Note: Descriptions are shown in the official language in which they were submitted.
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Substituted pyrazoline compounds, their preparation and use as medicaments
The present invention relates to substituted pyrazoline compounds, methods for
their
preparation, medicaments comprising these compounds as well as their use for
the
preparation of a medicament for the treatment of humans and animals.
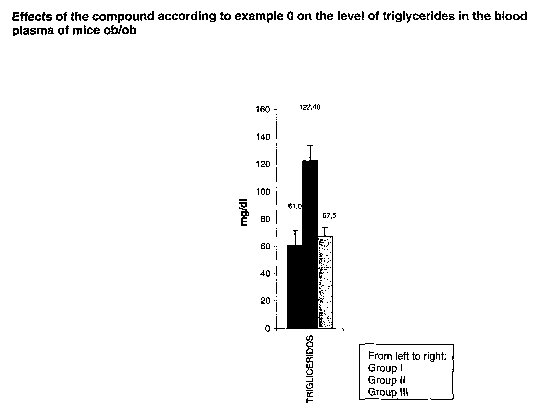
Triglycerides are the chemical form in which most fat exists in food as well
as in the
body. Triglycerides are present in blood plasma and, in association with
cholesterol,
form the plasma lipids. Triglycerides in blood plasma are derived from fats
consumed
directly or are synthesized from e.g. carbohydrates. Superfluous food intake
is
converted to triglycerides and transported to fat cells to be stored. Elevated
triglycerides may also be a consequence of disease states, such as untreated
diabetes mellitus. Excess of triglycerides in plasma (hypertriglyceridemia) is
linked to
the occurrence of coronary artery disease and possibly other disorders.
Therefore, compounds, which have an effect on triglycerides, especially in
blood
plasma are useful in the prevention and/or treatment of related disorders.
Thus, it was an object of the present invention to provide novel compounds for
use
as active substances in medicaments, which are suitable for the regulation
especially
the reduction of triglyceride levels in the blood plasma.
Said object was achieved by providing the substituted pyrazoline compounds of
general formula I given below, their stereoisomers, corresponding salts and
corresponding solvates thereof.
It has been found that these compounds have a marked effect on the level of
triglycerides in the blood plasma.
Thus, in one of its aspects the present invention relates to substituted
pyrazoline
compounds of general formula '
CONFIRMATION COPY
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
wherein
R1 represents hydrogen or a linear or branched C1_a-alkyl group,
R2, R3 and R4 independently of each other represent hydrogen, a linear or
branched C~_s-alkyl group, a linear or branched Ci_s-alkoxy group, a halogen
atom, CHEF, CHF2, CF3, CN, OH, N02, -(C=O)-R8, SH, SRB, SORs, S02R8,
NH2, NHRB, NR8R9, -(C=O)-NH2, -(C=O)-NHR8 or -(C=O)-NR8R9 whereby R8
and R9 for each substituent independently represent linear or branched Ci-s
alkyl,
R5 and Rs independently of each other represent a linear or branched Ci-s-
alkyl group, a linear or branched C~_s-alkoxy group, a halogen atom, CH2F,
CHF2, CF3, CN, OH, N02, -(C=O)-R1°, SH, SR1°, SOR1°,
NH2, NHRio,
NR'°R'1, -(C=O)-NH2, -(C=O)-NHR'° and -(C=O)-
NRi°R11, whereby Ri° and
2
R~ '
Rs
Rs
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
optionally R" for each substituent independently represent linear or branched
C~.s alkyl;
R' represents hydrogen, a linear or branched Ci~-alkyl group, a linear or
branched Ci_s-alkoxy group, a halogen atom, CH2F, CHF2, CF3, CN, OH, N02,
-(C=O)-R'°, SH, SR'°, SOR'°, NH2, NHR'°,
NR'°R", -(C=O)-NH2, -(C=O)-
NHR'° and -(C=O)-NR'°R", whereby R'° and optionally
R" for each
substituent independently represent linear or branched C~_s alkyl;
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide hereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
It is very preferred if to these compounds according to formula I the
following provisio
applies:
with the proviso that
if R' and R' are H and R5 and Rs both represent CI in the 3- and 4-position of
the phenyl ring neither of R2, R3 and R4 may represent F in the 4-position of
the phenyl ring if the other two of R2, R3 and R4 both represent H.
These compounds had a surprising effect on the blood levels of diet relevant
substances, e.g. Triglycerides.
Preferred linear or branched, saturated or unsaturated aliphatic groups, which
may
be substituted by one or more substituents, may preferably be selected from
the
group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-butyl,
sec-butyl, tert-
butyl, n-pentyl, n-hexyl, n-heptyl, n-octyl, n-nonyl, n-decyl, vinyl, ethinyl,
propenyl,
propinyl, butenyl and butinyl.
In the context of this invention, alkyl and cycloalkyl radicals are understood
as
meaning saturated and unsaturated (but not aromatic), branched, unbranched and
cyclic hydrocarbons, which can be unsubstituted or mono- or polysubstituted.
In
3
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
these radicals, Ci_2-alkyl represents C1- or C2-alkyl, C~_3-alkyl represents
C1-, C2- or
C3-alkyl, Ci~-alkyl represents C1-, C2-, C3- or C4-alkyl, C~_5-alkyl
represents C1-,
C2-, C3-, C4-, or C5-alkyl, Ci.s-alkyl represents C1-, C2-, C3-, C4-, C5- or
C6-alkyl,
C1_~-alkyl represents C1-, C2-, C3-, C4-, C5-, C6- or C7-alkyl, C~_s-alkyl
represents
C1-, C2-, C3-, C4-, C5-, C6-, C7- or C8-alkyl, Ci-,o-alkyl represents C1-, C2-
, C3-,
C4-, C5-, C6-, C7-, C8-, C9- or C10-alkyl and Ci_i8-alkyl represents C1-, C2-,
C3-,
C4-, C5-, C6-, C7-, C8-, C9-, C10-, C11-, C12-, C13-, C14-, C15-, C16-, C17-
or
C18-alkyl. Furthermore, C3~-cycloalkyl represents C3- or C4-cycloalkyl, C3_5-
cycloalkyl represents C3-, C4- or C5-cycloalkyl, C3~-cycloalkyl represents C3-
, C4-,
C5- or C6-cycloalkyl, C3_~-cycloalkyl represents C3-, C4-, C5-, C6- or C7-
cycloalkyl,
C3_s-cycloalkyl represents C3-, C4-, C5-, C6-, C7- or C8-cycloalkyl, C4_5-
cycloalkyl
represents C4- or C5-cycloalkyl, Ca-s-cycloalkyl represents C4-, C5- or C6-
cycloalkyl,
C4_~-cycloalkyl represents C4-, C5-, C6- or C7-cycloalkyl, C5_s-cycloalkyl
represents
C5- or C6-cycloalkyl and C5_~-cycloalkyl represents C5-, C6- or C7-cycloalkyl.
In
respect of cycloalkyl, the term also includes saturated cycloalkyls in which
one or 2
carbon atoms are replaced by a heteroatom, S, N or O. However, mono- or
polyunsaturated, preferably monounsaturated, cycloalkyls without a heteroatom
in
the ring also in particular fall under the term cycloalkyl as long as the
cycloalkyl is not
an aromatic system. The alkyl and cycloalkyl radicals are preferably methyl,
ethyl,
vinyl (ethenyl), propyl, allyl (2-propenyl), 1-propinyl, methylethyl, butyl, 1-
methylpropyl, 2-methylpropyl, 1,1-dimethylethyl, pentyl, 1,1-dimethylpropyl,
1,2-
dimethylpropyl, 2,2-dimethylpropyl, hexyl, 1-methylpentyl, cyclopropyl, 2-
methylcyclopropyl, cyclopropylmethyl, cyclobutyl, cyclopentyl,
cyclopentylmethyl,
cyclohexyl, cycloheptyl, cyclooctyl, and also adamantyl, (if substituted also
CHF2,
CF3 or CH20H) as well as pyrazolinone, oxopyrazolinone, [1 ,4]-dioxane or
dioxolane.
Here, in connection with alkyl and cycloalkyl - unless expressly defined
otherwise -
the term substituted in the context of this invention is understood as meaning
replacement of at least one hydrogen radical by F, CI, Br, I, NH2, SH or OH,
"polysubstituted" radicals being understood as meaning that the replacement
takes
effect both on different and on the same atoms several times with the same or
different substituents, for example three times on the same C atom, as in the
case of
CF3, or at different places, as in the case of -CH(OH)-CH=CH-CHCI2.
Particularly
preferred substituents here ark F, CI and OH. In respect of cycloalkyl, the
hydrogen
4
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
radical can also be replaced by OCi_3-alkyl or Ci-3-alkyl (in each case mono-
or
polysubstituted or unsubstituted), in particular methyl, ethyl, n-propyl, i-
propyl, CF3,
methoxy or ethoxy.
The term (CH2)3_6 is to be understood as meaning -CH2-CH2-CH2-, -CH2-CH2-CH2-
CH2-, -CH2-CH2-CH2-CH2-CH2- and -CH2-CH2-CH2-CH2-CHZ-CH2-, (CH2)1_4 is to be
understood as meaning -CH2-, -CH2-CH2-, -CH2-CH2-CH2- and -CH2-CHI-CH2-CH2-,
(CH2)4_5 is to be understood as meaning -CH2-CH2-CH2-CH2- and -CH2-CH2-CH2-
CHZ-CH2-, etc.
An aryl radical is understood as meaning ring systems with at least one
aromatic ring
but without heteroatoms even in only one of the rings. Examples are phenyl,
naphthyl, fluoranthenyl, fluorenyl, tetralinyl or indanyl, in particular 9H-
fluorenyl or
anthracenyl radicals, which can be unsubstituted or monosubstituted or
polysubstituted.
A heteroaryl radical is understood as meaning heterocyclic ring systems which
have
at least one unsaturated ring and can contain one or more heteroatoms from the
group consisting of nitrogen, oxygen and/or sulfur and can also be mono- or
polysubstituted. Examples which may be mentioned from the group of heteroaryls
are furan, benzofuran, thiophene, benzothiophene, pyrrole, pyridine,
pyrimidine,
pyrazine, quinoline, isoquinoline, phthalazine, benzo-1,2,5-thiadiazole,
benzothiazole, indole, benzotriazole, benzodioxolane, benzodioxane, carbazole
and
quinazoline.
Here, in connection with aryl and heteroaryl, substituted is understood as
meaning
substitution of the aryl or heteroaryl by R, OR, a halogen, preferably F
and/or CI, a
CF3, a CN, an N02, an NRR, a Ci_6-alkyl (saturated), a Ci_6_alkoxy, a C3_e-
cycloalkoxy, a C3_S-cycloalkyl or a C2_6-alkylene.
The term "salt" is to be understood as meaning any form of the active compound
used according to the invention in which it assumes an ionic form or is
charged and
is coupled with a counter-ion (a cation or anion) or is in solution. By this
are also to
be understood complexes of tt~ active compound with other molecules and ions,
in
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
particular complexes which are complexed via ionic interactions.
The term "physiologically acceptable salt" means in the context of this
invention any
salt that is physiologically tolerated (most of the time meaning not being
toxic-
especially not caused by the counter-ion) if used appropriately for a
treatment
especially if used on or applied to humans and/or mammals.
These physiologically acceptable salts can be formed with cations or bases and
in
the context of this invention is understood as meaning salts of at least one
of the
compounds used according to the invention - usually a (deprotonated) acid - as
an
anion with at least one, preferably inorganic, cation which is physiologically
tolerated
especially if used on humans and/or mammals. The salts of the alkali metals
and
alkaline earth metals are particularly preferred, and also those with NH4, but
in
particular (mono)- or (di)sodium, (mono)- or (di)potassium, magnesium or
calcium
salts.
These physiologically acceptable salts can also be formed with anions or acids
in the
context of this invention is understood as meaning salts of at least one of
the
compounds used according to the invention - usually protonated, for example on
the
nitrogen - as the cation with at least one anion which are physiologically
tolerated -
especially if used on humans and/or mammals. By this is understood in
particular, in
the context of this invention, the salt formed with a physiologically
tolerated acid, that
is to say salts of the particular active compound with inorganic or organic
acids which
are physiologically tolerated - especially if used on humans and/or mammals.
Examples of physiologically tolerated salts of particular acids are salts of:
hydrochloric acid, hydrobromic acid, sulfuric acid, methanesulfonic acid,
formic acid,
acetic acid, oxalic acid, succinic acid, malic acid, tartaric acid, mandelic
acid, fumaric
acid, lactic acid or citric acid.
The term "solvate" according to this invention is to be understood as meaning
any
form of the active compound according to the invention in which this compound
has
attached to it via non-covalent binding another molecule (most likely a polar
solvent)
especially including hydrates and alcoholates, e.g. methanolate.
i
6
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Unless otherwise stated, the compounds of the invention are also meant to
include
compounds which differ only in the presence of one or more isotopically
enriched
atoms. For example, compounds having the present structures except for the
replacement of a hydrogen by a deuterium or tritium, or the replacement of a
carbon
by'3C- or '4C-enriched carbon or'SN-enriched nitrogen are within the scope of
this
invention.
In a preferred embodiment of the invention for a compound according to formula
I at
least one of R2, R3 or R4 represents hydrogen, while at least one of R2, R3 or
R4 is
different from hydrogen.
In a preferred embodiment of the invention for a compound according to formula
I R'
represents hydrogen.
In a preferred embodiment of the irivention for a compound according to
formula I R2,
R3 and R4 independently of each other represent hydrogen, a linear or branched
Ci-s-
alkyl group, a halogen atom, or CF3, preferably R2, R3 and R4 independently of
each
other represent hydrogen, methyl, ethyl, F, CI, Br and CF3.
In a preferred embodiment of the invention for a compound according to formula
I R5
and Rs independently of each other represent a linear or branched Ci.s-alkyl
group, a
halogen atom, or CF3, preferably R5 and Rs independently of each other
represent
methyl, ethyl, F, CI, Br and CFA.
In a preferred embodiment of the invention for a compound according to formula
I R2
represents a chlorine atom in the 4-position of the phenyl ring, while R3 and
R4
represent hydrogen.
In a preferred embodiment of the invention for a compound according to formula
I R5
and Rs each represent a chlorine atoms in the 2- and 4-position of the phenyl
ring,
while R' represents hydrogen.
In a preferred embodiment of the invention for a compound according to formula
I R'
represents hydrogen, methyl o~ ethyl, preferably hydrogen.
7
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
R1
Ria
n
In a highly preferred further aspect of the invention the compound of general
formula
I is represented by a compound of general formula II
wherein
Ri represents hydrogen or a linear or branched Ci~-alkyl group,
R'2 or Ri3 independently of each other represent a linear or branched C~_s-
alkyl group, a linear or branched Ci.s-alkoxy group, a halogen atom, CH2F,
CHF2, CF3, CN, OH, N02, SH, NH2, hydrogen, methyl, ethyl, F, CI, Br and CF3,
R14 or R15 independently of each other represent a linear or branched Ci.s-
alkyl group, a linear or branched C~.s-alkoxy group, a halogen atom, CH2F,
CHF2, CF3, CN, OH, N02, SH, NH2, methyl, ethyl, F, CI, Br and CF3,
optionally in form of one of the stereoisomers, preferably enantiomers, or
diastereomers, a racem~te or in form of a mixture of at least two of the
8
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
In a preferred embodiment of the invention for a compound according to formula
II
R'2 and R'3 independently of each other represent hydrogen, a linear or
branched Ci_
6-alkyl group, a halogen atom, or CF3, preferably R'2 and R'3 independently of
each
other represent hydrogen, methyl, ethyl, F, CI, Br and CF3.
In a preferred embodiment of the invention for a compound according to formula
II
R'4, and R'S independently of each other represent a linear or branched Ci.s-
alkyl
group, a halogen atom, or CF3, preferably R'4 and R'S independently of each
other
represent methyl, ethyl, F, CI, Br and CF3.
In a preferred embodiment of the invention for a compound according to formula
II
R'3 represents CI and R'2 represents hydrogen.
In a preferred embodiment of the invention for a compound according to formula
II
R'4 and R'S each represent CI.
In a preferred embodiment of the invention for a compound according to formula
II R'
represents hydrogen, methyl or ethyl, preferably hydrogen.
In another preferred embodiment the compound according to formula I or II is
selected from the group consisting of:
5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazol-3-
carboxylic
acid,
optionally in the form of a corresponding N-oxide, a corresponding salt or a
corresponding solvate.
Another preferred embodiment of the invention covers also any prodrug of the
compounds of the invention described above as well as any medicament
comprising
9
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
this and any use thereof; especially including their esters and ethers.
Examples of
well known methods of producing a prodrug of a given acting compound are known
to those skilled in the art and can be found e.g. in Krogsgaard-Larsen et al.,
Textbook
of Drugdesign and Discovery, Taylor & Francis (April 2002).
Another aspect of the invention is a combination of compounds comprising at
least
one substituted pyrazoline compound of general formula I
wherein
R' represents hydrogen or a linear or branched Ci_4-alkyl group,
R2, R3 and R~ independently of each other represent hydrogen, a linear or
branched Ci_s-alkyl group, a linear or branched Ci_s-alkoxy group, a halogen
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-RB, SH, SRB, SORB, S02R8,
NH2, NHRB, NRBR9, -(C=O)-NH2, -(C=O)-NHRB or -(C=O)-NRBR9 whereby RB
and R9 for each substituent independently represent linear or branched Ci_s
alkyl,
R5, RB and R' independently of each other represent hydrogen, a linear or
branched Ci_s-alkyl grog, a linear or branched Ci_s-alkoxy group, a halogen
R~ '
R5
Rs
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-R'°, SH, SR'°,
SOR'°, NH2,
NHR'°, NR'°R", -(C=O)-NH2, -(C=O)-NHR'° and -(C=O)-
NR'°R", whereby
R'° and optionally R" for each substituent independently represent
linear or
branched Ci_6 alkyl;
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof;
and at least one substituted pyrazoline compound of general formula X
R1s
R'~
X
wherein
R's represents an optionally at least mono-substituted phenyl group,
R" represents an optionally at least mono-substituted phenyl group,
R'S represents a saturated or unsaturated, optionally at least mono-
substituted, optionally at least one heteroatom as ring member containing
cycloaliphatic group, which may be condensed with an optionally at least
mono-substituted mono- or polycyclic ring system, or an optionally at least
mono-substituted aryl or heteroaryl group, which may be condensed'with an
i
11
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
optionally at least mono-substituted mono- or polycyclic ring system, or an -
NR'9R2°-moiety,
R'9 and R2°, identical or different, represent a hydrogen atom, an
unbranched
or branched, saturated or unsaturated, optionally at least mono-substituted
aliphatic radical, a saturated or unsaturated, optionally at least mono-
substituted, optionally at least one heteroatom as ring member containing
cycloaliphatic group, which may be condensed with an optionally at least
mono-substituted mono- or polycyclic ring system, or an optionally at least
mono-substituted aryl or heteroaryl group, which may be condensed with an
optionally at least mono-substituted mono- or polycyclic ring system and/or
bonded via a linear or branched alkylene group, an -S02-R2'-moiety, or an -
NR22R2s-moiety, with the proviso that R'9 and R2° do not identically
represent
hydrogen,
R2' represents a linear or branched, saturated or unsaturated, optionally at
least mono-substituted aliphatic group, a saturated or unsaturated, optionally
at least mono-substituted, optionally at least one heteroatom as ring member
containing cycloaliphatic group, which may be condensed with a mono- or
polycyclic ring-system, or an optionally at least mono-substituted aryl or
heteroaryl group, which may be condensed with a mono- or polycyclic ring
system and/or bonded via a linear or branched alkylene group,
R22 and R23, identical or different, represent a hydrogen atom, an unbranched
or branched, saturated or unsaturated, optionally at least mono-substituted
aliphatic radical, a saturated or unsaturated, optionally at least mono-
substituted, optionally at least one heteroatom as ring member containing
cycloaliphatic group, which may be condensed with an optionally at least
mono-substituted mono- or polycyclic ring system, or an optionally at least
mono-substituted aryl or heteroaryl group, which may be condensed with an
optionally at least mono-substituted mono- or polycyclic ring system and/or
bonded via a linear or branched alkylene group,
i
12
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I at least one of R2, R3 or R4
represents hydrogen, while at least one of R2, R3 or R4 is different from
hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I at least on of R5, Rs or R'
represents
hydrogen, while at least one R5, R6 or R' is different from hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I R2, R3 and R4 independently of
each
other represent hydrogen, a linear or branched Ci.s-alkyl group, a halogen
atom, or
CF3, preferably R2, R3 and R4 independently of each other represent hydrogen,
methyl, ethyl, F, CI, Br and CF3.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I R5, R6 and R' independently of
each
other represent hydrogen, a linear or branched Ci_6-alkyl group, a halogen
atom, or
CF3, preferably R5, R6 and R' independently of each other represent hydrogen,
methyl, ethyl, F, CI, Br and CF3.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I R2 represents a chlorine atom
in the
4-position of the phenyl ring, while R3 and R4 represent hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I R5 and R6 each represent a
chlorine
atom in the 2- and 4-position of the phenyl ring, while R' represents
hydrogen.
r
13
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula I R' represents hydrogen, methyl
or
ethyl, preferably hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention the compound of general formula I is represented by a compound of
general formula II
R1
R14
II
wherein
R' represents hydrogen or a linear or branched Ci_4-alkyl group,
R72, R13, R14 or R'S independently of each other represent a linear or
branched
C1_6-alkyl group, a linear or branched Ci_6-alkoxy group, a halogen atom,
CH2F, CHF2, CF3, CN, OH, N02, SH, NH2, hydrogen, methyl, ethyl, F, CI, Br
and CF3,
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferak~y enantiomers and/or diastereomers, in any mixing
14
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula II R'2 and R'3 independently of
each
other represent hydrogen, a linear or branched Ci_6-alkyl group, a halogen
atom, or
CF3, preferably R'2 and R'3 independently of each other represent hydrogen,
methyl,
ethyl, F, CI, Br and CF3.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula II R'4, and R'S independently of
each
other represent hydrogen, a linear or branched C~_s-alkyl group, a halogen
atom, or
CF3, preferably R'4 and R'S independently of each other represent hydrogen,
methyl,
ethyl, F, CI, Br and CF3. ~n
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula II R'3 represents CI and R'2
represents hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula II R'4 and R'S each represent
CI.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula II R' represents hydrogen,
methyl or
ethyl, preferably hydrogen.
In a preferred embodiment of the combination of compounds according to the
invention the compound according to formula I or II is selected from the group
consisting of:
5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazol-3-
carboxylic
acid,
i
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
optionally in the form of a corresponding N-oxide, a corresponding salt or a
corresponding solvate.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X Ris represents a phenyl group,
which is optionally substituted by one or more substituents independently
selected
from the group consisting of a linear or branched Ci_6-alkyl group, a linear
or
branched Ci_6-alkoxy group, a halogen atom, CH2F, CHF2, CF3, CN, OH, N02, -
(C=O)-R', SH, SR', SOR', S02R', NH2, NHR', NR'R", -(C=O)-NH2, -(C=O)-NHR' and
-(C=O)-NR'R" whereby R' and R" for each substituent independently represent
linear
or branched Ci_6 alkyl, preferably R16 represents a phenyl group, which is
optionally
substituted by one or more substituents selected from the group consisting of
methyl,
ethyl, F, CI, Br and CF3, more preferably Ris represents a phenyl group, which
is
mono-substituted with a chlorine atom in the 4-position.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X R" represents a phenyl group,
which is optionally substituted by one or more substituents independently
selected
from the group consisting of a linear or branched Ci_6-alkyl group, a linear
or
branched C1_s-alkoxy group, a halogen atom, CH2F, CHF2, CF3, CN, OH, N02, -
(C=O)-R', SH, SR', SOR', SO2R', NH2, NHR', NR'R", -(C=O)-NHz, -(C=O)-NHR' and
-(C=O)-NR'R", whereby R' and optionally R" for each substituent independently
represent linear or branched C,_s alkyl, preferably R" represents a phenyl
group,
which is optionally substituted by one or more substituents independently
selected
from the group consisting of methyl, ethyl, F, CI, Br and CF3, more preferably
Ri'
represents a phenyl group, which is di-substituted with two chlorine atoms in
its 2-
and 4-position.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X R'8 represents a saturated or
unsaturated, optionally at least mono-substituted, optionally at least one
heteroatom
as ring member containing C3_$ cycloaliphatic group, which may be condensed
with
an optionally at least mono-substituted mono- or polycyclic ring system, or an
optionally at least mono-substi~r..ited, 5- or 6-membered aryl or heteroaryl
group,
16
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
which may be condensed with an optionally at least mono-substituted mono- or
polycyclic ring system, or an -NR'9R2°-moiety, preferably R'8
represents a saturated,
optionally at least mono-substituted, optionally one or more nitrogen-atoms as
ring
member containing C3_8 cycloaliphatic group, which may be condensed with an
optionally at least mono-substituted mono- or polycyclic ring system, or an -
NR'9R2o-
moiety, more preferably R'$ represents a pyrrolidinyl group, a piperidinyl
group or a
piperazinyl group, whereby each of these groups may be substituted with one or
more C,-s-alkyl groups, or an -NR'8R'9-moiety.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X R'9 and R2°, identical
or different,
represent a hydrogen atom, an unbranched or branched, saturated or
unsaturated,
optionally at least mono-substituted Ci_6-aliphatic radical, a saturated or
unsaturated,
optionally at least mono-substituted, optionally at least one heteroatom as
ring
member containing C3_8-cycloaliphatic group, which may be condensed with an
optionally at least mono-substituted mono- or polycyclic ring system, or an
optionally
at least mono-substituted, 5- or 6-membered aryl or heteroaryl group, which
may be
condensed with an optionally at least mono-substituted mono- or polycyclic
ring
system andlor bonded via a methylene (-CH2-) or ethylene (-CH2-CH2)-group, an -
S02-R2'-moiety, or an -NR22R2s-moiety, preferably one of these residues R'9
and R2o
represents a hydrogen atom and the other one of these residues R'9 and
R2°
represents a saturated or unsaturated, optionally at least mono-substituted,
optionally
at least one heteroatom as ring member containing C3_$-cycloaliphatic group,
which
may be condensed with an optionally at least mono-substituted mono- or
polycyclic
ring system, or an optionally at least mono-substituted, 5- or 6-membered aryl
or
heteroaryl group, which may be condensed with an optionally at least mono-
substituted mono- or polycyclic ring system, an -SO~-R2'-moiety, or an -
NR22R2s-
moiety, or R'9 and Rz°, identical or different, each represent a Ci_6
alkyl group, more
preferably one of these residues R'9 and R2° represents a hydrogen atom
and the
other one of these residues R'9 and RZ° represents an optionally at
least mono-
substituted pyrrolidinyl group, an optionally at least mono-substituted
piperidinyl
group, an optionally at least mono-substituted piperazinyl group, an
optionally at least
mono-substituted triazolyl group, an -SOZ-R2'-moiety, or an -NR~2R23-moiety,
or R'9
17
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
and R2°, identical or different, represent a methyl group, an ethyl
group, an n-propyl
group, an isopropyl group, an n-butyl group, a sec-butyl group or a tert.-
butyl group.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X R2' represents a linear or
branched,
saturated or unsaturated, optionally at least mono-substituted C~_s aliphatic
group, a
saturated or unsaturated, optionally at least mono-substituted, optionally at
least one
heteroatom as ring member containing C3_s cycloaliphatic group, which may be
condensed with a mono- or polycyclic ring-system, or an optionally at least
mono-
substituted, 5- or 6-membered aryl or heteroaryl group, which may be condensed
with a mono- or polycyclic ring system and/or bonded via a methylene (-CH2-)
or
ethylene (-CH2-CH2)-group, preferably R2' represents a Ci_s-alkyl group, a
saturated,
optionally at least mono-substituted cycloaliphatic group, which may be
condensed
with a mono- or polycyclic ring-system, or a phenyl group, which is optionally
substituted with one or more C1_s alkyl groups.
In a preferred embodiment of the combination of compounds according to the
invention for a compound according to formula X R~ and R23, identical or
different,
represent a hydrogen atom, an unbranched or branched, saturated or
unsaturated,
optionally at least mono-substituted Ci_s aliphatic radical, a saturated or
unsaturated,
optionally at least mono-substituted, optionally at least one heteroatom as
ring
member containing C3_$ cycloaliphatic group, which may be condensed with an
optionally at least mono-substituted mono- or polycyclic ring system, or an
optionally
at least mono-substituted, 5- or 6 membered aryl or heteroaryl group, which
may be
condensed with an optionally at least mono-substituted mono- or polycyclic
ring
system and/or bonded via a methylene (-CH2-) or ethylene (-CH2-CH2)-group,
preferably R22 and R23, identical or different, represent a hydrogen atom or a
C1-s
alkyl radical.
In a preferred embodiment of the combination of compounds according to the
invention the compound according to general formula X is represented by a
structure
wherein
18
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
R's represents a phenyl ring, which is mono-substituted with a halogen atom,
preferably a chlorine atom, in its 4-position,
R" represents a phenyl ring, which is di-substituted with two halogen atoms,
preferably chlorine atoms, in its 2- and 4-position,
R'8 represents a pyrrolidinyl group, a piperidinyl group, a piperazinyl group,
a
homo-piperazinyl group, a morpholinyl group, or an -NR'9R2°-moiety,
R'9 represents a hydrogen atom or a linear or branched C~.6-alkyl group,
R~° represents a linear or branched Ci.s alkyl group, an -S02-R2'-
moiety, a
pyrrolidinyl group, a piperidinyl group, a piperazinyl group, a homo-
piperazinyl
group, a morpholinyl group, a triazolyl group, whereby each of the
heterocyclic
rings may be substituted with one or more, identical or different, Ci.s-alkyl
groups, and
R2' represents a phenyl group, which is optionally substituted with one or
more
C1_s alkyl groups, which may be identical or different,
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers andlor diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
In a preferred embodiment of the combination of compounds according to the
invention the combination of compounds comprises at least one compound
according
to formula X selected from the group consisting of:
N-piperidinyl-5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-
pyrazol-3-carboxamide,
19
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid-[1,2,4]-triazole-4-yl-amide,
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid-(4-methyl-piperazin-1-yl)-amide,
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid diethylamide,
[5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-yl]-
piperidine-1-yl-methanone,
N-[5-(4-Chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazole-3-
carbonyl]-4-methylphenylsulfonamide,
optionally in the form of a corresponding N-oxide, a corresponding salt or a
corresponding solvate.
In a preferred embodiment of the combination of compounds according to the
invention the combination of compounds comprises at least one compound
according
to formula X selected from
5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazol-3-
carboxylic
acid,
and
N-piperidinyl-5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-
pyrazol-3-carboxamide;
each optionally in the form of a corresponding N-oxide, a corresponding salt
or
a corresponding solvate.
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
fn another aspect the present invention also provides a process for the
preparation of
substituted pyrazoline compounds of general formula I or II, wherein R' is
hydrogen,
given above, in that at least one benzaldehyde compound of general formula III
O H
R2
~J
~u R3
wherein R2, R3 and R4 have the meaning mentioned above, is reacted with a
pyruvate compound of general formula (IV)
(IV),
wherein G represents an OR group with R being a branched or unbranched
Ci_6 alkyl radical or G represents an O-K group with K being a cation,
preferably an anorganic kation, more preferably an alkali metal kation, most
preferably sodium, to yield a compound of general formula (V)
21
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
H
(V)
which is optionally isolated and/or optionally purified, and which is reacted
with
an optionally substituted phenyl hydrazine of general formula (VI)
,NH2
HN
R5
~J
R6
(VI)
or a corresponding salt thereof, wherein R5, R6 and R' have the meaning
mentioned above, under inert atmosphere, to yield a compound of general
formula (VII)
22
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
..
(VII)
wherein R2, R3, R4, R5, R6 and R' have the meaning as given above, which is
optionally isolated and/or optionally purified, and optionally esterified to
an
alkyl-ester if in the substituted pyrazoline compound of general formula I or
II
according to the invention R' is a linear or branched Ci_4-alkyl group.
The inventive process is also illustrated in scheme I given below:
23
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Scheme I:
Os , H
CH3C(C NH2-NH-Ph(R5R6R~) HO
R2 R4
~, J R3 ~~ ~
R ~u~ 3 ~ N
~A/
R2
R7
Rs R
The reaction of the benzaldehyde compound of general formula III with a
pyruvate compound of general formula IV is preferably carried out in the
presence of at least one base, more preferably in the presence of an alkali
metal hydroxide such as sodium hydroxide or potassium hydroxide or an alkali
metal methoxide such as sodium methoxide, as described, for example, in
Synthetic communications, 26(11 ), 2229-33, (1996). The respective description
is hereby incorporated by reference and forms part of the disclosure.
Preferably
said reaction is carried out in a protic reaction medium such as a Ci_4 alkyl
alcohol or mixtures of these.
Reaction temperature as well as the duration of the reaction may vary over a
broad
range. Preferred reaction temperatures range from -10 °C to the boiling
point of the
reaction medium. Suitable reaction times may vary for example from several
minutes
to several hours.
Also preferred the reaction of the benzaldehyde compound of general formula
III with a pyruvate compound of general formula IV is carried out under acid
catalysed conditions, more preferably by refluxing the mixture in
dichloromethane in the presence of copper(II)trifluoromethanesulfonate as
described, for example, in Synlett, (1 ), 147-149, 2001. The respective
24
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
description is hereby incorporated by reference and forms part of the
disclosure.'
The reaction of the compound of general formula (V) with an optionally
substituted phenyl hydrazin of general formula (VI) is preferably carried out
in a
suitable reaction medium such as C1_4-alcohols or ethers such as dioxane or
tetrahydrofurane or mixtures of at least two of these afore mentioned
compounds. Also preferably, said reaction may be carried out in the presence
of an acid, whereby the acid may be organic such as acetic acid and/or
inorganic such as hydrochloric acid. Furthermore, the reaction may also be
carried out in the presence of a base such as piperidine, piperazine, sodium
hydroxide, potassium hydroxide, sodium methoxide or sodium ethoxide, or a
mixture of at least two of these bases may also be used.
Reaction temperature as well as the duration of the reaction may vary over a
broad
range. Suitable reaction temperatures range from room temperature, i.e.
approximately 25 °C to the boiling point of the reaction medium.
Suitable reaction
times may vary for example from several minutes to several hours.
The carboxylic group of the compound of general formula (VII) may be activated
for
further reactions by the introduction of a suitable leaving group according to
conventional methods well known to those skilled in the art. Preferably the
compounds of general formula (VII) are transferred into an acid chloride, an
acid
anhydride, a mixed anhydride, a Ci_4 alkyl ester, an activated ester such as p-
nitrophenylester. Other well known methods for the activation of acids include
the
activation with N,N-dicyclohexylcarbodiimide or benzotriazol-N-
oxotris(dimethylamino) phosphonium hexafluorophosphate (BOP)).
If said activated compound of general formula (VII) is an acid chloride, it is
preferably prepared by reaction of the corresponding acid of general formula
(VII) with thionyl chloride or oxalyl chloride, whereby said chlorinating
agent is
also used as the solvent. Also preferably an additional solvent may be used.
Suitable solvents include hydrocarbons such as benzene, toluene or xylene,
halogenated hydrocarbons such as dichloromethane, chloroform or carbon
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
tetrachloride, ethers such as diethyl ether, dioxane, tetrahydrofurane or
dimethoxyethane. Mixtures of two or more solvents from one class or two or
more solvents from different classes may also be used. Preferred reaction
temperature range from OQ C to the boiling point of the solvent and reaction
times from several minutes to several hours.
If said activated compound of general formula (VII) is a mixed anhydride, said
anhydride may preferably be prepared, for example, by reaction of the
corresponding
acid of general formula (VII) with ethyl chloroformiate in the presence of a
base such
as triethylamine or pyridine, in a suitable solvent.
Following that the activated compound can be reacted with an alkyl-alcohol to
arrive
at compounds according to general formulas I or II with R' being a a linear or
branched Ci_4-alkyl group.
During the processes described above the protection of sensitive groups or of
reagents may be necessary and/or desirable. The introduction of conventional
protective groups as well as their removal may be performed by methods well-
known
to those skilled in the art.
If the substituted pyrazoline compounds of general formula I or II themselves
are
obtained in form of a mixture of stereoisomers, particularly enantiomers or
diastereomers, said mixtures may be separated by standard procedures known to
those skilled in the art, e.g. chromatographic methods or fractionalized
crystallization
with chiral reagents. It is also possible to obtain pure stereoisomers via
stereoselective synthesis.
In a further aspect the present invention also provides a process for the
preparation
of salts of substituted pyrazoline compounds of general formula I or II and
stereoisomers thereof, wherein at least one compound of general formula I or
II
having at least one basic group is reacted with at least one inorganic and/or
organic
acid, preferably in the presence of a suitable reaction medium. Suitable
reaction
26
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
media include, for example, any of the ones given above. Suitable inorganic
acids
include hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid,
nitric acid,
suitable organic acids are e.g. citric acid, malefic acid, fumaric acid,
tartaric acid, or
derivatives thereof, p-toluenesulfonic acid, methanesulfonic acid or
camphersulfonic
acid.
In yet a further aspect the present invention also provides a process for the
preparation of salts of substituted pyrazoline compounds of general formula I
or II or
stereoisomers thereof, wherein at least one compound of general formula I or
II
having at least one acidic group is reacted with one or more suitable bases,
preferably in the presence of a suitable reaction medium. Suitable bases are
e.g.
hydroxides, carbonates or alkoxides, which include suitable cations, derived
e.g. from
alkaline metals, alkaline earth metals or organic cations, e.g. [NH~R4_~~+,
wherein n is
0, 1, 2, 3 or 4 and R represents a branched or unbranched Ci_4-alkyl-radical.
Suitable
reaction media are, for example, any of the ones given above.
Solvates, preferably hydrates, of the substituted pyrazoline compounds of
general
formula I or II, of corresponding stereoisomers, of corresponding N-oxides or
of
corresponding salts thereof may also be obtained by standard procedures known
to
those skilled in the art.
Substituted pyrazoline compounds of general formula I or II, which comprise
nitrogen-atom containing saturated, unsaturated or aromatic rings may also be
obtained in the form of their N-oxides by methods well known to those skilled
in the
art.
The purification and isolation of the inventive substituted pyrazoline
compounds of
general formula I or II, of a corresponding stereoisomer, or salt, or solvate
or any
intermediate thereof may, if required, be carried out by conventional methods
known
to those skilled in the art, e.g. chromatographic methods or
recrystallization.
The substituted pyrazoline compounds of general formula I and II given below,
their
stereoisomers, corresponding N-oxides, corresponding salts thereof and
i
27
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
corresponding solvates are toxicologically acceptable and are therefore
suitable as
pharmaceutical active substances for the preparation of medicaments.
It has been found that the substituted pyrazoline compounds of general formula
(I)
and (II) given below, stereoisomers thereof, N-oxides thereof, corresponding
salts
and corresponding solvates have the property to regulate triglyceride levels
in blood
plasma.
The combination of compounds comprising substituted pyrazoline compounds of
general formula (I) and (II) given below, their stereoisomers, corresponding N-
oxides,
corresponding salts thereof and corresponding solvates and of substituted
pyrazoline
compounds of general formula (X) given below, their stereoisomers,
corresponding
N-oxides, corresponding salts thereof and corresponding solvates are
toxicologically
acceptable and are therefore suitable as pharmaceutical active substances for
the
preparation of medicaments.
It has been found that the substituted pyrazoline compounds of general formula
X
given below, stereoisomers thereof, N-oxides thereof, corresponding salts and
corresponding solvates have a high affinity to cannabinoid receptors,
particularly
cannabinoid 1 (CBS)-receptors, i.e. they act as antagonists on these
receptors. In
particular these pyrazoline compounds show litte or no development of
tolerance
during treatment particularly with respect to food intake. After ending the
treatment
with the pyrazoline compounds, reduced increase of body weight is found
compared
to the pre-treatment level.
Thus, another aspect of the present invention relates to a Medicament
comprising at
least one substituted pyrazoline compound of general formula I or II according
to the
invention and optionally one or more pharmaceutically acceptable excipients.
Thus, another aspect of the present invention relates to a Medicament
comprising at
least one substituted pyrazoline compound of general formula I
28
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
wherein
R' represents hydrogen or a linear or branched Ci_~-alkyl group,
R2, R3 and R4 independently of each other represent hydrogen, a linear or
branched Ci_s-alkyl group, a linear or branched C1_s-alkoxy group, a halogen
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-Rs, SH, SRB, SOR8, S02R8,
NH2, NHRB, NR8R9, -(C=O)-NH2, -(C=O)-NHR$ or -(C=O)-NR8R9 whereby R8
and R9 for each substituent independently represent linear or branched Gi_s
alkyl,
R5, Rs and R' independently of each other represent hydrogen, a linear or
branched C~_s-alkyl group, a linear or branched Ci_s-alkoxy group, a halogen
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-R'°, SH, SR'°,
SOR'°, NH2,
NHR'°, NR'°R", -(C=O)-NH2, -(C=O)-NHR'° and -(C=O)-
NR'°R", whereby
R'° and optionally R" for each substituent independently represent
linear or
branched C1_s alkyl;
29
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof;
and optionally one or more pharmaceutically acceptable excipients.
In an embodiment of the medicament according to the invention for the compound
according to formula I at least one of R2, R3 or R4 represents hydrogen, while
at least
one of R2, R3 or R4 is different from hydrogen.
In an embodiment of the medicament according to the invention for the compound
according to formula I at least one of R5, R6 or R' represents hydrogen, while
at least
one R5, R6 or R' is different from hydrogen.
In an embodiment of the medicament according to the invention for the compound
according to formula I R2, R3 and R4 independently of each other represent
hydrogen,
a linear or branched Ci_6-alkyl group, a halogen atom, or CF3, preferably R2,
R3 and
R4 independently of each other represent hydrogen, methyl, ethyl, F, CI, Br
and CF3.
In an embodiment of the medicament according to the invention for the compound
according to formula I R5, R6 and R' independently of each other represent
hydrogen, a linear or branched Ci_6-alkyl group, a halogen atom, or CF3,
preferably
R5, R6 and R' independently of each other represent hydrogen, methyl, ethyl,
F, CI,
Br and CF3.
In an embodiment of the medicament according to the invention for the compound
according to formula I R~ represents a chlorine atom in the 4-position of the
phenyl
ring, while R3 and R4 represent hydrogen.
In an embodiment of the medicament according to the invention for the compound
according to formula I R5 and R6 each represent a chlorine atoms in the 2- and
4-
position of the phenyl ring, while R' represents hydrogen.
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
In an embodiment of the medicament according to the invention for the compound
according to formula I R' represents hydrogen, methyl or ethyl, preferably
hydrogen.
In an embodiment of the medicament according to the invention the compound
according to formula I is represented by a compound of general formula (II)
R1
R14
II
wherein
R' represents hydrogen or a linear or branched C,.4-alkyl group,
R12, R13, R14 or R'S independently of each other represent a linear or
branched
Ci_6-alkyl group, a linear or branched Ci_6-alkoxy group, a halogen atom,
CHZF, CHF2, CF3, CN, OH, N02, SH, NH2, hydrogen, methyl, ethyl, F, CI, Br
and CF3,
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferaklly enantiomers and/or diastereomers, in any mixing
31
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof.
In an embodiment of the medicament according to the invention for the compound
according to formula II R'2 and R'3 independently of each other represent
hydrogen,
a linear or branched Ci_6-alkyl group, a halogen atom, or CF3, preferably R'2
and R'3
independently of each other represent hydrogen, methyl, ethyl, F, CI, Br and
CF3.
In an embodiment of the medicament according to the invention for the compound
according to formula II R'4, and R'S independently of each other represent
hydrogen,
a linear or branched C~_6-alkyl group, a halogen atom, or CF3, preferably R'4
and R'S
independently of each other represent hydrogen, methyl, ethyl, F, CI, Br and
CF3.
In an embodiment of the medicament according to the invention for the compound
according to formula II R13 represents CI and R'2 represents hydrogen.
In an embodiment of the medicament according to the invention for the compound
according to formula II R'4 and R'S each represent CI.
In an embodiment of the medicament according to the invention for the compound
according to formula II Ri represents hydrogen, methyl or ethyl, preferably
hydrogen.
In an embodiment of the medicament according to the invention the compound
according to formulas I or II is selected from the group consisting of:
5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazol-3-
carboxylic
acid,
optionally in the form of a corresponding N-oxide, a corresponding salt or a
corresponding solvate.
Another aspect of the invention is a medicament comprising at least one
combination
of compounds according to the invention and optionally one or more
pharmaceutically acceptable e~cipients.
32
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
In an embodiment of the medicaments according to the invention the medicament
is
for the regulation of triglyceride levels in the blood plasma and for the
prophylaxis
and/or treatment of disorders of the central nervous system, especially
stroke, of
disorders of the cardiovascular system and of of food intake disorders,
preferably
bulimia, anorexia, cachexia, obesity, type II diabetus mellitus (non-insuline
dependent diabetes mellitus), preferably obesity and diabetis.
In an embodiment of the medicament comprising the compound according to
formula
I or I I according to the invention the medicament is for the prophylaxis
andlor
treatment of disorders of the central nervous system, disorders of the immune
system, disorders of the cardiovascular system, disorders of the endocrinous
system,
disorders of the respiratory system, disorders of the gastrointestinal tract
or
reproductive disorders.
In an embodiment of the medicament comprising the combination according to the
invention the medicament is for the modulation of cannabinoid-receptors,
preferably
cannabinoid 1 (CBi) receptors, for the prophylaxis and/or treatment of
disorders of
the central nervous system, disorders of the immune system, disorders of the
cardiovascular system, disorders of the endocrinous system, disorders of the
respiratory system, disorders of the gastrointestinal tract or reproductive
disorders.
In an embodiment of the medicaments according to the invention the medicament
is
for the prophylaxis and/or treatment of food intake disorders, preferably
bulimia,
anorexia, cachexia, obesity, type II diabetus mellitus (non-insuline dependent
diabetes mellitus), preferably obesity.
In an embodiment of the medicaments according to the invention the medicament
is
for the prophylaxis and/or treatment of psychosis.
In an embodiment of the medicaments according to the invention the medicament
is
for the prophylaxis and/or treatment of alcohol abuse and/or addiction,
nicotine abuse
and/or addiction, drug abuse and/or addiction and/or medicament abuse and/or
33
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
addiction, preferably drug abuse and/or addiction and/or nicotine abuse and/or
addiction.
In an embodiment of the medicaments according to the invention the medicament
is
for the prophylaxis and/or treatment of one or more disorders selected from
the group
consisting of schizophrenia, anxiety, depression, epilepsy, neurodegenerative
disorders, cerebellar disorders, spinocerebellar disorders, cognitive
disorders, cranial
trauma, panic attacks, peripheric neuropathy, glaucoma, migraine, Morbus
Parkinson, Morbus Huntington, Morbus Alzheimer, Raynaud's disease, tremblement
disorders, compulsive disorders, senile dementia, thymic disorders, tardive
dyskinesia, bipolar disorders; bone disorders including osteoporosis or
Paget's
disease of bone; cancer, preferably for the prophylaxis and/or treatment of
one or
more types of cancer selected from the group consisting of brain cancer, bone
cancer, lip cancer, mouth cancer, esophageal cancer, stomach cancer, liver
cancer,
bladder cancer, pancreas cancer, ovary cancer, cervical cancer, lung cancer,
breast
cancer, skin camcer, colon cancer, bowl cancer and prostate cancer, more
preferably
for the prophylaxis and/or treatment of one or more types of cancer selected
from the
group consisting of colcon cancer, bowel cancer and prostate cancer;
medicament-
induced movement disorders, dystonia, endotoxemic shock, hemorragic shock,
hypotension, insomnia, immunologic disorders, sclerotic plaques, vomiting,
diarrhea,
asthma, memory disorders, pruritus, pain, or for potentiation of the analgesic
effect of
narcotic and non-narcotic analgesics, or for influencing intestinal transit.
Said medicaments may also comprise any combination of one or more of the
substituted pyrazoline compounds of general formula I given above,
stereoisomers
thereof, corresponding N-oxides thereof, physiologically acceptable salts
thereof or
physiologically acceptable solvates thereof.
Particularly preferably said medicaments are suitable for the prophylaxis
andlor
treatment of alcohol abuse, drug abuse andlor medicament abuse, preferably
drug
abuse and the treatment of obesity.
34
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Medicaments andlor drugs, which are frequently the subject of misuse include
opioids, barbiturates, cannabis, cocaine, amphetamines, phencyclidine,
hallucinogens and benzodiazepines.
The medicament according to the present invention may be in any form suitable
for
the application to humans and/or animals, preferably humans including infants,
children and adults and can be produced by standard procedures known to those
skilled in the art. The composition of the medicament may vary depending on
the
route of administration.
The medicament of the present invention may for example be administered
parentally in combination with conventional injectable liquid carriers, such
as water or
suitable alcohols. Conventional pharmaceutical excipients for injection, such
as
stabilizing agents, solubilizing agents, and buffers, may be included in such
injectable
compositions. These medicaments may for example be injected intramuscularly,
intraperitoneally, or intravenously.
Solid oral compositions (which are preferred as are liquid ones) may be
prepared by
conventional methods of blending, filling or tabletting. Repeated blending
operations
may be used to distribute the active agent throughout those compositions
employing
large quantities of fillers. Such operations are conventional in the art. The
tablets may
for example be prepared by wet or dry granulation and optionally coated
according to
the methods well known in normal pharmaceutical practice., in particular with
an
enteric coating.
The mentioned formulations will be prepared using standard methods such as
those
described or referred to in the Spanish and US Pharmacopeias and similar
reference
texts.
Medicaments according to the present invention may also be formulated into
orally
administrable compositions containing one or more physiologically compatible
carriers or excipients, in solid or liquid form. These compositions may
contain
conventional ingredients such as binding agents, fillers, lubricants, and
acceptable
wetting agents. The compositigns may take any convenient form, such as
tablets,
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
pellets, capsules, lozenges, aqueous or oily solutions, suspensions,
emulsions, or
dry powdered forms suitable for reconstitution with water or other suitable
liquid
medium before use, for immediate or retarded release.
The liquid oral forms for administration may also contain certain additives
such as
sweeteners, flavoring, preservatives, and emulsifying agents. Non-aqueous
liquid
compositions for oral administration may also be formulated, containing edible
oils.
Such liquid compositions may be conveniently encapsulated in e.g., gelatin
capsules
in a unit dosage amount.
The compositions of the present invention may also be administered topically
or via a
suppository.
The daily dosage for humans and animals may vary depending on factors that
have
their basis in the respective species or other factors, such as age, sex,
weight or
degree of illness and so forth. The daily dosage for humans may preferably be
in the
range froml to 2000, preferably 1 to 1500, more preferably 1 to 1000
milligrams of
active substance to be administered during one or several intakes per day.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I or II according to the invention or at least
one
combination of compounds according to the invention (and optionally one or
more
pharmaceutically acceptable excipients), for the preparation of a medicament
for the
regulation of triglyceride levels in the blood plasma and for the prophylaxis
and/or
treatment of disorders of the central nervous system, especially stroke, of
disorders
of the cardiovascular system and of food intake disorders, especially bulimia,
anorexia, cachexia, obesity, type II diabetus mellitus (non-insuline dependent
diabetes mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formaula I or II according to the invention (and
optionally one
or more pharmaceutically acceptable excipients,) for the preparation of a
medicament for the prophylaxis and/or treatment of disorders of the central
nervous
system, disorders of the immure system, disorders of the cardiovascular
system,
36
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
disorders of the endocrinous system, disorders of the respiratory system,
disorders of
the gastrointestinal tract or reproductive disorders.
Another preferred aspect of the invention is the use of at least one
combination of
compounds according to the invention (and optionally one or more
pharmaceutically
acceptable excipients,) for the preparation of a medicament for the modulation
of
cannabinoid-receptors, preferably cannabinoid 1 (CBi) receptors, for the
prophylaxis
andlor treatment of disorders of the central nervous system, disorders of the
immune
system, disorders of the cardiovascular system, disorders of the endocrinous
system,
disorders of the respiratory system, disorders of the gastrointestinal tract
or
reproductive disorders.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I or II according to the invention or at least
one
combination of compounds according to the invention (and optionally one or
more
pharmaceutically acceptable excipients), for the preparation of a medicament
for the
prophylaxis andlor treatment of food intake disorders, preferably bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I or II according to the invention or at least
one
combination of compounds according to the invention (and optionally one or
more
pharmaceutically acceptable excipients), for the preparation of a medicament
for the
prophylaxis and/or treatment of psychosis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I or II according to the invention or at least
one
combination of compounds according to the invention (and optionally one or
more
pharmaceutically acceptable excipients), for the preparation of a medicament
for the
prophylaxis and/or treatment of alcohol abuse andlor addiction, nicotine abuse
and/or
addiction, medicament abuse and/or addiction and/or drug abuse and/or
addiction,
preferably drug abuse and/or addiction or nicotine abuse and/or addiction. -
37
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I or II according to the invention or at least
one
combination of compounds according to the invention (and optionally one or
more
pharmaceutically acceptable excipients), for the preparation of a medicament
for the
prophylaxis and/or treatment of one or more disorders selected from the group
consisting of schizophrenia, anxiety, depression, epilepsy, neurodegenerative
disorders, cerebellar disorders, spinocerebellar disorders, cognitive
disorders, cranial
trauma, panic attacks, peripheric neuropathy, glaucoma, migraine, Morbus
Parkinson, Morbus Huntington, Morbus Alzheimer, Raynaud's disease, tremblement
disorders, compulsive disorders, senile dementia, thymic disorders, tardive
dyskinesia, bipolar disorders; bone disorders including osteoporosis or
Paget's
disease of bone; cancer, preferably for the prophylaxis and/or treatment of
one or
more types of cancer selected from the group consisting of brain cancer, bone
cancer, lip cancer, mouth cancer, esophageal cancer, stomach cancer, liver
cancer,
bladder cancer, pancreas cancer, ovary cancer, cervical cancer, lung cancer,
breast
cancer, skin camcer, colon cancer, bowl cancer and prostate cancer, more
preferably
for the prophylaxis and/or treatment of one or more types of cancer selected
from the
group consisting of colcon cancer, bowel cancer and prostate cancer;
medicament-
induced movement disorders, dystonia, endotoxemic shock, hemorragic shock,
hypotension, insomnia, immunologic disorders, sclerotic plaques, vomiting,
diarrhea,
asthma, memory disorders, pruritus, pain, or for potentiation of the analgesic
effect of
narcotic and non-narcotic analgesics, or for influencing intestinal transit.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of general formula I
38
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
wherein
R' represents hydrogen or a linear or branched Ci_4-alkyl group,
R2, R3 and R4 independently of each other represent hydrogen, a linear or
branched Ci_s-alkyl group, a linear or branched C1_s-alkoxy group, a halogen
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-R8, SH, SR8, SORs, S02Rs,
NH2, NHRs, NRaR9, -(C=O)-NH2, -(C=O)-NHR$ or -(C=O)-NRaR9 whereby R8
and R9 for each substituent independently represent linear or branched Ci_s
alkyl,
R5, Rs and R' independently of each other represent hydrogen, a linear or
branched C1_6-alkyl group, a linear or branched C~_s-alkoxy group, a halogen
atom, CH2F, CHF2, CF3, CN, OH, N02, -(C=O)-R'°, SH, SRi°,
SOR'°, NH2,
NHR'°, NR'°R", -(C=O)-NH2, -(C=O)-NHR'° and -(C=O)-
NR'°R", whereby
Ri° and optionally R" for each substituent independently represent
linear or
branched Ci_s alkyl;
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racem~te or in form of a mixture of at least two of the
39
RL I,
R'
~~~/~Rs
R6
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof;
and optionally one or more pharmaceutically acceptable excipients, for the
preparation of a medicament for the regulation of triglyceride levels in the
blood plasma and for the prophylaxis andlor treatment of disorders of
disorders of the central nervous system, especially stroke, of disorders of
the
cardiovascular system and of food intake disorders, especially bulimia,
anorexia, cachexia, obesity, type II diabetus mellitus (non-insuline dependent
diabetes mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use for the manufacture of a
medicament of at least one substituted pyrazoline compound of formula I for
which at
least one of R2, R3 or R4 represents hydrogen, while at least one of R2, R3 or
R4 is
different from hydrogen for the manufacture of a medicamentfor the regulation
of
triglyceride levels in the blood plasma and for the prophylaxis and/or
treatment of
disorders of disorders of the central nervous system, especially stroke, of
disorders of
the cardiovascular system and of food intake disorders, especially bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I for which at least on of R5, Rs or R'
represents
hydrogen, while at least one R5, R6 or R' is different from hydrogen for the
manufacture of a medicamentfor the regulation of triglyceride levels in the
blood
plasma and for the prophylaxis and/or treatment of disorders of disorders of
the
central nervous system, especially stroke, of disorders of the cardiovascular
system
and of food intake disorders, especially bulimia, anorexia, cachexia, obesity,
type II
diabetus mellitus (non-insuline dependent diabetes mellitus), preferably
obesity and
diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formu~ I for which R2, R3 and R4 independently of each
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
other represent hydrogen, a linear or branched C~_6-alkyl group, a halogen
atom, or
CF3, preferably R2, R3 and R4 independently of each other represent hydrogen,
methyl, ethyl, F, CI, Br and CF3 for the manufacture of a medicamentfor the
regulation
of triglyceride levels in the blood plasma and for the prophylaxis and/or
treatment of
disorders of disorders of the central nervous system, especially stroke, of
disorders of
the cardiovascular system and of food intake disorders, especially bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I for which R5, R6 and R' independently of each
other represent hydrogen, a linear or branched C~_6-alkyl group, a halogen
atom, or
CF3, preferably R5, Rs and R' independently of each other represent hydrogen,
methyl, ethyl, F, CI, Br and CF3 for the manufacture of a medicamentfor the
regulation
of triglyceride levels in the blood plasma and for the prophylaxis andlor
treatment of
disorders of disorders of the central nervous system, especially stroke, of
disorders of
the cardiovascular system and of food intake disorders, especially bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I for which R2 represents a chlorine atom in
the 4-
position of the phenyl ring, while R3 and R4 represent hydrogen for the
manufacture
of a medicamentfor the regulation of triglyceride levels in the blood plasma
and for
the prophylaxis and/or treatment of disorders of disorders of the central
nervous
system, especially stroke, of disorders of the cardiovascular system and of
food
intake disorders, especially bulimia, anorexia, cachexia, obesity, type II
diabetus
mellitus (non-insuline dependent diabetes mellitus), preferably obesity and
diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I for which R5 and R6 each represent a chlorine
atoms in the 2- and 4-position of the phenyl ring, while R' represents
hydrogen for
the manufacture of a medicamentfor the regulation of triglyceride levels in
the blood
plasma and for the prophylaxis and/or treatment of disorders of disorders of
the
41
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
central nervous system, especially stroke, of disorders of the cardiovascular
system
and of food intake disorders, especially bulimia, anorexia, cachexia, obesity,
type II
diabetus mellitus (non-insuline dependent diabetes mellitus), preferably
obesity and
diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula I for which R1 represents hydrogen, methyl or
ethyl,
preferably hydrogen for the manufacture of a medicamentfor the regulation of
triglyceride levels in the blood plasma and for the prophylaxis and/or
treatment of
disorders of disorders of the central nervous system, especially stroke, of
disorders of
the cardiovascular system and of food intake disorders, especially bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity and diabetis.
Another preferred aspect of the invention is the use of at least one
substituted
pyrazoline compound of formula wherein the compound of general formula (I) is
represented by a compound of general formula (II)
R1
R14
II
wherein
i
42
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
R' represents hydrogen or a linear or branched Ci~-alkyl group,
R12~ R13~ R14 ~r R15 independently of each other represent a linear or
branched
C~_s-alkyl group, a linear or branched C1.~-alkoxy group, a halogen atom,
CH2F, CHF2, CF3, CN, OH, N02, SH, NH2, hydrogen, methyl, ethyl, F, CI, Br
and CF3,
optionally in form of one of the stereoisomers, preferably enantiomers or
diastereomers, a racemate or in form of a mixture of at least two of the
stereoisomers, preferably enantiomers and/or diastereomers, in any mixing
ratio, or a corresponding N-oxide thereof, or a corresponding salt thereof, or
a
corresponding solvate thereof
for the manufacture of a medicamentfor the regulation of triglyceride levels
in
the blood plasma and for the prophylaxis and/or treatment of disorders of
disorders of the central nervous system, especially stroke, of disorders of
the
cardiovascular system and of food intake disorders, especially bulimia,
anorexia, cachexia, obesity, type II diabetus mellitus (non-insuline dependent
diabetes mellitus), preferably obesity and diabetis.
Another embodiment of the invention is the use of at least one substituted
pyrazoline
compound of formula II for which R12 and R'3 independently of each other
represent
hydrogen, a linear or branched C1_6-alkyl group, a halogen atom, or CF3,
preferably
R'2 and R'3 independently of each other represent hydrogen, methyl, ethyl, F,
CI, Br
and CF3 for the manufacture of a medicamentfor the regulation of triglyceride
levels
in the blood plasma and for the prophylaxis and/or treatment of disorders of
disorders
of the central nervous system, especially stroke, of disorders of the
cardiovascular
system and of food intake disorders, especially bulimia, anorexia, cachexia,
obesity,
type II diabetus mellitus (non-insuline dependent diabetes mellitus),
preferably
obesity and diabetis.
Another embodiment of the invention is the use of at least one substituted
pyrazoline
compound of formula II for which R'4, and R'S independently of each other
represent
hydrogen, a linear or branched C1_6-alkyl group, a halogen atom, or CF3,
preferably
43
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
R'4 and R'S independently of each other represent hydrogen, methyl, ethyl, F,
CI, Br
and CF3for the manufacture of a medicamentfor the regulation of triglyceride
levels
in the blood plasma and for the prophylaxis andlor treatment of disorders of
disorders
of the central nervous system, especially stroke, of disorders of the
cardiovascular
system and of food intake disorders, especially bulimia, anorexia, cachexia,
obesity,
type II diabetus mellitus (non-insuline dependent diabetes mellitus),
preferably
obesity and diabetis.
Another embodiment of the invention is the use of at least one substituted
pyrazoline
compound of formula II for which R'3 represents CI and R'2 represents hydrogen
for
the manufacture of a medicamentfor the regulation of triglyceride levels in
the blood
plasma and for the prophylaxis andlor treatment of disorders of disorders of
the
central nervous system, especially stroke, of disorders of the cardiovascular
system
and of food intake disorders, especially bulimia, anorexia, cachexia, obesity,
type II
diabetus mellitus (non-insuline dependent diabetes mellitus), preferably
obesity and
diabetis.
Another preferred embodiment of the invention is the use of at least one
substituted
pyrazoline compound of formula II for which R'4 and R'S each represent CI for
the
manufacture of a medicamentfor the regulation of triglyceride levels in the
blood
,plasma and for the prophylaxis and/or treatment of disorders of disorders of
the
central nervous system, especially stroke, of disorders of the cardiovascular
system
and of food intake disorders, especially bulimia, anorexia, cachexia, obesity,
type II
diabetus mellitus (non-insuline dependent diabetes mellitus), preferably
obesity and
diabetis.
Another preferred embodiment of the invention is the use of at least one
substituted
pyrazoline compound of formula II for which R' represents hydrogen, methyl or
ethyl,
preferably hydrogen for the manufacture of a medicamentfor the regulation of
triglyceride levels in the blood plasma and for the prophylaxis and/or
treatment of
disorders of disorders of the central nervous system, especially stroke, of
disorders of
the cardiovascular system and of food intake disorders, especially bulimia,
anorexia,
cachexia, obesity, type II diabetus mellitus (non-insuline dependent diabetes
mellitus), preferably obesity and diabetis.
44
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Another embodiment of the invention is the use of at least one substituted
pyrazoline
compound of formula I or II for which the compound according to formulas I or
II is
selected from the group consisting of:
5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-pyrazol-3-
carboxylic
acid,
optionally in the form of a corresponding N-oxide, a corresponding salt or a
corresponding solvate;
for the manufacture of a medicamentfor the regulation of triglyceride levels
in the
blood plasma and for the prophylaxis and/or treatment of disorders of
disorders of the
central nervous system, especially stroke, of disorders of the cardiovascular
system
and of food intake disorders, especially bulimia, anorexia, cachexia, obesity,
type II
diabetus mellitus (non-insuline dependent diabetes mellitus), preferably
obesity and
diabetis.
The present invention is illustrated below with the aid of examples. These
illustrations
are given solely by way of example and are not intended to limit the present
invention.
i
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Examples:
Example 0 represent a compound according to formula I or II.
Example 0:
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic
acid
a) 4-(4-chlorophenyl)-2-oxo-3-butenoic acid
COOH
CHO
O
~ CH3COC02Et
CI CI
In a three neck flask p-chlorobenzaldehyde (13,3 g, 95 mmoles) and ethyl
pyruvate
(10 g, 86 mmoles) were dissolved in 150 ml of absolute ethanoLThe solution was
ice-
cooled to 0°C and an aqueous solution of NaOH (3.8 g in 45 mL water)
was added
dropwise keeping the temperature below or equal to 10°C, whereby a
yellow-orange
colored precipitate was formed. The reaction mixture was stirred for 1 hour at
0°C
and an additional 1.5 hours at room temperature (approximately 25 °C).
Afterwards
the reaction mixture was cooled down to approximately 5°C and the
insoluble sodium
salt of 4-(4-chlorophenyl)-2-oxo-3-butenoic acid was isolated by filtration.
The filtrate was left in the refrigerator overnight, whereby more precipitate
is formed,
which was filtered off, combined with the first fraction of the salt and
washed with
diethyl ether. The sodium salt of 4-(4-chlorophenyl)-2-oxo-3-butenoic acid was
then
treated with a solution of 2N HCI, stirred for some minutes and solid 4-(4-
chlorophenyl)-2-oxo-3-butenoic acid was separated via filtration and dried to
give
12.7 g of the desired product (70% of theoretical yield).
1R (KBr, cm 1 ) : 3500-2500, 1719,3, 1686,5, 1603,4, 1587,8, 1081,9.
tH NMR(CDCI3, 8) : 7,4 (d, J=8,4Hz, 2H), 7,5 (d, J=16,1 Hz, 1H), 7,6 (d,
J=8,4Hz,
2H), 8,1 (d, J=16,1 Hz, 1 H).
46
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
a2) 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazofe-3-
carboxylic
acid
In an alternative route instead of using ethylpyruvate the salt CH3-C(O)-C(O)-
O'
Na+ (sodiumpyruvate) was used, dissolved ethanolic water.
b) 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic
acid
COOH
'O COOH
N.N
~ NHNHZ ' CI
CI
CI CI CI
CI
4-(4-chlorophenyl)-2-oxo-3-butenoic acid obtained according to step a) (12.6
g, 60
mmoles), 2,4-dichlorophenylhydrazine hydrochloride (12.8 g, 60 mmoles) and
glacial
acetic acid (200 mL) were mixed under a nitrogen atmosphere and heated to
reflux
for 4 hours, cooled down to room temperature (approximately 25 °C) and
given into
ice-water, whereby a sticky mass was obtained, which was extracted with
methylene
chloride. The combined methylene chloride fractions were washed with water,
dried
with sodium sulfate, filtered and evaporated to dryness to give a pale yellow
solid
(12.7 g, 57% of theoretical yield).
1R (KBr, cm-1 ) : 3200-2200, 1668,4, 1458, 1251,4, 1104,8.
' H NMR (CDCI3, 8) : 3,3 (dd, 1 H), 3,7 (dd, 1 H), 5,9 (dd, 1 H), 7,09-7,25
(m, 7H).
The Examples 1 to 6 represent compounds according to formula X.
47
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Example 1:
N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1 H-
pyrazole-3-carboxamide
(a) 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazole-3-
carboxylic
acid chloride
COOH COCI
N SOCI2
~N~
CI ~ ;I
CI
CI
CI
Under nitrogen atmosphere 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-
dihydro-
pyrazole-3-carboxylic acid (2.5 g, 6.8 mmols) obtained according to Example 0
was
dissolved in 4 mL of in thionyl chloride and heated to reflux for 2.5 hours.
The excess
thionyl chloride is removed from the reaction mixture under reduced pressure
and the
resulting crude residue (2.6 g) is used without any further purification.
1R (KBr, cm-') : 1732,3, 1700, 1533,3, 1478,1, 1212,9, 826,6.
Starting from this compound compounds according to general formulas I and II
wherein R' is a linear or branched C1_4-alkyl group can be prepared reacting
this
compound with the appropriate alkyl alcohol.
(b) N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-
dihydropyrazole-
3-carboxamide
48
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
CI
O
'NH-N
\ ,
~N
CI
CI
Under nitrogen atmosphere N-aminopiperidine (0.6 mL, 5.6 mmoles) and
triethylamine (4 mL) were dissolved in methylene chloride (25 mL). The
resulting
mixture was ice-cooled down to 0°C and a solution of 5-(4-chlorophenyl)-
1-(2,4-
dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic acid chloride obtained in
step (c) in
methylene chloride (15 mL) was added dropwise. The resulting reaction mixture
was
stirred at room temperature (approximately 25 °C) overnight. Afterwards
the reaction
mixture was washed with water, followed by a saturated aqueous solution of
sodium
bicarbonate, then again with water, dried over sodium sulfate, filtered and
evaporated
to dryness in a rotavapor. The resulting crude solid was crystallized from
ethanol.
The crystallized solid was removed via filtration and the mother liquors were
concentrated to yield a second fraction of crystallized product. The two
fractions were
combined to give a total amount of 1.7 g (57% of theoretical yield) of N-
piperidinyl-5-
(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydropyrazole-3-carboxamide
having a
melting point of 183-186°C.
1R (KBr, cm'1) : 3222,9, 2934,9, 1647,4, 1474,7, 1268,3, 815,6.
1H NMR ( CDCI3, 8) : 1,4 (m, 2H), 1,7 (m, 4H), 2,8 (m, 4H), 3,3 (dd, J=6,1 y
18,3Hz,
1 H), 3,7 (dd, J=12,5 and 18,3 Hz, 1 H), 5,7 (dd, J=6,1 and 12,5 Hz, 1 H), 7,0-
7,2 (m,
6H), 7,4 (s, 1 H).
The compounds according to the following examples 2-6 have been prepared
analogously to the process described in Example 1 in combination with Example
0.
49
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Example 2:
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid-[1,2,4]triazol-4-yl amide
Melting point: 134-138 °-C.
1R (IfBr, cm''): 3448, 1686, 1477, 1243, 1091, 821.
'H NMR(CDCI3, 8): 3,1 (dd, J=6,2 and 17,9Hz, 1 H), 3,7 (dd, J=12,3 and
17,9Hz, 1 H), 5,9 (dd, J=6,2 and 12,3 Hz, 1 H), 7,2-7,5 (m, 7H), 8,7 (s, 2H),
12,0
(bs, 1 H).
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Example 3:
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid-(4-methyl-piperazin-1-yl)-amide hydrochloride
Melting point: 150-155°-C.
1R (KBr, crri') : 3433, 1685, 1477, 1296, 1246, 1088, 1014, 825.
'H NMR (CDCI3, 8): 2,7 (d, J=4,2Hz, 3H), 3,0-3,4 (m, 9H), 3,6 (dd, J=11,9 and
17,9 Hz, 1 H), 5,8 (dd, J=5,5 and 11,9 Hz, 1 H), 7,1 (d, J=8,4Hz, 2H), 7,25
(2d,
J= 8,4 and 8,7 Hz, 3H), 7,4 (d, J=2,2Hz, 1 H), 7,5 (d, J=8,7Hz, 1 H), 9,8 (s,
1 H),
11,2 (bs).
Example 4:
5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carboxylic acid diethylamide
This compound was obtained in form of an oil.
1R (film, cm'') : 2974, 1621, 1471, 1274, 1092, 820.
'H NMR (CDCI3, S): 1,2 (m, 6H), 3,3-3,9 (m, 6H), 5,6 (dd, J=5,8 and 11,7 Hz,
1 H), 7-7,25 (m, 7H).
Example 5:
[5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazol-3-yl]-
piperidin-1-yl-methanone
Melting point: 105-110°-C.
1R (KBr, cm'1) : 2934, 1622, 1470, 1446, 1266, 1010, 817.
'H NMR ( CDCI3, 8): 1,7 (m, 6H), 3,4 (dd, J=5,7 and 17,9Hz, 1H), 3,7 (m, 3H),
3,9 (m, 2H), 5,6 (dd, J=6,1 y 11,9 Hz, 1H), 7-7,25 (m, 7H).
51
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Example 6:
N-[5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4,5-dihydro-1 H-pyrazole-3-
carbonyl~-4-methyl-phenylsulfonamide
This compound was obtained in form of an amorph solid.
1R (KBr, cm'') : 1697, 1481, 1436, 1340, 1169, 1074, 853.
'H NMR (CDCI3, 8): 2,4 (s, 3H), 3,2 (dd, J=6,6 and 18,3Hz, 1H), 3,6 (dd,
J=12,8 and
18,3Hz, 1 H), 5,8 (dd, J=6,6 and 12,8Hz, 1 H), 7 (d, J=8,2Hz, 2H), 7,2 (s, 1
H), 7,3-7,4
(m, 6H), 8 (d, J=8,1 Hz, 2H), 9 (s, 1 H).
Example 7:
N-oxide of N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-
dihydropyrazole-3-carboxamide
Under nitrogen gas as an inert atmosphere N-piperidinyl-5-(4-chlorophenyl)-1-
(2,4-
dichlorophenyl)-4,5-dihydropyrazole-3-carboxamide (0,15 g, 332 mmoles) was
dissolved in 7 ml of dichloromethane. The resulting solution was ice-cooled to
0 °C
and m-chloroperbenzoic acid (0,204 g, 0,83 mmoles) added in several portions.
After
stirring for 15 minutes a control via thin layer chromatography showed that no
starting
material was remaining. A saturated solution of sodium bicarbonate was then
slowly
added, the organic phase separated, washed with water, dried over sodium
sulfate
and filtered. The filtered solution was evaporated to dryness and the crude
product
was purified via column chromatography yielding 78 mg (50 % of theoretical
yield) of
the N-oxide of N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-
dihydropyrazole-3-carboxamide in form of a white solid having a melting point
of 115-
120 °C.
1R (KBr, cm''): 3202, 1678, 1654, 1474, 1309, 1107.
'H-NMR (CDCI3, 8): 1.6 (m, 2H), 1.8-2.0 (m, 4H), 2.55 (m, 2H), 3.3 (dd, J =
6.3 Hz
and 18.2 Hz, 1 H), 3.7 (m, 3H), 5.8 (dd, J = 6.3 Hz and 12.5 Hz, 1 H), 7.0-7.3
(m, 7H),
8.5 (s, 1 H.)
52
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
Pharmacological Data/Testing:
The compound according to example 0 is an inhibitor of high blood levels of
triglicerides. This effect has been probed in obese mice fed with high fat
diet. In the
following paragraphs it is described the method and the results obtained in
this study.
1. In-vivo testing for regulation of triglycerides in blood plasma
The study was done using six weeks old male mice B6 Lep ob/ob, obtained from
Charles River (France). Mice were divided in 3 groups : I (control), II
(vehicle), III
(example 0).
Group I:
The animals of the group I received the standard diet (D-12450B, Research
Diets,
NJ, USA).
Group II:
The animals of the groups II and III were fed with a High Fat Diet (D-12492,
Research Diets, NJ, USA), in both cases for 7 weeks (References 1 and 2).
Group III:
The animals of the groups III were fed with a High Fat Diet (D-12492, Research
Diets, NJ, USA), in both cases for 7 weeks (References 1 and 2).
At the end of the feeding period of 7 weeks, it was started the treatment
period (14
days): Group II mice received the vehicle (10 ml/kg/day, po, of the aqueous
solution
of acacia gum, 5% WN). Group III was administered with 30 mg/kg/day, po, of
the
inventive compound 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-
pyrazole-
3-carboxylic acid according to Example 0. Group I didn't received any
treatment. The
three groups of mice had the same diet than in the previous period.
At the end of the 14 days period of treatment, the blood levels of
triglicerides of the
animals were determined.
53
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
The analysis of the whole blood samples was done using test strips "Lipid
panel" and
the photometric Analyzer Cardio-Check Test System, from PA Instruments Polymer
Technology Systems Indianapolis, IN-46268, USA (Distributed in Spain by
Novalab
Iberica S.A.L, Madrid, Spain).
The results obtained were the following
Group Diet Treatment Triglicerides, Relative
whole
blood levels levels
(mg/dl)
I Standard 61 100%
II High Vehicle 122.4 (*) 200.6%
Fat
10 ml/kglday
po
III High Example 0 67.5 (N.S.) 110.6%
Fat
30 mg/kg/day
0
(*) : p<0.05, Anova followed Bonferroni t-test, compared with Group I.
NS : Not significant diference, compared with Group I.
The results showed that Group II mice receiving high fat diet had
significantly higher
triglicerides blood levels than the control Group I. But the administration of
the
compound according to Example 0 (Group III) improved the triglicerides blood
levels,
which were not different of the levels of the group I, which received standard
diet.
Figure 1 shows the clear reduction of triglyceride levels in blood plasma. The
level
(Group III) returns to the control level of Group I compared to the clearly
raised levels
found in the Group II without the treatment with the compound according to
Example
0.
Thus, it has been proved the inhibitory effect of the inventive compound 5-(4-
chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic acid
according to Example 0. on the high blood levels of triglicerides.
References
54
CA 02556568 2006-08-16
WO 2005/077909 PCT/EP2005/001465
1.-Lambert P.D. et al.:'Cyliary neurotrophic factor activates leptin-like
pathways and
reduces body fat"
P.N.A.S. 2001, 28 (8) : 4652-4657
2.- Grasa M.M. et al "Oleoyl-Estrone lowens the body weight of both ob/ob and
db/db
mice.
Hozm. Metab. Res 2000, 32 : 246-250
II. In-vivo testing for regulation of triglycerides in blood plasma
In a second set of experiments carried out similar to the tests shown above
the TG
(triglyceride) levels of diet-induced obese mice in blood were determined.
Mice receiving a high fat diet were - after a feeding period of 6 days -
either treated
p.o. with vehicle (0,5 % HPMC) or with the compound according to example 0 (30
mglkg/day p.o.).
TG levels in blood were determined on day 28 after beginning of the treatment.
TG (triglyceride) levels were 1.28 t 25 mmoles/I in the group treated with
vehicle and
only 0.80 ~ 0.07 mmoles/I in the group treated with the inventive compound 5-
(4-
chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic acid
according to Example 0. The results were statistically highly significant with
an
ANOVA factorial, Fisher s post-hoc test of *** p<0.005 vs. vehicle.
Thus, again the inhibitory effect of the inventive compound 5-(4-chlorophenyl)-
1-(2,4-
dichlorophenyl)-4,5-dihydro-pyrazole-3-carboxylic acid according to Example 0
on
the high blood levels of triglicerides was demonstrated.