Note: Descriptions are shown in the official language in which they were submitted.
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
ALKYL- AND PIPERIDINE-SUBSTITUTED
BENZIMIDAZOLE-DERIVATIVES
BACKGROUND OF THE INVENTION
TECHNICAL FIELD
This invention relates generally to novel benzimidazole-derivatives and their
use as
modulators of chemokine receptor activity, pharmaceutical compositions
containing the
same, and methods of using the same as agents for treatment and prevention of
inflammatory diseases such as asthma and allergic diseases, as well as
autoimmune
pathologies such as rheumatoid arthritis and atherosclerosis.
BACKGROUND INFORMATION
Chemokines are chemotactic cytokines, of molecular weight 6-15 kDa, that are
released by
a wide variety of cells to attract and activate, among other cell types,
macrophages, T and
B lymphocytes, eosinophils, basophils and neutrophils (reviewed in Luster, New
Eng. J
Med., 338, 436-445 (1998) and Rollins, Blood, 90,909-928 (1997)).
There are two major classes of chemokines, CXC and CC, depending on whether
the first
two cysteines in the amino acid sequence are separated by a single amino acid
(CXC) or
are adjacent (CC). The CXC chemokines, such as interleukin-8 (IL-8),
neutrophil-
activating protein-2 (NAP2) and melanoma growth stimulatory activity protein
(MGSA)
are chemotactic primarily for neutrophils and T lymphocytes, whereas the CC
chemokines,
such as RANTES, MIP-la, MIP-1 (3, the monocyte chemotactic proteins (MCP-1,
MCP-2,
MCP-3, MCP-4, and MCP-5) and the eotaxins (-1,-2, and-3) are chemotactic for,
among
other cell types, macrophages, T lymphocytes, eosinophils, dendritic cells,
and basophils.
There also exist the chemokines lymphotactin-1, lymphotactin-2 (both C
chemokines), and
fractalkine (a CXXXC chemokine) that do not fall into either of the major
chemokine
subfamilies.
The chemokines bind to specific cell-surface receptors belonging to the family
of G-
protein-coupled seventransmembrane-domain proteins (reviewed in Horuk, Trends
Pharm.
Sci., 15,159-165 (1994)) which are termed "chemokine receptors." On binding
their
cognate ligands, chemokine receptors transduce an intracellular signal through
the
associated trimeric G proteins, resulting in, among other responses, a rapid
increase in
intracellular calcium concentration, changes in cell shape, increased
expression of cellular
adhesion molecules, degranulation, and promotion of cell migration. There are
at least ten
-1-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
human chemokine receptors that bind or respond to CC chemokines with the
following
characteristic patterns: CCR1 (or"CKR-l"or"CC-CKR-1") [MIP-la, MCP-3, MCP-4,
RANTES] (Ben-Barruch, et al., Cell, 72,415-425 (1993), Luster, New Eng. J.
Med.,
338,436-445 (1998)); CCR-2A and CCR-2B (or "CKR-2A"/"CKR-2B"or"CC-CKR-
2A"/"CC-CYR-2B") [MCP-1, MCP2, MCP-3, MCP-4, MCP-5] (Charo et al., Proc. Natl.
Acad. Sci. USA, 91,2752-2756 (1994), Luster, New Eng. J. Med., 338,436-445
(1998));
CCR-3 (or"CY-R-3"or"CC-CKR-3") [eotaxin-1, eotaxin-2, RANTES, MCP-3, MCP-4]
(Combadiere, et al., J. Biol. Chem., 270,16491-16494 (1995), Luster, New Eng.
J. Med.,
338,436-445 (1998)); CCR-4 (or"CKR-4" or"CC-CKR-4") [TARC, MIP-la, RANTES,
MCP-1] (Power et al., J. Biol. Chem., 270,19495-19500 (1995), Luster, New Eng.
J. Med.,
338,436-445 (1998)); CCR-5 (or"CKR-5"OR"CCCY-R-5") [MIP-la, RANTES, MIP-lp]
(Sanson, et al., Biochemistry, 35,3362-3367 (1996)); CCR-6 (or"CKR-6"or "CC-
CKR-6")
[LARC] (Baba et al., J. Biol. Chem., 272, 14893-14898 (1997)); CCR-7 (or"CKR-
7"or"CC-CKR-7") [ELC] (Yoshie et al., J. Leukoc. Biol. 62,634-644 (1997)); CCR-
8
(or"CY.R-8"or"CC-CY-R-8") [1-309, TARC, MIP-lp] (Napolitano et al., J.
Immunol.,
157,2759-2763 (1996), Bernardini et al., Eur. J. Immunol., 28,582-588 (1998));
and CCR-
10 (or"CKR-10"or"CC-CKR-10") [MCP-1, MCP-3] (Bonini et al, DNA and Cell Biol.,
16,1249-1256 (1997)).
In addition to the mammalian chemokine receptors, mammalian cytomegaloviruses,
herpes
viruses and poxviruses have been shown to express, in infected cells, proteins
with the
binding properties of chemokine receptors (reviewed by Wells and Schwartz,
Curr. Opin.
Biotech., 8, 741-748 (1997)). Human CC chemokines, such as RANTES and MCP-3,
can
cause rapid mobilization of calcium via these virally encoded receptors.
Receptor
expression may be permissive for infection by allowing for the subversion of
normal
immune system surveillance and response to infection. Additionally, human
chemokine
receptors, such as CXCR-4, CCR-2, CCR-3, CCR-5 and CCR-8, can act as
coreceptors for
the infection of mammalian cells by microbes as with, for example, the human
immunodeficiency viruses (HIV).
Chemokine receptors have been implicated as being important mediators of
inflammatory,
infectious, and immunoregulatory disorders and diseases, including asthma and
allergic
diseases, as well as autoimmune pathologies such as rheumatoid arthritis and
atherosclerosis. For example, the chemokine receptor CCR-3 plays a pivotal
role in
attracting eosinophils to sites of allergic inflammation and in subsequently
activating these
cells. The chemokine ligands for CCR-3 induce a rapid increase in
intracellular calcium
concentration, increased expression of cellular adhesion molecules, cellular
degranulation,
and the promotion of eosinophil migration. Accordingly, agents which modulate
chemokine receptors would be useful in such disorders and diseases. In
addition, agents
which modulate chemokine receptors would also be useful in infectious diseases
such as
-2-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
by blocking infection of CCR-3 expressing cells by HIV or in preventing the
manipulation
of immune cellular responses by viruses such as cytomegaloviruses.
BACKGROUND ART
- WO 01 66525 discloses substituted benzimidazoles or benzotriazoles for the
modulation of the CCR5 receptor for the treatment i.e. HIV1, inflammatory or
immunoregulatory. One can also find in the description the assumption that the
io disclosed compounds have potential in the treatment of asthma or COPD.
BRIEF SUMMARY OF THE INVENTION
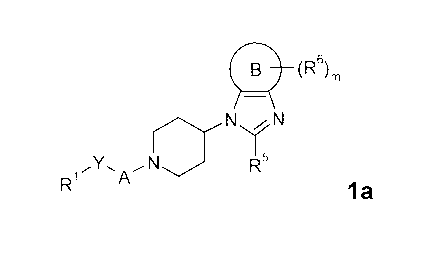
is A compound of general formula la
(R6)
N'//N
'Y. ~N NIR's
R~ '4
la
wherein Rl, R5, R6, A, B, Y and m are defined as below.
20 It is another object of the present invention to provide pharmaceutical
compositions
comprising a pharmaceutically acceptable carrier and a therapeutically
effective amount of
at least one of the compounds of the present invention or a pharmaceutically
acceptable
salt thereof. These and other objects, which will become apparent during the
following
detailed description.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a compound of general formula la
(Rs)m
N'//N
~Y' A ~NN IR'a
R~
la
wherein
-3-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
Rl is aryl, het or a annelated species thereof, wherein het is a heterocyclic
ring and the
annelated species comprises aryl-het-, het-aryl- or het-het-annelations, each
of said
aryl or het may be substituted with one, two or three R 2;
R 2 are each independently -C1_6-alkyl, -C3_6-cycloalkyl, -C1_6-haloalkyl, -
C1_6-aralkyl,
halogen, -CN, -COOR3, -COR3, -CONR3R4, -NR3R4, -NR3COR4, -NR3SO2R4,
-OR3, -NO2, -SR3, -SOR3, -S02R3 or -SO2NR3R4;
R3 is H, -C1_6-alkyl;
R4 is H, -C1_6-alkyl;
R5 is -C1_6-alkyl
R6 are each independently -C1_6-alkyl, -C1_6-alkoxy, -C1_6-acyloxy, -C1_6-
aralkyl,
-C3_6-cycloalkyl, -C1_6-haloalkyl, -C1_6-thioalkyl, halogen, -OR3, -SR3, -CN, -
NO2,
-COOR3, -COR3, -CONR3R4, -NR3R4, -NR3COR4, -NR3SO2R4, -SOR3, -S02R3,
-SO2NR3R4, aryl or het;
A is -C2_8-alkylene, optionally substituted with -C1_3-alkyl, halogen or -OH;
B is aryl or het;
Y is -CF2-, -NR4-, -0-, -S(O)n ;
n is 0, 1 or 2;
m is 0, 1, 2, 3 or 4;
and pharmaceutically acceptable salts thereof.
The compounds herein described may have asymmetric centres. Compounds of the
present
invention containing an asymmetrically substituted atom may be isolated in
optically
active or racemic forms. It is well known in the art how to prepare optically
active forms,
such as by resolution of racemic forms or by synthesis from optically active
starting
materials. Many geometric isomers of olefins, C=N double bonds, and the like
can also be
present in the compounds described herein, and all such stable isomers are
contemplated in
the present invention. Cis and trans geometric isomers of the compounds of the
present
invention are described and may be isolated as a mixture of isomers or as
separated
isomeric forms. All chiral, diastereomeric, racemic forms and all geometric
isomeric forms
of a structure are intended, unless the specific stereochemistry or isomeric
form is
specifically indicated.
USED TERMS AND DEFINITIONS
Terms not specifically defined herein should be given the meanings that would
be given to
them by one of skill in the art in light of the disclosure and the context. As
used in the
specification, however, unless specified to the contrary, the following terms
have the
meaning indicated and the following conventions are adhered to.
-4-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
In the groups, radicals, or moieties defined below, the number of carbon atoms
is often
specified preceding the group, for example, -C1-6 alkyl means an alkyl group
or radical
having 1 to 6 carbon atoms. In general, for groups comprising two or more
subgroups, the
last named group is the radical attachment point, for example, "thioalkyl"
means a
monovalent radical of the formula HS-Alk-. Unless otherwise specified below,
conventional definitions of terms control and conventional stable atom
valences are
presumed and achieved in all formulas and groups.
In general, all tautomeric forms and isomeric forms and mixtures, whether
individual
io geometric isomers or optical isomers or racemic or non-racemic mixtures of
isomers, of a
chemical structure or compound is intended, unless the specific
stereochemistry or
isomeric form is specifically indicated in the compound name or structure.
The term "substituted" as used herein, means that any one or more hydrogens on
the
designated atom is replaced with a selection from the indicated group,
provided that the
designated atom's normal valence is not exceeded, and that the substitution
results in a
stable compound.
The phrase "pharmaceutically acceptable" is employed herein to refer to those
compounds,
materials, compositions, and/or dosage forms which are, within the scope of
sound medical
judgment, suitable for use in contact with the tissues of human beings and
animals without
excessive toxicity, irritation, allergic response, or other problem or
complication,
commensurate with a reasonable benefit/risk ratio.
As used herein, "pharmaceutically acceptable salts" refer to derivatives of
the disclosed
compounds wherein the parent compound is modified by making acid or base salts
thereof.
Examples of pharmaceutically acceptable salts include, but are not limited to,
mineral or
organic acid salts of basic residues such as amines; alkali or organic salts
of acidic residues
such as carboxylic acids; and the like. The pharmaceutically acceptable salts
include the
conventional non-toxic salts or the quaternary ammonium salts of the parent
compound
formed, for example, from non-toxic inorganic or organic acids. For example,
such
conventional non-toxic salts include those derived from inorganic acids such
as
hydrochloric, hydrobromic, sulfuric, sulfamic, phosphoric, nitric and the
like; and the salts
prepared from organic acids such as acetic, propionic, succinic, glycolic,
stearic, lactic,
malic, tartaric, citric, ascorbic, pamoic, maleic, hydroxymaleic,
phenylacetic, glutamic,
benzoic, salicylic, sulfanilic, 2-acetoxybenzoic, fumaric, toluenesulfonic,
methanesulfonic,
ethane disulfonic, oxalic, isethionic, and the like.
The pharmaceutically acceptable salts of the present invention can be
synthesized from the
parent compound which contains a basic or acidic moiety by conventional
chemical
-5-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
methods. Generally, such salts can be prepared by reacting the free acid or
base forms of
these compounds with a stoichiometric amount of the appropriate base or acid
in water or
in an organic solvent, or in a mixture of the two; generally, non-aqueous
media like ether,
ethyl acetate, ethanol, isopropanol, or acetonitrile are preferred. Lists of
suitable salts are
found in Reminqton's Pharmaceutical Sciences, Mack Publishing Company, a
standard
reference text in this field. which release an active parent drug of the
present invention in
vivo when such prodrug is administered to a mammalian subject. Prodrugs the
present
invention are prepared by modifying functional groups present in the compound
in such a
way that the modifications are cleaved, either in routine manipulation or in
vivo, to the
io parent compound. Prodrugs include compounds of the present invention
wherein a
hydroxy, amino, or sulfhydryl group is bonded to any group that, when the
prodrug of the
present invention is administered to a mammalian subject, it cleaves to form a
free
hydroxyl, free amino, or free sulfhydryl group, respectively. Examples of
prodrugs include,
but are not limited to, acetate, formate and benzoate derivatives of alcohol
and amine
functional groups in the compounds of the present invention.
The term "aryl" as used herein, either alone or in combination with another
substituent,
means either an aromatic monocyclic system or aromatic multicyclic systems
containing
carbon atoms. For example, aryl includes a phenyl or a naphthyl ring system,
wherein aryl
means generally an aromatic system, for example phenyl.
The term "het" as used herein, either alone or in combination with another
substituent,
means a monovalent substituent derived by removal of a hydrogen from a five-,
six- or
seven-membered saturated or unsaturated (including aromatic) heterocycle
containing
carbon atoms and one, two, three or four ring heteroatoms selected from
nitrogen, oxygen
and sulfur. Examples of suitable heterocycles include: tetrahydrofuran,
thiophene,
diazepine, isoxazole, piperidine, dioxane, morpholine, piperazine or
0-11
Although generally covered under the term "het", the term "heteroaryl" as used
herein
precisely defines an unsaturated heterocycle for which the double bonds form
an aromatic
system. Suitable example of heteroaromatic system include: pyridine,
pyrimidine,
N I I <N I' ~ I I
~ g N/ N-N . N~ . LN .~ N .N N_N
, ;or
The term "annelated species of aryl or het" as used herein, either alone or in
combination
with another substituent wherein the annelated species presents as a aryl-het
(a), a het-aryl
-6-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
(b) or a het-het (c) annelation means a monovalent substituent derived by
removal of one
hydrogen from
a) an aromatic monocyclic system or aromatic multicyclic systems containing
carbon
atoms, which is annelated to a five-, six- or seven-membered saturated or
unsaturated
(including aromatic) heterocycle containing carbon atoms and one, two, three
or four
ring heteroatoms selected from nitrogen, oxygen and sulfur or
b) a five-, six-, or seven-membered saturated or unsaturated (including
aromatic)
heterocycle containing carbon atoms and one, two, three or four ring
heteroatoms
selected from nitrogen, oxygen and sulfur, which is annelated to an aromatic
monocyclic system or aromatic multicyclic systems containing carbon atoms or
c) a five-, six-, or seven-membered saturated or unsaturated (including
aromatic)
heterocycle containing carbon atoms and one, two, three or four ring
heteroatoms
selected from nitrogen, oxygen and sulfur, which is annelated to a five-, six-
, or seven-
membered saturated or unsaturated (including aromatic) heterocycle containing
carbon
atoms and one, two, three or four ring heteroatoms selected from nitrogen,
oxygen and
sulfur.
Suitable examples of an annelated species of aryl or het include: quinolinyl,
1-indoyl, 3-
indoyl, 5-indoyl, 6-indoyl, indolizinyl, benzimidazyl or purinyl.
The term "halogen" as used herein means a halogen substituent selected from
fluoro,
chloro, bromo or iodo.
The term "-C1_6-alkyP" as used herein, either alone or in combination with
another
substituent, means acyclic, straight or branched chain alkyl substituents
containing from
one to six carbon atoms and includes, for example, methyl, ethyl, propyl,
butyl, hexyl, 1-
methylethyl, 1-methylpropyl, 2-methylpropyl or 1,1-dimethylethyl.
The term "-C3_8-cycloalkyl" as used herein, either alone or in combination
with another
substituent, means a cycloalkyl substituent containing from three to six
carbon atoms and
includes cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
The term "-C1_6-haloalkyP" as used herein, either alone or in combination with
another
substituent, means acyclic, straight or branched chain alkyl substituents
containing up to
six carbon atoms having one or more hydrogens substituted for a halogen
selected from
bromo, chloro, fluoro or iodo. Accordingly "-Ca_6-haloalkyP" has the same
meaning with
exception that the chain contains two to six carbon atoms. Preferably the term
-Cl-6-haloalkyl represents -C1_6-fluoroalkyl such as 2-fluorethyl, 2,2,2-
trifluorethyl or
perfluorethyl.
-7-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
The term "-C1_6-alkoxy" as used herein, either alone or in combination with
another
substituent, means the substituent -Cl-6-alkyl-O- wherein alkyl is as defined
above
containing up to six carbon atoms. Alkoxy includes methoxy, ethoxy, propoxy, 1-
methylethoxy, butoxy or 1,1-dimethylethoxy. The latter substituent is known
commonly as
t-butoxy.
The term "-Cl_6-acyloxy" as used herein, either alone or in combination with
another
substituent, means the substituent -Cl-6-alkyl-(CO)O- wherein alkyl is as
defined above
containing up to six carbon atoms. Acyloxy includes MeCOO-, EtCOO-, nPrCOO-,
'PrCOO-, nBUCOO-, secBUCOO- or tertBUCOO-.
The term "-C1_6-aralkyl" as used herein, either alone or in combination with
another
substituent, means the substituent -Aryl-Cl-6-alkyl- wherein alkyl is as
defined above
containing up to six carbon atoms. Aralkyl includes benzyl, phenylethyl,
phenylpropyl, 1-
phenyl- 1 -methylethyl, phenylbutyl or 1 -phenyl- 1, 1 -dimethylethoxy.
--C1_6-thioalkylThe term "-C1_6-thioalkyl" as used herein, either alone or in
combination
with another substituent, means acyclic, straight or branched chain alkyl
substituents
containing up to six carbon atoms and a thiol (HS) group as a substituent. An
example of a
thioalkyl group is a thiopropyl, e.g., HS-CH2CH2CH2-.
The term "-C2_8-alkylene" as used herein means a divalent alkyl substituent
derived by the
removal of one hydrogen atom from each end of a saturated straight or branched
chain
aliphatic hydrocarbon containing from two to eight carbon atoms and includes,
for
example, CH2CH2C(CH3)2CH2CH2-. Accordingly "-C1_3-alkylene" has the same
meaning
with exception that the chain contains one to three carbon atoms.
PREFERRED EMBODIMENTS
The compounds of the instant application are useful for manufacturing a
medicament for
the prevention and/or treatment of diseases wherein the activity of a CCR-3-
receptor is
involved.
Preferred is the manufacturing of a medicament for treatment of a wide variety
of
inflammatory, infectious, and immunoregulatory disorders and diseases,
including asthma
and allergic diseases, infection by pathogenic microbes (which, by definition,
includes
viruses), as well as autoimmune pathologies such as the rheumatoid arthritis
and
atherosclerosis.
-8-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
Most preferred is the manufacturing of a medicament for the treatment of e.g.
inflammatory or allergic diseases and conditions, including respiratory
allergic diseases
such as asthma, allergic rhinitis, hypersensitivity lung diseases,
hypersensitivity
pneumonitis, eosinophilic cellulitis (e.g., Well's syndrome), eosinophilic
pneumonias (e.g.,
Loeffler's syndrome, chronic eosinophilic pneumonia), eosinophilic fasciitis
(e.g.,
Shulman's syndrome), delayed-type hypersensitivity, interstitial lung diseases
(ILD) (e.g.,
idiopathic pulmonary fibrosis, or ILD associated with rheumatoid arthritis,
systemic lupus
erythematosus, ankylosing spondylitis, systemic sclerosis, Sjogren's syndrome,
polymyositis or dermatomyositis); systemic anaphylaxis or hypersensitivity
responses,
io drug allergies (e.g., to penicillin, cephalosporins), eosinophilia-myalgia
syndrome due to
the ingestion of contaminated tryptophan, insect sting allergies; autoimmune
diseases, such
as rheumatoid arthritis, psoriatic arthritis, multiple sclerosis, systemic
lupus erythematosus,
myasthenia gravis, juvenile onset diabetes; glomerulonephritis, autoimmune
thyroiditis,
Behcet's disease; graft rejection (e.g., in transplantation), including
allograft rejection or
graftversus-host disease; inflammatory bowel diseases, such as Crohn's disease
and
ulcerative colitis; spondyloarthropathies; scleroderma; psoriasis (including
Tcell mediated
psoriasis) and inflammatory dermatoses such as an dermatitis, eczema, atopic
dermatitis,
allergic contact dermatitis, urticaria; vasculitis (e.g., necrotizing,
cutaneous, and
hypersensitivity vasculitis); eosinophilic myositis, eosinophilic fasciitis;
cancers with
leukocyte infiltration of the skin or organs.
Preferred are compounds of the general formula la wherein:
Rl is aryl or het, each optionally substituted with one, two or three R2;
R2 are each independently -C1_6-alkyl, -C1_6-haloalkyl, halogen, -CN, -OR3 or -
NOZ;
R3 is H or Methyl;
R5 is -C1_6-alkyl
R6 are each independently -C1_6-alkyl, -C1_6-alkoxy, -Cl_6-haloalkyl, halogen,
-OR3, -CN
or -NO2,
A is -C2_8-alkylene;
B is aryl;
Y is -NH-, -0-, -S(O)n ;
n is O or 2;
mis 0, 1 or 2;
and pharmaceutically acceptable salts thereof.
Particularly preferred are compounds of general formula 1b, wherein R1, R5,
R6, A, B, Y
and m are defined as above.
-9-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
~ (R6)m
N'//N
'Y' N NIR5
R~ A
lb
and pharmaceutically acceptable salts thereof. Most preferred are compounds of
general
formula lc,
s
~_(HaI)o2
N'//N
SN ~R5
2
(R )02 /
1c
wherein R5 is defined as above and
R2 is -CF3 or halogen;
Hal is halogen
and pharmaceutically acceptable salts thereof.
Most preferred are compounds of the general formula la, lb or lc wherein:
- Rl is aryl , both optionally substituted with one, two or three R2; more
preferred Rl is
phenyl, optionally substituted with one, two or three R2, or
- R2 is -CF3 or halogen, in particular fluoro; or
- R6 is preferably halogen, in particular fluoro; or
- A is -C2_8-alkylene or more preferred -CH2-CH2-CH2-; or
- B is phenyl: or
- Y is -NH-, -0-, -S-, -SOz-;
- m is l or 2;
and pharmaceutically acceptable salts thereof.
Particular preferred are compounds of the general formula 1c wherein
R2 is fluoro;
R5 is -C1_6-alkyl, preferably Methyl, Ethyl, Propyl and Butyl, more preferably
Ethyl and
Propyl;
Hal is fluoro or chloro;
and pharmaceutically acceptable salts thereof.
-10-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
Most preferred is a compound of formula ld
F
0
N sN
S N Y
\
F ~ / 1d
and pharmaceutically acceptable salts thereof.
The compounds of formula la, lb, lc or ld can be prepared using the reactions
and
techniques described below. The reactions are performed in a solvent
appropriate to the
reagents and materials employed and suitable for the transformations being
effected. It will
be understood by those skilled in the art of organic synthesis that the
functionality present
on the molecule should be consistent with the transformations proposed. This
will
sometimes require a judgment to modify the order of the synthetic steps or to
select one
particular process scheme over another in order to obtain a desired compound
of the
invention. It will also be recognized that another major consideration in the
planning of
any synthetic route in this field is the judicious choice of the protecting
group used for
protection of the reactive functional groups present in the compounds
described in this
invention. An authoritative account describing the many alternatives to the
trained
practitioner is Greene and Wuts (Protective Groups In Organic Synthesis, Wiley
and Sons,
1991).
PREPARATION
Compounds of the general formula la are prepared by adding a ring B,
substituted at least
by one nitro-function and a suitable leaving group (LG = i. e. F, Cl) in ortho-
position.
N02
LG / H N02
NH2 B (Rs)m N / s
Ph PN B (R )m
N~__ N
-I1-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
After coupling reaction, the nitro function is reduced by e.g. Hydrogen in
presence of a
Platinum / Charcoal catalyst, Fe/HC1, SnC12 or Sodiumdithionite (Na2S2O4) to a
free amine
group.
H N02 H NHz
~ s reduction N s
Ph~gN
B (R B (R )m
Ph~N
In the next step of the reaction, the resulting amine function is converted
into an amide
group and thereafter in the same or a separate step transformed in a ring-
closing reaction to
an imidazole derivative.
(Rs)m
H NH~ 1. peptide coupling
N ~ 2. ring closing ~ 30 Phu N
B (Rs)m PhaN R
~
wherein the whole process R5, R6, B, W, i, j, k,1 and m are defined as above.
After this, the protecting benzyl group of the piperidine is removed and
preferably, the
compounds of general formula la are prepared by reaction of a compound of
general
formula 2
(Rs)m
N'//N
HN ~
R 5 2
with a compound of the general formula 3.
R~ ~Y.A, LG
wherein R1, R5, R6, A, B, X, W, i, j, k, l and m are defined as for general
formula la above
and LG is a suitable leaving group e.g. a halogen, triflate, tosylate or
brosylate.
As will be appreciated by one of skill in the art, numerous modifications and
variations of
the present invention are possible in light of the above teachings. It is
therefore to be
understood that within the scope of the appended claims, the invention may be
practiced
otherwise than as specifically described herein.
-12-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
EXAMPLE 1
1-Benzyl-4-(4-fluoro-2-nitro-phenylamino)-piperidine
F
0
/-N rH N02
Ph ~/
2,5-Difluoro-nitrobenzene (21.5g), 1-benzyl-4-amino-piperidine (52.3g) and N-
methyl-
piperidone (100 ml) were stirred at 100 C for 3 h. After cooling the mixture
was
partitioned between water and ethyl acetate and the aqueous layer was
extracted twice with
ethyl acetate. The combined organic layers were extracted 5 times with water,
and the
solvent was evaporated in vacuo. The target compound was crystallized from
methanol to
yield 38.7g of the title compound as orange crystals (mp. 86-87 C).
EXAMPLE 2
1-B enzyl-4-(2-amino-4-fluorophenylamino)-piperidine:
F
Ph
\- No- N N
1-Benzyl-4-(4-fluoro-2-nitrophenylamino)-piperidine (10.1g) were dissolved in
1:1 TEF-
methanol (150m1) and hydrogenated with Pt/C (1.5g, 10%) at room temperature
for 1.5 h.
The catalyst was then removed by filtration, and the filtrate evaporated to
yield 9.1 g of the
title compound as an oil. The compound was used without further purification.
EXAlVIl'LE 3
N-[2-(1-Benzyl-piperidin-4-ylamino)-5-fluorophenyl]-propionamide
-13-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
F
0 (jNQN
To a stirred solution of 1-Benzyl-4-(2-amino-4-fluoro-phenylamino)-piperidine
(6g) in
ethyl acetate (50ml) at room temperature was added acetic anhydride (3.3ml).
After 2 h,
the reaction was concentrated in vacuo, and the crude oil partitioned between
2:1 DCM
and water (150m1) which was made alkaline with NaOH(aq). The organic layer was
separated, washed with water and dried followed by in vacuo concentration to
yield 7g of
the title compound as a yellow oil. The compound was used without further
purification.
EXAMPLE 4
1-(1-Benzyl-piperidin-4-yl)-2-ethyl-5-fluoro-lH-benzimidazole
F
0
N /N
\ I N~
N-[2-(1-Benzyl-piperidin-4-ylamino)-5-fluorophenyl]-propionamide (7g) in
glacial acetic
acid (70m1) was heated at reflux for 1 h. After cooling the residue was
dissolved in ethyl
acetate (100m1) and water (50m1) was then added. The mixture was made alkaline
with
K2C03, and the organic layer separated, washed with water, then dried and
concentrated
in vacuo. The resulting oil was purified by flash chromatography (9:1
DCM:MeOH) to
yield 6.4g of the title compound.
EXAIVII'LE 5
2-Ethyl-5-fluoro-l-piperidin-4-yl-lH-benzimidazole
-14-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
F
0
~N ~N
N
1-(1-Benzyl-piperidin-4-yl)-2-ethyl-5-fluoro-lH-benzimidazole (6.4g) in
methanol
(100m1) was hydrogenated with Pd/C (10%, 1.5g) at room temperature for 8 h.
The
catalyst was removed and the filtrate was evaporated and dissolved in ethanol.
The solution
was acidified with conc. HCl(aq) and recrystallised by the addition of
acetone. This
yielded 5.3g of the title compound as colourless crystals.
1H-NMR (DMSO-d6) 8[ppm] = 1.40 (314), 2.09-2.15 (2H), 2.81-2.94 (2H), 3.10-
3.22
(2H), 3.22-3.29 (2H), 3.41-3.51 (21-1), 4.99-5.11 (1H), 7.40 (1H), 7.69 (1H),
8.56 (1H),
9.30 (11-1), 10.03 (1H).
EXAIVIPLE 6
1-(3-Bromo-propylsulfanyl)-4-fluoro-benzene:
I ~ S~/Br
'\%
F
4-Fluorothiophenol (5.3m1), 1,3-dibromo-propane (15.3m1), K2C03 (14g) were
dissolved
in CH3CN (100 n-il) and refluxed for 3 h. The insoluble salts were removed,
the solvent
was evaporated and the residue dissolved in ethyl acetate (70 ml). The
solution was
extracted with diluted NaOH and water, dried and the solvent evaporated in
vacuo. The
residue was purified by distillation to yield 8g of the title compound as a
colourless oil. Bp.
150-155 C @ 20mmHg.
EXAMPLE 7
2-Ethyl-5-fluoro-1-{ 1-[3-(4-fluorophenylsulfanyl)-propyl]-piperidin-4-yl}-1H-
benzimidazole:
-15-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
F
0
N
N J
F
To a solution of 2-Ethyl-5-fluoro-l-piperidin-4-yl-lH-benzimidazole (5.4g) in
DMF
(30m1) was added 1-(3-bromo-propylsulfanyl)-4-fluoro-benzene (5.7g), K2C03
(3.2g) and
KI (150mg). The reaction was stirred at 90 C for 1 h. After cooling the
mixture was poured
into water (50m1) and was extracted with ethyl acetate (2x50m1). The organic
layer was
washed with water (2x40m1), dried and concentrated in vacuo. The resulting oil
was
purified by flash chromatography (97:3 DCM:MeOH), and the HCl salt (7.5g) of
the title
compound obtained by treatment of the purified oil with conc. HCl(aq) in an
isopropanol-
acetone solvent mixture, followed by recrystallisation from acetone-MeOH. Mp.
252-
255 C.
According to the synthetic route above the following examples were prepared:
EXAMPLE 8
2-Ethyl-l-{ 1-[3-(4-fluorophenylsulfanyl)-propyl]-piperidin-4-yl}-1H-
benzimidazole
/ \
F S N N
To a stirred solution of 1-(3-bromopropylsulfanyl)-4-fluorobenzene (0.5g) and
2-ethyl-l-
piperidin-4-yl-lH-benzimidazole (0.5g) in DMF (5m1) was added K2C03 (1.0g) and
KI
(0.05g). The mixture was heated at 100 C for 1 h, and then allowed to cool to
rt. Water
(50m1) was added and the mixture extracted with ethyl acetate (80m1), and the
organic
layer separated and washed with water. This was further dried and concentrated
in vacuo.
The oil was purified by flash chromatography (9:1 DCM:MeOH) to yield 0.6 g of
the title
compound as colourless crystals. Mp. 182-184 C.
-16-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
EKAIVIPLE 9
2-Butyl-l-{ 1-[3-(4-fluorophenylsulfanyl)-propyl]-piperidin-4-yl}-1H-
benzimidazole
F S N N
To a stirred solution of 1-(3-bromopropylsulfanyl)-4-fluorobenzene (0.2g) and
2-butyl-l-
piperidin-4-yl-lH-benzimidazole (0.3g) in DMF (5m1) was added K2C03 (0.6g) and
KI
(0.05g). The mixture was heated at 100 C for 1 h, and then allowed to cool to
rt. Water
(50m1) was added and the mixture extracted with ethyl acetate (80m1), and the
organic
layer separated and washed with water. This was further dried and concentrated
in vacuo.
The oil was purified by flash chromatography (9:1 DCM:MeOH) to yield 0.3 g of
the title
compound as colourless crystals. Mp. 159-161 C.
EXAMPLE 10
{ 3-[4-(2-Ethylbenzimidazol-1-yl)-piperidin-1-yl]-propyl}-(4-fluorophenyl)-
amine
F N / \
N /N
No-
To a stirred solution of 2-ethyl-l-piperidin-4-yl-lH-benzimidazole (0.6g) and
(3-
chloropropyl)-(4-fluorophenyl)amine (0.4g) in DMF (7m1) was added K2C03 (l.lg)
and
KI (0.1g). The mixture was heated at 100 C for 2 h, and then allowed to cool
to rt. Water
(50m1) was added and the mixture extracted with ethyl acetate (80m1), and the
organic
layer separated and washed with water. This was further dried and concentrated
in vacuo.
The oil was purified by flash chromatography (95:5 DCM:MeOH), and the solid
recrystallised from ether to yield 0.3 g of the title compound as colourless
crystals. Mp.
121-124 C.
-17-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
EKAMPLE 11
2-Ethyl-l-{ 1-[3-(4-fluorobenzenesulfonyl-)propyl]-piperidin-4-yl}-1H-
benzimidazole
11
F \ / So -
p
~N ,N
N
To a stirred solution of 2-ethyl-l-piperidin-4-yl-lH-benzimidazole
dihydrochloride (3.02g)
and 1-(3-chloropropane-l-sulfonyl)4-fluorobenzene (2.6g) in 3:1 THF-DMF (25m1)
was
added NaHCO3 (2.9g), KI (0.3g) and HMPT (5m1). The mixture was heated at
reflux for 2
h, and then allowed to cool to rt. Water (100ml) was added and the mixture
extracted with
ethyl acetate (2x80m1), and the organic layer separated and washed with water.
This was
further dried and concentrated in vacuo. The oil was purified by flash
chromatography and
the free base converted to the hydrochloride salt by action of HCl in ethanol.
The solid was
recrystallised from ethanol-petroleum ether to yield 2.0 g of the title
compound as
colourless crystals. Mp. 212-216 C.
EXAMPLE 12
1-{ 1-[3-(4-Fluorophenoxy)-propyl]-piperidin-4-yl}-1H-benzimidazole
F \ / O / \
_ \-~Na N vN
To a stirred solution of 1-piperidin-4-yl-lH-benzimidazole (4.02g) and 1-(3-
chloropropoxy)-4-fluorobenzene (4.16g) in DMF (50m1) was added K2C03 (4.1g)
and KI
(0.2g). The mixture was heated at 90 C for 2 h, and theri allowed to cool to
rt. Water
(100m1) was added and the mixture extracted with ethyl acetate (2x80m1), and
the organic
layer separated and washed with water. This was further dried and concentrated
in vacuo.
The free base converted to the hydrochloride salt by action of HCl in ethanol.
The solid
was recrystallised from ethanol-diethylether to yield 4.7g of the title
compound as
colourless crystals. MP. 233-235 C.
-18-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
METHOD OF TREATMENT
Accordingly, the present invention is directed towards compounds which are
useful in the
prevention and/or treatment of a wide variety of inflammatory, infectious, and
immunoregulatory disorders and diseases, including asthma and allergic
diseases, infection
by pathogenic microbes (which, by definition, includes viruses), as well as
autoimmune
pathologies such as the rheumatoid arthritis and atherosclerosis.
For example, an instant compound which inhibits one or more functions of a
mammalian
chemokine receptor (e.g., a human chemokine receptor) may be administered to
inhibit (i.
e., reduce or prevent) inflammation or infectious disease. As a result, one or
more
inflammatory process, such as leukocyte emigration, adhesion, chemotaxis,
exocytosis
(e.g., of enzymes, histamine) or inflammatory mediator release, is inhibited.
For example,
eosinophilic infiltration to inflammatory sites (e.g., in asthma or allergic
rhinitis) can be
inhibited according to the present method. In particular, the compound of the
following
examples has activity in blocking the migration of cells expressing the CCR-3
receptor
using the appropriate chemokines in the aforementioned assays.
Similarly, an instant compound which promotes one or more functions of the
mammalian
chemokine receptor (e.g., a human chemokine) as administered to stimulate
(induce or
enhance) an immune or inflammatory response, such as leukocyte emigration,
adhesion,
chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator
release,
resulting in the beneficial stimulation of inflammatory processes. For
example, eosinophils
can be recruited to combat parasitic infections. In addition, treatment of the
aforementioned inflammatory, allergic and autoimmune diseases can also be
contemplated
for an instant compound which promotes one or more functions of the mammalian
chemokine receptor if one contemplates the delivery of sufficient compound to
cause the
loss of receptor expression on cells through the induction of chemokine
receptor
internalization or the delivery of compound in a manner that results in the
misdirection of
the migration of cells.
In addition to primates, such as humans, a variety of other mammals can be
treated
according to the method of the present invention. For instance, mammals,
including but not
limited to, cows, sheep, goats, horses, dogs, cats, guinea pigs, rats or other
bovine, ovine,
equine, canine, feline, rodent or murine species can be treated. However, the
method can
also be practiced in other species, such as avian species. The subject treated
in the methods
above is a mammal, male or female, in whom modulation of chemokine receptor
activity is
desired. "Modulation" as used herein is intended to encompass antagonism,
agonism,
partial antagonism and/or partial agonism.
-19-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
Diseases or conditions of human or other species which can be treated with
inhibitors of
chemokine receptor function, include, but are not limited to: inflammatory or
allergic
diseases and conditions, including respiratory allergic diseases such as
asthma, allergic
rhinitis, hypersensitivity lung diseases, hypersensitivity pneumonitis,
eosinophilic cellulitis
(e.g., Well's syndrome), eosinophilic pneumonias (e.g., Loeffler's syndrome,
chronic
eosinophilic pneumonia), eosinophilic fasciitis (e.g., Shulman's syndrome),
delayed-type
hypersensitivity, interstitial lung diseases (ILD) (e.g., idiopathic pulmonary
fibrosis, or
ILD associated with rheumatoid arthritis, systemic lupus erythematosus,
ankylosing
spondylitis, systemic sclerosis, Sjogren's syndrome, polymyositis or
dermatomyositis);
systemic anaphylaxis or hypersensitivity responses, drug allergies (e.g., to
penicillin,
cephalosporins), eosinophilia-myalgia syndrome due to the ingestion of
contaminated
tryptophan, insect sting allergies; autoimmune diseases, such as rheumatoid
arthritis,
psoriatic arthritis, multiple sclerosis, systemic lupus erythematosus,
myasthenia gravis,
juvenile onset diabetes; glomerulonephritis, autoimmune thyroiditis, Behcet's
disease; graft
is rejection (e.g., in transplantation), including allograft rejection or
graftversus-host disease;
inflammatory bowel diseases, such as Crohn's disease and ulcerative colitis;
spondyloarthropathies; scleroderma; psoriasis (including Tcell mediated
psoriasis) and
inflammatory dermatoses such as an dermatitis, eczema, atopic dermatitis,
allergic contact
dermatitis, urticaria; vasculitis (e.g., necrotizing, cutaneous, and
hypersensitivity
vasculitis); eosinophilic myositis, eosinophilic fasciitis; cancers with
leukocyte infiltration
of the skin or organs. Other diseases or conditions in which undesirable
inflammatory
responses are to be inhibited can be treated, including, but not limited to,
reperfusion
injury, atherosclerosis, certain hematologic malignancies, cytokine-induced
toxicity (e.g.,
septic shock, endotoxic shock), polymyositis, dermatomyositis. Infectious
diseases or
conditions of human or other species which can be treated with inhibitors of
chemokine
receptor function, include, but are not limited to, HIV.
Diseases or conditions of humans or other species which can be treated with
promoters of
chemokine receptor function, include, but are not limited to:
immunosuppression, such as
3o that in individuals with immunodeficiency syndromes such as AIDS or other
viral
infections, individuals undergoing radiation therapy, chemotherapy, therapy
for
autoimmune disease or drug therapy (e.g., corticosteroid therapy), which
causes
immunosuppression; immunosuppression due to congenital deficiency in receptor
function
or other causes; and infections diseases, such as parasitic diseases,
including, but not
limited to helminth infections, such as nematodes (round worms);
(Trichuriasis,
Enterobiasis, Ascariasis, Hookworm, Strongyloidiasis, Trichinosis, filariasis)
; trematodes
(flukes) (Schistosomiasis, Clonorchiasis), cestodes (tape worms)
(Echinococcosis,
Taeniasis saginata, Cysticercosis); visceral worms, visceral larva migraines
(e.g.,
Toxocara), eosinophilic gastroenteritis (e.g., Anisaki sp., Phocanema sp.),
cutaneous larva
migraines (Ancylostona braziliense, Ancylostoma caninum). The compounds of the
-20-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
present invention are accordingly useful in the prevention and treatment of a
wide variety
of inflammatory, infectious and immunoregulatory disorders and diseases. In
addition,
treatment of the aforementioned inflammatory, allergic and autoimmune diseases
can also
be contemplated for promoters of chemokine receptor function if one
contemplates the
delivery of sufficient compound to cause the loss of receptor expression on
cells through
the induction of chemokine receptor internalization or delivery of compound in
a manner
that results in the misdirection of the migration of cells.
In another aspect, the instant invention may be used to evaluate the putative
specific
agonists or antagonists of a G protein coupled receptor. The present invention
is directed to
the use of these compounds in the preparation and execution of screening
assays for
compounds that modulate the activity of chemokine receptors. Furthermore, the
compounds of this invention are useful in establishing or determining the
binding site of
other compounds to chemokine receptors, e.g., by competitive inhibition or as
a reference
is in an assay to compare its known activity to a compound with an unknown
activity. When
developing new assays or protocols, compounds according to the present
invention could
be used to test their effectiveness.
Specifically, such compounds may be provided in a commercial kit, for example,
for use in
pharmaceutical research involving the aforementioned diseases. The compounds
of the
instant invention are also useful for the evaluation of putative specific
modulators of the
chemokine receptors. In addition, one could utilize compounds of this
invention to
examine the specificity of G protein coupled receptors that are not thought to
be
chemokine receptors, either by serving as examples of compounds which do not
bind or as
structural variants of compounds active on these receptors which may help
define specific
sites of interaction.
The CCR-3 receptor binding test is based on a K562 cell line (leukemia
myelogenic blast
cells ) transfected with the human chemokine receptor CCR-3 ( hCCR-3-C1 ).
The cell membranes were prepared by disrupting the hCCR-3 transfected K562
cells by
nitrogen decomposition and centrifugation at 40000 g , 4 C for lh. The
membranes were
re-suspended in the SPA incubation buffer without bovine serum albumin for
storage in
aliquots at -80 C .
The CCR-3 receptor binding assay with the radioligand 125Jodine-eotaxin-1 was
performed
in a Scintillation Proximity Assay ( SPA ) design. Cell membranes of hCCR-3 Cl
cells
were diluted in suitable concentrations ( 0.5 -5 ug protein / well ) in 96
well microtiter
plates ( 1450-401, Wallac).
-21-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
The test incubation mixture comprising 60 l of the membrane suspension, 80 1
of the
Wheat Germ Agglutinin coated PVT beads (organic scintillator, Amersham
Pharmacia
biotech) in a concentration of 0,4 mg and 40 l of radiolabelled 125 J
rhEotaxin (Amersham,
IM290) were incubated with 20 l of the test compound (dissolved in DMSO
dilutions )
for 2 hours. The SPA incubation buffer contained 25mM HEPES, 25mM MgClZ 6xH2O,
ImM CaC12 2xH2O and 0,1% bovine serum albumin. Included were controls for
specific
binding ( no displacer added ) and non-specific binding by adding unlabelled
rhEotaxin
(R&D Systems) or a test compound. Bound radioactivity was determined by
scintillation
counter (Micro Beta "Trilux", Wallac).
Determination of affinity of test compounds ( dissociation constant K; ) was
calculated by
iterative fitting of experimental data using the law of mass action based
program "easy sys"
(Schittkowski, Num Math 68, 129-142 (1994)).
The utility of the compounds in accordance with the present invention as
modulators of
chemokine receptor activity may be demonstrated by methodology known in the
art, such
as the assays for CCR-2 and CCR-3 ligand binding, as disclosed by Ponath et
al., J. Exp.
Med., 183,2437-2448 (1996) and Uguccioni et al., J. Clin. Invest.,
100,11371143 (1997).
Cell lines for expressing the receptor of interest include those naturally
expressing the
chemokine receptor, such as EOL-3 or THP-1, those induced to express the
chemokine
receptor by the addition of chemical or protein agents, such as HL-60 or
AML14.3D10
cells treated with, for example, butyric acid with interleukin-5 present, or a
cell engineered
to express a recombinant chemokine receptor, such as CHO or HEK-293. Finally,
blood or
tissue cells, for example human peripheral blood eosinophils, isolated using
methods as
described by Hansel et al., J. Immunol. Methods, 145,105-110 (1991), can be
utilized in
such assays. In particular, the compounds of the present invention have
activity in binding
to the CCR-3 receptor in the aforementioned assays. As used herein, "activity"
is intended
to mean a compound demonstrating an IC50 of 10 MM or lower in concentration
when
measured in the aforementioned assays. Such a result is indicative of the
intrinsic activity
of the compounds as modulators of chemokine receptor activity.
PHARMACEUTICAL FORMS
The compounds are administered to a mammal in a therapeutically effective
amount. By
"therapeutically effective amount" it is meant an amount of a compound of
formula la that,
when administered alone or in combination with an additional therapeutic agent
to a
mammal, is effective to prevent or ameliorate diseases, wherein the activity
of a CCR-3-
receptor is involved, or the progression of this disease.
-22-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
The compounds of this invention can be administered in such oral dosage forms
as tablets,
capsules (each of which includes sustained release or timed release
formulations), pills,
powders, granules, elixirs, tinctures, suspensions, syrups, and emulsions.
They may also be
administered in intravenous (bolus or infusion), intraperitoneal,
subcutaneous, or
intramuscular form, all using dosage forms well known to those of ordinary
skill in the
pharmaceutical arts. They can be administered alone, but generally will be
administered
with a pharmaceutical carrier selected on the basis of the chosen route of
administration
and standard pharmaceutical practice.
The dosage regimen for the compounds of the present invention will, of course,
vary
depending upon known factors, such as the pharmacodynamic characteristics of
the
particular agent and its mode and route of administration; the species, age,
sex, health,
medical condition, and weight of the recipient; the nature and extent of the
symptoms; the
kind of conculTent treatment; the frequency of treatment; the route of
administration, the
renal and hepatic function of the patient, and the effect desired. A physician
or veterinarian
can determine and prescribe the effective amount of the drug required to
prevent, counter,
or arrest the progress of the disorder.
By way of general guidance, the daily oral dosage of each active ingredient,
when used for
the indicated effects, will range between about 0.001 to 1000 mg/kg of body
weight,
preferably between about 0.01 to 100 mg/kg of body weight per day, and most
preferably
between about 1.0 to 20 mg/kg/day. Intravenously, the most preferred doses
will range
from about 1 to about 10 mg/kg/minute during a constant rate infusion.
Compounds of this
invention may be administered in a single daily dose, or the total daily
dosage may be
administered in divided doses of two, three, or four times daily.
Compounds of this invention can be administered in intranasal form via topical
use of
suitable intranasal vehicles, or via transdermale routes, using transdermale
skin patches.
When administered in the form of a transdermale delivery system, the dosage
administration will, of course, be continuous rather than intermittent
throughout the dosage
regimen.
The compounds are typically administered in admixture with suitable
pharmaceutical
diluents, excipients, or carriers (collectively referred to herein as
pharmaceutical carriers)
suitably selected with respect to the intended form of administration, that
is, oral tablets,
capsules, elixirs, syrups and the like, and consistent with conventional
pharmaceutical
practices.
For instance, for oral administration in the form of a tablet or capsule, the
active drug
component can be combined with an oral, non-toxic, pharmaceutically
acceptable, inert
-23-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
carrier such as lactose, starch, sucrose, glucose, methyl cellulose, magnesium
stearate,
dicalcium phosphate, calcium sulfate, mannitol, sorbitol and the like; for
oral
administration in liquid form, the oral drug components can be combined with
any oral,
non-toxic, pharmaceutically acceptable inert carrier such as ethanol,
glycerol, water, and
the like. Moreover, when desired or necessary, suitable binders, lubricants,
disintegrating
agents, and colouring agents can also be incorporated into the mixture.
Suitable binders
include starch, gelatine, natural sugars such as glucose or beta-lactose, corn
sweeteners,
natural and synthetic gums such as acacia, tragacanth, or sodium alginate,
carboxymethylcellulose, polyethylene glycol, waxes, and the like. Lubricants
used in these
io dosage forms include sodium oleate, sodium stearate, magnesium stearate,
sodium
benzoate, sodium acetate, sodium chloride, and the like. Disintegrators
include, without
limitation, starch, methyl cellulose, agar, bentonite, xanthan gum, and the
like.
The compounds of the present invention can also be administered in the form of
liposome
delivery systems, such as small unilamellar vesicles, large unilamellar
vesicles, and
multilamellar vesicles. Liposomes can be formed from a variety of
phospholipids, such as
cholesterol, stearylamine, or phosphatidylcholines.
Compounds of the present invention may also be coupled with soluble polymers
as
targetable drug carriers. Such polymers can include polyvinylpyrrolidone,
pyran
copolymer, polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspart-
amidephenol, or polyethyleneoxidepolylysine substituted with palmitoyl
residues.
Furthermore, the compounds of the present invention may be coupled to a class
of
biodegradable polymers useful in achieving controlled release of a drug, for
example,
polylactic acid, polyglycolic acid, copolymers of polylactic and polyglycolic
acid,
polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters,
polyacetals,
polydihydropyrans, polycyanoacylates, and cross linked or amphipathic block
copolymers
of hydrogels.
Dosage forms (pharmaceutical compositions) suitable for administration may
contain from
about 1 milligram to about 100 milligrams of active ingredient per dosage
unit.
In these pharmaceutical compositions the active ingredient will ordinarily be
present in an
amount of about 0.5-95% by weight based on the total weight of the
composition.
Gelatine capsules may contain the active ingredient and powdered carriers,
such as lactose,
starch, cellulose derivatives, magnesium stearate, stearic acid, and the like.
Similar diluents
can be used to make compressed tablets. Both tablets and capsules can be
manufactured as
sustained release products to provide for continuous release of medication
over a period of
-24-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
hours. Compressed tablets can be sugar coated or film coated to mask any
unpleasant taste
and protect the tablet from the atmosphere, or enteric coated for selective
disintegration in
the gastrointestinal tract.
Liquid dosage forms for oral administration can contain colouring and
flavouring to
increase patient acceptance.
In general, water, suitable oil, saline, aqueous dextrose (glucose), and
related sugar
solutions and glycols such as propylene glycol or polyethylene glycols are
suitable carriers
io for parenteral solutions. Solutions for parenteral administration
preferably contain a water
soluble salt of the active ingredient, suitable stabilizing agents, and if
necessary, buffer
substances. Antioxidizing agents such as sodium bisulfite, sodium sulfite, or
ascorbic acid,
either alone or combined, are suitable stabilizing agents. Also used are
citric acid and its
salts and sodium EDTA. In addition, parenteral solutions can contain
preservatives, such as
benzalkonium chloride, methyl-or propyl-paraben, and chlorobutanol.
Suitable pharmaceutical carriers are described in Reminqton's Pharmaceutical
Sciences,
Mack Publishing Company, a standard reference text in this field.
Where two or more of the foregoing second therapeutic agents are administered
with the
compound of formula la, generally the amount of each component in a typical
daily
dosage and typical dosage form may be reduced relative to the usual dosage of
the agent
when administered alone, in view of the additive or synergistic effect of the
therapeutic
agents when administered in combination.
Particularly when provided as a single dosage unit, the potential exists for a
chemical
interaction between the combined active ingredients. For this reason, when the
compound
of formula la and a second therapeutic agent are combined in a single dosage
unit they are
formulated such that although the active ingredients are combined in a single
dosage unit,
the physical contact between the active ingredients is minimized (that is,
reduced). For
example, one active ingredient may be enteric coated. By enteric coating one
of the active
ingredients, it is possible not only to minimize the contact between the
combined active
ingredients, but also, it is possible to control the release of one of these
components in the
gastrointestinal tract such that one of these components is not released in
the stomach but
rather is released in the intestines. One of the active ingredients may also
be coated with a
material which effects a sustained-release throughout the gastrointestinal
tract and also
serves to minimize physical contact between the combined active ingredients.
Furthermore, the sustained-released component can be additionally enteric
coated such that
the release of this component occurs only in the intestine. Still another
approach would
-25-
CA 02572740 2007-01-02
WO 2006/013073 PCT/EP2005/008294
involve the formulation of a combination product in which the one component is
coated
with a sustained and/or enteric release polymer, and the other component is
also coated
with a polymer such as a low viscosity grade of hydroxypropyl methylcellulose
(HPMC)
or other appropriate materials as known in the art, in order to further
separate the active
s components. The polymer coating serves to form an additional barrier to
interaction with
the other component.
These as well as other ways of minimizing contact between the components of
combination products of the present invention, whether administered in a
single dosage
io form or administered in separate forms but at the same time by the same
manner, will be
readily apparent to those skilled in the art, once armed with the present
disclosure.
-26-