Note: Descriptions are shown in the official language in which they were submitted.
CA 02571914 2007-01-08
Auto-oxidation and Internal Heating Type
Reforming Method and Apparatus for Hydrogen Production
This application is a division of co-pending Canadian Patent Application No.
2,323,728, filed October 18, 2000.
Technical Field
The present invention relates to improvement in a method of and an apparatus
for producing hydrogen by a reforming reaction. In particular, it relates to
providing
an auto-oxidation and internal heating type refonning method and apparatus for
hydrogen production.
Background Art
So far in producing hydrogen, a reforming method and apparatus using a
reforming reaction has advantageously been adopted in which a mixture of a
liydrocarbon or an aliphatic alcohol with water vapor is fed onto and
contacted with a
reforming catalyst. In such niethods and apparatus, it is customary that a
quantity of
heat needed for the reforming reaction is provided externally via wall
surfaces of the
refonning reactor.
In such conventional methods and apparatus as described, the necessary
amount of heat for the reforming reaction is supplied externally via the wall
surfaces
of the reforming reactor with a sensible heat of a combustion gas burnt
outside of the
reforming reactor or a sensible heat of a heating medium. It has therefore
been
necessary that an external combustion furnace or burner unit for heat supply
or
therrno-circulating unit for the heating mediunl or thermal catalyst be
separately
provided. As a result, the entire apparatus has had to be large in size and
yet it has
been difficult to obtain well satisfactory thennal efficiency. Also, in the
case of
hydrocarbons use, methane whose refoiming reaction temperature is required for
700
to 750 C is usually used. And if methane is heated externally to reach that
temperature in the reforming reactor, the wall surfaces of the reforming
reactor must
be limited in temperature to 1000 C or less, otherwise the material that makes
up
those wall surfaces tends to deteriorate, rendering the refonning reactor not
usable
over a long period of time and thus causing a difficulty in its workability.
1
CA 02571914 2007-01-08
Disclosure of Invention
The present invention is contrived to solve the foregoing problems. The
present invention is directed towards the provision of a reforming method for
hydrogen production which makes a reforming reactor apparatus compact, which
largely reduces energy cost needed for refonning, and which is capable of
bringing
development of NOX, an environmental pollutant, almost or unlimitedly to zero.
Sueh
method is claimed in the parent filing. The present invention also is directed
to the
provision of a reforming apparatus for carrying out such a method. This
apparatus is
claimed in the divisional filing.
In accordance with one aspect of the present invention, there is provided an
auto-oxidation and internal heating type reforming reactor apparatus for
hydrogen
production for use in a process in wliich a small amount of an oxidizing
catalyst is
admixed with a mass of a reforming catalyst, a small amount of oxygen is
admixed
with a gaseous mixture of a hydrocarbon or an aliphatic alcohol with water
vapor, and
the gaseous mixture is fed into contact with the reforming catalyst in the
mass to bring
about a reforming reaction of the gaseous mixture therewith to produce
1lydrogen and
an exothennal oxidization of a portion of the hydrocarbon or aliphatic alcohol
to
generate an amount of heat required to reform the gaseous mixture of the
hydrocarbon
or aliphatic alcohol with water vapor, wherein the apparatus comprises: a
reforming
reaction catalyst loaded bed having a refonning reaction catalyst and a small
amount
of an oxidizing catalyst distributively admixed therewith; and a thin layer of
an
oxidizing catalyst disposed at least one of behind and ahead of the reforming
reaction
catalyst loaded bed.
These and other features and advantages of the present invention will become
more readily apparent to those of ordinary skill in the art from the following
detailed
description of the preferred forms of embodiment thereof as illustrated in the
various
drawing Figures.
Brief Descri-ption of Drawings
In the accompanying drawings,
Fig. I is a cross sectional view in elevation illustrating a refol-ming appai-
atus
for use in carrying out a method according to the present invention, and as
implemented in a first form of embodiment thereof;
2
CA 02571914 2007-01-08
Fig. 2 is a diagrammatic view illustrating the refonning apparatus in the
first
form of embodiment of the invention shown in Fig. 1 and its related peripheral
equipment;
Fig. 3 is a cross sectional view in elevation illustrating a reforming
apparatus
for use in carrying out a method according to the present invention, and as
implemented in a second form of embodiment thereof;
Fig. 4 is a cross sectional view taken along the line IV-IV as viewed fi=om
top
in Fig. 3; and
Fig. 5 is a cross sectional view in elevation illustrating ai-eforming
apparatus
for use in carrying out a method according to the present invention, and zis
implemented in a third form of embodiment thereof.
Best Modes for Carrying Out the Invention
Research and investigations by the present inventor have revealed that feeding
a bed or layer of a reforming reaction catalyst with a gaseous mixture a
hydrocarbon
or an aliphatic alcohol with water vapor, if the gaseous mixture has a small
anlouat of
oxygen added thereto and the catalytic layer has a small amount of an
oxidizinc,
catalyst added thereto, permits a portion of the hydrocarbon or aliphatic
alcohol to be
exothermally oxidized and to internally derive an amount of heat as required
to
refonn the liydrocarbon or aliphatic alcohol.
In this case, a small amount of oxygen may be added to the gaseous mixture of
a hydrocarbon or aliphatic alcohol with water vapor either in advance or after
the
gaseous mixture on contacting with the reforming catalyst is in part reformed.
Alternatively, a portion of the small amount of oxygen may be added to the
gaseous
mixture of a hydrocarbon or aliphatic alcohol with watei- vapor in advance,
ancl a
reminder the small amoLmt of oxygen may be added after the resultant gaseous
mixture on contacting with the reforming catalyst is part reformed.
By the way, a large difference exists between a hydrocarbon and an aliphatic
alcohol in the amount of an oxidizing catalyst used, because of a difference
in
temperature between their respective reforming reactions as required.
Thus, a hydrocarbon in order for its reforming reaction to be aecomplished
requires a temperature of 700 to 750 C. In the conventionaI external heating
refon-n.er, a hydrocarbon commences its reforming reaction when its gaseous
niixtul-e
with water vapor is externally heated to a temperature arotmd 500 C, the
reforming
3
CA 02571914 2007-01-08
reaction coming to an end substantially when the temperature of the reformin-
reaction is raised up to 700 to 750 C.
Since the refonning reaction is an endothermic reaction, it is important that
an
amount of heat commensurate with the heat absorbed be replenished enough so
that
the reaction temperature may not fall but be maintained in the reforming i-
eaction
catalyst.
According to the method of the present invention, an exothennic oxidizing
i-eaction that is caused to occur inside of the catalyst bed or layer has been
foLmd to
produce a sensible heat which is sufficient to maintain the temperature
substantially in
a range between 700 C and 800 C and thus is capable of maintaining a
temperature as
required for the reforming reaction. To wit, if low temperature hydrogen
produced bv
the refonning reaction brings about a temperature drop in the gaseous inixtLu-
e of its
raw material hydrocarbon with water vapor, oxygen that i-emains in the gaseous
mixture will react with the hydrocarbon again in the presence of the oxidizing
catalyst
in the catalytic bed or layer to restore the gaseous mixture to a temperature
of about
800 C, thus continuing the reforming reaction repetitively. In this way, the
method of
the present invention, by giving rise to a state such as if a multi-stage fine
catalytic
combustion took place in a catalytic layer, is found to largely reduce even
the amount
of a refonning reaction catalyst and to make it possible to reduce the size of
the
apparatus as well. An amount of an oxidizing catalyst that ranges between 1
and 10%
of a refonning reaction catalyst (e. g., for methane, 3% 2%, namely in a
range of I
to 5%) has been found to be suitable for use. For an oxidizing catalyst use
may be
made of any catalyst that can withstand a temperature in this range, although
use is
typically made of platinum, palladium or the like as distributed in the
reforming
catalyst.
An aliphatic alcohol is markedly lower in reforniing temperature than a
hydrocarbon. Not only does its refonning reaction go on at a temperature as
low as
250 to 350 C, but it absorbs less amount of heat for the refonning reaction,
pennitting
the amount of oxygen being fed into a gaseous mixture of the aliphatic alcohol
with
water vapor to be considerably reduced. As a consequence, the reforming
reactor can
even more be reduced in size. For an aliphatic alcohol, an amount of an
oxidizing
catalyst that ranges between 1 and 5 % of a reforming reaction catalyst has
been
found to be suitable for use (e.g., for methanol, 2% 1% is preferable).
4
CA 02571914 2008-03-17
For instance, if the reforming reaction catalyst has a space velocity SV of
approximately 3,000h-I or so,an oxidizing catalyst having an SV of
approximately
100,000h"lor sowill achieve its purposes. Then, it is important that the
oxidizing
catalyst be dispersed and distributed enough evenly in the reforming reaction
catalyst.
Besides, to facilitate commencement of the reaction it has been found
advantageous to
dispose a thin layer of the oxidizing catalyst in front of the reforming
reaction
catalytic layer containing the oxidizing catalyst. Also, for concluding the
reaction
more quickly, it has been found that a thin layer of the oxidizing catalyst
may
advantageously be disposed behind the composite (mixed) refoiining reaction
catalytic layer.
A hydrocarbon or aliphatic alcohol fed into a refomiing reaction catalytic bed
or layer having an oxidizing catalyst admixed therein reacts with oxygen by
the
admixed oxidizing catalyst and is exothermally oxidized. A resultant gaseous
mixture
has its temperature elevated and, upon contacting with the reforming reaction
catalyst, -
brings about a reforming reaction thereby to produce hydrogen. As mentioned
before,
the reforming reaction being an endothermic reaction causes the gaseous
mixture to
reduce in temperature, but the presence of the oxidizing catalyst in the
reforming
reaction catalytic bed or layer downstreams permits an unreacted portion of
the
hydrocarbon or aliphatic alcohol still further to react with such residual
oxygen, thus
generating heat that tends to maintain the temperature of the gaseous mixture
substantially constant. In this way, the reforming reaction is allowed to
continue in
steps and in succession until the amount of oxygen in the gas #'ed may
completely be
consumed. In this case, dimensioning the thickness of the reforming reaction
catalytic
layer traversed by the gaseous mixture to be commensurate with the amount
(rate) of
feed of the gaseous mixture permits the reforming reaction to be concluded at
the end
of a way out of the reforming reaction catalytic bed or layer. From the
standpoint of
environmental control, it is desirable that the residual amount of oxygen be
made
completely nil by providing the exit end of the catalytic layer with a thin
layer made
up only of the oxidizing catalyst.
The ranges of SV suitable for the reactions in the present invention have been
found to lie in 1,500 to 8,000 for a hydrocarbon and 2,000 to 8,000 for an
aliphatic
alcohol.
In the case of hydrocarbons as a raw material in the present invention,
methane (CH4), ethane tC2H6), propane (C3H8), kerosene, gasolim and so forth
can be
CA 02571914 2007-01-08
used, and typically methane (CH4) is used. Then, the reforming reaction
generally
proceeds at a temperature of 750 to 800 C. For aliphatic alcohol for use in
the pi-esent
invention, use may be made of methanol, ethanol and so forth, and typically
used is
methanol. Then, the reforming reaction is carried out usually at a temperature
of 250
to 350 C.
The proportion of water vapor to a hydrocarbon: (H20/C) used typically
ranges between 2.5 and 3.5. The proportion of water vapor to an aliphatic
alcohol:
(H20/C) used typically ranges between 2 and 3.
For a reforming reaction catalyst, use may be made of any one coninioniy
used. Well often used may include NiS-SiOz = A1203, WSz-SiOz = A1703 and NiS-
WS?
Si02 = A1203.
Post-reforming gases include hydrogen, carbon dioxide, carbon monoxide and
water vapor. To increase the hydrogen concentration, a shift reaction may be
employed. As a shift catalyst, ordinarily Fe203 or Fe304 is used, but if the
reaction
temperature exceeds 700 C, it is desirable to use Crz03.
For an oxidizing catalyst, preference is given of platinum (Pt) or palladium
(Pd) which is resistant to a deleterious change at an elevated temperature.
For oxygen to be added, preference is given of pure oxygen. For pure oxygen,
use is made of oxygen produced from a water electrolytic cell or bath that
concurrently produces hydrogen which is advantageously used here as admixed
with
hydrogen obtained from the reforming reactor apparatus in the present
invention.
Noting that a water electrolytic bath or cell has an efficiency nowadays
increased up
to 80 to 90 %, this combination is found to be industrially valuable. hi
economical
view point, it might be possible to use air as a necessary oxygen resource.
However,
use of air reduces the concentration of hydrogen obtained by the reforming
reaction.
Thus, if air is used, it is desirable that an oxygen separation membrane be
eniployed
to increase the oxygen concentration in such air for feeding in the present
invention.
Also, if air is used in reforming a gaseous mixture of a hydrocarbon with
water vapor, such air should desirably have its operating pressure raised to 6
kg/cm'
to 8 lcg/cm2 . Then, the reforming reaction temperature may also be raised
froni the
aforementioned range of 700 to 750 C, fiirther to a range of 800 to 900 C.
This is because of the fact that if air is so used, it is necessary to later
remove
nitrogen (N2) from a reaction gas in order to raise its hydrogen
concentration. This is
attained by cooling the reaction gas after both the reforming and shift
reactions to a
6
CA 02571914 2007-01-08
temperature around 55 C and passing it through a hollow-fiber type separator
membrane apparatus using, e.g., polysulfone. In this case, since the operating
pressure for the reforming reaction to be raised in the range of 6 to 8
kg/cni', the
reforming reaction temperature inevitably should be raised from the noi-mal
range of
700 to 750 C to a higher range of 800 to 900 C.
If an oxygen-enriched air which oxygen concentration was raised by using the
nsembrane-type separator apparatus, and an assumption is made that air is
supplied
under its pressure ranging between 8.4 kg/cm2 and 9.0 kg/cm2, and that the
yield of
oxygen is to be 90 %, the resulted oxygen will have a concenti-ation of 30 to
And if the yield of oxygen is 70%, the resulted oxygen will have a
concentration of 6O
to 63%. Operating conditions may be selected from these i-anges depending on
an
oxygen concentration as required.
An explanation of the present invention is shown as below, specifically in
respect of two forms of embodiment thereof. It should be noted, however, that
these
forms of embodiment are illustrative and not intended to limit the present
invention
thereto.
First Form of Embodiment
Mention is made of the first fonn of embodiment of the present invention with
reference to Figs. 1 and 2. This form of embodiment of the invention is
charactei-ized
in that a gaseous mixture of a hydrocarbon or aliphatie alcohol, with water
vapoi- has
oxygen added therein and admixed therewith in advance.
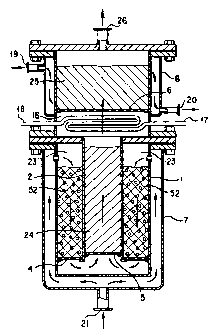
Mention is first made of a reforming reactor apparatus for practicing this
form
of the invention.
The reforming reactor apparatus includes a reforming i-eactor's outer cylinder
I
closed at bottom and a refonning reactor's inner cylinder 2 which define a
space
therewith, in which space a mixture 52 of a reforming reaction catalyst with a
small
amotuit of an oxidizing catalyst is loaded or packed in bed. The space has its
lower
end provided with a refonning reaction catalyst supporting lattice 4 as shown.
A
gaseous mixture liaving undergone a reforming reaction is allowed to pass
through the
lattice 4 and then pass a lower space to flow through an inner flow passage
loaded
with a high temperature shift catalyst 24, during which it is subjected to a
shift
reaction. The shift reaction is a reaction by which a mixture of carbon
monoxide and
water vapor is converted into hydrogen and carbon-dioxide gases and, it being
an
exothemiic reaction, an amount of heat then produced acts to heat, via an
outer wall 2
7
CA 02571914 2007-01-08
of the inner flow passage, an outer pack bed or catalytic layer of the
refonning
reaction catalyst and oxidizing catalyst mixture 52, namely, is fed into the
latter.
Using such a construction makes it possible, with heat generation anticipated,
to
reduce the amount of oxygen being added to the gaseous mixture of hydrocarbon
or
aliphatic alcohol with water vapor.
A further explanation in detail is given below along the gas flow in respect
of
the first form of embodiment shown in Figs. 1 and 2.
A feed gas consisting of the gaseous mixture of hydrocarbon or aliphatic
alcohol with water vapor and a small amoLuit of oxygen added thereto and
admixed
therewith is fed through a reforming gaseous mixture pre-heater inlet 19 and,
then
flowing inside of a low temperature shift reactor jacket 8, is allowed to flow
out
throtigh a refonning gaseous mixture pre-heater outlet 20. The feed gas then
flows
through a reforming gaseous mixture inlet 21 into a reforming reactor outer
cylinder
jacket 7. The feed gas while flowing through the low temperature shift reactor
jacket
8 and the reforming reactor apparatus outer cylinder jacket 7 is pre-heated
and then
entering a reforming reactor cylinder or annular space 23 loaded with a
reforming
i-eaction catalyst and oxidizing catalyst mixture 52, undergoes a refoi-ming
reaction
while in contact with the catalyst.
A gaseous mixture produced in the reforming reaction is passed thorougli the
reforming reaction catalyst supporting lattice 4, and then through a high
temperature
shift catalyst supporting lattice 5, and finally flowing into a high
temperattire shift
catalyst loaded cylinder 24. With the layer of the reforming reaction catalyst
and
oxidizing catalyst mixture 52 in the reforming reaction cylinder or aivlular
space 23
having a small amount of the oxidizing catalyst admixed therewitli in
dispersion, the
gaseous mixture is raised in temperature with a heat generated by a nlulti-
step
oxidizing reaction that it undergoes when it passes through the layer, and
thus permits
the reforming reaction to continues in succession. A reaction gas having undei-
gone
the reforming reaction is reduced in temperah.ire on exchanging heat in the
lower
space of the reforming reactor apparatus with the reforming gaseous mixture
flowing
througli the outer cylinder jacket 7 of the reforming reactor appai-atus and
flows into
the high temperature shift catalyst cylinder 24. A heat is generated by a
shift reaction
in the high temperature shift catalyst cylinder 24 and is transferred into the
reforming
reaction cylinder or annular space 23 though an inner wall thereof, thtts
serving to
reduce the temperature drop there by the endothermic reaction. As a result, it
is nlade
8
CA 02571914 2007-01-08
possible to reduce the amount of oxygen being added to the gaseous of
hydrocarbon
or aliphatic alcohol with water vapor. Since the shift reaction is generally
insufficient
by a high temperature shift reaction, in practice a low temperature shift
reaction is
additionally carried out. Also, since a reaction gas leaving the high
temperature shift
reaction is raised significantly in temperature, it is desirable to cool the
same in an
heat-exchanging arrangement such that as shown in Fig. 2, water raised in
temperature by exchanging heat in a water cooler C is made to flow tllrough a
gas
cooler medium inlet 17 into a high temperature shift reaction cylinder outlet
cooler
16. The cooled reaction gas is passed through a low tenlperature shift
catalyst layer or
bed 25 to conclude the shift reaction and is taken out of the system through a
refonned gas outlet 26. An amount of water vapor produced by 11eat exchange in
the
cooler 16 is taken out through a gas cooler medium outlet 18 for use in the
refonning
gaseous mixture by mixing it with hydrocarbon or aliphatic alcohol and oxygen
in a
mixer F as shown in Fig. 2.
Second Forin of Embodiment
Mention is made of the second form of embodimeiit of the present invention
witlz reference to Figs. 3 and 4. Tliis form of embodiment of the invention is
characterized in that oxygen is added to a gaseous mixture of a hydrogen
containing
i-eformable reactant or composition, here constituted by a hydrocarbon or
aliphatic
alcohol, with water vapor, after the gaseous mixture is in part reformed.
Mention is first made of a reforming reactor apparatus for practicing this foi-
m
of the invention.
The reforming reactor apparatus includes a reforming reactor's outer cylindei-
I
basically closed at bottom aiid more than one refoiming reactor's inner
cylinciers 2
which are disposed spaced apart from each other in the outer cylinder 1. The
outer
and inner cylinders 1 and 2 define a space therewith, in which space a
reforming
reaction catalyst 51 is loaded or packed in bed. The packed or loaded
refonning
reaction catalyst 51 is supported by a reforming reaction catalyst support or
carrier 4,
e. g., in the forrn of a lattice, disposed in the region of the lower ends of
the reforming
reactor's inner cylinder 2. Loaded or packed in each of the refonning
reactor's inner
cylinder 2 are, to say in the order from upstream to downstream of the gas
flow, a
reforming reaction catalyst and oxidizing catalyst mixture 52 containing a
small
aniount of an oxidizing catalyst, a high temperature shift catalyst 24 and a
low
temperature shift catalyst 25 which are supported by the reforming reaction
catalyst
9
CA 02571914 2007-01-08
support 4, a high temperature shift catalyst support 5 and a low temperattu-e
shi tt
catalyst support 6, respectively.
Disposed underneath each of the reforming reactor's inner cylinders 2 is a
mixer 38 for adding oxygen into the reformable gaseous mixture. Each mixer 38
lias
an opening to which oxygen blowing nozzle 39 sits opposite. While as
illustrated in
Fig. 3, the mixer 38 is shown as attached to an inner wall of the refonning
reactor's
inner cylinder 2 by means of a mixer fastening pin 55, it should be noted that
the
mixer niay be of any other form and may be attached in any other way.
As illustrated in Fig. 3, two elongate reforming reactor's inner cylinders 2
are
sllown as disposed left and right, each extending vertically, and are also
shown as
having a refonning gas collecting tube 33 disposed above them as can also be
seen
from the top plan view of Fig. 4. The refonning gas collecting tube 33 so
disposed
and arranged has at its top center a branch tube such that a reforming gas may
be
collected through it upwards.
A further explanation in detail is given below along the gas flow in respect
ol'
the second form of embodiment shown in Figs. 3 and 4.
A gaseous mixture of hydrocarbon or aliphatic alcoliol with water vapor is
introduced through reforming gaseous niixture inlet pipe 49 and allowed to
(low
through the space defined by the reforming reactor's outer cylinder I and the
refonning reactor's inner cyliriders 2.
This space is loaded with the reforming reaction catalyst 51.
A reforming reaction caused by the reforming reaction catalyst and oxidizing
reaction catalyst mixture 52 loaded in the inner cylinders 2 results in a high
temperature gas whose heat is transferred via walls of the inner cylinders 2
into the
refonning reaction catalyst 51 where a portion of the refonnable gaseous
mixture of
hydrocarbon or aliphatic alcohol with water vapor is reformed.
The reformable gaseous mixture in part reformed and passed through the
reforming catalyst support 4, then enters into the mixers 38 where it is
admixed with
oxygen or air from the oxygen or air blowing nozzles 39. The admixture then
passes
through the reforming catalyst support 4 and flows into the space loaded with
the
reforming reaction catalyst and oxidizing reaction catalyst mixture 52 in the
inner
cylinders 2.
In this space, the refonnable gaseous admixture in part reformed is raised in
temperature by the action of the oxidizing reaction catalyst to a target
reaction
CA 02571914 2007-01-08
teriniilation temperature and is then refonned by the action of the refonning
reaction
catalyst, thus terminating the reforming reaction.
The temperature the fully reformed gas is higli and a heat held in that gas is
transferred through the walls of the innei- cylinders 2 to the reformable
gascous
mixture, i. e., to the refonning reaction catalyst 51 in the space between the
refonning
reactor's outer cylinder I and the refonning reactor's inner cylinders 2, thus
reforming
the refonnable gaseous mixture in part.
The fiilly reformed gas is passed through the reforming reaction catalyst and
high temperature shift catalyst support 5 enters the region of the high
temperature
shift catalyst 24 where it undergoes a shift reaction.
Heat generated in this reaction is transferred as in the preceding step though
the walls of the reforming reactor's inner cylinders 2 to the reformable
gaseous
mixh.ire where it is utilized to raise the temperature of the reformable
gaseous
mixture.
The regions whose temperatures are lower and insufficient to cause the
reformable gaseous mixture to be reformed may be simply loaded with ceramic oi-
the
like particles in lieu of the reforming reaction catalyst.
Next, the fully refonned gas whose heat is transferred to the reformable
gaseous mixture and thus which is lowered in temperature flows passed through
thc
low temperature shift catalyst support 6 into the region loaded with the low
temperature shift catalyst 25 where it completes the shift reaction.
The amount of heat that acts to lower any heat or sensible heat generated in
the meantime is transferred via the walls of the reforniing reactor's inner
cylinders 2 to
the reformable gaseous mixture for utilization to raise its temperature as
well as have
been mentioned.
Third Fonn of Embodiment
Mention is made of the second form of embodiment of the present invention
with reference to Fig. 5. This form of embodiment of the invention is
characterized in
that a portion of a small amount of oxygen is added in advance to a gaseous
mixture
of a hydrocarbon or aliphatic alcohol with water vapor coming into contact
with
i-efonning catalyst, and the remainder of the small amount of oxygen is added
to the
gaseous mixture after the gaseous mixture is in part reformed.
Mention is first made of a reforming reactor apparatus for practicing this
('orm
of the invention.
11
CA 02571914 2007-01-08
The reforming reactor apparatus for use in this form of embodiinent is shown
in Fig. 5 as one shown in Figs. 3 and 4 and in part modified. In the modified
arrangement, upstreams of the reforming catalyst bed 51 in the space foniied
between
the refonning reactor's outer cylinder 1 and refonning reactor's inner
cylinder 2, a
region is provided loaded with a refining catalyst and oxidizing catalyst
mixture 52'
that contains a small amount of an oxidizing catalyst.
In the modified arrangement shown in Fig. 5, a gaseous mixture of a
hydrocarbon or an aliphatic alcohol with water vapor having a poi-tion of a
small
amount of oxygen admixed therewith is brought into contact with the reforming
catalyst and oxidizing catalyst mixture 52' and is thereby in part exothei-
mallv
oxidized and reformed while rising its temperature. The gaseous mixture in
part
reformed is passed through the reforming reaction catalyst 51 and is thereby
ftn-tlier
reformed. Thereafter, the mixer 38 acts to admix a remainder of the small
amount of
oxygen into the further reformed gaseous mixture. Then, the resultant gaseous
mixture past the reforming reaction catalyst and oxidizing catalyst mixture 52
raises
its temperature to an aimed reaction termination temperature while being
reformed by
the action of the refining reaction catalyst, thus completing the refonning
reaction.
Once the temperature rises occurs in it, the refonning reactor apparatus
ceases
feeding oxygen for admixing with the gaseous mixture.
The third fonn of enzbodiment of the invention described provides largely
reducing the time required for starting the operation of the refining reactor
apparatus.
With the system so designed as have been described, it is made possible to
reduce to minimum the total quantity of heat required for reformation and to
reducc to
an ultimate limit the amount of oxygen or air required for reformation while
obtainino
an increased concentration of hydrogen produced.
Although the present invention has been described hereinbefore in terms of the
presently preferred forms of embodiments in respect of an auto-oxidation and
internal
heating type reforming method and apparatus for hydrogen production, it is to
be
understood that such disclosure is purely illustrative and is not to be
interpreted as
liniiting. Consequently, without departing from the spirit and scope of the
invention,
various alterations, modifications, and/or alternative applications of the
invention will,
no doubt, be suggested to those skilled in the art after having read the
preceding
disclosure. Accordingly, it is intended that the following claims be
interpreted as
12
CA 02571914 2007-01-08
compassing all alterations, modifications, or alternative applications as fall
within the
trlie spirit and scope of the invention.
13