Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 292
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 292
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE:
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
PGD2 RECEPTOR ANTAGONISTS FOR THE TREATMENT OF INFLAMMATORY DISEASES
BACKGROUND OF THE INVENTION
[0001] CRTH2 is a G protein-coupled chemoattractant receptor expressed on Th2
cells (Nagata
et al., J. Immuraol., 1999, 162, 1278-1286), eosinophils, and basophils (Hirai
et al., J. Exp. Med.,
2001, 193, 255-261). Prostaglandin D2 (PGD2) is a natural ligand for CRTH2,
and is the major
inflammatory mediator produced from mast cells. It has been shown that
activation of CRTH2 by
PGD2 induces migration and activation of Th2 cells (Hirai et al., J. Exp. Med.
2001, 193, 255-261;
Gervais et al., J. Allergy Clin. Irnrnunol. 2001, 108, 982-988) which in turn
are involved in the
orchestration of an allergic inflammatory response by directly or indirectly
inducing migration,
activation, priming and prolonged survival of effector cells, such as
eosinophils and basophils (Sanz
et al., J. Imrnunol. 1998, 160, 5637-5645; Pope et al., J. Allergy Clin.
Immunol. 2001, 108, 594-601;
Teran L. M., Clin. Exp. Allergy 1999, 29, 287-290). The role of PGD2 in the
initiation and
maintenance of allergic inflammation has also been demonstrated in mouse
models of asthma by
showing that overproduction of PGD2 in vivo by PGD2 synthase exacerbates
airway inflammation
(Fujitani et al., J. Immunol. 2002,168, 443-449).
[0002] Accordingly, compounds which are modulators, preferably inhibitors, of
the interaction
between CRTH2 and PGD2 should be useful for the treatment of diseases and
disorders that are
mediated by CRTH2, PGD2, Th2 cells, eosinophils, and/or basophils. These
diseases include but are
not limited to allergic disorders, asthmatic disorders, and inflammatory
disorders such as allergic
rhinitis, allergic asthma, bronchoconstriction, atopic dermatitis and systemic
inflammatory disorders.
DETAILED DESCRIPTION OF THE INVENTION
[0003] Compounds of this invention, and pharmaceutically acceptable
compositions thereof, are
effective as inhibitors of the interaction between CRTH2 and its natural
ligand PGD2. Thus,
compounds of the invention and pharmaceutical compositions thereof are useful
for treating
inflammatory disorders and/or disorders with an inflammatory component.
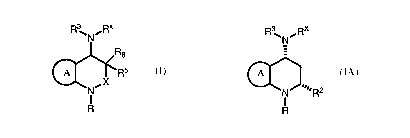
[0004] 1. Description of Compounds of Ge~aeral Forinula 1 (and subsets
thereo,~:
[0005] In one embodiment, the present invention relates to a compound of
formula I:
R \N~Rx
Rs
~ ~Rs
,X
N
I
R
I
-1-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
or a pharmaceutically acceptable salt thereof, wherein:
Ring A is an optionally substituted monocyclic aromatic ring;
R is -XI-R', wherein:
Xl is a bond, S(O), S(O)2, C(O) or C(O)NH, provided that when Xl is a bond,
SO or SO2, then R' is not H; and
R' is H or an optionally substituted, cycloaliphatic group, aromatic group or
non-
aromatic heterocyclic group;
X is -C(O)- or -C(RZ)Z-, wherein:
each RZ is independently -H, -Xa-R$ or an optionally substituted, aliphatic
group, cycloaliphatic group, aromatic group or a non-aromatic heterocyclic
group;
R" is -XZ-R4, wherein:
XZ is a bond, S(O), S(O)2, C(O) or C(O)NH; and
R4 is -H, -X6-R'° or an optionally substituted, aliphatic group,
cycloaliphatic
group, aromatic group or non-aromatic heterocyclic group;
provided that when XZ is a bond, SO or SOZ, then R4 is not H;
R3 is an optionally substituted, cycloaliphatic group, aromatic group or non-
aromatic
heterocyclic group; or -NR"R3, taken together, is an optionally substituted
non-aromatic
nitrogen containing heterocyclic group;
X4 and X6 are each independently a straight or branched hydrocarbyl group
optionally
substituted with one or more groups selected from the group consisting of
halo, -OH, =O, Cl-C3 alkoxy,
nitro and cyano;
RS and R6 are each independently H or Cl-C3 alkyl;
R8 and R'° are each independently H, -C(O)OR" or an optionally
substituted, cycloaliphatic
group, aromatic group or non-aromatic heterocyclic group;
R" is H or R'3; and
R'3 is CI-C6 alkyl or C3-C8 cycloalkyl.
[0006] In another embodiment, the present invention relates to a compound of
formula I:
or a pharmaceutically acceptable salt thereof, wherein:
Ring A is an optionally substituted monocyclic aromatic ring;
R is -Xl-R';
R" is -XZ-R4, and R3 is an optionally substituted aromatic group; or -NR"R3,
taken together, is
an
optionally substituted non-aromatic nitrogen containing heterocyclic group;
X is -C(O)- or -C(RZ)a-;
-2-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Xl and Xz are each independently a bond, S(O), S(O)2, C(O) or C(O)NH;
R' is H or an optionally substituted, cycloaliphatic group, aromatic group or
non-aromatic
heterocyclic group;
provided that when Xl is a bond, SO or SOz, then Rl is not H;
each RZ is independently H, -X4-R8 or an optionally substituted, aliphatic
group, cycloaliphatic
group, aromatic group or non-aromatic heterocyclic group;
R4 is H, -X~-R'° or an optionally substituted, aliphatic group,
cycloaliphatic group, aromatic
group
or non-aromatic heterocyclic group;
provided that when XZ is a bond, SO or SO2, then R4 is not H;
X4 and X6 are each independently a straight or branched hydrocarbyl group
optionally
substituted
with one or more groups selected from the group consisting of halo, -OH, =O,
Cl-C3 alkoxy, nitro and
cyano;
RS and R6 are each independently H or C~-C3 alkyl; and .
R$ and R'° are each independently H, -C(O)OR" or an optionally
substituted, cycloaliphatic
group, aromatic group or non-aromatic heterocyclic group;
R" is H or R13; and '
R'3 is Cl-C6 alkyl or C3-C$ cycloalkyl.
[0007] In one embodiment, compounds of formula I include compounds other than:
2-Methyl-
N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(2-methyl-1-oxobutyl)-4-quinolinyl]-
butamide; N-(1-
Acetyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-phenyl-heptamide; N-phenyl-
N-[1,2,3,4-
tetrahydro-2-methyl-1-(1-oxo-3-phenylpropyl)-4-quinolinyl]-
benzenepropanamide; N-phenyl-N-
[1,2,3,4-tetrahydro-2-methyl-1-(3-nitrobenzoyl)-4-quinolinyl]- hexanamide; N-
[l,l'-biphenyl]-3-yl-
N-[1,2,3,4-tetrahydro-1-(4-methoxybenzoyl)-2-methyl-4-quinolinyl]-acetamide; N-
(1-benzoyl-
1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-(4-nitrophenyl)- heptanamide; N-(1-
benzoyl-1,2,3,4-
tetrahydro-2-methyl-4-quinolinyl)-N-(4-methoxyphenyl)-2-methyl- propanamide; N-
[1-(4-
fluorobenzoyl)-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl- butanamide;
N-phenyl-N-
[1,2,3,4-tetrahydro-1-(3-methoxybenzoyl)-2-methyl-4-quinolinyl]-pentanamide; 2-
ethyl-N-[1-(2-
ethyl-1-oxobutyl)-1,2,3,4-tetrahydro-2,8-dimethyl-4-quinolinyl]-N-(2-
methylphenyl)-butanamide; N-
[1-[(4-fluorophenyl)acetyl]-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-
propanamide; . N-
phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(4-nitrobenzoyl)-4-quinolinyl]-
octanamide; N-cyclohexyl-4-
[(cyclohexylamino)carbonyl]phenylamino]-3,4-dihydro-2-methyl-1(2H)-
quinolinecarboxamide; N-[1-
(4-ethylbenzoyl)-1,2,3,4-tetrahydro-2,8-dimethyl-4-quinolinyl]-N-(2-
methylphenyl)-3-(4-
nitrophenyl)- 2-propenamide; 3-(4-methoxyphenyl)-N-phenyl-N-[1,2,3,4-
tetrahydro-1-[3-(4-
-3-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
methoxyphenyl)-1-oxo-2-propenyl]-2-methyl-4-quinolinyl]-2-propenamide; 4-
[(ethoxyoxoacetyl)phenylamino]-3,4-dihydro-2-methyl-d-oxo-ethyl ester-1(2H)-
quinolineacetic acid;
N-[1-(3-cyclohexyl-1-oxopropyl)-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-
phenyl-
cyclohexanepropanamide; 4-(acetylphenylamino)-3,4-dihydro-2-methyl-gamma-oxo-
1(2H)-
quinolinepentanoic acid; N-(1-benzoyl-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-2,2-dimethyl-N-
phenyl- propanamide; N-(1-benzoyl-6-bromo-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-N-phenyl-
pentanamide; N-[1-(2-furanylcarbonyl)-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl]-N-phenyl-
acetamide; 2-methyl-N-phenyl-N-[1,2,3,4-tetrahydro-1-(3-methoxybenzoyl)-2-
methyl-4-quinolinyl]-
propanamide; N-[1-[(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)acetyl]-1,2,3,4-
tetrahydro-2-methyl-4-
quinolinyl]-N-phenyl-acetamide; 2,2,2-trifluoro-N-phenyl-N-[1,2,3,4-tetrahydro-
1-(3-
methoxybenzoyl)-2-methyl-4-quinolinyl]- acetamide; 2-ethyl-N-[1-(2-ethyl-1-
oxobutyl)-1,2,3,4-
tetrahydro-2-methyl-4-quinolinyl]-N-phenyl- butanamide; N-(1-benzoyl-1,2,3,4-
tetrahydro-2-methyl-
4-quinolinyl)-N-(3-methoxyphenyl)- acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-
methyl-1-(1-
oxohexyl)-4-quinolinyl]- acetamide; N-(1-acetyl-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-N-phenyl-
2-thiophenecarboxamide; N-[1-(2-fluorobenzoyl)-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl]-N-
phenyl- hexanamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(4-methoxybenzoyl)-2-
methyl-4-quinolinyl]-
hexanamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(4-methoxybenzoyl)-2-methyl-4-
quinolinyl]-
hexanamide; N-[1-(cyclopropylcarbonyl)-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl]-N-phenyl-
cyclopropanecarboxamide; N-(1-benzoyl-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-N-(4-
methylphenyl)- acetamide; 2-methyl-N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-
(2-methyl-1-
oxopropyl)-4-quinolinyl]- propanamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(4-
methoxybenzoyl)-2-
methyl-4-quinolinyl]- 2-thiophenecarboxamide; 1-(3,5-dinitrobenzoyl)-N-formyl-
1,2,3,4-tetrahydro-
2-methyl-N-phenyl-4-quinolinamine; N-[1-(4-chloro-3-nitrobenzoyl)-1,2,3,4-
tetrahydro-2-methyl-4-
quinolinyl]-N-phenyl- acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(3-
nitrobenzoyl)-4-
quinolinyl]-acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(3-methoxybenzoyl)-2-
methyl-4-
quinolinyl]- hexanamide; N-[1-(2-furanylcarbonyl)-1,2,3,4-tetrahydro-2-methyl-
4-quinolinyl]-N-
phenyl-2-furancarboxamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(1-
oxopropyl)-4-
quinolinyl]-acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-[3-(4-methoxyphenyl)-1-
oxo-2-propenyl]-2-
methyl-4-quinolinyl]-acetamide; 3-(2-furanyl)-N-[1-[3-(2-furanyl)-1-oxo-2-
propenyl]-1,2,3,4-
tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-2-propenamide; N-[1-[2-(1,3-dihydro-
1,3-dioxo-2H-
isoindol-2-yl)-1-oxo-3-phenylpropyl]-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-
N-phenyl-
octanamide; N-[1-(3-chlorobenzoyl)-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-
phenyl-acetamide;
Relative stereochemistry N-phenyl-N-[(2R,4S)-1,2,3,4-tetrahydro-2-methyl-1-(1-
oxopropyl)-4-
quinolinyl]- acetamide; Relative stereochemistry N-[(2R,4S)-1-benzoyl-1,2,3,4-
tetrahydro-2-methyl-
4-quinolinyl]-2-methyl-N-phenyl-propanamide; Relative stereochemistry N-
[(2R,4S)-1-acetyl-
1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-hexanamide; Relative
stereochemistry N-
-4-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[(2R,4S)-1-acetyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-
propanamide; Relative
stereochemistry N-[(2R,4S)-1-acetyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-
N-phenyl-
heptanamide; Relative stereochemistry N-[(2R,4S)-1-benzoyl-1,2,3,4-tetrahydro-
2-methyl-4-
quinolinyl]-2,2-dimethyl-N-phenyl-propanamide; N-[1-(3-fluorobenzoyl)-1,2,3,4-
tetrahydro-2-
methyl-4-quinolinyl]-N-phenyl-acetamide; N-[1-[4-(1,1-dimethylethyl)benzoyl]-
1,2,3,4-tetrahydro-2-
methyl-4-quinolinyl]-N-phenyl- acetamide; N-(1-acetyl-1,2,3,4-tetrahydro-2-
methyl-4-quinolinyl)-2-
methyl-N-phenyl- propanamide; 2,2,2-trifluoro-N-phenyl-N-[1,2,3,4-tetrahydro-2-
methyl-1-
(trifluoroacetyl)-4-quinolinyl]- acetamide; Relative stereochemistry N-
[(2R,4S)-1-acetyl-1,2,3,4-
tetrahydro-2-methyl-4-quinolinyl]-2,2-dimethyl-N-phenyl-propanamide; Relative
stereochemistry N-
[(2R,4S)-1-acetyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-
butanamide; Relative
stereochemistry N-[(2R,4S)-1-benzoyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-
N-phenyl-
acetamide; Relative stereochemistry N-phenyl-N-[(2R,4S)-1,2,3,4-tetrahydro-2-
methyl-1-(1-
oxoheptyl)-4-quinolinyl]-acetamide; Relative stereochemistry N-phenyl-N-
[(2R,4S)-1,2,3,4-
tetrahydro-2-methyl-1-(1-oxohexyl)-4-quinolinyl]-acetamide; Relative
stereochemistry N-[(2R,4S)-1-
acetyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-N-phenyl-pentanamide; N-
phenyl-N-[1,2,3,4-
tetrahydro-2-methyl-1-(1-oxo-3-phenyl-2-propenyl)-4-quinolinyl]-acetamide;
Relative
stereochemistry N-[(2R,4S)-1-benzoyl-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl]-
N-phenyl-
heptanamide; Relative stereochemistry N-[(2R,4S)-1-acetyl-1,2,3,4-tetrahydro-2-
methyl-4-
quinolinyl]-N-phenyl-acetamide; Relative stereochemistry N-[(2R,4S)-1-benzoyl-
1,2,3,4-tetrahydro-
2-methyl-4-quinolinyl]-N-phenyl-pentanamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-
methyl-1-
(tricyclo[3.3.1.13,7]dec-1-ylcarbonyl)-4-quinolinyl]-acetamide; N-phenyl-N-
[1,2,3,4-tetrahydro-2-
methyl-1-(1-oxopropyl)-4-quinolinyl]- propanamide; N-phenyl-N-[1,2,3,4-
tetrahydro-2-methyl-1-(2-
thienylcarbonyl)-4-quinolinyl]- acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(4-
methoxybenzoyl)-2-
methyl-4-quinolinyl]- 2-furancarboxamide; N-phenyl-N-[1;2,3,4-tetrahydro-1-(4-
methoxybenzoyl)-2-
methyl-4-quinolinyl]- acetamide; N-[1-(3,5-dinitrobenzoyl)-1,2,3,4-tetrahydro-
2-methyl-4-
quinolinyl]-N-phenyl-acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(4-
nitrobenzoyl)-4-
quinolinyl]-acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(2-iodobenzoyl)-2-
methyl-4-quinolinyl]-
acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-(2-methyl-1-oxopropyl)-4-
quinolinyl]-
acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-[(4-
methylphenyl)sulfonyl]-4-quinolinyl]-
acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-2-methyl-1-[(4-nitrophenyl)methyl]-4-
quinolinyl]-
acetamide; N-phenyl-N-[1,2,3,4-tetrahydro-1-(3-methoxybenzoyl)-2-methyl-4-
quinolinyl]-
acetamide; N-(1-acetyl-1,2,3,4-tetrahydro-Z-methyl-4-quinolinyl)-N-phenyl-
butanamide; N-phenyl-
N-[1,2,3,4-tetrahydro-2-methyl-1-(1-oxobutyl)-4-quinolinyl]- acetamide; N-(1-
benzoyl-1,2,3,4-
tetrahydro-2-methyl-4-quinolinyl)-N-phenyl-hexanamide; N-(1-benzoyl-1,2,3,4-
tetrahydro-2-methyl-
4-quinolinyl)-N-phenyl-pentanamide; N-(1-benzoyl-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-N-
phenyl-propanamide; 1-benzoyl-1,2,3,4-tetrahydro-4-(N-
phenylacetamido)quinaldine; N-(1-acetyl-6-
-5-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
bromo-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-phenyl-acetamide; N-(1-
acetyl-1,2,3,4-
tetrahydro-2-methyl-6-nitro-4-quinolyl)-acetanilide; N-(1-acetyl-6-chloro-
1,2,3,4-tetrahydro-2-
methyl-4-quinolyl)-acetanilide; N-(1-acetyl-1,2,3,4-tetrahydro-2-methyl-4-
quinolinyl)-N-phenyl-
acetamide; N-(1-benzoyl-6-bromo-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-
phenyl-acetamide; N-
(1-benzoyl-6-chloro-1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-phenyl-
acetamide; N-(1-benzoyl-
1,2,3,4-tetrahydro-2-methyl-4-quinolinyl)-N-phenyl- butanamide; N-phenyl-N-
[1,2,3,4-tetrahydro-1-
(3-fluorobenzoyl)-2-methyl-4-quinolinyl]-hexanamide. N-[1-(3-Chloro-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide; N-[1-(4-Fluoro-benzoyl)-2-methyl-
6-nitro-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide; Pentanoic.acid (1-benzoyl-6-
bromo-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-phenyl-amide; N-(1-Benzoyl-6-chloro-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl)-N-phenyl-acetamide; N-[6-Chloro-1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide; N-[6-Bromo-1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide; N-(1-Benzoyl-6-nitro-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-
N-phenyl-acetamide; N-(1-Benzoyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-butyramide;
or N-[1-(3-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-2,2-
dimethyl-N-phenyl-
propionamide.
[0008] In a preferred embodiment of the present invention, X is' -CHRZ-, RZ is
-H, methyl or
ethyl; R3 is a substituted or unsubstituted aromatic group; RS and R6 are -H;
and the remainder of the
variables in Structural Formula (n are as defined above. More preferably, the
compound is
represented by a structural formula selected from Structural Formulas (II)-
(VIII):
R'
(In (IIn (IV)
-6-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
R'
(vn
Ra
(VII) (VIII)
[0009] The. variables in Structural Formulas (In-(VIIn are as described above
for Structural
Formula (n. Preferred values for these variables are provided below.
[0010] Phenyl Ring A is a substituted or unsubstituted phenyl group. Unless
otherwise indicated
suitable substituents for Phenyl Ring A are provided in the section below
describing suitable aryl ring
substituents.
[0011] R' in Structural Formulas (II)-(I~ and (VI)-(VIII) is -H, optionally
substituted,
cycloaliphatic group, aromatic group or non-aromatic heterocyclic group,
provided that R' in
Structural Formulas (III) is not -H.
[0012] Rz in Structural Formulas (II)-(VIII) is -H, methyl or ethyl.
[0013] R3 in Structural Formulas (II)-(VIII) is an optionally substituted
phenyl group.
[0014] R4 in Structural Formulas (II)-(Vn and (VIII) is -H, -CHzC(O)R'4, -
CH2R15, -CHZOR'4
or an optionally substituted CI-C3 alkyl group or an optionally substituted
cycloalkyl group, aromatic
group or non-aromatic heterocyclic group, provided that R4 in Structural
Formula (VI) is not -H; and
R4 in Structural Formulas (VIn is -(CH2)ri R'3°.
[0015] R'3° is -H, -CHzC(O)R'4, -CHZR'S, -CHZOR'4 or an optionally
substituted Cl-C3 alkyl
group or an optionally substituted cycloalkyl group, aromatic group or non-
aromatic heterocyclic
group.
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0016] Each R'4 is independently an -H or an optionally substituted alkyl
group, aromatic group,
cycloalkyl group or non-aromatic heterocyclic group.
[0017] Each R'S is independently an optionally aromatic group, cycloalkyl
group or non-aromatic
heterocyclic group.
[0018] n is 0, 1, 2 or 3.
[0019] More preferred values for R', R4 arid R'3a in Structural Formulas (In-
(VIII) are R' and
R'3a are an optionally substituted, phenyl, pyridyl, furanyl, thiophenyl,
isoxazolyl, imidazolyl,
pyrazolyl, pyrrolyl, benzofuranyl, tetrazolyl, thiazolyl, benzyl,
benzothiazolyl, benzoimidazolyl,
benzotriazolyl, benzomorpholinyl, benzopyrazolyl, indolyl, -CHZ-(N pyridyl), -
CHZ-furanyl,
-CHZ-thiophienyl, -CH2-isoxazolyl, -CHz-imidazolyl, -CHZ-pyrazolyl, -CHZ-
pyrollyl,
-CHZ-benzofuranyl, -CHZ-tetrazolyl, -CHZ-thiazolyl, -CHZ-tetrazolyl, . -CHz-
benzothiazolyl,
-CHZ-benzimidazolyl, -CHz-O-phenyl, -CHzC(O)-phenyl, naphthalimidyl,
tetrahydrofuranyl,
cyclohexyl, cyclopentyl or cyclopropyl group; and R4 is Cl-C4 alkyl, -CH20H, -
CHZOCH3,
-CHZOCHZCH3, -CHZCHzOCH3, -CHzCH20CH2CH3 or an optionally substituted, phenyl,
pyridyl,
furanyl, thiophenyl, isoxazolyl, imidazolyl, pyrazolyl, pyrrolyl,
benzofuranyl, tetrazolyl, benzyl,
benzothiazolyl, benzoimidazolyl, benzotriazolyl, benzomorpholinyl,
benzopyrazolyl, indolyl,.
-CHZ-(N-pyridyl), -CHZ-furanyl, -CHZ-thiophienyl, -CHZ-isoxazolyl, -CHZ-
imidazolyl,
-CHZ-pyrazolyl, -CHZ-pyrollyl, -CHZ-benzofuranyl, -CHZ-tetrazolyl, -CHz-
thiazolyl, -CHz-tetrazolyl,
-CHZ-benzothiazolyl, -CHz-benzimidazolyl, -CHZ-O-phenyl, -CHzC(O)-phenyl,
naphthalimidyl,.
tetrahydrofuranyl, cyclohexyl, cyclopentyl or cyclopropyl group, wherein R',
R4 and R'3 are
independently selected; and Ring A is optionally substituted at the five, six,
seven and/or the eight
position.
[0020] Even more preferably, the compounds in Structural Formulas (II)-(VIIn
have one of the
following features and preferably all of the following features: Phenyl Ring A
is optionally
substituted at the five, six, seven and/or eight position with R", wherein R"
is selected from
substituents provided in the section below describing suitable aryl ring
substituents unless otherwise
indicated herein.
[0021] R' is phenyl, thiophenyl, furanyl, pyridyl, oxazolyl, benzotriazole,
pyrimidinyl,
isoxazolyl or benzomorpholinyl, each group being optionally substituted with
R"; R3 is [R"]-phenyl;
and R4 is methyl, ethyl, propyl, iso-propyl, n-butyl, sec-butyl, tert-butyl, -
CHZOCH3 or
-CHZOCHaCH3.
[0022] Especially preferred are compounds represented by Structural Formulas
(II)-(VIIn
wherein Phenyl Ring A is optionally substituted at the six and/or seven
position with R"; R' is
thiophenyl, [R"]-thiophenyl, oxazolyl, [R"]-oxazolyl, pyridinyl, [R"]-
pyridinyl, benzotriazolyl,
[R"]-benzotriazolyl, benzomorpholinyl, [R"]-benzomorpholinyl, phenyl or phenyl
substituted with
_S-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
one to four groups selected from the group consisting of halo, -OR° and
-N(RI1)Z, [Rn]-oxazolyl,
N-Ri ~
oxazolyl and
[0023] R3 is phenyl substituted with one to four atoms or groups selected from
the group
consisting of Br, Cl, -CH3, -N(R16)2, -NHC(O)OR", -S(O)ZCH3, -S(O)zN(R'6)2 and
-R13C(O)N(R16)2,
where R16 is Cl-C6 alkyl.
[0024] In third preferred embodiment, Ring A in Structural Formulas (I) is a
monocyclic
heteroaryl group such as thiophene, furan, pyridine, pyrazole, pyrrole,
[2,3]pyrimidine,
[3,4]pyrimidine, [4,5]pyrimidine, [5,6]pyrimidine, oxazole, isoxazole or 1,2,3-
triazole, each group
being optionally substituted with Rl'. When Ring A has these values, then the
compound preferably
has at least one and preferably all of the following features: X is -CHRZ-, Rz
are -H, methyl or ethyl;
RS and R6 are -H; and R3 is a substituted or unsubstituted phenyl group. When
the compound has at
least one or all of these features, then preferably Rl and R4 are
independently -H, -CHZC(O)R'4,
-CHZR'S or -CHZOR'4 or an optionally substituted alkyl group, cycloalkyl
group, aromatic group or
non-aromatic heterocyclic group; and R'4 and R'S are as described above for
Structural Formula (In.
[0025] ' When Ring A in Structural Formula (I) is a monocyclic heteroaryl, as
described in the
preceding paragraph, commonly selected values for Xl and XZ are as follows: XI
and XZ are both
C(O); Xl is S(O)2 and XZ is C(O); Xl is C(O)NH and XZ is C(O); Xl is a bond
and XZ is C(O); and XZ
is C(O); Xl is C(O) and XZ is S(O)z; Xl is C(O) and; Xl is C(O) and Xz is a
bond; or Xl is C(O) and
XZ is C(O)NH. Alternatively, Phenyl Ring A in Structural Formulas (II)-(VIII)
is replaced with one
of the monocyclic aromatic groups described in the preceding paragraph and the
remainder of the
variables are as described above.
[0026] '~ In a fourth preferred embodiment, RZ in Structural Formulas (I)-
(VIII) is H, Cl-C4 alkyl,
halogentated Cl-C6 alkyl, C3-C$ cycloalkyl, substituted C3-C8 cycloalkyl,
phenyl, substituted phenyl, -
C(O)OR'6, benzyl, substituted benzyl or -(CHZ)"O(CHZ)m; Ris is Cl-C6 alkyl; n
and m are positive
integers such n+m=6; and the remainder of the variables are as described
above.
[0027] Also disclosed herein is a compound represented by Structural Formula
(II) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R' has the value corresponding to
any one of the
compounds in Table 1- 6 and R3 and R4 are as described above for Structural
Formula (II).
[0028] Also disclosed herein is a compound represented by Structural Formula
(In and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R3 has the value corresponding to
any one of the
compounds in Table 1- 6 and R' and R4 are as described above for Structural
Formula (II).
-9-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0029] Also disclosed herein is a compound represented by Structural Formula
(II) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R4 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R3 are as described above for Structural
Formula (II).
[0030] Also disclosed herein is a compound represented by Structural Formula
(III) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R3 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R4 are as described above for Structural
Formula (III).
[0031] Also disclosed herein is a compound represented by Structural Formula
(III) and methods
of use thereof for inhibiting CRTHZ in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R4 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R3 are as described above for Structural
Formula (IIn.
[0032] Also disclosed herein is a compound represented by Structural Formula
(III) and methods
of,use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R' has the value corresponding to
any one of the
compounds in Table 1 - 6 and R3 and R4 are as described above for Structural
Formula (IIn.
[0033] Also disclosed herein is a compound represented by Structural Formula
(IV) and methods
of use thereof, for inhibiting CRTH2 in a, subject in need ,of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R3 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R4 are as described above for Structural
Formula (IV).
[0034] Also disclosed herein is a compound represented by Structural Formula
(IV) and methods
of use thereof for inhibiting CRTH2, wherein R4 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R3 are as described above for Structural
Formula (IV).
[0035] Also disclosed herein is a compound represented by Structural Formula
(IV) and methods
of use thereof for inhibiting CRTH2, wherein R' has the value corresponding to
any one of the
compounds in Table 1 - 6 and R3 and R4 are as described above for Structural
Formula (IV).
[0036] Also disclosed herein is a compound represented by Structural Formula
(V) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R3 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R4 are as described above for Structural
Formula (V).
[0037] Also disclosed herein is a compound represented by Structural Formula
(V) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R4 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R3 are as described above for Structural
Formula (V).
-10-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0038] Also disclosed herein is a compound represented by Structural Formula
(V) and methods
of use thereof for inhibiting CRTfi2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R' has the value corresponding to
any one of the
compounds in Table 1 - 6 and R3 and R4 are as described above for Structural
Formula (V).
[0039] Also disclosed herein is a compound represented by Structural Formula
(VI) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R3 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R4 are as described above for Structural
Formula (VI).
[0040] Also disclosed herein is a compound represented by Structural Formula
(VI) and methods
of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R4 has the value corresponding to
any one of the
compounds in Table 1 - 6 and R' and R3 are as described above for Structural
Formula (VI).
[0041] Also disclosed herein is a compound represented by Structural Formula
(Vn and methods
of use thereof for inhibiting CRTHZ in a subject in need of treatment
therefore and pharmaceutical
compositions comprising the same, wherein R' has the value corresponding to
any one of the
compounds in Table 1 - 6 and R3 and R4 are as described above for Structural
Formula (VI).
[0042] Also disclosed herein is a compound represented by Structural Formula
(VII) and '
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
pharmaceutical:compositions comprising the same, wherein R3 has the value
corresponding to any one
of the compounds in Table 1- 6 and R' and R4 are as described above for
Structural Formula (VII).
[0043] Also. disclosed herein is a compound represented by Structural Formula
(VII) and
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
pharmaceutical compositions comprising the same, wherein R4 has the value
corresponding to any one
of the compounds in Table 1 - 6 and R' and R3 are as described above for
Structural Formula (VIn.
[0044] Also disclosed herein is a compound represented by Structural Formula
(VII) and
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
pharmaceutical compositions comprising the same, wherein R' has the value
corresponding to any one
of the compounds in Table 1 - 6 and R3 and R4 are as described above for
Structural Formula (VII).
[0045] Also disclosed herein is a compound represented by Structural Formula
(VIII) and
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
pharmaceutical compositions comprising the same, wherein R3 has the value
corresponding to any one
of the compounds in Table 1 - 6 and R' and R4 are as described above for
Structural Formula (VIII).
[0046] Also disclosed herein is a compound represented by Structural Formula
(VIIn and
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
-11-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
pharmaceutical compositions comprising the same, wherein R4 has the value
corresponding to any one
of the compounds in Table 1 - 6 and R' and R3 are as described above for
Structural Formula (VIII).
[0047] Also disclosed herein is a compound represented by Structural Formula
(VIII) and
methods of use thereof for inhibiting CRTH2 in a subject in need of treatment
therefore and
pharmaceutical compositions comprising the same, wherein R' has the value
corresponding to any one
of the compounds in Table 1- 6 and R3 and R4 are as described above for
Structural Formula (VIIn.
[0048] Specific examples of compounds of general formula I are shown in the
Exemplification
Section herein.
[0049] It will be understood that the immediately following definitions and
description apply to
compounds of general formula I (and subsets thereof described above in this
section 1 entitled
"Description of Compounds of Gerteral Formula 1 (and subsets thereo,~" As used
herein, the
following definitions shall apply unless otherwise indicated. For purposes of
this invention, the
chemical elements are identified in accordance with the Periodic Table of the
Elements, CAS version,
Handbook of Chemistry and Physics, 75''' Ed. Additionally, general principles
of organic chemistry
are described in "Organic Chemistry", Thomas Sorrell, University Science
Books, Sausalito: 1999,
and "March's Advanced Organic Chemistry", 5''' Ed., Ed.: Smith, M. B. and
March, J., John Wiley &
Sons, New York: 2001.
[0050] Many of the disclosed CRTH2 inhibitors contain one or more chiral
centers. The presence
of chiral centers in a molecule gives rise to stereoisomers. For example, a
pair of optical isomers,
referred to as "enantiomers", exist for every chiral center in a molecule; and
a pair of diastereomers
exist for every chiral center in a compound having two or more chiral centers.
Even though Structural
Formulas (1]-(VIII) do not explicitly depict stereochemistry, it is to be
understood that these formulas .
encompass enantiomers free from the corresponding optical isomer, racemic
mixtures, mixtures
enriched in one enantiomer relative to its corresponding optical isomer, a
diastereomer free of other
diastereomers, a pair of diastereomers free from other diasteromeric pairs,
mixtures of diasteromers,
mixtures of diasteromeric pairs, mixtures of diasteromers in which one
diastereomer is enriched
relative to the other diastereomer(s) and mixtures of diasteromeric pairs in
which one diastereomeric
pair is enriched relative to the other diastereomeric pair(s).
[0051] A preferred diastereomeric pair is when RZ and NR"R3 in Structural
Formulas (1)-(VIII)
are cis relative to one another. By way of example, the cis diastereomeric
pair for the compound
represented by Structural Formula (II) is shown below in Structural Formulas
(I~ and (X):
-12-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
O O
~~ R3
RvN~R4 ~N~Ra
I 4~
N 2 Rz N a ....Rz
Ri ~O R1 ~O
(1X)
[0052] The preferred configuration for Rz and NR"R3 (depicted by N(R3)(COR4)
in Structural
Formulas (IX) and (X) is (2R, 4S), as shown in Structural Formula (IX). Thus,
Structural Formula
(IX) represents a preferred optical isomer for the compound represented by
Struetural Formula (II).
Similarly, the corresponding (2R,4S) optical isomer for the compounds
represented by Structural
Formulas (I) and (III)- (VIII) and Tables 1-6 are also specifically disclosed.
The more preferred
configuration for R2 and NR"R3 (depicted by N(R3)(COR4)) in Structural
Formulas (IX) and (X) is
(2S, 4R), , as shown in Structural Formula (~. Thus, Structural Formula (X)
represents a more
preferred optical isomer for the compound represented by Structural Formulas
(n and (III)-(VIIn
and in Tables 1- 6.
[0053] As used herein, a structure depicting one optical isomer or a reference
to one optical
isomer is meant to include enantiomeric mixtures which are enriched with the
depicted or referenced
enantiomer relative to its optical isomer, for example, an enantiomeric excess
of at least 50%, 75%,
90%, 95% 99% or 99.5%. As used herein, a structure depicting a diastereomeric
pair or a reference to
one diasteromeric pair is meant to include mixtures which are enriched with
the depicted or
referenced diastereomeric pair relative to other diastereomers or
diastereomeric pairs) for the
compound, for example, a molar excess of at least 50%, 75%, 90%, 95% 99% or
99.5%.
[0054] The enantiomers of the present invention may be resolved by methods
known to those
skilled in the art, for example by formation of diastereoisomeric salts which
may be separated, for
example, by crystallization; formation of diastereoisomeric derivatives or
complexes which may be
separated, for example, by crystallization, gas-liquid or liquid
chromatography; selective reaction of
one enantiomer with an enantiomer-specific reagent, for example enzymatic
esterification; or gas-
liquid or liquid chromatography in a chiral environment, for example on a
chiral support for example
silica with a bound chiral ligand or in the presence of a chiral solvent.
Where the desired enantiomer
is converted into another chemical entity by one of the separation procedures
described above, a
further step is required to liberate the desired enantiomeric form.
Alternatively, specific enantiomers
may be synthesized by asymmetric synthesis using optically active reagents,
substrates, catalysts or
solvents, or by converting one enantiomer into the other by asymmetric
transformation.
-13-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0055] The diastereoisomeric pairs may be separated by methods known to those
skilled in the
art, for example chromatography or crystallization and the individual
enantiomers within each pair
may be separated as described above. Specific procedures for
chromatographically separating
diastereomeric pairs of precursors used in the preparation of compounds
disclosed herein are provided
in Scheme 1 and 2.
[0056] In certain instances compounds of the present invention may be
associated in isolated
form with solvent or water, as in a "solvate" or "hydrate". References to the
disclosed compounds or
structural formulas depicting the disclosed compounds are meant to include
such solvates and
hydrates.
[0057] The term "aliphatic" as used herein means straight-chain or branched
hydrocarbons which
are completely saturated or which contain one or more units of unsaturation,
but which are not
aromatic. An aliphatic group is typically CI_$, more typically Cl$. For
example, suitable aliphatic
groups include substituted or unsubstituted linear or branched alkyl, alkenyl,
alkynyl groups and
hybrids thereof. The terms "alkyl", "alkoxy", "hydroxyalkyl",
"alkoxyalkylene", and
"alkoxycarbonyl", used alone or as part of a larger moiety includes both
straight and branched
saturated chains containing one to eight carbon atoms. The terms "alkenyl" and
alkynyl" used alone
or as part of a larger moiety shall include both straight and branched chains
containing two o eight
carbon atoms and one or more double and/or triple bonds, respectively.
[0058] The term "cycloaliphatic" used alone or as part of a larger moiety
shall include cyclic C3-
Cio hydrocarbons which are completely saturated or which contain one or more
units of unsaturation,
but which are not aromatic. Cycloaliphatic groups are typically C3_lo, more
typically c3_~. A
"cycloalkyl" is an cyclic aliphatic group that is completely saturated.
[0059] "Alkoxy" means (alkyl)-O-; "alkoxyalkylene" means (alkyl)-O-(alkylene)
such as
methoxymethylene (CH30CH2); "hydroxyalkyl" means hydroxy substituted alkyl
group; "alkoxy
carbonyl means a carbonyl substituted with a carbonyl as in (alkyl)-O-C(O)-;
and "aralkyl" mean
alkyl substituted with an aromatic group. A "CI-G4 aralkyl group", for
example, has a Cl-C4 alkyl
group substituted with an aromatic group.
[0060] The term "heteroatom" means nitrogen, oxygen, or sulfur and includes
any oxidized form
of nitrogen and sulfur, and the quaternized form of any basic nitrogen. Also
the term "nitrogen"
includes a substitutable nitrogen of a heterocyclic ring. As an example, in a
saturated or partially
unsaturated ring having 0-3 heteroatoms selected from oxygen, sulfur or
nitrogen, the nitrogen may be
N (as in 3,4-dihydro-2H-pyrrolyl), NH (as in pyrrolidinyl) or NR~ (as in N-
substituted pyrrolidinyl).
[0061] The term "aromatic group" used alone or as part of a larger moiety as
in "aralkyl",
"aralkoxy", or "aryloxyalkyl", includes to carbocyclic aromatic ring groups
and heteroaryl rings
groups. The term "aromatic group" may be used interchangeably with the terms
"aryl", "aryl ring" or
"aromatic ring".
-14-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0062] Carbocyclic aromatic ring groups have only carbon ring atoms and
include monocyclic
aromatic rings such as phenyl and fused polycyclic aromatic ring systems in
which two or more
carbocyclic aromatic rings are fused to one another. Examples include 1-
naphthyl, 2-naphthyl, 1-
anthracyl and 2-anthracyl. Also included within the scope of the term
"carbocyclic aromatic ring", as
it is used herein, is a group in which an aromatic ring is fused to one or
more non-aromatic rings
(aliphatic or heterocyclic), such as in an indanyl, phthalimidyl,
naphthimidyl, phenantriidinyl, or
tetrahydronaphthyl, where the radical or point of attachment is on the
aromatic ring.
[0063] The term "heteroaryl" or "heteroaromatic", used alone or as part of a
larger moiety as in
"heteroaralkyl" or "heteroarylalkoxy", refers to heteroaromatic ring groups
having five to fourteen
members, including monocyclic heteraromatic rings and polycyclic aromatic
rings in which a
monocyclic aromatic ring is fused to one or more other carbocyclic or
heteroaromatic aromatic rings .
Examples of heteroaryl rings include 2-furanyl, 3-furanyl, N-imidazolyl, 2-
imidazolyl, 4-imidazolyl,
5-imidazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, 2-oxadiazolyl, 5-
oxadiazolyl, 2-oxazolyl, 4-
oxazolyl, 5-oxazolyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, pyxazolyl, 2-
pyridyl, 3-pyridyl, 4-pyridyl, 2-
pyrimidyl, 4-pyrimidyl, 5-pyrimidyl, 3-pyridazinyl, 2-thiazolyl, 4-thiazolyl,
5-thiazolyl, 2-triazolyl, 5-
triazolyl, tetrazolyl, 2,-thienyl, 3-thienyl, carbazolyl, benzinaidazolyl,
benzothienyl, benzofuranyl,
indolyl, , quinolinyl, benzotriazolyl, benzothiazolyl, benzooxazolyl,
benzimidazolyl, isoquinolinyl,
indolyl, isoindolyl, acridinyl, or benzoisazolyl. Also included within the
scope of the term
"heteroaryl", as it is used herein, is a group in which a heteroaryl ring is
fused to one or more
cycloaliphatic or non-aromatic heterocyclic groups where the radical or point
of attachment is on the
heteroaromatic ring. Examples include tetrahydroquinolinyl,
tetrahydroisoquinolinyl, and pyrido [3,
4-d] pyrimidinyl. The term "heteroaryl" may be interchangeably with the term
"heteroaryl ring" or
the term "heteroaromatic".
[0064] The term "non-aromatic heterocyclic ring", used alone or as part of a
larger moiety as in
"hetercyclylalkyl", refers to non-aromatic ring systems typically having five
to fourteen members, .
preferably five to ten, in which one or more ring carbons, preferably one to
four, are each replaced by
a heteroatom such as N, O, or S. Examples of non-aromatic heterocyclic rings
include 3-1H-
benzimidazol-2-one, 3-tetrahydrofuranyl, 2-tetrahydropyranyl, 3-
tetrahydropyranyl, 4-
tetrahydropyranyl, [1,3]-dioxalanyl, [1,3]-dithiolanyl, [1,3]-dioxanyl, 2-
tetrahydrothiophenyl, 3-
tetrahydrothiophenyl, 2-morpholinyl, 3-morpholinyl, 4-morpholinyl, 2-
thiomorpholinyl, 3-
thiomorpholinyl, 4-thiomorpholinyl, 1-pyrrolidinyl, 2-pyrrolidinyl, 3-
pyrorolidinyl, 1-piperazinyl, 2-
piperazinyl, 1-piperidinyl, 2-piperidinyl, 3-piperidinyl, 4-piperidinyl, 4-
thiazolidinyl, diazolonyl, N-
substituted diazolonyl, 1-pthalimidinyl, benzoxanyl, benzopyrrolidinyl,
benzopiperidinyl,
benzoxolanyl, benzothiolanyl, and benzothianyl.
[0065] A "hydrocarbyl group" is a polymethylene group,, i.e., -(CHZ)ri ,
wherein n is a positive
integer. Preferably, n is an integer from 1 to 6, more preferably from 2 to 4
and more preferably from
-15
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
2 to 3. A "substituted hydrocarbyl" is a hydrocarbyl group in which one or
more methylene hydrogen
atoms are replaced with a substituent. Suitable substituents are as described
below for a substituted
aliphatic group. Preferred substituents for the hydrocarbyl groups represented
by X3-X6 are halo, -OH,
=O, Cl-C3 alkyl, Cl-C3 alkoxy, nitro and cyano.
[0066] A hydrocarbyl group can be optionally interrupted by one or more
functional groups. A
hydrocarbyl is interrupted by a functional group when one of the internal
methylenes is replaced with
the functional group. Examples of suitable "interrupting functional groups"
include -O-, -S-, -N(Ra)-,
-S(O)-, -SOz-, -C(O)-, -OC(O)-, -N(Ra)C(O)-, -C(O)N(Ra)-, -SOZN(Ra)-, arid -
N(Ra)SOz-. Ra is H
or a Cl-C3 alkyl group.
[0067] An aromatic group (including Ring A, carbocyclic aromatic, heteroaryl,
aralkyl, aralkoxy,
aryloxyalkyl and heteroaralkyl and the like) group may contain one or more
substituents. Examples
of suitable substituents on an unsaturated carbon atom of an aromatic group
include a halogen -R°, -
OR°, -SR°, 1,2-methylene-dioxy, 1,2-ethylenedioxy, protected OH
(such as acyloxy), phenyl (Ph),
substituted Ph, -O(Ph), substituted -O(Ph), -CHz(Ph), substituted -CHz(Ph), -
CHZCHz(Ph),
substituted -CHZCHz(Ph), -NOz, -CN, -N(R')z, -NR'COzR°, -
NR'C(O)R°, -NR'NR'C(O)R°,
-N(R')C(O)N(R')z, -NR'NR'C(O)N(R')z, -NR'NR'C02R°, -C(O)C(O)R°, -
C(O)CHZC(O)R°, -COZR°,
-C(O)R°, -C(O)N(R°)z, -OC(O)N(R°)z, -S(O)zR°, -
SOzN(R')z, -S(O)R°, -NR'SOzN(R')z, -NR'SOZR°,
-C(=S)N(R')z, -(CHz)yN(R')z, -C(=NH)-N(R')z, -(CHz)yNHC(O)R°, -
(CHz)yNHC(O)CH(V-R°)(R°).
R' is R°, -COZR°, -SOZR° or -C(O)R° and preferably
hydrogen, C1.6 aliphatic, COZR°, SOZR° or
C(O)R°. R° is hydrogen or substituted or unsubstituted
aliphatic, cycloaliphatic, arpmatic, aralkyl or
non-aromatic heterocyclic group, and preferably hydrogen, Cl_6 alkyl, phenyl
(Ph), -CHz (Ph), aralkyl,
non-aromatic heterocyclic group or heteroaryl; y is 0-6; and V is Ci-C6
alkylene group. Examples of
substituents on the aliphatic group or the phenyl ring of R° include
amino, alkylamino, dialkylamino,
aminocarbonyl, halogen, alkyl, aminoalkyl, alkylaminocarbonyl,
dialkylaminocarbonyl,
allcylaminocarbonyloxy, dialkylaminocarbonyloxy, alkoxy, nitro, cyano,
carboxy, alkoxycarbonyl,
alkylcarbonyl, hydroxy, haloalkoxy, or haloalkyl.
[0068] An aliphatic group or a non-aromatic heterocycle may contain one or
more substituents.
Examples of suitable substituents on the saturated carbon of an aliphatic
group of a non-aromatic
heterocycle include those listed above for the unsaturated carbon of an
aromatic group and the
following: =O, =S, =NNHR*, =NN(R*)z, =NNHC(O)R*, =NNHCOz(alkyl), =NNHSOz
(alkyl), or
=NR*. Each R* is independently selected from hydrogen, an unsubstituted
aliphatic group or a
substituted aliphatic group. Examples of substituents on the aliphatic group
represented by R* include
amino, alkylamino, dialkylamino, aminocarbonyl, halogen, alkyl,
alkylaminocarbonyl,,
dialkylaminocarbonyl, alkylaminocarbonyloxy, dialkylaminocarbonyloxy, alkoxy,
nitro, cyano,
carboxy, alkoxycarbonyl, alkylcarbonyl, hydroxy, haloalkoxy, or haloalkyl.
-16-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0069] Suitable substitutents on the substitutable nitrogen of a heteroaryl or
non-aromatic
heterocyelic group include -R+, -N(R+)Z, -C(O)R+, -COZ R~, -C(O)C(O)R+, -
C(O)CHZ C(O)R+, -SOz
R'~, -SOz N(R~)2, -C(=S)N(R~)z, -C(=NH)-N(R~)2, and -NR+ SOZ R''~; wherein R+
is hydrogen, an
aliphatic group, a substituted aliphatic group, phenyl (Ph), substituted Ph, -
O(Ph), substituted -O(Ph),
CHZ(Ph), or an unsubstituted heteroaryl or non-aromatic heterocyclic ring.
Examples of substituents
on the aliphatic group or the phenyl ring represented by R+ include amino,
alkylamino, dialkylamino,
aminocarbonyl, halogen, alkyl, alkylaminocarbonyl, dialkylaminocarbonyloxy,
alkoxy, nitro, cyano,
carboxy, alkoxycarbonyl, alkylcarbonyl, hydroxy, haloalkoxy, or haloalkyl.
[0070] 2. Description of Compounds of General Formula 1-A (and subsets
thereof):
[0071] Another embodiment of the present invention is a compound represented
by Structural
Formula I-A:
R ~N~Rx
A
NJ....Ra
1
R
I-A
or a pharmaceutically acceptable salt thereof, wherein:
Ring A is an optionally substituted monocyclic aromatic;
R is Xl-Rz;
Rx is -Xz-R4;
Xl and XZ are each independently -S(O)2-, -C(O)-, or -C(O)NH-;
R' is:
A) an aromatic group or heteroaromatic group having 5-6 ring atoms, fused to a
monocyclic non-aromatic heterocyclic ring or monocyclic aromatic or
heteroaromatic
ring wherein the non-aromatic heterocyclic ring, the aromatic ring, or the
heteroaromatic ring are optionally substituted; or
B) an aromatic group or heteroaromatic group having 5-6 ring atoms,
substituted by:
i) Tz-V-T-RY;
ii) Tz-V-T-M-RY; or
iii) V-R9, wherein R9 is an optionally substituted non-aromatic carbocyclic or
heterocyclic group;
-17-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and wherein the aromatic or heteroaromatic group having 5-6 ring atoms
optionally is further substituted by 1-2 independently selected groups
represented by
RZ.
each RZ is independently selected from halogen, haloalkyl, R°, -
OR°,
-O(haloalkyl), -SR°, -NOz, -CN, -N(R')z, -NR'COZR°, -
NR'C(O)R°,
-NR'NR'C(O)R°, -N(R')C(O)N(R')z, -NR'NR'C(O)N(R')z, -
NR'NR'COZR°,
-C(O)C(O)R°, -C(O)CHZC(O)R°, -COzR°, -C(O)R°, -
C(O)N(R°)z, -OC(O)R°,
-OC(O)N(R°)z~ -S(O)zR°~ -SOzN(R')z~ -S(O)R°~ -
NR'S02N(R')z~ -NR'SOZR°~
-C(=S)N(R')z, and -C(=NH)-N(R')z;
each R' is independently hydrogen, alkyl, -C(O)OR°, S(O)zR°, or
-C(O)R°;
each R° is independently hydrogen or an alkyl group, non-aromatic
heterocyclic group or aromatic group and the alkyl, non-aromatic heterocyclic
group
and aromatic group represented by R° is optionally substituted with one
or more
independently selected groups represented by R#;
R# is R+, -OR+, -O(haloalkyl), -SR+, -NOz, -CN, -N(R+)z,
-NHCOZR+, -NHC(O)R+, -NHNHC(O)R+, -NHC(O)N(R+)z, -NHNHC(O)N(R+)z,
-NHNHCOzR~, -C(O)C(O)R+, -C(O)CHZC(O)R+,
-COzR*~ -C(O)R+, -C(O)N(R+)z~ -OC(O)R+, -OC(O)N(R+)z~ -S(O)zR+~ -S~zN(R+)z~
-S(O)RB, -NHSOZN(R+)z, -NHSOzR+, -C(=S)N(R'')z, or -C(=NH)-IV(R~z;
R+ is H, a Cl-C3 alkyl group, a monocyclic heteroaryl group, a non-aromatic
heterocyclic group or a phenyl group optionally substituted with alkyl,
haloalkyl,
alkoxy, haloalkoxy, halo, -CN, -NOz, amine, alkylamine or dialkylamine; or
N(R~)z
is a non-aromatic heterocyclic group, provided that non-aromatic heterocyclic
groups
represented by R+ and N(R+)z that comprise a secondary ring amine are
optionally
acylated or alkylated;
V is a covalent bond, -O-, -C(O)-, -N(R )-, -S-, -S(O)-, -C(O)NRS-, -
NRSC(O)-, -S(O)2NR5-~ -NRSS(O)2-~ Or-S(O)z-i
T is Cl_~o is a straight chain alkylene;
T' is a covalent bond, or a Cl_io straight chain alkylene, wherein T and T'
together contain no more than 10 carbon atoms, and wherein T and TI are
optionally
and independently substituted at any one or more substitutable carbon atoms
with
halide, alkyl, gem dialkyl, gem dihalo, haloalkyl, alkoxy, haloalkoxy, spiro
cycloalkyl, optionally N-substituted nitrogen containing spiro non-aromatic
heterocyclic group, O-containing spiro non-aromatic heterocyclic group, amine,
alkylamine, dialkylamine, alkoxy, or hydroxyl;
-18-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
M is an optionally substituted group selected from monocyclic aromatic,
heteroaromatic, monocyclic non-aromatic carbocyclic, or heterocyclic group;
RY is -C(O)ORS, -C(O)R5, -OC(O)R5, -C(O)N(RS)z, -NRSC(O)R5,
-NRSC(O)ORS, -S(O)zRS, -S(O)zCORS, -S(O)zN(RS)z, -NRSS(O)zRs,
-NRSS(O)zRs, S(O)zORS, -S(O)ORS, -S(O)R5, -SRS, -C(O)NRSS(O)zRs, -CN, _
NRSC(O)N(R5)z, -OC(O)N(RS)z, -N(RS)z, -ORS, an optionally substituted non-
aromatic heterocyclic group or an optionally substituted heteroaryl group;
provided that T is Cz_io when V is a covalent bond, and T is Cz_IO when V is -
O-, -S-, or -N(R')- and RY is -CN, -OH, -SH, -N(RS)z
each RS is independently -H, alkyl, haloalkyl, hydroxyalkyl, carboxyalkyl,
C(O)OCHZC6H5, S(O)zCH3, -C(O)OH, -C(O)OMe, -C(O)OEt, C(O)NHz, benzyl,
pyrrolidinyl, morpholinyl, or -N(RS)z is a nitrogen-containing non-aromatic
heterocyclic group;
Rz is C~_3 alkyl;
R3 is an optionally substituted monocyclic or bicyclic group selected from
aromatic,
heteroaromatic, non-aromatic carbocyclic, or non-aromatic heterocyclic; and
R4 is optionally substituted Cl_6alkyl, C~Ahydroxyalkyl, or optionally
substituted C3_
6cycloalkyl.
[0072] In one embodiment, compounds of formula I-A, or a pharmaceutically
acceptable salt
thereof, are provided, wherein:
Ring A is an optionally substituted monocyclic aromatic;
R is Xl-R1;
Rx is Xz-Rø;
XI and Xz are each independently -S(O)z-, -C(O)-, or -C(O)NH-;
R' is: A) an aromatic group or heteroaromatic group having 5-6 ring atoms,
substituted by:
i) T'-V-T-RY;
ii) Tl-V-T-M-RY; or
iii) V-R9 wherein R9 is an optionally substituted non-aromatic carbocyclic or
heterocyclic group;
and wherein the aromatic or heteroaromatic group represented by Rl optionally
is
further substituted by 1-2 independently selected groups represented by Rz; or
B) an aromatic group or heteroaromatic group having 5-6 ring atoms, fused to a
monocyclic non-aromatic heterocyclic ring or monocyclic aromatic ring wherein
the
non-aromatic heterocyclic ring or the aromatic ring are optionally
substituted;
-19-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
each Rz is independently selected from halogen, haloalkyl, R°, -
OR°, -O(haloalkyl), -SR°, -NOz,
-CN, -N(R')z, -NR'COZR°, -NR'C(O)R°, -NR'NR'C(O)R°, -
N(R')C(O)N(R')z, -NR'NR'C(O)N(R')z,
-NR'NR'COzR°, -C(O)C(O)R°, -C(O)CHzC(O)R°, -C02R°,
-C(O)R°, -C(O)N(R°)z, -OC(O)R°,
-OC(O)N(R°)z, -S(O)zR°, -SOzN(R')z, -S(O)R°, -
NR'SOZN(R')z, -NR'SOZR°, -C(=S)N(R')z, and
-C(=NH)-N(R')z;
each R is independently hydrogen, alkyl, -C(O)OR°, S(O)zR°, or -
C(O)R°;
each R° is independently hydrogen or an alkyl group, non-aromatic
heterocyclic group or
aromatic group and the alkyl, non-aromatic heterocyclic group and aromatic
group represented by R°
is optionally substituted with one or more independently selected groups
represented by R#;
R# is R+, -OR+, -O(haloalkyl), -SR+, -NOz, -CN, -N(R+)z, -NHCOZR+, -NHC(O)R+,
-NHNHC(O)R+, -NHC(O)N(R~z, -NHNHC(O)N(R+)z, -NHNHCOZR+, -C(O)C(O)R+,
-C(O)CHzC(O)R+, -COZR+, -C(O)R+, -C(O)N(R~)z, -OC(O)R+, -OC(O)N(R+)z, -
S(O)zR+, -SOZN(R+)z,
-S(O)RB', -NHSOZN(R'~)z, -NHSOZRk, -C(=S)N(R''~z, or -C(=NFTj-N(R~)z;
R+ is -H, a CI-C3 alkyl group, a monocyclic heteroaryl group, a non-aromatic
heterocyclic
group or a phenyl group optionally substituted with alkyl, haloalkyl, alkoxy,
haloalkoxy, halo, -CN,
-NOz, amine, alkylamine or dialkylamine; or -N(R+)z is a non-aromatic
heterocyclic group, provided
that non-aromatic heterocyclic groups represented by R~ and -N(R~)z that
comprise a secondary ring
amine are optionally acylated or alkylated;
Ry is -C(O)ORS, -C(O)R5, -OC(O)R5, -C(O)NRsz, -NRSC(O)R5, -NRSC(O)ORS, -
S(O)zRS, _
S(O)zCORs, -S(O)zN(RS)z, -NRSS(O)zRs, -NRSS(O)zRs, S(O)zORs, -S(O)ORS, -
S(O)R5, -SRS, -
C(O)NRSS(O)zRs, -CN, -NRSC(O)N(RS)z, -OC(O)N(RS)z, -N(RS)z, -ORS, an
optionally substituted
non-aromatic heterocyclic group or an optionally substituted heteroaryl group;
V is a covalent bond, -O-, -C(O)-, -N(R )-, -S-, -S(O)-, -C(O)NRS-, -NRSC(O)-,
-S(O)zNRS-, -
NRSS(O)z-, or-S(O)z-;
T is Cl_~o is a straight chain alkylene; provided that T is Cz_~o when V is a
covalent bond, and
T is Cz_io when V is -O-, -S-, or -N(R~)- and RY is -CN, -OH, -SH, -N(RS)z;
T' is a covalent bond, or a C~_lo straight chain alkylene, wherein T and T'
together contain no
more than 10 carbon atoms, and wherein T and T' are optionally and
independently substituted at any
one or more substitutable carbon atoms with halide, alkyl, gem dialkyl, gem
dihalo, haloalkyl, alkoxy,
haloalkoxy, spiro cycloalkyl, optionally N-substituted nitrogen containing
spiro non-aromatic
heterocyclic group, amine, alkylamine, dialkylamine, or hydroxyl;
each RS is independently -H, alkyl, haloalkyl, hydroxyalkyl, carboxyalkyl, -
C(O)OCHzC~iS,
S(O)zCH3, -C(O)OH, -C(O)OMe, -C(O)OEt, C(O)NHz, benzyl, pyrrolidinyl,
morpholinyl, or N(RS)z
is a nitrogen-containing non-aromatic heterocyclic group;
M is an optionally substituted monocyclic aromatic, heteroaromatic or an
optionally
substituted monocyclic non-aromatic carbocyclic or heterocyclic group;
-20-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Rz is Cl_3 alkyl;
R3 is an optionally substituted aromatic group having 5-6 ring atoms; and
R4 is Cl_3 alkyl or Cl_3 hydroxyalkyl.
[0073] The sections below defining the terms "aromatic group", "heteroaromatic
group", "non-
aromatic carbocycle" and "non-aromatic heterocyclic group" provide specific
examples of suitable
values for M. Suitable substituents for aromatic rings represented by M are as
defined for R'i;
suitable substituents for carbocyclics and non-aromatics are as described in
sections below defining
suitable substituents for these two groups.
[0074] In certain aspects compounds of the invention are compounds other than
one of the
following compounds:
[0075] When Rl is phenyl para substituted with V-T-RY, RZ is methyl, R3 is
para chlorophenyh
Rx is C(O)CH3, Xl is -C(O) and V is -O-, T-R'' is -CHZ-CHZ-C(CH3)z-CONH2, -CHZ-
CHZ-C(CH3)2-
OH, -CHz-CHz-CHz-C(CH3)2-OH, -CHZ-CHZ-C(CH3)z-COOH, -CHz-C (spiro cyclopropyl)-
CHZ-
COOH; or -CHz-C(CH3)Z-COOH.
[0076] When R' is phenyl para substituted with V-T-RY, RZ is methyl, R3 is
para chlorophenyl,
Rx is C(O)CH3, XI is -C(O) and V is a covalent bond then, T-RY is -CHZ-CHZ-
COOH.
[0077] When RZ is methyl, R3 is para chlorophenyl, RX is C(O)CH3, Xl is C(O)
and R' is phenyl
para substituted with V-R9 wherein R9 is an optionally substituted non-
aromatic heterocyclic group, V
is a covalent bond R' is N morpholinyl, N ethoxycarbonyl-4-piperdinyl, N-
acetyl-4-piperdinyl, N-
ethoxycarbonyl-4-piperid-3-enyl, N-pyrrolidinyl, N-ethyl-N'-piperazinyl, N-
acetyl-N'-piperazinyl, N-
(2'-hydroxyacetyl)-N'-piperazinyl, N (CHZC(O)OH-N'-piperazinyl, or N-
(CHZC(O)NHZ)-N'-
piperazinyl.
[0078] When RZ is methyl, R3 is para chlorophenyl, R" is C(O)CH3, Xl is C(O)
and Rl is phenyl
para substituted with V-R9 wherein R9 is an optionally substituted non-
aromatic heterocyclic group, V
is -O-, R' is N-ethoxycarbonyl-4-piperdinyl or N-acetyl-4-piperdinyl.
[0079] When RZ is methyl, R3 is phenyl, R" is C(O)CHZCH3, Xl is C(O) and R' is
phenyl para
substituted with a non-aromatic heterocyclic group, R' is N-acetyl-4-
piperdinyl, or N-morpholinyl.
[0080] Wlien RZ is methyl, R3 is para chlorophenyl, R" is C(O)CH3, XI is C(O)
and Rl is phenyl
fused to an optionally substituted non-aromatic heterocyclic group, R'
tetrahydrofuranyl, or N'-
methyl morpholinyl.
[0081] When RZ is methyl, R3 is para chlorophenyl, R" is C(O)CH3, Xl is C(O)
and Rl is
pyridinyl para substituted with a non aromatic heterocyclic group, R' is N-
morpholinyl.
[0082] When RZ is methyl, R3 is para chlorophenyl, R" is C(O)CH3, Xl is C(O)
and RI is phenyl
fused to an optionally substituted aromatic group, Rl is N-isopropyl
triazolyl, N-methyl triazolyl, N-
-21 -
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
isopropyl imidazolyl, 2, methyl N hydroxyethyl imidazolyl, 2, methyl N
carboxymethyl imidazolyl, N
carboxyethyl triazolyl N-isopropyl pyrazolyl.
[0083] When RZ is methyl, R3 is para chlorophenyl, RX is C(O)CH3, Xl is C(O)
and RI is
imidazolyl fused to an optionally substituted aromatic group, Rl is phenyl.
[0084] When Rz is methyl, R3 is para chlorophenyl, R" is C(O)CH3, Xt is C(O)
and Rl is
thiazolyl fused to an optionally substituted aromatic group, RI is phenyl.
[0085] In some embodiments compounds of the invention are compounds other than
compounds
disclosed in our U.S. Patent Application 10/678,872 filed October 4, 2003 (the
entire contents of
which are incorporated herein by reference).
[0086] In other embodiments, compounds of the invention are compounds other
than:
(~)-Cis-N-[1-(1H-indole-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
N-phenyl-
propionamide; (~)-Cis-N-[1-(benzofuran-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-pxopionamide; (~)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-
dihydro-2H-quinoline-
1-carbonyl]-phenoxy}-acetic acid ethyl ester; (~)-Cis--{4-[2-Methyl-4-(phenyl-
propionyl-amino)-3,4-
dihydro-2H-quinoline-1-carbonyl]-phenoxy}-acetic acid; (~)-Cis-N-{2-methyl-1-
[4-(2-morpholin-4-
yl-ethoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-N-phenyl-propionamide;
(~)-Cis-N-[1-(4-
carbamoylmethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
propionamide; (~)-
Cis-N-{ 1-[4-(2-hydroxy-2-methyl-propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl }-N-
phenyl-pxopionamide; (~)-Cis-N-[1-(4-dimethylcarbamoylmethoxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide; (~)-Cis-N-[1-
(benzo[b]thiophene-3-carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide; (~)-Cis-N-[1-
(benzo[b]thiophene-2-
carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide;
(~)-Cis-{4-[4-(acetyl-
phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-1-carbonylJ-phenylamino}-
acetic acid; (~)=Cis-
N-[1-(1-isopropyl-1H-benzotriazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-ylJ-N-
phenyl-propionarnide; (~)-Cis-4-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-
dihydro-2H-
quinoline-1-carbonyl]-phenyl}-piperidine-1-carboxylic acid ethyl ester; (~)-
Cis-N-[2-methyl-1-(4-
piperidin-4-yl-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
propionamide; (~)-Cis-N-{ 1-[4-
(1-acetyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-
N-phenyl-
propionamide; (~)-Cis-N-{ 1-[4-(1-ethyl-piperidin-4-yl)-benzoyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide; (~)-Cis-N-{2-methyl-1-[4-(4-methyl-
piperazin-1-yl)-
benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-N-phenyl-propionamide; (~)-Cis-N-[2-
methyl-1-(4-
methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
propionamide; (~)-Cis-N-[2-Methyl-1-(4-morpholin-4-yl-benzoyl)-1,2,3,4-
tetrahydro-quinolin-4-y1J-
N-phenyl-propionamide; (~)-Cis-N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide; (~)-Cis-2-{4-[4-(acetyl-phenyl-annino)-2-
methyl-3,4-dihydro-
2H-quinoline-1-carbonyl]-phenylamino}-propionic acid methyl ester; (~)-Cis-2-
{4-[4-(acetyl-phenyl-
-22-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
amino)-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl]-plienylamino}-
propionamide; (~)-Cis-N-[1-
(2,3-dihydro-benzo[1,4]dioxine-6-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
propionamide; (~)-Cis-N-[1-(benzo[c]isoxazole-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide; (~)-Cis-4-(4-{4-[(4-chloro-phenyl)-propionyl-amino]-
2-methyl-3,4-
dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyric acid ethyl ester; (~)-Cis-4-
(4-{4-[(4-chloro-
phenyl)-propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenoxy)-butyric acid;
(~)-Cis-N-(4-chloro-phenyl)-N-{2-methyl=1-[4-(1H-tetrazol-5-ylmethoxy)-
benzoyl]-1,2,3,4-
tetrahydro-quinolin-4-yl}-propionamide; (~)-Cis-N-(4-chloro-phenyl)-N-{1-[4-(3-
hydroxy-2,2-
dimethyl-propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-
acetamide; (~)-Cis-3-(4-{4-
[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl
}-phenoxy)-2,2-
dimethyl-propionic acid methyl ester; (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-
cyclopentyloxy-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide; (~)-Cis-N-{ 1-[4-(4-
Acetyl-piperazin-1-yl)-
benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-N-(4-chloro-phenyl)-
acetamide; (2S ,4R)- N-(4-
Chloro-phenyl)-N-[2-methyl-1-(4-morpholin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-
acetamide; (2S ,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenoxy)-butyric acid; (2S ,4R)- N-(4-Chloro-phenyl)-N-[2-methyl-1-
(6-morpholin-4-yl-
pyridine-3-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide; (2S ,4R)- 4-
(4-{4-[Acetyl-(4-
chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-
piperidine-1-
carboxylic acid ethyl ester; (2S ,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(2-
morpholin-4-yl-
ethoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide; (2S ,4R)-(4-{4-
[Acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-acetic
acid; (2S ,4R)- N-
(4-Chloxo-phenyl)-N- { 2-methyl-1-[4-( 1H-tetrazol-5-ylmethoxy)-benzoyl]-
1,2,3,4-tetrahydro-
quinolin-4-yl}-acetamide; (2S ,4R)-N-{1-[4-(1-Acetyl-piperidin-4-yloxy)-
benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide; (2S ,4R)- N-(4-Chloro-
phenyl)-N-{2-
methyl-1-[4-(pyridin-4-ylmethoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-
acetamide; (2S ,4R)-
4-(3-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-
carbonyl }-
phenoxy)-butyric acid; (2S, 4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-
methyl-3,4-dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-1-carboxylic acid ethyl ester;
(2S,4R)-N-(4-Chloro-phenyl)-
N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide; (2S,4R)-N-
(4-Chloro-phenyl)-N-[ 1-( 1-isopropyl-1 H-benzotriazole-5-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide; (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-
methyl-3,4-dihydro-
2H-quinoline-1-carbonyl}-phenyl)-propionic acid; (2S,4R)-3-(4-{4-[Acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenyl)-acrylic acid; N-
{(2S,4R)-1-[4-(1-
acetylpiperidin-4-yl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}-N-
phenylpropanamide; N-
{ (2R,4S)-1-[4-( 1-acetylpiperidin-4-yl)benzoyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl }-N-
phenylpropanamide; N-[(2S,4R)-2-methyl-1-(4-morpholin-4-ylbenzoyl)-1,2,3,4-
tetrahydroquinolin-4-
-23-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
yl]-N-phenylpropanamide; N-[(2R,4S)-2-methyl-1-(4-morpholin-4-ylbenzoyl)-
1,2,3,4-
tetrahydroquinolin-4-yl]-N-phenylpropanamide; N-{(2S,4R)-1-[4-(1-
acetylpiperidin-4-yl)benzoyl]-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl}-N-phenylpropanamide; N-{(2R,4S)-1-[4-
(1-acetylpiperidin-
4-yl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}-N-phenylpropanamide; N-
{(2S,4R)-1-[4-(1-
acetylpiperidin-4-yl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl }-N-(4-
chlorophenyl)acetamide; (4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-
3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)butanoic acid; N-{(2S,4R)-1-[4-(2-
amino-2-.
oxoethoxy)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}-N-(4-
chlorophenyl)acetamide; Ethyl-
4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-
1(2H)-
yl]carbonyl}phenyl)-3,6-dihydropyridine-1(2H)-carboxylate; N-[(2S,4R)-1-(1,3-
benzodioxol-5-
ylcarbonyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-N-phenylacetamide; N-{
(2S,4R)-2-methyl-1-
[(3-methyl-1-benzofuran-2-yl)carbonyl]-1,2,3,4-tetrahydroquinolin-4-yl}-N-
phenylacetamide; 4-(4-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-
yl)carbonyl }phenoxy)-N-ethylbutanamide; N-(4-Chlorophenyl)-N-[(2S,4R)-1-(3-
ethyl-4-
fluorobenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide; 4-(4-{
[(2S,4R)-4-[Acetyl(4-'
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-yl]carbonyl }phenoxy)-
2,2-
dimethylbutanamide; N-(4-chlorophenyl)-N-{(2S,4R)-2-methyl-1-[4-(4-oxo-4-
pyrrolidin-1-
ylbutoxy)benzoyl]-1,2,3,4-tetrahydroquinolin-4-yl}acetamide; 4-(4-{[(2S,4R)-4-
[Acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)butanamide; 4-(4-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-
yl]carbonyl }phenoxy)-N-(methylsulfonyl)butanamide; Ethyl 4-(4-{ [(2S,4R)-4-
[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)butanoate; 4-(4-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-
yl]carbonyl }phenoxy)-N-hydroxybutanamide; N-(4-chlorophenyl)-N-{ (2S,4R)-1-(4-
(3-
cyanopropoxy)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}acetamide; N-(4-
chlorophenyl)-N-
((2S,4R)-2-methyl-1-{4-[3-(1,2,4-oxadiazol-5-yl)propoxy]benzoyl}-1,2,3,4-
tetrahydroquinolin-4-
yl)acetamide; 3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-
yl]carbonyl}phenoxy)propanamide; N-{(2S,4R)-1-[4-(3-aminopropoxy)benzoyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl }-N-(4-chlorophenyl)acetamide; N-{ (2S,4R)-1-[4-(2-
amino-2-
oxoethoxy)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl }-N-(4-
chlorophenyl)acetamide; N-(4-
chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-[2-(methylamino)-2-oxoethoxy]benzoyl }-
1,2,3,4-
tetrahydroquinolin-4-yl)acetamide; 2-(4-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)-N,N-dimethylacetamide; N-(4-
chlorophenyl)-N-
{ (2S,4R)-2-methyl-1-[4-(2-morpholin-4-yl-2-oxoethoxy)benzoyl]-1,2,3,4-
tetrahydroquinolin-4-
yl } acetamide; N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{ 4-[2-(2-
oxopyrrolidin-1-
yl)ethoxy]benzoyl}-1,2,3,4-tetrahydroquinolin-4-yl)acetamide; N-(4-
chlorophenyl)-N-((2S,4R)-1-{4-
-24-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[2-(1H-imidazol-1-yl)ethoxy]benzoyl}-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl)acetamide; N-(4-
chlorophenyl)-N-{ (2S,4R)-2-methyl-1-[4-(2-pyrrolidin-1-ylethoxy)benzoyl]-
1,2,3,4-
tetrahydroquinolin-4-yl } acetamide; N-(4-chlorophenyl)-N-[(2S,4R)-1-(2,3-
dihydro-1-benzofuran-5-
ylcarbonyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide; N-(4-
Chlorophenyl)-N-{(2S,4R)-2-
methyl-1-[4-(3-pyrrolidin-1-ylpropoxy)benzoyl]-1,2,3,4-tetrahydroquinolin-4-
yl}acetamide; N-(4-
chlorophenyl)-N-{ (2S,4R)-2-methyl-1-[4-(3-morpholin-4-ylpropoxy)benzoyl]-
1,2,3,4-
tetrahydroquinolin-4-yl}acetamide; ' N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-
{4-[(4-
oxopentyl)oxy]benzoyl}-1,2,3,4-tetrahydroquinolin-4-yl)acetamide; N-(4-
Chlorophenyl)-N-{(2S,4R)-
1-[4-(3-hydroxy-3-methylbutoxy)benzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl}acetamide; N-
(4-Chlorophenyl)-N-((2S,4R)-1-{4-[(4-hydroxy-4-methylpentyl)oxy]benzoyl }-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl)acetamide; N-(4-chlorophenyl)-N-((2S,4R)-1-{4-[(1-
ethylpiperidin-4-
yl)methoxy]benzoyl}-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl)acetamide; N-(4-
chlorophenyl)-N-
((2S,4R)-1-{4-[3-(1H-imidazol-1-yl)propoxy]benzoyl }-2-methyl-1,2,3;4-
tetrahydroquinolin-4-
yl)acetamide; (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-methyl-3,4-dihydro-
2H-
benzo[1,4]oxazine-7-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide; N-
(4-chlorophenyl)-N-
{ (2S,4R)-2-methyl-1-[(4-methyl-3,4-dihydro-2H-1,4-benzoxazin-6-yl)carbonyl]-
1,2,3,4-
tetrahydroquinolin-4-yl}acetamide; N-(4-chlorophenyl)-N-{(2S,4R)-1-[4-(4-
ethylpiperazin-1-
yl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}acetamide; N-{(2S,4R)-1-
[4-(4-acetylpiperazin-
1-yl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}-N-(4-
chlorophenyl)acetamide; N-(4-
chlorophenyl)-N-{ (2S,4R)-1-[4-(4-glycoloylpiperazin-1-yl)benzoyl]-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}acetamide; N-(4-chlorophenyl)-N-{(2S,4R)-2-methyl-1-[4-
(3-morpholin-4-
ylprop-1-yn-1-yl)benzoyl]-1,2,3,4-tetrahydroquinolin-4-yl}acetamide; 4-(4-
{[(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-yl]carbonyl}phenyl)but-
3-ynoic acid; N-
[(2S,4R)-1-(1H-benzimidazol-2-ylcarbonyl)-2-methyl-1,2,3,4-tetrahydroquinolin-
4-yl]-N-(4-
chlorophenyl)acetamide; N-[(2S,4R)-1-(1,3-benzothiazol-2-ylcarbonyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-chlorophenyl)acetamide; N-(4-chlorophenyl)-N-
{(25,4R)-2-methyl-1-
[(1-methyl-1H-1,2,3-benzotriazol-5-yl)carbonyl]-1,2,3,4-tetrahydroquinolin-4-
yl}acetamide; N-(4-
chlorophenyl)-N-{(2S,4R)-1-[(1-isopropyl-1H-benzimidazol-5-yl)carbonyl]-2-
methyl-1,2,3,4- .
tetrahydroquinolin-4-yl}acetamide; [4-(4-{[(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenyl)piperazin-1-yl]acetic acid; N-
((2S,4R)-1-{4-[4-(2-amino-
2-oxoethyl)piperazin-1-yl]benzoyl }-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl)-
N-(4-
chlorophenyl)acetamide; 3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)propanoic acid; 4-(4-{[(2S,4R)-4-
[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-yl]carbonyl } phenoxy)-
2,2-
dimethylbutanoic acid; { 1-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)methyl]cyclopropyl}acetic acid; (2E)-
4-(4-{[(2S,4R)-4-
- 25 -
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)but-2-
enoic acid; 3-(4-{[(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)-2,2-dimethylpropanoic acid; (2E)-4-(4-{[(2S,4R)-4-
[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)-2-
methylbut-2-
enoic acid; N-(4-chlorophenyl)-N-{ (2S,4R)-2-methyl-1-[4-(3-
{[(trifluoromethyl)sulfonyl]amino}propoxy)benzoyl]-1,2,3,4-tetrahydroquinolin-
4-yl}acetamide; N-
(4-chlorophenyl)-N-((2S,4R)-1-{ [1-(2-hydroxyethyl)-2-methyl-1H benzimidazol-5-
yl]carbonyl }-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl)acetamide; 5-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-
methyl-3,4-dihydroquinolin-1(2H)-yl]carbonyl}-2-methyl-1H-benzimidazol-1-
yl)acetic acid; 3-(5-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-
yl]carbonyl }-1H-
1,2,3-benzotriazol-1-yl)propanoic acid; (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(1-
isopropyl-1H-indazole-
5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide; and (2S,4R)-
N-(4-Chloro-
phenyl)-N-(2-methyl-1-{4-[3-(1H-tetrazol-5-yl)-propoxy]-benzoyl}-1,2,3,4-
tetrahydro-quinolin-4-yl)-
acetamide.
[0087] The classes and subclasses described in more detail below and in the
specification herein
apply to each of the embodiments for compound I-A as described above.
[0088] In one embodiment, the present invention is a compound represented by
Structural
Formulas (II-A), (III-A) or (IV-A).
Rv ~ 4 Rte.
N R N R
V-T-RY -T-Ar-RY
(RZ)o,l, or 2
(II-A) (III-A)
-26-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
O
R3 ~
~N~Ra
~s
N ''~R2
0~~~1
V-T-Cy-RY
eJ
(RZ~O,i, or 2
(~-A)
[0089] Ar in Structural Formula (III-A) is a monocyclic aromatic ring. Cy in
Structural Formula
(1V) is a monocyclic non-aromatic carbocyclic or heteroeyclic group. All other
variables in Structural
Formulas (II-A)-(IV-A) are as described for Structural Formula (I-A).
Preferably, V in Structural
Formulas (II-A)-(IV-A) is a covalent bond, -O-, or N(R')-, and T in Structural
Formulas (II-A)-(IV-
A) is a CI_6 straight chain alkylene optionally substituted at any one or more
substitutable carbon
atoms with halide, alkyl, gem dialkyl, gem dihalo, haloalkyl, spiro
cycloalkyl, optionally N
substituted nitrogen containing spiro non-aromatic heterocyclic group, amine,
alkylamine,
dialkylamine, or hydroxyl.
[0090] Alternatively, in Structural Formula (II-A)-(IV-A) V is a covalent
bond, -NR'- or =O-;
and T is a Cl_lo straight chain alkylene (preferably Cl_5, more preferably
Cl_3) optionally mono=
substituted at any substitutable carbon atom with halide, alkyl, haloalkyl,
amine, dialkylamine, or
hydroxyl.
[0091] Preferably, RY in Structural Formula (I-A) and (II-A) is -C(O)ORS, -
C(O)N(RS)z,
-NRSC(O)R5, -NRSC(O)ORS, -S(O)zN(RS)z, -NRSS(O)zRs, -ORS, -CN, -NRSC(O)N(RS)z,
-N(Rj)z, an
optionally substituted non-aromatic heterocyclic group represented by R', or
an optionally substituted
heteroaryl group represented by R8. R~ is an optionally substituted
piperidinonyl, oxazolidinyl,
oxazolidinonyl, thiazolidinyl, tetrahydrofuranyl, tetrahydropyranyl,
thiazolidinyl, tetrahyrothiophene,
morpholinyl, thiomorpholinyl, imidazolidinyl, imidazolidinonyl, dioxanyl,
dioxolanyl, dithiolanyl,
pyrrolidinyl, pyrrolidinonyl, piperazinyl, or piperidinyl. R$ is an optionally
substituted furanyl,
tetrazolyl, oxazolyl, isoxazolyl, oxadiazolyl, pyrrolyl, pyrazolyl, pyridinyl,
pyrimidyl, thiazolyl,
thienyl, or imidazolyl, and all the remainder of the variables are as
described in the previous
paragraph.
[0092] More preferably, RY in Structural Formula (I-A) and (II-A) is -C(O)ORS,
-C(O)N(RS)z, -
NRSC(O)R5, -NRSC(O)ORS, -S(O)zN(R5)z, -NRSS(O)zRS, -NRSC(O)N(RS)z, -OH, an
optionally
-27-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
substituted non-aromatic heterocyclic group represented by R' or an optionally
substituted heteroaryl
group represented by R8. R' is piperidinonyl, morpholinyl, imidazolidinonyl,
pyrrolidinyl,
pyrrolidinonyl, piperazinyl, or piperidinyl. R$ is tetrazolyl, oxazolyl,
oxadiazolyl, pyrrolyl, pyrazolyl,
pyridinyl, or imidazolyl. RS is independently H or alkyl, or N(RS)2 is a
nitrogen-containing non-
aromatic heterocyclic group. V is a covalent bond, or -O-; and T is a Cl_5
straight chain alkylene
optionally substituted at the carbon atom adjacent to RY with halide, alkyl,
gem dialkyl, gem dihalo,
haloalkyl, spiro cycloalkyl, optionally N-substituted nitrogen containing
spiro non-aromatic
heterocyclic group, amine, dialkylamine, or hydroxyl.
[0093] More preferably, Rl in Structural Formula (I-A)-(III-A) is a phenyl
ring optionally
substituted by at the »ieta and para positions (more preferably para) by V-T-
RY or V-T-M-RY. Even
more preferably RY is -C(O)ORS, -C(O)N(RS)2, -OH, N-morpholinyl, 2-
morpholinyl, 3-morpholinyl,
N substituted 2-morpholinyl, N-substituted 3-morpholinyl, N-imidazolidinyl, 2-
imidazolidinyl, 4-
imidazolidinyl, 5-imidazolidinyl, N-substituted 2-imidazolidinyl, N'-
substituted N-imidazolidinyl, N-
substituted 4-imidazolidinyl, N substituted 5-imidazolidinyl, N-
imidazolidinonyl, 4-imidazolidinonyl;
5-imidazolidinoriyl, N-substituted 4-imidazolidinonyl, N-substituted 5-
imidazolidinonyl, N-
pyrrolidinyl, 2-pyrrolidinyl, 3-pyrrolidinyl, N-substituted 2-pyrrolidinyl, N-
substituted 3-pyrrolidinyl,
N-pyrrolidin-2-onyl" 3-pyrrolidin-2-onyl, 4-pyrrolidin-2-onyl, 5-pyrrolidin-2-
onyl, N-substituted 3-
pyrrolidin-2-only, N-substituted 4-pyrrolidin-2-only, N-substituted 5-
pyrrolidin-2-onyl, N-pymolidin-
3-onyl, 2-pyrrolidin-3-onyl, 4-pyrrolidin-3-onyl, 5-pyrrolidin-3-onyl, N-
substituted 2-pyrrolidin-3-
onyl N-substituted 4-pyrrolidin-3-onyl, N-substituted 5-pyrrolidin-3-onyl, N-
piperidinyl, 2-
piperidinyl, 3-piperidinyl, 4-piperidinyl, N-substituted 2-piperidinyl, N
substituted 3-piperidinyl, N-
substituted 4-piperidinyl, N-piperidin-2-onyl, 3-piperidin-2-onyl, 4-piperidin-
2-onyl, 5-piperidin-2-
onyl, 6-piperidiri-2-onyl, N-substituted 3-piperidin-2-onyl, N substituted 4-
piperidin-2-onyl, N-
substituted 5-piperidin-2-onyl, N-substituted 6-piperidin-2-onyl, N piperidin-
3-onyl, 2-piperidin-3-
onyl, 4-piperidin-3-onyl, 5-piperidin-3-onyl, 6-piperidin-3-onyl, N
substituted 2-piperidin-3-onyl, N-
substituted 4-piperidin-3-onyl, N-substituted 5-piperidin-3-onyl, N-
substituted 6-piperidin-3-onyl, N-
piperidin-4-onyl, 2-piperidin-4-onyl, 3-piperidin-4-onyl, 5-piperidin-4-onyl,
6-piperidin-4-onyl, N-
substituted 2-piperidin-4-onyl, N substituted 3-piperidin-4-onyl, N-
substituted 5-piperidin-4-onyl, N-
substituted 6-piperidin-4-onyl, N-piperazinyl, 2-piperazinyl, N'-substituted N-
piperazinyl, N
substituted 2-piperazinyl, furanyl, N-tetrazolyl, 5-tetrazolyl, N-substituted
5-tetrazolyl, 4-
(1,2,3)oxadiazolyl, 5-(1,2,3)oxadiazolyl, 3-(1,2,4)oxadiazolyl, 5-
(1,2,4)oxadiazolyl, 3-
(1,2,5)oxadiazolyl, 4-(1,2,5)oxadiazolyl, 2-(1,3,4)oxadiazolyl, 5-
(1,3,4)oxadiazolyl, N-pyrrolyl, 2-
pyrrolyl, 3-pyrrolyl, N-substituted 2-pyrrolyl, N-substituted 3-pyrrolyl, N-
pyrazolyl, 3-pyrazolyl, 4-
pyrazolyl, 5-pyrazolyl, N-substituted 3-pyrazolyl, N-substituted 4-pyrazolyl,
N-substituted 5-
pyrazolyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, N-imidazolyl, 2-imidazolyl,
4-imidazolyl, 5-
imidazolyl; N-substituted 2-imidazolyl, N-substituted 4-imidazolyl, or N-
substituted 5-imidazolyl. V
-28-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
is -O-, and T is a Cl_3 straight chain alkylene substituted at the carbon
adjacent to RY with fluoro,
methyl, gem dimethyl, gem difluoro fluoromethyl, spiro cyclopropyl, spiro
cyclobutyl, optionally N-
substituted spiro azetidinyl, optionally N-substituted spiro aziridinyl,
optionally N-substituted sprio
pyrrolidinyl, optionally N substituted spiro piperidinyl, amine, methylamine,
dimethylamine, or
hydroxyl.
[0094] Still more preferably RY is -C(O)ORS, -C(O)N(R5)z, -OH, N tetrazolyl, 5-
tetrazolyl, N-
substituted 5-tetrazolyl, N-imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-
imidazolyl; N-substituted 2-
imidazolyl, N-substituted 4-imidazolyl, or N-substituted 5-imidazolyl. RS is
H, methyl, or ethyl. . R'
in Structural Formula (I-A)-(III-A) is a phenyl ring optionally substituted by
at the para position by
V-T-RY or V-T-M-RY.
[0095] In another preferred embodiment, the present invention is a compound
represented by
Structural Formula (V-A):
O
R3
~N~Ra
N ''R2
Of. ~ \~
V-Rs.
~J
~Rz)o,i, ore
(V-A)
[0096] All variables in Structural Formula (V-A) are as described for
Structural Formula '(I-A).
Preferably R9 in Structural Formula (V-A) is an optionally substituted
cyclohexanyl, oxazolidinyl,
oxazolidinonyl, thiazolidinyl, tetrahydrofuranyl, tetrahydropyranyl,
thiazolidinyl, tetrahydrothienyl,
morpholinyl, thiomorpholinyl, imidazolidinyl, imidazolidinonyl, dioxanyl,
dioxolanyl, dithiolanyl,
pyrrolidinyl, pyrrolidinonyl, piperazinyl, isothiazolidinyl S,S, dioxide,
piperidinyl, or 1,2,5-
thiadiazolidine S, S-dioxide. In other embodiments, R9 in Structural Formula
(V-A) is an optionally
substituted cyclohexanyl, oxazolidinyl, oxazolidinonyl, thiazolidinyl,
tetrahydrofuranyl,
tetrahydropyranyl, thiazolidinyl, tetrahydrothienyl, morpholinyl,
thiomorpholinyl, imidazolidinyl,
imidazolidinonyl, dioxanyl, dioxolanyl, dithiolanyl, pyrrolidinyl,
pyrrolidinonyl, piperazinyl,
isothiazolidinyl S,S, dioxide, or piperidinyl. R9 is preferably meta or para
to the carbon atom bonded
to the carbonyl, more preferably para. V is preferably a covalent bond or -O-,
more preferably a
covalent bond.
-29-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[0097] More preferably, R9 in Structural Formula (V-A) is oxazolidinyl,
thiazolidinyl,
tetrahydrofuranyl, morpholinyl, imidazolidinyl, imidazolidinonyl,
pyrrolidinyl, pyrrolidinonyl,
piperazinyl, or piperidinyl, each optionally substituted by alkyl, halide,
haloalkyl, hydroxyalkyl, -
C(O)ORIZ, -C(O)Riz~ -OC(O)Riz~ -RizC(O~ORiz~ -C(O)N(Riz)z~ -NRIZC(O)Riz~ -
yzC(O)ORiz~ _
S(O)zRiz~ -S(O)zCOR'z, -S(O)zN(Riz)z~ -S(O)zOR'z~ -S(O)OR'z~ -ORIZ, -SRIZ, -
CN, _
yzC(O)N(Riz)z, -OC(O)N(R'z)z~ -(CHz)nCOzH~ -(CHz)nC(O)~°~ -
(CHz)nC(CH3)zCOzH~
-(CHz)nC(CH3)zC(O)NR° or -N(Rlz)z, n is an integer from 1 to 4, and
each Rlz is independently -H,
alkyl, haloalkyl, or hydroxyalkyl; and all of the other variables are as
described in the previous
paragraph. Alternatively, for those groups represented by R9 which have a
substitutable ring nitrogen,
the group is N substituted with Tz-RY', which is defined below, and optionally
substituted at one or
more substitutable ring carbon atoms with one or more groups listed above in
this paragraph.
[0098] More preferably, R9 in Structural Formula (V-A) is N-morpholinyl, 2-
morpholinyl, 3-
morpholinyl, N-substituted 2-morpholinyl, N substituted 3-morpholinyl, N-
pyrrolidinyl, 2-
pyrrolidinyl, 3-pyrrolidinyl, N substituted 2-pyrrolidinyl, N-substituted 3-
pyrrolidinyl, N piperazinyl,
2-piperazinyl, N'-substituted N piperazinyl, N substituted 2-piperazinyl, N-
piperidinyl, 2-piperidinyl, .
3-piperidinyl, 4-piperidinyl, N-substituted 2-piperidinyl, N substituted 3-
piperidinyl, N substituted 4-
piperidinyl, each optionally substituted at any substitutable carbon atom by
alkyl, halide, haloalkyl,
hydroxyalkyl, -C(O)OR'z, -C(O)Rlz, -OC(O)R'z, or -C(O)N(Rlz)z, and wherein the
N-substituents are
alkyl, haloallcyl, hydroxyalkyl, -C(O)ORIZ, -C(O)R'z, -(CHz)°COzH, -
(CHz)"C(O)NR°, _
(CHz)"C(CH3)zCOzH, -(CHz)"C(CH3)zC(O)NR° or -C(O)N(R'z)z. For these
groups represented by R9
which have a substitutable ring N, one preferred N substituent is Tz-RYI,
which is defined below.
[0099] Even more preferably, R9 in Structural Formula (V-A) is N-piperidinyl,
2-piperidinyl; 3-
piperidinyl, 4-piperidinyl, N-substituted 2-piperidinyl, N substituted 3-
piperidinyl, N-substituted 4-
piperidinyl, N-piperazinyl, 2-piperazinyl, N'-substituted N-piperazinyl, or N
substituted 2-piperazinyl,
and is optionally substituted by at any substitutable carbon atom by chloride,
fluoride, bromide,
methyl, ethyl, -C(O)OR'z, -OC(O)Rlz, -C(O)R'z or C(O)NHz, and wherein the N
substituents are
methyl, ethyl, -C(O)ORIZ, _C(p)Riz~ _(CHz)°C02H~ -(CHz)nC(O)~°~ -
(CHz)nC(CHs)zCOzH~ _
(CHz)"C(CH3)zC(O)NR°, or -C(O)NHz, and each R~z is independently -H,
methyl, or ethyl. For these
groups represented by R9 which have a substitutable ring N, one preferred N
substituent is Tz-RY',
which is defined below.
[00100] In another preferred embodiment, the present invention is a compound
represented by
Staructural Formula (VI-A):
-30-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
O
RvN~R4
A ~,,
\ N ~'R2
O/ ~ \
B
(VI-A)
[00101] B is an optionally substituted monocyclic non-aromatic heterocyclic
ring represented by
R'°, or a monocyclic aromatic ring (preferably heteroaromatic)
represented by R'3; all other variables
in Structural Formula (VI-A) are as described for Structural Formula (I-A).
[00102] Preferably, R'° in Structural Formula (VI-A) is oxazolidinyl,
oxazolidinonyl, dioxolanyl,
thiazolidinyl, tetxahydrofuranyl, tetrahydropyranyl, tetrahydrothiopyranyl,
tetrahydrothienyl,
morpholinyl, thiomorpholinyl, imidazolidinyl, imidazolidinonyl, dioxanyl,
dithiolanyl, pyrrolidinyl, '
piperazinyl, piperidinyl, piperidinyl, tetrahydrothienyl S,S dioxide,
thiomorpholinyl S,S dioxide,
tetrahydrothiopyranyl S,S dioxide, each of which are optionally substituted.
R'3 is pyrazolyl,
triazolyl, imidazolyl, furanyl, pyrrolyl, thienyl, cyclopentadienyl, and
thienyl S,S dioxide, each of
which are optionally substituted. All other variables in Structural Formula
(VI-A) are as described in
the previous paragraph.
[00103] Other examples of suitable values for R'° include
tetrahydrofuranyl, tetrahydropyranyl,
morpholinyl, imidazolidinyl, imidazolidinonyl, pyrrolidinyl, piperazinyl, or
piperidinyl each of which
is optionally substituted at any substitutable carbon ring atom with alkyl,
halide, haloalkyl,
hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -OC(O)R'z, -R'zC(O)OR'z-, -C(O)N(R'z)z, -
NR'zC(O)R'z,
-yzC(O)ORiz~ -S(O)zRiz~ -S(O)zCOR'z, -S(O)zN(Riz)z, -S(O)zORlz~ -S(O)ORIZ~ -
ORIZ~ -SR~z~ -CN
-~12C(O)N(R12)2~ -OC(O)N(R'z)z~ -N(RIZ)z~ -(CHzO-aCOzRlz~ -O(CHzO-aCOzRlz~ -
(CHz)t-
~CON(R'z)z~ -O(CHz)mCON(R'z)z, -(CHz)o-3(C(CH3)2)C02R12~ -O(CHz)o-
3(C(CH3)2)C02R'Ze -(CHz)o-
3(C(CH3)z)CON(R'z)z, or -O(CHz)o-3(C(CH3)z)CON(R'z)z, and each of which is
optionally substituted
at any substitutable ring nitrogen atom with alkyl, haloalkyl, hydroxyalkyl, -
C(O)OR'z, -C(O)R'z, -
R'zC(O)OR'z-, -C(O)N(R'z)z; and other examples of suitable values for R'3
include triazolyl,
imidazolyl, furanyl, pyrrolyl, thienyl, each of which is optionally
substituted at any substitutable ring
carbon atom with alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -
OC(O)R'z,
-C(O)OR'z-, -C(O)N(R'z)z~ -NR'zC(O)Riz~ _yzC(O)OR~z~ -S(O)zRiz~ -S(O)zCOR'z, -
S(O)zN(R~z)z~ -
S(O)zORIZ~ -S(O)ORIZ~ -ORIZ~ -SRIZ~ -CN~ -yzC(O)N(Riz)z, -OC(O)N(R'z)z~ -
N(R'z)z~ -(CHz)i-
aCOzR'z, -O(CHZO-aCOzR'z~ -(CHzh-aCON(R'z)z, -O(CHzh-aCON(R'z)z, -(CHz)o-
s(C(CH3)z)COzRIZ~ _
-31-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
O(CHz)o-s(C(CH3)2)C02R'2~ -(CHz)o-3(C(CHs)z)CON(R'z)z, or -O(CHz)o-
s(C(CH3)z)CON(R'z)z, and
each of which is optionally substituted at any substitutable ring nitrogen
atom with alkyl haloalkyl,
hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -R'zC(O)OR'z, -S(O)zR'z, S(O)zN(R'z)z, -
C(O)N(R'z)z. Each
R'z is independently H, alkyl, haloalkyl, or hydroxyalkyl.
[00104] Still other examples of suitable values for R'° include
tetrahydrofuranyl,
tetrahydropyranyl, morpholinyl, imidazolidinyl, imidazolidinonyl,
pyrrolidinyl, piperazinyl, or
piperidinyl each of which is optionally substituted at any substitutable
carbon ring atom with alkyl,
halide, haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -OC(O)R'z, -R'zC(O)OR'z-
, -G(O)N(R'z)z, -
yzC(O)Riz~ -yzC(O)ORiz~ _S(p)zRiz~ -S(O)zCOR'z, -S(O)zN(Riz)z, -S(O)zOR'z~ -
S(O)OR'z~ _
OR'z, -SR'z, -CN, -NR'zC(O)N(R'z)z, -OC(O)N(R'z)z, or -N(R'z)z, and each of
which is optionally
substituted at any substitutable ring nitrogen atom with alkyl haloalkyl,
hydroxyalkyl, -C(O)OR'z,
-C(O)R'z; -RIZC(O)OR'z-, -C(O)N(R'z)z; and other examples of suitable values
for R'3 include
triazolyl, imidazolyl, furanyl, pyrrolyl, thienyl, each of which is optionally
substituted at any
substitutable 'ring carbon atom with alkyl, halide, haloallcyl, hydroxyalkyl, -
C(O)OR'z, -C(O)R'z, -
OC(O)R'z, -C(O)OR'z-, -C(O)N(R'z)z~ -NR'zC(O)Riz~ -yzC(O)ORiz~ _S(O)zRiz~ -
S(O)zCOR'z, _
S(O)zN(Riz)z~ -S(O)zOR'z~ -S(O)OR'z~ -OR'z~ -SRIZ~ -CN~ -NR'zC(O)N(Riz)z, -
OC(O)N(R'z)z~ or -
N(R'z)z, and each of which is optionally substituted at any substitutable ring
nitrogen atom with alkyl
haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -R'zC(O)OR'z, -S(O)zR'z,
S(O)zN(R'z)z,
-C(O)N(R'z)z. Each R'z is independently H, alkyl, haloalkyl, or hydroxyalkyl.
[00105] Even more preferably, in Structural Formula (VI-A) R'° is
dioxolanyl, tetrahydrofuranyl,
morpholinyl, each optionally substituted at any substitutable carbon atom by
alkyl, halide, haloalkyl,
hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -OC(O)R'z, or -C(O)N(R'z)z, or each
optionally substituted at
any substitutable nitrogen atom by alkyl, haloallcyl, hydroxyalkyl, -C(O)OR'z,
-C(O)R'z, or -
C(O)N(R'z)z. R'3 1S triazolyl, imidazolyl, or pyrrolyl each optionally
substituted at any substitutable
carbon atom by alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)R'z, -
OC(O)R'z, or
-C(O)N(R'z)z, and each optionally substituted at any substitutable nitrogen
atom by alkyl, haloalkyl,
hydroxyalkyl, C(O)OR'z, -C(O)R'z, -R'zC(O)OR'z, -S(O)zR'z, S(O)zN(R'z)z, -
C(O)N(R'z)z.
[00106] Still more preferably, in Structural Formula (VI-A) R'° is
morpholinyl and is optionally
N-substituted by methyl, ethyl, -C(O)OR'z, C(O)NHz or -C(O)R'z, R'3 is
triazolyl and is optionally N-
substituted by methyl, ethyl, -C(O)OR'z, C(O)NHz or -C(O)R'z, and each R'z is
independently -H,
methyl, or ethyl.
[00107] In another preferred embodiment, in Structural Formula (VI-A) Ring B a
monocyclic
non-aromatic heterocycle or a monocyclic heteroaryl group comprising a ring
nitrogen atom that is
substituted with Tz-RY'. These monocyclic non-aromatic and heteroaryl groups
are optionally further
substituted. Preferably, the monocyclic non-aromatic heterocyclic ring is
represented by R'°,
Preferably, the monocyclic heteroaromatic ring is represented by R'3. Examples
of suitable values for
-32-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
RI° include oxazolidinyl, oxazolidinonyl, thiazolidinyl, morpholinyl,
thiomorpholinyl, imidazolidinyl,
imidazolidinonyl, pyrrolidinyl, piperazinyl, or piperidinyl, each of which is
N substituted with Tz-RYI
and optionally further substituted at any one or more ring carbon atoms by
alkyl, halide, haloalkyl,
hydroxyalkyl, -C(O)ORIZ, -C(O)R'z, -OC(O)R'z, -R'zC(O)OR'z-, -C(O)N(Rlz)z, -
NR'zC(O)R'z,
-yzC(O)ORiz~ -S(O)zRiz~ -S(O)zCOR'z, -S(O)zN(Riz)z~ -S(O)zOR'z~ -S(O)OR'2~ -
OR'z, -SR'z~ -CN
-yzC(O)N(Riz)z, -OC(O)N(R~z)z, -N(R'z)z, -(CHzh-aCOzR'z, -O(CHz)t-aCOzR'z, -
(CHz)i-
4CON(RIZ)z~ -O(CHz)i-aCON(R'z)z, -(CHz)o-s(C(CH3)z)COzR'z~ -O(CHz)o-
3(C(CH3)2)C02R'z~ -(CHz)o-
s(C(CHs)z)CON(R'z)z~ or -O(CHz)o-3(C(CHs)z)CON(Rlz)z.
[00108] Other examples of suitable values for Rl° include oxazolidinyl,
oxazolidinonyl,
thiazolidinyl, morpholinyl, thiomorpholinyl, imidazolidinyl, imidazolidinonyl,
pyrrolidinyl,
piperazinyl, or piperidinyl, each of which is N-substituted with Tz-RY' and
optionally further
substituted at any one or more ring carbon atoms by alkyl, halide, haloalkyl,
hydroxyalkyl, -
C(O)OR'z, -C(O)Riz~ -OC(O)Riz~ -RizC(O)ORiz-~ -C(O~N(Riz)z~ -NR'zC(O)Riz~ -
yzC(O)ORiz~ _.
S(O)zRiz~ S(O)zCOR'z, -S(O)zN(Riz)z~ -S(O)zOR'z~ -S(O)OR'z~ -OR'z~ -SR'z~ -CN
-NRizC(O)N(R'z)z~ -OC(O)N(R'z)z~ or -N(R'z)z~
[00109] Examples of suitable values for R'3 include pyrazolyl, triazolyl,
imidazolyl, or pyrrolyl,
each of which is N-substituted with Tz-RY' and optionally further substituted
at any one or more ring
carbon atoms with alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)Rlz,
-OC(O)Rlz, -
R'zC(O)OR'z-, -C(O)N(R'z)z~ -NR'zC(O)Ria~ -yzC(O)ORiz~ _S(O)zRiz~ -S(p)zCOR'z,
_
S(O)zN(Riz)z~ -S(O)zOR'z, -S(O)OR'z, -OR'z, -SR'z; -CN, _yzC(O)N(Riz)z, -
OC(O)N(Rlz)z~
-N(Riz)z~ -(CHzO-aCOzR'z~ -O(CHz)i-aCOzR'z~ -(CHz)mCON(RIZ)z~ -
O(CHz)mCON(RIZ)z, -(CHz)o-
3(~(~H3)2)C~2R'2~ -O(CHz)o-s(C(CH3)z)COzR'z, -(CHz)o-s(C(CHs)z)CON(R'z)z~ or -
O(CHz)o-
3(C(CH3)2)CON(R~2)2.
[001,10] Other examples of suitable values for R13 include pyrazolyl,
triazolyl, imidazolyl, or
pyrrolyl, each of which is N-substituted with Tz-RY' and optionally further
substituted at any one or
more ring carbon atoms with alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR'z,
-C(O)R'z, -
OC(O)Rlz, _RizC(O)ORiz_~ -C(O)N(Riz)z~ -NRIZC(O)Riz~ -~tzC(O)ORiz~ -S(O)zRiz~ -
S(O)zCORIZ, _
S(O)zN(Ria)z~ -S(O)zOR'z~ -S(O)OR'z~ -OR'z, -SR'2, -CN~ -NRIZC(O)N(Riz)z~ -
OC(O)N(R'z)z~ or -
N(R'z)z. Each Rlz is independently H, alkyl, haloalkyl, or hydroxyalkyl. All
other variables are as
described above for Structural Formula (VI-A).
[00111] Preferably, Tz in Structural Formula (VI-A) is Cl_6 is a straight
chain alkylene optionally
substituted at any one or more substitutable carbon atoms with halide, alkyl,
gem dialkyl, gem dihalo,
haloalkyl, spiro cycloalkyl, optionally N-substituted nitrogen containing
spiro non-aromatic
heterocyclic group, amine, alkylamine, dialkylamine, or hydroxyl. RYl is -
C(O)ORS, -C(O)N(RS)2,
-NRSC(O)R5, -NRSC(O)ORS, -S(O)zN(RS)z, -NRSS(O)zRs, -ORS, -CN, -NRSC(O)N(RS)z,
-N(R.S)z, an
optionally substituted non-aromatic heterocyclic group represented by R', or
an optionally substituted
-33
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
heteroaryl group represented by R8. R' is an optionally substituted
piperidinonyl, oxazolidinyl,
oxazolidinonyl, thiazolidinyl, tetrahydrofuranyl, tetrahydropyranyl,
thiazolidinyl, tetrahyrothiophene,
morpholinyl, thiomorpholinyl, imidazolidinyl, imidazolidinonyl, dioxanyl,
dioxolanyl, dithiolanyl,
pyrrolidinyl, pyrrolidinonyl, piperazinyl, or piperidinyl. R8 is an optionally
substituted furanyl,
tetrazolyl, oxazolyl, isoxazolyl, oxadiazolyl, pyrrolyl, pyrazolyl, pyridinyl,
pyrimidyl, thiazolyl,
thienyl, or imidazolyl.
[00112] Other examples of suitable values for R'° include morpholinyl,
thiomorpholinyl,
imidazolidinyl, imidazolidinonyl, pyrrolidinyl, piperazinyl, or piperidinyl
each of which is . N-
substituted with Tz-RYA and further optionally substituted at any
substitutable carbon ring atom with
alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR'z, -C(O)Rlz, -OC(O)Rlz, -
R'zC(O)OR'z-, -
C(O)N(R'z)z~ -NR'zC(O)Riz~ _NRizC(O)ORiz~ _S(O)zRiz~ _S(O)zCORIZ, -
S(O)zN(Riz)z, -S(O)zOR'z~
-S(O)ORIZ, -OR'z, -SR'z, -CN, -NRIZC(O)N(Rlz)z, -OC(O)N(R'z)z, or -N(R'z)z.
Other examples of
suitable values for R13 include triazolyl, imidazolyl, or pyrrolyl, each of
which is N substituted with
Tz-RY' and further optionally substituted at any substitutable ring carbon
atom with alkyl, halide,
haloalkyl, hydroxyalkyl,. -C(O)ORIZ, -C(O)RIZ, -OC(O)Rlz, -RIZC(O)ORIZ-, -
C(O)N(R'z)z,
-yzC(O)Riz~ -yzC(O)ORiz~ _S(O)zRiz~ -S(O)zCORIZ, -S(O)zN(R~z)z~ -S(O)zORl2, -
S(O)ORIZ~ _
ORIZ, -SRIZ, -CN, -NRIZC(O)N(Riz)z, -OC(O)N(Rlz)z, or -N(Rlz)z. RYl is -
C(O)ORS, -C(O)N(RS)z, -
NRSC(O)R5, -NRSC(O)ORS, -S(O)zN(RS)z, -NRSS(O)zRS, -NRSC(O)N(RS)z, -OH, an
optionally
substituted .non-aromatic heterocyclic group represented by R' or an
optionally substituted heteroaryl
group represented by R8. Each RS is independently H or alkyl, or N(RS)z is a
nitrogen-containing non-
aromadc heterocyclic group. R' is piperidinonyl, morpholinyl,
imidazolidinonyl, pyrrolidinyl,
pyrrolidinonyl,-piperazinyl, or piperidinyl. R8 is tetrazolyl, oxazolyl,
oxadiazolyl, pyrrolyl, pyrazolyl,
pyridinyl, or imidazolyl. Tz is a C~_5 straight chain alkylene optionally
substituted at the carbon atom
adjacent to RY with halide, alkyl, gem dialkyl, gem dihalo, haloalkyl, spiro
cycloalkyl, optionally N-
substituted nitrogen containing spiro non-aromatic heterocyclic group, amine,
dialkylamine~ or
hydroxyl.
[00113] Preferably, other examples of suitable values for Rl° include
morpholinyl, pyrrolidinyl,
piperazinyl, or piperidinyl each of which is N-substituted with Tz-Ryl and
further optionally
substituted at any substitutable carbon atom by alkyl, halide, haloalkyl,
hydroxyalkyl, -C(O)OR'z, -
C(O)Rlz, -OC(O)Rlz. Other examples of suitable values for R'3 include
imidazolyl, or pyrrolyl each
of which is N-substituted with Tz-RYl and further optionally substituted at
any substitutable carbon
atom by alkyl, halide, haloalkyl, hydroxyalkyl, -C(O)OR~z, -C(O)R'z, -
OC(O)R'z, or -C(O)N(R'z)z,
and each optionally substituted at any substitutable nitrogen atom by alkyl,
haloalkyl, hydroxyalkyl,
C(O)OR'z, -C(O)R~z~ _RizC(O)ORiz~ _S(O)zR~z~ S~O)zN(Riz)z~ -C(O)N(R'z)z. RYl
is -C(O)ORS, -
C(O)N(RS)z, -OH, N-morpholinyl, 2-morpholinyl, 3-morpholinyl, N-substituted 2-
morpholinyl, N
substituted 3-morpholinyl, N imidazolidinyl, 2-imidazolidinyl, 4-
imidazolidinyl, 5-imidazolidinyl, N-
-34-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
substituted 2-imidazolidinyl, N'-substituted N imidazolidinyl, N substituted 4-
imidazolidinyl, N-
substituted 5-imidazolidinyl, N-imidazolidinonyl, 4-imidazolidinonyl, 5-
imidazolidinonyl, N-
substituted 4-imidazolidinonyl, N-substituted 5-imidazolidinonyl, N-
pyrrolidinyl, 2-pyrrolidinyl, 3-
pyrrolidinyl, N-substituted 2-pyrrolidinyl, N-substituted 3-pyrrolidinyl, N-
pyrrolidin-2-onyl" 3-
pyrrolidin-2-onyl, 4-pyrrolidin-2-onyl, 5-pyrrolidin-2-onyl, N-substituted 3-
pyrrolidin-2-only, N
substituted 4-pyrrolidin-2-only, N-substituted 5-pyrrolidin-2-onyl, N-
pyrrolidin-3-onyl, 2-pyrrolidin-
3-onyl, 4-pyrrolidin-3-onyl, 5-pyrrolidin-3-onyl, N-substituted 2-pyrrolidin-3-
onyl N-substituted 4-
pyrrolidin-3-onyl, N-substituted 5-pyrrolidin-3-onyl, N piperidinyl, 2-
piperidinyl, 3-piperidinyl, 4-
piperidinyl, N-substituted 2-piperidinyl, N substituted 3-piperidinyl, N
substituted 4-piperidinyl, N-
piperidin-2-onyl, 3-piperidin-2-onyl, 4-piperidin-2-onyl, 5-piperidin-2-onyl,
6-piperidin-2-onyl, N-
substituted 3-piperidin-2-onyl, N-substituted 4-piperidin-2-onyl, N-
substituted 5-piperidin-2-onyl, N
substituted 6-piperidin-2-onyl, N-piperidin-3-onyl, 2-piperidin-3-onyl, 4-
piperidin-3-onyl; 5-
piperidin-3-onyl; 6-piperidin-3-onyl, N-substituted 2-piperidin-3-onyl, N-
substituted 4-piperidin-3-
onyl, N substituted 5-piperidin-3-onyl, N-substituted 6-piperidin-3-onyl, N
piperidin-4-onyl, 2-
piperidin-4-onyl, 3-piperidin-4-onyl, 5-piperidin-4-onyl, 6-piperidin-4-onyl,
N-substituted 2-
piperidin-4-onyl, N substituted 3-piperidin-4-onyl, N substituted 5-piperidin-
4-onyl, N substituted 6-
piperidin-4-onyl, N piperazinyl, 2-piperazinyl, N'-substituted N-piperazinyl,
N substituted 2- .
piperazinyl, furanyl, N-tetrazolyl, 5-tetrazolyl, N-substituted 5-tetrazolyl,
4-(1,2,3)oxadiazolyl, 5-
(1,2,3)oxadiazolyl, 3-(1,2,4)oxadiazolyl, 5-(1,2,4)oxadiazolyl, 3-
(1,2,5)oxadiazolyl, 4-
(1,2,5)oxadiazolyl, 2-(1,3,4)oxadiazolyl, 5-(1,3,4)oxadiazolyl, N pyrrolyl, 2-
pyrrolyl, 3-pyrrolyl, N-
substituted 2-pyrrolyl, N-substituted 3-pyrrolyl, N-pyrazolyl, 3-pyrazolyl, 4-
pyrazolyl, 5-pyrazolyl, N-
substituted 3-pyrazolyl, N-substituted 4-pyrazolyl, N-substituted 5-pyrazolyl,
2-pyridinyl, 3-pyridinyl,
4-pyridinyl, N-imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazolyl; N-
substituted 2-imidazolyl, N-
substituted 4-imidazolyl, or N-substituted 5-imidazolyl. T2 is a Cl_3 straight
chain alkylene substituted
with fluoro, methyl, gem dimethyl, gem difluoro fluoromethyl, spiro
cyclopropyl, spiro cyclobutyl,
optionally N-substituted spiro azetidinyl, optionally N-substituted spiro
aziridinyl, optionally N-
substituted spiro pyrrolidinyl, optionally N-substituted spiro piperidinyl,
amine, methylamine,
dimethylamine, or hydroxyl. '
[00114] More preferably, other examples of suitable values for R'°
include morpholinyl, N-
substituted with TZ-RY' and further optionally substituted at the carbon alpha
to the nitrogen atom
with methyl or gem dimethyl. Other examples of suitable values for R'3 include
imidazolyl N-
substituted with TZ-RY' and further optionally substituted at the carbon alpha
to the nitrogen atom
with methyl or gem dimethyl. RY' is -C(O)ORS, -C(O)N(RS)Z, -OH, N-tetrazolyl,
5-tetrazolyl, N-
substituted 5-tetrazolyl, N-imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-
imidazolyl; N-substituted 2-
imidazolyl, N-substituted 4-imidazolyl, or N-substituted 5-imidazolyl.
-35-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00115] In one embodiment, in Structural Formula (VI-A) Rl is an optionally
substituted phenyl
ring fused to the group represented by Rl° as represented by the
following Structural Formulas:
I\ o i\ N I\ N I\ N
~~
_N ,N _O
I\ I\ I\ ,N I\
N
N ~ OJ
\ o Ss
I~ ~ i% I% I\
0
I\ N I\ N I\
oso ~So
I\ o (\ N \ s
I
I, I, I, N
p ~N
\ o \ OSO
I~ y I\
0
[00116] In one embodiment in Structural Formula (VI-A) R' is an optionally
substituted phenyl
ring fused to the group represented by R'3 as represented by the following
Structural Formulas:
I\ N/N I\ NJ I\ \N I\
N / N / N / O/
-36-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~ \ \ ~ \ \ ~ \ NI ~ \ oI
/ S~ / N / /
\ S I \ N II ~ \ N~ ~ \ N ~N
/ ~ / N / N /
\ OSO \ \ \
/ I ~/ S~ ~/ \
O/~O
[00117] The Structural Formulas shown above for the fused bicyclic rings can
be further
optionally substituted at one or more substitutable carbon atoms or nitrogen
atoms. Examples of
suitable substituents as described above for the groups represented by
R'° and RI3. In a more specific
embodiment, any one of the fused bicyclic ring systems shown above that have
substitutable nitrogen
atoms are N-substituted with Tz-RY'. Tz-RY' is as described above.
As described above, Ring A (preferably phenyl ring A) in Structural Formulas
(I-A)-(VI-A) is
optionally substituted (preferably at the six and seven positions) with one or
more groups represented
by R'4; in Structural Formulas (I-A)-(V-A) RI is an aromatic group (preferably
a phenyl ring)
optionally substituted by 1-2 independently selected groups represented by RZ;
and in Structural
Formulas (I-A)-(VI-A) R3 is an aromatic group (preferably e.g., a phenyl ring)
optionally substituted
with one or more groups represented by R'1. Suitable values for R'4, RZ, and
R" are ones that do not
substantially decrease the ability of the compound of the invention to inhibit
CRTH2. Examples of
suitable substituents are halogen, haloalkyl, R°, -OR°, -
O(haloalkyl), -SR°, -NOz, -CN, -N(R')z, -
NR'COzR°, -NR'C(O)R°, -NR'NR'C(O)R°, -N(R')C(O)N(R')z, -
NR'NR'C(O)N(R')z, -NR'NR'COZR°,
-C(O)C(O)R°, -C(O)CHZC(O)R°, -COzR°, -C(O)R°, -
C(O)N(R°)z, -OC(O)R°, -OC(O)N(R°)z, -S(O)zR°,
-SOzN(R')z, -S(O)R°, -NR'SOZN(R')z, -NR'SOzR°, -C(=S)N(R')a, and
-C(=NH)-N(R')z. Additional
values for R'1 include 3,4-methylene-dioxy and 3,4-ethylene-dioxy. In other
embodiments, suitable
substituents for RI' also include halogen, haloalkyl, R°, -OR°, -
O(haloalkyl), -SR°, 3,4-methylene-
dioxy, 3,4-ethylene-dioxy, -NOz, -CN, -N(R')z, -NR'COZR°, -
NR'C(O)R°, -NR'NR'C(O)R°,
-N(R')C(O)N(R')z, -NR'NR'C(O)N(R')z, -NR'NR'COZR°, -C(O)C(O)R°, -
C(O)CHZC(O)R°, -COZR°,
-C(O)R°~ -C(O)N(R°)z~ -OC(O)R°, -OC(O)N(R°)z~ -
S(O)zR°, -SOzN(R')z~ -S(O)R°~ -NR'SOZN(R')z~ -
NR'SOZR°, -C(=S)N(R')z~ -(CHz)i-aCOzR°~ -O(CHzh-aCOaR°, -
(CHz)i-aCON(R°)z~ -O(CHz)mCON(R°)z.
-(CHz)o-s(C(CH3)z)COzR°~ -O(CHz)o-s(C(CHs)z)COzR°s -(CHz)o-
s(C(CH3)z)CON(R°)a~ -O(CHz)o-
3(C(CH3)z)CON(R°)z, or -C(=NH)-N(R')z.
[00118] Each R is independently hydrogen, alkyl, -C(O)OR°,
S(O)zR°, or -C(O)R°. Each R° is
independently hydrogen or an alkyl group, non-aromatic heterocyclic group or
aromatic group and the
-37-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
alkyl, non-aromatic heterocyclic group and aromatic group represented by
R° is optionally substituted
with one or more independently selected groups represented by R#. R# is R+, -
OR+, -O(haloalkyl),
-SR+, -NOz, -CN, -N(R+)z, -NHCOZR+, -NHC(O)R+, -NHNHC(O)R+, -NHC(O)N(R~z,
-NHNHC(O)N(R+)z, -NHNHCOzR+, -C(O)C(O)R+, -C(O)CHZC(O)R+, -COZR+, -C(O)R+,
-C(O)N(R+)z, -OC(O)R+, -OC(O)N(R+)a, -S(O)zR+, -SOzN(R+)z, -S(O)R+, -
NHSOzN(R+)a, -NHSOzR+,
-C(=S)N(R+)z, or -C(=NH)-N(R+)z. R+ is -H, a Cl-C3 alkyl group, a monocyclic
heteroaryl group, a
non-aromatic heterocyclic group or a phenyl group optionally substituted with
alkyl, haloalkyl,
alkoxy, haloalkoxy, halo, -CN, -NOz, amine, alkylamine or dialkylamine; or -
N(R+)z is a
non-aromatic heterocyclic group, provided that non-aromatic heterocyclic
groups represented by R+
and -N(R+)z that comprise a secondary ring amine are optionally acylated or
alkylated.
[00119] Preferred values for RZ and R" are halogen, haloalkyl, -R°, -
OR°, -O(haloalkyl), -COzR°,
-C(O)R°, =NR'SOZR°, -C(O)N(R°)z, -OC(O)R°, and -
OC(O)N(R°)z. 3,4-methylene-dioxy and 3,4-
ethylene-dioxy, and N(R')z are also preferred values for R". Each R° is
independently hydrogen,
haloalkyl or an alkyl group, each R' is independently H or alkyl.
[00120] Preferably, R3 is a phenyl ring optionally substituted at the meta or
para positions with
one or more groups represented by R". More preferred values for R" and RZ are
halogen, haloalkyl, -
R°, -OR°, and-O(haloalkyl). -NR'SOZR° and N(R')z are also
preferred values for R".
[00121] More preferably, R3 is a phenyl ring optionally substituted at the
para position with R".
R' is a phenyl ring optionally substituted at the zrzeta position by RZ. Even
more preferred values for
RZ and R" are chloride, fluoride, bromide, -OR°, or R°. -
NR'SOZR° and -N(R')z are also preferred
values for R". Each R° is independently hydrogen, haloalkyl or a Cl_3
alkyl group. Each R' is
independently hydrogen or a CI_3 alkyl group.
[00122] R'4 is an optional substituent, preferably at the six an seven
positions on phenyl ring A;
preferred values are halogen R°, -OR°, -COZR°, -
C(O)R°, -C(O)N(R°)z, -CN, -OC(O)R°,
(CHz)nCOzR°, O(CHz)"COzR°, NHSOZR°, NHC(O)N(R°)z,
(CHz)"OH, O(CHz)"OH,
(CHz)"C(O)N(R°)z, or O(CHz)"C(O)N(R°)z. R° is hydrogen,
haloalkyl or a Cl_3 alkyl group.
[00123] Another embodiment of the present invention, is a compound represented
by Structural
Formula (X-A):
-3S-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI
O
N-
_T_RY
(X-A)
V is a covalent bond or -O-.
T is an unsubstituted straight chained Cl_lo allcylene.
RY is -C(O)ORS, -C(O)R5, -OC(O)R5, -C(O)N(RS)z, -NRSC(O)R5, -NRSC(O)ORS, -
S(O)zRS, _
S(O)zCORs, -S(O)zN(RS)z, -NRSS(O)z, -NRSS(O)zRS, S(O)zORS, -S(O)ORs, -SRS, -
C(O)NRSS(O)zRS, _
CN, -NRSC(O)N(RS)z, -OC(O)N(RS)z, -N(RS)z, -ORS, an optionally substituted non-
aromatic
heterocyclic group or an optionally substituted heteroaryl group.
[00124] In some embodiments, the compound of Structural Formula (X-A),
excludes compounds
where T-RY is-CHZRz°, -CHZCHZRzI, or -(CHz)3Rzz. Rzo is -COOH, -
C(O)NHz, -C(O)NHCH3,
C(O)N(CH3)z, 5-tetrazolyl, 4-pyridinyl, N-ethyl-4-piperidinyl, or C(O)N
morpholinyl. Rz' is -COOH,
N-morpholinyl, C(O)NHz, N-pyrrolidin-2-onyl, N-imidazolyl, or N-pyrrolidinyl.
Rzz is -COOH,
C(O)N(CHZCH3)z, C(O)NH(CHzCH3), C(O)NHz, C(O)NHS(O)zCH3, C(O)NHOH,
C(O)OCHzCH3,
NHz, C(O)CH3, CN, NHS(O)zCF3, C(O)N-pyrrolidinyl, N-pyrrolidinyl, 5-
tetrazolyl, 5-
(1,2,4)oxadiazolyl, N morpholinyl, or N-imidazolyl.
[00125] In other embodiments, compounds of Structural Formula (X-A) (or a
pharmaceutically
acceptable salt thereof ) are provided:
CI ~ O
N
V-T-RY
(X-A)
or a pharmaceutically acceptable salt thereof, wherein:
-39-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
V is a covalent bond or -O-;
T is an straight chained Cl_IO alkylene substituted with alkyl, gem dialkyl,
haloalkyl, spiro
cycloalkyl, or an optionally N-substituted nitrogen containing spiro non-
aromatic heterocyclic group;
RY is Ry is -C(O)ORS, -C(O)R5, -OC(O)R5, -C(O)N(RS)z, -NRSC(O)R5, -NRSC(O)ORS,
-
S(O)zRS, -S(O)zCORS, -S(O)zN(RS)z, 'NRSS(O)2, -NRSS(O)zRs, S(O)zORS, -S(O)ORS,
-SRS,
-C(O)NRSS(O)zRS, -CN, -NRSC(O)N(RS)z, -OC(O)N(RS)z, -N(RS)z, -ORS, an
optionally substituted
non-aromatic heterocyclic group or an optionally substituted heteroaryl group;
and
each RS is independently -H, alleyl, haloalkyl, hydroxyalkyl, carboxyalkyl, -
C(O)OCHZC6I35,
S(O)zCH3, -C(O)OH, -C(O)OMe, -C(O)OEt, C(O)NHz, benzyl, pyrrolidinyl,
morpholinyl, or -N(RS)z
is an optionally substituted nitrogen-containing non-aromatic heterocyclic
group.
[00126] In yet other embodiments, compounds of Structural Formula (X-A) (or a
pharmaceutically acceptable salt thereof ) are provided:
CI ~ ~ O
N-
~,
s~~~~
N
o~ ~
V-T-RY
wherein:
V is-O-;
T is an straight chained CI_lo alkylene optionally substituted at any one or
more substitutable
carbon atoms with halide, alkyl, gem dialkyl, gem dihalo, haloalkyl, alkoxy,
haloalkoxy, spiro
cycloalkyl, optionally N-substituted nitrogen containing spiro non-aromatic
heterocyclic group,
amine, alkylamine, dialkylamine, or hydroxyl;
RY is RY is -C(O)ORS, -C(O)R5, -OC(O)R5, -C(O)N(RS)z, -NRSC(O)R5, -NRSC(O)ORS,
S(O)zRS, -S(O)zCORs, -S(O)zN(RS)z, -NRSS(O)z, -NRSS(O)zRS, S(O)zORs, -S(O)ORS,
-SRS,
-C(O)NRSS(O)zRS, -CN, -NRSC(O)N(RS)z, -OC(O)N(RS)z, -N(RS)z, -ORS, an
optionally substituted
non-aromatic heterocyclic group or an optionally substituted heteroaryl group;
and
-40-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
each RS is independently -H, alkyl, haloalkyl, hydroxyalkyl, carboxyalkyl, -
C(O)OCHZC~Iis,
S(O)zCH3, -C(O)OH, -C(O)OMe, -C(O)OEt, C(O)NHz, benzyl, pyrrolidinyl,
morpholinyl, or -N(RS)z
is an optionally substituted nitrogen-containing non-aromatic heterocyclic
group.
[00127] Compounds of general formula I-A (and subsets thereof as described
directly above in
section 2) include those described generally above, and are further
illustrated by the classes,
subclasses, and species disclosed above and herein. As used herein, the
following definitions shall
apply unless otherwise indicated. For purposes of this invention, the chemical
elements are identified
in accordance with the Periodic Table of the Elements, CAS version, Handbook
of Chemistry and
Physics, 75"' Ed. Additionally, general principles of organic chemistry are
described in "Organic
Chemistry", Thomas Sorrell, University Science Books, Sausalito: 1999, and
"March's Advanced
Organic Chemistry", 5''' Ed., Ed.: Smith, M. B. and March, J., John Wiley &
Sons, New York: 2001.
[00128] The term "alkylene" as used herein means a straight chained
hydrocarbon which is
completely saturated. An alkylene group is typically Cl_lo, more typically
Cl_6. more preferably from
Cl_5 and more preferably from C~_3. A "substituted alkylene" is an alkylene
group in which one or
more methylene hydrogen atoms are replaced with a substituent. Suitable
substituents are as
described below for a substituted alkyl group. Preferred substituents for the
alkylene group
represented by T are fluoro, methyl, gem dimethyl, gem difluoro fluoromethyl,
spiro cyclopropyl,
spiro cyclobutyl, optionally N-substituted spiro azetidinyl, optionally N-
substituted spiro aziridinyl,
optionally N substituted spiro pyrrolidinyl, optionally N-substituted spiro
piperidinyl, amine,
methylamine, dimethylamine, or hydroxyl. A "substitutable alkylene carbon
atom" is an alkylene
carbon atom that is bonded to one or more hydrogen atoms. The hydrogen atoms
can therefore
optionally be replaced with the substituent.
[00129] The terms "alkyl", "hydroxyalkyl", "carboxyalkyl", "haloalkyl",
"alkylamine",
"dialkylamine", used alone or as part of a larger moiety include both straight
and branched saturated
chains containing one to ten carbon atoms, preferably one to six, more
preferably one to five, and
even more preferably one to three.
[00130] The term "allyl" as used herein has the formula -CHZCH=CHz, and may be
optionally
substituted at any substitutable carbon atom. A "substitutable allyl carbon
atom" is an allyl carbon
atom that is bonded to one or more hydrogen atoms. The hydrogen atom can
therefore optionally be
replaced with the substituent. Suitable substituents are as described for
alkyl group.
[00131] The terms "gem dialkyl", and "gem dihalo" includes compounds where two
alkyl
substituents or two halo substituents, respectively, are attached to the same
carbon atom, e.g., -
C(CH3)z- or C(F)z-.
-41 -
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00132] A "spiro cycloalkyl" or "spiro non-aromatic heterocyclic" group is a
cycloalkyl or non-
aromatic heterocyclic group which shares one ring carbon atom with a carbon
atom in an alkylene
group or alkyl group.
[00133] The terms "non-aromatic carbocyclic" or "cycloaliphatic" shall include
cyclic C3_io
hydrocarbons which are completely saturated or which contain one or more units
of unsaturation, but
which are not aromatic. Cycloaliphatic groups are typically C3_io, more
typically C3_~.
[00134] "Alkoxy" means (alkyl)-O-; "haloallcoxy", means (halide)-O-;
"alkoxyalkylene" means
.(alkyl)-O-(alkylene) such as methoxymethylene (CH30CH2); "hydroxyalkyl" means
hydroxy
substituted alkyl group, acylated means "-C(O)-(alkyl)".
[00135] The term "heteroatom" means nitrogen, oxygen, or sulfur and includes
any oxidized form
of nitrogen and sulfur, and the quaternized form of any basic nitrogen. Also
the term "nitrogen"
includes a substitutable nitrogen of a heterocyclic ring. As an example, in a
saturated or partially
unsaturated ring having 0-3 heteroatoms selected from oxygen, sulfur or
nitrogen, the nitrogen may be
N (as in 3~4-dihydro-2H-pyrrolyl), NH (as in pyrrolidinyl) or NR+ (as in N-
substituted pyrrolidinyl).
[00136] The term "aromatic group", includes carbocyclic aromatic ring groups
and heteroaryl ring
groups. The term "aromatic group" may be used interchangeably with the terms
"aryl", "aryl ring" or
"aromatic ring".
[00137] Carbocyclic aromatic ring groups have only carbon ring atoms and
include monocyclic
aromatic rings such as phenyl and fused polycyclic aromatic ring systems in
which two or more
carbocyclic aromatic rings are fused to one another. Examples include 1-
naphthyl, 2-naphthyl, 1-
anthracyl and 2-anthracyl. Also included within the scope of the term
"carbocyclic aromatic ring", as
it is used herein, is a group in which an aromatic ring is fused to one or
more non-aromatic rings
(aliphatic or heterocyclic), such as in an indanyl, phthalimidyl,
naphthimidyl, phenantriidinyl; or
tetrahydronaphthyl, where the radical or point of attachment is on the
aromatic ring.
[00138] The term "heteroaryl" or "heteroaromatic", refers to heteroaromatic
ring groups having
five to fourteen members, including monocyclic heteroaromatic rings and
polycyclic aromatic rings in
which a monocyclic aromatic ring is fused to one or more other carbocyclic or
heteroaromatic
aromatic rings . Examples of heteroaryl rings include 2-furanyl, 3-furanyl, N-
imidazolyl, 2-
imidazolyl, 4-imidazolyl, 5-imidazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-
isoxazolyl, oxadiazolyl, 2-
oxadiazolyl, 5-oxadiazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, N-pyrrolyl, 2-
pyrrolyl, 3-pyrrolyl,
pyrazolyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 2-pyrimidyl, 4-pyrimidyl, 5-
pyrimidyl, 3-pyridazinyl,
2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-triazolyl, 5-triazolyl, tetrazolyl, 2-
thienyl, 3-thienyl, carbazolyl
benzimidazolyl, benzothienyl, benzofuranyl, indolyl, quinolinyl,
benzotriazolyl, benzothiazolyl,
benzooxazolyl, benzimidazolyl, isoquinolinyl, indolyl, isoindolyl, acridinyl,
or benzoisazolyl.
[00139] Also included within the scope of the term "heteroaryl", as it is used
herein, is a group in
which a heteroaryl ring is fused to one or more cycloaliphatic or non-aromatic
heterocyclic groups
-42
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
where the radical or point of attachment is on the heteroaromatic ring.
Examples include
tetrahydroquinolinyl, tetrahydroisoquinolinyl, and pyrido [3, 4-d]
pyrimidinyl. The term "heteroaryl"
may be interchangeably with the term "heteroaryl ring" or the term
"heteroaromatic".
[00140] The term "non-aromatic heterocyclic group", or "non-aromatic
heterocyclic ring" refers
to non-aromatic ring systems typically having three to fourteen members,
preferably three to six, in
which one or more ring carbons, preferably one to four, are each replaced by a
heteroatom such as N,
O, or S. Examples of non-aromatic heterocyclic rings include 3-
tetrahydrofuranyl, 2-
tetrahydropyranyl, 3-tetrahydropyranyl, 4-tetrahydropyranyl, [1,3]-dioxalanyl,
[1,3]-dithiolanyl, [1,3]-
dioxanyl, 2-tetrahydrothiophenyl, 3-tetrahydrothiophenyl, 2-morpholinyl, 3-
morpholinyl, N
morpholinyl, 2-thiomorpholinyl, 3-thiomorpholinyl, 4-thiomorpholinyl, N
pyrrolidinyl, 2-
pyrrolidinyl, 3-pyrorolidinyl, N-piperazinyl, 2-piperazinyl, N-piperidinyl, 2-
piperidinyl, 3-piperidinyl,
4-piperidinyl, piperidinonyl, 4-thiazolidinyl, diazolonyl, N substituted
diazolonyl, oxazolidinyl,
oxazolidinyl, tetrahydrothienyl, imidazolidinyl, imidazolidinonyl,
pyrrolidinonyl, isothiazolidinyl S,S,
dioxide, piperidinyl, 1-pthalimidinyl, 3-1H-benzimidazol-2-one, benzoxanyl,
benzopyrrolidinyl,
benzopiperidinyl, benzoxolanyl, benzothiolanyl, and benzothianyl.
[00141,] A "substituted aromatic group" is an aromatic group with a
substituent at one or more
substitutable ring carbon atoms or ring nitrogen atoms. Each substituent is
independently selected.
Examples of suitable substituents on an unsaturated carbon atom or
substitutable carbon atom of an
aromatic group, are as described above for Rz, R", and R14.
[00142] A. "substitutable ring carbon atom" in an aromatic ring (including the
aromatic groups
represented by Ring A, phenyl ring A, R' and R3) is a ring carbon atom that is
bonded to a hydrogen
atom. The hydrogen atom can therefore optionally be replaced with the
substituent. The term
"substitutable ring carbon atom" in an aromatic ring therefore excludes ring
carbon atoms that.are
fused with other rings or that are depicted as already being bonded to a
substituent.
[00143] , An alkyl group or a non-aromatic carbocycle or heterocycle may
contain one or more
substituents on any substitutable carbon atom. A "substitutable alkyl carbon
atom" is an alkyl carbon
atom that is bonded to one or more hydrogen atoms. The hydrogen atoms can
therefore optionally be
replaced with the substituent. Examples of suitable substituents on the
saturated carbon of an alkyl
group or a non-aromatic heterocycle include those listed above for the
unsaturated carbon of an
aromatic group and, at the internal carbon atoms of an alkyl group or on ring
carbon atoms of a non-
aromatic heterocyclic group, the following: =O, =S, =NNHR*, =NN(R*)2,
=NNHC(O)R*,
=NNHCOZ(alkyl), =NNHSOZ (alkyl), or =NR*. Each R* is independently selected
from hydrogen, an
unsubstituted alkyl group or a substituted alkyl group. Examples of
substituents on the alkyl group
represented by R* include amino, alkylamino, dialkylamino, aminocarbonyl,
halogen, alkyl,
allcylaminocarbonyl, dialkylaminocarbonyl, alkylaminocarbonyloxy,
dialkylaminocarbonyloxy,
alkoxy, vitro, cyano, carboxy, alkoxycarbonyl, alkylcarbonyl, hydroxy,
haloalkoxy, or haloalkyl.
-43-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00144] Suitable substituents on the substitutable nitrogen of a heteroaryl or
non-aromatic
heterocyclic group include -Rlz, -N(R'z)z, -C(O)R'z, -COZRiz, _C(O)C(O)R'z, -
C(O)CHz C(O)R'z, -
SOz Rzz, -SOZN(R'z)z, -C(=S)N(R'z)z, -C(=NH)-N(Rlz)z, and -NRIZSOzR'z; wherein
RIZ is hydrogen,
an alkyl group, a substituted alkyl group, phenyl (Ph), substituted Ph, -
O(Ph), substituted -O(Ph),
CHz(Ph), or an unsubstituted heteroaryl or non-aromatic heterocyclic ring.
Examples of substituents
on the alkyl group or the phenyl ring represented by Rlz include amino,
alkylamino, dialkylamino,
aminocarbonyl, halogen, alkyl, alkylaminocarbonyl, dialkylaminocarbonyloxy,
alkoxy, nitro, cyano,
carboxy, alkoxycarbonyl, alkylcarbonyl, hydroxy, haloalkoxy, or haloalkyl. A
"substitutable ring
nitrogen atom" in a heteroaryl or nitrogen-containing non-aromatic
heterocyclic group is a ring
nitrogen atom that is bonded to a hydrogen atom. The hydrogen atom can
therefore optionally be
replaced with the substituent. The term "substitutable ring nitrogen atom"
therefore excludes ring
nitrogen atoms that are depicted as already being bonded to a substituent, and
ring nitrogen atoms that
are ring atoms in two fused rings (as in, e.g., indolizine) and ring nitrogen
atoms that have three
covalent bonds to other ring atoms (as e.g., pyridine).
[00145] In certain instances compounds of the present invention may associated
in isolated fornn
with solvent or water, as in a "solvate" or "hydrate". References to the
disclosed compounds or
structural formulas depicting the disclosed compounds are meant to include
such solvates and
hydrates.
[00146] 3. Uses, Fonnulatian azzd Adrninistratiorz
[00147] As discussed above, the present invention provides compounds that are
useful as
inhibitors of CRTH2, and thus the present compounds are useful for treating
(therapeutically or
prophylactically) disorders with an inflammatory component and allergic
conditions. They can also
be used to inhibit inflammatory disorders and allergic conditions mediated by
°Th2 cells, eosinophils
and basophils.
[00148] Accordingly, in another aspect of the present invention,
pharmaceutically acceptable
compositions are provided, wherein these compositions comprise any of the
compounds as described
herein, and optionally comprise a pharmaceutically acceptable carrier,
adjuvant or vehicle. In certain
embodiments, these compositions optionally further comprise one or more
additional therapeutic
agents.
[00149] It will also be appreciated that certain of the compounds of present
invention can exist in
free form for treatment, or where appropriate, as a pharmaceutically
acceptable derivative thereof.
According to the present invention, a pharmaceutically acceptable derivative
includes, but is not
limited to, pharmaceutically acceptable prodrugs, salts, esters, salts of such
esters, or any other adduct
or derivative which upon administration to a patient in need is capable of
providing, directly or
indirectly, a compound as otherwise described herein, or a metabolite or
residue thereof.
_ q.q. _
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00150] As used herein, the term "pharmaceutically acceptable salt" refers to
those salts which
are, within the scope of sound medical judgement, suitable for use in contact
with the tissues of
humans and lower animals without undue toxicity, irritation, allergic response
and the like, and are
commensurate with a reasonable benefit/risk ratio. Pharmaceutically acceptable
salts are well known
in the art. For example, S. M. Berge et al., describe pharmaceutically
acceptable salts in detail in J.
Phannaceutical Sciezzces, 1977, 66, 1-19, incorporated herein by reference.
Pharmaceutically
acceptable salts of the compounds of this invention include those derived from
suitable inorganic and
organic acids and bases. Examples of pharmaceutically acceptable, nontoxic
acid addition salts are
salts of an amino group formed with inorganic acids such as hydrochloric acid,
hydrobromic acid,
phosphoric acid, sulfuric acid and perchloric acid or with organic acids such
as acetic acid, oxalic
acid, malefic acid, tartaric acid, citric acid, succinic acid or malonic acid
or by using other methods
used in the art such as ion exchange. Other pharmaceutically acceptable salts
include adipate, alginate,
ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, . borate,
butyrate, camphorate,
camphorsulfonate, citrate, cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate,
formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate,
heptanoate, hexanoate,
hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl
sulfate, malate, maleate,
malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate,
oleate, oxalate, palmitate,
pamoate, pectinate, persulfate, 3-phenylpropionate, phosphate, picrate,
pivalate, propionate, stearate,
succinate, sulfate, tartrate, thiocyanate, p-toluenesulfonate, undecanoate,
valerate salts, and the like.
Salts derived from appropriate bases include alkali metal, alkaline earth
metal, ammonium and N+(Ci-
4alkyl)4 salts. This invention also envisions the quaternization of any basic
nitrogen-containing groups
of the compounds disclosed herein. Water or oil-soluble or dispersable
products may be obtained by
such quaternization. Representative alkali or alkaline earth metal salts
include sodium, lithium,
potassium, calcium, magnesium, and the like. Further pharmaceutically
acceptable salts include, when
appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed
using
counterions such as halide, hydroxide, carboxylate, sulfate, phosphate,
nitrate, loweralkyl sulfonate
and aryl sulfonate.
[00151] As described above, the pharmaceutically acceptable compositions of
the present
invention additionally comprise a pharmaceutically acceptable carrier,
adjuvant, or vehicle, which, as
used herein, includes any and all solvents, diluents, or other liquid vehicle,
dispersion or suspension
aids, surface active agents, isotonic agents, thickening or emulsifying
agents, preservatives, solid
binders, lubricants and the like, as suited to the particular dosage form
desired. Remington's
Pharmaceutical Sciences, Sixteenth Edition, E. W. Martin (Mack Publishing Co.,
Easton, Pa., 190)
discloses various carriers used in formulating pharmaceutically acceptable
compositions and known
techniques for the preparation thereof. Except insofar as any conventional
carrier medium is
incompatible with the compounds of the invention, such as by producing any
undesirable biological
-45
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
effect or otherwise interacting in a deleterious manner with any other
components) of the
pharmaceutically acceptable composition, its use is contemplated to be within
the scope of this
invention. Some examples of materials which can serve as pharmaceutically
acceptable carriers
include, but are not limited to, ion exchangers, alumina, aluminum stearate,
lecithin, serum proteins,
such as human serum albumin, buffer substances such as phosphates, glycine,
sorbic acid, or
potassium sorbate, partial glyceride mixtures of saturated vegetable fatty
acids, water, salts or
electrolytes, such as protamine sulfate, disodium hydrogen phosphate,
potassium hydrogen phosphate,
sodium chloride, zinc salts, colloidal silica, magnesium trisilicate,
polyvinyl pyrrolidone,
polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers, wool fat,
sugars such as
lactose, glucose and sucrose; starches such as corn starch and potato starch;
cellulose and its
derivatives such as sodium carboxymethyl cellulose, ethyl cellulose and
cellulose acetate; powdered
tragacanth.malt; gelatin; talc; excipients such as cocoa butter and
suppository waxes; oils such as
peanut oil, cottonseed oil; safflower oil; sesame oil; olive oil; corn oil and
soybean oil; glycols; such a
propylene glycol or polyethylene glycol; esters such as ethyl oleate and ethyl
laurate; agar; buffering
agents such as magnesium hydroxide and aluminum hydroxide; alginic acid;
pyrogen-free water;
isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer
solutions, as well as other non-
toxic compatible lubricants such as sodium lauryl sulfate and magnesium
stearate, as well as coloring
agents, releasing agents, coating agents, sweetening, flavoring and perfuming
agents, preservatives
and antioxidants can also be present in the composition, according to the
judgment of the formulator.
[00152] In another aspect, a method for the treatment of an inflammatory
disease or a disease with
an inflammatory component is provided comprising administering an effective
amount of a
compound, or a pharmaceutical composition thereof to a subject in need
thereof. Compounds and
compositions of the invention are inhibitors of CRTH2, and thus, without
wishing to be bound by any
particular theory, the compounds and compositions are particularly useful for
treating or lessening the
severity of a disease, condition, or disorder where activation of one or more
of CRTH2, PGD2
(including DP activity), Th2 cells, eosinophils, and/or basophils is
implicated in the disease,
condition, or disorder. When activation of one or more of CRTH2, PGD2
(including DP activity), Th2
cells, eosinophils, and/or basophils is implicated in a particular disease,
condition, or disorder, the
disease, condition, or disorder may also be referred to as a "CRTH2-mediated
disease" or disease
symptom. Accordingly, in another aspect, the present invention provides a
method for treating or
lessening the severity of a disease, condition, or disorder where activation
of one or more of CRTH2,
PGD2 (including DP activity), Th2 cells, eosinophils, and/or basophils is
implicated in the disease
state.
[00153] In certain embodiments of the present invention an "effective amount"
of the compound
or pharmaceutically acceptable composition is that amount effective for
treating an inflammatory
disease or disease with an inflammatory component. In other embodiments, an
"effective amount" of
-46-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
a compound is an amount which inhibits binding of PGD2 to its receptor CRTH2
and thereby inhibits
one or more processes mediated by the binding in a subject, for example, the
release of
proinflammatory mediators. An "effective amount" of a compound can achieve a
desired therapeutic
and/or prophylactic effect, such as an amount which results in the prevention
of or a decrease in the
symptoms associated with an inflammatory disease or a disease mediated by one
or more of CRTH2,
PGD2 (including DP activity), Th2 cells, eosinophils, and basophils.
[00154] In one embodiment, the inflammatory disease is an allergic condition.
Examples of
allergic conditions for which the disclosed compounds, pharmaceutical
compositions and methods are
believed to be particularly effective include atopic dermatitis, allergic
rhinitis, rheumatoid arthritis,
chronic obstructive pulmonary disorder, or allergic asthma. Other allergic
conditions include systemic
anaphylaxis or hypersensitivity responses, drug allergies (e.g., to
penicillin, cephalosporins), insect
sting allergies and dermatoses such as dermatitis, eczema, atopic dermatitis,
allergic contact dermatitis
and urticaria.
[00155] ~ Examples of diseases with an inflammatory component for which the
disclosed
compounds, pharmaceutical composition and methods are believed to be
particularly effective include
osteoarthritis, inflammatory bowel disease [e.g., such as ulcerative colitis,
Crohn's disease, ileitis,
Celiac disease, nontropical Sprue, enteritis, enteropathy associated with
seronegative arthropathies,
microscopic or collagenous colitis, eosinophilic gastroenteritis, or pouchitis
resulting after
proctocolectomy, and ileoanal anastomosis] and disorders of the skin [e.g.,
psoriasis; erythema,
pruritis, and acne].
[00156] ' Many autoimmune diseases also have an inflammatory component.
Examples include
multiple sclerosis, systemic lupus erythematosus, myasthenia gravis, juvenile
onset diabetes,
glomerulonephritis and other nephritides, autoimmune thyroiditis, Behcet's
disease and graft rejection
(including allograft rejection or graft-versus-host disease). The inflammatory
component of these
disorders is believed to be mediated, at least in part, by CRTH2.
[00157] Diseases characterized by repurfusion have an inflammatory component
that is believed
to be mediated, at least in part by, by CRTH2. Examples include stroke,
cardiac ischemia, and the
like. The disclosed compounds and compositions also can be used to treat these
disorders.
[00158] Other diseases and conditions with an inflammatory component believed
to be mediated
by CRTH2 include mastitis (mammary gland), vaginitis, cholecystitis,
cholangitis or pericholangitis
(bile duct and surrounding tissue of the liver), chronic bronchitis, chronic
sinusitis, chronic
inflammatory diseases of the lung which result in interstitial fibrosis, such
as interstitial lung diseases
(ILD) (e.g., idiopathic pulmonary fibrosis, or ILD associated with rheumatoid
arthritis, or other
autoimmune conditions), hypersensitivity pneumonitis, collagen diseases and
sarcoidosis. Yet other
diseases or conditions with inflammatory components which are amendable to
treatment according to
methods disclosed herein include vasculitis (e.g., necrotizing, cutaneous, and
hypersensitivity
-47
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
vasculitis), spondyloarthropathies, scleroderma, atherosclerosis, restenosis
and myositis (including
polymyositis, dermatomyositis), pancreatitis and insulin-dependent diabetes
mellitus.
[00159] In a preferred embodiment, the invention provides a method of treating
asthma
comprising administering an effective amount of a compound of general formula
I (and subsets
thereof as described herein) to a subject in need thereof. .
[00160] The compounds and compositions, according to the method of the present
invention, may
be administered using any amount and any route of administration effective for
treating an
inflammatory disease or allergic condition. The exact amount required will
vary from subject to
subject, depending on the species, age, and general condition of the subject,
the severity of the
infection, the particular agent, its mode of administration, and the like. The
compounds of the
invention are preferably formulated in dosage unit form for ease of
administration and uniformity of
dosage. The expression "dosage unit form" as used herein refers to a
physically discrete unit of agent
appropriate for the patient to be treated. It will be understood, however,
that the total daily usage of
the compounds and compositions of the present invention will be decided by the
attending physician
within the scope of sound medical judgment. The specific effective dose level
for any particular
patient or organism will depend upon a variety of factors including the
disorder being treated and the
severity of the disorder; the activity of the specific compound employed; the
specific composition
employed; the age, body weight, general health, sex and diet of the patient;
the time of administration,
route of administration, and rate of excretion of the specific compound
employed; the duration of the
treatment; drugs used in combination or coincidental with the specific
compound employed, and like
factors well known in the medical arts. The term "patient", as used herein,
means an animal,
preferably a mammal, and most preferably a human.
[00161] The pharmaceutically acceptable compositions of this invention can be
administered to
humans and other animals orally, rectally, parenterally, intracisternally,
intravaginally,
intraperitoneally, topically (as by powders, ointments, or drops), bucally, as
an oral or nasal spray, or
the like, depending on the severity of the infection being treated. In certain
embodiments, the
compounds of the invention may be administered orally or parenterally at
dosage levels of about 0.01
mg/kg to about 50 mg/kg and preferably from about 1 mg/kg to about 25 mg/kg,
of subject body
weight per day, one or more times a day, to obtain the desired therapeutic
effect.
[00162] Liquid dosage forms for oral administration include, but are not
limited to,
pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions,
syrups and elixirs. In
addition to the active compounds, the liquid dosage forms may contain inert
diluents commonly used
in the art such as, for example, water or other solvents, solubilizing agents
and emulsifiers such as
ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl benzoate,
propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular,
cottonseed, groundnut,
corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl
alcohol, polyethylene glycols
-48
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and fatty acid esters of sorbitan, and mixtures thereof. Besides inert
diluents, the oral compositions
can also include adjuvants such as wetting agents, emulsifying and suspending
agents, sweetening,
flavoring, and perfuming agents.
[00163] Injectable preparations, for example, sterile injectable aqueous or
oleaginous suspensions
may be formulated according to the known art using suitable dispersing or
wetting agents and
suspending agents. The sterile injectable preparation may also be a sterile
injectable solution,
suspension or emulsion in a nontoxic parenterally acceptable diluent or
solvent, for example, as a
solution in 1,3-butanediol. Among the acceptable vehicles and solvents that
may be employed are
water, Ringer's solution, U.S.P. and isotonic sodium chloride solution. In
addition, sterile, fixed oils
are conventionally employed as a solvent or suspending medium. For this
purpose any bland fixed oil
can be employed including synthetic mono- or diglycerides. In addition, fatty
acids such as oleic acid
are used in the preparation of injectables.
[00164] The injectable formulations can be sterilized, for example, by
filtration through a
bacterial-retaining filter, or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable medium
prior to use.
[00165] In order to prolong the effect of a compound of the present invention,
it is often desirable
to slow the absorption of the compound from subcutaneous or intramuscular
injection. This may be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with poor water -
solubility. The rate of absorption of the compound then depends upon its rate
of dissolution that, in
turn, may depend upon crystal size and crystalline form. Alternatively,
delayed absorption of a
parenterally administered compound form is accomplished by dissolving or
suspending the compound
in an oil vehicle. Injectable depot forms are made by forming microencapsule
matrices of the
compound in biodegradable polymers such as polylactide-polyglycolide.
Depending upon the ratio of
compound to polymer and the nature of the particular polymer employed, the
rate of compound
release can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the compound in
liposomes or microemulsions that are compatible with body tissues.
[00166] Compositions for rectal or vaginal administration are preferably
suppositories which can
be prepared by mixing the compounds of this invention with suitable non-
irritating excipients or
carriers such as cocoa butter, polyethylene glycol or a suppository wax which
are solid at ambient
temperature but liquid at body temperature and therefore melt in the rectum or
vaginal cavity and
release the active compound.
[00167] Solid dosage forms for oral administration include capsules, tablets,
pills, powders, and
granules. In such solid dosage forms, the active compound is mixed with at
least one inert,
pharmaceutically acceptable excipient or carrier such as sodium citrate or
dicalcium phosphate and/or
-49-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol,
and silicic acid, b) binders
such as, for example, carboxymethylcellulose, alginates, gelatin,
polyvinylpyrrolidinone, sucrose, and
acacia, c) humectants such as glycerol, d) disintegrating agents such as agar--
agar, calcium carbonate,
potato or tapioca starch, alginic acid, certain silicates, and sodium
carbonate, e) solution retarding
agents such as paraffin, fj absorption accelerators such as quaternary
ammonium compounds, g)
wetting agents such as, for example, cetyl alcohol and glycerol monostearate,
h) absorbents such as
kaolin and.bentonite clay, and i) lubricants such as talc, calcium stearate,
magnesium stearate, solid
polyethylene glycols, sodium lauryl sulfate, and mixtures thereof. In the case
of capsules, tablets and
pills, the dosage form may also comprise buffering agents.
[00168] Solid compositions of a similar type may also be employed as fillers
in soft and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high molecular weight
polyethylene glycols and the like. The solid dosage forms of tablets, dragees,
capsules, pills, and
granules can be prepared with coatings and shells such as enteric coatings and
other coatings well
known in the pharmaceutical formulating art. They may optionally contain
opacifying agents and can
also be of a composition that they release the active ingredients) only, or
preferentially, in a certain
part of the intestinal tract, optionally, in a delayed manner. Examples of
embedding compositions that
can be .used include polymeric substances and waxes. Solid compositions of a
similar type may also
be employed as fillers in soft and hard-filled gelatin capsules using such
excipients as lactose or milk
sugar as well as high molecular weight polethylene glycols and the like.
[00169] The active compounds can also be in micro-encapsulated form with one
or' more
excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills, and granules can
be prepared with coatings and shells such as enteric coatings, release
controlling coatings and other
coatings well known in the pharmaceutical formulating art. In such solid
dosage forms the active
compound may be admixed with at least one inert diluent such as sucrose,
lactose or starch. Such
dosage forms may also comprise, as is normal practice, additional substances
other than inert diluents,
e.g., tableting lubricants and other tableting aids such a magnesium stearate
and microcrystalline
cellulose. In the case of capsules, tablets and pills, the dosage forms may
also comprise buffering
agents. They may optionally contain opacifying agents and can also be of a
composition that they
release the active ingredients) only, or preferentially, in a certain part of
the intestinal tract,
optionally, in a delayed manner. Examples of embedding compositions that can
be used include
polymeric substances and waxes.
[00170] Dosage forms for topical or transdermal administration of a compound
of this invention
include ointments, pastes, creams, lotions, gels, powders, solutions, sprays,
inhalants or patches. The
active component is admixed under sterile conditions with a pharmaceutically
acceptable carrier and
any needed preservatives or buffers as may be required. Ophthalmic
formulation, ear drops, and eye
drops are also contemplated as being within the scope of this invention.
Additionally, the present
-50
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
invention contemplates the use of transdermal patches, which have the added
advantage of providing
controlled delivery of a compound to the body. Such dosage forms can be made
by dissolving or
dispensing the compound in the proper medium. Absorption enhancers can also be
used to increase
the flux of the compound across the skin. The rate can be controlled by either
providing a rate
controlling membrane or by dispersing the compound in a polymer matrix or gel.
[00171] It will also be appreciated that the compounds and pharmaceutically
acceptable
compositions of the present invention can be employed in combination
therapies, that is, the
compounds and pharmaceutically acceptable compositions can be administered
concurrently with,
prior to, or subsequent to, one or more other desired therapeutics or medical
procedures. The
particular combination of therapies (therapeutics or procedures) ~to employ in
a combination regimen
will take into account compatibility of the desired therapeutics andlor
procedures and the desired
therapeutic effect to be achieved. It will also be appreciated that the
therapies employed may achieve
a desired effect for the same disorder (for example, an inventive compound may
be administered
concurrently with another agent used to treat the same disorder), or they may
achieve different effects
(e.g:, control of any adverse effects). As used herein, additional therapeutic
agents which are normally
administered to treat or prevent a particular disease, or condition, are known
as "appropriate for the
disease, or condition, being treated".
[00172] For example, compounds of the invention can also be administered in
combination with
one or more additional therapeutic agents, such as, theophylline, (3-
adrenergic bronchodilators,
corticosteroids, antihistamines, antiallergic agents, immunosuppressive agents
(e.g., cyclosporin A,
FK-506, prednisone, methylprednisolone), hormones (e.g., adrenocorticotropic
hormone (ACTH)),
cytokines (e.g., interferons (e.g., IFN(3-la, IFNB-lb)) and the like.
[00173] The amount of additional therapeutic agent present in the compositions
of this invention
will be no more than the amount that would normally be administered in a
composition comprising
that therapeutic agent as the only active agent. Preferably the amount of
additional therapeutic agent
in the presently disclosed compositions will range from about 50% to 100% of
the amount normally .
present in a composition comprising that agent as the only therapeutically
active agent.
[00174] The compounds of this invention or pharmaceutically acceptable
compositions thereof
may also be incorporated into compositions for coating implantable medical
devices, such as
prostheses, artificial valves, vascular grafts, stems and catheters.
Accordingly, the present invention,
in another aspect, includes a composition for coating an implantable device
comprising a compound
of the present invention as described generally above, and in classes and
subclasses herein, and a
carrier suitable for coating said implantable device. In still another aspect,
the present invention
includes an implantable device coated with a composition comprising a compound
of the present
invention as described generally above, and in classes and subclasses herein,
and a carrier suitable for
coating said implantable device.
-51-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00175] Another aspect of the invention relates to inhibiting CRTH2 activity
in a biological
sample or a patient, which method comprises administering to the patient, or
contacting said
biological sample with a compound of formula I or a composition comprising
said compound. The
term "biological sample", as used herein, includes, without limitation, cell
cultures or extracts thereof;
biopsied material obtained from a mammal or extracts thereof; and blood,
saliva, urine, feces, semen,
tears, or other body fluids or extracts thereof.
[00176] Inhibition of CRTH2 activity in a biological sample is useful for a
variety of purposes that
are known to one of skill in the art. Examples of such purposes include, but
are not limited to, blood
transfusion, organ-transplantation, biological specimen storage, and
biological assays.
[00177] 4. General Syntlaetic Methods:
[00178] Aother embodiment of the present invention is a method of preparing an
amino acid
compound represented by Structural Formula (XI-A):
Ark
NH
R2 j~COOH
(XI-A)
[00179] The method comprises the step of reacting Ar-NHZ with a lactone
represented by
Structural Formula (XXII-A):
O
__
O
R2
(XII-A).
[00180] Ar is an optionally substituted monocyclic aromatic group and RZ is Cl-
C3 alkyl.
Preferably, Ar is an optionally substituted phenyl group and RZ is methyl or
ethyl. Suitable
substituents for Ar are as described above for Ring A or Phenyl Ring A,
provided, however, that
functional groups which can interfere with the reaction are protected.
Functional groups which require
protection will be readily apparent to the skilled artisan and include amines,
alcohols, carboxylic
acids, and the like. Examples of preferred substituents include halo, cyano,
R°, -OR3°, -COzR3',
C(O)R°, -C(O)N(R")z, -OC(O)R°, (CH2)"COzR3', O(CHZ)"COzR3',
NHSOzR°, NHC(O)NR"z,
(CHZ)°OR3°, O(CHZ)"OR3°, (CHZ)nC(O)N(R")Z,
O(CHZ)"C(O)N(R")z; n is an integer from 1-4; R° is
independently hydrogen, CI_3 haloalkyl or a Cl_3 alkyl group; one R" is -H or
CI-C3 alkyl and the other
is an amine protecting group; R3° is an alcohol protecting group; and
R3' is a carboxylic acid
protecting group. Suitable protecting groups are well know in the art and are
disclosed in, for
example, in Greene and Wuts, "Protective Groups in Organic Synthesis", John
Wiley & Sons (1991).
-52
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
The entire teachings of Greene and Wits are incorporated herein by reference.
More commonly, Ar is
a phenyl group.
[00181] Also encompassed within the present invention is the corresponding
reaction with the
enantiomer of the amino acid compound represented by Structural Formula (XII-
A), thereby forming
the enantiomer of the compound represented by Structural Formula (XI-A).
[00182] The reaction of the aryl amine and cyclic lactone above can be carried
out in solvents in
which both reagents are soluble. Examples include protic solvents (e.g., water
and methanol) and
polar aprotic solvents (dimethylformamide, dimethyl sulfoxide"
hexamethylphosphoramide and the
like). An excess of one reagent relative to the other can be used (e.g., up to
a ten fold excess),
however equimolar amounts are more typical. The reaction is typically carried
out at the boiling point
of the solvent being used, but can also commonly carried out at temperatures
ranging from ambient
temperature to temperatures as high as 200° C..Temperatures from
70° C to 90° C are most commonly
used.
[00183] Another embodiment is a method of preparing an intermediate compound
represented by
Structural Formula (XIII-A) from the amino acid compound represented by
Structural Formula (XI-
A):
Ark
NH O O
R2~N~OiR
H
(XIII-A).
[00184] The method comprises the step of amidating the carboxylic acid group
of the amino acid
compound with NHZC(O)ORZ. The group -C(O)ORZ is an amine protecting group that
taken together
with -NHZ forms a carbamate. Thus, RZ is a substituted or unsubstituted alkyl,
allyl or aryl group.
Substituents that can be present on the alkyl, allyl or aryl group represented
by RZ are those which do
not interfere :in the reactions being carried and are readily recognizable to
the skilled artisan.
Examples include alkyl, halogen and alkoxy. Thus, suitable values for RZ are
well known to the
skilled artisan and are described, for example, in Green and Wuts, "Protective
Groups in Organic
Synthesis", John Wiley & Sons (1991). Specific examples include, but are not
limited to, benzyl,
methyl, ethyl, allyl, 2,2,2; trichloromethyl, 2,2,2-trichloro-tart-butyl, tart-
butyl or fluorenylmethyl.
[00185] The term "amidating a carboxylic acid with NHZC(O)ORZ refers to
converting a
carboxylic acid (-COOH) to the amide -C(O)NHC(O)ORZ in one or more reaction
steps. Many
methods for converting a carboxylic acid to an amide are known in the art.
Typically, the carboxylic
acid is first converted into a group that is more readily displaced by an
amine or amide than -OH.
Thus, -OH is converted into a better leaving group. A "leaving group" is a
group which can readily be
displaced by a nucleophile. In the present invention, the amino acid compound
can be converted
-53-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
directly to the intermediate compound by activating the carboxylic acid of the
amino acid compound
and then reacting with NHZC(O)ORZ. Alternatively, the carboxylic can be first
be converted to
carboxamide (-C(O)NHZ) by activating the carboxylic acid group of the amino
acid compound and
then reacting with NH3 or a functional equivalent thereof (e.g., NH4C1) and
then protecting the
resulting carboxamide. When NHZC(O)ORZ is used as a nucleophile, the amidation
is preferably
carried out in the presence of at least one equivalent of a non-nucleophilic
base such as an alkoxide
(lithium ter-butoxide, potassium tert butoxide, ilithium isopropoxide and
potassium isopropoxide) or
amide base (e.g., lithium or potassium isopropylamide or
hexamethylpiperidide).
[00186] In one example, -OH of the carboxylic acid is converted into a better
leaving group by
replacing it with a halogen, typically with chloride. The carboxylic acid is
thereby converted into an
acid halide, e.g., an acid chloride. Reagents suitable for preparing acid
chlorides from carboxylic acids
are well known in the art and include thionyl chloride, oxalyl chloride,
phosphorus trichloride and
phosphorus pentachloride. Typically, each carboxylic acid group is reacted
with about one equivalent
or a slight excess of thionyl chloride, oxalyl chloride, phosphorus.
trichloride and phosphorus
pentachloride in an inert solvent such as an ethereal solvent (e.g., diethyl
ether, tetrahydrofuran or 1,4-
dioxane), a halogenated solvent (e.g., methylene chloride or 1,2-
dichloroethane) or aromatic solvent
(e.g., benzene or toluene). When oxalyl chloride is used, a tertiary amine is
often added to accelerate
the reaction in quantities ranging from a catalytic amount to about one
equivalent relative to oxalyl
chloride.
[00187] Alternatively, the carboxylic acid is first converted into an
"activated ester". An ester -
COOR is said to be "activated" when -OR is readily displaced by an amine or
amide than -OH. -OR is
more easily displaced as R becomes more electron withdrawing. Some activated
esters are sufficiently '
stable that they can be isolated, e.g., esters wherein R is phenyl or
substituted phenyl. For example,
diphenylmalonate can be prepared from malonyl chloride and phenol, both
commercially available
from Aldrich Chemical Co., Milwaukee, WL, by procedures described above Other
activated esters
are more reactive and are generally prepared and used in situ.
[00188] Formation of an activated ester in situ requires a "coupling agent",
also referred to as a
"carboxylic acid activating agent", which is a reagent that replaces the
hydroxyl group of a carboxyl
acid with a group which is susceptible to nucleophilic displacement. Examples
of coupling agents
include 1,1'-carbonyldiimidazole (CDI), isobutyl chloroformate,
dimethylaminopropylethyl
carbodiimide (EDC), dicyclohexyl carbodiimide (DCC). When amidating by in situ
generation of an
activated ester, an excess of either the carboxylic acid or amine can be used
(typically a 50% excess,
more typically about a 10-15% excess). However, it is more common when
carrying out the present
invention to use equimolar amounts of both reagents. Generally, from about 1.0
equivalent to about
equivalents of coupling agent are used relative to each carboxylic acid group,
preferably from
about 1.0 equivalent to about 1.5 equivalents. When DCC is used, a weak acid
such as 1
-54
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
hydroxybenzotriazole (HOBt) is often added to accelerate the reaction.
Typically, about between one
to about 1.5 equivalents of HOBt relative to DCC is used, preferably between
about one to about 1.2
equivalents. The reaction is generally carried out in inert, aprotic solvents,
for example, halogenated
solvents such as methylene chloride, dichloroethane and chloroform, ethereal
solvents such as
tetrahydrofuran, 1,4-dioxane and diethyl ether and dimethylformamide. Suitable
reaction temperature
generally range from between about 0° to about 100°, but the
reaction is preferably carried out at
ambient temperature.
[00189] Yet another embodiment of the present invention is a method of
preparing a product
compound represented by Structural Formula (XIV-A):
O
~ Z
HN~O~R
A
NJ....R2
H
(XIV-A).
[00190] The method comprises the step of reducing the amide carbonyl of the
intermediate
compound to form a second intermediate and then cyclizing the second
intermediate to form the
product compound. The variables in Structural Formula (XIV-A) are as described
above for Structural
Formulas (XI-A)-(XIII-A). The "amide carbonyl" is understood to be the
carbonyl between the
methylene carbon and nitrogen atom and not the carbonyl that is bonded to both
a nitrogen and
oxygen atom.
" [00191] To carry out the reduction step, a reducing agent is used which can
reduces the amide
carbonyl but not the carbamate group. Sodium borohydride together with a Lewis
Acid such as
magnesium chloride or calcium chloride is one common example. The reduction
step is typically
carried out in an alcoholic solvent such as methanol or ethanol. An excess of
sodium borohydride and
Lewis Acid of up to 50% can be used, but typically from 0.5 to 1.0 equivalents
of sodium borohydride
and 0.5 to 2.0 equivalents of Lewis Acid are used.
[00192] The cyclization step is carried out in dilute aqueous or alcoholic
acid, using, for example,
0.1 N to 10 N HCI, HzS04, H3P04 or a sulfonic acid such as methane sulfonic
acid, toluene sulfonic
acid or phenyl sulfonic acid. More typically, between 0.8 N and 1.2 N acid is
used. Commonly, an
organic acid such acetic acid, benzoic acid, citric acid, and the like is also
be present, for example
between 0.5 equivalents to 10 equivalents. Typically, a co-solvent immiscible
in water or alcohol is
used. Common co-solvents include halogentated solvents such as dichloromethane
or chloroform and
ethereal solvents such as tetrahydrofuran and diethyl ether.
-55-
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00193] Another embodiment of the present invention is a method of preparing
the product
compound represented by Structural Formula (XIV-A) from the cyclic lactone
represented by
Structural Formula (XII-A). The method comprises combining the three reaction
steps described
above. Alternatively, the enantiomer of the compound represented by Structural
Formula (XIV-A) is
prepared using the same three reaction steps, provided, however, that the
enantiomer of the starting
lactone represented by Structural Formula (XII-A) is used.
[00194] Yet another embodiment of the present invention is a compound
represented by any one
of Structural Formulas (XI-A)-(XIV-A).
[00195] The invention is illustrated by the following examples which are not
intended to be
limiting in any way.
-56-
<IMG>
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
EXEMPLIFTCATION
[00196] General. All reactions involving air-sensitive reagents were performed
under a nitrogen
atmosphere. Reagents were used as received from commercial suppliers unless
otherwise noted. 'H
NMR data were recorded using the Bruker UltraShield 300 MHz/54mm instrument
equipped with
Bruker B-ACS60 Auto Sampler or the Varian 300 MHz instrument. Intermediates
and final
compounds were purified by flash chromatography using one of the following
instruments: 1.
Biotage 4-channel Quad UV Flash Collector equipped with a Quad 1 Pump Module
and the Quad
12/25 Cartridge module. 2. Biotage 12-channel Quad UV Flash Collector equipped
with a Quad 3
Pump Module and a Quad 3 Cartridge module. 3. ISCO combi-flash chromatography
instrument.
LC/MS spectra were obtained using a MicroMass Platform LC (Phenomenex C18
column, 5 micron, .
50x4.6 mm) equipped with a Gilson 215 Liquid Handler. Standard LC/MS
conditions is as follows:
Formic acid-Standard conditions:
% C (Water) 95.0 HP1100
LC
Pump
Gradient
Timetable
% D (Acetonitrile)5.0
% Formic Acid 0.1 The hich
gradient are
Timetable
contains
5
entries
w
Flow (ml/min) 3.500
Stop Time (mins)4.4 Time A% B% C% D% Flow Pressure
Min Pressure 0 0.00 0.0 0.0 95.05.0 3.500400
(bar)
Max Pressure 400 3.50 0.0 0.0 0.0 100.03.500400
(bar)
Oven Temperature25.0 4.30 0.0 0.0 0.0 100.03.500400
Left(C)
Oven Temperature 4.40 0.0 0.0 95.05.0 4.000400
Right(C) 25.0
5.00 0.0 0.0 95.05.0 4.000400
Scheme 1
O
NH
I , H~ Ethanol
NH2 rt, 12 h
~ N
H
1 = traps
2=cis
[00197] (~)-Cis- and (~)-traps-(2-ethyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
phenyl-amine (1) &
(2): A 250 mL, flask under nitrogen atmosphere was charged with aniline (1.0
g, 10.7 mmol, 1.0
equiv), acetaldehyde (0.599 mL, 10.7 mmol), benzotriazole (0.255 g, 2.1 mmol,
0.2 equiv) and dry
toluene (100 mL) (Caution: an exotherm was observed). The precipitated
benzotriazole/aldehyde
-58
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
adduct was observed immediately. The solution was allowed to stir at room
temperature for 12h. The
precipitate that forms after stirring over night was filtered and washed with
minimal diethyl ether, to
afford the cis-isomer exclusively. The trazzs-isomer could be obtained by
concentration of the filtrate.
The residue was purified by Biotage flash system (95% hexanel5% diethyl ether)
to yield the cis and
trans isomers as a mixture. The resulting oily residue was then trituated with
hexane to separate the
cis isomer as a white solid and the filtrate was concentrated to give the
trarzs isomer.
(~)-Cis-isomer -'H-NMR (CDCl3) 8: 1.24 (d, 3H), 1.52 (q, 1H), 2.38 (dddd, 1H),
3.63 (m, 1H),
3.75 (bs, 2H, -NH), 4.83 (dd, 1H), 6.51 (d, 1H); 6.68 (m, 4H), 7.05 (m, 1H),
7.19-7.26 (m, 2H), 7.39
(d, 1H).
(~)-Trans-isomer - 1H-NMR (CDCl3) 8: 1.22 (d, 3H), 1.56 (m, 1H), 2.20 (dt,
1H), 3.4 (m, 1H),
3.89 (bs, 2H, -NH), 4.55 (dt, 1H), 6.56 (dd, 1H), 6.66-6.75 (m, 4H), 7.08 (m,
1H), 7.19-7.26 (m, 3H).
Scheme 2
w I OII O'I W I CI ~ ~ W I O O
NH H C~O~CH NH N
3 3 Q
N~ Benzene, 50 °C ~ , N~ TEA, Toluene ~ , N
H \ ~O 90°C ~O
3 4
[00198] Cis-(~)-1-(2-methyl-4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-
ethanone (3) '
[00199] A 30 mL flask under nitrogen atmosphere was charged with (~)-cis-(2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-phenyl-amine (0.520 g, 2.2 mmol, 1.0 equiv), and
acetic anhydride (0.209
mL, 2.2 mmol, 1.0 equiv) and dry toluene (31 mL). The solution was heated to
50 °C for 15h. The
reaction mixture was evaporated in vacuo. The residue was purified by Biotage
flash system (70%
hexane/30% ethyl acetate) to yield the 2-acetyl cis isomers 67 % yield.
'H-IVMR (CDC13) b: 1.17 (d, 3H), 1.25 (q, 1H), 2.19 (s, 3H), 2.22(6s, 1H),
2.65 (m, 1H), 4.21 (dd,
1H), 4.96 (m, 1H), 6.65 (d, 2H), 6.75 (t, 1H), 7.12-7.33 (m, 6H).
[00200] Cis-(~)-furan-2-carboxylic acid (1-acetyl-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl)-
phenyl-amide (4)
[00201] A round bottom flask under nitrogen atmosphere was charged with cis-1-
(2-methyl-4
phenylamino-3,4-dihydro-2H-quinolin-1-yl)-ethanone (0.163 g, 0.58 mmol, 1.0
equiv) and 2-furoyl
chloride (0.285 mL, 2.9 mmol, 5.0 equ), pyridine (1.0 equiv.) and dry toluene
(3 mL). The solution
59
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
was heated to 90 °C for 15h. The reaction mixture was evaporated irz
vacuo. The residue was
purified by Biotage flash system (50% hexanel50% ethyl acetate) to yield the
cis isomer 40 % yield.
'H-NMR (CDC13) 8: 1.08 (d, 3H), 1.63 (m, 1H), 2.14 (s, 3H), 2.2 (bs, 1H), 4.77
(m, 1H), 5.75 (bs,
1H), 6.23 (dd, 1H), 7.12-7.45 (m, 10H).
Scheme 3
0
NH
NH CI ~ ~ ~ N O
CH3COCI ~ O O
~N Pyridine,r.t. I i N Pyridine, Toluene
H ~ 50°C
O O
6
[00202] (~)-Trans-1-(2-Methyl-4-phenylamino-3,4-dihydro-2Ii-quinolin-1-yl)-
ethanone (5)
[00203] A 30 mL flask under nitrogen atmosphere was charged with (~)-trarzs-(2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-phenyl-amine (0.260 g, 1.1 mmol, 1.0 equiv) and
acetyl chloride (0.075 mL,
1.0 mmol, 0.95 equ) in pyridine (5 mL). The solution was allowed to stir at
room
temperature for 6 h. The reaction mixture was evaporated in vacuo. The residue
was purified by
Biotage flash system (70% hexane/30% ethyl. acetate to 60% hexane/40% ethyl
acetate to 50%
hexane/50% ethyl acetate) to yield the 2-acetyl traps isomers 35 % yield.
1H-NMR (CDC13) 8: 1.19 (d, 3H),_ 1.76 (m, 1H), 2.17(x, 3H), 2.52 (dd, 1H),
4.60(t, 1H), 4.93 (m,
1H), 6.67 (d, 2H), 6.71 (t, 1H), 7.13-7.36 (m ,6H), 7.41 (d, 1H).
[00204] (~)-Traps-furan-2-carboxylic acid (1-acetyl-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl)-phenyl-amide (6)
[00205] A round bottom flask under nitrogen atmosphere was charged with (~)-
traps-1-(2-methyl-
4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-ethanone (0.110 g, 0.39 mmol, 1.0
equiv) and 2-furoyl
chloride (0.193 mL, 1.9 mmol, 5.0 equ), pyridine (1.0 equ.) and dry toluene (5
mL). The solution
was heated to 50 °C for 5h. The reaction mixture was evaporated in
vacuo. The residue was purified
by Biotage flash system (30% hexane/70 % ethyl acetate to 50% hexane/50% ethyl
acetate) to yield
the trarzs isomer 34 % yield.
'H-NMR (CDCl3) 8: 1.11(d, 3H), 1.76 (s, 3H), 2.07 (dd, 1H), 2.37 (m, 1H), 5.00
(m, 1H), 5.48 (d,
1H),6.14 (dd, 1H), 6.29 (t, 1H), 6.90 (m, 1H), 6.99 (m, 1H), 7.22-7.32 (m,
6H), 7.34 (d, 1H), 7.54 (dd,
1H).
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00206] (~)-Cis-N-(1-Acetyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-4-
tluoro-N-phenyl-
benzamide (7)
[00207] A 30 mL flask under nitrogen atmosphere was charged with (~)-cis- (2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-phenyl-amine (0.520 g, 2.2 mmol, 1.0 equiv) and
acetic anhydride (0.209
mL, 2.2 mmol, 1.0 eq.) and dry toluene (31 mL). The solution was heated to 50
°C for 15h. The
reaction mixture was evaporated in vacuo. The residue was purified by Biotage
flash system (70%
hexanel30% ethyl acetate) to yield the 2-acetyl cis isomers 67 % yield.
'H-NMR (CDCl3) 8: 1.17 (d, 3H), 1.25 (q, 1H), 2.19 (s, 3H), 2.22(bs, 1H), 2.65
(m, 1H), 4.21 (dd,
1H), 4.96 (m, 1H), 6.65 (d, 2H), 6.75 (t,1H), 7.12-7.33 (m, 6H).
[00208] A round bottom flask under nitrogen atmosphere was charged with (~)-
cis-1-(2-methyl-4-
phenylamino-3,4-dihydro-2H-quinolin-1-yl)-ethanone (1.0 equiv) and 2-
fluorobenzoyl chloride (5.0
equ), pyridine (1.0 equ.) and dry toluene (3 mL). The solution was heated to
90 °C for 15h. .The
reaction mixture was evaporated in vacuo. The residue was purified by Biotage
flash system (50%
hexane/50% ethyl acetate) to yield the cis isomer 40 % yield.
1H-NMR (CDC13) 8: 1.1 (3H, d), 1.2 (1H, m), 2.1 (3H, s), 2.1 (1H, m), 4.8 (1H,
m), 5.4 (1H, m), 6.8
(2H, m), 6.9-7.4 (9H, m), 7.5 (1H, m).
MS m/z: 403 (M+1).
[00209] (~)-Traps-N-(1-Acetyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-4-
fluoro-N-phenyl-
benzamide (8)
[00210] A 30 mL flask under nitrogen atmosphere was charged with (~)-traps-(2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-phenyl-amine (0.260 g, 1.1 mmol, 1.0 equiv) and
acetyl chloride (0.075 mL,
1.0 mmol, 0.95 equ) in pyridine (5 mL). The solution was allowed to stir at
room
temperature for 6 h. The reaction mixture was evaporated ira vacuo. The
residue was purified by
Biotage flash system (70% hexane/30% ethyl acetate to 60% hexanel40% ethyl
acetate to 50%
hexane/50% ethyl acetate) to yield the 2-acetyl traps isomers 35 % yield.
1H-NMR (CDCI3) 8: 1.19 (d, 3H), 1.76 (m, 1H), 2.17(s, 3H), 2.52 (dd, 1H),
4.60(t, 1H), 4.93 (m,
1H), 6.67 (d, 2H), 6.71 (t,1H), 7.13-7.36 (m ,6H), 7.41 (d, 1H).
[00211] A round bottom flask under nitrogen atmosphere was charged with (~)-
traras-1-(2-methyl-
4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-ethanone (1.0 equiv) and 4-
fluorobenzoyl chloride
(5.0 equ), pyridine (1.0 equ.) and dry toluene (5 mL). The solution was heated
to 50 °C for 5h. The
reaction mixture was evaporated in vacuo. The residue was purified by Biotage
flash system (30%
hexane/70 % ethyl acetate to 50% hexane/50% ethyl acetate) to yield the traps
isomer 34 % yield.
61
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDCl3) 8: 1.2 (3H, d), 1.9 (3H, s), 2.0 (1H, m), 2.3 (1H, m), 5.0 (1H,
m), 6.2 (1H, m), 6.6-
6.8 (4H, m), 7.1 (3H, m), 7.3 (4H, m), 7.6 (1H, m).
MS mlz: 403 (M+1).
General Procedure A
Scheme 4
NH CI ~ ' \ NH \ N' \
CH3COCI
N~ DIEA, DCM, I ~ N~ DIEA, DCM, I ~ N
H ~~ rt.
O O
1 O ~ ~ O
A-1
[00212] (~)-eis-N-[1-(furan-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-1)
[00213] To a solution of (~)-cis-(2-Methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
phenyl-amine (430
mg, 1.83 mmol) in dichloromethane (18 mL,) at room temperature was added
diisopropylethylamine
(318 uL, 1.83 mmol) followed by 2-furoyl chloride. It was allowed to let stir
at room temperature for
12h. The mixture was poured into water and extracted with dichloromethane. The
extracts' were
washed with 1 M(aq) NaOH and brine, dried over magnesium sulfate, filtered and
concentrated. The
crude residue was purified by silica gel chromatography (80% hexanes/20% ethyl
acetate) to afford
the amide (500 mg, 83 %).
[00214] To a solution of (~)-cis-furan-2-yl-(2-methyl-4-phenylamino-3,4-
dihydro-2H-quinolin-1-
yl)-methanone (360 mg, 1.0 mmol) in methylene chloride (5 mL) was added
diisopropylethylamine
(1.9 mL, 10 mmol) followed by acetyl chloride (388 uL, 5 mmol). The mixture
was stirred at room
temperature over night. The mixture was poured into water and extracted with
dichloromethane. The
extracts were washed with 1 M (aq) NaOH and brine, dried over magnesium
sulfate, filtered and
concentrated. The crude residue was purified by silica gel chromatography (50%
hexanes/50% ethyl
acetate) to afford the amide (230 mg, 57 %).
'H-NMR (CDC13) 8: 1.12 (d, 3H), 1.25 (t, 1H), 2.01 (s, 3H), 2.32 (m, 1H), 4.12
(sextet, 1H), 5.49
(bs, 1H), 6.22 (m, 2H), 6.84(d, 1H), 7.10 (t, 1H), 7.28-7.31 (m, 4H), 7. 38
(m, 4H).
MS xn/z: 375 (M+1).
62
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00215] (~)-Cis-2-methoxy-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-
4-yl]-N-phenyl-acetamide (A-2)
[00216] (~)-Cis-2-methoxy-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 3-
methoxybenzoyl
chloride for 2-furoyl chloride and methoxyacetyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.14 (d, 3H), 1.25 (t, 1H), 2.33 (m, 1H), 3.39 (s, 3H),
3.60(s, 3H), 3.85(d, 1H),
3.98 (d, 1H), 4.79 (sextet, 1H), 5.62 (bs, 1H), 6.53 (d, 1H), 6.72 (s, 1H),
6.81(d, 1H), 6.92 (t, 1H),
7.08 (t, 1H), 7.16 (t, 1H), 7.29 (m, 2H), 7. 35-7.42 (m, 3H).
MS m/z: 445 (M+1).
[00217] (~)-Cis-4-chloro-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-benzamide (A-3)
[00218] (~)-Cis-4-Chloro-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-benzamide was made following general procedure A, substituting 3-
methoxybenzoyl
chloride for 2-furoyl chloride and 4-chlorobenzoyl chloride for acetyl
chloride.
,.'H-NMR,(CDC13) 8: 1.24 (d, 3H), 1.26 (m, 1H), 2.29 (m, 1H), 3.60 (s, 3H),
4.84 (sextet, 1H), 5.92
(bs, 1H), 6.58 (d, 1H), 6.78(d, 2H), 6.82 (s, 1H), 6.95 (t, 1H), 7.08 (t, 2H),
7.16-7.25 (m, 7H), 7.34 (d,
.2H), 7.53 (d, 1H).
MS mlz: 511.0 (M+1).
[00219) : (~)-Cis-N-[1-(3-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-isobutyramide (A-4)
[00220] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
isobutyramide was made following general procedure A, substituting 3-
methoxybenzoyl chloride for
2-furoyl chloride and isobutyryl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.14 (d, 9H), 1.23 (t, 1H), 2.28 (m, 1H), 2.65 (sextet, 1H),
3.65 (s, 3H), 4.77
(sextet, 1H), 5.63 (bs, 1H), 6.51 (d, 1H), 6.67(d, 1H), 6.78 (d, 1H), 6.86 (m,
2H), 7.01 (t, 1H), 7.14 (t,
1H), 7.24-7.37 (m, 6H).
MS m/z: 443.0 (M+1).
[00221] (~)-Cis-N-[2-Methyl-1-(thiophene-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-5)
[00222] (~)-Cis-N-[2-methyl-1-(thiophene-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 2-
thiophene carbonyl
chloride for 2-furoyl chloride.
63
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00223] (~)-Cis-N-[2-methyl-1-(thiophene-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was separated by chiral HPLC using a chiral cel OD column and
eluting with 90%
hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)- N-[2-methyl-
1-(thiophene-2-
carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-11 & A-10,
respectively).
IH-NMR (CDC13) ~: 1.15 (d, 3H), 1.25 (m, 1H), 2.02 (s, 3H), 2.31 (m, 1H), 4.73
(sextet, 1H), 5.53
(bs, 1H), 6.68 (dd, 1H), 6.77 (t, 1H), 6.88 (d, 1H), 7.06 (t, 1H), 7.25-7.32
(m, 4H), 7.39 (m, 4H).
MS m/z: 391.0 (M+1).
[00224] (~)-Cis-N-[1-(4-tert-butyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-6)
[00225] (~)-Cis-N-[1-(4-tert-butyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting 4-tert-
butylbenzoyl chloride for 2-
furoyl chloride.
[00226] (~)-Cis-N-[1-(4-tent-butyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
acetamide was separated by chiral HPLC using a chiral cel OD column and
eluting with 90%
hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-N-[1-(4-
tert-butyl-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-8 & A-9,
respectively).
'H-NMR (CDCl3) S: 1.14 (d, 3H), 1.16 (m, 1H), 1.23 (s, 9H), 2.04 (s, 3H), 2.33
(m, 1H), 4.78 (sextet,
1H), 5.62 (bs, 1H), 6.53 (d, 1H), 6.91 (t, 1H), 7.15-7.40 (m, 11H).
MS m/z: 441 (M+1).
[00227] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide (A-7) , .
[00228], ,, (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting 4-fluorobenzoyl
chloride for 2-
furoyl chloride.
[00229] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was separated by chiral HPLC using a chiral cel OD column and
eluting with 90%
hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-N-[1-(4-
fluoro-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-52 & A-44,
respectively).
1H-NMR (CDCI3) 8: 1.13 (d, 3H), 1.25 (m, 1H), 2.03 (s, 3H), 2.32 (m, 1H), 4.78
(sextet, 1H), 5.62
(bs, 1H), 6.47 (d,1H), 6.83-6.95 (m, 3H), 7.16-7.40 (m, 9H).
MS m/z: 403 (M+1).
64
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00230] (~)-Cis-N-[2-methyl-1-(5-methyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide (A-12)
[00231] (~)-Cis-N-[2-methyl-1-(5-methyl-thiophene-2-carbonyl)-1,2,3,4-
tctrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 5-
methyl-2-
thiophenecarbonyl chloride for 2-furoyl chloride.
[00232] (~)-Cis-N-[2-methyl-1-(5-methyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting with
90% hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)- cis-N-[2-
methyl-1-(5-methyl-
thiophene-2-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-
59 & A-60,
respectively).
1H-NMR (CDC13) 8: 1.07 (m, 1H), 1.12 (d, 3H), 2.01 (s, 3H), 2.31 (m, 1H), 2.39
(s, 3H), 4.69 (sextet,
1H), 5.50 (bs, 1H), 6.44 (s, 1H), 6.51 (d, 1H), 6.94 (d, 1H), 7.09 (t, 1H),
7.21-7.30 (m, 3H), 7.39-7.41
(m, 4H).
MS m/z: 405 (M+1)
[00233] (~)-Cis-N-[2-methyl-1-(4-methyl-2-pyrazin-2-yl-thiazole-5-carbonyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-13)
[00234] . (~)-Cis-N-[2-ethyl-1-(4-methyl-2-pyrazin-2-yl-thiazole-5-carbonyl)-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide was made following general procedure A,
substituting 4-methyl-
2-(2-pyrazinyl)-1,3-thiazole-5-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDCl3) S: 1.18 (d, 3H), 1.77 (bs, 1H), 2.03 (s, 3H), 2.10 (s, 3H),
2.32 (m, 1H), 4.79
,(sextet,1H), 5.50 (bs, 1H), 6.74 (d, 1H), 7.03 (t,1H), 7.26-7.41 (m, 7H),
8.55 (d, 1H), 9.32 (s, 1H).
MS m/z: 484 (M+1).
[00235] (~)-Cis-N-[2-methyl-1-(3-methyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide (A-14)
[00236] (~)-Cis-N-[2-methyl-1-(3-methyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 3-
methyl-2-
thiophenecarbonyl chloride for 2-furoyl chloride.
'H-NMR (CDC13) S: 1.13 (d, 3H), 1.16 (m, 1H), 1.80 (s, 3H), 2.00 (s, 3H), 2.29
(m, 1H), 4.73 (sextet,
1H), 5.49 (bs, 1H), 6.56 (d, 1H), 6.66 (d, 1H), 6.97 (t, 1H), 7.16 (d, 2H),
7.25 (d, 2H), 7.32 (d, 1H),
7.38 (bs, 3H).
MS m/z: 405 (M+1).
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00237] (~)-Cis-N-[2-methyl-1-(5-phenyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide (A-15)
[00238] (~)-Cis-N-[2-methyl-1-(5-phenyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 5-
phenyl-2-
thiophenecarbonyl chloride for 2-furoyl chloride.
IH-NMR (CDC13) 8: 1.15 (d, 3H), 1.17 (m, 1H), 2.03 (s, 3H), 2.31 (m, 1H), 4.73
(sextet, 1H), 5.55
(bs,1H), 6.59 (s, 1H), 6.95 (d, 2H), 6.99 (s, 1H), 7.10 (t, 1H), 7.26-7.44 (m,
9H), 7.53 (d, 2H).
MS mlz: 467 (M+1).
[00239] (~)-Cis-N-[2-methyl-1-(4-methyl-2-phenyl-thiazole-5-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-16)
[00240] (~)-Cis-N-[2-methyl-1-(4-methyl-2-phenyl-thiazole-5-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide was made following general procedure A,
substituting 4-methyl-
2-phenyl-1,3-thiazole-5-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDC13) S: 1.16 (d, 3H), 1.18 (m, 1H), 2.03 (s, 3H), 2.14 (s, 3H), 2.32
(m, 1H), 4.74 (sextet,
1H), 5.53 (bs, 1H), 6.77 (d, 2H), 7.04 (t, 1H), 7.24-7.28 (m, 3H), 7.38-7.40
(m, 7H), 7.83 (d, 2H).
MS m/z: 482 (M+1).
[00241] (~)-Cis-N-[2-methyl-1-(4-methyl-[1,2,3]thiadiazole-5-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-17)
[00242] (~)-Cis-N-[2-methyl-1-(4-methyl-[1,2,3]thiadiazole-5-carbonyl)-1,2,3,4-
tetrahydro-.
quinolin-4-yl]-N-phenyl-acetamide was made following general procedure A,
substituting 4-methyl-
[1,2,3]thiadiazole-5-carbonyl chloride for 2-furoyl chloride.
1H-NMR (CDC13) 8: 1.17 (d, 3H), 1.21 (m, 1H), 2.01 (s, 3H), 2.36 (s, 3H), 2.24
(m, 1H), 4.81 (sextet,
1H), 5.48 (bs, 1H), 6.52 (d, 1H), 6.98 (t, 1H), 7.22-7.26 (rn, 3H), 7.37-7.42
(m, 4H).
MS m/z: 407 (M+1).
[00243] (~)-Cis-N-[1-(5-isopropyl-thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-19)
[00244] (~)-Cis-N-[1-(5-isopropyl-thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 5-
isopropylthiophene
carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDC13) 8: 1.11 (d, 3H), 1.15 (m, 1H), 1.19-1.25 (m, 6H), 2.01 (s, 3H),
2.30 (m, 1H), 2.70
(m, 1H), 4.69 (sextet, 1H), 5.51 (bs, 1H), 6.45 (s, 1H), 6.55 (s, 1H), 6.87-
6.95 (m, 1H), 7.04-7.08 (m,
1H), 7.27 (s, 3H), 7.38 (s, 4H).
66
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 433 (M+1).
[00245] (~)-Cis-N-[2-methyl-1-(3,4,5-tritluoro-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-20)
[00246] (~)-Cis-N-[2-methyl-1-(3,4,5-trifluoro-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 3,4,5-
trifluorobenzoyl
chloride for 2-furoyl chloride.
'H-NMR (CDC13) b: 1.12 (d, 3H), 1.21 (m, 1H), 2.03 (s, 3H), 2.31 (m, 1H), 4.71
(sextet, 1H), 5.55
(bs, 1H), 6.50 (d, 1H), 6.82 (t, 1H), 6.99 (t, 1H), 7.06 (t, 1H), 7.2,4-7.27
(m, 3H), 7.39 (m, 3H), 7.46
(d,1H). . ,
MS mlz: 439 (M+1).
[00247] . (t)-Cis-N-[1-(4-fluoro-3-methyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-acetamide (A-21)
[00248] (~)-Cis-N-[1-(4-fluoro-3-methyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 4-fluoro-
3-methyl benzoyl
chloride for 2-furoyl chloride.
IH-NMR (CDC13) 8: 1.12 (d, 3H), 1.22 (m, 1H), 2.04 (s, 3H), 2.15 (s, 3H), 2.29
(m, 1H), 4.75 (sextet,
1H), 5.60 (bs, 1H), 6.50 (d, 1H), 6.73 (t, 1H), 6.86 (s, 1H), 6.93 (t, 1H),
7.15-7.39 (m, 8H).
MS m/z: 417 (M+1).
[00249] (~)-Cis-N-[1-(4-tluoro-3-trifluoromethyl-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-22)
[00250] (~)-Cis-N-[1-(4-fluoro-3-trifluoromethyl-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide was made following general procedure A, substituting
4-fluoro-3-
(trifluoromethyl)-benzoyl chloride for 2-furoyl chloride.
'H-NMR (CDCl3) 8: 1.15 (d, 3H), 1.24 (m,1H), 2.04 (s, 3H), 2.33 (m,1H), 4.75
(sextet, 1H), 5.58
(bs, 1H), 6.46 (d,1H), 6.87-6.96 (m, 3H), 7.10-7.41 (m, 6H), 7.49 (d,1H), 7.74
(d, 1H).
MS m/z: 471 (M+1).
[00251] (*)-Cis-N-[1-(3-chloro-4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-acetamide (A-23)
[00252] (~)-Cis-N-[1-(3-chloro-4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 3-chloro-
4.-fluorobenzoyl
chloride for 2-furoyl chloride.
67
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDCI3) S: 1.13 (d, 3H), 1.24 (m, 1H), 2.04 (s, 3H), 2.31 (m, 1H), 4.76
(sextet, 1H), 5.59
(bs, 1H), 6.50 (d, 1H), 6.85 (d, 2H), 6.96 (t, 1H), 7.21 (t, 1H), 7.27 (m,
2H), 7.39 (m, 4H), 7.50 (d,
1H).
MS m/z: 437 (M+1).
[00253] (~)-Cis-N-[2-methyl-1-(2,4,6-trifluoro-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-24)
[00254] (~)-Cis-N-[2-methyl-1-(2,4,6-trifluoro-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetannide was made following general procedure A, substituting 2,4,6-
trifluorobenzoyl
chloride for 2-furoyl chloride.
1H-NMR (CDC13) 8: 1.13 (d, 3H), 1.21 (m, 1H), 2.05 (s, 3H), 2.29 (m, 1H), 4.86
(sextet, 1H), 5.45
(bs,1H), 6.35 (t, 1H), 6.70 (d, 2H), 6.95 (t,1H), 7.2 -7.5 (m, 7H).
MS mlz: 439 (M+1).
[00255] (~)-Cis-N-[1-(4-chloro-benzoyl)-2-methyl-1,2;3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-25)
[00256] (~)-Cis-N-[1-(4-chloro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A, substituting 4-
chlorobenzoyl chloride for 2-
furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.09 (t, 3H), 1.12 (d, 3H), 1.22 (m, 1H), 2.23 (m, 3H), 4.73
(sextet, 1H), 5.58
(bs, 1H), 6.46 (d, 1H), 6.78 (d, 1H), 6.88 (t, 1H), 6.98 (t, 1H), 7.15 (t,
1H), 7.18-7.44 (m, 8H).
MS m/z:433 (M+1).
[00257] ~, (~)-Cis-N-[2-methyl-1-(4-trifluoromethoxy-benzoyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide (A-26) .
[00258] (~)-Cis-N-[2-methyl-1-(4-trifluoromethoxy-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 4-
(trifluoromethoxy)benzoyl
chloride for 2-furoyl chloride.
1H-NMR (CDC13) 8: 1.16 (d, 3H), 1.24 (m, 1H), 2.28 (m, 3H), 4.78 (sextet, 1H),
5.61 (bs, 1H), 6.46
(d, 1H), 6.91 (t, 1H), 6.92 (t, 1H), 7.02 (d, 2H), 7.18 (t, 1H), 7.23-7.27 (m,
4H), 7.33 (d, 1H), 7.39 (s,
3H).
MS m/z: 469 (M+1).
[00259] (~)-Cis-N-[2-methyl-1-(3-trifluoromethoxy-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-acetamide (A-27)
68
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00260] (~)-Cis-N-[2-methyl-1-(3-trifluoromethoxy-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 3-
(trifluoromethoxy)benzoyl
chloride for 2-furoyl chloride.
'H-NMR (CDCl3) 8: 1.14 (t, 3H), 1.15 (d, 3H), 1.25 (m, 1H), 2.25 (m, 3H), 4.78
(sextet, 1H), 5.59
(bs, 1H), 6.46 (d, 1H), 6.91 (t, 1H), 6.95 (d, 1H), 7.12 -7.27 (m, 6H), 7.34
(d, 1H), 7.39 (s, 3H).
MS m/z: 469 (M+1).
[00261] (~)-Cis-N-[2-methyl-1-(3-phenyl-isoxazole-5-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide (A-28)
[00262] (~)-Cis-N-[2-methyl-1-(3-phenyl-isoxazole-5-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A, substituting
3-phenyl-5-
isoxazole carbonyl chloride fox 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDCl3) 8: 1.14 (t, 3H), 1.19 (d, 3H), 1.61 (m, 1H), 2.24 (m, 3H), 4.78
(sextet, 1H), 5.49
(bs, 1H), 6.34 (bs, 1H), 6.85 (d, 1H), 7.10 (t, 1H), 7.26 (s, 3H), 7.32 (t,
1H), 7.40 (m, 6H), 7.67 (s,
2H).
MS m/z: 466 (M+1).
[00263] (~)-Cis-N-{2-methyl-1-[4-(5-methyl-tetrazol-1-yl)-benzoyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-29)
[00264]~ (~)-Cis-N-{2-methyl-1-[4-(5-methyl-tetrazol-1-yl)-benzoyl]-1,2,3,4-
tetrahydro-quinolin-
4-yl }-N-phenyl-propionamide was made following general procedure A,
substituting 4-(5-methyl-1H-
tetrazole-1-yl)-benzoyl chloride for 2-furoyl chloride and propionyl chloride
for acetyl chloride.
'H-NMR (CDC13) S: 1.16 (t, 3H), 1.17 (d, 3H), 1.24 (m, 1H), 2.26 (m, 3H), 2.55
(s, 3H), 4.82 (sextet,
1H), 5.64 (bs,1H), 6.50 (d,1H), 6.94 (t,1H), 7.21-7.41 (m, 11H).
MS xnlz: 481 (M+1).
[00265] (~)-Cis-N-{1-[3-(4-chloro-phenyl)-isoxazole-5-carbonyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-30)
[00266] (~)-Cis-N-{ 1-[3-(4-chloro-phenyl)-isoxazole-5-carbonyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide was made following general procedure A
substituting 3-(4-
chlorophenyl)-5-isoxazole carbonyl chloride for 2-furoyl chloride and
propionyl chloride for acetyl
chloride.
'H-NMR (CDC13) 8: 1.21 (m, 6H), 1.24 (m, 1H), 2.23 (m, 3H), 4.76 (sextet, 1H),
5.48 (bs, 1H), 6.28
(s, 1H), 6.84 (d, 1H), 7.07 (m, 2H), 7.26-7.67 (m, 7H), 7.78 (d, 1H), 8.03 (t,
2H).
MS m/z: 500 (M+1).
69
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00267] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-2-
hydroxy-N-phenyl-acetamide (A-31)
[00268] (~)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
2-hydroxy-N-
phenyl-acetamide was made following general procedure A substituting 4-
fluorobenzoyl chloride for
2-furoyl chloride and acetoxyacetyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1.22 (m, 1H), 2.39 (m, 1H), 3.42 (s, 1H), 3.85
(d, 1H), 4.04 (d,
1H), 4.77 (sextet, 1H), 5.54 (bs, 1H), 6.49 (d, 1H), 6.85 (t, 2H), 6.94 (t,
1H), 7.18-7.27 (m, 5H), 7.33
(d,1H), 7.43 (s, 3H).
MS mlz: 419 (M+1).
[00269] (~)-Cis-N-[1-(1H-indole-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]=N-
phenyl-propionamide (A-32)
[00270] (~)-Cis-N-[1-(1H-indole-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A substituting indole-
2-carbonyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) b: 1.25 (t, 3H), 1.26 (d, 3H), 1.27 (m, 1H), 2.36 (m, 3H), 4.86
(sextet, 1H), 5.62
(bs, 1H),.5.95 (s,1H), 7.11 (t, 1H), 7.18 (t, 2H), 7.29 (t, 1H), 7.37 (m, 4H),
7.44-7.55 (m, 5H).
MS m/z: 438 (M+1).
[00271] (t)-Cis-N-[2-methyl-1-(4-pyrazol-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-33)
[00272] (~)-Cis-N-[2-methyl-1-(4-pyrazol-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A substituting 4-(1H-
pyrazol-1-yl)-
benzoyl chloride for 2-furoyl chloride and propionyl chloride for acetyl
chloride.
'H-NMR (CDC13) 8: 1.03 (t, 3H), 1.11 (d, 3H), 1.20 (m, 1H), 2.19 (m, 3H), 4.73
(sextet, 1H), 5.62
(bs, 1H), 6.39 (s,1H), 6.48 (d,1H), 6.86 (t,1H), 7.10-7.34 (m, 9H), 7.48 (d,
2H), 7.65 (s, 1H), 7.81 (s,
1H).
MS m/z: 465 (M+1).
[00273] (t)-Cis-N-[1-(benzofuran-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-34)
[00274] (~)-Cis-N-[1-(benzofuran-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A substituting 2-
benzofuran carbonyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 1.04 (t, 3H), 1.07 (d, 3H), 1.18 (m, 1H), 2.19 (m, 3H), 4.69
(sextet, 1H), 5.54
(bs, 1H), 6.41 (d, 1H), 6.70-7.39 (m, 12H), 7.43 (d, 1H).
MS m/z: 439 (M+1).
[00275] (~)-Cis-N-[1-(3-chloro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-35)
[00276] (~)-Cis-N-[1-(3-chloro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was following general procedure A made substituting 3-
chlorobenzoyl chloride for 2-
furoyl chloride and propionyl chloxide for acetyl chloride.
iH-NMR (CDC13) 8: 1.09 (t, 3H), 1.12 (d, 3H), 1.22 (m, 1H), 2.23 (m, 3H), 4.73
(sextet, 1H), 5.58
(bs, 1H), 6.46 (d,1H), 6.78 (d,1H), 6.88 (t, lIT), 6.98 (t,1H), 7.15 (t, 1H),
7.18-7.44 (m, 8H).
MS m/z433 (M+1).
[00277] (+_)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenoxy}-acetic acid ethyl ester (A-36)
[00278] (~)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-carbonyl]-
phenoxy}-acetic acid ethyl ester was. made from (~)-N-[1-(4-Hydroxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. (~)-N-[1-(4-Methoxy-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl propionamide (0.147 g) was dissolved in DMF
(5 mL) at room
temperature. Sodium hydride (60 % in oil, 0.021g) was added and the mixture
allowed to stir 30 min.
Ethyl 4-bromoacetate (0.065 g) was added and the reaction was allowed to stir
over night. Ethanol
was added and the reaction was concentrated irz vacuo. The crude residue was
purified by silica gel
chromatography (80/20 hexanes/ethyl acetate - 50/50 hexanes ethyl acetate
gradient) to afford the
product (130 mg, 73 %).
iH-NMR (CDC13) 8: 1.08-1.16 (m, 9H), 1.21 (t, 1H), 2.24 (m, 3H), 4.09 (q, 2H),
4.53 (s, 2H), 4.74
(sextet, 1H), 5.59 (bs,1H), 6.48 (d,1H), 6.67 (d, 2H), 6.89 (t,1H), 7.11-7.37
(m, 9H).
MS m/z: 500 (M+1).
[00279] (*)-Cis-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-propionamide (A-37)
[00280] (~)-Cis-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
propionamide was made from (~)-cis-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide substituting 3-chlorobenzoyl chloride.
(~)-Cis-N-[1-(4-
71
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
propionamide was (0.548 g,
0.001 mol) was dissolved in dichloromethane and a solution of BBr3 (1.0 M in
dichloromethane, 10
mL) was added; the reaction was allowed to stir at room temperature for 4h or
until no starting
material remained. The reaction was carefully washed with sat NaHC03 and
brine. The organics
were dried over MgS04, filtered and concentrated under reduced pressure. The
phenol was
concentrated and the residue was purified by Biotage flash chromatography
using 100 % EtOAc to
give a white solid, 68 % yield.
'H-NMR (CDC13) 8: 1.09 (d, 3H), 1.11 (t, 3H), 1.19 (m, 1H), 2.26 (m, 3H), 4.74
(sextet, 1H), 5.54
(bs, 1H), 6.46 (d,1H), 6.53 (d, 1H), 6.96 (t, 1H), 7.14-7.40 (m, 9H).
MS mlz: 415 (M+1)
[00281] (~)-Cis-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-propionamide (A-38)
[00282] (~)-Cis-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
propionamide was made following general procedure A substituting 4-
methoxybenzoyl chloride for 2-
furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDCl3) b: 1.12 (d, 3H), 1.15 (t, 3H), 1.17 (m, 1H), 2.23 (m, 3H), 3.74
(s, 3H), 4.74 (sextet,
1H), 5.61 (bs, 1H), 6.52 (d, 1H), 6.67 (d, 2H), 6.92 (d, 1H), 7.17 (d, 2H),
7.25-7.34 (m, 4H), 7.39 (6s,
3H).
MS m/z: 429 (M+1).
[00283] (~)-Cis--{4-[2-Methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenoxy}-acetic acid (A-39)
[00284] (~)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-carbonyl]-
phenoxy}-acetic acid was made from (~)-cis-{4-[2-methyl-4-(phenyl-propionyl-
amino)-3,4-dihydro-
2H-quinoline-1-carbonyl]-phenoxy}-acetic acid ethyl ester. (~)-Cis-{4-[2-
methyl-4-(phenyl-
propionyl-amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenoxy }-acetic acid
ethyl ester was
dissolved in ethanol (5 mL) and 0.5 mL of 1N NaOH was added at room
temperature. The reaction
was allowed to stir for 4h. The ethanol was removed in vacuo and the aqueous
solution was acidified
with 1N HCl to give a white precipitate which was filtered to give the desired
product in 88 % yield.
'H-NMR (CDCl3) b: 1.12 (d, 3H),1.16 (t, 3H), 1.15 (m, 1H), 2.28 (m, 3H), 4.52
(s, 2H), 4.74 (sextet,
1H), 5.63 (bs, 1H), 6.50 (d, 1H), 6.68 (d, 2H), 6.91 (t, 1H), 7.16 (t, 1H),
7.18 (d, 2H), 7.26-7.32 (m,
4H), 7.40 (bs, 2H).
MS mlz: 473.0 (M+1).
72
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00285] (~)-Cis-N-{2-methyl-1-[4-(2-morpholin-4-yl-ethoxy)-benzoyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-40)
[00286] (~)-Cis-N-{2-methyl-1-[4-(2-morpholin-4-yl-ethoxy)-benzoyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide was made from (~)-cis-N-[1-(4-hydroxy-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. (~)-Cis-N-[1-(4-
methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide was dissolved
in DMF (5 mL) at
room temperature. Sodium hydride (60 % in oil, 0.061g) was added and the
mixture allowed to stir
30 min. 4-(2-chloroethyl)morpholine hydrochloride (0.143 g) was added and the
reaction was
allowed to stir over night. Ethanol was added and the reaction was
concentrated in vacuo. The
residue was partition between ethyl acetate and water, then extracted 3x with
ethyl acetate, dried over
MgS04, filtered and concentrated. The crude residue was purified by silica gel
chromatography (2/98
methanol/dichloromethane - 5/95 methanol/dichloromethane gradient) to afford
the product (200 mg).
1H-NMR (CDC13) 8: 1.09 (d, 3H), 1.12 (m, 4H), 1.22 (s, 4H), 2.23 (m, 3H), 2.50
(s, 4H), 2.70 (m;
2H), 4.01 (t, 2H), 4.70 (sextet, 1H), 5.59 (bs, 1H), 6.49 (d, 1H), 6.64 (d,
2H), 6.89 (t, 1H), 7.13 (d,
2H), 7.23-7.36 (m, 7H).
MS m/z: 528.1 (M+1).
(00287] (~)-Cis-N-[1-(4-carbamoyhnethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-propionamide (A-41)
[00288] (~)-Cis-N-[1-(4-carbamoylrnethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide was made from (~)-cis-N-[1-(4-hydroxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. (~)-Cis-N-[1-(4-hydroxy-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (0.120 g) was
dissolved in DMF (5 mL) at
room temperature. Sodium hydride (60 % in oil, 0.70 g) was added and the
mixture allowed to stir 30
min. 2-Bromoacetamide (0.320 g) was added and the reaction was allowed to stir
over night. Ethanol
was added and the reaction was concentrated in vacuo. The residue was
partitioned between ethyl
acetate and water, then extracted 3x with ethyl acetate, dried over MgS04,
filtered and concentrated
down. The crude residue was purified by silica gel chromatography (2/98
methanol/dichloromethane
-10/90 methanol/dichloromethane gradient) to afford the product (20 mg,15%).
'H-NMR (CDC13) 8: 1.12 (d, 3H), 1.14 (t, 3H), 1.24 (t, 1H), 2.25 (m, 3H), 4.42
(s, 2H), 4.73 (sextet,
1H), 5.61 (bs,1H), 5.79 (s,1H), 6.49 (d, 2H), 6.70 (d, 2H), 6.92 (t,1H), 7.14-
7.39 (m, 8H).
MS mlz: 472.0 (M+1).
(00289] (~)-Cis-N-{1-[4-(2-hydroxy-2-methyl-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamude (A-42)
73
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00290] (~)-Cis-N-{ 1-[4-(2-hydroxy-2-methyl-propoxy)-benzoyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide was made from (~)-Cis-{4-[2-methyl-4-
(phenyl-propionyl-
amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenoxy}-acetic acid ethyl ester.
(~)-Cis-{4-[2-
methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-
phenoxy}-acetic acid
ethyl ester (0.170 g) was dissolved in THF and cooled to 0 °C.
Methylmagnesium bromide (3.0M sol
in diethyl ether, 0.5 mL) was added and the reaction was allowed to stir at 0
°C for 30 min. The
reaction was quenched with a saturated solution of ammonium chloride and
diluted with ethyl acetate.
The organics were seperated and washed with brine, dried over MgS04, filtered
and concentrated
down. The crude residue was purified by silica gel chromatography (50/50
hexanes/ethyl acetate -
75/25 hexanes ethyl acetate gradient) to afford the product (132 mg, 80 %).
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.14 (t, 3H), 1.23 (t, 1H), 1.29 (s, 6H), 2.24
(m, 3H), 3.70 (s, 2H),
4.74 (sextet, 1H), 5.61 (bs, 1H), 6.50 (d, 1H), 6.66 (d, 2H), 6.91 (t, 1H),
7.13 (t, 1H), 7.14 (d, 2H)~
7.25 (d, 1H), 7.32 (d; 1H), 7.37 (bs, 4H).
MS.m/z: 487.1 (M+1).
[00291] (~)-Cis-N-[1-(4-dimethylcarbamoyhnethoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-43)
[00292] (~)-Cis-N-[1-(4-dimethylcarbamoylmethoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made from (~)-cis-{4-[2-methyl-4-
(phenyl-propionyl-'
amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenoxy}-acetic acid. (~)-Cis-{-4-
[2-Methyl-4-
(phenyl-propionyl-amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenoxy}-acetic
acid (0.146 g) was
dissolved in THF (2 mL) at room temperature. HOBt (0.063 g), EDCI (0.071 g),
and dimethylamine
(2.0M solution in THF, 0.162 mL) was added along with 2 drops of DMF and
stirred at room
temperature for 11 h. The reaction was diluted with ethyl acetate, washed with
1N NaOH, 1N HCl
and brine. The organics were dried over MgS04, filtered and concentrated down.
The crude residue
was purified by silica gel chromatography (100% ethyl acetate) to afford the
product (84 mg, 54%).
'H-NMR (CDC13) 8: 1.10 (d, 3 H), 1.13 (t, 3 H), 1.22 (t, 1 H), 2.23 (m, 3 H),
2.94 (s, 3 H), 3.00 (s; 3
H), 4.60 (s, 2 H), 4.71 (sextet, 1 H), 5.58 (bs, 1 H), 6.49 (d, l H), 6.70 (d,
2 H), 6.89 (t, 1 H), 7.13 (d,
1 H), 7.24 (d, 2 H), 7.30 (d, l H), 7.37 (bs, 7 H).
' MS m/z: 500.1 (M+1).
[00293] (~)-Cis-N-[1-(3-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-45)
[00294] (~)-Cis-N-[1-(3-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A, substituting 3-
dimethylaminobenzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
74
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (300 MHz, CDCl3) 8: 1.11-1.24 (m, 7 H), 2.12 - 2.40 (m, 3 H), 2.83 (s,
6 H), 4.80 (ddd, 1
H), 5.59 (br s, 1 H), 6.49 (d, 1 H), 6.55 - 6.69 (m, 3 H), 6.92 (dd, 1 H),
7.00 (ddd, 1 H), 7.15 (ddd, 1
H), 7.23 - 7.34 (m, 3 H), 7.35 - 7.44 (m, 3 H).
MS mlz: 442 (M + 1).
[00295] (~)-Cis-N-[1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-46)
[00296] (~)-Cis-N-[1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A substituting 4-
dimethylaminobenzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (300 MHz, CDC13) b: 1.09 -1.28 (m, 7 H), 2.12 - 2.39 (m, 3 H), 2.93 (s,
6 H), 4.73 (ddd, 1
H), 5.61 (br s, 1 H), 6.47 (d, 2 H), 6.62 (d, l H), 6.96 (dd, 1 H), 7.12 -
7.20 (m, 3 H), 7.26 - 7.36 (m,
3 H), 7.38 - 7.46 (m, 3 H).
MS m/z: 442 (M + 1).
[00297] (~)-Cis-N-[2-methyl-1-(pyridine-3-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-47)
[00298] (~)-Cis-[2-ethyl-1-(pyridine-3-carbonyl)=1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A substituting 3-pyridinyl
chloride for 2-furoyl
chloride and propionyl chloride for acetyl chloride.
'H-NMR (300 MHz, CDCl3) 8: 1.08 -1.32 (m, 7 H), 2.16 - 2.44 (m, 3 H), 4.84
(ddd, 1 H), 5.62 (br s,
1 H), 6.53 (d, 1 H), 6.97 (dd, 1 H), 7.11 (dd, 1 H), 7.20 - 7.51 (m, 8H), 8.55
(dd, 1 H), 8.68 (br s, 1
~.
MS m/z: 400 (M + 1).
[00299] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-4-
methoxy-N-phenyl-butyramide (A-48)
[00300] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-4-methoxy-
N-phenyl-butyramide was made following general procedure A substituting 4-
fluorobenzoyl chloride
for 2-furoyl chloride and 4-methoxy-butyryl chloride for acetyl chloride.
'H-NMR (300 MHz, CDC13) 8: 1.08 -1.20 (m, 4 H), 1.86 - 2.02 (m, 2 H), 2.21-
2.41 (m, 3 H), 3.26
(m, 3 H), 3.28 - 3.44 (m, 2 H), 4.76 (ddd, 1 H), 5.64 (br s, 1 H), 6.43 (d, 1
H), 6.83 - 6.96 (m, 3 H),
7.17 - 7.34 (m, 5 H), 7.36 - 7.51 (m, 4 H).
MS mlz: 461 (M + 1).
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00301] (~)-Cis-2-(acetyl-methyl-amino)-N-[1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-49)
[00302] (~)-Cis-2-(acetyl-methyl-amino)-N-[1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide was made following general procedure A
substituting 4-
fluorobenzoyl chloride for 2-furoyl chloride and (acetyl-methyl-amino)-acetyl
chloride for acetyl
chloride.
'H-NMR (300 MHz, CDC13) 8: 1.10 - 1.18 (m, 4 H), 2.13 (s, 3 H), 2.27 - 2.43
(m, 1 H), 3.14 (m, 3
H), 3.77 (d, 1. H), 4.03 (d, 1 H), 4.76 (ddd, 1 H), 5.55 (br s, 1 H), 6.45 (d,
1 H), 6.81- 6.95 (m, 3 H),
7.15 - 7.26 (m, 3 H), 7.31- 7.49 (m, 5 H), 7.54 (d, 1 H).
MS m/z = 474 (M + 1).
[00303] (~)-Cis-cyclohexanecarboxylic acid [1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-phenylamide (A-54)
[00304] (~)-Cis-cyclohexanecarboxylic acid [1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro=
quinolin-4-yl]-phenylamide was made following general procedure A substituting
3-methoxybenzoyl
chloride for 2-furoyl chloride and cyclohexane carbonyl chloride for acetyl
chloride.
IH-NMR (CDC13) S: 0.8 (8 H, m), 1.5-1.8 (5 H, m), 2.0-2.4 (3 H, m), 3.7 (3 H,
d), 4.8 (1 H, m)~ 5.6
(1 H, d), 6.2-6.6 (2 H, m), 6.6-7.5 (11 H, m).
MS mlz: 483 (M+1).
[00305] (~)-Cis-isoxazole-5-carboxylic acid [1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-phenyl-amid (A-55)
[00306] (~)-Cis-isoxazole-5-carboxylic acid [1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-phenyl-amide was made following general procedure A
substituting 3-methoxybenzoyl
chloride for 2-furoyl chloride and isoxazole-5-carbonyl chloride for acetyl
chloride.
1H-NMR (CDC13) 8: 1.2 (3 H, d),1.2 (1 H, m), 2.4 (1 H, m), 3.6 (3 H, s), 4.9
(1 H, m), 5.8 (1 H, m),
6.4 (1 H, d), 6.7-7.7 (12 H, m), 8.2 (1 H, s), 8.4 (1 H, m).
MS m/z: 468 (M+1).
[00307] (~)-Cis-N-[1-(furan-3-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-56)
[00308] (~)-Cis-N-[1-(furan-3-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure A substituting 3- furoyl
chloride for 2-furoyl
chloride.
76
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) ~: 1.1 (3 H, d),1.2 (1 H, m), 2.0 (3 H, s), 2.2 (1 H, m), 4.7
(1 H, m), 5.5 (1 H, m),
5.9 (l H,s),6.9(lH,d),7.1(2H,m) 7.2-7.4 (7 H, m).
MS mlz: 375 (M+1).
[00309] (~)-Cis-N-[1-(3-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide (A-61)
[00310] (~)-Cis-N-[1-(3-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure A substituting 3-fluorobenzoyl
chloride for 2-furoyl
chloride.
'H-NMR (CDC13) ~: 1.1 (3 H, d), 1.1 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 4.7
(1 H, m), 5.6 (1 H, m),
6.4(lH,d),6.8(lH,d),6.9-7.4(llH,m).
MS m/z: 403 (M+1).
[00311) (~)-Cis-N-[1-(3,4-difluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-62)
[00312] (~)-Cis-N-[1-(3,4-difluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A substituting 3,4-
difluorobenzoyl chloride
for 2-furoyl chloride.
'H-NMR (CDCl3) 8: 1.1 (3 H, d), 1.1 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 4.7
(1 H, m), 5.6 (1 H, m),
6.5 (1 H, d), 6.8-7.0 (4 H, d), 7.3-7.5 (7 H, m).
MS m/z: 421 (M+1). '
[00313] (~)-Cis-N-[1-(benzo[b]thiophene-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide (A-63)
[00314] (~)-Cis-N-[1-(benzo[b]thiophene-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-
N-phenyl-acetamide was made following general procedure A substituting
benzo[b]thiophene-3-
carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDCI3) 8: 1.2 (3 H, d), 1.3 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 4.9
(1 H, m), 5.7 (1 H, m),
6.5(lH,d),6.8(lH,m),7.1-7.5(lOH,m),7.8(lH,d),8.0(lH,d).
MS m/z: 442 (M+2).
[00315] (~)-Gis-N-[1-(3,5-dimethyl-thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-64)
77
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00316] (~)-Cis-N-[1-(3,5-dimethyl-thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide was made following general procedure A substituting
3,5-dimethyl-
thiophene-2-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDC13) 8: 1.1 (3 H, d), 1.1 (1 H, m), 1.7 (3 H, s), 2.0 (3 H, d), 2.0
(1 H, m), 2.3 (3 H, s),
4.7(lH,m),5.5(lH,m),6.2(lH,s),6.7(lH,d),7.0(lH,t),7.1-7.4 (7 H, m).
MS mlz: 419 (M+1).
[00317] (~)-Cis-N-[1-(3-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
isobutyramide (A-65)
[00318] (~)-Cis-N-[1-(3-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
isobutyramide was made following general procedure A substituting 3-
fluorobenzoyl chloride for 2-
furoyl chloride and isopropyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.0-1.2 (10 H, m), 2.3 (1 H, m), 2.7 (1 H, m), 4.8 (1 H, m),
5.6 (1 H, m), 6.5 (1
H, m), 6.8-7.6 (12 H, m).
MS m/z: 431 (M+1).
[00319] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
isobutyramide (A-66)
[00320] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
isobutyramide was made following general procedure A substituting 4-
fluorobenzoyl chloride for 2-
furoyl chloride and isopropyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.0-1.2 (10 H, m), 2.3 (1 H, m), 2.6 (1 H, m), 4.8 (1 H, m),
5.6 (1 H, m), 6.5 (1
H, d), 6.8-7.0 (3 H, m), 7.1-7.4 (9 H, m).
MS m/z: 431 (M+1).
[00321] (~)-Cis-N-[1-(2,4-dimethyl-thiazole-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-67)
[00322] (~)-Cis-N-[1-(2,4-dimethyl-thiazole-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A substituting 2,4-
dimethyl-thiazole-
5-carbonyl chloride for 2-furoyl chloride. '
'H-NMR (CDC13) 8: 1.2 (3 H, d), 1.2 ( 1 H, m), 2.0 (3 H, s), 2.2 (3 H, s), 2.3
(1 H, m), 2.6 (3 H, s),
4.7 (1 H, m), 5.4 (1 H, m), 6.8 (1 H, d), 7.1 (2 H, m), 7.2-7.5 (6 H, m).
MS m/z: 420 (M+1).
78
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00323] (~)-Cis-N-[1-(furan-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-68)
j00324] (~)-Cis-N-[1-(furan-2-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A substituting propionyl
chloride for acetyl
chloride.
'H-NMR (CDC13) S: 1.0-1.2 (7 H, m), 2.2-2.4 (3 H, m), 4.7 (1 H, m), 5.4 (1 H,
m), 6.2 (2 H, m), 6.8
(1 H, d), 7.0-7.4 (9 H, m).
MS mlz: 389 .(M+1).
[00325] (~)-Cis-N-[1-(4-tluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
butyramide (A-69)
[00326] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
butyramide was made following general procedure A substituting 4-fluoxobenzoyl
chloride for 2-
furoyl chloride and butyryl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 0.8 (3 H, t), 1.2 (3 H, d), 1.2 (1 H, m), 1.5 (2 H, m), 2.0
(3 H, m), 4.7 (1 H, m),
5.4 (1 H, m), 6.5 (1 H, d), 6.6-6.8 (4 H, m), 6.9-7.3 (8 H, m).
MS m/z: 432 (M+2).
[00327] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-2-
phenoxy-N-phenyl-acetamide (A-72)
[00328] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinalin-4-
yl]-2-phenoxy-
N-phenyl-acetamide was made following general procedure A substituting 4-
fluorobenzoyl chloride
for 2-furoyl chloride and 1-chloro-3-phenoxy-propan-2-one for acetyl chloride.
'H-NMR (CDC13) 8: 1.1 (3 H, d), 1.1 (1 H, m), 2.3 (1 H, m), 4.5 (2 H, s), 4.7
(1 H, m), 5.7 (1 H, m),
6.4(lH,d),6.7-6.9(7 H,m),7.1-7.4 (9 H,m),10.0(lH,m).
MS m/z: 496 (M+2).
[00329] (*)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-3,N-
diphenyl-propionamide (A-73)
[00330] (~)-Cis-N-[1-(4-Fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-3,N-
diphenyl-propionamide was made following general procedure A substituting 4-
fluorobenzoyl
chloride for 2-furoyl chloride and 3-phenylpropionyl chloride for acetyl
chloride.
'H-NMR (CDCl3) 8: 1.2 (3 H, d), 1.2 (1 H, m), 2.2 (1 H, m), 2.7 (2 H, t), 3.1
(2 H, t), 4.7 (1 H, m),
5.7(lH,m),6.6(lH,d),6.8-7.6 (l7 H, m).
MS mlz: 494 (M+2).
79
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00331] (+_)-Cis-N-[1-(benzo[b]thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide (A-75)
[00332] (~)-Cis-N-[1-(benzo[b]thiophene-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A substituting
benzo[b]thiophene-2-
carbonyl chloride for 2-furoyl chloride and propionyl chloride for acetyl
chloride.
'H-NMR (CDC13) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, m), 4.8 (1 H, m), 5.6 (1 H,
m), 6.9 (1 H, d), 7.0
(2H,m),7.2-7.5 (9 H,m),7.6(lH,d),7.8(lH,d).
MS m/z: 456 (M+2).
[00333] (~)-Cis-N-[1-(4-cyano-benzoyl)-2-methyl-1,2,3,4.tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide (A-76)
[00334] (~)-Cis-N-[1-(4-cyano-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A substituting 4-
cyanobenzoyl chloride for 2-
furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.1-1.3 (7 H, m), 2.2-2.4 (3 H, m), 4.8 (1.H, m), 5.6 (1 H,
m), 6.4 (1 H, d), 6.9
(1 H, t), 7.2-7.6 (11 H, m).
MS mlz: 424 (M+1).
[00335] (~)-Cis-N-[1-(3-fluoro-4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-ghenyl-propionamide (A-77)
[00336]. (~)-Cis-N-[1-(3-fluoro-4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A substituting 3-
fluoro-4-
methoxybenzoyl chloride for 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDC13) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, m), 3.8 (3 H, s), 4.8 (1 H,
m), 5.6 (1 H, m), 6.5 (1
H,d),6.7(lH,t),6.8(lH,d),6.9(lH,t),7.2-7.5 (S H, m).
MS mlz: 447 (M+1).
[00337] (*)-Cis-N-[1-(4-methoxy-3-methyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-propionamide (A-78)
[00338] (~-)-Cis-N-[1-(4-methoxy-3-methyl-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A substituting 3-
methyl-4-
methoxybenzoyl chloride for 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDCI3) 8: 0.9-1.1 (7 H, m), 1.8-2.2 (6 H, m), 3.8 (3 H, s), 4.8 (1 H,
m), 5.6 (1 H, m), 6.5 (2
H, m), 6.7-7.8 (10 H, m).
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 443 (M+1).
[00339] (~)-Cis-N-[1-(4-etho~ry-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-propionamide (A-79)
[00340] (~)-Cis-N-[1-(4-ethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A substituting 4-
ethoxybenzoyl chloride fox 2-
furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) s: 1.1-1.3 (7 H, m), 1.4 (3 H, t), 2.2-2.4 (3 H, m), 4.0 (2 H,
q), 4.8 (1 H, m), 5.6 (1
H,m),6.5(lH,d),6.9(2H,d),6.9(lH,t),7.2-7.6 (9 H, m).
MS m/z: 443 (M+1).
[00341] (+_)-Cis-N-[2-methyl-1-(4-trifluoromethyl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-80)
[00342] (~)-Cis-N-[2-methyl-1-(4-trifluoromethyl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A substituting 4-
trifluoromethylbenzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 1.1-1.3 (7 H, m), 2.2-2.4 (3 H, m), 4.8 (1 H, m), 5.6 (1 H,
m), 6.4 (1 H, d)~ 6.9
(1 H, t), 7.2-7.6 (11 H, m).
MS m/z: 319 (M-147).
[00343] (~)-Cis-N-[1-(4-benzyl-morpholine-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-81)
[00344] (~)-Cis-N-[1-(4-benzyl-morpholine-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A substituting
4-benzyl-
morpholine-2-carbonyl chloride for 2-furoyl chloride and propionyl chloride
for acetyl chloride.
1H-NMR (CDC13) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (4 H, m), 2.6 (3 H, m), 3.5 (2 H,
m), 3.9 (1 H, m), 4.2
(lH,m),4.7(lH,m),5.2(lH,m),7.1-7.5 (l4 H, m).
MS m/z: 498 (M+1).
[00345] (~)-Cis-N-[1-(4-ethyl-morpholine-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide (A-82)
[00346] (~)-Cis-N-[1-(4-Ethyl-morpholine-2-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A substituting
4-ethyl-
morpholine-2-carbonyl chloride for 2-furoyl chloride and propionyl chloride
for acetyl chloride.
81
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 1.1-1.2 (10 H, m), 2.1-2.4 (6 H, m), 2.6 (2 H, m), 3.6 (1 H,
t), 3.9 (1 H, m), 4.2
(1 H, m), 4.7 (1 H, m), 5.2 (1 H, m), 7.2-7.5 (14 H, m).
MS mlz: 436 (M+1).
[00347] (~)-Cis-N-[2-methyl-1-(4-phenoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-propionamide (A-83)
[00348] (~)-Cis-N-[2-methyl-1-(4-phenoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
propionamide was made following general procedure A substituting 4-phenoxy
benzoyl chloride for
2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.0-1.2 (7 H, m), 2.2-2.4 (3 H, m), 4.7 (1 H, m), 5.6 (1 H,
m), 6.5 (1 H, d), 6.5
(1 H, d), 6.8 (2 H, d), 7.0-7.4 (15 H, m).
MS m/z: 491 (M+1).
j00349] (~)-Cis-N-[1-(4-fluoro-3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-propionamide (A-S4)
[00350] (~)-Cis-N-[1-(4-fluoro-3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A substituting 4-
fluoro-3-methoxy
benzoyl chloride for 2-furoyl chloride and propionyl chloride for acetyl
chloride. 1H-NMR (CDC13)
8:1.0-1.2(7H,m),2.2-2.4(3H,m),3.6(3H,s),4.7(lH,m),5.6(lH,m),6.4(lH,d),6.7-
6.9(4
H, m), 7.1-7.4 (7 H, m). MS m/z: 447 (M+1).
[00351] (+_)-Cis-N-[1-(4-methoxy-3-tritluoromethyl-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-85)
[00352] (~)-Cis-N-[1-(4-methoxy-3-trifluoromethyl-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made following general procedure A
substituting 4-
methoxy-3-trifluoromethyl benzoyl chloride for 2-furoyl chloride and propionyl
chloride for acetyl
chloride.
1H-NMR (CDC13) 8: 1.0-1.2 (7 H, m), 2.2-2.4 (3 H, m), 3.8 (3 H, s), 4.7 (1 H,
m), 5.6 (1 H, m), 6.5 (1
H,d),6.7(lH,d),7.0(2H,m),7.2-7.4 (7 H,m),7.8(lH,s).
MS mlz: 497 (M+1).
[00353] (~)-Cis-N-[1-(2,3-dihydro-benzofuran-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-86)
82
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00354] (~)-Cis-N-[1-(2,3-dihydro-benzofuran-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide was made following general procedure A
substituting 2,3-dihydro-
benzofuran-5-carbonyl chloride for 2-furoyl chloride and propionyl chloride
for acetyl chloride.
1H-NMR (CDCl3) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, m), 4.5 (2 H, t), 4.8 (1 H,
m), 5.6 (1 H, m), 6.5 (2
H, m), 6.9 (2 H, m), 7.1-7.4 (7 H, m).
MS m/z: 441 (M+1).
[00355] (~)-Cis-N-{2-methyl-1-[4-(3-methyl-ureido)-benzoyl]-1,2,3,4-tetrahydro-
quinolin-4-
yl}-N-phenyl-acetamide (A-87)
[00356] (~)-Cis-N-{2-methyl-1-[4-(3-methyl-ureido)-benzoyl]-1,2,3,4-tetrahydro-
quinolin-4-yl}-
N-phenyl-acetamide was made from (~)-cis-N-[1-(4-amino-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide. (~)-Cis-N-[1-(4-amino-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made following general procedure A,
substituting 4-
nitrobenzoyl chloride for 2-furoyl chloride and propionyl chloride for acetyl
chloride. The resulting
nitro analog was reduced with Pd/C (10%) in ethanol in a Parr shaker at 35
psi. (~)-Cis-N-[1-(4-
annino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide
(150 mg,
0.376mmo1) was dissolved in 10 ml toluene and 64 mg methylisocyanate
(1.13mmo1) was added. The
resulting reaction mixture was stirred at room temperature for 2 hours, then
was heated to 50 °C
overnight. The mixture was concentrated under vacuum. The residue was purified
by silica gel
chromatography, eluting with methanol-dichloromethane (1:19) to give the title
compound (87 mg,
51 %).
'H-NMR (CDC13) b: 1.1 (3 H, d), 1.1 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 2.7
(3 H, s), 4.7 (1 H, m),
5.1 (2 H, m), 5.6 (1 H, m), 6.5 (1 H, d), 6.9-7.0 (6 H, m), 7.2 (1 H, t), 7.2-
7.4 (5 H, m).
MS m/z: 457 (M+1).
[00357] (~)-Cis-N-[1-(4-diethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-88)
[00358] (~)-Cis-N-[1-(4-diethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made from (~)-cis-N-[1-(4-amino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide. (~)-Cis-N-[1-(4-amino-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide was dissolved in methylene chloride and
ethyl iodide (1.5 equiv.)
was added followed by KzC03. The reaction was allowed to stir at room
temperature for 12h. The
reaction mixture was filtered and concentrated under vacuum. The residue was
purified by silica gel
chromatography, eluting with methanol-dichloromethane (1:19) to give the title
compound.
83
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) ~: 1.0-1.2 (10 H, m), 2.0 (3 H, s), 2.4 (1 H, m), 3.3 (4 H, q),
4.7 ( 1 H, m), 5.6 (1
H,m),6.4(2H,d),6.6(lH,d),6.9(lH,t),7.0-7.4 (9 H, m).
MS xnlz: 456 (M+1).
[00359] (~)-Cis-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl]-
phenylamino]-acetic acid (A-89)
[00360] (~)-Cis-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl]-
phenylarriino}-acetic acid was made from (~)-cis-N-[1-(4-amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide. (~)-Cis-N-[1-(4-amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide was dissolved in
dimethylformamide and bromoacetic
acid ethyl ester was added followed by KzC03'. The reaction was allowed to
heat to 90 °C for 12h.
The reaction mixture was filtexed and concentrated under vacuum. The residue
was purified by silica
gel chromatography, eluting with methanol-dichloromethane (2:18) to give the
ester. The ester was
hydrolyzed using NaOH (aqueous) in methanol and water to give the title
compound.
1H-NMR (CDC13) 8: 1.1 (3 H, d), 1.1 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 3.6
(1 H, s), 4.7 (3 H~ b),
5.6 (l H, m), 6.3(lH,m),6.6(lH,d),6.8-7.4(llH,m).
MS m/z: 458 (M+1).
[00361] (~)-Cis-{N-[1-(4-methanesulfonylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-90)
[00362] (~)-Cis-{N-[1-(4-methanesulfonylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide was made from (~)-cis-N-[1-(4-amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide. (~)-cis-N-[1-(4-amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (50 mg, 0.12mmo1) was dissolved
in 5 ml DMF and
methanesulfonic anhydride (21 mg, 0.12mmo1) was added. The resulting reaction
mixture was heated
to 45 °C and stirred for 1 hour. The mixture was concentrated under
vacuum. The residue was purified
by silica gel chromatography, eluting with methanol-dichloromethane (1:9) to
give the title compound
(15 mg, 25%).
1H-NMR (CDCI3) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, rn), 3.0 (3 H, s), 4.7 (1 H,
m), 5.6 (1 H, m), 6.5
(lH,d),6.7(2H,d),6.9(lH,t),7.1(2H,m),7.2-7.4 (7 H, m).
MS mlz: 491 (M).
[00363] (t)-Cis-N-[6-Fluoro-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-(4-fluoro-ghenyl)-propionamide (A-91)
84
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00364) (~)-Cis-N-[6-fluoro-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
(4-fluoro-phenyl)-propionamide was made following general procedure A,
substituting 4-
fluarobenzoyl chloride for 2-furoyl chloride, (~)-cis-(6-fluora-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-
yl)-(4-fluoro-phenyl)-amine for (~)-cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl)-phenyl-amine and
propionyl chloride for acetyl chlaride. (~)-Cis-(6-fluoro-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-
(4-fluoro-phenyl)-amine was synthesized following the reactions detailed in
scheme 1, substituting 4-
fluoroaniline for aniline.
'H-NMR (CDC13) 8: 1.1-1.2 (6H,m), 2.2-2.4 (4H, m), 4.8 (1H, dd), 5.4-5.6 (1H,
br), 6.4 (1H, dd), 6.6
(1H, td), 6.8-7.0 (2H, m), 7.0-7.4 (6H, m).
MS m/z: 453 (M + 1).
[00365] (~)-Cis-N-[6-bromo-1-(4-tluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
qulnolin-4-yl]-
N-(4-bromo-phenyl)-propionamide (A-92)
[00366) (~)-Cis-N-[6-bromo-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
(4-bromo-phenyl)-propionamide was made following general procedure A,
substituting 4-
fluorobenzoyl chloride for 2-furoyl chloride, (~)-cis-(6-bromo-2-methyl-
1,2,3,4-tetrahydro-quinolin-
4-yl)-(4-bromo-phenyl)-amine for (~)-cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl)-phenyl-amine
and propionyl chloride for acetyl chloride. (~)-cis-(6-broma-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl)-(4-bromo-phenyl)-amine was synthesized following the reactions detailed in
scheme 1,
substituting 4-bromoaniline far aniline.
'H-NMR (CDCl3) ~: 1.1-1.2 (6H,m), 1.6 (1H, m), 2.2-2.4 (3H, m), 4.8 (1H, m),
5.4-5.6 (1H, br), 6.4
(1H, d), 6.8 (2H,.m), 7.0-7.4 (6H, M), 7.8-7.9 (2H, m).
MS m/z: 573 (M + 1).
[00367) (~)-Cis-N-[1-(3-ethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-93)
[00368] (~)-Cis-N-[1-(3-ethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure A, substituting 3-ethoxybenzoyl
chloride for 2-
furoyl chloride.
'H-NMR (CDC13) 8: 1.2 (3H,m), 1.4 (4H, m), 2.1 (3H, s), 2.4 (1H, m), 4.0 (2H,
m), 4.9 (1H, m), 5.6
(1H, br), 6.6 (1H, d), 6.9 (2H, m), 7.0 (1H, m), 7.2 (1H, m), 7.3 (1H, m), 7.4
-7.5 (7H, m).
MS mlz: 429 (M+1).
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00369] (~)-Cis-N-[1-(4-isopropoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-94)
[00370] (~)-Cis-N-[1-(4-isopropoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-pr0pionamide was made following general procedure A, substituting 4-
isopropoxybenzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 0.9-1.2 (l2H,m), 1.4(1H, m), 2.0 (3H, m), 4.3 (1H, m), 4.5
(1H, m), 5.4 (1H,
br), 6.3 (1H, d), 6.4 (2H, d), 6.7 (1H, m), 6.9-7.2 (9H, m).
MS m/z: 457 (M+1).
[00371] (~)-Cis-N-[1-(1-isopropyl-1H-benzotriazole-5-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-95)
[00372] (~)-Cis-N-[1-(1-Isopropyl-1H-benzotriazole-5-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made foil~wing general procedure A,
substituting 1-
isopropyl-1H-benzotriazole-5-carbonyl chloride for 2-furoyl chloride and
propionyl chloride for
acetyl chloride.
1H-NMR (CDCl3) 8: 1.3 (7H,m), 1.8 (6H, m), 2.4 (3H, m), 5.0 (1H, m), 5.1 (1H,
m), 5.7 (1H, br), 6.6
(1H, d), 7.0 (1H, m), 7.2-7.5 (9H, m), 8.3 (1H, s).
MS m/z: 482 (M+1).
[00373] (+)-Cis-N-[1-(3-Ethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-96)
[00374] (~)-Cis-N-[1-(3-ethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydxo- quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A, substituting 3-
ethoxybenzoyl chloride for 2-
furoyl chloride and propionyl chloride for acetyl chloride.'H-NMR (CDC13) b:
1.2 (6H,m), 1.5 (4H,
m), 2.4 (3H, m), 4.0 (2H, m), 4.9 (1H, m), 5.7 (1H, br), 6.6 (1H, d), 6.8 (1H,
d), 6.9 (1H, m), 7.1 (2H,
m), 7.2 (1H, m), 7.3-7.6 (7H, m).
MS mlz: 443 (M+1).
[00375] (~)-Cis-4-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenyl}-piperidine-1-carboxylic acid ethyl ester (A-97)
[00376] (~)-Cis-4-{4-[2-methyl-4-(phcnyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenyl }-piperidine-1-carboxylic acid ethyl ester was made following
general procedure A,
substituting 4-(4-chlorocarbonyl-phenyl)-piperidine-1-carboxylic acid ethyl
ester for 2-furoyl chloride
and propionyl chloride fox acetyl chloride.
86
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) b: 1.1-1.3 (10H, m), 1.5 (2H, m), 1.7 (2H, m), 2.3 (3H, m), 2.6
(1H, m), 2.8 (2H,
t), 4.1 (2H, m), 4.2 (2H, m), 4.8 (1H, m), 5.6 (1H, br), 6.5 (1H, d), 6.9 (1H,
m), 7.2 (2H, m), 7.3-7.4
(9H, m).
MS m/z: 554 (M+1).
[00377] (~)-Cis-N-[2-methyl-1-(4-piperidin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-98)
[00378] (~)-Cis-N-[2-methyl-1-(4-piperidin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was prepared from (~)-cis-4-{4-[2-methyl-4-(phenyl-
propionyl-amino)-3,4-
dihydro-2H-quinoline-1-carbonyl]-phenyl}-piperidine-1-carboxylic acid ethyl
ester. (~)-Cis-4-{4-[2-
methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenyl}-
piperidine-1-
carboxylic acid ethyl ester (96 mg, 0.17 mmol) was dissolved in acetonitrile
(2 mL).
Iodotrimetylsilane (74 uL, 0.51 mmol) was added and the reaction was allowed
to stir at room
temperature over night. Excess reagent was quenched by the addition of
methanol (1 mL) and the
mixture was concentrated under reduced pressure. The crude residue was
partitioned between ethyl
acetate and saturated aqueous sodium bicarbonate. The extracts were washed
with 1 M sodium
hydroxide, saturated aqueous sodium thiosulfate and brine, dried over sodium
sulfate, filtered,
concentrated and purified by silica gel chromatography (3:1 methylene chloride
/ methanol) (77 mg,
94 %).
'H-NMR (CDCl3) S: 1.1 (6H,m), 1.3 (1H, t), 1.6 (2H, m), 1.7 (2H, d), 2.3 (3H,
m), 2.6 (1H, m), 2.7
(2H, t), 3.2 (2H, d), 4.8 (1H, m), 5.6 (1H, br), 6.5 (1H, d), 6.9 (1H, m), 7.0
(2H, d), 7.2 (3H, m), 7.3-
7.4 (6H, m).
MS m/z: 482 (M+1).
[00379] (~)-Cis-N-[1-(4-Bromo-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-99)
[00380] (~)-Cis-N-[1-(4-bromo-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A, substituting 4-
bromobenzoyl chloride for 2-
furoyl chloride and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 1.2 (6H,m),1.25 (1H, m), 2.3 (3H, m), 4.8 (1H, m), 5.6 (1H,
br), 6.4 (1H, d), 6.9
(1H, m), 7.1 (2H, d), 7.2 (1H, m), 7.3-7.4 (8H, m).
M5 m/z: 477 (M+1).
[00381] (~)-Cis-N-{1-[4-(1-acetyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-100)
87
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00382] To a solution of (~)-cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
phenyl-amine (636
mg, 2.70 mmol) in dichloromethane (10 mL) at room temperature was added
diisopropylethylamine
(1.04 g,1.44 mL, 2.98 mmol) followed by freshly prepared 4-(4-chlorocarbonyl-
phenyl)-piperidine-1-
carboxylic acid tert-butyl ester (2.98 mmol). The mixture was stirred at room
temperature over night,
poured into water and extracted with dichloromethane. The extracts were washed
with 1 M(aq)
NaOH and brine, dried over magnesium sulfate, filtered dried and concentrated.
The crude residue
was purified by silica gel chromatography (100 % hexanes to 70/30 hexanes
ethyl acetate gradient) to
afford the pure amide (827 mg, 58 %).
[003$3] The (~)-cis-4-[4-(2-methyl-4-phenylamino-3,4-dihydro-2H-quinoline-1-
carbonyl)-
phenyl]-piperidine-1-carboxylic acid tert-butyl ester (827 mg, 1.57 mmol) thus
formed was dissolved
in methylene chloride (50 mL). Trifluoroacetic acid (3 mL) was added and the
mixture was stirred at
rt 70 min. Solvent and excess acid were removed under reduced pressure. The
crude residue was
dissolved .in ethyl acetate and neutralized with 1 M sodium hydroxide (to pH =
10.5). The aqueous
phase was extracted twice with additional ethyl acetate. The extracts were
combined and washed with
brine, dried over sodium sulfate, filtered and concentrated to afford the
crude diamine (676 mg, 100
%) as an oil.
[00384] To a solution of the piperidine amine obtained above (676 mg, 1.59
mmol) in methylene
chloride (25 mL) was added diisopropylethylamine (616 mg, 849 uL, 4.77 mmol),
followed by acetyl
chloride (162 mg, 156 uL, 2.06 mmol). The mixture was stirred at room
temperature over night. The
reaction mixture was poured into saturated aqueous sodium bicarbonate and
extracted with additional
methylene chloride. The extracts were combined, washed with brine, dried over
sodium sulfate,
filtered, dried and concentrated to afford the piperidine acetamide (844 mg, >
100 %).
[00385] The crude piperidine acetamide obtained above (844 mg) was dissolved
in methylene
chloride (25 mL) to which was then added diisopropylethylamine (205 mg, 283
uL, 1.59 mmol)
followed by propionyl chloride (4.42 g, 4.2 mL, 47.7 mmol). The resulting
reaction mixture was
stirred at room temperature 96 h and concentrated. The resulting residue was
partitioned between
ethyl acetate and saturated aqueous sodium bicarbonate. The extracts were
washed with brine and
dried over sodium sulfate, filtered, dried and concentrated. The crude residue
was purified by silica
gel chromatography (50150 ethyl acetate/hexanes to 100% ethyl acetate
gradient) to afford the product
(437 mg, 52 %).
[00386] (~)-Cis-N-{ 1-[4-(1-acetyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl }-N-phenyl-propionamide was separated by chiral HPLC using a chiral cel
OD column and
eluting with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and
(2S,4R)-cis-N-{ 1-[4-(1-
acetyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl }-N-
phenyl-propionamide
(A-51 & A-50, respectively).
88
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 1.2 (7H, m), 1.6 (2H, m), 1.8 (2H, d), 2.1 (3H, s), 2.3 (3H,
m), 2.6 (2H, m), 3.1
(1H, t), 3.9 (1H, m), 4.8 (2H, m), 5.6 (1H, br), 6.5 (1H, d), 6.9 (1H, m), 7.0
(2H, d), 7.1 (2H, d), 7.2-
7.4 (7H, m).
MS m/z: 524 (M+1)
[00387] (~)-Cis-N-{1-j4-(1-ethyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-101)
[00388] (~)-Cis-N-{ 1-[4-(1-ethyl-piperidin-4-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl}-N-phenyl-propionamide was made from (~)-cis-N-[2-methyl-1-(4-piperidin-4-
yl-benzoyl)-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. (~)-Cis-N-[2-methyl-1-
(4-piperidin-4-yl-
benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl propionamide was dissolved
in dichloromethane
(3 mL). Acetaldehyde (18 uL, 0.33 mmol) was added in a single portion. The
mixture was stirred at
room temperature 30 minutes and then a solution sodium triacetoxyborohydride
(35 mg, 0.165 mmol)
in dichloromethane (1 mL) was slowly added, followed by 1 drop acetic acid.
The mixture was
allowed to stir'at room temperature over night and was quenched by aqueous
sodium bicarbonate.
The biphasic mixture was extracted three times with methylene chloride (20
mL); the combined
extracts were washed with brine, dried over magnesium sulfate, filtered,
concentrated and purified by
HPLC to afford the product (35 mg, 62 %).
'H-NMR (CDC13) 8: 1.0-1.2 (9H, m), 1.3 (1H, m), 1.8 (4H, br), 2.0 (2H, m), 2.3
(3H, m), 2.5 (2H,
m), 3.1 (3H, m)~ 4.8 (1H, m), 5.6 (1H, br), 6.5 (1H, d), 6.9 (1H, m), 7.0 (2H,
d), 7.1-7.4 (9H, m).
MS m/z: 511 (M+2).
[00389] (~)-Cis-N-[2-Methyl-1-(4-vitro-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide (A-102)
[00390] (~)-Cis-N-[2-methyl-1-(4-vitro-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made following general procedure A, substituting 4-
nitrobenzoyl chloride fox 2-
furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) b: 1.2 (7H,m), 2.3 (3H, m), 4.8 (1H, m), 5.6 (1H, br), 6.4 (1H,
d), 6.9 (1H, m), 7.2-
7.4(9H, m), 8.0 (2H, d).
MS m/z: 444 (M+1).
[00391] (~)-Cis-N-[1-(4-amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide (A-103)
[00392] (~)-Cis-N-[1-(4-amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was prepared from (~)-cis-N-[2-methylmethyl-1-(4-vitro-benzoyl)-
1,2,3,4-tetrahydro-
89
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
quinolin-4-yl]-N-phenyl-propionamide. (~)-Cis-N-[2-methyl-1-(4-nitro-benzoyl)-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (200 mg, 0.45 mmol) was dissolved in
ethanol (20 mL).
Palladium on carbon (10 %) was carefully added and the resulting suspension
was shaken under
hydrogen gas (40 psi) over night. The suspension was filtered through Celite ~
to remove solids, and
the filter cake washed three times with ethanol. Concentration of the solution
afforded pure product
(160 mg, 86 %).
1H-NMR (CDCl3~ 8: 1.2 (7 H, m), 2.3 (3 H, m), 3.9 (2 H, br), 4.7 (1 H, m), 5.6
(1 H, br), 6.4 (2 H, d),
6.6(lH,d),6.9(lH,m),7.0(2H,d),7.1(lH,m),7.2-7.4 (6 H, m).
MS mlz: 414 (M+1).
[00393] (~)-Cis-N-[2-methyl-1-(4-pyrrol-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-104)
[00394] (~)-Cis-N-[2-methyl-1-(4-pyrrol-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A, substituting 4-
pyrrol-1-yl-benzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDCI3) 8: 1.2 (6 H, m), 1.3 (1 H, m), 2.3 (3 H, m), 4.8 (1 H, m), 5.6
(1 H, br), 6.3 (2 H, s),
6.6 (1 H, d), 6.9 (1 H, m), 7.1 (2 H, s), 7.2-7.4(11 H, m).
MS m/z: 464 (M+1).
[00395] (~)-Cis-N-[1-(4-acetylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl)-N-
phenyl-propionamide (A-105)
[00396] (~)-Cis-N-[1-(4-acetylamino benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was prepared from (~)-cis-N-[1-(4-amino-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. To a solution of (~)-cis-N-[1-
(4-amino-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (100mg,
0.24mmo1) in 2.5m1
tetrahydrofuran was added acetyl chloride (44 p,L, 0.63mmo1) followed by
triethylamine (88 p.L
,0.63mmo1). The reaction was stirred at room temperature overnight. The
mixture was concentrated
under reduced pressure. The residue was purified by silica gel chromatography,
eluting with hexane-
ethyl acetate (3:1) to give the title compound (51 mg, 46%).
1H-NMR (CDCI3) b: 1.1 (7 H, m), 2.2 (3 H, s), 2.3 (3 H, m), 4.8 (1 H, m), 5.6
(1 H, br), 6.5 (1 H, d),
6.9 (1 H, m), 7.1 (2 H, d), 7.2 (1 H, d), 7.3-7.4 (8 H, m), 8.4 (1 H, br).
MS m/z: 456 (M+1)
[00397] (~)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenyl}-carbamic acid ethyl ester (A-106)
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00398] (~)-Cis-{4-[2-methyl-4-(phenyl-propionyl-amino)-3,4-dihydro-2H-
quinoline-1-carbonyl]-
phenyl }-carbamic acid ethyl ester was made from (~)-cis-N-[1-(4-aminoamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide, following the method
described above in
the synthesis of (~)-cis-N-[1-(4-acetylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-
phenyl-propionamide, substituting ethyl chloroformate for acetyl chloride.
'H-NMR (CDC13) S: 1.1 (6 H,m), 1.3 (4 H,m), 2.3 (3 H, m), 4.2 ( 2 H, m), 4.8
(1 H, m), 5.6 (1 H, br),
6.5(lH,d),6.7(lH,br),6.9(lH,m),7.1-7.4(lOH,m).
MS m/z: 486 (M+1). .
[00399] (~)-Cis-N-{2-methyl-1-[4-(4-methyl-piperazin-1-yl)-benzoyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-107)
(00400] (~)-Cis-N-{2-methyl-1-[4-(4-methyl-piperazin-1-yl)-benzoyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide was made from (~)-cis-N-[1-(4-bromo-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. (~)-Cis-N-[1-(4-bromo-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (100 mg, 0.22
mmol) was combined
with cesium carbonate (355 mg, 1.09 mmol), racemic BINAP (25 mg, 0.04 mmol),
Pd2dba3 (36 mmol,
0.04 mmol) and 1-methyl piperazine and dissolved in toluene (10 mL). The
reaction mixture was
heated at 100 °C under argon overnight. The reaction was cooled to room
temperature, filtered and
the solids washed with ether. The filtrate was washed with water and brine,
dried over magnesium
sulfate, filtered and concentrated. The crude product was purified by HPLC.
'H-NMR (CDC13) 8: 1.2 (6 H,m), 1.3 (1 H,m), 2.2 (3 H, m), 2.3 (3 H, s), 2.5 (4
H, m), 3.2 (4 H, m),
4.7(lH,m),5.6(lH,bs),6.6(lH,d),6.7(2H,d),7.0(lH,m),7.2-7.4 (9 H, m).
MS m/z: 498 (M+2)
[00401] . (~)-Cis-N-[2-methyl-1-(4-pyrimidin-2-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-108)
(00402] (~)-Cis-N-[2-methyl-1-(4-pyrimidin-2-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A, substituting 4-
pyrimidin-2-yl-benzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.2 (7 H, m), 2.3 (3 H, m), 4.8 (1 H, m), 5.6 (1 H, br), 6.5
(1 H, d), 6.9 (1 H, m),
7.2-7.4 (10 H, m), 8.3 (2 H, d), 8.8 (2 H, d).
MS m/z: 478 (M+2).
[00403] (~)-Cis-N-[2-methyl-1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-
carbonyl)-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (A-109)
91
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00404] (~)-Cis-N-[2-methyl-1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide was made following general
procedure A,
substituting 4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-carbonyl chloride for
2-furoyl chloride
and propionyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.1 (6H, m),1.3 (1H, t), 2.3 (3H, m), 2.8 (3H, s), 3.3 (2H,
t), 4.2 (2H, t), 4.7 (1H,
m), 5.6 (1H, br), 6.3 (1H, d), 6.5 (1H, d), 6.6 (1H, d), 6.9 (1H, s), 7.0 (1H.
m), 7.1 (1H, m), 7.3-7.4
(7H, m).
MS m/z: 471 (M+2).
[00405] (~)-Cis-N-[2-Methyl-1-(4-morpholin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-110)
[00406] (~)-Cis-N-[2-methyl-1-(4-morpholin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made from (~)-cis-N-[1-(4-bromo-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide, following the procedure used to make (~)-
cis-N-{2-methyl-1-
[4-(4-methyl-piperazin-1-yl)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl }-N-
phenyl-propionamide
substituting morpholine for 1-methyl piperazine.
[00407] (~)-Cis-N-[2-methyl-1-(4-morpholin-4-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was separated by chiral HPLC using a chiral cel OD column
and eluting with
90% hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)- cis-N-[2-
methyl-1-(4-
morpholin-4-yl-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N=phenyl-
propionamide (A-120 & A-119,
respectively).
'H-NMR (CDC13) S: 1.1 (7H, m), 2.3 (3H, m), 3.1 (4H, t), 3.8 (4H, t), 4.7 (1H,
m), 5.6 (1H, br), 6.6
(1H, d), 6.7 (2H, d), 6.9 (1H, m), 7.2-7.4 (9H, m).
MS m/z: 485 (M+2).
[00408] (~)-Cis-N-{1-[4-(2,5-dimethyl-pyrrol-1-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-111)
[00409] (~)-Cis-N-{ 1-[4-(2,5-dimethyl-pyrrol-1-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl }-N-phenyl-propionamide was prepared from (~)-cis-N-[1-(4-amino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. A solution of (~)-cis-
N-[1-(4-amino-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (150
mg, 0.36 mmol),
and propionic acid (0.5 ml) in dry benzene (20 ml) was heated at reflux under
argon in a flask
equipped with a Dean-Stark trap while stirring with the exclusion of light.
The resulting solution was
cooled to room temperature, and concentrated under vacuum. Recovered oil was
purified by silica gel
chromatography, eluting with hexane-ethyl acetate (3:1) to give the title
compound (140 mg, 80%).
92
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
IH-NMR (CDC13) 8: 1.2 (7H, m), 2.0 (6H, s), 2.3(3H, m), 4.8 (1H, m), 5.6 (1H,
br), 5.9 (2H, s), 6.5
(1H, d), 6.9 (1H, m), 7.0 (1H, d), 7.2 (2H, m), 7.3-7.4 (8H, m).
MS m/z: 493 (M+2).
[00410] (~)-Cis-N-{1-[4-(2-ethyl-butylamino)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl}-N-phenyl-propionamide (A-112)
[00411] (~)-Cis-N-{ 1-[4-(2-ethyl-butylamino)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-phenyl-propionamide was prepared from (~)-cis-N-[1-(4-amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. To a solution of (~)-cis-N-[1-
(4-Amino-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-phenyl-propionamide (75 mg,
0.145mmo1) in
dichloromethane (3 mL) was added 2-ethylbutyraldehyde (26uL, 0.2mmo1) in one
portion. The
mixture was stirred at room temperature for a 0.5 h before a solution of
sodium triacetoxyborohydride
(74 mg, 0.348 mmol) 1 ml DCM was added slowly. A single drop of acetic acid
was added and the
reaction was allowed to stir at room temperature over night. Excess reagent
was quenched by .the
addition of saturated aqueous sodium bicarbonate. The resulting mixture was
extracted three times
with 20 mL dichloromethane. The combined extracts were W ashed with brine,
dried over magnesium
sulfate, filtered. and concentrated. Crude product was purified by HPLC to
afford the title compound
(60 mg, 83%).
'H-NMR (CDC13) S: 0.9 (6H, m), 1.2 (7H,m), 1.4 (5H, m), 2.3 (3H, m), 3.0 (2H,
d), 4.7 (1H, m); 5.6
(1H, br),.6.3 (2H, d), 6.6 (1H, d), 7.0 (1H, m), 7.1 (2H, d),7.2 (1H~ m), 7.3-
7.4 (6H, m).
MS m/z: 499 (M+2).
[00412] (~)-Cis-N-[2-Methyl-1-(4-propylamino-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-113)
[00413] (~)-Cis-N-[2-methyl-1-(4-propylamino-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was prepared from (~)-cis-N-[1-(4-amino-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide utilizing the reductive
amination conditions
described for the synthesis of (~)-cis-N-{ 1-[4-(2-ethyl-butylamino)-benzoyl]-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-phenyl-propionamide. Propionaldehyde was
substituted for 2-
ethylbutyraldehyde. The reaction was poorly selective and afforded
approximately equivalent
amounts of mono- and di-alkylated products (i.e., (~)-cis-N-[l-(4-
dipropylamino-benzoyl) -2-methyl-
1,2,3,4- tetrahydro- quinolin- 4- yl]-N-phenyl propionamidel see below).
1H-NMR (CDC13) S: 1.0 (3H, m), 1.1 (7H,m), 1.6 (2H, m), 2.3 (3H, m), 3.0 (2H,
d), 4.0 (1H, br), 4.7
(1H, m), 5.6 (1H, br), 6.3 (2H, d), 6.6 (1H, d), 6.9 (1H, m), 7.06 (2H, d),
7.14 (1H, m), 7.3-7.4 (6H,
m).
MS m/z: 457 (M+2).
93
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00414] (~)-Cis-N-[1-(4-dipropylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-114)
[00415] (~)-Cis-N-[1-(4-Dipropylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was prepared as a by-product in the synthesis of (~)-cis-N-
[2-methyl-1-(4-
propylamino-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide
described above.
'H-NMR (CDC13) 8: 1.0 (6H, t), 1.1 (6H,m), 1.4 (1H, m), 1.5 (4H, m), 2.3 (3H,
m), 3.2 (4H, t), 4.7
(1H, m), 5.6 (1H, br), 6.4 (2H, d), 6.7 (1H, d), 7.0 (1H, m), 7.1-7.2 (3H, m),
7.3-7.4 (6H, m).
MS m/z: 499 (M+2).
[00416] (~)-Cis-N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-phenyl-propionamide (A-115)
[00417] (~)-Cis-N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made from (~)-cis-N-[1-(4-bromo-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide following the procedure used to make (~)-
cis-N-{2-methyl-1-
[4-(4-methyl-piperazin-1-yl)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl }-N-
phenyl-propionamide
substituting pyrollidine for 1-methylpiperazine.
'H-NMR (CDC13) 8: 1.1 (7H, m), 2.0 (4H, m), 2.3 (3H, m), 3.2 (4H, m), 4.7 (1H,
m), 5.6 (1H, br); 6.3
(2H, d), 6.6 (1H, d), 6.9 (1H, m), 7.1-7.4 (9H, m).
MS m/z: 468 (M+1).
[00418] (~)-Cis-N-[2-methyl-1-(4-ureido-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-propionamide (A-116)
[00419] (~)-Cis-N-[2-methyl-1-(4-ureido-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
propionamide was made from (~)-cis-N-[1-(4-amino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide. A mixture of (~)-cis-N-[1-(4-Amino-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (100mg, 0.24mmo1) and
trimethylsilyl isocyanate
(120p,L, 30.72mmo1) in dry DMF (0.5mL) was stirred at room temperature for 3
days and then
concentrated under reduced pressure at 30 °C to dryness. °The
residual syrup was stirred with ethyl
acetate to which was added an additional lOmL of ethyl acetate with lOmL
water. The pH was
adjusted to 3.0 with 3 N HCl, and the separated aqueous layer was extracted
with ethyl acetate. The
combined ethyl acetate extracts were washed with water and brine, dried over
magnesium sulfate and
concentrated in vacuo, yielding the product (lOmg, 9% yield).
'H-NMR (CDCI3) 8: 1.2 (7H, m), 2.3 (3H, m), 4.7 (1H, m), 5.1 (2H, br), 5.6
(1H, br), 6.5 (1H, d), 6.9
(5H, m), 7.2 (7H, m), 7.9 (1H, br).
94
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 457 (M+1).
[00420] (~)-Cis-2-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenylamino}-propionic acid methyl ester (A-117)
[00421] (~)-Cis-2-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinoline-1-carbonyl]-
phenylamino}-propionic acid methyl ester was prepared from (~)-cis-N-[1-(4-
amino-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide. A mixture of
(~)-cis-N-[1-(4-
amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
propionamide (210 mg, 0.53
mmol), potassium carbonate (123 mg, 0.89 mmol), and methyl 2-bromopropionate
(70 uL,
0.63mmo1) in dry dimethylformamide (2 mL) was heated at 100°C for 6h,
then cooled to room
temperature and stirred with 20m1 water until all of the salts dissolved. The
aqueous layer was
separated and was extracted with ethyl acetate. The combined extracts were
washed with water and
brine, dried, over magnesium sulfate, filtered and concentrated under reduced
pressure. The resulting
oil was purified by silica gel chromatography, eluting with (97:3 methylene
chloride/methanol) to
afford the title compound (220 mg, 87°l0).
'H-NMR (CDCl3) 8: 1.2 (4H, m), 1.4 (3H, d), 2.0 (3H, s), 2.3 (1H, br), 3.7
(3H, s), 4.1 (1H, m), 4.7
(1H, m), 5.6 (1H, br), 6.3 (2H, d), 6.6 (1H, d), 6.9 (1H, m), 7.0 (2H, d), 7.3-
7.4 (7H, m).
MS m/z: 487 (M+2).
[00422] (~)-Cis-2-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenylamino}-propionamide (A-118)
[00.423] (~)-Cis-2-{4-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinoline-1-carbonyl]-
phenylamino}-propionamide was prepared from (~)-cis-2-{4-[4-(acetyl-phenyl-
amino)-2-methyl=3,4-
dihydro-2H-quinoline-1-carbonyl]-phenylamino}-propionic acid methyl ester.
[00424] To concentrated ammonium hydroxide (2 mL, 2.0 M) were added crude (~)-
cis-2-{4-[4-
(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl]-
phenylamino}-propionic acid
methyl ester (180 mg, 0.37 mmol) and trace amount ammonium chloride; the
mixture was heated at
100 °C for 6 h in a pressure reactor with good mixing. After cooling to
0 °C, the resulting precipitate
was filtered and washed with ice-water and extracted with ether. The combined
extracts were washed
with water and brine, dried over magnesium sulfate and concentrated under
reduced pressure. The
crude product was purified by HPLC to give the title compound (10 mg, 6%).
1H-NMR (CDC13) 8: 1.2 (4H, m), 1.5 (3H, d), 2.1 (3H, s), 2.3 (1H, br), 3.8
(1H, s), 4.4 (2H, br), 4.7
(1H, m), 5.6 (2H, m), 6.3 (2H, m), 6.6 (2H, d), 7.0 (1H, m), 7.1 (2H, d), 7.2
(1H, m), 7.3-7.4 (5H, m).
MS m/z: 471 (M+1)
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00425] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-acetamide (A-123)
[00426] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting 3-
methoxybenzoyl chloride for 2-
furoyl chloride.
[00427] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
acetamide was separated by chiral HPLC using a chiral cel OD column and
eluting with 90%
hexane/10°Io ethanol isocratic system to give (2R,4S)- and (2S,4R)- cis-
N-[1-(3-methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-126 ~ A-127,
respectively).
'H-NMR (CDCl3) 8: 1.15 (3 H, d; overlapping 1 H, t), 2.05 (3 H, s), 2.33 (1 H,
m), 3.60 (3 H,s), 4.80
(1 H, m), 5.65 (1 H, m), 6.55 (1 H, d), 6.75-6.85 (3 H, complex), 6.95 (1 H,
t), 7.15 (1 H, t), 7.25 (1 H,
t), 7.25-7.55 (6 H, m).
MS m/z: 415 (M +1).
[00428] (~)-Trans-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-124)
[00429] (~)-Trans-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting 3-
methoxybenzoyl chloride
for 2-furoyl chloride, and trazzs-(2-ethyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
phenyl-amine for cis-(2-
ethyl-1,2,3,4-tetrahydro-quinolin-4-yl)-phenyl-amine.
[00430] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-propionamide (A-128)
[00431] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl=
propionamide was made following general procedure A, substituting 3-
methoxybenzoyl chloride for
2-furoyl chloride, and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 1.15 (3H, d; overlapping 3H, t, 1H, t), 2.20 (2H,q), 2.33
(1H, m), 3.65 (3H,s),
4.80 (1H, m), 5.60 (1H, m), 6.55 (1H, d), 6.75-6.85 (3H, complex), 6.95 (1H,
t), 7.15 (1H, t), 7.20
(1H, t), 7.25-7.55 (6H, m) .
MS m/z:429 (M + 1).
[00432] (~)-Cis-N-[6-chloro-1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-(4-chloro-phenyl)-acetamide (A-129)
[00433] (~)-Cis-N-[6-chloro-1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-(4-chloro-phenyl)-acetamide was made following general procedure A,
substituting 3-
methoxybenzoyl chloride for 2-furoyl chloride, and (~)-cis-(6-chloro-2-methyl-
1,2,3,4-tetrahydro-
96
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
quinolin-4-yl)-(4-chloro-phenyl)-amine for (~)-cis-(2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-
phenyl-amine. (~)-Cis-(6-chloro-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-(4-
chloro-phenyl)-amine
was synthesized following the reactions detailed in scheme l, substituting 4-
chloroaniline for aniline.
1H-NMR (CDC13) r5: 1.15 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.35 (1H,
m), 3.65(3H,s), 4.80
(1H, m), 5.60 (1H, m), 6.42 (1H, d), 6.65-6.95 (overlapping 1H, d; lH,dd; 1H
dd ), 7.15 (1H, t), 7.20-
7.30 (6H, m), 7.40 (1H, d).
MS m/z: 484(M + 1).
[00434] (~)-Cis-N-[2-methyl-1-(1-methyl-1H-pyrrole-2-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-130)
[00435] (~)-Cis-N-[2-methyl-1-(1-methyl-1H-pyrrole-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-acetamide was made following general procedure A, substituting 1-
methyl-1H-pyrrole-
2-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDCl3) 8: 1.12 (3H, d; overlapping 1H, t), 2.00 (3H, s), 2.35 (1H, m),
3.80 (3H,s)., 4.70
(1H, m), 5.50 (1H, m), 5.80 (1H, d), 6.55 (lH,d), 6.80 (1H, d), 7.00 (lH,t),
7.20-7.50 (6H, m).
MS m/z: 388 (M + 1).
[00436] (~)-Cis-N-[2-methyl-1-(2-methyl-pyridine-4-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide (A-131)
[00437] : (~)-Cis-N-[2-methyl-1-(2-methyl-pyridine-4-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A, substituting 2-
methyl-isonicotinoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 1.11-1.16 (3H, d; overlapping 3H, t, and 1H, t), 2.20-2.35
(overlapping 2H, q;
and 1H, m), 2.47 (3H, s), 4.80 (1H, m), 5.60(1H, m), 6.48 (1H, d), 6.65
(lH,d), 6.85 (1H, t), 7.10-
7.40 (8H, m), 8.30 (1H, d).
MS m/z: 414 (M + 1).
[00438] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-3-methyl-
N-phenyl-butyramide (A-132)
[00439] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-3-methyl-N-
phenyl-butyramide was made following general procedure A, substituting 4-
fluorobenzoyl chloride
for 2-furoyl chloride and 3-methyl-butyryl chloride for acetyl chloride.
97
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDCl3) S: 0.90 (2 x 3H, d), 1.15 (3H, d; overlapping 1H, t), 2.15 (1H,
m), 2.20-2.35
(overlapping 2H, m; 1H, m), 4.80 (1H, m), 5.65 (1H, m), 6.50 (1H, d), 6.90
(4H, complex), 7.20-7.60
(8H, m).
MS m/z: 445 (M + 1).
[00440] (~)-Cis-N-[2-methyl-1-(6-methyl-pyridine-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide (A-133)
[00441] (~)-Cis-N-[2-methyl-1-(6-methyl-pyridine-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-.
N-phenyl-propionamide was made following general procedure A, substituting 6-
methyl-nicotinoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8:1.11-1.16 (3H, d; overlapping 3H, t, and 1H, t), 2.20-2.40
(overlapping 2H, q;
and 1H, m), 2.49 (3H, s), 4.80 (1H, m), 5.60(1H, m), 6.48 (1H, d), 6.80-7.00
(lH,d; 1H, t), 7.10-7.50
(9H, m), 8.60 (1H, d).
MS m/z: 414 (M + 1).
[00442] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-2-
morpholin-4-yl-N-phenyl-acetamide (A-134)
[00443] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-2-morpholin-
4-yl-N-phenyl-acetamide was made following general procedure A, substituting 4-
fluorobenzoyl
chloride for 2-furoyl chloride and morpholinoacetyl chloride for acetyl
chloride.
[00444] (~)-Cis-N-[1-(2,3-dihydro-benzo[1,4]dioxine-6-carbonyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (A-135)
[00445] (~)-Cis-N-[1-(2,3-dihydro-benzo[1,4]dioxine-6-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made following general procedure A,
substituting (~)-cis-
2,3-dihydro-benzo[1,4]dioxine-6-carbonyl chloride for 2-furoyl chloride and
propionyl chloride for
acetyl chloride.
'H-NMR (CDC13) 8: 1.10 (3H, d; overlapping 3H, t; 1H, t), 2.10 (2H, q, 1H, m),
4.10 (2x2H, m),
4.70 (1H, m), 5.65 (1H, m), 6.50-6.60 (2X1H, d), 7.20-7.40 (7H, m).
MS mlz: 457 (M + 1).
[00446] (~)-Cis-N-[2-methyl-1-(5-trifluoromethyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-136)
[00447] (~)-Cis-N-[2-methyl-1-(5-trifluoromethyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made following general procedure A,
substituting 5-
98
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
trifluoromethyl-thiophene-2-carbonyl chloride for 2-furoyl chloride and
propionyl chloride for acetyl
chloride.
'H-NMR (CDC13) 8: 1.10-1.15 (3H, d; overlapping 3H, t; 1H, t), 2.15- 2.35 (2H,
q, 1H, m), 4.70
(1H, m), 5.55 (1H, m), 6.45 (1H, d), 6.85 (1H, d), 7.00 - 7.20 (overlapping
lH,d; 1H, t), 7.20-7.60
(7H, m).
MS m/z: 473 (M+ 1).
[00448] (~)-Cis-N-[2-methyl-1-(6-trifluoromethyl-pyridine-3-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide (A-137)
[00449] (~)-Cis-N-[2-methyl-1-(6-trifluoromethyl-pyridine-3-carbonyl)-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-propionamide was made following general procedure A,
substituting 6-
trifluoromethyl-nicotinoyl chloride for 2-furoyl chloride and propionyl
chloride for acetyl chloride.
'H-NMR (CDC13) ~: 1.10 (3H, d; overlapping 3H, t; 1H, t), 2.00-2.40 (2H, q,
1H, m), 4.80 (1H, m),
5.65 (1H, m), 6.40 (1H, d), 7.00 (1H, d), 7.20-7.50 (9H, m), 8.70 (1H).
MS m/z: 468 (M + 1).
[00450] (~)-Cis-N-[2-methyl-1-(3-methyl-isoxazole-5-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide (A-138)
[00451] (~)-Cis-N-[2-methyl-1-(3-methyl-isoxazole-5-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A, substituting
3-methyl-
isoxazole-5-carbonyl chloride for 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDC13) 8: 1.10 (overlapping 3H, d; 3H,t; 1H, t), 2.10- 2.40
(overlapping 3H, s; 2H, q; 1H,
m), 4.80 (1H, m), 5.50 (1H, m), 6.80 (1H, d), 7.10 (1H, t), 7.20-7.50 (9H, m).
MS m/z: 404 (M + 1).
[00452] (~)-Cis-N-[2-methyl-1-(4-oxazol-5-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-139)
[00453] (~)-Cis-N-[2-Methyl-1-(4-oxazol-5-yl-benzoyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide was made following general procedure A, substituting 4-
oxazol-5-yl-benzoyl
chloride for 2-furoyl chloride and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.00-1.20 (overlapping 3H,t; 3H, d; 1H, t), 2.20-2.40 (2H,
q; 1H, m), 4.80 (1H,
m), 5.65 (1H, m), 6.55 (1H, d), 6.90 (1H, t), 7.20-7.60 (12H, m), 7.90 (1H,
s).
MS m/z: 466 (M + 1).
99
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00454] (~)-Cis-N-[1-(benzo[c]isoxazole-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide (A-140)
[00455] (~)-Cis-N-[1-(benzo[c]isoxazole-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-
N-phenyl-propionamide was made following general procedure A, substituting
benzo[c]isoxazole-3-
carbonyl chloride for 2-furoyl chloride and propionyl chloride for acetyl
chloride.
1H-NMR (CDCl3) 8: 1.14 (3H, t); 1.23 (3H, d), 2.20 (2H, q), 2.40 (1H, m), 4.80
(1H, m), 5.60 (1H,
m), 6.60 (1H, d), 7.00 (3H, complex), 7.00-7.40 (8H, m), 7.55 (1H, d).
MS m/z: 440 (M + 1).
[00456] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
succinamic acid methyl ester (A-141)
[00457] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
succinamic acid methyl ester was made following general procedure A,
substituting 4-fluorobenzoyl
chloride for 2-furoyl chloride and 3-chlorocarbonyl-propionic acid methyl
ester for acetyl chloride.
[00458] (~)-Cis-N-{1-[5-(4-chloro-phenyl)-furan-2-carbonyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-142)
[00459] (~)-Cis-N-{ 1-[5-(4-chloro-phenyl)-furan-2-carbonyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl }-N-phenyl-propionamide was made following general procedure A,
substituting 5-(4-
chloro-phenyl)-furan-2-carbonyl chloride for 2-furoyl chloride and propionyl
chloride for acetyl
chloride.
'H-NMR (CDCI3) 8: 1.08-1.36(7H, m), 2.15-2.35 (3H, m), 4.72 (1H, q), 5.40-5.60
(1H, br), 6.53 (2H,
d), 6.89 (1H, d), 7.04-7.09 (1H, m), 7.17-7.40 (10H, m).
MS m/z: 499 (M+1).
[00460] (~)-Cis-N-{1-[5-(2-chloro-4-trifluoromethyl-phenyl)-furan-2-carbonyl]-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl}-N-phenyl-propionamide (A-143)
[00461] (~)-Cis-N-{ 1-[5-(2-chloro-4-trifluoromethyl-phenyl)-furan-2-carbonyl]-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-phenyl-propionamide was made following general
procedure A,
substituting 5-(2-chloro-4-trifluoromethyl-phenyl)-furan-2-carbonyl chloride
for 2-furoyl chloride and
propionyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 1.08-1.36(7H, m), 2.15-2.35 (3H, m), 4.72 (1H, q), 5.40-5.60
(1H, br), 6.78-6.87
(2H, m), 7.05-7.49 (11H, m).
MS,m/z: 567 (M+1).
100
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00462] (~)-Cis-N-{2-methyl-1-[5-(4-vitro-phenyl)-furan-2-carbonyl]-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-phenyl-propionamide (A-144)
[00463] (~)-Cis-N-{ 2-methyl-1-[5-(4-vitro-phenyl)-furan-2-carbonyl]-1,2,3,4-
tetrahydro-quinolin-
4-yl}-N-phenyl-propionamide propionamide was made following general procedure
A, substituting 5-
(4-vitro-phenyl)-furan-2-carbonyl chloride for 2-furoyl chloride and propionyl
chloride for acetyl
chloride.
1H-NMR (CDC13) S: 1.13-1.22(7H, m), 2.20-2.36 (3H, m), 4.70 (1H, q), 5.40-5.60
(1H, br), 6.70 (2H,
d), 6.87 (1H, d), 7.03 (1H, t), 7.25-7.47 (8H, m), 8.15 (2H, d).
MS m/z: 510 (M+1).
[00464] (~)-Cis-N-[2-methyl-1-(5-methyl-isoxazole-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide (A-145)
[00465] (~)-Cis--N-[2-methyl-1-(5-methyl-isoxazole-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A, substituting
5-methyl-
isoxazole-3-carbonyl chloride for 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDC13) 8: 1.10-1.27(7H, m), 2.13-2.35 (6H, m), 4.78 (1H, q), 5.40-5.60
(1H, br), 6.84-6.86
(1H, d), 7.05 (1H, t), 7.22-7.38 (7H, m).
MS m/z: 404 (M+1).
[00466] (~)-Cis-N-[2-methyl-1-(2-methyl-thiophene-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-propionamide (A-146)
[00467] (~)-Cis-N-[2-methyl-1-(2-methyl-thiophene-3-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-phenyl-propionamide was made following general procedure A, substituting
2-methyl-
thiophene-3-carbonyl chloride for 2-furoyl chloride and propionyl chloride for
acetyl chloride.
'H-NMR (CDCl3) 8: 1.01-1.27(7H, m), 2.13-2.39 (6H, m), 4.62-4.78 (lH,m), 5.40-
5.60 (1H, br),
6.31-6.45 (2H, m), 6.60-6.83 (2H, m), 7.02-7.38 (6H, m).
MS m/z: 420 (M+1).
[00468] (~)-Cis-but-3-enoic acid [1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-phenyl-amide (A-147)
[00469] (~)-Cis-but-3-enoic acid [1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-phenyl-amide was made following general procedure A, substituting 4-
fluorobenzoyl chloride for
2-furoyl chloride and but-3-enoyl chloride for acetyl chloride.
'H-NMR (CDC13) 8: 0.98-1.17(4H, m), 2.13-2.29 (1H, m), 2.98-3.15 (2H, m), 4.60-
4.78 (1H, m),
4.98-5.20 (2H, m), 5.40-5.60 (1H, m), 5.70-5.91 (1H, m), 6.40 (1H, d), 6.75-
7.46 (11H, m).
101
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 429 (M+1).
[00470] (~)-Cis-N-{1-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazole-5-carbonyl]-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-phenyl-propionamide (A-148)
[00471] (~)-Cis-N-{ 1-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazole-5-carbonyl]-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-phenyl-propionamide was made following general
procedure A,
substituting 3-(4-fluoro-phenyl)-[1,2,4]oxadiazole-5-carbonyl chloride for 2-
furoyl chloride and
propionyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.14 (3H, t), 1.23-1.25 (4H, m), 2.17-2.39 (3H, m), 4.78-
4.80 (1H, m), 5.40-5.60
(1H, br), 7.03-7.09 (3H, m), 7.10-7.22 (4H, m), 7.24-7.40 (4H, m), 7.97-8.02
(2H, m).
MS m/z: 485 (M+1).
[00472] (~)-Cis-N-(1-benzoyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-acetamide'
(A-150)
[00473] (~)-Cis-N-(1-benzoyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-acetamide
was made following general procedure A, substituting benzoyl chloride for 2-
furoyl chloride.
'H-NMR (CDCl3) ~: 1.14 (3H, d), 1.58-1.69 (1H, m), 2.03 (3H, s), 2.22-2.37
(1H, m), 4.72-4.86 (1H,
m), 5.62 (1H, br s), 6.49 (1H, d), 6.88 (1H, t), 7.13-7.46 (12H, m).
MS m/z: 385 (M+1).
[00474] (~)-Cis-N-[1-(4-chloro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-151)
[00475] (~)-Cis-N-[1-(4-chloro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure A, substituting 4-chlorobenzoyl
chloride for 2-
furoyl chloride.
'H-NMR (CDC13) 8: 1.14 (3H, d), 1.61 (1H, br s), 2.03 (3H, s), 2.24-2.36 (1H,
m), 4.71-4.83 (1H,
m), 5.51-5.69 (1H, m), 6.48 (1H, d), 6.93 (1H, t), 7.12-7.28 (7H, m), 7.35-
7.40 (4H, m).
MS mlz: 419 (M)
[00476] (t)-Cis-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-acetamide (A-152)
[00477] (~)_Cis-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting 4-
methoxybenzoyl chloride for 2-
furoyl chloride.
102
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
IH-NMR (CDC13) 8: 1.12 (3H, d), 1.65 (1H, br s), 2.03 (3H, s), 2.24-2.37 (1H,
m), 3.74 (3H, s),
4.66-4.84 (1H, m), 5.53-5.70 (1H, m), 6.50-6.54 (1H, d), 6.68 (2H, d), 6.89-
6.96 (1H, m), 7.05-7.55
(9H, m).
MS m/z: 415 (M+1).
[00478] (~)-Cis-N-[2-methyl-1-(2-methyl-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-153)
[00479] (~)-Cis-N-[2-methyl-1-(2-methyl-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure A, substituting 2-toluoyl
chloride for 2-furoyl
chloride.
'H-NMR (CDC13) ~: 1.11 (3H, d), 1.60-1.64 (1H, m), 1.97 (3H, s), 2.03-2.3 (4H,
m), 4.77-4.89 (1H,
m), 5.41-5.58 (1H, m), 6.38-6.44 (1H, m), 6.79 (1H, t), 6.91-7.14 (4H, m),
7.16-7.28 (4H, m), 7.28-
7.41 (3H, m).
MS m/z: 399 (M+1).
[00480] (~)-Cis-N-[1-(3,5-dimethyl-isoxazole-4-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-154)
[00481] (~)-Cis-N-[1-(3,5-dimethyl-isoxazole-4-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-phenyl-acetamide was made following general procedure A, substituting
3,5-dimethyl-
isoxazole-4-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDC13) 8: 1.13 (3H, d), 1.57-181 (3H, m), 1.96-2.03 (5H, m), 2.15-2.63
(3H, m), 4.66-
4.81 (1H, m), 5.41-5.50(1H, m), 6.12 (1H, d), 7.03-7.15 (1H, m), 7.24-7.48
(7H, m).
MS m/z: 404 (M+1).
[00482] (~)-Cis-N-[1-(isoxazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-155)
[00483] (~)-Cis-N-[1-(isoxazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was made following general procedure A, substituting
isoxazole-5-carbonyl
chloride for 2-furoyl chloride.
[00484] (~)-Cis-N-[1-(isoxazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was separated by chiral HPLC using a chiral cel OD column and
eluting with 90%
hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)- cis-N-[1-
(isoxazole-5-carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide (A-70 & A-71,
respectively).
'H-NMR (CDCl3) 8: 1.12 (3H, d), 1.64 (1H, s), 1.96 (3H, s), 2.21-2.31 (1H, m),
4.63-4.75 (1H, m),
5.34-5.44 (1H, s), 5.98 (1H, s), 6.70 (1H, d), 7.04 (1H, t), 7.21-7.35 (7H,
m), 8.04-8.08 (1H, m).
103
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS xn/z: 376 (M+1).
[00485] (~)-Cis-N-(1-cyclohexanecarbonyl-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl)-N-
phenyl-acetamide (A-157)
[00486] (~)-Cis-N-(1-cyclohexanecarbonyl-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl)-N-phenyl-
acetamide was made following general procedure A, substituting
cyclohexanecarbonyl chloride for 2-
furoyl chloride.
'H-NMR (CDCI3) S: 0.97 (3H, d), 1.13-1.27 (3H, m), 1.31-1.47 (2H, m), 1.58-
1.89 (7H, m), 1.99
(3H, s), 2.14-2.24 (1H, m), 2.62-2.71 (1H, m), 4.70-4.78 (1H, m), 5.24-5.29
(1H, m), 7.07-7.10 (1H,
m), 7.21-7.24 (2H, m), 7.28-7.33 (2H, m), 7.34-7.42 (4H, m).
MS m/z: 391 (M+1).
[00487] (~)-Cis-N-[2-methyl-1-(pyridine-4-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-158)
[00488] (~)-Cis-N-[2-methyl-1-(pyridine-4-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting isonicotinoyl
chloride for 2-furoyl
chloride.
'H-NMR (CDC13) 8:1.16 (3H, d), 2.04 (3H, s), 2.25-2.35 (1H, m), 4.75-4.83 (1H,
m), 5.56-5.67 (1H,
m), 6.45-6.48 (1H, m), 6.92 (1H, t), 7.08 (2H, d), 7.19-7.27 (3H, m), 7.34-
7.42 (4H, m), 8.49 (2H, d).
MS m/z: 386 (M+1).
[00489] (~)-Cis-N-[1-(2,5-dimethyl-2H-pyrazole-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide (A-159)
[00490] (~)-Cis-N-[1-(2,5-dimethyl-2H-pyrazole-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-phenyl-acetamide was made following general procedure A,
substituting 2,5-
dimethyl-2H-pyrazole-3-carbonyl chloride for 2-furoyl chloride.
'H-NMR (CDCI3) 8: 1.14 (3H, d), 2.02 (3H, m), 2.07 (3H, m), 2.23-2.32 (2H, m),
4.68-4.76 (1H, m),
5.50 (1H, s), 6.66 (1H, d), 7.04 (1H, t), 7.21-7.28 (4H, m), 7.34-7.48 (4H,
m).
MS m/z: 404 (M+1).
[00491] (~)-Cis-N-[2-methyl-1-(pyridine-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide (A-160)
[00492] (~)-Cis-N-[2-methyl-1-(pyridine-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-phenyl-
acetamide was made following general procedure A, substituting pyridine-2-
carbonyl chloride for. 2-
furoyl chloride.
104
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) 8: 1.17 (3H, d), 1.93-2.03 (1H, m), 2.02 (3H, s), 2.32 (1H, br
s), 4.78-4.86 (1H, m),
5.60-5.61 (1H, m), 6.51 (1H, d), 6.86 (1H, t), 6.99 (1H, d), 7.14-7.50 (9H,
m), 8.53 (1H, d).
MS m/z: 385 (M+1).
[00493] (~)-Cis-N-[1-(isoxazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide (A-161)
[00494] (~)-Cis-N-[1-(isoxazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-propionamide following general procedure A, substituting isoxazole-5-
carbonyl chloride for
2-furoyl chloride.
'H-NMR (CDC13) 8: 0.95-1.20 (5H, m), 2.10-2.30 (4H, m), 4.69-4.74(1H, m), 5.30-
5.43 (1H, m),
5.96 (1H, s), 6.75 (1H, d), 7.75 (1H, t), 7.25-7.38 (8H, m), 8.06 (1H, s).
MS m/z: 390 (M+1).
Scheme 5
'I ~~I
~NH ' o o v 'NH
t , °~ ~ CH3COCi
i~ I -,
N~ DIEA, DCM " N DIEA DCM
H n
1 rt O O I W Ow
A-162
[00495] (~)-Cis-N-[1-(3-Methoxy-benzenesulfonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-N-phenyl-acetamide (A-162)
[00496] (~)-Cis-N-[1-(3-methoxy-benzenesulfonyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
phenyl-acetamide was synthesized using general procedure A, substituting 3-
methoxy-
benzenesulfonyl chloride for 2-furoyl chloride.
'H-NMR (CDCl3) 8: 1.4 (3H, d), 1.4 (1H, m), 1.9 (3H, s), 2.0 (1H, m), 3.6 (3H,
s), 4.1 (1H, m), 6.4
(1H, m), 6.9-7.4 (12H, m), 7.7 (1H, d).
MS m/z: 451 (M+1).
Scheme 6
105
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~
NH
CH
COC1
3
K2C03, KI DIEA, DCM
DMF, rt rt
1
28 A-164
[00497] (~)-Cis-N-[1-(3-Methoxy-benzyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (A-164)
[00498] (~)-Cis-N-[1-(3-methoxy-benzyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was synthesized by dissolving (~)-cis-(2-methyl-1,2,3,4-
tetrahydroquinol-4-yl) aniline in
dimethylformamide and adding potassium carbonate (1.0-10.0 equiv.),and the 1-
bromomethyl-3-
methoxy-benzene (1.1-3.0 equiv), catalytic potassium iodide and was stirred at
room temperature for
18 hours. The reaction mixture was filtered for removal of inorganic salts and
concentrated. The
crude mixture was purified by flash chromatography on silica gel using
gradient elution hexane-ethyl
acetate (5-20%). The corresponding aniline was then acylated as previously
described in general
procedure A to give (~)-cis-N-[1-(3-methoxy-benzyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-
phenyl-acetamide.
1H-NMR (CDCl3) 8: 1.15 (3H, d; overlapping 1H, t), 1.90(lH,m; 2H, m), 2.00
(3H, s), 3.33 (1H,
m), 3.60(3H,s), 4.30 (1H, m), 6.30 (1H, complex), 6.90 (1H, t), 6.90-7.40
(10H, m).
MS m/z: 443 (M +1).
[00499] (~)-Cis-N-(1-Benzyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-acetamide
(A-165)
[00500] (~)-Cis-N-(1-benzyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-acetamide was
made following the procedure describing the synthesis of A-164, substituting
benzyl bromide for 1-
bromomethyl-3-methoxy-benzene.
'H-NMR (CDC13) 8: 1.15 (3H, d; overlapping 1H, t), 1.90(lH,m; 2H, m), 2.00
(3H, s), 3.33 (1H,
m), 4.30 (1H, m), 6.30 (1H, m), 6.70 (1H, t), 6.90-7.40 (11H, m).
MS m/z: 413 (M +1).
[00501] (~)-Cis-N-(1-Ethyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-phenyl-
acetamide
(A-166)
[00502] (~)-Cis-N-(1-Ethyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-phenyl-
acetamide was
made following the procedure describing the synthesis of A-164, substituting
ethyl bromide for 1-
bromomethyl-3-methoxy-benzene.
106
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDCl3) S: 1.01 (3H, t), 1.15 (3H, d; overlapping 1H, t), 1.40 (1H, m),
1.90-2.00
(overlapping 3H, s; 1H, m), 3.20 (1H, m), 3.40 (1H, q), 3.60 (1H, m), 4.60
(1H, s), 6.20 (1H, br,m),
6.60- 6.80 (2H, m), 7.00-7.50 (7H, m).
MS m/z: 309 (M +1).
[00503] (~)-Cis-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-
yl]-aceticacid
methyl ester (A-167)
[00504] (~)-Cis-[4-(acetyl-phenyl-amino)-2=methyl-3,4-dihydro-2H-quinolin-1-
yl]-aceticacid
methyl ester was made following the procedure describing the synthesis of A-
164, substituting
bromo-acetic acid methyl ester for 1-bromomethyl-3-methoxy-benzene.
'H-NMR (CDC13) 8:: 1.20 (3H, d; overlapping 1H, t), 1.80(lH,m, 2.00 (3H, s),
3.40 (1H, m),
3.70(3H,s), 3.90(2H, s), 4.50 (1H, m), 6.10 (1H, t), 6.20 (lH,d), 6.75 (1H,
m), 6.90-7.10 (3H,
complex), 7.20-7.50 (3H, m).
MS m/z: 353 (M +1).
[00505] (~)-Cis-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-
yl]-acetic acid
(A-168)
[00506] (~)-Cis-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-
yl]-acetic
acid was made from (~)-cis-[4-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinolin-1-yl]-
aceticacid methyl ester. To a solution of (~)-cis-[4-(acetyl-phenyl-amino)-2-
methyl-3,4-dihydro-2H-
quinolin-1-yl]-acetic acid methyl ester was added 1.0 N aqueous sodium
hydroxide and heated to
80°C for 1 hr. The reaction mixture was concentrated and aqueous
mixture was acidified to pH 6.0
using hydrochloric acid (1N) followed by extraction with ethyl acetate twice.
Organics were dried
over sodium sulfate, filtered and concentrated to yield the desired product.
'H-NMR (CDC13) 8: 1.20 (3H, d; overlapping 1H, t), 1.80(lH,m, 2.00 (3H, s),
3.40 (1H, m),
3.90(2H, s), 4.50 (1H, m), 6.10 (1H, t), 6.20 (lH,d), 6.75 (1H, m), 6.90-7.10
(3H, m), 7.20-7.50 (3H,
m).
MS m/z: 339(M +1).
Scheme 7
107
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
NH I ~ \ I NH \ ' N
\ , \ CH3COCI \
N~ Toluene. 90 °C v 'N DIEA DCM I / N
H n ~
O NH O' 'NH
~o
29 I o
A-169 I
[00507] (~)-Cis-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-1-
carboxylicacid
(3-methoxy-phenyl)-amide (A-169)
[00508] (~)-Cis-(acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinoline-1-
carboxylicacid (3-
methoxy-phenyl)-amide was synthesized using general procedure A, substituting
3-
methoxyphenylisocyanate for 2-furoyl chloride using the following procedure.
To a solution of (~)-
cis-(3-methoxy-phenyl)-(2-methyl-4-anilino-3,4-dihydro-2H-quinolin-1-yl)-
methanone (0.1 g, 0.42
mmol) in toluene was added 3-methoxyphenylisocyanate (0.056 mL, 0.4255 mmol)
and the reaction
mixture was heated to 90°C for 18 hours. Reaction was cooled to room
temperature and concentrated.
The crude mixture was purified by flash chromatography on silica gel using
gradient elution hexane-
ethyl acetate (80%/20%) to give 38% of the desired product. .
1H-NMR (CDC13) 8: 1.1 (3H, d),1.2 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 3.8 (3H,
s), 4.5 (1H, m), 5:4
(lH,nri), 6.6 (1H, d), 6.8 (2H, m) 7.1-7.5 (11H, m).
MS m/z: 430 (M+1).
Scheme 8
\ NH NH ~w,0 \ N.S,R2
R~COCI \
\ I R2S~C1 I \
/ N~ DIEA, DCM ~ N DIEA, DCM i ~ N
H tt
1 O R~ O~R~
30 3~
[00509] (~)-Cis-N-(1-alkyl/aroyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-
phenyl-
alkyl/aryl sulfonamide
[00510] (~)-Cis-1-(2-Methyl-4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-
alkanone or (~)-cis-
(2-Methyl-4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-aryl-methanone can be
prepared from
108
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
compound 1 using general procedure A, substituting the corresponding sulfonyl
chloride for acetyl
chloride.
Scheme 9
i i
\ I \ NH \ N.Rz
~NH
R~COCI \ R2X
I I~ ~ I
N DIEA, DCM N K2C03, KI ~ N
rt O R DMF, rt O R
1
34 35
[00511] (~)-Cis-1-[4-(alkyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-
yl]-alkanone
or (~)-Cis-1-[4-(alkyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-yl]-
aryl methanone
[00512] (~)-Cis-1-[4-(alkyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-
yl]-alkanone or
(~)-cis-1-[4-(alkyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-quinolin-1-yl]-aryl
methanone may be
prepared from compound 1 using general procedure A, substituting the
corresponding alkyl chloride
for acetyl chloride and using the alkylation procedure in the synthesis of A-
164. Representative
examples of compound 35 are shown in the table below.
Scheme 10
0II
NH v -NH ~N~H~\
R~COCI I y Err~o
N DIEA, DCM / N Tolue~ / N
H rt
036\ O\ O I\
A-170
[00513] (~)-Cis-3-ethyl-1-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
1-phenyl-urea (A-170)
[00514] (~)-Cis-3-ethyl-1-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-1-
phenyl-urea was synthesized using general procedure A, substituting ethyl
isocyanate for acetyl
chloride using the following procedure. To a solution of (~)-cis-(3-methoxy-
phenyl)-(2-methyl-4-
anilino-3,4-dihydro-2H-quinolin-1-yl)-methanone in DMF was added ethyl
isocyanate and reaction
mixture was heated to 90°C for 1~ hours. The reaction was cooled to
room temperature and
109
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
concentrated. Crude mixture was purified by flash chromatography on silica gel
using gradient elution
hexane-ethyl acetate (5-20%).
'H-NMR (CDC13) 8: 1.05-1.20 (3H,t; overlapping 3H, d; and 1H, t), 2.35 (1H,
m), 3.30 (2H,q), 3.67
(3H,s),4.36 (1H, t), 4.80 (1H, m), 5.65 (1H, m), 6.50 (1H, d), 6.65 (1H, d),
6.80 (lH,d), 6.85 (2H,
complex), 7.00 (1H, t), 7.18 (1H, t), 7.35-7.50 (6H, m).
MS m/z: 444 (M + 1).
[00515] Compounds A-163, A-171- A-232 can be prepared by the schemes set forth
in Schemes
1- 10 and by the general procedures A and others described herein. Those
skilled in the art will be
able to recognize, or be able to ascertain, using no more than routine
experimentation, many
equivalents to the specific embodiments of the invention described herein.
[00516] Table 1: Compounds Derived from General Procedure A
110
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. No. -. No. No.
I I % ~aH
A-1 A-11
I A-21 I A-31
I/ \
I~ F I
F
\ I ~ \ I ra~ \ I \ I r~
A-2 I \ . A-12 ~ A-22 ~ A-32 I \
~I
CFA
I~ I/\
F
O
I , \ I ra~ ~~ ~H~
I °~
A_3 A_13 I ~ A_23 I
A-33
\I
/ \ / F
\I \ I ~ \ I ~I ~ o
A-4 I ~ , A-14 ~ A-24 ~ A-34
\I F
I~ F I \
/ I r~
A 5 I A-IS I , A-25 ~ A-35 I \
~~'~j~ a
I I / \ / ~a
A-6 ~ A-16 ~ A-26 I ~ A-36
\
I / \ / F 0.i
F
O O
\ I H~ ~r.~ ~,.~ \ I
A-7 A-17 ~ A-27 ~ A-37
~F ~N I ~ F I ~ OH
I
I
A-6 A-18 I , ~ ..", A-26 A-38
I=
d
QLI~
A-9 ~,. ,A-19 I , A-29 I ~ A-39 ,~ ~\/~
I I / OH
'I !~ ~''~ \ I H NI
A-10 I \ , ,~ A-20 I \ A-30 ~ A-40
\ F ~ ~ ~
F CI
~rlF
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. No. No, No.
,
\
A-41 A-51 A-61~ A-71\ I
Ni4
F
I /
I
I \ A I
-42 -52 -62 \ I / I
-72 \
I ~ON \ F
/ F F F
~I \I o
A-43~ A ~
53
- \ A-63 A-73
~'lf'~ I I
o F
F
I
~
A-44\ "Y A-54 \ I A-64\ I A-74
w
I \ \ I
F !
O
\ I r~ \ I \ I
I\ I N
~
A-45 A-55 / I A-65\ I A-75\ I
I/
F
I ~
I/
O
\ I rr~
-46 I A
-56 I , -66 I 76
- I
I o \ \
rf
I ~ I I \ _
F _N
N~ \ I ~ \ I ~ ~N~
-47 O O
I ~ / I~
A-57 , A-67~ I A-77
s \ F
N
F I
O o
\ A I \ I N~ \ I
-48 58
I - I A-68' I A-78~ I
\ \ \
I / F ~F ~! O/
\I \I
A-49~ A-59 A-69\ I A-79
I / F I / I /
F
\I
A-50I "'~ A-60 I \ x A-70\ I A-80\ I
"q
I/ F
F F
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. No. - No. No.
~H~. aN~ aN~.
A-81 ~ I A-91 \~ A-101 ~ A-111 I~" \
I \ ~F
\ I H~' B~NJ~ \ I NJ~ aN~
A-82 ~ I A-92 e~ A-102 ~ A-112
~'rf~ I
I ~Ng
F
~ I ~ ~ I ~ ~ I
A-83 A-93 I \ A-103 I , A-113 I \
I, I, I~ ~ I\ I\
NH,
I ~~' Q~' C~,~ G~N~..
A-84 \ I A-94 ~ A-104 I \ A-114
I~ ~ I
F
~NJ~ \ I Hue' I N~ ~,~
\
A-85 ~ I A-95 ~ A-105 ~ A-115 I \
F
I\ ~ I
d
QN~ Q o
A-86 '~ A-96 I , A-106 I A-116
I I \ 0.' ~ oII
~N~NI,
H
C~.,~ Q,~ C~,~
A-87 \ I A-97 A-107 ~ A-117
I \ t~t1' ~~ I ' ~ '~f~0.
0
I ~ NFL- ~.
A-88 \ I A-98 A-108 I / A-118
I, ~ I,
~/ I N
~NH N
~N~ ~N~ I ' rah ~d~
A-89 ~ A-99 ~ A-109 ~ A-119
I
I~~H I ~ &
o I
I
I
A-90 \ I A-100 A-110 ~ A-120
~NY
113
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No: No. No. . No.
o\ ~ I N
0
A-121 ~"° A-131 I , A-141
A-151
N F I
p \I /I op
I I v N
A-122 A-132 I , A-142
I A-152
~I~
v 'F 1 I i
~O
~I o I
~I
A-123 I , A-133 ~ A-143 A-153 I
I
I
~N~ : I '~'~ ~ ~ I rah
A-124 I~ A-134 ~ A-144 A-154
I i F NCe
A-125 ~.." A-135 I ~ A-145 I ~ ~ A-155
"'F I ".\ R
~N
i O
~ I N~ ~N~
A-126 I A-136 I ~ A-146 I ~ A-156
I
p' F
~I
A-127 I ~ ~ ,.,. A-137 I ~ ~ A-147 I , A-157 I ~
,'~/~p/~ N~
<JwF
~'FF F
A-128 A-138 I ~ A-148 I A-158
I ~ °' F
O- \ / ~N
O
p ~ I N
A-129 ° I ~ A-139 I ~ A-149 ~- A-159
v °~ I ,
~/
I I ~~ I
A-130 I ~ A-140 ~ A-150 I ~ A-160
4
I
114
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. StructureNo. StructureNo. StructureNo, Structure
:.
~-.
A-161 A-171~~ A-181 A-191
N
O ~&
I !
N' - N~ ~ ~ ~N~
A-162~ A-172~ A-182~ A-192I ~
N N N
O=A O
I i o=b~~
Q~ ~N~
A-163 A-173~ A-183~ A-193
N
$ ~ 8
i O
I
vI I N O ~ ~F
-164I -174 -184 I -194
~F
, ~
I ~ ~
01
o
w N~ /~ !I I o'1
N ~N v aN~
~
A-165~ , A-175~ A-185I ~ A-195
N N N
=g1
~ Q
N' ' N
A-166~ A-176~s A-186~ A-196I
::ll,,,,~~ ~N N
~ O~
~ N NH NH
I
N
~N~ C~,~
A-167I , A-177~~ A-187~ A-197
N H
O I
I N~ ~. ~l. QN~
N
A-168I ~ N A-178I ~ ~ A-188 A-198
0
Ii
N ~
A-169~ A-179~ A-189N A-199N
~ N F F~
NH ~ 1 ~(
~ O~NN N
D~YJ~~~
~CI
~ I N~~,~ ~. R
~
A-170I A-180~ A-190~ A-200
oJ.
~
115
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. StructureNo. StructureNo: gtru~ureNo. Structure
~,~J~ ~N~ QN-w p~
A-201~ A-211~ A-221~ A-231
N F
~N~ ~ 1~
A-202~ A-212~ A-222 A-232
F
O
~N \
~
A-203~ A-213~ A-223
F
L
~J ~ O
A-204 A-214~~ / A-224
L
Q
A-205~ A-215 A-225
O '9"F
~N~~ _
~
H~
A-206~ A-216 A-226
N
,
u_
/
\ I N'9~ Q ~~~\
~ ~
A-207~ A-217~ A-227
/
N
~Ov
O~1
~
A-208 A-218~ A-228
~~cn
A-209'~l~ A-219~ A-229
~rars9~
A-210~ A-220~ A-230
Scheme 11
116
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
NHCbz
OII 1. Na2S03, CH2CI2
i + H~CH
NH2 3 2. ~NHCbz . ~ N~CH3
H
BF30Et 11
(Exclusively cis)
[00517] (~)-Cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-carbamic acid
benzyl ester (11)
[00518] Aniline (3.64 mL, 39.97 mmol, 1.0 equ) was dissolved in methylene
chloride (100 mL)
and Na2S04 (2 g) was added and cooled to -25 °C. Acetaldehyde (2.23 mL,
39.97 mmol, 1.0 equ.)
was added to the solution and stirred for 1h at -25 °C. Sodium sulfate
was filtered off and N-vinyl-
carbamic acid benzyl ester (7.07g, 39.97 mmol, 1.0 equiv) was added to the
filtrate at -25°C, followed
by boron triflouride diethyl etherate (0.50 mL, 3.9 mmol, 0.1 equ). The
reaction was allowed to stir at
-25°C for 1h and then warmed to room temperature and stirred for 10h.
The reaction was evaporated ..
in vacuo and the residue was purified by Biotage flash system (20% ethyl
acetate/80% hexane) to
yield 4.0 g, 33 % of (~)-cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid benzyl ester as
a white solid.
H' NMR (300 MHz, CDC13) 5:7.38 (m, 5H), 7.17 (d, 1H), 7.02 (t, 1H, C7-H), 6.68
,(t, 1H), 6.47 (d,
1H), 5.17 (6s, 2H), 5.07 (m, 1H), 4.92 (d, 1H), 3.57 (m, 1H), 2.30 (m, 1H),
1.47 (q,1H), 1.21 (d, 3H).
General Procedure S ,.
Scheme 12
°
NHCbz i . °. NH2
_ Pd/C (10 %) w
I i DIF~4, DCM, EtOH
CHs n ~ H2 N CHs
» O
(Exclusively cis) % ~~, N~
-.- ._ cis-13
CI\
scan.
AcCI, DIEA
---. --
Cu(OAc)2, pyr DCM, rt
DMF, 60 °C
N~
cis-15
117
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00519] (~)-Cis-[1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
carbamic acid benzyl ester (12)
[00520] To a solution of (~)-cis-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid benzyl
ester (500 mg, 1.68 mmol) in methylene chloride (20 mL) at room temperature
was added
diisopropylethylamine (542 mg, 749 uL, 4.2 mmol) followed by 4-
dimethylaminobenzoyl chloride
and stirred at rrom temperature until no starting material was present. The
mixture was poured into
water and extracted with ethyl acetate. The extracts were washed with 1 M (aq)
NaOH and brine,
dried over sodium sulfate, filtered dried and concentrated. The crude residue
was purified by silica
gel chromatography (100% hexanes to 70% hexanes/30% ethyl acetate gradient) to
afford the amide
(665 mg, 89 %).
1H-NMR (300 MHz, CDCl3) ~: 1.24 (d, 3 H), 1.36 (m, l H), 2.75 (ddd, 1 H), 2.91
(s, 6 H), 4.79 - 4.92
(m, 3 H), 5.22 (s, 2 H), 6.43 (d, 2 H), 6.65 (d, 1 H), 6.90 (dd, 1 H), 7.07 -
7.18 (m, 5 H), 7.2 - 7.48
(m, 4 H).
1VIS m/z: 444 (M + 1).
[00521] (~)-Cis-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-4-
aminoquinoline
(13)
[00522] (~)-Cis-[1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
carbamic
acid benzyl ester (665 mg, 1.49 mmol) was dissolved in ethanol (30 mL). The
resulting solution was
evacuated and backfilled with argon. A catalytic amount of palladium on carbon
(10 %) was added.
The vessel was once again evacuated and this time was backfilled with hydrogen
from a balloon. The
reaction was then allowed to react at room temperature over night under a
hydrogen atmosphere.
Reaction was complete after 18h. The mixture was carefully filtered and
concentrated to 10%
volume. The resulting concentrated solution was filtered through an Acrodisc ~
and concentrated to
afford the crude amine (423 mg, 92 %).
'H-NMR (300 MHz, CDC13) 8: 1.19 - 1.40 (m, 4 H), 2.76 (ddd, 1 H), 2.95 (s, 6
H), 4.08 (dd, 1 H),
4.81 (m, 1 H), 6.42 (d, 2 H), 6.64 (d, 1 H), 6.99 (dd, 1 H), 7.08 - 7.23 (m, 5
H), 7.52 (d, l H).
MS m/z: 310 (M + 1).
[00523] (~)-Cis-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-4-(N 4-
chlorophenyl)aminoquinoline (14)
[00524] To a solution of (~)-cis-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-4
aminoquinoline (423 mg, 1.36 mmol) in DMF (15 mL, dry) was added 4-
chlorophenylboronic acid
(425 mg, 2.72 mmol), pyridine (322 mg, 330 uL, 4.08 mmol) and copper(Inacetate
(494 mg, 2.72
mmol). The heterogeneous green mixture was stirred open to air for 1 h and
then warmed to 60 °C
118
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and stirred over night (14 h). The mixture was then cooled to rt, poured into
rapidly stirred ethyl
acetate (150 mL); solids were removed by filtration. The extracts were washed
several times with
water and then once with brine. The extracts were then dried over anhydrous
sodium sulfate, filtered,
and concentrated under reduced pressure. The crude residue was purified by
silica gel
chromatography (100 % hexanes to 50/50 hexanes/ethyl acetate gradient) to
afford the aniline product
(120 mg, 22 %) as a yellow oil.
'H-NMR (300 MHz, CDC13) 8: 1.22 (d, 3 H), 1.36 (ddd, 1 H), 2.82 (ddd, 1 H),
2.95 (s, 6 H), 4.90 (6r
s, 1 H), 4.41 (br d, 1 H), 4.87 (ddd, 1 H), 6.65 (d, 2 H), 6.62 - 6.76 (m, 3
H), 6.97 - 7.11 (m, 2 H),
7.17 - 7.29 (m, 5 H).
MS m/z: 420 (M + 1)
[00525] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (15)
[00526] To a solution of (~)-cis-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-4-(N-
4-chlorophenyl)aminoquinoline (120 mg, 0.29 mmol) in methylene chloride (2 mL)
was added
diisopropylethylamine (37 mg, 0.051 mL, 0.29 mmol) followed by acetyl chloride
(2 mL). The
mixture was stirred at rt 4 h. The mixture was concentrated under reduced
pressure, dissolved in ethyl
acetate, washed with sat. aqueous sodium bicarbonate, brine and dried over
sodium sulfate. The
drying agent was removed by filtration under reduced pressure, concentrated
and purified by silica gel
chromatography (100 % hexanes - 25/75 hexanes/ethyl acetate gradient) to
afford pure (~)-cis-N-(4-.
chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
acetamide (45 mg, 34 %).
'H-NMR (300 MHz, CDC13) S: 1.14 -1.33 (m, 4 H), 2.13 (s, 3 H), 2.24 - 2.39 (m,
1 H), 2.94 (s, 6 H),
4.75 (ddd, 1 H), 5.61 (br s, 1 H), 6.44 (d, 2 H), 6.63 (d, 1 H), 6.96 (dd, 1
H), 7.07 - 7.36 (m, 6 H), '
7.40 (d, 2 H).
MS mlz: 420 (M + 1 )
[00527] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-o-
tolyl-acetamide (B-1)
[00528] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-o-tolyl-
acetamide was made following general procedure B, substituting 3-
methoxybenzoyl chloride for 4-
dimethylaminobenzoyl chloride and 2-tolylboronic acid for 4-
chlorophenylboronic acid.
'H-NMR (CDCl3) 8: 1.14 (d, 3H), 1.26 (s, 1H), 1.58 (s, 3H), 1.97 (s, 3H), 2.08
(m, 1H), 3.63 (s, 3H),
4.80 (sextet, 1H), 5.55 (bs, 1H), 6.53 (d, 1H), 6.76 (s, 1H), 6.83 (t, 2H),
6.93 (t, 1H), 7.10 (t, 1H),
7.15-7.37 (m, 6H).
119
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 429 (M+1 )
[00529] N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (B-2)
[00530] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
3-methoxybenzoyl
chloride for 4-dimethylaminobenzoyl chloride.
[00531] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting
with 90% hexanell0% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-
N-(4-chloro-phenyl)-
N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide
(B-9 & B-8,
respectively)
'H-NMR (CDC13) 8: 1.17 (d, 3H), 1.25 (t, 1H), 2.03 (s, 3H), 2.29 (m, 1H), 3.62
(s, 3H), 4.80 (sextet, ~:
1H), 5:60 (bs, 1H), 6.54 (d, 1H), 6.74(s, 1H), 6.80 (t, 1H), 6.93 (t, 1H),
7.08 (t, 1H), 7.14-7.30 (m,
5H), 7.38 (d, 2H).
MS m/z: 449 (M+1 )
[00532] ' (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(thiophene-2-carbonyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-3)
[00533] (~)-Cis-N-(4-chloro-phenyl)-N'-[2-methyl-1-(thiophene-2-carbonyl)-
1,2,3,4-tetrahydro-
quinoliii-4-yl]-acetamide was made following general procedure B, substituting
2-thiophenecarbonyl
chloride for 4-dimethylaminobenzoyl chloride.
[00534] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(thiophene-2-carbonyl)-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting
with 90% hexanell0% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-
N-(4-chloro-phenyl)-
N-[2-methyl-1-(thiophene-2-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide (B-7 & B-6,
respectively).
1H-NMR (300 MHz, CDCl3) 8: 1.11-1.24 (m, 4 H), 2.03 (s, 3 H), 2.22 - 2.35 (m,
1 H), 4.73 (ddd, 1~
H), 5.52 (br s, 1 H), 6.69 (dd, 1 H), 6.67 (dd, 1 H), 6.89 (d, 1 H), 7.08 (dd,
1H), 7.21 (d, 2 H), 7.27 -
7.43 (m, 5 H).
MS m/z: 425 (M + 1).
[00535] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-isobutyramide (B-4)
120
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00536] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-isobutyramide was made following general procedure
B, substituting 5-
methyl-2-thiophenecarbonyl chloride for 4-dimethylaminobenzoyl chloride and
isobutyryl chloride
for acetyl chloride.
[00537] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-isobutyramide was separated by chiral HPLC using a
chiral cel OD column
and eluting with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and
(2S,4R)-cis-N-(4-
Chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-
isobutyramide (B-11 & B-10 respectively).
'H-NMR (CDCl3) 8: 1.13 (d, 6H), 1.16 (d, 3H), 1.25 (m, 1H), 2.23 (m, 3H), 2.39
(s, 1H), 2.60
(septet, 1H), 4.66 (sextet, 1H), 5.50 (bs, 1H), 6.42 (s, 1H), 6.51 (s, 1H),
6.93 (d, 1H), 7.08 (t, 1H),
7.21 (d, 2H), 7.27 (d, 2H), 7.37 (bs, 2H).
MS m/z: 468 (M+1)
[00538] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
fluoro-phenyl)-propionamide (B-5)
[00539] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-fluoro-
phenyl)-propionamide was made following general procedure B, substituting 4-
fluorobenzoyl
chloride for 4-dimethylaminobenzoyl chloride, 4-fluorophenylboronic acid for 4-
chlorophenylboronic
acid, and propionyl chloride for acetyl chloride.
1H-NMR (CDCl3) 8: 1.14 (t, 3H), 1.15 (d, 3H), 1.24 (m, 1H), 2.26 (m, 3H), 4.75
(sextet, 1H), 5.61
(bs, 1H), 6.46 (d, 1H), 6.87 (m, 3H), 7.10-7.26 (m, 8H).
MS m/z: 435 (M+1)
[00540] (~)-Cis-N-(4-chloro-3-methyl-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-12)
[00541] (~)-Cis-N-(4-chloro-3-methyl-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-
fluorobenzoyl chloride for 4-dimethylaminobenzoyl chloride, propionyl chloride
for acetyl chloride
and 4-chloro-3-tolylboronic acid for 4-chlorophenylboronic acid.
'H-NMR (CDC13) 8: 1.08 (t, 3H), 1.09 (d, 3H), 1.18 (m, 1H), 2.18 (m, 3H), 2.31
(s, 3H), 4.69 (sextet,
1H), 5.49 (bs, 1H), 6.42 (d, 1H), 6.79 (t, 2H), 6.86 (t, 1H), 6.96 (dd, 1H),
7.05-7.22 (m, 6H).
MS m/z: 465 (M+1).
121
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00542] (~)-Cis-N-[1-(4-Fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
tritluoromethyl-phenyl)-propionamide (B-13)
[00543] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
trifluoro-methyl-phenyl)-propionamide was made following general procedure B,
substituting 4-
fluorobenzoyl chloride for 4-dimethylaminobenzoyl chloride, propionyl chloride
for acetyl chloride
and 4-trifluoromethylphenylboronic acid for 4-chlorophenylboronic acid.
'H-NMR (CDC13) 8: 1.15 (t, 3H), 1.17 (d, 3H), 1.20 (m, 1H), 2.29 (m, 3H), 4.79
(sextet, 1H), 5.62
(bs, 1H), 6.49 (d,1H), 6.87 (m, 3H), 7.19-7.28 (m, 6H), 7.41 (d, 1H), 7.69 (d,
1H).
MS m/z: 485 (M+1).
[00544] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide (B-14)
[00545] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-
methoxybenzoyl chloride for 4-dimethylaminobenzoyl chloride and propionyl
chloride for acetyl
chloride.
[00546] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide was separated by chiral HPLC using a chiral cel OD
column and eluting
.with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-
N-(4-chloro-phenyl)-
N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide (B-18 & B-17,
respectively).
'H-NMR (CDC13) 8: 1.14 (t, 3H), 1.15 (d, 3H), 1.25 (t, 1H), 2.29 (m, 3H), 3.74
(s, 3H), 4.74 (sextet,
1H), 5.61 (bs, 1H), 6.53 (d, 1H), 6.68 (d, 2H), 6.93 (t, 1H), 7.14-7.28 (m,
6H), 7.38 (d, 2H).
MS m/z: 463 (M+1).
[00547] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (B-15)
[00548] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
4-methoxybenzoyl
chloride for 4-dimethylaminobenzoyl chloride.
[00549] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting
with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-
N-(4-Chloro-phenyl)-
N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide
(B-34 & B-35,
respectively).
122
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDCl3) 8: 1.14 (d, 3H), 1.25 (t, 1H), 2.04 (s, 3H), 2.29 (m, 1H), 3.74
(s, 3H), 4.74 (sextet,
1H), 5.61 (bs, 1H), 6.53 (d, 1H), 6.68 (d, 2H), 6.93 (t, 1H), 7.14-7.28 (m,
6H), 7.38 (d, 2H).
MS m/z: 449 (M+1).
[00550] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide (B-16)
[00551] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide was made from (~)-cis-N-(4-chloro-phenyl)-N-[1-(4-
methoxy-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide. (~)-Cis-N-[1-(4-
methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-propionamide (0.548 g, 0.001
mol) was dissolved
in dichloromethane and a solution of BBr3 ( 1.0 M in dichloromethane, 10 mL)
was added; the reaction
was allowed to stir at room temperature for 4h or until no starting material
remained. The reaction
was washed with sat NaHC03 carefully and brine. The organics were dried over
MgS04, filtered and
coneentrated_down. The phenol was concentrated down and the residue was
purified by Biotage flash
chromatography using 100 % EtOAc to give a white solid , 74 % yield.
'H-NMR (CDCl3) 8: 1.09 (d, 3H), 1.11 (t, 3H), 1.19 (m, 1H), 2.26 (m, 3H), 4.74
(sextet, 1H), 5.54
(bs, 1H), 6.46 (d,1H), 6.53 (d, 1H), 6.96 (t, 1H), 7.14-7.40 (m, 9H).
MS m/z: 415 (M+1).
[00552] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-p-tolyl-
propionamide (B-21)
[00553] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,. 4-tetrahydro-quinolin-
4-ylJ-N-p-tolyl
propionamide was made following general procedure B, substituting 4-
fluorobenzoyl chloridefor 4-
dimethylaminobenzoyl chloride, propionyl chloride for acetyl chloride and 4-
tolylboronic acid for 4-
chlorophenylboronic acid.
'H-NMR (300 MHz, CDC13) 8: 1.05-1.21 (m, 7 H), 2.11- 2.54 (m, 6 H), 4.73 (ddd,
1 H), 5.56 (br s, 1
H), 6.37 d, 1 H), 6.8 - 7.0 (m, 3 H), 7.1- 7.4 (m, 8 H).
MS m/z: 431 (M + 1).
[00554] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (B-22)
[00555] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
4-fluorobenzoyl
chloride for 4-dimethylaminobenzoyl chloride.
123
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00556] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting
with 95% hexane/5% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-N-
(4-chloro-phenyl)-
N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide
(B-26 ~ B-27,
respectively).
'H-NMR (CDC13) 8: 1.1 (d ,3H); 1.1 (m, 1 H), 2.0 (d ,3 H), 2.3 (m, 1 H), 4.7
(m, l H), 5.6 (m, 1 H),
6.5 (d, 1H),6.7-7.0 (m, 3H), 7.1-7.4 (m, 8 H).
MS m/z: 436 (M+1).
[00557] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-24)
[00558] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1;2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure B,
substituting 5-methyl-
2-thiophenecarbonyl chloride for 4-dimethylaminobenzoyl chloride.
[00559] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was separated by chiral HPLC using a
chiral cel OD column and
eluting with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and
(2S,4R)-cis-N-(4-Chloro-
phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide
(B-28 & B-25, respectively).
1H-NMR (CDC13) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, d), 2.3 (1H, m), 2.4 (3H,
s), 4.7 (1H, m), 5.6
(1H, m), 6.4 (1H, m), 6.6 (1H, m), 7.0 (1H, m), 7.1 (1H, m), 7.2-7.4 (6H, m).
MS m/z: 439 (M+1).
[00560] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-29)
[00561] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting 5-
methyl-2-thiophenecarbonyl chloride for 4-dimethylaminobenzoyl chloride and
propionyl chloride for
acetyl chloride.
'H-NMR (CDCl3) 8: 1.1-1.2 (7H, m), 2.1-2.3 (3H, m), 2.3 (3H, s), 4.8 (1H, m),
5.6 (1H, m), 6..2-6.4
(2H, m), 6.8-7.4 (8H, m).
MS m/z: 452 (M+2).
[00562] (~)-Cis-4-(4-{4-[(4-chloro-phenyl)-propionyl-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid ethyl ester (B-30)
124
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00563] (~)-Cis-4-(4-{4-[(4-chloro-phenyl)-propionyl-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid ethyl ester was prepared from (~)-
cis-N-(4-chloro-
phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide. (~)-
Cis-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-
propionamide (140 mg, 0.31 mmol) was dissolved in DMF (5 mL) at room
temperature. Sodium
hydride (60 % in oil, 32 mg, 0.81 mmol) was added and the mixture allowed to
stir 30 min. Ethyl 4-
bromobutyrate (207 mg, 1.06 mmol) was added and the reaction was allowed to
stir over night.
Ethanol was added and the reaction was concentrated in vacuo. The crude
residue was purified by
silica gel chromatography (80/20 hexanes/ethyl acetate - 50/50 hexanes ethyl
acetate gradient) to
afford the product (171 mg, 0.304 rmnol, 98 %).
'H-NMR (CDCl3) b: 1.l-1.2 (7H, m), 1.3 (3H, t), 2.1 (2H, m), 2.3 (3H, m), 2.5
(2H, t), 3.9 (2H, t),
4.2 (2H, q), 4.8 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9 (1H, t),
7.1-7.3 (6H, m), 7.4 (2H,
m).
MS m/z: 563 (M+1).
[00564] (~)-Cis-4-(4-{4-[(4-chloro-phenyl)-propionyl-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid (B-31)
[00565] (~)-Cis-4-(4-{4-[(4-chloro-phenyl)-propionyl-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid was prepared from (~)-cis-4-(4-{4-
[(4-chloro-phenyl)-
propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-
butyric acid ethyl ester. '
Potassium carbonate (300 mg) was dissolved in water (5 mL) and (~)-cis-4-(4-{4-
[(4-chloro-phenyl)-
propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-
butyric acid ethyl ester
(171 mg, 0.303 mmol) was dissolved in methanol (5 mL) was added. The reaction
was allowed to stir
over night at room temperature. The methanol was removed in vacuo and
hydrochloric acid (1 N)
was added until acidic. Dichloromethane was added, extracted 2x; the combined
organics were dried
over magnesium sulfate, filtered and concentrated to afford the carboxylic
acid (50 mg, 31 %).
'H-NMR (CDCl3) 8: 1.1-1.2 (7H, m), 2.0 (2H, m), 2.3 (2H, m), 2.4 (3H, m), 3.3
(1H, s), 4.0 (2H, t),
4.8 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9 (1H, t), 7.1-7.3 (3H,
m), 7.4-7.6 (5H, m).
MS m/z: 535 (M+1).
[00566] (~)-Cis-N-(4-chloro-phenyl)-N-{2-methyl-1-[4-(1H-tetrazol-5-
ylmetho~ry)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-propionamide (B-32)
[00567] (~)-Cis-N-(4-chloro-phenyl)-N-{2-methyl-1-[4-(1H-tetrazol-5-ylmethoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-propionamide was prepared from (~)-cis-N-(4-
chloro-phenyl)-N-[1-
(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide.
(~)-Cis-N-(4-chloro-
phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide (700
125
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
mg, 1.42 mmol) was dissolved in DMF (10 mL) at room temperature. Sodium
hydride (60 % in oil,
227 mg, 5.68 mmol) was added and the mixture allowed to stir 30 min.
Bromoacetonitrile (850 mg,
7.11 mmol) was added and the reaction was allowed to stir over night. Ethanol
was added and the
reaction was concentrated irt vacuo. ' The crude residue was purified by
silica gel chromatography
(30/70 ethyl acetate/dichloromethane) to afford the product (320 mg, 42 %).
[00568] The nitrite (140 mg, 0.25 mmol) was dissolved in toluene, sodium azide
(160 mg, 2.5
mmol) and triethylammonium hydrochloride (345 mg, 2.5 mmol) were added and the
mixture was
heated to 80 °C over night. Reaction was cooled to room temperature and
water was added, followed
by hydrochloric acid (1 N) until acidic. The aqueous solution was extracted
three times with
dichloromethane. The combined extracts were dried over magnesium sulfate,
filtered, dried and
concentrated. The crude product was triturated with ethyl ether/hexanes to
yield a white solid (82.mg,
63 %).
'H-NMR (CDCl3) 8: 1.0-1.2 (7H, m), 2.2-2.4 (3H, m), 4.8 (1H, m), 5.2 (2H, dd),
5.6 (1H, m), 6.7
(2H, m), 6.9 (1H, t), 7.1 (2H, d), 7.2-7.6 (7H, m).
MS m/z: 531 (M+1).
(00569] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-isobutoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide (B-33)
[00570] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-isobutoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
4-isobutyloxybenzoyl
chloride for. 4-dimethylaminobenzoyl chloride.
'H-NMR (CDCl3) ~: 0.9-1.0 (8H, m), 1.2 (3H, d), 2.0 (3H, s), 2.3 (1H, m), 3.6
(2H, d), 4.7 (1H, m),
5.6 (1H; m); 6.5 (1H, d), 6.6 (2H, d), 6.9 (1H, m), 7.1-7.4 (8H, m).
MS m/z: 491 (M+1).
[00571] (~)-Cis-N-(4-chloro-phenyl)-N-{1-[4-(3-hydroxy-2,2-dimethyl-propoxy)-
benzoyl]-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (B-37)
[00572] (~)-Cis-N-(4-chloro-phenyl)-N-{ 1-[4-(3-hydroxy-2,2-dimethyl-propoxy)-
benzoyl]-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was prepared from (~)-cis-N-
(4-chloro-phenyl)-
N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide. (~)-Cis-N-(4-
chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-propionamide
(210 mg, 0.484 mmol) was dissolved in DMF (10 mL) at room temperature.
Potassium carbonate
(lg, 7.1 mmol) was added, followed by 3-bromo-2,2-dimethyl-propan-1-of (813
mg, 4.84 mmol), the
reaction was heated to 95 °C and stirred over night. The reaction
mixture was cooled to room
temperature, filtered and concentrated in vacuo. The crude product was
purified by silica gel
126
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
chromatography (95/5 dichloromethane/ethyl acetate - 70/30
dichloromethane%thyl acetate) to afford
the pure ester (110 mg, 44 %).
'H-NMR (CDC13) 8: 1.0 (6H, s), 1.1 (3H, d), 1.1 (1H, m), 1.7 (1H, br), 2.0
(3H, s), 2.3 (1H, m), 3.5
(2H, s), 3.7 (2H, s), 4.8 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9
(1H, t), 7.1-7.3 (7H, m), 7.4
(1H, d).
MS xn/z: 521 (M+1).
[00573] (~)-Cis-3-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl}-phenoxy)-2,2-dimethyl-propionic acid methyl ester (B-38)
[00574] (~)-Cis-3-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-2,2-dimethyl-propionic acid methyl ester was prepared from
(~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide. (~)-
Cis-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
propionamide (400 mg, 0.92 mmol) was dissolved in DMF (25 mL) at room
temperature. Potassium
carbonate (1g, 7.1 mmol) was added, followed by 3-bromo-2,2-dimethyl-propionic
acid methyl ester
(400 mg, 0.92 mmol), the reaction was heated to 95 °C and stirred over
night. The reaction mixture
was cooled to room temperature, filtered and concentrated in vacuo. The crude
product was purified
by silica gel chromatography (95/5 dichloromethane/ethyl acetate - 70/30
dichloromethane/ethyl
acetate) to afford the pure ester (40 mg, 8 %).
'H-NMR (CDC13) b: 1.1 (3H, d), 1.1 (1H, m), 1.3 (6H, s), 2.0 (3H, s), 2.3 (1H,
m), 3.7 (3H, s), 3.9
(2H, dd), 4.8 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9 (1H, t), 7.1-
7.3 (7H, m), 7.4 (1H, d).
MS m/z: 549 (M+1).
[00575] (~)-Cis-(4-{acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-amino}-phenyl)-acetic acid (B-39)
[00576] (~)-Cis-(4-{acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-phenyl)-acetic acid was made from (~)-cis-N-(4-cyanomethyl-phenyl)-N-[1-
(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (~)-Cis-N-(4-
cyanomethyl-phenyl)-
N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide
was made following
general procedure B, substituting 4-methoxybenzoylchloride for 4-
dimethylaminobenzoyl chloride
and 4-(phenylboronic acid)-acetonitrile for 4-chlorophenylboronic acid. (~)-
Cis-N-(4-cyanomethyl-
phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide was
dissolved in ethanol (4 mL), potassium hydroxide (120 mg in 0.3 mL water) was
added and the
reaction was heated at 80 °C over night. The ethanol was removed in
vacuo and hydrochloric acid (1
N) was added until acidic. Dichloromethane was added, extracted 2x; the
combined organics were
127
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
dried over magnesium sulfate, filtered and concentrated to afford the
carboxylic acid (30 mg) after
HPLC purification.
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s),.2.3 (1H, m), 3.6 (2H,
s), 3.8 (3H, s), 4.8
(1H, m); 5.7 (1H, m), 6.5 (1H, m), 6.6 (2H, m), 6.9 (1H, m), 7.1-7.3 (8H, m).
MS m/z: 495 (M+23).
[00577] (~)-Cis-3-{acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-benzoic acid (B-40)
[00578] (~)-Cis-3-{acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-benzoic acid was made following the procedure for (~)-cis-(4-{acetyl-[1-
(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino}-phenyl)-acetic
acid, substituting 3-
cyanophenylboronic acid for 4-(phenylboronic acid)-acetonitrile.
[00579] Basic nitrite hydrolysis using 1N NaOH in methanol and water afforded
both the
carboxylic acid and the primary amide, (~)_cis-3-{Acetyl-[1-(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-amino}-benzamide.
'H-NMR (CDCI3) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 3.8 (3H,
s), 4.8 (1H, m), 5.6
(1H, m), 6.5 (1H, d), 6.6 (2H, d), 6.9 (2H, m), 7.1-7.5 (5H, m), 7.9-8.2 (2H,
m).
MS m/z: 481 (M+23).
[00580]~ (~)-Cis-3-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-benzamide (B-41)
'H-NMR (CDC13) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 3.8 (3H,
s), 4.8 (1H, m), 5.7
(1H, m), 6.5 (1H, m), 6.6 (2H, m), 6.9 (1H, m), 7.1-7.3 (4H, m), 7.4-7.6 (2H,
m), 7.7-7.8 (2H, m).
MS m/z: 480 (M+23).
[00581] (~)-Cis-N-(4-Chloro-phenyl)-N-[1-(isoxazole-5-carbonyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-44)
[00582] (~)-Cis-N-(4-Chloro-phenyl)-N-[1-(isoxazole-5-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting 5-
isoxazolecarbonyl chloride for 4-dimethylaminobenzoyl chloride and propionyl
chloride for acetyl
chloride.
'H-NMR (CDCI3) $: 1.14 (3H, d; overlapping 3H, t, and 1H, t), 2.30
(overlapping 2H, q; and 1H,
m),4.75 (1H, m), 5.45 (1H, m), 6.00 (1H, d), 6.80 (lH,d), 7.10-7.40 (7H, m),
8.05 (1H, s).
MS m/z: 424 (M + 1).
128
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00583] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-cyclopentyloxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-45)
[00584] (~)-Cis-N-(4--chloro-phenyl)-N-[1-(4-cyclopentyloxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared from (~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-hydroxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide. To a
solution of (~)-cis-N-(4-
Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-acetamide in
dimethylformamide was added cyclopentyl bromide, potassium carbonate (3.0
equiv), potassium
iodide (catalytic) and heated to 65°C overnight. Reaction mixture was
filtered for removal of
inorganic salts and concentrated. Crude mixture was purified by flash
chromatography on silica gel
using gradient.elution ethyl acetate- methanol ( 2-20% methanol)
'H-NMR (CDC13) 8:: 1.15 (3H, d; overlapping 1H, t), 1.57 (2H, m), 1.79 (3x2H,
m), 2.04(3H,s),
2.30(lH,m), 4.60- 4.80 (1H, q, 1H, m), 5.60 (1H, m), 6.50 (1H, d), 6.62 (1H,
d), 6.90 (1H, t), 7.10-
7.30 (9H, m), 7.40 (lH,d).
MS m/z: 504 (M + 1).
[00585] (~)-Cis-N-{1-[4-(4-Acetyl-piperazin-1-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (B-46)
[00586] (~)-Cis-N-{ 1-[4-(4-Acetyl-piperazin-1-yl)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide was made from (~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-
hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide. (~)-
Cis-N-(4-Chloro-
phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide (1.07 g,
2.39 mmol) was dissolved in pyridine (5 mL) and trifluoro-methanesulfonic
anhydride (703 uL, 2.5
mmol) was added. The reaction was stirred at room temperature 3 h. The
reaction was partitioned
between ether and water, and the aqueous was extracted three times with ether.
The combined
extracts were dried over sodium sulfate, filtered and concentrated. The crude
triflate was purified by
silica gel chromatography (70/30 hexanes/ethyl acetate - 40/60 hexanes/ethyl
acetate gradient) to
afford (1.0g 74%) of pure material.
[00587] To the triflate, Pd2(dba)3, BINAP, cesium carbonate, 18-crown-6 ether
in toluene was
added N-acetyl piperazine and reaction mixture was heated to reflux for 18
hours. Reaction mixture
was cooled to room temperature and filtered through Celite ~ and concentrated.
Crude mixture was
purified by flash chromatography on silica gel using gradient elution ethyl
acetate-methanol (2-20%).
IH-NMR (CDCl3) 8: 1.13 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.10 (3H, s),
2.35 (1H, m),
3.20(2x2H,m), 3.60 (2H, t), 3.70 (2H, t), 4.80 (1H, m), 5.65 (1H, m), 6.55
(1H, d), 6.70 (1H, d), 6.95
(1H, t), 7.10-7.40 (9H, m).
MS m/z: 546 (M + 1).
129
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00588] (~)_Cis-N-(3-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (B-50)
[00589] (~)-Cis-N-(3-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
4-
fluorobenzoylchloride for 4-dimethylaminobenzoyl chloride and 3-
chlorophenylboronic acid for 4-
chlorophenylboronic acid.
'H-NMR (CDC13) 8: 1.16-1.26 (4H, m), 2.05 (3H, s), 2.25 - 2.39 (1H, m), 4.69 -
4.88 (1H, m), 5.47 -
5.68 (1 H, broad), 6.49 (1 H, d), 6.84-6.97 (4 H, m), 7.18 - 7.42 (7 H, m).
MS m/z 437(M+), 439(M+2).
[00590] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
phenoxy-phenyl)-acetamide (B-51)
[00591] (~)-Cis-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
phenoxy-phenyl)-acetamide was made following general procedure B, substituting
4-
fluorobenzoylchloride for 4-dimethylaminobenzoyl chloride and 4-
phenoxyphenylboronic acid for 4-
chlorophenylboronic acid.
1H-NMR (CDC13) 8: 1.16-1.18 (4H, m), 2.06 (3H, s), 2.34-2.38 ( 1H, m), 4.74-
4.82 (1H, m), 5.29
(1H, br), 6.47 (1H, d), 6.83-7.40 (16H, m).
MS m/z: 496 (M+1).
[00592] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,Z,3,4-tetrahydro-quinolin-
4-yl]-N-
pyridin-2-yl-acetamide (B-52)
[00593] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3~4-tetrahydro-quinolin-
4-yl]-N-pyridin-
2-yl-acetamide was made following general procedure B, substituting 3-methoxy
benzoyl chloride for
4-dimethylaminobenzoyl chloride and synthesis of the N-pyridinyl instead of
the 4-chlorophenyl was
accomplished using the following procedure.
[00594] Pdz(dba)3 (0.05 equ.), and rac-BINAP (0.1 equ.) were added to a flask
with degassed .
toluene and stirred for 1h. To the above solution was added 2-bromopyridine
(1.1 equ.) and Na0'Bu
(1.1 equ.) and stirred for 30 min. (~)-Cis-(4-amino-2-methyl-3,4-dihydro-2H-
quinolin-1-yl)-(3-
methoxy-phenyl)-methanone was dissolved in degassed toluene and added to the
solution and heated
to 100 °C for 17h. The reaction was diluted with ether and filtered
through celite and concentrated
down. The compound was purified by Biotage with 20% EtOAc/80% Hexane to 30%
EtOAc/70
%Hexane to 50 % EtOAc/50% Hexane to give 43% of the product. (~)-Cis-(3-
methoxy-phenyl)-[2-
methyl-4-(pyridin-2-ylamino)-3,4-dihydro-2H-quinolin-1-yl]-methanone was
acetylated with acetyl
130
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
chloride as previously described to give (~)-cis-N-[1-(3-methoxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-pyridin-2-yl-acetamide.
1H-NMR (CDCI3) 8: 1.16 (d, 3H), 1.24 (t, 1H), 2.02 (s, 3H), 2.43 (m, 1H), 3.61
(s, 3H), 4.81 (sextet,
1H), 5.65 (bs, 1H), 6.52 (d, 1H), 6.75 (s, 1H), 6.79 (d, 2H), 6.90 (t, 1H),
7.07 (t, 1H), 7.14 (t, 1H),
7.25-7.33 (m, 2H), 7.49 (d, 1H), 7.77 (t, 1H), 8.56 (s, 1H).
MS m/z: 416.0 (M+1).
[00595] (~)-Cis-N-cyclohexyl-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (B-53)
[00596] (~)-Cis-N-cyclohexyl-N-[1-(3-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-acetamide was made following general procedure B, substituting 3-methoxy
benzoyl chloride for
4-dimethylaminobenzoyl chloride and synthesis of the N-cyclohexyl instead of
the 4-chlorophenyl
was accomplished using the following procedure.
[00597], . (~)-Cis-(4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-yl)-(3-methoxy-
phenyl)-
methanone (1.0 equ.) , and cyclohexanone (1.0 equ.) were dissolved in ethanol
and a catalytic amount
of acetic acid was added. The reaction was stirred for ~30 minutes and NaBH4
(1.0 equ.) was added
and stirred for an additional 2 h at room temperature. Additional NaBH4 was
added (1.0 equ.) and
stirred for an additional 12h. The reaction was concentrated down and
partitioned between CHZC12
and 1N NaOH. The organics were separated and dried over Na2S04, filtered and
concentrated down:
The compound was purified by Biotage with 30% EtOAc/70% hexane to 50 %
EtOAc/50% hexane
give 96 % of the product. Cis-(~)-N-(4-cyclohexylamino-2-methyl-3,4-dihydro-2H-
quinolin-1-yl)-(3-
methoxy-phenyl)-methanone was acetylated with acetyl chloride as previously
described to give cis-
(~)-N-cyclohexyl-N-[1-(3-methoxy-benzoyl)-2-methyl-1,,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide.
1H-NMR (CDCl3) 8: 1.1-1.45 (m, 6H), 1.5-1.75 (m, 3H), 1.85-2.1 (m, 3H), 2.3
(s, 3H), 2.4 (m, 1H),
2.7 (m, 1H), 3.5 (q, 1H), 3.63 (s, 3H), 3.7 (m, 1H), 4.3 (dd, 1H), 4.90
(sextet, 1H), 6.6 (t, 1H), 6.7 (d,
1H)< 6.8 (s, 1H), 6.85 (m, 2H), 7.0 (m,.3H).
MS m/z: 421 (M+1).
[00598] (~)-Cis-N-(5-chloro-pyridin-2-yl)-N-[1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (S-54)
[00599] (~)-Cis-N-(5-chloro-pyridin-2-yl)-N-[1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure B,
substituting 3-methoxy
benzoyl chloride for 4-dimethylaminobenzoyl chloride and addition of the N-4-
chloropyridinyl
instead of the 4-chlorophenyl was accomplished using the following procedure
[00600] To a flask was added Pd2(dba)3 (molar 0.05 equ.), and rac-BINAP (0.1
equ.) in degassed
toluene and stirred for 1h. To the above solution was added 2,5-
dichloropyridinepyridine (1.1 equ.)
131
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and Na0'Bu (1.1 equ.) and stirred for 30 min. The corresponding amine, (~)-cis-
(4-amino-2-methyl-
3,4-dihydro-2H-quinolin-1-yl)-(3-methoxy-phenyl)-methanone was dissolved in
degassed toluene and
added to the solution and heated to 60 °C for 40 h. The reaction was
diluted with ether and filtered
through celite and concentrated down. The compound was purified by Biotage
with 20% EtOAc/80%
Hexane to give 45 % of the product. (~)-Cis-[4-(5-chloro-pyridin-2-ylamino)-2-
methyl-3,4-dihydro-
2H-quinolin-1-yl]-(3-methoxy-phenyl)-methanonewas acetylated with propionyl
chloride as
previously described to give (~)-cis-N-(5-chloro-pyridin-2-yl)-N-[1-(3-methoxy-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide.
'H-NMR (CDC13) S: 1.14 (t, 3H), 1.15 (d, 3H), 1.22 (m, 1H), 2.31 (m, 3H), 4.79
(sextet, 1H), 5.64
(bs, 1H), 6.44 (d, 1H), 6.81-6.92 (m, 3H), 7.10-7.22 (m, 4H), 7.43 (d, 1H),
7.72 (dd, 1H), 8.50 (d,
1H).
.MS m/z: 452 (M+1).
[00601] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,5-dimethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-55)
[00602] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,5-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
3-toluidine for aniline
and 4-methoxybenzoyl chloride for 4-dimethylaminobenzoyl chloride. The
reaction was non-
selective and also (~)-cis- N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,7-
dimethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was obtained in a 1:1 mixture with the
product.
'H-NMR (CDC13) 8: 1.07 (d, 3H), 1.25 (t, 1H), 1.91 (s, 3H), 2.15 (m, 1H), 2.43
(s, 3H), 3.76 (s, 3H),
4.26 (sextet, 1H), 6.28 (d, 1H), 6.33 (t, 1H), 6.58 (t, 1H), 6.62 (d, 2H),
6.77 (t, 1H), 6.88 (d, 3H), 7.28
(m, 2H), 7.44 (d, 1H).
MS m/z: 463.0 (M+1 )
[00603] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,7-dimethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-56)
[00604] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,7-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
3-toluidine for aniline
and 4-methoxybenzoyl chloride for 4-dimethylaminobenzoyl chloride. The
reaction was non-
selective and also (~)-cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,5-
dimethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was obtained in a 1:1 mixture with the
titled compound.
[00605] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2,7-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was separated by chiral HPLC using a chiral cel OD
column and eluting
with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and (2S,4R)-cis-
N-(4-chloro-phenyl)-
132
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
N-[1-(4-methoxy-benzoyl)-2,7-dimethyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide (B-58 & B-57,
respectively).
1H-NMR (CDC13) 8: 1.13 (d, 3H), 1.26 (t, 1H), 2.03 (s, 3H), 2.05 (s, 3H), 2.27
(m, 1H), 3.76 (s, 3H),
4.75 (sextet, 1H), 5.59 (bs, 1H), 6.35 (s, 1H), 6.68 (d, 2H), 6.95 (d, 1H),
7.18 (m, 1H), 7.20 (d, 2H),
7.37 (d, 2H).
MS m/z: 463.5 (M+1)
[00606] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-59)
[00607] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-
anisidine for aniline, 4-fluorobenzoyl chloride for 4-dimethylaminobenzoyl
chloride and propionyl
chloride for acetyl chloride.
'H-NMR (300 MHz, CDCl3) 8: 1.08 -1.22 (m, 7 H), 2.09 - 2.38 (m, 3 H), 3.79 (s,
3 H), 4.77 (ddd, 1
H), 5.58 (br s, 1 H), 6.41- 6.50 (m, 2 H), 6.82 - 6.94 (m, 3 H), 7.16 - 7.32
(m, 4 H), 7.35 - 7.44 (m, 2
H).
MS m/z = 481 (M + 1 ).
[00608] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-hydroxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-60)
[00609] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-hydroxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made from (~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-fluoro-
benzoyl)-6-methoxy-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide
using the procedure
described previously for the preparation of (~)-cis-N-(4-chloro-phenyl)-N-[1-
(4-hydroxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide.
'H-NMR (300 MHz, CDCl3) 8: 1.04 -1.18 (m, 7 H), 2.07 - 2.41 (m, 5 H), 4.76
(ddd, 1 H), 5.50 (br s,
1 H), 6.27 (d, 1 H), 6.36 (d,1 H), 6.65 (s, 1 H), 6.70 - 6.91 (m, 3 H), 7.03 -
7.44 (m, 4 H).
MS m/z: 467 (M + 1).
[00610] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2,7-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide (B-61)
[00611] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2,7-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting 3-toluidine for
aniline, 4-fluorobenzoyl chloride for 4-dimethylaminobenzoyl chloride, and
propionyl chloride for
acetyl chloride.
133
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (300 MHz, CDCl3) 8: 1.10 (m, 7 H), 2.04 (s, 3H), 2.14 - 2.32 (m, 3 H),
4.74 (ddd, 1H), 5.57
(br s, l H), 6.26 (s, 1 H), 6.81- 6.98 (m, 4 H), 7.11- 7.33 (m, 4 H), 7.31-
7.43 (m, 2 H).
MS m/z: 465 (M + 1).
[00612] (~)-Cis-[4-[(4-Chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-
methyl-
1,2,3,4-tetrahydro-quinolin-6-yloxy]-acetic acid methyl ester (B-62)
[00613] (~)-Cis-[4-[(4-Chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-6-yloxy]-acetic acid methyl ester was made from (~)-cis-N-
(4-chloro-phenyl)-N-
[1-(4-fluoro-benzoyl)-6-hydroxy-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide following
the phenol alkylation procedure used to make (~)-cis-4-(4-{4-[(4-chloro-
phenyl)-propionyl-amino]-2-
methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyric acid ethyl ester.
Methyl
bromoacetate was substituted for ethyl-4-bromobutyrate.
1H-NMR (300 MHz, CDC13) 8: 1.07 -1.22 (m, 7 H), 2.10 - 2.38 (m, 3 H), 3.80 (s,
2 H), 4.58 (s, 3 H),
4.75 (m, 1 H), 5.54 (br s, 1 H), 6.39 (m, 2 H), 6.81 - 6.94 (m, 3 H), 7.18 -
7.35 (m, 5 H, 7.36 - 7.44
(m, 2 H).
MS m/z: 539 (M + 1).
[00614] (~)-Cis-N-(4-Chloro-phenyl)-N-[6-(2-diethylamino-ethoxy)-1-(4-fluoro-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-63)
[00615] (~)-Cis-N-(4-Chloro-phenyl)-N-[6-(2-diethylamino-ethoxy)-1-(4-fluoro-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide was made from (~)-cis-N-
(4-chloro-phenyl)-
N-[1-(4-fluoro-benzoyl)-6-hydroxy-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
propionamide
following the phenol alkylation procedure used to make (~)-cis-4-(4-{4-[(4-
chloro-phenyl)-propionyl-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyric acid
ethyl ester. . (2-
Bromo-ethyl)-diethyl-amine was substituted for ethyl-4-bromobutyrate.
'H-NMR (300 MHz, CDC13) 8: 0.95 - 1.11 (m, 13 H), 2.09 - 2.38 (m, 3 H), 2.51 -
2.77 (m, 4 H),
2.79 - 2.92 (m, 2 H), 3.86 - 4.08 (m, 2 H), 4.76 (ddd, 1 H), 5.58 (br s, l H),
6.34 - 6.51 (m, 2 H), 6.78
- 6.94 (m, 3 H), 7.14 - 7.31 (m, 4 H), 7.37 - 7.42 (m, 2 H).
MS m/z: 566 (M + 1).
[00616] (~)-Cis-2-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-6-yloxy]-2-methyl-propionic acid ethyl ester (B-
64)
[00617] (~)-Cis-2-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-6-yloxy]-2-methyl-propionic acid ethyl ester was made from
(~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-fluoro-benzoyl)-6-hydroxy-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-propionamide
134
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
following the phenol alkylation procedure used to make (~)-cis-4-(4-{4-[(4-
chloro-phenyl)-propionyl-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-butyric acid
ethyl ester. 2-Bromo-
2-methyl-propionic acid ethyl ester was substituted for ethyl-4-bromobutyrate.
'H-NMR (300 MHz, CDC13) 8: 1.13 -1.28 (m, 10 H), 1.56 (s, 3 H), 1.58 (s, 3 H),
2.16 - 2.29 (m, 3
H), 4.73 (ddd, H), 5.56 (br s, 1 H), 6.31 - 6.39 (m, 2 H), 6.76 - 6.88 (m, 3
H), 7.16 - 7.22 (m, 4 H),
7.38 - 7.41 (m, 2 H).
MS m/z: 581 (M + 1).
[00618] (~)-Cis-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-
methyl-
1,2,3,4-tetrahydro-quinolin-6-yloxy]-acetic acid (B-65)
[00619] ~)-Cis-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-6-yloxy]-acetic acid was prepared from (~)-cis-[4-
[(4=chloro-phenyl)-propionyl-
amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-6-yloxy]-
acetic acid methyl ester.
To a solution of (~)-cis-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-6-yloxy]-acetic acid methyl ester (83 mg, 0.155
mmol) in methanol (3
mL) was added sodium hydroxide (1 M in water, 310 uL, 0.310 mmol). The
reaction was stirred at
room temperature 3 h and concentrated under reduced pressure to remove
methanol. The pH of the
remaining aqueous solution was adjusted to 6 with 1 M hydrochloric acid. The
suspension was
extracted twice with ethyl acetate. The combined extracts were washed with
brine, dried over sodium
sulfate, filtered and concentrated to afford the carboxylic acid (76 mg, 94
%).'
'H-IVMR (300 MHz, CDCl3) 8: 1.09 - 1.26 (m, 7 H), 2.08 - 2.1.8 (m, 3 H), 4.58
(AB q, 2 H), 4.79
(ddd, 1 H), 5.57 (br s, 1 H), 6.40 (m, 2 H), 6.86 (m, 3 H), 7.09 - 7.30 (m, 4
H), 7.35 - 7.46 (m, 2 H),
8.18 (br s, l H).
MS m/z: 523 (M -1).
[00620] (~)-Cis-2-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-6-yloxy]-2-methyl-propionic acid (B-66)
[00621] (~)-Cis-2-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-6-yloxy]-2-methyl-propionic acid was prepared from (~)-cis-
2-[4-[(4-chloro-
phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-6-yloxyJ-2-
methyl-propionic acid ethyl ester. The saponification conditions detailed in
the procedure for the
synthesis of (~)-cis-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-
benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-6-yloxy]-acetic acid were used.
'H-NMR (300 MHz, CDCl3) 8: 1.04 -1.21 (m, 7 H), 1.54 -1.66 (m, 6 H), 2.12 -
2.37 (m, 3 H), 4.77
(ddd, 1 H), 5.53 (br s, 1 H), 6.37 (d, 1 H), 6.48 (d, 1 H), 6.66 - 6.92 (m, 1
H), 7.12 - 7.26 (m, 4 H),
7.43 (m, 2 H), 9.00 (br s, 1 H).
135
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 553 (M + 1).
[00622] (~)-Cis-N-[6-carbamoylmethoxy-1-(4-tluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-(4-chloro-phenyl)-propionamide (B-67)
[00623] (~)-Cis-N-[6-carbamoylmethoxy-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-(4-chloro-phenyl)-propionamide was prepared from (~)-cis-4-
[(4-chloro-phenyl)-
propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-6-
yloxy]-acetic acid
methyl ester. To solid (~)-cis-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-
fluoro-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-6-yloxy]-acetic acid methyl ester (76 mg,
0.14 mmol) was added
a solution of ammonia in methanol (2 M, 10 mL). The resulting solution was
stirred over night at
room temperature and concentrated. The resulting crude amide was purified by
silica gel
chromatography (100 % hexanes - 100 % ethyl acetate gradient) to afford pure
product (59 mg, 76
%).
'H-NMR (300 MHz, CDC13) 8: 1.10 -1.23 (m, 7 H), 2.16 - 2.39 (m, 3 H), 4.44 (s,
2 H), 4/77 (ddd, 1
H), 5.56 (br s, 1 H), 6.25 (br s, 1 H), 6.40 - 6.62 (m, 3 H), 7.16 - 7.26 (m,
4 H), 7.35 - 7.48 (m, 2 H).
MS m/z: 524 (M + 1).
[00624] (~)-Cis-N-[6-Bromo-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
N-(4-chloro-phenyl)-propionamide (B-69)
[00625] (~)-Cis-N-[6-bromo-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-
(4-chloro-phenyl)-propionamide was made following general procedure B,
substituting 4-
bromoaniline for aniline and 4-fluorobenzoyl chloride for 4-
dimethylaminobenzoyl chloride and
propionyl chloride for acetyl chloride.
IH-NMR (CDCl3) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, m), 4.8 (1 H, m), 5.6 (1 H,
m), 6.4 (1 H, d), 6.9
(3 H,t),7.1 (l H,m),7.2(4H,m),7.4(3H,m).
MS m/z: 531 (M+2).
[00626] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-6-
morpholin-4-yl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-70)
[00627] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-6-
morpholin-4-yl-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made from (~)-cis-N-[6-bromo-1-(4-
fluoro-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-phenyl)-propionamide. (~)-
Cis-N-[6-bromo-1-
(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-
phenyl)-propionamide
was dissolved in toluene, followed by Pd2(dba)3, BINAP, sodium tert-butoxide,
and morpholine. The
reaction mixture was heated to 90 °C for 24 hours. The reaction mixture
was cooled to room
136
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
temperature and filtered through Celite ~ and concentrated. Crude mixture was
purified by flash
chromatography on silica gel using a gradient elution of hexane-ethylacetate
(10-50%).
1H-NMR (CDCI3) 8: 1.1-1.2 (7 H, m), 2.1-2.3 (3 H, m), 3.1 (4 H, t), 3.8 (4 H,
t), 4.8 (1 H, m), 5.6 (1
H,m),6.3(lH,d),6.4(lH,m),6.7(lH,s),6.9(3H,m),7.1-7.4 (S H, m).
MS m/z: 536 (M+1).
[00628] (~)-Cis- -(4-chloro-phenyl)-N-[6-diethylamino-1-(4-fluoro-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-71)
[00629] (~)-Cis-N-(4-chloro-phenyl)-N-[6-diethylamino-1-(4-fluoro-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made in the same way as (~)-cis-N-
(4-chloro-phenyl)-N-
[1-(4-fluoro-benzoyl)-2-methyl-6-morpholin-4-yl-1,2,3,4-tetrahydro-quinolin-4-
yl]-propionamide
except diethylamine was substituted for morpholine. The reaction was non-
selective and yielded (~)-
cis-N-[6-diethylamino-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-(4-
diethylamino-phenyl)-propionamide in addition to the titled compound.
1H-NMR (CDCI3) b: 1.1-1.3 (13 H, m),1.6 (1 H, m), 2.1-2.3 (3 H, m), 3.3 (4 H,
m), 4.7 (1 H, m), 5.6
(lH,m),6.2(lH,m),6.3(lH,m),6.5(lH,s),6.9(2H,m),7.3(4H,m),7.4(2H,m).
MS mlz: 523 (M+2).
[00630] (~)-Cis-N-[6-diethylamino-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N(4-diethylamino-phenyl)-propionamide (B-72)
[00631] (~)-Cis-N-[6-diethylamino-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N(4-diethylamino-phenyl)-propionamide was made in the same way as (~)-cis-
N-(4-chloro-
phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-6-morpholin-4-yl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
propionamide except diethylamine was substituted for morpholine. The reaction
was non-selective
and yielded (~)-cis-N-(4-chloro-phenyl)-N-[6-diethylamino-1-(4-fluoro-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide in addition to the titled compound.
1H-NMR(CDCl3)8: 1.1-1.3(l9H,m),2.3(3H,m),3.3(8 H,m),4.7(lH,m),5.6(lH,m),6.1 (1
H,m),6.2(lH,m),6.6(3H,m),6.9(lH,m),7.1(3H,m),7.3(2H,m).
MS m/z: 560 (M+2).
[00632] (~)-Cis-3-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-6-yl]-acrylic acid (B-73)
[00633] (~)-Cis-3-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-6-yl]-acrylic acid was made from (~)-cis-N-[6-bromo-1-(4-
fluoro-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-(4-chloro-phenyl)-propionamide. To
a solution of (~)-cis-
137
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
N-[6-bromo-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-
(4-chloro-phenyl)-
propionamide (250 mg, 0.47mmo1), TEA (0.2 ml, l.4mmo1), palladium acetate (11
mg, 0.047mmol),
1,3-Bis(diphenylphosphino)propane (39mg, 0.094mmol), in 10 ml DMF was added
0.13 ml methyl
acrylate (1.41mmol). The resulting reaction mixture was heated to 80 °C
overnight. The mixture was
filtered through celite and the filtrate was concentrated under vacuum. The
residue was purified by
silica gel chromatography, eluting with ethyl acetate-hexane (2:3) to give (~)-
cis-3-[4-[(4-chloro-
phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-6-yl]-acrylic
acid methyl ester (110 mg, 44%).
[00634] To a solution of (~)-cis-3-[4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-
fluoro-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-6-yl]-acrylic acid methyl ester (110 mg,
0.21mmol) in 4 ml
methanol was added 50 mg KzC03,(0.36mmol, in 2 ml water). The resulting
reaction mixture was
stirred at room temperature overnight. The methanol was removed under vacuum.
1M HCl was added
until the mixture was acidic. Dichloromethane (25 ml) was added. Organic layer
was dried with
magnesium sulfate. Dichloromethane was removed under vacuum. The residue was
purified by
HPLC to give 10 mg title compound.
'H-NMR (CDCl3) S: 1.0-1.2 (7 H, m), 2.4 (2 H, m), 2.5 (1 H, m), 3.3 (1 H, br),
4.8 (1 H, m), 5.6 (1 H,
m), 6.4 (1 H, d), 6.6 (1 H, d), 7.0 (2 H, t), 7.2-7.6 (9 H, m).
MS m/z: 522 (M+2).
[00635] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2,8-dimethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-74) ,
[00636] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2,8-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
2-toluidine for aniline
and 3-methoxybenzoyl chloride for 4-dimethylaminobenzoyl chloride.
1H-NMR (CDC13) 8: 1.11 (3H, d; overlapping 1H, t), 1.76 (3H,s), 2.00 (3H, s),
2.35 (1H, m),
3.55(3H,s), 5.00 (1H, m), 5.60 (1H, m), 6.65 (1H, s), 6.80 (1H, t), 6.85
(lH,t), 6.95 (1H, t), 7.15 (1H,
t), 7.25 (1H, t), 7.25-7.55 (6H, m)
MS m/z: 429(M + 1).
[00637] (~)-Cis-N-(4-Chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2,6-dimethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-75)
[00638] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2,6-dimethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure B, substituting
4-toluidine for aniline
and 3-methoxybenzoyl chloride for 4-dimethylaminobenzoyl chloride.
138
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) 8: 1.12 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.33-2.35
(3H,s; overlapping 1H,
m), 3.63 (3H,s), 4.80 (1H, m), 5.60 (1H, m), 6.44 (1H, d), 6.70-6.85 (3H,
complex), 7.05 (1H, t), 7.15
(1H, s), 7.25-7.55 (6H, complex).
MS m/z: 429(M + 1).
[00639] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2-methyl-6-
trifluoromethyl-
1,2,3,4-tetiahydro-quinolin-4-yl]-acetamide (B-76)
[00640] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(3-methoxy-benzoyl)-2-methyl-6-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure B, substituting 4-
trifluoromethylaniline for aniline and 3-methoxybenzoyl chloride for 4-
dimethylaminobenzoyl
chloride.
'H-NMR (CDCl3) 8: 1.15 (3H, d; overlapping 1H, t), 2.03 (3H, s), 2.38 (1H, m),
3.63(3H,s), 4.80
(1H, m), 5.60 (1H, m), 6.60 (1H, d), 6.70 (1H, d), 6.80 (lH,dd), 7.15 (1H, t),
7.25-7.40 (6H, m), 7:60
(1H, s).
MS m/z: 483 (M + 1).
[00641] (~)-Cis-N-(4-chloro-phenyl)-N-[6-methoxy-1-(3-methoxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-77)
[00642] ~)-Cis-N-(4-chloro-phenyl)-N-[6-methoxy-1-(3-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure B,
substituting 4-
methoxyaniline for aniline and 3-methoxybenzoyl chloride for 4-
dimethylaminobenzoyl chloride.
'H-NMR (CDC13) 8: 1.12 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.35 ( 1H,
m), 3.63 (3H, s), 3.76
(3H,s),.4.80 (1H, m), 5.60 (1H, m), 6.44 (1H, s), 6.70-6.95 (4H, complex),
7.15 (1H, t), 7.25-7:55
(6H,m).
MS m/z: 445 (M + 1).
[00643] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(thiophene-2-carbonyl)-6-
trifluoromethyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-78)
[00644] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(thiophene-2-carbonyl)-6-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure B, substituting 4-
trifluoromethylaniline for aniline and 2-thiophene carbonyl chloride for 4-
dimethylaminobenzoyl
chloride.
1H-NMR (CDC13) 8: 1.14 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.35 (1H, m),
4.80 (1H, m), 5.65
(1H, m), 6.65 (1H, d), 6.80 (lH,d), 7.00 (1H, d), 7.20 (overlapping 2xlH,d),
7.24-7.42 (3H, m), 7.60
(1H, s).
139
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 539 (M + 1).
[00645] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-6-
trifluoromethyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (E-79)
[00646] ~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(5-methyl-thiophene-2-
carbonyl)-6-
trifluoromethyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following
general procedure B,
substituting 4-trifluoromethylaniline for aniline and 5-methyl-2-thiophene
carbonyl chloride for 4-
dimethylaminobenzoyl chloride.
'H-NMR (CDC13) 8: 1.14 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.35 (1H, m),
2.40(3H,s), 4.80
(1H~ m), 5.65 (1H, m), 6.45 (1H, d), 6.55 (lH,d), 7.00 (1H, d), 7.20
(overlapping 2xlH,d), 7.24-7.42
(3H, m), 7.55 (1H, s).
MS m/z: 554 (M + 1).
[00647] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-7-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-80)
[00648] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-7-
trifluoromethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting 3-
trifluoromethylaniline for aniline, 4-fluorobenzoyl chloride for 4-
dimethylamin0benzoyl chloride and
propionyl chloride for acetyl chloride. A mixture of the 5 and 7 position
isomer was obtained.
'H-NMR (CDCl3) 8: 1.15 (3H, d; overlapping 1H, t), 2.20-2.40 (2H, q; 1H, m),
4.80 (1H, m), 5.65
(1H, m), 6.70 (1H, s), 6.95 (2xlH, t), 7.10-7.60 (8H, m)
MS m/z: 519 (M + 1).
[00649] (~)-Cis-N-[7-bromo-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-(4-chloro-phenyl)-acetamide (B-81)
[00650] (~)-Cis-N-[7-bromo-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-(4-chloro-phenyl)-acetamide was made following general procedure B,
substituting 3-
bromoaniline for aniline. A mixture of the 5 and 7 position isomer was
obtained.
[00651] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-isopropyl-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-82)
[00652] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-isopropyl-
2-methyl-
1;2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure B, substituting 3-
isopropylaniline for aniline. A mixture of the 5 and 7 position isomer was
obtained.
140
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 0.89 (2x3H, t), 1.15 (3H, d; overlapping 1H, t), 2.01 (3H,
s), 2.33 (1H, m),
2.60(1H, m), 2.87(2x3H, s), 4.80 (1H, m), 5.65 (1H, m), 6.40 (overlapping 1H,
s, 2H, d), 6.90 (1H, d),
7.10 (1H, d), 7.15-7.35 (5H, m) 7.40(lH,d).
MS m/z: 505 (M + 1).
[00653] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-7-
morpholin-
4-yl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-83)
[00654] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-7-
morpholin-4-
yl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made from (~)-cis-N-[7-
bromo-1-(4-
dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-
phenyl)-acetamide.
(~)-Cis-N-[7-bromo-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-(4-
chloro-phenyl)-acetamide was dissolved in toluene, followed by Pd2(dba)3,
BINAP, sodium tert-
butoxide, and morpholine. The reaction mixture was heated to 90°C for
24 hours. The reaction
mixture was cooled to room temperature and filtered through Celite ~ and
concentrated. Crude
mixture was purified by flash chromatography on silica gel using gradient
elution hexane-ethylacetate
(10-50%).
'H-NMR (CDCl3) 8: 1.11 (3H, d; overlapping.lH, t), 1.99 (3H, s), 2.33 (1H, m),
2.60-2.80 (2x2H,
m), 2.89(2x3H, ), 3.70(2x2H, m), 4.70 (1H, m), 5.60 (1H, m), 6.10 (1H, s),
6.44 (2xlH, d), 7.00-7.40
(8H, m).
MS m/z: 548 (M + 1).
[00655] (~)-Cis-N-[7-diethylamino-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-(4-diethylamino-phenyl)-acetamide (B-84)
[00656] (~)-Cis-N-[7-diethylamino-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-N-(4-diethylamino-phenyl)-acetamide was made in the same way as
(~)-cis-N-(4-
chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-7-morpholin-4-yl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide except diethylamine was substituted for morpholine.
The reaction was non-
selective and yielded (~)-cis-N-(4-chloro-phenyl)-N-[7-diethylamino-1-(4-
dimethylamino-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide in addition to the titled
compound.
'H-NMR (CDC13) 8: 0.78 (2x3H, t), 1.15 (overlapping 3H, d; 1H, t), 1.98 (3H,
s), 2.33 (1H, m),
2.87(2x3H, s), 2.90-3.10(2x2H, q), 4.70 (1H, m), 5.60 (1H, m), 5.90(1H, s),
6.46 (3xlH, d),7.00-7.40
(7H, m) .
MS m/z: 557(M + 1).
141
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00657] (~)-Cis-N-(4-chloro-phenyl)-N-[7-diethylamino-1-(4-dimethylamino-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-85)
[00658] (~)-Cis-N-(4-chloro-phenyl)-N-[7-diethylamino-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made in the same way as (~)-
cis-N-(4-chloro-
phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-7-morpholin-4-yl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-acetamide except diethylamine was substituted for morpholine. The reaction
was non-selective
and yielded (~)-cis-N-[7-diethylamino-1-(4-dimethylamino-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-N-(4-diethylamino-phenyl)-acetamide in addition to the titled
compound.
'H-NMR (CDCl3) 8:: 0.78 (2x3H, t), 1.15 (overlapping 2x3H, t; 3H, d; 1H, t),
2.00 (3H, s), 2.33
(1H, m), 2.76(2x3H, s), 2.80-3.00(2x2H, q), 3.24(2x2H, q), 4.60 (1H, m), 5.60
(1H, m), 5.90(1H, s),
6.46 (2xlH, d), 6.60 (1H, m), 6.90 (2xlH, d), 7.00-7.20 (6H, m).
MS m/z: 609(M + 1).
[00659] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-5-methoxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-86)
[00660] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-5-methoxy-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was prepared following general
procedure B, substituting 3-
anisidine for aniline, 4-fluorobenzoyl chloride for 4-dimethylaminobenzoyl
chloride and propionyl
chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.09-1.14 (6H, m), 1.50-1.66 (1H, m), 1.97-2.34 (3H, m),
3.83 (3H, s), 4.65 (1H,
q), 5.70-5.80 (1H, br), 6.08 (1H, d), 6.68 (1H, d). 6.81-6.89 (3H, m), 7.14-
7.18 (4H, m), 7.33-7.36
(2H, m).
MS m/z: 481 (M+1).
[00661] (~)-Cis-2,2-dimethyl-propionic acid 4-[acetyl-(4-chloro-phenyl)-
amino]=1-(4-
dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-7-yl ester (B-87)
[00662] (~)-Cis-2,2-dimethyl-propionic acid 4-[acetyl-(4-chloro-phenyl)-amino]-
1-(4-
dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-7-yl ester was
prepared following
general procedure B, substituting 2,2-dimethyl-propionic acid 3-amino-phenyl
ester for aniline.
1H-NMR (CDC13) 8: 1.11-1.25 (13H, m), 2.02 (3H, s), 2.20-2.40 (1H, m), 2.92
(6H, s), 4.60-4.72 (1H,
m), 5.45-5.55 (1H, br), 6.26 (1H, s), 6.46 (2H, d), 6.85 (1H, d), 7.09-7.39
(7H, m).
MS m/z: 562 (M+1).
[00663] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-hydroxy-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-88)
142
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00664] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-hydroxy-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made from (~)-cis-2,2-dimethyl-
propionic acid 4-
[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
7-yl ester. (~)-Cis-2,2-dimethyl-propionic acid 4-[acetyl-(4-chloro-phenyl)-
amino]-1-(4-
dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-7-yl ester (100
mg, 0.178 mmol) was
dissolved in tetrahydrofuran and sodium hydroxide (1 M, 356 uL, 0.356 mmol)
was added. The
mixture was stirred at room temperature 4 hours, then heated at reflux 2 h.
The mixture was cooled to
rt, acidified, concentrated and purified by silica gel chromatography (20 mg,
23 %).
1H-NMR (MeOD) 8: 1.06-1.08 (4H, m), 2.00 (3H, s), 2.35-2.45 (1H, m), 2.93 (6H,
s), 4.65-4.68 (1H,
m), 5.42-5.50 (1H, br), 6.07 (1H, s), 6.53 (2H, d), 6.63 (1H, d), 7..10-7.20
(3H, m), 7.35-7.48 (4H, m).
MS m/z:478 (M+1).
[00665] (~)-Cis-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetic acid ethyl ester (B-89)
[00666] (~)-Cis-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetic acid ethyl ester was prepared from
(~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-hydroxy-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
acetamide, following the alkylation conditions described for the synthesis of
(~)-cis-4-(4-{4-[(4-
chloro-phenyl)-propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenoxy)-butyric
acid ethyl ester, substituting ethyl bromoacetate for ethyl 4-bromobutyrate.
'H-NMR (MeOD) 8: 1.10-1.38 (7H, m), 2.00 (3H, s), 2.39-2.45 (1H, m)~.2.94 (6H,
s), 4.04-4.20 (2H,
m), 4.29 (2H, s), 4.60-4.75 (1H, m), 5.40-5.50 (1H, br), 6.16 (1H, s), 6.54
(2H, d), 6.79 (1H, d), 7.08
(2H, d), 7.20-7.48 (5H, m).
MS m/z:564 (M+1).
[00667] (~)-Cis-2-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetamide (B-90)
[00668] (~)-Cis-2-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetamide was prepared from (~)-cis-[4-
[acetyl-(4-chloro-
phenyl)-amino]-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-7-yloxy]-acetic
acid ethyl ester, via the same amidation procedure used in the synthesis of
(~)-cis-N-[6-
carbamoylmethoxy-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-chloro-
phenyl)-propionamide.
143
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (MeOD) 8: 1.09-1.15 (4H, m), 2.00 (3H, s), 2.39-2.45 (1H, m), 2.94 (6H,
s), 4.04-4.20 (2H,
m),4.60-4.75 (1H, m), 5.40-5.50 (1H, br), 6.14 (1H, s), 6.53 (2H, d), 6.81
(1H, d), 7.09 (2H, d), 7.20-
7.48 (5H, m).
MS m/z:535 (M+1).
[00669] (~)-Cis-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetic acid (B-91)
[00670] (~)-Cis-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-acetic acid was prepared from (~)-cis-[4-
[acetyl-(4-chloro-
phenyl)-amino]-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-7-yloxy]-acetic
acid ethyl ester following the saponification procedure described above for
the synthesis of (~)-cis-[4-
[(4-chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-6-
yloxy]-acetic acid.
1H-NMR (MeOD) 8: 1.08-1.10 (4H, m), 1.98 (3H, s), 2.39-2.45 (1H, m), 2.93 (6H,
s), 4.20 (2H, s),
4.61-4.70 (1H, m), 5.40-5.50 (1H, br), 6.17 (1H, s), 6.53 (2H, d), 6.79 (1H,
d), 7.08 (2H, d), 7.28-7.48
(5H, m).
MS m/z:536 (M+1).
[00671] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-(2-
hydroxy-2-
methyl-propoxy)-2-methyl-1;2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-92)
[00672] ~)_Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-(2-hydroxy-
2-methyl-
propoxy)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was prepared
from (~)-cis-[4-[acetyl-
(4-chloro-phenyl)-amino]-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-7-
yloxy]-acetic acid ethyl ester was using the same alkylation procedure
described for the synthesis of
(~)-cis-N-{ 1-[4-(2-hydroxy-2-methyl-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl }-
N-phenyl-propionamide. .
1H-NMR (CDC13) 8: 1.01-1.20 (4H, m), 1.30 (6H, s), 2.01 (3H, s), 2.20-2.40
(1H, m), 2.92 (6H, s),
3.70 (2H, s), 4.65-4.72 (1H, m), 5.45-5.55 (1H, br), 6.13 (1H, s), 6.45 (2H,
d), 6.65 (1H, d), 7.12-7.46
(7H, m).
MS m/z:551 (M+1).
[00673] (~)-Cis--N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-ethoxy-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (B-93)
[00674] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-7-ethoxy-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made from (~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-
144
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
dimethylamino-benzoyl)-7-hydroxy-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide using the
same alkylation procedure described for the synthesis of (~)-cis-4-(4-{4-[(4-
chloro-phenyl)-
propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-
butyric acid ethyl ester,
substituting ethyl iodide for ethyl-4-bromobutyrate.
'H-NMR (CDC13) 8: 1.01-1.20 (7H, m), 2.01 (3H, s), 2.20-2.40 (1H, m), 2.92
(6H, s), 3.60 (2H, q),
4.65-4.72 (1H, m), 5.45-5.55 (1H, br), 6.15 (1H, s), 6.44 (2H, d), 6.69 (1H,
d), 7.11-7.46 (7H, m).
MS m/z: 506 (M+1).
[00675] (~)-Cis-4-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-7-yloxy]-butyric acid ethyl ester (B-94)
[00676] (~)-Cis-4-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-butyric acid ethyl ester was made from
(~)-cis-N-(4-chloro-
phenyl)-N-[1-(4-dimethylamino-benzoyl}-7-hydroxy-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
acetamide using the same alkylation procedure described for the synthesis of
(~)-cis-4-(4-{4-[(4-
chloro-phenyl)-propionyl-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenoxy)-butyric
acid ethyl ester.
'H-NMR (CDCl3) S: 1.09-1.11 (4 H, m), 1.23 (3 H, t), 1.81-1.85 (2 H, m), 2.01
(3 H, s), 2.30-2.33 (3
H, m), 2.92 (6 H, s), 3.50-3.54 (1 H, m), 3.72-3.76 (1 H, m), 4.09 (2 H, q),
4.66-4.73 (1 H, m), 5.57-
~5.63(lH,m),6.14(lH,s),6.46(2H,d),6.68(lH,d),7.11-7.39 (7 H, m).
MS mlz: 593 (M+1).
[00677] (~)-Cis-4-[4-[acetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-7-yloxy]-butyric acid (S-95)
[00678] (~)-Cis-4-[4-[aeetyl-(4-chloro-phenyl)-amino]-1-(4-dimethylamino-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-7-yloxy]-butyric acid was made from (~)-cis-4-[4-
[acetyl-(4-chloro-
phenyl)-amino]-1-(4-dimethylamino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-7-yloxy]-butyric
acid ethyl ester following the saponification conditions described for the
synthesis of (~)-cis-[4-[(4-
chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-6-
yloxy]-acetic acid.
'H-NMR (CDC13) 8: 1.08-1.11 (4H, m), 1.80-1.86 (2H, m), 1.99 (3H, s), 2.28-
2.35 (3H, m), 2.89 (6H,
s) 3.37-3.46 (1H, m), 3.66-3.73 (1H, m), 4.64-4.72 (1H, m), 5.54-5.63 (1H, m),
6.07 (1H, s), 6.52
(2H, d), 6.67 (1H, d), 7.08-7.36 (7H, m).
MS mlz: 564 (M+1).
145
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00679] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2,7-
dimethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-96)
[00680] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2,7-
dimethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure B,
substituting 3-toluidine
for aniline. Both the 5 and 7-position isomers were obtained in this
procedure.
1H-NMR (CDC13) 8: 1.11 (3H, d), 1.45-1.59 (4H, m), 2.02-2.07 (3H, m), 2.24-
2.28 (1H, m), 2.92
(6H, s) 4.67-4.74(1H, m), 5.52-5.59 (lH,m), 6.43-6.45 (3H, m), 6.95 (1H, d),
7.13-7.22 (6H, m), 7.35-
7.43 (1H, m).
MS m/z: 307 (M).
[00681] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-phenethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide (B-97)
[00682] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-phenethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-fluorobenzoyl
chloride for' 4-dimethylaminobenzoyl chloride, 3-phenyl-propionaldehyde for
acetaldehyde and
propionyl chloride for acetyl chloride.
1H-NMR (CDC13) b: 1.16 (dt, 3H), 1.25 (m, 1H), 1.54 (m, 1H), 1.97 (m, 1H),
2.30 (m, 3H), 2.56 (t,
2H), 4.85 (sextet 1H), 5.66 (bs, 1H), 6.44 (d, 1H), 6.86 (t, 2H), 6.93 (m,
2H), 7.03 (d, 2H), 7.12-7.29
(m, 8H), 7.37 (d, 2H).
MS m/z: 542 (M+1).
[00683] (~)-Cis-N-(4-chloro-phenyl)-N-[2-(2-cyano-ethyl)-1-(4-fluoro-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-98)
[00684] (~)-Cis-N-(4-chloro-phenyl)-N-[2-(2-cyano-ethyl)-1-(4-fluoro-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-
fluorobenzoyl chloride for 4-dimethylaminobenzoyl chloride, 4-
oxobutyrylnitrile for acetaldehyde
and propionyl chloride for acetyl chloride.
'H-NMR (300 MHz, CDC13) 8: 1.19 -1.23 (m, 4 H), 1.65 -1.79 (m, 2 H), 2.07 -
2.57 (m, 5 H), 4.90
(ddd, 1 H), 5.61 (6r s, 1 H), 6.61 (d, l H), 6.86 (m, 2 H), 6.95 (dd, 1 H),
7.14 - 7.43 (m, 8 H).
MS m/z = 490 (M + 1).
[00685] (~)-Cis-N-[2-ethyl-1-(3-methoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-
phenyl-acetamide (B-99)
[00686] (~)-Cis-N-[2-ethyl-1-(3-methoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide was made following general procedure B, substituting 3-
methoxybenzoyl chloride for 4-
146
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
dimethylaminobenzoyl chloride, propionyl aldehyde for acetaldehyde and
phenylboronic acid for 4-
chlorophenylboronic acid.
'H-NMR (CDC13) S: 0.8 (3H, t),1.3 (2H, m), 1.6 (1H, m), 2.0 (3H, s), 2.3 (1H,
m), 3.7 (3H, s), 4.7
(1H, m), 5.7 (1H, m), 6.5 (1H, d), 6.7 (1H, s), 6.8 (2H, m), 6.9-7.4 (9H, m)
MS mlz: 429 (M+1).
[00687] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-phenyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-
phenyl-acetamide (B-100)
[00688] (~)-Cis-N-[1-(3-methoxy-benzoyl)-2-phenyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-phenyl-
acetamide was made following general procedure B, substituting 3-
methoxybenzoyl chloride for 4-
dimethylaminobenzoyl chloride, benzaldehyde for acetaldehyde and phenylboronic
acid for 4-
chlorophenylboronic acid.
'H-NMR (CDC13) 8: 1.5 (1H, m), 2.0 (3H, s), 2.5 (1H, m), 3.6 (3H, s), 5.7 (1H,
t) 5.8 (1H, m); 6.6
(1H, d), 6.9 (2H, m), 6.9-7.4 (15H, m).
MS m/z: 494 (M-18).
[00689] (~)-Cis-4-(acetyl-phenyl-amino)-1-(4-fluoro-benzoyl)-1,2,3,4-
tetrahydro-quinoline-2-
carboxylic acid ethyl ester (B-101)
[00690] (~)-Cis-4-(acetyl-phenyl-amino)-1-(4-fluoro-benzoyl)-1,2,3,4-
tetrahydro-quinoline-2-
carboxylic acid ethyl ester was made following general procedure B,
substituting 4-fluorobenzoyl
chloride for 4-dimethylaminobenzoyl chloride, ethyl glyoxylate for
acetaldehyde and phenylboronic
acid for 4-chlorophenylboronic acid.
'H-NMR (CDC13) 8: 1.2 (3H, t), 1.2 (1H, m), 2.0 (3H, s)~ 2.5 (1H, m), 4.1 (2H,
q), 5.0 (1H, t), 5.7
(1H, m), 6.6 (1H, d), 6.8-7.0 (4H, d), 7.1-7.4 (8H, m).
MS m/z: 461 (M+1).
[00691] (~)-Cis-4-(acetyl-phenyl-amino)-1-(4-fluoro-benzoyl)-1,2,3,4-
tetrahydro-quinoline-2-
carboxylic acid (B-102)
[00692] (~)-Cis-4-(acetyl-phenyl-amino)-1-(4-fluoro-benzoyl)-1,2,3,4-
tetrahydro-quinoline-2-
carboxylic acid was made from (~)-cis-4-(acetyl-phenyl-amino)-1-(3-methoxy-
benzoyl)-1,2,3,4-
tetrahydro-quinoline-2-carboxylic acid ethyl ester by basic hydrolysis with 1N
sodium hydroxide,
ethanol and water.
'H-NMR (CDC13) 8: 1.2 (1H, m), 2.0 (3H, s), 2.6 (1H, m), 5.0 (1H, t), 5.6 (1H,
m), 6.6 (1H, d), 6.9-
7.0 (3H, m), 7.2 (2H, m), 7.3-7.5 (7H, m).
MS m/z: 433 (M+1).
147
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00693] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-propyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide (B-103)
[00694] (~)_Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-propyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting 4-fluorobenzoyl
chloride for 4-dimethylaminobenzoyl chloride, butyryl aldehyde for
acetaldehyde and propionyl
chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 0.8 (3H, t), 1.1-1.2 (7H, m), 1.4 (1H, m), 2.1-2.3 (3H, m),
4.8 (1H, m), 5.6 (1H,
m), 6.7 (1H, d), 6.9-7.1 (4H, m), 7.2-7.5 (7H, m).
MS m/z: 479 (M+1).
[00695] (~)-Cis-propionic acid 4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-
fluoro-benzoyl)-
1,2,3,4-tetrahydro-quinolin-2-ylmethyl ester (B-104)
[00696] (~)-Cis-propionic acid 4-[(4-chloro-phenyl)-propionyl-amino]-1-(4-
fluoro-benzoyl)-
1,2,3,4-tetrahydro-quinolin-2-ylmethyl ester was prepared following general
procedure B, substituting
propionic acid 2-oxo-ethyl ester for acetaldehyde, 4-fluorobenzoyl chloride
for 4-
dimethylaminobenzoyl chloride and propionyl chloride for acetyl chloride.
1H-NMR (CDC13) 8: 0.8 (3H, t), 1.1 (3H, t), 1.1 (1H, m), 2.1 (2H, m), 2.2 (3H,
s), 3.8 (1H, m), 4.2
(1H, m), 5.0 (1H, m), 5.4 (1H, m), 6.4 (1H, d), 6.8 (3H, m), 7.1-7.4 (8H, m).
MS m/z: 523 (M+1).
[00697] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-hydroxymethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-105)
[00698] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-hydroxymethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was prepared from (~)-cis-propionic
acid 4-[(4-chloro-
phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-1,2,3,4-tetrahydro-quinolin-2-
ylmethyl ester using the
saponification conditions utilized in the synthesis of (~)-cis-[4-[(4-chloro-
phenyl)-propionyl-amino]-
1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-6-yloxy]-acetic
acid.
'H-NMR (CDCl3) 8: 1.1 (3H, t), 1.3 (1H, m), 1.8 (1H, m), 2.1 (2H, m), 3.4 (1H,
t), 3.6 (2H, m), 4.2
(1H, m), 6.2 (1H, m), 6.4 (1H, d), 6.7 (2H, t), 6.8-7.0 (5H, m), 7.1-7.3 (4H,
m).
MS mlz: 367 (M-99).
[00699] (~)-Cis-N-(4-chloro-phenyl)-N-[2-diethylaminomethyl-1-(4-fluoro-
benzoyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-106)
148
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00700] (~)-Cis-N-(4-chloro-phenyl)-N-[2-diethylaminomethyl-1-(4-fluoro-
benzoyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was made following general procedure B,
substituting
diethylamino-acetaldehyde for acetaldehyde, 4-fluorobenzoyl chloride for 4-
dimethylaminobenzoyl
chloride, and propionyl chloride for acetyl chloride.
'H-NMR (CDC13) S: 0.8 (6H, m),1.1 (3H, t), 1.1 (1H, m), 1.8 (2H, m), 2.2-2.5
(6H, m), 2.6 (1H, m),
4.8 (1H, m), 5.7 (1H, m), 6.4 (1H, d), 6.9 (3H, m), 7.1-7.4 (8H, m).
MS m/z: 523 (M+2).
[00701] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methoxymethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide (B-107)
[00702] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methoxymethyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-propionamide was prepared' following general
procedure B, substituting
methoxyacetaldehyde for acetaldehyde, 4-fluorobenzoyl chloride for 4-
dimethylamino-benzoyl
chloride, and propionyl chloride for acetyl chloride.
'H-NMR (CDCl3) 8: 1.1 (3H, t), 1.3 (1H, m), 1.8 (1H, m), 2.1 (1H, m), 3.4 (4H,
m), 3.6 (2H, m), 4.2
(1H, m), 6.3 (1H, m), 6.5 (1H, d), 6.7 (1H, m), 6.8-7.0 (4H, m), 7.1-7.4 (6H,
m).
MS m/z: 381 (M-99).
[00703] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-phenyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide (B-108)
[00704] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-phenyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-propionamide was made following general procedure B,
substituting benzaldehyde for
acetaldehyde, 4-fluorobenzoyl chloride for 4-dimethylaminobenzoyl chloride,
and propionyl chloride
for acetyl chloride.
'H-NMR(CDCl3)8:1.1-1.2 (3 H, m), 1.2-1.4(lH,m),2.2-2.4 (2 H,m),2.4-2.6 (l
H,m),5.6(1
H,t),5.8(lH,m),6.6(lH,d),6.8(2H,m),7.0(lH,m),7.2-7.4 (l3 H, m).
MS m/z: 513 (M + 1).
[00705] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-109)
[00706] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide was prepared following general
procedure B,
substituting N-(4-chloro-phenyl)-N-vinyl-propionamide for N-vinyl carbamic
acid benzyl ester and
trifluoroacetaldehyde for acetaldehyde in the synthesis of 11 and 4-
fluorobenzoyl chloride for 4-
dimethylaminobenzoyl chloride and propionyl chloride for acetyl chloride.
149
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) 8: 1.1-1.2 (3 H, m), 1.6 (1 H, br), 2.2 - 2.4 (3 H, m), 3.8 (3
H, s), 5.5 (1 H, m), 5.6
(lH,m),6.5(lH,s),6.8(lH,s),6.9(2H,t),7.1-7.3 (4 H,m),7.4(2H,d).
MS m/z: 535 (M+1).
[00707] (~)-Cis-N-(4-chloro-phenyl)-N-[6-methoxy-1-(3-methoxy-benzoyl)-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-110)
[00708] (~)-Cis-N-(4-chloro-phenyl)-N-[6-methoxy-1-(3-methoxy-benzoyl)-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide was made following the
procedure for the synthesis of
(~)-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-2-trifluoromethyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-propionamide, substituting 3-methoxybenzoyl chloride for 4-
fluorobenzoyl chloride.
IH-NMR (CDC13) 8: 1.1 - 1.2 (3 H, m), 1.6 (1 H, br), 2.2 - 2.4 (3 H, m), 3.7
(3 H, s), 3.8 (3 H, s); 5.5
lH,m),5.6(lH,m),6.5(2H,m),6.6(lH,m),6.8(3H,m),7.1(lH,t),7.2(2H,d),7.4(2H,d).
MS m/z: 547 (M+1).
9] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(furan-2-carbonyl)-6-methoxy-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide (B-111)
[00710] (~)-Cis-N-(4-chloro-phenyl)-N-[1-(furan-2-carbonyl)-6-methoxy-2-
trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide propionamide was made following
the procedure for
the synthesis of (~)-cis-N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-6-methoxy-
2-trifluoromethyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide, substituting 2-furoyl chloride
chloride for 4-
fluorobenzoyl chloride.
'H-NMR (CDC13) ~: 1.1- 1.2 (3 H,m), 1.6 (1 H, br), 2.2 - 2.4 (3 H, m), 3.8 (3
H, s), 5.4 (2 H, m), 6.0
(lH,m),6.3(lH,m),6.8(lH,m),6.9(lH,s),7.0(lH,m),7.2(2H,m),7.4(3H,m).
MS m/z: 507 ( M+1).
[00711] (~)-Cis-N-[2-benzyl-1-(4-fluoro-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-
chloro-phenyl)-propionamide (B-112)
[00712] (~)-Cis-N-[2-benzyl-1-(4-fluoro-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-(4-chloro-
phenyl)-propionamide was made following general procedure B, substituting
phenylacetaldehyde for
acetaldehyde, 4-fluorobenzoyl chloride for 4-dimethylamionbenzoyl chloride,
and propionyl chloride
for acetyl chloride.
'H-NMR (CDC13) 8: 1.14 (3 H, t), 2.05 - 2.52 (5 H, m), 3.18 - 3.24 (1 H, m),
4.89 - 4.93 (1 H, m) 5.45
- 5.55 (1 H, br), 6.46 (1 H, d), 6.83 - 7.37 (16 H, m).
MS m/z: 528 (M+1).
150
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00713] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(3-methyl-isoxazole-5-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (B-113)
[00714] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(3-methyl-isoxazole-5-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure B,
substituting 3-
methylisoxazole-5-carbonyl chloride for 4-dimethylamionbenzoyl chloride.
[00715] (~)-Cis-N-(4-chloro-phenyl)-N-[2-methyl-1-(3-methyl-isoxazole-5-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was separated by chiral HPLC using a
chiral cel OD column and
eluting with 90% hexane/10% ethanol isocratic system to give (2R,4S)- and
(2S,4R)-cis-N-(4-chloro-
phenyl)-N-[2-methyl-1-(3-methyl-isoxazole-5-carbonyl)-1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide
(B-42 & B-36, respectively)
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.2 (3H, s), 2.3 (1H,
m), 4.7 (1H, m), 5.4
(1H, m), 5.8 (1H, s), 6.8 (1H, d), 7.1-7.4 (7H, m).
MS m/z: 424 (M+1).
[00716] Compounds B-114- B-147 can be prepared by the schemes set forth in
Scheme 13 and 14
and by the general procedures B and others described herein. Those skilled in
the art will be able to
,recognize, or be able to ascertain, using no more than routine
experimentation, many equivalents to
the specific embodiments of the invention described herein.
151
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Table 2: Compounds Derived from General Procedure B
No: - . No. ructure
Jl
ructure
CI O
N
B-1 B-11
O~OMe
IS
CI~ O CI~ O
I-~N~ v I N~
B-2 I , N B-12
O I ~
OMe F
CI I % F3C
N~
B-3
B-13 i
N
O Ii
F
CI ~ O
\ I N~ CI~
B4
N B-14 N
O IS
O'
F I ~ CI ~
N i O
~N~
B-5 I , N B-15
O O
F O
CI~ 0~ CI ~
IJ~N I O
~N~
B-6 I ~ N B 15
, N
O OOH
CI~N~ CI~ O
V- V~N'~
B-7 I ~ N B-1 ~ ~ ,
N
O O~~
C1~ ~ CI~ O
N ~ai~
N'v
., B-18
N
I ~ O' O
O
CI~ ~ CI
V-N N
I ~ N B-19 I ~ N
~..
0 I ~ O
O
CI O CI~
N N
~
8-10 B-20
N N
C S O
S
152
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No: StrcrcfaraNo, 3Yructurg
..
~
'
N N
-21 I ~ 31
N N
o1
O I ~ ~
F ~~OH
O
CI~ ~ CI~
N
B-22 I , B-32 I N
N
p I~ I~ H
F
CI I ~ p
N~
B-23 \ I B-33
N
C IS
CI I ~ CI~ O
~ ~ N~
N
B-24 \ I B-34
N
~ ~
I S O
6
CI \ I Cy
~ N
N
B-25 ' B-35 ~~~.
~ I N o I,~
..,
s
p \
CI %/ CI \ I N
N"
B-26 w I N B-36 ~ I N "
p I ~ p I aN
F
CI~
N N
B-27 ~ I ",o B-37
N
~
~I ~
v _F '' ~ ~1
~O'~OH
CI \ I CI
N~
B-28 \ I B-38
N
\ / C
O S
CI \ I HOp I % N~
N~
B-29 I g-3g ~ I
N
O S p
\ / ' p
OH
c~H~ N
B-30 ~ B-40
0
153
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. SYiricture--'No 3Yructure
':
N~
I
B-41 B-51 I ~ N
o, I ,
F
CI \ I \ ~ O
~ ~N~
N
B-42 ~ I B-52
N ~
o IoN N
O
i
cl ~ ~N~
B-43 B-53 ~I
N
O
o ~
O I ~
i
CI
w I ~ CI~~i''N~~
N LJ-N
B-44 ~ I B-54 ' I
N
O ION C
CI~ p
~J-N'' ' N
B-45 I , N B-55 ~ I
N
~
o'
CI I ,
N
B-46 B-56 ~ I
N
N~NO
0~
CI~
~
B-47 I B-57 N
N
o~
N
O~OMe
cI~N~ ~N''
o CI
B-48 ~ .", B-56
N
~a, O
o~ I
OMe
c1 o
\ I N~ CI \ I N~
B-49 ~ I N B-59 ~ I ~
N
O
F
CI CI \ I N
B-50 ~ B-6p HO
N
~N ~
F O
~
F
154
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No' SYrticturra', N~.. , 5ti'uctore
~ " v
CI \ I N
~
N ,
I
-6'I ~ -71 N
w.N~
. J~N
~~
F ~F
CI~N~ , v I N
l N
~ ~'
8-62 ' B-72 ~
~ I
N N
~~ ~~ O I i
JJ
~
F F
N~ C ~ I N
B-63 J ~~ B-73 H I
I N
~
F I i
F
I CI I ~ N
B-64
N B-74 \ I
~~~ '~'~ N
~F y Ov
CI~N~ CI~N
~
B-65 No B-75
I ~
~0.
F
o.cl~N~ FFI I \ N
~
B-66 B-76 F \ I
N
F
p
CI'~ I
C ~ N
o ~N~
B-s7 "=N''''~ B-n ' \ I
F N I w .
i
cl~ ~ FI
N
B-68 o~ B-78 F \ I
f~~ N
F IS
OI ~
N
I ~ I ~ N
B-69 Br ~ B-79 p
I , ~I
N
N
I
F I
CI , I
N N
B-70 ~'N I B-80 F I
~ ~
F F
I ~ N
F I ~
F
I55
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'No. StructureNo. Structure
cl~ ~ c1
N N
B-81 B ~ I B-91 HO~. v I
N N
I~
N N
I
CI I % CI I N
N"
B-82 ~ I N B-92 lio~C. ~
I N
I ~ O I ~
' ~
N N
CI~N~ CI~N
J~ I~J
B-83 ~ ~ B-93 i.
o I
N Ni
CI I ~ N~
B-84 ~ \ B-94 ~o 0
I
N
I
CI I /
N
B-SS ~ v I B-95 Ho~~o
N
O I
~N'
CI~ ~ CI~
N N
g-86 Me0 g-96
C I
F I
N
I
CI I ~ CI ~ I
~ ~ N
N
B-87 ~ ~ B-97 I ~
N
I
I
F
CI I % CI \ I
~ N
N
B-BB H ~ I B98 I , N
N N
c~~~' I i F
~~~
~N~
Ct~a,
~N~ I ~ N
B-89 goo ~~ B-99 ~ I
~O~
I
N~ I \ N
a~
B-9O ,~ B-lOD ~ I
HzNO y
I N
I/
156
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. Shvature jVv. Strttcture
s I ~ CI i I
N N
B-101 ~pr B-111 ' ~I ~ F
~
O~ F
'V~~F
I N~ CI \ I
N
B-102 I ~ N OH B-112 I ~
N
I i F O
F
CI \ I N~ I N~
B-103 I ~ B-113
I I~N
F
CI \ I
N o
~
B-104 ~o~ B-114
~ I
o ~ crP
F
o
CI~N B
-105 I p 115
~oN
F -
CI I
N
B-106 I ~ J B-118
I
F
CI , I
N
B107 I ~ ~\ B-117
N
I
F
CI~
~
I~
B-108 N B-118
~N
O~F
CI N~/
B109 ~FF B-119
N
~
V 'F
Cl.n
1~N
cy
B-110 '-Ti 'T l B-120
FF
BEN
~O~
157
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~~ t~a~' ....;~I~.~trtictifrs~~~ ~,s . :" Na.~~~ . :_
stru~cure .r ,s'~~ ~ ~~.
4 B-121 B-131
Q
B-122 B-132
B-123 B-133
B-124 B-134
~b
B-125 B-135
B-126 B-136
B-127 B-137
Q
B-126 ~ B-136 0
B-129 B-139 0~
Q
B-130 B-140
15~
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~ ~ ~~d '~ ~'~~~ ~u " E f ~ ~ a
f ~ ~ ~~~ ~6'~,it ~ A.
~ ~~ ~ =S'~nlclare
k~:..',
~v~lre : ~
t ~ ~
a
pp
~
B-141
B-142 ~o~
8-143
8-144
B-145
B-146
Q~~
B-147
159
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Scheme 13
NHp 1: Boc2O NHBoc NaCN NHBoc 1. MeS03H N_ H~
j~OH OMs ----~ ' CN ~ CN
2. MsCI, TMEDA DMF, Bu4NBr ~ THF
(S) 16 1~ 2. NaOH 18
O
\ NHZ Cu(OAc)2, Pyr \ N HzSOr~/Hz0 \ ~NH2
/ B(OH)2 + RCN DM~ N '~~ Tol~ I / N 'CH3
18 H H
19 2
0
O
O 'I
CIO \ ~ \ ~ 1. NaBH4; MgC1~6H20 HN~ I \
N O ~ /
H ~ / 2. NCI, citric acid ~( \
LIOtBu H CHg ~''s,
21 H
22
(Exclusively cis)
[00717] Methanesulfonic acid 2-(S)-tert-butoxycarbonylamino-propyl ester (16)
[00718) To a room temperature solution of S-2-amino-propan-1-of (28.23 g,
0.375 mol) in ethyl
acetate (300 mL) was added BOC anhydride (86.13 g, 0.395 mol) dissolved in 30
mL of ethyl
acetate via an addition funnel (exothermic). The solution turns cloudy then
clear. The reaction
mixture was stirred for approximately 30 minutes. Tetramethylethylenediamine
(TMEDA) (59.6 mL,
0.395 mol) was added and the reaction mixture was cooled to approximately
0°C. Methanesulfonyl
chloride (30.6 mL, 0.395 mol) was added to the reaction mixture over a 30-
minute period. After
stirring for 2.5 hour at 0°C, during which time a white precipitate
formed. The reaction mixture was.
filtered and the filtrate was concentrated to'/a volume and poured into
hexanes (800mL) and rapidly
stirred. The mixture was cooled in an ice-bath for 2 h and then filtered to
give 82 g (86%) of
methanesulfonic acid 2-(S)-tert-butoxycarbonylamino-propyl ester.
tH NMR (300 MHz, CDC13) 51.23 (d, 3H), 1.44 (s, 9 H), 3.03 (s, 2 H), 3.96 (m,
1 H), 4.15 (rid, 1 H),
4.23 (rid, 1 H), 4.58 (bs, 1 H).
[00719] (S)-(2-Cyano-1-methyl-ethyl)-carbamic acid tert-butyl ester (17)
[00720] Sodium cyanide (48.928, 0.421 mol) was added to dimethylformamide
(DMF) (420 mL )
and the mixture was stirred at 35°C for 30 minutes. Tetrabutylammonium
bromide (5.228, 0.016 mol)
was added and the reaction mixture was stirred for an additional 2 h at
35°C. Methanesulfonic acid 2-
(S)-tert-butoxycarbonylamino-propyl ester (82.03 g, 0.324 mol) was added and
the reaction mixture
was stirred at 35°C overnight. Add an additional 5.228 of
tetrabutylammonium bromide (0.016 mol)
was added and stirred overnight at 35°C. The mixture was then
partitioned between 1200 mL water
160
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and 1600 mL of ethyl acetate. The resulting organic and aqueous phases were
separated and extracted
sequentially 2 times with 800 mL of ethyl acetate. The combined extracts were
washed 3 times with
500mL of water and a saturated solution of sodium chloride in water. The
organic layer was dried
over magnesium sulfate, filtered and concentrated to afford a solid in 84% of
(S)-(2-cyano-1-methyl-
ethyl)-carbamic acid tert-butyl ester.
[00721] (S)-3-Amino-butyronitrile (18)
[00722] To a solution of (S)-(2-cyano-1-methyl-ethyl)-carbamic acid tert-butyl
ester (50.29 g,
0.273 mol) dissolved in THF (550 mL) was added methanesulfonic acid (44 mL,
0.682 mol) and
stirred for 20 minutes. The reaction mixture was heated to 65°C for
approximately 3 h (make sure the
reaction is vented during this time). The mixture was allowed to cool to
ambient temperature. The
resulting solids were isolated by filtration to afford the title compound. The
solids were suspended in
dichloromethane, and 300 mL of sat. Na2C03 and the pH was adjusted to 13 with
6M NaOH (~20
mL). Extract 2X 500mL dichloromethane. Combine the organics and wash with a
saturated solution
of sodium chloride in water. The organic layer was dried over sodium sulfate,
filtered and
concentrated to give (S)-3-amino-butyronitrile in 64% yield.
'H NMR (300 MHz, CDCl3) 51.23 (d, 3 H),1.46 (bs, 2 H), 2.34 (dd, 1 H), 2.43
(dd, 1 H), 3.34 (sextet,
1 H). ,
[00723] (S)-3-Phenylamino-butyronitrile (19)
[00724] (S)-3-Amino-butyronitrile (2.518, 0.030 mol) was dissolved in 40 mL of
DMF, phenyl
boronic acid (4.73g, 0.0389 mol), Cu(OAc)2 (7.06g, 0.0389 mol) and pyridine
(6.29 mL, 0.077 mol)
were added and the reaction was heated to 65 °C open to the air until
no starting material was apparent
by LCMS (It is very important that this reaction not be run under argon or
nitrogen, it needs the air to
catalyze the reaction. Also, the reaction should be stirred very vigourously
to allow the air to mix
with the reaction.) Once the starting material was gone (~ 18 h), the reaction
was allowed to cool to
room temperature and poured into ethyl acetate and filter. Wash the
precipitate well with ethyl
acetate. The filtrate is washed 2 times with Hz0 and dried over Na2S04,
filtered and concentrated.
Isco chromatography (100% hexane to 30% ethyl acetate/ 70% hexane gradient)
afforded the N-
phenyl nitrite in 2.13g (41%) as a white solid.
'H NMR (300 MHz, CDCl3) 81.44 (d, 3 H),2.61 (d, 2 H), 3.64(bs, 1 H), 3.90 (bs,
1 H), 6.60 (d, 2 H),
6.77 (t, 1 H), 7.18-7.26 (m, 2 H)
[00725] (S)-3-Phenylamino-butyramide (20)
[00726] To a solution of (S)-3-phenylamino-butyronitrile (6.06g, 0.0378mo1) in
toluene (150 mL)
was added a cooled solution of conc. sulfuric acid in H20 ( 20.12 mL HzSOq/ 3
mL)- (The ratio of
161
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
toluene to acid/H20 is very important and should be followed strictly). Stir
the biphasic mixture at
room temperature for 0.5 h and warm to 35 °C and stir for 22h. The
reaction was cooled to room
temperature and quenched with 13g of Na2C03 in water (add slowly some
foaming). Separate the
organic and extract 2 x EtOAc. Combine all the organics and wash the organics
with brine, dry over
MgSO4, filter and concentrate to give the desired product in 2.11g (90%)
'H NMR (300 MHz, CDC13) 8:1.29 (d, 3 H), 2.40 (dd, 1 H), 2.48 (dd, 1 H), 3.73
(bs, 1 H), 3.92
(sextet, 1 H), 5.52 (bs, 1 H), 6.00 (bs, 1 H), 6.66 (d, 2 H), 6.74 (t, 1 H),
7.19 (m, 2 H)
[00727) (S)-(3-Phenylamino-butyryl)-carbamic acid benzyl ester (21)
[00728] A clean, dry and nitrogen gas purged flask was charged with (S)-3-
phenylamino-
butyramide (3.25g, 0.018 mmol) in THF (65 mL) and the mixture was cooled to -
10°C. Benzyl
chloroformate (3.12 mL, 0.022 mmol) was then added followed by the slow
addition of 1.0 M lithium
tert-butoxide in THF solution (18 mL). The lithium tert-butoxide solution was
added at such a rate
that the internal temperature remained below 0°C. Fifteen minutes after
the completion of base
addition, the reaction (starting material gone by TLC) was quenched by adding
EtOAc (65 mL) and
1.0 M hydrochloric acid (10 mL). The aqueous phase was then basified with 1N
NaOH. The aqueous
phase was extracted 3 x EtOAc. The organics were collected together and with
saturated aqueous
sodium chloride solution (130 mL). The phases were separated, the organic
layer was dried (MgS04),
filtered, and concentrated. Flash chromatography using a Biotage system (10%
EtOAc/ 90% heieane
to 20% EtOAc/ 80% Hexane) afforded.the title compound in 82% yield.
'H NMR (300 MHz, CDC13) 8: 1.30 (d, 3,H), 2.87 (dd, 1 H), 3.04 (dd, 1 H), 3.80
(6s, 1 H), 4.02 (m, 1
H), 5.17 (s, 2 H), 6.62 (d, 2 H), 6.73 (t, 1 H), 7.17 (t, 2 H), 7.37 (s, 5 H),
8.13 (bs, 1 H).
[00729] (2S, 4R)-(2-Methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-carbamic acid
benzyl ester (22)
[00730] A clean, dry flask was charged with (S)-(3-phenylamino-butyryl)-
carbamic acid benzyl
ester (0.821 g, 2.63 mmol) followed by reagent grade ethanol (20 mL) and
cooled to -10 °C. Sodium
borohydride (0.070 g, 1.84 mmol) was added to the solution in one portion.
Nitrogen gas purging is
maintained for 5 minutes. A solution of 3.3 M aqueous magnesium chloride
solution (0.561 g MgCl2
6H20 in 1.5 mL water) was added at such a rate that the internal temperature
did not exceed -5 °C.
Once addition was completed, the reaction solution was warmed to 0 °C
for 30 min. The reaction was
quenched with methylene chloride (10 mL), and 1 M hydrochloric acidlcitric
acid solution (10.52 mL
1 N HCl, and 1.38 g citric acid). This bilayer was stirred at room temperature
for six hours. The
reaction mixture was diluted with ethyl acetate (200 mL) and neutralized with
sat. aqueous NaHC03
solution (pH = 10). The organics were collected together and washed with sat.
NaCI solution and
dried over NazS04, filtered and concentrated. Flash chromatography using an
Isco system (100%
hexane to 50% EtOAc/ 50% hexane gradient) afforded the title compound (0.733
g). (91 %).
162
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (300 MHz, CDCl3) ~: 7.38 (m, 5 H), 7.17 (d, 1 H), 7.02 (t, 1 H), 6.68
(t, 1H, C6-H), 6.47 (d,
1H), 5.17 (bs, 2H), 5.07 (m, 1H), 4.92 (d, 1H), 3.78 (bs, 1H), 3.57 (m, 1H),
2.30 (m, 1H), 1.47 (q,
1H), 1.21 (d, 3H).
General Procedure C
Scheme 15
cl~
CI O NHCbz \ I N_ H O
NHCbz I ~ F : 1. H2 Pd/C ~ DIPEA, CI'
N 'o., ~~~'CH3 2~ Cu(OAc)2, Pyridine ~~"CH3 CH2CI2
H CH2CI2, DIPEA CI
22 ,
23 / F ~ H)2 24 F 25
[00731] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (25)
[00732] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid
benzyl ester (1.0 g, 3.38 mmol) in methylene chloride (50 mL) at room
temperature was added
diisopropylethylamine (650 uL, 3.72 mmol) followed by 4-fluorobenzoyl
chloride. The reaction was
stirred over night at room temperature. The mixture was poured into water and
extracted with ethyl
acetate. The extracts were washed with 1 M (aq) NaOH and brine, dried over
magnesium sulfate,
filtered dried and concentrated. The crude residue was purified by silica gel
chromatography (75%
hexanes l 25 % ethyl acetate) to afford the pure amide (720 mg, 51 %).
[00733] (2S,4R)-[1-(4-fluorobenzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-carbamic acid
benzyl ester (720 mg, 1.73 mmol) was dissolved in ethanol (30 mL). The vessel
in which resided the
resulting solution was evacuated and backfilled with argon. A catalytic amount
of Palladium on
Carbon (10 %) was added. The vessel was once again evacuated and this time was
backfilled with
hydrogen and shaken in a Parr bottle at 40 psi hydrogen. Reaction Was complete
after 4 h. The
mixture was carefully filtered and concentrated to 10% volume. The resulting
concentrated solution
was filtered through an Celite ~ and concentrated to afford the crude amine.
[00734] To a solution of (2S, 4R)- (4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-
yl)-(4-fluoro-
phenyl)-methanone (1.0 g, 3.5 mmol) in DMF (20 mL, dry) was added 4-
chlorophenylboronic acid
(1.1 g, 7.0 mmol), pyridine (850 uL, 10.5 mmol) and copper(II)acetate (1.27 g,
7.0 mmol). The
heterogeneous green mixture was stirred open to air for 1 h and then warmed to
60 °C and stirred over
night (14 h). The mixture was then cooled to rt, poured into rapidly stirred
ethyl acetate (150 mL);
163
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
solids were removed by filtration through Celite ~. The extracts were washed
several times with
water and then once with brine. The extracts were then dried over anhydrous
magnesium sulfate,
filtered, and concentrated under reduced pressure. The crude residue was
purified by silica gel
chromatography (95 % methylene chloride / 5 % ethyl acetate) to afford the
aniline product (250 mg,
18 %) as a yellow oil.
[00735] To a solution of (2S, 4R)- [4-(4-chloro-phenylamino)-2-methyl-3,4-
dihydro-2H-quinolin-
1-yl]-(4-fluoro-phenyl)-methanone (250 mg, 0.636 mmol) in methylene chloride
(5 mL) was added
diisopropylethylamine (120 uL, 0.70 mmol) followed by acetyl chloride (90 uL,
1.27 mmol). The
mixture was stirred at rt 4 h. The mixture was concentrated under reduced
pressure, dissolved in ethyl
acetate, washed with sat. aqueous sodium bicarbonate, brine and dried over
magnesium sulfate,
filtered, and concentrated under reduced pressure. The residue was purified by
silica gel
chromatography (25/75 hexanes/ethyl acetate gradient) to afford pure N-(4-
chloro-phenyl)-N-[1-(4-
fluoro-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (200 mg,
71 %).
'H-NMR (CDC13) b: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, d), 2.3 (1H, m), 4.7 (1H,
m), 5.6 (1H, m), 6.5
(1H, d),6.7-7.0 (3H, m), 7.1-7.4 (8H, m).
MS m/z: 436 (M+1).
[00736] (2S ,4R)- N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-morpholin-4-yl-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-1)
[00737] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-morpholin-4-yl-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure C,
substituting 4-
bromobenzoyl chloride for 4-fluorobenzoyl chloride. Further elaboration to the
morpoline was done
I following the same procedure as described for(~)-N-(4-chloro-phenyl)-N-[1-(4-
fluoro-benzoyl)-2-
methyl-6-morpholin-4-yl-1,2,3,4-tetrahydro-quinolin-4-yl]-propionamide.
1H-NMR (CDCl3) 8: 1.13 (d, 3H), 1.22 (t, 1H), 2.03 (s, 3H), 2.29 (s, 1H), 3.31
(t, 4H), 3.80 (t, 4H),
4.75 (sextet, 1H), 5.61 (bs, 1H), 6.58 (d, 1H), 6.64 (d, 2H), 6.94 (t, 1H),
7.15 (d, 2H), 7.18 (t, 1H),
7.21 (d, 2H), 7.28 -7.39 (m, 3H).
MS mlz: 505.4 (M+1).
[00738] (2S ,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2Ii-
quinoline-1-carbonyl}-phenoxy)-butyric acid (C-2)
[00739] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl }-phenoxy)-butyric acid was prepared was made following general
procedure C,
substituting 4-methoxybenzoyl chloride for 4-fluorobenzoyl chloride. Further
elaboration to the acid
164
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
was done following the same procedure as described for (~)-4-(4-{4-[(4-chloro-
phenyl)-propionyl-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyric acid.
'H-NMR (CDC13) ~: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.1 (2H, m), 2.3 (1H,
m), 2.5 (2H, m), 3.9
(2H, m), 4.7 (1H, m), 5.6 (1H, m), ), 6.5 (1H, d), 6.7 (2H, d), 6.9 (1H, t),
7.1-7.3 (7H, m), 7.4 (1H, d).
MS m/z: 522 (M+2).
[00740] (2S ,4R)- N-(4-Chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-3)
[00741] (2S,4R)- N-(4-Chloro-phenyl)-N-[1-(4-dimethylamino-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared following general procedure
C, substituting 4-
dimethylaminobenzoyl chloride fox 4-fluorobenzoyl chloride.
'H-NMR (300 MHz, CDC13) 8: 1.14 -1.33 (m; 4 H), 2.13 (s, 3 H), 2.24 - 2.39 (m,
1 H), 2.94 (s, 6 H),
4.75 (ddd, 1 H), 5.61 (br s, 1 H), 6.44 (d, 2 H), 6.63 (d, 1 H), 6.96 (dd, 1
H), 7.07 - 7.36 (m, 6 H),
7.40 (d, 2 H).
MS mlz: 420 (M + 1).
[00742] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-isopropoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-4)
[00743] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-isopxopoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 4-
isopropoxybenzoyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (300 MHz, CDC13) 8: 1.14 (d, 3 H), 1.23 -1.31 (m, 7 H), 2.03 (s, 3 H),
2.23 - 2.35 (m, 1
H), 4.45 (sept., 1 H), 4.74 (ddd,1 H), 5.61 (br s,1 H), 6.55 (d, l H), 6.64
(d, 2 H), 6.92 (dd,1 H), 7.09
- 7.24 (m, 5 H), 7.29 (d, 1 H), 7.34 - 7.41 (m, 2 H).
MS mfz: 477 (M + 1).
[00744] (2S ,4R)- N-(4-Chloro-phenyl)-N-[2-methyl-1-(6-morpholin-4-yl-pyridine-
3-
carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (C-5)
[00745] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(6-morpholin-4-yl-pyridine-3-
carbonyl)-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was prepared following general
procedure C, substituting
2-chloronicotinoyl chloride for 4-fluorobenzoyl chloride. Prior to removal of
the benzyl carbamate,
the chloronicotinamide was converted to the 2-morpholinonicotinamide as
follows. A solution of the
(2S,4R)-[1-(6-chloro-nicotinoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
carbamic acid benzyl
ester (525 mg, 1.20 mol) was dissolved in morpholine (5 mL). The resulting
solution was heated at
70 °C over night. Upon completion of reaction (12 h), the solution was
concentrated under reduced
165
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
pressure; the crude residue was dissolved in ethyl acetate and washed with
water and brine to remove
remaining morpholine. The extracts were dried over sodium sulfate, filtered
and concentrated to
afford the crude morpholinonicotinate (639 mg, > 100%). The resulting product
was carried on to
fully elaborated (2S,4R)-N-(4-chloro-phenyl)-N-[2-methyl-1-(6-morpholin-4-yl-
pyridine-3-carbonyl)-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide as described in general procedure
C.
'H-NMR (300 MHz, CDC13) 8: 1.11 -1.22 (m, 4 H), 2.03 (s, 3 H), 2.24 - 2.38
(m,1 H), 3.48 = 3.56
(m, 4 H), 3.74 - 3.80 (m, 4 H), 4.73 (ddd, 1 H), 5.56 (br s, 1 H), 6.30 (d, 1
H), 6.66 (d, 1 H), 7.02 (dd,
1 H), 7.12 (dd, 1 H), 7.16 - 7.25 (m, 3 H), 7.32 (d, l H), 7.40 (d, 2 H), 8.24
(br s, 1 H).
MS mlz: 505 (M + 1).
[00746] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethyl-isoxazole-5-carbonyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-6)
[00747] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethyl-isoxazole-5-carbonyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared following general procedure
C, substituting 3-
ethylisoxazole carbonyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (300 MHz, CDC13) 8: 1.06 -1.23 (m, 7 H), 2.02 (s, 3 H), 2.21- 2.37 (m,
1 H), 2.52 - 2.66
(m, 2 H), 4.72 (ddd, 1 H), 5.34 - 5.56 (br s, 1 H), 5.88 (s, 1 H), 6.80 (d, 1
H), 7.11 (dd, 1 H), 7.20 (d,
2 H), 7.28 - 7.43 (m, 4 H).
MS m/z: 438 (M + 1).
[00748] (2S,4R)-N-[1-(3-Benzyl-isoxazole-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-
4-yl]-N-(4-chloro-phenyl)-acetamide (C-7)
[00749] (2S,4R)-N-[1-(3-Benzyl-isoxazole-5-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-N-(4-chloro-phenyl)-acetamide was prepared following general procedure C,
substituting 3-
benzylisoxazole carbonyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (300 MHz, CDC13) S: 1.06 -1.43 (m, 4 H), 2.01 (s, 3 H), 2.16 - 2.35
(rn, 1 H), 3.81- 4.01
(m, 2 H), 4.70 (ddd, 1 H), 5.40 (br s, 1 H), 5.83 (s, 1 H), 6.75 (d, 1 H),
7.02 (dd, 1 H), 7.10 (m, d, 2
H), 7.14 - 7.22 (m, 2 H), 7.22 - 7.34 (m, 5 H), 7.38 (d, 2 H).
MS m/z: 500 (M + 1).
[00750] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-methoxymethyl-isoxazole-5-
carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (C-8)
[00751] ZS,4R)-N-(4-Chloro-phenyl)-N-[1-(3-methoxymethyl-isoxazole-5-carbonyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was prepared following general
procedure C, substituting
3-methoxymethyl ether isoxazole carbonyl chloride for 4-fluorobenzoyl
chloride.
166
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
IH-NMR (300 MHz, CDC13) 8: 1.11 -1.24 (m, 4 H), 2.02 (s, 3 H), 2.22 - 2.39 (m,
1 H), 3.28 (s, 3
H), 4.42 (s, 2 H), 4.73 (ddd, 1 H), 5.46 (6r s, 1 H), 6.09 (s, 1 H), 6.79 (d,
1 H), 7.10 (d, 1 H), 7.10 (d, 2
H), 7.27 - 7.42 (m, 4 H).
MS m/z: 454 (M + 1).
[00752] (2S ,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-piperidine-1-carboxylic acid ethyl ester (C-9)
[00753] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-piperidine-1-carboxylic acid ethyl ester was prepared
following general
procedure C, substituting 4-(4-chlorocarbonyl-phenoxy)-piperidine-1-carboxylic
acid ethyl ester for
4-fluorobenzoyl chloride.
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m),1.2 (3H, t),1.? (2H, m),1.9 (2H,
m), 2.0 (3H, s), 2_3
(1H, m), 3.3 (2H, m), 3.7 (2H, m), 4.1 (2H, q), 4.4 (1H, m), 4.8 (1H, m), 5.6
(1H, m), 6.5 (1H, d),' 6.7
(2H, d), 6.9 (1H, t), 7.1-7.3 (7H, m), 7.4 (1H, d).
MS mlz: 590 (M).
[00754] (2S ,4R)- 2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-acetamide (C-10)
[00755] (2S,4R)-2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenoxy)-acetamide was made from (2S,4R)-N-(4-chloro-phenyl)-N-[1-(4-
methoxy-
benz0yl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,4R)-N-(4-
Chloro-phenyl)-N-[1-
(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was
prepared following
general procedure C, substituting 4-methoxybenzoyl chloride for 4-
fluorobenzoyl chloride. Further
elaboration to the amide was done following the same procedure as described
for (~)-N-[1-(4-
carbamoylmethoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
propionamide
1H-NMR (CDC13) 8: 1.1(3H, m), 1.8(1H, s), 2.0 (3H, s), 2.3 (1H, m), 4.4 (2H,
s), 4.7 (1H, m), 5.6
(1H, br), 5.9 (2H, brs ) 6.5 (2H, d), 6.7 (2H, d), 6.9 (1H, t), 7.2-7.4( 7H,
m).
MS mlz: 492 (M+1).
[00756] (2S ,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(2-morpholin-4-yl-
ethoxy)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (C-11)
[00757] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(2-morpholin-4-yl-ethoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was made from (2S,4R)-N-(4-chloro-
phenyl)-N-[1-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,4R)-
N-(4-Chloro-
phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide was
167
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
prepared following general procedure C, substituting 4-methoxybenzoyl chloride
for 4-fluorobenzoyl
chloride. Further elaboration to the morpholine was done following the same
procedure as described
for (~)-N-{2-methyl-1-[4-(2-morpholin-4-yl-ethoxy)-benzoyl]-1,2,3,4-tetrahydro-
quinolin-4-yl}-N-
phenyl-propionamide.
'H-NMR (CDC13) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 2.6 (4H,
m), 2.8 (2H, m), 3.7
(4H, m), 4.1 (2H, m), 4.7 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9
(1H, t), 7.1-7.3 (7H, m),
7.4 (1H, d).
MS xn/z: 549 (M+2).
[00758] (2S ,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl}-phenoxy)-acetic acid (C-13)
[00759] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl }-phenoxy)-acetic acid was made from (2S,4R)-N-(4-chloro-phenyl)-N-[1-
(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,4R)-N-(4-
Chloro-phenyl)-N-[1-
(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was
prepared following
general procedure C, substituting 4-methoxybenzoyl chloride for 4-
fluorobenzoyl chloride. Further
elaboration, to the acid was done following the same procedure as described
for { (~)-4-[2-methyl-4- .
(phenyl-propionyl-amino)-3,4-dihydro-2H-quinoline-1-carbonyl]-phenoxy }-
acetic.
'H-NMR (CDC13) S: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 4.3 (2H,
s), 4.6 (1H, m), 5.6
(1H, m), 6.4-6.9 (5H, m), 7.0-7.4 (7H, m).
MS m/z: 494 (M+2).
[00760] (2S ,4R)- N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(1H-tetrazol-5-
yhnethoxy)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (C-14)
[00761] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(1H-tetrazol-5-ylmethoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was made from (2S,4R)-N-(4-chloro-
phenyl)-N-[l-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,4R)-
N-(4-Chloro-
phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide was
prepared following general procedure C, substituting 4-methoxybenzoyl chloride
for 4-fluorobenzoyl
chloride. Further elaboration to the tetrazole was done following the same
procedure as described for
(~)-N-(4-chloro-phenyl)-N-{2-methyl-1-[4-(1H-tetrazol-5-ylmethoxy)-benzoyl]-
1,2,3,4-tetrahydro-
quinolin-4-yl }-propionamide.
'H-NMR (CDC13) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 4.8 (1H,
m), 5.2 (2H, dd), 5.6
(1H, m), 6.4 (1H, m), 6.5 (1H, d), 7.0 (2H, m), 7.1-7.4 (8H, m).
MS m/z: 517 (M+1).
168
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00762] (2S ,4R)-N-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (C-15)
[00763] (2S,4R)-N-{ 1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide was prepared from (2S,4R)-4-(4-{4-
[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-
piperidine-1-carboxylic
acid ethyl ester, followed by removal of the ethoxy carbamate using basic
hydrolysis and then
acetylation.
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m), 1.6-2.1 (4H, m), 2.0 (6H, s), 2.3
(1H, m), 3.4 (1H, m),
3.5-3.8 (3H, m), 4.4 (1H, m), 4.7 (1H, m), 5.6 (1H, m), 6.5 (1H, d), 6.7 (2H,
d), 6.9 (1H, t), 7.1-7.3
(7H, m), 7.4 (1H, d).
MS m/z: 560 (M+1).
[00764] (2S ,4R)- N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(pyridin-4-ylmethoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (C-16)
[00765] (2S ,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(pyridin-4-ylmethoxy)-
benzoyl]-1,2,3,4-
tetrahydro-quinolin-4-yl}-acetamide (2S,4R)- N-(4-Chloro-phenyl)-N-{2-methyl-1-
[4-(1H-tetrazol-5-
ylmethoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was made from
(2S,4R)-N-(4-
chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-acetamide.
(2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-
acetamide was prepared following general procedure C, substituting 4-
methoxybenzoyl chloride for .
4-fluorobenzoyl chloride. (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was dissolved in dichloromethane and a
solution of BBr3 (1.0 M
in dichloromethane, 10 mL) was added; the reaction was allowed to stir at room
temperature for until
no starting material remained. The reaction was washed with sat NaHCO3
carefully and brine. The .
organics were dried over MgSOø, filtered and concentrated down. The residue
was purified by
Biotage flash chromatography using 100 % EtOAc to give a white solid.
[00766] The phenol was dissolved in DMF (5 mL) at room temperature. Sodium
hydride (60 % in
oil) was added and the mixture allowed to stir 30 min. 4-Bromomethyl-pyridine
was added and the
reaction was allowed to stir over night. Ethanol was added and the reaction
was concentrated ira
vacuo. The residue was partitioned between ethyl acetate and water, then
extracted three times with
ethyl acetate, dried over MgS04, filtered and concentrated down. The crude
residue was purified by
silica gel chromatography (2/98 methanol / dichloromethane - 5/95
methanol/dichloromethane
gradient) to afford the product.
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 4.7 (1H,
m), 5.0 (2H, s), 5.6
(1H, m), 6.5 (1H, d), 6.7 (2H, d), 6.9 (1H, t), 7.0-7.4 (10H, m), 8.6 (2H, d).
MS m/z: 526 (M+1).
169
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00767] (2S ,4R)- 4-(3-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid (C-17)
[00768] (2S,4R)-4-(3-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-butyric acid was prepared following general procedure C,
substituting 3-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. Further elaboration to
the acid was done
following the same procedure as described for (~)-4-(4-{4-[(4-chloro-phenyl)-
propionyl-amino]-2-
methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyric acid.
'H-NMR (CDCl3) 8: 1.1 (3H, d), 1.1 (1H, m), 1.8-2.0 (2H, m), 2.0 (3H, s), 2.3
(1H, m), 2.4 (2H, m),
3.8 (2H, m), 4.8 (1H, m), 5.7 (1H, m), 6.4 (1H, m), 6.5 (1H, d), 6.8 (1H, m),
7.0 (1H, t), 7.1-7.4 (7H,
m), 7.5 (1H, m).
MS mJz: 521 (M+1).
[00769] (2S,4R)- N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-18)
[00770] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared was made following general procedure C,
substituting 3-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. Further elaboration to
the phenol was done
following the same procedure as described for (~)-N-[1-(4-hydroxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-propionamide.
'H-NMR (CDCl3) S: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 4.7 (1H,
m), 5.6 (1H, m), 6.4
(2H, d), 6.5 (1H, d), 6.9 (3H, m), 7.1-7.3 (4H, m), 7.4 (2H, m), 8.0 (1H, br).
MS m/z: 435 (M+1).
[00771] (2S, 4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenyl)-piperidine-1-carboxylic acid ethyl ester (C-19)
[00772] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenyl)-piperidine-1-carboxylic acid ethyl ester was prepared
following general procedure
C, substituting 4-(4-chlorocarbonyl-phenyl)-piperidine-1-carboxylic acid ethyl
ester for 4-
fluorobenzoyl chloride.
1H-NMR (CDC13) 8: 1.1 (4 H, m), 1.3 (3 H, m), 1.5 (2 H, m), 1.7 (2 H, m), 2.0
(3 H, s), 2.3 (1 H, m),
2.6 (1 H, m), 2.8 (2 H, t), 4.1 (2 H, m), 4.2 (2 H, m), 4.8 (1 H, m), 5.6 (1
H, br), 6.5 (1 H, d), 6.9 (1 H,
m), 7.0 (2 H, d), 7.1 (2 H, d), 7.3 (5 H, m), 7.4 ( 2H, m).
MS m/z: 474 (M+1).
170
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00773] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (C-20)
[00774] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 3-ethoxybenzoyl
chloride for 4-fluorobenzoyl chloride.
1H-NMR (CDC13) 8: 1.2 (3 H, m), 1.3 (4 H, m), 2.0 (3 H, s), 2.2 (1 H, m), 3.9
(2 H, m), 4.8 (1 H, m),
5.6(lH,br),6.5(lH,d),6.7(lH,d),6.8(2H,m),6.9(lH,m),7.0(lH,m),7.1-7.3 (4
H,m),7.4
(2 H, d).
MS m/z: 463 (M+1).
[00775] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-
1-carbonyl}-phenyl)-carbamic acid ethyl ester (C-22)
[00776] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl }-phenyl)-carbamic acid ethyl ester was prepared following general
procedure C, substituting
(4-chlorocarbonyl-phenyl)-carbamic acid ethyl ester for 4-fluorobenzoyl
chloride.
1H-NMR (CDCl3) 8: 1.1 (3 H, m), 1.3 (4 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 4.2
(2 H, m), 4.8 (1 H, m),
5.6(lH,br),6.5(lH,d),6.9(lH,m),7.1-7.3(BH,m),7.4(2H,d).
MS m/z: 506 (M + 1).
[00777] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-oxazol-5-yl-benzoyl)-
1,2,3,4- ..
tetrahydro-quinolin-4-yl]-acetamide (C-24)
[00778] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-oxazol-5-yl-benzoyl)-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 4-oxazol-5-yl-
benzoyl chloride for 4-fluorobenzoyl chloride.
1H-NMR (CDCl3) 8: 1.2 (3 H, m), 1.3 (1 H, m), 2.1 (3 H, s), 2.3 (1 H, m), 4.8
(1 H, m), 5.6 (1 H, br),
6.5 (1 H, d), 6.9 (1 H, m), 7.1-7.3 (8 H, m), 7.4 (1 H, d), 7.5 (2 H,d), 7.9
(1H, s).
MS m/z: 486 (M+1).
[00779] (2S,4R)-N-(3,4-Dichloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-25)
[00780] (2S,4R)-N-(3,4-Dichloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was made following general procedure C, substituting
3,4-
dichlorophenylboronic acid for 4-chlorophenylboronic acid and 4-methoxybenzoyl
chloride for 4-
fluorobenzoyl chloride.
171
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDCl3) 8: 1.2 (3 H, m), 1.3 (1 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 3.7
(3 H, s), 4.8 (1 H, m),
5.6(lH,br),6.6(lH,d),6.7(2H,d),7.0(lH,m),7.2(3H,m),7.3(2H,d),7.4(lH,s),7.5(1H,
d).
MS mlz: 483 (M+1).
[00781] (2S,4R)-N-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-N-[1-(4-methoxy-benzoyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (C-26)
[00782] (2S,4R)-N-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-N-[1-(4-methoxy-benzoyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure C, substituting
2,3-dihydro-benzo[1,4]dioxine-6-carboxylic acid for 4-chlorophenylboronic acid
and 4-
methoxyphenylbenzoyl chloride for 4-fluorobenzoyl chloride.
1H-NMR (CDC13) 8: 1.2 (3 H, m),1.3 (1H, m), 2.0 (3 H, s), 2.4 (1 H, m), 3.7 (3
H, s), 4.3 (4 H, s), 4.8
(1 H, m), 5.6 (1 H, br), 6.5 (1 H, d), 6.68 (2 H, d), 6.7-6.9 (3 H, m), 7.10-
7.3 (5 H, m).
MS m/z: 474 (M+2).
[00783] (2S,4R)- N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-p-
tolyl-acetamide (C-27)
[00784] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-p-tolyl-
acetamide was prepared following general procedure C, substituting 4-
methoxybenzoyl chloride for
4-fluorobenzoyl chloride and 4-toluene boronic acid for 4-chlorophenylboronic
acid
IH-NMR (CDCl3) 8: 1.15 (3H, d; overlapping.1H, t), 2.01 (3H, s), 2.33-2.36
(overlapping 1H, m,
lH,s), 3.73(3H,s), 4.70 (1H, m), 5.65 (1H, m), 6.50 (1H, d), 6.68 (2xlH, d),
6.95 (1H, t), 7.00-7.40
(8H, m).
MS m/z: 429 (M+ 1).
[00785] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-28)
[00786] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-pyrrolidin-1-yl-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared following general procedure
C, substituting 4-
pyrrolidin-1-yl-benzoyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (CDC13) 8: 1.11-1.15 (4H, m), 1.94-1.98 (4H, m), 2.03 (3H, s), 2.24-
2.34 (1H, m), 3.21-3.25
(4H, m), 4.68-4.75 (1H, m), 5.61-5.65 (1H, br), 6.30 (2H, d), 6.63 (1H, d),
6.92-7.52 (9H, m).
MS m/z: 488 (M+1).
172
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00787] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(1-isopropyl-1H-benzotriazole-5-
carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (C-29)
[00788] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(1-isopropyl-1H-benzotriazole-5-
carbonyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure C, substituting 1-
isopropyl-1H-benzotriazole-5-carbonyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (CDC13) b: 1.19-1.27 (4 H, m), 1.68 (6 H, d), 2.04 (3 H, s), 2.30-2.40
(1 H, m), 4.83 (1 H,
q), 4.98 (1 H, q) 5.45-5.55 (1 H, br), 6.48 (1 H, d), 6.83 (1 H, t), 7.10-7.41
(8 H, m), 8.13 (1 H, br).
MS mlz: 503 (M+1).
[00789] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(1-hydroxy-1-methyl-ethyl)-
benzoyl]-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (C-30)
[00790] (2S;4R)-N-(4-Chloro-phenyl)-N-{ 1-[4-(1-hydroxy-1-methyl-ethyl)-
benzoyl]-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was prepared from (2S, 4R)-N-[1-(4-
acetyl-benzayl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-phenyl)-acetamide.
(2S,4R)-N-[1-(4-Acetyl-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-phenyl)-
acetamide (112 mg, 124
mmol) was dissolved in THF (5 mL) and cooled to 0 °C. Methyl magnesium
bromide (1.4 M in ether,
2 mL, 2.4 mmol) was added and the mixture stirred at 0 °C for 2 h. The
reaction was warmed to rt
and stirred an additional 2 h. The reaction was poured into saturated aqueous
ammonium chloride.
The phases were separated and the aqueous was extracted with ethyl acetate.
The extracts were
washed with brine, dried over magnesium sulfate, filtered, dried and
concentrated. The crude alcohol
was purified by silica gel chromatography to afford pure product (20 mg, 24
%).
'H-NMR (CDCl3) ~: 1.12-1.21 (4 H, m), 1.48 (6 H, d), 2.02 (3 H, s), 2.25-2.34
(1 H, m), 4.70-4.80 (1
H, m), 5.45-5.54 (1 H, br), 6.50 (1 H, d), 6.88 (1 H, t), 7.11-7.38 (10 H, m).
MS m/z: 478 (M+1).
[00791] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethoxy-isoxazole-5-carbonyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-31)
[00792] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(3-ethoxy-isoxazole-5-carbonyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared following general procedure
C, substituting 3-
ethoxy-isoxazole-5-carbonyl chloride for 4-fluorobenzoyl chloride.
'H-NMR (CDC13) ~: 1.16 (3H, d),1.33 (3H, t), 1.69 (1H, br s), 2.00 (3H, s),
2.21-2.38 (1H, m), 4.21
(2H, q), 4.66-4.73 (1H, m), 5.65 (1H, s), 6.86 (1H, d), 7.13-7.39 (8H, m).
MS m/z: 454 (M).
173
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00793] (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenyl)-propionic acid (C-32)
[00794] (2S,4R)-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenyl)-propionic acid was prepared from (2S,4R)- 3-(4-{4-[acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenyl)-acrylic acid. A
solution of (2S,4R)-
3-(4-{ 4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-
carbonyl }-phenyl)-
acrylic acid (50 mg, 0.102 mmol) in EtOH (2 ml) and CHZC12 (10 drops for
solubility) was subjected
to Pd-C (10%, ca. 50 mg) and 1 atm H2 gas. After 1 hour, the mixture was
filtered, concentrated and
subjected to silica gel chromatography (2% MeOH in EtOAc to 10% MeOH in
EtOAc), to afford the
title compound (50 mg, 99 %).
1H-NMR (CDC13 300 MHz) b 1.09 (3H, d), 1.17-1.18 (1H, m), 2.00 (3H, s), 2.20-
2.35 (1H, m), 2.46-
2.60 (2H, m), 2.80-2.90 (2H, m), 4.65-4.80 (1H, m), 5.40-5.71 (1H, m), 6.48
(1H, d), 6.89 (1H, t), 7.0
(2H, d), 7.12 (2H, d), 7.20-7.48 (5H, m), 7.72 (1H, d).
MS mlz: 322 (M - C8H~N0).
[00795] (2S,4R)-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenyl)-acrylic acid (C-33)
[00796] (2S,4R)-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenyl)-acrylic acid was prepared following general procedure C,
substituting 3-(4-
chlorocarbonyl-phenyl)-acrylic acid methyl ester for 4-fluorobenzoyl chloride.
The ester was
hydrolyzed as follows. To a solution of (2S,4R)- 3-(4-{4-[acetyl-(4-chloro-
phenyl)-amino]-2-rriethyl-
3,4-dihydro-2H-quinoline-1-carbonyl}-phenyl)-acrylic acid methyl ester (112
mg, 0.239 mmol) in
THF/MeOH (2 ml, 2 ml) was added LiOH (4 ml: 1.0 M in H20). Upon consumption of
the starting
unit (1 hour), the mixture was neutralized with aq. HCl (1.0 M), partioned
with EtOAc (10 ml) and
separated. The organic layer was separated and concentrated whereby the
resulting oil was subjected
to silica gel chromatography (2% MeOH in EtOAc to 10% MeOH in EtOAc) to afford
the title
compound (110 mg, 99 %).
'H-NMR (MeOD, 300 MHz) b 0.85-0.95 (1H, m),1.12 (3H, d), 2.04 (3H, s), 2.40-
2.53 (1H, m), 4.70-
4.80 (1H, m), 5.50-5.71 (1H, m), 6.46 (1H, d), 6.57 (1H, d), 6.96 (1H, t),
7.20-7.55 (8H, m), 7.60 (2H,
d), 7.81 (1H, d).
MS m/z: 320 (M - C8H~N0).
[00797] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-(4-
methoxy-phenyl)-acetamide (C-34)
[00798] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-(4-
methoxy-phenyl)-acetamide was prepared following general procedure C,
substituting 4-
174
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
methoxybenzoyl chloride for 4-fluorobenzoyl chloride and 4-
methoxyphenylboronic acid for 4-
chlorophenylboronic acid.
1H-NMR (CDCl3 300 MHz) 8 1.12 (3H, d), 1.20-1.23 (1H, m), 2.09 (3H, s), 2.30-
2.42 (1H, m), 3.71
(3H, s), 3.81 (3H, s) 4.70-4.81 (1H, m), 5.50-5.80 (1H, m), 6.52 (1H, d), 6.67
(2H, d), 6.80-6.94 (4H,
m), 7.10-7.40 (5H, m).
MS mlz: 280 (M- C9HIONOz).
[00799] (2S,4R)-N-(4-Isopropyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-35)
[00800] (2S,4R)-N-(4-Isopropyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 4-methoxybenzoyl
chloride for 4-fluorobenzoyl chloride and 4-isopropylphenylboronic acid for 4-
chlorophenylboronic
acid.
1H-NMR (CDC13 300 MHz) 8 1.21 (6H, d), 1.20-1.23 (1H, m), 1.23 (3H, d), 2.09
(3H, s), 2.30-2.42
(1H, m), 2.80-2.95 (1H, m), 3.74 (3H, s), 4.65-4.83 (1H, m), 5.50-5.80 (1H,
m), 6.53 (1H, d), 6.67
(2H, d), 6.72 (2H, d), 6.92 (1H, t), 7.02-7.12 (3H, m), 7.21 (2H, d), 7.38
(1H, d).
MS m/z: 280 (M- C~IHI4N0). .
[00801] (2S,4R)-N-(4-Bromo-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (C-36)
[00802] (2S,4R)-N-(4-Bromo-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 4-methoxybenzoyl
chloride for 4-fluorobenzoyl chloride and 4-bramophenylboronic acid for 4-
chlorophenylboronic acid.
'H-NMR (CDCl3 300 MHz) 8 1.12 (3H, d), 1.20-1.24 (1H, m), 2.05 (3H, s), 2.20-
2.38 (1H, m), 3.72
(3H, s), 4.66-4.81 (1H, m), 5.50-5.75 (1H, m), 6.52 (1H, d), 6.67 (2H, d),
6.92 (1H, t), 7.10-7.18 (5H,
m), 7.26 (1H, t), 7.48-7.58 (2H, m).
MS mlz: 493 (M + 1).
[00803] (2S,4R)-4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-amino}-benzoic acid (C-37)
[00804] (2S,4R)-4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-benzoic acid was made from (2S,4R)- 4-{acetyl-[1-(4-methoxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-amino}-benzoic acid methyl ester. (2S,4R)- 4-{acetyl-
[1-(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino}-benzoic acid methyl
ester prepared
following general procedure C, substituting 4-methoxybenzoyl chloride for 4-
fluorobenzoyl chloride
175
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and 4-phenylboronic acid methyl ester for 4-chlorophenylboronic acid. (2S,4R)-
4-{ acetyl-[1-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino}-benzoic
acid methyl ester was
converted to the acid using the following procedure. To a solution of 4-
{acetyl-[1-(4-methoxy-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino}-benzoic acid methyl
ester (10 mg, 0.038
mmol) in 4 ml methanol was added 100 mg KzC03 (0.72 mmol, in 0.5 ml water). ~
The resulting
reaction mixture was stirred at room temperature overnight. The methanol was
removed under
vacuum. 1M HCl was added until the mixture is acidic. Dichloromethane (20 ml)
and 5 ml water was
added. Organic layer was dried with magnesium sulfate. Dichloromethane was
removed under
vacuum to give the title compound (15 mg, 86%)
1H-NMR (CDC13) 8: 1.1 (3H, d), 1.1 (1H, m), 2.0 (3H, s), 2.3 (1H, m), 3.8 (3H,
s), 4.8 (1H, m), 5.6
(1H, m), 6.5 (1H, d), 6.6 (2H, d), 6.9 (1H, t), 7.1-7.4 (6H, m), 8.1 (2H, d).
MS mlz: 460 (M+2).
[00805] . (2S,4R)-N-(3-Aminomethyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (C-38)
[00806] (2S,4R)-N-(3-Aminomethyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared from (2S, 4R)-N-(3-cyano-
phenyl)-N-[1-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,
4R)-N-(3-cyano-
phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide was
prepared following standard procedure C, substituting 3-cyanophenylboronic
acid for 4-fluorobenzoyl
chloride. To a mixture of (2S, 4R)-N-(3-cyano-phenyl)-N-[1-(4-methoxy-benzoyl)-
2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (48mg,0.llmmol) in 2m1 ethanol was added
cobalt chloride (14
mg, O.llmmol). Sodium borohydride (12 mg, 0.33 mmol) was added at 0 °C,
and the temperature
was held at 0 ° for 30 min. The. mixture was then warmed to rt, and
stirred overnight. The reaction
was quenched by adding saturated aqueous ammonium chloride. The separated
aqueous layer was
extracted with ethyl acetate. The combined extracts were washed with water and
brine, dried over
magnesium sulfate, filtered and concentrated under reduced pressure. The crude
oil was purified by
HPLC to give the title compound (lOmg,10%).
'H-NMR (CDC13) 8: 1.1-1.2 (4 H, m), 2.0 (3 H, s), 2.3 (1 H, m), 3.4 (2 H, br),
3.8 (3 H, s), 4.3 (1 H,
d), 4.8 (2 H, d), 5.6 (1 H, br), 6.4 (1 H, m), 6.6 (2 H, m), 6.9 (1 H, m), 7.1-
7.4 (8H, m).
MS m/z: 444 (M+1)
[00807] (2S,4R)-N-(4-Butyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (C-39)
176
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00808] (2S,4R)-N-(4-Butyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure C,
substituting 4-methoxybenzoyl
chloride for 4-fluorobenzoyl chloride and 4-butylphenylboronic acid for 4-
chlorophenylboronic acid.
1H-NMR (CDC13) 8: 0.9 (3 H, m), 1.2 (3 H, d), 1.4 (3 H, m), 1.6 (2 H, m), 2.0
(3 H, s), 2.4 (1 H, m),
2.6(2H,m),3.8(3H,s),4.8(lH,m),5.6(lH,br),6.5(lH,d),6.7(2H,d),7.0(l H,m),7.1-
7.2(7
H, m), 7.4 (1 H, d).
MS m/z: 471 (M+1).
[00809] Compounds C-40- C-147 can be prepared by the schemes set forth in
Schemes 15-16 and
by the general procedures C and others described herein. Those skilled in the
art will be able to
recognize, or be able to ascertain, using no more than routine
experimentation, many equivalents to
the specific embodiments of the invention described herein.
177
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Table 3: Compounds Derived from General Procedure C
o., ' tructure No. Sinrcture
' -
CI~N~ cI~N~
I~I~ ~
G1 ~. C-11 ...,
yo ~o~.N~'J
N~ CyN
a ~
C-2 . C-12 ~.
,.
~ O~' T~
~ ~~OH
~ ~ 'I ~.S
~o~H
o
CI~N~ ~ o
f
C-3 I ~ N ." C-13
0 ~
I ~
'
i N~ ~oH
I o
o
CI~N~ Ct~N~
O I O
C-4 . C-14 ~.
ct~ ~
N N
C-5 ' C-15 I
.."
N
O~
~
OI~N~ cI~N
I
~
C.g I C-16 ~ ..
~
N ',.
I N
I N
Cl~d~ ~ ~W
N
r.
7 o 17 '
.,
o
~
~
r~~OH
~
d
o N
CI
C-8 C-18 ! \
OH
cy~
CI~N 'ls~~
~
G9 I C-19 ~
~N~~
Nxo~
CI~N~ CI
N
C-10 C-20 ~,
~o O
NHa
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~Vo- structure lVa. grruc~ure
CI~N
N
C-21 C-31 I , , a
I N
p
' T'0
rvffx
~tJ~CI CI v I
C-22 I ~ " C-32 I ~ .
I
~
NH O
o Jo
CazH
J~ C ~ I
N N
C-23 ~"' C-33 I ~ "
~ N
~NIfO./ O
o I ' i Co2H
I CI I
~N~ ~ I N_
C-24 I ~ N .". C ~ I .."
c N
A
O-~N I
i D'
CI
CI \ I O ~ I N
C-25 ~ N C-35 I
..e.
N '' N
O~ , I ~ o.
a
Co ~ I JL ~ I
N
C-26 I , N .." C-36 I
~N~ Ho s I
N
C-27 C-37
. ~ ..,.
0
O '~~ p
~
CI
~ I N~ HZN v I N
C-28 C-38
-, N
p O
NJ
CI o
~ I N'' ~NJOI.
C-29 ., C-39
0 I o.
CI O
N'~
C-30 ., C-40
O
I
~
OH
179
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. ~ StructureNa.. Structure
Njl
O
C-41 C-51
'
d
~d~
C-42 C-52 I\ ,
d d
Hf!
0
C-43 C-53
I~s
d
u
~
~
C-44 ~~ C-54
I
d
~H~~ H~
( , P'~ IId'
ill' '
C-45 i \ C-55 '
d
d
I
[/ uJ7 \
H
C-46 ~~ C-56 \
d
C-47 ~ C-57
~~d
Ho'~~u I J'
N
:
C_48 I . C_58 I \
y
\
d
d
~~N~l, d
C-49 ~ C-59
~
C-50 ~ C-60
I
d
l
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. Strucfuie N~i, Stractr.~re
::
H H~ ~ ~
N
C-61 ~.", C-71
d
d~
N
C-62 I C-72 ~,",
~d
d
N
C-63 I " C 73 I y
- H~ N
N
C-64 ~," C-74 ~I
" ~
I d
~
~
d
CI~ ypp I H
erv'N-
N
C-65 ~ ", C-75
~ ~."
~d I~
Br H N
~~/_~I~,
OII
C~N
C-66 ~,"" C-76
d ~d
Cy H~
~
C-67 N C-77 I
~~
I ~NH. d
0
H~
C-68 ~." C-78 I ,
~lfN~
d
- I
N
C-69 ~? C-79 , ,,
I NH,
0
INS
C-70 C-8~
I
i d
an.
101
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. Structure No. Structure
, , .
0n ~I~ II
N~ c~N
C-81 ~~ C-91 ~,"
"
I ~~////
I ~ ~NH~
c~~ C
C-82 ~ C-92
~"'1r~' d~~NH,
C-83 ~~~~ C-93 ~.",
~
NHi
~
'' ~~O
CI~
CC~~XXy~'I
C-84 C-94
~N
c N
C-85 ~~~ C-95 ~.."
I , dlrd.
a
C-86 M~~ I ~ C-96
""
d
0
H C O
C-87 ~ ~ C-97
c~N
C-88 C-98 ~."
~"
I d ,u
c~N~
C-89 C-99
I 'y'~~
I
d N
N- '
C-90 ~ C-100
~
182
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
~o --. . ~r~,G~ureNo. : Structui~
~
cl~~ C
' 7'~N
C-101 I 1
C-11
,y~
d~H
C~~~ C~~
/'~ r'~
JJy
~~
C-102 ~ C-112 ~.w~
,"
"
C-103 C-113 I, a
C-104 C-114
c~N~
C-105 ~ C-115
I ~oH
o
CI~N
yes( . I
C-106 ~- ~q
C-116 I
H NH,
~- IIIIO
CI~~
IN~
C-107 ~-.., C-117
I S'
al~~
CI~ _ II
~~I,
Jtt
~
C-108 ., C-118 ~''
~ 1
~
CI~
~a
C-109 I1 . C-119
I
,
I
C-110 '~ C-120
~
~'~11 H
1~3
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No. . >~ruc~ureNo, ;>3lracture
~.
c~ea
C-i , C-131 ~""
21
O"0H
CI\p
fUf~XX(~' I
C-122 ~.y, C-132
O OH HH~
a~~
c~~
C-123 C-133 i/
HN-L
a~~
C-124 ~, C-134 I \
H / \ ~OH
'' 1~O
I
C-125 I C-135 I ,
I/ H
O
O OI
C-126 I ~ C-136 I ,
I/ / H
O
c a
C-127 C-137 i
N ~ pj[
~~H
G~
C-128 ~..,, C-138 ~w.
~H
H
CI~H
7'~~
LL
C-129 ~ C-139
.", ~~~
I
'
~/
p o f.
c~n~
a
C-130 ~.., C-140 [~.,,
~''y~~~
R
~
'\~'~o c5
1~4
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Nc. Structcrre . Structure
OI , I O r
C-141
CI ~ I H
C-142
"",
W '~-
~OH
C-143
~OH
~O
C-144 I , .."
,
~OH
N_
C-145
I ~ ~N
cl~~
ut~1(~I
C-146 I
HH
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
General Procedure D
Scheme 15
0
NHCbz CI I ~ NHCbz 1, Hz pdlC
OMe
w _ ~ .
2. Pd (dba)3, Cs2C0
N ''~. CH2CI2, DIPEA I ~ N ~'CH3 2 ° 3
H
22 ~ ~ \ \°~° ~ ~ sr
OMe Toluene
26
O ~ O ~O~O ~ O
I NH DIPEA, CI~ I N
I ~ CH2CIp
N ~~~CHg ~ N ''CH3
O
OMe OMe
27 p_g
[00810] (2S,4R)-((4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-amino}-phenoxy)-acetic acid methyl ester (D-9)
[00811] (2S,4R)-((4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
amino}-phenoxy)-acetic acid methyl ester was prepared from (2S,4R)-(2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl)-carbamic acid benzyl ester as shown below. (2S,4R)-(2-Methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl)-carbamic acid benzyl ester (7.6 g, 25.65 mmol) was dissolved in
dichloromethane (50
mL) and the resulting solution was cooled to 0 °C. Triethylamine (14.3
mL) followed by freshly
distilled anisoyl chloride (8.75 mL, 51.3 mmol) dissolved in dichloromethane
(15 mL) were added
dropwise to this solution. The resulting reaction mixture was allowed to warm
to room temperature
and stir over night. The mixture was partitioned between dichloromethane and 1
M sodium
hydroxide. The extracts were washed with brine, dried over magnesium sulfate,
filtered and
concentrated. The crude amide was purified by silica gel chromatography (2:1
hexane:ethyl acetate)
to afford pure product (10 g, 91 %).
[00812] The (2S,4R)-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
carbamic acid benzyl ester thus formed (10 g) was dissolved in ethanol (400
mL). Palladium (10 %
on Carbon) was added. The black suspension was stirred under an atmosphere of
hydrogen for 3 h.
The mixture was filtered and concentrated. The crude amine was purified by
filtration through a short
silica plug (elution with ethyl acetate to 90/10 ethyl acetate/methanol
gradient) to afford pure amine
(5.17 g, 72 %).
1~6
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00813] (2S,4R)-(4-Amino-2-methyl-3,4-dihydro-2H-quinolin-1-yl)-(4-
methoxyphenyl)-
methanone (100 mg, 0.34 mmol), methyl-2-(4-bromophenoxy)-acetate (91 mg, 0.37
mmol), Pd2(dba)3
(17 mg, 0.02 mmol), 2-dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl (8
mg, 0.00002 mol)
and cesium carbonate (0.163 g, 0.0005 mol) were taken in a round bottom flask
which was then
flushed with nitrogen gas through a rubber septum. Toluene (2 mL) was injected
into the flask
through the rubber septum and the reaction mixture was stirred at 100
°C for 24 h. After cooling to
room temperature the reaction mixture was filtered through Celite~ and
evaporated to give the crude
product (0.236g). This crude product was purified by silica gel chromatography
eluting with 100%
hexanes to 50/50 hexanes/ethyl acetate gradient give the title compound (37mg,
24%).
[00814] Freshly distilled acetyl chloride (0.5 mL) was added to a solution of
the aniline thus
prepared (0.037g, 0.00008 mol) followed by diisopropylethylamine (0.0114g,
0.015 mL, 0.088 mmol)
in dichloromethane (0.5 mL); the mixture was stirred at room temperature for 2
days. The reaction
mixture was neutralized with 1 M sodium bicarbonate. The organic layer was
separated, washed
thrice with water, brine, dried over magnesium sulfate and evaporated. The
resulting crude product
was purified by silica gel chromatography eluting with (0% to 70% ethyl
acetate in hexanes to afford
the titled compound (l5mg, 38%).
1H- NMR (CDC13) S: 1.12-1.14 (4 H, m), 2.02 (3 H, s), 2.18 - 2.43(1 H, m),
3.75 (3 H, s) 3.82 (3 H,
s), 4.65 (2 H, s), 4.67 - 4.82 (1 H, m), 5.45 - 5.73 (1 H, broad), 6.52 (1 H),
6.68 (2 H, d), 6.89 - 6.95 '(3
H,m),7.13-7.21 (5 H,m),7.32(lH,d).
MS m/z: 504 (M + 1).
[00815] (2S,4R)-(4-{Acetyl-[1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-
amino}-phenoxy)-acetic acid (D-10)
(00816] (2S,4R)-(4-{Acetyl-[1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-
amino}-phenoxy)-acetic acid was prepared from (2S,4R)-(4-{acetyl-[1-(4-
methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]-amino}-phenoxy)-acetic acid methyl ester (15
mg, 0.03 mmol). The
methyl ester was dissolved in methanol (1 mL), sodium hydroxide (1 mL, 0.1 M
in water) was added
and the resulting solution was stirred at room temperature for 18 h. The
reaction mixture was
acidified with hydrochloric acid (1 M) and concentrated under reduced
pressure. The residue was
extracted with ethyl acetate and the extract was washed thrice with water,
brine, dried over sodium
sulphate, filtered and concentrated to yield the title compound (13 mg, 89%).
1H- NMR (CDC13) ~: 1.07 (4 H, m), 1.99 (3 H, s), 2.12 - 2.38(1 H, broad), 3.7
(3 H, s), 4.61(2 H, s),
4.66 - 4.78(1 H, m), 5.47 - 5.75 (1 H, broad), 6.49 (1 H, d), 6.64(2 H, d),
6.86 - 6.9(3 H,m), 7.09 -
7.16(5 H, m), 7.27 (1 H,d).
MS m/z 489 (M+), 490(M+1)
187
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00817] (2S,4R)-2-(4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-phenyl)-2-methyl-propionic acid (D-1)
[00818] (2S,4R)-2-(4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-phenyl)-2-methyl-propionic acid was prepared via saponification of
2-(4-{acetyl-[1-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino }-phenyl)-2-
methyl-propionic
acid methyl ester, as described in the synthesis of (2S,4R)-(4-{acetyl-[1-(4-
methoxybenzoyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]-amino}-phenoxy)-acetic acid. The
methyl ester was prepared
following general procedure D, substituting 2-(4-bromo-phenyl)-2-methyl-
propionic acid for methyl-
2-(4-bromophenoxy)-acetate.
'H-NMR (300 MHz, CD30D) 8: 1.07- 1.18 (m, 4 H), 1.58 (s, 6 H), 2.02 (s, 3 H),
2.42 - 2.56 (m, 1
H), 3.76' (s, 3 H), 4.74 (ddd, 1 H), 5.55 (br s, 1 H), 6.56 (d, 1 H), 6.75 (d,
2 H), 6.97 (dd, 1 H), 7.13 -
7.27 (m~-3 H), 7.36 (d, 2 H), 7.42 - 7.55 (m, 3 H).
[00819] (2S,4R)-4-(4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-2-chloro-phenyl)-4-oxo=butyric acid (D-2)
[00820) (2$4R)-4-(4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-
yl]-annino}-2-chloro-phenyl)-4-oxo-butyric acid was prepared from the
corresponding methyl ester
following the procedure above for the synthesis of (2S,4R)-(4-{acetyl-[1-(4-
methoxybenzoyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]-amino}-phenoxy)-acetic acid. The
corresponding methyl
ester was prepared following general procedure D, substituting 4-(4-bromo-2-
chloro-phenyl)-4-oxo-
butyric acid methyl ester for methyl-2-(4-bromophenoxy)-acetate.
1H-NMR (300 MHz, CD30D) ~: 1.10 -1.19 (m, 4 H), 2.08 (br s, 3 H), 2.41 - 2.56
(m, 1 H), 2.69 -
2.74 (m, 2 H), 3.20 - 3.26 (m, 2 H),' 3.75 (s, 3 H), 4.74 (ddd, 1 H), 5.45 -
5.62 (br s, 1 H), 6.57 (d, 1
H), 6.74 (d, 2 H), 6.98 (dd, 1 H), 7.16 (d, 2 H), 7.20 - 7.27 (m, 1 H), 742 -
7.49 (m, 2 H), 7.60 (br s,
1 ), 7.73 (d,1 H).
MS m/z: 549 (M ~-1).
[00821] (2S,4R)-N-(4-Dimethylsulfamoyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (D-3)
[00822] (2$4R)-N-(4-Dimethylsulfamoyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was prepared following general procedure
D, substituting 4-
bromo-N,N-dimethyl-benzenesulfonamide for methyl-2-(4-bromophenoxy)-acetate.
188
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) S: 1.1 (3 H, d), 1.1 ( 1H, m), 2.0 (3 H, s), 2.3 (1 H, m), 2.8
(6 H, s), 3.8 (3 H, s),
4.8(lH,m),5.6(lH,m),6.6(lH,d),6.7(2H,d),6.9(lH,t),7.2(3H,m),7.3(lH,m),7.5(2H,
d), 7.8 (2 H, d).
MS m/z: 522 (M+1).
[00823] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-[4-
(pyrrolidine-1-sulfonyl)-phenyl]-acetamide (D-4)
[00824] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-[4-
(pyrrolidine-1-sulfonyl)-phenyl]-acetamide was prepared following general
procedure D, substituting
1-(4-bromo-benzenesulfonyl)-pyrrolidine for methyl-2-(4-bromophenoxy)-acetate.
1H-NMZt (CDC13) b: 1.1 (3 H, d), 1:1 (1 H, m),1.7 (4 H, m), 2.0 (3 H, s), 2.3
(1 H, m), 3.3 (4 H, m),
3.7(3H,s),4.7(lH,m),5.6(lH,m),6.5(lH,d),6.6(2H,d),6.9(lH,t),7.3(4 H,m),7.4(2H,
d), 7.9 (2 H, d).
MS mlz: 548 (M+1).
[00825] (2S,4R)-N-(4-Methanesulfonyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-qainolin-4-yl]-acetamide (D-5)
[00826] (2S,4R)-N-(4-Methanesulfonyl-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide was made following general procedure D,
substituting 1-bromo-
4-methanesulfonyl-benzene for methyl-2-(4-bromophenoxy)-acetate.
'H-NMR (CDC13) 8: 1.1 - 1.2 (3 H, m), 2.0 - 2.2 (4 H, m), 2.3 (1 H, m); 3.1 (3
H, s), 3.7 (3 H, s), 4.8
(1 H), 5.6-5.8 (1 H, br), 6.5 (1 H, d), 6.6 (2 H, d), 6.9 (1 H, t), 7.1-7.3 (4
H, m), 7.4 (2 H, d), 8.0 (2 H,
d).
MS xnlz: 493 (M+1).
[00827] (2S,4R)-3-(4-{Acetyl-[1-(4-metho~ry-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-phenyl)-propionic acid (D-6)
[00828] (2S,4R)-3-(4-{Acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-phenyl)-propionic acid was prepared from (2S,4R)- N-(4-bromo-
phenyl)-N-[1-(4-
methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. (2S,4R)-
N-(4-Bromo-
phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide was
converted to the acrylic acid using the same procedure described in the
synthesis of (~)-3-[4-[(4-
chloro-phenyl)-propionyl-amino]-1-(4-fluoro-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-6-yl]-
acrylic acid. The reduction and saponification were carried out as in the
procedure describing the
189
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
preparation of (2S,4R)-3-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-propionic acid.
'H-NMR (CDC13 300 MHz) 8 1.12 (3H, d), 1.20-1.24 (1H, m), 2.00 (3H, s), 2.22-
2.38 (1H, m), 2.52
(2H, t), 3.00 (2H, t), 3.72 (3H, s), 4.64-4.79 (1H, m), 5.44-5.70 (1H, m),
6.50 (1H, d), 6.65 (2H, d),
6.90 (1H, t), 7.10-7.28 (7H, m), 7.32 (1H, d).
[00829] (2S,4R)-3-(4-{Acetyl-[1-(4-methoay-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]-amino}-phenyl)-propionamide (D-7)
[00830) (2S,4R)-3-(4-{Acetyl-[l-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin=4-
yl]-amino}-phenyl)-propionamide was prepared from (2S,4R)-3-(4-{Acetyl-[1-(4-
methoxy-benzoyl)-
2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-amino }-phenyl)-propionic acid. To
a solution of 3-(4-
{ acetyl-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
amino }-phenyl)-
propionic acid (21 mg, 0.042 mmol) in dimethylformamide (200 p,1) was added
HATU (24 mg,,0.063
mmol), HOBt (8.5 mg, 0.063 mmol), NH4Cl (4.5 mg, 0.084 mmol) and DIPEA (29
p.1, 0.168 mmol).
Upon consumption of the starting unit (2.5 hours), the mixture was diluted
with EtOAc (10 ml) and
washed with sat. NaHC03 (4 X 10 ml). The EtOAc layer was collected, dried over
NazS04, filtered,
and concentrated to afford the title compound ( 17.2 mg, 82 %).
'H-NMR (CDC13 300 MHz) 8 1.09 (3H, d), 1.20-1.24 (1H, m), 2.02 (3H, s), 2.22-
2.38 (1H, m), 2.52
(2H, t), 3.00, (2H, t), 3.73 (3H, s), 4.64-4.79 (1H, m), 5.30-5.70 (3H, m),
6.50 (1H, d), 6.68 (2H, 'd),
6.91 (1H, t), 7.10-7.28 (7H, m), 7.32 (1H, d).
[00831) , (2S,4R)-N-[1-(4-Methoay~benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-(4-
nitro-phenyl)-acetamide (D-8)
[00832] (2S,4R)-N-[1-(4-Methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-N-(4-
nitro-phenyl)-acetamide was made following general procedure D, substituting 4-
bromonitrobenzene
for methyl-2-(4-bromophenoxy)-acetate.
'H-NMR (CDCl3 300 MHz) 8 1.12 (3H, d), 1.20-1.24 (1H, m), 2.07 (3H, s), 2.20-
2.35 (1H, m), 3.73
(3H, s), 4.66-4.81 (1H, m), 5.50-5.78 (1H, m), 6.55 (1H, d), 6.68 (2H, d),
6.96 (1H, t), 7.10-7.32 (4H,
m), 7.46 (2H, d), 8.28 (2H, d).
MS m/z: 460 (M + 1).
Table 4: Compounds Derived from General Procedure D
190
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Na.: structure
-.
off ~ N
D-1 .."
0
pio
O OH
~N~
D-2
P
Q
N
D-3
0
~N~~~
0
i
~~N~
D-5
.",
'
o
D-6
~.
D-7
d
oxN w I
N
D-8 I ~
I,
D-9
D-10
191
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
General procedure E
Scheme 16
OEt O
O O OEt O
BY ~ / O OEt
/ OEt . OEt / O OEt I i
O N
O NH2 O H
38 I a
39
O O O
KOtBu / I OEt 1. 4M HCUDioxane / I
---,.
2. 4N HCI O N
Et20 N
I,
b
40 41
I O
~R
R ~ I NH R/ N~R3
I /
~NH2 / R3COC1 /
I
N
Na(OAc)3BH, DCE, AcOH N DCM, DIPEA
42 I / 43
i I OII / ~ OII
H2 Pd/C R N~R3 R~COCI R N~Rg
MeOH / ~ DIEA, DCM
O H O N
O~Rt
44
[00833] (~)-N-(7-acetyl-6-methyl-4,5,6,7-tetrahydrohetero[2,3-b]pyridin-4-yl)-
N-substituted
phenylacetamides (45)
[00834] 2-(2-Ethoxycarbonyl-1-methyl-ethylamino)-furan-3-carboxylic acid ethyl
ester (38)
[00835] 2-Amino-furan-3-carboxylic acid ethyl ester and but-2-enoic acid ethyl
ester is dissolved
in ethanol and heat to reflux in the presence of A1z03, until no starting
material remains and filter and
concentrate down. The residue is purified by flash chromatography to afford
the corresponding
diketone.
[00836] 2-[Benzyl-(2-ethoxycarbonyl-1-methyl-ethyl)-amino]-furan-3-carboxylic
acid ethyl
ester (39)
192
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00837] The synthesis is accomplished using the alkylation described for the
synthesis of A-164,
substituting benzyl bromide for the 3-methoxybenzyl bromide.
[00838) 7-Benzyl-6-methyl-4-oxo-4,5,6,7-tetrahydro-faro[2,3-b]pyridine-5-
carbo~rylic acid
ethyl ester (40)
[00839] To a solution of the diester in ethyl ether at room temperature is
added potassium tert-
butoxide and the mixture is allowed to stir for 1 hour. The mixture is
filtered to remove any
hydrolyzed material. The solvent was removed in vacuo to afford the potassium
salt of the bicyclic
ester.
[00840] 7-Benzyl-6-methyl-6,7-dihydro-5H-faro[2,3-b]pyridin-4-one (41)
[00841] The oc-keto-ester is dissolved in 4M HCl in dioxane and stirred for 2
hours at room
temperature. Then 4 N HCl is added and the mixture was heated in a 100
°C oil bath for 12 hours. The
mixture is then cooled to room temperature and neutralize with 1N NaOH. The
aqueous layer is
extracted with ethyl acetate, dried over magnesium sulfate and filter.
Evaporate the solvent iu vacuo
and purify the residue by flash chromatography to afford the corresponding
ketone.
[00842] (~)- (7-Benzyl-6-methyl-4,5,6,7-tetrahydro-faro[2,3-b]pyridin-4-yl)-
substituted
phenyl-amine (42)
[00843] Synthesis of the substituted phenyl amine is accomplished using the
procedure described
for F-1, substituting aniline for the corresponding aniline.
[00844] (~)-N-(7-Benzyl-6-methyl-4,5,6,7-tetrahydro-faro[2,3-b]pyridin-4-yl)-N-
substituted
phenyl-substituted amide (45)
[00845] Synthesis of the corresponding phenyl amide is accomplished using the
hydrogenation
and acylation procedures described in general procedure B with the
corresponding acid chlorides.
Representative examples of compound 45 are shown in the table below.
[00846] Compounds E-1- E-30 can be prepared by the schemes set forth in
Schemes 18 and by
the general procedures E and others described herein. Those skilled in the art
will be able to
recognize, or be able to ascertain, using no more than routine
experimentation, many equivalents to
the specific embodiments of the invention described herein.
193
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Table 5: Examples using General Procedure E
No. Stracture No,-> Structum.
,
.
N
E-1 ~ I E-11N
N
I ~ O~y
~ ~ N
E-2 ~ I E-12~ I
N
N O
I W
~ ~ N
N
N
E-3 / ~ E-13~ I
N
N o~
o I u~u
l
\ N~ ~ I
I
E-4 ; I E-14N
I ~ ~
/
~N~
N
E-5 KN I E-15N
0.
v / I ~
N'\ v I
N
E-6 N I E-16
N
\ /
E-7 I E-17
0 0 0
~N~
E-g E-18
N 0v
00.
N
E-9 '~ E-19
~N O ,N ~
O~Ov 0~0~
N
E-10 E-20
N N N.O N
00. 0~
194
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
No, 9truetureIVo. StYUCture
..: .
\ I
E-21
N
o O
E-22
o~o.
E-23~
'
Ci
o 'Ld
o.
E-24
I O ~N
O'
IL1I
E-25~N
~~
N
CI ~O~
O
/
I N
E-26~
cy I
%
0
E-27
0
I
/ I N
E-28
o c
E29
0 0
I
E-30
~
o~o.
195
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
General Procedures F
[00847] N-[1-(3-Methoxy-benzoyl)-2,2-dimethyl-1,2,3,4-tetrahydro-quinoliu-4-
yl]-N-phenyl-
acetamide (F-1)
[00848] N-[1-(3-Methoxy-benzoyl)-2,2-dimethyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-N-phenyl-
acetamide from 4-(hydroxy-2,2-dimethyl-3,4-dihydro-2H-quinolin-1-yl)-(3-
methoxy-plienyl)-
methanone which was synthesized according to reference Hamann, L. G.; Higuchi,
R. I. ; Zhi, L.;
Edwards, J. P.; Wang, X.; Marrschke, K. B. ; Kong, J. W.; Farmer, L. J.;
Jones, T. D. J. Med. Chern
1998, 41, 623. This was further elaborated to N-[1-(3-methoxy-benzoyl)-2,2-
dimethyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide using an in-situ formation of the
iodide and
displacement with the aniline according to the following procedure
[00849] To a chilled solution of (4-hydroxy-2,2-dimethyl-3,4-dihydro-2H-
quinolin-1-yl)-(3-
methoxy-phenyl)-methanone (500 mg, l.6mmo1) in 10 ml dichloromethane was added
slowly 0.8 ml
iodotrimethylsilane (5.6mmo1) at 0 °C. The resulting reaction mixture
was stirred at 0 °C for 6 hours.
Then the mixture was concentrated under vacuum. The residue was dissolved in
12 ml THF. BaC03
(630mg, 3.2mmol) and aniline (0.17 ml, 1.92mmo1) was added. The mixture was
stirred at RT
overnight. The mixture was filtered and the filtrate was concentrated under
vacuum. The residue was
purified by silica gel chromatography, eluting with ethyl acetate-hexane (1:4)
to give (2,2-dimethyl-4-
phenylamino-3,4-dihydro-2H-quinolin-1-yl)-(3-methoxy-phenyl)-methanone (150
mg, 24%).
[00850] To a solution of (2,2-dimethyl-4-phenylamino-3,4-dihydro-2H-quinolin-1-
yl)-(3-
methoxy-phenyl)-methanone in methylene chloride (5 mL) was added
diisopropylethylamine
followed by acetyl chloride. The mixture was sturred at room temperature over
night. The mixture
was poured into water and extracted with dichloromethane. The extracts were
washed with 1 M (aq)
NaOH and brine, dried over magnesium sulfate, filtered, dried and
concentrated. The crude residue
was purified by silica gel chromatography (50% hexanes/50% ethyl acetate) to
afford N-[1-(3-
methoxy-benzoyl)-2,2-dimethyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
acetamide.
'H-NMR (CDC13) 8: 1.5 (1H, m), 1.6 (3H, s), 1.7 (3H, s), 1.9 (1H, m), 2.0 (3H,
3), 3.7 (3H, m), 5.8
(1H, m), 6.5 (1H, d), 6.6-7.1 (8H, m), 7.2 (1H, m), 7.3-7.5 (3H, d).
MS m/z: 429 (M+1).
[00851] (2S,4R)-4-Chloro-N-ethyl-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-benzamide (F-2)
[00852] (2S,4R)-4-Chloro-N-ethyl-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-benzamide was synthesized as described in general procedure C,
except following
benzyl carbamate removal the amine was modified in the following manner. To a
solution of (2S,4R)-
(4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-yl)-(4-methoxy-phenyl)-methanone
(200 mg,
196
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
0.68mmo1) in 20 ml dichloromethane was added acetaldehyde (0.042 mL,
0.75mnnol). The reaction
mixture was stirred 30 min at room temperature. Then sodium
triacetoxyborohydride (0.156 g,
0.75mmo1) was added and the resulting reaction mixture was stirred at room
temperature for 6 hours.
N,N-diisopropylethylamine (0.3 mL, 2.3mmol) and 4-chlorobenzoyl chloride (0.4
mL, 3.lmmol) was
added and stixred at room temperature overnight. Dichloromethane (40 ml) was
added. The mixture
was washed with 30 ml sodium hydroxide (1N). The organic layer was dried with
magnesium sulfate,
filtered and concentrated i~z vacuo. The residue was purified by silica gel
chromatography, eluting
with ethyl acetate-dichloromethane (1:4) to give 80 mg (24 %) title compound
'H-NMR (CDCl3) 8: 1.2-1.4 (7H, m), 1.7 (1H, m), 2.7 (1H, m), 3.1 (1H, m), 3.8
(3H, s), 4.2 (1H, m),
4.8 (1H, m), 6.5 (1H, d), 6.6 (2H, d), 6.8 (2H, m), 6.9 (1H, m), 7.1-7.5 (6H,
m)
MS m/z: 463 (M+1).
[00853] N-[1-(3-Methoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
acetamide (F-
3)
[00854] N-[1-(3-Methoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-
acetamide was
made from 1-(4-methoxy-benzoyl)-2,3-dihydro-1H-quinolin-4-one which was
synthesized according
to reference Bellassou-Fargeau, M. C.; Graffe, B.; Sacquet, M. C.; Maitte, P.
J. of Heter. Chern.
1985, 22(3), 713. This was further elaborated to (4-hydroxy-3,4-dihydro-2H-
quinolin-1-yl)-(3-
methoxy-phenyl)-methanone by reduction of the ketone to the alcohol and in-
situ formation of the
iodide and displacement with the aniline according to the following procedure
the following
procedure. To a solution of 1-(3-methoxy-benzoyl)-2,3-dihydro-1H-quinolin-4-
one (310 mg,
l.lmmol) in 5 ml methanol was added 410 mg sodium borohydride (4.4mmo1). The
resulting
reaction.mixture was stirred at room temperature for 3 hours. The mixture was
concentrated under
vacuum and the residue was purified by silica gel chromatography, eluting with
ethyl acetate-hexane
(1:2) to give (4-hydroxy-3,4-dihydro-2H-quinolin-1-yl)-(3-methoxy-phenyl)-
methanone (215 mg,
69%). This was further elaborated to (3-methoxy-phenyl)-(4-phenylamino-3,4-
dihydro-2H-quinolin-
1-yl)-methanone using the following procedure. To a chilled~solution of (4-
hydroxy-3,4-dihydro-2H-
quinolin-1-yl)-(4-methoxy-phenyl)-methanone in dichloromethane was added
slowly
iodotrimethylsilane at 0 °C. The resulting reaction mixture was stirred
at 0 °C for 6 hours. Then the
mixture was concentrated under vacuum. The residue was dissolved in THF. BaC03
and aniline was
added. The mixture was stirred at room temperature overnight. The mixture was
filtered and the
filtrate was concentrated under vacuum. The residue was purified by silica gel
chromatography,
eluting with ethyl acetate-hexane (1:4) to give (4-phenylamino-3,4-dihydro-2H-
quinolin-1-yl)-(3-
methoxy-phenyl)-methanone
[00855] To a solution of (4-phenylamino-3,4-dihydro-2H-quinolin-1-yl)-(4-
methoxy-phenyl)-
methanone in methylene chloride was added diisopropylethylannine followed by
acetyl chloride. The
197
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
mixture was stirred at room temperature over night. The mixture was poured
into water and extracted
with dichloromethane. The extracts were washed with 1 M (aq) NaOH and brine,
dried over
magnesium sulfate, filtered dried and concentrated. The crude residue was
purified by silica gel
chromatography (50% hexanes/50% ethyl acetate) to afford (~)-N-[1-(3-methoxy-
benzoyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-N-phenyl-acetamide. '
'H-NMR (CDC13) 8: 1.2 (1H, m),1.9 (3H, s), 2.1 (1H, m), 2.3 (1H, m), 3.5 (1H,
m), 3.7 (3H, m), 4.1
(1H, m), 6.4 (2H, m), 6.6 (1H, m), 6.8-7.3 (6H, m), 7.4 (3H, m), 7.5 (1H, d).
MS m/z: 401 (M+1).
Table 6: Structurally Diverse Series
: r ~w''t~'ilctnr~'~ ~ : Stiuoture
No' < . ..~: Nab,!.. -
~
r
~o
,o
~N~
G
F_2 ~.."
N
3
i
,o
198
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00856] The Disclosed Compounds Inhibit Binding of PGDZ to CRTH2
[0085?] This radioligand membrane binding assay evaluates the ability of
compounds to inhibit
[3H] Prostaglandin Dz (PGDz) binding to the cloned human CRTH2 receptor stably
expressed in
HEK-293 cells (expressing human CRTh2 receptor and a subunit or the
heterotrimeric G protein 16
were prepared by Biosignal Company) using Scintillation Proximity Assay.
[00858] A binding buffer containing 50mM Tris-HCl (pH 7.5), 5mM MgCl2 and 1mM
EDTA was
prepared immediately prior to performing the assay. A bead/membrane solution
at twice the final
assay concentration comprising membranes (membranes bought from Biosignal)
from the HEK-293
cells cloned to express CRTH2 receptor bound to and [3H] PGDZ at twotimes the
final assay
concentration were prepared and stored on ice before adding to wells. Cold
PGD2 at twenty times the
final assay concentration was prepared and stored on ice before adding to
wells defining non-specific
binding (NSB) corning plates #3653 were used for this assay.
[00859] 10 mM stock concentrations of compounds in 100% DMSO were prepared and
stored at
room temperature. A 10 point concentration response curve was then constructed
for each compound,
starting at 10 pM (final assay concentration). The compounds were prepared at
40 times final assay
concentrations with nine consequent-3-fold dilutions.
[00860) 0.1 p,1 of each concentration of compound were added to the
appropriate well of the 384
plate and 2 p1 of cold PGDZ was added into the wells defining NSB. 20 p1 of
[3H] PGDz and then 20
p,1 of 2x of bead/membrane solution were then added to each well.
[00861) The plates were allowed to incubate at room temperature for
approximately 2 hours and
then counted on Packard Topcount using SPA tritium protocol for 1 minute/well.
[00862] The percent inhibition of PGDz binding (PGDZ used at the KD value or
lower) to the
HEK-293 cell membranes was determined, the assay was always run as duplicate
for n=1 for a total of
n=2.
[00863] Compounds A-3, A-11, A-16, A-17, A-20, A-24, A-35, A-49, A-51, A-54, A-
55, A-67,
A-70, A-72, A-73, A-81, A-82, A-120, A-130, A-131, A-132, A-143, A-144, A-147,
A-153, A-156,
A-157, A-159, B-7, B-9, B-11, B-13, B-18, B-20, B-26, B-28, B-34, B-39, B-40,
B-47, B-51, B-58,
B-59, B-63 to B-66, B-68, B-70, B-73, B-74, B-84, B-86, B-97, B-101 to B-112,
C-33, C-37, C-38,
D-1, D-2, D-6, D-10, F-3 have K; <10 uM.
[00864] Compounds A-8, A-53, A-58, A-124, A-126, A-154, B-53, B-100, F-1 have
K; <60 uM.
[00865] All remaining compounds have K; <1 uM
199
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
Ammonium acetate-standard conditions:
% A (Water) 95.0 HP1100 LC Pump Gradient Timetable
% B (Acetonitrile) 5.0
% Ammonium acetate 0.1 The gradient Timetable contains
4 entries which are:
Flow (ml/min) 2.500
Stop Time (mins) 3.8 Time A% B% C% D% Flow Pressure
Min Pressure (bar) 0 0.00 95.0 5.0 0.0 0.0 2.500 400
Max Pressure (bar) 400 2.00 0.0 100.0 0.0 0.0 2.500400
Oven Temperature 10.0 3.00 0.0 100.0 0.0 0.0 2.500400
Left(C)
Oven Temperature 3.05 95.0 5.0 0.0 0.0 2.000 400
Right(C) 10.0
LC-MS data were acquired using the "Ammonium acetate-standard" method unless
otherwise noted.
Scheme 17
1) 1.1 eq CDI, THF
p Ph. 2) 1.0 eq H2NC(O)OBn, - 10 C
1) PhNH2,H20, 80 C NCO H 3) Charge i.5 eq 1 M LiOtBu in THF
'; . ~ 2
93.7 % ee 1, 72% yield
94 % ee
1 ) 10 vol -EtOH, - 10 C, 0.7 eq NaBH4 NHCbz
Ph, 2) 1.05 eq MgCl2 (aq.)
N 3) DCM, 1 N HCI, 2.5 eq citric acid
N~CbZ ~ N 'o,,
H H
2, 92% crude yield 3, 85% crude yield
94% ee
NHCbz
Recryst
EtOAclheptane
,~~~.
H
99% ee
60% recovery
Scheme 18
200
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1) 1.0 equiv ~O (93.7% ee)
~~O
Me'~ charged over 1.5h at 80 C Ph
2 equiv Aniline NH
in 20 vol H20 ~C02H
2) K2C03 to pH 10 then MTBE extract
3) 6 N HCI to pH 5 then EtOAc extract 1
72% Mass Recovery
94% ee
[00$66] (S)-3-Phenylamino-butyric acid (1)
[00867] A jacketed 30-L 3-neck bottom-valve cylindrical reactor equipped with
overhead stirrer,
reflux condenser, addition funnel, and NZ inlet was flushed with N2. Water
(8.7 L) was charged
followed by aniline (423 L, 4.9 mol). Stirring was initiated and the internal
temperature set for 80
°C. After the internal temperature reached 77 °C, (R)-(3-
butyrolactone was charged over 1.5 h via
addition funnel. The internal temperature was maintained between 80 - 81.7
°C during the course of
addition. Once addition was completed, reaction was cooled to 20 °C
over 1 h. KZC03 (250 g, 1.8
mol, 0.75 equiv) was charged as a solid, and the aqueous pH was determined to
be 10. The aqueous
phase was extracted with MTBE (3 x 2 L), and the extracts were discarded.
After adjusting the pH of
the aqueous phase to 5 with 6 N HCl (225 mL), it was extracted with EtOAc (3 x
2 L). After each
extraction, the pH was checked and was readjusted to 5 with 6 N HCl as
necessary. The combined
EtOAc extracts were washed with saturated brine (2 L), dried with MgSO4, and
concentrated via
rotary evaporation to produce a pink oil. Recovery: 300 g (72 % ).
Enantiomeric excess was
determined by chiral HPLC to be 94%.
'H-NMR (CDC13) 8: 7.24 (t, 2 H); 6.81 (t, 1 H); 6.73 (d, 2 H); 3.93 (m, 1 H);
2,68 (dd, 1 H); 2.51 (dd,
1 H); 1.30 (d, 3 H).
Scheme 19
Ph Ph
~NH 1 ) 1.1 equiv CDI, THF NH O OII
j~C02H ~N~O
2) 1.0 equiv HZNC(O)OBn, -10 C H
1 2
3) 1.5 equiv 1 M LiOtBu in THF
98% Crude Yield
201
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00868] (S)-(Phenylamino-butyryl)-carbamic acid benzyl ester (2)
[00869] A 5-L, jacketed, glass reactor equipped with overhead stirrer,
addition funnel and
thermocouple was flushed with N2. A solution of (S)-3-phenylaminobutyric acid
(1,1008, 558 mmol)
in 800 mL THF (8 volumes relative to amino acid) was charged to the reactor.
The internal
temperature of the reaction mixture was set to 20 °C. 1,1'-
Carbonyldiimidazole (99.5 g, 614 mmol)
was charged to the stirred solution to produce a clear solution along with a
mild exotherm (5 °C) and
much gas evolution. After 1 h, benzyl carbamate (84.3 g, 558 mmol) was
charged, and the internal
temperature was set for -15 °C. At -15 °C, a 1 M solution of
LiOt-Bu in THF (837 mL, 837 mmol)
was charged to the reaction via addition funnel in 30 rnin so as to maintain
the internal temperature
between -10 and -15 °C. The solution becomes opaque pale brown and more
viscous. The internal
temperature is maintained at about -10 °C for another 15 min and then
allowed to warm to ambient
temperature. After 16 h, the reaction was quenched by the addition of 1000 mL
EtOAc (10 volumes)
followed by the 280 mL 1N HCl and 1000 mL Hz0 (10 volumes). The resulting
aqueous phase was
pH 9. The phases were separated, and the aqueous phase was extracted with
another 600 mL EtOAc
~' (6 volumes). The organic phases were combined and washed with 1000 mL 10%
brine (10 volumes).
The resulting organic solution of crude product is distilled down to a minimal
volume of 500 to 700
mL at 170 to 200 torn and a pot temperature of 35 to'40 °C. This crude
product was partitioned
between 2.0 L 1N HCl (20 volumes) and 1.0 L MTBE. An amber oily phase formed
which was the
HCI salt of the product and was kept with the aqueous acid phase. The MTBE
phase was extracted
with another 1000 mL 1N HCI (10 volumes), and the combined HCl phases were
backwashed with
another 500 nnL MTBE. The pH of the aqueous solution was adjusted to 9-10 by
the addition of 495
mL saturated aqueous K2CO3. The aqueous basic solution was extracted twice
with 800 mL EtOAc
(8 volumes), and the organic phases were combined, washed with 500 mL brine,
dried over NazS04
and evaporated to produce 161 g (92 %) of crude amber oil.
'H-NMR (CDC13) 8: 7.35 (m, 5 H); 7.15 (t, 2 H); 6.72 (t, 1 H); 6.61 (d, 2 H);
5.15 (s, 2 H); 4.03 (m, 1
H); 3.02 (dd, 1 H); 2.84 (dd, 1 H); 1.28 (d, 3 H).
Scheme 20
O
Ph
NH O O HN~O
~~ 1) 10 vol EtOH, 0.7 equiv NaBH4, -10 C
~H O ~ ~ ~ ~
2) 1.05 equiv aqueous MgCl2 H ~Me
2
3) CH2CI2, 1 N HCI, 2.5 equiv citric acid g g5%, 8q.% ee
[00870] (2S,4R)- (2-Methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-carbamic acid
benzyl ester (3)
202
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00871] A 5-L, jacketed, glass reactor equipped with overhead stirrer,
addition funnel and
thermocouple was flushed with N2. A solution of (S)-(Phenylamino-butyryl)-
carbamic acid benzyl
ester (2) (160g, 512 mmol) in EtOH (960 mL, 6 volumes) was charged to the
reactor, and the internal
temperature was set to -15 °C. The NaBH4 (13.68, 359 mmol) was charged
to the stirred solution in 3
portions. An aqueous solution of MgC12~6H20 (109g, 538 mmol in 110 mL water)
was charged to the
reactor via addition funnel over 1 h to maintain the internal temperature
between -10 to -5 °C. After
the addition was complete, the internal temperature was adjusted to 0
°C and the mixture was allowed
to stir another 30 min. The reactor was charged with 960 mL CHzCIz (6
volumes), citric acid (246g,
1280 mmol), and 960 mL 1N HCl (6 volumes). The internal temperature was set
for 20 °C and the
mixture was allowed to stir for 16 h. The reaction mixture was diluted with
960 mL EtOAc and the
aqueous pH was adjusted to 9 by the addition of 470 mL saturated aqueous
KZC03. The phases were
separated and the organic phase washed twice with 640 . mL water and then 640
mL brine. The
organic phase was dried over Na2S04 and evaporated to provide 130 g of a solid
(86% yield). The
crude product was recrystallized from 20 volumes of a 9/1, heptane/EtOAc
mixture. The solid was
slurried with 260 mL EtOAc (2 volumes) and 780 mL heptane (6 volumes) and
heated to 75 °C to
provide a clear solution. At 75 °C, another 260 mL heptane (2 volumes)
was slowly added followed
by gradual cooling. Crystallization usually begins around 58 to 68 °C.
At ambient temperature,
another 1300 mL heptane (10 volumes) was slowly added. After stirring at
ambient another 2 hr, the
solids are filtered and washed with 4 volumes of 9l1 heptane/EtOAc. The solids
were dried in a
vacuum oven to provide 90 g (60% recovery). The product had an enantiomeric
excess of 99.2% .as
determined by chiral HPLC.
1H-NMR (CDCl3) 8: 7.38 (m, 5H); 7.22 (d, 1H); 7.07 (t, 1 H); 6.78 (t, 1~H);
6.69 (d, 1 H); 5.17 (s, 2
H); 5.07 (m, 1 H); 4.94 (d, 1 H); 3.58 (m, 1 H)2.33 (m, 1 H); 1.60 (q, 1 H);
1.30 (d, 3 H).
Scheme 21
cl~
\ ~ N~.
NHCbz CI \ N_ HCbz \ N_ H
~~ , F \ 1. H2 PdIC I \ D1PEA, I \
\
I ~ N ~~'CH 2~ Cu(OAc)2, Pyridine ~ N ~"CH3 O ~ N ''CH3
CH2CI2, DI PEA II
\ CI~ O \
I ~ i ' i F
6
4 F B(OH)2 5 F
203
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00872] General Procedure G: (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-fluoro-
benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (6)
[00873] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid
benzyl ester (1.0 g, 3.38 mmol) in methylene chloride (50 mL) at room
temperature was added
diisopropylethylamine (650 uL, 3.72 mmol) followed by 4-fluorobenzoyl
chloride. The reaction was
stirred over night at room temperature. The mixture was poured into water and
extracted with ethyl
acetate. The extracts were washed with 1 M (aq) NaOH and brine, dried over
magnesium sulfate,
filtered dried and concentrated. The crude residue was purified by silica gel
chromatography (75%
hexanes / 25 % ethyl acetate) to afford the pure amide (720 mg, 51 %).
[00874] (2S,4R)-[1-(4-fluorobenzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-carbamic acid
benzyl ester (720 mg, 1.73 mmol) was dissolved in ethanol (30 mL). The vessel
in which resided the
resulting solution was evacuated and backfilled with argon. A catalytic amount
of Palladium on
Carbon (10 %) was added. The vessel was once again evacuated and this time was
backfilled with
hydrogen and shaken in a Parr bottle at 40 psi hydrogen. Reaction was complete
after 4 h. The
mixture was carefully filtered and concentrated to 10% volume. The resulting
concentrated solution
was filtered through an Celite ~ and concentrated to afford the crude amine.
[00875] To a solution of (2S, 4R)- (4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-
yl)-(4-fluoro-
phenyl)-methanone (1.0 g, 3.5 mmol) in DMF (20 mL, dry) was added 4-
chlorophenylboronic acid
(1.1 g, 7:0 mmol), pyridine (850 uL, 10.5 mmol) and copper(II)acetate (1.27 g,
7.0 mmol). The
heterogeneous green mixture was stirred open to air for 1 h and then warmed to
60 °C and stirred over
night (14 h). The mixture was then cooled to rt, poured into rapidly stirred
ethyl acetate (150 mL);
solids were removed by filtration through Celite ~. The extracts were washed
several times with
water and then once with brine. The extracts were then'dried over anhydrous
magnesium sulfate,
filtered, and concentrated under reduced pressure. The crude residue was
purified by silica gel
chromatography (95 % methylene chloride / 5 % ethyl acetate) to afford the
aniline product (250 mg,
18 %) as a yellow oil.
[00876] To a solution of (2S, 4R)- [4-(4-chloro-phenylamino)-2-methyl-3,4-
dihydro-2H-quinolin-
1-yl]-(4-fluoro-phenyl)-methanone (250 mg, 0.636 mmol) in acetyl chloride (5
mL) was added
diisopropylethylamine (120 uL, 0.70 mmol). The mixture was stirred at rt 4 h.
The mixture was
concentrated under reduced pressure, dissolved in ethyl acetate, Washed with
sat. aqueous sodium
bicarbonate, brine and dried over magnesium sulfate, filtered, and
concentrated under reduced
pressure. The residue was purified by silica gel chromatography (25/75
hexanes/ethyl acetate
gradient) to afford pure N-(4-chloro-phenyl)-N-[1-(4-fluoro-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide (200 mg, 71 %).
204
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 1.1 (d, 3H), 1.1 (m, 1H), 2.0 (d, 3H), 2.3 (m, 1H), 4.7 (m,
1H), 5.6 (m, 1H), 6.5
(d, 1H),6.7-7.0 (m, 3H), 7.1-7.4 (m, 8H).
MS m/z: 436 (M+1).
[00877] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenyl)-piperazine-1-carboxylic acid ethyl ester (G-1)
[00878] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quirioline-
1-carbonyl}-phenyl)-piperazine-1-carboxylic acid ethyl ester was made
following general procedure
G, substituting 4-(4-chlorocarbonyl-phenyl)-piperazine-1-carboxylic acid ethyl
ester for 4-
fluorobenzoyl chloride. The rest of the procedure is followed as indicated in
general procedure G to
yield (2S,4R)- 4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenyl)-piperazine-1-carboxylic acid ethyl ester.
[00879] 4-(4-Chlorocarbonyl-phenyl)-piperazine-1-carboxylic acid ethyl ester
was prepared by the
following procedure. Piperdine and 4-fluoro-benzoic acid methyl ester were
heated at 65°C in DMSO
for 48 h (US Patent 6069143). The reaction was quenched with NaHCO3 and
extracted 3x ethyl
acetate was dried over MgS04, filtered and concentrated down. The ester was
used directly. 4- .
Piperazin-1-yl-benzoic acid methyl ester was acetylated with ethyl
chloroformate and DIEA in
CHaClz to give 4-(4-methoxycarbonyl-phenyl)-piperazine-1-carboxylic acid ethyl
ester. The methyl
ester was hydrolyzed with to the acid by dissolving in tetrahydrofuran and
ethanol and sodium
hydroxide (1N) was added. The mixture was stirred at room temperature 2 hours.
The mixture was
cooled to 0 °C, acidified to form a' white precipitate. The solid was
filtered to give 4-(4-
methoxycarbonyl-phenyl)-piperazine-1-carboxylic acid. The acid was converted
to the acid chloride
by reaction with oxalyl chloride.
'H-NMR (CDC13) 8: 1.10 (d, 3H), 1.24 (m, 4H), 2.00 (s, 3H), 2.27 (m, 1H), 3.13
(t, 4H), 3.55 (t, 4H),
4.12 (q, 2H), 4.70 (m,1H), 5.56 (brs, 1H), 6.54 (d,1H), 6.62 (d, 2H), 6.91 (t,
1H), 7.09 (d, 2H), 7.14
d, 2H), 7.27 (m, 2H), 7.36 (d, 2H).
MS m/z: 575.16 (M+1).
[00880] N-{3-[(glycoloylamino)methyl]phenyl}-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide (G-2)
[00881] N-{3-[(glycoloylamino)methyl]phenyl}-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide was prepared following general
procedure G, substituting
4-methoxybenzoyl chloride for 4-fluorobenzoyl chloride, and (3-
cyanophenyl)boronic acid for 4-
chlorophenylboronic acid. The rest of the procedures were followed as
indicated in general procedure
205
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
A to afford N-(3-cyanophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide.
[00882] N-(3-cyanophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was further treated with cobalt(II) chloride
and sodium
borohydride (leq, 3 eq) to afford N-[3-(aminomethyl)phenyl]-N-[(2S,4R)-1-(4-
methoxybenzoyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide.
[00883] N-[3-(aminomethyl)phenyl]-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was then further coupled with glycolic acid
using coupling reagent
such as EDCI with HOBt to afford N-{ 3-[(glycoloylamino)methyl]phenyl }-N-
[(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide
'H -NMR (CDC13) 8: 1.1-1.2 (m, 4H), 2.0 (s, 3H), 2.3 (m, 1H), 3.8 (s, 3H), 4.2
(m, 1H), 4.5 (m, 2H),
.-4.7 (m, 2H), 5.6 (br, 1H), 6.5 (m, 1H), 6.6 (m, 2H), 6.9 (m, 1H), 7.1-7.4
(m, 8H).
MS m/z: 502 (M + 1).
[00884] .. N-(4-chloro-2-fluorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-3)
[00$85] N-(4-chloro-2-fluorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was made following general procedure G,
substituting 4-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. The amine-aryl coupling
was performed
differently to what is described in procedure G. Therefore (2S,4R)-1-(4-
methoxybenzoyl)-2-methyl- '
1,2,3,4-tetrahydroquinolin-4-amine (obtained from the hydrogenation step, 200
mg, 0.6 mmol, 1 equ.)
was dissolved in ethylene glycol dimethyl ether (1 mL) in a Schlenk tube. To
this solution was added
sequentially 1-bromo-4-chloro-2-fluorobenzene (105 uL, 0.84 mmol,1.4 equ.),
cesium carbonate (274
mg, 0.84 mmol, 1.4 equ.), palladium acetate (16 mg, 0.024 mmol, 0.04 equ.) and
2-
(Dicyclohexylphosphino)-2',4',6'-tri-i-propyl-1,1'-biphenyl (23 mg, 0.048
mmol, 0.08 equ.). The
reaction mixture was flushed with nitrogen and heated to 95°C in the
Schlenk tube for 48 h. Reaction
mixture was concentrated to leave a residue which was partitioned between
water and ethyl acetate
and extracted. The aqueous layer was separated and the organic layer was
washed with brine, dried
over magnesium sulfate, filtered and concentrated to give a black oil. The
crude product was purified
by silica gel chromatography (methylene chloride/methanol: 100/0 to 99/1
gradient) to provide
(2S,4R)-N-(4-chloro-2-fluorophenyl)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
amine (60 mg, 24 %).
[00886] To a solution of (2S,4R)-N (4-chloro-2-fluorophenyl)-1-(4-
methoxybenzoyl)-2-methyl
1,2,3,4-tetrahydroquinolin-4-amine (60 mg, 0.14 mmol, 1 equ.) in acetyl
chloride (0.5 mL) was added
diisopropylethylamine (25 uL, 0.14 mmol, 1 equ.). The mixture was stirred at
room temperature for
16 h. The mixture was concentrated under reduced pressure, dissolved in ethyl
acetate, washed with
206
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
sat. aqueous sodium bicarbonate, brine and dried over magnesium sulfate,
filtered, and concentrated
under reduced pressure. The residue was purified by silica gel chromatography
(methylene
chloride/methanol 99.5/0.5) to afford pure N-(4-chloro-2-fluorophenyl)-N
[(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide (140 mg, 92
%).
'H-NMR (CDC13) 8: 1.15 (d, 3H), 2.05 (s, 3H), 2.30 (m, 1H), 3.75 (s, 3H), 4.75
(m, 1H), 5.65 (m,
1H), 6.50 (d,1H), 6.70 (d, 2H), 7.00 (t,1H), 7.15-7.20 (m, 4H), 7.25-7.40 (m,
3H).
MS m/z: 467 (M+1).
[0088?] 4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}benzamide (G-4)
[00888] N (4-chlorophenyl)-N [(2S,4R)-1-(4-cyanobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquiriolin-
4-yl]acetamide (143 mg, 0.33 mmol) and potassium hydroxide (55 mg, 1.00 mmol))
were dissolved in
water (150 mL), ethanol (3 mL) and heated to 70 °C for 4h. The slurry
was portioned between 1N
HCl (until acidic) and methylene chloride. The organic layer was collected,
concentrated and
subjected to flash chromatography (EtOAc to 20% MeOH, EtOAc) to afford the
title compound as a
white solid.
'H-NMR (CDCl3) 8: 1.15-1.25 (m, 1H), 1.17 (d, 3H), 2.11 (s; 3H), 2.20-2.34 (m,
1H), 4.70-4.84 (m,
1H)" 5.50-5.80 (m, 1H), 5.80-6.00 (m, 1H), 6.40-6.60 (m, 1H), 6.40 (d, 1H),
6.83 (t, 1H), 7.08-7.30
(m, 8H), 7.60 (d, 2H).
MS m/z: 462 (M +1)
[00889] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-2-fluoro-phenoxy)-2,2-dimethyl-butyric acid methyl ester
(G-5)
[00890] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid
benzyl ester in methylene chloride at room temperature was added
diisopropylethylamine followed by
3-fluoro-4-methoxy-benzoyl chloride. The reaction was stirred over night at
room temperature. The
mixture was poured into water and extracted with ethyl acetate. The extracts
were washed with 1 M
(aq) NaOH and brine, dried over MgS04, filtered dried and concentrated. The
crude residue was
purified by silica gel chromatography (75% hexanes / 25 % ethyl acetate) to
afford the pure amide.
[00891] (2S,4R)-[1-(3-Fluoro-4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-
carbamic acid benzyl ester was dissolved in ethanol (30 mL). The vessel in
which resided the
resulting solution was evacuated and backfilled with argon. A catalytic amount
of Palladium on
Carbon (10 %) was added. The vessel was once again evacuated and this time was
backfilled with
hydrogen and shaken in a Parr bottle at 40 psi hydrogen. Reaction was complete
after 4 hours. The
mixture was carefully filtered and concentrated to 10% volume. The resulting
concentrated solution
was filtered through an Celite ~ and concentrated to afford the crude amine.
207
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00892] To a solution of (2S,4R)-(4-Amino-2-methyl-3,4-dihydro-2H-quinolin-1-
yl)-(3-fluoro-4-
methoxy-phenyl)-methanonein in DMF (dry) was added 4-chlorophenylboronic acid,
pyridine and
copper(I~acetate. The heterogeneous green mixture was stirred open to air for
1 hour and then
warmed to 60 °C and stirred over night (14 h). The mixture was then
cooled to room temperature,
poured into rapidly stirred ethyl acetate (150 mL); solids were removed by
filtration through Celite ~.
The extracts were washed several times with water and then once with brine.
The extracts were then
dried over anhydrous MgS04, filtered, and concentrated under reduced pressure.
The crude residue
was purified by silica gel chromatography (95 % methylene chloride / 5 % ethyl
acetate) to afford the
aniline product as a yellow oil.
[00893] To a solution of (2S,4R)-[4-(4-chloro-phenylamino)-2-methyl-3,4-
dihydro-2H-quinolin-1'=
yl]-(3-fluoro-4-methoxy-phenyl)-methanone in methylene chloride was added
diisopropylethylamine
followed by acetyl chloride. The mixture was stirred at rt 4 h. The mixture
was concentrated undex
reduced pressure, dissolved in ethyl acetate, washed with sat. aq. NaHC03,
brine and dried over
magnesium sulfate, filtered, and concentrated under reduced pressure. The
residue was purified by
silica,gel chromatography (25!75 hexanes/ethyl acetate gradient) to afford
pure the final product.
[00894] N-(4-Chloro-phenyl)-N-[1-(3-fluoro-4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (115 mg, 0.256 mmol) was dissolved in DMF (5 mL) at
room temperature.
KZCO3 (175 mg, 1.28 mmol) and 5-bromo-2,2-dimethyl-pentanoic acid ethyl ester
(100 mg, 0.511
mmol) was added and the reaction mixture was allowed to stir over night. The
mixture was
partitioned between methylene chloride and water; the methylene chloride layer
was dried over
MgS04, filtered and concentrated. The crude residue was purified by silica gel
chromatography (2/1
hexanes/ ethyl acetate - ethyl acetate gradient) to afford the product. -
1H-NMR (CDCl3) b: 1.1 (d, 3H),1.2 (s, 6H), 1.2 (m, 1H), 2.0 (s, 3H), 2.0 (t,
1H), 2.3 (m, 1H), 3.8 (s~
3H), 4.0 (t, 2H), 4.7 (m, 2H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.6 (t, 1H), 6.7 (d,
1H), 6.9 (t, 1H), 7.2 (m;
5H), 7.4 (d, 2H).
MS m/z: 581 (M+1).
[00895] (2S,4R)-N-(4-chloro-phenyl)-N-[1-(3,4-dihydro-2H-benzo[1,4]oxazine-6-
carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (G-6)
[00896] 6-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-carbonyl}-
2,3-dihydro-benzo[l,4]oxazine-4-carboxylic acid ethyl ester was dissolved in
methylene chloride (3
mL) and iodotrimethylsilane (1 mL). After 4 days, the reaction was quenched
with sat. aq. NaHC03.
The residue was partitioned between methylene chloride and water, then
extracted three times with
methylene chloride, dried over MgS04, filtered and concentrated. The crude
residue was purified by
silica gel chromatography (ethyl acetate) to afford the product.
208
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) 8: 1.1 (d, 3H), 1.2 (m, 1H), 2.0 (s, 3H), 2.3 (m, 1H), 3.3 (m,
2H), 4.2 (m, 2H), 4.7
(m, 1H), 5.5 (m, 1H), 6.4 (d,1H), 6.5 (d, llTj, 6.6 (d, 2H), 6.96 (t,1H), 7.2
(m, SH), 7.4 (d, 2H).
MS m/z: 476 (M+1).
[00897] N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-[3-(trifluoromethyl)-1H-
pyrazol-1-
yl]benzoyl}-1,2,3,4-tetrahydroquinolin-4-yl)acetamide (G-7)
[00898] N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-[3-(txifluoromethyl)-1H-
pyrazol-1-
yl]benzoyl}-1,2,3,4-tetrahydroquinolin-4-yl)acetamide was prepared following
general procedure A,
substituting 4-[3-(trifluoromethyl)-1H-pyrazol-1-yl]benzoyl chloride for 4-
fluorobenzoyl chloride. (4-
[3-(trifluoromethyl)-1H-pyrazol-1-yl]benzoyl chloride was prepared in one step
from 4-[3-
(trifluoromethyl)-1H-pyrazol-1-yl]benzoic acid . 4-[3-(Trifluoromethyl)-1H-
pyrazol-1-yl]benzoic acid
was treated with oxalyl chloride in DCM and catalytic DMF to afford 4-[3-
(trifluoromethyl)-1H-
pyrazol-1-yl]benzoyl chloride in decent yield). The rest of the procedures
were followed as indicated
in general procedure A to afford N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-
[3-(trifluoromethyl)-
1H-pyrazol-1-yl]benzoyl }-1,2,3,4-tetrahydroquinolin-4-yl)acetamide.
'H-NMR (CDC13) 8: 1.20 (d, 3H ; t; 1H), 2.03 (s, 3H), 2.33 (m, 1H), 4.80 (m,
1H), 5.62 (m, 1H),
6.47 (d, 1H), 6.77 (d, 1H), 6.90 (t, 1H), 7.00-7.40 (m, 10H)., 7.66 (d,1H)
MS m/z: 553 (M + 1).
[00899] (2S,4R)-N-(4-Chloro-phenyl)-N-[2-methyl-1-(4-methyl-3,4-dihydro-2H
benzo[1,4]oxazine-7-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (G-
8)
[00900] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid
benzyl ester (400 mg, 1.36 mmol) in methylene chloride (4 mL) at room
temperature was added
triethylamine (0.320 mL, 2.3 mmol) followed by 4-methyl-3,4-dihydro-2H-
benzo[1,4]oxazine-7-
carbonyl chloride (1.5 mmol). The reaction was stirred over night at room
temperature. The mixture
was poured into water and extracted with ethyl acetate. The extracts were
washed with 1 M (aq)
NaOH and brine, dried over MgS04, filtered and concentrated. The crude residue
was purified by
silica gel chromatography (75% hexanes / 25 % ethyl acetate) to afford the
pure amide.
[00901] (2S,4R)-[2-methyl-1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-
carbonyl)-1,2,3,4-
tetrahydro-quinolin-4-yl]-carbamic acid benzyl ester (300 mg) was dissolved in
ethanol. The vessel
in which resided the resulting solution was evacuated and backfilled with
argon. A catalytic amount
of Palladium on Carbon (10 %) was added. The vessel was once again evacuated
and this time was
backfilled with hydrogen and shaken in a Parr bottle at 40 psi hydrogen.
Reaction was complete after
4 hours. The mixture was carefully filtered and concentrated to 10% volume.
The resulting
concentrated solution was filtered through an Celite ~ and concentrated to
afford the crude amine.
209
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00902] To a solution of (2S, 4R)-(4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-
yl)-(4-methyl-
3,4-dihydro-2H-benzo[1,4]oxazin-7-yl)-methanone (662 mg, 1.87 mmol) in
methylene chloride (8
mL) was added 4-chlorophenylboronic acid (583 mg, 3.74 mmol), triethylamine
(1.81 ml, 13,09
mmol) and copper(I)]acetate (681 mg, 3.74 mmol). The heterogeneous green
mixture was stirred
open to air for 1 hour and then warmed to 60 °C and stirred over night
(14 h). The mixture was then
cooled to room temperature, poured into rapidly stirred ethyl acetate (150
mL); solids were removed
by filtration through Celite ~. The extracts were washed several times with
water and then once with
brine. The extracts were then dried over anhydrous MgS04, filtered, and
concentrated under reduced
pressure. The crude residue was purified by silica gel chromatography (95 %
methylene chloride / 5
% ethyl acetate) to afford the aniline product as a yellow oil.
[00903] To a solution of (2S,4R)-[4-(4-Chloro-phenylamino)-2-methyl-3,4-
dihydro-2H-quinolin-
1-yl]-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazin-7-yl)-methanone (540 mg, 1.25
mmol) in
methylene chloride (5 mL) was added diisopropylethylamine (0.240 mL, 1.37
mmol) followed by
acetyl,chloride (2 mL). The mixture was stirred at room temperature 4 hours.
The mixture was
concentrated under reduced pressure, dissolved in ethyl acetate, washed with
sat. aq. NaHC03, brine
and dried over magnesium sulfate, filtered, and concentrated under reduced
pressure. The residue was
purified by silica gel chromatography (25/75 hexaneslethyl acetate gradient)
to afford the final
product.
1H-NMR (CDC13) &: 1.1 (d, 3H),1.1 (m,1H), 2.0 (d, 3H), 2.3 (m, 1H), 2.8 (s,
3H), 3.2 (t, ZH), 4.2 (t;
2H), 4.7 (m, 1H), 5.6 (m, 1H), 6.3 (d, 1H),~6.5 (d, 1H), 6.6 (d, 1H), 6.8 (s,
1H), 6.9 (t, 1H), 7.2 (m,
5H), 7.4 (d, 2H).
MS m/z: 490 (M+1).
[00904] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(2,2-difluoro-benzo[1,3]dioxole-5-
carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (G-9)
[00905] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(2,2-difluoro-benzo[1,3]dioxole-5-
carbonyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was prepared following
general procedure G,
substituting 2,2-difluoro-1,3-benzodioxole-5-carbonyl chloride for 4-
fluorobenzoyl chloride. The
rest of the procedures were followed as indicated in general procedure G to
afford (2S,4R)-N-(4-
Chloro-phenyl)-N-[1-(2,2-difluoro-benzo[1,3] dioxole-5-carbonyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide.
'H-NMR (CDC13) 8: 1.15 (3H, d; overlapping 1H, t), 2.02 (3H, s), 2.33(1H, m),
4.78(1H, m),
5.60(1H, m), 6.44 (1H, d), 6.68 (1H, d), 6.95 (1H, t), 7.00-7.40 (8H, complex.
MS mlz: 499 (M + 1).
210
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00906] 5-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-
yl]carbonyl}phenyl)pentanamide (G-10)
[00907] Benzyl [(2S,4R)-1-(4-iodobenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-
4-yl]carbamate
was prepared following general procedure G, substituting 4-iodo-benzoyl
chloride for 4-fluorobenzoyl
chloride.
[00908] To a solution of benzyl [(2S,4R)-1-(4-iodobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-
4-yl]carbamate (0.6 g, 1.14 mmol) in DMF (15 mL) at room temperature was added
pent-4-enoic
acid ethyl ester (0.292 g, 2.28 mmol), potassium acetate (0.67 g, 6.84 mmol),
palladium acetate (0.05
g, 0.228 mmol); tetrabutylammonium chloride (0.32 g, 1.14 mmol), and
triphenylphosphine (0.06 g,
0.228 mmol). The mixture was stirred at room temperature for 1 hour and then
heated to 70 °C for 3
. hours:. The mixture was concentrated and ethyl acetate was added. The
solution was washed with
water, dried and concentrated. The residue was purified by silica gel
chromatography (70 % CHZCl2
/30%EtOAc) to afford ethyl (4E)-5-(4-{ [(2S,4R)-4-{ [(benzyloxy)carbonyl]amino
}-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenyl)pent-4-enoate (0.5 g, 83%), which was
further converted
into . ethyl 5-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-
yl]carbonyl}phenyl)pentanoate following general procedure G.
[00909] Ethyl 5-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenyl)pentanoate (0.135 g, 0.25 mmol) was hydrolyzed to the
acid by dissolving
in 6 ml methanol and potassium carbonate (0.2 g in 4 ml water) was added: The
mixture was heated
to 40 °C for 24 hours and methanol was removed in vacuo. The mixture
was acidified to form a white
precipitate. The solid was filtered to give the acid. To a solution of the
acid in DMF (10 mL) at room
temperature was added HATU (0.143 g, 0.375 mmol), DIEA (0.18 mL, 1 mmol), HOBt
(0.057 g,
0.375 mmol), and ammonium chloride (0.027 g, 0.5 mmol) was added. The mixture
was stirred for
18 hours and concentrated. The residue was dissolved in CHZC12 and washed with
water. The
organic layer was concentrated and purified by silica gel chromatography (87 %
CHZC12
/13%methanol) to afford 5-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1 (2H)-yl]carbonyl } phenyl)pentanamide.
'H-NMR (CDC13) 8: 1.16 (d, 3H), 1.18 (m, 1H), 1.56 (m, 3H), 1.98 (s, 3H), 2.05
(m, 1H), 2.14 (m,
2H), 2.26 (m, 1H), 2.53 (m, 2H), 4.74 (m, 1H), 5.59 (br, 2H), 5.76 (m, 1H),
6.48 (d, 1H), 6.88 (t, 1H),
6.94 (m, 2H), 7.19 (m, 6H), 7.32 (d, 2H).
MS m/z: 519 (M+1)
[00910] (2S,4R)- N-(4-Chloro-phenyl)-N-[1-(2,3-dihydro-benzofuran-5-carbonyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (G-11)
(00911] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(2,3-dihydro-benzofuran-5-carbonyl)-2-
methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was made following general
procedure G, substituting
211
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
2,3-dihydro-benzofuran-5-carbonyl chloride for 4-fluorobenzoyl chloride. The
rest of the procedure
is followed as indicated in general procedure G to yield (2S,4R)- N-(4-chloro-
phenyl)-N-[1-(2,3-
dihydro-benzofuran-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide.
'H-NMR (CDCl3) 8: 1.09 (d, 3H), 1.22 (t, 1H), 1.99 (s, 3H), 2.25 (s, 1H), 3.04
(m, 2H), 4.49 (t, 2H),
4.70 (m, 1H), 5.61 (bs, 1H), 6.50 (m, 2H), 6.88 (m, 2H), 7.10 (d, 2H), 7.18
(d, 2H), 7.26 (d,1H), 7.34
(d, 2H).
MS m/z: 461.2 (M+1).
[00912] N-(4-chlorophenyl)-N-[(2S,4R)-2-methyl-1-(pyridin-3-ylcarbonyl)-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-12)
[00913] N (4-chlorophenyl)-N [(2S,4R)-2-methyl-1-(pyridin-3-ylcarbonyl)-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure G,
substituting
nicotinoyl chloride hydrochloride for 4-fluorobenzoyl chloride. The rest of
the procedures were
followed as indicated in general procedure G to afford N-(4-chlorophenyl)-N
[(2S,4R)-2-methyl-1-
(pyridin-3-ylcarbonyl)-1,2,3,4-tetrahydroquinolin-4-yl]acetamide in decent
yield.
1H-NMR (CDC13, 300MHz) 8: 1.07-1.09 (d, 3H), 2.01 (s, 3H), 2.21-2.33 (m, 2H),
4.05-4.12 (q, 1H),
4.76-4.81 (m, 1H), 6.54-6.74 (d,1H), 6.93-7.38 (m, 9H), 8.49-8.56 (d, 2H).
MS mlz: 420 (M+1).
[00914] N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]-N-
phenylacetamide (G-13)
[00915] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure G,
substituting 4-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. The rest of the
procedures were followed as
indicated in general procedure G to afford (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-
methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was obtained in decent
yield.
[00916] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (200 mg, 0.36 mmol) was dissolved in MeOH and a
catalytic amount of
Palladium on Carbon (10%) was added. The round bottom flask in which resided
the resulting
solution was evacuated and backfilled with hydrogen. The reaction was stirred
under the hydrogen
atmosphere overnight. The mixture was carefully filtered through a Celite ~
plug and concentrated to
afford crude product. The crude product was purified by silica gel
chromatography (hexanes/ethyl
acetate system) to afford N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]-N-phenylacetamide (142 mg, 95%).
212
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDCl 3) 8: 1.10 (d, 3H), 1.60 (s,1H), 2.02 (s, 3H), 2.30 (m,1H), 3.74
(s, 3H), 4.75 (m,1H),
5.45 (b, 1H), 6.50 (d, 1H), 6.67 (d, 2H), 6.92 (t, 1H) 7.13-7.18 (m, 3H), 7.28
(d, 1H), 7.32-7.40 (m,
5H).
MS m/z: 415 (M+1)
[00917] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quiuoline-1-carbonyl}-2-fluoro-phenoxy)-2,2-dimethyl-butyric acid (G-14)
[00918] (2S,4R)-4-(4-t4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-2-fluoro-phenoxy)-2,2-dimethyl-butyric acid methyl ester (100 mg)
was dissolved in
methanol/THF (1:l, 5 mL), and lithium hydroxide (1.0N, 1 mL) was added. After
1 hour, the reaction
was acidified (HCl) and extracted from with methylene chloride. The organic
layer was dried,
filtered, and concentrated. The crude residue was purified by silica gel
chromatography ethyl acetate
- 5%MeOH/ethylacetate gradient) to afford the product.
1H-NMR (CDCl3) S: 1.1 (d, 3H), 1.2 (s, 6H), 1.2 (m, 1H), 2.0 (s, 3H), 2.0 (t,
1H), 2.3 (m, 1H), 4.0 (t,
2H), 4.7 (m, 2H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.6 (t, 1H), 6.7 (d, 1H), 6.9 (t,
1H), 7.2 (m, 5H), 7.4 (d,
2H), 11.1 (bs, 1H).
MS m/z: 567 (M+1).
[00919] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(1-isopropyl-1H-indazole-5-carbonyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (G-15)
[00920] (2S,4R)-N-(4-Chloxo-phenyl)-N-[1-(1-isopropyl-1H-indazole-5-carbonyl)-
2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was prepared following general
procedure G, substituting
1-Isopropyl-1H-indazole-5-carbonyl chloride for 4-fluorobenzoyl chloride. (1-
Isopropyl-1H-indazole-
5-carbonyl chloride was prepared in three steps from 1H-Indazole-5-carboxylic
acid ethyl ester. 1H-
Indazole-5-carboxylic acid ethyl ester was alkylated using 2-bromopropane in
presence of sodium
hydride in DMF at room temperature to yield the desired 1-isopropyl-1H-
indazole-5-carboxylic acid
ethyl ester. Ester hydrolysis using 1N sodium hydroxide in ethanol at
80°C gave 1-Isopropyl-1H-
indazole-5-carboxylic acid and subsequent treatment of this carboxylic acid
with oxalyl chloride and
catalytic DMF afforded 1-isopropyl-1H-indazole-5-carbonyl chloride in decent
yield). The rest of the
procedures were followed as indicated in general procedure G to afford (2S,4R)-
N-(4-Chloro-phenyl)-
N-[1-(1-isopropyl-1H-indazole-5-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-
4-yl]-acetamide.
(The first exception being in the deprotection of the benzyloxycarbonyl group
by treatment with 30%
HBr in AcOH substituting for palladium on carbon(10%) and the other in the
step of N-arylation of
(2S,4R)-(4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-yl)-( 1-isopropyl-1H-
indazol-5-yl)-methanone
with 4-chlorophenylboronic acid, in presence of copper acetate, triethylamine
was substituted for
pyridine, and the reaction was carried out in dichloromethane at room
temperature to afford the
213
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
(2S,4R)-N-(4-chloro-phenyl)-N-[1-(1-isopropyl-1H-indazole-5-carbonyl)-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide).
'H-NMR (CDC13) 8: 1.15 (3H, d; overlapping 1H, t), 1.60 (2x3H, d), 2.02 (3H,
s), 2.33(1H, m),
4.78(2xlH, m, overlapping), 5.60(1H, m), 6.44 (1H, d), 6.95 (1H, t), 7.00-7.40
(9H, complex).
ESI-MS m/z: 501 (M + 1).
[00921] N-(4-Chlorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-2-methylpropanamide (G-16)
[00922] N (4-Chlorophenyl)-N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-2-methylpropanamide was synthesized according to
general procedure G by
replacing 4-fluorobenzoyl chloride with 4-methoxybenzoyl chloride and by
substituting acetyl
chloride with 2-methyl propanoyl chloride. The rest of the procedure was
followed as indicated in
general procedure G to yield N (4-chlorophenyl)-N-[(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-2-methylpropanamide.
'H-NMR (CDCl3) 8: 1.12-1.26 (m, 10H), 2.20-2.28 (m, 1H), 2.61 (sp, 1H), 3.72
(s, 3H), 4.69-4.79
(m, 1H), 5.61 (br s, 1H), 6.52 (d,1H), 6.67 (d, 2H), 6.92 (t,1H), 7.11-7.41
(m, 8H).
MS m/z: 477 (M+1).
[00923] N-(4-Chlorophenyl)-N-{(2S,4R)-1-[(1,3-dimethyl-1H-thienoj2,3-c]pyrazol-
5-
yl)carbonyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-y1}acetamide (G-17)
[00924] N-(4-Chlorophenyl)-N-{(2S,4R)-1-[(1,3-dimethyl-1H-thieno[2,3-c]pyrazol-
5-
yl)carbonyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl}acetamide was prepared
following the
procedure for N-(4-chlorophenyl)-N-{ (2S,4R)-1-[(1-isopropyl-1H-pyrazol-4-
yl)carbonyl]-2-methyl-
1,2,3',4-tetrahydroquinolin-4-yl}acetamide substituting 1,3-dimethyl-1H-
thieno[2,3-c]pyrazole-5-
carbonyl chloride for 1-isopropyl-1H pyrazole-4-carbonyl chloride. (1,3-
Dimethyl-1H-thieno[2,3-
c]pyrazole-5-carbonyl chloride was prepared from commercially available 1,3-
dimethyl-1H-
thieno[2,3-c]pyrazole-5-carboxylic acid by treatment with oxalyl chloride and
catalytic DMF in
dichloromethane).
'H-NMR (CDC13) 8: 1.15 (d, 3H), 1.17 (t, 1H), 2.02 (s, 3H), 2.25 (s, 3H),
2.25(m, 1H), 3.80 (s, 3H),
4.62-4.74 (m, 1H), 5.48 -5.60 (m, 1H), 6.55 (s, 1H), 7.00-7.40 (m, 8H).
MS m/z: 493 (M + 1).
[00925] N-(4-chlorophenyl)-N-{(2S,4R)-1-[(1-isopropyl-1H-pyrazol-4-
yl)carbonyl]-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl}acetamide (G-18)
[00926] N (4-Chlorophenyl)-N {(2S,4R)-1-[(1-isopropyl-1H-pyrazol-4-
yl)carbonyl]-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl}acetamide was prepared following general
procedure G, substituting
214
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1-isopropyl-1H-pyrazole-4-carbonyl chloride fox 4-fluorobenzoyl chloride. (1-
Isopropyl-1H-
pyrazole-4-carbonyl chloride was prepared from commercially available 1-
isopropyl-1H-pyrazole-4-
carboxylic acid by treatment with oxalyl chloride and catalytic DMF in
dichloromethane). Other
modifications to general procedure A were inclusive of the deprotection of the
benzyloxycarbonyl
group via treatment with 30% HBr in acetic acid instead of palladium on carbon
(10%) and the use of
triethylamine instead of pyridine using dichloromethane as solvent at room
temperature during the N
arylation sequence.
IH-NMR (CDCl3) 8: 1.11 (d, 3H), 1.13 (t, 1H), 1.36 (d, 6H), 2.00 (s, 3H), 2.21-
2.28 (m, 1H), 4.25=
4.34 (m, 1H), 4.65-4.76 (m, 1H), 5.36-5.56 (br, 1H), 6.95 (d, 1H), 7.00-7.40
(m, 9H).
MS m/z: 451 (M + 1).
[00927] N-1H-indol-4-yl-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-19)
[00928] N 1H indol-4-yl-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-
4-yl]acetamide was made following general procedure G, substituting 4-
methoxybenzoyl chloride for
4-fluorobenzoyl chloride.
[00929] A mixture of (2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
amine (0.12 g, 0.4 mmol) (obtained after the hydrogenation step),
tart-butyl 4-bromo-1H-indole-1-carboxylate (0.099 g, 0.39 mmol), palladium dba
(0.018 g, 0.02
mmol), biphenyl-2-yl(di-tart-butyl)phosphine (0.006 g, 0.02 mmol) and cesium
carbonate (0.13 g, 0.4
mmol) in dimethoxy ethane (3 mL) was stirred at 80C under nitrogen for 15 h.
The reaction mixture
was filtered. The filtrate was evaporated to yield the crude product (0.222 g)
which was then cleaned
by silica gel chromatography (10% ethyl acetate: hexane) to give desired tart-
Butyl 4-{ [(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]amino}-1H-indole-1-
carboxylate (0.054
g, 32%).
'H-NMR (CDCl3) 8: 1.32 (d, 3H), 1.47-1.57 (m, 1H), 1.71 (s, 9H), 2.87-2.96 (m,
1H), 3.81 (s, 3H),
4.63-4.69 (m, 1H), 4.88-5.01 (m, 1H), 6.46 (d, 1H), 6.56-6.63 (m, 2H), 6.75-
6.78 (m, 2H), 6.94-7.09
(m, 2H), 7.2-7.36 (m, 4H), 7.59-7.66 (m, 2H).
MS m/z: 512 (M+1 )
[00930] A mixture of methyl tart-butyl 4-{ [(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]amino}-1H-indole-1-carboxylate (0.054 g, 0.1 mmol),
freshly distilled acetyl
chloride (0.5 mL) , diisopropylethylamine (0.015 g, 0.12 mmol) and 4-N,N-
dimethylaminopyndine
(catalytic amount) was sowed at room temperature for 43 h. Ice was added to
the reaction mixture and
stirred for 1 h. The reaction mixture was neutralized with solid sodium
bicarbonate and extracted
with ethyl acetate. The ethyl acetate extract was washed rivice with water,
brine, dried over sodium
215
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
sulphate and evaporated. The crude product (0.055 g) thus obtained was cleaned
by silica gel
chromatography (ethyl acetate: hexane) to give clean tent-butyl 4-
{acetyl[(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]amino}-1H-indole-1-
carboxylate (0.035
g, 60%).
'H-NMR (CDC13) 8: 1.04 (d, 3H), 1.47-1.57 (m, 1H), 1.69 (s, 9H), 1.97 (s, 3H),
2.87-2.96 (m, 1H),
3.75(s, 3H), 4.64-4.77 (m, 1H), 4.88-5.01 (m, 1H), 6.53 (d, 1H), 6.66 (d, 1H),
6.69 (d, 2H), 6.92-6.97
(m, 1H), 7.14-7.29 (m, 5H), 7.45 (d,1H), 7.73 (d, 1H), 8.21 (d,1H).
MS mlz: 411, 280.
[00931] tert-Butyl 4-{acetyl[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-
4-yl]amino}-1H-indole-1-carboxylate (0.035 g, 0.068 mmol) was stirred in 4 N
HCl in dioxane (1
mL) for 24 h and then evaporated to dryness. The residue was dissolved in
ethyl acetate, washed with
1N NaOH, water, brine, dried over sodium sulphate and evaporated to dryness to
give the crude
product (0.023 g). The crude was cleaned by silica gel chromatography to give
clean N-1H-indol-4-yl-
N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]acetamide (0.008 g,
28%).
'H-NMR (CDC13) b: 1.03 (d, 3H), 2.01 (s, 3H), 3.75(s, 3H), 4.08-4.16 (m, 1H),
4.61-4.79 (m, 1H),
6.52-6.55 (m, 2H), 6.67-6.7 (m, 2H), 6.92-7.51 (m, 9H), . 8.62 (bs,1H).
MS m/z: 454 (M+1).
[00932] 4-[(4-{[(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(21~-yl]carbonyl}phenyl)(methyl)amino]-2,2-dimethylbutanoic acid (G-20)
[00933] Methyl 4-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenyl)(methyl)amino]-2,2-dimethylbutanoate was dissolved in
methanol/tetrahydrofuran/water (2/1/1) then sodium hydroxide (3 equivalents)
was added and reaction
mixture heated to 40°C for 2 h. The mixture was concentrated, the
residue acidified with a 1N HCl
aqueous solution and extracted with ethyl acetate. The organic layer was
washed with brine, dried
over magnesium sulfate, filtered and concentrated to give 4-[(4-{ [(2S,4R)-4-
[Acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-yl]carbonyl
}phenyl)(methyl)amino]-2,2-
dimethylbutanoic acid.
'H-NMR (CDC13) 8: 1.09-1.12 (m, 1H), 1.11 (d, 3H), 1.22 (d, 6H), 1.69-1.74 (m,
2H), 2.01 (s, 3H),
2.24-2.31 (m, 1H), 2.83 (s, 3H), 3.27 (t, 2H), 4.65-4.76 (m, 1H), 5.60 (br s,
1H), 6.39 (d, 2H), 6.62 (d,
1H), 6.95 (t, 1H), 7.07-7.16 (m, 3H), 7.18-7.28 (m, 3H), 7.37 (d, 2H).
MS m/z: 562 (M+1).
216
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00934] N-(4-chlorophenyl)-2-hydroxy-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-21)
[00935] N-(4-chlorophenyl)-2-hydroxy-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was made following general procedure G,
substituting 4-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. Procedure A was followed
further substituting
acetoxyacetylchloride for acetyl chloride in the last step to yield 2-{(4-
chlorophenyl)[(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]amino}-2-oxoethyl
acetate.
[00936] 2-{(4-Chlorophenyl)[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]amino}-2-oxoethyl acetate (496 mg, 0.98 mmol, 1 eq.)
was dissolved in
methanol (12 m1). A solution of potassium carbonate (1.08 g, 7.84 mmol, 8 eq.)
in water (5 ml) was
added and reaction mixture was stirred at room temperature for 4 h. The
mixture was concentrated
and the residue dissolved in ethyl acetate and washed with water, brine, and
then dried over
magnesium sulfate, filtered and concentrated. The crude residue was purified
by silica gel
chromatography (methylene chloride/methanol: 98/2) to afford N (4-
chlorophenyl)-2-hydroxy-N
[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]acetamide (380 mg, 84 %).
1H-NMR (DMSO) 8: 1.05 (d, 3H), 2.45 (m, 1H), 3.70 (s, 3H), 3.80-3.95 (dd, 2H),
4.60 (m, 1H), 4.80
(m, 1H), 5.40 (m, 1H), 6.55 (d, 1H), 6.75 (d, 2H), 6.95 (t, 1H), 7.10 (d~ 2H),
7.20 (t, 1H), 7.40-7.60
(m, SH).
MS m/z: 465 (M+1).
[00937] (2S,4R)-6-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-
1-carbonyl}-2,3-dihydro-benzo[1,4]oxazine-4-carboxylic acid ethyl ester (G-22)
[00938] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic. acid
benzyl ester (990 mg, 3.49 mmol) in methylene chloride at room temperature was
added triethylamine
(1.21 mL, 8.71 mmol) followed by 6-chlorocarbonyl-2,3-dihydro-
benzo[1,4]oxazine-4-carboxylic
acid ethyl ester (3.49 mmol). The reaction was stirred over night at room
temperature. The mixture
was poured into water and extracted with ethyl acetate. The extracts were
washed with 1 M (aq)
NaOH and brine, dried over MgS04, filtered dried and concentrated. The crude
residue was purified
by silica gel chromatography (75% hexanes / 25 % ethyl acetate) to afford the
pure amide.
[00939] (2S,4R)-6-(4-Benzyloxycarbonylamino-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl)-
2,3-dihydro-benzo[1,4]oxazine-4-carboxylic acid ethyl ester (697 mmol) was
dissolved in ethanol (30
mL). The vessel in which resided the resulting solution was evacuated and
backfilled with argon. A
catalytic amount of Palladium on Carbon (10 %) was added. The vessel was once
again evacuated
and this time was backfilled with hydrogen and shaken in a Parr bottle at 40
psi hydrogen. Reaction
was complete after 4 hours. The mixture was carefully filtered and
concentrated to 10% volume. The
217
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
resulting concentrated solution was filtered through an Celite ~ and
concentrated to afford the crude
amine.
[00940] To a solution of (2S,4R)-6-(4-Amino-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl)-
2,3-dihydro-benzo[1,4]oxazine-4-carboxylic acid ethyl ester (470 mg, 1.18
mnnol) in methylene
chloride was added 4-chlorophenylboronic acid (368 mg, 2.36 mmol),
triethylamine (1.31 mL, 9.42
mmol) and copper(Il)acetate (429 mg, 2.36 mmol). The heterogeneous green
mixture was stirred
open to air for 1 hour and then warmed to 60 °C and stirred over night
( 14 hours). The mixture was
then cooled to room temperature, poured into rapidly stirred ethyl acetate
(150 mL); solids were
removed by filtration through Celite ~. The extracts were washed several times
with water and then
once with brine. The extracts were then dried over anhydrous MgS04, filtered,
and concentrated
under reduced pressure. The crude residue was purified by silica gel
chromatography (95 %
methylene chloride / S % ethyl acetate) to afford the aniline product as a
yellow oil.
[00941] To a solution of (2S, 4R)-6-[4-(4-Chloro-phenylamino)-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl]-2,3-dihydro-benzo[1,4]oxazine-4-carboxylic acid ethyl
ester (343 mg, 0.673
mmol) in methylene chloride (2 mL) was added diisopropylethylamine (0.129 mL,
0.739 mmol)
followed by acetyl chloride (2 xnL). The mixture was stirred at room
temperature 4 hours. The
mixture was concentrated under reduced pressure, dissolved in ethyl acetate,
washed with sat. aq.
sodium NaHC03, brine and dried over MgS04, filtered, and concentrated under
reduced pressure.
The residue was purified by silica gel chromatography (25/75 hexanes/ethyl
acetate gradient) to afford
pure product.
'H-NMR (CDC13) 8: 1.1 (d, 3H), 1.2 (m, 1H), 1.3 (t, 3H), 2.0 (s, 3H), 2.3 (m~
1H), 3.3 (m, 2H), 4.0
(m, 2H), 4.3 (m, 2H), 4.7 (m, 1H), 5.5 (m, 1H), 6.4 (d, 1H), 6.5 (d, 1H), 6.6
(d, 2H), 6.96 (t, 1H), 7.2
(m, 4H), 7.4 (d, 2H).
MS m/z: 548 (M+1).
[00942] N-[(2S,4R)-6-chloro-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]-N-(4-chlorophenyl)acetamide (G-23)
[00943] N [(2S,4R)-6-chloro-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-
(4-chlorophenyl)acetamide was synthesized as described for (2S, 4R)-(2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl)-carbamic acid benzyl ester, substituting 4-chloroaniline for
aniline. Further elaboration
following general procedure A, substituting 4-methoxy-benzoyl chloride for 4-
fluorobenzoyl chloride,
yielded N-[(2S,4R)-6-chloro-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-
chlorophenyl)acetamide.
'H-NMR (CDCI3) 8: 1.13 (d, 3H), 1.15 (t, 1H), 2.03 (s, 3H), 2.27 (m, 1H), 3.76
(s, 3H), 4.72 (sextet,
1H), 5.58 (bs, 1H), 6.43 (d, 1H), 6.71 (d, 2H), 6.93 (d, 1H), 7.14-7.29 (m,
5H), 7.41 (d, 2H).
MS m/z: 483 (M+1)
218
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00944] [(5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-
yl]carbonyl}isoxazol-3-yl)oxy]acetic acid (G-24)
[00945] Ethyl [(5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(21~-yl]carbonyl}isoxazol-3-yl)oxy]acetate was prepared following the
procedure for N (4-
chlorophenyl)-N {(2S,4R)-1-[(1-isopropyl-1H pyrazol-4-yl)carbonyl]-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}acetamide substituting ethyl {[5-
(chlorocarbonyl)isoxazol-3-yl]oxy}acetate
for 1-isopropyl-1H pyrazole-4-carbonyl chloride. (Ethyl { [5-
(chlorocarbonyl)isoxazol-3-
yl]oxy}acetate was prepared in 4 steps from methyl 3-hydroxy 5-isoxazole
carboxylate. Methyl 3-
hydroxy 5-isoxazole carboxylate (1.00 g, 6.95 mmol) was alkylated using ethyl
bromoacetate (0.850
mL, 7.64 mmol) in presence of potassium carbonate (1.05 g, 7.64 mmol) and
catalytic potassium
iodide in DMF (3.00 mL) at room temperature overnight. Water was added to the
reaction mixture
and extracted with ethyl acetate. The organics were washed with brine, dried
over anhydrous sodium.
sulfate, filtered and concentrated in vacuo to yield the desired methyl 3-(2-
ethoxy-2-
oxoethoxy)isoxazole-5-carboxylate (60%). (Ref: WO 03/063800 PCT/LTS03/03224).
Methyl 3-(2-
ethoxy-2-oxoethoxy)isoxazole-5-carboxylate was further treated with aqueous 5%
sodium hydroxide
in methanol to yield 3-(carboxymethoxy)isoxazole-5-carboxylic acid (86%). This
diacid (0.700 g,
3.76 mmol) was selectively esterified in the presence of catalytic p-
toluenesulfonic acid monohydrate
(100 mg) in ethanol at room temperature. Water was added to the reaction and
the mixture was
extracted with ethyl acetate. The organics were washed with brine, dried over
anhydrous sodium
sulfate, filtered and concentrated in vacuo to give the desired 3-(2-ethoxy-2-
oxoethoxy)isoxazole-5-
carboxylic acid (90%). 3-(2-Ethoxy-2-oxoethoxy)isoxazole-5-carboxylic acid was
treated with oxalyl
chloride and catalytic DMF in dichloromethane to yield the desired ethyl { [5-
(chlorocarbonyl)
isoxazol-3-yl]oxy}acetate). Ethyl [(5-{[(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}isoxazol-3-yl)oxy]acetate was treated with a
solution of lithium
hydroxide monohydrate (aq) in methanol at room temperature overnight to afford
[(5-{ [(2S,4R)-4-
[aeetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(21-
yl]carbonyl}isoxazol-3-
yl)oxy]acetic acid in 60% yield.
'H-NMR (CDC13) S: 1.15 (d, 3H), 1.17 (t, 1H), 2.02 (s, 3H), 2.42-2.68 (m, 1H),
4.69 (s, 1H) 4.72-
4.78 (m, 1H), 5.44-5.58 (br, 1H), 6.80 (d, 1H), 7.00-7.40 (m, 8H).
MS m/z: 484 (M + 1).
[00946] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-cyanobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-25)
[00947] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-cyanobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin
4-yl]acetamide was made following general procedure G, substituting 4-
cyanobenzoyl chloride for 4
219
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
fluorobenzoyl chloride. The rest of the procedure is followed as indicated in
general procedure G to
yield N (4-chlorophenyl)-N [(2S,4R)-1-(4-cyanobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]acetamide.
'H-NMR (CDCl3) S: 1.10-1.20 (m, 1H), 1.16 (d, 3H), 2.03 (s, 3H), 2.20-2.36 (m,
1H), 4.70-4.84 (m,
1H), 5.48-5.70 (m, 1H), 6.40 (d, 1H), 6.91 (t, 1H), 7.16-7.32 (m, 6H), 7.38
(d, 2H), 7.47 (d, 2H).
MS mlz: 444 (M +1)
[00948] Ethyl 4-[(5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}isoxazol-3-yl)oxy]-2,2-dimethylbutanoate (G-
26)
[00949] Ethyl 4-[(5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}isoxazol-3-yl)oxy]-2,2-dimethylbutanoate was prepared
following the procedure
for . N (4-chlorophenyl)-N {(2S,4R)-1-[(1-isopropyl-1H-pyrazol-4-yl)carbonyl]-
2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl}acetamide, substituting ethyl 4-{[5-
(chlorocarbonyl)isoxazol-3-yl]oxy}-2,2-
dimethylbutanoate for 1-isopropyl-1H-pyrazole-4-carbonyl chloride. (Ethyl 4-{
[5-(chlorocarbonyl)
isoxazol-3-yl]oxy}-2,2-dimethylbutanoate was prepared in three steps from
methyl 3-hydroxy 5-
isoxazole carboxylate. Methyl 3-hydroxy 5-isoxazole carboxylate (1.00 g, 6.94
mmol) was alkylated
using ethyl 4-bromo-2,2-dimethylbutanoate (1.59 g, 7.63 mmol) in the presence
of potassium
carbonate .(1.05 g, 7.64 mmol) and catalytic potassium iodide in DMF (5.00 mL)
at room temperature
overnight. Water was added to the reaction mixture and the mixture was
extracted with ethyl acetate.
The organics were washed with brine, dried over anhydrous sodium sulfate,
filtered, concentrated in
vacuo and purified on silica gel by flash chromatography using hexane/ethyl
acetate (10-50% .
gradient) to afford methyl 3-(4-ethoxy-3,3-dimethyl-4-oxobutoxy)isoxazole-5-
carboxylate in 35%
yield. Selective hydrolysis of the methyl ester by subsequent treatment with
lithium hydroxide (aq)
(1.50 equiv.) in methanol at room temperature afforded 3-(4-ethoxy-3,3-
dimethyl-4-
oxobutoxy)isoxazole-5-carboxylic acid which was then treated with oxalyl
chloride and catalytic
DMF in dichloromethane to yield the desired ethyl 4-{[5-
(chlorocarbonyl)isoxazol-3-yl]oxy}-2,2-
dimethylbutanoate).
'H-NMR (CDCl3) 8: 1.18 (d, 3H), 1.18 (t, 1H), 1.19 (s, 6H), 1.20(t, 3H), 1.99-
2.02 (s, 3H; m, 1H),
2.24-2.36 (m, 1H), 4.15 (q, 2H), 4.17-4.22 (m, 2H), 4.64-4.74 (m, 1H), 5.60-
5.68 (br, 1H), 6.80 (d,
1H), 7.00-7.40 (m, 8H).
MS m/z: 568 (M + 1).
[00950] 4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}benzoic acid (G-27)
[00951] Methyl 4-((2S,4R)-4-(N-(4-chlorophenyl)acetamido)-2-methyl-1,2,3,4
tetrahydroquinoline-1-carbonyl)benzoate was made following general procedure
G, substituting
220
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
methyl 4-(chlorocarbonyl)benzoate for 4-fluorobenzoyl chloride. The rest of
the procedure is
followed as indicated in general procedure A to yield Methyl 4-((2S,4R)-4-(N-
(4-
chlorophenyl)acetamido)-2-methyl-1,2,3,4-tetrahydroquinoline-1-
carbonyl)benzoate, which was
saponified with excess lithium hydroxide in MeOH/I~IF/H20 (2:1:2). The slurry
was acidified with
1N HCl and the crude product was extracted using methylene chloride. The
organic portion was
concentrated, and the resulting slurry was subjected to preparatory HPLC to
afford the title compound
as a white solid.'H-NMR (CDC13 8: 1.15-1.22 (m, 1H), 1.16 (d, 3H), 2.06 (s,
3H), 2.20-2.36 (m, 1H),
3.50-4.05 (bs, 1H), 4.72-4.88 (m, 1H), 5.50-5.75 (m, 1H), 6.45 (d, 1H), 6.85
(t, 1H), 7.08-7.30 (m,
6H), 7.40 (d, 2H), 7.87 (d, 2H).
MS m/z: 463 (M +1)
[00952] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]cyclopropanecarboxamide (G-28)
[00953] N (4-chlorophenyl)-N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]cyclopropanecarboxamide was made following general
procedure G,
substituting 4-methoxybenzoyl chloride for 4-fluorobenzoyl chloride. Procedure
G was followed
further substituting cyclopropane carbonyl chloride for acetyl chloride in the
last step to yield N-(4-
chlorophenyl)-N-[(2S;4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]cyclopropanecarboxamide.
'H-NMR (CDC13) 8: 0.75 (m, 2H), 1.00-1.20 (m, 5H), 1.45 (m, 1H), 2.30 (m, 1H),
3.75 (s, 3H), 4.75
(m, 1H), 5.60 (m,1H), 6.50 (d,1H), 6.60 (d, 2H), 6.90 (t,1H), 7.10-7.45 (m,
8H).
MS m/z: 475 (M+1).
[00954] N-[(2S,4R)-1-(4-metho~rybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]-N-[4-
(1F1-pyrrol-1-yl)phenyl]acetamide (G-29)
[00955] N-(4-amino-phenyl)-N-[(2S,4R)-1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared similarly to General Procedure G: To a
solution of (2S, 4R)-
(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-carbamic acid benzyl ester (1.00
equiv.) in methylene
chloride at room temperature was added diisopropylethylamine (1.50 equiv.)
followed by 4-anisoyl
chloride (1.15 equiv). The reaction was stirred over night at room
temperature. The mixture was
poured into water and extracted with ethyl acetate. The extracts were washed
with 1 M (aq) NaOH
and brine, dried over magnesium sulfate, filtered, dried and concentrated. The
crude residue was
purified by silica gel chromatography (75% hexanes / 25 % ethyl acetate) to
afford 2S,4R)-[1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-carbamic acid
benzyl ester.
[00956] (2S,4R)-[1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-carbamic acid
benzyl ester (1.00 equiv.) was dissolved in ethanol (30 mL). The vessel in
which resided the resulting
221
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
solution was evacuated and bacl~illed with argon. A catalytic amount of
Palladium on Carbon (10 %,
0.10 equiv. by wt.) was added. The vessel was once again evacuated and this
time was backfilled
with hydrogen and shaken in a Parr bottle at 40 psi hydrogen. Reaction was
complete after 4 h. The
mixture was carefully filtered and concentrated to 10% volume. The resulting
concentrated solution
was filtered through a Celite ~ and concentrated to afford the crude amine,
(2S, 4R)- (4-amino-2-
methyl-3,4-dihydro-2H-quinolin-1-yl)-(4-methoxy-phenyl)-methanone.
[00957j To a solution of (2S, 4R)- (4-amino-2-methyl-3,4-dihydro-2H-quinolin-1-
yl)-(4-methoxy-
phenyl)-methanone (1.00 equiv.) in DMF was added 4-nitrophenylboronic acid
(2.00 equiv.), pyridine
(2.50 equiv.) and copper(II)acetate (2.00 equiv.). The heterogeneous green
mixture was stirred open
to air for 1 h and then warmed to 60 °C and stirred over night (14 h).
The mixture was then cooled to
rt, poured into rapidly stirred ethyl acetate (150 mL); solids were removed by
filtration through Celite
~. The extracts were washed several times With water and then once with brine.
The extracts were
then dried over anhydrous magnesium sulfate, filtered, and concentrated under
reduced pressure. The
crude residue was purified by silica gel chromatography (95 % methylene
chloride I 5 % ethyl acetate)
to afford . (4-methoxy-phenyl)-[(2S,4R)-2-methyl-4-(4-nitro-phenylamino)-3,4-
dihydro-2H-quinolin-
1-yl]-methanone as a yellow solid.
[00958] To a solution of (2S, 4R)- [4-(4-nitro-phenylamino)-2-methyl-3,4-
dihydro-2H-quinolin-1-
yl]-(4-methoxy-phenyl)-methanone (1.00 equiv.) in methylene chloride was added
diisopropylethylamine (1.05 equiv) followed by acetyl chloride (l.OOequiv).
The mixture was stirred
at rt 48 h. The mixture was concentrated under reduced pressure, dissolved in
ethyl acetate, washed
with sat. aqueous sodium bicarbonate, brine and dried over magnesium sulfate,
filtered, and
concentrated under reduced pressure. The residue was purified by silica gel
chromatography (25175
hexanes/ethyl acetate gradient) to afford pure N (4-vitro-phenyl)-N [1-(4-
methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide, which was reduced to N-(4-
amino-phenyl)-N-[1-
(4-methoxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]- acetamide
using excess NH4COZH,
catalyic Pt(sulfided), in ethanol at reflux for 30 m., followed by filtration
and concentration. The
amine was used without further purification due to inherent chemical
instability.
[00959] N (4-amino-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]- acetamide (22mg, 0.05 mmol) was dissolved in MeOH:THF (1 mL each) and
cooled to 10 °C.
2,5-dimethoxy-tetrahydrofuran (1.25 equiv.) was dissolved in THF and catalytic
HZS04, and added
drop wise to the aniline mixture. The mixture was poured into sat. NaHC03 and
extracted with ethyl
acetate. The organic layer was dried, filtered and concentrated, and the crude
residue was purified by
preparative HPLC to afford the title compound as an off white solid.
'H-NMR (CDC13) 8: 1.00-1.22 (m, 1H), 1.12 (s, 3H), 2.04 (s, 3H), 2.22-2.42 (m,
1H), 3.72 (s, 3H),
4.64-4.84 (m, 1H), 5.50-5.80 (m, 1H), 6.38 (s, 2H), 6.53 (d,1H), 6.67 (d, 2H),
6.94 (t, 1H), 7.05-7.48
(m, 10H).
222
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS mlz: 480 (M +1)
[00960] Methyl 4-[(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinalin-1(21~-yl]carbonyl}phenyl)(methyl)amino]-2,2-dimethylbutanoate
(G-30)
[00961] Methyl 4-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1 (2I~-yl]carbonyl }phenyl)(methyl)amino]-2,2-dimethylbutanoate was prepared
according to
procedure G substituting 4-nitrobenzoyl chloride for 4-fluorobenzoyl chloride
in the first step and, to
avoid complications, the CBZ protecting group was cleaved by treatment of a
solution of the
protected amine, benzyl [(2S,4R)-2-methyl-1-(4-nitrobenzoyl)-1,2,3,4-
tetrahydroquinolin-4-
yl]carbamate, in dichloromethane with hydrogen bromide in acetic acid (30 wt%)
followed by
precipitation of the product from the reaction mixture by the addition of
hexanes. The rest of general
procedure A was followed as indicated to yield N-(4-chlorophenyl)-N [(2S,4R)-2-
methyl-1-(4-
nitrobenzoyl)-1,2,3,4-tetrahydroquinolin-4-yl]acetamide.
[00962]. This material was reduced by exposure to sulfided platinum (10 wt%)
and ammonium
formate (4.0 equivalents) in ethanol at 70 °C until all starting
material was consumed to afford N-
[(2S,4R)-1-(4-aminobenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-N (4-
chlorophenyl)acetamide.
[00963] This material underwent reductive alkylation first with methyl 2,2-
dimethyl-4-
oxobutanoate then formaldehyde. (Methyl 2,2-dimethyl-4-oxobutanoate was
prepared in 3 steps from
methyl 4,4-dimethoxybutanoate. Methyl 4,4-dimethoxybutanoate underwent lithium
enolate
formation with lithium diisopropyl amide (1.1 equivalents) in THF at -78
°C followed by quenching
with methyl iodide (2.0 equivalents) and aqueous work up; repeating the same
protocol yielded, after
standard chromatography (5% ethyl acetate/hexanes), methyl 4,4-dimethoxy-2,2-
dimethylbutanoate.
Subsequent treatment of this material with aqueous 6 normal hydrochloric acid
in acetone afforded,
after standard aqueous work up, methyl 2,2-dimethyl-4-oxobutanoate). A
solution of methyl 2,2-
dimethyl-4-oxobutanoate (1.0 equivalents), sodium triacetoxyborohydride (1.2
equivalents) and
aniline in THF with acetic acid (1.0 equivalent) was stirred until all
starting material had been
consumed. The reaction was then diluted with methanol, acidified with
concentrated hydrochloric
acid (3 drops) and an excess formaldehyde (37 weight % in H20) and sodium
cyanoborohydride (5
equivalents) were added. The reaction mixture stirred at room temperature
until all starting material
had been consumed, then standard aqueous work up followed by chromatography
(50% ethyl
acetate/hexanes) afforded methyl 4-[(4-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2Fn-yl]carbonyl }phenyl)(methyl)amino]-2,2-
dimethylbutanoate.
'H-NMK (CDC13) 8: 1.09-1.14 (m, 1H), 1.13 (d, 3H), 1.20 (d, 6H), 1.71-1.79 (m,
2H), 1.98 (s, 3H),
2.24-2.28 (m, 1H), 3.02 (s, 3H), 3.30 (t, 2H), 3.57 (s, 3H), 4.69-4.76 (m,
1H), 5.51 (br s, 1H), 6.39 (d,
1H), 6.84 (t, 1H), 7.16 (d, 2H), 7.24-7.29 (m, 4H), 7.34 (d, 2H), 7.54 (d,
2H).
223
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS mlz: 576 (M+1).
[00964] N-[3-(acetylamino)phenyl]-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-31)
[00965] N-[3-(acetylamino)phenyl]-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure G,
substituting 4-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride, and 3-
acetamidophenylboronic acid for 4-
chlorophenylboronic acid. The rest of the procedures were followed as
indicated in general procedure
G to afford N-[3-(acetylamino)phenyl]-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-y1]acetamide
'H -NMR (CDCl3) S: 1.1-1.2 (m, 4H), 2.0 (s, 3H), 2.3 (m, 4H), 3.7 (s, 3H), 4.8
(m, 1H), 5.6 (br, 1H),
6.5 (d, 1H), 6.7 (m, 2H,), 6.9 (m, 1H,), 7.1-7.4 (m, 7H,), 7.5 (m, 1H)
MS m/z: 472 (M + 1 ).
[00966] N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]-N-{4-
[(methylsulfonyl)amino]phenyl}acetamide (G-32)
[00967] N (4-amino-phenyl)-N [1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl]- acetamide (32 mg, 0.075 mmol) was dissolved in methylene chloride (1 mL)
and triethylamine
(10 equiv.), then cooled to -30 °C. Methanesulfonyl chloride (5 equiv.)
was added; after 30min, TLC
showed complete consumption of the starting aniline. The mixture was poured
into water and
extracted with EtOAc. The organic layer was dried, filtered and concentrated
to afford the
corresponding bis-methanesulfonated adduct.
[00968] The crude residue was dissolved in MeOH and ~20 equiv. of Cs2C03 was
added. After
stirring for 5 min, the mixture was poured into sat. NaHC03 and EtOAc. The
organic layer was dried,
filtered and concentrated. The resulting crude material was subjected to flash
chromatography
(EtOAc) to afford the title compounds as a white solid.
'H-NMR (CDCl3) 8: 1.00-1.22 (m, 1H), 1.11 (s, 3H), 1.20-1.28 (m, 1H), 2.01 (s,
3H), 2.20-2.40 (m,
1H), 3.01 (s, 3H) 3.72 (s, 3H), 4.64-4.82 (m, 1H), 5.45-5.75 (m, 1H), 6.51 (d,
1H), 6.63 (d, 2H), 6.91
(t, 1H), 7.10-7.35 (m, 7H), 7.38-7.44 (m, 1H).
MS m/z: 508 (M +1)
[00969] N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]-N-
pyridin-4-ylacetamide (G-33)
[00970] N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]-N-pyridin
4-ylacetamide was made following general procedure G, substituting 4-
methoxybenzoyl chloride for
224
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
4-fluorobenzoyl chloride. The amine-aryl coupling was performed differently to
what is described in
procedure G. Therefore (2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-amine
(obtained from the hydrogenation step, 356 mg, 1.2 mmol, 1 equ.) was dissolved
in ethylene glycol
dimethyl ether (4 mL) in a Schlenk tube. To this solution was added
sequentially 4-bromopyridine
hydrochloride (280 mg, 1.44 mmol, 1.2 equ.), cesium carbonate (940 mg, 2.88
mmol, 2.4 equ.),
palladium acetate (32 mg, 0.048 mmol, 0.04 equ.) and 2-(Dicyclohexylphosphino)-
2',4',6'-tri-i-propyl-
1,1'-biphenyl (48 mg, 0.096 mmol, 0.08 equ.). The reaction mixture was flushed
with nitrogen and
heated to 100°C in the Schlenk tube for 48 h. Reaction mixture was
concentrated to leave a residue'
which was partitioned between water and ethyl acetate and extaracted. The
aqueous layer was
separated and the organic layer was washed with brine, dried over magnesium
sulfate, filtered and
concentrated to give a brown oil. The crude product was purified by silica gel
chromatography
(methylene chloride/methanol: 99/1 to 90/10 gradient) to provide (2S,4R)-1-(4-
methoxybenzoyl)-2-
methyl-N-pyridin-4-yl-1,2,3,4-tetrahydroquinolin-4-amine (125 mg, 28 %).
[00971] To a solution of (2S,4R)-1-(4-methoxybenzoyl)-2-methyl-N-pyridin-4-yl-
1,2,3,4-
tetrahydroquinolin-4-amine (90 mg, 0.24 mmol, 1 equ.) in methylene chloride
(0.8 mL) was added.
diisopropylethylamine (84 uL, 0.48 mmol, 2 equ.) followed by acetyl chloride
(340 uL, 4.80 mmol,
20 equ.). The mixture was stirred at room temperature for 2 h. The mixture was
concentrated under
reduced pressure, dissolved in ethyl acetate, washed with sat. aqueous sodium
bicarbonate, brine and
dried over magnesium sulfate, filtered, and concentrated under reduced
pressure. The residue was
purified by silica gel chromatography (methylene chloride/methanol 97/3) to
affoxd pure N-[(2S,4R)-
1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-N-pyridin-4-
ylacetamide (60 mg, 61
%).
'H-NMR (CDCl3) 8: 1.20 (d, 3H), 2.20 (s, 3H), 2.30 (m, 1H), 3.75 (s, 3H), 4.80
(m, 1H), 5.65 (m, .
1H), 6.60 (d,1H), 6.70 (d, 2H), 7.05 (t, 1H), 7.10-7.20 (m, 4H), 7.25-7.40 (m,
2H), 8.70 (d, 2H).
M5 m/z: 416 (M+1).
[00972] N-(4-chloro-2-methylphenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (G-34)
[00973] N-(4-chloro-2-methylphenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was made following general procedure G,
substituting 4-
methoxybenzoyl chloride for 4-fluorobenzoyl chloride. The amine-aryl coupling
was performed
differently to what is described in procedure G. Therefore (2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-amine (obtained from the hydrogenation step, 500
mg,1.5 mmol, l equ.)
was dissolved in ethylene glycol dimethyl ether (5 mL) in a Schlenk tube. To
this solution was added
sequentially 2-bromo-5-chlorotoluene (400 mg,1.95 mmol,1.3 equ.), cesium
carbonate (684 mg, 2.10
mmol, 1.4 equ.), palladium acetate (40 mg, 0.06 mmol, 0.04 equ.) and 2-
(Dicyclohexylphosphino)-
225
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
2',4',6'-tri-i-propyl-1,1'-biphenyl (60 mg, 0.12 mmol, 0.08 equ.). The
reaction mixture was flushed
with nitrogen and heated to 90°C in the Schlenk tube for 48 h. Reaction
mixture was concentrated to
leave a residue which was partitioned between water and ethyl acetate and
extracted. The aqueous
layer was separated and the organic layer was washed with brine, dried over
magnesium sulfate,
filtered and concentrated to give a black oil. The crude product was purified
by silica gel
chromatography (methylene chloride/methanol: 99/1) to provide (2S,4R)-N-(4-
chloro-2-
methylphenyl)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-amine
(200 mg, 32 %).
[00974] To a solution of (2S,4R)-N-(4-chloro-2-methylphenyl)-1-(4-
methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-amine (140 mg, 0.33 mmol, 1 equ.) in acetyl
chloride (1.0 mL) was
added ' diisopropylethylamine (58 uL, 0.33 mmol, 1 equ.). The mixture was
stixred at room
temperature for 5 h. The mixture was concentrated under reduced pressure,
dissolved in ethyl acetate,
washed with sat. aqueous sodium bicarbonate, brine and dried over magnesium
sulfate, filtered, and
concentrated under reduced pressure. The residue was purified by silica gel
chromatography
(methylene chloride/methanol 99/1) to afford pure N (4-chloro-2-methylphenyl)-
N [(2S,4R)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide (140 mg, 92
%).
1H-NMR (CDC13) 8: 1.10 (d, 3H), 1.95 (s, 3H), 2.15 (m, 1H), 2.35 (s, 3H), 3.70
(s, 3H), 4.75 (m,
1H), 5.60 (m,1H), 6.50 (d, 1H), 6.65 (d, 2H), 6.95 (t,1H), 7:15-7.30 (m, 6H),
7.40 (s,1H).
MS m/z: 463 (M+1).
Table 7: Exemplary Compounds:
c, ~
~N~cH3 H O
_ _ ~I
HO~N~N~CH3
O
I / N ..CHs
O
O
Et
CH3
G-1 I G-2
226
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI / O
CI / F O \ (
~ N~CH3
~N~CH3 \
~ ~~'CH3
~~'CH3 O \
O ( \ ( i NHZ
/ O~CH3 O
G-3 G-4
CI
c1 ~ o
N CH3 \ (
N~CH3
N ''CH3 ~(
F ~~'CH3 H
O ' H3CCH3 N
O ( \
CH3 ~ O
O
G-5 G-6
CI c1 ~ ( o
H C~N \ ( \ N~CH3
3
\ (\
( , ~~ ~.,CH3
CH3
p ~ O'
o I\
.N N
N= CF3 CH3
G_7 G_8
227
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
O / I CI
CI , I O
H3C~'N
N~CH3
i ~ ~,.CHa
~~'CHa p w
O I .~ OXF ~ / NH2
~O F O
G-9 G-10
c1 p ~ CI \ I o
N~CH3 N~'CH3
__ \
N ,'CHs
N ~'CH3
O W O ~w
N
G-11 G-12
O CI / O
~ I N~CH3 ~ I N~CH3
~ ~ N ~'CH ( ~ N ~'CH
3 3
O ~ ~ O ~ ~ F H- sCVCHs
O'CH3 ~ O~COOH
G-13 G-14
228
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
c1 \ I N~ I
CI \ N~iPr
I
N ~~~~
N
O ( / vN O I
O
iPr
G-15 G-16
CI \ I o CI
N~CH3 N
~I
~..CH ( / N ....
3
O $
I N.CHs O ' ;N
~N N
I
CHs iPr
G-17 G-18
CI ~ o
~I
~N~CH3
HN ~
~I o ~I _
N ~'CH3
N~CH3 O
I / .CHs
~ N
~.~CH3
O I w CH3
i O~CHs HOOC CHs
G-19 G-20
229
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI
CI \ I N~OH w I N
., I i N .,,~ O O. Et
N CH3 ~'
p Iw ° I~ NJ
/ .CH3
O O
G-21 G-22
CI ~ I p
CI , I O ~ N~'CH3
N~CHs I w
CI ~
~~ ~~'CH3
O,
'~~'CHs
O ~ ~N
O ( / ~CH3 O-~
O COOH
G-23 G-24
CI ' I N_
CI / I O
~N~CHg I / -.n
N
O O
,
I i N .,CH3 ~ ~ N
O I ~ O
O,
Et
CN O
G-25 G-26
230
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI , ' O CI , I O
N
s N~CH3
N ,.CHs ~ i N ..CHs
O I~ O
.CHs
COOH O
G-27 G-28
~I / ~ p
N~CH3
~N ~.
O N CHs
~ N~CH3 O
I w i N.CHs
N ~'CHs
CHs
O ~ i .CHs HsC~O CHs
O O
G-29 G-30 ,
O H
O HsC~ .N
H3C N~N~CH3 O=~ ~ ~ O
H O ~N~CHs
W
/ N .,CHs W
N ~'CHs
O
O \
O
.CHs
CHs O
G-31 G-32
231
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI / CI~
N ~ O ~N~CH3
~N~CH3
N ~'CH3
~ / N ..CHs O
O ( ~ O
/ O.CH3 CH3
G-33 ~ G-34
Table 8: Names of Compounds Exemplified in Table 7:
G-1 Ethyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4
dihydroquinolin-1(2H)-yl]carbonyl}phenyl)piperazine-1-carboxylate
G-2 N-{3-[(Glycoloylamino)methyl]phenyl}-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide
G-3 N-(4-Ghloro-2-fluorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide
G-4 4-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-
1 (2H)-yl]carbonyl }benzamide
G-5 Methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-yl]carbonyl }-2-fluorophenoxy)-2,2-dimethylbutanoate
G-6 N-(4-Chlorophenyl)-N [(2S,4R)-1-(3,4-dihydro-2H-1,4-benzoxazin-6-
ylcarbonyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide
G-7 N (4-Chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-[3-(trifluoromethyl)-1H-
pyrazol-
1-yl]benzoyl }-1,2,3,4-tetrahydroquinolin-4-yl)acetamide
G-8 N-(4-Chlorophenyl)-N { (2S,4R)-2-methyl-1-[(4-methyl-3,4-dihydro-2H-1,4-
benzoxazin-7-yl)carbonyl]-1,2,3,4-tetrahydroquinolin-4-yl }acetamide
G-9 N (4-Chlorophenyl)-N-{(2S,4R)-1-[(2,2-difluoro-1,3-benzodioxol-5-
yl)carbonyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl } acetamide
G-10 5-(4-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1 (2H)-yl]carbonyl }phenyl)pentanamide
G-11 N-(4-Chlorophenyl)-N-[(2S,4R)-1-(2,3-dihydro-1-benzofuran-5-ylcarbonyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide
G-12 N (4-Chlorophenyl)-N [(2S,4R)-2-methyl-1-(pyridin-3-ylcarbonyl)-1,2,3,4-
232
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
tetrahydroquinolin-4-yl]acetamide
G-13 N-[(2S,4R)-1-(4-Methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-
N-
phenylacetamide
G-14 4-(4-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoic acid
G-15 N-(4-Chlorophenyl)-N {(2S,4R)-1-[(1-isopropyl-1H-indazol-5-yl)carbonyl]-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl }acetamide
G-16 N-(4-Chlorophenyl)-N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-2-methylpropanamide
G-17 N-(4-Chlorophenyl)-N-{(2S,4R)-1-[(1,3-dimethyl-1H-thieno[2,3-c]pyrazol-5
yl)carbonyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl } acetamide
G-18 N (4-Chlorophenyl)-N {(2S,4R)-1-[(1-isopropyl-1H-pyrazol-4-yl)carbonyl]-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl }acetamide
G-19 N 1H-Indol-4-yl-N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide
G-20 4-[(4-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-yl]carbonyl }phenyl)(methyl)amino]-2,2-
dimethylbutanoic acid
G-21 N-(4-Chlorophenyl)-2-hydroxy-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide
G-22 Ethyl 6-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-yl]carbonyl }-2,3-dihydro-4H-1,4-benzoxazine-4-
carboxylate
G-23 N-[(2S,4R)-6-Chloro-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinoliri-4-yl]-N-(4-chlorophenyl)acetamide
G-24 [(5-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}isoxazol-3-yl)oxy]acetic acid
G-25 N-(4-Chlorophenyl)-N [(2S,4R)-1-(4-cyanobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide
G-26 Ethyl 4-[(5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-yl]carbonyl }isoxazol-3-yl)oxy]-2,2-dimethylbutanoate
G-27 4-{ [(2S,4R)-4-[Acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-
1(2H)-yl]carbonyl}benzoic acid
G-28 N-(4-Chlorophenyl)-N [(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]cyclopropanecarboxamide
G-29 N-[(2S,4R)-1-(4-Methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-
N-
233
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[4-(1H-pyrrol-1-yl)phenyl]acetamide
G-30 Methyl 4-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenyl)(methyl)amino]-2,2-
dimethylbutanoate
G-31 N-[3-(Acetylamino)phenyl]-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-y1]acetamide
G-32 N-[(2S,4R)-1-(4-Methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-
N-
{ 4-[(methylsulfonyl)amino]phenyl } acetamide
G-33 N [(2S,4R)-1-(4-Methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]-
N-
pyridin-4-ylacetamide
G-34 N (4-Chloro-2-methylphenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide
Scheme 22
c1 c1 ,
CF3 ~ ~ ~ ~ N
NHCbz CI I ' NHCbz NH
1. HZ Pd/C ~ CI _
CF3
I / I ~ N ''CH 2. Cu(OAc)Z, Pyridine ~ N ~"CH3 DIPEA, N 'CHs
CH2CI2, DIPEA 3 CI ~ CFs O ~ CF3
CF3 O
I ~ /
CF B(OH)2 s CF3 B-31 CF3
3
~% yield 3-steps
CI Ct
O IN
N ~CF3
KOH I ~ . CHZCI2,
DIPEA
.
78% 2-steps ~ H ~'CH3
[00975] General Procedure H:
[00976] N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{[6-(tritluoromethyl)pyridin-
3-
yl]carbonyl}-1,2,3,4-tetrahydroquinolin-4-yl)acetamide (H-$3)
[00977] To a solution of (2S, 4R)-(2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic acid
benzyl ester (20.0 g, 0.067 mol) in methylene chloride (150 mL) at room
temperature was added
diisopropylethylamine ( 40.2 mL, 0.288 mol) at 0 °C followed by
addition of 3,5
bis(trifluoromethyl)benzylchloride (24.2 g, 15.8 mL, 0.087 mol). A catalytic
amount of 4
dimethylaminopyridine was added and the reaction turned a dark brown and was
allowed stir
overnight at room temperature. The mixture was partitioned between sodium
bicarbonate (saturated)
and methylene chloride. The organic layer was separated and dried over sodium
sulfate, filtered and
234
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
concentrated to a brown solid. The crude material was taken up in 150 mL of
methanol and stirred
with 15 g of cesium carbonate for 20 min. 160 mL of methylene chloride and 150
mL of water was
added to the solution and separated. The aqueous layer was extracted 2
additional times with
methylene chloride. The organics were collected together and dried over sodium
sulfate, filtered and
concentrated down to give a light orange solid (36 g, quint.).
[00978] Benzyl (2S,4R)-1-(3,5-bis(trifluoromethyl)benzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-
4-ylcarbamate (36 g, 0.067 mol) was dissolved in ethanol (135 mL). The vessel
in which resided the
resulting solution was evacuated and backfilled with argon. A catalytic amount
of Palladium on
Carbon (10 %) was added. The vessel was once again evacuated and this time was
backfilled with
hydrogen and shaken in a Parr bottle at 10 psi hydrogen. Reaction was allowed
to shake until no
starting material remained (1 day). The mixture was carefully filtered through
a Celite ~ pad and
concentrated to afford the crude amine (26.6g, 99 %).
[00979] (3,5-bis(trifluoromethyl)phenyl)((2S,4R)-4-amino-2-methyl-3,4-
dihydroquinolin-1(2H)-
yl)methanone (26.6 g, 0.066 mol) was dissolved in 1.0 L of dry methylene
chloride. To the solution
was also added .4-chlorophenylboronic acid (20.7 g, 0.132 mol, 2 equ.),
triethylamine (71.2 mL,
0.496mo1, 7.5 equ) and copper(I~acetate (24 g, 0.132 mol, 2 equ.). Finally 20
gm of molecular sieves
was added. The heterogeneous green mixture was stirred open to air for 1 h, an
additional 2 equivalent
of 4-chlorophenylboronic acid (20.7 gm, 0.132 mol) Was added. The mixture was
allowed to stir at
room temperature overnight and was then filtered through Celite ~. The
filtrate was diluted with
ethyl acetate to precipitate the copper salts, the mixture was filtered a
second time through Celite ~ to
give a brown solution which was concentrated down. The residue was purified by
flash
chromatography (95 % methylene chloride / 5 % ethyl acetate) to afford 19.54 g
of the product in 57.
% yield as a white solid.
[00980] A solution of (3,5-bis(trifluoromethyl)phenyl)((2S,4R)-4-(4-
chlorophenylamino)-2-
methyl-3,4-dihydxoquinolin-1(2H)-yl)methanone (19.44 g, 0.037 mol) in acetyl
chloride (5 mL) was
cooled to 0 °C and triethylamine (6.59 mL, 0.037 mol) was added
dropwise over ~30 min, a
precipitate forms during this time. An additional 250 mL of methylene chloride
was added to
completely dissolve all the precipitate. The reaction was allowed to stir
overnight at room
temperature. The mixture was concentrated under reduced pressure, partitioned
between ethyl acetate
and 1N sodium hydroxide while cooled to 0 °C. The aqueous layer was
extracted 3 times with ethyl
acetate washed with sat. aqueous sodium bicarbonate, brine and dried over
magnesium sulfate,
filtered, and concentrated under reduced pressure. The residue was purified by
silica gel
chromatography (25/75 hexanes/ethyl acetate gradient) to afford pure N-
((2S,4R)-1-(3,5-
bis(trifluoromethyl)benzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl)-N-(4-
chlorophenyl)acetamide
(H-31) (18.2 g, 86 %).
235
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
'H-NMR (CDC13) 8: 1.20 (d, 3H), 1.10-1.20 (m, 1H), 2.03 (s, 3H), 2.24-2.40 (m,
1H), 4.72-4.86 (m,
1H), 5.40-5.70 (m, 1H), 6.41 (d, 1H), 6.94 (t, 1H), 7.18-7.28 (m, 4H), 7.37
(t, 2H), 7.59 (s, 2H), 7.76
(s, 1H).
MS m/z: 555 (M +1).
[00981] To a solution of N-((2S,4R)-1-(3,5-bis(trifluoromethyl)benzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl)-N-(4-chlorophenyl)acetamide (5.5 g, 9.9 mmol) in
ethanol (60 mL) and
water (10 mL) was added potassium hydroxide (3.00g, 53.5 mmol). The mixture
was heated to 70 °C.
After 1 hour, an additional portion of potassium hydroxide (3.00g, 53.5 mmol)
was added and the
reaction was stirred for an additional 30m. The mixture was partitioned
between ethyl acetate and
sodium bicarbonate (sat. aq.). The organic layer was separated, washed twice
with brine, dried over
sodium sulfate, filtered and concentrated. The crude matarial was subjected to
flash chromatography
(2l1 hexanes/ethyl acetate gradient), resulting in pure N-(4-chlorophenyl)-N-
((2S,4R)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl)acetamide as a white solid (2.85 g, 91 %).
'H-NMR (CDCI3) 8: 1.10 (d, 3H), 1.30 (m, 1H), 1.80 (m, 1H), 1.90 (s, 3H), 3.50
(m, 1H), 3.60 (m,
1H), 6.30 (s broad, 1H), 6.45 (d, 1H), 6.70 (t, 1H), 6.90 (d, 2H), 7.00 (t,
1H), 7.15-7.25 (m, 3H). MS
m/z: 315 (M+1).
[00982] ~To a solution of N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl)acetamide (104 mg, 0.33 mmol, 1 equ.) in methylene chloride (2.0 mL) at
room temperature was
added diisopropylethylamine (86 uL, 0.49 mmol, 1.50 equ.) followed by 6-
trifluoromethyl nicotinyl
chloride (104 mg~ 0.49 mmol, 1.50 equ.). The reaction was stirred over night
at room temperature.
The mixture was concentrated, then poured into water and extracted with ethyl
acetate. The extracts
were washed with 1 M (aq) NaOH and brine, dried over magnesium sulfate,
filtered, dried and
concentrated, The crude residue was purified by silica gel chromatography
(ethyl acetate/hexane 1:1)
to afford the pure N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{ [6-
(trifluoromethyl)pyridin-3-
yl]carbonyl }-1,2,3,4-tetrahydroquinolin-4-yl)acetamide (140 mg, 87 %).
'H-NMR (CDCl3) 8: 1.16-1.18 (m, 1H), 1.17 (d, 3H), 1.99 (s, 3H), 2.20-2.28 (m,
1H), 4.77 (sextet,
1H), 5.50 (bs, 1H), 6.44 (d, 1H), 6.93 (t, 1H), 7.16-7.22 (m, 3H), 7.45-7.30
(m, 5H), 8.65 (s, 1H).
MS m/z = 465 (M+1).
Scheme 23
236
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
CI \ ( ~ CI \ I N~ OECI \ ~ N
N ~~\\,, Br~ .
\ BBr3, CH2CIz I \ O
~~~'CH3 ~~~~~''CH3 K2C03, DMF
O%~~~ O \
OMe I ~ OH
1Z 73 m
[00983] General Procedure I:
[00984] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin_4-yl]-acetamide (13)
[00985] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-methoxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was prepared following general procedure G,
substituting 4-methoxybenzoyl
chloride for 4-fluorobenzoyl chloride. (2S,4R)-N-(4-chloro-phenyl)=N-[1-(4-
methoxy-benzoyl)-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide was dissolved in
dichloromethane and a solution
of BBr3 (1~0 M in dichloromethane, 10 mL) was added; the reaction was allowed
to stir at room
temperature for until no starting material remained. The reaction was washed
with sat NaHCO3
carefully and brine. The organics were dried over MgS04, filtered and
concentrated down. The
residue was purified by Biotage flash chromatography using 100 % EtOAc to give
(2S,4R)-N-(4-
chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-
yl]-acetamide
'[00986] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl}-phenoxy)-acetic acid ethyl ester (14)
[00987] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.147 g) was dissolved in DMF at room temperature
and KzCO3 was
added. Ethyl 4-bromoacetate (0.065 g) was added and the reaction was allowed
to heat to 80°C
overnight. The reaction mixture was concentrated i~z vacuo. The residue was
partitioned between
ethyl acetate and water, then extracted three times with ethyl acetate, dried
over MgS04, filtered and
concentrated down. The crude residue was purified by silica gel chromatography
(80/20
hexanes/ethyl acetate - 50/50 hexanes ethyl acetate gradient) to afford the
product in 130 mg, 73 %.
[00988] (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenylamino)-propionamide (H-1)
[00989] (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl}-phenylamino)-propionamide was prepared from (2S,4R)- 3-(4-{4-
[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenylamino)-
propionic acid. The
acid (0.060 g, 0.118 mmol) was dissolved in DMF (1.5 mL) at room temperature
and HOBt (0.024 g,
237
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
0.177 mmol), HATU (0.068 g, 0.177 mol), and diisopropylethylamine (0.082 mL,
0.472 mmol) was
added followed by ammonium chloride (0.014 g, 0.236 mmol) and stirred at room
temperature for 18
h. The reaction was diluted with ethyl acetate, washed with 1N NaOH, 1N HCl
and brine. The
organics were dried over MgS04, filtered and concentrated down. The crude
residue was purified
HPLC purification.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.23 (m, 1H), 1.64 (br, 1H), 2.01 (s, 3H),
2.27 (m, 1H), 2.44 (t,
2H), 3.37 (t, 2H), 4.69 (m, 1H), 5.40 (br, 1H), 5.61 (brs, 1H), 5.80 (br, 1H),
6.31 (d, 2H), 6.59 (d, 1H),
6.94 (t, 1H), 7.02 (d, 2H), 7.10-7.23 (m, 4H), 7.36 (d, 2H).
MS m/z: 505.3 (M+1).
[00990] '4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoic acid (H-2)
[00991] To the solution of methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoate
(176mg, 0.30 mmol) in
MeOH/Z'HF, (1 mL/1mL) was added excessive LiOH (1N aqueous solution). The
reaction mixture
was stirred at r.t. overnight. The reaction was quenched by adding 6N HCl to
PH 2.,The mixture was
concentrated under reduced pressure to remove MeOH ,and THF. DCM (30 mL) was
added. The
reaction mixture was washed with brine (30 mL). The organic layer was dried
over magnesium
sulfate, filtered, and concentrated under reduced pressure. The crude residue
was purified by silica
gel chromatography (hexanes-ethyl acetate system) to afford slightly yellow
solid product (120 mg,
70%).
1H-NMR (CDCl3, 300MHz) 8: 1.11-1.13 (d, 3H), 1.25 (s, 6H), 2.02-2.08 (m, SH),
2.22-2.40 (m, 2H),
3.99-4.04 (t, 2H), 4.71-4.76 (q, 1H), 5.46 (b, 1H), 6,51-6.75 (m, 3H), 6.92-
7.06 (m, 2H), 7.16-7.38
(m, 5 H).
MS m/z: 567 (M+1).
[00992] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-
1-carbonyl}-phenoxy)-fluoro-acetic acid ethyl ester (H-3)
[00993] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (202 mg, 0.46 mmol) was dissolved in DMF (2 mL) at
room temperature.
CszC03 (760 mg, 2.33 mmol) was added followed by bromo-fluoro-acetic acid
ethyl ester (0.070 mL,
0.583 mmol) and the reaction was allowed to stir overnight. The mixture was
partitioned between
methylene chloride and water; the organic layer was dried over NazS04,
filtered and concentrated.
238
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
The crude residue was purified by silica gel chromatography (2/1 hexanesl
ethyl acetate) to afford the
product.
1H-NMR (CDC13) 8: 1.1 (d, 3H), 1.2 (m, 1H), 1.3 (t, 3H), 2.0 (s, 3H), 2.3 (m,
1H), 4.3 (q, 2H), 4.8
(m, 1H), 5.6 (bs, 1H), 5.9 (d, 1H), 6.5 (d,1H), 6.9 (m, 3H), 7.2 (m, 6H), 7.4
(d, 2H).
MS m/z: 539 (M+1).
[00994] (2S,4R)- 2-[4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperazin-1-yl]-acetamide (H-4)
[00995] (2S,4R)- 2-[4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperazin-1-yl]-acetamide was made from (2S ,4R)-
[4-(4-{4-[Acetyl-
(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenyl)-piperazin-1-yl]-
acetic acid. The acid (0.120 g, 0.21 mmol) was dissolved in DMF (2 mL) at room
temperature and
HOBt (0.043 g, 0.32 mmol), HATU (0.122 g, 0.32 mol), and diisopropylethylamine
(0.15 mL; 0.64
mmol) was added followed by ammonium chloride (0.023 g, 0.42 mmol) and stirred
at room
temperature. for 18 h. The reaction was diluted with ethyl acetate, washed
with 1N NaOH, 1N HCl
and brine. The organics were dried over MgS04, filtered and concentrated down.
The crude residue
was purified HPLC purification.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.20 (m, 1H), 2.00 (s, 3H), 2.24 ( m, 1H),
3.04 (t, 4H), 3.50 (m,
6H), 4.70 (m, 1H), 5.40(br, 1H), 5.56 (brs, 1H), 5.80 (br, 1H), 6.51 (d, 1H),
6.63 (d, 2H), 6.89 (t; 1H),
7.07-7.25 (m, 6H), 7.35 (d, 2H).
MS mlz: 560 (M+1)
[00996] 4-(4-{[(2S,4R)-4-[(4-Chlorophenyl)(isobutyryl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2Fn-yl]carbonyl}phenoxy)-2,2-dimethylbutanoic acid (H-5)
[00997] Methyl 4-(4-{ [(2S,4R)-4-[(4-ehlorophenyl)(isobutyryl)amino]-2-methyl-
3,4-
dihydroquinolin-1(21~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoate was dissolved
in
methanolltetrahydrofuran/water (2/1/1) then sodium hydroxide (3 equivalents)
was added and reaction
mixture stirred at 40 °C overnight. The mixture was concentrated, the
residue acidified with a 1N HCl
aqueous solution and extracted with ethyl acetate. The organic layer was
washed with brine, dried
over magnesium sulfate, filtered and concentrated to give 4-(4-{ [(2S,4R)-4-
[(4-
Chlorophenyl)(isobutyryl)amino]-2-methyl-3,4-dihydroquinolin-1 (21~-
yl]carbonyl }phenoxy)-2,2-
dimethylbutanoic acid.
'H-NMR (CDC13) 8: 1.11-1.21 (m, 10H), 1.25 (s, 6H), 2.03 (t, 2H), 2.21-2.29
(m, 1H), 2.61 (sp, 1H),
3.95 (t, 2H), 4.69-4.76 (m, 1H), 5.60 (br s, 1H), 6.51 (d, 1H), 6.62 (d, 2H),
6.91 (t, 1H), 7.11-7.43 (m,
8H).
MS m/z: 577 (M+1).
239
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[00998] {[3-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(21~-yl]carbonyl}phenoxy)propyl]amino}acetic acid (H-6)
[00999] { [3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}phenoxy)propyl]amino}acetic acid was prepared from (2S,4R)-N-{1-[4-
(3-amino-
propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl }-N-(4-chloro-
phenyl)-acetamide.
(2S,4R)-N-{ 1-[4-(3-amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl }-N-(4-
chloro-phenyl)-acetamide (0.032g, 0.065 mmol) was dissolved in
dimethylformamide, ethyl
bromoisobutyrate (0.05mL, 0.26 mmol) and potassium carbonate (0.018g, 0.13
mmol) were added.
The reaction was heated to 50°C for 17h. The reaction was concentrated
down and purified using
50%ethyl acetatel 50% hexane to 100% ethyl acetate to give 0.007g, 12% yield
of { [3-(4-{ [(2S,4R)-4-
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2I~-yl]carbonyl
}phenoxy)propyl]
amino}acetic ethyl ester.
[001000]
'H-NMR (CDCI3) 8: 1.13 (d, 3H), 1.17 (t, 3H),1.23 (m, 1H),1.55 (s, 6H), 2.02
(s, 3H), 2.06 -2.12 (m,
1H), 2.28 (m, 1H), 3.63 (q, 2H), 3.70-3.77 (m, 2H), 3.91 -3.95 (m, 1H), 4.13
(q, 2H), 4.74 (sextet,
1H), 5.58 (bs, 1H), 6.49-6.68 (m, 3H), 6.92 (t, 1H), 7.05-7.28 (m, 6H), 7.37
(d, 1H). MS m/z = 606.1
(M+1).
[001001] { [3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2F~-
yl]carbonyl}phenoxy)propyl] amino}acetic ethyl ester was hydrolyzed to the
acid by dissolving in
tetrahydrofuran and ethanol and sodium hydroxide (1N) was added. The mixture
was stirred at room
temperature overnight. The mixture was cooled to rt, acidified to form a white
precipitate. The solid
was filtered to give { [3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-dihydroquinolin-
1(21~-yl]carbonyl}phenoxy)propyl]amino}acetic acid 0.005g, 75% yield.
MS m/z : 578.3 (M+1).
[001002] N-[(2S,4R)-1-(4-tent-butylbenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-
chlorophenyl)acetamide (H-7)
[001003] N-[(2S,4R)-1-(4-tart-Butylbenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-
chlorophenyl)acetamide was synthesized according to general procedure H
substituting 4-tert-
butylbenzoyl chloride for 6-trifluoromethyl nicotinyl chloride. The rest of
the procedure was followed
as indicated in general procedure H to yield N-[(2S,4R)-1-(4-tart-
butylbenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N (4-chlorophenyl)acetamide.
IH-NMR (CDC13) 8: 1.13-1.22 (m, 1H), 1.14 (d, 3H), 1.22 (s, 9H), 2.03 (s, 3H),
2.24-2.35 (m, 1H),
4.72-4.80 (m, 1H), 5.61 (br s, 1H), 6.52 (d, 1H), 6.89 (t, 1H), 7.10-7.29 (m,
8H), 7.37 (d, 2H).
240
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS mlz: 475 (M+1).
[001004] (2S,4R)- N-(4-Chloro-phenyl)-N-{1-[4-(3-hydroxy-3-methyl-butoxy)-
benzoyl]-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-8)
[001005] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF (5 mL) at room temperature.
KZC03 was added
followed by 4-bromo-2-methyl-butan-2-of and the reaction was allowed to stir
at 90°C over night.
The reaction mixture was concentrated ira vacuo. The residue was partitioned
between ethyl acetate
and water, then extracted three times with ethyl acetate, dried over MgS04,
filtered and concentrated
down. The crude residue was purified by HPLC to afford the product.
[001006] 4-bromo-2-methyl-butan-2-of was prepared from 3-bromo-propionic acid
ethyl ester. 3-
Bromo-propionic acid ethyl ester (1.0 g, 5.5 mmol) was dissolved in 10 mL. of
ether and 3.7 mL of
methyl magnesium bromide (3.0 M in ether) was added at 0°C. The raction
was stirred at 0°C until no
starting material remained. The reaction was quenched with a sat'd ammonium
chloride solution and
extracted 3 x with ether. The organic were collected together and dried over
MgSO4, filtered and
concentrated down to give 4-bromo-2-methyl-butan-2-ol.
'H-NMR (CDCl3) 8: 1.12 (d, 3H), 1.24 (s+m~7H), 1.90 (t, 2H), 2.01 (s, 3H),
2.22 (m, 2H), 4.07 (t,
2H), 4.72 (m, 1H), 5.60 (brs, 1H), 6.50 (d, 1H), 6.65 (d, 2H), 6.90 (t, 1H),
7.10-7.20 (m, 5H), 7.25 (t,
1H), 7.35 (d, 2H).
MS m/z: 521 (M+1).
[001007] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-iodobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-9)
[001008] Purification of crude material in the last step of the synthesis of 5-
(4-{(2S,4R)-4-[acetyl~
(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenyl)-
2,2-dimethyl-
pentanoic acid also allowed to isolate N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-
iodobenzoyl)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]acetamide as a by-product in the synthesis (45
mg).
'H-NMR (MeOD) 8: 1.15 (d, 3H), 2.05 (s, 3H), 2.45 (m, 1H), 4.75 (m, 1H), 5.55
(m, 1H), 6.55 (d,
1H), 6.95 (t, 1H), 7.25 (m, 5H), 7.30-7.55 (m, 5H).
MS m/z: 545 (M+1).
[001009] (2S,4R)-N-{1-[4-(3-Acetylamino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (H-10)
[001010] (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamide (99 mg, 0.202 mmol) was dissolved in
methylene chloride (2 mL)
241
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
and triethylamine (0.056 mL, 0.404 mmol) and cooled to -40 °C. Acetyl
chloride (15 drops via pipet)
was added and the mixtrue was warmed to 0 °C for 30 minutes. The
mixture was partitioned between
methylene chloride and water; the methylene chloride layer was dried over
MgS04, filtered and
concentrated. The crude residue was purified by silica gel chromatography (1/1
hexanes/ ethyl acetate
- ethyl acetate gradient) to afford the product.
'H-NMR (CDC13) 8: 1.1 (s, 3H), 1.2 (m, 1H), 1.9 (s, 3H), 2.0 (m, 2H), 2.0 (s,
3H), 2.3 (m, 1H), 3.4
(q, 2H), 4.0 (t, 2H), 4.7 (m, 1H), 5.6 (bs, 1H), 5.7 (bs, 1H), 6.5 (d, 1H),
6.6 (d, 2H), 6.9 (t, 1H), 7.2
(m, 6H), 7.4 (d, 2H).
MS m/z: 534 (M+1).
[001011] (2S,4R)- N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(4,4,4-trifluoro-3-
hydroxy-3-
trifluoromethyl-butoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide
(H-11)
[001012] . (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-
1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF (5 mL) at room temperature.
KzC03 Was added
followed by toluene-4-sulfonic acid 4,4,4-trifluoro-3-hydroxy-3-
trifluoromethyl-butyl ester and the
reaction was allowed to stir at 90 °C over night. The reaction mixture
was concentrated in vacuo.
The residue was partitioned between ethyl acetate and water, then extracted
three times with ethyl
acetate, dried over MgS04, filtered and concentrated down. The crude residue
was purified by HPLC
to afford the product.
[001013] Toluene-4-sulfonic acid 4,4,4-trifluoro-3-hydroxy-3-trifluoromethyl-
butyl ester' was
prepared from 4,4,4-trifluoro-3-hydroxy-3-trifluoromethyl-butyric acid. 4,4,4-
trifluoro-3-hydroxy-3-
trifluoromethyl-butyric acid (1.74g, 7.0 mmol) was dissolved in THF (2 mL) at
0°C and BH3 SMe3
(2.6 mL, 26 mmol) was added dropwise (Ref: Tetrahedron, 2002, 9839). The
reaction was allowed to
warm to room temperature and stir for. 16h. The reaction was quenched with
methanol and the
solvent was removed to give the crude 4,4,4-trifluoro-3-trifluoromethyl-butane-
1,3-diol. The alcohol
was converted to the tosylate by addition of 4,4,4-trifluoro-3-trifluoromethyl-
butane-1,3-diol
dissolved in pyridine (2 mL) to tosyl chloride (1.39 mL) in 2 mL of CHzCIz and
a catalytic amount of
DMAP. The reaction was stirred at room temperature for 2 h and quenched.
'H-NMR (CDC13) 8: 1.12 (m, 4H), 2.01 (s, 3H), 2.24 (m, 1H), 2.25 (t, 2H), 3.94
(t, 2H), 4.70 (m,
1H), 5.53 (brs, 2H), 6.48 (d, 1H), 6.61 (d, 2H), 6.93 (t, 1H), 7.09-7.27 (m,
6H), 7.37 (d, 2H).
MS m/z: 629 (M+1).
[001014] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxy)-cyclohexanecarboxylic acid amide (H-12)
[001015] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1
carbonyl }-phenoxy)-cyclohexanecarboxylic acid (0.070g, 0.125 mmol) was
converted to the amide by
242
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
dissolving in THF (1 mL) at room temperature. HOBt (0.025 g), EDCI (0.035 g),
and ammonium
chloride (0.014 g, 0.25 mmol) was added along with 2 drops of DMF and stirred
at room temperature
for 11 h. The xeaction was diluted with ethyl acetate, washed with 1N NaOH, 1N
HCl and brine. The
organics were dried over MgS04, filtered and concentrated down. The crude
residue was purified by
silica gel chromatography (50% ethyl acetate/50% methanol) to afford the
product as a white powder
in a 71 % yield.
'H-NMR (CDCl3) 8: 1.13 (d, 4H), 1.19-1.60 (m, 7H), 2.01 (s, 4H), 2.20-2.39 (m,
2H), 4.72 (sextet,
1H), 5.60 (bs, 1H), 6.54 (d, 1H), 6.63 (d, 2H), 6.91 (t, 1H) 7.11-7.38 (m,
8H).
MS m/z: 560 (M+1).
[001016] (2S,4R)- N-(4-Chloro-phenyl)-N-(2-methyl-1-{4-[3-(1H-tetrazol-5-yl)-
propoxy]-
benzoyl}-1,2,3,4-tetrahydro-quinolin-4-yl)-acetamide (H-13)
[001017] The nitrile was prepared from (2S,4R)- N-(4-chloro-phenyl)-N-{ 1-[4-
(3-cyano-propoxy)-
benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide by dissolving in
toluene, sodium
azide and triethylammonium hydrochloride were added and the mixture was heated
to 80 °C over
night. Reaction was cooled to room temperature and water was added, followed
by hydrochloric acid
(1 N) until acidic. The aqueous solution was extracted three times with
dichloromethane. The
combined extracts were dried over magnesium sulfate, filtered, dried and
concentrated. The crude
product was triturated with ethyl ether/hexanes to yield a white solid in 63%
yield.
'H-NMR (CDCl3) 8: 1.02 (d, 3H), 1.19 (m, 1H), 1.89 (s, 3H), 1.96 (m, 2H), 2.11
(m, 1H), 2.79 (m,
2H), 3.63 (m, 2H), 4.59 (sextet, 1H), 5.42 (bs, 1H), 6.35 (m, 3H), 6.77 (t,
1H), 6.91-7.08 (m, 7H),
7.22 (d, 1H).
MS mlz: 545 (M+1).
[001018] Methyl 4-(4-{[(2S,4R)-4-[(4-chlorophenyl)(cyclopropylcarbonyl)amino]-
2-niethyl-
3,4-dihydroquinolin-1(21~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoate (H-14)
[001019] To a solution of N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]cyclopropanecarboxamide (200 mg, 0.42 mmol, 1 equ.) in
methylene chloride
(0.3 mL) was added a 1 M solution of boron tribromide in methylene chloride
(1.2 mL, 1.26 mmol, 3
equ.). The reaction mixture was stirxed at room temperature for 4 h, then the
reaction was quenched
with methanol and concentrated. The residue was partitioned between water and
ethyl acetate and
extracted. The aqueous layer was separated and the organic layer was washed
with brine, dried over
magnesium sulfate, filtered and concentrated to give N-(4-chlorophenyl)-N-
[(2S,4R)-1-(4-
hydroxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]cyclopropanecarboxamide as a beige
powder (190 mg, 98 %).
243
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001020] Methyl 4-(4-{ [(2S,4R)-4-[(4-chlorophenyl)(cyclopropylcarbonyl)amino]-
2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoate was made from
N-(4-
chlorophenyl)-N-[(2S,4R)-1-(4-hydroxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]cyclopropanecarboxamide following general procedure I, substituting methyl
4-bromo-2,2-
dimethylbutanoate for ethyl 4-bromoacetate to yield methyl 4-(4-{ [(2S,4R)-4-
[(4-
chlorophenyl)(cyclopropylcarbonyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2Fn-
yl]carbonyl }phenoxy)-2,2-dimethylbutanoate.
'H-NMR (CDC13) 8: 0.75 (m, 2H), 1.10 (m, 2H), 1.20 (d, 3H), 1.25 (s, 6H), 1.45
(m, 1H), 2.05 (t,
2H), 2.30 (m, 1H), 3.65 (s, 3H), 3.95 (t, 2H), 4.75 (m, 1H), 5.60 (m, 1H),
6.50 (d, 1H), 6.60 (d, 2H),
6.90 (t, 1H), 7.10-7.45 (m, 8H).
MS m/z: 589 (M+1).
[001021] Methyl 5-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}-2-tluorophenyl)-2,2-dimethylpentanoate (H-
15)
[001022] Methyl 5-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(21~-yl]carbonyl}-2-fluorophenyl)-2,2-dimethylpentanoate was prepared
following general
procedure B, substituting methyl 5-[4-(chlorocarbonyl)-2-fluorophenyl]-2,2-
dimethylpentanoate for
6-trifluoromethyl nicotinyl chloride. (Methyl 5-[4-(chlorocarbonyl)-2-
fluorophenyl]-2,2-
dimethylpentanoate was prepared in five steps from 4-bromo-3-fluorobenzoic
acid. To a solution of 3-
bromo-3-fluorobenzoic acid in toluene/methanol was added dropwise a 2M
solution of trimethylsilyl
diazomethane until slight yellow coloration persists indicating reaction had
gone to completion.
Reaction mixture was concentrated to give methyl-3-bromo-3-fluorobenzoate. To
a solution of
methyl-3-bromo-3-fluorobenzoate in dimethylformamide was added sequentially
palladium acetate,
triphenylphosphine, tetrabutylammonium chloride, potassium acetate and methyl
2,2-dimethylpent-4-
enoate. Reaction mixture was heated under microwave irradiation at
130°C for 10 m. to give methyl
3-fluoro-4-[(lE~-5-methoxy-4,4-dimethyl-5-oxopent-1-en-1-yl]benzoate.
Hydrogenation of this
diester gave methyl 3-fluoro-4-(5-methoxy-4,4-dimethyl-5-oxopentyl)benzoate
which benzoic ester
was selectively hydrolyzed using lithium hydroxide to give 3-fluoro-4-(5-
methoxy-4,4-dimethyl-5-
oxopentyl)benzoic acid. Subsequent treatment of this carboxylic acid with
oxalyl chloride and
catalytic DMF afforded methyl 5-[4-(chlorocarbonyl)-2-fluorophenyl]-2,2-
dimethylpentanoate in
decent yield). The rest of the procedures were followed as indicated in
general procedure H to afford
methyl 5-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (21~-
yl]carbonyl }-2-fluorophenyl)-2,2-dimethylpentanoate.
'H-NMR (CDC13) 8: 1.15 (d, 3H), 1.45 (m, 4H), 2.05 (s, 3H), 2.30 (m, 1H), 2.55
(m, 2H), 3.65 (s,
3H), 4.80 (m, 1H), 5.60 (m, 1H), 6.55 (d, 1H), 6.75 (d, 1H), 6.90-7.05 (m,
3H), 7.15-7.25 (m, 3H),
7.30-7.40 (m, 3H).
244
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 579 (M+1).
[001023] (2S,4R)-N-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-propionic acid (H-16)
[001024] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.32 g, 0.74 mmol) was dissolved in 10 ml DMF at
room temperature and
KZCO3 (0.51 g, 3.7 mmol) was added. 3-Chloro-2,2-dimethyl-propionic acid ethyl
ester (0.25 g, 1.52
mmol) was added and the reaction was allowed to heat to 90°C for 6
days. The reaction mixture was
concentrated in vacuo. The residue was partitioned between ethyl acetate and
water, then extracted
three times with ethyl acetate, dried over MgS04, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography (60% EtOAc/ 40% Hexane) to afford
the (2S,4R)-3-(4-{4-
[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl
}-phenoxy)-2,2-
dimethyl-propionic acid ethyl ester (0.13 g, 32%).
[001025] The ester was hydrolyzed to the acid by dissolving in 8 ml
tetrahydrofuran/methanol (1/1)
and potassium hydroxide (0.052 g in 2.5 ml water) was added. The mixture was
heated to 40 °C for 3
hours. The mixture was cooled to rt, acidified to form a white precipitate.
The solid was filtered to
give the product (0.098 g, 79%).
IH-NMR (CDC13) 8: 1.13 (d, 3H), 1.15 (t, 1H), 1.31 (s, 6H), 2.03 (s, 3H), 2.27
(m, 1H), 3.88 (q, 2H),
4.72 (sextet, 1H), 5.58 (bs, 1H), 6.52 (d, 1H), 6.69 (d, 2H), 6.83 (t, 2H),
7.08-7.35 (m, 6H), 7.38 (d,
2H). _
MS m/z: 535 (M+1)
[001026] (2S,4R)-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxy)-propane-1-sulfonic acid (H-17)
[001027] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (207 mg, 0.481 mmol) was dissolved in DMF (5 mL) at
room temperature.
NaH (58 mg, 2.40 mmol) was added followed by 3-chloro-propane-1-sulfonic acid
(sodium salt, 135
mg, 0.60 mmol) and the reaction was allowed to stir over night. The mixture
was partitioned between
methylene chloride and HCl (1.ON/water), then extracted three times with
methylene chloride, dried
over MgS04, filtered and concentrated. The crude residue was purified by
preparatory HPLC to
afford the product.
'H-NMR (CDCl3) 8: 1.1 (d, 3H), 1.1 (m, 1H), 2.0 (s, 3H), 2.2 (m, 3H), 3.2 (t,
2H), 3.9 (t, 2H), 4.8 (d,
1H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.6 (d, 2H), 6.9 (t, 1H), 7.2 (m, 6H), 7.4 (d,
2H), 10.6 (bs, 1H).
MS m/z: 557 (M+1).
245
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001028] Methyl 4-(5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoate (H-
18)
[001029] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-fluoro-3-hydroxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (476 mg, 1.05 mmol) was dissolved in DMF at
room temperature
and Cs2C03 (854 mg, 2.63 mmol) was added. Methyl 4-bromo-2,2-dimethylbutanoate
(702 mg, 1,58
mmol) was added and the reaction was stirred at r.t. overnight. The reaction
mixture was
concentrated under reduced pressure. The residue was partitioned between ethyl
acetate and water,
then extracted three times with ethyl acetate, dried over MgS04, filtered and
concentrated down. The
crude residue was purified by silica gel chromatography (hexanes-ethyl acetate
system) to afford
methyl 4-(5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (21~-
yl]carbonyl }-2-fluorophenoxy)-2,2-dimethylbutanoate (286 mg, 52%).
'H-NMR (CDCl3, 300MHz) S: 1.11-1.14 (d, 3H), 1.18-1.19 (d, 6H), 2.00 (s, 3H),
2. 22-2.27 (m, 1H),
3.64 (s, 3H), 3.88-3.95 (m, 1H), 4.69-4.77 (m, fH), 5.57 (b, 1H), 6.48-6.50
(d, 1H), 6.72-6.84 (m, 3
H), 6.91-6.96 (m, 1H), 7.12-7.37 (m, 6H).
MS m/z: 581 (M+1).
[001030] N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-fluoro-3-hydroxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-19)
[001031] N [(2S,4R)-1-(3-{ [tent butyl(dimethyl)silyl]oxy}-4-fluorobenzoyl)-2-
methyl-1,2,3,4=
tetrahydroquinolin-4-yl]-N-(4-chlorophenyl)acetamide (543 mg, 0.95 mmol) was
dissolved in
dichloromethane and a solution of TBAF (1.0 M in THF, 5.0 mL) was added; the
reaction mixture
was stirred at room temperature for until no starting material remained. The
reaction was washed
with sat. NaHC03 and brine carefully. The organic layer was dried over MgSOø,
filtered and
concentrated. The residue was purified by flash chromatography using He>zanes-
EtOAc system to
give N-(4-chlorophenyl)-N [(2S,4R)-1-(4-fluoro-3-hydroxybenzoyl)-2-methyl-
1;2,3,4-
tetrahydroquinolin-4-yl]acetamide (477 mg, 100%).
iH-NMR (CDCI 3) 8: 1.15 (d, 3H), 1.25 (m, 2H), 2.07 (s, 3H), 2.30 (b, 1H),
4.75 (m, 1H), 6.55 (d,
1H), 6.68 (d, 1H), 6.62-6.70 (m, 3H) 7.15-7.25 (m, 4H), 7.35-7.42 (m, 2H).
MS m/z: 453 (M+1)
[001032] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-N,N-diethyl-butyramide (H-20)
[001033] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline
1-carbonyl}-phenoxy)-N,N-diethyl-butyramide was prepared from (2S,4R)- 4-(4-{4-
[acetyl-(4-chloro
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-butyric
acid ethyl ester.
(2S,4R)- 4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-carbonyl}
246
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
phenoxy)-butyric acid ethyl ester (0.511 g, 0.93 mmol) was hydrolyzed to the
acid by dissolving in
tetrahydrofuran and ethanol and sodium hydroxide (1N) was added. The mixture
was stirred at room
temperature 4 hours. The mixture was cooled to rt, acidified to form a white
precipitate. The solid
was filtered to give (2S,4R)- 4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-
methyl-3,4-dihydro-2H-
quinoline-1-carbonyl}-1-ethylidene-pentG-2,4-dienyloxy)-butyric acid in 74%
yield. The acid
(0.050g, 0.09mma1) was converted to the amide by dissolving in THF (2 mL) at
room temperature.
HOBt (0.019 g), EDCI (0.022 g), and diethylamine (0.010 mL) was added along
with 2 drops of DMF
and stirred at room temperature for 11 h. The reaction was diluted with ethyl
acetate, washed with 1N
NaOH, 1N HCl and brine. The organics were dried over MgS04, filtered and
concentrated down. The
crude residue was purified by silica gel chromatography (50% ethyl acetate/50%
hexane to 100%
ethyl acetate) to afford the product (0.023 mg, 54%).
1H-NMR (CDC13) 8: 0.91 (d, 3H), 0.96 (t, 6H), 1.06 (m, 1H), 1.58 (m, 2H), 1.86
(s, 3H), 2.11 (m,
1H), 2.29(m, 2H), 3.11-3.17 (m, 4H), 3.78 (m, 2H), 4.56 (sextet, 1H), 5.43
(bs, 1H), 6.34 (d, 1H), 6.48
(d, 2H), 6.76 (t, 1H), 6.59-7.19 (m, 8H).
MS m/z: 576 (M+1).
[001034] N-{(2S,4R)-6-chloro-2-methyl-1-[(3-methylisoxazol-5-yl)carbonyl]-
1,2,3,4-
tetrahydroquinolin-4-yl}-N-(4-chlorophenyl)acetamide (H-21)
[001035] N-{(2S,4R)-6-chloro-2-methyl-1-[(3-methylisoxazol-5-yl)carbonyl]-
1,2,3,4-
tetrahydroquinolin-4-yl}-N-(4-chlorophenyl)acetamide was prepared following
the procedure
described ., for N {(2S,4R)-1-[3,5-bis(trifluoromethyl)benzoyl]-6-chloro-2-
methyl-1;2,3,4-
tetrahydroquinolin-4-yl}-N (4-chlorophenyl)acetamide substituting 3-
methylisoxazole-5-carbonyl
chloride for 3,5-bistrifluoromethyl benzoyl chloride.
'H-NMR (CDC13) 8 : 1.10 (d, 3H), 1.10 (1H, m), 2.02 (s, 3H), 2.20 (s, 3H),
2.24-2.32 (m, 1H), 4.68-
4.74 (m, 1H), 5.45-5.50 (m, 1H), 5.80 (s, 1H), 6.80 (d, 1H), 7.10-7.40 (m,
7H).
MS m/z: 458 (M + 1).
[001036] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2Ii-
quinoline-1-carbonyl}-phenoxy)-cyclohexanecarboxylic acid (H-22)
[001037] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-cyclohexanecarboxylic acid ethyl ester (0.060, 0.10 mmol)
was hydrolyzed to the
acid by dissolving in tetrahydrofuran and ethanol and sodium hydroxide (1N)
was added. The
mixture was stirred at room temperature 10 hours. The mixture was cooled to
room temperature,
acidified to form a white precipitate. The solid was filtered to give (2S,4R)-
4-(4-{4-[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-
cyclohexanecarboxylic
acid as a white powder in 62% yield.
247
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDCl3) b: 1.13 (d, 4H), 1.24-1.60 (m, SH), 2.01 (s, 6H), 2.20-2.39 (m,
2H), 4.72 (sextet,
1H), 5.60 (bs,1H), 6.53 (d,1H), 6.64 (d, 2H), 6.91 (t,1H) 7.11-7.38 (m, 8H).
MS miz: 561 (M+1).
[001038] Methyl 5-(4-{[(2S,4R)-4-[[(acetyloxy)acetyl](4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(21~-yl]carbonyl}phenyl)-2,2-dimethylpentanoate (H-23)
[001039] Methyl 5-(4-{ [(2S,4R)-4-[[(acetyloxy)acetyl](4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(2F1)-yl]carbonyl}phenyl)-2,2-dimethylpentanoate was prepared
following general
procedure B, substituting acetoxyacetylchloride for acetyl chloride in step 3
to provide 2-[{(2S,4R)-1-
[3,5-bis(trifluoromethyl)benzoyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl }
(4-chlorophenyl)amino]-
2-oxoethyl acetate.
[001040] To a solution of 2-[{(2S,4R)-1-[3,5-bis(trifluoromethyl)benzoyl]-2-
methyl-12,3,4-
tetrahydroquinolin-4-yl } (4-chlorophenyl)amino]-2-oxoethyl acetate (500 mg,
0.82 mmol,' 1 equ:) in
ethanol (6 mL) and water (1 mL) was added potassium hydroxide (229 mg, 4.1
mmol, 5 equ.). The
mixture was heated to 70 °C for 4h. The mixture was neutralized with 1N
aqueous HCl and extracted
with ethyl acetate. The organic layer was separated, washed with a saturated
aqueous solution of
sodium bicarbonate and twice with brine, dried over sodium sulfate, filtexed
and concentrated to give
crude N (4-chlorophenyl)-2-hydroxy-N-[(2S,4R)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide
used as such in the next step.
[001041] N-(4-Chlorophenyl)-2-hydroxy-N-[(2S,4R)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]acetamide (300 mg, 0.91 mmol, 1 equ.) was dissolved in methylene chloride
(3 mL). To this
solution was' added EDC (464 mg, 2.7 mmol, 3 equ.) and acetic acid (164 mg,
2.7 mmol, 3 equ:) and
the reaction mixture was stirred at room temperature for 20h. Reaction mixture
was concentrated and
the residue dissolved in ethyl acetate and washed with water, brine, and then
dried over magnesium
sulfate, filtered and concentrated. The crude residue was purified by silica
gel chromatography
(methylene chloride/methanol: 99/1 to 98/2 gradient) to afford 2-{(4-
chlorophenyl)[(2S,4R)-2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl]amino}-2-oxoethyl acetate (240 mg, 84 %).
[001042] To a solution of 2-{(4-chlorophenyl)[(2S,4R)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]amino}-2-oxoethyl acetate (170 mg, 0.45 mmol, 1 equ.) in methylene chloride
(2.0 mL) at room
temperature was added diisopropylethylamine (127 uL, 0.73 mmol, 1.60 equ.)
followed by methyl 5-
[4-(chlorocarbonyl)phenyl]-2,2-dimethylpentanoate (196 mg, 0.73 mmol, 1.60
equ.). The reaction
was stirred over night at room temperature. The mixture was concentrated, then
poured into water
and extracted with.ethyl acetate. The extracts were washed with brine, dried
over magnesium sulfate,
filtered dried and concentrated. The crude residue was purified by silica gel
chromatography (ethyl
acetate/hexane 25/75 to 1:1 gradient) to afford the pure methyl 5-(4-{
[(2S,4R)-4-
248
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[[(acetyloxy)acetyl](4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2F~-
yl]carbonyl}phenyl)-2,2-dimethylpentanoate (110 mg, 40 %).
1H-NMR (CDCl3) 8: 1.10 (s, 6H), 1.15 (d, 3H), 1.45 (m, 4H), 2.15 (s, 3H), 2.35
(m, 1H), 2.50 (m,
2H), 3.65 (s, 3H), 4.40-4.55 (q, 2H), 4.80 (m, 1H), 5.55 (m, 1H), 6.55 (d,
1H), 6.90-7.0 (m, 3H), 7.05-
7.20 (m, 3H), 7.30-7.55 (m, 5H).
MS mlz: 619 (M+1).
[001043] 4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2I~-
yl]carbonyl}phenoxy)-1-methylpyrrolidine-2-carboxylic acid (H-24)
[001044] 4-Hydroxy-1-methyl-pyrrolidine-2-carboxylic acid (0.27 g, 1.85 mmol)
was dissolved in
ml methanol at room temperature and (trimethylsilyl)diazomethane ( 2M solution
in hexane ) was
added until solution become yellow. The mixture was concentrated down to
afford crude 4-hydroxy-
1-methyl-pyrrolidine-Z-carboxylic acid methyl ester, which was converted to 4-
(4-{ [(2S,4R)-4-
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2I~-yl]carbonyl
}phenoxy)-1-
methylpyrrolidine-2-carboxylic acid following the same procedure as for the
preparation of (1R,2R)-
2-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-
1 (21~-
yl]carbonyl }phenoxy)methyl]cyclopentanecarboxylic acid.
[001045] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-butyramide (H-25)
[001046] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-butyramide was prepared from
(2S,4R)-N-4-(4-{4-
[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-
phenoxy)-2,2-
dimethyl-butyric acid by coupling NH4C1, HATU, DIEA, HOBt in DMF at room
temperature to yield
(2S,4R)-N-4-(4-{ 4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl}-phenoxy)-butyramide. The reaction mixture was concentrated down and
partitioned
between ethyl acetate and water, then extracted three times with ethyl
acetate, dried over magnesium
sulfate, filtered and concentrated down. The residue was purified by silica
gel chromatography (10%
methanol / 90 % dichloromethane) to afford pure (2S,4R)-N-4-(4-{4-[acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-butyramide
(63%).
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1.15 (t, 1H), 1.25 (s, 6H), 1.98 (t, 2H), 2.04
(s, 3H), 2.27 (m, 1H),
3.96 (t, 2H), 4.72 (sextet, 1H), 5.52 (br, 2H), 5.58 (bs, 1H), 6.52 (d, 1H),
6.67 (d, 2H), 6.86 (t, 1H),
7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS m/z: 548 (M+1)
249
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001047] (2S,4R)- N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-pyridin-3-yl-
propoxy)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-26)
[001048] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF (5 mL) at room temperature.
KzC03 was added
followed by 3-(3-bromo-propyl)-pyridine and the reaction was allowed to stir
at 90°C over night. The
reaction mixture was concentrated in vacuo. The residue was partitioned
between ethyl acetate and
water, then extracted three times with ethyl acetate, dried over MgS04,
filtered and concentrated
down. The crude residue was purified by HPLC to afford the product. .
'H-NMR (CDC13) 8: 1.12 (m, 4H), 1.83 (m, 2H), 2.01 (s, 3H), 2.24 (m, 1H), 2.76
(t, 2H), 3.89 (t,
2H), 4.73 (m, 1H), 5.60 (brs, 1H), 6.52 (d, 1H), 6.64 (d, 2H), 6.93 (t, 1H),
7.12-7.29 (m, 7H), 7.37 (d,
2H), 7.48 (d, 1H), 8.45 (m, 2H).
MS mlz: 554.36 (M+1).
[001049] (2S,4R)-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxymethyl)-furan-2-carboxylic acid amide (H-27)
[001050] (2S,4R)-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenoxymethyl)-furan-2-carboxylic acid (0.065g, 0.12mmo1) was
converted to the amide
by dissolving in THF (1 mL) at room temperature. HOBt (0.024 g), EDCI (0.033
g), and ammonium
chloride (0.013g, 0.232 mmol) was added along with 2 drops of DMF and stirred
at room temperature
for 11 h. The reaction was diluted with ethyl acetate, washed with 1N NaOH, 1N
HCl and brine. The
organics were dried over MgS04, filtered and concentrated down. The crude
residue was purified by
silica gel chromatography (10 ethyl acetate: 1 methanol) to afford the product
as a white solid in 61°l0
yield.
'H-NMR (CDC13) 8: 1.13-1.20 (m, 4H), 2.01 (s, 3H), 2.23-2.29 (m, 1H), 4.72-
4.74 (m, 1H), 4.99 (s,
2H), 5.59 (bs,1H) 6.47-6.51 (m, 2H), 6.71 (d, 2H), 6.91 (t,1H), 7.09-7.38 (m,
9H).
MS mlz: 558 (M+1).
[001051] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-piperazin-1-yl-
propoxy)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-28)
[001052] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-piperazin-1-yl-
propoxy)-benzoylJ-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide was prepared from (2S,4R)-4-[3-(4-
{4-[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-propyl]-
piperazine-1-
carboxylic acid tert-butyl ester. (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (0.40g, 0.92 mmol) was dissolved
in DMF at room
temperature and KZC03 (0.127g, 0.921 mmol) was added. 4-(3-Chloro-propyl)-
piperazine-1-
carboxylic acid tert-butyl ester (0.242g, 0.921 mmol) was added and the
reaction was allowed to heat
250
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
to 80°C overnight. The reaction mixture was concentrated in vacuo. The
residue was partitioned
between ethyl acetate and water, then extracted three times with ethyl
acetate, dried over MgSO~,
filtered and concentrated down. The crude residue was purified by silica gel
chromatography to
afford the product as colorless oil in 50% yield.
(2S,4R)-4-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl }-phenoxy)-propyl]-piperazine-1-carboxylic acid tent-butyl ester (246
mg) was dissolved in
4M HCl in dioxane (2 xnL). The mixture was stirred for 2h at room temperature.
After concentration,
the white solid was washed with EtOAc to provide the title compound in 100%
yield.
'H-NMR (DMSO, 2HCl salt) 8: 1.00-1.02 (m, 4H), 1.88-1.94 (m, 3H), 2.10-2.15
(m, 1H), 3.15;3.80
(m, 12H), 4.02 (t, 2H), 4.56-4.59 (m, 1H), 5.49 (bs, 1H), 6.50 (d, 1H), 6.76
(d, 2H), 6.96 (t, 1H), 7.04
(d, 2H), 7.16 (t, 1H), 7.39-7.53 (m, 5H).
MS m/z: 561 (M+1).
[001053] Methyl 4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4..
dihydroquinolin-1(21~-yl]carbonyl}-2,6-difluorophenoxy)-2,2-dimethylbutanoate
(H-29)
[001054] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure H,
substituting 3,5-
difluoro-4-methoxybenzoyl chloride for 6-trifluoromethyl nicotinyl chloride.
The rest of the
procedures were followed as indicated in general procedure H to afford N-(4-
chlorophenyl)-N-
[(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-
4-yl]acetamide was
obtained in decent yield.
[001055] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (200 mg, 0.41 mmol) was dissolved in
dichloromethane and a
solution of BBr3 (1.0 M in dichloromethane, 10 mL) was added; the reaction was
allowed to stir at
room temperature for until no starting material remained. The reaction was
washed with sat. NaHC03
and brine. The organic layer were dried over MgS04, filtered and concentrated
down. The residue
was purified by flash chromatography using hexanes-ethyl acetate system to
give N-(4-chlorophenyl)-
N-[(2S,4R)-1-(3,5-difluoro-4-hydroxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide
(152 mg, 78%).
[001056] N (4-chlorophenyl)-N [(2S,4R)-1-(3,5-difluoro-4-hydroxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (150 mg, 0.32 mmol) was dissolved in DMF at
room temperature
and CsZC03 (259 mg, 0.80 mmol) was added. Methyl 4-bromo-2,2-dimethylbutanoate
(157 mg, 0.48
mmol) was added and the reaction was stirred at room temperature overnight.
The reaction mixture
was concentrated under reduced pressure. The residue was partitioned between
ethyl acetate and
water, then extracted three times with ethyl acetate, dried over MgS04,
filtered and concentrated. The
251
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
crude residue was purified by silica gel chromatography (hexanes-ethyl acetate
system) to afford the
product (118 mg, 61%).
iH-NMR (CDC13, 300MHz) 8: 1.12-1.14 (d, 3H),1.21-1.24 (m, 6H),1.48-1.55 (m,
2H), 2.02 (s, 3H),
2.20-2.32 (m, 1H), 3.64 (s, 3H), 4.11-4.16 (m, 1H), 4.65-4.75 (m, 1H), 5.45-
5.55 (m, 1H), 6.51-6.54
(d, l H), 6.70-6.73 (m, 1H), 6.76-6.90 (m,1H), 7.18-7.39 (m, 7H).
MS m/z: 599 (M+1).
[001057] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-4-carboxylic acid amide (H-30)
[001058] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-piperidine-4-carboxylic acid amide was prepared from
(2S,4R)- 1-(4-{4-[acetyl-
(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenyl)-piperidine-4-
carboxylic acid. The acid (0.120 g, 0.22 mmol) was dissolved in DMF (2.5 mL)
at room temperature
and HOBt (0.044 g, 0.33 mmol), HATU (0.125 g, 0.33 mol), and
diisopropylethylamine (0.15 mL,
0.88 mmol) was added followed by ammonium chloride (0.024 g, 0.44 mmol) and
stirred at room
temperature for 16 h. The reaction was diluted with ethyl acetate, washed with
1N NaOH, 1N HCl
and brine. The organics were dried over MgS04, filtered and concentrated down.
The crude residue
was purified HPLC purification.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.23 (m, 1H), 1.79(m, 4H), 2.01 (m, 3H), 2.27
(m, 1H), 2.48 (m,
1H), 2.96 (m, 1H), 3.13 (m, 1H), 3.35 (m, 1H)~ 3.52 (m, 1H), 4.70 (m, 1H),
5.50 (6r, 2H), 6.24 (br,
1H), 6.56 (d,1H), 6.67 (d, 2H), 6.92 (t,1H), 7.08-7.28 (m, 6H), 7.37 (d, 2H).
MS m/z: 545.4 (M+1).
[001059] 2-{[3-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(21T)-yl]carbonyl}phenyl)propanoyl]amino}-2-methylpropanoic acid (H-32)
[001060] Methyl 3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2I~-yl]earbonyl}phenyl)propanoate (90 mg, 0.15 mmol, 1 equ.) was dissolved
in
methanol/tetrahydrofuran (2/1) (1 ml). A solution of sodium hydroxide (12 mg,
0.30 mmol, 2 eq.) in
water (0.5 ml) was added and reaction mixture was stirred at room temperature
fox 20 h. The mixture
was concentrated and the residue was acidified with a 1N HCl aqueous solution
and extracted with
ethyl acetate. The organic layer was washed with brine, dried over magnesium
sulfate, filtered and
concentrated to give 2-{ [3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(21~-yl]carbonyl}phenyl)propanoyl]amino}-2-methylpropanoic
acid (76 mg, 87
%).
'H-NMR (CDC13) 8: 1.15 (d, 3H), 1.45 (d, 6H), 2.05 (s, 3H), 2.30 (m, 1H), 2.40
(t, 2H), 2.85 (t, 2H),
4.75 (m, 1H), 5.60 (m, 1H), 6.30 (s, 1H), 6.55 (d, 1H), 6.90 (t, 1H), 6.90-
7.15 (m, 8H), 7.40 (d, 2H).
252
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
MS m/z: 576 (M+1).
[001061] Methyl 4-[5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-yl]carbonyl}-2-oxopyridin-1(2I~-yl]-2,2-
dimethylbutanoate (H-33) and
methyl 4-[(5-{[(2S,4R)-4-[acetyl(4-chloropheuyl)amino]-2-methyl-3,4-
dihydroqninolin-1 (2Fn-
yl]carbonyl}pyridin-2-yl)oxy]-2,2-dimethylbutanoate (H-143)
[001062] Methyl 4-[(5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2F1)-yl]carbonyl}pyridin-2-yl)oxy]-2,2-dimethylbutanoate was prepared from
(2S ,4R)- N-(4-
chloro-phenyl)-N-[1-(6-methoxy-pyridine-3-carbonyl)-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-yl]-
acetamide by deprotection of the methoxy and elaboration. (2S ,4R)- N-(4-
chloro-phenyl)-N-[1-(6-
methoxy-pyridine-3-carbonyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-
acetamide (0.062g, 0.13
mmol) was dissolved in methylene chloride and TMSI (0.020mL, 0.13 mmol) was
added and stirred
at room temperature for 14h. The reaction mixture was concentrated down and
methanol was added
and stirring was continued for 10h, concentrated down and used directly. N (4-
chlorophenyl)-N-
{ (2S,4R)-1-[(6-hydroxypyridin-3-yl)carbonyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl }acetamide
(0.060g, 0.13 mmol) was dissolved in 2 mL of dimethylformamide, followed by
0.0468 of methyl 4-
bromo-2,2-dimethylbutan0ate (0.20 mmol) and 0.0578 of silver carbonate (0.20
mmol). The flask
was covered with aluminum foil and the lights were turned off in the
enclosure. The reaction was
allowed to heat to 80°C for 24 h. Additional methyl 4-bromo-2,2-
dimethylbutanoate (0.0468, 0.20
mmol) was added and continued stirring at 80°C for 24h. The reaction
was concentrated down and
partitioned between ethyl acetate and water. The organics were collected
together and dried over
MgS04, filtered and concentrated. The residue was purified with 2%
methanol/98%methylene
chloride to 5% methanol/95% methylene chloride to 10% methanol/90%methylene
chloride. Two
products were obtained the O- and N- alkylated product, methyl 4-[(5-{
[(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (21~-yl]carbonyl } pyridin-
2-yl)oxy]-2,2-
dimethylbutanoate (0.0258, 32%) and methyl 4-[5-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-
methyl-3,4-dihydroquinolin-1 (21~-yl]carbonyl }-2-oxopyridin-1 (21~-yl]-2,2-
dimethylbutanoate
(0.0258, 32%).
O-alkylated'H-NMR (CDC13) 8: 1.13 (m, 1H), 1.14 (d, 3H), 1.22 (s, 6H), 1.99
(t, 2H), 2.01 (s, 3H),
2.29 (m, 1H), 3.62 (s, 3H), 4.27 (t, 2H), 4.74 (sextet, 1H), 5.58 (bs, 1H),
6.37 (d, 1H), 6.56 (d, 1H),
7.00 (t, 1H), 7.28-7.16 (m, 5H), 7.37 (d, 2H), 8.14 (bs, 1H).
MS mlz = 564 (M+1).
N-alkylated'H-NMR (CDCl3) 8: 1.11 (m, 1H), 1.12(d, 3H), 1.22 (s, 6H), 1.72-
1.90 (m, 2H), 2.01 (s,
3H), 2.25 (m, 1H), 3.67(s, 3H), 3.71-3.88 (m, 2H), 4.64 (, sextet, 1H), 5.51
(bs, 1H), 6.15 (d, 1H),
6.74 (d, 2H), 7.09 (t, 1H), 7.15 -7.33 (m, 4H), 7.37 (d, 2H), 7.57 (s,1H).
MS mlz : 564 (M+1).
253
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001063] N-{(2S,4R)-1-[4-(aminomethyl)benzoyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl}-
N-(4-chlorophenyl)acetamide (H-34)
[001064] N-{(2S,4R)-1-[4-(aminomethyl)benzoyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl}-N (4-
chlorophenyl)acetamide was made following general procedure H, substituting 4-
cyanobenzoyl
chloride for 6-trifluoromethyl nicotinyl chloride. The rest of the procedure
is followed as indicated in
general procedure H to yield the corresponding nitrite, N (4-chloro-phenyl)-N
[(2S,4R)-1-(4-cyano-
benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide. N-(4-Chloro-
phenyl)-N [(2S,4R)-1-
(4-cyano-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (225
mg, 0.51 mmol) was
dissolved in ethanol ( 7 xnL) and cobalt dichloride (0.079 g, 0.61 mmol) and
NaBH4 (0.059 g, 1.57
mmol) were added and the mixture was stirred at room temperature for 2h. The
slurry was filtered,
concentrated, and subjected to flash chromatography (1% NH40H, 15%MeOH, EtOAc)
to yield the
title compound (188 mg, 83%) as a white solid.'H-NMR (CDC13) 8: 1.00-1.20 (m,
1H), 1.14 (s, 3H),
1.48 (bs, 2H), 2.02 (s, 3H), 2.20-2.36 (m, 1H), 3.79 (s, 2H), 4.73-4.83 (m,
1H), 5.35-5.70 (m, 1H),
6.49 (d, 1H), 6.89 (t, 1H), 7.11-7.29 (m, 8H), 7.37 (d, 2H).
MS m/z: 448 (M +1)
[001065] (2S,4R)-N-[3-(4-{4-[Acetyl-(4-chloro-phenyl)=amino]-2-methyl-3,4-
dihydro-2H-
quunoline-1-carbonyl}-phenoxy)-propyl]-2,2-dimethyl-propionamide (H-35)
[001066] (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamide (60 mg, 0.122 mmol) was dissolved in
methylene chloride (2 mL)
and triethylamine (0.034 mL, 0.243 mmol) and cooled to -40 °C. Pivaloyl
chloride (10 drops via
'pipet) was added and the mixture was warmed to 0 °C for 30 minutes.
The mixture was partitioned
between methylene chloride and water; the methylene chloride layer was dried
over MgS04, filtered
and concentrated. The crude residue was purified by silica gel chromatography
(1/1 hexanes/ ethyl
acetate - ethyl acetate gradient) to afford the product.
'H-NMR (CDC13) b: 1.1 (s, 3H), 1.1 (s, 9H), 1.2 (m, 1H), 2.0 (m, 2H), 2.0 (s,
3H), 2.3 (m, 1H), 3.4
(q, 2H), 4.0 (t, 2H), 4.7 (m, 1H), 5.6 (bs, 1H), 6.0 (s, 1H), 6.5 (d, 1H), 6.6
(d, 2H), 6.9 (t, 1H), 7.2 (m,
6H), 7.4 (d, 2H).
MS m/z: 576 (M+1).
[001067] Methyl 4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoate (H-
36)
[001068] N (4-chlorophenyl)-N [(2S,4R)-1-(3-fluoro-4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure H,
substituting 3-fluoro-
4-methoxybenzoyt chloride for 6-trifluoromethyl nicotinyl chloride. The rest
of the procedures were
254
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
followed as indicated in general procedure H to afford N-(4-chlorophenyl)-N-
[(2S,4R)-1-(3-fluoro-4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide.
[001069] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3-fluoro-4-methoxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was dissolved in dichloromethane and a
solution of BBr3 (1.0 M in
dichloromethane, 10 mL) was added; the reaction was allowed to stir at room
temperature for until no
starting material remained. The reaction was washed with sat. NaHC03 and
brine. The organic layer
were dried over MgS04, filtered and concentrated down. The residue was
purified by flash
chromatography using hexanes-ethyl acetate system to give N (4-chlorophenyl)-N
[(2S,4R)-1-(3-
fluoro-4-hydroxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide in
decent yield.
[001070] N (4-chlorophenyl)-N-[(2S,4R)-1-(3-fluoro-4-hydroxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (317 mg, 0.70 mmol) was dissolved in DMF at
room temperature
and Cs2C03 (567 mg, 1.75 mmol) was added. Methyl 4-bromo-2,2-dimethylbutanoate
(465 mg, 1.05
mmol) was added and the reaction was stirred at room temperature overnight.
The reaction mixture
was concentrated under reduced pressure. The residue was partitioned between
ethyl acetate and
water, then extracted three times with ethyl acetate, dried over MgS04,
filtered and concentrated. The
crude residue was purified by silica gel chromatography (hexanes/ethyl acetate
system) to afford the
product (176 mg, 43%).
IH-NMR (CDCI3, 300MHz) 8: 1.11-1.13 (d, 3H), 1.22-1.29 (m, 6H), 1.51-1.64 (m,
2H), 2.07-2.09
(m, 5H), 2.25-2.29 (m, 2H), 3.65 (s, 3H), 3.95-3.99 (m, 2H), 4.70-4.75 (q,
1H), 5.27 (b, 1H), 6.51-
6.75 (m, 3H), 6.92-7.03 (m, 2H), 7.14-7.38 (m, 6 H).
MS m/z: 581 (M+1).
[001071] (2S,4R)-N-(4-chloro-phenyl)-N-{2-methyl-1-[4-(2-oxo-imidazolidin-1-
yl)-benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-37)
[001072] N-[1-(4-Amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-
(4-chloro-
phenyl)-acetamide (156 mg, 0.373 mmol) was dissolved in methylene chloride (3
mL) and was added
triethylamine (0.078 mL, 0.559 mmol) and 1-chloro-2-isocyanato-ethane 0.038
mL, 0.448 mmol).
The reaction was stirred at room temperature over night, and the reaction was
quenched with sat. aq.
NaHC03. The residue was partitioned between methylene chloride and water, then
extracted three
times with methylene chloride, dried over MgS04, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography (ethyl acetate) to afford the
product.
'H-NMR (CDCl3) 8: 1.2 (d, 3H), 1.2 (m, 1H), 2.0 (s, 3H), 2.3 (m, 1H), 4.0 (t,
2H), 4.4 (t, 2H), 4.8 (m,
1H), 5.6 (m, 1H), 6.5 (d, 1H), 6.9 (t, 1H), 7.2 (m, 9H), 7.4 (d, 2H).
MS m/z: 503 (M+1).
255
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001073] (2S,4R)- 4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-methyl-amino]-butyric acid (H-38)
[001074] (2S,4R)- 4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-methyl-amino]-butyric acid was prepared from (2S,4R)- 4-
[(4-{4-[acetyl-(4-
chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenyl)-
methyl-amino]-
butyric acid methyl ester was hydrolyzed to the acid by dissolving in
tetrahydrofuran and ethanol and
lithium hydroxide (1N) was added and heated 50°C for 2h. The mixture
was cooled to room
temperature, acidified to form a white precipitate. The solid was filtered to
give to afford the product
after HPLC purification.
'H-NMR (CDC13) 8: 1.10 (d, 3H), 1.18 (m, 1H), 1.81 (t, 2H), 2.02 (s, 3H), 2.28
(m, 3H), 2.86 (s, 3H),
3.28 (t, 2H), 4.70 (m, 1H), 5.58 (brs, 1H), 6.39 (d, 2H), 6.61 (d, 1H), 6.94
(t, 1H), 7.06-7.27 (m, 6H),
7.36 (d, 2H); 8.90 (br, 1H).
MS m/z: 534 (M+1).
[001075] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(4-hydroxy-4-methyl-pentyloxy)-
benzoyl]-2-
methyl-1,2;3,4-tetrahydro-quinolin-4-yl}-acetamide (H-39)
[001076] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.1g, 0.23 mmol) was dissolved in DMF (5 mL) at room
temperature.
KzC03 (0.317g, 2.3 mmol) was added. 5-Bromo-2-methyl-pentan-2-of (0.092g, 0.51
mmol) was
added and the reaction was allowed to heat to 80°C overnight. The
reaction mixture was concentrated
irz vacuo. The residue was parkitioned between ethyl acetate and water, then
extracted three times
with ethyl acetate, dried over MgSO~, filtered and concentrated down. The
crude residue was purified
by silica gel chromatography (70% EtOAc/ 30% Hexane) to afford the product
(0.097g, 79%).
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1:15 (t,1H), 1.26 (s, 6H), 1.52-1.90 (m, 5H),
2.04 (s, 3H), 2.27 (m,
1H), 3.91 (t, 2H), 4.72 (sextet, 1H), 5.48 (bs, 1H), 6.52 (d, 1H), 6.67 (d,
2H), 6.86 (t, 1H), 7.08-7.35
(m, 6H), 7.38 (d, 2H).
MS m/z: 535 (M+1)
[001077] (2S,4R)- 1-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-propyl]-1H-imidazole-2-carboxylic acid amide (H-
40)
(2S,4R)- 1-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1
carbonyl }-phenoxy)-propyl]-1H-imidazole-2-carboxylic acid amide was prepared
from (2S,4R)- 1-[3
(4-{ 4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-
carbonyl }-phenoxy)
propyl]-1H-imidazole-2-carboxylic acid ethyl ester. The ester (0.100g, 0.16
mmol) was hydrolyzed to
the acid by dissolving in tetrahydrofuran and ethanol and sodium hydroxide
(1N) was added. The
mixture was stirred at room temperature 4 hours. The mixture was cooled to rt,
acidified to form a
256
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
white precipitate. The. solid was filtered to give (2S,4R)- 1-[3-(4-{4-[Acetyl-
(4-chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-propyl]-1H-
imidazole-2-
carboxylic acid in 65% yield. The acid (0.066g, 0.09mmol) was converted to the
amide by dissolving
in DMF (2 mL) at room temperature. HATU (0.061 g, 0.16mmo1), HOBt (0.021g,
0.15 mmol), and
diisopropylethylamine (0.074 mL, 0.43 mmol) were added and stirred for 5 min.
followed by
ammonium chloride (O.Ollg, 0.20mmo1). °The reaction was stirred at room
temperature for 12h and
concentrated down the residue was diluted with ethyl acetate and washed with
1N HCl and dried over
MgS04, filtered and concentrated down. °The crude residue was purified
by silica gel chromatography
(5% MeOH/ 95% CHZC12/NH40H to 10% MeOH/ 90% CHZCIz/NH~OH to 15% MeOH/ 85%
CHZCIz/N~I40H ) to afford the product (0.050 mg, 81%).
'H-NMR (CDC13) S: 1.11 (d, 3H), .1.37 (m, 1H), 2.00 (s, 3H), 2.22 (m, 3H),
3.82 (t, 2H), 4:57 (t,
2H), 4.72 (sextet, 1H), 5.56 (bs, 1H), 6.49 (d, 1H), 6.60 (d, 2H), 6.90 (t,
1H), 6.89 (t, 1H), 6.93 (d,
2H), 7.11 (d, 2H), 7.15-7.27 (m, 5H), 7.34 (d, 1H).
MS m/z: 586 (M+1).
[001078] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(2-oxo-oxazolidin-5-
ylmethoxy)-
benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-41)
[001079] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.5 g, 1.14 mmol) was dissolved in DMF at room
temperature and KzC03
(1.26 g, 9.13 mmol) was added. 2-Chloromethyl-oxirane (0.42g, 4.57 mmol) was
added and the
reaction was allowed to heat to 80°C overnight. The reaction mixture
was concentrated irz vacuo. The
residue was partitioned between ethyl acetate and water, then extracted three
times with ethyl acetate,
dried over MgSO~, filtered and concentrated down. The crude residue was
purified by silica gel
chromatography (50% EtOAc/ 50% Hexane) to afford (2S,4R)-N-(4-chloro-phenyl)-N-
[2-methyl-1-
(4-oxiranylmethoxy-benzoyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide
(0.55g, 77%), which was
further elaborated to the product following ref. (Tetralzedrorz Lett 2002,
43(46), 8327).
'H-NMR (CDCI3) b: 1.13 (d, 3H), 1.15 (t, 1H), 2.03 (s, 3H), 2.27 (m, 1H), 3.57
(m, 1H), 3.68. (m,
1H), 4.05 (m, 2H), 4.73 (m, 1H), 4.86 (m, 1H), 5.58 (bs, 1H), 6.52 (d, 1H),
6.67 (d, 2H), 6.86 (t, 1H),
7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS m/z: 535 (M+1)
[001080] 5-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl]phenyl)-2,2-dimethylpentanoic acid (H-42)
[001081] 5-(4-{ (2S,4R)-4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-1
carbonyl }-phenyl)-2,2-dimethyl-pentanoic acid methyl ester (500 mg, 0.89
mmol, 1 eq.) was
dissolved in methanol/tetrahydrofuxan (2/1) (4 ml). A solution of sodium
hydroxide (71 mg, 1.8
257
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
mmol, 2 eq.) in water (1 ml) was added and reaction mixture heated to
60°C for 20 h. The mixture
was concentrated and the residue was acidified with a 1N HCl aqueous solution
and extracted with
ethyl acetate. The organic layer was washed with brine, dried over magnesium
sulfate, filtered and
concentrated to give the crude acid. Purification by silica gel chromatography
gave pure 5-(4-
{ (2S,4R)-4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl }-
phenyl)-2,2-dimethyl-pentanoic acid (292 mg, 60 %).
1H-NMR (MeOD) ~: 1.10 (d, 3H), 1.13 (s, 6H), 1.50 (m, 4H), 2.05 (s, 3H), 2.45
(m, 1H), 2.55 (t,
2H), 4.75 (m,1H), 5.55 (m, 1H), 6.55 (d, 1H), 6.95 (t, 1H), 7.05-7.20 (dd,
4H), 7.25 (t, 1H), 7.40-7.55
(m~ 5~.
MS m/z: 547 (M+1).
[001082] Methyl 4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-yl]carbonyl}phenyl)-2,2-dimethylbutanoate (H-43)
[001083] Methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenyl)-2,2-dimethylbutanoate was prepared according to
general procedure B,
substituting methyl 4-[4-(chlorocarbonyl)phenyl]-2,2-dimethylbutanoate for 6-
trifluoromethyl
nicotinyl chloride. (Methyl 4-[4-(chlorocarbonyl)phenyl]-2,2-dimethylbutanoate
was prepared in 5
steps from 4-(4-iodophenyl)butanoic acid. 4-(4-iodophenyl)butanoic acid was
converted to methyl 4-
(4-iodophenyl)butanoate by treatment with trimethylsilyl diazomethane (1.5
equivalents) in
benzene/methanol (4/1) at room temperature. Lithium enolate formation with
lithium diisopropyl °
amide (1.l equivalents) in THF at -78 °C followed by quenching with
methyl iodide (2.4 equivalents)
and aqueous work up then repeating the same protocol afforded, after standard
chromatography (10°l0
ethyl acetate/hexanes) methyl 4-(4-iodophenyl)-2,2-dimethylbutanoate.
Subsequent treatment of this
material with catalytic palladium(II) acetate (0.05 equivalents) and 1,3-
bis(diphenylphosphino)propane (0.05 equivalents) in the presence of water (5.0
equivalents) and
triethylamine (2.0 equivalents) in DMF at 80 °C under a carbon monoxide
atmosphere afforded, after
aqueous work up, 4-(4-methoxy-3,3-dimethyl-4-oxobutyl)benzoic acid. This
material was directly
converted to methyl 4-[4-(chlorocarbonyl)phenyl]-2,2-dimethylbutanoate by
treatment with excess
thionyl chloride in dichloromethane at room temperature for 2 hours then
removal of the volatiles in
vaccuo). The rest of the procedures were followed as indicated in general
procedure B to afford
methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-
yl]carbonyl }phenyl)-2,2-dimethylbutanoate.
'H-NMR (CDCl3) 8: 1.12-1.21 (m, 1H), 1.13 (d, 3H), 1.18 (s, 6H), 1.73 (ddd,
2H), 2.01 (s, 3H), 2.23-
2.30 (m, 1H), 2.43 (ddd, 2H), 3.64 (s, 3H), 4.71-4.79 (m, 1H), 5.60 (br s,
1H), 6.49 (d, 1H), 6.89 (t,
1H), 6.95 (d, 2H), 7.08-7.29 (m, 6H), 7.37 (d, 2H).
MS m/z: 547 (M+1).
258
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001084] 3-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-6-chloro-2-methyl-3,4-
dihydroquinolin-1(21i~-yl]carbonyl}phenoxy)-2,2-dimethylpropanoic acid (H-44)
[001085] 3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-6-chloro-2-methyl-3,4-
dihydroquinolin-
1(21~-yl]carbonyl}phenoxy)-2,2-dimethylpropanoic acid was synthesized from
(2S,4R)-N-3-(4-{4-
[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-
phenoxy)-2,2-
dimethyl-propionic acid (0.0308, 0.057 mmol) by addition of 1 mL of
dimethylformamide and TCCA
(0.00448, 0.018 mmol, 0.33 equ.) and stirred at room temperature for 40 min.
The reaction mixture
was diluted with water and acidified with 1N HCl to form a white precipitate.
The solid was dried on
the lyopholizer and purified with 5% methanol/dichloromethane to 10 %
methanol/90%
dichloromethane to yield 0.4008, 87% of 4-(4-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-6-chloro-2-
methyl-3,4-dihydroquinolin-1(2H)-y1]carbonyl}phenoxy)-2,2-dimethylbutanoic
acid.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.13 (m , 1H), 1.29 (s, 6H), 2.03 (s~ 3H),
2.27 (m, 1H), 3.92-3.85
(m, 2H), 4.72 (sextet, 1H), 5.51 (bs, 1H), 6.43 (d, 1H), 6.67 (d, 2H), 6.90
(d, 1H), 7.11 (s, 1H), 7.13
(d, 2H), 7.19 (d, 2H), 7.38 (d, 2H).
MS mlz : 569 (M+1).
[001086] N-(4-aminophenyl)-N-[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-4~
[001087] Benzyl (4-{acetyl[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]amino}phenyl)carbamate was dissolved in MeOH and was added a catalytic
amount of Palladium
on Carbon (10 %). The system was purged by Hydrogen gas, the subjected to 1
atm of Hz for 2h.
The reaction was quenched with air and filtered to give the title compound as
a white solid.
'H-NMR (CDCl3) ~: 1.10-1.60 (m, 1H), 1.10 (d, 3H), 2.01 (s, 3H), 2.22-2.38 (m,
1H), 3.70-3.95 (bs,
2H), 3.72 (s, 3H), 4.64-4.80 (m, 1H), 5.45-5.72 (m, 1H), 6.49 (d, 1H), 6.54-
6.70 (m, 2H), 6.64 (d,
2H), 6.89 (t,1H), 6.99 (d, 2H), 7.08-7.20 (m, 3H), 7.30 (d, 1H).
MS mlz: 430 (M +1)
[001088] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(4-methanesulfonylamino-3,3-
dimethyl-4-oxo-
butoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-46)
[001089] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1
carbonyl }-phenoxy)-2,2-dimethyl-butyric acid (200 mg, 0.37 mmol) was
dissolved in methylene
chloride (5 mL) and oxalyl chloride (2 mL). A single drop of DMF was added,
and the mixture was
stirred at room temperature until gas evolution ceased. All volatiles were
removed. The slurry was
dissolved in methylene chloride (10 mL) and triethylamine (2 mL).
Methanesulfonamide (200 mg,
2.10 mmol) was added and the reaction mixture was stirred for 3 hours. The
mixture was partitioned
259
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
between methylene chloride and water; the organic layer was dried over Na2S04,
filtered and
concentrated. The crude residue was purified by preparatory HPLC.
1H-NMR (CDCl3) S: 1.1 (d, 3H),1.2 (m,1H), 1.2 (s, 3H), 1.3 (s, 3H), 2.0 (s,
3H), 2.1 (t, lI~, 2.3 (m,
1H), 3.4 (m, 4H), 3.9 (m, 2H), 4.7 (m, 1H), 5.6 (bs, lI~, 5.9 (d, 1H), 6.5 (d,
1H), 6.4 (d, 2H), 6,9 (t,
1H), 7.2 (m, 6H), 7.4 (d, 2H).
MS m/z: 626 (M+1).
[001090] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-3-carboxylic acid (H-47)
[001091] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-piperidine-3-carboxylic acid was prepared from (2S,4R)- 1-
(4-{4-[acetyl-(4-
chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenyl)-
piperidine-3-
carboxylic acid ethyl ester (0.300g, 0.522 mol) was hydrolyzed to the acid by
dissolving in
tetrahydrofuran and ethanol and lithium hydroxide (1N) was added and heated
50°C for 2h. The
mixture was cooled to room temperature, acidified to form a white precipitate.
The solid was filtered
to give to afford the product after HPLC purification.
'H-NMR (CDCI3) ~: 1.11 (d, 3H), 1.23 (m, 1H), 1.77 (m, 2H), 1.94 (m, 2H), 2.02
(s, 3H), 2.27 (m,
1H), 2.43 (m, 1H), 2.78 (t, 2H), 3.60 (m, 2I~, 4.71 (m, 1H), 5.58 (brs, 1H),
6.57 (d, 1H), 6.63 (d,
2H), 6.93 (t, 1H), 7.07-7.28 (m, 6H), 7.37 (d, 2H), 9.30 (br, 1H).
MS m/z: 546.3 (M+1).
[001092] N-{(2S,4R)-6-Chloro-1-[(6-ethylpyridin-3-yl)carbonyl]-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}-N-(4-chlorophenyl)acetamide (H-48)
[001093] N-{(2S,4R)-6-Chloro-1-[(6-ethylpyridin-3-yl)carbonyl]-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}-N (4-chlorophenyl)acetamide was prepared following
the procedure
described for N {(2S,4R)-1-[3,5-bis(trifluoromethyl)benzoyl]-6-chloro-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}-N (4-chlorophenyl)acetamide substituting 6-ethyl
nicotinyl chloride for 3,5-
bistrifluoromethyl benzoyl chloride. (6-Ethyl nicotinyl chloride was prepared
in two steps from
methyl 6-chloronicotinate as described in the procedure for N-(4-chlorophenyl)-
N {(2S,4R)-1-[(6-
ethylpyridin-3-yl)carbonyl]-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl }
acetamide).
'H-NMR (CDCI3) 8: 1.26 (d, 3H); 1.28 (t, 3H), 1.28-1.31 (m, 1H), 2.02 (s, 3H),
2.22-2.36 (m, 1H),
2.81(q, 2H), 4.70-4.80 (m, 1H), 5.42-5.56 (br, 1H), 6.50 (d, 1H), 6.90-7.00
(m, 2H), 7.20-7.40 (m,
7H), 8.50 (br s, 1H).
MS m/z: 482 (M + 1).
260
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001094] 4-(4-{[(2S,4R)-4-[(4-chlorophenyl)(cyclopropylcarbonyl)amino]-2-
methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoic acid (H-49)
[001095] Methyl 4-(4-{ [(2S,4R)-4-[(4-chlorophenyl)(cyclopropylcarbonyl)amino]-
2-methyl-3,4-
dihydroquinolin-1(2I~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoate (100 mg, 0.17
mmol, 1 equ.)
was dissolved in methanol/tetrahydrofuran (2l1) (1 ml). A solution of sodium
hydroxide (21 mg, 0.51
mmol, 3 eq.) in water (0.5 ml) was added and reaction mixture heated to
40°C for 2 h. The mixture
was concentrated and the residue was acidified with a 1N HCl aqueous solution
and extracted with
ethyl acetate. The organic layer was washed with brine, dried over magnesium
sulfate, filtered and
concentrated to give the crude acid. Purification by silica gel chromatography
(methylene
chloride/methanol 98/2) gave pure 4-(4-{ [(2S,4R)-4-[(4-
chlorophenyl)(cyclopropylcarbonyl)amino]-
2-methyl-3,4-dihydroquinolin-1(2I~-yl]carbonyl}phenoxy)-2,2-dimethylbutanoic
acid (85 mg, 87 %).
'H-NMR (CDCl3) 8: 0.75 (m, 2H), 1.10 (m, 2H), 1.20 (d, 3H), 1.25 (s, 6H), 1.45
(m, 1H), 2.05 (t,
2H), 2.30 (m, 1H), 3.95 (t, 2H), 4.75 (m, 1H), 5.60 (m, 1H), 6.50 (d, 1H),
6.60 (d, 2H), 6.90 (t, 1H),
7:10-7.45 (m, 8H).
MS m/z: 575 (M+1).
[001096] N-(4-Chlorophenyl)-N-[(2S,4R)-1-(3-ethyl-4-fluorobenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-50)
[001097] N (4-Chlorophenyl)-N [(2S,4R)-1-(3-ethyl-4-fluorobenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure B,
substituting 4-fluoro-
3-ethylbenzoyl chloride for 6-trifluoromethyl nicotinyl chloride.
[001098] (4-fluoro-3-ethylbenzoyl chloride was prepared in 5 steps from 3-
bromo-4-fiuorobenzoic
acid. 3-bromo-4-fluorobenzoic acid was converted to methyl 3-bromo-4-
fluorobenzoate by treatment
with trimethylsilyl diazomethane (1.5 equivalents) in benzene/methanol (4/1)
at room temperature.
Subsequent reaction with tributyl(vinyl) tin (1.2 equivalents) in DMF in the
presence of catalytic
dichlororbis(triphenylphosphine) palladium(In (0.1 equivalents) at 80
°C under an argon atmosphere,
followed by aqueous work up and standard chromatography (10% ethyl
acetate/hexanes), yielded
methyl 4-fluoro-3-vinylbenzoate. Hydrogenation of the vinyl group by treatment
of this material with
palladium on carbon (10% palladium on carbon, 10 % by mass) in methanol under
an hydrogen
atmosphere then afforded methyl 3-ethyl-4-fluorobenzoate. This material was
dissolved in
methanol/tetrahydrofuran/water (2/1/1) then lithium hydroxide (5.0
.equivalents) was added and
reaction mixture stirred at room temperature for 2 hours. The mixture was
concentrated, the residue
acidified with a 1N HCl aqueous solution and extracted with ethyl acetate. The
organic layer was
washed with brine, dried over magnesium sulfate, filtered and concentrated to
give 4-fluoro-3
ethylbenzoic acid. This material was directly converted to 4-fluoro-3-
ethylbenzoyl chloride by
treatment with thionyl chloride (2.2 equivalents) in dichloromethane at room
temperature for 2 hours
261
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
followed by removal of the volatiles in vaccuo). The rest of the procedures
were followed as indicated
in general procedure B to afford N-(4-chlorophenyl)-N-[(2S,4R)-1-(3-ethyl-4-
fluorobenzoyl)-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide.
'H-NMR (CDC13) 8: 1.03 (t, 3H), 1.15 (d, 3H), 1.22-1.26 (m, 1H), 2.03 (s, 3H),
2.24-2.32 (m, 1H),
2.42-2.58 (m, 2H), 4.70-4.82 (m, 1H), 5.61 (br s, 1H), 6.48 (d, 1H), 6.78 (t,
1H), 6.92 (t, lI-i), 6.97-
7.02 (m, 1H), 7.07 (d,1H), 7.13-7.30 (m, 4H), 7.38 (d, 2H).
MS m/z: 465 (M+1).
[001099] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(3-imidazol-1-yl-3-methyl-butoxy)-
benzoyl]-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-51)
[001100] 3-Imidazol-1-yl-3-methyl-butan-1-of was dissolved in benzene at room
temperature with
PPh3 (0.088g, 0.33 mmol) added (2S,4R)-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-
benzoyl)-2-methyl-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (0.133g, 0.30 mmol) and stirred
for 5 min. DEAD
(0.058g, 0.33 mmol) was added and the reaction was stirred for 18 h at room
temperature. The
reaction was concentrated and purified by silica gel chromatography (4 %
MeOH/96 % CHZClz to 5 %
MeOH/95 % CHZC12 ~° 6 % MeOH/94 % CHZCl2) to afford the product in
46% yield
[001101] Preparation of 3-Imidazol-1-yl-3-methyl-butan-1-of '
[001102] 3-Imidazol-1-yl-3-methyl-butan-1-of was prepared from ethyl-2,2-
dimethylacrylate.
Ethyl-2,2-dimethylacrylate (2.04 mL, 14.7mmo1) and imidazole (0.500g, 7.34
,mmol) were mixed and
heated to 90°C for 48 h and cooled to room temperature. The reaction
mixture was diluted with
CHzCl2 and water. The aqueous layer was extracted 3x CHzClz and dried over
MgSO4, filtered and
concentrated down. The crude residue was purified by silica gel chromatography
(10% MeOH! 90%
CHaCl2) to give 35% yield of 3-imidazol-1-yl-3-methyl-butyric acid ethyl
ester. The ester was
reduced to the alcohol (US Patent application W003047586). 3-imidazol-1-yl-3-
methyl-butyric acid
ethyl ester (O.SOOg, 2.5 mmol) was dissolved in THF (40 mL) and cooled to
0°C and 2.55 mL of
LiAlH4 (1.0 M in ether) was added dropwise over 15 min. The reaction was
allowed to warm up to
room temperature over 1 h and quenched with dropwise addition of a sat'd
Na2S04 solution. The
resulting slurry was dried over solid NaZS04 and diluted with ethyl acetate,
filtered through a plug of
Celite and concentrated down.
1H-NMR (CDCl3) 8: 1.09 (d, 3H), 1.11 (m, 1H), 1.56 (s, 6H), 1.97 (s, 3H), 2.08
(m, 1H), 2.16 (t,
2H), 3.67 (m, 2H), 4.67 (sextet, 1H), 5.51 (bs, 1H), 6.49 (m, 3H), 6.87 (t,
1H), 6.96-7.61 (m, 10 H),
7.64 (m, 1H).
MS m/z: 568 (M+1).
[001103] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(1-ethyl-piperidin-4-ylmethoxy)-
benzoyl]-2-
methyl-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-52)
262
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001104] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxymethyl)-piperidine-1-carboxylic acid benzyl ester
(0.25 g) was
converted to the piperdine by hydrogenation with Pd/C (0.075 g) under hydrogen
in ethanol (17 ml).
The reaction mixture was filtered through ceilite and concentrated down to
give (2S,4R)-N-(4-Chloro-
phenyl)-N-{2-methyl-1-[4-(piperidin-4-ylmethoxy)-benzoyl]-1,2,3,4-tetrahydro-
quinolin-4-yl }-
acetamide (0.17 g, 85%).
[001105] The piperdine was reacted with acetaldehyde, Na(OAc)3BH in
dichloromethane at room
temperature overnight. Then washed with 1N NaOH, dried over MgS04, filtered
and concentrated
down. The crude residue was purified by silica gel chromatography (10%
methanol / 90 %
dichloromethane) to afford the product (55%).
1H-NMR (CDCl3) 8: 0.98-1.18 (m, 7H), 1.37 (m, 2H), 1.72-1.93 (m, 5H), 2.04 (s,
3H), 2.27 (m, 1H),
2.37 (q, 2H), 2.95 (m, 2H), 3.73 (d, 2H), 4.72 (sextet, 1H), 5.58 (bs, 1H),
6.52 (d, 1H), 6.67 (d, 2H),
6.86 (t, 1H), 7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS m/z: 560. (M+1 )
[001106] (2S,4R)- 4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-methyl-amino]-butyramide (H-53)
[001107] (2S,4R)- 4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-methyl-amino]-butyramide was made from (2S,4R)- 4-(4-{4-
[Acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenyl)-
piperazine-1-carboxylic
acid: The acid (0.050g~ 0.096mmo1) was dissolved in DMF (1 mL) at room
temperature and HOBt
(0.020 g, 0.144 mmol), HATU (0.055 g, 0.144 mol), and diisopropylethylamine
(0.067 mL, 0.384
mmol) was added followed by ammonium chloride (0.011g, 0.192 mmol) and stirred
at room
temperature for 16 h. The xeaction was diluted with ethyl acetate, washed with
1N NaOH, 1N HCI
and brine. The organics were dried over MgS04, filtered and concentrated down.
The crude residue
was purified HPLC purification.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.18 (m, 1H), 1.80 (t, 2H), 2.00 (s, 3H), 2.13
(t, 2H), 2.27 (m, 1H),
2.85 (s, 3H), 3.27 (t, 2H), 4.70 (m, 1H), 5.65 (br, 2H), 5.85 (brs, 1H), 6.39
(d, 2H), 6.59 (d, 1H), 6.93
(t, 1H), 7.03-7.34 (m, 6H), 7.36 (d, 2H).
MS m/z: 533 (M+1).
[001108] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxy)-3-(S)-hydroxy-butyric acid (H-54)
[001109] (2S,4R)-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (200 mg, 0.46 mmol) was dissolved in DMF (5 mL) at
room temperature.
CszC03 (374 mg, 1.15 mmol) was added followed by 4-bromo-3-(S)-(tart-butyl-
dimethyl-silanyloxy)-
263
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
butyric acid methyl ester (214 mg, 0.69 mmol) and the reaction was stirred at
room temperature for 18
hours. The mixture was concentrated under reduced pressure and dissolved in
ethyl acetate (15 mL).
The reaction mixture was washed with sat. aq. NaHC03 (15 mL), water (15 mL)
and brine (15 mL).
The organic phase was dried over MgSOd, filtered, and concentrated under
reduced pressure. The
crude residue was purified by silica gel chromatography (5195 ethyl
acetate/hexane - 50/50 ethyl
acetatelkiexane gradient) to afford slightly yellow solid product (74 mg,
37%).
[001110] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-3-(S)-(tert-butyl-dimethyl-silanyloxy)-butyric acid methyl
ester (99 mg, 0.148
mmol) was dissolved in THF (4 mL). To this solution was added tetrabutyl
ammonium fluoride (1.0
M in THF, 1 mL). The reaction mixture was stirred at room temperature for 3
hours. The mixture was
concentrated under reduced pressure and dissolved in DCM (15 mL). The reaction
mixture was
washed with sat. aq. NaHC03 (15 mL), water (15 mL) and brine (15 mL). The
organic phase was
dried over MgS04, filtered, and concentrated under reduced pressure. The
residue was purified by
silica gel chromatography (2/98 methanol / dichloromethane - 10/90
methanol/dichloromethane
gradient) to afford white solid product (41 mg, 51%).
[001111] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-3-(S)-hydroxy-butyric acid methyl ester (41.2 mg, 0.075
mmol) was dissolved in
MeOH/THF, (2:1, 3 mL). To this solution was added 2N LiOH (2 mL). The reaction
was stirred at
room temperature for 18 hours. The reaction mixture was concentrated in vacuo
to remove MeOH and
THF. Then 6N HCl aqueous solution was added to acidify the reaction mixture to
pH 2-3. The
reaction mixture was extracted with DCM (5 mL x 3). The extract was washed
with brine (15 mL)
and dried over MgS04, filtered and concentrated down. The crude residue was
purified by
preparatory HPLC to afford the product (35 mg, 87%).
'H-NMR (CDCl3, 300MHz) 8: 1.05-1.14 (xn, 4H), 2.0 (s, 3H), 2.25-2.27 (m, 1H),
2.60-2.62 (m, 2H),
3.87-3.88 (m, 2H), 4.28-4.34 (m, 1H), 4.70-4.77 (m, 1H), 5.58-5.62 (broad,
1H), 6.49-6.93 (m, 4H),
7.12-7.39 (m, 8H).
MS m/z: 538 (M+1).
[001112] (2S,4R)-N-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-propionamide (H-55)
[001113] (2S,4R)-N-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-propionamide was prepared from
(2S,4R)-N-3-(4-{4-
[Acetyl-(4-chloro-phenyl)-amino)-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl
}-phenoxy)-2,2-
dimethyl-propionic acid by coupling NH4C1, HATU, DIEA, HOBt in DMF at room
temperature to
yield (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl }-phenoxy)-butyramide. The reaction mixture was concentrated down and
partitioned
264
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
between ethyl acetate and water, then extracted three times with ethyl
acetate, dried over magnesium
sulfate, filtered and concentrated down. The residue was purified by silica
gel chromatography (10%
methanol / 90 % dichloromethane) to afford the product in a 88% yield.
'H-NMR (CDC13) 8: 1.13 (d, 3H),1.15 (t,1H), 1.31 (s, 6H), 1.71 (bs,1H), 2.03
(s, 3H), 2,27 (m, 1H),
3.83 (q, 2H), 4.72 (sextet, 1H), 5.58 (bs, 1H), 6.17(br, 1H), 6.52 (d, 1H),
6.69 (d, 2H), 6.83 (t, 2H),
7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS m/z: 534 (M+1)
[001114] (2S,4R)-N-(1-{4-[2-(1-Acetyl-piperidin-4-yl)-ethoxy]-benzoyl}-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl)-N-(4-chloro-phenyl)-acetamide (H-56)
[001115] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-(4-(2-piperidin-4-yl-
ethoxy)-benzoyl]=
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (0.085g, 0.15 mmol) was dissolved
in dichloromethane at
room temperature and DIEA (0.1 g; 0.56 mmol) was added. Acetyl chloride (0.2
mL, 2.8 mmol) was
added and the reaction was stirred at room temperature for 4 hours. The
reaction mixture was
concentrated ifa vacuo. The crude residue was purified by silica gel
chromatography (10% methanol /
90 % dichloromethane) to afford the product (0.068 g, 75%).
'H-NMR (CDCl3) 8: 1.15 (d, 3H), 1.15 (t, 1H), 1.21 (m, 2H), 1.58-1.77 (m, 5H),
1.98 (s, 3H), 2.04 (s,
3H), 2.27 (m, 1H), 2.51 (t, 1H), 2.98 (t, 1H), 3.72 (m, 1H), 3.93 (t, 2H),
4.51 (m, 1H), 4.72 (sextet,
1H), 5.58 (bs, 1H), 6.52 (d, 1H), 6.69 (d, 2H), 6.83 (t, 2H), 7.08-7.35 (m,
6H), 7.38 (d, 2H).
MS m/z: 588 (M+1)
[001116] 4-(5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoic acid ()Ei-57)
[001117] To the solution of Methyl 4-(5-{ [(2S,4R)-4-[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(217)-yl]carbonyl}-2-fluorophenoxy)-2,2-dimethylbutanoate in
MeOH/THF (1
mLllmL) was added excessive LiOH (1N aqueous solution). The reaction mixture
was stirred at r.t.
for overnight. The reaction was quenched by adding 6N HCl to PH 2. The mixture
was concentrated
under reduced pressure to remove MeOH and THF. DCM was added. The reaction
mixture was
washed with brine. The organic phase was dried over magnesium sulfate,
filtered, and concentrated
under reduced pressure. The crude residue was purified by preparative HPLC to
afford pure product.
'H-NMR (CDC13, 300MHz) 8: 0.90-0.94 (m, 2H), 1.12-1.14 (d, 3H), 1.21-1.24 (m,
3H), 1.60-1.72
(m, 2H), 1.95-2.03 (m, 1H), 2.09 (s, 3H), 2.20-2.27 (m, 1H), 3.45-3.59 (m,
1H), 3.60-3.75 (m, 1H),
4.65-4.75 (m, 1 H), 5.72-5.80 (m, 1H), 6.31-6.34 (m, 1H), 6.45-6.47 (m, 1H),
6.90-6.96 (m, 1H),
7.04-7.08 (m, 1H), 7.15-7.20 (m, 3H), 7.24-7.36 (m, 6H).
MS m/z: 567 (M+1).
265
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001118] N-(4-chlorophenyl)-N-((2S,4R)-2-methyl-1-{4-
[(methylsulfonyl)amino]benzoyl}-
1,2,3,4-tetrahydroquinolin-4-yl)acetamide (H-58)
[001119] To a solution of N-[(2S,4R)-1-(4-aminobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]-N-(4-chlorophenyl)acetamide (208 mg, 0.48 mmol) in methylene chloride (4.0
mL) at 0 °C was
added methanesulphonic anhydride (175 mg, 1.01 mmol) and N,N
diisopropylethylamine (0.460 mL,
2.63 mmol). The reaction was warmed to room temperature and stirred for 4
days. (Analysis by
LCMS indicated a mixture of starting aniline, mono-sulfonamide, and bis-
sulfonamide). Additional
methanesulphonic anhydride (170 mg, 0.98 mmol) was added and the reaction was
stirred an
additional 2 days at room temperature. The resulting reaction was diluted with
dichloromethane (25
mL) and poured into a 1:1 mixture of water and brine (25 mL). The organic
layer was separated,
dried over anhydrous magnesium sulfate, filtered, and concentrated under
reduced pressure to afford
crude N ((2S,4R)-1-{4-[bis(methylsulfonyl)amino]benzoyl}-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
.y1)-N-(4-chlorophenyl)acetamide (273 mg) as a yellow foam. The crude material
was used directly in
subsequent reactions.
[001120] To a solution of N-((2S,4R)-1-{4-[bis(methylsulfonyl)amino]benzoyl}-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl)-N-(4-chlorophenyl)acetamide (259 mg, 0.44 mnnol) in
tetrahydrofuran (5.0
mL) at room temperature was added 1.0 M sodium hydroxide (0.880 mL, 0.88
mmol). The reaction
was stirred at room temperature for 2 days. The resulting reaction mixture was
poured into a 1:1
mixture of water and saturated sodium bicarbonate (25 mL) and extracted with
ethyl acetate (1 x 25
mL). The aqueous layer was neutralized via addition of 1N HCl to pH ~8 and
extracted with ethyl
acetate (2 x 25 mL). The combined organic layers were washed once with brine,
dried over
anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure
to afford N-(4-
chlorophenyl)-N ((2S,4R)-2-methyl-1-{4-[(methylsulfonyl)amino]benzoyl}-1,2,3,4-
tetrahydroquinolin-4-yl)acetamide (190 mg, 76%) as a light-yellow, foamy
solid.
'H-NMR (CDC13) 8: 1.14 (d, 3H), 2.04 (s, 3H), 2.05-2.12 (m,.lH), 2.20-2.35 (m,
1H), 2.95 (s, 3H),
4.65-4.80 (m, 1H), 5.44-5.66 (m, 1H), 6.50 (d, 1H), 6.88-7.01 (m, 3H), 7.07-
7.32 (m, 6H), 7.35-7.46
(m, 3H).
MS m/z: 512 (M+1).
[001121] (2S,4R)-1-Methyl-pyrrolidine-2-carboxylic acid [3-(4-{4-[acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-propyl]-amide (H-
59)
[001122] (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamide (100 mg, 0.204 mmol) was dissolved in
methylene chloride (2
mL) and pyridine (2 mL). N-methyl proline (33 mg, 0.255 mmol) and EDC (63 mg,
0.255 mmol)
were added and the reaction was stirred for 1 hour. The mixture was
partitioned between methylene
266
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
chloride and water; the methylene chloride layer was dried over MgS04,
filtered and concentrated.
The crude residue was purified by silica gel chromatography (ethyl acetate) to
afford the product.
IH-NMR (CDCl3) 8: 1.1 (s, 3H), 1.2 (m, 1H), 1.8 (m, 2H), 2.0 (m, 2H), 2.0 (s,
3H), 2.2 (m, 2H), 2.3
(m, 1H), 2.4 (s, 3H), 2.6 (m, 1H), 3.2 (m, 2H), 3.4 (q, 2H), 4.0 (t, 2H), 4.1
(m, 1H), 4.8 (m 1H), 5.6
(bs,1H), 6.5 (d,1H), 6.6 (d, 2H), 6.9 (t,1H), 7.2 (m, 6H), 7.4 (d, 2H).
MS m/z: 603 (M+1).
[001123] 4-(4-{[(2R,4S)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}phenoxy)-2,2-dimethylbutanoic acid (H-60)
[001124] 4-(4-{ [(2R,4S)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}phenoxy)-2,2-dimethylbutanoic acid was prepared following the
procedure to prepare 4-
(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1
(21~-
yl]carbonyl}phenoxy)-2,2-dimethylbutanoic acid, substituting N (4-
chlorophenyl)-N [(2R,4S)-1-(4-
methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide for N (4-
chlorophenyl)-N-
[(2S,4R)-1-(4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]acetamide.
'H-NMR (CDCI3) 8: 1.14 (d, 3H), 1.15 (t, 1H), 1.25 (s, 6H), 2.02 (t, 2H), 2.05
(m, 2H), 2.27 (m, 1H),
:3.96 (t, 2H), 4.72 (sextet, 1H), 5.52 (br, 2H), 5.58 (bs, 1H), 6.52 (d, 1H),
6.67 (d, 2H), 6.86 (t, 1H),
7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS mlz: 549 (M+1).
[001125] (2S,4R)-N-(1-{4-[3-(Acetyl-ethyl-amino)-propoxy]-benzoyl}-2-methyl-
1,2,3,4- "
tetrahydro-quinolin-4-yl)-N-(4-chloro-phenyl)-acetamide (H-61)
[001126] (2S,4R)-N-{ 1-[4-(3-Acetylamino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (30 mg, 0.056 mmol) was dissolved
in DMF (1 mL).
Sodium hydride (20 mg, 0.833 mmol) and iodoethane (10 drops via pipet) were
added and the
reaction was stirred at room temperature over night. The mixture was
partitioned between methylene
chloride and water; the methylene chloride layer was dried over MgS04,
filtered and concentrated.
The crude residue was purified by preparatory HPLC.
1H-NMR (CDCl3) 8: 1.1 (m, 7H), 2.0 (m, 8H), 2.3 (m, 1H), 3.3 (q, 1H), 3.4 (q,
1H), 3.4 (t, 2H), 3.9
(t, 2H), 4.7 (m, 1H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.7 (d, 2H), 6.9 (t, 1H), 7.2
(m, 6H), 7.4 (d, 2H).
MS m/z: 562 (M+1).
[001127] N-(4-chlorophenyl)-N-{(2S,4R)-1-[4-(4-hydroxy-4-methylpentyl)benzoyl]-
2-methyl-
1,2,3,4-tetrahydroquinolin-4-yl}acetamide (H-62)
[001128] N-(4-chlorophenyl)-N { (2S,4R)-2-methyl-1-[4-(4-oxopentyl)benzoyl]-
1,2,3,4-
tetrahydroquinolin-4-yl}acetamide was prepared following general procedure H,
substituting 4-(4
267
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
oxopentyl)benzoyl chloride for 6-trifluoromethyl nicotinyl chloride to afford
N-(4-chlorophenyl)-N-
{(2S,4R)-2-methyl-1-[4-(4-oxopentyl)benzoyl]-1,2,3,4-tetrahydroquinolin-4-
yl}acetamide 1H-NMR
(CDC13): 1.14 (d, 4H), 1.87 (q, 2H), 2.01 (s, 3H), 2.04 (m, 1H), 2.06 (s, 3H),
2.32 (t, 2H),2.52 (t, 2H),
4.74 (q, 1H), 5.60 (br, 1H), 6.47 (d, 1H), 6.96 (m, 3H), 7.20 (m, 8H). MS m/z:
503 (M+1). (4-(4-
oxopentyl)benzoyl chloride was prepared in six steps from pent-4-en-2-ol. (as
follows: Pent-4-en-2-of
(4.0g, 46.44 mmol) was dissolved in CH2CI2 (30 mL). TBDMSCI (13.93 g, 92.88
mmol) and
imidazole (6.31g, 92.88 mmol) were added at 0°C. The reaction mixture
was stirred at RT for
overnight. The -organic phase was washed with sat. NaHC03 and brine, dried
over MgS04 and
concentrated. The residue was purified by column chromatography (hexane) to
give 5.14g of desired
product as a colorless oil (55%yield). 1H-NMR (CDC13) S: 0.02 (s, 6H), 0.89
(s,9H), 1.11 (d,3H),
2.14(q,2H), 3.80 (q, 1H), 5.03 (m, 2H), 5.80 (m,lH). MS m/z: 200 (M+1)
[001129] To a solution of ethyl 4-iodobenzoate (4.15g, 15.03 mmol) in DMF (20
xnL), was added
tert-butyl(dimethyl)[(1-methylbut-3-en-1-yl)oxy]silane (3.0g, 15.03 mmol) and
Pd(OAc)2 (342 mg,
1.50mmol),, triethyl amine (2 mL, 30.06 mmol) and DPPP (1.058, 2.55 mmol)
under Argon. The
mixture was stirred at 80°C for overnight. After concentrating, the
xesidue was dissolved in EtOAc
(20 mL). The organic phase was washed with Sat. NaHC03 and brine, dried over
MgS04 and
concentrated. The residue was purified by column chromatography (Hexane:
EtOAc, 20:1) to give
3.15g of desired product as a yellow oil (60% yield).1H-NMR (CDCl3) 8: 0.02
(s, 6H), 0.86 (s,9H),
1.16 (d,3H), 1.36 (t, 3H), 2.34(q, 2H), 3.89 (q, 1H), 4.34 (q, 2H), 6.38 (m,
2H), 7.36 (d, 2H), 7.95 (d,
2H). MS mlz: 348 (M+1).
[001130] To a solution of ethyl ethyl 4-(E-4-{[tert-
butyl(dimethyl)silyl]oxy}pent-1-en-1-
yl)benzoate (3.15 g, 9.07 mmol) in THF (10 mL), was added TBAF ( 18.15 mL,
18.15 mmol, 1M
solution in THF) at RT. The reaction mixture was stirred at RT for 2h. The
organic phase was washed
with Sat. NaHC03 and brine, dried over MgSO~ and concentrated. The residue was
purified by
column chromatography (hexane: EtOAc, 4:1) to give 1.258 of desired product as
a yellow oil (58%
yield). 'H-NMR (CDC13) 8: 1.19 (d,3H), 1.25 (t, 3H), 2.34(m, 2H), 3.91 (m,
1H), 4.34 (q, 2H), 6.38
(m, 2H), 7.36 (d, 2H), 7.93 (d, 2H). MS m/z: 237 (M+1).
[001131] A solution of ethyl 4-[(lE)-4-hydroxypent-1-en-1-yl]benzoate (1.25 g,
5.29 mmol) and
Pd/C (10% weight, 0.125g) in EtOH (20 mL) was degassed and bubbled through Hz.
The mixture was
stirred at RT for overnight. After filtering through celite, the organic
solution was concentrated to give
a colorless oil which was used in the next step without purification (1.1g,
88% yield). 'H-NMR
(CDCI3) s: 1.15 (d,3H), 1.35 (t, 3H), 2.70(m, 2H), 2.66 (t, 2H), 3.80
(quan.,1H), 4.33 (q, 2H), 7.23 (d,
2H), 7.94 (d, 2H). MS m/z: 239 (M+1).
[001132] To a solution of ethyl 4-(4-hydroxypentyl)benzoate (1.1g, 4.62 mmol)
and triethyl amine
(1.9 mL, 13.86mmol) in CHZCIz (20 mL), was added sulfur trioxide pyridine
complex (2.2g, 13.86
268
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
mmol) in DMSO (2 mL) at 0°C. The mixture was stirred at 0°C for
2h. The reaction mixture was
washed with 1N HCI solution, dried over MgS04 and concentrated. The residue
was purified by
column chromatography (Hexane: EtOAc, 3:1) to give 0.5 g of desired product as
a colorless oil (50%
yield).'H-NMR (CDC13) 5:1.36 (t, 3H), 1.86 (q, 2H), 2.09 (s, 3H), 2.40 (t,
2H), 2.67 (t, 2H), 4.32 (q,
2H), 7.23 (d, 2H), 7.95 (d, 2H). MS xnlz: 237 (M+1).
[001133] To a solution of ethyl 4-(4-oxopentyl)benzoate (500mg, 2.llmmol) in
THF/BtOHlH20 (5
mL, 10:1:1), was added NaOH (254 mg, 6.33mmo1). The mixture was reflux for
overnight. After
acidification, the mixture was extracted with EtOAc. The combined organic
phases were dried over
MgS04 and concentrated. The residue was purified by column chromatography
(EtOAc) to give 0.31
g of desired product as a white solid (70% yield).'H-NMR (CDCI3) 1.87 (q, 2H),
2.10 (s, 3H), 2.50 (t~
2H), 2.70 (t, 2H), 7.24 (d, 2H), 8.01 (d, 2H). MS m/z: 207 (M-1, ES-).
[001134] To a solution of N-(4-chloxophenyl)-N-((2S,4R)-2-methyl-1-(4-(4-
oxopentyl)benzoyl)-
1,2,3,4-tetrahydroquinolin-4-yl)acetamide (40 mg, 0.079mmo1) in THF (2mL), was
added MeMgBr
(68 uL, 0.095 mmol, 1.4 M solution in THF) at 0°C. The mixture was
stirred at 0°C for 2h. After
quenching with sat. NH4Cl solution, the mixture was extracted with EtOAc. The
combined organic
phases were dried over MgS04 and concentrated. The residue was purified by
column
chromatography (EtOAc) to give 16 mg of desired product as a white solid (40%
yield).
'H-NMR (CDC13): 1.14 (m, 10H), 1.37 (m, 2H), 1.61 (m, 2H), 2.01 (s, 3H), 2.22
(m, 1H), 2.49 (t,
2H), 4.74 (q, 1H), 5.60 (br, 1H), 6.49 (d, 1H), 6.97 (m, 3H), 7.20 (m, SH).
MS mlz: 519 (M+1).
[001135] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3,5-difluoro-4-hydroxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-63)
[001136] N-(4-chlorophenyl)-N [(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (200 mg, 0.41 mmol) was dissolved in
dichloromethane and a
solution of BBr3 (1.0 M in dichloromethane, 3.O~mL) was added; the reaction
mixture was stirred at
room temperature for until no starting material remained. The reaction mixture
was washed with sat.
NaHC03 and brine carefully. The organic layer was dried over MgS04, filtered
and concentrated.
The residue was purified by flash chromatography using Hexanes-EtOAc system to
give N-(4-
chlorophenyl)-N [(2S,4R)-1-(3,5-difluoro-4-hydroxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4--
yl]acetamide (152 mg, 78%).
'H-NMR (CDCI 3) 8: 1.12 (d, 3H), 1.24 (m, 2H), 2.07 (s, 3H), 2.25 (b, 1H),
4.70 (m, 1H), 6.52 (d,
1H), 6.78 (d, 2H), 7.18 (t, 1H), 7.20-7.40 (m, 6H).
MS m/z: 471 (M+1)
269
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001137] (2S,4R)-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-propyl]-carbamic acid methyl ester (H-64)
[001138] (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamide (150 mg, 0.30 mmol) was dissolved in DCM (1
mL). To this
solution was added TEA (36.4 mg, 0.36 mmol) followed by methyl chloroformate
(34 mg, 0.36
mmol). The reaction mixture was stirred at room temerature for 18 hours. The
mixture was
concentrated under reduced pressure and dissolved in DCM (15 mL). The reaction
mixture was
washed with sat. aq. NaHC03 (15 mL), water (15 mL) and brine (15 mL). The
organic phase was
,dried over MgSO~, filtered, and concentrated under reduced pressure. The
residue was purified by
silica gel chromatography (5/95 ethyl acetate/hexane - 50/50 ethyl
acetatelhexane gradient) to afford
white solid product (71 mg, 52%).
.'H-NMR (CDC13, 300MHz) 8: 0.68-0.88 (m, 2I~, 1.00-1.05 (m, 4H), 1.23 (s, 3H),
1.61-1.64 (broad,
1H) 2.02 (s, 3H), 2.22-2.33 (m, 1H), 3.31-3.37 (m, 1H), 3.62 (s, 3H), 3.93-
3.97 (m, 1H), 4.73-4.75
(m, 1H) 5.28-5.31 (broad, 1H), 6.50-6.93 (m, 4H), 7.12-7.39 (m, 8H).
MS m/z: 551 (M+1).
[001139] Methyl 5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2~-yl]carbonyl}thiophene-2-carboxylate (H-65)
[001140] Methyl 5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2F~-yl]carbonyl}thiophene-2-carboxylate was prepared following general
procedure H, substituting
methyl 5-(chlorocarbonyl)thiophene-2-carboxylate for 6-trifluoromethyl
nicotinyl chloride. (methyl 5-
(chlorocarbonyl)thiophene-2-carboxylate was prepared in one step from
thiophene-2,5-dicarboxylic
acid monomethyl ester. Treatment of this carboxylic acid with oxalyl chloride
and catalytic DMF
afforded methyl 5-(chlorocarbonyl)thiophene-2-carboxylate in decent yield).
The rest of the
procedures were followed as indicated in general procedure H to afford methyl
5-{ [(2S,4R)-4-
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2F~-yl]carbonyl
}thiophene-2-
carboxylate.
1H-NMR (CDCl3) 8: 1.10 (d, 3H), 2.05 (s, 3H), 2.20 (m, 1H), 3.80 (s, 3H), 4.65
(m, 1H), 5.50 (m,
1H), 6.45 (d~ 1H), 6.80 (d, 1H), 7.00 (t, 1H), 7.15 (d, 2H), 7.20-7.40 (m,
5H).
MS m/z: 483 (M+1).
[001141] (2S,4R)-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxymethyl)-furan-2-carboxylic acid (H-66)
[001142] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.28g, 0.64 mmol) was dissolved in DMF at room
temperature and KZC03
(0.125g, 0.89 mmol) was added. 5-chloromethyl-furan-2-carboxylic acid methyl
ester (0.130g, 0.64
270
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
mmol) was added and the reaction was allowed to heat to 80°C overnight.
The reaction mixture was
concentrated in vacuo. The residue was partitioned between ethyl acetate and
water, then extracted
three times with ethyl acetate, dried over MgSO4, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography to afford the product as colorless
oil in 53% yield.
[001143] (2S,4R)-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenoxymethyl)-furan-2-carboxylic acid methyl ester (0.145g, 0.25
mmol) was hydrolyzed
to the acid by dissolving in tetrahydrofuran and ethanol and sodium hydroxide
(1N) was added. The
mixture was stirred at room temperature 4 hours. The mixture was cooled to rt,
acidified to form a
white precipitate. The solid was filtered to give (2S,4R)-5-(4-{4-[Acetyl-(4-
chloro-phenyl)-amino]-2-
methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxymethyl)-furan-2-carboxylic
acid as a white
solid.
1H-NMR (CDC13) b: 1.12-1.19 (m, 4H), 2.03 (s, 3H), 2.23-2.29 (m, 1H), 4.73-
4.75 (m, 1H), 4.95 (s,
2H), 5.59 (bs, 1H) 6.48-6.51 (m, 2H), 6.69 (d, 2H), 6.91 (t, 1H), 7.12-7.37
(m, 9H).
MS m/z: 559 (M+1).
[001144] (2S,4R)-N-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quipolive-1-carbonyl}-phenoxy)-pentanoic acid amide (H-67)
[001145] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.146 g, 0.34 mmol) was dissolved in 5 ml DMF at
room temperature and
KZC03 (0.186 g, 1.35 mmol) was added. 5-Bromo-pentanoic acid ethyl ester (0.14
g, 0.67 mmol)
was added and the reaction was allowed to heat to 80°C overnight. The
reaction mixture was
concentrated i~z vacuo. The residue was partitioned between ethyl acetate and
water, then extracted
three times with ethyl acetate, dried over MgS04, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography (50% EtOAc/ 50% Hexane) to afford
the (2S,4R)-N-5-(4-
{ 4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-
carbonyl }-phenoxy)-
pentanoic acid ethyl ester (0.148 g, 78%).
(001146] The ester was hydrolyzed to the acid by dissolving in 10 ml
tetrahydrofuran/methanol
(1/1) and potassium hydroxide (0.182 g in 5 ml water) was added. The mixture
was heated to 40 °C
for 3 hours. The mixture was cooled to rt, acidified to form a white
precipitate. The solid was filtered
to give (2S,4R)-N-5-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-1-
carbonyl }-phenoxy)-pentanoic acid (0.128 g, 91 %).
[001147] (2S,4R)-N-5-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline
1-carbonyl}-phenoxy)-pentanoic acid amide was prepared from the acid by
coupling NH4CI, HATU,
DIEA, HOBt in DMF at room temperature to yield (2S,4R)-N-1-[2-(4-{4-[acetyl-(4-
chloro-phenyl)
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-ethyl]-
cyclobutanecarboxylic acid
amide. The reaction mixture was concentrated down and partitioned between
ethyl acetate and water,
271
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
then extracted three times with ethyl acetate, dried over magnesium sulfate,
filtered and concentrated
down. The residue was purified by silica gel chromatography (10% methanol / 90
%
dichloromethane) to afford pure product in 69% yield.
1H-NMR (CDC13) ~: 1.13 (d, 3H), 1.15 (t, 1H), 1.68 (m, 4H), 1.95 (s, 3H), 2.16-
2.37 (m, 3H), 3.86 (t,
2H), 4.72 (sextet, 1H), 5.58 (bs, 1H), 5.79 (bs, 1H), 6.52 (d, 1H), 6.67 (d,
2H), 6.86 (t, 1H), 7.08-7.35
(m, 6H), 7.38 (d, 2H).
MS mlz: 534 (M+1)
[00114$] N-(4-chlorophenyl)-N-{(2S,4R)-2-methyl-1-[(2-methylpyrimidin-5-
yl)carbonyl]-
1,2,3,4-tetrahydroquinolin-4-yl}acetamide (H-68)
[001149] N (4-chlorophenyl)-N-{(2S,4R)-2-methyl-1-[(2-methylpyrimidin-5-
yl)carbonyl]-1,2,3,4-
tetrahydroquinolin-4-yl ) acetamide was prepared following general procedure
H, substituting 2-
methylpyrimidine-5-carbonyl chloride for 6-trifluoromethyl nicotinyl chloride.
(2-methylpyrimidine-
5-carbonyl chloride was prepared in four steps. To a solution of 3,3-
dimethoxypropionate in ethylene
glycol dimethyl ether was added sodium hydride at 0°C, then methyl
formate. The reaction mixture
was warmed up to 50°C for 30 m. and then stirred at room temperature
for 20h. Anhydrous diethyl
ether was added and the precipitate was filtered to give sodium 2-
(dimethoxymethyl)-3-methoxy-3-
oxoprop-1-en-1-olate. To a solution of acetamide hydrochloride in
dimethylformamide was added
preformed sodium 2-(dimethoxymethyl)-3-methoxy-3-oxoprop-1-en-1-olate.
Reaction mixture was
heated to 100°C for 1h. to give methyl 2-methylpyrimidine-5-
carboxylate. Hydrolysis of the ester with
sodium hydroxide gave 2-methylpyrimidine-5-carboxylic acid. Subsequent
treatment of this
carboxylic acid with oxalyl chloride and catalytic DMF afforded 2-
methylpyrimidine-5-carbonyl
chloride in decent yield). The rest of the procedures were followed as
indicated in general procedure
H to afford N-(4-chlorophenyl)-N-{(2S,4R)-2-methyl-1-[(2-methylpyrimidin-5-
yl)carbonyl]-1,2,3,4-
tetrahydroquinolin-4-yl } acetamide.
'H-NMR (CDCI3) 8: 1.15 (d, 3H), 2.05 (s, 3H), 2.30 (m, 1H), 2.70 (s, 3H), 4.75
(m, 1H), 5.55 (m,
1H), 6.50 (d, 1H), 7.00 (t, 1H), 7.20-7.45 (m, 6H), 8.40 (s, 2H).
MS m/z: 435 (M+1).
[001150] N-(4-chlorophenyl)-N-{(2S,4R)-1-[4-(1,1-dioxidoisothiazolidin-2-
yl)benzoyl]-2-
methyl-1,2,3,4-tetrahydroquinolin-4-yl}acetamide (H-69)
[001151] To a solution of N [(2S,4R)-1-(4-aminobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4
yl]-N-(4-chlorophenyl)acetamide (321 mg, 0.74 mmol) in methylene chloride (7.0
mL) at 0 °C was
added triethylamine (0.654 mL, 4.70 mmol) and 3-chloropropane-1-sulfonyl
chloride (0.610 mL, 4.90
mmol). The reaction was warmed to room temperature and stirred for 2 days. To
the resulting
reaction was added potassium carbonate (496 mg, 3.59 mmol) and N,N
dimethylformamide (2.0 mL).
272
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
The reaction was stirred overnight (14 h) at room temperature. Next, 1N sodium
hydroxide (1.5 mL)
was added and the reaction stirred an additional 3 days at room temperature.
The resulting reaction
was diluted with methylene chloride (15 mL) and poured into water (25 mL). The
aqueous layer was
extracted with methylene chloride (2 x 25 mL) and the combined organic layers
were washed once
with brine. The organics were then dried over anhydrous magnesium sulfate,
filtered, and
concentrated under reduced pressure. The crude residue was purified by silica
gel chromatography
(0-5% methanol / methylene chloride gradient) to afford 85% pure product (220
mg). A portion of
this material (100 mg) was further purified via HPLC to afford pure N-(4-
chlorophenyl)-N-{(2S,4R)-
1-[4-(1,1-dioxidoisothiazolidin-2-yl)benzoyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl } acetamide
(73 mg, 18%).
'H-NMR (CDCI3) 8: 1.10-1.14 (m, 1H), 1.14 (d, 3H), 2.02 (s, 3H), 2.22-2.34 (m,
1H), 2.45-2.56 (m,
2H), 3.30-3.40 (m, 2H), 3.66-3.76 (m, 2H), 4.67-4.82 (m, 1H), 5.54-5.64 (m,
1H), 6.52 (d, 1H), 6.93
(t, 1H), 6.98-7.05 (m, 2H), 7.12-7.31 (m, 5H), 7.34-7.42 (m, 3H).
MS m/z: 538 (M+1).
[001152] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3,5-dichloro-4-ethylbenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (H-70)
[001153] N (4-chlorophenyl)-N [(2S,4R)-1-(3,5-dichloro-4-ethylbenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure H,
substituting 4-ethyl-
3,5-dichlorobenzoyl chloride for 6-trifluoromethyl nicotinyl chloride. (4-
ethyl-3,5-dichlorobenzoyl
chloride was prepared in two steps from 3,5-dichlorobenzoic acid. To a
solution of 3,5-
dichlorobenzoic acid in tetrahydrofuran was added lithium diisopropyl amide at
-78°C. After the
reaction mixture was stirred at -78°C for 1 h. ethyl iodide was added
and reaction mixture stirred at
room temperature for 2h. to give 4-ethyl-3,5-dichlorobenzoic acid. Subsequent
treatment of this
carboxylic acid with oxalyl chloride and catalytic DMF afforded 4-ethyl-3,5-
dichlorobenzoyl chloride
in decent yield). The rest of the procedures were followed as indicated in
general procedure B to
afford N (4-chlorophenyl)-N-[(2S,4R)-1-(3,5-dichloro-4-ethylbenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide.
1H-NMR (CDC13) 8: 1.00 (t, 3H), 1.10 (d, 3H), 2.05 (s, 3H), 2.20 (m, 1H), 2.80
(q, 2H), 4.65 (m,
1H), 5.50 (m, 1H), 6.50 (d, 1H), 6.95 (d, 1H), 7.00 (s, 2H), 7.2 (m, 3H), 7.25-
7.40 (m, 3H).
MS m/z: 517 (M+1).
[001154] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-butyric acid ethyl ester (H-71)
[001155] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF at room temperature and KZC03
was added. Ethyl-
273
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
4-bromobutyrate was added and the reaction was allowed to heat to 80°C
overnight. The reaction
mixture was concentrated in vacuo. The residue was partitioned between ethyl
acetate and water, then
extracted three times with ethyl acetate, dried over MgS04, filtered and
concentrated down. The
crude residue was purified by silica gel chromatography (50% EtOAc/ 50%
Hexane) to afford the
product.
'H-NMR (CDC13) 8: 1.10 (d, 3H),1.11 (m, 1H), 1.20 (t, 3H), 2.43 (t, 2H), 1.99
(s, 3H), 2.24(m, 1H),
3.90 (t, 2H), 4.09 (q, 2IT)~ 4.72 (sextet, 1H), 5.55 (bs, 1H), 6.49 (d, 1H)<
6.62 (d, 2H), 6.90(t, 1H),
7.11 (d, 2H), 7:16 (m,1H), 7.18 (d, 2H), 7.26 (d, 1H), 7.34 (d, 2H).
MS mlz: 549 (M+1).
[001156] (2S,4R)-3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxymethyl)-benzoic acid methyl ester (H-72)
[001157] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (150 mg, 0.35 mmol) was dissolved in DMF (5 mL) at
room temperature.
CsZC03 (283 mg, 0.87 mmol) was added followed by methyl 3-
(bromomethyl)benzoate (119 mg, 0.52
mmol) and the reaction was stirred at room temperature for 18 hours. The
mixture was concentrated
under reduced pressure and dissolved in ethyl acetate (15 mL). The reaction
mixture was washed with
sat. aq. NaHC03 (15 mL), water (15 mL) and brine (15 mL). The organic phase
was dried over
MgS04, filtered, and concentrated under reduced pressure. The crude residue
was purified by silica
gel chromatography (5195 ethyl acetate/hexane - 50150 ethyl acetate/hexane
gradient) to afford
slightly yellow solid product (111 mg, 54%).
1H-NMR (CDC13, 300MHz) S: 1.02-1.08 (d, 3H), 1.55 (s, 1H), 2.02 (s, 3H), 2.24-
2.32 (m, 1H,),
3.88-3.91 (s; 3H), 4.70-4.78 (m, 1H), 5.02-5.05 (s, 2H), 5.58-5.61 (b, 1H),
6.51-6.53 (m, 3H), 6.89-
7.29 (m, 8H), 7.36-7.46 (m,' 2H), 7.56-7.58 (m,1H), 7.97-8.04 (m, 2H).
MS m/z: 584 (M+1).
[001158] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-4-carboxylic acid ethyl ester (H-73)
[001159] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl }-phenyl)-piperidine-4-carboxylic acid ethyl ester was prepared
from (2S,4R)-trifluoro-
methanesulfonic acid 4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-1-
carbonyl }-phenyl ester by adding piperidine-4-carboxylic acid ethyl ester (4
eq.), Cs2C03 (3 eq.), 10
% Pd2(dba)3, BINAP (0.20 equ), and 10 % 18-Crown-6 (0.10 eq.) in toluene at
110 °C over night.
The reaction mixture was concentrated in vacuo. The residue was partitioned
between ethyl acetate
and water, then extracted three times with ethyl acetate, dried over MgS04,
filtered and concentrated
down. The crude residue was purified by HPLC to afford the product.
274
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDC13) r5: 1.10 (d, 3H),1.24 (m, 4H), 1.54-1.74 (mm, 4H), 2.00 (s,
3H), 2.27 (m,1H), 2.57
(m, 1H), 2,80 (m, 1H), 3.01 (m, 1H), 3.48 (m, 1H), 3.71 (m, 1H), 4.10 (q, 2H),
4.70 (m, 1H), 5.56
(brs,1H), 6.56 (d, 1H), 6.64 (d, 2H), 6.92 (t,1H), 7.07-7.28 (m, 6H), 7.35 (d,
2H).
MS mlz: 574.31 (M+1).
[001160] N-(4-Chlorophenyl)-N-{(2S,4R)-1-[(6-ethylpyridin-3-yl)carbonyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl}acetamide (H-74)
[001161] N (4-Chlorophenyl)-N {(2S,4R)-1-[(6-ethylpyridin-3-yl)carbonyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl } acetamide was prepared following general procedure
H, substituting 6-ethyl
nicotinyl chloride for 6-trifluoromethyl nicotinyl chloride. (6-Ethyl
nicotinyl chloride was prepared in
two steps from methyl 6-chloronicotinate. To a solution of methyl 6-
chloronicotinate (2.50 g, 14.6
mmol) in tetrahydrofuran (80 mL) and N-methyl pyrrolidone (3 mL) was added
iron (IIn acetyl
acetonate (500 mg), followed by dropwise addition of a solution of ethyl
magnesium bromide in ether
(9.80 mL,,17.5 mmol). The reaction was stirred at room temperature for 2 hours
(JAGS 2002,124,
13856-13863). The resulting reaction was quenched using (saturated) aqueous
ammonium chloride.
The mixture was extracted with ethyl acetate, and the organics were separated
and washed with brine,
then dried over anhydrous sodium sulfate, filtered and concentrated in vacuo.
The crude product was
purified on silica gel by flash chromatography using hexanes/ethyl acetate (10-
30%) to afford methyl
6-ethylnicotinate in 66% yield. The methyl ester was then hydrolysed in the
presence of aqueous
lithium hydroxide in methanol at room temperature overnight to give 6-
ethylnicotinic acid. Further
treatment of 6-ethyl nicotinic acid with oxalyl chloride and catalytic DMF in
dichloromethane gave
the desired 6-ethyl nicotinyl chloride).
'H-NMR (CDCl3) 8: 1.16 (d, 3H), 1.24 (t, 3H), 1.24-1.26 (m, 1H), 2.02 (s, 3H),
2.24-2.38 (m, 1H);
2.70-2.85 (q, 2H), 4.70-4.80 (m, 1H), 5.44-5.64 (m, 1H), 6.50 (d, 1H), 6.92-
6.98 (t, 2H), 7.20-7.40
(m, 8H), 8.50 (br s,1H).
MS m/z: 448 (M + 1).
[001162] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-
trifluoromethanesulfonylamino-
propoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-75)
[001163] (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamnde (34 mg, 0.069 mmol) was dissolved in
methylene chloride (0.5
mL) and triethylamine (0.019 mL, 0.138 mmol) and cooled to
-40 °C. Trifluoromethanesulfonic anhydride (0.015 mL, 0.086 mmol) was
added and the mixture was
warmed to 0 °C for 30 minutes. The mixture was partitioned between
methylene chloride and water;
the methylene chloride layer was dried over MgS04, filtered and concentrated.
The crude residue was
275
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
purified by silica gel chromatography (1l1 hexanes/ ethyl acetate - ethyl
acetate gradient) to afford the
product.
1H-NMR (CDCl3) 8: 1.1 (s, 3H), 1.2 (m, 1H), 1.9 (m, 2H), 2.0 (s, 3H), 2.3 (m,
1H), 3.6 (m, 2H), 4.0
(t, 2H), 4.7 (m, 1H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.6 (t, 1H), 6.7 (d, 1H), 6.9
(t, 1H), 7.2 (m, 7H), 7.4 (d,
2H).
MS m/z: 624 (M+1).
[001164] (2S,4R)-N-(4-Chloro-phenyl)-N-(2-methyl-1-{4-[3-(2-oxo-oxazolidin-3-
yl)-propoxy]-
benzoyl}-1,2,3,4-tetrahydro-quinolin-4-yl)-acetamide (H-76)
[001165] (2S,4R)-N-{ 1-[4-(3-Amino-pxopoxy)-benzoyl]-2-methyl-1,2,3,4-
tetrahydro-quinolin-4-
yl}-N-(4-chloro-phenyl)-acetamide (50 mg, 0.102 mmol) was dissolved in
methylene chloride (3 mL)
and triethylamine (0.150 mL, 1.08 mmol) and cooled to -40 °C. 2-
Bromoethylchloroformate (0.016
mL, 0.153 mmol) was added and the reaction was allowed to warm to room
temperature. After 1
hour at room temperature, DMF (2 mL) and CsZC03 (100 mg, 0.307 mmol) were
added and the
mixture was stirred over night. The mixture was partitioned between methylene
chloride and water;
the methylene chloride layer was dried over MgS04, filtered and concentrated.
The crude residue was
purified by silica gel chromatography (1/1 hexanes/ ethyl acetate = ethyl
acetate gradient) to afford the
product.
iH-NMR (CDCl3) 8: 1.1 (s, 3H), 1.2 (m, 1H), 1.9 (m, 2H), 2.0 (s, 3H), 2.3 (m,
1H), 3.4 (t, 2H); 3.5
(t, 2H), 3.9 (t, 2H), 4.3 (t, 2H), 4.7 (m, 1H), 5.6 (bs, 1H), 6.5 (d, 1H), 6.6
(d, 2H), 6.9 (t, 1H), 7.2 (m,
6H), 7.4 (d, 2H).
MS m/z: 562 (M+1).
[001166] (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenylamino)-propionic acid (H-77)
[001167] (2S,4R)- 3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenylamino)-propionic acid was prepared following the procedure
for (2S,4R)- 1-(4-{4-
[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-
phenyl)-
piperidine-4-carboxylic acid ethyl ester, substituting 3-amino-propionic acid
methyl ester for
piperidine-4-carboxylic acid ethyl ester to yield the (2S,4R)- 3-(4-{4-[Acetyl-
(4-chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenylamino)-propionic
acid methyl ester.
The ester was hydrolyzed to the acid by dissolving in tetrahydrofuran and
ethanol and lithium
hydroxide (1N) was added and heated 50°C for 2h. The mixture was cooled
to room temperature,
acidified to form a white precipitate. The solid was filtered to give to
afford the product after HPLC
purification.
276
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
1H-NMR (CDCl3) b: 1.10 (d, 3H),1.23 (m, 1H), 2.03 (s, 3H), 2.27 (m,1H), 2.51
(s, 2H), 3.30 (t, 2H),
4.71 (m, 1H), 5.61 (brs,1H), 6.27 (d, 2H), 6.59 (d, 1H), 6.93 (t, 1H), 7.01
(d, 2H), 7.02-7.27 (m, 4H),
7.37 (d, 2H), 8.90 (br,1H).
MS m/z: 506.3 (M+1).
[001168] N-(4-chlorophenyl)-N-[(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetannide (H-78)
[001169] N-(4-chlorophenyl)-N [(2S,4R)-1-(3,5-difluoro-4-methoxybenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide was prepared following general procedure H,
substituting 3,5-
difluoro-4-methoxybenzoyl chloride for 6-trifluoromethyl nicotinyl chloride.
(3,5-Difluoro-4-
methoxybenzoyl chloride was prepared in one step from 3,5-difluoro-4-
methoxybenzoic acid: 3,5-
difluoro-4-methoxybenzoic acid was treated with oxalyl chloride and catalytic
amount of DMF to
afford 3,5-difluoro-4-methoxybenzoyl chloride in decent yield). The rest of
the procedures were
followed as indicated in general procedure H to afford N-(4-chlorophenyl)-N
[(2S,4R)-1-(3,5-
difluoro-4-methoxybenzoyl)-2-methyl-1,2,3,4-tetrahydroquinolin-4-yl]acetamide
in decent yield.
'H-NMR (CDCl 3) 8: 1.13 (d, 3H), 1.24 (m, 2H), 2.02 (s, 3H), 2.28 (b, 1H),
3.94 (s, 3H), 4.70 (m,
1H), 6.52 (d,~lH), 6.72 (d, 2H), 6.90 (t; 1H), 7.17-7.23 (m, 3H), 7.28-7.38
(m, 3H).
MS m/z: 484 (M+1).
[001170] (2S,4R)-4-{4-[4-(Acetyl-phenyl-amino)-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl]-phenoxy}-2,2-dimethyl-butyric acid (H-79)
[001171] 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl}-phenoxy)-2,2-dimethyl-butyric acid (300 mg, 0.545 mmol) was
dissolved in ethanol. PdlC
(10% Palladium) was added, followed by HZ gas (1 atm--balloon). After 3 hours,
the reaction mixture
was filtered and concentrated. The crude residue was purified by silica gel
chromatography (100%
ethyl acetate to 5% methanol/ethyl acetate gradient) to afford the product.
'H-NMR (CDCl3) b: 1.1 (d, 3H), 1.2 (m, 1H), 1.2 (s, 3H), 1.3 (s, 3H), 2.0 (s,
3H), 2.1 (t, 1H), 2.3 (m,
1H), 3.9 (m, 2H~, 4.7 (m, 1H), 5.6 (bs, 1H), 5.9 (d, 1H), 6.5 (d, 1H), 6.4 (d,
2H), 6.9 (t, 1H), 7.2 (m,
7H), 7.4 (d, 2H), 10.8 (bs,1H).
MS m/z: 515 (M+1).
[001172] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-
1-carbonyl}-phenoxy)-fluoro-acetic acid (H-80)
[001173] (2S,4R)-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenoxy)-fluoro-acetic acid ethyl ester (100 mg) was dissolved in
methanol/THF (1:1, 5
mL), and potassium hydroxide (1.0N, 1 mL) was added. After 1 hour, the
reaction was acidified and
277
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
extracted with methylene chloride. The organic layer was dried, filtered, and
concentrated. The crude
residue was purified by silica gel chromatography 100% ethyl acetate to 5%
methanol/ethyl acetate
gradient to afford the product.
'H-NMR (CDC13) 8: 1.1 (d, 3H), 1.2 (m, 1H), 2.0 (s, 3H), 2.3 (m,1H), 4.8 (m,
1H), 5.6 (bs, 1H), 5.9
(d,1H), 6.5 (d, 1H), 6.9 (m, 3H), 7.2 (m, 6H), 7.4 (d, 2H),11.1 (bs,1H).
MS m/z: 511 (M+1)
[001174] (2S,4R)-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxy)-cyclohexanecarboxylic acid ethyl ester (H-81)
[001175] To a solution of diethyl azodicarboxylate (120 mg) in 5 ml THF at
0°C, was added PPh3
(181 mg). The mixture was stirred for 10 min at 0°C. Then (2S,4R)-N-(4-
Chloro-phenyl)-N-[1-(4-
hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide (200 mg)
was added. The
texture was stirred for 20 min at 0°C. 4-Hydroxy-cyclohexanecarboxylic
acid ethyl ester (80 mg) was
added. The final reaction mixture was stirred for overnight at RT. The mixture
was concentrated
under reduced pressure, dissolved in ethyl acetate, washed with sat. aqueous
sodium bicarbonate,
brine and dried over magnesium sulfate, filtered, and concentrated under
reduced pressure. The
residue was purified by silica gel chromatography (30/70 hexanes/ethyl acetate
gradient) to afford
pure (2S,4R)-4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl}-phenoxy)-cyclohexanecarboxylic acid ethyl ester (100mg, 37%).
'H-NMR (CDC13) b: 1.13 (d, 4H), 1.22 (t, 3H), 1.26-1.60 (m, 5H), 2.00-2.18 (m,
SH), 2.20-2.39 (m,
3H), 4.11 (q, 2H), 4.72 (sextet, 1H), 5.60 (bs, 1H), 6.53 (d, 1H), 6.64 (d,
2H), 6.91 (t, 1H) 7.11-7.38
(m, 8H).
MS mlz: 589 (M+1).
[001176] (1R,2R)-2-[(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-yl]carbonyl}phenoxy)methyl]cyclopentanecarboxylic acid
(H-82)
[001177] Trans-2-Hydroxymethyl-cyclopentanecarboxylic acid methyl ester (0.127
g, 0.81 mmol)
was dissolved in 10 ml toluene at room temperature with PPh3 (0.211g, 0.81
mmol), then added N-(4-
chlorophenyl)-N-[(2S,4R)-1-(4-hydroxybenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]acetamide
(0.1g, 0.23 mmol) and stirred for 5 min. DEAD (0.141g, 0.81 mmol) was added
and the reaction was
stirred for 18 h at room temperature. The reaction was concentrated and
purified by silica gel
chromatography (45 % dichloromethane /55% ethyl acetate) to afford methyl 2-
[(4-{ [(2S,4R)-4-
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-
yl]carbonyl}phenoxy)methyl]cyclopentanecarboxylate (0.12 g, 90%).
[001178] 2-[(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)
yl]carbonyl }phenoxy)methyl]cyclopentanecarboxylate was hydrolyzed to the acid
by dissolving in 5
278
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
ml methanol and potassium carbonate (0.100 g in 4 ml water) was added. The
mixture was stirred for
2 days. The mixture was cooled to room temperature and methanol was removed
irz vacuo. The
mixture was acidified to form a white precipitate. The solid was filtered to
give (1R,2R)-2-[(4-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (21~-
yl]carbonyl}phenoxy)methyl]cyclopentanecarboxylic acid (0.012 g,10%)
'H-NMR (CDCl3) ~: 1.12 (m, 1H), 1.18 (d, 3H), 1.51 (m, 1H), 1.74 (m, 2H), 1.96
(m, 3H), 2.02 (s,
3H), 2.28 (m, 1H), 2.65 (m, 2H), 3.89 (m, 2H), 4.77 (m, 1H), 5.60 (m, 1H),
6.48 (d, 1H), 6.61 (m,
2H), 6.88 (t, 1H), 7.19 (m, 6H), 7.37 (d, 2H).
MS m/z: 562 (M+1)
[001179] (ZS,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-ureido-propoxy)-
benzoyl]-1,2,3,4-
tetrahydro-quinolin-4-yl}-acetamide (H-84)
[001180] ; To a solution of (2S,4R)-N-{ 1-[4-(3-Amino-propoxy)-benzoyl]-2-
methyl-1,2,3,4-
tetrahydro-quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (50 mg, 0.10 mmol) in
DCM (1 mL) was
added trimethylsilyl isocyanate (23 mg, 0.20 mmol). The reaction mixture was
stirred at room
temperature for 18 hours. The reaction was quenched by adding 0.2 mL of water
and concentrated
under reduced pressure. The crude residue was purified by preparatory HPLC to
afford the product (9
mg,.l7%).
MS m/z: 536 (M+1).
[001181] (2S,4R)-N-(4-Chloro-phenyl)-N-{1-[4-(2-imidazol-1-yl-ethozty)-
benzoyl]-2-methyl-
1,2,3,4-tetrahydro-qwlnolin-4-yl}-acetamide (H-85)
[001182] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.065g, 0.15 mmol) was dissolved in DMF at room
temperature and
K2C03 (0.12 g, 0.87 nnmol) was added. 1-(2-Bromo-ethyl)-1H-imidazole (0.088,
0.45 mmol) was
added and the reaction was allowed to heat to 80°C overnight. The
reaction mixture was concentrated
in vacuo. The residue was partitioned between ethyl acetate and water, then
extracted three times
with ethyl acetate, dried over MgS04, filtered and concentrated down. The
crude residue was purified
by silica gel chromatography (10% methanol / 90 % dichloromethane) to afford
the product (0.06g,
76%).
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1.15 (t, 1H), 1.98 (s, 3H), 2.27 (m, 1H), 4.13
(t, 2H), 4.28 (t, 2H),
4.72 (sextet, 1H), 5.58 (bs, 1H), 6.48 (d, 1H), 6.64 (d, 2H), 6.91 (t, 1H),
6.98 (s, 1H), 7.03 (s, 1H),
7.08-7.35 (m, 6H), 7.38 (d, 2H), 7.55.(s, 1H).
MS m/z: 529 (M+1)
279
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001183] (ZS,4R)-1-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-propyl]-1H-imidazole-2-carboxylic acid ethyl
ester (H- 86
[001184] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF at room temperature and KZC03
was added. 1-(3-
Bromo-propyl)-1H-imidazole-2-carboxylic acid ethyl ester (prepared from the
dibromide and the
corresponding imidazole with NaH in THE was added and the reaction was allowed
to heat to 80°C
overnight. The reaction mixture was concentrated in vacuo. The residue was
partitioned between
ethyl acetate and water, then extracted thxee times with ethyl acetate, dried
over MgS04, filtered and
concentrated down. The crude residue was purified by silica gel chromatography
(5% MeOH/ 95%
CHZCIz/N~I40H to 10% MeOH/ 90% CHZCl2/NHaOH) to afford the product.
'H-NMR (CDC13) 8: 1.10 (d, 3H), 1.11 (m, 1H), 1.38 (t, 3H), 2.01 (s, 3H), 2.22
(m, 2H), 3.83 (m,
2H), 4.35 (q, 2H), 4.55 (m, 2H), 4.71 (sextet, 1H), 5.58 (bs, 1H), 6.49 (d,
1H), 6.63 (d, 2H), 6.90 (t,
1H), 7.08-7.28 (m, 9H), 7.36 (d, 1H).
[001185] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-butyric acid (H-87)
[001186] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.126 g, 0.242 mmol) was dissolved in 3 ml DMF at
room temperature and
KZC03 (0.267 g, 1.94 mmol) was added. 4-Bromo-2,2-dimethyl-butyric acid methyl
ester ( 0.202 g,
0.969 mmol, prepared according to the procedure from Tetrahedron 1994, 50(32),
9825-30 ) was
added and the reaction was allowed to heat to 80°C overnight. The
reaction mixture was concentrated
in vacuo. The residue was partitioned between ethyl acetate and water, then
extracted three times
with ethyl acetate, dried over MgS04, filtered and concentrated down. The
crude residue was purified
by silica gel chromatography (50% EtOAcI 50% Hexane) to afford the (2S,4R)-4-
(4-{4-[acetyl-(4-
chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-
2,2-dimethyl-
butyric acid methyl ester (0.105 g, 77%).
[001187] The ester was hydrolyzed to the acid by dissolving in 6 ml
tetrahydrofuran/methanol (1/1)
and potassium hydroxide (0.042 g in 2 ml water) was added. The mixture was
heated to 40 °C for 3
hours. The mixture was cooled to rt, acidified to form a white precipitate.
The solid was filtered to
give (2S,4R)-N-4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-quinoline-1-
carbonyl}-phenoxy)-2,2-dimethyl-butyric acid (0.085 g, 83%).
'H-NMR (CDCl3) 8: 1.14 (d, 3H), 1.15 (t, 1H), 1.25 (s, 6H), 2.02 (t, 2H), 2.05
(m, 2H), 2.27 (m, 1H),
3.96 (t, 2H), 4.72 (sextet, 1H), 5.52 (br, 2H), 5.58 (bs, 1H), 6.52 (d, 1H),
6.67 (d, 2H), 6.86 (t, 1H),
7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS mlz: 549 (M+1).
280
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001188] N-(4-Chlorophenyl)-N-{(2S,4R)-1-[4-(1,1-difluoroethyl)benzoyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl}acetamide (H-88)
[001189] N-(4-Chlorophenyl)-N-{(2S,4R)-1-[4-(1,1-difluoroethyl)benzoyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl}acetamide was prepared following general procedure H,
substituting 4-(1,1-
difluoroethyl)benzoyl chloride for 6-trifluoromethyl nicotinyl chloride. (4-
(1,1-difluoroethyl)benzoyl
chloride was prepared in 3 steps from 1-(4-iodophenyl)ethanone. Treatment of 1-
(4-
iodophenyl)ethanone with neat [Bis(2-methoxyethyl)amino]sulfur trifluoride
(1.7 equivalents) at 85
°C under argon in the presence of ethanol (0.1 equivalents), then
quenching with saturated aqueous
sodium bicarbonate followed by standard aqueous work up and chromatography
(hexanes) afforded 1-
(1,1-difluoroethyl)-4-iodobenzene. Subsequent lithium-halogen exchange with n-
butyl lithium (1.2
equivalents) in THF at -78 °C under argon and quenching of the
resultant organolithium species by
bubbling carbon dioxide through the reaction mixture afforded 4-(1,1-
difluoroethyl)benzoic acid
which was converted to 4-(1,1-difluoroethyl)benzoyl chloride by treatment with
thionyl chloride (2.2
equivalents) in dichloromethane at room temperature for 2 hours followed by
removal of the volatiles
in vaccuo). The rest of the procedures were followed as indicated in general
procedure H to afford N-
(4-chlorophenyl)-N {(2S,4R)-1-[4-(1,1-difluoroethyl)benzoyl]-2-methyl-1,2,3,4-
tetcahydroquinolin-4-
y1 } acetamide.
'H-NMR (CDCl3) 8: 1.16 (d, 3H), 1.22-1.26 (m, 1H), 1.82 (t, 3H), 2.03 (s, 3H),
2.24-2.35 (m, 1H),
4.72-4.83 (m, 1H), 5.63 (br s,1H), 6.47 (d, 1H), 6.90 (t, 1H), 7.16-7.46
(m,10H).
MS mlz: 483 (M+1).
[001190] N-[(2S,4R)-1-(3-{[tart-butyl(dimethyl)silyl]oxy}-4-fluorobenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-chlorophenyl)acetamide (H-89)
[001191] N-[(2S,4R)-1-(3-{[tart-butyl(dimethyl)silyl]oxy}-4-fluorobenzoyl)-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N (4-chlorophenyl)acetamide was prepared following
general procedure H,
substituting 3-{[tart-butyl(dimethyl)silyl]oxy}-4-fluorobenzoyl chloride for 6-
trifluoromethyl
nicotinyl chloride. (3-{[tertbutyl(dimethyl)silyl]oxy}-4-fluorobenzoyl
chloride was prepared in one
step from 3-{[tent-butyl(dimethyl)silyl]oxy}-4-fluorobenzoic acid. 3-{[tert-
butyl(dimethyl)silyl]oxy }-4-fluorobenzoic acid was treated with oxalyl
chloride and catalytic amount
of DMF to afford 3,5-difluoro-4-methoxybenzoyl chloride in decent yield). The
rest of the
procedures were followed as indicated in general procedure H to afford N-
[(2S,4R)-1-(3-{ [tert-
butyl(dimethyl)silyl]oxy }-4-fluorobenzoyl)-2-methyl-1,2,3,4-
tetrahydroquinolin-4-yl]-N-(4-
chlorophenyl)acetamide
'H-NMR (CDCI 3) 8: 0.1(s, 9H), 0.9 (s, 6H), 1.15 (d, 3H), 2.07 (s, 4H), 2.30
(b, 1H), 4.75 (m, 1H),
6.55 (d,1H), 6.68 (d, 1H), 6.62-6.70 (m, 3H) 7.15-7.25 (m, 4H), 7.35-7.42 (m,
2H).
MS m/z: 568 (M+1)
281
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001192] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-3-carboxylic acid amide (H-90)
[001193] (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-piperidine-3-carboxylic acid amide was prepared from
(2S,4R)-1-(4-{4-[Acetyl-
(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-
phenyl)-piperidine-3-
carboxylic acid. The acid (0.2 g, 0.366 mmol) was dissolved in DMF (3 mL) at
room temperature and
HOBt (0.74 g, 0.55 mmol), HATU (0.209 g, 0.55 mol), and diisopropylethylamine
(0.25 mL,) was
added followed by ammonium chloride (0.040 g, 0.74 mmol) and stirred at room
temperature for 16
h. The reaction was diluted with ethyl acetate, washed with 1N NaOH, 1N HCl
and brine. The
organics were dried over MgS04, filtered and concentrated down. The crude
residue was purified
HPLC purification.
'H-NMR (CDC13) 8: 1.10 (d, 3H), 1.20 (m, 1H), 1.73 (m, 2H), 1.84(m, 2H), 2.01
(s, 3H), 2.24 (m,
2H), 2.65 (m, 2H), 3.66 (m, 2H), 4.70 (m, 1H), 5.62 (br, 2H), 6.93 (br, 1H),
6.56 (d, 1H), 6.60 (d,
2H), 6.92 (t, 1H), 7.05-7.27 (m, 6H), 7.37 (d, 2H).
MS m/z: 545.3 (M+1).
[001194] (2S;4R)- N-{1-[4-(1-Acetyl-piperidin-4-yhnethoxy)-benzoyl]-2-methyl-
1,2,3,4-
tetrahydro-quinolin-4-yl}-N-(4-chloro-phenyl)-acetamide (H-91)
[001195] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(piperidin-4-ylmethoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (0.075 g, 0.14 mmol) was dissolved
in dichloromethane
at room temperature. DIEA (0.1 mL, 0.56 mmol) and acetyl chloride (0.2 mL, 2.8
mmol) was added.
The reaction was stirred at room temperature overnight. The reaction mixture
was concentrated in
vacuo. The crude residue was purified by silica gel chromatography (10%
methanol J 90 %
dichloromethane) to afford the product (0.049g, 49%).
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1.15 (t, 1H), 1.24 (m, 2H), 1.81 (m, 2H), 1.93
(m, 1H), 2.04 (s,
3H), 2.09 (s, 3H), 2.27 (m, 1H), 2.55 (t, 1H), 3.09 (t, 1H), 3.73 (m, 2H),
3.85 (m, 1H), 4.63 (m, 1.H),
4.72 (sextet, 1H), 5.58 (bs, 1H), 6.52 (d, 1H), 6.67 (d, 2H), 6.86 (t, 1H),
7.08-7.35 (m, 6H), 7.38 (d,
2H).
MS m/z: 574 (M+1)
[001196] (2S,4R)-N-5-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-dimethyl-pentanoic acid (H-92)
[001197] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (0.12 g, 0.28 mmol) was dissolved in 5 ml DMF at room
temperature and
KZC03 (0.155 g, 1.12 mmol) was added. 5-Bromo-2,2-dimethyl-pentanoic acid
methyl ester (0.123 g,
282
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
0.56 mmol, prepared according to the procedure from Tetrahedron 1994, 50(32),
9825-30 ) was
added and the reaction was allowed to heat to 80°C overnight. The
reaction mixture was concentrated
in vacuo. The residue was partitioned between ethyl acetate and water, then
extracted three times
with ethyl acetate, dried over MgSO~, filtered and concentrated down. The
crude residue was purified
by silica gel chromatography (50% EtOAcI 50% Hexane) to afford (2S,4R)-N-5-(4-
(4-[acetyl-(4-
chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-
2,2-dimethyl-
pentanoic,acid methyl ester (0.076 g, 48%).
[001198] The ester Was hydrolyzed to the acid by dissolving in 8 ml
tetrahydrofuran/methanol (1l1)
and potassium hydroxide (0.03 g in 2 ml water) was added. The mixture was
heated to 40 °C for 3
hours. The mixture was cooled to rt, acidified to form a white precipitate.
The solid was filtered to
give the product (0.057 g, 79%).
'H-NMR (CDC13) 8: 1.13 (d, 3H), 1.15 (t, 1H), 1.27 (s, 6H), 1.70 (m, 4H),
1.1.98 (s, 3H), 2.28 (m,
1H), 3.89 (t, 2H), 4.72 (sextet, 1H), 5.58 (bs, 1H), 6.52 (d, 1H), 6.67 (d,
2H), 6.86 (t, 1H), 7.08-7.35
(m, 6H), 7.38 (d, 2H).
MS m/z: 535 (M+1)
[001199] (2S,4R)-4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-methyl-amino]-butyric acid methyl ester (H-93)
[001200] (2S,4R)- 4-[(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-methyl-amino]-butyric acid methyl ester was prepared from
(2S,4R)- (4-{4-
[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl
}-phenyl)-methyl-
carbamic acid ethyl ester. (2S,4R)- (4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-
methyl-3,4-dihydro-2H-
quinoline-1-carbonyl}-phenyl)-methyl-carbamic acid ethyl ester was dissolved
in acetonitrile (2, mL).
Iodotrimetylsilane was added and the reaction was allowed to stir at room
temperature over night.
Excess reagent was quenched by the addition of methanol (1 mL) and the mixture
was concentrated
under reduced pressure. The crude residue was partitioned between ethyl
acetate and saturated
aqueous sodium bicarbonate. The extracts were washed with 1 M sodium
hydroxide, saturated
aqueous sodium thiosulfate and brine, dried over sodium sulfate, filtered,
concentrated and purified by
silica gel chromatography to yield N-(4-chloro-phenyl)-N-[2-methyl-1-(4-
methylamino-benzoyl)-
1,2,3,4-tetrahydro-quinolin-4-yl]-acetamide .
[001201] (2S,4R)- (N-(4-chloro-phenyl)-N-[2-methyl-1-(4-methylamino-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (0.015g, 0.033 mmol) was dissolved in
CHzCl2 (0.5 mL) at room
temperature. Sodium borohydride (0.007g, 1.5 eq.) was added followed by 4-oxo-
butyric acid methyl
ester (0.020 g, 4 eq.) and the reaction was allowed to stir over night. The
reaction mixture was
concentrated in vacuo. The residue was partitioned between ethyl acetate and
water, then extracted
283
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
three times with ethyl acetate, dried over MgS04, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography (33% Hexane / EA gradient) to afford
the product.
'H-NMR (CDC13) S: 1.09-1.24 (m, 4H), 1.84 (m, 2H), 2.00 (s, 3H), 2.27 (m, 3H),
2.86 (s, 3H), 3.29
(t, 2H), 3.62 (s, 3H), 4.70 {m, 1H), 5.58 {brs, 1H), 6.39 (d, 2H), 6.59 (d,
1H), 6.96 (t, 1H), 7.05-7.21
(m, 5H), 7.25 (d,1H), 7.36 (d, 2H).
MS m/z: 548 (M+1).
[001202] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-2,2-difluoro-butyric acid (H-94)
[001203] 2,2-Difluoro-4-hydroxy-butyric acid methyl ester (0.27 g, 3.21 mmol,
prepared according
to US Patent 4421690 procedure) was dissolved in toluene at room temperature
with PPh3 (0.421g,
3.21 mmol), then added (2S,4R)-N-(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-
methyl-1,2,3;4-
tetrahydro-quinolin-4-yl]-acetamide (0.20g, 0.46 mmol) and stirred for 5 min.
DEAD (0.028g, 3.21.
mmol) was added and the reaction was stirred for 18 h at room temperature. The
reaction was
concentrated and purified by silica gel chromatography (45 % CHzCl2 /55%EtOAc)
to afford (2S,4R)-
N-4-(4-{ 4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-
1-carbonyl }-
phenoxy)-2,2-difluoro-butyric acid methyl ester (0.23 g, 87%).
[001204] The ester was hydrolyzed to the acid by dissolving in 8 ml
tetrahydrofuran/methanol (1/1)
and potassium hydroxide (0.1 g in 4 ml water) was added. The mixture was
heated to 40 °C
overnight. The mixture was cooled to rt, acidified to form a white
precipitate. The solid was filtered
to.give the product (0.038 g, 97%).
IH-NMR (CDCI3) 8: 1.13 (d, 3H), 1.15 (t, 1H), 2.04 (s, 3H), 2.18-2.44 (bs,
4H), 3.95 {t, 2H); 4.72
(sextet,1H), 5.58 (bs,1H), 6.52 (d,1H), 6.67 (d, 2H), 6.86 (t,1H), 7.08-7.35
(m, 6H), 7.38 (d, 2H).
MS mlz: 557 (M+1).
[001205] {[3-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenoxy)propyl]amino}acetic acid (H-95)
[001206] { [3-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)propyl]amino}acetic . acid was prepared from (2S,4R)-N-{1-
[4-(3-amino-
propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl }-N-(4-chloro-
phenyl)-acetamide.
(2S,4R)-N-{ 1-[4-(3-amino-propoxy)-benzoyl]-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl }-N-(4-
chloro-phenyl)-acetamide (0.036g, 0.07 mmol) was dissolved in
dimethylformamide, ethyl
bromoacetate (0.008mL, 0.07 mmol) and potassium carbonate (0.021g, 0.14 mmol)
were added. The
reaction was heated to 50°C for 17h. The reaction was concentrated down
and purified using 50%
ethyl acetate/ 50% hexane to 100% ethyl acetate.
284
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001207] 'H-NMR (CDC13) 8: 0.91 (t, 1H), 1.12 (d, 3H), 1.24 (t, 3H), 2.02 (s,
3H), 2.04 (m, 2H),
2.28 (m, 1H), 3.47-3.53 (m, 2H), 3.94-4.02 (m, 3H), 4.06 (m, 1H), 4.10-4.22
(q, 2H), 4.54 (d, 2H),
4.72 (sextet, 1H), 5.61 (bs, 1H), 6.51 (d, 1H), 6.64 (d, 2H), 6.92 (t, 1H),
7.12 (d, 2H), 7.16-7.21 (m,
3H), 7.27 (d,1H), 7.36 (d, 2H). MS mlz = 577 (M+1).
[00120$] { [3-(4-{ [(2S,4R)-4.-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)propyl]amino}acetic ethyl ester was hydrolyzed to the acid
by dissolving in
tetrahydrofuran and ethanol and sodium hydroxide (1N) was added. The mixture
was stirred at room
temperature overnight. The mixture was cooled to rt, acidified to form a white
precipitate. The solid
was filtexed to give { [3-(4-{ [(25,4R)-4-[acetyl(4-chlorophenyl)amino]-2-
methyl-3,4-dihydroquinolin-
1(2H)-yl]carbonyl}phenoxy)propyl]amino}acetic acid (0.003g, 60% yield).
MS m/z : 564 (M+1).
[001209] (2S,4R)-N-4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H- '
quinoline-1-carbonyl}-phenoxy)-N-ethyl-butyramide (H-96)
[001210] (2S,4R)-N-4-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-1-ethylidene-pentG-2,4-dienyloxy)-butyric acid (0.064g, 0.123mmol)
was converted to
the amide by dissolving in THF (2 mL) at room temperature. HOBt (0.025 g),
EDCI (0.028 g), and
ethylamine (0.36 mmol) was added along with 2 drops of DMF and stirred at room
temperature for 11
h. The reaction was diluted with ethyl acetate, washed with 1N NaOH, 1N HCl
and brine. The
organics were. dried over MgSOa, filtered and concentrated down. The crude
residue was purified by
silica gel chromatography (50% ethyl acetate/50% hexane to 100% ethyl acetate)
to afford the product
(0.050 g, 74%).
'H-NMR (CDC13) 8: 1.06 (t, 3H), 1.13 (d, 3H), 1.15 (t, 1H), 1.81 (s, 1H), 2.04
(s, 3H), 2.06 (m; 2H),
2.27 (m, 1H), 2.32 (t, 2H), 3.28 (q, 2H), 3.91 (t, 2H), 4.72 (sextet, 1H),
5.66 (bs, 1H), 6.52 (d, 1H),
6.67 (d, 2H), 6.88 (t,1H), 7.08-7.35 (m, 6H), 7.38 (d, 2H).
MS m/z: 548 (M+1)
[001211] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-pyrazol-1-yl-propoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-97)
[001212] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide (75 mg, 0.17 mmol) was dissolved in DMF (1 mL) at
room temperature.
KaC03 (47 mg, 0.34 mmol) was added followed by 1-(3-bromopropyl)-pyrazole (64
mg, 0.34 mmol)
(prepared from the reaction of 1,3-dibromopropane and pyrazole with sodium
hydride in
tetrahydrofuran). The reaction was allowed to stir at 70°C for 3hrs.
The reaction mixture was
concentrated in vacuo. The residue was partitioned between ethyl acetate and
water, then extracted
three times with ethyl acetate. Organic layers were washed with brine, dried
over MgS04, filtered and
285
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
concentrated down. The crude residue was purified by silica gel chromatography
(1199 methanol /
dichloromethane - 3/97 methanol / dichloromethane gradient) to afford the
product.
'H-NMR (CDCl3) 8: 1.1 (d, 3H), 1.2 (m, 1H), 2.0 (s, 3H), 2.3 (m, 1H), 2.3 (m,
2H), 3.8 (t, 2H), 4.3
(t, 2H), 4.7 (m, 1H), 5.6 (m, 1H), 6.2 (t, 1H), 6.5 (d, 1H), 6.65 (d, 2H), 6.9
(t, 1H), 7.1-7.4 (m, 9H),
7.5 (s,1H).
MS m/z: 4431445 (M+1).
[001213] .N-(4-chlorophenyl)-N-{(2S,4R)-1-[(2-ethylpyrimidin-5-yl)carbonyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl}acetannide (H-98)
[001214] N-(4-chlorophenyl)-N { (2S,4R)-1-[(2-ethylpyrimidin-5-yl)carbonyl]-2-
methyl-1,2,3,4-
tetrahydroquinolin-4-yl } acetamide was prepared following general procedure
H, substituting 2-
ethylpyrimidine-5-carbonyl chloride for 6-trifluoromethyl nicotinyl chloride.
(2-ethylpyrimidine-5-
carbonyl chloride was prepared in four steps. To a solution of 3,3-
dimethoxypropionate in ethylene
glycol dimethyl ether was added sodium hydride at 0°C, then methyl
formate. The reaction mixture
was warmed up to 50°C fox 30 m. and then stirred at room temperature
for 20h. Anhydrous diethyl
ether was added and the precipitate was filtered to give sodium 2-
(dimethoxymethyl)-3-methoxy-3-
oxoprop-1-en-1-olate. To a solution of propionamide hydrochloride in
dimethylformamide was added
preformed sodium 2-(dimethoxymethyl)-3-methoxy-3-oxoprop-1-en-1-olate.
Reaction mixture was
.heated to 100°C for 1h. to give methyl 2-ethylpyrimidine-5-
carboxylate. Hydrolysis of the ester with
sodium hydroxide gave 2-ethylpyrimidine-5-carboxylic acid. Subsequent
treatment of this carboxylic
acid with oxalyl chloride and catalytic DMF afforded 2-ethylpyrimidine-5-
carbonyl chloride in decent
yield). The rest of the procedures were followed as indicated in general
procedure H to afford N-(4-
chlorophenyl)-N {(2S,4R)-2-methyl-1-[(2-ethylpyrimidin-5-yl)carbonyl]-1,2,3,4-
tetrahydroquinolin-
4-yl}acetamide.
1H-NMR (CDC13) S: 1.15 (d, 3H), 1.25 (t, 3H), 2.05 (s, 3H), 2.30 (m, 1H), 2.90
(q, 2H), 4.75 (m,
1H), 5.55 (m, 1H), 6.50 (d,1H), 7.00 (t, 1H), 7.20-7.45 (m, 6H), 8.40 (s, 2H).
MS m/z: 449 (M+1).
[001215] (2S,4R)- 3-[3-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-propyl]-5-methyl-3H-imidazole-4-carboxylic acid
(H-99)
[001216] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro-
quinolin-4-yl]-acetamide was dissolved in DMF at room temperature and KzC03
was added. 3-(3-
Bromo-propyl)-5-methyl-3H-imidazole-4-carboxylic acid ethyl ester (prepared
from the dibromide
and the corresponding imidazole with NaH in THE was added and the reaction was
allowed to heat to
80°C overnight. The reaction mixture was concentrated ira vacuo. The
residue was partitioned
between ethyl acetate and water, then extracted three times with ethyl
acetate, dried over MgS04,
286
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
filtered and concentrated down. The crude residue was purified by silica gel
chromatography (2%
MeOH/ 95% CHzCl2 to 10% MeOH/ 90% CHZCl2 ) to afford (2S,4R)-3-[3-(4-{4-
[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-propyl]-
5-methyl-3H-
imidazole-4-carboxylic acid ethyl ester. The ester (0.080g, 0.12 mmol) was
hydrolyzed to the acid by
dissolving in tetrahydrofuran and ethanol and sodium hydroxide (1N) was added.
The mixture was
stirred at room temperature 16 hours. The mixture was cooled to room
temperature, acidified to form
a white precipitate. The solid was filtered to give (2S,4R)- 3-[3-(4-{4-
[Acetyl-(4-chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-propyl]-5-
methyl-3H-imidazole-4-
carboxylic acid in 61 % yield.
'H-NMR (CDCl3) 8: 1.13 (d, 3H), 1.29 (m, 1H), 2.05 (s, 3H), 2.34 (m, 2H), 2.45
(m, 1H), 2.61 (s,
3H), 4.08 (m, 2H), 4.43 (m, 2H), 4.73 (sextet, 1H), 5.53 (bs, 1H), 6.55 (d,
1H), 6.73 (d, 2H), 6.96 (t,
1.H), 7.18 (m, 3H), 7.41 (m, 5H), 9.08 (s, 1H).
MS.mlz: 601 (M+1).
[001217] 5-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(21~-
yl]carbonyl}thiophene-2-carboxylic acid (H-100)
[001218] Methyl 5-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydraquinolin-
1(21~-yl]carbonyl}thiophene-2-carboxylate (80 mg, 0.17 mmol, 1 eq.) was
dissolved in methanol (6
ml). A solution of potassium carbonate (200 mg, 1.4 mmol, 8 eq.) in water (2
ml) was added and
reaction mixture was stirred at room temperature for 20 h. The mixture was
concentrated and the
residue was acidified with a 1N HCI aqueous solution and extracted with ethyl
acetate. The organic
layer was washed with brine, dried over magnesium sulfate, filtered and
concentrated to give 5-
{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(2Fn-
yl]carbonyl}thiophene-2-carboxylic acid (76 mg, 99 %).
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 2.05 (s, 3H), 2.25 (m, 1H), 4.70 (m~ 1H), 5.55
(m, 1H), 6.55 (d,
1H), 6.85 (d, 1H), 7.05 (t, 1H), 7.15-7.45 (m, 7H).
MS m/z: 469 (M+1).
[001219] (2S,4R)-N-1-[2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-ethyl]-cyclobutanecarboxylic acid amide (H-101)
[001220] (2S,4R)-N-1-[2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenoxy)-ethyl]-cyclobutanecarboxylic acid amide was
prepared from
(2S,4R)-N-1-[2-(4-{4-[acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-2H-
quinoline-1-
carbonyl }-phenoxy)-ethyl]-cyclobutanecarboxylic acid by coupling NH4Cl, HATU,
D1EA, HOBt in
DMF at room temperature to yield (2S,4R)-N-1-[2-(4-{4-[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-
3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-ethyl]-cyclobutanecarboxylic
acid amide. The
287
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
reaction mixture was concentrated down and partitioned between ethyl acetate
and water, then
extracted three times with ethyl acetate, dried over magnesium sulfate,
filtered and concentrated
down. The residue was purified by silica gel chromatography (10% methanol / 90
%
dichloromethane) to afford pure product (63%).
1H-NMR (CDC13) 8: 1.16 (d, 3H), 1.15 (t, 1H), 1.77-1.96 (m, 4H), 2.04 (s, 3H),
2.16-2.48 (m, 5H),
3.96 (t, 2H), 4.72 (sextet, 1H), 5.58 (bs, 1H), 6.52 (d, 1H), 6.67 (d, 2H),
6.86 (t, 1H), 7.08-7.35 (m,
6H), 7.38 (d, 2H).
MS m/z: 560 (M+1)
[001221] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(2-oxo-oxazolidin-3-yl)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-102)
[001222] N-[1-(4-Amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-
(4-chloro-
phenyl)-acetamide (61 mg, 0.142 mmol) was dissolved in methylene chloride (2
mL) and was added
triethylamine (0.100 mL, 0.700 mmol). 2-Bromoethylchloroformate (0.023 ml,,
0.213 mmol) was
added and the reaction was allowed to warm to room temperature. After 1 hour
at room temperature,
DMF (2 mL) and CsZC03 (100 mg, 0.307 mmol) were added and the mixture was
stirred over night.
The mixture was partitioned between methylene chloride and water; the
methylene chloride layer was
dried over MgS04, filtered and concentrated. The crude residue was purified
by. silica gel
chromatography (1l1 hexaneslethyl acetate to 100% ethyl acetate gradient) to
afford the product.
1H-NMR (CDC13) 8: 1.2 (d, 3H),1.2 (m,1H), 2.0 (s, 3H), 2.3 (m,1H), 3.8 (t,
2H), 4.4 (t, 2H), 4.8 (m,
1H), 5.6 (m,1H), 6.5 (d,1H), 6.9 (t,1H), 7.2 (m, 8H), 7.4 (d, 2H).
MS mlz: 504 (M+1).
[001223] 4-(4-{[(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1(2H)-
yl]carbonyl}phenoxy)-2-(diethylamino)butanoic acid (H-103)
[001224] 2-tert-Butoxycarbonylamino-4-hydroxy-butyric acid (0.79 g, 3.6 mmol)
was dissolved in
15 ml methanol at room temperature and (trimethylsilyl)diazomethane (2M
solution in hexane ) was
added till solution become yellow. The mixture was concentrated down to afford
crude 2-tert-
butoxycarbonylamino-4-hydroxy-butyric acid methyl ester.
[001225] The ester was dissolved in 20 ml toluene at room temperature with
PPh3 (0.948, 3.6
mmol), then added N-(4-chlorophenyl)-N-[(2S,4R)-1-(4-hydroxybenzoyl)-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl]acetamide (0.318, 0.71 mmol) and stirred for 5 min.
DEAD (0.6268, 3.6
mmol) was added and the reaction was stirred for 18 h at room temperature. The
reaction was
concentrated and purified by silica gel chromatography (45 % CHZCIz /55%EtOAc)
to afford crude
methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-1 (2H)-
yl]carbonyl }phenoxy)-2-[(tert-butoxycarbonyl)amino]butanoate (0.47 g ).
288
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001226] Methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenoxy)-2-[(tert-butoxycarbonyl)amino]butanoate was
dissolved in 20 ml
dichloromethane and 4 ml HCl in dioxane (4M) was added. The mixture was
stirred 2 hours and
concentrated down. The residue was washed with ether, then partition between
1M NaOH and
dichloromethane. The dichloromethane layer was removed and dried with MgS04.
Sodium
triacetoxyborohydride ( 0.61 g, 2.9 mmol ) and acetaldehyde (0.33 ml, 5.8 mmol
) was added. The
mixture was stirred 2 days and washed with 1M NaOH. The mixture was then
concentrated and
purified by silica gel chromatography (40 % CHZCIz /60%EtOAc) to afford methyl
4-(4-{ [(2S,4R)-4-
[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1 (2H)-yl]carbonyl
}phenoxy)-2-
(diethylamino)butanoate ( 0.075 g, 17% ).
[001227] Methyl 4-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2H)-yl]carbonyl}phenoxy)-2-(diethylamino)butanoate was hydrolyzed to the
acid by dissolving in 6
ml tetrahydrofuran/methanol (1/1) and potassium hydroxide (0.04 g in 2 ml
water) was added. The
mixture was heated to 50 °C for 18 hours. The mixture was cooled to
room temperature and
concentrated-. Then neutralized with 1M HCl and extracted with
dichloromethane. The
dichloromethane solution was dried and concentrated. The residue was purified
by silica gel
chromatography (87 % CHZCIz /13%methanol) to afford 4-(4-{ [(2S,4R)-4-
[acetyl(4-
chlorophenyl)amino]-2-methyl-3,4-dihydroquinolin-1(21-yl]carbonyl}phenoxy)-2-
(diethylamino)butanoic acid ( 0.04 g, 55% ).
1H-NMR (CDCl3) 8: 1.14 (m, 1H), 1.19 (d, 3H), 1.38 (m, 7H), 2.01 (m, 1H), 2.03
(s, 3H), 2.31 (m,
2H), 2.92 (m, 2H), 3.26 (m, 2H), 3.75 (m, 1H), 4.23 (m, 2H), 4.70 (m, 1H),
5.58 (br, 2H), 6.48 (d,
1H), 6.61 (d, 2H), 6.88 (t,1H), 7.19 (m, 6H), 7.37 (d, 2H).
MS mlz: 593 (M+1)
[001228] 3-(4-{(2S,4R)-4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-benzylamino)-propionic acid tent-butyl ester (H-104)
[001229] N {(2S,4R)-1-[4-(aminomethyl)benzoyl]-2-methyl-1,2,3,4-
tetrahydroquinolin-4-y1}-N-(4-
chlorophenyl)acetamide (145 mg, 0.32mmol) was dissolved in methylene chloride
( 2.5 mL) and tert-
butyl acrolate (0.052 mL, 0.36 mmol) and stirred at room temperature
overnight. The mixture was
concentrated then subjected to flash chromatography (50% hexanes / 50% ethyl
acetate) to afford the
title compound (159 mg, 85%) as a white solid.
'H-NMR (CDC13) 8: 1.13 (s, 3H), 1.15-1.20 (m, 1H), 1.41 (s, 9H), 1.73-2.05 (m,
1H), 2.02 (s, 3H),
2.20-2.37 (m, 1H), 2.40 (t, 2H), 2.77 (t, 2H), 3.72 (s, 2H), 4.69-4.82 (m,
1H), 5.42-5.76 (m, 1H), 6.48
(d, 1H), 6.87 (t, 1H), 7.08-7.31 (m, 8H), 7.37 (d, 2H).
MS m/z: 576 (M +1)
289
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001230] N-{(2S,4R)-1-[3,5-Bis(trifluoromethyl)benzoyl]-6-chloro-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}-N-(4-chlorophenyl)acetamide (H-105)
[001231] N-{(2S,4R)-1-[3,5-Bis(trifluoromethyl)benzoyl]-6-chloro-2-methyl-
1,2,3,4-
tetrahydroquinolin-4-yl}-N-(4-chlorophenyl)acetamide was prepared following
general procedure H
up to intermediate 4, substituting benzyl [(2S,4R)-6-chloro-2-methyl-1,2,3,4-
tetrahydroquinolin-4-
yl]carbamate for benzyl [(2S,4R)-2-methyl-1,2,3,4-tetrahydroquinolin-4-
yl]carbamate.
'H-NMR (CDCl3) 8: 1.16 (3H, t),1.20 (1H, m), 2.02 (s, 3H), 2.25-2.37 (m, 1H),
4.70-4.80 (m, 1H),
5.42-5.56 (m,1H), 6.40 (d, 1H), 6.90(dd, 1H), 7.20-7.40 (m, 5H), 7.60 (s, 2H),
7.80 (s, 1H).
MS m/z: 589 (M + 1).
[001232] N-(4-Chloro-phenyl)-N-[(2S,4R)-2-methyl-1-(4-pyrrolidin-2-yl-benzoyl)-
1,2,3,4-
tetrahydro-quinolin-4-yl]-acetamide (H-106)
[001233] Benzyl 2-(4-{ [(2S,4R)-4-[acetyl(4-chlorophenyl)amino]-2-methyl-3,4-
dihydroquinolin-
1(2F~-yl]carbonyl}phenyl)pyrrolidine-1-carboxylate (237mg, 0.381 mmol) was
dissolved in
HBr/Acetic acid (5 mL) and stirred for 2h. The reddish slurry was partitioned
between EtzO and 1N
HCI. The HCl/water layer was washed 3X with EtzO to rid the benzyl bromide.
The water layer was
neutralized with 1N NaOH, and washed 3X with methylene chloride. The organic
layer was dried
over MgSO4, filtered and concentrated to afford the title compound (170 mg,
91%) as a white
solid.'H-NMR (CDC13) S: 1.05-1.20 (m, 1H), 1.13 (s, 3H), 1.50-1.62 (m, 1H),
1.76-1.90 (m, 2H), 2.02
(s, 3H), 2.05-2.20 (m, 2H), 2.20-2.38 (m, 1H), 2.93-3.03 (m, 1H), 3.09-3.18
(m, 1H), 4.05 (t, 1H),
4.72-4.82 (m,1H), 5.45-5.75 (m, 1H), 6.49 (d,1H), 6.88 (t,1H), 7.11-7.29 (m,
8H), 7.37 (d, 2H).
MS m/z: 488 (M +1)
[001234] (2S,4R)-N-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2)-I-
quinoline-1-carbonyl}-phenyl)-N-methyl-succinamic acid methyl ester (H-107)
[001235] (2S, 4R)-N-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline
1-carbonyl}-phenyl)-N-methyl-succinamic acid methyl ester was prepared from
(2S, 4R)-N-[1-(4
Amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl]-N-(4-chloro-phenyl)-
acetamide. To a
solution of (2S, 4R)-N-[1-(4-Amino-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-N-(4
chloro-phenyl)-acetamide (115 mg, 0.265 mmoles) in dichloromethane (5.0 mL)
was added
diisopropylethylamine (50 uL, 0.265 mmoles) followed by the addition of methyl
succinyl chloride
(44 mg, 0.291 mmoles) and was stirred at room temperature overnight. Reaction
mixture was diluted
with dichloromethane and extracted with saturated aqueous sodium bicarbonate,
brine, dried over
sodium sulfate, filtered and concentrated under reduced pressure. Product was
purified on silica gel
by flash chromatography using hexane/ethyl acetate (1:1), ethyl acetate (100%)
and ethyl
acetatelmethanol (10%) to afford (2S, 4R)-N-(4-{4-[Acetyl-(4-chloro-phenyl)-
amino]-2-methyl-3,4
290
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
dihydro-2H-quinoline-1-carbonyl}-phenyl)-succinamic acid methyl ester (55 mg,
35%).1H-NMR
(CDCl3) 8: 1.11 (3H, s; overlapping 1H, t), 2.00 (3H, s), 2.30 (1H, m), 2.60-
2.80 (2x2H, m), 3.70
(3H, s), 4.70 (1H, m), 5.55 (1H, m), 6.55 (1H, d), 6.90(1H, dd), 7.10-7.40
(9H, complex), 7.80 (1H,
br,s). ESI-MS mlz: 548 (M + 1).
[001236] To a solution of (2S, 4R)-N-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-
methyl-3,4-
dihydro-2H-quinoline-1-carbonyl}-phenyl)-succinamic acid methyl ester ( 55 mg,
0.100 mmoles) in
DMF was added sodium hydride (60% dispersion in oil). After 30 minutes,
iodomethane (16 uL', 0.11
mmoles) was added to the reaction mixture and stirred at room temperature
overnight. Reaction was
quenched with water and extracted with ethyl acetate. Combined organics were
washed with saturated
aqueous sodium bicarbonate, brine, dried over sodium sulfate, filtered and
concentrated under reduced
pressure. Product was purified on silica gel by flash chromatography using
100% ethyl acetate and
90% ethyl acetate/ 10% methanol to afford . (2S,4R)-N-(4-{4-[Acetyl-(4-chloro-
phenyl)-amino]-2-
methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenyl)-N-methyl-succinamic acid
methyl ester (16
mg, 26%). .
'H-NMR (CDCl3) S: 1.11 (3H, s; overlapping 1H, t), 2.00 (3H, s), 2.30 (1H, m,
2H, m), 2.60 (2H,
m), 3.18 (3H, s), 3.65 (3H, s), 4.80 (1H, m), 5.60 (1H, m), 6.55 (1H, d),
6.90(1H, dd), 7.00-7.40 (10H;
complex).
ESI-MS m/z: 562 (M + 1)
[001237] (2S,4R)-N-(4-Chloro-phenyl)-N-{2-methyl-1-[4-(3-pyrrol-1-yl-propoxy)-
benzoyl]-
1,2,3,4-tetrahydro-quinolin-4-yl}-acetamide (H-108)
[001238] (2S,4R)-N-(4-Chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-
tetrahydro=
quinolin-4-yl]-acetamide (75 mg, 0.17 mmol) was dissolved in DMF (1 mL) at
room temperature.
KzC03 (47 mg, 0.34 mmol) was added followed by 1-(3-bromopropyl)-pyrrole and
the reaction was
allowed to stir at 70°C for 3hrs. The reaction mixture was concentrated
in vacuo. The residue was
partitioned between ethyl acetate and water, then extracted three times with
ethyl acetate. Organic
layers were washed with brine, dried over MgS04, filtered and concentrated
down. The crude residue
was purified by silica gel chromatography (1/1 ethyl acetate/hexanes) to
afford the product.
1H-NMR (CDC13) 8: 1.1 (d, 3H), 1.2 (m, 1H), 2.0 (s, 3H), 2.1 (m, 2H), 2.3 (m,
1H), 3.8 (t, 2H), 4.1
(t, 2H), 4.8 (m, 1H), 5.6 (m, 1H), 6.1 (d, 2H), 6.5 (d, 1H), 6.6 (d, 2H), 6.65
(d, 2H), 6.9 (t, 1H), 7.1-
7.3 (m, 6H), 7.4 (d, 2H).
MS m/z: 442/444 (M+1).
[001239] (2S,4R)-2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-dihydro-
2H-
quinoline-1-carbonyl}-phenoxy)-N-isopropyl-acetamide (H-109)
291
CA 02561564 2006-09-27
WO 2005/100321 PCT/US2005/011643
[001240] (2S,4R)- 2-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenoxy)-N-isopropyl-acetamide was made from (4-{4-[Acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl}-phenoxy)-acetic acid
ethyl este (0.050g,
0.096 mmol) by addition of isopropylamine (0.680 mL, 8 mmol) with trace sodium
cyanide in
ethanol (1 mL) to give 43 % Yield of (2S,4R)- 2-(4-{4-[acetyl-(4-chloro-
phenyl)-amino]-2-methyl-
3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-N-isopropyl-acetamide after
HPLC purification.
'H-NMR (CDC13) S: 1.12-1.19 (m, 10H), 2.02 (s, 3H), 2.28 (m, 1H), 4.11 (m,
1H), 4.37 (s, 2H), 4.73
(m, 1H), 5.59 (brs, 1H), 6.24 (br, 1H), 6.48 (d, 1H), 6.70 (d, 2H), 6.90 (t,
1H), 7.12-7.30 (m, 6H), 7.37
(d, 2H).
MS m/z: 534 (M+1).
[001241] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperazine-1-carboxylic acid tert-butyl ester (H-
110)
[001242] (2S,4R)- 4-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-quinoline-
1-carbonyl}-phenyl)-piperazine-1-carboxylic acid tert-butyl ester was prepared
following the
procedure for (2S,4R)- 1-(4-{4-[Acetyl-(4-chloro-phenyl)-amino]-2-methyl-3,4-
dihydro-2H-
quinoline-1-carbonyl}-phenyl)-piperidine-4-carboxylic acid ethyl ester,
substituting piperazine-1-
carboxylic acid tert-butyl ester for piperidine-4-carboxylic acid ethyl ester
to yield the product.
'H-NMR (CDCl3) 8: 1.10 (d, 3H), 1.24 (m, 1H), 1.45 (s, 9H), 2.00 (s, 3H), 2.27
(m, 1H), 3.12 (f~ 4H),
3.52 (t, 4H), 4.71 (m, 1H), 5.56 (brs, 1H), 6.54 (d, 1H), 6.62 (d, 2H), 6.92
(t, 1H), 7.09-7.28 (m, 6H),
7.36 (d, 2H).
MS m/z: 603.4 (M+1).
[001243] (2S,4R)-N-(4-Chloro-phenyl)-N-(1-{4-[2-(1-methanesulfonyl-piperidin-4-
yl)-ethoxy]-
benzoyl}-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-acetamide (H-111)
[001244] 4-(2-Hydroxy-ethyl)-piperidine-1-carboxylic acid tert-butyl ester
(0.119 g, 0.52 mmol)
was dissolved in toluene at room temperature with PPh3 (0.136g, 0.52 mmol),
then added (2S,4R)-N-
(4-chloro-phenyl)-N-[1-(4-hydroxy-benzoyl)-2-methyl-1,2,3,4-tetrahydro-
quinolin-4-yl]-acetamide
(0.150g, 0.35 mmol) and stirred for 5 min. DEAD (0.090g, 0.52 mmol) was added
and the reaction
was stirred for 18 h at room temperature. The reaction was concentrated and
purified by silica gel
chromatography (45 % CHzCIz /55%EtOAc) to afford (2S,4R)-4-[2-(4-{4-[acetyl-(4-
chloro-phenyl)-
amino]-2-methyl-3,4-dihydro-2H-quinoline-1-carbonyl }-phenoxy)-ethyl]-
piperidine-1-carboxylic
acid tert-butyl ester (0.2 g, 90%)
[001245] The ester was convert to (2S,4R)-N-(4-chloro-phenyl)-N-{2-methyl-1-[4-
(2-piperidin-4
yl-ethoxy)-benzoyl]-1,2,3,4-tetrahydro-quinolin-4-yl }-acetamide by reacting
with HCI (4M in
dioxane) in dichloromethane at room temperature for 3 hours. The piperidine
was dissolved in
292
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 292
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 292
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE: