Note: Descriptions are shown in the official language in which they were submitted.
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
Electrode assembly for the electrochemical treatment of
liquids with a low conductivity
The invention relates to an electrode assembly for the
electrochemical treatment of liquids with a low
conductivity, said assembly having electrodes which
have a polymeric solid electrolyte arranged between
them, are pressed against one another by means of a
pressure-exerting device and are formed in such a
manner that the liquid can flow through the assembly.
A main area of use for such an electrode assembly is in
water systems in which pure water or ultrapure water is
to be sterilized and rendered free of algae, in
particular. In this case, the water system may comprise
pipelines, collection tanks, open baths etc.
Electrode assemblies of the type mentioned initially
are used, in particular, to disinfect rainwater, to
disinfect ultrapure water circuits in the semiconductor
industry and pharmaceuticals industry, to remove
organic pollutants in rinse water, to purify water for
the food industry and cosmetics industry, and in all
types of industrial cooling-water circuits in order to
prevent the growth of algae or the growth of bacteria
or, in the case of high levels of contamination, to
reduce the latter.
Such an electrode assembly can be used to generate
oxidizing agents which oxidize germs and thus destroy
or inactivate them.
The electrochemical generation of oxidizing agents has
the advantage that it is possible, in principle, to
adapt to the respective application. A large amount of
oxidizing agent is thus required when a water system
has already been contaminated with algae or
biologically affected and is intended to be purified
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 2 -
and disinfected. In contrast, once this operation has
been concluded, the water system can be permanently
kept in the disinfected and purified state, for which
only a small amount of oxidizing agent is required from
time to time.
A varying amount of oxidizing agent is also required
when a water system has a high organic load as a result
of an accident. A similar situation applies to the
operation of filling a tank, in which a high level of
oxidizing agent production is initially required in
order to effect initial disinfection, while only
relatively small amounts of oxidizing agent are then
sufficient to maintain the disinfected state.
In principle, electrochemical methods are suitable for
satisfying the different demands imposed on the
production of oxidizing agents since the production of
oxidizing agents can be controlled by supplying
current.
In order to treat liquids with a low conductivity, for
example ultrapure water, it is necessary, on account of
the high resistance of the water, to use high voltages
in order to generate the current densities required for
the production of the oxidizing agents. This problem is
partly solved by using polymeric solid electrolytes
which, preferably in the form of a membrane having a
thickness of a few tenths of a millimeter to a few
millimeters, bridge the gap between the electrodes on
the basis of their ion conductivity and are suitable as
an interlayer between the electrodes in order to avoid
a short circuit . On account of the relatively good ion
conductivity of the polymeric solid electrolyte, the
electrical potential of one electrode is brought very
close to the other electrode, a film of water which is
thus exposed to high current densities being situated
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 3 -
between the surface of the polymeric solid electrolyte
and the directly adjacent electrode.
Such electrode assemblies have been implemented for
decades in the same manner in principle using the
design of a "Fischer cell". In this case, a pressure-
exerting device which is formed from a surrounding
housing is used to press the flat electrodes flat
against the membrane which is situated between the
electrodes and comprises a polymeric solid electrolyte.
A sufficient contact pressure is produced by screwing
flat pressure plates of the housing, which must take
place with a minimum torque.
On account of the high level of required stability of
the pressure plates of the housing, the construction of
such a cell is high and necessitates involved handling.
In addition, adaptation to higher throughputs is
problematic since the effective electrode area of the
cell would have to be enlarged for this purpose or the
flow of liquid would have to be divided up and passed
through a plurality of cells.
The Fischer cells were originally constructed using
lead oxide electrodes . In this case, the use of a lead
oxide anode has the further disadvantage that the
electrode decomposes in water if it is not held at a
protective potential. The use of an electrode assembly
having a lead oxide anode is therefore possible only
during continuous operation, with the result that the
option of using the corresponding cell only when
required does not apply.
DE 100 25 167 A1, for example, discloses the practice
of using an electrode through which a liquid can flow
on account of numerous groove-shaped channels and which
has a surface comprising a doped diamond layer. Such
electrodes have likewise been arranged in a cell
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 4 -
constructed like a Fischer cell (cf. DE 295 04 323 U1).
The associated handling disadvantages have been
accepted for decades by those skilled in the art as
being unalterable.
The present invention is based on the object of
designing an electrode assembly of the type mentioned
initially in such a manner that it enables an effective
design of a corresponding electrolysis cell and
nevertheless is simple to construct and handle.
In order to achieve this object, an electrode assembly
of the type mentioned initially is characterized,
according to the invention, in that the pressure-
exerting device is supported on the electrodes.
The electrode assembly according to the invention thus
does not require a special housing arrangement with
complicated pressure plates to press the electrodes
against the polymeric solid electrolyte used between
the electrodes but rather merely requires a pressure-
exerting device which is directly connected to the
electrodes and obtains the contact-pressure force from
the rather relatively low mechanical stability of the
electrodes. The invention is based on the knowledge
that an effective electrode assembly - in contrast to
the idea of those skilled in the art which has existed
for decades - can be implemented even without a very
high force pressing the electrodes against the
polymeric solid electrolyte. For suitable electrodes,
it is sufficient if only a certain relatively low
contact-pressure force of the electrodes is exerted on
the polymeric solid electrolyte, so that the
corresponding contact-pressure force does not have to
be generated in a complicated manner using specially
constructed housing parts but rather can be directly
exerted on the electrodes themselves in a simple
manner.
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 5 -
It is thus possible, for example, to use an expanded
metal grid which is coated, for example, with a doped
diamond layer as the support material for an electrode.
A plastic screw can be inserted through the grid
openings in the expanded metal grid until the head of
the plastic screw rests against the electrode. The two
electrodes can then be clamped in the direction of the
polymeric solid electrolyte by screwing a nut onto the
screw bolt which projects through the two electrodes
and the solid electrolyte lying in between.
In this case, an intensive flow through the electrode
assembly can be ensured by the polymeric solid
electrolyte, which is preferably in the form of a
membrane, also having through-flow openings. It is also
possible to ensure the flow through the interspace
between the electrodes by virtue of the polymeric solid
electrolyte being arranged in strips, which are at a
distance from one another, in the interspace between
the electrodes. In one development of this idea, the
polymeric solid electrolyte may also be arranged in
area pieces, which are at a distance from one another
on all sides, in the interspace, so that the ability to
flow through the interspace in different directions is
ensured.
The polymeric solid electrolyte may be inserted between
the electrodes in the form of a membrane. However, when
designed, in particular, in the form of area pieces
which are at a distance from one another on all sides,
it will be expedient if the polymeric solid electrolyte
is applied to one of the electrodes as a surface layer.
Since the electrode assembly according to the invention
does not require any complicated generation of contact
pressure, it is readily possible to use the electrode
assembly to construct a stack which enables an
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 6 -
effective electrolysis device even for relatively high
flow rates. Since the pressure-exerting device is
supported on the electrodes themselves, it is readily
possible to arrange numerous electrodes, with a
polymeric solid electrolyte arranged between them, to
form a stack. In this case, it is particularly
expedient if the electrodes are provided with the aid
of contact lugs, which project over their joint area,
for the purpose of electrical contact-connection. In
this case, the contact lugs of the anodes in the stack,
on the one hand, and of the cathodes in the stack, on
the other hand, may be formed such that they are
aligned with one another in order to simplify joint
contact-connection, for example by means of a contact
rod which is inserted through openings in the contact
lugs.
The electrode assembly according to the invention also
allows departure from the hitherto customary flat
electrodes in a surprisingly simple manner. It is thus
possible, for example, to design two electrodes in the
form of rods and to realize the polymeric solid
electrolyte between the electrodes by virtue of the
solid electrolyte in the form of a strip alternately
wrapping around the electrodes under prestress. In this
case, the strip may be fitted in such a manner that it
respectively wraps around the two electrodes in the
shape of a figure of eight, the wrapping-around process
taking place with a certain amount of prestress in
order to ensure intimate contact. The two electrodes
may be pressed against the strip sections of the
polymeric solid electrolyte between the electrodes
using, for example, a material which is in the form of
a wire, is wrapped around the electrodes and has ends
which are twisted together in order to generate the
pressure. In this case, the material which is in the
form of a wire may preferably be an insulating material
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
or may rest against the electrodes via. an insulating
layer.
The invention shall be explained in more detail below
with reference to exemplary embodiments which are
illustrated in the drawing, in which:
figure 1 shows a diagrammatic illustration of two
electrodes and a membrane which is arranged
between the latter and comprises a solid
electrolyte,
figure 2 shows a stack which is formed using the
assembly according to figure 1,
figure 3 shows a perspective illustration of the stack
according to figure 2,
figure 4 shows another embodiment of two electrodes
with a solid electrolyte in the form of
strips which are arranged parallel to one
another,
figure 5 shows a plan view of a stack which is formed
using the assembly according to figure and in
which each electrode is contact-connected,
figure 6 shows a stack which is formed using the
assembly according to figure 4 with only the
outer electrodes being contact-connected,
figure 7 shows a variant of the assembly according to
figure 4, in which the electrode plates are
provided with through-openings in the form of
slots,
figure 8 shows a stack which is formed using the
assembly according to figure 7,
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
g _
figure 9 shows an assembly comprising two electrodes,
one of which is coated with applied area
sections of the polymeric solid electrolyte
on its surface which faces the other
electrode,
figure 10 shows a stack which is formed using the
assembly according to figure 9,
figure 11 shows a perspective illustration which is
similar to figure 3 with contact lugs on the
electrodes which are polarized differently,
figure 12 shows a diagrammatic illustration of a
treatment cell which has been charged with an
electrode stack, and
figure 13 shows a view of an electrode assembly having
two electrodes in the form of rods.
Figure 1 shows two electrodes 1, 2 in the form of
expanded metal grids 11, 21. A first electrode 1 is
used as a cathode, while the second electrode 2 acts as
an anode. The two electrodes 1; 2 are flat, have a
rectangular cross section and have the same area shape.
A polymeric solid electrolyte 3 in the form of a
membrane 31 whose area corresponds to the area of the
electrodes 1, 2 is situated between the two electrodes
1, 2. The membrane 31 is provided with a respective
passage opening 4 in its four corner regions. The
membrane has a thickness of between 0.4 and 0.8 mm, for
example.
Outside the rectangular area of the expanded metal
grids 11, 21, the electrodes 1, 2 are each provided
with a contact lug 5, 6 which projects out of the area.
Both contact lugs have a passage opening 7, 8.
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 9 -
Figure 2 illustrates that the electrodes l, 2 which are
formed from the expanded metal grids 11, 21 and have a
respective solid electrolyte 3 between them are pressed
against one another using a clamping device 9, the
clamping device 9 extending over four electrode
assemblies 1, 2, 3 which have been joined to form a
stack. Clamping is effected using nuts 10 which can be
clamped, against the electrodes 1, 2, on the threaded
bolt 9.
According to figure 1, provision is made of four
threaded bolts 9 which are inserted through interspaces
in the expanded metal grids 11, 21 and through the
passage openings 4 in the polymeric solid electrolyte
3.
Figure 3 illustrates, in a perspective illustration,
that the electrodes 1, 2 are each connected to
different poles of the supply voltages. In the
exemplary embodiment illustrated in figures 1 to 3, the
electrodes 1, 2 are formed with a support in the form
of an expanded metal grid 11, 21 and are coated with a
doped diamond layer. It is also possible to apply
supply voltages of different magnitudes to the
electrodes 1, 2.
Figure 4 shows a modified exemplary embodiment in which
the electrodes 1, 2 are formed with metal plates 12, 22
that are coated with a doped diamond layer. The
electrodes have passage openings 41 in their corner
regions, through which threaded bolts 9 can be inserted
in the manner described with reference to figures 2 and
3.
In this exemplary embodiment, the polymeric electrolyte
3 is formed from vertical strips 32 which are arranged
parallel to, and at a distance from, one another. The
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 10 -
plan view in figure 5 illustrates that a flow can pass
through the electrode assemblies in the formed stack
perpendicular to the plane of the drawing on account of
the strips 32.
The stack assembly illustrated in figure 6 comprises
four identical electrodes 1 which are separated from
one another by a respective solid electrolyte 3 in the
form of the strips 32 here. In this case, contact-
connection is effected with different polarities only
at the two outer electrodes 1, as a result of which the
middle electrodes assume correspondingly stepped
potentials. Such an assembly in which the middle
electrodes act both as an anode (to one side) and as a
cathode is also referred to as a bipolar assembly.
The exemplary embodiment illustrated in figure 7
differs from the exemplary embodiment according to
figure 4 only by virtue of the fact that with metal
plates 13, 23 are used as the supports for the
electrodes 1, 2, said metal plates being provided with
horizontal passage openings 42 which are in the form of
slots and make it possible for a flow to pass through
the electrodes 1, 2. Accordingly, the arrows in figure
8 show that, in addition to the vertical f low
(perpendicular to the plane of the drawing), a flow can
pass through the electrode assemblies in the stacking
direction.
In the exemplary embodiment illustrated in figure 9,
the polymeric solid electrolyte 3 is applied, in the
form of circular area sections 33, to the surface of
the second electrode 2 which faces the first electrode
1. The polymeric electrolyte 3 is thus directly
laminated to the electrode 2. The plan view of a
multiple electrode assembly in figure 10 shows that a
flow can pass through the interspace between the
electrodes 1, 2 horizontally and vertically since the
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 11 -
area sections 33 are at a distance from one another on
all sides, thus resulting in through-flow regions in
the gaps.
Figure 11 shows an enlarged diagrammatic illustration
of the contact-connection of the electrodes 1, 2 using
the contact lugs 5, 6 and the passage openings 7, 8
situated in the latter. The contact lugs 5, 6 of the
respective identically polarized electrodes 1, 2 are
aligned with one another (figure 11 depicts contact
lugs 5, 6 only for the two rear electrodes l, 2 of the
stack). The contact lugs 5 of the first electrodes 1
can be contact-connected to one another by means of a
contact bolt (not illustrated), which is inserted
through the passage openings 7 which are aligned with
one another, and can thus be jointly connected to a
pole of the supply voltage . The other electrodes 2 are
contact-connected in the same manner using the contact
lugs 6 and the passage openings 8 which are situated in
the latter and are aligned with one another.
Figure 12 illustrates the structure of a treatment cell
100, only the anodes 2 of the electrode assemblies,
which are contact-connected using their contact lugs 5
which are aligned with one another, being illustrated
for the sake of clarity. The cell 100 has a housing 101
having an inlet opening 102 for the water to be
purified. In the housing 101, the water to be purified
flows into the region of the electrodes 2 from bottom
to top and emerges from the region of the electrodes 2
at the side in order to leave the housing 101 in
purified form via the outlet openings 103. Ventilation
slots 104 are situated in the upper region of the
housing 101.
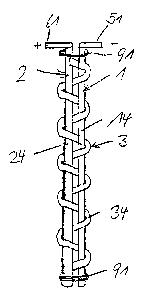
Figure 13 shows a different arrangement of the
electrodes 1, 2 which are designed as electrodes 14, 24
in the form of rods in this exemplary embodiment. The
CA 02560910 2006-09-22
WO 2005/095282 PCT/DE2005/000556
- 12 -
solid electrolyte 3 which, in the form of a long strip
34, form the shape of a "figure of eight" in a
meandering fashion is wound around the electrodes 1, 2
with prestress, so that the strip 34 already pulls the
electrodes l, 2 against one another, is used as a
spacer between the electrodes 1, 2. The electrodes are
pressed against one another and against the sections of
the solid electrolyte 3 between them using two loops 91
of an insulating material in the form of a wire which
are placed around the electrodes 1, 2 and can be pulled
together using twisted ends in order to thus pull the
electrodes 1, 2 against one another.
The electrodes 1, 2 are contact-connected at ends using
contact pieces 51, 61. Such a design of the electrode
assembly is particularly suitable for purifying water
in tube systems.