Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 53
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 53
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE:
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
TITLE OF THE INVENTION
ISOLATED NUCLEIC ACID MOLECULES ENCODING A NOVEL PHOSPHOPROTEIN- DARPP-
32, ENCODED PROTEIN AND USES THEREOF
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
60/572,634 filed
May 19, 2004, the contents of which are incorporated herein by reference in
their entirety.
FIELD OF THE INVENTION
The invention relates to the field of neurobiology. The invention provides
novel nucleic
acid molecules, encoded proteins and methods of using either in identifying
therapeutic moieties for use
in treating psychotic disorders. Specifically, the invention provides nucleic
acid molecules encoding a
phosphoprotein designated herein as DARPP-32 derived from a non-human source,
e.g., guinea pig,
encoded proteins and use of the sequences in the study, diagnosis, and
treatment of diseases affecting
brain function.
BACKGROUND OF THE INVENTION
Protein phosphorylation appears to be an important mechanism in neuronal
signal
transduction, whereby extracellular stimuli are relayed to the interior of
cells and subsequently these
stimuli regulate diverse cellular processes. The triggering mechanisms for
activation of protein
phosphorylation include many established second messengers (CAMP, cGMP,
calcium), which are
generated by interaction of neurotransmitters with their receptors ands which,
in turn, activate protein
kinases (protein-phosphorylating enzymes) that transfer phosphate from
adenosine triphosphate (ATP) to
substrate proteins. These substrate proteins, in turn, mediate many of the
physiological effects attributed
to the transmitter-receptor interaction.
Studies of signal transduction mediated by protein phosphorylation have
demonstrated a
central role for one particular phosphoprotein designated - DARPP-32 (dopamine-
and cyclic AMP
(cAMP)-regulated phosphoprotein having a molecular weight of 32 kilo daltons).
This protein is a
cystolic protein that is selectively enriched in medium-sized spiny neurons in
neostriatum, which is a,
major target for midbrain dopaminergic neurons. Ouimet et al., 1984. Since,
medium spiny neurons are
the only known projection neurons in the neostriatum, these function to
integrate all input to this brain
region. The spiny neurons contain both D1 class (D1, DS) and D2 class (D2, D3,
D4) dopamine receptors
(Sibley, D.R. and Monsma, F.J.Jr. ( 1992). Molecular biology of dopamine
receptors. Trends Pharmacol.
Sci 13, 61-69.). (Surmeier, D.J., Song, W.J., and Yan, Z. (1996).
-1-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Dopamine is a neurotransmitter in the brain. Since its discovery in the 1950s,
its function
in the brain has been intensely explored. It is well settled that dopamine is
essential in several aspects of
brain function.
Dopamine produces its biological effects on the neurons via activation of a
biochemical
cascade involving stimulation of D1 receptors, activation of adenylyl cyclase,
increased cAMP formation
and increased activity of PKA (Walaas and Greengard, 1984). Briefly, dopamine
released from
nigrostriatal nerve terminals acts on Dl dopamine receptors to increase the
activity of adenylyl cyclase,
increase cAMP formation, and stimulate cAMP-dependent protein kinase (PKA). In
turn, PKA
phosphorylates DARPP-32 at a single threonine residue (Thr34), convening the
protein into a potent
inhibitor of PP-1, which is a major multifunctional serine/threonine protein
phosphatase in the brain. PP-
1, in turn, regulates the phosphorylation state and activity of many
downstream physiological effectors,
including various neurotransmitter receptors and voltage-gated ion channels.
To date several classes of
ion channels, including Na+ channels and the L-, N- and P-classes of Ca2+
channels, the NMDA-R1
class of glutamate receptor, and the electrogenic ion pump, Na+,K+-ATPase,
have been shown to be
regulated by PP-1 Since protein phosphatase-1 is a major protein phosphatase
in the brain, this inhibitory
role of DARPP-32 has considerable physiological significance Conversely,
dopamine, acting on D2-like
receptors, through both inhibition of PKA and activation of calcium/calmodulin-
dependent protein
phosphatase (protein phosphatase 2B - PP-2B/calcineurin), causes the
dephosphorylation of DARPP-32.
PP-2B also acts synergistically with PP-2A to regulate the dephosphorylation
of DARPP-32 (Nishi et al.
1999.). Various studies have confirmed that the DARPP-32/PP-1 cascade is a
major target for psycho
stimulants and anti schizophrenic drugs. Consequently, modulators of
dopaminergic function will find
use in the treatment of a wide range of disorders affecting brain functions.
The role of DARPP-32 in the DARPP-32/protein phosphatase-1 (PP-1) cascade in
integrating the neurotransmitter pathways in medium spiny neurons of the
neostriatum is well
documented. For example, Forskolin, a drug which directly activates adenylyl
cyclase to increase cAMP
formation, mimics the effects of Dl receptor stimulation, resulting in
increased phosphorylation of
DARPP-32 in neostriatal slices. N-methyl-D-aspartate (NMDA) receptors, which
are present on all
medium spiny neurons, have been shown to participate in glutamate-mediated
dephosphorylation of
DARPP-32, probably through Ca2+-dependent activation of PP-2B. The
dephosphorylation of DARPP-
32 in response to NMDA has been shown to be blocked by cyclosporin A, a highly
specific PP-2B
inhibitor, confirming the involvement of PP-2B as an enzyme that
dephosphorylates Thr34 in intact cells.
Thus, dopamine and glutamate have opposing actions on the phosphorylation
state and activity of
DARPP-32, which may contribute to certain of the antagonistic effects of these
two neurotransmitters on
neostriatal neuron excitability. In addition to PP2B, PP2C and CK1, cyclin-
dependent kinase 5 (cdk5)
and PP2A are also involved in regulating the state of phosphorylation of DARPP-
32.
-2-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Likewise, studies in mice lacking the DARP-32 gene, have revealed that
inactivation of
the DARPP-32 gene markedly reduced, and in some cases abolished, various
responses to dopaminergic
agonists and antagonists. In some instances, the impairment of responses could
be overcome by
increasing the concentration of the test substance used. These studies thus
confirm that this protein plays
an essential role in mediating the actions and interactions of dopamine and
other neurotransmitters that
act on dopaminoceptive neurons. See Greengard et al., Neuron, 23: 435-447 (
1999).
Other studies have also confirmed that a cascade involving dopamine-mediated
receptor
activation of DARPP-32, inhibition of PP-1, and potentiation of
phosphorylation of neuronal substrates
plays a major role in regulating the efficacy of dopaminergic
neurotransmission under physiological
conditions. See Hemmings, Jr. et al. Nature 310 ( 1984), pp. 503-505 which
shows that dopamine-
dependent changes in either the phosphorylation or regulation of NR1 NMDA
receptors, Na+,K+-
ATPase, and N- and P-type Ca2+ channels are attenuated in the DARPP-32
knockout mice.
Depression is among the most debilitating psychiatric disorders and is
generally
associated with heterogeneous dysregulation of the biogenic amines. While few
pharmacological
approaches exist for treating depression, most of the current treatment
paradigms for depression involve
modulation of serotonergic neurotransmission through the usage of
compounds/agents that alter the level
of serotonin in the synaptic cleft. Recently, dopamine has been implicated in
the pathophysiology of
depression. The suggestion has been made that dopamine may be reduced in
depression and increased in
mania. See Guitart, X and E.J. Nestler, J. Neurochem. 59: 1164-1167 (1992),
who show a nexus between
the serotonergic pathways in the brain and DARPP-32. Two prevalent theories
regarding dopamine and
depression are that the mesolimbic dopamine pathway may be dysfunctional in
depression and that the
dopamine type 1 (D1) receptor may be hypoactive in depression (Ch.9. Mood
Disorders, in: CONCISE
TEXTBOOK OF CLINICAL PSYCHIATRY. Ed. by H I Kaplan and B J Sadock. Williams &
Wilkins,
Baltimore, Md., 1996, pp. 159-188). For example, drugs that reduce dopamine
concentrations (e.g.,
reserpine) and diseases that reduce dopamine concentrations (e.g., Parkinson's
disease) are associated
with depressive symptoms. As well, drugs that increase dopamine concentrations
(e.g., tyrosine,
amphetamine and bupropion) reduce the symptoms of depression. Consequently, in
view of the high
expression levels of DARP-32 in prefrontal cortex and the striatum, and the
nexus between DARP-32
and depression, it is believed that the novel sequences disclosed herein will
provide means to develop
novel assays that can be used to develop novel therapeutic moieties that can
be used to treat disorders
related to a dysfunctional serotonergic intracellular signaling pathways, more
particularly depression.
Dopamine has also been implicated in the pathophysiology of schizophrenia,
which is a
multi-factorial disease characterized by multiple genetic susceptibility
elements, each likely contributing
a modest increase in risk (Karaylorgou, M. & Gogos, J. A. ( 1997) Neuron 19,
967-79). The dopamine
hypothesis for the pathophysiology of schizophrenia maintains that dysfunction
of the dopamine
-3-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
neurotransmitter system plays a key role in the abnormalities that occur in
schizophrenia. (Seeman, P.
(1987) Synapse I, 133-52; Carlsson, A., et al., (2001) Annu Rev Pharmacol
Toxicol 41, 237-60).
Schizophrenia is characterized as having both "positive symptoms"
(hallucinations,
delusions, and conceptual disorganization) and "negative symptoms" (apathy,
social withdrawal, affect,
and poverty of speech). While many drugs effective in treating schizophrenia
share the common property
of blocking dopamine receptors, such neuroleptics are only effective for
treating the positive symptoms of
schizophrenia, but have little or no effect on the negative symptoms. As well,
neuroleptics-resistant
negative symptoms account for most of the social and vocational disability
caused by schizophrenia.
Further, neuroleptics cause extrapyramidal symptoms, including rigidity,
tremor, bradykinesia (slow
movement), and bradyphrenia (slow thought), as well as tardive dyskinesias and
dystonias. It is
believed that the selection of a preferred therapy for a particular subject
may prevent the subject from
having to endure possibly irreversible side effects from therapies that are
ineffective for that subject.
Reduced side effects of the preferred therapy compared to other therapies may
result in greater patient
compliance, further increasing the likelihood of therapeutic benefit from the
therapy. Consequently, the
dysfunctional dopamine hypothesis strongly argues for the use of the herein
disclosed sequences in
identifying therapeutic moieties that may play a role in the DARPP-32/protein
phosphatase-1 (PP-1)
cascade as a means of treating Schizophrenia.
Abnormalities in dopaminergic neurotransmission have also been implicated in
supranuclear palsy, Tourette's syndrome and obsessive-compulsive disorder.
Finally, drugs of abuse
such as cocaine, amphetamine, and opiate classes, as well as nicotine and
alcohol, achieve some of their
addictive actions by modifying dopaminergic transmission.
However, currently available dopaminergic pharmaceuticals have severe side
effects,
such as extra pyramidal side effects and tardive dyskinesia in dopaminergic
antagonists used as
antipsychotic moieties, and dyskinesia and psychosis in dopaminergic agonists
used as anti-Parkinson's
agents. In addition, the therapeutic effects are unsatisfactory as a whole.
Collectively, the data favor the suggestion that regulation of DARPP-32 via
phosphorylation or dephosphorylation is probably the major molecular mechanism
by which information
received through dopaminergic and other signaling pathways is integrated in
these neurons, which
constitute the principal efferent pathway from the striatum. For example, in
neostriatum, dopamine-
mediated effects on the function of calcium channels (Surmeier et al., 1994),
voltage-dependent sodium
channels (Surmeier et al., 1992; Schiffman et al., 1994) and Na<sup></sup>+,K<sup></sup>+ -
ATPase (Aperia et al.,
1991) are regulated directly or indirectly by protein phosphatase-1.
Therefore, there is a need in the art to provide new methods of screening that
can be used
to develop novel compositions or drugs that can be used to treat psychotic
diseases or disorders. In
addition, there is a need for simple tests of intracellular consequences of
antipsychotic action. Since all
-4-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
anti- psychotics act upon multiple receptors, with widely varying downstream
effects in terms of both
effective relief of symptoms and unwanted side effects, analysis of the
intracellular integration of these
signals provides a straightforward, cost-effective, and mechanism-based
comparison useful for
development of the next generation of therapeutic drugs. As well, there is
also a need to develop
treatments for such diseases or disorders that are~due, at least in part, to
an aberration or dysregulation of
an intracellular signaling pathway regulated by DARPP-32.
Consequently, therapeutic moieties that mimic or block the inhibitory effects
of DARPP-
32 on PP-1 should find use in the treatment of Parkinson's disease,
schizophrenia, drug addiction, and
other neuropsychiatric disorders involving abnormal dopaminergic function.
Such moieties would have
their use in the activation of downstream components of the dopamine signaling
cascade. Significantly,
the selective enrichment of DARPP-32 in dopaminoceptive neurons and its
regulation by dopamine
strongly indicate that DARPP-32, by regulating protein phosphatase-1 activity,
plays a key role in
mediating the effects of dopamine on these cells. As well, the control of
protein phosphatase- I activity
by DARPP-32 is likely to have a significant role in the regulation of neuronal
excitability. Consequently,
the sequences of the present invention aim to remedy the void attending the
current treatment paradigms
for treating various psychotic disorders by enabling the identification of
compounds that modulate .the
dopaminergic signaling pathway as an effective means for treating various
disorders of the brain.
SUMMARY OF THE INVENTION
In its broadest aspect, invention features isolated and substantially purified
polynucleotides that encode a mammalian phosphoprotein. An illustrative
nucleic acid molecule has the
nucleotide sequence of SEQ ID NO:1 of 896 nucleotides, of which the coding
sequence encompasses
nucleotides lto 567 nucleotides. The encoded polypeptide has the amino acid
sequence as set forth in
SEQ >D N0:2. The polypeptide is designated herein as nhDARPP-32. Preferably,
the polynucleotide
sequences of the invention is/are derived from a guinea pig.
The invention additionally features fragments, portions or antisense molecules
of the
disclosed sequences thereof, and expression vectors and host cells comprising
polynucleotides that
encode nhDARPP-32. Variants of the polynucleotide as well as a polynucleotide
sequence comprising
the complement of SEQ ID NO:1 are also included within the scope of the
invention.
In addition, the invention features polynucleotide sequences which hybridize
under
stringent conditions to SEQ ID NO:1.
The present invention also features antibodies which bind specifically to
DARPP-32
and/or various phosphorylated fragments thereof, i.e., Thr34phopshorylated
DARPP-32, and preferably
the amino acids sequences) of the invention. Pharmaceutical compositions
comprising substantially
purified nhDARPP-32 are also contemplated.
-5-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
The use of the herein disclosed nhDARPP-32 polypeptide, and of the nucleic
acid
sequences which encode it, is also based on the amino acid and structural
homologies between the herein
disclosed nhDARPP-32 and the other known DARPP-32 polypeptides as well as on
the known
associations and functions of such types of phosphoproteins. The disclosed
polynucleotides of the
invention share 90 percent of sequence identity with a mammalian counterpart
e.g., human DARPP-32,
which has been sequenced. See Brene et al., J. Neuroscience, 14: 985-998
(1994). Consequently, the
nhDARPP-32 of the invention may be used to treat disorders or diseases based,
in part, on an aberant or
dysregulated intracellular signaling pathway regulated by DARPP-32. Thus,
sequences of the invention
will find use in in vitro or in vivo assays to identify modulators of a
dysfunctional dopaminergic signally
pathway associated disorder, particularly those that are regulated by DARPP-
32. In this respect, it is
understood that the proteins of the inventions for use in any in vivo and in
vitro assays proposed herein.,
will comprise the amino acid sequences of SEQ 1D NO: 2 or encoded by the
nucleotide sequence
comprising SEQ ID NO:1 or biologically equivalent fragments thereof. For
example, in vivo assays of
the invention propose transforming cells with the cDNA of SEQ ID NO: 1 such
that the cell expresses a
functional protein of SEQ ID NO: 2 or a biologically equivalent fragment
thereof.
As would be appreciated by those skilled in the relevant art, and as mentioned
above, the
dopaminergic signaling pathways generally involve checks and balances, e.g., a
cascade of
phosphorylations and dephosphorylations. Hence, one way to address the current
voids in anti-pychotics
is to identify therapeutic moieties that either mimic or facilitate the
effects of D1 receptor stimulation or
mimic or block the inhibitory effects of DARPP-32 on PP-1. For example.
therapeutic moieties that mimic
or block the inhibitory effects of DARPP-32 on PP-1 should be useful in
treating Parkinson's disease,
schizophrenia, drug addiction, and other neuropsychiatric disorders involving
aberant or abnormal
dopaminergic function mediated by DARPP-32. Such moieties would have their use
in the activation of
downstream components of the dopamine signaling cascade.
A broad aspect of the invention provides a method for identifying a compound
to be
tested for its ability to modulate the activity of a dopaminergic
intracellular signaling pathway in a cell
comprising: (a) determining a first level of dopamine activity in said cell;
(b) contacting said cell with a
test compound; and (c) determining a second level of dopamine activity,
respectively, in said cell,
wherein a difference in said first level and said second level of dopamine
activity is indicative of the
ability of said test compound to modulate the dopaminergic intracellular
signaling pathway. In preferred
embodiments, dopamine activity entails determining the level of
phosphorylation of Thr34-
phosphorylated DARPP-32 . The same assays can be used to determine the ability
of other kinases and
phosphatases to phosphorylate DARPP-32 (SEQ ID N0:2) at distinct sites well
known to a skilled
artisan. Such kinases and phosphatases include CdkS, PP-1, PP2C, PP2B and
PP2A.
-6-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
The proposed assay further comprises the additional step of determining
whether the
dopaminergic intracellular signaling pathway is modulated.
A representative embodiment proposes a method of identifying a therapeutic
moiety to be
tested for its ability to modulate the activity of a dopaminergic
intracellular signaling pathway regulated
by DARPP-32 in a cell comprising: (a) determining a first level of
phosphorylated Thr34-DARPP-32 in
said cell; (b) contacting said cell with the therapeutic moiety under
investigation, and (c) determining a
second level of phosphorylated Thr34-DARPP-32, respectively, in said cell,
wherein a difference in said
first level and said second level of phosphorylated Thr34-DARPP-32 is
indicative of the ability of said
test compound to modulate the dopaminergic intracellular signaling pathway.
The amount of
dephosphorylated DARPP-32 versus phosphorylated DARPP-32 can also be used as
the end point
according to the above assays. As well, the level of phosphorylated DARPP-32
at other residues such as
Thr75, Ser137 etc. can also be used. It is noted that DARPP-32 can also be
phosphorylated by casein 2
(CK2) at serine 102.
In another aspect, the present invention provides a method for identifying
therapeutic
moieties that can modulate the activity of a dopaminergic intracellular
signaling pathway via modulation
of PKA, CK1, CK2, CdkS, protein phosphatase 1 ("PP-1), protein phosphatase-2C
("PP2C"), protein
phosphatase-2B ("PP2B") or protein phosphatase-2A ("PP2A") activity.
An illustrative method for modulating one of the above comprises contacting
the a
transformed cell with an effective amount of a compound that alters the
activity of a dopaminergic
receptor intracellular signaling molecule, wherein contact of the cell with
the compound results in a
modulation of the activity of PKA, CK1, CK2, CdkS, PP-1, PP2C, PP2B and/or
PP2A, whose modulation
may be quantified via the determination of the rate of phosphorylation or
dephosphorylation of DARPP-
32 of SEQ ID N0:2 at distinct residues known to one skilled in the art.
A representative method contemplates detecting the increase (or decrease) in
the amount
of phosphorylation (or dephosphorylation) of Thr34-phosphorylated DARPP-32,
Ser137-phosphorylated
DARPP-32, or Thr75-phosphorylated DARPP-32. Preferably, the DARPP-32
polypeptide comprises the
amino acid sequence of SEQ >D N0:2 or a functionally effective fragment
thereof. Detecting an increase
or decrease in the phosphorylation of other residues mediated by the
modulation of any one of PKA,
CK1, CK2, CdkS, AMPA receptor, PP-1, PP2C, PP2B and/or PP2A are well known to
one skilled in the
art.
The invention also provides methods of screening potential therapeutic
moieties (or drugs
or compounds) that are capable of potentially ameliorating and/or being used
in the treatment of a
dysfunctional dopaminergic signaling pathway preferably mediated by DARPP-32.
Methods for identifying therapeutic moieties (or drugs or compounds), e.g.,
drug
screening assays, to identify those moieties that may be used in therapeutic
methods for the treatment of a
_7_
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
dysfunctional dopaminergic intracellular signaling pathway preferably mediated
or regulated by DARPP-
32 are also provided.
Methods of treating a disorder or disease due in part by the aberration or
dysregulation of
an intracellular pathway regulated or mediated by DARPP-32 are also provided.
The proposed method
proposes administering to a patient in need thereof a therapeutic moiety that
alters the phosphorylation of
phosphorylated DARPP-32, wherein the therapeutic moiety modulates the activity
of PKA, CKI, CK2,
CdkS, AMPA receptor, PP-1, PP2C, PP2B and/or PP2A.
An alternative embodiment provides a method for regulating phosphorylation-
dependent
activation of one or more dopamine receptors, such as the D1 receptors in a
cell. The method proposes
administering an effective amount of a compound that modulates activity of
PKA, CK1, CK2, CdkS, PP-
1, PP2C, PP2B and/or PP2A, wherein modulation of the activity of one of PKA,
CK1, CK2, CdkS, PP-1,
PP2C, PP2B and/or PP2A results in an alteration in the phosphorylation-
dependent activation of the D1
receptors in the cell, e.g., DARPP-32.
A representative embodiment provides a method for treating a disorder
characterized by
dysfunctional dopaminergic intracellular signaling pathway mediated by DARPP-
32 in a patient in need
thereof comprising administering to the patient therapeutic moiety that
inhibits the dephosphorylation of
Thr34-phosphorylated DARPP-32, wherein the therapeutic moiety decreases PP2B
activity.
In accordance with the above, the invention provides a method for identifying
a
therapeutic moiety for use in the treatment of dopamine mediated disorder
regulated by DARPP-32 in a
patient in need of such treatment comprising: (a) contacting the potential
therapeutic moiety with PP2B
and Thr34-phosphorylated DARPP-32; and (b) detecting the amount of
dephosphorylation of Thr34-
phosphorylated DARPP-32; wherein a decrease in the dephosphorylation of Thr34-
phosphorylated
DARPP-32 in the presence of the therapeutic moiety is indicative that the
therapeutic moiety has
therapeutic utility in the treatment of a dopamine mediated disorder.
In yet another embodiment, the invention provides for a method treating a
dysfunctional
dopaminergic signaling pathway related disorder in a patient in need thereof
comprising administering to
the patient a therapeutic moiety that decreases the dephosphorylation of Thr34-
phosphorylated DARPP-
32, wherein the therapeutic moiety decreases PP-1 activity.
In accordance with the above, the invention provides a method for identifying
therapeutic
moiety for use in the treatment a patient presenting a disease or disorder
characterized by dysfunctional
dopaminergic intracellular signaling pathway regulated b by DARPP-32
comprising (a) contacting a
potential therapeutic moiety with PP-1 and Thr34-phosphorylated DARPP-32 of
SEQ >I7 N0:2; and (b)
detecting the amount of dephosphorylation of Thr34-phosphorylated DARPP-32;
wherein the therapeutic
moiety is identified if a decrease in the dephosphorylation of Thr34-
phosphorylated DARPP-32 is
detected in the presence of the potential therapeutic moiety.
_g_
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
A similar embodiment provides administering to the patient a therapeutic
moiety that
increases the dephosphorylation of Thr75-phosphorylated DARPP-32, wherein the
therapeutic moiety
increases PP2A activity. The therapeutic moiety is identified using the herein
disclosed DARPP-32
polypeptide.
In accordance with the above, the invention provides a method for identifying
therapeutic
moiety for use in the treatment a patient presenting a disease or disorder
characterized by dysfunctional
dopaminergic intracellular signaling pathway regulated b by DARPP-32
comprising (a) contacting the
potential therapeutic moiety with PP2A and Thr75-phosphorylated DARPP-32; and
(b) detecting the
amount of dephosphorylation of Thr75-phosphorylated DARPP-32; wherein an
increase in the
dephosphorylation of Thr75-phosphorylated DARPP-32 in the presence of the
potential therapeutic
moiety is indicative that the therapeutic moiety has therapeutic utility in
the treatment of the disorder .
In another embodiment, the invention provides a method for identifying
therapeutic
moiety for use in the treatment of a psychotic disorder mediated by DARPP-32
in a patient in need of
such treatment comprising: (a) contacting a potential therapeutic moiety with
CdkS and Thr75-
dephosphorylated DARPP-32; and (b) detecting the amount of phosphorylation of
Thr75-
dephosphorylated DARPP-32; wherein the therapeutic moiety is identified if a
decrease in the
phosphorylation of Thr75-dephosphorylated DARPP-32 is detected in the presence
of the potential
therapeutic moiety.
Likewise, an embodiment provides a method for identifying therapeutic moiety
to be
tested for an ability to treat a patient presenting symptoms consistent with a
disease or disorder
characterized by dysfunctional dopaminergic intracellular signaling pathway
regulated b by DARPP-32
comprising: (a) contacting a potential therapeutic moiety with dopamine and
The34-dephosphorylated
DARPP-32 of SEQ m NO: 2; and (b) detecting the amount of phosphorylation of
Thr34-
dephosphorylated DARPP-32; wherein an increase in the phosphorylation of Thr34-
dephosphorylated
DARPP-32 in the presence of the potential therapeutic moiety indicates that
the test therapeutic moiety is
capable of treating a dopamine mediated disorder.
In another aspect, the invention provides a method for identifying a potential
therapeutic
moiety to be tested for an ability to treat a psychotic disorder in a patient
in need of such treatment
comprising the steps of:
(a) contacting, in a transformed cell or one expressing the DARPP-32 of the
invention the potential therapeutic moiety with a Thr-75 dephosphorylated
DARPP-32 and detecting the
amount of phosphorylation of Thr-75 dephosphorylated DARPP-32, or
(b) contacting, in a transformed cell or one expressing the DARPP-32 of the
invention the potential therapeutic moiety with Thr-75 phosphorylated DARPP-32
and detecting the
amount of dephosphorylation of Thr-75 phosphorylated DARPP-32, wherein the
therapeutic moiety is
-9-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
identified as a potential atypical anti-psychotic compound if: (i) an increase
in the level of
phosphorylation of Thr-75 dephosphorylated DARPP-32 is detected in step (a),
ii) a decrease in the level
of dephosphorylation of Thr-75 phosphorylated DARPP-32 is detected in step
(b), respectively , relative
to a control level, in the presence of the potential test therapeutic moiety.
In another embodiment, the invention provides a method for treating a
dysfunctional
dopaminergic signaling pathway related disorder in a patient in need thereof
comprising administering to
the patient therapeutic moiety that decreases the dephosphorylation of Serl37-
phosphorylated DARPP-
32, wherein the therapeutic moiety decreases PP2C activity.
Accordingly, the method for identifying the therapeutic moiety for use in the
treatment of
dysfunctional dopaminergic signaling pathway related disorder in a patient in
need of such treatment
comprises: (a) contacting a potential therapeutic moiety with PP2C and Ser137-
phosphorylated DARPP-
32; and (b) detecting the amount of dephosphorylation of Ser137-phosphorylated
DARPP-32; wherein the
therapeutic moiety is identified if a decrease in the dephosphorylation of
Ser137-phosphorylated DARPP-
32 is detected in the presence of the potential therapeutic moiety.
Another embodiment provides a method for identifying a therapeutic moiety to
be tested
for an ability to treat a dysfunctional dopaminergic signaling pathway
mediated disorder in a patient in
need of such treatment comprising: (a) contacting a potential therapeutic
moiety with a Dl receptor
agonist, such as dopamine and Thr34-dephosphorylated DARPP-32; and (b)
detecting the amount of
phosphorylation of Thr34-dephosphorylated DARPP-32; wherein a decrease in the
phosphorylation of
Thr34-dephosphorylated DARPP-32 in the presence of the potential therapeutic
moiety indicated the
therapeutic potential of said moiety in its ability to treat said disorder.
The same assay may be performed using CdkS instead of dopamine and monitoring
a
decrease in phosphorylation of Thr75 dephosphorylated DARP-32 of SEQ )D N0:2.
Compounds identified herein for modulating the activity of PKA, CK1, CK2,
CdkS, PP-
1, PP2C, PP2B and/or PP2A are also encompassed by the invention as are
pharmaceutical compositions
of the therapeutic moieties (or drugs or compounds) for use in treating
disease or disorders due in part to
an aberration or dysregulation of an intracellular signaling pathway regulated
by DARPP-32.
The invention also encompasses pharmaceutical compositions for treating
disorders of
brain function mediated by DARPP-32.
brief description of the drawings
Figure 1 depicts the results of a sandwich ELISA (sELISA) for rat DARPP-32.
Figure 2 represents the nucleotide sequence (SEQ ID NO:1) encoding a DARPP-32
polypeptide derived from a guinea pig.
- 10-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Figure 3 depicts the deduced amino acid sequence (SEQ )D N0:2) of the DARPP-32
disclosed herein.
Figure 4 depicts the amino acid sequence alignment between DARPP-32 (SEQ >D
N0:2)
and the corresponding protein derived from a human, cow, rat and mouse.
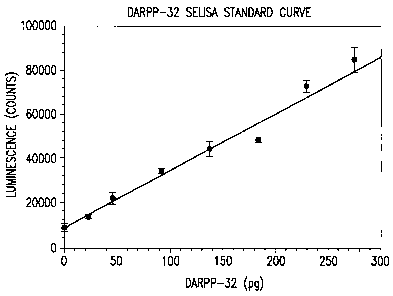
Figure 5 shows the standard curve for determination of pT34-DARPP-32 by
sELISA.
detailed description of the invention
Before the present proteins, nucleotide sequences, and methods are described,
it is to be
understood that the present invention is not limited to the particular
methodologies, protocols, cell lines,
vectors, and reagents described, as these may vary. It is also understood that
the terminology used herein
is for, the purpose of describing particular embodiments only, and is not to
limit the scope of the present
invention.
The singular forms "a," "an," and "the" include plural reference unless the
context clearly
dictates otherwise.
All technical and scientific terms used herein have the same meanings as
commonly
understood by one of ordinary skill in the art to which this invention
pertains. The practice of the present
invention will employ, unless otherwise indicated, conventional techniques of
protein chemistry and
biochemistry, molecular biology, microbiology and recombinant DNA technology,
which are within the
skill of the art. Such techniques are explained fully in the literature.
Although any machines, materials, and methods similar or equivalent to those
described
herein can be used to practice or test the present invention, the preferred
machines, materials, and
methods are now described. All patents, patent applications, and publications
mentioned herein, whether
supra or infra, are each incorporated by reference in its entirety.
Definitions
"Nucleic acid sequence" as used herein refers to an oligonucleotide,
nucleotide, or
polynucleotide, and fragments or portions thereof, and to DNA or RNA of
genomic or synthetic origin
which may be single-or double-stranded, and represent the sense or antisense
strand. Similarly, "amino
acid sequence" as used herein refers to an oligopeptide, peptide, polypeptide,
or protein sequence and
fragments or portions thereof, of a naturally occurring or synthetic molecule.
Where "amino acid sequence" is recited herein to refer to an amino acid
sequence of a
naturally occurring protein molecule, "amino acid sequence" and like terms,
such as "polypeptide" or
"protein" are not meant to limit the amino acid sequence to the complete,
native amino acid sequence
associated with the recited protein molecule.
-11-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
As used herein, "DARPP-32" or "nhDARPP-32" or "DARPP -32 of the invention" or
"invention protein" or grammatical equivalents thereof are used
interchangeably to refer to "Dopamine-
and cyclic AMP (cAMP)-regulated phosphoprotein having a molecular weight of 32
kilodaltons". The
term refer to the amino acid sequences of substantially purified DARPP-32
obtained from any species and
from any source whether natural, synthetic, semi-synthetic, or recombinant.
Preferably the DARPP-3
comprises the amino acid sequence as depicted in SEQ ID NO: 2 and derived from
a guinea pig.
As used herein, the term "Thr34 DARPP-32" is used interchangeably with "Thr34
DARPP32," "thr34 DARPP-32" 'Threonine-34 DARPP-32" and "threonine-34 DARPP-32"
along with
analogous abbreviations and denotes the thirty-fourth amino acid residue of
the amino sequence of
DARPP-32 in SEQ ID NO: 2 or in the human counterpart as disclosed by Brene et
al. (J. Neurosci.
14:985-998(1994)) having the GenBank Accession No. of AAB30129.1, which is a
threonine residue
that can be phosphorylated by the cyclic AMP dependent protein kinase (PKA).
Likewise, the term "phospho-Thr34 DARPP-32," or analogous abbreviations as
disclosed
above, denotes the phosphorylated form of Thr34 DARPP-32.
As used herein, the term "Thr75 DARPP-32" is used interchangeably with "Thr75
DARPP32," "thr75 DARPP-32", "Threonine-75 DARPP-32" and "threonine-75 DARPP-
32" along with
analogous abbreviations, and denotes the seventy-fifth amino acid residue in
the amino sequence of
DARPP-32 as shown in SEQ >D NO: 2. or in the human counterpart as disclosed in
Brene et al. supra,
having the GenBank Accession of AAB30129.1, which is a threonine residue that
can be phosphorylated
by CdkS.
As used herein, the term "phospho-Thr75 DARPP-32," or analogous abbreviations
as
disclosed above, denotes the phosphorylated form of Thr75 DARPP-32.
As used herein, the term "Serl37 DARPP-32" is used interchangeably with
"Ser137
DARPP32," "ser137 DARPP-32", "Serine-137 DARPP-32" and "serine-137 DARPP-32"
along with
analogous abbreviations denotes the one-hundred and thirty-seventh amino acid
residue of the amino
sequence of DARPP-32 - SEQ ID NO: 2 or as disclosed by Brene et al. (J.
Neurosci. 14:985-998 ( 1994))
having the GenBank Accession No. of AAB30129.1, which is a serine residue that
can be phosphorylated
by CK 1.
Likewise, the term "phospho-Ser137 DARPP-32" or analogous abbreviations as
disclosed above, denotes the phosphorylated form of Serl37 DARPP-32.
As used herein, the terms "CK1," "casein kinase I" or "CKI," are used
interchangeably
with or "casein kinase 1." CK1 is a member of the serine/threonine protein
kinases. CK1 includes, but is
not limited to members of the CKl (CKI) family of multiple isoforms. See
Desdouits, F. et al. 1995. J.
Biol. Chem. 270:8772-8778; Gross et al., 1998, Cell Signal 10(10): 699-711;
Vielhaber et al., 2001,
ItTBMB Life 51(2), 73-8).
-12-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Fragment of DARPP-32 can be between about 5 and 100 residues, or more
preferably
between about 10 and 50 residues. Methods of synthesizing fragments are well
known to a skilled
artisan.
A "CK1 phosphorylatable fragment of DARPP-32" refers to a protein fragment of
DARPP-32 of SEQ >D NO: 2 that contains a serine residue that, when in the
dephosphorylated form, can
be phosphorylated by CK1. For the nhDARPP-32 having the amino acid sequence as
ser forth in SEQ 1D
NO: 2 the serine residue is preferably Ser137 DARPP-32. Such fragments can be
between about 5 and
100 residues, or more preferably between about 10 and 50 residues. Methods of
synthesizing fragments
are well known to a skilled artisan. A CK1 phosphorylatable fragment of DARPP-
32 can be prepared by
any method commonly known in the art, e.g., cleaving (such as with a protease)
and dephosphorylating
the phosphorylated fragment from a larger fragment of phospho-Ser137 DARPP-32
protein or from the
full-length phospho-Serl37 DARPP-32 protein.
As used herein, the term "Ser102-DARPP-32" is used interchangeably with
"Ser102
DARPP32," "ser102 DARPP-32", "Serine-1302 DARPP-32" and "serine-102 DARPP-32"
along with
analogous abbreviations denotes the one-hundred and second amino acid residue
of the amino sequence
of DARPP-32 - SEQ ID NO: 2 or as disclosed by Brene et al. (J. Neurosci.
14:985-998 ( 1994)) having
the GenBank Accession No. of AAB30129.1, which is a serine residue that can be
phosphorylated by
CK2.
Likewise, the term "phospho-Ser102 DARPP-32" or analogous abbreviations as
disclosed above, denotes the phosphorylated form of Ser102 DARPP-32.
As used herein, the terms "CK2," "casein kinase 2" or "CKI," are used
interchangeably
with or "casein kinase 2." CK2 is a member of the serine/threonine protein
kinases.
Fragment of DARPP-32 can be between about 5 and 100 residues, or more
preferably
between about 10 and 50 residues. Methods of synthesizing fragments are well
known to a skilled
artisan.
A "CK2 phosphorylatable fragment of DARPP-32" refers to a protein fragment of
DARPP-32 of SEQ ID NO: 2 that contains a serine residue that, when in the
dephosphorylated form, can
be phosphorylated by CK2.
The terms "CDKS", "CdkS" or "cdk5" are used interchangeably with "cyclin-
dependent
kinase 5," which is also known as neuronal cyclin-dependent-like protein
(Nclk) and tau protein kinase II
(TPKII). CdkS is a member of the cyclin dependent kinases but atypically CdkS
employs a non-cyclin
cofactor called neuronal cyclin-dependent-like kinase 5 associated protein
(NckSa) rather than a cyclin. It
is a protein kinase that phosphorylates DARPP-32 on Threonine-75 but not on
Threonine-34.
Likewise, "CdkS phosphorylatable fragment of DARPP-32" refers to a protein
fragment
of DARPP-32 that contains a threonine residue that when in the
dephosphorylated form can be
-13-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
phosphorylated by CdkS. For the non-human DARPP-32 having the amino acid
sequence of SEQ ID
N0:2, the threonine residue is preferably Thr75 DARPP-32. Fragments can be
between about 5 and 100
residues, or more preferably between about 10 and 50 residues. For example, in
a particular embodiment,
the peptide fragment comprises 5 consecutive amino acids from SEQ )D NO: 2
including Thr75.
The term "PP2C dephosphorylatable fragment of DARPP-32" refers to a protein
fragment of DARPP-32 that contains a serine residue that when in the
phosphorylated form can be
dephosphorylated by PP2C. For the non-human DARPP-32 having the amino acid
sequence of SEQ )D
N0:2, the serine residue is preferably Ser137 DARPP-32. Fragments can be
between about 5 and 100
residues, or more preferably between about 10 and 50 residues. All of the
peptide fragments of DARPP-
32 can be prepared by any method commonly known in the art, e.g., cleaving
(such as with a protease)
and by phosphorylating the dephosphorylated fragment or by cleaving (such as
with a protease) the
phosphorylated fragment from a larger fragment of phospho-Ser137 DARPP-32
protein or from the full-
length phospho-Ser137 DARPP-32 protein.
As used herein, the term "PP2B dephosphorylatable fragment of DARPP-32" is a
protein
fragment of DARPP-32 that contains a threonine residue that when in the
phosphorylated form can be
dephosphorylated by PP2B. For the DARPP-32 of SEQ ID NO: 2 , the threonine
residue is preferably
Thr34 DARPP-32. Preferred fragments can be between about 5 and 100 residues,
or more preferably
between about 10 and 50 residues and may be prepared as any of the above
fragments.
Likewise, the term "PP2A dephosphorylatable fragment of DARPP-32" is a protein
fragment of DARPP-32 that contains a threonine residue that when in the
phosphorylated form can be
dephosphorylated by PP2A. For the DARPP-32 of SEQ 1D N0:2, the threonine
residue is preferably
Thr75 DARPP-32.
The amount and/or rate of phosphorylation of DARPP-32 or of a phosphorylatable
fragment of DARPP-32, as described hereinabove, as a kinase reaction is
"significantly changed" when
the amount and/or rate of phosphorylation of DARPP-32 or the phosphorylatable
fragment of DARPP-32
is increased or decreased by at least about 10-25%, relative to the control
reaction. Preferably, a
significant change in rate of the phosphorylation of DARPP-32 by a molecule of
interest (e.g., dopamine)
observed in the presence of a potential modulator is at some point correlated
with the Michaelis constants
(e.g., the Vmax or K~ of the reaction. For example, in the case of an
inhibitor, a Ki can be determined.
Thus, in certain embodiments, it may be preferable to study various
concentrations of a modulator in a
reaction mixture to allow the identification of the potential modulator as a
modulator.
As used herein, the amount and/or rate of dephosphorylation of DARPP-32 or of
a
dephosphorylatable fragment of DARPP-32, as described hereinabove, in a
phosphatase reaction is
"significantly changed" when the amount and/or rate of dephosphorylation of
DARPP-32 or the
dephosphorylatable fragment of DARPP-32 is increased or decreased by at least
about 10-25%, relative to
-14-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
the control reaction. Preferably, a significant change in rate of the
dephosphorylation of DARPP-32 by a
molecule of interest (e.g., PP2C, PP2B or PP2A) observed in the presence of a
potential modulator is at
some point correlated with the Michaelis constants (e.g., the Vmax or K~ of
the reaction. For example,
in the case of an inhibitor, a Ki can be determined. Thus, in certain
embodiments, it may be preferable to
study various concentrations of a modulator in a reaction mixture to allow the
identification of the
potential modulator as a modulator.
As used herein, the term "dopaminergic signaling pathway mediated disorder" is
used
interchangeably with the terms "dysfunctional dopamine regulated disorder"
refers collectively to a
disorder characterized by a "dysfunctional" or "dysregulation" of a
intracellular signaling pathway,
preferably a dopaminergic signaling pathway that is mediated by DARPP-32. Such
disorders include, but
are not limited to, depression, manic-depressive disorder, obsessive-
compulsive disorder, eating disorder,
post-traumatic stress syndrome, Parkinson's disease, schizophrenia, or a
neurodegenerative disorder.
Such a disorder also includes, but is not be limited to, a disease (e.g.,
depression) or a condition (e.g.,
addiction to cocaine) that involves an aberration or dysregulation of a signal
transmission pathway,
including, but not limited to, neurotransmission mediated by dopaminergic
receptors in excitable cells,
tissues or organs (e.g., neurons, brain, central nervous system, etc.). A
dysregulated serotonergic pathway
is also included within the meaning of this disorder. Preferably, the pathway
affected includes the
phosphorylation and/or dephosphorylation of DARPP-32, with the corresponding
treatment of the
dysregulation involving the stimulation and/or inhibition of the
phosphorylation and/or dephosphorylation
of one or more specific threonine and/or serine residues of DARPP-32 (see,
e.g., Greengard et al., Neuron
23:435-447 (1999); Bibb et al., Proc. Natl. Acad. Sci. 97:6809-68 14 (2000).
A "variant" of nhDARPP-32, as used herein, refers to an amino acid sequence
that is
altered by one or more amino acids. The variant may have "conservative"
changes, wherein a substituted
amino acid has similar structural or chemical properties, e.g., replacement of
leucine with isoleucine.
More rarely, a variant may have "nonconservative" changes, e.g., replacement
of a glycine with a
tryptophan. Similar minor variations may also include amino acid deletions or
insertions, or both.
Guidance in determining which amino acid residues may be substituted,
inserted, or deleted without
abolishing biological or immunological activity may be found using computer
programs well known in
the art, for example, DNASTAR software.
"Alterations" in the polynucleotide of SEQ )D NO:l, as used herein, comprise
any
alteration in the sequence of polynucleotides encoding the protein of the
invention including deletions,
insertions, and point mutations that may be detected using hybridization
assays. Included within this
definition is the detection of alterations to the genomic DNA sequence which
encodes nhDARPP-32 (e.g.,
by alterations in the pattern of restriction fragment length polymorphisms
capable of hybridizing to SEQ
>D NO:1), the inability of a selected fragment of SEQ 1D NO:1 to hybridize to
a sample of genomic DNA
-15-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
(e.g., using allele-specific oligonucleotide probes), and improper or
unexpected hybridization, such as
hybridization to a locus other than the normal chromosomal locus for the gene
encoding DARPP-32 (e.g.,
using fluorescent in situ hybridization "FISH" to metaphase chromosomes
spreads).
A "deletion", as used herein, refers to a change in either amino acid or
nucleotide
sequence in which one or more amino acid or nucleotide residues; respectively,
are absent.
An "insertion" or "addition", as used herein, refers to a change in an amino
acid or
nucleotide sequence resulting in the addition of one or more amino acid or
nucleotide residues,
respectively, as compared to the naturally occurnng molecule.
A "substitution", as used herein, refers to the replacement of one or more
amino acids or
nucleotides by different amino acids or nucleotides, respectively.
The term "biologically active" as used herein, refers to a protein having
structural,
regulatory, or biochemical functions of a naturally occurring molecule.
Likewise, "immunologically
active" refers to the capability of the natural, recombinant, or synthetic
nhDARPP-32, or any oligopeptide
thereof, to induce a specific immune response in appropriate animals or cells
and to bind with specific
antibodies.
As used herein, the term "modulate" or "modulation" shall have its usual
meaning, and
encompasses the meanings of the words "enhance," "inhibit," and "mimic."
"Modulation" of activity may
be either an increase or a decrease in protein activity, a change in the
degree or amount of
phosphorylation, change in binding characteristics, or any other change in the
biological, functional, or
immunological properties of DARPP-32.
As used herein, an "agonist" is any compound that acts directly or indirectly
through or
upon a receptor to produce a pharmacological effect. The terms "antagonist" or
"inhibitor" is any moiety
or compound that blocks the stimulation of a target molecule, e.g., DARPP-32
and its resulting
pharmacological effect.
As used herein, an "effective amount" of a modulatory compound is an amount
that can
be determined by one of skill in the art based on data from studies using
methods of analysis such as
those disclosed herein. Such data may include, but not be limited to, results
from IC50 determinations etc.
The term "derivative",~as used herein, refers to the chemical modification of
a nucleic
acid encoding nhDARPP-32 or the encoded nhDARPP-32. Illustrative of such
modifications would be
replacement of hydrogen by an alkyl, acyl, or amino group. A nucleic acid
derivative would encode a
polypeptide which retains essential biological characteristics of the natural
molecule.
The term "substantially purified", as used herein, refers to nucleic or amino
acid
sequences that are removed from their natural environment, isolated or
separated, and are at least 60%
free, preferably 75% free, and most preferably 90% free from other components
with which they are
naturally associated.
-16-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
The term "hybridization", as used herein, refers to any process by which a
strand of
nucleic acid binds with a complementary strand through base pairing.
The term "hybridization complex", as used herein, refers to a complex formed
between
two nucleic acid sequences by virtue of the formation of hydrogen bonds
between complementary G and
C bases and between complementary A and T bases; these hydrogen bonds may be
further stabilized by
base stacking interactions. The two complementary nucleic acid sequences
hydrogen bond in an
antiparallel configuration. A hybridization complex may be formed in solution
(e.g., CO t or RO t analysis)
or between one nucleic acid sequence present in solution and another nucleic
acid sequence immobilized
on a solid support (e.g., membranes, filters, chips, pins or glass slides to
which cells have been fixed for
in situ hybridization).
The terms "complementary" or "complementarity", as used herein, refer to the
natural
binding of polynucleotides under permissive salt and temperature conditions by
base-pairing. For
example, for the sequence "A-G-T" binds to the complementary sequence "T-C-A".
Complementarity
between two single-stranded molecules may be "partial", in which only some of
the nucleic acids bind, or
it may be complete when total complementarity exists between the single
stranded molecules. The degree
of complementarity between nucleic acid strands has significant effects on the
efficiency and strength of
hybridization between nucleic acid strands. This is of particular importance
in amplification reactions,
which depend upon binding between the nucleic acids strands.
The term "homology", as used herein, refers to a degree of complementarity.
There may
be partial homology or complete homology (i.e., identity). A partially
complementary sequence is one
that at least partially inhibits an identical sequence from hybridizing to a
target nucleic acid; it is referred
to using the functional term "substantially homologous." The inhibition of
hybridization of the completely
complementary sequence to the target sequence may be examined using a
hybridization assay (Southern
or northern blot, solution hybridization and the like) under conditions of low
stringency. A substantially
homologous sequence or probe will compete for and inhibit the binding (i.e.,
the hybridization) of a
completely homologous sequence or probe to the target sequence under
conditions of low stringency.
This is not to say that conditions of low stringency are such that non-
specific binding is permitted; low
stringency conditions require that the binding of two sequences to one another
be a specific (i.e.,
selective) interaction. The absence of non-specific binding may be tested by
the use of a second target
sequence which lacks even a partial degree of complementarity (e.g., less than
about 30% identity); in the
absence of non-specific binding the probe will not hybridize to the second non-
complementary target
sequence.
As known in the art, numerous equivalent conditions may be employed to
comprise
either low or high stringency conditions. Factors such as the length and
nature (DNA, RNA, base
composition) of the sequence, nature of the target (DNA, RNA, base
composition, presence in solution or
-17-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
immobilization, etc.), and the concentration of the salts and other components
(e.g., the presence or
absence of formamide, dextran sulfate and/or polyethylene glycol) are
considered and the hybridization
solution may be varied to generate conditions of either low or high stringency
different from, but
equivalent to, the above listed conditions.
The term "stringent conditions", as used herein, is the "stringency" which
occurs within a
range from about Tm-S° C. (S° C. below the melting temperature
(Tm) of the probe) to
about 20° C. to 25° C. below Tm. As will be understood by those
of skill in the art, the
stringency of hybridization may be altered in order to identify or detect
identical or related polynucleotide
sequences.
The term "antisense", as used herein, refers to nucleotide sequences which are
complementary to a specific DNA or RNA sequence. The term "antisense strand"
is used in reference to a
nucleic acid strand that is complementary to the "sense" strand. Antisense
molecules may be produced by
any method, including synthesis by ligating the genes) of interest in a
reverse orientation to a viral
promoter which permits the synthesis of a complementary strand. Once
introduced into a cell, this
transcribed strand combines with natural mRNA produced by the cell to form
duplexes. These duplexes
then block either the further transcription of the mRNA or its translation. In
this manner, mutant
phenotypes may be generated. The term "antisense strand" is used in reference
to a nucleic acid strand
that is complementary to the "sense" strand. The designation "negative" is
sometimes used in reference to
the antisense strand, and "positive" is sometimes used in reference to the
sense strand.
The term "portion", as used herein, with regard to a protein (as in "a portion
of a given
protein") refers to fragments of that protein. The fragments may range in size
from four amino acid
residues to the entire amino acid sequence minus one amino acid. Thus, a
protein "comprising at least a
portion of the amino acid sequence of SEQ ID NO: 2" encompasses the full-
length nhDARPP-32 and
fragments thereof.
"Transformation", as defined herein, describes a process by which exogenous
DNA
enters and changes a recipient cell. It may occur under natural or artificial
conditions using various
method well known in the art. Transformation may rely on any known method for
the insertion of foreign
nucleic acid sequences into a prokaryotic or eukaryotic host cell. The method
is selected based on the host
cell being transformed and may include, but is not limited to, viral
infection, electroporation, lipofection,
and particle bombardment. Such "transformed" cells include stably transformed
cells in which the
inserted DNA is capable of replication either as an autonomously replicating
plasmid or as part of the host
cell chromosome. They also include cells which transiently express the
inserted DNA or RNA for limited
periods of time.
The term "sample", as used herein, is used in its broadest sense. A biological
sample
suspected of containing nucleic acid encoding nhDARPP-32 or fragments thereof
may comprise a cell,
-18-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
chromosomes isolated from a cell (e.g., a spread of metaphase chromosomes),
genomic DNA (in solution
or bound to a solid support such as for Southern blot analysis), RNA (in
solution or bound to a solid
support such as for northern blot analysis), cDNA (in solution or bound to a
solid support), extract from
cells or a tissue, and the like.
As used herein, the term "antibody" refers to intact molecules as well as
fragments
thereof, such as Fa, F(ab')<sub>2</sub>, and Fv, which are capable of binding the
epitopic determinant.
Antibodies that bind nhDARPP-32 polypeptides can be prepared using intact
polypeptides or fragments
containing small peptides of interest as the immunizing antigen. The
polypeptide or peptide used to
immunize an animal can be derived from translated cDNA or synthesized
chemically, and can be
conjugated to a carrier protein, if desired. Commonly used carriers that are
chemically coupled to peptides
include bovine serum albumin and thyroglobulin. The coupled peptide is then
used to immunize the
animal (e.g., a mouse, a rat, or a rabbit).
The term "humanized antibody", as used herein, refers to antibody molecules in
which
amino acids have been replaced in the non-antigen binding regions in order to
more closely resemble a
human antibody, while still retaining the original binding ability.
The term "antigenic determinant" as used herein, refers to that portion of a
molecule that
makes contact with a particular antibody (i.e., an epitope). When a protein or
fragment of a protein is used
to immunize a host animal, numerous regions of the protein may induce the
production of antibodies
which bind specifically to a given region or three-dimensional structure on
the protein; these regions or
structures are referred to as antigenic determinants. An antigenic determinant
may compete with the intact
antigen (i.e., the immunogen used to elicit the immune response) for binding
to an antibody.
As used herein, the term "about" means within 10 to 15%, preferably within 5
to 10%.
For example an amino acid sequence that contains about 60 amino acid residues
can contain between 51
to 69 amino acid residues, more preferably 57 to 63 amino acid residues.
The nhDARPP-32 Coding Sequences
The nucleic and deduced amino acid sequences of nhDARPP-32 are shown in SEQ ID
NOS:1 and 2 respectively. In accordance with the invention, any nucleotide
sequence which encodes the
amino acid sequence of nhDARPP-32 can be used to generate recombinant
molecules which express
nhDARPP-32.
Methods for DNA sequencing are well known to a skilled artisan and may employ
such
enzymes as the Klenow fragment of DNA polymerase I Sequenase® (US
Biochemical Corp,
Cleveland Ohio)), Taq polymerase (Perkin Elmer, Norwalk Conn.), thermostable
T7 polymerase
(Amersham, Chicago Ill.), or combinations of recombinant polymerases and
proofreading exonucleases
such as the ELONGASE Amplification System marketed by Gibco BRL (Gaithersburg
Md.). As well,
-19-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
methods to extend the DNA from an oligonucleotide primer annealed to the DNA
template of interest
have been developed for both single-stranded and double-stranded templates.
Chain termination reaction
products were separated using electrophoresis and detected via their
incorporated, labelled precursors.
Recent improvements in mechanized reaction preparation, sequencing and
analysis have permitted
expansion in the number of sequences that can be determined per day.
Preferably, the process is
automated with machines such as the Hamilton Micro Lab 2200 (Hamilton, Reno
Nev.), Peltier Thermal
Cycler (PTC200; MJ Research, Watertown Mass.) and the ABI Catalyst 800 and 377
and 373 DNA
sequencers (Perkin Elmer).
The quality of any particular cDNA library may be determined by performing a
pilot
scale analysis of the cDNAs and checking for percentages of clones containing
vector, lambda or E. coli
DNA, mitochondrial or repetitive DNA, and clones with exact or homologous
matches to public
databases.
Extending the Polynucleotide Sequence:
The polynucleotide sequence - SEQ ID NO:1 or biologically equivalent sequences
thereof may be extended utilizing partial nucleotide sequence and various
methods known in the art to
detect upstream sequences such as promoters and regulatory elements. Gobinda
et al (1993; PCR
Methods Applic 2:318-22) disclose "restriction-site polymerase chain reaction
(PCR)" as a direct method
which uses universal primers to retrieve unknown sequence adjacent to a known
locus. According to the
process, initially, a genomic DNA is amplified in the presence of primer to a
linker sequence and a primer
specific to the known region. Thereafter, the amplified sequences are
subjected to a second round of PCR
with the same linker primer and another specific primer internal to the first
one. Products of each round
of PCR are transcribed with an appropriate RNA polymerase and sequenced using
reverse transcriptase.
Inverse PCR may also be used to amplify or extend the target sequences using
divergent
primers based on a known region (Triglia T. et al( 1988) Nucleic Acids Res
16:8186). The primers may
be designed using Oligo 4.0 (National Biosciences Inc, Plymouth Minn.), or
another appropriate program,
to be 22-30 nucleotides in length, to have a GC content of 50% or more, and to
anneal to the target
sequence at temperatures about 68°-72°C. The method proposes
using several restriction enzymes to
generate a suitable fragment in the known region of a gene. The fragment is
thereafter circularized by
intramolecular ligation and used as a PCR template.
Capture PCR (Lagerstrom M. et al ( 1991) PCR Methods Applic 1:111-19) is drawn
to a
method for PCR amplification of DNA fragments adjacent to a known sequence in
human and yeast
artificial chromosome (YAC) DNA. Capture PCR also requires multiple
restriction enzyme digestions
and ligations to place an engineered double-stranded sequence into an unknown
portion of the DNA
molecule before PCR.
-20-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Likewise, Parker J. D. et al (1991; Nucleic Acids Res 19:3055-60), teach
walking PCR, a
method for targeted gene walking which permits retrieval of unknown sequence.
PromoterFinderTM a new
kit available from Clontech (Palo.Alto Calif.) uses PCR, nested primers and
PromoterFinder libraries to
walk in genomic DNA. This process avoids the need to screen libraries and is
useful in finding
intron/exon junctions.
Another PCR method, "Improved Method for Obtaining Full Length cDNA Sequences"
by Guegler et al, patent application Ser. No. 08/487,112, filed Jun. 7, 1995
and hereby incorporated by
reference, employs XL-PCR.TM. (Perkin-Elmer) to amplify and/or extend
nucleotide sequences.
Preferred libraries for screening for full length cDNAs are ones that have
been size-
selected to include larger cDNAs. Also, random primed libraries are preferred
in that they will contain
more sequences which contain the 5' and upstream regions of genes. A randomly
primed library may be
particularly useful if an oligo d(T) library does not yield a full-length
cDNA. Genomic libraries are useful
for extension 5' of the promoter binding region.
A newer method for analyzing either the size or confirming the nucleotide
sequence of
sequencing or PCR products is commonly known as "capillary electrophoresis".
Systems for rapid
sequencing are available from Perkin Elmer, Beckman Instruments (Fullerton
Calif.), and other
companies. In general, capillary sequencing employs flowable polymers for
electrophoretic separation,
four different fluorescent dyes (one for each nucleotide) which are laser
activated, and detection of the
emitted wavelengths by a charge coupled devise camera. Outputllight intensity
is converted to electrical
signal using appropriate software (eg. GenotyperTM and Sequence NavigatorTM
from Perkin Elmer) and
the entire process from loading of samples to computer analysis and electronic
data display is computer
controlled. Capillary electrophoresis is particularly suited to the sequencing
of small pieces of DNA
which might be present in limited amounts in a particular sample. The
reproducible sequencing of up to
350 by of M 13 phage DNA in 30 min has been reported (Ruiz-Martinez M. C. et
al ( 1993) Anal Chem
65:2851-8).
Expression of the Nucleotide Sequence:
In accordance with the present invention, the polynucleotide sequences) - SEQ
ID NO:1
or biologically equivalent fragment/sequences thereof or functional
equivalents thereof, may be used to
generate recombinant DNA molecules that direct the expression of DARPP-32 in
appropriate host cells.
Due to the inherent degeneracy of the genetic code, other DNA sequences which
encode substantially the
same or a functionally equivalent amino acid sequence, may be used to clone
and express the invention
protein- nhDARPP-32. As will be understood by those of skill in the art, it
may be advantageous to
produce the nhDARPP-32 -encoding nucleotide sequences possessing non-naturally
occurring codons.
Codons preferred by a particular prokaryotic or eukaryotic host (Murray E. et
al (1989) Nuc Acids Res
-21-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
17:477-508) can be selected, for example, to increase the rate of GPG
expression or to produce
recombinant RNA transcripts having desirable properties, such as a longer half-
life, than transcripts
produced from naturally occurring sequence.
Also included within the scope of the present invention are polynucleotide
sequences that
are capable of hybridizing to the nucleotide sequence of SEQ B7 NO:1 under
conditions of intermediate
to maximal stringency. Hybridization conditions are based on the melting
temperature (Tm) of the
nucleic acid binding complex, as taught in Berger and Kimmel (1987, Guide to
Molecular Cloning
Techniques, Methods in Enzymology, Vol 152, Academic Press, San Diego Calif.)
incorporated herein by
reference, and confer a defined "stringency" as explained below.
"Maximum stringency" typically occurs at about Tm-5°C. (5°C.
below the Tm of the
probe); "high stringency" at about 5°C. to 10°C. below Tm;
"intermediate stringency" at about 10°C. to
20°C. below Tm; and "low stringency" at about 20°C. to
25°C. below Tm. As will be understood by
those of skill in the art, a maximum stringency hybridization can be used to
identify or detect identical
polynucleotide sequences while an intermediate (or low) stringency
hybridization can be used to identify
or detect similar or related polynucleotide sequences. The term
"hybridization" as used herein shall
include "the process by which a strand of nucleic acid joins with a
complementary strand through base
pairing" (Coombs J. (1994) Dictionary of Biotechnology, Stockton Press, New
York N.Y.) as well as the
process of amplification has carned out in polymerase chain reaction
technologies as described in
Dieffenbach C. W. and G. S. Dveksler (1995, PCR Primer, a Laboratory Manual,
Cold Spring Harbor
Press, Plainview N.Y.) and incorporated herein by reference.
As used herein a "deletion" is defined as a change in either nucleotide or
amino acid
sequence in which one or more nucleotides or amino acid residues,
respectively, are absent. As used
herein an "insertion" or "addition" is that change in a nucleotide or amino
acid sequence which has
resulted in the addition of one or more nucleotides or amino acid residues,
respectively, as compared to
the naturally occurring DARPP-32. As used herein "substitution" results from
the replacement of one or
more nucleotides or amino acids by different nucleotides or amino acids,
respectively.
Altered DARPP-32 encoding polynucleotide sequences of the invention that may
be used
in accordance with the invention include deletions, insertions or
substitutions of different nucleotide
residues resulting in a polynucleotide that encodes the same or a
functionally/biologically equivalent
DARPP-32. The protein may also show deletions, insertions or substitutions of
amino acid residues
which produce a silent change and result in a functionally equivalent nhDARPP-
32. Deliberate amino
acid substitutions may be made on the basis of similarity in polarity, charge,
solubility, hydrophobicity,
hydrophilicity, and/or the amphipathic nature of the residues as long as the
biological activity of a
nhDARPP-32 is retained. For example, negatively charged amino acids include
aspartic acid and
glutamic acid; positively charged amino acids include lysine and arginine; and
amino acids with
-22-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
uncharged polar head groups having similar hydrophilicity values include
leucine, isoleucine, valine;
glycine, alanine; asparagine, glutamine; serine, threonine phenylalanine, and
tyrosine.
Also included within the scope of the present invention are alleles of the
nhDARPP-32.
As used herein, an "allele" or "allelic sequence" is an alternative form of
nhDARPP-32, e.g. the
nhDARPP-32 isoform. Alleles result from a mutation, i.e., a change in the
nucleic acid sequence, and
generally produce altered mRNAs or polypeptides whose structure or function
may or may not be altered.
Any given gene may have none, one or many allelic forms. Common mutational
changes which give rise
to alleles are generally ascribed to deletions, additions or substitutions of
amino acids. Each of these
types of changes may occur alone, or in combination with the others, one or
more times in a given
sequence.
The nucleotide sequences of the present invention may be engineered in order
to alter a
nhDARPP-32 coding sequence for a variety of reasons, including but not limited
to, alterations, which
modify the cloning, processing and/or expression of the gene product. For
example, mutations may be
introduced using techniques which are well known in the art, e.g., site-
directed mutagenesis to insert new
restriction sites, to alter glycosylation patterns, to change codon
preference, etc.
Yet another embodiment of the invention proposes ligating a DARPP-32 natural,
modified or recombinant sequence to a heterologous sequence to encode a fusion
protein. For example,
for screening of peptide libraries for inhibitors of DARPP-32 activity, it may
be useful to encode a
chimeric DARPP-32 protein expressing a heterologous epitope that is recognized
by a commercially
available antibody. A fusion protein may also be engineered to contain a
cleavage site located between a
DARPP-32 sequence and the heterologous protein sequence, so that the nhDARPP-
32 may be cleaved
and purified away from the heterologous moiety.
In an alternate embodiment of the invention, the coding sequence of nhDARPP-32
(SEQ
)D NO:1) could be synthesized, whole or in part, using chemical methods well
known in the art (see
Caruthers M. H. et al (1980) Nuc Acids Res Symp Ser 215-23, Horn T. et
a1(1980) Nuc Acids Res Symp
Ser 225-32, etc). Alternatively, the protein itself could be produced using
chemical methods to synthesize
a nhDARPP-32 amino acid sequence, whole or in part identical to that embodied
in SEQ ID N0:2. For
example, peptides can be synthesized by solid phase techniques, cleaved from
the resin, and purified by
preparative high performance liquid chromatography (e.g., Creighton ( 1983)
Proteins Structures And
Molecular Principles, W. H. Freeman and Co, New York N.Y.). The composition of
the synthetic
peptides may be confirmed by amino acid analysis or sequencing (eg, the Edman
degradation procedure;
Creighton, supra).
Direct peptide synthesis can be performed using various solid-phase techniques
(Roberge
J. Y. et al ( 1995) Science 269:202-204) and automated synthesis may be
achieved, for example, using the
ABI 431A Peptide Synthesizer (Perkin Elmer) in accordance with the
instructions provided by the
- 23 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
manufacturer. Additionally the amino acid sequence of nhDARPP-32, or any part
thereof, may be altered
during direct synthesis and/or combined using chemical methods with sequences)
from other .calcium
channel subunits, or any part thereof, to produce a variant polypeptide.
Expression S std:
In order to express a biologically active nhDARPP-32 of SEQ )D NO: 2 including
fragments, and biologically equivalent fragments thereof, the nucleotide
sequence coding for nhDARPP-
32, or a functional equivalent, is inserted into an appropriate expression
vector, i.e., a vector which
contains the necessary elements for the transcription and translation of the
inserted coding sequence.
Conventional methods, e.g., which are well known to those skilled in the art
can be used
to construct expression vectors containing a nhDARPP-32 coding sequence and
appropriate
transcriptional or translational controls. These methods include in vitro
recombinant DNA techniques,
synthetic techniques and in vivo recombination or genetic recombination. Such
techniques are described
in Maniatis et al (1989) Molecular Cloning, A Laboratory Manual, Cold Spring
Harbor Press, Plainview
N.Y. and Ausubel F. M. et al. (1989) Current Protocols in Molecular Biology,
John Wiley & Sons, New
York N.Y.
A variety of expression vector/host systems may be utilized to contain and
express a
nhDARPP-32 coding sequence. These include but are not limited to
microorganisms such as bacteria
transformed with recombinant bacteriophage, plasmid or cosmid DNA expression
vectors; yeast
transformed with yeast expression vectors; insect cell systems infected with
virus expression vectors (eg,
baculovirus); plant cell systems transfected with virus expression vectors
(eg, cauliflower mosaic virus,
CaMV; tobacco mosaic virus, TMV) or transformed with bacterial expression
vectors (eg, Ti or pBR322
plasmid); or animal cell systems.
The "control elements" or "regulatory sequences" of these systems vary in
their strength
and specificities and are those nontranslated regions of the vector,
enhancers, promoters, and 3'
untranslated regions, which interact with host cellular proteins to carry out
transcription and translation.
Depending on the vector system and host utilized, any number of suitable
transcription and translation
elements, including constitutive and inducible promoters, may be used. For
example, when cloning in
bacterial systems, inducible promoters such as the hybrid lacZ promoter of the
Bluescript®
phagemid (Stratagene, LaJolla Calif.) and ptrp-lac hybrids and the like may be
used. The baculovirus
polyhedrin promoter may be used in insect cells. Promoters or enhancers
derived from the genomes of
plant cells (eg, heat shock, RUBISCO; and storage protein genes) or from plant
viruses (eg, viral
promoters or leader sequences) may be cloned into the vector. In mammalian
cell systems, promoters
from the mammalian genes or from mammalian viruses are most appropriate. If it
is necessary to
-24-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
generate a cell line that contains multiple copies of nhDARPP-32, vectors
based on SV40 or EBV may be
used with an appropriate selectable marker.
In bacterial systems, a number of expression vectors may be selected depending
upon the
use intended for nhDARPP-32 of SEQ 1D N0:2 or variant or fragment thereof
(collectively referred to as
"nhDARPP-32". For example, when large quantities of nhDARPP-32 are needed for
the induction of
antibodies, vectors which direct high level expression of fusion proteins that
are readily purified may be
desirable. Such vectors include, but are not limited to, the E. coli cloning
and expression vector
Bluescript® (Stratagene), in which the nhDARPP-32 coding sequence may be
ligated into the vector
in frame with sequences for the amino-terminal Met and the subsequent 7
residues of (3-galactosidase so
that a hybrid protein is produced; pIN vectors (Van Heeke G. & S. M. Schuster
(1989) J Biol Chem
264:5503-5509); and the like. pGEX vectors (Promega, Madison Wis.) may also be
used to express
foreign polypeptides as fusion proteins with glutathione S-transferase (GST).
In general, such fusion
proteins are soluble and can easily be purified from lysed cells by adsorption
to glutathione-agarose beads
followed by elution in the presence of free glutathione. Proteins made in such
systems are designed to
include heparin, thrombin or factor XA protease cleavage sites so that the
cloned polypeptide of interest
can be released from the nhDARPP-32 moiety at will.
In the yeast Saccharomyces cerevisiae, a number of vectors containing
constitutive or
inducible promoters such as alpha factor, alcohol oxidase and PGH may be used.
For a review of the
vectors and promoters, see Ausubel et al (supra).
In cases where plant expression vectors are used, the expression of a nhDARPP-
32
coding sequence may be driven by any of a number of promoters. For example,
viral promoters such as
the 35S or 19S promoters of CaMV (Rhodes C. A. et al (1988) Science 240:204-
207) may be used alone
or in combination with the omega leader sequence from TMV (Takamatsu N. et al
( 1987) EMBO J 6:307-
311). Alternatively, plant promoters such as the small subunit of RUBISCO
(Coruzzi G. et al (1984)
EMBO J 3:1671-79; Brogue R. et al (1984) Science 224:838-43); or heat shock
promoters (Winter J. and
Sinibaldi R. M. (1991) Results Probl Cell Differ 17:85-105) may be used. These
constructs can be
introduced into plant cells by direct DNA transformation or pathogen-mediated
transfection. Refer to
Hobbs S or Murry L E in McGraw Yearbook of Science and Technology (1992)
McGraw Hill New York
N.Y., pp 191-196 for reviews of such techniques.
An alternative expression system which could be used to express nhDARPP-32
encoding
sequence is an insect system. In one such system, Autographa californica
nuclear polyhedrosis virus
(AcNPV) is used as a vector to express foreign genes in Spodoptera frugiperda
cells or in Trichoplusia
larvae. The nhDARPP-32 coding sequence may be cloned into a nonessential
region of the virus, such as
the polyhedrin gene, and placed under control of the polyhedrin promoter.
Successful insertion of
nhDARPP-32 will render the polyhedrin gene inactive and produce recombinant
virus lacking coat
- 25 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
protein coat. The recombinant viruses are then used to infect S. frugiperda
cells or Trichoplusia larvae in
which nhDARPP-32 is expressed (Smith G. et al (1983) J Virol 46:584; Engelhard
E. K. et al (1994) Proc
Nat Acad Sci 91:3224-7).
In mammalian host cells, a number of viral-based expression systems may be
utilized. In
cases where an adenovirus is used as an expression vector, a nhDARPP-32 coding
sequence may be
ligated into an adenovirus transcription/translation complex consisting of the
late promoter and tripartite
leader sequence. Insertion in a nonessential E1 or E3 region of the viral
genome will result in a viable
virus capable of expressing nhDARPP-32 in infected host cells. (Logan and
Shenk (1984) Proc Natl Acad
Sci 81:3655-59). In addition, transcription enhancers, such as the rous
sarcoma virus (RSV) enhancer,
may be used to increase expression in mammalian host cells.
Specific initiation signals may also be required for efficient translation of
an inserted
nhDARPP-32 sequence. These signals include the ATG initiation codon and
adjacent sequences. In
cases where nhDARPP-32, its initiation codon and upstream sequences are
inserted into the appropriate
expression vector, no additional translational control signals may be needed.
However, in cases where
only coding sequence, or a portion thereof, is inserted, exogenous
transcriptional control signals including
the ATG initiation codon must be provided. As well, the initiation codon must
be in the correct reading
frame to ensure transcription of the entire insert. Exogenous transcriptional
elements and initiation
codons can be of various origins, both natural and synthetic. The efficiency
of expression may be
enhanced by the inclusion of enhancers appropriate to the cell system in use
(Scharf D. et al (1994)
Results Probl Cell Differ 20:125-62; Bittner M. et al (1987) Methods in
Enzymol 1 53:51 6-544).
In addition, a host cell strain may be chosen for its ability to modulate the
expression of
the inserted sequences or to process the expressed protein in the desired
fashion. Such modifications of
the polypeptide include, but are not limited to, acetylation, carboxylation,
glycosylation, phosphorylation,
lipidation and acylation. Post-translational processing which cleaves a
"prepro" form of the protein may
also be important for correct insertion, folding and/or function. Different
host cells such as CHO, HeLa,
MDCK, 293, WI38, etc have specific cellular machinery and characteristic
mechanisms for such post-
translational activities and may be chosen to ensure the correct modification
and processing of the
introduced, foreign protein.
For long-term, high-yield production of recombinant proteins, stable
expression is
preferred. For example, cell lines which stably express nhDARPP-32 may be
transformed using
expression vectors which contain viral origins of replication or endogenous
expression elements and a
selectable marker gene. Following the introduction of the vector, cells may be
allowed to grow for 1-2
days in an enriched media before they are switched to selective media. The
purpose of the selectable
marker is to confer resistance to selection and its presence allows growth and
recovery of cells which
-26-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
successfully express the introduced sequences. Resistant clumps of stably
transformed cells can be
proliferated using tissue culture techniques appropriate to the cell type.
Any number of selection systems may be used to recover transformed cell lines.
These
include, but are not limited to, the herpes simplex virus thymidine kinase
(Wigler M. et al (1977) Cell
11:223-32) and adenine phosphoribosyltransferase (Lowy I. et al (1980) Cell
22:817-23) genes which can
be employed in tk.- or aprt- cells, respectively. Also, antimetabolite,
antibiotic or herbicide resistance can
be used as the basis for selection; for example, dhfr which confers resistance
to methotrexate (Wigler M.
et al (1980) Proc Natl Acad Sci 77:3567-70); npt, which confers resistance to
the aminoglycosides
neomycin and G-418 (Colbere-Garapin F. et al (1981) J Mol Biol 150:1-14) and
als or pat, which confer
resistance to chlorsulfuron and phosphinotricin acetyltransferase,
respectively (Murry, supra). Additional
selectable genes have been described, for example, trpB, which allows cells to
utilize indole in place of
tryptophan, or hisD, which allows cells to utilize histinol in place of
histidine (Hartman S. C. and R. C.
Mulligan (1988) Proc Natl Acad Sci 85:8047-51). Recently, the use of visible
markers has gained
popularity with such markers as anthocyanins, (3 glucuronidase and its
substrate, GUS, and luciferase and
its substrate, luciferin, being widely used not only to identify
transformants, but also to quantify the
amount of transient or stable protein expression attributable to a specific
vector system (Rhodes C. A. et
al (1995) Methods Mol Biol 55:121-131).
Although the presence/absence of marker gene expression suggests that the gene
of
interest is also present, its presence and expression may need to be
confirmed. For example, if the
sequence encoding nhDARPP-32 is inserted within a marker gene sequence,
recombinant cells containing
sequences encoding nhDARPP-32 can be identified by the absence of marker gene
function.
Alternatively, a marker gene can be placed in tandem with an nhDARPP-32
sequence under the control of
a single promoter. Expression of the marker gene in response to induction or
selection usually indicates
expression of the tandem nhDARPP-32 as well.
Transformed cells containing the polynucleotide sequence encoding nhDARPP-32
can be
detected by DNA-DNA or DNA-RNA hybridization or amplified using probes or
portions or fragments
of polynucleotides encoding nhDARPP-32. Conventional nucleic acid
amplification based assays
generally involve using oligonucleotides or oligomers based on the nhDARPP-32-
encoding sequence to
detect transfectants containing DNA or RNA encoding the target sequence, e.g.,
nhDARPP-32.
Consequently, as used herein "oligonucleotides" or "oligomers" refer to a
nucleic acid sequence of at least
about 10 nucleotides and as many as about 60 nucleotides, preferably about 15
to 30 nucleotides, and
more preferably about 20-25 nucleotides, which can be used as a probe or
amplimer.
A variety of protocols for detecting and measuring the expression of nhDARPP-
32, using
either polyclonal or monoclonal antibodies specific for the protein are known
in the art. Examples
include enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA) and
fluorescent
-27-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
activated cell sorting (FACS). A two-site, monoclonal-based immunoassay
utilizing monoclonal
antibodies reactive to two non-interfering epitopes on the invention protein
is preferred, but a competitive
binding assay may be employed. These and other assays are described, among
other places, in Hampton
R. et al (1990, Serological Methods, a Laboratory Manual, APS Press, St. Paul
Minn.) and Maddox D. E.
et al (1983, J Exp Med 158:1211).
Likewise, the prior art is replete with references teachings a wide variety of
labels and
conjugation techniques useful in various nucleic acid and amino acid assays.
Means for producing labeled
hybridization or PCR probes for detecting sequences related to polynucleotides
encoding nhDARPP-32
include oligolabeling, nick translation, end-labeling or PCR amplification
using a labeled nucleotide are
detailed in the art. Alternatively, target sequences - those encoding nhDARPP-
32, or any portion of it,
may be cloned into a vector for the production of an mRNA probe. Such vectors
are known in the art, are
commercially available, and may be used to synthesize RNA probes in vitro by
addition of an appropriate
RNA polymerase such as T7, T3, or SP6 and labeled nucleotides. These
procedures may be conducted
using a variety of commercially available kits (Pharmacia Upjohn, (Kalamazoo,
Mich.); Promega
(Madison, Wis.) and U.S. Biochemical Corp., Cleveland, Ohio). Suitable
reporter molecules or labels,
which may be used, include radionuclides, enzymes, fluorescent,
chemiluminescent, or chromogenic
agents as well as substrates, cofactors, inhibitors, magnetic particles, and
the like. Patents teaching the use
of such labels include U.S. Pat. Nos. 3,817,837; 3,850,752; 3,939,350;
3,996,345; 4,277,437; 4,275,149
and 4,366,241. Also, recombinant immunoglobulins may be produced as shown in
U.S. Pat. No.
4,816,567 incorporated herein by reference.
Purified nhDARPP-32 polypeptides:
Host cells transformed with a nhDARPP-32 encoding nucleotide sequence may be
cultured under conditions suitable for the expression and recovery of the
encoded protein from cell
culture. The protein produced by a recombinant cell may be secreted or may be
contained intracellularly
depending on the sequence and/or the vector used. As will be understood by
those of skill in the art,
expression vectors containing nhDARPP-32 can be designed with signal sequences
which direct secretion
of nhDARPP-32 through a particular prokaryotic or eukaryotic cell membrane.
Other recombinant
constructions may join nhDARPP-32 to nucleotide sequence encoding a
polypeptide domain which will
facilitate purification of soluble proteins (Kroll D. J. et al (1993) DNA Cell
Biol 12:441-53; see also
above discussion of vectors containing fusion proteins). Such purification
facilitating domains include,
but are not limited to, metal chelating peptides such as histidine-tryptophan
modules that allow
purification on immobilized metals, protein A domains that allow purification
on immobilized
immunoglobulin, and the domain utilized in the FLAGS extension/affinity
purification system (Immunex
Corp., Seattle Wash.). The inclusion of a cleavable linker sequences such as
those specific for Factor XA
-28-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
or enterokinase (Invitrogen, San Diego, Calif.) between the purification
domain and nhDARPP-32 may
be used to facilitate purification. One such expression vector which may be
used provides for expression
of a fusion protein containing a nhDARPP-32 and a nucleic acid encoding 6
histidine residues followed
by thioredoxin and an enterokinase cleavage site. The histidine residues
facilitate purification on IMIAC
(immobilized metal ion affinity chromatography as described in Porath, J. et
al. ( 1992) Prot. Exp. Purif. 3:
263-281) while the enterokinase cleavage site provides a means for purifying
nhDARPP-32 from the
fusion protein. A discussion of vectors which contain fusion proteins is
provided in Kroll, D. J. et al.
(1993) DNA Cell Biol. 12:441-453.
On the other hand, suitable host cells that contain the coding sequence for
nhDARPP-32
and express nhDARPP-32 may be identified by a variety of procedures known to
one of skill in the art.
Such procedures include, but are not limited to, DNA-DNA or DNA-RNA
hybridizations, fluorescent
activated cell sorting and protein bioassay or immunoassay techniques which
include membrane, solution,
or chip based technologies for the detection and/or quantification of the
nucleic acid or protein.
Eukaryotic cells expressing heterologous nhDARP-32 of the invention may be
used in
assays to assay for DARPP-32 modulators. The recombinant cells of the
invention may be used to assess
D1 or D2 receptor function or DARPP-232 tissue distribution and to identify
compounds that modulate
the activity of , for example, DARPP-32. Because DARPP-32 is a member of the
dopamine regulated
signaling cascade and is thus involved in regulating the intracellular effects
of dopamine within the
nervous system and other fundamental processes, assays designed to assess such
activities and assays to
identify modulators of these activities provides a means to understand
fundamental physiological
processes and also a means to identify new drug candidates for an array of
disorders.
In addition to recombinant methods, fragments of nhDARPP-32 may be also
produced by
direct peptide synthesis using solid-phase techniques (cf Stewart et al.
(1969) Solid-Phase Peptide
Synthesis, W. H. Freeman Co., San Francisco, Calif.; Merrifield J. (1963) J.
Am. Chem. Soc. 85:2149-
2154). In vitro protein synthesis may be performed using manual techniques or
by automation.
Automated synthesis may be achieved, for example, using Applied Biosystems
431A Peptide Synthesizer
(Perkin Elmer). Various fragments of nhDARPP-32 may be chemically synthesized
separately and
combined using chemical methods to produce the full length molecule.
In another aspect, the recombinant cells of the invention contain heterologous
genes)
(foreign to the cell- cDNA encoding DARPP-32 of SEQ >D N0:2) with a
transcriptional control element,
which is active in the cell and responsive to an ion or molecule capable of
entering the cell through a
functional calcium channel and linked operatively for expression to a
structural gene for an indicator
protein, can also be employed for assaying a compound for calcium channel
agonist or antagonist activity.
The preferred method comprises exposing a culture of such recombinant cells to
a
solution of a compound being tested for such activity, together with an ion or
molecule, which is capable
-29-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
of entering the cells through a functional calcium channel and affecting the
activity of the transcriptional
control element controlling transcription of the genes for the indicator
protein, and comparing the level of
expression, in the cells of the culture, of the genes for the indicator
protein with the level of such
expression in the cells of another, control culture of such cells.
A "control culture," as clearly understood by the skilled, will be a culture
that is treated,
in substantially the same manner as the culture exposed to the compound being
assayed except that the
control culture is not exposed to the compound being assayed. Alternatively,
control culture may
comprise cells expressing a dysfunctional calcium channel. Levels of
expression of the genes for the
indicator proteins are ascertained readily by the skilled by known methods,
which involve measurements
of the concentration of indicator protein via assays for detectable compounds
produced in reactions
catalyzed by the indicator protein.
As indicated above, indicator proteins are enzymes which are active in the
cells of the
invention and catalyze production of readily detectable compounds (e.g.,
chromogens, fluorescent
compounds).
The role of DARPP-32 in the mobilization of Ca++ as part of the signal
transduction
pathway can be assayed in vitro. It requires preloading calcium channel
expressing cells with a
fluorescent dye such as FURA-2 or BCECF (Universal Imaging Corp, Westchester
Pa.) whose emission
characteristics have been altered by Ca++ binding. When the cells are exposed
to one or more activating
stimuli artificially or physiologically, Ca++ flux takes place. This flux can
be observed and quantified by
assaying the cells in a fluorometer or fluorescent activated cell sorter. The
measurement of Ca++
mobilization in mobilization assays is well known. Briefly, in a calcium
mobilization assay, cells
expressing the target receptor are loaded with a fluorescent dye that chelates
calcium ions, such as FURA-
2. Upon addition of a calcium channel modulator to the cells expressing a
calcium channel, the target
modulator binds to the calcium channel and calcium is released from the
intracellular stores. The dye
chelates these calcium ions. Spectrophotometric determination of the ratio for
dye:calcium complexes to
free dye determine the changes in intracellular calcium concentrations upon
addition of the target
modulator. Hits from screens and other test compounds can be similarly tested
in this assay to
functionally characterize them as agonists or antagonists. Increases in
intracellular calcium
concentrations are expected for compounds with agonist activity while
compounds with antagonist
activity are expected to block target modulator stimulated increases in
intracellular calcium
concentrations. See U.S. patent Number 6,420,137 and similar patents.
Pr~osed Uses of the various DARPP-32 Sequences of the Invention:
In another embodiment of the invention, the DARPP-32 protein or fragments
thereof
detailed herein may be used for therapeutic purposes.
-30-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Based on the chemical and structural homology that exists among nhDARPP-32
protein
(SEQ )D N0:2) and its human counterpart as disclosed in Brene et al., supra,
the DARPP-32 of SEQ >D
NO: 2 or a functionally equivalent fragment thereof, this protein is a cAMP-
regulated phosphoprotein
and is believed to function in the signal transduction pathway of
neurotransmitters in brain tissue From
the homology information provided above, it appears that nhDARPP-32 plays a
role in the modulation of
neurotransmitter signal transduction and cell development. Consequently, the
herein provided sequences
may be used in assays to identify anti-psychotics for use in treating human
disorders.
The collective data suggest that controlling DARPP-32 activity may provide a
novel
approach to degenerative neuronal disease treatment and may be especially be
useful in combination
therapy with other, conventional therapeutic moieties. This is so because
combinations of therapeutic
moieties having different cellular mechanisms of action often have synergistic
effects allowing the use of
lower effective doses of each therapeutic moiety thus lessening side effects.
Accordingly, in one embodiment of the invention, the modulation of nhDARPP-32
by
agonists and antagonists may play a role in reconstructing signal transduction
pathways that have been
interrupted by degenerative neuronal disease. In another embodiment of the
invention, nhDARPP-32 or
derivatives thereof, may be used for regenerating and enhancing the survival
of nerve cells by supplying
nhDARPP-32 or stimulating residual nhDARPP-32 with nhDARPP-32 agonists to stop
the degenerative
process in certain brain diseases such as Parkinson's and Huntington's
disease.
In an alternative therapeutic embodiment, antagonists which block or modulate
the effect
of DARPP-32 may be used in those situations where such inhibition or
modulation is therapeutically
desirable. Such situations may include the down-regulation of DARPP-32
activity to regulate cell growth
or to suppress abnormal signal transduction in diseased tissue. For example,
in one aspect, antibodies
which are specific for DARPP-32 (SEQ )D N0:2) may be used as an agonist,
antagonist, or as part of a
targeting or delivery mechanism so as to bring a pharmaceutical agent to cells
or tissue which express
DARPP-32.
The antibodies may be generated using methods that are well known in the art.
Such
antibodies may include, but are not limited to, polyclonal, monoclonal,
chimeric, single chain, Fab
fragments, and fragments produced by a Fab expression library. Neutralizing
antibodies, (i.e., those which
inhibit dimer formation) are especially preferred for therapeutic use.
For the production of antibodies, various hosts including goats, rabbits,
rats, mice,
humans, and other, may be immunized by injection with the protein of SEQ ID
N0:2 or immunologically
active fragments thereof or any functionally equivalent fragment or
oligopeptide thereof. Depending on
the host species, various adjuvants may be used to increase immunological
response. Such adjuvants
include, but are not limited to, Freund's, mineral gels such as aluminum
hydroxide, and surface active
substances such as lysolecithin, pluronic polyols, polyanions, peptides, oil
emulsions, keyhole limpet
-31-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
hemocyanin, and dinitrophenol. Among adjuvants used in humans, BCG (bacilli
Calmette-Guerin) and
Corynebacterium parvum are especially preferable. Preferably, the functionally
equivalent peptides or
fragments thereof used to induce antibodies to nhDARPP-32 have an amino acid
sequence consisting of
at least 5 amino acids, and more preferably at least 10 amino acids. It is
also preferable that they are
identical to a portion of the amino acid sequence of the natural protein, and
they may contain the entire
amino acid sequence of a small, naturally occurring molecule. Methods of
determining antigenic
determinants are well known and may be employed to identify those sequences
which will induce an
appropriate immune response. See Geysen et al. U.S Patent Nos. 5595915,
5998577 including
references cited therein. Short stretches of nhDARPP-32 amino acids may be
fused with those of another
protein such as keyhole limpet hemocyanin and antibody produced against the
chimeric molecule.
Monoclonal antibodies to DARPP-32 of SEQ >D NO: 2 may be prepared using any
technique which provides for the production of antibody molecules by
continuous cell lines in culture.
These include, but are not limited to, the hybridoma technique, the human B-
cell hybridoma technique,
and the EBV-hybridoma technique (Koehler et al. (1975) Nature 256:495-497;
Kosbor et al. (1983)
Immunol. Today 4:72; Cote et al. (1983) Proc. Natl. Acad. Sci. 80:2026-2030;
Cole et al. (1985)
Monoclonal Antibodies and Cancer Therapy, Alan R. Liss Inc., New York, N.Y.,
pp. 77-96).
In addition, techniques developed for the production of "chimeric antibodies",
the
splicing of mouse antibody genes to human antibody genes to obtain a molecule
with appropriate antigen
specificity and biological activity can be used (Morrison et al. ( 1984) Proc.
Natl. Acad. Sci. 81:6851-
6855; Neuberger et al. (1984) Nature 312:604-608; Takeda et al. (1985) Nature
314:452-454).
Alternatively, techniques described for the production of single chain
antibodies may be adapted, using
methods known in the art, to produce nhDARPP-32-specific single chain
antibodies. Antibodies with
related specificity but of distinct idiotypic composition may be generated by
chain shuffling from random
combinatorial immunoglobin libraries (Burton D. R. (1991) Proc. Natl. Acad.
Sci. 88:11120-3).
Antibodies may also be produced by inducing in vivo production in the
lymphocyte
population or by screening recombinant immunoglobulin libraries or panels of
highly specific binding
reagents as disclosed in the literature (Orlandi et al. (1989) Proc. Natl.
Acad. Sci. 86: 3833-3837 and
Winter et al. ( 1991 ), Nature 349:293-299).
Antibody fragments which contain specific binding sites for nhDARPP-32 may
also be
generated using well known techniques. For example, such fragments include,
but are not limited to, the
F(ab')2 fragments which can be produced by pepsin digestion of the antibody
molecule and the Fab
fragments which can be generated by reducing the disulfide bridges of the
F(ab')2 fragments.
Alternatively, Fab expression libraries may be constructed to allow rapid and
easy identification of
monoclonal Fab fragments with the desired specificity (Huse et al. (1989)
Science 256:1275-1281).
-32-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Various immunoassays may be used for screening to identify antibodies having
the
desired specificity. Numerous protocols for competitive binding or
immunoradiometric assays using
either polyclonal or monoclonal antibodies with established specificities are
well known in the art. Such
immunoassays typically involve the measurement of complex formation between
nhDARPP-32 and its
specific antibody. A two-site, monoclonal-based immunoassay utilizing
monoclonal antibodies reactive to
two non-interfering epitopes on a specific DARPP-32 protein is preferred, but
a competitive binding
assay may also be employed (Maddox et al. (1983) J. Exp. Med. 158:1211).
Proposed Diagnostic Assays Using DARPP 32 Specific Antibodies of the
Invention:
In another embodiment, antibodies which are specific for nhDARPP-32 may be
used for
the diagnosis of conditions or diseases characterized by expression of DARPP-
32, or in assays to monitor
patients being treated with DARPP-32, agonists, antagonists or inhibitors. The
antibodies useful for
diagnostic purposes may be prepared in the same manner as those described
above for therapeutics.
Diagnostic assays for DARPP-32 include methods which utilize the antibody and
a label to detect
DARPP-32 in human body fluids or extracts of cells or tissues. The antibodies
may be used with or
without modification, and may be labeled by joining them, either covalently or
non-covalently, with a
reporter molecule. A wide variety of reporter molecules which are known in the
art may be used, several
of which are described above.
A variety of protocols for measuring DARPP-32 expression, using either
polyclonal or
monoclonal antibodies specific for the respective protein are known in the
art. Examples include enzyme-
linked immunosorbent assay (ELISA), radioimmunoassay (RIA), and fluorescence
activated cell sorting
(FACS). A two-site, monoclonal-based immunoassay utilizing monoclonal
antibodies reactive to two
non-interfering epitopes on nhDARPP-32 is preferred, but a competitive binding
assay may be employed.
In order to provide a basis for diagnosing abnormal levels of DARPP-32
expression,
normal or standard values for DARPP-32 expression are established. Standard
values may be obtained by
combining body fluids or cell extracts taken from normal mammalian subjects,
preferably human, with
antibody to DARPP-32 under conditions suitable for complex formation which are
well known in the art.
The amount of standard complex formation may be quantified by comparing
various artificial membranes
containing known quantities of DARPP-32 with both control and disease samples
from biopsied tissues.
Thereafter, standard values obtained from normal samples may be compared with
values obtained from
samples from subjects which are symptomatic for the disease. Deviation between
standard and subject
values establishes the parameters for diagnosing the disease.
In an alternative embodiment of the invention, the polynucleotides encoding
nhDARPP-
32 (SEQ ID NO:1) may be used for diagnostic purposes. The polynucleotides
which may be used
include oligonucleotide sequences, antisense RNA and DNA molecules. The
polynucleotides may be
-33-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
used to detect and quantitate gene expression in biopsied tissues in which
expression of DARPP-32 may
be implicated. The diagnostic assay may be used to distinguish between
absence, presence, and excess
expression of DARPP-32 relative to normal, and to monitor regulation of DARPP-
32 activity levels
during therapeutic intervention.
In one aspect, hybridization or PCT probes which are capable of detecting
polynucleotide
sequences, including genomic sequences encoding human DARPP-32 or closely
related molecules, may
be used to identify nucleic acid sequences which encode DARPP-32. The
specificity of the probe,
whether it is made from a highly specific region, e.g., 10 unique nucleotides
in the 5' regulatory region, or
a less specific region, e.g., especially in the 3' region, and the stringency
of the hybridization or
amplification (maximal, high, intermediate, or low) will determine whether the
probe identifies only
naturally occurring sequences encoding DARPP-32, alleles, or related
sequences.
Probes may also be used for the detection of related sequences, and should
preferably
contain at least 50% of the nucleotides from any of these nhDARPP-32 encoding
sequences. The
hybridization probes of the subject invention may be derived from the
nucleotide sequence of SEQ )D
NO:1 or from genomic sequence including promoter, enhancer elements, and
introns of the naturally
occurring DARPP-32.
Other means for producing specific hybridization probes for DNAs encoding
DARPP-32
include the cloning of nucleic acid sequences encoding nhDARPP-32 or nhDARPP-
32 derivatives into
vectors for the production of mRNA probes. Such vectors are known in the art,
commercially available,
and may be used to synthesize RNA probes in vitro by means of the addition of
the appropriate RNA
polymerase as T7 or SP6 RNA polymerase and the appropriate radioactively
labeled nucleotides.
Hybridization probes may be labeled by a variety of reporter groups, for
example, radionuclides such as
32P or 355, or enzymatic labels, such as alkaline phosphatase coupled to the
probe via avidin/biotin
coupling systems, and the like.
Polynucleotide sequences encoding the DARPP-32 of SEQ >D NO: 2 may also be
used
for the diagnosis of conditions or diseases which are associated with
expression of DARPP-32. The
polynucleotide sequences encoding DARPP-32 of SEQ )D NO:1 or derivatives
thereof may be used in
hybridization or PCR assays of fluids or tissues from patient biopsies to
detect DARPP-32 expression,
e.g., human DARPP-32 based, in part, upon the close homology between the
nhDARPP-32 sequences
disclosed herein and the corresponding human sequences. The form of such
qualitative or quantitative
methods may include Southern or Northern analysis, dot blot, or other membrane-
based technologies;
PCR technologies; dip stick, pin, chip, and ELISA, all methods which are well
known in the art:
Considering the high degree of sequence homology between the sequences
disclosed
herein and the human DARPP-32 noted supra, the nucleotide sequences encoding
of the invention may
be useful in assays that detect activation or inactivation of human DARPP-32
associated with various
-34-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
degenerative neuronal diseases. Accordingly, the nucleotide sequence encoding
nhDARPP-32 of SEQ ID
NO:1 may be labeled by standard methods, and added to a fluid or tissue sample
from a patient under
conditions suitable for the formation of hybridization complexes. After a
suitable incubation period, the
sample is washed and the signal is quantitated and compared with a standard
value. If the amount of
signal in the~biopsied or extracted sample is significantly elevated over that
of a comparable control
sample, the nucleotide sequence has hybridized with nucleotide sequences in
the sample, and the presence
of elevated levels of nucleotide sequences encoding DARPP-32 in the sample
indicates the presence of
the associated disease. Such assays may also be used to evaluate the efficacy
of a particular therapeutic
treatment regimen in animal studies, in clinical trials, or in monitoring the
treatment of an individual
patient.
In order to provide a basis for the diagnosis of disease associated with
aberrant (high or
low levels relative to normal) expression of DARPP-32 for example, in a human,
a normal or standard
profile for expression is established. This may be accomplished by combining
body fluids or cell extracts
taken from normal subjects, either animal or human, with DARPP-32, or a
fragment thereof, under
conditions suitable for hybridization or amplification. Standard hybridization
may be quantified by
comparing the values obtained from normal subjects with a dilution series of
DARPP-32 measured in the
same experiment, where a known amount of a substantially purified DARPP-32 is
used. Standard values
obtained from normal samples may be compared with values obtained from samples
from patients who
are symptomatic for disease associated with DARPP-32. Deviation between
standard and subject values
is used to establish the presence of disease.
Once disease is established and a treatment protocol is initiated,
hybridization assays may
be repeated on a regular basis to evaluate whether the level of expression in
the patient begins to
approximate that which is observed in the normal patient. The results obtained
from successive assays
may be used to show the efficacy of treatment over a period ranging from
several days to months.
Additional diagnostic uses for oligonucleotides encoding nhDARPP-32 may
involve the
use of PCR. Such oligomers may be chemically synthesized, generated
enzymatically, or produced from
a recombinant source. Oligomers will preferably consist of two nucleotide
sequences, one with sense
orientation (5'.fwdarw.3') and another with antisense (3'.rarw.5'), employed
under optimized conditions
for identification of a specific gene or condition. The same two oligomers,
nested sets of oligomers, or
even a degenerate pool of oligomers may be employed under less stringent
conditions for detection and/or
quantification of closely related DNA or RNA sequences.
Methods which may also be used to quantitate the expression of DARPP-32
include
radiolabeling or biotinylating nucleotides, coamplification of a control
nucleic acid, and standard curves
onto which the experimental results are interpolated (Melby, P. C. et al.
(1993) J. Immunol. Methods,
159:235-244; Duplaa, C. et al. (1993) Anal. Biochem. 229-236.) The speed of
quantitation of multiple
-35-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
samples may be accelerated by running the assay in an ELISA format where the
oligomer of interest is
presented in various dilutions and a spectrophotometric or calorimetric
response gives rapid quantitation.
In other embodiments of the invention, the nucleotide sequences of the
invention may be
used in molecular biology techniques that have not yet been developed,
provided the new techniques rely
on properties of nucleotide sequences that are currently known, such as the
triplet genetic code, specific
base pair interactions, and the like.
In another embodiment of the invention, the nucleic acid sequence of SEQ >D
NO:1 may
also be used to generate hybridization probes which are useful for mapping the
naturally occurring
genomic sequence encoding human DARPP-32. The sequence may be mapped to a
particular
chromosome or to a specific region of the chromosome using well known
techniques. Such techniques
include in situ hybridization to chromosomal spreads, flow-sorted chromosomal
preparations, or artificial
chromosome constructions, such as yeast artificial chromosomes, bacterial
artificial chromosomes,
bacterial P1 constructions or single chromosome cDNA libraries as reviewed in
Price C. M. (1993) Blood
Rev. 7:127-134, and Trask B. J. (1991) Trends Genet 7:149-154.
The technique of fluorescent in situ hybridization of chromosome spreads, as
described in
Verma et al. (1988) Human Chromosomes: A Manual of Basic Techniques, Pergamon
Press, New York,
N.Y., may also be used. Fluorescent in situ hybridization of chromosomal
preparations and other physical
chromosome mapping techniques may be correlated with additional genetic map
data. Examples of
genetic map data can be found in the 1994 Genome Issue of Science (265:1981f).
Correlation between the
location of the gene encoding nhDARPP-32 on a physical chromosomal map and a
specific disease (or
predisposition to a specific disease) may help delimit the region of DNA
associated with that genetic
disease. The nucleotide sequences of the subject invention may be used to
detect differences in gene
sequences between normal, carrier, or affected individuals.
In situ hybridization of chromosomal preparations and physical mapping
techniques such
as linkage analysis using established chromosomal markers may be used for
extending genetic maps.
Often the placement of a gene on the chromosome of another mammalian species,
such as mouse, may
reveal associated markers even if the number or arm of a particular human
chromosome is not known.
New sequences can be assigned to chromosomal arms, or parts thereof, by
physical mapping. This
provides valuable information to investigators searching for disease genes
using positional cloning or
other gene discovery techniques. Once the disease or syndrome has been crudely
localized by genetic
linkage to a particular genomic region, for example, AT to l 1q22-23 (Gatti et
al. (1988) Nature 336:577-
580), any sequences mapping to that area may represent associated or
regulatory genes for further
investigation. The nucleotide sequence of the subject invention may also be
used to detect differences in
the chromosomal location due to translocation, inversion, etc. among normal,
carrier, or affected
individuals.
-36-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Alternatively, the DARPP-32 of the invention, its catalytic or immunogenic
fragments or
oligopeptides thereof, can be used for screening libraries of compounds in any
of a variety of drug
screening techniques with the aim of identifying therapeutic moieties useful
for treating neurological
diseases in humans. . The fragment employed in such a test may be free in
solution, affixed to a solid
support, borne on a cell surface, or located intracellularly. The formation of
binding complexes, between
the invention protein and the therapeutic moiety being tested, may be
measured.
Another technique for drug screening which may be used provides for high
throughput
screening of compounds having suitable binding affinity to the protein of
interest as described in
published PCT application W084/03564. In this method, as applied to nhDARPP-
32, large numbers of
different small test compounds are synthesized on a solid substrate, such as
plastic pins or some other
surface. The test compounds are reacted with nhDARPP-32, or fragments thereof,
and washed. Bound
nhDARPP-32 is then detected by methods well known in the art. Purified nhDARPP-
32 can also be
coated directly onto plates for use in the aforementioned drug screening
techniques. Alternatively, non-
neutralizing antibodies can be used to capture the peptide and immobilize it
on a solid support.
In yet another embodiment, one may use competitive drug screening assays in
which
neutralizing antibodies capable of binding nhDARPP-32 specifically compete
with a test compound for
binding nhDARPP-32 or human DARPP-32. In this manner, the antibodies can be
used to detect the
presence of any peptide which shares one or more antigenic determinants with
nhDARPP-32.
In additional embodiments, the nucleotide sequences of the invention (SEQ ID
NO:1)
may be used in any molecular biology techniques that have yet to be developed,
provided the new
techniques rely on properties of nucleotide sequences that are currently
known, including, but not limited
to, such properties as the triplet genetic code and specific base pair
interactions.
Methods for Screening for Compounds that Modulate the Activity of Dopamine
Activation of dopamine per se and therapeutic moiety /agents that enhance
dopaminergic
neurotransmission act on cell-surface receptors. Without wishing to be bound
by any particular theory, in
one aspect of the invention, dopamine D1 receptors mediate the phosphorylation
of DARPP-32 via
dopamine D1 receptor intracellular signaling pathways. As noted supra,
dopamine via the D1 receptors,
activates adenylyl cyclase and increased CAMP, which, in turn, activates
protein kinase A (PKA; cAMP
dependent protein kinase), which phosphorylates (or modulates phosphorylation
of) downstream elements
in intracellular signaling pathways, including but not limited to DARPP-32,
cAMP responsive element
binding protein (CREB), AMPA receptor (e.g., GluR1 AMPA receptor), cAMP, cGMP,
CK1, CK2,
CdkS, PKA, PKG, PP-2C, PP-2B and PP-1. Activation of PKA leads to the
phosphorylation of DARPP-
32 at Thr34. This phosphorylation event converts DARPP-32 into an inhibitor of
PP1. Consequently,
-37-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
the phosphorylation state of Thr34137-DARPP-32 can be modulated by modulation
of PKA via the
dopaminergic intracellular signaling pathway.
Alternatively, dopamine D2 receptor activation leads to adenylyl cyclase
inhibition (and
decreased cAMP). Intracellular concentration of cGMP also are unchanged or
inhibited after D2 receptor
activation: cGMP activates protein kinase G (PKG; cGMP-dependent protein
kinase), which
phosphorylates downstream signal transduction pathway elements, including but
not limited to
downstream elements in intracellular signaling pathways, including but not
limited to, DARPP-32 .
Since dopamine mimics the activity of other substances that modulate DARPP-32
phosphorylation, such as activators of CK1 or CK2, inhibitors of cdk5,
inhibitors of PP-1, inhibitors of
PP2C, inhibitors of PP2B, or activators of PP2A, and since dopaminergic
intracellular signaling pathways
are involved in the etiology of Parkinson's disease, depression,
schizophrenia, compounds that alter
activity of dopaminergic intracellular signaling molecules; preferably PKA but
also including, but not
limited to CK1, CK2, CdkS, AMPA receptor, PP-1, PP2C , PP2B and/or PP2A, may
be identified using
the herein disclosed sequences as a means of identifying compounds having anti-
psychotic activity. See
US Patent Application No. 2003/0109419, which is incorporated herein by
reference in its entirety.
Likewise, since dopamine plays an important role in controlling levels of
cAMP, and
since the cAMP-PKA pathway interacts with many other signaling pathways in the
brain, modulation of
dopamine will, in certain embodiments, ameliorate the symptoms and/or be used
in the treatment of
disorders including, but not limited to, Parkinson's disease, Huntington's
disease, attention deficit disorder
(ADD), attention deficit hyperactivity disorder (ADHD), neurodegenerative
disorder, Tourette's
syndrome, tic disorder, Lesch-Nyans disease, substance or drug abuse,
schizophrenia, depression, manic-
depressive disorder and obsessive-compulsive disorder.
For example, dopamine via the D1 receptors, activates PKA formation and
activates CK-
1. CK-1, in turn, phosphorylates DARPP-32 at Ser137. This phosphorylation
event converts DARPP-32
into an inhibitor of PP2B (i.e., calcineurin). Since PP2B dephosphorylates
DARPP-32 at Thr34, the
serotonin-mediated increase in DARPP-32 phosphorylation at Ser 137 potentiates
the serotonin/PKA-
mediated phosphorylation at Thr34-DARPP-32 and the subsequent inhibition of PP-
1. In other
embodiments, the phosphorylation state of Thr34-DARPP-32 can be modulated by
modulation of PP2C
via the dopaminergic intracellular signaling pathway. According to this
embodiment, the
phosphorylation of Thr34DARPP-32 increases via a decrease in the activity,
e.g., inhibition, of PP2C.
In the following examples, it is understood that the high degree of sequence
homology
between the nhDARPP-32 protein of SEQ ID NO: 2 and the human DARPP-32
disclosed in Brene et al.,
supra, suggest that the herein disclosed protein of SEQ ID N0:2 may be used in
various assay methods to
ultimately identify compounds useful in treating various neurological
disorders involving an aberrant
dopaminergic signaling pathway regulated DARP-32.
- 38 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
In a broad aspect, the invention provides a method for modulating activity of
an
intracellular signaling molecule, preferably, DARPP-32 comprising contacting
an amount of a compound
sufficient to alter activity of an intracellular signaling pathway, including
but not limited to a dopamine
D1 receptor, dopamine D2 receptor, serotonin, or glutamate (e.g., NMDA
receptor, AMPA receptor).
The intracellular signaling molecule may also include any one or more of cAMP
responsive element
binding protein (CREB), AMPA receptor (e.g., GluR1 AMPA receptor), cAMP, cGMP,
CK1, CK2,
CdkS, PKA, PKG, PP-2C, PP-2B, PP-l, calcium channels, Na/K ATPase and NMDA
receptor.
Methods of modulating casein kinase 1 ("CK1 " or "CK1"), casein kinase 2
("CK2"),
cyclin-dependent kinase 5 ("CdkS," "cdk5" or "CDKS"), protein phosphatase 1
("PP-1), AMPA receptor
("AMPA"), protein phosphatase-2C ("PP2C"), protein phosphatase-2B ("PP2B") or
protein phosphatase-
2A ("PP2A") activity in a cell are also encompassed.
A representative embodiment features a method for modulating DARPP-32 activity
in a
cell comprising contacting said cell with an amount of a compound sufficient
to alter activity of an
intracellular signaling pathway, including but not limited to the dopamine Dl
receptor intracellular
signaling, wherein contact of said cell or tissue with the compound results in
modulation of DARPP-32
activity.
Contact, of the cell with the compound results in a modulation of the activity
of PKA,
CKl, CdkS, PP-1, PP2C, PP2B and/or PP2A, whose modulation may be quantified
via the determination
of the rate of phosphorylation or dephosphorylation of DARPP-32 of SEQ >D N0:2
at distinct residues
known to one skilled in the art. It is understood that the cell expresses the
protein of SEQ )D NO: 2 or a
functionally equivalent fragment thereof. In other embodiments, the
phosphorylation of an element
downstream in an intracellular signaling pathway, including but not limited to
a calcium channel, Na/K
ATPase, NMDA receptor, and CREB, is modulated via modulation of dopamine. In
certain embodiments,
the compound is a compound identified by the methods of the invention, wherein
the compound
modulates DARPP-32 activity and wherein modulation this activity results in an
alteration in the activity
of said intracellular signaling molecule in a cell. In certain embodiments,
the compound binds to
dopamine. In other embodiments, the compound alters expression of dopamine.
A specific method contemplates detecting the increase (or decrease) in the
amount of
phosphorylation (or dephosphorylation) of Thr34-phosphorylated DARPP-32,
Serl37-phosphorylated
DARPP-32, or Thr75-phosphorylated DARPP-32. Detecting an increase or decrease
in the
phosphorylation of other residues mediated by the modulation of any one of
PKA, CK1, CdkS, AMPA
receptor, PP-l, PP2C, PP2B and/or PP2A are well known tone skilled in the art.
Another embodiment proposes a method for identifying a compound to be tested
for its
ability to modulate the activity of a dopaminergic intracellular signaling
pathway in a cell comprising:
(a) determining a first level of dopamine activity in said cell;
-39-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
(b) contacting said cell with a test compound; and
(c) determining a second level of dopamine activity, respectively, in said
cell,
wherein a difference in said first level and said second level of dopamine
activity is indicative of the
ability of said test compound to modulate the dopaminergic intracellular
signaling pathway.
Dopamine activity is quantified via the determination of the rate of
phosphorylation or
dephosphorylation of DARPP-32 of SEQ ID N0:2 at distinct residues known to one
skilled in the art. In
preferred embodiments, the phosphorylation of Thr34 of DARPP-32 is modulated
via modulation of
dopamine.
In a preferred embodiment, a difference in dopamine activity is indicative of
the ability of
said test compound to modulate phosphorylation-dependent activation of an
intracellular signaling
molecule, representative members of which include DARPP-32 (dopamine and cAMP-
regulated
phosphoprotein-32), cAMP responsive element binding protein (CREB), AMPA
receptor (e.g., GIuR1
AMPA receptor), CK1, CK2, CdkS, PKA, PKG, PP-2C, PP-2B, PP-I, calcium
channels, Na/K ATPase
and NMDA receptor. Preferably, phosphorylation of DARPP-32 is modulated.
According to the invention, a control level means a separate baseline level
measured in a
comparable cell or tissue not contacted with a test compound or a level that
is measured in a cell or tissue
prior to contacting it with a test compound.
In furtherance of the above, the invention provides an exemplary embodiment
that
provides a method of identifying a compound that modulates dopamine activity
in a dopamine D1
receptor intracellular signaling pathway in a cell or tissue comprising:
(a) determining a level of dopamine activity in said cell or tissue prior to
contact
with the compound to obtain a first level; and determining a second level of
dopamine after contact with
said compound to in said cell or tissue, wherein a difference in said first
level and said second level of
dopamine activity is indicative of the ability of said test compound to
modulate dopamine activity. In
certain embodiments, the difference in dopamineactivity is indicative of the
ability of said test compound
to modulate activity of the dopamine D1 receptor intracellular signaling
pathway.
However, in a preferred embodiment the difference in dopamine activity is
indicative of
the ability of the test compound to modulate phosphorylation-dependent
activation of an intracellular
signaling pathway molecule, wherein said molecule is DARPP-32
An alternative embodiment of the invention provides a method of identifying a
compound that modulates dopamine activity in a dopamine D1 receptor
intracellular signaling pathway in
a cell or tissue comprising:
(a) contacting said cell or tissue with a test compound; and (b) determining a
level of
dopamine activity in said cell or tissue; wherein a difference in said level
and a control level of dopamine
activity in a comparable cell or tissue not contacted with the test compound
is indicative of the ability of
-40-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
said test compound to modulate dopamine activity. Preferably, the difference
in dopamine activity is
indicative of the ability of said test compound to modulate phosphorylation-
dependent activation of a
DARPP-32. Phosphorylation/dephosphorylation activity of other members such
cAMP responsive
element binding protein (CREB), AMPA receptor (e.g., GIuRIAMPA receptor),
cAMP, cGMP, CK1,
CK2, CdkS; PKA, PKG, PP-2C, PP-2B, PP-l, calcium channels, Na/K ATPase and
NMDA receptor may
also be determined folwing any one or more of the assays detailed herein. .
Consequently, a specific embodiment provides a method for identifying a
therapeutic
moiety to be tested for an ability to treat a dopamine related disorder or a
dopamine D1 intracellular
signaling pathway disorder, in a patient in need of such treatment comprising:
(a) contacting a potential therapeutic moiety with dopamine and Thr34-
dephosphorylated DARPP-32; and
(b) detecting the amount of phosphorylation of Thr34-dephosphorylated DARPP-
32,
wherein said therapeutic moiety has therapeutic utility for treating said
disorder if an increase in the
phosphorylation of Thr34-dephosphorylated DARPP-32 is detected in the presence
of the potential
therapeutic moiety.
Alternatively, the assay may measure the rate of dephosphorylation of Thr34-
phosphorylated DARPP-32 in the presence of PP2B.
Another embodiment provides a method for identifying an therapeutic moiety to
be tested
for an ability to modulate activity of a dopamine D1 receptor, dopamine D2
receptor, serotonin or ,
glutamate (e.g., NMDA receptor, AMPA receptor) intracellular signaling pathway
in a cell or tissue
comprising:
(a) contacting said cell or tissue with a potential therapeutic moiety; and
(b)
determining a level of dopamine activity in said cell; wherein a difference in
said level and a control
level of dopamine activity in a comparable cell or tissue not contacted with
the test compound is
indicative of the ability of said test compound to modulate of the
intracellular signaling pathway.
Preferably, modulation of a dopamine D1 receptor intracellular signaling
pathway is modulated by
dopamine.
In certain embodiments, the method comprises the additional step of: (c)
determining
whether said intracellular signaling pathway is modulated.
As would be clearly understood by a person of ordinary skill in the art, any
and/or all of
the embodiments disclosed herein for identifying an therapeutic moiety, drug
or compound that can
modulate the activity of dopamine including such procedures that incorporate
rational drug design, as
disclosed herein, can be combined to form additional drug screens and assays,
all of which are
contemplated by the present invention.
-41 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
In certain embodiments, the compound modulates the activity of DRPP-32 by
binding to
DARPP-32. Binding may be measured under any standard art-known physiological
conditions, according
to methods well known in the art.
In another embodiment, the method comprises determining a first level of CK1,
CK2,
CdkS, AMPA receptor, PP-1, PP2C, PP2B and/or PP2A activity in a cell ;
contacting the cell or tissue
with a test compound; and determining a second level of CK1, CK2, CdkS, AMPA
receptor, PP-1, PP2C,
PP2B and/or PP2A activity in the cell or tissue, wherein a difference in the
first level and the second level
of CK1, CK2, CdkS, AMPA receptor, PP-1, PP2C, PP2B and/or PP2A activity is
indicative of the ability
of the test compound to modulate CK1, CK2, CdkS, AMPA receptor, PP-1, PP2C,
PP2B and/or PP2A
activity. According to the methods of the invention, patterns and/or levels of
DARPP-32 phosphorylation
may also be determined both before and after treatment of cells or tissues
with a test compound.
One of skill would understand that according to the invention, once a compound
is
identified as capable of producing, e.g., altered patterns and/or levels of
DARPP-32 phosphorylation
and/or dephosphorylation similar to known ameliorative compounds, the compound
may be used to treat
a dopamine-related disorder, a dopamine D1 receptor intracellular signaling
pathway disorder, as well as
other conditions in which dopaminergic systems are involved such as a
dysfunctional serotonergic
signaling mediated disorder exemplified by depression. In the context of the
present invention, the
compounds identified would be administered as an effective dose or amount
which can be determined by
one of skill in the art based on data from studies such as presented in this
specification. Such data would
include, but not be limited to, results from IC50 determinations.
Methods of treating a subject presenting symptoms consistent with a disorder
characterized by aberrant or dysregulation of a intracellular signaling
pathway regulated by DARPP-32
are also provided. Preferably, the signaling pathway is a dopaminergic
signaling pathway although a
serotonergic signaling pathway is also included considering that serotonin has
also been shown to mediate
phosphorylation of DARPP-32.
The method proposes administering to a subject in need thereof an amount of a
compound sufficient to alter activity of an intracellular signaling pathway
such as the dopamine D1
receptor, dopamine D2 receptor, serotonin, or glutamate (e.g., NMDA receptor,
AMPA receptor)
intracellular signaling pathway.
In preferred embodiments, the compound is a compound identified by the methods
of the
invention, wherein the compound modulates dopamine activity and wherein
modulation of dopamine
activity results in an alteration in the activity of said intracellular
signaling molecule in a cell or tissue.
In another embodiment, the invention provides a method for regulating
phosphorylation-
dependent activation of an intracellular signaling molecule comprising
administering an amount of a
compound sufficient to modulate dopamine activity, wherein modulation of the
dopamine activity results
-42-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
in an alteration in the phosphorylation-dependent activation of said
intracellular signaling molecule in the
cell, preferably DARPP-32.
In another embodiment, dopamine activity in cells or tissues of interest is
modulated in
situ or in vivo. The in vitro, in situ and in vivo applications may include,
but are not limited to modulating
activity in any of the cells disclosed hereinabove.
An exemplary in vivo method comprises administering the potential therapeutic
moiety to
a non-human mammal. The amount (and/or rate) of activation of dopamine is then
determined. A
therapeutic moiety is identified as capable of modulating the activity of an
intracellular signaling
pathway, via modulation of dopamine, when the amount (and/or rate) of dopamine
activation is increased
or decreased in the presence of the therapeutic moiety relative to in the
absence of the therapeutic moiety
. In preferred embodiments, the non-human mammal is a rodent. Preferably,
modulation of dopamine
results in an increase or decrease in the phosphorylation of DARPP-32.
Methods of testing a potential therapeutic moiety (e.g., a candidate drug,
potential
modulator, etc.) in animals or animal models are well known in the art. Thus
potential therapeutic moietys
can be used to treat whole animals
The potential efficacy of these compounds in relieving pathological symptoms
of a
disorder, including but not limited to, a dopamine-related disorder and/or a
dopamine D1 or D2
intracellular signaling pathway disorder, can be assessed in animal models for
disease
A still further aspect of the invention is a method for selecting a
therapeutic moiety for
possible use in the treatment of a psychotic disorder characterized by an
aberrant dopaminergic
intracellular signaling pathway regulated by DARPP-32, which comprises
administering a suspected
therapeutic moiety to an animal model for a disorder and measuring and/or
determining the putative
therapeutic moiety's effect on any of the phenotypic characteristics outlined
above which may be believed
to be related to said disorder.
In some embodiments, the therapeutic moiety is administered along with a D1
receptor
agonist. The amount (and/or rate) of modulation of dopamine activity is then
determined. Since the
administration of e.g., a D1 receptor agonist, in the absence of the
therapeutic moiety, should result in an
increase in DARPP-32 activity, a therapeutic moiety is identified as capable
of modulating the activity of
dopamine when the amount (and/or rate) of activation is significantly
increased or decreased in the
presence of the moiety relative to in the absence of the moiety.
In other embodiments, the therapeutic moiety is administered along with a D1
receptor
antagonist. The amount (and/or rate) of modulation of dopamine activity is
then determined. Since the
administration of a D1 receptor antagonist in the absence of the therapeutic
moiety should result in a
decrease in DARPP-32 activity, a therapeutic moiety is identified as capable
of modulating the activity
- 43 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
of dopamine when the amount (and/or rate) of activation is significantly
increased or decreased in the
presence of the therapeutic moiety relative to in the absence of the
therapeutic moiety .
Once a drug candidate is selected, structural variants of the drug candidate
can be tested.
These compounds can also be scrutinized and modified with parameters such as
membrane permeability,
specificity of effects, and toxicity. The selected (e.g., the most potent)
compounds of this secondary
screening can then be evaluated in situ and in animal models to determine
whether the selected
compounds alter the activity of dopamine, and/or induce predicted behavioral
alterations with minimal to
no side-effects. Such behavioral abnormalities are welll known to a skilled
artisan. In specific
embodiments, methods for testing for antidepressant efficacy commonly known in
the art, e.g., a rodent
tail-suspension test, can be used. These tests can be then be followed by
human trials in clinical studies.
Alternatively, in certain embodiments, human trials in clinical studies can be
performed without animal
testing. Compounds affecting targets other than dopamine can also be similarly
screened, using
alternative targets exemplified below.
Alternatively, modulators (e.g., activators or inhibitors) of dopamine
activity can be
obtained by screening, e.g., a random peptide library produced by recombinant
bacteriophage (see, e.g.,
Scott and Smith, Science 249:386-390 (1990); Cwirla et al., Proc. Natl. Acad.
Sci. USA 87:6378-6382
(1990); Devlin et al., Science 249:404-406 (1990)) or a chemical library.
Using the "phage method" very
large libraries can be constructed ( 106-108 chemical entities). A second
approach may be to use chemical
methods, of which the Geysen method (Geysen et al., Molecular Immunology
23:709-715 (1986); Geysen
et al. J. Immunologic Method 102:259-274 (1987)) and the method of Fodor et
al. (Science 251:767-773
(1991)) are examples. Furka et al. (14th international Congress of
Biochemistry, Volume 5, Abstract
FR:013 (1988); Furka, Int. J. Peptide Protein Res. 37:487-493 (1991)),
Houghton (U.S. Pat. No.
4,631,211, issued December 1986) and Rutter et al. (U.S. Pat. No. 5,010,175,
issued Apr. 23, 1991)
disclose methods to produce a mixture of peptides. Such peptides can be tested
as potential modulators of
dopamine activity.
Synthetic libraries (Needels et al., Proc. Natl. Acad. Sci. USA 90:10700-4
(1993);
Ohlmeyer et al., Proc. Natl.. Acad. Sci. USA 90:10922-10926 (1993); Lam et
al., International Patent
Publication No. WO 92/00252; Kocis et al., International Patent Publication
No. WO 94/28028, each of
which is incorporated herein by reference in its entirety), and the like can
also be used to screen for
modulators of dopamine activation, according to the present invention. Once a
potential modulator is
identified, chemical analogues can be either selected from a library of
chemicals as are commercially
available (e.g., from Chembridge Corporation, San Diego, Calif. or Evotec OAI,
Abingdon, UK), or
alternatively synthesized de novo. The prospective therapeutic moiety (drug)
can be placed into any
standard assay to test its effect on the activity of PDE1B activation. A drug
is then selected that modulates
the activity of dopamine activation.
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Screens for small molecules, analogs thereof are also encompassed by the
invention, as
are screens for natural modulators of dopamine, such as those molecules that
bind to and inhibit or
activate, e.g., D1 receptors or dopamine in vivo. Such modulation is
preferably determined via
phosphorylation or dephosphorylation of DARPP-32 of SEQ )D N0:2.
Alternatively, natural products
libraries can be screened using assays of the invention for molecules that
modulate e.g., D1 or D2
receptors activation or dopamine activity or DARPP-32 modulation.
Preferably, a potential modulator can be assayed for its ability to modulate
the
phosphorylation of Thr34 DARPP-32 by PKA or its dephosphorylation by PP2B, or
the phosphorylation
of Ser845-GluR1 AMPA receptor by PKA, or the dephosphorylation of Ser845-GluR1
AMPA receptor,
either independently, or subsequent to, a binding assay as disclosed herein.
In one such embodiment, the amount and/or rate of phosphorylation or
dephosphorylation of Thr34 DARPP-32, or a fragment thereof comprising the
Thr34 residue, is
determined. Such assays are known in the art. See for example U.S Patent
Application No.
20030211040 ('040), which is incorporated by reference herein in its entirety.
For example, various enzymatic assays for kinases and phosphatases are known
to a
skilled artisan and may be used in determining the amounbrate of
phosphorylation or dephosphorylation
of a phosphorylated or dephosphorylated DARPP-32 fragment. Kinase activity may
be measured as
described in Parker, Law, et al., 2000, Development of high throughput
screening assays using
fluorescence polarization: nuclear receptor-ligand-binding and
kinase/phosphatase assays, J. Biomolec.
Screening 5(2): 77-88; Bader et al. (2001, Journal of Biomolecular Screening
6(4): 255-64); Liu, F., X. H.
Ma, et al. (2001). "Regulation of cyclin-dependent kinase 5 and casein kinase
1 by metabotropic
glutamate receptors." Proceedings of the National Academy of Sciences of the
United States of America
98(20): 11062-8; Evans, D. B., K. B. Rank, et al. (2002). "A scintillation
proximity assay for studying
inhibitors of human tau protein kinase I1/CdkS using a 96-well format."
Journal of Biochemical &
Biophysical Methods 50(2-3): 151-61.
Likewise, activities of protein phosphatases may be monitored by a variety of
methods
known to those skilled in the art, e.g., the methods disclosed in Cohen et
a1.(1988, Protein phosphatase-1
and protein phosphatase-2A from rabbit skeletal muscle, Methods Enzymol
159:390-408) or Stewart and
Cohen (1988, Protein phosphatase-2B from rabbit skeletal muscle: a Ca<sup>2</sup>+-
dependent, calmodulin-
stimulated enzyme, Methods Enzymol 159:409-16).
The clinical use of neuroleptics (anti-psychotics) has provided a means for
treating
patients suffering from psychotic disorders. Known neuroleptic agents,
regardless of their chemical
structures, are pharmacologically active with a large number of central
monoaminergic neurotransmitter
receptors, including dopaminergic, serotonergic, adrenergic, muscarinic, and
histaminergic receptors. It is
believed that the therapeutic and adverse effects of these drugs are mediated
by distinct receptor subtypes.
- 45 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
With respect to the dopamine receptor system, current neuroleptic agents
generally act on
the dopamine receptor as dopamine antagonists. Neuroleptics are generally
characterized as an agent that
produces sedative or tranquilizing effects, and which also produces motor side
effects, such as catalepsy
or extrapyramidal symptomatology. The prevailing theory as to the mechanism of
action of neuroleptics
antipsychotic drugs proposes the antagonism of dopamine D2 receptors. This is
based on the observation
that these drugs have high affinity for this receptor in vitro, and that a
correlation exists between their
potency to block D2 receptors and their clinical efficacy. See, e.g.,
Silverstone T., Acta Psychiatr Scand
Suppl 1990;358:88-91).
At the present time, nine major classes of antipsychotics have been developed
and are
widely prescribed to treat psychotic symptoms irrespective of their etiology.
Continuous long-term use of
neuroleptics is indicated in many psychotic disorders, such as (for more than
six months) (i) primary
indications such as Schizophrenia, Paranoia, Childhood psychoses, some
degenerative or idiopathic
neuropsychiatric disorders (notably, Huntington's disease and Gilles de la
Tourette's syndrome); (ii)
secondary indications such as extremely unstable manic-depressive or other
episodic psychoses (unusual),
otherwise unmanageable behavior symptoms in dementia, amentia, or other brain
syndromes; and (iii)
questionable indications such as chronic characterological disorders with
schizoid, "borderline," or
neurotic characteristics; substance abuse; or antisocial behavior, recurrent
mood disorders. See, e.g.,
Baldessarini, Chemotherapy in Psychiatry, Revised and Enlarged Edition,
Harvard University Press,
Cambridge, Mass., (1985), the contents of which is entirely incorporated
herein by reference.
However, clinical use of these common neuroleptics is limited, however, not
only
because of their inability to reduce symptoms in a substantial number of
patients, i.e., schizophrenia but
also by their side effect profiles. In fact, nearly all of the "typical" or
older generation compounds have
significant adverse effects on human motor function such as persistent and
poorly reversible motoric
dysfunctions (e.g., tardive dyskinesia) in a significant number of patients.
For example, classical
neuroleptic agents, as exemplified by the butyrophenones and phenothiazines,
can, upon long-term
administration, produce severe motoric symptomatology, termed tardive
dyskinesia a movement disorder
characterized by involuntary writhing movements of the tongue and oral
musculature seen with long-term
administration of these agents. Tardive dyskinesia is usually reversible upon
discontinuation of the
chronic neuroleptic, if the drug is discontinued soon after symptoms of
tardive dyskinesia appear.
Otherwise symptoms may also persist. Pharmacological intervention for
treatment of tardive dyskinesia
is only moderately successful. Such motor abnormalities are known to occur in
as high as 10% of the
patients who are maintained on these drugs for several years; the incidence is
much greater in certain
groups, such as middle-aged females.
Because of the severity of these side effects and the low therapeutic-to-toxic
index of
conventional neuroleptics, other neuroleptics, called atypical neuroleptics,
have been recently developed.
- 46
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
Atypical neuroleptics have a lower incidence of extrapyramidal symptoms and
tardive dyskinesia;
however, they are still associated with weight gain and effects on blood
pressure and liver function, as
observed for conventional neuroleptics. This adds considerably to the cost and
limits the availability of
this treatment. Also, the mechanism of action of atypical anti-psychotics, is
not well understood.
Notwithstanding the limitations attending newer atypical anti-psychotics,
considerable effort has been
expended to find an improved therapeutic moieties with similar antipsychotic
properties but without much
success.
Consequently, there is an unmet need in the art to provide new methods of
screening that
can be used to develop novel therapeutic moieties or drugs that can be used to
treat psychotic diseases or
disorders. In addition, there is a need for simple tests of intracellular
consequences of antipsychotic
action. Since all anti-psychotics act upon multiple receptors, with widely
varying downstream effects in
terms of both effective relief of symptoms and unwanted side effects, analysis
of the intracellular
integration of these signals will provide a straightforward, cost-effective,
and mechanism-based
comparison useful for development of the next generation of therapeutic drugs.
Also, there is a need to
develop treatments for such diseases or disorders that are due, at least in
part, to an aberration or
dysregulation of an intracellular signaling pathway regulated by DARPP-32. The
herein disclosed
sequences aim to overcome the aforementioned drawbacks attending conventional
therapeutic moieties
and fulfills an the unmet needs noted above.
For example, in one aspect, the herein disclosed sequences may be used to
screen for
candidate therapeutic moiety based upon its ability to phosphorylate DARPP-32.
Thus, a test therapeutic
moiety nay be classified as an anti-psychotic based, in part upon its ability
and phosphorylation pattern
of DARPP-32 of SEQ )D N0:2 when compared the the ability and phosphorylation
pattern of a
conventional atypical anti-psychotic.
Thus, an increase in the sae the phosphorylataion pattern ability of this
moiety to said ,
which relies on determining levels and pattern of phosphorylation of DARPP-32
of SEQ ID N0:2 , by a
test compound and by a conventional. Atypical anti-psychotic drug fur use in
the proposed assays
includes, but is not limited to clozapine, risperidone, iloperidone,
olanzapine, quetiapine zotepine,
perospirone and ziprasidone.
Thus, in one embodiment, an effective atypical anti-psychotic therapeutic
moiety is one
which increases the phosphorylation of a DAARP-32 fragment, e.g., Thr34-
dephosphorylated DARPP-
32 or decreases the dephosphorylation of Thr34-phosphorylated DARPP-32
relative to a conventional
anti-psychotic. In another embodiment , the ability to treat a psychotic
disorder is tested so that if the
therapeutic moiety ameliorates the psychotic disorder, an atypical anti-
psychotic moiety is identified.
Preferably, the psychotic disorder is Parkinson's disease, depression or
schizophrenia. The ability to
-47-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
treat a psychotic disorder is tested in one of a Parkinson's disease,
depression or a schizophrenic animal
model.
Likewise, the sequences of the invention may also be used in methods for
classifying
drugs with unknown pharmacological activity relative to conventional anti-
psychotics, both typical and
atypical. For example, cells expressing a functional DARPP-32 of SEQ ID N0:2
are contacted with a
therapeutic moiety with unknown pharmacological activity, and the
level/pattern of phosphorylation of
DARPP-32, in said cell is determined and compared to the pattern of
phosphorylation of DARPP-32 by
conventional therapeutic moieties whose pattern of phosphorylation and known
pharmacological activity
are well known, such that identification of a similar pattern of
phosphorylation of the unknown
therapeutic moiety with a pattern of phosphorylation of a therapeutic moiety
with known
pharmacological activity results in classification of the unknown drug.
Preferably, treatment of a subject in vivo with a potential therapeutic moiety
for use as an
anti-psychotic drug produces a distinct phosphorylation pattern of
intracellular signaling protein DARPP-
32 at two sites (Thr34 and Thr75). It being understood that PP2B
dephosphorylates DARPP-32 at Thr34,
while PKA phosphorylates DARPP-32 at Thr34.
According to the invention, in the case of DARPP-32 phosphorylation, all three
categories of drugs (typical anti-psychotic, atypical anti-psychotic and
selective dopamine D2 receptor
antagonist) preferably will increase phosphorylation at Thr34 site of DARPP-32
of SEQ ID N0:2. With
administration of a typical anti-psychotic such as haloperidol, Thr 34
phosphorylation will increase for up
to 30 minutes, but at 60 minutes, there will be no statistical difference from
controls. However, in the
case of phosphorylation at the Thr-75 site of DARPP-32, preferably only
treatment with an atypical anti-
psychotic, e.g., clozapine, will significantly increase phosphorylation levels
of DARPP-32 at 15, 30 and
60 minutes. A selective dopamine D2 receptor antagonist, e.g. eticlopride,
preferably will decrease
DARPP-32 phosphorylation at Thr75 of DARPP-32 30 minutes after administration,
while a typical anti-
psychotic e.g., haloperidol, preferably will be without effect.
Determining the levels of phosphoproteins in a cell is well known to a skilled
artisan. For
example, e.g. cultured neuronal cells, aliquots of brain homogenate or of
homogenates of cultured cells,
may be separated by SDS/PAGE analysis according to standard methods, e.g.,
SDS/PAGE analysis using
10% polyacrylamide gels. The separated proteins may be analyzed by any method
known in the art. For
example, proteins are analyzed by immunoblot analysis. Other methods are well
known.
Likewise, the effect of the potential therapeutic moiety, whether known or
unknown on
the phosphorylation of DARPP-32 at either of two sites (Thr34 and/or Thr75)
may be assed using
conventional methods including phosphorylation state-specific antibodies.
Whether the cell-based screens measure dephosphorylation or phosphorylation
may
depend on the extent to which the substrate is normally phosphorylated in the
cell. Thus, in some
- 48 -
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
embodiments, the cell is treated with a compound that results in increased
phosphorylation of the
DARPP-32 prior to performing the assay. In certain, embodiments of the
invention quantitative methods
for detecting the extent or rate of dephosphorylation (e.g., ELISA) are
employed.
An alternative embodiment of the invention provides a cell-based assay for
phosphorylation. In a specific embodiment, signal transduction based on
protein phosphorylation may be
visualized in vivo, e.g., in single living cells using fluorescent indicators,
using methods such as those
disclosed in Sato et al. (2002, Fluorescent indicators for imaging protein
phosphorylation in single living
cells, Nature Biotechnology 20(3): 287-94). Such sensors consist of two
fluorescent protein molecules,
separated by a flexible linker. The linker peptide contains a phosphorylation
site and a phosphoprotein
recognition element. Phosphorylation of the linker causes a conformational
change that brings the two
fluorescent proteins into close proximity, allowing FRET to occur and changing
the fluorescent output of
the system.
Pharmaceutical Compositions/Dosage:
An additional embodiment of the invention relates to the administration of a
pharmaceutical composition, in conjunction with a pharmaceutically acceptable
carrier, for any of the
therapeutic effects discussed above. Such pharmaceutical compositions may
consist of nhDARPP-32,
antibodies to DARPP-32, agonists, antagonists, or inhibitors of DARPP-32. The
compositions may be
administered alone or in combination with at least one other agent, such as
stabilizing compound, which
may be administered in any sterile, biocompatible pharmaceutical carrier,
including, but not limited to,
saline, buffered saline, dextrose, and water. The compositions may be
administered to a patient alone, or
in combination with other agents, drugs or hormones.
The pharmaceutical compositions utilized in this invention may be administered
by any
number of routes including, but not limited to, oral, intravenous,
intramuscular, infra-arterial,
intramedullary, intrathecal, intraventricular, transdermal, subcutaneous,
intraperitoneal, intranasal,
enteral, topical, sublingual, transdermal, or rectal means.
In addition to the active ingredients, these pharmaceutical compositions may
contain
suitable pharmaceutically-acceptable carriers comprising excipients and
auxiliaries which facilitate
processing of the active compounds into preparations which can be used
pharmaceutically. Further details
on techniques for formulation and administration may be found in the latest
edition of Remington's
Pharmaceutical Sciences (Maack Publishing Co., Easton, Pa.).
After pharmaceutical compositions have been prepared, they can be placed in an
appropriate container and labeled for treatment of an indicated condition.
Pharmaceutical compositions
suitable for use in the invention include compositions wherein the active
ingredients are contained in an
-49-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
effective amount to achieve the intended purpose. The determination of an
effective dose is well within
the capability of those skilled in the art.
A therapeutically effective dose refers to that amount of active ingredient,
for example
agonist, antibodies to DARPP-32, antagonists, or inhibitors of DARPP-32, which
ameliorates the
symptoms or condition. Therapeutic efficacy and toxicity may be determined by
standard pharmaceutical
procedures in cell cultures or experimental animals, e.g., ED50 (the dose
therapeutically effective in 50%
of the population) and LD50 (the dose lethal to 50% of the population). The
dose ratio between
therapeutic and toxic effects is the therapeutic index, and it can be
expressed as the ratio, LD50/BD50.
Pharmaceutical compositions which exhibit large therapeutic indices are
preferred. The data obtained
from cell culture assays and animal studies is used in formulating a range of
dosage for human use. The
dosage contained in such compositions is preferably within a range of
circulating concentrations that
include the ED50 with little or no toxicity. The dosage varies within this
range depending upon the
dosage form employed, sensitivity of the patient, and the route of
administration.
Further details on techniques for formulation and administration may be found
in the
latest edition of "Remington's Pharmaceutical Sciences" (Mack Publishing Co,
Easton Pa.). Although
local delivery is desirable, there are other means, for example, oral;
parenteral delivery, including intra-
arterial (directly to the tumor), intramuscular, subcutaneous, intramedullary,
intrathecal, intraventricular,
intravenous, intraperitoneal, or intranasal administration.
The examples below are provided to illustrate the subject invention. These
examples are
provided by way of illustration and are not included for the purpose of
limiting the invention.
EXAMPLE 1
Cloning of Guinea Pig DARPP-32
Total RNA (1.2 mg) was prepared from 1.2 g of guinea pig whole brain tissue
using the
TRIZOL reagent (Invitrogen # 15596-026) according to the manufacturer's
instructions. The Oligolex kit
(Qiagen # 70022) was used to purify poly-A RNA from 500 pg of total RNA with a
yield of 12 p,g
according to the manufacturer's instructions.
RACE ready cDNA was synthesized using the SMART RACE cDNA Amplification Kit
(BD Bioscience # K1811-1). Guinea pig brain mRNA (1 ~.g in 3 p,1), 3'-CDS
primer (1 p1) and RNase-
free water ( 1 p,1) from the kit were mixed in an 0.5 ml microcentrifuge tube.
The contents was incubated
at 70°C for 2 min and then cooled on ice for 2 min. First-Strand buffer
(2 ~,1 of Sx concentrate), 20 mM
DTT (1 ~.l), 10 mM dNTP mix (1 p1), and PowerScript Reverse Transcriptase from
the kit (1 ~,1) were
added. The tube was incubated at 42°C for 1.5 hr. The RACE ready cDNA
sample was diluted with 250
~,l of Tricine-EDTA buffer from the kit and stored at - 20°C.
-50-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
PCR primers for the cloning and amplification of the guinea pig DARPP-32 cDNA
were
designed based on the 5' and 3' ends of the consensus sequence of the human,
mouse and rat DARPP-32
cDNAs. A PCR reaction was carned out using the RACE ready cDNA prepared above
as template (3 p.l),
5' primer (NB426: 5'-ATGGACCCCAAGGACCGCAAGAAG-3' (SEQ 1D N0:3), 1 ~,1 of a 20
~,M
solution), 3' primer (NB428: 5'-TTATGTGCCGGACTCAGGGGGG-3' (SEQ ID N0:4), 1 p1
of a 20
~,M solution), RNase-free water (45 p1), and two puRETaq Ready-To-Go PCR beads
(Amersham
Bioscience # 27-9557-O1) in a PCR tube with 30 rounds of PCR (94°C for
10 s, 60°C for 10 s and 72°C
for 1 min). An amplicon from this reaction was purified using a Qiaquick
column (Qiagen # 28104). The
purified amplicon was cloned into PCRscript vector, and four E. coli
transformant plasmid DNAs were
sent to sequencing. Three of four were the same and contained an open reading
frame with high
homology to the consensus sequence of known DARPP-32 cDNAs.
Since the PCR primers NB426 and NB428 were designed based on the consensus
sequence of known DARPP-32 cDNA sequences, the portions of the cDNA cloned
above that are derived
from the primer sequences may not exactly equal the guinea pig sequence. In
order to verify the.5' and 3'
sequences of the cloned cDNA, two non-coding region primers (NB468: 5'-
CGAGACCCCACGACGCGCGCCCCGCCCGCC-3' (SEQ ID NO:S) and NB464: 5'-
TTTCCCCAGATCTTAGGGTCCTGCCCTGT-3' (SEQ ID N0:6)) were designed according to the
consensus sequences of the 5' and 3' non-coding regions of human, mouse and
rat DARPP-32. Two
primers internal to the guinea pig DARPP-32 cDNA (NB448: 5'-
CTCTGGCTCAGTGAGTGCTGGGC-
3' (SEQ >17 N0:7) and NB445: 5'-ACCACCTCAAGTCCAAGAGACCCAA-3' (SEQ m N0:8))
were
also used for this verification. The PCR reaction settings were the same as
above except that the
annealing temperature was 65°C instead of 60°C and 35 instead of
30 cycles were performed. The two
amplicons were cloned into TA cloning vector and sequenced. The sequencing
results showed that the 5'
end primer (NB426) sequence was the same as the guinea pig sequence, but that
the 3' end primer
(NB428) sequence differed from the guinea pig sequence by two bases. These
corrections are
incorporated into the reported guinea pig cDNA sequence.
EXAMPLE 2
DARPP-32 specific antibodies can be used to detect a given target in a variety
of
standard assay formats. Such formats include immunoprecipitation, Western
blotting, ELISA,
radioimmunoassay, and immunometric assays. See Harlow & Lane, Antibodies, A
Laboratory Manual
(CSHP NY, 1988). Immunometric or sandwich assays (sELISA) are a preferred
format (see U.S. Pat. No.
4,376,110, 4,486,530, 5,914,241, and 5,965,375). Such assays use one antibody
or population of
antibodies immobilized to a solid phase, and another antibody or population of
antibodies in solution.
Typically, the solution antibody or population of antibodies is labeled. If an
antibody population is used,
-51-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
the population typically contains antibodies binding to different epitope
specificities within the target
antigen. Accordingly, the same population can be used for both solid phase and
solution antibody. If
monoclonal antibodies are used, first and second monoclonal antibodies having
different binding
specificities are used for the solid and solution phase. Solid phase and
solution antibodies can be
contacted with target antigen in either order or simultaneously. If the solid
phase antibody is contacted
first, the assay is referred to as being a forward assay. Conversely, if the
solution antibody is contacted
first, the assay is referred to as being a reverse assay. If target is
contacted with both antibodies
simultaneously, the assay is referred to as a simultaneous assay. After
contacting the target with antibody,
a sample is incubated for a period that usually varies from about 10 min to
about 24 hr and is usually
about 1 hr. A wash step is then performed to remove components of the sample
not specifically bound to
the antibody(ies) being used as a diagnostic reagent. When solid phase and
solution antibodies are bound
in separate steps, a wash can be performed after either or both binding steps.
After washing, binding is
quantified, typically by detecting label linked to the solid phase through
binding of labelled solution
antibody. Usually for a given pair of antibodies or populations of antibodies
and given reaction
conditions, a calibration curve is prepared from samples containing known
concentrations of target
antigen. Concentrations of antigen in samples being tested are then read by
interpolation from the
calibration curve. Analyte can be measured either from the amount of labelled
solution antibody bound at
equilibrium or by kinetic measurements of bound labelled solution antibody at
a series of time points
before equilibrium is reached. The slope of such a curve is a measure of the
concentration of target in a
sample. Suitable detectable labels for use in the above methods include any
moiety that is detectable by
spectroscopic, photochemical, biochemical, immunochemical, electrical,
optical, chemical, or other
means. See Handbook of Fluorescent Probes and Research Chemicals (6th Ed.,
Molecular Probes, Inc.,
Eugene Oreg.). Radiolabels can be detected using photographic film or
scintillation counters, fluorescent
markers can be detected using a photodetector to detect emitted light.
Enzymatic labels are typically
detected by providing the enzyme with a substrate and detecting the reaction
product produced by the
action of the enzyme on the substrate, and colorimetric labels are detected by
simply visualizing the
colored label.
Referring to Figure 5, detailed therein are the results of a detection Assay -
Luminescence Measurements using a Sandwich ELISA (sELISA)
Briefly, a black 96-well flat-bottom plate (Costar #3925) was treated with 100
p.1 per well
of a 1 ~g/ml solution of an anti-DARPP-32 antibody, MS2551, in 0.05 M sodium
carbonate/bicarbonate
pH 9.6 for approximately 16 hr at 4 °C with constant agitation. The
antibody MS2551 was prepared
according to standard methods under contract with Covance Research Products
Inc. by immunizing a
rabbit with a peptide corresponding to amino acids 2-13 of rat DARPP-32
coupled to KLH. The reactive
antibodies were purified on a column of immobilized peptide antigen. Wells
were rinsed three times with
-52-
CA 02565014 2006-10-26
WO 2005/113572 PCT/US2005/017356
0.01 M phosphate-buffered saline pH 7.4 (PBS) at room temperature (RT), and
treated with 250 p1 per
well of 0.2% casein in PBS for 2 hr at RT with constant agitation. Solutions
containing the indicated
concentrations of purified recombinant rat DARPP-32 with 0.2% casein in PBS
plus 0.05% tween-20
(PBST) (100 p.1 per well) were incubated in the wells for 2 hr at RT with
constant agitation, followed by
three rinses with 200 ~.1 per well of PBST. Next wells were treated with 100
~.1 per well of 1 p,g/ml of sc-
11365-AP in 0.2 % casein, PEST for 2 hr at RT with constant agitation. The
antibody, sc-11365-AP was
prepared by chemical conjugation of alkaline phosphatase (using a kit from
Pierce Chemical Co., #31493)
to sc-11365, a commercial purified rabbit polyclonal anti-DARPP-32 antibody
raised to a C-terminal
portion of DARPP-32 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Wells
were rinsed five times
with 200 ~,1 per well of PBST at RT, followed by incubation for 30 min at RT
with 100 ~I per well of
CDP Star (Applied Biosystems), a solution containing an alkaline phosphatase
substrate whose product is
luminescent. Luminescence was measured using a LJL Biosystems Analyst AD96-384
luminometer.
-53-
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 53
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 53
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE: