Note: Descriptions are shown in the official language in which they were submitted.
CA 02561622 2006-09-29
IN-LINE VESSEL SEALER AND DIVIDER
BACKGROUND
The present disclosure relates to an electrosurgical forceps and
more particularly, the present disclosure relates to an elongated endoscopic
combination bipolar and monopolar electrosurgical forceps for sealing and/or
cutting tissue.
Technical Field
Electrosurgical forceps utilize both mechanical clamping action and
electrical energy to affect hemostasis by heating tissue and blood vessels to
coagulate, cauterize and/or seal tissue. As an alternative to open forceps for
use with open surgical procedures, many modern surgeons use endoscopes and
endoscopic instruments for remotely accessing organs through smaller,
puncture-like incisions. As a direct result thereof, patients tend to benefit
from
less scarring and reduced healing time.
1
CA 02561622 2006-09-29
Endoscopic instruments are inserted into the patient through a
cannula, or port, which has been made with a trocar. Typical sizes for
cannulas
range from three millimeters to twelve millimeters. Smaller cannulas are
usually
preferred, which, as can be appreciated, ultimately presents a design
challenge
to instrument manufacturers who must find ways to make endoscopic
instruments that fit through the smaller cannulas.
Many endoscopic surgical procedures require cutting or ligating
blood vessels or vascular tissue. Due to the inherent spatial considerations
of
the surgical cavity, surgeons often have difficulty suturing vessels or
performing
other traditional methods of controlling bleeding, e.g., clamping and/or tying-
off
transected blood vessels. By utilizing an endoscopic electrosurgical forceps,
a
surgeon can either cauterize, coagulate/desiccate and/or simply reduce or slow
bleeding simply by controlling the intensity, frequency and duration of the
electrosurgical energy applied through the jaw members to the tissue. Most
small blood vessels, i.e., in the range below two millimeters in diameter, can
often be closed using standard electrosurgical instruments and techniques.
However, if a larger vessel is ligated, it may be necessary for the surgeon to
convert the endoscopic procedure into an open-surgical procedure and thereby
abandon the benefits of endoscopic surgery. Alternatively, the surgeon can
seal
the larger vessel or tissue.
It is thought that the process of coagulating vessels is
fundamentally different than electrosurgical vessel sealing. For the purposes
herein, "coagulation" is defined as a process of desiccating tissue wherein
the
2
CA 02561622 2006-09-29
tissue cells are ruptured and dried. "Vessel sealing" or "tissue sealing" is
defined
as the process of liquefying the collagen in the tissue so that it reforms
into a
fused mass. Coagulation of small vessels is sufficient to permanently close
them, while larger vessels need to be sealed to assure permanent closure.
In order to effectively seal larger vessels (or tissue) two
predominant mechanical parameters must be accurately controlled - the
pressure applied to the vessel (tissue) and the gap distance between the
electrodes or tissue sealing surfaces - both of which are affected by the
thickness of the sealed vessel. More
particularly, accurate application of
pressure is important to oppose the walls of the vessel; to reduce the tissue
impedance to a low enough value that allows enough electrosurgical energy
through the tissue; to overcome the forces of expansion during tissue heating;
and to contribute to the end tissue thickness which is an indication of a good
seal. It has been determined that a typical jaw gap for fusing vessel walls is
optimum between 0.001 and 0.006 inches. Below this range, the seal may shred
or tear and above this range the lumens may not be properly or effectively
sealed.
With respect to smaller vessels, the pressure applied to the tissue
tends to become less relevant whereas the gap distance between the
electrically
conductive surfaces becomes more significant for effective sealing. In other
words, the chances of the two electrically conductive surfaces touching during
activation increases as vessels become smaller.
3
CA 02561622 2006-09-29
Many known instruments include blade members or shearing
members which simply cut tissue in a mechanical and/or electromechanical
manner and are relatively ineffective for vessel sealing purposes. Other
instruments rely on clamping pressure alone to procure proper sealing
thickness
and are not designed to take into account gap tolerances and/or parallelism
and
flatness requirements which are parameters which, if properly controlled, can
assure a consistent and effective tissue seal. For example, it is known that
it is
difficult to adequately control thickness of the resulting sealed tissue by
controlling clamping pressure alone for either of two reasons: 1) if too much
force is applied, there is a possibility that the two poles will touch and
energy will
not be transferred through the tissue resulting in an ineffective seal; or 2)
if too
low a force is applied the tissue may pre-maturely move prior to activation
and
sealing and/or a thicker, less reliable seal may be created.
As mentioned above, in order to properly and effectively seal larger
vessels or tissue, a greater closure force between opposing jaw members is
required. It is known that a large closure force between the jaws typically
requires large actuation forces which are necessary to create a large moment
about the pivot for each jaw. This presents a design challenge for instrument
manufacturers who must weigh the advantages of manufacturing an overly-
simplified design against the disadvantages of a design that may require the
user to exert a large closure force to effectively seal tissue. As a result,
designers must compensate for these large closure forces by either designing
instruments with metal pins and/or by designing instruments which at least
4
CA 02561622 2006-09-29
partially offload these closure forces to reduce the chances of mechanical
failure
and reduce fatigue for the end user (i.e., surgeon).
Increasing the closure forces between electrodes may have other
undesirable effects, e.g., it may cause the opposing electrodes to come into
close contact with one another which may result in a short circuit and a small
closure force may cause pre-mature movement of the tissue during compression
and prior to activation. As a result thereof, providing an instrument which
consistently provides the appropriate closure force between opposing electrode
within a preferred pressure range will enhance the chances of a successful
seal.
As can be appreciated, relying on a surgeon to manually provide the
appropriate
closure force within the appropriate range on a consistent basis would be
difficult
and the resultant effectiveness and quality of the seal may vary. Moreover,
the
overall success of creating an effective tissue seal is greatly reliant upon
the
user's expertise, vision, dexterity, and experience in judging the appropriate
closure force to uniformly, consistently and effectively seal the vessel. In
other
words, the success of the seal would greatly depend upon the ultimate skill of
the surgeon rather than the efficiency of the instrument.
It has been found that the pressure range for assuring a consistent
and effective seal is between about 3 kg/cm2 to about 16 kg/cm2 and,
preferably,
within a working range of 7 kg/cm2 to 13 kg/cm2. Manufacturing an instrument
which is capable of providing a closure pressure within this working range has
been shown to be effective for sealing arteries, tissues and other vascular
bundles.
CA 02561622 2014-01-24
Various force-actuating assemblies have been developed in the
past for providing the appropriate closure forces to effect vessel sealing.
For
= example, one such actuating assembly has been developed by Valleylab,
Inc. of
Boulder, Colorado, a division of Tyco Healthcare LP, for use with Valleylab's
-vessel sealing and dividing instrument commonly sold under the trademark
LIGASURE ATLAS . This assembly includes a four-bar mechanical linkage, a
spring and a drive assembly which cooperate to consistently provide and
maintain tissue pressures within the above working ranges. Co-pending U.S.
Application Serial Nos. 10/179,863 entitled "VESSEL SEALER AND DIVIDER"
(now U.S. Patent No. 7,101,371), 10/116,944 entitled "VESSEL SEALER AND
DIVIDER" (now U.S. Patent No. 7,083,618), 10/472,295 entitled "'VESSEL
SEALER AND DIVIDER" (now U.S. Patent No. 7,101,372) and PCT Application
Serial Nos. PCT/US01/01890 entitled "VESSEL SEALER AND DIVIDER and
PCT/US01/11340 entitled "VESSEL SEALER AND DIVIDER" all describe in
detail various operating features of the LIGASURE ATLAS and various methods
relating thereto.
Other force-actuating mechanisms or assemblies are described in
commonly-owned U.S. Application Serial Nos. 10/460,926, published December 16,
2004 as U.S. Patent Publication No. 2004/0254573, entitled "VESSEL SEALER
AND DIVIDER FOR USE WITH SMALL TROCARS AND CANNULAS" and
10/953,757 published May 19, 2005 as U.S. Patent Publication No. 2005/0107785,
entitled "VESSEL SEALER AND DIVIDER HAVING ELONGATED KNIFE STROKE
AND SAFETY FOR CUTTING MECHANISM". As
6
CA 02561622 2006-09-29
described therein, simpler and more mechanically advantageous actuating and
drive assemblies are described therein which facilitate grasping and
manipulating vessels and tissue and which reduce user fatigue.
In certain surgical operations, a bipolar forceps is used in
combination with a monopolar forceps or monopolar coagulator to treat tissue
and control bleeding during the surgery. As such and during the course of a
particular operation, a surgeon may be required to substitute a monopolar
instrument for the bipolar instrument which would typically involve
substitution
through the trocar or cannula. As can be appreciated this may occur on more
than one occasion over the course of the operation which can be quite time
consuming and which may unnecessarily subject the instruments to possible
non-sterile environments.
It would be desirous to develop a small, simple and cost effelitive
combination bipolar and monopolar instrument which can be utilized with small
cannulas. Moreover,
it would be desirous to provide an instrument which
includes an easily manipulatable handle and instrument body which includes a
mechanically advantageous force-actuating assembly to reduce user fatigue.'
SUMMARY
The present disclosure relates to an endoscopic forceps having a
housing with a shaft attached thereto, the shaft including a pair of jaw
members
disposed at a distal end thereof. The forceps also includes a drive assembly
7
CA 02561622 2006-09-29
disposed in the housing which is configured to move the jaw members relative
to
one another from a first position wherein the jaw members are disposed in
spaced relation relative to one another to a second position wherein the jaw
members are closer to one another for manipulating tissue. A pair of handles
is
operatively connected to the drive assembly and the handles are configured to
move relative to the housing to actuate the drive assembly to move the jaw
members. Each jaw member is adapted to connect to a source of electrical
energy such that the jaw members are capable of conducting energy for treating
tissue.
A first switch is disposed on the housing and is activatable to
selectively deliver energy of a first electrical potential to at least one jaw
member
for treating tissue in a monopolar fashion. A second switch is disposed on the
housing and is activatable to selectively deliver energy of a first electrical
potential to one jaw member and selectively deliver energy of a second
electrical
potential to the other jaw member for treating tissue in a bipolar fashion.
In one embodiment according to the present disclosure, the forceps
also includes a knife assembly which is operatively associated with the
housing.
The knife assembly is selectively actuatable to advance a knife through tissue
disposed between the jaw members when the jaw members are disposed in the
second position. In yet another embodiment, at least one of the jaw members
may include a monopolar extension which extends beyond the insulative housing
of the jaw member to permit delicate dissection of tissue.
8
CA 02561622 2006-09-29
In one particularly useful embodiment, at least one of the handles
includes a knife lockout which prevents the knife assembly from being actuated
when the jaw members are disposed in the second position. The knife lockout
mechanism may include a mechanical interface extending from at least one of
the handles. The mechanical interface is dimensioned to impede movement of
the knife assembly when the handles are disposed in a first (i.e., open)
position
relative to the housing and the mechanical interface is dimensioned to permit
actuation of the knife assembly when the handles are disposed in a second
position relative to the housing.
In another embodiment according to the present disclosure, the
forceps includes a monopolar lockout which prevents activation of the first
switch
when the jaw members are disposed in the first position. In one particularly
useful embodiment, the monopolar lockout includes a mechanical interface
disposed on at least one of the handles which prevents activation of the first
switch when the handles are disposed in a first position relative to the
housing
and permits activation of the first switch when the handles are disposed in a
second position relative to the housing. The monopolar lockout may include a
pressure activated switch disposed in the housing such that movement of the
handles from a first position relative to the housing to a second position
relative
to the housing closes the pressure activated switch to allow activation of the
first
switch.
In still yet another embodiment according to the present disclosure,
the handles of the forceps are disposed on opposite sides of the housing and
are
9
CA 02561622 2006-09-29
movable from a first, spaced position relative to the housing to a second
closer
position relative to the housing. The housing may also be configured to
include
a pair of slits defined on opposite sides of the housing and the handles may
be
dimensioned to move relative to the housing within the slits. In one
particularly
useful embodiment, the housing includes a longitudinal axis defined
therethrough and the handles are disposed at an angle "a" relative to the
longitudinal axis to facilitate handling.
In yet another embodiment according to the present disclosure, an
intensity controller is included which regulates the intensity of
electrosurgical
energy to the forceps during activation. In a particularly useful embodiment,
the
intensity controller is a slide potentiometer and is operable only in a
monopolar
mode.
In still another embodiment, the forceps may include an electrical
safety which regulates the forceps to operating in either a bipolar fashion or
a
monopolar fashion during any given time. In a particularly useful embodiment,
the first switch and the second switch are independently and exclusively
activatable relative to one another.
The present disclosure also relates to an electrosurgical system
having an electrosurgical generator and an endoscopic forceps. The forceps
includes a housing having a shaft attached thereto with a pair of jaw members
disposed at a distal end thereof. The jaw members are adapted to connect to
the electrosurgical generator. The forceps also includes a drive assembly
CA 02561622 2006-09-29
disposed in the housing which moves the jaw members relative to one another
from a first position wherein the jaw members are disposed in spaced relation
relative to one another to a second position wherein the jaw members are
closer
to one another for manipulating tissue. A pair of handles is operatively
connected to the drive assembly to actuate the drive assembly to move the jaw
members.
A first switch is disposed on the housing and is activatable to
selectively deliver energy of a first electrical potential to at least one jaw
member
for treating tissue in a monopolar fashion. A second switch is disposed on the
housing and is activatable to selectively deliver energy of a first electrical
potential to one jaw member and selectively deliver energy of a second
electrical
potential to the other jaw member for treating tissue in a bipolar fashion.
In one embodiment, the generator includes a control circuit having
a safety circuit which permits independent and exclusive activation of the
forceps
in either a bipolar or monopolar fashion. The safety circuit may be electrical
or
electro-mechanical and activated upon movement to the pair of handles relative
to the housing. The generator may also include a control circuit having an
isolation circuit operably connected to the second switch which regulates the
energy to the jaw members while bypassing the second switch to protect the
integrity of the second switch from current overload.
11
CA 02561622 2006-09-29
BRIEF DESCRIPTION OF THE DRAWINGS
Various embodiments of the subject instrument are described
herein with reference to the drawings wherein:
Fig. 1A is a top, perspective view of an endoscopic forceps shown
in an open configuration and including a housing, a handle assembly, a shaft
and an end effector assembly according to the present disclosure;
Fig. 1B is a top, perspective view of the endoscopic forceps of Fig.
1A showing the end effector assembly in a closed configuration according to
the
present disclosure;
Fig. 2 is a bottom, perspective view of the endoscopic forceps of
Fig. 1A;
Fig. 3A is an enlarged left, perspective view of the end effector
assembly of Fig. 1A;
Fig. 3B is an enlarged left, perspective view of the end effector
assembly of Fig. 1B;
Fig. 3C is an enlarged side view of the end effector assembly of
Fig. 1A;
12
CA 02561622 2006-09-29
Fig. 3D is an enlarged end view of the end effector assembly of
Fig. 1A;
Fig. 4 is a top, internal perspective view of the forceps of Fig. 1A
shown without a housing cover;
Fig. 5A is an enlarged, top view of the forceps of Fig. 1A showing
the disposition of the internal components when the forceps is in an open
configuration;
Fig. 5B is an enlarged, top view of the forceps of Fig. 1B showing
the disposition of the internal components when the forceps is in a closed
configuration;
Fig. 6A is an enlarged perspective view of the internal working
components of the forceps of Fig. 1B showing a knife actuator in an un-
actuated
position;
Fig. 6B is an enlarged perspective view of the internal working
components of the forceps of Fig. 1B showing a knife actuator being actuated;
Fig. 7 is an enlarged side view of the knife actuator in an un-
actuated position;
13
CA 02561622 2006-09-29
Fig. 8A is a greatly-enlarged, top cross sectional view of an end
effector of the end effector assembly showing a knife of the knife actuator in
a
proximal-most or unactuated position;
Fig. 8B is a greatly-enlarged, top cross sectional view of the end
effector assembly of Fig. 8A showing the position of the knife after
actuation;
Fig. 9A is an enlarged, top view showing the handle assembly in an
unactuated position;
Fig. 9B is an enlarged, top view showing the handle assembly after
actuation;
Fig. 10A is a greatly-enlarged, side cross sectional view of the end
effector assembly shown in an open configuration;
Fig. 10B is a greatly-enlarged, side cross sectional view of the end
effector assembly shown in a closed configuration;
Fig. 10C is a greatly-enlarged, front perspective view of a bottom
jaw member of the end effector assembly showing the knife of the knife
actuator
in a proximal-most or unactuated position;
Fig. 10D is a greatly-enlarged, front perspective view of the bottom
jaw member of Fig. 10C showing the position of the knife after actuation;
14
..
CA 02561622 2006-09-29
Fig. 11A is an enlarged, top view similar to Fig. 9B showing the
knife actuator after actuation;
Fig. 11B is a greatly-enlarged, side cross sectional view of the end
effector assembly showing the position of the knife after actuation;
Fig. 12 is top, perspective view of the forceps of Fig. 1B showing
rotation of the end effector assembly;
Fig. 13 is a top, perspective view of the forceps with parts
separated;
Fig. 14 is an enlarged, perspective view of the housing with parts
separated;
Fig. 15A is a greatly-enlarged, perspective view of the bottom jaw
of the end effector assembly with parts separated;
Fig. 15B is a greatly-enlarged, perspective view of the top jaw of
the end effector assembly with parts separated;
Fig. 16 is an enlarged, perspective view of a circuit board for use
with the forceps according to the present disclosure;
CA 02561622 2006-09-29
Fig. 17 is a greatly-enlarged, perspective view of the elongated
shaft for housing various moving parts of the drive assembly and knife
assembly;
Fig. 18 is a top, perspective view of an alternate safety lockout
mechanism for use with the forceps of Fig. 1A;
Fig. 19 is a top view of a flex circuit board for use with the forceps
of Fig. 1A;
Fig. 20 is a schematic diagram showing the operational features of
a safety switch of the flex circuit board of Fig. 19;
Fig. 21 is an internal perspective view showing the assembly of the
safety switch of Fig. 19 in the housing of the forceps;
Fig. 22A-22C are internal views showing the operational
movements of the safety lockout mechanism of Fig. 18 as the lockout
mechanism engages the safety switch of the flex circuit board; and
Fig. 23 is a schematic electrical diagram of the electrical switching
assembly.
16
CA 02561622 2006-09-29
DETAILED DESCRIPTION
Turning now to Figs. 1A-2, one embodiment of a combination
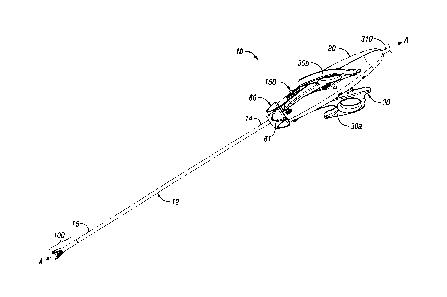
endoscopic bipolar and monopolar forceps 10 is shown for use with various
surgical procedures and generally includes a housing 20, a handle assembly 30,
a rotating assembly 80, a knife trigger assembly 70 and an end effector
assembly 100 which mutually cooperate to grasp, seal and divide tubular
vessels
and vascular tissue (Figs. 10A and 10B). Although the majority of the figure
drawings depict a forceps 10 for use in connection with endoscopic surgical
procedures, the present disclosure may be used for more traditional open
surgical procedures. For the purposes herein, the forceps 10 is described in
terms of an endoscopic instrument; however, it is contemplated that an open
version of the forceps may also include the same or similar operating
components and features as described below.
Forceps 10 includes a shaft 12 which has a distal end 16
dimensioned to mechanically engage the end effector assembly 100 and a
proximal end 14 which mechanically engages the housing 20. Details of how the
shaft 12 connects to the end effector are described in more detail below. The
proximal end 14 of shaft 12 is received within the housing 20 and the
connections relating thereto are also described in detail below. In the
drawings
and in the descriptions which follow, the term "proximal, as is traditional,
will
refer to the end of the forceps 10 which is closer to the user, while the term
"distal" will refer to the end which is further from the user.
17
CA 02561622 2006-09-29
Forceps 10 also includes an electrosurgical cable 310 which
connects the forceps 10 to a source of electrosurgical energy, e.g., a
generator
500 (See Fig. 16). Generators such as those sold by Valleylab - a division of
Tyco Healthcare LP, located in Boulder Colorado may be used as a source of
both bipolar electrosurgical energy for sealing vessel and vascular tissues as
well as monopolar electrosurgical energy which is typically employed to
coagulate or cauterize tissue. It is
envisioned that the generator 500 may
include various safety and performance features including isolated output,
impedance control and/or independent activation of accessories. The
electrosurgical generator 500 may also be configured to include Valleylab's
Instant ResponseTM technology which provides an advanced feedback system to
sense changes in tissue two-hundred (200) times per second and adjust voltage
and current to maintain appropriate power. The Instant ResponseTM technology
is believed to provide one or more of the following benefits to surgical
procedure:
= Consistent clinical effect through all tissue types;
= Reduced thermal spread and risk of collateral tissue damage;
= Less need to "turn up the generator"; and
= Designed for the minimally invasive environment.
As best show in Fig. 16, cable 310 is divided into cable leads 310a
and 310b which are configured to connect the forceps to the electrosurgical
generator 500 by virtue of one or more connectors or by virtue of separate so-
called "flying leads" which are configured to connect to the generator 500 at
a
18
CA 02561622 2014-01-24
single location and provide either bipolar, monopolar (or a combination
thereof)
energy as desired or based upon the particular instrument configuration set up
by the surgeon prior to surgery. One example of a universal electrical
connector
is being currently developed by Valleylab, Inc. of Boulder, Colorado a
division of
Tyco Healthcare, LP and is the subject of U.S. Patent Application Serial No.
10/718,114, published May 26, 2005 as U.S. Patent Publication No.
2005/0113818, entitled "CONNECTOR SYSTEMS FOR
ELECTROSURGICAL GENERATOR".
Handle assembly 30 includes two movable handles 30a and 30b
disposed on opposite sides of housing 20. Handles 30a and 30b are movable
relative to one another to actuate the end effector assembly 100 as explained
in
more detail below with respect to the operation of the forceps 10.
As best seen in the exploded view of Fig. 13, housing 20 is formed
from two (2) housing halves 20a and 20b which each include a plurality of
interfaces 205 which are dimensioned to mechanically align and engage one
another to form housing 20 and enclose the internal working components of
forceps 10. It is envisioned that a plurality of additional interfaces (not
shown)
may disposed at various points around the periphery of housing halves 20a and
20b for ultrasonic welding purposes, e.g., energy direction/deflection points.
It is
also contemplated that housing halves 20a and 20b (as well as the other
components described below) may be assembled together in any fashion known
in the art. For example, alignment pins, snap-like interfaces, tongue and
groove
interfaces, locking tabs, adhesive ports, etc. may all be utilized either
alone or in
combination for assembly purposes.
19 =
CA 02561622 2006-09-29
Rotating assembly 80 is mechanically coupled to housing 20 and is
rotatable approximately 90 degrees in either direction about a longitudinal
axis
"A" (See Figs. 1A-2 and 12). Details of the rotating assembly 80 are described
in
more detail with respect to Figs. 12-14. Rotating assembly 80 includes two
halves 81a and 81b which, when assembled, form the rotating assembly 80
which, in turn, supports the elongated shaft 12 which houses drive assembly 60
and the knife assembly 70. Halves 81a and 81b are mechanically engaged to
housing 20 atop flanges 82a and 82b, respectively, during assembly and may
include other mechanical interfaces dimensioned to securely engage the two
halves 81a and 81b of the rotating assembly 80, e.g., alignment pins, snap-fit
interfaces, ultrasonic welding points, etc.
As mentioned above, end effector assembly 100 is attached at the
distal end 16 of shaft 12 and includes a pair of opposing jaw members 110 and
120 (See Figs. 3A-3D). Handles 30a and 30b of handle assembly 30 ultimately
connect to drive assembly 60 which, together, mechanically cooperate to impart
movement of the jaw members 110 and 120 from an open position wherein the
jaw members 110 and 120 are disposed in spaced relation relative to one
another, to a clamping or closed position wherein the jaw members 110 and 120
cooperate to grasp tissue (Figs. 10A and 10B) therebetween.
It is envisioned that the forceps 10 may be designed such that it is
fully or partially disposable depending upon a particular purpose or to
achieve a
particular result. For example, end effector assembly 100 may be selectively
CA 02561622 2006-09-29
and releasably engageable with the distal end 16 of the shaft 12 and/or the
proximal end 14 of shaft 12 may be selectively and releasably engageable with
the housing 20 and the handle assembly 30. In either of these two instances,
the forceps 10 would be considered "partially disposable" or "reposable",
i.e., a
new or different end effector assembly 100 (or end effector assembly 100 and
shaft 12) selectively replaces the old end effector assembly 100 as needed. As
can be appreciated, the presently disclosed electrical connections may have to
be altered to modify the instrument to a reposable forceps.
Turning now to the more detailed features of the present disclosure
as described with respect to Figs. 1A - 16, handles 30a and 30b each include
an
aperture 33a and 33b, respectively, defined therein which enables a user to
grasp and move each respective handle 30a and 30b relative to one another.
Handles 30a and 30b also include ergonomically-enhanced gripping elements
39a and 39b, respectively, disposed along an outer edge thereof which are
designed to facilitate gripping of the handles 30a and 30b during activation.
It
is envisioned that gripping elements 39a and 39b may include one or more
protuberances, scallops and/or ribs to enhance gripping.
As best illustrated in Figs. 1A and 7, handles 30a and 30b are
configured to extend outwardly on opposite sides from a transverse axis "B"
defined through housing 20 which is perpendicular to longitudinal axis "A".
Handles 30a and 30b are movable relative to one another in a direction
parallel
to axis "B" to open and close the jaw members 110 and 120 as needed during
surgery. This forceps style is commonly referred to as an "in-line" or
hemostat
21
CA 02561622 2006-09-29
style forceps as compared to a so-called "pistol grip" style forceps or
endoscopic
instrument. In-line hemostats or forceps are more commonly manufactured for
open surgical procedures and typically include a pair of shafts having
integrally
coupled handles which are movable relative to one another to open and close
the jaw members disposed at the distal end thereof.
As best illustrated in Fig. 5A and as mentioned above, handles 30a
and 30b mechanically couple to the housing 20 and are movable relative to the
housing (and each other) to affect movement of the jaw members 110 and 120
from the open or spaced configuration to a closed position about tissue. Each
handle, e.g., handle 30a shown in Fig. 7, is also configured to extend
downwardly at an angle alpha (a) relative to the longitudinal axis "A". It is
envisioned that manufacturing the handles 30a and 30b to extend in this
fashion
facilitates and enhances gripping and manipulation of the forceps 10 during
operating conditions. It is envisioned that the angle (a) of the handles 30a
and
30b of forceps 10 may be adjustable to allow different users to essentially
"customize" the handles 30a and 30b for a particular use of for a particular
hand
size. Alternatively, different forceps 10 may be manufactured with different
pre-
fixed angles (a) for use with specific surgical procedures, for particular
hand
sizes (i.e., small, medium and large) and/or for other surgical purposes. It
is
further contemplated that in a particularly useful embodiment, the angle (a)
of the
handle ranges from about zero degrees (0 ) degrees to about thirty-five
degrees
(350).
22
CA 02561622 2006-09-29
As best seen in Figs. 5A, 5B, 13 and 14, the distal end 34 and 37
of each handle 30a and 30b, respectively, is selectively moveable about pivot
pins 34a and 34b attached to a distal end 21 of the housing 20. As explained
in more detail below, movement of the handles relative to one another imparts
movement of the jaw members 110 and 120 relative to one another. The distal
ends 34 and 37 are configured to include complimentary gear teeth 34a' and
34b' which are configured to intermesh with one another to facilitate
consistent
movement of the handle members 30a and 30b relative to one another and to
enhance actuation of the jaw members 110 and 120.
In Fig. 14, the proximal end 30a' and 30b' of the each handle 30a
and 30b, respectively, includes a flange 31a and 31b which extends from the
proximal end 30a' and 30b' of each handle 30a and 30b towards the housing 20.
Each of the flanges 31a and 31b includes an aperture 36c' and 36d' disposed
therein for receiving an end 36c and 36d of a toggle link 35a and 35b,
respectively. The opposite ends 36a and 36b of the toggle links 35a and 35b
are
configured to attached to an actuating or drive collar 69 of the drive
assembly 60
through corresponding apertures 36a' and 36b' defined therethrough. It is
envisioned that the toggle links 35a and 35b may be dimensioned in a generally
S-shaped configuration to attach the handles 30a and 30b to the drive collar
69
or the toggle links 35a and 35b may be generally U-shaped (as disclosed) to
accomplish this purpose. It is contemplated that dimensioning the toggle links
35a and 35b in a U-shaped configuration may reduce buckling during actuation.
23
CA 02561622 2006-09-29
As can be appreciated, movement of the handles 30a and 30b from
an open or spaced apart configuration to a closed position towards the housing
forces the actuating collar 69 proximally against a spring 63 which, in turn,
translates a drive shaft 17 proximally to close the jaw members 110 and 120
(see Figs. 7-9). The operative relationship of the drive collar 69 and the
handle
assembly 30 is explained in detail below with respect to the operation of the
forceps 10.
The handles 30a and 30b force the toggle links 35a and 35b to
rotate along the longitudinal axis "A" beyond a parallel orientation with
shaft 17
or longitudinal axis "A" such that, upon release, the force of spring 63
maintains
the toggle links 35a and 35b in an over center or an over-extended (or past
parallel) configuration thereby locking the handles 30a and 30b (and therefore
the jaw members 110 and 120) relative to one another (Fig. 9B). Movement of
the handles 30a and 30b away from one another (and the housing 20) unlocks
and opens the handles 30a and 30b and, in turn, the jaw members 110 and 120
for subsequent grasping or re-grasping of tissue. In one
embodiment, the
handles 30a and 30b may be biased in an open configuration to facilitate
handling and manipulation of the forceps within an operative field. Various
spring-like mechanisms are contemplated which may be utilized to accomplish
this purpose.
Handle 30a also includes a locking flange 32 which is disposed
between the distal and proximal ends 34a' and 30a', respectively, which
extends
towards the housing 20 and moves relative thereto when handle 30a is actuated.
24
CA 02561622 2006-09-29
Locking flange 32 includes a lockout element 32' (Fig. 14) which is
dimensioned
to prevent actuation of the knife assembly 70 when handle 30a is disposed in a
spaced-apart or open configuration. Actuation or movement of the handle 30a
towards the housing 20 disengages the lockout element 32 to allow movement of
the knife assembly 70 (e.g., collar 74) to separate tissue as explained in
more
detail below.
Movable handles 30a and 30b are designed to provide a distinct
lever-like mechanical advantage over conventional handle assemblies due to the
unique position of the toggle links 35a and 35b which, when actuated, rotate
along the longitudinal axis "A" to displace the actuation or drive collar 69.
In
other words, it is envisioned that enhanced mechanical advantage for actuating
the jaw members 110 and 120 is gained by virtue of the unique position and
combination of several inter-cooperating elements (i.e., opposing handles 30a,
30b, toggle links 35a, 35b and gear teeth located at the distal ends 34 and 37
of
the handle members 30a, 30b, respectively) which reduce the overall user
forces
necessary to obtain and maintain the jaw members 110 and 120 under ideal
operating pressures of about 3 kg/cm2 to about 16 kg/cm2. In other words, it
is
envisioned that the combination of these elements and their positions relative
to
one another enables the user to gain lever-like mechanical advantage to
actuate
the jaw members 110 and 120 enabling the user to close the jaw member's 110
and 120 with lesser force while still generating the required forces necessary
to
effect a proper and effective tissue seal. The details relating to the various
movements of the above-identified elements are explained below with respect to
the operation of the forceps 10.
CA 02561622 2006-09-29
As shown best in Figs. 3A-3D, 10A-10D and 15A-15D, the end
effector assembly 100 includes opposing jaw members 110 and 120 which
cooperate to effectively grasp tissue for sealing purposes. The end effector
assembly 100 is designed as a bilateral assembly, i.e., both jaw members 110
and 120 pivot relative to one another about a pivot pin 185 disposed
thereth rough.
A reciprocating drive sleeve 17 (See Fig. 17) is slidingly disposed
within the shaft 12 and is remotely operable by the drive assembly 60 as
explained in more detail below. Drive sleeve 17 includes a bifurcated distal
end
composed of halves 17a and 17b, respectively, which define a cavity 17'
therebetween for receiving jaw members 110 and 120. More particularly and as
best illustrated in Figs. 15A and 15B, jaw members 110 and 120 include
proximal
flanges 113 and 123 (See Figs. 15A and 15B), respectively, which each include
an elongated angled slot 181a and 181b, respectively, defined therethrough. A
drive pin 180 (See Figs. 10A and 10B) mounts jaw members 110 and 120 to the
end of a rotating shaft 18 and within cavity 17' disposed at the distal ends
17a
and 17b of drive sleeve 17.
Upon actuation of the drive assembly 60, the drive sleeve 17
reciprocates which, in turn, causes the drive pin 180 to ride within slots
181a and
181b to open and close the jaw members 110 and 120 as desired. The jaw
members 110 and 120, in turn, pivot about pivot pin 185 disposed through
respective pivot holes 186a and 186b disposed within flanges 113 and 123.
26
CA 02561622 2006-09-29
As can be appreciated, squeezing handles 30a and 30b toward the housing 20
pulls drive sleeve 17 and drive pin 180 proximally to close the jaw members
110
and 120 about tissue 420 grasped therebetween and pushing the sleeve 17
distally opens the jaw members 110 and 120 for grasping purposes.
As best shown in Fig. 15B, jaw member 110 also includes a
support base 119 which extends distally from flange 113 and which is
dimensioned to support an insulative plate 119' thereon. lnsulative plate
119', in
turn, is configured to support an electrically conductive tissue engaging
surface
or sealing plate 112 thereon. It is contemplated that the sealing plate 112
may
be affixed atop the insulative plate 119' and support base 119 in any known
manner in the art, snap-fit, over-molding, stamping, ultrasonically welded,
etc.
Support base 119 together with the insulative plate 119' and electrically
conductive tissue engaging surface 112 are encapsulated by an outer insulative
housing 114. Outer housing 114 includes a cavity 114a which is dimensioned to
securely engage the electrically conductive sealing surface 112 as well as the
support base 119 and insulative plate 119'. This may be accomplished by
stamping, by overmolding, by overmolding a stamped electrically conductive
sealing plate and/or by overmolding a metal injection molded seal plate. All
of
these manufacturing techniques produce jaw member 110 having an electrically
conductive surface 112 which is substantially surrounded by an insulating
substrate 114.
For example and as shown in Fig. 15B, the electrically conductive
sealing plate 112 includes a mating portion 112a which surrounds the periphery
27
CA 02561622 2014-01-24
of the sealing plate 112. Flange 112a is designed to matingly engage an inner
lip 117 of the outer insulator 114. It is envisioned that lead 325a extending
from
circuit board 170 or generator 500 (See Fig. 16) terminates within the outer
insulator 114 and is designed to electro-mechanically couple to the sealing
plate
112 by virtue of a crimp-like connection 326a. For example, the insulator
119',
electrically conductive sealing surface 112 and the outer, non-conductive jaw
housing 114 are preferably dimensioned to limit and/or reduce many of the
known undesirable effects related to tissue sealing, e.g., flashover, thermal
spread and stray current dissipation.
It is envisioned that the electrically conductive sealing surface 112
may also include an outer peripheral edge which has a pre-defined radius and
the outer housing, 114 meets the electrically conductive sealing surface 112
along an adjoining edge of the sealing surface 112 in a generally tangential
position. At the interface, the electrically conductive surface 112 is raised
relative to the outer housing 114. These and other envisioned embodiments are
discussed in co-pending, commonly assigned Application Serial No.
PCT/US01/11412 entitled "ELECTROSURGICAL INSTRUMENT WHICH
REDUCES COLLATERAL DAMAGE TO ADJACENT TISSUE" by Johnson et al.
and co-pending, commonly assigned Application Serial No. PCT/US01/11411
entitled "ELECTROSURGICAL INSTRUMENT WHICH IS DESIGNED TO
REDUCE THE INCIDENCE OF FLASHOVER" by Johnson et al.
28
CA 02561622 2006-09-29
The electrically conductive surface or sealing plate 112 and the
outer housing 114, when assembled, form a longitudinally-oriented slot 115a
defined therethrough for reciprocation of the knife blade 190. It is
envisioned
that the knife channel 115a cooperates with a corresponding knife channel 115b
defined in jaw member 120 to facilitate longitudinal extension of the knife
blade
190 along a preferred cutting plane to effectively and accurately separate the
tissue along the formed tissue seal. As best illustrated in Figs. 8A, 8B, 15A
and
15B, knife channel 115 runs through the center of the jaw members 110 and
120, respectively, such that a blade 190 from the knife assembly 70 can cut
the
tissue grasped between the jaw members 110 and 120 when the jaw members
110 and 120 are in a closed position. More particularly and as mentioned above
with respect to the discussion of the handle assembly 30, handle 30a includes
a
lockout flange which prevents actuation of the knife assembly 70 when the
handle 30a is open thus preventing accidental or premature activation of the
blade 190 through the tissue.
As explained above and as illustrated in Figs. 15A and 15B, the
knife channel 115 is formed when the jaw members 110 and 120 are closed. In
other words, the knife channel 115 includes two knife channel halves - knife
channel half 115a disposed in sealing plate 112 of jaw member 110 and knife
channel half 115b disposed sealing plate 122 of jaw member 120. It is
envisioned that the knife channel 115 may be configured as a straight slot
with
no degree of curvature which, in turn, causes the knife 190 to move through
the
tissue in a substantially straight fashion. Alternatively, the knife channel
115 may
be dimensioned to include some degree of curvature to cause the knife 190 to
29
CA 02561622 2014-01-24
move through tissue in a curved fashion. Insulating plate 119' also forms part
of
the knife channel 115 and includes a channel 115a' defined therein which
extends along insulating plate 119' and which aligns in vertical registration
with
knife channel half 115a to facilitate translation of distal end 192 of the
knife 190
thereth rough.
The electrically conductive sealing plate 112 of jaw member 110
also includes a monopolar extension 112a which allows a surgeon to selectively
coagulate tissue when disposed in a monopolar activation mode as explained in
more detail below with respect to the operation of the forceps 10. Monopolar
extension 112a is preferably integrally associated with conductive sealing
plate
112 but may also be selectively extendible depending upon a particular
purpose.
The shape and dimension of the monopolar extension 112a may be
dimensioned to match the overall contour of the curving contour of the jaw
member 110 or the jaw housing 114. The edges of the monopolar extension
112a may be dimensioned to include radii specifically dimensioned to reduce
current density along the edges thereof, e.g., smooth curves and transition
points. The thickness of the monopolar extension 112a is preferably within a
range of about 0.010 inches +/-.005 inches. The width of the monopolar
extension 112a is preferably about 0.084 inches +/-.010 inches to permit the
creation of an enterotomy that the jaw member(s) may pass therethrough for the
purposes of mechanically spreading tissue. The length is preferably about
0.040
inches +/-.010 inches. Commonly-owned U.S. Application Serial No. 10/970,307,
published May 26, 2005 as U.S. Patent Publication No. 2005/0113827,
entitled "BIPOLAR FORCEPS HAVING MONOPOLAR EXTENSION" and
U.S. Application Serial No. 10/988,950 published June 23, 2005 as U.S.
Patent Publication No. 2005/0137590 entitled "BIPOLAR FORCEPS
HAVING
CA 02561622 2014-01-24
MONOPOLAR EXTENSION" disclose various embodiments of a monopolar
extension which may be configured for use with forceps 10 of the present
disclosure.
Jaw member 120 includes similar elements to jaw member 110
such as jaw housing 124 which encapsulates a support plate 129, an insulator
plate 129 and an electrically conductive sealing surface 122. Likewise, the
electrically conductive surface 122 and the insulator plate 129', when
assembled,
include respective longitudinally-oriented knife channels 115a and 115a'
defined
therethrough for reciprocation of the knife blade 190. As mentioned above,
when the jaw members 110 and 120 are closed about tissue, knife channels
115a and 115b form a complete knife channel 115 to allow longitudinal
extension
of the knife 190 in a distal fashion to sever tissue along a tissue seal. It
is also
envisioned that the knife channel 115 may be completely disposed in one of the
two jaw members, e.g., jaw member 120, depending upon a particular purpose.
It is also envisioned that jaw member 120 may be assembled in a similar manner
as described above with respect to jaw member 110.
As best seen in Fig. 15A, jaw member 120 includes a series of stop
members 90 disposed on the inner facing surface of the electrically conductive
sealing surface 122 to facilitate gripping and manipulation of tissue and to
define
a gap "G" (Fig. 10B) between opposing jaw members 110 and 120 during sealing
and cutting of tissue. It is envisioned that the series of stop members 90 may
be
employed on one or both jaw members 110 and 120 depending upon a particular
31
CA 02561622 2014-01-24
purpose or to achieve a desired result. A detailed discussion of these and
other
envisioned stop members 90 as well as various manufacturing and assembling
processes for attaching and/or affixing the stop members 90 to the
electrically
conductive sealing surfaces 112, 122 are described in commonly-assigned, co-
pending U.S. Application Serial No. PCT/US01/11413 entitled "VESSEL
=
SEALER AND DIVIDER WITH NON-CONDUCTIVE STOP MEMBERS" by
Dycus et al.
Jaw member 120 is connected to a second electrical lead 325b
extending from circuit board 170 or generator 500 (See Fig. 16) which
terminates
within the outer insulator 124 and is designed to electro-mechanically couple
to
the sealing plate 122 by virtue of a crimp-like connection 326b. As explained
in
more detail below, leads 325a and 325b allow a user to selectively supply
either
bipolar or monopolar electrosurgical energy to the jaw members 110 and 120 as
needed during surgery.
Jaw members 110 and 120 are electrically isolated from one
another such that electrosurgical energy can be effectively transferred
through
the tissue to form a tissue seal. For example and as best illustrated in Figs.
15A
and 15B, each jaw member, e.g., 110, includes a uniquely-designed
electrosurgical cable path disposed therethrough which transmits
electrosurgical
energy to the electrically conductive sealing surface 112. Cable lead 325a is
held loosely but securely along the cable path to permit rotation of the jaw
members 110 and 120. As can be appreciated, this isolates electrically
conductive sealing surface 112 from the remaining operative components of the
32
CA 02561622 2006-09-29
end effector assembly 100, jaw member 120 and shaft 12. The two electrical
potentials are isolated from one another by virtue of the insulative sheathing
surrounding the cable leads 325a and 325b.
As mentioned above, jaw members 110 and 120 are engaged to
the end of rotating shaft 18 by pivot pin 185 such that rotation the rotating
assembly 80 correspondingly rotates shaft 18 (along with sleeve 17 and knife
drive rod 71) which, in turn, rotates end effector assembly 100 (See Fig. 12).
More particularly, the distal end of rotating shaft 18 is bifurcated to
include ends
18a and 18b which define a channel therein for receiving jaw members 110 and
120. Pivot pin 185 secures the jaw members 110 and 120 to ends 18a and 18b
through aperture 186a and 186b defined through jaw members 110 and 120,
respectively. As best seen in Figs. 13 and 17, rotating shaft 18 is
dimensioned
to slidingly receive knife drive rod 71, knife 190 and a knife guide 197
therein.
Rotating shaft 18, in turn, is rotatingly received within drive sleeve 17
which as
mentioned above connects to the drive assembly 60. The details with respect to
the knife assembly are explained in more detail with respect to Figs. 5A, 5B,
6A,
6B, 7, 8A and 8B.
Rotating shaft 18 and drive shaft 17 are fixed to the rotating
assembly 80 by two rotating tabs which are engaged through slot 18c in the
rotating shaft 18 such that rotating of the rotating member correspondingly
rotates the rotating shaft 18. It is envisioned that the drive shaft and the
rotating
shaft may be affixed to the rotating assembly in other ways known in the art,
snap-fit, friction fit, etc.
33
CA 02561622 2006-09-29
Figs. 13 and 14 show the details of the forceps 10 and the
component features thereof, namely, the housing 20, the drive assembly 60, the
rotating assembly 80, the knife assembly 70 and the handle assembly 30. More
particularly, Fig. 13 shows the entire forceps 10 along with the above-
identified
assemblies and components thereof in an exploded condition and Fig. 14 shows
an exploded view of the housing 20 and the components contained therein.
Housing 20 includes housing halves 20a and 20b which, when
mated, form housing 20. As can be appreciated, housing 20, once formed,
forms an internal cavity 25 which houses the various assemblies identified
above
which will enable a user to selectively manipulate, grasp, seal and sever
tissue in
a simple, effective, and efficient manner. Each half of the housing, e.g.,
half
20b, includes a series of mechanical interfacing components, e.g., 205 which
align and/or mate with a corresponding series of mechanical interfaces (not
shown) to align the two housing halves 20a and 20b about the inner components
and assemblies. The housing halves 20a and 20b may then be sonic welded or
otherwise matingly engaged to secure the housing halves 20a and 20b once
assembled.
As mentioned above, the handle assembly 30 includes two
movable handles 30a and 30b which each cooperate with a toggle link 35a and
35b, respectively, to actuate the actuating or drive collar 69 of the drive
assembly
60. The drive collar, in turn, reciprocates drive sleeve 17 to open and close
the
jaw members 110 and 120 as described above. Movable handles 30a and 30b
34
CA 02561622 2006-09-29
are designed to provide a distinct lever-like mechanical advantage over
conventional handle assemblies due to the unique position of the toggle links
35a and 35b which, when actuated, rotate along the longitudinal axis "A" to
displace the actuation collar 69. More particularly and as mentioned above, it
is
envisioned that enhanced lever-like mechanical advantage for actuating the jaw
members 110 and 120 is gained by virtue of the unique position and combination
of various inter-cooperating elements such as the toggle links 35a and 35b and
the gear teeth 34a and 34b at the distal end of the handles 30a and 30b which
cooperate to reduce the overall user forces necessary to obtain and maintain
the
jaw members under ideal operating pressures of about 3 kg/cm2 to about 16
kg/cm2.
As mentioned above, movement of the handles 30a and 30b from
an open or spaced apart configuration to a closed position towards the housing
20 forces the actuating collar 69 proximally against spring 63 which, in turn,
translates drive sleeve 17 proximally to close the jaw members 110 and 120.
Moreover, as the handles 30a and 30b rotate to a closed position, the handles
30a and 30b force the toggle links 35a and 35b to rotate along the
longitudinal
axis "A" beyond a parallel orientation with longitudinal axis "A" such that
upon
release of the handles 30a and 30b from a closed position, the force of spring
63
maintains the toggle links 35a and 35b in an over-extended\over-centered
(i.e.,
past parallel) configuration thereby locking the handles 30a and 30b (and
therefore the jaw members 110 and 120) relative to one another (See Figs. 9A
and 9B). To unlock the jaw members 110 and 120, the handles 30a and 30b are
moved away from one another (and the housing 20) to return the toggle links
i
CA 02561622 2006-09-29
35a and 35b to at least a parallel orientation with respect to longitudinal
axis "A"
which unlocks and opens the handles 30a and 30b and, in turn, the jaw
members 110 and 120 for subsequent grasping or re-grasping of tissue. Once
the handles 30a and 30b are opened past parallel the force of spring 63
facilitates opening of the handles 30a and 30b and the jaw members 110 and
120.
As mentioned above, handle 30a also includes a locking flange 32
which is dimensioned to prevent actuation of the knife assembly 70 when handle
30a is disposed in a spaced-apart or open configuration. Actuation or movement
of the handle 30a towards the housing 20 disengages the lockout element 32 to
allow movement of the knife assembly 70 to separate tissue as explained in
more detail below.
As best seen in Fig. 14, the drive assembly includes drive collar 69,
spring 63 and locking sleeve 62. Toggle links 35a and 35b operatively connect
the drive collar 69 to the handles 30a and 30b, respectively. The locking
sleeve
62 is dimensioned to fit through an opening 67 defined through the drive
collar
69 and the spring 63 is dimensioned to fit over the locking sleeve 62. The
spring
63, in turn, is biased between and against the drive collar 69 and a pair of
locking bolts 62a and 62b which to the locking sleeve 62. Upon actuation of
the
handles 30a and 30b, the toggle links 35a and 35b force the drive collar 69
proximally to compress the spring 63 against the locking bolts 62a and 62b.
36
CA 02561622 2006-09-29
As best seen in Figs. 9A and 9B, the locking sleeve 62 and sleeve
17 are clamped or welded together at assembly. Locking sleeve 62 includes a
distal collar 62' which abuts drive collar 69 to ensure axial translation of
the
driving collar 69 upon actuation of the handles 30a and 30b. Locking sleeve 62
and sleeve 17 are also dimensioned to reciprocate through locking nuts 62a and
62b during actuation of handles 30a and 30b which enables the spring 63 to
compress against locking nuts 62a and 62b which as mentioned above,
facilitates locking the forceps 10 in a closed orientation within desired
force
ranges and facilitates opening of the handles 30a and 30b after activation of
the
forceps 10.
Fig. 14 also shows the rotating assembly 80 which includes two C-
shaped rotating halves 81a and 81b which, when assembled about shaft 17,
form a generally circular rotating member 81. More particularly, each rotating
half, e.g., 81b, includes a series of mechanical interfaces 83 which matingly
engage a corresponding series of mechanical interfaces (not shown) in half 81a
to form rotating member 81. Half 81b also includes a tab or protrusion (Not
shown) which together with a corresponding tab or protrusion (not shown)
disposed on half 81a cooperate to matingly engage slots 17c and 18c on the
drive shaft 17 and rotating shaft 18, respectively. As can be appreciated,
this
permits selective rotation of the end effector assembly 100 about axis "A" by
manipulating the rotating member 80 in the direction of the arrow "R" (see
Figs.
1A and 12).
37
CA 02561622 2006-09-29
As mentioned above, the jaw members 110 and 120 may be
opened, closed and rotated to manipulate tissue until sealing is desired. This
enables the user to position and re-position the forceps 10 prior to
activation and
sealing. It is envisioned that the unique feed path of the cable leads 325a
and
325b through the rotating assembly 80, along shaft 18 and, ultimately, to jaw
members 110 and 120 enables the user to rotate the end effector assembly 100
about 170 degrees in both the clockwise and counterclockwise direction without
tangling or causing undue strain on cable leads 325a and 325b.
As best shown in Figs. 5A, 5B, 6A, 6B, 7, 11A, 11B and 14, the
knife assembly 70 mounts atop housing 20 and is configured to selectively
translate a knife bar 71 which, in turn, translates knife 190 through tissue.
More
particularly, the knife assembly 70 includes a finger actuator 76 having an
elongated support base 72 affixed thereto which is selectively moveable
parallel
to longitudinal axis "A". Elongated support base 72 includes a proximal end
which is configured as a gear rack having a series of gear teeth 72a which
depend downwardly therefrom. Gear teeth 72a are configured to mesh with a
corresponding pinion gear 77 mounted for rotation on the housing 20. The
pinion gear 77 also meshes with a second gear track 75 having a plurality of
gear teeth 75a disposed on a collar 74 which is slid ingly translatable atop
sleeve
17. As best shown in Figs. 9A, 9B and 11A, a pin 78 attaches the collar 74 to
a
proximal end 71b of knife bar 71 through slot 17d defined through sleeve 17.
Proximal translation of the finger actuator 76 in the direction "F" rotates
the
pinion gear 77 in a clockwise direction which, in turn, forces the second gear
track 75a distally in the direction "H" (see Fig. 7). A spring 79 biases the
collar
38
CA 02561622 2006-09-29
74 against the housing 20 to automatically return the knife assembly 70 to a
pre-
firing position after the finger actuator 76 is released.
As mentioned above, the knife assembly 70 is prevented from
being actuated when the jaw members 110 and 120 are opened by virtue of
flange 32 disposed on handle 30a being positioned to prevent distal activation
of
the collar 74 when handles 30a and 30b are opened. Upon movement of the
handles 30a and 30b to a closed position, the flange 32 is positioned to allow
distal translation of collar 74 to actuate the knife bar 71.
The operating features and relative movements of the internal
working components of the forceps 10 are shown by phantom representation in
the various figures. As the handles 30a and 30b are squeezed, the drive collar
69, through the mechanical advantage of the in-line toggle links 35a and 35b,
is
moved proximally which, in turn, compresses a spring 63 against the locking
nuts
62a and 62b. As a result thereof, the drive collar 69 reciprocates locking
sleeve
62 proximally which, in turn, reciprocates drive sleeve 17 proximally to
closes jaw
members 110 and 120. Once the jaw members 110 and 120 are closed about
tissue the user can selectively energize the electrically conductive sealing
plates
for either monopolar activation or bipolar activation to treat tissue.
As best shown in Figs. 6A, 14 and 16, the forceps 10 includes two
switches 250 and 260 which are mounted within or atop the housing 20 and
which allow a user to selectively activate the forceps 10 to selectively
transmit
bipolar energy to the jaw members 110 and 120 or selectively transmit
39
CA 02561622 2006-09-29
monopolar energy to the jaw members 110 and 120 or to a single jaw member,
e.g., jaw member 110. For the purposes herein, it is envisioned that either
switch, e.g., switch 250, may be configured for monopolar activation and the
other switch, e.g., switch 260, may be configured for bipolar activation.
Further
the switches 250 and 260 may include indicia or other identifying elements,
e.g.,
raised protuberances, scallops, different shapes, etc., to distinguish the two
switches 250 and 260 from one another which may prove especially useful
during wet operating conditions.
In one particularly useful embodiment and as best shown in Fig.
6A, switches 250 and 260 are mounted within the housing 20 on opposite sides
of longitudinal axis "A" and on opposite sides of the knife assembly 70. As
can
be appreciated, the knife assembly 70 (and actuation thereof) and the switches
250 and 260 (and the activation thereof) are conveniently located to
facilitate
actuation/activation by the user during operating conditions. For example, it
is
contemplated that the user may utilize the same finger to both activate the
switches 250 and 260 to treat tissue and actuate the knife assembly 70 to cut
tissue once treated.
As shown in Figs. 6A and 16, cable 310 is fed through the housing
20b on one side of the drive assembly 60 and electromechanically connects to a
printed circuit board 172 of the switch assembly 170. More particularly, cable
310 is internally divided into a plurality of leads 311a-311f which are
secured by
a crimp-like connector 174 to a series of corresponding contacts 176a-176f
extending from the printed circuit board 172 or to other electrically
conductive
CA 02561622 2006-09-29
leads which ultimately connect to the jaw members. Other electromechanical
connections are also envisioned which are commonly known in the art, e.g., IDC
connections, soldering, etc. It is envisioned the various leads 311a-311f are
configured to transmit different electrical potentials or control signals to
the
printed circuit board 172 which, in conjunction with generator 500, regulates,
monitors and controls the electrical energy to the jaw members 110 and 120. As
mentioned above with respect to the description of the jaw members, electrical
leads 325a and 325b extend through the rotating member 80, along shaft 18 to
ultimately connect to the jaw members 110 and 120.
Fig. 23 shows a schematic representation of a control circuit 510
for use with the presently disclosed forceps 10. As mentioned above, forceps
10
is configured to operate in two independent modes of operation ¨ bipolar mode
and monopolar mode for different surgical procedures. When one of the
switches 250 (Si in Figs. 19 and 23) or 260 (S2 in Figs. 19 and 23) of switch
assembly 170 is depressed, a contact (not shown) on the switches 250 and 260
activates the appropriate electrical potential (or potentials) to the jaw
members
110 and 120 which is (are) carried through leads 325a and/or 325b. For
example, if switch 250 (LigaSure TM activation) is depressed, the circuit
board 172
signals the generator 500 to configures the forceps 10 as a bipolar forceps
and
lead 325a carries a first electrical potential to jaw member 110 and lead 325b
carries a second electrical potential to jaw member 120. As such the jaw
members 110 and 120 conduct bipolar energy through the tissue upon activation
to create a tissue seal. Fig. 23 shows one example of contemplated electrical
circuitry which may be utilized to accomplish this purpose.
41
CA 02561622 2014-01-24
If switch 260 (monopolar activation) is depressed, the circuit board
172 configures the forceps as a monopolar forceps and lead 325a caries a first
electrical potential to jaw member 110 to coagulate or otherwise treat tissue
in a
monopolar fashion. As mentioned above, jaw member 110 includes a
monopolar extension which facilitates monopolar treatment of various tissue
types, e.g., avascular tissue structures, and/or allows quick dissection of
narrow
tissue planes. Activation of the monopolar extension may be controlled by an
activation circuit which allows the user to selectively apply monopolar energy
or
bipolar energy as needed during surgery. One envisioned activation circuit is
disclosed in commonly-owned U.S. Patent Application Serial No. 10/970,307,
published May 26, 2005 as U.S. Patent Publication No. 2005/0113827,
entitled "BIPOLAR FORCEPS HAVING MONOPOLAR EXTENSION" and
U.S. Application Serial No. 10/988,950, published June 23, 2005 as U.S.
Patent Publication No. 2005/0137590, entitled "BIPOLAR FORCEPS
HAVING MONOPOLAR EXTENSION".
Alternatively and as best shown in Fig. 23, during the monopolar
mode when switch 260 is depressed, the generator (or the printed circuit
board)
can direct both leads 325a and 325b to carry the same electrical potential to
jaw
members 110 and 120 depending upon a particular purpose or depending upon
a desired surgical treatment, e.g., so-called "coagulative painting". As can
be
appreciated, in a monopolar mode, a return pad would be necessarily placed in
contact with the patient to act as a return path (not shown) for the
electrical
energy. The return pad in this instance would connect to the generator 500
directly or though a return pad control mechanism (not shown) which may be
42
CA 02561622 2014-01-24
configured to monitor certain parameters of the return pad. Various
envisioned control systems are disclosed in commonly-owned U.S. Patent
Application Serial No. 10/918,984, published January 27, 2005 as U.S.
Patent Publication No. 2005/0021022, entitled "MULTIPLE RE RETURN
CABLE PAD CONTACT DETECTION SYSTEM", U.S. Patent Application
Serial No. 09/310,059 entitled "ELECTROSURGICAL RETURN
ELECTRODE MONITOR" (now U.S. Patent No. 6,258,085), and U.S. Patent
Publication No. 2006/0079872 entitled "DEVICE FOR DETECTING
HEATING UNDER A PATIENT RETURN ELECTRODE".
In a bipolar mode, the circuit 510 (schematically-illustrated in Fig.
23) electrical routes energy to the two jaw members 110 and 120. More
particularly, when switch 250 Is depressed an isolated circuit 520 of the
circuit
510 recognizes a resistance drop thereacross which is recognized by the
generator to initiate electrosurgical energy to supply a first electrical
potential to
jaw member 110 and a second electrical potential to jaw member 120. Switch
520 acts as an insolated control circuit and is protected by circuitry within
the
generator from the higher current loop which supplies electrical energy to the
jaw
members 110 and 120. This reduces the chances of electrical failure of the
switch 260 due to high current loads during activation.
As best shown in Fig. 14, handle 30a also includes a switch lockout
mechanism 255 which may be configured to prevent activation of one or both
43
CA 02561622 2006-09-29
switches 250 and 260 when the jaw members 110 and 120 are disposed in an
open configuration. More particularly, lockout mechanism 255 extends from
handle 30a towards housing 20 and is selectively moveable with the handle 30a
from a first position wherein the lockout mechanism 255 prevents one or both
switches 250 and 260 from being depressed to contact the circuit board 172 to
a
second position closer to the housing 20 wherein the lockout mechanism 255 is
positioned to allow activation of switch 250 (or switches 250 and 260). It is
envisioned that the lockout mechanism 255 may be configured as a purely
mechanical lockout which physically prevents movement of one or both switches
250 and/or 260 or may be configured as an electromechanical lockout which
includes a mechanical element which activates a safety switch to allow
activation. Moreover, the switch lockout mechanism 255 may be configured
such that one or both switches may be independently and exclusively
activatable, i.e., only one switch may be activated at a time.
For example, flex circuit 170 may include a safety switch 171 which
is activated when lockout mechanism 255 physically engages safety switch 171
to close the circuit to permit electrosurgical activation. In other words, the
safety
switch 171 is deflected or physically engaged (i.e., by virtue of the movement
of
lockout mechanism 255 when the handles 30a and 30b are closed) to close the
electrical path and permit electrosurgical activation. Further details with
respect
to various embodiments of the safety switch are described below with respect
to
Figs. 18 -21D. It is also envisioned that a purely electrical safety switch
(See
Fig. 23) may be included which allows activation based upon the satisfaction
of
an electrical condition, e.g., optical alignment of points on the handle 30a
(or
44
CA 02561622 2006-09-29
handles (30a and 30b), magnetic or electromagnetic alignment (or misalignment)
to close a switch, proximity sensors, scanners, mercury (or the like)
switches,
etc. Again, the safety switch 171 may be configured such that one or both
switches 250 and/or 260 may be independently and exclusively activatable,
i.e.,
only one switch may be activated at a time.
As can be appreciated, locating the switches 250 and 260 on the
housing 20 is advantageous during operating conditions since this positioning
reduces the amount of electrical cable in the operating room and eliminates
the
possibility of activating the wrong instrument or wrong switch during a
surgical
procedure due to "line-of-sight" activation. An automatic safety circuit or an
electro-mechanical or mechanical safety lock (not shown) may be employed
which prevents the switches 250 and 260 from energizing the jaw members 110
and 120 in a different mode (i.e. bipolar or monopolar mode) without de-
activating a safety circuit or other safety mechanism, i.e., independent and
exclusive activation. For example, it may be desirable to configure the switch
assembly 70 such that it must be re-set before switching between electrical
modes. Re-setting may be accomplished by re-grasping tissue, re-opening the
handles 30a and 30b, a reset switch or re-set lever, or other ways customary
in
the art.
As can be appreciated various switching algorithms (See Fig. 23)
may be employed to activate both the bipolar mode for vessel sealing and the
monopolar mode for additional tissue treatments (e.g., coagulation,
dissection,
etc.). It is also envisioned that the safety or lockout mentioned above may be
CA 02561622 2006-09-29
employed as part of an algorithm to either electrically, mechanically or
electromechanically lock out" one electrical mode during activation of the
other
electrical mode. In addition, it is contemplated that a toggle switch (or the
like)
may be employed to activate one mode at a time for safety reasons.
The safety switch 171 when assembled (and when the handles
30a' and 30b and jaws 110 and 120 are opened) is secured against an interior
wall or ledge 173 of housing 20b as shown in Fig. 22A. Upon movement of the
handle 30a toward housing 20b, safety lockout 255 moves inwardly relative to
the housing 20b toward the safety switch 171 as shown in Fig. 22B. As the
handles 30a and 30b move toward the closed position (as described in detail
above), the safety lockout 255 engages the safety circuit 171' (S3 in Figs. 19
and
23) to complete circuit and allow selective activation of the forceps 10 (see
also
Fig. 23).
As best shown in Figs. 14 and 23, the switching assembly may
include an intensity control 150 which electromechanically connects to the
circuit
board 172 and which is configured to allow the user to selectively regulate
the
intensity of the electrosurgical energy during operating conditions. It is
envisioned that the intensity control 150 is particularly configured to
regulate the
intensity control when the forceps is configured in a monopolar mode. In one
particularly useful embodiment, intensity control 150 is elongated and
includes a
contact 154 which extends transversally therefrom to electro-mechanically
interface with the circuit board 172 through the housing 20. An actuating knob
151 extends transversally from the opposite side of the intensity control 150
and
46
CA 02561622 2006-09-29
is dimensioned to protrude from the side of housing 20 when assembled (see
Figs. 5A, 5B, 6A and 6B). In one
particularly useful embodiment, intensity
control 150 is configured to slide along housing 20 to regulate the intensity
level
as desired.
It is envisioned that the intensity control 150 may be configured to
slide along the housing 20 in a discreet or continuous manner depending upon a
particular purpose. Moreover,
various types of indicia 155 and/or tactile
feedback elements (not shown) may be utilized to denote the position and/or
intensity level of the electrical energy, e.g., numbers, graphical indicia,
mechanical interfaces, etc. It is also envisioned that the user may configure
the
initial intensity level on the generator 500 (See Fig. 16) and the intensity
control
150 on the forceps 10 may be utilized to increase or decrease the pre-set
level
by a certain percentage by moving knob 151.
Intensity controller 150 may be configured to function as a slide
potentiometer, sliding over and along the flexible or printed circuit board
(which
may be configured to function as a voltage divider network or "VDN"). For
example, the intensity controller 150 may be configured to have a first
position
wherein knob 151 is disposed at a proximal-most position (e.g., closest to the
user) which corresponds to a relative low intensity setting, a second position
wherein knob 151 is disposed at a distal-most position (e.g., furthest from
the
user) corresponding to a relative high intensity setting, and a plurality of
intermediate positions wherein knob 151 is disposed at varying positions
therebetween corresponding to various intermediate intensity settings. As can
be
47
CA 02561622 2014-01-24
appreciated, the intensity settings from the proximal end to the distal end
may be reversed, e.g., high to low. One embodiment of an intensity
controller 150 is disclosed in commonly-owned U.S. Patent Serial No.
11/337,990, published August 10, 2006 as U.S. Patent Publication No.
2006/0178667, entitled "ELECTROSURGICAL PENCIL WITH ADVANCED
ES CONTROLS".
As illustrated in Fig. 14 and as mentioned above, knob 151 may be
dimensioned to ride along a guide channel 157 disposed within housing 20a
which is provided with a series of discreet or detented positions defining a
series
of positions, e.g., five, to allow easy selection of the output intensity from
the low
intensity setting to the high intensity setting. The series of cooperating
discreet
or detented positions also provide the surgeon with a degree of tactile
feedback.
Accordingly, in use, as intensity controller 150 slides distally and
proximally, a
mechanical interface 158 disposed atop contact 154 selectively engages a
series of corresponding detents (not shown) to set the intensity level as well
as
to provide the user with tactile feedback as to when the intensity controller
150
has been set to the desired intensity setting. Alternatively, audible feedback
can
be produced from intensity controller 150 (e.g., a "click"), from
electrosurgical
energy source 500 (e.g., a "tone") and/or from an auxiliary sound-producing
device such as a buzzer (not shown).
Intensity controller 150 may also be configured and adapted to
adjust the power parameters (e.g., voltage, power and/or current intensity)
and/or the power verses impedance curve shape to affect the perceived output
intensity. For example, the greater intensity controller 150 is displaced in a
distal
direction the greater the level of the power parameters transmitted to the jaw
48
CA 02561622 2006-09-29
members 110 and 120 (or simply jaw member 110 when disposed in a
monopolar configuration). When the forceps is disposed in a monopolar mode,
current intensities can range from about 60 mA to about 240 mA with tissue
having an impedance of about 2k ohms. An intensity level of 60 mA may provide
very light and/or minimal cutting/dissecting/hemostatic effects. An intensity
level
of 240 mA provides very aggressive cutting/dissecting/hemostatic effects.
Intensity settings are typically preset and selected from a look-up
table based on a desired surgical effect, surgical specialty and/or surgeon
preference. The selection may be made automatically or selected manually by
the user.
It is envisioned that when the forceps 10 is changed from one
mode to another mode, the intensity controller 150 may be configured such that
it must be reset (e.g., the knob 151 is re-positioned to the proximal-most end
of
guide channels 157 thus re-setting the intensity level to the preset
configuration.
After being reset, intensity controller 150 may be adjusted as needed to the
desired and/or necessary intensity level for the mode selected.
It is envisioned and contemplated that the circuit board 172 or
generator 500 may also include an algorithm which stores the last intensity
level
setting for each mode. In this manner, intensity controller 150 does not have
to
be reset to the last operative value when the particular mode is re-selected.
49
CA 02561622 2006-09-29
The present disclosure also relates to a method for treating tissue
with electrosurgical energy from the electrosurgical generator 500 which
includes
the steps of: providing an endoscopic forceps 10 including a housing 20 having
a shaft 12 affixed thereto. The shaft 12 includes first and second jaw
members,
110 and 120, respectively, attached proximate a distal end of the shaft 12. An
actuator or handle assembly 30 is included for moving jaw members 110 and
120 relative to one another from a first position wherein the jaw members 110
and 120 are disposed in spaced relation relative to one another to a second
position wherein the jaw members 110 and 120 cooperate to grasp tissue
therebetween. The switch assembly 170 is included on the housing 20 which
permits the user to selectively energize the jaw members 110 and 120 in a
monopolar or bipolar mode to treat tissue.
As can be appreciated and as mentioned above, the switch
assembly 170 includes switches 250 and 260, printed circuit board 172 and
connectors 176a-d. An intensity control 150 may also be included with the
switch assembly 170 to regulate the intensity level of the electrosurgical
energy
when disposed in either mode. In this particular method, the steps further
include: grasping tissue between the jaw members 110 and 120; selectively
activating the jaw members 110 and 120 to treat tissue disposed between the
jaw members 110 and 120 in a bipolar or monopolar fashion; and selectively
regulating the intensity of the electrosurgical energy by controlling the
intensity
control 150.
CA 02561622 2006-09-29
Other steps of the method may include the steps of: providing a
knife assembly 70 which is configured for selective actuation of a knife and
the
step of selectively actuating the knife assembly 70 to advance the knife 190
to
divide tissue after tissue treatment. Still other steps may include: adjusting
the
intensity of the electrosurgical energy as needed during operating conditions;
un-
locking the knife assembly 70 prior to actuation or unlocking the knife
assembly
70 simultaneously when actuating the handles 30a and 30b from the first and
second positions.
As best shown in Fig. 17, the distal end 71a of the elongated knife
bar 71 of the knife assembly 70 attaches to the knife 190 at a proximal end
thereof. It is envisioned that the knife 190 may be attached to the knife bar
71 in
any way known in the art, e.g., snap-fit, fiction-fit, pinned, welded, glued,
etc. In
the particular embodiment shown in Fig. 17, a clamp collar 197 is used to
retain
the knife 190 securely engaged with the knife bar 71.
Switches 250 and 260 are typically push-button-type and
ergonomically dimensioned to seat within respective apertures 250' and 260' of
housing 20 (once assembled). It is envisioned that the switches 250 and 260
permit the user to selectively activate the forceps 10 for surgical treatment
of
tissue. More particularly, when either switch 250 or 260 is depressed,
electrosurgical energy is transferred through leads 325a and/or 325b to
respective jaw members 110 and 120.
51
CA 02561622 2014-01-24
Again and as noted above, a safety switch 255 (or circuit or
algorithm (not shown)) may be employed such that one or both of the switches
250 and 260 cannot fire unless the jaw members 110 and 120 are closed and/or
unless the jaw members 110 and 120 have tissue held therebetween. In the
latter instance, a sensor (not shown) may be employed to determine if tissue
is
grasped between jaw members. In addition, other sensor mechanisms may be
employed which determine pre-surgical, concurrent surgical (i.e., during
surgery)
and/or post surgical conditions. These sensor mechanisms may also be utilized
with a closed-loop feedback system coupled to the electrosurgical generator to
regulate the electrosurgical energy based upon one or more pre-surgical,
concurrent surgical or post surgical conditions. Various sensor mechanisms and
feedback systems are described in commonly-owned co-pending U.S.
Patent Application Serial No. 10/427,832, published January 22, 2004 as
U.S. Patent Publication No. 2004/0015163, entitled "METHOD AND
SYSTEM FOR CONTROLLING OUTPUT OF RF MEDICAL GENERATOR".
Turning back to Fig. 14 which shows the exploded view of the
housing 20, rotating assembly 80, drive assembly 70, handle assembly 30 and
switch assembly 170, it is envisioned that all of these various component
parts
along with the shaft 12 and the end effector assembly 100 are assembled during
the manufacturing process to form a partially and/or fully disposable forceps
10.
For example and as mentioned above, the shaft 12 and/or end effector assembly
100 may be disposable and, therefore, selectively/releasably engagable with
the
housing 20 and rotating assembly 80 to form a partially disposable forceps 10
and/or the entire forceps 10 may be disposable after use.
52
CA 02561622 2006-09-29
It is envisioned that the opposing jaw members 110 and 120 may
be rotated and partially opened and closed without unlocking the knife
assembly
70 which, as can be appreciated, allows the user to grip and manipulate the
tissue without premature activation of the knife 190. As mentioned below, only
a
substantially fully closed position of the handles 30a and 30b will unlock the
knife
assembly 70 for actuation.
Once the desired position for the sealing site is determined and the
jaw members 110 and 120 are properly positioned, handles 30a and 30b may be
squeezed to actuate the drive assembly 60 to close the jaw members 110 and
120 about tissue. As mentioned above, when the handles 30a and 30b are fully
closed about tissue the toggle links 35a and 35b over-rotate past parallel
with
the longitudinal axis "A" such that slightly releasing the handles 30a and 30b
biases the spring 63 to lock the handles 30a and 30b relative to one another.
As
can be appreciated, when the handles 30a and 30b lock relative to one another,
the jaw members 110 and 120, in turn, lock and secure about tissue within a
pressure range of about 3kg/crre to about 16kg/cm2 and, preferably, with a
pressure range of about 7kg/cm2 to about 13 kg/cm2. The forceps 10 is now
ready for selective application of electrosurgical energy and subsequent
separation of the tissue (if desired).
It is envisioned that the combination of the mechanical advantage
gained by the disposition of the toggle links 35a and 35b relative to the
longitudinal axis "A" along with the mechanical advantage gained by
configuring
53
CA 02561622 2006-09-29
the distal ends 34a' and 34b' as inter-engaging gear teeth will facilitate and
assure consistent, uniform and accurate closure pressure about the tissue
within
the desired working pressure range of about 3 kg/cm2 to about 16 kg/cm2 and,
in
one particularly useful embodiment, about 7 kg/cm2 to about '13 kg/cm2. By
controlling the intensity, frequency and duration of the electrosurgical
energy
applied to the tissue, the user can either cauterize, coagulate/desiccate,
seal
and/or simply reduce or slow bleeding by activating either or both switches
250
and 260.
In one or more particularly useful embodiments, the electrically
conductive sealing surfaces 112, 122 of the jaw members 110, 120,
respectively,
are relatively flat to avoid current concentrations at sharp edges and to
avoid
arcing between high points. In addition and due to the reaction force of the
tissue when engaged, jaw members 110 and 120 are preferably manufactured to
resist bending. For example, the jaw members 110 and 120 may be tapered
along the width thereof which is advantageous since the thicker proximal
portion
of the jaw members 110 and 120 will resist bending due to the reaction force
of
the tissue.
As mentioned above, at least one jaw member, e.g., 120, may
include one or more stop members 90 which limit the movement of the two
opposing jaw members 110 and 120 relative to one another. The stop
member(s) 90 may be dimensioned to extend from the sealing surface 122 a
predetermined distance according to the specific material properties (e.g.,
compressive strength, thermal expansion, etc.) to yield a consistent and
accurate
54
CA 02561622 2006-09-29
gap distance "G" during sealing. The gap distance between opposing sealing
surfaces 112 and 122 during sealing ranges from about 0.001 inches to about
0.006 inches and, in one particularly useful embodiment, between about 0.002
and about 0.003 inches. The non-conductive stop member(s) 90 may be molded
onto the jaw members 110 and 120 (e.g., overmolding, injection molding, etc.),
stamped onto the jaw members 110 and 120 or deposited (e.g., deposition) onto
the jaw members 110 and 120. For example, one technique involves thermally
spraying a ceramic material (or the like) onto the surfaces of one or both jaw
members 110 and 120 to form the stop member(s) 90. Several thermal spraying
techniques are contemplated which involve depositing a broad range of heat
resistant and insulative materials on various surfaces to create stop members
90
for controlling the gap distance between electrically conductive surfaces 112
and
122.
As energy is being selectively transferred to the end effector
assembly 100, across the jaw members 110 and 120 and through the tissue, a
tissue seal forms isolating two tissue halves. At this point and with other
known
vessel sealing instruments, the user must remove and replace the forceps 10
with a cutting instrument (not shown) to divide the tissue halves along the
tissue
seal. As can be appreciated, this is both time consuming and tedious and may
result in inaccurate tissue division across the tissue seal due to
misalignment or
misplacement of the cutting instrument along the ideal tissue cutting plane.
As explained in detail above, the present disclosure incorporates
knife assembly 70 which, when activated via the trigger knob 76, progressively
CA 02561622 2006-09-29
and selectively divides the tissue along an ideal tissue plane in precise
manner
to effectively and reliably divide the tissue into two sealed halves with a
tissue
gap therebetween. The knife assembly 70 allows the user to quickly separate
the tissue immediately after sealing without substituting a cutting instrument
through a cannula or trocar port. As can be appreciated, accurate sealing and
dividing of tissue is accomplished with the same forceps 10.
It is envisioned that knife blade 190 may also be coupled to the
same or an alternative electrosurgical energy source to facilitate separation
of
the tissue along the tissue seal. Moreover, it is envisioned that the angle of
the
knife blade tip 192 may be dimensioned to provide more or less aggressive
cutting angles depending upon a particular purpose. For example, the knife
blade tip 192 may be positioned at an angle which reduces "tissue wisps"
associated with cutting. More over, the knife blade tip 192 may be designed
having different blade geometries such as serrated, notched, perforated,
hollow,
concave, convex etc. depending upon a particular purpose or to achieve a
particular result. It is also contemplated that the forceps 10 may be
activated in
a monopolar mode to divide tissue after formation of a tissue seal.
Once the tissue is divided into tissue halves, the jaw members 110
and 120 may be opened by re-grasping the handles 30a and 30b moving each
handle 30a and 30b outwardly relative to the housing 20. It is envisioned that
the knife assembly 70 generally cuts in a progressive, uni-directional fashion
(i.e., distally).
56
CA 02561622 2014-01-24
As best shown in Figs. 3A ¨ 3C, the proximal portions of the jaw
members 110 and 120 and the distal end 16 of shaft 12 may be covered by a
resilient or flexible insulating material or boot 220 to reduce stray current
concentrations during electrosurgical activation especially in the monopolar
activation mode. More particularly, the boot 220 is flexible from a first
configuration (See Fig. 3B) when the jaw members 110 and 120 are disposed in
a closed orientation to a second expanded configuration (See Figs. 3A and 3C)
when the jaw members 110 and 120 are opened. As can be appreciated, when
the jaw members 110 and 120 open, the boot flexes or expands at areas 220a
and 220b to accommodate the movement of the proximal flanges 113 and 123.
Figs. 18-22C show one particularly useful embodiment of a safety
lockout mechanism 255' for use with a flex circuit 170'. Much like the above
described safety lockout 255, lockout mechanism 255' is disposed on handle
30a' at a point distal to trigger lockout 32'. This particular safety lockout
255' is
configured to extend normally to the longitudinal axis "A" as shown best in
Fig.
18. Movement of handle 30a' towards housing 20 causes the safety lockout 255'
to move towards the housing 20 in a similar manner as described above. Safety
lockout 255' is configured to engage a safety switch 171' of the flex circuit
170' to
allow activation only when handle 30a' (and, in turn, jaw members 110 and 120)
57
CA 02561622 2006-09-29
is moved relative to housing 20 (i.e., both handles 30a' and 30b are closed to
grasp tissue).
As best shown in schematic illustration of Fig. 20, safety switch
171' is designed as part of a circuit 400 such that circuit 400 remains open
until
the safety switch 171' is activated. Fig. 21 shows the position of safety
switch
171' prior to and after assembly. More particularly, upon assembly, the safety
switch 171' is flexed into position (see phantom representation) by the top
portion 20a of housing 20 such that the distal portion of the safety switch
171' is
biased and wedged against an interior wall or ledge 173' disposed within
housing 20b. It is envisioned that the safety switch 171' will remain secured
in
place for the useful life of the forceps 10.
Figs. 22A -22C show the activation sequence of the safety switch
171'. More particularly and as mentioned above, the safety switch 171' when
assembled (and when the handles 30a' and 30b and jaws 110 and 120 are
opened) is secured against an interior wall or ledge 173' of housing 20b as
shown in Fig. 22A. Upon
movement of the handle 30a' toward housing 20b,
safety lockout 255' moves inwardly relative to the housing 20b toward the
safety
switch 171' as shown in Fig. 22B. As the handles 30a' and 30b move toward the
closed position (as described in detail above), the safety lockout 255'
engages
the safety circuit 171' to complete circuit 400 and allow selective activation
of the
forceps 10.
58
,
CA 02561622 2006-09-29
It is envisioned that the safety switch 171' may be configured to
allow both bipolar and monopolar activation once closed or configured in a
more
restrictive fashion, e.g., only permit one type of electrical activation at a
time
without re-setting the safety switch 171' (i.e., opening and re-grasping the
handles 30a' and 30b, a separate toggle switch (not shown), etc.). Moreover,
it
is also envisioned that the safety switch 171' may be configured to simply
safeguard against the activation of one of the modes (i.e., the monopolar
mode)
depending upon a particular purpose and the other mode (i.e., the bipolar
mode)
is not restricted by the safety switch 171'.
From the foregoing and with reference to the various figure
drawings, those skilled in the art will appreciate that certain modifications
can
also be made to the present disclosure without departing from the scope of the
same. For example, it may be preferable to add other features to the forceps
10,
e.g., an articulating assembly to axially displace the end effector assembly
100
relative to the elongated shaft 12.
It is also contemplated that the forceps 10 (and/or the
electrosurgical generator used in connection with the forceps 10) may include
a
sensor or feedback mechanism (not shown) which automatically selects the
appropriate amount of electrosurgical energy to effectively seal the
particularly-
sized tissue grasped between the jaw members 110 and 120. The sensor or
feedback mechanism may also measure the impedance across the tissue during
sealing and provide an indicator (visual and/or audible) that an effective
seal has
been created between the jaw members 110 and 120. Examples of such sensor
59
CA 02561622 2014-01-24
systems are described in commonly-owned U.S. Patent Application Serial
No. 10/427,832, published January 22, 2004 as U.S. Patent Publication No.
2004/0015163, entitled "METHOD AND SYSTEM FOR CONTROLLING
OUTPUT OF RF MEDICAL GENERATOR".
Moreover, it is contemplated that the knife assembly 70 may
include other types of recoil mechanisms which are designed to accomplish the
same purpose, e.g., gas-actuated recoil, electrically-actuated recoil (i.e.,
solenoid), etc. It is also envisioned that the forceps 10 may be used to cut
tissue
without sealing. Alternatively, the knife assembly 70 may be coupled to the
same or alternate electrosurgical energy source to facilitate cutting of the
tissue.
Although the figures depict the forceps 10 manipulating an isolated
vessel, it is contemplated that the forceps 10 may be used with non-isolated
vessels as well. Other cutting mechanisms are also contemplated to cut tissue
along the ideal tissue plane.
It is envisioned that the outer surface of the end effector assembly
100 may include a nickel-based material, coating, stamping, metal injection
molding which is designed to reduce adhesion between the jaw members 110
and 120 with the surrounding tissue during activation and sealing. Moreover,
it is
also contemplated that the conductive surfaces 112 and 122 of the jaw members
110 and 120 may be manufactured from one (or a combination of one or more)
of the following materials: nickel-chrome, chromium nitride, MedCoat 2000
manufactured by The Electrolizing Corporation of OHIO, Inconel 600 and tin-
CA 02561622 2006-09-29
nickel. The tissue conductive surfaces 112 and 122 may also be coated with
one or more of the above materials to achieve the same result, i.e., a "non-
stick
surface". As can be appreciated, reducing the amount that the tissue "sticks"
during sealing improves the overall efficacy of the instrument.
One particular class of materials disclosed herein has
demonstrated superior non-stick properties and, in some instances, superior
seal
quality. For example, nitride coatings which include, but not are not limited
to:
TiN, ZrN, TiAIN, and CrN are preferred materials used for non-stick purposes.
CrN has been found to be particularly useful for non-stick purposes due to its
overall surface properties and optimal performance. Other classes of materials
have also been found to reducing overall sticking. For
example, high
nickel/chrome alloys with a Ni/Cr ratio of approximately 5:1 have been found
to
significantly reduce sticking in bipolar instrumentation. One particularly
useful
non-stick material in this class is Inconel 600. Bipolar instrumentation
having
sealing surfaces 112 and 122 made from or coated with Ni200, N1201 (-100%
Ni) also showed improved non-stick performance over typical bipolar stainless
steel electrodes.
While the drawings show one particular type of monopolar lockout
or safety mechanism 255 for use with the presently disclosed forceps, Figs. 18
and 19 show an alternative safety lockout mechanism which may be employed
with the forceps 10.
61
CA 02561622 2006-09-29
While several embodiments of the disclosure have been shown in
the drawings, it is not intended that the disclosure be limited thereto, as it
is
intended that the disclosure be as broad in scope as the art will allow and
that
the specification be read likewise. Therefore, the above description should
not
be construed as limiting, but merely as exemplifications of preferred
embodiments. Those skilled in the art will envision other modifications within
the
scope and spirit of the claims appended hereto.
62