Note: Descriptions are shown in the official language in which they were submitted.
CA 02559843 2006-09-13 Fi~ 6 - W - C A
DESCRIPTION
CORROSION-RESISTANT STEEL EXCELLENT IN TOUGHNESS OF BASE
METAL AND WELD PORTION, AND
METHOD OF MANUFACTURING THE SAME
Technical Field
The present invention relates to a corrosion-
resistant steel excellent in toughness of a base metal
and a weld portion, and a method of manufacturing the
same, and more specifically, a corrosion-resistant steel
used in various forms under various corrosive
environments, such as various containers, vacuum vessels,
low-temperature heat exchangers and bathroom components
used under corrosive environment with dewing or under
indoor environment; such as bridge, support columns,
tunnel reinforcing components, interior and exterior
materials for buildings, roof materials and fittings used
under aerial corrosive environment; such as various
reinforcing structures and support columns used under
corrosive environment with concrete; and such as marine
vessels, bridges, piles, sheet piles and marine
structures used under corrosive environments with
seawater.
Background Art
Steels used under various corrosive environments,
such as high-temperature and high-humidity corrosive
environment, corrosive environment with dewing, aerial
1
CA 02559843 2006-09-13
corrosive environment, corrosive environment with city
water, corrosive environment with soil, corrosive
environment with concrete, and corrosive environment with
seawater, are generally provided with some anti-corrosion
measures. In recent years, in view of improving
reliability, simplifying manufacturing and application
processes, achieving maintenance-free, saving resources
and the like, there have been increasing trends in using
Cr-containing steels and stainless steels, for the
purpose of improving corrosion resistance of the steel
base. Most of conventional techniques have, however,
failed in providing a practical measure from an
economical point of view, because improvement in the
corrosion resistance has resulted in increase in material
cost, and have sometimes resulted in only a limited range
of applications due to poor strength when austenitic
steels were used.
As seen in the above-described examples, any steels
containing certain levels of Cr generally became more
likely to cause local corrosion as the corrosive
environment became more severe, so that as a
countermeasure for this problem, further increase in the
concentration of Cr or Mo has been a most general
technical means for improving the resistivity against
corrosion.
In recent years, there have been proposed steels
added with Al besides Cr, aiming at improving the
corrosion resistance, or both of the corrosion resistance
and workability, as disclosed in Japanese Patent
2
CA 02559843 2010-08-18
Application Laid-Open Nos. 5-279791, 6-179949, 6-179950,
6-179951, 6-212256, 6-212257, 7-3388 and 11-350082 and
the like. These steels may be effective to some degree
in terms of improvement in the corrosion resistance or
both of the corrosion resistance and the workability, but
poor in toughness of the base metal and the heat affected
zone (HAZ), and this raises a tough obstacle for the
steel to be applied to weld structures.
Summary of the Invention
After considering the above-described situations,
the present invention relates to a low-cost,
corrosion-resistant steel showing a large corrosion
resistance under various corrosive environments such as
corrosive environment with dewing, aerial corrosive
environment, corrosive environment with city water, and
corrosive environment with seawater, and excellent in the
toughness in the heat affected zone (HAZ).
The present inventors made extensive studies from every
aspect, in order to develop a steel showing excellent
corrosion resistance under various corrosive environments
such as corrosive environment with dewing, aerial
corrosive environment, corrosive environment with city
water, corrosive environment with concrete, and corrosive
environment with seawater. First, after extensive
investigations into techniques for improving the
corrosion resistance under the above-described various
environments, as well as the toughness of the weld
3
CA 02559843 2006-09-13
portion, the present inventors found that a steel
containing 3 to 11% of Cr, added with 0.1 to 2% of Al,
showed a very excellent corrosion resistance under the
above-described various corrosive environments. However,
this sort of steel typically produces coarse ferrite when
heated at 1200 C or above during welding, due to its wide
range of ferrite phase transformation, so that the
toughness may degrade to a considerable degree, and may
cause cracks and the like after welding. The present
inventors then further went through a series of
experiments, and found out that a mode of generation of
the coarse ferrite phase transformation during welding
can be estimated based on a parameter Tp below, expressed
using amounts of addition of alloying elements. The
parameter Tp can be expressed using concentrations of
ferrite-forming elements (Cr, Al) and austenite-forming
elements (Mn, Ni, for example) which suppress production
of ferrite phase. The present inventors found out that
production of ferrite at higher temperatures can be
suppressed, when the parameter Tp has a value of not
smaller than a predetermined level.
On the other hand, addition of some austenite-
forming elements described in the above can suppress
production of the coarse ferrite phase in the weld
portion, but addition of large amounts of the alloying
elements may promote formation of a low-temperature-
transformation-forming phase with poor toughness in the
process of cooling after rolling of the base metal, and
thereby tends to lower the toughness of the base metal.
4
CA 02559843 2008-04-04
The present inventors then made extensive studies on
preventing such embrittlement, defined a parameter Tc
which specifies concentrations of the alloying elements
capable of ensuring a desirable level of toughness of the
base metal after rolling, and found out that a desirable
level of toughness can be ensured when the parameter Tc
has a value of not smaller than a predetermined level.
Basic concepts of the present invention are as
follows.
(1) A corrosion-resistant steel excellent in
toughness of a base metal and a weld portion, containing,
in % by weight:
C: 0.2% or less;
Si: 0.01 to 2.0%;
Mn: 0.1 to 4%;
P: 0.03% or less;
S: 0.01% or less;
Cr: 3 to 11%;
Al: 0.1 to 2%; and
N: 0.02% or less, and
having values of 1150 or above, and 600 or above
respectively for Tp and Tc expressed by the equations
below using concentrations of Cr, Al, C, Mn, Cu and Ni
respectively given as %Cr, %Al, %C, %Mn, %Cu and %Ni.
Tp = 1601 - (34%Cr + 287%A1) + (500%C + 33%Mn +
60%Cu + 107%Ni); and
Tc = 910 + 80%Al - (300%C + 80%Mn + 15%Cr + 55%Ni).
(2) The corrosion-resistant steel excellent in
CA 02559843 2008-04-04
toughness of a base metal and a weld portion according to
(1), further containing, in % by weight, any one of, or
two or more elements selected from the group consisting
of:
Cu: 0.1 to 4%;
Ni: 0.1 to 4%;
Mo: 0.01 to 1%;
V: 0.01 to 0.1%;
Nb: 0.005 to 0.050%;
Ti: 0.005 to 0.03%;
Ca: 0.0005 to 0.05%;
Mg: 0.0005 to 0.05%; and
REM: 0.001 to 0.1%.
(3) A method of manufacturing a corrosion-resistant
steel excellent in toughness of a base metal and a weld
portion, including the steps of:
heating a steel slab;
the steel slab containing, in % by weight:
C: 0.2% or less;
Si: 0.01 to 2.0%;
Mn: 0.1 to 4%;
P: 0.03% or less;
S: 0.01% or less;
Cr: 3 to 11%;
Al: 0.1 to 2%; and
N: 0.02% or less, and
having values of 1150 or above, and 600 or above
respectively for Tp and Tc expressed by the equations
6
CA 02559843 2006-09-13
below using concentrations of Cr, Al, C, Mn, Cu and Ni
respectively given as %Cr, %Al, %C, %Mn, %Cu and %Ni,
forming a steel plate by hot rolling of the steel
slab; and
cooling the steel plate by air.
Tp = 1601 - (34%Cr + 287%Al) + (500%C + 33%Mn +
60%Cu + 107%Ni); and
Tc = 910 + 80%Al - (300%C + 80%Mn + 15%Cr + 55%Ni).
(4) The method of manufacturing a corrosion-
resistant steel excellent in toughness of a base metal
and a weld portion according to (3), wherein the steel
slab further contains, in % by weight, any one of, or two
or more elements selected from the group consisting of:
Cu: 0.1 to 4%;
Ni: 0.1 to 4%;
Me: 0.01 to 1%;
V: 0.01 to 0.1%;
Nb: 0.005 to 0.050%;
Ti: 0.005 to 0.03%;
Ca: 0.0005 to 0.05%;
Mg: 0.0005 to 0.05%; and
REM: 0.001 to 0.1%.
(5) The method of manufacturing a corrosion-
resistant steel excellent in toughness of a base metal
and a weld portion according to (3), further including,
after the step of cooling the steel plate by air,
tempering the steel plate at a temperature of A,1
7
CA 02559843 2010-08-18
transformation point or below.
(6) The method of manufacturing a corrosion-
resistant steel excellent in toughness of a base metal
and a weld portion according to (4), further including,
after the step of cooling the steel plate by air,
tempering the steel plate at a temperature of Act
transformation point or below.
The invention relates to a corrosion-resistant steel
containing, in % by weight:
C: 0.2% or less;
Si: 0.01 to 2.0%;
Mn: 0.1 to 4%;
P: 0.03% or less;
S: 0.01% or less;
Cr: 3 to 11%;
Al: 0.56 to 2%; and
N: 0.02% or less, and
and optionally containing, in % by weight:
Cu: 0 to 4%; and
Ni: 0 to 4%; and
having values of 1150 or above, and 600 or above
respectively for Tp and Tc expressed by the equations below
using concentrations of Cr, Al, C, Mn, Cu and Ni
respectively given as %Cr, %Al, %C, %Mn, %Cu and %Ni, in %
by weight:
+
Tp = 1601 - (34%Cr + 287%Al) + (500%C + 33%Mn + 60%Cu
107oNi); and
Tc = 910 + 80%Al - (300%C + 80%Mn + 15%Cr + 55oNi).
8
CA 02559843 2010-08-18
The invention also relates to a method of manufacturing
a corrosion-resistant steel, the method comprising the steps
of:
heating a steel slab;
the steel slab containing, in % by weight:
C: 0.2% or less;
Si: 0.01 to 2.0%;
Mn: 0.1 to 4%;
P: 0.03% or less;
S: 0.01% or less;
Cr: 3 to 11%;
Al: 0.56 to 2%; and
N: 0.02% or less, and
and optionally containing, in % by weight:
Cu: 0 to 4%; and
Ni: 0 to 4%; and
having values of 1150 or above, and 600 or above
respectively for Tp and Tc expressed by the equations below
using concentrations of Cr, Al, C, Mn, Cu and Ni
respectively given as %Cr, %Al, %C, %Mn, %Cu and %Ni, in %
by weight:
Tp = 1601 - (34%Cr + 287%Al) + (500%C + 33%Mn + 60%Cu +
107%Ni); and
Tc = 910 + 80%Al - (300%C + 80%Mn + 15%Cr + 55%Ni),
forming a steel plate by hot rolling of the steel slab; and
cooling the steel plate by air.
8a
CA 02559843 2009-07-29
Brief Description of the Drawings
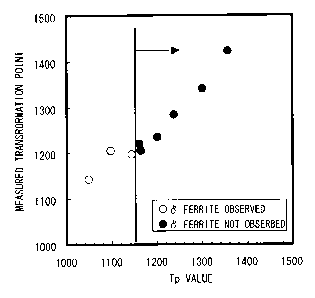
Fig. 1 is a graph showing relations between the Tp
values (calculated values of A4 transformation point) and
measured transformation points, and relations between the
Tp values and presence or absence of 6 ferrite; and
Fig. 2 is a graph showing relations between the Tc
values and the toughness (vE_5) of the base metal.
Detailed Description of the Preferred Embodiments
Paragraphs below will explain constitutive elements
of the inventive corrosion-resistant steel,
concentrations thereof and the like.
C: C is an element improving the strength, but
addition to an amount exceeding a predetermined level
results in degradation of the toughness in the heat
affected zone (HAZ). The upper limit of the C
concentration is therefore set to 0.20.
Si: Si is effectively added to a steel containing
2% or more of Cr, as a deoxidizer and a strengthening
element, wherein the concentration thereof less than
8b
CA 02559843 2006-09-13
0.01% results in only an insufficient effect of
deoxidization, whereas the concentration exceeding 2.0%
not only saturates the effect but also adversely degrades
the toughness of the heat affected zone (HAZ). The range
of Si concentration is therefore limited from 0.01% and
2.0%, both ends inclusive.
Cr: Cr is added in order to ensure a desirable
level of corrosion resistance, similarly to Al, wherein
an amount of addition of 36 or more exhibits the effect,
whereas the amount of addition exceeding 11% not only
increases the cost, but also impairs again the toughness
of the heat affected zone (HAZ). The upper limit of the
Cr concentration is set to 11%.
Al: Al is an important element, similar to Cr, in
view of ensuring a desirable level of corrosion
resistance in the present invention, wherein the
concentration of Al is necessarily set to 0.1% or more in
view of ensuring a desirable level of corrosion
resistance. On the other hand, the amount of addition
exceeding 2% extremely widens a temperature range causing
the ferrite phase transformation. The concentration of
Al is therefore limited to 0.1% to 2%, both ends
inclusive.
Mn: Mn in the present invention functions mainly as
improving the strength and as an austenite-forming
element, and is added to suppress generation of coarse
ferrite promoted by Cr and Al added in view of improving
the corrosion resistance. More specifically, Cr and Al
are ferrite-forming elements as well-known, wherein large
9
CA 02559843 2006-09-13
amounts of addition of these elements may give a ferrite
single phase structure over a range from solidification
point to room temperature, without causing
transformation, and may considerably degrade the
toughness not only in the base metal, but also in the
heat affected zone (HAZ). The present inventors made
systematic experiments aiming at improving the toughness
of the heat affected zone (HAZ) without causing the
corrosion resistance, and found out that addition of Mn
can avoid the problem. Specific conditions for
limitation therefor will be described later, wherein Mn
is necessarily added to as much as 0.1% or more, but the
amount of addition exceeding 4% enhances the hardening
property, so that the addition is limited up to 4%.
N: The less N is contained, the more preferable,
because a large amount of addition thereof to steel plate
may lower the toughness of the base metal and the heat
affected zone (HAZ), so that the upper limit of
concentration thereof is set to 0.02%.
P: The less P is contained, the more preferable,
because abundance thereof lowers the toughness, so that
the upper limit of concentration thereof is set to 0.03%.
The concentration thereof ascribable to inevitable
contamination is preferably minimized as possible.
S: The less S is contained, the more preferable,
too, because abundance thereof lowers pitting resistance,
so that the upper limit of concentration thereof is set
to 0.01%. Similarly to P, also the concentration of S
ascribable to inevitable contamination is preferably
CA 02559843 2006-09-13
minimized as possible.
The present invention further allows addition of
the elements below.
Cu, Ni: Both of Cu and Ni exhibit effects of
improving the strength, and of suppressing the ferrite
generation. In particular, Ni has an effect of improving
the toughness of the base metal and the heat affected
zone (HAZ). Addition to as much as 0.1% or more is
necessary for both of Cu and Ni in order to obtain these
effects, wherein the amounts of addition of the both
exceeding 4% enhances the hardenability and causes
embrittlement. Both of the concentrations of Cu and Ni
are therefore set to 0.1 to 4%.
Mo: Mo added to as much as 0.01% or more to a steel
added with Cr and Al exhibits an effect of suppressing
generation and growth of pitting, without impairing the
toughness of the base metal, whereas an amount of
addition exceeding 1.0% not only saturates the effect but
also degrades the toughness. The concentration of Mo is
therefore set to 0.01% to 1.0%.
Nb: Nb is an element improving the strength and
toughness without impairing the corrosion resistance,
wherein the effect thereof is recognizable at a
concentration of as small as 0.005%, whereas the
concentration exceeding 0.05% considerably degrades the
toughness of the heat affected zone (HAZ). The
concentration of Nb is therefore set to 0.005% to 0.05%.
V: V is an element improving the strength without
impairing the corrosion resistance, similar to Nb,
11
CA 02559843 2006-09-13
wherein the effect thereof is recognizable at a
concentration of as small as 0.01% or more, whereas a
large amount of addition degrades the toughness as well-
known. The upper limit of the V concentration is set to
0.1%.
Ti: Ti is an element contributive to refinement of
crystal grains at high temperatures through production of
nitride, and can particularly improve the toughness of
the heat affected zone (HAZ), without impairing the
corrosion resistance. Both of refinement of the crystal
grains and improvement in the toughness are recognizable
at a concentration of as small as 0.005% or more, whereas
addition to as much as exceeding 0.03% adversely degrades
the toughness of the base metal and the heat affected
zone (HAZ), due to deposition of a large amount of
carbide. The range of concentration is therefore set to
0.005% to 0.03%.
Ca, Mg: Ca and Mg are elements capable of improving
the corrosion resistance in a steel containing Cr and Al.
Although much of the mechanism thereof remain unclear at
present, it has been made clear that improvement in the
corrosion resistance is recognizable at a concentration
of as small as 5 ppm or more for the both, whereas the
amount of addition exceeding 500 ppm not only saturates
the effect of improving the corrosion resistance, but
also tends to degrade the toughness. The concentrations
of these elements are therefore set to 5 ppm to 500 ppm,
both ends inclusive.
REM: In the present invention, also appropriate
12
CA 02559843 2006-09-13
addition of rare earth metals (REM) can improve the
toughness of the base metal and the weld portion, without
impairing the corrosion resistance. An amount of
addition of 0.001% or more is necessary, whereas a large
amount of addition degrades the toughness, so that the
upper limit thereof is set to 0.1%.
In the present invention, the parameter Tp
expressed by the equation (1) is introduced, in order to
improve the toughness of the weld portion, as one major
object of the present invention.
Equation (1)
Tp = 1601 - (34%Cr + 28V%Al) + (500%C + 33%Mn +
60%Cu + 107 oNi )
where, %Cr, %Al, %C, %Mn, %Cu and %Ni are concentrations
of Cr, Al, C, Mn, Cu and Ni (o by weight), respectively.
Fig. 1 shows results of measurement and observation
of transformation point and generation behavior of coarse
ferrite, obtained when materials composed of a 0.015oC-
0.15oSi steel (P, S and N are within the ranges of the
present invention) as a base, added with Mn, Cr and Al,
and for some cases with Cu and/or Ni, were subjected to
welding cycles. It is found from Fig. 1 that generation
of the coarse ferrite phase is suppressed when value of
the parameter Tp, plotted on the abscissa, reaches and
exceeds 1150.
The present inventors further investigated into
relations between concentrations of the alloying elements
and the toughness, for the purpose of ensuring a
desirable level of toughness of the base metal, and found
13
CA 02559843 2006-09-13
out that the toughness of the base metal can be evaluated
based on the parameter Tc expressed by the equation (2).
Equation (2)
Tc = 910 + 80%Al - (300%C + 80%Mn + 15%Cr + 55%Ni)
Fig. 2 shows, together with values of the parameter
Tc, results of measurement of the toughness of a 0.02 to
0.05%C-0.25%Si steel as a base, added with Mn: 1.50 to
3.72%, Cr: 5.1 to 10.3% and Al: 0.8 to 1.5%, wherein a
20-mm-thick steel plate was manufactured by hot rolling,
and test pieces were collected from a portion having a
quarter thickness (5 mm) in the longitudinal direction.
It is found from Fig. 2 that a range of the parameter Tc
of 600 or above can ensure an absorption energy at -5 C
(vE_5) of as desirable as 100 J or above. The present
invention therefore sets the lower limit thereof to 600.
As for corrosion-resistant steel of the present
invention, the steel slab can be made by the ingot
making/breaking down method, continuous casting method
and the like. The steel slab may further be processed to
give a steel plate by hot rolling, hot forging or the
like, or may be hot-worked to give an arbitrary geometry
corresponding to a user's need, such as steel pipes
represented by seamless steel pipe, shape steels and the
like. The hot working can be followed by air-cooling,
for example. Tempering at a temperature not higher than
A,1 transformation point, aiming at further improving the
strength, will never interfere the effects of the present
invention.
The corrosion-resistant steel according to the
14
CA 02559843 2006-09-13
present invention is applicable, for example, to various
corrosive environments, such as high-temperature and
high-humidity corrosive environment, corrosive
environment with dewing, aerial corrosive environment,
corrosive environment with city water, corrosive
environment with soil, corrosive environment with
concrete, corrosive environment with seawater, and
corrosive environments based on any combinations of them.
(Examples)
Each of steels having compositions listed in Table
1 was melted and cast, hot rolled to give a 15-mm-thick
steel plate, wherein some of them were further tempered,
and subjected to the tests described below.
(1) Toughness Evaluation Test for Heat Affected Zone
(HAZ)
All test pieces were collected from the center-
thickness portion of the plate in the longitudinal
direction.
Evaluation of Toughness of Base Metal: Evaluation
was carried out based on absorbed energy observed in the
Charpy test at -5 C.
Evaluation of Toughness of Heat Affected Zone
(HAZ): Impact test of the heat affected zone (HAZ) after
being subjected to the welding heat cycles was carried
out. The maximum heating temperature and the cooling
rate in the test were set to 1400 C and 15 C/s,
respectively. The base metal was also subjected to the
impact test. Transition temperatures were determined for
the both, and L\vTrs=([transition temperature of base
CA 02559843 2006-09-13
metal]-[transition temperature after heat cycles]) was
determined.
(2) Corrosion Test
5-mm-thick corrosion test pieces were collected by
cutting from a test steel plate, wherein some of them
were provided with Zn-base coating (coating thickness: 15
to 25 gm), and then subjected to the test under
conditions described below.
Indoor Environment: The uncoated pieces were
subjected to a one-hundred-day exposure test in a room
with an air conditioner.
Humid Environment: The test pieces were kept at
-20 C for 2 hours, and then kept in an environment of 95%
humidity at 25 C for 4 hours, and this cycle was repeated
13000 times. Size of rust spot was scored for all
samples.
Salt Damage Environment: The test pieces were
exposed to a coastal splash zone for 17 months.
Results of these tests are shown in Table 2. All of
steels marked with A to K are those within the scope of
the present invention, and every one of them showed a
toughness of the base metal of 100 J or above, and a
toughness of the heat affected zone (HAZ), evaluated in
terms of LvTrs, of -15 C or above, proving only a small
lowering in the toughness. As for corrosion resistance,
only a slight rusting of as small as 2 mm or less was
observed on some of the pieces, and all pieces showed
desirable characteristics.
On the contrary, all of the steels marked with L to
16
CA 02559843 2006-09-13
U are those according to comparative examples out of
scope of the present invention. More specifically,
steels L, M and N, having the concentrations of C, Si and
Mn, respectively, exceeding the upper limits specified by
the present invention, showed almost desirable corrosion
resistance, but showed considerable degradation in the
toughness. The steel marked with L showed a toughness
(AvTrs) of the heat affected zone (HAZ) of -40 C,
indicating a large decrease. The steels marked with 0
and P, having amounts of addition of Cr and Al, which are
elements contributive to improvement in the corrosion
resistance, fallen below the lower limits, showed
considerable decrease in the corrosion resistance. The
steel marked with Q, having the Al concentration
exceeding the upper limit, showed a desirable corrosion
resistance, but was degraded in the toughness of the base
metal. The steel marked with R, having Ni added as
exceeding the upper limit, again showed a desirable
corrosion resistance, but was poor in the toughness of
the base metal. All of the steels marked with S, T and U
have the concentrations of the individual element fallen
within the ranges of the present invention, but have
value(s) of the parameter(s) Tp and/or Tc out of the
ranges of the present invention. More specifically, the
steel marked with S is an example having only the
parameter Tp fallen out of the range of the present
invention, showing a degraded toughness of the heat
affected zone (HAZ) of -55 C. The steel marked with T is
an example having only the parameter Tc fallen out of the
17
CA 02559843 2006-09-13
range of the present invention, showing a degraded
toughness of the base metal of 83 J. The last steel
marked with U is an example having both of the parameters
Tp and Tc fallen out of the ranges of the present
invention, showing degradation both in the toughness of
the base metal and the heat affected zone (HAZ).
Industrial Applicability
The present invention can provide, at low costs, a
steel excellent not only in the corrosion resistance in
corrosive environment with dewing, and in other various
corrosive environments such as indoor environment, aerial
corrosive environment and corrosive environment with
seawater, but also in the toughness of the heat affected
zone (HAZ) which is important for weld structures, and
can make a huge contribution to industrial development.
18
CA 02559843 2006-09-13
LL- uj
Z O N Co
F-J
D d LL) Q Q
N = Z O W
U
U) r- c0 LO Ln to CO Ln CO N 0) U) M co co 00 CC) 00 00 co
_
s f r- LO elf) 00 co M rLO M cO(D LC)CO)C.LO CD to Cn
ICD> 0 0 0 C) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 ca
6 6 000 00 0 61616 0 0 0 0 0 C7 C5 O
lf)U)CO CO N N co tD N[b to CO M Co M to ~NtC) N.-
00r-00.-t`M - LC) OnU N to N Co M LO
C> C> CD C> 6 C)
CO NNMMCO (TN V"ctN NOON MOONC0 (bM
m MCO -V' -4, -CDC) NOO .-N M CO
U) co MO U) tD V LC) U) CO Lo C') U M CO CO tD CO
N M .- N 0 ) N LA N L` co co
O N M
0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0 0
0 C. 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
O O O
O O
. . . . . . . . . . .
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 61c:) 0 0
M'- C V-- C D N N c 0 M C O 'IT
c D M N N M M N C O
C3 C> C> C. C, C> C> C> C> c:l C> C> C> CD C> CD Co CD
0 0 0 0 0 0 0 0 CD 0 C) 0 C) C) 0 0 0 0 0 0 0
. . . . . . . . . .
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 C. 0 0 0 0 0
tD M tO CV cO M CO CT co co et m N CD co N MCO Lf') M(D
C O LC) 000 NCO~MVO M~N~~lf IC)Mr-U)
N N M N O N N N N N N N N -0 N N M O M M M
CD (7) tD co CO CO N r cD U) cD CD U) to CT CO M 00 M
r N r ~Y O CV N CV CO M U) I- N M C) N CV N CO
0 0 0 0 O 0 0 0 -6 0 cli 6 6 0 0 0 0 0 0
N U) M CV M I~ OO h- O) ~ U) LC) M co U) co C CO to U)
N N C M CD ^ C) N M N C) N C V M'
U O O 0 0 0 0 0 0 0 0 lc:)l '- 0 0 0 0 0 0 0
~. 0 0 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0
W O
m Q M U O W L.L_ C7 S- Y J Z O 4 O Cn F-O
19
CA 02559843 2006-09-13
O co ) O J
W H d
~ C) d ll~ W Q Q
U W Z m W
M O
H- U
O O W~- O~ L O C) t o- for M M U) M c o CO r- ~
N O =- O C) CO CO t- CO C) ~ C) r - C) C) 0)
N- N- t0 c0 N- c0 to (0 t0 C0 co c0 CO n CO CD Si CO U) U)
C))O U)N C))sf .-O U) CO CC) N N- (.0 MM C0 c0Mt0 U)N CO 0 m U) C) M U') 1000
U)0) N-0
F- N ~ N N M M N C') q* N sT C') C) N N C`) - U) M.-
~.-.-~~r'-.-err- =-,---~r~r-=-=-=---~
CO N to
ae
M M ti
LAJ C> O O O N
O O O
O
O
ca N
O O O
U
O O O
N r
co
N
O O O
O O O
N CO
O O
O O
U) N
F- O O
O O
N U)
0
O O
4) 11.01 N CO 00 N M cli UC -
LO N N Cn CO CO N U) r-
66 M N O
N c0 N
N M
N U
O O O
W O
m Q O] U O W L.. C7 M Y J m O 0- O V) I-O
I-
CA 02559843 2006-09-13
W
w LLJ C/> :m
C¾Q Qo
@@@ O 00400 xx 00 @<@
oo @
W
to co Q
LL.
W
U
LW CD W
_
m? OO 101 ---
O 4 d ww.W.J
W p C O J J d W
N U Q d g OC
m m N W
O Z Z C/) w O
W - OC of O
- @@@ @000@ 0 O 0 x x I I
C) Z N LO -J
O
Ii LL. LL_
Cl) O O Op
CD LLI
OC W w F- ~ F--
N
Op CD O O OO QO O O 00 0 O O /Q OO 000 ~w
Z Z Oa, x
-
1 ~ Z
C7 O
Z O
p I- O
UiWn'
W W a
W MQ LIJ O LO O L[") O Lo l17 O LC) O O Lt) Ln l!") l!) N O LO LO
QU l~ 11 11 I I 1 1 I 11 I I I I I I I I
L <5
LL LL >
NQ C> C/>.<
W W Ly
CD ui CD
O = O =
O J
-CO W C") C') N N 00 M O M Cl) 00 M L n CO O M M M M M .O
W N
W N M N - N N C V O C O L c') N N Q co N co r
= Q >
O m
I-
C-D
LLJ ppp + p p Cl Cm c) + m pp + pp + Op p0
O W W W p w W W W W LL) W Cl W W W W in M W W p W W W W W
JJ JLLJ J J J J .J J J J W J J W J J J J
1--- p LLJ J J J J w J J J J J J w J J J J L J J J w J J J J
U O O C) O J O O O O O J O O O J O O J 0 0 0 0
QS w w w O d w OC x w 00. OC mmOa OC=O~ W OC Q'
= W N Cl) C/3.0 1 W Cl) Cl) Cl) Cl) V) wlJ-1 C.) C/)C- W J, C) i W Cl) V) C/)
Cl)
QQ
ZMQ¾~ddQQdQ~QQQ¾~"dd¾~Qd
Cl)
Q
m
W Q OO U Cl W LI.. C.7 S- M ]C J Z O O_ d Q C/) I- O
co
Z
LL- c:) LU C)
0 - W Q
W Q
V> =Z 0W
an
r v
21