Note: Descriptions are shown in the official language in which they were submitted.
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
')C'-TYF~ CALCIUM CHANNEL SPLhCE YARIA~iT ~I~MP(~SrTIUNS AhTI~
M~THI~DS
Cross~ksference to Related Application
[00111] This application claims benefit of U.S. F3tavisiQnal Application
601549,879 filed 10 lvxay
2004. The contents of this application are incorporated herein by reference in
their entirety.
Technical Field
[0002] The invention relates to Cav3.l calciunn channel splice variants and
their involvement in
cell proliferative c4nditions, and to a nov$1 interaction between a newly
discovered c~a,,3.1 calcium
channel splice variant and annexin Bf. The invention also relates to methods
and composition for
pragnosing, diagnosing and treating cell proliferative conditions.
Sacksround
[OD03] 1~"rimary l7rain tumors are classified according to the presurrted cell
of origin. Glia-derived
brain tumors are categorized as glianna and acCOUnt far the majority of
primary brain tu~nQrs
(Sutherland et al., 1987). High-grade gliorna are often associated with a poor
pxognosis, as the
tumor may proliferate rapidly, invade neighboring areas of the brain and often
recurs following
surgical resection andlor radiotherapy (reviewed in Walker and I~ye, 2001).
Tumor fornnation
alters genies involved in cell proliferation, difFexentiation, migration arid
apoptosis. Ion cf~annels are
important mediators of such functions and recent findings eoni~irmed are,
altered regulation and
expression of chloride, patassiurn arid sodium channels in gliorna (0lsen et
al., 2003, Ransom et al.,
20(12, Schrey er al , 20D2).
[0004] Calcium channels, mostly T-types, are also regulated during cell
differentiation and tumor
fbnnation (l3ertolesi et al., 2.003, Chemin et cxl, 2002, I~iroaka ea aL,
2002, Mariot er al" 20U2,
Toyota et al.,1999). To date, tkucee different genes encoding distinct cx!
subunits of the T-type
channel have been identified (Ga"3.1, Ca"3.2 and Ca,,3.3) (Cribbs et al.,
1998, Lee et a~, 1999,
l~cltory et rcl., 2001, Perez-Reyes et al., 1998). Alternative splicing
generates additional isafaxms
of these channels with distinct biophysical properties such as kinetics and
voltage-dependence of
activation, inactivation and deactivation (Chemin et al., 2f~ia, Monteil ez
al., 2000}.
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
[OOUS~ Ca"3.1 channels ara expressed abundantly in CN5 neurons and were
recently shown to be
ptpsent in 2~strocytes (Klugl~auer et aL,1999, Latour et at., 2Cl113).
However, their function in
astrocytes remains poorly understood.
[OU06j The function and rnetnbrane expression of a nutttber of higlx voltage-
activated calcium,
channels is regulated by interaction with other Cellular proteins. FQr
example, N- and P!Q-type
channel function is regulated by synaptic proteins, such as syntaxin, SNAP-25
or cysteine string
protein (88,89), (U.S Pat Nos. 5,23,051 and 6,Q94,631). L.-type channels are
rztQdulated through
interactions witk~ calmadulin (Liang, H., DeMaria, C.D., Erickson, M.C'.r.,
lVXori, M.X., Alseikhan,
B.A., and'Yue, D.T. 2003. I3nified mechanisms of Ca''"' regulation across the
~az'~ channel family.
,Neuron 39, 951-9ti0.) as well as A-Kinase anchoring proteins (Alder, C.,
Dubel, S.J., Earrexe, C.,
Jarvis, S.E., Statz, a,~., Spaetgens, R.L., Scott, .T.D., Carnet, Y., De
'Waard, M. ~amponi, G.W.,
NargeQt, J., and Baurinet, E. 2002 Traf>:<aking of ~.-type calcium channels
mediated by the
gostsynaptic scaffolding pz~otein AR.Ai'79. J. ,i3ia1 Chem. 277 33f98-336113),
The close association
of calmadulin with the channels 2~Llows channels to directly activate
calciumlcalznodulin activated
signaling cascades (Dalmetsch, It"E., Pajvani,1(3., Fife, I~., Spans, J.M. and
Cn-eenbert, Irr.C.E. 21101.
Signaling to the nucleus by an L-type calciuttt channel-calmodulin complex
through the MAP kinase
pathway. Science 294, 333-3~9.). To date, with the exception of G protean ~3z
subunit interactions
with ~a"3.2 (Wolfe, J.T., Wang, kL, Howard, f., Garrison, J.C., and Barren,
P.C,). 20U3. T-type
calcium channel regulation by speck g-protein betagamma subunits. Nature 424,
2119-213.),
proteins interacting with T-type channels have not been clearly identified.
Disclosure of the Invention
tOtl~~l] In humans, three isofoems of the T Type (Cav3.1) calcium channel a,
subunit have been
reported as a result of alternate splicing of exans 25 and 26 in the ITt-N
linacer region (Cav3.1a,
Ca,,3.lb or ~Ca"3.lbc). According to the invention, human gliomas express
Cav3.1 channels in situ,
splicing of these exons is uniquely regulated, and there is expression of a
glioma-specific novel T-
type variant (Gav3.lac). As discussed below, seven human glionta, samples
were. collected at the
time of sutgexy, ftNA was extracted and cDNA was produced forl~.T-1'CIt
analysis. In addition,
three glioma cell lines (U87, IJ~63 and. U251N), primary cultures of humian
fetal astrocytes as well
as adult and Fetal human brain cDN'A were used. Previously described Ca"3.1
splice varianks were
present in glioma samples, cultured cells and whole brain. The results
xevealed that in the normal
adult brain, Cav3.la transexipts predominate while Ca"3.1b is ntostiy fetal-
specil~e_ 'V'itT-PCR
2
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
results an gtlama and gliotna cell lines showed that Ca"3.1 expression in
tumor cells resembles fetal
brain expression pattern as Cav3.lbc is pcedorninantly expressed. In addition,
a novel splice variant,
Cay3.1 ac, was identified, as expressed in three glioma biopsies and one
glioma cell line, but not in
normal brain or fetal astrocytes. Transient expression of this variant
revealed that Ca,,3.lac displays
similar current-voltage and steady-state inactivation properties compared to
Ca"3.1b, but a slower
recavexy from inaCLlvatlan. Io addition, a navel interaetio~n between the
Cav3.lae splice variant and
annexin III was discovered. Thus, provided is a xnechanisrn which can be
targeted fc~r tumor growkh
inhibition.
(OOOSj It has been discovered that Ca,,3.1 splice variants axe associated with
cell praliferative
conditions, such as brain cancers, gliatna, breast cancers, eye cancers and
retinablastoma.. Far
example, Cay3.1 splice variant rr~NA and protein are present in glioma sales
and giioma cell
lines. It alsr~ has been discovered that gliainas express a splice variant of
Cav3.1 that is normally
mainly found in fetal cells (Ca"3.1b~, as well as a. navel, gliama-specific
Cav3_1 splirx isaform
(Ca~3.lac). l;lectmphysiological oharacteriaation of Ca,,3.lac indicates
similar gating
characteristics to other ~av~.1 splice isofonns. In addition, it was
discovered that the protein
annexin 1II (ANA III interacted with a T-type calcium channel.
[0009] Thus, provided herein is a method for screening cells irr a human for
abnarnral expression
of Cav3. 1. T~type isoforms, thus deterniining the turnorigenic gatential of
the cells. one aspect is a
method far detecting a risk of, or the presence of, a cell prolifexative
disorder in a subject, which
oonlprises determining the presence ar absence of an abnormal expression of
Ca"3.1 T-type
isafoxms, whereby said abnormal expression indicates the risk or ~resenae of
this disorder. ~y
"expression" of a Ca"~.1 splice variant or isofonn is meant the presence of
nxR.l~A encoding the
variant or isofarzn and/or the presence of the protein itself. The expression
levels of these splice
variants can be datermi~aed in sannples comprising cells taken from the
subject. The simples may be
biopsies, tissues, ar circulating cells. The presence of rnRNA carr he
detected try a variety of
methods, including prlar formation of cDNAvnd hybridizatia~n or I~T-P'CR.
Narthvern Blot rnay also
be USed. ~~e presence 4f the pratain may be dcteated in sorr~c instances by
iruuaunoreaction with
antibodies specific far the isaform.
[Qpl4] At least three indicaiior~s of abnormal expression may be assessed. In
one aspect, the
determination is rr~de of ttte presence car absence of an isofarm uniquely
associated with abnornsally
proliferative cells, such as gliornas, i.e., the Cav3.iac form. In another
method to determine
3
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
abnormal expression, the distribution of isaforms is determined whereby the
frresence of an
abnormally high percentage of the Cav3.lbc form is indicative of the risk or
presence of a cellular
proliferative condition. The presence of the Cav~.lb fo~n is also an
indication. zn these,
embodiments, the cell proliferative disorder sometimes is selected from the
gz°oup consisting oi= brain
cancer, glioma, breast saucer, eye cancer and retinoblastoma. °
[0011.] The methods described above sometimes further comprise determining
whether thez'e is
increased cell pmliferatio~n within a subject identified as having the
presence of a nucleotide
sequence that encodes a Cav3.1 ae calcium channel or the presettce of a Cav~.1
ac calcium channel
protein in cells. The presence or absence of increased cell proliferation
sometimes is detected in a
tissue biopsy front the subject, and sametirnes increased cell proliferation
is detected ire vlvo. The
previously described methods sometimes further comprise administering a
molecule that reduces
cell proliferation in a subject identified as having the presence of a
nucleotide sequence that encodes
a Ca"3, l ac calcium channel, the presence of a Cav3.1 ac calcium channel
protein in cells, andlar the
presence of increased cell proliferation, in an amount effective to reduce the
cell proliferation. The
molecule that reduces cell proliferation in the subject sometimes is a
Cav3.lac calcium channel
antagonist molecule andlcr a molecule that inhibits an interaction between a
Cav3.iac Calcium
channel and ,AhT~~ III.
[0012] ,Also provided is a rr~ethaci. foc creating a cell proliferative
disorder chaxaeteri~ed by
expression Ca"3.1 ae, which comprises adnninistering a T-type calcium channel
blocker to a subject
in need thereof in an amount effective tv treat the cell proliferative
disorder. Specific embodiments
are directzd to a metttad for treating a cell proliferative disorder in a
subject, which comprises
administering a Cav3.1 ac calcium channel antagonist molecule or a molecule
that inhibits the
interaction of a Cav3.lac calcium channel with ANX iir to a subject in need
thereof in an amount
effc~tive to treat tyre cell proliferative disvrdef. These substances may be
used in the preparation of
rnedicaxnents for such treatment as well,
[QQL3] Provided also is a composition which comprises a cancer cell in
combination with an
antibody that specifically binds to a Ca~r3.lac calcium channel protein, or a
synthetic nucleic acid
C4mpPfSi1'l~ a nucleotide Sequence complementary to a polynueleotide sequence
in a cancer cell
nucleic acid that encodes a Cav3.lac calcium channel. The synthetic nucleic
acid sometimes is
linked to a sokid sugpor<, and the nucleic acid sometimes is arranged on the
solid support in an arxay.
.A.lsa provided is a composition which comprises a cancer cell in combination
with a Cav3.lae
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
calcium channel antagonist molecule or a molecule that inhibits the
interaction of a Ca,,3.1 ac
calcium channel with AhTX iII. Also featured is a composition which comprises
an isolated
Ca~,3.lac calcium channel-encadir~g nucleic acid or protein and 2rzt isolated
ANX 17I-encoding
nucleic acid or protein.
[004] Also provided are a variety of methods and compositions related to
screening cornpaunds
for the ability to inhibit tl~ interaction between Ca~'J.Iac arid ANX
IIh(e.g., by a compound's ability
to bind to a selected Cav3.lac-like peptide). One aspect is a. method for
identifying a molecule that
inhibits cell proliferation, which comprises contactirAg ozte or mare cells
comprising a Ca~3.lac
calcium channel encoding nucleic acid andlor a Ca"3.l.aa calcium channel
polypeptide with a test
molecule, and determining whether the test molecule decreases cell
proliferation, whereby a test
molecule that decreases cell proliferation is identified a.~ a molecule that
inhibits cell prcsliferation.
Another aspect is a zrzethad of screening for a molecule that it~tiEaits cell
proliferation, one of which
comprises: (a) incubating a Ca"3.lac palypeptide or substantially identical
polypeptide thereof with
a test molecule under conditions sufficient to permit binding between tlae
polypeptide and the test
molevule irr a reaction mixture, (b) contacting A~ III with the xeaotion
mixture antler conditions
sufficient to permit binding between the polypeptide and ANX T.>I, and (c)
detecting the laresence or
absence of deat~ased binding between the polypeptide and ANX 1TI, whereby the
preseneE of
decreased binding between the polypeptide and ,A.l~l~ III identifies the test
molecule as a molecule
that inhibits tl~e interaction between Cav3.I ac and ANX III. Analogous
methods are described for
detecting compounds that inhibit the intet'action of ~-type calcium channels
with syntaxin and
51'TAP-25 in U.S. Pat Nas. 5,623,051. and 6,090,631. A ~a"~.lac polypeptide is
a polypeptide that is
unique to this isoforrn. The Car3.lac polypeptide sometimes comprises ~5 ox
more sequential
amino acids selected from a region spanning amino acid 156 to amino acid 1570
of a Ca"3.Iac T-
type calcium channel, and the polypegtide sometimes consists of the amino acid
s~uence
SK.E1~(~MAIaL.h~C.DDYIAS~S$ASAAS.
~1101~j ri~ethods and compositions described herein sometimes pertain to a
Ca,,3.lb calcium
channel instead of, or sometimes in addition ta, a ~aV3.lac calcium channel.
For example, methods
for detecting a risk or presence of a cell proliferative condition in a
subject sometimes is perforEned
by detecting the pz~esence of a Ca"3.1b calcium channel encoding nucleotide
sequence or Cay3.lb
calcium channel protein in a sample instead of, or in addition to, detecting
the presence of a
Cay3. l ac calcium channel component. The Ca~3. i b calcium cha~anel
sotr~titnes is targeted in other
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
eutbodiments, such as in a method for treating a cell prolifexative condition
by administering a
Cav3. x b calcium channel antagonist to a subject in need there~af in an
amount cuff eient to treat the
cell praliferative condition instead af, ar in addition to, administering a
Ca"3.lac calcium channel
antagonist.
[p01~6] These and other aspects of the present invention are evident upon
reference to the
following detailed description and attached drawings.
Brief Description of the Drawinss
[iJa~.7] Figures 1A td lE depict immunofiuorescen( a studies showing Ca"3.1
calcium channel
expression in glioma. Irnmunocytochemistry of Ca"3.1 channels on T~251N
glian~a. cells is assessed
by confoca! microscopy at40~ (Figure lA) and lkl0~'. (Figure 1B). A paraffin-
embedded section of
a malignant astracytorna showing OFAZy expression is,in Figure 1C and showing
Ca,,3.1 channel
expression is in Figure 1D. Coexpressian of T-type channels on GFAF-stained
astroeytes is shown
in Figure 1E.
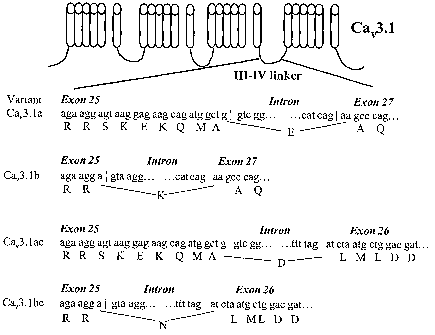
loty~.~) Figures 2A and 2.f3 shaves that specific isQforms of the Cav3.l ~I-IV
linl~er generated by
alternative splicing of exon 25 and 26 are differentially expmssed in the
human brain at various
develapme~ntal stages and in glioma. Figure 2A is a schematic rept'esentation
of alternative splicing
mechanisms in the Ca"3.1 FII-ATV linker. Inh~an-exon boundaries and splice
donor and acceptor sites
are shown in lower case letters, artlino acid sequence is shaven in upper case
lettering. The
sequences at tlae beginning of exans 27 and 26 are shown. The entire axon 2G
contains ttae amino
acid sequence LML.DDVIASGSSASAAS, such that inclusion of this exon results in
a 50 by
increase, as shown in Figure 2B. Fxan 2S can either produce a long (CaV3.la)
ar short isofarm
(Ca"3.1b), wktile exon 26, when present, gives rise t4 two additiox~a.!
variants (Cav3.lac or
Ca~3.lbc). Figure 2B is an agamse gel showing Iii-N linker 1tT-PCIZ results in
the normal adult
(lane 1), fetal brain (lane 2), u251.'bT gliorna cells (lane ~) and a glioma
sample (lane 4). The first
lane represents the molecular weight marker (MW weight is indicated by armws)_
[~Ox9~ Figures 3A to 3D show biophysical properties of Ca"3.lac (~r) and
Cav3.lb (4). Error
bars reflect standard errors. Figure 3A shows current-voltage relationships
far Ca"3.lac (n=13) and
Ca.V3. l b (n=10) vax-iants. The data were fitted with the Boltzmanri relation
(solid line). Figure 3l~
shows steady-state inactivation relationships, fitted with the Baitztx~ann
relation. Far CaY3.lac
(n=11) and Ca"3.1b (n=8). Note that there is no difference in slope or half
inactivation potential
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
with, these two channel isafarms. Figure 3C shawl time constants far
inactivation at various test
potentials. The time constants were obtained by tnonaexpaztential fits to fla;
raw data. Only at +2U
mV is there a statistical differez~oe between the two channel isofarnrs
(asterisk). A total of 13 and 10
experiments are included far the ac and b variants, respectively. Inset:
Current records obtained
from CaY3.16 and Cav3.lac, elicited by a 150 ms step depolarization. The
cumenks were scaled to
overlap at peak, the peak curxent amplitudes of the raw two currents, were,
respectively 6$0 pA and
574 pA. Note the similar inactivation kinetics, Figure 3D depicts a time
constant of recovery from
inactivarion. The data were obtained by using an inactivating prepulse
followed by a test
depolarization at various recovery intervals. The data normalized and fitted
rnonoexpanetially. Note
that the ac variant shows an increase in the time canstar~t of recovery from
inactivation. Ca"3.1b:
°r=1~0C1.(IOrns(n= 9), Cav3.lac: ~15A~.52rr~s (n=9).
~acrza] 1~igures ~4A antl X13 illustrate Ca,,3.1 III-IY linker splice isaform
distribution in normal
brain, gliama cell lines and glioma samples. lligure 4A shows the nutxaber of
clones far each
canditiota corresponding to the various isoforms. Figure ~4B is a schematic
representation a~ the
percentage of each isaform in fetal and adult brain, fetal astracytes and
gliarrta (gliazna cell lines and
,glioma samples combined).
[0021] Figure 5 shows the identification and confirmation of annexin IIf as a
liinding partner of
tk~e Ca"3.lac splice isoform IIl-kV' linker.
Detailed Description '
[0022] The present invention offers several strategies for pmgnosing,
diagnosing and treating
cell prolifez~ative conditions. .1'n prognostic and diagnostic methods, Cav3.
l splice variant-encoding
nucleotide sequences ar proteins are detected in a sample from the subject,
and such methods
sometimes are coupled with furthex diagnostic inforrxLatian andlor procedures,
andlor treatment
pxacEdures. In methods of treating cell proliferative disorders, T-type
calcium channel bkyckers can
be used to prevent entry of eaiciurn into mitogenic cells, thus preventing
initiation of cell
proliferation. Alternatively, a molecule that inhibits the interaction between
Cav3.lac and ANA III
can tae administered to reduce cell proliferation. Ca"3.1 splice variant
antagonists, such as antisense
molecules, ribozyrnes, si~IA molecules, compounds or antibodies directed
specifically to Cav3.lac
ar C:av3.l,b splice variants, can also be adtrAinistered to reduce cell
proliferation. Alternatively, A1VX
ZXI antagonists cane be administered to reduce cell proliferation and treat
Gel1 praliferative disorders.
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Itx certain embodiments, the cancer cell is from a brain tumor ar glioma, and
in other embodiments
the cancer cell is Pram a breast cancer tumor or eye cancer tumor (e.g.,
retinablastanraa).
[0023] ,A characteristic of the cells of the invention that exhibit undesired
proliferation is the
presence trf abnormal expression of at least one Cav3. l calcium channel
splice variant. This
abnormal expression can manifest itself in a number of ways so as to meet the
definition of
"abnormal expression.°' 1~irst, the presence of the sglice variant
Cav3.lac, either in the farm of
m~NA or protein or bath, is indicative of such cells and of such abnartttal
expression. Second, the
presence of the splice variant Cav3.lb also indicates abnormal expression;
such expression is
represented by either dr both the presence of tnRiV'.A ar protein
corresponding to this splice variant.
In addition, the proportion of the total CaY3.1 expression attributable to
Cav3.lbc indicates abnormal
expressxnn if the cells are non-fetal cells and the percentage measured as a
mole percent o;F mRlwdA
or pr~rtein is greater than 20%n of the total Cav3.1 calcium channel mRNA or
protein level. i These
percentages are an a male basis.
Cantaositions
[U02~j liven the association of certain Cav3.1 splice variants with cell
praliferative disorders,
provided is a composition which comprises a cancer cell in combination with an
anti>~vdy that
specifically binds to a Cav3.1 calcium channel protein splice variant
associated with the cancer (s.y.,
a ~Ca."3.1 ac ar ('av3.lla caloium channel protein). Examples of antibodies
are described hereafter. In
some embodiments, the cancer cell is intact, is in association with Other
cells (e.g., the cancer cell is
in a cell culture or in a tissue) or is separated from other cells (e.g., the
cancer cell is dispersed in a
liquid medium). In other embodiments, the cancer cell is not intact (e.g., it
is a cell lysate). The
cancer cell sometimes is isolated from a subject having a'cell praliferative
condition, sometimes is
isolated from a tissue of the subject, and sometimes is isolated from ar is
part of a cell Lire
Established from a subject having a cell praliferative condition.
X11025] Cell prolifexative disorders also include but are not limited to
cancers of the calarectum,
bmast, lung, liver, pancreas, lymph node, colon, prostate, brain, head and
r~eclc, skin, liver, kidney,
and heart. The cell praliferative condition sometimes is a brain cancer such
as glioma., sometimes is
an eye cancer such as rctinablastoma, and sametimcs is breast cancer. A cell
prali~Grative condition
sometimes is a hematopoietic neaplastic disorder, which is a disease involving
hyperplastic/neoplastic cells of hematopaietic origin (e.g., arising from
tltyeloid, Lymphoid or
erythroid lineages, or precursor cells thereof). The disease can arise from
poorly differentiated acute
leukemias, e.g., erythroblastic leukemia and acute megakaryabIastic leukemia.
,Additional myeloid
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
disorders include, but ate oat limited ta, acute promyeiaid leukemia (APML),
acute myela,genaus
leukemia (AML) aid chronic myelogenous leukemia (CML) (reviewed in Vaickus,
Grit. l~ev_ in
OncolJHernatol. 11:267..97 (1991)); lymphoid malignancies include, but are oat
limited to acute
lymphoblastic leukemia (ALL), which includes B-lineage ALL and T-lineage ALL,
chronic
lymphacytic leukemia (~LL~, gralymphacytic leukemia (PLL), hairy cell leukemia
(HLL) and
Waldenstrorn's macroglobulinemia (WM). Additional farms of malignant lymphomas
include, but
are riot limited to non-.~Iodgkin lymphoma and va~ciants thereof, peripheral T
cell tymphaxnas, adult
T cell leukemia/iymphoma (ATL), eutaneous T-cell lymphasna (CTCL)> urge
granular lymphacytic
leukemia (Ltig'), Hadghin's disease and Reed-Sternherg disease.
[O(126~ Tn certain embodiments, the composition catnprises a cancer cell and a
synthetic nucleic
acid comgnising a nucleotide sequence complementary to a palynucleatide
ser~uence in a cancer cell
nucleic acid that encodes a Ca,~3.1 calcium channel splice variaa~t associated
with a cancer (e.,g., a
Ca,,3.lac or Ca~3.l~ calcium channel). As used herein, the term "nucleic acid"
includes DNA
molecules (e_~., a complementary DNA (eDNA) and genamic DN'A (gDNA)), RNA
molecules (~.g.,
rr~RNA ar siRNA) az~d analogs c~f DNA ar RNA (e.g., RNA or DNA comprising or
consisting of
nucleotide analogs). The ~nueleic acid molecule sometimes is single-stranded
and often is dnuble-
stranded. The nucleic acid sometimes is a fragment, which sometimes is 50,100,
ar 200 or more
base pairs in length, and is sometimes about 300, 40f?, 500, 600, 700, 800,
900, 1400, 1 i 00,1200,
1300, ar 1400 base pairs in length. An example of a nucleic acid fragment is
a~n oligonucleatide. As
used herein, the term "oli~cnttcleotide" refers to a nucleic acid ca;mprising
about $ to about S0
covalently linked nucleotides, often eornprising from about $ to about 35
nucleotides, and mare
often Exam about 10 to about 25 nucleotides. The backbone and nucleotides
within an
aligonucleatide rnay be the same as those of naturally occurring nucleic
acids, ar analogs ar
derivatives of naturally occurring nucleic acids, provided that
oligonucleatides having-such analogs
ar derivatives retain the ability to hybridize specifically to a nucleic acid
comprising a targeted
polytrzorphisrn. c~ligc~nucleatides described herein may be used as
hybridization probes or as
components of prognostic or diagnostic assays, for example, as described
herein.
[O~D~7] Oligonuclentides often are syxwchesiaxd using standard methods and
equipment, such as
the A13ITM39001-Tigh Ttn~oughput DNA Synthesizer and the EDTTETM89o9 N'uafeic
Acid
Synthesizer, bath of which one available from. Applied Biosystems (Foster
City, CA). Analogs and
derivatives are exemplified its LT.S. Fat. Nos. 4,469,863; 5,535,$21;
5,541,306; 5,637,683;
5,637,fr84; ~,70U,922; S,7f'7,~83; 5,719,262; 5,739,308; 5,773,401; 5,886,1
b5; 5,929,26;
5,977,296; 6,140,482; WO QD/56746; Wp 01114398, and related puf~licatians.
Methods fox
synthesizing aligonucleatides comprising such analogs or derivatives are
disclosed, for example, in
9
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
the patent publications cited above and in iT.S. Ixat. Nos. 5,614,622;
5,739,314; 5,955,599;
5,962,674.; 6,117,992; in WO 00175372; and in related publications.
(3ligt~nt~cleotides sometimes
are linked to a second moiety. The second moiety may be an additional
nucleotide Sequence such as
a tail sequence (e.g., a polyadenosine tail), an adapter sequence (e.g.,~phage
M13 universal tail
sequence), arid others. Alternatively, the second moiety nnay be a non-
nual~eotide moiety such as a
moiety which facilitates linkage to a solid support or a label to facilitate
detection of the
oligocrucleotide, ~'he second moiety may be attached to any position of the
oligonucleotide, and
labels include but are not limited to a radioactive label, a fluorescent
label, a chemiluminescent
label, a paramagnetic Label, and the Like.
[(11128] The synthetic nucleic acid may be linked to a solid support, and the
nucleic acid may be
arranged on the solid support in an array. An array, sometimes referred to as
a "microarray,"
sometimes includes an oligonucleotides described herein, and methods for
txraking aced using
oligonucleotide microarrays are disclosed in ~fJ.S. Pat. Nos. 5,492,$06;
5,525,464; 5,589,330;
5,695,90; 5,849,4$3; 6,01$,041; 6,1145,996; 6,136,541; 6,142,681; 6,156,501;
6,197,506;
6,223,127; 6,22.5,625; 6,22,9,911; 6,239,273: WO 00152625; WA 01!254$5; and
W~J 01129259. The
microarray typically comprises a solid support and the oligonucleokides
sometimes are linked to the
solid support by covalent or non-covalent inter~.ctions. ~'he oligonucleatides
sometimes are linked
to the solid support directly or by a spacer molecule.
[1ID29~ The invention further includes cotrepositinns wllieh comprise an
isolated Cav3.lac
calcium channel-encoding nucleic acid or proCeirr. The term "isolated" refers
to substances that are
separated from their natural environments or from the materials present in the
natural source. For
example, with rEgard to genoxnic »NA, the term "isolated" includes nucleic
acids which are
separated from the chromosome with which the genomic DNA is naturahy
associated. An
"isolated" nucleic acid is often free of sequences which naturally flank the
nucleic acid (i.e.,
sequences located at the 5' andlor 3' ends of the nucleic acid) in the genomic
DNA of the organism
from which the nucleic acid is derived. For example, in various ennbodiments,
the isolated nucleic
acid molecule can contain less than about S kb, 4 kb, 3 kb, 2 kb, l kb, 0.5 kb
or O.l kb of 5' and/or
3" nucleotide sequences whick~ flank the nucleic acid molecule in genomic DNA
of the cell from
which the nucleic acid is derived. An "isolated" nucleic acid molecule, such
as a cl:)NA molecule,
sometimes is substantially fi~e of other cellular material, ar culture medium
when produced by
recombinant techniques, car substantially free of chemical precursors or other
chemicals when
chemically syritl~esixed. An "isolated" polypeptide car protein is
substantially free of cellular
material or other contaminating proteins from the cell or tissue source from
which the protein is
derived, or~srtbstantially free frown chemical precursors or other chemicals
when chemically
synthesized. "Substantially free" means a preparation of a substance having
less than about 30°la,
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
20/0, 10°lo and mare preferably 5~'0 (by dry weight), of material from
its source of derivation. Wkren
the palypeptide or a biologically active portion thereof is produced
recaznbinantly, it often is
substantially free of culture medium, specifically, where culture medium
represents less thin about
20%, sometimes less than about 10~, arid often less than about 5°!a of
the volume of the polypeptide
preparation.
((130] Alsa provided is a composition which comprises a cancer cell in
combination with a
molecule that antagonizes a Ca~3.1 oalcium channel splice variant associated
with a cell
proliferative disorder (e.g., antagonizes a t'av3.lac ox Ca~3.Ik~ calcium
channel) ar a molecule that
inhibits ttte interaction of a Ga"3.1 calcium channel splice variant with ANA
III (e.g., Ca~3.lac).
Examples c~f such molecules include but tire not limited to cotnpaunds,
antisense nucleic acids,
ribozyme nucleic acids, inhibitory ItNA, and antibodies, which are deserlbed
in greater detail
h~~~~.
Compounds
(40.31] Compounds can he obtained using any of the numerous approaches iz~
combinatorial
library methods la~own in the art, including biological libraries; peptoid
libraries (libraries of
molecules having the functionalities of peptides, but with a xtovel, hors-
peptide backbone which are
resistant to enzymatic degradation but which nevertheless remain biaactive
(see, e.g., 2uckexmann et
al., J. Med. Chem37: 2b78-SS (1994)); spatially addressable parallel solid
phase ox solution phase
libraries; synthetic library methods requiring decanvalution; "one-bead one-
compound" library
methods; and synthetic library methods using affinity chromatography
selection. Biological library
and peptoid library approaches are typically limited to peptide libraries,
while the other approaches~~
am applicable to peptide, non-peptide rrligomer ar small molecule libraries of
compounds (LJam,
Anticancer Drug Ides. 12: 145, ( 1997)). Examples of methods for synthesizing
molecular libraries
'ara described, far example, in DeWitt et al., Proc. Nod. Acad. Sci. U'.S.A.
9(1: 69CI9 (1993); Exb er
ai:, Froc. Natl. Acad. Sci. USA 91: 11422 (1994); 2;uckermaivi et al., J. Med.
Chem. 37: 267$
(l994); Cho et ad., Science 261: 1303 (1993); Carrell et aL, Angew. Gksem.
lot. Ed. )~~ngl. 33: 2059
(1994); Care!! er al., Angew. Ohem. Irrt. Ed. Engl. 33: 2061 (1994); and in
Gallop et aL, J. Med.
Cbem. 37: 1233 ( 1994).
~00~2] Libraries of compounds may be presented itl solution (e.g., Houghten,
Bioteahniques 1.3:
41.2-421 (1992)), or on beads (Lam, Nature 354: $2-$4 (19~9i)), chips (Fodor,
Nature 364: SSS-556
(1993)), bacteria ar spores (Ladner, United States Patent No. 5,223,409),
plasmids (Cuh er al., l'roc.
Natl. Acad. Sci. USA 89: 1865-1869 (1992)) or on phage (Scott and Smith,
Science 249: 3$6-390
11
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
(1994}; l~evlin, Science 249: JL04-4.t?~6 (19917): Cv~rirla ed al., Proc.
~atl. Aced. Sci. 87: 4378-6382
(1990}; Feiici, J. Mol. Blol. 2?2: 301-310 (1991}; l.~:dner supra.).
[4033] A compound sometimes modulates expression or activity of a palypeptides
and often is a
small molecule. Small molecules include, but are not litrated to, peptides,
peptidomimetics {e.g.,
peptoids}, amino acids, atnina acid analogs, pofynucleotides, polynucleatide
analogs, nucleotides,
nucleotide analogs, organic ar ina~rganic compounds (e.e., including
heceroarganic and
organometallic compounds} having a molecular w~lght less than about 14,000
grams per mole,
organic or inorganic cax~c~paunds having a molecular weight less than about
5,000 grams per mole,
organic ar inorganic compounds having a molecular weight less than about 1,000
grams prer mote,
organic or inorganic compounds having a molecular weight less Chart about 500
grams per male, and
salts, esters, and other pharmaceutically acceptable forms of such compounds.
10434] Fxamgles of'f'-type calcium channel small molecule antagonists and
methods for
determining their effect on calcium channel funetir~n are disclosed in CJ~.S.
patent application
publication no. fJ'S-2fl04-0034035-A1 published February 19, 2004; U.~. patent
application
publication no, us-2004-0044004-A1 published March 4, 2004; T.T.~. patent
application no.
10/763,9?4 filed January 22, 2004 and U.B. patent application tta. 6111474,$64
filed May 30, 2003.
Antisense. Ribazyme~R~Ai, silitVA and Modified Nucleic Acid Molecules
[11035] An "antise~nse" nucleic acid refers to a nucleotide sequence
complementary to a "sense"
nucleic acid encoding a palypeptide, e.g., complementary to the codartg strand
of a double-stranded
a'I~NA molecule or complerne~ntary to an mRNA sequence. 'fhe antisense nucleic
acid Cart be
complementary to an ex~kire coding strand, or to only a portion thereof. In
another embodiment, the
antisense nucleic acid molecule is ancisense to a "noncading zegiori' of the
coding strand of a
nucleotide sequence encoding the calciut:n channel ar AN'~ III protein.
[0031i] An antisense nucleic acid can be designed such that it is
complementary to the entire.
coding region, and often the antiset~se nucleic acid is an aligonucleatide
ant3sense to only a gartion
of a coding ar noneoding region of rnRNA. Far example, the antisense of
igoz~ucleatide can be
complementary to the region surrounding the translation start site of tnRNA,
e.g., between the -10
and f10 regioins of tt~e target gene nucleotide sequence of interest. An
antisense aligonucleatide can
be, for example, about 7, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, ~0, GS, 70,
75, $0, or mare
nucleotides in length. The antisense nucleic acids, which include the
rihozynnes described hereafter,
can kre designed to target calcium chanxxel a»d AhIX IF nucleic acids.
[Q0~3T] An antisense nucleic acid can he constructed using chemical synthesis
and enzymatic
ligatian reactions using standard procedures. k'or example, an antisense
nucleic acid {e.~ , an
antisense aliganucleatide} can be chemically synthesized using naturally
occurring nucleotides or
12
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
variously modified nucleotides designed to increase the biological stability
of the m~ol~ules yr to
increase the physics! stability of the duplex formed between the antisensE and
sense zlucleie acids,
e.g., phasphorathiaate derivatives and acridine substituted nucleotides can be
used. Antisense
nucleic acid else can be produced biologically using an expression vector into
whick~ a nucleic acid
has beon subcloned in an antisense orientation (i.e., RNA transcribed from the
inserted nucleic acid
will be of an antisense arAez~tation to a target nucleic acid of interest,
described further in the
following suf~section).
~fl038] When utilized as therapeutics, antisense nucleic acids often are
administered to a subject
(e.g.,1~y direct injection at a tissue site) err generated in situ such
tl~at,they hybridize with or bind to
cellulax rnltLTA andlor genornic DNA, encoding a polypeptide and thefeby
inhibit expression of the
palypeptide, for example, by inhibiting transcription a~ndlor translation.
Alternatively, anti~nse
nucleic acid molecules can be tnodifle~i to target selected cells and then are
administered
systemically. For systemic administration, antisense molecules can be modified
such that they
specifically bind to receptors or antigens expressed an a selected ce1!
surface, for e~arnple, by
linking antisense nucleic acid molecules tc~ peptides or antibodies which bind
to cell surface
receptors ar antigens. Antisense nucleic acid molecules can also be delivered
to cells using the
vectors described herein, Sufficient intraceliuiar concentrations of antisense
molecules are achieved
by incorporating a strong promoter, such as a poi 11 or poi III promoter, in
the vector construct.
m
[0039 Atttisensc nucleic acid molecules sometimes are alpha-auomeric nucleic
acid molecules.
An alpha-anameric nucleic acid molecule forms speeif a double-stranded hybrids
with
complementary RNA in which, contrary to the usual beta-units, the strands run
parallel to each Other
(Cxaultier et al., Nucleic Acids. Res.15; 6625-6641 ( 19$7)). Antisense
nucleic acid molecules can
also comprise a 2'-o-methylribantrcleotide (Inane et al., Nucleic Acids Res.
15: X131-6I4$ (19$7)7
or a chirneric ItNA-3~NA analogue (moue e~r al., FI~S ~,ett. X15: 327-33U
(1987)). A,ntisense
nucleic acids sometimes are composed of DNA or PNA or any other nucleic acid
derivatives
described previously.
[OiI~IU] In another embodiment, an antisense nucleic acid Is a ribazyxtae. A,
ribazyme having
speci~~city ~or a calcium channel or ANX III-encoding nucleic acid can include
one or more
sequences complementary to the nucleotide sequence of a calcium channel or ANX
Zli sequence,
and a sequence having a ltttawn catalytic region responsible for mRNA cleavage
(see e.,g., U.S. Pat.
No. 5,093,246 or Haselhoff and Oerlach, Nature 334: 5$5-591 (1988)). For
ercample, a derivative of
a TeGrahymena L-19 IVS RNA is sometimes utilized in which the nucleotide
sequeraie of the active
site is complementary to the nucleotide sequence to be cleaved in the target
rnRNA (see e,g., Gech et
ar. U.S. Patent 1~1'a. 4,987,071; and Leah er al. U.S. Patent No. 5,11G,74Z).
Also, target mRNA can
13
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
be eased to select a catalytic RNA having a specific ribanuclease activity
from x pawl of RNA
molecules see e; g., Barrel & Szostak, science 261: 14i i-1418 (1993)).
[t1Q41] Antagonists include in certain embodiments nucleic acids that can farm
triple helix
structures with a calcium chaxanel nncleotitle sequence, especially ore that
inoludas a regulatory
region that controls calcium channel expression. Caldittm channel gene
expressicm can be inhibited
1~y targeting nucleotide sequences complementary to the regulatory region c~f
the channel (e.g.,
promoter andlor erthancers) to form triple helical structures that prevent
transcription of the channel
gene in target cells (see e.g., Ilelene, Antioat~cer f7rug I?es. 6(6): 669-84
(1991); Helene at r~l., Ann.
N.Y. Aced. Sci. 6b0: 27-36 (1992); amdMaher, Bioassays 14(12): 807-i~ (1992).
Potential
sequences that can be targeted for triple helix formation can be increased by
creating a so-palled
"switehback"" nuoleic acid rnaleoule. Switchback nnalecules are synthesized in
an alternating S'-3',
3'-~' manner, such that they base pair with first one strand of a duplex and
then tl~e other,
eliminating the necessity for a sizeable stretch of either purines or
pyrimidines to be present on one
strand caf a duplex.
[Ot142~ Caloium channel antagonists also include RNAi and siRNA nucleic acids.
Gene
expression may be inhibited by the introduction of double-stranded 1~NA
(dsltNA), which induces
potent and specific gene silencing, a phenomenon called RNA interference nor
RNAi. See, e.g., Fire
et aL, US Patent Number G,5(IG,559; Tuschl et aL PCT >:nternatianal
Publication Na. WO 01175164;
day et rxL PCf International Publication Na. WO 03/0101 St7Al; or Basher dM,
Labduesse, Nat Cell
13io12000 Feb;2(2):831-6. This process has been improved by decreasing the
size of the dauble-
stranded RNA to 20-24 base pairs (to create small-interfering RNAs ar sil~NAs)
that "switched off"
genes in marnz3nalian cells without initiating an acute phase response, i. e.,
a host defense mechanism
that often results in cell death (see, e.g., Caplen et ett. Proc Natl Aced Sci
U ~ .A.. 2()01 Aug
14;98(17):9742-'7 and Elbashir et at. Methods 2bIl2 Fe6;26(Z):199-213). There
is increasing
evidence of past-transcriptianal gene silencing by RNA interference (I~NAi)
for inhibiting targeted
expression in mammalian cells at the mltNA level, in human cells. There is
additional evidence of
effective methods for inhibiting the proliferation and migration of tumax
Dells in human patients, and
far inhibiting metastatic cancer development (,see, e,g,, U.S. Patent
.Application No.
US2001000993183; Gaplen et al. Prat Natl Acad Sci U S A; and Ahderrahmani ~t
at. Mo1 Cell Bial
2,OOi Nov21(21):7256-67).
[01143 An ''siRNA'" or "RNAi" refers to a nucleic acid that forms a double
stranded )2.NA and
has the ability to reduce or inhibit expr~essian of a gene or target gene when
the siRNA is delivered
to or expressed in the same cell as the gene or target gene. "siRNA" refers to
short double-stranded
Rl'iA fanned by the complementary strands. Complementary portions of the
siltNA tltat hybridize
to form the double stranded molecule often have substantial or complete
identity to the target
14
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
molecule sequence. In one embodiment, azt siF~IriA refers to a nucleic acid
that has substantial or
complete identity to a target gene and farms a double stranded siRNA, such as
a calcium channel
encoding nucleotide sequence, far example.
j0044] When designing the siRNA molecules, the targeted region often; is
selected from a given
I1N'A sequence beginning 54 to lOty nucleotides downstream of the start codan.
See, e.g., Elbashir
et ai,. Methods 2b;199-213 (2002), hnitially, 5' or 3' L3TRs and regions
nearby the start cadan were
avoided assuming that UTR-binding proteins and/or translation initiation
complexes may interfere
with binding of the siRNP or RISC endr~nuclease complex_ Sometimes regions of
the target 23
nucleotides in length carifarming to the secluenee motif AA(N19)TT' (N, axe
nucleotide), and raglans
with approximately 309to to 70% G/C-content (often about 50% C'x!C-content)
often are selected. If
na suitable sequences are found, the search often is extended using the motif
NA(N21). The
sequence of the sense siRIVA sometimes corresponds to (N19) TT ar N21
(position 3 to 23 of the
23-nt motif), respectively. In the latter case, the 3' end of the sense siRNA
often is converted to TT. ,
The rationale for this sequence conversion is to generate a symmetric duplex
with respect to the
sequence composition of the sense and antisense 3' overhangs. 'T'he antisense
SiRNA is synthesized
as the complement to position 1. to 2I of the 23-nt motif because position 1.
of the 23-nt motif is
not recognized sequence-specifically by the antisense sirtNA, the 3'-mast
nucleotide residue of the
antisense siRNA can be chosen deliberately. However, the penultimate
nucleotide of the antisense
siltNA (complementary to position 2 of the 23,nt motif) often is complementary
to the targeted
sequence. For simpiifyi~ng chemical synttzesis, TT' often is utilized,
siRl'rTAs corresponding to the
target motif NAR(N17)YNN, where R is patina (A,Cr) and Y is pyrimidine (C,C.~,
often are selected.
Respective 21 nucleotide sense and antisense siRNAs often begin with a patina
nucleotide and can
also be expressed from pol IlI expression vectors without a change in
targeting site. Expression of
'RNAs from pal III promoters often is efficient when the first transcribed
nucleotide is a patina.
j004S] The sequence of the sil2NA can correspond to the full length target
gene, or a
subsequenee thereof: Often, the sit~NA is about 15 to about 50 nucleotides in
length (e.g., each
complementary sequence of the double stranded siRNA is 15-50 nucleotides in
lengtth, and the
double stranded siRNA is about I ~-50 base pairs in length, sometimes about 20-
30 nucleotides in
length or about 2(1-25 nucleotides in length., e.g., 2U, 21, 22, 23, 24, 25,
2~6, 27, 2$, 2q, or 30
nucleotides in length. ~'he siRhTA sometimes is about 21 nucleotides in
length. Methods of using
siRl~'~1. are well laxovcm in the axt, and specific siRNA molecules ~.y be
purchased from a number of
companies including Dhaxsnacon Rcseareh, Inc.
j0046] Antisense, ribazyme, RNAi and siRNA nucleic acids can be altered to
form modified
nucleic acid molecules. The nucleic acids can be altered at base moieties,
sugar moieties or
phosphate backbone moieties to improve stability, hybridization, ar salability
of the molecule. Far
1.5
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
example, the deoxyribose phosphate backbone of nucleic said nr~olec~tles can
be modified to
generate peptide nucleic acids (see Hyrup et at., Bioorganic & Medicinal
Chemistry 4 (1}: 5-23
(1996}). AS used herein, the farms "pegtide nucleic acid" ar ''PNA" refers to
a nucleic acid mimic
such as a DIVA n~inaxo, in which the deoxyribase phosphate bactcbane is
replaced by a pseudopeptide
Backbone and only the fou~c natural nucleaBases are retained. The neutral
Backbaae of a PICA can
allow far specific hybridization to DNA and RNA under conditions of law ionic
strength. Synthesis
of PNA otigotners can be performed using standard solid phase peptide
synthesis protocols as
described, for example, inn Hyrup et al., (1996) supr~x and'Perry-O'Kee~
etal., Prat. Nati. Aced. Sci.
93: 14470-675 (1996).
[4~D47j PNAs of nucleic acids can be used in pragnc~stic, diagnostic, and
tlzerapeatie applications.
For example, PNAs can be used as antisense or antlgene agents fQr sequence-
specific modulation of
gene expression by, for example, inducitlg transcription or translation arrest
or inhibiting replication.
>?lVAs of nucleic acid molecules can also be used in the analysis of single
base pair mutations in a
gene, (e.g., by PNA-directed 1'CR clamping); as "artificial restriction
enzymes" when used in
cornbinatian wikh other ez~yEnes, (e.g., S1 nucleases (Hyrup (t~9G} supra));
or as probes Qr primers
far DNA sequencing ar hybridization (Hyrup et at, (1996} supra; Pet~ry-
C1'Keefe supra}.
[U048j fn other embodiments, aliganucleotides may include other appended
groups such as
peptides (e.g., for targeting' host cell receptors in viva), or agents
facilitating transport across cell
membranes (see e.g., Letsinger et ul., Proc. Natl. Aced. Sci. USA 86:
6553~655b (19$9); T emaitre ei
al., F'xac. Natl. Aced. Sci. rl"SA. 84: 6~I8-652 (198'7); I~T P'ublicatian NQ.
WU881t?981U) or the
blood-brain barrier (sue, e.g., FCT' Publication No. W089f Ifl134). In
addition, oligonucleatides can
be modified with hybrldi~ation-triggered cleavage agents (see, e.g., Krol et
.al., Bio-.'T'echniques 6:
~5$-976 (1988)) ar intercalating agents. (See, e.g., Zan, Pharm, Res. 5: 539-
54~ (19$8) }. To this
end, the oligonucieotide may be conjugated t0 another molecule, (e.g., a
peptide, hybridization
triggered crass-linking agent, transport agent, or hybridization-triggered
cleavage agent).
Anti-Calcium Channel and A'NX in Antibodies
X0049] 'file term "antibody" as used herein refers t0 an imsmunoglobulin
molecule aF
immunologically active portion thereof, i.e., an antigen-binding portion.
Examples of
irtununologically active portions of irrimunaglobulin molecules include Flab)
and 1~(ab')2 fragments
which can 6e generated by treating the ~ntiBody with an enzyme such as pepsin.
An ~ntibvdy
sometimes is a palyclonal, monoclan,al, recombinant (e.g., a chimeric or
humanized}, fully human,
non-hurnan (e.g., marine), or a single chain antibody. A,n antibody rnay have
effeCtor fu;action and
can i'wx complement, and is sometimes caapled to a toxin ar imaging agent.
1G
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
[$050] A full-length calcium channel or ANX III polypeptide or antigenic
peptide fragment can
be used as an immunogezl or can be used to identify anti-calcium channel or
ANA III antibodies
made with other imnnunogens, e.g., cells, membrane preparations, and the like.
Arr antigenic peptide
often includes at least 8 amino acid residues of the target protein and
encompasses an epitape of the
protein. Antigenic peptides sometimes include 10 or more amino acids, 15 or
snore amino acids, ZO
ar mare amino acids, or 30 or more amino acids. Hydrophilic and hydrophobic
fragments of target
polypeptides sometimes are used as irnmunogens. Epitopes encompassed by the,
antigenic peptide
often are regit~ns located on the surface of tk~e polypeptide (e.g.,
hydrophilic regions) and regions
with high antigenioity. Por example, an Emini suxface probability analysis of
human polypeptide
sequences can be used to indicate the regions that have a particularly high
probability of being
localized to the surface of calcium channel and ANX ILI polypeptide and are
thus likely to constitute
surface residues u.~eful four targeting antibody production. The antibody may
bind an epitope on any
domain or region of calcium channel ox ANA !~ poiypeptides. An antibody can be
made by
immunizing with a purified calcium channel or ANX lII antigen, or a fragment
thereof, a membrane
associated antigen, tissues, e.g., crude tissue preparations, whole cells
(e.g., living cells), lysed cells,
or cell fractions.
[0051] Chimeric, humanized, and completely human antibodies are useful for
applications which
include repeated administration to subjects. Chimeric and humanized monoclonal
antibodies,
comprising both human and non-human portions, can be Wade using standard
recombinant DNA
techniques. Such chimeric and humanized monoclonal antibodies can be produced
by recombinant
DNA techniques known in the art, for example using methods described in
Robinson et a1
International Application hlo. PC'I'fI~'S86I02z~9; Akira, et al European
Patent Application 1$4,1.$7;
Taniguchi, M., European Patent Application 171,4~b; Morrison et al European
Patent Application
173,494; Neuberger et ai FC"T International Fublicatlan No. WO 8b/01533;
Cabilly et al U.S. Patent
No. 4,$15,57; Cabilly et aI European Patent Application 125,023; Better et
al., Science 240: 1041-
1043 (1988); Liu .et ad., F'roc_ Natl. Acad. Sci. USA $4: 3439-3443 (1987; Liu
et ai., r. Immunol.
139: 3521-3526 (19$7); Sun et tel" Froc:. Natl. Acad. Sci. USA 84: 214-218
(t987~; Nishimura et al.,
Canc. Res. 47: 999-1005 (1987; Wood et ad., Nature 314: 446-449 (19$5); az~d
Shaw et ul., Y. Natl.
CaneerTnst. $0: 1553-1559 (1988); Mon7son, S. L., Science 229: 1202-1207
(19$5); Cti eta~,
BivTechniques 4: 214 (198b); Winter I:I.S. Patent 5,225,539; Jones er al.,
Nature 321: 552-SZ5
(195; ~'erttoeyan et al_, Science 239: 1534; and Beidler et al., J. Imntunol.
141: 4053-405U (1988).
[OOS2) Completely human antibodies are desirable for therapeutic treatment of
human patients.
Such antibodies can be produced using transgenio mice that are incapable of
expressing endogenous
immunoglobulin heavy and light chains genes, but which can e~cpress human
heavy and light chain
genes. See, fox example, Lonberg and Huszar, Int. Rev. Ixnmuz~ol. 13: 65-93
(1995); and U.S. Patent
17
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Nas. 5,625,126; 5,633,425; 5,569,825; 5,661,016; and 5,545,800. In addition,
companies such as
Abgenix, hnc. (Fre~nont, CA) and Medarex, Inc. (F~rincetan, NJ}, can be
engaged to provide human
antibodies directed against a selected antigen using tecltnolagy similar to
that described above-
Campletely l~ur~n antibodies that recognize a selected epitape also can be
gexterated using a
technique referred to as "guided selection:' In !isle approach a selected non-
human ~nnanaclonal
antibody (e.g., a marine aatibady) is used to guide the selection of a
completely human antibody
recognizing the same epitape. This technology is described far example by
Jespers et al.,
»iofi'echnolagy 12: 899-903 (1994).
[U05~] Aa anti-calcium channel ar AblX rII antibody can be a sitxgle chain
antibody. A single
chain antibody (scFV) can be engineered (see, c_g., Calcher et rzl., Axon. N
~l Acad. Sci. 880: 263-80
(19990; and L;Geitsr, Clin. Catxcer P.es. 2: 245-52 (1996)). Single chain
antibodies can be dimerized
~ar multimerized to generate multivalent antibodies having speci:facities for
different epitapes of the
same target palypeptide.
[11054] Antibodies also may be selected ar nwdified so that they exhibit
reduced or no ability to
bind an Fc receptor. 1~ar example, an antilyady !nay be an isotype or subtype,
fragment ar ether
rnuta~at, which does not support binding to an Fc receptor (e.g., it has a
mutagenized ar deleted 1~c
receptor binding region). '
[~U05Sa Also, an antibody (ar fragment thereof) may be conjugated to a
therapeutic tzraiety such
as a c~rtatoxin, a therapeutic agent ar a radioactive mete! ion. A cytotoxin
ar cytotaxic agent
includes any agent that is detrimental to cells, Examples include taxol,
aytochalasin B, gramicidin
D, etltidium bromide, emetinc, mitomycix~, etopasirle, tenoposide,
vincristine, viublastine, calchicin,
daxarubicin, daunorubicin, dihydraxy anthracin diane, mitoxantrone,
mitlaz~mycin, acti~namycin D,
1 dehydratestostero~ne, glucocorticaids, procaine, tetxacaine, lidocaine,
propranolol, arid puramycin
and analogs ar homalags thereof. Therapeutic agents include, but are not
limited to, antimetabalites
(e.,~., raetborrexate, 6-mercaptapurine, 6-thiaguaniae, cytarabiae, 5-
fluorouracil ~leca3cbazine),
alkylating agents (e.g., tkaeGhlorethamine, thiotepa. chlorambucil, melphalan,
carmustine (BCNU}
and lamustine (~CNU), cyclophosphamide, busuifan, dibromornannitol,
streptazatacin, znitamycin
C, and cis-dichloradiamine platl~num (fl} (DDl'} cisplatia), anthracyclines
(e.g., dauaorubicin
(formerly ~daunomycin} and doxorubicin), antibiotics (e.~., dactinamycin
(formerly actinornycin),
bleort~ycin, mithramycin, and anthramyoi~n (AMC)}, and anti-mitotic agents
(e.g., vincristine and
vinblastine).
[01156] Antibody conjugates can be used far modifying a given biological
response. For
example, the drug moiety may be a protein ar palypeptide possessing a desired
biological activity.
Such proteins tray include, for example, a toxin such as abrin, ricin A,
Qseudomonas exataxia, ar
diphtheria t4xin; a polypeptide such as tumor necrosis factor, ~y-interferon,
a-interferon, nerve
1$
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
growth factor, platelet derived growth factor, tissue plasminogen activator;
or, biological response
modifiers such as, far example, lyxnphokines, interleukiz~-1 ("IL-1"),
interls;ukin-2 ("IIr~,"),
interieukin-6 ("IL,-b"), granulocyte macrophage colony stimulating factor ~"GM-
CSF'), granuiocyte
colony stimulating factor ("G-CSF'), or other growth factors. Also, an
antibody can be conjugated
to a second antibody to form an antibody heteroconjugate as described by Sega!
in U.S.1'atent No.
4,b76,980, for example.
[0057] An anti-calciunn channel ar AN3~ III antibody (e.g., monoclonal
antibody) can be used to
isolate calcium channel or AN'~ III polypeptides by standard techniques, such
as affinity
chromatography or immunaprecipitatian. An anti-calciutn~ channel or ANX rII
antibody can be used
to detect a calcium channel or AN'X III polypeptide (~.g., in a cellular
lysate or cell supernatant) to
evaluate the abundance and pattern of e~eprcssion of the polypeptide. Anti-
calcium channel or AIVX
III antibodies can be used diagnostically to monitor polypeptide levels in
tissue as part of a clinical
testing procedure, e. g., to determine the efficacy of a given treatmezrt
regimen. Detection can be
facilitated by coupling (i.e., physically linieing) the antibody to a
detectable substance (i.e., antibody
labeling). Examples of detectable substances include various enzymes,
prosthetic groups,
fluorescent materials, luminescent nnaterials, bioluminescent materials, and
radioactive materials.
Examples ol'suitable enzymes Include horseradish pem~cidase, alkaline
phosphakase, ~-
gaiactosidase, ar aeetylchviinesterase; examples of suita#~le prosthetic group
complexes include
streptavidinlbiatin and avidin/biotin; examples of suitable fluorescent
materials include
umbelliferane, ftuorescein, fluoreseein isothiocyanate, rhodamine,
dichlorotriazinylamine
fluarescein, dansyl chloride or phycoerythrin; an example of a luminescent
material includes
luminol; examples of bioluminescent materials include luciferase, luciferin,
and aequorin, and
examples of suitable radioactive material include ~~I, is~I, 35S or 3I~ or any
radioactive molecule
suitable for andlor used in in vaVa nuclear medicine proeedu~nes. Also, an
anti-calcium channel or
A1VX III antibody can be utilized as a test molecule for deternvning whether
it can txeat a cell
praliferative disordez~, and as a therapeutic for administration to a subject
for treat3x~g a cell
praliferative disorder.
r0(iS~] Included x~re antlbadies that bind only a native calcium channel
splice variant or ANX III
l~lyPePtide, only denatured or otherwise non-native calcium channel or A1V'X
III polypeptide, or
which bind both, as well as those having linear or canfarmaxional epitopes.
Conformational
epitopes sometimes can be identified by selecting antibodies that bind to
native but not deztatured
polypeptide.
19
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Methods For l7etermininQ a Disk of or i"resence of a Ceii FraliferativE
Disorder
[U059] Frovided herein are methods for detecting a risk of, ar the presence
of, a cell proliferative
disorder in a subject, which methods comprise determining whether an abnormal
patterns of
expression of Cav~.1 caleiutn channel m'f~TA, ar protein exists. l;xpressian
of a particular splice
variant can be determined by detecting the presence of m~t'A encoding the
splice variant andlor by
detecting the protein splice variant itself. As demonstrated below, cells that
undergo abnornnal
proliferation uniquely produce the splice variant Cav3.lae, and thus the
presence or absence of
expression of this splice variant is indicative of the propensity of the cei!
for proliferation. The
presence of this splice variant expression when found in the cells in a sample
from a subject
indicates that the subject is at risk for, or is afflicted with, a cell
praliferative disorder. Similarly, the
presence of Cav3.1 b splice variant is characteristic of hyperproliferative
cells and the presence of
this splice variant expression i;n cells of a sample derived from a subject
indicates that the subject is
at risk far or is afflicted with a cell pmliferative disorder. Also diagnostic
is the propartian of the
splice variant Ca~3.l bc, where the presence of more than about 2c~9~,
especially more than about
3096, in particular more than about 4~'0, 5~%, or 60%n of Cav3.lbc as a
percentage of the total
erav3.1 expression in the cells indicates the presence of a cell prolifetative
disorder. As noted,
expression levels tnay be measured either at the mRI~A level or the protein
level.
[Oi~60j The mRNA or protein is detected in a biological sample from the
subject. For example,
the biological sample may be bleed, saliva, sputum, urine, cell scrapings,
andlar biopsy tissue. The
tez~n "subject" as used herein refers primarily to a human but else refers tv
other mammals such as
aog$~ Cats, and ungulates (e.g., cattle, sheep, and swine). Subjects also
include avians (e.g., chickens
and turkeys), reptiles, and fish (e-g., salmon), as embodixments described
herein can be adapted to
nucleic acid samples isolated frarn any of these organisms. A nucleic acid,
protein or biological
sample may be isolated from the subject and then directly utilized in a method
for determining the
presence of a calcium channel splice variant associated with a cell
praliferative condition, or
alternatively, the sample may be isaiated and then stored (e.g., frozen) for a
period of time before
being subjected to analysis.
t0I161] The nucleotide sequence or polypeptide sequence detected sometimes is
substantially
identical to the nucleotide sequence ar amino acid sequence of the calcium
channel associated with a
cell proliferative condition. Jn some embodiments, the n~ucleatirle sequence
or pc~lypeptide sequence
detected is substantially identical to a Ca~3.iao calcium cba~nnel or Cav3.lb
calcium channel
encoding nucleotide sequence ar polypeptide sequence, as allelic variants may
occur- The sequence
detected at ti~t7,es is $0%a or snare, 81 % or znore...85% ar more... $9% Qr
rnore, or 9Q% tar more
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
identical to $ Cav3.lac calcium channel or Cav3.lb calcium channel encoding
nucleotide sequence
ar palypeptide sequence, and often is 91~'o ar more, 92% or more...9a% or
mare...9'~~ ar more,
98% or more, or 999'o ar more identical to a Ca,,3.lac calcium channel or
Cav3.lb calcium channel
encoding nucleotide sequancc or poIypeptide sequence. Also, an intracellular
loop region bet~coveer~
conserved transmetnbrane zegions, such as an intracellular it~og between
region I and iZ, between II
and fEl or between lIi and N in a detected nucleotide sequence or pcrlypeptide
sequence sametinnes
bears less sequence identity to a corresponding loop in a Cav3.lac calcium
channel Qr Cav3.lb
calcium channel as compared to the rest of the sequence. In such loop regions,
the detected
nucleotide sequence ar palypeptide sequence st~metimes bears SO% ar mare,
51%'0 or more...,fiU% or
rnare...70% or rnare...8Q9O car more...90%Q ar rnore...9~% ar more_ ..97% ar
more, 98% or more or
999'0 or snore sequence identity to a corresponding loop sequence in a Cav3.1
ac calcium channel ar
Cav3.I~a calcium channel.
(0(rf2] Any suitable method for detecting the Ca,,~.1 calcium channel splice
variant nucleotide
Sequence or amino acid sequence is utilised. Far examrple, irt a process far
detecting a specific
Ca"3.1 calcium chatmel splice variant protein, a biological sample from a
subject sometimes is
processed (e.g" processed to disrupt cell membranes) and then contacted with
an antibody that
specifically binds to the particular Cav3.1 calcium channel splice variant
being detected (e.g., a
Cav3.lac or Cav3.1 b caiciutn channtel splice variant). A variety of methods
are knov~n f~,r detecting
floe presence or absence of a particular Cav3.1 calcium channel splice variant
nucleotide sequence
assaciaced with a cell praliferative condition, v~rhich include the ti.T-PCIZ,
techniques descriherl
hem,.after.
(~lHS3j xl= it is determined that a subject is at risk of a particular cell
praliferative disorder, such as
one described previously, the risk may be expressed in any lazown and useful
man~rter. Far example,
risk of a cell pmliferative disorder sorrtetimes is expressed as a
probability, such as an odds ratio,
percentage. or risk factor, The risk assessment is based upon the presence or
absence c~f a Ca"~.1
calcium channel nucleotide sequence or polypeptide associated with ttie cell
proliferative condition
(e.~,, x Ca"3.lac or Cav3.lh calcium channel splice variant), and also may be
teased in part upon
phenotypic traits of the individual being tested. Ivlethads for calculating
risks based upon patient
data ate known (see, ~.,~., Agresti, Categorical Data Analysis, 2nd ~d. 2Q02.
Wiley).
j00fr4] I~rocesses for identifying a nucleotide sequence encoding a Cay3.I
calciurct chanr~ei splice
variant associated with a sell praliferative disorder or the encoded protein
in a sample from the
21
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
subject sametitx~s is combined with other infarcnatiort ar processes.
1~ombining with other
information or frrocesses often enhances the abilit~r of a health care
provider to diagnose ar treat the
cell pxoliferative disorder. Other information sotnetitxces is the presence or
absence of another
calcium cltannei splice variant nucleotide sequence or palypeptide in a sample
frarn the subject;
phenotypic information pertaining to the subject (e.g., family history of a
cell proliferative disorder
and personal history of a teal proliferative disorders); andlor information
from a further process far
diagnosing a cell proliferative disorder, For example, another process for
diagrnasing a cell
proliferative disorder sometimes is detecting tha presence or absence of
increased eel! protiferatian
within a subject ittentified as having the presence of a nucleotide sequence
that encodes a Ca~3.1
calcium channel or the presence of a CaY3.I calcium channel protein assaoiated
with a cell
pxaliferative disorder. The presence or absence of increased cell
praliferatian sametitnes is detected
in a tissue biopsy from the su6jeet. In other embodiments, the presence 4r
absence of increased. cell
proliferation is detected in viva, such as by nuclear medicine procedures, far
example.
[~fh~] In ether embodiments. the processes described above satnetimes are
combined with a
further process far treating the cell proliferative disorder. Any treatment of
the cell praliferative
disorder can be adr~aenisterec~ to the subject- A general rJtethad for
treating a cell praliferative
disorder sometimes is prescribed, such as administering a chemotherapy or
radiation therapy
regimen to the subject that reduces cell pratifaratian. l4lethads that
specifically target tha cell
praliferative condition also may he prescribed, such as removing one ar more
tumors frarn the
subject in surgery, andlar treatment with an antagonist of a specific
rr~alecule that causes the cell
proliferative disorder. Compositions and methods for treating cell
proliferative disorders by
antagonizing a T-type calcium chaxmet (e.,g" a Ca"3.1 cai~cium channel splice
variant associated with
a cell proliferative disorder) are described in greater detail hereafter.
~,ethods to Inhibit Cellular Proliferatiari
[01156] Cellular pxaliferation may be inhibited itt cells that express
abnormal patterns of Ca~,~.i
calcium channel splice variants using a number of techniques. As noted above,
the abnormal pattern
ctzay reflect itself in the ratio of the various splice variants or the
presence of Cav3.lb or the presence
of Cav3.lac.
[b067] In orte approach, bioclcers of T-kype calcium channels rnay be used;
however, a more
nuartced approach focuses an the T-type channels that are abnormally expressed
- e.g., Ca,.3, i bt
Cav3.lac, and Cav3.lbc, rnhibltion of either expression or activity of these
chatanels or both t~.y be
effective. Expression of these splice variants rnay be negatively affected
using, for example,
2~
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
antisense oligonucleotides, inb;ibitory lx,~TA, ar ribozyrnes, all pf which
are designed tp be speck
for these sQlice variants to be targeted. Alternatively, specific antagonists,
such as antibodies that
are direceed toward the splice variant in question are useful. In the ease c~f
Ca"3.lac, inhibitors of its
ixtteractian with the ANX III protein or substances that bind specifically to
AhTX ~ protein may also
be used.
[006$] As dempttstrated below, the spline variants differ in the tinker
sequence between
regions III attd TV' of the ai protein characteristic of Cav3.I calcium
chanrteIs (see Pig. 2). Thus,
ribo~ymes, inhibitory RIBA, and antisense ~quences targeted to the mRl'fA
zegion specific for the
sequences characteristic of the undesired splice variant in t,~cis raglan rnay
be used, as wail as
antibodies specifically itnmunoreactive with the protein encoded in this
region.
[0069] As further described below, these approaches to inhibiting the
ptnliferation of abnormally
proliferating cells e~.hibiting abnormal ~a"3.1 splice variant exptesssian can
be applied to cells that
exist in a subject as well as cells in cultetre or biopsy.
Cell proliferative Treatment Cannposit~rws and Methods
[007(1] Provided are zr~thods for treating a cell pr4liferative disorder
characterized by expression
of a Ca"3.1 calcinrn channel splice variant assaciatcd with the cell
praliferative disorder (e.g.,
Cav3. tar andlor Cav3.Ib and/or Ca"3.lbc~, which cpmprise administering a T-
type calcium channel
bfocker to a subject in need thereof in an atrtount effective to treat the
oell proliferative disorder. An
antagonist molecule specific far a Cav3. t calcium channel splice variant
associated with the cell
pratife~'ative disorder (e.g., Ca"3.lac andic~r Ca"3,1b andlor Cav3.lbc) is
preferred, or a rnatecerl$
that inhibits the interaction between AbTX IIi with a. Ca"3. t calcium channel
associated with the cell
proliferative disorder (e.,g., Cav3.lac), t4 a subject in need thereof in an
amount effective to treat the
cell praliferative disorder.
[00?1] In addition, expression of undesired splice variants n toy be inhibited
by an antisense,
ribo~yme, Rt~FAi, siIthTA, or triple helix-forming nucleic acid. Activity may
be inhibited by an
antibody ax compound small molecule type. The antagonist sometis blocks the
calcium channel
and sometimes interferes with and inhibits an interaction between a Cav3.1
calcium channel splice
variant and Ahl'X III (e.g., inhibiting biztdirig l,~etween a Cav3.1 aalciurn
channel splice variant and
AhTX TII), Inhibition of binding between a Ca"3.1 calcium channet splice
variant and AbIX zIf
sometitr~s is effected by binding of the antagpnist to the calcium, channel,
to ANX IZI or to a
complex fornned by the calcium channel and ANX ItI. In some embodiments, a
nnolecule
~3
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
administered is a compound described in U.S. patent application publication
no. US-20Q4-0034~35-
l ,
A1 published February 19, 2t7Q4; U.$. patent apglicatun publication no. U5-
2(14-00~4004~A1
published March 4, 2004; U.S. patent application no. 101763,974 filed Ianuary
22, 2004 and U.S.
patent application no. ~01474,~64 filed May 30, x.003. In other embodiments, a
rnafecuta
administered is art antibody that specifically binds to a Ca"3.1 aatcium
charuael associated with a cell
proliferative disorder (~ ~., Ca"3..t ac andior Cav3.l b), ar an antibody that
specifically binds to .ANA
lII. An antiba~ly that binds to a calcium channel often intribits or blocks
the function of the
molecule andlor inhibits the interaction between the calcium channel and A1VX
III. An antibody that
binds to ANX III often inhibits tire interaction between tfre calcium channel
and ANX III.
j0U72~ The arttaganist often is formulated as a pharmaceutical cant~rsition
with a
pharmaceutically ~acceptat~(e carrier. As used herein, the terrrt
"pharmaceutically acceptat~le carrier"
inc;;adc.s sofveras, ulnpesmiun u~dia, coatings, antibacterial and antifungat
agents, isotonic and
absorption delaying agents, and the like, compatible with pharmaceutical
adrrrinistratian.
Supplementary active compounds sometimes are incorporated into the
compositions.
[0073] A phsrfnaceutical composition is formulated to be compatible with its
intended route of
administration. Examples of routes of administration include parenteral, ~.g.,
intravenous,
intradenxraf, sut~utanenus, oral (~.g., inhalation), transdermal (topical),
transmucosal, and rectal
adrrtinistratian. Solutions or suspensions used for patr~nteral, intradern~al,
or subcutaneous
apglicatian can include the following eaxrarponents: a sterile difuent such as
water far injection, saline
solution, f red ails, polyethylene glycals, glycerin, propylene glycol or
other synthetic solvents;
antibacterial agents such as benzyl alcohol or methyl parabens; antioxidants
such as ascorbic acid ar
sodium bisulfate; chelating agents such as ethylcnediarrtinetetraacetic acid;
buffers such as acetates.
citrates or phosphates and agents for the adjustment of tonicity such as
sodium chloride ar dextrose.
gI~ can be adjusted with acids or bases, such as hydrtichlaric acrd or sodium
hydroxide. The
Qarenteral preparation can be enclosed in ampoules, disposable syringes ar
multiple dose vials made
of glass ar plastic.
~0474J t,~ra1 catrrpasitians generally include an inert diluent ar an edible
carrier. 1~or the ptupc~se
of oral ttrerapeutic administration, the active compound can be incorporated
with excipieiats and
used in the farm of tablets, troches, or capsules, e.g., gelatin capsules.
Oral compositions can else
be preparred using a fluid carrier for use as a rrroutlewasft,
k'harrrAaceutically compatible binding
agents, andlar adjuvant materials can be included as part of the composition.
The tablets, hills,
capsules, r~roches and the Like cart contain any of the following ingredients,
ar eompourxds of a
similar nature: a binder such as micracrystalline cellulose, gum tragacantll
or gelatin; an excipient
such as starch or lacdyse, a disintegrating agent such as alginic acid,
Primogel, or com starch; a
24
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
lubricant such as magnesium stearate or Sterotss; a glidant such as colloidal
siliean dioxide; a
sweetening agent such as sucrose or saccharin; or a Elevating agent such as
peppermint, nnethyl
salicylate, ar orarfge flavoring.
[OO~Sj Pheutical comgasitions suitable fdr inject~ble use include sterile
aqueous solutions
(where warEr soluble) or dispersions and sterile powders for the
exGerx~paraneaus preparation of
sterile injectabla solutions or dispersion. For intravenous administration,
suitable carriers include
physiological saline, baeteriostatic water, Cremophor FL'rM (I~ASF,
Parsippany, lad) or phosphate
buffered saline (PAS). In alt cases, the camposidan must be sterile and should
be fluid to the extent
that easy syringability exists. It should be stable under tile conditzans of
manufacture and starags
and must be pzeserved against the contaminating action of microorganisms such.
as bacteria and
fungi. The carrier can be a solvexit or dispersion r»edium ac~ntaining, far
example, water, ethanol,
polyol (for e~cample, glycerol, propylene glycol, and liquid galyethylene
glycol, and the like), and
suitable nxixtures there Proper fluidity can be maintained. for example, by
the use of a coating
such as lecithin, by the maintenance of the required particle size in the case
of dispersion and by the
use of surfactants. Prevention of the action of micrat~rganisms can be
achieved by various
antibacterial and antifungai agents, far example, parabens, Chloxabutanol,
phenol, ascorbic acid,
thimsrosal, and the like. In many cases, it is preferable to include isotonic
agents, for exarnpie,
sugars, polyslcahols such as mattnital, sarbitol, sodium chloride in the
campositic~n" Prc~tanged
absorption of the injectahle compositions can be brought about by including in
the composition an
ag$nt which delays absorption, for example, alun~inunrt moriostearate and
gelatin.
[007~~ Sterile injectable solutions can be prepared by incorporating the
active compound in the
required amount in an appropriate solvent with one or a cambinatian of
ingredients described shave,
as required, fallawed by filtered sterilization_ Generally, dispersions are
prepared by incorporating
the active compound into a sterile Vehicle which contains a basic dispersion
medium and the
required ether ingredian~ from those described. In the case of sterile powders
for the preparation of
stirrile injectable solutions, the methods of preparation often utili2ed a~
vacuum drying and freexe-
drying which yieids a powder of the active ingredient plus any additional
desired ingredient from a
previausIy steriIe~filtered solution thereof.
[Oif~'T.i Far administration by inhalstion, the compounds are delivered in the
form of err aerosol
spray from pressured container ar dispenser which contains a suitable
propellant, e.g., a gas such as
carbon dioxide, ar a nebulizer. Systemic administration can else be by
transmucosal or transdermaI
rneans. Far trazzsmucosal or transderntal administration, penetrants
appropriate to the barrier to be
petymeated are used in tile formuiation_ Such penet~rants aro generally Known
in the art, and include,
far example, far transmucasal administration, detergents, bile salts, and
fusidic acid derivatives.
Transmucosal administration can be accomplished through the use of nasal
sprays ar suppositories.
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
For transdermal administration, the active compounds are formulated into
ointrraents, salves, gels, ar
creates (e.g., sunscreen) as generally known in the art. N~ol~cules can also
be prepared in the farm
of suppositories (e.g., with conventional suppository bases such as cocoa
butter and ether
glyceridas) or retention srtemas far rectal delivery.
[Df1?g] Itt one embodir~aent, active molecules are prepared vrith carriers
that will protect the
campCVnd against tepid elimination Pram the body, such as a controlled release
formulation,
including implants and tnicroencapsulated delivery systems, biodegradable,
laiacornpatible
polymers can he used., such as ethylene vinyl acetate, polyanhydrides,
polyglycolic acid, cc~~iagen,
polyarthaesters, and polylactie acid. Methods for preparation of such
forrr~ulations will lee apparent
to those skilled a the art. Materials can also lx; obtained camrnercially from
Alza Corpo~xatian anal
Nova Pharmaceuticals, hoc. Liposamal suspensions (including liposames targeted
to infected cells
with znanoclonal antibodies to viral antigens) can also be used as
pharzna~ceutically acceptable
carriers. These cart be prepared according to methods known to those skilled
in. the art, far example,
as descrilaed in U.S. l~atettl hTo, 4,~22,$i i.
[0079] It is advantageous zo formulate oral or parenteral compositions in
dosage unit form for
ease of administration and uniformity of dosage. Dosage unit faun as used
herein refers to
physically discrete units suited as unitary dosages for the subject to be
treated, where each unit
aantains a predetermined quantity of active compound calculated to produce the
desh~d therapeutic
effect in association with the required pharmaceutical carrier,
[OU80] Toxicity and therapeutic efficacy of molecules and formulations can be
determined dy
standard phartr~ceuticxl procedures in cell cultures Qr experimental animals
in which ~,Dso values
(the dose lethal to 50'0 of the poputation) and Eb~, values (the dose
therapeutically effective in 5!~
pf the population) sometirnss era determined. The dose ratio between toxic
arid therapeutic effects
is the therapeutic index and it can be expressed as ttwe ratio ~.D~~D~.
MoleGUles which exhibit
high therapeutic indices often are utilized. 'While molecules that exhibit
toxic side effects may be
used, care should be taken to design a delivery systettt that targets such
compounds to the site of
affected tissue for minimizing potential clamagr~ to uninfected cells and
reducing side effects.
[OOgI,3 Data attained Pram cell culture assays and animal studies can be used
irt.formuIating a
range 4f dosages far use in humans. A dosage of such molecules Lies ~ferably
within a range of
circulating coneer~tratinns that include the ):DSO ~vitla little or na
toxicity. The dosage may vary
within this range depending upon the dosage form employed and the route of
administration utilized.
Par any mt~lecules used in the method, the therapeutically effective dose can
be estimated initially
from cell culture assays. .A dace may be.forn~ulated in animal models to
achieve a circulating
plasma concentration range that includes the ICSO (i.e., the concentration of
the test compound which
achieves a half maxixral inhibition of sytnptams) as determined in cell
culture. Such ittfarrraation
2B
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
can be used to rn~are accurately determine useful doses in humans. Levels in
plasma naay be
measured, far example, by high performance liquid ehromatography_
@452] A therapeukically effective amount of protein ar polypeptide (i.e., an
effective dosage)
ranges from abaat U.001 to 3p ~/kg body weight, sometimes about 0.0 t to 25
mglkg body weight,
often about Q.1 to 20 tnglkg body weight, and more often about 1 to lU mglkg,
2 to 9~ mglkg, 3 to 8
mgtkg, 4 to 7 mglkg, or 5 to ~r mglkg body weight. The prc~tain or polypeptide
can be administered
one tune per week for between about I to 10 weeks, sometimes between 2 to S
weeks, often between
about 3 to 7 weeks, and more often for about ~l, 5, or 6 weeks. The skilled
artisan will xpprecia~
that certain factors may influence the dQSage and tuning reguired to
effectively treat a subject,
including but not limited to the severity of the disease or disorder, previous
treatments, the general
health audlor age of the subject, and other diseases present. Moreover,
treatment of a subject with a
therapeutically effective amount of a protein, palypeptide, ar antibody cats
include a single treatment
or can include a series of treatments.
[OQ85] Iu fotrnulation ambodirnents comprising an antibody, a dosage of 0.1
rngrlc,8 of body
weight (,generally 10 mglkg to 20 mg/kg) is often utilized. If the antibody is
to act in the brain, a
dosage of 50 mglkg to 1 t» mgtkg often is appropriate. Generally, partially
human antibodies and
fully human antibodies have a Longer half life within the human body than
other antibodies.
Accordingly, lower dosages and less frerluent adnninistration is often
possible. Modifications such
as lipidatiatt can be used to stabilize antibodies and to enhance uptake and
tissue penetration (e,g.,
into the brain). .,4 method far lipidatian of antibodies is desciabed by
Cruikslaank et al., 1. Acquired
Immune Deficiency Syndxames and.I3uman Retravirolagy I4: X~3 (197).
00084] Antibody conjugates can be used for modifying a given biological
response, and the drug
moiety is not to.be construed as limited to classical chemical therapeutic
agents. For example, the
drug moiety may be a protein or polypeptide possessing a desired biological
activity. Such proteins
may include, far example, a toxin such as abrin, ricin A, pseudarnonas
exotoxin, ar diphtheria toxin;
a polypeptide such as tumor necrosis factor, .alpha.-.interferon, .beta.-
interferon, nerve growth factor,
platelet derived growth factor, tissue plasminogen activator; ar, biological
response rrtodifiers sucte
as, for example, lymphokines, interleukin-1 ("Il;-1»}, interleukin-2 ("Ih-2"),
interleukin-~ ("IL-f"),
granulocyte macrophage colony stimulat4~ng factor ("GM-CS)~''~, granulocyte
colony stimulating
factor ("CS-CSF'~, or other growth factors. Alternatively, an antibody can be
conjugated to a se,.cond
antibody to form an antibody heteroconj~tgate as descKibed in rJ.S. Patent No.
A.,b7b,g80.
[d055] Far compounds, exemplary doses include milligram or microgram amounts
of the
compound per kilogram of subject or sarr~ple weight, for example, about i
nrricrograsn per kilogram
to about 5011 m~illigraens per l~ilogram, about I00 micrograms per kilogram to
about 5 milligrams per
kilagraFn, or about 1 microgram per kilogram to about 51~ micrograms per
kilogram. It is understood
z~
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
that appropriate doses of a small molecule depend upon the potency of the
small molecule with
respect to the expression or activity to be modulated. When one or more of
these small molecules is
to be administered to an animal (e.g., a human) in order to modulate
expression or activity of a
polypeptide or nucleic acid, a physician, veterinarian, or researcher may, for
example, prescribe a
relatively low dose at first, subsequently increasing the dose until an
appropriate response is
obtained. In addition, it is understood that the specific dose level for any
particular animal subject
will depend upon a variety of factors including the activity of the specific
compound employed, the
age, body weight, general health, gender, and diet of the subject, the time of
administration, the
route of administration, the rate of excretion, any drug combination, and the
degree of expression or
activity to be modulated.
[0086] Pharmaceutical compositions of active ingredients can be administered
by any of the
paths described herein for therapeutic and prophylactic methods for treating a
cell proliferative
disorder. With regard to both prophylactic and therapeutic methods of
treatment, such treatments
may be specifically tailored or modified, based on knowledge obtained from
pharmacogenomic
analyses. As used herein, the term "treatment" is defined as the application
or administration of a
therapeutic agent to a patient, or application or administration of a
therapeutic molecule to an
isolated tissue or cell line from a patient, who has a disease, a symptom of
disease or a
predisposition toward a disease, with the purpose to cure, heal, alleviate,
relieve, alter, remedy,
ameliorate, improve or affect the disease, the symptoms of disease or the
predisposition toward the
disease.
Screening Methods
[0087] Provided herein are methods for identifying a molecule that inhibits
cell proliferation,
which comprise contacting one or more cells comprising a Ca~3.1 calcium
channel encoding nucleic
acid and/or a Ca~3.1 calcium channel polypeptide with a test molecule, and
determining whether the
test molecule decreases cell proliferation, whereby a test molecule that
decreases cell proliferation is
identified as a molecule that inhibits cell proliferation. The Ca~3.1 calcium
channel utilized in the
process is associated with a cell proliferative disorder, and sometimes is a
Ca~3.lac and/or Ca~3.lb
and/or an excess of CaV3.lbc calcium channel splice variant. Thus, in one form
of these assays,
compounds may be evaluated by their effects on expression of splice variants
characteristic of
abnormal cellular proliferation or by their ability to inhibit the activity of
these splice variants in
transport of calcium ion. Also provided are methods of screening for a
molecule that inhibits the
interaction between Cav3.lac and ANX III. In one embodiment, this method
comprises detecting
28
SUBSTITUTE SHEET (RULE 26)
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
the 1ev$I of interaction of the calcium channel splice variant (lac) and AN.~
~I protein the presence
arid absence of the substance to be tested. A decrease in the interaction in
the presence as compared
to~ the absence of the test substance indicates that the test substance is a
successful candidate
molecule for treatment of cell proliferative disorders and fox inhibiting,
cell proliferation generally.
In lieu of using the splice variant ,Fer se, a poiypeptide representative of
the splice variant, i.e., that
encoded by the diFferentiatin~ portion c~f the rnRN,A encoding the 1~ N
linlaer, can be used. As
hated in .Figure ~, the sequence SKI;xCt7MAD,per.se is unique to Ca,,3.lac and
the extended faun of
this segmept into exan 2~5 includes sequences shared with the abnormally hi
ftxly expressed
Ca,,3.lbc. xn one spec:ifie embodiment, rlxe method comprises: (a) incubating
a Cav3. lac
palypeptide or substantially identical polypeptide thereof with a test
molecule under conditions
suf~tcie~at to pernnit binding between the polypeptide and the test molecule
in a reaction mixture, (b)
contacting AlrtJ~ 'flI with the reaction mixture under conditions sufficient
to permit binding betwc~n
the polypeptide and Abl'X III, and (e) detecting the presence or absence of
decreased binding
between the palypeptide and AI~1'.~~ IfI, whereby the presence of decreased
binding between the
polypeptide and ANA IlI identifies the test molecule as a nnolecule that
inhibits the interaction
between t~aV3.lac and ANA III. In such methods, the CaY3.lac polypeptide
soznetinxes camprisEs
?5 ar more sequential anxino acids selected from a region spanning amino acid
1545 to amino acid
1570 of a Ca,~3.I ac T-type calcium channel, and in certain embodiments, the
poiypeptide consists of
the amint~ acid sequence S1~EKC~MAI7LMLI7I~VL4SGSSASAAS.
[t108$a A reaction mixture or system soexietimes is a cell free in vitro
environment and sometimes
is a celi~based environment such as a collection of cells, a tissue, as organ,
or an organism. A
system is "contacted" with a test molecule in a variety of manners, including
adding' rnalecules in
solution and allowing theta to interact with one anotlt~er by diffusion, cell
in3ection, and any
administration routes in an animal. As used herein, the terns "interaction"
refers to an effect of a test
molecule on a calcium channel nucleic acid or paiygeptide, At~t~ III nucleic
acid or palypeptide~ ar
complex between a calcir~m channel and ANX III, svltere the effect is
sometimes binding between
the test malececle and the nucleic acid or polypeptide, and sometiKnes is an
observable change in
cells, tissue, or an organism.
~OtlB~a Any rnethcxl far determining whether a calcium channel is inhibited or
blocked can be
utilized, and exarnpies of such processes are described in U.S, patent
application publication na. US-
2004-0034035-A1 published February 19, ~OU4; LT.S. patent application
publication rta. US-2.t70~-
004hOC14-A 1 published lVlarch 4, 2(IQ4; I3.S. patent application na.
101763,9"74 filed January ~2,
2Cx?4 and U.S. fratent application no. 60!474,864 filed May 30, 203. In an
embodiment, a standard
patch clamp technique is etnplayed td identify blockers of'I'-type calcium
channel curFenrs. Briefly,
2~
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
HEK cell tines stably expressing a human a1G T-type channel are used for
recordings (passage #: Q.
20, 37"C, 5°lu COQ. To obtain T type currents, plastic dishes
containing semi confluent cells axe
positioned on the stage of a ZEhSS A~rUVERT 5100 microscope after replacing
the cntture
rxxedium with external solution. Whale cell patches are obtained using
pipettes (borosilicate glass
with filament, tJ.D.: 1.5 mm, LD.: 0.86 mm, 10 cm length), fabricated on a
SLITTER F 97 pullet
with resistance values of ~S Nls2. increases or decreased in currents ors
detected, to determine
whether a test nnolecuIe modulates the si,~nal of a T-type calcium channel.
Examples of other aSSays
are described in Example 4 hereafter.
[It090) Any other z~nethod for determining whether the test molecule
interaucts with a calcium
channel or for determining whether an interaction between a ealciucn channel
splice variant and
ANA III is inhibited can be utilized, Examples of suoh methods include, for
example, titrametric,
acidirnetric, radiometric, NMft" txeonolayer, polarographic,
spectrophotvarretric, fEuoresaent, and
ESR assays. Specific embodiments include fluorescence resonance energy
transfer 1:FRE'T) assays,
surface plasmon resonance assays, and certain heterogeneous assays.
[OU91] In FRET assay embodirr~nts, a fluorvphore label on a first, "donor"
molecule (s.g., the
calcium channel) is selected such that its emitted fluorescent energy is
absorbed by a fluorescent
label an a second, "acceptor" molecule (e.~., ANX IIIy, which in turn is able
to fluoresce due to the
absorbed energy frvrn the donor (e.g., ):,afcvwiez er al., U.S. P'atent No,
5,631,169; Stavrianopoulos
et cal_ I3.S. Patent No. 4,868, lU3). Alternately, a donor of a non
derivatized palypsptide may be
natural fluorescence energy of tryptoph~an residues. When Iabels are utilized,
they are chosen to
emit a different wa~eleztgth of light, such that the acceptor label may be
differentiated from that of
the donor. Since the efficien~ey of energy transfer between the labels is
related to the distance
separating the molecules, the spatial relationship between the molecules can
i~ assessed. In a
situation in which 'binding occurs between the molecules, the fluorescence
emission of the
°'acceptox" rnalecule label in the assay should be maximal. ,A h~ET
>~inding event can be
conveniently measured by standard fluvrometrac detectors.
[092] rn surface plastnon resonance assay embodiments, biospeCific
interactions are detected in
real time without labeling any of the ,interactants with detectable chemicals
(e.g., Sjvlander &
TJrbarwic2k, .A,nal. them. 63: 233$-2345 ( 1991) and S2abo et at., Ctur. Opin.
Struct, Bial. 5: 8~9~-705
(1995)). Changes in mass at the binding surface, which axe indicative of
binding events, result in
aiteratians ~f the reftactlve index of light near the surface (the optical
phenvmentsn of surface
plasmon resonance (SFR)), which is a detectable signal used to monitor real-
time interactions
between biological mQleeules. This type of assay sometimes is referred to as
biomolecular
interaction analysis (BIA). In such assays, the calcium channel splice variant
protein or ANA ILI
protein sometimes i$ IinlcEd to a solid support surface and the effect of a
test molecule an the binding
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
of the other added binding partner (e.g., the added binding partner is ANX III
where the calcium
channel is linked to the solid support) is determined by detecting changes in
SPR.
[0093] In other embodiments, the calcium channel, ANX III or test molecule is
anchored to a
solid surface in a heterogeneous assay. The target calcium channel or ANX III
molecule often is
anchored to a solid surface, and the non-anchored molecule sometimes is
directly labeled and
sometimes is indirectly labeled. The anchored molecule may be linked to any
suitable solid surface,
examples of which include a surface of a microtiter plate, test tube, micro-
centrifuge tube or silicon
chip. In certain embodiments, the molecule to be anchored may be produced by
recombinant
processes as a product that includes a contiguous and heterologous polypeptide
region, where the
heterologous region is capable of binding to a molecule linked to a solid
surface. For example, a
calcium chamiel splice variant polypeptide or ANX III polypeptide may be fused
to glutathione-S-
transferase and then adsorbed onto glutathione sepharose beads (Sigma
Chemical, St. Louis, MO) or
glutathione-derivatized microtiter plates. The molecule to be conjugated to
the solid surface (e.g., a
calcium channel splice variant) may be linked before, during or after the
other molecules (e.g., ANX
III and/or a test molecule) are added to the system, which are combined under
conditions conducive
to complex formation (e.g., at physiological conditions for salt and pH).
Other techniques for
immobilizing a calcium channel or ANX III molecule on a solid support include
using biotin and
streptavidin. For example, a biotinylated calcium channel or ANX III
polypeptide can be prepared
from biotin-NHS (N-hydroxy-succinimide) using known techniques (e.g.,
biotinylation kit, Pierce
Chemicals, Rockford, IL), and immobilized in wells of a streptavidin-coated
microtiter plate (Pierce
Chemical).
[0094] In heterogeneous assay embodiments, one or more non-immobilized
components are
added to the coated surface containing the anchored component or components
under conditions
conducive to binding. After the reaction is complete, unreacted components are
removed (e.g., by
washing) under conditions allowing for any molecules bound to the anchored
molecule or molecules
to remain immobilized on the solid surface. The detection of molecules
anchored on the solid
surface can be accomplished in a number of ways. Where the non-immobilized
component is pre-
labeled, the detection of label immobilized on the surface indicates that
complexes were formed.
Where a previously non-irninobilized component is not pre-labeled, an indirect
label can be used to
detect complexes anchored on the surface. In certain embodiments, an indirect
label is a labeled
antibody that specifically binds to the immobilized, non-anchored component,
or an antibody that
specifically binds to the immobilized, non-anchored component, which in turn
is labeled directly, or
is labeled indirectly with a labeled anti-Ig antibody, for example. In the
latter embodiments, the
label sometimes is an enzyme utilized in an enzyme-linked format. In
embodiments incorporating
an antibody, the antibody often specifipally binds to a calcium channel splice
variant or ANX III
31
SUBSTITUTE SHEET (RULE 26)
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
molecule without significantly interfering W th binding o~ the catciura
channel to ANA III
polypeptide or binding oaf the test molecule, Such antibodies can be anchored
to the solid. support.
j1109S] In alternative embodiments, a hotttogeneous assay conducted in a
liquid. phase without a
solid support can be utilized. In such an assay, the reaction products are
separated from unreacted
components by standard techniques that include but are not lirr~ited ea
differential centrifugation
(see, for example, Itivas, G., and Mirlton, A. P., Trends Biachem act
Aug;1.8(~): 284-7 (1993));
chrarnatograptty (~ ~ , gel filtration ekxromatagraphy, ion-exchange
chrornstography);
el~ectr~aphoresis (e.g., Ausubel et crL, eds. Current Frotocals in Molecular
Biology , J. Wiley: htew
'York (1999)); irrurtunopreeipitation (e.g., A,usubel, F. et aL, eds. Current
Protocols in lwloIecular
Biology , d. 'Whey: New York (1999)) and mass sgectraxrtetry (e.g., r.T.S.
Pat. lVos. 5,547,$35;
~,605,7~5; 5,691.141; 5,849,542; 5,8fi9,2A.2; .x,928,906; 6,043,031, and
x,194,144).
[i1p,96] Tlxe assay embodiments described above sometimres are conducted in a
direct format or a
compeEitive format. It1 the latter farnnat, an interaction betweetl a calcium
channel splice variant,
AN'X lII andlor a test molecule is determined by detecting an interaction of
one component with a
target component in the presence of another carnpnnent that interacts with the
target component. In
an emhodimertt, a competitive assay sometimes is conducted by monitoring the
amount of an
antibody bound to a calcium channel splice variant in the presence of ANA ~
andlor a test
rnniecule, where the atztilxady and AY~IX III compete for binding to the
calcium channel splice
variant. In the assay embodiments described herein, a convenient measure of
binding affinity can be
calculated from signals generated by the assays using known methods. For
example, a .K~, I~;, I~m,
pre-steady sta#e kinetic constant, ICS, LDSO or Eb~ parameter can be utili~d
to rank tesk molecules
in the assays.
[pi197~ In certain entbodimetuts, modulators of calcium channel sptice variant
or ANX III
expression are identified. For example, a cell or cell-free mixture is
contacted with a rest molecule
anal the expression of tlxe calcium channel splice variant ar A1~X III mRNA or
poEypeptide is
evaluated relative to the Eevel of such products in the absence of the
candidate compound. When
expt~ssian of the calcium channel splice variant or AlII mh.~'A or polypeptide
is less in the
presence of the test molecule than iri its absence, the candidate compH~und is
identified as a
stimulator of calcium channel or ANA III expression, for ex2~mpie. Calcium
channel splice variant
or ANX IIx mR.N'A~. or palypeptide expression levels can be determined by
standard methods, such as
thane described hereirk.
[UQ913J Where membrane-hound farms of a calcium channel era used, it trAay tae
desirable to
utilize a solubiIizing agent. Examples of such solubilizing agents include non-
ionic detergents such
a5 n-octylgiucoside, n-dodeeylglueasida, n-dodecylzt~alt4side, actanayl-N-
metltylglucarnide,
decanoyl-N-methylglucamide, Tritons ~~-104, Tritons ~-1,14,'1'~hesit~,
Isotridecypoly(Ethylene
32
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
glycol ethEr)n, 3-[(3-cholaxrtidapropyl)dixnethylamminio3-1-propane sulfonate
(CHAPS), 3-[{3-
cholamidaprapYltItYlammittiol-~-hydmxy-1-propane sultanate (CHAP~Q), ar N'-
dadecyl-
~t,N-diznethyf-3-atrumanio-1-propane sulfonate.
[0099] The foliowin~ examples are intended to Illustrate hut not tcs Iirnit
the invention.
Exarno_le 1
fiissue samples
[0100] Glioma samples were obtained with informed consent from seveat patients
who
underwent craniQt~amy far brain tumor resection. The extent of tk~e resection
and standard
neurosurgical methods were not modified for the purpose of this study. Part of
tlxe specimen was
removed, quickly frozen in liquid nitrageta and stared at-$0°C until
RNA preparation. Another part
ores seat for routine neurapathalogicai evaluation. The clinicapathalagical
data of the patients are
presented in Table 1. fix of the tumors were eategarized as astrocytoma and
One was
aligodendrogliarna (case 01 ). The astraocytocnas ranged in World Health
OrgartizatiQn (WHU) grade
(Kleihe~es et csl. 1~~4) from ZI (increased aelluIarity and xniid atypic
without mitoses, endothelial
proliferation and necrosis) to IV (marked cellutar heteragenity, cytaplasmic
and nuclear
pleomorphism, mitotic figures including atypical farcKts, endothelial
proliferation and necrosis)
Grade ITI tumors were differentiated fxom Grade fV by the absence by
endothelial proliferation amd
necroses. The dligadendroglioma was anaplastic WHO Orade III (high
cellularity, widespread
nuclear atylaia and pleamorphisrn, high mytotic index, endothelial
prolifaratian and necrosis).
Table x. Clinuopat~~olrrgical d$t~a of patients undergoing xesechau of a
glivrita
p'atien~t 'Vi~tO Olassi$cationbest Recurrence
01 III M Yes
02 III F ~ Yes
43 III M ' 'Yes
04 IV 142 Na
0~ III M Na
O8 IEx M ~'es
.-
11 1T M hla
* World Health organization {I~leihues et ,~L, 189).
Fxam_pte ~
Cell culture and tran~xent tratl5fecticxn
[01(il~ primary cultures of human fetal astracytes (~99~'o purity) are
prepared and maintained
as described ict Corley et ctl. (2000). The glioma cell lines LT87, L?2Sl.N
and U56~ are prepared and
33
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
u$ed as previously reported (l3esson and'Yong, 200x). These cells were
cultured iz~ I7ulbecco's
modified Eagle's nzediurn (l~MEhri) containing I O%a (vJv) fetal calf serum
with L-glutaxnine (~~,
radium pyruvate (t~ and 1% (viv) null-essential amino acids. They were
maintained at 37°C in
a~hurnidified atmosphere with 9~% air and 5% CC7~andgrawn until cvnfluency.
The medium was
changed every two days. Tissue culture and transfection of tsA-X01 cells is
described. in detail in
l~eedle et aL (2002). ~ricfly,1~EI~ tails were ,grown to 85% contluency at
37°C (5~'o Cc~27 in
»ivIEIVI (tl0~ro fetal bovine serum, 20(li1/rnI penicillin and f).2mgerrU
strept~amycin, Life
Technoltrgies, Tnc.). Cells were dissociated with txypsin (4.~~%)-BDTA and
plated an glass
caverslips. All of the above solutions used far cell culture were purchased
from Gibca~~RL. The
Ca,,3.iac and Ca~3.lb variants (~pg) and green fluorescent ~rt~tein (I~,g) DNA
were trarlsfected into
teals using the calcium phosphate method. Cells were transferred to 28RC 2~1.
hours after
transfectian and recordings were performed two days later.
Example 3
RNA, isofatian and RT-PCR
[~Ia~;] Total RNA from gliaxna sazreples and cell tines as wet! as from
primary cultures of fetal
astrocytes was extracted following a ~'rizol protocol (Life Technolagles),
l7Nase treated, extracted
using phendtlchloroform extraction and resuspended irt bFPC-treated water. The
RT-13CR forward
(5'CA. ,t"rTTACCGGTC'x~GGT~CCGCACAA_3') and reverse
(5'GAATG"FGGGGGCTGCTGfiiTA..t",.TG~.'fCCAT3°) primers were directed to
the I~I-TV' lintker and
were based on the hun~arl CAChiIG sequence (Genbank accession number
A~1269f5). The
detailed protocol for RT-F'CR is described irt Latour et aZ. (2f'l03).
[d7,(I~] As illustrated in Figure ~A, splicing of axon 25 to axon 27 results
in CaV3.la or the
Ca,,3.ib variant. Splicing of axon 25 to axon 26 ~esuit~s in tire Ca"3.lac ar
Cav3.lbc variant. To
determine Ca"3.1 gene expzussion in human whole brain, glioxna samples and.
glioma call lines, RT-
PCIt analysis was performed using printers directed to the Ill-IV Iinker
regiaxr of Ca"3,1 which
contains these axons. Agarose gel analysis ofRT-PCR results can hurnxa adult
brain (Figure 2B lane
1) reveals the expression c~f two different Ca,,3_1 mRNA transcripts. The
Iawer molecular weight
prodr~ct likely carresgonds tc~ CaV3.1 a while the higher weight product
probably aamespands t4 the
Ca,,~,lbc isaform. ftesuits obtained with RT-Pof fetal brain (Figure 2~ lane
2) suggest
expression of Ca"3.lbc (top band), but not of Ca,.3.I a. The faint lower band
indicates mRNA
expressxan of Ca,,3.lb in fetal tissue. These results are carlsisterxt with
previous findings by {i)
34
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
showing mRl~TA expression ~7f Ca"3.1a, b and be in the brain and (ii) slaawing
differential expression
of exam 25 variants, with Cav3.lb being stra~n~ly fetal-specific and Cav3.la
being mire abundant in
the adult brain (Manteil et aL, 2Ci00). To determine Ga"3.1 expression in
glionna, RT-I'~It, analysis
was parfornxed an U251N giiama cells (Figure 2B lane 3) and gliozna biopsies
(Figure 2B lane 4)
using the same primer pairs. Agarase gel analysis revealed the presence of a
higher molecular
weight ~rocluct in lanes 3 and 4, suggesting iha presence of a novel, longer
isoform of the II1-IV
linlter expressed in glxoma, Sequencing of the 1t~'-1'CR product confirmed the
presence of a novel.
variant, ~av3.lac. These results are evidence of tumor specific expression of
a Cav3.1 channel
splice variant in human trrain.
[01U4] To further investigate the differential Ca~3.1 gene expression in,
adult and fetal normal
brain and glic~rna, ,ten clones were sequenced from each RT FCR reaction and
the relative abundance
of Cav3.la, b, be aztd ac were determined for each condition, pfistributiort
of the variants is shown in
Figure 4A. Consistent with the literature (ManEeil et al., 2()CIO), sequencing
revealed the exclusive
expression of Ca"3.lbc in, fetal brain and a predominant expression of Cav3.la
in adult brain. since
glioma frequently arises from astroeytes, RT-l~CR analysis was performed on
human fetal astro~cyte
cultures to evaluate Ca"3.1 gene expression in normal astracytes. As
illustrated in Figure ~,
Cav3.11~ is predo~minaritly expressed in fetal astrocytes, consistent with
fetal whole brain RT-PCIt
results. Sequencing analysis of the IT563, U$7 and IJ2~lhl' gliama cell lines
demonstrated that these
cells mostly express Cav3,lb and Cav3.tbc. Since these cells are derived from
adult 6rair~ tissue, a
predarninant expression of Cav3.la was expected. The presence of the Cav3.lac
variant in the
LTZS1N Dell line and in three giioma sarreples was identified. 'his variant
was observed only nn
gliorna and not in normal tisS~ue, suestic,g a gliaxna-specific expression of
Cav3.lac, which is
evidence pf a differential gene expression of Cav3. I in gliorna, as gliotnas
mostly express fetal
isoforms and the Cav3.lac spline variant.
Examale 4
~lectJah,~ol~x
(llxOS] Glass coverslips carrying transfected ce(Is were transferred to a 3 cm
culture dish
containing the recording solution (2IimM BaCl2, 1mM MgCl2, ltfmM I~EF'E~,
4UnnM
tetraethylammanium chloride,1t?mtvl gluctsse, 65miM CsCt, pH 7.2 with TEA-
Qki). Calcium
channel activity in tratwsfeeted tsA~201 cells was characterized via whole-
cell patch clamp
3S
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
recordings using an .Axapatch 2(fOB amEplif'~r (Axon Instrmnents, Faster City,
t:A) linked to a
persanai computer equipped with pC~.AMP v8Ø Patch pipettes (Sutler
barasilicate glass, EF15U-
SG-15) were pulled (Sutler P'-87 micraelectrode pullet), fire polished
(Narishige) and showed typical
resistances of 3 to 4 MS2 when Tilted wish pipette Solution (in cnM: 108
CsMeSQ~, h lVlg~lx, 9
EG~'A, pH 7.2). All data fig~erss, fits and statistics were completed using
SigrnaPlat 2004 (51'SS
Inc).
[QlOGj Splicing of the III-.TV linlter has prGViausly been shown to alter the
biophysical
properties of T-type channels (Chemin et aL., 2001a). The IfI-fV linker region
of CaW3.l ac was
subclaned into a full-length Ca"3.1 channel, and transiently expressed the
variant in tsA-2ta1 Bells
for electraphysialogical characterization. Both clones expz~essed well in HEK
cells arLd produced
typical fi-type eurxent densities and wavefarrns. Figure 3 compares the
biophysical pxapertie~ of
Cav3.lac to the Cav3,lb variant (Beedle et al., 2002). As shown in Figure 3,
there was no statistical
difference in the position of the current voltage-relations (Figure 3A), the
voltage-dependences Qf
inactivation (Figure 3$), oar the majority of time constants of inactivation
(Figure 3C). Current
densities also were similar fox bath variants. "there was, however, a
statistically significant slowing
in the time course of recovery from inactivation far the Cav3.lac variant
(1~igure 3D). These
observations suggest that the gIiama-.specifio Ca~3.lac variant displays
electraphysiological
cha~,Cte~stics that are similar to those observed with Cav3.lb.
[0107j ?here results pxavide evidence far law voltage-activated calcium
channels in human
gliama. Liven that a range of different calcium-mediated intracellular
cascades are involved in
cancers, this suggests that tha activation of those channels and subsequent
calcium influx likely
contribute to calcium signaling irt gtioma cells. Since Car,3.1 ac is Likely
ea contribute to tumor
,growth, it is interesting that the laiophysical properties of this channel
are virtually indistinguishable
from Ca~3.lb expressed in tsA-2A1 ceps. However, it has been spawn that Ca"3.3
T-type calcium
channels expressed in a nenranal cellular baclcgraund can show different
electraphysialagical
characteristics compared tQ channels expressed in tsA-201 cells (Chemin er
r~L, ZOClIb). xhis
difference can be due to the presence of neuron-specific xnteraeting proteins.
The ~a~3.lac variant
contains the longest amino acid seqt.~ence of all domain l~-IV linker splice
variants, thus it is
possible that sptioittg of this region could lead to the creation of an
intcractian site far neuronigtial
specific regulatory proteins which may affect channel function, aT be involved
in intracellular
signaling events mediated by channel activity. Tt zs spawn in Example G that a
Cav3.lac variant
interacts with annexin T1I while other Cav3.I isafoz~ms da r<ot.
36
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Exam le S
Invuunofluarescence
~l To determine iP gliama cells express T-type calcium clsannels,
irnrraunostaining was
performed an gliarna samples and on a gliom~a cell line using a rabbit
polyclonal Cav3.l antibody
directed to the rZ-11I Iirnlcer region 4f the oc, subunit (Latour ~x al"
2003). The presence of T-type
channels art U251x3 ,gliarua cells was assessed using Cy~3-conjugated anti-
rabbit secondary
antibody. As shown in representative 40X (Figure I,A) and 100X (Figure 1B)
cactfacal images. mast
of the r3251N cells display abun~:iant expression of the Ca~3.1 channel.
bauble-imrnunc~staining ~svas
performed on paraffin-ezni~r3ded glictma sections using the giial ~brillary
aaidlc protein (CxFAF)
mouse monoclonal antibody in addition to the T-type channel antibody. The
fluorescent anti-manse
CyTM2-con,~ugated secondary anti6ady was used against the manse primary
(CxFAP) antibody. This
dual staining canf'n-med the expression of ~ay3. I channels vn astracytes fn
sine. A confocal image of
T-typE channel inamunafluarescance in a malignant astrocytama is shown in
Figuze 1C, The staining
reveals clear expression of ~a~3.1 chatutels in the tumoz. The pt~senee c~f
astraeytes in the tissue
was canfirtned by GrFAP itnmunoiabeling (Figure 1b). The ca-expccessiaxt of
GFAP and T-type
char;nals is assessed in Figure lE. CelIs were pre-incubated with the Ca"3.1
aantrol peptide and
stained following the same pratoool to car4~'trm antibody specificity. 'this
procedure significantly
decreased the brightness of the immunofluorescence signal in glioma. ~,s an
additional caritrol,
gliatna cells and sections also were ir~ubated in the presence of the
fluorescent secondary an~,tibody
alone and no signal was detected. This evidence shows expression of a voltage-
Baked talciurn
channel in human gliatna cells and demonstrates that glioma and cultured
glianna cells abundantly
eMpress ~av3.1 channels,
[Oi~j The U251N glioma cells tvez~ grwwn until contluencp in a culture dish
and detached
fxam the dish with a 3-5 min. 37°'C incabatian with trypsin. culture
medium was added to the
resuspension sc~le~ian and cells were plated directly onto glass Coverslips in
2~F-well plates. Cells
were grown far an additional 24h, fixed with a 15 rain, irteubatian period. in
4~'o paraforrnaldehyde at
4° C and stained using a pratQCal described in Latour et al., 0003).
Paraf~r~-embedded glioma
sections obtained from tlm Foothills I~nspitai pathology services wets
deparafiFirdzed in xylene and
taktydrated irt a descending ethanol ,series (1(IQ~o, gStYo, SO~o and 70'0).
Tutttar sections were then
washed twice itt ~'BS and incubated for Ih at room temperature in a fcnsh
f''BS solution coatainlng
59'o normal donkey serum (3acksan Tmmunol~esearch). Slides were ttaen
transferred to a fresh >?BS
solution containing 0.0'1°~o BaA (Sigma), mrtuse anti-gNal fibrillary
acidic prcaiein (GFA,P) (1:3000)
and rabbit anti-~ocla (1:5000) overnight at 4°C. The next clay, the
sections wexe washed tluee effnes in
37
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
PBS and were incubated at 4°C overnight in a firesh FBS solution
containing Cy~"°'3-conjugated
donkey anki-rabbit 1gG (1:2UD0, Jackson .Isnmuna~esearch) and Cy~'"2-
conjugated donkey anti-
rnause Igt:'x (1:2!700, Jaeksan rmmunaResearch). The next day, the sections
were washed again tlvee
tim~a with cold P$S and xnaunted with Fiuarsave (~~lbiochem).
Example 6
Interaction of Ca,,3.lac with Annexin Z1I
[01,10] liT PCR was used tc~ examine the presence of Cay3..! T-type channels
in human gtlotrta.
Using this approach, a splice variant of Cav3.1 (Cav3.lac) was identified that
contains both crops 25
and 26 in the intracellular loop connecting domains Ill and IV (Figure 2).
This variant was seen in
surgery samples from gliama patients, and in the LT251N human gtioma cell
line. Moreover, the
variant was detected in the human retinoblastama cell line Y 79, rn contrast,
this variant was pat
present in normal human brain ar auitured human astrocytes, which expressed
predominantly the
previously identified Cav3.la anti CaV3.lbc variants. The full length cl~hTA
of Ca"3.lac was
assembled and functionally characterized in tsA-~O1 cells. In a pul! dawn
assay using GST-fusion
proteins Qf various da~ain ).lI-IV linker splice variants, the ac variant was
selectively able to pull
down a protein band in the 30-40 kDa range (Figure 5). This band was excised,
digested and
subjected to fingerprinting via mass spectrometry, with a positive
identification of the band as
annexln ITI (AI'T~ lIi). The identifiaatian and specificity for Cav3.lac was
subsequently aonf~u~ed
via additional pul! downs and Western blotting with an ANX Ill antibody
(Figure 5). Her~ee, a
Ca"3_1 splice variant that appears to be selectively expressed in mitagenic
cells can form a complex
wills ANX IfI.
[Olli~ Human anxxexins are a family of 13 di~'ererlt calcium binding proteins
with wide
distribution across different tissues (Oerl~, V. and Moss, S.B. 2002.
Annexins: prom sttretare to
function. FhysioZ. Rev. 82, 331-371). They appear to share an ability to bind
lrhasphaligids
(Raynal, P., arid Follard, H.B.199d.. Annexins: the problem of assessing the
biological role far a
gene family oi' multi~unctianal calcium- and phosphalipids-binding proteiats.
Bivchim. Biaphys.
Actor 1197, ~3-93. Swairjo, M.A., and Season, B.A. 1,994.. Annexin structure
and membrane
interactions; a tnalecular perspective. Annec, l~ev. Biophys. Biomol. Srruct,
23,193-213. F'erron, B.,
Lewi-T3entley. A., ~"xeny,13.. and Russa-Marie, F.1997. Can, enzymatic
activity, ar otherwise, be
inferred fCOrn structural studies of annexin 1II? ,l. Biol. Chetrz. 272,11321-
LI32~_ Sapkova, J.,
ltaguenes-Nicoi., C., Vincent, M., Chevalier, A., Lewit-Bentley, A., Retsso-
Marie, 'F., and Gaitay, J.
20x2. c:a(2+) and membrane binding to annexin 3 modulate; the structure and
dyn~nics of its N
3$
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
terminus and domain Iii. l~ratei» ~'ei. xi,161~-1625.), and some ;members of
the annexin family
have been shown to form calcium perrr~able pares in bilayers, while others
have been linked to cell
signaling in tumors llfygaaxd, S.T., Flaugiand, H.I~., Kwistoffersen, E.K.,
Lund-datZansen, M.,
Laerum, t~.D., an;d Tysnes, C>.1~.1998. Expression of artnexin II in gliotna
cell lines and in brain
tumor biopsies. J. Neuro~o»ca~ a8,11-18.). ANA-I, IV and VI have been Shown to
modulate
calcium, potassium and/or chloride channel activity (I~aciff, J.M., Behbehani,
M.M., Kaetzel, M.A.,
and Dedman,1~~ t 996. Annexin YI Enadulatcs Ca~~ and I~.+ conductances of
spinal cord and dorsal
rootganglion,neurons, Am, T. Fhysioh Cell Fhysiol. 271, C2()04-C2CJ15.
Kaet2el, M.A,., Chang
Chars, .Ii.> Dubinsky, W .P., I)edman> J.R., and Nelson, D.J. 1994. A role for
annexin IV in
epithelial cell function. Inhibition of calcium-activated chloride
conductance. ~: Bial, Cherra. 2b9,
5297-53(15.). Although not neuron specific, A,NX III is expressed in DRS
neurons and in astrocytes
(Naciff, J.M., Kastzle, M.A,> Behbehani, M.M. and Dedman, J.It. 1996.
Differential expression of
annexin,s I-IV in rat dorsaE root ganglia and spinal oord. J Comp, ,NeurQl,
368, 356-37fi.), and has
been linked to a host of intracellular signaling events, such as inhibition of
phpspholipase A2
activity (itaynal, P., and I?otlard, ?~LB. X994. Annexins: the problem of
assessing the biological role
for a gene family of multifunctional calcium- and phospholipids-binding
proteins. ~iochim.
Biophys. Acts 1197, 63-93.). AbrX III is comprised of faun homologous domains
and has a
predicted molecular weight of about ~6 kDa (Favier-pearon,'E_, Lewit-.Bentley,
~A~., and Russo-
Marie, F.199~6. The high-resolution crystal structure a~ humane anttexin III
shows subtle differences
with annexin V. Biochemistry 35, 1740-1744.x. ANX III snay regulate calcium
chanuael activity,
and calcium influx through Cav3,1ac channels may stimulate cell praliferati<nn
via ANX III.
[01X2] Calcium entry via L-type channels mediates the dissociation of
preassociated
calmadulin from the channel, wlxich results in a downstream activation of CREB
mediated gene
transcription. Considering that AriTX-III is also a calcium binding protein,
its association with
CaV3.1 channels is similar to this mechanisrrt. As tire expression of Ca,,3. l
ac channel isofarm is
specific to znitogetxic cells, it is possible that Cav3.1 ac is re,~uired far
proliferation, and that the
,ANA-IIIICav~.lac interaction plays a role in proliferation. It has been shown
that T-type channel
blockers and knockdown of T-type ahan,nels can inhibit prali~eration of Y-79
cells (Bertotesi, G.E.,
Shi, C., Elbaum, L., 3o11icnore, C., Rozenberg, G_, Barnes, 5., Kelly, M.E.
2Q02. The Ca(2+)
ehazlnei antaganist5 mibefradil and pimozide ii~liibit cell ,growth via
different cytoxic naeChaniszns.
.A~foL .lslurmrntu~ol. 62, 210-219.).
39
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Cited Documents
Beedle AIVI, Hamid J, ~amponi GW . 2002. Inhibition of transiently expressed
Iaw- end high-
voltage-activated calcium ck~annels by trivalent metal catians. J Nlembr $iol
187:225-38.
Bertalesi ~rE, JollimQ~re CA, SZxi O, Elbaum L, Denavan-Wright EM, Barnes S,
Kelly IvIB. 2003.
.l~egulatian of alphal~ T-type calcium ahanr~l gene (CACNAIO) expression
during neuronal
differentiation. Eur J Neumsci 17:1802-lU.
Bessan A, 'Sfong 'VW (2000} ~valvemenE of p21~'~'~'P' in protein iCinase C ~-
induced cell cycle
progression. Mal Cell Bioi 20: 4580-4590.
Chenaiti 3, l~orneil A, Eaurinet E, Nargeot J, Lory P. 2001x_ AEternatively
spliced alpha(iG)
(Ca(V)3.1) intracellular loops promote speo~c T type Ca(2+) channel gating
properties. Biaphys J
80x238-S0.
Ct~emin J,1'~onteil A, Dubel S, Nargeat J, Lory P. 2001h. The alphalI T-type
calciutn channel
exhibits faster gating properties when overexpressed in neurobiastomalglioFna
Nti 108-15 cells. Eur
J Neurosci t4;1678-86.
Chemin J, Nargeot J, Lory P. 2Gb2. Neuronal T-type alpha 1 H calcium channels
induce
net~ritagenesis and expression of high-voltage-activated calcium cIaannels in
the NG108-i5 cell line.
J rileurosci 22:6856-62.
Corley SM,.Ladiwala U, Benson A and Yong VW, 200I. A,strocytes attenuate
aligodandrocyte
death in vitro through an a(~} intsgrin-laminin-dependent rnecltanistn. Giia
36. 281-2~4.
Cribbs ~.L, ~,ee J.H, Yang J, Satin J, Zhang 'Y, T~aud A, l3arctay J,
l~illiatnspn NCp, Fox M, Reel
M,1'ere~-l~eyes E. 1995. Cloning and charac~erizatian of alphalH from human
heart, a member of
the 'F-type Ca2+ c4anrtei ,gene family. ~irc Res 83;103-9.
b'Asaenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. 2004.
Electraphysiologicai and mc~Iecttlar evidence of L-(Ca"1), N- (CaY2.2), and R-
{Cay2.3} type Caz+
channels in rat cartiCal astracytes. Glia 45;354-t3.
Hiroaka K, Bertalesi GE, Kelly ME, Denavan-VCrright EM, Sure X, Har~tid J,
Zamponi GW,
Juhasz AE, Haynes LVP, Barnes 5. 2002. T Type calcium channel aiphalCs and
alphalli subunits ire
human retinablastoma cells and ttaeir Loss after differentiation. J
Neurophysiol $8:19G-205.
Kleihues P, Burger PC, Scheithauer BW 1993.'I'he new'VYI3p classification of
brain tumors.
Brain Patha1.3:255-68.
Klugbauer N, lVlarais )r, Lacinava L, kIofmans~ p'. 1999. A T-type calcium
channel from mouse
brain.1'flugers Arch 437:'7F0-5.
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
Kunert l~adeit J, Stepien kI, Radelc A, Lyson K,1'awlilcowSlci M. 1989.
It3hibitory effect of
calcium channel blaclcers on proliferation of human, gliotna cells in vitro.
Acta Neurol Stand
79;16fr9.
Latoux I, Haznid J, Beedle AM, ~mpoz~i GW, Macvicar BA. 2003. Expression of
voltage-gated
Ca~+ ck~annel subtypes in oultured astrocytes. Glia 4'I :347-53.
Lee JI"I, Daud AN, Cribbs LL, Lacerda AE, J"erevetaev .A, Klockner U,
Sohneider T, Ferez-Reyes E.
1999. Cloning and expression ~rf a novel member of the lnw voltage-activated T-
type calcium
channel family. J Neurt~sei X9:19I~-21.
Mariat P, V$naverbexghe K, T~levee N, Idossier MF, Prevarslcaya N. 2002.
Overexpressaan of an
alpha i~I (Ca"3.~) T-type calcium channel daring neuroenttocrine
differentiation of hunnan prostate
cancer cells. J $iol Cherxi 2'77:10824-33.
McRory rE, Sand CM, Hamming IBS, Mezeyava J, Sutton I~~, Baillie DL, Stea A,
Snutch T.P.
2001. Molecular and functional characterization of a family of rat brain T-
type calciumi channels. J
l~iol Chem 276:3999-401,1.
Monteil A, Chemin J, Bouri~aet E, Mennessier G, Loxy P, NarBeot J. 2100.
Molecular and
functional properties of the human alpha (1G) subunit that forms T-type
~calciutrt channels, J )3io1
Chern 275:6090-100.
(?lsen ML, Schade S, Lyons SA" Amoral M),3, Santheitr~r l;i. 2003. .Expression
of voltage-gated
ohIoride channels in human glionaa cells.1 I~eurosci 23:5572-S~.
Fez~ez-Reyes E, Crilabs L>;-, Daud A, L,acerda AE, Barclay J, 'Williamson MF,
Fax M, Rees Ivi,
T..ee JH.1998. Molecular eharacteri~ation of a neuronal low-voltage-activated
T-type calcium
channel. Nature 391.:$96.900.
Ransom t:B, Liu 3~, Sontheimer H. ~CI~. BK chanrAels in human g1 ioma cells
have enhanced
calcium sensitivity. Glia 3$:281-91.
Schrey M, Coding C, Kraft R, Beetz C, Kalff R, Wolfl S,1'att S, 20f12.
Molecular
characterization of voltage-gated sodiunn channels in human glivma. Neumreport
13:?~93-8.
Sontheimer, JI. 2003. ll~alig~aant glioma: perverting glutamate and ion
homeostasis for selective
advantage. Trends IVeurosci 26:543-9,
Sutherland .~xR, Florell R, Louw T3, Chai NW, Sitna Atl1~.1987, Epiden~iAlogy
of pxirnaxy
ir~tracranial r~eoplasms in Manitoba, Canada. Can J Neurol Sci 14:586-92.
Toyota. M, .~1'o C, ahe-Toyota M, Baylin SB, lssa JP. 1939. Inactivation off'
~rCNt~,IG, a T-type
~calclum channel gene, by aberrant methylation~ t~f its 5' GpG island in human
tumors. Dancer l2es
~59:d~535-41.
Walker D~'r, Kaye AH. 2001. Diagnosis and management of astrocytomas,
oiigodendroglioma
and mixed glioma: a xevievv. Australas RadiQl 45:72-82.
4~
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
3ar~is, S.E. and ~mgoni, G.W. 2001. Irtteractians between presyttaptic ~a2+
channels,
cytr~plastnic messengers arid proteins o~ tiae synaptic vesicle release
complex. ~"rertds Fharnmcol
Sci. ~2, 519-525.
Magga, J.M. Jatvis, S.E., A~tot, M.L, Zampou,i, G.W. and Braun, 3.8. 2000.
Cysteine stritx~
protein regulates G protein znodutation of iV-type cal~eiam ch,attttels.
Neuron '8,195-ZU4.
Nucleotide Sectuenre and Amino Acid Sectuence $mbodime~2ts
z3uman aZpha~.G ao splia~ variant (SEQ ZD N0, 11
~p,~~GGACGArGAGGAGGATGGAGCGGGCC',,CCGAGGAGTCGGGFICAC',,GCCCGGAGCTTCAfiCCGGC'1"C
AACGACCT
GTC GGGCCGGCCGGGGCCGGGGTCAGCAGAAAAGGACC!CGGGCAGCGCGGACTCCGAGGCCGAGG
GGCTGCCG1'ACCCGGCGCTGGCCCCGGTGGT'i'TTCTTCTACfiTG~I.GCCAGGACAGCCGCCCGCGGAGCfiGGT
GT
CTCCGCACGGTCTG'1'AACCCCTGGTfiTGAGCGCA'z'CAGC~.1,',C'G7:
I'GGTCATCCT'.T'CTGAACTGCG'T'GACCCTGGG
CATG'.~TCCGGCCATGCGt~GGACATCGCCTGTGACTCCCAGCGCTGCGGGATCCTGCACGCCTTTGAfiGACTTCA
TCTTTGCCTTCT'I'TC~CCGmGGP~.GATGGTGGTGAA,GATGGTGGCCTTGGGCATCTTTGGGAAAAAGTGTTAGCT
G
C~,SAGACACT~'GGA,ACCGGCTTGACT'TTTTCATCGTC,ATCGCAGGGATGCfiGGI~GTACTCGCTGGACCTGCA
GAA
CGTCAGCTZ'CTCAGCTGTCAGGACAGTCCGTGTGCTGCGACCGCTCAGGGCCATTAACGGGGTGCCCAGCA2'GC
GCATCCTTGTCACGTTGCTGCTGGATACGCTGCCCA't'GCTGGGCAACGTCCTGCTGC't'CTGCTTCTTCGTCTTC
'L'TCATCT'TCGGCATCGTCGGCGTCCAt~CTGTGGGCAGGGCfiGCTTCGGAACCGAfiGCTTCC~'ACCTGAGAAT
TT
CAGCCTCCCCCTGA4s'CGTGGA,G:CTGOA,GCGCTZi,'LTACC~'sGAGA(",'TAGAACGAGGA'X'GAGAC7CC
CC'x'TCATCTGCT
CGCAGCCACGCGAGA1~CGGCAfiGCGG2'CCTGC~.GAAGCGTGCCCACGCTGCGCGGGGACGGGGGCGGTGGCCCA
CCTTGGGGTC'TGGACTA'z'GAGGCCTACAACAGGTCCAGCAACACCACCTGTGTCAACTGG13ACCAGTACTACAC
C,AACTGC'T,'CAGCGGGGGAGCACAACCCGTTCAACaGGCGCCA'~CAACT'I'TGACAACAT'Z'GGCTATGGCTG
GATCG
CCATC'I'TCCAGGTC.~1TCACGC'T'GGAGGGCTGGGTCGACATGATGTACTT'TGTGATGC,,ATGCTCATTCCTZ
'CTAG
AATTTCATCTAGTTCATCCTCCTCATCATCGTGGGCTCCTTC'I~TCATGATCAACG2'GTC~CGTGGTGGTGATTGC
CACGCAGTTCAGTGAfsA,CCAAGCA,raCGGGAAAGCCAGCTGATGCGGGAGCAGCGfiGTGCGGTTCCTGTCCAACG
CCAGCACGCTGGC~'AGCTTCTCTGAGCCCGGCAGCTGCTATGAGGAGCTGC'~CAA,c'.,TACCTGGTGTACATCCT
fi
CGTA1~GGCAGCCCGCAGGCTGGCTCAGG'I'CTCTCGGGCAGCAGGZ'GTGCGGGTTGGGCTGCTCAGCAGCCCAGC
ACCCCTC
,CxGGGGCGAGGAG1~CCCAGCCCAGC.?~GCAGCTGCTCTCGCTCCCACC(~CCGCCT.ATCCG'x'CCACCACC
TGGTGCACCACCACCACCACCA'x'CACCACCAGTACCACGTGGGCAATGGGACGCx'CAGGGCGCCCGGGGCCAGC
CCGGAGATCCAGGAGAGGGATGCCAATGGG'~CCCGCA.GGCTCATGCTGCCACCACCC'~CGAGGCGTGCCCTCTC
CGGGGCCCCCCCTGGTGGCGCAGAGTCTGTGCACAGCTTCTAGGATGGCGACTGCCAGTTAGAGCCAGTCCGCT
GCCAGGCGCCCCCTCCCAGGTCCCCATGTGAGGGATCCGGCAGGAC"t'GTGGGCAGCGGGAAGC",TGTATCGCACC
GTCC.A,CACCP,GCCCTCGACCGGAGACGCTGAh~GGAGAAGGCACTAGTAGA(',GTG
.~,aC'!,'GC4"AGCTt,~~'1,'GCiGCCCCC
AAGCCTCACCAGCCTCAACATCCCACCGGGGCCCTACAGCTCCA,TGCACAAGCTGCTGGAGACACAGAGTAC.t~G
GTGCCTGCCAAAGCTCTTGCAAGATCTCCAC~,CCCTTGCTTGAAAGCAGACAGTGGAGCCTGTGGTCCF1GACAGC
TGCCCCTACTGTGCCCGGGCCGGGGCAGGGGAGGTGGAGCTGGGCGACCGTf",AA23Te;CCfiGACTCAGACAGCGA
GGCAGTTTATGAGTTCACACAGGATGGCCAGCACAGCGACCTGCGGGACCCCCACAGCCGGCGGCA~,CGGAGCC
TGGGCCCAGATGCAGAGCCCA.GCTCTGTGCTGGCCTTCTCSGAGGC'tAATC°t'GTGACACCTTCC,(',
A~1AGATTGTG
GACAGGAAGTAC'~TTGGCCGGGGAATCATGATCGCCATGCTGGTCAACACACTCAGGATGGGCATC.n.~AATACCA
CGAGCAGCCCGAGGAGCTTACCAACGCCCTAGAAATGAGCAACATCGTCTTG,ACCAGCCTCTTTGCCG'tGGAGA
TGCTGCTGAA,GCTGCTmGTGTATGGTCCCTTTGGC'i'ACATCAAGAATCCCTACAACATCTmCGATGGTGTCATT
GTGGmCATCAGCGTG'~C,GGAGATCGTGGGCC~1,GCAGGGGGGCGGCCTGTCGCTGCTGCGGACCTfiGCGCCTGAT
CGTCACTGTCT'I'TCAGATCCTGACCCAGGAGGAC'.PGGAACAAAGTCCTCTACAATGGTATGGCCTCCACGT'CGT
CCTGGGCGGCCCT'.~T,bITT'z'CAT'1'GCCCTCATGACCTTGGGCAACTACGTGCTCTTCAA~'TTGCTGGTCGC
CATT
CT
'GG'TGGAGGGCT2'CCAGGCGGAC7GGAGfITGCGA.~1CAAGr'I'CCGAATCA,rrAGCCCGATT'.~CTTCTCAC
GCAGCC'f'
GGATGGTGA'rGGGGACAGGAAGAAGTGGTfiGc,CCTTGGTGTCCCTGGGAGAGCACCCGGACyCTGCGGAAGAGCC
TGGTGCCGCCTCTCATGATCCACACGGCCGCGACACCCATC~'CGGZ'GCCCAAGAGCACCAGCACGGGCCTGGGC
GAGGCGCTGGGCCCTGCGTCGCGCCGCACCAGCAGCAGCGGGTCGGCAGAGCCTGGGGCGGCCCACGAGATGA.~,
GTCACCGCCCAGCGCCCGCAGCTCTCCGGACAGCCCCTGGAGCGCTGCAAGCAGCTGGACCAGCAGGCGCTGCA
42
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
GCCGGAACA~'',CCTCGGCCGTGCACCCAGCCTCaAAGCGGAGAAGCCCAAGTGG2aGAGCGGCGGTCG'CTGTTGTC
G
GGAGAAGGCCAC,GAGAGCCAGGATGAAGAGGAGAGCTCAGAAGAGGAGCGGGCCAGCGCfiGCGGGCAGTGAt~CA
fiCGCCACAGt3GGGTCCCTGGAGCGGGAGGCCAAGAGTTCCfiTTGrr,CCTGCCAGACACACTtAGGTGCCAGGGC
TGCA'~CCaCACT
,~"aCCAG'~C~GCCGAGGGTCTGCTTCTGAGCACCP,GGACTGCAATGGCAAGTCGGC'I'TL~AGGC'zCGC
CTGGCGCGGGCCCTGGGGCCTGA~'GACCCCCCACT'GGATGGGGATGACCCCGATGACGAGGGGAACCT'GAGCAA
AGGGGAACGGGTCCGCGCG'I~aGATCCGAGCCCGACTCCCTGCCTGCTGCCTCGAGCGAGACTCCTGGTCAGCCT
ACATCTTCCCTCC~'CAGTCCAGGTTCCGCCTCCTGTGTCACGGGATCAfiCACCCACAAGATGT'T'CGAGCACGTG
GTCCTTGTCATCAfiCTTCCTTAACTGCATCACCATCGCCATGGZ.1GCGCCCCAAAATTGACCCCC3?,CAGCGCTGA
ACGCA.TCTTCGTGACGCTCTCGAATTACRTCTTC.fi,CCGCAGTCTTTCTGGCTGAAATGACAGTGAAGGTGGTGCs
i<ACTGGGCTGGTGCTTCGGGGAGCAGGCGTACCTGGGGAGCAGTTGGAACGTGCTGGACGGGCTGTTGGTGCTC
ATCTCCGTCA'T'CGAvCA'.tTCTGGTGTCCATGGTCTCTGACAGCGGCACCAAGATCCTGGt;CATGCTGAGGG'~G
CT
GCGGC'Z'GCTGCGGACCCTGCGCCCGGTCAGGGTGATCAGCCGGGCGCAGGGGCTGAAGCTGGTGGTGGAGACGC
TGATGTCCTCACTGAAACCCATCGGGAACATTGTAGTCATCTGCTGTGCCTTCTTCATCATTTTCGGCATCTTG
GGGGTC'aC~.GCTC'.I'TCAAP~GGGAAGTTTTTCGTGT6CCAGGGCGAGGATACGAGGAACATCACCAATAAATCG
GA
C',L'GTGCCGAGGCCAGTTACCGGTGGGTCCGGCACAAGTACAACTTTGACAACCTTGGCCAGGCCCTGATGfiCCC
TGTTC
,t'"xTTTTGGCCTGGAAGGATGGTTGGGTGGACA'.G'CATGTACGATGGGGTGGATGCZ'GTC'aGGCGTGGACCA.
G
CAGCCCA,TC.'~TGAACCACAACCCCTGGATGCTGCTGTAC'1'~'CATC'~'CGTTCCTGC'1"CATTGTGGCCTTC
T'~'TGT
CGTGAACA'rGTT'3,'GTGGG'~'GTGG'x'GGTGGAGAACTTCCACAAGTGTCGGCAGCACCAGGAGGAAGAGGAGG
CCC
GGCGGCGGGAGGAGAAGCGCCTACGAA,GACTGGAGAAAAAGA,GAAGGAGTAAGGAGAAGCA,t',7ATGGCTGATC'
.l'A
ATGCTGGACGATGTAATTGC'I'fiCCGGC1~1GCTCAGCCAGGGCTGGGTCAGAAGCCCAGTGCAAACCTTACTAC'I
~
CGACTAGTCCCGCTTCCGGCTCCTCCa~~CCACGACfiTGTGCACGAGCCACfiACCTGGACCTCTTCATCACAGGTG
TCA,TCGGGC'TGAACGTGG'1'CACCATGGCCATGGAGCA.CTACCAGCAGCCCCA,GATTGTGGATGP.GGCTCTGA
AG
ATCTGCAACTACATCT'TC~.C'I'GTCAT'CTTTGTGTTGGAGTCAGTT~"TCAAACTTGTCiGCCTTTGGTTTCCC;
Z'CG
GTTCxTCCA~GGACAGG'I'GGAACCAGCTGGACCTGGCCAT'~GTGCTGCTGTCCATCAfiGGGCATCACGCTGGAGG
AAATCGAGGTCAACGCC'.C'CGCTGCCCATCAACCCCACCA'rCATCCGCATCATGAG(~GTGCTGCGCA'~'TGCCC
GA
GTGCTGAAGCTGCTGAAGATGGCTGTGGGCATGGc~GGCCCTGCTGGACACGGTGATGCAGGCCCTGCCCCAGGT
GGGfiAACCfiGGGAC~i'CTCTTGATGTTGTTGTTTTTGATCTTTGCAGGTCTGGGCGTGGAGC1'CTT'~GGAGI3.
CC
TGGAGTGTGACGAGAGACACGCCTGTGAGGGGCTGGGCCGTCATGCCACCT'T'TCGGAACTfiTGGCATGGCCTTC
CTAACCCTCTTCCGAGTCTCCACAGGTCaACAAT~,'GGAATGGCATTATC~AAGGAC,ACCGfiCCGGGACTGTGACC
A
Gts'T;GTCCACC."TGC'PACAA,CACGGTCATC'x'CGCC'.C'ATCTI'.CT'I'TGTGTCCTTCGTGCTGACGGC
CCAG'~'TCGTGC
TAGfiCAACGTGGT'GATGGCCGTGCTGATGAAC',CACCTGGAGGAGAGCAACA,AGGAGGCCAAGGAGGAGGCCGAG
CTAGAGGCTGAGGTGGAGCTGGAGA'f'GAP,GACCCTCAGCCCCGAGCCCCACTGGCCAC'I'GGGCAGGCCCTTCCT
CTGGCCTGGGGTCGAGGGCCGCGACAGCCCCGACAGCC4CAAGCCTGt"aGGCTCTGCACCCAGCGGCGCACGCGA
GATCAGCCTCCCAC2~TTTCCC7,'GGAGGACCCGACGATGCAGCCCCACCCCACGGAGCTGCCAGGACCAGAC2~A
CTGACTGTGCGGAAGTCTGGtaGTCAGCCGAACGCACTCTCTGCCCAATGACAGCTACATGTGTCGGCATGGGAG
CACTGCCGAGGGGCCCCTGGGACACAGGGGCTGGGGGCTCCCCAAAGCTCAGTCAGGCTCCGTCTfiGTCCGTTC
ACTCCCAGCCAGCAGATACCAGCTACkITCCTGCAGCTTCCCAAAGATGCACCTGATC~'GCTCCAGCCCCACAGC
GCCCCAACC'~'GGGGCACCAT'CCCCAAACTGCCCCCACCAGGACGCTCCCCTT~'GGCTCAGAGGCCACTG.AGGCG
CCAGGCAGCa~IA.TAAGGACTGACTCCT'x'GGACGTTCAGGGTCTGGGCAGCCGGG.~lAGACCTGCTGGC'AC"sA
Gfx'TGA
CxTGGGCCCTCCCCGCCCCTGC,CCCrGGCCTACTC~'~'TCTGGGGCCAGTCAAGTACCCAGGCACAGCAGCACTCC
CGCAGCCACAGCAAGATCTGCAAGCACATGACCCCGCCAGCCCCTTGGCGAGGCCCAGAACCCAACTGGGGCAA
GGGCCCTCCAGAGAr''CAGAAGC.AGCTTAGAG'x"TGGG'ACAC
.t'aGAGCTGAGC'~'GGATTTCAGGAK~ACCTCC'TGCCCC
CTGGCGGCCAGGAGGA,GCCCCCATCCCCACGGGACCTGAAGAAGTGCTACAGCGTGGAGGCCCAGAGCTGCCAG
CGCCGGGCTACGTCC'1'GGCTGGATGAGCAGAGGAGACACTCTATCGCCGTCAGCTGCCT'GGACAGCGGCTCCCA.
ACCCCACCTGGGGACAGACCCCTCTAACCTTGGGGGCCAGCCTCTTGGGGGGGCTGGGAGCCGGCCCAAGAAZaA
AACTCAGCCCGGCTAG'Y'ATCACCATAGACCCCCCCGAGAGCCAAGG2'CCTCGGACCCCGCCCAGCCCTCiGTATG
~'GGCTCGGGAGGAGGGCTCCGTCCAGCGACTCCAAGGATCCCTTGGCCTCTGGCCCCCCTGACAGCATGGCTGG
CTCGCCCTCCCCAAAGAAAc~ATGfiGCTGAG'Z'CTCTCCGGTTTATCCTCTGACCCAGCAGACCTGGACCGG"xGA
i~Iuma~z alphalG ac splice ~tra~iant Witk~ amino arid txanslatio~, tSEQ TD
A1C: 21
atrgg~acgag9'aggag'9'atggacrcg9gc3c~ga.ggagtcg9gacag~cccggag~tx~atg
!~I D E E E D G A G A E E S G Q P R S F M
~9gotcaaag~,cctgtcgggggc~ggg9'3~c99eCg9fggG~ggggtcagcagaaaae~gac
R 2~ N D ~ S G A G G R ~' G P G s A E K D
43
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
acgggcagcgcggactecgaggcggaggggctgcagtaccoggcgatggaaceggtggtt
P G S A D S E A E G L P Y P A L .~. P V 'V
ttettctacttgagccaggacagccgcccgcggagCtggtgtctccgcacggtctgtaac
F F ~'' L 8 ~ t1 S R P R fiu W ~.' Tr R 'T V C lIi
ecctggtttgagcgcatcagcatgttg9tcatccttctcaactgcgtgaCCCtgggcatg
pWFE~,'.I SMLVZLIrNCV2'LC~M
ttecggceatgcgaggacatcgcctgtgactcaaagcgatgccggatcctgcaggccttt
F Ft Q C E D I A C I7 S Q ~t C ~t I L S~ A F
gatgacttcatctttgccttctttgccgtggagatggtggtgaagats~gtggcciagggc
D D F t F A F P A V E I~ V V If M V A L C,t
atetttgggaa~.aagtgttacctgggac~acacttggaaccg~gettgactttttcatcgtc
2 F G kt K C Y L G D T W N R L D F F z V
atagcaggcfatgctggagtactcgctggacCtgcac~aacgtcagcttctcagctgtcagg
r A G M ?a .E Y S L h L t~7 N V S F $ A V R
acagtccgtgtgctgcgacogctcag$gccaetaaccgg9t9'cacagcatgcgcaCcatt
T V R V L R P L R A I N R V P S M R I L
gtcaec~ttgatgctgga,tacgctg~cccatgetgggaaacg'teetgetgctctgettcttc
'V '.~' L L T., D T L P ~i L G N U T~ z. L ~ C F F
gtcttcttcatcttcggcatcgtcggcgtcCagctgtgggcagg9ct9cttcggaaccga
V F F I F G I V G V Q L W A G L L R N R
tgcttcetaeatgagaatttcagcotccccctqagcgtggacctggagcgctattaacag
C F L P E N F S L F L $ V D L E R $
acagagaaegagc~atgagagcaccttcatotgetcGCagccacgcgagaaeggaatgcgg
T E 1V &: I7 'E S P F Z C S Q P R E N G I~ R
tactgcagaagcgtgcccacgctgrcgcgg9gacS'Sgg9'cJStg9cccacettgcggtctg
S G R S V P T L 1~ ~'r D G G G G P P C G I.
gactatgaggactacaacagatacagcaacaccaactgtgtcaactggaacc~.gt~.ctaa
D Y E .A Y N S S S N T T C V N W N' Q Y Y
accaactgc~.oagcgggggagcacaaccccttcaag~ggcg~acataaa.cttt.gacaacatt
T ~3 C.' S A G ~.'' H N Y' F K G A I I3 F D I3 I
ggctatgcctggatagccatGttccaggtcatcacgctggagggctgggtcgaoatcacg
G Y A tn1! T A I F ~ '~ I T L E G W V D I M
tactttgtgatggatgctcattcottctacaatttcatctaattaatectcateG.tcatc
F V M D A I3 6 ~' Y' N F° I Y F I L r, I I
gtgggctccttcttca.t$atCaacCtgtgCCtgg'tggtc,~attgCaacgcagttaa.gtgag
V G $ F F M I I3 L C L V V I A T ~ F S E
aceaagcagegggaaagccagctgatgcgggag~cagc9'tgtgcB9ttcatgtccaaagcc
TKQRES QLMREQ~tVI~FL SN.A
agcaccatggctagcttctctgagccCCfgsagCtgctatga.ggagctgatcaagtacatc,~
S T L A S F S E P 6 S C Y E E L L K Y L
gtgtaaatGCtGCgtaaggcagCCCgcagg~atggctCaggtCtctCgggCagcagc,~tgtg
V Y' I L R K A A R R L A Q V' S R h. A G V
aJggttgggctgCtCagaagcGCac~'cacCCetcgggggacaggagaaccagcacagcagc
R V G L L S S P A. P L G 6 Q E 'x" Q P 5 S
agetgetGtegctcceacegccgcctatecgtcCa~caacctggtgcaaeaccaccaccac
a~ C B R S FI R R L S V H H T~ V H H ti H H
catcaccaccaetaecacatgggaaatggga~getGagggcec~eegggccagcccggag
H H H FI Y H L G N G T L R A p R A S P E
atccaggacagggatgacaatgggtcccgcaggctcatgctgacaccaccatagacgcat
I Q D R D A N G S R R L M L P P P $ T P
gacctatccggggcceaccctggtggcgGagagtatgtgcacagcttctaccatgccga,c
AtrSt'aAI~P~"aGAESVH~"FYHAD
tgGGa4ttagagCCaC~tcGgCtr~CCaggCC~CGCCCtCecaggt~CCcatCtgclggCaCcc
C H L E P V R C Q A P P P 1~ S F S E A, S
g JCagg~;1.ctcftgggCa9'Cgg9aaggtgtatcCGacCgt GCacaGCagCC ctCCa4Cc~ga.g
G R T V G S G it V ~'' P T V H 'i' S P P F E
acgcCgaaggagaac~gcactagtagaggGg9ctgccagctctgggcccecaaccctaacc
T L k E K A L V E V A A $ 5 G P P T L T
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
agcctcaacatCCCacCCgggccctacagctccatgcacaagctgctggagacacagagt
SLNI1~~PGFYS SMIiIi.LLETS~S
acaggtgcctgccaaagetcttgcaagatctccagcccttgcttgaaagcagacagtgga
T G A c Q s s a K z s s P c L ~e A D s G
gectgtggtacagacagctgcccctactgt9ccCgJgecJg9gca39JgaJgt~'gagctc
A C G P D S C P Y C A R A G A G E V E L
gccgaccgtgaaatgactc~actca.gaeagcgaggcagtttatg~agttcacacaggatgcc
A D Vii. L M P D S D S E A V Y E F T Q D A
eageaeagcgacctccgggaccaecacagccggcggcaaeggagCCtgggCCCag'atgca
Q 'H S If L R D P H S F2 R Q R S L G P D A
gagcccagctctgtgctggccttctggaggctaat~tgtgacaccttcCgaaagattgtg
E P S S V L A F W R T~ I C D T F R K . I V
gaCagcaagtaetttggccggggaatcatgatcgccatcctc~gtaaacacactcagcatg
D S R Y F G R G 2 M I A T T~ V N T L S M
ggcatcgaataccaagagcag4ccgaggagcttaccaacgcectagaaatcagcaacatc
G~ I E Y H E Q P E E L. T ~T A L E Y S N I
gtcttcaccagcctctttgccctggagatgctgctgaagctgcttgtgtatggtcecttt
V F T S L F A L E M L L EC L L V Y G P F
ggctac~.tcaagaatCCCtacaacatcttegatggtgteattgkggtcatea.gcg~tgtgg
G Y Z If ~T P X N I F L1 G V I V V I S V W
gaga.tcgtgggcCagCagggsgggeggcctgtaggtgctgcgga.CattccgcCtg~atgcgt
E I V 6 Q Q G G G L S V L R 't' F R L M R
gtgctgaagctggtgcgattcctgccggcgctgcageggaagctggtggtgctcatgaag
V L K L V R F Ta P A L Q R Q L V V L I~2 K
accatggacaacgtggccaccttetgcatgctgcttatgctcttcatCttcatcttcage
fi M D N V A '~ F C M L L M L F I F I F S
atCCtgggcatgcatctcttcggCtgcaagtttgcCtCtgag~g'ggatggggaeaccCtg
I L G M H L F G C TC F A S E R D G ~7 T L
ccagaccggaagaattttgactccttgctctgggccatcgtcactgtctttcagatcctg
D R IC N k' D S L L W A T V T V F Q I L
acccaggaggactggaacaaagtcctctacaatggtatggcctccacgtcgtCCtgggcg
'1'QEDWNKVLYf3GMAS~'SSWA
gccctttatttcattgecctcatgacctteggcaacta.cgtgcttttcaatttgctggta
A L Y F I A L M T F G N Y V L F N L L V
gccattctggtggagggcCtccaggcggaggg~agatgccaacaagtacgaatcagagcce
A I J~ V E G F Q A E G D ,,~ N K S E S E P
gatttcttctcacccagcctggatggtgatggggacage,~aagaagtgcttggccttggtg
D F F S P S L D G 1~ G D R K K C L A L V
tccctgggagagcacccggagctgcggaagagectgctgccgcctctcatcatccacacg
S L G E F~ P E L R K S L L p P ~r I 2 H T .
gccgccacacccatgtcgctgcccaagagcaccagaacgggcctgggcgagc~cJct9ggc
A A T P M S L P K S '~' S T G L fr E A L Ca
CctgcgtCgcgccgcaccagcagcagcgggtCggcagagcctggggcggcccacgagatg
P A S R R T S S S G S A E P G A A H E M
aagtcaccgcccagcgcocgcagctctccgcaoagcccctggagcgctc~Gaagcagctgg
K S P p S A R S S P H S P W S A A S S W
accagcaggcgctccagccggaacagcctcggccgtgcaccaagcctgaagcggagaagc
T S R R S S kt N S L G R A 1~ S L T~ R R S
cc~gtggagagcggcg9'tccctgttgtcgB Jagaaggcc~.9'gagagccaggatgaaga.g
P S G E R R S T, L S G E G Q E S Q D E E
gagagctcagaagaggagcgggccagccaCgcgggcac~tgaccatC~ccacagggggtcc
E S S E E E R ,A $ P' A G S 17 H R H R G S
Gts~gagegggaggcaaagagttcctttga,CCtgccagaaaeactgcaggtgCCagggCtg
L E R E A K S S F D L P D T L c~ V P G ~,
catcgcactgccagtggccgagggtctgattctgagcaccaggactgcaatggcaagtcg
H R T A S G R G S A S E H Q D C N G K S
gcttaagggcgcctggccegggccctgcggcctgatgacaccccactggaCggggatgac
A S G R L A R A L R P D p p p L p G D p
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
gcagatgacgagggcaacctgagcaaaggggaacgggtccgcgegtggatcCgagccGga
A D D E G N L S K G E R V R A W I !2 A R
etaectgcatgctgcctcgagccgagactcctggtcagcctacatcttccetcctcagtcc
L F A C C L E R. D S W S A '~' I F ~' P S2 S
aggttcegcctcctgtgtcaccggateatcaccoa.caagatgttcgaccacgtggteett
R F R L L C H R I I T H K M F D H V V L
gtoatcatcttcvttaactgcatcaccatcgcCatggagcgcoccaaaattgaccccaac
V I Z F L N C I T I A M E R P Ti I D P H
agcgatgaaagcatattcctgaccctctcoaattacatcttcaccgcagtctttctggct
S ~,, E ft I F' h Z' L S P1 Y I ~' T A V F L A
gaaa.tgacagtgaaggtggtggcactgggct99't8attcggg3agcaggc3tacotgagg
E M T V K V V ,A L G W C F G E Q A Y L R
ag~cagtt9~gaacgtg'ctggaagggctgttggtgatcatetccgtca,tcgacaCtatggtg
S S V~I Id 'tt' L D G L L V L I S V I D I L V
tccatggtctctgacagcggcaccaagatcctgg'gcatgctgagggtgctgcggctgctg
S M V S D S G T K I L G M Ir R V L Ti L L
Cggacca~.gegcccgctcagggtgatcagccgg~tcgca9'gggctgaagctggt3gtggag
R T L R F' L R V I S R A Q ~G L IC L V V E
acgctgatgtaatcactgaaacccatCggcaaCc~ttgtagtcatctgctgtgccttcttc
'1' L M S S L K P I G N I V V' I C C A F F
atcattttcggcatctY~gggggtgcagctattcaaagggaagGtttxcgtgtgccagg9c
t I F' G I L G V Q L F K G I~ F F V C Q G
gaggataccaggaacatcaecaataaatcggaetgtgccgac~gccagttaccggtgggtc
E D T R N I T D1 K S D C ~ E A S Y, kt W V
cggcaeaagt~acaaCtttgacaaccttggGCaggccctgatgtocctgttcgttttggcc
R H K 'Y N F D N L G Q A L M S L F V IJ A
tccaaggat9'Jttgggtcgacatcatgtacgatgggat9'9'at9ct9'tgggcgtggacCag
S K D G W V D I M 'Y D G L D A V G V D Q
cagcccatcatgaaccacaacccctggatgatgctgtacttcatetcgttcctgctcatt
Q P I M N H N P W M L L Y F I S F L L x
gtc~gccttctttgtcetgaacatgtttgtgggtgtggtggtggagaacttccacaagGgt
V A F F V L N N! F V' G V V V ~.' N F H K C
cggcagcaccag~'aggaagaggaggcccggcggagggaggagaagcgcctacgaagactg
R Q H Q E E E E A R R R E E K R L R R L
gagaaaaagagaaggagtaaggagaagc~.gatggctgatetaatgctggaagatgtaatt
E K K R R S iC E IC Q M A D L M L i! D Y I
gcttccggcagctcagacagcgaCgcgtcagaagccGa,gtgcaaaccttaotactccgac
A 8 1~a ~ S A G A A S E A Q G Ff J.' Y Y S D
tactcacgcttcaggctcctcgtacaecacttgtgcaccagcCactacctggacctcttc
Y S R F R L L V H H L C T S H Y L D L F
ateaoaggtgtcatcgggctgaacgtggtaacca.tggacatggagca.ctaccagaagcac
I T G V I G L N V V T M. A M E H Y Q Q P
cagattctggatgaggctctgaa.gatctgcaactacatattcactgtaa.tctttgtcttg
Q I L D E A L fC 2 C N Y I F T V 2' F V L
gagtcagttttcaaacttgtggcctttggtttacgtcggttctCacaggacaggtggaaa
E S V F Tt L V A F G F R R F F Q D R W, N
cagetggacctggccattgtgctgctgtccatcatgggcatcacgctggaggaaatcgag
Q L D L A I V L L S I M G I T L E E I E
gtcaact~cctagctgcecatcaaCCCCaacatcatCCgcatcatgagggtgctgcgcatt
V N A S L P I N P T Z I R z M R V' L R I
gcccgagtgctgaagctgctgaagatggctgtgggcatgcgggcgctgctggacacggtg
A Ii V L K L L ff M A V G M R A L L D T V
atgcaggccctgccccaggtggggaacctgggacttctattcatgtCgttgtttttcatc
M i,~ A L P f~ V G N L G L L F M L L F F Z
tttgcagctetgggcgtggagctctttggagacctggagtgtgacgagacacacccctgt
F A A L G V E .r. F G D L E C b E T I3 P C
g'agggcctgggcogtcatgccacctttcggaactttggcatggtcttactaaccctct~tc
EGLGRf~AT~'RN~'GMA~'LTLF
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
cgagtctCCacaggtgacaattggaatggcattatgaagg~.caccctocgggaatgtgac
R V S T G D 13 W 1~T G I M K D T xa R I1 .~'y' D
caggaS-tacacctgctacaacaaggtcatctcgcctatctactttgtgtacttcgtgctg
QESTC'~N~'V I SPIYk'VSFVL
acggeccagttcgtgctagtcaacgtggtgat:egccgtgotJatgaagcacctggaggag
T A Q fi V fa V L3 V V I A V L M IC H L E E
dt~Caacdag~a.ggCaaaggaggaggocgagctagaggctgagctggagctggag2ttgaag
S N YC E A K E E A E L E A E L E Ir E M K
accctcagcocCCagCCCCactcgccactgg9cagccacttcctotggcetggggteJa9
T L 8 P Q P ~j S P L G S P k~ L W P G V E
ggccccg~cagccccgacagccccaag~ctggggctctgCacccagcggcccaagcgaga
G P I1 S P D S P K P G A Ta H P A A H A R
tcagactccaacttttacctggagcaccccaogatgcagceccaccccacggagctgc~a
S A S H F S L E ti P ~' M Q F H P 'f E L F
ggaccagacttactgactgtgcggaagtctggggtcagccgaacgcaGtctctgcacaat
G P D L L T V R K S G V S It T H S L F R
gacagctacatgtgtcggeatgggagcactgocgaggggcccctgggacacaggggctgg
D S Y M C R $ G S T A E G P L. G" 'ri R G W
gggCtCCCCaaagCCCagtC;~r~gCtccgtottgtcCg~.tcactCCCagecagCagat~CC
G 7r P 1'C A Q $ G S V L S Y kI S ~,,7 P A D 'P
agatacat.cotgcagettcccaaagatgcaoctcat:ctgatccagccccacagcgcccca
$ Y I L Q L P K 3:1 A F ~I L L Q P #i S A 1?
acetggggcacCatccocaaactgcccccaccaggaegctcccctttggctca,gaggcca
T Tnl' G T I F K L P P F G R S P L t1 Q R P
ctcaggcgccaggcagcaata~.9'gactgaatcettggacgttca~~gtctgggcagccgg
L R R Q A A I R T D S L '17 V Q G L G S R
gaagaCatgctggcagaggtgagtggJccctccccgccectggcccgBgceta.ctctttc
E 17 L L A 'E V S G P S P P L A R A '1' S F
tggggccagtoaagt.aeacaggcacageagcactcacgcagcc.~cagcaagatctceaag
W G ~ S S T Q A Q ø H S R S H S ~~ z S It
cacatgaccccgccagccccCCgcccaggGccagaacacaactggggc~.agggccatcca
YL M T P P A P C $ G P E P N W G K ,ra P P
gagacaagaagcagattagagttggacacggag~ctgagctggatttaaggagacctcctg~
E T 'R S 9 L 8 L D T E L S W I S G D L L
ccccctggcggccag9'aggagaccacatcaccacgggacCtgaagaagtgrcta.cagcgtg
$ P G G Q E E F P S F R D L K K C Y S V
gaggccCagagctgccagcs~cCggcctacgtcvt9Jct9gatgaJcagaggagacactat
E A Q S C Q R R P ~' S G~1 L D E Q R R H S
atagcegtcag4tgcctgga.cagaggctcccaaccccacctgggcacagaccectctaac
I A V S c L D $ G 8 Q P H L G T D .L~ S N
C~tgg~J9gCCagCCtCt'~g95<JgJ'catg9'gagCCggCCCa~gaaaaaaC~caC3CCCgc.ct
LGGt,~PLGC'aP .(~xS~P~Fi.KKLS PP
agtatC?.CCa'tags'i..'cCCCCCG~$gagCCl,aggt:CCtC JCJa.CCG'CtgCCCa~GCGtC,~gCat'.G
s z m I n ~ F ~ s Q ~ ~ R T P F s ~ c ~
tgcatccggaggagggcteagtccag~gactcCaaggatccattggactCtggC~C~cCt
C L R R R A P S S L1 S K D $ L A S G P P
gacagcatggctgcctcgccctacecaaagaaagatgtgctgagtctctccggtttatcc
D S M .'~ A S P S P K K D V L S L 8 G L S
tctgaccaagcagacctggacccatga
S D F' A D ,T, D P -
X0113] Modifieatians rnay 1>e r~r~ade to the foregoing without degarrit~p Pram
the basic aspects of
the invention. Although the invention has l5eeit described in substantial
detail with reference to one
or more specific em6oditnents, those of skill in the art will recc~nize Shat
chat~~e$ may be made to
the embodiments specifically disclosed in this application, yet these
madi~icatians aid
47
CA 02566041 2006-11-07
WO 2005/108575 PCT/CA2005/000713
irnprtrvements are within the scope and spirit of the invention, as set forth
in the clainr~s which
follow. Citatir~n of the above publications and documents is not intended as
an admission that any
of the foregoing is pertinent pria~r art, rior.does it constitute any
admission as to the contents' or date
of these pu'blieations or documents. Each patent, patent application, document
and publication
referenced herein is hereby incorporated by reference in its entirety.
4$