Note: Descriptions are shown in the official language in which they were submitted.
CA 02562842 2006-10-06
1 "SYSTEM FOR RECOVERING GAS PRODUCED DURING
2 ELECTRODIALYSIS"
3
4 FIELD OF THE INVENTION
The invention generally relates to water treatment systems and in
6 particular to the recovery of hydrogen gas produced during desalination
by
7 electrodialysis, with the hydrogen gas being useful as a fuel source
within the unit
8 operations of the water treatment system.
9
BACKGROUND OF THE INVENTION
11 As fresh water demands increase along with the confounding
12 impacts of global warming on water, the potential for a global water
crisis is
13 imminent because of the decrease in fresh water quality, availability,
and supply
14 for human consumption and other commercial, industrial, agricultural
sectors.
Therefore, integrated water resource management, including water treatment,
16 has become one of the most urgent issues of the 21st century.
17 Depending upon the natural or anthropogenic sources, saline water
18 may generally contain dissolved metals, organic contaminants and a
complex
19 mixture of salts, ranging in a total dissolved solids (TDS)
concentration from
about 1000 mg/L to 250,000 mg/L. Typical sources of saline water are sea
21 water, naturally occurring saline surface water or brackish ground
water, fertilizer
22 salt run-off (from irrigation), salt retention ponds (from the storage
of de-icer salts
23 for transportation network maintenance), produced water (from oil and
gas
24 exploration and production, depressurizing coal bed methane or mine
operations
and drainage) and brines generated from various industrial processes.
CA 02562842 2006-10-06
1 Saline water can be treated by various desalination processes,
2 such as thermal, prissier or electrically driven, to remove dissolved
salts and
3 minerals and produce de-mineralized water for various uses, such as for
the
4 production of drinking water, effluent treatment and water reclamation.
However,
conventional desalination processes are energy intensive and can cause
6 significant operational and environmental impact.
7 Therefore, there is a need in the art for an improved
desalination
8 system and a water treatment process.
9
2
CA 02562842 2006-10-06
1 SUMMARY OF THE INVENTION
2 Embodiments of the present invention comprise a water treatment
3 process in which hydrogen gas, as a byproduct of water desalination by
4 electrodialysis, is captured. Further, the hydrogen can be used as a
source of
energy for an advanced alternative power generating device, such as a fuel
cell
6 or bio-fuel generator, to contribute to the water treatment process to
thereby
7 reduce energy consumption of the overall water treatment process.
8 More particularly, gas produced during electrodialysis (ED) of
saline
9 water is recovered from a two phase gas/liquid electrolyte solution that
flows
through an electrode compartment of a conventional ED unit. Specifically,
11 hydrogen gas is entrained in a catholyte solution circulating through a
cathode
12 compartment, while oxygen gas is entrained in an anolyte solution
circulating
13 through an anode compartment. The catholyte and anolyte solutions are
each
14 fed to separate catholyte and anolyte tanks or towers (electrolyte towers),
respectively, in which the entrained gas separates from the solution in a
16 headspace of the towers and is collected at a gas outlet within the
headspace, at
17 ambient temperature and pressure.
18 Notably, bench-scale experiments have found that attempts to
19 recover the gas directly from the electrode compartments results in
extreme pH
imbalances within the ED unit and deterioration of the ED unit's ion exchange
21 membranes, while the use of the electrolyte towers obviates that
problem.
22 Preferably, the alkaline catholyte solution and the acidic anolyte
solution
23 circulating from the towers are mixed in an electrolyte mixing tank to
neutralize
24 the pH before returning the electrolyte solution to the ED unit.
3
CA 02562842 2006-10-06
1 As opposed to some prior art systems which require the use of
2 relatively inert gas, such as nitrogen gas, to dilute or sweep the
hydrogen from
3 electrolyte solution and some others which simply vent hydrogen gas to
the
4 atmosphere, the present system captures a relatively pure hydrogen gas
stream.
Further, as the hydrogen gas is spatially separated from the oxygen gas when
it
6 is recovered, cross-contamination of the usable gas is reduced while also
7 minimizing the risk of explosion. The hydrogen gas can be further
processed to
8 increase hydrogen gas purity as required for the intended advanced power
9 generating device.
Embodiments of the invention reduce the impact of the desalination
11 process on the environment and act to recover a useful fuel source.
12 While it is particularly contemplated that the invention recovers
13 hydrogen and oxygen gas, other gases that may be produced during a
particular
14 operation of an ED unit can also be recovered.
Accordingly, in a broad aspect of the invention there is provided a
16 system for recovering gas produced during electrodialysis comprising an
17 electrodialysis unit comprising at least one electrode compartment for
circulating
18 an electrolyte solution therethrough, with the gas being entrained in
the
19 electrolyte solution, and at least one electrolyte tower fluidly
connected to the at
least one electrode compartment. The at least one electrolyte tower comprises
21 an upper headspace portion and a gas outlet positioned in the upper
headspace
22 portion. The electrolyte solution is to be circulated between the at
least one
23 electrode compartment and the at least one electrolyte tower and wherein
the
24 entrained gas is to be recovered into the head space portion and
collected from
the gas outlet.
4
CA 02562842 2006-10-06
1 In one embodiment, an electrolyte solution inlet is positioned
within
2 the headspace portion to enhance efficient separation of the gas from the
3 electrolyte solution.
4 The recovered gas can be further processed to increase the gas
purity, such as by a gas scrubber coupled with a coiled tube bubbler. Devices
6 for purifying the gas can be positioned within the tower or external to
the tower.
7 In another broad aspect of the invention, there is provided an
8 energy efficient water treatment system comprising a hydrogen powered
device
9 for providing at least a portion of power to operate the unit operations
of the water
treatment system, and an electrodialysis unit for treating a salt-containing
11 feedwater to produce a desalinated water stream and a concentrated brine
12 stream. The electrodialysis unit comprises a cathode compartment for
circulating
13 a catholyte solution therethrough, with a hydrogen gas being entrained
in the
14 catholyte solution. The system further comprises a catholyte tower
fluidly
connected to the cathode compartment, the catholyte tower comprising an upper
16 head space portion and a hydrogen gas outlet positioned within the
headspace
17 portion. The catholyte solution is to be circulated between the cathode
18 compartment and the catholyte tower and wherein the entrained gas is to
be
19 recovered into the head space portion of the catholyte tower and
collected from
the gas outlet for use in the hydrogen powered device.
21 Other embodiments of the invention are described herein.
5
CA 02562842 2006-10-06
1 BRIEF DESCRIPTION OF THE DRAWINGS
2 In drawings which are intended to illustrate embodiments of the
3 invention and which are not intended to limit the scope of the invention:
4 Figure 1 is a flow diagram of a system for recovering gas produced
by electrodialysis according to an embodiment of the present invention;
6 Figure 2 is flow diagram of a flow diagram of a water treatment
7 process using the system of Fig. 1, with recovered hydrogen gas being
used as a
8 fuel source for an advanced power generating device used in the system;
9 Figure 3 is perspective view of an embodiment of an electrolyte
tower according to Fig. 1
11 Figure 4A is a perspective view of another embodiment of a
12 catholyte tower according to Fig. 1, with the catholyte tower including
additional
13 components for purifying hydrogen gas;
14 Figure 4B is a perspective view of the electrolyte solution inlet
illustrating electrolyte solution flowing to the drain port and separated
gases
16 according to Fig. 4A;
17 Figure 5 is a perspective view of yet another embodiment of a
18 catholyte tower according to Fig. 1, with the electrolyte tower
including additional
19 components for purifying hydrogen gas and collecting fugitive gases;
Figure 6 is a flow diagram of downstream processing steps for
21 further purifying hydrogen recovered according to Fig. 1;
22 Figure 7 is a gas chromatogram of the ED process cathode gas
23 (hydrogen concentration results);
24 Figure 8 is a gas chromatogram of the ED process anode gas
(oxygen concentration results);
6
CA 02562842 2006-10-06
1 Figure 9 is
a combined table with a graphical representation of the
2
characterization of the hydrogen from the ED electrode gas tower analyzed over
3 a process run of 34 hours, as represented by eight samples;
4 Figure 10 is
a combined table with a graphical representation of the
characterization of the oxygen from the ED electrode gas tower analyzed over a
6 process run of 15 hours, as represented by six samples; and
7 Figure 11 a
combined table with a graphical representation of five
8 simulated
sample illustrating the characterization of the inlet and outlet gas
9 before and
after scrubbing Hydrogen gas with solid adsorbents at ambient
temperature and low (atmospheric) pressure (simulating the gas liquid
separation
11 chamber).
12
7
CA 02562842 2006-10-06
1 DETAILED DESCRIPTION OF THE INVENTION
2 It is to be
understood by one of ordinary skill in the art that the
3 following is
a description of exemplary embodiments only and is not intended as
4 limiting the broader aspects of the invention.
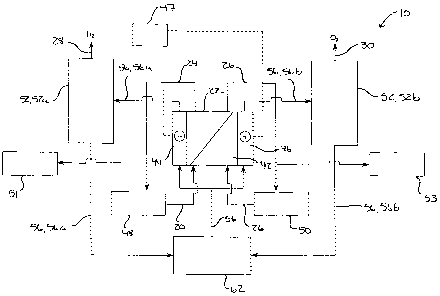
With reference to Fig. 1, a system 10 for recovering gas produced
6 during
electrodialysis is generally shown in which salt-containing feed water 20 is
7 fed through
an electrodialysis (ED) cell 22 to produce an low TDS or desalinated
8 water stream
24 and a concentrated TDS brine stream 26, while recovering
9 separate streams of hydrogen gas 28 and oxygen gas 30.
With reference to Fig. 2, the system is particularly useful as part of
11 an energy
efficient water treatment system 32 in which the recovered gas, in
12 particular
hydrogen gas 28, is used as a fuel source for a power generating
13 device 34
(e.g. solid oxide fuel cells (SOFC), bio fuel, gas or alternative energy
14 powered
generators, hydrogen proton exchange membrane (PEM) fuel cells) that
provides power 36 to the unit operations of the water treatment system 32. For
16 example, the
power 36 can be used to augment or meet the power requirements
17 of the ED
unit 22, water pre-treatment processes 38 (e.g. to remove total
18 suspended
solids, hardness ions, metals, organics and other contaminants), or
19 other
conventional water treatment processes 40 (e.g. distillation, evaporation,
partial vapour pressure processes, ion exchange, pressure driven membrane
21 processes and other electrically driven membrane processes).
22 In detail
and with particular reference to Fig. 1, the ED unit 22
23 generally
includes a membrane compartment 42 positioned between a cathode
24 compartment
44 and an anode compartment 46, as is generally known in the art.
The membrane compartment 42 contains a stack of alternating anion exchange
8
CA 02562842 2011-04-07
1 membranes and cation exchange membranes (not detailed), and the cathode
and
2 anode compartments 44, 46 contain a cathode and anode, respectively (not
shown).
3 In the known and usual operation of the ED unit 22, the feed water
20
4 passes through the membrane compartment 42 while an electrical field is
imposed
under the influence of an external direct current power source 47 connected to
the
6 anodes and cathodes. Selective cation and anion movement across the
membranes
7 produces the desalinated water stream 24 and the concentrated brine
stream 26,
8 which then exit the ED unit 22. The desalinated water stream 24 is
circulated
9 through the membrane compartment 42 and a feed water recycle tank 48. The
brine
concentrate stream 26 is circulated through the membrane compartment 42 and a
11 brine recycle tank 50.
12 The feed water 20 can be subjected to additional cycles of
13 electrodialysis until an acceptable concentration of TDS has been
achieved to
14 produce a final product water 51, as determined by a conductivity sensor
or other
means. Similarly, the brine concentrate 26 achieves the desired TDS to produce
a
16 final brine concentrate 53 which can be reused, such as pre-wetting
transportation
17 roadways in winter maintenance operations.
18 Any suitable ED unit 22 can be used as would be contemplated
by
19 one skilled in the art. It is particularly contemplated to use the HEED
ED unit (EET
Corporation, Harriman, TN and as described in US patent 6,824,662) as the
21 desalinated water 24 recovery is high (about 85%) and the waste brine 26
recovery
22 is low (about 15%), operated in either batch or semi-continuous mode.
9
CA 02562842 2006-10-06
1 The system
10 further comprises electrolyte towers 52, and
2 specifically
a catholyte tower 52a and an anolyte tower 52b, that provide for the
3 separate recovery of the produced gases as recovered gases G, namely
4 hydrogen gas
28 and the oxygen gas 30, respectively, produced during operation
of the ED unit 22. In particular, the produced gas is entrained in an
electrolyte
6 solution 56, e.g. aqueous sodium sulphate, which circulates between the
7 electrode
compartments 44, 46 and the electrolyte towers 52a, 52b. More
8
specifically, the hydrogen gas 28 is entrained in a catholyte solution 56a
that is
9 fed from the
cathode compartment 44 to the catholyte tower 52a, and the oxygen
gas 30 is entrained in an anolyte solution 56b that is fed from the anode
11 compartment
46 to anolyte tower 52b. Importantly, because the hydrogen gas 28
12 is separated
from the oxygen gas 30 by the respective anolyte and catholyte and
13 anolyte
towers 52a, 52b, the risk of explosion of the recovered hydrogen gas 28
14 is reduced, and the use of an inert gas for venting is eliminated.
Generally, the pH of the catholyte solution becomes alkaline (e.g.
16 pH 10.0 to
12.0) while the pH of the anolyte solution becomes acidic (e.g. pH 2.0
17 to 4.0).
Therefore, the catholyte and anolyte solutions 56a, 56b are mixed in an
18 electrolyte
mixing tank 62 including to neutralize the pH before the electrolyte
19 solution 56
is returned to the electrode compartments 44, 46. Alternatively, the
catholyte and anolyte solutions 56a, 56b can be circulated independently, with
21 the pH of each being adjusted as necessary.
22 With further
reference to Fig. 3, each electrolyte tower 52 is a
23 closed tank
having a lower electrolyte solution portion 66 that contains circulating
24 electrolyte
solution 56 and an upper head space portion 68. Electrolyte solution
56 flows from the cathode compartments 44,46 and is discharged to their
CA 02562842 2006-10-06
1 respective
towers 52 through an electrolyte solution inlet 70. As the electrolyte
2 solution 56 passes through the inlet 70 and into the headspace portion 68,
the
3 entrained
hydrogen or oxygen gas 28, 30 separates from the electrolyte solution
4 56.
As shown in Figs. 4A and 4B, the electrolyte solution inlet 70 is a
6 conduit
comprising an electrolyte solution drain port 84 and a gas discharge 85.
7 The drain
port 84 can comprise a liquid sump or trap including fit with a
8 perforated
frit 86, with the drain port 84 being appropriate positioned for
9 discharge of
the electrolyte solution 56 for collection such as in the electrolyte
solution portion 66. As the electrolyte solution 56 flows out of the drain
port 84
11 and into the
electrolyte solution portion 66, the entrained gas separates from the
12 electrolyte solution 56 and the resulting separated gas 28 flows into the
13 headspace 68
without adversely affecting the upstream ED unit membrane
14 dynamics.
Preferably the solution inlet 70 and drain port 84 are arranged to
retain the electrolyte solution 56 to maximize separation of the recovered
gases
16 G from the
entrained gases while allowing the electrolyte solution 56 to drain into
17 the
electrolyte solution portion 66. In one case, a perforated frit 86 is employed
18 to
controlling drainage of the electrolyte solution 56 while enabling recovered
gas
19 G to separate from solution.
Preferably the solution inlet 70 is positioned within the headspace
21 portion 68
and an electrolyte solution outlet 72 is positioned within the electrolyte
22 solution
portion 66. The entrained hydrogen or oxygen gas 28, 30 flows into the
23 headspace
portion 66, while the electrolyte solution 56 flows into the electrolyte
24 solution
portion 66 for discharge from the electrolyte solution outlet 72. The
hydrogen or oxygen gas 28, 30 in the headspace portion 68 is directed out a
gas
11
CA 02562842 2006-10-06
1 outlet 74
adjacent a top 76 of the tower 52 for collection and use, as desired.
2 The recovered gas G, 28, 30 can be further processed in serially arranged
3 devices either independently arranged or incorporated in the towers 52
4 themselves.
The electrolyte towers 52 are of any suitable construction as would
6 be
appreciated by one skilled in the art. Preferably, the towers 52 are columnar,
7 constructed
of non-corrosive material such as polyethylene terephtalate (PET)
8 copolymer
plastic. An exemplary electrolyte tower 52 is a vertically elongated
9 vessel with
the electrolyte solution portion 66 making up about the bottom 10-
15% of volume. A removable top 76 that can be secured with a detachable metal
11 seal flange permits access to the electrolyte tower 52 as required.
12 While the
electrolyte towers 52 generally serve to provide for
13 gas/liquid
separation of gas entrained in an electrolyte solution, the separated
14 gas can be
further processed to achieve a desired purity according to various
techniques known in the art. For example, the electrolyte towers 52 can also
16 include
various additional components for purifying the recovered gas. This is
17 particularly
important when recovering hydrogen gas 28 to reduce cross-over
18 contaminants
and to increase the purity of the hydrogen gas 28 for use as a fuel
19 source.
Particularly, to avoid approaching the hydrogen lower explosive limit
(LEL), it is preferably to remove oxygen cross-contamination,
21 Accordingly
and with reference again to Fig. 4A, an embodiment of
22 the
catholyte tower 52a further includes a chamber 80 positioned above the
23 electrolyte solution portion 66 and within the headspace portion 68. The
24 electrolyte
solution inlet 70 is in fluid communication with the chamber 80.
Located within the chamber 80 is a gas scrubber 88, positioned above the
12
CA 02562842 2006-10-06
1 electrolyte solution inlet 70, which selectively removes cross-
contamination as
2 the recovered hydrogen gas 28 flows upwardly through the gas scrubber 88
and
3 out of the gas outlet 74. For example, for removing contaminating oxygen
and
4 nitrogen gases from hydrogen gas 28, the gas scrubber 88 can be one or
more of
a mixed bed carbon molecular sieve and an oxygen scavenging adsorbent such
6 as iron powder. An access door 90 is provided on the chamber 80 to remove
and
7 replace the gas scrubber 88 as necessary.
8 The chamber 80 also includes a liquid trap 91 positioned at the top
9 92 of the chamber 80 at the gas outlet 74, with the liquid trap 91 being
a conical
porous strainer. The liquid trap 91 serves to coalesce vapor from the
recovered
11 hydrogen gas 28 into large droplets which then drips back into the
chamber 80.
12 The droplets may include scrubbing liquor from the adsorbent which are
collected
13 at a bottom 94 of the chamber 80. Collected liquid, which may contain
trace high
14 density gas contaminants, exits the chamber 80 such as though a porous
alumina frit 96 and into a U-tube drain trap 98 and preferably is directed
through
16 an outlet 100 out of the catholyte tower 52a.
17 Alternatively, the chamber 80 and associated gas purification
18 devices could be located external to the catholyte tower 52a, as would
be evident
19 to one skilled in the art.
Also at the top end 76 of the catholyte tower 56a one can monitor a
21 lower explosive limit (LEL) 102 and provide an emergency exhaust and
pressure
22 relief vent line 104.
23 With reference to Fig. 5, to aid in further recovery of residual
24 hydrogen gas 28 from the catholyte solution 56a, a partial sleeve 106 is
positioned around a lower portion of the chamber 80. An annular space 108 is
13
CA 02562842 2011-04-07
1 formed between the chamber 80 and the sleeve 106 in which the electrolyte
solution drain
2 port 84 is located. A bottom end 110 of the sleeve 106 receives the
catholyte solution 56a
3 and provides additional residence time for recovery of any residual
separated gases 28.
4 The bottom end 110 includes a secondary drain port 112 for outflow of
spend catholyte
solution 56a into the electrolyte solution portion 66. A top end 114 of the
sleeve 106
6 encloses the annular space 108 and a secondary gas inlet 116 to the chamber
80 is
7 positioned below the scrubber 88. Residual gas 28 is collected within the
annular space
8 108 and directed into the chamber 80 through the secondary inlet 116.
9 With reference to Figs. 4A and 5, a conduit 118 is provided between
the
headspace portion 68 and the electrolyte solution inlet 70 to recycle fugitive
gas in the
11 catholyte tower that is outside of chamber 80 and sleeve 106 back to the
chamber 80.
12 With reference to Fig. 6, the recovered hydrogen gas 28 can be
subjected to
13 additional downstream processing steps. In particular, the hydrogen gas
28 is directed to a
14 coiled, deionized water tube gas bubbler 120 immersed in degassed,
deionized water 122
(approximately pH 6.0) within a closed vessel 124.
16 One form of suitable bubbler includes a flat plate 2 pm ceramic
pore sparger
17 128 positioned at the outlet 126 of the bubbler 120. A steady stream of
hydrogen gas micro
18 bubbles 130 are produced in the deionized water 122. The preferred
coiled tubing is
19 Vinylidene polyfluoride (PVDF), polyamide or Polychlorotrifluoroethylene
(PCTFE) selected
for low hydrogen gas permeation. At a low flow-rate (about 1.0 Uminute) the
micro-bubbles
21 (about 1 to 2 mm in diameter) create the interfacial surface area to
scrub out the ultra-trace
22 amounts
23
14
CA 02562842 2006-10-06
1 of oxygen gas cross-contamination (specific gravity 1.105 at 21 C
solubility 13.8
2 mg/L at 25 C) and nitrogen (specific gravity 0.967 at 21 C and
solubility 8.9
3 mg/L at 25 C) while the lighter hydrogen gas (specific gravity about
0.0696 at 21
4 C and approximate solubility 0.0182 vol/vol at 25 C) achieves limited
or no
mass transfer efficiency.
6 A gas outlet 132 at the top of the vessel 124 includes a
hydrophobic
7 membrane 136 (e.g. ZeflourTM, 2 m 44 mm hydrophobic TeflonI'm membrane
8 (Pall Gelman P5PJ047)) on a conical PTFE support 138. The hydrophobic
9 membrane 136 blocks transport of water to remove humidity from the
hydrogen
gas 28 while permitting the free flow of gas 28 into a discharge duct and hood
11 140. Coalesced water then drips off the conical support 138 and back
into the
12 vessel 124.
13 The gas bubbler 120 can be positioned within the catholyte tower
14 52a or outside the catholyte tower 52a.
For moderately high purity gas applications (>98% purity)
16 downstream from the hood and duct 140 is a sample port and gas discharge
142
17 followed by a water coalescer 144, a flash back arrestor 146, LEL and
gas purity
18 sensor 148, flow meter/controller 150 and a sample and discharge line
152 to a
19 burner/bio fuel powered generator (not shown). These moderately high
purity
gas applications may include advanced power generating devices such as SOFC
21 fuel cells, bio fuel, gas or alternative energy powered generators.
22 For extremely high purity gas applications (>99.999% hydrogen)
23 the sample line 152 is shut off and diverted through a separate line
flash back
24 arrestor 154 into a cryogenic purification system 156 and gas purity
sensor 158.
The hydrogen gas 28 is compressed 160 and stored 162 for use (fuel cell).
These
CA 02562842 2011-04-07
1 extremely high purity gas applications may include advanced power generating
2 devices such as the hydrogen PEM fuel cells.
3 Figs. 7-
11 exemplify the characterization of gases recovered using the
4 system 10 of the invention or simulations thereof.
Fig. 7 is a gas chromatograph of the gas leaving the cathode
6
compartment of the electrodialysis unit. As shown, the gas entering the
catholyte
7 tower
comprises hydrogen, oxygen and nitrogen, with hydrogen gas being the major
8 constituent.
9 Fig. 8
is a gas chromatograph of the gas leaving the anode
compartment of the electrodialysis unit. As shown, the gas entering the
anolyte
11 tower
comprises hydrogen, oxygen and nitrogen, with oxygen has being the major
12 constituent.
13 Fig. 9
is a graphical representation of the electrode gases at the
14 cathode.
From eight samples taken over a period of 34 hours, the concentration of
hydrogen gas is between the range of about 73 ¨ 93 mole percent, while the
16
concentration of oxygen gas is between the range of about4 ¨ 12 mole percent,
and
17 the concentration of nitrogen gas is between the range of about 2 ¨ 17
mole percent.
18 Fig. 9
demonstrates that the major constituent of the gas entering the cathode is
19 hydrogen gas.
Fig. 10 is a graphical representation of the gas constituents of the gas
21 entering
the anolyte tower. From six samples taken over a period of 15 hours, the
22 average
concentration of hydrogen is less than about 2 mole percent, the average
23
concentration of oxygen is greater than about 78 mole percent, and the average
16
CA 02562842 2011-04-07
24 concentration of nitrogen gas is less than about 17 mole percent. Fig.
10 illustrates
25 that the major constituent of the gas entering the anolyte tower is
oxygen gas.
26 Fig. 11 is a graphical representation of five stimulated
sample
27 experiments illustrating the characterization of the inlet and outlet
gas before and
28 after scrubbing hydrogen gas with oxygen scavengers and solid carbon
molecular
29 sieves adsorbents at ambient temperature and pressure. Mix1 and Mix 2
were
30 hydrogen gas of less than 80% purity while mix 3, 4 and 5 were hydrogen
gas of
31 greater than 80% purity. As shown, scavenging and adsorption of the
hydrogen gas
32 of less than 80% purity at ambient temperature were not successful.
However,
33 scavenging and adsorption of the hydrogen gas of greater than 80% purity
at
34 ambient temperature was successful.
35 Although preferred embodiments of the invention have been
described
36 in some detail herein above, those skilled in the art will recognize
that various
37 substitutions, and modifications of the invention, may be made without
departing
38 from the scope of the invention.
17