Note: Descriptions are shown in the official language in which they were submitted.
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
PROCESS AND CATALYSTS FOR THE OPENING OF NAPHTHENE RINGS
The present invention relates to a process for the up-
grading of distillates having a boiling point ranging from
100 to 450°C. The process comprises the opening of the ring
of the naphthene compounds contained in these distillates
to obtain prevalently branched, open-chain paraffiniC com-
pounds, mainly having the same number of carbon atoms as
the starting naphthene.
The process is carried out in the presence of a bi-
functional catalytic system comprising one or more metals
selected from Pt, Pd, Ir, Ru, Rh and Re, and a silico-
aluminate of an acidic nature, selected from a micro-
mesoporous silico-alumina having a suitable composition,
and a zeolite belonging to the MTW group.
The present invention also relates to particular Cata-
lytic compositions. The production of clean fuels for use
in new generation engines which reduce exhaust emissions,
is one of the main problems of the refining industry.
The definition of future specifications of fuels is
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
still subject to discussion, however in order to meet with
the increasingly strict regulations with respect to emis-
sions, medium distillates will undoubtedly be requested
with significantly different characteristics from those
currently used, also from the point of view of composition.
As far as gas oil for motor vehicles is concerned, in
addition to a reduction in the sulfur content, other impor
tant aspects linked to the quality of diesel fuel and which
will probably be the object of stricter regulations in the
near future, are: the content of aromatic products, the
density, the T95 (temperature at which 950 of the product
distills) and the cetane number.
Most probably, not all of the characteristics listed
above will be the object of future regulations, it is cer
taro however that from a compositional point of view, the
reduction in the content of aromatic products by saturation
of the ring to naphthene compounds represents an effective
method for improving the properties of diesel fuel, as it
leads to a reduction in the density, the boiling point with
the same molecular weight and an increase in the cetane
number. A further improvement in the characteristics in the
above sense, can be obtained through the opening reaction
of the naphthene ring to open-chain aliphatic compounds.
Paraffins are the best compounds as, with the same molecu-
lar weight, they have a higher cetane number, a lower den-
- 2 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
city and boiling point.
Generally speaking, there are two processes commer
cially available for obtaining medium distillates with a
reduced content of aromatic products: dearomatization and
hydrocracking.
In the former case, the conversion of the aromatic
structures into naphthene structures causes a significant
reduction in the density and the boiling point, and an in-
crease in the cetane number with low yields to cracking
products. It should be noted, however, that in this case
there is a high hydrogen consumption, with rather limited
increases in the cetane number and reduction in the den-
city.
A typical process for the production of distillates is
hydrocracking. The catalysts used are of the bifunctional
type, i.e. consisting of metals which have a hydro-
dehydrogenating function, supported on a phase which can
have various acidity characteristics, generally containing
zeolite. Under the typical operating conditions, there is a
significant reduction in the content of aromatic rings, but
with much high yields to light products.
A method which has been quite recently proposed for
considerably improving the characteristics of medium dis-
tillates, envisages saturation of the aromatic rings fol-
lowed by the selective opening of the naphthene ring into
- 3 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
the corresponding aliphatic chains with the minimum possi-
ble loss of carbon into lighter products. In this case, in
the ideal situation in which all the aromatic structures
are transformed into open-chain aliphatic compounds, there
would be a product essentially consisting of a mixture of
iso and normal paraffins, thus obtaining the maximum bene-
fit in terms of density, boiling point and Cetane number.
Whereas the hydrogenation of aromatic structures is a rela-
tively simple operation, and there are various catalysts
commercially available, the selective opening of the naph-
thene ring is, on the other hand, much more complex. Gener-
ally speaking, the opening of the naphthene ring can be ef-
fected according to two mechanisms:
- breakage of the C-C bond by means of a mechanism of
the Carbo-cationic type; this mechanism, operating
with classical bifunctional catalysts consisting of a
metal which has a hydro/dehydrogenating function on an
acidic support, is generally characterized by a low
selectivity, due to the presence of dealkylation reac-
tions and the secondary cracking of the alkanes
formed;
- breakage of the C-C bond of the ring via hydro-
genolysis, catalyzed by a metal such as platinum, rho-
dium or iridium, on a non-acidic support. One of the
characteristics of this mechanism is that the conver-
- 4 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
sion of rings with six chain-ends is much slower and
less selective with respect to rings with five chain-
ends.
In the case of the naphtha cut, there are various pro-
cesses in which the charge is upgraded by means of the hy-
dro-decyclization of the naphthene compounds. These proc-
esses are characterized in that they are carried out in two
steps: in the first step, the opening of the naphthene ring
is obtained, and in the second step the paraffins formed
are isomerized in order to increase the octane number.
US 5,463,155, for example, describes a combined proc-
ess for increasing the content of isoparaffins in naphtha,
which comprises:
- a treatment step of naphtha, giving a paraffin-
enriched intermediate mixture, effected with a non-
acidic catalyst comprising at least one metal of the
platinum group and a support selected from metal ox-
ides and large-pore zeolites, wherein the support is
made non-acidic by means of suitable impregnation or
ion exchange treatment with solutions of alkaline or
alkaline-earth salts;
- a second step in which the mixture thus obtained is
subjected to isomerization in the presence of an acid
catalyst containing at least one metal of the platinum
group.
- 5 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
US 5,382,731 describes a two-step process in which the
charge is put in contact, in the first reactor, with a
ring-opening catalyst, consisting of a component which has
a hydro-dehydrogenating function and an acidic solid con-
s silting of zirconia modified with tungstate. The second re-
actor operates in such a way as to favour isomerization. In
this case, the catalyst consists of platinum deposited on
alumina and the reaction takes place in the presence of a
chlorinated compound. US 5,763,731 describes a process for
selective ring-opening in compounds of the naphthene type,
giving paraffinic-type compounds. The process uses cata-
lysts containing a metal selected from Ir, Ru or their mix-
tures and is capable of reducing the number of cyclic
structures in the product by the opening of the ring, with-
out dealkylation of the alkyl substituents linked to these
cycles. As the end-product has an increased cetane number,
the paraffins thus obtained prove to be mainly linear or
with low branchings. This patent also specifies that the
use of platinum on a Y-type zeolitic support leads to very
low selectivities towards the opening of the ring.
EP 875288 describes a process for the ring-opening of
organic compounds containing cycles using a catalyst con-
taming a support selected from alumina, silica, zirconia
or their mixtures, a metal selected from Pt, Pd, Rh, Re,
Ir, Ni, cobalt and their mixtures, and a metal selected
- 6 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
from W, Mo, La and rare earth metals. The product obtained
mainly contains n-paraffins, whereas skeleton isomeriza-
tion, cracking and dehydrogenation reactions are substan-
tially suppressed.
When the reaction is effected on bifunctional cata-
lysts, the splitting of the C-C bond present in paraffinic
chains or naphthene compounds takes place by the formation
of a carbo-ration and subsequent (3-splitting reaction. The
most important difference between paraffinic and naphthene
compounds is the splitting rate of the C-C bond which, in
the case of naphthene compounds is lower in orders of mag-
nitude (Weitkamp, J. and Ernst, S., Catal. Today 19,107
(1994)). As a result of this difference in reactivity, the
subsequent cracking reactions of the products formed, cause
an extremely low selectivity towards the opening of the
naphthene ring.
The patent EP 582347 describes a bifunctional catalyst
which comprises:
a) a component of an acidic nature consisting of a gel of
silica and alumina amorphous to X-rays, having a molar ra-
do SiOz/A1z03 ranging from 30 to 500, a surface area rang-
ing from 500 to l, 000 m2/g, with a porosity of 0 .3 to 0 . 6
mg/g, a pore diameter prevalently within the range of 10-30
A;
b) one or more metals belonging to group VIII.
- 7 _
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
This material is capable of catalyzing the hydro-
isomerization of n-paraffins.
The Applicant has now surprisingly found a process for
obtaining the opening of the naphthene rings to give paraf
finic compounds, preferably branched, in a single reaction
step, with high conversions and selectivities, using cata-
lytic compositions having suitably calibrated acidity char-
acteristics. Contrary to what is currently known, it has
been found that by the appropriate selection of the acidic
component and metal, it is possible to obtain, also in the
case of a bifunctional catalyst, conversions of naphthene
rings to paraffins with significantly higher selectivities
with respect to the known art.
A first object of the present invention therefore re-
fates to a process for the upgrading of distillates having
a boiling point ranging from 100 to 450°C, by the ring
opening of the naphthene compounds contained in the distil
fates to give paraffin mixtures, said process consisting in
treating said distillates, in the presence of hydrogen,
with a catalytic system comprising:
a) one or more metals selected from Pt, Pd, Ir, Ru, Rh
and Re
b) a silico-aluminate of an acidic nature selected from a
zeolite belonging to the MTV~1 group and a completely amor
phous, micro-mesoporous silico-alumina, having a molar ra
_ g _
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
do Si02/A1203 ranging from 30 to 500, a surface area
greater than 500 m2/g, a pore volume within the range of
0.3-1.3 ml/g, an average pore diameter of less than 40 A.
The distillates thus treated prove to be enriched in
paraffins mainly having the same number of carbon atoms as
the starting naphthenes.
Isoparaffinic compounds prevail in the paraffinic mix-
tures thus obtained.
The acid component (b) of the catalytic composition
can be selected from zeolites of the MTW type: the MTW
group is described in Atlas of zeolite structure types,
W.M.Meier and D.H.Olson, 1987, Butterworths. ZSM-12 zeo
lite, described in US 3,832,449, is preferably used for the
process of the present invention.
When the component of an acidic nature (b) is a
silico-alumina, a preferred aspect is that the Si02/A1203
molar ratio ranges from 50/1 to 300/1 and the porosity from
0.4 to 0.5 ml/g.
Micro-mesoporous, completely amorphous silico
aluminas, useful for the present invention, called MSA, are
described in US 5,049,536, EP 659,478, EP 812,804. Their
XRD spectrum from powders does not have a crystalline
structure and does not show any peak.
Catalytic compositions which can be used in the pres-
ent invention, in which the acid component is a silico-
- g _
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
alumina of the MSA type, are described in EP 582,347.
As far as the metallic component of the catalytic Com-
positions used in the process of the present invention are
concerned, this is selected from Pt, Pd, Ir, Ru, Rh, Re and
their mixtures. According to a particularly preferred as-
pect of the present invention, the metal is platinum, irid-
ium or their mixtures.
The metal or mixture of metals is preferably in a
quantity ranging from 0.1 to 5% by weight with respect to
the total weight of the catalytic composition, and prefera-
bly ranges from 0.3 to 1.5%.
The weight percentage of the metal, or metals, refers
to the content of metal expressed as a metallic element; in
the end-catalyst, after Calcination, said metal is in the
form of an oxide.
The catalytic compositions containing one or more met-
als selected from Pt, Pd, Ir, Ru, Rh and Re, and, as acidic
component completely amorphous micro-mesoporous silico-
aluminas of the MSA type, are new and are a particular as-
pest of the present invention.
Before being used, the catalyst is activated by means
of the known techniques, for example by means of a reduC-
tion treatment, and preferably by drying and subsequent re-
duction. The drying is effected in an inert atmosphere at
temperatures ranging from 25 to 100°C, whereas the reduc-
- 10 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
tion is obtained by thermal treatment of the catalyst in a
reducing atmosphere (H2) at a temperature ranging from 300
to 450°C.
The acidic component (b) of the catalyst adopted in
the process of the present invention can be used as such or
in extruded form with traditional binders, such as for ex-
ample aluminum oxide, bohemite or pseudo-bohemite. The
acidic component (b) and the binder can be premixed in
weight ratios ranging from 30:70 to 90:10, preferably from
50:50 to 70:30. At the end of the mixing, the product ob-
tamed is consolidated in the desired end-form, for example
in the form of extruded pellets or tablets.
With respect to the metal phase (a) of the catalyst,
this can be introduced by means of impregnation or ion ex-
change. According to the first technique, the acidic compo-
vent (b), also in extruded form, is wetted with an aqueous
solution of a compound of the metal, operating, for exam-
ple, at room temperature, and at a pH ranging from 1 to 4.
The aqueous solution preferably has a concentration of the
metal expressed as g/1 ranging from 0.2 to 2Ø The result-
ing product is dried, preferably in air, at room tempera-
ture, and is calcined in an oxidizing atmosphere at a tem-
perature ranging from 200 to 600°C.
In the case of alcohol impregnation, the acidic compo-
vent (b) is suspended in an alcohol solution containing the
- 11 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
metal. After impregnation, the solid is dried and calcined.
According to the ion exchange technique, the acidic
component (b) is suspended in an aqueous solution of a com
Alex or salt of the metal, operating at room temperature
and at a pH ranging from 6 to 10. After the ion exchange,
the solid is separated, washed with water, dried and fi-
nally thermally treated in an inert or oxidizing atmos-
phere. Temperatures useful for the purpose range from 200
to 600°C.
Metal compounds well suited for the preparations de-
scribed above are : H2PtCl6,
Pt
(NH3)
4 (OH)
2,
Pt
(NH3)
4C12,
Pd (NH3) 4 (OH) 2, PdCl~, H2IrCl6,RuCl3, RhCl3 When the cata-
.
lytic composition comprises more than one metal, the im-
pregnation is carried out follows: the acidic component
as
(b), also in extruded form, is wetted witha solution of
a
compound of a first metal, the resulting product is dried,
optionally calcined, and is impregnated with a solution of
a compound of a second metal. The product is dried and cal-
cination is then effected in an oxidizing atmosphere at a
temperature ranging from 200 to 600°C. Alternatively, a
single aqueous solution containing two or more compounds of
different metals can be used for contemporaneously intro-
during said metals.
The catalytic compositions of the present invention in
which the acidic component is a silico-alumina of the MSA
- 12 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
type with a pore volume greater than 0.6 ml/g, are new and
are a further object of the present invention.
The catalytic compositions of the present invention in
which the acidic component is of the ZSM-12 type, are new
and are a further aspect of the present invention.
The distillates which can be subjected to this upgrad-
ing process are mixtures having boiling points within the
range of 100 to 450°C. In particular, they can be hydrocar-
bon cuts selected from naphthas, diesel, kerosene, jet
fuel, light cycle oil, HVGO, heavy FCC fraction.
The process of the present invention is carried out at
a temperature ranging from 240 to 380°C, at a pressure
ranging from 20 to 70 atm, at a WHSV ranging from 0.5 to 2
hours-1 and a ratio between hydrogen and charge (H~/HC)
ranging from 400 to 2,000 Nlt/kg. It is preferable to oper-
ate at a pressure higher than 40 atm and lower than or
equal to 70 atm, whereas the temperature preferably ranges
from 240 to 320°C when the acidic component ((b) is a zeo-
lite of the MTW type, whereas it preferably ranges from 300
to 380°C when the acidic component (b) is a silico-alumina.
The following experimental examples are provided for a
better illustration of the present invention.
Example 1
Preparation of catalyst A: ZSM-12/0.50 Pt
a) Preparation of ZSM-12 zeolite
- 13 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
127 grams of tetra-ethyl ammonium hydroxide at 40% by
weight, in aqueous solution, are added to 24 grams of de-
mineralized water. 4 grams of sodium aluminate at 56% by
weight of A1203 are then added. The limpid solution thus
obtained is poured, under stirring, into 350 grams of Ludox
HS 400 colloidal silica. After brief stirring, a homogene-
ous limpid gel is obtained, which is poured into a 1 litre
autoclave made of AISI 316 steel, equipped with an anchor
stirrer. The gel is left to crystallize under hydro-thermal
conditions at 160°C for 60 hours. At the end of this phase,
the autoclave is cooled to room temperature. The slurry ob-
tamed is homogeneous with a milky appearance. The slurry
is centrifuged. The solid discharged is washed by re-
dispersion in water, centrifuged again, dried at 120°C and
Calcined at 550°C for 5 hours. Upon .X-ray diffraction
analysis, the solid obtained proves to consist of pure ZSM
12. The solid obtained is subsequently exchanged in ammonia
form by treatment with a 3 M solution of ammonium~acetate.
Upon subsequent calcination at 550°C for 5 hours, the zeo
lite is obtained in acidic form.
b) Deposition of platinum (0.5o by weight of Pt)
In order to disperse the platinum onto the zeolite
prepared in the previous step (a), an aqueous solution of
hexachloroplatinic acid (H2PtCl6), hydrochloric acid and
acetic acid was used, in the following molar ratios:
- 14 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
H2PtCl6/HCL/CH3COOH = 1/0.84/0.05, having a platinum Concen-
tration of 0.75 g/1. A volume of 200 ml of this solution
was added to 30 g of the zeolite prepared as described
above, so that all the solid was covered by the solution,
to avoid heterogeneity in the platinum distribution. The
suspension thus obtained was maintained under stirring for
about an hour at room temperature and subsequently degassed
by suction under vacuum (about 18 mmHg) at room tempera-
ture. The solvent was subsequently removed by heating to
about 70°C under vacuum. The dry product was finally Cal-
tined under a stream of air with the following temperature
profile 25-350°C in two hours, 360°C for 3 hours.
A ZSM-12 zeolite is obtained, containing 0.5% by
weight of platinum.
Example 2
Preparation of catalyst B: ZSM-12/lo Pt
A quantity of platinum equal to 1 o by weight, is de-
posited on a ZSM-12 zeolite, prepared as described in step
(a) of the previous example 1. The same procedure described
in step (b) of Example 1 is adopted for the deposition, us-
ing 400 ml of the same aqueous solution of hexachloro-
platiniC acid and 30 g of ZSM-12 zeolite.
At the end, a ZSM-12 zeolite is obtained, containing to of
platinum.
Example 3
- 15 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Preparation of catalyst C: ZSM-12/1% Pt
A quantity of platinum equal to to by weight is depos-
ited on a ZSM-12 zeolite prepared as described in the pre-
vious Example l, using an aqueous solution of platinum
tetra-amine hydroxide Pt(NH3)4(OH)z having a platinum Con-
Centration of 0.861 g/1. A volume of 180 ml of this solu-
tion was added to 15.5 g of ZSM-12 prepared as described in
step (a) of the previous Example l, so that all the solid
is covered by the solution, in order to avoid heterogeneity
in the platinum distribution. The suspension thus obtained
is maintained under stirring for about an hour at room tem-
perature and subsequently degassed by suction under vacuum
(about 18 mmHg) at room temperature. The solvent is subse-
quently removed by heating to about 70°C under vacuum. The
dry product is finally Calcined under a stream of air with
the following temperature profile 25-380°C in two hours,
380°C for 3 hours.
A ZSM-12 zeolite is obtained, containing 01o by weight
of platinum.
Example 4
Preparation of catalyst D: MSA 100/1% Pt
a) Preparation of the MSA acidic component
23.5 litres of demineralized water, 19.6 kg of aqueous
solution at 14.4% by weight of TPA-OH and 600 g of aluminum
tri-isopropoxide are introduced into a 100 litre reactor.
- 16 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
The mixture is heated to 60°C and maintained under stirring
at this temperature for 1 hour, in order to obtain a limpid
solution. The temperature of the solution is then brought
to 90°C and 31.1 kg of tetra-ethyl silicate are rapidly
added. The reactor is closed and the stirring rate is regu-
fated at about 1.2 m/s, the mixture being maintained under
stirring for three hours at a temperature ranging from 80
to 90°C, with thermostatic control to remove the heat pro-
duced by the hydrolysis reaction. The pressure in the reac-
for rises to about 0.2 Mpag. At the end, the reaction mix-
ture is discharged and cooled to room temperature, obtain-
ing a homogeneous and relatively fluid gel (viscosity 0.011
Pa~s) having the following composition molar ratios:
Si02/A1203 = 101
TPA.OH/Si02 = 0.093
HZO/SiO~ = 15
The product is left to rest for about 6-8 hours and is
then dried by maintaining it in a stream of air at 100°C
until the weight becomes constant. It is finally calcined
in a muffle at 550°C for 8 hours in air.
In this way, a porous solid is obtained, with acidic
characteristics, essentially consisting of silica-alumina
with a molar ratio Si02/A1203 - 100, a BET surface area of
740 mz/g, a pore volume of 0.49 ml/g, an average diameter
of 2.3 nm. Upon X-ray analysis, the solid proves to be sub-
- 17 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
stantially amorphous, the XRD spectrum from powders does
not have a crystalline structure and does not show any
peak.
b) Deposition of platinum (1o by weight of Pt)
In order to disperse the platinum onto the acidic com-
ponent, an aqueous solution of hexachloroplat.inic acid
(HZPtCl6), hydrochloric acid and acetic acid was used, in
the following molar ratios: H~PtCl6/HCl/CH3COOH -
1/0.84/0.05, having a platinum concentration of 0.75 g/1. A
volume of 400 ml of this'solution was added to 30 g of the
zeolite prepared as described in the previous step (a), so
that all the solid was covered by the solution, to avoid
heterogeneity in the platinum distribution. The suspension
thus obtained was maintained under stirring for about an
hour at room temperature and subsequently degassed by suc-
tion under vacuum (about 18 mmHg) at room temperature. The
solvent was subsequently removed by heating to about 70°C
under vacuum. The dry product was finally calcined under a
stream of air with the following temperature profile 25-
350°C in two hours, 350°C for 2 hours, 350-400°C in 50
min., 400°C for 3 hours.
A silico-alumina of the MSA type is obtained, contain-
ing 1% by weight of platinum.
Example 5
Preparation of catalyst E: MSA 50/1% Pt
- 18 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
a) Preparation of the MSA acidic component
23.5 litres of demineralized water, 19.6 kg of aqueous
solution at 14.40 by weight of TPA-OH and 1,200 g of alumi-
num tri-isopropoxide are introduced into a 100 litre reac-
tor. The mixture is heated to 60°C and maintained under
stirring at this temperature for 1 hour, in order to obtain
a limpid solution. The temperature of the solution is then
brought to 90°C and 31.1 kg of tetra-ethyl silicate are
rapidly added. The reactor is closed and the stirring rate
is regulated at about 1.2 m/s, the mixture being maintained
under stirring for three hours at a temperature ranging
from 80 to 90°C, with thermostatic control to remove the
heat produced by the hydrolysis reaction. The pressure in
the reactor rises to about 0.2 MPag. At the end, the reac-
tion mixture is discharged and cooled to room temperature,
obtaining a homogeneous and relatively fluid gel (viscosity
0.011 Pa~s) having the following composition molar ratios:
SiO~/A1203 = 50.5
TPA.OH/Si02 = 0.093
H20/Si02 = 15
The product is left to rest for about 6-8 hours and is
then dried by maintaining it in a stream of air at 100°C
until the weight becomes constant. It is finally calcined
in a muffle at 550°C for 8 hours in air.
In this way, a porous solid is obtained, with acidic
- 19 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
characteristics, essentially consisting of silica-alumina
with a molar ratio Si02/A1203 - 50.2, a BET of 794 m2/g, a
pore volume of 0.42 ml/g, an average diameter of 2.1 nm.
Upon X-ray analysis, the solid proves to be substantially
amorphous, the XRD spectrum from powders does not have a
crystalline structure and does not show any peak.
b) Deposition of platinum (1.0% by weight of Pt)
The same procedure is adopted as described in Example
4, using 400 ml of the same aqueous solution of hexachloro-
platinic acid, which are added to 30 g of the solid pre-
pared under point (a).
A silico-alumina of the MSA type is obtained, contain-
ing 1% of platinum.
Example 6
Catalyst F: MSA 50/1% of Pt
A quantity of Pt equal to 1% by weight is deposited on
an MSA acid component prepared according to step (a) of Ex-
ample 5, using the same deposition procedure as that de-
scribed in Example 3: 180 ml of an aqueous solution of
Pt(NH3)4(OH)2 are used, whose platinum titer is 0.861 g/1,
and added to 15.5 g of MSA acid solid prepared as described
in step (a) of Example 5.
A silico-alumina of the MSA type is obtained, contain-
ing 1% of platinum.
Example 7
- 20 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Catalyst G: MSA 50/1% of Pt
A quantity of Pt equal to 1.5% by weight is deposited
on an MSA acid component prepared according to step (a) of
Example 5, using the same deposition procedure as that de-
scribed in Example 4: 400 ml of an aqueous solution of
hexachloroplatiniC acid, with. a platinum concentration of
1.125 g/1, are added to 30 g of MSA acid component.
A silico-alumina of the MSA type is obtained, contain-
ing 1.5% of Pt.
Example 8
Catalyst H: MSA 50/1% of Ir
A quantity of Ir equal to 1% by weight is deposited on
an MSA acid component prepared according to step (a) of Ex-
ample 5, using an aqueous solution of hexachloroiridic acid
(HZIrCl6), hydrochloric acid and acetic acid in the follow-
ing molar ratios: H2IrC16/HCl/CH3COOH - 1/0.84/0.05, having
a concentration of iridium of 0.75 g/1. A volume of 400 ml
of this solution is added to 30 g of the solid prepared as
described in the previous step (a) , so that all the solid
is covered by the solution, in order to avoid heterogeneity
in the platinum distribution. The suspension thus obtained
is maintained under stirring for about an hour at room tem-
perature and subsequently degassed by suction under vacuum
(about 18 mmHg) at room temperature. The solvent is subse-
quently removed by heating to about 70°C in a stream of
- 21 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
air. The dry product is finally calcined under a stream of
air with the following temperature profile 25-350°C in two
hours, 350°C for 2 hours, 350-400°C in 50 min., 400°C for
3
hours.
A silico-alumina of the MSA type is obtained, contain-
ing 1% by weight of Ir.
Example 9
Catalytic test
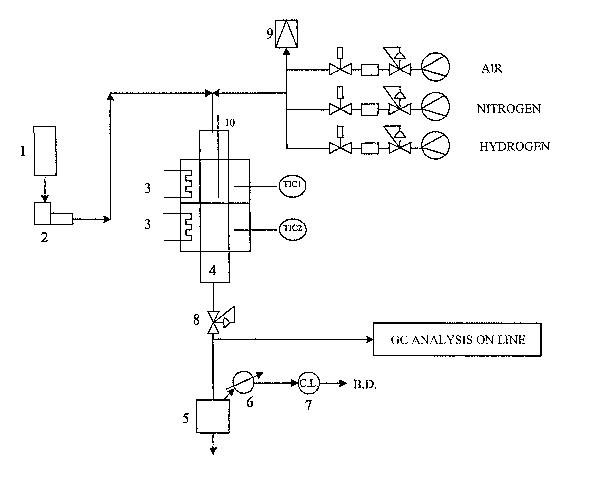
The catalytic tests were carried out on a continuous
laboratory plant shown in Figure 1. The system consisted of
a tubular fixed bed reactor (4) with a useful volume of the
charge of 20 cm3 corresponding to a height of the catalytic
bed in the isotherm section of 10 cm. The feeding of the
charge, contained in the tank (1) and hydrogen to the reac-
for are effected by means of a dosage pump (2) and a mass
flow meter, respectively. The system is also equipped with
two gas lines (air and nitrogen) which are used in the re-
generation phase of the catalyst. The reactor operates in
an equicurrent down flow system. The temperature of the re-
actor is regulated by means of an oven with two heating
elements (3) whereas the temperature control of the cata-
lytic bed is effected by means of a thermocouple (10) posi-
tioned inside the reactor. The pressure of the reactor is
regulated by means of a valve (8) situated downstream of
the reactor. The reaction products are collected in a sepa-
- 22 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
rator (5) which operates at room temperature and atmos-
pheric pressure. The products leaving the separator (5)
pass into a condenser (6) cooled to 5°C and are subse-
quently sent to a gas meter (C.L.) (7) and then to the
blow-down (B.D. ) . (9) is the breakage disk. The distribu-
tion of the products and conversion level are determined by
means of mass balance and gas chromatographic analysis of
the reaction products.
Catalysts A, B and C were tested in the process of the
present invention, in the equipment described above, using
methyl-cyclohexane as substrate.
Before being tested, the catalysts were activated as
follows:
1) 1 hour at room temperature in a nitrogen stream;
2) 1 hour at 50°C in a hydrogen stream;
3) heating from room temperature to 380°C with a profile
of 3°C/min in a hydrogen stream;
4) the temperature is kept constant at 380°C for 3 hours
in a hydrogen stream, and is then cooled to 200°C.
During the activation, the pressure in the reactor is
maintained at between 2.0 and 6.0 MPa (20 and 60 atm).
The results are indicated in Table 1 and were obtained
under the following conditions:
WHSV = 1 hour-1
H2/HC = 1143 Nlt/kg
- 23 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Pressure = 60 atm
Table 1
Cat. A Cat. B Cat. C
Temp:300C Temp:320C Temp:310C
Conv. % 74.4 79.9 80.4
Sel. R0 n-parafFins3.6 7.9 8.1
%
Sel. R0 iso-paraffins32.3 37.2 37.9
%
Sel. Npth C, % 46.3 39.5 37.6
Sel. Crack % 17.2 14.5 16.2
With reference to Table 1, the meaning of the abbre-
viations is provided hereunder:
Conv. o: [(g/h Methyl-CyClO hexane fed - g/h Methyl-
cyclohexane in the products)/(g/h Methyl-cyclohexane fed)]
x 100
RO . ring opening
Sel. R0 n-paraffins %: [(g/h of C6 and C~ n-paraffins in
the products)/(g/h of methyl-cyclohexane converted)] x 100
Sel. R0 iso-paraffins %: [ (g/h of C6 and C~ iso-paraffins
in the products)/(g/h of methyl-cyclohexane converted)] x
100
Sel . Crack % : [ (g/h of C1, Cz, C3, C4 and CS iso-alkanes and
n-alkanes) ] x 100
Sel . Npth C~ o : [ (g/h of ethyl-oyclopentane and isomers of
dimethyl cyclopentane)/(g/h of methyl-cyclohexane con
verted) ] x 100 .
- 24 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
The ethyl-cyclopentane and isomers of dimethyl cyclo-
pentane are first formation products which in turn give
rise to the formation of n-paraffins and iso-paraffins by
the ring opening.
Unless otherwise specified, the complement to 100 of
the selectivities consists of minority reaction products
such as methyl-cyclopentane, cyclopentane and cyclohexane.
In the examples indicated in Table 1 and in those in-
dicated below, the (iso-C6 + n-C6) / (iso-C~ + n-C~) ratio is
lower than 0.1.
Example 10
Catalytic test
Following the procedure described in Example 9, cata-
lysts D (MSA 100/1% Pt), E (MSA 50/1.0% Pt), F (MSA 50/1.0%
Pt) and G (MSA 50/l.5% Pt) were evaluated. The results are
provided in Table 2 and were obtained under the following
conditions:
WHSV = 1 hour-1
H~/HC = 1143 Nlt/Kg
2 0 P = 6 0 atm
- 25 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Table 2
Cat. Cat. E Cat. F Cat. G
D 340C 320C 340C
360C
Conv. % 94.8 91.4 81.8 91.0
Sel. R0 n-paraffins14.2 13.5 10.6 10.8
%
Sel. R0 iso-paraffins58.3 60.3 48.8 61.3
%
Sel. Npth C, % 10.2 14.9 33.9 14.4
Sel. Crack % 15.5 11.2 6.7 10.3
Example 11
Catalytic test with MSA 100/1% Pt - Pressure effect
Following the procedure described in Example 9, the
performances of catalysts D (MSA 100/10 Pt) and F (MSA
50/1.0% Pt) were determined with variations in the pres-
sure.
In all cases the WHSV was 1 hour-1 and the HZ/HC molar
ratio was 1143 Nlt/Kg. The results indicated in Table 3
show that the increase in pressure causes an increase in
the conversion together with a considerable increase in the
selectivity to C6-C~ iso-paraffins and n-paraffins.
- 26 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Table 3
Cat. D Cat. F
Temp: 360C Temp:
320C
Pressure (atm) 20 70 40 60
Conv. % 74.6 98.1 76.8 81.8
Sel. R0 n-paraffins4.2 15.7 8.6 10.6
%
Sel. R0 iso-paraffins22.2 57.4 29.5 48.8
%
Sel. Npth. C, 59.0 3.6 55.3 33.9
%
Sel. Crack % 7.3 18.6 6.6 6.7
Example 12
Catalytic test with catalyst C: ZSM-12/1% Pt
Following the procedure described in Example 9, cata-
lyst C was used, varying the pressure from 20 to 70 atm.
The results are indicated in Table 4.
The tests were effected under the following operating
conditions: Temperature: 310°C; WHSV - 1.0 hour-l; Hz/HC -
1143 Nlt/Kg.
In this case, unlike the behaviour shown by the rata-
lytic system containing MSA as acidic component, the in-
crease in pressure had no effect on the conversion, it in-
creases the selectivity to C6 and C~ iso-paraffins and n-
paraffins and at the same time considerably decreases the
selectivity to cracking products.
- 27 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Table 4
Pressure (atm) 20 40 60 70
Conv. % 78.9 80.1 80.4 80.9
Sel. R0 n-paraffins6.6 7.6 8.2 10.7
%
Sel. R0 iso-paraffins29.7 35.2 37.8 39.1
%
Sel. Npth. C, 38.9 38.5 37.6 35.3
%
Sel. Crack % 23.8 18.4 16.2 14.3
Example 13
Catalytic test
Following the procedure described in Example 9, cata-
lyst H (MSA 50/1.0 Ir) was evaluated.
The results are indicated in Table 5 and were obtained
under the following conditions:
WHSV = 1 hour-1
Hz/HC = 1143 Nlt/Kg
P = 20 atm
Table 5
Cat. H
2 0 260C
Conv. % 81
Sel. R0 n-paraffins % 3.4
Sel. R0 iso-parafFins % 84.2
Sel. Npth. C~ % 4.3
2 5 Sel. Crack % 8.0
- 28 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Example 14 (comparative)
Preparation of catalyst I: Mordenite/l.Oo Pt
Engelhard HSZ-690HOA mordenite is used, characterized
by an Si02/A1203 ratio = 200.
In order to disperse the platinum onto this zeolite,
an aqueous solution of hexachloroplatinic acid (HzPtCl6),
hydrochloric acid and acetic acid was used, in the follow-
ing molar ratios: HZPtClg/HC1/CH3COOH = 1/0.84/0.05, having
a platinum concentration of 0.75 g/1. A volume of 200 ml of
this solution was added to 30 g of the zeolite so that all
the solid was covered by the solution, to avoid heterogene-
ity in the platinum distribution. The suspension thus ob-
tamed was maintained under stirring for about an hour at
room temperature and subsequently degassed by suction under
vacuum (about 18 mmHg) at room temperature. The solvent was
subsequently removed by heating to about 70°C under vacuum.
The dry product was finally calcined under a stream of air
with the following temperature profile 25-350°C in 2 hours,
360°C for 3 hours.
A mordenite is obtained, containing 1.0 % by weight of
platinum.
Example 15 (comparative)
Preparation of catalyst L: ZSM-23/1.0% Pt
a) Preparation of ZSM-23
The ZSM-23 zeolite is prepared according to Ernst,
- 29 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Verified Syntheses of Zeolitic Materials H. Robson Ed., El-
sevier, (2001) page 217:
6.4 g of Cab-o-Sil were dispersed in 78.2 g of an 0.55
M aqueous solution of NaOH. A solution containing 9.5 g of
H20, 3.4 g of pyrrolidine and 0.7 g of A1~(S04)3*16H20 was
added to the slurry obtained. Finally, 1.7 g of HzS04 were
added dropwise under vigorous stirring. The slurry ob-
tamed, having a pH value of 11.6, was left to crystallize
under hydro-thermal conditions at 180°C for 2 days in an
AISI 316 steel autoclave, subjected to a rotating movement.
The ZSM-23 zeolite at this stage of the synthesis is
in soda form, in order to obtain the acidic form, it is
subjected to the treatment described hereunder.
100 g of ZSM-23 are redispersed in 1000 g of an 0.2 M
solution of ammonium acetate. The mixture is left under
stirring at 40°C for about 3 hours. The solid phase is then
separated and the operation is repeated twice. The solid is
finally redispersed in demineralized water for a last wash-
ing.
The damp panel obtained consists of ZSM-23 in ammonia
form. In order to obtain the zeolite in acidic form, the
solid is dried at 150°C, and is then calcined at 550°C for
5 hours in air, thus eliminating the ammonia and possible
present of organic templating agent still blocked.
b) deposition of the platinum (1.0 % Pt)
- 30 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
A quantity of platinum equal to 1% by weight is deposited
on a ZSM-23 zeolite prepared as described in the previous
step (a), using an aqueous solution of platinum tetra-amine
hydroxide Pt(NH3)4(OH)2, having a platinum concentration of
0.861 g/1. A volume of 180 ml of this solution was added to
15.5 g of ZSM-23 prepared as described in the previous step
(1) , so that all the solid is covered by the solution, to
avoid heterogeneity in the platinum distribution. The sus-
pension thus obtained was maintained under stirring for
about an hour at room temperature and subsequently degassed
by suction under vacuum (about 18 mmHg) at room tempera
ture. The solvent was subsequently removed by heating to
about 70°C under vacuum. The dry product was finally cal
cined under a stream of air with the following temperature
profile 25-380°C in 2 hours, 380°C for 3 hours.
A ZSM-23 zeolite is obtained, containing 1% of plati-
num.
Example 16 (comparative)
Preparation of catalyst M
For the preparation of this catalyst, a commercial
amorphous silico alumina (PK-200 of Kalichemie) is used,
having the following characteristics:
~ weight % composition: 90% SiOz, 10o A12O3
~ molar ratio Si02/A1z03: 15.3
~ surface area: 450 m2/g
- 31 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
In order to disperse the platinum onto this silico-
alumina, an aqueous solution of hexachloroplatinic acid
(HzPtCl6), hydrochloric acid and acetic acid was used, in
the following molar ratios: HZPtCl6/HCl/CH3COOH -
1/0.84/0.05, having a platinum concentration of 0.75 g/1. A
volume of 200 ml of this solution was added to 30 g of the
silico-alumina so that all the solid was covered by the so-
lution, to avoid heterogeneity in the platinum distribu-
tion. The suspension thus obtained was maintained under
stirring for about an hour at room temperature and subse-
quently degassed by suction under vacuum (about 18 mmHg) at
room temperature. The solvent was subsequently removed by
heating to about 70°C under vacuum. The dry product was fi-
nally calcined under a stream of air with the following
temperature profile 25-350°C in 2 hours, 360°C for 3 hours.
A silico-alumina is obtained, containing 1.0 % by
weight of platinum.
Example 17 (comparative)
Catalytic test
The catalytic tests were carried out as described in
Example 9, on the continuous laboratory plant shown in Fig-
ure 1.
Catalysts I, L and M are tested in the process of the
present invention using methyl cyclohexane as substrate.
Before being tested the catalysts were activated as fol-
- 32 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
lows:
1) 1 hour at room temperature in a nitrogen stream;
2) 1 hour at 50°C in a hydrogen stream;
3) heating from room temperature to 380°C with a profile
of 3°C/min in a hydrogen stream;
4) the temperature is kept constant at 380°C for 3 hours
in a hydrogen stream, and is then cooled to 200°C.
During the activation, the pressure in the reactor is
maintained at between 2.0 and 6.0 MPa (20 and 60 atm).
The results are indicated in Table 6 and were obtained
under the following conditions:
WHSV = 1 hour-1
HZ/HC = 1143 Nlt/kg
Table 6
Cat. M Cat. Cat. L Cat. D Cat. B
I
PIC-200/PtMOR/PtZSM-23/PtMSA-100/PtZSM-12/Pt
360C 290C 320C 360C 320C
20 atm 20 60 atm 20 atm 60 atm
atm
Conv. % 70.2 70.4 67.4 74.6 79.9
2 Sel. R0 n-paraffins2.2 3.4 5.2 4.2 7.9
0 %
Sel. R0 iso-paraffins8.0 18.2 24.4 22.2 37.2
%
Sel. Npth C~ % 77.5 55.3 48.1 59.0 39.5
Sel. Crack % 3.8 16.4 31.3 7.3 14.5
An examination of the data of Table 6 demonstrates
that the catalytic systems based on Pk-200, mordenite (MOR)
- 33 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
and ZSM-23 show much lower performances in terms of selec-
tivity towards the ring opening or, as in the case of ZSM-
23 a much higher selectivity to cracking products with re-
spect to the performances of the catalytic systems object
of the present invention, in particular B and D.
Example 18 (comparative)
In this example, the results obtained in US 5,763,731,
Example 11 are indicated as a comparison, wherein an
Ir/amorphous Si02-A1203 catalyst, containing 0.9% by weight
of Ir and having an Si02/A1203 ratio of 85/15, is tested in
the ring opening of n-butyl-cyclohexane (BCH). The test
preparation and conditions are described in Example 1 of
said patent.
At 275°C and a total LHSV of 4.2, a conversion of BCH
of 42.20 is obtained, together with a selectivity of prod-
ucts deriving from the ring opening of 0.45.
A comparison between these results and those obtained
in Example 13 of the present patent application shows that
the catalyst containing iridium, representative of the in-
vention, provides much higher conversion and selectivity
results than those of the comparative catalyst. The diver-
sity of the results is such that only a minimum part can be
attributed to the diversity of the substrates.
Example 19
In this example, the results obtained in US 5,763,731,
- 34 -
CA 02562492 2006-10-05
WO 2005/103207 PCT/EP2005/004157
Example 7 are indicated as a comparison, wherein a Pt/ECR-
32 catalyst, containing 0.9% by weight of Ir is tested in
the ring opening of n-butyl-CyClohexane (BCH). The test
preparation and conditions are described in Example 6 of
said patent. The ECR-32 zeolite is described in US
4,931,267
At 275°C and a total LHSV of 2.9, a conversion of BCH
of 90.0% is obtained, together with a selectivity of prod-
ucts deriving from the ring opening of 0.01.
On comparing these results with those obtained in Ex-
ample 12, it can be observed that the catalyst containing
10 of Pt and ZSM-12 zeolite of the present invention pro-
vides much higher selectivity results, not proportional to
the diversity of the substrates.
20
- 35 -