Note: Descriptions are shown in the official language in which they were submitted.
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
METHOD AND DEVICE TO PROCESS, LABEL AND CONCENTRATE ANALYTES
Field of the Invention
The present invention relates to a device and methods for the preparation, in
fluid
samples, for the detection and quantification of an analyte that may be
present in the fluid
sample.
Background of the Invention
Micro-organisms, including toxic bacteria, yeast and mould account for several
million cases of food-related illnesses and 9,000 deaths per year in the
United States
alone. Contaminated poultry and meat products are a major cause of these
deaths and
illness. The four most common pathogens infecting poultry and meat products
are E. coli
0157:H7, Campylobacter jejuni/coli, Salmonella species and Listeria species
and L.
monocytogenes.
Contaminated water supplies are also a health hazard. The United States
Environmental Protection Agency has determined that the level of E. coli in
water supply
is a good indicator of health risk. Other common indicators are total
coliforms, fecal
coliforms, fecal streptococci and enterococci.
Many disease conditions, such as bacterial and viral infections, cancers,
heart
attacks and strokes for example, may be detected through the testing of blood
and tissues,
including interstitial fluid, saliva, urine, semen, feces, genetic coding and
tissue sections,
for markers that are associated with particular conditions. Early and rapid
diagnosis and
measurement of treatment efficacy is desired to enhance successful treatment.
The accurate quantitation and rapid detection of micro-organisms, such as
bacteria, viruses, fungi or other infectious organisms and indicators in food
and water, on
surfaces where food is prepared and on other surfaces that should meet
sanitary standards
is a pressing need. There is also a serious need for the accurate and rapid
identification of
micro-organisms and disease markers of a patient.
In a typical test assay, a fluid sample is mixed with a reagent, such as an
antibody,
specific to a particular analyte, such as an antigen. The reaction of the
analyte with the
-1-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
reagent may result in a color change that may be visually observed, or in
chemiluminescent, bioluminescent or fluorescent species that may be observed
with a
microscope or detected by a photodetecting device, such as a
spectrophotometer. The
reagent may also be a fluorescent or other such detectable labelling reagent
that binds to
the analyte. Irradiation scattered, reflected, transmitted or absorbed by the
fluid sample
may also be indicative of the identity and type of analyte in the fluid
sample.
In a commonly used assay technique, two types of antibodies are used, both
specific to the analyte. One type of antibody is immobilized on a solid
support. The
other type of antibody is labelled by conjugation with a detectable marker and
mixed with
the sample. A complex between the first antibody, the substance being tested
for and the
second antibody is formed, immobilizing the marker. The label to identify the
specific
marker may also be an enzyme or a fluorescent or radioactive marker, which may
then be
detected. The reaction ingredients are all mixed in a single step for reaction
in a single
reaction liquid. See, for example, U.S. Patent No. 5,610,077. The reaction
liquid is
simultaneously contacted with the immobilized capture antibody to allow for
adsorption
of analyte and marker antibody to the immobilized capture antibody.
Competitive
interference between the binding reactions typically induces instability
during the testing
process. The ratio of marker antibody to analyte has to be optimized depending
on the
analyte concentration likely to be present in the test fluid. These types of
assays, known
in the art as "sandwich" immunoassays, typically provide only a threshold
concentration
for the analyte of interest and are read as qualitative, positive or negative
results results.
Fluid samples must often be concentrated to increase the number of analyte
particles to a level that may be reliably detected. The fluid sample may then
be
transferred to a container, such as a test tube, for mixing with the reagents.
The reacted
fluid sample is transferred to an assay device for analysis or to another
container for
mixing with other reagents. Such transfers, which are often conducted with a
pipette are
time consuming, labour intensive and expose the fluid sample and the people
conducting
the analysis to contamination. There is a need for a more convenient aseptic
method to
concentrate analyte, transfer fluid samples and mix fluid samples with
reagents and other
compounds required for testing while minimizing competitive interference.
-2-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
Summary of the Invention
The invention provides a sample processing and labelling applicator device for
transferring a fluid sample, including an applicator body defining a hollow
bore with an
open end and an outlet, a plunger having a first end and being effective to
fit within the
hollow bore, and a second end extending out of the open end where the first
end is located
within the hollow bore; and a reagent located between the piston and the
outlet where the
piston is located within the hollow bore.
According to another aspect of the present invention, there is provided a
method
for concentrating an analyte present in a liquid sample, including the step of
providing an
applicator body defining a hollow bore with an open end and an outlet, a
plunger having a
first end at one end and being effective to fit within the hollow bore, and a
second end
extending out of the open end where the piston is located within the hollow
bore; and a
reagent located between the first end and the outlet where the first end is
located within
the hollow bore; mixing and incubating the analyte and the reagent for a
period of time
sufficient to react the analyte with the reagent and expelling a controlled
amount of the
portion through the outlet.
According to another aspect of the present invention, there is provided a
method
for reacting an analyte present in a liquid sample, including the step of
providing an
applicator body defining a hollow bore with an open end and an outlet, a
plunger having a
first end at one end and being effective to fit within the hollow bore, and a
second end
extending out of the open end where the first end is located within the hollow
bore; and a
reagent located between the first end and the outlet where the piston is
located within the
hollow bore, mixing and incubating the portion with the reagent for a period
of time
sufficient to react the analyte in the portion with the reagent; and expelling
an amount of
the portion through the outlet.
Brief Description of the Drawings
Figure 1 is a front perspective view of a sample applicator of the present
invention;
Figure 2 is a cross-sectional view of the sample applicator taken along lines
2-2 of
Figure 1; and
-3-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
Figure 3 is a perspective view of a cap of the present invention detached from
the
sample applicator.
Detailed Description of the Invention
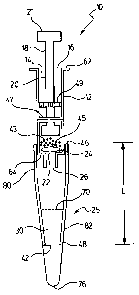
Sample applicator 10 is shown in Figure 1. The sample applicator 10 comprises
an
applicator body that is preferably syringe body 12, and which is preferably
cylindrical in
shape. The syringe body 12 has a first end 62 and a second end 64. The syringe
body 12
defines a hollow bore 14 with a first open end 16. A plunger 18 has a first
end that
preferably defines a piston 20 at its first end within the bore 14 and a
second end 21
extending out of the first open end 16 of the bore 14. The second end 21 is
preferably
widened for engagement by a user. The syringe body 12 defines an outlet 22 at
the
second end 64 of the syringe body 12. A flange 24 is preferably attached to
the second
end 64 of the syringe body 12. The flange 24 is preferably cylindrical. The
flange defines
outlet 26 that is in fluid communication with the outlet 22 of the syringe
body 12. The
outer surface of the flange 24 may have the shape of a Luer lock or taper.
Reagents 43,
and preferably a concentrating material 45 to facilitate the analysis of the
fluid sample,
are provided between the plunger 20 and the outlet 22. The concentrating
material 45 is
preferably super absorbent polymer particles. The reagents are preferably
monoclonal
antibodies that are specific for a given analyte. Most preferably the reagents
are
lyophilized monoclonal antibodies that rapidly reconstitute upon mixing with a
sample
fluid. The reagents may also be frozen, in liquid form, freeze dried into a
pellet or may be
dried on the interior surfaces of the syringe body 12.
Volume indicator graduation lines 47, 49 are preferably provided for ease of
use.
On a 1 ml syringe body, the first volume line 47 may correspond to drawing 200
ml from
the source and the second volume line 49 may correspond to drawing an
additional 100-
150 ml into the syringe bore 14, for example.
The syringe applicator 10 includes a preferably cylindrical inlet/outlet
assembly
25 comprising an upper region 80 and a lower region 82. The inner diameter of
the upper
portion 80 is about the same as or is slightly larger than the outer diameter
of the lower
portion 82. The upper region 80 has a first end 46 coupled to the second end
64 of the
syringe body 12. The lower region has an end 48 being for receiving a fluid
sample. The
inlet/outlet assembly 25 defines an opening 42 at the end 48 for passage of
sample fluid.
-4-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
The inlet/outlet assembly 25 is provided to extend the distance from the
flange 24 to the
source for ease of use and to avoid insertion of syringe body 12 into the
source. Fluid
sample on the syringe body could contaminate the user and the surroundings.
A length L for the inlet/outlet assembly 25 of at least about 2 cm is
preferred
whereas 3 cm is more preferred. A preferred range is from about 2 cm to about
7 cm.
The configuration of the inlet/outlet assembly 25 may vary according to the
application to
be carried out using the syringe applicator 10.
A cap 30 is preferably attached to the end 48 of lower portion 82 of the
inlet/outlet
assembly 25 thereby covering the opening 42 and sealing the syringe applicator
10 to
prevent contamination prior to use. The cap 30 preferably has a first
outwardly tapering
cone-shaped cylindrical shaped section and a flat tip 76. The cap 30 is
preferably attached
to the inlet/outlet assembly 25 by means of perforations 70. The cap must
therefore be
broken off from the inlet/outlet assembly 25. An intact attached cap indicates
that the
syringe applicator 10 has not been used. The cap 30 is adapted to engage the
lower region
82 in a fluid tight fit. The cap 30 is preferably a rigid moulded plastic such
as polystyrene,
an acrylic or polypropylene. A semi-rigid material may also be used in
alternate
embodiments.
To use the sample applicator 10 of the present invention, the cap 30 is torn
off the
lower region 82 of the inlet/outlet assembly 25 and a fluid sample is drawn
from a fluid
source. The fluid source may be a culture medium or media, depending on the
concentration of the analyte in the fluid sample and the nature of the source
matrix. For
example, if the source is a food sample, culturing is typically required. If
the source is
feces, culturing may not be necessary. The lower region 82 is inserted into
the source and
the plunger 18 is withdrawn to draw a fluid sample from the source through the
inlet/outlet assembly 25 and into the bore 14 of the syringe body 12. About
300-350 l is
an appropriate amount, for example. If the volume lines 47 and 49 are
provided, the first
volume 47 is preferably positioned so that an appropriate amount of the fluid
sample is
drawn into the syringe applicator when plunger 18 is withdrawn up to the first
volume
line 47. The sample applicator 10 is then removed from the source and the
plunger 18 is
preferably further withdrawn, to the second volume line 49. The cap 30 is
placed over the
inlet/outlet assembly 25 to cap and seal the fluid inside the syringe body 12.
The syringe
-5-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
applicator 10 is then inverted several times to reconstitute the reagents 43,
and to
adequately mix the reagents with the fluid sample. If the concentrating
material 45 is
present, the fluid sample is allowed to incubate for about 20 minutes. If the
concentrating
material 45 is not present, about 5 minutes of incubation is sufficient to
reconstitute the
reagents which are typically lyophilized antibodies thereby enabling only the
now
hydrated and conjugated marker to react specifically with the analyte in a
controlled
environment, thereby preventing interference and maintaining excess, viable
marker
antibody which are required for labelling the calibration arrays also
immobilized on the
same solid support as the capture antibody. After sufficient incubation, the
cap 30 is
removed and the plunger is advanced to expel a sufficient amount of the fluid
sample
from the bore 14 through the inlet/outlet assembly 25 and into an assay device
for
analysis. Instead of being transferred to an assay device, the fluid sample
may be
transferred to another location, such as into another container for reaction
with other
reagents.
If only the concentrating material 45 is provided in the syringe body 12, the
fluid
sample may be transferred into a reaction chamber for reaction with reagents.
Even if
reagents are present in the reaction chamber, further reaction with additional
reagents
may be required.
The fluid sample once exposed to the reagents in the sample applicator 10 may
be
transferred to an assay device to be analyzed for the presence of analytes.
The analyte
may be a microbe such as a bacterium, a virus or a fungus, for example. The
analyte may
also be a biological substance such as a peptide, an amino acid, a nucleic
acid, or a
protein, such as an enzyme, an antibody, an antigen, an immuno-globulin or a
hormone,
for example. The analyte may also be a chemical substance such as a chemical
element,
chemical compound or a polymer, for example.
The fluid sample may also be transferred to a reaction vessel. The source of
the
fluid sample may be a culture medium containing a suspect food, water or fecal
matter,
for example. The source may be uncultured, as well. The sample applicator 10
includes
reagents for reacting with and enabling the identification of an analyte in
the fluid sample.
The reagents may be labelled with a fluorescent chemical, colorimetric or
radioactive
label, for example, as is known in the art. The label can also be a latex
bead, metal
-6-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
colloidal particle, dye, enzyme or Stoke effect transducer. Other types of
reagents may
also be used, as is known in the art.
If the analyte is present and the reagents are antibodies, the reagent
antibodies
bind to the analyte and label the analyte with the detectable marker. The
fluid sample and
the labelled analyte may then be transferred to an appropriate assay device
for analysis. A
preferred assay device disclosed in U.S. Patent Application Publication No.
20020019062
entitled "Assay Devices", which is incorporated by reference herein in its
entirety.
In one application, the analyte is E. coli 0157:H7. The sample applicator
contains
lyophilized monoclonal mouse-anti-E. coli 0157:H7 antibody, available from
Biodesign
International, Saco, Maine, U.S.A., for example. The antibodies may be pre-
conjugated
with Alexa Fluor 647 fluorescent dye available from Molecular Probes
Incorporated,
Eugene, Ohio, U.S.A., for example. Molecular Probes also provides an Alexa
Fluor
647 Protein Labeling Kit (A-20173) for conjugating the antibodies to the dye.
The
labelled antibodies are preferably lyophilized as is known in the art.
Alternatively, frozen
labelled antibodies may be used. In another alternative, an aliquot of the
labelled
antibodies in a liquid suspension may be drawn into or otherwise placed within
the
syringe body and the sample applicator 10 may be placed in a freezer to freeze
the
suspension. The sample applicator 10 may be used after removal from the
freezer.
In another example, the reagents may be a luciferin/luciferase complex for
detecting ATP as an indicator of the presence of biological contamination of a
sample.
ATP reacts with a luciferin/luciferase complex, in the presence of oxygen and
magnesium
ions (Mg+2 ) to produce visible light at a wavelength of 562 nanometers. Since
all living
cells contain ATP, detection of light in 562 nanometers is indicative of the
presence of
living cells. Luciferin/luciferase is commonly extracted from fireflies.
Luciferin/luciferase reagents are commercially available. An Adenosine 5'
Triphosphate
(ATP) Bioluminescent Assay Kit, FL-AAM, available from the Sigma-Aldrich
Corporation, Milwaukee, Wisconsin, U.S.A., may be used, for example. FL-AAM is
a
lyophilized powder containing firefly luciferase, luciferin, MgSO4 , EDTA,
dithiothreitol
("DTT") and bovine serum albumin ("BSA") in a tricine buffer. After mixing
with the
reagents, the fluid sample can be transferred to an appropriate assay device
for detection
-7-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
of luminescent species resulting from the reaction. A device containing a
photomultiplier
tube may be used, for example.
In addition to the labelled antibodies or other such reagents specific to the
analyte,
a second type of labelled antibody or other such reagent may be provided
specific to a
control spot in an assay device. Application of the fluid sample to the assay
device
results in the second type of antibody binding to the control spot. This
provides an
indication that the assay device has been used, even if an insufficient amount
of analyte is
present in the fluid sample for detection of the analyte.
In addition to the labelled antibodies or other such reagents specific to the
analyte
and a second type of labelled antibody or other such reagent, a fluid dye may
be added to
the pre-lyophilization mix to confirm the presence and successful transfer of
the dyed
fluid sample to another vessel or assay device. A typical dye is Bromophenol
Blue,
Sigma Chemicals, St. Louis, Missouri 63178.
The concentrating materia145 absorbs liquid from the fluid sample, increasing
the
concentration of the analyte, such as bacteria, in the fluid sample. The
concentrating
material may be any material that absorbs fluid and does not react with the
analyte in the
fluid sample. Super-absorbent polymers, such as polyacrylates, cellulose
derivatives and
hydrogels, for example, are preferred. Super-absorbent polymers are discussed
in
Absorbent Polymer Technology, Studies in Polymer Science 8, edited by Lisa
Brannon-
Peppes and Ronald S. Harland, Elsevier Sciences, 1990, which is incorporated
by
reference herein, in its entirety. A suitable commercially available super-
absorbent
polymer is Favor -Pac 100, from Stockausen Inc., Greensborough, North
Caroline,
U.S.A. Favor -Pac 100 is said to be a salt of crosslinked polyacrylic acid and
grafted
copolymer. According to the manufacturer, the carboxylic groups of the polymer
are
solvated when brought into contact with water or water based liquid. As a
result, the
groups partially dissociate into negatively charged carboxylic ions. In this
state, the
polymer chain contains a large number of similarly charged ionic groups that
repel each
other. The polymer coils become more bulky and thus extend their propensity to
absorb
aqueous fluid. Due to the cross-linking between the polymer chains, a gel is
formed.
Water is strongly bonded by hydrogen bonds in the gel. The physical
characteristics of
the Favor -Pac 100, as provided by the manufacturer, are:
-8-
CA 02573925 2007-01-12
WO 2006/007711 PCT/CA2005/001129
Physical Form White granules
Particle Size 100-850 microns
Product Density 540g/ +/-30
Sifting Properties Free Flowing
Moisture Content 5% +/-2
pH value (1% gel in 0.9% NaC1) 6.0 +/- 0.5
Storage > 1 year under dry conditions
Thirty (30) milligrams of Favor -Pac 100 has been found to be an appropriate
amount of Favor -Pac 100 to use with a fluid sample of about 300-350
microliters, to
increase analyte concentration by a factor of three (3). Excessive
concentration, such as
10 times or more, is generally not desirable. If the concentrating material is
being
provided with the lyophilized antibodies, it may be desirable to add
additional antibodies
to compensate for antibodies that may be absorbed by the concentrating
material along
with the fluid and for the initial high level of fluid in the sample. As
mentioned above,
the super-absorbent polymer particles have a size preferably greater than
about 0.80
millimeters. Smaller particles may be removed using a mechanical separation
process,
such as a sieve. Another example of a super-absorbent polymer is carboxymethyl
cellulose.
Depending on the sensitivity of the detection system and the concentration of
the
analyte in the fluid sample, concentration of the fluid sample may not be
required.
The concentrating material 45 may be provided without the reagents. For
exainple, concentration may be advantageous but it may be desired to label the
analyte
afterwards. The analyte could be labelled in the assay device, for example. In
some
applications, it is necessary to concentrate the fluid sample prior to
labelling.
The present invention is not intended to be limited in scope by the specific
embodiments described herein. Although the present invention has been
described in
detail for the purpose of illustration, various modifications of the invention
as disclosed,
in addition to those described herein, will become apparent to those of skill
in the art for
the foregoing description. Such modifications are intended to be encompassed
within the
scope of the present claims.
-9-