Note: Descriptions are shown in the official language in which they were submitted.
CA 02570809 2007-01-03
WO 02/16235 PCT/GB01/03648
-1-
SPRAY DEVICE
The present invention relates to a respiratory.aid
adapted to combat more reliably the symptoms of a subject
with a respiratory disorder, in particular to a spray device
(eg an inhaler) comprising an aerosol forinulation
administered to the respiratory or nasal tract.
Various respiratory aids are now widely available to.a
subject wishing to self-administer therapeutic and.
preventative agents to combat the symptoms of a respiratory
disorder such as asthma. These aids come in a range of shapes
and sizes to suit the age and ability of the subject. Such
respiratory aids include inhalers and auxiliary devices such
as spacers,optimisation chambers, reservoirs, expansion
chambers.and deceleration chambers. ~.
A common example of a respiratory aid is the inhaler
which is a manually operated device used to dispense into the
respiratory passages a discrete amount of a therapeutic or
preventative agent (eg in the form of a spray). One of the
benefits of an inhaler is that the subject is able to manage
the respiratory disorder through self-administration of a
preventative agent. A successful preventative regime relies
on regular self-administration of the preventative agent to
avert breathing difficulties and other respiratory symptoms.
Breathing difficulties may occur suddenly and
indiscriminately and their onset frequently leads to a loss
in co-ordination. A second benefit of an inhaler is that
sudden respiratory attacks may be treated by immediate self-
administration of the desired therapeutic agent. The
preventative and therapeutic benefits of the inhaler rely on
the subject being able to locate reliably and effortlessly an
inhaler with an at least partially charged container.
CONFIRMATION COPY
CA 02570809 2007-01-03
WO 02/16235 PCT/GB01/03648
-2-
In principle inhalers are available in two types,
namely an aerosol device powered by a propellant (eg a
metered dose inhaler of the type described in inter alia GB-
A-2293110) qr a powder containing device (eg a metered dose
powder inhaler). The therapeutic or preventative agent may
be in dry powder or liquid (eg suspension) form and generally
speaking is drawn into the respiratory passages by
simultaneously dispensing the agent and taking a sharp intake
of breath.
Most forms of aerosol inhaler comprise a metal
container for the therapeutic or preventative agent and a
discharge valve through which the agent may be dispensed
continuously or discretely via a nozzle. For example,
conventional metered dose inhalers comprise a metal canister
secured to a metered dose valve. A determination of the
number of doses remaining in the canister requires a manual
record of the number of doses which have been dispensed (for
example using a mechanical counter). Many counter devices of
a mechanical type have been proposed.
US-A-3505870 discloses a metal aerosol container with a
transparent window in a small circular opening in the base.
The present invention is based on the recognition that
the welfare of a subject having a respiratory disorder (such
as asthma) may be improved by assisting them to rapidly and
reliably assess the status of the container. More
particularly, the present invention relates to a respiratory
aid such as an inhaler adapted so that the subject may
rapidly determine how much of the preventative or therapeutic
agent remains within the container.
Thus viewed from one aspect the present invention
provides a respiratory aid (eg a medical aerosol device) for
CA 02570809 2008-09-18
use in sel.f-adminiatration of an agent for combatting (eg
preventing or treating) the symptoms of a respiratory
disorder, eaid respiratory aid comprising: a container for
the agent operatively connected to a discharge valve through
which a therapeutically or preventatively effective amount
of said agent may be dispensed via a nozzle and
an actuator body adapted to actuate the valve, wherein
the container comprises :
a glass vial coated with a coating of polymeric material,
the coating of polymeric material having at least one clear or
translucent portion arranged to permit observation of
level of agent in the containex.
In accordance with a further aspect of the present
invention, there is provided a medical aerosol device
comprising a container and a discharge valve through which
fluid may be dispensed via a nozzle, an actuator adapted to
actuate the valve, the container comprising a glass vial
coated with a layer of polymeric ma.terral, the polymeric
material having at leaat one clear or translucent portion
extended axially of the vial and arranged to permit
observation of the level ot liquid in the container.
The respiratory aid may be any type of inhaler
ira.cluding one of the group consisting of a pressurised
metered doae inhaler (both manually operable and breath
actuated) , an aerosol inhaler and a dry powder inhaler.
Preferably the actuator is adapted to directly or
iruiirectly administer a therapeutically or preventatively
effective am4unt of said agent into the respiratory passages
of a subject through a delivery outlet, aaid delivery outlet
being adapter to fit in the subject's mouth or nose or into
an auxiliary device (such as a spacer or an optitnisation
chamber).
CA 02570809 2007-01-03
WO 02/16235 PCT/GB01/03648
-4-
Glass vials have not previously been used for medical
aerosol devices because of the risk of breakage if the device
is dropped, a risk which is increased in distressed subjects
suffering from an asthma attack. Not only are the shards of
glass hazar(ious but the patient may not have a replacement
device readily to hand. Thus the use of glass in accordance
with the invention is somewhat contrary to conventional
wisdom. Application of a polymeric coating may advantageously
reduce the ri'sk of injury and distress in the event of
breakage.
The polymeric coating may be composed of a.polyolefin
such as polyethylene, polypropylene, polystyrene or
copolymers or blends thereof. Alternative polymers include
ABS, acetyl, acrylic and other polymers. Polypropylene is
preferred.
In a first embodiment the clear or translucent portion
may comprise a window of reduced thickness of polymer
coating. The window may be formed by polishing the portion
of reduced thickness or by polishing the polymer coating to
form a polished portion of reduced thickness.
Alternatively the clear or translucent portion may
comprise a portion of increased thickness, the portion being
polished to.provide a transparent or translucent surface.
Two windows may be provided on opposite sides of the
vial to advantageously facilitate transmission of light.
In a preferred embodiment, the clear or translucent
portion extends axially of the vial. Gradations or other
markings may be provided on or adjacent to the window to
indicate the number of doses available.
CA 02570809 2007-01-03
WO 02/16235 PCT/GB01/03648
The device is preferably a metered dose inhaler device
or nasal spray.
The container may comprise a conventional metered dose
inhaler or nasal spray casing, a window being provided to
facilitate observation of the liquid level within the vial.
Gradations or other markings may be provided on the casing in
addition to or instead of markings on the vial.
The invention is further described by means of example
but not in any limitative sense with reference to the
accompanying drawings of which:
Figure 1 is an elevation of a vial in accordance with
the invention;
Figure 2 is a perspective view of the vial shown in.
Figure 1;
Figure 3 shows the vial and a metered dose inhaler
body;
Figure-4 shows the vial and an alternative metered dose
inhaler body; and
Figure 5 shows the vial with a nasal spray dispenser.
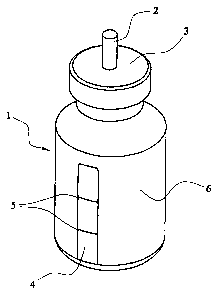
Figures 1 and 2.illustrate a vial in accordance with an
embodiment of this invention. A glass vial 1 has a
conventional aerosol outlet.2 secured by means of a crimped
cap 3. The vial 1 is formedof glass with an outer coating
of polypropylene 6. A window 4 extending axially of the vial
is formed by polishing a portion of the polypropylene coating:
so that'the level of liquid within the vial 1 can be.
observed. Gradations 5 allow a user to check whether the
quantity of liquid available for dispensing is within
predetermined maximum.and minimum limits.
CA 02570809 2007-01-03
WO 02/16235 PCT/GBO1/03648
-6-
Figure 3 shows the vial of Figures 1 and 2 inverted for
insertion into a conventional metered dose inhaler body 7.
The body 7 includes a nozzle 8 defining a mouthpiece 8. The
portion 9...of the body 7 into which the vial 1 is inserted may
be composed of transparent or translucent material so that
the vial 1 can be observed.without removal from the body 7.
Figure 4 illustrates the vial 1 inverted for insertion
into a Norton EASI-BREATHE (Registered Trade Mark) metered
dose inhaler. The inhaler comprises a body portion 10
adapted to receive the vial 1 and having a pivotable cap 11
which may be opened to facilitate dispensing of the drug. A
cap 12secured to the body 10 may be twisted to prime the
metered dose inhaler mechanism (not shown).
In use,=the cap 12 may be removed from the body 10 to
allow inspection of the vial 1. Alternatively a window (not
shown) may be provided in the side of the body 10 to
facilitate inspection of the vial 1 without removal from the
body 10.
Figure 5 illustrates a nasal spray device in accordance
with an embodiment of this invention. The vial 1 is engaged
in a cap 21 having an outlet 22 for insertion into the nasal
cavity. The base 20 into which the body of the vial 1 is
received incorporates a window 24 having gradations 25. In
use the window 4 is aligned with the window 24 so that the
level of liquid within the vial 1 may be observed without
removing the latter from the base 20.
In this embodiment, the gradations 5 are optional.but
may serve to confirm to a patient by alignment with the
gradations 25 that the vial is correctly inserted into the
spray cap 21 and base 20.