Note: Descriptions are shown in the official language in which they were submitted.
CA 02572791 2007-O1-17
02-123
PREPARATION OF STABLE EMULSION
USING DYNAMIC OR STATIC MIXERS
BACKGROUND OF THE INVENTION
The invention relates to the preparation of emulsions and,
more particularly, to a process for preparing such emulsions and
mixtures of emulsions wherein gelling of additives such as
surfactants is avoided and wherein properties of the emulsion
can be tailored to desired requirements.
This application is a divisional application of
application Serial No. 2,430,203, filed May 28, 2003.
Viscous hydrocarbons such as natural bitumen are found in
significant amounts in Canada, the United States, China, Nigeria
and Venezuela. These hydrocarbons typically are liquid having
viscosities from 1,000 to 600,000 cP at room temperature. This
viscosity, and the relative low reactivity of such materials,
make for substantial difficulties in handling. One method for
addressing such problems is to make an emulsion in water of such
materials thereby reducing viscosity of same and consequently
improving properties of the product as a fuel.
Different methods have been proposed for preparing such
emulsions, and these emulsions must be stabilized using
emulsifiers or surfactants which can be added and/or activated
from within the hydrocarbon. Additionally, the emulsion should
remain stable upon inclusion of other substances or additives as
desired.
1
CA 02572791 2007-O1-17
The present invention seeks to provide a process for
preparing an emulsion of viscous hydrocarbon in water which
produces a stable and useful end product.
Other advantages of the present invention will appear
hereinbelow.
SUMMARY OF THE TNVENTION
In accordance with this invention, there is provided a
process for preparing a hydrocarbon-in-water emulsion,
comprising the steps of:
providing a substantially homogeneous solution of a
liquid additive in water;
mixing said solution with a hydrocarbon in a mixer so
as to provide a first emulsion of hydrocarbon in water
having a ratio by volume of hydrocarbon to water of at
least about 90:10;
adding additional additives to at least one of said
mixer and said first emulsion; and
diluting said emulsion with water so as to provide a
final emulsion having a ratio by volume of hydrocarbon to
water of less than 90:10.
la
CA 02572791 2007-O1-17
02-123
In another aspect, a process is provided for
preparing a hydrocarbon in water emulsion, which process
comprises the steps of providing a liquid additive that tends to
gel when mixed with water at temperatures less than a gelling
temperature T~; providing a stream of water at a temperature T~
less than said gelling temperature TG; feeding said stream to a
mixer having a mixer inlet so as to impart energy to said
stream; adding said liquid additive to said stream downstream of
said mixer inlet, whereby said liquid additive mixes with said
stream and said energy inhibits gelling of said liquid additives
so as to provide a substantially homogeneous solution of said
liquid additive in water; and mixing said solution with
hydrocarbon in a mixer so as to form a hydrocarbon-in-water
emulsion.
The water solution preparation step in accordance with the
present invention advantageously avoids the formation of gel
without excessive use of heating and cooling.
According to a further embodiment of the invention, a
process is also provided which allows for fine tuning the
emulsion to have desirable properties.
BRIEF DESCRIPTION OF THE DRAWINGS
A detailed description of preferred embodiments of the
present invention follows, with reference to the attached
drawings, wherein:
Figure 1 schematically illustrates a process in accordance
with the present invention;
Figure 2 illustrates the gel temperature profile for a
typical surfactant material at different concentrations in
water;
Figure 3 illustrates a heat-only process that can be used
to avoid gelling;
2
CA 02572791 2007-O1-17
02-123
Figure 4 illustrates a preferred embodiment of the present
invention wherein some heat is applied, and mixing energy is
used to avoid geI formation;
Figure 5 schematically illustrates a preferred mixture in
accordance with the present invention, along with preferred
placement of an additive injector;
Figure 6 schematically illustrates a process in accordance
with the present invention;
Figure 7 schematically illustrates a portion of the process
of Figure 6;
Figure 8 schematically illustrates a further portion of the
process of Figure 6;
Figure 9 illustrates droplet size distribution obtained
using static and dynamic mixers for large droplet size
emulsions;
Figure 10 illustrates droplet size distribution obtained
using static and dynamic mixers for small droplet size
emulsions;
Figure 11 illustrates droplet size distribution obtained by
mixing large and small droplet size emulsions in optimal
proportions; and
Figure 12 illustrates droplet size distribution obtained
after incorporation of additives and water in order to fine tune
the resulting emulsion properties.
DETAILED DESCRIPTION
The invention relates to a process for preparing
hydrocarbon-in-water emulsions using a process for preparing
solutions of additives and surfactants wherein heating and a
static mixer are used to avoid geI formation of the additives.
The invention further relates to a process for preparing
hydrocarbon-in-water emulsions using a process for preparing
concentrated emulsions, and diluting and/or mixing them with
3
CA 02572791 2007-O1-17
02-123
other emulsions so as to create emulsions with specific
geometric and rheological properties.
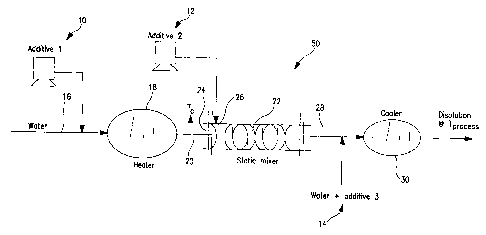
Figure 1 schematically illustrates a surfactant solution
preparation process wherein several additives 10, 12, 14 are to
be added to a stream 16 of water. In accordance with this
embodiment of the present invention, additives 10 and 14 are
water soluble, and do not gel, and can therefore be added at any
convenient point.
Additive 12, however, is an additive such as a surfactant
which tends to gel, or have properties similar to those
illustrated in Figure 2, if mixed with water below certain
temperatures, fox example at ambient temperature. Stream 16 is
therefore fed to a heater 18 to increase the temperature of
stream 16 from ambient temperature to a temperature T~ which is
greater than ambient temperature, and which is preferably less
than the maximum gelling temperature T~ of additive 12. The
heated stream 20 is then fed to a static mixer 22, through a
static mixer inlet 24, to impart mixing energy to the stream.
Once at least some energy has been imparted to the stream,
additive 12 is then added to static mixer 22, preferably at an
additive inlet 26 which is schematically illustrated in Figure
1.
The mixing energy imparted to stream 20 within mixer 22 in
accordance with the invention has advantageously been found to
be sufficient to prevent gel formation of additive 12, despite
the fact that the temperature of stream 20 has not been heated
to a temperature above the gelling temperature T~.
Stream 28 exiting static mixer 22 advantageously comprises
a substantially homogeneous and gel-free mixture of water 16 and
additive 12, along with any other additives 10 and the like
which may have been provided as desired.
As set forth above, additives 10 and 14 are water soluble,
and can be added at any point. Thus, in the embodiment
4
CA 02572791 2007-O1-17
02-123
illustrated in Figure 1, additive 10 is added to stream 16
upstream of heater I8 and static mixer 22, while additive 14 is.
added downstream of mixer 22.
Still referring to Figure 1, stream 28 can itself be fed,
at temperature T~, to further processing steps such as an
emulsion forming step or the like, particularly when such
process is effective at temperature T~. This is advantageous
since the heat used to form the solution can be used again in
such emulsion preparation, thereby enhancing process efficiency.
For other processes, wherein lower temperatures are
required, stream 28 can be fed to a cooler 30 as schematically
illustrated so as to reduce the temperature to a temperature TP
which is more suitable to the desired process.
Referring to Figures 2-4, Figure 2 shows a typical gel
temperature profile for a liquid additive having gelling
tendencies, and shows the gelling temperature T~ at
concentrations of the additive in water. As shown, at high
concentrations the additive is liquid at substantially any
temperature. As should also be clear, however, if such material
is merely added to water, so as to reduce concentration at a low
temperature, the additive will gel and cause various problems.
One class of additives which has a gelling profile as
illustrated in Figure 2 are surfactants for use in making
oil/water emulsions. For example, ethoxylated nonylphenol (NPE)
has a profile as illustrated. NPE is typically provided
commercially having a concentration in water of about 90~ or
higher, which generally corresponds to point 32 shown in Figure
2, Another example of such a surfactant is ethoxylated alcohol
(TDE). It is typical to use such surfactants at a reduced
concentration, preferably of about 0.2%, which corresponds to
point 34 shown on Figure 2. In accordance with the present
invention, the process provided allows for dilution from point
CA 02572791 2007-O1-17
02-123
32 to point 34 without the need to heat in excess of temperature
T~, and without the formation of gel.
Of course, NPE is one example only of an additive having a
gelling profile as illustrated in Figure 2. Other additives
behave similarly and can therefore advantageously be used in
accordance with the process of the present invention.
Furthermore, the various additives which can be used in
accordance with the present invention advantageously include at
least one surfactant selected from the group consisting of
nonionic surfactants, anionic surfactants, inactive surfactants,
bioactive surfactants, activating additives for activating
inactive surfactants, polymers, clay particles and the like, and
combinations thereof. In connection with activating additives
for activating inactive surfactants, the inactive surfactants
can be naturally occurring or separately added, or a combination
of both. The activating additive can advantageously comprise a
pH buffer solution selected so as to provide the resulting water
solution of the additive with a pH of at least about l0.
Furthermore, a preferred activating additive is an alkaline
additive selected from the group consisting of sodium, potassium
and lithium salts, amines and combinations thereof.
Figure 3 illustrates the heating and cooling that would be
necessary to go from ambient temperature to a processing
temperature while heating to a temperature above T~. While this
would avoid formation of gel, it should readily be appreciated
that the heating and cooling costs would be substantial.
Turning now to Figure 4, the preferred process of the
present invention is shown wherein the additive is diluted with
water at a temperature that is heated to a temperature T~ that is
greater than ambient temperature, but less than the maximum gel
formation temperature T~. This moves the additive sufficiently
high on the gel formation profile that energy imparted from the
6
CA 02572791 2007-O1-17
02-123
static mixer can successfully prevent formation of gel and allow
effective mixture with the liquid base or water as desired.
Tt should readily be appreciated that the heating and
cooling costs in the process of the present invention are
substantially reduced as compared to that in Figure 3. Further,
a static mixer which is used to provide the energy desired is
likewise efficiently operated, reliable and inexpensive.
Turning now to Figure 5, a preferred placement of additive
inlet is illustrated. Figure 5 schematically shows a static
mixer wherein mixer 22 has a series of swirling flow imparting
members 36 each having a length Lm corresponding to a 360°
rotation along mixer 22. Mixer 22 and member 36 also have a
diameter do. In accordance with the present invention, a
surfactant or additive inlet 38, or preferably a plurality of
inlets 38, are advantageously positioned downstream of the
beginning of a swirling flow imparting member 36 by a distance Lb
which is preferably approximately equal to Lm/4. Furthermore,
inlet or inlets 38 advantageously extend inwardly into mixer 22
by a distance h which is preferably equal to about do/4. This
advantageously injects the additive into the stream at a point
where sufficient swirling energy has been imparted that gel
formation can be avoided at temperatures less than the gel
formation temperature. This advantageously provides for the
excellent results obtained in accordance with the present
invention.
It should readily be appreciated that the surfactant
solution preparation process provided can be carried out in a
continuous manner, and provides for manufacture of downstream
products such as viscous hydrocarbon in water emulsions with a
high degree of quality since surfactant concentration is
homogeneously distributed through the water phase. Furthermore,
it should readily be appreciated that this process provides such
excellent results with a minimum amount of energy used for
7
CA 02572791 2007-O1-17
02-123
heating and/or cooling, and utilizing a mixer which requires a
minimum amount of maintenance.
Figure 6 schematically illustrates a process in accordance
with the present invention for preparing an emulsion in
accordance with the present invention. Figure 6 shows module 50
for preparing an additive and surfactant solution as described
above in connection with Figures 1-5.
As shown in Figure 6, modules 52, 54 are also provided for
preparation of two or more different emulsions, and a module 56
is provided for combining the different emulsions to produce the
desired final emulsion.
Still referring to Figure 6, in accordance with the present
invention, a solution stream 58 from module 50 is advantageously
divided into a first portion 60 and a second portion 62. First
portion 60 may be further diluted with water 64 if desired, and
then mixed with a hydrocarbon 66 in a mixer in module 52 so as
to form a first emulsion 68 having a large droplet diameter
size, for example in the range of about 30 microns.
Second portion 62 is mixed with hydrocarbon 70 in a mixer
of module 54 so as to form a second emulsion 72 which has a
small average droplet size. Emulsions 68 and 72 are then
combined in module 56 to form the desired end emulsion. In this
regard, additional water 74 may advantageously be added to the
system, for example by adding to small droplet diameter emulsion
72, so as to provide a final bimodal emulsion having a desired
water content, for example of greater than or equal to about
29%, and/or further water can be added downstream of the
emulsion mixing process as well.
Figure 7 schematically illustrates the portion of Figure 6
relating to module 52 for forming the large droplet diameter
emulsion according to the embodiment of Figure 6. Figure 7
shows portion 60 of the surfactant and additive solution being
mixed with water 64 and fed to a first mixer 100 which in this
8
CA 02572791 2007-O1-17
02-123
case is shown as a static mixer. The resulting mixture is then
combined with hydrocarbon 66 and fed to a further mixer 76 which
in this case is also shown as being a static mixer. It should
be appreciated that mixers 100 and 76 as shown herein can
alternatively be dynamic mixers if preferred. From static mixer
76, the hydrocarbon-water mixture is then further fed to a
further mixer 78 which may be a dynamic mixer or a static mixer,
or both, and which is preferably operated at a shear sufficient
to provide the desired droplet size emulsion. In this regard,
shear with a dynamic mixer is controlled by operating the mixer
at particular rates, while shear in a static mixer is controlled
by adjusting the flow rate and temperature for a given mixer
diameter.
Figure 8 is substantially similar to Figure 7 and shows in
greater detail module 54 for preparation of the small droplet
diameter emulsion of the embodiment of Figure 6. As shown, a
second portion 62 of surfactant additive solution is mixed with
additional water 80 and fed to a mixer 82 which is also shown in
this case as a static mixer. The resulting diluted solution is
then mixed with hydrocarbon 70 and fed to a further mixer 84
which may also be a static or dynamic mixer, and which is shown
in this case as a static mixer_ The resulting pre-mixed
combination is then fed to a further mixer 86 which can be
either a dynamic mixer, a static mixer or both, and which is
operated at shear selected to provide the desired small droplet
size, for example of 3 microns. The resulting emulsion may be
diluted with further water 74 as shown, and is then fed to
module 56 (Figure 6) for final preparation of the desired
emulsion.
As set forth above, the formed emulsion can advantageously
be diluted so as to provide a desired ratio of hydrocarbon to
water in the final product. In this regard, the additional
mixing step is preferably carried out so as to provide an
9
CA 02572791 2007-O1-17
02-123
emulsion having a ratio by volume of hydrocarbon to water of at
least about 90:10, and emulsion dilution advantageously is
carried out so as to provide a final emulsion having a ratio by
volume of hydrocarbon to water of less than about 90:10. More
preferably, the initial emulsion is formed having a ratio of
hydrocarbon to water, by volume, of at least about 97:3, and the
step of adding water to dilute the emulsion is carried out so as
to provide a final emulsion having a ratio of hydrocarbon to
water, by volume, of about 70:30. Of course, other ratios may
be desired. However, the ratios as set forth above
advantageously provide for efficient and effective mixing of
emulsions and a particularly stable and desirable final product.
Figures 9 and 10 show shear rates used with static and
dynamic mixers in order to obtain large and small droplet sizes,
respectively, for emulsions as desired. Figure 9 shows that, in
order to obtain droplet sizes in the range of 12-35 microns,
static and dynamic mixers both provide acceptable results at
shear rates of less than 300 1/s, and these mixers appear to be
interchangeable as compared to results obtained.
Figure 10 shows that, for the small droplet size emulsion,
acceptable mean droplet sizes in the range of 3.5 to 4.75
microns can be obtained with static or dynamic mixers at shear
rates between about 800 and about 1200 1/s, and also shows that
static and dynamic mixers are substantially interchangeable for
providing such droplet sizes.
The foregoing description is given in terms of preparation
of a final emulsion from two or more different emulsions. It
should of course be appreciated that the process of the present
invention, including additive solution preparation and operation
of mixers, is equally well suited to preparation of a single
emulsion as well. In this case, and referring to Figure 6,
module 54 would not be needed. Further, module 56 for combining
would not be necessary, and only a dilution module for diluting
to
CA 02572791 2007-O1-17
02-123
the final emulsion to the desired water content would be
desired.
As set forth above, the hydrocarbon phase from which the
emulsions can be prepared in accordance with the process of the
present invention is any of a wide variety of hydrocarbons.
This process can most advantageously be used with viscous
hydrocarbons since the emulsion provides beneficial reduction of
viscosity.
Additives to be added in the solution in module 50 may
typically include nonionic surfactants, anionic surfactants,
bioactive surfactants, inactive surfactants, activating
additives for activating inactive surfactants, polymers, clay
particles and the like, and combinations thereof. The process
of the present invention advantageously allows for such
additives and surfactants, including those which gel upon
contact with water, to be used in the desired small
concentrations, through dilution with water, without adverse
gelling and the like. This is particularly advantageous in that
a savings in material costs is experienced when the small
concentrations of surfactant are used. Furthermore the use of
excessive surfactant, in addition to being a cost, can be
undesirable in the final product as well.
Turning now to Figure 11, bimodal emulsions can
advantageously be formed by first making a plurality of
monomodal emulsions, and then mixing the emulsions in accordance
with the process described above. Figure 11 shows two different
monomodal emulsions in terms of droplet size distributions, and
also shows the droplet size distribution for a bimodal or final
emulsion product prepared from the two monomodal emulsions.
This final product is stable and has desired properties.
In further accordance with the invention, it has been found
that emulsions prepared in accordance with the present invention
are particularly stable when mixed with additional additives.
II
CA 02572791 2007-O1-17
02-123
In order to prepare a final emulsion product, various additives
are typically required, many of which can adversely impact upon
stability of the emulsion. Emulsions prepared in accordance
with the present invention, however, show remarkable stability
even after such additives have been mixed into the emulsion
product. Figure 12 shows mean droplet size for an emulsion
before and after mixing with additional additives. As can be
seen, the droplet size distribution is substantially identical
before and after addition of these additives. This stability
allows for fine-tuning of the emulsion product to desired
properties which is a particular advantage of the process of the
present invention.
The following examples demonstrate the excellent results
obtained using the surfactant solution preparation process in
accordance with the present invention.
Example 1
In this example, a Kenics~' mixer having % inch X12 elements
was used to mix TDE with water at a temperature of 35°C. This
water had been heated to 35°C from ambient temperature. Mixing
was carried out at various water flow rates and additive flow
rates, with mixing energy imparted by the static mixer being
determined based upon the materials fed to the mixer, the
process temperature and specifics of the mixer. Table 1 below
sets forth the amounts of dissolution obtained in each case.
m~Hio ,
Water FlowAdditive Flow Mixing Energy Dissolution Degree
(1/s) (ml/min.) (W/Kg) (grs dissolved/total
grs)
0.42 303 199 0.99
0.33 240 104 0.98
0.24 180 40 0.94
0.12 84 4 0.78
As shown, excellent dissolution was obtained at mixing
energy of 40 W/Kg and above for the flows shown. At a mixing
12
CA 02572791 2007-O1-17
02-123
energy of only 4 W/Kg only 78% dissolution was obtained. Thus,
the mixing energy provided by the static mixer in accordance
with the present invention clearly helps to avoid gel formation
and enhances complete dissolution of the additive.
Example 2
In this example, a stream of heated water was mixed with
surfactant in three different locations along the mixer in order
to demonstrate the advantageous position of injectors for the
additive.
In the first instance, the additive was injected at the
entrance to the mixer, along with the water. In the second
evaluation, the additive was injected through a single injector
at a point as selected according to the illustration of Figure
5. Finally, in a third evaluation, additive was injected
through two injectors positioned at a point as illustrated in
Figure 5.
With the additive introduced at the entrance to the mixer,
only 72% dissolution was obtained. With additive introduced
through a single injector downstream of -the inlet, 80%
dissolution was obtained. With the additive injected through
two injectors downstream of the inlet as illustrated in Figure
5, 94% dissolution was obtained. Thus, positioning of the
injector or inlet for the additive in accordance with the
present invention provides for enhanced dissolution as desired.
It should readily be appreciated that a process has been
provided in accordance with the present invention for preparing
hydrocarbon-in-water emulsions, wherein liquid additives are
prepared for incorporation into the emulsion without gelling,
and wherein a final emulsion can be prepared to meet specific
requirements, all as desired.
Gelling of additives is avoided without use of excessive
amounts of mixing energy, thereby allowing small concentrations
13
CA 02572791 2007-O1-17
02-123
of additives such as surfactants and the like to be used without
gelling. Further, the process provides a versatility in
adapting emulsions to desired end uses, including a potentially
wide variety of additives, without affecting emulsion stability
and without gelling of the additives.
It is to be understood that the invention is not limited to
the illustrations described and shown herein, which are deemed
to be merely illustrative of the best modes of carrying out the
invention, and which are susceptible of modification of form,
size, arrangement of parts and details of operation. The
invention rather is intended to encompass all such modifications
which are within its spirit and scope as defined by the claims.
14