Note: Descriptions are shown in the official language in which they were submitted.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
METHOD AND APPARATUS PROVIDING IMPROVED THROUGHPUT AND
OPERATING LIFE OF SUBMERGED MEMBRANES
TECHNICAL FIELD
[1] The present invention relates to methods and devices for processing,
refining,
and/or treating liquid compositions. More specifically, the invention relates
to membrane
separation methods and devices employing a selective, semi-permeable,
microporous, or
other partitioning membrane for processing, refining, and/or treating liquid
compositions,
for example membrane waste-water purification processes and apparatus.
BACKGROUND OF THE INVENTION
Background Pertaining to Vertical Bioreactors
[2] High efficiency wastewater treatment has become increasingly important as
the
world's population continues to grow. The quantity of water needed for human
consumption and other uses has increased at a rapid pace, while the amount of
naturally
available water remains unchanged. The ever-increasing demand for usable,
clean water
has made reclamation of wastewater an essential component of growth and
development of
human populations.
[3] In the United States and other developed nations, as existing metropolitan
areas
become overcrowded, developers are encouraged or required to construct new
housing in
previously undeveloped areas. Many of these undeveloped areas lack sufficient
water for
consumption, irrigation and similar purposes, necessitating reclamation and
reuse of
available water resources. For development in these areas to be successful,
sewage from
the residential use of water, commonly referred to as wastewater, ~is
therefore a primary
target for reclamation.
[4] Residential wastewater has a high water content, but requires substantial
processing before it can be reused because of the human waste and other
contaminants
mixed with it. To achieve reclamation of residential wastewater in many new
development areas, isolated from existing sewage treatment facilities, on-site
wastewater
treatment and reclamation is highly advantageous or essential.
[5] A wide variety of different wastewater treatment systems have been
proposed for
reclaiming residential sewage and other categories of wastewater. One such
system
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
disclosed in U.S. Pat. No. 2,528,649, incorporates a simple sedimentation tank
for
separating solid waste, or "sludge", from wastewater. After sedimentation, the
sludge is
passed to a digestion system where it is allowed to settle so that clear
aqueous liquid
separates from the sludge. The clarified liquid is redirected back to the
sedimentation
tank. Unfortunately, this system suffers from a number of shortcomings that
make it
inefficient. In particular, the system incorporates a relatively crude
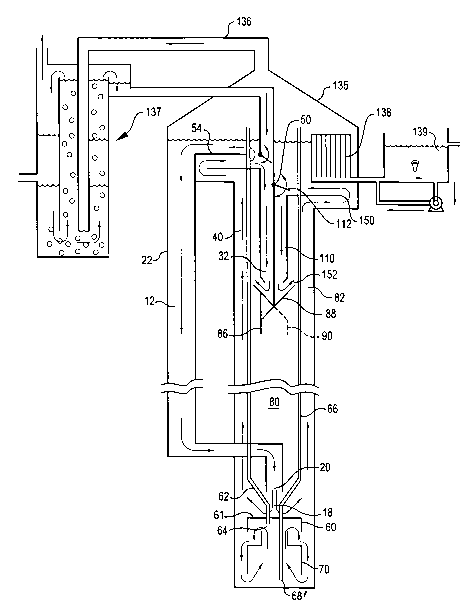
sedimentation system
that merely allows the influent sewage to separate and does not aerate or
facilitate
processing of the sewage in any other way.
[6] A number of wastewater treatment processes comprise "biological" systems
utilizing microorganisms contained in an activated biomass, or sludge for the
removal of
COD, phosphorous and/or nitrogen from wastewater. These treatment processes
typically
incorporate multiple treatment phases or "zones", namely: ( 1 ) a preliminary
treatment area;
(2) a primary treatment area; and (3) a secondary treatment area. Preliminary
treatment is
primarily concerned with the removal of solid inorganics from untreated
wastewater.
Typically, this preliminary treatment encompasses a two-stage treatment
process in which
the debris is removed by screens and/or settling. Organic matter is carried
out in the fluid
stream for subsequent treatment. Primary treatment entails a physical process
wherein a
portion of the organics, including suspended solids such as feces, food
particles, etc. is
removed by flotation or sedimentation. Secondary treatment typically
encompasses a
biological treatment process where microorganisms are utilized to remove
remaining
organics, nitrogen and phosphorous from the wastewater fluid stream.
Microorganism
growth and metabolic activity are exploited and controlled through the use of
controlled
growth conditions.
[7] In large scale municipal or industrial applications, biological treatment
processes
typically utilize a basin or other reservoir in which the wastewater is mixed
with a
suspension of biomass/sludge. Subsequent growth and metabolism of the
microorganisms,
and the resultant treatment of the wastewater, is carried out under aerobic
and/or
anaerobic/anoxic conditions. In most large scale municipal or industrial
treatment
systems, the various components of the treatment process are performed in
discrete basins
or reactors. As such, there is a continuous flow of the wastewater from one
process step to
the next. Biomass containing the active microorganisms may be recycled from
one
process step to another. The conditioning of such biomass to enhance growth of
2
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
particularized subgroups of microorganisms possessing a proclivity for
performing a
specific type of metabolic process, e.g. phosphate removal, nitrogen removal
has been the
subject matter of numerous patents, including: U.S. Pat. No. 4,056,465; U.S.
Pat. No.
4,487,697; U.S. Pat. No. 4,568,462; U.S. Pat. No. 5,344,562. The optimization
of other
components or aspects of biological wastewater treatment has also engendered a
variety of
patents, including: U.S. Pat. No. 2,788,127; U.S. Pat. No. 2,875,151; U.S.
Pat. No.
3,440,669; U.S. Pat. No. 3,543,294; U.S. Pat. No. 4,522,722; U.S. Pat. No.
4,824,572;
U.S. Pat. No. 5,290,435; U.S. Pat. No. 5,354,471; U.S. Pat. No. 5,395,527;
U.S. Pat. No.
5,480,548; U.S. Pat. No.4,259,182; U.S. Pat. No. 4,780,208; U.S. Pat. No
5,252,214; U.S.
Pat. No. 5,022,993; U.S. Pat. No. 5,342,522; U.S. Pat. No. 3,957,632; U.S.Pat.
No.
5,098,572; U.S. Pat. No. 5,290,451; Canadian Patent # 1,064,169; Canadian
Patent #
1,096,976; Canadian Patent # 1,198,837; Canadian Patent # 1,304,839; Canadian
Patent #
1,307,059; Canadian Patent # 2,041,329.
[8] Biological removal of organic carbon, nitrogen and phosphorus compounds
from
waste water requires attention to special environmental conditions within the
processing
equipment. For instance, for bacteria and other microbes to convert organic
carbon
compounds (measured as BOD) to carbon dioxide and water, a well mixed aerobic
environment is required. Approximately one pound of oxygen is required for
each pound
of BOD removed. To convert nitrogen compounds to nitrogen gas and carbon
dioxide,
nitrosomas and nitrobacter operate in an aerobic environment consuming
inorganic carbon.
Approximately 4.6 pounds of oxygen is required for each pound of ammonia-N
converted
to nitrate-N (assuming alkalinity is sufficient). Subsequently, facultative
bacteria operate
in an anoxic environment consuming organic carbon and liberating nitrogen gas.
Approximately 2.6 pounds of oxygen is recovered for each pound of nitrate-N
converted to
nitrogen gas. To biologically tie up phosphate in the cell mass, an anaerobic
step to
produce volatile fatty acids is required. This is followed by Poly P microbes
consuming
large amounts of phosphorus required to metabolize the volatile fatty acids in
an aerobic
environment thus concentrating the phosphate in the biomass (see, e.g.,
Abstract by Dr. W.
Wilson Western Canada Water and Wastewater Conference, Calgary AB. Jan 2002).
[9] The combination of these many biological processes ideally results in a
Biological
Nutrient Removal (BNR) process, sometimes called tertiary treatment. However,
a well-
designed tertiary treatment operation requires coordination and sequencing of
a complex
3
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
assemblage of components, processes and conditions. Each of the constituent
biological
processing steps proceeds at its own rate, with specific environmental
parameters required.
Efficient tertiary processing also requires the correct amounts of specialty
microbes to
sustain the microbial populations and perform specific processing functions.
[10] Current wastewater treatment systems which attempt to provide tertiary
treatment
include Upflow Sludge Bed Filter (USBF), Sequencing Batch Reactor (SBR) and
Membrane Separation Activated Sludge (MSAS) systems. The Sequencing Batch
Reactor
(SBR) process is a modification of the conventional activated sludge process.
U.S. Pat.
No 5,503,748 discloses a long vertical shaft aerator applied to the SBR
technology. The
SBR process employs a number of discrete steps, typically comprising
sequential fill,
reaction, settlement and decantation of wastewater with biomass in an enclosed
reactor. In
the initial step of this process, wastewater is transferred into a reactor
containing biomass,
and combined to form a mixed liquor. In the reaction step of the treatment
process the
microorganisms of the biomass utilize and metabolize and/or take up the
nitrogen,
phosphorous and/or organic sources in the wastewater. These latter reactions
may be
performed under anaerobic conditions, anoxic conditions, aerobic conditions,
or a
combination thereof to manipulate organism growth, population dynamics and
contaminant processing. The length of this stage will be dependent on the
waste's
characteristic, concentration of the biomass, and other factors. Following the
reaction
cycle, the biomass in the mixed liquor is allowed to settle out. A sludge
blanket settles on
the bottom of the reactor leaving a treated effluent supernatant. The treated
and clarified
wastewater (i.e. effluent) is subsequently decanted and discharged. The
reactor vessel is
then refilled and the treatment process cycle reinitiated. Thus, the
sequencing batch
reactor's process is based on discrete operation in time, whereas other
wastewater
treatment processes are based on distinct operations in space, e.g., by
performance of
different reactions in separate vessels.
[11] A number of additional wastewater treatment designs feature an air-lift
reactor,
which is a mechanically simple, combined gas-liquid flow device characterized
by fluid
circulation in a defined cyclic pattern through a set of specifically designed
channels.
Fluid motion is due to the mean density difference in an upflow (riser) and
downflow
(downcomer) sections of the reactor. The air-lift reactor is ordinarily
comprised of distinct
zones with different flow patterns. The riser is typically the zone where the
gas is injected
4
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
creating a fluid density difference, resulting in upward flow of both liquid
and gas phases.
At the top of the reactor, there is a gas-liquid separator section, which is
typically a region
of horizontal fluid flow and flow reversal where gas bubbles disengage from
the liquid
phase. The downcomer is the zone where the gas-liquid dispersion or degassed
liquid
ordinarily recirculates to the riser. The downcomer zone exhibits either
single-phase, two-
phase cocurrent, or two-phase mixed cocurrent-countercurrent downward flow,
depending
on whether the liquid velocity is greater than the free-rise velocity of the
bubbles. The
base section at the lower end of the vessel communicates the exit of the
downcomer to the
entrance of the riser.
[12] The air-lift reactor has predominantly been used for microorganism
fermentation
processes such as the ICI single cell protein production. Nonetheless, a
number of systems
are known which utilize air-lift reactors for wastewater treatment. Among
these examples
is the Betz reactor (Gasner, Biotech. Bioeng. 16:1179-1195, 1974), and "deep
shaft"
bioreactors for effluent treatment (see, e.g., Hines et al., Chem. Eng. Sym.
Ser. U.K.
41:D1-D10, 1975).
[13] Following the original development of deep shaft bioreactor, technology,
recent
efforts have led to improvements in long vertical shaft bioreactor systems for
wastewater
treatment. Among these improvements, U.S. Pat. No. 4,279,754, 5,645,726, and
5,650,070 issued to Pollock each disclose a modified vertical shaft bioreactor
system for
the treatment of biodegradable wastewater and/or sludge. Generally, these
vertical shaft
bioreactor systems comprise a bioreactor, a solid/liquid separator and
intervening
apparatus in communication with the bioreactor and separator. The bioreactor
comprises a
circulatory system which includes two or more vertical, side-by-side or
coaxial chambers,
a downflow chamber (downcomer) and an upflow chamber (riser). These chambers
are
connected at their upper ends through a surface basin and communicate at their
lower ends
via a common "mix zone" adjacent the lower end of the downcomer.
[14] In addition to the mix zone, these reactors feature a "plug flow zone"
located below
the mix zone and communicating therewith. As previously described, the term
"plug
flow" has referred to a net downward migration of solid particles from the mix
zone
toward an effluent outlet located at the lower end of the reactor. In one
application to
sludge digestion the net downward migration has been reported by Guild et al.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
(Proceedings WEF conf., Atlanta Ga., Oct 2001), to include local back mixing
only, but
over extended periods of operation (e.g., about 16 hours), inter-zonal mixing
occurs.
[15] The waste-containing liquor ("mixed liquor") is driven through the
circulating
system (i.e., between the downflow and upflow chambers, the surface basin and
the mix
S zone) by injection of an oxygen-containing gas, usually air, near the bottom
of the reactor
(e.g., at the mix zone and plug flow zone). A portion of the circulating flow
is directed to
the plug flow zone and is removed at the lower end thereof as effluent. In
wastewater
treatment reactors, the air is typically injected 5-10 feet above the bottom
of the reactor
and, optionally, immediately below the lower end of the downcomer. The deepest
air
injection point divides the plug flow zone into a quasi plug flow zone with
localized back
mixing above the deepest point of air injection, and a strict plug flow zone
with reportedly
no mixing below the deepest point of air injection.
[16] At start-up of the bioreactor, air is injected into the riser in the
nature of an air lift
pump, causing liquor circulation between and through the upflow and downflow
chambers. Fluid in the downcomer has a higher density than the liquid-bubble
mixture of
the riser and thereby provides a sufficient lifting force to maintain
circulation.
[17] Once the bioreactor circulation is thus initiated, all of the air
injection is diverted to
the mix zone and/or plug flow zone. The air bubbles that rise out of these
zones are
trained into the upflow chamber and are excluded from the downflow chamber
where the
downward flow of liquor exceeds the rise rate of the bubbles. Dissolved oxygen
in the
circulating mixed liquor is the principal reactant in the biochemical
degradation of the
waste. As the liquor ascends in the riser to regions of lower hydrostatic
pressure, this and
other dissolved gases separate and form bubbles. When the liquid/bubble
mixture from
the riser enters the basin, gas disengagement occurs. To facilitate this
purpose, the surface
basin is ordinarily fitted with a horizontal baffle at the top of the upflow
chamber to force
the mixed liquor to traverse a major part of the basin and release spent gas
before re
entering the downflow chamber for further treatment.
[18] U.S. Pat. No. 5,650,070 discloses a process where influent waste water is
introduced at depth into the riser chamber through an upwardly directed outlet
arm of an
influent conduit. A zone of turbulence is created at the lower end of the
downflow
chamber by the turn-around velocity head as the circulating flow reverses from
downward
to upward flow. This mix zone is not well defined but typically is between 1 S-
25 feet
6
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
deep. A portion of the mixed liquor in the mix zone flows downwardly into the
top of the
plug flow zone in response to an equal amount of treated effluent being
removed from the
lower end of the plug flow zone into an effluent line, as discussed above.
During
operation of the bioreactor the flow of influent liquor to and effluent liquor
from the
S bioreactor are controlled in response to changes in level of liquid in the
connecting upper
basin.
[19] Reaction between waste, dissolved oxygen, nutrients and biomass
(including an
active microbial population), substantially takes place in an upper
circulating zone of the
bioreactor defined by the surface basin, the upflow and downflow chambers and
the mix
zone. The majority of the contents of the mix zone circulate upwardly into the
upflow
chamber. In this upflow chamber undissolved gas, mostly nitrogen, expands to
help
provide the gas lift necessary to drive circulation of the liquor in the upper
part of the
reactor. The spent gas is released from the liquor as it traverses the
horizontal baffle in the
surface basin. The plug flow zone located below the upper circulating zone
provides a
final treatment or "polish" to the mixed liquor flowing downward from the mix
zone to
effluent extraction at the lower end of the reactor.
[20] The injected oxygen-containing gas dissolves readily under pressure in
the liquor in
the plug flow zone where there is localized back mixing resulting in a slow
net downward
movement of liquor. Undissolved gas (bubbles) migrate upward to the very
turbulent mix
zone under pressure. The gas to liquid transfer in this zone is very high,
reaching overall
reactor oxygen transfer efficiencies in excess of 65%. The products of the
reaction are
carbon dioxide and additional biomass which, in combination with unreacted
solid
material present in the influent wastewater, forms a sludge (or biosolids).
(21] In addition to aerobic digestion of BOD, it is becoming more and more
important
to couple biological nutrient removal (BNR) of nitrogen and phosphorous
compounds with
conventional wastewater treatment. As the demand for higher quality liquid
effluent
discharges increase, the need for technologies as provided by the present
invention has
become increasingly more compelling. The old Secondary Biological treatment
standard
of 30 mg/L BOD and 30 mg/L TSS is no longer adequate in many jurisdictions and
limits
are now often placed on nitrogen and phosphorus as well. Effective removal of
these
nutrients is essential in view of existing and developing environmental laws
aimed at
7
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
preventing eutrophication of natural waters and the attendant ecosystem
damages that
result therefrom.
(22] In basic terms, nitrogen removal is accomplished by converting ammonia
contained
in a mixed liquor stream to nitrites and nitrates, in the presence of oxygen,
which is known
as an aerobic nitrifying stage. Ammonia conversion to nitrite is carned out by
microbes
known as Nitrosomonas, while the conversion of nitrite to nitrate is
accomplished by
Nitrobacters. Nitrate conversion to nitrogen gas occurs in an anoxic
denitrifying stage that
takes place in a suspended growth environment devoid of dissolved oxygen.
Nitrogen,
carbon dioxide and water is produced, with the gas being vented from the
system.
Nitrification rates can be optimized by regulating interdependent waste stream
parameters
such as temperature, dissolved oxygen levels (DØ), pH, solids retention time
(SRT),
ammonia concentration and BOD/TKN ratio (Total Kjeldahl Nitrogen, or TKN, is
organic
nitrogen plus the nitrogen from ammonia and ammonium). Higher temperatures and
higher dissolved oxygen levels tend to promote increased nitrification rates,
as does pH
levels in 7.0 to 8.0 range. Sludge retention times of from 3.5 to 5, and
preferably 5-8, days
dramatically increase nitrification efficiency, after which time efficiencies
tend to remain
constant. Increases in ammonia concentration increases the nitrification rate
but only to a
maximum level attainable after which further ammonia concentration increases
do less to
increase the rate of nitrification. Rates have also been shown to be maximized
at
BOD/TKN ratios of less than 1.0 (see, e.g., Abstract by Dr.W. Wilson, Western
Canada
Water and Wastewater BNR conference ,Calgary AB Canada Jan.2002).
[23] Physical/Bio-Chemical phosphorous removal typically requires an anaerobic
suspended growth zone at the start of the system, and a sludge fermentation
tank to supply
volatile fatty acids (VFA's) for the energy needs of the phosphorous ingesting
organisms
(Acinetobacters). Recently it has been reported that anaerobic force mains can
generate
sufficient volatile acids to permit substantial biological phosphorus removal.
[24] Refractory treatment and polishing stages may be added to the process,
downstream of the final clarification stage. In many waste streams, the
majority of organic
compounds (80%-90%) are easily biodegraded. The remaining fraction biodegrade
more
slowly and are termed "refractory" compounds. Prior art biological nutrient
removal
designs incorporate a single sludge and a single clarifies, for example, U.S.
Pat. No.
8
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
3,964,998 to Barnard, but in that case the overall oxidation rate of the
system has to be
reduced to satisfy the slowest compound to oxidize.
[25] Biological nutrient removal (BNR) systems can take various process
configurations. One such embodiment is the five stage Modified BardenphoTM
process,
which is based upon U.S. Pat. No. 3,964,998 to Barnard. It provides anaerobic,
anoxic and
aerobic stages for removal of phosphorous, nitrogen and organic carbon. More
than 24
BardenphoTM treatment plants are operational, with most using the five stage
process as
opposed to the previously designed four stage process. Most of these
facilities require
supplemental chemical addition to meet effluent phosphorous limits of less
than 1.0 mg/L.
Plants using this process employ various aeration methods, tank
configurations, pumping
equipment and sludge handling methods. WEF Manual of Practice No. 8, "Design
of
Municipal Wastewater Treatment Plants", Vol. 2, 1991.
[26] In the context of vertical bioreactor technology, Pollock (U.S. Pat. No.
5,651,892,
issued July 29, 1997, incorporated herein by reference) discloses an
innovative process
utilizing a vertical bioreactor linked to a flooded filter via a flotation
separator. According
to this design, improved reaction rates are achieved by separating the biomass
into a high
rate aerobic organic carbon removal step, followed by an aerobic nitrification
step using a
separate nitrifying biomass. These steps are then followed by a high rate
denitrification
step in an anoxic environment created by feeding influent and return mixed
liquor or
effluent into that zone to provide a source of organic carbon and consume the
oxygen.
[27] Incorporation of an anaerobic processing step for phosphate removal is
typically
done in a separate reactor~lue to the long fermentation time required for
volatile fatty
acid production. Furthermore, phosphorus removal in single mixed liquor
systems is
difficult to implement because the phosphate rich biomass produced in the
aerobic portion
of the process should not contact the anaerobic fermentation reactor product
due to the risk
of re-solubilizing the entrapped phosphate. In other instances, biological
phosphorus
removal is augmented by addition of metal salts such as ferric chloride or
alum. These can
be added directly into the aerobic zone of the reactor to chemically bind the
phosphate.
[28J Thus, a variety of treatment systems, including coupled vertical shaft
reactors and
SBR's, have been successfully used to provide tertiary wastewater treatment.
However,
these tertiary treatment systems involve a single mixed liquor process wherein
all of the
9
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
specialty microbes involved in the process are mixed together. These include
autotrophic
organisms that utilize energy from inorganic material (e.g., the nitrifiers
Nitrosomonas and
Nitrobacters), and heterotrophs which utilize organic energy sources and
include the
aerobic BOD removers and the Acinetobacter biological phosphorous removers
(Bio-P
S organisms). Therefore, in all of these types of systems, the rate of
treatment is controlled
by the slowest performing microbe, usually nitrosomas which converts ammonium
to
nitrite. Due to the slow overall rate of treatment, these single mixed liquor
systems are
called extended aeration systems and are quite energy intensive.
[29] Despite the foregoing developments and advancements in wastewater
treatment
technologies, there remains an urgent need in the art for improved wastewater
treatment
systems that can satisfy a broadened range of uses and perform expanded and
enhanced
functions not satisfied by existing wastewater treatment systems. For example,
there is a
long unmet need in the art for a simplified wastewater treatment process and
apparatus that
provides enhanced biological nutrient removal (BNR) and which, in certain
embodiments,
can produce class A bio-solids required for unrestricted land applications. In
addition,
there remains an unfulfilled need for wastewater treatment systems and methods
that
satisfy these expanded functions while minimizing the costs and environmental
impacts
that attend conventional wastewater treatment plant installation and
operation.
Background Pertaining to Membrane Separation Technologies
and Use of Membranes in Bioreactors for Waste Water Treatment
[30] Membrane separation, which employs a selective, semi-permeable, or
partitioning
membrane is a rapidly evolving aspect of industrial separation technology for
processing,
refining, and/or treating liquid compositions, for example as employed in
modern
membrane waste-water purification processes and apparatus. In general membrane
separation devices and processes, a first liquid composition, for example an
influent liquid
waste water stream or flow, contact one surface of the membrane, and one or
more
constituents of the first liquid composition typically pass through the
membrane, often as a
result of a driving force or forces, for form a second liquid composition, for
example an
effluent flow stream at a second surface of the membrane, whereby one or more
separated
or partitioned components of the first liquid composition are excluded or left
behind (i.e.,
they are partitioned or retained at the first membrane surface to remain in
solution,
suspension, or contact, with the first liquid composition.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[31] Membrane separation technologies that can be employed within the methods
and
devices of the invention for processing, refining or treating liquid
compositions include
microfiltration, ultrafiltration, nanofiltration, reverse osmosis,
electrodialysis,
electrodeionization, pervaporation, membrane extraction, membrane
distillation,
S membrane stripping, membrane aeration, and other membrane-based processes.
Various
driving forces may be used principally, or in combination with other driving
forces
disclosed herein, to effectuate or enhance membrane function, depending on the
type of the
membrane separation employed. Pressure-driven membrane filtration, also known
as
membrane filtration, includes microfiltration, ultrafiltration, nanofiltration
and reverse
osmosis, and uses pressure as a driving force, whereas electrical driving
force is used in
electrodialysis and electrodeionization.
[32] Historically, membrane separation processes or systems have not been
considered
cost effective for water treatment due to the adverse impacts that membrane
scaling,
membrane fouling, membrane degradation and the like impose on the efficiency
of
removing solutes from aqueous water streams. More recently, however,
advancements in
technology have made membrane separation a more commercially viable technology
for
treating aqueous compositions suitable for use in industrial and residential
water treatment
processes.
[33] The technology of solids-liquid separation using membranes has been
rapidly
developing the in the wastewater treatment industry and in other membrane
separation
fields of use. For early membrane wastewater treatment plants, the predicted
useful
lifespan of membranes was between about 5-7 years. Currently, useful membrane
lifespan
in waste water treatment applications is often as long as 8 years or greater.
[34] In North America and other areas of the world, water rationing has become
increasingly common, even in cities that normally having good water resources,
such as
Vancouver, Seattle, and Calgary. Water rationing has become critical in many
parts of the
prairie and desert states. The moisture content in the soil in some areas is
already less than
in the "dirty thirties." The primary factor in progressive water rationing
restrictions has
been attributed to the inability of existing potable water treatment plants to
produce
enough potable water to satisfy increasing domestic and commercial demands.
Associated
with this problem, there is a need for improved wastewater treatment capacity
to increase
11
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
production of mid-quality water for irngation that is currently produced more
expensively
by potable water plants.
[35] Presently, there are a number of membrane bio-reactor plants operating at
over
eight million gallons per day (8 MGD), and a 12 MGD plant is reportedly under
construction in Europe. Newer hotels have been engineered to have two sets of
plumbing,
one for potable water and one for recycle water for such uses as toilet
flushing.
[36] Most cities in North America that are growing have segregated surface
drainage
lines and sewer lines. Wherever there is an existing surface water drain line,
it is feasible
to run a small diameter recycle water return line inside the much larger drain
line, without
the high costs associated with excavation and new line placement. In this
development
model, cross contamination is not a significant concern, because when it is
raining the
recycle water is not required. Furthermore, the recycle line is pressurized
with a higher
quality water than the runoff water. The less expensive recycle water can be
delivered to
most locations in the city for use in irngation and/or maintenance of streets,
golf courses ,
1 S parks, sod farms, nurseries, lawns, etc.
[37] Alternatively, small treatment plants, such as those using improved long
vertical
shaft bio-reactors that provide tertiary treatment, could be strategically
placed throughout
the urban areas and could be privately owned and operated without municipal
involvement. In low demand periods, they could discharge directly into the
surface water
drains, thereby substantially reducing loads on municipal plants.
[38] The improved long vertical shaft bio-reactors accomplish BNR treatment in
a
single integrated bioreactor that uses sequential zones, each dedicated to a
specific part of
the total treatment. Therefore each zone may be optimized individually.
[39] Technological advances in membrane separation, processing, and treatment
technologies have been occurnng at a rapid pace. The flux rate of membranes
(flow rate
per sq. feet of membrane surface) has been increasing while the cost per sq.
feet has
steadily decreased. In addition, costs of membranes used in modern treatment
and
processing plants has been decreasing, and will decrease even more
significantly over the
next decade. These factors, taken together, will further encourage the use of
membranes in
the treatment of recycle wastewater, among other processes. For example,
recent
membrane bio-reactor (MBR) pilot plant trials at San Diego indicate that
recycle water
12
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
will cost $3.05/1000 gal and $1.92/1000 gal when produced in plants of 1 and S
MGD size
respectively. This cost includes amortization of capital, operating and
maintenance costs
based on year-round operations. At least one golf course in Seattle pays
$3.96/100 cu. feet
of potable water ($5.29/1000 gal) on a seasonal demand basis.
[40] Membrane bioreactors require periodic cleaning to maintain their
performance.
The cleaning frequency depends on the type of membranes and their operating
environment, and is typically as frequent as every few months. The existing
reactors
typically are not operational during membrane cleaning, causing a temporary
and
reoccurring loss of wastewater treatment capacity. Further, cleaning often
involves use of
expensive, specialized chemicals requiring compliance with environmental
regulations in
use and disposal.
[41] The improved long vertical shaft bio-reactors offers distinct process
advantages
over other bioreactors, and there is a need for a method and apparatus
incorporating
membranes in such reactors.
[42] Aspects of the present invention satisfy these needs and fulfill
additional objects
and advantages that will become apparent from the following description and
appended
drawings.
SUMMARY OF THE INVENTION
[43] The invention provides methods and apparatus having improved through-put
and
operating life of submerged membranes used in biological treatment of waste
waters, and
increased time between cleaning and maintenance of the membranes. More
specifically,
the invention relates to membrane separation methods and devices employing a
selective,
semi-permeable, microporous, or other partitioning membrane for processing,
refining,
and/or treating liquid compositions, for example membrane waste-water
purification
processes and apparatus. Other aspects of the invention improve diffusion of a
gas in a
liquid by creating a substantially uniform pressure differential between
opposite sides of a
membrane.
[44] Within one aspect of the invention a submerged membrane assembly and
associated methods and apparatus are provided . The submerged assembly
typically
includes a membrane having at least a first surface and a second surface,
which most often
comprise opposing faces of a planar membrane. In certain embodiments the
opposing
13
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
surfaces of the membrane are square or rectangular, and the membrane has a
vertical axis
(e.g., a vertical defined by one side of a square-configured membrane or an
elongated side
of a rectangular membrane). The membrane is permeable between the first and
second
surfaces by molecules of less than a predetermined size.
[45] Within other aspects of the invention, the submerged membrane assembly
includes
a first fluid compartment that contains a first fluid having a first specific
gravity in fluid
communication with the first membrane surface. The assembly also includes a
second
fluid compartment that contains a second fluid having a second specific
gravity in fluid
communication with the second membrane surface.
[46] Additionally, the membrane assembly typically includes means for imposing
a
differential hydraulic head between the first fluid contained in the first
compartment and
the second fluid contained in the second compartment, and means for changing
the second
specific gravity. The differential hydraulic head imposing means may include
the first
fluid compartment, wherein the first fluid compartment defines a first column
height, and
the second fluid compartment, wherein the second fluid compartment defines a
second
column height. The second column height may be selected relative to the first
column
height to produce a selected pressure differential across the membrane along
the vertical
axis of the membrane at the first specific gravity and a changed second
specific gravity
(i.e., the second specific gravity altered from an initial second specific
gravity value to the
changed second specific gravity value by operation of said means for changing
the second
specific gravity). The first column height and the second column height may
each be
established solely by gravity and construction and design of the first and
second fluid
compartments (typically by having an outflow or overflow port or opening in
the second
fluid compartment that is lower in correspondence to the membrane vertical
axis than a
fluid column height in the first fluid compartment). The differential
hydraulic head
imposing means may alternatively include a means for applying a pressure
differential
between the first and second fluid compartments. For example, a negative
pressure
generating means or vacuum may be applied to the second compartment or fluid
to
generate a reduced pressure in the second fluid compared to fluid pressure of
the first fluid
in the first compartment. Alternatively, a positive pressure generating means
or
pressurizing device may be applied to the first compartment or fluid to
generate an
14
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
elevated pressure in the first fluid compared to fluid pressure of the second
fluid in the
second compartment.
[47) Within various embodiments of the invention, the second specific gravity
changing
means may include a means for directly or indirectly introducing gas into the
second fluid
in the second compartment. For example, gas can be directly dissolved in the
second fluid
or directly introduced into the second fluid in the form of bubbles, thereby
reducing the
second specific gravity to the desired, changed second specific gravity value.
Typically,
the first fluid contains a dissolved gas, and gas is introduced from the first
fluid to the
second fluid by passing through the membrane from the first side to the second
side, either
in solution or in the form of microbubbles or larger gas bubbles. In certain
embodiments,
dissolved gas (e.g., air or oxygen) in the first fluid passes between the
first and second
surfaces of the membrane and, at or near the second surface, nucleates to form
gas bubbles
that are incorporated in the second fluid. When the gas introducing means thus
involves
transfer of dissolved gas from the first fluid into the second fluid, the gas
can nucleate at or
near the second membrane surface, which may include nucleation between the
first and
second membrane surfaces, at the second membrane surface, within the second
fluid
compartment, and/or dissolution of the gas within the second fluid. The gas
introducing
means can alternately achieve dissolved gas introduction from the first to the
second fluid
without dissolution of the gas and formation of bubbles, which can
alternatively take place
after the gas introduction or not at all. In yet additional embodiments, the
dissolved gas of
the first fluid may nucleate in response to a mechanical action imparted by
passing through
the membrane, in response to a pressure differential across the membrane, or
in the second
fluid in response to a difference in dissolved gas levels between the first
fluid and the
second fluid. In certain other embodiments, the means for changing the second
specific
gravity may include a gas introduction port coupled to the second fluid
compartment for
introduction of gas into the second fluid. Gas can be introduced into the
second fluid via
this gas introduction port in the form of pressurized gas or in other forms,
for example by
introducing a gas-saturated fluid that mixes with the second fluid.
[48] Another aspect of the invention provides a submerged membrane assembly.
The
submerged membrane assembly includes a membrane having a first surface, a
second
surface, and a vertical axis, and which is permeable between the surfaces by
molecules of
less than a predetermined size. The assembly further includes a first fluid
compartment in
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
fluid communication with the first membrane surface that contains a first
fluid having a
first specific gravity at a first column height, a second fluid compartment in
fluid
communication with the second membrane surface that contains a second fluid
having a
second specific gravity at a second column height, and means for changing the
second
specific gravity. The second column height selected relative to the first
column height to
produce a selected pressure differential across the membrane along the
vertical axis at the
first specific gravity and the changed second specific gravity. The second
specific gravity
changing means may include a gas added to the second fluid, and the gas may be
added by
direct or indirect introduction of gas into the second fluid (typically in
bubble form, but
optionally in an initially dissolved form). In exemplary embodiments, the
second specific
gravity changing means includes a gas added to the second fluid by a dissolved
gas of the
first fluid permeating through the membrane and nucleating proximate to, or
within, the
second fluid. The gas may nucleate at or near at least a portion of the second
surface of the
membrane and optionally impart a desired scouring action on the membrane by
nucleation
1 S (either between the first and second membrane surfaces in the event
nucleation occurs
within the membrane, or more typically at or near the second membrane surface)
and/or by
the mechanical effects of bubbles rising in the second fluid.
[49] The membrane assembly may optionally include a gas inlet port coupled to
the
second fluid compartment for direct introduction of gas (e.g., dissolved in a
fluid, or in
pressurized gas form) into the second fluid..
[50] The assembly may further include a fluid collector that collects fluid
from the
second compartment, for example through an overflow port at or near the second
fluid
column height. In certain embodiments, the first fluid compartment may be a
head tank or
a saddle tank of a vertical bioreactor or other wastewater treatment
apparatus.
[51] For use in wastewater treatment applications, the membrane assembly of
the
invention typically includes a semi-permeable membrane that excludes particle
exchange
between the first and second surfaces (permeation) by particles of a size
greater than a
selected size indicated for the processed (effluent) water. For most treated
wastewater, the
selected membrane pore size will be less than or equal to about 2 microns,
more typically
less than or equal to 0.5 microns, and often less than or equal to 0.1 micron.
The
membrane may include any of a variety of commercially available membranes for
use in
16
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
wastewater treatment applications, for example a flat plate membrane, or a
hollow fiber
membrane.
[52] In related aspects of the invention, the submerged membrane assembly
includes a
membrane having a first surface, a second surface, and a vertical axis, and is
permeable
between the first and second surfaces by molecules of less than a
predetermined size. The
assembly includes a first fluid compartment in fluid communication with the
first
membrane surface which contains a first fluid having a first specific gravity
at a first
column height. The assembly also includes a second fluid compartment in fluid
communication with the second membrane surface which contains a second fluid
having a
second specific gravity at a second column height. The second fluid contains,
or is altered
to contain, a gas in an amount sufficient to adjust the second specific
gravity to more
closely approximate the first specific gravity. In exemplary embodiments, the
gas
contained in the second fluid is in the form of gas bubbles. A fluid collector
is fluidly
connected to the second compartment at the second fluid column height to
collect fluid
from the second compartment. The second column height is selected relative to
the first
column height to produce a selected pressure differential across the membrane
along the
vertical axis. The first fluid compartment further may include a first fluid
outflow at the
first column height. The first fluid may include dissolved gas. The gas in the
second fluid
may include bubbles formed by a dissolved gas of the first fluid that has
permeated the
membrane and nucleated (within or proximate to the second fluid, for example
by
nucleating at or near the second membrane surface). A gas bubble rising in the
second
fluid may impart a cleaning action on the second membrane surface. The second
fluid
compartment may include a gas inlet port to introduce gas directly into the
second fluid (as
an alternate, or complementary gas introduction means to gas that permeates
between the
first and second membrane surfaces from the first fluid. The first column
height and the
second column height may be established without a mechanical device, e.g.,
solely as
determined by gravity, or by application of negative pressure to the second
fluid or positive
pressure to the first fluid.
[53] The methods and devices of the invention are broadly applicable within
fluid
treatment methods and devices. In various treatment processes and devices
where
membranes are employed, where fluids containing solids tend to foul the
membranes or
where clean fluids have a slow permeate rate, the invention provides
substantial
17
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
advantages. In the case of drinking water, membrane run time can be extended
by adding
C02 to the first and/or second fluids, which is also desirable for pH
adjustment of the
water. Industrial filters, for example filters to remove sediment and
precipitated protein
from chilled beer, this will also be advantageous for recarbonation prior to
bottling. Inert
S gas filtration, such as gasoline purification using nitrogen gas, is also
amenable to
optimization using the methods and devices of the invention. In this case, a
gas recovery
system is provided downstream of the membrane, and a repressurization system
may also
be employed. Nitrous oxide may also be employed as an added gas (e.g., as a
gas
introduced into the second fluid) to yield desired fuels/additives.
[54] In the case of viscous fluids, such as lubricants, processing of such
fluids will also
be facilitated by the methods and devices of the invention, particularly by
using an inert
gas within said methods and devices. Inert gases, such as nitrogen, argon,
helium, carbon
dioxide, are all candidates for such applications. Active gasses, such as
methane, are only
sparingly soluble in water, and therefore will have more limited uses within
the invention.
Some gasses are sensitive to pH changes. For instance, bicarbonate of soda
dissolves in
water without pressure but a shift in pH will release C02 in the same fashion
that pressure
changes do.
[55] Other fluid processing technologies to which the methods and devices of
the
invention can be applied include, for example, desalinization plants,
biotechnical and
biomedical separation procedures (e.g., dialysis of blood and other body
fluids), and
environmental decontamination processes (e.g., oil and other petroleum
contaminant
removal from marine and fresh water sites).
[56] In more detailed aspect of the invention, methods for treating fluids by
membrane
separation are provided that employ a selective, semi-permeable, microporous,
or other
partitioning membrane for processing, refining, and/or treating liquid
compositions, for
example membrane waste-water purification processes and apparatus. These
methods
include containing a first fluid having a first specific gravity, containing a
second fluid
having a second specific gravity, separating the first fluid from the second
fluid with a
permeable membrane having a first surface in fluid communication with the
first fluid, a
second surface in fluid communication with the second fluid, the membrane
further having
a vertical axis and being permeable between the surfaces by molecules of less
than a
predetermined size. The method further includes imposing a differential
hydraulic head
1~
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
(e.g., passively by gravity and differential chamber overflow levels, or
actively by
application of positive or negative pressure as described herein) between the
first fluid and
the second fluid, adjusting the second specific gravity (typically by
introduction of gas),
and collecting the second fluid. Imposing the differential hydraulic head may
further
include containing the first fluid at a first column height, and containing
the second fluid at
a second column height, wherein the second column height is selected relative
to the first
column height to produce a selected pressure differential across the membrane
along the
membrane vertical axis at the first specific gravity and the adjusted second
specific gravity.
[57] Another aspect of the invention provides a method of treating a fluid by
membrane
separation employing a selective, semi-permeable, microporous, or other
partitioning
membrane for processing, refining, and/or treating liquid compositions, for
example
membrane waste-water purification processes and apparatus. The method includes
containing a first fluid having a first specific gravity at a first column
height, and
containing second fluid having a second specific gravity at a second column
height. The
method includes separating the first fluid from the second fluid with a
permeable
membrane having a first surface in fluid communication with the first fluid,
and a second
surface in fluid communication with the second fluid. The membrane has a
vertical axis
and is permeable between the surfaces by molecules of less than a
predetermined size.
The method further includes adjusting the second specific gravity to more
closely
approximate the first specific gravity in value. Alternate normalization of
specific
gravities between the first and second fluids can be achieved in other ways,
for example by
introduction of non-gaseous solutes into the first fluid. In certain
embodiments, the second
specific gravity is adjusted to within approximately +/- 5 percent of the
first specific
gravity (i.e., to a value that is 95% of the value of the first specific
gravity). In another
embodiment, the second specific gravity is adjusted to within approximately +/-
2.5
percent of the first specific gravity. The method also includes production of
a selected
pressure differential across the membrane along its vertical axis at the
adjusted second
specific gravity, for example by providing or selecting a second column height
that differs
from the first column height. In more detailed embodiments, the method further
includes
collecting the second fluid, for example by overflowing or off draining the
second fluid as
a processed effluent.
19
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[58] Another aspect of the invention provides an improved vertical shaft
bioreactor and
associated methods for treatment of wastewater. The vertical bioreactor and
associated
methods are as described herein, above. The bioreactor receives an influent of
wastewater
containing biodegradable matter for treatment and produces an effluent flow
which is
directed to a submerged membrane assembly of the invention. The improvement in
the
bioreactor includes a membrane-adapted head tank that functions as a normal
vertical shaft
bioreactor head tank but is modified to receive and contain the effluent flow
and
removably receive the submerged membrane. The submerged membrane includes a
permeable membrane having a first surface, a second surface, and a vertical
axis, and
which is permeable between the surfaces by molecules of less than a
predetermined size.
The first membrane surface is in fluid communication with the effluent flow in
the head
tank, and the second membrane surface is in fluid communication with a second
fluid
having a second specific gravity and contained in a second fluid compartment.
The
improvement includes a means for imposing a differential hydraulic head
between the
1 S effluent flow contained in the tank and the second fluid contained in the
second fluid
compartment, and a means for adjusting the second specific gravity. In more
detailed
embodiments, the improvement also includes a fluid collector that collects the
second
fluid.
(59] In other detailed aspects the invention provides an improved bioreactor
for
treatment of wastewater, the bioreactor receiving an influent of wastewater
containing
biodegradable matter for treatment and producing effluent flow having a first
specific
gravity. The improvement includes a tank that receives and contains the
effluent flow at a
first column height, and that removably mounts a submerged membrane assembly,
and a
fluid collector that collects the second fluid. The submerged membrane
assembly includes
a permeable membrane having a first surface, a second surface, and a vertical
axis, and
which is permeable between the surfaces by molecules of less than a
predetermined size.
The first membrane surface is in fluid communication with the effluent flow. A
second
fluid compartment (separated by the membrane from the head tank) contains a
second fluid
having a second specific gravity at a second column height, and the second
membrane
surface is in fluid communication with the second fluid. The improvement
further
includes a means for adjusting the second specific gravity. The second column
height is
selected relative to the first column height to produce a selected pressure
differential
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
across the membrane along the vertical axis at the changed second specific
gravity. A
portion of the contained effluent flow may be exposed to a normal atmospheric
pressure.
[60] In yet additional detailed aspects the invention provides a submerged
membrane
gas diffusion apparatus. The apparatus includes a membrane having a first
surface and a
S second surface, and a vertical axis, and which is permeable between the
surfaces by
molecules of less than a predetermined size. The apparatus includes a first
containment
member, typically a tubular containment member, having a bubble capture
aperture, a first
membrane mounting portion in fluid communication with the first surface of the
membrane, and a first chamber in fluid communication with the first membrane
mounting
portion and the bubble capture aperture, the chamber including a rising gas
bubble capture
portion proximate to the bubble capture aperture and having a first vertical
length. The
apparatus further includes a second containment member, typically a tubular
containment
member, having a gas release aperture, a second membrane mounting portion in
fluid
communication with the first surface of the membrane, and a second chamber in
fluid
1 S communication with the second membrane mounting portion and the gas
release aperture,
the chamber including a gas reservoir portion proximate to the gas release
aperture and
having a second vertical length that is less than the first vertical length.
Notably, the first
and second containment members can be constructed and dimensioned according to
a
variety of designs to function in the manner disclosed herein below, whereas
the tubular
design described herein is provided for exemplary purposes only.
[61] Another aspect of the invention provides a submerged membrane gas
diffusion
assembly. The assembly includes a membrane having a first surface and a second
surface,
and a vertical axis, and which is permeable between the surfaces by molecules
of less than
a predetermined size. The assembly includes an aeration compartment that
contains a first
fluid and rising bubbles of a gas, a static fluid compartment that contains a
second fluid,
and a fluid treatment compartment that contains a fluid to be treated in fluid
communication with the second membrane surface. The assembly also includes a
first
tubular member having a bubble capture aperture located in the aeration
compartment, a
first membrane mounting portion in fluid communication with the first surface
of the
membrane, and a first chamber in fluid communication with the first membrane
mounting
portion and the bubble capture aperture, the chamber including a rising gas
bubble capture
portion proximate to the bubble capture aperture and having a first vertical
length. The
21
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
assembly further includes a second tubular member having a gas release
aperture located
in the static fluid compartment, a second membrane mounting portion in fluid
communication with the first surface of the membrane; and a second chamber in
fluid
communication with the second membrane mounting portion and the gas release
aperture,
the chamber including a gas reservoir portion proximate to the gas release
aperture and
having a second vertical length that is less than the first vertical length.
[62] A further aspect of the invention provides a method for diffusing a gas
into a target
fluid. The method includes permeably separating the target fluid from the gas
with a
membrane, the membrane having a first surface in contact with the gas, a
second surface in
contact with the target fluid, and which is permeable between the surfaces by
molecules of
less than a predetermined size. The method also includes capturing the gas by
receiving a
first fluid that includes rising bubbles of the gas into a bubble capture
aperture of a first
chamber, the first chamber including a rising gas bubble capture portion
proximate to the
bubble capture aperture and having a first vertical length. The method further
comprises
imposing a hydraulic head on the gas in the first chamber using a buoyancy of
the gas in
the first fluid to displace the first fluid from the bubble capture portion.
Imposition of the
hydraulic head forces the gas to flow between the gas bubble capture portion
of the first
chamber and a first membrane mounting portion of the first chamber, which is
in fluid
communication with the first surface of the membrane. The method further
includes
permeation of at least a portion of the gas through the membrane and into the
target liquid
in response to imposition of the hydraulic head. In addition, the gas flows
between a
second membrane mounting portion, which is in fluid communication with the
first
surface of the membrane, and a second chamber. The second chamber has a gas
reservoir
portion proximate to a gas release aperture and a second vertical length that
is less than the
first vertical length. The method automatically releases the gas through the
gas release
aperture when the hydraulic head displaces a second fluid from the gas
reservoir portion.
[63] Additional aspects of the invention are set forth in detail in the
following
description and appended drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[64] Figure 1 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment.
22
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[65] Figure 2 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment. This embodiment
features a
conventional sedimentation clarifier followed by an aerated polishing
biofilter followed by
an ultra violet light disinfection chamber and back wash tank.
[66] Figure 3 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment. This embodiment
features an
integrated circular sedimentation clarifier surrounding the circular zone 2
head tank which
surrounds the circular zone 1 head tank. All three tanks being concentric with
the vertical
reactor. A provision is made to return settled activated sludge by gravity to
either zone 1
or zone 2.
[67] Figure 4 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment. This embodiment
features
moving bed media circulating in zone 2 or alternately fixed media suspended in
the head
tank of zone 2.
[68] Figure 5 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment. This embodiment
features a
pressurized head tank, an off gas collector means, said off gas driving an air
lift influent
pump required to overcome said head tank pressure, a membrane filtration
cartridge
operating under pressure to separate biomass from liquid and a clean water
ultraviolet
(UV) disinfecting chamber also serving as back wash storage for membrane
backwashing.
[69] Figure 6 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in waste water treatment. This embodiment
features an
integrated clarifier followed by an aerated polishing biofilter followed by an
ultra violet
light disinfection chamber and filter back wash tank.
[70] Figure 7 is a diagrammatic vertical section through one embodiment of a
bioreactor
according to the invention for use in treatment of biosolids. This embodiment
features an
inter zonal self batching air lock at the bottom of the bioreactor. In this
case, zone 2 head
tank is concentric and internal to zone 1 head tank.
[71] Figure 8 is an isometric vertical section through one embodiment of the
bioreactor
according to the invention for use in waste water treatment. This section
shows typical
23
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
arrangement of various channels and the position of the aeration distribution
header, zone
1 head tank, zone 2 head tank and an integral sedimentation clarifier.
[72] Figure 9 is an isometric vertical section of a portion of reactor
internal channels
and downcomer flanged and bolted. This figure shows a downcomer expansion tool
which is used during insertion of the assembly into the reactor casing.
(73] Figure 10 is a diagrammatic end view of the reactor internal section
showing the
downcomer and radial baffles. The element in the center represents the
expansion tool in
its relaxed position. The downcomer is also in its relaxed position. The
removable
expansion tool which is operated by actuation means from the ground level, is
inserted in
its relaxed position during fabrication.
[74] Figure 11 is a diagrammatic end view of the reactor internal section
showing the
downcomer forced out of round by the expansion tool. The radial baffles
connected to the
downcomer are shown relaxed from the casing wall, allowing easy insertion.
[75] Figure 12 provides a graphical representation of the EPA time and
temperature
requirements for class A bio-solids.
[76] Figure 13 provides an exemplary block flow diagram of the present
invention
adapted to produce recycle quality water, Class A bio-solids, and clean
odorless off gas.
The following key applies to the Figure 13:
Preliminary treatment
A Fine screens
B Solids hopper-Screenings and washed grit
C Hyrdaclone degritter
Waste water BNR treatment as described herein
D Deoxygenation unit (channel 32+40 )
E Denitrification (head tank 16 )
F Anoxic/anaerobic unit (channel 12 )
G Aerobic unit (zone 1 channel 80 )
H Nitrification (zone 2 head tank, 110 and 82)
24
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
I Sedimentation clarifier (120 )
J Waste activated sludge float thickener
K Alum or ferric chloride feeder
L Process air compressor
Recycle quality water (units required by law)
M Flocculating tank
N Cloth disk filter
0 Chlorination
P Ultraviolet disinfection
Q Backwash pump
Thermophilic aerobic digestion as described herein
class A biosolids
R Zone 1 thermophilic aerobic digester
S Zone 2
T Acid feeder
U Polymer feeder
V Centrifuge de-watering
W Flotation cell
X Air compressor
Y Off gas collection system
Z Class A bio-solids collection
[77] Figures 14-1 through 14-7 illustrate a presence of nucleated dissolved
air or applied
dispersed air on the clean water (or permeate) side of a permeable membrane,
creation of
an equalized pressure differential along a vertical axis of a submerged
permeable
membrane assembly, and scouring the clean water side of the membrane with
rising
bubbles, according to an embodiment of the invention.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[78] Figure 1 S is a top perspective view of a bioreactor head tank, and a
membrane
bioreactor head having plurality of saddle tanks mounting membrane bioreactor
assemblies, according to an embodiment of the invention.
[79] Figure 16A is a top view of the saddle tank of the membrane bioreactor
head of
Figure 1 S illustrating a top membrane bioreactor assembly that includes a
plurality of flat
plate permeable membranes, according to an embodiment of the invention
[80] Figure 16B is a cross-sectional side view of the bioreactor head tank of
Figure 15,
and of the saddle tank having a stack of four membrane bioreactor assemblies
positioned
vertically above each other, according to an embodiment of the invention.
[81] Figure 17 illustrates a folded saddle tank system that includes a first
folded saddle
tank and a second folded saddle tank that collectively carry the membrane
assemblies,
according to an embodiment of the invention.
[82] Figure 18 illustrate results of a series of membrane throughput tests
conducted on
bench test apparatus of under varying condition and levels of diffused gas in
water,
according to an embodiment of the invention.
[83] Figure 19 illustrates results of a series of temperature vs. viscosity
tests conducted
on the bench test apparatus.
[84] Figure 20 illustrates a cross-sectional view of a gas diffusion apparatus
that
maintains equal pressure differentials across a plurality membranes in a gas-
liquid system,
according to an embodiment of the invention.
[85] Figure 21 illustrates several aspects of the gas diffusion apparatus of
Figure 20,
according to an embodiment of the invention.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS
[86] As illustrated in the attached Figures, the instant invention provides a
long vertical
shaft bioreactor 10 for wastewater treatment. The bioreactor of the invention
shares a
number of structural and functional characteristics with previously described
vertical shaft
bioreactor systems (see, e.g., U.S. Pat. Nos. 4,279,754, 5,645,726, and
5,650,070 issued to
Pollock, each incorporated herein by reference), but departs in several
important and novel
aspects therefrom.
[87] In reference to Figure 1, the vertical shaft bioreactor 10 of the
invention features a
wastewater circulation system which includes two or more substantially
vertical channels,
26
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
including at least one downflow channel, or downcomer channel 12, fluidly
interconnected
in a circuitous, open or closed, path with at least one upflow channel, or
riser channel 14.
The downcomer and riser channels are typically interconnected at their upper
ends via a
surface basin or head tank 16, which may be open or closed, and at a lower
junction
corresponding to a mix zone 18 situated below a lower port or aperture 20 of
the
downcomer.
[88] The downcomer 12 and riser 14 channels are typically defined by separate
conduits, for example by separate, cylindrical-walled pipes. Alternatively,
they may be
defined as interconnected compartments or channels sharing one or more walls,
for
example as parallel channels separated by partitioning structures (e.g.,
radial partitions or
septa) within an elongate, compartmentalized reactor vessel or frame. The
downcomer
and riser channels are preferably oriented substantially parallel to one
another, for example
in a side-by-side or coaxial relative configuration.
[89] Typically, the downcomer 12 and riser 14 channels are defined as separate
conduits
over at least a portion of their lengths. In one example, the downcomer
channel is defined
by a separate, cylindrical-walled downcomer conduit (e.g., a steel pipe) 22
nested coaxially
within a larger diameter, cylindrical walled riser conduit 24 (which will
often correspond
to an outer wall or casing of the entire bioreactor assembly). As such, the
attached Figures
are generally to be interpreted as schematic illustrations, wherein for ease
of illustration
the drawings which show the downcomer conduit laterally displaced relative to
the riser
conduit are intended also to schematically illustrate an alternative, parallel
or coaxially
nested configuration of the downcomer conduit within the larger riser conduit.
[90] In one embodiment of the invention adapted for residential use, the
wastewater
treatment bioreactor 10 of the invention is constructed to service a small
residential
community of about 5,000 population. Typically, two parallel bioreactors are
installed in
accordance with EPA redundancy requirements, in vertical in-ground shafts
bored using
conventional drilling technology. In various embodiments, the bioreactor of
the invention
can be constructed, configured with secondary features, or adjusted to provide
the
secondary and/or tertiary levels of treatment, listed below.
a) Secondary treatment (BOD and TSS removal) only.
27
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
b) Secondary treatment with nitrification of ammonia (conversion of ammonia
to nitrate).
c) Secondary treatment with nitrification and denitrification (removal of
ammonia and nitrate).
d) Secondary treatment with nitrification, denitrification, and chemical
phosphorus removed (tertiary treatment). Some biological phosphorus removal
will occur
at low loads.
e) Thermophilic aerobic digestion and pasteurization of sewage sludges to
produce class A biosolids.
(91] In brief reference to the following description, the secondary treatment
of a) above
may be completely aerobic both in the zone 1 head tank 16 and downcomer
channel 12 of
zone 1, and in the zone 2 upflow channels) 82 and head tank 15. This
configuration
requires a shaft of about 30 inches diameter and 250 feet deep, a zone 1 head
tank of about
6 feet diameter x 10 feet deep and a concentric zone 2 head tank of about 12
feet diameter
x 10 feet deep. The concentric clarifier is about 28 feet diameter x 10 feet
deep and is
fitted with a rake mechanism to assist in sludge removal. In more detailed
embodiments,
this reactor will treat residential sewage from at least a 2,500 member human
population
and produce < 30 mg/L TBOD and <30 mg/L TSS.
[92] The secondary treatment process of b) is also completely aerobic and of
the same
general dimensions as a) except the zone 2 head tank is about 16 feet in
diameter. A larger
portion of the air originating at the bottom of zone 1 is diverted into zone 2
using a
diverter mechanism 84. The treatment system of c) above is designed for anoxic
conditions in the head tank and downcomer of zone 1. In certain embodiments,
this
reactor will treat residential sewage from at least a 2,500 member human
population and
produce < 1 mg/L ammonia-N, <15 mg/L TBOD, and < 15 mg/L TSS.
[93] Only a small fraction of air from the lower portion of zone 1 is diverted
into the
zone 1 upflow channels) 40. In addition to raw influent feed in the upper end
of zone l,
recycled nitrified effluent or return activated sludge from the clarifier or,
alternatively from
zone 2 head tank, is added to the raw influent to create the anoxic
conditions.
[94] In this treatment process the reactor is enlarged to approximately 36
inches in
diameter, zone 1 head tank is increased to about 8 feet diameter, zone 2 head
tank is
28
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
increased to about 16 feet in diameter. The concentric clarifier has an
outside diameter of
about 30' and is fitted with a rake mechanism. In more detailed embodiments,
this process
will treat residential sewage from a human population of 2,500 or greater to <
5 mg/L
TKN, <10 mg/L TBOD, and < 10 mg/L TSS.
[95] The treatment system of d) above is the same general dimension of c).
Within the
treatment process of d), alum of fernc chloride may be added into zone 2 for
chemical
precipitation of phosphorus. It is usually uneconomic to use only a biological
phosphorus
removal process alone to achieve a high degree of phosphorus removal (e.g., 2-
3 mg/L
residual) on small plants, since a pre-fermentation step to produce volatile
fatty acids
(VFA) may be required. Typical characteristics of effluent from this plant
are: TBOD < 10
mg/L; TSS<10 mg/L; TN < 5 mg/L; P04< 1 mg/L.
[96] In the case of sludge treatment e), the reactor is reconfigured such that
zone 1
surrounds zone 2, or may be adjacent to zone 2 throughout the major portion of
the reactor
length and zone 2 head tank 15' surrounds the zone 1 head tank 16'. Zone 1 and
zone 2
are hydraulically connected at the bottom of zone 2 through a self hatching
air lock device
which precludes zone 1 contents from entering zone 2 while processing each
batch. The
thermophilic aerobic digester volume of configuration e) is about one half the
volume of
the wastewater treatment reactor producing the biomass. Because sludge storage
provision
is more economic to build than redundancy in reactors, only one digester is
required for
two treatment reactors. Accordingly the small town of about 5000 people
requires 2
treatment reactors and 1 sludge digester all of the same size. The foregoing
example is a
typical design for small communities of about 5000 people.
[97] Since about 80% of the voidage (air lift) occurs in the top 80-100 feet
of any air lift
reactor, the superior channels can be effective between 150 and 50 feet deep,
preferably
80-88 feet which is the standard length of two joints of double random length
pipe. Off the
shelf air compressors are readily available in 100, 125 and 150 psi models
corresponds to
shaft depth of 200, 250 and 300 feet. Although airlift bioreactors have been
built between
60 feet and 500 feet depths, a more common range is 150 to 350 feet depth and
a range of
200 feet to 300 feet is now most common.
[98] Conventional water well rigs can drill holes up to about 48 inches and
deep
foundation equipment for pilings can drill up to about ten feet in diameter.
Augers (where
29
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
geology permits) can drill up to about 20-feet diameter but are limited to
about 200-feet
depth. Mined shafts can be up to 30 feet diameter and of virtually any depth.
[99] Small municipal plant reactors (5000 population) will typically be placed
with
conventional water well rigs and preferably be about 24 to 48 inches in
diameter.
S [100] Larger communities (10,000-50,000 population) may require shafts of 5
to 10 feet
diameter x 200 feet depth placed by deep foundation piling machines and
augers, whereas
very large industrial plants (e.g. pulp mills) may require shafts placed by
mining
techniques.
[101] The long vertical shaft bioreactor 10 of the invention receives
influent, typically
wastewater or sludge, through an influent conduit 30 which introduces the
influent into an
influent channel 32. The influent flows downward to the bottom of the influent
channel,
where it exits through a shielded influent port 34 and combines with upflow in
a zone 1
upflow channel 40 delineated at its lower end by the influent port. The
influent port is
upturned or otherwise shielded to prevent admission of bubbles from below the
zone 1
upflow channel from entering the influent channel.
[102] In alternate embodiments of the invention, the influent channel 32 can
optionally
accept recycle flow of liquor from the head tank 16 portion of zone 1 of the
bioreactor 10.
This flow is regulated by a zone 1 recycle flow regulator 50, for example a
manual or
motor-actuated baffle, valve or other flow-regulating apparatus. In this
context, the
influent flow through the zone 1 recycle regulator 50 is ordinarily throttled
via an influent
flow throttling control mechanism. This can include, for example, a system
control unit S 1
(e.g., a system control microprocessor) operatively linked to a valve or
baffle actuator 52
and an optional flow sensor 53 or 53' for determining influent and/or zone 1
recycle flow
or alternatively dissolved oxygen DO probe 49 to monitor oxygen levels.
Control of
influent flow through the regulator functions in part to adjust the air lift
in zone 1 upflow
channel 40 and facilitate gravity influent flow. The combined flow in the zone
1 upflow
channel contains some anoxic air bubbles (see below) and is therefore lighter
than the fluid
in influent channel 32, and rises. By anoxic air bubbles is meant bubbles
predominately
containing gasses other than useable oxygen. Flow in the zone 1 upflow channel
40
traverses a horizontal degas plate 54 and descends substantially free of
entrained bubbles
in the downcomer channel 12 under gravity and enters the main riser channel 14
in the
vicinity of the mix zone 18, where it is intensively aerated.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[103] The compressed air or other oxygen-containing gas or liquid serving as
the
oxygenation source for the bioreactor 10 is typically delivered through one or
more
dedicated oxygenating lines, typically compressed air lines 62. A dedicated
compressed
air line is connected to a compressed air supply at the surface and runs
downward parallel
to the riser channel (e.g., nested within the riser conduit 24) extending to
an oxygenation
port, typically an air delivery port 64, that opens in fluid connection with
the riser channel
14. The air delivery port 64 is generally positioned beneath the air
distribution header 60
to release the compressed air for dispersal by the header, as described above.
Within
certain embodiments of the invention, compressed air (or other oxygen-
containing gas or
liquid) is optionally, or additionally, delivered within the bioreactor by a
dual-service
aeration/solids extraction line 66. Functioning of this line can be
controlled, e.g., by a
system control unit 51 as described above, to optionally deliver compressed
air or other
oxygen-containing gas or liquid and, in a second operation mode, serve as a
waste solids
extraction line 66 to purge waste solids from a sump 67 portion of the reactor
located at
the bottom of the riser channel. The waste solids extraction line extends from
the surface
(e.g., from a surface-located, waste-solids extraction/flotation reservoir) to
a
aeration/waste solids extraction port 68 opening in fluid connection with the
sump. Solid
particles that settle into the sump will accumulate over a period of hours of
operation. For
the majority of the bioreactor's operation time, the aeration/solids
extraction line is
continuously purged by flow of compressed air, and therefore the sump 67 is
substantially
mixed and aerated and forms a functional part of the mix zone 18.
Periodically, the
aeration/extraction line can be depressurized, whereby settled solids within
the sump will
rush to the top of the reactor to be purged therefrom. These solids are highly
aerated, well
stabilized (odor free) and because of the high gas content will spontaneously
float to a
thickened sludge.
[104] In related embodiments of the invention, the improved vertical shaft
bioreactor 10
features two simultaneously-operating aeration lines or ports to enhance the
formation of
small, dispersed bubbles to generate upflow currents and supply process air
within the
bioreactor. The use of two aeration lines is exemplified by the dedicated
compressed air
line 62 and dual-function aeration/solids extraction line 66, which each
operate at least for
a majority of the bioreactor process time in a compressed air delivery mode.
In this mode,
the two lines in concert provide a cooperative, multiple source compressed air
injection
31
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
mechanism of the invention, which serves to enhance the turbulence and small
bubble-
forming capacity within the mixing zone 18 of the reactor, which is in turn
expanded by
the cooperation of multiple compressed aeration lines or ports. In one aspect
of this
enhanced mixing/bubble forming mechanism, a first aeration line opening,
exemplified by
the air delivery port 64 of dedicated air line 62, is positioned below the air
distribution
header 60 and above a second aeration line opening, exemplified by
aeration/extraction
port 68 of the dual-function aeration/solids extraction line. Compressed air
released from
this lower aeration port stimulates fluid mixing and bubble formation near the
bottom of
the riser channel 14 to set up a first circulation path or vector. The
resultant circulating
fluid-bubble mixture impinges upwardly and/or transversely against mixed fluid
and
bubbles generated by the introduction of compressed air from the first, upper
air line 62.
This results in increased shear forces and the production of smaller air
bubbles in an
enlarged mixing zone, compared to the results achieved by operation of a
single aeration
line (see, Figure 1).
[105] In conjunction with the above-described use of a cooperative, multiple
source
compressed air circulation regime, certain embodiments of the invention
incorporate a
modified (typically stepped, chambered, or baffled) header, or a mufti-
component header
complex, to augment the enhanced mixing/bubble forming mechanism provided by
multiple, interactive aeration sources. In one aspect, a second, cooperating
shear header 70
is mounted within the riser chamber 14 below the main bubble distribution
header 60 and
works in conjunction with two, vertically tiered aeration sources generally as
described
above. The shear header can be any flow diverting or channeling device that
enhances an
upward and/or transverse or radial flow component within the mixing zone
generated by a
second, lower-positioned aeration source (exemplified by the aeration/solids
extraction
port 68). In one exemplary embodiment, the shear header comprises an
internally stepped
draught tube (Figure 1) attached by vertical struts to the underside of the
distribution
header. Compressed air fed into the aeration/solids extraction line 66 causes
an air lift
effect in the stepped draught tube, thus establishing a separate circulation
pattern or vector
in the lower portion of the mix zone as shown in Figure 1. This upward and/or
transverse
or radial circulating flow impinges against mixed fluid and bubbles generated
by the
introduction of compressed air from the first, upper air line 62 near the
perimeter of the
distribution header, which interaction is regulated in part by air delivered
though the
32
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
aeration/solids extraction port, while the balance of process air is delivered
though the
dedicated air delivery port 64. This creates very high flow rates inside the
serrated skirt in
increased shear at the perimeter of the distribution header which aids
substantially in
shearing bubbles to a smaller size. Whereas previous bioreactors typically
generate
bubbles at the site of distribution in the range of about a half inch to three
quarters of an
inch in diameter, the novel interactive flow mechanism and cooperative header
design of
the invention generates substantially smaller bubbles, typically about one
quarter to one
half inch, often less than one quarter inch, down to as small as one-fifth to
one-eighth inch
or less in diameter. For example, studies published in the water Environment
Research
Journal May/June 1999 pgs. 307-315 (incorporated herein by reference)
determined that
bubbles about 2mm are the optimum diameter for mixing and oxygen transfer.
However
bubbles of this size do not form naturally at an orifice without some
mechanism for
shearing the bubble. The bubble size is determined when the buoyancy force
equals the
attraction forces at the orifice and bubble size is not necessarily a function
of orifice size.
Since bubbles of this size range have a rise rate of about 0.8-1.0 feetlsec.
in water, a
downward circulation velocity of greater than 1 feet/sec. in the vicinity of
the serrated skirt
60 will cause the bubble to be sheared from the orifice. The circulation
velocity is
regulated by the amount of air injected in line 68 and can be adjusted
independently of the
air being applied at orifice 64. Samples extracted periodically in line 66 can
be measured
for dissolved oxygen. The circulation velocity between aerator elements 60 and
70 can be
adjusted to maximize the oxygen transfer. This novel design provides enhanced
mixing
and bubble distribution without unacceptable risk of clogging. When the
aeration/solids
extraction line is being used for biomass wasting, air-flow in the dedicated
air line
maintains reactor circulation. At this point, when the aerator barrel of the
shear header is
depressurized a new batch of waste biomass transfers from the mix zone 18 to
the sump
and aeration of biomass within the aeration barrel of the shear header begins
again.
[106] Yet additional embodiments of the invention are distinguished by virtue
of their
novel features for channeling, circulating, and segregating fluid, air and/or
biomass within
the reactor 10. These features are in turn variable, combinable in alternative
reactor
configurations, and/or adjustable within additional aspects of the
inventior~allowing use
or modification of the reactor for different wastewater treatment applications
and results.
In general aspects, the bioreactor of the invention features a first treatment
or processing
33
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
"zone" designated zone 1, wherein the majority (e.g., greater than 80%, up to
90-95% or
greater) of the primary reaction between waste, dissolved oxygen, nutrients
and biomass
(including an active microbial population), takes place. Within certain
embodiments, this
zone is defined to include an upper circulating zone of the bioreactor
comprising the
surface basin or head tank 16, a primary reaction chamber 80 comprising a
central volume
of the riser channel 14, the downcomer channel 12, and the mix zone 18.
[107] The majority of the contents of the mix zone 18 represent a fluid-bubble
mixture
that is less dense than the fluid in the downcomer channel 12 and therefore
circulates
upwardly from the mix zone into the primary reaction chamber 80. Undissolved
gas,
mostly nitrogen, expands to help provide the gas lift necessary to drive
circulation of the
liquor in the upper part of the reactor 10 in the patterns as shown by the
arrows throughout
the Figures. The products of this primary reaction are carbon dioxide and
additional
biomass which, in combination with unreacted solid material present in the
influent
wastewater, forms a sludge (or biosolids).
[108] In certain embodiments of the invention, as illustrated in Figure 1,
upflow of fluid
in the primary reactor channel 80 is segregated into multiple, smaller upflow
channels in
an upper section of the bioreactor 10. In one exemplary embodiment, upflow
from the
primary reactor channel is diverted into at least two discrete superior upflow
channels, as
exemplified by the zone 1 upflow channel 40 and a zone 2 (typically operated
as a
polishing zone) upflow channel 82 depicted in Figure 1. In one exemplary
construction
design, flow diversion from the primary reactor channel into multiple,
superior channels is
achieved by employing a fixed or adjustable diversion plate 84 or comparable
flow
diverting device that is anchored near the top of the primary reactor channel.
[109] The diversion plate 84 is configured and dimensioned to segregate the
primary
reactor channel 80 upflow into multiple superior channels. Typically, the
diverter plate is
configured and dimensioned to intercept and divert a larger fraction of total
upflow
volume of the fluid-bubble mixture from the primary reactor channel into a
selected
"aerobic" upflow channel, depending on the desired mode of operation of the
bioreactor
10, as further explained below. In the exemplary embodiment shown in Figure 1,
the
diverter plate features a vertical baffle 86 that facilitates segregation and
channeling of the
fluid-bubble mixture flowing upward in the primary reactor channel toward an
upwardly
angled, laterally or radially extending flow diverting extension 88 of the
diverter plate that
34
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
diverts a larger fraction of the total upflow volume of fluid and bubbles from
the primary
reactor channel into one or the other of the first zone upflow channel 40, or
second zone
upflow channel 82. Accordingly, a smaller fraction of the total upflow volume
of fluid
and bubbles is allowed to pass into the remaining superior upflow channel 40,
thereby
limiting as a primary process determinant the flow of aerated fluid into this
remaining
channel so as to contribute to generation of anoxic conditions in this
channel, if desired.
[110] Selection, positioning and adjustment of the flow diverter mechanism
depends on
the selected mode of operation of the bioreactor 10. In alternative
embodiments, the
diverter plate 84 can be positioned, shaped, dimensioned and/or adjusted to
channel
upflow of the fluid-bubble mixture from the primary reactor channel 80 into
one or more
superior channels to achieve higher aerobic environmental conditions in the
selected
channel(s), while limiting the upflow (particularly of high oxygen-containing
fluid) into
one or more superior channels selected for lower aerobic, even anoxic,
environmental
conditions. By way of example, the following steady state functionality of
adjustable
baffles 86 and 84 is described. In Figure 1, 10 bubbles are depicted as rising
uniformly at
the top of zone 1 immediately below baffle 86. The baffle is adjusted so that
3 bubbles are
segregated into area 39 and 7 are segregated into area 81. However the flow
into area 81 is
approximately equal to Q, influent/effluent flow + 1.75 Q nitrated recycle
flow =2.75 Q.
In this exemplary design, the flow into area 39 is controlled to 5 Q.
Therefore the flow per
bubble in area 39 is 5/3=1.7 Q/bubble and in area 81 it is 2.75/7=0.4 Q/
bubble. Similarly
the oxygen demand and supply in the superior channels and head tanks can be
calculated.
Typically the average BOD in the area 39 and 81 is about 10 mg/L and the
average
ammonia -N concentration to be removed is 1 S mg/L (after ammonia used in cell
synthesis) and the denitrified recycle flow is 1.75Q. Therefore the average
ammonia
concentration would be 15/1.75 = 8.57mg/L. This level of ammonia-N is equal to
8.75
mg/L-Nx 4.6 # oxygen/# N =39 mg/L of BOD equivalent. The total load into zone
2 is
therefore = 2.75Q (10+39) = 134 Q oxygen units. Since there are 7 bubble
oxygen units
the load per bubble is 134/7 = 19 oxygen units required/bubble. Similarly the
load into
area 39 is SQxlO mg/L BOD = SOQ oxygen units required. However in channel 40
above
port 34 the load increases to SOQunits + Qx200 units (assuming the influent
BOD is 200
mg/L) for a total load of 250 Q units of oxygen required. Since there are only
3 bubble
oxygen units available, the oxygen required per bubble is 250/3 = 83 oxygen
units.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Therefore the oxygen demand per bubble oxygen unit is higher in head tank 16
than in
head tank 15 by 83/19 = 4.3 times. Consequently, if there is measurable
dissolved oxygen
in head tank 16 there will be surplus DO in head tank 15, and if there is
surplus DO in
head tank 16 there will substantially more DO at any level below baffle 86
down to the
mix zone 18. Thus baffle 86 can be adjusted to accommodate a wide range of
load and
flow criteria.
[111] Thus, in one aspect of the invention, the improved long vertical shaft
bioreactor
functions for mufti-purpose waste treatment by providing aerobic digestion of
BOD as
well as single mixed liquor processing BNR treatment. Refernng to Figure 2,
the flow
diverter 84 is constructed and configured as shown (compare alternate diverter
configuration/setting shown by phantom line 90) to divert a majority fraction
of total
upflow volume of the fluid-bubble mixture from the primary reactor channel
into the zone
2 upflow channel 82, while limiting the upflow volume of fluid and bubbles
from the
primary reactor channel 80 into the zone 1 upflow channel 40. Volume ratio in
influent
1 S channel 32 and flow down and into the zone 1 upflow channel (which
intercepts only a
small fraction of the bubbles from the primary reactor channel) can be finely
controlled.
Thus, a relatively small amount of air lift and a slow circulation rate can be
provided the
zone 1 upflow channel compared to the lift and circulation in the zone 2
upflow channel in
this diverter configuration. The residence time of the fluid mixture in the
zone 1 upflow
channel is therefore increased, and the oxygen transfer capability in zone 1
upflow channel
40 is reduced due to the reduced bubble upflow. Notably, the bubbles in the
zone 1
upflow channel are mostly nitrogen, because the oxygen is largely consumed in
the lower
and middle part of zone 1 (particularly including the mix zone 18 and the
primary reactor
channel 80 below the diverter).
[112] Within this embodiment and adjustment/operation mode of the bioreactor
10, the
superior channel referred to as the zone 1 upflow channel 40, can be selected
to provide an
anoxic environment, achieved in part by the low relative influx of oxygen and
the high
oxygen demand of the raw influent stream. This anoxic zone continues
throughout the
circulation path between the zone 1 upflow channel and the downcomer channel
12, as
approximately indicated by the arrows in Figure 2. Within this anoxic zone, a
final step of
BNR processing, denitrification of nitrate initially contained in the mixture
of fluid in the
zone 1 upflow channel, occurs. When this mixture, following the path
indicated, reaches
36
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
the mix zone 18, re-aeration of the anoxic flow exiting the lower downcomer
port 20
occurs, and residual BOD that was not removed in the anoxic zone is oxidized
in the lower
part of zone 1 (including the mix zone and primary reactor channel 80).
Thereafter, a
portion of the uprising flow in the primary reactor channel flows upward into
the zone 1
upflow channel 40, because this top portion of zone 1 is designed to be
anoxic, the number
of bubbles required for bio-oxidation is reduced. The airlift effect is also
greatly reduced
to slow the upflow in this part of the reactor. In addition, the ability to
control influent
flow via the zone 1 recycle flow regulator 50 also allows adjustment of air
lift and flow in
the zone 1 upflow channel.
[113] Within the foregoing operation mode of the bioreactor 10, a major
portion of the
uprising air flow in the primary reactor channel 80 flows upward into the
other superior
upflow channel(s), exemplified by the zone 2 upflow channel 82. The relative
lower liquid
upflow fraction thus segregated includes the majority of bubbles originating
at the lower
end of zone 1 (e.g., bubbles generated by the dedicated air line 62 and
optional multi-
purpose aeration/waste solid extraction line 66, functioning in concert with
the bubble
distribution header 60 and optional shearing enhancer mechanism exemplified by
the shear
header 70). This active, fluid-bubble mixture segregated into zone 2 by
operation of the
diverter 84 enters the zone 2 upflow channel, then mixes with vigorous re-
circulating flow
entering zone 2 through a zone 2 recirculation channel 110 (which recycles
liquor from the
zone 2 head tank 15). This recirculation flow is optionally regulated by a
zone 2
recirculation flow regulator 112, for example a manual or motor-actuated
baffle, valve or
other flow-regulating apparatus. This recycle flow regulator is also
optionally controlled
by the system control unit 51 (e.g., system control microprocessor)
operatively linked to a
valve or baffle actuator 52 and optional flow sensor 53 for determining zone 2
recycle
flow).
[114] When the bioreactor 10 is thus configured and/or adjusted for BNR
removal,
nitrification of mixed liquor can be efficiently conducted and controlled
within zone 2 of
the bioreactor, in accordance with the above-described construction and
operation details.
Some of the mixed liquor from zone 2 may be discharged to a detached 120 or
integrated
120' solids-liquid separator (clarifier) (see, e.g., Figures 2-4, and 6). Some
of the mixed
liquor from zone 2 may be returned to the influent channel 32, where it
undergoes de-
nitrification, as described above, and the cycle repeats. Optionally, some
clarified effluent
37
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
may be returned to channel 32 during low flow periods, thereby removing more
nitrogen
compounds overall.
[115] In more detailed embodiments of the invention, influent, return
clarified effluent
(e.g., recycled from a separate clarifies 120 or integrated clarifier120'),
and return
activated sludge are combined in a preselected ratio to facilitate operation
of the bioreactor
10. This can be achieve using various flow control features of the invention,
and is
facilitated in part by incorporation and controlled operation of a zone 1
activated sludge
return channel 122 and a zone 2 activated sludge return channel 124 which
receive
activated sludge (e.g., via a sludge extractor line 126 connected to the
clarifies) and direct
the sludge into the zone 1 influent channel 32 or zone 2 recycle channel 110,
respectively
(see, e.g., Figures 2-4, and 8). Flow control within and between each of the
illustrated
feed, flow and drain lines and ports throughout the appended Figures is
readily achieved
using flow regulators 50 operatively interconnected with valve or baffle
actuators 52
and/or flow sensors, all of which are operatively integrated and controlled by
one or more
system control units) 52.
[116] The selected mix ratio per volume of influent of typical municipal waste
may be as
high as 3 volumes of clarified effluent and 1 volume of return activated
sludge to as low as
1 volume of clarified effluent and 1 volume of return activated sludge.
Approximately
85% of total nitrogen will be converted to Nz with 1.75 volumes of either
clarified effluent
or mixed liquor per volume of influent (see, e.g., Naohiro Taniguchi et al.
report on air lift
recirculation for nitrification and denitrification , R&D Division, Japan
sewage works
agency 1987, incorporated herein by reference.) It should be noted, however,
that some
industrial wastes may require 100 or more recycled volumes per volume of
influent.
[117] With respect to the nitrification process functions of the bioreactor
10, this can be
further modified or enhanced by selection or adjustment of the various reactor
features and
operation parameters described above. In addition, the system can readily
incorporate, or
be coupled with, additional system features or components to enhance BNR
process
functions. Because the BOD is low in zone 2, growth of BOD-removing organisms
is
generally minimized, which allows nitrifying bacteria to dominate the biomass.
In
addition to this advantage, a substantial improvement in the rate of
conversion of
ammonium to nitrite and nitrate can also be realized by increasing the
concentration of
nitrifying bacteria. Since nitrifiers are attachment organisms, the provision
of attachment
38
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
sites in a mixed liquor in the form of sponge balls, suspended media, bits of
small diameter
plastic or rubber (elastomeric) polyethylene tubing, hanging strings of porous
fabric in the
liquor, etc., can be used quite effectively within the devices and methods of
the invention
(see, e.g., Keith Ganze "Moving Bed Aerobic Treatment" Industrial Waste Water
Nov/Dec
1998, incorporated herein by reference.) For example, referring to Figure 4,
the BNR
processes of the bioreactor can be substantially improved by including
suspended media
130 that encapsulate or provide substrate for nitrifying bacteria within the
recycling
circulation path of zone 2 (see, also, T Lessel et al" Erfahrungen mit
getauchten
Festbettreaktorn fur die Nitrifikation" 38.Jahrgang, Heft 12/1991, Seite 1652
bis 1665,
incorporated herein by reference), which modification is facilitated by the
novel relative
positioning and interzonal separation between zone 1 and zone 2. The moving
bed media
can be prevented from escaping in the effluent, for example by simple screens.
Alternatively, fixed media 132 can be secured within in the head tank to
increase the
biomass of microorganisms adapted for BNR processing. These modifications
yield a
1 S superior BNR performance. For example, the combination of a zone 2 regime
that
minimizes BOD-removing bacteria along with the increased attached growth
biomass of
nitrifying bacteria (e.g. 15-20 g/L equivalent nitrifiers) provides for highly
effective BNR
processing within the bioreactor of the invention. A single sludge extended
aeration
process typically contains 15-20% of nitrifying bacteria (by weight or
population
percentage of sludge mass). However, when attachment media are used within the
present
invention, the biomass of nitrifiers can be expanded up to greater than 30%,
often up to
60-70%, as much as 75-85% or more of nitrifiers in the system population. This
relates to
the relative exhaustion of BOD in this process stage and zone of the system,
as well as to
the effective use of fixed or circulating attachment media within zone 2.
These novel
features and characteristics distinguish the modified single sludge system of
the present
invention from other single sludge processes.
[118J Within additional aspects of the invention, a novel nitrification
process is provided
which relies substantially or entirely upon residual dissolved oxygen
originating near the
bottom of zone 1 as the source of oxygen to drive the process. Yet another
important
benefit and distinction that arises by using the unspent gases from zone 1 in
this fashion is
the high level of C02 available, which is also required by nitrifying bacteria
as a source of
inorganic carbon. In other nitrification systems, the primary inorganic carbon
source
39
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
depends on alkalinity of the wastewater and is typically determined by the
presence of
CaC03. The bioreactor process systems of the invention are therefore more
compact and
require less energy than current, extended aeration systems. Bioreactors
constructed and
operated according to the invention also produce a better quality biomass
(including class
A biosolids if desired) that is easier to separate from the mother liquor.
[119] To further enhance the functions and operation of the bioreactor 10 of
the
invention, various coupled or integrated features can be incorporated with the
bioreactor
for enhanced processing of waste water. As illustrated in Figure 2, the
bioreactor
according to the invention for use in waste water treatment may incorporate a
conventional, stand-alone sedimentation clarifies 120. The bioreactor is
further optionally
fluidly connected with an aerated polishing biofilter 133 and/or an ultra
violet light
disinfection chamber 134 and/or back wash tank. In certain embodiments, line
136 returns
backwash to the influent.
[120] Alternatively, Figures 3 and 8 (schematically and by partial sectional
perspective
views, respectively) illustrate an additional embodiment of the bioreactor 10
according to
the invention-featuring an integrated circular sedimentation clarifies 120'
surrounding a
circular zone 2 head tank 15 which in turn surrounds a circular zone, l head
tank 16 (all
three tanks being concentric in this vertical reactor). In these embodiments,
settled
activated sludge is returned by gravity to either zone 1 or zone 2.
[121] Alternate embodiments of the bioreactor 10 illustrated in Figure 4
feature moving
bed media 130 circulating in zone 2 and, additionally or alternatively, fixed
media 132
suspended in the head tank 15 of zone 2. Another embodiment, as illustrated in
Figure 5,
incorporates a pressurized head tank 135, and an optional off gas collector
136 (see, e.g.,
U.S.Pat. No. 4,272,379 to Pollock, incorporated herein by reference), for
example with
off gas driving an air lift influent pump 137 required to overcome the head
tank pressure,
as well as an optional membrane filtration cartridge 138 (see, e.g., George
Heiner et al
"Membrane Bioreactors" Pollution Engineering Dec 1999, incorporated herein by
reference) operating under pressure to separate biomass from liquid and a
clean water,
ultraviolet (UV) disinfecting chamber 139 also serving as back wash storage
for membrane
backwashing. Still other embodiments, as shown in Figure 6, feature an
integrated
clarifies 120' fluidly connected to an aerated polishing biofilter 133 and an
ultra violet
light disinfection chamber 134 and filter back wash tank.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[122] Typically, for long vertical shaft bio-reactors, the optimum biological
air supply
rate required for bio-oxidation process creates excessive "voidage" at the top
of the
reactor, comparable in the present case to the superior upflow channels
exemplified by the
zone 1 upflow channel 40 and zone 2 upflow channel 82. Excessive voidage
produces
undesirable slugging (water hammer), which can cause reactor damage attributed
to
vibration. The occurrence of slugging air voidage also signifies poor oxygen
transfer
characteristics within the circulating fluids. The invention addresses these
problems in a
number of ways, including by providing novel means for regulating circulation
velocities
and modulating gas content in selected parts or channels of the reactor.
[123] Since oxygen transfer rate and oxygen utilization rates are relatively
slower than
upward hydraulic velocities in the reactor 10, increasing velocity only
reduces the
operating efficiency of the reactor. Increased flow decreases bubble contact
time and
slows oxygen transfer, thus more aeration is required to optimize the process.
Similarly,
reducing aeration reduces reactor capacity. One proposed method for resolving
air voidage
and related problems is presented in U.S. Patent Application Serial No.
09/570,162, filed
May 11, 2000 (incorporated herein by reference) describing the "VerTreat II"
bioreactor.
In this disclosure, flow velocity is beneficially reduced by incorporation of
an orifice plate
in the lower section of the riser channel. However, this solution does not
substantially
resolve the problem of slugging, and the orifice plate creates additional
problems including
risk of fouling and flow aberrations particularly in small municipal plants.
[124] The bioreactor 10 of the present invention resolves these problems in
part by
incorporating a novel relative configuration of zone 1 and zone 2. Unlike the
previously
described "VerTreat I" bioreactor (see, e.g., U.S. Pat. No. 5,650,070, issued
July 22, 1997,
incorporated herein by reference), where zone 2 is below zone 1 and therefore
no voidage
control in zone 2 is possible, the present invention can control flow and gas
content in each
zone, independently. Conventional prior art "Deep Shaft" reactors start
slugging at a
upflow velocity of about 2 feet per second. The above-noted VerTreat II
reactors with
orifice plates can operate down to about one and a quarter feet per second.
Within the
present bioreactor, this value can be dampened to as little as one quarter to
one half feet
per second in the lower part of the riser channel. At lower riser velocities,
some heavier
solid particles will settle into the sump 67. These solids are conveniently
extracted, along
41
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
with surplus biomass (e.g., circulating within the shear header 70 and
surrounding mix
zone 18) when desired, by purging of the dual-purpose aeration/solids
extraction line 66.
[125] The invention provides substantially more efficient new features and
methods for
slowing velocity over prior art methods, which includes the ability to dilute
the air lift
stream in one or more superior upflow channels) of the reactor with bubble
free fluid, as
described above. The advantage of these features and methods over the VerTreat
II
technology includes the elimination of potential plugging of the orifice plate
in the lower
and inaccessible section of the riser channel, which is particularly
problematic in smaller
diameter reactors.
[126] In long vertical air lift reactors such as the bioreactor 10 of the
invention, where
fluid/gas mixtures are caused to circulate in vertical channels, the volume of
gas in a
defined volume of liquid changes with the pressure (gas laws). Consequently at
the
bottom of the reactor, the volume of gas in liquid (voidage) is small, whereas
at the top of
the reactor the same expanded gas volume to liquid volume ratio is many times
larger.
Since 34 feet of water is equivalent to about one atmosphere of pressure, it
can be readily
calculated that 1 cubic foot of air on the surface (1 scf) becomes 0.5 cubic
feet at 34 feet
depth and 0.33 cubic feet at 68 feet and 0.25 cu. feet at 102 feet. Therefore
integrating the
area under the volume vs. depth curve shows 78% of the gas volume voidage
occurs in the
top 102 feet of the reactor.
[127] Many studies on air-lift pumps and other bubble/water columns show that
slugging
in water occurs at 11-14% voidage. Slugging is undesirable because the bubbles
coalesce
into large air pockets which set up vibrations in the reactor, and most
importantly, large
bubbles have very poor oxygen transfer characteristics. Proposed controls of
voidage to
ameliorate these effects have been attempted in at least two different ways.
One proposed
control is to increase the reactor cross section sufficiently to allow
disengaging the gas
from the gas/liquid mixture. Alternatively, efforts have been undertaken to
maintain
residual pressure on the gas/liquid mixture at the top of the reactor. Each of
these
proposed controls have attendant drawbacks making them undesirable for use
within the
bioreactor of the present invention. For example, head tank designs of some
air-lift
reactors are provided where liquid depths of'/2 atmosphere (17 feet) are used.
This
reduces the maximum voidage by 17%, but head tank depths much deeper than 17
feet are
42
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
difficult to construct. In addition, tall head tanks above ground require
pumping influent
against a significant hydraulic head, wasting substantial energy.
[128] The invention provides novel features and method for controlling voidage
and
ameliorating the adverse effects of slugging. Briefly, these features and
methods reduce
the quantity of bubbles per unit of fluid in one or more selected channels or
chambers of
the reactor 10, either by adding more fluid or reducing the gas. In more
detailed aspects,
liquid flow in one or more superior upflow channels of the reactor is
increased by
recycling liquor from an upper segment (e.g., 60-90') of the reactor, through
a degas step,
and back down to a lower, recycling influx point near the bottom of the upper
segment
(e.g., 60-90 feet below the surface). It is generally considered that total
gas flow (air flow)
is determined by biological optimization requirements, however this total gas
flow can
also be proportioned into selected, superior upflow channels in the upper part
of the
reactor using novel flow control mechanisms described herein.
[129] Because approximately 75-80% of the voidage occurs in the top 60-90 feet
of the
reactor, the recycle channels (exemplified by the influent channel 32 which
optionally
nested receives zone 1 recycle input from zone 1 recycle port 140, and the
zone 2 recycle
channel 110), are only about 25-35% of the total depth of a typical bioreactor
and occupy
only a small fraction of the reactor cross section area and volume. In
practice, zone 1 and
zone 2 of the reactor comprise approximately equal fluid volume, but in the
case of BNR
removal zone 2 is expanded in volume for nitrification by increasing the
diameter of the
zone 2 head tank 15. The voidage in the zone 2 recycle channel can be readily
controlled
under a wide range of operating conditions by designing for sufficient,
adjustable recycle
flow of degassed liquor from the zone 2 head tank 15 as regulated by the zone
2 recycle
regulator 112. The bubble volume in the zone 1 upflow channel 40 can therefore
be
diluted by degassed liquor to the extent limited by the acceptable range of
minimum and
maximum values for influent flow, which is somewhat limited. To resolve this
limitation,
a regulated amount of liquor may be diverted through the zone 1 recycle port
by
adjustment of the zone 1 recycle flow regulator 50 (effectuated by operation
of the system
control unit 51). Controlling flow from the head tank in this coordinated
manner is
necessary to maintain gravity feed of the effluent.
[130] The instant invention therefore provides a number of separate and
optionally
cooperative mechanisms and methods to alleviate the problems of slugging at
low
43
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
bioreactor 10 flow velocities. In another aspect, this problem is alleviated
by providing a
choice of adjustable diverter or baffle devices, exemplified by the fixed or
adjustable
diverter mechanism 84. The configuration (including size, shape, location and
orientation)
of this exemplary diverter plate can be fixed at the time of construction and
installation of
S the reactor. Alternatively, these and other flow diverter parameters can be
selectably
altered, for example by employing a manual or motorized diverter plate
adjustment
mechanism optionally integrated for functional control (e.g., to control
positional and
orientation parameters) by the system controller 51. Operation of the flow
diverter serves
to direct a greater or lesser fraction of air bubbles entrained in the upflow
from the primary
reactor channel 80 into one or more selected superior channels, for example to
divert a
greater fraction of the fluid-bubble mixture toward the zone 2 upflow channel
82, allowing
a lesser to pass upward into the zone 1 upflow channel 40.
[131] Once the desired fraction of bubbles have been thus diverted into the
zone 2
upflow channel 82, the voidage in this channel can be easily corrected by
changing the
amount of zone 2 recycle flow through adjustment of the zone 2 recycle flow
regulator
112. The circulatory loop (following arrows between zone 2 upflow channel 82,
across
zone 2 degas plate 150, through zone 2 recycle regulator 112, down zone 2
recycle channel
110, and through zone 2 shielded recirculation port 152), together with a
surface basin or
zone 2 head tank 15 at the top, comprise zone 2 and represent the polishing
process and
optional nitrification features of the bioreactor which are driven by waste
gas from zone 1.
The configuration of the diverter which segregates flow into the superior
upflow channels
prevents liquor transfer from zone 2 into zone l, since both liquid and air
flow in the zone
2 upflow channel 82 is unidirectionally upward. In this regard, as noted
above, zone 2
circulation characteristics are ideal for the application of fixed media 132
(Figure 4) and,
alternatively or cooperatively, membrane separation components (Figure 5).
Moving bed
media 130 (Figure 4) can also be used, since zone 2 circulates completely
separately from
zone 1, to enhance nitrification within alternative process modes of the
reactor.
[132] Hydraulically, any influent flow into zone 1 of the bioreactor 10 (and
any required
external recycle streams from the clarifier 120 or zone 2 head tank 15) that
enter zone 1
must leave zone 1 by entering the bottom of zone 2. Since zone 1 is a closed
loop, namely
zone 1 upflow channel 40, zone 1 head tank 16, downcomer 12 and primary
reactor
channel 80, the number of recycles in this loop and the liquid velocity
depends directly on
44
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
the volume of air bubbles diverted by diverter plate 84 into zone 1 upflow
channel 40. For
example, in a typical municipal effluent of 200 mg/L of BOD, the number of
internal
recycles is approximately the BOD in mg/L divided by the OZ potential in the
reactor,
divided by the oxygen transfer efficiency. In a 250 feet deep reactor, oxygen
is injected at
S about 7.3 atmospheres of pressure. Solubility of OZ in water at 1 atmosphere
and 20° C is
about 8 mg/L. This means the dissolved oxygen potential at 7.3 atmospheres is
7.3 x 8 =
59 mg/L or about 40 mg/L at an oxygen transfer efficiency of 70%. Therefore,
the
minimum number of recycles is 200 divided by 59 x .70 = about 5. In practice 6
or 7
recycles might be used as a safety factor. A hydraulic loss calculation will
determine the
fraction of air required for 6 or 7 internal recycles; e.g., 30% of the air
that is applied at the
bottom of zone 1. As the organic load to the plant increases or decreases, the
air rate is
adjusted accordingly, causing the number of internal recycles to increase or
decrease to
satisfy the BOD requirement. However, 30% of the air applied remains
consistent,
constant as determined by diverter plate 84 placement. Field trimming is
achieved, for
example, by adjusting regulator valve 50, which changes recycle flow within
the air lift
section at zone 1 upflow channel 40, thus reducing or increasing its air lift
capability.
(133] Similarly, any flow from zone 1 that enters zone 2 must leave as
effluent from zone
2. Since the lower portion of zone 2 comprising upflow channel 82 and adjacent
downflow channel 110 typically has no internal recycle connection with zone 1,
any air
diverted from zone 1 into zone 2 will simply cause circulation in the superior
channels) of
zone 2 with no change in the circulation rate of zone 1 (change in air rate in
zone 1 does,
however, affect the circulation rate in zone 2, but not vice versa).
[134] Therefore, within certain aspects of the invention, diverting for
example 70% of
the air originating at the bottom of zone 1 into zone 2 only affects the
circulation in zone 2
which can be easily controlled by the zone 2 recycle regulator 112.
Hydraulically, influent
flow into zone 1 upflow into zone 2 and effluent from zone 2 within the
reactor 10 are
equal in quantity, i.e., influent flow entering the reactor in zone 1 exits
through zone 2.
With reference to prior art vertical bioreactors treating municipal waste, the
internal
recycle flow is about ten to twelve times the influent flow, or effluent flow.
The present
process, which features novel air lift controls as described above, can reduce
this flow by
about a 2-3 fold reduction, often a 5-6 fold or even greater reduction.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[135] By adjusting the configuration of the diverter (generally referring to
any diverter
device for segregating flow from the primary reactor channel 80 into a
plurality of superior
upflow channels), the selected bubble fraction only (not typically the same as
the liquid
flow fraction) in the primary reactor channel can be segregated among any
desired number
of channels (typically 2, 4 or 6, depending on reactor size and purpose) in
any ratio
selected to achieve optimum operation of zone 1 and zone 2 (note that each
superior
channel shown in Figure 8 has a companion channel opposite it, which is a
typical layout
for larger reactors using two or more clarifiers. Smaller reactors have only 4
channels and
a center downcomer, as illustrated in Figure 7). For example, typical flow
values in the
zone 1 upflow channel 40 may be selected to be 6-8 times (alternatively, 2-3
times with
BNR) the flow entering zone 2 at the top of zone 1 at the level of the
diverter plate 84
(immediately below the zone 2 upflow channel 82), but only require 20-30% the
amount of
air to produce a non slugging air lift effect. Alternatively, when not using
BNR, the flow
into the zone 2 upflow channel may be selected to be about one sixth the flow
in the zone
1 upflow channel, but conversely receive about 75-85% of the air. Air flow
settings into
the zone 2 upflow channel can thus be set over a broad range of flow settings,
for example
10-15%, 20-30%, 30-50%, SO-75%, 75-90% or greater.
[136] After diluting the zone 2 upflow, for example using 8 to 10 times the
recycle flow
from the zone 2 head tank 15 via the zone 2 recycle regulator 112, the air
lift effect in the
zone 2 upflow channel can be readily controlled. This control depends on the
novel
mechanisms and methods set forth above for segregating flow in an aerated and
flowing
vertical column, providing for selectable channeling of flow in different
proportions into
two or more other superior vertical columns, while the air bubbles may be
split in a
completely different ratio among these vertical columns. This novel ability to
control air
lift allows a better biological match between oxygen supply (dependent on the
time
available at pressure to dissolve oxygen, which is in turn a function of flow
velocity) and
oxygen utilization which is a function of respiration rate, (dependent on
dissolved
oxygen---not primarily upon the amount of bubbles present).
[137] Within yet another aspect of the invention, novel features and methods
are
provided for addressing the challenges involved in the disposal of by-product
sludge
and/or surplus bio-solids from the bioreactor 10 treatment processes.
Recognizing the
nutrient value of these biosolids, the EPA in the US adopted 40 CFR 503 in
1993, which
46
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
proscribes various process criteria to achieve class A bio-solids for
unrestricted use as a
soil supplement. Whenever possible, beneficial reuse of bio-solids is
encouraged. One set
of criteria for Class A bio-solids requires a minimum volatile solids
reduction, as well as a
Time-Temperature relationship, for example a 38% volatile Solids reduction and
a 60°C
S temperature for 5 hours qualifies as a Class A product. Figure 12.
[138] Within a modified embodiment of the invention, referring to Figure 7,
the
bioreactor 10 is designed to function alternatively as a waste sludge digester
and to meet
the minimum volatile solids reduction and Time-Temperature relationship
criteria for
Class A biosolids production. In this regard, the reactor is specially
designed and operated
with a unique flow and zonal separation regime that provides for production of
Class A
biosolids in as little as 5-6 days, often in 3-4 days or less, using
thermophilic bacteria
operating at 58--65°C but typically 58°-62°C and often
60°C. The 38% volatile solids
reduction is a measure of stability of the biomass or vector attraction
reduction (VAR),
while the elevated temperatures pasteurize the product to control E-coli and
virtually
eliminate salmonellae. Consuming 38% of the volatile matter minimizes odor
potential
and provides enough food energy for Thermophilic bacteria to raise the
temperature of the
reactor to over 60°C, without applying exogenous heat.
[139] Published data demonstrate two areas of concern for existing vertical
shaft
bioreactors that seek to produce class A biosolids (see, e.g., Report on
VerTad operations
King County WA, project 30900 May 20001, incorporated herein by reference.)
First,
small vertical bioreactors (e.g., "VerTad reactors", as described for example
in U.S. Patent
Application Serial No. 09/570,162, filed May 11, 2000 (incorporated herein by
reference),
feature a relative disposition of zone 2 (polishing zone) below zone 1. These
reactors have
a comparatively large surface area to volume ratio, and excessive heat is lost
to the
surrounding geology. Small reactors therefore require supplemental heat to
support class
A biosolids production, which is available at additional cost by recapturing
the waste heat
from the compressor or from the hot effluent stream.
[140] A second area of concern for previous vertical bioreactors directed to
high quality
biosolids production is that there is insufficient liquid to liquid separation
between zones 1
and 2. Published data of tracer studies in VerTad reactors show that the zone
2 (polishing
zone) behaves as a plug flow reactor, with a critical feature of localized
back-mixing.
Over a period of about 8 hours, zone 2 begins to mix with zone 1 and the whole
system
47
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
(zone 1 and zone 2) is mixed in 16-20 hrs. Accordingly, some solid particles,
potentially
containing salmonellae or other prohibited contaminants, can settle from zone
1 into zone
2 without being exposed to the required retention time at pasteurizing
temperature to meet
class A biosolids requirements.
[l41] The improved bioreactor/digester 10' of the present invention is
configured in a
distinct manner with zone 1 surrounding zone 2 (Figure 7), such that for any
given volume
of reactor the surface to volume ratio is smaller than in previously described
reactors
directed to quality biosolids production, whereby the heat lost to the
surrounding geology
is much less. The improved bioreactor/digester provides enhanced liquid to
liquid
separation at a transfer point between zone 1 and zone 2. The transfer point
is delineated
by an air lock mechanism 172 (e.g., a diaphragm-less air operated valve)
typically
including an air lock baffle 170 as depicted in Figure 7. The baffle extends
upward into an
air pocket formed by the introduction of clean, pressurized air from a
dedicated air line 62
with air delivery port 64 or aeration/solids extraction line with
corresponding port 68
located near sump 67. Zone 1 is aerated through port 69.
[142] Within this aspect of the invention, it is considered critical that when
the apparatus
is being used as an aerobic thermophilic sludge digester, bubbles from zone 1
must not
enter zone 2 because of the risk of re-inoculating the pasteurized product in
zone 2. To
prevent this from occurring, pressure in the air lock is maintained by fresh
clean
compressed air, and there is no liquid flow or contact between zone 1 and zone
2 or
transmission of contaminated air from zone 1 to zone 2. The air lock is
designed to
prevent inter-zonal mixing of liquid between batches, ensuring that zone 1
does not re-
inoculate the pasteurized biomass in zone 2 with pathogenic bacteria during
batch
processing. As an example, one batch of sludge may be processed every 5-8
hours, thus
ensuring that the critical time temperature of 60°C for five hours is
always met within each
batch.
[143] In operation of this embodiment of the invention, waste biomass is fed
continuously or intermittently into the reactor/digester 10', e.g., into the
zone 1 head tank
16'. As the head tank level in zone 1 rises above that of the zone 2 head tank
15' level, a
pressure differential develops across the center baffle 170 in the air lock.
Eventually the
zone 1 liquid level in the air lock exceeds the baffle height and fluid
transfers from zone 1
to zone 2. Line 64 air supply is placed slightly below the liquid level of
zone 2 within the
48
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
airlock, whereby at the first onset of flow between zone 1 and zone 2, the
bubbles are
swept away into zone 2 and the air lock collapses. Flow stops when the head
tank levels
are again equal and the airlock re-establishes itself. A batch can also be
initiated by
draining the zone 2 head tank 15'. Figure 7 shows zone 2 head tank being
drained and the
air lock approaching batch transfer. The size of the batch is the change in
head tank level
multiplied by the surface area of the tank. Therefore the baffle 170 need only
penetrate
into the air lock 172 by a foot or two because 1-2 feet of liquid level change
in the head
tank would typically represent a full batch. The additional hydraulic
considerations in this
aspect of the invention are similar to those set forth for the preceding
embodiments.
[144] When the bioreactor 10' functions as a waste sludge digester (see, e.g.,
Figure 7),
thickened waste sludge, generally 4-5 % solids by weight, is fed into the
reactor, for
example through influent conduit 30. The feed can be continuous, or batch
wise,
depending on the operation of the waste water treatment system generating the
sludge.
The raw sludge typically descends into the reactor through influent channel
32, and is met
with a zone 1 upflow stream 40' containing an elevated percentage of air
bubbles (e.g., 10-
15%). The combined streams are less dense than the influent stream 32' or flow
in the
downcomer channel 12' and as a result, downward circulation is induced in the
downcomer channel and in the influent channel. In this way influent is drawn
into the
reactor and circulation and aeration occur in zone 1. In Figure 7, it is
important to realize
that the head tank circulation from zone 1 upflow channel 40' to channel
downcomer
channel 12 is behind the zone 2 head tank 15' as indicated by the broken
arrows.
[145] In addition to zone 1 and zone 2 being hydraulically separated by a
diaphragm-less
air valve (air lock 172), the lower portion of each zone functions as a pseudo
plug flow
zone while the top portion of each zone is circulated in the superior channels
and is well
mixed. As a result each of zone 1 and 2 is further divided into two additional
smaller
zones to double guard against reinocculation of the finished product with the
raw influent.
When the present invention is used as a sludge digester, baffle 86 extends to
about 70-90
of the reactor depth and baffle 84 completely seals off the bottom of zone 2
from zone
1. For certainty that no cross contamination can occur, zone 2 may be further
sealed with
second outer wall 197 in close proximity to the outer casing 196 as shown in
Figure 10 and
Figure 11. The air locks 170 are shown penetrating the septa wall between
zonel and zone
49
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
2 at a location above baffle 84, but below ports 34 and 152. Zone 1 has an
aerated volume
below zone 2 of at least one batch volume and preferably two.
[146] The reactor/digester 10' of Figure 7 is thus very similar in its
operation to the waste
water treatment reactor illustrated in Figure 1, but differs in four principal
aspects:
1. The zone 1 surrounds zone 2;
2. Zone 2 extends downward about 70-90% of the depth of the reactor within
zone 1;
3. Each zone has its own aeration means;
4. There is liquid to liquid separation between zone 1 and zone 2 through use
of the airlock 172.
S. Each of zone one and zone two is further divided into an upper circulating
zone and a lower pseudo plug flow zone.
[147] Once sludge enters the reactor/digester 10' it has a mean residence time
of
approximately 2 to 3 days in zone 1, and 2 to 3 days in zone 2. The EPA
criteria for the
production of class A bio-solids dictates the time between batches, which
varies with
temperature--as an example the minimum residence time for a batch at
60° C is 5 hours, or
about 4.8 batches per day. Therefore, zone 1 and zone 2 theoretically contain
between 9.6
and 14.4 batches each. In practice, however, each batch would be about 8
hours, and
therefore zone 1 and zone 2 would contain between 6 to 9 batches each. The
overall
residence time is determined by the biodegradability of the sludge. For class
A bio-solids,
the process must achieve a minimum of 38% volatile solids reduction which
typically
takes 3.5-5 days. The hatching time is determined by the temperature (see,
e.g., Figure
12). The preferred operating temperatures of 58°C-62°C require
approximately 8-4 hours.
[148] As noted above, the air line 62 can be operated to maintain the air
pressure in the
air lock 172 of the reactor/digester 10' to control hatching. Stopping the air
flow in line 62
will also trigger a batch discharge after the appropriate processing time has
elapsed. A
batch can also be triggered by lowering the liquid level in the zone 2 head
tank 15'. Once
the batch in zone 2 is discharged, the head tank level in zone 1 is
automatically lowered an
equal amount by the action of the automatic hatching valve located between the
bottoms
of zone 1 and 2, and the cycle repeats. When a batch is processed through the
reactor, it is
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
reduced in solids content from approximately 4-5% down to about 2-3%. This
product
(class A biosolids) may then be de-watered.
[149] Published research by The University of Washington (Guild et al.,
Proceedings of
WEF Conference, Atlanta GA, 2001, incorporated herein by reference) indicates
that when
thermophilic aerobic digested sludge from a vertical shaft reactor having
certain features in
common with the reactor of the present invention was fed to a mesophilic
anaerobic
digester, the retention time in the anaerobic digester was reduced, the
overall volatile
solids reduction was better, the dewaterability was better and required less
polymer. The
thermophilic aerobic digester is operated with a about a 2 day retention time
and can
generate enough heat to comply with Class A biosolids.
[150] It is well documented that during the aerobic thermophilic digestion of
biomass,
there is minimal nitrification of ammonia at temperatures above 42°C.
It is also well
documented that in anaerobic digestion of biomass (where there is no air
stripping),
ammonia and carbon dioxide react to form ammonium bicarbonate. In a vertical
aerobic
thermophilic digester, it is reasonable to believe that ammonium bicarbonate
also forms,
due to large amounts of both ammonia and carbon dioxide remaining in solution
due to
pressure.
[151J The selection of operating temperatures is very important in long,
vertical
thermophilic aerobic digesters because ammonium bicarbonate decomposes at
about 60°C.
Ammonium bicarbonate is very important in the efficiency of the solids liquid
separation
(dewatering) step of the process. For instance, when operating a deep vertical
thermophilic aerobic digester at 55°C to 58 °C, the digested
sludge samples were very
granular before drying the sample but not after drying at about 104°C.
On one occasion
when the head tank was opened without cooling the reactor (for emergency
repair of a
float switch), the inside surface, particularly the uninsulated access cover,
was coated with
tiny white angular crystals much like white sugar or salt. These crystals
subsequently
disappeared and were not found again at the higher operating temperatures.
Another
observation that is common, is that when a batch of product is transferred
into the soak
zone at about 58°C (where there is negligible biological activity), the
temperature
increases and holds constant for about 2 hours, then cools at the cool-down
rate of the
reactor when operating on hot water. The heat of crystallization of 10,000
mg/L of
ammonium bicarbonate would account for the apparent heat generated in the soak
zone.
51
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Empirically, these observations would suggest the formation of ammonium
bicarbonate
crystals below 60° C. This is contradicted by the fact that ammonium
bicarbonate is very
soluble in water, but less so in the presence of high levels of other
dissolved solids, and
perhaps the surface chemistry of the microbiology facilitate the
crystallization process.
For instance, Struvite (magnesium ammonium phosphate) is readily formed in
anerobic
digesters of plants using biological phosphorus removal but not in plants
using chemical
phosphorus removal. Controlling the reactor temperature to below 60°C
may allow
ammonium bicarbonate crystals to form which would easily float separate with
the sludge.
[152] Table 1 compares the performance of floatation, nutrient fractionation,
and
dewaterability of thermophilic aerobic digested sludge that was taken from a
deep vertical
thermophilic aerobic digester similar to the present invention. It is known
that
thermophilically digested sludge will dewater better than anaerobically
digested biosolids
however at much higher polymer dose. Previous studies investigated the cause
of the high
polymer requirement and found that monovalent ions such as sodium, potassium,
and
particularly ammonium ions can interfere with the charge-bridging mechanisms
in the floc.
In conventional thermophilic aerobic digesters the nitrification of ammonia is
inhibited
over 42°C and therefore the ammonia produced is in largely in solution,
as evidenced by
typically high pH. The carbon dioxide produced is substantially stripped out
by the large
air flows required in these digestors and less carbon dioxide remains in
solution to form
ammonium bicarbonate. Since the air bubble contact is in the order of seconds,
and the
rate of solution of ammonia is much faster than that of carbon dioxide, the
environment
does not favor the formation of ammonium bicarbonate.
52
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Table 1
Nutrient Fractionation
CF is Concentration Factor
pH 7.8-8.0
T °C Under 60 (59-60.5)
4.80% Digested Vertad Sludge
StreamTS% CF TN CF NH3 CF ORG-N CF TP CF Cake Poly
mg/L mg/L mg/L mg/L % #/T
Digest4.8 4780 1163 3095 970
ed
2.2 2.4 1.6 3.1 2.8
Float10.7 11347 1860 9487 2750
7.1 1.2 50 24
0
RecyclClear 1589 1570 19 115
a
pH 8.5-8.8
T °C Over 60 (61.5-63.5)
3.80% Digested Vertad Sludge
StreamTS% CF TN CF NH3 CF ORG-N CF TP CF Cake Poly
mg/L mg/L mg/L mg/L % #/T
Digest3.8 1851 802 1049 548 26-3050-70
ed
1.5 1.7 1.2 2.1 1.3
Float5.6 3185 948 2238 704 31-3414
3.4 1.8 9.9 1.6
RecyclTurbid 927 702 225 442
a
[153] It is believed that below 60°C ammonium bicarbonate forms in a
deep vertical
bioreactor due to the high level of carbon dioxide and ammonia in contact and
under
pressure for long periods of time. Above 60°C ammonium bicarbonate
decomposes and
the carbon dioxide and ammonia are stripped out with the air stream, very
similarly to the
53
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
conventional thermophilic aerobic processes. When the final product, processed
below
60°C, is acidified with sulfuric acid, alum, or ferrous sulphate, etc,
ammonium sulfate is
formed and COZ is released, thus floating the sludge. Unexpectedly, the
floated product
dewaters exceptionally well. In recent reports by Murthy et al. (Mesophilic
Aeration of
Auto Thermal Thermophilic Aerobically Digested Biosolids to Improve Plant
Operations,
Water Environment Research 72, 476, 2000; Aerobic Thermophilic Digestion in A
Deep
Vertical Reactor, Project 30900, Prepared for King County Department of
Natural
Resources, March 28, 2001, each incorporated herein by reference) the
concentration of
biopolymer (proteins and polysaccharides) in thermophilically areobic
digestion could be
minimized by limiting the residence time of the thermophilic digestion. The
present
invention has 1/3 tol/2 the residence times of conventional thermophilic
aerobic digesters.
The presence of biopolymer and monovalent ions, particularly ammonia, in
solution
correlates well to an increase of polymer consumption. The formation of
ammonium
bicarbonate would significantly reduce ammonium ions.
[154] Lowering the pH with acid to about 5.0, causes the biosolids to float to
about 10-
12% concentration. Lowering the pH to 4.5-4.0 and lower yields a faster float
separation
but may require adjustment, e.g., to pH 5.5-6.0, which is the pH range of the
sludge before
digestion. Digestion below 60°C controls the reactor pH to 7.8-8.0
while digestion over
60°C results in an operating pH of 8.6-8.8, reflecting the effect of
more free ammonia due
to the decomposition of the ammonia bicarbonate. Flotation separating is
better below
60°C than above 60°C, in all categories, where the less acid
used yields a thicker float
blanket and better nutrient fractionation. These biosolids can be further
centrifuged to 30-
35% solids concentration using a low polymer dose of about 1 S pounds polymer
per ton
dry weight biomass. The acidification process may cause some cell lysis, which
will also
help dewater the sludge.
[155] These results are substantially better than conventional thermophilic
aerobic
digestion processes which require 30-50 pounds polymer per ton dry weight
biosolids and
centrifuge to only 20-25% solids. Acidifying the conventional thermophilic
aerobic
digester product does not float separate the solids, presumably due to the
lack of
ammonium bicarbonate.
[156] Examination of the data in Table 1 shows the profound effect on
flotation,
dewatering, and nutrient fractionation, between operating the reactor under 60
°C and over
54
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
60°C. Operation under 60°C generates less free ammonia and more
ammonium
bicarbonate, therefore the pH is lower and there is less ammonia in the off
gas. In order to
get a common base for a comparison between the two sets of data, a
concentration factor is
calculated. The concentration factor (CF) is the ratio of the final
concentration to the
S starting concentration.
(157] Looking at the "under 60°C "set of data the float solids were 2.2
times more
concentrated compared to the digested sludge solids; the total nitrogen in the
float was 2.4
times as concentrated; the ammonia in the float was 1.6 times as concentrated;
the organic
nitrogen was 3.1 times as concentrated; and the total phosphorus was 2.8 times
as
concentrated. Except for ammonia the nutrient concentration factor ranged from
2.4 to 3.1
when the solids concentration factor was 2.2.
[158] Looking at the "over 60°C" set of data the float solids were 1.5
times more
concentrated compared to the digested sludge solids; the total nitrogen in the
float was 1.7
times as concentrated; the ammonia in the float was 1.2 times as concentrated;
the organic
nitrogen was 2.1 times as concentrated; and the total phosphorus was 1.3 times
as
concentrated. The nutrient concentration factor, including ammonia, ranged
from 1.2 to
2.1 when the solids concentration factor was 1.5.
[159] These data strongly suggest that the nutrient fractionates into the
sludge solids in
nearly the same ratio as the solids concentration factor (except for ammonia
under 60 °C
which is explained later). It is expected that the same fractionation will
also occur during
dewatering of the floated solids.
[160] However, looking at the float solids concentration factor compared to
the subnatent
or recycle stream, a completely different and surprising discovery emerges.
[161] The "under 60°C" set of data shows the total nitrogen in the
float was 7.1 times as
concentrated as in the recycle; the ammonia in the float was 1.2 times as
concentrated; the
organic nitrogen was 500 times as concentrated; and the total phosphorus was
24 times as
concentrated. Except for ammonia all the nutrients shifted dramatically from
the clear
recycle into the sludge solids. In other words, except for ammonia, the other
nutrients are
substantially removed from the recycle streams thus benefiting the operation
of the
treatment plant and improving the nutrient value of the bio-solids.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[162J The "over 60°C" set of data shows the total nitrogen in the float
was 3.4 times as
concentrated than in the recycle; the ammonia in the float was 1.8 times as
concentrated;
the organic nitrogen was 10 times as concentrated; and the total phosphorus
was 1.6 times
as concentrated. Except for ammonia and phosphorus, the nutrient shifted
significantly,
but less dramatically from the turbid recycle into the solids.
[163] A possible explanation of the minimal shift of ammonia into the solids
is that the
acidification of ammonium bicarbonate results in ammonium sulphate which is
very stable
but very soluble. The shift in the organic nitrogen to the sludge solids is
likely because
organic nitrogen is present in the particulate matter of digested sludge and
would likely
float separate. The ammonium bicarbonate crystals, if any remain after
acidification,
might also float separate as particulate matter. The shift in phosphorus to
the sludge solids
by acidification of the sludge can be explained by the formation of insoluble
precipitates in
the presence of a high concentration of metals occurnng naturally in the
sludge. This
effect is not so pronounced over 60°C, probably because the float
separation was poor and
1 S the tiny particles formed in the precipitate are difficult to float.
[164] In constructing and installing the improved vertical shaft bioreactor 10
of the
invention, twin bioreactors (to satisfy EPA redundancy requirements) will
often be placed
in cased and grouted steel shafts approximately 36 inches in diameter and 250
feet deep.
The exemplary scope and reactor design described here for illustration
purposes is suited
for a community of about 5000 people requiring a tertiary treatment plant with
biological
nutrient removal would proceed as follows. Also described here for
illustration purposes
is a novel, modular bioreactor assembly design, while it will be understood
that the use of
a modular assembly method is not necessary to practice the invention.
[165] The inner head tank for this exemplary installation is about 8 feet in
diameter and
approximately 12 feet high. The shop fabricated reactor internals include 6
flanged tube
bundles each about 40-feet long. The bottom 40-feet length (first length) is
made up of the
aeration distributor 60, the shear header 70, the airlines 62 and 66, attached
to a short
length of downcomer 12. The second, third and fourth tube bundles, include 40
feet,
modular sections 190 typically including a central downcomer conduit 22 with
airlines 62
and 66 attached (see, e.g., Figures 9-11). These sections are joined, e.g.,
bolted, together
sequentially at modular section joints 192 to the preceding section as the
sections are
sequentially lowered into the shaft. The top two sections, 5 and 6, comprise
the
56
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
downcomer air lines and superior channels formed as a unit by using the
central
downcomer 22 and radial channel partitions 194. After installation, the radial
partitions
will assume a light press fit in the reactor shell (e.g., against an inner
wall 196 of the riser
conduit 24.
S [166] To facilitate modular construction of the bioreactor 10, the superior
channel-
forming radial partitions 194 are relaxed from the inner wall 196 of the
reactor during
insertion by expanding the diameter of the central (e.g., downcomer 22)
conduit in a
direction generally perpendicular to the radial partition (see, e.g., Figure
11). To expand
the downcomer conduit in this manner, Figure 9 depicts a novel conduit
expansion device
198, which is provided, for example, in the form of a spreader sized and
dimensioned for
insertion within the downcomer conduit. The spreader typically has paired,
opposed and
reciprocating spreader parts 200, 202, which can be manually, reciprocatingly
repositioned
between relaxed and expanded configurations (e.g., by remotely turning a
threaded
expansion driver 204 that engages each of the reciprocating spreader parts and
causes them
to spread in the direction of the outwardly directed arrows in Figure 9, or to
cooperatively
relax in the opposite direction). Thus, Figure 10 provides a diagrammatic end
view of the
reactor internal section showing the downcomer and radial baffles. The
expansion tool
198 in the center of the downcomer conduit 22 is shown in its relaxed
position.
Accordingly, in this Figure the downcomer is also in its relaxed position.
Figure 11
provides a diagrammatic end view of the reactor internal section showing the
downcomer
forced out of round by the expansion tool in its expanded configuration,
wherein the radial
baffles 194 connected to the downcomer are forcibly retracted away from the
inner casing
wall 196 to allow insertion of the reactor section 190 therein. When the
invention is used
as a digester, a sealed zone 2 can be provided by adding a second outer wall
197 on half
the assembly. Because this second wall is applied to only half the
circumference, it does
not prevent the spreaders from deforming the center tube thus relaxing the
wall pressure of
the septa partitions during installation.
[167] After assembly to this stage is complete, the zone 1 head tank 16 is
bolted to the
top of the last section. The zone 2 head tank 15 is field-erected from pre-
fabricated
sections. The modular reactor tube bundles can be delivered to a site for
installation by a
single truck, and the head tanks by a second truck. The clarifies 120 shell
can be cast in
place using concrete or made from prefabricated steel sections. The clarifies
is fitted with
57
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
a conventional skimmer mechanism. Finally the compressors and other ancillary
equipment are connected. Because of the small footprint these small plants can
easily be
housed in a building.
(168] To further understand the distinct and diverse methods of waste water
treatment
employing the novel apparatus provided herein, Figure 13 provides an exemplary
block-
flow diagram which can be used to identify the various flow patterns and
further
understand the inter-relationship of unit processes. Figure 13 is divided into
four areas, as
delineated by the broken lines. The bottom left area is a conventional
preliminary
treatment area where the waste water is passed through a fine screen in unit A
and is
degritted in a hydroclone separator C. The screenings and grit are deposited
in a hopper B
and sent to landfill.
[169] The upper left area of figure 13 is the wastewater treatment and BNR
part of the
bio-reactor of the invention and represents certain exemplary components
thereof. Unit D
represents a deoxygenation step or pre-denitrification step and references
channel 40
channel 32 and recycle 50 of Figure 1. The unit D is agitated by the anoxic
waste gas
originating in lower zone 1 (channel 80 of Figure 1. The line 301
schematically represents
the waste gas transfer from lower zone 1 (channel 80) to upper zone 1 (channel
40) but in
this aspect of the invention the lower zone 1 is immediately below upper zone
l and no
transfer line is needed. Unit D receives raw influent (channel 30) from unit
C, recycle
from head tank E and nitrified recycle from zone 2 (unit H). The purpose of
unit D is to
remove any useable molecular oxygen, accept nitrates from recycle and ammonia
and
BOD from the raw influent.
1. Unit E represents the head tank 16. This unit receives anoxic gas (309)
from unit D which serves to mix the contents of head tankl6. Unit E also
accepts raw waste water containing about 25mg/L of ammonia and 1.75
volumes of nitrated recycle containing no ammonia or appreciable BOD. After
mixing, the nitrate in the 1.75 volumes of nitrated recycle are converted to
nitrogen gas and the influent concentrations are thus diluted by, e.g., 1
Qx25mg/L ammonia+1.75xni1 ammonia /2.75Q=25/2.75=9 mg/L ammonia and
similarly 200/2.75 =72 mg/L BOD. The denitrification process liberates, e.g.,
about 2.6 mg oxygen/mg of nitrate denitrified and some of the alkalinity is
recovered. These quantities are exemplary and beneficial to the process.
58
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Denitrification is quite a fast reaction and is accomplished by the microbes
naturally occurnng in the waste water.
2. Unit F receives, e.g., about 2.75 volumes of denitrified wastewater
containing approximately 9 mg/L ammonia and 72 mg/L BOD. Since there is
no molecular oxygen or bound oxygen, the biomass will become anaerobic and
start using some of the proteins in the raw sewage to make amino acids. The
poly P microbes in the system will give up their phosphorus and load up on
VFA's. There is some evidence that VFA's can be produced in anaerobic
sewer lines where anaerobic slime is allowed to accumulate on the pipe wall.
A rope like open weave tube (131) may be hung from the head tank down
inside the clean bore channel 12. There is minimal risk of plugging the
channel
because unlike other prior reactors there are no airlines or other pipes to
become entangled with. It is to be expected that anaerobic biomass will
accumulate on the rope and some VFA's will be produced allowing some
biological phosphorus to be removed. Monitoring the weight of the rope will
give some indication of the amount of biomass present. The flexibility of the
rope and the velocity of the water should cause excess biomass to fall off and
drop into the chamber 67 sump where it can be removed as waste sludge.
3. Unit G represents the lower portion of zone 1. This area is highly
aerated and is designed to reaerate the anaerobic mixed liquor as quickly as
possible. Since the mixed liquor that enters the lower portion of zone 1 is
rich
in BOD, ammonia and sufficient VFA's, the oxygen demand in the lower
portion of zone 1 will be the maximum for any part of the reactor. The BOD
removal step requires ammonia of cell synthesis which is 5% of the BOD or
about 4 mg/L. There is a feed forward stream of 2.75 Q which is transferred
into zone 2 containing about (9 in zone 1-4 consumed in cell synthesis )=5
mg/L of ammonia . Experience with vertical bio-reactors has shown that some
of the ammonia is actually nitrified in the lower zone 1. It is not uncommon
to
find 2-3 mg/L of nitrate in a bio-reactor designed not to nitrify. In the case
of a
BNR plant designed to nitrify, some of the nitrifying bacteria will end up in
zone 1 because of the 1.75 Q recycle stream from zone 2 to zonel.
Additionally there is 5Q flow (containing 2 mg/L nitrate) from zone 1 to the
59
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
deoxygenation Unit D. These flows will be denitrified further removing
nitrogen from the system. Conservatively the effluent from zone 1 to zone 2
will contain no more than 5 mg/L BOD, 3mg/L ammonia, and 2 mg/L nitrate.
the 3 mg/L of ammonia will be fully converted to nitrate in zone 2. Therefore
the effluent will end up being about <10 mg/L BOD, <10 mg/L TSS and< 5
mg/L total Nitrogen.
4. Unit H represents head tank 15 and operates under very low loading
rates. The feed rate into zone 2 head tank is 2.75Q containing 3mg/L ammonia
and 10 mg/LBOD. Zone 2 receives its air supply from zone 1 (shown
schematically as line 302). Because of the low BOD the biomass production
will be low and the biomass produced by nitrification is 1/5 - 1/3 that of BOD
reduction. Because of the slow growth of nitrifying bacteria, they cannot be
permitted to be washed out of zone 2 in the 1.75 recycle flow to zone 1.
Fortunately these bacteria are attachment microbes and will grow on any fixed
1 S or moving bed media. In the present invention moving bed media can
advantageously be used, because the lower end of zone 2 is designed not to
allow any back-flow into zonel, and simple screening will prevent the media
from escaping at the top. Fixed media may also be employed but fixed media
tends to plug up occasionally and requires cleaning or changing. Moving bed
media tends to be self cleaning but does wear out over time.
S. Unit I is a conventional sedimentation clarifies which separates the bio-
solids from the effluent and~returns these biosolids (activated sludge, R.AS)
to
unit D or E. In a BNR plant the RAS should never become anoxic because the
nitrate in the effluent and RAS will denitrify causing the sludge to start
floating
in the clarifies. In the present invention there is the potential to provide
an
effluent from zone 2 with a high DO but a low oxygen demand, thereby
preventing anoxic conditions in the clarifies. Very high DO in the effluent is
discouraged because there could be some resolubleizing of ammonia and
phosphate in the clarifies.
[170] Membrane separation, although expensive, eliminates many of the
operational
problems of clarifiers in BNR plants. In the present invention membrane
separation allows
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
much higher MLSS and a smaller reactor. Membrane separation provides a better
quality
recycle water than the present standards require.
[171] The upper right of Figure 13 is the final chemical treatment of tertiary
water to
meet recycle quality standards. By current law, chemical flocculation,
filtration and
residual chlorine must be used. Unit M is a flocculating tank with mechanical
mixer. Unit
N is a rotating cloth disk filter. Unit P is a ultra violet disinfection
channel and combined
back wash tank. Unit O is a chlorination step where just enough chlorine is
added to
maintain a residual in the pipe line. Unit Q is a back wash pump which can be
used to
backwash the cloth filter or the membranes if required.
[172] The lower right of Figure 13 is the thermophilic aerobic digestion
section of the
plant. Unit R represents the first aerobic stage (zone 1) of the two step
process. Unit S
represents the second stage of the digestion or zone 2. These two zones are
connected
through an air lock valve. Unit W represents the acid flotation thickening
step. Unit T is
an acid feeder. Unit V represents the dewatering step, in this case a
centrifuge, with a unit
polymer feeder U.
[173] The BNR process above has been examined in detail in Figure 13 in order
to
illustrate process advantages that are not reported in previous bioreactor
designs. Among
these novel process advantages are that screened and degritted influent is fed
into
deoxygenating channel 40 and is mixed with denitrified liquor from head tank
16. The
head tank 16 is agitated with anoxic gas produced in channel 40 and with
DO<.05.
Denitrified liquor from head tank 16 descends in channel 12 under anoxic or
optionally
anaerobic conditions completing the denitrification process or optionally
creating VFA's.
[174] In addition, it is notable that downflow in channel 12 enters the bottom
of zone 1 in
the vicinity of the aeration distributor in an area of vigorous mixing.
Channel 80 which is
the major portion of zone 1 is highly aerobic, removes the BOD, rapidly
oxidizes the
VFA's consuming phosphorus and in some cases nitrifies a portion of the
ammonia.
[175] Further notable is the fact that rising liquor in channel 80 splits into
the
deoxygenation area and a portion passes upward into zone 2. Zone 2
substantially
degrades the remainder of the BOD and converts the remainder of the ammonia to
nitrate.
61
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[176] In additional aspects, waste gas from channel 80 circulates via
deoxygenation
channels 32 and 40 and also provides the oxygen for bio-oxidation of BOD and
ammonia
in zone 2.
[177] Also noted, a portion of nitrified liquor can be returned to the
denitrification step
where the nitrate -N is converted to nitrogen gas while a second portion goes
to a
clarification step where the biomass is separated from the effluent. The
biomass is
returned to the denitrification step and the clarified effluent is discharged.
[178] In related embodiments, anoxic gas is used for mixing anoxic liquor.
Unit D
deoxygenates not only the various liquid streams, but the gas stream passing
through the
unit. This deoxygenated gas can be used subsequently to mix the contents of
the
denitrification unit E. This eliminates the need for mechanical mixers saving
energy,
maintenance and capital.
[179] Additional embodiments of the invention provided novel anaerobic
processes.
Unit F is a long vertical channel which may converted to an anaerobic chamber
for the
purpose of creating VFA's. In the present invention there are no airlines or
extraction
lines in unit F. This allows the use of media such as open weave rope or tubes
to be
suspended in the reactor without the fear of plugging the channel or becoming
entwined
with other pipes. The purpose of the fixed media is to accumulate attached
growth
anaerobic bacteria (acid formers). The amount of fixed media and anaerobic
biomass can
be adjusted from the surface by rolling up a portion of the rope or fabric
tube. The amount
of media can be monitored on line by measuring the weight of the rope. The
liquid
velocity downward in channel 12 keeps excess biomass from forming and any
excess will
fall off. Since channel 12 is open at the bottom waste anaerobic biomass would
collect in
sump 67 and be removed through the flotation tank Unit J.
[180] In still additional embodiments, wasting sludge through an air line 66
or 69
provides instant spontaneous flotation upon depressurization. Wasting sludge
(WAS)
from a well aerated and mixed part of zone 1, a process not contemplated in
previous
designs, favors the capture of phosphate in the sludge. Float solids are
suitable for
digestion without any further thickening.
62
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Membrane Separation System
[181] This description next addresses membrane separation systems, methods and
devices employing a selective, semi-permeable, microporous, or other
partitioning
membrane for processing, refining, and/or treating liquid compositions, for
example
membrane waste-water purification processes and apparatus. These systems,
methods, and
devices provide improved throughput and/or improved operating life of
submerged
membranes, particularly membrane bioreactors providing biological treatment of
wastewaters.
[182] There are several technical considerations for incorporating membrane
bioreactors
in wastewater treatment facilities, including long vertical shaft bioreactors.
A first
consideration is a popular misconception that the membranes alone produce an
exceptional
quality effluent. This is not necessarily accurate because membranes, in
themselves, do
not produce recycle quality water. The treatment of wastewater to recycle
quality is
primarily the result of biological treatment, however a micro filtration
membrane is
1 S responsible for physically separating substantially all the microorganisms
from the water,
down to about 0.1 micron in diameter. Viruses smaller than 0.1 micron are also
typically
removed because about 99% of viruses stick to host bacteria. The better the
bioreactor, the
better the quality of effluent.
[183] In cases where inorganic dissolved solids must also be removed, the
effluent from
the biological treatment membrane reactor can be fiuther treated by using
ultrafiltration,
nanofiltration , or reverse osmosis (R0). This quality of water is suitable
for aquifer
recharging etc.
[184] A second consideration is that recycle quality water not only requires
the removal
of biological oxygen demand (BOD) and total suspended solids (TSS), but also
requires
the removal of the nutrients, nitrogen and phosphorus, (N & P) to low levels
that will not
support aquatic growth. This requires the use of a good biological nutrient
removal (BNR)
process. Typical existing membrane bioreactor processes operate on a single
sludge back-
mixed bioreactor, which is less efficient and more expensive to build and
operate than the
improved long vertical shaft bio-reactors.
[185] For example, a presently proposed installation of twin 0.25 MGD (0.5 MGD
total)
conventional membrane biological reactors is estimated to cost about 1.2
million dollars,
63
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
(reactor and membranes only), occupy about 8000 sq. feet, draw about 75 HP,
and require
1000 standard cubic feet per minute (scfm) of air. By comparison, twin
improved long
vertical shaft 0.25 MGD reactors would cost about 1.0 million dollars
including the price
of the membranes estimated at $400,000. The improved long vertical shaft bio-
reactors
would occupy about 1000 sq. feet and draw about 30 HP. Only 100 scfm of air is
required
for the improved long vertical shaft bio-reactors, reducing the process off
gas flow to the
equivalent of a household kitchen or bathroom fan. The improved long vertical
shaft bio-
reactors operate in a plug flow configuration with internal recycle streams.
Plug flow
reactors are known to produce a better quality effluent than back-mixed
reactors. This is
because in a plug flow reactor the effluent is at the lowest possible
concentration
achievable with that biomass. In a back-mix reactor, the effluent constituents
are at the
same concentration as the contents of the reactor. Indeed, in some cases in a
back-mixed
reactor, a portion of the influent may short circuit directly to the effluent.
It is also known
that with a single sludge bio-reactor, where specialty microbes such as
nitrifiers must
compete with more robust and faster growing BOD microbes, larger quantities of
biomass
are required (to prevent wash-out of the nitrifiers). This leads to larger
reactors.
[186] An additional consideration is that, aside from the biological
advantages of the
improved long vertical shaft bio-reactors, there are certain hydraulic
advantages that are
not possible with other reactors. Several unique hydraulic characteristics
observed in
existing long shaft vertical aeration reactors suggest that membrane
separation systems
will operate better in a vertical aeration reactor than in a surface back-
mixed reactor
because of substantial concentrations of supersaturated dissolved gases.
[187] To confirm this prediction regarding supersaturated dissolved gases, a
membrane
separator was adapted to an existing long vertical shaft aeration reactor. A
principal
hydraulic characteristic of vertical aerators is that the reactor circulates a
mixture of bio-
solids, liquid, dissolved gasses and dispersed gas (bubbles), in a very long
vertical
pathway. The pressure at the lower end of this pathway can be up to 1 SO psi.
As a result
of the pressure, there are substantial concentrations of supersaturated
dissolved gasses in
the liquid even when brought to the surface. These supersaturated gasses
represent a
significant resource of stored energy. For example, in a 0.25 MGD improved
long vertical
shaft bio-reactor, the surface area in contact with the moving fluid in the
reactor changes
from about 4000 sq. feet in the reactor to about 20,000 thousand sq. feet in a
membrane
64
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
cell. When liquids containing supersaturated dissolved gasses contact a large
surface, the
dissolved gas tends to come out of solution and create a scouring action. This
is like using
soda water to remove spots on clothing. Actually, there are many cleaners that
use
foaming agents to improve scouring action.
[188] In the case of long vertical shaft bioreactors with submerged membrane
bioreactors, it is predicted that the action of supersaturated dissolved gas
in the mixed
liquor will help keep a membrane surface proximate to the mixed liquor
sufficiently clean,
thus increasing the flux rates (rate of liquid flow through the membrane) and
the time
between cleaning. There are several observed factors of long vertical shaft
bioreactors that
provide support for this prediction. For example, a vertical bioreactor that
had run 22
years was recently dismantled. The head tank was made of steel plate, sand
blasted and
coated with 6-mil (.006") epoxy. The remainder of the reactor was bare steel.
The epoxy
coated surfaces were exceptionally clean and even the bolts in the epoxy
coated head tank
could be easily undone. There was no evidence of any biomass buildup on the
epoxy
surface, even near the downcomer end of the head tank where the flow velocity
would be
very slow (perhaps 0.1-0.5 ft/sec). The dissolved gas content at that point
would be about
25-35 Mg/L and the colloidal gas content would be about 40-50 ml/L (50-65
Mg/L).
There were, a few locations where the epoxy coating had been damaged resulting
in a
localized accumulation of biomass attached to the bare steel. The bare steel
surfaces in the
rest of the reactor were coated with a gray slime layer, even in the areas of
high turbulence
and high dissolved gas content. This gray biomass slime, typically found on
metal
surfaces in these types of reactors, contains phospholipids and is useful in
protecting the
bare steel against corrosion, referred to as bio-passavation.
[189] To further validate these findings, a rubber hose about 100 ft long,
weighted at its
lower end with 90 feet of steel pipe was used in a vertical shaft aerator for
an air line in the
downcomer. The liquid flow velocity in the upper end of the downcomer was in
the order
of 3-4 feet/sec. The hose could be reeled up to change the point of air
injection in the
downcomer. On the upper end of the hose there was no biomass build up in the
zone
where dissolved gasses were present, but there was a significant biomass build
up in the
zone where these gasses were re-dissolved due to increasing pressure in the
downcomer.
The liquid velocity was the same for both the upper and lower zones in the
downcomer.
This hose was designed for air service and was not permeable to air from the
inside. This
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
observation also shows that in the absence of dissolved gas in the downcomer
biomass will
build up to provide an anoxic /anaerobic zone.
[190] In additional studies, an early design vertical bio-reactor was equipped
with a
fiberglass downcomer. This plant is still in service with no report of any
failures. Another
plant built at the same time, also using a fiberglass downcomer, was shutdown
and filled
with clean water. Video inspection showed no build up of biomass on the wall
of the
fiberglass tube and no delamination of the resin and fibers. Fiberglass is
typically not
permeable to dissolved gas.
[191] In a separate study, a small vertical aeration shaft was inspected after
about 26
months of service. The ABS downcomer was in good condition with no biomass
build up.
A similar vertical aerator was fitted with a steel downcomer. Inspection
revealed a
phospholipid biomass coating commonly found in steel reactors.
[192] Further validating the present findings, during the early development of
vertical
bioreactors rubber downcomer tubes were installed to reduce the suspended
weight and to
prevent flow reversal. Three of these downcomers failed due to de-lamination
of the tube
wall between the rubber surface and the reinforcing fabric. These tubes were
designed for
water service and were permeable to dissolved air. The maker of the tube
claimed that
dissolved air had become entrapped between the inner and outer rubber layers
causing the
failure. This phenomenon is seen in tubeless radial tires where the air in the
tire leaks into
the cord layer and causes delamination.
[193] In a separate study, , another vertical aeration reactor was examined
for corrosion
after 20 years of service. The only part of the reactor that had any
significant wear was at
the outlet of the air-lift influent pump that was located in the riser section
of the reactor. It
would appear in this extreme duty, the air /water velocity is sufficient to
remove the
protective phosphate coating allowing corrosion of the bare metal.
[194] A frequent observation regarding surface condition of several head tanks
examined
after long periods of operation is that in locations featuring an abrupt
change in fluid flow,
such as immediately following a baffle, the epoxy coating is often
deteriorated. These
areas may be considered "hydraulic shadows."
[195] Releasing a dissolved gas and its stored energy provides a powerful
scouring effect
on the epoxy and/or metal surfaces. This energy level is sufficient to remove
the epoxy
66
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
but not enough to significantly damage metal surfaces. However the bacterial
slime
coating found elsewhere in a reactor, even adjacent to the shadow, is removed
as
evidenced by the formation of a light rust coating on the metal surface.
[196] Similarly when the test membrane was installed in the Y branch of a
vertical shaft
reactor described below, there was an air line, which was in close proximity
to one corner
of the membrane. The air line did not touch the membrane but acted as a baffle
and
caused a downstream "hydraulic shadow" over about 10-15% of the surface area
at one
corner and on one side of the membrane. In this "hydraulic shadow", the
membrane had
begun to delaminate slightly. The membrane is made of non-woven polyolefin
strands,
perhaps 10-20 microns in diameter, compressed and sintered together by some
means,
probably heat and pressure. Under the microscope there were hair-like
whiskers,
approximately 1/8 inch long, protruding perpendicularly to the surface. These
whiskers
were found on the membrane only on one side, and only in the proximity, of the
air line. It
is likely that the abrupt change in flow causes the dissolved gas to nucleate
and to erode
/wear the polymeric surface. Cavitation may be occurnng because the whiskers
are
protruding outward and appears to have been lifted from the surface. The
remainder of the
membrane was unaffected by the high levels of dissolved gas and had no
evidence of
surface deterioration when examined under the microscope.
[197] The amount of dissolved gas is surprising. As an example, the solubility
of air in
water is about 21 mg/L at one atmosphere of pressure. A 500 ft. deep vertical
shaft reactor
could theoretically dissolve 287 mg/L of air. Assuming a dissolving efficiency
of 70% and
a recovery efficiency of 70%, there would be about 140 mg/L of air in the
liquid in the
head tank of the reactor. Since 1 ml of air weighs 1.29 mg, this translates to
about 10% by
volume of the liquid would be derived from dissolved gas. This represents
substantially
more dissolved (stored) bubble volume than the dispersed bubble volume used to
circulate
the contents of the vertical bioreactor. Surprisingly it is more dissolved
bubble volume
than the dispersed bubble volume (4-6%) required to circulate either a Kubota
or Zenon
membrane reactor. Furthermore this stored bubble volume represents
considerable stored
energy.
[198] It is predicted that by releasing this stored energy at the critical
time and controlled
rate across the membrane surface a very powerful cleaning action can be
created. In fact,
there is enough energy stored in this manner to delaminate/cavitate the
membrane if
67
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
released in an uncontrolled way, such as can occur in the proximity of the air
line. This
phenomenon now explains observations of failed rubber down-comers.
[199] The total dissolved air may be calculated quite accurately in the liquid
in a vertical
reactor by using a dissolved oxygen probe. Under no load conditions, i.e., no
BOD load,
the total dissolved air is about 2.61 times the dissolved oxygen reading.
Under load, the
oxygen readings are reduced but the oxygen consumption can be calculated from
the BOD
values. At the time of this study, the vertical bioreactor was operating under
a typical
diurnal organic load patterns. Note that when the riser air is maintained at
substantially a
constant value (55-65 scfm) the dissolved oxygen values increase by a factor
of nearly two
when only 43 scfin of down-comer air is applied. This indicates that the down-
comer air
is mainly responsible for dissolved gas while the riser air is mainly
responsible for
dispersed. More importantly, the dissolved oxygen level in the permeate (even
though
reduced 50-60% by the BOD reaction) reaches supersaturated values (nitrogen
gas and
carbon dioxide gas would therefore be even higher) proving that supersaturated
gasses in
the liquid easily pass easily through the membrane. As an example, if the
residual
dissolved oxygen in the permeate is 10 mg/L and 50% of the oxygen was consumed
in the
reaction, then the starting value would have been at least 20 mg/L. Therefore
the starting
dissolved air would then be 2.6 x 20 = 52 mg/L and the nitrogen fraction would
be 32
mg/L. This is conservatively, the amount of dissolved gas going through the
membrane.
Remember that some of the dissolved gas, perhaps half, is also precipitating
on the outside
of the membrane.
[200] In consideration of the magnitude of the observed scouring effect of
uncontrolled
gas nucleation on polymeric surfaces, the flow redistribution device located
between each
level of membranes has been redesigned within the present invention. The new
design
consists of a series of adjustable and/or removable baffles, which will create
low level but
controlled "hydraulic shadow" effect across the membrane. This controlled
effect is
similar to, but much less intense than, the one inadvertently
created/discovered in the
proximity of the air line.
(201] The importance of this discovery is that, where it was thought this type
of vertical
aeration reactor could supply only a fraction of the air required to operate
the membrane;
there is actually more than enough air in the "stored energy" form (i.e.,
dissolved). It is
6~
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
now possible to get the stored gas out of solution in the right amount and at
the critical
location to achieve the novel objects and advantages disclosed herein.
[202] Thus, the invention provides for the employment of supersaturated
dissolved
gasses in fluid processing methods and devices to clean surfaces that the
subject fluids
contact. Various observations that validate these results include:
a) Polymeric surfaces submerged in liquid flowing at wide range of velocities
from about 0.1 to 4.0 feet / sec. do not experience a build up of biomass in
the presence
about 20-30 Mg/L of dissolved gas and /or about 30-50 Mg/L or colloidal gasses
in the
liquid.
b) Non polymeric surfaces, (bare metal exposed by damage to polymeric
coating) submerged in liquid flowing at wide range of velocities from about
0.1 to 4.0 feet
/ sec. do experience a build up of biomass even when there is about 20-30 Mg/L
of
dissolved gas and /or about 30-50 Mg/L of colloidal gasses present in the
liquid.
c) Biomass build up is experienced in the absence of dissolved gasses even at
relatively high liquid velocities of 3-4 feet / sec.
d) Biomass build up can occur on metallic (steel) surfaces at flow velocities
up to about 4 feet/sec. This biomass contains phospholipids that protect the
metal by bio-
passivation.
e) Flow velocities over about 10-feet/sec and in the presence of large amounts
of air (over about 100 mg/L) prevent the build up of biomass and the build up
of the
corrosion inhibiting phospholipids. As a result, metal corrosion and metal
erosion occur.
f) Non-permeable polymeric membranes can delaminate if the pores do not go
right through the wall.
g) The high airflow rates suggested by membrane manufacturers are not
necessary for efficient operation of submerged membranes in a presence of
supersaturated
dissolved gases. Kubota, a leading membrane manufacturer, states in its
literature on
membranes used for solid-liquid separation of mixed liquor that a thin film
biomass is
allowed to form on the surface of the membrane to increase its effectiveness
in removing
small particles. At a flux rate of about 0.5 gal/hr/sq. feet the time between
cleaning
membranes is about 6 months. A minimum air rate of about 40 scfm / 1000 sq.
feet is
required and a minimum cross-flow liquid velocity of about 1 feet/sec is
required.
69
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Zenon membranes operate at a nearly double the flux rate of the Kubota
membranes, but provision is made for pulse reverse flow cleaning. In one mode
of
operation a ten-second pulse is applied for every ten minutes of operation.
Zenon also use
a mechanically-applied vacuum to draw on the membrane. Overall, the Zenon
technology
requires a lower air rate to stimulate and clean the membrane than the Kubota
membrane.
The airflow rates suggested by these two leading membrane manufacturers
is 8 to 10 times higher than the airflow rate typically available in improved
long vertical
shaft bioreactors. Both Kubota and Zenon have designed their membranes to
operate in
relatively shallow basins. The improved long vertical shaft bio-reactor is
configured on a
vertical axis, and allows membranes to be stacked 2-5 units high and still
maintain enough
driving head in the reactor to circulate the system. In shallow tanks the
driving head that
causes air/liquid circulation through the membranes amounts to a few inches at
best. In an
improved long vertical shaft bio-reactor plant, the driving head might be 10-
12 feet. A re-
distribution header is located between each deck of membranes thus allowing
the same air
1 S to be used 4-5 times. By stacking membranes, the superficial cross-flow
(actually up-
flow) liquid velocity across the membrane increases as the cross-sectional
(footprint) area
decreases. Although not optimized, a first trial design of a 0.25 MGD improved
long
vertical shaft bio-reactors plant incorporating membrane bioreactor technology
indicates it
would supply about half the air and about 1/3 liquid flow velocities
recommended by the
membrane manufacturers. For the reasons stated above and the evidence
gathered, the
dissolved air fraction in the liquid flow is a far more important factor in
keeping the
membranes clean than either the air rate or the liquid rate. Over-design is to
be avoided
because it is possible to clean the membranes too well and destroy the
required thin bio-
film. The scouring action can be adjusted by using fewer decks of membranes or
less air.
Conversely, one can always add air and more decks if more velocity and/or
scouring are
needed.
h) Dissolved salts and particles smaller than 0.04 microns pass through
microfiltration membranes and therefore there is no reason to suspect that
dissolved gasses
will not pass through. The dissolved gasses that do pass through the membrane
may help
in keeping the inside of the membrane clean.
i) The membranes can be cleaned with bleach and therefore the material that
is blocking the pores is probably mostly organic.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
j) Typically membrane reactors need fine screening of the raw influent
because the plant may be dealing with whole raw sewage.
[203] The foregoing findings and conclusions were further validated by
installing
membrane bioreactors in a deep shaft vertical reactor at Virden, Manitoba,
Canada,
beginning in August of 2003. The Virden Reactor was the first commercial deep
shaft
vertical reactor installed in North America in 1978. The treatment plant was
started up in
1980, and has been in continuous service since then. The plant is one of the
older deep
shaft designs where both downcomer and riser air is used in the circulation
and aeration of
the shaft contents. The reactor is 30" in diameter and 500' deep. The
downcomer is 18" in
diameter and the riser is formed by the annular space between the casing wall
and the
downcomer. At the top of the reactor there is a Y branch to allow the mixed
liquor to
transfer from the riser to the downcomer via a head tank. The head tank is
approximately
25 feet long, 6 feet wide and 4.5 feet deep. The configuration of this reactor
is ideal for
tests since it allows the ratio of dissolved to dispersed air to be
selectively changed.
Applying more downcomer air results in more dissolved air while applying more
riser air
results in more dispersed (bubbles) air. As shown later, the ratio of
dissolved to dispersed
air makes up to a nine-fold difference in membrane flow rates at the same
hydraulic head.
[204] In order to install membrane bioreactors in the Virden reactor, it was
necessary to
cut an access-way into the top of the head tank. The access-way is located
directly over
the 21" ID Y branch. When the head tank was opened after 23 years of
continuous
operation, the same pattern of bio-fouling was discovered on the epoxy coating
as found in
another long vertical shaft bioreactor opened after 22 years of operation. The
patterns
were almost identical. In each case, the floor of the head tank, where both
the liquid
velocity and the bubble content is the lowest, had a minimum of attached
biomass. This is
contrary to the teaching of the membrane manufacturers, who recommend a much
higher
velocity and higher bubble content. However it should be noted that although
there would
be few, if any, bubbles on the floor, the fluid would be supersaturated with
dissolved gas.
The conclusion that it is the dissolved gas nucleating on the polymeric
surface that reduces
bio-fouling is further supported by this observation. The fact that the shaft
of the other
examined bioreactor was used for treating high strength warm industrial waste,
while the
Virden shaft was treating cold low strength municipal wastewater, appears to
have little
influence on this phenomenon.
71
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[205] A test membrane solidlliquid separator was installed in the Virden deep
shaft
reactor for one month to assess improvements in through-put and operating life
of the
submerged membrane assemblies of the invention. The test membrane was removed
from
the reactor and carefully examined. Notably, the membranes were clean, and any
matter
on the exterior surface easily washed off in water despite having been
operated in a thick
concentration of sticky mixed liquor for a month. Additionally, it was
observed that
different air rates in the reactor produced different effluent water flow
rates from the
membrane. When downcomer air was increased, (more dissolved air in
circulation), the
reactor circulation flow rate decreased, but the flow through the membrane
increases.
Conversely, when more riser air was applied (more dispersed air) the
circulation velocity
in the reactor increased but flow out of the membrane decreases. This is
contrary to
conventional understanding of membrane function and operation, as evinced by
operation
instructions of membrane manufacturers. In conventional membrane plants, a
high
aeration rate is required to maintain circulation velocity across the
membrane. Typically a
conventional plant would use (as a minimum) about 2 times the trans-membrane
velocity
and 8-10 times the airflow that is available in a deep shaft type reactor.
[206] In additional studies to clarify the disclosure herein, the test
bioreactor was fitted
with a sample port in the head tank located close to the outlet of the
membrane. Dissolved
gas concentrations across the membrane were measured with a dissolved oxygen
meter
(DO meter) and reactor circulation velocities across the membrane are
calculated from the
time to circulate tracers such as soap. Permeate flow out of the membrane was
measured
in a calibrated flask, and the hydraulic head is maintained by an overflow to
the flotation
tanks. In this test case, the head over the membrane was maintained at 1 foot.
A drop leg
was provided to cause a siphon effect of one meter, a typical operating value
for this type
of membrane. The membrane support frame can hold a lower membrane submerged
between, about 6-9 feet, and an upper membrane submerged between about 1-4
feet. The
overflow heights are the same for both membrane locations.
72
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[207] The following Tables 2 and 3 shows the effect of various air rates on
the membrane
performance:
TABLE 2
Date Riser Down Total Flow MLSS Membrane
Air Comer Air ml/min mg/L Effluent
scfm Air D.O. mg/L
scfm
Aug-263:00 PM 65 43 108 440 9347
Aug-278:00 AM 65 0 65 310 7058
Aug-272:00 PM 65 43 108 450 7058 10
Aug-279:00 PM 65 43 108 450 7058
Aug-287:00 AM 65 43 108 425 6027
Aug-288:00 AM 65 0 55 310 6027 5.4
Aug-2810:00 65 43 108 412 6027 8.8
AM
Sep-22Before 75 0 75 180 10226
Inspection
Sep-23After 75 55 130 500 7800
Inspection
Sep-24After 108 0 108 <50 9200
Inspection
73
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
TABLE 3
O1 108/0 = Riser Air/Downcomer Air
100
90 use
~'9i~
8p X5/0 ~ ~ No ~~c ~~G P~~ O X5/55
~9ir
0 ~\se~ 0 0 c~/as
4 50
a~
~' 40
20
0 100 200 300 400 500
ml/min/membrane
0 38 76 114 152 190
US gal/day/membrane
0 4,4 8.8 13.2 17.6 22,0
US gal/sq ff/day
[208] Table 3 is a plot of data points of Table 2. The data of Table 2 do not
reflect the
5 importance of the dissolved air fraction. However, the effect of varying the
air rates was
noted and the information provided in Tables 2 and 3. There are two data sets
that
illustrate the importance of the downcomer air (dissolved air). Point 1 and
point 2 have
the same total volume of air applied (108 scfin). Point 1 has 108 scfm in the
riser (mostly
dispersed) and no downcomer air (i.e. no dissolved air). Remember that the
conventional
10 teaching says that high velocity and high air rates yield highest flow
rates in the
membrane. However, in the trial run, (point 1), the highest air rate and the
highest
circulation rate yields the lowest flow rate out of the membrane.
[209] At point 2, there is a total air rate of 108 scfm but this time, 43 scfm
is applied to
the downcomer. The effect of downcomer air is to slow the circulation
velocity.
Conventional teaching predicts that the flow out of the membrane would also
slow but in
the trial run the flow out of the membrane increased nine fold.
74
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[210] Further, at points 3 and 4, both points have 75 scfin of air in the
riser but point 4
has 55 scfm in the downcomer that serves to slow the circulation velocity. The
output
from the membrane increases three fold.
[211] Therefore, the dissolved air fraction in the wastewater has a dominant
effect on the
throughput of the membrane. Other factors will also influence the performance
of the
membrane. Among these factors are the concentration of biomass, the sludge
age, the
biological health of the sludge, the amount of exo-cellular polymer present,
the condition
of the membrane, etc. These factors are expected to have a minor impact on
overall results
since most of the results are from one-day's operation during which sludge
conditions are
not predicted to change much during the subject period.
[212] One important cause of increased membrane through-put within the present
invention relates to gas dynamics of vertical bioreactors. In particular, deep
shaft reactor
systems provide significant advantages over other bioreactors and fluid
treatment
apparatus by providing a high dissolved air fraction. In addition, they
involve distinct
biochemical and physicochemical processes, for example oxidation of organic
carbon, and
dissolution of oxygen and other gases that result in supersaturated levels of
desired gases,
e.g., carbon dioxide.
[213] In fluid dynamics, the term "rheology" describes a complex, non-linear
relationship
between fluid deformation and stress occurring in fluid flow patterns. The
increased
throughput phenomena is believed related to a change in rheology on a membrane
surface.
Because of high amounts of dissolved gas in the fluid, the rheology of both
the biomass
(solids containing fluid and gas) and the fluid media change on contact with
the
membrane, perhaps making the membrane more permeable.
[214] The test membrane was fitted with clear vinyl tubing, which allowed
visual
observation of the permeate stream. The permeate stream contained a
significant amount
of bubbles, perhaps 1/16 to 1/8 inch in diameter. It is estimated that as much
as 10-15
of the permeate flow is made up of discrete bubbles. It is believed that the
dissolved gas
passes through the membrane unimpeded and then nucleates at or near the
membrane
surface, which may include nucleation between the surfaces, at the membrane
surface, or
within a fluid proximate to the membrane surface, and causes an air-lift
effect proximate to
the membrane surface. It is reasonably expected that discrete bubbles will not
pass at high
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
levels through a semi-permeable membrane. Unless there is dissolved air
present in the
water passing through the membrane, (or alternatively air bubbled into the
clean water side
of the membrane) no air-lift can be expected on the permeate side of the test
membrane.
This air-lift caused by the bubbles has a significant pumping effect because
during the
installation of the permeate line, permeate flow from the membrane can be
raised almost to
the surface of the liquid level in the head tank. Since the liquid being
filtered located
outside the membrane contains about 9% air voidage, the nucleating gas volume
inside the
membrane would be likewise be about 9% gas voidage in order for liquid to flow
out of
the membrane at similar interior and exterior hydraulic heads. It is apparent
that the
dissolved gas fraction helps keep the membrane outside surface clean and
therefore, the
dissolved gas fraction inside the membrane will also help keep the inside of
the membrane
clean.
[215] In a conventional membrane application, the water inside the membrane
(on the
permeate side) contains very little air and is much more dense than the water
outside the
membrane which contains the air required for scouring. Therefore, in
conventional
systems the water will not flow out of the membrane unless a slight vacuum is
applied to
the effluent side (Zenon uses a vacuum pump) or the influent is pressurized
(Kubota uses
compressed air). When a vacuum is applied, water tends to flow preferentially
through the
pores closest to the top of the membrane. When pressure is applied with
compressed air,
the resulting head is the sum of the heads due to density difference between
the water
inside and outside the membrane plus the head required to cause flow through
the pores
plus any hydraulic losses due to fluid motion. In the improved long vertical
shaft bio-
reactors system, the head due to density differences is largely eliminated and
potentially
enough dissolved gas could enter the membrane to cause enough air-lift to
overcome the
head loss through the pores as well.
[216] Figures 14-1 through 14-7 illustrate several aspects of a submerged
permeable
membrane assembly 400 for membrane separation according to the invention. In
this
exemplary embodiment, the membrane assembly is a "U-shaped" assembly, while it
will
be appreciated that various alternative designs and configurations of the
assembly can be
constructed and operated according to the disclosure herein. As illustrated in
Figure 14-1
(a cross-sectional view along a vertical axis 402 of the submerged membrane
assembly),
the exemplary membrane assembly includes a "U-shaped" container 405 that is 6
feet tall
76
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
and has a first fluid compartment 420 and a second fluid compartment 430. Also
in this
exemplary embodiment, the compartments are separated by a separator member 414
and a
membrane 410 that is 3 feet high and installed at the bottom of the "U" where
the two
fluid compartments connect. The membrane 410 has a first surface 411, a second
surface
412, and a vertical axis 402. The first fluid compartment 420 is configured to
contain a
first fluid 424 in fluid communication with the first surface 411 of the
membrane 410.
The second fluid compartment 430 is configured to contain a second fluid 434
in fluid
communication with the second surface 412 of the membrane 410. The first fluid
424 has
a first specific gravity, or density, and the second fluid 434 has a second
specific gravity.
(217] The membrane 410 schematically represented in Figures 14-1 through 4-7
may be
any membrane structure, including plate and frame, tubular, hollow fiber, and
spiral
wound. The membrane may be any selective, semi-permeable, microporous, or
other
partitioning membrane for processing, refining, and/or treating liquid
compositions, for
example membrane waste-water purification processes and apparatus. The
membrane may
be made from any material, and may include one or more selected from cellulose
acetate,
polyvinyl chloride, polysulfones, polycarbonates, and polyacrylonitriles. The
membrane
410 is generally permeable by molecules of less than a predetermined size, and
includes
pores 415 between the surfaces 411 and 412 having a pore size permitting
movement of
molecules smaller than a removal size between the first and second surfaces
411, 412 and
rejecting movement of larger molecules. The particle removal size for semi-
permeable
membranes used in membrane bioreactor applications typically range between
10.0 and
0.05 microns. While a particle removal size may be selected in conjunction
with other
parameters relevant to a particular use of the membrane, in a certain
embodiment a semi-
permeable membrane having a particle removal size in a range of between
approximately
0.05 to 0.1 microns generally produced good results filtering wastewater. This
range
removes most viruses, most long-chain molecules (macromolecules), and all
bacteria. In
another embodiment, a membrane that substantially removes particles larger
than 0.1
microns is generally expected to produce satisfactory results filtering
wastewater.
[218] Figures 14-1 through 14-7 also illustrate the fluids 420 and 430 being
contained at
various vertical column heights in the assembly 400. The exemplary, "U-shaped"
assembly has a maximum column height of six feet, and the Figures include
other
illustrative dimensions of the vertical column height from zero to six feet
along the vertical
77
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
axis 402, with zero feet starting at the maximum height of the assembly 400,
and six feet at
the maximum depth of the membrane 410. In Figure 14-1, the first fluid
compartment 420
contains the first fluid 424 at a first column height 422 of six feet. Also,
the second fluid
compartment 430 contains the second fluid 434 at a second column height 432 of
six feet.
[219] As further illustrated in Figures 14-1 through 14-7, the vertical axis
402 of the
membrane 410 is typically aligned with a corresponding first chamber vertical
axis 423
and a second chamber vertical axis 433. Generally, the first chamber vertical
axis 423 and
second chamber vertical axis are approximately parallel and correspond to an
effective
vertical gravitational axis that is roughly coincident with a direction of
bubble rise in the
first andlor second chambers. Typically, the direction of bubble rise is
vertical within the
first and second chambers. When the membrane is oriented vertically, the
membrane
vertical axis is roughly parallel to the first chamber vertical axis 423 and
second chamber
vertical axis. However, in certain embodiments the membrane may not be
oriented
vertically, for example it may be positioned with the first and second
surfaces tilted
relative to the direction of gas bubble rise and vertical axes of the first
and second
chambers. In these embodiments, the membrane vertical axis 402 is not parallel
to the first
and second membrane surfaces, and instead corresponds to the direction of
bubble rise in
the first and/or second chambers.
[220] As illustrated in Figure 14-l, the first fluid compartment 420 contains
the first fluid
424 for filtration, such as dirty water, wastewater, or sewage to be filtered,
and the second
fluid compartment 430 contains the second fluid 434 as filtrate, such as clean
water,
recyclable water, or permeate. The submerged membrane assembly 400 is
illustrated with
the first fluid 424 illustrated as dirty water, and the second fluid 434
illustrated as clean
water. Both fluids (420, 430) have a specific gravity of one. In Figure 14-1,
neither the
second fluid 434 in the second fluid compartment 430 nor the first fluid 424
in first fluid
compartment 420 have any air or bubbles present. It can be easily calculated
that the
pressure at the surface of each fluid ("0" fluid column height) is 0 psig, the
pressure at 3'
depth is 1.298 psig, and the pressure at 6' is 2.597 psig. At any particular
depth on the
membrane there is equal pressure on each side of the membrane. Pressure at any
depth in
a liquid column is the average density times the height of the column. For
example, the
density of water is 62.4 #/cu. feet. A column of 6 feet of water would have a
pressure of
6x62.4=374.4 #/ sq. feet or 2.6 #/ sq in. Gauge pressure does not take into
account
78
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
atmospheric pressure so the pressure at the bottom of a column of water in
this case would
be approximately 2.6 psig.
[221] As illustrated in Figure 14-2, gas in the form of air bubbles 426 is
present in the
first fluid 424 contained in the first fluid compartment 420. The air bubbles
426 may be
added to scour and clean the first membrane surface 411. In a typical
conventional
membrane installation, the amount of air bubbles 426 present in the first
fluid 424 (dirty
water) to adequately scour the first surface 411 of the membrane 410 reduces
the specific
gravity of the first fluid 424 from 1.0 to about 0.9. Again, it can be
calculated that the
pressures at the top of the assembly 400 is 0 psig. The pressure on the second
membrane
surface 412 (the clean water side) at the top of the membrane 410, i.e., at
the three-foot
elevation on the column height, is 1.298 psig, and the pressure on the first
membrane
surface 411 (the dirty water side) is 1.168 psig. Similarly, the pressure on
the second
membrane surface 412 (the clean water side) at the bottom of the membrane 410,
i.e., at
the six-foot elevation on the column height, is 2.597 psig, and on the
pressure on the first
membrane surface 411 (the dirty water side) is 2.337 psig. In this static
water test, the
second fluid 434 (clean water) will try to flow through the membrane 410 into
first fluid
424 (dirty-water) of the membrane 410 because of the reverse pressure
differential. Also
note that the pressure differential across the top of the membrane 410 is 0.13
psig while
the pressure differential across the bottom of the membrane is 0.26 psig. Not
only will
water try to flow in the wrong direction, but more water will flow across the
membrane at
the bottom than at the top.
[222] Figure 14-3 shows that, if the second column height 432, or liquid
level, on the
second fluid 434 contained in the second fluid compartment 430 (clean water)
is reduced
by 0.62 feet with respect to the first column height 422, then the pressure on
both the
second membrane surface 412 (clean water side) and on the first membrane
surface 411
(dirty water side) at the bottom, i.e., six-foot elevation of the column
height, will be equal
at 2.337 psig. Note however that the pressure on the second membrane surface
412 (the
clean water side) at the top of the membrane 410, i.e., at the three-foot
elevation on the
column height, is 1.03 psig, while the pressure on the first membrane surface
411 (the dirty
water side) is 1.168 psig. This creates a pressure differential of 0.13 psig
at the three-foot
elevation. Under the above-described conditions, fluid will flow the correct
way, from the
first membrane surface 411 (dirty water side) of the membrane 410 to the
second
79
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
membrane surface 412 (clean water side). Since the pressure differential at
the bottom of
the of the membrane 410 is 0.0, no water will flow either way, but at the top
of the
membrane the water will flow from the dirty water side (430) of the membrane
410 to the
clean water side (420). As a point of interest, if the second fluid column
height 432 is
reduced by 0.4 feet water will flow the correct way at the top of the membrane
410 and the
wrong way at the bottom of the membrane. The second fluid column height 432
may be
varied with respect to the first fluid column height 422 by any suitable
method, device, or
means, including providing an outlet or overflow for the second fluid 434 at a
selected
elevation, applying a vacuum to the second fluid 434, andlor applying a
pressure to the
first fluid 424.
[223] Figure 14-4 illustrates a pressure differential across the membrane 410
resulting
from a change in the specific gravity of the second fluid 434 of the membrane
assembly
400 of Figure 14-3, according to an embodiment of the invention. In Figure 14-
4, a gas, in
the form of air bubbles 426, is present in the first fluid 424 (dirty water)
contained in the
first fluid compartment 420 and forms aerated water. Sufficient air bubbles
436 may be
added to the second fluid 434 (clean-water) contained in the second fluid
compartment 430
to change or adjust the specific gravity of the second fluid to more closely
approximate the
first specific gravity of the first fluid 424 contained in the first
compartment 420. This
reduces the second specific gravity of the second fluid 434 to the first
specific gravity of
the first fluid 424. As in Figure 14-1, the pressures with respect the
membrane 410 at
various depths along a column height can be calculated. The pressures will be
90% of the
pressures in Figure 14-1 because, in this case, the aerated water (434)
specific gravity is
90% of unaerated water specific gravity. Note that the pressure differential
across the
membrane 410 at all elevations is zero. In addition to creation of an
equalized pressure
differential along a vertical axis of the submerged permeable membrane 410,
the presence
of rising bubbles of the air 436 proximate to the second surface 412 (clean
water or
permeate side) of the permeable membrane imparts a scouring action on the
second surface
of the membrane 412, according to an embodiment of the invention.
[224] Figure 14-5 illustrates a submerged membrane assembly 401 having a
selected
differential hydraulic head 452 imposed between the first fluid 424 contained
in the first
fluid compartment 420 and the second fluid 434 contained in the second fluid
compartment 430, according to an embodiment of the invention. Alternative
embodiments
SO
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
for imposing the differential hydraulic head are described below. If the
specific gravity of
the second fluid 434 is adjusted to more closely approximate the specific
gravity of the
first fluid 424, and a selected differential hydraulic head 452 is imposed
between the first
fluid 424 and the second fluid 434, a selected pressure differential across
the membrane
410 results along the vertical axis of the membrane 410. As illustrated in
Figure 14-S, the
second specific gravity is adjusted to equal the first specific gravity, and a
2.0-foot
differential head 452 is additionally imposed between the first fluid 424 and
the second
fluid 434. As before, the pressures at the top and bottom of the membrane 410
can be
calculated. The pressure differential across the membrane 410 is uniform
(0.779 psig)
along its vertical axis, from top to bottom. Now, each pore on the membrane
410 sees
approximately the same driving pressure, and each pore will transmit about the
same
amount of water. Using the entire membrane surface, and every pore equally,
the
membrane assembly 401 typically produces more flow than the membrane
assemblies
having unequal pressure differentials of Figure 14-3 and Figure 14-6 for
example. If the
adjusted or changed second specific gravity does not closely equal the first
specific gravity,
the selected pressure differential across the membrane is expected to vary
only a minor
degree along the vertical axis of the membrane. For example, variation of the
pressure
differential along the vertical axis is expected to be generally uniform,
i.e., not vary more
than +/- 30% per vertical linear foot, when the second specific gravity is
adjusted to within
approximately +/- 5 percent of the first specific gravity.
[225] In the embodiment illustrated in Figure 14-5, the differential hydraulic
head 452 is
imposed by selecting the second fluid column height 432 with respect to the
fist fluid
column height 422 to produce a selected pressure differential across the
membrane 410
along the vertical axis at the first specific gravity and the adjusted or
changed second
specific gravity. Figure 14-5 illustrates a selected second column height 432
of 4.0 feet
and a first column height 422 of 6.0 feet producing a selected differential
hydraulic head
452 of 2.0 feet. As described in conjunction with Figure 14-4, the second
fluid column
height 432 may be varied with respect to the first fluid column height 422 by
any suitable
method, device, or means, including providing an outlet or overflow for the
second fluid
434 at a selected elevation, applying a vacuum to the second fluid 434, and/or
applying a
pressure to the first fluid 424. In an embodiment using gravity, the column
heights 422 and
~1
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
432 may be established by providing fluid outlets or overflows from the fist
fluid
compartment 420 at 6.0 feet and from second fluid compartment 430 at 4.0 feet.
(226] In an alternative embodiment, the differential hydraulic head 452 can be
imposed
by enclosing the first fluid compartment 420 and applying a pressure, such as
by
compressed air generated by a mechanical compressor, thus increasing the first
column
height 422 without physically increasing the vertical dimension of the first
fluid
compartment. In another alternative embodiment, the differential hydraulic
head 452 can
imposed by applying a vacuum, such as generated by a mechanical vacuum pump,
to the
second fluid compartment 430, thus decreasing the second column height 432
without
physically decreasing the vertical dimension of the second fluid compartment.
Using
gravity solely to impose the differential hydraulic head 452 may be considered
preferable
because gravity does not require any mechanical devices that consume power and
require
maintenance, such as pumps. In addition, using gravity solely eliminates any
problems
associated with maintaining an enclosed fluid compartment.
[227] Additional features of the embodiment illustrated in Figure 14-5 include
flowing
the first fluid 424 past the first surface 411 of the membrane 410 while
maintaining the
first column height 422. This embodiment also allows the second fluid 434 to
be collected
from the second fluid compartment 430 as filtered, clear, or clean water while
still
maintaining the selected second column height 432 to impose the differential
hydraulic
head 452.
[228] Figure 14-6 illustrates a comparison of how existing Zenon and Kubota
membranes
typically react with the 2.0-foot differential hydraulic head 452 imposed as
illustrated in
Figure 14-5. The existing apparatus and methods for operating these membranes
do not
change or adjust the specific gravity of the second fluid 434 to closely
approximate the
specific gravity of the first fluid 424. Simply imposing the differential
hydraulic head 452
across the membrane 410 does not achieve a generally uniform pressure
differential across
the membrane along the vertical axis. It only results in a pressure
differential that is
considerably higher at the top of the membrane than at the bottom. In other
words, the
pressure differential varies along the vertical axis of the membrane.
[229] This description will next address embodiments for changing or adjusting
the
second specific gravity by including diffused gas or air bubbles 436 in the
second fluid 434
as previously described in conjunction with Figure 14-5. As described in
conjunction with
~2
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Figure 14-5, an aspect of the invention includes changing and/or adjusting the
second
specific gravity to more closely approximate the first specific gravity in
value. In a certain
embodiment, the second specific gravity is adjusted to within approximately +/-
5 percent
of the first specific gravity. In another embodiment, the second specific
gravity is adjusted
to within approximately +/- 2.5 percent of the first specific gravity.
[230) Figures 14-S and 14-7 illustrate alternative embodiments of the
invention for
including bubbles 436 in the second fluid 434 to change the second specific
gravity, and
optionally to impart a scouring action to the second surface 412 of the
membrane 410. In
an embodiment illustrated in Figure 14-5, the bubbles 436 are sourced from
supersaturated
dissolved gases present in the first fluid 424. As previously described, long
shaft vertical
reactors receive at their head tank substantial concentrations of fluid having
supersaturated
dissolved gases. If the fluid 424 is such a fluid having a substantial
concentration of
supersaturated dissolved gases, a portion of the supersaturated dissolved gas
will nucleate
on the first surface 411 of the membrane 410. This nucleated gas will impart a
scouring
action on the first surface 411 as the nucleated bubbles rise in the fluid
424. Another
portion of the supersaturated dissolved gases of the fluid 424 permeate the
membrane 410
by passing from the first surface 411 through the pores of the membrane and
emerging on
or proximate to the second surface 412 and in the second fluid 434. A portion
of this
passed-through supersaturated dissolved gas will nucleate and form gas bubbles
436, thus
adding diffused gas to the second fluid 434. The mechanism by which the
supersaturated
dissolved gas nucleates in the second fluid 434 is not fully understood. The
nucleation
may be caused in whole or in part by a mechanical action of the dissolved gas
passing
through the membrane 410. Alternatively, the nucleation may be caused in whole
or in
part by the pressure differential between the first fluid 424 in the first
compartment 420
and the second fluid 434 in the second fluid compartment 430 imposed by the
differential
hydraulic head 452. Also alternatively, the nucleation may be caused by a
difference in
dissolved gas levels between the first fluid 424 and the second fluid 434. The
nucleation
may be on the second surface 412, within the second fluid 434, within the
second fluid 434
proximate to the second surface 412, or within the membrane 410. The gas
bubbles 436
nucleate on or proximate to the second surface 412, and impart a scouring
and/or cleaning
action on the second surface as they rise in the second fluid 434.
g3
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[231] Figure 14-7 illustrates a submerged membrane assembly 402 with
differential
hydraulic head 452 and gas inlet 438, in accordance with an embodiment of the
invention.
The assembly is substantially similar to the membrane assembly 401 of Figure
14-5, with
an added optional inlet 438 coupled to the second fluid compartment 430. The
optional
inlet 438 includes configuration for adding air or gas into the second fluid
compartment
430, and forming bubbles 436 in the second fluid 434. The air may be added by
providing
air or a gas to the inlet 438, and diffusing the air or gas within the second
fluid
compartment 430. A diffusing device may be included with the inlet 438 to
assist bubble
formation within the second fluid compartment. Alternatively, the air or gas
may be first
diffused in another liquid, which is then flowed through the inlet 438 into
the second fluid
compartment 430 and added to the second liquid 434 in sufficient quantities to
adjust the
second specific gravity to closely approximate the first specific gravity, and
optimally,
equalize the first and second specific gravities. In a further alternative
embodiment, the
bubbles 436 of air or gas may be proved by other sources, such as a chemical
reaction, an
ultrasonic device, and a microwave device.
[232] The Zenon and Kubota submerged membrane processes of Figure 14-6 can be
improved by adding air or gas to the second fluid compartment 430 (clean water
side) of
the membrane 410 using the submerged membrane assembly 402 with the gas inlet
438 as
illustrated in Figure 14-7. While adding a gas directly to the second fluid
compartment
430 of the Zenon and Kubota processes comprises an improvement to those
processes, it is
not expected to produce a similar degree of scouring of the second membrane
surface 412
in the clean water side to that produced by bubble nucleation on the second
surface
resulting from a supersaturated mixed liquor media as is present in long
vertical shaft
bioreactors.
[233] Figures 15 and 16 illustrate an improved long vertical shaft bio-reactor
500 for
treatment of waste waters having a membrane bioreactor head 503 that includes
plurality
of submerged membrane bioreactor assemblies 510, according to an embodiment of
the
invention. The long vertical shaft bioreactor may be any type of long vertical
shaft
bioreactor that has substantial concentrations of supersaturated dissolved gas
at the head
tank 502 level, such as the bioreactors of Figure 5 or Figure 8. Figure 15 is
a top
perspective view of a bioreactor head tank 502, and a membrane bioreactor head
503
having plurality of saddle tanks 506A-I7 mounting the membrane bioreactor
assemblies
84
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
510. Figure 16A is a top view of saddle tank 506A of the membrane bioreactor
head 503,
illustrating the top membrane bioreactor assembly S l OD that includes a
plurality of flat
plate semi-permeable membranes 511. Figure 16B is a cross-sectional side view
of the
bioreactor head tank 502, and of the saddle tank 506A having a stack of four
membrane
bioreactor assemblies S l0A-D positioned vertically above each other.
[234] Figures 15 and 16 illustrates an embodiment of a membrane bioreactor
head 503,
having four stacks or columns of membrane bioreactor assemblies 510 arranged
circumferentially around the outside of and in fluid communication with the
head tank
502. In practice, eight saddle tanks typically would be used to entirely
surround the
periphery of the head tank 502 and maximize membrane filtration. In Figure 15,
each
saddle tank 506 includes four tiers of submerged membrane assemblies S l0A-D
positioned
vertically above each other. Each assembly 510 is approximately 4 feet high,
for a total
membrane bioreactor head 503 column height 424 of approximately 16 feet. If a
membrane fails, it may be replaced by shutting down only one of the saddle
tanks 506,
thus allowing the reactor and the other seven saddle tanks to continue
operation. The
uppermost submerged membrane assembly S l OD can be serviced from the top of
the
saddle tank 506, while the lower three submerged membrane assemblies S l0A-C
can be
serviced through tip out (mail box like) drawers as illustrated in Figure 1 S
for assembly
S 10A and in Figure 16B for assembly S l OC.
[235] Each membrane bioreactor assembly 510 includes a plurality of flat plate
semi-
permeable membranes 511 coupled by a membrane output line 512 to an exterior
manifold
S 14. The exterior manifold 514 is coupled by a collection line 516 to a
collection trough
538. The plate membranes 511 are typically include a frame that supports two
rectangular
semi-permeable membranes having their second surfaces 412 facing each other
and
defining in cooperation with the fame an interior second fluid compartment 430
between.
The first surfaces 411 of the semi-permeable membrane are exposed to a fluid
surrounding
the exterior of the membrane assembly 510. The collection line 516 may be made
of any
tubular member suitable for carrying permeate or fluid outputted by the plate
membranes
511. The collection line 516 may be transparent or clear, allowing a user to
visually
inspect the bubble 436 content and clarity of the output from each individual
plate
membrane 511.
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[236] The collection lines S l0A-C flow permeate upward into the collection
trough 538
as illustrated in Figure 16B. The collection line $ l OD is formed into a
siphon that flows
permeate from the membrane 511 downward, discharging into the trough 538. The
first
column height 422 is defined between the lowest point of the lowest membrane S
11 and
the level of the outflow 528 from the saddle tank 506. The second column
height 432 is
defined between the lowest point of the lowest membrane 511 and the level of
the trough
538.
[237] In an embodiment of the invention, each membrane assembly 510 includes
75 flat
plate membranes. Using eight separate saddle tanks 506A-D and 506E-H (not
shown)
around a head tank 75 provides a total number of flat plate membrane
bioreactors 511 in
this configuration of 75/ tier x 4 tiers /saddle tank x 8 tanks = 2400 flat
plate membranes.
Experience with the plate membrane indicates that this arrangement would
process about
0.3 MGD on average and 0.6 MGD at peak flow. The head tank 502 diameter in
this
embodiment is approximately 9 feet, and with the saddle tanks 506 makes the
reactor
about 13 feet in diameter.
[238] In operation, the first fluid 424 as inflow 526 of effluent from the
long vertical
shaft bioreactor flows into the bottom of the saddle tank 506A from a long
vertical shaft
bioreactor (not shown). The first fluid 424 has a first specific gravity, and
includes
bubbles 426 and supersaturated dissolved air. The first fluid 424 rises
through the saddle
tank 506A past the column of submerged membrane bioreactor assemblies S l0A-D,
and
becomes outflow 528 as it overflows the saddle tank at a 12 foot elevation.
The outflow
528 returns to the long vertical shaft bioreactor for further processing or
removal from the
reactor. The individual flat plate membranes 511 filter the first fluid 424 as
described in
conjunction with Figures 14-1 through 14-7, and primarily as described in
conjunction
Figure 14-S. The first fluid 424 has a first column height 422 of 16 feet
between the
bottom of the bottom flat plate membranes 511 and the out flow 528. The second
fluid
434 has a second vertical column height 434 of 12 feet established by the
collection trough
538 and the collection lines 516 leading into it. As a result, a differential
hydraulic head
452 is imposed between the first fluid 424, the effluent, and the second fluid
434, the
permeate or filtered water.
[239] Furthermore, by nucleating the dissolved gas of the first fluid 424 in
the second
fluid 434 as described in conjunction with Figures 14-1 through 14-7, and
creating a gas
86
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
fraction on the second surface 411 (clean side) of the membranes (including
the vertical
conduits leading to the collector trough) equal to the gas fraction on the
first surface 410
(dirty side) of the membrane, it is possible to maintain a generally uniform
pressure
differential along the vertical axis of each membrane 511 of each submerged
membrane
bioreactor assembly 510 at 1.168 psig. As in Figure 14-5, the pressure
differential at the
top of each membrane 511 is the same as it is at the bottom, and the pressure
differential
across the top tier of membranes S l OD (which is under a siphon head) is
exactly the same
as the pressure differential across each of the other three tiers of membranes
S l0A-C. As a
result, it is expected that each and every membrane in the saddle tank 506
will produce the
same flow. The pressure differential of 1.168 psig is equivalent to about 33
inches of
water.
[240] In conventional systems, there is no gas nucleation in the second fluid
434, and
therefore the difference in pressure differential between the top and the
bottom on a 1
meter (40") high membrane 511 is 40"x 10%= 4 inches of water. This may not
appear
significant, but it is enough to cause unequal flow through any particular
membrane.
[241] For peak flows, the pressure differentials across all the membranes 511
can be
raised equally by simply increasing the height of the dirty water column, the
first column
height 422. Note that the air bubbles 426 are used four times as they travel
from the
bottom membrane assembly 5 10A to the top tier S 1 OD.
[242] In an alternative embodiment, the membrane bioreactor assemblies may be
arranged within the head tank 502, and the saddle tanks 506A-H eliminated.
[243] Figure 17 illustrates a folded saddle tank system S50 that includes a
first folded
saddle tank 556A and a second folded saddle tank 556B that collectively carry
the
membrane assemblies S l0A-C, according to an embodiment of the invention. In
some
cases, four vertical tiers of submerged membrane assemblies S l0A-C, for
example, as
illustrated in Figures 15 and 16, may create a plant that is too high. In that
case, a folded
saddle tank, such as the folded saddle tank 556 can be used advantageously. In
the
configuration of Figure 17, the membrane assemblies S l0A-B are contained in a
first
saddle tank 556A, and the membrane assemblies S l OC-D are contained in a
second saddle
tank 556B. The second saddle tank 556A includes an inlet 568 for fluid
coupling the
inflow 526 of effluent from a bioreactor (not shown). A fluid coupling member
558
couples the out flow 558 of the first saddle tank 556A into the second saddle
tank 556B.
87
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[244] The second saddle tank 556B is open to the atmosphere, but the first
saddle tank
556A is not. The second column height 432 exists in two segments across the
folded
saddle tank system SSO, a fist portion 432A across the first saddle tank 556A,
and a second
portion 432B across the second saddle tank 556B. The first column height 422
is not
shown in Figure 17, but its effective dimension is from the out flow level 558
of the
second saddle tank 556B at atmospheric pressure to the lowest point of a
membrane plate
of submerged bioreactor assembly SlOA of the first saddle tank 556A. The
system 550
includes two collection troughs 538A and 538B receiving permeate or clean
water (532)
from the submerged membrane bioreactor assemblies S l0A-D of the first and
second
saddle tanks 556A and 556B respectively.
[245] In operation, the folded saddle tank system 550 functions substantially
similarly to
the system 500 of Figures 15 and 16. Inflow 526 enters the first saddle tank
556A through
inlet 568, and flows upward past the submerged membrane bioreactor assemblies
S 10A
and S l OB. The liquid overflow and pressurized off gas 559 are piped through
the fluid
coupling member 558 into the bottom of the second saddle tank 556B, and flows
upward
past the submerged membrane bioreactor assemblies S l OC and 51 OD. The
hydraulic
calculations are the same as for the four tier high arrangement. As before
each membrane
sees the same pressure differential top to bottom and from tier to tier. A
generally uniform
pressure differential of approximately 1.75 psig is created between the first
and second
surfaces 411, 412 of the membranes of the membrane assemblies The outside
diameter of
the head tank 502 (not shown) remains at 9 feet, but the overall outside
diameter with the
folded saddle tank system 550 increases to 18 feet. Again, the air bubbles 436
(not shown)
are used four times as it passes through each of the four tiers of membranes
510.
[246] Figure 17 illustrates several pressure gages [P] 571 and valves 570
introduced for
clarity and understanding. The pressure, in psig, at each gage location is
shown next to the
gage. There are no pressure gages or valves in an actual plant because when
the valves are
closed the dissolved air would come out of solution and change the density of
the liquid in
the membrane discharge lines. However for this illustration, assume that, at
any moment in
time under normal operation, the valves may be closed momentarily, resulting
in the
pressures shown on the gages. The selection of 1.75 psig is the nominal
pressure exerted
by 4 ft. of water, which is typical for this type of saddle tank design.
88
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[247] Note that all the gage pressures on the discharge lines from the
membranes are
equal. The pressure (head) in the discharge lines of the membranes in saddle
tank 556A is
due to the 3.5 psi of off gas pressure (equivalent to 8 ft. of water)
superimposed on the
liquid in tank 556A. The pressure at the collection trough 538A is reduced by
the 1.75 psi
(4 feet of liquid standing in the discharge line of membranes S l OB), and 3.5
psi (8 ft. of
water) standing in the discharge line of membrane S l 0A.
[248] Similarly, the discharge line from membrane S l OC is under a hydraulic
head of
1.75 psi (4 feet) and the discharge line from membrane S l OD is under a
siphon (vacuum)
of 1.75 psi. Experience in the field shows that air bubbles are permitted in a
siphon line
provided the lines are sized properly to maintain adequate discharge flow
velocities,
generally of greater than 2 ft./sec.
[249] To further elucidate various aspects of the invention, a bench test
apparatus was
constructed according to the teachings herein and was used to conduct a series
of bench
tests of membrane throughput under varying membrane conditions and levels of
diffused
gas in water. Figure 18 illustrates results of a series of tests conducted on
the bench test
apparatus.
[250] Field observations show that the membrane permeability increases with an
increase
in dissolved gas. For example, see Table 3 above where adding dissolved air
resulted in a
significant increase in the permeate throughput. Other field observations
demonstrate a
scouring effect that the pressurized gasses in the reactor liquor exert on
membrane and
other surfaces. In vertical shaft bioreactors, a significant cleaning action
occurs at strategic
locations within the reactor, which typically are locations where dissolved
gasses come out
of solution.
(251] As noted above, the bench test apparatus was devised using a Kubota
membrane of
the same type used in field tests described in Table 3. These tests
demonstrated that
increasing the dissolved gas content by adding downcomer air in the reactor
liquor had a
large effect on permeate flow. Figure 18 shows the performance of a section of
the
Kubota membrane that had been previously used in a reactor for more than two
months. A
series of eight permeability tests were done over a period of a week on the
Kubota
membrane using the bench test apparatus. The test apparatus membrane section
was
89
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
approximately 1/130 of the area of both sides of a full size (1/2 m x 1 m)
Kubota
membrane.
[252] The test apparatus was configured like an aeration shaft with an outer
casing of
3.488" m with a downcomer of 1" inside diameter. The liquid circulation was
driven with
a large aquarium air pump with two injection ports near the bottom of the
downcomer. The
membrane was located in a machined recess at the bottom of the 3.5 " diameter
tube and a
removable bottom cover supports the membrane from movement in the downward
direction. The bottom cover plate included a series of machined grooves
dimensioned
similarly to the grooves in the Kubota membrane. A piece of coarse felt
blotter membrane,
taken from the field trial membrane unit, is installed between the membrane
and the
permeate collection system. Membrane discharge tubes were installed both
vertically
upward and downward from lower surface of the membrane. Additionally, the
lower tube
can be used as a siphon or drain to remove permeate from the lower side of the
membrane.
[253] Permeability tests were conducted to measure the effect of dissolved gas
on flow
rates through the membrane. In order to do this the influence of air-lift
effect in the
membrane discharge line must be separated from the effect of increased flow
due to
degassing. As a result the membrane is oriented horizontally at the bottom of
a Plexiglas
tube 24" tall and 3.5" in diameter. The first tests used a membrane glued to
the bottom of
the cylinder. This test simply determined that gas saturated liquid would pass
through the
membrane but there was no provision for the effect of vacuum, the effect of
the felt
wicking layer under the membrane skin, or the effect of the permeate channeled
collection
system.
[254] The test apparatus used a porous felt layer under the membrane and a
channeled
permeate collection system similar to the Kubota design. The area of the test
membrane is
9.5 sq. in. or 1/130 of the area of both sides of the field test Kubota
membrane. The
membrane used in the field was ('/Z meter x 1 meter) and had a rated surface
area of 8.6
sq.ft. or 1238 sq.in.
[255] In the field trials, only 1 foot of positive head was available. In
order to get flows
over 150 U.S. gal per membrane per day, a vacuum of up to 28" was successively
applied
to the permeate side. Actually ,the field unit will self prime the siphon by
using only the 1
ft. of positive head. However, in the test apparatus, thel/8 inch diameter
clear vinyl
discharge line from the membrane worked quite well when used as a siphon on
test runs
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
1,3,and 4 when there was little or no gas in the permeate. In fact, on run 3
the number and
size of bubbles in the siphon could be visually estimated.
[256] In the saddle tank design described above in conjunction with Figure 15,
the top
tier of membranes operates under siphon flow. Test data was collected from the
test
apparatus data under negative head. Note that on run S (fresh soda water )
there was
enough dissolved gas transfer through the membrane to interrupt the operation
of the
siphon however using soda water about 12 hours old, (run 7) the siphon effect
worked well
again.
[257] Air-lift circulation through a riser and downcomer: Initially, it was
thought that tap
water and/or soda water would permeate the membrane over long periods of time
without
loss of throughput. This was not the case. When tap water is left standing in
the test
apparatus, the flow slows over time. When soda water is left to stand in the
test apparatus
there is virtually no deterioration in flow. However when an air-lift
circulation was
employed with tap water there was no noticeable deterioration in flow perhaps
due to
surface scouring of the membrane. The soda water product likely uses reverse
osmosis
water while the city water is sand filtered. Initially it was thought that
there might be a
measurable difference in the quality of water but that turned out to not be
the case on run
8. Later it is shown that the gas nucleation effect on the downstream side of
the membrane
has the dominate effect on permeate flow.
[258] Flow calibrated in micro-litres/min: The method employed to measure flow
involved measuring the change in liquid height in a small diameter cylindrical
catch tube
and a stopwatch. This method is quite accurate with a high degree of
repeatability (+/- 25
micro litters.) as demonstrated by the good fit of the curves to the data
points.
[259] Figure 18 plots the test run results. A first step was to establish. The
first two
runs were to establish permeate flow base line data similar to that observed
in the field.
Run 1 was on tap water and used the dirty membrane. The water was airlift
circulated. Run
2 was done in the same way but using fresh soda water as the liquid. Air-lift
circulation
was not used in run 2 because it caused too much foam.
[260] A second step was to clean the membrane according to field observations.
Soda
water was air circulated across the face of the membrane overnight. This
simulated the
bubble nucleation concept seen in the field.
91
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[261] A third step was to establish permeate base line flows on clean
membranes. Run 3
used the stale soda water that had been aerated overnight. Run 4 used tap
water.
[262] A fourth step was to determine the effect of gas content (function of
soda water "
out of the bottle" age) on permeate flow compared to tap water. Run 5 was on 1
hour-old
soda water. Run 6 was on tap water. Run7 was on 12 hr old soda water.
[263] A fifth step was to approximate the gas content of the liquor in a
typical bioreactor.
Run 8 was on 50% tap water and 50% soda water. The soda water / tap water
mixture was
changed frequently to keep the age of the soda water to less than 30 minutes
out "of the
bottle." In the field the C02 in a long shaft vertical reactor is replenished
every 6-10
minutes, so run 8 is conservative.
[264] The plot of test run results in Figure 18 illustrates several aspects of
the invention.
For the purpose of comparisons between runs, a 24" hydraulic head is used as a
common
pressure.
1 ) Tap water with, air circulation, was run through a dirty membrane and at
24" of head pressure and about 1050 micro liters per min of permeate was
produced. There
were no bubbles visible in the siphon line and a vacuum was easily maintained.
2) Fresh soda water was then processed on the same dirty membrane and
about 1875 micro liters of permeate was produced or about a 78% gain in flow.
This is
approximately the same gain in performance as in the field trial when the bio-
reactor fluid
was supersaturated with dissolved air, (i.e. downcomer air was added). It is
interesting to
note that the soda water used was fresh, between 1 and 5 hours old, yet the
degree of
nucleation on the membrane was sufficient to preclude the use of a siphon from
24 to 32
inches of head. This was interpreted as proof that the dissolved gas permeates
the
membrane easily.
3) Soda water was then air circulated across the membrane face for a further
12 Hours. The permeate lines were blocked off so that the dissolved gas
impinged on the
membrane surface and very little, if any, fluid or gas transferred through.
This simulates
the conditions in the reactor where it is alleged that a polymeric surface can
be effectively
cleaned by bubble effervescence. The permeate discharge lines were then
unplugged and
the permeate flow reached 2200 micro liters / min at 24" of head. Stale soda
water (run 3)
achieved a 40% increase in flow over tap water (run4) when both were processed
on a
92
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
clean membrane. Note that the test runs illustrate that it does not matter
whether the tap
water or the fresh soda water is run first, the fresh soda water always
outperforms the tap
water. Note also that the lack of dissolved gas allowed full siphon effect and
no bubbles
were observed in the discharge lines. Also remember that the differential
transmembrane
pressure effect must be ignored in all of these runs because the membrane is
horizontal.
When the membrane is clean, the improvement in permeate flow appears to be
related only
to the effect of dissolved gas nucleating in or on the membrane. It is
predicted that these
results are related to a substantial change in the partial pressure of the gas
in the fluid. The
dissolved gas is at super-saturation pressure in the liquid on the upstream
side of the
membrane, but is at atmospheric pressure on the down stream side of the
membrane.
Consequently, the gas is moving from high pressure to low pressure across the
membrane
and possibly taking the fluid with it.
The Zenon membrane produces about 50 % more flow per sq. ft. than the
Kubota membrane but the Zenon membrane uses a vacuum on the permeate discharge
line.
It may be that Zenon membranes are influenced by the drop in partial pressure
across the
membrane thus causing a nucleating gas effect. From a differential density
across the
membrane perspective, a 40" tall Kubota membrane should perform better than a
60" tall
Zenon membrane.
4) To quantify the effect of the membrane cleaning process of step 3, tap
water
was re-run on the alleged cleaned membrane. This time the permeate flow
increased from
1050 micro liters per min. in test 1) to1575 micro liters in test 4. This
represents a SO%
increase in permeate flow due to impingement /nucleating gas cleaning.
5) Fresh soda water (1-6 hr old) was processed on the clean membrane and the
permeate flow (2400 micro liters per minute) was marginally better (9%) than
run 3 (2200
micro liters per minute) which used soda that had been air stripped for 12
hrs. Again it is
seen that extremely high levels of dissolved gas are not needed to create an
effect.
6) Run 6 was on tap water and the permeate flow rate increased (44%) to 2275
micro liters per min. from 1575 micro liters per min. over the earlier run 4
also on tap
water, both using a clean membrane. Run 6 on tap water produced slightly less
permeate
flow (5% ) than fresh soda water in Run S.
93
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
7) Stale soda water (12 hrs old) was run on a clean membrane. The permeate
flow (1950 micro liters per min.) was 23% less than the 1-6 hr old soda
permeate rate of
(2400 micro liters per min.). The permeate flow rate for 12 hr old soda (1950
micro liters
per min.) was surprisingly (16%) lower than for the tap water run 6 (2275micro
liters per
min.). It would appear that when the filters are clean the rheological
properties of the stale
soda water and the tap water behave similarly. The data indicates that the
difference in
permeate flow of the two soda water runs, is related to the age of the soda
which in turn is
a function of the amount of dissolved gas present. However, in this case the
tap water
permeate flow exceeded the stale soda water run indicating that there is
really no
difference in the rheology of the two fluids when processed on a clean
membrane. This
gives credence to the idea that the increase in permeate flow is indeed a
function of the gas
nucleation phenomena rather than a difference in the physical/chemical
properties of the
two fluids.
8) Having determined that the rheology and the physical/chemical properties
of the two water sources are similar (once the dissolved gases are
equilibrated), a final run
(8) of a SO% tap water and 50% fresh soda water was 'evaluated. In this case
the mixture
was replenished often, at less than 30-minute intervals, to more closely
approximate the
nature of a vertical shaft bioreactor. In this run 8, the permeate flow
reached 2600 micro
liters per min. for a 15% increase over tap water alone, and 8% over fresh
soda water
alone. Run 8 at 2600 micro liters per minute is equivalent to 130 U.S. gal per
day per full
size membrane. Also keep in mind that these figures are at only 24" of head,
while in the
field up to 39 inches were run.
[265] The above observations strongly indicate that dissolved gas nucleation
does play a
role in membrane flow rate. These data also strongly indicate that the
dissolved gas is
instrumental in the cleaning process. The amount of dissolved gas effects the
flow rate but
the amount of dissolved gas in the bench test apparatus is time dependent.
Fortunately the
dissolved gas content of the liquor in the field test is constant unless
changed purposely.
[266] Another test run was performed to correlate a relationship between
dissolved gas
content and time of exposure to the atmosphere. Fresh soda water was processed
on the
dirty membrane at three pressure heads. The flow rate changed as follows:
'TABLE 4
At 18" head
94
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
Elapsed Time (minutes)micro liters per minutechange in rate of
flow-
micro liters per
minute
3 1790
8 1650 28
20 1500 12.5
40 1450 .625
60 1435 .75
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
At 8.5 "head
TABLE 5
Elapsed Time (minutes)micro liters per minutechange in rate of
flow-
micro liters per
minute
900
18 700 25
38 650 2.5
108 625 .35
TABLE 6
At 7" of head (Same soda water as above, slight change of head pressure from
8.5" to 7".)
Elapsed Time (minutes)micro liters per minutechange in rate of
flow-
micro liters per
minute
300 615
360 590. .4
10 [267] These tests indicate that soda water more than 1 hour old is fairly
stable (less than
.75 micro liter per minute) and therefore all the data points on the curves
(except for run 8)
are for fresh soda water at least 60 min old. On the clean membrane the
difference in
permeate flow between 1-5 hr old soda and 12+-hour-old soda is about 25% at
24" of
head. In the field, the fluid in circulation is always freshly saturated with
C02 every 6-10
1 S minutes, and may therefore achieve a much larger throughput than these
tests indicate.
[268] These tests also indicate that soda water, either stale or fresh,
outperforms tap
water in all cases on dirty or semi clean membranes. Once the membrane is
clean with
soda water (which also contains a small amount of citric acid) there is not
much difference
between tap water and soda water. The membrane in the test apparatus was
visibly cleaner,
after 5 days of exposure to soda water and tap water, than at the start of the
test.
[269] The pressure differential variation from top to bottom of a Kubota
membrane is 4-
6" of water and for a Zenon membrane it is about 6-8". Run 8 using a mixture
of 50% tap
water and SO% soda water on a clean membrane shows a throughput of 2600 micro
liters
/min at a head of 24". A 4,"6" and 8" pressure differential accounts for 15%,
25%, and
35% of the total permeate flow respectively. The influence on flow due to the
pressure
differential variation from top to bottom of the vertically oriented membrane
is in addition
96
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
to the increase of flow due to the degassing phenomenon, cited above,
occurring at the face
of the membrane. Combined, these two effects could potentially double membrane
throughput. Based in part on the above, it is contemplated that the increase
in flow of
permeate through the membrane is due to one or more factors selected from a
change in
partial pressure of the gas effect, a nucleating gas effect, or a release of
stored energy
effect.
[270] Figure 19 illustrates results of a series of temperature, viscosity, and
flow tests
conducted on the bench test apparatus. Several trials were performed on a test
apparatus to
see what difference temperature would make on membrane permeate flow.
Viscosity and
temperature are inversely related, and throughput fluid flow was expected to
be strongly
related to temperature. Figure 19 quantifies these factors based on several
trials on the test
apparatus and confirms these expected relationships. An important point is
that viscosity
varies about 10% between 15 and 25°C. However, between 15 and
25°C, the fluid flow
varies almost 50%, or 550 micro liters /min. As illustrated in Figure 19, the
membranes
are sensitive to temp and viscosity changes in the 15-25 degree range.
[271 ] The vertical long shaft bioreactors are installed in the ground, and
develop over
time a huge thermal flywheel effect. That is to say, the effluent temperatures
are much less
variable than a conventional plant and therefore should have much less
difficulty dealing
with temperature variations than conventional treatment processes.
[272] While the above description describes with respect to Figures 14-19
aspects of the
invention using submerged membranes to separate useable water from wastewater,
sewage
or sludge, the invention are not so limited. The methods and devices of the
invention are
also readily employed for membrane separation of other desired fluids from a
stream
containing the untreated fluid and any unwanted matter. For example, aspects
of the
invention may be used to improve membrane throughput and/or membrane self
cleaning in
saltwater desalination, separation in a chemical process, or in any other
situation where
membranes are used to separate solute particles, suspended materials and other
contaminants from a fluid or solvent.
[273] The invention therefore includes treating an influent that includes
removal of a
targeted fluid from the influent with increased membrane throughput. The
method
typically involves a flowing influent stream that includes a fluid that
includes a dissolved
gas, and a flowing permeate stream that consists essentially of the fluid and
the gas. The
97
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
two streams are separated with a permeable membrane having a first surface in
fluid
communication with the influent stream, and a second surface in fluid
communication
with the permeate stream. The membrane is permeable between the surfaces by
molecules
of less than a predetermined size, the permeability size being selected to
allow the targeted
fluid to pass and reject unwanted components of the influent stream. The gas
may be
dissolved in the fluid by any manner or means, for example by injection and as
a result of a
chemical process occurring within the influent. The amount of the dissolved
gas in the
fluid of the influent stream is an amount that increases the permeate stream
flow over the
permeate stream flow when the fluid of the influent stream does not include
the dissolved
gas. This amount may vary depending on the nature of the fluid, the gas, and
operating
parameters of a system performing the membrane separation. The amount of
dissolved gas
in the fluid of the influent stream may be at least the saturation level of
the gas, or may be
a supersaturation level of the gas. The dissolved gas may include air, or a
component of
air such as carbon dioxide. The targeted fluid may be water, blood, or any
other fluid.
1 S (274] Another aspect of the invention includes treating an influent that
includes
imparting a self cleaning action on membrane surfaces. The method includes a
flowing
influent stream that includes a fluid that includes a dissolved gas, and a
flowing permeate
stream that consists essentially of the fluid and the gas. The two streams are
separated
with a permeable membrane having a first surface in fluid communication with
the
influent, and a second surface in fluid communication with the permeate. The
membrane
is permeable between the surfaces by molecules of less than a predetermined
size, the
permeability size being selected to allow the targeted fluid to pass and
reject unwanted
components of the influent stream. The fluid of the influent stream includes
the dissolved
gas in an amount that permeates the membrane and nucleates proximate to the
second
surface. The fluid of the influent stream may include the dissolved gas in an
amount that
imparts a scouring action on the first surface. The fluid of the influent
stream may include
the dissolved gas in an amount that nucleates on the second surface and
imparts a scouring
action on the second surface. The nucleation of the gas proximate to membrane
surface
imparts a scouring action on the surface that helps clean the surface. This
increases
operating life of the membranes by increasing time between scheduled membrane
cleaning
cycles that remove the membrane from service. Previous Figures 14 through 17
describe
aspects of the invention creating a selected pressure differential across
membranes along a
9g
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
vertical axis in a liquid-liquid system. However, an embodiment of the present
apparatus
can be used for creating a selected pressure differential along a vertical
axis of membranes
in a gas-liquid or a gas-gas system.
Membrane Diffuser
[275] A common conventional technology uses low-pressure horizontally
orientated
membrane diffusers, typically flat plate membranes placed horizontally on a
floor of an
aeration tank. The floor area, even if completely covered with membranes, has
a relatively
small area compared to the tank volume to be aerated. In such horizontal
applications, a
liquid being aerated is contained above the membrane. This liquid subjects the
entire
membrane surface to a hydrostatic pressure. A disadvantage of this horizontal
membrane
design is that bubbles generated are quite large when they leave the surface
of the
membrane. This is because a bubble must grow in low-pressure horizontal
membrane
systems until buoyancy exceeds attraction force before the bubble is released.
Low-
pressure, horizontal membrane systems typically generate bubbles about 1-2
millimeters in
diameter. Current practice is to force the bubble from the surface of the
horizontal
membrane by increasing the internal gas pressure to about twice the static
liquid pressure.
This makes small, fine bubbles, but requires substantially more energy in
compressing the
gas.
[276] An emerging design places membranes in a vertical configuration, and
allows the
liquid being aerated to flow between the membranes. The membrane surface area
in an
aeration tank is greatly increased by arranging the membranes vertically, and
the bubbles
generated are smaller due to the shearing action of the liquid flow between
membranes.
Very low energy requirements that are 20-30% of conventional horizontal
membrane
systems have been reported. However, in the vertical layout, a top portion of
the
membrane sees a lower pressure from the liquid than a bottom portion of the
membrane
because the bottom portion is at a greater depth. This results in an unequal
airflow along a
vertical axis of the membrane surface. A common complaint in this design is
that
vertically orientated membranes "wet out" and cease air flow through the
membrane. The
"wet out" generally begins with a portion of the membrane at the greatest
depth, and
proceeds upward. The lack of airflow in the lower membranes allows water to
enter the
membrane, which restricts or stops gas diffusion by the membrane.
99
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[277] Figure 20 schematically illustrates a submerged membrane gas diffusion
apparatus
600, according to an embodiment of the invention. Figure 21 is a partial cross-
sectional
front view of the gas diffusion apparatus 600 of Figure 20 and illustrates
several aspects of
the apparatus, according to an embodiment of the invention. The membrane gas
diffusion
apparatus 600 includes three separate compartments, a fluid treatment
compartment 601, a
bubbling fluid compartment 602, and a static fluid compartment 603. The
compartments
(601, 602, 603) are preferably located proximate to each other for
convenience. The
membrane gas diffusion apparatus 600 also includes at least one membrane
bundle that
diffuses a gas into a liquid. In the exemplary embodiment illustrated in
Figure 20, three
hollow tube membrane bundles 610A-C are positioned at different elevations in
the fluid
treatment compartment 601 of the gas diffusion apparatus 600. This embodiment
of the
invention can alternately employ one or more membranes. The membranes can be
of any
type suitable for membrane gas diffusion, such as plate and frame, tubular,
hollow fiber,
and spiral wound membranes. Further, the membranes can be made from any
suitable
1 S material, such as cellulose acetate, polyvinyl chloride, polysulfones,
polycarbonates, and
polyacrylonitriles.
[278] Elements of the submerged membrane gas diffusion apparatus 600 include a
membrane bundle 610, a membrane-mounting member 612, a fluid treatment
compartment
601, a bubbling fluid compartment 602, and a static fluid compartment 603. For
clarity in
viewing Figure 20, detailed reference numbers are generally provided only for
the bottom
membrane bundle 610A and its associated membrane-mounting member 612. Membrane
bundles 610B and 610C are substantially similar to membrane bundle 610A.
Typically,
each membrane bundle is about 6 inches in diameter and about 30 inches long,
and
typically includes a plurality of hollow tubular membranes. The hollow tubular
membranes have a typical inside diameter of about one inch. Figure 21
illustrates the
membrane bundle as including three hollow tubular membranes 610A-1, 610A-2,
and
610A-3. However, there may be any number of tubular membranes in each tier of
membrane bundles 610. The membrane bundles 610A-610C are oriented such that
the
fluid to be treated 634, such as a mixed liquor, flows among tubular membrane
bundles of
each of the several tiers during aeration. Each tubular membrane has a first
surface, a
second surface, and is permeable between the surfaces by molecules of less
than a
predetermined size, such as described in conjunction with Figures 14-1 through
14-7.
100
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
[279] Each membrane-mounting member 612, which is a tubular member with a
right
hand 6128 and a left hand 612L portion in a preferred embodiment, mounts or
carries a
respective end of the membrane bundle 610 at a membrane-mounting portion. Each
membrane-mounting member 612 includes a chamber 614 that provides the fluid
communication FC between the bubbling fluid compartment 602, the first surface
411 of
each membrane of the membrane bundle 410 mounted to the mounting member, and
the
static water compartment 603. The chamber 614L of left-hand portion 612L of
the
membrane mounting member 612 includes a substantially vertically orientated
bubble
capture chamber 617 and a bubble capture aperture 619, which are illustrated
in Figure 21
as part of a rising gas bubble capture member 615. The member 615 is coupled
with the
mounting member 612L to form an assembly. The chamber 6148 of the right-had
portion
6128 of the membrane mounting member 612 includes a substantially vertically
orientated
gas reservoir chamber 618 and gas release aperture 611, which are illustrated
in Figure 21
as part of a release member 616. The member 616 is coupled with the mounting
member
6128 to form an assembly. The chambers 617 and 618 each have a vertical
length, the
vertical length 654 of the chamber 617 being greater than the vertical length
656 of
chamber 618.
[280] For purposes of describing an embodiment of the invention, a fluid to be
diffused
620 is described as air 620. In other embodiments, the fluid 620 to be
diffused may be
any type of gas, or may be a liquid. Diffusion will be described herein as
aeration, but the
invention is not so limited. Further, a liquid 634 to be treated into which
the diffusion
occurs will be described as wastewater or water. In other embodiments, the
fluid 634 to be
treated may be any type of liquid or gas.
[281] The fluid treatment compartment 601 includes a configuration that
contains the
wastewater 634, such as a reactor basin tank that contains high concentrations
of
suspended solids or mixed liquor for aeration in conjunction with treatment.
Typically, the
wastewater 634 flows into the fluid treatment compartment 601 for aeration,
receives
aeration, and flows out, usually for further processing or disposal.
[282] The bubbling fluid compartment 602 includes a configuration that
contains a first
fluid 632 and the rising bubbles 626 of the air 620. The first fluid 632 will
be described as
clean water 632, but may be any fluid having a specific gravity greater than
the air 620.
The compartment 602 optionally includes a source for the bubbles 626, which
may include
101
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
a gas inlet port 622 that receives the air 620 to be formed into air bubbles
626 in the water
632. The port may receive the air 620 from an external source that, upon entry
into the
bubbling fluid compartment 602 and the clean water 632, forms the bubbles 626.
Alternatively, the port 622 may receive the clean water 632 including the
bubbles 626 into
the compartment 602. The gas inlet 622 may include any apparatus that forms
the air
bubbles 626 in the water 632.
[283) The static fluid compartment 603 includes a configuration that contains
a static
fluid 636, described as clean water 636, but which may be any fluid, but may
be any fluid
having a specific gravity greater than the air 620. Optionally, the
compartment 603
includes a configuration allowing a user to visually observe whether any
bubbles of the gas
620 are being discharged from the gas release aperture 611 of the gas release
member 616,
or are otherwise present.
[284] Figure 20 illustrates the assembly 600 arranged with the bubbling fluid
compartment 602 and the static water compartment 603 each abutting the fluid
treatment
compartment 601. The compartments may be defined in a single tank or
structure.
Alternatively, the compartments may be separate tank structures, one of more
of which
abuts another. In an alternative arrangement, the compartments 602 and 603 can
also abut
each other. In another alternative arrangement, one compartment may be a
distance from
another compartment. Figure 19 also illustrates a "zero" elevation at a lowest
point in the
apparatus 600, with the elevation increasing in an upward or vertical
direction. In the
assembly 600, the three tiers of hollow tube membrane bundles 610A-C are
mounted in a
fluid treatment compartment 601 at elevations 4.0, 6.5, and 9.0 feet
respectively. In
practice, any suitable number of the membrane bundles 610 may be used, the
membrane
bundles may have any separation, and can be only inches apart.
[285] As illustrated in Figures 20 and 21, the rising bubble capture portion
of the first
chamber 614L, shown as capture member 615 and bubble capture aperture 619, are
located
in the bubbling fluid compartment 602. The gas reservoir portion of the second
chamber
6148, shown as release member 616 and gas release aperture 61 l, are located
in the static
water compartment 603. The rising bubble capture members 615 are illustrated
with a 2.5
foot-long vertical length measured from the bubble capture aperture 619 to the
lowest
elevation of the respective membrane bundles 610 to which they are coupled.
Gas release
members 616 are illustrated with a 2.0 foot-long vertical length measured from
the gas
102
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
release aperture 611 to the lowest elevation of the respective membrane
bundles 610 to
which they are coupled. The rising bubble-capture members 615 and the gas
release
member 616 may be any length. However, the gas release members 616 are shorter
that
the rising bubble-capture members 615. A length differential of 0.5 feet is
expected to
provide satisfactory results. If there is a significant difference in the
specific gravity of the
aerated clean water 632 and the static clean water 636, the length
differential between the
gas release member 616 and the bubble-capture member 616 is adjusted to
provide the
automatic gas release functionality described below.
[286] In use, the bubbling fluid compartment 602 is filed with aerated clean
water 632,
and the static water compartment 603 is filled with static clean water 636.
The fluid
treatment compartment 601 is filled with the wastewater 634 to be aerated to a
level
sufficient to submerge the membranes 610A-C. The wastewater 634 optimally is
flowed
through the compartment 601 from a low elevation to a high elevation proximate
to the
second surfaces of the membranes in a manner that facilitates aeration, and
then flowed
1 S from the compartment.
[287] Figure 20 illustrates an initial static water level of 12 feet in the
assembly 600,
which then increases to 12.6 feet in the compartments 601 and 602 as the water
632 and
wastewater 634 are aerated. The air 620 is pumped at a relatively low pressure
into the
bubbling fluid compartment 602 through port 622, and the air bubbles 626 are
formed in
the clean water contained in the compartment to form the aerated water 632.
Only a small
amount air pressure is required to pump the air 620 through the port 622 and
into the
compartment 602, saving energy compared to existing systems requiring an
increased
pressure to force air bubbles from diffusion membranes. The bubbles 626 are
formed in a
diameter sufficient to cause the bubbles to rise in the aerated water 632. The
bubbles 626
rise in the aerated water 632, and a portion of the bubbles rise through the
capture member
bubble capture aperture 619 and are captured in the rising bubble capture
member chamber
617. In the chamber 617, the rising bubbles 626 coalesce and ultimately
release the air
620 above an aerated water 632/air 620 interface 658 within the capture member
chamber
617. Because the capture member chamber 617 is in fluid communication with
membrane-mounting member portion of the chamber 614, which is in turn in fluid
communication with the first surface of the membranes of the membrane bundle
610, the
103
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
released air 620 flows or is communicated with the first surface of the
membranes along
the fluid communication path FC.
[288] The vertical position of the aerated water 632/air 620 interface 658
within the
capture member chamber 617 with respect to a lowest elevation of the membranes
of the
membrane assembly defines a gas column 652 having a vertical length, which can
also be
described as a hydraulic head or differential hydraulic head. The gas column
652 imposes
a hydraulic head on the air 620, which is a function of the buoyancy of the
air 620 in the
aerated water 632. That imposed hydraulic head is transmitted to the portion
of the air 620
in fluid communication with the first surface of the membrane of the tube
membrane
bundle 610. If the specific gravities of the aerated water 632 and the
wastewater 634 are
substantially similar, the hydraulic head between the first membrane surfaces
411 exposed
to the chamber 614 and the second membrane surfaces 412 of the membranes of
the
membrane bundle 610 exposed to the fluid 634 in the fluid treatment
compartment 601
will approximate the hydraulic head created by the gas column 652. Figure 20
illustrates
the gas column length 652 as one foot of the water 632, establishing hydraulic
head equal
to one-foot of water. The one-foot hydraulic head applies a pressure to the
molecules of
the air 620 in fluid communication FC with the first surface 411 of the
membranes of the
membrane bundles 610, forcing some of the air molecules through pores of the
membranes
to form aeration air bubbles 628 in the water 634.
[289] The gas column 652 vertical length and resulting differential hydraulic
head are
established by the amount of the bubbles 626 in the bubbling fluid compartment
602 that
enter the bubble capture aperture 619. Increasing the number of air bubbles
626 formed in
the aerated water 632 increases the number of air bubbles rising into the
bubble capture
aperture 619, thus increasing the flow of air into the membrane-mounting
member
chamber 614. This increased air flow will exceed that which can permeate the
membranes
610 at the existing imposed hydraulic head. The air 620 will accumulate in the
chambers
614, 617, and 618, and the vertical elevation of the aerated water 632/air 620
interface 658
will decrease. This increases the gas column length 652, and increases the
imposed
hydraulic head on the released air 620, thus increasing the air flow through
the membranes
until an equilibrium is reached in response to the amount of bubbles 626 in
the bubbling
fluid compartment 602. The internal air pressure of the membrane bundles 610
self
adjusts to the air flow provided by the bubbles 626. The higher the air flow
provided by
104
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
the bubbles 626, the lower the water 632 level in the rising bubble capture
member 615,
and the greater the differential hydraulic head 652.
[290] If a hollow tube of the membrane bundle 610 becomes blocked, or if the
captured
bubbles 626 produce more air 620 than the membranes of the membrane bundle 610
can
diffuse, the air will build up in the tube membrane bundle 610 until the air
fills and
overflows the air release member chamber 618 from the gas release aperture
611,
transfernng the air to the static water compartment 603. This release occurs
because the
air release member chamber 618 has a smaller vertical length 656 than the
rising bubble
capture member chamber 617 vertical length 654, and will vent the air 620
before the air
620 fills and overflows the rising bubble capture member chamber. An
appearance of air
bubbles in the clean water 636 of the compartment 603 indicates that excessive
air 620 is
being supplied to the membrane bundle610, or that the membrane bundle needs
cleaning.
Because the membrane bundle 610 is connected to clean water compartments 602
and 603,
no internal fouling of the membranes should occur.
[291] On startup, the membrane surfaces of the membranes of the tubular
membrane
bundle 510 have differing vertical elevations. Using the membrane bundle 610C
as an
example, a top hollow tube membrane of the bundle is at elevation 9.0 feet and
a bottom
hollow tube is at 8.5 feet. Initially, the top membrane in the tube membrane
610C bundle
will see a little greater pressure differential than the bottom membrane
because it is at a
lesser depth, and will therefore produce a little more air bubbles 628 until
its maximum
flow rate is achieved, thus increasing the internal .pressure on the air 620
and causing the
bottom membrane to approach maximum transfer as well.
[292] The hydraulic head created by the gas column 652 can be calculated as
follows:
Since the water 634 in the fluid treatment compartment 601 is aerated as a
result of its
processing, there is a voidage of between about 2-10 %. For purposes of
describing the
system 600, a voidage of S% will be assumed. The dynamic water levels in both
the fluid
treatment compartment 601 and the bubbling fluid compartment 602 are
established at 12
feet x 105% = 12.6 feet. The hydraulic head across the membrane surfaces of
the top
bundle tubes of the membrane bundle 610C is the pressure of the water 634
outside the
second membrane surface 412 minus the pressure of the air 620 inside at the
first
membrane surface 411. The outside water 634 pressure is (12.6-9.5)/2.31 x
.95=1.27 psig
while the inside air 620 pressure is (12.6-8)/2.31 x .95=1.89 psig. The
hydraulic head is
105
CA 02560193 2006-09-18
WO 2005/100264 PCT/US2005/010976
0.62 psig. Similarly the outside water 634 pressure on the bottom membrane
bundle 610A
is (12.6-4.5)12.31 x.95=3.33psig and the inside air 620 pressure is (12.6-
3)/2.31 x.95=3.94.
Again, the hydraulic head is 0.62 psig. These calculations illustrate an
aspect of the
invention providing a selected hydraulic head or pressure differential across
all the
membranes of the assembly 600.
[293] Occasionally it will be necessary to shut down the gas diffusion
apparatus 600, and
clean water 632 and 636 will enter the membranes 610. When the air 620 is
restarted, the
water will be forced out of the air release members 616 and into the static
water
compartment 603, thus self purging the airways of the tubular membranes of the
membrane bundles 610. In an alternative embodiment, the compartment 603 could
be
filled with a cleaning fluid for periodic cleaning of the membranes by
stopping the air
bubbles 626.
[294] It should be noted that there are many applications where the apparatus
600 could
be used. Some examples are ozonation (03), chlorination (C~2), or
recarbonation (C02) of
1 S drinking water, disinfection of wastewater or re-oxygenation of effluent
using pure Oz, or
biochemical nutrient addition or feedstock, such as NH3, CH4, SOz, etc.
[295] Although the foregoing invention has been described in detail by way of
example
for purposes of clarity of understanding, it will be apparent to the artisan
that certain
changes and modifications are comprehended by the disclosure and may be
practiced
without undue experimentation within the scope of the invention that is
described herein
by way of illustration not limitation. All publications, patents, and patent
applications
cited herein are hereby incorporated by reference in their entirety for all
purposes.
106