Note: Descriptions are shown in the official language in which they were submitted.
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
DETECTION OR MEASUREMENT OF ANTIBODIES TO ANTIGENIC
PROTEINS IN BIOLOGICAL TISSUES OR SAMPLES
This application is based on US 60/584,374, filed June 30, 2004, which is
entirely incorporated here by reference.
Field of the Invention:
The present invention in the field of biotechnology and medical diagnostics,
relates to methods for detecting and/or measuring therapeutic or induced
antibodies to
antigenic proteins in a sample, comprising (a) adding a labeled or unlabeled
antigenic
protein or fragment thereof to a sample expected to contain therapeutic or
induced
antibodies, and (b) measuring differences in at least one characteristic
between (i) a
labeled antibody-antigenic protein complex; (ii) an unlabeled antibody-
antigenic protein
complex in the sample; and/or (iii) displaced labeled or unlabeled antibody,
antigenic
protein or fragment thereof.
Background of the Invention:
In response to the presence of foreign or mis-recognized endogenous proteins,
the body can produce antibodies, termed herein as "induced antibodies" which
include
antibodies to antigenic proteins or therapeutic proteins, such as therapeutic
antibodies,
or antibodies to endogenous proteins that are involved, e.g., in inflammatory,
infectious, autoimmune, aging, or neurological diseases or pathologies and
related
conditions. Meanwhile, a therapeutic antibody may form an immune complex with
its
target. One important aspect of such medical treatment is to detect the
presence
and/or measure the amount of induced antibodies or immune complexes in a
patient's
immune response to such therapy or autoimmune condition.
Such detection or measurement is important as a tool in the diagnosis and/or
evaluation of treatment parameters to determine which and how much therapeutic
protein, antibody or other treatment should be used. For example, if a patient
is given
a therapeutic protein for treatment and the patient subsequently produces
induced
antibodies against the therapeutic protein, the amount of induced antibodies
in the
serum could be determined to find out how to modify the dosage or type of
therapeutic
protein administered. Alternatively, the presence and amount of induced
antibodies to
endogenous proteins in an autoimmune patient can be evaluated to diagnose
and/or
1
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
determine appropriate treatment for particular diseases and pre-pathological
or
pathological conditions.
Prior methods have utilized known immunoassay methods to attempt to
measure induced antibody responses to particular therapeutic or endogenous
proteins.
However, these methods have been unreliable. One problem associated with the
methods involves using reagent antibodies to detect and distinguish induced
antibodies from other non-immune antibodies. In addition, there are challenges
in
specifically detecting complexes consisting of the induced antibody and the
antigenic
protein, e.g., where the complexes are not clearly distinguishable from the
uncomplexed induced antibody or other non-immune antibodies present in patient
serum. These difficulties have made previous methods less useful in diagnosis
or
evaluation of treatment of pathological conditions or effects associated with
biologic
therapies.
Accordingly, there is a need to provide alternative methods for detecting
and/or
3.5 measuring therapeutic or induced antibodies to antigenic proteins that are
suitable for
diagnosis or evaluation of treatment in patients having autoimmune conditions
or
conditions that can be treated using therapeutic proteins.
Summary of Invention:
The present invention provides at least one method for the detection and/or
measurement of induced antibodies to antigenic proteins. Such antigenic
proteins or
fragments thereof can include endogenous, foreign or administered proteins,
such as,
but not limited to, antibodies or fragments, such as therapeutic antibodies,
therapeutic
proteins, genetically engineered proteins and labeled or derivatized proteins.
The present invention provides a new method utilizing at least one detectably
labeled or unlabeled antigenic protein or fragment thereof, where the
detectable label
can include, inter alia, at least one radiolabel and/or at least one other
suitable marker,
or any combination thereof. Such method of the present invention can include,
but is
not limited to, the use of characteristic differences (e.g., size, physical or
chemical
characteristic, and/or label differences) between (1) the induced antibody-
antigenic
protein complex; (2) the labeled induced antibody-antigenic protein complex;
and/or (3)
displaced components thereof, to detect or measure induced antibody in
biological
samples, e.g., but not limited to, serum, plasma, whole blood, cerebrospinal
fluid
(CSF), lymph or tissue homogenates,
2
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
In a preferred embodiment of the present invention, radioiabeled and/or
detectably labeled antigenic protein or fragments thereof can be used to
displace
unlabeled antigenic protein from the induced antibody-antigenic protein
complex. The
labeled induced antibody- antigenic protein complex can then be distinguished
and/or
resolved from the unlabeled protein complex, and/or free unlabeled antigenic
protein
by different retention times using chromatography or other methods (e.g., HPLC
size
exclusion chromatography), indicating changed molecular weight. Since human
serum
contains many serum proteins, it can be difficult to distinguish the labeled
induced
antibody-antigenic protein complex from other high molecular weight endogenous
components in the serum via UV absorbance, dynamic light scattering or other
known
methods. The labeled induced antibody- antigenic protein complex is detected
based
on molecular size, label, tag, amplification of the label or tag, and/or the
ability of the
labeled antigenic protein to bind to at least one detectable substrate.
Description of the Drawings
Figure 1 shows a counts per minute (CPM) chromatogram of a radiolabeled
antigenic
protein that has been resolved by size on an HPLC column and detected via the
radiolabel.
Figure 2 shows a CPM chromatogram of an immune complex of an antigenic protein
and a radiolabeled monoclonal antibody to the antigenic protein that has been
resolved by size on an HPLC column and detected via the radiolabel.
Figure 3 shows a CPM chromatogram of an immune complex of an antigenic protein
and a monoclonal antibody to the antigenic protein which has been incubated in
the
presence of excess radiolabeled antigenic protein. The profile of these
proteins is
shown following separation by size on an HPLC column and detection via the
radiolabel.
Figure 4 shows a CPM chromatogram of an immune complex of an antigenic protein
and a monoclonal antibody to the antigenic protein, which has been incubated
in the
presence of excess radiolabeled non-immune IgG.
Figure 5 shows a CPM chromatogram of an immune complex of an antigenic protein
and a polyclonal antibody to the antigenic protein, which has been incubated
in the
presence of excess radiolabeled antigenic protein. The profile of these
proteins is
3
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
shown following separation by size on an HPLC column and detection via the
radiolabel.
Figure 6A is a CPM chromatogram of baseline patient serum sample taken prior
to the
initiation of an infliximab (anti-TNF antibody) treatment regimen. Figure 6B
is a CPM
chromatogram of patient serum taken from the same patient 28 weeks after the
initiation of the treatment (8 weeks after the latest infliximab infusion).
Each sample
was incubated with radiolabeled infliximab followed by separation on an HPLC
column
and detected via the radiolabel.
Figure 7 shows a CPM chromatogram of serum taken from a patient 62 weeks after
the initiation of the treatment (8 weeks after the latest infliximab
infusion). The sample
was incubated with radiolabeled infliximab followed by separation on an HPLC
column
and detected via the radiolabel.
Figure 8 shows a CPM chromatogram of serum taken from a patient 110 weeks
after
the initiation of the treatment (more than 8 weeks after the latest infliximab
infusion).
The sample was incubated with radiolabeled infliximab followed by separation
on an
HPLC column and detected via the radiolabel.
Figure 9 is a graphical representation showing PCR amplification of an anti-
biotin
antibody-DNA conjugate bound to biotinylated infliximab.
Figure 10 shows an expanded CPM chromatogram of an induced antibody-antigenic
protein complex. The antigenic protein is infliximab. The complex is shown in
the
presence or absence of infliximab Fab (iFab) and detected via the radiolabel.
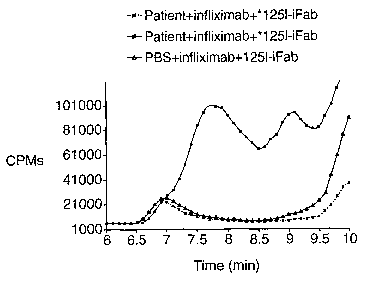
Figure 11 shows an expanded CPM chromatogram of serum taken from a patient
positive with induced antibody against infliximab. The sample was first
incubated with
unlabeled infliximab and then with 1251-labeled infliximab Fab fragment (1251-
iFab). It
was separated on an HPLC column and detected via the radiolabel.
Detailed Description:
The present invention in the field of biotechnology and medical diagnostics,
relates to methods for detecting and/or measuring therapeutic or induced
antibodies to
antigenic proteins in a sample, comprising (a) adding a labeled or unlabeled
antigenic
protein or fragment thereof to a sample expected to contain therapeutic or
induced
antibodies, and (b) measuring differences in at least one characteristic
between (i) a
4
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
labeled antibody-antigenic protein complex; (ii) an unlabeled antibody-
antigenic protein
complex in the sample; and/or (iii) displaced labeled or unlabeled antibody,
antigenic
protein or fragment thereof.
Citations
All publications or patents cited herein are entirely incorporated herein by
reference as they show the state of the art at the time of the present
invention and/or
to provide description and enablement of the present invention. Publications
refer to
scientific or patent publications, or any other information available in any
media format,
including all recorded, electronic or printed formats. The following
references are
Zo entirely incorporated herein by reference: Ausubel, et al., ed., Current
Protocols in
Molecular Biology, John Wiley & Sons, Inc., NY, NY (1987-2005); Sambrook, et
al.,
Molecular Cloning: A Laboratory Manual, 2"d Edition, Cold Spring Harbor, NY
(1989);
Harlow and Lane, Antibodies, a Laboratory Manual, Cold Spring Harbor, NY
(1989);
Colligan, et al., eds., Current Protocols in Immunology, John Wiley & Sons,
Inc., NY
l.s (1994-2005); Colligan et al., Current Protocols in Protein Science, John
Wiley & Sons,
NY, NY, (1997-2005). Furuya, D., et al., Journal of Immunological methods 238
(2000): 173-180.
The antigenic proteins include, for example, therapeutic proteins, diagnostic
proteins, antibodies, natural or genetically engineered proteins, protein
complexes,
20 labeled and derivatized proteins, peptides, and peptide mimetic. The
proteins and
related molecules can be either endogenous or foreign to the animal or human.
The
present invention also applies to antigenic substances such as small
molecules,
nucleic acids, carbohydrates, and lipids. The antigenic substances can be
involved in
therapy and diagnosis of, for example, therapeutic antibody treatable
diseases,
25 autoimmune, neurological and other diseases, aging and the like. The
present
invention applies to sample types including, but not limited to sera, plasma,
isolated
blood cells, lymph, CSF, tissues, tissue homogenates, and the like, as well
known in
the art.
The characteristics that can be measured in the present invention include, but
30 not limited to, retention time, molecular weight, buoyant density,
fluorescence
polarization, poly-ethylene glycol (PEG) precipitation, and/or those known in
the art.
The labels that can be used include, but not limited to, radiolabels (1123,
1125, C14,
H3, etc.), DNA labels, nucleic acid labels, fluorescent labels, enzymatic
labels,
chemiluminescence or other labels. The labeled displaced antibody amounts may
be
5
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
quantitatively correlated with the type, amount and affinity of the induced
antibody.
Labeled or unlabeled proteins and complexes can be separated by chromatography
(HPLC, TLC, etc.), mass spectroscopy, ultracentrifugation, sucrose density
gradient
ultracentrifugation, analytical ultracentrifugation, electrophoresis, and/or
other methods
known in the field. See, e.g., Ausubel, Harlow and Lane and Colligan, et al.,
supra,
and the like, which are entirely incorporated herein by reference.
According to the present invention, antibody titer may be determined by any
method known to the art using standard techniques, including, but not limited
to,
ELISA, RIA, EIA, and other solid phase immunoassays, radioimmunoassay,
nephelometry, rocket electrophoresis, Western blot, immunofluorescence, cell
based
assays, etc. See, e.g., Ausubel, Harlow and Lane and Colligan, et al., supra,
and the
like, which are entirely incorporated herein by reference.
In the following non-limiting examples, samples were analyzed using an
Integral HPLC Workstation (Applied Biosystems, Foster City, CA) configured in
the
is single column mode with a BioSep 3000 size exclusion column (Phenononex,
Torrance, CA) and detected using an ABI dual UV detector at 280 and 220 nm
followed by a radioactivity detector (Packard Instrument Company, Downers
Grove,
IL). These techniques are by way of example only, and the invention can
include any
known method, technique, or material, as well known in the art, based on the
teaching
and guidance presented herein.
Example 1: Detection of experimentally formed antigen and monoclonal
antibody immune complex by intercolation of labeled antigen into the immune
complex
The immune complex of antigenic protein (infliximab, 15.3 ug/mL) and induced
murine monoclonal antibody to the antigenic protein (5.1 ug/mL, 3:1 molar
ratio) was
experimentally formed in normal human serum. At these specified
concentrations, the
induced monoclonal antibody was completely bound by the excess antigenic
protein
and not detectable using current in vitro assay formats.
The CPM chromatogram in figure 1 shows that the retention time of 1251-
labeled antigenic protein (infliximab) is approximately 16.4 minutes, which is
characteristic of the protein's size and shape. The retention time remains
relatively
constant when the HPLC column, flow parameters and mobile phase buffer, are
left
unchanged.
6
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
The immune complex of an antigenic protein and its induced antibody is larger
in size than each of the individual component. Accordingly, its retention time
should
be shorter than that of the uncomplexed antigenic protein or induced antibody.
As
shown in figure 2, the retention time of the immune complex of infliximab
(15.3 ug/mL)
and the radiolabeled induced monoclonal antibody against infliximab (5.1
ug/mL) is
approximately 14.8 minutes. This is shorter than 16.4 minutes, the retention
time of
the 1251-labeled infliximab (Figure 1).
To demonstrate that induced monoclonal antibody can be detected through
radiolabeled antigenic protein, serum containing immune complex of infliximab
and
induced monoclonal antibody against infliximab was incubated in the presence
of
excess 1251-labeled infliximab for 1 hour at 37 degrees. Figure 3 shows the
CPM
chromatogram with peaks at 24.8, 16.8 and 14.8 minutes. These are retention
times
characteristic of free 1251 not associated with protein (24.8 minutes),
uncomplexed
1251-labeled infliximab (16.8 minutes), and immune complex of 1251-labeled
infliximab
and induced murine antibody (14.8 minutes), respectively. It indicates that a
portion of
1251-labeled infliximab was able to integrate into the unlabeled preformed
immune
complex (retention time at 14.8 minutes), while the excess labeled antigenic
protein
remained unbound (retention time at 16.8 minutes). Therefore, induced murine
antibody to infliximab was detected via the ability of excess 1251-labeled
infliximab to
displace unlabeled infliximab in the existing immune complex.
As a control, serum containing immune complex of infliximab and induced
monoclonal antibody against infliximab was incubated in the presence of excess
1251-
labeled normal non-immune monkey IgG for 1 hour at 37 degrees. The normal non-
immune monkey IgG is non-specific for either component of the preformed immune
complex, i.e., it is not capable of binding to either infliximab or the
induced monoclonal
antibody. Its retention time is the same as that of infliximab (which is also
an IgG1
antibody). Figure 4 shows the CPM chromatogram with a single peak at 16.4
minutes,
which the retention time characteristic of 1251-labeled normal non-immune
monkey
IgG. It indicates that non-specific 1251-labeled protein is not able to
integrate into the
unlabeled preformed immune complex.
Example 2: Detection of experimentally formed antigen and polyclonal antibody
immune complex by intercolation of labeled antigen into the immune complex
The immune complex of antigenic protein (infliximab, 15.3 ug/mL) and induced
monkey polyclonal antibody to the antigenic protein (5.1 ug/mL, 3:1 molar
ratio) was
7
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
experimentally formed in normal human serum. At these specified
concentrations, the
induced polyclonal antibody was completely bound by the excess antigenic
protein and
not detectable using current in vitro assay formats.
To demonstrate that induced polyclonal antibody can be detected through
radiolabeled antigenic protein, serum containing immune complex of infliximab
and
induced polyclonal antibody against infliximab was incubated in the presence
of
excess 1251-labeled infliximab for 1 hour at 37 degrees. Figure 5 shows the
CPM
chromatogram with peaks at 24.8, 16.8, 14.4 and 13.2 minutes. These are
retention
times characteristic of free 1251 not associated with protein (24.8 minutes),
uncomplexed 1251-labeled infliximab (16.8 minutes), and complexes with
variable
sizes and stoichiometry of 1251-labeled infliximab and induced polyclonal
antibody
(14.4 and 13.2 minutes), respectively. It indicates that a portion of 1251-
labeled
infliximab was able to integrate into the unlabeled preformed immune complex
(retention time at 14.4 and 13.2 minutes), while the excess labeled infliximab
remained
unbound (retention time at 16.8 minutes). Therefore, induced polyclonal
antibodis to
infliximab were detected via the ability of excess 1251-labeled infliximab to
displace
unlabeled infliximab in the existing immune complex.
Example 3: Detection of infliximab and induced anti-infliximab antibody immune
complexes in patient serum
Serum samples were taken from patient A at week 0 and week 28 after the
initiation of infliximab treatment (8 weeks after the latest infliximab
infusion). Both
were determined by double antigen EIA analysis to be negative for induced
antibodies
to infliximab. No circulating infliximab was detectable using a validated
ELISA in either
sample.
The serum was incubated with approximately 15 g/mL of'251-labeled
infliximab for at least one hour at 37 degrees on a shaking platform. For
serum
sample taken at week 0 (figure 6A), a single peak was detected at 16.4
minutes, the
retention time of uncomplexed 125 I-labeled infliximab. There is no
significantly visible
peak at less than 16.4 minutes (the percentage of the area under the
chromatogram of
retention time less than 16.4 minutes over the total chromatogram area is
approximately 11.6%), which suggests that no complex with higher molecular
weight is
present. Similar pattern was observed for serum sample taken at week 28
(figure 6B)
with a single peak at 16.4 minutes, and the area under the chromatogram of
retention
8
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
time less than 16.4 minutes represents approximately 14.9% of the total
chromatogram area. Therefore, the HPLC analysis confirms the absence of an
induced immune response.
In another experiment, serum samples were taken from patient B at week 62
after the initiation of infliximab treatment (8 weeks after the latest
infliximab infusion).
It was determined by double antigen EIA analysis to be negative for induced
antibodies to infliximab. However, circulating infliximab was not detectable
using a
validated ELISA in this sample. Accordingly, this serum sample is considered
inconclusive, i.e., no detectable induced antibody, but circulating antigenic
protein
(infliximab) is present.
The serum was incubated with approximately 15 g/mL of1251-labeled
infliximab for at least one hour at 37 degrees. The CPM chromatogram (figure
7)
shows a single peak was detected at 16.4 minutes and no significantly visible
peak at
less than 16.4 minutes, which suggests that no complex with higher molecular
weight
is present. This pattern is similar to those in figures 6A and 6B, in which
the sera were
known to be negative for antibodies to infliximab by ELISA. Therefore, the
HPLC
analysis shows that the serum sample which was inconclusive based on ELISA is
negative of an induced immune response.
In another experiment, serum samples were taken from patient C at week 110
after the initiation of infliximab treatment (more than 8 weeks after the
latest infliximab
infusion). It was determined by double antigen EIA analysis to be positive for
induced
antibodies to infliximab (titer 1:10).
The patient serum was incubated with approximately 15 g/mL of1251-labeled
infliximab for at least 1 hour at 37 degrees. The CPM chromatogram (figure 8)
shows
peaks at retention times of 16.4, 14.0 and 11.6 minutes. The retention times
of 14.0
and 11.6 minutes are indicative of immune compiexes of'25I-labeled infliximab
and
induced antibodies against infliximab. Therefore, the HPLC analysis confirms
the
presence of an induced immune response.
Example 4: lmmuno-PCR amplification system
A non-radioactive, immuno-PCR system was developed to detect the presence
of induced antibody. In this assay format, if the immune complex is present in
serum
sample, biotinylated infliximab, which displaces the unlabeled infliximab in
the
9
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
complex, can be detected using an anti-biotin antibody-DNA conjugate, followed
by
PCR amplification of the conjugates DNA label.
Serial dilutions of biotinylated infliximab were coated onto NUNC
polycarbonate
immuno-PCR wells. The plate was then blocked with nonfat dried milk in buffer
s containing salmon sperm DNA to block nonspecific DNA binding. The blocked
plate
was probed for biotinylated infliximab using a mouse anti-biotin antibody
conjugated to
a 5'-amidated 227 base pair, double-stranded DNA molecule. After extensive
washing, internal primers and PCR reagents were added directly to the wells
and the
plate was subjected to PCR amplification. A biotinylated infliximab dose
dependent
specific amplification was shown in figure 9. The detection limit was around
450fg (1.7
x 106 molecules) biotinylated infliximab.
Example 5: Detection of induced anti-infliximab antibodies using 1251-Iabeled
infliximab or 1251 -labeled Fab fragment of infliximab
In this example, patient serum was determined by a double antigen EIA
analysis to be positive for induced antibodies to infliximab. However, no free
infliximab
was detectable in this sample.
In one experiment, the serum was incubated with 70 g/mL of'25I-labeled
infliximab at 37 degrees for at least 1 hour to form 125I-labeled infliximab-
induced
antibody complexes. The sample was reanalyzed in the double antigen EIA and
was
rendered to inconclusive due to the absence of signal. An excess of infliximab
Fab
fragment (iFab) was added to the preformed immune complex and incubated for at
least 1 hour at 37 degrees. The sample was separated and counted on an HPLC
system. Fractions (0.25 mL) were collected using a Gilson fraction collector
and
aliquots were then counted using a Topcount Microscintillation Counter. The
retention
time of1251-labeled infliximab (molecular weight = 149kD) was approximately 10
minutes, consistent for a human lg on this HPLC system. The 125 I-labeled
infliximab-
induced antibody complex resolved as a smaller series of peaks that eluted
between 7
to 9 minutes (figure 10). Following incubation with iFab, the height of the
immune
complex peak was reduced, indicating that iFab displaced some of the'251-
labeled
infliximab in the immune complex. This suggests that antigenic protein-induced
antibody complex can be detected through fragment (iFab) displacement of
labeled
antigenic protein (infliximab).
CA 02572263 2006-12-22
WO 2006/004958 PCT/US2005/023378
In another experiment, the serum was incubated with an excess of unlabeled
infliximab at 37 degrees F for at least 1 hour to form unlabeled infliximab-
induced
antibody complexes. An excess of 125 I-labeled iFab was added to the preformed
immune complex and incubated for at least 1 hour at 37 degrees F. The sample
was
separated and counted on an HPLC system. The retention time for'25I-iFab is
approximately 11.3 minutes. As shown in figure 11, following the addition
of'25I-iFab,
distinctive peaks were observed in the 7 to 9 minute region, indicating
that1251-iFab
was incorporated into the unlabeled complexes. This suggests that antigenic
protein-
induced antibody complex can be detected using labeled protein antigenic
fragment
(iFab).
It will be clear that the invention can be practiced otherwise than as
particularly
described in the foregoing description and examples. Numerous modifications
and
variations of the present invention are possible in light of the above
teachings and,
therefore, are within the scope of the claims.
11