Note: Descriptions are shown in the official language in which they were submitted.
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
METHOD FOR CONSOLIDATING TOUGH
COATED HARD POWDERS
FIELD OF THE INVENTION
[001] A method of consolidating Tough-Coated Hard Powders (TCHP)
to essentially full density at low or no pressure, and articles consolidated
using this method, is disclosed. The method is a cost-effective method of
making sintered bodies of TCHP materials based on liquid phase sintering
that provides increased value over conventional hard articles and tool
materials known presently in the art.
BACKGROUND OF THE INVENTION
[002] Sintering may be defined as the thermal treatment of a powder
or compact for the purpose of bonding the particles together to create a solid
article.
[003] In certain applications where the powder is comprised of a
mixture of powders of at least two distinct materials with different melting
points, the powder mixture is compacted into a porous ("green") body. This
body is heated above the melting point of the lowest melting constituent and a
portion of the compacted loose powder mixture is liquified. After maintaining
the body at the sintering temperature for a predetermined time, the material
is
allowed to cool and the liquid solidifies and "cements" the body into a
densified useful structure. Examples of such systems are copper/tin,
iron/copper, and tungsten carbide/cobalt.
[004] In such processes, the densification of the compacted body
takes place in the presence of a liquid phase, and such sintering processes
are termed "liquid phase sintering" (LPS). In some systems, particularly the
consolidation of "hard metals" such as tungsten carbide and other ceramic
particles, LPS is sometimes called conventional sintering. In LPS processes it
is beneficial to have a certain minimum amount of liquid phase present at
sintering temperature to assure transport of the binder phase to accomplish
uniform distribution and densification. It is also generally beneficial to
restrict
the amount of liquid phase present in order to avoid part shape deformation
and grain growth.
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
[005] This liquefaction enables, for instance, increased mass
transport, particle rearrangement, development of a skeletal structure, and
densification. It is generally thought that this is accomplished by rounding
of
the particles as the external irregularities are liquefied, and by the
migration of
this liquid to fill the voids. Upon cooling, recrystallization and often grain
growth occur. Porosity, as a percentage of the whole volume, may decrease
due to densification of the structure. The rate of densification may be
influenced by, for example, sintering temperature, sintering time, sintering
pressure, sintering atmosphere, and weight fraction of the binder constituent
present.
[006] Liquid phase sintering of conventional hardmetals such as
tungsten carbide - cobalt (WC-Co) compacts is generally performed at
sintering temperatures that range from 1325 C to 1475 C.
[007] As the WC-Co compact is heated during sintering of WC-Co
hardmetals, the cobalt will start to behave like a very viscous liquid at
about
700 C and diffusion will increase with increasing temperature as Co viscosity
correspondingly decreases. The grease-like behavior and viscosity of Co
metal is believed to create capillary attractor forces resulting from the
strong
propensity of Co to wet as much WC surface as possible. This results in a
rearrangement of WC particles and the composite begins to shrink even
before the first liquid phase has formed.
[008] At 1275 C, the Co binder metal begins to dissolve the WC
particles and a ternary eutectic reaction begins to form a Co-W-C alloy. As
temperature continues to increase, the increased surface wetting,
liquefication, and capillary forces cause continued particle rearrangement and
shrinkage of the powder mass into the shape of desired articles as grain
boundaries move through the interface between the WC grains and Co binder
phase.
[009] High density, uniformity, and WC stoichiometry in the sintered
part are basic requirements for WC-Co microstructural integrity and strength.
Ensuring proper local carbon balance during liquid phase sintering, which
eliminates the formation of the brittle carbon-deficient Co3W3C eta phase and
carbon porosity caused by too much carbon is also important in providing the
2
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
fracture toughness of WC-Co materials. Eliminating strength-robbing porosity
and grain growth in the microstructure can be accomplished through selection
of an appropriate sintering temperature and pressure. For example, the
temperature must be high enough to liquefy an adequate amount of material
to accomplish the mass transfer necessary to fill the pores between particles
while maintaining the temperature low enough to avoid WC overdissolution
that causes grain growth. To the extent that capillary forces are insufficient
to
provide densification to close to theoretical density, external pressure may
be
applied.
[010] In conventional sintering, typically small percentages (3-18wt%)
of cobalt are mixed with WC. The cobalt binder plays a role in densification
and its uniform distribution is desired in order to achieve uniformity in WC-
Co
microstructures. Microstructural defects are commonly found in sintered WC-
Co parts. A general cause is inherently imperfect blending (even for long
periods of time) of WC and Co powders that are of approximately equal
diameters. It is desired that this process will encapsulate (or at least
associate) each WC particle with just the right amount of Co such that the Co-
to-WC ratio is essentially uniform throughout the mix. Statistically, it is
highly
unlikely that this result can be achieved because cobalt is not available in
small enough nanoparticles to blend uniformly with the WC particles. Cobalt
oxygenation, explosive pyrophoric reactions, and particle agglomeration are
among the barriers to their availability.
[011] The consequence is a WC-Co mixture with Co-rich and Co-poor
areas. The liquid phase occurs first in the Co-rich zones, and the cobalt,
unsaturated with WC, seeks thermodynamic equilibrium by (a) consuming
nearby smaller WC crystals (the smallest ones may be totally consumed) and
(b) by mobilizing unsaturated Co over long distances toward Co-poor zones to
dissolve more and more WC until saturation is reached. Thus, a higher
temperature than that necessary to create the liquid phase is needed to
liquefy and transport the cobalt to Co-poor zones where it is required for
equilibrium and for sufficient Co liquid to wet the WC particles.
[012] Combating the effects of this uneven Co distribution is typically
done using (a) very long ball-milling times, (b) higher sintering
temperatures,
3
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
and (c) longer sintering times. The ball milling tends to reduce many of the
WC particles into fines, which are preferentially dissolved by Co during
heating. The latter two measures do help spread the binder phase and
normalize the distribution of the liquid Cobalt during sintering, but they
also
increase the dissolution of WC. In addition, some of the Co will penetrate the
WC particles along their grain boundaries because of the WC/WC interface
energy is higher (more positive) than the interface energy of WC/Co, at least
as long as grain boundaries are present with interface angles nearly
perpendicular to the surface. Upon cooling, the saturated WC-Co solution
precipitates WC, preferentially nucleating and recrystallizing WC onto the
adjacent remaining larger undissolved WC crystals, creating the undesirable
Ostwald ripening (grain growth) phenomenon as solidification takes place.
This grain growth proceeds until the temperature is decreased to below the
1275 C ternary eutectic of the Co-W-C system. Figure 1 shows the
pseudobinary WC-Co phase diagram. Sintered densities of nearly 100% are
commonplace for WC-Co materials.
[013] Increasing sintering temperatures thus aids binder mobility but
also causes excessive WC dissolution, resulting in unwanted grain growth.
There is a tradeoff between sintering temperature and sintering time that must
be carefully balanced. The maximum temperature must be high enough to
liquefy enough material to accomplish the mass transfer necessary to fill the
pores between particles (compromises structural strength) while trying to
avoid too high a temperature for too long a time to avoid grain growth (which
also reduces structural strength).
[014] Since control of sintering temperature is one major aspect for
high quality hardmetal microstructures, alternative sintering techniques have
been employed. These techniques include the investigation of shortened
sintering times (e.g. microwave sintering) and use of gas pressures (e.g. hot
pressing, hot isostatic pressing [HiP], and the Ceracon and Roc-Tec sinter-
forging methods) to achieve consolidation at lower temperatures.
[015] Another approach used in consolidating conventional
hardmetals is to increase the weight fraction of the binder such as cobalt.
This
can be in the range of 18-25 wt%. This not only increases the amount of liquid
4
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
present but can have the beneficial effect of increasing the toughness of the
structure. However, this approach has two significant drawbacks and is
therefore generally avoided. First, increasing the weight percent of binder
diminishes the weight percent of WC (the wear-resistant phase) in the
structure and diminishes wear resistance accordingly. Second, increasing the
amount of binder also dissolves more WC, contributing significantly to grain
growth during cooling.
[016] Further, the only means to improve the wear resistance of
conventional carbides (while retaining the high fracture toughness of the WC-
Co substrate) for the past seventy years has been to (a) continuously refine
and improve conventional powder and consolidation processing methods, (b)
to add thin wear-resistant coatings, and (c) to laminate harder materials onto
a WC-Co substrate. Improving conventional WC-Co microstructures is a
delicate balance of time, temperature, grainsize, and other product and
process parameters. Incremental improvements in conventional carbides
have been achieved over the past fifty years through better sintering
temperature control and the use of higher purity, highly uniform WC and Co
starting powders. Since the introduction of external coatings over thirty
years
ago, improvements in wear resistance of materials with the toughness of WC-
Co has been slowed almost to a halt.
[017] While these techniques have reduced the problems that occur in
liquid phase sintering of conventional hardmetals, there nevertheless remains
an unmet need for a method of producing particles with properties that allow
for uniform properties throughout the WC and binder powders upon sintering
and articles formed from such particles.
[018] To avoid the previously described drawbacks, the invention
provides a method of consolidating by liquid phase sintering a new class of
designed-microstructure particulate materials with unprecedented
combinations of property extremes called Tough-Coated Hard Powders
(TCHPs, or EternAloy ). This novel family of sintered particulate materials is
comprised of one or more types of superhard Geldart Class C or larger
ceramic or refractory alloy core particles having extreme wear resistance,
lubricity, and other properties which are (1) individually coated with
CA 02570671 2011-04-26
WO 30061001791 PCTIUS2004/0 13445
nanolayers of a metal compound having a relatively higher fracture
toughness, such as WC or TaC, and (2) coated again with a second layer
comprising a binder metal, such as Co or Ni. The combination of
multiproperty alloys within the TCHP sintered structure allows the combination
of normally conflicting performance extremes, including, but not limited to
toughness, abrasiveness, chemical wear resistance, and light weight, at levels
heretofore to provide materials with superior properties unavailable from the
sintered homogeneous powders. TCHP materials are disclosed in U.S.
Patent 6,372,346 to Toth,
[099] The process of the present invention allows the integration of
thermodynamically incompatible material phases and property extremes in a
single material. Thus, TCHP materials can be engineered to combine
hardness approaching that of diamond with fracture toughness greater than
that of tungsten carbide, and weight approximately that of titanium. As a
result, TCHPs can significantly exceed the wear resistance of conventional
metal cutting and forming tools; abrasives; friction and wear products and
thermal coatings; and automotive, aerospace, heavy industrial, and defense
components.
SUMMARY OF THE INVENTION
[020] In view of the foregoing, there are provided methods of forming
an article from particulate material. The method comprises providing a
plurality of core particles comprised of one core particle material, or a
plurality
of different core particle materials chosen from metal and metalloid nitrides,
metal and metalloid carbides, metal and metalloid carbonitrides, metal and
metalloid borides, metal and metalloid oxides, metal and metalloid sulfides,
metal and metalloid silicides, and diamond.
[0211 An intermediate layer is provided on a majority of the core
particles. The intermediate layer comprises a second compound, different in
composition from the core particle Material and having a higher relative
fracture toughness. The second compound is capable of bonding with the
core particle, material and capable of bonding with a metal chosen from iron,
6
CA 02570671 2006-11-29
WO 2006/001791 PCTIUS2004/018445
cobalt, nickel, copper, titanium, aluminum, magnesium, lithium, beryllium,
silver, gold, platinum and their mixtures. The combination of the core
particle
and the intermediate layer forms coated particles.
[022] An outer layer is applied to the coated particles. The outer layer
comprises a metal chosen from iron, cobalt, nickel, and their mixtures and
forms a substantially continuous outer layer on the intermediate layer. The
combination of the coated particles and the outer layer forms component
particles.
[023) A plurality of the component particles are shaped into an article.
[024] The article is sintered to substantially full density without
significant external consolidation pressure at a temperature sufficient to
liquefy at least a portion of the outer layer, and for a time sufficient to
dissolve
a portion of the intermediate layer in the liquid formed from the outer layer.
[025] Liquids formed from the outer layer and the intermediate layer
are solidified prior to significant detrimental interaction of the liquids
with the
core particles.
[026] In one embodiment, the core particle material has the formula
MaXb, where M is a metal chosen from titanium, zirconium, hafnium,
vanadium, niobium, tantalum, chromium, molybdenum, tungsten, aluminum,
magnesium, copper, and silicon; X is an element chosen from nitrogen,
carbon, boron, sulfur, and oxygen; and a and b are numbers greater than zero
up to and including fourteen.
[027] In another embodiment, the core particle material is selected
from TiN, TiCN, TiC, TiB2, ZrC, ZrN, ZrB2, HfC, HfN, HfB2, TaB2, VC, VN,
cBN, hBN, A1203, Si3N4, SIB6, SiAICB, B4C, B203, W2B5, WB2, WS2, AIN,
AIMgB14, MoS2, MoSi2i Mo2B5, and MoB2.
[028] Metalloid elements are those elements located along the line
between the metals and nonmetals in the periodic table. Metalloids generally
include boron, silicon, germanium, arsenic, antimony, and tellurium. Polonium
is often considered a metalloid, too. Non-limiting examples of nitride
metalloids are cubic boron nitride (cBN) and SI3N4. An example of a carbide
metalloid is B4C. An example of a bimetalloid compound is SiB6.
7
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
[029] Also disclosed herein is a method forming an article from
particulate material that comprises providing a plurality of core particles
comprised of one core particle material, or a plurality of different core
particle
materials, such as those selected from TIN, TiCN, TiC, TiB2, ZrC, ZrN, ZrB2,
HfC, HfN, HfB2, TaB2, VC, VN, cBN, hBN, AI203, Si3N4, SiB6, SiAICB, B4C,
B203, W2B5, WB2, WS2, AIN, AIMgB14, MoS2, MoSi2, Mo2B5, MoB2, and
diamond; and
[030] providing an intermediate layer on a majority of these core
particles in an amount ranging from 10% to 80% by weight of the article. The
intermediate layer generally comprises a second compound, different in
composition from the core particle material and has a higher relative fracture
toughness, wherein the second compound is selected from WC, TaC, W2C,
and a mixture of WC and W2C, thereby forming coated particles.
[031] The coated particles are typically treated as previously
described, which includes applying an outer layer to the coated particles, the
outer layer comprising a metal chosen from iron, cobalt, nickel, and their
mixtures to form a substantially continuous outer layer on the intermediate
layer, thereby forming component particles;
[032] shaping a plurality of the component particles into an article;
[033] sintering the article at a temperature sufficient to liquefy at least
a portion of the outer layer, and for a time sufficient to dissolve from 5 to
90
volume % of the intermediate layer in the liquid formed from the outer layer
to
provide an effective amount of liquid to achieve substantially full density
without significant external consolidation pressure, the solid portion of said
intermediate layer preventing chemical interaction of the liquid with the core
particles; and
[034] solidifying liquids formed from the outer layer and the
intermediate layer prior to significant detrimental interaction of the liquids
with
the core particles.
[035] The sintering temperature and time are such that they do not
result in complete dissolution of the intermediate layer, but at most, lead to
the
dissolution of some part of the intermediate layer, such as 5-50% dissolution
or 50-99% dissolution of the intermediate layer. Indeed, it is the solid
portion
8
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
of the intermediate layer that prevents chemical interaction of the liquid
with
said core particles.
BRIEF DESCRIPTION OF THE DRAWINGS
[036] Fig. I is the pseudo-binary WC-Co phase diagram.
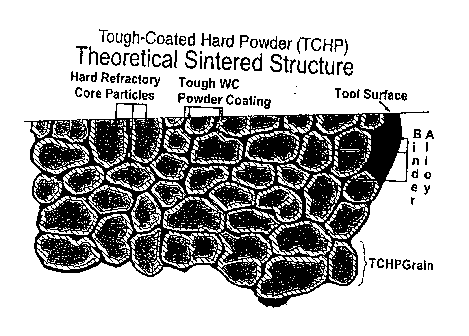
[037] Fig. 2 represents a typical TCHP sintered article.
[038] Fig. 3 is an SEM photograph showing that the TCHP structure is
intact even when excessive Co is included.
[039] Fig. 4 is an SEM photograph showing effective prevention of
WC layer dissolution during and after sintering.
[040] Fig. 5 represents a model of different TCHP materials at various
sintering temperatures. This compares particle dissolution under various
liquid phase sintering conditions.
[041] Fig. 6 is a table of calculated WC-Co solid and liquid phase
compositions at various temperatures and cobalt contents.
[042] Fig. 7 are microstructural photographs of liquid phase sintered
TCHP.
DETAILED DESCRIPTION
[043] The present disclosure describes methods of encapsulating and
sintering fine particles having desired sets of properties with grain boundary
modifiers having other properties, thus allowing for the design of previously
impossible material-property combinations. The TCHP "building block" particle
contains elements, such as hardness + wear resistance + toughness + binder
metal + other designer properties, and gives the materials engineer thousands
of new material grades with engineered properties simultaneously optimized
at the nano-, micro-, macro- and functional levels.
[044] This merging of nanoencapsulation with the sintering of fine
particles creates pseudoalloy structures integrating thermodynamically
incompatible material phases and properties. Such integration allows these
phases and properties to operate, for example, at working surfaces and edges
of tools, as complex components, and as thermally-applied coatings.
Combination of multiple properties, such as, for example, low weight, low
9
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
coefficient of friction, high/low thermal conductivity, lubricity, and
lubrication, is
accomplished without the traditional limitations imposed by alloys,
laminations, mechanical property enhancement, and heat treatment.
[045] The methods described herein comprise the formation of an
article from particulate material. For example, the particulate material, or
TCHPs, comprises a plurality of core particles, an intermediate coating on a
majority of the particles, and an outer coating on the particles.
[046] In their powdered embodiments, the core particles can be a
unique composite particulate material class that is comprised, for example, of
one core particle material, or a plurality of different core particles
materials
chosen from metals or metalloids of nitrides, carbides, carbonitrides,
borides,
oxides, sulfides, and silicides, or diamond. The core particle materials is
often
a metal compound, having the formula MaXb, where M is chosen from at least
one element chosen from titanium, zirconium, hafnium, vanadium, niobium,
tantalum, chromium, molybdenum, tungsten, aluminum, magnesium, copper,
boron, and silicon, while X is chosen from at least one element chosen from
nitrogen, carbon, boron, sulfur, silicon, and oxygen.
[047] The letters a and b in the formula MaXb are numbers that range
from greater than zero to fourteen. Non-limiting examples of such compounds
include, TiN, TiCN, TiC, ZrC, ZrN, VC, VN, A1203, Si3N4, SiB6, SiAICB, W2B5,
AIN, AIMgB14, MoS2r MoSi2, Mo2B5, and Mo2B. In another embodiment, the
plurality of core particles comprise at least one particle selected from
diamond, cubic boron nitride, and hexagonal boron nitride, and their mixtures
with each other or any of the above-described materials.
[048] "Chosen from" or "selected from" as used herein refers to
selection of individual components or the combination of two (or more)
components. For example, X may comprise only one of nitrogen, carbon,
boron, sulfur, silicon, and oxygen, or it may comprise a mixture of any or all
of
these components.
[049] In other embodiments, a majority of the particles contain an
intermediate layer comprising WC, W2C, tool steel, glassy and devitrified
nanosteel alloys, silicon nitride, or tantalum carbide. Such materials have a
fracture toughness greater than that of cubic boron nitride. It is to be
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
understood, however, that the material of the intermediate layer only need to
have a higher relative fracture toughness than that of the material comprising
the core particles, as well as being capable of bonding with the metal
compound(s) or materials forming the core particles and is also capable of
bonding with a metal chosen from iron, cobalt, nickel, copper, titanium,
aluminum, magnesium, lithium, beryllium, silver, gold, and platinum.
[050] In one non-limiting embodiment, the coated particles have an
average particle size less than about 1000 microns. In another embodiment,
the coated particles may have an average particle size of less than 100
microns, for example, less than about 50 microns, even less than 2 microns
and, further, for example, of less than about 1 micron. In yet another
embodiment, the coated particles may have an average particle size in the
range of 100-1000 nanometers.
[051] In another non-limiting embodiment, the intermediate layer may
have a thickness, after sintering, in the range of from 5% to 50% of the
diameter of the core particles. The thickness of the intermediate layer has an
effect on the mechanical properties of the articles made therefrom. In one
embodiment, when the coated particles (the core with an intermediate layer
thereon) have an average particle diameter as measured graphically in a
photomicrograph of a cross-section using the mean free path method of less
than about 2 microns, the resistance to dislocation movement within adjacent
sintered particles is enhanced, improving the mechanical properties of the
sintered article. Even using a classic mechanical approach, using finite
element analysis, it is apparent that increasing the thickness of a spherical
shelf WC surrounding a TiN sphere from about 0.1 micron to about 0.4 micron
can increase the theoretical toughness over 40%. As the WC, TaC, W2C, or
WC and W2C coatings are decreased below from about 150 nanometers, it is
believed that image stresses begin to progressively increase fracture
toughness well above that predicted by a finite element analysis. As
discussed by N. Louat, Acta Metallurgica, Vol. 33, No. 1, p. 59-69 (1985),
"image stresses" are defined as intrinsic Newtonian resistance to
microstructural dislocation glide.
11
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
[052] Such an intermediate layer may be deposited by at least one
method chosen from chemical vapor deposition, physical vapor deposition,
plasma deposition, laser cladding or deposition process, plasma cladding,
magnetic plasma deposition, electrochemical plating, electroless plating,
sputtering, solid phase synthesis, solution chemistry deposition processes,
and combinations of such processes.
[053] In certain embodiments depending on the compound or
compounds being deposited, the various precursors used for a given
compound deposited, the layer deposition method used from the previous
paragraph, the core particle chemistry, the intermediate layer thickness, and
the desired properties of the coating, the intermediate layer is deposited at
a
temperature that may range from 20 C to about 8000 C, such as, for
example, from 20 C to 125 C. In other embodiments, the intermediate layer is
deposited at a temperature that may range from 125 C to 1800 C, from
1800 C to about 8000 C and further, for example, from 200 C to 800 C.
[054] Additionally, in certain embodiments, the intermediate layer
comprises a material selected from, for example, WC, TaC, W2C, or WC and
W2C in an amount that may range from, for example, 60% to 98% by weight
of the article. In another embodiment, the intermediate layer comprises WC,
TaC, W2C, or WC and W2C in an amount that may range from, for example,
10% to 60% by weight of the article. In yet another embodiment, the
intermediate layer comprises WC, TaC, W2C, or WC and W2C in an amount
that may range from, for example, 5% to 10% by weight of the article.
[055] In certain embodiments, the majority of coated TCHP particles
can then encapsulated by an outer binder layer that may, for example, be
continuous. This layer may comprise cobalt, nickel, iron, their mixtures,
their
alloys, or their intermetallic compounds deposited on the outer surface of the
second metal compound layer. The outer layer typically has a thickness after
sintering in the range of from 3% to 12% of the diameter of the coated
particles. Such outer layer may further comprise at least one layer chosen
from other metals, or ceramic, binder, sintering aid, and polymeric material.
[056] The outer layer may be deposited by at least one of the
following methods: chemical vapor deposition, physical vapor deposition,
12
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
plasma deposition, laser cladding or deposition process, plasma cladding,
magnetic plasma deposition, electrochemical plating, electroless plating,
sputtering, solid phase synthesis, or solution chemistry deposition process,
and combinations thereof. In one embodiment of TCHP, the previously
mentioned outer layer comprises at least one compound selected from metal,
ceramic, binder, sintering aid, waxes, or polymeric materials. In the case of
binders, sintering aids, waxes, or polymeric materials, coating may be
accomplished by means of mixing or blending, with or without the addition of
heat in the range of 50 to 150 C.
1057] TCHP coating layers may be deposited throughout a wide range
of temperatures, using many different processes, with CVD being the most
common. The most common temperature range for CVD coating deposition
is 200 C to 800 C. However, much higher temperatures (1800 C to about
8000 C) are typical for processes such as plasma deposition, magnetic
plasma deposition, pulsed laser deposition and electric arc discharge.
Furthermore, much lower temperatures (20 C to 200 C) are typical for
processes such as sol-gel solution chemistry, electrochemical and electroless
depositions.
[058] As with the intermediate layer, the various outer layer
embodiments are deposited at different temperatures depending on the
compound or compounds being deposited, the various precursors used for a
given compound deposited, the layer deposition method used from the
previous paragraph, the core particle chemistry, the intermediate layer
thickness, and the desired properties of the coating, the outer layer may be
deposited at temperatures ranging from 20 C to 650 C. In one embodiment,
the outer layer is deposited at a temperature ranging from, for example, 20 C
to 125 C. In another embodiment, the outer layer is deposited at a
temperature ranging from, for example, 125 C to 650 C. In another
embodiment, the outer layer is deposited at a temperature that may range
from, for example, 200 C to 550 C.
[059] As stated, the outer layer of the particle generally has a
thickness after sintering in the range of from 3% to 12% of the diameter of
the
coated particles. The thickness of the outer layer may allow strain fields
13
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
associated with dislocations in one coated particle to be transmitted through
the outer binder layer to the immediately adjacent intermediate layer.
[060] In one embodiment, the outer layer comprises an amount, for
example, up to 45% by weight of the article and further, for example, from
about 0.5% to 3.0% by weight of the article. In another embodiment, the outer
layer comprises an amount ranging from greater than 3.0% to 18% by weight
of the article, and in yet another the outer layer comprises an amount ranging
from greater than 18% to 45% by weight of the article.
[061] The combination of the core particles, intermediate layer, and
outer layer may form a coated particle, having an average particle size of,
for
example, less than about 1 micron.
[062] By using the above-described powders, a sintered TCHP
embodiment comprising a plurality of sintered TCHP coated composite
particle variants having a plurality of core particle compounds or elements as
described above can be engineered to simultaneously reside in a common
contiguous microstructure of high fracture toughness comprised of the particle
intermediate coatings and binder layers. It is these combinations and
permutations of over 30 different core particle compounds and elements that
gives the TCHP family such a profound diversity of property variance each
with unique property combinations.
[063] Generally, TCHPs are fabricated for eventual consolidatation
into or clad onto articles. Consolidated TCHP articles are designed for
numerous applications, such as those demanding both extreme wear
resistance and high toughness. In their consolidated embodiments, TCHPs
are a unique material class essentially comprised of plural composite TCHP
coated particles sintered into a unified whole. In certain embodiments, the
TCHP-coated particles are sintered into articles utilizing liquid phase
sintering.
In one embodiment, the articles are liquid phase sintered utilizing cobalt as
the binder phase. In other embodiments, nickel or iron or alloys of cobalt,
nickel, and iron bay be used as binders. Consolidation during this sintering
process may occur primarily from capillary forces.
[064] Liquid phase sintering of TCHPs may be facilitated by several
factors. One factor is a substantially uniform distribution of the material
14
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
comprising the outer layer throughout the powder. For purposes of describing
the distribution of this material, "uniform" means that the outer layer on the
surface of the intermediate layer of the particles is such that the material
of
the outer layer is evenly distributed throughout the body of the unsintered
compacted powder. This may be achieved, in certain embodiments, by
adding cobalt (or other material comprising the outer layer on the particle)
atom-by-atom during coating, to encapsulate the surface of the highly
contiguously WC-coated TCHP particle with the targeted Co:WC ratio. This
continues until the desired Co:WC ratio is uniformly distributed on the TCHP
particles and throughout the powder. This feature of TCHP allows the
conditions to be adapted to suit many different targeted TCHP compositions,
such as, for example, by (a) protecting the core particles from dissolution by
the binder and (b) providing a contiguous tough support structure. The result
is higher sintering temperatures than those used for conventional WC-Co
materials, while reducing the requirement for high external pressure, without
risking WC grain growth and loss of strength. More uniform Co distribution
also results in far better microstructural consistency and a homogeneous
distribution of wear-resistant phase core particles. This resulting TCHP
homogeneous microstructure has superior microstructural integrity. This
leads to fewer structural defects and further translates into better, more
consistent material properties, with a concomitant increase in performance.
[065] In certain embodiments, sintering may occur at conditions, such
as temperature, and/or consolidation pressure, for a time sufficient to obtain
a
liquid phase in the outer layer, the intermediate layer, or both in an amount
up
to, for example, 99.5%, such as 70% by volume of the layers, not including
the core particle volume and further, for example, up to 45% by volume of the
layers, not including the core particle volume.
[066] In certain embodiments, sintering temperatures may range, for
example, from 600 C to about 8000 C. In one embodiment, the sintering
temperature may range from 600 C to 1700 C, such as, for example, from
1250 C to 1700 C. In another embodiment, the sintering temperature may
range, for example, from 1700 C to about 8000 C.
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
[067] In one non-limiting embodiment, the sintering temperature may
range, for example, from 600 C to 1700 C and the amount of the liquid phase
may range, for example, from 6 to 44% by volume of the layers, not including
the core particle volume.
[068] Generally, TCHP consolidation takes place at some pressure
higher than absolute zero pressure, such as in the range from zero absolute
pressure to atmospheric pressure.
[069] Typically "vacuum" sintering pressures take place in the range of
I - 760 torr (760 torr = I atmosphere), and this is commonly referred to as
"pressureless" sintering. In this instance, the use of lower-than-atmospheric
pressure is generally for two purposes: control of chemical reaction rates and
control of physical processes during the various temperature ranges
employed during the sintering process. The gases may include but are not
limited to nitrogen, argon, helium, hydrogen, neon, krypton, xenon, methane,
acetylene, carbon monoxide, carbon dioxide, and their mixtures and related
compounds.
[070] It is to be understood that "pressureless" sintering only refers to
the sintering or consolidation at sintering temperatures, not the forming of
pre-
fired (or "green") articles during cold or warm compacting processes, such as
cold isostatic pressing (CIP). During compacting procedures, external
consolidation pressure is generally applied in an amount sufficient to form a
"green" article. It would be clear to one of ordinary skill in the art that
sintering
does not occur during warm or cold compacting processes.
[071] Binders typically used to add green strength to articles formed
from the TCHP described herein, include, but are not limited to, paraffin
waxes, stearic acid, ethylene bis-stearamide (EBS), plasticizers (such as
polyvinyl alcohol, polyethylene glycol, or synthetic resins), and similar
related
organic compounds.
[072] Certain TCHP core powders, such as nitrides, including but not
limited to TIN, ZrN, and HfN, react to high sintering temperatures by off-
gassing nitrogen. The liberation of N2 frees Ti atoms, which can deplete the
WC coating of carbon, creating an off-stoichiometric condition that is harmful
to TCHP mechanical properties. Examples of chemical TCHP reactions that
16
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
can be prevented or promoted through use of lower-than-atmospheric
pressures include oxidation and reduction reactions (such as decarburization,
deoxidation, denitrification, offgassing, or chemical decomposition of various
constituents in the core powder or coatings). Control of these oxidation and
reduction reactions is desired for consistency in sintered parts and for
stabilizing processes that further aid in densification.
[073] Some TCHP core particles are very irregularly shaped and may
require the addition of lubricants to aid in their consolidation since they
are not
rounded off by dissolution. In addition, the thin WC and Co TCHP coatings
require protection from airborne oxygen and moisture, and this may require an
additional polymeric protective coating. Examples of physical TCHP
processes that can be controlled through use of lower-than-atmospheric
pressures include transport of polymeric materials (e.g. debinding or delubing
rates), volatilization rates, heat transfer rates, and possible thermal
decomposition of constituent materials.
[074] Polymeric materials are used in these TCHP applications as
fugitive binders and lubricants, for protective encapsulation, and for shelf-
life
enhancement, for example, include those previously mentioned, e.g., paraffin
waxes, stearic acid, ethylene bis-stearamide (EBS), plasticizers (such as
polyvinyl alcohol, polyethylene glycol, or synthetic resins), and similar
related
organic compounds.
[075] Pressures below atmospheric pressure are typically not used for
consolidation purposes. One purpose of absolute pressures above
atmospheric is to consolidate the PM part. However, gas pressures above
atmospheric may also address control of the chemical reactions listed above.
[076] It is understood that the volume of the liquid phase in the outer
layer or the intermediate layer may be increased by increasing at least one
parameter chosen, for example, from the sintering temperature, the sintering
pressure, and the binder material content. A non-limiting example of the
binder material is cobalt.
[077] The very uniform distribution of Co, both locally and throughout
the entire TCHP body, reduces the requirement for high external pressure by
permitting an increase in the sintering temperature up to that needed above
17
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
the 1275 C eutectic point to obtain the amount of liquid phase necessary for
mass transport and TCHP densification.
[078] In sintering TCHP, even at sub-eutectic temperatures, the
wetting angle of the cobalt layer on the WC coating may, for example, be
small and, further, for example, may be zero. In one embodiment, the cobalt
in TCHP, coated directly on the WC layers, need only travel extremely short
distances to wet and cover the WC coatings. During heating of TCHP, the
outer layers of atoms in each WC layer first diffuse and then dissolve into
the
outer Co layer. The WC layer is uniformly dissolved from the outside inwards.
In TCHP, these layers achieve thermodynamic equilibrium and liquid phase
with greatly reduced cobalt mobility needed.
[079] In certain embodiments, cobalt does not penetrate the coatings
to the core particle. For example, a highly contiguous WC(,-),) coating
surface
structure typical of the contiguity of CVD coatings on tool inserts and other
articles may be present. The CVD-deposited WC(,,) polycrystals at
deposition temperatures can be up to two orders of magnitude smaller and
more tightly packed than those found in conventional milled WC-Co particles.
During carburization of the WC(1_x) coating to stoichiometry, there is grain
growth within the coating polycrystals (depending on carburization
temperature). However, the intimate proximity of cobalt to these polycrystals
is such that the coating polycrystals will be dissolved uniformly around the
WC
coating and the equilibrium may limit grain growth. In Figures 3 and 4, it can
be seen in the WC coating structures after sintering that the polycrystals may
be one order of magnitude smaller than conventional milled WC-Co
polycrystals. In another embodiment, grain growth up to about 1 micron may
occur in zones where significant Co-pooling occurs.
[080] The imperviousness of the TCHP WC coatings to Co attack may
be due at least partly to the following explanation. It is axiomatic that the
WC
and Co in TCHP will behave chemically essentially like the WC and Co in
conventional hardmetal blends. By evaluating the WC-Co phase diagram (see
Figure 5), it can be determined (see Figure 6) that while sintering a typical
TCHP target matrix consisting of 50v% of the particle (75wt%) WC coating
with a coating composition of 94wt%WC - 6wt%Co at 1500 C, 87.1wt% of the
18
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
WC coating (or 92.7% of the original 50v% WC coating) remains as protective
solid WC in the TCHP coating on the TCHP core particles. Since the WC
coating dissolves from the outside in, the remaining solid WC can only be
present as the targeted core-protective and structural coating.
[081] As the cobalt softens and approaches liquid phase, some
particle rearrangement would be expected, but rearrangement alone will be
insufficient to provide full densification, so additional WC must be
liquefied.
Densification can be obtained even with very low volumes of liquid phase.
Since the liquid phase Co is uniformly distributed in TCHP, almost completely
along all WC surfaces, without pooling or gradients, a very low volume of
liquid Co binder may at provide a major part of liquid phase sintering. It is
believed that dissolution of WC must provide the remaining part of the liquid
phase sintering.
[082] As stated, the WC coatings of TCHP particles generally dissolve
from the outside in leaving an undissolved protective and structural layer
around the core particles, and re-precipitate and nucleate to reinforce the
existing particle coatings or as kinetically-transported pore and interstitial
filler
material. As used herein "interstitial filler" means a material that fills the
interstices (small spaces) between adjacent particles. Only partial
dissolution
of the WC coating layer in the Co binder is necessary for densification, WC re-
precipitation/recrystallization, and creation of contiguous TCHP
microstructural matrix integrity. The only Co and WC mobility required is that
needed to transport material to fill the diminishing nearby interstitices
between
coated core particles.
[083] In theory, there are at least three avenues for increasing the
dissolution of a solute in a solvent: (1) increasing the amount of solvent
present (in one embodiment the Co:WC wt% ratio), (2) increasing the
temperature of the solvent and solute, and (3) reducing the pressure on the
solvent and solute. In reality, there are only two avenues to increasing the
amount of liquid phase present during sintering TCHPs. The first two
avenues are discussed.
[084] A certain number of core particles, for example, a transition
metal carbides and nitrides, will interact chemically with cobalt, nickel and
19
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
other binders. These core particles are referred to as the "soluble core"
group
particles. With respect to increasing the temperature, even if the TCHP
sintering temperature were to be considerably increased to an amount
sufficient to provide the necessary amount of liquid-phase for LPS - a thick
WC layer will still be present to protect the "soluble core" group particles
from
attack by cobalt. It should be possible to increase the temperature as high as
required to get any additional liquid phase ("lubricant + interstitial filler
+
capillary attractor material") needed for full density with minimal concern
about
grain growth.
[085] For example, in one embodiment, such as a 1 micron core
TCHP, TiN particle, with the WC and TiN v% being equal, the initial WC
coating (spherical model) will be almost 129 nm thick, and will comprise about
75 wt% of the total particle. Dissolution of the WC at 1500 C will remove only
7.9 nm, or about 6% of the coating thickness, leaving about 121 nm, or about
94% of the original coating thickness for core particle protection, inter-core
particle distance uniformity, and structural toughness.
[086] Because of this feature of TCHP, increasing the amount of
binder phase solvent present, by for example, increasing the cobalt layer
thickness is another feasible sintering method that can be used according to
the methods described herein. For example, increasing cobalt weight
percentages higher than are customary in WC-Co sintering become feasible
as a means of providing the needed dissolution, capillarity, WC kinetics, and
densification for TCHP.. It is to be remembered that in TCHP, the WC is
primarily present as a tough matrix material because the real wear resistance
is being provided by the core particles. Added cobalt will therefore add to
the
amount of liquid phase during sintering while at the same time increasing
fracture toughness after cooling.
[087] Sintering may occur in a process chosen from sintering press,
vacuum, powder injection molding, plastified extrusion, hot press, hot
isostatic
press (HIP), sinter-HIP, sintering furnace, laser cladding process, plasma
cladding, high velocity oxygen-fueled (HVOF), spark plasma sintering,
pressure plasma sintering, pressure-transmission medium, dynamic/explosive
compaction, sinter forge, rapid prototyping, electron beam, and electric arc.
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
[088] In TCHP, the WC coating protects the core particles. First,
during sintering, especially in the "soluble core" group, the WC coating can
protect the core particles from dissolution by the binder metal and can also
protect the matrix from harmful pollution such as, for example, by TIN, ZrN,
NbC. During use, the highly wear resistant TCHP core particles can protect
the WC-Co support matrix from wear after sintering while the support matrix
protects the brittle phases from fracture and pullout. Figure 2 depicts the
sintered microstructure of a typical TCHP material.
[089] The TCHP structure with small hard core particle size and
tough, nanoscale shells separated by thin cobalt ligaments below one
micrometer between grains, improves, for example, elasticity, hardness,
fracture toughness, and strength. In one non-limiting embodiment, even with
a low hardness material (such as cobalt) the image stresses from dislocations
near the surface (and all are near surfaces with submicrometer grains), the
composite properties are higher than possible in abrasive composites.
[090] According to the methods described herein, TCHP provides
sinterable metal particulate materials that can be engineered to afford an
optimum balance of properties, such as, for example, toughness, strength,
low frictional coefficient, and hardness. In one non-limiting embodiment,
operating improvements that can be observed in dies and other tooling
fabricated from TCHP's are, for example: (a) a lower friction coefficient at
the
interface between the work piece and tool, yielding reduced heat, wear, and
cratering, and requiring less processing power and auxiliary use of external
lubricants, ultimately resulting in longer tool life and better process
control; (b)
a low reactivity with iron, reducing sticking and diffusion, flank, or die
wear,
and in turn extending the service life of the drawing die; and (c) a sintered
tool
microstructure in which the tough, strong coating material (e.g., WC) on the
particles forms a cellular support macrostructure for the tool while, at the
same time, providing a surface conforming and tightly-bound protective layer
for the hard particulate cores (of, for example, TiN), holding them in
position
and permitting optimal exposure and hard phase retention at the wear-
resistant tool surface. This is in contrast to articles produced by
conventional
methods wherein Ti-Co-WC alloying drastically lowers binder strength that
21
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
exists between the particles and the binder itself lowers the level of
toughness
and bending strength, or in which a sintered article is wholly coated to
impart
hardness, wherein the thin coating has limited life or cracks.
[091] Placing the hard-phase alloys inside, as the core particle instead
of at the outside, distributes hard-phase alloys (exposed at the external
surfaces after finish grinding) throughout the sintered microstructure in much
greater proportions or thicknesses than is possible in any known conventional
material. This in itself can, for example, increase wear resistance, reduce
chemical interaction with the work piece, and lower coefficient of friction
significantly. Tool life may be enhanced by the constant renewal of surface
grains that wear or are pulled away by the opposing sliding surface.
[092] Also, the wear resistance and adhesion characteristics of many
of the possible core materials are known from their performance in
conventional materials, so their performance as core particle materials is, in
light of the present disclosure, predictable. Since, in certain non-limiting
embodiments, the core particles are coated with known materials (e.g., WC)
blending and sintering together coated particles having several different core
materials will facilitate enhancement of multiple characteristics.
Accordingly,
the cost of development and testing are reduced while providing a final
material with unique properties. Thus, designing a sintered microstructure
where each particle has a tough shell (the intermediate layer) that can adhere
very strongly to its neighbor particles to form a tough cellular support
system
throughout the sintered article substrate, produces a sintered article with a
high combination of strength, high elastic modulus, fracture toughness, and
hard alloy content.
[093] In certain embodiments, resultant article macrostructure is a
cellular microstructure framework composed of tough, strong, tightly
interbonded coated particle shells, each containing and supporting at least
one material chosen from mechanically and chemically-bonded core particles,
crystals, fibers, and whiskers, exposed in cross-section at the external
surfaces during finish grinding and polishing. This principle of optimizing
the
combination of different materials for the core particles and the surrounding
intermediate layer allows the combination of normally conflicting article
22
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
performance characteristics, such as, for example, strength and hardness, at
levels not achievable with conventional materials.
[094] This concept can give a material designer multiple tools that
may be used singly or in combination and a straightforward method providing
easy and total control in adapting the TCHP particle structure (intermediate
layer thickness, size, and core materials) and mix (integrating different
powders into tool and article zones) to meet many different unique, combined,
and specialty demand conditions with a single article or tool.
[095] Moreover, using a standard strong material (such as WC, for
example) as a tough outer particle shell dramatically reduces the research,
development and industrialization effort because only one material reaction
precursor gas (for example, tungsten carbide) will have to be used to coat the
powder particles, instead of the many dozens of complicated precursor and
reactant gases used in multiple external substrate coatings. Such particulate
materials will sinter as if made of tungsten carbide particles, for example,
which are already known to bond very strongly to neighboring tungsten
carbide particles with a binder such as cobalt. The tungsten carbide coating
thickness on the particle may be increased, for example, to meet more
challenging strength applications or may be decreased, for example, in more
critical wear applications to solve most design challenges. For instance, the
core particle size can readily be increased to meet more severe requirements
for wear resistance or decreased for higher strength applications. Using
different core particle materials with characteristics such as, for example,
hardness and coefficient of friction, known or found to perform better in
specific applications, such as, for example, for flank wear or crater wear,
may
also be accomplished by selection of the core material. It is also possible to
blend the above thickness, diameter, and core material powder parameters to
solve most multiple criteria applications.
[096] Articles made from TCHP particles combine the best mechanical
properties of strength, hardness, high elastic modulus, fracture toughness,
low interaction with the work piece, and low coefficient of friction that
exist
separately in conventional materials into an article of unmatched combined
properties. TCHPs have virtually unlimited uses in the manufacture, surface
23
CA 02570671 2006-11-29
WO 2006/0111791 PCT/US2004/018445
modification, or repair of components, assemblies, and machines. One
component group includes cutting, forming, grinding, measuring, petroleum,
and mining and construction tools. Nontool components include biomedical,
military, electronic, sports, thermal management, and cosmetic applications.
Extensive industrial applications will be found in the agricultural, civil,
lumber
and paper, petrochemical, rubber and plastic, transportation,
aircraft/aerospace, maritime, architectural, and energy sectors. Thus this
material is well-suited for use in a broad array of articles including, for
example:
[097] tooling, such as wire drawing dies, extrusion dies, forging dies,
cutting and stamping dies, forms, forming rollers, injection molds, shears,
drills, milling and lathe cutters, saws, hobs, broaches, reamers, taps and
dies;
[098] individual mechanical parts, such as gears, cams, journals,
nozzles, seals, valve seats, pump impellers, capstans, sheaves, bearing and
wear surfaces;
[099] integrated co-sintered components to replace mating parts
internal combustion engine connecting rods, bearings and/or to provide hard
surface zones in powdered metal (P/M) mechanical parts substituted for
forged or machined steel parts with heat treated zones, such as camshafts,
transmission parts, printer/copier parts;
[0100] heavy industrial articles such as deep well drilling bits, teeth for
mining and earthmoving equipment, hot rolls for steel mills; and
[0101] electromechanical components such as memory drive reading
heads, specialized magnets.
[0102] The fact that consolidated TCHP articles are macroscopically
homogeneous, rather than externally coated, can offer users or suppliers the
opportunity of economically regrinding and reusing the initially worn
articles.
This is especially important for tools such as wire drawing dies, twist
drills,
milling cutters, and water jet nozzles.
[0103] It will be appreciated by those skilled in the art that changes
could be made to the embodiments described above without departing from
the broad inventive concept thereof. It is understood, therefore, that this
invention is not limited to the particular embodiments disclosed, but it is
24
CA 02570671 2006-11-29
WO 2006/001791 PCT/US2004/018445
intended to cover modifications which are within the spirit and scope of the
invention, as defined by the appended claims.
[0104] Unless otherwise indicated, all numbers expressing quantities of
ingredients, reaction conditions, and so forth used in the specification and
claims are to be understood as being modified in all instances by the term
"about," which intended to mean +/- 5% of the number expressed.
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in the following specification and attached claims are approximations
that
may vary depending upon the desired properties sought to be obtained by the
present invention.