Note: Descriptions are shown in the official language in which they were submitted.
CA 02569615 2006-12-18
-1-
Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues
of
purin-2,6-diamine and their uses
This application is a divisional application of Canadian Patent Application
No. 2,416,631
filed on 31 July 2001.
The present invention is in the field of nucleic acid binding compounds
comprising 7-
substituted 7-deaza-8-aza-2,6-diamino-purine bases, compounds useful for the
preparation of such compounds, various uses thereof and methods for the
determination
to of nucleic acids using said compounds in the field of diagnostics.
Background of the Invention
Nucleic acids have been found tv be useful analytes for the determination of
the presence
or absence of genes or microorganisms in human body fluids, food or
environment in the
15 field of health care. Nucleic acid analysis has found widespread use after
the introduction of
nucleic acid amplification, like the Polymerase Chain Reaction (PCR, see US-A-
4,683,202).
Thus, sufficient amounts of nucleic acids are available from each sample. The
nucleic acids
can be determined from this pretreated sample using a variety of different
techniques,
dependent on the particular purpose. Most assays require the use of.a probe
which is either
2o immobilized or immobilizable or is labelled by attachment of one or more
reporter groups.
A reporter group has the characteristics to be itself capable to be determined
or it can be
reacted with reagents that make the probe determinable via said reporter
group. Thus, for
example, probes that are labelled by reporter groups can be determined, as can
be hybrids
25 that contain the probe and a nucleic acid to be determined. In case of
immobilized probes,
the hybrid between the probe and the nucleic acid to be determined is
determined at the
solid phase to which the probe is bound. In a particular farm of assays, not
only one
nucleic acid having a specific sequence, but a large number of nucleic acids
of different
sequence is determined. For this purpose, the probes are immobilized in tiny
spots in an
3o array on a flat surface such as a glass chip (EP-A-0 476 014 and TIBTECH
(1997), Vol.15,
465-469, W089/10977, W089/11548, US 5,202,231, US5,002,867, WO 93/17126).
Further
development has provided methods for making very large arrays of
oligonucleotide probes
CA 02569615 2006-12-18
- la-
in very small axeas.(US 5,143,854, W0 90/15070, WO 92/10092). Microfabricafied
arrays of
large numbers of oligonucleotide probes, called "DNA chips" offer great
promise for a wide
variety of applications (see e.g. US 6,156,501 and US 6,022,963).
However, nucleic acid determinations often suffer from the problem that the
base pairing
possibilities between the natural bases A and T and C and G have different
stability. This
can be attributed to the different capability of these bases to form hydrogen
bonding. Thus,
CA 02569615 2006-12-18
-2-
the dA-dT-base pair has two hydrogen bridges, while the dG-dC-base pair has
three
hydrogen bridges. This results in different melting temperatures (Tm) of
hybrids,
depending on the GC content [1 - 3]. The higher the GC content, the higher the
Tm. The
hybridisation strength or fine degree of hybridization may be investigated by
the
measurement of the Tm of the resulting duplex. This can be done by exposing a
duplex in
solution to gradually increasing temperature and monitoring the denaturation
of the
duplex, for example, by absorbance of ultraviolet light, which increases with
the unstacking
of base pairs that accompanies denaturation. The Tm is generally defined as
the
temperature midpoint of the transition from a fully duplex structure to
complete
denaturation, i.e. the formation of two isolated single strands.
Therefore in routine nucleic acid analysis, there is often the wish to change
the T~, of a
nucleic acid molecule. For example, for certain purposes it may be
advantageous to
equalize or harmonize the Tm of nucleic acids of the same length or to make it
even
independent from the length of the nucleic acid or the binding region in order
to be in the
position to apply similar hybridization conditions for all assays. This is
particularly
necessary for assays using arrays, as on such arrays the hybridizing
conditions for each
probe must be identical. one solution was the use of low hybridization
temperatures.
Under such conditions, many nucleic acids having a low degree of base sequence
2o complementarity will bind to the probe. This is called unspecific binding
which does not
allow discrimination between similar sequences. Another proposal was directed
to the use
of chemical reagents in the hybridization mixture, for example the addition of
.
tetramethylammonium chloride (TMAC). This reagent reduces the difference
between the
stability of dG-dC and dA-dT base pairs but the effect is insufficient for
short
oligonucleotides. Further the addition of salts such as TMA.C may not be
appreciated as it
complicates the optimization of the assay. Another proposal was directed to
the use of
different concentrations of each different (immobilized) probe in one assay.
This was
found to be technically complex if not impossible on a chip surface. As a
further option the
substitution of ribonucleotides in an oligonucleotide composed of
deoxyribonucleotides,
az~d vice versa was applied for the adaptation of DNA stability, Hoheisel
(1996), Nucleic
Acids Res. 24, 430-432.
However, it may be also advantageous to increase the Tm of a given nucleic
acid. This is
interesting in the field of nucleic acids used for antisense therapy, mismatch
discrimination
and for nucleic acids used in diagnostics. The nucleic acids may be used as
prirners or
probes. The aim is to allow a more simple design of primers and probes used in
multiplex
CA 02569615 2006-12-18
-3-
reactions and to synthesize shorter capture probes used on chips, as the
chemical synthesis
of oligonucleotides on a chip surface used for arrays is not as effective as
in routine
oligonucleotide synthesis. The relative contribution of each base pair to the
melting
temperature of a hybrid is the higher the shorter an oligonucleotide is. In
consequence, the
difference in stability between a mismatch and a perfect match is higher for
shorter
oligonucleotides. However, short oligonucleotides hybridize weakly and,
therefore, the
hybridization reaction has to be performed at low stringency. In consequence,
the potential
higher ability of discrimination between different sequences by shorter
oligonucleotides
can only be used under conditions of low stringency. It would be of
considerable advantage
to provide bases which allow to achieve a high level of mismatch
discrimination under
stringent conditions, in particular for short oligonucleotides at temperatures
used e.g. in
amplification reactions. Further, there is the desire in the state of the art
to use short
oligoxiucleotides with high discriminatory power in arrays as the chemical
synthesis of
oligonucleotides on solid supports used for arrays is not as effective as in
routine synthesis.
Therefore, the ability to use shorter oligonucleotides under stringent
conditions would be
of considerable advantage. If bases are found that lead to an increase of the
Tm of an
oligonucleotide hybridized to its complementary strand, other bases may then
be used in
the same oligonucleotide to further adjust the Tm according to the preferences
of the test
system to be used.
zo
Theoretically, oligonucleotide duplexes forming other tridentate base pairs
should exhibit a
similar or higher stability, e.g, those with 2-aminoadenine opposite to
thymine.
Nevertheless, it has been shown that 2-aminoadenine-thymine/uracil base pairs
exhibit
only a low thermal stability [4 -10]. From the data published so far one can
conclude that
the additional NI IZ-group of 2'-deoxyadenosin-2-amine (molecule 1 (see
below); n2Ad)
contributes very little to the base pair stability of a DNA duplex. The Tm-
increase is in the
range of only 1-2° C. Furthermore, this stabilization does not
correspond to the total
number of nzAd -residues incorporated in the duplex instead of dA [11]. A
stronger
stabilization as reported for duplex DNA is found for duplex RNA or for DNA-
RNA
3o hybrids [9J [10] [12]. A rather high base pair stability is observed when 2-
aminoadenine is
introduced into PNA [13] or hexitol nucleic acids [14J. Modified backbones
other than of
DNA or of RNA appear to enhance the stability of the 2-aminoadenine-
thymine/uracil
pair.
The unusual behavior of oligonucleotide duplexes containing n2Ad-dT residues
is
interesting fox the development of an adenine-thymine recognition motif
showing the same
CA 02569615 2006-12-18
-4-
or even a higher stability than a guanine-cytosine base pair. In the following
compounds
the purine moiety of compound 1 is replaced by an 8-aza-7-deazapurine
(pyrazolo[3,4-
d]pyrimidine) or a 7-deazapurine (pyrrolo[2,3-d]pyrimidine) leading to
nucleosides (2a
[15], 2b, 2c or 3 [16], [17] see below).
NH2
7 i
1 N~
~w ~ ~ 8 2 6
li2N' L _N N 9
3
2a: R = H 3
2b: R = Br
2c: R =
purine numbering systematic numbering systematic numbering
Compounds of similar chemical architecture were investigated in the prior art.
The
synthesis of 7-substituted-7-deaza and 8-aza-7-deazapurine 2'-
deoxyribonucleotides, their
incorporation into oligonucleotides, and the stability of the corresponding
duplexes has
been investigated (Seela et al. (1997) Nucleosides & Nucleotides 16, 963-966).
This
document does not contain a disclosure of 7-substituted 7-deaza-8-aza-diamino-
purines.
Stabilization of duplexes by pyrazolopyrimidine base analogues have been
reported (Seela
et al. (1988) Helv. Chim. Acta 71, 1191-1198; Seela et al. (I988) Helv. Chim
Acta 71, 1813-
1823; and Seela et al. (1989) Nucleic Acids Res. 17, 901-910)
Pyrazolo[3,4-d)pyrimidine residues in oligonucleotides are also useful as
sites of
attachment of various groups (W090/14353). Oligonucleotides having
incorporated one or
more pyrazolo[3,4-d]pyrimidine have an enhanced tendency to form triplexes
(Belousov et
2o al. (1998). Nucleic Acids Res. 26, 1324-1328).
The compounds 7-iodo, 7-cyano and 7-propynyl-7-deaza-2-amino-2'-deoxyadenosine
were synthesized by Balow et al. ( 1997, Nucleosides & Nucleotides 16; 941-944
) and
incorporated into oligonucleotide sequences. These oligonudeotides exhibit
enhanced
binding affinities to RNA complements relative to unmodified sequences.
However, no
CA 02569615 2006-12-18
-$-
corresponding 8-aza-compounds were made and investigated. Seela et al. (1999,
Nucleosides & Nucleotides 18, 1399-1400) disclose 7-substituted 8-aza-7-
deazapurine
DNA, its synthesis and duplex stability. The authors do not address possible
uses of the
disclosed compounds,
WO 90!03370 discloses 3,4-disubstituted and 3,4,6-trisubstituted pyrazolo-[3,4-
d]-
pyrimidines, more particularly 4,6-diamino-pyrazolo-[3,4-d]-pyrimidines with a
linker at
the C3-position to which an intercalator, an electrophilic cross linker or a
reporter group is
attached. These compounds may be attached to sugars or incorporated into
oligonucleotides and thereby used for the identification, isolation,
localization and/ or
detection of complementary nucleic acid sequences of interest. US 5,594,121
discloses
novel oligomers with enhanced abilities to form duplexes or triplexes. The
oJigomers may
contain 7-substituted 8-aza-7-deaza-diamino-purirres with propinyl and aryls
as
substituents at the 7-position. Compositions containing these oligorners may
used for
diagnostic purposes.
There is still a need to provide probes with a high discriminatory power and
with a short
length, the Tm of which is high under stringent conditions and which can be
used in various
methods useful in the field of diagnostics as e.g. in the Lightcycler~ system
(Roche,
Mannheim, Germany), TaqMan~ (W092/02638 and corresponding US patents US
5,210,015, US 5,804,375, US 5,487,972) or other applications involving
fluorescence energy
transfer.
Terms and definitions
z5 Conventional techniques of molecular biology and nucleic acid chemistry,
which are within
the skill of the art, are fully explained fully in the literature. See, for
example, Sambrook et
al., 1989, Molecular Cloning--A Laboratory Manual, Cold Spring Harbor
Laboratory, Cold
Spring Harbor, New York; Oligonucleotide Synthesis (M. J. Gait, ed., 1984);
Nucleic Acid
Hybridization (B. D. Hames and S. J. Higgins. eds.> 1984); and a series,
Methods in
Enzymology (Aca.demic Press, Inc.), all of which are incorporated herein by
reference.
The terms "nucleic acid" and "oligonucleotide" refer to
polydeoxyribonucleotides
(containing 2-deoxy-D-ribose), to polyribonucleotides (containing D-ribose),
and to any
other type of polynucleotide which is an N glycoside of a purine or pyrimidine
base, or
CA 02569615 2006-12-18
-6-
modified purine or pyrimidine base. There is no intended distinction in length
between the
terms "nucleic acid" and "oligonucleotide'", and these terms will be used
interchangeably.
These terms refer only to the primary structure of the molecule. Thus, these
terms include
double- and single-stranded DNA, as well as double- and single-stranded RNA.
The term
"polynucleotide"shah be used interchangably for "nucleic acid".
The term "backbone" or "nucleic acid backbone" for a nucleic acid binding
compound
according to the invention refers to the structure of the chemical moiety
linking
nucleobases in a nucleic acid binding compound. The bases are attached to the
backbone
and take part in base pairing to a complementary nucleic acid binding compound
via
hydrogen bonds. This may include structures formed from any and all means of
chemically
linking nucleotides, e.g. the natural occuring phosphodiester ribose backbone
or unnatural
linkages as e.g. phosphorthioates, methyl phosphonates, phosphoramidates and
phosphortriesters. Peptide nucleic acids have unnatural linkages. Therefore, a
"modified
i5 backbone" as used herein includes modifications to the chemical linkage
between
nucleotides as described above, as well as other modifications that may be
used to enhance
stability and affinity, such as modifications to the sugar structure. For
example an a-
anomer of deoxyribose may be used, where the base is inverted with xespect to
the natural
f3-anomer. In an embodiment, the 2'-OH of the sugar group may be altexed to 2'-
O-allcyl or
2'-O-allcyl-n(O-allcyl), which provides resistance to degradation without
comprising
affinity. An unmodified nucleotide sequence having a phosphodiester backbone
is
"comparable" to a nucleobase-containing sequence having a modified backbone if
the two
sequences have identical base sequencing. Thus, the backbones of such
sequences are also
comparable.
2S
The term "nucleic acid binding compound" refers to substances which associate
with
nucleic acids of any sequence and are able to function as binding partner to a
substantially
complementary nucleic acid. The binding preferably occurs via hydrogen bonding
between
complementary base pairs when the nucleic acid binding compound is in a single-
stranded
form. Preferably, non-natural bases, the subject of the invention, attached to
the backbone
of the nucleic acid binding compound may be also involved in hydrogen-bonding,
however, these may also be able to form hydrogen bonds to only some or all
natural
occuring bases as e.g, inosine. The expert in the field recognizes that the
most well-known
"nucleic acid binding compounds" are nucleic acids as DNA or RNA.
CA 02569615 2006-12-18
_7_
The term "probe" refers to synthetic or biologically produced nucleic acids
(DNA or RNA)
which, by design or selection, contain specific nucleotide sequences that
allow them to
hybridize under defined predetermined stringencies, specifically (i.e.,
preferentially) to
target nucleic acids. A "probe" can be identified as a "capture probe" meaning
that it
"captures" the target nucleic acid so that it can be separated from
undesirable materials
which might obscure its detection. Once separation is accomplished, detection
of the
captured target nucleic acid can be achieved using a suitable procedure.
"Capture probes"
are often akeady attached to a solid phase.
The term "hybridization" refers the formation of a duplex structure by two
single-stranded
nucleic acids due to complementary base pairing. Hybridization ca.n occur
between fully
complementary nucleic acid strands or between "substantially complementary"
nucleic acid
strands that contain minor regions of mismatch. Conditions under which only
fully
complementary nucleic acid strands will hybridize are referred to as
"stringent
hybridization conditions" or "sequence-specific hybridization conditions".
Stable duplexes
of substantially complementary sequences can be achieved under less stringent
hybridization conditions; the degree of mismatch tolerated can be controlled
by suitable
adjustment of the hybridization conditions. Those skilled in the art of
nucleic acid
technology can determine duplex stability empirically considering a number of
variables
including, for example, the length and base pair concentration of the
oligonucleotides,
ionic strength, and incidence of mismatched base pairs, following the guidance
provided by
the art (see, e.g., Sambrook et al., 1989, Molecular Cloning--A Laboratory
Manual, Cold
Spring Harbor Laboratory, Cold Spring Harbor, N.Y.; and Wetmur, 1991, Critical
Review
in Biochem, and Mol. Biol. 26(3/4):227-259).
The texm "primer" refers to an oligonucleotide capable of acting as a point of
initiation of
DNA synthesis under conditions in which synthesis of a primer extension
product
complementary to a nucleic acid strand is induced, i.e., either in the
presence of four
different nucleoside triphosphates and an agent for extension (e.g., a DNA
polymerase ox
reverse transcriptase) in an appropriate buffer and at a suitable temperature.
As used
herein, the term "primer" is intended to encompass the oligonucleotides used
in ligation-
mediated amplification processes, in which one oligonucleotide is "extended"
by ligation to
a second oligonucleotide which hybridizes at an adjacent position. Thus, the
term "primer
extension", as used herein, refers to both the polymerization of individual
nucleoside
3s triphosphates using the primer as a point of initiation of DNA synthesis
and to the ligation
of two primers to form an extended product. A primer is preferably a single-
stranded DNA.
CA 02569615 2006-12-18
_8_
The appropriate length of a primer depends on the intended use of the primer
but typically
ranges from 6 to 50 nucleotides. Short primer molecules generally require
cooler
temperatures to form sufficiently stable hybrid complexes with the template. A
primer need
not reflect the exact sequence of the template nucleic acid, but must be
sufficiently
complementary to hybridize with the template. The design of suitable primers
for the
amplification of a given target sequence is well known in the art and
described in the
literature cited herein. Primers can incorporate additional features which
allow for the
detection or immobilization of the primer but do not alter the basic property
of the primer,
that of acting as a point of initiation of DNA synthesis. Por example, primers
may contain
l0 an additional nucleic acid sequence at the 5' end which does not hybridize
to the target
nucleic acid, but which facilitates cloning of the amplified product. The
region of the
primer which is sufficiently complementary to the template to hybridize is,
refered to herein
as the hybridizing region.
The terms "target, "target sequence", "target segment", "target region', and
"target nucleic
acid" refer to a region or subsequence of a nucleic acid which is to be
amplified or
investigated.
As used herein, a primer is "specific" for a target sequence if the number of
mismatches
present between the primer sequence and the target sequence is less than the
number of
mismatches present between the primer sequence and non-target sequences which
may be
present in the sample. Hybridization conditions can be chosen under which
stable duplexes
are formed only if the number of mismatches present is no more than the number
of
mismatches present between the primer sequence and the target sequence. Under
such
conditions, the primer can form a stable duplex only with a target sequence.
Thus, the use
of target-specific primers under suitably stringent amplification conditions
enables the
specific amplification of those target sequences which contain the target
primer binding
sites. The use of sequence-specific amplification conditions enables the
specific
amplification of those target sequences which contain the exactly
complementary primer
binding sites.
Halogen means a fluoro, chloro, bromo or iodo group. The most preferred
halogen groups
are -I and -Br.
Alkyl groups are preferably chosen from allcyl groups containing from 1 to 10
carbon
atoms, either arranged in linear, branched or cyclic form. The actual length
of the alkyl
CA 02569615 2006-12-18
-9-
group will depend on the steric situation at the specific position where the
alkyl group is
located. If there are steric constraints, the allcyl group will generally be
smaller, the methyl
and ethyl group being most preferred. All allcyl, allcenyl and alkynyl groups
can be either
unsubstituted or substituted. Substitution by hetero atoms as outlined above,
will help to
increase solubility in aqueous solutions.
Alkenyl groups are preferably selected from alkenyl groups containing from 2
to 10 carbon
atoms. For the selections similar considerations apply as for allcyl groups.
They also can be
linear, branched and cyclic. The most preferred alkenyl group is the ethylene
group.
Alkynyl groups have preferably from 2 to 10 carbon atoms. Again, those carbon
atoms can
be arranged in linear, branched and cyclic manner. Further, there can be more
than one
triple bond in the allcynyl group. The most preferred alkynyl group is the 3-
propargyl-
group.
Alkoxy groups preferably contain from 1 to 6 carbon atoms and are attached to
the rest of
the moiety via the oxygen atom. For the allcyl group contained in the allcoxy
groups, the
same considerations apply as for allcyl groups. The most preferred allcoxy
group is the
methoxy group.
By "aryl" and "heteroaryl" (or "heteroaromatic") is meant a carbocyclic or
heterocydic
group comprising at least one ring having physical and chemical properties
resembling
compounds such as an aromatic group of from 5 to 6 ring atoms and comprising 4
to 20
carbon atoms, usually 4 to 9 or 4 to 12 carbon atoms, in which one to three
ring atoms is N,
S or O, provided that no adjacent ring atoms are O--O, S--S, O--S or S--O.
Aryl and
heteroaryl groups include, phenyl, 2-, 4- and 5-pyrimidinyl, 2-, 4- and 5-
thiazoyl, 2-s-
triazinyl, 2-, 4-imidazolyl, 2-, 4- and 5-oxazolyl, 2-, 3- and 4-pyridyl, 2-
and 3-thienyl, 2-
and 3-furanyl, 2- and 3-pyrrolyl optionally substituted preferably on a ring C
by oxygen,
alkyl of 1-4 carbon atoms or halogen. Heteroaryl groups also include optional
substitution
on a ring N by alkyl of 1-4 carbon atoms or haloalkyl of 1-4 carbon atoms and
1-4 halogen
atoms. Exemplary substituents on the aryl or heteroaryl group include methyl,
ethyl,
trifluoromethyl and brorno. Such substituted aryl and heteroaryl groups
include benzyl and
the like. "Heteroaryl" also means systems having two or more rings, including
bicydic
moieties such as benzimidazole, benzotriazole, benzoxazole, and indole. Aryl
groups are
the phenyl or naphtyl moiety, either unsubstituted or substituted by one or
more of amino,
-cyano, -aminoalkyl, -O-(Ci-CIO)-allcyl, -S-(Cl-Clo)-alkyl, -(Cl-Clo)-alkyl,
sulfonyl,
CA 02569615 2006-12-18
- 10-
sulfenyl, sulfinyl, nitro and nitroso. Most preferred aryl group is the phenyl
group,
Preferred aiylallcyl group is the benxyl group. The preferred alkylamino group
is the
ethylamino group. The preferred -COO(Cl-C4) alkyl group contains one or two
carbon
atoms in the alkyl moiety (methyl or ethyl esters). Other aryl groups are
heteroarylgroups
as e.g. pyrimidine, purine, pyrrol, or pyrazole. Aryl and heteroaryl.
According to the
present invention the term aryl shall also include all heteroaryls.
Aryloxy groups preferably contain from 6 to ZO carbon atoms. Those carbon
atoms may be
contained in one or more aromatic rings and further in side chains (for
example, alkyl
chains) attached to the aromatic moiety. Preferred aryloxy groups are the
phenoxy and the
benzoxy group.
A "protecting group" is a chemical group that is attached to a functional
moiety (for
example to the oxygen in a hydroxyl group or the nitrogen in an amino group,
replacing
the hydrogen) to protect the functional group from reacting in an undesired
way. A
protecting group is further defined by the fact that it can be removed without
destroying
the biological activity of the molecule formed, here the binding of the
nucleic acid binding
compound to a nucleic acid. Suitable protecting groups are known to a man
skilled in the
art. Especially preferred protecting groups for example for hydroxyl groups at
the 5'-end of
a nucleotide or oligonucleotide are selected from the trityl groups, for
example
dimethvxytrityl. Preferred protecting groups at exocyclic amino groups in
formula I are
aryl groups, most preferred the benzoyl group (Bz), phenoxyacetyl or acetyl or
formyl, and
the amidine protecting groups as e.g. the N,N-diallcylformamidine group,
preferentially the
dimethyl-, diisobutyl-, diisobutyryl and the di-n-butylformamidine group.
Preferred O-
protecting groups are the amyl groups, the diphenylcarbamoyl group, the aryl
groups, and
the silyl groups. Among these most preferred is the benzoyl group. Preferred
silyl groups
are the trialkylsilyl groups, like, trimethylsilyl, triethylsilyl and tertiary
butyl-dimethyl-silyl.
Another preferred silyl group is the trimethylsilyl-oxy methyl group (TOM)
(WO 99/09044). Further, preferred protecting groups are groups as ortho nitro-
benzyl protecting groups like 2-(4-nitrophenyl)ethoxycarbonyl (NPEOC) or
photoactivable compounds as 2-nitrophenylpropyloxycarbonyl (NPPOC) (Giegrich
et al.,
Nucleosides & Nucleotides 1998,17,1987). According to the invention, also the
phthaloyl
group maybe used as protecting group.
Any atom in the definitions within the formulae presented herein is not
liraited to a specific
isotope. Thus, a phosphorous atom (P) can either mean the regular 31P or the
radioactive
CA 02569615 2006-12-18
-11-
szp or a mixture thereof. The same applies for hydrogen (H/D/T), carbon (C),
iodine (Cl,
Br, I) and nitrogen (N).
During chemical synthesis, any reactive groups as e.g. -OH, -SH, -NHz, -NH-
alkyl, -NH-
alkenylene, -NH-alkynylene, or -NH-aryl (including those groups in reporter
groups)
should be protected by suitable protecting groups, i.e. that the present
invention
contemplates compounds for the synthesis of olignucleotides wherein the
formulas or
substituents are chosen with the proviso that one or two hydrogen atoms of any-
OH, -SH,
-NHz, -NH-alkyl, -NH-alkenylene, -NH- alkynylene, or NH-aryl group are
substituted by
1o a protecting group. Further, during chemical synthesis, the compound will
be attached fox
convenience to a solid phase. In these cases, the definitions of the
substituents given above
will be selected accordingly.
Reporter groups are generally groups that make the nucleic acid binding
compound as well
as anynucleic acids bound thereto distinguishable from the remainder of the
liquid, i.e. the
sample (nucleic acid binding compounds having attached a reporter group can
also be
termed labeled nucleic acid binding compounds, labeled probes or just probes).
The term
reporter group and the specific embodiments preferably include a linker which
is used to
connect the moiety intended to be used (the actual solid phase or the
fluorophoric moiety)
2o to the position of attachment as the reporter group. The linker will
provide flexibility such
that the nucleic acid binding compound can bind the nucleic acid sequence to
be
determined without major hindrance by the solid phase. Linkers, especially
those that are
not hydrophobic, for example based on consecutive ethylenoxy units, for
example as
disclosed in DE 3943522 are known to a man skilled in the art.
By "array" is meant an arrangement of addressable locations on a device. The
locations can
be arranged in two dimensional arrays, three dimensional arrays, or other
matrix formats.
The number of locations can range from several to at least hundreds of
thousands. Most
importantly, each location represents a totally independent reaction site.
Each location
3o carries a nucleic acid binding compound which can serve as a binding
partner for a second
nucleic acid binding compound, a nucleic acid, in particular a target nucleic
acid.
The term "building block" or "subunit" refers to a compound which can be used
in
oligonucleotide synthesis wherein subsequently single building blocks are
chemically linked
to form a more complex structure, i.e, an oligonucleotide precursor. Examples
for building
blocks are phosphoramidites or phosphonates.
CA 02569615 2006-12-18
-12-
The term "substituted compound" shall mean that a compound carries further
chemical
groups, moieties or substituents other than the compound itself. These
substituents shall in
principle include but axe not limited to halogens or alkyl, allcenyl,
allcynyl, or aryl
compounds optimally substituted with further heteroatoms
Figure legends
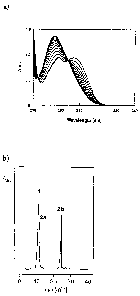
Figure la: Ultraviolett spectra of demination of compound 2a
Figure lb: High pressure liquid chromatography (HPLC) profile of compounds 1,
2a and
io 2b
Figure 2a: High pressure liquid chromatography (HPLC) profile of a snake venom
diesterase/ allcaline phosphatase hydrolysate of an oligonucleotide containing
2a
Figure 2b: High pressure liquid chromatography (HPLC) profile of a snake venom
diesterase/ alkaline phosphatase hydrolysate of an oligonucleotide containing
2b
15 Figure 3: Circular dichroism spectra of antiparallel DNA/DNA duplexes
(Figure 3a) and
antiparallel DNA/ RNA duplexes (Figure 3b)
Figure 4: Circular dichroism spectra of parallel DNA/DNA duplexes (Figure 4a)
and
parallel DNA/ RNA duplexes (Figure 4b)
Figure 5. HPLC profiles of the nucleosides 1, 2a-c (a) and 3a-c and dT (b).
The mixtures of
20 the nucleosides were analyzed on reversed-phase HPLC at 260 nm (RP-18,
gradiemt: 0-30
min in O.1M (Et3NH)OAc (pH7.0) / MeCN (95:5), 0.7 ml/rnin). HPLC profile of
oligonucleotides 100 (c) and 304 (d) after enzymatic hydrolysis with snake-
venom
phosphodiesterase followed by allcaline phosphatase in 0.1 M Tris-HCl buffer
(pH 8.3) at
37°. HPLC was performed on a RP-18 column (200 x 10 mm), 0-30 min in
0.1 M
25 (EtsNH)OAc (pH 7.0) / MeCN (95:5), 0.7 ml/min. Peak detection: 260 nm.
Figure 6. The tendency of Tm -increase vs. the number of 2b residues.
Figure 7. (a) CD spectra of duplexes 102 ~ 103,108 ~ 109, 110 ~ 111, 104 ~ 105
( in 100
mM NaCI, 100 mM MgClz, and 60 mM Na-cacodylate (pH 7.0). (b) CD Spectra of
duplexes I02 ~ 403, 108 ~ 403, 110 ~ 403, 104 ~ 403 in 100 mM NaCI, 100 mM
MgClz, and
CA 02569615 2006-12-18
_13_
60 mM Na-cacodylate (pH 7.0), the concentration of the oligonucleotides is 5
ACM + 5 pM
(single strand concentration).
Description of the Invention
The above-mentioned problem could be solved by the findings of the present
invention
which discloses nucleic acid binding compounds wherein 7-substituted 8-aza-7-
deaza-2,6-
diamino-purines are incorporated, derivatives of these oligonucleotides and
compounds
useful for the synthesis thereof. Further, the present invention discloses
methods wherein
the compounds according to the present invention may be used.
io
Surprisingly, an oligonucleotide having incorporated a 7-iodo or 7-bromo-8-aza-
7-deaza-
2,6-diamino-purine in place of an adenine residue shows an unexpected increase
in the
melting temperature. This fording is in contrast to the behaviour of 5-halogen
substituted
pyrimidines. This effect can be observed fox parallel as well as for
antiparallel duplexes.
15 These findings could not be foreseen on the basis of the state of the art,
however , the
surprising finding can be generalized to other 8-aza-7-deaza-2,6-diamino-
purines
substituted at the 7-position (purine numbering) with a hydrophobic residue as
the
halogen substituent is hydrophobic or a residue containing a hydrophobic
moiety attached
to the 7-position. Further, the teachings can be generalized to an electron-
withdrawing
20 substituent. Therefore, preferred other substituents at this position as
e.g. alkyl, allcenyl and
allcinyl, preferably with a length of 6 carbon atoms, more preferably with a
length of 3
carbon atoms, are suitable in the present invention. The 7-position allows the
substituents
to extend into the major groove of the DNA where they find enough space and do
not
disturb the DNA double helix. A possible explanation for this effect could be
removal of
25 water from the major groove and the concomitant hydrophobisation of the
major groove
thereby increasing the stacking of the DNA bases. Substituents fulfilling
these requirements
may also carry other functional groups as e.g. reporter groups.
CA 02569615 2006-12-18
- 14-
In summary, the present invention discloses nucleic acid binding compounds
comprising
7-substituted 7-deaza-8-aza-2,6-diamino-purine bases, compounds useful for the
preparation of such compounds, various uses thereof and methods for the
determination
of nucleic acids using said compounds in the field of diagnostics.
The subject of the present invention is a nucleic acid binding compound
comprising a
backbone, said backbone having attached heterocyclic groups capable of base
pairing to
nucleobases characterized in that a heterocyclic group is a group of the
general formula I
Formula I
R1
Ni
N
N/
R3FiN
1o wherein
RI is independent from X, RZ or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
15 (3) Cyano
(4) -COO
(5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(C2-Cloy-alkenyl substituted according to (10)
(7) -(CZ-Cloy-alkynyl substituted according to ( 10)
20 (8) -(C6-C22)-aryl substituted according to (10)
(g) -W-(Cl-Cio)-~'h -W-(Ca-Clo)-alkenyl, -W-(CZ-Cio)-~Yl> -W-(Ce-
CZZ)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NI3-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHZ)n-[O-
(CH2)rJs -, where r and s are, independently of each other, an integer
25 between 1. to 18 and n is 0 or 1 independently from r arid s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl, allcynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -N02, -CN, -S-(Cl-C6)-allcyl, -NRSR6, -N+RSR6R12, -ORl2,
-CORIl, -NH-CO-NR5R6, -NH-CS-NRSR6 and-(CHz)n [O-(CH2)r]$
30 NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
CA 02569615 2006-12-18
- IS -
wherein Rll is selected from the group consisting of -NHRIZ, OR'z, and -
SRIz
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Coo)-alkyl, -{Cz-Clo)-alkenyl, -(Cz-Cloy-allcinyl, -
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said alkyl, alkenyl, alkynyl or aryl in substituents (5) to ( 10) being
l0 unsubstituted or substituted by one or more moieties selected from the
group
consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -
COR", -NH-CONRSR6, -NH-CSNRSR6 and -(CHz)"-[O-(CHz)r]S-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
15 with the proviso that R5, R6 or Riz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHR~2, ORIZ, or SR12;
Rz, R3 is independent from X, R', Rz and R3 and is selected from the group of,
( 1 ) --H
(2) (CrCio)-alkyl,
20 (3) (Cz-Clo)-~enyl,
(4) (Ca-Cio)-~Yh
(5) (Cs-C2z)-aryh
(d) -Z-(CuCio)-~Yh -Z-{C2-Cio)-alkenyl, -Z-(Cz-Cio)-~ynyh -Z-(Cs_
Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)"-
25 [O-(CHz)r)S -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, allcenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
30 NOz, -ORIZ, -CN, -SH, -S-(Cl-Cs)-alkyl, -NRSR6, -N+RSR6R'z, -
CORII, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n [O-{CHz)r]9-
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rii is selected from the group consisting of -NHRIZ and
35 OR~z,
CA 02569615 2006-12-18
-16-
wherein R5, R6 and R12 are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Coo)-alkenyl, -{Cz-Clo)-
alklmyl, -(C6-Cz2)-aryl and a reporter group,
said alkyl, allcenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of
halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-allcoxy, -OH, -NRSR6, -CORI', -NH-
CONR5R6, -NH-CSNRSR6 and -(CHz)" [O-(CHz)r]s-NRSR6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
X is independent fron-~ Rl, RZ or R3 and is selected from the group consisting
of N
and CH
D is the position of attachment of the group to the rest of the nucleic acid
binding
compound.
and any salts thereof.
The nucleobases, to which the nucleic acid binding compound according to the
invention
may bind, can be nucleobases occuring in nature, as e.g. adenosine, guanosine,
uridin or
cytidin or the 2'-desoxyderivatives thereof, or nucleobases not occuring in
nature as e.g.
nucleobases with heteroryclic groups according to the invention, heterocyclic
groups as
pyrrolo-(2,3-d]-pyrimidine or pyrazolo[3,4-d]-pyrimidine or analogues thereof,
preferably
the said analogues of guanine or adenine or 7-deaza-guanine. Other non-natural
heterocyclic groups are known to the person skilled in the art and need not to
be
mentioned explixitly herein.
In another preferred embodiment, the invention relates to nucleic acid binding
compounds
with the general formula I and residues Rz and R3 as defined above, that have
electron-
withdrawing or hydrophobic substituents at the 7-position of 7-deaza-8-aza-2,6-
diamino-
purine. Therefore, in addition to the preferred halogen substituents other
preferred
substituents at the 7-position are hydrophobic in nature as e.g. alkyl,
allcenyl and alkinyl
residues. It is, however, sufficient if the first residues extending into the
major groove are
hydrophobic. In detail, other preferred substituents at the 7-position are the
residues
(1) -(Cl-Cio)-allcyl substituted according to (5)
(2) -(Cz-Cio)-~enyl substituted according to (5)
(3) -(Cz-Cio)-~yl substituted according to (5)
(4) -(C6-Czz)-aryl substituted according to (5)
CA 02569615 2006-12-18
-17-
(5) substituents (1) to (4) wherein any alkyl, allcenyl, allcynyl or aryl can
be
substituted by one or more moieties selected from, the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NR5R6, -N~R5R6R'z, -OR'z,
-COR", -NH-CO-NRSR6, -NH-CS-NR5R6 and -(CHa)n (O-(CHz)ras-
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein R" is selected from the group consisting of -NHRIZ, OR'z, and -
SR'z
wherein R5, R6 and R'z are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Cloy-allcenyl, -(Cz-Cloy-allcinyl, -
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking ox cleaving,
said alkyl, alkenyl, allcynyl or aryl in substituents (1) to (4) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -COR", -NH-
CONRSR6, -NH-CSNRSR6 and-(CHz)"(O-(CHz)r]s NR5R6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1.
independently from r and s,
with the proviso that R5, R6 or R'z is not a reporter group if the radicals
(1) to (3) are
substituted by -NR5R6, NHRIZ, ORIZ, or SR'z;
More preferably, the residue ( 1) has a length of between 1 and 6 carbon
atoms, more
preferably a length of between 1 to 3 carbon atoms, and the residues (2) and
(3) have a
length of between 2 and 6 carbon atoms, more preferably a length of between 2
to 3 carbon
atoms.
In the most preferred embodiment the heterocyclic group is 7-bxomo-7-deaza-8-
aza-2,6-
diamino-purine or 7-iodo-7-deaza-8-aza-2,6-diamino-purine. The nucleic acid
binding
compounds according to the invention further possess the advantage of having a
very stable
glycosidic bond in contrast to 2-amino-adenosine which has an extremely acid
labile
glycosidic bond and may only be used in oligonucleotide synthesis if specific
conditions are
used. In a further embodiment of the invention, the halogenides in the 7-
position of the 7-
deaza-8-aza-2,6-diamino-purine may be substituted by pseudohalogenides as e.g.
-SCN or
-CN.
CA 02569615 2006-12-18
-18-
The preferred substituents mentioned above are also preferred in the methods
and uses
according to the present invention.
The heterocyclic groups of formula I are mainly characterized by the following
properties:
- The base is linked to the backbone, preferred to a sugax moiety, via the 9-
position
(purine numbering).
The base contains an aromatic ~-electron system which is capable to form
stacking
interactions with other nucleic acid constituents.
l0 - The base contains donor and/ or acceptor sites) for hydrogen bonding to
the natural
nucleoside T.
In order to increase the Tm, in a nucleic acid binding compound one or more A
in a strand
complementary to a T in the nucleic acid to be determined could be replaced by
the
is heteroryclic groups according to the invention. The oligonucleotide would
then bind
specifically to the target sequence containing T opposite to the bases
according to the
invention with a binding energy in the order of a G-C base pair but with
higher stability
than a A-T base pair. This works for antiparallel or parallel duplexes equally
well whereby
natural A-T base pairs have equal abilities to bind in parallel or
antiparallel duplexes buff
20 with a lower binding energy in the parallel duplex. However, the
heterocyclic group
according to the invention, when incorporated into a nucleic acid binding
compound, will
bind to a T in the opposite strand equally well in a parallel or in an
antiparallel duplex. In
order to effect antiparallel binding in a duplex, natural G or C bases
normally forming G-C
base pairs in antiparallel duplexes have to be substituted by non-natural base
pairs as e.g.
25 G-iC (isocytosine) or better G meiC (rnethylated isocytosine) or C-iG
(isoguanosine) as
disclosed above. A summary of parallel and antiparallel duplexes can be found
in EP 0 624
161 or Seela et al. (Tetrahedron 55 ( 1999), 9481-9500) and is shown
schematically in
scheme 11 and 12 in example 4.
3o This general principle of course is not limited, as bases showing the same
characteristics in
the 6-membered ring would be expected to have the same properties based on the
above
explanation due to their containing the structure according to the invention.
Particularly,
the farer the part of the heterocyclic group from the part participating in
the base pairing,
the more tolerant will the oligomer be over modifications in the chemical
structure, for
35 example the attachment of groups to this part of the heteroryclic rings. In
the following,
CA 02569615 2006-12-18
-19-
when reference is made to the heterocyclic group of the invention, there is
made reference
to a heterocyclic group according to general formula I.
The present invention also contemplates tautomeric forms and salts of
heterocyclic groups
of formula I.
The nucleic acid binding compound according to the invention preferably has a
length of
less than 100 subunits, more preferably of from 10 to 30 subunits. In order to
be active as
nucleic acid binding compound, the substituents should be chosen such that
hydrogen
to bonds to heterocyclic groups at the nucleic acid to be bound are enabled,
preferably by base
pairing, for example by Watson-Crick or Hoogsteen base pairing. Compounds in
which the
substituents do not enable such preferred hydrogen bonding, can be useful as
intermediates
for the preparation of nucleic acid binding compounds. Preferred nucleic acid
binding
compounds of the invention are those which are chemically synthesized.
The nucleic acid binding compound will be constructed such that it contains a
nucleobase
sequence which is substantially complementary to the nucleic acid to be
determined or the
nucleic acid to which it is intended to be bound by base pairing dependent on
the
formation of a parallel or an antiparallel duplex. As those nucleic acids will
usually contain
at Ieast once any of the naturally occurring nucleobases Ade, CyC> Gua and Thy
or Ura, the
nucleic acid binding compound according to the invention will also contain any
of those
four bases. However, acccording to the invention, at least one of the
heterocyclic groups is
replaced by the heterocyclic base of formula I.
If the nucleic acid binding compound is to be used as a probe for the
determination of a
nucleic acid, or any other identification of the compound or the nucleic acid
is intended,
any of the substituents are selected such as to contain a reporter group.
While as many
reporter groups can be attached as useful to label the nucleic acid binding
compound
sufficiently, it is preferred to attach only a limited number of reporter
groups to a single
3o subunit, such that recognition of nucleic acids, affinities to nucleic
acids and solubility is
not affected such that the probe would not be useful in hybridization assays.
In a very
preferred case, there will be only from 1 to 4, most preferably 1 or 2 or most
preferred only
one reporter group in each nucleic acid binding compound. There are formats
for the
nucleic acid determination which require more than one reporter group attached
to the
3S probe. An example for such formats is disclosed in W092/02638. In this
case, one of the
reporter groups will be a fluorescence emitter, while the other is a
fluorescence quencher.
CA 02569615 2006-12-18
The reporter group may also be attached to a heterocyclic base which is not
according to
formula I.
In a preferred embodiment of the invention, Rl is alkynyl-amino -C--__C-E-
NRSR6, alkenyl-
amino -CH=CH-E-NR5R6 and -E-NR5R6 wherein E is -[(CHZ)rF]S (CHa)r- wherein F =
O
or S and r and s are independently from one another an integer from 1 to 18,
wherein RS
and R6 are selected independently from the group consisting of -H, -(Cl-Clo)-
alkyl, -(CZ-
Clo)-allcenyl, -(CZ-Cloy-alkynyl, -(C6-CZZ)-aryl and a reporter group, wherein
R" is selected
from the group consisting of -NHRIZ and OR12, wherein R5, R6 and R'2 are
selected
to independently from the group consisting of -H, -(Cl-Clo)-alkyl, -(CZ-Clo)-
allcenyl, -(CZ-
Clo)-alkynyl, -(C6-C22)-aryl and a reporter group, said alkyl, alkenyl,
alkynyl or aryl being
unsubstituted or substituted by one or more moieties selected from the group
consisting of
-halogen, -SH, -S-(C~-C6)-alkyl, -(Cl-Cs)-alkoacy, -OH, -NRSR6, -COR", -NH-
CONRSR6, -
NH-CSNRsR6 and -(CHZ)n [O-(CHZ)r]S-NRSR6, r and s are independently of each
other an
integer of from 1 to 18 and n is 0 or 1 independently from r and s.
Reporter groups are generally groups that make the nucleic acid binding
compound as well
as any nucleic acids bound thereto distinguishable from the remainder of the
liquid, i.e. the
sample (nucleic acid binding compounds having attached a reporter group can
also be
2o termed labeled nucleic acid binding compounds, labeled probes or just
probes). This
distinction can be either effected by selecting the reporter group from the
group of directly
or indirectly detectable groups or from the groups of immobilized or
immobilizable
groups. Directly detectable groups are for example fluorescent compounds, like
fluorescein
and its derivatives, like hexachlorofluorescein and hexafluorofluorescein,
rhodamines,
psoralenes squaraines, porphyrines, fluorescent particles, bioluminescent
compounds, like
acridinium esters and luminol, ox the cyanine dyes, Like Cy-5. Examples of
such
compounds are disclosed in EP 0 680 949. Further, spin labels like TEMPO,
electrochemically detectably groups, ferrocene, viologene, heavy metal
chelates and
electrochemiluminescent labels, like ruthenium bispyridyl complexes, and
3o naphthoquinones, quencherdyes, like dabcyl, and nuclease active complexes,
for example
of Pe and Cu, are useful detectable groups. Other examples of such compounds
are
europium complexes. Indirectly detectable groups are groups that can be
recognized by
another moiety which is directly-or indirectly labelled. Examples of such
indirect detectable
groups are haptens, like digoxigenin or biotin. Digoxigenin for example can be
recognized
by antibodies against digoxigenin. Those antibodies may either be labelled
directly or can
be recognized by labelled antibodies directed against the (antidigoxigenin)
antibodies.
CA 02569615 2006-12-18
-21-
Formats based on the recognition of digoxigenin are disclosed in EP-B-0 324
474. Biotin
can be recognized by avidin and similar compounds, like streptavidin and other
biotin
binding compounds. Again, those compounds can be labelled directly or
indirectly. Further
interesting labels are those directly detectable by atomic force microscopy
(AFM) or
scanning tunneling microscopy (STM). The reporter group can further be a
nucleotide
sequence which does not interfere with other nucleotide sequences in the
sample. The
sequence can therefore be specifically recognized by nucleotide containing a
complementary sequence. This nucleotide sequence can be labelled directly or
indirectly or
can be immobiIizable or immobilized. A reporter group can further be a solid
phase.
Attachment of the nucleic acid binding compound with solid phase can be either
directly or
indirectly as pointed out above for the detectable group. Examples of such
solid phases are
latex beads or gold particles. In another embodiment of the invention, a
further reporter
group attached to the nucleic acid binding compound may be any positively or
negatively
charged group, preferably a carboxylate group or an ammonium N+R5R6R12 with
1S substituents as specified under formula I as described above. These may be
attached e.g. via
a propargylen linker to the base and enhance the sensitivity of MALDI-TOF mass
spectroscopy (MALDI-TOF: matrix-assisted laser desorptionlionization time-of
flight) in
the positive or negative mode. The substituents of the ammonium group are
preferably
introduced into the oligonucleotide via post-labelling, i.e. binding compounds
can be
postlabeled with reporter groups when a suitable reactive group is introduced
during
oligonucleotide synthesis, for example, an amino group protected in the
oligonucleotide
synthesis precursor with a phthaloyl group.
Direct labelling can be effected by covalent coupling of a nucleic acid
binding compound to
a reactive group on the solid phase, i.e. preferably via a linker. Indirect
labelling can be
made similar as disclosed above for the detectable groups. Preferably,
indirect attachment is
non-covalently by biospecific interactions, for example those selected from
the group of
hapten-antibody, vitamin-receptor and nucleic acid-complementary nucleic acid.
Again,
those interactions and their use in nucleic acid assays is known to a man
skilled in the art.
Solid phases that are useful for immobilization of the probe according to the
invention are
preferably selected from the group of polystyrene, polyethylene,
polypropylene, glass, SiOz
and Ti02. The formats of such solid phases can be selected according to the
needs of the
instrumentation and format of the assay. For example, a solid phase may assume
the form of a bead or a vessel.
CA 02569615 2006-12-18
-. 22 -
The most popular backbone is the naturally occurring sugar phosphate backbone
of nucleic
acids containing either ribonucleoside subunits (RNA) or deoxyribonucleoside
subunits
(DNA). Therefore, in a preferred embodiment, the backbone of the nucleic acid
binding
compound comprises phosphodiester linkages and ribose. In the last years,
there were
s descriptions of nucleic binding compounds that have similar properties like
oligonucleotides, but differ in their backbone, which have structures formed
from any and
all means of chemically linking nucleotides in contrast to the natural
occuring
phosphodiester ribose backbone. Therefore, it is evident that the invention
would still
work, even if the backbone of the nucleic acid binding compound is not an
oligonucleotide
in the strict sense, i.e. it has a modified backbone. The backbone may include
e.g.
phosphorothioates, methyl phosphonates, phosphoramidates and
phosphorotriesters
linkages. Peptide nucleic acids also have unnatural linkages. The
modifications in the
backbone may vary the properties of the nucleic acid binding compound, i.e. it
may
enhance stability and affinity. Therefore, in a preferred embodiment, the
nucleic acid
binding compounds are those, wherein the backbone comprises one or more
moieties of
the general formula II
Formula Il g
A
R14
I
U-P-V
~T
wherein
A is selected from the group consisting of O, S, CH2, N-CO-(Cl-Clo)-alkyl,
L is selected from the group consisting of oxy, sulfanediyl, -CHZ- and -NRza-,
T is selected from the group consisting of oxo, thioxo and selenoxo,
U is selected from the group consisting of -OH, O-, -O-reporter group, -SH, -S
reporter group -SeH, -(Ci-Clo)-alkoxy, (Cl-Clo)-alkyl, -(C6-Cue)-aryl, -(C6-
Cm) a 1-(Cl-Clo)-ally I, -NR23Rz4, and -O- Ci-CIo
rY Y -( ( )-alkyl-)n R25, wherein n
can be any integer between l and 6, or wherein -NR23R2~ can together with N
be a 5-6-membered heterocyclic ring,
CA 02569615 2006-12-18
-23-
V is selected from the group consisting of oxy, sulfanediyl, -CHz-, or -NRzz-,
R14 is selected from the group consisting of -H, -OH, -(Cl-C6)-alkyl, -(Cl-
Clo)-
allcoxy, -(Cz-Cloy-allcenyloxy, -halogen, -azido, -O-allyl, -O-alltinyl, and -
NHz,
Rzz is independently selected from the group of -H and -(Cl-Clo)-allcyl,
Rz3 and Rz~ are independently selected from the group consisting of -(Cl-Clo)-
alkyl, -
(Cl-Czo)-aryh -(Cs-Cl4)-aryl~(Cl-Clo)-a~'1~ -(Cl-Cs)-~yl-INH(CHz)~~a-
NRz6Rz~ and a reporter group,
Rzs is selected fram the group consisting of -H, -OH, -halogen, -amino,
-(Cl-Cl8)-alkylamino, -COOH, -CONHz and -COO(Cl-C4)-alkyl and a
reporter group,
Rzs and Rz~ are independently selected from the group consisting from -H,
-(Cl-C6)-alkyl, and -(Cl-C4)-alkoxy-(Cl-C6)-alkyl and a reporter group,
c is an integer from 2 to 6,
d is an integer from 0 to 6, and
B is a moiety of formula T
Formula I
s R1
N~
N
N/
X I
R3HI3
D
wherein
Rl is independent from X, Rz or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
( 3 ) Cyano
(4) -COO-
(5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(Cz-Clo)-allcenyl substituted according to (IO)
(7) -(Cz-Clo)-allcynyl substituted according to ( 10)
(8) -(C6-Czz)-aryl substituted according to (10)
(9) -W-(Cl-Cto)-~'1~ -W-(Cz-Clo)-alkenyl, -W-(Cz-Clo)-~Yh -W-(C6-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-,
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri [O-
(CHz)r)s -, where r and s are, independently of each other, an integer
between 1 to I8 and n is 0 or 1 independently from r and s,
CA 02569615 2006-12-18
-24-
(10) substituents (5) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(CI-C6)-allcyl, -NR5R6, -N+RSR6Rlz, -ORIZ,
-CORII, -NH-CO-NRSR6, -NH-CS-NRSR6 and -(CHz)n-[O-(CHz)r]S
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein R" is selected from the group consisting of -NHRIZ, ORIZ, and -
SRIz
wherein R5, R6 and RIZ are selected independently from the group
1o consisting of -H, -(CI-CIO)-alkyl, -(Cz-CIO)-allcenyl, -(Cz-CIO)-alkinyl,
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
15 said allcyl, allcenyl, allcynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(CI-C6)-alkyl, -(CI-C6)-allcoxy, -OH, -NRSR6, -
CORII, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)t)s-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
zo independently from r and s,
with the proviso that RS, R6 or RIZ is not a reporter group if the radicals
(5) to
(7) are substituted by -NR5R6, NHRIZ, ORIZ, or SRIZ;
Rz, R3 is independent from X, RI, Rz and R3 and is selected from the group of,
(1) -H
25 (2) (CI-CIO)-alkyl,
(3) (Cz-CIO)-allcenyl,
(4) (Cz-CIO)-a~Yh
(S) (C6-Czz)-aryl,
(6) -Z-(CI-CIO)-alkyl, -Z-(Cz-CIO)-alkenyl, -Z-(Cz-CIO)-~Yh -Z-(Ce-
3o Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)n-
[O-(CHz)r]5 -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, allcenyl, alkynyl or aryl can be substituted by one
35 or more moieties selected from the group consisting of -halogen,
NOz, -ORIZ, -CN, -SH, -S-(CI-C6)-alkyl, -NR5R6, -N+R5R6RIZ, _
CA 02569615 2006-12-18
- 25 -
CORI', -NH-CONRSR6, -NH-CSNR5R6 and-(CHZ)"-[O-(CHZ)r]S-
NR5R6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHR12 and
OR12,
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Cio)-allcyl, -(C2-Clo)-alkenyl, -(CZ-CIO)-
alkynyl, -(C6-C22)-aryl and a reporter group,
said alkyl, allcenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted
to or substituted by one or more moieties selected from the group consisting
of -
halogen, -SH, -S-(Cl-C6)-alkyl, -(CI-C6)-aIkoxy, -OH, -NRsR6, -CORM, -NH-
CONRSR6, -NH-CSNR5R6 and-(CHZ)n [O-(CH2)r]S NRSR6, r and s are
independently of each other an integer of from x to 18 and n is 0 or 1
independently from r and s;
15 X is independent from Rl, RZ or R~ and is selected from the group
consisting of N
and CH; and
D is the position of attachment of the group to the rest of the nucleic acid
binding compound
20 or any salts thereof.
The preferred definitions of the groups as defined under formula I apply to
formula II and
the following formulae, if not indicated otherwise.
25 Preferably, in compounds of formula II, R14 is hydrogen. Preferred
definition of L is oxy.
Preferred definition of U is -OH and -O-reporter group. Preferred definition
of V is oxy.
Preferred definition of c is an integer from 2 to 4, and of d an integer from
0 to 2.
Compounds of formula II are especially suited to contain the heterocyclic
moiety of the
invention as an integrated part (preferably not at one of the termini) of the
nucleic acid
3o binding compound. The group NR23R24 is preferably selected from the group
consisting of
dialkylamino groups. In case of this group together with the forming of 5- or
6-membered
heterocyclic ring, it assumes preferably the definition of morpholinyl,
pyrrolidinyl or
piperidinyl.
35 In a further preferred embodiment, the sugar configuration is selected from
the group
consisting of the a-D-, (3-D-, a-L- and [i-L-configurations, most preferred
the compound
CA 02569615 2006-12-18
-26-
contains at least one 2'-deoxy-(i-D-ezythro-pentofuranosyl moiety or one (3-D-
ribofuranosyl moiety. In a preferred embodiment of the invention, D is the
glycosid C-1 of
a sugar moiety of the compound according to the invention.
s In another embodiment of the invention the sugar is in a locked
conformation. LNA
(Locked Nucleic Acid) is a novel class of nucleic acid analogue. LNA oligomers
obey the
Watson-Crick base pairing rules and hybridize to complementary
oligonucleotides.
However, when compared to DNA and other nucleic acid derivatives, LNA provides
vastly
improved hybridization performance. LNA/DNA or LNA/RNA duplexes are much more
to thermally stable than the similar duplexes formed by DNA or RNA. In fact,
LNA has the
highest affinity towards complementary DNA and RNA ever to be repoxted. In
general, the
thermal stability of a LNA/DNA duplex is increased 3°C to 8°C
per modified base in the
oligo. Within the fields of general molecular biology and molecular
diagnostics, five major
'fields for the application of LNA have been identified which are capture
probes, sample
15 preparation, detection of SNP's (Single Nucleotide Polymorphisms), allele
specific PCR,
and hybridization probes, Molecular Beacons, Padlock probes, Taqman probes
(W092/02638 and corresponding US patents US 5,210,015, US 5,804,375, US
5,487,972)
and probes for in-situ hybridizations. In most respects, LNA may be handled
like DNA.
LNA is at least as stable as DNA and is soluble in aqueous buffers. LNA can be
ethanol
2o precipitated, dried and resuspended, and can be analyzed on gels, HPLC and
MALDI-TOF.
LNAs are novel nucleic acid analogs that can dramatically increase the
performance of not
only diagnostic assays that probe and evaluate genetic information but also of
antisense and
other genetic medicine approaches. These analogs, which can be utilized in
most
applications just like their natural counterparts, lock the nucleic acid into
the most
25 productive conformation for hybridization: Hybridizafiion, or complementary
docking of
genetic probes, is the predominant form of evaluation of genetic information
in
diagnostics. A broad variety of applications for LNA have been developed
including a
number of extremely sensitive and specific assays able to detect specific
disease-causing
single base mutations in an individual's genes In the detection of SNPs
(Single Nucleotide
30 Polymorphisms), which are the small variations in our genes, that may cause
a
predisposition to disease, there are data to show that LNA capture probes of
only eight
nucleotides in length are able to more effectively discriminate between
mutated and
wildtype genes in a sample than much longer conventional nucleic acid capture
probes.
Therefore the invention also contemplates compounds according to the invention
wherein
35 e.g. at least one atom of the sugar moiety e.g. a carbon or an oxygen atom
is connected to at
least one other atom of the sugar moiety via at least one bridging moiety
containing at least
CA 02569615 2006-12-18
_Z'J-
one atom whereby a conformationally constrained sugar is formed as outlined
above.
Thereby the sugar is fixed in a locked conformation.
For the synthesis of the compounds according to the invention, the reader is
referred to
Chemistry of Nucleosides and Nucleotides Part 1, edited by L. B. Townsend,
Plenum Press
New York ( 1988), Chapter 2: Synthesis and Properties of Purine Nucleosides
and
Nucleotides, page 113-281 or to US patent 5,594,121. However, more information
is
provided below.
l0 Different chemical structures can be used in the backbone of the nucleic
acid binding
compound. The expert skilled in the field appreciates the fact that the
nucleic acid binding
compound may also possess a modifed 3'-end. Therefore, a preferred subject of
the
invention is a nucleic acid binding compound as outlined above, wherein the
backbone
comprises one or more moieties of the general formula III, wherein t is 0 or
1,
B
Formula III A
R14
Mt
I
R15
wherein in the case that t=1,
A is selected from the group consisting of O, S, CHz and N-(Cl-C6)-alkyl,
M is selected from the group consisting of oxy, sulfanediyl, -NRzz-, -(Cl-
Clo)-alkyl-, or -O-(Cl-Clo)-alkyl-O-, and -S-(Cl-Clo)-alkyl-O- and -
2o NRzz-(Cl-Cs)-alkyl-O-,
Rzz is selected from the group of -H, -(Cl-Clo)-allcyl, a protecting group
and a reporter group,
Rl4 is selected from the group consisting of -H, -OH, -(Cl-C3)-alkyl, -(Cl-
Cs)-~Yh -(Cl-Clo)-alkoxy, -(Cz-Clo)-allcenyloxy, -(Cz-Clo)-a~Yloxf,
-(O-CHz)n- wherein n may be an integer of from 1 to 18, -halogen, -
azido, SH, -(Cl-Cloy-allcylmercapto, O-reporter group, O-solid phase
and -NHz,
Rls is selected from the group consisting of -H, -(Cl-C6)-alkyl, -(Cz-Clo)-
allcenyl, -(Cz-Clo)-alkynyl, -(Cz-Clo)-alkyl-carbonyl, -(C3-Cl9)-alkenyl-
CA 02569615 2006-12-18
- 28 -
carbonyl, -(C~-Cl9)-allrynyl-carbonyl, -(C6-C14)-aryl-(Cl-Clo)-alkyl, a
solid phase and a group of formula IV
Formula IV
U-~P-R29
T
wherein
T is selected from the group consisting of oxo, thioxo and selenoxo,
and
U is selected from the group consisting of -OH, O-, -O-reporter
group, -SH, -SeH, -(Cl-Clo)-allcoxy, -(Cl-Clo)-alkyl, -(C6-C2a)-
aryl, -(C;6-Cl4)-aryl-(Cl-Cloy-~'1~ -NRZ3Rz4~ and -(-O-(Cl-Clo)-
allcyl-)-RZS, wherein N can be any integer between 1 and 6, or
to wherein NRz3Rz4 can together with N be a S-6-membered
heterocyclic ring,
R'~ and R''~ are independently selected from the group consisting of -
(CmClo)-~'h -(CrCzo)-aryh -(Cs-Cl4)-aryl-(Cl-Cloy-~yh -(Cl_
C6)-a~l-~~(CH2~c~ d-NRz6R2T
Rzs is selected from the group consisting of -H, -OH, -halogen, -amino,
-(Cl-Cl8)-alkylamino, -COOH, -CONHz and -COO(Cl-C4)-alkyl,
Rz6 and Rz' are independently selected from the group consisting from -H,
-(Cl-Cs)-~Yh and -(Cl-C4)-alkoxy-(Cl-C6)-allcyl
Rz9 is selected from the group consisting of -OR3o and -SR3o,
2o R3o is selected from the group consisting of -H, -(Cl-Clo)-alkyl, -(C.i-
Clo)-
allcenyl, -(C6-C2z)-aryl, a protecting group, a solid phase and a reporter
group
c is an integer from 2 to 6,
d is an integer from 0 to 6, and
B is a moiety of formula I
CA 02569615 2006-12-18
-29-
Formula I
NRx Rl
N/
~N
N/
X
R3HN
wherein
D
Rl is independent from X, RZ ox R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO'
(5) -(C~-Clo)-alkyl substituted according to (10)
(6) -(CZ-Cloy-alkenyl substituted according to (10)
to (7) -(CZ-C~o)-alkynyl substituted according to (10)
(8) -(C6-C22)-aryl substituted according to ( 10)
(9) -W-(Cl-Cio)-a~y'1~ -'w-(C2-C~o)-alkenyl, -W-(C2-C~o)-~Yh -W-(Cs-
C22)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-,
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHZ)"-[O-
(CHZ)r]S -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NO2, -CN, -S-(Cr-C6)-alkyl, -NRSR6, -N+R5R6RlZ, -ORl2,
-CORM, -NH-CO-NR5R6, -NH-CS-NR5R6 and-(CH2)"-(O-(CHZ)r]s-
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein Rl l is selected from the group consisting of -NHR'2, ORIZ, arid -
SR'2
wherein R5, R6 and Rl2 are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(C2-Clo)-allcenyl, -(CZ-Clo)-alkinyl, _
(C6-CZZ)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
3o binding, cross-linking or cleaving,
CA 02569615 2006-12-18
-30-
said alkyl, allcenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Ci-Ce)-alkyl, -(C1-C6)-alkoxy, -OH, -NR5R6, -
CORI', -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)r)S-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NR5R6, NHRIZ, ORIZ, or SRIZ;
Rz, R3 is independent from X, Rl, Rz and R3 and is selected from the group of,
to (1) -H
(2) (Ci-Cto)-alkyl,
(3) (Cz-Cio)-alkenyl,
(4) (Cz-Cio)-~Yh
(5) (C6-Czz)-aryh
15 (6) -z-(Ci-Cio)-~'1~ -z-(Cz-Cio)-allcenyh -Z-(Cz-Cio)-a~yh -Z-(Cs-
Czz)-aryl or Z-H, whereirn Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)ri
[O-(CHz)r]S -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
2o wherein any alkyl, alkenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -ORIZ, -CN, -SH, -S-(Cl-C6)-allcyl, -NRSR6, -N'~RSR6R'z, _
CORM, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)r]S
NR5R6, r and s are independently of each other an integer of from 1
25 to 18 and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHRIZ and
ORIZ,
wherein R5, R6 and R'z are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-alkenyl, -(Cz-Clo)-
3o alkynyl, -(C6-Czz)-aryl and a reporter group,
said alkyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -CORII> -NH-
CONRSR6, -NH-CSNR5R6 and-(CHz)"-[O-(CHz)r)s-NR5R6, r and s are
35 independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
CA 02569615 2006-12-18
-3I-
X is independent from Rl, RZ or R3 and is selected from the group consisting
of N
and CH; and
D is the position of attachment of the group to the rest of the
nucleic acid binding compound
and wherein in the case that t=0, Rls is -H,
or any salts thereof.
to
For the definitions and preferences the particulars apply as outlined for the
substituents
under formulae I and II, if not specified otherwise specifically for formula
III. In a preferred
embodiment the 3'-end possesses a 2',3'-didesoxyribose, i.e. wherein t=0, R15
is -H and R14
is -H, or an analogue thereof. This is of interest if an enzymatical
termination is necessary
15 when the nucleic acid binding compound according to the invention is
extended with
triphosphate compounds also occuring in nature as the triphosphates of
adenosine,
guanosine, uridin, cytidine or thymidine or the desoxyderivates of the
triphosphates of
adenosine, guanosine, cytidine or thymidine. However, the invention also
relates to the
extension of primers containing only nucleotides occuring in nature or nucleic
acid
2o binding compounds according to the invention with triphosphate compounds
according to
the invention with the general formula VIII.
Nucleic acid binding compounds, wherein the group of formula I is attached to
subunits,
for example the nucleotide, at the 3'-terminus of the compound, are useful
either as
25 starting compound for the synthesis of longer compounds orland as end-
labeled probes.
This group of compounds is especially preferred because the terminal position
of probes
generally is the most tolerant in view of attachment of chemical moieties.
In view of the modifications to the 3'-end of the nucleic acid binding
compound, it is
3o evident that also the 5'-end of the nucleic acid binding compound may be
modified.
Therefore, another preferred subject of the invention is a nucleic acid
binding compound
as outlined above comprising a backbone moiety of the formula V
CA 02569615 2006-12-18
-32-
Formula V
wherein
~~M' B
A
R14
A is selected from the group consisting of O, S, CHz and N-(C~-C6)-alkyl,
M' is selected from the group consisting of oxy, sulfanediyl, -NRzz-, -(Cl-
C~o)-
alkyl, or -O-(Cl-Clo)-alkyl-O-, and -S-(Cl-Clo)-alkyl-O- and -NRzz-(Ci-Cs)-
alkyl-O-,
Rzz is selected from the group of -H, a protecting group, a reporter group and
-(Cl-Clo)-alkyl,
R14 is selected from the group consisting of -H, -OH, -(Cl-Clo)-alkoxy,
to -(CrClo)-allcenyloxy, -(Cz-Clo)-allcynyloxy, -halogen, azido, -SH, -S-(CI-
C6)-
alkylinercapto, O-reporter group, O-solid phase and NHz,
Ri6 is selected from the group consisting of -H, -(Cl-C$)-alkyl, -(Cz-Cl8)-
alkenyl> -
(Cz-Ci8)-allcynyl, -(Cz-C18)-alkyl-carbonyl, -(C3-Cl9)-allcenyl-carbonyl, -
(C3_
Ci9)-alkynyl-arbonyl, -(C6-C14)-aryl-(Cl-C$)-alkyl, a protective group or a
compound of formula TV
wherein
Formula IV I
U_~-Ras
T
T is selected from the group consisting of oxo, thioxo and selenoxo,
U is selected from the group consisting of -OH, -SH, -SeH, -(Cl-Cla)-allco~cy,
-(C~-Cio)-alkyl, -(C6-Czz)-aryl, -(C6-Ci4)-aryl-(Cl-Cio)-~'h -NRz3Rz4! and -(-
2o O-(Cl-Clo)-alkyl-)-Rzs, wherein n can be any integer between 1 and 6,
wherein
NRz3Rz4 can together with N be a 5-6-membered heterocyclic ring,
Rz3 and Rz4 are independently selected from the group consisting of -(Cx-Clo)-
alkyl, -
(CrCzo)-aryl, -(C6-C14)-aryl-(Cl-Cio)-~yh -(CrCs)-~'1°~~(CHz)~)d-
NRz6Rz~,
Rzs is selected from the group consisting of -H, -OH, -halogen, -amino,
-(Cl-CI$)-alkylamino, -COOH, -CONHz and -COO(Cl-C4)-alkyl,
CA 02569615 2006-12-18
-33-
Rz6 and R2' are independently selected from the group consisting from -H,
-(Ci-C6)-alkyl, and -(Cl-C4)-allcoxy-(Cl-Cs)-alkyl
Rz9 is selected from the group consisting of -OR3° and -SR3o,
R3° is selected from the group consisting of -H, -. (Cl-Cloy-allcyl, -
(Cz-Clo)-allcenyl, -
(C6-Czz)-aryl, a protecting group, a solid phase and a reporter group, and
c is an integer from 2 to 6,
d is an integer from 0 to 6, and
B is a moiety of formula I
Formula i
RI
N~
'N
N/
R3HN
D
wherein
Ri is independent
from X, RZ
or R3 and
is selected
from the group
consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO-
(5) -(Cl-Cl)-allcyl substituted according to ( 10)
(6) -(Cz-Clo)-allcenyl substituted according to
(10)
(7) -(Cz-Glo)-allcynyl substituted according to
(10)
(8) -(C6-Czz)-aryl substituted according to (10)
(9) -W-(Ci-Cio)-alkyl, 'W-(Cz-Clo)-allcenyl, -W-(Cz-Cl)-allcynyl,
-W-(C6-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-,
-S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)n-(O-
(CHz)t]s -, where r and s are, independently
of each other, an integer
between 1 to 18 and n is 0 or 1 independently
from r and s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl,
alkynyl or aryl can be
substituted by one or more moieties selected
from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(C1-C6)-allcyl,
-NRSR6, -N~RSR6Rlz, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NRSR6 and-(CHz)n-(O-(CHz)r]s-
NR5R6, r and s are independently of each other
an integer of from 1 to 18
3o and n is 0 or 1 independently from r and s,
CA 02569615 2006-12-18
-34-
wherein Rll is selected from the group consisting of -NHR~z, ORIZ, and -
SRIz
wherein R5> R6 and Rlz are selected independently from the group
consisting of -H, -(Ci-Cio)-alkyl, -(Cz-Clo)-alkenyl, -(Cz-Cio)-alkinyl, -
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said alkyl, alkenyl; alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-Cs)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -
COR", -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)n-[O-(CHz)r]5-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHR12, ORIZ, or SRIZ;
Rz, R3 is independent from X, Rl, R2 and R3 and is selected from the group of,
(1) -H
(2) (Ci-Cio)-alkyl,
(3) (Cz-Cio)-allcenyl,
(~) (CZ-Cio)-alkynyl,
(5) (Cs-Czz)-aryl,
(6) -Z-(Ci-Cio)-a~'1~ -Z-(Cz-Clo)-allcenyl, -Z-(Ca-Cio)-~Yh -Z-(Ce-
Cue)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)n-
[O-(CHz)=]g-, where r and s are, independently of each other, an
integer between 1 to 18 anal n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, alkenyl, allcynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
3o NOz, -OR'z, -CN, -SH, -S-(Cl-C6)-allcyl, -NRSR6, -1~T"RSR6Rlz, -
COR~1, -NH-CONR5R6, -NH-CSNR5R6 and--(CHz)n [O-(CHz)r]s-
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rl' is selected from the group consisting of -NHRiz and
OR12,
CA 02569615 2006-12-18
-35-
wherein R5, R6 and R'Z are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(CZ-Clo)-alkenyl, -(CZ-CIO)-
allcynyl, -(C6-CZZ)-aryl and a reporter group,
said alkyl, alkenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Ci-C6)-alkyl, -(Cl-C6)-allcoxy, -OH, -NR$R6, -CORI1, -NH_
CONR5R6, -NH-CSNRSR6 and-(CHZ)"-[O-(CHZ)r)$ NR5R6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
l0 X is independent from Rl, RZ or R3 and is selected from the group
consisting of N
and CH; and
A is the position of attachment of the group to the rest of the nucleic acid
binding compound
and any salts thereof.
A very preferred compound is a compound of formula V, wherein M' is O, R16 is
H and R'4
is selected from the group consisting of hydrogen and hydroxyl.
Those compounds can for example be used as 5'-terminally labeled probes.
Regarding the
definitions of the substituents, the definitions as given above apply if not
indicated
otherwise.
The backbone of,the nucleic acid binding compound has the function to bear the
base
pairing heterorycles such that the compound can bind to a nucleic acid having
a
complementary sequence. Preferably, the degree of complementarity in the
naturally
occurring bases will be in the range from 70 % up to 100 % in a stretch of
bases in a region
effecting binding, compared to the stretch of same length in the region of the
nucleic acid
to be bound. Deletions and insertions of subunits in each sequence will
therefor, in this
calculation, be counted as gaps until the next fitting base and thus reduce
complementarity
by as many bases as the gap contains.
Preferred backbone contains sugar-phosphate moieties. Prom these, deoxy sugar
containing backbones are further preferred.
Pach moiety in the backbone bearing a moiety capable of base pairing to a
nucleic acid of
complementary sequence, including the moieties of the invention, are termed a
subunit.
CA 02569615 2006-12-18
-36-
Compounds are known that have backbones mixed of different kinds of subunits.
Recently,
a new kind of non-natural nucleic acid binding compounds was described. They
are termed
Peptide Nucleic Acids (PNA), as they contain at least one peptide bond between
the
subunits (WO 92120702). The nucleic acid binding compound of the present
invention can
have any length. However, due to the convenience of chemical synthesis,
compounds of a
length of less than 100, more preferably from 10 to 30 subunits, for example
nucleosides,
are preferred.
Altering the thermal stability (Tm) of a duplex formed between a nucleic acid
binding
to compound according to the invention, e.g. used as a probe, and a second
nucleic acid
binding compound using the heterocyclic groups according to the invention and
other
analogues allows for optimization of duplex stability and mismatch
discrimination (see e.g.
Kwok, Shirley; Chang, Sheng Yung; Sninsky, John J.; Wang, Alice. A guide to
the design
and use of mismatched and degenerate primers. PCR Methods AppL (1994), 3 (4),
39-47).
15 One useful aspect of altering the T~, arises from the fact that Adenine-
Thymine (A-T)
duplexes have a lower Tm than Guanine-Cytosine (G-C) duplexes, due in part to
the fact
that the A-T duplexes have 2 hydrogen bonds per base-pair, while the G-C
duplexes have 3
hydrogen bonds per base pair. For example in heterogeneous oligonucleotide
arrays, in
which there is a non-uniform distribution of bases, it can be difficult to
optimize
20 hybridization conditions for all probes simultaneously. Thus, in some
embodiments, it is
desirable to destabilize G-C-rich duplexes and/or to increase the stability of
A-T-rich
duplexes while maintaining the sequence specificity of hybridization. This
results in a
harmonization or equalization of the contribution of each base pair to the
melting
temperature of a duplex. This is accomplished, e.g. by replacing one or more
of the
25 heterocyclic groups in the nucleic acid binding compound used as a probe
(or as the target
nucleic acid) with certain modified, non-standard bases. Therefore, in another
embodiment, the invention relates to nucleic acid binding compounds according
to the
invention wherein the nucleic acid binding compounds in addition to a
heterocyclic group
of formula I further contain a heteroryclic group different from the group of
the general
30 formula I, i.e. at least one other heterocyclic group. Substitufiion of
guanine residues with
7-deazaguanine, for example, will generally destabilize duplexes, whereas
substituting
adenine residues with 2,6-diaminopurine will enhance duplex stability. A
variety of other
modified bases are also incorporated into nucleic acids to enhance or decrease
overall
duplex stability while maintaining specificity of hybridization. The
incorporation of 6-aza-
3s pyrimidine analogs into oligonucleotide probes generally decreases their
binding affinity
for complementary nucleic acids. Many 5-substituted pyrimidines substantially
increase the
CA 02569615 2006-12-18
-37-
stability of hybrids in which they have been substituted in place of the
native pyrimidines in
the sequence. Examples include 5-bromo-, 5-methyl-, 5-propynyl-, 5-(ixnidazol-
2-yl)-and
5-(thiazol-2-yl)-derivatives of cytosine and uracil. Preferably the additional
heterocyclic
gxoup is a pyrrolo-[2,3-d]-pyrimidine or a pyrazolo[3,4-d]-pyrimidine or an
analogue
thereof, in, particular the said analogues of adenine or guanine. It should be
emphasized
that the invention also relates to the case where all other heterocyclic
groups are those
occuring in nature as adenine, guanine, uracil, rytasin or thymin. Many
modified
nucleosides, nucleotides and various bases suitable for incorporation into
nucleosides are
commercially available from a variety of manufacturers, including the SIGMA
chemical
company (Saint Louis, Mo.), R&D systems (Minneapolis, Minn.), Pharmacia LICE
Biotechnology (Piscataway, N.J.), CLONTBCH Laboratories, Inc. (Palo Alto,
Calif.), Chem
Genes Corp., Aldrich Chemical Company (Milwaukee, Wis.), Glen Research, Inc.,
GI$CO
BRL Life Technologies, Inc. (Gaithersberg, Md.), Fluka Chemica-Biochemika
Analytika
(Fluka Chemie AG, Buchs, Switzerland), Invitrogen, San Diego, Calif., and
Applied
Biosystems (Foster City, Calif.), as well as many other commercial sources
known to one of
skill. Methods of attaching bases to sugar moieties to form nucleosides are
known. See, e.g.,
Lukevics and Zablocka (1991), Nucleoside Synthesis: Organosilicon Methods
Bllis
Hvrwood Limited Chichester, West Sussex, England and the references therein.
Methods of
phosphorylating nucleosides to form nucleotides, and of incorporating
nucleotides into
oligonucleotides are also known. See, e.g., Agrawal (ed) (1993) Protocols for
Oligonucleotides and Analogues, Synthesis and Properties, Methods in Molecular
Biology
volume 20, F-Iumana Press, Towota, N.J., and the references therein. See also,
Crooke and
Lebleu, and Sanghvi and Cook, and the references cited therein, both supra.
In yet another embodiment, the invention relates to nucleic acid binding
compounds
according to the invention wherein the nucleic acid binding compound according
to the
invention further contains at the 3'-end in addition to a heterocyclic group
of formula I a
heteroryclic group different from the group of the general formula I, i.e. at
least one other
hetexocyclic group. Preferably, the nucleic acid binding compound according to
the
invention additionally comprises a heterocyclic group which is a pyrrolo-[2,3-
d]-
pyrimidine or a pyrazolo[3,4-d]-pyrimidine or an analogue thereof, preferably
the said
analogues of guanine or adenine.
The invention furthex contemplates the binding product of a nucleic acid
binding
compound according to the invention and a second nucleic acid binding
compound, the
nucleic acid binding compound according to the invention and the second
nucleic acid
CA 02569615 2006-12-18
-38-
binding compound being bound to each other by base pairing in parallel or
antiparallel
orientation. In addition to heterocyclic groups with formula I with
substituents as defined
above, the nucleic acid binding compound according to the invention may
contain other
natural nucleobase or nucleobases not occuring in nature as e.g. nucleobases
with
heterocyclic groups according to the invention, heterocyclic groups as pyrrolo-
[2,3-d]-
pyrimidine or pyrazolo[3,4-d]-pyrimidine or analogues thereof, preferably the
said
analogues of guanine or adenine or 7-deaza-guanine. Further non-natural
heterocyclic
groups are known to the person skilled in the art.
Another embodiment of the invention is a nucleic acid binding compound wherein
the
heterocyclic group of formula I as defined above is incorporated to compensate
for a
decrease of the melting point created by the attachment of the reporter
groups, preferably 1
to 5 nucleotides separated from the nucleotide to which a reporter group is
attached. This
is because a reporter group leads to disturbations of the hybridization
efficiency of a nucleic
acid binding compound close to the point or nucleotide whereto the reporter
group is
attached.
Another embodiment of the invention is a nucleic acid binding compound wherein
the
heterocyclic group of formula I as defined above is incorporated to compensate
for a
2o decrease of the melting point created by mismatches discrimination. This
problem has been
discussed by Kwok, Shirley; Chang, Sheng Yung; Sninsky, John J.; Wang, Alice.
A guide to
the design and use of mismatched and degenerate primers. PCR Methods Appl.
(1994),
3 (4), 39-47. This is particlarly useful for the amplification of viral
subtypes where the
hybridization stretch does not contain complementary bases over the total
length of the
stretch.
In an embodiment, the invention relates to nucleic acid binding compounds
according to
the inventionwherein a protecting group substitutes one or two hydrogen atoms
of a -OH,
-SH, -NH2, -NH-alkyl, -NH-allcenylene, -NH- alkynylene, or a NH-aryl group,
although it
3o is preferred that the nucleic acid binding compounds according to the
invention only
contain a few protecting groups or even none.
One particular preferred embodiment, is the use of the nucleic acid binding
compounds in
field of arrays of nucleic acid binding compounds bound to a solid surface
(see e.g.
US5,I43,854, US 6,022,963, US 6,156,501, W090/15070, WO 92!10092), which has
the
properties as described in these references and can be manufactured as
described therein or
CA 02569615 2006-12-18
-39-
by Niemeyer and Blohm (Angew. Chem. Int. Ed.1999, 38, 2865-2869). Therefore,
in a
preferred embodiment, the invention relates to a composition for analyzing
interactions
between nucleic acid binding compounds whereby one nucleic acid binding
compound is a
target nucleic acid. The composition comprises an array of a plurality of
nucleic acid
binding compounds having different sequences, wherein said plurality of
nucleic acid
binding compounds are coupled to a solid substrate at known locations and are
selected to
bind to complementary nucleic acid binding compounds or target nucleic acids
whereby
only the nucleic acid binding compounds or the nucleic acid binding compounds
and the
complementary nucleic acid binding compounds (or target nucleic acids)
together are
nucleic acid binding compounds comprising a backbone, said backbone having
attached
heterocyclic groups capable of base pairing to nucleobases, wherein a
heterocyclic group is
a substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof. Different
kinds of
supports are possible as e.g. nonporous supports or other solid supports less
porous than
typical peptide synthesis supports; however, for certain applications of the
invention, quite
IS porous beads, resins, or other supports work well and are often preferable.
One such
support is a resin in the form of beads. In general, the bead size is in the
range of 1 nm to
100 prn, but a more massive solid support of up to 1 mm in size may sometimes
be used.
Particularly preferred resins include Sasrin resin (a polystyrene resin
available from
Bachem Bioscience, Switzerland); and TentaGel S AC, TentaGel PHB, or TentaGel
S NHZ
resin (polystyrene-polyethylene glycol copolymer resins available from Rappe
Polymere,
Tubingen, Germany). Other preferred supports are commercially available and
described
by Novabiochem, La Jolla, Calif. In other embodiments, the solid substrate is
flat, or
alternatively, may take on alternative surface configurations. For example,
the solid
substrate may contain raised or depressed regions on which synthesis takes
place or is
coated with porous SiO~/ glass. In some embodiments, the solid substrate will
be chosen to
provide appropriate light-absorbing characteristics, For example, the
substrate may be a
polymerized Langmuir $lodgett film, functionalized glass, Si, Ge, GaAs, GaP,
Si02, SiN4,
modified silicon, or any one of a variety of gels or polymers such as
(poly)tetrafluorethylene, (poly)vinylidendiffuoride, polystyrene,
polycarbonate, or
combinations thereof. Other suitable solid substrate material will be readily
apparent to
those of skill in the art. Preferably, the surface of the solid substrate will
contain reactive
groups, which could be carboxyl, amino, hydroxyl, thiol, or the like. More
preferably, the
surface will be optically transparent and will have surface Si--OH
functionalities, such as
are found on silica surfaces. Particularly preferred is therefore a
composition, wherein the
3s solid substrate is selected from the group consisting of silica, polymeric
materials, glass,
porous glass, beads, chips, and slides.
CA 02569615 2006-12-18
Preferred is a composition according to the invention wherein only the nucleic
acid
binding compounds or the nucleic acid binding compounds and the complementary
nucleic acid binding compounds (or target nucleic acid) are nucleic acid
binding
s compounds according to the invention, i.e. they contain a heterocyclic group
with formula
I as described above. The complementary nucleic acid binding compounds (or
target
nucleic acid) may contain a heterocyclic group according to formula I as
described above
when they are amplified with e.g. the polymerase chain reaction in the
presence of a
triphosphate according to the invention containing a heterocyclic group of
formula I as
described above.
Preferred is a composition according to the invention, wherein the nucleic
acid binding
compounds comprise a backbone, said backbone having attached heterocyclic
groups
capable of base pairing to nucleobases characterized in that a heterocyclic
groups is a
substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof. In a preferred
embodiment
the substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof is a
substituted 7-deaza-
8-aza-2,6-diamino-purine or a derivative thereof or a 7-substituted 7-deaza-8-
aza-2,6-
diamino-purine or a derivative thereof. In another embodiment of the invention
the
substituted pyrazolo[3,4-d]pyrimidine analogue is a substituted pyrazolo(3,4-
dJpyrimidine
2o analogue of adenine or guanine or a 7-substituted pyrazolo[3,4-d]pyrimidine
analogue of
adenine or guanine, wherein the adenine or guanine analogues may preferably
carry the
same substituents R' in the 7-position or N-substituents R2 and R3 as set out
directly below
for the substituted 7-deaza-8-aza-2,6-diamino-purine or a derivative thereof
or a 7-
substituted 7-deaza-8-aza-2,6-diamino-purine or a derivative thereof.
In a very preferred embodiment of the invention the substituted 7-deaza-8-aza-
2,6-
diamino-purine or a derivative thereof or the 7-substituted 7-deaza-8-aza-2,6-
diamino-
purine or a derivative thereof has the general formula I
Formula I
R~ R~
N~
N
N
X
R~RN
D
R' is independent from X, Rz or R3 and is selected from the group consisting
of
CA 02569615 2006-12-18
-41
( 1 ) -Yr, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -coo'
(5) -(Cl-Cjo)-alkyl substituted according to ( 10)
(6) -(C2-Cloy-allcenyl substituted according to (10)
(7) -(CZ-Cloy-alkynyl substituted according to (10)
(8) -(C6-Cue)-aryl substituted according to ( 10)
(9) -W-(Cl-Clo)-~yl> -W-(Ca-Cio)-alkenyl, -W-(Cz-Cio)-~Yh -W-(C6-
to Cue)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)n [O-
(CHz)r]a -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
( 10) substituents (5) to (9) wherein any alkyl, allcenyl, alkynyl or aryl can
be
15 substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Ci-Cs)-alkyl, -NRSR6, -N+RSR6Rlz, -OR12,
-CORM, -NH-CO-NRSR6, -NH-CS-NRSR6 and -(CHZ)n-[O-(CHZ)r]s-
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
2o wherein Rl' is selected from the group consisting of -NHR'2, OR12, and -
SR'z
wherein R5, R6 and R12 are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(CZ-Clo)-alkenyl, -(CZ-Clo)-alkinyl,
(C6-CZZ)-aryl and a reporter group, a group which facilitates intracellular
2s uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said alkyl, allcenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
30 consisting of -halogen, -SH, -S-(Cl-C6)-allcyl, -(Cl-C6)-allcoxy, -OH, -
NR5R6,
CORII, -NH-CONRSR6, -NH-CSNRSR6 and-(CH2)~ [O-(CHZ)r)$-NR5R6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or RIZ is not a reporter group if the radicals
(5) to
35 (7) are substituted by -NR5R6, NHR12, OR'Z, or SR12;
R2, R3 is independent from X, Rl, RZ and R3 and is selected from the group of,
CA 02569615 2006-12-18
-42-
(1) -H
(2) (CrCio)-alkyh
(3) (Cz-Clo)-alkenyl,
(Cz-Cio)-~Yh
(5) (Cs-Czz)-aryl,
-Z-(CmCio)-~'1~ -Z'(~-Cio)-alkenyl, -Z-(CZ-Cio)-~Yl> -Z-(Cs-
Czz)-aryl or Z-H> wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)"-
[O-(CHZ)r]s -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, allcenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -ORl2, -CN, -SH, -S-(Cl-Cs)-alkyl, -NRSRs, -NtR5R6Riz, _
CORM, -NH-CONRSRs, -NH-CSNRSRs and-(CHz)n-[O-(CHz)r]S-
NRSRs, r and s axe independently of each other an integer of from i
to 18 and n is 0 or 1 independently from r and s,
wherein Rl' is selected from the group consisting of -NHRIZ and
OR'z,
wherein R5, Rs and R12 are selected independently from the group
2o consisting of -H, -(C1-Cloy-alkyl, -(Cz-Cloy-alkenyl, -(Cz-Cio)~
alkynyl, -(Cs-Cz~)-aryl and a reporter group,
said alkyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of
halogen, -SH, -S-(Cx-Cs)-alkyl, -(Cl-Cs)-alkoxy, -OH, -NRSRs, -CORI1, -NH_
CONRSRs, -NH-CSNRSRs and-(CHZ)"-[O-(CHz)r]s-NRSRs, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
X is independent from Rl, R2 or R3 and is selected from the group consisting
of N
and CH
3o D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
In a very preferred embodiment, the nucleic acid binding compound is a nucleic
acid
3s binding compound according to the invention, preferably R'=Br or R'=I.
Further preferred
CA 02569615 2006-12-18
- 43 -
is a composition which comprises an array of nucleic acid binding compounds 5
to 20
nucleotides in length.
The invention is further related to a binding product of a first nucleic acid
binding
compound according to the invention or a composition according to the
invention with a
second nucleic acid binding compound or a second a nucleic acid binding
compound
according to the invention, wherein the first nucleic acid binding compound or
the
composition and the second nucleic acid binding compound being bound to each
other by
base pairing in parallel or antiparallel orientation.
The invention is further related to methods for the synthesis of the nucleic
acid binding
compounds according to the invention and to compounds useful in these methods.
The
nucleic acid binding compound of the present invention can be prepared in
solution or,
preferably, on a solid phase, where appropriate using an automatic synthesis
device. The
I5 oligomers can be assembled stepwise by successively condensing a
mononucleotide, which
in each case possesses a nucleotide base, onto an appropriately derivatized
support or onto
a growing oligomer chain. Alternatively, the nucleic acid binding compounds
can be
assembled by joining dinucleotides or trinucleotides together [S. Beaucage et
al.,
Tetrahedron, 48 (12), 2223-2311, (1992); and Tetrahedron, 48 (28), 6123-6194,
(1993)].
This is particularly advanteous when synthesizing oligonucleotides which
posses modified
phosphate bridges.
The oligonucleotides are assembled using methods which are known to the person
skilled
in the art, such as the triester method, the H-phosphonate method or the
phosphoramidite
method [E. Sonveaux, (1986), Bioorganic Chemistry, 14, 274-325; S. L. Beaucage
et al.,
( 1992), Tetrahedron, 48, 2223-2311 J .
The compounds according to the present invention can be advantageously used in
oligonucleotide synthesis as the ammonia hydrolysis of the protecting groups
of the
pyrazolo[3,4-dJpyrimidine nucleosides is quicker than the slow ammonia
hydrolysis of 2-
amino-adenosine which takes several days.
A further subject of the invention is therefore a method for the chemical
synthesis of
nucleic acid binding compounds of the present invention using activated
subunits, wherein
said subunit contains a group of formula I. The most preferred method of
chemical
synthesis uses the phosphoramidite approach. A particularly preferred method
uses a
CA 02569615 2006-12-18
-44-
activated subunit one or more compounds of general formula VII. This method
has the
advantage that it is very convenient and the reagents necessary, for example a
phosphoramidite containing a group of formula I, are possible to be included
easily.
A further subject of the invention are therefore compounds of the general
formula VII
Formula Vl( R~M'
A
M R14
P~ NR32R17
R18
wherein
to A is selected from the group consisting of O, S, CHZ and N-(Cl-C6)-alkyl,
M and M' are independently selected from the group consisting of oxy,
sulfanediyl, -
NRZZ, -(Cl-Clo)-allcyl, or -O-(Cl-Clo)-alkyl-O-, and -S-
(Cl-Clo)-alkyl-O- and -NRZZ-(C1-C6)-alkyl-O-,
R22 is selected from the group of -H and -(Cl-Clo)-alkyl,
15 Rl4 is selected from the group consisting of -H, -OR~1, -(Cl-Clo)-alkoxy,
-(CZ-Cloy-alkenylaxy, -(CZ-Cloy-alkynyloxy, -halogen, -azido, NHR31, SR31,
Rsl is a protecting group or a reporter group,
R3Z and Rl~ are independently selected from the group consisting of -H, -(Cl-
Clo)-
alkyl, -(CZ-Clo)-alkenyl, -(C6-C22)-aryl, or wherein NR32Rl' can form together
2o with N a 5-6-membered heterocyclic ring,
Rle is selected from the group consisting of -(CZ-C6)-alkenyloxy, substituted
or
unsubstituted -(Cl-C6)-allryl, unsubstituted -(Cl-C6)-alkoxy or -(Cl-C6)-
alkoxy
substituted one or more times by a group selected from the group consisting of
-halogen, p-nitroaryloxy and -cyano, and
25 B is a group of formula I
CA 02569615 2006-12-18
Formula I R, R,
N~ ~\
N
N/
x I
R3HN
D
Rl is independent from ~C, Rz or R3 and is selected from the group consisting
of
(1) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO-
(5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(Cz-Clo)-alkenyl substituted according to (10)
(7) -(Cz-Clo)-alkynyl substituted according to (10)
(8) -(C6-Czz)-aryl substituted according to ( 10)
(9) W-(Cl-Cm)-~'h -W-(Cz-Clo)-alkenyl, -W-(Cz-Cm)-~Yh 'W-(Cs-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri (O-
(CH2)~]S -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, allcenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NRSR6, -N+RSR6Rlz, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NR5R6 and -(CHZ)"-[O-(CHz)r]s
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein Rl1 is selected from the group consisting of -NHRIZ, ORIZ, and -
SRIz
2s wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-alltenyl, -(CZ-Cloy-allcinyl, -
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
, binding, cross-linking or cleaving,
(11)
CA 02569615 2006-12-18
-46-
O
Nuc-(CH2)~ N
O
0
Nuc-CH=CH-(CHZ)"-N
O
O
Nuc-C=C-(CH2)~-N
O
wherein Nuc is the link to formula I and n is any integer from 1 to 18
said alkyl, alkenyl, allcynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(GI-C6)-alkyl, -(Ci-C6)-alkoxy, -OH, -NR5R6, -
CORI1, -NH-CONR5R6, -NH-CSNRSR6 and-(CHZ)"[0-(CHZ)rJS NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or R'2 is not a reporter group if the radicals
(5) to (7)
are substituted by -NR5R6, NHRl2, ORi2, or SRIZ;
said alkyl, alkenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Ci-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NR5R6, -
CORI1, -NH-CONRSR6, -NH-CSNRSR6 and-(CHZ)p-tO-(CHz)r]a NR5R6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or R'2 is not a reporter group if the radicals
(5) to (7)
are substituted by -NRSR6, NHR~2, OR12, or SRIZ;
Rz, R3 is independent from X, Rs, RZ and R3 and is selected from the group of,
(1) -H
(CmC~o)-~'h
(3) (CZ-Go)-alkenyl,
(~-Cio)-~Yh
(5) (C6-CzZ)-aryl,
CA 02569615 2006-12-18
-47-
(6) -Z-(Ci-Clo)-~Yh -Z-(C2-Cio)-alkenyl, -Z-(C2-Cio)-~yh -Z-(C6-
Caa)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHZ)n-
[O-(CHz)r~s-~ Where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, alkenyl, allcynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
N02, -OR12, -CN, -SH, -S-(Cl-Cs)-~Yh -NR5R6, -N+RSR6R'2, _
CORM, -NH-CONRSR6, -NH-CSNRSR6 and-(CHZ)n-j0-(CHZ)r~s-
l0 NR5R6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or I independently from r and s,
wherein Rl l is selected from the group consisting of -NHR'Z and
ORIZ,
wherein R5, R6 and R12 are selected independently from the group
15 consisting of -H, -(Cl-Cio)-allcyl, -(CZ-Cloy-alkenyl, -(C2-Cla)-
alkynyl, -(C6-Cz2)-aryl and a reporter group,
said alkyl, alkenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted or
substituted by one or more moieties selected from the group consisting of -
halogen, -
SH, -S-(Ci-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -CORM, -NH-CONRSR6, -NH-
2o CSNR5R6 and -(CHz)"-j0-(CHZ)r]S-NR5R6, r and s are independently of each
other
an integer of from 1 to I8 and n is 0 or 1 independently from r and s;
X is independent from Rl, RZ or R3 and is selected from the group consisting
of N
and CH
25 D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
with the proviso that one or two hydrogen atoms of any-OH, -SH, -NH2, -NH-
alkyl, -NH-
allcenylene, -NH- alkynylene, or -NH-aryl group are substituted by a
protecting group,
and any salts thereof.
After suitable protective groups for the amino groups at position 2 and 6 and
for the free
5'-hydroxyl group of the sugar moiety have been introduced, the monomers are
converted
into the corresponding phosphonate or phosphoramidite derivatives. Suitable
amino
protective groups, for example in the form of acyl protective groups (e.g.
isobutyryl, acetyl
CA 02569615 2006-12-18
-48-
or phenoxyacetyl), are inserted using well-known methods [J. C. Schulhof, D.
Molko, R.
Teoule, ( 1987), Nucleic Acids Res., 15, 397-416]. An example of a suitable
protective group
for the free 5'-OH group of the sugar is the 4,4'-dimethoxytrityl residue,
whose insertion is
likewise effected using known methods [C. B. Reese (1978), Tetrahedron, 34,
3143; D.
Flockerzi et al., (1981), Liebigs Ann, Chem., 1568]. The monomers which have
been
protected in this way can be converted into the corresponding phosphonates in
accordance
with a protocol due to Froehler et al. [B. C. Froehler et al., ( 1986),
Nucleic Acids Res., 14,
5399]. Cyanoethyl-phosphoramidite derivatives can, for example, be prepared by
reacting
the monomers with chloro-~i-cyanoethoxy-(N,N-diisopropylamino)phosphane in
anhydrous dichlormethane [N. D. Sinha et al., (1984), Nucleic Acids Res., 12,
4539].
Further subject of the invention are compounds of the general formula IX
R32R17
Formula IX
8P\ M~ B
R1 A
R3\ M R14
is
wherein
A is selected from the group consisting of O, S, CHZ and N-(Cl-C6)-alkyl,
M and M' are independently selected from the group consisting of oxy,
sulfanediyl, -
2o NRzz, -(Cl-Clo)-alkyl, or -O-(Cl-Clo)-alkyl-O-, and -S-(Cl-Cloy-alkyl-O-
and -
NRZZ-(Cl-C6)-alkyl-O-,
Rz2 is selected from the group of -H and -(Cl-Clo)-alkyl,
Rl4 is selected from the group consisting of -H, -OR31, -(Cl-Clo)-allcoxy,
-(C2-Clo)-alkenyloxy, -(CZ-Cloy-alkynyloxy, -halogen, -azido, NHR31, SR31, or
25 O-reporter group,
R31 is a protecting group or a reporter group,
R32 and Rl' are independently selected from the group consisting of -H, - (Cl-
Clo)-
alkyl, -(C2-Cloy-alkenyl, -(C6-C22)-aryl, or wherein NR32Rl' can form together
with N a 5-6-membered heterocyclic ring,
CA 02569615 2006-12-18
-49-
RI$ is selected from the group consisting of-(Cz-C6)-alkenyloxy, substituted
or
unsubstituted -(Cl-C6)-alkyl, unsubstituted -(CI-C6)-alkoxy ox -(CI-C6)-alkoxy
substituted one or more times by a group selected from the group consisting of
-halogen, p-nitroaryloxy and -cyano, and
B is a group of formula I
Formula I
nn A1
N
N
N
X
ALAN
D
RI is independent from X, Rz or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
to (3) Cyano
(4) -COO-
(5) -(CI-CIO)-alkyl substituted according to (10)
(6) -(Cz-CIO)-a~enyl substituted according to (10)
(7) -(Cz-CIO)-alkynyl substituted according to ( 10)'
(8) -(C~-Czz)-aryl substituted according to (10)
(9) -W-(CI-CIO)-~'1~ -W-(Cz-CIO)-alkenyl, -W-(C2-CIO)-~Yh -W-(C6-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)n [O-
(CHz)~]9 -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(CI-C6)-alkyl, -NRSR6, -N+RSR6Rlz, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NRSR6 and-(CHz)n-[O-(CHZ)r~s-
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein RII is selected from the group consisting of -NHRIZ, ORI2, and -
SRiz
wherein R5, R6 and RIZ are selected independently from the group
consisting of -H, -(CI-CIO)-alkyl, -(Cz-CIO)-alkenyl, -(C2-CIO)-alkinyl, -
(C6-Cz2)-aryl and a reporter group, a group which facilitates intracellular
CA 02569615 2006-12-18
-50-
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
(11)
0
Nuc-(CH2)n N
0
0
Nuc-CH=CH-(CH2)~ N
0
O
Nuc-C=C-(CH2)n N
O
wherein Nuc is the link to formula I and n is any integer from 1 to 18
said alkyl, alkenyl, allcynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Ci-C6)-alkyl, -(C,-C6)-alkoxy, -OH, -NRSR6,
IO CORI', -NH-CONRSR6, -NH-CSNRSR6 and -(CHz)n [O-(CHz)r]S NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or R'z is not a reporter group if the radicals
(5) to
(7) are substituted by -NR5R6, NHRIZ, OR~z, or SRIZ;
is Rz, R3 is independent from X, R', Rz and R3 and is selected from the group
of,
(1) -H
(CmCio)-~'1~
(3) (Cz-Clo)-alkenyl,
(Cz-Cio)-~Yh
20 (5) (C6-Czz)-aryl,
(6) -Z-(Ci-Go)-~'h -Z-(Cz-Cio)-alkenyl, -Z-(Cz-Cio)-~Yh -Z-(C6-
Czz)-aryl or Z-H, wherein Z = -GO-, -CO-NH-, -CS-NH-, -(CHz)"--
[O-(CHz)r]s -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
2s (7) substituents (Z) to (6)
CA 02569615 2006-12-18
-51-
wherein any allcyl, allcenyl, allcynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -OR'2, -CN, -SH, -S-(Cl-C6)-alkyl, -NRSR6, -N~R5R6RlZ, -
CORII, -NH-CONR5R6, -NH-CSNR$R6 and-(CHZ)n [O-(CHZ)r~s
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rl l is selected from the group consisting of -NHRIZ and
OR12
wherein R5, R6 and R12 are selected independently from the group
~ consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-alkenyl, -(CZ-Clo)-
allcynyl, -(C6-CZa)-aryl and a reporter group,
said alkyl, alkenyl, allrynyl or aryl in substituents (2) to (7) being
unsubstituted or substituted by one or more moieties selected from the
group consisting of -halogen, -SH, -S-(Cl-C6)-allcyl, -(Cl-C6)-allcoxy, -OH,
-NRSR6, -CORI', -NH-CONRSR6, -NH-CSNRSR6 and-(CHZ)n-[O-
(CHZ)r]S-NRSR6, r and s are independently of each other an integer of from
1 to 18 and n is 0 or 1 independently from r and s;
X is independent from Rl, RZ or R3 and is selected from the group consisting
of N
and CH
2o D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
with the proviso that one or two hydrogen atoms of any-OH, -SH, -NHz, -NH-
alkyl, -NH-
alkenylene, -NH- allcynylene, or -NH-aryl group are substituted by a
protecting group,
and any salts thereof.
Those compounds can be used like those of formula VII in chemical synthesis.
A further subject of the invention are compounds of the general formula X
wherein
CA 02569615 2006-12-18
-52-
Formula X
R
M
A
M R14
I
H-P=p
O
M and M' are independently selected from the group consisting of oxy,
sulfanediyl, -NRzz, -
(Cl-Clo)-~T1~ or -O-(Cl-Cio)-~Yl-O-~ and -S-
s (Cl-Cio)-~T1-O- and -NRzz-(Cl-C6)-alkyl-O-,
Rzz is selected from the group of -H and -(Cl-Clo)-allcyl,
Rl4 is selected from the group consisting of -H, -OR31, -(Cl-Clo)-alkoxy,
-(Cz-Clo)-allcenyloxy, -(Cz-Clo)-alkynyloxy, -halogen, -azido, NHR31, SR31, or
O-reporter,
io R31 is a protecting group or a reporter group,
Formula I
nn R1
N/
N
N
x
R~1W
n
Rl is independent from X, Rz or R3 and is selected from the group consisting
of
(1) -F, -Cl, -Br or -I,
(2) Nitro
is (3) Cyano
(4) -COO'
(5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(Cz-Cloy-alkenyl substituted according to (10)
(7) -(Cz-Cloy-alkynyl substituted according to (10)
20 (8) -(C6-Czz)-aryl substituted according to (10)
(9) -W-(Cl-Cio)-~yh -W-(Cz-Clo)-alkenyl, -W-(Cz-Clo)-~yh 'W-(Ce-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CA 02569615 2006-12-18
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri [O-
(CH2)r]s-, where r anal s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NR5R6, -Ni'R5R6R12, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NRSR6 and -(CHz)n [O-(CHz)r]s
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
to wherein Rl1 is selected from the group consisting of -NHRIZ, ORIZ, and -
SRl z
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Cio)-alkenyl, -(Cz-Cio)-~Yh _
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
15 uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
(11)
O
Nuc-(CH2)"-N
O
O
Nuc-CH=CH-(CH2)~ N
O
O
Nuc-C=C-(CHZ)~ N
O
20 wherein Nuc is the link to formula I and n is any integer from 1 to 18
said alkyl, alkenyl, allcynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(C~-Cs)-alkyl, -(Ci-C6)-allcoxy, -OH, -NR5R6, -
CORiI, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)r]s-NR5R6, r
CA 02569615 2006-12-18
-54-
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHRIZ, ORrz, or SRIZ;
Rz, R3 is independent from X, Rl, Rz and R3 and is selected from the group of,
(1) -H
(2) (CmCio)-a~'1~
(3) (Cz-Cio)-allcenyl,
(4) (Cz-Cio)-~Yh
l0 (5) (C6-Cue)-aryl,
(6) Z-(Cl-Cio)-~'h -Z-(Cz-Cio)-alkenyl, -Z-(Cz-Cio)-~Yh -Z-(C6-
Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)n
[O-(CHz)r]S -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
is (7) substituents (2) to (6)
wherein any alkyl, allcenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -ORIZ, -CN, -SH, -S-(Cl-C6)-alkyl, -NRSR6, -N+RSR6Rlz, _
CORK, -NH-CONR5R6, -NH-CSNRSR6 and-(CHz)n-(O-(CHz)r)s
20 NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rl' is selected from the group consisting of -NHR'z and
ORIZ,
wherein R5, R6 and R'z are selected independently from the group
25 consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Cloy-alkenyl, -(Cz-Clo)-
allrymyl, -(C6-Czz)-aryl and a reporter group,
said alkyl, allcenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted or substituted by one or more moieties selected from the
group consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-aIkoxy,
30 OH, -NR5R6, -CORM, -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)"-~O_
(CHz)r~s NR5R6, r and s are independently of each other an integer of
from 1 to 18 and n is 0 or 1 independently from r and s;
X is independent from Rl, R2 or R3 and is selected from the group consisting
of N
and CH
35 D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
CA 02569615 2006-12-18
with the proviso that one or two hydrogen atoms of any-OH, -SH, -NHZ, -NH-
allcyl, -NH-
allcenylene, -NH- alkynylene, or -NH-aryl group are substituted by a
protecting group, and
any salts thereof. Those compounds are useful in chemical synthesis of nucleic
acid binding
compounds as mentioned above and the precursors thereof.
In another option which is more suited for long oligomers and those based on
natural
backbones, the oligomers are produced enzymatically. In this case, a starting
oligomer
is reacted with a polymerase and a triphosphate or modified triphosphate such
that a
monophoshate or a modified monophosphate is attached to a terminus of the
oligomer,
thus elongating the oligomer. Also for this method, the man skilled in the art
will know
several possible formates, like the nick-translation approach, or the simple
primer
extension (J. Sambrook. E.F. Fritsch, T. Maniatis, Molecular Cloning - A
laboratory
Manual, Cold Spring Harbor Laboratory Press 1989).
A further subject of the invention is therefore a method for the enzymatic
synthesis of a
nucleic acid binding compound according to the invention comprising reacting a
triphosphate subunit with a primer using a nucleic acid as a template for the
elongation of
the primer, wherein the triphosphate subunit contains a heterocyclic group of
formula I.
Preferably, the triphosphate subunit has the formula VI. For example, 7- or 8-
substituted
7-deaza-2'-deoxyadenosine and guanosine-triphosphates can be easily
incorporated
enzymatically into DNA by various DNA polymerases (WO 00/68422).
A further subject of the present invention are therefore compounds of the
general formula
VI
Formula VI RAM'
B
A
R14
R15
CA 02569615 2006-12-18
-56-
wherein
A is selected from the group consisting of O, S, CHz and N-(Cl-C6)-allcyl,
M and M' are independently selected from the group consisting of o~cy,
sulfanediyl,
NRzz-, -(C1-CIO)-alkyl-, or -O-(C1-Coo)-alkyl-O-, and -S-(CI-Coo)-alkyl-O- and
_NRzz_(Ci_Cs)_~'1_O_~
Rzz is selected from the gxoup of -H, -(Cl-Cio)-alkyl, a protecting group and
a
reporter group,
R14 is selected from the group consisting of -H, -OR31, -(Cl-Cio)-allcoxy, O-
to protecting group, S-protecting group, NHz-protecting group, -(Cz-Clo)-
allcenyloxy, -(Cz-Clo)-aikymyloxy, -halogen, -azido, SH, -(Cl-Clo)-
alkylmercapto, and -O-solid phase,
R15 and R16 are independently selected from the group consisting of -H, -(Cl-
C6)-
~yh -(Cz-Cio)-allcenyl, -(Cz-Cio)-~Yh -(Cz-Coo)-~'1-carbonyl, -(Cs-Ci9)-
alkenyl-carbonyl, -(C3-C19)-alkynyl-carbonyl, -(C6-C14)-aryl-(Cl-Clo)-alkyl,
protecting group and a solid phase
B is the link to a moiety of formula I,
Formula t
HRH R1
N
N
N
R~HN x I
D
2o Ri is independent from X, Rz or R3 and is selected from the group
consisting of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO-
(5) -(Cl-Cio)-alkyl substituted according to (10)
(6) -(Cz-Coo)-allcenyl substituted according to (10)
(7) -(Cz-Clo)-alkynyl substituted according to ( 10)
(8) -(C6-Czz)-aryl substituted according to (10)
(g) W-(C~-Cio)-~Yh -W-(Cz-Cio)-alkenyl, -W-(Cz-Cio)-~Yh -W-(C6-
3o Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri [O-
CA 02569615 2006-12-18
- 57 -
(CHz)r]S-, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, allcenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NO2, -CN, -S-(Cl-C6)-alkyl, -NRSR6, -N+RSR6R'2, -OR12,
-CORM, -NH-CO-NR5R6, -NH-CS-NR5R6 and-(CHZ)"-[O-(CHZ)rJs
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein RI1 is selected from the group consisting of -NHRIZ, OR'Z, and -
to SR'2
wherein R5, R6 and R12 are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(C2-Clo)-alkenyl, -(Cz-Cio)-alkinyl, _
(C6-Cz2)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
15 hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
(I1)
O
Nuc-(CH2)~-N
O
O
Nuc-CH=CH-(CHZ)n N
0
O
Nuc-C-'-C-(CHz)"-N
O
wherein Nuc is the link to formula I and n is any integer from 1 to 18
20 said alkyl, alkenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Gl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NR5R6,
CORII, -NH-CONRSR6, -NH-CSNRSR6 and -(CH2)~ [O-(CHZ)rls NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
25 independently from r and s,
CA 02569615 2006-12-18
-58-
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5)
to (7) are substituted by -NRSR6, NHRIZ, ORIZ, or SRIZ;
Rz, R~ is independent from X, Rl> Rz and R3 and is selected from the group of,
(1) -H
(2) (CmCio)-alkyh
(3) (Cz-Clo)-allcenyl,
(4) (Cz-Cio)-~Yh
(5) (C6-Czz)-aryl,
(6) -Z-(Cl-Clo)-alkyl, -Z-(Cz-Clo)-alkenyl, -Z-(Cz-Clo)-alkynyl, -Z-(C6-
to Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)"-
[O-(CHz)r)s -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, allcenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
N02, -OR'z, -CN, -SH, -S-(Cl-C6)-alkyl, -NRSR6, -N+R5R6Rlz, _
CORM, -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)"-(O-(CHz)r]s
NR5R6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein R'1 is selected from the group consisting of -NHRIZ and
ORIZ,
wherein RS, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Cio)-alkyl, -(Cz-Clo)-alkenyl, -(Cz-Clo)-
alkynyl, -(Cb-Czz)-aryl and a reporter group,
said alkyl, allcenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted or substituted by one or more moieties selected from the
group consisting of -halogen, -SH, -S-(Ci-C6)-alkyl, -(Ci-C6)-alkoxy, -OH, -
NR5R6, -COR", -NH-CONR5R6, -NH-CSNR5R6 and -(CHz)n-[O-(CHz)r]s-
NRSR6, r and s axe independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s;
X is independent from Rl, Rz or R3 and is selected from the group consisting
of N
and CH
D is the position of attachment of the group to the rest of the nucleic acid
binding
compound.
CA 02569615 2006-12-18
-59-
whereby optionally at least one protecting group substitutes one or two
hydrogen
atoms of a -OH, -SH, NHZ, NH-alkyl, -NH-allcenylene, -NH-alkynylene, or a -NH-
aryl group, and any salts thereof.
Most preferred in these compounds -M'R16 is a triphosphate group and -MR'S is
OH. The
most preferred compound is the one in which R14 is -H.
Most preferred compounds for enzymatic synthesis of a nucleic acid binding
compound
according to the invention are of formula VIII
to
Formula VIII
PPP -O
R3g R14
wherein
PPP is a triphosphate group, a thiotriphosphate group or analogues thereof,
R'4 is selected from the group consisting of -H, -OH, -(Cl-Clo)-alkoxy,
-(C2-C,o)-alkenyloxy, -(CZ-Cloy-alkynyloxy halogen, -azido and NH2,
R36 is selected from the group of -H and -OH, and
B is a group of formula I.
2o
Formula i
HRH R1
N
N
N
x
R~NN
D
wherein
Rl is independent from X, R2 or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO-
CA 02569615 2006-12-18
-60-
(5) -(Cl-Clo)-allcyl substituted according to (IO)
(6) -(Cz-Coo)-alkenyl substituted according to (10)
(7) -(Cz-Clo)-allcynyl substituted according to (10)
(8) -(C6-Czz)-aryl substituted according to (10)
(9) -W-(Cl-Cio)-alkyl, -W-(Cz-Cloy-allcenyl, -W-(Cz-Cio)-~ynyh -W-(Cs-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-,
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri [O-
(CHz)r)s -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any allcyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-allcyl, -NRSR6, -N+RSR6Rlz, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NR5R6 and-(CHz)n-[O-(CHz)r)5-
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHRIZ, ORIZ, and -
SRlz
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Cio)-alkenyl, -(Cz-Clo)-alkinyl, _
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said allcyl, alkenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-C6)-allryl, -(Cl-C6)-alkoxy, -OH, -NR5R6, -
CORI', -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)n-[O-(CHz)~]S-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHRIZ, ORIZ, or SRIZ;
Rz, R3 is independent from X, R', R2 and R3 and is selected from the group of,
(1) -H
(2) (CI-Clo)-alkyl,
(3) (Cz-Clo)-alkenyl,
(4) (Cz-Cio)-alkynyl,
CA 02569615 2006-12-18
-61-
(5) (C6-Czz)-aryh
(6) -Z-(Cl-Clo)-~'h -Z-(Cz-Cio)-allcenyl, -Z-(Cz-Cio)-~yh -Z-(C6-
Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)"-
[O-(CHz)r)s -~ where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any allcyl, allcenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -OR12~ -CN~ -SH~ -S-(Ci-Cs)-~yh -NRSR6~ -N+R5R6Rlz, _
COR", -NH-CONR5R6, -NH-CSNRSR6 and -(CHz)"-(O-(CHz)T)5-
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein R" is selected from the group consisting of -NHR'z and
OR'2,
wherein R5, R6 and R'z are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-alkenyl, -(CZ-Clo)-
alkynyl, -(Ce-Czz)-aryl and a reporter group,
said alkyl, alkenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Cl-C6)-allcyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -COR", -NH-
CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHZ)r~s NR5R6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
X is independent from R', Rz or R3 and is selected from the group
consisting of N and CH
D is the position of attachment of the group to the rest of the nucleic
acid binding compound.
whereby optionally at least one protecting group substitutes one or two
hydrogen
atoms of a -0H, -SH, NHz, NH-alkyl, -NH-alkenylene, -NH-aIkynylene, or a -NH-
aryl group, and any salts thereof.
3' deoxy- and 2'-3'-didesoxytriphosphate subunits according to formula VIII
for example
can be used as terminating nucleotides in sequencing methods.
CA 02569615 2006-12-18
-62-
More preferable, above mentioned method for enzymatic synthesis uses as a
triphosphate
subunit a compound of formula VIII as defined above.
By the above methods, it is principally possible to introduce only one monomer
containing
the moiety of the invention into one nucleic acid binding component, but also
more than
one, as the case may be. This is especially possible using chemical methods
for the synthesis
of nucleic acid binding compounds,
These nucleic acid compounds according to the invention can be usefully
applied in
l0 hybridization methods. Therefore, a further subject of the invention is a
method for the
determination of a nucleic acid comprising the steps of providing a sample
suspected to
contain said nucleic acid, providing a nucleic acid binding compound, which is
essentially
complementary to a part or all of said nucleic acid, contacting said sample
with said nucleic
acid binding compound under conditions for binding said nucleic acid binding
compound
i5 to said nucleic acid, and determining the degree of hybridization or the
binding product
formed from said nucleic acid and said nucleic acid binding compound as a
measure of the
presence of said nucleic acid.
Methods for determination of nucleic acids by hybridization are generally
known, for
20 example from Sambrook et al. (cited above). They can easily adopted for the
use of probes
of the present invention.
Probes of the present invention also allow the determination of pathogens like
bacteria or
viruses, for example hepatitis A, B or C virus (HBV, HCV), the human
immunodeficiency
25 virus (HIV), the human papilloma virus or parvovirus B19. However, any
other viruses are
possible.
In a preferred embodiment of the invention, a nucleic acid binding compound,
hereinafter
termed a first nucleic acid binding compound, is used in a hybridization
reaction to form a
30 parallel or antiparallel duplex with a second nucleic acid binding compound
wherein the
first and/or the second nucleic acid binding compound comprise a backbone,
said
backbone having attached heterocyclic groups capable of base pairing to
nudeobases
characterized in that a heteroryclic groups, i.e, at least one of said
heterocyclic groups, is a
substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof. Preferably, the
hybridization
35 reaction is a multiplex hybridization reaction, i.e. multiple target
nucleic acids as second
nucleic acid binding compounds and multiple first nucleic acid binding
compounds are
CA 02569615 2006-12-18
-63-
present. This is done preferably in the form of an array, i.e. the first
nucleic acid binding
compound comprises a multitude of different nucleic acid binding compounds
with
different sequences and is attached in the form of an array to a solid phase
on different
addressable locations. In a further embodiment, a nucleic acid binding
compound is used
as a capture probe, whereby the nucleic acid binding compound has a backbone
whereto
heterocyclic groups capable of base pairing to nucleobases are attached
characterized in
that a heterocyclic group, i.e. at least one of said heterocyclic groups, is a
substituted
pyrazolo[3,4-d)pyrimidine or an analogue thereof. Most preferred the nucleic
acid binding
compound is a nucleic acid binding compound with a heterocyclic group of the
formula I
and the substituents described therefor. Most preferred are the halogen
substituents in the
7-position as e.g. brom and iod.
In another preferred embodiment, the substituted pyrazolo[3,4-d]pyrimidine or
the
analogue thereof is used in place of a heteroryclic group in a first nucleic
acid binding
compound to increase the melting temperature of a parallel or antiparallel
duplex with a
second nucleic acid binding compound whereby the increase in melting
temperature is
increased in comparison to the melting temperature of a duplex of the first
nucleic acid
binding compound with the second nucleic acid binding compound whezein the
heterocyclic group in the first nucleic acid binding compound is complementary
to a
heterocyclic group in the second nucleic acid binding compound. Preferably, a
natural
heterocyclic group as an adenine base is substituted in the first nucleic acid
binding
compound by the heteroryclic group according to the invention.
In another preferred embodiment, the substituted pyrazolo[3,4-d]pyrimidine or
the
analogue thereof is used in place of a heterocyclic group in a first nucleic
acid binding
compound used as a probe in an amplification reaction, to increase the melting
temperature of a duplex with a second nucleic acid binding compound in
comparison to
the melting temperature of a primer used in the amplification reaction,
whereby the
increase in melting temperature is compared to the melting temperature of a
duplex of the
3o first nucleic acid binding compound with the second nucleic acid binding
compound
wherein the heterocyclic group in the first nucleic acid binding compound is
complementary to a heterocyclic group in the second nucleic acid binding
compound.
Preferably, the amplification reaction is in the TaqMan~ format which is
described in more
detail below. Preferably, a naturally occuring heterocyclic group as an
adenine base is
substituted by the heterocyclic group according to the invention.
CA 02569615 2006-12-18
-64-
In another embodiment the substituted pyrazolo[3,4-d]pyrimidine or the
analogue thereof
is used in place of a heterocyclic group in a first nucleic acid binding
compound to
harmonize the contribution of each base pair to the melting temperature of a
parallel or
antiparallel duplex with a second nucleic acid binding compound. This is
particularly
interesting when other non-natural compounds are present which contribute to
the
melting temperature in the order of the contribution of the heteroryclic group
acccording
to the invention. Then it is of interest to use the heterocyclic group
according to the
invention to equalize (or harmonize) the contribution of each heterocyclic
group or base.
This has already been described supra. This use is particularly interesting
for multiplex
l0 reactions and in arrays.
In a further preferred embodiment, the substituted pyrazolo[3,4-d]pyrimidine
or the
analogue thereof is used in place of a heterocyclic group in a first nucleic
acid binding
compound for enhanced detection of sequences in a second nucleic acid binding
15 compound having mismatches in a duplex with the first nucleic acid binding
compound.
Preferably the second nucleic acid binding compound is a target nucleic acid
e.g. different
subtypes of a virus. The substituted pyrazolo(3,4-d]pyrimidine or the analogue
thereof can
in principle be positioned anywhere in the nucleic acid binding compound. This
use is of
particular interest when the second nucleic acid binding compound is a target
nucleic acid,
20 in particular a viral target, and different subtypes of a virus have to be
amplified and
detected. Therefore, in another embodiment of the invention the substituted
pyrazolo[3,4-
d]pyrimidine or an analogue thereof is used in place of a heterocyclic group
in a nucleic
acid binding compound for enhanced detection of subtypes in a target nucleic
acid.
25 In another embodiment, the substituted pyrazolo[3,4-d]pyrimidine or an
analogue thereof
is used in place of a heteroryclic group in a nucleic acid binding compound to
increase the
melting temperature of an intramolecular duplex or hairpin of the nucleic acid
binding
compound whereby the increase in melting temperature is compared to the
melting
temperature of the intramolecular duplex of the nucleic acid binding compound
wherein
30 the heteroryclic group in the nucleic acid binding compound is
complementary to a
heterocyclic group in the hybridizing part of the nucleic acid binding
compound. This is
particularly interesting in the Molecular beacons, Scorpion and TaqMan
technology
(W092102638 and corresponding US patents US 5,210,015, US 5,804,375, US
5,487,972)
when two fluorescent labels have to be brought into close proximity for
efficient
35 quenching.
CA 02569615 2006-12-18
-65-
Selecting the length of nucleic acid binding compounds or probes is also an
important
consideration when optimizing hybridization specificity. In general, shorter
probe
sequences are more specific than longer ones, in that the occurrence of a
single-base
mismatch has a greater destabilizing effect on the hybrid duplex. However, as
the overall
thermodynamic stability of hybrids decreases with length, in some embodiments
it is
desirable to enhance duplex stability for short probes globally. Therefore, in
a further
embodiment the substituted pyrazolo [3,4-d]pyrimidine or an analogue thereof
is used in
place of a heterocyclic group in a nucleic acid binding compound to reduce the
length of
the nucleic acid binding compound in detection reactions as the heterocyclic
group
to according to the invention has a high contribution to the melting
temperature and
therefore duplex stability of short probes.
Preferably, in the above described uses, at least one, preferably one or two,
reporter groups
are attached to the nucleic acid binding compound or a probe. Preferably, the
substituted
pyrazolo[3,4-d]pyrimidine or the analogue thereof used in place of a
heterocyclic group is 1
to 5 nucleotides separated from the point of attachment of one or of all of
the reporter
groups. Preferably, the substituted pyrazolo[3,4-d]pyrimidine or the analogue
thereof is
used in place of an adenine in the nucleic acid binding compound.
All uses described above are preferably performed in the form of multiplex
hybridization
reactions, i.e. multiple target nucleic acids as second nucleic acid binding
compounds and
multiple first nucleic acid binding compounds are present. This is done
preferably in the
form of an array, i.e. the first nucleic acid binding compound comprises a
multitude of
different nucleic acid binding compounds with different sequences and is
attached in the
form of an array to a solid phase on different addressable locations.
In all the uses of the invention, the substituted pyrazolo[3,4-d]pyrimidine
analogue is
preferably a substituted pyrazolo[3,4-d]pyrimidine analogue of adenine or
guanine or a 7-
. substituted pyrazolo[3,4-d]pyrimidine analogue of adenine or guanine,
wherein the
adenine or guanine analogues may preferably carry the same substituents RI in
the 7-
position or N-substituents Rz and R3 as set out directly below for the
substituted 7-deaza-8-
aza-2,6-diamino-purine or a derivative thereof or a 7-substituted 7-deaza-8-
aza-2,6-
diamino-purine or a derivative thereof. More preferably, the substituted
pyrazolo[3,4-
d]pyrimidine or an analogue thereof is a substituted 7-deaza-8-aza-2,6-diamino-
purine or
a derivative thereof ar a 7-substituted 7-deaza-8-aza-2,6-diamino-purine or a
derivative
thereof. Even more preferred, the substituted 7-deaza-8-aza-2,6-diamino-purine
or a
CA 02569615 2006-12-18
-66-
derivative thereof or the 7-substituted 7-deaza-8-aza-2,6-diamino-purine or a
derivative
thereof has the formula I with the substituents as defined in the following.
In the uses described above, in the most preferred embodiment the substituted
7-deaza-8
aza-2,6-diamino-purine or a derivative thereof or the 7-substituted 7-deaza-8-
aza-2>6
diamino-purine or a derivative thereof has the general formula I
Formula I
NR Rl
N
N
N/
X
R~HN
D
wherein
R' is independent from X, Rz or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) --COO-
is (5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(Cz-Clo)-alkenyl substituted according to (10)
(7) -(CZ-Cloy-alkynyl substituted according to (10)
(8) -(C6-C22)-aryl substituted according to (10)
(9) -W-(Cl-Clo)-alkYl, -W-(Cz-Cio)-alkenyl, -W-(CZ-Clo)-~ynyh -W-(Cs-
Cz2)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHZ)n [O-
(CHZ)~]s -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (S) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NO2, -CN, -S-(Cl-C6)-allcyl, -NRSR6, -N'~R5R6R'Z, -OR'Z,
-COR", -NH-CO-NR5R6, -NH-CS-NR5R6 and -(CHZ)"-[O-(CHZ)r]6-
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein R" is selected from the group consisting of -NHR'2, OR'2, and --
SR'z
CA 02569615 2006-12-18
-67-
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-allcenyl, -(Cz-Clo)-alkinyl, _
(C6-Cue)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said alkyl, alkenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH> -NRSR6, -
COR'1, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHZ)r]s-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or R12 is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHRIZ, ORIZ, or SRIZ;
Rz, R3 is independent from X, R', Rz and R3 and is selected from the group of,
(1) -H
(2) (Ci-Cio)-alkyh
(3) (Cz-Clo)-alkenyl,
(4) (Cz-Cio)-~Yh
(5) (C6-Cue)-aryl,
(6) -Z-(CmCio)-~'1~ -Z-(Cz-Cio)-alkenyl, -Z-(Cz-Cio)-~Yh -Z-(Cs-
Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)"-
[O-(CHz)~]e-, where r and s are, independently of each other, an
integer between I to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, alkenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NO2, -ORIZ, -CN, -SH, -S-(Cl-C6)-allcyl, -NRSR6, -N+RSR6Rlz, _
CORI', -NH-CONRSR6, -NH-CSNR5R6 arid -(CHz)n-[O-(CHz)r]$
NR5R6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -N~iRlz and
ORIZ,
wherein R5, R6 and Rtz are selected independently from the group
consisting of -H, -(C~-Clo)-alkyl, -(Cz-Clo)-allcenyl, -(C2-Clo)-
allcynyl, -(C6-Czz)-aryl and a reporter group,
CA 02569615 2006-12-18
_6g_
said alkyl, allcenyl, allcynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of
halogen, -SH, -S-(Cl-C6)-alkyl, -(C1-C6)-alkoxy, -OH, -NRSR6, -CORM, -NH-
CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)r]s NR5R6> r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
X is independent from Rl, Rz or R3 and is selected from the group consisting
of N
and CH; and
D is the position of attachment of the group to the rest of the nucleic acid
binding
compound
or any salts thereof.
In the most preferred embodiment, the nucleic acid binding compound is a
nucleic acid
binding compound according to the invention preferably wherein Rl=Br or Rl=I.
Nucleic acid binding compounds according to the present invention also can be
applied in
nucleic acid determination methods in the case the nucleic acid to be
determined is
amplified. Since the original publication of nucleic acid amplification,
various primer-
based nucleic acid amplification methods have been described including, but
are not
limited to, Ligase Chain Reaction (LCR, Wu and Wallace, 1989, Genomics 4:560-
569 and
Barany, 1991, Proc. Natl. Acad. Sci. USA 88:189-193); Polymerase Ligase Chain
Reaction
(Barany, 1991, PCR Methods and Applic. 1:5-16); Gap-LCR (PCT Patent
Publication No.
WO 90/01069); Repair Chain Reaction (European Patent Publication No. 439,182
A2), 3SR
(Kwoh et al., 1989, Proc. Natl. Acad. Sci. USA 86:1173-1177; Guatelli et al.,
1990, Proc.
Natl. Acad. Sci. USA 87:1874-1878; PCT Patent Publication No. WO 9210880A),
and
NASBA (U.S. Pat. No. 5,130,238). Further, there are strand displacement
amplification
(SDA), transciption mediated amplification (TMA), and Q(i-amplification (for a
review see
e.g. Whelen and Persing (1996). Annu. Rev. Microbiol. 50, 349-373; Abramson
and Myers,
1993, Current Opinion in Biotechnology 4:41-47). A preferred method is the
polymerase
chain reaction (PCR). The invention is also related to the amplification of
the target nucleic
acid in the presence of the triphophates of heterocyclic groups according to
the invention
as pyrazolo-[3,4-d]-pyrimidines, substituted variants thereof or analogues
thereof,
particularly preferred are 7-substituted variants thereof. Most preferred are
the
triphosphates of the heterocylic groups according to formula I with the
substituents as
defined above.
CA 02569615 2006-12-18
-69-
The nucleic acid binding compounds according to the present invention can be
used as
primers and probes as e.g. as a capture probe. In the case, that the nucleic
acid binding
compound should be used as probe, it will preferably contain a detectable
reporter group.
Any hybrids formed from the nucleic acid binding compound and a nucleic acid
can then
be determined via the detectable reporter group. This group of assays can
further be
divided into two groups, one being the group of homogeneous assays and the
other being
the heterogeneous assays. In heterogeneous assays, preferably the hybrid
(binding product)
will be determined when bound to a solid phase. This embodiment has the
advantage that
any excess of probe and other components can be removed easily from the
hybrid, thus
1o make the determination easier. The hybrid formed can be captured to a solid
phase either
covalently, noncovalently, specifically or unspecifically. There are several
embodiments
which are known to a man skilled in the art.
In the so-called homogeneous assays, the hybrid formed will not be bound to a
solid phase,
Is but will be determined either directly or indirectly in solution. A
preferred example of such
assays is disclosed in WO 92/02638.
In particular, when using several nucleic acid binding compounds, for example
when
conducting PCR-, multiplex-PCR- or multiplex-hybridization-methods it is often
difficult
2o to find appropriate hybridization conditions ensuring a good specificity
without loosing
some specific hybridization complexes resulted from a lower Tm, which also
means a lower
stability. In the case of diagnostic methods this can lead to false negative
results, which
should be avoided. A further di.~culty lies in the complexity of biological
samples, for
example blood or sputum. Such samples often have background nucleic acids,
which may
25 disturb the determination method, for example leading to false positive
results.
Therefore the heterocyclic groups of formula I can also be used in multiplex
hybridization
methods in order to increase the Tm of one or more hybridization complexes
formed in an
assay. By introducing a heterocyclic group of formula I instead of a natural
base contained
30 in a nucleic acid binding compound used in that assay the Tm of the
hybridization complex
formed with its target nucleic acid can be increased. Such changes of the Tm
still allows the
specific hybridization of the nucleic acid compound with its target nucleic
acid at a
different temperature. A preferred application field are multiplex
hybridization methods on
chips which often use hundreds to thousands hybridization probes. '
CA 02569615 2006-12-18
Also included in the present invention are intermediates and precursor
compounds for the
chemical synthesis of the described nucleic acid binding compounds. Preferred
intermediates and precursor compounds are described below.
Preferred is a solid phase bound precursor for the synthesis of a nucleic acid
binding
compound comprising a backbone , wherein the backbone comprises a moiety of
the
general formula VI
Formula VI RAM'
B
A
M R14
I
R15
1 o wherein
A is selected from the group consisting of O, S, CHZ and N-(Cl-C6)-alkyl,
M and M' are independently selected from the group consisting of oxy,
sulfanediyl, -
NRzz-, -(Cl-Cloy-~-~ or -O-(Cl-Clo)-~Yl-O-~ and -S-(Cl-Clo)-~'1-O- and
-NRzz-(Cl-Cs)-a~''1-O-~
is Rzz is selected from the group of-H, -(Cl-Clo)-alkyl, a protecting group
and a
reporter group,
R14 is selected from the group consisting of -H, -OR31, -(Cl-Clo)-alkoxy, O-
protecting group, S-protecting group, NH2-protecting group, -(Cz-Clo)-
allcenyloxy, -(Cz-Clo)-alkynyloxy, -halogen, -azido, SH, -(Cl-Clo)-
2o allcylmercapto, and -O-solid phase,
Rls and Rlb are independently selected from the group consisting of -H, -(Cl-
C6)-
alkyl, -(Cz-Clo)-alkenyl, -(Cz-Clo)-alkynyl, -(Cz-Clo)-~'1-carbonyl, -(C3-Cl9)-
allcenyl-carbonyl, -(C3-Cl9)-alkynyl-carbonyl, -(C6-CI4)-aryl-(Ci-Clo)-alkyl,
protecting group and a solid phase
25 B is the link to a moiety of formula I,
CA 02569615 2006-12-18
-71-
Formula I
Ri R~
N
N
N,
x
R~RN
D
wherein
Rl is independent from X, Rz or R3 and is selected from the group consisting
of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO'
(5) -(Cl-Clo)-aryl substituted according to (10)
(6) -(Cz-Clo)-alkenyl substituted according to ( 10)
l0 (7) -(Cz-Clo)-all~ynyl substituted according to (10)
(8) -(C6-Czz)-aryl substituted according to (10)
(9) W-(Cl-Clo)-~'h -W-(Cz-Cio)-alkenyl, -W-(Cz-Cio)-~Yh -W-(C6-
Czz)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-> -NH-CO-NH-, -NH-CS-NH-, -(CHz)ri (O-
(CHz)r]S -, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
(10) substituents (5) to (9) wherein any alkyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NRSR6, -N'~RSR6Ri2, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NR5R6 and -(CHz)"-(O-(CHz)r~s
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein Rl l is selected from the group consisting of -NHRIZ, ORIZ, and -
SRIz
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(C2-Cloy-alkenyl, -(Cz-Clo)-alkinyl, _
(C6-Cue)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridixes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
(11)
CA 02569615 2006-12-18
_72_
O
Nuc-(CHz)n-N
0
0
Nuc-CH=CH-(CHz)~ N
O
O
Nuc-C=C-(CH2)~-N
O
wherein Nuc is the Iink to formula I and n is any integer from 1 to 18
Rz, R3 is independent from X, RI, Rz and R3 and is selected from the group of,
(1) -H
(2) (CmCio)-~'h
(3) (Cz-Clo)-allcenyl,
(4) (Cz-Cio)-~Yh
(5) (C6-Czz)-aryl>
(6) -Z-(CmCio)-~Yl~ -Z-(Cz-Cio)-allcenyl, -Z-(Cz-Cio)-alkynyl, -Z-(C6-
l0 Czz)-aryl or Z-H> wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)n
[O-(CHZ)r)S -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or Z independently from r and s,
(7) substituents (2) to (6)
wherein any alkyl, alkenyl, alkynyl or aryl can be substituted by one
15 or more moieties selected from the group consisting of -halogen, -
NOz, -ORIZ, -CN, -SH, -S-(Ci-Cs)-alkyl, -NRSR6, -N+RSR6Rlz, _
COR", -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)"[O-(CHz)as-
NR5R6, r and s are ilidependently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
20 wherein Rll is selected from the group consisting of -NHR'z and
ORIZ,
wherein R5, R6 and Rlz are selected independently from the group
consisting of-H,.-(Cl-Clo)-alkyl, -(Cz-Clo)-allcenyl, -(Cz-Clo)-
alkynyl, -(C6-Czz)-aryl and a reporter group,
CA 02569615 2006-12-18
-
said alkyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted or
substituted by one or more moieties selected from the group consisting of -
halogen, -
SH, -S-(Cl-C6)-alkyl, -(C,-C6)-allcoxy, -OH, -NR5R6, -CORM, -NH-CONR5R6, -NH-
CSNR5R6 and -(CHz)"-(O-(CHZ)r~s-NRSR6, r and s are independently of each other
an integer of from 1 to 18 and n is 0 or 1 independently from r and s;
X is independent from Rl, RZ or R3 and is selected from the group consisting
of N
and CH
1o D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
with the proviso that one or two hydrogen atoms of any-0H, -SH, -NHZ, -NH-
alkyl, -NH-
allcenylene, -NH- alkynylene, or -NH-aryl group are substituted by a
protecting group, and
I5 any salts thereof. Such compounds of Formula VI can be used for chemical
synthesis of
nucleic acid binding compounds according to the invention as precursors. In
this case the
compounds are linked to a solid phase, preferred R14, Rls, or Ri6is O-solid
phase, most
preferred RIS is solid phase. It is also preferred that reactive groups are
protected by
protective groups.
Also included in the present invention are precursors and intermediates of a
nucleic acid
binding compound, wherein the backbone comprises a moiety of the general
formula III
B
Formula I11 A
~R14
M
I
R15
as
wherein
A is selected from the group consisting of O, S, CHz and N-(C1-C6)-alkyl,
CA 02569615 2006-12-18
-74-
M is selected from the group consisting of oxy, sulfanediyl, -NRZZ-, -(Cl-Clo)-
alkyl-, or -O-(Cl-Clo)-alkyl-O-, and -S-(Cl-Clo)-allcyl-O-, O-CO-, -NR22-(Cr-
C6)-alkyl-O-,
R22 is selected from the group of -H, -(C1-Clo)-alkyl, a protecting group and
a
S reporter group,
Rl4 is selected from the group consisting of -H, -OH, -(Cl-Cloy-alkoxy,
-(CZ-Cloy-alkenyloxy, -(CZ-Cloy-alkynyloxy, -halogen, -azido, SH, -(Cl-Clo)-
allcylmercapto, O-reporter group, O-solid phase and -NHz linked to a
protecting group,
Rls is selected from the group consisting of -H, -(Cl-C6)-alkyl, -(Cz-Clo)-
allcenyl, -
(Cz-Clo)-a~ynyl, -(Cz-Clo)-alkyl-carbonyl, -(C3-Clg)-alkenyl-carbonyl, -(C3-
Cl9)-alkynyl-carbonyl, -(C6-Cl4)-aryl-(Cl-Clo)-alkyl and a solid phase,
B is the link to a moiety of formula I,
Formula l
euc Ri
N~
N
N/
X
R~HN
IS D
wherein
R' is in dependent from X, RZ or R3 and is selected
from the group consisting of
( 1 ) -F, -Cl, -Br or -I,
(2) Nitro
(3) Cyano
(4) -COO-
(5) -(Cl-Clo)-allcyl substituted according to (
10)
(6) -(Cz-Clo)-alkenyl substituted according to
(10)
2S (7) -(CZ-Cloy-alkynyl substituted according to
(10)
(8) -(C6-C22)-aryl substituted according to (10)
(9) -W-(Cl-Cloy-alkyl, -W-(Ca-Clo)-alkenyl, -W-(C2-Cloy-~Yh
-W-(C6-
C22)-aryl or W-H, wherein W= -S-, -O-, -NH-,
-S-S-, -CO-, -COO-, -
CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHZ)rt
[O-
(CHz)r]S -, where r and s are, independently
of each other, an integer
between 1 to 18 and n is 0 or 1 independently
from r and s,
CA 02569615 2006-12-18
_75-
( 10) substituents (5) to (9) wherein any alkyl, allcenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NR5R6, -N+RSRbRI2, -ORIZ,
-CORII, -NH-CO-NRSR6, -NH-CS-NRSR6 and-(CHz)"-[O-(CHz)r)s-
NR5R6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein R' 1 is selected from the group consisting of -NHRiz, ORIZ, and -
SRIz
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Clo)-alkenyl, -(Cz-Cloy-alkinyl, -
(C6-Czz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
is (11)
O
Nuc-(CHZ)"~N
O
O
Nuc-GH=CH-(CH2)"-N
1 w
O
O
Nuo-C=C-(CH2)~ N
O
wherein Nuc is the link to formula I and n is any integer from 1 to 18
said alkyl, alkenyl, alkynyl or aryl in substituents (5) to ( 10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-allcoxy, -OH, -NRSR6, -
CORII, -NH-CONRSR6, -NH-CSNR5R6 and -(CHz)n (O-(CHz)T)6=NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHRIZ, ORIZ, or SRIZ;
CA 02569615 2006-12-18
-76-
R2, R3 is independent from X, Rl, RZ and R3 and is selected from the group of,
(1) -H
(2) (C~-Cn)-~Yh
(3) (Cz-Cio)-alkenyl,
s (~) (Cz-Cio)-~Yh
(5) (Cs-Cz2)-aryl,
(6) -Z-(Cl-Cio)-~Yh -Z-(C2-Cio)-alkenyl, -Z-(CZ-Cio)-~'nYh -Z-(CS-
C22)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHZ)"-
[O-(CHZ)r]$-, where r and s are, independently of each other, an
to integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
wherein any allcyl, alkenyl, allcynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOZ, -OR'z, -CN, -SH, -;S-(Cl-C6)-alkyl, -NRsRb, -N+R5R6R'2, _
15 CORM, -NH-CONRSR6, -NH-CSNR5R6 and-(CH2)~ [O-(CHZ)r]s-
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHRIZ and
OR'2,
20 wherein R5, R6 and R12 are selected independently from the group
consisting of -H, -(C1-Cloy-alkyl, -(CZ-Cloy-allcenyl, -(CZ-Clo)-
alkynyl, -(C6-Cz2)-aryl and a reporter group,
said alkyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
25 halogen, -SH, -S-(CI-Cs)-alkyl, -(Ci-C6)-alkoxy, -OH, -NRSR6, -CORM, -NH-
CONR5R6, -NH-CSNR5R6 and-(CH2)"-[O-(CHZ)rj8 NRSR6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
30 X is independent from Rl, RZ or R3 and is selected from the group
consisting of N
and CH
D is the position of attachment of the group to the rest of the nucleic acid
binding
compound
with the proviso that one or two hydrogen atoms of any-OH, -SH, -NH2, -NH-
alkyl, -
35 NH-alkenylene, -NH- alkynylene, or -NH-aryl group are substituted by a
protecting
group, and any salts thereof,
CA 02569615 2006-12-18
-77-
wherein R14 is O-solid phase or R15 is solid phase.
Solid phases for chemical synthesis of a nucleic acid binding compound
according to the
invention preferably also include linkers to fix the growing nucleic acid
binding compound.
Such linkers are known in the art. Preferably such linkers can be cleaved
after synthesis to
free said nucleic acid binding compound and can for example also be used to
generate a
free 3'-hydroxy group in said nucleic acid binding compound. Such linkers are
known in
the art, for example succinic acid linked via an amide bond to the solid phase
and via an
ester to the precursor or intermediate, Preferred R'S is solid phase, but in
the precursor for
to chemical synthesis of a nucleic acid binding compound according to formula
III
alternatively Rl~ may also be solid phase. Reactive groups of said compound
are preferably
protected by a protective group.
A more general formula of preferred precursors and intermediates according to
the present
15 invention are compounds comprising a backbone, said backbone having
attached
heteroryclic groups characterized in that a heterocyclic group is a group of
the general
formula I
Formula 1
HR2 Ri
N~
N
N/
R~HN
I
D
Rl is independent from X, RZ or R3 and is selected from the group consisting
of
20 (I) -F, -Cl, -Br or -I,
(2) I3itro
(3) Cyano
(4) -COO-
(5) -(Cl-Clo)-alkyl substituted according to (10)
25 (6) -(CZ-Clo}-alkenyl substituted according to (IO)
(7) -(CZ-Cloy-allcynyl substituted according to (10)
(8) -(C6-Cue)-aryl substituted according to (10)
(9) -W-(Ci-Cio)-~'1~ -W-(Cz-Cio)-alkenyl, -W-(Cz-Cio)-~Yl> -W-(C6-
C22)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-, -
3o CO-NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CH~)ri [O-
CA 02569615 2006-12-18
_7$_
(CHz)r]S-, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
( 10) substituents (5) to (9) wherein any alkyl, allcenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -NOz, -CN, -S-(Cl-C6)-alkyl, -NRSR6, -N+R5R6R12, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NR5R6 arid-(CHz)n [O-(CHz)r]s
NRSR6, r and s are independently of each other an integer of from 1 to 18
and n is 0 or 1 independently from r and s,
wherein Ril is selected from the group consisting of -NHRiz, ORIZ, and -
l0 SRIz
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Coo)-allcenyl, -(Cz-Clo)-alkinyl, _
(C6-Gzz)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
15 hybridizes to its complementary nucleic acid, attacks the latter while
binding, cross-linking or cleaving,
said alkyl, alkenyl, alkynyl or aryl in substituents (51 to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -
2o CORK, -NH-CONRSR6, -NH-CSNRSR6 and -(CHZ)n [O-(CHZ)r]S-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that R5, R6 or R'z is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHR12, ORIZ, or SRIZ;
25 Rz, R3 is independent from X, Rl, Rz and R3 and is selected from the group
of,
(1) H
(2) (Ci-Cio)-alkyl,
(3) (CZ-Clo)-allcenyl,
(4) (Ca-Cio)-~Yh
30 (5) (C6-Cz2)-aryl,
(6) -Z-(Cl-Clo)-alkyl, -Z-(CZ-Clo)-alkenyl, -Z-(Cz-Clo)-alkynyl, -Z-(C6-
CZZ)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)ri
[O-(CHZ)~]s -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
35 (7) substituents (2) to (6)
CA 02569615 2006-12-18
-79-
wherein any alkyl, alkenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -OR12~ -CN~ -SH~ -S-(CmCs)-~Yh -~5R6~ -N+R5R6Ria, _
CORM, -NH-CONRSR6, -NH-CSNR5R6 and-(CHz)n [O-(CHz)r~s
NRSR6, r and s are independently of each other an integer of from 1
to 18 and n is 0 or 1 independently from r and s,
wherein RI1 is selected from the group consisting of -NHRIZ and
ORIZ,
wherein R5, R6 and Rlz are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(Cz-Cloy-alkenyl, -(Cz-Clo)-
alkynyl, -(C6-Czz)-aryl and a reporter group,
said allcyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-allcoxy, -OH, -NRSR6, -COR'1, -NH-
CONR5R6, -NH-CSNRSR6 and -(CHz)"-[O-(CHz)r]S-NRSR6, r and s are
independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
X is independent from Rl> Rz or R3 and is selected from the group consisting
of N
and CH
2o D is the position of attachment of the group to the rest of the nucleic
acid binding
compound.
wherein said backbone is solid phase bound;
with the proviso that one or two hydrogen atoms of any-OH, -SH, -NHz, -NH-
allcyl,
-NH-alkenylene, -NH- alkynylene, or -NH-aryl group are substituted by a
protecting
group,
and any salts thereof.
Beside the possibility that the precursor compound or intermediate is coupled
to the solid
phase at the backbone, it can also be linked at the heterocyclic group of
formula I included
in said compound, for example using a -OH, -SH or -NHZ groups as attachment
site.
Preferably the other reactive groups of said compound are protected by
protective groups.
A preferred embodiment of the invention is a method for the determination of
the
presence, absence or amount of a nucleic acid comprising the steps of
providing a sample
CA 02569615 2006-12-18
-80-
suspected to contain the nucleic acid, providing a nucleic acid binding
compound
compound comprising a backbone, said backbone having attached heterocyclic
groups
capable of base pairing to nucleobases characterized in that a heterocyclic
group is a
substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof, which is
essentially
complementary to a part or all of the nucleic acid, contacting said sample
with the nucleic
acid binding compound under conditions for binding the nucleic acid binding
compound
to the nucleic acid, determining the binding product or the degree of
hybridization
between the nucleic acid and the nucleic acid binding compound as a measure of
the
presence, absence or amount of the nucleic acid.
A further embodiment of the invention is a metr~od for the determination of
the presence,
absence or amount of a nucleic acid wherein a nucleic acid binding compound is
used as a
capture probe, wherein the nucleic acid binding compound comprises a backbone,
said
backbone having attached heterocyclic groups capable of base pairing to
nucleobases
characterized in that a heterocyclic group is a sul>stituted pyrazolo[3,4-
d]pyrimidine or an
analogue thereof.
Yet another embodiment of the invention is a method for distinguishing related
nucleotide
sequences in a nucleic acid, the method comprising the steps of providing a
nucleic acid
binding compound comprising a backbone, said backbone having attached
heteroryclic
groups capable of base pairing to nucleobases characterized in that a
heterocyclic group is a
substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof and having a
defined
sequence, providing a nucleic acid with two related nucleotide sequences, each
of which
comprises a target sequence, wherein one of the nucleotide sequence is a
target sequence
that is perfectly complementary to the nucleic acid binding compound and at
least one
other of the segments is a related target sequence, incubating the nucleic
acid with the
nucleic acid binding compound under hybridization conditions, and determining
the
degree of hybridization between the nucleic acid binding compound and each of
the
segments.
Preferably any substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof is
located in
said compound to pair with dT and to increase the melting point of the nucleic
binding
compound hybridized to its complementary nucleic acid. The expert in the field
is aware of
the fact that the increase of the melting point of a nucleic acid binding
compound
according to the invention is influenced by the environment of the
heterocyclic group
CA 02569615 2006-12-18
- 81 .
according to the invention. Preferably however, the melting point is 4.5 to 7
°C higher,
preferably 5 or 5.5 to 7 °C, more preferably 6 to 7 °C than the
Tm of a dA-dT pair.
In a very preferred embodiment, in the methods according to the invention the
nucleic
acids are isolated from biological material, preferably from a human or an
animal.
Preferably the methods according to the invention are used in the diagnostical
field.
In still another embodiment of the invention a method as described above is
contemplated
wherein the nucleic acid binding compound according to the invention comprises
a
l0 reporter group which is a fluorescent label, preferably fluorescein.
Preferably, the nucleic
acid binding compound according to the invention comprises multiple
fluorescent labels
wherein the emission wavelengths of one of the fluorescent labels overlaps the
absorption
wavelengths of another of the fluorescent labels. The nucleic acid binding
compound may
further comprise a quenching agent which quenches the fluorescence emission of
the
15 fluorescent label, which can be fluorescein. Preferably the quenching agent
is a fluorescent
rhodamine or ryanine. Preferably the method further comprises the step of
altering the
spatial relationship between the fluorescent label and the quenching agent
subsequent to
hybridization, preferably by exonuclease hydrolysis of the nucleic acid
binding compound
whereby release of label occurs as a result of exonuclease hydrolysis. In a
preferred
20 embodiment, the degree of hybridization between the nucleic acid binding
compound and
the nucleic acid is determined by the quantity of label that is released from
the nucleic acid
binding compound subsequent to hybridization.
In a preferred embodiment of the invention, a method for distinguishing
related nucleotide
25 sequences is disclosed, wherein the related sequences preferably differ by
a single
nucleotide. Preferably, the degree of hybridization between the nucleic acid
binding
compound and the nucleic acid is determined by the priming ability of the
nucleic acid
binding compound, wherein most preferably priming occurs as part of an
amplification
reaction which may be an amplification reaction described above. The
amplification
30 reaction is preferably a polymerase chain reaction.
In methods for the determination of the presence, absence or amount of a
nucleic acid or
the method for distinguishing related nucleotide sequences more than one
nucleic acid
binding compound may be used, wherein the nucleic acid binding compound
comprises a
35 backbone, said backbone having attached heterocyclic groups capable of base
pairing to
nucleobases characterized in that a heterocyclic group is a substituted
pyrazolo [3,4
CA 02569615 2006-12-18
-82-
d]pyrimidine or an analogue thereof. Preferably, two nucleic acid binding
compounds are
used. In a preferred embodiment the first of the two nucleic acid binding
compounds
comprises a fluorescence donor and the second of the two nucleic acid binding
compounds
comprises a fluorescence acceptor, wherein the emission wavelengths of the
fluorescence
donor overlap the absorption wavelengths of the fluorescence acceptor. Then,
the degree of
hybridization can be measured by the quantity of light transferred between the
fluorescence
donor and the fluorescence acceptor and emitted by the fluorescence acceptor.
In another
embodiment the degree of hybridization is determined by the measurement of the
melting
temperature between the nucleic acid binding compound and the nucleic acid.
to
In yet another embodiment of the invention, a method for detecting the
presence of a
target sequence in a nucleic acid is disclosed, the method comprising the
steps of providing
a nucleic acid which is to be tested for the presence of the target sequence,
providing a
nucleic acid binding compound having a sequence that is substantially
complementary to
15 the target sequence and comprising a backbone, said backbone having
attached heterocyclic
groups capable of base pairing to nucleobases characterized in that a
heteroryclic groups is
a substituted pyrazolo[3,4-d]pyrimidine or an analogue thereof, incubating the
nucleic acid
and the nucleic acid binding compound under hybridization conditions; and
identifying
hybridized nucleic acids. Preferably, multiple nucleic acids are tested for
the presence of the
2d target sequence, whereby the nucleic acids have related target sequences.
Most preferably,
the nucleic acids differ from one another by a single nucleotide within the
target sequence.
Preferably, the nucleic acid binding compound is a primer comprising an
extendible 3'-
hydroxyl group. In a preferred embodiment, the hybridized nucleic acids are
identified by
extending the primer with a polymerizing enzyme, which can be a thermostable
enzyme
25 and wherein the nucleic acid binding compound is a primer in an
amplification reaction,
preferably a polymerase chain reaction. Preferably the thermostable enzyme is
the DNA
polymerase from Thermus aquafiicus, the so-called Taq-Polymerase.
In still another embodiment of the invention, a method for primer extension is
disclosed
3o which comprises the steps of providing a nucleic acid containing a target
sequence,
providing one or more nucleic acid binding compounds complementary to the
target
sequence, wherein nucleic acid binding compound comprises a backbone, said
backbone
having attached heterocyclic groups capable of base pairing to nucleobases
characterized in
that a heterocyclic group is a substituted pyrazolo[3,4-d]pyrimidine or an
analogue thereof,
35 providing a polymerizing enzyme and nucleotide substrates, and incubating
the nucleic
acid, the nucleic acid binding compounds, the enzyme and the substrates under
conditions
CA 02569615 2006-12-18
-83-
favorable for polymerization. Preferably, the method is part of an
amplification reaction,
most preferably a polymerase chain reaction. The method can be used in the
synthesis of a
cDNA molecule.
Another embodiment of the invention is a method for determining the nucleotide
sequence of a nucleic acid, the method comprising the steps of providing an
array of
nucleic acid binding compounds having different known sequences and comprising
a
backbone, said backbone having attached heterocyclic groups capable of base
pairing to
nucleobases characterized in that a heterocyclic group is a substituted
pyrazolo(3,4-
1o d]pyrimidine or an analogue thereof, with the proviso that the nucleic acid
binding
compounds do not contain a reporter group, incubating the nucleic acid with
the array
under hybridization conditions, and determining to which of the nucleic acid
binding
compounds in the array the nucleic acid hybridizes.
Still another embodiment of the invention is a method for determining the
nucleotide
sequence of a target sequence in a nucleic acid, the method comprising the
steps of
providing a nucleic acid comprising the target sequence, providing at least
two nucleic acid
binding compounds with a known sequence comprising a backbone, said backbone
having
attached heterorydic groups capable of base pairing to nucleobases
characterized in that a
heteroryclic group is a substituted pyrazolo[3,4-d]pyrimidine or an analogue
thereof, and
wherein one of the at least two nucleic acid binding compounds has a sequence
that is
perfectly complementary to the target sequence and at least one other of the
nucleic acid
binding compounds has a related target sequence, incubating the nucleic acid
binding
compounds with the nucleic acid under hybridization conditions, and
determining the
degree of hybridization between each of the nucleic acid binding compounds and
the
nucleic acid. Preferably, the at least one other nucleic acid binding
compounds has a single-
nucleotide mismatch with the target sequence.
Further, the invention contemplates a method for examining gene expression in
a cell, the
method comprising the steps of providing a population of nucleic acids
representative of
the genes expxessed in the cell, providing an array of nucleic acid binding
compounds
comprising a backbone, said backbone having attached heterocyclic groups
capable of base
pairing to nucleobases characterized in that a heteroryclic group is a
substituted
pyrazolo(3,4-d]pyrimidine or an analogue thereof, with the proviso that the
nucleic acid
binding compounds do not contain a reporter group, incubating the population
of nucleic
acids with the array under hybridization conditions, and determining which of
the nucleic
CA 02569615 2006-12-18
-84-
acid binding compounds in the array become hybridized to nucleic acids which
is
optionally labelled by incorporation of e.g. of adenosinetriphosphate coupled
to a label.
Still another embodiment of the invention is a method for identifying a
mutation in a
target sequence of a gene of interest, the method comprising the steps of
providing a
nucleic acid that comprises the target sequence, providing an array of nucleic
acid binding
compounds of different sequences, wherein the different sequences include the
wild-type
target sequence and different mutant target sequences, wherein the nucleic
acid binding
compounds comprise a backbone, said backbone having attached heterocyclic
groups
io capable of base pairing to nucleobases characterized in that a heterocyclic
group is a
substituted pyrazolo [3,4-d]pyrimidine or an analogue thereof with the proviso
that the
nucleic acid binding compounds do not contain a reporter group, incubating the
nucleic
acid with the array under hybridization conditions, and determining which of
the nucleic
acid binding compounds in the array become hybridized to the nucleic acid.
In all methods presented above the substituted pyrazolo [3,4-d]pyrimidine
analogue may be
preferably the substituted pyrazolo [3,4-d]pyrimidine or an analogue thereof
is a
substituted 7-deaza-8-aza-2,6-diamino-purine or a derivative thereof or a 7-
substituted 7-
deaza-8-aza-2,6-diamino-purine or a derivative thereof. In another embodiment
of the
invention the substituted pyrazolo[3,4-d]pyrimidine analogue is a substituted
pyrazolo[3,4-d]pyrimidine analogue of adenine or guanine or a 7-substituted
pyrazolo[3,4-
d]pyrimidine analogue of adenine or guanine, wherein the adenine or guanine
analogues
rnay preferably carry the same substituents Rl in the 7-position or N-
substituents RZ and R3
as set out directly below for the substituted 7-deaza-8-aza-2,6-diamino-purine
or a
derivative thereof or a 7-substituted 7-deaza-8-aza-2,6-diamino-purine or a
derivative
thereof.
In a preferred embodiment the substituted 7-deaza-8-aza-2,6-diamino-purine or
a
derivative thereof or the 7-substituted 7-deaza-8-aza-2,6-diamino-purine or a
derivative
thereof has the general formula I
CA 02569615 2006-12-18
_8$-
Formula I
ga Ri
N~
,
N
N/
X I
R~HN
wherein
D
Rl is independent from X, RZ or R3 and is selected from the group consisting
of
(1) -F, -Cl, -$r or -I,
s (2) Nitro
(3) Cyano
(4) -COO'
(5) -(Cl-Clo)-alkyl substituted according to (10)
(6) -(CZ-Cloy-alkenyl substituted according to (10)
(7) -(CZ-Cloy-allcynyl substituted according to (IO)
(8) -(C6-CZZ)-aryl substituted according to ( 10)
) w-(CmCio)-~Yh -W-(Ca-Clo)-allcenyl, -W-(CZ-Clo)-~Yh -W-(C6-
C22)-aryl or W-H, wherein W= -S-, -O-, -NH-, -S-S-, -CO-, -COO-,
CO~NH-, -NH-CO-, -NH-CO-NH-, -NH-CS-NH-, -(CHZ)"[O-
(CHZ)rJs-, where r and s are, independently of each other, an integer
between 1 to 18 and n is 0 or 1 independently from r and s,
( 10) substituents (S) to (9) wherein any allcyl, alkenyl, alkynyl or aryl can
be
substituted by one or more moieties selected from the group consisting of
-halogen, -SH, -N02, -CN, -S-(Cl-C6)-aIkyl, -NRSR6, -N+RSR6ltlz, -ORIZ,
-CORM, -NH-CO-NRSR6, -NH-CS-NRSR6 and-(CHZ)"[O-(CH2)rJs-
NRSR6, r and s are independently of each other an integer of from Z to 18
and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHRl2, OR12, and -
SRIz
wherein R5, R6 and RlZ are selected independently from the group
consisting of -H, -(Cl-Clo)-alkyl, -(CZ-Cloy-allcenyl, -(CZ-Cloy-alkinyl,
(C6-CZa)-aryl and a reporter group, a group which facilitates intracellular
uptake or a group that, when the nucleic acid binding compound
hybridizes to its complementary nucleic acid, attacks the latter while
3o binding, cross-linking or cleaving,
CA 02569615 2006-12-18
-86-
said alkyl, alkenyl, alkynyl or aryl in substituents (5) to (10) being
unsubstituted or substituted by one or more moieties selected from the group
consisting of -halogen, -SH, -S-(Cl-C6)-alkyl, -(Cl-C6)-alkoxy, -OH, -NRSR6, -
COR'1, -NH-CONRSR6, -NH-CSNR5R6 and -(CHz)n-[O-(CHz)r]6-NRSR6, r
and s are independently of each other an integer of from 1 to 18 and n is 0 or
1
independently from r and s,
with the proviso that RS, R6 or Rlz is not a reporter group if the radicals
(5) to
(7) are substituted by -NRSR6, NHRIZ, ORIZ, or SRIZ;
Rz, R3 is independent from X, RI, Rz and R3 and is selected from the group of,
io (1) -H
(2) (Ci-Gio)-a~'1~
(3) (Cz-Clo)-alkenyl,
(4) (Gz-Cio)-~Yh
(5) (G6-Czz)-aryl,
15 (6) -Z-(Cl-Cio)-allcyl, -Z-(Cz-Cio)-alkenyl, -Z-(Cz-Cio)-allrynyl, -Z-(C6-
Czz)-aryl or Z-H, wherein Z = -CO-, -CO-NH-, -CS-NH-, -(CHz)n
[O-(CHz)~]S -, where r and s are, independently of each other, an
integer between 1 to 18 and n is 1 or 2 independently from r and s,
(7) substituents (2) to (6)
20 wherein any alkyl, alkenyl, alkynyl or aryl can be substituted by one
or more moieties selected from the group consisting of -halogen, -
NOz, -ORIZ, -CN, -SH, -S-(C1-C6)-alkyl, -NR5R6, -N+R5R6R'z,
COR'1, -NH-CONRSR6, -NH-CSNRSR6 and-(CHz)n-[O-(CHz)r]s-
NRSR6, r and s are independently of each other an integer of from 1
25 to 18 and n is 0 or 1 independently from r and s,
wherein Rll is selected from the group consisting of -NHRIZ and
ORIZ,
wherein R5, R6 and R'z are selected independently from the group
consisting of -H, -(C1-Clo)-alkyl, -(Cz-Clo)-aIkenyl, -(C2-Clo)-
30 alkynyl, -(C6-Czz)-aryl and a reporter group,
said alkyl, alkenyl, alkynyl or aryl in substituents (2) to (7) being
unsubstituted
or substituted by one or more moieties selected from the group consisting of -
halogen, -SH, -S-(Cl-C6)-alkyl, -(CI-C6)-alkoxy, -OH, -NRSR6, -CORM, -NH-
CONRSR6, -NH-GSNRSR6 and -(CHz)n-[O-(CHz)r]5-NRSR6, r and s are
35 independently of each other an integer of from 1 to 18 and n is 0 or 1
independently from r and s;
CA 02569615 2006-12-18
_87_
X is independent from Rl, Rz or R3 and is selected from the group consisting
of N
and CH; and
D is the position of attachment of the group to the rest of the nucleic acid
binding
compound
or any salts thereof.
In still another preferred embodiment, the nucleic acid binding compound is a
nucleic acid
binding compound according to the invention, preferably R' is a hydrophobic or
electron-
withdrawing substituent as defined previously, preferably a halogen
substituent whereby Br
l0 or I is most preferred.
In all the methods or uses of the present invention the nucleic acid binding
compound
having incorporated a heterocylic group according to the present invention can
bind to an
opposite strand to form a parallel or antiparallel duplex.
The invention furthermoxe relates to pharmaceutical compositions comprising
one or
more nucleic acid binding compounds containing a heterocyclic group of formula
I,
together with a physiologically acceptable excipients and, where appropriate,
suitable
additives and/or conventional auxiliary substances. Therefore, one embodiment
of the
invention is a pharmaceutical composition comprising a nucleic acid binding
compound
according to the invention. Another embodiment is a nucleic acid binding
compound
according to the invention for use in medicine. In a quite general manner, the
present
invention extends to the use of such nucleic acid binding compounds in
therapeutically
effective compositions. Such compositions are understood to mean to include
the nucleic
acid binding compounds according to the invention as antisense
oligonucleotides, triple
helix forming oligonucleotides, aptamers or ribozymes, in particular antisense
oligonucleotides.
The invention further contemplates protecting groups derived from phthalic
acid (see e.g.
Ramzaeva et al. (1999). Nucleosides & Nucleotides 18,1439-1440; Rich B. Meyer
in
Methods in Molecular Biology 26 ( 1994), pp. 73-91, Humana Press Inc. Totowa,
NJ, USA;
Griffey et al. (1996). J. Med. Chem. 39, 5100-5109; Gibson and Benkovic
(1987). Nucl.
Acids Res. 15, 6455-6467). Aminoallcinyl or aminoalkenyl side chains of the
pyrazolo [3,4-
d]pyrimidin analogues of desoxyadenosine, desoxy-guanosine or desoxy
isoguanosine may
be protected with phthalic whereby an imide is formed. In contrast to the
trifluoroacetyl
protecting group which may be removed relatively easy from an oligonucleotide,
the
CA 02569615 2006-12-18
_$8_
removal of a phthaloyl group from aliphatic side chains is not easy as e.g.
for peptides.
Therefore, hydrazine would have to be used which cannot however be used in the
case of
oligonucleotides, as pyrimidine nucleosides are degraded. The amino groups of
alkynyl
compounds, in particular of propargylamine as well as those which are in allyl
position on
other positions in the chain are less basic. Thereby, a phthaloyl group may be
more easily
removed and no methyl amine has to be used. The deprotection is performed
under
standard conditions, i.e. concentrated ammonia solution at 60 °C, 12
hours. The advantage
of the phthaloyl group is that both H-atom positions of the amino group are
acylated. For
normal aryl groups as e.g. the tri~luoroacetyl (TFA) group used in US
5,151,507, the amino
l0 group is capped by acylation and is monofunctionalixed thereafter. Later
the TFA group is
xemoved whereby the acetyl group remains bound and is difficult to remove. The
advantage of the phthaloyl group for such puzposes is obvious.
Hence, in one embodiment of the invention a building block for the synthesis
of an
oligonucleotide comprising the nucleosides adenosine or desoxyadenosine,
guanosine or
desoxyguanosine, isoguanosine or desoxyisoguanosine, cytidine or
deoxycytidine, uridine
or desoxyuridine, thymidine or desoxythymidine, ox analogues of these
nucleosides
wherein a substituent is attached to the base moiety selected from the group
of
phthalimidoalkyl, phthalimidoallcenyl, or phthalimidoalkynyl groups with the
following
formulas
O
Nuc-(CH2)n-N
O
0
Nuc-CH=CH-(CH2)n N
O
O
Nuc-C=C-(CHZ)n-N
O
wherein Nuc is the position of attachment of the substituent to the base
moiety and n is
any integer from 1 to 18, with the proviso that the phthalimidoalkynyl or
phthaiimidoalkyl
group is not attached to the C5-atom of deoxyuridine and the proviso that the
phthalimidoalkynyl group is not attached to the C7-atom of 7-deaza-
deoxyguanosine. In a
CA 02569615 2006-12-18
-89-
preferred embodiment the analogue of these nucleosides is a pyrazolo[3,4-
dJpyrimidine
analogue. In a further preferred embodiment, the building block according to
the invention
is a phosphoramidite derivative.
Further the invention contemplates the use of a building block according to
the invention
for the synthesis of an oligonucleotide. Further, the use of a phthaloyl group
as a protecting
group is contemplated in a method for the synthesis of an oligonucleotide from
building
blocks comprising the nucleosides adenosine or desoxyadenosine, guanosine or
desoxyguanosine, isoguanosine or desoxyisoguanosine, cytidine or
deoxycytidine, uridine
or desoxyuridine, thymidine or desoxythymidine, or analogues of these
nucleosides
wherein the amino groups of -(CHZ)n--NH2, -CH=CH-(CHZ)ri NHZ, or -C---C-(CHZ)n
NHz
attached to the base moiety are derivatized with the phthaloyl group and n is
any integer
from 1 to 18, with the proviso that the -(CHZ)n-NHz or -C---C-(CHZ)n NHZ group
is not
attached to the C5-atom of deoxyuridine and the proviso that the -C-C-(CHz)n
NHZ
group is not attached to the C7-atom of 7-deaza-deoxyguanosine. In a preferred
is embodiment the analogue of these nucleosides is a pyrazolo[3,4-d]pyrimidine
analogue. In
a further preferred embodiment, the building block is a phosphoramidite
derivative.
In yet another embodiment of the invention, a method for the synthesis of an
oligonucleotide from building blocks according to the invention is disclosed.
These examples are intended to illustrate possible applications and should not
limit the
scope of the invention.
CA 02569615 2006-12-18
-90-
The present invention is explained in more detail by the following examples:
Examples
Example 1: Synthesis and data on the 7-bromo-8aza-7-deazapurin-2,6-diamine
nucleosides
1.1. Synthesis and Properties of Monomers
- The allcoxy nucleosides 4a,b ( 18J served as precursor for the synthesis of
the 8-aza-7-
deazapurin-2,6-diamine (pyrazolo[3,4-d)pyrimidin- 4,6-diamine) nucleosides
2a,b (purine
numbering is used throughout the discussion section) The amination was
performed in a
steel bomb (4 days, 25% aq. NH3, 70°). Both nucleosides (2a,b) were
isolated crystalline. A
few related 8-aza-7-deazapurin-2,6-diamine nucleosides have been prepared
earlier [I9 -
22J.
Treatment of compound 2a with adenosine deaminase (ADA) resulted in the
formation of
8-aza-7-deaza-2'-deoxyguanosine [18J. The reaction was followed W-spectrophoto-
metrically (Figure la).
The time-dependent spectra show two isosbestic points (~, = 234 rim and 267
nm)
indicating the conversion of the starting material into only one reaction
product. The
deamination of 2a occurs much slower than that of the purine nucleoside 1. The
7-bromo
derivative 2b [18J was not deaminated under these conditions even at high
enzyme
concentrations as it was observed earlier [20].
Scheme 1
CA 02569615 2006-12-18
-91-
0
H~ I \~
HzN ~N N
(~Ha)2~H R NHz R
0
N ~ I ~N o ~ I ~N H
HZN' _ N N aq. 25 /° NHg, 4 d HyN N N ADA HO
O 68 - 72% O Trls HCI, 20° O X
HO ~ HO
HN \\
HO Hp ~ ~ ~N
HZN N N
4a: R = H 2a: R = H HO
4b: R = Br 2b: R = Br
4c: R = I 2c: R = I
HO
X=Br,i
Next, the half lives of the N-glycosylic bonds of compounds 2a,b in acidic
medium were
measured and compared with that of compound 1. The reaction was performed in
0.5 N
HCl at room temperature and was followed UV-spectrophotometrically as well as
by
HPLC-analysis (Tablel),
Table 1. Half life Values (i) of 2'-Deoxyadenosine Derivatives in HCl at
25°.
i [min] a) ~,(nm) ~ Compound i [~n~ a) ~
6 252 I 2b Stable ") 242
2a 91 255 ~2b 87') 242
in 0.5 N HC1. °) Within 4 h. ') Measured in 2
d) Determined by HPLC.
From the data of the table it is apparent that the 8-aza-7-deazapurin-2,6-
diamine
nucleoside (2a) shows an about 15-fold higher glycosylic bond stability than
the parent 2-
amino-2'-deoxyadenosine (1). The glycosylic bond of 2b is stable under these
conditions.
Hydrolysis occurs when the reaction is performed in 2 N HCl (Table 1
).Furthermore, the
7-bromo substituent increases the lipophilic character of the molecule thereby
decreasing
ZS the chromatographic mobility of compound 2b, in comparison to compounds 2a
or 1 on
revexse phase HPLC (Figure lb).
In order to study the influence of the nucleobases on the N ~ S
pseudorotational
equilibrium of the sugar moiety, the 1H-NMR spectra of the nucleosides 1- 3
were
measured in DZO. The analysis was performed on the basis of five vicinal'H,1H
coupling
CA 02569615 2006-12-18
-92-
constants using the PSEUROT program [23]. According to Tale 2 the 8-aza-7-
deazapurin-
2,6-diamine nucleoside 2a shows a more pronounced N-conformer population than
the
corresponding purine nucleoside 1, while the N-type population of the related
7-
deazapurine nucleoside 3 is decreased. This is in line with an increase of the
!7-electron
deficiency of the 8-aza-7-deazapurine system. An additional effect of the
electron-
withdrawing 7-bromo substituent is not observed (2b) but was found for the
more
electron-attracting cyano group introduced into the 7-position of 8--aza-7-
deaza-2'-
deoxyguanosine [24]. The conformation around the C(4')-C(5') bond indicates
that the 8-
aza-7-deazapurin-2,6-diamine nucleosides 2a and b as the 8-aza-7-deaza-Z'-
1o deoxyguanosines [24], prefer the Y'-(-sc)-rotamer population, while for the
regular purine
nucleosides the ~y~+~g-(+sc)- or the Y~'~g-(ap)-conformation is predominant
[25].
Table 2. 3J (H, H) Coupling Constants of the Sugar Moieties and N/S-Conformer
Populations of the 2'-Deoxyribonucleosides 1- 3 at 303 K. a)
'JH,H/Hz Conformation
1',2'1',2"2',3'2",3'3',4'4',5'4',5"%N %S
Y~*~gY' Y~
~g
1 7.30 6.10 7.00 3.10 3.403.20 4.30 31 69 62 25 13
2a 6.60 6.80 6.90 3.70 3.604.00 5.80 37 63 36 42 22
2b 6.60 6.70 6.80 3.70 3.904.30 5.90 37 63 32 43 25
3 7.90 6.40 6.20 3.10 3.003.87 4.82 25 75 48 32 20
$) Solvent D20; r.m.s. < 0.4 Hz; ~ 0 Jm"~ ~ < 0.4 Hz.
In the past several laboratories have reported on a straightforward protection
protocol for
2-amino-2'-deoxyadenosine ( 1 ). Drastic hydrolysis conditions are necessary
for the
complete removal of benzoyl protecting groups [4] while the more labile
phenoxyacetyl
(pac) residues were removed without difficulty [26], [27). Nevertheless, the
formation of
2o the N-bis-acylated derivatives is encountered with difficulties due to the
monoprotection of
the molecule [28] and an increased tendency of the acylated derivatives to be
subjected to
depurination [4]. According to the observation that the nucleosides 2a,b are
significantly
more stable than the purine nucleoside 1 (Table 1) the N-aryl derivatives of
2a,b should
show similar properties. Therefore, the phenoxyacetyl derivative 5a as well as
the benzoyl
z5 compound 5b were prepared employing the protocol of transient protection
[29J. 2,4,5-
Tzichlorophenylphenoxy acetate [27] or benzoyl chloride [30] were used as
acylation
reagents The bis-phenoxyacylated derivative 5a was formed as it was described
for the
purine compound 1 [ 19]. However, the yield of 5a was rather low (30%). The
bis-
CA 02569615 2006-12-18
-93-
benzoylated nucleoside 5b was isolated in much better yield (63%) (Scheme 2;
a) 6a:
Pyridine; Me3SiCl, 2,4,5-trichlorophenylphenoxyacetate, 40°,12 h. 6b:
Pyridine, Me3SiCl,
PhCOCI, r.t., 12 h. b) Pyridine/(Me0)zTr-Cl, r.t., 4 h. c) THF, 2-cyanoethyl
diisopropylphosphoramidochloridite, r.t. 30 min). Both compounds (5a,b) were
converted
into the DMT-derivatives 6a,b using the standard reaction conditions [31].
Phosphitylation
in the presence of 2-cyanoethyl diisopropylphosphoroamidochloridite and
('Pr)ZEtN
afforded the phosphoramidites 7a,b [32]. However, as the benzoylated
derivative 6b is
poorly soluble in THF-solution, a large volume had to be used. According to
this the
phosphitylation was less effective and the yield of the phosphoramidite which
is normally
in the range of 80% was decreased to 67% (Scheme 2).
Scheme 2
NHR
N~ \
~\~N
RHN~N~ a~ b)
O 6a:25% 7a:43%
HO ~~~ 6b:49% 7b:67%
HO
5a: R = Pac 6a: R = Pac 7a: R = Pac
5b: R = Bz 6b: R = Bz 7b: R = Bz
Deprotection of compound 5a (25% aq. NH3, 40°, HPLC-monitoring) showed
that the first
pac-group is removed witlun 5 min, while the removal of the second pac-group
afforded 20
min. At r.t. a complete deprotection takes place in less than one hour. The
complete
removal of the two benzoyl groups of compound 5b (25% aq. NH3, 40°,
HPLC
monitoring) requires 8 - 9 h while the deprotection of the bis-benzoylated
purine
nucleoside 1 afforded several days [4] [7]. From this point of view the
benzoyl-protected
phosphoramidite 7b represents a useful. building block for the incorporation
of compound
2a in oligonucleotides. Nevertheless, the low solubility of the intermediate
6b represents a
problem.
In spite of this the synthesis of the N,N'-diallcyhnethyliden derivatives was
8a,b undertaken.
Original attempts to introduce N,N'-dimethylaminomethylidene residues into the
purine
nucleoside 1 failed due to the instability of the protecting groups [28J. The
N,N-
dibutylaminomethyliden group [33] for the protection of the exoryclic amino
function of
an 8-aza-7-deazapurine analogue of 2'-deoxyisoguanosine was used previously
[34]. The
CA 02569615 2006-12-18
-94-
same group was now introduced into the nucleosides 2a,b. The bis-amidines 8a,b
were
obtained as the major products (50% yield), while the mono adducts (9a,b) were
formed as
minor components (17%). The formation of the mono adduct might be circumvented
when the more vigorous conditions are used. For the protected nucleoside 8a,b
the time of
complete deprotection (conc, ammonia, 40°, HPLC-monitoring at 260 nm)
was
determined to be 440 min for 8a and 450 min for 8b. A half live value cannot
be given as a
mono-protected intermediate is formed.
Scheme 3
(n-Bu)ZNHC=N
N~ \
,N
~ N
(n-Bu)zNHC=N- -N~
HO
HO
Sa: R = H
8b: R = Br
a~ 10a:77%
10b: 61
(n-Bu)2NHC=
w
O H NON
a
11a: 53%
DMTO 11 b: 72%
NL(:HpGH20 N(i-Pry
10a:R=H 11a:R=H
lOb:R=Br 11b:R=Br
Subsequently, the 4,4'-dimethoxytrityl residues were introduced. After the
work-up of the
reaction followed by silica gel flash-chromatography one N,N'-
dibutylaminomethylidene
residue was hydrolyzed to give a formyl group (Scheme 3; a) Pyridine/(Me0)2Tr-
Cl, r.t., 4
h. b) THF, 2-cyanoethyl diisopropyl-phosphoramidochloridite, r.t., 30 min).
This group is
now protecting the 2-amino function, while the 6-amino group is still carrying
the
dibutylaminomethylidene residue. The structure of compounds l0a,b was
established on
the basis of NMR spectra. The amino group ( 10.77 ppm) and the proton of the
foxmyl
residue (9.56 ppm) are split in doublets with J = 9.88 Hz. This characteristic
coupling
CA 02569615 2006-12-18
-95-
pattern has already been observed for the formyl derivatives of 5-aza-7-deaza-
2'-
deoxyguanosine [35] and 2-amino-8-aza-7-deazapurine 2'-deoxynucleoside [36].
Phosphitylation of the DMT-derivatives l0a,b was performed in THF in the
presence of
chloro(2-cyanoethyl)-(diisopropylamino)phosphine furnishing the
phosphoramidites
lla,b (Scheme 3). These phosphoramidites as well as the corresponding building
block 7b
carrying benzoyl protecting groups can be efficiently used in solid-phase
oligonucleotide
synthesis resulting in high coupling yields. All compounds were characterized
by 1H-,13C-,
and 31P-NMR spectra and by elemental analysis (Table 3 and experimental part).
1o Table 3.13C-NMR Chemical Shifts of Pyrazolo[3,4-d]pyrimidine 2'-
Deoxyribonucleosides
a~
C(2)'C(4)) C(5) C(6))C(7) C=O C=O C(1'C(2'C(3'C(4' C(5'
) C(7a) C(3a C(4)C(3) / / ) ) ) ) )
C(6) ) =CH =CH
')
2a 156.9158.3 95.5 162.7133.3--- --- 83.338.071.387.4 62.7
2b 157.4157.6 94.5 162.7119.2--- --- 83.037.570.987.3 62.4
5a 152.2154.8 100.5156.1136.1168.5169.183.637.771.187.7 62.5
5b 153.3155.2 102.2156.1134.0165.6166.383.537.670.987.5 62.3
6a 152.2154.8 100.5155.9135.9168.5169.283.738.170.885.6 64.3
6b 153.3155.3 102.3156.1132.2165.7166.483.738.070.985.6 64.4
8afl 157.1157.6 106.0158.9134.9164.1166.286.141.273.789.1 64.3
8b 156.1156.1 103.6157.9121.4162.1164.483.337.570.887.5 62.2
8b ~ 157.1157.7 104.7159.2122.9163. 166.786.241.073.889.0 64.2
9 156.6157.3 102.4162.3133.7162.9--- 83.137.871.287.3 62.6
e)
l0afl 155.5155.5 107.1156.8134.9161.8163.784.238.373.686.7 64.9
10b 156.0156.1 103.8157.8121.3162.1164.483.5. 70.585.3 64.1
e)
a) Measured ) )
in Purine Systematic
(D6)DMSO numbering. numbering.
at
298
K.
d) Tentative. ) Superimposed DMSO. ured
by f) in
(D6) Meas CDCl3.
1.2. Oligonucleotides
1.2.1 Synthesis and Characterization. - Automated solid-phase synthesis of the
oligonucleotides 102 - 116 (Tables 4 - 6) was performed using the
phosphoramidites 7b
and l lb as well as standard phosphoramidites. The oligonudeotides were
detritylated and
purified on oligonucleotide purification cartridges or by reversed-phase HPLC
(conditions
for puzification see experimental part). The homogeneity of the compounds was
proven by
ion-exchange chromatography on a 4 X 250 mm NucleoPac PA-100 column (DIONEX,
CA 02569615 2006-12-18
-96-
P/N 043018, USA). The composition of the oligonucleotides was determined by
tandem
hydrolysis with snake venom phosphodiesterase and allcaline phosphatase as
described (see
Figure 2a,b) [2].
The 8-aza-7-deazapurin-2,6-diamine nucleosides 2a migrates slightly slower
than dA. The
bromo compound 2b is much more hydrophobic as it can be seen from the Figure
2b.
From the modified oligonucleotides MALDI-TOF mass spectra were taken (Table 6,
Experimental part). The correct mass was found in all cases which underlines
that all
protecting groups were split off during a 10 h ammonia treatment at
60°.
1.2.2 Duplex Stability
Because of the presence of three hydrogen bonds the n2Ad-dT base pair (II) is
expected to
show the same or a similar stability as a dG-dC pair (III). Experimental data
obtained from
DNA-melting experiments show that the thermodynamic stability of duplexes
containing
an n2Ad-dT base pair is somewhat higher than that with a dA-dT pairs but still
remains far
below that of a dG-dC pair. Various studies have been performed to explain
this dilemma
but a convincing answer has not been given. A rather detailed examination of
this matter
has been undertaken by Sagy et al [ 11]. This author compared the thermal
stabilities of a
bidentate base pair represented by the motifs dA-dU, dI-dC, dA-dT and dI-mSCd
with
those of tridentade pairs such as n2Ad-dT, dG-dC, dG-mSCd and others. The
experiments
were performed with alternating polynucleotides synthesized enzymatically. As
long as a 2-
amino group was absent (bidentate bases pairs), the various duplexes show all
a very
similar stability independently from the particular base structure. By
insertion of a 2-amino
group, which leads to the tridentate base pair this similarity disappears. The
dG-dC base
pair is now much more stable than the nZAd-dT pair. The authors noted also a
significant
effect of the 5-substituents attached to the pyrimidine moiety; while the
presence of a 5-
methyl group results in a rather small stabilization; larger alkyl groups
destabilize the DNA.
Scheme 4
H H
'N N-H ~~~~ O CH3 ~~~~ CH3
N ~~N ~~~~ H-N~ ~> , H-.
N h--N
O ~ ~~~~p
Watson Crick Base Pair I Watson Crick Base Pair Il
CA 02569615 2006-12-18
_97_
H
.. H-N ~... p CH3
~ .... N~ ~> , H_
h--N
.. ~~ O ....0
H
Watson Crick Base Pair III Watson Crick Base Pair IV
R = H, Br
In order to evaluate the influence of the 8-aza-7-deazapurin-2,6-diamine
nucleosides 2a,b
on the base pair stability in comparison to the purine nucleoside 1 all three
compounds
were incorporated into the non-self complementary duplex f -d(TAGGTCAATACT) (
102)
and 5'-d(AGTATTGACCTA) ( 103). This duplex is used as a standard in our
laboratory to
study the influence of modified nucleosides on the thermal stability and the
structural
behavior of the helical formation. The Tm-value of (102~I03) is 46° in
0.1 M NaCI in the
presence of 10 mM MgCl2 and 10 mM Na-cacodylate buffer. The incorporation of
six
nZAa-residues instead of six dA residues increases the Tm value by only
4° (see duplex
104~105, Table 4 ). This corresponds to a 0.7° Tm increase per residue.
Similar findings
have been reported from experiments performed in other laboratories [5 - 12].
When four
8-aza-7-deazapurin-2,6-diamine nucleosides 2a were replacing dA an increase of
the Tm-
value already from 46° to 52° was measured, which corresponds to
a 1.5° increase per
modified residue (compare duplexes 102~103 vs. 108~109). As the stability
increase caused
by an n2Ad-residue was only 0.7°, the 8-aza-7-deazapurin-2,6-diamine
forms a more stable
tridentate base pairs with dT than that of the purin-2,6-diamine.
Table 4. T~, Value and Thermodynamic Data of Antiparallel Stranded
Oligonucleotides
Containing Purin-2,6-diamine 2'-Deoxyribonudeoside and Related Pyrazolo[3,4-d]-
pyrimidine Analogs. e)
Tm 0H° ~S° DG°31o
[°C] [kcal/mol] [cal/mol. K] [kcal/mol]
5'-d(TAGGTCAATACT) (102) ~ (103) 46 -86 -230 -10.3
3'-d(ATC CAG TTA TGA)
5'-d(T1G GTC 11T 1CT) (104) ~ (105) 50 -58 -155 -11.2
3'-d(ATC C1G TTl TGA)
5'-d(TAG GCC GGC ACT) (106) ~ (107) 65 -92 -247 -16.0
CA 02569615 2006-12-18
-98-
3'-d(ATC CGG CCG TGA) '
5'-d(TAG GTC 2a2aT ACT) (108) ~ (109) 52 -105 -299 -12.7
3'-d(ATC C2aG TT2a TGA)
5'-d(TAG GTC 2b2bT ACT) (110) ~ (111) 68 -110 -297 -17.9
3'-d(ATC C2bG TT2b TGA)
5'-d(TAG GTC 2b2bT ACT) (110) ~ (103) 56 -85 -232 -12.9
3'-d(ATC CAG TTA TGA)
5'-d(TAG GTC AAT ACT) (102) ~ (111) 58 -88 -239 -13.4
3'-d(ATC C2bG TT2b TGA)
a) Measured at 260 nm in 0.1 M NaCI, 0.1 M MgCIZ, and 10 mM Na-cacodylate
buffer, pH 7.0
with 5 + 5 ltM oligomer concentration.
It has been shown that 7-substituents of 7-deazapurines as well as of 8-aza-7-
deazapurine
are well accommodated in the major.groove of DNA [1-3]. In particular, halogen
substituents show very favorable properties with regard to duplex stability.
This prompted
us to replace dA-residues now by the 7-bromo derivative 2b. According to Table
4 the
duplex (20~21) shows a remarkable stability. The Tm-value was 68°
compared to 46° of the
duplex with dA-dT pairs. The duplex stability is strengthened by 5.5 °
per modified base.
The duplex (106~107) containing four dG-residues at the same positions lead to
a Tm-value
of 65° which corresponds to a 5° Tm-increase. Thus, the
stability of a base pair motif IV
to (Scheme 4) which follows a dA-dT recognition motif is now as stable as a dG-
dC pair (base
pair III).
An explanation for this unexpected observation can be given on the basis of
the basicity
differences of the amino groups of the 8-aza-7-deazapurin-2,6-diamine
nucleosides 2a,b
compared to that of the purin-2,6-diamine nucleoside 1. As discussed above the
third
15 hydrogen bond of compound 1 contributes very little to the nZAd-dT base
pair stability
(Scheme 4, base pair II) while the 2-amino group of dG makes a large
contribution to the
stability of a dG-dC pair. With regard to the chemical structure of the
molecule, both, the
amino group of dG and isoGd are part of an actylated guanidine moiety which is
expected
to be considerably less basic than the 2-amino group of n2Ad which is also a
part of
2o guanidine system being now aromatic but is not connected to an electron-
attracting
moiety. These differences cause the low acidity of the 2-amino group of 1
compared to dG
or isoGd. This property is already noticeable in the extraordinary resistance
of the arylated
2-amino group of 1 against alkaline deprotection (see above). Next, the
question has to be
answered why compounds 1 and 2a,b are different. Contrary to compound 1, one
can draw
CA 02569615 2006-12-18
-99-
mesomeric structures of the base of compounds 2a,b with a positive charge
either at the 2-
or the 6-amino group and a negative one at the pyrazole nitrogen-2.
These mesomeric formulas explain the acidity (reduced basicity) of the 2-amino
group of
2a over that of 1. When a bromo or other electron-attracting substituents are
introduced at
the 7-position this effect is even strengthened. Thus, the 2-amino-group of 2a
will become
a better donor for hydrogen bonds with dT than the 2-amino group of 1. The
additional
bromo substituent can cause several favorable effects: (i) It acidifies the 2-
amino group
further; (ii) it represents a hydrophobic group in the major groove of the DNA-
duplex; (iii)
it can form a hydrogen bond with the 2-amino group. Apart from these
properties of the
l0 monomeric units the environmental conditions of the base within the nucleic
acid duplexes
have to modulate the stability of the n2A-dT base pairs. Otherwise, it cannot
be explained
why the stability of such a base pair is different in DNA-duplexes, DNA-RNA
hybrids or
PNA-duplexes.
Scheme 5
O NH2
HN ~ ~ N/
H2N- 'N N H2N- -N N
NH2 r NH2 Br ~ H2 r
N'~ ~ \ N ~ ~' N i
~N ~ ~ ~ ~ ENO ~> ~ ENO
HZN N N H2N N N H2N N N
Yet, it has not been proven whether the Tm value increases linearly with the
number of
incorporated 8-aza-7-deazapurine residues. In the case of compound 1 such a
study has
been undertaken, which showed that in poly(dA-dT) a linear relationship exists
when the
replacement of dA by the n2Aa-residues did not exceed 50/0. However, there was
no further
Tm-increase at higher n2Aa- incorporation when the Tm-values were measured at
low salt
concentration. A similar observation as made on synthetic oligonucleotides was
obtained
for DNA of the ryano phage S-2L which contains nZAdinstead of dA [38].
CA 02569615 2006-12-18
- 100 -
In order to identify the helical structure of the duplexes CD-spectra of the
duplexes
102~x03 to 110~111 were measured [39J. The CD-spectra of all duplexes indicate
a B-like
DNA structure, with a positive Cotton effect around 270 nm to 290 nm and a
negative lobe
around 250 nm (see Figure3a).
It has been reported that the purine nucleoside 1 forms more stable duplexes
with
complementary RNA than with DNA [40J. Therefore, oligonucleotides containing 1
or 2a,b
were hybridized with a complementary oligoribonucleotide 114 (Table 5).
According to
Table 5 the DNA-RNA hybrid 104~114 shows a Tm increase over that of the parent
l0 102~114. The 8-aza-7-deazapurin-2,6-diamine residue 2a shows a similar
effect as that of 1.
An additional stabilization is observed when the bromo compound 2b was
incorporated.
All CD-spectra of the DNA-RNA hybrids (see Figure 3b) adopt the form of an A-
DNA.
Table 5. Tm Value and Thermodynamic Data of RNA-DNA and DNA - DNA hybrids.a)
Tm eHo QS~ ~Gp310
[kcai/molJ [~mol ~ [k~umol)
Kl
5'-d(TAG GTC AAT ACT) (102) ~ (114) 45 -92 -264 -10.2
3'-(AUC CAG UUA UGA)
5'-d(T1G GTC 11T 1CT) (104) ~ (114) 48 -60 -162 -11.0
3'-(AUC CAG UUA UGA)
5'-d(TAG GTC 2a2aT ACT) (108) ~ (114) 48 -89 -263 -11.0
3'-(AUC CAG UUA UGA)
5'-d(TAG GTC 2b2bT ACT) (110) ~ (114) 53 -93 -258 -12.4
3'-(AUC CAG UUA UGA)
a) See Table 4.
Finally, the base pairing properties of the nucleosides l and 2a,b were
studied in parallel-
stranded DNA (ps-DNA) [37J, [42J. For this purpose it was necessary to
exchange the dC-
dG pair by a mSiCa-dG and/or dC-iGd pair. As standard duplexes the hybrids
(102~115) or
(116~103) were chosen. In both series of experiments the purin-2,6-diamine (1)
destabilized the ps-hybrids (104~I15 and 116~105), The nucleoside 2a already
led to
slightly more stable duplexes (see Table 6). As in the case of antiparallel
DNA the bromo
derivative 2b resulted in a significant increase of the Tm value (110~115 and
116~111). The
CA 02569615 2006-12-18
-101=
two sets of CD-spectra (Figure4a,b) of the ps-duplexes are rather different to
those of aps-
hybrids (see Figure4a).
From these results it is apparent that the 2-amino group causes
destabilization in the series
of purine compounds and reduces the stabilizing effect observed in aps-DNA
significantly.
This behavior is not surprising at it was already observed that mono-
substituted purin-2-
amine or 8-aza-7-deazapurin-2-amine have a rather unfavorable influence on the
stability
of the ps-DNA [36J [42J.
Table 6. Tm value, Thermodynamic Data of Parallel Stranded Oligonucleotides
Formed by
to Oligonucleotides Containing iGd and mSiCd. a~ b)
Tm ~H° C~S° ~G°3~o
(kcal/mol] [~]/moI ~ [kcal/mol
KJ 1
5'-d(TAGGTCAATACT) (102)(115)39 -74 -211 -8.8
[37J
5'-d(ATiC iCAiG TTA TiGA)
5'-d(T1G GTC 11T 1CT) (104) 36 -76 -200 -7.9
(115)
5'-d(ATiC iCAiG TTA TiGA)
5'-d(TAG GTC 2a2aT ACT) (108)41 -62 -171 -9.0
(115)
5'-d(ATiC iCAiG TTA TiGA)
5'-d(TAG GTC 2b2bT ACT) (110)45 -67 -184 -10.2
(115)
5'-d(ATiC iCAiG TTA TiGA)
5'-d(TiCA TAA iCTiG iGAT) 44 -85 -242 -10.3
(116)
(103)[37J
5'-d(AGT ATT GAC CTA)
5'-d(TiCA TAA iCTiG iGAT) 39 -61 -170 -8.4
(116) (105)
5'-d(AGT 1TT G1C CTA)
5'-d(TiCA TAA iCTiG iGAT) 45 -80 -230 ' -10.3
( 116) ( 109)
5'-d(AGT 2aTT G2aC CTA)
5'-d(TiCA TAA iCTiG iGAT) 48 -68 -186 -10.9
(116) (111)
5'-d(AGT 2bTT G2bC CTA)
a) Measured at 260 nm in 1 M NaCl, 0.1 M 12, 60 mM Na-cacodylate buffer, pH
7.0 with
5 + 5 p.M oligomer concentration. b) d(iC) = mSiCd = 2'-deoxy-5-
methylisocytidine.
CA 02569615 2006-12-18
- 102 -
The two amino groups of the 8-aza-7-deazapurine nucleosides 2a,b can be
protected with
either benzoyl residues or dibutylaminomethylene groups without causing
depurination
problems during the acidic detritylation or the deprotection under standard
conditions in
ammonia. The problems of depurination and an extraordinary high stability of
the 2-
amino protecting groups of the purin-2,6-diamine nucleoside 1 is not observed.
1.3 Experimental Part
Monomers: General. See [2]. Flash chromatography (FC): 0.4 bar on silica gel
60 H
Merck, Darmstadt, Germany). Thin-layer chromatography (TLC): Aluminium sheets
silica
gel 6O F254 (0.2 mm, Merck, Germany). TLC Scanning: CS-930 TLC scanner
(Shimadzu,
Japan).Solvent systems for flash chromatography (FC) and TLC: CHzCl2 / MeOH
98:2 (A)
CHZCl2 f MeOH 95:5 (B), CHZC121 MeOH 9:1 (C), CHZCl2 ! acetone 95:5 (D),CHZClz
l
acetone 9:1 (E), CHZC12 / EtOAc 85:15 (F). M.p.: Biichi-SMP-20 apparatus
(Biichi,
Switzerland); uncorrected. NMR Spectra: Avance -DPX-250 and AMX-500
spectrometers
(Broker, Germany); 8 values are in ppm downfield from internal SiMe4 (1H, mC).
Microanalyses were performed by Mikroanalytisches Labor Beller (Gottingen,
Germany).
Oligonucleotides:
Synthesis and purification of Oligonudeotides 102 -116. The synthesis was
carried out on
an automated DNA synthesizer (Applied Biosystems, model ABI 392-08 for
phosphoramidite chemistry) in a lpmol scale with 3'-phosphoramidites of
[(Me0)ZTrJibZGd, [(Me0)2Tr]bz6Ad, [(Me0)ZTr]bz4Cdand [(Me0)2Tr]Td,togetherwith
the phosphoramidites of the derivatives 7b and l lb. The synthesis of the
oligomers
followed the regular protocol of the DNA synthesizer for phosphoramidites.
After cleavage
from the solid-support, the oligonucleotides were deprotected in 25% NH3/H20
(12 -15 h
at 60°). The purification of the 5'-(dimethoxytrityl)-oligomers were
performed by OPC
cartridges as well as by reversed-phase HPLC (RP-18). The following solvent
gradient was
used (A, MeCN; B, 0.1 M (Et3NH )OAc (pH 7.0)/ MeCN 95:5): 3 rnin 20% A in B,
12 min
15 - 40% A in B with a flow rate of 1.0 ml/min. To remove the 4;4'-
dimethoxytrityl
residues they were treated with 2.5% CHCIzCOOH/CHZC12 for 5 min at r.t. The
detritylated oligomers were purified by reversed-phase HPLC with the gradient:
20 min 0 -
20% A in B with a flow rate of 1 ml/min. The oligomers were desalted on a
short column
(RP-18, silica gel) using H20 for the elution of the salt, while the oligomers
were eluted
with MeOH/HZO 3:2. The oligomers were lyophilized on a Speed-Vac evaporator to
yield
colorless solids which were frozen at -24°.
Table 7, Molecular Masses M+ of Oligonucleotides 12,13 and 18 - 21 determined
by
CA 02569615 2006-12-18
-103-
MALDI-TOF Mass Spectroscopy
Oligomer M+ (talc.) M+ (found)
5'-d(TAG GTC AAT ACT) ( 102) 3644.4 3645
5'-d(AGT ATT GAC CTA) 3644.4 3645
( 103)
S'-d(TAG GTC 2a2aT ACT) 3674.4 3677
(108)
5'-d(AGT 2aTT G2aC CTA) 3674.4 3675
(109)
5'-d(TAG GTC 2b2bT ACT) 3832.5 3830
(110)
5'-d(AGT 2bTT G2bC CTA) 3832.5 3832
(111)
The MALDI-TOF-spectra were measured on a Biflex III by Dr. T, Wenzel (Broker
Saxonia,
Germany). Nucleoside-Composition Analysis. The analyses were performed as
described in
[37]. Extinction coefficients of the nucleoside constituents: sz6fl: dA 15400,
dT 8800, dG
11700, dC 7600, iGd 7400, mSiCd 6300, 2a 8800, 2b 8700. Snake-venom
phosphodiesterase
(EC 3.1.4.x., Cratallus durissus) and alkaline phosphatase (EC 3.1.3.1., E.
coli) were
obtained from Roche Diagnostics GmbH, Germany.
Determination of Tm Values and Thermodynamic Data. Absorbance vs. temperature
profiles were measured on a Cary-1llE IIV/VIS spectrometer (Varian, Australia)
equipped
with a Cary thermoelectrical controller. The Tm values were determined in the
reference
cell with a Pt-100 resistor, and the thermodynamic data were calculated using
the Meltwin
3.0 program [43].
Circular Dichroism Spectra. The CD-spectra were recorded with a Jasco-600
(Jasco, Japan)
spectropolarimeter with thermostatically (Lauda-RCS-6 bath) controlled lcm
cuvettes.
W-Spectra: 150-20 spectrometer (Hitachi, Japan).
3-Bromo-1-[2-deoxy-[3-D-erythro-pentofuranosyl]-1H-pyrazolo[3,4-d]-pyrirnidin-
4,6-
diamine (2b). A sole, of compd 4b [18] (1.0 g, 2.6 mmol) in aq. 25% NH3 (80
ml) was
heated at 70° for 4 d in a steel vessel. The solvent was evaporated,
and the residue was
dissolved in hot H20, Crystallization occurred upon cooling. Colorless needles
(646 mg,
72%). M.p. 155°. TLC (C): Rf0.2. W (MeOH): 260 (8700), 278 (9000).1H-
NMR
((D6)DMSO): 2.16, 2.68 (2m, HZ-C(2')); 3.38, 3.48 (2m, HZ-C(5')); 3.77 (m, H-
C(4')); 4.36
(m, H-C(3')); 4.72 (t, J = 4.9, HO-C(5')); 5.19 (d, J = 4.1, HO-C(3')); 6.32
('t', J = 6.5, H-
C(1')); 6.39 (s, NH2); 6.77 (br, NHZ). Anal. talc. for CloHlsBrN6O3 (345.2): C
34.80, H 3.80,
N 24.35; found: C 34.97, H 3.97, N 24.21.
CA 02569615 2006-12-18
- 104 -
N4,N6-Bis(phenoxyacefiyl)-1-[2-deoxy-(3-D-erythro-pentofuranosylJ-1H-
pyrazolo[3,4-d]-
pyrimidin-4,6-diamine (5a). Compd. 2a [ 15] ( 1.5 g, 5.6 mmol) was dried by
coevaporation
with pyridine. The residue was dissolved in pyridine (25 ml) and
trimethylsilyl chloride
(3.3 ml, 26 mmol) was added at r.t while stirring. Stirring was continued for
15 min and a
sole. of 2,4,5-trichlorophenyl phenoxyacetate (5.4 g,16.4 mmol) [27] in
pyridine (15 ml)
was added in one portion. The reaction mixture was stirred for 16 h at
40°. It was cooled
(ice bath) and H20 (4.2 ml) was added. After 5 min, aq. 25% NH3 (6 ml) was
introduced,
and the mixture was concentrated to dryness. The residue was applied FC
(eluant: A -~ C)
yielding a colorless foam (900 mg, 30%). TLC (C): RE 0.4. LTV (MeOH): 266
(9100).1H-
NMR ((D6)DMSO): 2.26, 2.82 (2m, Hz-C(2')); 3.34, 3.48 (2m, HZ-C(5')); 3.82 (m,
H-
C(4')); 4.44 (m, H-C(3')); 4.73 (t, J = 5.5, HO-C(5')); 5.14 (s, OCHZ)); 5.17
(d, J = 4.3, HO-
C(3')); 6.60 ('t', J = 6.3, H-C(1')); 6.92 - 7.32 (m, arom. H); 8.41 (s, H-
C(3)); 10.77 (s, NH);
11.30 (s, NH). Anal. talc. for C26H2sNs0~ (534.5): C 58.42, H 4.90, N 15.72;
found: C 58.68,
H 4.78, N 15.20.
N4,N6-Bis(phenoxyacetyl)-1-[2-deoxy-5-O-(4,4'-dimethoxytrityl)-(3-D-erythro-
pentofuranosyl]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (6a). Compd. 5a (0.8
g, 1.5
mmol) was coevaporated twice with pyridine. The residue was dissolved in
pyridine (2 ml)
and 4,4'-dimethoxytrityl chloride (0.6 g, 1.77 mmol) was added. Stirring at
r.t. was
continued for 4 h than the soln. was diluted with MeOH (5 ml) and washed with
5% aq.
sodium bicarbonate (3 x 20 rnl). The organic layer was dried (Na2S04) and
concentrated to
dryness. The residue was purified by FC (eluant: D-~E) yielding a colorless
foam (310 mg,
25%). TLC (E): Rf 0.3. W (MeOH): 266 (8900). 1H NMR: ((D6)DMSO): 2.32, 2.81
(2m,
HZ-C(2')); 2.99 (2m, H2-C(5')); 3.67 (2s, OCH3); 3.95 (m, H-C(4')); 4.53 (m, H-
C(3'));
5.15 (s, OCHZ)); 5.33 (d, J = 4.7, HO-C(3')); 6.62 ('t', J = 6.3, H-C(1'));
6.69 - 7.31 (m,
arom. H); 8.36 (s, H-C(3)); 10.83 (s, NH); 11.56 (s, NH). Anal. talc. for
C4~H44N6O9
(836.9): C 67.45, H 5.30, N 10.04; found: C 66.95, H 5.41, N 10.15.
N4,N6-Bis(phenoxyacetyl)-1-[2-deoxy-5-O-(4,4'-dimethoxytrityl)-(3-D-erythro
pentofuranosyl]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3'-[(2-Cyanoethyl)
N,N
Diisopropyl-phosphoramidite] (7a). To a sole. of compd. 7a (0.15 g, 0.18 mmol)
in THF
(0.5 ml), (IPr)ZEtN (0.12 ml, 0.71 mmol) and 2-cyanoethyl
diisopropylphosphoramidochloridite (64 mg, 0.27 mmol) were added. Stirring
under
argon atmosphere at r.t. was continued fox 30 min. Then, the mixture was
diluted with
3s CH2C12 (20 ml) and 5% aq. NaHC03. The mixture was extracted with CHZCIz (3
x 15 ml).
CA 02569615 2006-12-18
- 105 -
The combined organic layers were dried (Na2S04) and evaporated to give an oil.
The
residue was submitted to FC (eluant: F) yielding (80 mg, 43%) of a colorless
foam. TLC
(F): Rf 0.8. 31P-NMR (CDCl3): 149.2, 149.4.
N4,N6-Bis(benzoyl)-1-[2-deoxy-(3-D-erythro-pentofuranosyl]-1H-pyrazolo[3,4-d]-
pyrimidin-4,6-diamine (5b). Compd. 2a [17] (1 g, 3.8 mmol) was coevaporated
twice with
toluene and dissolved in pyridine (40 ml) and TMS-Cl (4.0 g, 36.8 mmol). The
reaction
mixture was stirred under argon atmosphere, cooled to 0° and PhCOCI
(6.6 ml, 57 mmol)
were added drop-wise to the sole, over a period of 30 min. After stirring
overnight at r.t.
the mixture was diluted in EtOAc (200 ml) and washed with a sat. sodium
bicarbonate
sole. (200 ml) and ice cold HZO (200 ml). The aq. layer was extracted with
EtOAc (2 x 400
ml). The combined org. layers were evaporated to dryness and dissolved in THF!
MeOH/
H20 5:4:1 (250 ml). The dark orange sole. was cooled to 0°, and 2 N
NaOH (25 ml) was
added while stirring was continued for 40 min. The residue was purified by FC
(eluant: A
1s --~ B) yielding 1.1 g (61%) of an amorphous solid. TLC (B): Rf0.3. UV
(MeOH): 245
(16400), 274 (15200). 1H-NMR ((D6)DMSO): 2.13, 2.67 (2m, HZ-C(2')); 3.38, 3.52
(2m,
HZ-C(5')); 3.84 (m, H-C(4')); 4.46 (m, H-C(3')); 4.72 (t, J = 5.7, HO-C(5'));
5.29 (d, J =
4.4, HO-C(3')); 6.66 ('t', J = 6.5, H-C(1')); 7.51 - 8.11 (m, arom. H); 8.40
(s, H-C3); 10.95
(s, NH); 11.49 (s, NH). Anal. calc. for C24HzaN60s (474.5): C 60.75, H 4.67, N
17.71; found:
2o C 61.03, H 4.70, N 17.58.
N4,N6-Bis (b enzoyl)-1- [2-deoxy-5-O-(4,4'-dimethoxytrityl)-(3-D-erythro-
pentofuranosyl] -
1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (6a). As described for 6a with 5a
(500 mg, 1.05
mmol) and DMT-Cl (460 mg, 1.35 mmol) in pyridine (3 ml). The residue was
purified by
25 FC (eluant: D --~ E) yielding 400 mg (49%) of a colorless foam. TLC (E): Rf
0.4. UV
(MeOH): 244 (16400), 275 (15200).1H-NMR ((D6)DMSO): 2.35, 2.67 (2m, HZ-C(2'));
3.07, 3.10 (2m, HZ-C(5')); 3.68,3.69 (2s, OCH3); 3.97 (m, H-C(4')); 4.56 (m, H-
C(3')); 5.35
(d, J = 4.8, HO-C(3')); 6.72 - 8.11 (m, arom. H); 8.36 (s, H-C(3)); 11.01 (s,
NH); 11.54 (s,
NH).
N'°,N6-Bis(benzoyl)-1-[2-deoxy-5-O-(4,4'-dimethoxytrityl)-[i-D-erythro-
pentofuranosyl]-
1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3'-((2-Cyanoethyl) N,N-
Diisopropylphosphoramidite] (7b). As described for 7a with 6b (110 mg, 0.14
rnmol ),
(IPr)2EtN (75~..i1, 0.43 mmol) and 2-cyanoethyl
diisopropylphosphoramidochloridite (38 E.tl,
CA 02569615 2006-12-18
-106-
0.17 mmol) in THF (3 ml) at 30°. The oily residue was submitted to FC
(eluant: F) yielding
92 mg (67%) of a colorless foam. TLC (F): Rf0.8. 31P-NMR (CDC13): 149.82,
149.63.
N4,N6-Bis((di-n-butylamino)methylene)-1-(2-deoxy-[i-D-erythxo-pentofuranosyl]-
1H-
pyrazolo[3,4-d]-pyrimidin-4,6-diamine (8a). To a stirred suspension of 2a (300
mg, 1.13
mmol) in MeOH (5 ml) N,N-di-n-butylformamide dimethyl acetal (790 ~l, 3.39
mmol)
was added. Stirring was confiinued for 2 h at 40°. The reaction mixture
was evaporated to
dryness and the residue adsorbed on silica gel. FC (eluant: A-~ B) afforded
two main
products. From the faster migrating zone a colorless foam of 8a (330 mg, 53%)
was
l0 isolated. TLC (B): Rf0.4. W (MeOH): 235 (22800), 274 (13800), 322 (25
700).'H-NMR
(CDCl3): 0.87 -0.95 (m, CHzCH3); 1.26 - 1.38 (m, CHZCH3); 1.57 - 1.60 (m,
CHZCH2); 2.42
- 2.92 (m, Hz-C(2')); 3.26 - 3.72 (2m, Hz-C{5'), NCHz); 3.88 (m, H-C(4'));
4.39 (m, H-
C(3')); 4.78 (t, J = 5.8, HO-C(5')); 5.21 (d, J= 4.8, HO-C(3')); 6.55 ('t', J=
6.9, H-C(1'));
7.93 (s, H-C(3(); 8.66 (s, N=CH); 8.88 (s, N=CH). Anal. talc. for C~H49NgO3
(544.7): C
15 61.74, H 8.88, N 20.57; found: C 61.71, H 8.91, N 20.57. From the slower
migrating zone 1-
[2-deoxy-(3-D-erythro-pentofuranosyl)-N6-((di-n-butylamino)-methylene)-1H-
pyrazolo[3,4-d]-pyrimidin-4,6-diamine (9a) (80 mg ,17%) was obtained. TLC (B):
Rf 0.3.
W (MeOH): 235 (23000), 275 (14900), 320 (22100).1H-NMR ((D6)DMSO): 0.88 -0.95
(rn, CHZCH3); 1.27 - 1.36 (m, CHZCH3); 1.53 - 1.62 (m, CHzCHz); 2.21 (m, H-
C(2')); 2.91
20 (m, H-C(2')); 3.38 - 3.59 (2m, Hz-C(5'), NCHz); 3.79 (m, H-C(4')); 4.40 (m,
H-C(3')); 4.78
(t, J = 5.8, HO-C(5')); 5.19 (d, J = 4.4, HO-C(3')); 6.36 (s, NHz); 6.40 ('t',
J = 6.5, H-C(1'));
7.77 (s, H-C(3)); 8.69 (s, N=CH). Anal. talc. for C19H31N703 (405.5): C 56.28,
H 7.71, N
24.18; found: C 55.98, H 7.52, N 24.05.
25 ~ N~,N6-Bis((di-n-butylamino)methylene)-3-bromo-1-[2-deoxy-(3-D-erythro-
pentofuranosyl)-1H-pyrazolo[3,4-d)-pyrimidin-4,6-diamine (8b). As described
for 8a,
with 2b (350 mg, l mmol) and N,N-di-n-butylformamide dimethyl acetal (720
E.~,1, 3.1
mmol) in MeOH (7 ml) for 2 h at 40°. After FC (eluant: A --~B) two main
products were
isolated. The faster migrating zone gave 8b as a foam (310 mg, 50%). TLC(B):
Rf 0.3. W
30 (MeOH): 235 (22500), 275 (14500), 320 (22100).1H-NMR ((D6)DMSO): 0.94 -
0.99 (m,
CHZCH3);1.32 -1.44 {m, CH2CH3);1.59 -1.73 (m, CHZCHz); 2.21 (m, H-C(2')); 2.91
(m,
H-C(2')); 3.32 - 3.78 (2m, Hz-C(5'), NCHz)> 3.89 (m, H-C(4')); 4.40 (m, H-
C(3')); 4.78 (t,
J = 5.8, HO-C(5')}; 5,19 (d, J = 4,4, HO-C(3')); 6.81 ('t', J = 6.5, H-C(1'));
8.69 (s, N=CH);
8.94 (s, N=CH).Anal. talc. for Cz8H4~BrN803 (623.6): C 53.93, H 7.60, N 17.97;
found: C
35 54.01, H 7.52, N 18.05. The slower zone furnished 3-bromo-1-[2-deoxy-(3-D-
erythro-
CA 02569615 2006-12-18
- 107 -
pentofuranosyl] -N6-((di-n-butylamino)methylene)-1H-pyrazolo [3,4-d]-pyrimidin-
4,6-
diamine (9b) (75 mg, 16%). TLC(B): Rf0.3. UV (MeOH): 236 (21600), 276 (14000),
320
(21900).1H-NMR ((D6)DMSO): 0.90 - 0.96 (m, CH2CHa); 1.30 - 1.39 (m, CHZCH3);
1.59
1.73 (m, CH2CH2); 2.23 (m, H-C(2')); 2.89 (m, H-C(2')); 3.35 - 3.78 {2m, HZ-
C(5'),
NCHZ); 3.84 (m, H-C(4')); 4.43 (m, H-C(3')); 4.80 (t, J = 5.7, HO-C(5')); 5.23
(d, J = 4.6,
HO-C(3')); 6.40 (s, NHz); 6.81 ('t', J= 6.4, H-C(1')); 8.65 (s, N=CH). Anal.
talc. for
C19H3oBrN~03 (484.4): C 47.11, H 6.24, N 20.24; found: C 47.23, H 6.52, N
20.35.
1-[2-Deoxy-5-O-(4,4'-dimethoxytriiyl)-(3-D-erythro-pentofuranosyl] -N4-( (di-n-
butylamino)methylene)-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine
(l0a). As
described for 6a with 8a (80 mg, 0.15 mmol), DMT-Cl (60 mg, 0.18 mmol) in
pyridine
(0.5 ml). The residue was purified by FC (eluant: D -~ E) yielding 85 mg (77%)
of a
colorless foam. TLC (E): Rf 0.3. W (MeOH): 235 (22800), 276 (20000), 320
(24800).1H-
NMR (CDC13): 0.98 - 1.05 (m, CHzCH3); 1.14 - 1.48 (m, CHzCH3); 1.65 - 1.78 (m,
CHZCHZ); 2.42 - 2.92 (m, HZ-C(2')); 3.35 - 3.70 (2m, HZ-C(5'), NCHZ); 3.71 (s,
OCH3);
4.06 (m, H-C{4')); 4.39 (m, H-C(3')); 5.21 (d, J = 4.8, HO-C(3')); 6.55 ('t',
J = 6.6, H-
C(1')); 6.76 - 7.96 (m, arom. H); 7.94 (d, J = 10.5, NH); 7.96 (s, H-C(3));
8.81 (s, N=CH);
9.57 (d, J = 10.5, CHO). Anal. talc. for C41H49N7O6 (735.9): C 66.92, H 6.71,
N 13.32;
found: C 66.85, H 6,56, N 13.40.
3-Bromo-1-[2-deoxy-5-O-(4,4'-dimefihoxytrityl)-(3-D-erythro-pentofuranosyl]-
N4-((di-n-
butylamino)methylene)-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine
(IOb). As
described for 6a with 8b {310 mg, 0.5 mmol), DMT-Cl (202 mg, 0.6 mmol) in
pyridine
{2 ml). The residue was purified twice by FC (eluant: D -~ E) yielding 250 mg
(61%) of a
colorless foam. TLC (E): Rf 0.3. UV (MeOH): 235 (22900), 276 (21100), 320
(25300).'H-
NMR (D6)DMSO): 0.90 - 0.96 (m, CHzCH3); 1.28 - 1.37 (m, CHZCH3); 1.61 - 1.68
(m,
CH2CH2); 2.21 (m, H-C(2')); 2.91 (m, H-C(2')); 3.03 (2m, Hz-C(5')); 3.35 -
3.52 (m,
NCH2); 3.69 (s, OCH3); 3.90 {m, H-C(4')); 4.48 (m, H-C(3')); 5.32 (d, J = 4.7,
HO-C(3'));
6.46 ('t', J = 6.2, H-C(1')); 6.73 - 7.31 (m, arom. H); 8.99 (s, N=CH); 9.56
(d, j = 9.89,
NH); 10.77 (d, j = 9.88, CHO). Anal. talc. for C41H4sBrN~06 (814.8): C 60.44,
H 5.94, N
12.03; found: C 60.36, H 5.73, N 11.85.
1- [ 2-D eoxy-5-O-(4,4'-dimethoxytrityl)-(3-D-erythro-pentofuranosyl] -N4- (
(di-n-
butylamino)methylene)-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3'-
[(2-
Cyanoethyl) N,N-Diisopropylphosphoramidite] ( l la). As described for 7a with
l0a (110
CA 02569615 2006-12-18
- 108 -
mg, 0.15 mmol), ('Pr)ZEtN (78 ~1, 45 mmol) and 2-cyanoethyl
diisopropylphosphoramidochloridite (47 pl, 0.2 mmol) in THF (2 ml). The oily
residue
was submitted to FC (eluant: F) yielding 75 mg (53%) of a colorless foam. TLC
(F): Rf 0.8.
3iP-NMR (CDC13): 149.58, 149.52.
3-Bromo-1-[2-deoxy-5-O-(4,4'-dimetho~rytrityl)-[3-D-erythro-pentofuranosyl]-N4-
((di-n-
butylamino)methylene)-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3'-
[(2-
Cyanoefihyl) N,N-Diisopropylphosphoramidite] (1lb). As described for 7a with
lOb (90
mg, 0.11 mural), ('Pr)ZEtN (63 ~.a, 0.36 mmol) and 2-cyanoethyl
diisopropylphosphoramido-chloridite (31 pl, 0.14 mmol) in THF (1 ml). The oily
residue
was submitted to FC (eluant: F) yielding 80 mg (72%) of a colorless foam. TLC
(F): Rf 0.8.
31P-NMR (CDCl3): 149.58, 149.53.
Example 2: Synthesis of the 7-iodo-derivatives
Oligonucleotides were synthesized containing halogenated "purine" and
pyrimidine bases. Bromo
and iodo substituents were introduced into the 7-position of 8-aza-7-
deazapurin-2,6-diamine (2b,
c) or into the 5-position of uracil residues (3b, c). Phosphoramidites were
synthesized using the
isobutyryl-residue for the protection of 2b and the benzoyl group for 2c.
Duplexes containing the
residues 2b or 2c gave always higher Tm values than those of the non-modified
counterparts
containing 2'-deoxyadenosine, the purin-2,6-diamine 2'-deoxyribonucleoside (1)
or 2a at the same
positions. Six 2b-residues replacing dA in the duplex 5'-d(TAGGTCAATACT) (102)
~ 5'-
d(AGTATTGACCTA) (103) raised the Tm value from 48° to 75°
(4.5° per modification). Contrary
to this, incorporation of the 5-halogenated 2'-deoxyuridines 3b or 3c into
oligonucleotide duplexes
showed very little influence on the thermal stability, regardless which
"purine" nucleoside is located
opposite to them. The positive effects on the thermal stability of duplexes
observed in DNA were
also found in DNA-RNA hybrids or DNA with parallel chain orientation.
The following data compare the effects of halogen substituents such as bromine
and iodine
introduced into the 7-position of 8-aza-7-deazapurin-2,6-diamine 2'-
deoxyribonucleosides 2b, c
and the 2'-deoxyuridine derivatives 3b, c (purine numbering is used ; see
Scheme 6). These
CA 02569615 2006-12-18
- 109 -
nucleosides will be incorporated into various positions of duplex DNA, and the
number of
incorporations will be increased, stepwise. As the halogen substituents are
directed into the major
groove, in both the series, the "purine" and pyrimidine nucleosides, it was of
interest to quantify the
effects. Furthermore, the effect of multiple incorporations will be
investigated as it is known from
other duplex-stabilizing nucleosides, that the stabilizing effects level
offwith an increasing number
of modified nucleoside incorporations (C. Bailly, M. J. Waning, Nucleic Acids
Res. 1998, 26, 4309
and references therein; J. S~gi, E. Szakonyi, M. Vorlickova, J. Kypr, J.
Biomolec. Struct. Dyn. 1996,
13, 1035.). Oligonucleotide duplexes incorporating 7-halogenated 7-
deazapurines (= pyrrolo [2,3-
d]pyrimidines) show differences in the series of 7-iodo and 7-bromo compounds
(F. Seela, M.
Zulauf, Chem. Eur. j. 1998, 4, 1781), which result from the spatial
requirements of the halogens
(van den Waals radii: Br = 1.85 tpr; I = 1.98 ~r (A. Bondi, J. Phys. Chem.
1964, 68, 441) and/or from
the more hydrophobic character of the iodo substituents compared to the bromo
residues. Thus,
the 7-iodo derivative of the 8-aza-7-deazapurin-2,6-diamine nucleoside (2c)
was synthesized,
converted into a phosphoramidite, and a number of oligonucleotides were
synthesized containing
the 7-iodinated 8-aza-7-deazapurine-2,6-diamine nucleoside 2c. A comparison of
the thermal
stability of these duplexes will illustrate the striking differences in duplex
stabilization among
halogens introduced into pyrimidine or 8-aza-7-deazapurine residues located in
a similar
environment of the major groove of a B-DNA.
Scheme 6
NH2
~N~6~ N ~ R
I ~> s 2
HzN G N Ns
3
2a: R = H 3a: R = H
2b: R = Br 3b: R = Br
2c: R = I 3c: R = I
purine numbering systematic numbering
CA 02569615 2006-12-18
-110
2.1. Synthesis and Properties of Monomers
The 7-iodinated 8-aza-7-deazapurin-2,6-diamine 2'-deoxyribonucleoside 2c was
prepared from the
6-isopropoxy compound 4 (F. Seela, G. Becher, Synthesis 1998, 2, 207) upon
treatment with 25010
aq. NHs at 70°C for 4 days in an autoclave. The halogenated nucleosides
2a, b F. (Seela, G. Becher,
Synthesis 1998, 2, 207) as well as the 2'-deoxyuridine derivatives 3a-c have
been described elsewhere
(J. Asakua, M. J. Robins, J. Org. Chem. 1990, 55, 4928). The halogen
substituents change the
mobility of the nucleosides on an RP-HPLC column with the iodinated 2c and 3c
as the slowest
migrating compounds (Figure Sa, b). The retention times refer to the
hydrophobic character of the
nucleosides; the data were used later for the composition analysis of the base-
modified
oligonucleotides.
Data of the Iodo compound - Table 8. Half life Values (a) of 2c at
25°.
Compound i [min] a) ~.(nm)
h'NHz2c'zsAd (2c) stable b) 242
1'NHZZC'zsAd (2c) 61 ') 242
a) Measured in 0.5 N HCl. n) Within 3h. ')
Measured in 2 N HCI.
The nucleobases of the modified nucleosides 2a-c and 3a-c influence the N H S
pseudorotational
equilibrium of the sugar moiety. This affects the conformation of the sugar
phosphate backbone of
DNA. Por this reason the 1H NMR spectra of the pyrazolo[3,4-d)pyrimidine
nucleoside 2c as well as
of the pyrimidine nucleosides 3a-c were measured in D20 and 3J [1H,'H] NMR
coupling constants
were determined. The conformational analysis was performed on the basis of 3J
[1H,'H] couplings
using the program PSEUROT (Van J. Wijk, C. Altona, 'PSEUROT 6.2 -- A Program
fox the
Conformational Analysis of the Five-Membered Rings', University of Leiden,
July 1993) According
to Table 9 the 8-aza-7-deazapurin-2,6-diamine nucleoside 2a shows a higher N-
conformer
population than the corresponding purine nucleoside 1. The conformation around
the C(4')-C(5')
bond indicates that the 8-aza-7-deazapurin-2,6-diamine nucleoside 2c as the 8-
aza-7-deaza-2'-
CA 02569615 2006-12-18
-11I-
deoxy-guanosines (F. Seela, G. Becher, H. Rosemeyer, H. Reuter, G. Kastner, I.
A. Mikhailopulo,
Helv. Chim. Acta 1999, 82,105) prefers the y'-(-sc)-rotamer population, while
for the regular purine
nucleosides the yt~~g-(+sc)- or the yt'~g-(ap)-conformation is predominant (G.
Blackburn, M. J. Gait,
'Nucleic Acids in Chemistry and Biology', IRL Press, Oxford University Press
1990, p. 28) The
conformation of the sugar moieties of the pyrimidine nucleosides 3a-c is also
influenced by the
substitution at the 5-position (Table 9). The main change is observed between
3a without a 5-
substituent and the derivatives 3b, c with Me or halogen substituents.
Table 9. 3J (H, H) Coupling Constants of the Sugar Moieties and NlS-Conformer
Populations of the 2 =Deoxyribonucleosides 9 - 3 at 303 K a)
3~H,H~HZ Conformation
1',2'1',2"2',3'2",3'3',4'4',5'4',5"%N %S yt''~Ay~ Yt-)s
1 7.30 6.107.003.103.403.204.3031 69 62 25 13
2a 6.60 6.806.903.703.604.005.8037 63 36 42 22
2c 6.60 6.706.853.903.854.305.9038 62 31 43 26
dT ___ ___ ___ ___ __. __ ___ 36 64 ___ __ ___
[16]
3a 6.70 6.606.504.304.103.605.2030 70 47 35 18
3b 6.45 6.506.504.704.503.404.7034 66 55 30 15
3c 6.50 6.506.504.504.403.404.8033 67 54 31 15
SOIVeht D20; ~.m.S. < 0.4 HZ; J D J'~~x ~ < 0.4 HZ.
[16] C. Thibaudeau, J. Plavec, J. Chattopadhyaya, J. Drg. Chem. 1996, 61, 266.
As the reactivity of the amino groups of the nucleoside 2b is rather different
to 1, various residues
were studied for the base protection. Earlier, the nucleoside 2b was protected
with N,N-di-(n-
butyl)formamidine (dnb) residue. Now, the isobutyryl group was introduced
employing the
protocol of transient protection (2b -> 13a) (G. S. Ti, B. L. Gaffney, R. A.
Jones, J. Am. Chem. Soc.
1982,104, 1316) As a side product the mono-protected nucleoside 14 was
isolated (220). The
formation of mono-acylated compounds has been observed in the case of other
2,6-diaminopurine
nucleosides (I. Luyten, A. V. Aerschot, J. Rozenski, R. Busson, and P.
Herdewijn, Nucleosides
Nucleotides 1997,16, 1649) When performing the allcaline hydrolysis of
compounds 13a or 14 in
25% aq. NH3 at 40°, a fast deprotection was observed (See Scheme 7).
The half life for 13a and 14
were determined and found to be 4.5 min and 20.5 min, respectively. It
indicates that this
protecting group is appropriate for the solid-phase oligonucleotide synthesis.
For the protection of
CA 02569615 2006-12-18
- 112 -
the iodo nucleoside 2c the transient protection protocol was used as in 2b,
but in this case a benzoyl
group was chosen (2c --~ 13b). The time for complete deprotection of 13b (25%
aq. NH3, 40°C,
HPLC-monitoring at 260 nm) was 450 min. A half life was not determined because
of the step-wise
reaction.
The base-protected nucleosides 13a, b as well as the pyrimidine nucleosides 3a-
c were converted
into the DMT-derivatives 15a, b, and 17a-c using standard reaction conditions
(Y. S. Sanghvi, G. D.
Hoke, S. M. Freier, M.C. Zounes, C. Gonzalez, L. Cummins, H. Sasmor, P. D.
Cook, NucleicAcids
Res. 1993, 21, 3197) Phosphitylation of the DMT-derivatives 15a, b was
performed in THP in the
presence of 2-cyanoethyl diisopropylphosphoramidochloridite furnishing the
phosphoramidites
16a, b (Scheme 7); the pyrimidine building blocks 18a-c were prepared from 17a-
c in
dichloromethane (Y. S. Sanghvi, G. D. Hoke, S. M. Freier, M.C. Zounes, C.
Gonzalez, L. Cummins,
H. Sasmor, P. D. Cook, NucleicAcids Res. 1993, 21, 3197) These
phosphoramidites (16a, b and 18a-
c) were employed in solid-phase oligonudeotide synthesis. All compounds were
characterized by
1H-, ~jC-, and 3'P-NMR spectra and by elemental analysis (Table 10 ). Table 11
summarizes the'3C
NMR data of 8-aza-7-deazapurine as well as those of pyrimidine nucleosides.
The assignment was
made according to gated-decoupled spectra. The NMR data of 3a-c were included
as a search of the
literature gave little information on that matter.
2o Scheme 7
CA 02569615 2006-12-18
-113..
iPrQ I R2HN R~ NH Z Br
.. ~ N N .r ~ N N y I 1N
N~ ~ ~N~
HEN N N (i) R HN N ibuHN N
HD D Hc~ D ' HC D
H~ HO Hc~
13a: R~ = Br, R2 = ibu
13b: R~ = I , Rz = b z
R2HN R~ RzHN R~
N! I ~N N~ ~ ~~N
.. z ~ r~ ~ ~ .~N,
(ii) R HN N N (iii) R HN N
--
0 0
DMTp DMTfJ
HO a
i
NCCH 2CH2o'P~'N(i-Pr) ~
15 a: R~ = Br, R~ = ibu lga: R~ = Br, R~ = ibu (-- 8a)
lib: R~ = I, Rz = b~ 16 b: R~ = I, RZ = bz
0 0
HN l R HN I R
C~~N (iii) . U-! ''N
~w
DMTO DMTo
Ho O
s
NCH2CHzCCG'P'N(i-Pr) ~
17a: R=H 18a: R=H
1Tb: R = Br 18b: R = Br
17c: R=I 18c: R=I
(i)13a: Me3SiCl, pyridine, isobutyric anhydride, r.t., 4h;13b: Me3SiCl,
pyridine, benzoyl chloride,
r.t., 24h. (ii) (MeC7)2TrCl, pyridine, r.t., 4h; (iii) 2-cyanoethyl
diisopropylphosphoramidochloridite,
CHgCl2, r.t., 30 min.
CA 02569615 2006-12-18
- 114 -
Table 10.'3C-NMR Chemical Shifts of Pyrazolo[3,4-d)-pyrimidine-4,6-diamine 2'-
Deoxyribo-nucleosidese)
C(2) C(4) C(5) C(6) C(7) C=OI C=01 C(2'C(3'C(4'C(5'
) ) ) e) d) C(1'
C(7) C(3) C(4) C(3) =CH =CH ) ) ) ) )
d) d) d) d)
2b 157.6157.4 94.5 162.7 119.2 83.0 37.570.987.362.4
2c 157.0157.6 91.2 162.2 98.3 83.1 37.670.987.362.4
3a 163.2150.5 101.8140.6 84.2 38.570.587.461.3
3b 159.1149.7 95.6 140.6 84.7 38.469.987.560.7
3c 160.4150.0 69.9 145.0 84.6 ~ 69.287.460.7
13a 155.8153.8 104.7156.2 121.4175.2176.5 37.570.787.762.2
83.6
13b ~ 152.1 105.7153.4 96.3 169.4177.5 38.771.688.663.1
149.6 84.4
14 156.0156.5 97.4 157.5 119.1175.383.3 37.570.887.562.2
15a 155.6154.9 98.5 157.5 117.2174.7175.2 37.672.285.463.6
83.5
15b 149.5152.1 105.8153.2 96.2 --- 177.5 f) 71.686.765.3
84.8
17a 163.0158.0 101.5144.7 84.1 fi) 69.985.363.4
17b 158.0159.1 96.0 144.7 84.9 38.470.385.763.6
17c 158.0160.5 69.8 144.2 84.8 38.470.485.863.7
a) Measured in (Ds)DMSO at 303 K. ') Purine numbering. d) Systematic
numbering. e) Tentative.
f) Superimposed by (D6)DMSO.
2.2. Oligonucleotides
2. 2.1 Synthesis and Characterization
Automated solid-phase synthesis of the oligonucleotides (Tables 11-17) was
performed using the
phosphoramidites 16a, b and 18a-c as well as the standard building blocks. The
syntheses followed
the standard protocol (S. L. Beauge, M. Caruthers, Tetrahedron Lett. 1981, 22,
1859) and the
coupling yields were always higher than 97%. Oligonucleotides containing
halogenated dU-residues
require the use of the 4-tert-butylphenoxyacetyl (tac) groups for the
protection of the canonical
phosphoramidites (E. Fewer, C. F~brega, R. G. Garcia, F. Azorin, R. Eritja,
Nucleosides Nucleotides
CA 02569615 2006-12-18
-ils-
1996, I5, 907; J. C. Schulhof, D. Molko, and R. Teoule, Nucleic Acids Res.
1987, I5, 397; R. D.
Sheardy and N. C. Seeman, j. Org. Chem. 1986, 51, 4301). In these cases the
deprotection was
performed with conc, ammonia at room temperature, while in all other cases the
deprotection was
carried out at 60°C. The oligonucleotides were detritylated and
purified on purification cartridges
(Applied Biosystems, 'User's Manual for Oligonucleotide Purification
Cartridges) ] or by reversed-
phase HPLC (conditions for purification see Exper. Part). The homogeneity of
the compounds was
proven by ion-exchange chromatography (see Exper. Part). The composition of
the
oligonucleotides was determined by tandem hydrolysis with snake venom
phosphodiesterase and
alkaline phosphatase followed by RP-18 HPLC as described (F. Seela, C. Wei,
Helv. Chim. Acta
1999, 82, 726). Typical examples are shown in Figure 5c,d. The newly
incorporated iodonucleosides
2c or 3c migrate slower than the canonical DNA constituents. The
oligonucleotides were also
characterized by MALDI-TOF mass spectra. The detected masses were in good
agreement with the
calculated values (Table 18).
Table 18. Molecular Masses (MN") of Oligonucleofides Measured by MALDI-TOF
Mass
Spectromefry.
Oligomer MH' (calc.) MH; (found)
5'-d(TAG GTC AAT ACT) (102) 3644.4 3645
5'-d(AGT ATT GAC CTA} (103) 3644.4 3645
5'-d(TAG GTC 2a2aT ACT) (108)3674,4 3677
5'-d(AGT 2aTT G2aC CTA) (109)3674.4 3675
5'-d(TAG GTC 2b2bT ACT) (110)3832.5 3830
5'-d(AGT 2bTT G2bC CTA) (111)3832.5 3832
5'-d(T2bG GTC 2b2bT 2bCT) 4020 4021
(201)
5'-d(AGT ATT G2cC CTA) (202) 3786 ? 3787
5'-d(AGT 2cTT GAC CTA} (203) 3786? 3792
5'-d(AGT 2cTT G2cC CTA} (100)3926.5 3927
5'-tf(TAG GTC 2c2cT ACT) (204)3927 3931
5'-d(T2cG GTC 2c2cT 2cCT) 4206.3 4210
(205)
CA 02569615 2006-12-18
- 116 -
5'-d(TAG G3aC AA3a ACT)3613.1 3616
(300)
5'-d(AGT A3a3a GAC CTA)3613.1 3615
(301 )
5'-d(TAG G3bC AA3b ACT)3774.1 3775
(302)
5'-d(AGT A3b3b GAC CTA)3774.1 3772
(303)
5'-d(TAG G3cC AA3c ACT)3868.1 3871
(304)
5'-d(AGT A3c3c GAC CTA)3868.1 3871
(305)
2.2.2 Base Pair Stabilities of the Oligonucleotides Duplexes
The 7-bromo nucleoside 2b was found to stabilize DNA duplexes strongly while
the non-
halogenated compound 2a contributes very little to the duplex stability (see
also F. Seela, G. Becher,
Helv. Chim. Acta 2000, 83, 928; F. Seela, G. Becher, M. Zulauf, Nucleosides
Nucleotides 1999, I8,
1399). The contribution of the purin-2,6-diamine nucleoside 1 on the duplex
stability is even lower
(C. Cheong, I. J. Tinoco, A. Chollet, Nudeic Acids Res. 1988, 16, 5115; J. D.
Hoheisel, H. Lehrach,
FEBS Lett. 1990, 274,103). Thus, DNA duplexes containing compound 1-thymine
base pairs are
only slightly more stable than those with dA-dT pairs. As it was not known
whether the stabilizing
effect of the bromo nucleoside 2b will increase continuously by an increasing
number of
incorporations or will level off by multiple incorporations - as it is
reported for other modified
nucleosides (C. Bailly, M. J. Waring, Nucleic Acids Res. 1998, 26, 4309 and
references therein)- a
series of oligonucleotides were synthesized containing the halogenated
compound 2b in a
consecutive manner or in distant position. The modified residues were
incorporated in one or both
strands of a double-stranded DNA. The total number of incorporations was
increased in duplexes
from 1 to 6. The non-halogenated duplexes containing compound 2a were prepared
for
comparison. Apart from the incorporation of the bromo nucleoside 2b, the iodo
compound 2c was
also studied. For all experiments the non-self complementary duplex 5'-
d(TAGGTCAATACT)
(102) ~ 5'-d(AGTATTGACCTA) (103) was chosen.
The non-halogenated nucleoside 2a increases the Tm value of the standard
duplex 102~103 by only
1° per modified residue (Table 11 and F. Seela, G. Becher, 2001,
submitted). Contrary, the bromo
compound 2b contributes a 4-5° stabilization per modif ed residue which
represents an outstanding
high stabilization induced by a non-canonical base. The strength of this
effect is sequence-
CA 02569615 2006-12-18
-117 -
dependent but the stability of the duplexes increases with an increasing
number of 2b -
incorporations as shown in Figure 6. When the iodo nucleoside 2c was replacing
the bromo
compound 2b, a similar effect regarding duplex stabilization is observed
(Table 11). The effects of
halogen substituents introduced into the pyrazolo[3,4-d]pyrimidine derivatives
of dG (F. Seela, G.
S Becher, Helv. Chim. Acta 1999, 82, 1640) or dA (F. Seela, M. Zulauf, J.
Chem. Soc. Perkin Trans. 1
1999, 479) amounts only to 2° per modified residue.
A similar set of experiments as described for the duplexes containing the
halogenated 8-aza-7-
deazapurine nucleosides 2b or 2c was performed with the halogenated 2'-
deoxyuridine derivatives
3b or 3c (Table 12). Neither the bromo nucleoside 3b nor the iodo nucleoside
3c increases the
stability of the duplexes significantly compared to that of the non-
halogenated 3a or dT. Thus, only
the base pairs of type I (Scheme 8) with halogen substituents located at the 7-
position of the 8-aza-
7-deazapurine (pyrazolo [3,4-d]pyrimidine) moiety are stabilized by the
halogen substituents while
those of the type II (Scheme 8) with the halogens attached to the 5-positions
of the pyrimidine base
exert little influence compared to those with dA-dT pairs.
Scheme 8
Fi R
H3C p~~~~~~~~H-N
/ ~ N R p~~,~««H-N
w
N-H~~~~~~~~ ~ N\ . /
N / ~N \ N N-H~~",~~~ ~N N
/ -~p~~~~~n~H- ~--N
H
base pair la: R = H base pair Ila: R = H
Ib: R = Br Ilb: R = Br
Ic: R = I ~ Ilc: R = I
Table 11. Tm Values and Thermodynamic Data of Duplex Formation of
Oligonucleotides
Containing the Pyrazolo(3,4-djpyrimidine Nucleosides Za-c Opposite to dT 8)
Compound Tm ~H ~S DG3~o
[C] (kcallmol][callmol [kcal/mol]
. Kl
5'-d(TAG GTC AAT 47 -83.8 -235.9 -10.6
ACT) (102)
3'-d(ATC CAG TTA
TGA) (103)
5'-d(TAG GTC Za2aT 50 -93.3 -263.09 -11.7
ACT) (108)
CA 02569615 2006-12-18
- 118 -
3'-d(ATC CAG TTA TGA)
(103)
5'-d(TAG GTC AAT ACT)50 -98.79 -279.63 -12.06
(102)
3'-d(ATC C2aG TT2a
TGA) (109)
5'-d(TAG GTC 2a2aT 51 -gg,g6 -278.58 -12.26
ACT) (108)
3'-d(ATC C2aG TT2a
TGA) (109)
54 _gg.gg -280.74 -12.89
5'-d(TAG GTC 2bAT
ACT) (200)
3'-d(ATC CAG TTA TGAj
(103)
5'-d(TAG GTC 2b2bT 56 -91.42 -251.66 -13.37
ACT) (110)
3'-d(ATC CAG TTA TGA)
(103)
5'-d(TAG GTC AT ACT) 5g -91.85 -251.25 -13.92
(102)
3'-d(ATC C2bG TT2b
TGA) (111)
5'-d(TAG GTC 2bAT 63 -100.70 -274.04 -15.70
ACT) (200)
3'-d(ATC C2bG TT2b
TGA) (111)
5'-d(TAG GTC 2b2bT 67 -105.40 -285.02 -17.00
ACT) (110)
3'-d(ATC C2bG TT26
TGA) (111)
5'-d(T2bG GTC 2b2bT g4 -99.08 -268.47 -15.81
2bCT)(201 )
3'-d(ATC CAG TTA TGAj
(103)
5'-d(T2bG GTC 2b2bT 75 -107.43 -283.41 -19.53
2bCT)(201 )
3'-d(ATC CZbG TT2b
TGA) (111 )
5'-d(TAG GTC AAT ACT)51 -90.65 -254.58 -11,69
(102)
3'-d(ATC C2cG TTA
TGA) (202)
5'-d(TAG GTC AAT ACT)5q, -93.22 -260.43 -12.45
(102)
3'-d(ATC CAG TT2c
TGA) (203)
5'-d(TAG GTC AAT ACT)57 -95.09 -263.29 -13.43
(102)
3'-d(ATC C2CG TT2c
TGA) (100)
5'-d(TAG GTC 2c2cT 55 -96.46 -268.72 -13.12
ACT)(204)
3'-d(ATC CAG TTA TGA)
(102)
5'-d(TAG GTC 2c2cT 5g -102.95 -284.63 -14.67
ACT)(204)
3'-d(ATC C2cGT T A
TGA) (202)
5'-d(TAG GTC 2c2cT 6g -104.93 -284.73 -16.62
ACT)(204)
3'-d(ATC C2cGT T2c
TGA) (100)
5'-d(TZcG GTC2c2cT 72 - - -
2cCTj
(205)
3'-d(ATC C2cG TT2c
TGA) (100)
a?Thermodynamic parameters are derived from the fitting of melting curves
measured at
260 ram in 0.1 M NaCI,10mM MgCl2, and 10 mM Na-cacodylate buffer, pH 7.0 with
5 pM
+ 5 pM single strand concentration. The 0G° are taken directly from the
program Meltwin
3.0 referring to 310°. Earlier publications of our laboratory using the
fitting program refer to
the same temperature and not to 298° as indicated. The thermodynamic
data determined
from the van't Hoff plots using the concentration dependence of the Tm-values
are
consistent with those obtained from the curare fitting within 15%. The van't
Hoff data of the
formation of the duplex 102~103 are the following: OH° = 86.8 kcal/mol;
4S° = 243.7 caI/K
mol; ~G°3~o= 11.3 kcallmol.
CA 02569615 2006-12-18
- 119 -
Table 12. Tm Values and Thermodynamic Dafa of Duplex Formulation of
Oligonucleotides
Containing the Pyrimidine Nucleosides 3a-c Opposite fo dA a)
Compound Tm DH° eS° eG°a,o
LC] [kcal/mol](callmol [kcal/mol]
K]
5'-d(TAG G3aC AA3a 47 -86.35 -244.74 -10.45
ACT) (300)
3'-d(ATC CAG TTA TGA)
(103)
5'-d(TAG GTC AAT ACT) 48 -92.11 -261.95 -10.86
(102)
3'-d(ATC CAG 3a3aA
TGA) (301 }
5'-d(TAG G3aC AA3a 46 -86.37 -245.42 -10.25
ACT) (300)
3'-d(ATC CAG 3a3aA
TGA) (301)
5'-d(TAG G3bC AA3b 48 -95.76 -271.88 -11.44
ACT) (302)
3'-d(ATC CAG TTA TGA)
(103)
5'-d(TAG GTC A A T 49 -97.43 -276.95 -11.53
ACT) (102}
3'-d(ATC CAG 3b3bA
TGA) (303)
5'-d(TAG G3bC AA3b 49 -94.25 -267.57 -11.26
ACT) (302)
3'-d(ATC CAG 3b3bA
TGA) (303)
5'-d(TAG G3cC AA3c 49 -96.38 -273.73 -11.48
ACT) (304)
3'-d(ATC CAG TTA TGA)
(103)
5'-d(TAG GTC AAT ACT) 50 -94.99 -269.20 -11.50
(102)
3'-d(ATC CAG 3c3cA
TGA) (305)
5'-d(TAG G3cC AA3c 50 -99.14 -281.47 -11.85
ACT) (304)
3'-d(ATC CAG 3c3cA
TGA) (305)
a) See Table 11.
As the stability of the base pairs of the halogenated pyriznidine nucleosides
3b or 3c with dA was
low compared to the halogenated pyrazolo [3,4-d]pyrimidine compounds 2b or 2c
incorporated
opposite to dT, tridendate base pairs are formed in which the halogenated
pyrimidine nucleosides
3a-c are located opposite to the purine-2.6-diamine nucleoside 1 instead of dA
(Scheme 9, by type
III). In this case a strengthening of the base pair can be expected if the
formation of a third
hydrogen bond is possible. However, the Tm-values of those duple-xes were also
not influenced
significantly by the replacement of dA-residues by the nucleoside 1 (Table
13). This indicates that
the 2-amino group does not participate in possible base pairs as shown in IIIa-
c (Scheme 9), a
finding which is similar to that observed for a base pair between 1 and dT (F.
Seela, G. Becher, 2001,
submitted; C. Cheong, I. J. Tinoco, A. Chollet, Nucleic Acids Res.1988,16,
5115; J. D. Hoheisel, H.
Lehrach, FEBS Lett. 1990, 274,103). A similar behaviour was found in the case
of duplexes
containing the base pair IVa (Scheme 9) formed between nucleoside 2a and the
pyrimidine
nucleosides 3a-c (Table 13). Nevertheless, the duplexes incorporating the base
pairs IVb,c are
slightly more stable than those containing the base pairs IIIb,c.
CA 02569615 2006-12-18
- 120 -
Scheme 9
pnu~u~H-- ~ R puuuu
~N-H~~~~m~ ~ N N-H
N - ~ N
p~m~~aH_N
H
H
base pair Illa: R = H base pair IVa: R = H
Iltb: R = Br IVb: R = 8r
Illc: R = I IVc: R = I
Table 13. Tm Values and Thermodynamic Data of Oligonucleofide Duplexes
Containing
s the Halogenated Pyrimidine Nucleosides 3a-c Opposite to the Purin-2,6-
diamine
Nucleoside ~ or the Pyrazoloj3,4-djpyrimidindin-4,6-diamine Nucleoside 2a a)
Tm eH eS eG3,o
[C] [kcaUmol] [callmol [kcallmol]
K]
5'-d(TAG GTC AAT ACT) 49 -83.15 -232.81 -10.94
(102)
3'-d(ATC C1 G TT1 TGA)
(105)
5'-d(TAG G3aC AA3a ACT) 48 -73.03 -202.44 -10.25
(300)
3'-d(ATC C1G TT1 TGA)
(105)
5'-d(TAG G3bC AA3b ACT) 49 -73.84 -203.94 -10.59
(302)
3'-d(ATC C1 G TT1 TGA)
(105)
5'-d(TAG G3cC AA3c ACT) 49 -77.60 -215.48 -10.76
(304)
3'-d(ATG C1 G TT1 TGA)
(105)
5'-d(T1G GTC 11T 1CT) 48 -54.74 -144.65 -9.88
(104)
3'-d(ATC CAG TTA TGA)
(103)
5'-d(T1 G GTC 11 T 1 48 -60.03 -161.49 -9.94
CT) (104)
3'-d(ATC CAG 3a3aA TGA)
(301 )
5'-d(T1G GTC 11T 1CT) 48 -60.09 -160.37 -10.35
(104)
3'-d(ATC CAG 3b3b TGA)
(303)
5'-d(T1G GTC 11T 1CT) 48 -62.35 -167.33 -10.45
(104)
3'-d(ATC CAG 3c3cA TGA)
(305)
5'-d(TAG GTC 2a2aT ACT) 49 -87.32 -245.54 -11.16
(108)
3'-d(ATC CAG 3a3aA TGA)
(301)
5'-d(T1 G GTC 2a2aT ACT)50 -89.11 -250.34 -11.47
(108)
3'-d(ATC CAG 3b3b TGA)
(303)
5'-d(TAG GTC 2a2aT ACT) 51 -81.32 -225.60 -11.35
(108)
3'-d(ATC GAG 3c3cA TGA)
(305)
5'-d(TAG G3aC AA3a ACT) 49 -90.30 -253.96 -11.53
(300)
3'-d(ATC C2aG TT2a TGA)
(109)
5'-d(TAG G3bC AA3b ACT) 51 -93.05 -261.91 -11.82
(302)
3'-d(ATC C2aG TT2a TGA)
(109)
5'-d(TAG G3cC AA3c ACT) 50 -90.58 -254.11 -11.77
(304)
3'-d(ATC C2aG TT2a TGAI
(1091
CA 02569615 2006-12-18
-121-
a) See Table 11.
A significant duplex stabilization was observed when the halogenated 8-aza-7-
deazapurine
nucleosides 2b or 2c were incorporated opposite to the pyrimidine nucleosides
3a-c (Table 14 and
Scheme 10, by of type V and VI). The Tm increase amounts to about 4-5°
per incorporated residue
of the halogenated nucleosides 2b or 2c. All duplexes containing the
halogenated nucleosides 2b or
2c (Scheme 10) give very similar Tm values, no matter which pyrimidine monomer
is located
opposite to it. Thus, only halogen substituents attached to the modified
purine residues lead to a
duplex stabilization while halogens linked to the 5-position of the 2'-
deoxyuridine moiety
contribute very little to the duplex stability (S. M. Freier and K.-H. Altman,
Nucleic Acids Res. 1997,
25, 4429; F. Seela, Y. He, Helv. Chim. Acfa 2000, 83, 2527; S. Wang, E. T.
Kool, Biochemistry 1995,
34, 4125)
Scheme 10
H Br H I
. R p~~~~~n~H- \ N R p««~~~~H-N 'N
I
N-H~~n~~~~ ~ N\ ~ N-H,~~~a"N ~ N\
N-~ ~N \ . N--~ ~= \N
p~~~~~~~~H-t~H
p~~~~~~~~H-N
H
base pair Vla: R = H
base pair Va: R = H Vlb: R = Br
Vb: R = Br
Vc: R = I Vlc: R = I
Table 14. Tm Values and Thermodynamic Data of Oligonucleotides Contalnlng the
Pyrazoio(3,4-dJpyrfmldin-~,6-diamine Nucleosides 2b-c Opposite to dT and 3a-c
a)
Duplex Tm eH ~S aGs~o
[C] kcal/mo1 callmol [kcallmol]
K1
5'-d(TAG GTC 2bAT ACT) 52 -92.88 -260.97 -11.94
(200)
3'-d(ATC CAG 3a3aA TGA)
(301 )
5'-d(TAG GTC 2b2bT ACT)55 -96.78 -269.67 -13.14
(110)
3'-d(ATC CAG 3a3aA TGA)
(301)
5'-d(T2bG GTC 2b2bT 64 -101.57 -276.51 -15.82
2bCT) (201)
3'-d(ATC CAG 3a3aA TGA)
(301 )
5'-d(TAG GTC 2bAT ACT) 54 -98.06 -275.03 -12.76
(200)
3'-d(ATC CAG 3b3bA TGA)
(303)
5'-d(TAG GTC 2b2bT ACT)55 -95.42 _26g,gg -12.71
(190)
3'-d(ATC CAG 3b3b TGA)
(303)
CA 02569615 2006-12-18
- 122 -
5'-d(T2bG GTC 2b2bT 2bCT) {201 ) 65 -106.49 -290.34 -16.44
3'-d(ATC CAG 3b3bA TGA) (303)
5'-d(TAG GTC 2bAT ACT) (200) 54 -87.97 -244,29 -12.20
3'-d(ATC CAG 3c3cA TGA) (305)
5'-d(TAG GTC 2b2bT ACT) (110) 55 -89.17 -246.44 -12.74
3'-d(ATC CAG 3c3cTGA) (305)
5'-d(T2bG GTC 2b2bT 2bCT) {201 ) 65 -101.57 -275.34 -16.18
3'-d(ATC CAG 3c3cA TGA) (305)
5'-d(TAG G3aC AA3a 56 -95.76 -265.45 -13.43
ACT) (300)
3'-d(ATC C2bG TT2b
TGA) (111 )
5'-d(TAG G3bC AA3b 56 -96.53 -268.70 -13.20
ACT) (302)
3'-d(ATC C2bG TT2b
TGA) (111)
5'-d(TAG G3cC AA3c 57 -97.07 -269.07 -13.62
ACT) (304)
3'-d(ATC C2bG TT2b
TGA) (111)
5'-d(TAG G3aC AA3a 55 -96.93 -270.69 -12.98
ACT) (300)
3'-d(ATC C2cG TT2c
TGA) (100)
5'-d(TAG G3bC AA3b 55 -100.82 -282.19 -13.29
ACT) (302)
3'-d(ATC C2cG TT2c
TGA) (100)
5'-d(TAG G3cC AA3c 55 -98.00 -273.26 -13.25
ACT) (304)
3'-d{ATC C2cG TT2c
TGA) (100)
a) See Table 11.
2.2.3 Base Discrimination
In order to investigate the discrimination of the iodo nucleoside 2c towards
the four canonical
DNA- constituents, hybridization experiments were performed according to Table
15. As expected,
the base pair 2c-dT is the strongest (102 ~ 100, Tm 59°) while those of
the duplexes forming
mismatches melt at a significantly lower temperature (Table 15). The
discrimination of the iodo.
nucleoside 2c is similar to that of the canonical nucleosides except that the
duplex 40I ~ 100 (Tm =
52°) shows a 7° lower Tm- value than the duplex 102~100 (Tm =
59°), while duplex 401 ~ 103 (Tm =
46°) has almost the same stability as the parent duplex I02 ~ 103 (Tm =
48°). The high Tm - value of
401 ~ 103 is the result of the formation of the dG-dA Hoogsteen pair which is
obviously not formed
between dG and compound 2c.
Table 15. Tm - Values of Oligonucieofides Containing the Pyrazolo[3,4-
djpyrimidin-4,6-
diamine Nucleoside 2c Opposite to fhe Four Canonical Nucleosides e)
Duplex Tm Tm
foCl ~Cl
5'-d(TAG GTC AAT ACT) 57 5'-d(TAG GTC AAT ACT) (102)
(102) 48
3'-d(ATC C2cG TT2c 3'-d(ATC CAG TTA TGA) (103)
TGA) (100)
5'-d{TAG GAC AAT ACT) 45 5'-d(TAG GAC AAT ACT) (400)
(400) 3$
3'-d(ATC C2cG TT2c 3'-d(ATC CAG TTA TGA) {103)
TGA) (100)
CA 02569615 2006-12-18
-123-
5'-d(TAG GGC AAT ACT) 50 5'-d(TAG GGC AAT ACT)
(401 ) (401 ) 46
3'-d(ATC C2cG TT2c TGA) 3'-d(ATC CAG TTA TGA)
(100) (103)
5'-d(TAG GCC AATACT) 45 5'-d(TAG GCC AATACT) (402)
(402) 36
3'-d(ATC C2cG TT2c TGA)~ 3'-d(ATC CAG TTA TGA)
(100) (103)
a) see Table 11.
The CD-spectra of the duplexes containing the 2-amino-8-aza-7-deazaadenine
derivatives 2a, b
were measured next. A B-like DNA structure can be deduced from the curves
displayed in Figure
7a. A positive Cotton effect around 270 to 290 nm and a negative lobe at 250
nm are observed for
the standard duplex (102~103). The CD spectrum of the duplex 104~105
containing 1 shows
significant differences similar to that of 110~111 while the duplex 108~109
shows a stronger
negative Cotton effect at 260 nm and a positive one at 235 nm. Similar
differences were reported for
the oligonucleotides containing 7-substituted 8-aza-7-deaza-7-iodoguanine (N.
Ramzaeva, F. Seela,
Helv. ehim. Acta 1996, 79, 1549).
2.4 DNA-RNA Hybrids
In order to study the influence of the 2,6-diamino nucleosides 1 and 2a,b on
the stability of DNA-
RNA hybrids, the oligodeoxyribonucleotides 110, 300, 302, 304, 104 and 102
were hybridized with
the oligoribonucleotide 403. Table 16 shows that the various modifications
exert a significant
influence on the duplex stability of the DNA-RNA hybrids. While the
incorporation of the non-
halogenated nucleosides 1 or 2a stabilizes the DNA-RNA structure very little,
the hybridization of
the oligonucleotide containing 2b with 403 ( 110 ~ 403) show a significant
increase of the duplex
stability as in DNA. Compounds 3b and 3c enhance the thermal stability of DNA-
RNA to a small
degree, which is also observed for other modifications of 2'-deoxyuridine (B.
C Froehler, S.
Wadwani, T. J. Terhorst, S. R. Gerrard, Tetrahedron 1992, 33, 5307; J. Sagi,
A. Szemzt3, K. $binger,
A. Szabolcs, G. Sagi, $. Ruff, L. C)tvos, Tetrahedron Lett. 1993, 34, 2191).
The hypochromicities of
the chimeric hybrids are slightly decreased over those of the DNA-DNA duplexes
(data not shown).
From the CD-spectra of Figure 7b it can be seen that the DNA-RNA hybrids adopt
the A-form (N.
Ramzaeva, C. IVIittelbach, F. Seela, Helv. Chim. Acta 1997, 80,1809).
CA 02569615 2006-12-18
- 124 -
Table 16. Tm Values and Thermodynamic Data of DNA-RNA Hybrids e)
Duplex Tm dH ~S 4G3to
[C] [kcallmol][callmol [kcallmol]
K]
5'-d(TAG GTC AAT ACT) 45 -92.1 -264.0 -10.2
(102)
3'-(AUC CAG UUA UGA) (403)
5'-d(T1 G GTC 11 T 1 CT) 48 -60.46 -162.12 -10.18
(104)
3'-(AUC CAG UUA UGA) (403)
5'-d(TAG GTC 2a2aT ACT) 48 _ _
(108)
3'-(AUC CAG UUA UGA) (403)
5'-d(TAG GTC 2b2bT ACT) 53 -98.64 -276.97 -12.74
(110)
3'-(AUC CAG UUA UGA) (403)
5'-d(TAG G3aC AA3a ACT) 44 -74.43 -208.26 -9.84
(300)
3'-(AUC CAG UUA UGA) (403)
5'-d(TAG G3bC AA3b ACT) 48 -77.19 -215.13 -10.47
(302)
3'-(AUC CAG UUA UGA) (403)
5'-d(TAG G3cC AA3c ACT) 47 -78.77 -220.52 -10.37
(304)
3'-(AUC CAG UUA UGA) (403)
a) See Table 11.
2.5 Duplexes with Parallel Chain Orientation
The base pairing of the nucleosides 1, 2a-c as well as 3b, c was also
investigated in parallel stranded
DNA [29]. For this purpose it was necessary to replace the dC-dG pair by a
mSiCd-iGd pair. The
duplexes 102 ~ 115 and 116 ~ 103 served as standards (F. Seela, C. Wei, G.
Becher, M. Zulauf, P.
Leonard, Bioorg. Med. Chem. Lett. 2000, ID, 289). Substitution of dA-residues
by the diamino
nucleoside 1 resulted in a slight stability decrease of the ps-duplexes 104 ~
115 and 116 ~ 105 (Table
17). The incorporation of 2a-c into the ps-DNA resulted in a significant
increase of the Tm values.
The incorporation of the substituted pyrimidine nucleosides 3b, c in place of
dT leads only to
minor changes of the Tm values. From this it can be concluded that similar to
DNA with
antiparallel chain orientation, the halogen substituents of the "purine"
nucleosides 2b, c stabilize
these duplexes while the halogen substituents of the pyrimidine nucleosides
contribute very little to
the duplex stability.
Table 17. Tm Values and Thermodynamic Data of Parallel-Stranded
Oligonucleotide
Duplexes Containing the Nucleosides 1, 2a-c and 3a-c 8~ b)
Duplex Tm ~H° dS° °
[°C] [kcal/moi] [cal/mol ~ K ~G ato
l [kcal/mol]
5'-d(TAG GTC AAT ACT) (102) 3g -74.4 -212.3 -8.5
CA 02569615 2006-12-18
-125-
5'-d(AtiC iCAiG TTA
TiGA) {115)
5'-d(T1 G GTC 11T 1 36 -48.12 -129.68 -7.90
CT) (104)
5'-d(ATiC iCAiG TTA
TiGA) (115)
5'-d(TAG GTC 2a2aT 41 -61.77 -170.95 -8.75
ACT) (108)
5'-d(AT1C iCAiG TTA
TiGA) (115)
5'-d(TAG GTC 2b2bT 45 -66,59 -183.94 -9.54
ACT) (110)
5'-d(ATiC iCAiG TTA
TiGA) (115)
5'-d(TAG G3bC AA3b 37 -50.64 -137.87 -7,88
ACT) (302)
5'-d(ATiC iCAiG TTA
TIGA) (115)
5'-d(TAG G3cC AA3c 36 -55.09 -152.14 -7.90
ACT) (304)
5'-d(ATiC iCAiG TTA
TiGA) (115)
5'-d(TiCA TAA iCTiG 44 -85.0 -242.0 -10.0
iGAT) (116)
5'-d(AGT ATT GAC CTA)
(103)
5'-d(TiCA TAA iCTiG 39 -61.00 -169.68 -8.38
iGAT) (116)
5'-d(AGT 1TT G1C CTA)
(105)
5'-d(TiCA TAA iCTiG 45 -80 -230 -10.3
iGAT) (116)
5'-d(AGT 2aTT G2aC
CTA) (109)
5'-d(TiCA TAA iCTiG 48 -68.34 -186.29 -10.56
iGAT) (116)
5'-d{AGT 2bTT G2bC
CTA) {111 )
5'-d(TiCA TAA iCTiG 43 -76.03 -215.51 -9.19
iGAT) (116)
5'-d(AGT A3b3b GAC
CTA) (303)
5'-d(TiCA TAA iCTiG 42 -67.15 -187.02 -9.15
iGAT) (116)
5' d(AGT A3c3c GAC
CTA) (305)
B) Measured at 260 uffer,
nm in 1 M NaCf, 100 pH 7.0
M MgClz, 60 mM Na-cacodylate mth 5
b + 5 pM
oligomer concentration.SiCd = 2'-deoxy-5-methylisocytidine.
) d(iC) = m
2.3. Conclusion
The halogen substituents introduced into the 7-position of the 8-aza-7-
deazapurine 2'-
deoxynucleoside (2b, c) or the 5-position of 2'-deoxyuridine residues (3a-c)
have very different
influences on the stability of nucleic acid duplexes. In the case of the 7-
halogenated 8-aza-7-
deazapurine 2'-deoxynucleosides 2b, c, each monomer contributes about
4°-5° to the duplex
stabilization, while the pyrimidine nucleosides 3a-c show very little
influence or even no effect. This
is surprising as the halogens in both series of nudeobases are directed into
the major groove of B-
DNA both being in a not identical but a very similar environment. When both,
the halogenated
pyrimidines and the halogenated 8-aza-7-deazapurine nucleosides are present in
a DNA-duplex
only the latter make a contribution to the duplex stabilization. Consequently,
the increase of the
hydrophobic character of the major groove induced by the lipophilic halogen
substituents and the
expelling of water molecules is not the major effect induced by the
halogenation of the major
CA 02569615 2006-12-18
- 126 -
groove. Apparently, stacking interaction between the nearest neighbours are
strengthened in the
case of 2b, c but not with 3b, c.
2.4. Bxperimental Part
Monomers. General. See preceeding manuscripts (F. Seela, G. Becher, Helv.
Chim. Acts 2000, 83,
928; F. Seela, G. Becher, M. Zulauf, Nucleosides Nucleotides 1999, 18, 1399;
F. Seela, M. Zulauf, J.
Chem. Soc. Perkin Trans. 1 1999, 479.) Flash chromatography (FC): 0.4 bar on
silica gel 60 H
Merck, Darmstadt, Germany). Thin-layer chromafiography (TLC): Aluminum sheets,
silica gel 60
F254 (0.2 mm, Merck, Germany). Solvent systems for FC and TLC: CHzCl2 / MeOH
9:1 (A), CHZC12 /
MeOH 95:5 (B), CHiCIz / acetone 9:1 (C), CHZC12 / EtOAc 85:15 (D), CH2Cl2 /
acetone 95:5 (E),.
M.p.: Btichi-SMP-20 apparatus (Btichi, Switzerland); uncorrected. NMR Spectra:
Avance -DPX-250
and AMX-500 spectrometers (Broker, Germany); 8 values are in ppm downfield
from internal
SiMe4 (1H,13C). Microanalyses were performed by Mikroanalytisches Labor Beller
(Gottingen,
Germany).
Oligonucleotides.- Oligonucleotide synthesis was performed on a DNA
synthesizer, model 392
(Applied Biasystems, Weiterstadt, Germany). Melting curves were measured with
a Cary-1/3
W/VIS spectrophotometer ( Varian, Australia) equipped with a Cary
thermoelectrical controller.
The temperature was measured continuously in the reference cell with a Pt-100
resistor, and the
thermodynamic data of duplex formation were calculated using the Meltwin 3.0
program (J. A.
McDowell, D. H. Turner, Biochemistry 1996, 35,14077). The CD-spectra were
recorded with a
jasco-600 (]asco, Japan) spectropolarimeter with thermostatically (Lauds-RCS-6
bath) controlled
lcm cuvettes. LJV-Spectra:150-20 spectrometer (Hitachi, Japan). The enzymatic
hydrolysis of the
oligomers was performed as describeda) (F. Seela, G. Becher, Helv. Chirn. Acts
2000, 83, 928; F.
Seela, G. Becher, M. Zulauf, Nucleosides Nucleotides 1999,18,1399) using the
following extinction
coefficients: s26o: ~Gd 2700, dT 8800, dC 7300, dA 15400, dG 11700, 2a 8800,
2b 8700, 2c 8700, 3a
10000 , 3b 4800, 3c 3700. Snake-venom phosphodiesterase (EC 3.1.15.1,
Crotallus durissus) and
allcaline phosphatase (EC 3.1.3.1, E. coli) were generous gifts from Roche
Diagnostics GmbH,
CA 02569615 2006-12-18
-127-
Germany. The MALDI-TOF-spectra were measured on a Biflex III spectrometer
(Bruker Saxonia,
Leipzig, Germany).
Synthesis and Purification of Oligonucleotides. - The synthesis was carried
out in a 1 - ~mol scale
using 3'-phosphoramidites of [(Me0)ZTr]ibZGd, [(Me0)ZTr]bzSAd, [(Me0)zTr]bz4Cd
and
[(Me0)ZTr]Td for the synthesis of oligonucleotides containing 2a-c. and
[(Me0)2Tr]tacZGd,
[(Me0)ZTr]tac6Ad, [(Me0)aTr]tac4Cdand [(Me0)ZTr]Td for the synthesis of
oligonucleotides
containing 3a-c. After cleavage of the oligonucleotides from the solid
support, the first were
deprotected in 25% aq. NH3 for 12 - 15 h at 60°. The latter were
incubated in 25% aq. NH3 for 1.5
2 h at r. t. for deprotection. The purification of the 5'-(dimethoxytrityl)-
oligomers was performed
by reversed-phase HPLC (RP-18). The following solvent gradient was used (A,
O.I M (Et3NH )OAc
(pH 7.0)/ MeCN 95:5; B, MeCN): 3 min 20% B in A, 12 min 20 - 40% B in A with a
flow rate of 1.0
ml/min. The concentrated oligonucleotide solutions were treated with 2.5%
CHCIzCOOHlCHZCl2
for 5 min at r.t, to remove the 4,4'-dimethoxytrityl residues. The
detritylated oligomers were
purified by reversed-phase HPLC with the gradient: 20 min 0 - 20% B in A with
a flow rate of
1 ml/min. The oligomers were desalted on a short column (RP-18, silica gel)
and then lyophilized
on a Speed-Vac evaporator to yield colorless solids which were frozen at -
24°. The purified oligmers
were dissolved in 100 l,tl of double-distilled HZO, and the purity was
controlled by ion-exchange
chromatography on a Dionex-Nucleopac-PA-I00 HPLC column (4 x 250 mm, P/N
043010; Dionex,
Idstein, Germany).
Nucleoside-Composition Analysis.- The oligonucleoddes were dissolved in
O.lt,~t Tris-HCl buffer (pH
8.3, 200 ~1), and treated with snake-venom phosphodiesterase (3 E.~l) at
37° for 45 min, and then
alkaline phosphatase (3 pl) at 37° for another 30 min. The reaction
mixtures were analyzed on
reversed-phase HPLC (RP-18, at 260 nm, gradient A, 0.7 mllmin). The zetention
time of 2a-c and
3a-c were used as standards (Figure 5). The extinction coefficients of the
nucleosides and the peak
areas were used for quantification of the composition of the oligonucleotides
(Figure Sa-d).
CA 02569615 2006-12-18
- 128 -
X-(2-Deoxy-~-D-erythro-pentofuranosyl)-3-iodo-IH-pyrazolo(3,4-dj-pyrimidin-4,6-
diamine (2c). A
soln. of compd. 6-amino-1-{2-deoxy-{i-D-erythro-pentofuranosyl]-3-iodo-4-
isopropoxy-1H-
pyrazolo{3,4-d]pyrimidine 12 (P. Seela, G. Becher, Synthesis 1998, 2, 207.) (1
g, 2.3 mmol) in a aq.
25% NH3 soln. (80 ml) was heated at 70°C for 4 d. The solvent was
evaporated to dryness, the
residue dissolved in hot water and crystallized. Colorless needles (640 mg,
71%). M.p. 154°. TLC
(A): Rf0.2. UV (MeOH): 223 (31800), 260 (8700), 278 (9100).'H-NMR ((D6)DMSO):
2.12 (m, Ha
C(2')); 2.67 (m, Hp-C(2')); 3.38, 3.44 (m, HZ-C(5')); 3.73 (m, H-C(4')); 4.33
(m, H-C(3')); 4.73 (t,
J= 5.7, OH-C(5')); 5.17 (d, J= 4.3, OH-C(3')); 6.27 ('t', J= 6.5, H-C(1'));
6.34 (br, NHZ); 6.62 (br,
NHZ). Anal. calc. for CIpH13~6~3 (392.2): C 30.63, H 3.34, N 21.43; found: C
30.91, H 3.61, N
21.27.
3-Bromo-1-(2-deoxy-,Q-D-erythro pentofuranosyl)-4,6-((2-methylpropanoyl)aminoj-
1H-
pyrazolo(3,4-djpyrimidine (12a). Compd. 2b (F. Seela, G. Becher, Synthesis
1998, 2, 207) (0.74 g,
2.14 mmol) was co-evaporated with anhyr. pyridine for three times and
dissolved in anhydr.
pyridine (5 ml) while stirring at r.t. Me3SiC1 (1.37 ml, 10.8 mmol), and after
15 min isobutyric
anhydride (3.56 ml, 21.5 mmol) were added. Stirring was continued for 3 h. The
reaction mixture
was cooled in an ice-bath and diluted with H20 (2.5 ml), 5 min later aq. 25%
NH3 (4.3 ml) was
added. After stirring for 30 min the reaction mixture was evaporated to
dryness and coevaporated
with toluene (three times). The residue was purified by FC (CHZCIZ/MeOH 9:1)
furnishing two
zones. Prom the fast migrating zone compound 13a was obtained as a colorless
amorphous solid
(500 mg, 48%). Rf (A) 0.4. U~ (MeOH): 284 (9500), 239 (33900).'H-NMR
((D6)DMSO): 1.15 (m,
2 CH(CH3)i); 2.25 (m , Ha C(2')); 2.75 (m , Hp-C(2')); 2.86 (m , 2 CH(CH3)z);
3.47 (m , HZ-C(5'));
3.81 (m, H-C(4')); 4.44 (m, H-C(3')); 4.73 (t, J= 5.5, OH-C(5')); 5.32 (d, J~
4.3, OH-C(3')); 6.54
('t', J= 6.6, H-C(1')); 10.59,10,72 (2 s, 2 NH). Anal. talc. for CI8Hz5BrN6O5
{485.3): C 44.55, H
5.19, N 17.32, found C 44.90, H 5.28, N 16.81.
4-Amino-3-bromo-1-(2-deoxy-a-D-erythro-pento ficranosyl)-6-((2-
methylpropanoyt)amino j-IH'-
pyrazoto(3,4-d)pyrimidine (14). The slower migrating zone from the above
reaction afforded
compound 14 as a colorless amorphous solid (0.2 g, 22%). RE (A) 0.36: W
(MeOH): 282 ( 10900),
CA 02569615 2006-12-18
- 129 -
237 (49500).'H-NMR ((D6)DMSO): 1.04, 1.07 (m, CH(CH~)z); 2.21 (m, Ha-C(2'));
2.7I (m, Hp-
C(2')); 2.90 (m, CH(CH3)z); 3.46 (m, Hz-C(5')); 3.78 (m, H-C(4')); 4.38 (m, H-
C(3')); 4.72 (t, J
=5.6, OH-C(5')); 5.28 (d, J= 4.3, OH-C(3')); 6.42 ('t', j=6.4, H-C(1')); 6.96,
7.76 (br, NHz); 10.08
(br, NH). Anal. talc. for C14H19BrNs0~ (415.2): C 40.49, H 4.61, N 20.24,
found C 40.58, H 4.72, N
19.93.
1-[2-Deoxy-,(i-D-erythro-pentof~ccranosyl]-4,6-dibenzamido-3-iodo-1H-
pyraxolo[3,4-d]-pyrimidine
(13b). Compound 2c (1.0 g, 2.55 mmol) was co-evaporated twice with toluene. It
was dissolved in
anhydr. pyridine (40 ml), and TMS-Cl (3.25 ml, 25.5 mmol) was added while
stirring. The reaction
mixture was stirred under argon atmosphere, cooled to 0°, and PhCOCI
(3.0 ml, 25.8 mmol) was
added dropwise within 30 min. After stirring overnight at r.t, the mixture was
diluted with EtOAc
(200 ml), washed with a sat. aq. NaHC03 soln. (200 ml) and ice-cold H20 (200
ml). The aq, phase
was extracted with EtOAc (2 x 400 ml). The combined org. phases were
evaporated to dryness and
the residue dissolved in THF/MeOHlHzO ( 250 ml, 5:4:1). The dark orange soln.
was cooled to 0°C,
then 2 N NaOH (25 ml) was added, and stirring was continued for another 40
min. The residue was
purified by FC (CH2Clz/MeOH, 98:2 -~ CHZCIz/MeOH, 95:5) to yield an amorphous
solid ( 1.I5 g,
75%). TLC (B): Rf0.4. W (MeOH): 244 (17400), 276 (14200). IH-NMR ((D6)DMSO):
2.13 (m,
Ha C(2')); 2.67 (m, Hp-C(2')); 3.38, 3.52 (m, Hz-C(5')); 3.84 (m, H-C(4'));
4.46 (m , H-C(3')); 4.72
(t, J= 5.7, OH-C(5')); 5.29 (d, J= 4.4, OH-C(3')); 6.66 ('t', J= 6.5, H-
C(1')); 7.5I - 8.11 (m, arom.
H);10.54,10.78 (s, 2 NH). Anal. talc. for Cz4HzuNeOs (586.4): C 48.01, H 3.53,
N 14.00; found: C
47.93, H 3.53, N 14.05.
3-Bromo-I-[2-deoxy-S-O-(4,4'-dimethoxytrityl)-J3-D-erythro pentofuranosyl]-4,6-
diisobutyrytamino-
1H-pyrazolo(3,4-d]pyrimidine (15a). Compound 13a (0.5 g, 1.03 mmol) was
coevaporated with
anhydr, pyridine for three times and dissolved in pyridine (1.5 mI). DMT-Cl
(0.45 g,1.33 mmol)
was added, and the mixture was stirred at r.t. for 3 h. The reaction was
quenched by addition of
MeOH and the mixture evaporated to dryness and coevaporated with toluene for
three times. FC
(CHZCIz: MeOH, 10:1)) gave 15a as a colorless foam (0.57 g, 70%). Rf (B) 0.3.
LJV (MeOH): 237
(52000), 283 (10500).'H-NMR ((D6)DMSO): 1.04--1.17 (m, 2 (CH3)zCH)); 2.29 (m ,
Ha C(2'));
CA 02569615 2006-12-18
-130 -
2.85 (m, Hp-C(2')); 2.89 (m, 2 (CH3)ZCH)); 3.07 (m, HZ-C(5')); 3.71 (s, 2
Me0); 3.94 (m, H-C(4'));
4.46 (m, H-C(3')); 5.35 (m, OH-C(3')); 6.57 (m, H-C(1')); 10.57, 10.74 (s, 2
NH). Anal. talc, for
C39H43BrNs0, (787.2): C 59.47, H 5,46, N 10.67; found: C 59.08, H 5.37, N
10.39.
3-Bromo-1-(2-deoxy-5-O-(4,4'-dimethoxytriphenylmethyl)-,l3-n-erythro-
pentofuranosyl)-4,6-
diisobutyrylamino-IH-pyrazolo(3,4-dJpyrimidine 3'-((2-Cyanoethyl)-N,N-
diisopropylphosphoramidite (16a). To a sole, of compound 15a (0.24 g, 0.3
mmol) in anhydr.
CHaCIz (3 rnl) (Ar) (iPr)2EtN (0.16 ml, 0.9 mmol) and 2-ryanoethyl
diisopropylphosphoramidochloridite (91 ~l, 0.41 mmol) was added, and the
mixture was stirred at
r. t. for 30 min. The reaction was monitored by TLC. Then, the reaction
mixture was diluted with
CHZCIz, and the sole. washed with a 5% aq. NaHC03 sole, twice and with brine.
The organic phase
was dried (NazS04) , concentrated, and the product was separated by FC to
yield a colorless foam
(0.25 g, 84%). Rf(E), 0.63, 0.69. W (MeOH): 282 (10000), 237 (49500). 3'P-NMR
(CDCl3),149.61,
149.65.'H-NMR ((D6)DMSO): 1.11-1.35 (m, 4 CH(CH3)z); 2.51 (m , Ha-C(2')); 2.66
(m, Hp-
C(2')); 2.95-3.91(m , HZ-C(5')); CH(CH3)2; CHZCHz); 3.80 (s, Me0), 4.26 (m, H-
C(4')); 4.82 (m,
H-C(3')); 6.71 (m, H-C(1')); 6.75-7.44 (m, atom. H); 8.39, 8.59 (br, 2 NH).
Anal. talc. for
C48~60BrNgOgP (921.2): C 58.36, H 6.08, N 11.35; found: C 58.86, H 6.18, N
11.55.
1-(2-Deoxy-5-O-(4,4'-dimethoxytrityl)-/j-D-erythro pentofuranosylJ-4,6-
dibenzamido-3-iodo-IH-
pyrazolo(3,4-dJ-pyrimidine (15b). Compound I3b (450 mg, 0.75 mmol) was
coevaporated twice
with anhydr. pyridine. The residue was dissolved in pyridine (2 ml) and
dimethoxytrityl chloride
(305 mg, 0.9 mmol) was added. After 4 h stirring, the soln. was diluted with 5
ml of MeOH and
washed with a 5% aq. NaHC03 soln. (3 x 20 ml). The organic phase was dried
(NaZS04) and
concentrated to dryness. The residue was purified by FC (CH~C12/acetone, 95:5 -
3 CHZC12/acetone,
9:1) yielding 375 mg (55%) of a colorless foam. TLC (CH2Clz/acetone 9:1):
Rf(B) 0.4. W (MeOH):
244 (16900), 276 (16200).1H-NMR ((D6)DMSO): 2.35 (m , Ha C(2')); 2.67 (m, Hp-
C(2')); 3.07,
3.09 (2 m, Hz-C(5')); 3.70 (s, 2 OCH3); 3.96 (m , H-C(4')); 4.56 (m , H-
C(3')); (d, j= 4.8, OH-
C(3')); 6.72 - 8.11 (m, atom. H);10.54,10.78 (2 s, 2 NH). Anal. talc. for
C45H39~607 (902.73): C
59.87, H 4.35, N 9.31; found: C 59.93, H 4.33, N 9.39.
CA 02569615 2006-12-18
-131-
1- j2-Deoxy-5-O-(dimethoxytrityl)-~i-t~-erythro-pentofuranosyl]-4,6-
dibenzamido-3-iodo-T H
pyrazolo j3,4-d] pyrimidine 3'- j(2-Cyanoethyl-N,N diisopropylphosphoramidite]
(16b). Compound
15b (330 mg, 0.37 mmol) was dissolved in THF (5 ml). (iPr)ZEtN (186 X11, 1.07
mmol) and 2-
cyanoethyl diisopropylphosphoramidochloridite ( 108 pl, 0.48 mmol) were added
under argon
atmosphere. After 30 min the mixture was diluted with CHZC12 {20 ml) and a 5%
aq. NaHC03 soln
(2 x 20 ml). The mixture was extracted with CH2C12 (3 x 15 ml). The combined
organic layer was
dried (Na2S04) and evaporated to an oil. The residue was submitted to FC
(CHzCIz/EtOAc, 85:15)
yielding 280 mg (69%) of a colorless foam. TLC (D): Rf 0.8. g1P-NMR (CDC13):
149.37, 149.38.
Example 3 -Investigation of array precursor compounds
According to standard methodology, the oligonucleotides shown in Table 19 were
synthesized from
the phosphoramidites accoxding to this invention 19 (= 8a and 16a) to 23 in
scheme 11 and
standard A,G,C and T phosphoramidites as already described above. The
phosphoramidites are also
available from Glen Research and were used according to the manufacturer's
instructions
(Orderungnumbers: 10-1906-02 (20), 10-1964-02 (21), 10-1056-02 (22), 10-1067-
02 (23)). The
hybridisation behaviour was investigated as described using W/VIS spectroscopy
measuring
temperature dependent absorption at a wavelength of 260 nm.
CA 02569615 2006-12-18
-132 -
i-BuHN Br
p 0
,. ,~ r
~I
I~BuHH N N "~
~ ~p_ ~ MMf HN ,~,r.,~a~,~. p ~, i~-Prh p
(Me0~Tr0~ ~OCHzCHZCN p ' I
0 ZO H NCI. P rk
~~0 _p
NCCHzCHiO' ~ANCI-Prh HzCN
19 ~=8a='16a~ 21
c~
23
NC
NHZ 8t
HgN ~I N H N'~'~../''a~l~H
O
Ho
HO ~~ ~..t r..,~ 'pH
2b 2~
0
0 0
HN -- H~'~..~.~ N CH3
~f
o N HzN''r N
HO D 2~ HO 0
HA HO
Table 19. Tm Values and Thermodynamic Data of Duplex Pormation ofAntiparallel
or Parallel
Oligonucleotide Dupelxes CarryingFluorescent Reporter Groups and/or Aminoalkyl
Linkers for the
Immobilization on Arrays °~.
Oligonucleotide Tm 0H° ~S° 0G°3to
CA 02569615 2006-12-18
- 133 -
[°C] [kcal/mol] [callK mol] [kcal/mol]
5'-d(TAG GTC AAT ACT) (102) 47 -86.8 -243.7 -11.3
(103)
3'-d(ATC CAG TTA TGA)
i
S'-d(TAG GTC 2b2bT ACT) ( 110)56 -91.4 -251.7 -13.4
( 103)
3'-d(ATC CAG TTA TGA)
i
5'-d(Z4 TAG GTC AAT ACT) (500)49 -90.0 -254.4 -11.1
( 103)
I 3'-d(ATC CAG TTA TGA)
I
5'-d(24 TAG GTC 2b2bT ACT) 56 -87.2 -239.3 -13.0
(501) (103)
3'-d(ATC CAG T T A TGA)
5'-d(24 TAG GTC AAT ACT) (500)49 -95.2 -270.8 -11.2
(502)
3'-d(ATC CAG TTA TGA 25)
5'-d(24 TAG GTC 2b2bT ACT) 56 -92.7 -256.6 -13.1
(501) (502)
3'-d(ATC CAG T T A TGA 25)
5'-d(24 TAG GTC AAT ACT) (500)50 -86.5 -242.7 -11.2
(503)
3'-d(ATC CAG TTA TGA 26)
5'-d(24 TAG GTC 2b2bT ACT) 5g -95.1 -262.1 -13.8
(SO1) (503)
3'-d(ATC CAG T T A TGA 26)
5'-d(T 27 A TAA 27 T 27 27 42 -77.1 -220.6 -8.7
TA) (504)(505)
5'-d(A G T ATT G A G G AT)
5'-d(24 T 27 A TAA 27 T 27 43 -71.9 -201.8 -9.3
27 TA)(506)(507)
5'-d(A G T ATT G A G G AT 26)
5'-d(24 T 27 A T2b2b 27 T 27 48 -g3.7 -265.4 -11.3
27 TA) (508).(507)
5'-d(A G T AT T G A G G AT
26)
~ Measured UV-spectrophotometricallyin 10 mM dylate,10
at 260 nm Na-caco mM MgClz,100
mM NaCl (pH 7) at 5 pM + 5 thermodynamic
E.~M of single strand concentration. data
The were
calculated using the program
Melton (3.0).
It could be shown that the melting behaviour is very similar in the parallel
and antiparallel mode
even when labelled with reporter groups. The situation is similar to the
situation when the nucleic
acid binding compound according to the invention is bound to surface or builds
up an array or
when it binds to an array of natural or non-natural nucleic acid binding
compounds exemplifying
the usefulness of the present invention. This is exemplified by the
oligonucleotide 501 containing a
linker by which it can attached to a solid phase and hybridizes to a labelled
target nucleic acid like
the oligonucleotide 503.
Example 4 - Visualization of antiparallel and parallel hybridization
CA 02569615 2006-12-18
-134-
The hydrogen bonding interaction pattern in parallel or antiparallel duplexes
is visualized
in scheme 12 and 13.
Scheme 12
H H
~N N-H ~ ~ ~ ~ 0 CH3 ~N N-H ~ ~ ~ ~ O
~--N
N~~~~ H-N ~> N ~~~~H-N
N~ N ~ N
O ~ O CH3
Watson Crick AT-Base Pair I Reverse Watson Crick AT-Base Pair II
antiparallel parallel
H H
.... O CH3 ,... O
~--N
H-N~ , ~H-N
h--N
.1....0 ~ ....0 CH3
H
Watson Crick n2AT-Base Pair III Reverse Watson Crick n2AT-Base Pair IV
antiparallel parallel
R H
.-....H . . .. O CH3 , . . . O
N ~N
.... H-N ~ ~ H N
~N
.~H . . . . O ~ . . . . p CHg
Watson Crick PT-Base Pair V Reverse Watson Crick PT-Base Pair V!
R=H,Br,I R=H,Br,I
parallel parallel
Scheme 13
CA 02569615 2006-12-18
-135-
H H
H-N I~~~~ O
~-.N
.... N
...~ N~
~N
. . . . O ~ ~ H-N
\H
Watson Crick GC-Base Pair VII Reverse Watson Crick iGC-Base Pair VIII
antiparallel parallel
H . H
O~~~~ H-N / ~~~~ H-N
~~--N ~--N
/ N-H ~~ ~~ N / -H ~~ ~~ N
N-=-~
N-H ~~ ~~ O -H ~~ ~~ O CH3
i
H
Reverse Watson Crick GfC-Base Pair IX Reverse Watson Crick G"'eiC-Base Pair X
parallel parallel
CA 02569615 2006-12-18
- 136 -
References
[1] N, Ramzaeva, F. Seela, Helv. Chim. Acta 1996, 79,1549.
[2] F. Seela, M. Zulauf, J. Chem. Soc., Perkin Trans. 1 1999, 479.
[3] F. Seela, G. Becher, Helv. Chim. Acta 1999, 82, 94.
[4] B. L. Gaffney, L. A. Marky, R. A. Jones, Tetrahedron 1984, 40, 3.
[5] C. Bailly, M. J. Waring, NucleicAcids Res. 1998, 26, 4309.
[6] A. Chollet, E. H. Kawashima, Nucleic Acids Res. 1988,16, 305.
[7] A. Chollet, A. Chollet - Damerius, E. H. Kawashima, Chem. Scri. 1986, 26,
37.
[8] F. B. Howard, H. T. Miles, Biochemistry 1983, 22, 597.
[9] G. M. Lamm, B. J. Blencowe, B. S. Sproat, A. M. Iribarren, U. Ryder, A. I.
Lamond,
Nucleic Acids Res. 1991,19, 3193.
[ 10] J. D. Hoheisel, H. Lehrach, FEBS Lett. 1990, 274, 103.
[11] J. Sagi, E. Szakonyi, M. Vorlickova, J. Kypr, J. Biomolec. Struct.
Dyn.1996,13,1035.
[ 12] S. Gryaznov, R. G. Schultz, Tetrahedron Lett. 1994, 35, 2489.
[ 13] G. Haaima, H. F. Hansen, L. Christensen, O. Dahl, P. E. Nielsen, Nucleic
Acids Res.
1997, 25, 4639.
[ 14] V. Boudou, L. Kerremans, B. De Bouvere, E. Lescrinier, G. Schepers, R.
Busson, A.
Van Aerschot, P. Herdewijn; Nucleic Acids Res. 1999, 27, 1450.
[15) F. Seela, H. Driller, Helv. Chim. Acta 1988, 71, 757.
[16] G. Balow, V. Mohan, E. A. Lesnik, J. F. Johnston, B. P. Monia, O. L.
Acevedo,
Nucleic Acids Res. 1998, 26, 3350.
[17] F. Seela, H. Steker, H. Driller, U. Bindig, LiebigsAnn. Chem. 1987, 1S.
[18] P. Seela, G. Becher, Synthesis 1998, 207.
[ 19] L. D. Garaeva, I. V. Yartseva, S. Y. Melnik, Nucleosides Nucleotides
1991,10, 1295.
[20] F. Oertel, H. Winter, Z. Kazimierczuk, J. A. Vilpo, P. Richter, F. Seela,
Liebigs Ann.
Chem. 1992, 1165.
[21] J. Davoll, K. A. Kerridge, J. Chern. Soc. 1961, 2589.
[22] L. D. Garaeva, I. A. Korbukh, Y. V. Dobrynin, T. G. Nikolaeva, M. N.
Preobrazhenskaya, Pharm. Chem. J. (Engl. Transl.) 1988, 22, 523.
[23] J. van Wijk, C. Altona, 'PSEUROT 6.2 -A Program for the Conformational
Analysis
of the Five-Membered Rings', University of Leiden, July 1993; C. A. G.
Haasnoot, F.
A. A. M. de Leeuw, C. Altona, Tetrahedron 1980, 86, 2783.
[24] F. Seela, G. Becher, H. Rosemeyer, H. Reuter, G. Kastner, I. A.
Mikhailopulo, Helv,
Chim. Acta 1999, 82, 105.
CA 02569615 2006-12-18
-137-
[25] G. Blackburn, M. J. Gait, in 'Nucleic Acids in Chemistry and Biology',
IRL press,
Oxford University Press 1990, p. 28.
[26] B. S. Sproat, A. M. Iribarren, R. G. Garcia, B. Beijer, Nucleic Acids
Res. 1991, I9, 733.
[27] A. Cano, M. F. Goodman, R. Eritja> Nucleosides Nucleotides 1994,13, 501.
[28] I. Luyten, A. Van Aerschot, J. Rozenski, R. Busson, P. Herdewijn,
Nucleosides
Nucleotides 1997, 16, 1649.
[29] G. S. Ti, B. L. Gaffney, R. A. Jones, J. Am. Chem. Soc. 1982,104,1316.
[30] K. Groebke, J. Hunzicker, W. Fraser, L. Peng, U. Diederichsen, K.
Zimmerman,
A. Holzner, Ch. Leumann, A. Eschenmoser, Helv. Chim. Acta 1998, 81, 175.
[31] B. C. Froehler, P. G. Ng, M. D. Matteucci, Nucleic Acids Res. 1986, I4,
5399.
[32] N. D. Sinha, J. Biernat, J. McManus, H. Koster, Nucleic Acids Res. 1984,
12, 4539.
[33] L. J. McBride, R. Kierzek, S. L. Beaucage, M. H. Caruthers, J. Am. Chem.
Soc.
1986,108, 2040.
[34] F. Seela, C. Wei, A. Melenewski, Y. He, R. Kroschel, E. Feiling,
Nucleosides
Nucleotides 1999, I8, 1543.
[35] F. Seela, A. Melenewski, Eur, j. Org. Chem 1999, 485.
[36] F. Seela, G. Becher, Helu Chim. Acta 2000, 83, 928.
[37] F. Seela, C. Wei, HeIv. Chim. Acta 1999, 82, 726.
(38] M. D. Kirnos, I. Y. Khudyakov, N. I. Alexandrushkina, B. F. Vanyushin,
Nature
1977, 270, 369.
[39] F. B. Howard, C. Chen, J. S. Cohen, H. T. Miles, Biochem. Biophys. Res.
Commun.
1984,118, 848.
[40] E. A. Lesnik, S. M. Freier, Biochemistry, 1995, 34, 10807.
[41] Ribozyme Pharmaceuticals Inc., 2950 Wilderness Place, Boulder, CO 80301,
USA.
[42] F. Seela, C. Wei, G. Becher, M. Zulauf, P. Leonard, Bio. Org. Med. Chem.
Lett. 2000,
10, 289.
[43] J. A. McDowell, D. H. Turner, Biochemistry, 1996, 35, 14077.
CA 02569615 2006-12-18
- 138 -
SEQUENCE LISTING
<110> F. HOFFMANN-LA ROCHE AG
<120> NUCLEIC ACID BINDING COMPOUNDS CONTAINING PYRAZOLO[3,4-D]-
PYRIMIDINE ANALOGUES OF PURIN-2,6-DIAMINE AND THEIR USES
<130> PAT 53750AW-1
<140> PCT/EPO1/08850
<141> 2001-07-31
<150> EP 00116816.0
<151> 2000-08-03
<160> 39
<170> PatentIn Ver. 2.1
<210> 1
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(loo)
<220>
<223> n in positions 4 and 8 denotes compound 2c
<400> 1
agtnttgncc to 12
<210> 2
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(102)
<400> 2
taggtcaata ct 12
<210> 3
<211> 12
<212> DNA
<213> Artificial Sequence
CA 02569615 2006-12-18
- 139 -
<220>
<223> Description of Artificial Sequence:oligonucleotide
(103)
<400> 3
agtattgacc to 12
<210> 4
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(104)
<220>
<223> n in positions 2, 7, 8 and 10 denotes compound 1
<400> 4
tnggtcnntn ct 12
<210> 5
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(105)
<220>
<223> n in positions 4 and 8 denotes compound 1
<400> 5
agtnttgncc to 12
<210> 6
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(106)
<400> 6
CA 02569615 2006-12-18
- 140 -
taggccggca ct 12
<210> 7
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(107)
<400> 7
agtgccggcc to 12
<210> 8
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(108)
<220>
<223> n in positions 7 and 8 denotes compound 2a
<400> 8
taggtcnnta ct 12
<210> 9
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(109)
<220>
<223> n in positions 4 and 8 denotes compound 2a
<400> 9
agtnttgncc to 12
<210> 10
<211> 12
<212> DNA
<213> Artificial Sequence
CA 02569615 2006-12-18
- 141 -
<220>
<223> Description of Artificial Sequence:oligonucleotide
(110)
<220>
<223> n in positions 7 and 8 denotes compound 2b
<400> 10
taggtcnnta ct 12
<210> 11
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(111)
<220>
<223> n in positions 4 and 8 denotes compound 2b
<400> 11
agtnttgncc to 12
<210> 12
<211> 12
<212> RNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(114)
<400> 12
aguauugacc ua 12
<210> 13
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(115)
CA 02569615 2006-12-18
- 142 -
<220>
<223> n in following positions denotes: 3 + 4 = isoC, 6
+ 11 = isoG
<400> 13
atnnanttat na 12
<210> 14
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(116)
<220>
<223> n in following positions denotes: 2 + 7 = isoC, 9
+ 10 = isoG
<400> 14
tnataantnn at 12
<210> 15
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(200)
<220>
<223> n in position 7 denotes compound 2b
<400> 15
taggtcnata ct 12
<210> 16
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(201)
<220>
<223> n in positions 2, 7, 8 and 10 denotes compound 2b
CA 02569615 2006-12-18
- 143 -
<400> 16
tnggtcnntn ct 12
<210> 17
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(202)
<220>
<223> n in position 8 denotes compound 2c
<400> 17
agtattgncc to 12
<210> 18
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(203)
<220>
<223> n in position 4 denotes compound 2c
<400> 18
agtnttgacc to 12
<210> 19
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(204)
<220>
<223> n in positions 7 and 8 denotes compound 2c
<400> 19
taggtcnnta ct 12
CA 02569615 2006-12-18
- 144 -
<210> 20
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(205)
<220>
<223> n in positions 2, 7, 8 and 10 denotes compound 2c
<400> 20
tnggtcnntn ct 12
<210> 21
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(300)
<220>
<223> n in positions 5 and 9 denotes compound 3a
<400> 21
taggncaana ct 12
<210> 22
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(301)
<220>
<223> n in positions 5 and 6 denotes compound 3a
<400> 22
agtanngacc to 12
<210> 23
<211> 12
CA 02569615 2006-12-18
- 145 -
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(302)
<220>
<223> n in positions 5 and 9 denotes compound 3b
<400> 23
taggncaana ct 12
<210> 24
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(303)
<220>
<223> n in positions 5 and 6 denotes compound 3b
<400> 24
agtanngacc to 12
<210> 25
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(304)
<220>
<223> n in positions 5 and 9 denotes compound 3c
<400> 25
taggncaana ct 12
<210> 26
<211> 12
<212> DNA
<213> Artificial Sequence
CA 02569615 2006-12-18
- 146 -
<220>
<223> Description of Artificial Sequence:oligonucleotide
(305)
<220>
<223> n in positions 5 and 6 denotes compound 3c
<400> 26
agtanngacc to 12
<210> 27
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(400)
<400> 27
taggacaata ct 12
<210> 28
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(401)
<400> 28
tagggcaata ct 12
<210> 29
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(402)
<400> 29
taggccaata ct 12
CA 02569615 2006-12-18
- 147 -
<210> 30
<211> 12
<212> RNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(403)
<400> 30
aguauugacc ua 12
<210> 31
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(500)
<220>
<223> n in position 1 denotes compound 24
<400> 31
ntaggtcaat act 13
<210> 32
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(501)
<220>
<223> n in position 1 denotes compound 24 and n in
positions 8 and 9 denotes compound 2b
<400> 32
ntaggtcnnt act 13
<210> 33
<211> 13
<212> DNA
<213> Artificial Sequence
CA 02569615 2006-12-18
- 148 -
<220>
<223> Description of Artificial Sequence:oligonucleotide
(502)
<220>
<223> n in position 1 denotes compound 25
<400> 33
nagtattgac cta 13
<210> 34
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(503)
<220>
<223> n in position 1 denotes compound 26
<400> 34
nagtattgac cta 13
<210> 35
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(504)
<220>
<223> n in positions 2, 7, 9 and 10 denotes compound 27
<400> 35
tnataantnn to 12
<210> 36
<211> 12
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(505)
CA 02569615 2006-12-18
- 149 -
<400> 36
agtattgagg at 12
<210> 37
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(506)
<220>
<223> n in positions 3, 8, 10 and 11 denotes compound 27
and n in position 1 denotes compound 24
<400> 37
ntnataantn nta 13
<210> 38
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(507)
<220>
<223> n in position 13 denotes compound 26
<400> 38
agtattgagg atn 13
<210> 39
<211> 13
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:oligonucleotide
(508)
<220>
<223> n in positions 3, 8, 10 and 11 denotes compound
27, n in positions 6 and 7 denotes compound 2b and
n in position 1 denotes compound 24