrical
shapes of buses allow for approximately uniform electrode resistance
distribution across
the array.
In some preferred embodiments of cell-substrate impedance measurement
devices, electrode structures comprise multiple electrode elements, and each
electrode
element connects directly to an electrode bus. Electrode elements of a first
electrode
structure connect to a first electrode bus, and electrode elements of a second
electrode
structure connect to a second electrode bus. In these embodiments, each of the
two
electrode buses connects to a separate connection pad via an electrical trace.
Although the
resistances of the traces contribute to the resistance at a location on the
array, for any two
locations on the array the trace connections from the first bus to a first
connection pad
and from the second bus to a second connection pad are identical. Thus, in
these
preferred embodiments trace resistances do not need to be taken into account
in designing
the geometry of the array to provide for uniform resistances across the array.
1i1 preferred embodiments of the present invention, a device for monitoring
cell-
substrate impedance has two or more electrode arrays that share a connection
pad.
Preferably one of the electrode structures of at least one of the electrode
arrays of the
device is connected to a connection pad that also connects to an electrode
structure of at
least one other of the electrode arrays of the device. Preferably for at least
two arrays of
the device, each of the two or more arrays has a first electrode structure
connected to a
connection pad that connects with an electrode structure of at least one other
electrode
array, and each of the two or more arrays has a second electrode structure
that connects to
a connection pad that does not connect with any other electrode structures or
arrays of the
device. Thus, in preferred designs of a device there are at least two
electrode arrays each
of which has a first electrode structure that is connected to a common
connection pad and
a second electrode structure that is connected to an independent connection
pad.
2~
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In some preferred embodiments of the present invention, each of the electrode
structures of an array is connected to an electrode bus that is connected to
one of the two
or more connection pads of the device via an electrically conductive trace. In
preferred
embodiments, each of the two electrode structures is connected to a single
bus, such that
each array connects to two buses, one for each electrode structures. In this
arrangement,
each of the two buses connects to a separate connection pad of the substrate.
The electrically conductive traces that connect a bus with a connection can be
fabricated of any electrically conductive material. The traces can be
localized to the
surface of the substrate, and can be optionally covered with an insulating
layer.
Alternatively the traces can be disposed in a second plane of the substrate.
Description of
arrangements and design of electrically conductive traces on impedance
measurement
devices can be found in parent U.S. Patent Application 10/705,447, herein
incorporated
by reference for all disclosure on fabrication and design of electrically
conductive trace
on substrates.
Appropriate electronic connection means such as metal clips engaged onto the
connection pads on the substrate and connected printed-circuit-boards can be
used for
leading the electronic connections from the connection pads on the devices to
external
electronic circuitry (e.g. an impedance analyzer). Description of the design
of cell-
substrate impedance devices and their manufacture can be~found in U.S. Patent
Application No. 10/705,447, herein incorporated by reference for all
description and
disclosure of the design, features, and manufacture of impedance device
comprising
electrode arrays.
Preferably the nonconducting substrate is planar, and is flat or approximately
flat.
Exemplary substrates can comprise many materials, including, but not limited
to, silicon
dioxide on silicon, silicon-on-insulator (SOI) wafer, glass (e.g., quartz
glass, lead glass or
borosilicate glass), sapphire, ceramics, polymer, fiber glass, plastics, e.g.,
polyimide (e.g.
Kapton, polyimide film supplied by DuPont), polystyrene, polycarbonate,
polyvinyl
chloride, polyester, polypropylene and urea resin. Preferably, the substrate
and the surface
of the substrate are not going to interfere with molecular binding reactions
that will occur at
the substrate surface. For cell-substrate impedance monitoring, any surface of
the
nonconducting substrate that can be exposed to cells during the use of a
device of the
29
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
present invention is preferably biocompatible. Substrate materials that are
not
biocompatible can be made biocompatible by coating with another material, such
as
polymer or biomolecular coating.
All or a portion of the surface of a substrate can be chemically treated,
including
but not limited to, modifying the surface such as by addition of functional
groups, or
addition of charged or hydrophobic groups.
Descriptions of electrode arrays used for impedance measurement that apply to
the devices of the present invention are described in parent U.S. Patent
Application No.
10/705,447, herein incorporated by reference for all disclosure relating to
electrode arrays
(or structural units), electrode structures, electrode materials, electrode
dimensions, and
methods of manufacturing electrodes on substrates.
Preferred electrode arrays for devices of the present invention include arrays
comprising two electrode structures, such as, for example, spiral electrode
arrays and
interdigitated arrays. In some preferred devices of the present invention,
electrode arrays
are fabricated on a substrate, in which the arrays comprises two electrode
structures, each
of which comprises multiple circle-on-line electrode elements, in which the
electrode
elements of one structure alternate with the electrode elements of the
opposite electrode
structure.
Preferably, the electrode elements (or electrode structures) of an array of
the
present device of the present invention are of approximately equal widths.
Preferably the
electrode elements (or electrode structures) of an array of the present device
of the
present invention are greater than 30 microns in width, more preferably from
about 50 to
about 300 microns in width, and more preferably yet about 90 microns in width.
Preferably, the electrode elements (or electrode structures) of an array of
the
present device of the present invention are approximately evenly spaced.
Preferably, the
gap between electrode elements (or electrode structures) of an array of the
present device
of the present invention is less than 50 microns in width, more preferably
from about 5 to
about 30 microns in width, and more preferably yet about 20 microns in width.
A device of the present invention can include one or more fluid-impermeable
receptacles which serve as fluid containers. Such receptacles may be
reversibly or
irreversibly attached to or formed within the substrate or portions thereof
(such as, for
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
example, wells formed as in a microtiter plate). In another example, the
device of the
present invention includes microelectrode strips reversibly or irreversibly
attached to plastic
housings that have openings that correspond to electrode structure units
located on the
microelectrode strips. Suitable fluid container materials comprise plastics,
glass, or plastic
coated materials such as ceramics, glass, metal, etc. Descriptions and
disclosure of devices
that comprise fluid containers can be found in parent U.S. Patent Application
No.
10/705,447, herein incorporated by reference for all disclosure of fluid
containers and fluid
container structures that can engage a substrate comprising electrodes for
impedance
measurements, including their dimensions, design, composition, and methods of
manufacture.
In preferred embodiments, each electrode array on the substrate of a device of
the
present invention is associated with a fluid-impermeable container or
receptacle, such as,
for example, a well. Preferably, the device of the present invention is
assembled to a
bottomless, multiwell plastic plate or strip with a fluid tight seal. The
device is
assembled such that a single array of the substrate is at the bottom of a
receptacle or well.
Preferably, each array of a device is associated with a well of a multiwell
plate. hl some
preferred embodiments, a multiwell device for cell-substrate impedance
measurement has
"non-array" wells that are attached to the substrate but not associated with
arrays. Such
wells can optionally be used for performing non-impedance based assays, or for
viewing
cells microscopically.
The design and assembly of rnultiwell impedance measurement devices is
described in parent U.S. Patent Application No. 10/705,447, and also in parent
application U.S. Patent Application No. 10/987,732, both herein incorporated
by
reference for disclosure of multiwell impedance measurement devices, including
their
design, composition, and manufacture. A device of the present invention
preferably has
between 2 and 1,536 wells, more preferably between 4 and 384 wells, and even
more
preferably, between 16 and 96 wells, all or less than all or which are
associated with
electrode arrays.
In some preferred embodiments, commercial tissue culture plates can be adapted
to fit a device of the present invention. Bottomless plates may also be custom-
made to
preferred dimensions. Preferably, well diameters are from about 1 millimeter
to about 20
31
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
millimeters, more preferably from about 2 millimeters to about 8 millimeters
at the
bottom of the well (the end disposed on the substrate). The wells can have a
uniform
diameter or can taper toward the bottom so that the diameter of the container
at the end in
contact with the substrate is smaller than the diameter of the opposing end.
Methods of Use
The present invention also includes methods of using a device of the present
invention that comprises fluid containers situated over electrode arrays to
measure cell-
substrate impedance. Such methods include: providing a device of the present
invention
that comprises fluid containers situated over electrode arrays, attaching an
impedance
analyzer to a device of the present invention, adding cells to one or more
fluid containers
of the device, and measuring impedance over one or more arrays of the device.
Methods
of performing cell assays using impedance measurement devices can be found in
parent
U.S. Patent Application No. 10/987,732 and U.S. Patent Application 10/705,447,
both
herein incorporated by reference for all disclosure of methods of using
impedance
measurement devices, as well as in Sections D and E of the present
application.
Cell-Substrate Impedance Measurement Systems
In another aspect, the present invention is directed to a cell-substrate
impedance
measurement system comprising a) at least one multiple-well cell-substrate
impedance
measuring device, in which at least two of the multiple wells comprise an
electrode array
at the bottom of the well; b) an impedance analyzer electronically connected
to the
multiple-well cell-substrate impedance measuring device; c) a device station
capable of
engaging the one or more multiple-well devices and comprising electronic
circuitry
capable of selecting and connecting electrode arrays within any of the
multiple wells to
the impedance analyzer; and d) a software program connected to the device
station and
impedance analyzer to control the device station and perform data acquisition
and data
analysis from the impedance analyzer.
In a cell-substrate impedance measurement system of the present invention, the
impedance analyzer engages connection pads of one or more multi-well devices
to
measure impedance.111 one embodiment of the above system, the impedance
analyzer is
32
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
capable of measuring impedance between 0.1 ohm and 105 ohm in frequency range
of
1Hz to 1 MHz. The impedance analyzer is preferably capable of measuring both
resistance and reactance (capacitive reactance and inductive reactance)
components of the
impedance. In a preferred embodiment of the above system, the impedance
analyzer is
capable of measuring impedance between 0.1 ohm and 103 ohm in frequency range
of
100 Hz to 100 kHz.
A cell-substrate measurement system can be used to efficiently and
simultaneously
perform multiple assays by using circuitry of the device station to digitally
switch from
recording from measuring impedance over an array in one well to measuring
impedance
over an array in another well. In one embodiment of the above system, the
system under
software control is capable of completing an impedance measurement for an
individual
well at a single frequency within less than ten seconds. In another
embodiment, the
averaged time used by the system to complete an impedance measurement for an
individual well at a single frequency is less than one second.
A multiple-well cell-substrate impedance measuring device in a system of the
present
invention can be any multiple-well cell-substrate impedance measuring device
in which
at least two of the multiple wells comprise an electrode array at the bottom
of the well,
and in which at least two of the multiple wells comprise an electrode array
are
individually addressed. In one embodiment of the above system, the mufti-well
device
takes the form of a specialized microtiter plate which has microelectronic
sensor arrays
integrated into the bottom of the wells.
A device used in a system of the present invention, when connected to an
impedance analyzer, can measure differences in impedance values that relate to
cell
behavior. For example, a cell-substrate impedance measuring device used in a
system of
the present invention can measure differences in impedance values when cells
are
attached to the electrode array and when cells are not attached to the
electrode array, or
can detect differences in impedance values when the number, type, activity,
adhesiveness, or morphology of cells attached to the electrode-comprising
surface of the
apparatus changes.
Preferred devices that can be part of a cell-substrate impedance monitoring
system can be those described in parent U.S. Patent Application No.
10/705,447, and in
33
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
U.S. Patent Application No. 10/987,732, both herein incorporated by reference
for
disclosure of cell-substrate impedance monitoring devices that comprise
electrode arrays,
including disclosure of their design, composition, and manufacture. Preferred
devices that
can be part of a cell-substrate impedance monitoring system can also be those
described
in the present application.
Preferably a multi-well device of a system of the present invention comprises
between 4 and 1,536 wells, some or all of which can comprise electrode arrays.
In some
embodiments of the present invention, a device station can comprise one or
more
platforms or one or more slots for positioning one or more multiwell devices.
The one or
more platforms or one or more slots can comprise sockets, pins or other
devices for
electrically connecting the device to the device station. The device station
preferably can
be positioned in a tissue culture incubator during cell impedance measurement
assays. It
can be electrically connected to an impedance analyzer and computer that are
preferably
located outside the tissue culture incubator.
The device station comprises electronic circuitry that can connect to an
impedance monitoring device and an impedance analyzer and electronic switches
that can
switch on and off connections to each of the two or more electrode arrays of
the
multiwell devices used in the system. The switches of the device station are
controlled by
a software program. The software program directs the device station to connect
arrays of
the device to an impedance analyzer and monitor impedance from one or more of
the
electrode arrays. During impedance monitoring, the impedance analyzer can
motitor
impedance at one frequency or at more than one frequency. Preferably,
impedance
monitoring is performed at more than one time point for a given assay, and
preferably,
impedance is monitored at at least three time points. The device station can
connect
individual arrays of a device to an impedance analyzer to monitor one, some,
or all of the
arrays of a device for a measurement time point. The switches of the device
station allow
the selected individual arrays to be monitored in rapid succession for each
desired
monitoring time point. Each monitoring time point is in fact a narrow time
frame (for
example from less than one second to minutes) of measurement in the assay
during which
impedance monitoring is performed. In some preferred embodiments of the
present
34
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
invention, the device station software is programmable to direct impedance
monitoring of
any of the wells of the device that comprise arrays at chosen time intervals.
The software of the impedance monitoring system can also store and display
data.
Data can be displayed on a screen, as printed data, or both. Preferably the
software can
allow entry and display of experimental parameters, such as descriptive
information
including cells types, compound concentrations, time intervals monitored, etc.
Preferably, the software can also analyze impedance data. In preferred
embodiments, the software can calculate a cell index (CI) for one or more time
points for
one or more wells of the multiwell device. In some preferred embodiments, the
software
can calculate a cell change index (CCI) from impedance measurements of one or
more
wells of the multiwell device. The software can preferably generate plots of
impedance
data and impedance values, such as but not limited to CI or CCI, with respect
to time.
The software may perform other analysis as well, such as calculate cell number
from CI,
generate dose-response curves based on impedance data, calculate IC values
based on
impedance values, and calculate kinetic parameters of cell growth or behavior
based on
impedance values and impedance value curves. The software of the impedance
monitoring system can also store and display analyses of the data, such as
calculated
impedance values and kinetic parameters derived therefrom, Data can be
displayed on a
screen, as printed data, or both.
C. Methods for Calculating Cell Index (CI) and Cell Change Index (CCI)
Celllradex
Based on the dependent relationship between the measured impedance, cell
number (more accurately, the viable cell number, or attached cell number) and
cell
attachment status, it is possible to derive a so-called "cell number index" or
"cell index"
from the measured impedance frequency spectra that provides a useful index for
quantitating and comparing cell behavior in the impedance-based assays of the
present
invention. In some applications of the present invention, "cell index" in the
present
application is the same as "cell number index" in PCT Application No.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
PCT/LTS03/22557,entitled "IMPEDANCE BASED DEVICES AND METHODS FOR
USE IN ASSAYS", filed on July 18, 2003 and in United States patent application
No.
10/705,447,entitled "IMPEDANCE BASED DEVICES AND METHODS FOR USE IN
ASSAYS," Attorney Docket No. ACE-OOlOI.P.l.1-US, filed on November 10, 2003,
U.S. Patent Application No. 10/987,732, filed November 12, 2004, U.S. Patent
application 10/705,447 and PCT Application No. PCT/LTS03/22557 are hereby
incorporated by reference for the discussions and disclosures of cell index
and cell
number index they contain.
Various methods for calculating such a cell number index can be used, some of
which are novel methods disclosed herein.
The present invention provides several methods of calculating cell index
numbers
for cells attached to two or more essentially identical arrays of a cell-
substrate impedance
device, where the cells are monitored for impedance changes. In preferred
embodiments
of the present invention, the methods calculate cell index number with better
accuracy
than previous methods of calculating cell index for cells on two or more
arrays of a cell-
substrate monitoring device. In some preferred methods of the present
invention, methods
of calculating a cell index rely on novel methods for calculating the
resistances of
electrical traces leading to two or more essentially identical arrays. The
present invention
therefore also includes methods of calculating resistances of electrical
traces leading to
two or more essentially identical arrays on a substrate.
By "essentially identical electrode arrays" or "essentially identical arrays"
is
meant that the dimensions and arrangement of electrodes, electrode structures,
and
electrode elements is the same for the referenced arrays. Thus, two
essentially identical
electrode arrays will have electrode structures of the same dimensions
(length, width,
thickness), where the electrode structures have the same number of electrode
elements,
and the arrangement of electrode structures and electrode elements in each
array are the
same. By arrangement is meant the distance between structures or elements (gap
width),
their physical position with respect to one another, and their geometry
(angles, degree of
curvature, circle-on-line or castellated geometries, etc.), including the same
features of
any electrode buses that may be connected to electrode structures or electrode
elements.
Electrodes of essentially identical arrays also comprise the same materials.
For the
36
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
purposes of calculating trace resistances and cell index number, a substrate
can have any
number of essentially identical arrays.
The following discussion provides novel methods of calculating cell index of
cells
adhered to arrays of a cell-substrate impedance monitoring device and novel
methods for
the calculation of the resistances of the electrical connection traces leading
to two or
more electrode arrays of a cell-substrate impedance monitoring device.
Impedance (Z) has two components, namely the resistance Rs and reactance
Xs. Mathematically, the impedance Z is expressed as follows,
Z= Rs + j Xs, (2)
where j = ~ , depicting that for the (serial) reactance component Xs, the
voltage applied over it is 90 degree phased-out from the current going through
it. For the
(serial) resistance, the voltage applied over it is in phase with the current
going through it.
As it is well-known in electronic and electrical engineering, the impedance
can also be
expressed in terms of parallel resistance Rp and parallel reactance Xp, as
follows,
z=Rp*G Xp)/(Rp+j Xp)~ (3)
where j = ~ . Nevertheless; these expressions (serial resistance and serial
reactance, or
parallel resistance and parallel reactance) are equivalent. Those who are
skilled in
electrical and electronic engineering can readily derive one form of
expression from the
parameter values in the other expression. For the sake of clarity and
consistency, the
description and discussion in the present invention utilizes the expression of
serial
resistance and serial reactaxice. For simplicity, serial resistance and serial
reactance are
simply called resistance and reactance.
As described in US patent application no. 10/705,447, entitled "Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
number PCT/LTS03/22557, entitled "Impedance based devices and methods for use
in
assays", filed on July 18, 2003, both of which are herein incorporated by
reference for
37
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
disclosures relating to cell-substrate impedance monitoring, monitoring cell-
substrate
impedance for detection or measurement of change in impedance can be done by
measuring impedance in any suitable range of frequencies. For example, the
impedance
can be measured in a frequency range from about 1 Hz to about 100 MHz. In
another
example, the impedance can be measured in a frequency range from about 100 Hz
to
about 2 MHz. The impedance is typically a function of the frequency, i.e., the
impedance
values change as frequency changes. Monitoring cell-substrate impedance can be
done
either in a single frequency or multiple frequencies. If the impedance
measurement is
performed at multiple frequencies, then a frequency-dependent impedance
spectrum is
obtained - i.e., there is an impedance value at each measured frequency. As
mentioned
above, the impedance has two components - a resistance component and a
reactance
component. A change in either resistance component or reactance component or
both
components can constitute a change in impedance.
As described in the US patent application no. 10/705,447,entitled "Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
number PCT/LTS03/22557,entitled "Impedance based devices and methods for use
in
assays", filed on July 18, 2003, herein incorporated by reference for
disclosure of
methods of measuring electrical impedance, the method for the measurement of
electrical
(or electronic) impedance is achieved by , (1) applying a voltage between or
among said
electrodes at a given frequency (or multiple frequencies, or having specific
voltage
waveform) and monitoring the electrical current through said electrodes at the
frequency
(or multiple frequencies, or having specific waveform), dividing the voltage
amplitude
value by the current amplitude value to derive the impedance value; (2)
applying an
electric current of a single frequency component (or multiple frequencies or
having
specific current wave form) through said electrodes and monitoring the voltage
resulted
between or among said electrodes at the frequency (or multiple frequencies, or
having
specific waveform), dividing the voltage amplitude value by the current
amplitude value
to derive the impedance value; (3) other methods that can measure or determine
electric
impedance. Note that in the description above of "dividing the voltage
amplitude value
by the current amplitude value to derive the impedance value", the "division"
is done for
the values of current amplitude and voltage amplitude at same frequencies. As
it is well-
38
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
known in electrical and electronic engineering, in such calculations (e.g.
divisions
mentioned above), the current amplitude and voltage amplitude are expressed in
the form
of complex numbers, which take into account of how big the current and the
voltage are
and what the phase difference between the sinusoidal waves of the current and
the
voltage is. Similarly, the impedance value is also expressed in a complex
form, having
both resistance and reactance component, as shown in equations above.
As described in the US patent application no. 10/705,447, entitled "Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
number PCT/LTS03/22557,entitled "Impedance based devices and methods for use
in
assays", filed on July 18,. 2003, both incorporated herein by reference for
disclosure
relating to Cell Index or Cell Number Index, the measured cell-substrate
impedance can
be used to calculate a parameter termed Cell Index or Cell Number Index.
Various
methods for calculating such a cell number index can be used based on the
changes in
resistance or reactance when cells are attached to the electrode structures
with respect to
the cases no cells are attached to the electrode structures. The impedance
(resistance and
reactance) of the electrode structures with no cell attached but with same
cell culture
medium over the electrode structures is sometimes referred as baseline
impedance. The
baseline impedance may be obtained by one or more of the following ways: (1)
the
impedance measured for the electrode structures with a cell-free culture
medium
introduced into the well containing the electrode structures, wherein the
culture medium
is the same as that used for the impedance measurements for the condition
where the cell
attachment is monitored; (2) the impedance measured shortly (e.g. 10 minutes)
after the
cell-containing medium was applied to the wells comprising the electrode
structures on
the well bottom (during the short period after cell-containing medium
addition, cells do
not have enough time to attach to the electrode surfaces. The length of this
short-period
may depend on cell type and/or surface treatment or modification on the
electrode
surfaces); (3) the impedance measured for the electrode structures when all
the cells in
the well were killed by certain treatment (e.g. high-temperature treatment)
and/or
reagents (e.g. detergent) (for this method to be used, the treatment and/or
reagents should
not affect the dielectric property of the medium which is over the
electrodes).
39
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In one example (A), the cell index or cell number index can be calculated by:
(Al) at each measured frequency, calculating the resistance ratio by dividing
the resistance of the electrode arrays when cells are present and/or attached
to the
electrodes by the baseline resistance,
(A2) finding or determining the maximum value in the resistance ratio over the
frequency spectrum,
(A3) and subtracting one from the maximum value in the resistance ratio.
TJsing a mathematically formula, Cell Index is derived as
Cell Index = max R~err (.f ) _ 1 (4)
r=i,z,...N Rb (. f )
Where N is the number of the frequency points at which the impedance is
measured. For
example, if the frequencies used for the measurements are at 10 kHz, 25 kHz
and 50 kHz,
then N=3, fl= 10 kHz, f2= 25 kHz, f3= 50 kHz. R~ell (.f ) is the resistance
(cell-substrate
resistance) of the electrode arrays or electrode structures when the cells are
present on the
electrodes at the frequency f and Rb ( f,. ) is the baseline resistance of the
electrode array
or structures at the frequency f .
The cell index obtained for a given well reflects: 1) how many cells are
attached to the
electrode surfaces in this well, 2) how well cells are attached to the
electrode surfaces in
the well._ In this case, a zero or near-zero "cell index or cell number index"
indicates
that no cells or very small number of cells are present on or attached to the
electrode
surfaces. In other words, if no cells are present on the electrodes, or if the
cells are not
well-attached onto the electrodes, R~efr (.f ) is about the same as R6 ( f; )
, leading to Cell
Index =0. A higher value of "cell number index" indicates that, for same type
of the
cells and cells under similar physiological conditions, more cells are
attached to the
electrode surfaces. In other words, under same physiological conditions, more
cells
attached on the electrodes, the larger the values R~err (.f ) is, leading to a
large value for
Cell Index. Thus Cell Index is a quantitative measure of cell number present
in a well.
A higher value of "cell index" may also indicate that, for same type of the
cells and same
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
number of the cells, cells are attached better (for example, cells spread out
more, or cell
adhesion to the electrode surfaces is stronger) on the electrode surfaces.
Thus, for same number of the cells present in the well, change in a cell
status will lead to
a change in cell index. For example, an increase in cell adhesion or a cell
spread leading
to large cell/electrode contact area will result in an increase in R~~rr ( f )
and a larger Cell
Index. On the other hand, a cell death or toxicity induced cell detachment,
cell rounding
up, will lead to smaller R~e« ( f ) and thus smaller Cell Index.
In another example (B), the cell number index can be calculated by:
(B1) at each measured frequency, calculating the reactance ratio by dividing
the
reactance of the electrode arrays when cells are present on and/or attached to
the
electrodes by the baseline reactance,
(B2) finding or determining the maximum value in the reactance ratio over the
frequency spectrum,
(B3) and subtracting one from the maximum value in the resistance ratio.
In this case, a zero or near-zero "cell number index" indicates that no cells
or
very small number of cells are present on or attached to the electrode
surfaces. A higher
value of "cell number index" indicates that, for same type of the cells and
cells under
similar physiological conditions, more cells are attached to the electrode
surfaces.
In yet another example (C), the cell index can be calculated by:
(C1) at each measured frequency, subtracting the baseline resistance from the
resistance of the electrode arrays when cells are present or attached to the
electrodes
to determine the change in the resistance with the cells present relative to
the baseline
resistance;
(C2) then fording or determining the maximum value in the change of the
resistance.
41
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In this case, "cell-number index" is derived based on the maximum change in
the resistance across the measured frequency range with the cells present
relative to the
baseline resistance. This cell index would have a dimension of ohm.
In yet another example (D), the cell index can be calculated by:
(D1) at each measured frequency, calculating the magnitude of the impedance
(equaling to RSz +XSz , where RS and XS are the serial resistance and
reactance,
respectively).
(D2) subtracting the magnitude of the baseline impedance from the magnitude of
the
impedance of the electrode arrays when cells are present or attached to the
electrodes
to determine the change in the magnitude of the impedance with the cells
present
relative to the baseline impedance;
(D3) then finding or determining the maximum value in the change of the
magnitude
of the impedance.
In this case, "cell-number index" is derived based on the maximum change in
the magnitude of the impedance across the measured frequency range with the
cells
present relative to the baseline impedance. This cell index would have a
dimension of
ohm.
In yet another example (E), the index can be calculated by:
(E1) at each measured frequency, calculating the resistance ratio by dividing
the
resistance of electrode arrays when cells are present or attached to the
electrodes by
the baseline resistance,
(E2) then calculating the relative change in resistance in each measured
frequency by
subtracting one from the resistance ratio,
(E3) then integrating all the relative-change value (i.e., surntning together
all the
relative-change values at different frequencies).
In this case, "cell-number index" is derived based on multiple-frequency
points, instead of single peak-frequency lilce above examples. Again, a zero
or near-zero
"cell number index" indicates that on cells are present on the electrodes. A
higher value
of "cell number index" indicates that, for same type of the cells and cells
under similar
physiological conditions, more cells are attached to the electrodes.
42
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In yet another example (F), the cell index can be calculated by:
(Fl) at each measured frequency, subtracting the baseline resistance from the
resistance of the electrode arrays when cells are attached to the electrodes
to
determine the change in the resistance with the cells present relative to the
baseline
impedance; (here the change in the resistance is given by
~(.f ) - Rs-cell (.f ) - Rs-baseline (.f ) for the frequency f , RS-cell ~d Rs-
bnseliue ~e the
serial resistances with the cells present on the electrode array and the
baseline serial
resistances, respectively);
(F3) analyzing the frequency dependency of the change of the resistance to
derive
certain parameters that can quantify such dependency. In one example, such
can be calculated as ~IOR( f,.)I . The parameters) are used as cell index or
cell
a
number index.
In this case, "cell-number index" is derived based on the analysis of the
frequency spectrum of the change in the resistance. Depending how the
parameters are
calculated, the cell index may have a dimension of ohm.
In yet another example (G), the cell index can be calculated by:
(G1) at each measured frequency, calculating the magnitude of the impedance
(equaling to RSZ +X52 , where RS and XS are the serial resistance and
reactance,
respectively).
(G2) subtracting the magnitude of the baseline impedance from the magnitude of
the
impedance of the electrode arrays when cells are attached to the electrodes to
determine the change in the magnitude of the impedance with the cells present
relative to the baseline impedance; (here, the change in the magnitude of the
impedance is given bplZ( f ) = I Z~ell (.f )I - Zbaseliue (J i )I for the
frequency f ,
parameters can be calculated as ~ ~OR( f )~2 . In another example, such
parameter
r
43
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Zcelf (f )~ - V Rs-cell (J i )Z + Xs_cell (J i )Z ~ Rs-cell ~d X s-cell being
the serial reSlStanCe
and reactance with the cells present on the electrode arrays, respectively,
Z~ell(.f )I is
the magnitude of the impedance of the electrode array with cells present on
the
electrode arrays, I Zbnselir:e (~ )I is the magnitude of the baseline
impedance of the
electrode array);
(G3) analyzing the frequency dependency of the change of the magnitude of the
impedance to derive certain parameters that can quantify such dependency. In
one
example, such parameters can be calculated as ~ ~tlZ( f,.)~z . In another
example,
1
such parameter can be calculated as ~ (4Z( fl )I . The ~parameter(s) are used
as cell
1
index or cell number index.
In this case, "cell-number index" is derived based on the analysis of the
frequency spectrum of the change in the magnitude of the impedance. Depending
how
the parameters are calculated, the cell index may have a dimension of ohm.
~15 As described in the US patent application no. 10/705,447, entitled
"Impedance
based devices and methods for use in assays", filed on November 10, 2003 and
PCT
application number PCT/IJS03/22557,entitled "Impedance based devices and
methods
for use in assays", filed on July 18,. 2003, and U.S. Patent Application No.
10/987,732,
all herein incorporated by reference for disclosure of Cell Index or Cell
Number Index
and its calculation, there are different methods for calculating the parameter
termed Cell
Index or Cell Number Index from the measured cell-substrate impedance
(resistance or
reactance). Cell Index or Cell Number Index is a quantitative measure of cells
in the
wells under cell-substrate impedance measurement.
It is worthwhile to point out that it is not necessary to derive such a "cell
number
index" for utilizing the impedance information for monitoring cell conditions
over the
electrodes. Actually, one may choose to directly use measured impedance (e.g.,
at a
single fixed frequency; or at a maximum relative-change frequency, or at
multiple
frequencies) as an indicator of cell conditions. If measured impedance values
are directly
44
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
used for monitoring cell conditions, then resistance, or reactance or both
resistance and
reactance can be used.
Still, deriving "cell index" or "cell number index" and using such index to
monitor cell conditions may have advantages. There are several advantages of
using
"cell number index" to monitor cell growth and/or attachment and/or viability
conditions.
First, one can compare the performance of different electrode geometries by
utilizing such cell number index.
Secondly, for a given electrode geometry, it is possible to construct
"calibration
curve" for depicting the relationship between the cell number and the cell
number index
by performing impedance measurements for different number of cells added to
the
electrodes (in such an experiment, it is important to make sure that the
seeded cells have
well-attached to the electrode surfaces). With such a calibration curve, when
a new
impedance measurement is performed, it is then possible to estimate cell
number from the
newly-measured cell number index.
Thirdly, cell number index can also be used to compare different surface
conditions. For the same electrode geometry and same number of cells, a
surface
treatment given a larger cell number index indicates a better attachment for
the cells to
the electrode surface and/or better surface for cell attaclunent.
As shown above, for some methods of calculating cell index or cell number
index,
it is important to know the impedance (resistance and/or reactance) of the
electrode
structures with and without cells present on them. Based on the equation (1),
the
impedance of the electrode array (with or without cells present on the
electrodes) is given
by
zelectrode-arrny= ztotnl - Ztrnce - switch
Where Zswuel, is the impedance of electronic switch at its on stage, Zt,.nee
is the
impedance of the electrical connection traces (or electrical conductive
traces) on the
substrate between the connection pads and the electrode buses, Ztotnt is the
total
impedance measured at the impedance analyzer. By choosing electronic switches
with
good quality, it is possible to have all the electronic switches have a
consistent on-
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
impedance (mainly resistance). For example, the on-resistance of electronic
switches can
be about 3 ohm (+/- 10%) with the on reactance being negligible (for example,
less than
0.2 ohm in the frequency range of interest). Thus, if the trace impedance is
determined or
calculated, then formula (5) can be used to calculate the impedance of the
electrode
arrays with or without cells present.
A method is invented in the present application to determine the impedance of
electrical conductive (electrical connection) traces (mainly trace resistance,
trace
reactance is very small for the thin conductive film trace) based on the
relationships
among two or more essentially identical arrays on a cell-substrate impedance
monitoring
device. In the following, the four electrode arrays A, B, C and D as indicated
in Figure 1,
are used to illustrate this method. The electrical reactance (serial
reactance) of the
electronic switches and the electrical reactance (serial reactance) of the
electrical
connection traces are small as compared with the corresponding electrical
resistances
(serial resistances). Thus, we focus on the analysis of the resistance of the
electrical
connection traces. The impedance determined from the impedance analyzer does
contain
both resistance (serial resistance, Rtorai ) and reactance (serial reactance).
For the
electrode arrays A - D, the measured total resistance RtOtal , the resistance
( R~.a~e ) of
electrical conductive (connection) trace, the switch resistance ( RSwitch )
and the resistance
( Re-array ) of the electrode array satisfy the following equations:
Re_a,.ray-~ = Rtotal-A - Rtra~e-A - Rswuch-A (6A)
Re-array-B = Rtotaf-B - Rtrace-B - Rswitch-s (6B)
Re-array-c = Rtotal-a - Rtrace-c - Rswitcls-c (6C)
Re-array-D - Rtotal-D Rtrnce-D Rswitch-D (6D)
With chosen electronic switches having consistent switch-on resistance,
Rs,~ir~,,-A ,
Rswrt~n-a ~ Rs,t,rt~n-c and Rsw,t~l,-D have very similar values and can be
assluned to be the
same, RS~VItCh ~ Thus, in above equations, the known parameters are Rtotal-A ~
Rtotai-s ~
Rtotar-c ~ and Rtotal-D ~ and Rs,~;t~n-,~ , Rsw,re,,-a , Rsw,r~l,-c and
Rs,vitcn-D ~ and there are eight
46
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
unkrlOWn parameters Re_nrrny-A ~ Re-nrrny-B ~ Re-nrrny-C ~ ~d Re-nrrny-D ~ and
Rtrnce-A, Rtrace-B ~
Rtrn~e-c ~d Rtrnce-D ~ It is impossible to solve these equations for the eight
unknown
variables from these four equations directly. Additional relationships between
these
variables are needed to solve for them. Each trace resistance (Rtrnce-A,
Rtrnce-B
Rtrnce-c and Rtrnce-D ) depends on the metal film type used, and the geometry
of the trace
such as the how many rectangular segments the trace has, the film
thickness(es) of the
segments, the widths) of the segments, the lengths) of the segment(s). For
example,
__ N LA-i
Rtrace-A ~ p ~ 7
i=1 tA-i dA-i
where N is the number of the segments of the trace-A, tA_Z, dA-t and LA_t is
the thickness,
width and length of the i-th segment of the traces for the electrode array A,
and p is the
resistivity of the thin film. The equation here applies to the film comprising
a single type
of metal. The equation can be readily modified to be applicable to the film
comprising
two or more metal types (e.g. gold film over chromium adhesion layer).
If the film thickness is reasonably uniform (for example, less than 10% in
thickness variation) across the substrate, then the relationship among the
trace resistances
is simply determined by the pre-determined geometrical shapes (e.g. the
length, width of
the segments). For example, it would be straightforward to calculate the ratio
aA_D
between the resistance of the electrically conductive traces for the electrode
array A to
the resistance of the electrically conductive traces for the electrode array D
as below,
where the film thickness is assumed to be the same everywhere on these traces
and the
resistivity is also the same everywhere on these traces,
N LA-i N LA_i
_ _ _ ~ ~ ~ d A-i
Rtrnce A i=1 tA-i d A-i _
aA D Rtrnce-D ~ p LD-i LD-i
i-1 tD-i dD_i i=1 dD_i
Similarly, one can determine the ratio aB_D and ac_D based on the pre-
determined
geometrical relationships for the traces of the electrode arrays B, C and D.
Note that
47
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
above equations can be similarly derived for the cases where the thin film in
these traces
comprises more than one metal type. Thus, based on the equalities
Rswitch-A - Rswilch-B Rswitcd-C Rswitch-D Rswitch ~ (9A)
Rtra~e-A = aA-D ' Rtrace-D ~ (9B)
Rtrace-B aB-D ' Rtrace-D ~
and Rtrace-c = ac-v ' Rtra~e-D (9D)
equations (6A)-(6D) can be re-written in the following format:
to Re-array-A - Rtotal-A aA-D ' Rtrace-D Rswitch (10A)
Re-array-B Rtotal-B aB-D ' Rtrace-D Rswitch 10B
Re-nrray-C - Rtotal-C aC-D ' Rtrace-D Rswitch 1 ~C'
Re-arrny-D - Rtotnl-D Rtrnce-D Rswitch-D (10D)
For equations (10A) through (10D), there are five unknown variables, Re-array-
A,
Re-array-B ~ Re-array-c ~ ~d Re-array-D and Rtra~e-D . Mathematically, these
unknown
variables cannot be determined from these equations. Additional information is
needed
to solve for these variables Re-army-A ~ Re-arrny-B ~ Re-array-C ~ ~d Re-array-
D ~d Rtrnce-D
One approach is invented and described in the present invention. In this
approach, same biological or chemical solutions or suspensions are applied to
the
electrode-arrays A through D. Because the electrode arrays A through D have
essentially
identical electrode structures, the electrode array resistances Re-array-A ~
Re-array-B ~
Re-array-c ~d Re-array-D should be of same, or very similar value for such a
condition
when all the electrode arrays are exposed to the same biological or chemical
solutions or
suspensions, i.e.: Re_array-A ~ Re-array-a ~ Re-array-c ~ Re-array-D . If we
assume the averaged
electrode array resistance is Re-array , then these approximate relationship
exists
48
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Re-array-A ~ Re-array-B 'v Re-array-C 'v Re-array-D "~ Re-array . Thus,
equations (1 OA -10D) Can
be changed to the following:
Re-array ~ Rtotal-A - aA-D ' Rtrace-D - Rswitch (11A)
Re-array ~ Rtotal-B - aB-D ' Rtraee-D - Rs,~~it~lr (11B)
Jr Re-arrny ~ Rlotal-C aC-D ' Rtrace-D Rswitrlr (1 1 C)
Re-array 'v Rtotal-D Rtrnce-D Rswitch-D (11D)
Thus, we would need to find Rtraee-D and Re-array that satisfy the above
approximate equality as close as possible. One mathematical approach is to
find Rtrace-D
and Re_array that would result in the minimum value for the following
expression - an
expression that quantifies the differences between the two sides of the
approximate
equality in ( 11 A, 11 B, 11 C and 11 D),
~ ( 2
F(Rtrace-D ~ Re-array ) [,Re-array \Rtotal-A aA-DRtrace-D Rswitch ~~
r r 2
LRe-array \Rtotal-B aB-DRtrace-D Rswitch ~~
l 2
[Re-array lRtotal-C aC-DRtrace-D Rswitch ~~
l 2
LRe-array \Rtotal-D Rtrace-D Rswitch ~~ 12
The expression F(Rt,.a~e-D , Re-array ) is the sum of the squared-differences
between the
two-sides of the approximate equality in (11A, 11B, 11C and 11D). The smaller
F(Rtraee-D,Re-array ), the closer the two sides of the approximate equality
(11A, 11B, 11C
and 11D). Thus, values of Rtrace-D and Re-arr~y that result in the minimum
value of
F(Rtrace-D , Re-arr~r,, ) should be determined. Mathematical approach involves
in the
calculation of the first order derivative of F(Rtrace-D , Re-array ) to Rhnee-
D and to Re-array
and let such first order derivatives equal to zero. The values of Rtrace-D and
Re-array that
result in zero for these first-order-derivatives are those that result in the
minimum value
of F(Rtrace-D , Re-array ) . The first order derivatives are as follows:
49
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
a~F(Rh.ace-D,Re-aaray )~ _ _( _ _
- 2' aA-D ' [Re-array \Rtotal-A aA-DRtrace-D Rswitch ~~+
a Rtrace-D
2' aB-D ' lRe-array lRtotal-B aB-DRtrace-D Rswitch ~~ +
2' aC-D ' LRe-army \Rtotal-C aC-DRtrace-D Rswitch ~~ +
2' LRe-array \Rtotal-D Rtrace-D Rswitch ~~
=0; (13A)
a~F(Rtrace-D,Re-aaray)~ _( _ _
= 2' [Re-array \Rtotal-A aA-DRtrnce-D Rswitcl, ~~+
(~ Re-a~.ray
2' LRe_array \Rtotal-B aB-DRtrace-D Rswitch ~~ +
2' ~Re-array lRtotal-C aC-DRtrace-D Rswitch ~~ +
2' lRe-arrny lRtotal-D Rh~ace-D Rswitch ~~
=0. (13B)
Equations (13A) and (13B) can be re-written as
1 S Re-arrny ' LaA-D -~- aB-D ~- GEC-D -~- 1,'f Rtrace-D ' ~,aA-DZ + GAB-DZ '-
~- GEC-DZ -f- 1 J=
aA-D ' LRtotal-A Rswitch ~+ aB-D ' LRtotal-B Rswitch ~+
aC-D ' LRtotal-c - Rswitch ~+ LRtotal-D - Rswitch ~ (14A)
4' Re-army + Rtrace-D ' LaA-D + aB-D + aC-D + 1]=
LRtotal-A - Rswitcl, J + LRtotal-B - Rs,ritch J+ LRtotal-c - Rswitch J +
LRtotal-D - Rswitch J
( 14B)
Thus, we can solve for Rtraee-D as follows:
50
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
4' si - Au ' Sz
Rtra~e-D = (15)
4'Aiz -A~i'B~z
where Al l = LaA-D + as-D + ac-D + 1~ ;
z z
Alz = ~aA-D +. aB-D + ac_D + 1 ;
S Sl = aA-D ~ LRtotal-A Rswitch J+ aB-D ~ LRtotal-B Rswitch J+
aC-D ' LRtotal-C Rswitch J+ LRtotal-D Rswitch J ~
Blz = LaA-D -~- (~',B-D + aC-D + 1] a
sz = LRtotal-A Rswitcli J+ LRtotal-B Rswitch J+LRtotal-C Rswitch J+ LRtotal-D
Rswitch J'
Thus, with the determined Rtrace-D , the trace resistances of Rtrace-A, Rtrace-
B , and Rra~~-c
can be calculated using equations (9B), (9C) and (9D). Furthermore, the
electrode array
resistance Re-arrny-A ~ Re-array-B ~ Re-array-C ~d Re-army-D c~ be calculated
from the
measured resistance Rtotal-~ ~ Rtatal-B ~ Rtotal-c and Rtotal-D respectively
using equations
(10A), (10B), (10C) and (10D).
Thus, one aspect of the present invention is directed to a method of
calculation of
the resistances of the electrical connection traces s from the measured, total
resistances
for two or more essentially identical electrode arrays (such as, for example
arrays A-D in
Figure 1), comprising the following steps:
(1) exposing the electrode arrays to the solutions having same or similar
solutions or suspensions;
(2) with an impedance analyzer or impedance measurement circuit, measuring
the resistance (serial resistance) for each electrode array, such resistance
being the sum of the resistance of electronic switches, the resistance of the
electrical connection traces between the connection pads and the electrode
~5 structures (for example, between the connection pads and the electrode
buses, for the electrode structures in Figure 1), and the resistance of the
electrode array with the solutions or suspensions present;
51
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
(3) solving for the resistances of electrical connection traces using equation
(15) and equations (9B), (9C) and (9D), noting in the calculation with
equation (15), the geometrical relationships between the electrode arrays
are used to determine the factor aA_~ , aa-D and ac-D
Another aspect of the present invention is directed to a method of calculating
the
resistance of the electrode arrays from the measured, total electrode
resistances for two ro
more essentially identical electrode arrays (such as, for example arrays A-D
in Figure 1)
if the same or similar solutions or suspensions are added to be in contact
with the
electrode assays, comprising the following steps:
(1) exposing the electrode arrays to the solutions having same or similar
solutions or suspensions;
(2) with an impedance analyzer or impedance measurement circuit, measuring
a the resistance (serial resistance) for each electrode array, such resistance
being the sum of the resistance of electronic switches, the resistance of the
electrical connection traces between the connection pads and the electrode
structures (for example, between the connection pads and the electrode
buses, for the electrode structures in Figure 1) and the resistance of the
electrode arrays with the solutions or suspensions present;
(3) solving for the resistances of electrical connection traces using equation
(15) and equations (9B), (9C) and (9D), noting in the calculation with
equation (15), the geometrical relationships between the electrode arrays
are used to determine the factor a~-D , aB-D and ac-D ;
(4) calculating the resistances of the electrode arrays using equations (10A,
10B, lOC and 10D) ).
In many applications, the solutions or suspensions (for example, cell
suspension)
applied to each electrode array may have different compositions. For example,
cell
suspensions of different cell numbers may be used so that the suspensions
applied to each
electrode array are quite different. Under such cases, the determination of
the resistance
52
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
of the electrode arrays,with the cells present would require the determination
of the
resistance of the electrical connection traces by performing a "reference run"
or
"calibration run" in which the electrode arrays are exposed to a same,
reference solution.
From the "reference run", the resistances of the electrical connection traces
can be
determined. In a separate test, the electrode arrays are exposed to the
solutions or cell
suspensions of interest and the resistances for the electrode arrays under
such conditions
are measured with an impedance analyzer or impedance measuring circuit. The
resistance of the electrode arrays with such cell suspensions present can be
determined
(or continuously determined) from the measured resistance by subtracting the
sum of the
resistance of the electronic switches and the resistance of the electrical
connection traces
for corresponding electrode arrays from the measured resistances.
Thus, another aspect of the present invention is directed to a method of
calculating the resistance of the electrode arrays from the total electrical
resistances
measured at an impedance analyzer for essentially identical electrode arrays
(such as
electrode arrays A-D in Figure 1 used as an example) if different solutions or
suspensions of interest are applied to the electrode assays, comprising the
following
steps:
(1) exposing the electrode arrays to the solutions having same or similar
solutions
or suspensions (reference solutions);
(2) with an impedance analyzer or impedance measurement circuit, measuring the
resistance (serial resistance) for each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
connection traces between the connection pads and the electrode structures
(for example, between the connection pads and the electrode buses, for the
electrode structures in Figure 1) and the resistance of the electrode arrays
with the reference solutions present;
(3) solving for the resistances of electrical connection traces using equation
(15)
and equations (9B), (9C) and (9D), noting in the calculation with equation
(15), the geometrical relationships between the electrode arrays of Figure 1
are used to determine the factor aA_D , aB-D and ac-D ;
53
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
(4) applying the solutions or suspensions of interest to each electrode array;
and
with an impedance analyzer or impedance measurement circuit, measuring the
resistance (serial resistance) of each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
connection traces between the connection pads and the electrode structures,
the resistance of the electrode arrays with the solutions or suspensions of
the
interest present,
(5) Calculating the resistance of the electrode arrays using equations (10A),
(1 OB), (10C) and (10D) by subtracting the electronic switch resistances and
the resistances of electrical connection traces from the measured resistances
in
the step (4).
Note that in above method, the steps of exposing the electrode arrays to
reference
solutions for the determination of the resistances of electrically conductive
traces (step
(1), (2) and (3)) may be performed before or after the steps of applying the
solutions or
suspensions of interest to the electrode arrays and measuring the total
electrical resistance
(step (4)). For example, step (4) may be performed first. After that, the
solutions or
suspensions of the interest may be removed from the electrode array. The
reference
solutions can then be added to the electrode arrays (step (1)). Step (2) and
step (3) can be
then performed to determine the resistances of electrical connection traces.
Finally, Step
(5) can be done.
In another approach, step (1) and (2) can be performed ahead of step (4).
Another aspect of the present invention is directed to a method of determining
the
resistance of the electrode arrays with the cells present for a cell-based
assay based on the
total electrical resistance measured at an impedance analyzer for essentially
identical
electrode arrays. In this method, the electrode arrays are exposed to a same,
reference
solution (for example, a same cell culture medium that does not contain any
cells) and
electrical measurement is conducted to determine the resistance of electrical
connection
traces. With the resistances of the electrical connection traces determined,
electrical
resistances of the electrode arrays with cell suspensions added to electrode
arrays can be
calculated from the total electrical resistances measured at an impedance
analyzer. Such
54
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
total electrical resistance would include the resistance of the electrode
arrays with cells
present, the resistance of electronic switches and the resistance of
electrical connection
traces. The method comprises following steps
(1) exposing the electrode arrays to the solutions having same or similar
solutions
or suspensions (reference solutions);
(2) with an impedance analyzer or impedance measurement circuit, measuring the
resistance (serial resistance) for each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
connection traces between the connection pads and the electrode structures
(for example, between the connection pads and the electrode buses, for the
electrode structures in Figure 1) and the resistance of the electrode arrays
with the reference solutions present;
(3) solving for the resistances of electrical connection traces using equation
(15)
and equations (9B), (9C) and (9D), noting in the calculation with equation
(15), the geometrical relationships between the electrode arrays in Figure 1
are used to determine the factor aA_~ , aB_D and a~_D ;
(4) applying the cell suspensions of interest to each electrode array; and
with an
impedance analyzer or impedance measurement circuit, measuring the
resistance (serial resistance) of each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
connection traces between the connection pads and the electrode structures,
the resistance of the electrode arrays with the cell suspensions of the
interest
present,
(5) Calculating the resistance of the electrode arrays using equations (10A),
(10B), (10C) and (10D) by subtracting the electronic switch resistances and
the resistances of electrical connection traces from the measured resistances
in
step (4).
Note that in above method, the steps of exposing the electrode arrays to
reference
solution for the determination of the electrical resistance of electrically
conductive traces
(step (1), (2) and (3)) may be performed before or after the steps of applying
the solutions
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
of interest or cell suspensions of interest to the electrode arrays and
measuring the total
electrical resistance (step (4)). For example, step (4) may be performed
first, followed by
steps (1) and (2). In one approach, after step (4), the cell suspensions of
the interest may
be removed from the electrode array. Then reference solutions can be added to
the
electrode arrays. In another approach, after step (4), the cells are all lysed
with some cell
lysis solutions so that the electrodes are exposed to the same, reference
solutions for the
measurement and calculation of step (2) and (3). And then, step (5) is
performed to
determine the electrical resistance of electrode arrays with the cell
suspensions of interest
present.
The determination of the resistances of the electrical conductive traces for
the
electrode arrays that essentially identical electrode arrays may be, or may
not be, part of
the monitoring of cell-substrate impedance for cell-based assays. It depends
on how the
impedance data (measured at a single frequency or multiple frequencies,
measured at
multiple time points) of the electrode arrays is analyzed.
In some assays, one is interested in the relative change in the resistance or
impedance of the electrode arrays with the cells present relative to the
baseline resistance
or impedance. For such cases, it is preferred to determine the resistance (or
impedance)
of the electrode arrays from the total, measures resistance (or impedance) by
subtracting
the resistance of the electrical conductive traces and the resistance of
electronic switches.
Thus, determination of the resistances or impedance of the electrically
conductive traces
may be required.
In some other assays, one is interested in the absolute changes in the
resistance (or
impedance) of the electrode arrays with cells present relative to the baseline
resistance (or
impedance). In these cases, one can directly subtract the measured resistance
or
impedance for the baseline condition from the measured resistance or impedance
for the
condition that the cells are present on the electrode arrays. The contribution
of the
resistance (or impedance) of the electronic switches and the resistance (or
impedance) of
the electrically conductive traces to the total measured resistance (or
impedance) values is
cancelled out in such subtractions. Thus, there is no need for determining the
resistances
of the electrically conductive traces.
56
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In some assays, one is interested in calculating the Cell Index or Cell Number
Index based on the monitored impedance values. Depending on which method is
used
for calculating the Cell Index, it may, or may not, be necessary to determine
the
resistances of the electrically conductive traces. For example, for the Cell
Index
calculation method (A) described above, the resistances of the electrically
conductive
traces are needed, in order to remove the effect of the resistance of the
electrically
conductive traces on the analysis of the relative change of the resistance or
impedance.
In another example, for the Cell Index calculation method (F) described above,
there is
no need to determine the resistances of the electrically conductive traces
since the effect
of the resistance of the electrically conductive traces is canceled out in the
calculations.
.The monitoring of the cell-substrate impedance may be or may not be based on
the change with respect to the baseline impedance (or resistance). For
example, a cell-
based assay is performed to assess the effect of a test compound on the cells.
One
method in performing such an assay is by monitoring of the cell-substrate
impedance and
determining the change in the cell-substrate impedance before and after the
addition of
the test compound to the cells. The monitoring of cell-substrate impedance can
be
performed at a single frequency point or multiple frequency points, at a
single time point
or multiple time points after drug addition. For example, the impedance is
first measured
at a single frequency or multiple frequencies for the electrode arrays with
the cells
present just before addition of test compound. The test compound is then added
to the
cells. The impedance is then measured again at the same single frequency or
multiple
frequencies for the electrode arrays with the cells after the addition of test
compound.
Such post-compound addition measurement may be performed for many time points
continuously in a regular or irregular time intervals. The change in the cell-
substrate
impedances can be determined or quantified by subtracting the impedance(s)
(resistance
and/or reactance) measured before addition of the test compound from the
impedance(s)
(resistance and/or reactance) measured after addition of the test compound. If
the
measurement is done at multiple frequencies, a single parameter or multiple
parameters
may be further derived for each time point after compound addition based on
the
calculated change in the cell-substrate impedances. Such parameters are used
to quantify
the cell changes after compound addition. Such approaches can be used further
to
57
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
analyze the responses of the cells to a test compound at multiple
concentrations to derive
dose-dependent response curves.
Normalized Cell Index, Delta Cell Index
A "Normalized Cell Index" at a given time point is calculated by dividing the
Cell
Index at the time point by the Cell Index at a reference time point. Thus, the
Normalized
Cell Index is 1 at the reference time point. Normalized cell index is cell
index normalized
against cell index at a particular time point. In most cases in the present
applications,
normalized cell index is derived as normalized relative to the time point
immediately
before a compound addition or treatment. Thus, normalized cell index at such
time point
(immediately before compound addition) is always unit one for all wells. One
possible
benefit for using such normalized cell index is to remove the effect from
difference in
cell number in different wells. A well having more cells may produce a larger
impedance
response following compound treatment. Using normalized cell index, it helps
to remove
such variations caused by different cell numbers.
A "delta cell index" at a given time point is calculated by subtracting the
cell
index at a standard time point from the cell index at the given time point.
Thus, the delta
cell index is the absolute change in the cell index from an initial time (the
standard time
point) to the measurement time.
Cell Change Index
The time-dependent cellular response (including cytotoxicity response) may be
analyzed by deriving parameters that directly reflect the changes in cell
status. For
example, time dependent celluler response may be analyzed by calculating the
slope of
change in the measured impedance responses (that is equivalent to the first
order
derivative of the impedance response with respect to time, impedance response
here can
be measured impedance data or derived values such as cell index, normalized
cell index
or delta cell index). In another example, the time-dependent cellular
responses
(including cytotoxicresposnes) responses may be analyzed for their higher
order
derivatives with respect to time. Such high order derivatives may provide
additional
58
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
information as for how cells responding to different compounds and as for the
mechanisms of compound action.
As an example, we describe how one can to derive a parameter, called Cell
Change Index, based on the real time, quantitative information (i.e., cell
index, CI) about
biological status of cells in the wells provided from RT-CES system. This new
parameter, Cell Change Index (CCI), can effectively link time dependent cell
index I with
cell status, is calculated as,
CCI(t) = dCl(t) . (5)
CI(t) ~ dt
Thus CCI is the normalized rate of change in cell index. CCI values can be
used to
quantify the cell status change. For cells in an exponential growth under
regular cell
culture condition, the cell index determined by a cell-substrate impedance
monitoring
system described herein is expected to be a proportionate measure of the cell
number in
the well since the cell morphology and average extent of cell adhesion to the
electrode
surfaces among the whole cell population do not exhibit significant changes
over time.
Thus, the cell index (C1] increase with time following an exponential
function, such that
_t
CI (t) = CI (0) * 2 DT (6)
where DT is the cell doubling time. For such exponential growth culture,
CCI(t) is a
constant, giving
0.693 0.7
CCI (t) _ ~ (7)
DT N DT
Thus, several types of CCI(t) can be classified as:
(1) If CCI is about 0.7/DT, cell index increases in the same rate as that
expected
for an exponential growth of the cells.
59
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
(2) If CCI » 0.7/DT, cell index increases faster than that expected for an
exponential growth (or log growth) of the cells. This indicates that cells may
grow faster than regular exponential growth, or cells may exhibit some
morphology change (e.g. cell spreading out or adhering better to the electrode
surfaces), leading to large impedance signal, or both of above effects, or
there
may be other cell behaviors occurnng particular to the assay or culture
conditions.
(3) If CCI is more than zero but somewhat smaller than 0.7/DT, then cell index
increases in the rate slowed than that expected for an exponential growth.
This indicates that cell growth rate may be slowed down relative to
exponential growth, or cell growth may be somewhat inhibited by chemical
compounds added to the culture media or by other cell culture parameters, or
that certain populations of cells are dying off and detaching from the
electrode surfaces, or there may be other cell behaviors occurnng particular
to
the assay or culture conditions.
(4) If CCI is about zero, then cell index shows a near constant value. This
may
indicate that the cell growth is nearly-completely inhibited. For example, all
the cells axe arrested at certain points of cell cycle and are not progressing
further. Or, this may indicate that the number of cells dying off in the
culture is nearly as the number of newly-divided cells. Alternatively this may
indicate that cells reach stationary phase of cell culture. Alternatively this
may indicate that number of cells are above the detection upper limit of the
cell-substrate impedance monitoring system. There is also the possibility of
other cell behaviors occurring particular to the assay or culture conditions.
(5) If CCI is negative, then the cell index is decreasing with time, showing
the
cells losing attachment to the electrode surface or changing their morphology.
(6) If CCI is very negative, then the cell index decreases rapidly with time,
showing that either cells lose attachment to the electrode surfaces quickly or
cells change their morphology very quickly.
60
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
D. Methods for performing real-time cell-based assays
The present invention provide cell-based assays that can be performed in real
time
to assess cell proliferation, cell growth, cell death, cell morphology, cell
membrane
properties (for example, size, morphology, or composition of the cell
membrane) cell
adhesion, and cell motility. Thus the assays can be cytotoxicity assays,
proliferation
assays, apoptosis assays, cell adhesion assays, cell activation or stimulation
assays, anti-
cancer compound efficacy assays, receptor-ligand binding or signal
transduction analysis,
assays of cytoskeletal changes, assays of cell structural changes (including
but not limited
to, changes in cell membrane size, morphology, or composition), cell
quantification, cell
quality control, time-dependent cytotoxicity profiling, assays of cell
differentiation or de-
differentiation, detection or quantitation of neutralizing antibodies,
specific T-cell
mediated cytotoxic effect assays, assays of cell adhesivity, assays of cell-
cell interactions,
analysis of microbial, viral, or environmental toxins, etc.
The assays are real-time assays in the sense that cell behavior or cell status
being
assayed can be assessed continuously at regular or irregular intervals. Cell
behaviors, cell
responses, or cell status can be assayed and the results recorded or displayed
within
seconds to minutes of their occurrence. The cell response during an assay can
be
monitored essentially continuously over a selected time period. For example, a
culture
can be monitored every five to fifteen minutes for several hours to several
days after
addition of a reagent. The interval between impedance monitoring, whether
impedance
monitoring is performed at regular or irregular intervals, and the duration of
the
impedance monitoring assay can be determined by the experimenter.
Thus, the cell-based impedance assays of the present invention avoid
inadvertently biased or misleading evaluation of cell responses due to the
time point or
time points chosen for sampling or assaying the cells. In addition, the assays
do not
require sampling of cell cultures or addition of reagents and thus eliminate
the
inconvenience, delay in obtaining results, and error introduced by many
assays.
Descriptions of cell-substrate monitoring and associated devices, systems and
methods of use have been provided in United States provisional application
number
60/379,749, filed on July 20, 2002; United States provisional application
number
601435,400, filed on December 20, 2002; United States Provisional application
61
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
601469,572, filed on May 9, 2003, PCT application number
PCT/LJS03/22557,entitled
"Impedance based devices and methods for use in assays", filed on July 18,
2003; PCT
application number PCT/US03/22537,entitled "Impedance based apparatuses and
methods for analyzing cells and particles", filed on July 18, 2003; United
States patent
application number 10/705,447,entitled "Impedance based devices and methods
for use in
assays", filed on November 10, 2003; U.S. Patent Application No. 10/987,732
United
States patent application numberl0/705,615,entitled "Impedance based
apparatuses and
methods for analyzing cells and particles", filed on November 10, 2003, all
incorporated
herein by reference for their disclosure of cell-substrate impedance devices,
systems, and
methods of use. Additional details of cell-substrate impedance monitoring
technology is
further disclosed in the present invention.
In brief, for measurement of cell-substrate or cell-electrode impedance using
the
technology of the present invention, cell-substrate impedance monitoring
devices are
used that have microelectrode arrays with appropriate geometries fabricated
onto the
bottom surfaces of wells such as microtiter plate wells, or have a similar
design of having
multiple fluid containers (such as wells) having electrodes fabricated on
their bottom
surfaces facing into the fluid containers. Cells are introduced into the fluid
containers of
the devices, and make contact with and attach to the electrode surfaces. The
presence,
absence or change of properties of cells affects the electronic and ionic
passage on the
electrode sensor surfaces. Measuring the impedance between or among electrodes
provides important information about biological status of cells present on the
sensors.
When there are changes to the biological status of the cells analogue
electronic readout
signals can be measured automatically and in real time, and can be converted
to digital
signals for processing and for analysis.
Preferably, cell-substrate impedance assays are performed using a system of
the
present invention that comprises a device of the present invention, an
impedance monitor,
a device station that comprises electronic circuitry and engages the device
and the
impedance analyzer, and a software program that controls the device station
and records
and analyzes impedance data.
Using a system of the present invention, a cell index can optionally be
automatically derived and provided based on measured electrode impedance
values. The
62
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
cell index obtained for a given well reflects: 1) how many cells are attached
to the
electrode surfaces in this well, and 2) how well (tightly or extensively)
cells are attached
to the electrode surfaces in this well. Thus, the more the cells of same type
in similar
physiological conditions attach the electrode surfaces, the larger the cell
index. And, the
better the cells attach to the electrode surfaces (e.g., the cells spread-out
more to have
larger contact areas, or the cells attach tighter to electrode surfaces), the
larger the cell
index.
In one aspect of the present invention, a method is provided for performing
cell-
based assays, comprising: a) providing a cell-substrate impedance monitoring
device of
the present invention that comprises two or more electrode arrays, each of
which is
associated with a fluid container of the device; b) attaching the device to an
impedance
monitor; c) introducing cells into one or more fluid containers of the device;
and d)
monitoring cell-substrate impedance of at least one of the fluid containers
that comprises
an electrode array and cells. Preferably, impedance is monitored from the at
least one
fluid container to obtain impedance measurements at at least three time
points.
Preferably, impedance measurements or impedance values derived from impedance
measurements from at least three time points are plotted versus time to
generate one or
more impedance curves for the one or more fluid containers.
In a related aspect of the present invention, a method is provided for
performing
cell-based assays in an impedance-monitoring system, comprising: a) providing
a cell-
substrate impedance monitoring system of the present invention that comprises
a device
having two or more electrode arrays, each of which is associated with a well
of the
device; b) introducing cells into one or more wells of the device; and c)
monitoring cell-
substrate impedance of at least one of the wells that comprises an electrode
array and
cells. Preferably, impedance is monitored from the one or more wells of the
device to
obtain impedance measurements at at least three time points. Preferably,
impedance
measurements or impedance values derived from impedance measurements from at
least
three time points are plotted versus time to generate one or more impedance
curves for
the one or more wells.
The method can be used to assay cell status, where cell status includes, but
is not
limited to, cell attachment or adhesion status (e.g. the degree of cell
spread, the
63
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
attachment area of a cell, the degree of tightness of cell attachment, cell
morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis and/or necrosis. The cell-based assays
that be
performed with above methods include, but are not limited to, cell adhesion,
cell
apoptosis, cell differentiation, cell proliferation, cell survival,
cytotoxicity, cell
morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, and cell-based assays for screening and measuring ligand-receptor
binding.
In preferred embodiments of this aspect of the present invention, cells are
added
to at least two fluid containers of a device, each of which comprises an
electrode array,
and impedance is monitored from at least two wells that comprise cells and an
electrode
array.
The cells used in the assay can be primary cells isolated from any species or
cells
of cell lines. Primary cells can be from blood or tissue. The cells can be
engineered cells
into which nucleic acids or proteins have been introduced. In some
embodiments,
different cell types are added to different wells and the behavior of the cell
types is
compared.
An impedance monitoring assay can be from minutes to days or.even weeks in
duration. Preferably, impedance is monitored at three or more time points,
although this
is not a requirement of the present invention. Impedance can be monitored at
regular or
irregular time intervals, or a combination of irregular and regular time
intervals. In one
embodiment of a cell-based impedance assay, the cell-substrate impedance is
monitored
at regular time intervals. In some embodiments of the present invention,
impedance is
monitored at irregular intervals and then at regular intervals during a
particular time
window of the assay. Impedance can be monitored at one frequency or at more
than one
frequency. For example, in some preferred embodiments, impedance is monitored
over a
range of frequencies for each time point at which impedance is monitored.
Preferably,
64
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
impedance is monitored at at least one frequency between about 1 Hz and about
100
MHz, more preferably at at least one frequency between about 100 Hz and about
2 MHz.
In yet another aspect, the present invention provides a method for performing
S real-time cell-based assay investigating the effect of a compound on cells,
comprising: a)
providing an above described system; b) seeding the cells to the wells of
multiple-well
devices; c) adding the compound to the wells containing cells; d) monitoring
cell-
substrate impedance before and after adding the compound at a regular or
irregular time
interval; wherein the time dependent impedance change provides information
about time
dependent cell status before addition of the compound and about time dependent
cell
status under the interaction of the compound. Information about cell status
includes, not
limited to, cell attachment or adhesion status (e.g. the degree of cell
spread, the
attachment area of a cell, the degree of tightness of cell attachment, cell
morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis andlor necrosis. Information about
cell status
may also include any compound-cell interaction leading to any change to one or
more of
above cell status indicators. For example, if the compound binds to a receptor
on the cell
surface and such binding leads to a change in cell morphology, then the
binding of
compound to the receptor can be assayed by the monitored cell-substrate
impedance. The
cell-based assays that be performed with above methods include, but not
limited to, cell
adhesion, cell apoptosis, cell differentiation, cell proliferation, cell
survival, cytotoxicity,
cell morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, cell-based assay for screening and measuring ligand-receptor binding.
In one embodiment of the above cell-based assay, the cell-substrate impedance
is
monitored at regular time intervals. In exemplary embodiments, the impedance
is
measured at a regular 2 hour, 1 hour, 30 min or 15 min time interval before
and after
adding the compound. In the present application, a real-time assay means that
one can
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
perform the measurement on cell-substrate impedance with various time
resolutions, for
example, measurement taking place at a longer time interval such as every hour
or every
two hours, or at a shorter time interval every minute or a few minutes. Real-
time assay
does not mean that the measurements are provided in a continuous,
uninterrupted fashion.
In another word, real-time assay does not mean that the measurements are
performed at
every single moment.
Figure 2 depicts results of the use of methods of the present invention to
monitor
cell proliferation. In this experiment, H460 cells were introduced into wells
of a 16 well
device of a cell-substrate impedance monitoring system of the present
invention, with
different wells receiving different initial cell seeding numbers. The device
was engaged
with a device station of the system that was in a tissue culture incubator
that kept a
temperature of 37 degrees C and an atmosphere of 5% C02. Cell-substrate
impedance
was monitored at 15 minute intervals for 125 hours. The cell index was
calculated by the
system for each time point and displayed as a function of time to give cell
growth
(proliferation) curves for each cell seeding number. The cell growth curves
were plotted
on a log scale showing exponential growth phases and stationary phases.
Figure 3 depicts results of real-time monitoring of cell attachment and
spreading
of NIH3T3 cells. The cells were seeded onto cell-substrate impedance
monitoring devices
of the present invention that were coated with either poly-L-lysine or
fibronectin. The
device was connected to a device station that was in a tissue culture
incubator that kept a
temperature of 37 degrees C and an atmosphere of 5% CO2. Cell attachment and
cell
spreading on the difference coating surfaces were monitored by measuring
impedance on
the cell-substrate monitoring system. Impedance was monitored in real time
every 3
minutes for 3 hours. The cell index for each time point was calculated by the
impedance
monitoring system and plotted as a function of time.
Figure 4 shows the results of an experiment monitoring morphological changes
in
Cos-7 cells in response to stimulation with epidermal growth factor (EGF).
Cells were
seeded in wells of a 16 well monitoring device of the present invention that
engaged a
device station of a cell-substate monitoring system. The device station was
positioned in
an incubator held at 37 degrees C and 5% COa. The cells were serum starved for
8 hours
66
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
and then stimulated with 50 nanograms/mL of EGF. Control cells did not receive
EGF.
Impedance was monitored at 3 minute intervals for 2 hours and then at 1 hour
intervals
for 14 hours. The cell index was calculated by the system and plotted as a
function of
time. An initial jump in cell index is seen in EGF-treated cells due to
membrane ruffling
and actin dynamics in response to EGF. The arrow indicates the point of EGF
addition.
D.1. Cell proliferation assays
The present invention provides methods for performing cell proliferation
assays.
In these assays, an increase in monitored impedance is indicative of an
increases cell
number. The impedance measurements or impedance values derived from impedance
measurements can be plotted versus time to obtain growth curves for cells
growing in a
fluid container of a cell-substrate monitoring device of the present
invention.
The present invention provides a method of generating at least one cell growth
curve, comprising: providing a device of the present invention having two or
more
electrode arrays, each of which is associated with a fluid container of the
device;
attaching the device to an impedance analyzer; adding cells to one or more
fluid
containers of the device; monitoring impedance from the one or more fluid
containers to
obtain impedance measurements at three or more time points after adding the
cells to the
one or fluid containers; and plotting the impedance measurements or values for
the three
or more time points versus time to generate at least one growth curve for the
cells in the
one or more fluid containers.
The present invention also provides a method of generating at least one growth
curve using a system of the present invention, where the system includes a
multi-well
cell-substrate impedance monitoring device, an impedance analyzer, a device
station, and
a software program. The method includes; providing a mufti-well cell-substrate
impedance measuring system; adding cells to one or more wells of the system;
monitoring impedance from the one or more wells to obtain impedance
measurements at
three or more time points after adding cells to the one or more wells; and
plotting
impedance measurements or impedance values for the three or more time points
versus
time to generate a growth curve for the cells in the one or more wells.
67
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Preferably, using a device or system of the present invention, impedance is
monitored at four or more time points, in which at least one of the four or
more time
points is measured from a fluid container prior to adding cells to the fluid
container.
Preferably, impedance is monitored at regular or irregular time intervals for
an assay
period of from minutes to days. In many cases, proliferation assays can be
performed by
monitoring impedance for a period of between several hours and several days.
It is preferable to perform replicate proliferation assays in which more than
one
fluid container is seeded with same number of cells of the same type. In this
case, a plot
can optionally be generated by plotting averaged impedance measurements of
values at
assayed time points for replicate wells versus time. Preferably, a standard
deviation for
the averaged values is also calculated.
A growth curve can be generated by plotting impedance measurements versus
time, or by plotting cell index values that are calculated from impedance
measurements,
such as normalized cell index values or delta cell index values versus time.
The
impedance measurement or cell index axis (typically the y-axis) can optionally
use a log
scale.
An impedance value can be any indices of impedance derived from impedance
measurement, including, as nonlimiting examples, a cell index, a normalized
cell index or
a delta cell index. In certain embodiment, impedance value can also be a "raw"
measured
or monitored impedance value. Cell index (including normalized and delta cell
index)
can be a useful value for plotting growth curves, as it relates impedance
measurements to
cell number. For cell growth curves, a delta cell index for a given time point
can be
derived by subtracting the cell index at a baseline point, such as a time
point after cell
attachment and just before log phase growth, from the cell index measurement
at the
given time point. Preferably, determinations of impedance values and
generating growth
curves based on impedance measurements or values can be performed by software,
and
preferably by software that interfaces directly with the impedance analyzer.
For example,
where the growth assays are performed by a system of the present invention,
impedance
values (where used) can be measured or derived or calculated and growth curves
generated by a software program that controls and receives data from the
impedance
analyzer.
6~
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
A growth curve generated from impedance measurements or cell index values
(including normalized cell index values and delta cell index values) can
optionally be
used to calculate one or more kinetic parameters of cell growth or behavior.
For example,
a growth curve can be used to calculate the length of a lag phase, cell
attachment time,
cell attachment rate, or a cell doubling time.
Figure 2 shows real-time monitoring of proliferation of H460 cells seeded at
different
initial cell seeding numbers on a cell substrate impedance monitoring system
of the
presnet invention. The cell proliferation was continuously recorded every 15
minutes for
over 125 hours. The cell growth curves in the log scale show exponential cell
growth or
cells in the stationary phase. The cell index curve shown here can be used to
calculate
cell doubling time (DT). For example, taking the cell index for initial
seeding density of
900 cells. It took appoximately 57 hrs (from about 55 hr to about 112 hr) for
cell index to
increase from 0.3 to 3Ø Thus, the cell index doubling time is about 17.2 hrs
(=log(2)*57). Assuming that there is a linear correlation between cell number
and cell
index in this range, then cell doubling time is the same as the cell index
doubling time.
Thus, the cell doubling time (DT) is about 17.2 hrs. Another simple method to
calculate
the cell index doubling time is just to figure out how long t takes cell index
to double.
For example, for the cell index curve with initial seeding density of 900
cells. It took
about 17 hrs for cell index to change from 1.0 (at about 82 hrs) to 2.0 (at
about 99 hrs).
Thus the clel index doublimng time is 17 lirs.
Figure 3 shows real time monitoring of cell attachment and spreading of NIH3T3
cells using a cell-substrate imepdnace monitoring system of the presnet
invention. The
cells were seeded onto devices coated with either poly- L-lysine or
fibronectin. The cell
attachment and cell spreading processes on the different coating surfaces were
monitored
every 3 minutes for over 3 hours in real time. Using the cell index curve
showing in
Figure 3, we can calculate the cell attachment time and cell attachment rate.
Initial cell
index increase immediately following cell addition to the ells (at time =0 in
Figure 3)
reflects the cell spreading and attachment process. The time it takes for cell
index to
increase from zero to a maximum value or a some-what constant value (assuming
that
there is no cell division or growth in this initial time period following cell
seeding) is the
cell attachment time. For NIH3T3 cells, cell attachment time in a fibronectin
coated well
69
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
is about 1.2 hrs, as compared with the attachment time of about 3.5 hrs for
the same cells
in a poly-L-lysine coated well. Cell attachment rate is defined as 1 over the
cell
attachment time. Thus, cell attachment rate is about 0.83 hr -1 and about 0.29
hr-1'
respectively, for NIH3T3 cells attaching to a fibronectin-coated well and a
poly-L-lysine
coated well.
Figure 4 shows real-time monitoring of morphological changes in Cos-7 cells
uisng a cell-substrate impedance monitoring system of the presnet invention.
The cells
were serum starved for 8 hours and stimulated with or without 50 ng/mL EGF.
Changes
in cell morphology were monitored at 3 min intervals for 2 hours and then 1
hour interval
for 14 hours. The initial jump in the signal in EGF-treated cells is due to
membrane
ruffling and actin dynamics in response to EGF. The arrow indicates the point
of EGF
stimulation. Using the cell index curve showing in Figure 4, we can calculate
the cell
attachment time and cell attaclunent rate. Initial cell index increase
immediately
following cell addition to the ells (at time =0 in Figure 4) reflects the cell
spreading and
attachment process. The time it takes for cell index to increase from zero to
a maximum
value or a some-what constant value (assuming that there is no cell division
or growth in
this initial time period following cell seeding) is the cell attachment time.
For Cos-7 cells
shown here, the cell attachment time is about 4 hrs. Cell attachment rate, as
defined: 1
over the cell attachment time, is about 0.25 hr -1 for Cos-7 cells.
Furthermore, we can
also calculate the length of lag phase. The lag phase corresponds to the time
it takes for
cells to enter the growth phase after the completion of cell attachment
process. Based on
the cell index curve in Figure, cell attachment was complete at about 4 hrs.
The cells
showed significant increase in cell index - indicating cell growth, at around
9 hrs. Thus,
the length of lag phase is about 5 hrs ( = 9 hr - 4 hr).
Comparing Growth Curves of Two of Mof~e Cell Types
Two or more cell types can be seeded to separate wells in a proliferation
assay
using the methods of the present invention to generate growth curves of the
two or more
cell types. The growth curves or kinetic parameters derived from the growth
curves of the
cell types can be compared.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In this aspect, the invention includes a method of generating growth curves
for at
least two cell types, comprising: providing a device of the present invention
having two
or more electrode arrays, each of which is associated with a fluid container
of the device;
attaching the device to an impedance analyzer; adding cells of two or more
cell types to
two or more fluid containers of the device, in which at least one of the two
or more fluid
containers receives one cell type and at least one other of the two or more
fluid containers
receives a different cell type, to provide two or more fluid containers
comprising two or
more different cell types; monitoring impedance from the two or more fluid
containers
comprising different cell types at three or more time points after adding the
two or more
cell types to the two or more fluid containers; and plotting impedance
measurements or
impedance values for the three or more time points versus time to generate a
growth
curve for the two or more cell types.
The present invention also provides a method of generating at least one growth
curve using a system of the present invention, where the system includes a
mufti-well
cell-substrate impedance monitoring device, an impedance analyzer, a device
station, and
a software program. The method includes; providing a mufti-well cell-substrate
impedance measuring system; adding cells of two or more cell types to two or
more wells
of the device, in which at least one of the two or more wells receives one
cell type and at
least one other of the two or more wells receives a different cell type, to
provide two or
more wells comprising two or more different cell types; monitoring impedance
from the
two or more wells comprising different cell types at three or more time points
after
adding the two or more cell types to the two or more wells; and plotting
impedance
measurements or impedance values for the three or more time points versus time
to
generate a growth curve for the two or more cell types.
As, described above for proliferation assays, impedance is preferably
monitored
using an impedance monitoring device or system at four or more time points, in
which at
least one of the four or more time points is measured from fluid containers
prior to adding
cells to the fluid containers. Preferably, impedance is monitored at regular
or irregular
time intervals for an assay period of from minutes to days, for example, for a
period of
between several hours and several days.
71
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
It is preferable to perform replicate proliferation assays in which more than
one
fluid container is seeded with same number of cells of the same type. In this
case, a plot
can optionally be generated by plotting averaged impedance measurements of
values at
assayed time points for replicate wells versus time. Preferably, a standard
deviation for
the averaged values is also calculated.
Growth curves for different cell types can be generated as described above.
Impedance or impedance values, such as cell index, normalized cell index, or
delta cell
index can be plotted versus time. The impedance measurement or cell index axis
(typically the y-axis) can optionally use a log scale.
A growth curve generated from impedance measurements or cell index values
(including normalized cell index values and delta cell index values) can
optionally be
used to calculate one or more kinetic parameters of cell growth or behavior.
For example,
a growth curve can be used to calculate the duration of a lag phase, cell
attachment time,
cell attachment rate, or a cell doubling time.
Preferably, the growth curves of the two or more different cell types, or
kinetic
parameters derived from the growth curves of the two or more different cell
types, are
compared to determine differences among the cell types in proliferation
patterns or rates,
or in kinetic parameters that can be derived from growth curves. The different
cell types
used can be any cell types, including primary cells isolated from blood or
tissue of an
animal or human, or cells from cell lines. For example, proliferation rates of
two types of
primary cancer cell can be compared, or of primary cancer cells of the same
type but
different grades. In another example, primary cells of individuals of
different genotypes
can be compared. In another example, proliferation rates of primary or cell
line stem cells
can be compared. In yet another example, growth curves or parameters of
control and
genetically modified cells of a cell line can be compared. In yet another
example, growth
curves or parameters of cells infected with virus and control cells can be
compared.
D.2.Quantifyin~ Cells Using Cell-Substrate Impedance Devices
The present invention also includes a method of quantifying cells, comprising:
providing a device of the present invention having two or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
72
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
impedance analyzer; adding cells to one or more fluid containers of the
device;
monitoring impedance from the one or more fluid containers to obtain impedance
measurements at one or more time points after adding the cells to the one or
more fluid
containers; deriving a cell index for the one or more time points; and using
the cell index
to determine the number of cells in the one or more fluid containers at at
least one of the
one or more time points. The cell index is used to determine the number of
cells using a
formula that relates cell index to cell number, in which the formula is
obtained by:
providing a device for cell-substrate monitoring, attaching the device to an
impedance
monitor; adding cells to one or more fluid containers of the device; measuring
impedance
of the one or more fluid containers comprising cells; calculating a cell index
from the
impedance measurements; determining the number of cells of said at least one
fluid
container at the time of impedance monitoring by a means other than impedance
monitoring; and deriving a formula that relates the number of cells of the one
or more
fluid containers at the two or more time points with the impedance
measurements at the
two or more time points.
In the embodiment of above method for obtaining the formula, sometime, the
number of cells introduced to the wells are pre-known or predetermined before
cells are
added in to the wells. Under such conditions, one assumes that there will be
no change in
cell number or little change in cell number when the impedance measurement for
obtaining the formula is performed.
The number of cells determined by a method other than impedance monitoring
can be determined by, for example, cell plating, hemacytometer counting, flow
cytometry, or Coulter counting.
The method can also be practiced using an impedance monitoring system of the
present invention, where the system includes a mufti-well cell-substrate
impedance
monitoring device, an impedance analyzer, a device station, and a software
program. The
method includes; providing a mufti-well cell-substrate impedance measuring
system;
adding cells one or more wells of the system; monitoring impedance from the
one or
more wells comprising cells at one or more time points after adding the cells
to the one or
more wells; deriving a cell index for the one or more time points; and using
the cell index
73
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
to determine the number of cells in said at least well at at least one of said
one or more
time points.
The cell index is used to determine the number of cells using a formula that
relates cell index to cell number, in which the formula is obtained by:
providing a system
for cell-substrate monitoring, where the system comprises at least one mufti-
well cell-
substrate impedance monitoring device, adding cells to one or more wells of a
device of
the system; measuring impedance of the one or more wells comprising cells at
two or
more time points; calculating a cell index from the impedance measurement at
the two or
more time points; determining the number of cells of the one or more wells at
the two or
more time points by a means other than impedance monitoring; and deriving a
formula
that relates the number of cells of the one or more wells at the two or more
time points
with the impedance measurements at the two or more time points.
In the embodiment of above method for obtaining the formula, sometime, the
number of cells introduced to the wells are pre-known or predetermined before
cells are
added in to the wells. Under such conditions, one assumes that there will be
no change in
cell number or little change in cell number when the impedance measurement for
obtaining the formula is performed.
The number of cells determined by a method other than impedance monitoring
can be determined by, for example, cell plating, hemacytometer counting, flow
cytometry, or Coulter counting.
Formulas relating cell index (including normalized cell index and delta cell
index,
which can also be used) to cell number for a given cell type can be used to
quantitate
cells of that type in assays using a cell-substrate impedance monitoring
device, such as a
device described herein. Generally, for a give cell type and for cells under
similar
physiological conditions, the derived formulas relating cell index to cell
number can be
used in subsequent assays. There is no need to obtain the formula each time
when an
assay is performed. However, it is worthwhile to point that the formula can
only be valid
as long as the cells are under same physiological conditions in the assays
where the
formula was derived and where the formula is used. If the cell condition is
different, for
example, the composition of culture mediais changed, or the cell attachment
surface is
altered, then the formula will not hold. In another example, if cells are in
log-growth
74
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
phase in one assay and are in stationary phase in another assay, then the
formula may not
hold. Another point worth mentioning here is that relates the fact the derived
cell index
or impedance also depends on cell attachment quality on the surface as well as
cell
morphology. If cell morphology or cell attachment changes during an assay,
then one
need to distinguish between the changes caused by change in cell number or in
cell
morphology or in cell attachment.
As an example, we can derive the correlation formula between cell index and
cell
number for NIH3T3 cells order the experimental conditions. The formula for
converting
cell index to cell number for this particular case is: Cell number = 2000*
Cell index -
145. To test this formula, we found the error in estimating cell number based
on the cell
index data shown in Figure ~ as compared to the seeded cell number is less
than 20% .
D.3. Cell-based assays to test the effects of compounds on cells
In yet another aspect, the present invention provides a method for performing
a
cell-based assay investigating the effect of one or more test compounds on
cells,
comprising: providing a device of the present invention having two or more
electrode
arrays, each of which is associated with a fluid container of the device;
attaching the
device to an impedance analyzer; introducing cells into two or more fluid
containers of
the device that comprise an electrode array; adding at least one test compound
to at least
one of the one or more of the fluid containers comprising cells and an
electrode array to
provide at least one test compound fluid container; providing at least one
control fluid
container to which cells are added that does not receive test compound; and
monitoring
cell-substrate impedance of the one or more test compound fluid containers and
the one
or more control fluid containers at at least three time points after adding
the one or more
test compounds, and analyzing impedance measurements from the one or more test
compound fluid containers and the one or more control fluid containers at at
least three
time points after adding the one or more test compounds, in which changes in
impedance
can provide information about cell responses to the one or more test
compounds.
In a related aspect the present invention also provides a method for
performing a
cell-based assay investigating the effect of one or more test compounds on
cells, where
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
the system includes a mufti-well cell-substrate impedance monitoring device,
an
impedance analyzer, a device station comprising electronic circuitry that
engages the
device and connects the two or more electrode arrays of the device to the
impedance
analyzer, and a software program that controls the device station and can
record and
analyze data from the impedance analyzer. The method includes; providing a
mufti-well
cell-substrate impedance measuring system; introducing cells into two or more
wells of
the device; adding at least one test compound to at least one of the one or
more of the
wells comprising cells to provide at least one test compound well; providing
at least one
control well to which cells are added that does not receive test compound;
monitoring
cell-substrate impedance of the one or more test compound wells and the one or
more
control wells at at least three time points after adding the one or more test
compounds;
and analyzing impedance measurements from the one or more test compound wells
and
the one or more control wells at at least three time points after adding the
one or more test
compounds, in which changes in impedance can provide information about cell
responses
to the one or more test compounds.
A test compound can be any compound, including a small molecule, a large
molecule, a molecular complex, an organic molecule, an inorganic molecule, a
biomolecule such as but not limited to a lipid, a steroid, a carbohydrate, a
fatty acid, an
amino acid, a peptide, a protein, a nucleic acid, or any combination of these.
A test
compound can be a synthetic compound, a naturally occurnng compound, a
derivative of
a naturally-occurring compound, etc. The structure of a test compound can be
known or
unknown.
Information about cell responses to the one or more test compounds includes,
but
is not limited to, information about cell attachment or adhesion status (e.g.
the degree of
cell spread, the attachment area of a cell, the degree of tightness of cell
attachment, cell
morphology) on the substrate including on the electrodes, cell growth or
proliferation
status; number of viable cells and/or dead cells in the well; cytoskeleton
change and re-
organization and number of cells going through apoptosis and/or necrosis.
Information
about cell status may also include any compound-cell interaction leading to
any change to
one or more of above cell status indicators. For example, if the compound
binds to a
receptor on the cell surface and such binding leads to a change in cell
morphology, then
76
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
the binding of compound to the receptor can be assayed by the monitored cell-
substrate
impedance.
The cells used in the assay can be primary cells isolated from any species or
can
be cells of cell lines. The cells can be genetically engineered cells (For
example, cells
from a genetically modified organism, such as for example from a "gene
knockout"
organism, or cells that have been engineered to over-express an endogenous
gene or a
transgene, or cells whose normal gene expression has been manipulated by use
of
antisense molecules or silencing RNA.) W some embodiments, different cell
types are
added to different wells and the behavior of the different cell types in
response to one or
more compounds is compared.
The cell-based assays that be performed with above methods include, but are
not
limited to, cell adhesion, apoptosis, cell differentiation, cell
proliferation, cell survival,
cytotoxicity, cell morphology detection, cell quantification, cell quality
control, time-
dependent cytotoxicity profiling, IgE-mediated cell activation or stimulation,
receptor-
ligand binding, viral, bacterial, or environmental toxin mediated cell
pathologic changes
or cell death, detection or quantification of neutralizing antibodies,
specific T-cell
mediated cytotoxic effect, and cell-based assay for screening or measuring
ligand-
receptor binding.
In the methods of the present invention that investigate test compound effects
on
cells, impedance is preferably monitored from at least one test compound well
at at least
one time point before adding said at least one test compound to said at least
one test
compound well. Preferably, impedance is monitored at four or more time points,
at least
one of which is prior to the addition of one or more test compounds.
Preferably,
impedance is monitored at regular or irregular time intervals for an assay
period of from
minutes to days, for example, for a period of between several hours and
several days. In
one embodiment of the above cell-based assay, the cell-substrate impedance is
monitored
at at least one time point prior to addition of the test compound, and at
regular time
intervals thereafter. For example, impedance can be measured at one or more
intervals
before adding the compound and at a regular 2 hour, 1 hour, 30 min or 15 min
time
intervals after adding the compound. Preferably, impedance is measured at
three or more
time points spaced at regular intervals. In the present application, a real-
time assay
77
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
means allows one to perform the measurement on cell-substrate impedance with
various
time resolutions, for example, measurement taking place at a longer time
interval such as
every hour or every two hours, or at a shorter time interval every minute or a
few
minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a range of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
It is preferable to perform replicate test compound assays in which more than
one
fluid container of cells receives the same compound at the same concentration.
In this
case, impedance measurements or values can be averaged for the assayed time
points for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
In the methods of the present invention, analyzing impedance can comprise
plotting cell impedance versus time to obtain at least one test compound
impedance curve
and at least one control impedance curve. Preferably, at least one test
compound
impedance curve and said at least one control impedance curve are compared to
identify a
time frame, if any, in which a test compound curve differs significantly from
a control
curve, indicating a time frame of an effect of a test compound on cells. For
example,
depending on the time frame at which a test compound curve differs
significantly from a
control curve, the test compound can be hypothesized to affect one or more of,
for
example, cell attachment or adhesion, cell growth or proliferation,
cytoskeleton
organization or function, or apoptosis or cell death.
Preferably, data from impedance monitoring of a well that comprises cells and
a
test compound is compared with data from impedance monitoring of a well that
comprises cells in the absence of a test compound, however, this is not a
requirement of
the present invention. For example, it is also possible to compare impedance
measurements from one or more time points prior to the addition of compound to
compare impedance measurements from one or more time points after the addition
of
compound. Such comparisons can be used directly to assess the cells' response
to a
78
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
compound. It is also possible to calculate a cell index (or cell number index)
using the
impedance values obtained.
Methods of calculating a cell index (cell number index) are disclosed herein
as
well as in parent application U.S. Patent Application 10/705,447, U.S. Patent
Application
No. l O1987,732,both herein incorporated by reference for disclosures relating
to cell
number index and its calculation. The cell index calculated from impedance
measurements of wells receiving compound can be compared with the cell index
calculated from impedance measurements of control wells to assess the effect
of a
compound on cells. Alternatively, cell index calculated from impedance
measurements of
wells from one or more time points after the addition of a compound can be
compared
with the cell index calculated from impedance measurements of wells from one
or more
time points prior to 'the addition of a compound to assess the effect of a
compound on
cells. In some preferred embodiments, the cell index can be used as an
indicator of
cytotoxicity.
The derivation of cell index from impedance measurements is provided in
Section
C of the present application. Cell index values (including normalized cell
index values
and delta cell index values) from at least three time points from at least one
test
compound well and at least one control well can be plotted versus time to
obtain one or
more test compound cell index curve and one or more control cell index curves.
The one
or more test compound cell index curves and the one or more control cell index
curves
can be compared to identify a time frame, if any, in which a test compound
curve differs
significantly from a control crave, indicating a time frame of an effect of a
test compound
on cells. For example, depending on the time frame at which a test compound
curve
differs significantly from a control curve, the test compound can be
hypothesized to
affect one or more of, for example, cell attachment or adhesion, cell growth
or
proliferation, cytoskeleton organization or function, or apoptosis or cell
death.
Cell index values at three or more assay time points for one or more test
compound wells and one or more control wells can be used to derive cell change
index
(CCl) values or a second order derivatives of cell index at three or more
assay time
points. The calculation of cell change index is provided in Section C of the
present
application. The value of CCI at a give time point can be determined to be
either
79
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
approximately equal to 0.7, much greater than 0.7, greater than zero and less
than 0.7,
approximately equal to zero, less than zero, or much less than zero. These
values can
indicate cell behavior at an assay time point, as CCI approximately equal to
0.7 indicates
log rate growth, a CCI much greater than 0.7 indicates faster than log rate
growth, a CCI
greater than zero and less than 0.7 indicates slower than log rate growth, a
CCI
approximately equal to zero indicates no growth (a constant cell index), a CCI
less than zero indicates cells are detaching from the substrate, and a CCI
much less than
zero indicates cell are detaching rapidly from the substrate.
For a given assay time point, differences in CCI value between control and
compound treated wells can indicate a time at which the compound has an effect
on cells,
as well as providing information on the type of effect the compound has.
The CCI can fixrther be used to obtain information on the effect of a test
compound by plotting CCI versus time for. at least three assay time points to
obtain a cell
change index curve (CCI curve) for at least one control container or well and
at at least
one test compound container or well. One or more test compound CCI curves can
be
compared with one or more control CCI curves to obtain information on cell
status or
behavior in response to said at least one test compound, wherein said cellular
status or
behavior is at least one of cell attachment or adhesion status; cell growth or
proliferation
status; the number of viable cells or dead cells; cytoskeleton change or re-
organization; ~or
the number of cells going through apoptosis or necrosis.
Cell-based Assays with More Than Oyae Cell Type
The present invention also provides methods of comparing the effects of a
compound on two or more cell types. In one aspect, the method comprises:
providing a device of the present invention having two or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
impedance analyzer; introducing cells into two or more fluid containers of the
device that
comprise an electrode array, wherein at least one of the two or more fluid
containers
receives one cell type and at least one other of the two or more fluid
containers receives a
different cell type; adding a test compound to the one or more fluid
containers receiving
one cell type and adding the test compound to the one or more fluid containers
receiving
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
a different cell type to provide at least two test compound fluid containers
that comprise
cells of different types; providing at least two control fluid containers that
do not receive
test compound, in which at least one of the control fluid containers receives
cells of the
one type and at least one of the control fluid containers receives cells of
the different
type; monitoring cell-substrate impedance of the two or more test compound
fluid
containers that comprise different cell types and the one or more control
fluid containers
at at least three time points after adding the one or more test compounds; and
analyzing
impedance measurements from the two or more test compound fluid containers
comprising different cell types and from the one or more control fluid
containers at at
least three time points after adding the one or more test compounds, in which
changes in
impedance can provide information about cell responses to the one or more test
compounds.
In a related aspect the present invention also provides a method for
performing a
cell-based assay investigating the effect of one or more test compounds on
cells using a
cell-substrate impedance monitoring system of the present invention, where the
system
includes a multi-well cell-substrate impedance monitoring device, an impedance
analyzer, a device station comprising electronic circuitry that engages the
device and
connects the two or more electrode arrays of the device to the impedance
analyzer, and a
software program that controls the device station and can record and analyze
data from
the impedance analyzer. The method includes: providing a mufti-well cell-
substrate
impedance measuring system; introducing cells into two or more wells of the
device that
comprise an electrode array, wherein at least one of the two or more wells
receives one
cell type and at least one other of the two or more wells receives a different
cell type;
adding a test compound to the one or more wells receiving one cell type and
adding the
test compound to the one or more wells receiving a different cell type to
provide at least
two test compound wells that comprise cells of different types; providing at
least two
control wells that do not receive test compound, in which at least one of the
wells
receives cells of the one type and at least one of the control wells receives
cells of the
different type; monitoring cell-substrate impedance of the two or more test
compound
wells that comprise different cell types and the one or more control wells at
at least three
time points after adding the one or more test compounds; and analyzing
impedance
81
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
measurements from the two or more test compound wells comprising different
cell types
and from the one or more control wells at at least three time points after
adding the one or
more test compounds, in which changes in impedance can provide information
about cell
responses to the one or more test compounds.
In the methods of the present invention that investigate test compound effects
on
cells, impedance is preferably monitored from at least two test compound wells
comprising different cell types at at least one time point before adding test
compound to
the at least one two compound wells. Preferably, impedance is monitored at
four or more
time points, at least one of which is prior to the addition of one or more
test compounds.
Preferably, impedance is monitored at regular or irregular time intervals for
an assay
period of from minutes to days, for example, for a period of between several
hours and
several days. In one embodiment of the above cell-based assay, the cell-
substrate
impedance is monitored at at least one time point prior to addition of the
test compound,
and at regular time intervals thereafter. For example, impedance can be
measured at one
or more intervals before adding the compound and at a regular 2 hour, 1 hour,
30 min or
15 min time intervals after adding the compound. Preferably, impedance is
measured at
three or more time points spaced at regular intervals. In the present
application, a real-
time assay means allows one to perform the measurement on cell-substrate
impedance
with various time resolutions, for example, measurement taking place at a
longer time
interval such as every hour or every two hours, or at a shorter time interval
every minute
or a few minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a range of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
As disclosed in an earlier section on compound assays, a test compound can be
any compound whose effect on cells can be investigated. A test compound used
in assays
comparing cell responses can be a compound whose effect on one or more of the
cell
types to be assayed is known, or can be a compound whose effects on any of the
cell
types to be assayed are unknown. In preferred methods of the present
invention, cells are
82
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
introduced into at least three wells of the device that each comprise an
electrode array,
and at least one well that comprises an electrode array and comprises cells
does not
receive a test compound. A control well that does not receive a test compound
can be
monitored, and its impedance data can be compared with that of wells that
receive a
compound to determine the effect of the test compounds on cells.
As disclosed in a previous section for compound assays, the cell types used in
the
assay can be primary cells isolated from any species or can be cells of cell
lines. In some
preferred embodiments, the different cell types are the same type of cell from
different
individuals, and thus have different genotypes. One or more of the cell types
can be
genetically engineered (For example, cells from a genetically modified
organism, such as
for example from a "gene knockout" organism, or cells that have been
engineered to
overexpress an endogenous gene or a transgene, or cells whose normal gene
expression
has been manipulated by use of antisense molecules or silencing RNA.) In these
cases,
genetically modified cells can be compared with control cells. In another
example the
cells can be, for example, stem cells from different stages of differentiation
or of different
genotypes whose response to growth factors is being compared. In other
examples the
cells can be cancer cells where the test compound is tested for its cytotoxic
effects. The
cells can be primary cancer cells of the same type isolated from different
individuals, for
example, or different cancer cell lines, or cancer cells of the same type but
of different
grades. In some embodiments, three or more different cell types are added to
different
wells and the behavior of the three or more different cell types in response
to one or more
compounds is compared. In preferred embodiments of the present invention, for
each cell
type tested there is a control performed in which the control does not receive
test
compound.
A variety of assays can be employed, where the effect of a test compound on
the
behavior of two or more cell types in the assay is under investigation. Such
assays
include, as nonlimiting examples, cell adhesion assays, apoptosis assays, cell
differentiation assays, cell proliferation assays, cell survival assays,
cytotoxicity assays,
cell morphology detection assays, cell quantification assays, cell quality
control assays,
time-dependent cytotoxicity profiling assays, IgE-mediated cell activation or
stimulation
assays, receptor-ligand binding assays, viral, bacterial, or environmental
toxin mediated
83
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
cell pathologic changes or cell death assays, detection or quantification of
neutralizing
antibodies, specific T-cell mediated cytotoxic effect assays, and cell-based
assays for
screening or measuring ligand-receptor binding.
In the assays of the present invention is preferable to perform replicate test
compound assays in which more than one fluid container of cells of the same
type
receives the same compound at the same concentration. In this case, impedance
measurements or values can optionally be averaged for the assayed time points
for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
Preferably, time-dependent responses of the first and second types of cells
are
compared to see how similar or different the responses from the two types of
cells are.
In one method of the present invention, impedance from a first cell type well
is plotted
versus time to give a first cell type impedance curve and impedance from a
second cell
type well is plotted versus time to give a second cell type impedance curve.
Cell index
(including normalized cell index or delta cell index) from wells comprising
cells of
different types can also be calculated from impedance data and plotted versus
time to
give cell index curves.
The impedance curves or cell index curves from the different cell types can be
compared to determine whether the time frame, magnitude, and duration of a
cells
response to a compound are similar or different. Preferably, impedance curves
or cell
index curves generated from control wells comprising each cell type in the
absence of
compound are compared with the test compound curves to assess the compound-
specific
effects on each cell type. The effects of the compounds on one or more of the
two or
more cell types can be effects on cell attachment or adhesion, cell growth or
proliferation;
the number of viable cells or dead cells; cytoskeleton organization or
function; or the
number of cells going through apoptosis or necrosis in response to a test
compound.
Assays can be designed to investigate the compound's effects on particular
cellular
processes or activities.
The effect of a compound on at least one of the cell types used in the assay
may
be known. The mechanism of action of a compound on at least one of the cell
types used
in the assay may be known. In such cases, comparison of the compound response
of one
84
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
or more different cell types with the compound response of a cell type whose
response to
the compound is characterized can give information as to the similarity or
difference in
response of a different cell type to the compound.
In one preferred embodiment of this method, time-dependent cytotoxic responses
of particular cell types to a compound are compared. Cytotoxicity assays can
provide
information on the sensitivity of one or more cell type to a compound.
Figures 10A and B show the responses of various cell types (listed in Table 1)
to
olomoucine treatment as monitored using a cell-substrate imepdnace monitoring
system
of the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
with olomoucine. Comparison among these cell index curves showed that certain
similarity does exist. Take the treatment of olomoucine at 100 uM as an
example. For a
significant number of cell types tested, olomoucine treatment resulted in a
near-constant
cell index for some length of time (for example: 10, 20 or 30 hrs) a long
time. This
relates to the fact olomoucine is a cell cycle resting compound and for some
time period
following compound addition, cells do not divide any more and so cell number
does not
change but cells remain "live". Thus, for such time period, cell index did not
change
with time. The "near-constant" cell index curves were also observed for cells
treated
with roscovitine, which is another compound causing cell cycle arrest. The
cell index
curves shown in Figures 10A and l OB are strikingly different from the cell
index curves
shown in Figure 9A and 9B, and Figure 11A and 11B, where compounds follow
different
mechanism of compound action.
The CI derived from impedance data from wells comprising different cell types
and a test compound can be used to derive cell change index (CCI) values for
assay time
points. CCI values of particular cell types at assay time points can be
compared. Such
comparisons can indicate whether different cell types are responding similarly
to a
compound. CCI can also be plotted versus time, and CCI curves of cells of
different types
assayed with one or more test compounds can be compared to determine the
similarities
or differences in cellular responses of different cell types to a test
compound.
~5
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Cell-based Assays with More Tlzan One Conapound
The present invention also provides methods of comparing the effects of two or
more different compounds on cells. In one aspect, the method comprises:
providing a device of the present invention having three or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
impedance analyzer; introducing cells into three or more fluid containers of
the device
that comprise an electrode array; adding at least one test compound to at
least one of the
three or more fluid containers comprising cells and adding at least one
different test
compound to at least one other of the three or more fluid containers
comprising cells to
provide at least two different test compound fluid containers; providing as a
control fluid
container at least one of the three or more fluid containers, in which the
control fluid
container receives cells but does not receive compound; attaching an impedance
analyzer
to the device; monitoring cell-substrate impedance of the two or more
different test
compound fluid containers that comprise different compounds and the one or
more
control fluid containers at at least three time points after adding the one or
more test
compounds; and analyzing impedance measurements from the two or more different
test
compound fluid containers and from the one or more control fluid containers at
at least
three time points after adding the one or more test compounds, in which
changes in
impedance can provide information about cell responses to the one or more test
compounds.
In a related aspect, the present invention provides a method for performing a
cell-
based assay investigating the effect of two or more test compounds on cells
using a cell-
substrate impedance monitoring system. The method includes: a) providing a
cell-
substrate impedance monitoring system of the present invention; b) introducing
cells into
at least two wells of the device that each comprise an electrode array; c)
adding to at least
one well of the device comprising cells and an electrode array a first test
compound; d)
adding to at least one other well of the device comprising cells and an
electrode array a
second test compound; and e) monitoring cell-substrate impedance of at least
one well
comprising cells and a first compound and at least one well comprising cells
and a second
compound, in which changes in impedance can provide information about cell
responses
to the first and second compounds.
86
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Preferably, time-dependent responses of cells to the first compound and the
second compound are compared to see how similar or different the responses
from the
two compounds are. In one preferred embodiment of this method, time-dependent
cytotoxic responses are compared.
The cells and test compound that can be used in the assay can be any as
described
above for assays testing effects of test compounds.
In the assays of the present invention is preferable to perform replicate test
compound assays in which more than one fluid container of cells of the same
type
receives the same compound at the same concentration. In this case, impedance
measurements or values can optionally be averaged for the assayed time points
for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
Impedance monitoring can be as described above for assays testing effects of
test
compounds. Preferably impedance is monitored from the at least two different
test
compound wells and at least one control well at at least one time point before
adding said
at least one test compound to said at least one test compound well.
Preferably, impedance
is monitored at four or more time points, at least one of which is prior to
the addition of
one or more test compounds. Preferably, impedance is monitored at regular or
irregular
time intervals for an assay period of from minutes to days, for example, for a
period of
between several hours and several days. In one embodiment of the above cell-
based
assay, the cell-substrate impedance is monitored at at least one time point
prior to
addition of the test compound, and at regular time intervals thereafter. For
example,
impedance can be measured at one or more intervals before adding the compound
and at
a regular 2 hour, 1 hour, 30 min or 15 min time intervals after adding the
compound.
Preferably, impedance is measured at three or more time points spaced at
regular
intervals. In the present application, a real-time assay means allows one to
perform the
measurement on cell-substrate impedance with various time resolutions, for
example,
measurement taking place at a longer time interval such as every hour or every
two hours,
or at a shorter time interval every minute or a few minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a range of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
87
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
Preferably, data from impedance monitoring of wells that comprise different
test
compounds are compared.
In one embodiment, for at least two different compound wells, impedance at
three
or more assay time points can be plotted versus time. Preferably, for a
control well that
does not receive compound, impedance at the same three or more assay time
points is
also plotted versus time. The impedance curves of different compound wells can
be
compared with the control impedance curve to determine whether the compounds
have a
similar or different effect on cells.
Cell index (including normalized cell index or delta cell index) from wells
comprising cells of different types can also be calculated from impedance data
and
plotted versus time to give cell index curves.
The impedance curves or cell index curves from the different cell types can be
compared to determine whether the time frame, magnitude, and duration the
response of
cells to different compounds are similar or different. Preferably, impedance
curves or cell
index curves generated from one or more control wells comprising cells in the
absence of
compound are compared with the test compound curves to assess the compound-
specific
effects of each compound. The effects of the compounds on cells can be for
example,
effects on cell attachment or adhesion, cell growth or proliferation; the
number of viable
cells or dead cells; cytoskeleton organization or function; or the number of
cells going
through apoptosis or necrosis in response to a test compound. Assays can be
designed to
investigate the compound's effects on particular cellular processes or
activities.
The effect on cells of one or more of the compounds used in the assay may be
known. The mechanism of action of one or more compounds used in the assay may
be
known. In such cases, comparison of the responses of cells to other test
compounds used
in the assay with cellular responses to the one or more compounds whose
effects are
characterized can give information as to the similarity or difference in
response of
different compounds to a known compound.
Information about cell responses to the compound includes, but is not limited
to,
information about cell attachment or adhesion status (e.g. the degree of cell
spread, the
88
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
attachment area of a cell, the degree of tightness of cell attachment, cell
morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis andlor necrosis. Information about
cell status
may also include any compound-cell interaction leading to any change to one or
more of
above cell status indicators. For example, if the compound binds to a receptor
on the cell
surface and such binding leads to a change in cell morphology, then the
binding of
compound to the receptor can be assayed by the monitored cell-substrate
impedance. The
cell-based assays that be performed with above methods include, but not
limited to, cell
adhesion, cell apoptosis, cell differentiation, cell proliferation, cell
survival, cytotoxicity,
cell morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, cell-based assay for screening and measuring ligand-receptor binding.
A plurality of compounds can be assayed with multiple cell types. In one
preferred embodiment of this method, time-dependent cytotoxic responses of
different
cell types to a set of compounds are compared.
The CI derived from impedance data from wells comprising different cell types
and a test compound can be used to derive cell change index (CCI) values for
assay time
points. CCI values of particular cell types at assay time points can be
compared. Such
comparisons can indicate whether different cell types are responding similarly
to a
compound. CCI can also be plotted versus time, and CCI curves of cells of
different types
assayed with one or more test compounds can be compared to determine the
similarities
or differences in cellular responses of different cell types to a test
compound.
For example, the time frame, magnitude, and duration of a difference in
response
as evidenced by the curves can indicate a difference in efficacy or mechanism
of
compounds. The impedance differences can reflect differences in, for example,
cell
attachment or adhesion, cell growth or proliferation; the number of viable
cells or dead
89
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
cells; cytoskeleton organization or function; or the number of cells going
through
apoptosis or necrosis in response to a test compound.
A variety of assays can be employed, where the effect of two or more test
compound on the behavior cells is under investigation. Such assays include, as
nonlimiting examples, cell adhesion assays, apoptosis assays, cell
differentiation assays,
cell proliferation assays, cell survival assays, cytotoxicity assays, cell
morphology
detection assays, cell quantification assays, cell quality control assays,
time-dependent
cytotoxicity profiling assays, IgE-mediated cell activation or stimulation
assays, receptor-
ligand binding assays, viral, bacterial, or environmental toxin mediated cell
pathologic
changes or cell death assays, detection or quantification of neutralizing
antibodies,
specific T-cell mediated cytotoxic effect assays, and cell-based assays for
screening or
measuring ligand-receptor binding.
In one preferred embodiment of this method, time-dependent cytotoxic responses
of cells to a set of compounds are compared. "Cytotoxicity profiling" in which
the
impedance responses of cells in response to a plurality of potentially
cytotoxic
compounds are compared, can provide information on the efficacy and mechanism
of a
test compound. Cytotoxicity profiling can be performed by comparing any
combination
of impedance plots, kinetic parameters derived from impedance plots, CI plots,
CCI
values, and CCI plots.
In one embodiment of the method, analyzing the cytotoxicity response may
include derivation of the slope of change in the time dependent cytotoxicity
response at a
given compound concentration. hi yet another embodiment of the method,
analyzing
real-time cytotoxicity response may include derivation of high-order
derivatives of the
time dependent cytotoxicity response with respect to time at a given compound
concentration.
Evaluating the Effect ofDiffe~ent Concentrations of a Compound on Cells
The present invention also includes methods of performing assays to test the
effect of different concentrations of one or more test compounds on cells.
In one aspect, a method for testing different concentrations of a test
compound on
cells comprises: providing a device of the present invention having three or
more
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
electrode arrays, each of which is associated with a fluid container of the
device;
attaching the device to an impedance analyzer; introducing cells into at least
two of the
three or more fluid containers of the device that comprise an electrode array;
adding
different concentrations of a test compound to the two or more fluid
containers of the
device that comprise cells; providing a control fluid container that comprises
cells but
does not receive compound; monitoring cell-substrate impedance of the two or
more
different test compound fluid containers that comprise different
concentrations of a test
compound and of the one or more control fluid containers at at least three
time points
after adding a test compound; and analyzing impedance measurements from the
two or
more different test compound fluid containers and one or more control fluid
containers at
at least three time points after adding a test compound, in which changes in
impedance
can provide information about cell responses to the test compounds.
In a related aspect, the present invention provides a method for performing a
cell-
based assay investigating the effect of two or more concentrations of a test
compound on
cells using a cell-substrate impedance monitoring system. The method includes:
providing a cell-substrate impedance monitoring system of the present
invention;
introducing cells into at least two of the three or more wells of the device
that comprise
an electrode array; adding different concentrations of a test compound to the
two or more
wells of the device that comprise cells; providing a control well that
comprises cells but
does not receive test compound; monitoring cell-substrate impedance of the two
or more
different test compound wells that comprise different concentrations of a test
compound
and the one or more control wells at at least three time points after adding a
test
compound; and analyzing impedance measurements from the two or more different
test
compound wells and the one or more control wells at at least three time points
after
adding a test compound, in which changes in impedance can provide information
about
cell responses to the test compounds.
The cells and test compound that can be used in the assay can be any as
described
above for assays testing effects of test compounds.
Impedance monitoring can be as described above for assays testing effects of
test
compounds. Preferably impedance is monitored from the at least two different
test
compound wells and at least one control well at at least one time point before
adding said
91
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
at least one test compound to said at least one test compound well.
Preferably, impedance
is monitored at four or more time points, at least one of which is prior to
the addition of
one or more test compounds. Preferably, impedance is monitored at regular or
irregular
time intervals for an assay period of from minutes to days, for example, for a
period of
between several hours and several days. In one embodiment of the above cell-
based
assay, the cell-substrate impedance is monitored at at least one time point
prior to
addition of the test compound, and at regular time intervals thereafter. For
example,
impedance can be measured at one or more intervals before adding the compound
and at
a regular 2 hour, 1 hour, 30 min or 15 min time intervals after adding the
compound.
Preferably, impedance is measured at three or more time points spaced at
regular
intervals. In the present application, a real-time assay means allows one to
perform the
measurement on cell-substrate impedance with various time resolutions, for
example,
measurements taking place at a longer time interval such as every hour or
every two
hours, or at a shorter time interval every minute or a few minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a range of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
In one embodiment, for at least two different compound concentrations,
impedance or, preferably, cell index (including normalized cell index or delta
cell index),
at three or more assay time points is be plotted versus time. Preferably, for
a control well
that does not receive compound, impedance at the same three or more assay time
points is
also plotted versus time. An impedance curve or cell index curve can give an
indication
of the time frame at which a compound affects cell response. In some preferred
embodiments, the cell index can be used as an indicator of cytotoxicity.
Figures 9A and B shows the responses of various cell types (listed in Table 1)
to
doxorubicin treatment as monitored using a cell-substrate imepdnace monitoring
system
of the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
92
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
with doxorubicin. Comparison among these cell index curves showed that certain
similarity does exist. Take the treatment of doxoorubincin at 3.13 uM as an
example.
For most of cell types tested, initially after the treatment, cell index
increased with time
in similar way to the cell index from DMSO control wells. After 10-20 hrs,
depending on
cell type, the cell index reached a peak and started decreasing with time.
From that time
on, the cell index monotonically decreases. Such cell index curves -
characterized by
"going up first and then going down" -were also observed for the cells treated
with 5-
Fluorouracil. Both Doxorubicin and 5-Fluorouacil act on cells through effects
on DNA
replication or topology.
Furthermore, such cell index curves are strikingly different from the cell
index
curves shown in Figure 10A and l OB, where 100 uM of olomoucine resulted in a
nearly
constant cell index value for 10, 20 even 30 hrs after compound addition. The
cell index
curves shown in Figure 9 are also strikingly different from the cell index
curves in Figure
11, where nM concentration of paclitaxel caused an intial cell index decrease
for about 15
hrs (it varies between cell types) and then a cell index increase. These
dynamic changes
in cell index curves reflect the fact that these different compounds interacts
with the cells
differently. Compounds that interact with cells in similar way or following
same
mechanism would result in a similar cell index response curves. One
application of this
is to investigate the mechanism of compound action based on the observed cell
index
curves. If cell index responses follow a certain pattern, then one may be able
to deduce
the mechanism of compound action. Alternatively, if two compounds showed
similar,
dynamic cell index response curves, then these two compounds may act on the
cells with
similar or same mechanism of compound action.
Figure 11A and 11S shows the responses of various cell types (listed in Table
1)
to paclitaxel treatment as monitored using a cell-substrate imepdnace
monitoring system
of the presnet invention. The indicated cell lines were seeded onto microtiter
devices
fabricated with electronic sensor arrays shown in Figure 1. The cellular
responses were
continuously monitored at 15 or 30 or 60 minutes time interval before and
after treatment
with paclitaxel. Comparison among these cell index curves showed that certain
similarity
does exist. Talce the treatment of palitaxel at 0.78 -12.5 nM range as
examples.
93
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Typically, such nM paclitaxel treatment resulted in an initial decrease in
cell index for
about 15 - 20 hrs. For one particular cell index curve, after the cell index
reached a
minimum, it then reversed its decreasing trend and started to increase. Such
"going down
and then going up" feature in cell index curves was also observed in cell
index curves for
cells treated with vinblastin or colcemid. Examples of cell index curve for
vinblastin-
treated cells are shown in Figure 16A and Figure 22. All these compounds -
i.e.,
paclitaxel, vinblastin and colcemid, are so called mitotic poisons and follow
similar
mechanism of drug action. For example, both vinblastin and paclitaxel act on
microtubule dynamics within a cell.
In addition, for a given assay time point, cell index (including normalized
cell
index or delta cell index), can be plotted versus compound concentration. Such
dose
response relationships can be used to derive a time-dependent ICS, IC10, IC20,
IC30,
IC40, IC50, IC60, IC70, IC80, IC90, or IC95. In some preferred embodiments, a
time-
dependent IC50 is calculated for a compound. Determining a range of time-
dependent
ICSOs for a compound provides information on when the effect of the compound
on cells
is maximal.
The CI derived from impedance data from wells comprising different cell types
and a test compound can be used to derive cell change index (CCI) values for
assay time
points. CCI values of particular cell types at assay time points can be
compared. Such
comparisons can indicate whether different cell types are responding similarly
to a
compound. CCI can also be plotted versus time, and CCI curves of cells of
different types
assayed with one or more test compounds can be compared to determine the
similarities
or differences in cellular responses of different cell types to a test
compound.
For example, the time frame, magnitude, and duration of a difference in
response
as evidenced by the curves can indicate a difference in efficacy or mechanism
of
compounds. The impedance differences can reflect differences in, for example,
cell
attachment or adhesion, cell growth or proliferation; the number of viable
cells or dead
cells; cytoskeleton organization or function; or the number of cells going
through
apoptosis or necrosis in response to a test compound.
Preferably, data from impedance monitoring of wells that comprise different
cell
types are compared. In one preferred embodiment impedance monitoring is
performed for
94
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
different cell types exposed to multiple dose concentrations of a compound. In
some
embodiments, multiple compounds can be tested with multiple cell types. In
some
embodiments, multiple compounds at multiple concentrations can be tested with
multiple
cell types.
Cytotoxicity Profiling
In another aspect, the present invention provides a method for performing real-
time
cytotoxicity assay of a compound, comprising: a) providing an above described
system;
b) seeding cells to the wells of multiple-well devices; c) adding the compound
to the
wells containing cells; d) monitoring cell-substrate impedance before and
after adding the
compound at a regular or irregular time interval; wherein the time dependent
impedance
change provides information about time dependent cytotoxicity of the compound.
In one
embodiment, the cell-substrate impedance is monitored at regular time
intervals. In
exemplary embodiments, the impedance is measured at a regular 2 hour, 1 hour,
30 min
or 15 min time interval before and after adding the compound.
Tn one embodiment of the above method, multiple wells with same cell types are
used, wherein each well is added with the compound of different
concentrations. The
method provides the time-dependent and concentration-dependent cytotoxic
responses.
In yet another aspect, the present invention provides a method for analyzing
and
comparing time-dependent cytotoxic effects of a first compound and a second
compound
on a cell type, comprising : a) performing a real-time cytotoxicity assay on a
cell type
with the first compound using the method described above; b) performing a real-
time
cytotoxicity assay on said cell type with the second compound using the method
described above; c) comparing the time-dependent cytotoxic responses of the
first
compomid and the second compound to see how similax or different the responses
from
the two compounds are. In one embodiment of this method, time-dependent
cytotoxic
responses are determined for the first compound at multiple dose
concentrations. In
another embodiment, time-dependent cytotoxic responses are determined for the
second
compound at multiple dose concentrations. In yet another embodiment, time-
dependent
cytotoxic responses are determined for both first compound and second compound
at
multiple dose concentrations.
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In another embodiment of above methods, the first compound is a compound with
a known mechanism for its cytotoxic effect and the second compound is a
compound
with an unknown mechanism for its cytotoxic effect. If the time dependent
cytotoxic
responses from the second compound are similar to that of the first one, the
second
compound may follow a similar mechanism for its cytotoxic effect to the first
compound.
Various approaches may be used in comparing the cytotoxic responses of the
compounds. A cell index (or cell number index) can optionally be calculated
using the
impedance values obtained. In one embodiment of the method described above,
time
dependent IC50 may be derived for the compounds and comparison between their
cytotoxic responses is done by comparing their time dependent IC50 curves
based on cell
index values. If the IC50 curves follow a similar time-dependent trend, the
two
compounds may follow a similar mechanism for inducing cytotoxicty effects. In
another
embodiment of the method described, direct comparison of time-dependent
cytotoxic
responses of two compounds are done where the concentrations for the two
compounds
may be the same or may be different. Direct comparison between time-dependent
cytotoxic responses may be done by analyzing the slope of change in the
measured
responses (that is equivalent to the first order derivative of the response
with respect to
time) and comparing the time-dependent slopes for the two compounds. lil
another
approach, the time-dependent cytotoxic responses may be analyzed for their
higher order
derivatives with respect to time. Comparing such high order derivatives may
provide
additional information as for the mechanisms of compotmd-induced cytotoxicity.
In one embodiment of the method, analyzing real-time cytotoxicity response may
include the derivation of time-dependent IC50 values for the compound on the
multiple
cell types. In another embodiment of the method, analyzing real-time
cytotoxicity
response may include derivation of the slope of change in the time dependent
cytotoxicity
response at a given compound concentration. In yet another embodiment of the
method,
analyzing real-time cytotoxicity response may include derivation of high-order
derivatives of the time dependent cytotoxicity response with respect to time
at a given
compound concentration.
In yet another embodiment, the above methods are applied to perform
cytotoxicity
profiling of multiple compounds on multiple cell types.
96
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In another embodiment of the method, analyzing real-time cytotoxicity response
may include derivation of the slope of change in the time dependent
cytotoxicity response
at a given compound concentration. In yet another embodiment of the method,
analyzing
real-time cytotoxicity response may include derivation of high-order
derivatives of the
time dependent cytotoxicity response with respect to time at a given compound
concentration.
Some examples of compound assays that can be performed using a cell-substrate
impedance system of the present invention are provided by way of illustration
with
reference to the figures. In these examples, cell index is calculated using
the sasi~e method
as the Cell Index calculation method (A) as described in Section C of the
present
application. In some of the figures of the present application, Normalized
Cell Index was
plotted. The Normalized Cell Index at a given time point is calculated by
dividing the
Cell Index at the time point by the Cell Index at a reference time point.
Thus, the
Normalized Cell Index is 1 at the reference time point.
As described in the present application, if the cell attachment conditions
remain
unchanged or exhibit little change over the course of an assay that uses
impedance
monitoring, then the larger the cell index, the larger the number of the cells
in the wells.
A decrease in cell index suggests that some cells are detaching from the
substrate surface
or dying under the influence of the compound. An increase in cell index
suggests that
more cells are attaching to the substrate surfaces, indicating an increase in
overall cell
number.
figure 5 shows curves that represent the time-dependent cell index for H460
cells
treated with different concentrations of the anticancer drug paclitaxel. In
this experiment,
H460 cells were introduced into wells of a 16X cell-substrate impedance
monitoring
device. The device was positioned on a device station that was located in an
incubator
maintaining conditions of 37 degrees C and 5% CO2. The cells were cultured and
treated
at their exponential growth phase with different concentrations of paclitaxel.
The
dynamic response of the cells to different doses of paclitaxel was monitored
by
monitoring cell-substrate impedance in real time every 15 minutes for 50 hours
after
treatment using a cell-substrate impedance monitoring system. The cell-
substrate
impedance monitoring system calculated the cell index at each time point
monitored and
97
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
plotted the cell index as a function of time. For paclitaxel concentrations
between 67
nanomolar and 500 nanomolar, H460 cells exhibited a gradual decrease in cell
index after
compound addition. However, the cell index reached a minimum at a time
dependent on
the compound concentration, between about 15 hours and 20 hours after compound
addition. After that point, there was a gradual increase in cell index in
these wells. The
cell index for compound concentration of 33 nanomolar exhibited a near-
constant value
for up to about 15 hours after compound addition. After 15 hours following
compound
addition, the cell index exhibited a gradual increase.
Information about cell responses to the compound includes, but is not limited
to,
information about cell attachment or adhesion status (e.g. the degree of cell
spread, the
attachment area of a cell, the degree of tightness of cell attachment, cell
morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis and/or necrosis. W formation about
cell status
may also include any compound-cell interaction leading to any change to one or
more of
above cell status indicators. For example, if the compound binds to a receptor
on the cell
surface and such binding leads to a change in cell morphology, then the
binding of
compound to the receptor can be assayed by the monitored cell-substrate
impedance. The
cell-based assays that be performed with above methods include, but not
limited to, cell
adhesion, cell apoptosis, cell differentiation, cell proliferation, cell
survival, cytotoxicity,
cell morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, cell-based assay for screening and measuring ligand-receptor binding.
Figure 6 shows curves that represent the time-dependent cell index for H460
cells
treated with anticancer drug AC101103. H460 cells were introduced into wells
of a 16X
cell-substrate impedance monitoring device. The device was positioned on a
device
station that was located in an incubator maintaining conditions of 37 degrees
C and 5%
COz. The cells were cultured and treated at their exponential growth phase
with different
concentrations of AC 101103. The dynamic response of the cells to different
doses of
9~
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
AC101103 was monitored by measuring impedance in real time every 30 minutes
for
about 20 hours after treatment on the cell-substrate monitoring system.
Notably, the time-dependent cell index in Figure 6 is significantly different
from
those shown in Figure 5. For compound concentrations at 3.125 microgram/ml,
6.25
microgram /ml and 12.5 microgram /ml, the cell index exhibited a near-constant
value for
about 5 hrs, about 15 hrs and > 20 hrs respectively. For compound
concentrations at
3.125 microgram /ml and 6.25 microgram/ml, the cell index started to increase
after
about 5 hrs and about 15 hrs following compound addition. For the compound
concentration of 25 microgram/ml, there was a gradual, yet slow decrease in
the cell
index after compound addition. For the compound concentration of 50 microgram
/ml,
there was an about 10 hr time period over which the cell index remained near-
constant,
and after that, the cell index decreased steadily.
Figure 7 shows dynamic drug response curves of A549 cells treated with
doxorubicin. 10,000 A549 cells were seeded into each well of a 16 X device.
The device
was positioned on a device station that was located in an incubator
maintaining
conditions of 37 degrees C and 5% C02. Cell attaclnnent and cell growth were
monitored
on a cell-substrate impedance system in real time before treatment by
monitoring
impedance at regular intervals. When the cells were in exponential growth
phase,
doxorubicin at different concentrations was added to the wells. The same
volume of the
solvent used to dissolve the drug was added to some wells as a control. The
time, and
drug dose dependent cell response (calculated as cell index) to doxorubicin
was recorded
in real time on the cell-substrate impedance monitoring system as shown in
this figure.
Example 1. Profiling of dynamic cell responses to anti-cancer drugs using ACEA
RT-CES system.
In this study, we used the RT-CES system to dynamically monitor cancer cell
responses
to chemotherapeutic compounds with characterized mechanisms, and to profile
the
specific cell response patterns. Thirteen cancer cell lines including cancers
of breast,
prostate, lung, colon, ovary, kidney, fibroblast, and central nervous system
were tested
(Table 1). Each cancer cell type was treated with 11 chemotherapeutic
compounds,
classified as DNA damaging agents, protein kinase inhibitors, anti-mitotic
drugs, cell
99
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
cycle specific inhibitors, protein synthesis inhibitors plus a compound of
unknown
category (Table 2). Dynamic and dose dependent cell-compound interaction
patterns
were characterized and summarized for all the tested cell lines and compounds.
The
profiles for three drugs, doxorubicin, olomoucine and paclitaxel against a
panel of 12
different cell lines are presented in Figures 9, 10 and 11= respectively. In
addition, we
characterized the biological effect of these compounds on cells by monitoring
cell cycle
progression, cell viability and morphological status of the cells in an
attempt to correlate
specific cellular response to the shape of the cell index trace (Figures 12,
13 and 14).
Furthermore we calculated the time-dependent IC-50 values for each compound
against
the various cell lines (Figure 15) and developed an algorithm to calculate
Cell Change
Index to profile the dynamic cell response of the different chemotherapeutic
agents across
the different cell lines. Cell Change Index was calculated for the dynamic RT-
CES
responses of different cell lines to different chemotherapeutic agents using
the definitions
described above. Based on the time-dependent values of CCI, each CCI value
region
across the time scale is represented by black-white shading-based coding. For
example,
if after compound addition, the CCI value (for a particular cell line under a
specific
compound treatment at the concentration of IC50 value) is nearly zero for
certain period
of time and then becomes positive, attaining a value about 0.7/DT (DT is
doubling).
Then the cell response to this compound is represented as a ~ rectangle
followed by a
rectangle. Examples of such analysis is shown in Figures 16. The overall black-
white
shading-based coding map representing the cell dynamic responses to various
compounds
is shown in Figure 17.
In summary of this study, we note that using the RT-CES system to screen
chemotherapeutic agents results in unique activity patterns that is dependent
on the
compound itself, the concentration, length of incubation and the cell type.
The
"signature" patterns of each drug correlates with specific biological
phenomenon such as
log growth, cell cycle rest, morphology change and cell death. Cell Change
Index was a
good parameter derived from RT-CES data to mathematically describe cell
changes. Cell
response profiling based on CCI value indicates that drugs with similar
mechanism of
action displays similar patterns. Thus, the similarity in the dynamic cell-
compound
interaction patterns may indicate similarity in mechanism of action, mode of
resistance
100
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
and possibly molecular targets. The RT-CES system can be readily adapted to
high
throughput dynamic screening and analysis of anti-cancer compounds and the
information-intensive approach presented in this study can be applied to
profile existing
cancer chemotherapeutic agents, screen new compounds and provide insight into
the
mechanism of action of anti-cancer agents.
Table I. List of cancer cell lines tested against a number of chemical
compounds.
4 y<-,.." ~ 4C~! ~6d " . ~.,~~
" ~ '
~ ;:~~ ~ &~~ r4? , 1 ~a
RM7~~~~ :
MDA.MB231 Breast Cancer
MCF7 Breast Cancer
NCI-H460 Non-Small Cell Lung Cancer
MV522 SW Non-Small Cell Lung Cancer
A549 Non-Small Cell Lung Cancer
HT29 Colon cancer
HCC2998 Colon cancer
A2780 Ovarian Cancer
OVCAR4 Ovarian Cancer
PC-3 Prostate Cancer
HepG2 Human Hepatosarcoma
A431 Epidermoid Cancer
HT1080 Fibrosarcoma
101
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Table II. List of chemical compounds used in the study of profiling cell
dynamic
responses to a number of anti-cancer compounds.
~I~ "~~-BdI~"' ~.
Blb ! f~ ~'~
~ ~~. a'~41 *~e ,
d . G)<"~ir ,F"
_. ~ r, r" ifk J , 3 ~ , . ..
~.~ a y- ~,', ,> .'wt~ a ,: v~ 're !r~. v
ar ; 1 .'~' ~'%T.6~' ~ 1.y~
' ~9' i
, ;..', f"
i a $ JI
~ ~~~,~~~w~~:~~lll
,n ,. ~.~..., ~ di5k:n~.~~~~. ~~.,.
~,~:....~, ~ .., b7 .... .k, a~~ ,. Y ww~~,F~~~m~~~~~li~S
~ ~, ..,a. < <. ..
u ,.~,~ .,<
, . a,. ..
Effect on DNA replication From High to Low (dilution
or Topology factor:2)
Doxorubincin 6.25 uM - 0.098 uM
5-Fluorouracil 50 uM - 0.78 uM
Mitotic Poisons
Colcemid 3.125 uM - 0.049 uM
Paclitaxol 0.0125 uM- 0.00019
uM
Vinblastin 1.56 uM - 0.024 uM
Cell Cycle Arrest
Olomoucine 100 uM - 1.56 uM
Roscovitine 50 uM - 0.78 uM
Kinase Inhibitors
Staurosporine 5 uM - 0.078 uM
Tamoxifan 50 uM - 0.78 uM
____.___ ___.._.._...
_..
Protein synthesis Inhibitor
Rifampicin 100 uM - 1.56 uM
Unknown type
ACEA-1
Example 2. Cytotoxicity Profiling
Methods
Cells. All the cells used in this study were obtained from ATCC and maintained
at 37°C
incubator with 5% C02 saturation. H460, HepG2 and HT1080 cells were maintained
in
RPMI media containing 5% FBS and 1% penicillin and streptomycin. NIH3T3 cells
were
maintained in DMEM media containing 10% FBS and 1% penicillin and
streptomycin.
Cell Pr-olifercztion Assays. For each of the cell type, the indicated number
of cells was
seeded per well in 96X microtiter plates (e-plateT~ with incorporated
electrode structures
in individual wells device in 100 ~,L of media. The attachment, spreading and
proliferation of the cells were continuously monitored every 30 minutes using
the RT-
102
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
CESTM system (a cell-substrate impedance monitoring system. Cell proliferation
was
monitored for a period of 48-72 hours depending on the experiment. The
electronic
readout, cell-sensor impedance is displayed as a parameter called Cell Index.
Drug Treattnent and Cytotoxicity Assessfnent. For each cell type the optimum
cell
concentration was chosen based on their respective proliferation pattern
(Figure 18). The
indicated cell numbers were seeded per well of ACEA's 16X or 96X e-plateTM (
exemplary devices of the present invention) in 100 pL final volume. The
attachment,
spreading and proliferation of the cells were continuously monitored every 30
minutes
using the RT-CES system (an exemplary system of the present invention).
Approximately
24 hours after seeding, when the cells were in the log growth phase, the cells
were treated
with 100 pL of the indicated compounds dissolved in cell culture media. The
cells were
also treated with DMSO, which served as vehicle control. Depending on the
experiment,
the final DMSO concentration in the media was in the range of 0.25%-0.5%.
MTTAssay. Increasing numbers ofNIH3T3 cells were seeded in 16X e-plate and
monitored by RT-CES to obtain the corresponding Cell Index. The media was
immediately aspirated and the cells were then assayed by using the standard
MTT assay
according to the manufacturer's protocol.
Flow Cytometry. A549 cells were seeded at a density of 500,000 cells/well in
60 mm
tissue culture dishes. Approximately, 24 hours after seeding, the cells were
treated with
the indicated final concentration of Olomoucine and 16 hours later the cells
were washed
with PBS, trypsinized, washed twice with PBS and fixed in 70% methanol and
stored at
4°C until the staining step. The cells were stained with propidium
iodide and analyzed by
FAGS using a wavelength of 488nm.
Monitoring Dynamic Cell Proliferation in Real-Time Using the RT-CES
In order to assess dynamic cell proliferation using the RT-CES system, H460
human lung cancex cells, H1080 fibrosaxcoma cells, HepG2 human hepatosarcoma
cells
and NIH3T3 mouse fibroblasts were seeded at 2500 and 10,000 cells per well in
triplicate
103
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
in ACEA's 96X e-plateTM. The cells were continuously monitored every 30
minutes
using the RT-CES system for the indicated period of time (Figure 18). As shown
in
Figure 1 ~, each cell type has its own characteristic kinetic trace, based on
the number of
cells seeded, the overall size and morphology of the cells and the degree to
which the
cells interact with the sensor surface. Also, the adhesion and spreading
kinetics as well as
time when the cells enter the log growth phase is characteristic of each of
the indicated
cell lines and therefore offers an excellent internal control and a way to
standardize and
validate stock cultures during different phases of the manufacturing process.
To ascertain that the RT-CES units of Cell Index correlates with the number of
the cells in the well, increasing numbers of NIH3T3 cells were seeded in ACEA
16X e-
plateTM and were monitored for up to 10 hours, at which time the Cell Index
was
acquired. Figure 19A shows a plot of Cell number seeded versus the Cell Index
obtained
and indicates that for this particular cell type the RT-CES system could
detect as little as
100 cells and the readout is linear by two orders of magnitude all the way up
to 10000
cells. In addition, at the end of the experiment described in Figure 19A, the
cells were
also assayed by the MTT assay. As shown in Figure 19B, even at up to 1000
cells the
MTT assay is not appreciably different than background values and for cell
numbers
exceeding 1000, then the MTT units correlates with the number of cells seeded
in a linear
fashion. However, it is important to remember that while the RT-CES system is
capable
of dynamic and continuous measurements, for comparative reasons the experiment
described in Figure 19 was only conducted at a single point, since MTT is a
single point
assay.
Assessment of Drug Interaction with Target Cells Using the RT-CESTM System
To assess drug potency using the RT-CES system, the IC-50 value of Tamoxifen
was
determined for different cell lines and compared with MTT assay at 4~ hours
after
Tamoxifen addition. According to Table III, the IC-50 values obtained for
Tamoxifen for
the different cell lines using the RT-CES system is very consistent with the
values
obtained by the MTT assay, indicating that the RT-CES system can be used to
assess the
potency of various drugs against different adherent cell lines.
104
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
In order to observe the kinetics of drug interaction with target cells, A549
non-small lung
cancer cells were seeded in ACEA 96X e-plateTM and continuously monitored
until the
cells reached the log growth phase at which point different concentrations of
paclitaxel
were added to the cells at the indicated final concentration. As shown in
Figure 20A,
paclitaxel at the highest concentration initially induces a cytotoxic effect
which is mainly
due to cell death as judged by Annexin V staining (Figure 20B). Remarkably,
the cells
recover from the initial cytotoxic effect of the drug and start to re-
proliferate. While it
remains to be determined if this phenomenon is due to metabolism and
inactivation of
paclitaxel or due to the emergence of paclitaxel-resistant subpopulation, this
experiment
clearly exemplifies the tremendous advantage of real-time measurement which is
offered
by the RT-CES system and allows the user to the opportunity to observe and
assess the
entire history of drug interaction with the target cells which provides
further information
in addition to cell viability or cytotoxicity. The phenomenon observed in
Figure 20A
would've been easily missed by traditional single-point assays such as MTT.
Yet another major advantage of using the RT-CES system to continually monitor
the
interaction of drugs with target cells is that the user can obtain insight
into the mechanism
of action of the drug of interest. To demonstrate this point, A549 cells were
seeded in
ACEA 96X microtiter device and continually monitored by the RT-CES. The cells
were
treated with either DMSO as the vehicle control or with 100 p,M Olomoucine
which is a
CDK inhibitor and induces cell cycle arrest either at G1-jS transition or at
the G2-jM
transition, depending on the cell line. As shown in Figure 21A addition of
Olomoucine to
exponentially growing A549 cells causes the trace of the Cell Index recordings
of the
cells to level off and remain in a steady state that is reminiscent of cell
cycle block, where
the cells are neither proliferating nor dying off. The control cells treated
with DMSO
continue to proliferate until they reach confluence, at which time they are
contact .
inhibited and the Cell Index recording levels off. To demonstrate that the
effect of
Olomoucine on A549 cells as monitored by the RT-CES was indeed due to an
arrest of
the cell cycle, A549 cells growing on tissue culture dish were treated with
the same
concentrations of DMSO and Olomoucine and subjected to flow cytometry
analysis. As
shown in Figure 21B, the flow cytometry analysis indicates that treatment of
A549 cells
105
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
with the indicated concentration of Olomoucine induces cell cycle arrest at
the G2~M
transition, where CDKs such as CDK2 is active. Taken together, using the RT-
CES
system to dynamically monitor drug interaction with the target cells offers
the user the
opportunity to understand the mechanism of drug action and its mode of
interaction with
the target cell.
In order to assess the RT-CES system for analysis of cytotoxicity, the
interaction of A549
cells was examined with cytotoxic agents with different mechanism of action.
Figure 22
shows the characteristic trace of A549 cells monitored by RT-CESTM and treated
with
different concentrations of 5-fluorouracil, vinblastine and staurosporine.
According to
Figure 22, dynamic monitoring of the interaction of the indicated cytotoxic
agents leads
to the generation of characteristic kinetic patterns that is dependent on the
cellular
background, the concentration of the drug, the duration of exposure and the
mechanism
of drug action. Since each compound has its own characteristic pattern, these
kinetic
traces could potentially be used to determine the mechanism of action of
compounds with
unknown targets by comparing the kinetic profile to the profile of compounds
with
known mechanism of action.
Label-free and dynamic monitoring of cell proliferation, viability and
cytotoxicity
using the RT-CES system offers very distinct and important advantages over
traditional
endpoint assays. It allows for built in internal quality control to assure
consistency and
reproducibility between the different assays. Dynamic monitoring allows for
observation
of the entire episode of drug interaction with target cells and the user can
therefore have a
better understanding of the mode and mechanism of drug interaction.
Furthermore, the
actual kinetic trace of the drug interaction with the target cell is very
significant because
it can offer clues as to the mechanism of drug interaction with the target
cell. Finally,
since each compound or drug has its own characteristic profile with respect to
its
interaction with target cells, the RT-CES system can be used as a way to
determine the
mechanism of action of drugs with unknown targets.
106
CA 02556219 2006-08-04
WO 2005/077104 PCT/US2005/004481
Table III. Comparison of IC-50 values for Tamoxifen treatment of different
cancer cell
lines using the RT-CES system versus MTT assay. The indicated cell lines were
seeded
in ACEA 16X devices and monitored by RT-CES. Approximately 24 hours later, the
cells were treated with increasing concentrations of Tamoxifen and then
continually
monitored by RT-CES. The experiment was stopped about 48 hours later and the
cells in
the 16X devices were assayed by using MTT. The IC-50 values derived from RT-
CES
system are time-dependent. In the table, the IC-50 values at about 48 hrs
after compound
treatment are shown for RT-CES system determination and MTT assay.
15
All of the references cited herein, including patents, patent applications,
and publications,
and including references cited in the Bibliography, are incorporated by
reference in their
entireties.
Headings are for the convenience of the reader and do not limit the scope of
the
invention.
107