Note: Descriptions are shown in the official language in which they were submitted.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
1
FIBROUS STRUCTURES COMPRISING A SURFACE TREATING COMPOSITION
AND A LOTION COMPOSITION
FIELD OF THE INVENTION
The present invention relates to fibrous structures comprising a surface
treating
composition and a lotion composition, single- or multi-ply sanitary tissue
products made
therefrom and processes for making same. More particularly, the present
invention
relates to fibrous structures comprising a user contacting surface comprising
a first region
and a second region, wherein the first region comprises a first composition
and the second
region comprises a second composition different from the first composition.
Even more
particularly, the present invention relates to fibrous structures comprising a
user
contacting surface comprising a first region comprising a surface treating
composition
and a second region comprising a lotion composition.
BACKGROUND OF THE INVENTION
Fibrous structures comprising a surface treating composition such as a
softening
composition and a lotion composition are known. However, such conventional
fibrous
structures have utilized full coverage of a surface of the fibrous structure
with the surface
treating compositions and/or lotion compositions in an attempt to maximize
transfer of
the lotion composition.
Formulators have added an anti-migration material, such as a quaternary
ammonium compound, into a fiber furnish such that the fibers are coated with
the
quaternary ammonium compound. The fibrous structure formed by the fiber
furnish
tends to mitigate the migration of a subsequently applied lotion composition
into the
fibrous structure. The function of the quaternary ammonium compound and the
level at
which it is used are not provide softening of the fibrous structure surface.
It is also known in the art to add quaternary ammonium compounds and/or
silicones and/or other types of agents into the fiber furnish for the purpose
of debonding
the fibers. -
None' of the known fibrous structures teach or suggest treating a surface of
the
fibrous structure with a surface treating composition and a lotion composition
such that a
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
2
user contacting surface comprising a first region comprising the surface
treating
composition and a second region comprising a lotion composition is produced on
the
surface of the fibrous structure.
Accordingly, there is a need for a fibrous structure that comprises a surface
treating composition such as a softening composition and a lotion composition
such that a
user contacting surface comprising a first region comprising the surface
treating
composition and a second region comprising the lotion composition is produced
on a
surface of the fibrous structure, a single- or multi-ply sanitary tissue
product made
therefrom and processes for making same.
SUMMARY OF THE INVENTION
The present invention fulfills the needs described above by providing a
fibrous
structure and/or sanitary tissue product comprising a surface treating
composition and a
lotion composition such that a user contacting surface comprising a first
region
comprising the surface treating composition and a second region comprising the
lotion
composition is produced on a surface of the fibrous structure and/or sanitary
tissue
product.
In one example of the present invention, a fibrous structure and/or single- or
multi-ply sanitary tissue product comprising a user contacting surface wherein
the user
contacting surface comprises a first region comprising a surface treating
composition and
second region comprising a lotion composition is provided.
In another example of the present invention, a fibrous structure and/or single-
or
multi-ply sanitary tissue product comprising a surface treating composition
and a lotion
composition, wherein the surface treating composition is present on a surface
of the
fibrous structure and/or single- or multi-ply sanitary tissue product at a
greater level by
weight than within the fibrous structure and/or single- or multi-ply sanitary
tissue product
and the lotion composition is present within the fibrous structure and/or
single- or multi-
ply sanitary tissue product at a greater level by weight than on the surface
of the fibrous
structure and/or single- or multi-ply sanitary tissue product, is provided.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
3
Relative concentration of the surface treating composition and/or lotion
composition on the surface can be determined using the Relative Concentration
on
Surface Test Method described herein.
In yet another example of the present invention, a fibrous structure and/or
single-
or multi-ply sanitary tissue product comprising:
a. a surface treating composition comprising a surface treating agent
selected
from the group consisting of: polymers, hydrocarbons, waxes, oils, silicones,
quaternary
ammonium compounds, fluorocarbons, substituted C10-C22 alkanes, substituted
C10-C22
alkenes, polyols, sugar derivatives and mixtures thereof; and
b. a lotion composition comprising a compound selected from the group
consisting of: oils, alcohol ethoxylates, fatty acid esters, hydrocarbons and
mixtures
thereof;
wherein the surface treating composition is present on a surface of the
fibrous
structure and/or single- or multi-ply sanitary tissue product and the lotion
composition is
present on less than the entire surface of the surface treating composition,
is provided.
In even another example of the present invention, a single- or multi-ply
sanitary
tissue product comprising a fibrous structure according to the present
invention is
provided.
In even yet another example of the present invention, a process for treating a
fibrous structure comprising the step of applying a lotion composition to a
surface
treating composition associated with a surface of a fibrous structure and/or
single- or
multi-ply sanitary tissue product, is provided.
In still yet another example of the present invention, a process for treating
a
fibrous structure and/or single- or multi-ply sanitary tissue product
comprising the steps
of:
a. applying a surface treating composition to a surface of a fibrous structure
and/or single- or multi-ply sanitary tissue product; and
b. applying a lotion composition to the surface treating composition, is
provided.
In even still yet another example of the present invention, a process for
treating a
fibrous structure and/or single- or multi-ply sanitary tissue product
comprising the steps
of:
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
4
a. applying a surface treating composition to a surface of a fibrous structure
and/or single- or multi-ply sanitary tissue product; and
b. applying a lotion composition to the surface of the fibrous structure
and/or
single- or multi-ply sanitary tissue product, is provided.
Accordingly, the present invention provides fibrous structures and/or single-
or
multi-ply sanitary tissue products comprising a surface treating composition
and a lotion
composition, products made therefrom and processes for making same.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic representation of a fibrous structure in accordance with
the
present invention;
Fig. 2 is a cross-sectional view of Fig. 1 taken along line 2-2;
Fig. 3 is a cross-sectional view of another example of a fibrous structure in
accordance with the present invention;
Fig. 4 is a cross-sectional view of another example of a fibrous structure in
accordance with the present invention;
Fig. 5 is a cross-sectional view of another example of a fibrous structure in
accordance with the present invention;
Fig. 6 is a schematic representation of another example of a fibrous structure
in
accordance with the present invention.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
"Fiber" as used herein means an elongate particulate having an apparent length
greatly exceeding its apparent diameter, i.e. a length to diameter ratio of at
least about 10.
Fibers having a non-circular cross-section are common; the "diameter" in this
case may
be considered to be the diameter of a circle having cross-sectional area equal
to the cross-
sectional area of the fiber. More specifically, as used herein, "fiber" refers
to
=
papermaking fibers. The present invention contemplates the use of a variety of
papermaking fibers, such as, for example, natural fibers or synthetic fibers,
or any other
suitable fibers, and any combination thereof.
CA 02563539 2009-08-25
Natural papermaking fibers useful in the present invention include animal
fibers,
mineral fibers, plant fibers and mixtures thereof. Animal fibers may, for
example, be
selected from the group consisting of: wool, silk and mixtures thereof. Plant
fibers may,
for example, be derived from a plant selected from the group consisting of:
wood, cotton,
cotton linters, flax, sisal, abaca, hemp, hesperaloe, jute, bamboo, bagasse,
kudzu, corn,
sorghum, gourd, agave, loofah and mixtures thereof.
Wood fibers; often referred to as wood pulps include chemical pulps, such as
kraft
(sulfate) and sulfite pulps, as well as mechanical and semi-chemical pulps
including, for
example, groundwood, thermomechanical pulp, chemi-mechanical pulp (CMP),
cherni-
thermomechanical pulp (CTMP), neutral semi-chemical sulfite pulp (NSCS).
Chemical
pulps, however, may be preferred since they impart a superior tactile sense of
softness to
tissue sheets made therefrom. Pulps derived from both deciduous trees
(hereinafter, also
referred to as "hardwood") and coniferous trees (hereinafter, also referred to
as
"softwood") may be utilized. The hardwood and softwood fibers can be blended,
or
alternatively, can be deposited in layers to provide a stratified and/or
layered fibrous
structure. U.S. Pat. Nos. 4,300,981 and U.S. Pat. No. 3,994,771
discloses layering of hardwood and softwood fibers.
Also applicable to the present invention are fibers derived from recycled
paper, which
may contain any or all of the above categories as well as other non-fibrous
materials such
as fillers and adhesives used to facilitate the original papermaldng.
The wood pulp fibers may be short (typical of hardwood fibers) or long
(typical of
softwood fibers). Nonlimiting examples of short fibers include fibers derived
from a fiber
source selected from the group consisting of Acacia, Eucalyptus, Maple, Oak,
Aspen,
Birch, Cottonwood, Alder, Ash, Cherry, Elm, Hickory, Poplar, Gum, Walnut,
Locust,
Sycamore, Beech, Catalpa, Sassafras, Gmelina, Albizia, Anthocephalus, and
Magnolia.
Nonlimiting examples of long fibers include fibers derived from Pine, Spruce,
Fir,
Tamarack, Hemlock, Cypress, and Cedar. Softwood fibers derived from the kraft
process and originating from more-northern climates may be preferred. These
are often
referred to as northern softwood kraft (NSK) pulps.
Synthetic fibers may be selected from the group consisting of: wet spun
fibers,
dry spun fibers, melt spun (including melt blown) fibers, synthetic pulp
fibers and
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
6
mixtures thereof. Synthetic fibers may, for example, be comprised of cellulose
(often
referred to as "rayon"); cellulose derivatives such as esters, ether, or
nitrous derivatives;
polyolefins (including polyethylene and polypropylene); polyesters (including
polyethylene terephthalate); polyamides (often referred to as "nylon");
acrylics; non-
cellulosic polymeric carbohydrates (such as starch, chitin and chitin
derivatives such as
chitosan); and mixtures thereof.
"Fibrous structure" as used herein means a structure that comprises one or
more
fibers. Nonlimiting examples of processes for making fibrous structures
include known
wet-laid papermaking processes and air-laid papermaking processes. Such
processes
typically include steps of preparing a fiber composition, oftentimes referred
to as a fiber
slurry in wet-laid processes, either wet or dry, and then depositing a
plurality of fibers
onto a forming wire or belt such that an embryonic fibrous structure is
formed, drying
and/or bonding the fibers together such that a fibrous structure is formed,
and/or further
processing the fibrous structure such that a finished fibrous structure is
formed. For
example, in typical papermaking processes, the finished fibrous structure is
the fibrous
structure that is wound on the reel at the end of papermaking, but before
converting
thereof into a sanitary tissue product.
"Sanitary tissue product" comprises one or more fibrous structures, converted
or
not, that is useful as a wiping implement for post-urinary and post-bowel
movement
cleaning (toilet tissue), for otorhinolaryngological discharges (facial tissue
and/or
disposable handkerchiefs), and multi-functional absorbent and cleaning uses
(absorbent
towels and/or wipes). In one example, a lotion composition-containing multi-
ply
disposable handkerchief having a caliper of from about 0.1 mm to about 0.4 mm
in
accordance with the present invention is provided.
"Ply" or "Plies" as used herein means an individual finished fibrous structure
optionally to be disposed in a substantially contiguous, face-to-face
relationship with
other plies, forming a multiple ply finished fibrous structure product and/or
sanitary
tissue product. It is also contemplated that a single fibrous structure can
effectively form
two "plies" or multiple "plies", for example, by being folded on itself.
"Surface of a fibrous structure" as used herein means that portion of the
fibrous
structure that is exposed to the external environment. In other words, the
surface of a
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
7
fibrous structure is that portion of the fibrous structure that is not
completely surrounded
by other portions of the fibrous structure.
"User Contacting Surface" as used herein means that portion of the fibrous
structure and/or surface treating composition and/or lotion composition
present directly
and/or indirectly on the surface of the fibrous structure that is exposed to
the external
environment. In other words, it is that surface formed by the fibrous
structure including
any surface treating composition and/or lotion composition present directly
and/or
indirectly on the surface of the fibrous structure that contacts an opposing
surface when
used by a user. For example, it is that surface formed by the fibrous
structure including
any surface treating composition and/or lotion composition present directly
and/or
indirectly on the surface of the fibrous structure that contacts a user's skin
when a user
wipes his/her skin with the fibrous structure of the present invention.
In one example, the user contacting surface, especially for a textured and/or
structured fibrous structure, such as a through-air-dried fibrous structure
and/or an
embossed fibrous structure, may comprise raised areas and recessed areas of
the fibrous
structure. In the case of a through-air-dried, pattern densified fibrous
structure the raised
areas may be knuckles and the recessed areas may be pillows and vice versa.
Accordingly, the knuckles may, directly and/or indirectly, comprise the lotion
composition and the pillows may comprise the surface treating composition and
vice
versa so that when a user contacts the user's skin with the fibrous structure,
the lotion
composition and surface treating composition both contact the user's skin. A
similar case
is true for embossed fibrous structures with the embossed areas may, directly
and/or
indirectly, comprise the lotion composition and the non-embossed areas may
comprise
the surface treating composition and vice versa.
In one example, the user contacting surface has to comprise regions of
sufficient
size such that two or more different regions (comprising different
compositions) are
exposed to an opposing surface during use. In other words, a surface of a
fibrous
structure that is substantially covered (on a microscopic scale) by a lotion
composition
but completely covered on a macro scale by such lotion composition such that a
user's
skin is only contacted by the lotion composition does not contain two
different regions in
its user contacting surface. In one example a user contacting surface may
comprise an
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
8
external layer of a multi-layer fibrous structure wherein the external layer
may comprise a
surface treating composition and/or a lotion composition.
The user contacting surface may be present on the fibrous structure and/or
sanitary tissue product before use by the user and/or the user contacting
surface may be
created/formed prior to and/or during use of the fibrous structure and/or
sanitary tissue
product by the user, such as upon the user applying pressure to the fibrous
structure
and/or sanitary tissue product as the user contacts the user's skin with the
fibrous
structure and/or sanitary tissue product.
All percentages and ratios are calculated by weight unless otherwise
indicated.
All percentages and ratios are calculated based on the total composition
unless otherwise
indicated.
Unless otherwise noted, all component or composition levels are in reference
to
the active level of that component or composition, and are exclusive of
impurities, for
example, residual solvents or by-products, which may be present in
commercially
available sources.
Fibrous Structure
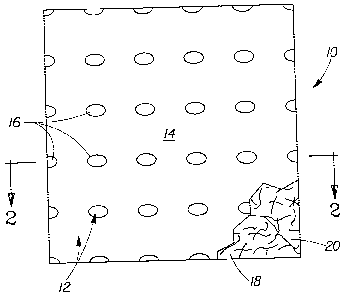
Fig. 1 is a schematic representation of a fibrous structure in accordance with
the
present invention. As shown in Fig. 1, a fibrous structure 10 comprising a
user contacting
surface 12 comprising a first region 14 and a second region 16. The user
contacting
surface 12 is associated with a surface of the fibrous structure 18. As shown,
the surface
of the fibrous structure 18 may comprise one or more fibers 20.
The first region 14 and/or second region 16 may be present on (associated
with)
the surface of the fibrous structure 18. When the first region 14 and/or the
second region
16 are present on the surface of the fibrous structure 18, one or both may be
present on
the surface of the fibrous structure 18 in the form of a continuous or
substantially
continuous network and/or in a plurality of discrete areas (also sometimes
known as
"islands").
When present on the surface of the fibrous structure 18, the first region 14
and/or
the second region 16 may be in contact with and/or cover the entire or
substantially the
entire surface area of the surface of the fibrous structure 18. In one
example, the first
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
9
region 18 is in contact with and/or covers the entire or substantially the
entire surface area
of the surface of the fibrous structure 18.
When present on the surface of the fibrous structure 18, the first region 14
and/or
the second region 16 may be in contact with and/or cover less than the entire
or
substantially the entire surface area of the surface of the fibrous structure
18. In one
example, the second region 16 is in contact with and/or covers less than the
entire or
substantially the entire surface area of the surface of the fibrous structure
18. When
either region covers less than substantially the entire surface area of the
surface of the
fibrous structure 18, that region may be in the form of a plurality of
discrete areas.
As shown in Fig. 2, the first region 14 is in contact with and/or covers
substantially the entire surface area of the surface of the fibrous structure
18 and the
second region 16 is in contact with and/or covers less than substantially the
entire surface
area of the surface of the fibrous structure 18. The first region 14 may be in
the form of a
continuous or substantially continuous network and the second region 16 may be
in the
form of a plurality of discrete areas dispersed throughout the continuous or
substantially
continuous network of the first region 14.
Either region may be in contact with the other region. As shown in Fig. 3, the
second region 16 is in contact with the first region 14 such that the first
region 14 is
positioned between the second region 16 and the surface of the fibrous
structure 18. The
second region 16 may be present on less than the entire surface area of the
first region 14.
The second region 16 may be present on the first region 14 in the form of one
or more
discrete areas. As shown in Figs. 1, 4 and 5, portions of the second region 16
are in
contact with the first region 14 such that both the second region 16 and the
first region 14
are in contact directly with the surface of the fibrous structure 18. Also as
shown in Figs.
4 and 5, a portion of the second region 16 is not in contact with the first
region 14.
Figs. 4 and 5 also show that less than substantially the entire surface area
of the
surface of the fibrous structure 18 is in contacted by or covered by the first
region 14 and
the second region 16. In these examples, the user contacting surface 12
comprises a third
region, namely, the surface of the fibrous structure 18 as well as the first
region 14 and
the second region 16.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
Fig. 6 is a schematic representation of another example of a fibrous structure
in
accordance with the present invention. The fibrous structure 10 comprises a
user
contacting surface 12 that comprises a first region 14, a second region 16 and
a third
region, in this case, the surface of the fibrous structure 18 which comprises
one or more
fibers 20.
The first region 14 comprises a surface treating composition.
The second region 16 comprises a lotion composition.
In one example, the surface treating composition and/or lotion composition may
be present on the surface of the fibrous structure 18 at a greater level by
weight than
within the fibrous structure.
In another example, the surface treating composition and/or lotion composition
may be present within the fibrous structure at a greater level by weight than
on the
surface of the fibrous structure 18.
The surface area coverage of the surface treating composition on the surface
of the
fibrous structure may be greater than about 10% and/or greater than about 30%
and/or
greater than about 50% to about 100% and/or to about 90% and/or to about 85%.
The surface area coverage of the lotion composition on the surface of the
fibrous
structure may be may be greater than about 1% and/or greater than about 5%
and/or
greater than about 10% and/or greater than about 20% to about 99% and/or to
about 90%
and/or to about 75% and/or to about 50%. .
In one example, the surface area of the fibrous structure and/or sanitary
tissue
product comprises greater than about 10% and/or greater than about 20% and/or
greater
than about 50% and/or greater than about 70% and/or greater than about 80%
and/or
greater than about 90% of the surface treating composition and from 0 to about
90%
and/or from 0 to about 80% and/or from 0 to about 50% and/or from 0 to about
30%
and/or from 0 to about 20% and/or from 0 to about 10% of the lotion
composition. When
the surface area of the surface of the fibrous structure and/or sanitary
tissue product
comprises 0% of the lotion composition, then the lotion may be within the
fibrous
structure and/or within the sanitary tissue product, such as between two plies
of the
sanitary tissue product.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
11
In another example, the surface area of the user contacting surface comprises
from
about 20% to about 97% and/or from about 50% to about 97% and/or from about
80% to
about 97% of the surface treating composition and from about 3% to about 80%
and/or
from about 3% to about 50% and/or from about 3% to about 20% and/or from about
3%
to about 15% of the lotion composition.
Surface area coverage of the fibrous structure and/or sanitary tissue product
may
be determined by the Surface Area Coverage Test Method described herein.
Each region may, within itself, exhibit differential concentrations of their
respective compositions and/or differential elevations (protrusions from the
surface of the
fibrous structure) of their respective compositions
The user contacting surface area may comprise from greater than about 10%
and/or greater than about 30% and/or greater than about 50% to about 100%
and/or to
about 90% and/or to about 85% of the surface treating comppsition and/or
greater than
about 1% and/or greater than about 5% and/or greater than about 10% and/or
greater than
about 20% to about 99% and/or to about 90% and/or to about 75% and/or to about
'50%
of the lotion composition.
The combination of the surface treating composition and lotion composition in
the
user contacting surface exhibits softness greater than a user contacting
surface comprising
either the surface treating composition or lotion composition alone.
The user contacting surface may be planar or may have protrusions of either
the
surface treating composition and/or lotion composition such that the user
contacting
surface exhibits differential elevations.
In another example, the user contacting surface may comprise areas of greater
concentration and/or greater elevation of the lotion composition, areas of
less
concentration and/or lesser elevation of the lotion composition, and areas of
the surface
treating composition.
The surface treating composition and the lotion composition may comprise one
or
more similar and/or identical ingredients so long as the user contacting
surface comprises
a first region comprising a different composition (at least one ingredient
differs in the
composition) than a composition present in a second region.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
12
Nonlimiting types of fibrous structures according to the present invention
include
conventionally felt-pressed fibrous structures; pattern densified fibrous
structures; and
high-bulk, uncompacted fibrous structures. The fibrous structures may be of a
homogeneous or multilayered (two or three or more layers) construction; and
the sanitary
tissue products made therefrom may be of a single-ply or multi-ply
construction.
The fibrous structures may be post-processed, such as by embossing and/or
calendaring and/or folding and/or printing images thereon.
The fibrous structures may be through-air-dried fibrous structures or
conventionally dried fibrous structures.
The fibrous structures may be creped or uncreped.
The fibrous structures and/or sanitary tissue products of the present
invention may
exhibit a basis weight of between about 10 g/m2 to about 120 g/m2 and/or from
about 12
g/m2 to about 80 g/m2 and/or from about 14 g/m2 to about 65 g/m2.
The fibrous structures and/or sanitary tissue products of the present
invention may
exhibit a total dry tensile strength of greater than about 59 g/cm (150 Win)
and/or from
about 78 g/cm (200 Win) and/or from about 98 g/cm (250 Win) to about 1182 g/cm
(3000
g/M) and/or to about 984 g/cm (2500 Win) and/or to about 787 g/cm (2000 Win)
and/or to
about 394 g/cm (1000 g/M) and/or to about 335 g/cm (850 Win).
The fibrous structure and/or sanitary tissue products of the present invention
may
exhibit a density of less than about 0.60 g/cm3 and/or less than about 0.30
g/cm3 and/or
less than about 0.20 g/cm3 and/or less than about 0.10 g/cm3 and/or less than
about 0.07
g/cm3 and/or less than about 0.05 g/cm3 and/or from about 0.01 g/cm3 to about
0.20 g/cm3
and/or from about 0.02 g/cm3 to about 0.10 g/cm3.
The fibrous structures and/or sanitary tissue products of the present
invention may
exhibit an average lint value of greater than about 0.1 and/or greater than
about 0.5 and/or
greater than about 1.0 and/or greater than about 1.5 and/or greater than about
2.0 and/or
greater than about 3.0 to about 20 and/or to about 15 and/or to about 13
and/or to about
and/or to about 8.
Surface Treating Composition
A surface treating composition, for purposes of the present invention, is a
composition that improves the tactile sensation of a surface of a fibrous
structure
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
13
perceived by a user whom holds a fibrous structure and/or sanitary tissue
product
comprising the fibrous structure and rubs it across the user's skin. Such
tactile
perceivable softness can be characterized by, but is not limited to, friction,
flexibility, and
smoothness, as well as subjective descriptors, such as a feeling like
lubricious, velvet, silk
or flannel.
The surface treating composition may or may not be transferable. Typically, it
is
substantially non-transferable.
The surface treating composition may increase or decrease the surface friction
of
the surface of the fibrous structure, especially the user contacting surface
of the fibrous
structure. Typically, the surface treating composition will reduce the surface
friction of
the surface of the fibrous structure compared to a surface of the fibrous
structure without
such surface treating composition.
The surface treating composition may have a wettability tension less than or
equal
to the surface tension of the lotion composition so as to minimize the
spreading of the
lotion composition that comes into contact with the surface treating
composition.
The surface treating composition comprises a surface treating agent. The
surface
treating composition during application to the fibrous structure may comprise
at least
about 0.1% and/or at least 0.5% and/or at least about 1% and/or at least about
3% and/or
at least about 5% to about 90% and/or to about 80% and/or to about 70% and/or
to about
50% and/or to about 40% by weight of the surface treating agent. In one
example, the
surface treating composition comprises from about 5% to about 40% by weight of
the
surface treating agent.
The surface treating composition present on the fibrous structure and/or
sanitary
tissue product comprising the fibrous structure of the present invention may
comprise at
least about 0.01% and/or at least about 0.05% and/or at least about 0.1% of
total basis
weight of the surface treating agent. In one example, the fibrous structure
and/or sanitary
tissue product may comprise from about 0.01% to about 20% and/or from about
0.05% to
about 15% and/or from about 0.1% to about 10% and/or from about 0.01% to about
5%
and/or from about 0.1% to about 2% of total basis weight of the surface
treating
composition.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
14
In one example, the surface treating composition of the present invention is a
microemulsion of a surface treating agent (for example an aminofunctional
polydimethylsiloxane) in water. In such an example, the concentration of the
surface
treating agent within the surface treating composition may be from about 3% to
about
60% and/or from about 4% to about 50% and/or from about 5% to about 40%.
Nonlimiting examples of such microemulsions are commercially available from
Wacker
Chemie, Dow Coming and/or General Electric Silicones.
Nonlimiting examples of suitable surface treating agents can be selected from
the
group consisting of: polymers such as polyethylene and derivatives thereof,
hydrocarbons, waxes, oils, silicones (polysiloxanes), quaternary ammonium
compounds,
fluorocarbons, substituted C10-C22 alkanes, substituted C10-C22 alkenes, in
particular
derivatives of fatty alcohols and fatty acids(such as fatty acid amides, fatty
acid
condensates and fatty alcohol condensates), polyols, derivatives of polyols
(such as esters
and ethers), sugar derivatives (such as ethers and esters), polyglycols (such
as
polyethyleneglycol) and mixtures thereof.
Nonlimiting examples of suitable waxes may be selected from the group
consisting of: paraffin, polyethylene waxes, beeswax and mixtures thereof.
Nonlimiting examples of suitable oils may be selected from the group
consisting
of: mineral oil, silicone oil, silicone gels, petrolatum and mixtures thereof.
Nonlimiting examples of suitable silicones (polysiloxanes) may be selected
from
the group consisting of: polydimethylsiloxanes, aminosilicones, cationic
silicones,
quaternary silicones, silicone betaines and mixtures thereof.
Nonlimiting examples of suitable polysiloxanes and/or monomeric/oligomeric
units may be selected from the compounds having monomeric siloxane units of
the
following structure:
R1
I',
wherein, RI and R2, for each independent siloxane monomeric unit can each
independently be hydrogen or any alkyl, aryl, alkenyl, alkaryl, arakyl,
cycloalkyl,
halogenated hydrocarbon, or other radical. Any of such radical can be
substituted or
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
unsubstituted. R1 and R2 radicals of any particular monomeric unit may differ
from the
corresponding functionalities of the next adjoining monomeric unit.
Additionally, the
polysiloxane can be either a straight chain, a branched chain or have a cyclic
structure.
The radicals R1 and R2 can additionally independently be other silaceous
functionalities
such as, but not limited to siloxanes, polysiloxanes, silanes, and
polysilanes. The radicals
R1 and R2 may contain any of a variety of organic functionalities including,
for example,
alcohol, carboxylic acid, phenyl, and amine functionalities. The end groups
can be
reactive (alkoxy or hydroxyl) or nonreactive (trimethylsiloxy). The polymer
can be
branched or unbranched.
Exemplary alkyl radicals are methyl, ethyl, propyl, butyl, pentyl, hexyl,
octyl,
decyl, octadecyl, and the like. Exemplary alkenyl radicals are vinyl, allyl,
and the like.
Exemplary aryl radicals are phenyl, diphenyl, naphthyl, and the like.
Exemplary alkaryl
radicals are toyl, xylyl, ethylphenyl, and the like. Exemplary aralkyl
radicals are benzyl,
alpha-phenylethyl, beta-phenylethyl, alpha-phenylbutyl, and the like.
Exemplary
cycloalkyl radicals are cyclobutyl, cyclopentyl, cyclohexyl, and the like.
Exemplary
halogenated hydrocarbon radicals are chloromethyl, bromoethyl,
tetrafluorethyl,
fluorethyl, trifluorethyl, trifluorotloyl, hexafluoroxylyl, and the like.
Viscosity of polysiloxanes useful for this invention may vary as widely as the
viscosity of polysiloxanes in general vary, so long as the polysiloxane can be
rendered
into a form which can be applied to the fibrous structures herein. This
includes, but is not
limited to, viscosity as low as about 25 centistokes to about 20,000,000
centistokes or
even higher.
Nonlimiting examples of suitable quaternary ammonium compounds may be
selected from compounds having the formula:
ER] _____________________________________ xe
4-m
wherein:
m is 1 to 3; each RI is independently a Ci -C6 alkyl group, hydroxyalkyl
group,
hydrocarbyl or substituted hydrocarbyl group, alkoxylated group, benzyl group,
or
mixtures thereof; each R2 is independently a C14 -C22 alkyl group,
hydroxyalkyl group,
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
16
hydrocarbyl or substituted hydrocarbyl group, alkoxylated group, benzyl group,
or
mixtures thereof; and X- is any quaternary ammonium-compatible anion.
In one example, each R1 is methyl and X- is chloride or methyl sulfate and
each R2
is independently C16 -C18 alkyl or alkenyl. Each R2 may be independently
straight-chain
C18 alkyl or alkenyl.
In another example, the quaternary ammonium compounds may be mono or
diester variations having the formula:
(R1)4_m ¨N+ ¨ [(CH2)n ¨Y¨R3 ]m )(-
wherein:
Y is 0 (0)C , or ¨C(0) 0 , Or ¨NH¨C(0) ¨, Or ¨C(0) ¨NH¨; m is 1
to 3; n is Oto 4; each R1 is independently a C1 -C6 alkyl group, hydroxyalkyl
group,
hydrocarbyl or substituted hydrocarbyl group, alkoxylated group, benzyl group,
or
mixtures thereof; each R3 is independently a C13 -C21 alkyl group,
hydroxyalkyl group,
hydrocarbyl or substituted hydrocarbyl group, aLkoxylated group, benzyl group,
or
mixtures thereof, and X- is any quaternary ammonium-compatible anion.
In one example, Y is ¨0 ____ (0)C , or ¨C(0) ¨0 ________________________ ; m-
2; and n=2, each R1 is
independently a C1 -C3, alkyl group, each R3 is independently C13 -C17 alkyl
and/or
alkenyl. In another example each R1 is methyl and each R3 is independently a
straight
chain C15-C17 alkyl and/or alkenyl.
In another example, the quaternary ammonium compound may be an
imidazolinium compound, such as an imidazolinium salt.
As mentioned above, X- can be any quaternary ammonium-compatible anion, for
example, acetate, chloride, bromide, methyl sulfate, formate, sulfate, nitrate
and the like
can also be used in the present invention. In one example, X- is chloride or
methyl sulfate.
The surface treating composition may comprise additional ingredients such as a
vehicle as described herein below which may not be present on the fibrous
structure
and/or sanitary tissue product comprising such fibrous structure. In one
example, the
surface treating composition may comprise a surface treating agent and a
vehicle such as
water to facilitate the application of the surface treating agent onto the
surface of the
fibrous structure.
Lotion Composition
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
17
The lotion composition may comprise oils and/or emollients and/or waxes and/or
immobilizing agents. In one example, the lotion composition comprises from
about 10%
to about 90% of an oil and/or liquid emollient and from about 10% to about 50%
of
immobilizing agent and/or from about 0% to about 60% of petrolatum and
optionally the
balance of a vehicle.
The lotion compositions may be heterogeneous. They may contain solids, gel
structures, polymeric material, a multiplicity of phases (such as oily and
water phase)
and/or emulsified components. It may be difficult to determine precisely the
melting
temperature of the lotion composition, i.e. difficult to determine the
temperature of
transition between the liquid form, the quasi-liquid from, the quasi-solid
form and the
solid form. The terms melting temperature, melting point, transition point and
transition
temperature are used interchangeably in this document and have the same
meaning.
The lotion compositions may be semi-solid, of high viscosity so they do not
substantially flow without activation during the life of the product or gel
structures.
The lotion compositions may be shear thinning and/or they may strongly change
their viscosity around skin temperature to allow for transfer and easy
spreading on a
user's skin.
The lotion compositions may be in the form of emulsions and/or dispersions.
In one example of a lotion composition, the lotion composition has a water
content of less than about 20% and/or less than 10% and/or less than about 5%
or less
than about 0.5%.
In another example, the lotion composition may have a solids content of at
least
about 15% and/or at least about 25% and/or at least about 30% and/or at least
about 40%
to about 100% and/or to about 95% and/or to about 90% and/or to about 80%.
Nonlimiting examples of suitable oils and/or emollients include glycols (such
as
propylene glycol and/or glycerine), polyglycols (such as triethylene glycol),
petrolatum,
fatty acids, fatty alcohols, fatty alcohol ethoxylates, fatty alcohol esters
and fatty alcohol
ethers, fatty acid ethoxylates, fatty acid amides and fatty acid esters,
hydrocarbon oils
(such as mineral oil), squalane, fluorinated emollients, silicone oil (such as
dimethicone)
and mixtures thereof.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
18
Suitable fatty acid ester type emollients include those derived from C12-C28
fatty
acids, such as C16-C22 saturated fatty acids, and short chain (C1-C8 and/or Ci-
C3)
monohydric alcohols. Representative examples of such esters include methyl
palmitate,
methyl stearate, isopropyl laurate, isopropyl myristate, isopropyl palmitate,
and
ethylhexyl palmitate. Suitable fatty acid ester emollients can also be derived
from esters
of longer chain fatty alcohols (C12-C28 and/or C12-C16) and shorter chain
fatty acids e.g.,
lactic acid, such as lauryl lactate and cetyl lactate. Suitable alkyl
ethoxylate type
emollients include C12-C18 fatty alcohol ethoxylates having an average of from
about 3 to
about 30 and/or from about 4 to about 23 oxyethylene units. Nonlimiting
examples of
such alkyl ethoxylates include laureth-3 (a lauryl ethoxylate having an
average of 3
oxyethylene units), laureth-23 (a lauryl ethoxylate having an average of 23
oxyethylene
units), ceteth-10 (acetyl ethoxylate having an average of 10 oxyethylene
units) and
steareth-10 (a stearyl ethoxylate having an average of 10 oxyethylene units).
These alkyl
ethoxylate emollients can be used in combination with other emollients, such
as
petroleum-based emollients, such as petrolatum, at a weight ratio of alkyl
ethoxylate
emollient to petroleum-based emollient of from about 1:1 to about 1:3 and/or
from about
1:1.5 to about 1:2.5.
Immobilizing agents include agents that are may prevent migration of the
emollient into the fibrous structure such that the emollient remain primarily
on the surface
of the fibrous structure and/or sanitary tissue product and/or on the surface
treating
composition on a surface of the fibrous structure and/or sanitary tissue
product and
facilitate transfer of the lotion composition to a user's skin. Immobilizing
agents may
function as viscosity increasing agents and/or gelling agents.
Nonlimiting examples of suitable immobilizing agents include waxes (such as
ceresin wax, ozokerite, microcrystalline wax, petroleum waxes, fisher tropsh
waxes,
silicone waxes, paraffin waxes), fatty alcohols (such as cetyl and/or stearyl
alcohol), fatty
acids and their salts (such as metal salts of stearic acid), mono and
polyhydroxy fatty acid
esters, mono and polyhydroxy fatty acid amides, silica and silica derivatives,
gelling
agents, thickeners and mixtures thereof.
In one example, the lotion composition comprises at least one immobilizing
agent
and at least one emollient.
CA 02563539 2009-08-25
19
In one example, the lotion composition comprises a sucrose ester of a fatty
acid.
The lotion composition may be added to a fibrous structure at any point during
the
papermalcing and/or converting process. In one example, the lotion composition
is added
to the fibrous structure during the converting process.
The lotion composition may be a transferable lotion composition. A
transferable
lotion composition comprises at least one component that is capable of being
transferred
to an opposing surface such as a user's skin upon use. In one example, at
least 0.1% of
the transferable lotion present on the user contacting surface transfers to
the user's skin
during use. The amount of transferable composition that transfers to a user's
skin during
use can be determined by known methods such as by tape stripping the skin 3
times, after
use of the fibrous structure and/or sanitary tissue product by the user, with
Tegaderir
Tapes, available from 3M, and analyzing the tapes for the transferable
composition or a
component within the transferable composition assuming all components of the
transferable composition transfer equally.
Other optional components that may be included in the lotion composition
include
vehicles, perfumes, especially long lasting and/or enduring perfumes,
antibacterial
actives, antiviral actives, disinfectants, pharmaceutical actives, film
formers, deodorants,
opacifiers, astringents, solvents, cooling sensate agents, and the like.
Particular examples
of lotion composition components include camphor, thymol, menthol, chamomile
extracts, aloe vera, calendula officinalis, alpha bisalbolol, Vitamin E,
Vitamin E acetate.
In one example of the lotion composition of the present invention, the lotion
composition has a melting point greater than about 35 C. For example, the
lotion
composition has to be subjected to a temperature of greater than about 35 C
before a
substantial amount (for example, greater than 30% and/or greater than 40%
and/or greater
than 50% and/or greater than 60%) of the lotion composition melts. This can be
expressed as:
(1) AH2/ AH1 is equal to or larger than about 1 and/or equal to or larger than
about 4
and/or equal to or larger than about 9; and/or
(2) AH2 is equal to or larger than about 30J/g, 40J/g and/or equal to or
larger than
about 60J/g (especially if Mil is 0)
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
wherein: AH1 is the energy required to raise the temperature of the lotion
composition
from 15 C to 35 C; AH2 is the energy required to raise the temperature of the
lotion
composition from 35 C to the temperature where the lotion composition is fully
liquid or
where no more melting occurs below 100 C in the case the lotion composition
contains
components only melting above 100 C.
AH is measured by DSC technique using standard parameters known to the one
skilled in the art. DSC data are obtained using a Thwing Albert DSC 2920
Instrument,
calibrated with an indium metal standard with a melting onset temperature of
156.6 C
and a heat of melting of 6.80 calories per gram, as reported in the
literature. The sample
is first heated to 100 C at a rate of 10 C/min, equilibrated for 5 minutes at
100 C, cooled
down to -30 C at a rate of -2.5 C/min, equilibrated at -30 C for 5 minutes and
then
finally heated from -30 C to +100 C at a rate of 2.5 C/min to evaluate the
melt
behaviour. For determination of AH1 and AH2 the final heating ramp is used.
AH1 is the
area between the DSC curve and the baseline between 15 C and 35 C and AH2 is
the area
between the DSC curve and the baseline between 35 C and the temperature where
the
lotion composition is fully liquid or where no more melting occurs below 100 C
in the
case the lotion composition contains components only melting above 100 C. By
way of
example, a lotion composition of the present invention that comprises about
40%
Stearylalcohol, about 30% Mineral oil and about 30% Petrolatum has a value of
AH2/ AH1
> 9 and a value of AH2 > 60J/g.
In one example, the lotion composition is present on the surface of the
fibrous
structure and/or sanitary tissue product and/or on the surface treating
composition present
on the surface of the fibrous structure and/or sanitary tissue product at a
level of at least
about 0.5 g/m2 and/or at least about 1.0 g/m2 and/or at least about 1.5 g/m2
per user
contacting surface. In another example, the lotion composition is present on
the surface
of the fibrous structure and/or sanitary tissue product and/or on the surface
treating
composition present on the surface of the fibrous structure and/or sanitary
tissue product
at a level of from about 0.5 g/m2 and/or from about 1.0 g/m2 and/or from about
1.5 g/m2
to about 10 g/m2 and/or to about 8 g/m2 and/or to about 6 g/m2 per user
contacting
surface.
Vehicle
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
21
As used herein a "vehicle" is a material that can be used to dilute and/or
emulsify
agents forming the surface treating composition and/or lotion composition to
form a
dispersion/emulsion. A vehicle may be present in the surface treating
composition and/or
lotion composition, especially during application of the surface treating
composition
and/or to the fibrous structure. A vehicle may dissolve a component (true
solution or
micellar solution) or a component may be dispersed throughout the vehicle
(dispersion or
emulsion). The vehicle of a suspension or emulsion is typically the continuous
phase
thereof. That is, other components of the dispersion or emulsion are dispersed
on a
molecular level or as discrete particles throughout the vehicle.
Suitable materials for use as the vehicle of the present invention include
hydroxyl
functional liquids, including but not limited to water. In one example, the
lotion
composition comprises less than about 20% and/or less than about 10% and/or
less than
about 5% and/or less than about 0.5% w/w of a vehicle, such as water. In one
example,
the surface treating composition comprises greater than about 50% and/or
greater than
about 70% and/or greater than about 85% and/or greater than about 95% and/or
greater
than about 98% w/w of a vehicle, such as water.
Process Aids
Process aids may also be used in the lotion compositions of the present
invention.
Nonlimiting examples of suitable process aids include brighteners, such as
TINOPAL
CBS-X , obtainable from CIBA-GEIGY of Greensboro, N.C.
Nonlimiting Examples of Lotion Compositions
Example 1 of Lotion Composition:
Stearyl Alcohol C01897 * 40% w/w
Petrolatum Snowwhite V28EP ** 30% w/w
Mineral oil Carnation ** 30% w/w
* Available from Procter&Gamble Chemicals, Cincinnati, USA
,
** Available from Crompton Corporation
The lotion composition has a melting point of about 51 C and a melt viscosity
at
56 C of about 17 m*Pas measured at a shear rate of 0.1 1/s. The mineral oil
used in this
formulation has a viscosity of about 21 mPa*s at 20 C. The lotion composition
can be
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
22
applied to one or both surfaces of the fibrous structure at total add-on
levels of 3.6g/m2,
4.2g/m2, 6g/m2, 7.2g/m2, 8.4g/m2 and 11.4g/m2.
Processes for Treating Fibrous Structures and/or Sanitary Tissue Products
a. Surface Treating Composition:
Any contact or contact free application suitable for applying the surface
treating
composition, such as spraying, dipping, padding, printing, slot extruding,
rotogravure
printing, flexographic printing, offset printing, screen printing, mask or
stencil application
process and mixtures thereof can be used to apply the surface treating
composition to the
fibrous structure and/or sanitary tissue product and/or to the lotion
composition present
on a surface of the fibrous structure and/or sanitary tissue product. Surface
treating
compositions can be applied to the fibrous structure and/or sanitary tissue
product before,
concurrently, or after the lotion composition application to the fibrous
structure and/or
sanitary tissue product. The surface treating composition can be applied
during
papermaking and/or converting, especially if applied to the outside layer of a
layered
fibrous structure and/or sanitary tissue product comprising such layered
fibrous structure.
In one example, the surface treating composition is applied by an application
process that provides a relatively high surface area coverage on the surface
of the fibrous
structure and/or sanitary tissue product. Examples of such suitable
application process
include, but are not limited to, printing, slot extruding and/or spraying with
fine particles
(although spraying has disadvantage of producing aero soles if high area
coverage is to be
achieved).
b. Lotion Composition:
Any contact or contact free application suitable for applying the lotion
composition, such as spraying, dipping, padding, printing, slot extruding,
rotogravure
printing, flexographic printing, offset printing, screen printing, mask or
stencil application
process and mixtures thereof can be used to apply the lotion composition to
the fibrous
structure and/or sanitary tissue product and/or surface treating composition
present on the
surface of the fibrous structure and/or sanitary tissue product. The lotion
composition can
be applied to the fibrous structure and/or sanitary tissue product before,
concurrently,
and/or after the surface treating composition application to the fibrous
structure and/or
sanitary tissue product. In one example, the lotion composition is applied to
the surface
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
23
treating composition present on the surface of the fibrous structure and/or
sanitary tissue
product.
In one example, the lotion composition is applied by an application process
that
provides a relatively low surface area coverage on the surface of the fibrous
structure
and/or sanitary tissue product and/or on the surface treating composition
present on the
surface of the fibrous structure and/or sanitary tissue product such that
regions of surface
treating composition and regions of lotion composition produce the user
contacting
surface. Example of such suitable application processes include, but are not
limited to,
spraying, especially spraying with rotating discs, printing, slot extruding in
stripes and/or
other patterns.
In one example, the surface treating composition may be added to a fiber
furnish
that will form an external layer of a multilayer fibrous structure. The lotion
composition
may be applied to the surface formed by the external layer of the multilayer
fibrous
structure.
In one example, the surface treating composition is applied to the surface of
the
fibrous structure during the fibrous structure making process, such as before
and/or after
drying the fibrous structure. The lotion composition may then be applied to
the surface
treating composition on the surface of the fibrous structure during the
converting process.
In one example, the surface treating composition contains less than about 5%
and/or less than about 3% and/or less than about 1% and/or less than about
0.5% moisture
at the time the lotion composition is applied to it.
Treated Fibrous Structure Examples
Fibrous Structure - Example 1:
A first fibrous structure is a conventional wet pressed, homogeneous, dry
creped
fibrous structure with a basis weight of about 15.4 g/m2. The fibrous
structure has a
composition of about 40% Northern Softwood Kraft and 60% Eucalyptus. Four
plies of
the fibrous structure are combined together in an off line combining operation
to produce
a sanitary tissue product. The 4-ply sanitary tissue product has a basis
weight of about
60g/m2, a thickness of about 0.3 mm, a machine direction strength of about
1280 g/in, a
cross direction strength of about 610 g/in, and a wet burst of about 200g. It
contains a
wet strength agent and a dry strength agent.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
24
Fibrous Structure - Example 2:
A second fibrous structure is a conventional wet pressed, layered, dry creped
fibrous structure with a basis weight of about 14.6 g/m2. The outer layer
contains about
100% Eucalyptus fiber whereas the inner layer is composed of a furnish mix of
about
85% Northern Softwood Kraft, 10% CTMP and about 5% Eucalyptus fiber. Both
layers
are of about equal basis weight (symmetrical layer split). Four plies of the
fibrous
structure are combined together in an off line combining operation to form a
sanitary
tissue product such that the Eucalyptus layer is present on the two outer
surfaces of the
combined 4-ply sanitary tissue product. The 4-ply sanitary tissue product has
a basis
weight of about 60g/m2, a thickness of about 0.3 mm, a machine direction
strength of
about 1180 Win, a cross direction strength of about 560 Win, and a wet burst
of about
200g. It contains a wet strength agent and a dry strength agent.
Fibrous Structure - Example 3:
A third fibrous structure is formed from an aqueous slurry of Northern
Softwood
Kraft (NSK) of about 3% consistency made up using a conventional pulper and
passed
through a stock pipe toward the headbox of the Fourdrinier. A 1% dispersion of
Hercules' Kymene 557 LX is prepared and is added to the NSK stock pipe at a
rate
sufficient to deliver about 0.8% Kymene 557 LX based on the dry weight of the
ultimately resulting sanitary tissue product. The absorption of the permanent
wet strength
resin i enhanced by passing the treated slurry through an in-line mixer. An
aqueous
solution of Carboxymethyl cellulose (CMC) dissolved in water and diluted to a
solution
strength of 1% is added next to the NSK stock pipe after the in-line mixer at
a rate of
about 0.1% CMC by weight based on the dry weight of the ultimately resulting
sanitary
tissue product. The aqueous slurry of NSK fibers passes through a centrifugal
stock
pump to aid in distributing the CMC. An aqueous dispersion of DiTallow
DiMethyl
Ammonium Methyl Sulfate (DTDMAMS) (170 F) at a concentration of 1% by weight
is
added to the NSK stock pipe at a rate of about 0.1% by weight DTDMAMS based on
the
dry weight of the ultimately resulting sanitary tissue product. An aqueous
slurry of
eucalyptus bleached haft fibrous pulp fibers (from Aracruz - Brazil) of about
1.5% by
weight is made up using a conventional repulper and is passed through a stock
pipe
toward the headbox of the Fourdrinier. This Eucalyptus furnish joins the NSK
slurry at
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
the fan pump where both are diluted with white water to about 0.2%
consistency. An
aqueous slurry of eucalyptus bleached kraft fibrous pulp fibers (from Aracruz -
Brazil) of
about 3% by weight is made up using a conventional repulper. The Eucalyptus
slurry
passes to the second fan pump where it is diluted with white water to a
consistency of
about 0.2%. The slurries of NSK/eucalyptus and eucalyptus are directed into a
multi-
channeled headbox suitably equipped with layering leaves to maintain the
streams as
separate layers until discharged onto a traveling Fourdrinier wire. A three-
chambered
headbox is used. The eucalyptus slurry containing 48% of the dry weight of the
ultimate
sanitary fibrous structure is directed to the chamber leading to the layer in
contact with
the wire, while the NSK/eucalyptus slurry comprising 52% (27-35% NSK and 17-
25%
eucalyptus) of the dry weight of the ultimate paper is directed to the chamber
leading to
the center and inside layer. The NSK/eucalyptus and eucalyptus slurries are
combined at
the discharge of the headbox into a composite slurry. The composite slurry is
discharged
onto the traveling Fourdrinier wire and is dewatered assisted by a deflector
and vacuum
boxes. The embryonic wet fibrous structure is transferred from the Fourdrinier
wire, at a
fiber consistency of about 17% by weight at the point of transfer, to a
patterned drying
fabric. The drying fabric is designed to yield a pattern-densified tissue
with
discontinuous low-density deflected areas arranged within a continuous network
of high
density (knuckle) areas. This drying fabric is formed by casting an impervious
resin
surface onto a fiber mesh supporting fabric. The supporting fabric is a 48 x
52 filament,
dual layer mesh. The thickness of the resin cast is about 8 mil above the
supporting
fabric. The knuckle area is about 35-50% and the open cells remain at a
frequency of
about 68-562 per square inch. Further de-watering is accomplished by vacuum
assisted
drainage until the fibrous structure has a fiber consistency of about 23-27%.
While
remaining in contact with the patterned forming fabric, the patterned fibrous
structure is
pre-dried by air blown through to a fiber consistency of about 60% by weight.
The semi-
dry fibrous structure is then adhered to the surface of a Yankee dryer with a
sprayed
creping adhesive comprising a 0.250% aqueous solution of polyvinyl alcohol.
The
creping adhesive is delivered to the Yankee surface at a rate of 0.1% adhesive
solids
based on the dry weight of the fibrous structure. The fiber consistency is
increased to
about 98% before the fibrous structure is dry creped from the Yankee with a
doctor blade.
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
26
After the doctor blade, the fibrous structure is calendared across all its
width with a steel
to rubber calendar roll operating at a loading of 300-500 psi. The resulting
tissue has a
basis weight of about 20-25 g/m2; a 1-ply total dry tensile between 250 and
370 g/in, a 1-
ply wet burst between 35 and 65 gr/in and a 2-ply caliper of about 0.015-0.020
inches.
The resulting tissue is then combined with a like sheet to form a two-ply,
creped, pattern-
densified tissue so that the eucalyptus fibers face the outside and it is
subjected to
calendaring between two smooth steel calendar rolls. The product is then ply-
bonded
using a mechanical plybond wheel to ensure that both plies stay together. The
resulting
two-ply tissue has a) a total basis weight of about 39-50 g/m2; b) a 2-ply
total dry tensile
between 450 and 700 gr/in; c) a 2-ply wet burst between 100 and 130 g/M; d) a
4-ply
caliper of about 0.51 and 0.89 mm.
The fibrous structures described above can be used in combination with any of
the
treatment processes and lotion compositions described below.
Converting of the Fibrous Structures of Examples 1-3:
The combined parent roll is subsequently converted into a sanitary tissue
product.
The multi-ply parent roll is unwound and subjected to calandering between two
smooth
steel calender rolls followed by high pressure embossing to achieve ply
bonding. The
majority of the fibrous structure remains unaffected by the high pressure
embossing. The
surface treating composition and the lotion composition are then applied to
the fibrous
structure as described in detail below. Finally the tissue was cut in machine
direction,
followed by cutting in cross direction into sheets of approximately 21cm x
21cm, folded,
stacked into stacks of 9 sheets and packed into individual pocket packs.
Applying Surface Treating Composition to Fibrous Structures of Examples 1-3:
Directly following the ply bond operation, the surface treating composition is
printed onto the surface of the 4-ply fibrous structure and/or sanitary tissue
product using
a roto-gravure printing process. About 1.5 g/m2 of the surface treatment
composition is
transferred to each side of the 4-ply product.
The printing station consists of two engraved anilox rolls facing each other
in a
horizontal arrangement and forming a gap in between through which the fibrous
structure
and/or sanitary tissue product is run. The geometry is arranged in a way that
the rolls
touch the fibrous structure and/or sanitary tissue product and transfer lotion
composition
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
27
macroscopically uniformly onto both surfaces of the 4-ply fibrous structure
and/or
sanitary tissue product but the fibrous structure and/or sanitary tissue
product does not
wrap any of the two anilox rolls. The anilox rolls are engraved to a cell
volume of about
3 ml per square meter and about 100 cells per square centimeter, and supplied
with lotion
composition from a closed supply chamber designed to fill the engraved volume
with
lotion composition. The gap between the two rolls is adjusted to achieve the
target add-
on level. Surface coverage of the surface treatment composition was
substantially 100%
and homogenious. Surface coverage can, e.g. be tested using a surface
treatment
composition that has 0.01% Tinopal CBS-X added, a fluorescent dye, available
from Ciba
Speciality Chemicals, Basel, Switzerland.
Samples have also been made using an identical application system as described
below for the application of lotion composition. The equipment was operated at
ambient
temperature at a disc speed of about 4000rpm. Surface coverage of this
application is
lower than using a printing process as described above. While still within the
scope of
this invention, this process is therefore less preferred.
Applying Lotion Composition to Fibrous Structures of Examples 1-3:
Directly following the surface treating composition, the lotion composition is
applied to the fibrous structure and/or sanitary tissue product. The fibrous
structure and/or
sanitary tissue product span between the two operations was about 5 meter. A
commercially available rotary spray application system RFT-Compact-III with
applicator
heads for the tissue and textile industry (available from Weitmann&Konrad GmbH
& Co
KG, Leinfelden Echterdingen, Germany) was modified to be used to practice the
present
invention. The application head is equipped with 5 sets of rotary disks (type
1/1) and has
an effective application width of 448mm. The housing of the application head
was
replaced with water heated walls on the top, the bottom and the rear side of
the
application head. The whole unit was then insulated towards the outside. Two
of these
modified application heads were used, installed facing each other so that both
sides of a
fibrous structure and/or sanitary tissue product can be treated
simultaneously. Heating
units with an integrated pump (Type W60/10-12/40, available from Kelviplast
GmbH,
Germany) are used to supply the application units with water of the desired
temperature.
In particular, the design of the heating elements was chosen so that the
temperature inside
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
28
the application head is within +/-2 C from the target temperature. The lotion
composition
infeed of the application heads are connected through a heat traced piping
system to a
heated pump that is connected through heat traced piping to a heated 1001iter
tank that
holds the melted lotion composition. The return lines of the applicator feed
back into the
heated tank. A heated flow meter was installed in the lotion composition
supply line
between pump and application heads. The flow meter (Promass 63M, available
from
Endress & Hauser, Switzerland) was connected to the control unit of the RFT-
Compact-
III system that was then used to control the lotion composition pump (Gear
pump of type
Labu Brox) to deliver the desired lotion composition flow to the application
heads.
No changes are made to the setup, shape and dimensions of the rotating
surfaces
in the commercially available application head. Each set of rotating surfaces
consisted of
2 rotating discs stacked on top of each other. The lotion composition supply
to the two
rotating surfaces of each stack is equally split. The discs have a diameter of
about 98mm.
The five individual stacks of rotating surfaces are spaced apart by about
112mm. The
first, third and fifth set of rotating surfaces is installed vertically
shifted versus the second
and fourth stack of rotating surfaces to avoid interference between the
horizontally
overlapping streams of droplets. The sets of rotating surfaces are
commercially available
from Weitmann & Konrad GmbH & Co, Germany (type 1/1, Art. No. 618996 [upper
set]
and 618997 [lower set]). The applicator is operated horizontally and with a
distance of
about 154mm between the fibrous structure and/or sanitary tissue product and
the center
of the disks. The fibrous structure and/or sanitary tissue product is run
vertically from top
to bottom between the two application heads. Controlled by the windows in the
housing
between the rotating surfaces and the fibrous structure and/or sanitary tissue
product,
each stack of rotating surfaces covers a cross direction width of about 224mm
on the
fibrous structure and/or sanitary tissue product with the exception of the two
outer stacks
of rotating surfaces of the applicator which only cover 112mm each. At each
position the
streams of two stacks of rotating surfaces are overlapping. Even distribution
to the
individual stacks of discs was achieved with throttles of lmm diameter,
installed between
the infeeds to the rotary discs and the central supply pipe of the applicator.
The lotion
composition temperature is controlled to a determined value through the
heating of the
tank, the piping and the temperature in the application heads to the desired
value. The
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
29
flow rate is adjusted to achieve the desired add-on level of the fibrous
structure. During
application, the fibrous structure and/or sanitary tissue product is typically
kept at room
temperature. Some samples were made where the fibrous structure was cooled or
heated
prior to application of the lotion composition. The lotion composition almost
instantaneously solidifies after impacting the fibrous structure and/or
sanitary tissue
product. Samples with add-on levels of 1.5 g/m2, 2.3 g/m2, 3g/m2 and 4.5g/m2
per fibrous
structure and/or sanitary tissue product side are made.
The surface area coverage of the lotion composition is about 15% for the
sample
made with 3g/m2 lotion application per fibrous structure and/or sanitary
tissue product
side, a disc speed of about 2500rpm, and a lotion composition temperature in
the
applicator of about 56 C.
Process Conditions for Treating of Fibrous Structures of Examples 1-3:
The rotating surfaces are operated at 2500rpm for the samples described below
but additional samples are made at speeds between 200rpm and 5000rpm.
The lotion composition is usually maintained at a temperature of about 5-10 C
above the melting point, for the lotion compositions described below all
temperature
settings are kept at 56 C. Products are made at temperatures less than 2 C
below and
more than 10 C above the melting point. The fibrous structure and/or sanitary
tissue
product speed for the examples below is 200m/min, but samples can be made at
fibrous
structure and/or sanitary tissue speeds between 10m/min and 400m/min.
Test Methods
A. Surface Area Coverage Test Method
The local surface treating composition and/or lotion composition basis weight
on
the surface of the fibrous structure can be determined by scanning IR/NIR
(infrared or
near infrared) spectroscopy in transmission mode (absorption spectroscopy)
using a
Perkin Elmer Spectrum Spotlight 300 instrument in combination with Spotlight
software
version 1.1.0 B38.
The following procedure is applicable to surface treating compositions and/or
lotion compositions comprising a linear hydrocarbon component of repeated ¨
(CH2) -
units. Adaptation of the procedure may become necessary if the composition is
composed
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
mostly or entirely of other materials. Such adaptations will depend on the
composition
and will usually be apparent to those skilled in the art.
The measurements are done with samples representative for the tissue. A 5 x 5
mm sample (or larger) is placed on the sample holder, which is mounted on a XY
table
and the spectral area used for analysis is scanned at a spatial resolution of
25 um in both x
and y dimension. For the analysis of materials containing linear chains of
¨CH2- groups
the region between 4000cm-1 and 4500cm-1 is scanned and the range between
4296cm-1
(W1) and 4368cm-1 (W2) is used for analysis. At least 16 scans are taken at a
resolution
of 1 cm-1. If more than 16 scans are used, care needs to be taken that the
sample does not
change structure as a result of heating up.
Next, a map of the local basis weight of the sample is generated. The
integrated
absorption between W2 and W1 and above a sloping linear baseline is determined
for
each pixel of 25um x 25um using the ChemiMap menu of the software. The
baseline is
defined by the absorbency at W1 and W2. The two base points option is chosen
in the
ChemiMap menu of the software and set at W1 and W2. Start and end point of the
integration are also set at W1 and W2. The scaling factor is set to a value V1
which is
defined as: V1 = F* DW where F is the factor described below and DW=W2-W1 is
the
delta in wave numbers between the upper (W2) and the lower (W1) wave number in
cm-1.
The scaling with the factor DW transforms the average absorbance above the
baseline within the wave number range W1 to W2 into an integrated absorption
above the
baseline. The factor F translates the integrated absorption into local basis
weight in g/m2.
The file, which is generated with the ChemiMap command, contains the local
basis weight for each pixel of 25um x 25um in area. The file is saved as a
text file (.txt
format) and also as a bitmap (.bmp format) in 8 bit grey scale format. The
text file is
imported into EXCEL and the first row and first column are removed (they do
not contain
image data, but position data). The resulting data are representing the array
of pixels of
local basis weight in g/m2. The maximum (MaxLBW) and minimum (MinLBW) value,
as
well as the average (AvgLBW) of the whole dataset is calculated in EXCEL.
The bitmap file (.bmp file) is imported into AnalySIS image analysis software
for
further processing (Analysis Pro version 3.1 (build 508), available from Soft
Imaging
GmbH, Germany). The imported grey scale file is still in ROB format with all
three color
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
31
channels set equal (in 8 bit resolution). In AnalySIS the file is color
separated to extract
one of the three identical color channels (red). The resulting file is now
scaled from G=0
to G=255, G=0 representing the minimum value (MinLBW) of the original
spotlight data
and 255 representing the maximum value (MaxLBW) of the original spotlight
data. The
image is calibrated in x-y by setting the pixel size in x and y dimension to
match the
original sample. The image is resealed in z-direction to display the local
basis weight
values in g/m2 but all calculations within AnalySIS have to be made in the G=0
to G=255
scale. The G values can be easily transformed into local basis weight numbers
by the
following relationship:
LLBW=A*(G+OFFSET), where A= (MaxLBW-MinLBW)/255 and
OFF SET=(255*MinLBW)/(MaxLBW-MinLBW)
The G values can be easily transformed into local lotion basis weight numbers
(LLBW) by the following relationship: G--(LLBW/A) - OFFSET
LLBW can be local lotion composition basis weight or local surface treating
composition basis weight depending upon what is being measured.
The average value of all local basis weight datapoints above 10g/m2 can be
calculated from the EXCEL datafile.
The area of fibrous structure and/or sanitary tissue product affected by the
composition is calculated in Analysis by setting a lower threshold at the G
value
equivalent to 3g/m2 and calculating the area above that threshold. The setting
"holes not
filled" is used. The areas of the composition is similarly determined by
setting the
threshold at a G value equivalent to 10g/m2 (10g/m2 equals G=10/A - OFFSET).
If the areas of the composition are defined to have a certain minimum and/or
maximum area is set as a filter. The area percentage of composition larger
than a certain
area is calculated by dividing the area of the composition calculated without
area filter,
divided by the area of the composition calculated with area filter.
The factor F to convert integrated absorption values into local lotion basis
weight
values is determined by the following procedure: A representative set of
calibration
samples of known average basis weight of the composition is scanned in the
spectral
range used for the analysis as described above and analyzed for integrated
peak area
between W1 and W2 (4296cm-1 and 4368cm-1 for mostly hydrocarbon like
materials).
CA 02563539 2006-10-18
WO 2005/106119 PCT/US2005/013684
32
The integrated peak area is obtained from the procedure above if the factor F
is set equal
to 1. The dataset is then imported to EXCEL and the average pixel value of
this dataset is
calculated. As the factor F was set equal to 1 this value is equal to the mean
integrated
peak area (AIPA) of the sample in the wave number range W1 to W2. The factor F
is then
calculated as F=1/slope of a linear least square fit through the origin of the
plot of AIPA
vs. average composition basis weight of the sample. Calibration samples to
determine
the factor F can either be prepared or an existing composition-containing
sample can be
used. If an existing sample is used the composition basis weight can be
determined by
extraction. An example for such a procedure is given below. Examples for how
the factor
F is determined by analyzing an existing sample (market product) and by
preparing
calibration samples is also given below. It is important, that the absorbency
in the
wavelength range used for analysis should never exceed about 1 to ensure a
linear
correlation between the infrared signal and the local composition basis weight
i. Determination of factor F by preparing calibration samples
Preparation of calibration samples: A suitable piece of the substrate of known
area, weight and basis weight is evenly treated with the composition. A
suitable type of
equipment is a hot wax cartridge spray gun type MK-DUO Line Art.No. 140101,
available from MK HeiBwachstechnik GmbH, Aichach, Germany. After the
application,
the composition is equilibrated in the sheet by placing the sample in an oven
at a
temperature of about 10 C above the mp (or at a temperature suitable to allow
for
sufficient equilibration of the composition in/on the sheet). For relatively
low viscosity
samples equilibration for about an hour is sufficient. The sample is then
cooled down to
room temperature and equilibrated for moisture content at 23 C (+-1 C) and 50%
(+-2%)
relative humidity and weighed again. The composition basis weight of that
sample [in
g/m2] is then calculated as (sample weight after composition treatment [in
grams] ¨
sample weight before composition treatment [in grams]) divided by area of the
sample [in
m2]. The samples are then analyzed by the procedure described above to
determine the
factor F. Preferably, calibration samples are prepared in a range of
composition basis
weights that include the range to be measured.
Determination of Factor F for a market product: The basis weight of the sample
is
determined by a standard procedure. The sample is then analyzed by the
procedure
CA 02563539 2009-08-25
33
described above for the average integrated peak area between 4296cm-1 and
4368cm-1.
The sample is then extracted by the procedure described below to determine the
composition add-on. The Factor F is then calculated as
Factor F = composition basis weight [g/m2] / average integrated peak area
If the composition does not contain a sufficient amount of linear hydrocarbon
like
material, or the substrate contains materials that do not allow for a
quantification of
composition between 4296cm-1 and 4368cm-1, a different wave number range in
the
infrared or near infrared range has to be identified that is suitable to
quantify the
composition by IR. spectroscopy. Any wave number range with a linear
correlation
between integrated absorption coefficient above base line and composition
basis weight
can be used. If more than one possible wave number range can be identified,
the range
with the best signal to noise ratio is used. Whenever the composition is based
on linear
hydrocarbon like materials with CH2 groups the absorption band between 4296cm-
1 and
4368cm-1 should be used.
B. Relative Concentration of Composition on Surface Test Method
Relative concentration of a composition on a surface of the fibrous structure
and/or sanitary tissue product may be determined by using near JR
spectroscopy,
especially if the sample contains a hydrocarbon-containing composition. The
near IR
spectroscopy method may use a filter photometer or other near JR instrument,
but it must
be configured for back scatter detection. Appropriate wavelengths are used.
The fibrous structure and/or sanitary tissue product is placed under the near
JR
instrument and a reading is obtained. The sample is then turned over to obtain
a reading
from the other side of the sample.
In addition to near IR, mid-JR spectroscopy with suitable equipment and
wavelengths may also be used to determine relative concentration of a
composition on a
surface of a fibrous structure and/or sanitary tissue product.
All documents cited in the Detailed Description of the Invention are,
not to be construed
as an admission that it is prior art with respect to the present invention.
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
CA 02563539 2012-08-13
34
modifications can be made without departing from the invention described
herein.