Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02567394 2012-04-26
[001] This application is a continuation-in-part of U.S. Application No.
11/578,562, filed October 16, 2006, which is a national stage application of
International Application No. PCT/US05/14109, filed April 26, 2005, which
claims the
benefit of priority to U.S. Provisional Application No. 60/566,171, filed
April 29, 2004.
DESCRIPTION OF THE INVENTION
Field of the Invention
[002] The present invention relates to antibodies generated against platelet
membrane glycoprotein VI (GPVI), fragments, or naturally-occurring variants
thereof,
to methods of producing the anti-GPVI antibodies, and to the use of these
antibodies
as research, diagnostic and immunotherapeutic agents, in particular, as
diagnostic
and therapeutic agents for the detection and treatment of thrombosis and other
vascular diseases.
Background of the Invention
[003] Platelets are small, anuclear blood cells that are essential to
hemostatic
control and wound healing. Circulating platelets are fairly quiescent under
normal
conditions. However, when a blood vessel is torn or damaged, platelets are
exposed
to various factors that instigate complicated and interconnected cellular
programs
leading to blood coagulation and clot formation, which are reviewed in
Mechanisms
of Platelet Activation and Control, K. S. Authi, S. P. Watson, and V. V. Kakar
(eds.)
Plenum Press, 1993. The activation of these cellular programs result in
dramatic
increases in membrane adhesive properties, platelet aggregation, and the
release of
vasoconstrictive and fibrinolytic factors. As a consequence, a clot forms at
the site of
trauma, plugging any breach in the vessel wall and providing a substrate for
fibroblast
invasion and repair.
[004] The early events in the clotting process can be functionally separated
into two primary components: adhesion and activation. Adhesion is the process
of
"sticking" platelets to the injured vascular wall, whereas activation
initiates complex
-2-
CA 02567394 2006-11-24
physiological changes inside the cell. Together, these two processes result in
platelet
aggregation, plug formation, and ultimately, in a mature clot. Although these
events
are crucial in limiting blood loss to the site of injury, platelet adhesion
and activation
may also contribute to exacerbation of a diseased state. For example, clotting
may
cause blockage of diseased blood vessels, leading to ischemia and resulting in
damage to vital tissues such as the heart and brain. The dual role of
platelets in
hemostasis and thrombogenesis is reviewed in Ruggeri, Nature Medicine, 8:1227-
1234 (2002).
[005] Most steps in these processes depend on the interaction of
extracellular ligands with specific receptors embedded in the platelet cell
membrane.
In vivo, the first visible change in platelet behavior is the adhesion of
platelets to an
area of denuded endothelium caused by endothelial injury. Among the
micromolecular constituents which become exposed at the denuded endothelium,
collagen is considered the most reactive with platelets. Collagen supports
platelet
adhesion through direct and indirect pathways and also activates platelets by
initiating platelet aggregation and generating coagulant activity necessary
for plug
formation. Baumgartner, Thromb Haemost. 37:1-16 (1977).
[006] The initial contact between the platelets and subendothelium involves
interaction of the platelet glycoprotein complex GPib-V-IX with von Willebrand
factor
(vWf) bound to the exposed subendothelium. This interaction appears to be a
reversible process and is insufficient for stable adhesion, as illustrated by
"rolling" of
platelets along the vessel wall. Ruggeri, Nature Medicine, 8:1227-1234 (2002).
Although the vWf interaction does not completely immobilize circulating
platelets, it is
essential to platelet adherence under high blood flow conditions. Subsequent
irreversible binding of platelets to subendothelial collagen through
glycoprotein GPIa-
Ila (also known as integrin a2131) stabilizes the vWf interaction event,
firmly anchoring
the platelet to the vessel wall. Unlike vWf, collagen adhesion appears to be a
slower
process and is effective only under low flow conditions, or after platelets
have been
partially arrested by vWf interactions. In addition, GPIa-Ila binding induces
the
flattening (spreading) of platelet against the vessel wall. Spreading promotes
the
binding of other subendothelial adhesion factors including fibronectin,
vitronectin and
-3-
CA 02567394 2006-11-24
thrombospondin. These post-spreading interactions further stabilize the
adhesion of
platelets to the vessel wall.
[007] GPIs-Ila is an integrin comprising an alpha and beta subunit. In their
normal conformation, integrins have low affinity for their natural ligand but
may be
converted to high affinity receptors through signals generated by other cell
receptors
Nieswandt B and Watson SP. Blood 102:449-461 (2003). Stimulation of GPIa-Ila
and other collagen receptors induces a host of physiological changes. Among
these
are altered cell surface adhesion properties that result in platelet-platelet
aggregation, and the secretion of various bioactive compounds. These compounds
include the vasoconstrictor, epinephrine, and proclotting factors, which
activate
thrombin and lead to polymerization of fibrinogen into the fibrin threads of a
mature
clot. In addition, activated platelets release ADP and thromboxane A2 (TXA2).
These
powerful thrombogenic factors amplify the initial activation signal,
recruiting additional
platelets into the activated state.
[008] In addition to GPIa-Ila, at least two other collagen receptors are
expressed on the platelet cell surface, namely, GPIV (CD36), and GPVI
(reviewed in
Farndale RW et al., J Thromb. Haemost 2:561-573, 2004). Clues to the functions
of
these platelet collagen receptors have come from the study of human patient
variants. Studies of human patient variants suggest that GPVI plays a major
role in
platelet-collagen interactions while contribution of GPIV remains minor. These
studies also showed that a substantial number of individuals lacking either
GPIa-I la
or GPVI exhibit slightly prolonged bleeding times as compared to those who
express
GPla-lIa or GPVI. GPIa-Ila deficiencies generally lead to more severe bleeding
disorders than those deficient in GPVI. Nevertheless, these patients rarely
present
such a severe bleeding tendency as that seen in individuals with Bernard
Soulier
Syndrome, which is caused by GPIb deficiency, or Glanzmann thromasthenia,
which
is caused by GPIIb-Illa deficiency.
[009] Observations of human variants, along with recent in vitro data, suggest
that the three collagen receptors act in concert to mediate collagen-platelet
interactions. In vitro, for instance, it is now possible to block the activity
of each
collagen receptor with antibodies specific for the collagen receptor sites.
Individually,
-4-
CA 02567394 2006-11-24
each antibody partially inhibits platelet adhesion to collagen and pairwise
combinations of antibodies are significantly more inhibitory, particularly
when GPIa-
Ila and GPVI are inhibited simultaneously. Moreover, these studies demonstrate
that
GPIV, GPIs-Ila, and GPVI contribute to thrombosis through two distinct
pathways,
mechanistically distinguishable by the requirement for divalent metal cations.
[010] Biochemical and sequence information indicates that GPla-11a is a
cation-dependent integrin-type receptor. In contrast, biochemical studies
reveal that
GPIV and GPVI do not require divalent metal cations and are thus of the non-
integrin
type. Of the non-integrin class, observations of human subjects clearly
suggest that
GPVI is more important than GPIV in the primary adhesion process. Indeed, in
vitro
experiments where GPIa-Ila function is blocked by chelating divalant cations,
antibodies directed against GPVI completely abolish collagen-platelet
interaction.
Nakamura et al. J. Biol. Chem. 273:4338-4344, (1998).
[011] GPVI was first identified about 30 years ago by isoelectric focusing and
electrophoresis. Until recently, its function was completely undefined and it
was
known merely as a platelet glycoprotein with a molecular mass of approximately
62
kDa under reducing condition. However, beginning around 1987, Dr. Minoru Okuma
and associates examined several patients with a form of thrombocytopenic
purpura,
a bleeding/bruising syndrome characterized by accelerated platelet destruction
and
decreased numbers of circulating platelets. The platelets in some of Dr.
Okuma's
patients aggregated normally in response to most agonists, including ADP,
thrombin,
ristocetin, and calcium ionophore (A23187) but were markedly unresponsive to
collagen. Moreover, these platelets were found to have reduced amounts, or
even
totally lack, the 62 kDa glycoprotein. Sugiyama et al., Blood 69:1712-20
(1987);
Moroi et al., J. Clin. Invest. 84:1440-45 (1989); Ryo et al., Am. J. Hematol.
39:25-31
(1992); and Arai et al., Brit. J. Haematol. 89:124-130 (1995).
[012] The key reagent in the early studies of GPVI function came from one of
Dr. Okuma's thrombocytopenic purpura patients. This patient presented with
massive, unexplained bleeding and was treated by transfusion with HLA-matched
platelets. Subsequent detailed examination of the patient's blood revealed a
total
lack of GPVI. Most surprisingly, because this patient totally lacked GPVI, her
-5-
CA 02567394 2006-11-24
immune system had identified the GPVI molecules on the transfused platelets as
foreign antigens and produced polyclonal antibodies against GPVI. Sugiyama et
al.,
Blood 69:1712-20 (1987).
[013] A naturally occurring antibody is composed of two identical binding
sites, specific for a single antigenic epitope. The two antigen-specific
portions are
linked by a common stem, or Fc domain, to form a complex capable of binding to
two
identical antigen molecules. Moreover, the divalent nature of the antibody, in
conjunction with aggregatory properties of the Fc domain, allow cross-linking
and
aggregation of many specific antigen molecules. Dr. Okuma found that the
divalent
antibodies from the patient's serum caused a massive aggregation response when
mixed with normal platelets. Conversely, when the antigen-specific domains are
rendered monovalent by enzymatic removal of linking Fc domain, the resulting
Fab
fragments completely abolished collagen-induced aggregation of normal
platelets
and inhibited platelet-collagen adhesion.
[014] Dr. Okuma has graciously made this rare serum available to the
scientific community. Unfortunately, the supply is limited, and the
circumstances
surrounding its discovery are virtually irreproducible. Although the Okuma
serum
made possible much of the research into the function of GPVI and had long
provided
the sole method of identifying a protein as GPVI, it has recently been
discovered that
the C-type lectin, convulxin, specifically binds to GPVI with high affinity
and can be
labeled as a probe to identify the GPVI protein. Francishetti et al., Toxicon
35:1217-
28 (1997); Polgaret al., J. Biol. Chem. 272(24):13576-83 (1997); Jandrot-
Perrus et
al., J. Biol. Chem. 272(2):27035-41 (1997). Convulxin is a venom component
from
the tropical rattlesnake Crotalus durissus terrificus. In its native,
multivalent form,
convulxin is a potent inducer of platelet aggregation and secretion of
proaggregatory
and proclotting factors. The multivalent nature of convulxin is critical to
the
aggregatory effect. Although the underlying physiology of the reaction is
unclear,
individual convulxin subunits still bind to GPVI, but inhibits, rather than
induces
aggregation. It has been suggested that monovalent convulxin blocks the
transmission of collagen-induced signals to the interior of the cell.
-6-
CA 02567394 2006-11-24
[015] Even more recently, the full GPVI sequence was determined.
Clemetson et al., J. Biol. Chem. 274:29019-24 (1999); WO 00/68377; Jandrot-
Perrus
et al., Blood 96:1798-807 (2000); Ezumi et al., Biochem Biophys Res Commun.
277:27-36 (2000). GPVI belongs to the immunoglobulin super family and is non-
covalently associated with the Fc receptor gamma chain (FcRy chain). Gibbins
et al.,
FEBS Lett. 413:255-259 (1997); Tsuji et al., J. Biol. Chem. 272:23528-23531
(1997).
It is currently believed that collagen binding to GPVI induces tyrosine
phosphorylation
of FcRy. Phosphorylated FcRy then recruits the Syk kinase, ultimately leading
to a
cascade of intracellular events including phospho-activation of Syk, and
phospholipase C-y2. These events ultimately result in increased intercellular
calcium
levels and the secretion of proaggregatory and proclotting factors. Therefore,
the
FcRy chain serves as the signal-transducing part of the receptor in humans and
mouse platelets. Clemetson et al., J. Biol. Chem. 274:29019-290 (1999);
Jandrot-
Perrus et al., Blood 96:1798-1807 (2000); Gibbins et al., FEBS Lett. 413:255-
259
(1997); Tsuji et al., J. Biol. Chem. 272:23528-23531 (1997).
[016] Platelet activation, including that mediated by GPVI, can also stimulate
platelet matrix metalloproteases (MMPs). Jurasz et al., Circ. Res. 90:1041-
1043
(2002). Furthermore, activation of platelets results in loss or down
regulation of
certain cell surface receptors. This loss or down regulation of surface
receptors often
entails the proteolytic cleavage or shedding of their extracellular domains,
mediated
by the activated platelet MMPs. Two specific examples of such shedding are the
proteolytic cleavage by MMPs of GPlba and GPVI on human platelets. Bergmeier
et
al., Circ. Res. 95:667-683 (2004); Bergmeier et al., Thromb. Haemost. 91:951-
958
(2004); Gardiner et al., Blood 104:3611-3617 (2004); Stephens et al., Blood
105:186-
191 (2005).
[017] Down-regulation of GPVI and shedding of its extracellular domain
(ectodomain; soluble GPVI; sGPVI) into plasma has been observed in murine
platelets and suggested also in human platelets. Boylan et al., Blood 104:1350-
1355
(2004); Nieswandt et al., J. Exp. Med. 193:459-470 (2001); Sugiyama et al.,
Blood
67:1712-1720 (1987). Two recent studies have described slow shedding of sGPVI
from platelets in reponse to activation by the GPVI-specific agonists
collagen,
-7-
CA 02567394 2006-11-24
collagen related peptide (CRP) and convulxin. Bergmeier et al., Thromb.
Haemost.
91:951-958 (2004); Gardiner et al., Blood 104:3611-3617 (2004). In both
studies,
MMP specific inhibitors blocked agonist-induced sGPVI shedding, at least under
in
vitro conditions.
[018] Shedding of sGPVI in response to platelet activation via GPVI also
appears to occur in vivo. This is supported by the observation that injection
of rat
anti-mouse GPVI antibodies in mice results in shedding of sGPVI. Nieswandt et
al., J.
Exp. Med. 193:459-469 (2001). It has also been shown that an anti-human GPVI
antibody can induce shedding of sGPVI from circulating human platelets in
NOD/SCID mice. Boylan et al., Blood 108:908-914 (2006). Furthermore, elevated
levels of GPVI on the platelet surface have been reported in patients with
severe
myocardial infarction. Samaha et al., Med. Sci. Monit. 11:CR224-CR229 (2005);
Bigalke et al., Eur. Heart J. 27:2165-2169 (2006). These investigators
proposed that
determination of GPVI levels on the platelet surface in patient blood, as a
platelet-
specific thrombotic marker, may help to identify high risk patients before
myocardial
ischemia becomes evident.
[019] Patients deficient in GPVI suffer from mild bleeding diathesis and their
platelets respond poorly to collagen. Sugiyama et al., Blood 69:1712-1720
(1987);
Moroi et al., J. Clin. Invest. 84:1140-1445 (1989); Arai et al., Br. J.
Haemtol. 89:124-
130 (1995). Studies with GPVI-deficient human platelets or platelets blocked
with
anti-GPVI Fab fragments (obtained from a patient serum) clearly demonstrated a
lack
of platelet interaction with immobilized collagen under high and low shear
rates and
reduced firm adhesion to immobilized vWf under high shear. Goto et al.,
Circulation
106:266-272 (2002).
[020] Studies with knockout mice deficient in the FcRy chain, which also
results in a GPVI-deficient phenotype, or with mice depleted of GPVI,
confirmed
these observations but these animals exhibited slightly prolonged tail
bleeding time.
Nieswandt et al., The EMBO Journal 20:2120-2130 (2001). GPVI-deficient, FcRy -
positive mice showed bleeding times similar to those of wild type and GPVI-
heterozygous mice. Platelets from the GPVI-deficient mice did not aggregate in
response to collagen and to convulxin, and showed dramatically reduced
adhesion to
-8-
CA 02567394 2006-11-24
immobilized collagen under flow conditions, thereby confirming that GPVI plays
a
major role in collagen-induced platelet functions and thrombosis. Kato et al.,
Blood
102:1701-1707 (2003).
[021] It is now accepted that GPVI is the principle receptor for collagen-
induced platelet activation, and is a critical conduit for signal
transduction. lchinohe
et al., J. Biol Chem. 270(47):28029-28036 (1995); Tsuji et al., J. Biol Chem.
272(28):23528-31 (1997). In contrast, the other major collagen receptor in
platelets,
GPla-Ila, is primarily involved with the cation-dependent processes for
effecting
stable adhesion and spreading leading to thrombus growth. Reviewed in
Nieswandt
and Watson, Blood 102:449-461 (2003).
[022] The need in the art for GPVI antagonists, such as antibodies against
GPVI, is highlighted by the unfortunate fact that inappropriate platelet
aggregation
and clot formation is a major etiologic factor in a wide range of human
diseases, most
commonly, vascular diseases. Excessive platelet deposition on the inner walls
of
arteries and veins contributes to atherosclerosis and arteriosclerotic
plaques, which
reduce the flow of blood to sensitive tissues. Ultimately, this platelet-
dependent
buildup may manifest as acute myocardial infarct, chronic unstable angina,
transient
ischemia, stroke, peripheral vascular disease, arterial thrombosis, pulmonary
embolism, restenosis, and various other conditions.
[023] These conditions typically begin with an abnormal clot that develops in
a blood vessel, called a thrombus. Once a clot has developed, continued flow
of
blood past the clot is likely to break it free from its attachment. Such
freely flowing
clots are known as emboli. Emboli generally travel through the circulation
until
trapped in a narrow point in the circulatory system. This occlusion may occur
in the
brain, lung or coronary arteries, resulting in pain, disability or death.
[024] Intravascular clots may result from naturally-occurring sclerosis,
septicemic shock, or physical damage to blood vessels. Indeed, the very
invasive
methods used to diagnose and treat vascular disease, (e.g. vascular grafts,
exploratory and in-dwelling catheters, stents, shunts, and other devices)
themselves,
damage vessel walls. This can activate platelets, stimulate aggregation, and
ultimately lead to the formation of thrombi and emboli, further endangering
the life
-9-
CA 02567394 2006-11-24
and health of the patient. Thus, methods for controlling or reducing platelet
aggregation and clot formation have been a long-sought goal in managing these
diseases.
[025] Collagen-induced platelet activation may also result in elevated plasma
levels of sGPVI via shedding. The measurement of sGPVI in patient-derived
plasma
may therefore directly relate to the severity of any existing vascular injury
or
thrombosis. Thus, there is a need for the development of improved methods for
the
detection and quantitation of sGPVI in plasma. Such methods may provide a
sensitive indicator of inappropriate platelet aggregation and clot formation
and allow
early diagnosis, monitoring and prevention of these conditions.
SUMMARY OF THE INVENTION
[026] The present invention provides GPVI specific antibodies that are more
potent inhibitors of collagen-induced platelet functions than those previously
reported
in the art. Thus, the GPVI specific antibodies of the invention may be useful
antithrombotic agents and may have reduced side effects often associated with
administration of other antithrombotic agents.
[027] One aspect of the present invention provides a monoclonal antibody
specific for a GPVI polypeptide, peptide, or naturally-occurring variant
thereof, that
inhibits collagen-induced platelet aggregation at an IC50 of less than about
7, 4, 3, 2,
1, 0.6, g/ml, or any value subsumed within this range. The monoclonal
antibody
specific for a GPVI polypeptide, peptide, or naturally-occurring variant
thereof may
also inhibit collagen-induced platelet adhesion at an IC50 of less than about
1, 0.5,
0.2, 0.1 g/ml, or any value subsumed within this range.
[028] Another aspect of the present invention provides a monoclonal antibody
specific for a GPVI polypeptide, peptide, or naturally-occurring variant
thereof, that
specifically binds to a GPVI polypeptide, peptide, or naturally-occurring
variant
thereof, at a Kd of equal to or lower than 10-8M. In another embodiment, the
anti-
GPVI antibodies of the invention specifically bind to a GPVI polypeptide,
peptide, or
naturally-occurring variant thereof, at a Kd of equal to or lower than 10-9M.
The
-10-
CA 02567394 2006-11-24
monoclonal antibodies of the present invention also inhibit collagen-induced
ATP
secretion and/or collagen-induced thromboxane A2 formation.
[029] The monoclonal antibodies include active antibody fragments. Active
antibody fragments may include chemically, enzymatically, or recombinantly
produced Fab fragments, F(ab)2 fragments, or peptides comprising at least one
complementarity determining region (CDR) specific for a GPVI polypeptide,
peptide,
or naturally-occurring variant thereof. In an embodiment of the invention, the
CDRs
comprise any one of the sequences of SEQ ID NOs. 1-24, or a variant thereof,
wherein the CDR variant specifically binds a GPVI polypeptide, peptide, or
naturally-
occurring variant thereof. Exemplary antibodies include OM1, OM2, OM3, and
OM4.
[030] The present invention also provides a method for inhibiting platelet
aggregation, collagen-induced ATP secretion, collagen-induced thromboxane A2
formation, and/or platelet adhesion, by contacting platelets with a monoclonal
antibody specific for a GPVI polypeptide, peptide, or naturally-occurring
variant
thereof.
[031] The invention further provides a method of producing a monoclonal
antibody specific for a GPVI polypeptide, peptide, or naturally-occurring
variant
thereof. The method comprises immunizing a GPVI-deficient host with a GPVI
antigen and obtaining the antibody. A GPVI-deficient host includes, for
example, a
GPVI heterozygous host and a homozygous GPVI knock-out host. Another aspect of
the invention provides monoclonal antibodies specific for a GPVI polypeptide,
peptide, or naturally-occurring variant, thereof produced by the method
described.
[032] The invention also provides an antithrombotic composition comprising a
pharmaceutically effective amount of a GPVI specific monoclonal antibody of
the
invention. The antithrombotic agent may be used to treat a patient. Thus, an
aspect
of the invention provides a method of treating a patient who, for example, is
in need
of treatment for vascular disease.
[033] The invention further provides a highly sensitive assay for detecting
and
quantifying soluble GPVI (sGPVI). This assay comprises a first GPVI-specific
antibody and a second GPVI-specific antibody. The first GPVI-specific antibody
of
the invention may capture the sGPVI and the second GPVI-specific antibody of
the
- 11 -
CA 02567394 2006-11-24
invention may detect the captured sGPVI. The first antibody may be immobilized
on
a solid support and the second antibody may be labeled. The two antibodies may
bind sGPVI non-competitively. This assay is useful for determining the levels
of
soluble GPVI in biological samples, for example in serum or plasma.
[034] The invention also provides a method and a kit for the quantitation of
sGPVI in samples obtained from individuals who may be at risk of developing or
who
have a vascular disease or thrombosis. Because the levels of sGPVI may relate
to
the severity of an existing vascular disease or thrombosis, the method and the
kit are
useful for the prevention, diagnosis, and treatment of such diseases. The
vascular
diseases include, for example, platelet disorders, thrombocytopenia, cerebral
vascular disease, pheripheral vascular disease and cardiovascular disease.
[035] The invention further provides a method for identifying antithrombotic
agents by contacting a GPVI antigen with a GPVI specific monoclonal antibody
of the
invention and a test compound, and measuring inhibition of the binding of the
monoclonal antibody to the GPVI antigen. The GPVI antigen, GPVI specific
monoclonal antibody, and the test compound may be added in any order. For
example, the GPVI antigen may be contacted with the test compound before
contacted with the GPVI specific monoclonal antibody. In another example, the
GPVI
antigen may be contacted with GPVI specific monoclonal antibody and the test
compound simultaneously.
[036] It is to be understood that both the foregoing general description and
the following detailed description are exemplary and explanatory only and are
not
restrictive of the invention, as claimed.
[037] The accompanying drawings, which are incorporated in and constitute a
part of this specification, illustrate several embodiments of the invention
and together
with the description, serve to explain the principles of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[038] Figure 1 is a schematic diagram of the generation of GPVI knock-out
mice. Fig. 1A shows the wild-type allele of GPVI, the targeting vector used
for
homologous recombination at exons 2 and 3, and the resulting mutant allele.
Fig. 1 B
-12-
CA 02567394 2006-11-24
shows the size differences of the 5' and 3' fragments derived by cleavage with
restriction enzymes in wild-type and mutant genomes.
[039] Figure 2 is a bar graph showing the effects of Fab fragments of OM1,
OM2, OM3, and OM4 at 0.1-100 g/ml on platelet (human) adhesion to fibrillar
collagen under static conditions.
[040] Figure 3 is a bar graph showing the effects of Fab fragments of OM1,
OM2, OM3, and OM4 at 0.001-1 .ig/ml on Mg2+-independent (GPVI-dependent)
human platelet adhesion to fibrillar collagen under static conditions.
[041] Figure 4 illustrates the effects of Fab fragments of OM1, OM2, OM,
OM4, and ReoPro on platelet (human) adhesion to acid insoluble collagen under
high shear stress (2600 sec-1) conditions.
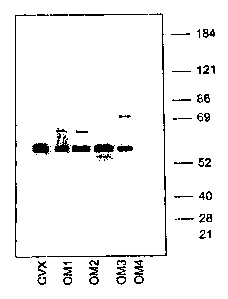
[042] Figure 5 is a Western blot of the OM series antibodies (OM1, OM2,
OM3, and OM4) and convulxin (CVX) reacting with GPVI in human platelet lysate.
[043] Figure 6 illustrates the complete lack of platelet aggregation induced
by
collagen and convulxin in GPVI knock-out animals.
[044] Figure 7 illustrates the lack of interaction of platelets from GPVI
knock-
out mice to acid insoluble collagen under high shear stress conditions.
[045] Figure 8 is a graph showing the effect of the OM2 Fab fragment and
ReoPro on ex vivo collagen-induced platelet aggregation in Cynomolgus
monkeys.
[046] Figure 9 is a graph showing the effect of the OM2 Fab fragment and
ReoPro on skin bleeding time in Cynomolgus monkeys.
[047] Figure 10 is a graph showing the anti-aggregation effect of the OM2
Fab fragment and ReoPro over time after bolus injection (0.4 mg/kg) in
Cynomolgus
monkeys.
[048] Figure 11 is a graph showing the time-course effect of the OM2 Fab
fragment and ReoPro on skin bleeding time after bolus injection (0.4 mg/kg)
in
Cynomolgus monkeys.
[049] Figure 12 illustrates the effects of the OM4 Fab and 7E3 F(ab')2
fragments on ex vivo collagen-induced platelet aggregation in rats.
-13-
CA 02567394 2006-11-24
[050] Figure 13 illustrates the effects of the OM4 Fab and 7E3 F(ab')2
fragments on bleeding time in rats. Figure 13A shows the effects on nail
bleeding
time and Figure 13B shows the effects on tail bleeding time.
[051] Figure 14 illustrates the effects of the OM4 Fab and 7E3 F(ab')2
fragments on platelet count in rats.
[052] Figure 15 is a bar graph showing the effect of the OM4 Fab fragment on
arterial thrombus formation in rats when administered before (Figure 15A) and
after
(Figure 15B) endothelial injury.
[053] Figure 16 is a graph showing the concentration-dependent binding of
biotinylated OM2 Fab fragment to human platelets in vitro.
[054] Figure 17 illustrates the production and purification of recombinant
sGPVI protein. Figure 17A illustrates the structure of the recombinant sGPVI.
Figure
17B shows the recombinant sGPVI in a SDS-polyacrylamide gel.
[055] Figure 18 is a schematic representation of an ELISA for detecting
sGPVI. Figure 18A illustrates the relationship of the epitopes recognized by
the
GPVI-specific antibodies OM1, OM2, OM3 and OM4. Figure 18B shows a schematic
diagram of the ELISA system.
[056] Figure 19 illustrates the quantitation of sGPVI in a biological sample
by
the ELISA, using purified recombinant sGPVI as the standard protein. The curve
shows the sensitivity and range of the ELISA.
[057] Figure 20 illustrates a method for detecting agonist-induced sGPVI
shedding from platelets in biological samples.
[058] Figure 21 is a graph showing the detection of sGPVI shed in platelet-
rich plasma from human donors in response to platelet activation by GPVI-
specific
and non-GPVI-specific agonists.
[059] Figure 22 shows the time course of sGPVI shedding from human
platelets upon activation by GPVI-specific and non-GPVI-specific agonists.
DESCRIPTION OF THE EMBODIMENTS
[060] This invention describes novel GPVI specific antibodies that are potent
inhibitors of collagen-induced platelet responses, including, but not limited
to, platelet
-14-
CA 02567394 2012-04-26
aggregation, adhesion, collagen-induced ATP release, and thromboxane A2 (TXA2)
formation. The invention also describes methods for producing the anti-GPVI
antibodies. The anti-GPVI antibodies of the invention may be useful for
inhibiting
thrombus formation and for treating patients in need of anti-thrombotic
treatment.
[061] The term "antibodies" includes monoclonal antibodies. The monoclonal
antibodies of the invention include active antibody fragments, such as
F(ab')2, and
Fab fragments, as well as any recombinantly produced binding partners.
Antibodies
are defined to be "specifically binding" if they bind a GPVI polypeptide,
peptide, or
naturally-occurring variant thereof, with a dissociation constant (Kd) equal
to or lower
than 10"7M. In an embodiment of the invention, the anti-GPVI antibodies
specifically
bind to a GPVI polypeptide, peptide, or naturally-occurring variant thereof,
at a Kd of
equal to or lower than 10-8M. In another embodiment, the anti-GPVI antibodies
of the
invention specifically bind to a GPVI polypeptide, peptide, or naturally-
occurring
variant thereof, at a Kd of equal to or lower than 10-9M. Affinities of
binding partners
or antibodies may be readily determined using conventional techniques, for
example
by measuring the saturation binding isotherms of 1251-labeled IgG or its
fragments, or
by homologous displacement of 1251gG by unlabeled IgG using nonlinear-
regression
analysis as described by Motulsky, in Analyzing Data with GraphPad Prism
(1999),
GraphPad Software Inc., San Diego, CA. Other techniques are known in the art,
for
example, those described by Scatchard et al., Ann. NY Acad. Sci., 51:660
(1949).
GPVI polypeptides, peptides, or naturally-occurring variants thereof, are
described in
U. S. Publication No. 2003/0186885,
[062] Antibodies may be readily generated from a variety of sources, for
example, horses, cows, goats, sheep, dogs, chickens, rabbits, mice, hamsters,
or
rats, using procedures that are well-known in the art. In an embodiment of the
invention, the host animals are Armenian hamsters. In another embodiment, the
host
animals are GPVI-deficient animals. As used herein, "GPVI-deficient" refers to
about
50% or greater reduction in endogenous GPVI production in an animal compared
to
a wild-type animal. The reduction in endogenous GPVI production may be such
that
GPVI production is completely inhibited. GPVI-deficient animals may be
generated
-15-
CA 02567394 2006-11-24
by a number of methods known in the art. These may include manipulation of
GPVI
production at the nucleic acid (DNA or RNA) level in an animal. GPVI-deficient
animals may be generated by methods including, but not limited to, knock-out
(see,
e.g., Galli-Taliadoros et al., J. Immunol. Methods 181:1-15, 1995; Robbins,
Circ. Res.
73:3-9, 1993; Hergueux et al., Transplant Proc. 25:30-32, 1993), knock-in
(Colucci-
Guyon et al., Cell 79:679-694, 1994; Le Mouellic et al., PNAS 87:4712-4716,
1990;
Hanks et al., Science 269:679-682, 1995; Wang et al., Nature 379:823-825,
1996),
mutation (Askew et al., Mol. Cell. Biol. 13:4115-4124, 1993; Stacey et al.,
Mol. Cell.
Biol. 14:1009-1016, 1995; Hasty et al., Nature 350:243-246, 1991; Valancius et
al.,
Mol. Cell. Biol. 11:1402-1408, 1991; Wu et al., PNAS 91:2819-2823, 1994; Horie
et
al., Gene 166:197-204, 1995; Toth et al., Gene 178:161-168, 1996), deletion
(You et
al., Nature Genet., 15:285-288, 1997; Holdener-Kenny et al., Bioessays 14:831-
839,
1992), antisense oligonucleotide technology (Wagner et al., Nature Biotechnol.
14:840-844, 1996; Kitajima et al., Science 258:1792-1795, 1992; Urban et al.,
Farmaco. 58:243-58, 2003; Orum et al., Curr Opin Mol Thera 3:239-43, 2001;
Sohail
et al., Curr Opin Mol Ther. 2:264-71, 2000; Smith et al., Eur J Pharm Sci.
11:191-8,
2000), interfering RNA (RNAi) technology (Scherr et al., Curr Med Chem. 10:245-
56,
2003; Nishikura, Cell 107:415-418, 2001; Hannon, Nature 4418:244-251, 2002;
U.S.
Patent No. 5,506,559), or by using any other chemicals, naturally-occurring,
recombinant, or synthetic peptides, polypeptides, proteins, polysaccharides,
small
molecules and other compounds designed to reduce or inhibit GPVI production in
a
host.
[063] Without being bound to theory, hosts that produce none or lower than
normal amounts of endogenous GPVI may mount a stronger immune reaction to
GPVI than those that make normal levels of GPVI. Thus, antibodies to GPVI that
are
more effective at inhibiting collagen-induced platelet responses such as
platelet
aggregation, thrombogenesis, and/or platelet activation at lower doses may be
produced compared to those obtained from normal hosts that make GPVI. Methods
for producing antibodies in knock-out animals have been described in Pass et
al.,
Scand. J. Immunol. 58:298-305 (2003); Zlot et al., J. Lipid Res. 40:76-84
(1999);
-16-
CA 02567394 2012-04-26
Declerck et a/., J. Biol. Chem. 270:8397-8400 (1995); and Castrop et al.,
Immunobiology 193:281-287 (1995).
[064] Hosts may be immunized as described in US Patent Publication No. US
2003/0186885 Al. Briefly, hosts may be immunized with "GPVI antigen" which
includes, but is not limited to, native GPVI polypeptides, peptides, or
naturally-
occurring variants thereof, isolated from platelets or other GPVI-expressing
cells;
recombinant GPVI polypeptides, peptides, or recombinant forms of naturally-
occurring
variants thereof, expressed from prokaryotic or eukaryotic cells; platelets
obtained from
various species, including human; cells expressing GPVI polypeptides,
peptides, or
naturally-occurring variants thereof; nucleic acids encoding GPVI
polypeptides,
peptides, or naturally-occurring variants thereof; or any combination thereof.
[065] Purified GPVI polypeptides, or a peptide based on the amino acid
sequence of GPVI polypeptides conjugated to an adjuvant or carrier, are
typically
administered to the host animal intraperitoneally. The immunogenicity of GPVI
polypeptides may be enhanced through the use of an adjuvant, for example,
Freund's complete or incomplete adjuvant. Following booster immunizations,
small
samples of serum are collected and tested for reactivity to GPVI polypeptides.
Examples of various assays useful for such determination include those
described in:
Antibodies: A Laboratory Manual, Harlow and Lane (eds.), Cold Spring Harbor
Laboratory Press, 1988; as well as procedures such as countercurrent immuno-
electrophoresis (CIEP), radioimmunoassay, radioimmunoprecipitation (RIP),
enzyme-
linked immuno-sorbent assays (ELISA), dot blot assays, and sandwich assays and
FACS. See U.S. Pat. Nos. 4,376,110 and 4,486,530.
[066] Monoclonal antibodies can be readily prepared using well-known
procedures, see for example, the procedures described in U.S. Pat. Nos. RE
32,011,
4,902,614, 4,543,439, and 4,411,993; Monoclonal Antibodies, Hybridomas: A New
Dimension in Biological Analyses, Plenum Press, Kennett, McKearn, and Bechtol
(eds.), 1980. Briefly, the host animals are injected intraperitoneally at
about 1 week
intervals with a GPVI antigen, optionally in the presence of adjuvant.
Immunizations
are carried out until desired titer of antibody is achieved.
-17-
CA 02567394 2012-04-26
[067] Mouse sera are then assayed for antibody titer by FACS analysis using
GPVI-FcRy chain transfected CHO cells, or any other method known in the art.
The
selected mice are given a booster dose of the GPVI antigen. Three days later,
the
mice are sacrificed and their spleen cells are fused with commercially
available
myeloma cells, P3U1 (ATCC), following established protocols. Myeloma cells are
washed several times in serum-free media and fused to mouse spleen cells. The
fusing agent is 50% PEG (Roche). Fusion is plated out into eight 96-well flat
bottom
plates (Corning) containing HAT supplemented DMEM media and allowed to grow
for
1-2 weeks. Supernatants from resultant hybridomas are collected and analyzed
for
the presence of anti-GPVI antibodies by performing FACS analysis using CHO
cells
expressing GPVI and FcRy-chain. FACS analysis is also performed using wild-
type
CHO cells to eliminate clones producing antibodies against CHO cell antigens.
Positive clones can be grown in bulk culture and supernatants are subsequently
purified over a Protein A or G SepharoseTM column (Pharmacia). It is
understood
that many techniques could be used to generate antibodies against GPVI
polypeptides and peptides and that this embodiment in no way limits the scope
of the
invention.
[068] The monoclonal antibodies of the invention may be produced using
alternative techniques, such as those described by Alting-Mees et al.,
"Monoclonal
Antibody Expression Libraries: A Rapid Alternative to Hybridomas", in
Strategies in
Molecular Biology 3:1-9 (1990). Similarly, binding partners constructed, for
example,
using recombinant DNA techniques to incorporate the variable regions of a gene
that
encodes a specific binding antibody, are included in the monoclonal antibodies
of the
invention. Such a technique is described in Larrick et al., Biotechnology,
7:394 (1989).
[0691 Other types of antibodies may be produced in conjunction with the state
of knowledge in the art. For example, antiidiotype antibodies may be obtained
by
immunizing a host with an antigen comprising the antigen binding site of a
purified
monoclonal anti-GPVI antibody, and testing the resultant sera or monoclonal
supernatant for activity as described in Knight et al., Mol. Immunol 32:1271-
81
(1995). In an embodiment of the invention, the antiidiotype antibodies may be
-18-
CA 02567394 2012-04-26
obtained by immunizing a host with a peptide comprising a complementarity
determining region (CDR) of an anti-GPVI antibody of the invention. The
antiidiotype
antibodies of the invention include active antiidiotype antibody fragments,
which refer
to chemically, enzymatically, or recombinantly produced fragments of an
antiidiotype
antibody, including, Fab, F(ab)2, or peptides comprising at least one
complementarity
determining region (CDR) that bind specifically to an anti-GPVI antibody. In
addition,
the invention comprises biosynthetic GPVI antibody binding sites, as described
by
Huston et al., Proc. Natl, Acad. Sci. USA 85:5879 (1988); single-domain
antibodies
comprising isolated heavy chain variable domains, as described by Ward et al.,
Nature 341:544 (1989); and antibodies that have been engineered to contain
elements of human antibodies that are capable of specifically binding GPVI
polypeptides. Anti-GPVI antibodies may also be generated using the phage
display
technology as described in Ventor et al., Ann Rev. Immunol. 12:43355 (1994)
and
the references cited therein.
[070] The antibodies of the invention also include active antibody fragments,
which refer to chemically, enzymatically, or recombinantly produced fragments
of an
antibody, including, Fab, F(ab)2, or peptides comprising at least one
complementarity
determining region (CDR) that bind specifically to a GPVI polypeptide,
peptide, or a
naturally-occurring variant thereof. A common enzymatic method utilizing
pepsin or
papain removes the Fc antibody domain to produce bivalent F(ab)2 and
monovalent
Fab fragments. These procedures are basically described in Gorini et al., J.
Immunol. 103:1132 (1969); Handbook of Experimental Immunology Vol 1: DM Wier
(ed), Blackwell Alden Press, Oxford, UK, 1997; and Antibodies: A Laboratory
Manual, Harlow and Lane (eds.), Cold Spring Harbor Laboratory Press, 1988; and
U.S. Pat. No. 4,470,925 (Auditore-Hargreaves).
[071] Intact GPVI-specific antibodies, and antibody fragments, such as Fab
and F(ab)2 fragments, may be covalently coupled to drugs or carrier molecules.
In
addition, the GPVI-specific antibodies of the invention may be cross-linked,
directly,
or through a suitable carrier molecule, to form multivalent complexes. In one
embodiment, F(ab)2 fragments are metabolically stabilized by covalent cross
linking
-19-
CA 02567394 2012-04-26
as described in Reno et al. (U.S. Pat. No. 5,506,342).
[072] Monoclonal antibodies specific for a GPVI polypeptide, peptide, or a
naturally-occurring variant thereof, may be tested for their ability to block
platelet
activation by ligand (collagen)-dependent binding. Monoclonal antibodies which
block platelet functions may be useful antithrombotic agents.
[073] The antibodies of the present invention may also be humanized.
Human and humanized antibodies are thus preferred for clinical use. See, for
example, LoBuglio et al., Proc. Natl. Acad. Sci. USA 86:4220-24 (1989);
Meredith et
al., J. Nucl. Med. 33, 23-29 (1992); Salah et al., Hum. Antibod. Hybridomas
3:19-24
(1992); Knight et al., Mol. Immunol 32:1271-81 (1995); and Lockwood et al., Q.
J.
Med. 89:903-12, (1996).
[074] Development of fully human antibodies generally require a suitable
source of human immune B lymphocytes. One method for generating human
antibodies involves the immunization and expansion of B lymphocytes with
suitable
specificities from pools of naive B cells obtained from non-immunized
individuals that
were placed in in vitro culture. Ohlin and Borrebaeck, in Methods of
Immunological
Analysis, vol. II, Masseyeff et al. (eds), VCH Verlagsgesellschaft mbH,
Weinheim, p.
298-325 (1992); Borrebaeck and Ohlin, in Protocols in Cell and Tissue Culture,
Doyle
et al. (eds), J. Wiley & Sons Ltd., Chichester 25E:1.1-7 (1993). Human
antibodies
against HIV-1 glycoproteins have been developed by this method. Ohlin et al.,
Immunology 68:325-331 (1989); Ohlin et al., Clin. Exp. Immunol. 89:290-295
(1992);
Duenas et al., Immunology 89:1-7 (1996). More recently, in vivo technologies
have
been developed that utilize animals either engrafted with human immune cells
or
those that have been introduced with the entire human immunoglobulin loci.
Ilan et
al., Curr. Opin. Mol. Ther. 4:102-109 (2002); Ishida et al., Cloning Stem
Cells 4:91-
102 (2002). Fully human antibodies have been produced upon immunization of
these animals with various human antigens.
[075] Humanized antibodies may be generated by replacing most, or all, of
the structural portions of a monoclonal antibody with corresponding human
antibody
sequences. Consequently, a hybrid molecule is generated in which only the
antigen-
-20-
CA 02567394 2012-04-26
specific variable, or complementarity determining region (CDR) is composed of
non-
human sequence. Various strategies for designing humanized antibodies are
reviewed in Winter and Milstein, Nature 349:293-99 (1991); Harris, BCSTBS5
23(4):1035-38 (1995); Morrison and Schlom, in Important Advances in Oncology,
J.
B. Lippincott Co. (1990); L. Presta, "Humanized Monoclonal Antibodies," in
Annual
Reports in Medicinal Chemistry, Academic Press, (1994); and A. Lewis and J.
Crowe,
"Generation of Humanized Monoclonal Antibodies by'Best Fit' Framework
Selection
and Recombinant Polymerase Chain Reaction " in Generation of Antibodies by
Cell
and Gene Immortalization. Year Immunol. 1993, vol 7, pp 110-118, (C. Terhorst,
F.
Malvasi, and A. Albertini (eds.) Basel, Karger.
[076] Antibodies specific for a GPVI polypeptide, peptide, or naturally-
occurring variant thereof, may also be humanized by selecting and purifying
anti-
GPVI antibodies by Ig-specific adsorption, such as Protein A chromatography,
or by
affinity chromatography using immobilized GPVI peptides. The heavy and light
chains may be dissociated by standard means, and the individual chains
purified. A
partial amino acid sequence of the individual chains may be determined and
degenerate oligonucieotides may be generated for each chain according to the
method of Lathe et al., J. Mol. Biol. 183:1-12 (1985). The DNA encoding these
antibody chains may then be cloned and sequenced from the anti-GPVI antibody-
producing cell by PCR or other standard methods.
[077] The antibody DNA and amino acid sequence may be analyzed and
compared with known sequences of human heavy and light chains. Based on the
sequence comparisons, the GPVI-specific antibody chains may be humanized by
replacing portions of the non-human DNA with human sequences, thus forming a
chimeric antibody with specificity to GPVI. In one embodiment, the GPVI-
specific
antibody is humanized with human J1 and K constant regions using the
expression
vectors described by Sun et al., Proc. Natl. Acad. Sci. USA 84:214-218 (1987).
Methods for the preparation of nonhuman-human hybrids are well known in the
art
and described in detail in, for example, Knight et al., Mol. Immunol 32:1271-
81
(1995); U.S. Pat. Nos. 5,705,154 (Dalie et al.); 5,693,322 (Creekmore et al.);
-21-
CA 02567394 2012-04-26
5,677,180 (Robinson et al.); 5,646,253 (Wallace et al.); 5,585,097 (Bolt et
al.);
5,631,349 (Diamantstein et al.); and 5,580,774 (Beavers et al.). To maximize
the
production of high affinity chimeric antibodies, the methods of Queen et al.,
(U.S. Pat.
No. 5,585,089) and Queen et at, Proc. Nat. Acad. Sci. USA, 86:10029-33 (1989),
may
be employed.
[078] Humanized antibodies may also be generated using the phage display
approach as taught in Rader et al., Proc. Nat. Acad. Sci. USA, 95:8910-8915
(1998)
and Steinberger et al., J. Biol. Chem. 275:36073-36078 (2000) and as
exemplified by
Son et al., J Immunol Methods. 286:187-201 (2004), Lee et al., J Immunother.
27:201-210 (2004), and by others skilled in the art.
[079] The part of the antibody molecule that binds to an antigen is comprised
of only a small number of amino acids in the variable (V) regions of the heavy
(VH)
and light (VL) chains. These amino acids are brought into close proximity by
folding
of the V regions. Comparisons of the amino acid sequences of the variable
regions of
lgG show that most of the variability resides in three regions called the
complementarity determining regions (CDRs). Each chain (H and L) contains
three
CDRs. Antibodies with different specificities have different CDR's while
antibodies of
the exact same specificity generally have identical or highly conserved CDR's.
The
present invention encompasses monoclonal antibodies or peptides comprising at
least one complementarity determining region (CDR), or a variant thereof, of
the
GPVI antibodies of the invention. The invention encompasses monoclonal
antibodies or peptides comprising at least one of the amino acid sequences of
SEQ
ID NOs. 1-24, or variants thereof.
[080] A "variant" of an antibody or a peptide comprising a CDR herein refers
to an antibody or peptide comprising an amino acid sequence substantially
identical
to SEQ ID NOs. 1-24, but which has an amino acid sequence different from that
of
SEQ ID NOs. 1-24 because of one or more deletions, insertions or
substitutions. The
variant also retains at least 70, 80, 90 or 100% of its binding affinity to a
GPVI
polypeptide, peptide, or naturally-occurring variant thereof, compared with an
antibody or peptide comprising its corresponding CDR. Binding affinities may
be
determined according to any method known in the art, for example, as taught by
-22-
CA 02567394 2012-04-26
Fujimura et al., Thromb. Haemost. 87:728-734 (2002) and as exemplified in
Example
4 below. A variant comprises a CDR that is preferably at least 60%, 65%, 70%,
80%,
85%, or 90% identical to SEQ ID NOs. 1-24. The percent identity can be
determined,
for example, by comparing sequence information using the GAP computer program,
version 6.0 described by Devereux et al. (Nucl. Acids Res. 12:387, 1984) and
available from the University of Wisconsin Genetics Computer Group (UWGCG).
The
GAP program utilizes the alignment method of Needleman and Wunsch (J. Mol.
Biol.
48:443, 1970), as revised by Smith and Waterman (Adv. Appl. Math 2:482,1981).
The preferred default parameters for the GAP program include: (1) a unary
comparison matrix (containing a value of 1 for identities and 0 for non-
identities) for
nucleotides, and the weighted comparison matrix of Gribskov and Burgess (Nucl.
Acids Res. 14:6745, 1986), as described by Schwartz and Dayhoff, eds., Atlas
of
Protein Sequence and Structure, National Biomedical Research Foundation, pp.
353-
358,1979; (2) a penalty of 3.0 for each gap and an additional 0.10 penalty for
each
symbol in each gap; and (3) no penalty for end gaps.
[081] Variants may comprise conservatively substituted sequences.
Conservative substitution refers to replacement of a given amino acid residue
with a
residue having similar physiochemical characteristics. Examples of
conservative
substitutions include substitution of one aliphatic residue for another, such
as Ile, Val,
Leu, or Ala for one another, or substitutions of one polar residue for
another, such as
between Lys and Arg; Glu and Asp; or Gln and Asn. Other such conservative
substitutions, for example, substitutions of entire regions having similar
hydrophobicity characteristics, are well known.
[082] Candidate anti-GPVI antibodies may be screened for effects on platelet
adhesion and activation using various assays known in the art. These include,
for
example, the platelet adhesion inhibitor assay described in U.S. Pat. No.
5,686,571;
a modified constant flow assay of Diaz-Ricart et al. (Blood 82:491-496, 1993)
that
allows the use of a smaller volume of blood and less antibody, as described in
Brown
and Larson (BMC Immunology 2:9-15, 2001); the plate assay described in Matsuno
et al. (British J. Haematology 92:960-967,1996) and in Nakamura et al. (J.
Biol.
Chem. 273(8):4338-44,1998) (the "Nakamura procedure"),
-23-
CA 02567394 2012-04-26
In each case, candidate GPVI agonists or antagonists may be pre- or co-
incubated
with the reaction components in the presence or absence of Mgt+. Incubation in
the
absence of Mg2+ blocks the function of GPIa/lIa such that the remaining
collagen-
dependent activity is primarily mediated by the GPVI receptor.
[083] A modified Nakamura procedure may be used to measure platelet
adhesion to immobilized acid insoluble fibrillar collagen under static
conditions. The
modified Nakamura assay is described briefly below. The major modification to
the
original assay includes the replacement of 51Cr-labeled platelets with
unlabeled
platelets and the measurement of adhesion by quantification of LDH activity
released
by adherent platelets using a commercially available kit. One of skill in the
art
recognizes how to make other modifications to the assay conditions in view of
the
particular anti-GPVI antibody tested.
[084] Adhesion Assay -- Microtiter wells are coated with type I acid-insoluble
equine tendon fibrillar collagen. Platelets at a concentration of 4x108/ml are
suspended in Tyrode-HEPES buffer or in Tyrode-HEPES buffer supplemented with
Mg2+ (1 mM), and adhesion assays are carried out as described previously
(Tandon
et al., Br. J. Haematol. 89:124-30, 1995). Briefly, the platelets are
incubated with a
sample such as an antibody solution for 30 minutes at room temperature prior
to their
addition to the collagen-coated wells. Adhesion is carried out for 60 minutes
at room
temperature in the presence and absence of Mg2+. Unattached platelets are
removed by repeated washing of the wells and the adhered platelets are
solubilized
in Triton X-100. A commercially available LDH measuring kit (CytoTox 96,
Promega,
Madison, WI, USA) based on a colorimetric assay is used to measure released
LDH
activity.
[085] Assay for ATP Release and Thromboxane A2 (TXA2) generation --
Collagen-induced ATP release is measured in a dual channel lumiaggregometer
(Model 650CA - Chronolog Corporation Havertown PA, USA). Briefly, platelet
rich
plasma (platelet count adjusted to 3x108/ml with platelet poor plasma) is
mixed with
luciferase-luciferin reagent (Chronolog Corporation). Platelets are incubated
at 37 C
for 5 minutes in the presence and absence of a test antibody solution, e.g.
Fab
-24-
CA 02567394 2006-11-24
fragments, prior to challenge with collagen. Aggregation and ATP release are
measured simultaneously. At desired times, the reaction is stopped by addition
of a
cocktail of inhibitors that inhibit synthesis of TXA2. The supernatant of
platelet
suspension is transferred to a small tube and frozen at -20 C until measured
for
collagen-induced TXA2 formation. TXA2 is measured as TXB2, a stable metabolite
of
TXA2.
[086] Platelet Aggregation Assay -- A simple assay for detecting or
determining antithrombotic activity is provided by the platelet aggregation
assay
described in Sun et al., J Cardivascular Pharmcol. 40:557-585 (2002). Anti-
GPVI
antibodies, e.g., intact IgG, F(ab')2, or Fab fragments, or control buffer
(0.15 M NaCl,
0.01 M Tris.HCI, pH 7.4), is added to a cuvette containing platelet-rich
plasma (200
pl). The mixture is incubated for 3 to 5 minutes at 37 C in the heating module
of an
aggregometer prior to inducing aggregation with collagen. The cuvette is
placed in a
four channel aggregometer (AG 10 Kowa, Japan), which measures the kinetics of
particle formation by laser scattering and aggregation by changes in light
transmission. Aggregation is initiated with 0.5-4 pg/ml of collagen. The
optimal
concentrations or collagen are those that give at least 70% change in light
transmission and are determined for each experiment. Aggregation is monitored
for
at least 8-10 minutes after the addition of collagen.
[087] In-vitro Assay --The GPVI specific antibodies or antibody fragments
may be further assayed using the systems developed by Diaz-Ricart and co-
workers
(Arteriosclerosis, Thromb. Vasc. Biol. 16:883-888, 1996). This assay
determines the
effect of GPVI antibodies on platelets under flow conditions using de-
endothelialized
rabbit aorta and human endothelial cell matrices.
[088] In vivo Assay -- The in vivo activity of GPVI antibodies or antibody
fragments may be assayed using standard models of platelet function as
described in
Coller and Scudder, Blood 66:1456-59 (1985); Colter et al., Blood 68:783-86
(1986);
Coller et al., Circulation 80:1766-74 (1989); Coller et al., Ann. Intern. Med.
109:635-
38 (1988); Gold et al., Circulation 77, 670-77 (1988); and Mickelson et al.,
J. Molec.
Cell Cardiol. 21:393-405 (1989).
-25-
CA 02567394 2006-11-24
[089] The above tests demonstrate that the GPVI specific antibodies of the
invention are more potent than those previously reported by others.
Specifically, the
GPVI specific antibodies of the invention inhibit collagen-induced platelet
aggregation
at a lower IC50 than antibodies in the art. The term "IC50" is known in the
art as the
concentration at which 50% inhibition is observed and is any positive value
greater
than zero. The IC50 for inducing inhibition of collagen-induced platelet
aggregation
was determined using a concentration of collagen that induced 70-90% platelet
aggregation within 5 minutes of its contact with platelets. The terms
"inhibition" or
"inhibit" refers to a decrease or cessation of any phenotypic characteristic
or to the
decrease or cessation in the incidence, degree, or likelihood of that
characteristic. In
the context of platelet aggregation, "inhibition" refers to a measurable
decrease or
cessation in the aggregation of platelets. Such inhibition may be detected by
the test
described above, or by any other method known in the art. Similarly,
"inhibition" in
the context of platelet adhesion refers to a measurable decrease or cessation
in the
adhesion of platelets to a surface and such inhibition may be detected by the
test
described above, or by any other method known in the art.
[090] The GPVI specific antibodies of the invention inhibit collagen-induced
platelet aggregation at an IC50 of less than about 7, 4, 3, 2, 1, 0.6 g/ml,
or any value
subsumed within this range. The GPVI specific antibodies of the invention also
inhibit collagen-induced platelet adhesion at an IC50 of less than or equal to
about 1,
0.5, 0.2, 0.1 g/ml, or any value subsumed within this range. The GPVI
specific
antibodies of the invention also inhibit collagen-induced ATP secretion and/or
collagen-induced thromboxane A2 formation. The GPVI specific antibodies of the
invention include active antibody fragments. Active antibody fragments include
chemically, enzymatically, or recombinantly produced Fab fragments, F(ab)2
fragments, and peptides comprising at least one complementarity determining
region
(CDR) specific for a GPVI polypeptide, peptide, or naturally-occurring variant
thereof.
[091] The GPVI specific antibodies of the invention may be formulated into
pharmaceutical compositions according to known methods. The pharmaceutical
composition of the invention comprises at least one GPVI specific antibody and
include, but are not limited to, intact monoclonal antibodies; Fab fragments;
F(ab)2
-26-
CA 02567394 2006-11-24
fragments; and peptides comprising at least one CDR sequence, or variant
thereof.
The GPVI specific antibody may be combined with other known active materials.
[092] Compositions of the invention include at least one GPVI specific
antibody admixed with pharmaceutically acceptable excipients, including, but
not
limited to, diluents (e.g., Tris-HCI, acetate, phosphate, water),
preservatives (e.g.,
Thimerosal, benzyl alcohol, parabens), emulsifiers, salts, polymers, buffers,
solubilizers, adjuvants and/or carriers. Suitable excipients and their
formulations are
described in Remington: The Science and Practice of Pharmacy, 20th ed, Mack
Publishing Co. (2000). In addition, such compositions may contain GPVI
specific
antibodies complexed with polyethylene glycol (PEG), metal ions, incorporated
into
polymeric compounds such as polyacetic acid, polyglycolic acid, hydrogels,
etc., or
incorporated into liposomes, microemulsions, micelles, unilamellar,
multilamellar, or
colamellar vesicles, erythrocyte ghosts or spheroblasts.
[093] The instant invention also comprises methods of inhibiting thrombosis,
for example, by inhibiting platelet aggregation or platelet adhesion,
comprising
contacting activated or resting platelets with antibodies directed against
GPVI.
"Inhibiting thrombosis" refers to a decrease or cessation of a thrombotic
event or to
the decrease in the incidence, degree, or likelihood of thrombotic events in a
patient,
patient population, or in vitro test systems. The invention also relates to
the
treatment of a patient, hereby defined as any person or non-human animal in
need of
anti-thrombotic treatment to reduce the incidence, likelihood, or degree of
thrombosis, or platelet aggregation, or platelet activation, or to any subject
for whom
treatment may be beneficial for the treatment of vascular disease, including
humans
and non-human animals. Such non-human animals to be treated include all
domesticated and feral vertebrates including, but not limited to: mice, rats,
rabbits,
fish, birds, hamsters, dogs, cats, swine, sheep, horses, cattle, and non-human
primates.
[094] The treatment of a patient comprises the administration of a
pharmaceutically effective amount of an anti-GPVI antibody-containing
composition
of the invention. One of ordinary skill in the art may empirically determine
the
optimum dosage and dosage schedule for administering these compositions.
-27-
CA 02567394 2006-11-24
Nevertheless, a pharmaceutically effective amount is that amount which
provides a
measurable anti-thrombotic effect, for example, a reduction in the incidence,
degree,
or likelihood of thrombosis, platelet aggregation, or platelet activation as
measured in
vivo or in vitro, or provides a measurable decrease in the likelihood,
incidence, or
degree of vascular disease, clot or emboli formation, or ischemic events in a
patient.
[095] A pharmaceutically effective amount may be administered as a single
dose or as multiple doses over the course of treatment. A kit within the scope
of the
invention comprises a container containing one or more doses of a
pharmaceutically
effective amount of an anti-GPVI antibody-containing composition of the
invention.
Such kits encompass anti-GPVI antibodies alone, admixed or suspended with a
suitable pharmaceutically acceptable diluent and/or other excipient, or
formulated to
be admixed or suspended in a suitably acceptable diluent and/or other
excipient prior
to administration.
[096] The compositions of the invention may be administered by any method
familiar to those of ordinary skill in the art, for example, intravenous
administration by
bolus injection, continuous, or intermittent infusion. In alternative
embodiments, the
compositions may be administered intraperitoneally, intracorporeally, intra-
articularly,
intraventricularly, intrathecally, intramuscularly, subcutaneously, topically,
tonsillarly,
mucosally, intranasally, transdermally, intravaginally, orally, or by
inhalation.
[097] The GPVI specific antibodies of the invention may also be used to
screen for compounds that may be useful as anti-thrombotic agents. The
screening
method comprises contacting a GPVI antigen with a test compound and a GPVI
specific antibody and measuring the inhibition of binding of the GPVI specific
antibody to the GPVI polypeptide, peptide, or a naturally-occurring variant
thereof.
The GPVI antigen, GPVI specific antibody, and the test compound may be added
in
any order. For example, the GPVI antigen may be contacted with the test
compound
before contacted with the GPVI specific monoclonal antibody. In another
example,
the GPVI antigen may be contacted with GPVI specific monoclonal antibody and
the
test compound simultaneously.
[098] Inhibition of binding suggests that the test compound competes for, or
otherwise interferes with, the same binding site on GPVI as the GPVI specific
-28-
CA 02567394 2006-11-24
antibody. "GPVI antigen" in the context of screening for anti-thrombotic
agents,
refers to, but is not limited to, native GPVI polypeptides, peptides, or
naturally-
occurring variants thereof, isolated from platelets or other GPVI-expressing
cells;
recombinant GPVI polypeptides, peptides, or naturally-occurring variants
thereof,
expressed from prokaryotic or eukaryotic cells; or cells expressing GPVI
polypeptides, peptides, or naturally-occurring variants thereof. A test
compound may
be any chemical, protein, peptide, polypeptide, or nucleic acid (DNA or RNA).
The
test compound may be naturally-occurring or may be synthesized by methods
known
in the art. The screening method of the invention may employ high-throughput
screening (HTS) methods. High-throughput screening methods are reviewed in
Khandurina et al., Curr Opin Chem Biol. 6:359-66 (2002); Kumble, Anal Bioanal
Chem. 377:812-819 (2003); and Bleicher et al., Nature Rev Drug Disc 2:369-378
(2003).
[099] A compound identified as inhibiting the binding of a GPVI specific
antibody, or an antibody fragment thereof, to a GPVI antigen, may be further
tested
for its effect on platelet functions by any of the methods disclosed herein,
or by other
methods known in the art. These platelet functions include collagen-induced
platelet
aggregation, collagen-induced platelet adhesion, collagen-induced ATP
secretion,
and collagen-induced thromboxane A2 formation.
[0100] The present invention also comprises a method for producing a
human GPVI ectodomain (sGPVI) by recombinant expression in host cells. A
suitable expression vector for sGPVI may be created by standard molecular
biology
methods. The recombinant sGPVI may comprise a signal peptide that facilitates
its
secretion. The signal peptide may be any signal peptide known in the art,
including
natural, artificial or heterologous signal peptides, for example, the signal
peptide of
the Ig K light chain. The expressed sGPVI may also comprise modifications,
such as,
for example, peptide tags that facilitate detection or purification. Such
peptide tags
include, among others, histidine tags, V5 tags, glutathione S-transferase
(GST) tags,
maltose-binding protein (MBP) tags, biotin acceptor peptide (BAP) tags,
streptavidin-
binding peptide (Strep-II) tags, calmodulin-binding peptide (CBP) tags,
hemagglutinin
(HA) tags, myc tags, and FLAG tags. The host cells used for expression of
sGPVI
-29-
CA 02567394 2006-11-24
may be prokaryotic or eukaryotic. For example, an eukaryotic host cell may be
a
CHO cell.
[0101] The recombinant sGPVI may be useful in multiple applications, for
example, as a standard protein for assays that detect or quantify GPVI
polypeptides,
peptides, or naturally-occurring variants thereof; as a competitive inhibitor
of platelet
interaction with GPVI interacting molecules (such as collagen, CRP, or
convulxin) in
vitro, ex vivo or in vivo; as an antigen for the immunization of animals for
production
of new antibodies directed against the extracellular domain of GPVI; and as a
tool for
affinity selection or purification of antibodies directed against the
extracellular domain
of GPVI.
[0102] The recombinant sGPVI may be used as a standard protein for an
ELISA of the invention, for example, an ELISA used for the detection of sGPVI
in
biological samples for preventive or therapeutic monitoring of sGPVI levels in
human
individuals. Recombinant sGPVI may also be used as a competitive inhibitor of
platelet interaction with GPVI interacting molecules, for example, collagen,
thereby
preventing or reducing platelet aggregation, blood clotting or other
undesirable
consequences of platelet interaction with injured vasculature. Recombinant
sGPVI
may also be covalently or non-covalently coupled to a solid support for
selection of
new or improved GPVI-specific antibodies or purification of any GPVI-specific
antibody, for example, by affinity chromatography. In an embodiment of the
invention, GPVI-specific antibodies may be selected or purified from samples
containing antibodies directed against a large number of different epitopes,
thereby
generating GPVI-specific antibody preparations that are subtantially free of
contaminating antibodies or other contaminating proteins.
[0103] The present invention further provides an assay that allows sensitive
detection and quantitation of sGPVI in biological samples. An embodiment of
such
an assay is a sandwich ELISA that uses a first and second GPVI-specific
antibody.
Suitable GPVI-specific antibodies include the monoclonal antibodies of the
present
invention, for example, OM1, OM2, OM3 and OM4 antibodies. The first and second
antibodies of the invention may be capable of inhibiting collagen-induced
platelet
aggregation at an IC50 of less than about 7, 4, 3, 2, 1, 0.6 pg/mi, or any
value
-30-
CA 02567394 2006-11-24
subsumed within this range, and specifically bind to the GPVI polypeptide,
peptide, or
naturally-occurring variant thereof, at a Kd of equal to or lower than 10"8M.
The first
and second antibodies may be different from each other. OM1 and OM2 antibodies
recognize non-overlapping epitopes of GPVI and, therefore, can bind
simultaneously
and non-competitively to the same sGPVI molecule. The ELISA may comprise a
first
antibody, for example, OM1, which is immobilized on a solid support, and a
second
antibody, for example, OM2, which is coupled directly or indirectly to a label
to
facilitate detection and quantitation of sGPVI. One example of such a label is
biotin,
which can be detected via its highly specific interaction with avidin. Other
examples
of labels include digoxigenin, fluorophores, metal complexes and enzymes.
Suitable
fluorophores may include Cy3, Cy5, phycoerythrin, fluorescein, rhodamine,
Texas
red, quantum dots, coumarin fluorophores, oxazole fluorophores, and
flurorescent
proteins such as Aequorea victoria green fluorescent protein, and variants
thereof.
Suitable metal complexes may include europium cryptates, metal carbonyl
complexes, and porphines. Suitable enzymes may include horseradish peroxidase,
alkaline phosphatase, R-galactosidase and luciferases. Soluble GPVI may be
captured onto the solid support by the first antibody and may be detected and
quantified upon binding of the second antibody. ELISAs of the invention are
capable
of detecting and quantifying as little as 0.2-5 ng/ml sGPVI in biological
samples. This
is sufficiently sensitive to detect and quantify sGPVI in the plasma of
healthy human
individuals whose levels are generally about 6 ng/ml sGPVI, as well as in the
plasma
of human patients containing elevated levels of sGPVI. The ELISA of the
invention
may be used to monitor sGPVI levels in the blood of human individuals at an
indicated time point or at multiple time points over an indicated period of
time.
[0104] The invention further provides methods and kits for detecting or
quantifying the presence of a GPVI polypeptide, peptide, or naturally-
occurring
variant thereof, in a biological sample or test sample. The methods comprise
contacting the sample with the first antibody of the invention described
above,
contacting the sample with the second antibody of the invention also described
above, capturing the soluble GPVI with the first antibody and detecting or
quantifying
the sGPVI with the second antibody, which may be labeled. The kits comprise
the
-31-
CA 02567394 2006-11-24
first and second antibodies of the present invention, and the first antibody
may be
immobilized and the second antibody may be labeleled. Such methods and kits
can
be used to determine if an individual is at increased risk of developing or is
already
suffering from a condition that is associated with abnormal levels of a GPVI
polypeptide, peptide, or naturally-occurring variant thereof. Such conditions
include
immunological, vascular and other disorders, such as, for example,
thrombocytopenia; platelet disorders; cardiovascular diseases, such as
unstable
angina, acute myocardial infarction, coronary artery disease, coronary
revascularization, ventricular thromboembolism, atherosclerosis, or plaque
formation;
cerebral vascular diseases, such as stroke and ischemia; pheripheral vascular
diseases, such as chronic arterial occlusive disease; venous thromboembolism
diseases, such as diseases involving leg swelling, ulceration, pulmonary
embolism,
or abdominal venous thrombosis; cancer metastasis, for example of tumor cells
derived from cancerous colon, breast or liver tissue; liver disorders; and
certain
embryonic disorders.
[0105] An embodiment of such a method or kit comprises the ELISA
described above. Kits may also include instructions for determining whether
the
tested individual is at increased risk of developing or is already suffering
from a
disorder associated with abnormal levels of a GPVI polypeptide, peptide, or
naturally-
occurring variant thereof, based on the measured amounts of the GPVI
polypeptide,
peptide or variant in the individual's sample(s).
[0106] The present invention is illustrated by the following Examples, which
are not intended to be limiting in any way.
EXAMPLE 1
PREPARATION OF MONOCLONAL GPVI ANTIBODIES
[0107] Normal mice (Balb/c, female), Armenian hamsters (male) and GPVI
knock-out mice (produced at Otsuka GEN institute) were immunized to produce
monoclonal antibodies as described below.
[0108] GPVI knockout mice were generated as previously described (Mori
et.al. Neurosci. Res. 43: 251-7, 2002). The targeting vector was constructed
by
-32-
CA 02567394 2006-11-24
replacing a genomic fragment of the GPVI gene (Ezumi Y. et at., Biochem
Biophys
Res Comm 277:27-36, 2000) from 129/Sv mouse genomic ;L clones containing the
last 5 bases of exon 2 to the first half of exon 3 (Clalsite) with the pMC1-
neo-polyA
(Stratagene) cassette as shown in Fig. 1A. The linearized construct was
electroporated into AB2.2 ES cells derived from 129/Sv mouse (Lexicon Genetics
Inc., The Woodlands, TX) and the cells were selected in G-418. G-418-resistant
ES
cell clones were screened for successful homologous recombination at exons 2
and
3 by probing Sphl- or Kpnl-digested genomic DNAs with 5' or 3' external
probes,
respectively (Fig. 1 B; Southern blotting data not shown). Chimeric mice
derived from
the homologous recombinant ES cells were mated with C57BL/6J mice to obtain
heterozygous mutants (F1). Homozygous mutants (F2) were derived by mating the
obtained heterozygous mutants and confirmed by Southern blotting. Mice were
genotyped by polymerase chain reaction (PCR) using genomic DNA extracted from
tail snips. No abnormalities in birth rate, birth weight, growth and
development,
Mendelian distribution, or bleeding disorders were observed in the homozygous
mutants. Background-matched wild-type and heterozygous mice were used as
controls. Armenian hamsters were obtained from Cytogen Research and
Development Inc. Boston, MA. All animals were kept and bred according to the
Institutional Animal Care and Use Committee (IACUC) protocol at Otsuka
Maryland
Medicinal Laboratories.
[0109] Normal mice were immunized with plasmid containing GPVI cDNA ("p-
target"), CHO cell line expressing GPVI-FcRy chain ("CGP6", wherein
transfection
was performed using LipofectamineTM 2000 from Invitrogen), GPVI purified from
GPVI-FcRy expressing CHO cells ("PGP6"), native GPVI purified from human
platelets ("nGP6") and recombinant partial GPVI (lacking the first Ig domain)
expressed in E. coli ("PAGP6"). Native GPVI from human platelets and PGP6 from
GPVI-FcRy transfected cells were purified by combining the lectin affinity,
ion-
exchange chromatography, and convulxin-affinity methods described in U.S.
Publication No. 2003/0186885.
[0110] CHO cells stably expressing GPVI and FcRy ("CGP6") were
established by co-transfection of pTarget vector (Promega) containing full-
length
-33-
CA 02567394 2006-11-24
human GPVI cDNA ("p-target") and pcDNA3.1(+)zeocin vector (Invitrogen)
containing full-length FcRy cDNA using Lipofectamine 2000 (Invitrogen). Cells
expressing both receptors were selected in medium supplemented with G418 and
zeocin. Expression of GPVI was detected by FACS analysis with the Epic Altra
FACS analyzer (Beckman Coulter) using a polyclonal human anti-GPVI antibody
(Dr.
Okuma's serum described earlier) or a FITC-labeled convulxin. Detection of
FcRy
expression was performed by immunoblotting using commercially available anti-
FcRy
polyclonal antibody (Upstate Biotechnology).
[0111] Recombinant partial GPVI was prepared by inserting into the pET21
vector (Novagen) a cDNA encoding a GPVI polypeptide lacking the entire first
Ig
domain of human GPVI. This partial protein was expressed in E. Coli strain
BL21(DE3). Expressed protein ("PAGP6") was purified from the inclusion bodies
as
described by the manufacturer.
[0112] Armenian hamsters were immunized with CGP6 and human platelets.
GPVI knock-out mice were also immunized with CGP6 and human platelets.
[0113] Immunogens except p-target were injected intraperitoneally. P-target
was injected intradermally. Recombinant or purified proteins were injected as
an
emulsion with adjuvant (Titermax Gold, Cytrx Corporation). Some of the
antigens
were immunized with mouse IL-6 (5000/injection) to boost the immune system.
Animals were immunized until a serum titer between 10-50,000 was obtained.
[0114] Monoclonal antibodies were produced by conventional hybridoma
technology. Asa fusion partner, P3U1 cells were used. For screening for
positive
hybridoma clones, FACS analysis was performed in the Epic Altra FACS analyzer
by
Beckman Coulter using GPVI-FcRy expressing CHO cells. For CGP6-immunized
animals, FACS analysis was performed with both GPVI-FcRy-transfected and non-
transfected wild-type CHO cells to distinguish clones that produced non-GPVI
antibodies e.g. antibodies to CHO cell-related antigens.
[0115] Hybridoma cells producing anti-GPVI antibodies were then grown in
medium containing 10% fetal calf serum (Invitrogen Corporation, CA) (which
contained negligible amounts of bovine IgG < 1 pg/ml serum). IgG was purified
from
the cell-free culture supernatant by protein G-SepharoseTM (Amersham
Biosciences,
-34-
CA 02567394 2006-11-24
NJ ) or Protein A-SepharoseTM (Amersham Biosciences, NJ) affinity
chromatography
on a Waters 650 system (Waters Corporation, MA). Mouse IgG was purified by
affinity chromatography on Protein G-SepharoseTM. Hamster IgG was purified on
Protein A-SepharoseTM. Protein G-bound IgG was eluted from the affinity matrix
with
low pH glycine (pH 2.75), collected into basic solution to neutralize the
acid, and
dialyzed in saline for use in functional assays. Protein - A bound hamster IgG
was
eluted with a pH gradient 7.5 -3Ø Antibody was eluted at pH 4.5. In most
cases,
antibody was >90% pure when analyzed in Agilent 2100 Bioanalyzer (Agilent
Technology).
[0116] Bivalent F(ab')2 fragments were prepared from intact IgG by papain
digestion using standard methods. A solution of IgG (5 mg/ml) was made in 100
mM
citric acid, pH 6.5 and 5.0 mM EDTA and digested for 15 hrs at 37 C with pre-
activated papain (cysteine free) at an enzyme to IgG ratio of 1:50 (wt/wt).
The
reaction was quenched with freshly prepared iodoacetamide and F(ab')2
fragments
were separated from undigested IgG and Fc by ion exchange chromatography on a
MonoQ column (Amersham Biosciences, NJ). Fractions containing F(ab')2 were
pooled, concentrated and reduced/alkylated to obtain monovalent Fab fragments
according to the method of Parham et al., J. Immunol. Meth. 53: 133-173
(1982).
Finally, Fab fragments were purified to homogeneity by size exclusion
chromatography on Superdex 75 (Amersham Biosciences, NJ). Direct conversion of
IgG into monovalent Fabs by papain in the presence of cysteine was avoided
because in a few cases, papain over-digested the IgG, giving rise to unstable
and
smaller-sized Fab fragments.
[0117] Initially, wild-type Balb/c mice were immunized as described above
with immunogens including purified GPVI from human platelets ("nGP6"), plasmid
containing GPVI cDNA ("p-target"), and CHO cells expressing the GPVI-FcRy
chain
complex ("CGP6"). See Table 1. After screening more than 8500 clones, 3 clones
with significant but moderate affinity to GPVI were identified (two from CGP6
and one
from PGP6). Surprisingly, more than 5500 clones arising from immunizations
with p-
target, nGPVI, and PAGP6 did not yield a single GPVI-positive clone with
biological
activity. In an attempt to obtain antibodies with enhanced affinity and
biological
-35-
CA 02567394 2006-11-24
activity, a different species of animals was immunized. Armenian hamsters were
immunized with CGP6 because this immunogen produced two positive clones in
wild-
type mice. Because human platelets express native GPVI on their surface,
washed
human platelets were also used as immunogens. Seven GPVI-positive clones were
obtained from the CGP6 immunizations and one clone from human platelet
immunization. See Table 2.
Table 1 Immunization of Wild-type Mice
Immunogen # of clones screened # of positive clones
p-target 1192 0
CGP6 1953 2
PGP6 1064 1
nGP6 3797 0
PAGP6 764 0
Total 8770 3
Table 2 Immunization of Hamsters
Immunogen # of clones screened # of positive clones
CGP6 1547 7
human platelets 1414 1 (OM3)
Total 2961 8
[0118] GPVI knock-out (GPVI-KO) mice were then used as hosts for
immunization. The GPVI-KO mice lack GPVI and do not respond to high doses of
collagen and to convulxin (a GPVI specific agonist). Therefore, it was
theorized that
injected GPVI may be more antigenic in GPVI-deficient mice and may therefore
-36-
CA 02567394 2006-11-24
produce GPVI antibodies having high affinity for GPVI-peptide. The results of
immunization of GPVI-KO mice are shown in Table 3. Immunization of GPVI-KO
mice with washed human platelet suspensions did not produce any positive
clones.
However, eight clones were obtained from the immunization of GPVI-KO mice with
CGP6, which had high affinity to GPVI as judged by the large rightward shift
in
fluorescence intensity in the FACS analysis.
Table 3 Immunization of GPVI Knock-Out Mice
Immunogen # of clones screened # of positive clones
CGP6 3889 8 (including OM1, OM2 and
OM4)
human platelets 397 0
Total 4286 8
[0119] IgG immunoglobulins were then obtained from the GPVI-positive
clones: 3 clones from wild type mice, 8 clones from hamster fusions, and eight
clones
from GPVI-KO mice fusions. IgG was purified by affinity chromatography using
either
Protein G (wild-type and GPVI-KO mice) or Protein A (hamsters), as described
above. Purified antibodies from all clones induced full platelet aggregation
at
relatively small concentrations of IgG at 0.1-7pg/ml (17 clones) and 10-30
pg/ml (2
clones), suggesting that these antibodies cross-linked GPVI and FcR IIA, or
cross-
linked GPVI molecules. To exclude the possibility of GPVI-FcRIIA cross
linking,
F(ab')2 fragments were prepared. In preliminary studies, F(ab')2 fragments
prepared
from two antibodies activated human platelets, although several fold higher
concentrations were needed compared to intact IgGs. This confirmed that GPVI
cross linking by the bivalent antibodies may be the cause of the observed
platelet
activation. In order to avoid GPVI cross linking, all F(ab')2 fragments were
converted
to Fab fragments by reduction/alkylation. Resulting Fab fragments did not
activate
-37-
CA 02567394 2006-11-24
human platelets when tested up to several fold higher concentrations at which
intact
IgG activates human platelets.
EXAMPLE 2
GPVI SPECIFIC ANTIBODIES ARE POTENT INHIBITORS OF COLLAGEN-
INDUCED PLATELET AGGREGATION AND ADHESION
[0120] The inhibitory potential of the Fab fragments prepared according to
Example 1 was tested on collagen-induced platelet functions, including
collagen-
induced platelet aggregation and adhesion of platelets to immobilized collagen
under
static and flow conditions.
[0121] The antibodies were tested for in vitro platelet aggregation as
follows.
A collagen dosing experiment was first performed to determine the amount of
collagen that would give 70-90% platelet aggregation within 5 minutes of its
addition
because collagen response varies among individuals. Moreover, the type of
collagen
used in the assays can dramatically affect the response. From experience, acid
insoluble equine tendon collagen (Nycomed, Germany) provided the greatest
platelet
aggregation response. Nieswandt (J. Biol. Chem. 275:23998-24002, 2000, and
U.S.
Patent Publication No. 2002/0141992), Lecut et al. (J. Thrombosis and
Haemostasis
1:2653-2662, 2003), and Moroi et al. (Thromb. Haemost. 89:996-1003, 2003) also
used acid insoluble equine tendon collagen, whereas others used a less
responsive
form of collagen, for example, bovine collagen type I fibers (Smethurst et
al., Blood
103:903-911, 2004, and WO 03/054020). In the assays exemplified below, 1-4
lag/ml
acid insoluble equine tendon collagen (Nycomed, Germany) was used to examine
the inhibitory effect of a particular Fab preparation on platelet aggregation.
The type
of collagen used is same as that used in Qian et al. (Human Antibodies 11:97-
105,
2002), WO 01/00810, WO 02/080968, and their related applications; but as
discussed below, the GPVI antibodies of the present invention exhibit
significantly
greater inhibitory effect compared to the GPVI antibodies disclosed in Qian,
WO
01/00810, and WO 02/080968.
[0122] The platelet aggregation assay was performed by collecting blood in
1/10 volume of 3.8 % trisodium citrate as an anticoagulant (Nakamura et al.,
J. Biol.
-38-
CA 02567394 2006-11-24
Chem. 273(8):4338-44, 1998). Platelet rich plasma (PRP) was obtained by
centrifugation of whole blood at 180 x g for 15-20 minutes at room
temperature.
Platelets were counted and adjusted to 3-4x108 platelets/ml in platelet poor
plasma
prior to performing platelet aggregation studies. All experiments were
performed
within 3-4 hrs after blood collection and PRP was maintained at room
temperature
the entire time. Aggregation studies were performed in a four channel
aggregometer
AG10 (Kowa, Japan) which measures the kinetics of particle formation by laser
scattering and aggregation by light transmission at 650 nm in the visible
region of the
light spectrum. PRP was incubated at 37 C with varying amounts of Fab
fragments
for 10 minutes (5 minutes without stirring followed by additional 5 minutes
with
stirring) prior to the addition of acid insoluble equine tendon collagen
(Nycomed,
Germany) and aggregation was monitored for an additional 5-10 minutes.
[0123] Platelet adhesion to collagen under static conditions was examined
using a modified procedure of Nakamura et al., J. Biol. Chem. 273:4338-4334
(1998)
described earlier. Briefly, washed platelets were incubated with desired
amounts of
Fab fragments for 30 minutes in the presence and absence of Mg2+ at room
temperature prior to their addition to collagen-coated wells (2 g/well with
acid
insoluble equine tendon collagen) (Nycomed, Germany). After 60-90 minutes of
incubation at room temperature, unadhered platelets were removed by gentle
washing with buffered saline and the adhesion was quantified by determining
the
LDH content of adhered platelets using a commercially available LDH kit
(Promega,
MA).
[0124] Platelet adhesion to immobilized collagen under flow conditions was
measured in a flow chamber developed by Glycotech (Rockville, MD). Whole
blood,
anticoagulated with recombinant hirudin (50units/ml), was incubated with a
solution
containing Fab fragment for 15 minutes at 37 C prior to being drawn through a
collagen-coated (5 g/cm2 acid insoluble equine tendon collagen) (Nycomed,
Germany) flow chamber at high shear (2600 sec 1, 2min). Unadhered platelets
were
washed with phosphate buffered saline and the adhered platelets were fixed
with
glutaraldehyde (0.5% w/v, 1 h) and stained with toludine blue/sodium borate
(0.05%,
min). Surface coverage by platelets was estimated by digital image analysis.
The
-39-
CA 02567394 2006-11-24
average from 10 non-overlapping images was used to determine percentage
surface
area coverage.
[0125] Of the antibodies selected, Fab fragments from four IgGs, OM1, OM2,
OM3, and OM4, inhibited collagen-induced platelet aggregation on human
platelets.
See Tables 2, 3, and 4. The order of inhibitory potency of the Fab fragments
against
collagen-induced platelet aggregation was OM1>OM2>OM3>OM4. Average IC50
and SD values of each antibody for inhibition of collagen-induced platelet
aggregation are shown in Table 4.
Table 4: Effect of Anti-GPVI Fab Fragments on Collagen-Induced
Aggregation of Human Platelets and Cross-Reactivity to Non-Human
Platelets
Fab IC50 (lag/ml)* Cross reactivity**
Fragment
Average SD Rat Dog Monkey
ID
OM1 0.575 0.049 (-) (-) (+)
OM2 1.69 0.506 (-) (-) (+)
OM3 3.02 0.768 (-) (-) (+)
OM4 7.0 5.5 (+) (-) (+)
ReoProR 1.71 0.345 (-) (+) (+)
7E3 F(ab')2 n/d*** n/d (+) n/d n/d
* Dose response curve were obtained using platelets from 3 different subjects.
IC50
values were calculated by non-regression analysis. Values are average SD
from 3
experiments.
** Cross reactivity with animal platelets was based on the ability of
individual Fab
fragments to inhibit collagen-induced platelet aggregation and by positive
rightward
shift in FACS analysis. A (+) sign indicates inhibition of collagen-induced
aggregation
by Fab fragments and positive rightward shift while a (-) sign indicates no
reaction in
both tests.
*** Not determined
-40-
CA 02567394 2006-11-24
[0126] Cross reactivity of each antibody to rat, dog, and monkey platelets
were also tested. Monkey and dog blood was purchased from Covance Research
Inc, Vienna, VA. Sprague Dowley rats were obtained from Charles River
Laboratories, Willmington, MA. All four Fab fragments inhibited collagen-
induced
aggregation of monkey platelets. Interestingly, OM4 cross-reacted with rat
platelets.
These GPVI specific antibodies are useful tools for testing the effect of GPVI
specific
antibodies in animal models. Hybridomas producing OM1, OM2, OM3, and OM4
antibodies were deposited with the American Type Culture Collection (Manassas,
VA) on April 29, 2004, as ATCC Nos. PTA-5938, PTA-5939, PTA-5940, and PTA-
5941, respectively.
[0127] For comparison, ReoPro (Centocor, Inc.), a widely used human-
mouse chimeric anti-GPIIb-Illa Fab fragment, and a F(ab')2 fragment of the
anti-
GPI I b-I I I b monoclonal antibody 7E3 (Collerand Scudder, Blood 66:1456-
1459,
1985), was tested on several of the same donors under the same conditions used
to
test the GPVI specific antibodies. ReoPro inhibited collagen-induced platelet
aggregation at an IC50 of 1.71 pg/ml. Thus, OM1 possesed greater inhibitory
potential than ReoPro , while OM2 was equipotent to ReoPro . ReoPro was 2-4
times more potent than OM3 and OM4 in this assay. 7E3 F(ab')2 cross-reacted
with
rat platelets, whereas ReoPro did not (see Table 4).
[0128] The effect of Fab fragments on platelet adhesion under static
conditions was also tested. Adhesion was carried out in the presence (GPIa-Ila
and
GPVI- dependent) and absence (GPVI-dependent) of Mg2+. Mg2+-independent
adhesion is solely dependent on the presence of GPVI and was inhibited by all
four
Fabs at relatively low concentrations (IC50 ranging from 0.1-1 pg/ml) as shown
in Fig.
2. OM1, OM2, and OM3 had similar inhibitory activity (IC50 range 0.1-0.2 pg
Fabs/ml) while OM4 required a slightly higher dose to achieve similar
inhibition (IC50
range 0.2-1 pg Fabs/ml). The Mg2+-dependent adhesion process required
relatively
higher doses of Fab fragments than those required for the Mg2+-independent
adhesion process (see Fig. 2). However, none of the Fabs were able to
completely
block the Mg2+- dependent adhesion of platelets to collagen.
-41-
CA 02567394 2006-11-24
[0129] The effect of Fab fragments on Mg2+-independent platelet adhesion
under static conditions was repeated using even lower doses (0.001-1 g
Fabs/ml).
As shown in Fig. 3, OM2 and OM4 Fab fragments inhibited platelet adhesion by
40-
60% at 0.1 g Fab/ml and OM1 and OM3 Fab fragments inhibited adhesion by 10-
20% at the same concentration. This discrepancy may be due to batch variation
and
donor variability. In conclusion, the OM series of Fab fragments effectively
inhibit
GPVI-dependent (Mg2+-independent adhesion) at concentrations lower than
0.5 g/ml.
[0130] The inhibitory effect of anti-GPVI Fab fragments on platelet adhesion
was also tested under conditions closer to in vivo situations, as described
above.
Fab fragments of OM1, OM2, OM3, OM4 significantly inhibited platelet adhesion
to
immobilized collagen under high shear conditions (2600sec 1). Compared to a
control sample, anti-GPVI Fabs induced dramatic changes in the size and
morphology of the aggregates (Fig. 4). In comparison, ReoPro (Centocor, Inc.)
also
prevented the formation of aggregates but a uniform layer of single platelets
was
observed on collagen fibers (Fig. 4).
[0131] The morphology of the aggregates is a result of two events: (1) the
area covered by primary monolayer of platelets and (2) subsequent formation of
aggregates thus adding a volume dimension to the over all picture. The
dramatic
reduction in aggregate formation along with surface coverage by the anti-GPVI
Fab
suggests that GPVI is not only involved in the primary adhesion process but
that it
also plays an important role in post-adhesion events, including platelet
activation and
subsequent thrombus growth.
[0132] Aggregation and adhesion assays demonstrate that the Fab
fragments of GPVI specific antibodies of the invention, such as OM1, OM2, OM3,
and OM4, are potent inhibitors of platelet functions. They are more potent at
inhibiting collagen-induced platelet aggregation than the Fab fragment of the
mouse
monoclonal antibody 9012.2 generated from mice immunized with a cDNA encoding
recombinant soluble GPVI-Fc (rsGPVI-Fc) fusion protein as described in Lucet
et al.
(J. Thrombosis and Haemostasis 1:2653-2662, 2003), WO 02/80968, and US Patent
-42-
CA 02567394 2006-11-24
Publication 2004/0253236. Additionally, the OM antibodies are more potent at
inhibiting Mg2+-indepedent adhesion to collagen than the 9012.2 Fab fragment.
[0133] Nieswandt (J. Biol. Chem. 275:23998-24002, 2000, and U.S. Patent
Publication No. 2002/0141992) reported a rat monoclonal antibody to mouse
GPVI,
JAQ1. However, saturating concentrations of JAQ1 (20 g/ml) only displayed a
limited inhibitory effect on collagen-induced platelet aggregation (see U.S.
Patent
Publication 2002/0141992, paragraph 29). Furthermore, JAQ1 did not recognize
human GPVI in FACS analysis or Western blotting in our hands or in others (see
Takayama et al., Jpn. J. Thromb. Hemost. 14: 75-81, 2003).
[0134] Others have generated single chain Fvs (ScFvs). For example, Qian
et al. (Human Antibodies 11:97-105, 2002) reported a single chain Fv (ScFv)
antibody of GPVI that had an IC50 of 80-90 pg/ml in a collagen-induced
platelet
aggregation study using 2 g/ml of the same collagen used in the present
invention.
Thus, the GPVI specific antibodies of the invention are significantly more
potent in
inhibiting collagen-induced platelet aggregation compared with Qian et al.
ScFvs
also reported in WO 01/00810, WO 02/80968, and their related applications
required
a significantly greater concentration (110-150 .ig/ml of ScFv) for inhibiting
collagen-
induced platelet aggregation compared with the GPVI specific antibodies of the
present invention.
[0135] Similarly, Smethurst et al. (Blood 103:903-911, 2004, and WO
03/054020), reported a ScFv antibody of GPVI that had an IC50 of 12-16 pg/ml
in
collagen-induced platelet aggregation. In comparison, OM1, OM2, OM3 and OM4
are more potent than Smethurt's ScFv. Additionally, although U.S. Patent Nos.
6,245,527 and 6,383,779 disclose anti-GPVI antibodies, they do not provide any
examples of anti-GPVI antibodies that are as potent at inhibiting collagen-
induced
platelet aggregation as those of the present invention.
-43-
CA 02567394 2006-11-24
EXAMPLE 3
GPVI SPECIFIC ANTIBODIES INHIBIT COLLAGEN-INDUCED SECRETION
AND THROMBOXANE A2 FORMATION
[0136] Fab fragments of the GPVI specific antibodies of the invention were
also tested for their effect on collagen-induced secretion and thromboxane A2
(TXA2)
formation. Secretion refers to agonist-induced release of bioactive contents
from
alpha and dense granules from platelets.
[0137] One way to quantify agonist-induced release is to measure ATP
content in the medium by luciferase assay using chemiluminescence method.
Platelet-rich plasma (PRP) was tested for collagen-induced ATP secretion using
a
Lumi-aggregometer (Chronolog Corporation, PA) and a luciferase-luciferin
reagent.
The Lumi-aggregometer simultaneously measures the agonist-induced platelet
aggregation and ATP secretion. Briefly, human blood was drawn directly into
3.8%
trisodium citrate with a syringe (9:1 volume blood:citrate). Platelet-rich
plasma (PRP)
was prepared by centrifugation at 180 x g for 15 minutes. PRP (360 pl) was
mixed
with 40 l luciferase-luciferin reagent (Chrono-lume; Chronolog Corporation,
PA) and
the mixture was incubated with varying amounts of test Fabs, ReoPro , or
control for
minutes at 37 C. 1-4 g/ml of collagen (acid insoluble equine tendon collagen)
(Nycomed, Germany) was added at five minutes and aggregation and ATP secretion
were monitored for eight minutes. At the end of the reaction (10-11 minutes),
a
known amount of ATP solution was added to obtain a deflection which was used
to
calculate the ATP amount secreted by platelets upon agonist challenge.
[0138] Thromboxane A2 formation was measured in parallel samples used
above. Ten minutes after the collagen addition step above, 500 pL of stop
solution
(50mM EDTA, 2mM Indomethacin in 130mM NaCl) was added to 200 pL of PRP to
terminate thromboxane A2 formation. The suspension was centrifuged at 1,000 x
g
for 10 min at 4 C. The supernatant was saved and frozen at -20 C until tested
for
thromboxane B2, which is a stable metabolite of thromboxane A2. Thromboxane B2
was quantified by using a commercially available kit (Thromboxane B2 Biotrak
Assay,
Amersham).
-44-
CA 02567394 2006-11-24
[0139] All Fab fragments of OM1, OM2, OM3, and OM4 antibodies potently
inhibited collagen-induced ATP release from human platelets (Table 5). OM1
showed inhibition of more than 90% at 1 pg/mL. OM2, OM3, and OM4 also attained
inhibition of more than 90% at 3 pg/mL. ReoPro was less effective at
inhibiting ATP
secretion than the anti-GPVI antibodies, suggesting that anti-GPVI Fabs are
better
inhibitors of collagen-induced ATP release. Secondary agonists released from
platelets are known to synergize thrombus growth. Therefore, inhibitors of
collagen-
induced secretion, such as the GPVI specific antibodies of the invention, are
potent
inhibitors of thrombus growth.
Table 5: Effect of Anti-GPVI Fab Fragments
on Collagen-Induced ATP Release From Human Platelets*
antibody concentration %inhibition
(pg/mL) (mean SD)
OM1 1 97.6 2.1
3 96.8 6.3
OM2 1 63.6 41.6
3 97.7 2.7
OM3 1 72.8 23.7
3 94.2 0.4
OM4 1 50.0 29.5
3 91.3 5.2
ReoPro 1 18.0 13.5
3 56.9 31.7
*Results were obtained from 4 different platelet donors.
-45-
CA 02567394 2006-11-24
[0140] Collagen-induced thromboxane A2 generation was also strongly
inhibited by Fab fragments of OM1, OM2, OM3, and OM4 (Table 6). Among the four
Fab fragments tested, OM1 showed inhibition of more than 90% at 1 pg/mL. OM2
and OM3 also attained inhibition of more than 90% at 3 pg/mL. OM4 inhibited
thromboxane A2 formation by about 86% at 3 pg/mL. In contrast, ReoPro showed
little or no inhibitory effect on thromboxane A2 formation at 1 and 3 pg/mL.
It has
also been shown that blockade of GPVI inhibits the generation of TXA2 and
expression of an activated Ilb-Ills complex on collagen-adhered platelets
(Nakamura
et al., J. Biol. Chem. 273:4338, 1998; Nakamura et al., J. Biol. Chem.
274:11879,
1999). Therefore, inhibition of TXA2 generation by anti-GPVI Fab fragments
suggests that the GPVI specific antibodies of the invention inhibit the
upstream
signaling events leading to the expression of activated Ilb-Ills complex and
ultimately,
followed by an attenuation in thrombus growth.
-46-
CA 02567394 2006-11-24
Table 6 - Effects of Fab Fragments of Anti-GPVI Antibodies
on Collagen-Induced Thromboxane A2 Formation in Human Platelets*
antibody concentration ng/3X10 platelets
(pg/mL) (mean SD)
control 104.3 37.4
OM 1 1 5.0 3.4
3 2.9 2.7
OM2 1 31.5 36.5
3 5.0 3.3
OM3 1 24.1 10.6
3 6.9 4.8
OM4 1 28.7 8.1
3 14.5 4.9
ReoPro 1 77.8 35.1
3 106.7 70.9
*Results were obtained using platelets from 4 different donors
EXAMPLE 4
BINDING AFFINITIES AND REACTIVITY OF Fab FRAGMENTS OF ANTI-
GPVI ANTIBODIES TO GPVI
[0141] Binding affinities were determined according to the method of
Fujimura et al. Thromb Haemost 87:728-34 (2002). 1251-labeled antibodies used
in
the determination of binding affinities were prepared from unlabeled IgGs
according
-47-
CA 02567394 2006-11-24
to the lodo-beads method (Pierce, IL). Briefly, one lodo-bead was soaked for 5
minutes in iodination buffer containing 0.5mCi carrier-free Na1251 (Amersham)
followed by the addition of a candidate IgG (100 pg). After 5 minutes of
incubation at
room temperature, the reaction mixture was applied to a PD10 column (Amersham)
to separate 1251 -bound IgG from free iodine. Fractions containing 1251-IgG
were
eluted from the column and a small volume (1 pl) was subjected to TCA
precipitation.
Both the precipitated pellets and the resulting supernatants were counted in a
gamma counter to quantify the incorporation of 1251 in IgG. Fractions with
maximal
counts in the precipitate (<95%) were pooled and read in a spectrophotometer
at
280nm to determine protein concentration. A known volume was recounted in a
gamma counter to obtain specific activity of the labeled IgG. The specific
activity
among various antibodies ranged from 0.33-0.97 pCi/ug IgG.
[0142] Binding affinities were determined by incubation of washed human
platelets (5x108/ml) with 1 nM 1251-IgG in the presence and absence of various
concentrations of unlabelled homologous IgG (0-500nM) for 1 hr. The free and
bound
radioactivities were separated by layering the mixture over BSA (10% solution
in
saline) solution and centrifuged at 15,000 x g for 10 minutes. After careful
removal of
both the supernatant and the BSA cushion, the tip of the tube was cut and
radioactivity of the pellet was counted in a gamma counter. Eight to ten
triplicate-
point competition binding isotherms were developed for evaluating the binding
of
individual IgG. The data was analyzed using the non-linear regression analysis
software, Prism (GraphPad Software Inc. CA).
[0143] Binding affinity experiments revealed that all four antibodies bound
avidly to platelets with affinities ranging from 0.7-1.7nM (Table 7). The
antibodies of
the invention were compared with the binding affinities of antibodies reported
in the
art: ReoPro (Kd=6.25 2.6x10-9M) (from Sassoli et al. Thromb Haemost 85:868-
902,
2001), scFv-10B12 (Kd=7.9x10-7M) (from Smethurst et al., Blood 103:903-91,
2004),
and 9012.2-IgG (Kd=18x10-9M) (from WO 02/080968). ReoPro and scFv-10B12
are monovalent fragments whereas the OM series used in this example and 9012.2
are IgGs. All four OM antibodies bound with higher affinity to human platelets
than
reported for ReoPro , scFv-10B12, and 9012.2-IgG (Table 7). Monovalent
fragments
-48-
CA 02567394 2006-11-24
generally have somewhat reduced affinities compared with their corresponding
intact
IgG; however, that reduction would not be expected to be significant.
Table 7. Binding Affinities of Anti-GPVI Antibodies
Antibody ID Kd pg IgG/ml
OM1 1.724 0.22x10 M 0.258 0.033
OM2 0.723 0.093x10 M 0.108 0.0103
OM3 0.8187 0.14x10 M 0.1220 022
OM4 0.785 0.25x10 M 0.117 0.037
ReoPro * 6.25 2.6x10 M 0.31 0.13
scFv - 10B12** 7.9x10 M 23.7
9012.2 - IgG*** 18x10 M 2.7
The binding study of the OM series is from a single experiment using platelets
from different
individuals. Each data point was run in triplicate.
* Data from Sassoli et al. Thromb Haemost 85:868-902 (2001).
** Data from Smethurst et al., Blood 103:903-911(2004). 1 OB1 2 is a human
specific scFv
antibody against GPVI obtained from a phage display method.
*** Data from WO 02/080968. 9012.2 is a monoclonal anti-GPVI antibody.
[0144] Western blotting analysis was performed to determine the reactivity of
the OM series antibodies to GPVI from various species. Platelets (1x108/ml)
from
various species were solubilized in 2% SDS containing EDTA, EGTA, PMSF and
NEM (1 mM each). Proteins were separated on 4-20% pre-cast Tris-glycine
gradient
mini gels (Invitrogen, Carlsbad, CA), and the resolved proteins were
transferred to
nitrocellulose membranes (Invitrogen, Carlsbad CA). Individual lanes were cut
and
blocked with 5% skim milk in TBS-T (10mM Tris.HCI pH 7.4, 150mM NaCl, and 0.5%
Tween 20) for 60 minutes. Nitrocellulose strips were incubated with OM
antibodies
(2pg/ml) or biotinylated convulxin overnight at 4 C. Membranes were washed
extensively with TBS-T and probed with either HRP-conjugated goat anti-mouse
IgG
-49-
CA 02567394 2006-11-24
(OM1, OM2, and OM4), HRP-conjugated goat anti-hamster IgG (OM3) or
streptavidin-HRP (biotinylated convulxin) for 1 hr at room temperature.
Membranes
were washed three times with a large excess of TBS-T. Immune reactive bands
were visualized using an enhanced chemiluminescence detection system (ECL-
Amersham Pharmacia Biotech, Little Chalfont, UK).
[0145] All four OM antibodies reacted with denatured GPVI from human
platelets on immunoblots (Fig. 5). Similar reactivity was seen with monkey
platelets
(blots not shown). None of the antibodies reacted with mouse, pig, dog, rabbit
or
guinea pig platelets. Only OM4 reacted positively with rat platelet lysate.
These data
suggest that all of the OM series antibodies recognize GPVI in platelets from
humans
and monkeys while OM4 additionally recognizes GPVI in rat platelets.
EXAMPLE 5
Complementarity Determining Regions (CDRs)
[0146] The sequences of the complementarity determining regions (CDRs) of
OM1, OM2, OM3 and OM4 were determined.
[0147] Total RNA was isolated from OM1, OM2, OM3, and OM4 hybridomas
using TRIzol (Invitrogen). cDNA was synthesized with the SuperScript First-
Strand
System (Invitrogen) using random primers. DNA sequences corresponding to the
variable regions of antibodies were then amplified by polymerase chain
reaction
using Platinum Pfx DNA Polymerase (Invitrogen) with Heavy Primers or Light
Primers
mix (Amersham). The amplified DNA was ligated into pCR4-TOPO vector
(Invitrogen) and the resulting construct was transformed into chemically
competent
cells using the TOPO TA Cloning kit (Invitrogen). Transformed cells were
cultured in
the presence of kanamycin and the amplified plasmid was isolated using the
E.Z.N.A.
Plasmid Miniprep Kit 11 (Omega Bio-tek). Sequencing of inserted DNA was
performed on the ABI PRISM 310 Genetic Analyser using the ABI PRISM BigDye
Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). DNA sequences were
analyzed and converted into amino acid sequences using the OMIGA 2.0 software
(Oxford Molecular).
[0148] The CDRs of OM1, OM2, OM3, and OM4 are shown in Table 8.
-50-
CA 02567394 2006-11-24
Table 8: CDR Sequences of Anti-GPVI Antibodies
OM1
H1: SYWMN (SEQ ID NO:1)
H2: MIHPSDSETTLNQKFKD (SEQ ID NO:2)
H3: DDYYDSSSHALDY (SEQ ID NO:3)
L1: RASQSVSTSTYSYIY (SEQ ID NO:4)
L2: FASYLES (SEQ ID NO:5)
L3: QHIWEIPWTF (SEQ ID NO:6)
OM2
H1: DHYIS (SEQ ID NO:7)
H2: WIYPGYGNIRYNEKFKG (SEQ ID NO:8)
H3: SADGYFRYFDV (SEQ ID NO:9)
L1: RASGNIHNYLA (SEQ ID NO:10)
L2: NSEILAD (SEQ ID NO:11)
L3: QHFWTAPFTF (SEQ ID NO:12)
OM3
H1: DFYMN (SEQ ID NO:13)
H2: SISGGSSDIKYADVVKG (SEQ ID NO:14)
H3: WGDHWDLDY (SEQ ID NO:15)
L1: QASQNIGNELN (SEQ ID NO:16)
L2: GASSLYP (SEQ ID NO:17)
L3: KQDLNYPITF (SEQ ID NO:18)
OM4
H1: SFGMH (SEQ ID NO:19)
H2: FISSGSSTIYYADIVKG (SEQ ID NO:20)
H3: SGYANAMDY (SEQ ID NO:21)
L1: KASQDVSPAVT (SEQ ID NO:22)
L2: WASTRHT (SEQ ID NO:23)
L3: QQHYSFPWTF (SEQ ID NO:24)
-51 -
CA 02567394 2006-11-24
EXAMPLE 6
GPVI AND THROMBOSIS
[0149] The role of GPVI in thrombosis had been shown in a prior study using
GPVI-depleted or FcRy -KO/GPVI deficient mice (Nieswandt et al., The EMBO
Journal 20:2120-2130 (2001)). Both the GPVI-depleted or FcRy -KO/GPVI
deficient
mice lack the FcRy chain. In this example, the involvement of GPVI in
thrombosis
was confirmed using GPVI-KO mice that do not lack the FcRy chain.
[0150] GPVI knock-out mice were developed as described above. Platelets
from these mice failed to respond to high dose collagen (20 pg/ml) and to the
GPVI-
specific agonist, convulxin (3pg/ml), but responded normally to ADP (5pM)
(Fig. 6).
Heterozygous GPVI-deficient mice which produce half the amount of GPVI
compared
with wild-type showed reduced responses to collagen and convulxin but normal
responses were observed upon increase in agonist dose (Fig. 6). These
observations confirm the role of GPVI as a dominant collagen receptor on the
platelet
surface.
[0151] The role of GPVI in thrombosis and homeostasis was first tested by
co-injection of collagen-epinephrine, which normally induces a lethal
pulmonary
thrombo-embolism (see Table 9). Mice were anaesthetized with ketamine/xylazine
(150/15 mg/kg, IP) and a mixture of collagen and epinephrine (800/60 pg/kg)
was
injected into the right jugular vein. The animals were then observed for 15
minutes
and categorized as follows: (a) animals that succumbed to death within 10
minutes of
the injection, and (b) animals that survived and showed transient respiratory
distress,
which was alleviated within 10 minutes. The surgical wounds of surviving
animals
were sutured and the animals were returned to the animal facility.
Approximately
83% (15 out of 18) of GPVI wild-type mice died within 5 minutes of injection.
All
heterozygous GPVI-deficient mice (18 out of 18) died within five minutes of
injection.
In contrast to wild-type and heterozygous animals, approximately 55% of the
GPVI-
KO mice (homozygous) survived the lethal injection of collagen and
epinephrine,
suggesting that GPVI plays an important role in pulmonary thromboembolism
induced by the injection of collagen.
-52-
CA 02567394 2006-11-24
Table 9. The Role of GPVI in Pulmonary Thromboembolism
Genotype number of animals % Survival
survived
Wild-type (+/+) 3/18 -16
Heterozygous (+1-) 0/18 0
Homozygous (-/-) 10/18 -55
[0152] As shown in Table 10, GPVI knock-out mice had essentially same tail
bleeding time as wild-type and heterozygous mice. Bleeding times of the GPVI
knock-out mice were compared to those of knock-out mice deficient in (33
integrin,
which is also found on platelets and is involved in platelet aggregation and
thrombosis (Kairbaan et al., J. Clin. Invest. 103:229-238, 1999). In contrast
to the
GPVI-deficient mice, the homozygous (33 knock-out mice bled continuously.
Therefore, administration of anti-GPVI Fab fragments in vivo may be safer as
they
should not significantly affect bleeding time because the bleeding time in
GPVI-
knockout mice is similar to that of wild-type mice.
Table 10. Tail Bleeding Time in Wild-Type, Heterozygous and Homozygous
Mice: Comparison With 03 Knock-Out Mice
Genotype Tail bleeding time (seconds)
GPVI-knock out 133-knock out*
Wild-type (+1+) 191 186(n=9) 156 89 (n=15)
Heterozygous (+1-) 190 193(n=14) 156 99 (n=15)
Homozygous (-/-) 165 110 (n=13) >600 (n=15)**
*J Clin Invest 103:229, 1999
** Significantly different from wild-type and heterozygous mice. In most
cases,
bleeding had to be stopped manually to prevent death
-53-
CA 02567394 2006-11-24
[0153] To further confirm the role of GPVI in thrombosis, whole blood from
wild-type, heterozygous and GPVI-KO mice were perfused onto type I collagen-
coated cover slips at a shear stress of 2600sec-1. Platelets from GPVI-KO mice
failed to adhere to collagen fibers while platelets from wild-type and GPVI-
heterozygous mice adhered to collagen fibers and formed large thrombi. There
was
no difference in surface coverage and thrombus morphology between wild-type
and
GPVI-heterozygous mice (Fig. 7).
EXAMPLE 7
EFFECT OF OM2 Fab FRAGMENT ON EX VIVO COLLAGEN-INDUCED
PLATELET AGGREGATION AND SKIN BLEEDING TIME IN CYNOMOLGUS
MONKEYS
[0154] Dose-escalation study. The OM2 Fab fragment was further evaluated
because it showed the strongest inhibitory effect among the OM antibodies in
an in
vitro collagen-induced platelet aggregation assay using platelets from
Cynomolgus
monkeys. The OM2 Fab fragment was administered by intravenous injection to
Cynomolgus monkeys in escalating doses and its effect on ex vivo collagen-
induced
platelet aggregation and skin bleeding time were evaluated. ReoPro was tested
in a
similar manner.
[0155] Under inhalation anesthesia, the OM2 Fab fragment or ReoPro was
injected intravenously into the cephalic vein of the forearm at 1 hr
intervals. Thirty
minutes after each injection, blood was collected for the measurement of
collagen-
induced platelet aggregation. Platelet rich plasma (PRP) and platelet poor
plasma
(PPP) were prepared by sequential centrifugation of blood that was anti-
coagulated
with trisodium citrate. Platelet aggregation was measured by the turbidimetric
method 1 hr after blood collection. Concentrations of collagen used to induce
platelet
aggregation were between 5 and 20 pg/mL, depending on the responsiveness of
the
platelets from each monkey. Skin bleeding time was measured 30 min after each
injection of the antibody by compressing the muscle of the forearm with a
manchette
at 40mmHg and incising the skin with a Triplett Bleeding time device (Helena
Laboratory). Blood flowing from the wound was absorbed with filter paper and
-54-
CA 02567394 2006-11-24
bleeding time was measured until bleeding stopped, or at 1800 sec. Cumulative
dose of antibody was used to evaluate the effects of the antibodies.
[0156] The OM2 Fab fragment exerted a dose-dependent inhibitory effect on
collagen-induced platelet aggregation (Fig. 8). At the cumulative dose of
0.2mg/kg or
higher, the OM2 Fab fragment inhibited platelet aggregation by more than 80%.
[0157] ReoPro also exerted a dose-dependent inhibitory effect on
collagen-induced platelet aggregation (Fig. 8) but inhibited platelet
aggregation by
more than 80% at a cumulative dose of 0.35mg/kg or higher.
[0158] OM2 Fab fragment prolonged skin bleeding time only slightly (2.4
times the baseline value) at a cumulative dose of 0.8mg/kg (Fig. 9). Although
the
OM2 Fab fragment slightly prolonged bleeding time at a cumulative dose of
18.8mg/kg, bleeding time did not exceed the bleeding time observed at
0.8mg/kg.
[0159] In contrast, ReoPro prolonged skin bleeding time dramatically (Fig 9).
At a cumulative dose of 0.35mg/kg, the average bleeding time was 5 times
longer
than the baseline value. Additionally, the prolongation of bleeding time by
ReoPro
was dose-dependent. At a cumulative dose of 1.55mg/kg, bleeding time was 9.5
times longer than baseline level.
[0160] In summary, the OM2 Fab fragment showed an equally potent
inhibitory effect on ex vivo collagen-induced platelet aggregation as
exhibited by
ReoPro . However, the effect of OM2 Fab fragment on skin bleeding time was
much
milder than that of ReoPro . These results suggest that blockade of GPVI has a
superior risk/benefit ratio when compared to that of GPIIb/Illa blockade and
therefore
may be better suited for clinical treatment. Moreover, a red spot was observed
at the
injection site in one monkey after administration of ReoPro at 0.4 and 0.8
mg/kg.
Although this spot disappeared after several days, no abnormalities were
observed
after OM2 Fab administration.
[0161] Pharmacodynamics study. The change in the effects of the OM2 Fab
fragment over time on ex vivo collagen-induced platelet aggregation and skin
bleeding time was evaluated in three Cynomolgus monkeys after bolus
intravenous
injection. The change in effects by ReoPro was similarly evaluated.
-55-
CA 02567394 2006-11-24
[0162] The dose of 0.4mg/kg was selected for this study because the OM2
Fab fragment and ReoPro inhibited ex vivo collagen-induced platelet
aggregation at
0.2mg/kg and 0.35mg/kg, respectively. After bolus intravenous injection of
antibody
into the cephalic vein of the forearm, blood was collected at 1, 2, 3, 6 and
24 hrs.
Platelet aggregation was measured 1 hr after each blood collection as
described
above under the dose-escalation study. Concentrations of collagen used to
induce
platelet aggregation were 5 or 10 pg/mL in this study, depending on the
responsiveness of the platelets from each monkey. Bleeding time was also
measured at 1, 2, 3, 6 and 24 hrs after antibody administration as described
under
the dose-escalation study.
[0163] The OM2 Fab fragment injected at 0.4mg/kg inhibited ex vivo
collagen-induced platelet aggregation by more than 80% at 1, 2, 3 and 6 hrs
after
administration (Fig. 10). At 24 hrs after injection, platelet aggregation
recovered
nearly to basal level.
[0164] Similarly, ReoPro injected at 0.4mg/kg inhibited ex vivo collagen-
induced platelet aggregation by more than 80% at 1 and 2 hrs after
administration
(Fig. 10). However, in contrast to the OM2 Fab fragment, platelet aggregation
recovered in a time-dependent manner: 73% inhibition at 3 hr, 47% inhibition
at 6 hr
and 6% inhibition at 24 hr after administration.
[0165] As shown in Fig. 11, the OM2 Fab fragment slightly prolonged skin
bleeding time between 1 and 6 hrs after administration (1.7 to 2.0 times
longer than
baseline level). Bleeding time returned to nearly baseline level at 24 hrs
after
injection, coincident with the recovery of platelet aggregation.
[0166] In contrast, ReoPro significantly prolonged bleeding time at 1 hr
after
administration (10.7 times longer than baseline level) (Fig. 11). Prolongation
of
bleeding time became less prominent in a time-dependent manner.
[0167] These results showed that the inhibitory half-life of the OM2 Fab
fragment on platelet aggregation is longer than that of ReoPro . In addition,
these
results again suggest that the risk/benefit ratio of the OM2 Fab fragment is
superior
to that of ReoPro .
-56-
CA 02567394 2006-11-24
EXAMPLE 8
EFFECT OF OM4 Fab FRAGMENT ON EX VIVO COLLAGEN-INDUCED
PLATELET AGGREGATION, BLEEDING TIMES, AND PLATELET COUNT IN
RATS
[0168] The OM4 Fab fragment was further tested for ex vivo collagen-
induced platelet aggregation, tail and nail bleeding time in rats. For
comparison, the
7E3 F(ab')2 fragment derived from an established murine antibody raised
against
human platelet glycoprotein complex GPIIb/Illa (Collar et al. J. Clin Invest.
72:325-
338, 1983) was tested in a similar manner. 7E3 IgG was obtained from large
cultures
using a 7E3 hybridoma obtained from the ATTC. F(ab')2 fragments were prepared
as
described in Collar et al. J. Clin Invest. 72:325-338, 1983. In preliminary
studies
using rats, the optimal dose of OM4 Fab and 7E3 F(ab')2 were determined to be
20
and 10mg/kg, respectively. The ex vivo collagen-induced platelet aggregation
remained inhibited by OM4 Fab for 30 minutes after which the inhibition
reversed at
60-90 minutes, suggesting a fast clearance of OM4 Fab in rats. All
observations
were made at 20 minutes after the administration of vehicle, test and
reference
antibody.
[0169] Adult male Sprague-Dawley rats were anesthetized with
ketamine/xylazine. Heparin-filled catheters were inserted into the femoral
vein,
femoral artery, and carotid artery for drug administration, blood
pressure/heart rate
recording, and blood sampling, respectively. After the equilibration period, a
small
sample of blood (-1.2 mL, anticoagulated with 10 U/mL heparin) was withdrawn
from
the carotid artery for determination of platelet count. The degree of platelet
aggregation elicited by 1 g/mL collagen was measured using a whole blood
aggregometer (Chrono-log, Havertown, PA). Nail-bleeding time was also
determined
at this time by cutting one of the hind limb toenails at a point that
transected the nail
pulp and by absorbing the blood every 15 sec onto a piece of filter paper,
without
touching the cut surface of the nail. Nail-bleeding time was defined as the
time
elapsed between cutting the nail and the point at which no further blood
absorbed
onto the filter paper.
-57-
CA 02567394 2006-11-24
[0170] Vehicle, OM4 Fab, or 7E3 F(ab')2 composition was administered into
the femoral vein. At 20 minutes, a second blood sample was withdrawn for
aggregometry and platelet count determination, as described above. Immediately
following withdrawal of the final sample, nail and tail bleeding times were
determined.
Tail-bleeding time was determined by removing the terminal 3 mm of the tail
using a
sharp scalpel blade and immersing the distal 2 - 3 cm tail into 37 C saline.
Tail-
bleeding time was defined as the time elapsed between cutting the tail and the
point
at which no more blood drew from the cut surface of the tail. Six rats were
used for
each group (vehicle, OM4 Fab and 7E3 F(ab')2 fragment).
[0171] Both OM4 Fab and 7E3 F(ab')2 fragments produced a statistically
significant degree of inhibition of platelet aggregation at 20 min after the
administration of the antibody fragments, in relation to the vehicle (P<0.05)
(Fig. 12).
The inhibition was highly reproducible, although the variability was slightly
greater in
the OM4 Fab group. Blood pressure and body core temperature remained
unchanged by the intravenous administration of vehicle, OM4 Fab, and 7E3
F(ab')2
fragment compounds.
[0172] Nail bleeding time was dramatically prolonged by the administration of
the 7E3 F(ab')2 fragment while the administration of OM4 Fab had no effect in
four
out of six animals tested (Fig. 13A). In two animals, the nail bleeding time
was
prolonged to 26 and 31 minutes by the administration of OM4 Fab. In contrast,
the
7E3 F(ab')2 fragment prolonged nail bleeding time beyond 30 minutes in all
cases
except one in which nail bleeding halted at 18 minutes (Fig. 13A). The mean
nail
bleeding time in animals receiving 7E3 F(ab')2 fragment was significantly
greater than
OM4 Fab and vehicle groups, as indicated by the Student's t-test. OM4 Fab did
not
induce any significant prolongation of nail bleeding time when compared to
vehicle.
[0173] Similar to the nail bleeding time, tail bleeding time was dramatically
prolonged in all animals by the administration of 7E3 F(ab')2 fragment while
administration of OM4 Fab had no effect in four out of six animals tested
(Fig. 13B).
In two animals, the tail bleeding time was prolonged to 26 and >30 minutes. In
contrast, the 7E3 F(ab')2 fragment prolonged tail bleeding time to beyond 30
minutes
-58-
CA 02567394 2006-11-24
in all cases (Fig. 13B). There was no significant effect of intravenous
administration
of vehicle, OM4 Fab and 7E3 F(ab')2 fragment on platelet count (Fig. 14).
[0174] The data obtained from this study clearly shows that OM4 Fab
(20mg/kg) and 7E3 F(ab')2 fragment (10mg/kg) elicit similar degrees of
inhibition of
collagen-induced platelet aggregation but 7E3 F(ab')2 significantly prolonged
bleeding time. OM4 Fab's ability to inhibit platelet function without
significantly
affecting bleeding time suggests that it would be therapeutically beneficial.
OM4
Fabs may provide similar positive benefits as currently available platelet
inhibitors
without their negative bleeding side effects.
EXAMPLE 9
EFFECT OF OM4 Fab FRAGMENT IN A RAT ARTERIAL THROMBOSIS MODEL
[0175] The effect of the OM4 Fab fragment was also studied in an in vivo
arterial thrombosis model in rats. Although various models of in vivo
thrombosis
have been reported in the literature, a model developed by Folts (see e.g.,
Circulation
83(6 Suppl):IV 3-14, 1991) has been widely used to test the efficacy of
antithrombotic
agents. This original model was developed in a canine coronary artery but for
this
study, the model was modified for testing on rat carotid artery. Briefly, the
carotid
artery was mechanically injured, followed by stenosis. Combination of vascular
injury
and narrowing of the blood vessel (two conditions mimicking the pathogenesis
of
thrombosis, i.e. arteriosclerosis and stenosis) results in the formation of a
thrombus.
The thrombus can then be mechanically dislodged and reformed by removal and
replacement of the vascular occluder, respectively. This leads to cyclic flow
reduction (CFR) as discussed in more detail below. Antithrombotic agents may
reduce the number of CFR or completely prevent the formation of CFR.
[0176] Rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and
mechanically ventilated with an intubation of the tracheal. A segment of the
femoral
vein was dissected and used for drug injection. The carotid artery was exposed
via a
midline incision in the ventral cervical area and dissected free of connective
tissue. A
small flow probe (Transonic, 1 RB, Transonic Systems Inc., Ithaca, NY) was
placed
distally on the artery to measure blood flow. Thrombosis was induced by
applying
-59-
CA 02567394 2006-11-24
two conditions. First, an injury was induced by three consecutive cross-clamps
of the
middle exposed segment of the artery for 10 seconds each, with a needle holder
having one ratchet click closed. Second, a 50% reduction of the baseline blood
flow
was applied by inflating a vascular occluder (1.5 mm inner diameter, In Vivo
Metric,
Healdsburg, CA), which consists of a C-shaped balloon that was placed on the
site of
the injury. Blood flow was gradually reduced to zero within about 2-5 minutes
due to
the formation of a thrombus. The thrombus was physically dislodged by
deflating the
balloon, and blood flow was immediately restored. One CFR is counted when the
blood flow reduces to zero after the 50% blood flow reduction and when blood
flow is
restored. After re-applying the 50% flow reduction, blood flow again gradually
decreased, resulting in the next CFR as a new thrombus formed. The number of
CFRs was counted during a 30 min observation period. The number was also
rounded to a half cycle.
[0177] In the pre-injury groups, either saline or OM4 Fab fragment at
20mg/kg was given by an intravenous bolus injection 2 min before mechanical
injury
was applied. CFRs were then recorded for 30 min.
[0178] In the post-injury groups, CFRs were initiated by endothelial injury
and
50% reduction of flow. CFRs were observed for 15 min followed by an
intravenous
bolus injection of vehicle or OM4 Fab fragment at 20mg/kg. CFRs were then
recorded for 30 min.
[0179] An un-paired t-test was used to compare the number of CFRs in
saline and OM4 pre- or post-treated groups. P<0.05 was considered significant.
[0180] Pre-injury groups. OM4 Fab fragment injected at 20mg/kg before
mechanic injury reduced the number of CFRs from 10.5 4.1 (mean SD) to 4.1
5.2 (Fig. 15A). This reduction was statistically significant (p<0.02, t-test).
[0181] Post-injury groups. OM4 Fab fragment injected at 20mg/kg injected
after establishing CFR also reduced the number of CFRs from 12.2 3.8 to 3.5
3.6
(Fig. 15B). This reduction was also statistically significant (p<0.003, t-
test). This
result demonstrates that the OM4 Fab fragment can inhibit thrombus formation
even
after initiation of the interaction between platelets and subendothelial
collagen.
Moreover, these results suggest that anti-GPVI antibodies can potently inhibit
in vivo
-60-
CA 02567394 2006-11-24
arterial thrombosis formation induced by endothelial injury/blood flow
perturbation
that are proposed triggers of thrombotic diseases in clinical situations.
EXAMPLE 10
RELATIONSHIP BETWEEN THE OCCUPANCY RATE OF GPVI AND INHIBITION
OF COLLAGEN-INDUCED PLATELET AGGREGATION
[0182] The relationship between GPVI occupancy rate on the platelet surface
and the inhibitory effect on collagen-induced platelet aggregation by
biotinylated OM2
Fab fragment in human platelets in vitro was examined.
[0183] OM2 Fab fragment was biotinylated with Sulfo-NHS-LC-Biotin
(Pierce). Biotin solution (150pL, 10 mg/mL in distilled water) was added to
OM2 Fab
fragment solution in Phosphate Buffered Saline (PBS) (5mg, 2mg/mL). Reaction
tube was incubated on ice for 2 hrs. To remove free biotin, the reaction
mixture was
dialyzed against saline in a cold room overnight.
[0184] Blood was collected from 3 healthy donors in 1/10 volume of 3.8 %
trisodium citrate anticoagulant and platelet rich plasma (PRP) obtained by
centrifugation at 180 x g for 15 min at room temperature. Platelets were
counted and
adjusted to 3x108 platelets/mL with platelet poor plasma. Measurement of
aggregation was performed within 4 hrs of blood collection. Aggregation
studies
were performed in an AG10 aggregometer (Kowa, Japan). PRP was incubated with
biotinylated OM2 Fab fragment (0, 0.1, 0.3, 0.5, 0.7, 1, 3, 10 and 20pg/mL)
for 10 min
at 37 C before collagen was added and aggregation was monitored for an
additional
5-10 min. The collagen dose required to induce 70-90 % aggregation was
determined for each donor prior to the evaluation of the antibody.
[0185] PRP (3x108 platelets/mL, 400pL each) was incubated with biotinylated
OM2 Fab fragment (0, 0.1, 0.3, 0.5, 0.7, 1, 3, 10 and 20pg/mL) for 10 min at
room
temperature. Then, washed platelets were prepared as follows. PRP was
supplemented with 1 pg/mL PGE1, 1 mM EDTA, and EGTA and centrifuged at
2,000 x g for 10 min. After discarding plasma, the platelet pellet was
suspended in
platelet wash buffer (PWB: phosphate-buffered saline supplemented with 1 mM
EDTA
and EGTA, 0.1% NaN3, 100 ng/mL PGE1 and 0.35% BSA, pH 7.4). Then, platelets
-61-
CA 02567394 2006-11-24
were centrifuged and washed again. The washed platelets were suspended in a
small volume of PWB and platelets were counted. Finally, the washed platelets
(1 X108 platelets/mL) were solubilized by mixing with an equal volume of 1%
Triton X-
100-containing phosphate-buffered saline.
[0186] Biotinylated OM2 Fab fragment was quantified by the ELISA method
using streptavidin (Pierce, 21125) as a capture reagent and goat anti-mouse
IgG
antibody-HRP (American Qualex, A106PU) as a detecting reagent.
[0187] In all three donors, collagen-induced platelet aggregation was
inhibited by more than 75% by biotinylated 0M2 Fab fragment at a concentration
of
3pg/mL.
[0188] In the occupancy rate assay, occupancy rate was assumed to be at
100% at 20pg/mL of biotinylated 0M2 Fab fragment (bound amount: 26.4
5.5ng/5X107 platelets). As shown in Fig. 16, the amount of bound 0M2 Fab
fragment
saturated at 3pg/mL (24.3 3.5ng/5X107 platelets). The occupancy rate of
biotinylated 0M2 Fab fragment at 3pg/mL was 92%. This result suggests that an
occupancy rate of more than 90% of GPVI on platelet surface is required to
exert
maximal inhibitory effects on collagen-induced platelet aggregation.
[0189] In conclusion, the GPVI specific antibodies of the invention may be
useful antithrombotic agents. As demonstrated above, the GPVI specific
antibodies
are potent inhibitors of platelet functions induced by collagen.
EXAMPLE 11
PREPARATION OF RECOMBINANT SOLUBLE GPVI
[0190] A recombinant soluble GPVI polypeptide comprising the ectodomain
of GPVI was produced as a standard protein for use in an ELISA to detect and
quantify shed sGPVI in biological samples.
[0191] The DNA sequence encoding the ectodomain of human GPVI (amino
acid residues 22-219) was amplified by standard PCR procedures. The PCR
product
was digested with EcoRl and Notl restriction enzymes and inserted into the
plasmid
pEF1/SecTagA/V5HisA via the EcoRl and Notl sites. This vector encodes the
signal
peptide of the Ig K light chain fused to the N-terminal end, and V5 and Hisx6
peptide
-62-
CA 02567394 2006-11-24
tags fused to the C-terminal end, of the polypeptide encoded by the inserted
DNA
sequence. Figure 17A illustrates the structure of the resulting sGPVI
polypeptide
which comprises the Ig signal peptide at the N-terminus, followed by the two
immunoglobulin (Ig) domains of the GPVI ectodomain, and the V5 and Hisx6
peptide
tags at the C-terminus.
[0192] The resulting plasmid was transfected into CHO-K1 cells using
Lipofectamine 2000 (Invitrogen) as described by the manufacturer. Transfected
CHO
cells were cloned by limiting dilution and individual, G418-resistant clones
were
expanded in the presence of the selection marker G418. The levels of secreted
sGPVI in the culture medium of individual cell clones were compared using a
sandwich ELISA (see Example 12). Clones producing maximal amounts of sGPVI
were selected for further use. Selected sGPVI-expressing CHO cells were
cultured
in roller bottles in DMEM/F12 (1:1) medium containing 2.0-2.5% fetal calf
serum
(FCS) and 100pg/ml G418 at 37 C in 5% C02-containing atmosphere.
[0193] The culture supernatant was harvested once the cells reached
confluence. The culture supernatant was centrifuged at 15,000 x g for 45
minutes at
4 C to remove cells and cellular debris. Cleared supernatant was concentrated
20-fold using an Amicon YM10 filter assembly (Millipore). The recombinant
sGPVI
was then purified by affinity chromatography using anti-GPVI antibody (OMI)-
coupled SepharoseTM. HiTrapTM NHS-activated HP columns from Amersham
Pharmacia were used to couple OM1 IgG to SepharoseTM beads according to the
manufacturer's instructions. The concentrated supernatant was loaded onto the
OM1-coupled SepharoseTM column which had been equilibrated in PBS. To remove
low affinity binding proteins the column was washed extensively with PBS until
the
OD280nm of the wash returned to the baseline (<0.01). The column-bound sGPVI
was eluted with 3M KSCN in PBS. The fractions containing protein (as assessed
by
measurement of OD280nm) were pooled, dialyzed in PBS, concentrated and stored
at -20 C. The purity and molecular weight of sGPVI was assessed by SDS-PAGE.
[0194] Figure 17B shows a Coomassie-stained SDS-polyacrylamide gel with
the molecular weight markers in the left lane and the recombinant sGPVI in the
right
lane. The molecular weights of the marker proteins are indicated (in kDa). The
-63-
CA 02567394 2006-11-24
recombinant sGPVI is substantially pure, as evidenced by the absence of
significant
contaminating polypeptides, and has an apparent molecular weight of 40 kDa
under
reducing conditions. The authenticity of the sGPVI was confirmed by
demonstrating
its reactivity with biotinylated convulxin, OM1 and OM2 IgGs in Western blot
and
ELISA assays. Furthermore, the presence of Hisx6 and V5 peptide tags was
confirmed by demonstrating reactivity with anti-His and anti-V5 monoclonal
antibodies in ELISA assays. These results show that a highly purified
recombinant
sGPVI preparation was obtained and may be used in a variety of applications,
including as a standard protein for a sGPVI-specific ELISA.
EXAMPLE 12
A SENSITIVE ELISA FOR MEASURING SOLUBLE GPVI IN BIOLOGICAL
SAMPLES
[0195] A sensitive method for detection and quantitation of sGPVI shedding
in biological samples was developed. This method is useful, for example, for
monitoring sGPVI levels in blood samples obtained from patients with acute or
chronic thrombosis or other vascular diseases.
[0196] This example provides a sandwich ELISA. Briefly, a capture GPVI-
specific antibody (OM1) was immobilized onto a solid support, such as plastic
wells.
After blocking the wells with non-specific protein, sGPVI was added. A second,
non-
competitive and labeled GPVI-specific antibody (biotinylated OM2 Fab) was
added
and captured sGPVI was detected and quantified by horseradish peroxidase (HRP)-
conjugated streptavidin. Figure 18 illustrates the basic properties of this
sandwich
ELISA. Figure 18A illustrates the overlap or lack of overlap of the GPVI
epitopes
recognized by the monoclonal GPVI-specific antibodies OM1, OM2, OM3 and OM4.
OM1 and OM2 antibodies recognize non-overlapping epitopes and hence do not
compete with each other for binding to GPVI. Figure 18B illustrates the
principle of
capture and detection of sGPVI by the ELISA.
[0197] Hybridomas producing OM1 and OM2 IgG were grown in DMEM/F12
medium containing 5% fetal bovine serum (containing negligible amounts of
bovine
IgG: < 1 pg/ml) at 37 C in roller bottles. Once cell growth reached optimal
density,
-64-
CA 02567394 2006-11-24
the cells were removed by centrifugation at 3500 x g for 30 minutes and the
resulting
supernatant was further clarified by filtration through a 0.2pm filter. The
filtered
supernatant was loaded at a flow rate of 5-6ml/min onto a Protein G-
SepharoseTM
column (Amersham Pharmacia) using a Waters 650 Protein Separation System. The
column was washed extensively until the OD280nm of the wash returned to the
baseline (<0.01). The bound IgG was eluted from the column with a low pH
glycine-
HCL buffer (pH 2.75) directly into 1/10 volume of 1 M Tris-HCI, pH 8Ø
Fractions
containing protein (as assessed by measurement of OD280nm) were pooled,
concentrated and dialyzed extensively against saline. The purity of each IgG
was
assessed by SDS-PAGE and ranged from 88-92%.
[0198] Fab fragments of the OM2 monoclonal antibody were prepared by
papain digestion. A solution of OM2 IgG (5mg/ml) in 100mM citric acid, pH 6.5,
and
5.0 mM EDTA, supplemented with cysteine (10mM), was digested for 3 hrs at 37 C
with papain (Sigma; 28mg/ml stock) at an enzyme:IgG ratio of 1:100 (w/w).
Digestion
was quenched with freshly prepared iodoacetamide (final concentration of 30mM)
and Fab fragments were separated from undigested IgG and Fc fragments by ion
exchange chromatography on a MonoQ column (Amersham Pharmacia Biotech).
Fab- containing fractions were pooled, concentrated and purified to
homogeneity by
size exclusion chromatography on a Superdex 75 column (Amersham Pharmacia
Biotech). Fab fragments were dialyzed extensively against isotonic saline
(0.9%),
concentrated to 10mg/ml using Amicon concentration filtration units with PM10
filter,
filtered through a 0.22pm filter and stored at 4 C. The purity of the OM2 Fab
preparation was assessed by SDS-PAGE and ranged from 95-98%.
[0199] OM2 Fab was biotinylated with NHS-biotin according to the
instructions provided by the supplier (Pierce Chemical Co.). Briefly, OM2 Fab
(1-
5mg/mi) was dialyzed against 100mM carbonate/300mM NaCl, pH 8Ø NHS-biotin
solution was added (IgG:NHS-biotin ratio of 1:10 (mol/mol)) and the mixture
was
incubated for 1-3 hours at room temperature. The conjugation reaction was
terminated by addition of 1/10 volume of 1M Tris-HCI, pH 8.0, and the reaction
mix
was dialyzed extensively against PBS (pH 7.4) to remove free biotin.
Biotinylated
OM2 Fab was stored at 4 C in small aliquots.
-65-
CA 02567394 2006-11-24
[0200] 96 well ELISA plates (Maxisorb, Nunc) were coated with OM1 IgG by
adding 100pl IgG solution to each well (IgG at 1 pg/ml in PBS/0.05% azide) and
incubating the mixture overnight at 4 C. The IgG solution was removed, wells
were
washed with PBST (PBS containing 0.05% Tween20) and unoccupied sites were
blocked with 300pl blocking buffer (for example, PBS/5% Sorbitol/1 % BSA/0.1 %
azide) for 1-2 hours at room temperature or overnight at 4 C. Wells were
rinsed once
with PBST prior to starting the sGPVI assay. 100pl of appropriately diluted
test
samples (blood plasma) or standards (recombinant sGPVI) were added to the
wells
and incubated for 1-2 hours at room temperature or at 4 C overnight to allow
capture
of plasma sGPVI or recombinant sGPVI by the OM1 capture antibody immobilized
to
the wells. After washing wells three times with PBST, biotinylated OM2 Fab
(100pl of
a 0.1 pg/ml solution) was added to each well and incubated for 1 hour at room
temperature. Wells were washed three times with PBST to remove unbound
biotinylated OM2 Fab. Streptavidin-HRP (100pl/well of a 1:6000 dilution;
Invitrogen)
was added to each well and incubated for 30 minutes at room temperature. The
plate was rinsed three times with PBST and 100 pl substrate solution
(Microwell
Peroxidase Substrate System, KPL) was added to each well. After 10 minutes
incubation at room temperature, the enzymatic reaction was terminated by the
addition of stop solution (50 pl/well of 1 M H2SO4). The color intensity
resulting from
the enzymatic reaction was determined in a plate reader at a wavelength of
450nm
(Power wave HT; BioTek Instruments, Inc.).
[0201] Figure 19 shows an example of results obtained by the ELISA.
Increasing concentrations of recombinant sGPVI (see Example 11) (within a
range of
0.1 ng/ml to 5.Ong/mi) were added to the wells and detected as described
above. The
resulting curve demonstrates that the developed ELISA can reliably measure as
low
as 0.2ng/ml sGPVI and as high as 2.5 ng/ml sGPVI in a biological sample. In
healthy
human volunteers the plasma concentration of soluble GPVI averaged about
6ng/ml.
Hence, these results demonstrate that the ELISA of the invention is
sufficiently
sensitive to detect and quantify sGPVI levels in plasma of healthy human
individuals
or in plasma of patients with conditions associated with elevated sGPVI
shedding.
-66-
CA 02567394 2006-11-24
EXAMPLE 13
MONITORING OF TIME-DEPENDENT AND AGONIST-INDUCED SHEDDING OF
SOLUBLE GPVI FROM HUMAN PLATELETS
[0202] The mechanism and time-course of agonist-induced sGPVI shedding
from human platelets was investigated using an in vitro assay system. The
effects of
GPVI-specific and non-GPVI-specific agonists on sGPVI shedding were compared.
[0203] Human platelet rich plasma derived from healthy donors was treated
with an optimal dose (producing about 90% platelet aggregation) of the
platelet
agonists ADP, thrombin receptor activating peptide (TRAP), collagen, collagen-
related peptide (CRP) and convulxin (rattlesnake venom protein) to induce
platelet
aggregation and sGPVI shedding. Collagen, CRP and convulxin activate platelets
mainly through their interactions with GPVI and are hence termed GPVI-specific
agonists, while ADP and TRAP utilize different receptors to activate platelets
and are
hence termed non-G PVl -specific agonists. Matsumoto et al., Thromb. Haemost.
96:176-175 (2006). The experiments were designed to determine maximal platelet
aggregation induced by the test agonists within ten minutes as well as agonist-
induced sGPVI shedding over a time period of 2 hours. The experimental design
is
illustrated in Figure 20.
[0204] Preparation of the agonists. ADP and TRAP (SFLLRN-NH2) were
purchased from Sigma Chemical Company as powders. ADP was dissolved in saline
at a concentration of 10mM and stored at -20 C in small aliquots. Working
concentrations of ADP solutions were made in saline on the day of use and
maintained on ice. Unused ADP was discarded at the end of the day.
[0205] A stock solution of TRAP (10mM) was made in dilute acetic acid
(5mM) and stored in small aliquots at -20 C. On the day of use, the stock
solution
was diluted with dilute acetic acid to the desired concentration and the
diluted TRAP
solution was maintained on ice. If used within 2-3 days, the diluted working
solution
of TRAP was stored at 4 C; otherwise it was discarded.
[0206] Acid-insoluble type I equine collagen, the most commonly used
collagen for performing in vitro and ex vivo collagen-induced platelet
aggregation
experiments, was purchased from Nycomed at 1 mg/ml. Prior to use, the stock
-67-
CA 02567394 2006-11-24
solution was diluted to 100-200pg/ml with the buffer provided by the supplier.
Diluted
solutions were stored at 4 C.
[0207] CRP, a 40-mer peptide containing repeated GPO (glycine, proline and
hydroxyproline) sequences, was kindly synthesized by Dr. Michinori Tanaka,
Medicinal Chemistry Research Institute, Otsuka Pharmaceutical Co., Ltd.
Monomeric
CRP was polymerized by cross-linking with EM grade gluteraldehyde (0.25%) at 4
C
for 2-3 hours by the method described by Morton et al., Biochem. J. 306:337-
344
(1995). This preparation of CRP induced maximal aggregation (70-90%) of human
and monkey platelets at 31.25-62ng/ml. CRP was stored in PBS at 1 mg/ml at 4 C
in
small aliquots. Very dilute working solutions of CRP were discarded at the end
of the
day.
[0208] Convulxin was prepared from lyophilized venom of tropical rattlesnake
(Crotalus durissus terrificus; Soerensen Laboratories, Brazil) by size
exclusion
chromatography as described by Polgar et al., J. Biol. Chem. 272:13576-83
(1997).
Purified convulxin was stored in collagen dilution buffer (5mM acetic
acid/145mM
NaCI/5mM glucose pH 3.0) at 4 C. The preparation of convulxin consistently
induced
maximal aggregation (70-90%) of human and monkey platelets at 10-20ng/ml.
[0209] During the experimental procedure, all working solutions of the
agonists were maintained on ice.
[0210] Preparation of samples and execution of the experiments. Blood was
collected from three healthy volunteer donors who had not ingested any drug
that
adversely affects agonist-induced platelet aggregation. One of the donors
provided
blood twice on two separate occasions. The blood was collected into 1/10
volume of
3.8% trisodium citrate as an anticoagulant using a two syringe method.
Platelet rich
plasma (PRP) was obtained by centrifugation at 180 x g for 20 minutes at room
temperature. Platelets were counted and adjusted to 3x1 08 platelets/ml with
platelet
poor plasma. All experiments were performed within 4 hours of blood
collection.
Aggregation studies were performed in an AG10 aggregometer (Kowa, Japan). PRP
was stirred (1100 rpm) at 37 C for 1-2 minutes in the aggregometer prior to
challenge
with the test agonists. Aggregation was followed for 10 minutes. The
aggregation
tubes were then removed and maintained at 37 C with stirring. Samples were
drawn
-68-
CA 02567394 2006-11-24
from the tubes at desired time intervals (0, 10, 30, 60, and 120 minutes after
addition
of agonists) and spun at 14,000 g for 5 minutes to obtain platelet-free
supernatants
and at 100,000 g for two hours to obtain platelet-free supernatants that were
also
free of microparticles formed during platelet activation. Resulting
supernatants were
stored at -20 C until sGPVI quantitation by the sandwich ELISA method
described in
Example 12.
[0211] Results of the experiments. All agonists induced time-dependent
shedding of sGPVI. However, GPVI-specific agonists (i.e. collagen, CRP and
convulxin) consistently induced significantly more sGPVI shedding than non-
GPVI-
specific agonists (i.e. ADP and TRAP).
[0212] Figure 21 shows the data obtained by the ELISA. The data represent
the average of 4 experiments performed with platelet rich plasma obtained from
three
donors, whereby blood was drawn twice from one of the donors at two separate
occasions. The quantitation of sGPVI shed in reponse to the agonists was based
on
correlating the OD450nm reading of the test samples with the linear portion of
the
standard curve generated by the software of the plate reader. The error bars
reflect
the standard deviations. Figure 21 shows the data for both platelet-free
supernatants
(Plt agg-removed) and for platelet- and microparticle-free supernatants (MP-
removed). The data for the platelet- and microparticle-free supernatants are
also
depicted in Figure 22, in which the sGPVI level is plotted over time. Table 12
provides the same data in table format.
[0213] The results show that there is a basal level of sGPVI present in human
plasma approximating 9.55 2.52 ng/ml (Figures 21-22 and Table 12; Control).
Furthermore, this value remained constant over the entire 2 hour test period
in the
absence of any added agonist. Hence, platelets do not shed any significant
amount
of sGPVI into the supernatant plasma under stirring conditions and saline
alone does
not induce sGPVI shedding.
[0214] However, when challenged with optimal doses of agonists, such as
ADP, TRAP, collagen, CRP and convulxin, a time-dependent shedding of sGPVI
from human platelets is observed. The non-GPVI-specific agonists ADP (10pM)
and
TRAP (15pM) induced strong platelet aggregation but induced only moderate
sGPVI
-69-
CA 02567394 2012-04-26
shedding, reaching a maximum level of 38-40ng/ml sGPVI by the 2 hour time
point
(Figures 21-22 and Table 12; ADP and TRAP). The GPVI-specific agonists
collagen
(2pg/ml), CRP (31.25ng/ml) and convuixin (10ng/ml) induced significantly more
sGPVI shedding (60-80ng/ml) during the same time period (Figure 21-22 and
Table
12; Collagen, CRP and Convulxin). Of note, the shedding of sGPVI appears to be
a
slow process that continues for at least 2 hours without subsiding.
Furthermore, the
GPVI-specific agonists induce significantly more shedding than non-GPVI-
specific
agonists.
[0215] In summary, these results confirm the utility of the sandwich ELISA of
the invention (see, e.g., Example 12) for measuring physiological levels of
sGPVI in
biological samples. Furthermore, the results demonstrate a specific
application of the
ELISA, namely in the detection and quantitation of sGPVI shedding in platelet
rich
plasma derived from human donors in response to platelet activation by GPVI-
specific agonists, including collagen, the most thrombogenic component of
injured
vasculature. The instant invention may thus be used for preventive monitoring
of
sGPVI levels in healthy individuals or individuals at increased risk of
developing a
vascular disease, or for diagnostic monitoring of sGPVI levels in patients
with
vascular disease, for example, during a treatment regimen.
[0216] The specification is most thoroughly understood in light of the
teachings
of the references cited within the specification. Other embodiments of the
invention will
be apparent to those skilled in the art from consideration of the
specification and
practice of the invention disclosed herein. It is intended that the
specification and
examples be considered as exemplary only.
-70-
CA 02567394 2006-11-24
Table 12: Agonist-induced, time-dependent sGPVI shedding
from human platelets
sGPVI shedding Ing/ml]
Control (saline) Collagen (2 g/ml) ADP (101M)
Time
Imin] Platelet free sup MP-free sup Platelet free sup MP-free sup Platelet
free sup MP-free sup
Aver* SD** Aver SD Aver SD Aver SD Aver SD Aver SD
0 12.22 5.12 7.99 2.52 15.22 6.80 13.10 8.05 10.78 3.30 12.20 5.33
11.58 4.66 7.84 2.68 19.85 8.42 22.10 12.50 13.68 6.60 18.21 7.84
30 11.50 4.03 9.70 3.24 19.94 10.19 37.97 28.58 18.36 6.20 24.64 9.92
60 10.23 4.83 9.27 2.19 32.99 11.12 46.62 26.50 21.15 5.74 30.20 7.94
120 10.04 3.98 9.55 2.52 44.36 20.88 61.15 27.83 29.29 7.24 38.69 8.83
sGPVI shedding ]ng/mIJ
CRP (31.25ng/ml) Convulxin (IOng/ml) TRAP (15 M)
Time Platelet free sup MP-free sup Platelet free sup MP-free sup Platelet free
sup MP-free sup
]min]
Aver SD Aver SD Aver SD Aver SD Aver SD Aver SD
0 15.43 6.75 14.46 7.05 13.58 5.36 13.71 6.98 12.08 5.16 15.07 10.05
10 21.29 8.63 24.40 10.13 19.41 7.35 20.18 9.13 17.08 7.37 21.42 13.35
30 34.69 13.44 33.07 15.40 31.87 9.07 34.85 13.76 28.82 15.69 3139 7.53
60 45.44 22.39 51.09 14.01 45.10 18.75 50.81 15.64 23.86 9.40 32.16 13.65
120 53.72 17.49 60.77 14.72 60.20 26.64 79.70 28.26 30.73 15.86 39.14 16.85
* Average of the ELISA results from 4 different samples, each run in
duplicate.
** Standard deviation of the ELISA results from 4 different samples, each run
in
duplicate.
-71-
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.