Note: Descriptions are shown in the official language in which they were submitted.
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
VISCOELASTIC SURFACTANT RHEOLOGY MODIFICATION
Background of the Invention
[0001] The invention relates to rheology enhancers for viscoelastic surfactant
fluid
systems (VES's). More particularly it relates to selection and optimization of
rheology
enhancers for fluid systems to be used over broad ranges of salinity and
temperature.
Most particularly it relates to rheology enhancers to shorten shear recovery
times and
increase the viscosity of VES's for use in oilfield treatment fluids.
[0002] Certain surfactants, when in aqueous solution, form viscoelastic
fluids. Such
surfactants are termed "viscoelastic surfactants", or "VES's". Other
components, such
as additional VES's, co-surfactants, buffers, acids, solvents, and salts, are
optional or
necessary (depending upon the specific VES fluid system used) and perform such
functions as increasing the stability (especially thermal stability) or
increasing the
viscosity of the systems by modifying and/or stabilizing the micelles; all the
components
together are called a viscoelastic surfactant system. Not to be limited by
theory, but
many viscoelastic surfactant systems form long rod-like or worm-like micelles
in
aqueous solution. Entanglement of these micelle structures gives viscosity and
elasticity
to the fluid. For a fluid to have good viscosity and elasticity under given
conditions,
proper micelles must be formed and proper entanglement is needed. This
requires the
surfactant's structure to satisfy certain geometric requirements and the
micelles to have
sufficient length or interconnections for adequate entanglements.
[0003] Many chemical additives are known to improve the rheological behavior
(greater
viscosity and/or greater stability and/or greater brine tolerance and/or lower
shear
sensitivity and/or faster rehealing if micelles are disrupted, for example by
shear). Such
materials are typically called co-surfactants, rheology modifiers, or rheology
enhancers,
etc., and typically are alcohols, organic acids such as carboxylic acids and
sufonic acids,
sulfonates, and others. We shall use the term rheology enhancers here. Such
materials
often have different effects, depending upon their exact composition and
concentration,
relative to the exact surfactant composition (for example hydrocarbon chain
lengths of
groups in the surfactant and co-surfactant) and concentration. For example,
such
1
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
materials may be beneficial at some concentrations and harmful (lower
viscosity,
reduced stability, greater shear sensitivity, longer rehealing times) at
others.
[0004] In particular, many VES fluid systems exhibit long viscosity recovery
times after
experiencing prolonged high shear. Slow recovery negatively impacts drag
reduction
and proppant transport capability, which consequently lead to undesirably high
treating
pressures and risks of near wellbore screen-outs. Although additives are known
that can
shorten VES shear recovery times and increase viscosities, there is a need for
additional
simple, inexpensive rheology enhancers.
Surhmary of the Invention
[0005] One embodiment is an oilfield treatment method consisting of preparing
and
injecting down a well a fluid containing a viscoelastic surfactant selected
from
zwitterionic, amphoteric, and cationic surfactants and mixtures of those
surfactants, and
a rheology enhancer in a concentration sufficient to shorten the shear
recovery time of
the fluid, in which the rheology enhancer is selected from the group
consisting of an
amphiphilic polymer, for example a homopolymer or copolymer containing at
least a
portion consisting of partially hydrolyzed polyvinyl ester, partially
hydrolyzed
polyacrylate or sulfonate-containing polymers. The rheology enhancer may also
increase the viscosity of the fluid.
[0006] The viscoelastic surfactant system may contain a zwitterionic
surfactant, for
example a surfactant or mixture of surfactants having the formula:
RCONH-(CHZ)a(CH2CH20),n(CH2)b-N+(CH3)2-(CH2)a~(CH2CH20)m>(CH2)b~C00~
in which R is an alkyl group that contains from about 17 to about 23 carbon
atoms which
may be branched or straight chained and which may be saturated or unsaturated;
a, b, a',
and b' are each from 0 to 10 and m and m' are each from 0 to 13, a and b are
each 1 or 2
if m is not 0 and (a + b) is from 2 to 10 if m is 0; a' and b' are each 1 or 2
when m' is not
0 and (a' + b') is from 1 to 5 if m' is 0; (m + m') is from 0 to 14; and
CH2CHz0 may
also be OCHZCH2. The zwitterionic surfactant may have the betaine structure:
2
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
H3 \ CH3 O
R N \~CH2)r/N ~(CH2)p O-
I
O
in which R is a hydrocarbon group that may be branched or straight chained,
aromatic,
aliphatic or olefinic and has from about 14 to about 26 carbon atoms and may
contain an
amine; n = about 2 to about 4; and p = 1 to about 5, and mixtures of these
compounds.
The betaine may be oleylamidopropyl betaine or erucylamidopropyl betaine and
may
contain a co-surfactant.
[0007] The viscoelastic surfactant system may contain a cationic surfactant,
for example
a surfactant or mixture of surfactants having the structure:
R1N ~R2)~3OR4) x
in which R1 has from about 14 to about 26 carbon atoms and may be branched or
straight chained, aromatic, saturated or unsaturated, and may comprise a
carbonyl, an
amide, a retroamide, an imide, a urea, or an amine; R2 , R3, and R4 are each
independently hydrogen or a C1 to about C6 aliphatic group which may be the
same or
different, branched or straight chained, saturated or unsaturated and one or
more than
one of which may be substituted with a group that renders the R2, R3, and R4
group
more hydrophilic; the R2, R3 and R4 groups may be incorporated into a
heterocyclic 5-
or 6-member ring structure which includes the nitrogen atom; the R2, R3 and R4
groups
may be the same or different; R1, R2, R3 and/or R4 may contain one or more
ethylene
oxide and/or propylene oxide units; and X is an anion; and mixtures of these
compounds. As a further example, R1 contains from about 18 to about 22 carbon
atoms
and may contain a carbonyl, an amide, or an amine; R2 , R3, and R4 contain
from 1 to
about 3 carbon atoms, and X is a halide. As a further example, Rl comprises
from
about 18 to about 22 carbon atoms and may comprise a carbonyl, an amide, or an
amine,
3
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
and R2 , R3, and R4 are the same as one another and comprise from 1 to about 3
carbon
atoms. The cationic viscoelastic surfactant system optionally contains amines,
alcohols,
glycols, organic salts, chelating agents, solvents, mutual solvents, organic
acids, organic
acid salts, inorganic salts, oligomers, polymers, co-polymers, and mixtures of
said
materials, present at a concentration of between about 0.01 and about 10
percent, for
example at a concentration of between about 0.01 and about 1 percent. The
amphoteric
surfactant may be, for example, an amine oxide, for example an amidoamine
oxide.
[0008] The rheology enhancer is present in the fluid at a concentration of
from about
0.0001 % to about 0.5%, for example at a concentration of from about 0.0001 %
to about
0.05%.
[0009] The rheology enhancer contains, as one example, a partially hydrolyzed
polyvinyl acetate having a percent hydrolysis between about 10% and about 95%.
The
molecular weight is, for example, from about 500 to about 100,000,000. Other
esters
may be used, for example C2 to CII esters (a.e. the partially hydrolyzed ethyl
to undecyl
esters of polyvinyl alcohol). As another example, the rheology enhancer
contains
partially hydrolyzed polyvinyl acetate having a percent hydrolysis between
about 30%
and about 88%, and the molecular weight is, for example, from about 500 to
about
100,000,000.
[0010] The rheology enhancer may also contain partially hydrolyzed
polyacrylates, or
partially hydrolyzed polymethacrylates or the like, for example, but not
limited to,
partially hydrolyzed polymethyl acrylate, partially hydrolyzed polyethyl
acrylate,
partially hydrolyzed polybutyl acrylate, partially hydrolyzed polymethyl
methacrylate,
and mixtures of these polymers. The rheology enhancer may also contain
sulfonate-
containing polymers.
[0011] The amphiphilic polymer or copolymer rheology enhancer may be linear,
branched, or have a comb, dendritic, brush, graft, star or star-branched
shape. It may
contain repeating units other than vinyl esters, acrylates, and the
corresponding
hydrolysed groups. The other repeating units are, for example, polyethylene
oxide/polyethylene glycol or polypropylene oxide/polypropylene glycol. The
copolymers may be random, alternating, or block copolymers.
4
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
[0012] The fluid further may optionally contain an acid selected from
hydrochloric acid,
hydrofluoric acid, formic acid, acetic acid, lactic acid, glycolic acid,
polylactic acid,
polyglycolic acid, sulfamic acid, malic acid, citric acid, tartaric acid,
malefic acid,
methylsulfamic acid, chloroacetic acid, and mixtures of these acids.
[0013] Another embodiment is a method of shortening the shear recovery time of
a
viscoelastic surfactant based fluid containing a viscoelastic surfactant
selected from
zwitterionic, amphoteric, and cationic surfactants and mixtures of those
surfactants,
consisting of adding a rheology enhancer, in a concentration sufficient to
shorten the
shear recovery time of the fluid, selected from the amphiphilic polymers
described
above.
[0014] Yet another embodiment is a composition containing a viscoelastic
surfactant
selected from zwitterionic, amphoteric, and cationic surfactants and mixtures
of those
surfactants; and a rheology enhancer in a concentration sufficient to shorten
the shear
recovery time of the fluid, selected from the partially hydrolyzed polyvinyl
acetates (or
other esters) and partially hydrolyzed polyacrylates described above.
[0015] In addition to oilfield uses, the rheology enhancer of the invention
may be used
in household and industrial cleaners, agricultural chemicals, personal hygiene
products,
cosmetics, pharmaceuticals, printing and other fields.
BriefDescription of the Drawings
[0016] Figure 1 shows viscosity as a function of temperature of fluids having
one
concentration of a viscoelastic surfactant and various concentrations of an
amphiphilic
rheology enhancer of the invention.
[0017] Figure 2 shows viscosity as a function of temperature of fluids
containing
varying amounts of a fixed ratio of a viscoelastic surfactant and an
amphiphilic rheology
enhancer of the invention.
[0018] Figure 3 shows viscosity as a function of temperature of a fluid
containing a
viscoelastic surfactant and an amphiphilic rheology enhancer of the invention
and
different clay stabilizers. TMAC is tetramethylammonium chloride.
s
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
[0019] Figure 4 shows viscosity as a function of temperature of a fluid
containing a
viscoelastic surfactant and an amphiphilic Theology enhancer of the invention
and high
salt brines.
[0020] Figure 5 shows viscosity as a function of temperature of a fluid
containing a
viscoelastic surfactant and an amphiphilic Theology enhancer of the invention
and a
high-density brine.
Detailed Description of the Invention
[0021] When fluids are viscosified by the addition of viscoelastic surfactant
systems, the
viscosity increase is believed to be due to the formation of micelles, for
example worm-
like micelles, which entangle to give structure to the fluid that leads to the
viscosity. In
addition to the viscosity itself, an important aspect of a fluid's properties
is the degree
and rate of viscosity-recovery or re-healing when the fluid is subjected to
high shear and
the shear is then reduced. For VES fluids, shear may disrupt the micelle
structure, after
which the structure reforms. Controlling the degree and rate of reassembling
(re-
pealing) is necessary to maximize performance of the surfactant system for
different
applications. For example, in hydraulic fracturing it is critical for the
fluid to regain
viscosity as quickly as possible after exiting the high-shear region in the
tubulars and
entering the low-shear environment in the hydraulic fracture. On the other
hand, it is
beneficial in coiled tubing cleanouts to impart a slight delay in regaining
full viscosity in
order to "jet" the solids more efficiently from the bottom of the wellbore
into the
annulus. Once in the annulus the regained viscosity ensures that the solids
are
effectively transported to the surface.
[0022] Viscoelastic surfactant fluid systems have been shown to have excellent
Theological properties for hydraulic fracturing applications; however, shear
recovery
time, not fluid viscosity, often dictates the minimum concentration of
surfactant
required. For example, a fluid made with a certain concentration of surfactant
may show
adequate viscosity for fracturing at a given temperature, but the minimal
usable
concentration may be high due to slow shear recovery with a lower
concentration. An
acceptable shear recovery time is considered to be 15 seconds. A time longer
than 15
seconds will negatively impact drag reduction and proppant transport.
Shortening the
6
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
viscosity-recovery time makes it possible to use VES fluid systems that would
otherwise
not be suitable in many applications. In addition, when a theology modifier
also
increases fluid viscosity, then less surfactant is needed to provide a given
viscosity.
Examples of theology enhancers are given in U. S. Patent Application Serial
No.
10/994,664, which is assigned to the same assignee as the present invention.
[0023] We have found that certain simple additives, when included in certain
viscoelastic surfactant fluid systems (such as cationic, amphoteric, and
zwitterionic
viscoelastic surfactant fluid systems, especially betaine viscoelastic
surfactant fluid
systems), in the proper concentration relative to the surfactant active
ingredient,
significantly shorten the shear recovery time of the systems, increasing the
viscosity at
the same time. In many cases, the shear recovery is nearly instantaneous.
[0024] We have found new classes of chemical additives that are effective for
shortening
the repealing time after high shear, and increasing the viscosity of VES
systems at a
given temperature, making the fluids more useful for many purposes, such as,
but not
limited to, uses as oilfield treatment fluids, especially stimulation fluids,
most especially
hydraulic fracturing fluids. We will call these materials "theology enhancers"
here. The
theology enhancers extend the conditions under which the VES systems can be
used, and
reduce the amount of surfactant needed, which in turn reduces the cost and
improves
clean-up.
[0025] Suitable theology enhancers of the invention include amphiphilic
polymers
(having some polar groups on an otherwise water-insoluble backbone, or having
side
chains that themselves are water soluble backbones, and/or having some
insoluble
groups or segments on the backbone or on the side chain(s), or on both, so
that the
polymer is soluble in both water and organic solvents and has an affinity to
both polar
and non-polar solvents), for example polymers or copolymers containing
partially
hydrolyzed polyvinyl acetates (PHPVA's) consisting of or containing the
following
structure or structure segment:
7
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
~n ~ ~~m
OAc OH
OAc OH OH OAc
typically abbreviated as in the first structure shown, with [m/(n + m)] 100 =
hydrolysis, although actually having the hydrolyzed sites randomly
distributed, as shown
in the second structure. (This structure is also sometimes known as partially
hydrolyzed
polyvinyl alcohol or as polyvinyl alcohol/polyvinyl acetate copolymer.)
Examples are
obtained from Synthomer Limited, Harlow, Essex, United Kingdom, under the
trade
names Alcotex WD100 and Alcotex WD200. Alcotex WD200 is an aqueous solution
containing approximately 20% of a copolymer containing polyvinyl acetate that
is
approximately 42-45% hydrolyzed, having an average molecular weight of about
25,000; it contains less than 2% methanol. For shortening of shear recovery
time,
suitable partially hydrolyzed polyvinyl acetates (PHPVA's) or PHPVA-containing
copolymers are from about 10% to about 95% hydrolyzed and have a molecular
weight
of from about 500 to about 100,000,000. For increasing fluid system rheology,
suitable
PHPVA's or PHPVA-containing copolymers are from about 30% to about 88%
hydrolyzed and have a molecular weight of from about 5000 to about
100,000,000.
Other partially hydrolyzed polyvinyl esters (sometimes referred to as
partially
hydrolyzed polyvinyl alcohols) may be used, for example those obtained from CZ
to Cl
esters (i.e. the partially hydrolyzed ethyl to undecyl esters of polyvinyl
alcohols). It
should be understood that when we refer to polymers, we include copolymers.
[0026] Other suitable amphiphilic polymers include partially hydrolyzed
polyacrylates,
or partially hydrolyzed polymethacrylates or the like, for example, but not
limited to,
partially hydrolyzed polymethyl acrylate, partially hydrolyzed polyethyl
acrylate,
partially hydrolyzed polybutyl acrylate, partially hydrolyzed polymethyl
methacrylate,
and mixtures of these polymers. The rheology enhancer may also contain
sulfonate-
8
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
containing polymers, such as polystyrene sulfonates and condensation products
of
naphthalene sulfonates.
[0027) Other polymers that may be used as the rheology enhancer of the
invention, or
may be part of the amphiphilic polymeric rheology enhancer of the invention,
include
those described in U. S. Patent Nos. 5,760,154 (except those containing
polysaccharides)
and 5,147,907 (the portion not containing dextrins). Also useful as part of
all of the
rheology enhancer are polymers shown in U. S. Patent No. 5,574,124 (such as
terpolymers of acrylic acid, malefic anhydride and vinyl acetate). Also useful
as part of
all of the rheology enhancer are polymers shown in U. S. Patent No. 6,207,780
(such as
polymers built up of a) monoethylenically unsaturated dicarboxylic acids
and/or their
salts, b) monoethylenically unsaturated monocarboxylic acids and/or their
salts, c)
monounsaturated monomers which, after hydrolysis or saponification, can be
converted
into monomers having a hydroxyl group covalently bonded at the C--C-chain, d)
monoethylenically unsaturated sulfonic acid groups or sulfate groups-
containing
monomers, and optionally e) further radically copolymerizable monomers).
[0028] The amphiphilic polymer or copolymer rheology enhancer may be linear,
branched, or have a comb, dendritic, brush, graft, star or star-branched
shape. It may
contain repeating units other than vinyl esters, vinyl acrylates, and the
corresponding
hydrolysed groups. The other repeating units are, for example, polyethylene
oxide/polyethylene glycol or polypropylene oxide/polypropylene glycol. The
copolymers may be random, alternating, or block copolymers.
[0029] Suitable concentrations (in the final fluid system) are from about
0.0001% to
about 0.5%, for example from about 0.0001% to about 0.05%. These are very low
concentrations for rheology enhancers.
[0030] The rheology enhancers of the present invention give the desired
results with
cationic, amphoteric, and zwitterionic viscoelastic surfactant systems. They
have been
found to be particularly effective with certain zwitterionic surfactants. In
general,
particularly suitable zwitterionic surfactants have the formula:
RCONH-(CH2)a(CH2CH20)m(CH2)b-N+(CH3)2-(CH2)a'(CH2CH20)m'(CH2)b'COO
in which R is an alkyl group that contains from about 17 to about 23 carbon
atoms which
may be branched or straight chained and which may be saturated or unsaturated;
a, b, a',
9
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
and b' are each from 0 to 10 and m and m' are each from 0 to 13; a and b are
each 1 or 2
if m is not 0 and (a + b) is from 2 to 10 if m is 0; a' and b' are each 1 or 2
when m' is not
0 and (a' + b') is from 1 to 5 if m is 0; (m + m') is from 0 to 14; and
CHZCH20 may also
be OCH2CH2.
[0031] Preferred zwitterionic surfactants include betaines. Two suitable
examples of
betaines are BET-O and BET-E. The surfactant in BET-O-30 is shown below; one
chemical name is oleylamidopropyl betaine. It is designated BET-O-30 because
as
obtained from the supplier (Rhodia, Inc. Cranbury, New Jersey, U. S. A.) it is
called
Mirataine BET-O-30 because it contains an oleyl acid amide group (including a
CI~H33
alkene tail group) and contains about 30% active surfactant; the remainder is
substantially water, sodium chloride, and propylene glycol. An analogous
material,
BET-E-40, is also available from Rhodia and contains an erucic acid amide
group
(including a C2IHat alkene tail group) and is approximately 40% active
ingredient, with
the remainder being substantially water, sodium chloride, and isopropanol. VES
systems, in particular BET-E-40, optionally contain about 1 % of a
condensation product
of a naphthalene sulfonic acid, for example sodium polynaphthalene sulfonate,
as a
rheology modifier, as described in U. S. Patent Application Publication No.
2003-
0134751. The surfactant in BET-E-40 is also shown below; one chemical name is
erucylamidopropyl betaine. As-received concentrates of BET-E-40 were used in
the
experiments reported below, where they will be referred to as "VES" and ''VES-
1".
BET surfactants, and other VES's that are suitable for the present Invention,
are
described in U. S. Patent No. 6,258,859. According to that patent, BET
surfactants make
viscoelastic gels when in the presence of certain organic acids, organic acid
salts, or
inorganic salts; in that patent, the inorganic salts were present at a weight
concentration
up to about 30%. Co-surfactants may be useful in extending the brine
tolerance, and to
increase the gel strength and to reduce the shear sensitivity of the VES-
fluid, in
particular for BET-O-type surfactants. An example given in U. S. Patent No.
6,258,859
is sodium dodecylbenzene sulfonate (SDBS), also shown below. Other suitable co-
surfactants include, for example those having the SDBS-like structure in which
x = 5 -
15; preferred co-surfactants are those in which x = 7 - 15. Still other
suitable co-
surfactants for BET-O-30 are certain chelating agents such as trisodium
hydroxyethylethylenediamine triacetate. The rheology enhancers of the present
Io
CA 02561814 2006-10-02
Attorney Docket N o. 56.0885
Inventors: Chen et al
invention may be used with viscoelastic surfactant fluid systems that contain
such
additives as co-surfactants, organic acids, organic acid salts, and/or
inorganic salts.
H HsC O_
+,(CHz)P
C17H33
\\ ~ H3
(CHz)r,
O
Surfactant in BET-O-30 (when n = 3 and p = I)
H HsC O_
+,(CHz)P
Czi Hai N ~ ~ O
'(CHz " CH3
O
Surfactant in BET-E-40 (when n = 3 and p = 1 )
SO3
i
~ (CHz)xCHs
SDBS (when x = I I and the counterion is Na+)
11
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
[0032] Preferred embodiments of the present invention use betaines; most
preferred
embodiments use BET-E-40. Although experiments have not been performed, it is
believed that mixtures of betaines, especially BET-E-40, with other
surfactants are also
suitable. Such mixtures are within the scope of embodiments of the invention.
[0033] Other betaines that are suitable include those in which the alkene side
chain (tail
group) contains 17 - 23 carbon atoms (not counting the carbonyl carbon atom)
which
may be branched or straight chained and which may be saturated or unsaturated,
n = 2 -
10, and p = 1 - 5, and mixtures of these compounds. More preferred betaines
are those
in which the alkene side chain contains 17 - 21 carbon atoms (not counting the
carbonyl
carbon atom) which may be branched or straight chained and which may be
saturated or
unsaturated, n = 3 - 5, and p = 1 - 3, and mixtures of these compounds. These
surfactants are used at a concentration of about 0.5 to about 10%, preferably
from about
1 to about 5%, and most preferably from about 1.5 to about 4.5%.
[0034] Exemplary cationic viscoelastic surfactants include the amine salts and
quaternary amine salts disclosed in U.S. Patent Nos. 5,979,557, and 6,435,277
which
have a common Assignee as the present application.
[0035] Examples of suitable cationic viscoelastic surfactants include cationic
surfactants
having the structure:
R1N+(R2)(R3)(R4) X
in which R1 has from about 14 to about 26 carbon atoms and may be branched or
straight chained, aromatic, saturated or unsaturated, and may contain a
carbonyl, an
amide, a retroamide, an imide, a urea, or an amine; R2 , R3, and R4 are each
independently hydrogen or a CI to about C6 aliphatic group which may be the
same or
different, branched or straight chained, saturated or unsaturated and one or
more than
one of which may be substituted with a group that renders the R2, R3, and R4
group
more hydrophilic; the R2, R3 and R4 groups may be incorporated into a
heterocyclic 5-
or 6-member ring structure which includes the nitrogen atom; the R2, R3 and R4
groups
may be the same or different; RI, R2, R3 and/or R4 may contain one or more
ethylene
12
CA 02561814 2006-10-02
Attorney Docket N o. 56.0885
Inventors: Chen et al
oxide and/or propylene oxide units; and X- is an anion. Mixtures of such
compounds are
also suitable. As a further example, RI is from about 18 to about 22 carbon
atoms and
may contain a carbonyl, an amide, or an amine, and R2 , R3, and R4 are the
same as one
another and contain from 1 to about 3 carbon atoms.
[0036] Cationic surfactants having the structure RIN+(R2)(R3)(R4) X may
optionally
contain amines having the structure RIN(R2)(R3). It is well known that
commercially
available cationic quaternary amine surfactants often contain the
corresponding amines
(in which R1, R2, and R3 in the cationic surfactant and in the amine have the
same
structure). As received commercially available VES surfactant concentrate
formulations,
for example cationic VES surfactant formulations, may also optionally contain
one or
more members of the group consisting of alcohols, glycols, organic salts,
chelating
agents, solvents, mutual solvents, organic acids, organic acid salts,
inorganic salts,
oligomers, polymers, co-polymers, and mixtures of these members. They may also
contain performance enhancers, such as viscosity enhancers, for example
polysulfonates,
for example polysulfonic acids, as described in copending U. S. Patent
Application
Publication No. 2003-0134751 which has a common Assignee as the present
application.
[0037] Another suitable cationic VES is erucyl bis(2-hydroxyethyl) methyl
ammonium
chloride, also known as (Z)-13 docosenyl-N-N- bis (2-hydroxyethyl) methyl
ammonium
chloride. It is commonly obtained from manufacturers as a mixture containing
about 60
weight percent surfactant in a mixture of isopropanol, ethylene glycol, and
water. Other
suitable amine salts and quaternary amine salts include (either alone or in
combination in
accordance with the invention), erucyl trimethyl ammonium chloride; N-methyl-
N,N-
bis(2-hydroxyethyl) rapeseed ammonium chloride; oleyl methyl bis(hydroxyethyl)
ammonium chloride; erucylamidopropyltrimethylamine chloride, octadecyl methyl
bis(hydroxyethyl) ammonium bromide; octadecyl tris(hydroxyethyl) ammonium
bromide; octadecyl dimethyl hydroxyethyl ammonium bromide; cetyl dimethyl
hydroxyethyl ammonium bromide; cetyl methyl bis(hydroxyethyl) ammonium
salicylate; cetyl methyl bis(hydroxyethyl) ammonium 3,4,-dichlorobenzoate;
cetyl
tris(hydroxyethyl) ammonium iodide; cosyl dimethyl hydroxyethyl ammonium
bromide;
cosyl methyl bis(hydroxyethyl) ammonium chloride; cosyl tris(hydroxyethyl)
ammonium bromide; dicosyl dimethyl hydroxyethyl ammonium bromide; dicosyl
13
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
methyl bis(hydroxyethyl) ammonium chloride; dicosyl tris(hydroxyethyl)
ammonium
bromide; hexadecyl ethyl bis(hydroxyethyl) ammonium chloride; hexadecyl
isopropyl
bis(hydroxyethyl) ammonium iodide; and cetylamino, N-octadecyl pyridinium
chloride.
[0038] Many fluids made with viscoelastic surfactant systems, for example
those
containing cationic surfactants having structures similar to that of erucyl
bis(2-
hydroxyethyl) methyl ammonium chloride, inherently have short re-heal times
and the
rheology enhancers of the present invention may not be needed except under
special
circumstances, for example at very low temperature.
[0039] Amphoteric viscoelastic surfactants are also suitable. Exemplary
amphoteric
viscoelastic surfactant systems include those described in U.S. Patent No.
6,703,352, for
example amine oxides. Other exemplary viscoelastic surfactant systems include
those
described in U.S. Patent Application Nos. 2002/0147114, 2005/0067165, and
2005/0137095, for example amidoamine oxides. Mixtures of zwitterionic
surfactants and
amphoteric surfactants are suitable. An example is a mixture of about 13%
isopropanol,
about 5% 1-butanol, about 15% ethylene glycol monobutyl ether, about 4% sodium
chloride, about 30% water, about 30% cocoamidopropyl betaine, and about 2%
cocoamidopropylamine oxide.
[0040] Viscoelastic surfactant fluids, for example those used in the oilfield,
may also
contain agents that dissolve minerals and compounds, for example in
formations, scale,
and filtercakes. Such agents may be, for example, hydrochloric acid, formic
acid, acetic
acid, lactic acid, glycolic acid, sulfamic acid, malic acid, citric acid,
tartaric acid, malefic
acid, methylsulfamic acid, chloroacetic acid, aminopolycarboxylic acids, 3-
hydroxypropionic acid, polyaminopolycarboxylic acids, for example trisodium
hydroxyethylethylenediamine triacetate, and salts of these acids and mixtures
of these
acids and/or salts. For sandstone treatment, the fluid also typically contains
a hydrogen
fluoride source. The hydrogen fluoride source may be HF itself or may be
selected from
ammonium fluoride and/or ammonium bifluoride or mixtures of the two; when
strong
acid is present the HF source may also be one or more of polyvinylammonium
fluoride,
polyvinylpyridinium fluoride, pyridinium fluoride, imidazolium fluoride,
sodium
tetrafluoroborate, ammonium tetrafluoroborate, and salts of
hexafluoroantimony. When
the formation-dissolving agent is a strong acid, the fluid preferably contains
a corrosion
inhibitor. The fluid optionally contains chelating agents for polyvalent
cations, for
14
CA 02561814 2006-10-02
Attorney Docket N o. 56.0885
Inventors: Chen et al
example especially aluminum, calcium and iron (in which case the agents are
often
called iron sequestering agents) to prevent their precipitation. Some of the
formation-
dissolving agents just described are such chelating agents as well. Chelating
agents are
added at a concentration, for example, of about 0.5% (of active ingredient).
When VES
fluids contain strong acids, they are typically not gelled and display low
viscosity; when
the pH increases as the acid reacts with the mineral, the system gels and the
viscosity
increases. Such fluids may be called viscoelastic diverting acids, or VDA's.
The
rheology enhancers of the present invention may be used in viscoelastic
surfactant fluid
systems containing acids and chelating agents.
[0041] Preparation and use (mixing, storing, pumping, etc.) of the improved
VES fluid
systems containing rheology enhancers of the invention are the same as for
such fluids
without the rheology enhancers. For example, the order of mixing is not
affected by
including these rheology enhancers. Optionally, the rheology enhancers may be
incorporated in surfactant concentrates (provided that they do not affect
component
solubilities or concentrate freezing points) so that the concentrates can be
diluted with an
aqueous fluid to make VES systems. This maintains the operational simplicity
of the
VES systems. As is normally the case in fluid formulation, laboratory tests
should be
run to ensure that the additives do not affect, and are not affected by, other
components
in the fluid (such as salts, for example). In particular, the rheology
enhancers of the
present invention may be used with other rheology modifiers. Adjusting the
concentrations of surfactant, rheology enhancer, and other fluid components to
account
for the effects of other components is within the scope of the invention.
[0042] The fluid may be used, for example in oilfield treatments. As examples,
the fluid
may be used as a pad fluid and/or as a carrier fluid and/or as a diverter in
hydraulic
fracturing, as a carrier fluid for lost circulation control agents, as a
carrier fluid for gravel
packing, and as a diverter or a main fluid in acidizing and acid fracturing.
The fluids
may also be used in other industries, such as in household and industrial
cleaners,
agricultural chemicals, personal hygiene products, cosmetics, pharmaceuticals,
printing
and in other fields.
[0043] The optimal concentration of a given rheology enhancing additive of the
invention for a given choice of VES surfactant fluid system at a given
concentration and
temperature, and with given other materials present, can be determined by
simple
CA 02561814 2006-10-02
Attorney Docket N o. 56.0885
Inventors: Chen et al
experiments. The total viscoelastic surfactant concentration must be
sufficient to form a
viscoelastic gel under conditions at which the surfactants have sufficient
aggregation
tendency. The appropriate amounts of surfactant and rheology enhancer are
those
necessary to achieve the desired viscosity and shear recovery time as
determined by
experiment. Again, tolerance for, and optimal amounts of other additives may
also be
determined by simple experiment. In general, the amount of surfactant (as
active
ingredient) is from about 0.15% to about 3%. Commercially available surfactant
concentrates may contain some materials that are themselves rheology
enhancers,
although they may be present for example for concentrate freezing point
depression, so
the amount of surfactant and rheology enhancer used is determined for the
specific
concentrate used. Mixtures of surfactants and/or mixtures of rheology
enhancers
(including mixtures of more than one rheology enhancer of the invention, and
mixtures
of one or more rheology enhancers of the invention with one or more other
rheology
enhancers) may be used. Mixtures of surfactants may include surfactants that
are not
viscoelastic surfactants when not part of a viscoelastic surfactant system.
All mixtures
are tested and optimized; for example, too much total rheology enhancer may
decrease
the beneficial effects.
[0044] Experimental: The present invention can be further understood from the
following examples. In the examples, the cationic surfactants Cat A and Cat B
were
cationic surfactant formulations containing the same cationic surfactant
R1N+(R2)(R3)(R4) X (in which RI has from about 18 to about 22 carbon atoms and
contains an amide; R2 , R3, and R4 are the same short-chained saturated alkyl
group, and
X is a halide). Cat A and Cat B contain differing choices and/or amounts of
the types of
additives commonly obtained in commercially available as-received surfactant
concentrates, including amines having the structure R1N(R2)(R3) in which RI,
R2 , and
R3 are the same as for the surfactant. The zwitterionic surfactants Zw A and
Zw B are
BET-E-40 and BET-E-40 containing about 1 % polynaphthalene sulfonate
respectively.
The concentrations given for the surfactants are for the as-received
concentrates.
[0045] Example 1: The table below shows the shear recovery times observed when
various amounts of WD200 were added to four surfactant systems. In these
experiments, approximately 200 mL of already-mixed VES fluid was sheared at no
less
16
CA 02561814 2006-10-02
Attorney Docket N o. 56.0885
Inventors: Chen et al
than 10,000 rpm for no less than 30 seconds and no more than 1 minute in a 1
l~ Waring
blender. The shearing was stopped and timing was begun. The fluid was poured
back
and forth between a beaker and the blender cup and the fluid recovery was
characterized
by two times, referred to as the initial and final recovery times; both were
estimated by
visual observation. The initial fluid recovery time was the time at which
fluid ''balling"
occurred (when the fluid showed the first signs of elasticity as indicated by
the fluid
taking a longer time to achieve a flat surface in the receiving beaker when
poured). The
final fluid recovery time was the time at which fluid "upping" occurred. The
fluid "lips"
when inclining the upper beaker or cup containing the fluid does not result in
fluid flow
into the container below, but rather the formation of a "lip", and pulling the
container
back to a vertical position pulls back the "lip". In fracturing fluid
practice, "upping" is
used to estimate when the fluid reaches its near-equilibrium elasticity. The
table shows
the final fluid recovery times for several systems and shows that 0.015% of
WD200
reduces the shear recovery times of two different cationic surfactant systems
from over
five minutes to 6 seconds or to too short to measure, and 0.005% WD200 reduces
the
shear recovery times of two different zwitterionic surfactants systems to too
short to
measure.
WD200 Shear Recovery
Surfactant S Concentration concentration Time
stem
C 1% 0 > 5 min
A
at 0.015% 6 sec
B 1 % 0 > 5 min
C
at 0.015% 0 sec
Z 1 % 0 > 1 hour
B
w 0.00$% 0 S2C
Z 1 % 0 > 1 hour
A
w 0.005% 0 sec
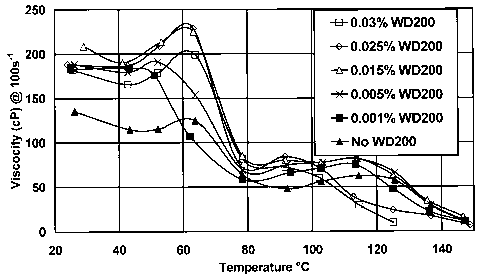
[0046] Example 2: In addition to dramatically shortening shear recovery times
at very
low concentrations, the rheology enhancer of the invention also increases
fluid system
viscosity. Varying amounts of WD200 were added to fluids made with 4.5% of as-
received surfactant concentrate Zw A without added salt. The viscosity
measured with a
Fann 50 at 100 sec I was measured at various temperatures. The results are
shown in
Fig. 1. At low temperatures, the viscosity was increased by all concentrations
of WD200
tested. At higher temperatures, the higher WD200 concentrations lowered the
viscosity
17
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventors: Chen et al
(although they still shortened shear recovery time). For this concentration of
this
surfactant, about 0.015% of this rheology enhancer is optimal for increasing
the viscosity
over the entire temperature range. For other surfactant/rheology enhancer
combinations
and other surfactant concentrations, the optimal rheology enhancer
concentration may be
different. Similarly, if optimization at a certain temperature is desired.
another
surfactant/rheology enhancer combination and/or other surfactant and rheology
enhancer
concentrations may be optimal. This is determined by simple experiments like
those of
this example.
[0047] Example 3: Because the rheology enhancer of the invention is suitable
over a
broad range of conditions, it is advantageous to prepackage a concentrate
containing a
viscoelastic surfactant and a rheology enhancer (or more than one of each) at
a fixed
concentration ratio. Fig. 2 shows the effect of varying the total
concentration of one
such package (as-received Zw A with WD200 added so that the ratio of
surfactant to
WD200 is 300) at varying concentrations.
[0048] Example 4: This system gels without added salt. Fluids injected into
subterranean formations typically contain salts as clay stabilizers. A
particularly suitable
formulation, 4.5% w A with 0.015% WD200, was tested with no added salt and
with two
common clay stabilizers: 2% KCl and 0.2% tetramethylammonium chloride (TMAC).
Fig. 3 shows that all three had essentially the same viscosity vs. temperature
profiles at
100 sec ~ .
[0049] Example 5: The water quality used to make up fluids in the oilfield,
for example
fracturing fluids, is a concern in many areas of the world. It is difficult to
obtain clean
water in some locations. Water with high hardness and other contaminants is
frequently
used. Produced water, typically high in calcium and chloride, is generated on
site as a
waste product and its disposal may be expensive. It is very advantageous if a
fracturing
fluid can be made with any water available, including produced water, and
reliably gels
without concerns about the water quality. Viscoelastic surfactant/rheology
enhancer
combinations of the invention, for example a Zw A/WD200 formulation containing
4.5% as-received Zw A and 0.015% WD200, show good compatibility with produced
water. Fig. 4 shows that the fluid viscosity is still good when the calcium
concentration
reaches 2000 ppm, even in combination with a high concentration of NaCI,
simulating
the major salinity contributors in typical produced water.
18
CA 02561814 2006-10-02
Attorney Docket No. 56.0885
Inventorsv Chen et al
[0050] Example 6: In wells that need pressure control, or for gravel pack
applications, a
gel in a high-density brine is commonly desired. Viscoelastic
surfactant/rheology
enhancer combinations of the invention, for example a Zw A/WD200 formulation
containing 4.5% as-received Zw A and 0.015% WD200, show excellent
compatibility
with heavy brine. As an example, shown in Fig. 5, not only was the fluid
viscosity
performance excellent in a 13 ppg (1.56 kg/L) CaBr2/CaCl2 brine, but also the
heavy
brine increased the viscosity and thermal stability (temperature limit) of the
fluid system.
19