Note: Descriptions are shown in the official language in which they were submitted.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-1-
"Treatment Of Alkaline Bayer Process Residues"
Field of the Invention
The present invention relates to a method for the treatment of residues from
the
Bayer process. More particularly, the present invention relates to a method
for
decreasing the pH of alkaline Bayer process residues to render such more
suitable for disposal. In one form, the present invention also provides a
means for
the concomitant treatment of flue gases.
Background Art
The Bayer process is widely used for the production of alumina from alumina
containing ores, such as bauxite. The process involves contacting alumina-
containing ores with recycled caustic aluminate solutions, at elevated
temperatures, in a process commonly referred to as digestion. Aluminate
solution
is separated from the resulting slurryand cooled.
During alumina extraction, lime and small quantities of chemical reagents are
also
added to the process. The entrained alkaline solution in the residual slurry
contains caustic soda and other soluble compounds resulting from the reaction
of
the caustic soda with bauxite ore (such as a range of sodium-organic species
and
dissolved alumina) and also from reaction with air (such as sodium carbonate).
Whilst the vast majority of the aluminate solution is removed prior to
disposal of
the solids, the remaining solution entrained in the residual solids, and the
resulting
elevated pH, causes difficulties for disposal.
Carbonation has been shown to be an effective means treating residual slurry
to
reduce its pH and hence the hazardous nature of the residue normally
attributed
to the high pH and corrosivity of the entrained liquor. Carbonation is the
addition
of gaseous CO2 to a residue slurry prior to the deposition of this slurry into
a
residue storage area. The CO2 reacts with the alkaline components within the
liquor, and if held in contact with the slurry for long enough, the adsorbed
and
solid forms of alkalinity are also reacted according to the following
equations:
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-2-
NaAI(OH)4 + CO2 ~- NaAICO3(OH)2 + H2O
NaOH + CO2 - NaHCO3
Na2CO3 + CO2 + H2O ~ 2NaHCO3
3Ca(OH)2.2AI(OH)3 +3CO2 ~ 3CaCO3 + A1203.3H20 + 3H20
International patent application WO 93/16003 (Alcoa of Australia Ltd)
describes
the direct injection of concentrated CO2 into thickened residue slurry.
However,
the effectiveness of this treatment at an operational scale is dependant on
the
level of solid alkalinity present in the slurry as tri-calcium aluminate,
which is slow
to react. Adequate retention time in pressurised reaction vessels and the
production of sufficient buffering, in the form of bicarbonate and sodium
aluminium
carbonate, is required to ensure a pH of 10.5 or less is sustained in the
final
residue deposit.
Whilst the use of concentrated CO2 adds significantly to the costs associated
with
the method described in International patent application WO 93/16003, it does
allow direct injection of CO2 into a residue slurry, obviating the need to use
elaborate dilute gas contacting systems. The presence of solids and dissolved
alumina in the residue slurry to be treated, presents a number of significant
issues
for the operation of these dilute gas contacting systems.
The preceding discussion of the background to the invention is intended to
facilitate an understanding of the present invention. However, it should be
appreciated that the discussion is not an acknowledgement or admission that
any
of the material referred to was part of the common general knowledge in
Australia
as at the priority date of the application.
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of
any other integer or group of integers.
CA 02556439 2010-09-29
-3-
Disclosure of the Invention
In accordance with the present invention there is provided a method for the
treatment of an alkaline Bayer process residue, the method comprising a step
of:
contacting the alkaline Bayer process residue with a bicarbonate solution.
A bicarbonate solution is a readily produced form of acidity that obviates the
need
for the elaborate gas-slurry contacting systems necessary for anything other
than
concentrated carbon dioxide streams. The bicarbonate solution may be
generated by any means. However, one advantage of the invention is that the
bicarbonate solution may be generated from a gas stream of relatively low
carbon
dioxide concentration and may be contacted with the Bayer process residue
without the need for elaborate gas-slurry contactor apparatus, which are prone
to
scaling.
Preferably, the bicarbonate solution is at an elevated temperature.
As discussed in the `Background Art' section, alkaline Bayer process residue
contains solid alkalinity in the form of tri-calcium aluminate. Elevated
temperatures facilitate the reaction of tri-calcium aluminate. However, it is
difficult
and expensive to adequately heat a thickened slurry, so the application of
elevated temperatures to the method described in International patent
application
WO 93/16003 is not generally considered practical. The use of a bicarbonate
solution at elevated temperature substantially overcomes this problem.
In one form of the invention, the bicarbonate solution is formed by the step
of:
contacting a solution with carbon dioxide.
Preferably, the solution is a carbonate solution. Economically, the solution
is a
sodium carbonate solution.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-4-
Without wishing to be bound by theory, where the solution is a sodium
carbonate
solution, the reaction of the carbon dioxide with the sodium carbonate
solution to
form sodium bicarbonate may be represented as follows:
Na2CO3 + CO2 + H2O -- 2NaHCO3
Further, where the solution is a sodium carbonate solution, the reaction of
the
sodium bicarbonate with the liquid and solid forms of alkalinity in the
alkaline
Bayer process residue may be represented as follows:
NaAI(OH)4 + 2NaHCO3 1 NaAICO3(OH)2 + Na2CO3 + 2H20
NaOH + NaHCO3 7 Na2CO3+ H2O
3Ca(OH)2.2AI(OH)3 + 6NaHCO3 7 3CaCO3 + A1203.3H20 + 3Na2CO3 + 6H20
In a more specific form of the invention, the bicarbonate solution is formed
by the
step of:
Contacting the solution with a gaseous stream containing carbon dioxide.
In one form of the invention, the gaseous stream may be provided in the form
of
flue gas.
Typically, flue gas is produced at elevated temperatures, and this
conveniently and
economically allows the production of a bicarbonate solution at elevated
temperatures. Conveniently, the flue gas is provided in the form of a flue gas
generated by the Bayer process. A variety of flue gases, such as calciner flue
gas, boiler flue gas and kiln flue gas are generated at Bayer process
refineries.
Conveniently, the gaseous stream comprises calciner flue gas, boiler flue gas
or
kiln flue gas, or a mixture of two or more of such.
The flue gas may be treated to increase carbon dioxide concentration prior to
contact with the solution.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-5-
Where the gaseous stream containing carbon dioxide is provided in the form of
a
flue gas, prior to the step of contacting the solution with a flue gas, the
present
invention preferably comprises the step of:
Cooling the flue gas.
Preferably still, where the gaseous stream containing carbon dioxide is
provided in
the form of a flue gas, prior to the step of contacting the solution with a
flue gas,
the present invention preferably comprises the steps of:
Cooling the flue gas and retaining the heat therefrom.
Where the gaseous stream containing carbon dioxide is provided in the form of
a
flue gas, prior to the step of contacting the solution with a flue gas, the
present
invention preferably comprises the steps of:
Dehumidifying the flue gas.
Some flue gases have an appreciable water content, and dehumidifying the flue
gas may appreciably increase the carbon dioxide concentration.
In one form of the invention, the steps of cooling the flue gas and
dehumidifying
the flue gas are concurrently achieved by the step of:
Contacting the flue gas with a cool water stream.
The step of cooling the flue gas prior to contacting such with the solution
enables
more efficient conversion to bicarbonate, with efficiencies diminishing at
elevated
temperatures.
In one form of the invention, where the bicarbonate solution is provided at
elevated temperature and method comprises the step of cooling the flue gas and
retaining the heat therefrom, the present invention preferably comprises the
step
of:
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-6-
Utilising the heat retained from the step of cooling the flue gas to produce
the bicarbonate solution at elevated temperature.
Thus, in a preferred form of the invention, the flue gas is contacted with the
solution at a temperature amenable to the efficient formation of the
bicarbonate
solution, whilst the bicarbonate solution is contacted with the alkaline Bayer
process residue at a temperature amendable to the efficient neutralisation of
solid
alkalinity in the form of tri-calcium aluminate.
Flue gas typically contains particulates, odour forming compounds, sulfurous
compounds and volatile organic carbon compounds. The use of flue gas as the
gaseous stream affords the further advantage of reducing the quantities of
particulates, odour forming compounds, sulfurous compounds and volatile
organic
carbon compounds from at least a portion of the flue gas. Thus, where the
gaseous stream comprises flue gas, the present invention also provides a means
for the concomitant treatment of flue gases.
Carbonation of residue using a bicarbonate solution in the particular
embodiment
where the bicarbonate solution is generated using flue gas provides the
additional
benefit of providing a sink for the CO2 which reduces the atmospheric emission
of
CO2 (and other substances) in the flue gas.
Preferably, the pH of the bicarbonate solution is between about 7.5 and 9Ø
Preferably still, the pH of the bicarbonate solution is between 7.5 and 8Ø
Preferably, the total alkali concentration of the bicarbonate solution is
substantially
identical to the total alkali concentration of the alkaline Bayer process
residue.
Prior to the step of contacting the alkaline Bayer process residue with a
bicarbonate solution, the method may comprise the step of:
Thickening the alkaline Bayer process residue.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-7-
The alkaline Bayer process residue produced by the digestion process contains
significant levels of soda and alumina. Thickening the alkaline Bayer process
residue reduces soda and alumina levels.
The extent to which the alkaline Bayer process residue is thickened prior to
contact with the bicarbonate solution is largely an economic consideration.
Higher
density results in higher soda and alumina recovery. Higher density also means
less caustic in solution to be treated with the bicarbonate.
Preferably, after the step of contacting the solution with a gaseous stream
containing carbon dioxide, the method comprises the step of:
Recovering a carbonate solution.
In one form of the invention, the step of contacting the alkaline Bayer
process
residue with a bicarbonate solution is performed in a reaction vessel having
an
overflow and an underflow, and the step of recovering a carbonate solution
comprises the step of:
Recovering the carbonate solution as an overflow from the reaction vessel.
In one form of the invention, the reaction vessel is a gravity separation
vessel,
such as a thickener.
In a preferred form of the invention, where the invention comprises the step
of
contacting a carbonate solution with carbon dioxide, the carbonate solution so
contacted is provided in the form of the recovered carbonate solution.
The recovered carbonate solution is low in alumina, the dissolved alumina
having
largely precipitated once the alkaline Bayer process residue is contacted with
the
bicarbonate solution. The low alumina levels substantially reduce scaling
issues
in any apparatus by which the recovered carbonate solution is contacted with
carbon dioxide.
CA 02556439 2010-09-29
-8-
In a specific form of the present invention, there is provided a method for
the
treatment of an alkaline Bayer process residue, the method comprising steps
of:
contacting a carbonate solution with a gaseous stream containing carbon
dioxide to form a bicarbonate solution;
contacting the alkaline Bayer process residue with the bicarbonate
solution;
recovering a carbonate solution; and
recycling the recovered carbonate solution by contacting the recovered
carbonate solution with a gaseous stream containing carbon dioxide to
form a bicarbonate solution.
In a more specific form of the invention, there is provided a method for the
treatment of an alkaline Bayer process residue, the method comprising steps
of:
cooling and dehumidifying a flue gas by contacting the flue gas with a cool
water stream and retaining the heat therefrom;
contacting a carbonate solution with the cooled, dehumidified flue gas to
form a bicarbonate solution ;
heating the bicarbonate solution utilising the heat recovered from the
cooled flue gas;
contacting the alkaline Bayer process residue with the heated bicarbonate
solution;
recovering a carbonate solution; and
CA 02556439 2010-09-29
-9-
recycling the recovered carbonate solution by contacting the recovered
carbonate solution with a cooled, dehumidified flue gas to form a
bicarbonate solution.
The steps of the method may be performed simultaneously in a continuous
process.
Best Mode (s) for Carrying Out the Invention
The best method of performing the present invention currently known to the
applicant will now be described by way of example only, with reference to
Figure
1.
It must be appreciated that the following description of the best method does
not
limit the generality of the preceding description of the invention.
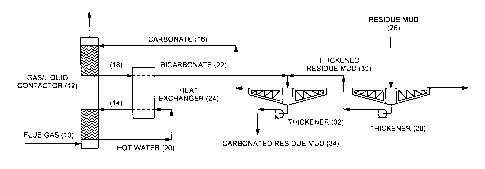
As shown in the accompanying drawing, a gaseous stream containing carbon
dioxide in the form of hot flue gas 10 is introduced into a gas-liquid
contactor 12.
The hot flue gas 10 is first contacted with a cold water stream 14, and thus
cooled and dehumidified. The cooled and dehumidified flue gas is then
contacted
with a carbonate solution 16, thereby generating a bicarbonate solution 18 of
pH
of approximately 7.5-8.
Heat recovered from the hot flue gas 10 produces a hot water stream 20, which
is
then used to produce a heated bicarbonate solution 22 to a temperature of
approximately 60 C, by way of a heat exchanger 24. A temperature of
approximately 60 C is readily achievable by heat transfer from flue gas, and
causes improved reaction of tri-calcium aluminate.
Alkaline Bayer process residue in the form of residue mud 26 from digestion is
introduced into a first thickener 28. Thickened alkaline Bayer process residue
in
the form of underflow 30 from the first thickener 28 is mixed with the heated
bicarbonate solution 22 in a second thickener 32, wherein the bicarbonate of
the
heated bicarbonate solution 22 reacts with the solid and liquid alkalinity of
the
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-10-
thickened alkaline Bayer process residue, thereby reducing the alkalinity
thereof.
The total alkali concentration of the bicarbonate solution 22 is substantially
identical to that of the thickened residue mud 30. Carbonated residue 34 from
the
second thickener 32 is removed as underflow, whilst the overflow 16 is
recycled to
the contactor 12 in the form of the carbonate solution 16 for generation of
the
bicarbonate solution 18.
Experimental
To further describe the invention, a series of experiments will now be
described.
However, it must be appreciated that the following description of those
experiments is not to limit the generality of the above description of the
invention.
The experiments were conducted in order to determine the feasibility of
residue
neutralisation using dual superthickeners with stack gas carbonation of the
second superthickener overflow. The objective was to produce a carbonated
residue slurry, which was considered to be a slurry where the pH has been
reduced to below pH 10.0, the majority of the solid alkalinity (primarily tri-
calcium
aluminate) has been reacted and there is sufficient bicarbonate in the final
solution to buffer reversion from any remaining unreacted solid alkalinity
An alkaline Bayer process residue in the form of a sample of superthickener
underflow (STUF) was collected from a Western Australian alumina refinery. The
STUF had a TC (total caustic concentration expressed as g/L sodium carbonate)
of 18.4 g/L, a TA (TA represents total alkali concentration expressed as g/L
sodium carbonate) of 23.4 g/L and an aluminate concentration of 7.6 g/L
(expressed as g/L AI203). Further, a carbonate solution in the form of a
sample of
superthickener overflow (STOF) was collected at the same time. The STOF had
a TC of 16.9 g/L, a TA of 24.1 g/L and an aluminate concentration of 8.5 g/L.
The superthickener overflow sample was carbonated, by bubbling through
concentrated C02, to produce a bicarbonate solution (STOFC), filtered on a
filter
press and shown to have a TC of -22.34 g/L, a TA of 21.99 g/L and an aluminate
concentration of <0.04 g/L.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-11-
The carbonated super-thickener overflow (STOFC) was then added to the
superthickener underflow (STUF) at ratios between 0.8 and 4.0 kL of STOFC per
kL of STUF.
Three sets of samples at each mixing ratio were added to a rotating water bath
at
60 C, a nominal temperature within the likely range achievable with heat
transfer
from the flue gas. A sample was taken off at 30 minutes, 6 hours and at 24
hours.
The liquors were analysed for pH TC, TA, A1203 and the solids for tri-calcium
aluminate (TCA6).
Results of the test work are summarised in the following table below and shown
graphically on Figures 2 to 4. Figure 2 shows the pH of the slurry after
mixing
with STOFC. Figure 3 shows the TC of the slurry after mixing with STOFC, TC
being the total caustic in solution (a positive number indicates NaOH, a
negative
number indicates NaHCO3). Figure 4 shows the alumina concentration of the
slurry after mixing with STOFC.
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-12-
Liquor Alkali TCA6 A1203 pH
(expressed as g/L Na2CO3)
NaOH Na2CO3 NaHCO3 Total
(g/L) (g/L) (g/LI) (g/L) (g/kg) (g/L)
Compositions Prior to Mixing
STOF 16.93 7.20 0 24.13 8.46 13.03
STOFC 0 0 22.34 21.99 <0.04 7.45
STUF 18.42 4.94 0 23.36 1.00 7.56 13.10
Compositions After Mixing
Mixing Ratio
Time STOFC/
(hours) STUF
0.5 0.8 5.78 15.67 0 21.45 0 4.14 12.17
6 0.8 6.83 14.41 0 21.24 0 3.86 12.36
24 0.8 6.73 14.42 0 21.15 0 3.21 12.44
0.5 1.0 3.35 18.17 0 21.52 0 2.93 11.89
6 1.0 3.87 17.38 0 21.25 0 2.47 12.13
24 1.0 3.93 17.26 0 21.19 0 2.06 12.25
0.5 1.4 0.15 21.55 0 21.70 0 1.44 10.95
6 1.3 0.71 20.76 0 21.47 0 1.09 11.37
24 1.3 0.57 20.85 0 21.41 0 0.79 11.50
0.5 1.8 0 19.24 2.40 21.64 0 0.62 10.40
6 1.7 0 19.95 1.70 21.65 0 0.47 10.57
24 1.7 0 19.85 1.75 21.59 0 0.32 10.61
0.5 2.1 0 17.16 4.28 21.43 0 0.21 10.02
6 2.1 0 17.91 3.77 21.68 0 0.28 10.23
24 2.1 0 17.83 3.82 21.65 0 0.18 10.23
0.5 2.5 0 15.36 6.05 21.41 0 <0.04 9.99
6 2.5 0 16.24 5.35 21.59 0 0.17 10.02
24 2.5 0 15.96 5.64 21.59 0 0.13 10.00
0.5 2.9 0 13.84 7.59 21.43 0 <0.04 9.86
6 2.9 0 14.60 6.92 21.52 0 <0.04 9.85
24 2.9 0 14.29 7.18 21.47 0 <0.04 9.83
0.5 3.3 0 12.52 8.98 21.50 0 <0.04 9.73
6 3.3 0 13.14 8.31 21.44 0 <0.04 9.72
24 3.3 0 13.17 8.22 21.39 0 <0.04 9.73
0.5 3.7 0 11.49 10.03 21.52 0 <0.04 9.65
6 3.8 0 12.07 9.35 21.41 0 <0.04 9.62
24 3.7 0 11.94 9.42 21.36 0 <0.04 9.62
0.5 3.9 0 10.82 10.66 21.48 0 <0.04 9.59
6 3.9 0 11.76 9.67 21.43 0 <0.04 9.60
24 3.9 0 11.48 9.84 21.32 0 <0.04 9.57
CA 02556439 2006-08-15
WO 2005/077830 PCT/AU2005/000182
-13-
The experiments demonstrate that with addition of a sufficient volume of
bicarbonate solution in the form of carbonated STOF, the alkaline Bayer
process
residue (STUF) was `carbonated' in that the sodium hydroxide, sodium carbonate
solution is converted to a sodium carbonate, sodium bicarbonate solution. It
can
be seen that a pH of less than 10 can be readily attained at appropriate
mixing
ratios, that the tri-calcium aluminate is fully reacted and, that with a
sufficiently
high mixing ratio, a high bicarbonate level can be retained with the settled
solids
providing a significant buffer in the settled mud.
The experiments also demonstrate that the dissolved alumina in the residue
slurry
(STUF) is precipitated and that the supernatant recovered after settling of
the
solids (STOF) is low in dissolved alumina, providing a low alumina
carbonate/bicarbonate solution for recirculation to the CO2 contactor, thereby
reducing scaling issues.
Data indicates that, at 60 C, the reaction is complete in less than 30
minutes, in
that there was no further change by holding for 6 and 24 hours.
Modifications and variations such as would be apparent to the skilled
addressee
are considered to fall within the scope of the present invention.