Note: Descriptions are shown in the official language in which they were submitted.
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
MEDICAMENT HOUSING APPARATUS HAVING OUTER AND INNER
CONTAINERS
Field and Background of the Invention
The invention generally relates to packaging for housing medicament.
Numerous examples of packaging pharmaceutical medicament present
in oral dosage form are disclosed. Specific examples include those intended
to accommodate packaging a variety of predetermined volumes of
medicament. To illustrate further, U.S. Patent Nos. 5,315,811; 5,197,602; and
io 6,243,936 disclose packaging systems for containing materials therein, and
methods for assembling packaging systems for containing materials.
Notwithstanding any potential advantages offered by the above-
proposed packaging systems, such systems may be potentially complicated
from an assembling perspective in that an individual packaging system is
made up of multiple components. It would be desirable to obtain a packaging
system that is simpler in structure, thus having the potential to reduce
multiple
steps in assembling a conventional packaging system.
Summary of the Invention
In view of the above, and in one aspect, the present invention provides
a packaging system. The packaging system includes a plurality of
substantially uniformly shaped containers comprising an outer container and
an inner container present within the outer container. The outer container
includes a flange and a container wall attached thereto and extending
downwardly from the flange. The inner container includes a container wall and
a bottom portion attached to the container wall which closes the inner
container. Both the outer container and the inner container each have
respective cross-sections. The outer container has a bottom portion that is
not enclosed, and a void space is present between the outer container and
the inner container.
The inner container further includes a neck having a base portion and
wherein the flange of said outer container is attached to the base portion. As
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
a result, and advantageously, the outer container and the inner container are
a unitary structure.
Brief Description of the Drawings
FIGS. 1 a and 1 b respectively are side and bottom cross-sectional
views of an embodiment of the packaging system.
FIG. 2 is a perspective view of an embodiment of the packaging
system.
FIGS. 3a and 3b respectively are side and bottom cross-sectional
io views of an embodiment of the packaging system.
FIG. 4 is a perspective view of an embodiment of the packaging
system.
FIG. 5 is a perspective view of an embodiment of an array of packaging
systems.
Detailed Description of the Embodiments
The present invention will now be described in reference to the
embodiments set forth herein, including, without limitation, those described
in
the drawings. It should be appreciated that these embodiments are for
illustrative purposes only, and are not intended to limit the scope of the
invention as defined by the claims.
All publications, patents, and patent applications cited herein, whether
supra or infra, are hereby incorporated herein by reference in their entirety
to
the same extent as if each individual publication, patent, or patent
application
was specifically and individually indicated to be incorporated by reference.
It must be noted that, as used in the specification and appended
claims, the singular forms "a", "an", "the", and "one" may include plural
referents unless the content clearly dictates otherwise.
A number of materials may be employed in forming the outer and inner
containers of the invention. For example, these may be formed from a
polymer, preferably one or more thermoplastic polymers. Exemplary
polymers include at least one material such as, without limitation,
2
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
polyvinyl chloride, polyvinylidene chloride, polypropylene, polyethylene,
polychlorotrifluoroethylene, and combinations thereof. The packaging
system of the present invention may be fabricated by employed accepted
techniques such as, without limitation, injection molding.
At least one medicament may be housed in the packaging system of
the present invention. For the purposes of the invention, the term
"medicament", as used herein, is meant to mean and include any substance
(i.e., compound or composition of matter) which, when administered to an
organism (human or animal) induces a desired pharmacologic and/or
io physiologic effect by local and/or systemic action. The term therefore
encompasses substances traditionally regarded as actives, drugs and
bioactive agents, as well as biopharmaceuticals (e.g., peptides, hormones,
nucleic acids, gene constructs, etc.) typically employed to treat a number of
conditions which is defined broadly to encompass diseases, disorders,
infections, and the like. Exemplary medicaments include, without limitation,
antibiotics, antivirals, H2-receptor antagonists, 5HT, agonists, 5HT3
antagonists, COX2-inhibitors, medicaments used in treating psychiatric
conditions such as depression, anxiety, bipolar condition, tranquilizers ,
medicaments used in treating metabolic conditions, anticancer medicaments,
medicaments used in treating neurological conditions such as epilepsy and
Parkinsons Disease, medicaments used in treating cardiovascular conditions,
non-steroidal anti-inflammatory medicaments, medicaments used in treating
Central Nervous System conditions, and medicaments employed in treating
hepatitis.
The term medicament also encompasses pharmaceutically acceptable
salts, esters, solvates, and/or hydrates of the pharmaceutically active
substances referred to hereinabove. Various combinations of any of the
above medicaments may also be employed.
In accordance with the present invention, the medicament is typically
3o employed in an oral pharmaceutical formulation. An oral pharmaceutical
formulation typically refers to the combination of at least one medicament and
one or more added components or elements, such as an "excipient" or
3
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
"carrier." As will be appreciated by one having ordinary skill in the art, the
terms "excipient" and "carrier" generally refer to substantially inert
materials
that are nontoxic and do not interact with other components of the
composition in a deleterious manner. Examples of normally employed
"excipients," include pharmaceutical grades of carbohydrates, including
monosaccharides, disaccharides, cyclodextrins and polysaccharides (e.g.,
dextrose, sucrose, lactose, raffinose, mannitol, sorbitol, inositol, dextrins
and
maltodextrins); starch; cellulose; salts (e.g., sodium or calcium phosphates,
calcium sulfate, magnesium sulfate); citric acid; tartaric acid; glycine;
leucine;
to high molecular weight polyethylene glyols (PEG); pluronics; surfactants;
lubricants; stearates and their salts or esters (e.g., magnesium stearate);
amino acids; fatty acids; and combinations thereof.
The oral pharmaceutical formulation may be utilized in a variety of unit
dosage forms including, without limitation, a tablet, a pill, a capsule, a
is lozenge, and combinations thereof. The unit dosage forms may encompass
hospital unit dosage forms, as well as others.
The invention will now be described with respect to the drawings. It
should be appreciated that the drawings are merely set forth to illustrate the
invention and do not serve to limit the scope of the invention as defined by
the
20 claims.
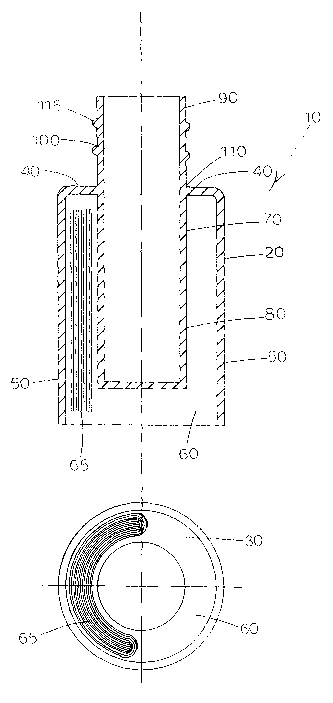
FIGS. 1a and 1b depict an embodiment of a packaging system 10
according to the present invention. As shown, the system 10 is present as a
unitary structure and includes an outer container 20. In this embodiment,
outer container 20 has a cross-sectional area 30 that is circular shaped. The
25 outer container 20 includes a flange 40 and a container wall 50 extending
downwardly from flange 40 and throughout the periphery of the flange 40.
The bottom of the container wall 50 is left unclosed such that it is open to
the
external environment, accordingly, a void space 60 is formed within the
container wall 50.
30 Within the void space 60, and as shown in FIG. 1a, an inner container
70 is present. The diameter of the inner container (d) may be selected from
various sizes. For example, in one embodiment, the diameter may range
4
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
from about 1 cm to about 10 cm. A first portion 80 of the inner container 70
extends below flange 40 and into the void space 60 and a second portion 90
.extending above the flange 40. A package outsert (depicted as 65) may be
present in the void space 60 formed between the inner container 70 and the
outer container 20. The inner container 70 includes a neck 100 having a base
portion 110. As depicted in FIG. 1, the flange 40 is attached to inner
container 70 at base portion 110 such that the outer container 20 and the
inner container 70 are joined and thus present as a unitary structure.
Optionally, and as shown in FIG. 1, the neck 100 of the inner container 70
io may be configured to accommodate a cap. In this embodiment, threads 115
present such that a corresponding cap (not shown) can fit thereon. It should
also be appreciated that other configurations may be used in place of, or in
combination with threads such as, without limitation, helical locks, lug
locks,
and the like.
FIG. 2 represents a perspective view of the packaging system 10
illustrated in cross-section in FIG. 1. As shown, in this particular
embodiment,
a bottle label 120 may be present on outer surface 130 of outer container 20.
Also, in place of, or in addition to label 120, a microelectronic chip having
an
antenna associated therewith (shown in FIG. 5 as 140) can be attached to
outer surface 130. Chip 140 is advantageous in that it allows for the
electronic storage and retrieval of information relevant to the product housed
within packaging system 10.
FIGS. 1 a, 1 b, and 2 depict the cross-section of outer container 20
being circular shaped. It should be appreciated that, for the purposes of the
invention, outer container may have other cross-sectional configurations
including, without limitation, circular, triangular, heptagonal, hexagonal,
octahedral, etc. As an example, FIGS. 3a, 3b and 4 depict the cross-section
of outer container 20 being rectangular (e.g., square) shaped.
FIG. 5 illustrates an array 10' of packaging systems 10 having square
cross-sectional areas. As shown, caps 150 are present on the packaging
systems 10. Such an embodiment is believed to be advantageous in that it
5
CA 02566313 2006-11-14
WO 2005/115869 PCT/US2005/017027
allows for multiple packaging systems to be stored and/or transported in
configuration with minimal wasted space between packaging systems, and
minimal movement of the packaging systems.
The present invention has been described with respect to the
embodiments set forth herein. Nonetheless, it should be noted that such
embodiments are merely set forth to illustrate the invention, and do not limit
its
scope as defined by the claims set forth herein.
6