Note: Descriptions are shown in the official language in which they were submitted.
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
PHARMACEUTICAL PACKAGE FOR SIMULTANEOUSLY MAINTAINING LOW MOISTURE AND LOW
OXYGEN LEVELS
Field Of The Invention
The present invention relates to a device for reducing the oxygen content of
the air
surrounding pharmaceutical dosage forms contained within an oxygen-permeable
bottle,
while also maintaining a relatively low moisture level in said air during the
shelf-life of the
product.
Background Of The Invention
Oxygen induced drug degradation is a factor that can limit the shelf life,
usually as
indicated by the expiration date, of a drug product. In the case of drugs that
are highly oxygen-
sensitive, such degradation may render a drug unmarketable or cause a
candidate to be
excluded from development.
In some cases, oxygen sensitivity occurs only in the presence of certain
excipients.
Since oxidation is often not accelerated by standard Arrhenius-based increased
temperature
studies (known in the art as "accelerated aging studies"), instances can occur
in which the
oxygen sensitivity of the drug is not recognized until drug development has
progressed into late
stages of development. At such later stages of development, reformulation and
addition of
standard antioxidants can require considerably more time and money. In
addition, more clinical
data may be necessary with a new formulation. Thus, a need for reducing or
eliminating
oxygen-based drug instability, without requiring a formulation change, has
existed in the art.
Often in drug development, a need may arise to reduce or prevent oxygen-
induced
degradation of a drug candidate or to provide adequate stability for initial
studies without
investing a lot of resources prior to proof of concept. Once a candidate has
been selected for
further development, oxygen-sensitivity can then be addressed by more
traditional strategies.
In addition to oxygen sensitivity of a pharmaceutically active ingredient in a
dosage form,
the dosage form itself can be sensitive to moisture. This sensitivity can be
due to direct reaction
(e.g., hydrolysis), or to physical effects such as plasticization of drug or
excipients, sticking of
dosage forms together ("twinning"), or to deliquescence (absorption of
atmospheric moisture).
For these reasons, many pharmaceutical dosage forms are packaged with added
desiccants.
The most common pharmaceutically acceptable desiccant is silica, which
controls the relative
humidity (RH) to below 20%.
The use of metal-based oxygen absorbers in the food industry for preservation
of foods
is well known. In such systems a metal in a reduced oxidation state reacts
with oxygen in the
presence of water to form a metal oxide. For example, Mitsubishi Gas
Corporation introduced
iron-plus-carbonate salt sachets under the trade name AgelessTM for use in
stabilizing packaged
foods by preventing oxidation. Other iron and metal-based oxygen absorbers
combined with
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-2-
various salts and other incremental improvements quickly followed suit,
usually with the metal in
the form of a powder or other subdivided form, and with all components of the
absorber being
contained within an oxygen permeable sachet. In a metal oxidation reaction,
water provides the
activation mechanism used in most such oxygen-scavenging applications. Oxygen-
absorbing
sachets are generally stored dry where they can be handled without consuming
oxygen. In the
presence of moist foods, the oxygen-absorber is activated and begins removing
oxygen.
Recently, companies in the food industry have introduced self-activated oxygen
absorbers
to provide oxygen absorption with dry food products. These have involved
combining moisture-
holding additives with the metals (usually iron) in sachets (See, e.g.,
Japanese Publications
SHO56-50618 and SH057-31449; and U.S. Patent No. 5,725,795). European Patent
Application Nos. 864630A1 and 964046A1 describe the use of iron iodide and
bromide to allow
oxygen absorption in a low humidity environment without the need to bring in
water; however,
commercial application of this technology has not been realized.
In the pharmaceutical industry, there have been some limited reports of using
oxygen
absorbers to stabilize drugs. For example, in 1984, tablets of an anti-
inflammatory drug were
stabilized in large glass (i.e., oxygen-impermeable) jars with oxygen
absorbing sachets for six
months at 50 C (Japanese Patent No. SHO59-176247). The source of the oxygen
being
removed was primarily from the headspace and not from ingress, i.e., due to
permeation of
oxygen through the walls of the jar. Similarly, Japanese Patent No. SH096-
253638 describes
cold remedy powders stabilized in impermeable bottles by either nitrogen
purging or with
oxygen absorbers in the bottle. In a 1990 publication, L-cysteine in an
ophthalmic ointment was
stored with an oxygen absorber. (See, i.e., Kyushu Yakugakkai Kaiho, "L-
Cysteine Ophthalmic
Solution Stabilized with Oxygen Absorber," 44, 37-41 (1990).) In 1995, tonic
solutions of
vitamin C were stabilized using a bottle cap having an oxygen absorber covered
with a
polyolefin (Japanese Patent No. SH094-17056). U.S. Patent No. 5,839,593
describes the
incorporation of an oxygen-absorber into the liner of a bottle cap. More
recently, U.S. Patent
Nos. 6,093,572; 6,007,529; and 5,881,534; and PCT publication WO 9737628
describe the use
of oxygen absorbers with parenterals and their particular benefit for
sterilization. Placement of
oxygen-absorbing sachets between an intravenous (IV) bag or blood bag and its
outer
packaging is commonly used in commercial applications. Pre-filled syringes
with absorbers
between the syringes and outer packaging are also known. EP 0 837 o69 Al
discloses the use
of oxygen absorbers to stabilize acarbose in gas-impermeable bottles.
United States Patent 6,688,468B2 and EP 1 243 524 A2 disclose the use of
oxygen
absorbers with pharmaceutical dosage forms in permeable packaging. The oxygen
absorbers
used in these patent applications are largely iron based with added moisture
controlled by salt
slurries. Although these systems perform well for many pharmaceutical
applications, they cause
the humidity in the bottle environment to be at 55 to 75% relative humidity,
since the oxygen
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-3-
consumption reaction requires humidity to operate. Although it is possible to
dry the bottle
environment somewhat using a desiccant, the oxygen absorber will, in general,
also be dried by
the desiccant and be less effective at removing the oxygen permeating through
the bottle walls.
The result is that the oxygen level in the bottle will not remain low enough
to provide for
beneficial stabilization of the pharmaceutically active ingredient over its
entire shelf life.
In all of the aforementioned documents, there is no disclosure or guidance
relating to the
issue of how to use a self-activated oxygen absorber to absorb oxygen in an
oxygen-permeable
pharmaceutical container while simultaneously maintaining the environment in
the container low
in moisture, for example through the use of a dessicant. Doing so would
require the
combination of a self-activated oxygen absorber, which requires water to
function, with a
dessicant which absorbs the water.
Non-iron based oxygen absorbers that do not increase the relative humidity
near the
absorbing unit have been marketed for use with pharmaceuticals under the
registered
trademark PharmaKeep by Mitsubishi Gas Corporation and Sud-Chemie
Corporation. These
absorbers, however, provide only a limited absorption capacity (typically less
than about 40-cc
of oxygen), which is not adequate to provide for protection of pharmaceuticals
in permeable
packages for a typical shelf life of at least two years. Although it is
possible, in theory, to use a
number of such units to provide for adequate oxygen absorption on an ongoing
basis, for
common bottle sizes of 30-250 cc, the sheer number needed to maintain a low
oxygen level
during the shelf life of the pharmaceutical would generally preclude filling
with dosage forms.
For all of the reasons noted above, there remains a need for an oxygen
absorber that is
capable of providing, in a convenient and cost effective manner, adequate
oxygen absorption
capacity to be usable with oxygen permeable pharmaceutical packaging for at
least two years of
shelf life, but which also allows the relative humidity inside the packaging
to be maintained
below 50%, preferably less than 40%, more preferably less than 30%.
Summary Of The Invention
The present invention provides a pharmaceutical package comprising an oxygen
permeable bottle containing therein at least one sub-container containing a
self-activated
oxygen absorber and at least one sub-container containing a desiccant. The sub-
containers
can be separate units or unitary, i.e., fabricated together as separate
compartments within a
single unit, termed herein a "cartridge", containing the self-activated oxygen
absorber in one
compartment and the desiccant in a separate compartment. The invention solves
a problem,
namely that the interior of the bottle is maintained at a low oxygen level to
protect oxygen-
sensitive pharmaceuticals and also at a low moisture level to protect moisture-
sensitive
pharmaceuticals and/or dosage forms. This dual protection occurs even though
the self-
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-4-
activated oxygen absorber requires moisture to function and the sub-container
or compartment
in which it resides is exposed to the interior of the bottle.
In one aspect the invention provides a method of maintaining the oxygen
content of the
air inside a pharmaceutical bottle at a reduced level relative to the oxygen
content of the air
outside the bottle, said bottle being fabricated at least in part of a
pharmaceutically acceptable
oxygen-permeable material, while simultaneously maintaining said inside air at
a relative
humidity of less than 50%, comprising the steps of:
disposing, within said bottle, a first and second sub-container,
said first sub-container containing a desiccant and being adapted to expose
said
desiccant to the interior of said bottle,
said second sub-container containing a self-activated metal-based oxygen-
absorber,
said absorber having sufficient oxygen-reducing capacity to reduce and to
maintain the
oxygen content of said inside air at a level that is less than the oxygen
level of the ambient (i.e.,
outside the bottle) air,
said second sub-container having an orifice that exposes said absorber to the
interior of
said bottle, said orifice having dimensions that allow oxygen scavenging by
said absorber inside
said bottle while simultaneously limiting the diffusion rate of water from
said second sub-
container such that the interior of said bottle is maintained below 50% RH,
preferably below
40% RH, more preferably below 30% RH.
In a second aspect the invention provides a pharmaceutical package comprising
a bottle
that maintains the oxygen content of the air within its interior volume at a
reduced level relative
to the oxygen content of the ambient air, comprising:
A) said bottle, which is fabricated at least in part of an oxygen permeable
material,
B) a desiccant disposed within a first sub-container disposed inside said
bottle,
said first sub-container being adapted to expose said dessicant to the
interior of
said bottle,
C) a self-activated metal-based oxygen-absorber disposed within a second sub-
container disposed inside said bottle,
said absorber having sufficient oxygen-scavenging capacity to reduce and to
maintain the interior of said bottle at an oxygen level less than the oxygen
level of the ambient
air,
said second sub-container having an orifice that exposes said absorber to the
interior of said bottle, said orifice having dimensions that allow oxygen
scavenging while limiting
the diffusion rate of water from said second sub-container such that the
interior of said bottle is
maintained below 50% RH, preferably below 40% RH, more preferably below 30%
RH.
In most embodiments the bottle is closed, and preferably sealed, although it
is possible
to implement the invention in the absence of a seal.
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-5-
The term "bottle" is intended to be general, and to include any type or shape
of
pharmaceutical container that is fabricated at least in part from an oxygen-
permeable material.
A "pharmaceutical bottle" is one wherein the oxygen-permeable material from
which it is
fabricated is pharmaceutically acceptable. Thus "bottle" includes traditional
square or round
plastic bottles, jars, bags, pouches, or other pharmaceutically-acceptable
containers.
"Relative humidity", sometimes abbreviated herein as "RH", has its usual
meaning, i.e.,
the ratio of the actual humidity over the saturated humidity at the same
temperature.
The "package" disclosed herein refers to the combination of a pharmaceutical
bottle
having disposed therein a self-activated oxygen absorber and a desiccant, each
contained in its
own sub-container, the bottle being intended to be filled by a (usually pre-
determined) number
of solid pharmaceutical dosage forms, typically tablets or capsules. The
"inside" or "interior" of
the bottle refers to'the free, i.e., unoccupied volume of the bottle once
filled and containing the
first and second sub-containers described in (B) and (C) above, or additional
sub-containers or
cartridges, as described below. The free volume, also referred to in the art
as "headspace", of
such filled bottles is generally between 10 and 100 cc. The amount of
headspace is not critical
since more than one oxygen-absorbing sub-container can be added to the bottle.
Generally,
given the typical size of a pharmaceutical bottle and the rate at which oxygen
permeates known
oxygen-permeable plastics used to fabricate pharmaceutical bottles, the oxygen-
absorbing sub-
container is implemented to have a hole (uncovered) that is 100- 700 microns
in diameter,
preferably 200-600 microns. The hole will generally be round since it can be
implemented with
a drill, although shape is not critical and other shapes having an equivalent
area can also be
used. In an alternate embodiment a larger hole can be implemented and covered
with a
microporous material having a porosity generally between 0.05 and 0.2, and a
thickness
between 0.5 and 2.5 mm. Suitable membranes are widely commercially available,
for example
from General Electric Osmonics (a division of GE Water Technologies, Trevose,
PA) and from
Millipore Corporation, (Billerica, MA). The total amount of pore area, defined
as the porosity
times the area, should be equivalent to the area of a hole having dimensions
as described
above.
An oxygen-permeable bottle generally refers to one made of a material that,
when
sealed or closed, will admit sufficient oxygen to cause oxidative degradation
of the contained
active pharmaceutical ingredient over a reasonable shelf life, a "reasonable
shelf life" usually
being between six months and three years, typically two years. Such materials
include any of
the pharmaceutically acceptable available plastics commonly used in the
industry and further
discussed and identified below. As stated above, the bottle is one that, as
part of the
manufacturing operation, is closed and preferably sealed once it has been
filled with
pharmaceutical dosage forms and the at least two sub-containers (B) and (C)
described above.
Any oxygen-permeable bottle that allows for oxidative degradation of more than
0.2% of the
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-6-
contained active pharmaceutical ingredient or compound during its reasonable
shelf life can
benefit from this invention. Bottle shape is not critical.
The term "self-activated oxygen absorber" refers to a metal-based substance
that
removes oxygen by reacting with it to chemically bind it, generally by forming
a metal oxide.
The term "activated" means that the metal-based substance requires the
presence of water (i.e.,
as a reactant) to drive the metallic oxide-forming reaction. The oxygen
absorbers useful in the
present invention are "self-activated", meaning that they are sold as a unit
that contains the
water needed to enable the oxide-formation, the water usually being present in
the form of a
humidity controlling substance, typically an aqueous slurry of a salt or a
sugar, such
compositions being designed to maintain a specific humidity in a closed
environment. The
preferred metal is elemental iron, powdered to increase its surface area.
Other metals that are
useful, although less preferred, include nickel, tin, copper and zinc.
The oxygen absorber reduces the oxygen content of the air within the bottle,
once the
bottle has been closed or sealed, to a level that is below the oxygen level of
the surrounding air
outside the bottle, for example the ambient air in a warehouse or shipping
hold, or other storage
environment or transportation means. Thereafter, the absorber maintains the
oxygen in the
headspace air at a level preferably below 10.0% (i.e., by volume, based on the
headspace
volume) preferably below 3.0 %, more preferably below 1.0%, most preferably
below 0.5%.
Sub-containers (B) and (C) can be implemented as physically separate
containers that
are added to the bottle separately in the manufacturing process. In a
preferred embodiment,
and as disclosed further below, sub-containers (B) and (C) are formed as
physically separate
compartments of a single unit, referred to herein as a "cartridge". When
discussing such a
cartridge, the compartments therein are designated as (B), (C), etc to have a
meaning
corresponding to the letter designations given above for sub-containers (B),
(C), and so forth.
The cartridge can advantageously be fabricated out of a plastic (including the
oxygen-
permeable ones disclosed herein) by a suitable molding operation.
A further preferred embodiment, illustrated below, relates to the inclusion,
in said bottle,
of a third sub-container or cartridge compartment (D) adapted to contain a
separate quantity of
self-activated metal-based oxygen absorber from that in sub-container or
canister compartment
(C). This third sub-container or compartment functions to rapidly reduce or
remove the oxygen
initially contained in the bottle headspace once the bottle has been closed or
sealed for storage,
transport, and/or sale. Preferably, (D) is engineered as a third compartment
in a cartridge also
containing, as individual compartments therein, (B) and (C). Because this
third sub-container or
compartment is designed to remove the oxygen initially present in the
headspace, it preferably
contains only enough metal and water to react approximately stoichiometrically
with the oxygen
initially present in the headspace once the bottle has been closed or sealed.
To facilitate
oxygen removal from the headspace, the third sub-container or compartment has
an orifice,
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-7-
preferably in the form of a porous membrane having a permeability such that
the flux of oxygen
allows the entire head space to be scavenged to below 3% (VN) of oxygen in
less than 3 days,
preferably to below 2% in 3 days relative to the orifice provided in the sub-
container or
compartment (C) that contains the self-activated oxygen-absorber. Providing a
much larger
pore area in sub-container or compartment (D) enables it to effect oxygen
removal rapidly and,
therefore, to quickly implement a relatively oxygen-free environment once the
bottle has been
closed or sealed. Thereafter, sub-container or compartment (C) maintains the
oxygen at a
relatively low level.
Hereinafter the invention will be described by reference to cartridges and the
compartments therein, it being understood that this is for convenience and
ease of description,
and that the cartridges and compartments described hereinafter can also be
implemented
equivalently as separate sub-containers.
The access opening to compartment (B) which contains the desiccant is also
relatively
much larger, hence more open to the bottle interior, than the access to the
bottle interior
provided by the orifice in compartment (C). The opening that exposes the
desiccant in
compartment (B) to the headspace is preferably in the form of a membrane,
having a large pore
area, to avoid spillage of the desiccant from compartment (B). Alternatively,
a plurality of small
orifices, such as drilled holes, can be separately implemented in lieu of a
membrane. By
making the total orifice surface area in compartment (B) relatively much
larger than the orifice in
canister compartment (C), the interior of the bottle is maintained relatively
dry, much drier than
the interior of the (water-containing) oxygen-absorbing compartment (C). This
area, however,
may be restricted to provide a relative humidity in bottle A at a somewhat
higher value than is
typically maintained with desiccants. This serves to minimize moisture loss
from compartment
(C). The total area of the orifice area in sub-container (B), whether the area
is in the form of
holes or a porous membrane, is typically at least 0.3 cm2, preferably between
0.3 cm2 and 0.4
cm2.
Brief Description Of The Drawings
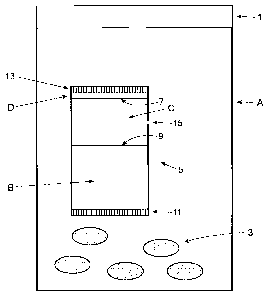
Figure 1 is a front view of a bottle having a cartridge disposed therein in a
preferred
embodiment according to the invention.
Figure 2 is a graph illustrating the rate of water removal by the desiccant as
a function of
orifice size in compartment (B).
Figure 3 is a graph illustrating the rate of oxygen consumption by an iron-
based self-
activated oxygen absorber in compartment (C) as a function of orifice size.
Detailed Description
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-8-
Figure 1 illustrates a preferred embodiment of the present invention designed
to provide
oxygen absorption with low moisture for an extended period in a packaged
product. In Figure 1,
"A" represents a pharmaceutical bottle, which is generally fabricated, in
whole or in part, of an
oxygen-permeable plastic. Bottle A is preferably sealed, most preferably with
a heat induction
seal (HIS) 1 made of a metal foil and an adhesive that effects bonding of the
bottle to the foil.
Disposed within pharmaceutical bottle A are pharmaceutical dosage forms 3,
preferably tablets,
capsules or the like. Also disposed within pharmaceutical bottle A is a
cartridge, designated
generally as 5 and comprised, for the sake of illustration only, of three
separate compartments
B, C, and D, separated from each other by dividers 7 and 9, which are walls
preferably
fabricated from the same material as the rest of canister 5 and manufactured
integrally
therewith, for example as part of a molding process. Compartment B contains a
desiccant (not
shown) such as silica gel and is exposed to the bottle interior by means of
porous membrane
11, thereby allowing relatively free exchange between compartment (B) and the
bottle
headspace, whereby moist air inside bottle A enters and dry air leaves the
compartment. A
second compartment D contains a self-activated oxygen absorber present in
sufficient quantity
to remove the initial head-space oxygen in bottle A. Compartment D contains
porous
membrane 13 which allows for relatively free access by compartment D to the
oxygen-
containing air in the headspace of bottle A, thereby effecting oxygen
scavenging. A third
compartment C contains sufficient self-activated iron absorber (i.e., metal
and moisture) to
scavenge oxygen permeating through the bottle walls during the shelf-life of
the product.
Compartment C contains an orifice 15 that can be implemented in the form of a
hole, tube or
microporous filter. The cross sectional area of the orifice is such that it
effects a rate of oxygen
scavenging sufficient to match the ingress rate of oxygen into bottle A, yet
the area is such that
the orifice limits the rate of moisture loss from compartment C so that there
is adequate
moisture in the compartment (i.e., to enable metallic oxide formation) during
the entire shelf-life
of the pharmaceutically active ingredient. Some moisture does escape from
compartment (C),
but the rate is small relative to the moisture-absorbing capacity of the
desiccant in subunit "D".
This is a critical feature of the invention, i.e., the cross-sectional area of
the orifice (or orifices) in
compartment (C). On one hand, the area is large enough to effect efficient
oxygen scavenging
from the interior of the bottle during the shelf life of the product, thereby
eliminating or reducing
oxidative degradation of the pharmaceutical product. On the other hand, the
area is small
enough to limit the amount of moisture that escapes compartment (C) to no more
than that that
can be removed by the desiccant during the shelf life.
As stated previously, if the orifice is implemented as a circular hole, it
should have a
diameter of from 100 to 700 microns, corresponding to a cross sectional area
of from about 0.8
x 10-4 to about 38 x 10-4 cm2. Orifice shape is not critical, and other shapes
having equivalent
cross-sectional areas can be implemented equally effectively.
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-9-
Figure 2 shows the (experimentally-determined) variation in the rate of
moisture
scavenging by the desiccant silica gel as a function of orifice diameter
(e.g., in compartment (C))
through a barrier having a single tube therein of varying diameter. The data
points in the graph
were measured while maintaining the external environment at 40 C and 75%
relative humidity
(RH). The graph demonstrates that the rate of moisture transfer out of a
cartridge with a high
humidity compartment (C) can be controlled in a predictable manner by picking
a suitable size
for the orifice.
Figure 3 shows the rate of oxygen scavenging by iron through a barrier having
a single
tube implemented therein, as a function of orifice (i.e., tube) cross
sectional area. The data
demonstrate that the rate of oxygen scavenging, i.e. by compartment (C) can be
controlled in a
predictable manner depending on orifice size.
In Figure 1, pharmaceutical container A is a bottle or other container for
dispensing
pharmaceutical dosage forms. The bottle is designed to protect a dosage form
from mechanical
harm and to limit exposure of the dosage forms contained therein to light and
contaminants in
the environment. Glass bottles can in some cases function effectively due to
the low
(essentially no) permeability of glass to oxygen and moisture, and are within
the scope of the
present invention. However, due to the risk of breakage and the added expense
of working with
glass, bottles are preferably made, usually entirely, of plastic, essentially
all of such plastics
being oxygen-permeable in varying degrees. Suitable plastics for use in
fabricating
pharmaceutical bottles generally involve such plastics as low density
polyethylene (LDPE), high
density polyethylene (HDPE), polypropylene (PP), polystyrene (PS) and
polycarbonate (PC).
The oxygen permeability of these materials ranges from 3500 cc mil/(m2 day
atm) for PS to 9500
cc mil/(mZday atm) for LDPE. Other suitable packaging materials include
polyesters (PET,
PEN), nylon, polyvinyl chloride (PVC), poly(vinylidine chloride) (PVDC),
poly(tetrafluoroethylene), etc., and laminates containing layers of one or
more such materials.
The present invention provides, in a preferred embodiment, for a cartridge
that can be added to
a pharmaceutical bottle and that provides for a significant reduction in the
oxygen and moisture
levels, including such a reduction at the permeation rates disclosed above.
Once an oxygen permeable bottle is filled with a pre-determined amount of
dosage
forms containing an oxygen-sensitive drug and a cartridge according to the
invention, the botle
is then closed, as by capping with a twist-on cap, or stoppering, or sealing.
If the bottle is
sealed, a preferred seal is a heat-induction seal (HIS). Other useful seals
include adhesives
such as pressure sensitive adhesives, thermal adhesives, photocured adhesives,
and binary
mixture adhesives such as epoxy resins. Adhesion can also be effected by such
techniques as
ultrasonic welding which do not require adhesives. A packing material (e.g.,
cotton) may be
optionally added to the bottle prior to sealing to prevent any damage to the
contents such as
chipping or cracking of the unit dosage forms. HIS is commonly used in the
pharmaceutical
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-10-
industry to seal plastic bottle tops, both as a means of protecting the dosage
form from the
environment and as a means of preventing (and making obvious) any tampering.
The induction
seal and the bottle are preferably matched to achieve an acceptable seal.
Procedures for
induction sealing are well known to those skilled in the art. For a detailed
description see
"Induction Sealing Guidelines", R. M. Cain (Kerr Group, Inc.), 1995 and W. F.
Zito "Unraveling
the Myths and Mysteries of Induction Sealing", J. Packaging Tech., 1990.
Any pharmaceutical dosage form 3 containing an oxygen-sensitive pharmaceutical
compound susceptible to degradation as a result of exposure to oxygen may be
disposed within
pharmaceutical bottle A. Examples of oxygen-sensitive materials that are
subject to
degradation due to oxygen exposure include materials such as amines either as
salts or as free
bases, sulfides, allylic alcohols, phenols, alcohols, aldehydes and the like.
In addition, some
basic pharmaceutically active materials or compounds, especially amines, with
pKa values in the
range from about 1 to about 10, more particularly in the range from about 5 to
about 9, are often
subject to oxygen degradation and may accordingly benefit from the present
invention, as well
as some pharmaceutically active materials or compounds having redox potentials
less than or
equal to about 1300 mV vs. Ag/Ag+, more preferably less than or equal to about
1000 mV vs.
Ag/Ag+. Suitable pharmaceutically active compounds include compounds such as
atorvastatin
(especially when used in an amorphous form), pseudoephedrine, tiagabine,
acitretin,
rescinnamine, lovastatin, tretinoin, isotretinoin, simvastatin, ivermectin,
verapamil, oxybutynin,
hydroxyurea, selegiline, esterified estrogens, tranylcypromine, carbamazepine,
ticlopidine,
methyldopahydro, chlorothiazide, methyldopa, naproxen, acetominophen,
erythromycin,
bupropion, rifapentine, penicillamine, mexiletine, verapamil, diltiazem,
ibuprofen, cyclosporine,
saquinavir, morphine, sertraline, cetirizine, N-[[2-methoxy-5-(1-
methyl)phenyl]methyl]-2-
(diphenylmethyl)-1-azabicyclo[2.2.2]octan-3-amine and the like. The invention
is particularly
suitable for stabilizing high-energy drug forms to oxidation. Examples of high-
energy drug forms
include amorphous forms and small particle sized drug forms. A preferred
example of a high-
energy form of a drug is prepared by spray-drying a drug as a dispersion in
combination with an
enteric polymer as described in EP 1027886A2 and EP 901786A2, each
incorporated herein by
reference. Suitable enteric polymers include those described in Patent
application Nos. WO
0147495 Al, EP 1027886 A2, EP 1027885 A2, and U.S. Pub. No.2002/0009494 Al,
incorporated herein by reference.
The present invention can additionally stabilize excipients in the dosage form
to oxidative
degradation. For example, degradation that leads to discoloration, harmful
reactivity with the
active component of the drug or changes in the dosage form performance, such
as dissolution
or disintegration rates. Nonexclusive examples of excipients commonly used in
pharmaceutical
formulations that could be stabilized by application of the present invention
include
poly(ethylene oxides), poly(ethylene glycols) and poly(oxyethylene) alkyl
ethers.
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-11-
The present invention provides a reduction in the degree of oxidative
degradation or
discoloration where such degradation or discoloration can be measured by light
absorption or
reflection spectroscopy and/or chromatographic analysis, in particular, HPLC
analysis. The
invention need not totally eliminate such degradation; however, practice of
the present invention
preferably reduces the degradation by at least about 20%, more preferably by
about 50% and
most preferably by about 75% when compared to samples stored in the absence of
the
cartridge/oxygen absorber as disclosed herein.
Although the shape of the cartridge is not critical, the cartridges described
herein can be
generally tubular in shape to facilitate high speed bottle insertion. The
cartridge can be in the
form of a canister, i.e., a tubular container with the desired number of
compartments and having
either or both end compartments openable to facilitate filling. The ends of
the tube can be flat or
circular, convex or concave, as desired. The cartridge can be fabricated as
known in the art by
using a suitable mold and molding process, typically injection molding with a
thermoformable
polymer.
The desiccant (sub-container or compartment B) provides for a low relative
humidity in
the pharmaceutical package. The desiccant for use in the practice of the
invention can be any
available desiccant. Preferred desiccants include those commonly used in the
pharmaceutical
industry that have adequate capacity to handle the combination of moisture
ingress into the
bottle and moisture given off by the self-activated oxygen absorber. Suitable
desiccants are
discussed in R. L. Dobson, J. Packaging Technol., 1, 127-131 (1987). A
preferred desiccant is
silica gel. The desiccant can be supplied in the form of a sachet, cartridge
or canister.
It is desirable to maintain the relative humidity in the bottle A at a level
that, while still
providing protection of the dosage forms from the adverse effects of humidity,
minimizes the
loss of moisture from compartment C. To this effect, the barrier 11 can be
made to limit the
moisture transfer rate. This rate limitation can be effected using a membrane
of somewhat
limited moisture permeation (by virtue of material or thickness) or by
suitable choice of a
material having an appropriate permeability. This material and surface area
selection can be
made based on experiments and depends on the particular moisture sensitivity
of the dosage
form used. In general, it is desirable that the permeability of barrier 11 to
moisture be such that
the relative humidity in the bottle A is maintained at or below 40% RH, more
preferably, below
30% RH, under, as a reference, storage conditions of 30 C, and 75% RH).
The amount of desiccant used is preferably sufficient to handle moisture
ingress through
the pharmaceutical bottle walls during the storage duration, which depends on
the humidity of
the external environment. For conditions of 30 C and 60% RH, the rate of water
permeation into
a 60-cc HDPE bottle with an internal humidity kept below 40% RH can be
estimated at about
0.25 mg/day (91 mg/yr). In addition, there is preferably enough desiccant to
handle moisture
loss from the oxygen absorber (estimated at about 146 mg/yr, as discussed
below). Silica gel
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-12-
has an approximate capacity to maintain a relative humidity below 40% at about
0.5 mg H20/mg
silica. Thus an amount of silica gel to place in sub-container or compartment
B is between 475
and 1100 mg, an amount that will absorb both moisture from external permeation
and moisture
escaping internally from absorber compartment (C), based on the orifice size
in the
compartment, during a reasonable shelf life. It will be recognized by those
skilled in the art that
similar calculations can be made for different bottle materials having
different rates of water
permeation, headspace volumes, and different conditions of temperature and
relative humidity.
The cartridge compartment is constructed such that it physically separates the
desiccant from
direct contact with the pharmaceutical ingredients, yet allows the moisture
from inside the
pharmaceutical package to be scavenged.
Compartment D of the cartridge contains a self-activated oxygen absorber
capable of
rapidly removing oxygen from the headspace in pharmaceutical bottle A. This
absorber is
preferably an iron-based absorber and can be the same material used as the
self-activated
oxygen absorber disposed within absorber compartment (C). To enable the metal
to scavenge
oxygen, a moisture source must be provided. In the present invention, and as
commercially
available, this moisture source is preferably provided in the form of a salt
or sugar slurry.
Because compartment D is designed to rapidly remove the oxygen from the
headspace inside
bottle A, it only needs to function for a few days, and thus requires only a
relatively small amount
of absorber. The absorber in compartment (D) can therefore be at a high
relative humidity,
though it will rapidly deplete its moisture as the desiccant in compartment B
competes for the
moisture. Preferably, the humidity source in compartment B maintains a
relative humidity (RH)
above about 50%; more preferably above 60%; still more preferably, above 65%.
Preferred
moisture sources are salts or salt mixtures. Particularly preferred salts are
sodium chloride,
potassium chloride and potassium sulfate. Compartment D is constructed such
that it physically
separates the self-activated oxygen absorber from direct contact with the
pharmaceutical
dosage forms, yet allows oxygen from inside the pharmaceutical package to be
scavenged.
Preferred compartments (D) contain sachets wherein the containment sack is
fabricated of
porous (e.g., woven) material. Alternatively the cartridge compartment (D) can
itself be porous,
as by having an open section covered with a porous fabric or membrane.
The amount of headspace oxygen in bottle A can be determined by measuring the
volume of the bottle, subtracting the volume of the dosage forms and dividing
the remaining
volume by five (to account for the oxygen abundance). For example, in an
approximately 60-cc
bottle that is half filled with dosage forms, the headspace volume will be
about 6-cc of oxygen.
The amount of iron used to remove the oxygen, and excluding any oxygen due to
ingress,
should be at least stoichiometrically sufficient. Since the oxygen-absorbing
capacity of the iron
is about 300-cc/g, the minimum amount of iron needed for removal of the
headspace oxygen of
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-13-
a 60-cc bottle is 20 mg. Therefore, the amount of iron for this subunit is
preferably between 20
and 100 mg.
Compartment C contains sufficient oxygen absorber to enable the oxygen level
in the
pharmaceutical bottle to remain low during the product's reasonable shelf life
by balancing the
oxygen ingress rate into the bottle with a comparable rate of oxygen
scavenging. At the same
time, the rate of loss of moisture from compartment C is sufficiently low that
the overall moisture
level in bottle A remains low due to the desiccant and there remains enough
water in the subunit
to provide for the needed relative humidity in the subunit for iron activity
during the duration of
the shelf-life. It has been determined that these contradictory and opposing
goals can be met
with an oxygen absorber and a moisture controlling element encased in a low
permeability
cartridge compartment in combination with a rate-controlling port (15 in
Figure 1).
Preferentially, the cartridge is made from a plastic or metal material
considered safe for contact
with pharmaceutical ingredients. Examples of preferred materials include
plastics such as
polyethylene (PE), polystyrene (PS) and polyvinylchloride (PVC). Although the
cartridge can be
made out of permeable plastics, the actual amount of oxygen and moisture that
transfers
through these materials (as opposed to the holes or membranes) is a low due to
the low surface
area and does not significantly impact the oxygen and moisture levels in
bottle A. The rate-
controlling port has the property of restricting moisture transfer while
allowing sufficient oxygen
transfer.
Figure 2 shows the rate of moisture transfer from a test environment
controlled to be at
40 C and 75% RH into a sub-container having a fixed amount of silica gel, the
sub-container
having a single orifice, implemented therein as a tube, as the only entrance
for moisture from
the environment. The amount of water entering through the tube was monitored
as a function of
time and the diameter of the tube. These data provide the basis for
calculating the moisture
transfer rate from the self-activated oxygen absorber compartment (C) to the
bottle A
headspace. Once this transfer rate has been calculated, it can in turn be used
to calculate the
amount of desiccant needed for compartment (B).
As an example, to function effectively in pharmaceutical applications, the
relative
humidity in bottle A is preferably maintained at below about 50%RH, more
preferably below 30%
RH, while the RH in compartment (C) is preferably 40-70%, more preferably 50-
60%.
Therefore, the rate of moisture transfer from the test system (75%RH to 10%RH)
can be
corrected to take into account the relative humidities in the product as
envisioned (60%RH to
30%RH) by dividing the value by
(75 - 15)/(60 - 30) = 2
wherein
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-14-
75 is the RH (in %) of the test environment
15 is the RH maintained by the desiccant
60 is the minimum desired RH for the oxygen absorber to function
30 is the desired RH of the headspace.
Figure 3 shows the rate of oxygen transfer through similar tubes into an iron
oxygen
absorber. In this case, the oxygen depletion of a fixed oxygen volume was used
for the
measurement. Again the oxygen transfer rate was monitored as a function of the
size of the
tube. The desired rate of oxygen scavenging and, therefore, the diameter of a
hole or tube
needs to take into account the fact that the oxygen scavenger will need to
handle the oxygen
permeation into the pharmaceutical package and to maintain a low oxygen level
(e.g., 1%). The
tube (or other orifice) must accordingly have a hole large enough to
compensate for the
incremental difference in the rate of oxygen diffusion as the difference in
pressure is increased
from that used in the test. Since this latter rate should be proportional to
the difference in
pressure, on going from 0 to 20.8% oxygen of the test to 0 to 1% oxygen in the
final product, the
corresponding oxygen rate found from the graph in Figure 3 should be
multiplied by 20.8.
To determine the amount of oxygen entering the pharmaceutical package, a round
bottle
made of high-density polyethylene (HDPE) with a labeled capacity of 60 cm3 and
a wall
thickness of 37 mils (0.94 mm) can be used as a representative sample. If the
bottle is 4 cm in
diameter and 7.3 cm in height (in reality the bottle will taper to give less
surface area than this
approximation), then the surface area will be approximately 100 cm2. If one
uses HDPE as the
bottle material and assumes the inside of the pharmaceutical package to be
maintained at 1 /o
oxygen, then the rate of oxygen permeation into the bottle can be calculated
as follows:
4000 cm3 mil/(m2 d atm) X (0. 18-0.009) atm X 0.01 m2/37 mil = 0.18 cm3 of
02/day
Using this value and the factor of 20.8 discussed above, it can be determined
that the hole size
to meet the oxygen demand for a 60-cm3 HDPE bottle (to bring the oxygen to 1%)
is about 500
m in diameter.
At this diameter, the amount of water loss can be estimated from the graph of
Figure 2 at
0.87 mg/day. Correcting this for the envisioned system as described above
brings this value to
0.43 mg/day.
Thus the use of data such as that exemplified in Figures 2 and 3 will be
appreciated by
those skilled in the art. Using Figure 3, one can calculate the size of the
orifice in (oxygen
absorber) compartment (C) to effectively scavenge oxygen. Figure 2 can be used
to determine
the corresponding moisture loss from the compartment and the amount of
desiccant needed.
The orifice in compartment (C) that controls the rate of moisture and oxygen
transfer ("15"
in Figure 1) can be produced in the following manners :
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-15-
(1) A single hole can be used as the orifice. The hole preferably has a
diameter of between
100 and 700,um; more preferably, between 200 and 600 m. The hole can be
cylindrical
(round with parallel sides), conical (round with sloping sides) or
rectangular. The hole
can be made by any technique known in the art. Particularly preferred methods
of
forming the hole include drilling through the cartridge wall using a
mechanical, ultrasonic
or laser drill, or forming the compartment hole in place by, for example,
injection molding.
A high porosity material or mesh can be used in conjunction with this hole to
prevent
powder from escaping from the cartridge of canister. The diameter of a mesh
should be
smaller than the fine particles in the subunit, preferably smaller than about
15 m.
(2) A tube is placed through the port area. The tube preferably has an
internal diameter of
between 100 and 700,um; more preferably, between 200 and 600 m. The tube is
preferably sealed into the cartridge in the port area using an adhesive or by
melting the
adjacent wall. The tube length can range from 1 to 25 mm. A high porosity
material or
mesh can be used in conjunction with this tube to prevent powder from escaping
from
the cartridge of canister. The diameter of a mesh should be smaller than the
fine
particles in the subunit, preferably smaller than about 15 m. -
(3) A microporous membrane is placed in the port area. This filter restricts
the moisture and
oxygen diffusion. Preferably, the microporous membrane has a porosity of
between 0.05
and 0.20 and a thickness of 0.5 to 2.5 mm. The preferred diameter of the
membrane is
between 100 and 1000 /im.
The active oxygen absorber in compartment (C) is preferably iron. The iron is
preferably in
its reduced form (that is, Fe ). The iron can be atomized, milled, pulverized,
electrolyzed or
otherwise treated to form a fine powder as is known in the art. The amount of
iron used in the
present invention can be optimized based on the permeability of the
pharmaceutical packaging
"A" and the storage duration. Using the round HDPE bottle described above as a
representative
example, the amount of oxygen that needs to be scavenged is about 66 cm3/yr.
Based on an
oxygen-absorption capacity for iron of about 300 cm3/g, the amount of iron
needed in
compartment (C) (Figure 1) is about 220 mg. To build in for losses, the
subunit therefore
preferably contains between about 225 and 500 mg of iron.
To enable the iron to scavenge oxygen, a moisture source must be provided. In
the
present invention, this moisture source is preferably provided in the form of
a salt or sugar
slurry. The moisture controlling material should be able to control the
moisture in the
compartment of the oxygen absorber. Since the rate of loss of moisture from
compartment (C)
is proportional to the difference in relative humidity between that in
compartment (C) and in the
pharmaceutical bottle headspace itself, it is desirable to make the relative
humidity in
compartment (C) as low as possible while still providing adequate moisture to
enable oxygen-
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-16-
scavenging activity. It is therefore preferred to control the humidity in
compartment (C) to
between 40 and 70% RH; more preferably, between 50 and 60% RH. The humidity-
controlling
salt or sugar slurry can be an inorganic or organic salt or salt mixture, a
sugar or sugar mixture,
or a mixture of salts and sugars, provided such materials can control the
relative humidity to the
desired range. Particularly preferred materials for controlling said relative
humidity include
sodium chloride, calcium nitrate, sodium bisulfate, sodium chlorate, potassium
iodide, sodium
bromide, magnesium acetate, sodium nitrate, ammonium chloride, potassium
nitrate, potassium
bromide and magnesium nitrate. The amount of salt or sugar used needs to be
sufficient to
provide for the desired control of the relative humidity even as some of the
water is removed in
the use of the present invention.
For the slurry to control the relative humidity in compartment (C) during the
shelf life of
the product, there must be sufficient water to handle the anticipated water
loss. Based on the
size of the rate-controlling port "I" (Figure 1), this rate can be kept to
about 157 mg per year. In
the practice of the present invention, the amount of water in the cartridge is
therefore preferably
between 150 and 400 mg; more preferably, between 180 and 360 mg.
To control the relative humidity with this much water, the amount of the salt
or sugar
used must be sufficient that at least some of the solid remains undissolved.
As such, one can
determine the amount of salt or sugar by multiplying the amount of water added
by the water
solubility of the salt or sugar. As an example, for the humidity controlling
additive magnesium
nitrate, this leads to a preferred amount of this additive of between 225 and
450 mg, based on a
solubility of 1250 mg/mL.
The present invention provides for the removal of oxygen not only from the
entrapped air
within the pharmaceutical bottle (Figure 1) but also oxygen that enters the
bottle via ingress. It
will be appreciated that in the use of the oxygen-absorbing cartridge, one can
engineer a unit
having the appropriate absorption capacity for the given bottle and desired
shelf-life. It is also
possible to engineer an oxygen-absorbing unit that is standard, but for which
the number of
such units actually applied will depend on the bottle design and shelf-life.
The oxygen-absorber need not remove 100% of the oxygen from the interior air
in the
bottle; however, it is preferred that the absorber be present in an amount
such that it is capable
of maintaining a level of oxygen less than or equal to about 10.0% preferably
less than or equal
to about 3.0%, more preferably less than or equal to about 1.0%, most
preferably less than or
equal to about 0.5%, for about 2 years inside the oxygen permeable bottle.
EXAMPLES
EXAMPLE 1
A 20 mm Flurotec Teflon stopper (West Pharmaceutical Services, Jersey Shore,
PA)
was drilled through the center with a 1.0 mm drill bit. HPLC tubing (Upchurch
Scientific, Oak
CA 02574102 2007-01-15
WO 2006/008651 PCT/IB2005/002446
-17-
Harbor, WA) was cut to 2 cm in length and forced through the drilled hole in
the stopper. The
tubing used included model 1520 (762,um inner diameter), model 1532 (508,um
inner diameter),
model 1531 B (254 pm inner diameter), model 1535 (127 ,um inner diameter) and
model 1560
(64 ym inner diameter), all from Upchurch Scientific (a division of Scivex
Inc., Oak Harbor, WA).
For the measurement of water vapor uptake, a 1 gram Sorb-It canister (Sud-
Chemie,
Belen, NM) was cut open and the silica gel contents poured into a 10-cc
tubular flint Type I glass
vial (Wheaton Science Products, Syracuse, NE). The stopper with tubing was
crimp-sealed to
the vial and the initial weight recorded. The test units were then placed in a
40 C/75% RH
stability chamber and periodically weighed over two weeks.
For the measurement of oxygen consumption, the contents of an oxygen scavenger
(DSR#4062B, 200-cc oxygen absorber from Multisorb Corp., Buffalo, NY) was cut
open and the
contents poured into a vial (same as above), and the stopper with tubing was
crimp-sealed to
the vial within 3 minutes. Each test unit was placed in a 250-cc HDPE bottle,
which was then
heat induction sealed under ambient air conditions. Thus each system initially
contained
approximately 21 % oxygen. The bottles were stored at ambient temperature and
RH
(approximately 20 C and 30% RH). At the end of two weeks, the oxygen level
inside the HDPE
bottle was measured using a Mocon PAC Check 450 (Mocon Inc., Minneapolis, MN),
which was
standardized with ambient air (21% oxygen) and a 0.5% oxygen standard from
Mocon Inc.
EXAMPLE 2
A cartridge is made by injection molding polyethylene into two compartments
with
cylindrical shape of diameter 0.5 inches (1.3 cm) and wall diameters of
approximately 1 mm.
The top compartment has a single 600 m diameter hole with a lattice (diameter
of openings of
m) on the side as part of the mold. The bottom compartment is 0.25 inches in
height (0.63
25 cm). The top compartment is 0.5 inches in height (1.3 cm). Into the bottom
compartment is filled
0.5 g of silica gel. A cap of sintered polyethylene (porosity of 0.1) is
adhered to the bottom
compartment to seal in the powder. Magnesium nitrate (1.0 kg) is slurried with
800 g of water to
give a 44% (w:w) slurry. The top compartment is filled with a combination of
300 mg of fine iron
powder (as described in U. S. Patent No. 5,725,795) and 450 mg of the
magnesium nitrate
slurry. A cap is formed by injection molding polyethylene into a cylinder with
a wall and a top
having a high porosity (0.4). The cap is 0.55 inches in diameter (1.4 cm) and
0.2 inches in
height (0.5 cm). The cap compartment is filled with 50 mg of self-activated
iron oxygen absorber
(available from Multisorb Corp., Buffalo, NY), then the porous top is adhered
to it. This entire
cap is then adhered to the top compartment of the above cylinder. A 60 cm3
polyethylene bottle
is loaded with pharmaceutically active tablets and one of the above
cartridges. The bottle is
sealed using a heat induction seal.